Note: Descriptions are shown in the official language in which they were submitted.
CA 02646311 2008-12-11
1811
PROCESS FOR PURIFYING WASTE SULFURIC ACID
FIELD OF THE INVENTION
The invention relates to a process for the regeneration of waste sulfuric acid
contaminated with recyclable products, such as methylsulfuric acid,
dimethylether,
methanol, aliphatic and olefinic hydrocarbons, chlorinated hydrocarbon, and
organosilicon
compounds.
BACKGROUND TO THE INVENTION
Chloromethane is used in the direct synthesis of methylchlorosilanes. The
chloromethane required is generally prepared from methanol and hydrogen
chloride, water
and, in small amounts, dimethyl ether being formed as by-products.
Chloromethane for
preparing methylchlorosilanes must be carefully dried and freed from dimethyl
ether.
The hydrogen chloride used for the preparation of chloromethane originates
from
hydrolysis reactions of methylchlorosilanes. Therefore, in the prepared
chloromethane,
minor constituents of methylchlorosilanes, such as aliphatic and olefinic
hydrocarbons and
chlorinated hydrocarbons, may also be found. If the hydrogen chloride from
hydrolysis
reactions of methylchlorosilanes is used directly without intermediate
purification in the
production of chloromethane, organosilicon compounds in accordance with the
vapor
pressure can additionally pass into the product.
Chloromethane can be freed from the above mentioned impurities by scrubbing
with concentrated sulfuric acid. In addition to water and dimethyl ether, the
remaining
minor constituents, particularly olefins and organosilicon compounds are
absorbed in the
sulfuric acid owing to their Lewis base properties. The used sulfuric acid is
approximately
75% strength by weight and must be ejected, when its absorption capacity for
dimethyl
ether is exhausted.
DE-A-25 03 610 describes a process for purifying sulfuric acid contaminated
with
methylsulfuric acid. For this purpose, used sulfuric acid originating from the
purification of
1
CA 02646311 2008-12-11
chloromethane is diluted with 10% to 25% by weight of water, based on the
sulfuric acid
content. Steam is introduced, at a temperature of 170 to 180 C. The
methylsulfuric acid
content is hydrolyzed to methanol and sulfuric acid and methanol is distilled
off.
The process of DE-A-25 03 610 is not applicable to sulfuric acid which, in
addition
to methylsulfuric acid, dimethyl ether and methanol further contains
additional impurities
described above, such as olefins and organosilicones, since cracking processes
occur owing
to the high temperature. The carbon formed is solid like coke and leads to
rapid blocking of
apparatus components. The high acid concentration causes a slight oxidation of
organic
contents. This in turn releases sulfur dioxide, which produces additional off-
gas pollution.
In the booklet "Schott Engineering, 1987" from Schott Engineering GmbH, Mainz,
pages 12 to 17, a process for the concentration and purification of waste
sulfuric acid is
described, in which oxidizing agents are added in a problem-specific manner to
the waste
acid.
The oxidation of organic impurities described in Schott is expensive at high
pollution rates, since large amounts of oxidizing agent have to be added. The
oxidation of
organosilicon compounds leads to colloidally distributed silica. Filtration,
owing to the
aggressive medium and owing to the small particle size, represents a
relatively great
problem. Furthermore, contacting relatively large amounts of an oxidizing
agent with the
sulfuric acid containing dimethyl ether comprises a considerable safety
hazard.
The addition of oxidizing agents following the process described in DE-A-25 03
610 is not expedient, since the coarse coke particles are oxidized slowly and
only with
difficulty, owing to the small surface area in comparison to the volume.
USP 5,683,671 discloses a method for purification of sulfuric acid
contaminated
particularly with methylsulfuric acid. This reference seeks to address the
shortcomings of
DE-A-25 03 610 by in a first step diluting the sulfuric acid to at least 45%,
but no more
than 55% concentration with liquid water, to a maximum of 135 C. Steam is then
injected
at a temperature of 120 to 140 C, which hydrolyses the methylsulfuric acid
component into
methanol and sulfuric acid, and wherein the methanol is distilled off. In a
second step an
oxidizing agent is added at a temperature of between 20 and 130 C.
However, USP 5,683,671 cannot be used for sulfuric acid that also contains, in
addition to methylsulfuric acid, dimethylether, and methanol, the above-
mentioned
2
CA 02646311 2008-12-11
contaminants because they are subjected to cracking at high temperatures. The
resulting
hydrocarbon is hard as coke and forms deposits on the equipment components.
Adding oxidation agents according to the method disclosed in DE 25 03 610 Al
is
also unreasonable because coarse coke particles, having a small surface area
compared to
the amount used, are oxidized only poorly and slowly.
United States Patent Application No. 2008/0293979A1 - Wacker Chemie AG,
published 27 November 2008, defines a method for the regeneration of waste
sulfuric acid
contaminated with admixtures from the group consisting of methylsulfuric acid,
dimethylether, methanol, aliphatic and olefinic hydrocarbons, chlorinated
hydrocarbons,
and organosilicon compounds, wherein the waste sulfuric acid is diluted by
adding water
and injecting steam to a concentration of not more than 55 wt. % based on
sulfuric acid and
heated at most to 135 C to boiling, concentrated at reduced pressure and at a
temperature of
at least 170 C to at least 80 wt. % and is subsequently reacted with an
oxidizing agent.
In prior art processes, organic compounds are released in a typical packed
stripping
column as a combined vapour/liquid stream as the waste sulfuric acid stream
enters the hot
column. Since the column is generally under an inert nitrogen blanket to avoid
a
flammable atmosphere, the organic materials tend to char/form carbon/coke
deposits when
the organic materials contact the hot packing. This charring requires the
packing to be
periodically cleaned but also results in the charred materials passing out of
the column with
the diluted acid for subsequent treatments.
However, although the aforesaid US2008/0293979A1 process may provide an
improvement in the art, there still remains the problem of charring and
particulate carbon
formation when spent sulfuric acid contacts the surfaces of the stripping
column and its
packing when the feed acid is diluted by water/steam therein, to release the
volatile organic
materials. There is, therefore, a need to provide an improved process for
purifying waste
sulfuric acid containing minor constituents of the aforesaid organic
contaminants.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide an improved process for
the
purification of waste sulfuric acid contaminated with recyclable products,
such as
3
CA 02646311 2008-12-11
methylsulfuric acid, dimethylether, methanol, aliphatic and olefinic
hydrocarbons,
chlorinated hydrocarbons, and organosilicon compounds, which process reduces
or
eliminates charring of the organic materials which contaminates the surface of
equipment,
particularly, stripping columns, as well as being passed on to subsequent
process steps.
Accordingly, in one aspect the invention provides a process for purifying used
sulfuric acid feed acid which contains, as minor constituents, methylsulfuric
acid, dimethyl
ether, methanol, aliphatic and olefinic hydrocarbons, chlorinated hydrocarbons
and
organosilicon compounds which comprises a two-stage organic compound
volatilization
process comprising
(a) diluting the used sulfuric acid with a stream selected from water, steam
and an
aqueous distillate mixture to a minimum temperature of about 100 C to effect
hydrolysis of
methylsulfuric acid to methanol and sulfuric acid and vaporization of methyl
chloride,
dimethyl ether and a portion of the released methanol to provide a first
diluted acid and a
first volatile organic vapour comprising methyl chloride and dimethyl ether
and a portion
of the released methanol;
(b) removing said methyl chloride and dimethyl ether and methanol vapour;
(c) passing said diluted acid to an acid stripping column in counter-current
flow
with steam and, optionally, water to provide a second volatile organics vapour
and a second
diluted sulfuric acid;
(d) removing said second volatile organics vapour; and
(e) collecting said second diluted sulfuric acid.
Preferably, the used sulfuric acid is diluted in step (a) to not less than 50
wt.% and
more preferably, not less than 57 wt.%.
Thus, in the practice of the present two-stage invention, contaminated waste
sulfuric acid is diluted external of the stripping column in a first stage
with water, steam
and/or, most preferably, distillate liquors to a temperature of about 115 C
generally
resulting from the heat of dilution. This external pre-dilution stage is,
preferably, effected
in a static mixer and subsequently in a small vessel and results in release of
organic
material vapours and venting thereof for subsequent combination with the
vapours leaving
the stripping column of use in the second dilution stage. Thus, these organic
materials
4
CA 02646311 2008-12-11
have no, or only a reduced, opportunity to contact the hot packing surfaces in
the inert
atmosphere of the stripping column.
The sulfuric acid regeneration and purification process and plant according to
the
invention, as hereinabove defined, includes a steam stripping column,
preferably, followed
by a two-stage, steam heated sulfuric acid concentrator (SAC) designed to
produce 96%
product acid or whichever strength is suitable for recycle to the precursor
process. The
contaminated organic vapours from the dilution and stripping steps are,
subsequently,
concentrated in an overheads fractionation column to recover an organic rich
overhead
stream and a bottom distillate stream containing only trace amounts of organic
components.
The organic rich overhead stream can be further separated by partial
condensation in a
methanol rich liquid stream for potential recycle and a dimethyl ether rich
vapour stream
for recycle or incineration. The distillate stream recovered from the bottom
of the
fractionation column can be used as dilution in the preceding dilution step.
Accordingly, in a preferred aspect, the invention further provides a process
as
hereinabove defined comprising
(f) collecting said vapour selected from the group consisting of step (b) and
step (d);
(g) fractionating said vapour of step (f) to produce both a liquid methanol
stream and a dimethyl ether rich vapour stream overhead and a liquid
distillate bottoms
stream.
Preferably, the process as hereinabove defined comprises the use of
distillates
mixture, defined as a portion of the condensed aqueous stream evaporated
during the
subsequent sulfuric acid concentration (SAC) stages.
Preferably, the process as hereinabove defined has said first diluted acid of
about
115 C and wherein said first diluted acid and said second diluted sulfuric
acid is at least
57wt. %.
In more detail, feed acid contaminated with volatile organic compounds is
diluted
by a stream of water, steam or, preferably, distillate or mixtures thereof in
a static mixer
and subsequently in a small, vented vessel to about 60 wt.%. It is believed
that the dilution
is sufficient to result in the hydrolysis of the methylsulfuric acid with the
formation of
methanol and sulfuric acid, wherein the heat of dilution raises the
temperature of the acid to
5
CA 02646311 2008-12-11
approximately 115 C. Most of the methyl chloride, and dimethyl ether and a
portion of the
released methanol are vaporized and removed from the system. If acid weaker
than design
is to be processed, steam can be injected into the acid in the static mixer to
ensure that the
acid fed to the stripping column is above 100 C.
The diluted acid is passed down the packed stripping column, counter current
to the
stripping steam. The temperature rise caused by dilution and steam addition
results in the
volatilization of any methanol and other organic compounds carried over from
the first
stage, in the packing, which are then carried to the top of the column by the
stripping
steam. Preferably, part of the stripping steam is generated internally in the
column, by
concentrating the column bottoms to approximately 65 wt.% in a natural
thermosyphon
stripping column reboiler. The reboiler circulation loop includes a stripper
retention tank
to give additional residence time for the 65% acid. Low pressure steam is
injected below
the packing of the column to supplement the steam generated in the reboiler.
Preferably, operation of the stripping column is carried out under partial
vacuum to
reduce processing temperatures and further reduction in charring of organic
compounds not
removed under stage 1.
Inclusion of a partial concentration step to 65 wt.% in the stripping column
enables
a high ratio of stripping steam/acid feed to be economically achieved which
still further
enhances organics material removal.
Fractionation of the combined overheads vapour from the dilution vessel and
the
stripping column provides a useful methanol liquid stream, a dimethyl ether
rich vapour
stream for recycle or incineration and an almost organic free bottoms
distillate stream for
recycle.
The acid leaving the bottom of the stripping column, which contains only small
amounts of residual organics, flows by gravity to an ODU (Organics Destruction
Unit)
First Reactor. In ODU First Reactor, provision is, preferably, made to,
optionally, add a
solution of oxidizing agent, generally hydrogen peroxide in acid, to the
stripper bottoms to
improve the colour and further reduce the organics level in the stripper
bottoms. Acid
overflows the ODU First Reactor and enters a first evaporation stage. There
are, preferably,
2 evaporation stages in series in the preferred SAC system but the number of
stages may
vary from one to multiple stages depending on circumstances. In the case of 2
stage
6
CA 02646311 2008-12-11
operation acid flows between the two stages by gravity and is concentrated to
about 88
wt.% H2SO4 in Stage 1 and overflows to Stage 2 at about 175 C via an ODU
Second
Reactor which provides additional residence time. Additional hydrogen peroxide
is,
optionally, added prior to ODU Second Reactor and the oxidation of any
remaining
organics is completed therein. The final product acid at 96 wt % H2S04 or
other desired
strength flows by gravity from the second stage via a feed/product
interchanges and into a
product drum. After cooling the product acid is, optionally, passed through a
filter to
remove any particles of silica which may have been formed by the oxidation of
the
organosilicon compounds, before being pumped to final storage
Vapours generated in the evaporator(s) are condensed in one or more overhead
condensers. These vapours are substantially water vapour containing small
amounts of
sulphuric acid. Design techniques familiar to those skilled in the art are
applied to
minimize the amount of sulphuric acid which reports to the final condensed
aqueous
condensate stream
The SAC system described above operates under vacuum most typically, although
not always, drawn by steam ejectors. The design of the SAC system and
selection of
operating conditions are optimized by means familiar to those skilled in the
art to achieve
the desired product strength and quality with minimum acid loss to effluent.
The combined
condensed aqueous stream from the acid concentration stages, together with
that from the
steam ejector condensers and the fractionation column bottoms stream,
comprises the
distillate stream, a portion of which is preferentially used for the initial
feed acid dilution
step in order to reduce water consumption and effluent load.
BRIEF DESCRIPTION OF THE DRAWINGS
In order that the invention may be better understood, preferred embodiments
will
now be described by way of example only with reference to the accompanying
drawing
wherein
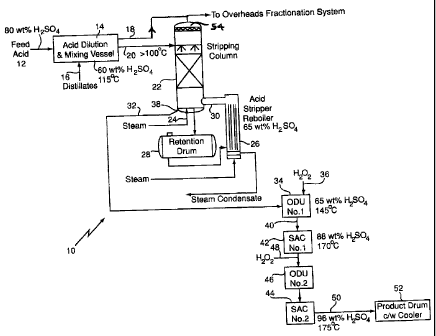
Fig. 1 is a diagrammatic process and plant layout according to the invention.
7
CA 02646311 2008-12-11
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Fig. 1 shows generally as 10, a two-stage process for eliminating or reducing
the
amount of charring and carbon particulate formation formed in sulfuric acid
purification
processes of the prior art.
Fig. 1 shows a spent 80 wt. % sulfuric acid feed stream 12 fed to an acid
mixing
and dilution vessel 14 into which is passed an aqueous distillate mixture 16
to generate a 60
wt. % sulfuric acid at about 115 C and effect hydrolysis of the methylsulfuric
acid.
Volatile organic materials, such as methanol, methyl chloride and dimethyl
ether, are
removed through conduit 18 and the diluted acid passed through conduit 20 to
packed
stripping column 22 in counter current flow to steam stream 24.
The volatile organic vapour stream 18 and the overheads stream 54 from column
22
are combined and enter the overheads fractionation system.
Part of the stripping steam is generated internally in column 22, by
concentrating
the column bottoms to approximately 65 wt.% in a natural thermosyphon
stripping column
reboiler 26. The reboiler circulation loop includes a stripper retention tank
or drum 28 to
give additional residence time for the 65% acid. Low pressure steam 24 is
injected below
the packing of column 22 to supplement steam 30 generated in reboiler 26.
The acid leaving the bottom of stripping column 22, which contains only small
amounts of residual organics, flows by gravity 32 to an ODU (Organics
Destruction Unit)
First Reactor 34. In ODU First Reactor 34, provision is, preferably, made to,
optionally,
add a solution of oxidizing agent, generally, hydrogen peroxide 36 in acid to
the stripper
bottoms 38 to improve the colour and further reduce the organics level in
stripper bottoms
38. Acid overflows 40 and enters a first evaporation stage 42. There are,
preferably, 2
evaporation stages 42, 44 in series in the preferred Sulphuric Acid
Concentration (SAC)
system. Acid flows between the two stages by gravity and is concentrated to
about 88
wt.% H2SO4 in Stage 1 and overflows to Stage 2 at about 175 C via an ODU
Second
Reactor 46 which provides additional residence time. Additional hydrogen
peroxide 48 is,
optionally, added prior to ODU Second Reactor 46 and the oxidation of any
remaining
organics is completed therein. The final product acid at 96 wt % H2SO4 flows
by gravity 50
8
CA 02646311 2008-12-11
from the second stage into a product drum 52. After cooling, this cooled 96
wt.% acid may
then be returned to a silicones plant on level control. The concentrated,
cooled acid may
optionally be passed through a filter to remove any residual particles of
inorganic matter
produced by the acid processing.
Each SAC evaporator 42, 44, preferably, consists of a tantalum bayonet heat
exchanger in a natural circulation thermosyphon loop with a separator vessel
mounted
above.
EXAMPLES
In the following examples a spent sulfuric acid, from the production of
silicones
was used having the following composition:-
Sulfuric acid 80.0% by weight
Water 3.5% by weight
Dimethyl ether 12.0% by weight
Methyl sulfuric acid 3.5% by weight
Methanol 1.0% by weight
Methyl chloride Trace
Organosilicon compounds Trace
Total organic carbon (TOC) > 7.0% by weight (70,000 ppm)
The process steps described were carried out continuously.
Example 1
Spent sulfuric acid and water streams at room temperature were metered
continuously into the top of a glass column at rates calculated to give a
diluted acid stream
of 57 wt % acid strength. The column was hot-oil jacketed and was 1" diameter
and 36'
length packed with 3/16' ceramic Intalox saddles. The column was operated at
slight
below atmospheric pressure under an inert atmosphere.
Live steam was injected at the bottom of the column at a temperature of about
105 C. The acid leaving the bottom of the column flowed by gravity into a
glass flask
located in a heating mantle which served as a reboiler. The reboiler was
maintained at a
temperature at which the acid was concentrated to about 65 wt % acid strength.
The steam
generated in this concentration step was returned to the bottom of the column
to act as
additional stripping steam.
9
CA 02646311 2008-12-11
The product acid from the stripping column/reboiler apparatus was a clear
light
amber colour compared to the clear dark amber colour of the acid feed.
Depending on the
exact combination of operating conditions and stripping steam/acid feed ratios
employed
the product acid Total Organic Carbon (TOC) content was reduced to between
from 650
ppm to 2700 ppm.
After completion of a number of experiments the column was dismantled and
evidence of charring and carbon deposition was observed at the feed point
locations at the
top of the column and on the heated walls of the column.
Example 2
Spent sulfuric acid and water streams were metered continuously at room
temperature into an agitated mixing vessel mounted externally to the stripping
column at
rates calculated to give a diluted acid stream of 57 wt. % acid strength at
about 115 C. The
vapours generated in the mixing vessel were vented from the vessel and
combined with
vapours leaving the top of the stripping column. The diluted acid formed in
the mixing
vessel flowed by gravity to the top of the stripping column which was operated
at
560 mmHg abs. pressure and under an inert atmosphere.
Live steam was injected at the bottom of the column at a temperature of about
105 C. The acid leaving the bottom of the column flowed by gravity into a
glass flask
located in a heating mantle which served as a reboiler. The reboiler was
maintained at a
temperature at which the acid was concentrated to about 65 wt. % acid
strength. This
temperature varied according to the pressure at which the column was operated.
The steam
generated in this concentration step was returned to the bottom of the column
to act as
additional stripping stream.
The product acid from the stripping column/reboiler apparatus was a clear
light
amber colour compared to the dark amber colour of the acid feed. Depending on
the exact
combination of operating conditions and stripping steam/acid feed ratios
employed the
product acid TOC was reduced to as low as 60 ppm.
This stripper product acid was then continuously concentrated by multi-stage
vacuum concentration to a 96 wt.% strength product suitable for recycling to a
silicones
manufacturing process. A suitable quality product in terms of TOC content was
obtained
without the necessity for the addition of an oxidizing agent.
CA 02646311 2008-12-11
After completion of a number of experiments, the column was inspected and no
charring or carbon deposition was found to have occurred.
Although this disclosure has described and illustrated certain preferred
embodiments of the invention, it is to be understood that the invention is not
restricted to
those particular embodiments. Rather, the invention includes all embodiments
which are
functional or mechanical equivalents of the specific embodiments and features
that have
been described and illustrated.
15
25
11