Note: Descriptions are shown in the official language in which they were submitted.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-1-
PROCESS FOR SUPERHEATED STEAM
BACKGROUND OF THE INVENTION
[0001] Many industrially significant chemical reactions are highly exothermic
and the heat of reaction is used to generate steam. Examples of steam
generating chemical processes include ethylene oxide production by partial
oxidation of ethylene, methanol production from synthesis gas, gasification or
partial oxidation of carbonaceous materials, formaldehyde production from
methanol, production of Fischer-Tropsch hydrocarbons or alcohois from
synthesis gas, ammonia production from hydrogen and nitrogen, and the water-
gas shift reaction to produce hydrogen from carbon monoxide and water. In
such processes, the saturated steam, that is steam at its dew point for the
prevailing pressure and temperature conditions, typically is generated by
cooling
of reactors or as a post-reaction heat removal technique. The amount of steam
generated, however, often can exceed the heating needs within the battery
limits
of the process itself.
[0002] In addition to its use as a heating medium, steam thus generated can
be used as a source of work to generate electricity in a turbogenerator or as
motive force to drive machinery such as a turbine-driven compressor or pump.
During the expansion process in turbomachinery, a portion of the enthalpy of
the inlet high pressure steam is converted to motive work, and is converted to
a
lower pressure, cooler steam that exits the turbine. Such processes are
described for example in "Steam, Its Generation and Use", Babcock and Wilcox
Co, New York, 37th Edition, 1960, Chapter 10, pp. 10-1 to 10-22.
[0003] Although either saturated or superheated steam can be used in
turbomachinery, it is well-known in the art that the thermodynamic efficiency
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-2-
(useful work energy out divided by enthalpy input) is proportional to the
amount
of superheat. An example of this phenomenon is shown in Figure 10, pg.10-8,
of the above reference, in which the thermodynamic efficiency is 39.7% for the
expansion of 100 bara saturated steam across a steam turbine to 0.485 bara. By
contrast, a 42.6% efficiency is achieved for the same pressure differential
with
the steam superheated by 167 C prior to introduction to the turbine.
[0004] Often during the expansion process, a fraction of the vaporous steam
feed condenses and forms liquid water. Generation of liquid water within the
turbine results in formation of water droplets, these droplets strike the
turbine
blades with great force and cause erosive wear over time. The amount of liquid
water generated in the turbine is a complex function of the degree of
superheat
of the inlet steam, the pressure differential across the turbine, and the
mechanical efficiency of the turbine. For example, if 100 bara saturated steam
is
expanded across a steam turbine to 0.485 bara at 85% efficiency, the quality
(i.e., the fraction of a wet steam that is in the vapor state) of the outlet
steam is
73.4%, whereas introduction into the same steam turbine of 100 bara saturated
steam superheated by 200 C results in an outlet quality of 87.5%.
Alternatively if
50 bara saturated steam is expanded to 0.485 bara, the outlet steam quality is
74.8%. If the degree of superheat is high enough no liquid water will form.
For a
100 bara to 0.485 bara expansion at 85% mechanical efficiency, the outlet
quality is 100% if the inlet steam is superheated by at least 495 C
[0005] It is well known that the use of saturated steam as the motive force in
turbomachinery causes increased erosive wear and resulting higher maintenance
costs as compared to the use of superheated steam. Typically, an outlet
quality
of at least 75% is preferred, an outlet quality of 85% or higher is more
preferable. With many steam generating chemical processes, however, no high
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-3-
temperature heat source of sufficient quantity is available to superheat the
steam thus generated. Although it is possible to burn a portion of either the
raw
material, product, by-product streams, or an externally supplied fuel to
provide
a high temperature heat source useful for superheating steam, this method is
hampered by the wasteful consumption of raw materials, insufficient
availability
of by-products, or requires the purchase of expensive fuels and additional
capital for the combustor and associated heat exchangers. Thus, there is a
need
to provide a means for superheating steam from steam generating chemical
processes without undue capital or fuel costs.
SUMMARY OF THE INVENTION
[0006] In one embodiment of the invention, I have discovered that high
pressure steam generated in a chemical process may be conveniently and
economically used to produce a superheated steam by reducing the pressure of
a portion of the high pressure steam to produce a lower pressure steam and
using the remaining portion of the high pressure steam to superheat the lower
pressure steam. Accordingly, a process for the preparation of superheated
steam is set forth comprising:
(a) recovering heat from at least one chemical process to produce a high
pressure steam;
(b) reducing the pressure of a portion of the high pressure steam of step (a)
to produce a lower pressure steam and a remaining portion of the high
pressure steam; and
(c) transferring heat from at least a fraction of the remaining portion of the
higher pressure steam of step (b) to the lower pressure steam to produce
a superheated steam from the lower pressure steam.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-4-
The process of the invention may be used in conjunction with a variety of
chemical processes. For example, the high pressure steam may be generated
from at least one chemical process selected from partial oxidation,
carbonylation, hydrogenation, and homologation. Representative examples of
chemical processes include, but are not limited to, gasification of
carbonaceous
materials to produce synthesis gas, hydrogenation of carbon monoxide or
carbon dioxide to produce methanol, partial oxidation of ethylene to produce
ethylene oxide, steam reforming of methane to produce synthesis gas, partial
oxidation of methanol to produce formaldehyde, production of Fischer-Tropsch
hydrocarbons or alcohols from synthesis gas, ammonia production from
hydrogen and nitrogen, autothermal reforming of carbonaceous feedstocks to
produce synthesis gas, hydrogenation of dimethyl terephthalate to
cyclohexanedimethanol, carbonylation of methanol to acetic acid, the water-gas
shift reaction to produce hydrogen and carbon dioxide from carbon monoxide
and water, or a combination thereof. The superheated, lower pressure steam can
be used to generate electricity in a steam turbine, operate a steam turbine
drive,
or as a heat source.
[0007] The process of the invention may be used advantageously with
processes that produce syngas by the partial oxidation of carbonaceous
materials. Such processes, either alone or in combination with other chemical
processes, frequently produce abundant or excessive amounts of high pressure
steam but are deficient in superheated steam. Hence, another aspect of the
invention is a process for the preparation of superheated steam, comprising:
(a) reacting a carbonaceous material with oxygen, water, or carbon dioxide
to produce heat and a syngas stream comprising hydrogen, carbon
monoxide, and carbon dioxide;
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-5-
(b) recovering the heat to produce a high pressure steam; and
(c) transferring heat from at least a fraction of the high pressure steam of
step (b) to a lower pressure steam by indirect heat exchange to produce a
superheated steam from the lower pressure steam.
The carbonaceous material may include, but is not limited to, methane,
petroleum residuum, carbon monoxide, coal, coke, lignite, oil shale, oil
sands,
peat, biomass, petroleum refining residues, petroleum cokes, asphalts, vacuum
resid, heavy oils, or combinations thereof, and can be reacted with oxygen in
a
gasifier, partial oxidizer, or reformer. The lower pressure steam may be
obtained by reducing the pressure of a portion of the high pressure steam or
by
recovery of heat from at least one chemical process such as, for example a
water-gas shift reaction, hydrogenation of carbon monoxide to produce
methanol, hydrogenation of nitrogen to produce ammonia, carbonylation of
methanol to produce acetic acid, Fischer-Tropsch processes, production of
alkyl
formates from carbon monoxide and alcohols, and combinations thereof.
[0008] In yet another aspect of the invention, the high pressure steam can
be produced by recovering heat from a gasifier and can be used to generate a
superheated steam which, in turn, can be used to drive a steam turbine. Thus,
the invention also provides a process-for driving a steam turbine, comprising:
(a) reacting a carbonaceous material with oxygen in a gasifier to produce
heat and a syngas stream comprising hydrogen, carbon monoxide, and
carbon dioxide;
(b) recovering the heat to produce a high pressure steam;
(c) transferring heat from at least a fraction of the high pressure steam of
step (b) to a lower pressure steam by indirect heat exchange to produce a
superheated steam from the lower pressure steam; and
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-6-
(d) passing the superheated steam to a steam turbine.
The steam turbine can be used to drive a generator to produce electricity or
drive a gas compressor. For example, the gasifier and turbine may be part of
an
integrated gasification combined cycle (abbreviated herein as "IGCC") power
plant, which may further comprise a chemical producing plant to convert excess
syngas into fuel or salable chemicals.
BRIEF DESCRIPTION OF DRAWINGS
[0009] FIGURES 1-5 are schematic flow diagrams that illustrate several
embodiments of the process of the invention.
DETAILED DESCRIPTION
[0010] in a general embodiment, the present invention provides a novel
process for superheating steam in which high pressure steam generated in a
chemical process can be used advantageously to produce a superheated steam
without the use of an external heat source. It has been discovered that a
portion
of the high pressure steam may be reduced in pressure to produce a lower
pressure steam and that the remaining portion of the high pressure steam can
be used to superheat the lower pressure steam to produce a superheated steam.
Accordingly, a process for the preparation of superheated steam is set forth
comprising:
(a) recovering heat from at least one chemical process to produce a high
pressure steam;
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-7-
(b) reducing the pressure of a portion of the high pressure steam of step (a)
to produce a lower pressure steam and a remaining portion of the high
pressure steam; and
(c) transferring heat from at least a fraction of the remaining portion of the
higher pressure steam of step (b) to the lower pressure steam to produce
a superheated steam from the lower pressure steam.
The high pressure steam can. be generated from a variety of chemical processes
such as, for example, partial oxidation, carbonylation, hydrogenation, and
homologation. Some representative examples of chemical processes include, but
are not limited to, gasification of carbonaceous materials to produce
synthesis
gas, hydrogenation of carbon monoxide or carbon dioxide to produce methanol,
partial oxidation of ethylene to produce ethylene oxide, steam reforming of
methane to produce synthesis gas, partial oxidation of methanol to produce
formaldehyde, production of Fischer-Tropsch hydrocarbons or alcohols from
synthesis gas, ammonia production from hydrogen and nitrogen, autothermal
reforming of carbonaceous feedstocks to produce synthesis gas, hydrogenation
of dimethyl terephthalate to cyclohexanedimethanol, carbonylation of methanol
to acetic acid, water-gas shift reaction to produce hydrogen and carbon
dioxide
from carbon monoxide and water, or a combination thereof. The superheated,
lower pressure steam may used to generate electricity with a steam turbine,
operate a steam turbine drive, or as a heat source.
[0011] Unless otherwise indicated, all numbers expressing quantities of
ingredients, properties such as molecular weight, reaction conditions, and so
forth used in the specification and claims are to be understood as being
modified in all instances by the term "about." Accordingly, unless indicated
to
the contrary, the numerical parameters set forth in the following
specification
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-8-
and attached claims are approximations that may vary depending upon the
desired properties sought to be obtained by the present invention. At the very
least, each numerical parameter,should at least be construed in light of the
number of reported significant digits and by applying ordinary rounding
techniques. Further, the ranges stated in this disclosure and the claims are
intended to include the entire range specifically and not just the
endpoint(s). For
example, a range stated to be 0 to 10 is intended to disclose all whole
numbers
between 0 and 10 such as, for example 1, 2, 3, 4, etc., all fractional numbers
between 0 and 10, for example 1.5, 2.3, 4.57, 6.113, etc., and the endpoints 0
and 10. Also, a range associated with chemical substituent groups such as, for
example, "Cl to Cs hydrocarbons", is intended to specifically include and
disclose C, and Cs hydrocarbons as well as C2, C3, and C4 hydrocarbons.
[0012] Notwithstanding that the numerical ranges and parameters setting
forth the broad scope of the invention are approximations, the numerical
values
set forth in the specific examples are reported as precisely as possible. Any
numerical value, however, inherently contains certain errors necessarily
resulting
from the standard deviation found in their respective testing measurements
and/or calculations.
[0013] As used in the specification and the appended claims, the singular
forms "a," "an" and "the" include their plural referents unless the context
clearly
dictates otherwise. For example, references to a "heat exchanger," or a "steam
flow," is intended to include the one or more heat exchangers, or steam flows.
References to a composition or process containing or including "an" ingredient
or "a" step is intended to include other ingredients or other steps,
respectively,
in addition to the one named.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-9-
[0014] The term "containing" or "including", as used herein, is intended to be
synonymous with the term "comprising"; that is at least the named compound,
element, particle, or process step, etc., is present in the composition or
article
or process, but does not exclude the presence of other compounds, catalysts,
materiats, particles, process steps, etc, even if the other such compounds,
material, particles, process steps, etc., have the same function as what is
named,
unless expressly excluded in the claims.
[0015] It is also to be understood that the mention of one or more process
steps does not preclude the presence of additional -process steps before or
after
the combined recited steps or intervening process steps between those steps
expressly identified. Moreover, the lettering of process steps or ingredients
is a
convenient means for identifying discrete activities or ingredients and the
recited lettering can be arranged in any sequence, unless otherwise indicated.
[0016] The process of the invention comprises recovering heat from a least
one chemical process to produce a high pressure steam. The high pressure
steam used in the instant invention may be saturated or superheated. The
recovery of heat may be from any chemical process which produces sufficient
heat to produce steam having a pressure of about 4 bara. The term bara, as
used herein means "bar absolute". Steam at about 4 bara or higher may be
dropped in pressure and superheated to useful levels by means laid out in this
invention. In such processes, typicatly saturated steam, i.e., steam at its
dew
point for the prevailing pressure and temperature conditions, is generated by
cooling of reactors or as a post-reaction heat removal technique.
Alternatively,
the lower pressure steam derived from the steam generating chemical process
may be superheated, but with a degree of superheat lower than desired. In this
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-10-
latter case, the lower pressure steam may be subjected to the steps of the
instant invention to further increase its degree of superheat.
[0017] Representative examples of such heat producing chemical processes
include, but are not limited to, partial oxidation, carbonylation,
hydrogenation,
water-gas shift reaction, steam reforming, and homologation. More specific,
non-limiting examples of chemical processes which may be used in the process
of the invention include gasification of carbonaceous materials to produce
synthesis gas, hydrogenation of carbon monoxide or carbon dioxide to produce
methanol, partial oxidation of ethylene to produce ethylene oxide, steam
reforming of methane to produce synthesis gas, partial oxidation of methanol
to
produce formaldehyde, production of Fischer-Tropsch hydrocarbons or alcohols
from synthesis gas, ammonia production from hydrogen and nitrogen,
autothermal reforming of carbonaceous feedstocks to produce synthesis gas,
hydrogenation of dimethyl terephthalate to cyclohexanedimethanol,
carbonylation of methanol to acetic acid,.water-gas shift reaction to produce
hydrogen and carbon dioxide from carbon monoxide and water, or a
combination thereof.
[0018] The chemical process can, for example, include the partial oxidation
of a carbonaceous material by reaction with oxygen, water, or carbon dioxide
to
produce heat and syngas stream comprising hydrogen, carbon monoxide, and
carbon dioxide. The term "carbonaceous", as used herein, means the various,
suitable feedstocks that contain carbon and is intended to include gaseous,
liquid, and solid hydrocarbons, hydrocarbonaceous materials, and mixtures
thereof. Substantially any combustible carbon-containing organic material, or
slurries thereof, may be included within the definition of the term
"carbonaceous". Solid, gaseous, and liquid feeds may be mixed and used
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-11-
simultaneously; and these may include paraffinic, olefinic, acetylenic,
naphthenic, and aromatic compounds in any proportion. Also included within
the definition of the term "carbonaceous" are oxygenated carbonaceous organic
materials including carbohydrates, cellulosic materials, aldehydes, organic
acids,
alcohols, ketones, carbon monoxide, oxygenated fuel oil, waste liquids and by-
products from chemical processes containing oxygenated carbonaceous organic.
materials, and mixtures thereof. The term "syngas", as used herein, is
intended
to be synonymous with the term "synthesis gas" and understood to mean a
gaseous mixture of varying composition comprising primarily hydrogen, carbon
monoxide, and various impurities depending on its method of generation. The
partial oxidation process, for example, may comprise steam or carbon dioxide
reforming of carbonaceous materials such as, for example, natural gas or
petroleum derivatives. These processes are well known to persons skilled in
the
art and are practiced commercially. In another example, the partial oxidation
process may comprise gasification of carbonaceous materials such as, for
example, methane, coal, coke, lignite, oil shale, oil sands, peat, biomass,
petroleum refining residues, petroleum cokes, asphalts, vacuum-resid, heavy
oils, or combinations thereof, by reaction with oxygen to produce syngas and
heat. The term "oxygen", as used herein, is intented to include substantially
pure
gaseous, elemental oxygen, or any reactive 02-containing gas, such as air,
substantially pure oxygen having greater than about 90 mole percent oxygen, or
oxygen-enriched air having greater than about 21 mole percent oxygen.
Substantially pure oxygen is preferred in the industry. To obtain
substantially
pure oxygen, air is compressed and then separated into substantially pure
oxygen and substantially pure nitrogen in an air separation plant. Such plants
are known in the industry.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-12-
[0019] Any one of several known gasification processes can be incorporated
into the process of the instant invention. These gasification processes
generally
fall into broad categories as laid out in Chapter 5 of "Gasification", (C.
Higman
and M. van der Burgt, Elsevier, 2003). Examples are moving bed gasifiers such
as the Lurgi dry ash process, the British Gas/Lurgi slagging gasifier, the
Ruhr
100 gasifier; fluid-bed gasifiers such as the Winkler and high temperature
Winkler processes, the Kellogg Brown and Root (KBR) transport gasifier, the
Lurgi
circulating fluid bed gasifier, the U-Gas agglomerating fluid bed process, and
the Kellogg Rust Westinghouse agglomeratirig fluid bed process; and-entrained-
flow gasifiers such as the Texaco, Shell, Prenflo, Noell, E-Gas (or Destec),
CCP,
Eagle, and Koppers-Totzek processes. The gasifiers contemplated for use in the
process may be operated over a range of pressures and temperatures between
about 1 to about 103 bar absolute and 400 C to 2000 C, with preferred values
within the range of about 21 to about 83 bara and temperatures between 500 C
to 1500 C. Depending on the carbonaceous or hydrocarbonaceous feedstock
used therein and type of gasifier utilized to generate the gaseous carbon
monoxide, carbon dioxide, and hydrogen, preparation of the feedstock may
comprise grinding, and one or more unit operations of drying, slurrying the
ground feedstock in a suitable fluid (e.g., water, organic liquids,
supercritical or
liquid carbon dioxide). Typical carbonaceous materials which can be oxidized
to
produce syngas include, but are not limited to, petroleum residuum,
bituminous, subbituminous, and anthracitic coals and cokes, lignite, oil
shale,
oil sands, peat, biomass, petroleum refining residues, petroleum cokes, and
the
like.
[0020] The heat produced in the chemical process may be recovered by any
heat exchange means known in the art including, but not limited to, radiant
heat
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-13-
exchange, convective heat exchange, or a combination thereof to produce a high
pressure steam. For example, in gasification processes, the heat may be
recovered using at least one of the following types of heat exchangers
selected
from steam generating heat exchangers (i.e., boilers), wherein heat is
transferred from the syngas to boil water; shell and tube; plate and frame;
spiral; or combinations of one or more of these heat exchangers. For example,
the heat from the gasification process can be recovered by radiant heat
exchange. Convective heating or cooling occurs by transfer of heat from one
point within a fluid (gas or liquid) by mixing of one portion of the fluid
with
another portion. A typical indirect heat exchange process will involve
transfer of
heat to or from a solid surface (often a tube wall) to a fluid element
adjacent to
the wall, then by convection into the bulk fluid phase. Radiant heat transfer
involves the emission of electromagnetic energy from matter excited by
temperature and absorption of the emitted energy by other matter at a distance
from the source of emission. For example, the raw syngas leaves the gasifier
and
can be cooled in a radiant syngas cooler. The recovered heat is used to
generate
high pressure steam. Radiant syngas coolers are known in the art and may
comprise, for example, at least one ring of vertical water cooled tubes, such
as
shown and described in U.S. Patent No's. 4,310,333 and 4,377,1 32.
[00211 The use of multiple steam generating heat exchangers also is
contemplated to be within the scope of the instant invention. Steam and
condensate generated within gas cooling zones may embody one or more steam
products of different pressures. The gas cooling zones optionally may comprise
other absorption, adsorption, or condensation steps for removal of trace
impurities, e.g., such as ammonia, hydrogen chloride, hydrogen cyanide, and
trace metals such as mercury, arsenic, and the like.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-14-
[00221 The high pressure steam can be saturated or superheated and
typically will have a pressure of about 4 to about 140 bara. in another
example,
the high pressure steam can have a pressure of about 20 to about 120 bara. A
portion of the high pressure steam can be reduced in pressure to produce a
lower pressure steam and a remaining portion of the high pressure steam. If
the
high pressure steam is superheated, the lower pressure steam also may be
superheated but to an insufficient degee. The terms "high pressure steam" and
"lower pressure steam", as used herein, are intended to indicate the relative
and
not absolute pressures of the various steam flows of the present invention. As
used herein in the context of the claims and description, "high pressure
steam"
is intended to mean steam from which heat is transferred, wherein the term
"lower pressure steam" means steam to which heat is transferred.
Representative
examples of portions or fractions of the high pressure steam that can be let
down or expanded to produce the lower pressure steam are about 40 to about
95 mass%, about 50 to about 80 mass%, about 60 to about 95 mass%, about 70
to about 95 mass%, and about 75 to about 95 mass%, based on the total mass of
the high pressure steam. Any means known in art may be used to reduce the
pressure of the high pressure steam; however, it will be evident to persons
skilled in the art that generation of a lower pressure steam will involve
expanding a portion of the the high pressure steam. For example, the process
of
the invention may comprise expanding a portion of the high pressure steam
through a valve, a turbine, or a combination thereof. Typically, the ratio of
the
pressures of the high to lower pressure steams will be 140:1 to 1.5:1, 100:1
to
2:1, 25:1 to 2:1, or 10:1 to 2:1. In addition, the higher presssure steam and
the
lower pressure steam typically will have a difference in water saturation
temperature of about 40oC to about 250oC.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-15-
[0023] According to the invention, heat can be transferred from at least a
fraction of the remaining portion of the higher pressure steam to the lower
pressure steam to produce a superheated steam from the lower pressure steam
or increase the degree of superheat if the lower pressure steam is already
superheated. The term "superheated", as used herein, is understood to mean
that the lower pressure steam is heated above its dew point at a given
pressure
or, if it is already superheated, its degree of superheat increased. The
amount of
superheat typically is at least 40 C. Other examples of superheat are from
about
20 C to about 250 C, from about 50 C to, at least 1 50 C, and at least 50 C to
about 125 C. The heat exchange between the low and high pressure steam can
occur by indirect methods using any device known in the art, including shell
and
tube heat exchangers, plate and frame exchangers, spiral exchangers, and
compact plate-fin exchangers. "Indirect heat exchange", as used herein, is
understood to mean the exchange of heat across a surface without mixing as
opposed to "direct heat exchange" in which the high and lower pressure steam
are mixed together. Typically, the heat exchanger is of shell and tube design,
with the condensing high pressure steam on the shell side. The heat exchange
process may be implemented as multiple heat exchangers in series.
[0024] The approach temperature, i.e., the temperature difference between
the superheated lower pressure steam and the temperature of the high pressure
steam, is typically from about 1 to about 20 C. Other examples of approach
temperatures are from about 1 to about 10 C and from about 1 to about 5 C.
Although, desired to be as low as possible, the practical limit to the
approach
temperature is strongly dictated by economics. The area required for heat
transfer increases logarithmically with a decrease in approach temperature.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-16-
[0025] The lower pressure steam subjected to heat exchange against the
remaining portion of high pressure steam may have a quality less than or equal
to unity depending on the temperature and pressure conditions of the inlet
high
pressure steam as well as the outlet pressure. The term "quality", as used
herein
with respect to steam, means the mass fraction of vapor in the vapor phase
with
respect to the total mass of water and vapor in the steam. If desired, liquid
water
may be removed from the lower pressure steam by any means known in the art
such as described in "Phase Segregation", Chapter 3, pp.1 29-148, LJ. Jacobs
and W.R. Penney, in Handbook of Separation Process Technology, R.W.
Rousseau, ed., Wiley & Sons, 1987, including knockout pots, pipe separators,
mesh pads, centrifugal vanes, tangential entry separators, demister or
coalescer
pads, wavy plates, packing, cyclone or venturi scrubbers, electrostatic
precipitators, and the like. - -
[0026] The superheated, lower pressure steam generated in the instant
invention may used to generate electricity in a steam turbine, operate a steam
turbine drive, or as a heat source. Typically, the superheated, lower pressure
steam can be passed to a steam turbine where is used to supply motive force to
operate a compressor or a generator. When passed to a steam turbine, the
degree of superheating of the lower pressure steam produced in the process of
the invention generally will produce outlet quality of about 75% to about
100%.
Other examples of outlet quality for the steam exiting the steam turbine are
about 80% to about 100% -and about 85/ to about 100%.
[0027] The superheated steam process of the present invention, in particular,
may be used in conjunction with processes that produce syngas by the partial
oxidation of carbonaceous materials such as, for example, gasification or
steam
reforming of methane. Such processes, either alone or in combination with
other
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-17-
chemical processes frequently produce abundant or excessive amounts of high
pressure steam but are deficient in superheated steam. Therefore, another
aspect of the invention is a'process for the preparation of superheated steam,
comprising:
(a) reacting a carbonaceous material with oxygen, water, or carbon dioxide
to produce heat and a syngas stream comprising hydrogen, carbon
monoxide, and carbon dioxide;
(b) recovering the heat to produce a high pressure steam; and
(c) transferring heat from at least a fraction of the high pressure steam of
step (b) to a lower pressure steam by indirect heat exchange to produce a
superheated steam from the lower pressure steam.
The above process is understood to include the various embodiments of heat
recovery, heat exchange, steam pressure, steam turbines, steam pressure
reduction, steam quality, etc., as set forth hereinabove in any combination.
For
example, the carbonaceous material can be reacted with oxygen, water, or
carbon dioxide to produce heat and.syngas stream comprising hydrogen, carbon
monoxide, and carbon dioxide. As described above, carbonaceous materials can
include, but are not limited to, methane, petroleum residuum, coal, coke,
lignite,
carbon monoxide, oil shale, oil sands, peat, biomass, petroleum refining
residues, petroleum cokes, asphalts, vacuum resid, heavy.oils, or combinations
thereof. The carbonaceous material may be reacted in any type partial
oxidation
reactor known in the art such as, for example, a gasifier, partial oxidizer,
or
reformer. In one embodiment, for example, the carbonaceous material can
comprise methane and is reacted with water in a reformer. In another example,
the carbonaceous material may comprise coal or petroleum coke and is reacted
with oxygen in a gasifier. In yet another example, the carbonaceous material
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-18-
comprises carbon monoxide and is reacted with water in a water-gas shift
reaction.
[0028J The heat produced by the syngas process can be recovered by radiant
heat exchange, convective heat exchange, or a combination thereof to produce a
high pressure steam as described previously. The high pressure steam can be
saturated or superheated and, typically, will have a pressure of about 4 to
140
bara or, in another example, about 20 to 120 bara. A portion of the high
pressure steam can be reduced in pressure to produce a lower pressure steam
and a remaining portion of the high pressure steam. Any means known in art
may be used to reduce the pressure of the high pressure steam such as, for
example, expanding a portion of the high pressure steam through a valve, a
turbine, or combination thereof.
[0029] The lower pressure steam also may be produced by recovering heat
from one or more chemicaf processes in addition to and distinct from the
process used to generate the high pressure steam. Representative examples of
chemical processes which can be used include the water-gas shift reaction,
hydrogenation of carbon monoxide or carbon dioxide to produce methanol,
hydrogenation of nitrogen to produce ammonia, carbonytation of methanol to
produce acetic acid, Fischer-Tropsch processes, production of alkyl formates
from carbon monoxide and alcohols, and combinations thereof. Heat recovery
can be accomplish by heat exchange techniques well known in the art and
described hereinabove. In another embodiment, the chemical process may
include the water-gas shift reaction, hydrogenation of carbon monoxide or
carbon dioxide to produce methanol, hydrogenation of nitrogen to produce
ammonia, or a combination thereof.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-19-
[0030] Typically the water-gas shift reaction is accomplished in a catalyzed
fashion by methods known in the art. The water gas shift catalyst is
advantageously sulfur-tolerant. For example, such sulfur tolerant catalysts
can
include, but are not limited to, cobalt-molybdenum catalysts. Operating
temperatures are typically 250 C to 500 C. Alternatively, the water-gas shift
reaction may be accomplished, after sulfur removal from the carbon monoxide-
containing reactant gas, using high or low temperature shift catalysts. High
temperature shift catalysts, for example iron-oxide promoted with chromium or
copper, operate in the range of 300 C to 500 C. Low temperature shift
catalysts,
for example, copper-zinc-aluminum catalysts, operate in the range of 200 C to
300 C. Alternatively, the water-gas shift reaction may be accomplished
without.
the aid of a catalyst when the temperature of the gas is greater than about
900 C. Because of the highly exothermic nature of the water-gas shift
reaction,
steam may be generated by recovering heat from the exit gases of the water-gas
shift reactor. The water-gas shift reaction may be accomplished in any reactor
format known in the art for controlling the heat release of exothermic
reactions.
Examples of suitable reactor formats are single stage adiabatic fixed bed
reactors; multiple-stage adiabatic fixed bed reactors with interstage cooling,
steam generation, or cold-shotting; tubular fixed bed, reactors with steam
generation or cooling; or fluidized beds.
[0031] The process of hydrogenation of carbon monoxide or carbon dioxide
to produce methanol can comprise any type of methanol synthesis plant that is
well known to persons skilled in the art, many of which are widely practiced
on a
commercial basis. Most commercial methanol synthesis plants operate in the
gas phase at a pressure range of about 25 to about 140 bara using various
copper based catalyst systems depending on the technology used. A number of
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-20-
different state-of-the-art technologies are known for synthesizing methanol
such as, for example, the ICI (Imperial Chemical Industries) process, the
Lurgi
process, and the Mitsubishi process. Liquid phase processes are also well
known
in the art. Thus, the methanol process according to the present invention may
comprise a fixed bed methanol reactor, containing a solid or supported
catalyst,
or liquid slurry phase methanol reactor, which utilizes a slurried catalyst in
which metal or supported catalyst particles are slurried in an unreactive
liquid
medium such asjor example, mineral oil.
[0032] Typically, a syngas stream is supplied to a methanol reactor at the
pressure of about 25 to about 140 bara, depending upon the process employed.
The syngas then reacts over a catalyst to form methanol. The syngas stream may
or may not contain carbon dioxide in addition to hydrogeri and carbon
monoxide. The reaction is exothermic; therefore, heat removal is ordinarily
required. The raw or impure methanol is then condensed and may be purified to
remove impurities such as higher alcohols including ethanol, propanol, and the
like or, burned without purification as fuel. The uncondensed vapor phase
comprising unreacted syngas feedstock typically is recycled to the methanof=
processfeed.
[0033] The chemical process also can include the hydrogenation of nitrogen
to produce ammonia. This process can be carried by the Haber-Bosch process
by means known in the art as exemplified by LeBlance et al in "Ammonia", Kirk-
Othmer Encyclopedia of Chemical Technology, Volume 2, 3rd Edition, 1978, pp.
494-500.
[0034] In another embodiment of the invention, the chemical process can
comprise a Fischer-Tropsch prdcess for the production of hydrocarbons and
alcohols from syngas as exemplified in U.S. Patent No's. 5,621,155 and
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
- 21 -
6,682,711. Typically, the Fischer-Tropsch reaction may be effected in a fixed
bed, in a slurry bed, or in a fluidized bed reactor. The Fischer-Tropsch
reaction
conditions may include using a reaction temperature of between 190oC and
340oC, with the actual reaction temperature being largely determined by the
reactor configuration. For example, when a fluidized bed reactor is used, the
reaction temperature is preferably between 300oC and 340()C; when a fixed bed
reactor is .used, the reaction temperature is preferably between 200oC and
250oC; and when a slurry bed reactor is used, the reaction temperature is
preferably between 1 90oC and 270oC.
[0035] In one embodiment, the process of the invention'can be used in an
integrated combined cycle power plant in which coal or petroleum coke is
reacted with oxygen to produce syngas and that syngas is used to fuel a
combustion turbine for the generation of electricity and for the coproduction
of
chemicals such as, for example, methanol, Fischer-Tropsch hydrocarbons, or
ammonia. Recovery of heat from the gasification process can be used to
generate a high pressure steam which, in turn, can be used to superheat a
lower
pressure steam that is generated by heat recovery from the chemical process or
by reducing a portion of the pressure of the high pressure steam.
[0036] As described above, heat can be transferred from at least a fraction of
the higher pressure steam to the lower pressure steam to produce a
superheated, lower pressure steam. The superheated lower pressure steam may
used to generate electricity in a steam turbine, operate a steam turbine
drive, or
as a heat source. When passed to a steam turbine, the degree of superheating
of
the lower pressure steam produced in the process of the invention generally
will
produce outlet quality of about 75% to about 100%. Other examples of outlet
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-22-
quality for the steam exiting the steam turbine are about 80% to about 100%
and
about 85% to about 100%.
[0037] The present invention also provides a process for driving a steam
turbine using superheated, lower pressure steam produced by exchanging heat
between a high pressure steam and a lower pressure steam as described
hereinabove. Thus, another aspect of the invention is a process for driving a
steam turbine, comprising:
(a) reacting a carbonaceous material with oxygen in a gasifier-to produce
heat and a syngas stream comprising hydrogen, carbon monoxide, and
carbon dioxide;
(b) recovering the heat to produce a high pressure steam;
(c) transferring heat from at least a fraction of the high pressure steam of
step (b) to a lower pressure steam by indirect heat exchange to produce a
superheated steam from the lower pressure steam; and
(d) passing the superheated steam to a steam turbine.
The above process is understood to include the various embodiments of heat
recovery, heat exchange, steam pressure, steam turbines, steam pressure
reduction, steam quality, etc., as set forth hereinabove in any combination.
Our
process comprises reacting a carbonaceous material such as, for example,
petroleum residuum, coal, coke, lignite, oil shale, oil sands, peat, biomass,
petroleum refining residues, petroleum cokes, asphalts, vacuum resid, heavy
oils, or combinations thereof, in a gasifier to produce a syngas stream.
Typically, the carbonaceous material will comprise coal or petroleum coke and
is
reacted with oxygen or oxygen-containing gas in a gasifier.
[0038] The heat produced by the gasification process can be recovered by
radiant heat exchange, convective heat exchange, or a combination thereof to
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-23-
produce a high pressure steam as described previously. Typically, the heat
from
the gasification process is recovered by radiant heat exchange. The high
pressure steam can be saturated or superheated and, typically, will have a
pressure of about 4 to 140 bara or, in another example, about 20 to 120 bara.
[0039] The lower pressure steam may be produced, as described above, by
reducing the pressure of a portion of the high pressure steam or by recovering
heat from one or more chemical processes in addition to and distinct from the
process used to generate the high pressure steam. Representative examples of
chemical processes which can be used have been described previously and
include the water-gas shift reaction, hydrogenation of carbon monoxide or
carbon dioxide to produce methanol, hydrogenation of nitrogen to produce
ammonia, carbonylation of methanol to produce acetic acid, Fischer-Tropsch
processes, production of alkyl formates from carbon monoxide and alcohols,
and combinations thereof. In another embodiment, the chemical process
comprises a water-gas shift reaction, hydrogenation of carbon monoxide or
carbon dioxide to produce methanol, hydrogenation of nitrogen to produce
ammonia, or a combination thereof. In yet another embodiment, the chemical
process comprises hydrogenation of carbon monoxide or carbon dioxide to
produce methanol. In yet another embodiment, the chemical process may
include the water-gas shift reaction, hydrogenation of carbon monoxide or
carbon dioxide to produce methanol, hydrogenation of nitrogen to produce
ammonia, or a combination thereof. Heat recovery can be accomplish by heat
exchange techniques well known in the art and described hereinabove.
[0040] Heat can be transferred from at least a fraction of the higher pressure
steam of to the lower pressure steam to produce a superheated, lower pressure
steam as described previously. The superheated, lower pressure steam may be
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-24-
passed to steam turbine, which may be used to drive a generator to produce
electricity or to drive a gas compressor. The degree of superheating of the
lower
pressure steam produced in the process of the invention generally will produce
outlet quality of about 75% to about 1001. Other examples of outlet quality
for
the steam exiting the steam turbine are about 80% to about 100% and about 85%
to about 100%.
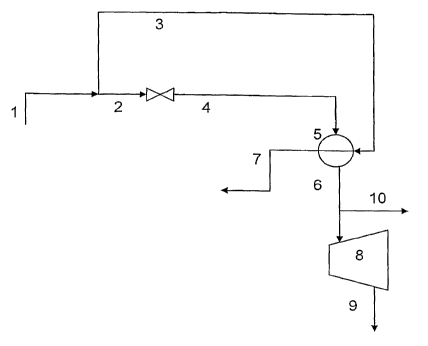
[0041] Several embodiments of the process of the invention are are illustrated
herein with particular reference to Figures 1-5. In the embodiment set forth
in
FIGURE 1, a portion of the high pressure steam flowing in conduit 1 is
directed
to conduit 2 and passed through a control valve to produce lower pressure
steam 4. Conduit 4 may comprise lower pressure steam with a quality less than
or equal to unity, depending on the temperature and pressure conditions of
steam 2. Steam 4 is passed through one side of a heat exchange device 5
wherein, steam 4 is superheated by indirect contact with the remaining portion
of the high pressure steam flowing via conduit 3 to the other side of heat
exchange device S. Superheated lovver pressure steam emerges from heat
exchange device 5 via conduit 6. Condensate and any remaining vapor fraction
of the high pressure steam exits via conduit 7. A fraction of superheated
steam
6 may be removed from the process via.conduit 10 and the remainder of steam
6 is passed on to a steam driven turbine 8 to provide motive force to produce
electrical or mechanical energy. The exhaust from turbine 8 exits via conduit
9.
[0042] In the embodiment set forth in FIGURE 2, a portion of the high
pressure steam flowing in conduit I is directed to conduit 2 and passed
through
a control valve to produce lower pressure steam 4. Conduit 4 may comprise
lower pressure steam with a quality less than or equal to unity, depending on
the temperarture and pressure conditions of steam 2.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-25-
[0043] Liquid water is separated from the lower pressure vaporous steam in
gas-liquid segregation zone 7. Lower pressure steam, essentially free from
liquid water, exits gas-liquid segregation zone 7 via conduit 5, while liquid
water is removed via conduit 6. Removal of liquid water may be accomplished by
any means known in the art, for example as described in "phase Segregation",
Chapter 3, pp.129-148, L J..Jacobs and W.R. Penney, in Handbook of Separation
Process Technology, R.W. Rousseau, ed., Wiley & Sons, 1987, including knockout
pots, pipe separators, mesh pads, centrifugal vanes, tangential entry
separators,
demister or coalescer pads, wavy plates, packing, cyclone or venturi
scrubbers,
electrostatic precipitators, and the like.
[0044] Steam 5 is passed through one side of a heat exchange device 8
wherein, steam 5 is superheated by indirect contact with the remaining portion
of the high pressure steam flowing via conduit 3 to the other side of heat
exchange device 8. Superheated, lower pressure steam emerges from heat
exchange device 8 via conduit 10. Condensate and any remaining vapor fraction
of the high pressure steam exits via conduit 9. A fraction of Superheated
steam
may be removed from the process via conduit 13 and the remainder of steam
10 is passed on to steam driven turbine 1 1 to provide motive force to produce
electrical or mechanical energy. The exhaust from turbine 11 exits via conduit
12.
[0045] In the embodiment set forth in FIGURE 3, a portion of the high
pressure steam flowing in conduit 1 is directed to conduit 2 and passed
through
a control valve to produce lower pressure steam 4. Conduit 4 may comprise
lower pressure steam with a quality less than or equal to unity, depending. on
the temperarture and pressure conditions of steam 2.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-26-
[0046] Liquid water is separated from the lower pressure vaporous steam in
gas-liquid segregation zone 7. Lower pressure steam, essentially free from
liquid water, exits,gas-liquid segregation zone 7 via conduit 5, while liquid
water is removed via conduit 6. Steam 5 is passed through one side of a heat
exchange device 8 wherein, steam 5 is superheated by indirect contact with the
remaining portion of the high pressure steam flowing via conduit 3 to the
other
side of heat exchange device 8. Superheated lower pressure steam emerges
from heat exchange device 8 via conduit 10. Condensate and any remaining
vapor fraction of the high press.ure steam exits via conduit 9. A fraction of
superheated steam 10 may be removed from the process via conduit 20 and the
remainder of steam 10 is passed on to steam driven turbine 11 to provide
motive force to produce electrical or mechanical energy. The exhaust from
turbine 1 1 exits via conduit 12 and is condensed in condenser 13 to produce
condensate 14. Condensate 14, stream 9, and condensate 6, may be combined
with make-up water 15 to produce a boiler feed water stream 16.
[0047] Steam generating zone 17 may comprise steam generating heat
exchangers (i.e., boilers) wherein heat is transferred from a heating medium
to
boil water and boiler feed water exchangers. Heat transfer within steam
generating zone 17 may occur by radiant and/or convective heat transfer
mechanisms. Heat is transferred into zone 17 via stream 19. Stream 19 may
represent heat flow such as, for example, from a chemical reaction, as
described
hereinabove, or a flow of matter. The use of multiple heat exchangers is
contemplated to be within the scope of the instant invention. High pressure
steam generated within zone 17 exits via conduit 1. A fraction of the steam
generated in zone 17 may be directed to conduit 18 to exit the process.
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-27-
[0048] In the embodiment set forth.in FIGURE 4, a portion of the high
pressure steam flowing in conduit 1 is directed to conduit 2 and passed
through
steam turbine 20 to produce lower pressure steam 4 and electricity. Conduit 4
may comprise a lower pressure steam with a quality less than or equal to
unity,
depending on the temperature and pressure conditions of steam 2.
'[0049] Liquid water is separated from the lower pressure vaporous steam in
gas-liquid segregation zone 7. Lower pressure steam, essentially free from
liquid water, exits gas-liquid segregation zone 7 via conduit 5, while liquid
water is removed via conduit 6. Steam 5 is passed through one side of a heat
exchange device 8 wherein, steam 5 is superheated by indirect contact with the
remaining portion of the high pressure steam flowing via conduit 3 to the
other
side of heat exchange device 8. Superheated lower pressure steam emerges
from heat exchange device 8 via conduit 10. Condensate and any remaining
vapor fraction of the high pressure steam exits via conduit 9. A fraction of
lower
pressure superheated steam 10 may be removed from the process via conduit
21 and the remainder of steam 10 is passed on to steam driven turbine 11 to
provide motive force to produce electrical or mechanical energy. The exhaust
from turbine 1 i exits via conduit 12 and is condensed in condenser 13 to
produce condensate 14. Condensate 14, stream 9, and condensate 6, may be
combined with make-up water 15 to produce a boiler feed water stream 16.
[0050] Steam generating zone 17 may comprise steam generating heat
exchangers (i.e., boilers) wherein heat is transferred from a heating medium
to
boil water, and boiler feed water exchangers. Heat transfer within steam
generating zone 17 may occur by radiant and/or convective heat transfer
mechanisms. Heat is transferred into zone 17 via stream 1 9. Stream 19 may
represent heat flow such as, for example, from a chemical reaction, as
described
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-28-
hereinabove, or a flow of matter. The use of multiple heat exchangers is
contemplated to be within the scope of the instant invention. High pressure
steam generated within zone 17 exits via conduit I. A fraction of the high
pressure steam generated in zone 17 may be directed to conduit 18 to exit the
process.
[00511 In the embodiment set forth in FIGURE 5, a high pressure steam
ffowing in conduit 1 is directed to one side of heat exchange device 2, such
that
heat is transferred to lower pressure steam 9 on the other side of device 2 to
produce superheated steam 10. Condensate and any remaining vapor fraction of
the high pressure steam exits device 2 via conduit 3. Condensate 3, may be
combined with make-up water 4 to produce a boiler feed water stream 19 to
steam generating zone 5.
[0052] High pressure steam generating zone 5 may comprise steam
generating heat exchangers (i.e., boilers) wherein heat is transferred from a
heating medium to boil water, and boiler feed water exchangers. Heat transfer
within steam generating zone 5 may occur by radiant and/or convective heat
transfer mechanisms. Heat is transferred- into zone 5 via stream 6. Stream 6
may
represent heat flow, for example from a chemical reaction, or a flow of
matter.
The use of multiple heat exchangers is contemplated to be within the scope of
the instant invention. High pressure steam generated within zone 5 exits via
conduit 1. A fraction of high pressure steam generated in zone 5 may be
directed to conduit 7 to exit the process.
[0053] A fraction of superheated steam 10 may be removed from the process
via conduit 20 and the remainder of steam 10 is passed on to steam driven
turbine 1 1 to provide motive force to produce electrical or mechanical
energy.
The exhaust from turbine 1 1 exits via conduit 12 and is condensed in
condenser
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-29-
13 to produce condensate 14. Condensate 14 may be combined with make-up
water 15 to produce a boiler feed water stream 16 for lower pressure steam
generating zone 8.
[0054] Steam generating zone 8 may comprise steam generating heat
exchangers (i.e., boilers) wherein heat is transferred from a heating medium
to
boil water, and boiler feed water exchangers. Heat transfer within steam
generating zone 8 may occur by radiant and/or convective heat transfer
mechanisms. Heat is transferred into zone 8 via stream 1 8. Stream 18 may
represent heat flow such as, for example, from a chemical reaction, as
described
hereinabove, or a flow of matter. The use of multiple heat exchangers is
contemplated to be within the scope of the instant invention. High pressure
steam generated within zone 8 exits via conduit 1. A fraction of the steam
generated in zone 8 may be directed to conduit 17 to exit the process.
EXAMPLES
[0055] General - A better understanding of the invention is provided with
particuiar reference to the examples given below. For Examples 1 -9 and
Comparative Examples 1-3, heat and material balance calculations were carried
out to illustrate the aspects of the instant invention by process simulation
software using methods described in "Program Computes Steam Rates and
Properties", by V. Ganapathy in Hydrocarbon Processing, November 1 988, pp.
105-108, and in standard engineering texts such as, for example, Perry's
Handbook of Chemical Engineering, 6th ed., New York, McGraw Hill, 1984. Also,
unless expressly stated otherwise, it should be understood that the high
pressure steam or heat used to generate the high pressure steam, as set forth
in
the Examples and Comparative Examples, may be obtained by recovering heat
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-30-
from any heat-producing chemical process as described hereinabove such as,
for example, the production of syngas by gasification of carbonaceous
materials
or by steam reforming of methane, the water-gas shift reaction, hydrogenation
of carbon monoxide or carbon dioxide to produce methanol, production of
ammonia by hydrogenation of nitrogen, or a combination of one or more of
these processes.
[0056] Comparative Example 7- 100,000 kg/hr of saturated high pressure
steam at 131 bara and 331 .45 C is fed to a,#steam turbine with an outlet
pressure of 0.12 bara and a mechanical efficiency of 86.5% to produce
electricity. The turbine generates 22.3 MW, with a steam quality of 69.3% at
the
outlet of the turbine.
[0057] Examples 1-5 - Examples 1 -5 illustrate the effect on turbine outlet
steam quality by changing the pressure to which the high pressure inlet steam
is
reduced as per Figure 1 of the instant invention. 100,000 kg/hr of''saturated
high pressure steam at 131 bara and 331.45 C is divided and a portion is
reduced in pressure. The resulting lower pressure steam is subjected to heat
exchange with the remaining portion of high pressu're steam. The approach
temperature in the heat exchanger is 5 C, i.e., the superheated lower pressure
steam temperature is 326.45 C in all cases. The superheated lower pressure
steam is fed to a steam turbine with an outlet pressure of 0.12 bara and a
mechanical efficiency of 86.5% to produce electricity. Table 1 shows results
per
the instant invention for various lower pressure values.
Table 1: Effect of Pressure
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-31 -
Turbine
Lower Amount of Degrees Outlet
Pressure Lower P Steam Superheat, Electricity, Steam
Steam, bara (thousands C MW Quality
K /hr)
Example 1 65.5 79.2 45.1 18.8 79.5%
Example 2 56.2 77.6 55.1 18.3 81.0%
Example 3 42.4 75.4 72.6. 17.5 83.3%
Example 4 28.6 73.6 95.2 16.4 86.2%
Example 5 14.6 72.2 128.7 14.7 90.5%
[0058] Comparative Example 2 - 100,000 kg/hr of saturated high pressure
steam at 131 bara and 331.45 C is fed to a steam turbine with an outlet
pressure of 0.12 bara and a mechanical efficiency of 86.5% to produce
electricity. The turbine generates 22.3 MW, with a steam quality of 69.3% at
the
outlet of the turbine. The wet steam from the outlet of the turbine is
condensed
at the saturation temperature of 0.12 bara steam (49.6 C), giving up 165.1
GJ/hr
during the condensation process. The condensed steam is pumped back up to
131 bara and subjected to heat transfer where 245.3 Gj/hr are transferred to
produce 100,000 kg/hr of saturated high pressure steam at 131 bara and
331.45 C, completing the steam cycle. The overall efficiency, E, of the steam
cycle is 32.7% , where:
E = (heat in -condensing duty)/heat in =(245.3GJ/hr-165.1 GJ/hr)/245.3 GJ/hr
[0059] Example 6 - Example 6, following the nomenclature of Figure 3,
illustrates the overall efficiency of a steam cycle. Heat input into steam
generating zone 17 via conduit 19 is 245.3 GJ/hr as in Comparative Example 2.
112,350 kg/hr of liquid water at 49.5 C, is boiled in heat transfer zone 17 to
produce 112,350 kg/hr of saturated high pressure steam at 131 bara and
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-32-
331.45 C in conduit 1. 1 7,550 Kg/hr is diverted via conduit 3, the remainder
of
94,800 kg/hr passes via conduit 2 and is flashed across a valve to produce
saturated steam at 42.4 bara, 253.8 C, 91.8% quality. The resulting lower
pressure steam is divided into 7800 kg/hr of saturated liquid in conduit 6 and
87,000 kg/hr of saturated vapor in conduit S. Conduit 5 subjected to heat
exchange with conduit 3 in exchanger 8. The approach temperature in the heat
exchanger is 5 C, producing superheated lower pressure steam temperature at
326.45 C, 42.4 bara in conduit 10 and condensed high pressure steam at
331.45 C in conduit 9. The superheated lower pressure steam in conduit 10 is
fed to steam turbine 1 1 with an outlet pressure of 0.12 bara and a mechanical
efficiency of 86.5% to produce 20.2 MW of electricity. The steam at the outlet
of
the turbine, conduit 12, has a quality of 83.3%. Conduit 12 is fully condensed
in
exchanger 13 by removal of 172.8 Gj/hr of energy, and exits as saturated
liquid
stream 14 at 49.5 C. Streams 14, 9, and 6 are combined and pumped back up to
131 bara, subjected to steam generating zone in zone 17 where 245.3 GJ/hr are
transferred via conduit 19 to produce 112,350 kg/hr of saturated high pressure
steam at 131 bara and 331.45 C, completing the steam cycle. The overall
efficiency, E, of the steam cycle is 29.6%. This efficiency is 90.5% of the
efficiency reported in Comparative Example 2, but with a much higher steam
turbine outlet quality, as per the objective of the instant invention.
[0060] Example 7- Example 7, following the nomenclature of Figure 4,
illustrates the overall efficiency of a steam cycle. Heat input into steam
generating zone 17 via conduit 19 is 245.3 GJ/hr as in Comparative Example 2.
115,290 kg/hr of liquid water via conduit 16 is boiled in heat transfer zone
17
to produce 115,290 kg/hr of saturated high pressure steam at 131 bara and
331.45 C, exiting via conduit 1. 16,410 Kg/hr is diverted via conduit 3, the
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-33-
remainder of 98,880 kg/hr passes via conduit 2 and is expanded in steam
turbine 20 with a mechanical efficiency of 86.5% to produce 4.4 MW of
electricity, and saturated steam at 42.4 bara, 253.8 C, 82.3% quality. The
resulting lower pressure steam is divided into 17510 kg/hr of saturated liquid
in
conduit 6 and 81,370 kg/hr of saturated vapor in conduit 5. Conduit 5
subjected to heat exchange with conduit 3 in exchanger 8. The approach
temperature in the heat exchanger is 5 C, producing superheated lower
pressure steam temperature at 326.45 C, 42.4 bara in conduit 10 and
condensed high pressure steam at 331.45 C in conduit 9. The superheated
lower pressure steam in conduit 10 is fed to steam turbine 11 with an outlet
pressure of 0.12 bara and a mechanical efficiency of 86.5% to produce 18.8 MW
of electricity. The steam at the outlet of the turbine, conduit 12, has a
quality of
83.3%. Conduit 12 is fully condensed in exchanger 13 by removal of 161.6 GJ/hr
of energy, and exits as saturated liquid stream 14 at 49.5 C. Streams 14, 9,
and
6 are combined and pumped back up to 131 bara, subjected to steam
.generating zone in zone 17 where 245.3 GJ/hr are transferred via conduit 19
to
produce 1 1 5,290 kg/hr of saturated high pressure steam at 131 bara and
331.45 C, completing the steam cycle. The overall efficiency, E, of the steam
cycle is 34.1 fo. This efficiency is 104.2% of the efficiency reported in
Comparative Example 2, and with a much higher steam turbine outlet quality, as
per the objective of the instant invention. Furthermore, the total electricity
production of 23.2 MW exceeds that of Comparative Example 2 (22.3 MW) by
4.1%.
[00611 Comparative Example 3 - 100,000 kg/hr of saturated high pressure
steam at 41.4 bara and 252.36 C is fed to a steam turbine with an outlet
pressure of 0.12 bara and a mechanical efficiency of 86.5% to produce
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
-34-
electricity. The turbine generates 20.75 MW, with a steam quality of 77.4% at
the
outlet of the turbine. The wet steam from the outlet of the turbine is
condensed
at the saturation temperature of 0.12 bara steam (49.6 C), giving up 184.5
GJ/hr
during the condensation process. The condensed steam is pumped back up to
41.4 bara and subjected to heat transfer where 259.3GJ/hr are transferred to
produce 100,000 kg/hr of saturated high pressure steam at 41.4 bara and
252.36 C, completing the steam cycle. The overall efficiency, E, of the steam
cycle is 28.9%, where:
E = (heat in -condensing duty)/heat in =(259.3G.J/hr-184.5 GJ/hr)/259.3 GJ/hr
[0062] Example 8 - Example 8, following the nomenclature of Figure 4,
illustrates the overall efficiency of a steam cycle. Heat input into steam
generating zone 17 via conduit 19 is 259.3 Gj/hr as in Comparative Example 3.
104,860 kg/hr of liquid water via conduit 16 is boiled in heat transfer zone
17
to produce 104,860 kg/hr of saturated high pressure steam at 41.4 bara and
252.36 C, exiting via conduit 1. 8,070 Kg/hr is diverted via conduit 3, the
remainder of 96,790 kg/hr passes via conduit 2 and is expanded in steam
turbine 20 with a mechanical efficiency of 86.5% to produce 6.00 MW of
electricity, and saturated steam at 10.34 bara, 181.35 C, 90% quality. The
resulting lower pressure steam is divided into 9,660 kg/hr of saturated liquid
in
conduit 6 and 87,130 kg/hr of saturated vapor in conduit 5. Conduit 5
subjected to heat exchange with conduit 3 in exchanger S. The approach
temperature in the heat exchanger is 5 C, producing superheated lower
pressure steam at 247.36 C,'10.34 bara in conduit 10 and condensed high
pressure steam at 252.36 C in conduit 9. The superheated lower pressure steam
in conduit 10 is fed to steam turbine 1 1 with an outlet pressure of 0.12 bara
and
a mechanical efficiency of 86.5% to produce 1 5.2 MW of electricity. The steam
at
CA 02645769 2008-10-17
WO 2007/127056 PCT/US2007/008919
- 35 -
the outlet of the turbine, conduit 12, has a quality of 88.1 %. Conduit 12 is
fully
condensed in exchanger 13 by removal of 173.34 GJ/hr of energy, and exits as
saturated liquid stream 14 at 49.5 C. Streams 14, 9, and 6 are combined and
pumped back up to 41.4 bara, subjected to steam generating zone in zone 17
where 259.3 GJ/hr are transferred via conduit 19 to produce 104,860 kg/hr of
saturated high pressure steam at 41.4 bara and 252.36 C, completing the steam
cycle. The overall efficiency, E, of the steam cycle is 29.5%. This efficiency
is
102.2 !0 of the efficiency reported in Comparative Example 2, and with a much
higher steam turbine outlet quality, as per the objective of the instant
invention.
Furthermore, the total electricity production of 21.21 MW exceeds that of
Comparative Example 2 (20.75 MW) by 2.2%.
[0063] Example 9- Example 9 illustrates the embodiment of the invention as
set forth in Figure 5. A syngas stream from an oxygen blown gasifier
comprising
57,242 lbmole/hr of carbon monoxide, hydrogen, water, and carbon dioxide is
subjected to a water gas shift reaction to produce a hot shifted syngas. A
portion of the heat of reaction is removed in heat transfer zone 5 by
generating
1 15,693 kg/hr of 37.6 bara steam at 246.7 C. The syngas is further cooled in
heat transfer zone .8 to produce 455,475 kg/hr of 4.5 bara steam at 147.6 C.
The lower pressure steam exits zone 8 via conduit 9 and is superheated in
exchanger 2 by heat exchange with 62,600 kg/hr of high pressure steam in
conduit 1. The approach temperature in exchanger 2 is 5 C. 455,475 kg/hr of
superheated steam at 241.65 C is passed via conduit 10 through turbine 11
(86.5% efficiency) to generate 66.7 MW of power. The outlet quality of the
steam
in conduit 12 is 92.8%. This compares to a power generation of 59.9 MW, with
an outlet quality of 86% if steam 9 had not been superheated.