Note: Descriptions are shown in the official language in which they were submitted.
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
METHODS AND APPARATUS FOR TISSUE MODIFICATION
CROSS-REFERENCES TO RELATED APPLICATIONS
100011 The present application claiins priority to U.S. Patent Application
Serial Nos.:
11/375,265 (Attoi-ney Docket No. 026445-000700US), titled "Methods and Appai-
atus for
Tissue Modification," filed March 13, 2006; 11/405,848 (Attorney Docket No.
026445-
000720US), titled "Mechanical Tissue Modification Devices and Methods," filed
April 17,
2006; 11/406,486 (Attor-ney Docket No. 026445-000721 US), titled "Powered
Tissue
Modification Devices and Methods," filed April 17, 2006; 11/405,859 (Attorney
Docket No.
026445-000722), titled "Tissue Modification Barrier Devices and Methods,"
filed April 17,
2006; and 11/429,377 (Attoi-ney Docket No. 026445-000723US), titled "Flexible
Tissue
Rasp," filed May 4, 2006. The full disclosures of all the above-cited
references are hereby
incorporated by reference.
STATEMENT AS TO RIGHTS TO INVENTIONS MADE UNDER
FEDERALLY SPONSORED RESEARCH OR DEVELOPMENT
100021 NOT APPLICABLE
REFERENCE TO A "SEQUENCE LISTING," A TABLE, OR A COMPUTER
PROGRAM LISTING APPENDIX SUBMITTED ON A COMPACT DISK.
[0003] NOT APPLICABLE
BACKGROUND OF THE INVENTION
100041 Field of the Invention. The present invention relates to methods and
apparatus for
modifying tissue in a patient.
100051 Many pathological conditions in the human body may be caused by
enlargement,
movement, displacement and/or a variety of other changes of bodily tissue,
causing the tissue
to press against (or "impinge on") one or more otherwise normal tissues or
organs. For
example, a cancerous tumor may press against an adjacent organ and adversely
affect the
functioning and/or the health of that organ. In other cases, bony growths (or
"bone spurs"),
arthritic changes in bone and/or soft tissue, redundant soft tissue, or other
hypertrophic bone
1
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
or soft tissue conditions may impinge on nearby nerve and/or vascular tissues
and
compromise functioning of one or mot-e nerves, i-educe blood flow through a
blood vessel, oi-
both. Other examples of tissues which may grow or move to press against
adjacent tissues
include ligaments, tendons, cysts, car-tilage, scar tissue, blood vessels,
adipose tissue, tumoi-,
hematoma, and inflammatory tissue.
100061 One specific example of a condition caused by tissue iinpingement is
spinal
stenosis. Spinal stenosis occurs when neural tissue and/or vascular tissue in
the spine become
iinpinged by one or more str-uctures pressing against them ("neural and/or
neurovascular
impingement"), causing one or more syinptoms. This impingement of tissue may
occur in
one or inore of several different areas in the spine, such as in the central
spinal canal (the
vertical passage through which the spinal cord and cauda equina extends), the
lateral recesses
of the spinal canal, or one or more intervertebral foramina (the openings
through which nerve
roots bi-anching from the spinal cord pass).
100071 For explanatory purposes, Fig. I is offered to show an approximate top
view of a
vertebra (one of the bones of the spinal column) with the cauda equina (the
horsetail-shaped
bundle of nerves that extends from the base of the spinal cord through the
central spinal
canal) shown in cross section and two nerve roots exiting the central spinal
canal and
extending through intervertebral foramina on either side of the vertebra.
(Fig. I is not drawn
to exact scale and is intended for exemplary purposes only. It should be
emphasized here that
the drawing figures appended to this application are not intended to be
precisely anatomically
correct and are provided for exemplary purposes to facilitate description.)
The spinal cord
and cauda equina run vertically along the spine through the central spinal
canal, while nerve
roots branch off of the spinal cord and cauda equina between adjacent
vertebrae and extend
through the intervertebral foramina.
10008J One common cause of spinal stenosis is buckling and thickening of the
ligamentum
flavum (one of the ligaments attached to and connecting the vertebrae), as
shown in Fig. I.
Buckling or thickening of the ligamentum flavum may impinge on one or more
neurovascular
structures, dorsal root ganglia, nerve roots and/or the spinal cord itself
Another common
cause of neural and neurovascular compression within the spine is disease of
one or more of
the intervertebral discs (the malleable discs between adjacent vertebrae),
which may lead to
collapse, bulging or hemiation of the disc. In Fig. 1, an intervertebral disc
is shown with
three solid-tipped arrows demonstrating how the disc might bulge or herniate
into the central
2
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
spinal canal to impinge upon the spinal cord, cauda equina and/or individual
nerve roots.
Other causes of neural and neui-ovascular impingement in the spine include:
hypertT-ophy of
one or more facet joints (also known as zygopophaseal joints, facet joints
provide articulation
between adjacent vertebrae--two vertebral facet superior articular processes
are shown in Fig.
1); formation of osteophytes (bony gi-owths or "bone spurs") on vertebi-ae;
spondylolisthesis
(sliding of one vertebra relative to an adjacent vertebra); and (facet joint)
synovial cysts.
Disc, bone, ligament or other tissue may impinge on the spinal cord, the cauda
equina,
branching spinal nerves and/or blood vessels in the spine to cause loss of
function, ischemia
(shortage of blood supply) and even permanent damage of neural or
neurovascular tissue. In
a patient, this may manifest as pain, impaired sensation and/or loss of
strength or mobility.
100091 In the United States, spinal stenosis occurs with an incidence of
between 4% and
6% of adults aged 50 and older and is the most frequent reason cited for back
surgery in
patients aged 60 and older. Conservative approaches to the treatment of
syinptoms of spinal
stensosis include systemic medications and physical therapy. Epidural steroid
injections
may also be utilized, but they do not provide ling lasting benefits. When
these approaches
are inadequate, current treatment for spinal stenosis is generally limited to
invasive surgical
procedures to remove vertebra] ligament, cartilage, bone spurs, synovial
cysts, cartilage, and
bone to provide increased rooin for neural and neurovascular tissue. The
standard surgical
procedure for spinal stenosis treatment includes laminectomy (complete removal
of the
lamina (see Fig. 1) of one or more vertebrae) or laminotomy (partial removal
of the lamina),
followed by removal (or "resection") of the ligamentum flavum. In addition,
the surgery
often includes partial or occasionally complete facetectomy (removal of all or
part of one or
more facet joints between vertebrae). In cases where a bulging intervertebral
disc contributes
to neural impingement, disc material may be removed surgically in a discectomy
procedure.
[0010] Removal of vertebral bone, as occurs in laminectomy and facetectomy,
often leaves
the effected area of the spine very unstable, leading to a need for an
additional highly
invasive fusion procedure that puts extra demands on the patient's vertebrae
and limits the
patient's ability to move. In a spinal fusion procedure, the vertebrae are
attached together
with some kind of support mechanism to prevent them from moving relative to
one another
and to allow adjacent vertebral bones to fuse together. Unfortunately, a
surgical spine fusion
results in a loss of ability to move the fused section of the back,
diminishing the patient's
range of motion and causing stress on the discs and facet joints of adjacent
vertebral
segments.
3
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100111 While laminectomy, facetectomy, discectoiny, and spinal fusion
frequently improve
symptoms of neural and ncui-ovascular impingement in the short term, these
procedures ai-e
highly invasive, diminish spinal function, drastically disrupt nonnal anatomy,
and increase
long-tenn morbidity above levels seen in untreated patients.
100121 Therefore, it would be desirable to have less invasive methods and
devices for
addressing neural and neurovascular impingement in a spine. Ideally, methods
and devices
for addressing impingement in spine would treat one or more target tissues
while preventing
unwanted effects on adjacent or nearby non-target tissues. Also ideally, such
methods and
devices would be minimally invasive and reduce impingement without removing
significant
amounts of vertebral bone, joint, or other spinal support structures, thereby
avoiding the need
for spinal fusion and, ideally, reducing the long-tenn morbidity levels
resulting from
currently available surgical treatinents. It may also be advantageous to have
less invasive
methods and devices for modifying target tissues in parts of the body other
than the spine
while preventing modification of non-target tissues. At least some of these
objectives will be
inet by the present invention.
100131 Description of Background Art. Flexible wire saws and chain saws, such
as
threadwire saws (T-saws) and Gigli saws, have been used since the late 1800s
to saw through
bone and other tissue in the human body. See, for example, Brunori A et al.,
"Celebrating the
Centenial (1894-1994): Leonardo Gigli and His Wire Saw," J Neurosurg 82:1086-
1090,
1995. An example of one such saw is described in U.S. Patent No. 8250, issued
to P. A.
Stohlmann on November 28, 1876. A description of using a T-saw to cut
vertebral bone is
provided in Kawahara N et al., "Recapping T-Saw Laminoplasty for Spinal Cord
Tumors,"
SPINE Volume 24, Number 13, pp. 1363-1370.
100141 Methods and apparatus for treating spinal stenosis are described, for
example, in
U.S. Patent Application Publication Nos. 2006/0264994 and 2004/0122459 and in
PCT
Patent Application Pub. No. WO 01/08571. A surgical instrument for removing
cartilage
from a knee cavity is described in U.S. Patent No. 3,835,859.
BRIEF SUMMARY OF THE INVENTION
[0015] In various embodiments, the present invention provides methods,
apparatus and
systeins for modifying tissue in a patient. Generally, the methods, apparatus
and systems
may involve using an elongate, at least partially flexible tissue modification
device having
4
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
one or niore tissue modification membei-s to modify one or more target
tissues. The tissue
modification device may be configured such that when the tissue inodification
member (or
members) is in a position for modifying target tissue, one or more sides,
surfaces or portions
of the tissue modification device configui-ed to avoid or prevent damage to
non-target tissue
will face non-tai-get tissue. In various embodiments, during a tissue
modification pi-ocedure,
an anchoring and/or tensioning force may be applied at or near either a distal
portion or a
proximal portion of the tissue modification device, either inside or outside
the patient.
Pulling or tensioning force may also be applied to the opposite end of the
device to urge the
tissue modifying member(s) against target tissue. The tissue modifying members
may then
be activated to modify tissue while being prevented from extending
significantly beyond the
target tissue in a proximal or distal direction. In some embodiments, the
tissue modifying
members may be generally disposed along a length of the tissue modification
device that
approxiinates a length of target tissue to be modified.
100161 By "applying an anchoring force," it is meant that a force is applied
to maintain a
portion of a device, or the device as a whole, substantially stable or motion-
free. Applying an
anchoring force is, therefore, not limited to preventing all movement of a
device, and in fact,
a device to which an anchoring force is applied inay actually move in one or
more directions
in some embodiments. In other embodiinents, an anchoring force is applied to
maintain a
portion of a device substantially stable, while another portion of the device
is allowed to
move more freely. As will be described in further detail below, applying an
anchoring force
in one einbodiment involves a usei- of a device grasping the device at or near
one of its ends.
In other embodiments, devices may use one or more anchoring members to apply
an
anchoring force. In a number of embodiments, an anchoring force may be applied
with or
against one or more tissues of a patient's body, and the tissue(s) may often
move even as they
apply (or help apply) the force. Thus, again, applying an anchoring force to a
device does not
necessarily mean that all motion of the device is eliminated. Of course, in
some
embodiinents, it may be possible and desirable to eliminate all movement or
substantially all
movement of a device (or portion of a device), and in some embodiments
anchoring force
may be used to do so.
100171 Methods, apparatus and systems of aspects of the present invention
generally
provide for tissue modification while preventing unwanted modification of, or
damage to,
surrounding tissues. Tensioning the tissue modification device by applying
anchoring force
at or near one end and applying tensioning or pulling force at or near the
opposite end may
5
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
enhance the ability of tissue modification members of the device to work
effectively within a
Iimited ti-eatment space. Applying tensioning force to a predominantly
flexible device may
also allow the device to have a relatively small profile, thus facilitating
its use in less invasive
procedures and in othei- procedures in which alternative approaches to target
tissue may be
advantageous.
100181 In the present application, "modifying tissue- or "tissue modification"
may include
any suitable modification to tissue, such as but not limited to cutting,
removing, abrading,
shrinking, burning, ablating, melting, cooling, heating, freezing,
administering medication to,
polishing, stenting or moving tissue. In some embodiments, the described
methods,
apparatus and systems may be used to inodify tissue in a spine, such as for ti-
eating neural
impingement, neurovascular impingement and/or spinal stenosis. In alternative
embodiments, target tissues in other parts of the body may be modified.
100191 In one aspect of the present invention, apparatus for inodifying one or
more tissues
in a patient inay include: an elongate, at least partially flexible body
having a proximal
portion and a distal portion; a tissue modifying meinber disposed along one
side of the body
for a limited length approximating a length of a target tissue to be treated;
an atraurnatic
surface located adjacent the tissue modifying member to face non-target
tissue; an actuator
coupled with the tissue modifying member and extending to the proximal portion
of the body
to activate the tissue modifying member without significantly translating the
elongate body
proximally or distally; and means at or near the proximal and distal portions
of the elongate
body for facilitating application of at least one of tensioning or anchoring
force to urge the
tissue modifying member against the target tissue. In some embodiments, a
flexible portion
of the elongate body may be configured to extend through an intervertebral
foramen of the
patient's spine while the proximal and distal portions of the device extend
out of the patient,
and the tissue modifying member may be configured to remove soft tissue and/or
bone to
treat or alleviate spinal stenosis.
100201 In some embodiments, the elongate body may have a width of not more
than 5 mm
at any point along its length. In some embodiments, the elongate body inay
include at least
one of a guidewire connector, a guidewire lumen, a rail, a track, or a
lengthwise impression
along which the device may be passed over or pulled behind a delivery device.
In some
embodiments, the tissue modifying member may be disposed along a length of the
body
measuring no longer than 3 cm.
6
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100211 Examples of tissue modifying members that may be included in the device
include
but are not limited to a rongeur, a curette, a scalpel, one or more cutting
blades, a scissors, a
forceps, a probe, a rasp, a file, an abrasive element, one or more small
planes, an
electrosui-gical device, a bipolar electrode, a unipolar electrode, a thermal
electrode, a rotary
powered mechanical shaver, a reciprocating powei-ed mechanical shaver, a
powered
mechanical burr, a laser, an ultrasound crystal, a cryogenic probe, and a
pressurized water jet.
In one embodiinent, the tissue modifying member may coinprise two opposing
blades, at
least one of which may be nioved to bring the blades together to cut target
tissue. In some
embodiments, the tissue modifying member may be mobile relative to the
elongate body,
while in alternative embodiments, the tissue modifying member may be
predominantly static
relative to the elongate body. In some embodiments, the tissue modifying
member may be
deployable out of a window on the elongate body.
100221 In some embodiments, the force application means may comprise proximal
and
distal handles configured to facilitate application of force from outside the
patient, and at
least one of the proximal or distal handles may be removably attachable to the
elongate body.
Some embodiments may optionally further include at least one shield member
removably
couplable with the elongate body to protect the non-target tissue froin damage
during a tissue
modification procedure. Some embodiments may optionally further include a
tissue
collection chamber housed in or coupled with the elongate body.
10023J In another aspect of the present invention, a system for modifying one
or more
tissues in a spine of a patient may include tissue modification apparatus, at
least one
guidewire, and instructions for use. The tissue modification apparatus may
include: an
elongate, at least partially flexible body having a proximal portion and a
distal portion; a
tissue modifying member disposed along one side of the body for a limited
length
approximating a length of a target tissue to be treated; an atraumatic surface
located adjacent
the tissue modifying member to face non-target tissue; an actuator coupled
with the tissue
modifying member and extending to the proximal portion of the body to activate
the tissue
modifying member without significantly translating the elongate body
proxiinally or distally;
and means at or near the proximal and distal portions of the elongate body for
facilitating
application of tensioning and/or anchoring force to urge the tissue modifying
member against
the target tissue.
7
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100241 In some embodiments, the system may include multiple tissue
modification devices.
In some embodiments, the system may further include a dilator and an
introducer sheath
through which the tissue modification device is advanced into the patient.
100251 In another aspect of the present invention, a device for modifying
tissue in a spine
may include: a shaft having a proximal portion and a distal portion, the
distal portion having
dimensions which allow it to be passed into an epidural space of the spine and
between target
and non-tai-get tissues; a distal force application member extending from the
distal portion of
the shaft and configured to facilitate application of at least one of
anchoring force or
tensioning force to the shaft; a movable tissue modifying member coupled with
the shaft at or
near its distal portion; a drive member coupled with the tissue modifying
member; and a
power transmission inember coupled with the drive member. In some
einbodiments, a
flexible portion of the distal portion of the shaft inay be configured to
extend through an
intervertebral foramen of the patient's spine while the proximal portion of
the shaft and the
distal force application inember extend out of the patient. Also in soine
embodiments, the
tissue modifying member is configured to remove at least one of soft tissue or
bone to treat or
alleviate spinal stenosis
[0026] Some embodiinents may further include a proximal force application
member
coupled with the shaft at or near the proximal portion and configured to
facilitate application
of at least one of anchoring or tensioning force to the shaft. In some
einbodiments, for
example, the proximal force application member may comprise a handle. Such an
embodiment may optionally further include an actuator coupled with the handle
for activating
the at least one drive member.
10027] In some embodiments, the distal portion of the shaft may have a width
of not more
than 7 mm. In some embodiments, the distal portion of the shaft may have a
height of not
more than 2 mm. In some embodiments, the distal force application member may
comprise a
distal extension of the shaft. Optionally, the distal force application member
may further
comprise a removable distal handle.
[0028] In some embodiments, the shaft may further comprise a window on the
distal
portion, and the tissue modifying member may modify tissue through the window.
Some
embodiments may optionally include a visualization member disposed at or near
the distal
portion of the shaft for facilitating visualization of a tissue to be
modified. Some
embodiments may further include at least one lumen extending through the
shaft, such as but
8
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
not limited to an irrigation fluid lumen, a suction lumen, a combined
irrigation/suction lumen,
a guidewire lumen, a fiber optic lumen, a lumen for passage of one or more
visualization
devices, a lumen for passage of the power transmission member(s) and/or a
lumen for
passage of one oi- more steering members.
100291 In various alternative embodiments, the power transmission member may
comprise
one or more radiofrequency, ultrasound, laser, microwave, water, thermal
and/or cryogenic
power transmission members. In some embodiments, the distal portion of the
shaft may
include a tissue protective surface disposed adjacent the tissue modifying
member for
preventing unwanted damage to non-target tissue during a tissue modification
procedure.
Some embodiments may further include at least one barrier device slidably
coupled with the
shaft for preventing unwanted damage to non-target tissue during a tissue
modification
procedure. Additionally, some embodiments may include at least one electrode
coupled with
the tissue protective surface or the barrier device to stimulate tissue in
contact with the tissue
protective surface or the barrier device. In some embodiments, the shaft may
inlcude a
hollow chamber at or near its distal end for collecting removed tissue.
100301 In another aspect of the present invention, a device for modifying
tissue in a patient
may include: an elongate, at least partially flexible body having a proximal
portion and a
distal portion, the distal portion having dimensions which allow it to be
passed between target
and non-target tissues in the patient; proximal and distal force application
meinbers coupled
with the proximal and distal portions of the elongate body and configured to
facilitate
application of at least one of anchoring force and tensioning force to the
elongate body; a
movable tissue modifying member coupled with the elongate body; a drive member
coupled
with the tissue modifying member; and a power transmission member coupled with
the drive
member. In some embodiments, the dimensions of the elongate body may allow a
flexible
portion of the body to extend through an intervertebral foramen of the
patient's spine while
the proximal and distal portions of the device extend out of the patient. Also
in some
embodiments, the tissue modifying member may be configured to remove at least
one of soft
tissue or bone to treat or alleviate spinal stenosis.
100311 In some embodiments, the proxiinal and distal force application
meinbers may each
comprise a handle, and at least one of the handles may be removable. Some
embodiments
may further include an actuator coupled with one of the handles for activating
the drive
9
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
member. In some embodiments, the dimensions of the elongate body allow at
least part of
the body to be passed through an intei-vertebral foramen of the patient's
spine.
100321 In another aspect of the present invention, a barrier device for
preventing unwanted
damage to tissue of a patient during a tissue modification procedure may
include: at least one
shape changing portion changeable from a collapsed configuration, to
facilitate passage into
the patient, to an expanded configuration, to facilitate protection of non-
target tissue; at least
one elongate portion extending from the shape changing portion and having a
low profile to
facilitate passage of the barrier device into the patient and a length
sufficient to extend from
an opening on the patient's skin to an area at or near target and non-target
tissues; and at least
one guide feature extending along at least a portion of the barrier to allow
the barrier to be
passed into the patient over at least one guide meinber. Generally, the
barrier device inay
have an overall length sufficient to pass from a first opening on the
patient's skin and
between the target and non-target tissues.
[0033] In some embodiments, the device may have an overall length, size and
configuration to allow it to extend froin the first opening on the patient's
skin, into an
epidural space of the patient's spine, and between target and non-target
tissues in the spine.
ln some embodiments, the overall length of the device may be sufficient to
allow it to further
extend from outside the patient, through the first opening, between the target
and non-target
tissues, and out the patient through a second opening on the patient's skin.
100341 In some embodiments, the at least one elongate portion may include a
proximal
elongate portion extending from a proximal end of the shape changing portion
and a distal
elongate portion extending from a distal end of the shape changing portion. In
some
embodiments, the device may further include a barrier delivery sheath through
which the
barrier device may be passed into the patient. In some einbodiments, the shape
changing
portion may change shape automatically when released from the barrier delivery
device. In
other embodiments, the device may further include at least one actuator
coupled with the
shape changing portion to change it from its collapsed to its expanded
configuration.
[0035] In some embodiments, the at least one guide feature is selected from
the group
consisting of guidewire lumens, split lumens, rails, tracks and lengthwise
impressions. In
some embodiments, at least one guide feature is configured to facilitate
guidance of one or
more tissue modification devices along the barrier member. Some embodiments
may further
include at least one conductive electrode coupled with the barrier device for
delivering
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
electi-ic current to at least one of the target tissue or non-target tissue.
The device may
optionally further include at least one monitoring device for inonitoring an
effect of the
electric current on the tissue, the monitoring device selected from the group
consisting of
electi-omyogr-aphy (EMG) inonitoring devices and somatosensory evoked
potential (SSEP)
monitoring devices.
100361 In some embodiments, the shape changing portion of the barrier device
may include
a front surface for facing a tissue modification device for performing a
procedure in the spine
and a back surface for facing non-target tissue. In some embodiments, the
shape changing
portion may be selected from the group consisting of an expandable scaffold, a
hydrogel
material, a wire mesh, an expandable stent and an inflatable bladder.
10037] In another aspect of the present invention, a device for modifying
tissue in a spine
of a patient to treat or alleviate spinal stenosis may include: an elongate,
at least partially
flexible body having a proximal portion and a distal portion; an abrasive
surface disposed
along a portion of one side of the elongate body; a non-abrasive surface
located adjacent the
abrasive surface so as to face non-target tissue when the abrasive surface is
positioned to face
tai-get tissue; a proximal tensioning member coupled with the elongate body at
or near the
proximal portion for facilitating application of tensioning force to, and
translation of, the
elongate body; and a distal tensioning member, coupled with the elongate body
at or near the
distal portion and not directly connected to the proximal tensioning member,
for facilitating
application of tensioning force to, and translation of, the elongate body.
100381 In one embodiment, the elongate body may have a width of not more than
5 mm and
a height of not more than 2 mm. In some embodiments, the elongate body may
include a
guidewire connector, a guidewire lumen, a rail, a track, and/or a lengthwise
impression along
which the device may be passed over or pulled behind a delivery device. In
some
embodiinents, each of the proximal and distal tensioning members may coinprise
a handle,
and at least one of the handles may be removably attachable to the elongate
body. In another
embodiinent, at least one of the proximal and distal tensioning members may be
deployable
from within the elongate body.
100391 Some embodiments may further include at least one shield member coupled
with
the elongate body to protect the non-target tissue from damage during a tissue
modification
procedure. In some embodiments, the shield member may include at least one
anchor for
anchoring the shield member outside the patient. In some embodiments, the
anchor may
11
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
include pi-oximal and distal anchors that may be removably couplable with the
shield member
at or near proximal and distal portions thereof. In some embodiments, the
shield member
may include at least one window along its length, through which the abrasive
surface may be
exposed to modify target tissue. Some einbodiments may optionally furthei-
include at least
one electrode coupled with the shield member for testing positioning of the
shield member.
Optionally, some embodiments inay also include at least one electrode coupled
with the
tissue modifying device at or near at least one of the abrasive surface and
the non-abrasive
surface for testing positioning of the device. In some embodiinents, the
device may also
include at least one lumen in the elongate body for providing at least one of
suction and
irrigation.
100401 In another aspect of the present invention, a device for modifying
tissue in a spine
of a patient to treat or alleviate spinal stenosis may include: an elongate,
at least partially
flexible shield member having a proximal portion, a distal portion and at
least one opening
along its length; an elongate, at least partially flexible tissue
inodification member disposed at
least partly within the shield inember, the tissue modification member having
a proximal
portion, a distal portion, and at least one abrasive surface; at least one
proximal tensioning
member at or near the proximal portion of at least one of the shield member
and the tissue
modification member for facilitating application of tensioning force in a
first direction; and at
least one distal tensioning member at or near the distal portion of at least
one of the shield
member and the tissue modification member and not directly connected to the
proximal
tensioning member, for facilitating application of tensioning force in a
second direction.
100411 In another aspect of the present invention, a method for modifying
tissue in a patient
may involve: advancing an elongate, at least partially flexible tissue
modification device into
a patient and between target tissue and non-target tissues; positioning a
tissue modifying
member of the tissue modification device adjacent the target tissue such that
the tissue
modifying member faces the target tissue and does not face the non-target
tissue; applying
tensioning forces to the tissue modification device at or near distal and
proximal portions of
the device, to urge the tissue modifying member against the target tissue; and
modifying the
target tissue, using the tissue modifying member, while preventing the tissue
modifying
member from extending significantly beyond the target tissue toward the
proximal or distal
portion of the tissue modification device during tissue modification.
12
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100421 In some embodiments, advancing the tissue modification device may
involve
advancing a flexible portion of the device into an epidural space and through
a spinal channel
of the patient s spine, such that the proximal and distal portions of the
device extend outside
the patient. In some einbodiments, advancing the tissue modification device
may involve
advancing the flexible portion along a curved path through an intervertebral
foramen. In
some embodiments, modifying the target tissue may involve removing soft tissue
and/or bone
in the spine to treat or alleviate spinal stenosis.
100431 These and other aspects and embodiments are described more fully below
in the
Detailed Description, with reference to the attached Drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
100441 FIG. I is cross-sectional view of a spine, showing a top view of a
lumbar vertebra, a
cross-sectional view of the cauda equina, and two exiting nerve roots;
100451 FIG. 2 is a cross-sectional view of a portion of a patient's back and
spine, showing
part of a vertebra and appai-atus in place for modifying tissue according to
one embodiment
of the present invention;
100461 FIG. 3A is a perspective view of a tissue modification device according
to one
embodiment of the present invention;
100471 FIG. 3B is a perspective view of a portion of the tissue modification
device of FIG.
3A;
[0048] FIG. 3C is a top view of the portion shown in FIG. 3B;
[0049] FIG. 3D is a side view of the portion shown in FIGS. 3B and 3C;
[0050] FIGS. 3E and 3F are cross-sectional views of a portion of the tissue
modification
device taken through lines A-A and B-B, respectively, shown in FIG. 3C;
[0051] FIG. 3G is a perspective view of a portion of the tissue modification
device of
FIGS. 3B-3F, shown with a blade of the device in a closed position according
to one
embodiment of the present invention;
100521 FIG. 3H is a top view of the portion shown in FIG. 3G;
100531 FIG. 31 is a side view of the portion shown in FIGS. 3G and 3H;
13
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100541 FIG. 4A is a perspective view of a tissue modification device according
to one
embodiment of the present invention;
100551 FIG. 4B is a perspective view of a portion of the tissue modification
device of FIG.
4A;
100561 FIG. 4C is a close-up, perspective view of a portion of the tissue
niodification
device of FIGS. 4A and 4B, showing a tissue modifying member according to one
embodiment of the present invention;
10057] FIGS. 5A-5D are cross-sectional views of a spine and demonstrate a
method for
using a tissue inodification device according to one embodiment of the present
invention;
100581 FIG. 6A is a cross-sectional view of a portion of a patient's spine and
back, with
apparatus for modifying tissue in position for inodifying spinal tissue and
with a distal
portion of the apparatus anchored outside the patient according to one
embodiment of the
present invention;
100591 FIG. 6B is a ci-oss-sectional view of a portion of a patient's spine
and back, with
apparatus for modifying tissue in position for modifying spinal tissue and
with a distal
portion of the apparatus anchored inside the patient according to one
embodiment of the
present invention;
100601 FIGS. 7A-70 are cross-sectional views of a portion of a patient's spine
and back,
demonstrating a method for introducing apparatus for modifying spinal tissue
to an area in
the spine for performing the tissue modification according to one embodiment
of the present
invention;
100611 FIGS. 8A-8F are cross-sectional views of a portion of a patient's spine
and back,
demonstrating a method for introducing apparatus for modifying spinal tissue
to an area in
the spine for performing the tissue modification according to an alternative
embodiment of
the present invention;
100621 FIGS. 9A-9B are cross-sectional views of a portion of a patient's spine
and back,
demonstrating a method for introducing apparatus for modifying spinal tissue
to an area in
the spine for performing the tissue modification according to an alternative
embodiment of
the present invention;
14
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100631 FIG. l0A is a perspective view of a distal portion of an introducer
sheath according
to one embodiment of the present invention;
100641 FIGS. 10B and I OC ai-e perspective and cross-sectional views,
respectively, of a
tissue shield device according to one einbodiment of the present invention;
and
100651 FIGS. l OD and ] OE ai-e perspective and ci-oss-sectional views,
respectively, of a
tissue shield device according to an alternative embodiment of the present
invention.
100661 FIGS. I 1 A and 1 I B are cross-sectional views of a spine with a
tissue modification
device in position for modifying tissue according to various einbodiments of
the present
invention.
[0067] FIG. 12 is a cross-sectional view of a portion of a spine with a tissue
modification
device in position for modifying tissue according to an altei-native
embodiment of the present
invention.
100681 FIGS. 13A-13E are cross-sectional views of a portion of a spine with a
tissue
modification device in position for modifying tissue according to various
alternative
embodiments of the present invention.
100691 FIGS. 13F and 13G are cross-sectional views of a portion of the tissue
modification
device of FIG. 13E, through line C-C on FIG. 13E, in various configurations
according to one
embodiment of the present invention.
[0070] FIG. 14 is a cross-sectional view of a portion of a spine with a tissue
modification
device having a steerable distal portion in position for modifying tissue
according to one
embodiment of the present invention.
[0071] FIG. 15A is a cross-sectional view of a portion of a spine with a
tissue modification
device in position for modifying tissue according to one embodiment of the
present invention.
[0072] FIG. 15B is a close-up of portion D-D of FIG. 15A.
[0073] FIG. 16 is a cross-sectional view of a portion of a spine with a tissue
modification
device in position for modifying tissue according to one embodiment of the
present invention.
[0074] FIGS. 17A-17E are cross-sectional views taken through line A-A of FIG.
16
according to various alternative embodiments of the present invention.
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
[0075] FIG. 18 is a cross-sectional view taken through line B-B of FIG. 16
according to
one embodiment of the present invention.
[0076] FIGS. 19A-22 are perspective or side views of a distal portion of a
tissue
modification device according to various alternative embodiments of the
present invention.
[0077] FIGS. 23A and 23B are perspective views of a distal portion of a tissue
modification
device according to alternative embodiments of the present invention.
[0078] FIGS. 24A and 24B are side views of a distal portion of a tissue
modification device
according to one embodiment of the present invention.
[0079] FIG. 25A is a perspective view of a mesh-type barrier device deploying
from a
sheath according to one embodiment of the present invention.
[0080] FIG. 25B is a top view of the mesh-type barrier device of Fig. 25A in
its free state,
prior to loading in a sheath.
[0081] FIG. 25C is a perspective view of a flexible tab-type barrier device
deploying from
a sheath according to an alternative embodiment of the present invention.
[0082] FIG. 25D is a top view of the flexible tab-type barrier device of Fig.
25C in its free
state, prior to loading in a sheath.
[0083] FIG. 25E is a perspective view of a slit-type barrier device deploying
from a sheath
according to an alternative embodiment of the present invention.
[0084] FIG. 25F is a top view of the slit-type barrier device of Fig. 25E in
its free state,
prior to loading in a sheath.
[0085] FIG. 25G is a perspective view of a rib-type barrier device deploying
from a sheath
according to an alternative embodiment of the present invention.
[0086] FIG. 25H is a top view of the rib-type barrier device of Fig. 25G in
its free state,
prior to loading in a sheath.
[0087] FIG. 251 is a perspective view of a sheet-type barrier device deploying
from a
sheath according to an alternative embodiment of the present invention.
[0088] FIG. 25J is a top view of the sheet-type barrier device of Fig. 251 in
its free state,
prior to loading in a sheath.
16
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
100891 FIG. 25K is a perspective view of a bar-type barrier device deploying
from a sheath
according to an altei-native embodiment of the present invention.
100901 FIG. 25L is a top view of the bar-type barrier device of Fig. 25K in
its free state,
prior to loading in a sheath.
100911 FIGS. 26A and 26B are perspective views of a woven tube barrier device
in low-
profile and expanded states, respectively, according to one embodiment of the
present
invention.
100921 FIGS. 27A and 27B are perspective views of a flat woven barrier device
in low-
profile and expanded states, respectively, according to one embodiment of the
pi-esent
invention.
100931 FIGS. 28A and 28B are perspective views of a barrier device with a pull-
mechanism
in low-profile and expanded states, respectively, according to one embodiment
of the present
invention.
100941 FIG. 29A is a perspective view of a cylindrical housing for a barrier
device in an un-
deployed state according to one embodiment of the pi-esent invention.
100951 FIG. 29B is a perspective view of the cylindrical housing of Fig. 29A
and a barrier
device deployed froin the housing according to one embodiment of the present
invention.
100961 FIGS. 30A-30C are perspective views of a hydrogel material barrier
device in the
process of unrolling/expanding after exposure to a fluid according to one
embodiment of the
present invention.
[0097] FIGS. 31 A-31 C are perspective and side views of a barrier device made
from a
plurality of curved elements according to one embodiment of the present
invention.
100981 FIGS. 32A and 32B are perspective views of a barrier device and a
tissue
modification device according to one embodiment of the present invention.
[0099] FIGS. 33A and 33B are perspective views of a barrier device and a
tissue
modification device according to an alternative embodiment of the present
invention.
[0100] FIG. 34 is a perspective view of a barrier device and a tissue
modification device
according to an alternative embodiment of the present invention.
17
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
101011 FIG. 35 is a perspective view of a ban-ier device and a tissue
modification device
according to an alternative embodiment of the present invention.
101021 FIGS. 36A and 36B ai-e end-on views of a barrier device according to
alternative
embodiments of the present invention.
101031 FIGS. 37A and 37B are end-on views of a barrier device according to
alternative
embodiinents of the present invention.
101041 FIG. 38 is an end-on view of a barrier device and delivery device
according to one
embodiment of the present invention.
101051 FIG. 39 is a side view of a tissue modification rasp device, shown with
a cross-
sectional view of a spine according to one embodiment of the present
invention.
101061 FIGS. 40A-40D are perspective views of various abrasive, tissue
modifying
portions of tissue modification rasp devices, according to various
enibodiments of the present
invention.
101071 FIG. 41 is a side view of a tissue modification rasp device including a
barrier
member according to one embodiment of the present invention.
101081 FIGS. 42A and 42B are perspective and partial side views, respectively,
of a tissue
modification rasp device according to an alternative embodiment of the present
invention.
DETAILED DESCRIPTION OF THE INVENTION
101091 Methods, apparatus and systems for modifying tissue in a patient are
provided.
Although the following description and accompanying drawing figures generally
focus on
tissue modification in spine, in various alternative embodiments any of a
number of tissues in
any of a number of anatomical locations in a patient may be modified.
101101 Referring to Fig. 2, in one embodiment a tissue modification device 102
may
include an elongate body 108 having a proximal portion 107 and a distal
portion 109, a
handle 104 with an actuator 106 coupled with proximal portion 107, one or more
tissue
modifying members 110, and one or more protective surfaces 112. In various
embodiments,
some of which are described further below, modification device 102 may be
introduced into
an area for perfonning a treatment, such as a spine, using any of a number of
different
introduction methods, devices and systems. In Fig. 2, for example,
modification device 102
18
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
extends thi-ough an introducer device 114 placed through a first incision 240
on the patient's
back and into the central spinal canal. Modification devicc 102 is advanced
along a guide
member 116, which extends through introducer member 114, through the
intervertebral
foi-amen between two adjacent vertebrae (only part of one vertebra is shown in
Fig. 2), and
out a second (or "distal") incision 242 on the back. In some embodiments, as
shown, guide
member has a beveled distal tip 1 1 7 for facilitating advancement of guide
member 1 16
through tissue.
101111 Generally, tissue modification device 102 may be advanced to a position
in the
spine such that tissue modifying member I 10 faces target tissue to be
modified, such as
buckled, thickened or otherwise impinging ligamentum flavum tissue as shown in
Fig. 2.
Modification device 102 is configured such that when tissue modifying member 1
10 faces the
target tissue, protective surface(s) 112 face non-target tissue. Protective
surface 112 may be
simply a length of elongate body 108 or may have one or more protective
features, such as a
widened diameter, protective or lubricious coating, extendable barrier, drug-
eluting coating
1 5 or ports, or the like. In some instances, protective surface(s) 1 12 may
act as "non-tissue-
modifying" surfaces, in that they may not substantially modify the non-target
tissue. In
alternative embodiments, protective surface(s) 112 may affect non-target
tissue by protecting
it in some active way, such as by administering one or more protective drugs,
applying one or
more forms of energy, providing a physical barrier, or the like.
[01121 In some embodiments, once tissue modification device 102 is positioned
such that
tissue modifying member 110 faces target tissue and protective surface 112
faces non-target
tissue, an anchoring force may be applied at or near distal portion 109 of
elongate body 108,
either inside or outside the patient's body. A tensioning force may also be
applied at or near
proximal portion 107 of elongate body 108, such as by pulling on handle 104
(one-directional
arrows), and actuator 106 may be used (two-headed arrow) to activate tissue
modifying
member(s) 110 to modify target tissue. In the example shown, anchoring force
is applied
near distal portion 109 by a user's hand 244, and handle 104 is pulled
proximally (arrows) to
apply tensioning force. In an alternative embodiment, hand 244 may grasp guide
member
116 at or near its distal portion 117 and thus apply anchoring force to it,
thus also applying
anchoring force to elongate body 108. In one variation of such an embodiment,
elongate
body 108 or handle 104 may optionally be adjustably clamped to guide member
116 to
further enhance or facilitate application of anchoring force to elongate body
108. Tissue
modification via tissue modifying members 110 may include cutting, ablating,
dissecting,
19
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
i-epairing, reducing blood flow in, shrinking, shaving, burring, biting,
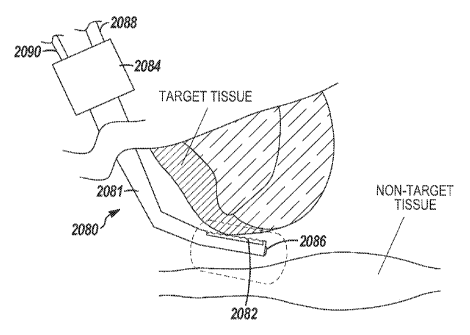
remodeling, biopsying,
debricling, lysing, debulking, sanding, filing, planing, heating, cooling,
vaporizing, delivering
a drug to, and/or retracting the target tissue. Once tissue has been modified,
tissue
modification device 102 and any introducer devices 1 14, guide members 116 or
other devices
may be removed from the patient.
101131 In various embodiments of the apparatus, tissue modifying member(s) 1
10 may be
disposed along any suitable length of body 108. In one embodiment, for
example, such as an
embodiment of the device to be used in a spinal treatment, tissue modifying
members 110
may be disposed along a length of the device measuring no longer than 10 cin,
and preferably
no more than 6 cm, and even more preferably no more than 3 cm. In various
embodiments,
tissue modifying member(s) 1 10 may include a rongeur, a curette, a scalpel,
one or more
cutting blades, a scissors, a forceps, a probe, a rasp, a file, an abrasive
eleinent, one or more
small planes, an electrosurgical device, a bipolar electrode, a unipolar
electrode, a thennal
electrode, a rotary powered mechanical shaver, a reciprocating powered
inechanical shaver, a
powered mechanical burr, a laser, an ultrasound crystal, a cryogenic probe, a
pressurized
water jet, a drug dispensing element, a needle, a needle electrode, or some
combination
thereof. In various embodiments, all tissue modifying members 1 10 may be
mobile relative
to the elongate body, all may be static, or some may be mobile and some may be
static.
These and other aspects and embodiments are described further below.
101141 Turning now to Fig. 3A-31, more detailed figures of one embodiment of
tissue
modification device 102 are shown. Referring to Fig. 3A, tissue modification
device 102
may include elongate body 108 having proximal portion 107 and distal portion
109, a
window 111 disposed along elongate body 108, two tissue modifying blades 110
exposed
through window I 11, and handle 104 with actuator 106 coupled with proximal
portion 107.
In the embodiment shown, the tissue modifying members comprise blades 110,
although in
alternative embodiments other tissue modifying members may be added or
substituted.
101151 In various embodiments, elongate body 108 may have any number of
dimensions,
shapes, profiles and amounts of flexibility. For example, distal portion 109
is shown having a
curved shape to demonstrate that at least a portion of elongate body 108 may
be flexible. In
various embodiments, elongate body 108 may have one or more of a round, ovoid,
ellipsoid,
flat, cambered flat, rectangular, square, triangular, symmetric or asymmetric
cross-sectional
shape. As shown in Figs. 3C and 3D, in the pictured embodiment, elongate body
108 has a
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
relatively flat configuration, which may facilitate placement of body 108
between target and
non-target tissues. Distal portion 109 of body 108 inay be tapered, to
facilitate its passage
into or through nai-row spaces as well as through small incisions on a
patient's skin. Body
108 may also include a slightly widened portion around the ai-ea of window I 1
1 and blades.
In one embodiment, such as an embodiment used for modifying tissue in a spine,
body 108
may have a sinall profile, such as having a height of not more than 10 min at
any point along
its length and a width of not inore than 20 mm at any point along its length,
or more
preferably a height not more than 5 mm at any point along its length and a
width of not more
than 10 mm at any point along its length, or even more preferably a height not
more than 2
mm at any point along its length and a width of not more than 4 mm at any
point along its
length. Body 108 may be long enough to extend through a first incision on a
patient, between
target and non-target tissue, and out a second incision on a patient.
Alternatively, body 108
may be long enough to extend thr-ough a first incision, between the target and
non-target
tissue, and to an anchoring location within the patient. In another
alternative embodiment,
body 108 may be long enough to extend through a first incision, between the
target and non-
target tissue, to a location nearby but distal to the target tissue within the
patient, with some
portion of tissue modification device 102 anchored to guide member 116. In
some
embodiments, elongate body 108 includes at least one feature for allowing
passage of the
body over a guidewire or other guide member or to allow passage of one or more
guide
members over or through body 108. For example, in various embodiments body 108
may
include one or more guidewire lumens, rails, tracks, lengthwise impressions or
some
combination thereof.
101161 In one embodiment, elongate body 108 is predominantly flexible along
its length
and comprises any suitable flexible material, such as thin, flexible metals,
plastics, fabrics or
the like. In some embodiments, it may be advantageous to include one or more
rigid sections
in elongate body 108, such as to impart pushability to a portion of body 108
or to facilitate
application of force to tissue modification members 110 without causing
unwanted bending
or kinking of elongate body 108. In such embodiments, rigidity may be
conferred by using
additional materials in body 108 or by making the rigid portions thicker or
wider or of a
different shape.
[0117] Handle 104 may have any suitable configuration according to various
embodiments.
Similarly, actuator 106 may include any of a number of actuation devices in
various
embodiinents. In the einbodiment shown in Fig. 3A, actuator 106 comprises a
trigger or
21
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
moving handle portion, which is grasped by a user and pulled or squeezed
toward handle 104
to bring blades 1 10 togethei- to cut tissue. In an alternative embodiment,
actuator 106 instead
may include a switch or button for activating a radiofrequency surgical
ablation tissue
modifying member. In yet another embodiment, actuator 106 may include a
combination
trigger and switch, one or mot-e pull wires, any suitable fonn of lever and/or
some
coinbination thereof.
101181 Figs. 3B-3D show in greater detail a portion of tissue modification
device 102. In
these figures, window 1 1 l and blades 1 10 are more clearly seen. In one
einbodiment, at least
a portion of elongate body 108 and blades l 10 may have a slightly curved
configuration. In
alternative embodiments, at least a portion of elongate body 108 and blades
110 may be flat.
In other alternative einbodinients, tissue inodification members such as
blades 1 10 may be
proud to elongate body 108.
101191 Blades 1 10 include a distal I l0a and a proximal blade I l Ob that
reside at the distal
and proximal edges, respectively, of window 1 11 of elongate body 108. Window
I 1 1 of
body 108 may accominodate both soft and hard tissue when the device is
forcibly applied to
the surface of a target tissue site. The top view of the distal portion of
elongate body 108,
shown in Fig. 3C, depicts the angled edges of distal blade 110a and proximal
blade 110b,
which facilitate shearing of target tissue. In alternative embodiments, blades
110 may have
any of a number of alternative shapes and configurations. The distal portion
of body 108
may have a very low profile (height compared to width), as shown in side view
Fig. 3D,
where only blades 110 protrude from the top surface of the elongate body 108.
In one
embodiment, also as shown in Fig. 3D, a guidewire tube 120 (or lumen) may
extend from (or
be coupled with) a lower surface of elongate body 108. The lower surface of
elongate body
108 is an example of a protective or non-tissue-modifying surface.
101201 In one embodiment, distal blade 110a is coupled with two pull-wires
118, as seen in
Figs. 3C, 3E and 3F. Pull-wires 118 coupled to and translated by actuator 106
on handle 104
may be used to drive distal blade 1 l Oa proximally to contact the cutting
edge of proximal
blade 110b, thus cutting tissue. Other alternative mechanisms for driving
blades 110, such as
gears, ribbons or belts, magnets, electrically powered, shape memory alloy,
electro magnetic
solenoids and/or the like, coupled to suitable actuators, may be used in
alternative
embodiments. As mentioned, in one embodiment distal blade II 0a and/or
proximal blade
110b may have an outwardly curvilinear shape along its cutting edge.
Alternatively, distal
22
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
blade I l0a may have a different blade shape, including flat, rectilineai-, v-
shaped, and
inwardly curvilinear (concave vs. convex). The cutting edge of either blade
110 may have a
sharp edge formed by a simple bevel or chamfer. Alternatively or in addition,
a cutting edge
may have tooth-like elements that interlock with a cutting edge of an opposing
blade, or may
have corrugated ridges, serrations, rasp-like features, or the like. In
various embodiments,
both blades l 10 may be of equal sharpness, or alternatively one blade 1 10
may be sharp and
the othei- substantially flat to provide a sui-face against which the sharp
blade 1 10 may cut.
Alternately or in addition, both cutting edges may be equally hard, or a first
cutting edge may
be harder than a second, the latter of which deflects under force from the
first harder edge to
facilitate shearing of the target tissue.
101211 Figs. 3E and 3F show cross-sectional views through elongate body at
lines A-A and
B-B, respectively, of Fig. 3C. In some embodiments, all or a portion of
elongate body 108,
such as the lower surface shown in Fig. 3E, may include a lubricious surface
for facilitating
manipulation of the too] in the surgical space and at the anatomical site. The
lubricious lower
surface also provides a barrier between blades 1 10 and non-target tissue in
the surgical space.
The lower surface may include a guide member lumen 120 to accommodate a
guidewire or
other access device or rail. Fig. 3E shows distal blade l 10 coupled with pull
wires 118. Fig.
3F shows proximal blade 110b, which is not coupled with pull wires 118 but
rather fixed to
body 108. In various alternative embodiments, proximal blade 110b may be
movable distally
while distal blade 110a is static, both blades may be moved toward one
another, or a different
number of blades may be used, such as one blade drawn toward a backstop or
more than two
blades, one or more of which may be mobile. In various alternative
embodiments, guide
member lumen 120 may be accommodated on a side surface or more centrally
within
elongate body 108. In further alternative embodiments, the one or more guide
member
lumens 120 may comprise one or more various cross sectional shapes, for
example
substantially round, substantially oval, or substantially rectabular, to
accommodate alternative
guide members, for example flat or rectangular guidewires, needles or rails.
In still other
alternative embodiments guide member lumen 120 may be adjustably coupled with
the
elongate body 108 to enable manipulation of the location of the elongate body
108 and
therefore the tissue modifying members 110 relative to the guiding member.
[01221 Referring now to Figs. 3G-3I, blades 110 are shown in their closed
position. In one
embodiment, when distal blade I l0a is drawn proximally to cut tissue, at
least some of the
cut tissue is captured in a hollow interior portion of elongate body 108.
Various
23
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
embodiments may further include a cover, a cut tissue housing portion and/or
the like for
collecting cut tissue and/or other tissue debris. Such collected tissue and
debi-is inay then be
removed from the patient during or after a tissue modification procedure.
During a given
tissue inodification procedure, distal blade II 0a may be drawn proximally to
cut tissue,
allowed to retract distally, and drawn proxinially again to further cut tissue
as many times as
desired to achieve a desired amount of tissue cutting.
101231 Blades l 10 may be made from any suitable metal, polyinei-, cei-amic,
or combination
thereof. Suitable metals, for example, may include but are not limited to
stainless steel (303,
304, 316, 316L), nickel-titanium alloy, tungsten carbide alloy, or cobalt-
chromium alloy, for
example, Elgiloy 1z (Elgin Specialty Metals, Elgin, IL, USA), Conichrome
(Carpenter
Technology, Reading, PA,USA), or Phynox (Imphy SA, Paris, France). In some
embodiments, materials for the blades or for portions or coatings of the
blades may be chosen
for their electrically conductive or thermally resistive properties. Suitable
polymers include
but are not limited to nylon, polyester, Dacron , polyethylene, acetal, Delrin
(DuPont,
Wilmington, DE ), polycarbonate, nylon, polyetheretherketone (PEEK), and
polyetherketoneketone (PEKK). In some embodiments, polymers may be glass-
filled to add
sti-ength and stiffness. Ceramics may include but are not limited to aluminas,
zirconias, and
carbides. In various embodiments, blades 110 may be manufactured using metal
injection
molding (MIM), CNC machining, injection molding, grinding and/or the like.
Pull wires 118
be made from metal or polyiner and may have circular, oval, rectangular,
square or braided
cross-sections. In some embodiments, a diameter of a pull wire 118 may range
from about
.001" - .050", and more preferably from about .010"-.020".
[0124] Depending on the tissue to be treated or modified, activating blades l
10 (or other
tissue modifying members in alternative embodiments) may cause them to modify
target
tissue along an area having any of a number of suitable lengths. In use, it
may also be
advantageous to limit the extent of action of blades 110 or other tissue
modifying members to
a desired length of tissue, thus not allowing blades 110 to affect tissue
beyond that length. In
so limiting the effect of blades, unwanted modification of, or damage to,
surrounding tissues
and structures may be limited or even eliminated. In one embodiment, for
example, where
the tissue modification device is used to modify tissue in a spine, blades 110
may operate
along a length of target tissue of no more than 10 cm, and preferably no more
than 6 cm, and
even more preferably no more than 3 cm. Of course, in other parts of the body
and to address
other tissues, different tissue modification devices may be used and tissue
modifying
24
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
meinbers may have many different lengths of activity. In one embodiment, to
facilitate
proper location of tissue modifying members, such as blades 1 10, relative to
tai-get tissue, the
tissue modifying members and/or the elongate body and/or one or more
additional features
intended for just such a purpose may be composed of a material i-eadily
identifiable via x-ray,
fluoroscopic, magnetic resonance or ultrasound imaging techniques.
101251 In various embodiinents, a number of different techniques may be used
to prevent
blades 1 10 (or other tissue modifying members) from extending significantly
beyond the
target tissue. In one embodiment, for example, preventing blades 110 from
extending
significantly beyond the target tissue involves holding tissue inodification
device 102 as a
whole predominantly stable to prevent device 102 from translating in a
direction toward its
proximal portion or toward its distal portion while activating blades 110.
Holding device 102
stable is achieved by anchoring one end of the device and applying tensioning
force at or near
the other end, as described further below.
101261 In the embodiment shown in Figs. 3A-31, pull wires 1 18 are retracted
proximally by
squeezing actuator 106 proximally. In an alternative embodiment, squeezing
actuator 106
may cause both blades 1 10 to translate inward so that they meet approximately
in the middle
of window 1 1 1 . In a further embodiment, distal blade II 0a may be returned
to it's starting
position by a pulling force generated from the distal end of device 102, for
example by using
a distal actuator that is attached to distal wires, or by pulling on the
distal guide inember
which is attached to distal blade 1 10a. In yet another alternative
embodiment, proximal blade
I l Ob may be moved to cut by a pulling force generated from the distal end of
device 102, for
example by using a distal actuator that is attached to distal wires, or by
pulling on the distal
guide 1nember which is attached to proximal blade 110b. In yet another
embodiment,
squeezing actuator 106 may cause proximal blade 110b to move distally while
distal blade
110a stays fixed. In other alternative embodiments, one or more blades 110 may
move side-
to-side, one or more blades 110 may pop, slide or bow up out of window I 11
when activated,
or one or more blades 110 may expand through window. In another embodiment,
one or more
blades 110 and/or other tissue modifying members of device 102 may be powered
devices
configured to cut, shave, grind, abrade and/or resect target tissue. In other
embodiments, one
or more blades may be coupled with an energy transmission device, such as a
radiofrequency
(RF) or thermal resistive device, to provide energy to blade(s) 110 for
cutting, ablating,
shrinking, dissecting, coagulating or heating and thus enhancing tissue
modification. In
another embodiment, a rasp or file may be used in conjunction with or coupled
with one or
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
niore blades. In any of these embodiinents, use of actuator 106 and one or
more moving
blades 1 10 provides for tissue modification with relatively little overall
translation or other
movement of tissue inodification device 102. Thus, target tissue may be
modified without
extending blades 1 10 or other tissue modification members significantly
beyond an area of
target tissue to be treated.
101271 Referring now to Figs. 4A-4C, in an alternative embodiment, a tissue
modification
device 202 may include an elongate body 208 having a proximal portion and a
distal portion
209, a handle 204 and actuator 206 coupled with proximal portion, and a window
211 and
tissue modifying inember 210 disposed near distal portion 209. As seen more
clearly in Figs.
4B and 4C, in the embodiment shown, tissue modifying member 210 comprises an
RF
electrode wire loop. Wire loop 210 may comprise any suitable RF electi-ode,
such as those
commonly used and known in the electrosurgical arts, and may be powered by an
internal or
external RF generator, such as the RF generators provided by Gyrus Medical,
Inc. (Maple
Grove, MN). Any of a number of different ranges of radio frequency may be
used, according
to various embodiments. For example, some embodiments may use RF energy in a
range of
between about 70 hertz and about 5inegahertz. In some embodiments, the power
range for
RF energy may be between about 0.5 Watts and about 200 Watts. Additionally, in
various
embodiments, RF current may be delivered directly into conductive tissue or
may be
delivered to a conductive medium, such as saline or Lactate Ringers solution,
which inay in
some einbodiments be heated or vaporized or converted to plasma that in turn
inodifies target
tissue. Distal portion 209 includes a tapered tip, similar to that described
above, to facilitate
passage of elongate body 208 into narrow anatomical sites. Handle 204 and
actuator 206 are
similar to those described above, although in the embodiment of Figs. 4A-4C,
actuator 206
inay be used to change the diameter of the wire loop 210. Using actuator 206,
wire loop 210
may be caused to extend out of window 211, expand, retract, translate and/or
the like. Some
embodiments may optionally include a second actuator (not shown), such as a
foot switch for
activating an RF generator to delivery RF current to an electrode.
[0128] Elongate body 208 may be fabricated from any suitable material and have
any of a
number of configurations. In one embodiment, body 208 comprises a metal tube
with a full-
thickness slit (to unfold the tube into a flat form--not shown) or stiffening
element (not
shown). The split tube provides for a simple manufacturing process as well as
a conductive
pathway for bi-polar RF operation.
26
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
101291 Refen-ing to Fig. 4C, insulators 222 may be disposed around a portion
of wire loop
210 so that only a desired portion of wire loop 210 may transfer RF current
into the tissue for
tissue inodifying capability. Wire loop 210, covered with insulators 222 may
extend
proximally into support tubes 218. In various alternative embodiments, an
electrode tissue
modifying member (of which wire loop 210 is but one example) may be bipolar or
monopolar. For example, as shown in Fig. 4C, a sleeve 224 housed toward the
distal portion
of window 211 may act as a return electrode for wire loop 210 in a bipolar
device. Wire loop
electrodes 210 may be made from various conductive metals such as stainless
steel alloys,
nickel titanium alloys, titanium alloys, tungsten alloys and the like.
Insulators 222 may be
made from a thermally and electrically stable polymer, such as polyimide,
polyetheretherketone (PEEK), polytetrafluoroethylene (PTFE), polyainide-imide,
or the like,
and inay optionally be fiber reinforced or contain a braid for additional
stiffness and strength.
In alternative embodiments, insulators 222 may be coinposed of a ceramic-based
material.
101301 In one embodiment, wire loop 210 may be housed within elongate body 208
during
delivery of tissue modification device 202 into a patient, and then caused to
extend up out of
window 211, relative to the rest of body 208, to remove tissue. Wire loop 210
may also be
flexible so that it may pop or bow up out of window 211 and may deflect when
it encounters
hard tissue surfaces. Wire loop 210 may have any of a number of shapes, such
as curved,
flat, spiral or ridged. Wire loop 210 may have a diameter similar to the width
of body 208,
while in alternative embodiments it may expand when extended out of window 211
to have a
smaller or larger diameter than that of body 208. Pull wires (not shown) may
be retracted
proxiinally, in a manner similar to that described above, in order to collapse
wire loop 210,
decrease the diameter and lower the profile of the wire loop 210, and/or pull
wire loop 210
proximally to remove tissue or be housed within body 208. The low profile of
the collapsed
wire loop 210, facilitates insertion and removal of tissue modification device
202 prior to and
after tissue modification. As the wire loop 210 diameter is reduced, support
tubes 218 deflect
toward the center of elongate body 208.
[0131] In an alternative embodiment (not shown), tissue inodification device
202 may
include multiple RF wire loops 210 or other RF members. In another embodiment,
device
202 may include one or more blades as well as RF wire loop 210. In such an
embodiment,
wire loop 210 may be used to remove or otherwise modify soft tissues, such as
ligamentum
flavum, or to provide hemostasis, and blades may be used to modify hard
tissues, such as
bone. In other embodiments, as described further below, two separate tissue
modification
27
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
devices (or more than two devices) may be used in one procedure to modify
different types of
tissue, enhance modification of one type of tissue or the like.
101321 In other alternative embodiments, tissue modification devices 202 may
include
tissue modifying members such as a rongeur, a curette, a scalpel, a scissors,
a forceps, a
pi-obe, ai-asp, a file, an abrasive element, one or more small planes, a
rotary powered
mechanical shaver, a reciprocating powered mechanical shaver, a powered
mechanical burr, a
laser, an ultrasound crystal a cryogenic probe, a pressurized water jet, a
drug dispensing
element, a needle, a needle electrode, or some combination thereof. In some
embodiments,
for example, it may be advantageous to have one or more tissue modifying
members that
stabilize target tissue, such as by grasping the tissue or using tissue
restraints such as barbs,
hooks, compressive members or the like. In one embodiment, soft tissue may be
stabilized
by applying a contained, low-temperature substance (for example, in the cryo-
range of
temperatures) that hardens the tissue, thus facilitating resection of the
tissue by a blade, rasp
oi- other device. In another embodiment, one or more stiffening substances or
members may
be applied to tissue, such as bioabsorbable rods.
101331 Refen-ing now to Figs. 5A-5D, one embodiment of a method for inodifying
tissue in
a spine is demonstrated in simplified, diagraminatic, cross-sectional views of
a portion of a
patient's back and spine. Fig. 5A shows a portion of the patient's back in
cross section, with
a portion of a vertebra, the spinal cord with branching nerve roots, and
target tissue, which in
this illustration is the ligamentum flavum and possibly a portion of the facet
capsule. The
target tissue is typically impinging directly on one or more of the group
including nerve roots,
neurovascular structures, dorsal root ganglia, cauda equina, or individual
nerves.
[0134] In Fig. 513, tissue modification device 102 has been positioned in the
patient's back
to perfonn a tissue modification procedure. Various methods, devices and
systems for
introducing device 102 into the patient and advancing it to the position for
modifying tissue
are described in further detail below. Generally, device 102 may be positioned
via a
percutaneous or open surgical procedure, according to various embodiments. In
one
embodiment, device 102 may be inserted into the patient through a first
incision 240,
advanced into the spine and between target tissue and non-target tissue (such
as spinal cord,
nerve roots, nerves and/or neurovascular tissue), and further advanced so a
distal portion of
elongate body 108 exits a second (or distal) incision 242 to reside outside
the patient. In
positioning device 102, one or more tissue modifying members (not shown) are
positioned to
28
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
face the target tissue, while one or more protective portions of elongate body
108 face non-
target tissue.
101351 Referring to Fig. 5C, once device 102 is positioned in a desii-ed
location, anchoring
force may be applied at or near the distal portion of elongate body 108. In
one embodiment,
applying anchoring force involves a user 244 grasping body 108 at or near its
distal portion.
In alternative embodiments, as described further below, anchoring force may be
applied by
deploying one or inore anchor members disposed at or near the distal portion
of body 108, or
by grasping a guidewire or other guide member extending through at least part
of body 108.
Once the anchoring force is applied, proximally-directed tensioning force may
be applied to
device 102, such as by pulling proximally on handle 104 (one-directional,
diagonal arrows).
This tensioning force, when applied to the substantially anchored device 102,
may help urge
the tissue modifying member(s) against the target tissue (one-directional,
vertical arrows near
target tissue), thus enhancing contact with the target tissue and facilitating
its modification.
With the tissue modifying meinber(s) contacting the target tissue, actuator
106 may be
squeezed or pulled (two-headed arrow) to cause the tissue modifying member(s)
to modify
tissue. (Alternative actuators may be activated in different ways in
alternative embodiments.)
101361 In various alternative embodiments, certain of the above-described
steps may be
carried out in different order. For example, in one embodiment the distal
portion of elongate
body 108 may be anchored within or outside the patient before the tissue
modifying members
are positioned adjacent the target tissue. In another alternative embodiment,
the proximal
portion of device 102 may be anchored, and the tensioning force may be applied
to the distal
portion of device 102. In yet another embodiment, tensioning force may be
applied to both
ends of the device. In yet another embodiment, a second handle and actuator
may be coupled
with the distal end of body 108 after it exits the patient's back, allowing
tensioning forces as
well as tissue modifying actuation to occur at both the proximal and distal
portions of device
102. By anchoring one end of device 102 and applying tensioning force to the
opposite end,
contact of the tissue modifying members with the target tissue is enhanced,
thus reducing or
eliminating the need for translating or otherwise moving device 102 as a whole
and reducing
the overall profile and the resulting access pathway required to position the
device. Reducing
movement and profile of device 102 and using tissue modifying members confined
to a
relatively small area of device 102 helps facilitate target tissue
modification while
minimizing or eliminating damage to surrounding tissues or structures.
29
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
[0137] As mentioned above, tissue may be modified using one tissue
modification device
or multiple devices, according to various embodiments. In one embodiment, for
example, an
RF electrosurgical tissue modification device may be used in the patient to
remove soft tissue
such as liganient, and a bladed tissue modification device such as a rongeur
may then be used
to i-emove additional sott tissue, calcified soft tissue, or hard tissue such
as bone. In some
embodiments, such multiple devices may be inserted, used and removed serially,
while in
alternative embodiments such devices may be inserted into the patient at the
same time to be
used in combination.
101381 Referring to Fig. 5D, using one or more tissue modification devices
102, a desired
amount of target tissue may be removed from more than one area in the spine.
Figs. 5A-5C
demonstrate removal of target tissue on one side of the spine, and that method
or a similar
method inay also be used to remove target tissue on an opposite side of the
spine, as shown in
Fig. 5D, where target tissue has been removed from both sides. That the
desired amount of
tissue has been removed may be confirmed by tactile feedback from the device
or from a
separate device, by testing nerve conduction through one or more previously
impinged
nerves, by testing blood flow through one or more previously impinged blood
vessels, by
passing (independently or over the guide member) a measurement probe or sound
through the
treated portion, through one or more radiographic tests, through some
combination thereof, or
by any other reasonable means.
101391 Referring now to Fig. 6A, tissue modification device 102 is shown with
one
embodiment of a distal anchoring member 250 deployed at the patient's skin. In
various
embodiments, anchoring members may include but are not limited to one or more
handles,
barbs, hooks, screws, toggle bolts, needles, inflatable balloons, meshes,
stents, wires, lassos,
backstops or the like. In some embodiments, anchoring members 250 may be
disposed at the
extreme distal portion 109 of elongate body 108, while in other embodiments
anchoring
members 250 may be located more proximally. In the embodiment shown, anchoring
members 250 are deployed at the patient's skin. In an alternative embodiment,
anchoring
may be achieved outside the patient by deploying one or more anchoring members
250 above
the skin and having a user grasp the anchoring members 250. In an alternative
embodiment,
anchoring may be achieved outside the patient by deploying one or more
anchoring members
250 above the skin and having a user grasp anchoring members 250, after tissue
modification
device 102 has been anchored to the guide member. In another alternative
embodiment,
anchoring may be achieved outside the patient by attaching anchoring member
250 to an
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
external device, for example one that is mounted on the patient or on the
procedure table. In
a further alternative embodiment, anchoring may be achieved outside the
patient by attaching
the guide member to an external device, for example one that is mounted to on
the patient or
on the procedure table, after tissue modification device 102 has been anchored
to the guide
meinber. Anchoring members 250 generally are deployable from a first,
contracted
configuration to facilitate delivery of device 102, to a second, expanded
configuration to
facilitate anchoring. This change in configuration may be achieved, for
exainple, by using
shape meinory or super-elastic materials, by spring loading anchoring members
250 into
body 108 or the like. In most embodiments, anchoring members 250 may also be
collapsed
down into the first, contracted configuration after a tissue modification
procedure has been
perfonned, to facilitate withdrawal of device 102 from the patient. In an
alternative
embodiment, anchoring members 250 may detach from body 108 and may be easily
removable from the patient"s skin.
101401 Fig. 6B shows tissue modification device 102 with an alternative
embodiment of a
distal anchoring member 260. Here, distal anchoring member 260 includes
multiple hooks or
barbs extended out the distal portion 109 of elongate body 108 within the
patient's back. In
using such an embodiment, it may not be necessary to pass guide member 117
through a
second, distal incision on the patient, although in some embodiments guide
member 117 may
extend significantly beyond distal portion 109. Anchoring inember(s) 260,
according to
various embodiments, may be deployed so as to anchor to bone, ligament,
tendon, capsule,
cartilage, muscle, or any other suitable tissue of the patient. They may be
deployed into
vertebral bone or other suitable tissue immediately adjacent an intervertebral
foramen or at a
location more distant from the intervertebral foramen. When a tissue
modification procedure
is complete, anchoring members 260 are retracted within elongate body for
removal of device
102 from the patient.
101411 Referring now to Figs. 7A-70, a system and method for introducing a
tissue
modification device into a spine is demonstrated. This system and method may
be referred to
as an "access system" or "access method," in that they provide or facilitate
gaining access to
a target tissue to be modified. Of course, the embodiment shown is merely one
exemplary
embodiment, and any of a number of other suitable methods, devices or systems
may be used
to introduce one or more devices for modifying tissue in spine. For example,
in one
alternative embodiment a spinal tissue modification procedure may be carried
out through an
open surgical approach. Therefore, the following description is provided
primarily for
31
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
exemplary purposes and should not be interpreted to limit the scope of the
invention as it is
defined in the claiins.
10142] Referring to Fig. 7A, in one embodiment a device delivcry method first
involves
advancing a loss of resistance syringe 304 including a plunger 3 10, barrel
308 and fluid
and/or air 306, coupled with the proximal portion of a needle 300 (or cannula)
into the
patient's back. The distal portion of cannula 300 is advanced through the
ligamentum flavum
until it enters the central spinal canal where a loss of resistance to
pressure placed on plunger
310 is encountered, and fluid and/or air 306 is injected into central spinal
canal to confirm
correct placement of cannula 300 as shown in fig. 7B. Syringe 304 is then
removed, as in
Fig. 7C, and a guidewire 312 with a non-rigid, atraumatic tip is advanced
through cannula
300 into the central spinal canal, as in Fig. 7D. Next, cannula 300 is
removed, as in Fig. 7E,
leaving behind guidewire 312. As shown in Figs. 7F and 7G, an introducer
sheath 114,
coupled with a dilator 314, is then advanced over guidewire 312 to position a
distal portion of
sheath 1] 4 at a desired location within the spine. Dilator 314 and guidewire
312 are then
removed, as in Fig. 7H.
10143] Once introducer sheath 114 is in place, one or more curved or steerable
guide
devices 318 may be advanced through it to desired positions in and/or through
the spine, as
shown in Figs. 71 and 7J. One or more guide members 116, may then be advanced
through
the guide device 318, as shown in Figs. 7J-7L. Finally, guide device 318 may
be removed, as
in Fig. 7M, and elongate body 108 of tissue modification device 102 may be
advanced over
guide meinber 116 and through introducer sheath 114 to a desired position in
the spine, as in
Fig. 7N. As shown in Fig. 70, elongate body 108 may be tensioned to urge
tissue modifying
members I 10 against target tissue, as shown with arrows at opposite ends of
device 102,
while distal portion 109 is anchored, in this case by hand 244. In an
alternative embodiment,
guide member 116 may be tensioned to urge tissue modifying members 110 against
target
tissue as shown in Fig. 7N.
101441 Once tissue modification device 102 is in a desired position, tissues
which may be
modified in various embodiments include, but are not limited to, ligament,
tendon, tumor,
cyst, cartilage, scar, "bone spurs," inflammatory and bone tissue. In some
embodiments,
modifying the target tissue reduces impingement of the tissue on a spinal
cord, a branching
nerve or nerve root, a dorsal root ganglia, and/or vascular tissue in the
spine. Actuator 106 on
handle 104 is activated to modify target tissue using tissue modification
member(s) 110,
32
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
while elongate body 108 is held relatively stable by hand 244 and by tension
force applied to
handle 104.
101451 In various embodiments, the system and method described immediately
above may
include additional features or steps, may have fewer features or steps, may
have an alternate
order of implementation of steps, or may have different features or steps. For
example, in
some embodiments placement of device 102 will be perfonned in a medial-to-
lateral
direction (relative to the patient), while in alternative embodiments device
placement will be
performed lateral-to-medial. In some embodiments, one or more components of
the system
described may be anchored to the patient, such as guide member 116 or
introducer sheath
114. In various embodiments, one or more guide members 116 may include one or
more
wires, rails or tracks and may be inserted thi-ough guide device 318,
introducer sheath 114
without guide device 318, cannula 300, an epidural needle, a lumen of an
endoscope, a lumen
of a tissue shield or barrier device, a curved guide device 318 placed through
a lumen of an
endoscope, or the like. In other embodiments, for example, guide device 318
may be placed
through introducer cannula 300 and then introducer sheath 114 inay be passed
over guide
device 318. Tissue modification device 102 may similarly be inserted with or
without using
any of these devices or components in various combinations. Various guidewires
312, guide
devices 318 and/or guide members 116 may be pre-shaped to have one or more
curves, may
be steerable, and/or may include one or more rails, tracks, grooves, Iuinens,
slots, partial
lumens, or some combination thereof.
101461 ln some embodiments, tissue modification device 102 is inserted through
one or
more hollow devices as described above (such as introducer sheath 114, as
shown, or cannula
300 in an alternative embodiment) in such a way that device 102 expands upon
extending out
of a distal portion of the hollow delivery device thereby assuming a wider
profile for
modifying a greater amount of target tissue from a single location. In an
alternative
embodiment, device 102 retains the same overall profile during insertion and
during use. In
some embodiments, one or more delivery devices will remain in the patient
during use of
tissue modification device 102, while in alternative einbodiments all delivery
devices are
removed from the patient when tissue modification device 102 is operating. In
some
einbodiments, tissue modification device 102 may be slidably coupled with one
or more
delivery devices during delivery and/or during use. In one embodiment, tissue
modification
device 102 is advanced through introducer sheath 114 and sheath 114 is used as
an irrigation
and evacuation lumen to irrigate the area of the target tissue and evacuate
removed tissue and
33
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
other debris, typically by applying a vacuum. In alternative embodiinents,
tissue
modification device 102 may include an irrigation and/or evacuation lumen to
irrigate an area
of the target tissue and evacuate removed tissue and other debris.
101471 Some embodiments of an access system for facilitating tissue
modification may
further include one or more visualization devices (not shown). Such devices
may be used to
facilitate placement of the access system foi- introducing the tissue
modification device, to
facilitate tissue modification itself, or any combination of these functions.
Examples of
visualization devices that may be used include flexible, partially flexible,
or rigid fiber optic
scopes, rigid rod and lens endoscopes, CCD or CMOS chips at the distal portion
of rigid or
flexible probes, LED illumination, fibers or transmission of an external light
source for
illumination or the like. Such devices inay be slidably couplable with one or
more
components of an access system or inay be slidably or fixedly coupled with a
tissue
modification device. In other embodiments, additional or alternative devices
for helping
position, use or assess the effect of a tissue modification device may be
included. Examples
of other such devices may include one or more neural stimulation electrodes
with EMG or
SSEP monitoring, ultrasound imaging transducers external or internal to the
patient, a
computed tomography (CT) scanner, a magnetic resonance imaging (MRI) scanner,
a
reflectance spectrophotometry device, and a tissue impedance monitor disposed
across a
bipolar electrode tissue modification inember or disposed elsewhere on a
tissue modification
device or disposed on the access system.
101481 Referring now to Figs. 8A-8E, in an alternative embodiment, a tissue
modification
device and optionally one or more introduction/access devices may be
positioned in a patient
using an open surgical technique. As shown in Fig. 8A, for example, in one
embodiment an
open surgical incision is made on a patient's back, and two retractors 402 are
used to expose
a portion of the patient's vertebra. As shown in Fig. 8B, an introducer sheath
414 may then
be inserted through the incision, between retractors 402. As in Fig. 8C, a
curved guide device
418 may then be inserted through introducer sheath 414. Guide device 418
extends into the
epidural space and through the intervertebral foramen as shown in Fig. 8D.
101491 In some embodiments, a curved and cannulated thin, blunt probe may be
placed
directly through the open incision into the epidural space of the spine, or
alternatively may be
placed through introducer sheath 414. The probe tip may be advanced to or
through a neural
foramen. Such a probe may be similar in shape, for example, to a Woodson
elevator,
34
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
Penfield 3, hockey stick probe, ball tipped pi-obe, or the like. In
alternative embodiinents,
probes that may be manually bent to change their shapes, or probes with
articulating tips, or
probes with shape lock portions, and/or probes having grooves instead of
cannulas may be
used.
101501 As shown in Figs. 8D-8E, a substantially straight, flexible guidewire
420 with a
sharp tip 422 may then be inserted through curved guide device 418 and
advanced so that its
distal portion with sharp tip 422 extends outside the patient's back at a
location separate froin
the open incision (Fig. 8E). Guide device 418 may then be removed, as in Fig.
8F, and in
subsequent steps a tissue modification device may be inserted over guide wire
420 and
through introducer sheath 414 and used to modify tissue as described in more
detail above.
In an alternative embodiment, a curved, flexible cannula may be inserted
through the curved
guide device, until it extends lateral to the neural foramen, after which a
substantially
straight, flexible guidewire with a sharp tip may then be inserted through
curved cannula and
advanced so that its distal portion with sharp tip extends outside the
patient's back.
101511 Referring now to Figs. 9A and 9B, another alternative open surgical
access method
is shown. In Fig. 9A, a curved guide device 446 is shown in place through the
epidural space
and intervertebral foramen, and a guidewire 440 with a beveled distal tip 442
is about to be
advanced through guide device 446. As shown in Fig. 9B, in this embodiment,
guidewire
440 is directed by guide device 446 back through the open incision through
which the various
access devices are introduced. In such an embodiment, then, only one incision
is created and
the proximal and distal portions of one or more devices extend out of the
patient's back
through the same incision.
101521 In various alternative embodiments, open surgical access may be through
exposure
down to a vertebral lamina, through ligamentum flavum without lamina removal,
through
ligamentum flavum with partial or complete lamina removal, through ligamentum
flavum
with or without lamina removal with partial or complete medial facet joint
removal, through
open exposure and out through skin laterally, through open exposure and back
out through
the open exposure, or through a lateral open exposure that accesses the neural
foramen from
the lateral side. One or more visualization devices may be used with open
surgical access
procedures as well as with percutaneous or other less invasive procedures. In
another
alternative embodiment (not shown), a tissue modification device may be placed
in the
patient directly, without any introduction devices.
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
101531 Referring now to Figs. I OA-10E, in the embodiments described above,
the tissue
modification devices 102, 202 include at least one non-tissue-modifying (or
"protective")
portion, side or surface. The non-tissue-modifying portion is located on
tissue modification
device 102, 202 so as to be positioned adjacent non-tai-get tissue when tissue
modifying
members 1 10, 210 are facing the target tissue. The non-tissue-modification
surface of the
device is configured so as to not modify or damage tissue, and thus the non-
target tissue is
protected from unwanted modification or damage during a tissue modification
procedure.
[0154] Optionally, in some embodiments, tissue modification devices or systems
may
further include one or inore tissue shields or barriers for further protecting
non-target tissues.
Such shields may be slidably coupled with, fixedly coupled with, or separate
fi-om the tissue
modification devices with which they are used. In various embodiments, a
shield may be
delivered between target and non-target tissues before delivering the tissue
modification
device, may be delivered along with the tissue modification device, or may be
delivered after
delivery of the tissue modification device but before the device is activated.
Generally, a
shield will be interposed between the non-target tissue and the tissue
modification device.
101551 Fig. l0A shows a distal portion of an introducer device 514 through
which a shield
may be introduced. Figs. I OB and l OC show one embodiment of a shield device
500 partially
deployed and in cross-section, respectively. Typically, shield 500 will have a
first, small-
profile configuration for delivery to an area near non-target tissue and a
second, expanded
configuration for protecting the non target tissue. Shield itself may be
configured as one
piece of super-elastic or shape-memory material, as a scaffold with material
draped between
the scaffolding, as a series of expandable wires or tubes, as a semicircular
stent-like device,
as one or more expandable balloons or bladders, as a fan or spring-loaded
device, or as any of
a number of different devices configured to expand upon release from a
delivery device to
protect tissue. As shown in Figs. l OB and l OC, shield 500 may comprise a
sheet of material
disposed with a first end 502a abutting a second end 502b within introducer
device 514 and
unfurling upon delivery. In an alternative embodiment, as shown in Figs. I OD
and 10E,
opposite ends 522a and 522b of a shield device 520 may overlap in introducer
device 514.
Generally, shield 500, 520 may be introduced via introducer device 514 in one
embodiment
or, alternatively, may be introduced via any of the various means for
introducing the tissue
modification device, such as those described in conjunction with Figs. 7A-7S,
8A-8F and 9A-
9B. In some embodiments, shield 500, 520 may be fixedly coupled with or an
extension of a
tissue modification device. Shield 500, 520 may also include one or more
lumens, rails,
36
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
passages or the like for passing a guidewire or other guide member, for
introducing,
removing or exchanging any of a variety of tissue modification, drug delivery,
or diagnostic
devices, for passing a visualization device, for providing irrigation fluid at
the tissue
modification site, and oi- the like. In some embodiments, shield 500, 520 is
advanced over
multiple guidewires and the guidewires remain in place during a tissue
modification
procedure to enhance the stability and/or maintain positioning of shield. 300,
320.
[0156] Referring now to Figs. I lA and 1 1B, in an alternative embodiment, a
powered
tissue modification device 2000 suitably includes an elongate shaft 2001
having a proximal
portion 2002, a distal portion 2003 and a longitudinal axis 2008, one or more
tissue
modifying members 2004 coupled with shaft 2001 at or near distal portion 2003,
and a handle
2006 coupled with shaft 2001 at or near proximal portion 2002. Optionally,
soine
embodiments may also include one or more power connectors 2010 foi- connecting
device
2000 with one or more power sources. In some embodiments, shaft 2001 has a
size and
shape that facilitate passage of at least distal portion 2003 into an epidural
space of the spine
and between target tissue, such as ligamentum flavum, and non-target tissue,
such as neural
and/or neurovascular tissue. In some embodiments, shaft 2001 may include one
or more
bends or curves at or near its distal portion 2003 to further facilitate
passage and positioning
of device 2000. In some embodiments, for example, a bend or curve inay
facilitate passage
of at least part of distal portion 2003 at least partway into an
intervertebral foramen.
[0157] In some embodiments, as shown in Fig. 1 l A, distal portion 2003 of
device 2000
may be advanced through the skin of the back of a patient, adjacent a spinous
process. Distal
portion 2003 inay then be advanced between the lamina of adjacent vertebral
bodies, into the
epidural space, and between target and non-target tissues to position tissue
inodifying
member(s) 2004 in a desired location for modifying target tissue. Power may
then be
provided to activate tissue modifying member(s) 2004 and thus to modify target
tissue. A
portion of device 2000 adjacent tissue modifying member(s) 2004 may be
configured to face
non-target tissue while tissue modifying member(s) 2004 face the target
tissue, thus
preventing unwanted damage or modification of the non-target tissue. In some
embodiments,
as described more fully above, the portion of device 2000 facing the non-
target tissue may be
configured to modify the non-target tissue in some way, such as to protect the
tissue with a
delivered substance, and/or to test the non-target tissue to confirm that it
is non-target tissue.
37
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
10158] In various embodiments, handle 2006 may have any suitable configuration
and
features. In some embodiments, handle 2006 includes one or more actuators for
activating
tissue modifying member(s) 2004. Power connector 2010 may have any suitable
configuration and may deliver any suitable type of energy from an external
power source (not
shown) to device 2000 in various embodiments, such as but not limited to
electric,
radiofrequency, ultrasound, laser or conductive energy. In alternative
embodiments, device
2000 may be battery operated or use any other suitable source of internal
power or energy,
and such internal energy source may be housed in handle 2006, for example.
From whatever
source, power is typically transmitted to tissue modifying member(s) 2004 to
activate them
and thus modify tissue.
101591 With reference now to Fig. 11 B, in various embodiments, tissue
modification device
2000 may be advanced into a patient using any of a number of suitable
techniques and
approaches, some of which have been described previously. Fig. 11 A
illustrates one
approach to advancing distal portion 2003 of device 2000 to a position between
target and
non-target tissue from a contralateral approach, while Fig. 1 1 B illustrates
an ipsilateral
approach. As shown in Fig. 1 1 B, distal portion 2003 may include two or more
bends and/or
may be at least partially flexible or steerable to facilitate a desired
approach angle, according
to various embodiments.
101601 Turning to Fig. 12, in another embodiment a tissue modification device
2020
includes an elongate shaft 2011 having a proximal portion 2012 and a distal
portion 2013,
one or more tissue modification member(s) 2014, a conductive electrode 2015
coupled with
shaft 2011, a handle 2016, a power connector 2030, and multiple additional
connection
members 2032, 2033. Electrode 2015 may be configured to deliver a non-target
frequency
and non-target amplitude of electrical current to non-target tissue. The non-
target frequency
and non-target amplitude may stimulate a response from a neural tissue. In one
embodiment,
a first connection member 2032 may provide power to electrode 2015, and if non-
target-
tissue is stimulated by current from the electrode, the observation of this
stimulation, as
measured by EMG, SSEP or watching for muscular activation, provides evidence
that
electrode 2015 is adjacent the non-target tissue. A target stimulating
electric current may
also be delivered through second connection member 2033 to tissue modifying
member(s)
2014 (e.g., composed of electrically conductive material) and/or to a target
stimulating
electrode located adjacent and on the same side of device 2020 as tissue
modifying
member(s) 2014. For example, if the target simulating electric current is
configured with a
38
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
frequency and amplitude to stimulate a response from neural tissue, the type
(e.g., neural,
non-neural) of the tissue immediately adjacent tissue modifying inember(s)
2014 may be
determined based on the tissue response or lack thereof. In one embodiment,
device 2020
may be configured to allow for control of the target stimulating electric
current and the non-
target stimulating electric current. For example, the target stimulating
electric current and the
non-target stimulating electric current may be sequentially delivered to
distal portion 2013 of
device 2020 to determine the location of neural tissue prior to activation of
tissue modifying
member(s) 2014, for example, to help ensure that tissue modifying member(s)
2014 do not
damage neural tissue.
101611 In various embodiments, tissue modifying member(s) 2014 may include one
or
more of a rongeur, a curette, a scalpel, one or more cutting blades, a
scissors, a forceps, a
probe, a rasp, a file, an abrasive element, one or more small planes, an
electrosurgical device,
a bipolar electrode, a unipolar electrode, a thermal electrode, a rotary
powered mechanical
shaver, a reciprocating powered mechanical shaver, a powered mechanical burr,
a laser, an
ultrasound crystal, a cryogenic probe, a pressurized water jet, or some
combination thereof.
Some embodiments include one tissue modifying member 2014, while others
include
multiple tissue modifying members 2014. As is described further below, tissue
modifying
member(s) 2014 may have any of a number of suitable sizes, shapes and
configurations and
may move or actuate in any suitable way.
[0162] Referring now to Fig. 13A, in an alternative embodiment, a tissue
modification
device 2040 includes an elongate shaft 2041, one or more tissue modifying
member(s) 2044,
a handle 2046 and a power connector 2050. Additionally, device 2040 may
include a
guidewire lumen (not shown) extending through all or part of shaft 2041, which
may allow
passage of a guidewire 2048 having proximal 2049 and distal 2047 ends
therethrough. In one
embodiment, for example, guidewire 2048 may extend from a proximal end of
shaft 2041
through a distal end of shaft 2041 and out the patient. In some embodiments,
as described
previously above, anchoring and/or tensioning force may be applied at or near
distal end
2047 and/or proximal end 2049 to help urge tissue modifying member(s) 2044
against target
tissue.
101631 In the embodiment shown in Fig. 13A, tissue modifying member 2044 is
shown
having relatively flat configuration. In many of the subsequent embodiments
described
herein, various embodiments of tissue modifying members are also shown as
having flat
39
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
configurations, primarily for ease of description. In alternative embodiments,
however, and
with reference now to Fig. 13B, tissue modification device may include a
curved and/or
flexible tissue modifying member 2045 (or multiple curved and/or flexible
members), having
a curved/flexible surface for contacting target tissue. Device 2040 may also
include a curved
and/or flexible shaft 2042. Such curved and/or flexible tissue inodifying
members 2045 (or
tissue modifying surfaces) and shafts 2042 may facilitate tissue modification
in some
embodiments, in that tissue modifying members 2045 may more readily conform to
target
tissue. In alternative embodiments, many if not all of the devices described
in the present
application may have such curved and/or flexible tissue modifying member(s).
101641 Referring now to Figs. 13C-13E, several alternative embodiments of
anchoring
members for use with a tissue modification device are shown. Fig. 13C, for
example, shows
tissue modification device 2040 including a wire anchor 2060 coupled with
guidewire 2048.
In various embodiments, wire anchor 2060 may be either removably or
permanently attached
to guidewire 2048 at or near its distal end 2047 to provide anchoring force
against the
patient's back from outside the patient. Wire anchor 2060 may minimize or
prevent
guidewire 2048 from moving through the patient's back towards proximal end
2049. In
alternative embodiments, additional anchoring and/or tensioning force may be
applied to
distal end 2047, such as by holding and/or pulling distal end 2047 by hand.
[0165] In an alternative embodiment, as shown in Fig. 13D, distal end 2047 of
guidewire
2048 may include one or more deployable anchoring members 2062, which may be
deployed
within the patient to anchor into tissue of the patient's back, such as
muscle, bone, ligament
or the like. In one embodiment, for example, guidewire 2048 may have one or
more lumens
(not shown), and anchoring members 2062 may be translated through the lumen(s)
to extend
out of distal end 2047. When a tissue modification procedure is complete,
anchoring
members 2062 may be retracted within the lumen(s) so that guidewire 2048 may
be more
easily removed from the patient.
(0166] Referring to Fig. 13E, in another embodiment tissue removal device 2040
may also
include one or more proximal shaft anchoring members 2066 and/or one or more
proximal
guidewire anchoring members 2064. Shaft anchoring member 2066 may, in
alternative
embodiments, either be coupled with or removably couplable with shaft 2041 to
facilitate
application of anchoring force. For example, in one embodiment, shaft
anchoring member
2066 may abut the patient's back to resist translation of shaft 2041 into the
patient.
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
101671 Proximal guidewire anchoring member 2064 may be included in handle
2046, as
shown, or in other einbodiments may be located proximal or distal to handle
2046.
Guidewire anchoring member 2064 may be used to lock or anchor guidewire 2048
to prevent
or ininimize its translation into or out of device 2040. This may help
facilitate application of
tensioning and/or anchoring force via guidewire 2048.
101681 Figs. 13F and 13G show cross-sectional views of handle 2046 and
proximal
guidewire anchoring member 2064 taken through line C-C on Fig. 13D. In one
embodiment,
handle 2046 may include proximal guidewire anchoring inember 2064, a tissue
modifying
drive 2068 in a first lumen, a guidewire 2048 in a guidewire lumen 2070,
multiple guiding
slots 2074 with corresponding guiding tabs 2075, and one or more lobes 2072.
As shown in
Fig. 13F, when guidewire anchoring member 2064 is disengaged, guidewire 2048
may freely
translate within guidewire lumen 2070, because lobe 2072 does not interfere.
As shown in
Fig. 13G, when guidewire anchoring member 2064 is engaged, for example by
translating
and/or rotating guidewire anchoring member 2064 with respect to handle 2046,
guidewire
2048 may friction fit (e.g., clamp, pinch) between lobe 2072 of guidewire
anchoring member
2064 and the wall of guide wire lumen 2070. Guidewire anchoring member 2064
may then
be translated and/or rotated back to the original position (Fig. 13F) to
disengage guidewire
2048.
[0169] Referring now to Fig. 14, in some embodiments a tissue removal device
2100 may
include an elongate shaft 2104 having proximal 2106 and distal 2105 portions,
one or more
tissue modifying members 2108, a movable handle 2103 coupled with proximal
portion 2106,
and a power connector 2107. In the embodiment shown, distal portion 2105 is at
least
partially steerable (shown in Fig. 14 as two, overlapping distal portions),
and movable handle
2103 may be used (two-headed, straight arrow) to steer distal portion 2105
while holding
shaft 2104 relatively stationary. A steerable distal portion 2105 may enhance
the ability of
tissue modifying members 2108 to contact and apply force against target
tissue. In some
embodiments, the location of tissue modifying inembers 2108 may be adjusted,
using
steerable distal portion 2105, without significantly moving shaft 2104. In
some
embodiments, steerable distal portion 2105 may move in multiple directions,
such as laterally
and up-and-down, relative to the longitudinal axis of shaft 2104. Movable
handle 2103 may
operate with a piston-like motion, in one embodiment, where a distal portion
of handle 2102
is attached to the shaft and a proximal portion handle 2103 is attached to a
tensioning
member. The tensioning member may translate tension to steerable distal end
2105 when
41
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
handle 2103 is actuated, which in turn deflects distal end 2105. In various
alternative
einbodiments, any other handle steering mechanisms and/or other steering
mechanisms, many
of which are known in the art, may be used.
101701 Figs. 15A and 15B show one embodiment of a tissue modification device
2080,
which includes an elongate shaft 2081, one or more tissue modifying members
2082, a handle
2084, a visualization device 2086, an optical cable 2090 and a power connector
2088.
Visualization device 2086 may include any suitable device, such as but not
limited to an
endoscope. An endoscope visualization device may have lenses and/or fiber
optics, for
example, for delivering light (or other energy) to illuminate the tissue and
capturing images.
As shown in Fig. 15B, in some embodiinents, visualization device 2086 may
include one or
more image capturing elements 2094 and one or more illuminating elements 2092.
Image
capturing element 2094 may include, for example, a CCD or CMOS chip, in some
embodiments. Illuminating element 2092 may include, for example, one or more
light
emitting diodes (LEDs) or fiber optics, in some embodiments.
[0171] In some embodiments, optical cable 2090 may include fiber optics. Some
or all of
the fiber optics may comprise or may be coupled with illuminating elements
2092.
Alternatively or additionally, some or all of the fiber optics may be
connected to a camera
(not shown). For example, such a camera may be attached to the proximal end of
tissue
modification device 2080. Optical cable 2090 may alternatively include one or
more
electrical wires connected to a power source (e.g., to power LED(s)) and/or an
image
capturing element 2094. Lenses, fiber optics, LED(s), or combinations thereof
may be used
for illumination with lenses, fiber optics, CCD, CMOS, or combinations thereof
used for
image capturing, according to various embodiments.
[0172] Referring now to Fig. 16, another embodiment of a tissue modification
device 2120
may suitably include an elongate shaft 2132, one or more tissue modifying
members 2130, a
handle 2124, a visualization device 2122, an optical cable 2128 and a power
connector 2126.
In this embodiment, visualization device 2122 is located proximal to tissue
modifying
member(s) 2130 on shaft 2132. In various embodiments, visualization device(s)
2112 may
be positioned along shaft 2132 at any desired location.
[0173] Figs. 17A-17E show cross-sectional views of various embodiments of
shaft 2132,
from the perspective of line A-A in Fig. 16. In the embodiment shown in Fig.
17A, for
example, shaft 2132 may include a tissue modifying drive 2134 within a tissue
modifying
42
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
drive lumen 2140, and a guidewire 2136 within a guidewire lumen 2138. Tissue
inodifying
drive 2134 may be configured to translate or rotate with respect to tissue
modifying drive
lumen 2140. Examples of tissue modifying drives 2134 include, but are not
limited to, a
drive shaft, one or inore conductive wires, one or more optical fibers, or the
like. Various
embodiments may also include a motor, which may be located in the handle of
the device,
near the device distal end, in a separate drive apparatus, or the like.
101741 In an alternative embodiment, as shown in Fig. 17B, shaft 2132 may
include tissue
modifying drive 2134 within tissue modifying drive lumen 2140, guidewire 2136
within
guidewire lumen 2138, conductive eleinents 2146 within a visualization lumen
2144, and
suction/irrigation lumen 2142. Suction/irrigation lumen 2142 may be used, for
example, to
deliver gas, fluid or pushable solids (e.g., granular solids) from outside the
patient to the
distal end of the device oi- to aspirate gas, fluids, tissue and/or other
material from the
targeted tissue region to the outside of the patient. Suction/irrigation lumen
2142 may also be
used, in some embodiments, to slidably pass instruments, such as a long
flexible needle or
biopsy forceps. Visualization lumen 2144, in some embodiments, may be
configured to
receive conductive elements 2146, such as elements to power LEDs at the distal
end of the
device, and/or to carry the signal from a CCD or CMOS chip located at the
distal end of the
device that has captured a visual image and converted it into an electronic
signal to a display
device located outside the patient.
[0175] In yet another alternative embodiment, shown in Fig. 17C, shaft 2132
may include
tissue modifying drive 2134 within tissue modifying drive lumen 2140,
conductive elements
and/or fiber optics 2152 within visualization lumen 2144, and separate suction
2150 and
irrigation 2148 lumens. Suction lumen 2150 and irrigation lumen 2148 may be
used, in some
embodiments, to simultaneously or sequentially deliver and remove gases,
fluids and/or
pushable solids to and from the distal end of a tissue modification device.
Delivery and
removal of gases, fluids and/or pushable solids may help clear detached and/or
non-detached
target tissue and other debris from the treatment site and/or maintain a clear
visualization of
the target and non-target tissue via the visualization element.
[0176] In another embodiment, illustrated in Fig. 17D, fiber optics 2152 may
be disposed
within a hollow shaft 2132, in between various lumens 2148, 2150, 2144, 2140.
Alternatively, fiber optics 2152 may be replaced with electrical conductors in
other
embodiments. Fiber optics 2152 may deliver light to illuminate the target
tissue and/or
43
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
deliver light from the tissue to a viewing device. Visualization lumen 2144,
in such an
embodiment, may transport light or electrical signals in proximal and/or
distal directions.
[0177] In yet another alternative embodiment, as in Fig. 17E, shaft 2132 may
include a
steering actuator 2154 within a steering lumen 2156. Steering actuator 2154
may be used, for
example, to help steer a distal portion of a tissue modification device, as
described in further
detail above.
[0178] Fig. 18 is an end-on cross-sectional view of tissue modification device
2120 of Fig.
16, shown from the perspective of line B-B. In this embodiment, visualization
device 2122
and illuminating elements 2158 are located proximal to tissue modifying member
2130. In
the embodiment shown, tissue modifying member 2130 includes a rotating disc
mounted on a
post in a bearing 2160, with multiple raised cutting edges 2131 on the disc.
Two
suction/irrigation lumens 2142 allow for introduction and suction of gas,
fluid and/or
pushable solids from an area of tissue modification.
[0179] Figs. 19A-23B illustrate a number of embodiments of a distal end of a
tissue
modification device having various different tissue modifying members. In any
of these
embodiments, the devices may be couplable distally with a guidewire or may
extend distally
from the tissue modifying portion shown, to extend the device out of the
patient distally.
Referring to Figs. 19A-19C, in another embodiment, a tissue modification
device 2310 may
include a tissue modifying member 2312 comprising a movable platform with an
abrasive
surface and coupled with a drive shaft 2314 extending out of a distal opening
2316. As
shown in the various figures, in some embodiments tissue modifying member 2312
may
move laterally (Fig. 19A), may translate back and forth (Fig. 19B) and/or may
vibrate (Fig.
19C).
[0180] In yet another embodiment, as in Fig. 20, a tissue modification device
2320 may
include a tissue modification member 2322 comprising an oval or round platform
with an
abrasive surface and coupled with a drive shaft 2324 extending out of a distal
opening 2326.
In such an embodiment, tissue modifying member 2322 may be made to rotate or
move in a
circular pattern, as well as translate, move laterally, oscillate and/or
vibrate according to
various embodiments.
[0181] Referring to Fig. 21, in another embodiment a tissue modification
device 2330
includes multiple tissue modifying members 2332, each including a movable
platform 2333
with an abrasive surface attached to a drive shaft 2334. Tissue modifying
members may
44
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
move back and forth i-elative to one another and to the device shaft in any
suitable pattern.
Moving tissue modifying inembers 2332 back and forth relative to one another
may help
them apply tensioning forces to target tissue, thereby enhancing the ability
of tissue
modifying members 2332 to cut, shear, tear and/or otherwise modify tai-get
tissues.
101821 As shown in Fig. 22, in another alternative embodiment, a tissue
modification
device 2340 may suitably include tissue modifying members comprising one or
more blades,
such as a distal blade 2344a and a proximal blade 2344b, each having a cutting
edge 2345a,
2345b. In the embodiment shown, proximal blade 2344b is movable and may
translated
distally toward the opposing distal blade 2344a. In alternative embodiments,
distal blade
2344a may be movable or both blades 2344a, 2344b may be movable. Alternative
embodiments may include one movable blade, more than two movable blades facing
in one
direction, more that two movable blades facing in different directions, a
movable blade and a
backstop against which the blade may be driven, or any other suitable
coinbination of
movable and/or immobile blades. Furthermore, any blade of any given embodiment
may
have any suitable shape, size and overall configuration. In some embodiments,
blades may
be flat, while in others they may be curved, squared off, ridged, bent,
serrated or the like.
Blades may be long or short, multiple blades may be aligned closely one after
the other, such
as in a typical multi-blade razor used for shaving a face, multiple blades may
be disposed
apart from one another by several millimeters or even centimeters, and/or the
like. Blades
may have any suitable amount of sharpness or dullness, and in some embodiment
a
combination of sharper and duller blades may be used. Therefore, although
exemplary
embodiinents of blades are described in detail above and below, any other
suitable blades or
combinations of blades may be substituted in various embodiments, without
departing from
the scope of the present invention.
101831 Blades 2344a, 2344b, or any other blades described in alternative
embodiments
herein, may be fabricated from metals, polymers, ceramics, any other suitable
material or
combination of materials. According to various embodiments, suitable metals
for blades may
include, but are not limited to, stainless steel (303, 304, 316, 316L), nickel-
titanium alloy, or
cobalt-chromium alloy, for example, Elgiloy (Elgin Specialty Metals, Elgin,
IL, USA),
Conichrome (Carpenter Technology, Reading, PA, USA), or Phynox (Imphy SA,
Paris,
France). Polymer materials include nylon, polyester, Dacron , polyethylene,
acetal, Delrin
(DuPont), polycarbonate, nylon, polyetheretherketone (PEEK), and
polyetherketoneketone
(PEKK). In some embodiments where polymers are used, such polymers may be
glass-filled
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
or carbon-filled to add strength and stiffness. Ceramics may include, but are
not limited to,
aluminas, zirconias, and carbides. Blades may be manufactured using skills
known in the art,
for example, inetal injection molding (MIM), CNC machining, injection molding,
grinding,
EDM, sheet metal bending, etching, or the like. Other portions of a tissue
modification
device, such as a cover over one or more blades or other features, may be
inade of any
suitable material now known or hereafter discovered. A blade cover, for
example, may be
fabricated in various embodiments of one or moi-e polymeric materials, such as
nylon,
silicone, polyetheretherketone (PEEK), polyetherketoneketone (PEKK),
polytetrafluoroethylene (PTFE), polyurethane (Tecothane,), Pebax (co, USA),
polycarbonate,
Delrin (co, USA), high-density polyethylene (HDPE), low-density polyethylene
(LDPE),
HMWPE, UHMWPE, or the like.
[01841 Referring now to Fig. 23A, in one embodiment a tissue modification
device 2350
may have a substantially cylindrical, circular, or otherwise curved shaft 2352
as well as one
or more substantially cylindrical, circular, or otherwise curved blades 2354.
In the
embodiment shown, blade 2354 protrudes out of a window 2358 of shaft 2352.
When blade
2354 is moved proximally (arrow), its cutting edge 2356 moves toward and
perhaps engages
with an opposing cutting edge 2356 of shaft 2352.
101851 In an alternative embodiment, as in Fig. 23B, a tissue modification
device 2360 may
have a substantially cylindrical, circular, or otherwise curved shaft 2362 as
well as one or
more substantially cylindrical, circular, or otherwise curved blades 2364. In
the embodiment
shown, blade 2364 protrudes out of a window 2368 of shaft 2362. Blade 2364 may
be
rotated (arrows), to cause its cutting edges 2366 cut target tissue. In some
embodiments, one
or more curved blades 2364 may be translated as well as rotated. In either of
the
embodiments shown in Figs. 23A and 23B, cut target tissue may optionally be
removed
through the inside of curved shaft 2352 or curved blade 2364.
101861 Referring now to Figs. 24A and 24B, in some embodiments, a tissue
modification
device may include one or more anchoring members 2374 coupled with a distal
shaft portion
2370 of the device. In various embodiments, any of a number of suitable
anchoring members
may be used. Some embodiments of anchoring members have been previously
described
above, and others will be described further below. In one embodiment, for
example,
anchoring members 2374 may comprise multiple needles, as shown in Figs. 24A
and 24B.
Needles 2374 may act not only to anchor distal shaft portion 2370 to tissue,
but may also
46
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
change one or more characteristics of the tissue. For example, in some
embodiments,
inserting multiple needles into tissue may stiffen the tissue and thus enhance
the ability of
one or inore tissue modifying members 2372 to cut or otherwise modify the
tissue. In one
embodiment, anchoring meinbers/needles 2374 may be deployable out of distal
shaft portion
2370 (arrow), such that needles 2374 are retracted during delivery of the
device into the
patient and then deployed into the target tissue when in a desired position.
In various
embodiments, anchoring members/needles 2374 may extend out of distal shaft
portion 2370
in an orientation substantially perpendicular to the longitudinal axis of
distal shaft portion
2370 or in any other suitable orientation relative to distal shaft portion
2370 or otherwise
non-parallel to the longitudinal axis. During use, the tissue stiffening
projections can extend
into the target tissue. Needles 2374 will typically have a modulus of
elasticity greater than
the modulus of elasticity of the target tissue, and thus may stiffen (i.e.,
increase the effective
modulus of elasticity) of the target tissue.
101871 In the embodiment shown in Figs. 24A and 24B, as well as in any
alternative
embodiments described herein, one or more members such as tissue anchoring
members/needles 2374 may be used to modify tissue in any of a number of
suitable ways.
For example, in some embodiments, energy may be transmitted to one or more
tissue
anchoring members/needles 2374 to cool, heat or otherwise transmit energy to
the tissue.
Such cooling or heating, for example, may further change the stiffness or
consistency of the
tissue, thus facilitating tissue modification by one or more tissue modifying
members. In one
embodiment, for example, it may be advantageous to cool or even freeze tissue
to increase its
stiffness so that it can be more easily cut or abraded. For example, in some
embodiments, a
cryogenic fluid may be delivered via an irrigation lumen and/or
suction/irrigation lumen to
directly reduce the temperature of the anchoring members 2374 or to separately
cool the
target tissue. Any other suitable change may alternatively be made to tissue
to enhance a
tissue modification procedure according to various embodiments.
101881 As shown in Fig. 24B, when anchoring members 2374 are in place within
target
tissue, tissue modifying member 2372 (a blade in the embodiment shown) may be
translated
out of distal shaft portion to cut or otherwise modify tissue. Tissue
modifying member 2372
may advance out of distal shaft portion 2370 in a direction perpendicular or
otherwise non-
parallel to anchoring members 2374. In some embodiments, anchoring
members/needles
2374 may retain target tissue after cutting, so that it may be removed from
the patient.
47
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
101891 Referring now to Figs. 25A and 25B, one embodiment of a barrier 602
comprising a
woven, braided or non-woven material with a lattice structure 604 is shown.
Fig. 25A shows
barrier 602 being deployed from delivery device 601, and Fig. 25B shows
barrier 602 in its
completely deployed (expanded, free) configuration. In various embodiments,
bari-ier 602
may have any of a number of suitable dimensions. For example, in some
embodiments,
barrier 602 may have a width ranging from about 0.100" to about 3.000", a
length ranging
from about 0.100" to about 72", and a thickness ranging from about 0.001 " to
about 0.250 '.
In some embodiments, as described in connection with Figs. I OB and l OD
above, barrier 602
may have a narrowed or tapered distal end. Barrier 602 may be manufactured by
methods
known in the art, such as in a single-layei- flat-form or a dual-layer tubular-
fonn that is
pressed flat. Material used to fabricate barrier 602, in various embodiments,
may be
composed of a weave of metallic wire, monofilament or braided. The metallic
wire may be
made from any suitable material, such as stainless steel (303, 304, 316,
316L), nickel-
titanium alloy, cobalt-chromium alloy, ElgiloyOO (Elgin Specialty Metals,
Elgin, IL, USA),
Conichrome g (Carpentei- Technology, Reading, PA,USA), Phynox (Imphy SA,
Paris,
France) or the like. A woven material may be composed of a weave of polymer
strands,
monofilament or braided material. Polymer strands in a woven, braided or non-
woven
material construction may be made from nylon, polyester, Dacron ,
polyethylene, Kevlar
(DuPont, ), acetal, Delrin (DuPont, ), polycarbonate, nylon, silicone,
polyetheretherketone
(PEEK), polyetherketoneketone (PEKK), polytetrafluoroethylene (PTFE),
polyurethane,
UHMWPE, or the like. In some embodiments, barrier 602 may self-expand after
being
released from a constrained configuration in delivery device 601. In some
embodiments,
such self-expansion may be achieved by forming barrier 602 from a shape-memory
or super-
elastic material.
101901 Referring to Figs. 25C and 25D, in an alternative embodiment, a barrier
612 may
include multiple slits 615 extending from opposite edges 613a, 613b toward the
longitudinal
center of barrier 612 to form 1nultiple tabs 616. Slits 615 may enhance
flexibility of barrier
612 by allowing tabs 616 to flex independently. Tabs 616 may then return to
their flat-form
state individually as delivery device 601 is pulled proximally, as shown in
FIG. 25D. Tabs
616 may also conform individually to surrounding tissue, thereby helping
protect non-target
tissue, in some embodiments. Barrier 612 may be made of any suitable material,
such as but
not limited to those described above, and slits 615 may be formed by any
suitable method,
such as die cutting, milling machining, laser cutting, EDM machining,
injection molded,
48
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
etching, water-jet cutting, and blade cutting. In an alternative embodiment,
barrier 612 may
be made by assembling multiple tabs 6] 6 to a central meinbei- by welding,
soldering, brazing,
or laser welding, for example.
10191] Figs. 25E and 25F illustrate another alternative embodiment of a
barrier 622 having
slits 627 disposed more centrally and not extending to the lateral edges 623a,
623b.
101921 In another alternative embodiment, shown in Figs. 25G and 25H, a
barrier 632
comprises a central support member 639 and multiple lateral ribs 638 that form
a skeleton-
like framework. ln various embodiments, central meinber 639 and ribs 638 may
either
comprise the same material or different materials, and any suitable materials
may be used,
such as but not limited to the materials listed above. In some embodiments,
ribs 638 may
retain a curvilinear shape after deployment that is heat-set in nickel-
titanium or mechanically
fonned, as shown in Fig. 25G.
101931 Referring to Figs. 251 and 25J, in another alternative embodiment, a
barrier 642 may
comprise a flat-fonn sheet made from polymer, porous polymer, woven or non-
woven fabric,
metal, porous metal, foam, hydrogel, a double-layer polyiner "bag" to create
an inflatable
bladder, or the like.
[0194] Referring to Figs. 25K and 25L, in another alternative embodiment, a
barrier 652
may comprise a central support inember 650 and ribs 659 that straighten
completely or nearly
completely upon deployment. Optionally, barrier 652 may also include a flex-
point 651 at
which barrier 652 may articulate.
[0195] Figs. 26A and 26B illustrate another alternative embodiment of a
barrier device 832,
in which device 832 comprises a tubular, woven mesh. Barrier device 832 may
assume an
elongate, low-profile configuration, as in Fig. 26A, to facilitate its
delivery to a treatment
area, and may also be compressed from one or both ends to assume a
widened/expanded
configuration for protecting tissue, as in Fig. 82B. In another embodiment, as
in Figs. 27A
and 27B, a bamer device may comprise a flat woven mesh.
101961 Another alternative embodiment of a barrier device 852 is depicted in
Figs. 28A and
28B. Here, a first pull wire 854 and a second pull wire 855, extending from
opposite ends of
a shape changing portion of barrier device 852, may be pulled to cause the
shape changing
portion to expand or widen (Fig. 28B). In some embodiments, when pull wires
854, 855 are
49
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
released, the shape changing portion may resume its original, narrower
configuration (Fig.
28A).
101971 Referring now to Figs. 29A-29B, in another embodiment, a barrier device
862 may
be housed in a housing 864 comprising two halves 866, 868, and a lumen for
allowing
passage of a guidewire 869. When halves 866, 868 are pulled apart, as in Fig.
29B, barrier
device 862 is free to expand.
101981 Referring now to Figs. 30A-30C, in another alternative embodiment, a
barrier
device 892 may include a piece of hydrogel material, which expands and/or
unrolls from a
collapsed/rolled configuration (Fig. 30A) to an expanded/unrolled
configuration (Fig. 30C)
when exposed to one or more fluids, such as saline, water, blood or the like.
In one
embodiment, hydrogel may be injected directly into an area between target and
non-target
tissues to form barrier device 892, and device 892 may be left in the
patient's body to
dissolve after a tissue modification procedure is complete. In other
alternative embodiments,
bamer device 892 may comprise one or more alternative self-expanding materials
or
materials that expand upon exposure to fluid.
101991 In another embodiment, as shown in Figs. 31 A-31 C, a barrier device
872 may
include a woven wire structure including lateral straight wires 874 coupled
with crossing
wires 877, 878 via multiple loops 876. In one einbodiment, lateral wires 874
slide freely
through loops 876, to allow barrier device 872 to collapse and expand. Wires
876, 877, 878
may be coupled with end caps 880, 881 at either end of barrier member 872.
Some
embodiments may also include pull tabs 879, 882 at either end of barrier
member 872. As
shown in Fig. 31 C, when pull tabs 879, 882 are pulled, barrier device 872 may
shorten and
expand to a wider configuration. As shown in Figs. 31 A and 31 C, when pull
tabs 879, 882
are pulled, an angle between cross wires 877, 878 decreases. In an alternative
embodiment,
pulling pull tabs 879, 882 may cause barrier device 872 to collapse. In some
embodiments,
wires 874, 877, 878 themselves may perforrn the protective function of barrier
member 872,
while in alternative embodiments a material or membrane may be coupled with
wires 874,
877, 878.
[0200] Figs. 32A and 32B illustrate how, in one embodiment, a barrier device
3020
extending through a delivery device 601 may help protect tissue during a
tissue modification
procedure involving use of a tissue modification device 3024. In various
embodiments,
tissue modification device 3024 may include, but is not limited to, a rongeur,
a curette, a
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
scalpel, one or more cutting blades, a scissors, a forceps, a probe, a rasp, a
file, an abrasive
element, one or more small planes, an electrosurgical device, a bipolar
electrode, a unipolar
electrode, a thermal electrodc a rotary powered mechanical shaver, a
reciprocating powered
mechanical shaver, a powered mechanical burr, a laser, an ultrasound crystal,
a cryogenic
probe, a pressurized water jet, or any combination of such devices. Tissue
modification
device 3024 may be advanced and retracted (double-headed arrows) freely on one
side of
ban-ier device 3020 and may be used to modify tissue, while barrier device
3020 protects
non-target tissue from sustaining unwanted damage. In some embodiments,
barrier device
3020 may also be used to help guide tissue modification device 3024 to and/or
from a
position for perfonning a tissue modification procedure. Such guidance may be
achieved by
a shape, surface characteristic and/or one or more guide features of barrier
device 3020,
according to various embodiinents.
102011 Turning to Figs. 33A and 33B, in another embodiinent, a barrier device
3030 may
include an open, shape-changing portion 3030, closed, elongate extensions 3034
extending
from either end of shape-changing.portion 3030, and at least one guide feature
3035
extending through its length. Guide feature 3035 may include, in various
embodiments, one
or more guidewires (as shown), rails, impressions, lumens, tracks or the like,
any of which
may facilitate guidance of a tissue modification device 3032 along and/or
through barrier
device 3030. In various embodiments, guide featiire 3035 may comprise a
separate device,
not attached to barrier member 3030, as in the guidewire of Figs. 33A and 33B.
Alternatively, one or more guide featui-es 3035 may be attached to, or
integral with, ban-ier
member 3030.
[02021 Fig. 34 shows an embodiment of a barrier device 3050 including a
central rail 3052
guide member along which a tissue modification device 3054 may be guided. Fig.
35 shows
an alternative embodiment of a barrier device 3060 including a central rail
3062 guide
member along which a tissue modification device 3064 may be guided. In some
embodiments, barrier devices 3050, 3060 and tissue modification devices 3054,
3064 may be
advanced through a delivery device 601, while other embodiments may not employ
such a
delivery device 601.
[02031 Referring to Fig. 36A, in one embodiment, a barrier device 3070 may
include a
central channel 3072, accessible by a slit 3076, and multiple flex grooves
3074. Multiple flex
grooves 3074 may facilitate collapsing of ban-ier device 3070. In another
embodiment, as in
51
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
Fig. 36B, a barrier device 3080 may have a smooth, non-grooved sui-face and a
central
channel 3082, accessible by a slit 3086. Slit 3076, 3086 may facilitate
coupling and
decoupling of a tissue modification device with barrier device 3070, 3080.
[0204] Figs. 37A and 37B show two additional alternative embodiments of
barrier devices
3190, 3200. Barrier device 3 190 includes a protruding central guide feature
3192, a flat
tissue protective portion 3193, and lateral support members 3194. Barrier
device 3200
includes a central impression guide feature 3202, a flat tissue protective
portion 3203, and
lateral support members 3204.
102051 As described immediately above, in any of a number of different
embodiments, a
barrier device may include one or more guide features. Such guide features
may, in various
embodiments, correspond with one or more guide features on a guide device or
guide
member for guiding the ban-ier member to a desired location and/or position in
a patient.
Alternative or additionally, one or more guide features on a barriei- device
may be used to
facilitate guidance of one or more tissue modification devices along, over
and/or through the
barrier device. Thus, in some embodiments, a barrier member may include
multiple guide
features for guiding the barrier device and for guiding a tissue modification
device. In other
embodiments, the same guide feature(s) on a barrier device may be used to
guide both the
barrier device and a tissue modification device. Any suitable combination of
guide feature(s)
having any size, shape, pattern or the like may be used according to various
einobdiments.
[0206] Fig. 38 illustrates one embodiment of a delivery device 3210 for
delivering a barrier
device 3220 to a location in a patient. In this embodiment, barrier device
3220 includes a
guidewire lumen 3221, through which a guidewire 3222 may extend, and a guide
feature
3223, over which one or more tissue modification devices (not shown) may be
passed.
Optionally, delivery device 3210 may include a visualization luinen 3216, thi-
ough which a
visualization device may be passed, a suction lumen 3214, and an irrigation
lumen 3216. In
alternative embodiments, delivery device 3210 many have any of a number of
suitable
different configurations and features. For example, in one embodiment suction
lumen 3214
and irrigation lumen 3216 may combined into one lumen, multiple visualization
lumens 3216
may be included, and or the like.
[0207] As is mentioned above, in many of the described embodiments, a barrier
device may
include one or more pieces of material. Such material may include any suitable
material or
combination, and in some embodiments may comprise a polymer, such as latex,
rubber
52
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
(viton), nylon, silicone, polyetheretherketone (PEEK), polyetherketoneketone
(PEKK),
polytetrafluoroethylene (PTFE), polyurethane (Tecothane,), Pebax (co, USA),
polycarbonate,
Deirin (DuPont, USA), high-density polyethylene (HDPE), low-density
polyethylene
(LDPE), high-molecular weight polyethylene (HMWPE), ultra-high-molecular
weight
polyethylene (UHMWPE), paraline coating, or the like. The material may be
coated,
laminated, impregnated, covered, or over-molded on a barrier- device, or
alternatively may be
attached to a barrier device by adhesives or cements, thermal bonding
techniques, with
fasteners such as clasps or thread, or by fonning pockets in the material
which fit over ribs of
the barrier.
102081 In other embodiments, one or more conductive wires may be included in a
barrier
device, such that the wires may be disposed and selectively activated/exposed
along either or
both of a target tissue surface or a non-target tissue surface of the barrier
device. In one
embodiment, for example, wires may be coupled with lateral support members of
a barrier
device. Conductive wires may be used, for example, to stimulate and thus
identify specific
tissues, such as nerves, and/or to monitor the position/location of the
barrier device by
measuring impedance and/or imparting electrical currents to induce stimulation
to the target
tissue. In one embodiment, an array of wire contact points along a barrier
device may be
implemented and independently activated to verify that the bam'er device is in
a desired
location/position.
102091 With reference now to Fig. 39, in some embodiments a tissue
modification device
3600 may include an elongate, at least partially flexible body 3602, an
abrasive tissue
modifying surface 3604, a proximal handle 3606 and a distal handle 3608. As
has been
mentioned above, in some embodiments abrasive surface 3604 may coinprise any
of a
number of various abrasive members, configurations or the like, such as but
not limited to a
rasp. Various abrasive surface/rasp embodiments, for example, are described in
further detail
in PCT Patent Application Pub. No. PCT/US2005/037136, which was previously
incorporated by reference. For example, embodiments including abrasive or rasp
surfaces are
described in Figs. 34, 35, 41, 42, 48, 61, 62, 64, 86-99, 101 and 102, and
their accompanying
detailed description in PCT Patent Application Pub. No. PCT/US2005/037136.
[0210] In use, the distal end of elongate body 3602 may be advanced through
the patient's
back, into the epidural space, between target and non-target tissue, and out
the patient's back,
as in Fig. 39. Distal handle 3608 may then be removably coupled with the
distal end of
53
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
elongate body 3602 (or near the distal end in alternative embodiinents). A
user may then
grasp proximal handle 3606 and distal handle 3608 and pull on both to apply
tensioning force
(solid-tipped, upward-pointing arrows) to urge abrasive surface 3604 against
the target tissue.
The user may also use handles 3606, 3608 to translate elongate body 3602 back
and forth
(double-headed arrows) to cause abrasive surface 3604 to abrade the target
tissue. During a
given tissue modification procedure, tensioning force may be applied, using
separate handles
3606, 3608, by pulling handles 3606, 3608 in different directions or in the
same direction
(i.e., parallel to one another). In some procedures, handles 3606, 3608 may be
moved about
to apply tensioning force from different angles and directions during the
procedure. As
mentioned above, By "separate handles," it is meant that handles 3606, 3608
are not
connected to one another by a common handle or other connecting device or
mechanism.
Obviously, however, handles 3606, 3608 may be coupled with (in some
embodiments
removably coupled with) elongate body 3602 (or a shield in other embodiments)
at or near its
distal and proximal ends or portions.
102111 Elongate body 3602 may have any suitable dimensions, according to
various
embodiments. In soine embodiments, elongate body 3602 is sufficiently long to
extend from
outside the patient, through a channel in the spine, such as an intervertebral
foramen, and out
of the patient through an exit point located apart from the entry point.
Elongate body 3602
will typically have a width sufficient to prevent abrasive surface 3604 from
cutting
completely through bone when tensioning force is applied and body 3602 is
translated. For
example, in one embodiment, body 3602 may have a width (at least along a
portion where
abrasive surface 3604 is disposed) of about 3 mm or less, and more preferably
about 5 mm or
less. Body 3602 may also have a height that facilitates its passage into the
patient and
between target and non-target tissues. For example, in one embodiment, body
3602 has a
height of about 4 mm or less, and more preferably about 2 mm or less.
102121 In some embodiments, abrasive surface 3604 may be disposed along one
side of
elongate body 3602 and along a limited length of elongate body 3602, to
prevent or minimize
unwanted damage to nearby non-target tissues as elongate body 3602 is
translated. For
example, in some embodiments, abrasive surface 3604 may be disposed along a
length of the
device measuring no longer than 10 em, and preferably no more than 6 cm, and
even more
preferably no more than 3 cm. In alternative embodiments, abrasive surface
3604 may
extend along a substantial majority or even the entire length of elongate body
3602 and/or
may reside on multiple sides of elongate body 3602. In one embodiment, for
example, all of
54
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
elongate body 3602 may comprise abrasive surface 3604, and at least a portion
of elongate
body 3602 may be disposed within a shield or barrier inember to protect non-
target tissues
from damage during a procedure. Some embodiments, however, include at least
one non-
abrasive side or surface adjacent abrasive surface 3604, to protect non-target
tissue from
unwanted damage. Such a non-abrasive surface may optionally be made of a
lubricious or
low-friction material and/or may be coated with a lubricious or low-friction
coating, in some
embodiments.
102131 Proximal handle 3606 and distal handle 3608 may have any size, shape or
configuration in various embodiments. In fact, in various embodiments, distal
handle 3608,
proximal handle 3606, or both may be left off altogether. In Fig. 39, proximal
handle 3606 is
shown as a squeezable handle with a trigger, as has been described previously
for use with a
bladed, RF or other movable tissue modifying member (or meinbers). Such a
squeezable
handle 3606 is not required in every embodiment, but may be used in some
embodiments,
such as when an abrasive/rasp device 3600 may be interchanged with a bladed
device, RF
device and/or the like during a tissue modification procedure. Thus, in soine
embodiments,
squeezable proximal handle 3606 is removably couplable with elongate body
3602, so that
various alternative tissue modifying members may be used with the same
proximal handle
3602. In such embodiments, for example, target tissue may be modified using
rasp elongate
body 3602 and then may be further modified using an RF device, bladed device,
powered
device or the like. In various embodiments, such devices may be used in any
order.
Similarly, distal handle 3608 may also be used with more than one device.
10214] In some embodiments, tissue modification device 3600 may further
include one or
more electrodes (not shown) coupled with or immediately adjacent abrasive
surface 3604
and/or non-abrasive surface(s) of elongate body 3602. Such electrodes may be
activated, for
example, via a trigger or button on proximal handle 3606 in order to test
positioning of
abrasive surface 3604 within the patient. For example, once a user believes
abrasive surface
3604 to be in position for treating target tissue, an electrode on abrasive
surface 3604 may be
activated. If abrasive surface 3604 is actually in contact with nerve tissue,
which the user
does not want to treat or damage, the patient's leg may twitch or jerk,
showing the user that
abrasive surface 3604 should be repositioned or the procedure aborted.
Alternatively or
additionally, an evoked EMG response of a patient may be monitored to
determine if the
activated electrode is touching or near nerve tissue. In another embodiment,
electrode may
be placed on a non-abrasive surface, so that when activated, it deinonstrates
that the non-
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
abrasive surface is facing non-target tissue, as intended. In various
embodiments, any
combination of electrodes may be used. Further description of such electrodes
and their use
can be found in PCT Patent Application Pub. No. PCT/US2005/037136.
102151 Referring now to Figs. 40A-40D, in various embodiments, a rasp or
abrasive surface
of a tissue modification device inay have any of a number of suitable
configurations, sizes,
numbers of rasp eleinents and/or the like. A number of such abrasive surfaces,
for example,
are described in previously incorporated PCT Patent Application Pub. No.
PCT/US2005/037136, such as in Figs. 90-96 and the accompanying detailed
description. The
embodiments shown in Figs. 40A-40D are further examples of rasp/abrasive
surface
configurations, according to various embodiments.
102161 In one embodiment, as shown in Fig. 40A, a diagonally patterned rasp
member
3624 having multiple notches 3626 may be disposed along one side of an
elongate body 3622
of a tissue modification device. Of course, in various embodiments, rasp
member 3624 may
have any number of bends or may have any other alternative shape or
configuration. In
alternative embodiments, rasp member 3624 may be made of any of the materials
listed in the
foregoing description for any alternative embodiments of tissue modifying
members. For
example, in some embodiments, rasp member 3624 may have hard edge and be
comprised of
a material like stainless steel or titanium, while in other embodiments rasp
meinber 3624 may
be fabricated as an abrasive surface of diamond, tungsten carbide or the like.
In yet another
embodiment, a braided wire, such as the braided wire used in a Gigli saw, may
be adhered to
a surface of elongate body 3622 to form rasp member 3624. Obviously, rasp
member 3624
may have any of a number of configurations and may be fabricated from any
suitable
material, and thus, rasp member 3624 is not limited to the examples described
here.
[0217] Fig. 40B shows an alternative embodiment, in which a rasp member 3634
and
multiple channel openings 3636 are disposed along an elongate body 3632 of a
tissue
modification device. In such an embodiment, tissue that is abraded off by rasp
member 3634
may enter channel openings 3636 into a hollow portion (or multiple hollow
portions) of
elongate body 3632. In various embodiments, removed tissue may be either
stored in such a
channel and removed when the tissue modification device is removed from the
patient, or
may alternatively be directed out of elongate body 3632 using imgation,
suction or a
combination thereof.
56
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
102181 In another embodiment, shown in Fig. 40C, a rasp portion 3644, disposed
along an
elongate body 3642, may include any number of rasp members 3646 and,
optionally, any
number of channel openings 3648. In some embodiments, rasp members 3646 may
have
cutting edges that face in the same direction. In such embodiments, rasp
members 3646
abrade or cut tissue when elongate body 3642 is translated in one direction
and do not abrade
or cut tissue when translated in the opposite direction. In various
embodiments, rasp
meinbers 3646 may also be configured to direct tissue in channel openings
3648.
102191 Fig. 40D shows another embodiment of a rasp portion 3654 disposed along
an
elongate body 3652 of a tissue modification device. Rasp portion 3654 again
includes
multiple rasp members 3656 and multiple channel openings 3658, but in this
embodiment,
rasp members 3656 have alternating rows of oppositely directed cutting edges.
Thus, when
elongate body 3652 is translated back and forth, rasp members 3656 abrade or
cut tissue as
elongate body 3652 travels in both directions.
[02201 With reference now to Fig. 41, in an alternative embodiment, a tissue
modification
device 3700 may include an elongate, at least partially flexible body 3702, at
least part of
which is disposed within a shield member 3710 (or "barrier member") having an
opening
3712 along its length. Elongate body 3702 may include at least one abrasive
surface 3704,
which may comprise a rasp or other abrasive surface as discussed above, and
which may be
exposed through opening 3712 to contact and abrade target tissue. Tissue
modification
device 3700 may also include a proximal handle 3706 and a distal handle 3708,
either or both
of which may be removably coupled with elongate body 3702, according to
various
embodiments. Shield member 3710 may optionally include a proximal anchoring
member
3714 and/or a distal anchoring member 3716 for anchoring shield member 3710
outside the
patient. In alternative embodiments, proximal handle 3706, distal handle 3708,
or both may
be coupled with shield member 3710, rather than with body 3702.
[02211 In use, shield member 3710 may be passed into the patient's back, into
the epidural
space, between target and non-target tissue, and out the patient's back. In
various
embodiments, elongate body 3702 may be passed into the patient along with
shield member
3710 or through shield member 3710 after it is in place. In another
embodiment, elongate
body 3702 may be passed into patient first, and shield member 3710 may be
passed over it
into the patient. Abrasive surface 3704 may be positioned so that it is
exposed and/or
protrudes through opening 3712 on shield member 3710 to contact target tissue.
Tensioning
57
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
force may be applied to shield member 3710, elongate body 3702, or both, to
urge abrasive
surface 3704 into the target tissue. For example, in some embodiments,
tensioning force may
be applied by grasping and pulling on handles 3706, 3708, while in other
embodiments,
tensioning force may be applied by grasping and pulling on distal and proximal
portions of
shield member 3710. At some point, either before or after applying tensioning
force,
anchoring members 3714, 3716 may be coupled with or deployed from shield
member 3710.
Various alternative embodiments may include only proximal anchoring member
3714 or only
distal anchoring ineinber 3716, and the unanchored end of shield ineinber 3714
may be
pulled to apply tensioning force. Anchoring members 3714, 3716 may include any
suitable
device for anchoring or leveraging against the patient's skin, some exemplary
embodiments
of which are described above in connection with Fig. 6A. In alternative
embodiments,
anchoring members 3714, 3716 may attach to one or more devices apart from the
patient,
such as a rail of an operating table or the like. In other alternative
embodiments, shield
meinber 3710 may be held relatively stationary by manually holding one or both
of its ends.
In other embodiments, shield member 3710 may be held relatively stable simply
by residing
in the patient's own tissue. In further alternative embodiments, both shield
member 3710 and
body 3702 may be held relatively stable, and one or more actuators on proximal
handle 3706
and/or distal handle 3708 may be used to move or otherwise activate abrasive
surface 3704 to
abrade the target tissue.
102221 Elongate body 3702 inay be translated back and forth through shield
member 3710
to cause abrasive surface 3704 to abrade target tissue. Because shield member
3710
generally protects non-target tissue from unwanted damage, abrasive surface
3704 may be
disposed along elongate body for any desired length and/or may be disposed
about all or
substantially all of the circumference of elongate body 3702. In some
embodiments, for
example, abrasive surface 3704 may extend the entire length of elongate body
3702. In fact,
in some embodiments, elongate body 3702 may comprise a rasp, braided wire saw
or the like.
In some embodiments, shield member 3710 may include one or more protective
materials,
added layers of material, or the like (not shown) along one or more edges of
opening 3712, to
prevent dainage to such edges of opening 3712 when elongate body 3702 is
translated back
and forth.
[0223] In various embodiments, shield member 3710, elongate body 3702, or both
may
include additional features to enhance a tissue modification procedure to
treat or alleviate
spinal stenosis. For example, in various embodiments, shield member 3710
and/or elongate
58
CA 02646251 2008-09-09
WO 2007/106740 PCT/US2007/063679
body 3702 may include one or more lumens for applying suction and/or
irrigation, to help
remove tissue debris from the patient. Such debi-is may be removed through one
or more
lumens in shield member 3710, one or more lumens in elongate body 3702, or
between shield
member 3710 and elongate body 3702, in various embodiments. Optionally, one or
more
electrodes may be positioned on shield member 3710, elongate body 3702,
abrasive surface
3704 or some combination thereof, to help allow a user to verify device 3700
is in a desired
location in the patient, as described above. In various embodiments, other
optional features
may also be added.
102241 Turning now to Figs. 42A and 42B, in another embodiment, a tissue
modification
device 800 may include an elongate body 802, a widened tissue modifying
portion 806
including an abrasive surface 808, tapered portions 810 and a non-abrasive
surface 816, a
proximal handle 812 and a distal handle 814. (Fig. 42B shows a side view of a
portion of
device 800.) In one embodiment, elongate body 802 inay comprise a metal wire,
and tissue
modifying portion 806 may comprise a widei- section coupled with the wire.
Body 802,
tissue modifying portion 806 and the like may have any suitable size and
configuration, and
abrasive surface 808 may have any suitable configuration, examples of which
have been
described in greater detail above and in PCT Patent Application Pub. No.
PCT/US2005/037136, which was previously incorporated by reference. In various
embodiments, body 802 may be coupled with tissue modifying portion 806 using
any
technique, such as welding, attaching with adhesive or the like. In an
alternative
embodiment, body 802 and tissue modifying portion are formed from one piece of
material.
Optionally, body 802 and/or tissue modifying portion 806 may include one or
more lumens,
such as a guidewire lumen, suction lumen, irrigation fluid lumen and/or the
like. Device 800
may also include a shield member, one or more electrodes, or any of the
additional features
described above in conjunction with other embodiments.
102251 Although various illustrative embodiments are described above, any of a
number of
changes may be made to various embodiments without departing from the scope of
the
invention as described by the claims. Therefore, the foregoing description
should not be
interpreted to limit the scope of the invention as it is set forth in the
claims.
59