Note: Descriptions are shown in the official language in which they were submitted.
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
INHIBITION OF BREAST CARCINOMA STEM CELL GROWTH AND
METASTASIS
This application claims priority to U.S. Provisional Application Serial No.
60/783,09 1, filed March 16, 2006, the disclosure of which is incorporated
herein by
reference.
FIELD OF THE INVENTION
The present invention relates generally to the field of cancer and more
particularly
to inhibiting the growth of breast carcinoma stem cells.
BACKGROUND OF THE INVENTION:
Cancers of epithelial origin are responsible for the majority of cancer-
related
deaths from incurable metastatic disease. The cancer stem cell hypothesis
(Reya et al.,
(2001) Nature 414:105) proposes that certain tumors originate from and persist
due to
mutations in tissue stem cells that result in unregulated, immortal
proliferation, and in this
state are referred to as cancer stem cells (CSC). It has long been recognized
that only a
very small percentage of cells in a tumor are capable of immortal growth
(approximately
1/1000 to 1/5000 cells in lung tumors and 1/1,000,000 in leukemia cells (Reya,
(2001);
Dick, J. E. (2003) Proc Natl Acad Sci 100:3547; Marx, J. (2003) Science
301:1308.).
There is now very good evidence that a number of cancers, including breast
Gudjonsson,
et al. (2002) Genes Dev 16:693; Al-Hajj, et al. Proc Natl Acad Sci
100:3983; Dontu, G., et al., (2004) Breast Cancer Res 6:R605; Ponti, D.,
(2005) Cancer
Res 65:5506); colon (Kim, et al. (2005) Cell 121:823), ovarian (Bapat, et al.
(2005)
Cancer Res 65:3025), lung (Kim, et al. (2005) Cell 121:823, and prostate
cancers
(Schalken, (2003) Urology 62:11.), leukemia (Dick, J. E. (2003), glioma
(Kondo, et al.
(2004) Proc Natl Acad Sci 101:78 1; Singh, S. K., et al. (2004) Nature
432:396), retinoblastoma (Reedijk, M., S. et al. (2005) Cancer Res 65:8530)
and
hepatocellular carcinoma (Rosner, A., K. et al. (2002) Am JPathol 161:1087)
proliferate
from cancer stem cells. Cancer stem cells have also been isolated from
established tumor
cell lines (Gudjonsson, et al. (2002) Genes Dev 16:693; Ponti, D., (2005)
Cancer Res
65:5506; Kondo, et al. (2004) Proc Natl Acad Sci 101:781), and retain the same
phenotype
as the tumors from which they were originally isolated. Evidence in several
types of
cancer shows that pathways prominent in normal stem cell function, notably
Wnt, Notch,
1
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
Ssh (sonic hedgehog), XIAP (X-linked inhibitor of apoptosis protein) become
`dysregulated' in cancer stem cells (CSC) (Reya et al., (2001) Nature 414:105;
Dontu, G.,
et al., (2004) Breast Cancer Res 6:R605; Rosner, A., K. et al. (2002) Am
JPathol
161:1087; Reya, T., et al. (2005) Nature 434:843; Li, Y., B. et al. Proc Natl
Acad Sci
100:15853; Yang, L., Z. et al. (2003) Cancer Res 63:6815; Liu, S., et al.
(2005) Breast
Cancer Res 7:86; Mikaelian, I., et al. (2004) Breast Cancer Res 6:R668).
Screening mammography is highly effective in identifying breast cancer in
women,
and it is estimated that in 2005, more than 211,000 new cases of invasive
breast cancer
and approximately 58,000 new cases of in situ breast cancer will be identified
in the U.S.
(Society, A. C. Breast cancer Facts and Figures American Cancer Society 2005).
Breast
cancer is the leading cause of cancer death in women (Sasco, A. J. (2003) Horm
Res 60
Suppl 3:50.) with more than 40,000 deaths annually (Society, A. C. Breast
cancer Facts
and Figures American Cancer Society 2005) due to recurrence of local and
distant
metastasis. Breast cancer recurrence has been linked to the presence of
systemic
micrometastases. The therapeutic resources are limited, since Herceptin which,
in
combination with radiotherapy and chemotherapy reduces the rate of recurrences
(Bapat,
S. A., et al. (2005) Cancer Res 65:3025) can be applied to only 30% of breast
cancer
patients (HER2 positive).
The high rates of recurrence and metastasis, even following surgery,
chemotherapy, radiation, targeted small molecule and antibody therapies - all
of which
shrink tumors but do not eliminate immortal tumor cells- underscore the need
to identify
new therapeutic strategies that specifically target and kill cancer stem cells
in order to
eliminate recurrence and metastatic disease. Therefore, there is an ongoing
need for
understanding the neoplastic changes that occur uniquely in cancer stem cells
can lead to
an understanding of how CSC tumors form, how they proliferate, how they evade
standard
treatments, and for the development of therapies that target cancer stem
cells.
SUMMARY OF THE INVENTION
The present invention provides a method for inhibiting the growth of breast
carcinoma stem cells. The breast carcinoma stem cells express High Molecular
Weight -
Melanoma Associated Antigen (HMW-MAA). The method comprises administering to
an
individual a composition comprising an antibody reactive with HMW-MAA in an
amount
effective to inhibit the growth of the breast carcinoma stem cells.
2
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
In another embodiment, a method is provided for inhibiting metastasis of a
breast
carcinoma where the breast carcinoma comprises HMW-MAA+ breast carcinoma stem
cells. The method comprises administering to the individuat a composition
comprising an
amount of an antibody reactive with HMW-MAA effective to inhibit metastasis of
the
breast carcinoma.
In another embodiment, a method is provided for detection of HMW-MAA+ breast
carcinoma stem cells. The method comprises administering to an individual, or
contacting
a biological sample obtained from the individual with, a combination of
antibodies, where
the combination of antibodies comprises an antibody directed HMW-MAA and at
least
one antibody directed to a breast cancer stem cell marker. Detecting the
binding of both
the HMW-MAA antibody and the at least one breast cancer stem cell marker
determines
the presence of an HMA-MAA+ breast carcinoma stem cell.
In particular embodiments, the antibody employed in practicing the invention
can
be the monoclonal antibody designated 225.28 and/or the monoclonal antibody
designated
763.74.
BRIEF DESCRIPTION OF THE FIGURES
Figures lA and 1B present a graphical representation of data obtained by
fluorescence activated cell sorting (FACS) of HMW-MAA expression by a
subpopulation
of breast carcinoma stem cells.
Figure 2 is a photographic representation of a Western blot analysis of HMW-
MAA expressed by MDA-MB-435 cells.
Figures 3A and 3B are graphical representations of data obtained from FACS
separation of MDA-MB-435s cells stained with antibodies to breast carcinoma
stem cell
markers.
Figure 4 is a graphical depiction of results obtained from inhibition by HMW-
MAA-specific mAb 763.74 and 225.28 of human breast cancer cell MDA-MB-435s
lung
metastases in SCID mice.
Figure 5 is a graphical depiction of results obtained from inhibition of post-
surgery
lung metastasis of human breast carcinoma stem cells by use of mAb 225.28
3
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
DETAILED DESCRIPTION OF THE IlVVENTION
The present invention is related to the discovery that HMW-MAA is present on
breast carcinoma stem cells. The invention provides a method of inhibiting the
growth of
breast carcinomas which comprise HMW-MAA+ breast carcinoma stem cells. The
method comprises administering to an individual a composition comprising an
antibody
reactive with HMW-1VIAA in an amount effective to inhibit the growth of the
breast
carcinoma stem cells.
Also provided is a method for inhibiting metastasis of a breast carcinoma in
an
individual, wherein the breast carcinoma comprises HMW-MAA+ breast carcinoma
stem
cells. The method comprises administering to the individual an amount of an
antibody
reactive with HMW-MAA effective to inhibit the metastasis.
In another embodiment, a method is provided for detection of HMW 1VIAA+ breast
carcinoma stem cells by administering a combination of antibodies to an
individual or a
biological sample obtained from the individual. The combination of antibodies
comprises
an antibody directed to HMW-MAA and at least one antibody directed to a breast
cancer
stem cell marker. Detection of the binding of the HMW-IVIAA antibody and the
antibody
to the breast cancer stem cell marker determines the presence of an HMA-MAA+
breast
carcinoma stem cell.
With respect to HMW-MAA, it is a highly glycosylated integral membrane
chondroitin sulfate. It consists of an N-linked 280 kDa glycoprotein component
and a 450
kDa chondroitin sulfate proteoglycan component. The two components share the
same
core protein. Through the use of mouse and human monoclonal antibodies, a
number of
its antigenic determinants have been identified. They display a heterogeneous
expression
on melanoma cells line and in melanoma lesions. HMW-MAA plays a role in the
growth
and metastatic potential of melanoma cells, and while one report observed HMW-
MAA
expression on breast carcinoma cells (Dell'Erba et al., (2001) Anticancer Res.
Mar-Apr;
21(2A):925-30), the present discovery that HMW-MAA is expressed on breast
carcinoma
stem cells is unique in that there is presently no evidence that antigens
expressed by tumor
cells are also expressed by cancer stem cells. In connection with this
finding, we
demonstrate that substantial percentages of breast carcinoma stem cells
obtained from
pleural effusions of breast cancer patients include breast carcinoma stem
cells that express
HMW-MAA. Further, we demonstrate that the method of the invention can be used
to
inhibit metastasis of carcinomas formed in an animal model inoculated with
human breast
4
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
carcinoma stem cells that we have determined express HMW-IViAA. Further still,
we
demonstrate that post-resection reoccurrence of carcinomas formed from human
breast
cancer stem cells that express HMW-MAA can be effectively inhibited using the
method
of the invention. Thus, the method is expected to provide a unique therapy for
breast
cancer patients by targeting breast carcinoma stem cells that express HMW-
1VIAA.
Breast carcinoma stem cells are considered those breast carcinoma cells that
express CD44 ("CD44+"), but do not express CD24 ("CD24-") or express low
amounts of
CD24 ("CD241o") relative to normal cells or non-stem cells. ESA is also known
to be a
marker of breast carcinoma stem cells, while B38.1 is known to be breast
cancer cell.
Non-stem cells are considered those which express one or more of CD2, CD3,
CD10,
CD 16, CD 18, CD31, CD45 CD64, and CD 140b. Accordingly, cells expressing any
of
CD2, CD3, CDIO, CD16, CD18, CD31, CD45, CD64 or CD140b are not considered
breast carcinoma stem cells. It will be recognized by those skilled in the art
that other
markers for identifying breast carcinoma stem cells may be known or identified
hereafter
and may be used in identifying breast carcinoma stem cells in connection with
the present
invention.
The aforementioned markers can be used to identify breast carcinoma stem cells
using conventional methods such as immunohisotchenistry or cell sorting. In
one
embodiment, breast carcinoma stem cells can be identified essentially using
the cell
sorting methods and markers described by Al-Hajj, et al. (PNAS (2003) Vol.
100, pp3984-
3983). The present invention provides an adaptation of this method such that
breast
carcinoma stem cells that express HMW-MAA can be identified using anti- HMW-
MAA
antibodies.
In one embodiment, identification of breast carcinoma stem cells can be
performed
by flow cytometry using standard cell sorting procedures. For example, cells
obtained
from patient effusions or biopsies using conventional techniques may be
processed by first
ficolling the fluid (typically 500 ML- 2L) to remove debris and red blood cell
contamination. Gating can also be carried out (for example, with antibody
directed to
CD45) to distinguish over blood cells. Flow cytometric staining for breast
carcinoma stem
cell phenotypic analysis can identify "lineage negative" cells (negative for
CD2,
3,10,16,18,31,45, 64,140b, for example, using PE labeled antibodies). For FACS
analysis,
CD44+-FITC labeled antibody/CD241o PerCP labeled antibody (all antibodies from
BD/Pharmingen, San Jose, CA) can be used to assay cells from human breast
cancer
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
patient pleural effusions. Cell sorting from malignant effusions can
optionally first use
anti-PE coated beads to deplete the lineage marker positive cells to greatly
reduce the
number of non-carcinoma stem cells and thereby reduce the cell sorting time.
In another embodiment, a patient sample can be assessed for the presence and
percentage of various cell populations by flow cytometry sorting of
ESA~CD44+CD24-n0W
cells, as per Al-Hajj et al. In combination or in series with this staining,
the
ESA+CD44+CD24"n0W cells can be stained with an anti-HMW-MAA antibody to
identify
breast carcinoma stem cells that express HMW-MAA. Optionally, staining can be
carried
out with more than one antibody directed toward HMW-MAA which are each
directed to
different epitopes of HMW-MAA. Non-limiting examples of monoclonal antibodies
suitable for use in this method include the anti- HMW-MAA antibodies
designated 225.28
and/or the monoclonal antibody designated 763.74.
HMW-MAA antibodies of the invention can be used be used for a variety of
diagnostic assays, imaging methodologies, and therapeutic methods in the
management of
breast cancer. For example, efficacy of the present method in inhibiting the
growth of, or
eliminating breast carcinoma stem cells in an individual could be ascertained
by analysis
of samples obtained from the individual before and after treatment, such as by
analysis of
pre- and post-treatment biopsies, immunohistochemical analysis, or cell
sorting analysis to
determine the presence of breast carcinoma stem cells that express HMW-MAA.
Anti- HMW-MAA antibodies can be conjugated to various moieties for diagnostic
or therapeutic applications related to HMW-MAA+ breast carcinoma stem cells.
For
example, anti- HMW-MAA antibodies may be conjugated to a therapeutic agent to
enable
localization of the therapeutic agent to breast carcinoma stem cells which
express HMW-
MAA. Examples of suitable therapeutic agents include, but are not limited to,
an anti-
tumor drug, a toxin, a radioactive agent, a cytokine, a second antibody or an
enzyme.
Examples of cytotoxic agents include, but are not limited to ricin, ricin A-
chain,
doxorubicin, daunorubicin, taxol, ethiduim bromide, mitomycin, and the like.
In another embodiment, the anti- HMW-MAA antibodies may be conjugated to a
radioactive agent. A variety of radioactive isotopes are available for
conjugating to mAbs
such that breast carcinoma stem cells that express HMW-MAA may be imaged or
selectively destroyed. For selective destruction of cells, the antibodies may
be conjugated
to a highly radioactive atom, such as Inl1t, Aj211, It31, I125, Y90, Re186,
ReIgg, Sm153, Bi212,
P32, Pb212 and radioactive isotopes of Lu.
6
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
When the antibody conjugates are used for identifying breast carcinoma stem
cells
which express HMW-MAA, the antibody conjugates may comprise any suitable
detectable
markers which include, but are not limited to, a radioisotope, a fluorescent
compound, a
bioluminescent compound, chemiluminescent compound, a metal chelator or an
enzyme.
For example, certain radioisotopes can be used for scintigraphic studies, such
as Tc99-
(metastable technetium-99), I123, or as spin labeled atoms for nuclear
magnetic resonance
(NMR) imaging (also known as magnetic resonance imaging, or "MRI"), such as
1123, I131 ,
I124 , F19, C13, Nls, Ol' or Gadlinium (III) or Manganese (II). Such labels
may be
incorporated in into the antibodies in known ways. "Monoclonal Antibodies in
Iminunoscintigraphy" (Chatal, CRC Press 1989) describes suitable methods in
detail.
In addition to the antibodies disclosed here, other antibodies to HMW-MAA can
also be produced. The methods for producing monoclonal and polyclonal antisera
are well
known in the art. The antibodies or fragments may also be produced by
recombinant
means. Alternatively, fully human monoclonal antibodies can also be produced
by
methods such as phage display and transgenic methods (Vaughan et al., 1998,
Nature
Biotechnology 16: 535-539). For example, fully human anti-HMW-MA monoclonal
antibodies may be generated using large human Ig gene combinatorial libraries
(i.e.;, phage
display); (Griffiths and Hoogenboom, Building an in vitro immune system: human
:
antibodies from phage display libraries. In: Protein Engineering of Antibody
Molecules
for Prophylactic and Therapeutic Applications in Man. Clark, M. (Ed.).
Nottingham
Academic, pp 45-64 (1993); Burton and Barbas, Human Antibodies from
combinatorial
libraries. Id., pp 65-82).
The anti-HMW-MAA antibodies may be administered by any suitable means,
including parenteral, subcutaneous, intraperitoneal, intraputmonary, and
intranasal.
Parenteral infusions include intramuscular, intravenous, intraarterial,
intraperitoneal,
intralymphatic or subcutaneous administration. In addition, the antibodies may
be
administered by pulse infusion, e.g., with declining doses of the antibody.
One may also administer other compounds, such as chemotherapeutic agents,
immunosuppressive agents and/or cytokines with the anti- HMW-MAA antibodies.
The
combined administration can include co-administration, using separate
formulations or a
single pharmaceutical formulation, and can also include consecutive
administration in
either order, wherein preferably there is a time period while both (or all)
active agents
simultaneously exert their biological activities.
7
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
Therapeutic formulations comprising anti- HMW-MAA antibodies may be
prepared by mixing with known pharmaceutically acceptable carriers, excipients
or
stabilizers. It will be recognized by one of skill in the art that the form
and character of
the pharmaceutically acceptable carrier or diluent is dictated by the amount
of active
ingredient with which it is to be combined, the route of administration and
other well-
known variables, such as the size of the individual and the stage of the
disease.
The following illustrative examples are provided to further describe, but not
to
limit the invention.
EXAMPLE 1
This Example demonstrates HMW-MAA expression by a subpopulation of breast
carcinoma stem cells in breast carcinoma stem cell lines.
Staining of seven human breast carcinoma cell lines (Figures 1A and IB) with
the
H1V1W-MAA-specific mAb 763.74, TP61.5 and VF1-TP41.2 demonstrates that at
least
80% of the CD44*, CD24 lo cells were stained by HMW-MAA-specific mAb in the
cell
lines MDA-MB-435, about 70 and 50% in the cell lines MDA-MB-231 and HS578T,
respectively, and less than 4 !o in the cell line MCF-7 and SLTM-149. It is
noteworthy that
the percentage of CD44+, CD241o cells stained by the three HMW-MAA-specific
niAb is
stable across multiple cell culture passages, which indicates that the
expression of HMW-
MAA by breast carcinoma stem cells is a stable characteristic.
EXAMPLE 2
This Example demonstrates the molecular profile of HMW-MAA expressed by
breast carcinoma stem cells. To characterize the molecular basis of the
staining of breast
carcinoma stem cells by HMW-MAA-specific mAb, a lysate of the human breast
carcinoma cell line MDA-MB-435 was tested with mAb 763.74 in Western blotting_
Specifically, and as shown in Figure 2, a lysate from CD44}CD24" breast
carcinoma cells
MDA-MB-435 was separated by 8% SDS-polyacrylamide gel for immunoblot analysis
with HMW-MAA-specific mAb 763.74 (lane 3) and isotype control mAb MK2-23 (lane
6). Human melanoma cells M14, which do not express HMW-MAA (lanes 1 and 4),
and
M14/fIMW cells, which express HMW-MAA following HMW-MAA cDNA transfection
8
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
(lanes 2 and 5), were used as controls. The two characteristic components of
the HMW-
MAA were identified as depicted in Figure 2.
EXAMPLE 3
This Example demonstrates HMW-MAA expression by CD44+/CD24-/low breast
carcinoma stem cells in the human breast cancer cell line MDA-MB-435s. As
depicted in
Figure 3A, staining of MDA-MB-435s cells with CD24-,CD44-specific mAbs showed
that
>80% of cells are CD44+/CD24-/low breast carcinoma stem cells as indicated. As
shown
in Figure 3B, staining of CD44+/CD24-/low putative breast carcinoma stem cells
with
HMW-MAA-specific mAb 225.28 (bottom panel) and with an isotype control mAb
(top
panel) showed that 99.1 % of CSC are HMW-MAA positive. Thus, a human breast
cancer
stem cell line is demonstrated to express HMW-MAA.
EXAMPLE 4
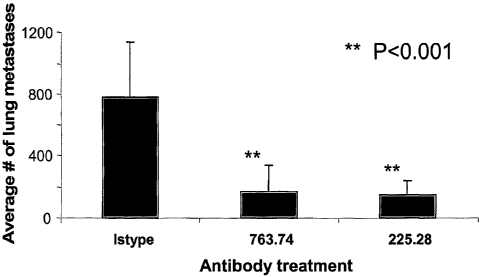
This Example demonstrates inhibition by HMW-MAA-specific mAb 763.74 and
225.28 of human breast carcinoma stem cell (MDA-MB-435s) lung metastases in
SCID
mice. Results are presented in Figure 4. To obtain the data shown in Figure 4,
human
breast cancer cell MDA-MB-435s (2x106) were injected i.v. into each SCID mouse
on day
0. Subsequently, all tumor bearing mice were randomized into three groups
(5/group).
Starting on day 3, one of the groups was injected i.p. with HMW-MAA-specific
mAb
763.74 and one with HMW-MAA-specific mAb 225.28 (1001ig/mouse) twice weekly
for a
total of 9 injections. The third group of mice was injected with an isotype
control
antibody. On day 34, all mice were euthanized and lung metastatic nodules were
counted.
Differences between HMW-MAA-specific mAb treated groups and isotype control
antibody treated group were significant (p<0.001).
Thus, this Example demonstrates that administration of either of two distinct
HMW-MAA-specific mAbs can inhibit metastasis from tumors produced in an animal
model by inoculation with human breast carcinoma stem cells that express HMW-
MAA,
while administration of an isotyped control mAb that does not bind to HMW-MAA
is
ineffective in inhibiting such metastasis.
EXAMPLE 5
9
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
This Example demonstrates inhibition of post-surgery lung metastasis of human
breast carcinoma stem cells by use of mAb 225.28. To obtain the data shown in
Figure 5,
the following regimen was employed:
Day 0: Mammary fat tumor s.c. inoculation; Day 7: mAb 225.28 treatment with
200 g/mouse, 2x weekly; Day 71: Surgically remove tumor; Day 103: stop
treatment; Day
134: sacrifice mice and collect lungs for metastasis analysis.
As can be seen from Figure 5, administration of mAb 225.28 results in a
statistically significant inhibition of lung metastasis after the removal of a
tumor obtained
by inoculation of an animal model with human breast carcinoma stem cells that
express
HMW-MAA.
EXAMPLE 6
This Example demonstrates inhibition of human breast carcinoma post-surgery
reoccurrences by mAbs directed to HMW-MAA.
To obtain the data presented in Table 1, human breast cancer stem cell MDA-MB-
435s (2x106) were injected into mammary fat pad of each SCID mouse on day 0.
Subsequently, all tumor bearing mice were randomized into three groups
(5/group).
Starting on day 7, one of the groups was injected i.p. with HMW-MAA-speciftc
mAb
763.74 and one with HMW-MAA-specific mAb 225.28 (200 g/mouse) twice weekly for
a
total of 18 injections. The third group of mice was injected with an isotype
control -
antibody. On day 71, all tumors were removed surgically from mice. The
treatment with
mAb was continued in the same regimen with additional 9 injections. On day
131, all mice
were sacrificed, local tumor reoccurrences and lung matastases were detected
and
analysed.
Table 1.
mAb 225.28 F3C25 763.74
# of tumor 0/5 3/5 1/5
reoccurrences/group (1 dead)
As can be seen from Table 1, administration of either of two distinct HMW-MAA-
specific mAbs results in inhibition of the recurrence of tumors obtained by
inoculation of
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
an animal model with human breast carcinoma stem cells that express HMW-MAA,
while
an isotype control (F3C25) which recognizes an irrelevant antigen does not
inhibit such
recurrence.
EXAlVII'LE 7
This Example demonstrates HMW-1VIAA expression by subpopulations of breast
carcinoma stem cells in pleural exudates from patients with breast cancer.
To obtain the data summarized in Table 2, pleural effusion cells from breast
cancer
patients were labeled with anti-HMW-MAA mAb (clone 225.28, 763.74, TP41.2, or
TP61.5), followed by PE-labeled anti-mouse IgG. After washing, cells were
stained with
FITC-labeled anti-CD24, PerCP-labeled anti-CD45, APC-labeled anti-CD44, and 7-
AAD.
Percentages of CD44+CD24- populations in CD45- 7AAD- cells or CD45- 7AAD-
HMW-MAA+ cells were analyzed by flow cytometry. Enrichment of CD44+CD24-
population by gating at HMW-MAA positive cells was calculated by dividing the
percentages of CD44+CD24- cells in CD45- 7AAD- HMW-MAA+ population with.that
in CD45- 7AAD- population and are shown in parenthesis in each well. The
highest fold
enrichment is shown at the right column for each patient's sample.
Table 2.
`96 af CtM4+6 in HMNI-MAA+=ecits CfptA enrichment)
¾3t(en6 Totai'ie11 niifribg'r' CD44 i CD24:(36~' mA6;r25.28 mAb'7634; mlW
TF4i.2^ mAb;;7P61:5 Average hipTi~
(1x106): " = =. . . .. . .. : : . . . fotd
anrirfi
6,4, 280 2.91 :8:69 (2:96) .5.7.(1.96T 10;2 (3:S1) 24:7, (8.49) 12.3:(4.23) E
PS: 4170: 16.3. 2G.8;(164)' 689..{4;23?. 23=l:(2:45) 1$:3i(f:12)'
34:4:.('2:11) ~
P6 =298 7:22 :4p70;(U.6S), 0.00.(0.A)= 3.57 (Oc50) 50.0;(6:93)' 14 fi'=(2.02)
!
380220 i9:2= 35.5,(1L85)' 95.4"{4.97)= 61.3,(3.19)= 75:4 (3:43) 56.9 (3.4$) !
P7
P8 18_, , 2.9Q (0:16)=, 60.7.,C3 37);= 20.9~(1:16) 31:9:(7:i74). 29 O(1.61) :
P9' ='98 3.3$ ;22E6'(6;69)' 40:2~(11:89) 3825 (21:39) 35:7 (10:56)
34.3:(10.13.; 11
P10 3300;, 31.fs 91 S,C2:9b)== 96'.$ (3:Ob) ' 93 7(2 97,) 44 8(3 OOj= 94 2(2
98) :
, 7b S, 81}` 76:fi (5.89)
P27 200 ' 13.. 67 4%(S 18): 93r3:{7:18);, 70:3 (S 41) (S
P12 = .1000' 4 4A 13 5;~2 73):, (19:47) 90.3 (18;28) 67:3 (13 62) .66
8(13.53,; `1!
P13 2515' 11:6 ;?!tid`(b:12)' 814 (7,02). 6$.7 (5 92) 7fi.).'(6 6i).. 94 S
(6:42j
P14= 47 ' 32:2 8,d0.{0:69j' 49:i=(4:02) 32:0;(2:62)45:3'(s;71)
P15 56' S8T 64.3`(1:18). 903:~1:54). nd n8 74:5(1.36)=
Averrage
(fCEtf
enrlchment) 878.83 '16 59 35:18 (2.73)=. :68.4'(6.02) 48.2 (5:25) .52:9'(S.86)
Thus, this Example demonstrates the presence of breast carcinoma stem cells
that
express HMW-MAA in human breast cancer patients.
11
CA 02646127 2008-09-16
WO 2007/109193 PCT/US2007/006736
This invention has been described through examples presented above. Routine
modifications to the methods and compositions presented herein will be
apparent to those
skilled in the art and are intended to be within the scope of the claims
appended hereto.
12