Note: Descriptions are shown in the official language in which they were submitted.
... . . ... .. ........ . .. . .... ... ... . i. .. . . ... ... ... .. .. . .
. .
CA 02639310 2008-09-04
COMPOSITE SEAL AND METHOD FOR MANUFACTURING
BACKGROUND
TECHNICAL FIELD
The present disclosure relates to surgical devices and, more particularly,
relates to a seal assembly for use with a surgical access device during a
minimally
invasive surgical procedure, for example, in both laparoscopic and endoscopic
procedures.
DESCRIPTION OF RELATED ART
Minimally invasive surgical procedures avoid open invasive surgery in
favor of closed or local surgery with less trauma. These procedures involve
use of
laparoscopic devices and remote-control manipulation of instruments with
indirect
observation of the surgical field through an endoscope or similar device, and
are carried
out through the skin or through a body cavity or anatomical opening.
Laparoscopic and
endoscopic procedures generally require that any instrumentation inserted into
the body
be sealed, i.e. provisions must be made to ensure that gases do not enter or
exit the body
through the incision as, for example, in surgical procedures in which the
surgical region
,
CA 02639310 2008-09-04
is insufflated. These procedures typically employ surgical instruments which
are
introduced into the body through a cannula. The cannula has a seal assembly
associated
therewith and provides a substantially fluid tight seal about the instrument
to preserve the
integrity of the established air or gas within the surgical region.
Minimally invasive procedures have several advantages over traditional
open surgery, including less patient trauma, reduced recovery time, reduced
potential for
infection, etc. However, minimally invasive surgery, such as laparoscopy, has
several
disadvantages. In particular, the frictional forces exerted on surgical
instruments inserted
through it, has proved to be difficult in procedures requiring extensive
manipulation of
the long narrow endoscopic instruments within a remote site because of the
restricted
mobility. In addition, known seal devices are deficient in resilience and in
rigidity for
affixing the seal within a cannula or trocar housing.
SUMMARY
A surgical access device is provided which includes a housing having an
interior wall defining a longitudinal axis and having at least one aperture
configured and
dimensioned to permit passage of a surgical instrument through the aperture.
The
surgical access device includes a seal member supported in the housing and
defining an
access channel through the seal member. The seal member includes a resilient
layer
forming a seal with the housing interior wall and defining an orifice through
it. The seal
member also includes a fabric layer substantially encapsulating the resilient
layer such
that a surface of the resilient layer which defines the orifice is covered by
the fabric layer.
The access channel is configured and dimensioned such that insertion of a
surgical
2
,
CA 02639310 2008-09-04
instrument into the access channel causes the seal member to form a
substantial sealing
relation with the surgical instrument when it is inserted therethrough.
A composite surgical seal is provided for use in a surgical access device
defining an access channel through the seal, and includes a seal member
configured and
dimensioned to form a seal with a housing interior wall of a surgical access
device. The
seal member includes a resilient layer defining an orifice therethrough and a
fabric layer
substantially encapsulating the resilient layer such that a surface of the
resilient layer
which defines the orifice is covered by the fabric layer. The access channel
is configured
and dimensioned such that insertion of a surgical instrument into the access
channel
causes the seal member to form a substantial sealing relation with the
surgical instrument
inserted therethrough.
A method of forming a composite seal assembly for use in a surgical
access device is also provided whereby the steps include initially providing
first and
second fabric ring assemblies each including a rigid ring having a fabric
layer secured to
it. The first and second fabric ring assemblies are then positioned in
opposing relation to
each other such that a gap is created between them preceding approximation of
opposing
central portions of each fabric layer. Subsequently, a gel material is
introduced between
the first and second fabric layers to fill the gap formed between the first
and second fabric
ring assemblies to form the seal assembly. An orifice is then formed through a
central
portion of the seal assembly such that the fabric layer of one or both of the
fabric ring
assemblies covers the surface of the orifice.
3
i
CA 02639310 2008-09-04
The seal member may also include a rigid ring layer attached to an outer
circumference of at least one of a proximal end or distal end of the seal
member and is
adapted for mounting to the housing.
BRIEF DESCRIPTION OF THE DRAWINGS
Various embodiments of the present disclosure will be described
hereinbelow with reference to the figures wherein:
FIGS. 1 and 2 are perspective views of an access assembly and a seal
assembly in accordance with the principles of the present disclosure;
FIG. 3 is a perspective view with parts separated of the access and seal
assemblies of FIG. 1;
FIG. 4 is a partial side cross-sectional view of the access and seal
assemblies;
FIG. 5 is a perspective view illustrating a seal assembly in accordance
with the present disclosure;
FIG. 6A is an enlarged side cross-sectional view of an embodiment of the
seal assembly of FIG. 1;
FIG. 6B is an enlarged view of the indicated area of detail of the seal
assembly of FIG. 6A;
FIG. 7A is a top plan view of the seal assembly;
4
. . . . . . . . . . . . . . . . i . _ .. .. . . . . .... . . .
CA 02639310 2008-09-04
FIG. 7B is a side cross sectional view of the seal assembly of FIG. 7A
taken along section line A-A; and
FIG. 8 is a flow chart illustrating the steps of a method for forming a seal
assembly in accordance with the present disclosure.
DETAILED DESCRIPTION
The seal assembly of the present disclosure, either alone or in combination
with a seal system internal to a cannula assembly, provides a substantial seal
between a
body cavity of a patient and the outside atmosphere before, during and after
insertion of
an object through the cannula assembly. Moreover, the seal assembly of the
present
disclosure is capable of accommodating objects of varying diameters, e.g.,
instruments
from about 4.5 mm to about 15 mm, while maintaining a fluid tight interface
about the
instrumentation adapted for insertion through a trocar and/or cannula assembly
to prevent
gas and/or fluid leakage so as to preserve the atmospheric integrity of a
surgical
procedure. The flexibility of the present seal assembly greatly facilitates
endoscopic
and/or laparoscopic surgery where a variety of instruments having differing
diameters are
often needed during a single surgical procedure. Specifically, the surgical
device includes
a seal assembly which facilitates lateral and/or angular manipulation of the
surgical
instrument while also maintaining a seal about the instrument. The seal
assembly is
further adapted to substantially close in the absence of a surgical instrument
to maintain
the integrity of the insufflated peritoneal cavity.
The surgical seal assembly of the present disclosure is additionally
adapted to decrease the frictional forces exerted on surgical instruments
inserted through
,
,
CA 02639310 2008-09-04
it which has proven to be difficult in procedures requiring extensive
manipulation of the
long narrow endoscopic instruments within a remote site because of the
restricted
mobility.
Moreover, the manufacturing of a durable seal assembly for use with a
surgical access device has proven to be expensive and lacking effectiveness
with
maintaining good seal properties. The present disclosure provides for a more
efficient
and cost effective way of manufacturing a seal assembly to provide good
sealing and
durable properties. Specifically, the manufacturing of the seal assembly of
the present
disclosure provides for effective sealing properties during on and off axis
motion while
reducing the frictional forces of the surgical instruments lodged
therethrough.
Examples of surgical instrumentation include clip appliers, graspers,
dissectors, retractors, staplers, laser probes, photographic devices,
endoscopes and
laparoscopes, tubes, and the like. Such instruments will be collectively
referred to herein
as "instruments" or "instrumentation".
The seal assembly may also be adapted dimensionally to receive and form
a seal about a physician's arm or hand during a hand-assisted laparoscopic
procedure. In
this application, the seal assembly is a component of an access member which
is
introduced within the body to provide access to underlying tissue in, e.g.,
the abdominal
cavity.
Moreover, the seal assembly may be readily incorporated into an access
device, such as a conventional trocar device or cannula housing to provide the
device
with zero-closure and/or sealing around an instrument or other object.
6
. . . . . . .. . . . . . ... .. . . . i . . . .. . . . . . . . . . . . .. .. .
.
CA 02639310 2008-09-04
The subject matter of this disclosure generally relates to the subject matter
of commonly assigned U.S. provisional application entitled SURGICAL PORTAL
WITH GEL AND FABRIC SEAL ASSEMBLY filed under Express Mail Certificate No.
EM 075410446 US on September 17, 2007, Attorney Docket No. H-US-00806 (203-
5424).
In the following discussion, the term "proximal" or "trailing" will refer to
the port'ion of the surgical device nearest to the clinician during operation
while the term
"distal" or "leading" will refer to that portion of the portal apparatus most
remote to the
clinician.
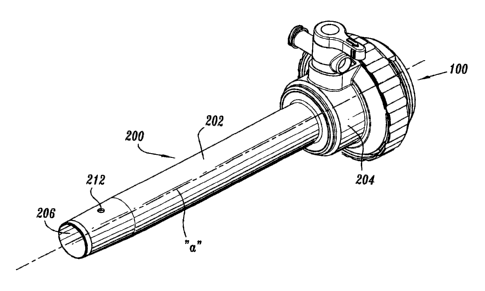
Referring now to the drawings, in which like reference numerals identify
identical or substantially similar parts throughout the several views, FIGS. 1-
2 illustrate
one embodiment of a seal assembly, i.e. seal assembly 100 of the present
disclosure
mounted to an access device 200 such as cannula or trocar assembly. Cannula
assembly
200 may be any conventional cannula suitable for the intended purpose of
accessing a
body cavity and typically defines a passageway permitting introduction of
instruments
therethrough. Cannula assembly 200 is particularly adapted for use in
laparoscopic
surgery where the peritoneal cavity is insufflated with a suitable gas, e.g.,
COZ, to raise
the cavity wall from the internal organs therein. Cannula assembly 200 is
typically used
with an obturator assembly'(not shown) which may be blunt, a non-bladed, or a
sharp
pointed instrument positionable within the passageway of the cannula assembly
200. The
obturator assembly is utilized to penetrate the abdominal wall or introduce
the cannula
assembly 200 through the abdominal wall, and then subsequently is removed from
the
7
i
CA 02639310 2008-09-04
access device to permit introduction of the surgical instrumentation utilized
to perform
the procedure through the passageway.
With reference to FIGS.1-4, cannula assembly 200 includes cannula
sleeve 202 and cannula housing 204 mounted to an end of the sleeve 202. Any
means for
mounting cannula sleeve 202 to cannula housing 204 are envisioned including
threaded
arrangements, bayonet coupling, snap-fit arrangements, adhesives, etc. Cannula
sleeve
202 and cannula housing 204 may be integrally formed. Cannula sleeve 202
defines a
longitudinal axis "a" extending along the length of sleeve 202. Sleeve 202
further defines
an internal longitudinal passage 206 dimensioned to permit passage of surgical
instrumentation. Sleeve 202 defines collar 208 which is mounted to cannula
housing 202
and an inner tapered wall 210 adjacent the collar 208. The sloped
configuration of
tapered wa11210 may assist in guiding the inserted instrument into
longitudinal passage
206. Adjacent the distal end of cannula sleeve 202 is aperture 212 which
extends through
the wall of the sleeve 202. Aperture 212 permits passage of insufflation gases
through
cannula sleeve 202 during the surgical procedure. Sleeve 202 may be formed of
stainless
steel or other rigid materials such as a polymeric material or the like.
Sleeve 202 may be
clear or opaque. The diameter of sleeve 202 may vary, but, typically ranges
from about
mm to about 15 mm for use with the seal assembly 100 of the present
disclosure.
Cannula housing 204 includes port opening 214 and luer fitting 216
positioned within the port opening 214. Luer fitting 216 is adapted for
connection to a
supply of insufflation gaseous is conventional in the art and incorporates
valve 218 to
selectively open and close the passage of the luer fitting 216. Cannula
housing 204
further includes duckbill or zero closure valve 220 which tapers distally and
inwardly to a
8
i
CA 02639310 2008-09-04
sealed configuration. Closure valve 220 defines slit 222 which opens to permit
passage of
the surgical instrumentation and closes in the absence of the instrumentation.
Closure
valve 220 is preferably adapted to close upon exposure to the forces exerted
by the
insufflation gases in the internal cavity. Other zero closure valves are also
contemplated
including single or multiple slit valve arrangements, trumpet valves, flapper
valves, etc.
Closure valve 220 rests upon internal shelf 224 of cannula housing 204 when
assembled.
Cannula housing 204 includes at least one locking recess 226 preferably
two recesses arranged in diametrical opposed relation. Locking recesses 226
serve to
releasably secure seal assembly 100 to cannula assembly 200.
With continued reference to FIGS. 1-4, seal assembly 100 will be
discussed in detail. Seal assembly 100 may be a separate component from
cannula
assembly 200 and, accordingly, adapted for releasable connection to the
cannula
assembly 200. Alternatively, seal assembly 100 may be incorporated as part of
cannula
assembly 200. Seal assembly 100 includes a seal housing, generally identified
as
reference numeral 102, and seal member 104 which is disposed within the seal
housing
102. Seal housing 102 houses the sealing components of the assembly and
defines the
outer valve or seal body of the seal assembly 100. Seal housing 102 defines
central seal
housing axis "b" which is preferably parallel to the axis "a" of cannula
sleeve 202 and,
more specifically, coincident with the axis "a" of the cannula sleeve 202.
Seal housing
102 incorporates three housing components, namely, first, second and third
housing
components 106, 108, 110, respectively, which, when assembled together, form
the seal
housing 102. Assembly of housing components 106, 108, 110 may be affected by
any of
the aforementioned connection means discussed with respect to cannula housing
204.
9
. .. .. . . . . . . .. . . ... . i . . . . .. . . . . . .
CA 02639310 2008-09-04
First housing component 106 defines inner guide wall 112 and outer wall
114 disposed radially outwardly of the inner guide wall 112. Inner guide wall
112 defines
central passage 116 which is dimensioned to receive a surgical instrument and
laterally
confine the instrument within seal housing 102. As best shown in FIG. 4, inner
guide
wall 112 defines sloped or tapered portion 118 adjacent its proximal end.
Sloped portion
118 is obliquely arranged relative to seal housing axis "b" and extends
radially inwardly
relative to the seal housing axis "b" in the distal direction. Sloped portion
118 assists in
guiding the inserted instrument into central passage 116, particularly, when
the
instrument is non-aligned or off-axis relative to the seal housing axis "b",
or introduced at
an angle relative to the seal housing axis "b". Sloped portion 118 provides
more
flexibility to the surgeon by removing the necessity that the instrument be
substantially
aligned with the seal housing axis "b" upon insertion. Inner guide wall 112 is
generally
cylindrical in configuration and terminates in a distal arcuate or rounded
surface 120.
Second housing component 108 includes transverse wall 122, inner
cylindrical wall 124 depending in a proximal direction outwardly from the
transverse
wall 120 and outer wall 126 depending in a distal direction outwardly from the
transverse
wall 120. Inner cylindrical wall 124 is dimensioned to mate with outer wall
114 of first
.
housing component 106, e.g., in a manner to be positioned within the interior
of the outer
wall 114 in frictional relation therewith. In the alternative, outer wall 114
of first housing
component 106 may be adhered to inner cylindrical wall 124 of second housing
component 108. Outer wall 126 defines scalloped outer surface 126a which is
dimensioned for gripping engagement by the user.
. . . . . . . .. . . . . ... . . . ~ . .
CA 02639310 2008-09-04
In one embodiment, seal member 104 is mounted to proximal housing
component 106 through, e.g., conventional means, such as by adhering the seal
mernber
104 to the housing component 106 or molding the seal member 104 in the housing
component 106. Seal member 104 may be fabricated from an elastomer such as a
soft
urethane gel, silicone gel, thermoplastic elastomer (TPE) or the like and
preferably has
compressible characteristics to permit the seal to receive objects having a
variety of sizes,
to conform and form a seal about the outer surface of the inserted object, and
to close
upon removal of the object. Seal member 104 may be capable of accommodating
instruments of varying diameters, e.g. from about 5 mm to about 12 mm, while
providing
a fluid tight seal with the outer diameter of each instrument. Seal member 104
may
include a central orifice 138 advantageously dimensioned to permit reception
and passage
of a surgical instrument. In particular, orifice 138 expands upon insertion of
the surgical
instrument to permit passage of the surgical instrument whereby the surface
portions
defining orifice 138 engage the instrument in sealed relation therewith. The
orifice 138 is
further adapted to assume a substantially reduced dimension upon removal of
the
instrument. In this position, the seal member 104 restricts the egress of
gaseous matter
through seal housing 102. Orifice 138 may have shapes other than that of a
circular cross
section opening, such as "t"-shaped, "x" shaped, helical, etc.
The use of the seal assembly 100 and cannula assembly 200 in connection
with introduction of a surgical instrument will be discussed. Seal assembly
100 is
mounted to cannula assembly 200 and cannula assembly 200 is introduced into an
insufflated abdominal cavity. An object, e.g., an instrument, is inserted into
seal
assembly 100 through orifice 138 whereby the portions defining the orifice 138
stretch to
11
CA 02639310 2008-09-04
accommodate the instrument diameter, as necessary. The instrument is distally
passed
through the valve 220 in sealed relation therewith and into the body cavity to
perform the
desired procedure. The instrument is removed and the orifice 138 of the seal
member
104 returns to a reduced diameter configuration to assist in maintaining the
integrity of
the established pneumoperitoneum. Other instruments may be introduced through
the
seal assembly 100 and access device to perform further operative techniques as
desired.
FIG. 5 illustrates a composite seal member 300 as an exemplary
embodiment of a seal member in accordance with the present disclosure. Seal
member
300 is configured and dimensioned to be supported within the housing of the
surgical
access device, e.g., between suitable surfaces of seal housing 102, and to
define an access
channel 312 therein. Seal member 300 includes a gel layer 304 which forms a
seal with
the housing interior wall and defining an orifice 338 therethrough. A fabric
layer 320 sits
above and below gel layer 304 such that a surface 320 of the gel layer 304
which defines
orifice 338 is covered by fabric layer 320. Access channe1312 is configured
and
dimensioned such that insertion of a surgical instrument into access channel
312 causes
seal member 300 to form a substantial sealing relation with the surgical
instrument
inserted therethrough.
Referring now to FIGS. 6A and 6B, composite seal member 300 includes
a seal assembly fabricated from a first generally soft gel layer 304 and an
elastic fabric
layer 320 which substantially encapsulates gel layer 304. FIG. 6B is an
exploded view
of the central portion of seal member 300 illustrating fabric layer 320
substantially
encapsulating gel layer 304 and wherein fabric layer 320 also covers the
surface of gel
layer 304 which defines orifice 338.
12
CA 02639310 2008-09-04
In one embodiment, gel layer 304 may be any suitable material identified
hereinabove in connection with the embodiment of FIGS. 1-4, including, for
example, a
thermoplastic elastomer (TPE) or other flexible lubricous material, urethane
gel, a silicon
gel, alginates, gum Arabic, polymer hydrogels or a polymeric thereof, or any
combination
of these materials. Fabric layer 320 may include a SPANDEX material
containing 20%
LYCRA available from Milliken. Alternatively, fabric layer 320 may be
disposed on
just one of either the proximally facing surface or the distally facing
surface of seal
member 300, as desired (not shown).
Fabric layer 320 provides a degree of rigidity to gel layer 304 and may
desirably assist in maintaining gel layer 304 in its disc-shaped
configuration. Moreover,
the combination of fabric layer 320 and gel layer 304 defines a seal having
enhanced
adaptability to a variety of different diameter objects, e.g., instruments,
and which
maintains a seal upon offset manipulation of the object. Fabric layer 320 also
serves as a
secondary seal supplemental to the sealing functions of gel layer 304.
In still fui-ther embodiments, the seal znember may include a coating to
reduce frictional forces on the surgical instrument. The coating may be, e.g.,
an
amorphous diamond coating, ion implantation coating, silicon or hydrogel
coating,
TEFLON etc. and allows for more efficient manipulation of instrumentation
through
the access channel of a cannula or trocar.
Referring now to FIGS. 7A and 7B, in one embodiment, seal member 300
illustrates a rigid ring layer 322 attached to an outer circumference of at
least one of a
proximal end or distal end of seal member 300. Ring layer 322 is adapted for
mounting
13
i
CA 02639310 2008-09-04
to the housing of seal member 300. Rigid ring layer 322 is made from a
material such as
nylon or any other rigid thermoplastic, e.g., polypropylene, polyethylene,
polycarbonate,
etc., and provides for a method for fixing the seal within a housing of a
surgical access
device.
In one embodiment of a method of forming a composite seal assembly for
use in a surgical access device as illustrated in the flow chart of FIG. 8,
includes the step
of initially providing first and second fabric ring assemblies each including
a rigid ring
having a fabric iayer secured thereto (Block 400). The first and second fabric
ring
assemblies are then positioned in opposing relation to each other such that a
gap is
created therebetween (Block 410). Thereafter, opposing central portions of
each fabric
layer are approximated (Block 420) prior to introducing a gel material between
the first
and second fabric layers to fill the gap formed between the first and second
fabric ring
assemblies which forms the seal assembly (Block 430). An orifice is then
formed
through a central portion of the seal assembly such that the fabric layer of
one or both of
the fabric ring assemblies covers the surface of the orifice (Block 440).
In more detail, in some embodiments, a composite seal assembly for use in
a surgical access device is formed by securing a first fabric ring assembly to
a first fabric
layer and a second fabric ring assembly to a second fabric layer via
overmolding the
fabric ring assemblies onto the fabric layers. Additionally, excess fabric on
the orifice of
the seal member is removed. The first and second fabric ring assemblies are
then
positioned in opposing relation to each other such that a gap is created
therebetween via
pressing the fabric ring assemblies into recesses of a mold.
14
1
CA 02639310 2008-09-04
The opposing central portions of each fabric layer are then pinched
together with mating core pins creating an access channel for instruments to
pass
therebetween having good sealing properties. A thin layer of fabric at the
central portion
of the seal assembly is thereafter removed to form the orifice 324 which
defines the
access channe1312 such that the orifice accepts surgical instruments
therethrough. In
further embodiments, a coating is applied to the seal member to reduce
frictional force
with the surgical instruments. The coating may be, e.g., an amorphous diamond
coating,
ion implantation coating, silicon or hydrogel coating, TEFLON coating etc.,
and
allows for more efficient manipulation of instrumentation through the access
channei of a
cannula or trocar.
VVhile the above description contains many specifics, these specifics
should not be construed as limitations on the scope of the disclosure, but
merely as
exemplifications of embodiments thereof. Those skilled in the art will
envision many
other possibilities within the scope and spirit of the disclosure as defined
by the claims
appended hereto.