Note: Descriptions are shown in the official language in which they were submitted.
CA 02640098 2016-02-15
FLAME RETARDANT COATING
TECHNICAL FIELD
[0001] This document relates to flame retardant coatings, and flame
retardant
polyurea elastomer coatings.
BACKGROUND
[0002] An intumescent is a substance which swells as a result of heat
exposure, thus
increasing in volume, and decreasing in density. Intumescents are typically
used in passive
fire protection and, in America, require listing and approval use and
compliance in their
installed configurations in order to comply with the law.
SUMMARY
[0003] A method of applying a flame retardant coating is disclosed. In an
embodiment, there is provided a method of adhering a flame retardant polyurea
elastomer
coating to a smooth surface, the method comprising providing a first part
containing
isocyanates, and a second part containing polyamines, in which at least one of
the first part
and the second part contains a flame retardant, a mineral matrix agent, and
both of spray
processable graphite and a graphite stabilizer agent; mixing the first part
and the second part
and spraying the first part and the second part together on to the smooth
surface to form the
flame retardant polyurea elastomer coating on the smooth surface.
[0004] In other embodiments, the smooth surface comprises metal, the
smooth
surface comprises phenolic composite panels, at least one of the first part
and the second part
comprises a graphite suspending agent, the graphite suspending agent comprises
hydrogenated castor oil and Octadecanamide,N,N'-1,2-ethanediylbis 12- hydroxy-
, the
graphite stabilizer agent is selected to form a polymer char that locks the
graphite together
under flame heat, the graphite stabilizer agent comprises at least one of
ammonium
polyphosphate, zinc polyborate, melamine polyphosphate, melamine
pyrophosphate, triethyl
phosphate, and zinc phosphate and the smooth surface comprises a floor in a
rail vehicle.
1
CA 02640098 2016-02-15
BRIEF DESCRIPTION OF THE FIGURES
[0005] Embodiments will now be described with reference to the figures, in
which
like reference characters denote like elements, by way of example, and in
which:
[0006] Fig. 1 is a perspective view of a flame retardant polyurea
elastomer coating.
[0007] Fig. 2 is a side elevation view of a transport vehicle, with a
partial cut-away
illustrating the interior of the vehicle.
[0008] Fig. 3 is a flow schematic of a method of manufacturing a flame
retardant
polyurea elastomer coating.
[0009] Fig. 4 is a flow schematic of a method of adhering a flame
retardant polyurea
elastomer coating to a smooth surface.
[0010] Fig. 5 is a further method of manufacturing a flame retardant
polyurea
elastomer coating comprising a graphite suspending agent.
[0011] Fig. 6 is a side elevation view, in section, of a flame retardant
polyurea
elastomer coating on structural steel.
[0012] Fig. 7 is a side elevation view, in section, of a flame retardant
polyurea
elastomer coating over rocks.
DETAILED DESCRIPTION
[0013] Immaterial modifications may be made to the embodiments described
here
without departing from what is covered by the claims.
[0014] Polyureas are commonly used as, for example, secondary containment
liners
because of their seamless application and rapid cure. The flammability of
existing polyurea
secondary containment linings may actually contribute to the intensity of a
fire, and thus
intumescents may be added to improve flame resistance.
[0015] In some embodiments, a coating is provided that has the surface
hardness,
flexibility, and chemical resistance of a sprayed polyurea. Tear strength may
be slightly
lower than regular polyureas. The coating may have the longevity of a polyurea
as well as
low flammability. Unlike many existing secondary containment liners, the
intumescent
polyurea do not require covering with sand for fire protection. This feature
minimizes
disposal issues due to contaminated sand from spills.
2
CA 02640098 2016-02-15
[0016] In some embodiments, a coating is provided that is a fire
resistant, zero VOC,
high performance polyurea elastomer. This coating may be designed to provide a
flame
resistant, durable, chemical resistant lining for secondary containment
applications and as a
surface coating for metal, concrete or wood for example. In some embodiments,
the coating
is a fast set system which allows a fast return to service. It may be intended
for use with
plural component spray equipment with a mix ratio of 1 to 1, for example.
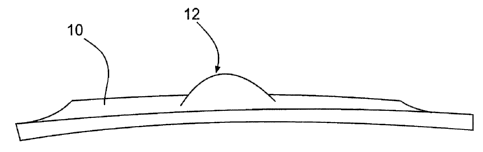
[0017] Referring to Fig. 1, a flame retardant coating 10, for example a
flame
retardant polyurea elastomer coating is illustrated. Coating 10 comprises
polyurea elastomer,
spray processable graphite, a graphite stabilizer agent, and a flame
retardant. As illustrated
coating 10 is flame retardant, and intumesces under flame heat, for example
forming an
expanded portion 12.
[0018] Spraying coating 10 may require on-site machine processing. Spray
processable graphite refers to the fact that the graphite is suitable to be
processed in the type
of machinery used to apply polymer formulations, for example a high pressure
proportioner
or a reaction injection molding machine. In order to achieve suitable
intumescent expansion
of the sprayed expandable graphite, a large enough particle size is required,
but if the particle
size is too large then it will not be spray processable in a machine. On the
other hand, if too
small of a size is used, the graphite won't expand enough or provide enough
fire protection.
Because larger graphite particle sizes give better fire retardant performance,
previous
systems employed the use of larger graphite particles. However, systems with
larger graphite
particles can't process through RIM or HP proportioners, and had to be mixed
by hand or in
a static mixer, which have reduced productivity relative to a spray device.
Thus, a sprayable
system that provides good fire retardant properties was needed.
[0019] In some embodiments, a blend of graphite small enough to machine
process,
but also large enough to give a more stable char is used. The combination of
two or more
types of graphite may improve the performance. In some embodiments, the spray
processable graphite is 50-110 mesh, with at least 50% of the spray
processable graphite
being between 50 and 70 mesh. In other embodiments, the spray processable
graphite is
100% 60 mesh. For reference, 50 mesh corresponds to a size of about 300
microns, while
100 mesh corresponds to a particle size of about 150 microns. Graphite is not
typically used
0
CA 02640098 2016-02-15
in elastomers because it causes problems and doesn't process well. In some
embodiments,
this document teaches a modified form of graphite that will avoid these
problems. In general,
the use of suitable densities of machine spray processable graphite produces a
coating 10 that
passes at least the flame spread test.
[0020] The graphite stabilizer agent is employed to form a polymer char
that locks
the graphite together under flame heat. The presence of the graphite
stabilizer agent insulates
the graphite as it expands, gluing the graphite together and forming a skin
that limits the
amount of oxygen that can access the graphite. Graphite, on its own, may form
a porous
material that falls off under turbulent flame conditions. The graphite
stabilizer agent may
comprise for example at least one of ammonium polyphosphate, zinc polyborate,
melamine
polyphosphate, melamine pyrophosphate, triethyl phosphate, and zinc phosphate.
[0021] The coating 10 is designed to pass Federal US regulations for the
ASTM E
1354 Test Method for Heat and Visible Smoke Release Rate, the ASTM E162 Test
Method
for Surface Flammability, and the ASTM E662 Test Method for Specific Optical
Density of
Smoke Generated by Solid Materials. An example of a suitable flame retardant
is TCPP. In
some embodiments the flame retardant comprises no brominated compounds, as
these are
known carcinogens. In some embodiments, coating 10 may be provided with or
without
halogenated flame retardants, depending on the application. In general,
halogenated flame
retardants may be used where high performance is required, although good
performance may
still be obtained using a halogen-free composition.
[0022] In some embodiments, coating 10 may further comprise a graphite
suspending
agent. Because the graphite comprises particulates, the graphite may settle
out of the liquid
formulation used to create coating 10. The suspending agent suspends the
graphite, allowing
the coating 10 formed by spraying and reacting the formulation to have
graphite suitably
uniformly dispersed throughout the coating 10. This gives the coating 10
better flame
retardant characteristics. This may also be important during processing of the
formulation
used to create coating 10, since any settled graphite may clog the spray
machinery. The
graphite suspending agent is to be contrasted with standard thickeners, or
viscosity
modifiers, in that it physically suspends the graphite beyond merely reducing
the rate of drop
out. Some thickeners, such as hydroxyethyl cellulose, have been found to be
not effective at
4
CA 02640098 2016-02-15
suspending the graphite. In some embodiments, the graphite suspending agent
comprises
thixatrolTM (hydrogenated castor oil and Octadecanamide,N,N'-1,2-ethanediylbis
12-
hydroxyl-).
[0023] In some embodiments, the polymeric composition further comprises a
mineral
matrix agent. The mineral matrix agent greatly aids the performance of coating
10 when
affixed to smooth surfaces, for example steel or metal. Under the intense heat
of a flame, the
mineral matrix agent leaves a mineral matrix that prolongs the life of the
char that is formed.
The mineral matrix formed prevents the pyrolysis of the carbon char, the
pyrolysis of which
may otherwise result in the char being blown off of the surface it is affixed
to, leaving bare
surface to be incinerated. This is important in steel applications, as the
longer the char is
maintained, the longer it takes for the surface of the material coating 10 is
affixed to reach its
critical temperature. In some embodiments, the mineral matrix agent comprises
at least one
of glass and perlite. The glass may be provided as small spheres, or as
processed shards for
example. Referring to Fig. 6, in some embodiments, coating 10 may be used as
fire
protection for structural steel 30.
[0024] Referring to Fig. 7, in some embodiments, coating 10 may be used as
a rock
reinforcing coating in, for example, an underground mine. In the example
shown, coating 10
is covering a pile of rocks 32. In other of these embodiments, coating 10 may
be sprayed on
the walls, roof, or floor of the interior of a mine, in order to reinforce it.
Coating 10 forms a
gas impermeable stop that prevents hazardous gases from leaching from the
formation and
into the mine shaft.
[0025] In some embodiments, coating 10 is formed by a two-part
polymerizable
system. In the case of polyurea elastomers, one component typically contains
isocyantes,
while the other component contains polyamines. The mixing of these two
components causes
the reaction that forms the coating 10.
[0026] Referring to Fig. 3, a method of manufacturing a flame retardant
polyurea
elastomer coating is illustrated. In a step 14, a two part polymerizable
system comprising a
first part containing isocyanates, and a second part containing polyamines, is
provided. At
least one of the first part and the second part contains a flame retardant,
and both of spray
CA 02640098 2016-02-15
processable graphite and a graphite stabilizer agent. In a second step 16, the
first part and the
second part are mixed to form the flame retardant polyurea elastomer coating
10.
[0027] Referring to Fig. 4, a method of preparing a flame retardant
polyurea
elastomer coating on a smooth surface is illustrated. In a step 18, a first
part containing
isocyanates, and a second part containing polyamines, is provided. At least
one of the first
part and the second part contains a flame retardant, a mineral matrix agent,
and both of spray
processable graphite and a graphite stabilizer agent. In a step 20, the first
part and the second
part are mixed to form the flame retardant polyurea elastomer coating 10 on
the smooth
surface. The smooth surface may comprise, for example, metal. The smooth
surface may
comprise, for example, phenolic composite panels (not shown).
[0028] Referring to Fig. 5, a method of manufacturing a flame retardant
polyurea
elastomer coating is illustrated. In step 22, a two part polymerizable system
comprising a
first part containing isocyanates, and a second part containing polyamines, is
provided. At
least one of the first part and the second part contains both of spray
processable graphite and
a graphite suspending agent. In step 24, the first part and the second part
are mixed to form
the flame retardant polyurea elastomer coating 10.
[0029] Referring to Fig. 2, the coating 10 may be used as flooring 26 in a
transport
vehicle 28. The coating 10 may be a spray applied polymer that cures rapidly
to form a
seamless, durable floor finish. This spray may be available in a range of
solid colors or with
optional accent colors for a designer finish. As a spray-in-place floor system
it offers the
design flexibility of color variations. Options include guided walkways to
ensure optimum
traffic flow, borders or outlines, safety markings and custom logos
permanently imbedded in
the floor. Flooring 26 is the only spray-in-place floor system that exceeds
all federal fire
safety standards for use in mass transit applications. Flooring 26 may be used
in passenger
railcar, bus and other transit vehicles, for example, as well as station and
other floor areas.
One advantage with using the disclosed flooring 26 is an improvement in
productivity
brought about by the ability to spray apply the system. Previous flooring
systems in railcars
and buses required that a rubber surface had to be glued down using contact
cement. This
type of procedure, including drying and installation time, could take at least
8 days to
complete. This can be contrasted with the installation of flooring 26, which
may be achieved
6
CA 02640098 2016-02-15
in a single day, allowing the vehicle to be back online in at most two days.
In some
embodiments, flooring 26 may be any type of flooring. Flooring 26 is highly
abrasion
resistant, long lasting and has superior impact resistance. Because it is an
elastomer, flooring
26 has no cracking or chipping. Flooring 26 remains flexible at low
temperatures and gives
excellent traction in cold weather. Flooring 26 is also stain resistant, which
reduces cleaning
time. In order to avoid graphite streaking, the two-component system may be
adjusted to
change the speed with which it is cured, allowing the graphite to settle below
the surface of
coating 10 before curing.
[0030] Table 1 below illustrates an exemplary two part polymerizable
system. As is
evident, there are numerous optional components that may be present, such as
moisture
scavengers, defoamers, thixotropic agents, solvents, coupling agents, and a
variety of other
species that may improve or tailor the resulting coating 10. In addition,
various coloring
agents may be used, such as for example Black Paste, Red Oxide, and Yellow
Oxide. In the
exemplary system, two different types of polymeric isocyanates are used, in
order to
carefully control the hardness of the resulting coating.
[0031] Table 1: exemplary two part polymerizable system
% by Range
Ingredients lbs Kg L Function
Weight (%)
COMPONENT A
Polypropylene diamine 53.21 24.14 24.21 Reactive amine
36.5 25-50
moisture scavenger 3.1
Zeolite Paste 4.55 2.06 1.79 - optional 0-5
Thixatrol ST 0.13 0.06 0.06 suspending agent 0.09 0-
0.2
AirexTM 900 0.09 0.04 0.04 defoamer 0.06 <1
0.08 0.05-
Fumed Silica 0.10 0.05 0.02 thixotropic agent 0.10
Ammonium 20.8
Polyphosphate 30.40 13.79 7.26 Forms
polymer char 0-30
Airex TM 900 0.03 0.01 0.01 Defoamer 0.02 <1
Diethyltoluene diamine 24.50 11.11 10.87 Reactive amine
16.8 0-25
coupling agent to 0.3
SilquestTM A-187 0.48 0.22 0.20 adhere to surface 0-1
graphite 3626 23.65 10.73 5.364 expander
16.2 0-30
graphite 3538 8.69 3.94 1.970 expander 6
0-30
sub total 145.82 66.14 51.80 100
COMPONENT B
TCPP 32.92 14.93 11.58 flame retardant
24.8 0-40
7
CA 02640098 2016-02-15
polymeric 37.6
HXI 1624 50.00 22.68 19.89
isocyanate 0-100
combo harder or 37.6
HXI 1644 50.00 22.68 19.89 softer
0-100
sub total 132.92 60.29 51.36 100
TOTAL 278.74 126.44 103.16
[0032] Table 2: Further exemplary two part polymerizable system
ingredients lbs Kg
COMPONENT A
D-2000 1359.17 616.51 618.37
Zeolite Paste 116.22 52.72 45.63
Thixatrol TM ST 3.19 1.45 1.42
Airex TM 900 2.30 1.04 1.10
R-972 2.55 1.16 0.53
APP 776.55 352.24 185.39
Tio2 0.00 0.00 0.00
Raven TM 410
Red Oxide
Yellow Oxide
Airex 900 0.64 0.29 0.30
Ethacure TM 100 625.78 283.85 277.74
Silquest A-187 12.26 5.56 5.20
graphite 3626 604.07 274.00 130.478
graphite 3538 221.86 100.63 47.921
sub total 3724.59 1689.46 1314.08
COMPONENT B
TCPP 840.84 381.40 295.66
HXI 1624 1277.10 579.29 522.35
HXI 1644 1277.10 579.29 514.01
sub total 3395.04 1539.98 1332.02
TOTAL 7119.63 3229.44 2646.10
[0033] In Table 2, a further exemplary system is illustrated. This table
is not
necessarily indicative of every species present in each component. Further,
the amounts of
pigment have not been indicated, as these will vary depending on the desired
color. This
system has been used to create the flooring in the trains of the BART
transport system in San
8
CA 02640098 2016-02-15
Francisco. In order to create component A (also known as the resin), a clean
resin blend tank
is placed on a platform scale, and D-2000 TM (polyamine -
polyoxypropylenediamine) and the
Zeolite paste are added. Next, the tank is placed under a mixer and blended at
high speed.
While mixing, the THIXATROL STTI" (hydrogenated castor oil and
Octadecanamide,N,N'-
1,2-ethanediyIbis 12- hydroxy-), AIREX 9001M (organo-modified polysiloxane
containing
fumed silica or dichlorodimethylsilane reaction products with silica), R972 TM
(fumed
silica), APP (Ammonium polyphosphate), Ti02, RAVEN 410 TM (carbon black /
black
paste), Red Oxide, and Yellow Oxide are added. Ethacure may be used (curing
agent, A 75-
81 - 3,5-diethyltoluene-2,4-diam ine, % 18-20 - 3,5-diethyltoluene-2,6-diam
ine, % 0.5-3 -
Dialkylated m-phenylenediamines, % <0.08 Water). The mixture is then blended
for 60
minutes, and the temperature is raised to 31 degrees Celsius. The blender is
then slowed to
medium speed, and A1REX 900Tm), SILQUEST Al87TM (Gamma-
Glycidoxypropyltrimethoxysilane) and graphite are added. At first only the
3538 Graphite is
added slowly and ground over 10 minutes. Then, the 3626 Graphite is added
slowly and
ground over another 5 minutes. The entire mixture is then blended at medium
speed for 15
minutes. A sample is then collected and submitted for quality control, and
upon approval, the
mixture is filled into storage drums.
[0034] In order to create component B (also called the Iso), a clean ISO
blend tank is
placed on a scale. DOP, if present, is removed from the tank, and HXI 1644,
HXI 1624, and
TCPP are added. The tank is then placed under a mixer and blended for 45
minutes. A
sample is then collected and submitted for quality control, and upon approval,
the mixture is
filled into storage drums. DOP (dioctyl phthalate) is put into the tank before
storage. A
single batch size used to create a coating 10 with these components may
comprise 1323 liters
of component A, and 1332 liters of component B. Table 3 illustrates some E162
test results
with exemplary coatings.
[0035] For application, a regulated high-pressure proportioner (1:1) and
spray gun
system capable of producing 2000 - 2500 psi may be used. Component A may be
thoroughly
mixed for 45 to 60 minutes prior to use. Component A may also be preheated to
achieve a
uniform mix. It should be checked that no residue is left on the bottom of
drum after mixing.
Recommended Heat Settings include: Line/Pre-Heaters 150- 160 F, Hose Heat 150-
160 F.
9
CA 02640098 2016-02-15
The components are then applied to a properly prepared substrate. A first coat
is applied at
less than 10 mm and allowed to become tack free before continuing. Following
coats are
then applied at 20 mm per coat, allowing the surface to become tack free
before the
application of subsequent coats. The coating is sprayed with a uniform motion,
allowing 50
to 75% overlap.
[0036] Table 3: E162 Flame Spread Data
COMPANY: Quantum Group VTEC# 100-2992-4
AL FOIL? YES
COLOR: Grey EXP TIME: 15 MIN.
DIMENSIONS: 6" X 18" DATE: 7/1112008
THICKNESS: 0.229"
SPECIAL
PREPARATION: none
OBSERVATIONS:
3 inches 6 inches 9 inches 12 inches 15 inches
Time To... (min) (min) (min) (min) (min)
Specimen
1 1.35 9.33 12.18 -
2 2.18 7.25 12.08 -
3 1.58 7.93 9.5 -
4 2.25 8.67 12.93 -
Max
Sample Wt Base Temp Temp deg Is
Specimen Fs Q kg deg C C INDEX
1 2.11 11.75 1.881 192.8 255.1 24.8
2 1.86 10.31 1.888 196.5 251.2 19.2
3 2.14 12.65 1.898 189 256.1 27.03
4 1.82 10.64 1.882 194 250.4 19.35
Average 1.9825 11.3375 1.88725
193.075 253.2 22.595
AVG. FLAMESPREAD FACTOR (FS) = 1.98
AVERAGE HEAT OF EVOLUTION (Q) = 11.34
AVERAGE FLAMESPREAD INDEX (Is) = 22.6
FLAMESPREAD INDEX RANGE TO
(Is) = 19.2 27.03
CA 02640098 2016-02-15
[0037] Table 4 illustrates some physical properties of an exemplary
coating used as
flooring material in the BART system. The exemplary finished spray flooring
coating
material with a thickness of 60 to 125 mil (1500 to 3000 micron) meets the
requirements
listed in Table 4.
[0038] Table 4: Physical properties of exemplary coating used as flooring
material
Tensile
Elongation,
ASTM D412: 100%
Tensile Strength,
ASTM D412: 600-1000 psi
45-50 before texture
Shore D is applied, with
Harndess, ASTM texture, hardness
A2240: shall be 30 minimum
Gel Time/Tack
Free: 3 to 10 seconds
Solids by
Volume: 100%
Abrasion
Resistance, 250 mg.wt.
ASTM D4060: loss/cycles
[0039] The exemplary flooring has been manufactured to meet the following
test
requirements for fire, smoke, toxicity, and friction. In the Standard Test
Method for Critical
Radiant Flux of Floor Covering Systems Using a Radiant Heat Energy Source
(Method:
ASTM E-648), the requirement was that the flooring have a Critical Radial Flux
of more
than or equal to 0.50 watts/sq. cm. In the Standard Method for Specific
Optical Density of
Smoke Generated by Solid Materials (Method: ASTM E-662), the requirements in
the
flaming and non-flaming modes were: Ds g 1.5 minutes - maximum: 100, DS g 4.0
minutes - maximum: 200, Dm @ 15.0 minutes - maximum: 300, and Dm g 20.0
minutes -
maximum: 300. In the Cone Calorimeter tests, three tests were required at each
of the
following: 25, 50, and 75 kW/sq meter using horizontal sample position
(Method: ASTM
1354). The requirements were a maximum heat release rate of less than 150
kW/sq meter, an
average heat release rate at 3 minutes of less than 100kW/sq meter, and an
average heat
release ignition to flameout of less than 50 kW/sq meter. In the toxicity
tests, (Bombardier
test method SMP800 or Boeing test method BSS-7238 "Test Method for Smoke
Generation
11
CA 02640098 2016-02-15
by Materials in Combustion"), the requirements were that the generation of
toxic gases
identified below do not exceed the indicated concentration in either the
flaming or non-
flaming modes: Carbon Monoxide (CO): 3500 ppm, Nitrogen Oxides (NO2): 100 ppm,
Sulphur Dioxide (S02): 100 ppm, Hydrogen Chloride (HCL): 500 ppm, Hydrogen
Fluoride
(HF): 200 ppm, and Hydrogen Cyanide (HCN): 150 ppm. In the slip Resistance
tests
(Method: ASTM C-I028), the requirements were that the Coefficient of friction
for both wet
and dry conditions is 0.6 or greater. In the Adhesion, Bonding tests, which
illustrate the
bonding strength of the spray coating to the fiberglass and steel substrate of
the floor
Method: ASTM D-4541), the requirement was that the Pull-off strength, using a
20mm (0.79
inches) diameter plug, must be 500 psi or greater.
[0040] Disclosed is a novel fire resistant polymer coating 10. This can be
spray and
injection moulded or spray applied to a wide range of substrates. The rapid
cure rate of this
coating allows a fast return to service in retrofit applications and rapid
deployment in OEM
applications. This coating may be used where all the benefits of durability
and performance
are needed, and where life safety is paramount, such as marine applications,
passenger rail
and mass transit. Some physical properties may include a Flame Spread
Classification
(ASTM E84) of 20, a Smoke Developed Classification (ASTM E84) of 115, a
Service
Temperature of-SO to 200 C, a Shore Hardness of 50D approximately, an
Elongation of
100%, and a Tensile Strength of 1000 psi.
[0041] In some embodiments, the polyurea elastomer may have no
polyurethane
present. This may be accomplished, for example, by providing component A with
no
polyols. In some embodiments, in order to achieve the desired targets for
flammability and
smoke densities, polyurethane must be avoided. Polyurethane is thermoplastic,
and thus it
may soften too much and fall away under the intense heat of flame, failing
fire tests.
[0042] In some embodiments, a coating is provided that is a fire
resistant,
polyaspartic polyurea coating. This coating may be designed to provide a flame
resistant,
UV resistant finish coat for application to metal, concrete or wood for
example. Table 5
illustrates an exemplary preparation for a coating 10 of this nature.
[0043] Table 5: Exemplary polyaspartic coating
I Ingredients lbs Kg L % by Range I
12
CA 02640098 2016-02-15
weight (%)
COMPONENT A
Desmophen TM 1420 985.13 446.85 421.56 41.7 0-50
DesmophenTM XP 7076 146.93 66.65 76.61 6.2 10-Feb
AcemattTM TS100 0 0 0 0-5
Byk 333 4.31 1.95 1.88 0.2 <1
Additive OF 26.72 12.12 13.46 1.1 0.5-2
Propylene Carbonate 0 0 0 0-20
TCPP 0 0 0 0-25
Borchi Gol 0011 30.39 13.78 16.03 1.3 2-Jan
Eversorb TM 74 41.74 18.93 16.18 1.8 0-5
Tio2 46.72 21.19 5.3 2 Varies
Raven TM 410 1.44 0.65 0.36 0.1 Varies
Red Oxide 2.61 1.18 0.26 0.1 Varies
Yellow Oxide 7.31 3.32 0.72 0.3 Varies
APP 396.73 179.96 94.71 16.8 0-
30
graphite 3626 316.6 143.61 71.805 13.4 0-25
graphite 3538 105.58 47.89 23.945 4.5 0-25
Eversorb TM 93 0 0 0
Desmophen TM 1520 250.46 113.61 107.18 10.6 0-50
sub total 2362.67 1071.7 850 100
COMPONENT B
TEP 523.62 237.51 221.97 32.2 0-
50
MEK 0 0 0 0 0-10
Desmodur TM N-3600 1102.01 499.87 430.92 67.8 50-80
Xylene 0 0 0 0 0-10
sub total 1625.63 737.38 652.9 100
[0044] The polyaspartic polyurea uses an aliphatic isocyanate rather than
for
example an aromatic isocyanate, in order to impart different characteristics
to the resulting
coating, such as UV resistance. Uv resistance makes the coating 10 more
resistant to
degradation by the sun. The desmodur N-3600 is an HDI, which may be contrasted
with the
MDIs used in other coating 10 embodiments. In some embodiments, a combination
of MDI
and HDI may be used.
[0045] There are numerous examples of applications for the coatings 10
disclosed
herein. Examples include, but are not limited to, roofing, siding, insulation,
wall covering,
13
CA 02640098 2016-02-15
structural element coating, exterior covering for buildings or vehicles,
floorways, walkways,
and reinforcing covering.
[0046] A flame retardant polymer composition is also disclosed comprising
polymer
matrix, spray processable graphite, a graphite stabilizer agent, and a flame
retardant. The
polymer matrix may comprise at least one of polyurethane and polyurea. In some
embodiments, the polymer matrix comprises polyurethane. It should be
understood that the
polymer composition may have all of the same characteristics, and be made in
similar ways
as, any of the coating 10 embodiments disclosed herein. A skilled worker would
understand
that all of the methods disclosed herein may be adapted to create these
polymer
compositions, by substituting at least one of the polyamines and isocyanates
in the
preparations. For example, a two part polymerizable system may be produced
that will yield
a polyurethane coating if polyols are used in component A. Similar to coating
10, the flame
retardant polymer composition may further comprise thixatrol as a graphite
suspending
agent. In some embodiments, the flame retardant polymer composition is a
coating. In some
embodiments, the polymer composition is a combination of polyurea and
polyurethane.
[0047] The system disclosed herein may be applied in the field using
existing high
output spray equipment. The system meets fire properties that haven't been met
in any
system known to the inventors.
[0048] In the claims, the word "comprising" is used in its inclusive sense
and does
not exclude other elements being present. The indefinite article "a" before a
claim feature
does not exclude more than one of the feature being present. Each one of the
individual
features described here may be used in one or more embodiments and is not, by
virtue only
of being described here, to be construed as essential to all embodiments as
defined by the
claims.
14