Note: Descriptions are shown in the official language in which they were submitted.
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-1-
"Hematite Precipitation at Elevated Temperature and Pressure"
Field of the Invention
The present invention relates to hematite precipitation from solutions
containing
nickel, cobalt and ferric iron at elevated temperature and pressure. In
particular,
the present invention relates to a hydrometallurgical method for co-treating a
pregnant leach solution ("PLS") resulting from an atmospheric leach, with a
typical
slurry for a high pressure acid leach ("HPAL") of a sulphide concentrate,
sulphide
ore or laterite ore. More particularly, the method of the present invention is
intended to allow the precipitation of iron as hematite from the PLS of an
atmospheric leach, whilst potentiating the leach of a nickel laterite and/or
sulphide
in a HPAL circuit.
Background Art
To date, nickel laterite and sulphide ores and sulphide concentrates have
typically
been leached under conditions of elevated temperature and pressure. The HPAL
process involves the use of specialised equipment resulting in a substantial
capital outlay, in addition to costly energy requirements.
Alternatively, US Patent 4,548,794 teaches that the atmospheric leaching of
laterite ores has been found to consume higher amounts of sulphuric acid
making
this process even less economical when compared to the HPAL circuit. This is
dominated by the readily extractable iron and aluminium achieved under
atmospheric pressure and temperature.
It has been found that leach solutions generated from an atmospheric leach
operation are a valuable source of readily available ferric iron (used as an
oxidant)
with the mutual benefit of releasing free acid when the solution is discharged
to an
autoclave treating high grade laterite ore or sulphide ore or concentrates via
a
typical HPAL processing route. This significantly improves the overall
economics
of treating a nickel containing ore utilising atmospheric technology and
allows for
the co-treatment of sulphide ores or concentrates.
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-2-
In the nickel industry, iron is most often rejected as a ferric oxyhydroxide
(typically
as a goethite) and as a hematite product from the high pressure acid leaching
process for nickel laterites. In some situations iron is also rejected as a
jarosite
product.
Unfortunately, the rejection of iron as a ferric oxyhydroxide requires the
addition of
considerable quantities of neutralising agent, such as limestone, which
neutralises
the freely available sulphuric acid plus the acid formed when the ferric
sulphate is
converted to ferric oxyhydroxide. This effectively results in the loss of
valuable
sulphuric acid, which is not economic to recover from the neutralised
solutions.
In one form, the present invention economically addresses the problem of acid
regeneration resulting from hematite precipitation by recycling the product
solution
to an atmospheric leach process, or back into the HPAL circuit. Additionally,
the
requirement for a neutralising agent in the precipitation of iron from an
atmospheric leach solution is substantially overcome, and the ferric iron
present
can be utilised as the oxidant when treating sulphide ores.
The preceding discussion of the background art is intended to facilitate an
understanding of the present invention only. It should be appreciated that the
discussion is not an acknowledgement or admission that any of the material
referred to was part of the common general knowledge in Australia as at the
priority date of the application.
Throughout the specification, unless the context requires otherwise, the word
"comprise" or variations such as "comprises" or "comprising", will be
understood to
imply the inclusion of a stated integer or group of integers but not the
exclusion of
any other integer or group of integers.
Throughout the specification, the term "atmospheric" when used with reference
to
leaching is to be understood to refer to any one or more of a vat, heap, thin-
layer,
tank, dump or in-situ leach, unless the context requires otherwise.
` f3 CA 02641919 2008-08-22 PCT/AU2007/000210
Received 21 September 2007
- 3/1 -
Disclosure of the Invention
In accordance with the present invention there is provided a
hydrometallurgical
method for precipitating iron as hematite at elevated temperature and pressure
from a pregnant leach solution ("PLS") containing nickel, cobalt and iron, the
method characterised by the steps of:
(i) leaching a low to medium grade nickel laterite ore to produce a PLS
containing nickel, cobalt and ferric iron;
(ii) subjecting the PLS to elevated temperature and pressure for a time
sufficient to precipitate iron as hematite;
(iii) passing the product of step (ii) through a solids/liquid separation
step to substantially remove the hematite precipitate, and produce a
substantially iron-free, acid containing solution;
(iv) recirculating at least a portion of the substantially iron-free, acid
containing solution of step (iii) to the leach circuit of step (i) to
facilitate further leaching; and
(v) recovering nickel and cobalt from the final substantially iron-free,
acid containing solution.
Preferably, the ferric iron is in the form of ferric sulphate.
Still preferably, hematite precipitation results in the regeneration of
sulphuric acid.
Preferably, the PLS directed to the precipitation step (ii) is maintained
within the
range of about 100 C and 260 C in order to convert substantially all of the
ferric
sulphate to hematite.
Still preferably, the temperature of the PLS is maintained within the range of
about
120 C and 260 C, during the precipit%kUet(?RhW'
1PEA/AU
cA 02641919 2008-08-22 PCT/AU2007/000210
Received 21 September 2007
- 3/2 -
The residence time required for conversion of substantially all of the ferric
sulphate to hematite is preferably within the range of about 5 minutes to 180
minutes.
Amended Sheet
IPEA/AU
.
~ PCT/AU2007/000210
CA 02641919 2008-08-22
Received 21 September 2007
-4-
The pressure during hematite precipitation is preferably maintained within the
range of about 100 kPa and 4500 kPa.
More preferably, the pressure during hematite precipitation is maintained
within
the range of about 200 kPa and 4500 kPa.
In one form of the present invention the precipitation step (ii) is carried
out in a
pipe reactor.
Preferably, the concentration of nickel, cobalt and iron in the PLS directed
to the
precipitation circuit of step (ii), is within the range of about 1 to 20 g/L,
0.1 to 5 g/L
and 1 to 40 g/L, respectively.
The free acid concentration after the precipitation of hematite is preferably
in the
range of about 20 g/L to 120 g/L.
More preferably, the free acid concentration after the precipitation of
hematite is
within the range of about 30 g/L to 100 g/L.
In one form of the present invention, the PLS results from a heap leach of a
low to
medium grade nickel ore.
Further, in one form of the present invention at least a portion of the
substantially
iron-free, acid containing solution of step (iii) is recirculated to the
precipitation
circuit of step (ii) at elevated temperature and pressure.
In accordance with the present invention there is further provided a
hydrometallurgical method for precipitating iron as hematite at elevated
Amended Sheet
IPEA/AU
J CA 02641919 2008-08-22 PCT/AU2007/000210
Received 21 September 2007
-5-
temperature and pressure from a leach solution containing nickel, cobalt and
iron,
and regenerating acid for application in a further leaching process, the
method
characterised by the steps of:
(i) leaching a low to medium grade nickel laterite ore to produce a
pregnant leach solution ("PLS");
(ii) directing the PLS of step (i) containing nickel, cobalt, and ferric iron
to a high pressure acid leach ("HPAL") circuit for the treatment of a
laterite ore and/or sulphide ore or concentrate, maintaining this
solution at a required temperature and residence time, to precipitate
iron as hematite, and regenerate acid, thereby producing an
autoclave discharge slurry;
(iii) passing the autoclave discharge sluny through a solids-liquid
separation circuit to remove the hematite precipitate, and produce a
substantially iron-free, acid containing solution;
(iv) recirculating at least a portion of the substantially iron-free, acid
containing solution of step (iii) to the leach circuit of step (i) to
facilitate further leaching; and
(v) recovering nickel and cobalt from the solution of step (iii).
Preferably, the PLS directed to the HPAL is heated to within the range of
about
160 C and 260 C in order to convert substantially all of the ferric sulphate
to
hematite.
Still preferably, the PLS directed to the HPAL is heated to within the range
of
about 240 C and 260 C in order to convert substantially all of the ferric
sulphate to
hematite.
Still further preferably, the temperature of the PLS is heated to within the
range of
about 255 C and 260 C. Amended Sheet
IPEA/AU
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-6-
The residence time required for conversion of substantially all of the ferric
sulphate to hematite in the HPAL circuit is preferably within the range of
about 5
minutes to 120 minutes.
Still preferably, the residence time required for conversion of the majority
of ferric
sulphate to hematite in the HPAL circuit is within the range of about 30
minutes to
90 minutes.
The pressure in the HPAL circuit is preferably maintained within the range of
about 610kPa and 4500kPa.
The pressure in the HPAL circuit is more preferably maintained within the
range of
about 3300kPa and 4500kPa.
Still further preferably, the pressure for the HPAL conditions is maintained
within
the range of about 4300kPa and 4500kPa.
Preferably, the concentration of nickel, cobalt and iron in the PLS is within
the
range of about 1 to 20 g/L, 0.1 to 5 g/L and 1 to 40 g/L, respectively.
The free acid concentration in the HPAL circuit after the precipitation of
hematite
is preferably in the range of about 50 g/L to 120 g/L.
More preferably, the free acid concentration in the HPAL circuit after the
precipitation of hematite is within the range of about 50 g/L to 100 g/L.
The PLS is preferably preheated using one ore more heat exchangers before
entering the HPAL circuit, thereby reducing energy requirements.
Still preferably, the temperature of the PLS achieved by heat exchange prior
to
entering the HPAL circuit is preferably within the range of about 60 C and 120
C.
. ~
CA 02641919 2008-08-22 PCT/AU2007/000210
Received 21 September 2007
-7-
Preferably, the autoclave discharge slurry is cooled by passing the solution
back
through a heat exchanger.
The cooled autoclave discharge slurry is preferably within the range of about
80 C
to 140 C after passing through the heat exchanger.
In one form of the present invention the leach of step (i) is provided in the
form of
a heap leach circuit.
Brief Description of the Drawings
The present invention will now be described, by way of example only, with
reference to a first and second embodiment thereof and the accompanying
drawings, in which;
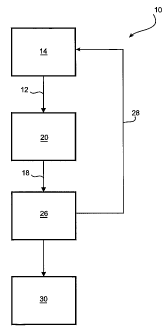
Figure 1 is a diagrammatic representation of a flow sheet depicting a
hydrometallurgical method for the precipitation of iron in the form of
hematite at elevated temperature and pressure from a pregnant leach
solution containing nickel, cobalt and iron in accordance with a first
embodiment of the present invention;
Figure 2 is a diagrammatic representation of a flow sheet depicting a
hydrometallurgical method for the precipitation of iron in the form of
hematite at elevated temperature and pressure from a pregnant leach
solution containing nickel, cobalt and iron in accordance with a second
embodiment of the present invention, the PLS being a product of a heap
leach;
Amended Sheet
IPEA/AU
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-8-
Figure 3 is a graph showing the change in iron concentration, free acid
concentration and hematite precipitation from a column leach solution,
wherein the leach liquor was heated to 140 C and held at 450 kPa in an
autoclave;
Figure 4 is a graph showing the change in iron concentration, free acid
concentration and hematite precipitation from a column leach liquor
wherein the leach liquor was heated to 200 C and held at 1600 kPa in an
autoclave; and
Figure 5 is a graph showing the change in iron concentration, free acid
concentration and hematite precipitation from a column leach liquor
wherein the leach liquor was heated to 240 C and held at 3100 kPa in an
autoclave.
Best Mode(s) for Carrying Out the Invention
In Figure 1 there is shown a hydrometallurgical method 10 for precipitating
iron in
the form of hematite at elevated temperature and pressure from a pregnant
leach
solution 12 ("PLS") containing nickel, cobalt and ferric iron in accordance
with a
first embodiment of the present invention.
The PLS 12, containing between 1 to 20g/L nickel, 0.1 to 5 g/L cobalt, and 1
to
40g/L iron, is the result of an atmospheric leach 14 of a low to medium grade
nickel laterite ore. The PLS 12 is then directed to a reactor vessel, for
example a
pipe reactor 20 in which it is heated to within the range of 100 C and 260 C,
for
example 120 C to 260 C, and maintained at a pressure within the range of 100
kPa and 4500 kPa, for example 200 kPa to 4500 kPa, for a residence time of
between 5 and 180 minutes, such that hematite is precipitated and acid
regenerated.
It is envisaged that the concentration of acid in a reacted PLS 18 resulting
from
hematite precipitation will be within the range of 20 to 120 g/L, for example
30 g/L
to 100 g/L. The reacted PLS 18 then proceeds to a solid liquid separation
circuit
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-9-
26 before the acid containing solution resulting therefrom is redirected to
the
atmospheric leach 14 to facilitate further leaching and/or being directed to
the
recovery circuit 30.
In Figure 2 there is shown a hydrometallurgical method 40 for precipitating
iron in
the form of hematite at elevated temperature and pressure from a pregnant
leach
solution 12 ("PLS") containing nickel, cobalt and ferric iron in accordance
with a
second embodiment of the present invention. The method 40 is substantially
similar to the method 10 described hereinabove and like numerals denote like
parts/steps.
The PLS 12 is collected from an atmospheric leach in the form of a heap leach
14
and is directed to a first heat exchanger 16 where it is preheated to between
about 60 C and 120 C by an autoclave discharge slurry 18 exiting a high
pressure
acid leach ("HPAL") circuit 20. The preheated PLS 22 is then directed to the
HPAL circuit 20 where it is integrated into the leach of a nickel sulphide, or
high
grade nickel laterite, or both. The ferric iron already present in the PLS 14
can be
utilised as the oxidant, thus reducing the requirement for adding an oxidant
to the
HPAL circuit 20.
The slurry in the HPAL circuit 20 is then maintained at an elevated
temperature of
between about 160 C and 260 C, for example 240 C and 260 C, or preferably
255 C and 260 C, and pressure of between about 610 kPa and 4500 kPa, for
example 3300 kPa and 4500 kPa, or preferably 4300 kPa and 4500 kPa, for the
required residence time, which is dependent on the operating conditions
adopted,
generally ranging between about 5 minutes and 120 minutes, for example
between 30 minutes to 90 minutes.
The autoclave discharge slurry 18 from the HPAL circuit 20 is cooled to
between
about 80 C and 140 C by passing it back through the heat exchanger 16. The
cooled slurry 24 then undergoes a solid/liquid separation 26 to remove the
precipitated hematite from the solution.
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-10-
It is understood by the inventors that the process of hematite precipitation
generates acid according to the following equation:
2Fe2(SO4)3 +3HZ0 H Fe203 +3H2SO4
The concentration of free acid in the separated solution 28 after the hematite
precipitation is generally within the range of about 50 g/L up to 120 g/L
sulphuric
acid, for example 50 g/L to 100 g/L. Thus the solution may be returned to the
heap leach 14 to aid further leaching, and/or it may proceed to the recovery
circuit
30.
The precipitation of hematite also at least reduces or may eliminate the
requirement for a neutralising agent, as is typically needed for the removal
of iron
as ferric hydroxide or ferric oxyhydroxide, under atmospheric conditions.
The present invention is further illustrated by way of the following non-
limiting
examples:
EXAMPLE 1
A pregnant leach solution containing high iron levels in the form of ferric
sulphate
was treated at 140 C and at 450 kPa to reduce the ferric sulphate to hematite.
The composition of the feed solution is set out in Table 1 below:
Table 1: Composition of Pregnant Leach Solution 1.
Element Concentration (mg/L)
Solution 1
Fe (total) 26,800
Fe (ferrous) 380
Fe (ferric) 26,420
Free Acid (g/1) 14.2
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-11-
Solution 1 was treated heated to 140 C with a pressure of 450 kPa and held for
120 minutes, as the iron in ferric form was converted to hematite. In addition
the
free acid concentration increased from 14.2 g/I to 32.1 g/I as the ferric
sulphate
was converted to hematite.
The composition of the resultant solution is set out in Table 2 below:
Table 2: Composition of Reduced Leach Solution 1.
Concentration (mg/L)
Element
Solution 1
Fe (total) 19,300
Fe (ferrous) 34
Fe (ferric) 19,266
Free Acid (g/1) 32.1
The change in iron concentration, free acid concentration and hematite
precipitation under these conditions are shown in Figure 3.
EXAMPLE 2
A pregnant leach solution containing high iron levels in the form of ferric
sulphate
was treated at 200 C and at 1,600 kPa to reduce the ferric sulphate to
hematite.
The composition of the feed solution is set out in Table 3 below:
Table 3: Composition of Pregnant Leach Solution 1.
Concentration (mg/L)
Element
Solution 1
Fe (total) 26,800
Fe (ferrous) 279
Fe (ferric) 26,521
Free Acid (g/1) 14.2
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-12-
Solution 1 was treated heated to 200 C with a pressure of 1,600 kPa and held
for
120 minutes, as the iron in ferric form was converted to hematite. In addition
the
free acid concentration increased from 14.2 g/I to 68.1 g/I as the ferric
sulphate
was converted to hematite. The composition of the resultant solution is set
out in
Table 4 below:
Table 4: Composition of Reduced Leach Solution 1.
Concentration (mg/L)
Element
Solution 1
Fe (total) 6,530
Fe (ferrous) 279
Fe (ferric) 6,251
Free Acid (g/1) 68.1
The change in iron concentration, free acid concentration and hematite
precipitation under these conditions are shown in Figure 4.
EXAMPLE 3
The pregnant leach solution containing high iron levels in the form of ferric
sulphate was treated at 240 C and at 3,100 kPa to reduce the ferric sulphate
to
hematite. The composition of the feed solutions is set out in Table 5 below:
Table 5: Composition of Pregnant Leach Solution 2.
Concentration (mg/L)
Element
Solution 2
Fe (total) 33,700
Fe (ferrous) 268
Fe (ferric) 33,432
Free Acid (g/1) 18.3
CA 02641919 2008-08-22
WO 2007/095689 PCT/AU2007/000210
-13-
Solution 1 was treated heated to 240 C with a pressure of 3,100 kPa and held
for
120 minutes, as the iron in ferric form was converted to hematite. In addition
the
free acid concentration increased from 18.3 g/I to 105.8 g/I as the ferric
sulphate
was converted to hematite. The composition of the resultant solution is set
out in
Table 6 below:
Table 6: Composition of Reduced Leach Solution 2.
Concentration (mg/L)
Element
Solution 2
Fe (total) 4,330
Fe (ferrous) 268
Fe (ferric) 4,062
Free Acid (g/1) 105.8
The change in iron concentration, free acid concentration and hematite
precipitation under these conditions are shown in Figure 5.
It can be seen from the above examples that significant quantities of iron can
be
removed from an atmospheric leach solution using the method hereinbefore
described. Acid has also been shown to be regenerated in sufficient quantities
to
aid further leaching, be it leaching to first generate the leach solution
containing
ferric iron or leaching at elevated temperature and pressure.
Modifications and variations such as would be apparent to the skilled
addressee
are considered to fall within the scope of the present invention.