Note: Descriptions are shown in the official language in which they were submitted.
CA 02643170 2012-03-23
29276-763D
-1-
Sterically Hindered Amine Ethers as Stabilizers Against Light. Oxygen and/or
Heat
This is a divisional application of Canadian patent application
serial No. 2,262,435, filed on February 23, 1999.
The invention relates to a new process for the preparation of sterically
hindered amine
ethers, new compounds of this class, their use as stabilizers for organic
material against
degradation by light, oxygen and/or heat and corresponding compositions.
A number of publications describe the stabilization of organic material using
specific sterically
hindered amine (HALS) compounds as stabilizers. A valuable class of sterically
hindered
amines are compounds wherein the nitrogen atom is part of a heterocyclic ring
and the
nitrogen atom carries an additional organic substituent linked over an oxygen
atom (NOR-
HALS; Kurumada et at., J.Polym.Sci, Poly.Chem. Ed. 22, 277-81 (1984); US-A-
5204473); the
oxygen-linked substituent is introduced in these compounds by etherification
of the free oxyl-
or hydroxylamine with suitable agents.
Some N-allyl nitroxides rearrange under certain conditions into amine ethers
(Meisenheimer
rearrangement; Chem. Ber. 52, 1667 (1919); Chem. Ber. 55, 513 (1922)).
Cleavage of the
nitroxide with formation of alkene and hydroxylamine (Cope elimination) is a
competing
reaction, the rate of which increases with increasing steric hindrance
(J.March, Advanced
Organic Chemistry, IV Ed., Wiley, 1992).
Now it has been found that, surprisingly, oxidation of a 1-allyl-
substituted sterically hindered amine effectively leads to the
corresponding 1-allyloxy-substituted product. According to one
aspect, the invention of the parent application pertains to a
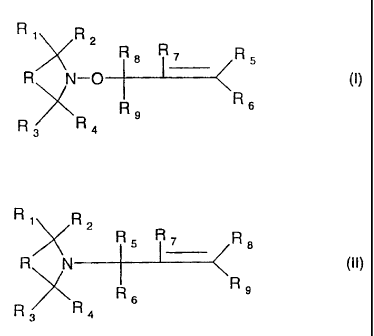
process for the preparation of a compound of the formula I
Ri R2
~X R8 7 R5
R N-0 (1)
R9 R6
R3 R4
CA 02643170 2008-10-23
29276-763D
- la -
wherein
R,, R2, R3 and R4, independently of each other, are C1-C8alkyl or C,-
C5hydroxyalkyl, or R,
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
R5, R6, R7, R8 and R9, independently of each other, are H, C1-C8alkyl, C2-
C8alkenyl, C5-
C12aryl, C1-C4haloalkyl, an electron withdrawing group, or C6-C12aryl which is
substituted by a
CA 02643170 2008-10-23
-2-
residue selected from C1-C4alkyl, C1-C4alkoxy, halogen; and R7 and R8 together
may also
form a chemical bond; and
R is an organic linking group containing 2-500 carbon atoms and forming,
together with the
carbon atoms it is directly connected to and the nitrogen atom, a substituted,
5-, 6 or 7-
membered cyclic ring structure; R preferably being a C2-C500hydrocarbon
optionally
containing 1-200 hetero atoms selected from nitrogen, oxygen, phosphorus,
sulfur, silicon
and halogen, and,
characterized in that a compound of the formula II
R1 R2
R R7 R8
R N (II)
R R
R3 R4 6
wherein all residues R and R1-R9 are as defined for formula I, is oxidized.
R7 and R8 together as a chemical bond form an allenic double bond in formula
I, and a triple
bond in formula II.
In the compounds of formula I and II and further products, R1, R2, R3 and R4
independently
preferably are methyl or ethyl, especially methyl.
R5, R6, R7, R8 and R9 as an electron withdrawing group include -CN, nitro,
halogen or -
COOR10 where R10 is C1-C12alkyl, C5-C12cycloalkyl, C7-C9phenylalkyl or phenyl.
Preferred as
electron withdrawing group are -CN or -COOR10 where R10 is C1-C12alkyl, C5-
C12cycloalkyl or
phenyl, especially wherein R10 is C1-C12alkyl or cyclohexyl.
Preferably, R5, R6, R7, R8 and R9 independently are H or methyl, especially H.
Also preferred
are compounds wherein R5 and R6 independently are H or methyl, especially H,
and R7, R8
and R9 independently are haloalkyl, phenyl, vinyl, nitro, CN, COOR10, or R7
and R8 together
form a chemical bond.
Further preferences for the linking group R are mainly as described below for
products of
formulae 111, IV and V.
CA 02643170 2008-10-23
-3-
Of special importance is a process for the preparation of a compound of the
formula I by
oxidation of a compound of the formula II,
wherein
R,, R2, R3 and R4, independently of each other, are C1-C8alkyl or C,-
C5hydroxyalkyl, or R,
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
R5, R6, R7, R8 and R9, independently of each other, are H, C,-C8alkyl, C3-
C8alkenyl, C5-
C12aryl, an electron withdrawing group, C6-C,2aryl which is substituted by C1-
C4alkyl, C1-
C4alkoxy, halogen; and
R is a C3-C5 hydrocarbon optionally containing 1-200 hetero atoms selected
from nitrogen,
oxygen, phosphorus, sulfur and halogen, and forming, together with the two
carbon and the
nitrogen atom, a substituted, 6-membered cyclic ring structure.
Aryl stands for a group obeying the Debye-Hueckel rule; preferred as C6-
C12aryl are phenyl
and naphthyl.
Alkyl is a branched or unbranched radical, embracing, within the definitions
given, methyl,
ethyl, propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, 2-
ethylbutyl, n-pentyl, isopentyl,
1-methylpentyl, 1,3-dimethylbutyl, n-hexyl, 1-methylhexyl, n-heptyl,
isoheptyl, 1,1,3,3-tetra-
methylbutyl, 1 -methylheptyl, 3-methylheptyl, n-octyl, 2-ethylhexyl, 1,1,3-
trimethylhexyl,
1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl, 1-methylundecyl, dodecyl,
1,1,3,3,5,5-hexa-
methylhexyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl,
octadecyl, eicosyl or do-
cosyl.
Alkanoyl is alkyl connected over a carbonyl linkage; thus, C2-C20alkanoyl
includes acetyl,
propionyl, butyryl, hexanoyl, steaoryl.
Haloalkyl is alkyl substituted by halogen, e.g. 1 or 2 halogen atoms. Halogen
atoms are
preferably chloro or bromo, especially bromo.
Cycloalkyl is a saturated monovalent monocyclic hydrocarbon residue, e.g.
cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, cyclododecyl; preferred is cyclohexyl:
Organic residues or hydrocarbons containing heteroatoms, such as alkyl or
alkylene
interrupted by hetero groups like oxygen or NH, usually contain these
heteroatoms as typical
CA 02643170 2008-10-23
-4-
functional groups like oxo, oxa, hydroxy, carboxy, ester, amino, amido, nitro,
nitrilo,
isocyanato, fluoro, chloro, bromo, phosphate, phosphonate, phosphite, silyl,
thio, sulfide,
sulfinyl, sulfo, heterocyclyl including pyrrolyl, indyl, carbazolyl, furyl,
benzofuryl, thiophenyl,
benzothiophenyl, pyridyl, chinolyl, isochinolyl, pyridazinyl, pyrimidinyl,
pyrazinyl, triazolyl,
benzotriazolyl, triazinyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, and
corresponding saturated
and/or substituted groups like, for example, piperidyl, piperazinyl,
morpholinyl etc.. They may
be interrupted by one or more of these groups; usually there are no linkages
of the type 0-0,
O-N (except nitro, cyanato, isocyanato, nitroso), N-N (except in heterocyclic
ring structures),
N-P or P-P present, regardless of the order.
Preferably, in organic residues or hydrocarbons containing heteroatoms such as
R there is
not more than one heteroatom attached by a single bond to the same carbon
atom. A spacer
consisting of one or more heteroatoms usually is embedded in a carbon chain or
ring or
inserted into a carbon-hydrogen bond.
Compounds of the formula I can be monomeric or polymeric. They contain 1 or
more groups
of the formula I'
R' R2
---~ R8 R7 R5
N-O (I')
R7'\ R R s R 6
3 4
In case that the compounds of the formula I' are polymeric, they contain a
group of the
formula I' in the repeating structural unit.
Starting compounds of the formula 11 are known in the art or can be obtained
in analogy to
known compounds. Present process can start from isolated compounds of the
formula Il or
can use the solution of these starting compounds as obtained directly after
synthesis.
In the process of present invention, the oxidation reaction can be carried out
using known
oxidants, e.g. oxygen, peroxides or other oxidizing agents such as nitrates,
permanganates,
chlorates; preferred are peroxides, such as hydrogen peroxide based systems,
especially
peracids such as perbenzoic acid or peracetic acid. The oxidant is
conveniently used in
CA 02643170 2012-03-23
29276-763D
-5-
stoiciometric amount or in excess, e.g. using 1-2 moles active oxyen atoms for
each group of
the formula I' in the desired product.
The reaction can be carried out in the presence of a suitable solvent, for
example an
aromatic or aliphatic hydrocarbon, alcohol, ester, amide, ether, or
halogenated hydrocarbon;
examples are benzene, toluene, xylene, mesitylene, methanol, ethanol,
propanol, butanol,
dimethylformamide, dimethylsulfoxide, methylene chloride; preferred is a C1-
C4alcohol,
benzene, toluene, xylene, or chlorinated C,-C6hydrocarbon.
Temperature and pressure are not critical and depend mainly on the oxidant
system used;
preferably, temperature is kept during the reaction in the range between -20 C
and +40 C.
Conveniently, the pressure is kept close to atmospheric pressure, e.g. between
0.5 and 1.5
bar; when oxidation is achieved with gaseous oxygen, the pressure of oxygen or
oxygen/inertgas may exceed atmospheric pressure.
The process of present invention can be followed by further process steps
known in the art,
e.g. hydrogenation of an ethylenic double bond, halogenation, e.g.
bromination, of an
ethylenic double bond and/or polymerization, with or without previous
isolation of the product
of formula I.
Hydrogenation of the ethylenic double bond (carbon-carbon double bond) in the
compound
of the formula I can be achieved by methods known in the art, e.g. reaction
with gaseous
hydrogen under catalytic conditions or reaction with hydrogenating agents.
Preferred is
catalytic hydrogenation; known catalysts can be employed such as Pt, Pt, Pd,
Ni, Ru, Rh, on
support such as carbon or without support, Raney-Ni etc.
Hydrogenation of an allenic double bond in a compound of formula I wherein R7
and R8
together are a chemical bond, proceeds in 2 steps. The first step leads to a
partly
hydrogenated product, which may be isolated or subjected to further
derivatization, and
which corresponds to formula IV
CA 02643170 2008-10-23
-6-
R1 R2
H/R s
R N-OH C\ (IV)
R
6
R R R s
3 4
where R, R,-R6 and R9 are as defined above.
Complete hydrogenation yields a product of the formula III
R1 R2
R8 R7 H/R5
R N-O H C\ (III)
R
s
R >(R R s
3 4
where R, R,-R6 and R9 are as defined above for formula I and R7 and R8 are H,
C,-C8alkyl,
C3-C8alkenyl, C5-C,2aryl, C,-C4haloalkyl, an electron withdrawing group, or C6-
C,2aryl which
is substituted by a residue selected from C,-C4alkyl, C,-C4alkoxy, halogen.
Halogenation is another follow-up reaction which may be carried out subsequent
to the
process of present invention, mainly according to methods known in the art and
using the
appropriate reactant, e.g. as summarized in J. March, Advanced Organic
Chemistry:
Reaction mechanisms and Structure, 4th Edn., Wiley, 1992, p. 812. Halogen
thereby is
added to the carbon-carbon double bond of a compound of formula I or IV,
resulting in an a,
3-dihalogenated product. The halogen reagent X2, where X is F, Cl, Br, I or
preferably Cl or
Br, especially Br, can be applied in gaseous, liquid or solid form, pure or as
a solution.
Halogen can also be released during the reaction in appropriate amounts using
a suitable
carrier substance or source.
Reactions can be carried out according to methods known in the art using
hydrogen
pressures in the common range, preferably between 0.5 and about 200 bar,
especially
between 1 and 100 bar (1 bar =105 Pa). Reactions can be carried out using
suitable
solbents, e.g. water, hydrocarbons like hexane, petrol fractions, toluene,
xylene, esters,
ethers, halogenated hydrocarbons or alcohols like methanol or ethanol.
Reactions can also
be carried out without solvent. Temperatures are uncritical and are mainly in
the range
CA 02643170 2008-10-23
-7-
between -10 C and about 150 C, e.g. between 0 C and the boiling point of the
solvent like
the range 0-100 C or 20-80 C.
Present invention therefore also pertains to a process for the preparation of
a compound of
the formula V
RR 2
R8 R' R5
R N-O R 6 (V)
R9 R20 R21
R3 R4
wherein R, R1, R2, R3 and R4, R5, R6, and R9 are as defined for formula I,
R7 and R8 are H, C1-C8alkyl, C3-C8alkenyl, C5-C12aryl, C1-C4haloalkyl,
halogen, an electron
withdrawing group, or C6-C12aryl which is substituted by a residue selected
from C1-C4alkyl,
C1-C4alkoxy, halogen; and
both of R20 and R21 are either hydrogen or halogen; characterized in that a
compound of the
formula II is oxidized and the resulting intermediate of formula I is
subjected to hydrogenation
and/or halogenation.
The above process can also be carried out in a way wherein the intermediate of
formula I is
derivatized to become another structure of formula I before subjecting it to
hydrogenation
and/or halogenation, e.g. by esterification, dimerization, trimerization or
polymerization; or
wherein the intermediate of formula I is first subjected to hydrogenation, and
then further
derivatized, e.g. by esterification, dimerization, trimerization or
polymerization.
Preferred is a process for the preparation of a compound of the formula V
wherein
R is an organic linking group containing 2-500 carbon atoms and forming,
together with the
carbon atoms it is directly connected to and the nitrogen atom, a substituted,
5-, 6 or 7-
membered cyclic ring structure;
R1, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
C5hydroxyalkyl, or R1
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
R5i R6, and R9, independently of each other, are H, C1-C8alkyl, C3-C8alkenyl,
C5-C12aryl, C1-
C4haloalkyl, an electron withdrawing group, or C6-Ct2aryl which is substituted
by a residue
selected from C1-C4alkyl, C1-C4alkoxy, halogen;
CA 02643170 2008-10-23
29276-763
-8-
R7 and R8 are H, C,-Cealkyl, C3-Cealkenyl, C5-C12aryl, C1-C4haloalkyl,
halogen, an electron
withdrawing group, or C6-C12aryl which is substituted by a residue selected
from C1-C4alkyl,
C1-C4alkoxy, halogen; and both of R20 and R21 are either hydrogen or halogen.
Most preferred products of the process of present invention are of formulae
llic, lVa and Va
described further below.
In general, all products of present process can be used as stabilizers for
organic material
against detrimental effects of light, oxygen and heat. Of special value are
the compounds of
formulae I, la, III, IV and V. Best results are achieved with compounds of
formula Ill wherein
R, R,, R2, R3 and R4, are as defined for formula I and
R5, R6i R7, R8 and R9, independently of each other, are H, C1-C8alkyl, C2-
C8alkenyl, C5-
C12aryl, CN, COOR10, where R10 is as defined for formula I, or are C6-C12aryl
which is
substituted by a residue selected from C,-C4alkyl, C1-C4alkoxy. Organic
material most
effectively stabilized by present compounds is organic polymeric material
described below,
e.g. coatings and thermoplastic bulk polymers, films or fibers. Where the
polymers come in
contact with a pesticide, e.g. a pesticide containing sulfur and/or halogen
atoms, the
products of present process achieve both a stabilization against light and
detrimental effects
of the pesticide. This is especially important for polymers, e.g. films, tapes
or fibers, used in
agricultural applications, mainly polyolefines such as PE or PP or polyolefin
copolymers.
Preferred compounds in this application are those of formula Ill, especially
111c.below.
An important utility for all products of present process is the stabilization
of paper and pulp,
especially paper or pulp still containing lignin, against yellowing.
Application of present
products can be done as described, for-example, in the international patent
application No.
WO 98/04381, and
publications cited therein. Most preferred for this application is the use of
a monomeric
compound of the formula Ill, e.g. one wherein R is C3-C12 alkylene, or C4-
C12alkylene
interrupted by 0, NH, OCO or NHCO, R1-R4 are methyl or ethyl, especially
methyl, and R5-R9
are each H or C1-C4alkyl, especially H; examples are 1-propyloxy-2,2,6,6-
tetramethylpiperidine, 1-propyloxy-2,2,6,6-tetramethylpiperidine-4-one, or the
product of
present example 4a (see below).
Further, the products of present process can be used with advantage as flame
retardants for
organic polymers. Thus, by using the products of present invention, an organic
polymer is
stabilized against detrimental effects of light, oxygen and heat, while the
same time the
inflammability of the polymer is effectively reduced. Application of present
products can be
done as described, for example, in the international patent application No. WO
98/13469
and publications cited therein,
CA 02643170 2008-10-23
29276-763D
9 -
as well as EP-A-792911 or US-A-5393812. Most preferred for this utility is the
use of a
compound of the formula III or V, especially those of formula Illc below or of
formula V
wherein at least one of R5, R6, R7, R8, R9, R20 or R21 is halogen, especially
bromo. Present
compounds can be used as flame retardants alone or in combination with known
flame
retardants like a flame retardant compound selected from the halogenated,
phosphorus,
boron, silicon and antimony compounds, metal hydroxides, metal hydrates and
metal oxides
or mixtures thereof.
It has been a further finding of this invention that some compounds of formula
III are
especially well suitable as stabilizers for organic material against
detrimental effects of light,
oxygen and heat.
According to another aspect of the invention, the present
divisional application provides compositions comprising
A) an organic polymer which is sensitive to oxidative, thermal and/or actinic
degradation, and
B) at least one compound of the formula Illa, IVa or Va
R1 R2
,i( R' N-O-C C-CH3 (Ilia)
H2 H2
R3 >(R R R2
R R
1 22 5
R N-O R 6 (IVa)
R R23
R3 R4
CA 02643170 2008-10-23
-10-
R1 R2
R8 R7 R5
R N-O R 6 (Va)
R9 R20 R21
R3 R4
wherein
H2
C-
Rand R each is an organic linking group of the formula E/ (VI);
E2
E2 is -CO- or -(CH2)P , where p is 0, 1 or 2;
E1 is a carbon atom carrying the two residues R24 and R25, or is >N-R25, or is
oxygen, and R24
and R25 are hydrogen or an organic residue, characterized in that the linking
group R in total
contains 2-500 carbon atoms and forms, together with the carbon atoms it is
directly
connected to it and the nitrogen atom, a substituted, 5-, 6 or 7-membered
cyclic ring
structure;
R1, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
Cshydroxyalkyl, or R,
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
R5, R6, R7, R8 and R9, independently of each other, are H, C1-C8alkyl, C2-
C8alkenyl, C5-
C12aryl, C1-C4haloalkyl, an electron withdrawing group, or C6-C12aryl which is
substituted by a
residue selected from C1-C4alkyl, C1-C4alkoxy, halogen;
R20 and R21 are halogen; and
R22 and R23 are hydrogen or together are a chemical bond.
H2
R C-
Usually R' in formula Iila is not the linking group R 24\ / wherein R24 and
R25 together
25 C-
H2
are =0 or wherein R24 is hydrogen and R25 is hydrogen or hydroxy.
Preferred is a formula Illa wherein His a C7-Cs00hydrocarbon containing 1-200
hetero atoms
selected from nitrogen, oxygen, phosphorus, sulfur and halogen, and forming,
together with
CA 02643170 2012-03-23
29276-763D
-11-
the two carbon and the nitrogen atom, a substituted, 5- or 6-membered cyclic
ring
structure, and
R1, R2, R3 and R4 are as defined above.
According to a still further aspect, the invention relates to compound of the
formula IIIc, IVa or Va
R1
R2
R N-O-C-C-CH3 (IIIc)
H2 H2
R3 R4
R1
R2
R22 R5
R N-O R6 (IVa)
R9 R23
R R4
R1
R2
R8 R7 R5
R N-O R6 (Va)
R9 R20 R21
R3 - R4
wherein R is the structure
H2 H2
H2 C- C-
R24xC (Via), R26-N (VIb) or O (VIC)
R25 (CH2)P O 0
CA 02643170 2012-03-23
29276-763D
- 11a -
where p is 0, 1 or 2;
R1, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
C5hydroxyalkyl,
or R1 and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl,
or R3 and R4 together with the carbon atom they are attached to are C5-
C12cycloalkyl;
R5 and R6 independently are H or methyl; and R7, R8 and R9 independently are
C1-C4haloalkyl, phenyl, vinyl, nitro, CN, COOR10, where R10 is C1-C12alkyl,
C5-C12cycloalkyl or phenyl;
R24 and R25 independently are hydrogen or form together with the remaining
structure of
(Vla) a divalent C7-C500 hydrocarbon or a C2-C5oohydrocarbon containing 1-200
hetero
atoms selected from nitrogen, oxygen, phosphorus, sulfur, silicon and halogen;
R26 is hydrogen or forms together with the remaining structure of formula
(Vlb) a
C2-C5oohydrocarbon containing 1-200 hetero atoms selected from nitrogen,
oxygen,
phosphorus, sulfur, silicon and halogen;
R20 and R21 are halogen; and
R22 and R23 are hydrogen or together are a chemical bond,
with the proviso that R in formula Illc is not the linking group
H2
R24: /C-
R25 'x\C-
H2
wherein R24 and R25 together are =0 or wherein R24 is hydrogen and R25 is
hydrogen,
OH, or alkanoyloxy which is substituted by phenoxy or alkylphenoxy.
CA 02643170 2012-03-23
29276-763D
-11b-
According to a still further aspect, the invention relates to compound of the
formula la
A A
fNN R12 N N
N~ INI R11 N~ INI
Y H3C CH3 Y
B B
H3C NI CH3
(Ia)
R9 R8
-R7
R6 R5 n
in which the index n ranges from 1 to 15;
R5, R6, R7, R8 and R9, independently of each other, are C1-C8alkyl, C3-
C8alkenyl,
C5-C12aryl, C6-C12aryl which is substituted by C1-C4alkyl, C1-C4alkoxy,
halogen, -CN,
nitro, halogen or -COOR1o where R10 is C1-C12alkyl, C5-C12cycloalkyl,
C7-C9phenylalkyl or phenyl;
R12 is C2-C12alkylene, C4-C12alkenylene, C5-C7cycloalkylene, C5-
C7cycloalkylene-
di(C1-C4alkylene), C1-C4alkylenedi(C5-C7cycloalkylene), phenylenedi(C1-
C4alkylene)
or C4-C12alkylene interrupted by 1,4-piperazinediyl, -0- or >N-X1 with X1
being
C1-C12acyl or (C1-C12alkoxy)carbonyl or having one of the definitions of R14
given
below except hydrogen; or R12 is a group of the formula (lb') or (Ic');
-CH2-CH-CH2- (Ib)
O
I
C=0
I
X2
CA 02643170 2012-03-23
29276-763D
-11c-
/O O
- X3--< )--- X3 (Ic')
O
X2 being C1-C18alkyl, C5-C12cycloalkyl which is unsubstituted or substituted
by 1, 2
or 3 C1-C4alkyl; phenyl which is unsubstituted or substituted by 1, 2 or 3 C1-
C4alkyl or
C1-C4alkoxy; C7-Cgphenylalkyl which is unsubstituted or substituted on the
phenyl
by 1, 2 or 3 C1-C4alkyl; and
the radicals X3 being independently of one another C2-C12alkylene;
the radicals A are independently of one another -OR13, -N(R14)(R15) or a group
of the
formula (Id');
H3C CH3
4R8 R7
-X N-O RS (Id')
R6
R9
H3C CH3
R13, R14 and R15, which are identical or different, are hydrogen, C1-C18alkyl,
C5-C12cycloalkyl which is unsubstituted or substituted by 1, 2 or 3 C1-
C4alkyl;
C3-C18alkenyl, phenyl which is unsubstituted or substituted by 1, 2 or 3 C1-
C4alkyl or
C1-C4alkoxy; C7-Cgphenylalkyl which is unsubstituted or substituted on the
phenyl
by 1, 2 or 3 C1-C4alkyl; tetrahydrofurfuryl or C2-C4alkyl which is substituted
in the 2, 3
or 4 position by -OH, C1-C8alkoxy, di(C1-C4alkyl)amino or a group of the
formula (le');
Y N- (le')
with Y being -0-, -CH2-, -CH2CH2- or >N-CH3,
or -N(R14)(R15) is additionally a group of the formula (le');
X is -0- or >N-R16;
CA 02643170 2012-03-23
29276-763D
-11d-
R16 is hydrogen, C1-C18alkyl, C3-C18alkenyl, C5-C12cycloalkyl which is
unsubstituted
or substituted by 1, 2 or 3 C1-C4alkyl; C7-Cgphenylalkyl which is
unsubstituted or
substituted on the phenyl by 1, 2 or 3 C1-C4alkyl; tetrahydrofurfuryl, a group
of the
formula (If'),
H3C CH3
R8 R7 R5
N-O 1-4 (If)
R R6
9
H3C CH3
or C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C1-
C8alkoxy, di(C1-
C4alkyl)amino or a group of the formula (le');
R11 has one of the definitions given for R16; and
the radicals B have independently of one another one of the definitions given
for A.
Current invention further provides for the use of compounds of the formula
Ilia for
stabilizing organic polymers against oxidative, thermal or actinic
degradation. The
invention likewise comprises a method of stabilizing organic polymers against
thermal, oxidative and/or actinic degradation, which comprises adding to the
polymer
at least one compound of the formula Ilia.
CA 02643170 2012-03-23
29276-763D
-11e-
More detailed examples of sterically hindered amines are described below under
classes (a')
to O').
(a') A compound of the formula (1 a)
CH3 G1
G-CH2
G1 N )-o G12
G-CH2 (1 a)
CH3
ni
in which n1 is a number from 1 to 4, G and G1, independently of one another,
are hydrogen or
methyl,
G11 is n-propoxy, O-CH=C=CH2, O-CH=CH-CH3 or halogenated n-propoxy, especially
n-
propoxy, or brominated n-propoxy;
G12, if n1 is 1, is hydrogen, C1-C18alkyl which is uninterrupted or
interrupted by one or more
oxygen atoms, cyanoethyl, benzoyl, glycidyl, a monovalent radical of an
aliphatic,
cycloaliphatic, araliphatic, unsaturated or aromatic carboxylic acid, carbamic
acid or
phosphorus-containing acid or a monovalent silyl radical, preferably a radical
of an aliphatic
carboxylic acid having 2 to 18 carbon atoms, of a cycloaliphatic carboxylic
acid having 7 to
15 carbon atoms, or an a,(3-unsaturated carboxylic acid having 3 to 5 carbon
atoms or of an
aromatic carboxylic acid having 7 to 15 carbon atoms, where each carboxylic
acid can be
substituted in the aliphatic, cycloaliphatic or aromatic moiety by-1 to 3 -
COOZ12 groups, in
which Z12 is H,*C1-C20alkyl, C3-C12alkenyl, C5-C7cycloalkyl, phenyl or benzyl,
CA 02643170 2008-10-23
-12-
G12, if n1 is 2, is C2-C12alkylene, C4-C12alkenylene, xylylene, a divalent
radical of an aliphatic,
cycloaliphatic, araliphatic or aromatic dicarboxylic acid, dicarbamic acid or
phosphorus-
containing acid or a divalent silyl radical, preferably a radical of an
aliphatic dicarboxylic acid
having 2 to 36 carbon atoms, or a cycloaliphatic or aromatic dicarboxylic acid
having 8-14
carbon atoms or of an aliphatic, cycloaliphatic or aromatic dicarbamic acid
having 8-14
carbon atoms, where each dicarboxylic acid may be substituted in the
aliphatic,
cycloaliphatic or aromatic moiety by one or two -COOZ12 groups,
G12, if n1 is 3, is a trivalent radical of an aliphatic, cycloaliphatic or
aromatic tricarboxylic acid,
which may be substituted in the aliphatic, cycloaliphatic or aromatic moiety
by
-COOZ12, of an aromatic tricarbamic acid or of a phosphorus-containing acid,
or is a trivalent
silyl radical,
and G12, if n1 is 4, is a tetravalent radical of an aliphatic, cycloaliphatic
or aromatic
tetracarboxylic acid.
The carboxylic acid radicals mentioned above are in each case taken to mean
radicals of the
formula (-CO)XR, where x is as defined above, and the meaning of R arises from
the
definition given.
Alkyl with up to 20 carbon atoms is, for example, methyl, ethyl, n-propyl, n-
butyl, sec-butyl,
tert-butyl, n-hexyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-undecyl, n-
dodecyl,
n-tridecyl, n-tetradecyl, n-hexadecyl or n-octadecyl.
Examples of several G12 radicals are given below.
If G12 is a monovalent radical of a carboxylic acid, it is, for example, an
acetyl, caproyl,
stearoyl, acryloyl, methacryloyl, benzoyl or (3-(3,5-di-tert-butyl-4-
hydroxyphenyl)propionyl
radical.
If G12 is a monovalent silyl radical, it is, for example, a radical of the
formula
-(C;H2j)-Si(Z')2Z", in which j is an integer in the range from 2 to 5, and Z'
and Z",
independently of one another, are C1-C4alkyl or C1-C4alkoxy.
If G12 is a divalent radical of a dicarboxylic acid, it is, for example, a
malonyl, succinyl,
glutaryl, adipoyl, suberoyl, sebacoyl, maleoyl, itaconyl, phthaloyl,
dibutylmalonyl,
CA 02643170 2008-10-23
-13-
dibenzylmalonyl, butyl(3,5-di-tert-butyl-4-hydroxybenzyl)malonyl or
bicycloheptenedicarbonyl
radical or a group of the formula
O
H3C-O --0- CH -C
C
O
If G12 is a trivalent radical of a tricarboxylic acid, it is, for example, a
trimellitoyl, citryl or
nitrilotriacetyl radical.
If G12 is a tetravalent radical of a tetracarboxylic acid, it is, for example,
the tetravalent radical
of butane-1,2,3,4-tetracarboxylic acid or of pyromellitic acid.
If G12 is a divalent radical of a dicarbamic acid, it is, for example,
hexamethylenedicarbamoyl
or 2,4-toluylenedicarbamoyl radical.
Preference is given to compounds of the formula (1 a) in which G and G1 are
hydrogen, G11 is
hydrogen or methyl, n1 is 2 and G12 is the diacyl radical of an aliphatic
dicarboxylic acid
having 4-12 carbon atoms.
(b') A compound of the formula (1 b)
CA 02643170 2008-10-23
-14-
CH3 G,
G-CH2 i13
Gõ -N N G14 (1 b)
G-CH2
CH3
n2
in which G and G1, independently of one another, are hydrogen or methyl,
Gõ is n-propoxy,
G13 is hydrogen, C1-C12alkyl, C2-C5hydroxyalkyl, C5-C7cycloalkyl, C7-
C8aralkyl,
C1-C18alkanoyl, C3-C5alkenoyl, benzoyl or a group of the formula (1 b-1)
CH3 G1
G-CH2
Gõ-N (1b-1)
G-CH2
CH3
n2 is the number 1, 2 or 3;
and G14, if n2 is 1, is hydrogen, C1-C18alkyl, C3-C8alkenyl, C5-C7cycloalkyl,
C1-C4alkyl which is
substituted by a hydroxyl, cyano, alkoxycarbonyl or carbamide group, glycidyl,
a group of the
formula -CH2-CH(OH)-Z or of the formula -CONH-Z, in which Z is hydrogen,
methyl or
phenyl;
G14, if n2 is 2, is C2-C12alkylene, C6-C12arylene, xylylene, a -CH2-CH(OH)-CH2
group or a
-CH2-CH(OH)-CH2-O-D-O- group, in which D is C2-C10alkylene, C6-C15arylene,
C6-C12cycloalkylene, or, provided that G13 is not alkanoyl, alkenoyl or
benzoyl, G14 can
alternatively be 1-oxo-C2-C12alkylene, a divalent radical of an aliphatic,
cycloaliphatic or
aromatic dicarboxylic acid or dicarbamic acid or alternatively the group -CO-,
G14, if n2 is 3, is a group
CA 02643170 2008-10-23
-15-
O
-CH2CH (OH)CH2 N N / CH2CH(OH)CH2
O N O
1
CH2CH(OH)CH2-
or, if n2 is 1, G13 and G14 together can be the divalent radical of an
aliphatic, cycloaliphatic or
aromatic 1,2- or 1,3-dicarboxylic acid.
Some examples for the radicals G13, G14 and D are given below.
Any alkyl substituents are as defined above for (a').
Any C5-C7cycloalkyl substituents are, in particular, cyclohexyl.
C7-C8aralkyl G13 is, in particular, phenylethyl or especially benzyl.
C2-C5hydroxyalkyl G13 is, in particular, 2-hydroxyethyl or 2-hydroxypropyl.
C1-C18alkanoyl G13 is, for example, formyl, acetyl, propionyl, butyryl,
octanoyl, dodecanoyl,
hexadecanoyl, octadecanoyl, but preferably acetyl, and C3-C5alkenoyl G13 is,
in particular,
acryloyl.
C2-C8alkenyl G14 is, for example, allyl, methallyl, 2-butenyl, 2-pentenyl, 2-
hexenyl or
2-octenyl.
G14 as a hydroxyl-, cyano-, alkoxycarbonyl- or carbamide-substituted C1-
C4alkyl can be, for
example, 2-hydroxyethyl, 2-hydroxypropyl, 2-cyanoethyl, methoxycarbonylmethyl,
2-ethoxycarbonylethyl, 2-aminocarbonylpropyl or 2-
(dimethylaminocarbonyl)ethyl.
Any C2-C12alkylene radicals are, for example, ethylene, propylene, 2,2-
dimethylpropylene,
tetramethylene, hexamethylene, octamethylene, decamethylene or
dodecamethylene.
CA 02643170 2008-10-23
-16-
Any C6-C15arylene substituents are, for example, o-, m- or p-phenylene, 1,4-
naphthylene or
4,4'-diphenylene.
C6-C12cycloalkylene is, in particular, cyclohexylene.
G14 as 1-oxo-C2-C12alkylene is preferably a group
II I CH3
-C-C-
I
CH3
Preference is given to compounds of the formula (1 b) in which n2 is 1 or 2, G
and G1 are
hydrogen, G13 is hydrogen, C1-C,2alkyl or a group of the formula
CH3 G1
G-CH2
Gt1-N
G-CH2
CH3
and G14, in the case where n2=1, is hydrogen or C1-C12alkyl, and, in the case
where n2=2, is
C2-C8alkylene or 1-oxo-C2-C8alkylene.
(c') A compound of the formula (1c)
CH G
G-CH2 1
G11-N G15 (1c)
O
G-CH2
CH3
n3
CA 02643170 2008-10-23
-17-
in which n3 is the number 1 or 2, G, G, and Gõ are as defined under (b'), and
G15, if n3 is 1,
is C2-C8alkylene, C2-C8hydroxyalkylene or C4-C22acyloxyalkylene, and if n3 is
2, G15 is the
(-CH2)2C(CH2-)2 group.
C2-C8alkylene or C2-C8hydroxyalkylene G15 is, for example, ethylene, 1 -
methylethylene,
propylene, 2-ethylpropylene or 2-ethyl-2-hydroxymethylpropylene.
C4-C22acyloxyalkylene G15 is, for example, 2-ethyl-2-acetoxymethylpropylene.
(d') A compound of the formula (1d-1), (1d-2) or (1d-3),
CH3 G16 p
G-CH2 G, "
N-C
G- N
CN G17 (1 d-1)
G-CH2 II
CH3 O
n4
CH3 T1
G, I
G-CH2 O-C-T2
GTj-- N I (1d-2)
N-C
G-CH2 I
O
CH3 H
CH3 Ti
G
GH2 3 (
1d-3)
n4
CA 02643170 2008-10-23
-18-
in which n4 is the number 1 or 2, G, G, and Gõ are as defined under (b'),
G16 is hydrogen, C,-C12alkyl, allyl, benzyl, glycidyl or C2-C6alkoxyalkyl, and
G17, if n4 is 1, is hydrogen, C1-C12alkyl, C3-C5alkenyl, C7-C9aralkyl, C5-
C7cycloalkyl,
C2-C4hydroxyalkyl, C2-C6alkoxyalkyl, C6-C10aryl, glycidyl or a group of the
formula
-(CH2)p COO-Q or -(CH2)p O-CO-Q, in which p is 1 or 2, and 0 is C1-C4alkyl or
phenyl, and
G17, if n is 2, is C2-C12alkylene, C4-C12alkenylene, C6-C12arylene, a group of
the formula
-CH2-CH(OH)-CH2-O-D'-O-CH2-CH(OH)-CH2-, in which D' is C2-Cloalkylene, C6-
C15arylene,
C6-C12cycloalkylene or a group of the formula -CH2CH(OD")CH2-(OCH2-
CH(OD")CH2)2-, in
which D" is hydrogen, C1-C18alkyl, allyl, benzyl, C2-C12alkanoyl or benzoyl,
T, and T2, independently of one another, are hydrogen, C1-C18alkyl or
unsubstituted or
halogen- or C1-C4alkyl-substituted C6-C10aryl or C7-C9aralkyl, or
T, and T2 together with the carbon atom bonding them form a C5-C14cycloalkane
ring.
A compound of the formula (1 d-3) is preferred.
Some examples of the several variables in the formulae (1 d-1), (1 d-2) and (1
d-3) are given
below.
Any C,-C12alkyl substituents are, for example, methyl, ethyl, n-propyl, n-
butyl, sec-butyl, tert-
butyl, n-hexyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-undecyl or n-
dodecyl.
Any C1-C18alkyl substituents can be, for example, the abovementioned groups
and in
addition, for example, n-tridecyl, n-tetradecyl, n-hexadecyl or n-octadecyl.
Any C2-C6alkoxyalkyl substituents are, for example, methoxymethyl,
ethoxymethyl,
propoxymethyl, tert-butoxymethyl, ethoxyethyl, ethoxypropyl, n-butoxyethyl,
tert-butoxyethyl,
isopropoxyethyl or propoxypropyl.
C3-C5alkenyl G17 is, for example, 1 -propenyl, allyl, methallyl, 2-butenyl or
2-pentenyl.
C7-C9aralkyl G17, T, and T2 are, in particular, phenethyl or especially
benzyl. If T, and T2
together with the carbon atom form a cycloalkane ring, this can be, for
example, a cyclo-
pentane, cyclohexane, cyclooctane or cyclododecane ring.
CA 02643170 2008-10-23
-19-
C2-C4hydroxyalkyl G17 is, for example, 2-hydroxyethyl, 2-hydroxypropyl, 2-
hydroxybutyl or
4-hydroxybutyl.
C6-C10aryl G17, T, and T2 are, in particular, phenyl or a- or R-naphthyl,
which are
unsubstituted or substituted by halogen or C1-C4alkyl.
C2-C12alkylene G17 is, for example, ethylene, propylene, 2,2-
dimethylpropylene,
tetramethylene, hexamethylene, octamethylene, decamethylene or
dodecamethylene.
C4-C12alkenylene G17 is, in particular, 2-butenylene, 2-pentenylene or 3-
hexenylene.
C6-C12arylene G17 is, for example, o-, m- or p-phenylene, 1,4-naphthylene or
4,4'-diphenylene.
C2-C12alkanoyl D" is, for example, propionyl, butyryl, octanoyl, dodecanoyl,
but preferably
acetyl.
C2-C10alkylene, C6-C15arylene or C6-C12cycloalkylene D' have, for example, one
of the
definitions given for D under (b').
(e') A compound of the formula (1e)
Gis
N N
GZ0 (1 e)
G19 N
n5
in which n5 is the number 1 or 2, and G18 is a group of the formula
CA 02643170 2008-10-23
-20-
CH3 G, CH3 G,
G-CH2 G-CH2\_G2
Gõ-N (A)x E- or Gõ-N N-(A)- E-
G-CH2 G-CH( I
CH3 CH3
in which G and G11 are as defined under (b'), and G, and G2 are hydrogen,
methyl or,
together, are a substituent =0,
E is -0- or -ND"'-,
A is C2-C6alkylene or -(CH2)3-0- and
x, is the number 0 or 1,
D"' is hydrogen, C1-C12alkyl, C2-C5hydroxyalkyl or C5-C7cycloalkyl,
G19 is identical to G18 or is one of the groups -N(G21)(G22), -OG23, -
N(H)(CH20G23) or
-N(CH20G23)2,
G20, if n = 1, is identical to G18 or G,9 and, if n = 2, is an -E-D'v-E-
group, in which D'v is
C2-C8alkylene or C2-C8alkylene which is interrupted by 1 or 2 -NG21- groups,
G21 is C1-C12alkyl, cyclohexyl, benzyl or C1-C4-hydroxyalkyl or a group of the
formula
CH3 G CH3 CH3
G-CH2 1 H3C NJ,-'N CH3
Gõ-N or GN N--L N N--Gõ
I N
G-CH2 H3C n-C4Hs n-C4H9
CH3 3
CH3 CH3
G22 is C,-C,2alkyl, cyclohexyl, benzyl or C1-C4hydroxyalkyl, and
G23 is hydrogen, C1-C12alkyl or phenyl, or G21 and G22 together are C4-
C5alkylene or
C4-C5oxaalkylene, for example -CH2CH2-0-CH2CH2-, or a group of the formula
-CH2CH2-N (G11)-CH2CH2-.
Some examples of the several variables in the formula (1 e) are given below.
Any C1-C12alkyl substituents are, for example, methyl, ethyl, n-propyl, n-
butyl, sec-butyl, tert-
butyl, n-hexyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-undecyl or n-
dodecyl.
CA 02643170 2008-10-23
-21-
Any hydroxyalkyl substituents are, for example, 2-hydroxyethyl, 2-
hydroxypropyl,
3-hydroxypropyl, 2-hydroxybutyl or 4-hydroxybutyl.
Any C5-C7cycloalkyl substituents are, for example, cyclopentyl, cyclohexyl or
cycloheptyl.
Cyclohexyl is preferred.
C2-C6alkylene A is, for example, ethylene, propylene, 2,2-dimethylpropylene,
tetramethylene
or hexamethylene.
If G21 and G22 together are C4-C5alkylene or oxaalkylene, they are, for
example, tetramethy-
lene, pentamethylene or 3-oxapentamethylene.
Examples of polyalkylpiperidine compounds from this class are the compounds of
the
following formulae:
O-CH2CH2CH3
H3C N 7 CH3
H3C CH3
N - n-C4Hs
CH3 CH3
H3C N N CH3
H3CCH2CH2 O- N i --- ' N J-- I N N- O-CH2CH2CH3
H3C CH3 n-C4Hs n-C4H9 CH CH3 3
(f') A compound of the formula (1 f)
CA 02643170 2008-10-23
-22-
H3C CH3
NN N-Gõ (1f)
H3C CH3 (NN) H
3C CH3
Gõ N NN--~
H3C CH3
wherein Gõ is as defined under (b').
(g') Oligomeric or polymeric compounds whose recurring structural unit
contains a 2,2,6,6-
tetraalkylpiperidinyl radical, in particular polyesters, polyethers,
polyarnides, polyamines,
polyurethanes, polyureas, polyaminotriazines, poly(meth)acrylates,
poly(meth)acrylamldes
and copolymers thereof which contain such radicals.
Examples of 2,2,6,6-polyalkylpiperidine compounds from this class are the
compounds of the
following formulae, where m, to m14 is a number from 2 to about 200,
preferably 2 to 100, for
example 2 to 50, 2 to 40 or 3 to 40 or 4 to 10.
The meanings of the end groups which saturate the free valences in the
oligomeric or
polymeric compounds listed below depend on the processes used for the
preparation of said
compounds. The end groups can also in addition be modified after the synthesis
of the
compounds.
Examples are compounds of the formula (1 g-1)
CA 02643170 2008-10-23
-23-
N
/ ~--- N R 12 N
N N
R11 H3C CH3
H3C A. CH3 0g-1)
CH2
1 H2
CH3 n
in which the index n ranges from 1 to 15, being especially from the range 3-9;
R12 is C2-C12alkylene, C4-C12alkenylene, C5-C7cycloalkylene, C5-
C7cycloalkylene-
di(C,-C4alkylene), C,-C4alkylenedi(C5-C7cycloalkylene), phenylenedi(C,-
C4alkylene) or
C4-C12alkylene interrupted by 1,4-piperazinediyl, -0- or >N-X1 with X, being
C,-C,2acyl or
(C1-C,2alkoxy)carbonyl or having one of the definitions of R14 given below
except hydrogen;
or R12 is a group of the formula (lb') or (Ic');
-CH2 i H-CH2 (lb')
O
1
C=0
X2
O 0
--X3__/ I--X3 (Ic')
O 0
with m being 2 or 3,
X2 being C1-C18alkyl, C5-C12cycloalkyl which is unsubstituted or substituted
by 1, 2 or 3
C,-C4alkyl; phenyl which is unsubstituted or substituted by 1, 2 or 3 C1-
C4alkyl or
C1-C4alkoxy; C7-C9phenylalkyl which is unsubstituted or substituted on the
phenyl by 1, 2 or 3
C,-C4alkyl; and
the radicals X3 being independently of one another C2-C12alkylene;
the radicals B are independently of one another Cl, -OR13, -N(R14)(RI5) or a
group of the
formula (Ilid);
CA 02643170 2008-10-23
-24-
H3C CH3
X N -O-C C CH3 (Ilid)
H2 H2
H3C CH3
R13, R14 and R15, which are identical or different, are hydrogen, C1-C,8alkyl,
C5-C,2cycloalkyl
which is unsubstituted or substituted by 1, 2 or 3 C1-C4aIkyl; C3-C,8alkenyl,
phenyl which is
unsubstituted or substituted by 1, 2 or 3 C1-C4alkyl or C1-C4alkoxy; C7-
C9phenyiaikyl which is
unsubstituted or substituted on the phenyl by 1, 2 or 3 C1-C4aIkyl;
tetrahydrofurfuryl or
C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C,-Cealkoxy,
di(C 1 -C4alkyl) amino or a group of the formula (le');
Y\--,N (le')
with Y being -0-, -CH2-, -CH2CH2- or >N-CH3,
or -N(R14)(R,5) is additionally a group of the formula (le');
X is -0- or >N-R16;
R16 is hydrogen, C,-C,8alkyl, C3-C,8alkenyl, C5-C12cycloalkyl which is
unsubstituted or
substituted by 1, 2 or 3 C1-C4alkyl; C7-Cgphenylalkyl which is unsubstituted
or substituted on
the phenyl by 1, 2 or 3 C1-C4alkyl; tetrahydrofurfuryl, a group of the formula
(Illf),
H3C CH3
N -O-C-C CH3 (uhf)
H2 H2
H3C CH3
or C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C1-
C8alkoxy,
di(C1-C4alkyl)amino or a group of the formula (le');
R11 has one of the definitions given for R16.
In these compounds, the end group bonded to the triazine residue can be, for
example, a
group B or -N(R11)-R12-B, such as chlorine or a group
CA 02643170 2008-10-23
-25-
N (CH2)6 N H
H3C CH3 H3C 4~ CH3
N
H3C CH3 H3C I CH3
Gõ Gil
and the end group bonded to the diamino group can be, for example, hydrogen or
a di-B-
substituted triazinyl group, such as a group
N~--CI or (N~--CI
N N CH3 CHi N, N
1 i
HN C-CI 12 i ---CM3 FIN
H
1 CH3 CH3
It may be convenient to replace the chlorine attached to the triazine by e.g. -
OH or an amino
group. Suitable amino groups are typically: pyrrolidin-1-yl, morpholino, -NH2,
-N(C,-C8alkyI)2
and -NY'(C,-C8alkyl) wherein Y' is hydrogen or a group of the formula
H3C CH3
N-Gõ
H3C CH3
In the above shown oligomeric and polymeric compounds,
examples of alkyl are methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl,
isobutyl, tert-butyl, 2-
ethylbutyl, n-pentyl, isopentyl, 1-methylpentyl, 1,3-dimethylbutyl, n-hexyl, 1-
methylhexyl, n-
heptyl, isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl, 3-methylheptyl, n-
octyl, 2-ethyl-
hexyl, 1,1,3-trimethylhexyl, 1,1,3,3-tetramethylpentyl, nonyl, decyl, undecyl,
1-methylundecyl,
dodecyl, 1,1,3,3,5,5-hexamethylhexyl, tridecyl, tetradecyl, pentadecyl,
hexadecyl,
heptadecyl, octadecyl, eicosyl and docosyl;
CA 02643170 2008-10-23
-26-
examples of cycloalkyl are cyclopentyl, cyclohexyl, cycloheptyl and
cyclooctyl;
an example of C7-C9phenylalkyl is benzyl; and
examples of alkylene are ethylene, propylene, trimethylene, tetramethylene,
pentamethylene,
2,2-dimethyltrimethylene, hexamethylene, trimethyihexamethylene, octamethylene
and
decamethylene.
(h') A compound of the formula (1 h)
CH3 0
G-CH2- P
G-N N G14
(1 h)
G-CH2
CH3
ns
in which n6 is the number 1 or 2, G and Gõ are as defined under (a'), and G14
is as defined
under (b').
(i') A compound of the formula (1 i)
G39\/N\T/G39
N N (1i)
G39
wherein the radicals G39, independently of one another, are a group of the
formula (1 i-1)
H3C
O CH3
N G41 N N-G42 (1i-1)
G40 H3C CH3
in which G40 is C1-C12alkyl or C5-C12cycloalkyl, G41 is C2-C12alkylene and G42
is hydrogen,
CA 02643170 2008-10-23
-27-
C1-C8alkyl, -O-, -CH2CN, C3-C6alkenyl, C7-C9phenylalkyl, CrCgphenylalkyl which
is
substituted on the phenyl radical by C,-C4alkyl; or C1-C8acyl.
Alkyl is for example C1-C4alkyl, in particular methyl, ethyl, propyl or butyl.
Cycloalkyl is preferably cyclohexyl.
Alkylene is for example ethylene, propylene, trimethylene, tetramethylene,
pentamethylene,
2,2-dimethyltrimethylene or hexamethylene.
Alkenyl is preferably allyl.
Phenylalkyl is preferably benzyl.
Acyl is preferably acetyl.
Especially preferred is a compound of the formula Illb
A /N~--N R 12 ' N iN II A
N~ N N~ N
R11 H3C CH3 Y
H3C i CH3 (Illb)
0
1
CH2
1 H2
CH3 n
in which
the radicals A are independently of one another -OR13, -N(R14)(R,5) or a group
of the
formula (Illd);
CA 02643170 2008-10-23
-28-
H3C CH3
X N -O-C C CH3 (Ilid)
H2 H2
H3C CH3
X is -0- or >N-R16;
R16 is hydrogen, C1-C18alkyl, C3-C18alkenyl, C5-C12cycloalkyl which is
unsubstituted or
substituted by 1, 2 or 3 C1-C4alkyl; CrCgphenylalkyl which is unsubstituted or
substituted on
the phenyl by 1, 2 or 3 C1-C4alkyl; tetrahydrofurfuryl, a group of the formula
(Illf),
H3C CH3
N -O-C-C CH3 (Il If)
4 H2 H2
H3C CH3
or C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C1-
C8alkoxy,
di(C1-C4alkyl)amino or a group of the formula (le');
R11 has one of the definitions given for R16; and
the radicals B have independently of one another one of the definitions given
for A; and
where formula (le') and all other symbols are as defined above for formula Ia.
The radicals B, R11 and R12 in the individual recurrent units may be identical
or different
within the meanings given.
(j') A compound of the formula (2a)
R2 0
R1`~/
CH3 CH3 CH3 O - N 0 (2a)
R3--~-j
R4
in which
R1, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
Cshydroxyalkyl, or R1
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are CS-C12cycloalkyl.
Specific examples for hydrogenated products of present invention include the
following
compounds:
CA 02643170 2008-10-23
-29-
1-propoxy-2,2,6,6-tetramethyl-4-piperidone,
1 -propoxy-2,2,6,6-tetramethyl-4-piperidol,
bis(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)-(3',5'-di-tert.butyl-
4'hydroxybenzyl)
butylmalonate,
bis(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)sebacate,
bis(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)succinate,
N,N'-bis(l -propoxy-2,2,6,6-tetramethyl-4-piperidyl) hexane-1,6-diamine,
N-butyl-1-propoxy-2,2,6,6-tetramethyl-4-piperidinamine,
5-(1 -propoxy-2,2,6,6-tetramethyl-4-piperidyl)-2-cyclo-undecyloxazole,
1,6-hexanediyl -N,N'-bis(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl-formamide),
1,5-dioxaspiro(5,5)undecane-3,3-dicarboxylic acid bis(1-propoxy-2,2,6,6-
tetramethyl-4-
piperidyl) ester,
1,5,8,12-tetrakis(2',4'-bis(1 "-propoxy-2",2",6",6"-tetramethyl-4"-
piperidyl(butyl)amino)-1',3',5'-
triazin 6' yI) 1,5,8,12-tetraazadodccanc,
1,3,5-tris(N-cyclohexyl-N-(1-propoxy-2,2,6,6-tetramethylpiperazine-3-one-4-
yl)amino)-s-
triazine,
linear or cyclic condensates of N,N'-bis(1-propoxy-2,2,6,6-tetramethyl-4-
piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-dichloro-1,3,5-
triazine,
tris(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)nitrilotriacetate,
tetrakis-(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)-1,2,3,4-butane-
tetracarboxylate,
1,1'-(1,2-ethanediyl)-bis-(4-propoxy-3,3,5,5-tetramethylpipe razi none),
4-benzoyl-1 -propoxy-2,2,6,6-tetramethylpiperidine,
4-stearyloxy-1-propoxy-2,2,6,6-tetramethylpiper idine,
3-n-octyl-8-propoxy-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decan-2,4-dione,
linear or cyclic condensates of N,N'-bis-(1-propoxy-2,2,6,6-tetramethyl-4-
piperi-
dyl)hexamethylenediamine and 4-morpholino-2,6-dichloro-1,3,5-triazine,
the condensate of 2-chloro-4,6-bis(4-n-butylamino-l -propoxy-2,2,6,6-
tetramethylpiperidyl)-
1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane,
8-propoxy-3-dodecyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-
dione,
3-dodecyl-1 -(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)pyrrolidin-2,5-dione,
a mixture of 4-hexadecyloxy- and 4-stearyloxy-1-propoxy-2,2,6,6-
tetramethylpiperidine,
a condensation product of N,N'-bis(1-propoxy-2,2,6,6-tetramethyl-4-
pipe(dyl)hexamethylenediamine and 4-cyclohexylamino-2,6-dichloro-1,3,5-
triazine,
a condensation product of 1,2-bis(3-aminopropylamino)ethane and 2,4,6-
trichloro-1,3,5-
triazine as well as 4-butylamino- 1 -propoxy-2,2,6,6-tetramethylpiperidine;
CA 02643170 2008-10-23
-30-
N-(1-propoxy-2,2,6,6-tetramethyl-4-piperidyl)-n-dodecylsuccinimid,
2-undecyl-8-propoxy-7,7,9,9-tetramethyl-1-oxa-3,8-diaza-4-oxo-
spiro[4,5]decane,
a reaction product of 8-propoxy-7,7,9,9-tetramethyl-2-cycloundecyl-1-oxa-3,8-
diaza-4-
oxospiro [4,5]decane and epichlorohydrin,
N,N'-bis-formyl-N,N'-bis(1-propoxy-2,2,6,6-tetramethyl-4-
piperidyl)hexamethylenediamine,
poly[methyl-pro pyl-3-oxy-4-(1-propoxy-2,2,6,6-tetramethyl-4-
piperidyl)]siloxane,
reaction product of maleic acid anhydride-a-olefin-copolymer with 1 -propoxy-
2,2,6,6-
tetramethyl-4-aminopiperidine; or the compound
CH2CH2CH3
O'CH2CH2CH3
~~ lYJ
R-NH-(CH2)3-N(R)-(CH2)2-N(R)-(CH2)3-NH-R, with R = C4H9 N_N-C4Hy
N~,N
Compounds of the formulae I, III and V, especially Ilia and Illb can be
employed with
advantage for stabilizing organic material against the damaging effect of
light, oxygen and/or
heat, especially for stabilizing synthetic organic polymers or compositions
containing them.
They are notable for high thermal stability, substrate compatibility and good
persistence in
the substrate.
Examples of polymers which can be stabilized in this way are the following:
1. Polymers of monoolefins and diolefins, for example polypropylene,
polyisobutylene, po-
lybut-1-ene, poly-4-methylpent-l -ene, polyisoprene or polybutadiene, as well
as polymers of
cycloolefins, for instance of cyclopentene or norbornene, polyethylene (which
optionally can
be crosslinked), for example high density polyethylene (HDPE), high density
and high mole-
cular weight polyethylene (HDPE-HMW), high density and ultrahigh molecular
weight poly-
ethylene (HDPE-UHMW), medium density polyethylene (MDPE), low density
polyethylene
(LDPE), linear low density polyethylene (LLDPE), (VLDPE) and (ULDPE).
Polyolefins, i.e. the polymers of monoolefins exemplified in the preceding
paragraph, prefe-
rably polyethylene and polypropylene, can be prepared by different, and
especially by the
following, methods:
CA 02643170 2008-10-23
-31-
a) radical polymerisation (normally under high pressure and at elevated
temperature).
b) catalytic polymerisation using a catalyst that normally contains one or
more than one
metal of groups IVb, Vb, Vlb or VIII of the Periodic Table. These metals
usually have
one or more than one ligand, typically oxides, halides, alcoholates, esters,
ethers,
amines, alkyls, alkenyls and/or aryls that may be either 7t- or a-coordinated.
These
metal complexes may be in the free form or fixed on substrates, typically on
activated magnesium chloride, titanium(III) chloride, alumina or silicon
oxide. These
catalysts may be soluble or insoluble in the polymerisation medium. The
catalysts
can be used by themselves in the polymerisation or further activators may be
used,
typically metal alkyls, metal hydrides, metal alkyl halides, metal alkyl
oxides or metal
alkyloxanes, said metals being elements of groups Ia, Ila and/or Illa of the
Periodic
Table. The activators may be modified conveniently with further ester, ether,
amine
or silyl ether groups. These catalyst systems are usually termed Phillips,
Standard
Oil 'Indiana, Ziegler (-Natta), TNZ (DuPont), metallocene or single site
catalysts
(SSC).
2. Mixtures of the polymers mentioned under 1), for example mixtures of
polypropylene with
polyisobutylene, polypropylene with polyethylene (for example PP/HDPE,
PP/LDPE) and
mixtures of different types of polyethylene (for example LDPE/HDPE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monomers,
for example ethylene/propylene copolymers, linear low density polyethylene
(LLDPE) and
mixtures thereof with low density polyethylene (LDPE), propylene/but-l -ene
copolymers,
propylene/isobutylene copolymers, ethylene/but-l -ene copolymers,
ethylene/hexene copo-
lymers, ethylene/methylpentene copolymers, ethylene/heptene copolymers,
ethylene/octene
copolymers, propylene/butadiene copolymers, isobutylene/isoprene copolymers,
ethy-
lene/alkyl acrylate copolymers, ethylene/alkyl methacrylate copolymers,
ethylene/vinyl ace-
tate copolymers and their copolymers with carbon monoxide or ethylene/acrylic
acid copo-
lymers and their salts (ionomers) as well as terpolymers of ethylene with
propylene and a
diene such as hexadiene, dicyclopentadiene or ethylidene-norbomene; and
mixtures of such
copolymers with one another and with polymers mentioned in 1) above, for
example
polypropylene/ethylene-propylene copolymers, LDPE/ethylene-vinyl acetate
copolymers
CA 02643170 2008-10-23
-32-
(EVA), LDPE/ethylene-acrylic acid copolymers (EAA), LLDPE/EVA, LLDPE/EAA and
alter-
nating or random polyalkylene/carbon monoxide copolymers and mixtures thereof
with other
polymers, for example polyamides.
4. Hydrocarbon resins (for example C5-C9) including hydrogenated modifications
thereof
(e.g. tackifiers) and mixtures of polyalkylenes and starch.
5. Polystyrene, poly(p-methylstyrene), poly((x-methylstyrene).
6. Copolymers of styrene or a-methylstyrene with dienes or acrylic
derivatives, for example
styrene/butadiene, styrene/acrylonitrile, styrene/alkyl methacrylate,
styrene/butadiene/alkyl
acrylate, styrene/butadiene/alkyl methacrylate, styrene/maleic anhydride,
styrene/acryloni-
trile/methyl acrylate; mixtures of high impact strength of styrene copolymers
and another
polymer, for example a polyacrylate, a diene polymer or an
ethylene/propylene/diene terpo-
lymer; and block copolymers of styrene such as styrene/butadiene/styrene,
styrene/iso-
prene/styrene, styrene/ethylene/butylene/styrene or
styrene/ethylene/propylene/ styrene.
7. Graft copolymers of styrene or (x-methylstyrene, for example styrene on
polybutadiene,
styrene on polybutadiene-styrene or polybutadiene-acrylonitrile copolymers;
styrene and
acrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile
and methyl meth-
acrylate on polybutadiene; styrene and maleic, anhydride on polybutadiene;
styrene, acrylo-
nitrile and maleic anhydride or maleimide on polybutadiene; styrene and
maleimide on poly-
butadiene; styrene and alkyl acrylates or methacrylates on polybutadiene;
styrene and acry-
lonitrile on ethylene/propylene/diene terpolymers; styrene and acrylonitrile
on polyalkyl acry-
lates or polyalkyl methacrylates, styrene and acrylonitrile on
acrylate/butadiene copolymers,
as well as mixtures thereof with the copolymers listed under 6), for example
the copolymer
mixtures known as ABS, MBS, ASA or AES polymers.
8. Halogen-containing polymers such as polychloroprene, chlorinated rubbers,
chlorinated
and brominated copolymer of isobutylene-isoprene (halobutyl rubber),
chlorinated or sulfo-
chlorinated polyethylene, copolymers of ethylene and chlorinated ethylene,
epichlorohydrin
homo- and copolymers, especially polymers of halogen-containing vinyl
compounds, for
example polyvinyl chloride, polyvinylidene chloride, polyvinyl fluoride,
polyvinylidene fluoride,
CA 02643170 2008-10-23
-33-
as well as copolymers thereof such as vinyl chloride/vinylidene chloride,
vinyl chloride/vinyl
acetate or vinylidene chloride/vinyl acetate copolymers.
9. Polymers derived from a,R-unsaturated acids and derivatives thereof such as
polyacry-
lates and polymethacrylates; polymethyl methacrylates, polyacrylamides and
polyacryloni-
triles, impact-modified with butyl acrylate.
10. Copolymers of the monomers mentioned under 9) with each other or with
other unsatu-
rated monomers, for example acrylonitrile/ butadiene copolymers,
acrylonitrile/alkyl acrylate
copolymers, acrylonitrile/alkoxyalkyl acrylate or acrylonitrile/vinyl halide
copolymers or acry-
lonitrile/ alkyl methacrylate/butadiene terpolymers.
11. Polymers derived from unsaturated alcohols and amines or the acyl
derivatives or ace-
tals thereof, for example polyvinyl alcohol, polyvinyl acetate, polyvinyl
stearate, polyvinyl
benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl phthalate or
polyallyl melamine; as
well as their copolymers with olefins mentioned in 1) above.
12. Homopolymers and copolymers of cyclic ethers such as polyalkylene glycols,
polyethy-
lene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
13. Polyacetals such as polyoxymethylene and those polyoxymethylenes which
contain
ethylene oxide as a comonomer; polyacetals modified with thermoplastic
polyurethanes,
acrylates or MBS.
14. Polyphenylene oxides and sulfides, and mixtures of polyphenylene oxides
with styrene
polymers or polyamides.
15. Polyurethanes derived from hydroxyl-terminated polyethers, polyesters or
polybutadi-
enes on the one hand and aliphatic or aromatic polyisocyanates on the other,
as well as
precursors thereof.
16. Polyamides and copolyamides derived from diamines and dicarboxylic acids
and/or from
aminocarboxylic acids or the corresponding lactams, for example polyamide 4,
polyamide 6,
polyamide 6/6, 6/10, 6/9, 6/12, 4/6, 12/12, polyamide 11, polyamide 12,
aromatic polyamides
CA 02643170 2008-10-23
-34-
starting from m-xylene diamine and adipic acid; polyamides prepared from
hexamethylenediamine and isophthalic or/and terephthalic acid and with or
without an ela-
stomer as modifier, for example poly-2,4,4,-trimethylhexamethylene
terephthalamide or poly-
m-phenylene isophthalamide; and also block copolymers of the aforementioned
polyamides
with polyolefins, olefin copolymers, ionomers or chemically bonded or grafted
elastomers; or
with polyethers, e.g. with polyethylene glycol, polypropylene glycol or
polytetramethylene
glycol; as well as polyamides or copolyamides modified with EPDM or ABS; and
polyamides
condensed during processing (RIM polyamide systems).
17. Polyureas, polyimides, polyamide-imides, polyetherimids, polyesterimids,
polyhydantoins
and polybenzimidazoles.
18. Polyesters derived from dicarboxylic acids and diols and/or from
hydroxycarboxylic acids
or the corresponding lactones, for example polyethylene terephthalate,
polybutylene
terephthalate, poly-l,4-diriiethylolcyclohexane terephthalate and
polyhydroxybenzoates, as
well as block copolyether esters derived from hydroxyl-terminated polyethers;
and also poly-
esters modified with polycarbonates or MBS.
19. Polycarbonates and polyester carbonates.
20. Polysulfones, polyether sulfones and polyether ketones.
21. Crosslinked polymers derived from aldehydes on the one hand and phenols,
ureas and
melamines on the other hand, such as phenol/formaldehyde resins,
urea/formaldehyde re-
sins and melamine/formaldehyde resins.
22. Drying and non-drying alkyd resins.
23. Unsaturated polyester resins derived from copolyesters of saturated and
unsaturated
dicarboxylic acids with polyhydric alcohols and vinyl compounds as
crosslinking agents, and
also halogen-containing modifications thereof of low flammability.
24. Crosslinkable acrylic resins derived from substituted acrylates, for
example epoxy acry-
lates, urethane acrylates or polyester acrylates.
CA 02643170 2008-10-23
-35-
25. Alkyd resins, polyester resins and acrylate resins crosslinked with
melamine resins, urea
resins, isocyanates, isocyanurates, polyisocyanates or epoxy resins.
26. Crosslinked epoxy resins derived from aliphatic, cycloaliphatic,
heterocyclic or aromatic
glycidyl compounds, e.g. products of diglycidyl ethers of bisphenol A and
bisphenol F, which
are crosslinked with customary hardeners such as anhydrides or amines, with or
without
accelerators.
27. Blends of the aforementioned polymers (polyblends), for example PP/EPDM,
Poly-
amide/EPDM or ABS, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS, PBTP/ABS, PC/ASA,
PC/PBT, PVC/CPE, PVC/acrylates, POM/thermoplastic PUR, PC/thermoplastic PUR,
POM/acrylate, POM/MBS, PPO/HIPS, PPO/PA 6.6 and copolymers, PA/HDPE, PA/PP,
PA/PPO, PBT/PC/ABS or PBT/PET/PC.
Of particular interest is the use of compounds of the formula Ilia as
stabilizers in
synthetic organic polymers, for example a coating or a bulk polymer or article
formed therefrom, especially in thermoplastic polymers and corresponding
compositions as well as in coating compositions. Thermoplastic polymers of
most
importance in present compositions are polyolefines and their copolymers, such
as
listed above under items 1-3, thermoplastic polyolefin (TPO), thermoplastic
polyurethan (TPU), thermoplastic rubber (TPR), polycarbonate, such as in item
19
above, and blends, such as in item 28 above. Of utmost importance are
polyethylene (PE), polypropylene (PP), polycarbonate (PC) and polycarbonate
blends such as PC/ABS blends, as well as in acid or metal catalyzed coating
compositions.
In general the compounds of present invention are added to the material to be
stabilized in amounts of from 0.1 to 10 %, preferably from 0.01 to 5 %, in
particular
from 0.01 to 2 % (based on the material to be stabilized). Particular
preference is
given to the use of the novel compounds in amounts of from 0.05 to 1.5 %,
especially from 0.1 to 0.5 %. Where compounds of present invention are used as
flame retardants, dosages are usually higher, e.g. 0.1 to 25 % by weight,
mainly
0.1 to 10 % by weight of the organic material to be stabilized and protected
against
inflammation.
CA 02643170 2008-10-23
-36-
Incorporation into the materials can be effected, for example, by mixing in or
applying the compounds of the formula I, Ilia, IV, V and, if desired, further
additives by the methods which are customary in the art. Where polymers are
involved, especially synthetic polymers, incorporation can take place prior to
or
during the shaping operation, or by applying the dissolved or dispersed
compound
to the polymer, with or without subsequent evaporation of the solvent. In the
case
of elastomers, these can also be stabilized as latices. A further possibility
for
incorporating the compounds of the formula Ilia into polymers is to add them
before, during or directly after the polymerization of the corresponding
monomers
or prior to crosslinking. In this context the compound of the formula Ilia can
be
added as it is or else in encapsulated form (for example in waxes, oils or
polymers). In the case of addition prior to or during the polymerization, the
compounds of the formula Ilia can also act as a regulator of the chain length
of the
polymers (chain terminator).
The compounds of the formula Ilia can also be added in the form of a
masterbatch
containing said compound in a concentration, for example, of from 2.5 to 25 %
by
weight to the polymers that are to be stabilized.
The compounds of the formula Illa can judiciously be incorporated by the
following
methods:
- as emulsion or dispersion (e.g. to latices or emulsion polymers),
- as a dry mixture during the mixing in of additional components
or polymer mixtures,
- by direct introduction into the processing apparatus (e.g. extruders,
internal
mixers, etc);
- as solution or melt.
Novel polymer compositions can be employed in various forms and/or processed
to give various products, for example as (to give) films, fibres, tapes,
moulding
compositions, profiles, or as binders for coating materials, adhesives or
putties.
CA 02643170 2008-10-23
-37-
In addition to the compounds of the formula Ilia the novel compositions may as
additional component C comprise one or more conventional additives such as,
for
example, those indicated below.
1. Antioxidants
1.1. Alkylated monophenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-
tert-butyl-4,6-di-
methylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-
butylphenol, 2,6-di-tert-bu-
tyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2-(a-methylcyclohexyl)-
4,6-dimethyl-
phenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexyiphenol, 2,6-di-tert-
butyl-4-
methoxymethylphenol, nonylphenols which are linear or branched in the side
chains, for
example, 2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-(1'-methylundec-l'-
yl)phenol, 2,4-di-
methyl-6-(1'-methylheptadec-1'-yl)phenol, 2,4-dimethyl-6-(1'-methyltridec-l'-
yl)phenol and
mixtures thereof.
1.2. Alkylthiomethylphenols, for example 2,4-dioctylthiomethyl-6-tert-
butylphenol, 2,4-dioc-
tylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-di-
dodecylthiomethyl-
4-nonylphenol.
1.3. Hydroguinones and alkylated hydroquinones, for example 2,6-di-tert-butyl-
4-methoxy-
phenol, 2,5-di-tert-butyihydroquinone, 2,5-di-tert-amyihydroquinone, 2,6-
diphenyl-4-octade-
cyloxyphenol, 2,6-di-tert-butyihydroquinone, 2,5-di-tert-butyl-4-
hydroxyanisole, 3,5-di-tert-
butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate, bis-(3,5-
di-tert-butyl-4-
hydroxyphenyl) adipate.
1.4. Tocopherols, for example a-tocopherol, (3-tocopherol, y-tocopherol, S-
tocopherol and
mixtures thereof (Vitamin E).
1.5. Hydroxylated thiodiphenyl ethers, for example 2,2'-thiobis(6-tert-butyl-4-
methylphenol),
2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol), 4,4'-
thiobis(6-tert-butyl-2-
methylphenol), 4,4'-thiobis-(3,6-di-sec-amylphenol), 4,4'-bis(2,6-dimethyl-4-
hydroxyphe-
nyl)disulfide.
CA 02643170 2008-10-23
-38-
1.6. Alkylidenebisphenols, for example 2,2'-methylenebis(6-tert-butyl-4-
methylphenol), 2,2'-
methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis[4-methyl-6-(a-
methylcyclohexyl)-
phenol), 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylenebis(6-
nonyl-4-me-
thyiphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-
ethylidenebis(4,6-di-tert-butyl-
phenol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-
methylenebis[6-((x-methylben-
zyl)-4-nonylphenol], 2,2'-methylenebis[6-(a,(x-dimethylbenzyl)-4-nonylphenol),
4,4'-methy-
lenebis(2,6-di-tert-butylphenol), 4,4'-methylenebis(6-tert-butyl-2-
methylphenol), 1,1-bis(5-tert-
butyl-4-hydroxy-2-methylphenyl)butane, 2,6-bis(3-tert-butyl-5-methyl-2-
hydroxybenzyl)-4-
methylphenol, 1,1,3-tris(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 1,1-
bis(5-tert-butyl-4-
hydroxy-2-methyl-phenyl)-3-n-dodecylmercaptobutane, ethylene glycol bis[3,3-
bis(3'-tert-
butyl-4'-hydroxyphenyl)butyrate], bis(3-tert-butyl-4-hydroxy-5-methyl-
phenyl)dicyclopentadi-
ene, bis[2-(3'-tert-butyl-2'-hydroxy-5'-methylbenzyl)-6-tert-butyl-4-
methylphenyl]terephthalate,
1,1-bis-(3,5-dimethyl-2-hydroxyphenyl)butane, 2,2, bis-(3,5-di-tert-butyl-4-
hydroxyphe-
nyl)propanc, 2,2-bis-(5-tert-butyl-4-hydroxyl-methylphenyl)-4-n-
dodecylmercaptobutane,
1,1,5,5-tetra-(5-tert-butyl-4-hydroxy-2-methylphenyl)pentane.
1.7. 0-. N- and S-benzyl compounds, for example 3,5,3',5'-tetra-tert-butyl-
4,4'-dihydroxydi-
benzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzylmercaptoacetate, tridecyl-
4-hydroxy-
3,5-di-tert-butylbenzylmercaptoacetate, tris(3,5-di-tert-butyl-4-
hydroxybenzyl)amine, bis(4-
tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithioterephthalate, bis(3,5-di-tert-
butyl-4-hydroxy-
benzyl)sulfide, isooctyl-3,5-di-tert-butyl-4-hydroxybenzylmercaptoacetate.
1.8. Hydroxybenzylated malonates, for example dioctadecyl-2,2-bis-(3,5-di-tert-
butyl-2-hy-
droxybenzyl)-malonate, di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)-
malonate, di-
dodecylmercaptoethyl-2,2-bis-(3,5-di-tert-butyl-4-hydroxybenzyl)malonate,
bis[4-(1,1,3,3-te-
tramethylbutyl)phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
1.9. Aromatic hydroxybenzyl compounds, for example 1,3,5-tris-(3,5-di-tert-
butyl-4-hydroxy-
benzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-
2,3,5,6-tetrame-
thylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
1.10. Triazine Compounds, for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-
butyl-4-hy-
droxyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-
hydroxyanilino)-1,3,5-
triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,3,5-
triazine, 2,4,6-
CA 02643170 2008-10-23
-39-
tris(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris-(3,5-di-
tert-butyl-4-hydroxy-
benzyl) isocyanu rate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dim
ethylbenzyl)isocyanurate, 2,4,6-
tris(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-triazine, 1,3,5-tris(3,5-di-
tert-butyl-4-
hydroxyphenylpropionyl)-hexahydro-1,3,5-triazine, 1,3,5-tris(3,5-dicyclohexyl-
4-hydroxyben-
zyl)isocyanurate.
1.11. Benzylphosphonates, for example dimethyl-2,5-di-tert-butyl-4-
hydroxybenzylphospho-
nate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl3,5-di-
tert-butyl-4-hy-
droxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy-3-
methylbenzylphosphonate,
the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-
hydroxybenzylphosphonic acid.
1.12. Acylaminophenols, for example 4-hydroxylauranilide, 4-
hydroxystearanilide, octyl N -
(3, 5-di-tert-butyl-4-hyd roxyphenyl)carbamate.
1.13. Esters of 0-(3.5-di-tert-butyl-4-hydroxyphenyl)gropionic acid with mono-
or polyhydric
alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-
hexanediol, 1,9-
nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene
glycol, diethy-
lene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)
isocyanurate, N,N'-bis(hy-
droxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethyihexanediol,
trimethylol-
propane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.14. Esters of 0-(5-tert-butyl-4-hydroxy-3-methylphenyl)gropionic acid with
mono- or poly-
hydric alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol,
octadecanol, 1,6-hexane-
diol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol,
thiodiethylene glycol,
diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl)
isocyanurate, N,N'-
bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol,
trimethylhexanediol,
trimethylolpropane, 4-hydroxymethyl-1 -phospha-2,6,7-
trioxabicyclo[2.2.2]octane.
1.15. Esters of Q-(3,5-dicvclohexyl-4-hydroxyphenyi)propionic acid with mono-
or polyhydric
alcohols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol,
1,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N'-
bis(hydroxyethyl)ox-
amide, 3-thiaundecanol, 3-thiapentadecanol, trimethyihexanediol,
trimethylolpropane, 4-hy-
droxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
CA 02643170 2008-10-23
-40-
1.16. Esters of 3.5-di-tert-butyl-4-hydroxyphenyl acetic acid with mono- or
polyhydric alco-
hols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-
nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol,
triethylene glycol, pentaerythritol, tns(hydroxyethyl)isocyanurate, N,N'-
bis(hydroxyethyl)ox-
amide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol,
trimethylolpropane, 4-hy-
droxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.17. Amides of 0-(3,5-di-tert-butt'-4-hydroxyphenyl)propionic acid e.g. N,N'-
bis(3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)hexamethylenediamide, N,N'-bis(3,5-di-tert-
butyl-4-hydroxy-
phenylpropionyl)trimethylenediamide, N,N'-bis(3,5-di-tert-butyl-4-
hydroxyphenylpropionyl)-
hydrazide, N,N'-bis[2-(3-[3,5-di-tert-butyl-4-
hydroxyphenyi]propionyloxy)ethyl]oxamide
(Naugard XL-1 supplied by Uniroyal).
1.18. Ascorbic acid (vitamin C)
1.19. Aminic antioxidants, for example N,N'-di-isopropyl-p-phenylenediamine,
N,N'-di-sec-
butyl-p-phenylenediamine, N,N'-bis(1,4-dimethylpentyl)-p-phenylenediamine,
N,N'-bis(l -
ethyl-3-methylpentyl)-p-phenylenediamine, N, N'-bis(1-methylheptyl)-p-
phenylenediamine,
N,N'-dicyclohexyl-p-phenylenediamine, N,N'-diphenyl-p-phenylenediamine, N,N'-
bis(2-naph-
thyl)-p-phenylenediamine, N-isopropyl-N'-phenyl-p-phenylenediamine, N-(1,3-
dimethylbutyl)-
N'-phenyl-p-phenylenediamine, N-(1-methylheptyl)-N'-phenyl-p-phenylenediamine,
N-cyclo-
hexyl-N'-phenyl-p-phenlenediamine, 4-(p-toluenesulfamoyl)diphenylamine, N,N'-
dimethyl-
N,N'-di-sec-butyl-p-phenylenediamine, diphenylamine, N-allyldiphenylamine, 4-
isopropoxy-
diphenylamine, N-phenyl-l -naphthylamine, N-(4-tert-octylphenyl)-1-
naphthylamine, N-phe-
nyl-2-naphthylamine, octylated diphenylamine, for example p,p'-di-tert-
octyldiphenylamine, 4-
n-butylaminophenol, 4-butyrylaminophenol, 4-nonanoylaminophenol, 4-
dodecanoylamino-
phenol, 4-octadecanoylaminophenol, bis(4-methoxyphenyl)amine, 2,6-di-tert-
butyl-4-dime-
thylaminomethylphenol, 2,4'-diaminodiphenylmethane, 4,4'-
diaminodiphenylmethane,
N,N,N',N'-tetramethyl-4,4'-diaminodiphenylmethane, 1,2-bis[(2-
methylphenyl)amino]ethane,
1,2-bis(phenylamino) propane, (o-tolyl)biguanide, bis[4-(1',3'-
dimethylbutyl)phenyl]amine, tert-
octylated N-phenyl-l-naphthylamine, a mixture of mono- and dialkylated tert-
butyl/tert-
octyldiphenylamines, a mixture of mono- and dialkylated nonyldiphenylamines, a
mixture of
mono- and dialkylated dodecyldiphenylamines, a mixture of mono- and
dialkylated isopro-
CA 02643170 2008-10-23
-41-
pyl/isohexyldiphenylamines, a mixture of mono- and dialkylated tert-
butyldiphenylamines,
2,3-dihydro-3,3-dimethyl-4H-1,4-benzothiazine, phenothiazine, a mixture of
mono- and dial-
kylated tert-butyl/tert-octylphenothiazines, a mixture of mono- and
dialkylated tert-octyl-phe-
nothiazines, N-allylphenothiazin, N,N,N',N'-tetraphenyl-l,4-diaminobut-2-ene,
N,N-bis-
(2,2,6,6-tetramethyl-piperid-4-yi-hexamethylenediamine, bis(2,2,6,6-
tetramethylpiperid-4-yl)-
sebacate, 2,2,6,6-tetramethylpiperidin-4-one, 2,2,6,6-tetramethylpiperidin-4-
ol.
2. UV absorbers and light stabilisers
2.1. 2-(2'-Hvdroxyphenvl)benzotriazoles, for example 2-(2'-hydroxy-5'-
methylphenyl)-benzo-
triazole, 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)benzotriazole, 2-(5'-tert-
butyl-2'-hydroxyphe-
nyl)benzotriazole, 2-(2'-hydroxy-5'-(1,1,3,3-
tetramethylbutyi)phenyl)benzotriazole, 2-(3',5'-di-
tert-butyl-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-2'-
hydroxy-5'-methylphe-
nyl)-5-chloro-benzotriazole, 2-(3'-sec-butyl-5'-tert-butyl-2'-
hydroxyphenyl)benzotriazole, 2-(2'-
hydroxy-4'-octyloxyphenyl)benzotriazole, 2-(3',5'-di-tert-amyl-2'-
hydroxyphenyl)benzotriazole,
2-(3',5'-bis-(a,(x-dimethylbenzyl)-2'-hydroxyphenyl)benzotriazole, 2-(3'-tert-
butyl-2'-hydroxy-
5'-(2-octyloxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-
5'-[2-(2-
ethylhexyloxy)-carbonylethyl]-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-
tert-butyl-2'-hy-
droxy-5'-(2-methoxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3'-tert-
butyl-2'-hydroxy-
5'-(2-methoxycarbonylethyl)phenyl)benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-
5'-(2-octyloxy-
carbonylethyl)phenyl)benzotriazole, 2-(3'-tert-butyl-5'-[2-(2-
ethylhexyloxy)carbonylethyl]-2'-
hydroxyphenyl)benzotriazole, 2-(3'-dodecyl-2'-hydroxy-5'-
methylphenyl)benzotriazole, 2-(3'-
tert-butyl-2'-hydroxy-5'-(2-isooctyloxycarbonylethyl)phenylbenzotriazole, 2,2'-
methylene-bis-
[4-(1,1,3,3-tetramethylbutyi)-6-benzotriazole-2-ylphenol]; the
transesterification product of 2-
[3'-tert-butyl-5'-(2-methoxycarbonylethyl)-2'-hydroxyphenyl]-2H-benzotriazole
with polyethy-
lene glycol 300; [R-CH2CH2 COO-CH2CH,+ where R = 3'-tert-butyl-4'-hydroxy-5'-
2H-
2
benzotriazol-2-ylphenyl, 2-[2'-hydroxy-3'-(a,a-dimethylbenzyl)-5'-(1,1,3,3-
tetramethylbutyl)-
phenyl]benzotriazole; 2-[2'-hydroxy-3'-(1,1,3,3-tetramethylbutyi)-5'-(a,a-
dimethylbenzyl)-
phenyl]benzotriazole.
2.2. 2-Hydroxybenzophenones, for example the 4-hydroxy, 4-methoxy, 4-octyloxy,
4-decyl-
oxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-
dimethoxy derivatives.
CA 02643170 2008-10-23
-42-
2.3. Esters of substituted and unsubstituted benzoic acids, as for example 4-
tertbutyl-phenyl
salicylate, phenyl salicylate, octyiphenyl salicylate, dibenzoyl resorcinol,
bis(4-tert-butylben-
zoyl) resorcinol, benzoyl resorcinol, 2,4-di-tert-butylphenyl 3,5-di-tert-
butyl-4-hydroxybenzo-
ate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-
butyl-4-hydroxy-
benzoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-
hydroxybenzoate.
2.4. Acrylates, for example ethyl a-cyano-[3,(3-diphenylacrylate, isooctyl a-
cyano-O,j-diphe-
nylacrylate, methyl a-carbomethoxycinnamate, methyl a-cyano-(3-methyl-p-
methoxy-cinna-
mate, butyl a-cyano-f3-methyl-p-methoxy-cinnamate, methyl a-carbomethoxy-p-
methoxycin-
namate and N-([3-carbomethoxy-[3-cyanovinyl)-2-methylindoline.
2.5. Nickel compounds, for example nickel complexes of 2,2'-thio-bis-[4-
(1,1,3,3-tetrame-
thylbutyl) phenol], such as the 1:1 or 1:2 complex, with or without additional
ligands such as
n-butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel
dibutyldithiocarbamate,
nickel salts of the monoalkyl esters, e.g. the methyl or ethyl ester, of 4-
hydroxy-3,5-di-tert-
butylbenzylphosphonic acid, nickel complexes of ketoximes, e.g. of 2-hydroxy-4-
methylphe-
nyl undecylketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-hydroxypyrazole,
with or
without additional ligands.
2.6. Sterically hindered amines, for example bis(2,2,6,6-tetramethyl-4-
piperidyl)sebacate,
bis(2,2,6,6-tetramethyl-4-piperidyl)succinate, bis(1,2,2,6,6-pentamethyl-4-
piperidyl)sebacate,
bis(1-octyloxy-2,2,6,6-tetramethyl-4-pipe(dyl)sebacate, bis(1,2,2,6,6-
pentamethyl-4-pi-
peridyl) n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensate of
1-(2-hydroxy-
ethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, linear or
cyclic condensates
of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-
octylamino-2,6-
dichloro-1,3,5-triazine, tris(2,2,6,6-tetramethyl-4-
piperidyl)nitrilotriacetate, tetrakis(2,2,6,6-
tetramethyl-4-piperidyl)-1,2,3,4-butane-tetracarboxylate, 1,1'-(1,2-
ethanediyl)-bis(3,3,5,5-
tetramethylpiperazinone), 4-benzoyl-2,2,6,6-tetramethylpiperidine, 4-
stearyioxy-2,2,6,6-
tetramethylpiperidine, bis(1,2,2,6,6-pentamethylpipe(dyl)-2-n-butyl-2-(2-
hydroxy-3,5-di-tert-
butylbenzyl)malonate, 3-n-octyl-7,7,9,9-tetramethyl-1,3,8-
triazaspiro[4.5]decan-2,4-dione,
bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)sebacate, bis(1-octyloxy-2,2,6,6-
tetrame-
thylpiperidyl)succinate, linear or cyclic condensates of N,N'-bis-(2,2,6,6-
tetramethyl-4-piperi-
dyl)hexamethylenediamine and 4-morpholino-2,6-dichloro-1,3,5-triazine, the
condensate of
2-chloro-4,6-bis(4-n-butylamino-2,2,6,6-tetramethylpiperidyl )-1,3,5-triazine
and 1,2-bis(3-
CA 02643170 2008-10-23
-43-
aminopropylamino)ethane, the condensate of 2-chloro-4,6-di-(4-n-butylamino-
1,2,2,6,6-pen-
tamethylpiperidyl)-1,3,5-triazine and 1,2-bis-(3-aminopropylamino)ethane, 8-
acetyl-3-dode-
cyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl-1-
(2,2,6,6-tetrame-
thyl-4-piperidyl)pyrrolidin-2,5-dione, 3-dodecyl-1-(1,2,2,6,6-pentamethyl-4-
piperidyl)pyrroli-
dine-2,5-dione, a mixture of 4-hexadecyloxy- and 4-stearyloxy-2,2,6,6-
tetramethylpiperidine,
a condensation product of N,N'-bis(2,2,6,6-tetramethyl-4-
piperidyl)hexamethylenediamine
and 4-cyclohexylamino-2,6-dichloro-1,3,5-triazine, a condensation product of
1,2-bis(3-ami-
nopropylamino) ethane and 2,4,6-trichloro-1,3,5-triazine as well as 4-
butylamino-2,2,6,6-te-
tramethylpiperidine (CAS Reg. No. [136504-96-6]); N-(2,2,6,6-tetramethyl-4-
piperidyl)-n-do-
decylsuccinimid, N-(1,2,2,6,6-pentamethyl-4-piperidyl)-n-dodecylsuccinimid, 2-
undecyl-
7,7,9,9-tetramethyl-1-oxa-3,8-diaza-4-oxo-spiro[4,5]decane, a reaction product
of 7,7,9,9-
tetramethyl-2-cycloundecyl-l-oxa-3,8-diaza-4-oxospiro [4,5]decane and
epichlorohydrin, 1,1-
bis(1,2,2,6,6-pentamethyl-4-piperidyloxycarbonyl)-2-(4-methoxyphenyl)ethene,
N,N'-bis-
formyl-N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine, diester
of 4-methoxy-
methylene-malonic acid with 1,2,2,6,6-pentamethyl-4-hydroxypiperidine,
poly[methylpropyl-3-
oxy-4-(2,2,6,6-tetramethyl-4-piperidyl)]siloxane, reaction product of maleic
acid anhydride-a-
olefin-copolymer with 2,2,6,6-tetramethyl-4-aminopiperidine or 1,2,2,6,6-
pentamethyl-4-
aminopiperidine, 2,4-bis[N-(1-cyclohexyloxy-2,2,6,6-tetramethylpiperidine-4-
yl)-N-butyl-
amino]-6-(2-hydroxyethyl)amino-1,3,5-triazine.
2.7. Oxamides, for example 4,4'-dioctyloxyoxanilide, 2,2'-diethoxyoxanilide,
2,2'-dioctyloxy-
5,5'-di-tert-butoxanilide, 2,2'-didodecyloxy-5,5'-di-tert-butoxanilide, 2-
ethoxy-2'-ethyloxanilide,
N,N'-bis(3-dimethylaminopropyl)oxamide, 2-ethoxy-5-tert-butyl-2'-ethoxanilide
and its mixture
with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide, mixtures of o- and p-methoxy-
disubstituted
oxanilides and mixtures of o- and p-ethoxy-disubstituted oxanilides.
2.8. 2-(2-Hydroxypheny_l)-1,3.5-triazines, for example 2,4,6-tris(2-hydroxy-4-
octyloxyphenyl)-
1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-
1,3,5-triazine, 2-
(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2,4-bis(2-
hydroxy-4-pro-
pyloxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-
octyloxyphenyl)-4,6-bis-
(4-methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-dodecyooxyphenyl)-4,6-bis(2,4-
dimethylphe-
nyl)-1,3,5-triazine, 2-(2-hydroxy-4-tridecyloxyphenyl)-4,6-bis(2,4-
dimethylphenyl)-1,3,5-tri-
azine, 2-[2-hydroxy-4-(2-hydroxy-3-butyloxy-propoxy)phenyl]-4,6-bis(2,4-
dimethyl)-1,3,5-tri-
azine, 2-[2-hydroxy-4-(2-hydroxy-3-octyloxy-propyloxy)phenyl]-4,6-bis(2,4-
dimethyl)-1,3,5-
CA 02643170 2008-10-23
-44-
triazine, 2-[4-(dodecyloxy/tridecyloxy-2-hydroxypropoxy)-2-hydroxy-phenyl]-4,6-
bis(2,4-di-
methylphenyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-dodecyloxy-
propoxy)phenyl]-4,6-
bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-hexyloxy)phenyl-4,6-
diphenyl-1,3,5-
triazine, 2-(2-hydroxy-4-methoxyphenyl)-4,6-diphenyl-1,3,5-triazine, 2,4,6-
tris[2-hydroxy-4-(3-
butoxy-2-hydroxy-propoxy)phenyl]-1,3,5-triazine, 2-(2-hydroxyphenyl)-4-(4-
methoxyphenyl)-
6-phenyl-1,3,5-triazine, 2-{2-hydroxy-4-[3-(2-ethylhexyl-1-oxy)-2-
hydroxypropyloxy]phenyl}-
4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-{2-hydroxy-4-[1-octyloxycarbonyl-
ethoxy]phe-
nyl}-4,6-bis(4-phenylphenyl)-1,3,5-triazine wherein the octyl moiety is a
mixture of different
isomers.
3. Metal deactivators, for example N,N'-diphenyloxamide, N-salicylal-N'-
salicyloyl hydrazine,
N,N'-bis(salicyloyl) hydrazine, N,N'-bis(3,5-di-tert-butyl-4-
hydroxyphenylpropionyl) hydrazine,
3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl dihydrazide,
oxanilide, isophthaloyl
dihydrazide, sebacoyl bisphenylhydrazide, N,N'-diacetyladipoyl dihydrazide, N,
N'-bis(salicyl-
oyl)oxalyl dihydrazide, N,N'-bis(salicyloyl)thiopropionyl dihydrazide.
4. Phosphites and phosphonites, for example triphenyl phosphite, diphenyl
alkyl phosphites,
phenyl dialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite,
trioctadecyl phos-
phite, distearyl pentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl)
phosphite, dilsodecyl
pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl) pentaerythritol
diphosphite, bis(2,6-di-
tert-butyl-4-methylphenyl)-pentaerythritol diphosphite,
diisodecyloxypentaerythritol di-
phosphite, bis(2,4-di-tert-butyl-6-methylphenyl)pentaerythritol diphosphite,
bis(2,4,6-tris(tert-
butylphenyl)pentaerythritol diphosphite, tristearyl sorbitol triphosphite,
tetrakis(2,4-di-tert-bu-
tylphenyl) 4,4'-biphenylene diphosphonite, 6-isooctyloxy-2,4,8,10-tetra-tert-
butyl-12H-di-
benz[d,g]-1,3,2-dioxaphosphocin, 6-fluoro-2,4,8,1 0-tetra-tert-butyl-1 2-
methyl-dibenz[d,g]-
1,3,2-dioxaphosphocin, bis(2,4-di-tert-butyl-6-methylphenyl) methyl phosphite,
bis(2,4-di-tert-
butyl-6-methylphenyl) ethyl phosphite, 2,2',2"-nitrilo[triethyltris(3,3',5,5'-
tetra-tert-butyl-1,1'-
biphenyl-2,2'-diyl)phosphite], 2-ethyihexyi(3,3',5,5'-tetra-tert-butyl-1,1'-
biphenyl-2,2'-di-
yl)phosphite.
Especially preferred are the following phosphites:
Tris(2,4-di-tert-butylphenyl) phosphite (irgafos 168, Ciba-Geigy),
tris(nonyiphenyl) phosphite,
CA 02643170 2008-10-23
-45 -
(CH3)3C C(CH3)3 (CH3)C C(CH)3
\ O
H3
C-CH P-F P-O-CH2CH2 N
O
LcH33jI C (CH3)3 C(CH3)3
(CH3)3C 3
(CH3)3C C(CH3)3
O
P - O -- CH2CH(CA)CH2CH3
O
(CH3)3C
C(CH3)3
O 0
(CH33C O--P P-O C(CH3)3
O 0
C(CH3)3 (CH3)3C
C(CH3)3 (CH3)3C
O 0
H3C O-P
\ :K / P-O CH3
O 0
C(CH3)3 (CH3)3C
CH3
r H3C -C -CH3
O O
H37C18-O-P P-O-C18H37 O P-OCH2CH3
O O H3C
LH3CC CH3
CH3 2
CA 02643170 2008-10-23
-46-
5. Hydroxylamines, for example, N,N-dibenzylhydroxylamine, N,N-
diethylhydroxylamine,
N,N-dioctylhydroxylamine, N,N-dilaurylhydroxylamine, N,N-
ditetradecylhydroxylamine, N,N-
dihexadecylhydroxylamine, N,N-dioctadecylhydroxylamine, N-hexadecyl-N-
octadecylhy-
droxylamine, N-heptadecyl-N-octadecylhydroxylamine, N,N-dialkylhydroxylamine
derived
from hydrogenated tallow amine.
6. Nitrones, for example, N-benzyl-alpha-phenyl-nitrone, N-ethyl-alpha-methyl-
nitrone, N-oc-
tyl-alpha-heptyl-nitrone, N-lauryl-alpha-undecyl-nitrone, N-tetradecyl-alpha-
tridcyl-nitrone, N-
hexadecyl-alpha-pentadecyl-nitrone, N-octadecyl-alpha-heptadecyl-nitrone, N-
hexadecyl-
alpha-heptadecyl-nitrone, N-ocatadecyl-alpha-pentadecyl-nitrone, N-heptadecyl-
alpha-hep-
tadecyl-nitrone, N-octadecyl-alpha-hexadecyl-nitrone, nitrone derived from N,N-
dialkylhy-
droxylamine derived from hydrogenated tallow amine.
7. Thiosynergists, for example, dilauryl thiodipropionate or distearyl
thiodipropionate.
8. Peroxide scavengers, for example esters of 0-thiodipropionic acid, for
example the lauryl,
stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc salt
of 2-mercapto-
benzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide,
pentaerythritol tetrakis(f -
dodecylmercapto)propionate.
9. Polyamide stabilisers, for example, copper salts in combination with
iodides and/or phos-
phorus compounds and salts of divalent manganese.
10. Basic co-stabilisers, for example, melamine, polyvinylpyrrolidone,
dicyandiamide, triallyl
cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides,
polyurethanes, alkali
metal salts and alkaline earth metal salts of higher fatty acids for example
calcium stearate,
zinc stearate, magnesium behenate, magnesium stearate, sodium ricinoleate and
potassium
palmitate, antimony pyrocatecholate or zink pyrocatecholate.
11. Nucleating agents, for example, inorganic substances such as talcum, metal
oxides such
as titanium dioxide or magnesium oxide, phosphates, carbonates or sulfates of,
preferably,
alkaline earth metals; organic compounds such as mono- or polycarboxylic acids
and the
CA 02643170 2008-10-23
-47-
salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic
acid, sodium succinate
or sodium benzoate; polymeric compounds such as ionic copolymers (ionomers).
12. Fillers and reinforcing agents, for example, calcium carbonate, silicates,
glass fibres,
glass bulbs, asbestos, talc, kaolin, mica, barium sulfate, metal oxides and
hydroxides, carbon
black, graphite, wood flour and flours or fibers of other natural products,
synthetic fibers.
13. Other additives, for example, plasticisers, lubricants, emulsifiers,
pigments, rheology
additives, catalysts, flow-control agents, optical brighteners, flameproofing
agents, antistatic
agents and blowing agents.
14. Benzofuranones and indolinones, for example those disclosed in U.S.
4,325,863;
U.S. 4,338,244; U.S. 5,175,312; U.S. 5,216,052; U.S. 5,252,643; DE-A-4316611;
DE-A-4316622; DE-A-4316876; EP-A-0589839 or EP-A-0591102 or 3-[4-(2-
acetoxyethoxy)-
phenyl]-5,7-di-tert-butyl-benzofuran-2-one, 5,7-di-tert-butyl-3-[4-(2-
stearoyloxyethoxy)phe-
nyl]benzofuran-2-one, 3,3'-bis[5,7-di-tert-butyl-3-(4-[2-
hydroxyethoxy]phenyl)benzofuran-2-
one), 5,7-di-tert-butyl-3-(4-ethoxyphenyl)benzofuran-2-one, 3-(4-acetoxy-3,5-
dimethylphe-
nyl)-5,7-di-tert-butyl-benzofuran-2-one, 3-(3,5-dimethyl-4-pivaloyloxyphenyl)-
5,7-di-tert-butyl-
benzofuran-2-one, 3-(3,4-dimethylphenyl)-5,7-di-tert-butyl-benzofuran-2-one, 3-
(2,3-di-
methylphenyl)-5,7-di-tert-butyl-benzofuran-2-one.
The conventional additives are judiciously employed in amounts of 0.1-10 % by
weight, for example 0.2-5 % by weight, based on the material to be stabilized.
Costabilizers optionally to be added to the stabilizer mixture of the
invention are preferably
further light stabilizers, for instance those of the 2-hydroxyphenyl-
benztriazole,
2-hydroxyphenyl-triazine, benzophenone or oxalanilide classes, e.g. as
described in EP-A-
453396, EP-A-434608, US-A-5298067, WO 94/18278, GB-A-2297091 and WO 96/28431,
and/or further hindered amines derived from 2,2,6,6-tetraalkylpiperidine
containing at least
one group of the formula
CA 02643170 2008-10-23
29276-763
-48-
GCH2 CH3
-N
G-CH2 CH3
in which G is hydrogen or methyl, especially hydrogen; examples of
tetraalkylpiperidine derivatives which can be used as costabilizers with
mixtures of
the invention are given in EP-A-356 677, pages 3 - 17, sections a) to Q.
Likewise of particular interest is the use of the novel mixtures comprising
compounds of the
formula lila as stabilizers for coatings, for example for paints. The
invention therefore also
relates to those compositions whose component (A) is a film-forming binder for
coatings.
The novel coating composition preferably comprises 0.01 - 10 parts by weight
of (B), in
particular 0.05 - 10 parts by weight of (B), especially 0.1 - 5 parts by
weight of (B), per
100 parts by weight of solid binder (A).
Multilayer systems are possible here as well, where the concentration of the
novel stabilizer
(component (B)) in the outer layer can be relatively high, for example from 1
to 15 parts by
weight of (B); in particular 3 - 10 parts by weight of (B), per 100 parts by
weight of solid
binder (A).
The use of the novel stabilizer in coatings is accompanied by the additional
advantage that it
prevents delamination, i.e. the flaking-off of the coating from the substrate.
This advantage is
particularly important in the case of metallic substrates, including
multilayer systems on
metallic substrates.
The binder (component (A)) can in principle be any binder which is customary
in industry, for
example those described in Ullmann's Encyclopedia of Industrial Chemistry, 5th
Edition,
Vol. A18, pp. 368-426, VCH, Weinheim 1991. In general, it is a film-forming
binder based on
a thermoplastic or thermosetting resin, predominantly on a thermosetting
resin. Examples
thereof are alkyd, acrylic, polyester, phenolic, melamine, epoxy and
polyurethane resins and
mixtures thereof. = .
CA 02643170 2008-10-23
-49-
Component (A) can be a cold-curable or hot-curable binder; the addition of a
curing catalyst
may be advantageous. Suitable catalysts which accelerate curing of the binder
are
described, for example, in Ullmann's Encyclopedia of Industrial Chemistry,
Vol. Al 8, p.469,
VCH Verlagsgesellschaft, Weinheim 1991.
Preference is given to coating compositions in which component (A) is a binder
comprising a
functional acrylate resin and a crosslinking agent.
Examples of coating compositions containing specific binders are:
1. paints based on cold- or hot-crosslinkable alkyd, acrylate, polyester,
epoxy or melamine
resins or mixtures of such resins, if desired with addition of a curing
catalyst;
2. two-component polyurethane paints based on hydroxyl-containing acrylate,
polyester or
polyether resins and aliphatic or aromatic isocyanates, isocyanurates or
polyisocyanates;
3. one-component polyurethane paints based on blocked isocyanates,
isocyanurates or
polyisocyanates which are deblocked during baking, if desired with addition of
a melamine
resin;
4. one-component polyurethane paints based on a Trisalkoxycarbonyltriazine
crosslinker and a hydroxyl group containing resin such as acrylate, polyester
or
polyether resins;
5. one-component polyurethane paints based on aliphatic or aromatic
urethaneacrylates or polyurethaneacrylates having free amino groups within the
urethane strukture and melamine resins or polyether resins, if necessary with
curing catalyst;
6. two-component paints based on (poly)ketimines and aliphatic or aromatic
isocyanates,
isocyanurates or polyisocyanates;
7. two-component paints based on (poly)ketimines and an unsaturated acrylate
resin or a
polyacetoacetate resin or a methacrylamidoglycolate methyl ester;
8. two-component paints based on carboxyl- or amino-containing polyacrylates
and
polyepoxides;
9. two-component paints based on acrylate resins containing anhydride groups
and on a
polyhydroxy or polyamino component;
10. two-component paints based on acrylate-containing anhydrides and
polyepoxides;
CA 02643170 2008-10-23
-50-
11. two-component paints based on (poly)oxazolines and acrylate resins
containing
anhydride groups, or unsaturated acrylate resins, or aliphatic or aromatic
isocyanates,
isocyanurates or polyisocyanates;
12. two-component paints based on unsaturated polyacrylates and polymalonates;
13. thermoplastic polyacrylate paints based on thermoplastic acrylate resins
or externally
crosslinking acrylate resins in combination with etherifled melamine resins;
14. paint systems based on siloxane-modified or fluorine-modified acrylate
resins.
In addition to components (A) and (B), the coating composition according to
the invention
preferably comprises as component (C) a light stabilizer of the sterically
hindered amine
type, the 2-(2-hydroxyphenyl)-1,3,5-triazine and/or 2-hydroxyphenyl-2H-
benzotriazole type,
for example as mentioned in the above list in sections 2.1, 2.6 and 2.8.
Further examples for
light stabilizers of the 2-(2-hydroxyphenyi)-1,3,5-triazine type
advantageously to be added
can be found e.g. in the publications US-A-4619956, EP-A-434608, US-A-5198498,
US-A-
5322868, US-A-5369140, US-A-5298067, WO-94/18278, EP-A-704437, GB-A-2297091,
WO-96/28431. Of special technical interest is the addition of the 2-(2-
hydroxyphenyl)-1,3,5-
triazines and/or 2-hydroxyphenyl-2H-benzotriazoles, especially the 2-(2-
hydroxyphenyl)-
1,3,5-triazines.
Component (C) is preferably used in an amount of 0.05 - 5 parts by weight per
100 parts by
weight of the solid binder.
Apart from components (A), (B) and, if used, (C), the coating composition can
also comprise
further components, examples being solvents, pigments, dyes, plasticizers,
stabilizers,
thixotropic agents, drying catalysts and/or levelling agents. Examples of
possible
components are those described in Ullmann's Encyclopedia of Industrial
Chemistry, 5th
Edition, Vol. A18, pp. 429-471, VCH, Weinheim 1991.
Possible drying catalysts or curing catalysts are, for example, organometallic
compounds,
amines, amino-containing resins and/or phosphines. Examples of organometallic
compounds
are metal carboxylates, especially those of the metals Pb, Mn, Co, Zn, Zr or
Cu, or metal
chelates, especially those of the metals Al, Ti or Zr, or organometallic
compounds such as
organotin compounds, for example.
CA 02643170 2008-10-23
-51 -
Examples of metal carboxylates are the stearates of Pb, Mn or Zn, the octoates
of Co, Zn or
Cu, the naphthenates of Mn and Co or the corresponding linoleates, resinates
or tallates.
Examples of metal chelates are the aluminium, titanium or zirconium chelates
of
acetylacetone, ethyl acetylacetate, salicylaldehyde, salicylaldoxime, o-
hydroxyacetophenone
or ethyl trifluoroacetylacetate, and the alkoxides of these metals.
Examples of organotin compounds are dibutyltin oxide, dibutyltin dilaurate or
dibutyltin
dioctoate.
Examples of amines are, in particular, tertiary amines, for example
tributylamine,
triethanolamine, N-methyldiethanolamine, N-dimethylethanolamine, N-
ethylmorpholine,
N-methylmorpholine or diazabicyclooctane (triethylenediamine) and salts
thereof. Further
examples are quaternary ammonium salts, for example trimethylbenzylammonium
chloride.
Amino-containing resins are simultaneously binder and curing catalyst.
Examples thereof are
amino-containing acrylate copolymers.
The curing catalyst used can also be a phosphine, for example
triphenylphosphine.
The novel coating compositions can also be radiation-curable coating
compositions. In this
case, the binder essentially comprises monomeric or oligomeric compounds
containing
ethylenically unsaturated bonds, which after application are cured by actinic
radiation, i.e.
converted into a crosslinked, high molecular weight form. Where the system is
UV-curing, it
generally contains a photoinitiator as well. Corresponding systems are
described in the
abovementioned publication Ullmann's Encyclopedia of Industrial Chemistry, 5th
Edition,
Vol. Al 8, pages 451-453. In radiation-curable coating compositions, the novel
stabilizers can
also be employed without the addition of sterically hindered amines.
The coating compositions according to the invention can be applied to any
desired
substrates, for example to metal, wood, plastic or ceramic materials. They are
preferably
used as topcoat in the finishing of automobiles. If the topcoat comprises two
layers, of which
the lower layer is pigmented and the upper layer is not pigmented, the novel
coating
composition can be used for either the upper or the lower layer or for both
layers, but
preferably for the upper layer.
CA 02643170 2008-10-23
-52-
The novel coating compositions can be applied to the substrates by the
customary methods,
for example by brushing, spraying, pouring, dipping or electrophoresis; see
also Ullmann's
Encyclopedia of Industrial Chemistry, 5th Edition, Vol. A18, pp. 491-500.
Depending on the binder system, the coatings can be cured at room temperature
or by
heating. The coatings are preferably cured at 50 - 150 C, and in the case of
powder coatings
or coil coatings even at higher temperatures.
The coatings obtained in accordance with the invention have excellent
resistance to the
damaging effects of light, oxygen and heat; particular mention should be made
of the good
light stability and weathering resistance of the coatings thus obtained, for
example paints.
The invention therefore also relates to a coating, in particular a paint,
which has been
stabilized against the damaging effects of light, oxygen and heat by a content
of the
compound of the formula F according to the invention. The paint is preferably
a topcoat for
automobiles. The invention furthermore relates to a process for stabilizing a
coating based
on organic polymers against damage by light, oxygen and/or heat, which
comprises mixing
with the coating composition a mixture comprising a compound of the formula F,
and to the
use of mixtures comprising a compound of the formula F in coating compositions
as
stabilizers against damage by light, oxygen and/or heat.
The coating compositions can comprise an organic solvent or solvent mixture in
which the binder is soluble. The coating composition can otherwise be an
aqueous
solution or dispersion. The vehicle can also be a mixture of organic solvent
and
water. The coating composition may be a high-solids paint or can be solvent-
free
(e.g. a powder coating material). Powder coatings are, for example, those
described in Ullmann's Encyclopedia of Industrial Chemistry, 5th Ed., A18,
pages
438-444. The additive of present invention can be used therein e.g. as
described
e.g. in EP-A-856563, especially page 22, line 21, until page 26, line 29, and
literature cited in this reference.The powder coating material may also have
the
form of a powder-slurry (dispersion of the powder preferably in water).
Examples of resins for powder coatings are:
CA 02643170 2008-10-23
-53-
1. Carboxy- or hydroxy-functionalised polyester resins, based on monomers such
as
terephthalic acid, isophthalic acid, neopentyl glycol, 2-methyl-1,3-
propandiol, tris-1,1,1-
(hydroxymethyl)propane etc.
2. Epoxy resins based on bisphenols, such as bisphenol A or Novolac epoxy
resins for
thermal or uv-cure with cationic photoinitiators.
3. Hydroxy-, carboxy- or glycidyl-functionalised acrylate polymers and
copolymers. Suitable
comonomers include styrene, alkyl methacrylates, acrylamide, acrylonitrile
etc.
4. Unsaturated polyester resins for uv-cureable powder coatings, typically
used in
conjunction with multifuntional vinyl ethers or acrylate esters.
Powder coating based on resins with carboxy functionality are typically used
together with
crosslinking agents of the following classes:
1) polyfunctional epoxy compounds, such as epoxy resins,
triglycidylisocyanurate,
epoxidisod unsaturated fatty acid esters (such as Uranox resins from DSM),
and esters
and ethers of glycidol (such as Araldit PT910 from Ciba Specialty Chemicals).
2) 0-hydroxyalkylamides, such as Primid types XL552 and QM1260 from Ems
Chemie.
3) derivatives of melamine, benzoguanimine and glycoluril, such as Powderlink
1174 from
American Cyanamid.
Crosslinking agents for resins of hydroxy functionality include anhydrides and
especially
blocked diisocyanates and uretdiones, etc.
Powder coatings based on resins with epoxy functionality are typically used
together with'
crosslinking agents such as diacids (such as 1,12-dodecanedioic acid), carboxy-
functional
polyesters, carboxy-functional copolymers of acrylates and methacrylates,
anhydrides (such
as the anhydride prepared from 1, 1 2-dodecanedioic acid).
Other additives that can be used together with the compounds of the invention
in powder
coatings include: degassing agents, flow promoters, tribocharging additives
cure catalysts,
sensitisers, cationic and free-radical photoinitiators, as well as typical
liquid paint additives.
A particular advantage of the compounds of the invention is their low
basicity, as basic
compounds often catalyse the crosslinking reactions of powder coatings to
cause poor flow
and degassing, and reduced storage stability. This is particularly useful in
formulations of
CA 02643170 2008-10-23
-54-
high reactivity, such as the glycidylmethacrylate-functionalised acrylics.
Here, the
combination of the compounds of the invention together with uv-absorbers,
especially of the
hydroxyphenyltriazine class, can be used to improve the weatherability without
causing
catalysis. In other binder systems and with other classes of uv-absorbers,
such as those
previously mentioned to be of particular use in automotive paints, synergistic
effects on the
weatherability are also found.
In powder coatings the compounds of the invention can also be used to improve
the
oxidative stability and reduce yellowing on curing and overbaking. Here not
only is the low
basicity advantageous, but also the ability of the hindered morpholinones to
withstand and
prevent yellowing caused by oxides of nitrogen in gas-fired ovens. Use
together particularly
with phosphite and phosphonite costabilisers, as disclosed in EP-A-816442, and
dialkylesters of dithiopropionic acid is particularly beneficial. The
compounds of the invention
can, whore appropriate also be used to stabilise polyester during manufacture
as well as at
all stages of its subsequent use.
The pigments can be inorganic, organic or metallic pigments. The novel coating
compositions preferably contain no pigments and are used as a clearcoat.
Likewise preferred is the use of the coating composition as a topcoat for
applications in the
automobile industry, especially as a pigmented or unpigmented topcoat of the
paint finish. Its
use for underlying coats, however, is also possible.
Some products of present process are novel compounds.
Present invention therefore also pertains to a compound of the formula 1,
especially a
compound of the formula Ia, wherein R5, R6, R7, Re and R9, independently of
each other, are
H, C1-C8alkyl, C3-C8alkenyl, C5-C12aryl, an electron withdrawing group, C6-
C,2aryl which is
substituted by C1-C4alkyl, C,-C4alkoxy, halogen, and wherein at least one of
R5, R6, R7, R8
and R9 is not H. Present invention also pertains to a compound of the formula
I, especially a
compound of the formula la, wherein R5, R6, R7, R8 and R9, independently of
each other, are
C1-C8alkyl, C3-C8alkenyl, C5-C12aryl, an electron withdrawing group, C6-
C12aryl which is
substituted by C1-C4alkyl, C,-C4alkoxy, halogen; and all other symbols are as
defined above.
Preferred compounds of the formula I are those of the formula la
CA 02643170 2008-10-23
-55-
A N R 12 N -A
N~ N N~ IIN
R11 H3C CH3 Y
H3C A.
CH3
O (la)
R9 Ra
fR7
6 RS
R n
in which the index n ranges from 1 to 15, being especially from the range 3-9;
R5-R9 are as defined for formula I;
R12 is C2-C12alkylene, C4-Ci2alkenylene, C5-C7cycloalkylene, C5-
C7cycloalkylene-
di(C1-C4alkylene), C1-C4alkylenedi(C5-C7cycloalkylene), phenylenedi(C1-
C4alkylene) or
C4-C12alkylene interrupted by 1,4-piperazinediyl, -0- or >N-X1 with X1 being
C1-C12acyl or
(C1-C12alkoxy)carbonyl or having one of the definitions of R14 given below
except hydrogen;
or R12 is a group of the formula (lb') or (Ic');
-CH2 j H-CHZ (lb')
C=0
1
X2
O O DC
(Ic')
O 0
.with m being 2 or 3,
X2 being C1-C18alkyl, C5-C12cycloalkyl which is unsubstituted or substituted
by 1, 2 or 3
C1-C4alkyl; phenyl which is unsubstituted or substituted by 1, 2 or 3 C1-
C4alkyl or
C1-C4alkoxy; C7-C9phenylalkyl which is unsubstituted or substituted on the
phenyl by 1, 2 or 3
C1-C4alkyl; and
CA 02643170 2008-10-23
-56-
the radicals X3 being independently of one another C2-C12alkylene;
the radicals A are independently of one another -OR13, -N(R14)(R,5) or a group
of the
formula (Id');
H3C CH3
R8 R7 R5
x N -0 (Id')
Rs
H3C CH3 9
R13, R14 and R15, which are identical or different, are hydrogen, C,-Ci8alkyl,
C5-C12cycloalkyl
which is unsubstituted or substituted by 1, 2 or 3 C1-C4alkyl; C3-C18alkenyl,
phenyl which is
unsubstituted or substituted by 1, 2 or 3 C1-C4alkyl or C1-C4alkoxy; C7-
C9phenylalkyl which is
unsubstituted or substituted on the phenyl by 1, 2 or 3 C1-C4alkyl;
tetrahydrofurfuryl or
C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C1-Cealkoxy,
di(C1-C4alkyl)amino or a group of the formula (le');
Y \--/ N (le')
with Y being -0-, -CH2-, -CH2CH2- or >N-CH3,
or -N(R14)(R15) is additionally a group of the formula (le');
X is -0- or >N-R16;
R16 is hydrogen, C,-Ct8alkyl, C3-C18alkenyl, C5-C12cycloalkyl which is
unsubstituted or
substituted by 1, 2 or 3 C1-C4alkyl; CrC9phenylalkyl which is unsubstituted or
substituted on
the phenyl by 1, 2 or 3 C1-C4alkyl; tetrahydrofurfuryl, a group of the formula
(If'),
H3C CH3
R8 R7 R5
N-O (if)
R R6
H3C CH3 s
or C2-C4alkyl which is substituted in the 2, 3 or 4 position by -OH, C,-
C8alkoxy,
di(C1-C4alkyl)amino or a group of the formula (le');
R11 has one of the definitions given for R16i and
the radicals B have independently of one another one of the definitions given
for A;
and where in the individual recurrent units of the formula (la), each of the
radicals B, R11 and
R12 may have identical or different meanings.
Further new products of present process correspond to formulae Illc, IVa and
Va
CA 02643170 2008-10-23
-57-
R1 X R 2
R N-O-C C-CH3 , (IIIc)
H2 H2
R3 R4
R1 R2
i~~ R22 Rs
R N-O R 6 (IVa)
R9 R23
R3 R4
R1 R2
X R 8 R
1 7 R5
R N-O R 6 (V)
R9 R20 R21
R3 XRH2
C-
wherein R is an organic linking group of the formula E/ (VI);
Ei-
E2 is -CO- or -(CH2)p , where p is 0, 1 or 2;
E, is a carbon atom carrying the two residues R24 and R25i or is >N-R25, or is
oxygen, and R24
and R25 are hydrogen or an organic residue, characterized in that the linking
group R in total
contains 2-500 carbon atoms and forms, together with the carbon atoms it is
directly
connected to it and the nitrogen atom, a substituted, 5-, 6 or 7-membered
cyclic ring
structure;
R,, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
C5hydroxyalkyl, or R,
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
R5, R6, R,, R8 and R9, independently of each other, are H, C1-C8alkyl, C2-
C8alkenyl, C5-
C12aryl, C1-C4haloalkyl, an electron withdrawing group, or C6-C12aryl which is
substituted by a
residue selected from C1-C4alkyl, C1-C4alkoxy, halogen;
CA 02643170 2008-10-23
-58-
R20 and R21 are halogen; and
R22 and R23 are hydrogen or together are a chemical bond,
H2
R 24 C-
with the proviso that R in formula Ilic is not the linking group wherein R24
and
R 25 C-
H2
R25 together are =0 or wherein R24 is hydrogen and R25 is hydrogen, OH, or
alkanoyloxy
which is substituted by phenoxy or alkylphenoxy.
When E1 is substituted carbon, E2 mainly is -(CH2)P , especially CH2; when E1
is oxygen or
NR25, E2 mainly is carbonyl.
Thus, a compound of formula Illc, lVa or Va is preferred, wherein R is a
divalent C7-C500
hydrocarbon or a C2-C500hydrocarbon containing 1-200 hetero atoms selected
from nitrogen,
oxygen, phosphorus, sulfur, silicon and halogen, and conforms to the structure
H2 H2
H2 C- C-
R z j (VIa), R 26 N\ (Vib) or 0 (VIc)
R 25 (C~H2)p O/j- 0
where p is 0, 1 or 2;
R1, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
C5hydroxyalkyl, or R1
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
R5 and R6 independently are H or methyl; and R7, Re and R9 independently are
C1-
C4haloalkyl, phenyl, vinyl, nitro, CN, COOR10, where R10 is C1-C12alkyl, C5-
C12cycloalkyl or
phenyl;
R24 and R25 independently are hydrogen or an organic residue as defined, and
R26 is hydrogen or an organic residue forming, together with the remaining
structure of
formula (VIb) a C2-C500hydrocarbon containing 1-200 hetero atoms selected from
nitrogen,
oxygen, phosphorus, sulfur, silicon and halogen.
Most preferred is a compound of formula Illc, IVa or Va, wherein
CA 02643170 2008-10-23
-59-
R5 and R6 independently are H or methyl; and R,, R8 and R9 independently are
C1-
C4bromoalkyl, phenyl, CN, COOR10, where R10 is C1-C,2alkyl, C5-C,2cycloalkyl
or phenyl;
especially wherein R5, R6, R7, R8 and R9 are hydrogen;
R20 and R21 are bromo; and
when R conforms to the structure of formula Via,
p is 1 and RI, R2i R3 and R4, independently of each other, are methyl or
ethyl;
when R conforms to the structure of formula VIb,
R,, R2, R3 and R4, independently of each other, are methyl or ethyl; or R, and
R2 together
with the carbon atom they are attached to are C5-C12cycloalkyl, or R3 and R4
together with
the carbon atom they are attached to are C5-C12cycloalkyl;
when R conforms to the structure of formula Vic,
R,, R2, R3 and R4, independently of each other, are C1-C8alkyl or C1-
C5hydroxyalkyl, or R,
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl.
Special emphasis is given to a compound of the formula illc
R1 R2
R N-O-C C-CH3 , (Ilic)
H2 H2
R3 R4
wherein R is a C7-C500hydrocarbon containing 1-200 hetero atoms selected from
nitrogen,
oxygen, phosphorus, sulfur and halogen, and forming, together with the two
carbon and the
nitrogen atom, a substituted, 5- or 6-membered cyclic ring structure, and
R1, R2, R3 and R4 are as defined above, with the proviso that R does not
complete formula
Illc to form a structure of the formula
R2 R,
O N -O-C C CH3
H
H2 H2
A
R4 R3
Especially preferred is a compound of the formula Illc shown above.
CA 02643170 2008-10-23
-60-
Thus, the new sterically hindered amine usually corresponds to the formulae
(1a), (1b) or
(2a) or contains at least one group of the formula (3) or (4)
G-CH CH3 G
CH3 G,
2 G - CH2 G13
Gõ-N O G12 J- I
G-CH2 (1a) Gõ-N N--G14 (1b),
CH3 G - CH2
n1 CH3
n2
R2 O
R, _)_\
G11- N 0 (2a)
R4
CH3 G, CH3 G,
G-CH2 G2 G-CH2 _4_4 G2
Gõ - N (3), Gil- N N- (4),
G - CH2 G - CH2 G3
CH3 CH3
in which
n, is a number from 1 to 4, G and G1, independently of one another, are
hydrogen or methyl,
G11 is n-propoxy, O-CH=C=CH2, O-CH=CH-CH3 or halogenated n-propoxy, especially
n-
propoxy, or brominated n-propoxy;
G12, if n, is 1, is C1-C18alkyl which is uninterrupted or interrupted by one
or more oxygen
atoms, cyanoethyl, benzoyl, glycidyl, a monovalent radical of an aliphatic,
cycloaliphatic,
unsaturated or aromatic carboxylic acid, carbamic acid or phosphorus-
containing acid or a
monovalent silyl radical, preferably a radical of an aliphatic carboxylic acid
having 2 to 18
carbon atoms, of a cycloaliphatic carboxylic acid having 7 to 15 carbon atoms,
or an a,R-
unsaturated carboxylic acid having 3 to 5 carbon atoms, where each carboxylic
acid can be
substituted in the aliphatic, cycloaliphatic or aromatic moiety, if present,
by 1 to 3 -COOZ12
groups, in which Z12 is H, C1-C20alkyl, C3-C12alkenyl, C5-C7cycloalkyl, phenyl
or benzyl,
CA 02643170 2008-10-23
-61-
G12, if n1 is 2, is C2-C12alkylene, C4-C12alkenylene, xylylene, a divalent
radical of an aliphatic,
cycloaliphatic, araliphatic or aromatic dicarboxylic acid, dicarbamic acid or
phosphorus-
containing acid or a divalent silyl radical, preferably a radical of an
aliphatic dicarboxylic acid
having 2 to 36 carbon atoms, or a cycloaliphatic or aromatic dicarboxylic acid
having 8-14
carbon atoms or of an aliphatic, cycloaliphatic or aromatic dicarbamic acid
having 8-14
carbon atoms, where each dicarboxylic acid may be substituted in the
aliphatic,
cycloaliphatic or aromatic moiety by one or two -COOZ12 groups,
G12, if n1 is 3, is a trivalent radical of an aliphatic, cycloaliphatic or
aromatic ticarboxylic acid,
which may be substituted in the aliphatic, cycloaliphatic or aromatic moiety
by
-COOZ12i of an aromatic tricarbamic acid or of a phosphorus-containing acid,
or is a trivalent
silyl radical,
and G12, if n1 is 4, is a tetravalent radical of an aliphatic, cycloaliphatic
or aromatic
tetracarboxylic acid;
R1, R2, R3 and R4, independently of each other, are C1-Cealkyl or C1-
C5hydroxyalkyl, or R1
and R2 together with the carbon atom they are attached to are C5-
C12cycloalkyl, or R3 and R4
together with the carbon atom they are attached to are C5-C12cycloalkyl;
G is hydrogen or methyl;
G1 and G2, independently of one another, are hydrogen, methyl or together are
a substituent
=0; and
G3 is a direct bond or methylene,
open bonds of formulae (3) and (4) are linked to a carbon, nitrogen or oxygen
atom of an
organic residue as defined above,
G13 is hydrogen, C1-C12alkyl, C2-C5hydroxyalkyl, C5-C7cycloalkyl, C7-
Cearalkyl,
C1-C18alkanoyl, C3-C5alkenoyl, benzoyl or a group of the formula (1 b-1)
CH3 G1
G -CH2
G11-N (1b-1)
G -CH2
CH3
n2 is the number 1, 2 or 3;
and G14, if n2 is 1, is hydrogen, C1-C18alkyl, C3-Cealkenyl, C5-C7cycloaikyl,
C1-C4alkyl which is
substituted by a hydroxyl, cyano, alkoxycarbonyl or carbamide group, glycidyl,
a group of the
CA 02643170 2008-10-23
29276-763
-62-
formula -CH2-CH(OH)-Z or of the formula -CONH-Z, in which Z is hydrogen,
methyl or
phenyl;
G14, if n2 is 2, is C2-C12alkylene, C6-C12arylene, xylylene, a -CH2-CH(OH)-CH2
group or a
-CH2-CH(OH)-CH2-O-D-O- group, in which D is C2-C10alkylene, C6-C15arylene,
C6-C12cycloalkylene, or, provided that G13 is not alkanoyl, alkenoyl or
benzoyl, G14 can
alternatively be 1-oxo-C2-C12alkylene, a divalent radical of an aliphatic,
cycloaliphatic or
aromatic dicarboxylic acid or dicarbamic acid or alternatively the group -CO-,
G14, if n2 is 3, is a group
0
-CHzCH(OH)CH2',/CH2CH(OH)CH2
N N
O1,11 N O
1
CH2CH(OH)CH2-
or, if n2 is 1, G13 and G14 together can be the divalent radical of an
aliphatic, cycloaliphatic or
aromatic 1,2- or 1,3-dicarboxylic acid.
Preferred new hindered amines of formula Illc are as described above in
sections
(b') - (j') and preferences indicated therein. The new compounds of formula
111c are
useful as stabilizers for organic material against degradation by light,
oxygen
and/or heat. The materials to be stabilized can, for example, be oils, fats,
waxes,
cosmetics or biocides. Particular interest attaches to use in polymeric
materials, as
in plastics, rubbers, coating materials, photographic materials or adhesives;
examples are organic polymers as described above, and reprographic, especially
color photographic material as described, for instance, in GB-A-2319523, DE-A-
19750906, page 23, line 20, until page 105, line 32, or in US-A-5 538 840,
column
25, line 60, to column 106, line 31.
Other preferences are as described above for the compounds of formulae I and
Ili.
The examples below illustrate the invention further. All parts or percentages,
in the
examples as in the remainder of the description and in the claims, are by
weight,
CA 02643170 2008-10-23
-63-
unless stated otherwise. Room temperature denotes a temperature in the range
20-30 C, unless stated otherwise. In the examples, the following
abbreviations
are used:
M.P. melting point or range;
Mn number average of molecular weight (g/mol);
Mw weight average of molecular weight (g/mol);
GPC gel permeation chromatography.
Example 1: Preparation of the starting compound of the formula
c4H9 H9C4
I N N ( -N N N -N N N
N~ C fN. n' N N
Fi3C
CF6 H Fl3C Ct t3 N04
CA F 3C N CH3 HtC N CF 3 C i Ot C i O
H H
N -C4H9 H9C4 - N H H H9C4 - N
CA H3C A a- C4H9
H3C i CH3
H
Step 1: A solution of 74.3 g (0.35 moles) of N-(2,2,6,6-tetramethyl-4-
piperidinyl)-n-butylamine
in 50 ml of water is added slowly at 0 C to a solution of 64.5 g (0.35 moles)
of cyanuric
chloride in 500 ml of xylene, keeping the temperature during the addition and
for further
1 hour.
After 2 hours at room temperature, the mixture is cooled to 0 C and an aqueous
solution of
14.7 g (0.368 moles) of sodium hydroxide in 50 ml of water is added.
After 1/2 hour at 0 C and for further 2 hours at room temperature, the aqueous
solution is
separated off and 69.2 g (0.175 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-
piperidinyl)-1,6-
hexanediamine are added.
The mixture is heated to 50 C for 1 hour and 48.4 g (0.35 moles) of ground
potassium
carbonate are added and heated to 60 C for 4 hours.
CA 02643170 2008-10-23
-64-
After washing with water, the organic phase is concentrated under vacuum at
60-70 C/10 mbar, being 250 ml of xylene recovered.
138.1 g (0.35 moles) of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-
hexanediamine are
added and the mixture is heated to 150 C for 2 hours, cooled again and added
with 14 g
(0.35 moles) of ground sodium hydroxide.
The mixture is heated to 140 C for further 4 hours, being the residual water
of reaction
eliminated off azeotropically and for further 4 hours at 160 C.
After cooling to 60 C, the mixture is diluted with 300 ml of xylene, filtered
and washed three
times with 100 ml of ethylene glycol.
This solution can be used for the isolation of the compound described in
Example 6.
Step 2: After concentrating under vacuum at 60 C/10 mbar, 54.4 g (0.147 moles)
of 2-chloro-
4,6-bis-(dibutylamino)-1,3,5-triazine are added.
The mixture is heated to 140 C for 3 hours and 20.3 g (0.147 moles) of ground
potassium
carbonate are added, being the mixture heated to reflux and being the reaction
water
eliminated off azeotropically.
The mixture is heated to 160 C for 4 hours, added to further 20.3 g (0.147
moles) of ground
potassium carbonate and heated again to 160 C for 2 hours. After cooling to 60
C, the
mixture is diluted with 300 ml of xylene, filtered and concentrated under
vacuum at
140 C/1 mbar. A solid is obtained with a melting range of 130-136 C after
drying; Mn (by
GPC): 2830 g/mol.
Example 2: Process of present invention; preparation of the compound of the
formula
CA 02643170 2008-10-23
-65-
CA NP4
N--riN~--N (Gtz)6N N~--N (Ct{Z)6---N N~--N
N~ N N~ N n' N~ N I
CF6 I
H3CC - H3C A CF6 C CF% H9Ca
CF~
CA NC N Chia F 3C N Chia NC N CN NC i Gt
O O p
I4:c4
CH3 CH3 h13C CH 3 C CH3 CA
hi3C N a-l
i
O
Chi
Step 1: In a 1 liter stainless steel autoclave with heating and mechanical
stirrer are charged:
a solution of 150 g (0.05 mol) of the product of example 1 in 400 ml of
xylene,
66.5 g (0.55 mol) of allyl bromide and 114 g (0.825 mol) of potassium
carbonate. The mixture
is heated to 150 C, left to react for 5 hours and cooled down to 60 C. 300 ml
of water are
added and the mixture is vigorously stirred. The organic layer is then
collected and charged
in a 1 liter round bottomed flask equipped with a mechanical stirrer,
thermometer and
dropping funnel. After cooling to -15 C, 128 g of a solution of 32% by weight
of peracetic acid
in acetic acid is added during 30 minutes under stirring. The temperature is
raised to 0 C and
the reaction mixture is left to react for 4 hours.
A solution of 250 g of K2CO3 in 500 ml of water is added and kept for 30
minutes at 0 C with
stirring. The organic layer is collected, washed 3 times with 100 ml portions
of water, and
dried over sodium sulfate.
Step 2: The solution is charged in a 1 I stainless steel autoclave. After
addition of 3 g of 5%
by weight platinum on carbon, the autoclave is filled with hydrogen of 40 bar
and maintained
at 70 C with stirring for a period of 6 hours. Subsequently, the autoclave is
cooled to 20 C
and vented. After removing the catalyst by filtration, the solution is
concentrated at 140 C
and 1 mbar. The product is obtained as a white solid, m.p. 127-135 C, Mn (GPC)
= 3580
g/mol, Mw/Mn = 1.33.
CA 02643170 2008-10-23
-66-
Example 3 : Process of present invention; preparation of the compound of the
formula
O- OH
In a round bottomed flask are charged : 20 g of 1 allyl-2,2,6,6-tetramethyl-
piperidin-4-ol, 40 ml
of methanol, 118 g of H202 solution at 35 % (v/v).
The mixture is heated to 65 C, left to react for 5 hours, concentrated under
vacuum until
methanol has been distilled off and 40 ml of CH2CI2 are added. The mixture is
stirred and the
organic layer is collected and concentrated. The product is obtained as a pale
yellow oil.
1H NMR (300 MHz, CDCI3)/ppm: 5.90 - 5.80 (m, 1H) ; 5.21 - 4.97 (m, 2H) ; 4.24
(m, 2H);
3.87 (m, 1 H) ; 1.75 (m, 2H) ; 1.42 ( m, 2H) ; 1.15 (s, 3H) ; 1.11 (s, 3H).
Example 4a : Preparation of the compound of the formula
O-N OH
In a stainless steel autoclave are charged : 20 g of the product of example 3,
0.2 g of nickel
raney and 100 ml of toluene; the autoclave is filled with hydrogen of 8 bar
and maintained at
25 C under stirring for a period of 8 hours. Subsequently the autoclave is
vented , the
catalyst is removed by filtration and the mixture is concentrated under
vacuum. The product
is obtained as a white solid. 1H NMR (300 MHz, CDCI3)/ppm: 3.95 (m, 1H); 3.66
(t, 2H); 1.57
(m, 4H); 1.22 (m, 2H); 1.13 (s, 12H); 0.89 (t, 3H).
Example 4b: Preparation of the compound of the formula
CA 02643170 2008-10-23
-67-
O O N-O
~-(CH2)8
O-N O O
In a round bottomed flask are charged : 20 g of product of example 4a), 10.6 g
of methyl
sebacate ,100 ml of xylene and 0.25 g of di-butyl-tin-oxide.
The mixture is heated to 145 C, left to react for 6 hours with stirring,
cooled down and
concentrated under vacuum, yielding the above product as an oil.
'H NMR (300 MHz, CDCI3)/ppm: 4.95 (m, 2H); 3.66 (t, 4H); 2.21 (t, 4H); 1.77
(m, 4H); 1.63 -
1.42 (m, 12H); 1.28 - 1.18 (m, 36H); 0.90 (t, 6H).
Other analytical data :
HPLC assay : 80 %
Elemental analysis : C measured 67.6 % calculated 68.4 %
H measured 10.6 % calculated 10.8 %
N measured 4.7 % calculated 4.7 %
Example 5 : Process of present invention; preparation of the compound of the
formula
O O N-O
--(CH2)8\
O-N O O
In a stainless steel autoclave are charged 20 g of sebacic acid bis-(2,2,6,6-
tetramethyl-
piperidin-4y1) ester (commercial name: Tinuvin 770), 24 g of allyl bromide, 6
g of potassium
carbonate and 100 ml of toluene; the mixture is heated to 120 C, left to
react for 5 hours
with stirring, cooled down and filtered to remove the salts. The excess of
ally) bromide is
removed by distillation.
CA 02643170 2008-10-23
-68-
The solution is charged in a round bottomed flask and 8 g of
metachloroperbenzoic acid
dissolved in 50 ml of toluene are added over a period time of 30 minutes
keeping the
temperature below 25 C.
Then a solution of 13 g of potassium carbonate in 100 ml of water is added to
the reaction
mixture and it is left under stirring for 30 minutes. The organic layer is
collected and washed
with a potassium carbonate solution prepared as described above.
The organic layer is then dried over sodium sulfate and charged in a stainless
steel
autoclave.
After addition of 1 g of 5 % by weight platinum on carbon, the autoclave is
filled with
hydrogen of 8 bar and maintained at 25 C with stirring for 4 hours. After
removing the
catalyst by filtration, the solution is concentrated under vacuum. The product
is obtained as a
pale yellow oil; 'H NMR (300 MHz, CDC13)/ppm: 4.95 (m, 2H); 3.66 (t, 4H); 2.21
(t, 4H); 1.77
(m, 4H); 1.63 - 1,42 (m, 12H); 1.28 - 1.18 (m, 36H); 0.90 (t, 6H).
Other analytical data :
HPLC assay : 78%
Elemental analysis : C measured 67.2 % calculated 68.4 %
H measured 10.3 % calculated 10.8 %
N measured 4.6 % calculated 4.7 %
Example 6: Intermediate of the formula
N (Q1)6- N
N 1Y--- N -- (CH2)6 - N H
--f-r
CF~ C
N, N H3C ri
CC
1-3C N CH3 H3C N CH3 F6c i CF6 H3C i CH3
H H H H
NC4 N
H3c CF 3
H3C N CH3
H
The preparation of the current compound follows the procedure described in
Example 1 up to
Step 1. The solution obtained from step 1 is then concentrated at 140 C and 1
mbar and it
yields a solid, mp 138-143 C, with an average Mn (by GPC) of 2555 g/mol.
CA 02643170 2008-10-23
-69-
Example 7:
N (CFQ6 - N N N (CH2)6 - N O-~\
N~ n
NC A CF~ NBA CF6 C CF6
H3C N at F6C N CF6 3C i at F13C N CH3
O p O p
NQ, N
H3C CF6
H3C N CH3
I
O
To a solution of 150 g of the product of example 6 in 400 ml of xylene are
added 66.5 g of
allyl bromide and 114 g of potassium carbonate. The mixture is heated to 150
C, left to react
for 5 hours and cooled down to 60 C. 300 ml of water are added and the mixture
is
vigorously stirred.
The organic layer is then collected, cooled to -15 C and 128 g of a solution
of 32% by weight
of peracetic acid .in acetic acid is added during 30 minutes under stirring.
The temperature is
raised to 0 C and the reaction mixture is left to react for 4 hours.
A solution of 250 g of K2CO3 in 500 ml of water is added while stirring and
left to react for 30
minutes at 0 C. The organic layer is collected, washed 3 times with 100 ml
portions of water,
and dried over sodium sulfate. The solution is charged in a 1 1 stainless
steel autoclave. After
addition of 3 g of 5% by weight platinum on carbon, the autoclave is filled
with hydrogen of
40 bar and maintained at 70 C with stirring for a period of 6 hours.
Subsequently, the
autoclave is cooled to 20 C and vented. After removing the catalyst by
filtration, the solution
is concentrated at 140 C and 1 mbar.. The product is obtained as a white
solid, m.p. 125 -
135 C, Mn (GPC) = 2979 g/mol.
Example 8: Compound of the formula
CA 02643170 2008-10-23
-70-
CI
N" \N
N
N
N N
O 0
To a solution of 93 g of 2-chloro-4,6-bis-(N-n-butyl-N-(2,2,6,6-
tetramethylpiperidin-4-yl))-
1,3,5-triazine in 300 ml of toluene are added 42 g of allyl bromide and 71.7 g
of potassium
carbonate. The mixture is heated to 150 C, left to react for 10 hours, cooled
down to 60 C
and 300 ml of water are added while stirring. The organic layer is then
collected and cooled
to -5 C, 64 g of a solution of 39% by weight of peracetic acid in acetic acid
is added during
30 minutes under stirring. The temperature is raised to 0 C and the reaction
mixture is left to
react for 2 hours.
A solution of 90 g of sodium carbonate in 500 ml of water is added and kept
for 30 minutes at
0 C with stirring. The organic layer is collected and dried over sodium
sulfate. The solution is
charged in a 1 I stainless steel autoclave. After addition of 3 g of 5% by
weight platinum on
carbon, the autoclave is filled with hydrogen of 40 bar and maintained at 70 C
with stirring for
a period of 6 hours. Subsequently, the autoclave is cooled to 20 C and vented.
After
removing the catalyst by filtration, the solution is concentrated at 140 C and
1 mbar. The
product is obtained as a white solid.
1 H NMR (300 MHz, CDCI3)/ppm: 4.97 (m, 2H) ; 3.67 (t, 4H) ; 3.29 (m, 4H); 1.80
- 1.30 (m,
20H) ; 1.31 - 1.08 (m, 28H) ; 0.92 - 0.80 ( m, 12H).
CA 02643170 2008-10-23
-71-
Example 9: Compound of the formula
~o- N N N N -O~
N-
N\ ~~HNN---~N AN
/N N"'\ N
` O II N N-0
N N
N N
N
A mixture composed of 64.5 q of the compound described in the example 8, 200
ml of
xylene, 20.4 g of potassium carbonate and 4.6 g of N-1-[2-(3-Amino-
propylamino)-ethyl]-
propane-1,3-diamine is heated up to 140 C for 10 hours, cooled down to 20 C
and washed
with 200 ml of water. The collected organic layer is concentrated at 140 C and
1 mbar. The
solid obtained has a m.p. in the range of 115 - 120 C.
Example 10: Compound of the formula
N N 0
I
N
N,O
To a solution of 30 g of N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,6-
hexanediamine in 240
ml of toluene are added 50 g of allyl bromide and 63 g of potassium carbonate.
The resulting
mixture is heated up to 150 C and left to react for 6 hours, cooled down to 20
C, filtered and
the volume of the solution is reduced at 100 ml by solvent evaporation at 110
C and 1 mbar
of vacuum. To the concentrate solution are added 150 ml of toluene, after
cooling down to -
C a solution of 82 g of m-chloro perbenzoic acid in 200 ml of toluene is added
over one
hour under stirring. After heating the mixture up to 0 C, a solution of 100 g
of potassium
carbonate in 300 ml of water is added under stirring and left to reach 20 C.
CA 02643170 2008-10-23
-72-
The organic layer is collected, dried over sodium sulfate and hydrogenated
with 40 bar of
hydrogen with 2 g of 5% platinum over carbon at 70 C for 8 hours. The
catalyst is recovered
by filtration and the solution is concentrated at 110 C under 1 mbar.
1 H NMR (300 MHz, CDCI3)/ppm: 3.75 (t, 4H) ; 3.54 (t, 4H) ; 2.82 (m, 2H); 2.63
(t, 4H) ; 1.78 -
1.45 (m, 20H) ; 1.42 - 1.23 ( m, 4H) ; 1.16 (s, 12H) ; 1.10 (s, 12H), 0.89 (m,
6H).
Example 11: Compound of the formula
O
O N-O
To a solution of 90 g of benzoic acid 1-but-2-enyl-2,2,6,6-tetramethyl-
piperidin-4-yl ester in
200 ml of toluene, are added 70 g of 4-Bromo-2-butene and 100 g of potassium
carbonate.
The mixture is heated to 140 C, left to react for 10 hours under stirring,
cooled down to 20 C,
poured in 200 ml of water and stirred. The organic layer is then collected,
concentrated by
removing 100 ml solvent at 110 C at 20 mbar, then added with 100 ml of fresh
toluene. The
resulting solution is cooled down to -15 C and a solution of 100 g of m-chloro-
perbenzoic
acid in 200 ml of toluene is added during 30 minutes under stirring. The
mixture is left to
react for 2 hours at 0 C then a solution of 40 g of potassium carbonate in 300
ml of water is
added under stirring. The organic layer is collected and the solvent
evaporated under
vacuum. The product obtained is a pale yellow liquid.
1 H NMR (300 MHz, CDCI3)/ppm: 7.99 (d, 2H) ; 7.49 (m, 1 H) ; 7.37 (m, 2H);
5.85 (m, 1 H) ;
5.26 (m, 1H);5.06-5.00(m,2H);4.28(m, 1H); 1.94 (m, 2H);1.71 (m,2H); 1.25-1.17
(m, 15 H).
Example 12: Compound of the formula 4N-0
To a solution of 52 g of 2,2,6,6, tetramethylpiperidine in 250 ml of hexane
are added 102 g of
potassium carbonate and 135 g of propargyl bromide. The mixture is heated to
140 C, left to
react for 10 hours under stirring, cooled down to 20 C, poured in 300 ml of
water and stirred.
The organic layer is then collected, dried over sodium sulphate, and distilled
under vacuum
collecting the fraction at 64 C and 10 mmHg which is then dissolved in 250 ml
of
CA 02643170 2008-10-23
-73-
dichloromethane and cooled to -15 C. To the resulting solution is added a
solution of 100 g
of m-chloro-perbenzoic acid in 200 ml of hexane in 30 minutes while stirring.
The mixture is
left to react for 2 hours at 0 C and a solution of 40 g of potassium carbonate
in 300 ml of
water is added while stirring. The organic layer is collected, dried over
sodium sulfate and the
solvent evaporated under vacuum. The product obtained is a pale yellow liquid.
1 H NMR (300 MHz, CDCI3)/ppm: 6.82 (t, 1 H) ; 5.49 (d, 2H) ; 1.52 (s, 6 H) ;
1.13 (s, 12H).
Example 13: Compound of the formula N-O,,~
A mixture of 16 g of the compound described in the example 11, 500 ml of
ethanol and 0.9 g
of Lindlar catalyst is charged in an autoclave. The autoclave is filled with
10 bar of hydrogen
and maintained at 40 C under stirring for a period of 6 hours, then it is
cooled to 20 C and
vented. After removing the catalyst by filtration, the solution is
concentrated under vacuum.
The resulting product is obtained as a pale yellow liquid.
1 H NMR (300 MHz, CDCI3)/ppm: 6.29 (d, 1 H) ; 4.01 (m, 1 H) ; 1.57 (d, 3 H) ;
1.45 (m, 6H) ;
1.12 (s, 12H).
Example 14: Compound of the formula 4N-0
A mixture of 38 g of the compound described in the example 11, 300 ml of
toluene and 1 g of
platinum supported on carbon at 5% by weight is charged in an autoclave. The
autoclave is
filled with 30 bar of hydrogen and maintained at 40 C under stirring for a
period of 6 hours,
then it is cooled to 20 C and vented. After removing the catalyst by
filtration, the solvent is
removed by vacuum concentration at 110 C and 35mbar. The resulting product is
obtained
as a pale yellow liquid.
1 H NMR (300 MHz, CDCI3)/ppm: 3.69 (t, 2H) ; 1.45 (m, 8 H) ; 1.15 (s, 12H) ;
0.92 (t, 3H).
CA 02643170 2008-10-23
-74-
Example 15: Compound of the formula
O-N 0---(CH2)34 O N-O~
207 g of 4-hydroxy-2,2,6,6-tetramethylpiperidine, 100 g of DBE-26 (a mixture
of 75% of
glutaric acid dimethyl ester and 25% of adipic acid dimethyl ester, from
DuPont-USA) are
dissolved in 500 ml of toluene, added with 2 g of lithium amide and heated and
maintained to
reflux for 6 hours, while the methanol formed during the reaction is distilled
off by
azeotropation. The mixture is then cooled to 20 C, washed with water, dried
over sodium
sulfate. The resulting solution is then reacted with ally) bromide, sodium
carbonate, peracetic
acid, hydrogen and 5% by weight platinum on carbon, following the same
procedure and the
same stoichiometric ratios described for the preparation of the compound in
example 5. The
product is obtained as a pale yellow oil.
1 H NMR (300 MHz, CDCI3)/ppm: 4.95 (m, 2H) ; 3.63 (t, 4H) ; 2.24 (m, 2H); 1.86
- 1.71 (m,
4H-6H); 1.57-1.44 (m,8H); 1.15 (m, 28H) ; 0.89 (m, 6H).
Example 16: Compound of the formula
\~~/ N~ N -O~
N ,
O- N N N N N -O~
Step 1: To a solution of 64 g of 2,4,6-tris-(N-n-butyl-N-(2,2,6,6-
tetramethylpiperidin-4-yl))-
1,3,5-triazine in 300 ml of toluene are added 48 g of ally) bromide and 55.8 g
of potassium
carbonate. The mixture is heated to 150 C, left to react for 5 hours, cooled
down to 60 C and
300 ml of water are added while stirring. The organic layer is then collected,
concentrated by
removing 100 ml of solvent at 110 C at 20 mbar, added with 400 ml of fresh
toluene, cooled
to -5 C and 57 g of a solution of 39% by weight of peracetic acid in acetic
acid is added
CA 02643170 2008-10-23
-75-
during 30 minutes under stirring. The temperature is raised to 0 C and the
reaction mixture is
left to react for 2 hours.
A solution of 80 g of sodium carbonate in 500 ml of water is added and kept
for 30 minutes at
0 C with stirring. The organic layer is collected and dried over sodium
sulfate.
Step 2: The solution is charged in a 1 I stainless steel autoclave. After
addition of 2 g
platinum supported on carbon at 5% by weight, the autoclave is filled with
hydrogen of 40 bar
and maintained at 70 C with stirring for a period of 6 hours, then cooled to
20 C and vented.
After removing the catalyst by filtration, the solution is concentrated at 140
C and 1 mbar.
The product is obtained as a white solid, m.p. 92 - 100 C.
Example 17: Compound of the formula
/~Br
N -o `-Br
N ~
Br I
i
~O- N N N N N-o~Br
Br Br
The solution obtained in step 1 of example 14 is added with 43 g of bromine in
30 minutes
while stirring at 25 C in the dark. The mixture is left to react for 6 hours
at 25 C, washed with
a solution of 54 g of potassium carbonate in 500 ml of water, collected, dried
with sodium
sulfate and concentrated at 100 C under reduced pressure (10 mbar). The
obtained pale
pink solid has a melting point 106-110 C.
CA 02643170 2008-10-23
-76-
Example 18: Compound of the formula
Cathy H9C4
I -F'N N N
N (C-6-N N-(Cs-N -jr- N
N. N N~ N jn' N N
C A CHs N c CHs N3C A CNa F6C CHs NsCa
CA HsC N CH. C N CI, KsC i CHs V N CN3
O O O O
N -C4H9 H9C4-N H9C4-N
& Br Br I
I alls
& FisC BI Br CA
NC N CHs
I
O
Br
Br
To the solution obtained in step 1 of example 2, 88 g of bromine is added
during 30 minutes
while stirring at 25 C in the dark. The mixture is left to react for 6 hours
at 25 C, washed with
a solution of 108 g of potassium carbonate in 1000 ml of water, collected,
dried with sodium
sulfate and concentrated at 120 C under reduced pressure (10 mbar). The
obtained pale
yellow solid has a melting point higher than 250 C (decomposition).
Bromine content : 32.1 % by weight; Mn (by GPC) : 2862.
Application Examples
Example 20: LIGHT STABILIZING ACTION IN PP TAPES
1 g of each compound of the list reported below and, 1 g of tris(2,4-di-ter-
butylphenyl)
phosphite, 0.5 g of pentaerythritol tetrakis (3-(3,5-di-tert-butyl-
4hydroxyphenyl)propionate) , 1
g of calcium stearate are mixed in a turbomixer with 1000 g of polypropylene
powder having
a melt index of 2.1 g/10 minutes (measured at 230 C and 2.16 Kg) and already
containing 1
g of tris(2,4-di-ter-butylphenyl phosphite) and 10.5 g of pentaerythritol
tetrakis (3-(3,5-di-tert-
butyl-4- hydroxyphenyl)propionate).
The mixture is extruded at 200-220 C to give polymer granules which are
subsequently
converted to stretched tapes of 50 microns thickness and 2.5 mm width, using a
semi
CA 02643170 2008-10-23
29276-763
-77-
industrial type of apparatus (Leonard-Sumirago(VA)-Italy) and working under
the following
conditions :
Extruder temperature : 210 - 230 C
Head temperature : 240 - 260 C
Stretch ratio : 1 : 6
The tapes thus prepared are mounted on a white card and exposed in Weather-O-
Meter 65
WR (ASTM G26-96D 2565-85) with a black panel temperature of 63 C.
The residual tensile strength is measured, by means of a constant velocity
tensometer, on a
sample taken after various light exposure times; from this, the exposure time
(in hours)
required to halve the initial tensile strength (T50) is calculated.
For the purpose of comparison, tapes prepared under the same conditions as
indicated
ahnvo, hr it without the addition of the stabilisers of the present invention,
are exposed.
The results obtained are shown in the table below.
Compound of the Invention T50 (hours)
none 340
compound of example 5 3040
Example 21: LIGHT STABILIZING ACTION IN LDPE FILMS
Each compound of the list reported below is mixed via master batch with LDPE
pellets
(Riblene FF 29, supplied by Enichem, Milano, Italy), characterised by a
density of 0.921
g/cm3 and a melt flow index (190 C/2.16Kg) of 0.60 g/10 minutes, in a slow
mixer.
The master batch had previously been prepared by extruding powdered LDPE and
10 % by
weight of the compounds of the list reported below.
The mixture is blow extruded at 210 C and films of 150 microns thickness are
obtained.
Films are mounted on a white cardboard in metal frames and exposed in Atlas Ci
65 Xenon
.Arc Weather-O-meter, at 63 C black panel temperature, continuos dry cycle,
according to
ASTM G 26-96.
CA 02643170 2008-10-23
-78-
During the exposure, the performance is periodically evaluated measuring the
carbonyl
increment (iCO; increase of carbonyl concentration) by means of a FT-IR
spectrophotometer
and testing the samples for embrittlement. For some samples, the residual
tensile strength is
measured by means of a constant velocity tensometer, on a sample taken after
various light
exposure times; from this, the exposure time (in hours) required to halve the
initial tensile
strength (T50) is calculated.
Results are compiled in the following table; a low increase of carbonyl
concentration and a
high T50 time indicate good stabilisation.
Table: Increase of carbonyl concentration (iCO) after 4760 hours exposure
and.T50
Compound T50/hours iCO
0.2 % of example 2 > 7050 0.08
without stabiliser 660 embrittled after 1560 h
Example 22: LIGHT STABILIZING ACTION IN LDPE FILMS TREATED WITH BORDEAUX
MIXTURE
Each compound of the list reported below is mixed via master batch with LDPE
pellets
(Riblene FF 29, supplied by Enichem, Milano, Italy), characterised by a
density of 0.921
g/cm3 and a melt flow index (190 C/2.16Kg) of 0.60 g/10 minutes, in a slow
mixer.
The master batch had previously been prepared by extruding powdered LDPE and
10 % by
weight of the compound of the list reported below.
The mixture is blow extruded at 210 C and films of 150 microns thickness are
obtained .
Films for pesticide treatment are kept 24 hours in a suspension of Bordeaux
mixture (widely
used pesticide based on copper sufate) and water (10 g of mixture in 1 litre
of water).
Treated films are put into quartz tubes and are exposed in Atlas Ci 65 Xenon
Arc Weather-0-
meter, at 63 C black panel temperature, continuos dry cycle, according to ASTM
G 26-96.
CA 02643170 2008-10-23
29276-763
-79-
During the exposure, the performance is periodically evaluated measuring the
carbonyl
increment (increase of carbonyl concentration; iCO) by means of a FT-IR
spectrophotometer.
Results are compiled in the following table.
Table: Increase of carbonyl concentration (iCO) after indicated hours of
weathering
Compound iCO after 0 h
0.15 % of example 2 0
without stabiliser 0
Example 23: LIGHT STABILIZING ACTION IN LDPE FILMS TREATED WITH VAPAM
Each compound of the list reported below Is mixed via master batch with LDPE
pellets
(Riblene FF 29, supplied by Enichem, Milano, Italy), characterised by a
density of 0.921
g/cm3 and a melt flow index (190 C/2.16Kg) of 0.60 g/10 minutes, in a slow
mixer.
The master batch had previously been prepared by extruding powdered LDPE and
10 % by
weight of the compound of the list reported below.
The mixture is blow extruded at 210 C and films of 150 microns thickness are
obtained.
Films for pesticide treatment are kept inside a dryer for 20 days at 30 C, in
presence of the
TM
vapours emitted by 2 it of an aqueous solution containing 50% of VAPAM
(Baslini S.p.A.,
Trevigiio/BG, Italy), which, in turn, in an aqueous solution of 382 g per
litre of metam-sodium,
having the formula CH3-NH-CS-SNa.
Treated films are put into quartz tubes and are exposed in Atlas Ci 65 Xenon
Arc Weather-0-
meter, at 63 C black panel temperature, continuos dry cycle, according to ASTM
G 26-96.
During the exposure, the performance is periodically evaluated measuring the
carbonyl
increment by means of a FT-IR spectrophotometer and testing the samples for
embrittlement. Results are compiled in the following table; a low increase of
carbonyl
concentration indicates good stabilisation.
CA 02643170 2012-03-23
29276-763D
-80-
Table: Increase of carbonyl concentration (iCO) after 1000 hours exposure
Compound Concentration iCO
example 2 0.2 % 0.38
without stabiliser 0 embritteled
Example 24: LIGHT STABILIZING ACTION IN GREENHOUSE FILMS
Each compound of the list reported below is mixed via master batch with LDPE
pellets
(Riblene FF 29, supplied by Enichem, Milano, Italy), characterised by a
density of 0.921
g/cm3 and a melt flow index (190 C/2.16Kg) of 0.60 g/10 minutes, in a slow
mixer.
The master batch had previously been prepared by extruding powdered LDPE and
10 % by
weight of the sterically hindered hydroxylamine ether of present Invention
(compound A) and
the relevant concentrations of component B (= oxo and or Itydioxyl group
containing metal
costabiliser) and C (further costabiliser; salt of carboxylic acid).
The mixture is blow extruded at 210 C and films of 150 microns thickness are
obtained .
The films are exposed on the south face roof of a greenhouse in Pontecchio
Marconi
(Bologna-Italy). The following pesticide are applied in the greenhouse :
TA1
VAPAM (Baslini S.p.A., Treviglio/BG, Italy), which, in turn, in an aqueous
solution of 382g per
litre of metam-sodium, having the formula CH3-NH-CS-SNa;
TM
SESMETRIN,(Bimex SpA, IsolaNi, Italy), which is a 23.75 % (%w/w) aqueous
solution of
permethrin having the formula
H3C CH3
CI X C
C-C CC-C
CI 2
The greenhouse is treated with a solution of 4 litres of VAPAM in 10 litres of
water every 6
months, and with SESMETRIN (5 g in 5 litres of water) every month.
CA 02643170 2008-10-23
-81-
During the exposure, the performance is periodically evaluated measuring the
carbonyl
increment by means of a FT-IR spectrophotometer. The exposure is measured in
kilolangley
(Klys; energy per unit area); 1100 Klys corresponds to 1 year of exposure.
Compound A Component B Ca-Stearate iCO after
0,4% of example 2 0.2 % ZnO 0.2
0,4% of example 2 0 0
none 0 0
Example 25: Wood Coating
a) Impregnation:
The substrate (pine) is impregnated using a a commercially available
impregnation
("Xylamon Incolore" solids content of 5,2% from Sepam).
The impregnation is applied by brush (1 application) and dried for 24 hours at
room
temperature.
b) Too Coat:
A top coat is prepared from:
73.8 parts of an Alkyd Resin (Jagol PS 21 , E. Jager KG),
0.52 parts of antiskinning agent (Exkin 2 , Servo Delden B. V.)
20.8 parts of aliphatic hydrocarbon solvent (Exxsol D 40 , Deutsche Exxon
Chemical
GmbH)
4.16 parts of a metal drier (Jager Antihydro - Trockner , E. Jager KG)
0,70 parts of a PE - wax, 21 % in solvent (Lanco Glidd AH , G. M. Langer &
Co)
The top coat is stabilized with 2% UVA (compound of the formula
CH3
HO 1-13C CH3
N
IN C-C O-CBH,7
H2 H2
UV-Absorber from Ciba Specialty Chemicals) and 1 % stabilizer according to the
invention as
indicated in the following table.
All concentrations are by weight based on binder solids.
CA 02643170 2008-10-23
-82-
The topcoat is applied by brush (2 applications) on the impregnated pine
panels and dried for
24 hours at room temperature after each application.
The panels are exposed to accelerated weathering:(QUV, 8h light at 70 C, 4h
condensation
at 50 C, UV - A lamps).
Gloss (600) is measured according to DIN 67530 every 400h weathering. An
unexposed pine
panel with unstabilized top coat is used as reference.
The results are presented in the following table.
Table: Gloss (600) after 2400h exposure
Unstabilized Topcoat 25
2%UVA 63
2% UVA + 1 % c pd. of example 5 86
Initial gloss (60 ) for all samples: 92 - 93
The results show a good gloss retainment achieved with the stabilizer of
present invention.
Example 26: Stabilization of a 2-coat metallic finish
The light-stabilizers to be tested are dissolved in 30 g of Solvesso 100 and
tested in a
clearcoat having the following composition (parts by weight):
Synthacryl SC 303 1) 27.51
Synthacryl SC 370 2) 23.34
Maprenal 650 3) 27.29
Butyl acetate/Butanol (37/8) 4.33
Isobutanol 4.87
Solvesso 150 4) 2.72
Crystal Oil K-30 5) 8.74
Levelling assistant Baysilon MA 6) 1.20
100.00
'Acrylate resin, Hoechst AG; 65 % solution in xylene/butanol (26:9)
2 Acrylate resin, Hoechst AG; 75 % solution in Solvesso 1004
3 Melamine resin, Hoechst AG; 55 % solution in isobutanol
4 aromatic hydrocarbon mixture, boiling range: 182-203 C (Solvesso 150) or
CA 02643170 2008-10-23
-83-
161 - 178 C (Solvesso 100); manufacturer: Esso
aliphatic hydrocarbon mixture, boiling range: 145 - 200 C; manufacturer:
Shell
61 % in Solvesso 150; manufacturer: Bayer AG
1 % of the stabilizer indicated in the following table and 1.5 % of the UVA of
example 25 are
added to the clearcoat, based on the solids content of the varnish. For
comparison, a
clearcoat containing no light-stabilizers is used.
The clearcoat is diluted with Solvesso 100 to spray viscosity and is applied
by spraying to a
prepared aluminium panel ( Uniprime Epoxy, silver-metallic basecoat) which is
baked at
130 C, for 30 minutes, to give a dry film thickness of 40 - 50 gm of
clearcoat.
The samples are then weathered in an Atlas Xe-Wom weatherometer (CAM 180) in a
cycle
as follows: 40' UV-Tight, 20' light with rain (front), 60' light, 60' dark
with rain (both sides), light
at 70 C, dark at 40 C (Filter: quartz / boro; 0.55 W/cm2 at 340 nm).
The surface gloss (20 gloss as defined in DIN 67530) of the samples is then
measured at
regular intervals; high values indicate a good stabilization. The results are
shown in the
following table.
Table:
Light-stabilizer 20 gloss (DIN 67530) after ... hours weathering
0 hours 800 hours 3200 hours
None 94 33 crack after 800h
1 % cpd. of example 5 + 92 92 72
1.5% UVA