Note: Descriptions are shown in the official language in which they were submitted.
CA 02646337 2008-09-17
WO 2007/110402 1 PCT/EP2007/052854
94346pct
PACKAGING MEANS FOR MULTI-DOSE POWDER INHALERS WITH
OPTIMIZED DISCHARGING PROPERTIES
The invention relates to a package for pharmaceutical compositions, mixtures
or
formulations for use in a powder inhaler.
Prior art
Medical aerosol therapy intended for pulmonary inhalation using nebulisers,
metered-dose
aerosols or powder inhalers plays an important part in the treatment of
numerous lung
diseases.
In the field of powder inhalers, single-dose and multi-dose devices are known.
The multi-
dose powder inhalers contain the pharmaceutical composition, mixture or
formulation
either in the form of a powder supply from which the single dose is taken from
a well by
means of a built-in metering unit, or pre-metered packaged single doses which
are either
stored together in the device (e.g. in the form of packaged amounts in
blisters) or are
placed individually in the device for use (e.g. in the form of capsules).
In multi-dose powder inhalers, in particular, the manner of packaging in which
the powder
formulation is present in the device is critical to the quality of the product
and hence to its
suitability for use by inhalation.
Therefore, a chief objective of the packaging is to keep the chemical
composition of the
atmosphere inside the packaging constant, in order to prevent physical or
chemical changes
to the pharmaceutical composition, mixture or formulation, or to stabilise
them.
In this context a distinction is made between stability directed to the short
term, which the
pharmaceutical composition, mixture or formulation has to have per se, even if
it is not
adequately protected by the packaging ("in-use stability") and the long-term
stability, i.e.
CA 02646337 2008-09-17
WO 2007/110402 2 PCT/EP2007/052854
the stability which has to be guaranteed for as long as the pharmaceutical
composition,
mixture or formulation is contained in the unopened package.
It is also important that the patient receives the correct dose of the
inhalable formulation on
inhaling.
The term "fine particle dose" refers to the dose that reaches the patient's
lungs. The fine
particle dose is influenced by the interactions of the micronised particles of
active
substance with one another and also the interactions with the excipients.
It is also known that particularly as a result of variations in the level of
moisture in the
interior of the packaging these interactions may increase such that the fine
particle dose is
significantly reduced. Such changes include the penetration of water into the
package and
also the elimination of water from the inside of the package.
It is also necessary for a package to have a shape and an opening pattern
(i.e. the places in
the packaging which allow the blister to be opened by piercing or cutting)
which enable an
optimum air supply and hence optimum delivery of the pharmaceutical
composition,
mixture or formulation.
Examples of multi-dose powder inhalers are known in the art. They are known
for example
from EP 0 703 800 B1 or EP 0 911 047 A1, which disclose a powder inhaler
consisting of
a cup-shaped lower part and an equally cup-shaped lid. After placing the
capsule in the
capsule holder, the patient can press an actuating member which is movable
from a resting
position and thereby interacts with at least one pin that can be pressed into
the capsule
holder. The capsule is pierced by the pin or pins and the drug is released.
Thus, for
example, DE 3348370 and DE 3336486 further disclose inhalers which contain a
disc-
shaped blister pack comprising a number of wells arranged in a circle. The
individual
wells each contain a dose of a medicament powder intended for inhalation. The
wells are
closed off on both sides by a sealing film, for example. To deliver the
medicament powder
the cavity is opened. An air channel connects the opened well with the
mouthpiece of the
CA 02646337 2008-09-17
WO 2007/110402 3 PCT/EP2007/052854
inhaler. By way of example the inhaler of DE 3336486 will be described in more
detail. It
comprises a housing in which there is a chamber (supply chamber) comprising an
air inlet
and in which there is a disc-shaped round blister with medicament pouches
packed therein.
The blister is loosely connected to a rotatable round disc. Around the disc
are formed
holes which are axially in contact with the medicament pouches, i.e. the
pouches and holes
are located above and below one another. The chamber has an air outlet. The
inhaler also
has a piston which is arranged so that it can open a pouch of medicament by
piercing it, so
that the medicament is released into the chamber and can be breathed in
through a
mouthpiece. Reference is made to the drawings in the patent application and US
patent
specification.
With regard to the packaging of medicament powders, a distinction is made
between the
primary packaging and the secondary packaging. The primary packaging is
characterised
in that it is in direct contact with the inhalable formulation. The primary
packaging may
optionally be surrounded by a second, outer protection, the secondary
packaging. The
primary packaging may be, for example, a capsule, a solid or flexible blister
with wells or
a disc comprising wells.
The secondary packaging may be a blister, a pouch, a bag or other container.
The
secondary packaging generally totally encloses the primary packaging.
Secondary
packaging is used particularly when the primary packaging does not provide
adequate
protection from moisture.
The primary packaging and optionally the secondary packaging have the task of
protecting
the active substance and also the entire inhalable formulation from chemical
or physical
change, so that it remains stable for long periods. The physical changes in
question may
be, in particular, changes which might affect the delivery of the intended
dose of fine
particles.
The choice of a suitable material for the packaging is determined by two
factors. On the
one hand the material must be able to perform the protective function
required. On the
CA 02646337 2008-09-17
WO 2007/110402 4 PCT/EP2007/052854
other hand the material must be such that the packaging can be made into the
form needed
for use in the powder inhaler and can perform the function expected of it.
The problem underlying the present invention is to optimise the shape of the
packaging,
the piercing pattern of the packaging and the fill level of a pharmaceutical
composition,
mixture or formulation, so as to improve the delivery of a pharmaceutical
composition,
mixture or formulation.
This problem is solved by a package according to claim 1. Advantageous further
features
are the subject-matter of the subsidiary claims.
Description of the invention
The present invention therefore relates to packages for inhalable powders,
which are
optimised in their shape, piercing pattern and fill level with a
pharmaceutical composition,
mixture or formulation.
Detailed description of the invention
The invention relates to optimised packages as described above which allow
improved air
flow and hence improved delivery of a pharmaceutical composition, mixture or
formulation by virtue of the shape of their opening pattern in their piercing
pattern and the
fill level with the pharmaceutical composition, mixture or formulation.
Preferably the
packages consist of a capsule, a blister, a blister disc or a blister strip.
These different
forms (with the exception of the capsules) will hereinafter be referred to
over all as blisters.
The capsule generally consists of two parts, a capsule body (body) and a
capsule cap
(cap), which fit telescopically inside one another. However, multi-part
capsules are also
known. It is preferable to use size 2-4 capsules, most preferably size 3
capsules.
The capsule material is non-digestible plastics or gelatine, particularly hard
gelatine.
CA 02646337 2008-09-17
WO 2007/110402 5 PCT/EP2007/052854
The blister disc may be, for example, a cylinder-like disc up to 5 mm high and
up to 15 cm
in diameter. In the disc are depressions or holes (wells) formed
perpendicularly to the
plane of the disc. A disc of this kind may for example be placed in an inhaler
according to
DE 3348370 or DE 3336486. An inhaler of this kind has a housing which contains
the
disc-shaped round blister with medicament pouches packed therein. The inhaler
comprises, inter alia, a pin which is arranged so that it can open one
medicament pouch, so
that the medicament is released into the chamber and can be breathed in
through a
mouthpiece.
The shape of the package according to the invention including the shape of the
well is
fundamentally determined by the powder inhaler which is to be used.
Preferably, the
packaging is teardrop-shaped or oval or in the shape of a figure of eight. The
inflow
surfaces and outflow surface(s) are as far away from one another as possible.
The package which is a blister first of all comprises a base element
consisting of a
thermoplastic plastics and at least two wells separated from one another by a
web. The
wells are open at least on one side, possible on two opposing sides. These
openings are
closed off in the packaging ready for use, e.g. by means of a sealing foil
which is attached
to the base element.
The package may consist of standard commercial materials. Preferably, it
consists of a
plastics material. Most preferably, the materials used as plastics selected
from among the
thermoplastic polymers such as e.g. polystyrenes, polyolefins, polyamides,
polyvinyl
chlorides, polyethylenes, polycarbonate, polyester, polypropylene,
polyethylene
terephthalate or polyurethane. These have the necessary rigidity or mobility
to enable them
to perform the mechanical tasks of the primary packaging. Also suitable are,
for example,
natural substances such as gelatine or composite materials of plastics and
metals, such as
aluminium.
CA 02646337 2008-09-17
WO 2007/110402 6 PCT/EP2007/052854
According to the invention it is not essential, though preferable, that all
the walls of the
well should consist of the same material. In a well, at least the wall that
closes off the
opening may be made of a different material from the other walls.
Further information regarding the composition or processing can be found in
the prior art,
particularly EP599690, EP432438 or EP400460.
The fill level of the pharmaceutical composition, mixture or formulation in
the package can
be optimised and will depend on the flowability of the pharmaceutical
composition,
mixture or formulation.
The term flowability indicates the ability of the pharmaceutical composition,
mixture or
formulation to flow easily as a loose material. In the art the flowability ffc
is defined as
follows:
ffc =6I /a".
Here, a, is the solidification tension and 6,, is the bulk strength. Normally
the flowability
is determined using a ring shear device.
For pharmaceutical compositions, mixtures or formulations with poor to no
flowability, the
flowability accords with the following: 4 _ ffc > 1. In this case, packages
are used which
are partly filled, such that there is a free passage for air between at least
one inlet opening
and an outlet opening. For readily flowable pharmaceutical compositions,
formulations or
mixtures the formula for the flowability is 4 < ffc . In this case, packages
which are partly
filled are used, such that there is a free passage of air between at least one
inlet opening
and an outlet opening, or which are completely filled so that an advantageous
(simple and
inexpensive) filling process can be used. In the latter case, the fill volume
is determined by
the shape of the packaging and there is no need for any special metering
process.
Examples of pharmaceutical compositions, formulations or mixtures include all
the
inhalable compounds, such as e.g. inhalable macromolecules, as disclosed in EP
1 003 478.
CA 02646337 2008-09-17
WO 2007/110402 7 PCT/EP2007/052854
The compounds listed below may be used in the device according to the
invention on their
own or in combination. In the compounds mentioned below, W is a
pharmacologically
active substance and is selected (for example) from among the betamimetics,
anticholinergics, corticosteroids, PDE4-inhibitors, LTD4-antagonists, EGFR-
inhibitors,
dopamine agonists, H1-antihistamines, PAF-antagonists and PI3-kinase
inhibitors.
Moreover, double or triple combinations of W may be combined and used in the
device
according to the invention. Combinations of W might be, for example:
- W denotes a betamimetic, combined with an anticholinergic, corticosteroid,
PDE4-
inhibitor, EGFR-inhibitor or LTD4-antagonist,
lo - W denotes an anticholinergic, combined with a betamimetic,
corticosteroid, PDE4-
inhibitor, EGFR-inhibitor or LTD4-antagonist,
- W denotes a corticosteroid, combined with a PDE4-inhibitor, EGFR-inhibitor
or LTD4-
antagonist
- W denotes a PDE4-inhibitor, combined with an EGFR-inhibitor or LTD4-
antagonist
- W denotes an EGFR-inhibitor, combined with an LTD4-antagonist.
The compounds used as betamimetics are preferably compounds selected from
among
albuterol, arformoterol, bambuterol, bitolterol, broxaterol, carbuterol,
clenbuterol,
fenoterol, formoterol, hexoprenaline, ibuterol, isoetharine, isoprenaline,
levosalbutamol,
mabuterol, meluadrine, metaproterenol, orciprenaline, pirbuterol, procaterol,
reproterol,
rimiterol, ritodrine, salmefamol, salmeterol, soterenol, sulphonterol,
terbutaline, tiaramide,
tolubuterol, zinterol, CHF-1035, HOKU-81, KUL-1248 and
- 3-(4- {6-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
hexyloxy}-
butyl)-benzyl-sulphonamide
- 5-[2-(5.6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1 H-quinolin-2-
one
- 4-hydroxy-7-[2- {[2- { [3-(2-phenylethoxy)propyl] sulphonyl } ethyl]-amino }
ethyl]-
2(3H)-benzothiazolone
- 1-(2-fluoro-4-hydroxyphenyl)-2-[4-(1-benzimidazolyl)-2-methyl-2-
butylamino]ethanol
- 1-[3-(4-methoxybenzyl-amino)-4-hydroxyphenyl]-2-[4-(1-benzimidazolyl)-2-
methyl-
2-butylamino] ethanol
CA 02646337 2008-09-17
WO 2007/110402 8 PCT/EP2007/052854
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-[3-(4-N,N-
dimethylaminophenyl)-
2-methyl-2-propylamino] ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-[3-(4-methoxyphenyl)-2-
methyl-2-
propylamino]ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-[3-(4-n-butyloxyphenyl)-2-
methyl-
2-propylamino]ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-{4-[3-(4-methoxyphenyl)-
1,2,4-
triazol-3-yl]-2-methyl-2-butylamino } ethanol
- 5-hydroxy-8-(1-hydroxy-2-isopropylaminobutyl)-2H-1,4-benzoxazin-3-(4H)-one
- 1-(4-amino-3-chloro-5-trifluoromethylphenyl)-2-tert.-butylamino)ethanol
- 6-hydroxy-8- { 1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-
ethyl } -
4H-benzo[ 1,4]oxazin-3-one
- 6-hydroxy-8- { 1-hydroxy-2-[2-( ethyl4-phenoxy-acetate)-1,1-dimethyl-
ethylamino]-
ethyl}-4H-benzo[ 1,4]oxazin-3-one
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-phenoxy-acetic acid)-1,1-dimethyl-ethylamino]-
ethyl } -4H-benzo[ 1,4]oxazin-3-one
- 8-{2-[1,1-dimethyl-2-(2.4.6-trimethylphenyl)-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-benzo [ 1,4] oxazin-3 -one
- 6-hydroxy-8- { 1-hydroxy-2-[2-(4-hydroxy-phenyl)-1,1-dimethyl-ethylamino]-
ethyl } -
4H-benzo[ 1,4]oxazin-3-one
- 6-hydroxy-8-{1-hydroxy-2-[2-(4-isopropyl-phenyl)-1.1dimethyl-ethylamino]-
ethyl}-
4H-b enzo [ 1,4] oxazin-3 -one
- 8- {2-[2-(4-ethyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl } -6-
hydroxy-4H-
benzo[ 1,4] oxazin-3 -one
- 8-{2-[2-(4-ethoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-
benzo[ 1,4]oxazin-3-one
- 4-(4- {2-[2-hydroxy-2-(6-hydroxy-3-oxo-3.4-dihydro-2H-benzo[ 1,4]oxazin-8-
yl)-
ethylamino]-2-methyl-propyl } -phenoxy)-butyric acid
- 8-{2-[2-(3.4-difluoro-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-
4H-benzo[ 1,4]oxazin-3-one
- 1-(4-ethoxy-carbonylamino-3-cyano-5-fluorophenyl)-2-(tert-butylamino)ethanol
CA 02646337 2008-09-17
WO 2007/110402 9 PCT/EP2007/052854
- 2-hydroxy-5-(1-hydroxy-2- {2-[4-(2-hydroxy-2-phenyl-ethylamino)-phenyl]-
ethylamino } -ethyl)-benzaldehyde
- N-[2-hydroxy-5-(1-hydroxy-2-{2-[4-(2-hydroxy-2-phenyl-ethylamino)-phenyl]-
ethylamino } -ethyl)-phenyl]-formamide
- 8-hydroxy-5-(1-hydroxy-2-{2-[4-(6-methoxy-biphenyl-3-ylamino)-phenyl]-
ethylamino } -ethyl)-1 H-quinolin-2-one
- 8-hydroxy-5-[1-hydroxy-2-(6-phenethylamino-hexylamino)-ethyl]-1H-quinolin-2-
one
- 5-[2-(2- {4-[4-(2-amino-2-methyl-propoxy)-phenylamino]-phenyl }-ethylamino)-
1-
hydroxy-ethyl]-8-hydroxy-1 H-quinolin-2-one
- [3-(4- {6-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
hexyloxy} -
butyl)-S-methyl-phenyl]-urea
- 4-(2- {6-[2-(2.6-dichloro-benzyloxy)-ethoxy]-hexylamino } -1-hydroxy-ethyl)-
2-
hydroxymethyl-pheno l
- 3-(4-{6-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
hexyloxy}-
butyl)-benzylsulphonamide
- 3-(3- {7-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
heptyloxy}-
propyl)-benzylsulphonamide
- 4-(2- {6-[4-(3-cyclopentanesulphonyl-phenyl)-butoxy]-hexylamino}-1-hydroxy-
ethyl)-
2-hydroxymethyl-phenol
- N-Adamantan-2-yl-2-(3- {2-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-
ethylamino]-propyl } -phenyl)-acetamide
optionally in the form of the racemates, enantiomers, diastereomers thereof
and optionally
in the form of the pharmacologically acceptable acid addition salts, solvates
or hydrates
thereof. According to the invention the acid addition salts of the
betamimetics are
preferably selected from among the hydrochloride, hydrobromide, hydriodide,
hydrosulphate, hydrophosphate, hydromethanesulphonate, hydronitrate,
hydromaleate,
hydroacetate, hydrocitrate, hydrofumarate, hydrotartrate, hydroxalate,
hydrosuccinate,
hydrobenzoate and hydro-p-toluenesulphonate.
CA 02646337 2008-09-17
WO 2007/110402 10 PCT/EP2007/052854
The anticholinergics used are preferably compounds selected from among the
tiotropium
salts, preferably the bromide salt, oxitropium salts, preferably the bromide
salt, flutropium
salts, preferably the bromide salt, ipratropium salts, preferably the bromide
salt,
glycopyrronium salts, preferably the bromide salt, trospium salts, preferably
the chloride
salt, tolterodine. In the above-mentioned salts the cations are the
pharmacologically active
constituents. As anions the above-mentioned salts may preferably contain the
chloride,
bromide, iodide, sulphate, phosphate, methanesulphonate, nitrate, maleate,
acetate, citrate,
fumarate, tartrate, oxalate, succinate, benzoate or p-toluenesulphonate, while
chloride,
bromide, iodide, sulphate, methanesulphonate or p-toluenesulphonate are
preferred as
counter-ions. Of all the salts the chlorides, bromides, iodides and
methanesulphonates are
particularly preferred.
Other preferred anticholinergics are selected from among the salts of formula
AC-1
.
ao N O
0
X HO
S
S
AC-1
wherein X denotes an anion with a single negative charge, preferably an anion
selected
from among the fluoride, chloride, bromide, iodide, sulphate, phosphate,
methanesulphonate, nitrate, maleate, acetate, citrate, fumarate, tartrate,
oxalate, succinate,
benzoate and p-toluenesulphonate, preferably an anion with a single negative
charge,
particularly preferably an anion selected from among the fluoride, chloride,
bromide,
methanesulphonate and p-toluenesulphonate, particularly preferably bromide,
optionally in
the form of the racemates, enantiomers or hydrates thereof. Of particular
importance are
those pharmaceutical combinations which contain the enantiomers of formula AC-
1-en
CA 02646337 2008-09-17
WO 2007/110402 11 PCT/EP2007/052854
0-0 O
p X-
AC-1-en
wherein X - may have the above-mentioned meanings. Other preferred
anticholinergics
are selected from the salts of formula AC-2
\
OH
,j,'
R X
AC-2
wherein R denotes either methyl or ethyl and wherein X may have the above-
mentioned
meanings. In an alternativen embodiment the compound of formula AC-2 may also
be
present in the form of the free base AC-2-base.
OH
N
AC-2-base
Other specified compounds are:
- tropeno12,2-diphenylpropionate methobromide,
- scopine 2,2-diphenylpropionate methobromide,
- scopine 2-fluoro-2,2-diphenylacetate methobromide,
- tropenol 2-fluoro-2,2-diphenyl acetate methobromide;
CA 02646337 2008-09-17
WO 2007/110402 12 PCT/EP2007/052854
- tropeno13,3',4,4'-tetrafluorobenzilate methobromide,
- scopine 3,3',4,4'-tetrafluorobenzilate methobromide,
- tropeno14,4'-difluorobenzilate methobromide,
- scopine 4,4'-difluorobenzilate methobromide,
- tropeno13,3'-difluorobenzilate methobromide,
- scopine 3,3'- difluorobenzilate methobromide;
- tropenol 9-hydroxy-fluorene-9-carboxylate methobromide;
- tropenol 9-fluoro-fluorene-9-carboxylate methobromide;
- scopine 9-hydroxy-fluorene-9- carboxylate methobromide;
- scopine 9-fluoro-fluorene-9- carboxylate methobromide;
- tropenol 9-methyl-fluorene-9- carboxylate methobromide;
- scopine 9-methyl-fluorene-9- carboxylate methobromide;
- cyclopropyltropine benzilate methobromide;
- cyclopropyltropine 2,2-diphenylpropionate methobromide;
- cyclopropyltropine 9-hydroxy-xanthene-9-carboxylate methobromide;
- cyclopropyltropine 9-methyl-fluorene-9-carboxylate methobromide;
- cyclopropyltropine 9-methyl-xanthene-9-carboxylate methobromide;
- cyclopropyltropine 9-hydroxy-fluorene-9-carboxylate methobromide;
- cyclopropyltropine methyl 4,4'-difluorobenzilate methobromide.
- tropenol 9-hydroxy-xanthene-9-carboxylate methobromide;
- scopine 9-hydroxy-xanthene-9-carboxylate methobromide;
- tropenol 9-methyl-xanthene-9-carboxylate -methobromide;
- scopine 9-methyl-xanthene-9-carboxylate -methobromide;
- tropenol 9-ethyl-xanthene-9-carboxylate methobromide;
- tropenol 9-difluoromethyl-xanthene-9-carboxylate methobromide;
- scopine 9-hydroxymethyl-xanthene-9-carboxylate methobromide,
The above-mentioned compounds may also be used as salts within the scope of
the present
invention, wherein instead of the methobromide the salts metho-X are used,
wherein X
may have the meanings given hereinbefore for X.
CA 02646337 2008-09-17
WO 2007/110402 13 PCT/EP2007/052854
As corticosteroids it is preferable to use compounds selected from among
beclomethasone,
betamethasone, budesonide, butixocort, ciclesonide, deflazacort,
dexamethasone,
etiprednol, flunisolide, fluticasone, loteprednol, mometasone, prednisolone,
prednisone,
rofleponide, triamcinolone, RPR-106541, NS-126, ST-26 and
- (S)-fluoromethy16,9-difluoro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-l6-
methyl-3-
oxo-androsta-1,4-diene-l7-carbothionate
- (S)-(2-oxo-tetrahydro-furan-3S-yl)6,9-difluoro-l1-hydroxy-16-methyl-3-oxo-17-
propionyloxy-androsta-1,4-diene-l7-carbothionate,
- cyanomethy16a,9a-difluoro-11(3-hydroxy-l6a-methyl-3-oxo-l7a-(2,2,3,3-
tertamethylcyclopropylcarbonyl)oxy-androsta-1,4-diene-17 f 3-carboxylate
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the salts and derivatives thereof, the solvates
and/or hydrates
thereof. Any reference to steroids includes a reference to any salts or
derivatives, hydrates
or solvates thereof which may exist. Examples of possible salts and
derivatives of the
steroids may be: alkali metal salts, such as for example sodium or potassium
salts,
sulphobenzoates, phosphates, isonicotinates, acetates, dichloroacetates,
propionates,
dihydrogen phosphates, palmitates, pivalates or furoates.
PDE4-inhibitors which may be used are preferably compounds selected from among
enprofyllin, theophyllin, roflumilast, ariflo (cilomilast), tofimilast,
pumafentrin, lirimilast,
arofyllin, atizoram, D-4418, Bay-198004, BY343, CP-325.366, D-4396 (Sch-
351591),
AWD-12-281 (GW-842470), NCS-613, CDP-840, D-4418, PD-168787, T-440, T-2585,
V-11294A, CI-1018, CDC-801, CDC-3052, D-22888, YM-58997, Z-15370 and
- N-(3,5-dichloro-l-oxo-pyridin-4-yl)-4-difluoromethoxy-3-
cyclopropylmethoxybenzamide
- (-)p-[(4aR*, l ObS *)-9-ethoxy-1,2,3,4,4a,10b-hexahydro-8-methoxy-2-
methylbenzo [s] [ 1,6]naphthyridin-6-yl]-N,N-diisopropylbenzamide
- (R)-(+)-1-(4-bromobenzyl)-4-[(3-cyclopentyloxy)-4-methoxyphenyl]-2-
pyrrolidone
- 3-(cyclopentyloxy-4-methoxyphenyl)-1-(4-N'-[N-2-cyano-S-methyl-
isothioureido]benzyl)-2-pyrrolidone
- cis[4-cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)cyclohexane-l-carboxylic
acid]
CA 02646337 2008-09-17
WO 2007/110402 14 PCT/EP2007/052854
- 2-carbomethoxy-4-cyano-4-(3-cyclopropylmethoxy-4-difluoromethoxy-
phenyl)cyclohexan-l-one
- cis[4-cyano-4-(3 -cyclopropylmethoxy-4-difluoromethoxyphenyl)cyclohexan-l-
ol]
- (R)-(+)-ethyl [4-(3 -cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-ylidene]
acetate
- (S)-(-)-ethyl[4-(3 -cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-ylidene]
acetate
- 9-cyclopentyl-5,6-dihydro-7-ethyl-3-(2-thienyl)-9H-pyrazolo[3.4-c]-1,2,4-
triazolo[4.3-
a]pyridine
- 9-cyclopentyl-5,6-dihydro-7-ethyl-3-(tert-butyl)-9H-pyrazolo[3.4-c]-1,2,4-
triazolo[4.3-
a]pyridine
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition salts
thereof, the
solvates and/or hydrates thereof. According to the invention the acid addition
salts of the
PDE4 inhibitors are preferably selected from among the hydrochloride,
hydrobromide,
hydriodide, hydrosulphate, hydrophosphate, hydromethanesulphonate,
hydronitrate,
hydromaleate, hydroacetate, hydrocitrate, hydrofumarate, hydrotartrate,
hydroxalate,
hydrosuccinate, hydrobenzoate and hydro-p-toluenesulphonate.
The LTD4-antagonists used are preferably compounds selected from among
montelukast,
pranlukast, zafirlukast, MCC-847 (ZD-3523), MN-001, MEN-91507 (LM-1507), VUF-
5078, VUF-K-8707, L-733321 and
- 1-(((R)-(3-(2-(6,7-difluoro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2- hydroxy-2-
propyl)phenyl)thio)methylcyclopropane-acetic acid,
- 1-(((1(R)-3(3-(2-(2,3-dichlorothieno[3,2-b]pyridin-5-yl)-(E)-ethenyl)phenyl)-
3-(2-(1-
hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid
- [2-[[2-(4-tert-butyl-2-thiazolyl)-5-benzofuranyl]oxymethyl]phenyl]acetic
acid
optionally in the form of the racemates, enantiomers or diastereomers thereof
and
optionally in the form of the pharmacologically acceptable acid addition
salts, solvates
and/or hydrates thereof. According to the invention these acid addition salts
are preferably
selected from among the hydrochloride, hydrobromide, hydroiodide,
hydrosulphate,
hydrophosphate, hydromethanesulphonate, hydronitrate, hydromaleate,
hydroacetate,
hydrocitrate, hydrofumarate, hydrotartrate, hydroxalate, hydrosuccinate,
hydrobenzoate
CA 02646337 2008-09-17
WO 2007/110402 15 PCT/EP2007/052854
and hydro-p-toluenesulphonate. By salts or derivatives which the LTD4-
antagonists may
optionally be capable of forming are meant, for example: alkali metal salts,
such as for
example sodium or potassium salts, alkaline earth metal salts,
sulphobenzoates,
phosphates, isonicotinates, acetates, propionates, dihydrogen phosphates,
palmitates,
pivalates or furoates.
EGFR-inhibitors which may be used are preferably compounds selected from among
cetuximab, trastuzumab, ABX-EGF, Mab ICR-62 and
- 4-[(3-chloro-4-fluorophenyl)amino]-6- {[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]-
amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6- {[4-(N,N-diethylamino)-1-oxo-2-buten-l-
yl]-
amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6- { [4-(N,N-dimethylamino)-1-oxo-2-buten-
l-
yl] amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6- {[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]amino } -7-
cyclopentyloxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-((R)-6-methyl-2-oxo-morpholin-4-
yl)-1-
oxo-2-buten-l-yl]amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-((R)-6-methyl-2-oxo-morpholin-4-
yl)-1-
oxo-2-buten-l-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {[4-((R)-2-methoxymethyl-6-oxo-
morpholin-4-
yl)-1-oxo-2-buten-l-yl]amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-((S)-6-methyl-2-oxo-morpholin-4-yl)-
ethoxy]-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-( {4-[N-(2-methoxy-ethyl)-N-methyl-
amino]-1-
oxo-2-buten-l-y1 } amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-1
-
yl] amino } -7-cyclopentyloxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-{[4-(N,N-bis-(2-methoxy-ethyl)-amino)-1-oxo-
2-
buten-l-yl]amino}-7-cyclopropylmethoxy-quinazoline
CA 02646337 2008-09-17
WO 2007/110402 16 PCT/EP2007/052854
- 4-[(R)-(1-phenyl-ethyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-ethyl-amino]-1-
oxo-2-
buten-l-yl} amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-amino]-1-
oxo-2-
buten-l-yl } amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-( {4-[N-(tetrahydropyran-4-yl)-N-methyl-
amino]-1-
oxo-2-buten-l-yl } amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-
yl] amino } -7-((R)-tetrahydrofuran-3-yloxy)-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-
yl] amino } -7-((S)-tetrahydrofuran-3-yloxy)-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-( {4-[N-(2-methoxy-ethyl)-N-methyl-
amino]-1-
oxo-2-buten-l-yl } amino)-7-cyclopentyloxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N-cyclopropyl-N-methyl-amino)-1-
oxo-2-
buten-l-yl]amino } -7-cyclopentyloxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-
yl]amino } -7-[(R)-(tetrahydrofuran-2-y1)methoxy]-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-
yl] amino } - 7- [(S)-(tetrahydro furan-2-yl)methoxy] -quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6.7-bis-(2-methoxy-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)-propyloxy]-6-
[(vinyl-
carbonyl)amino]-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-(4-hydroxy-phenyl)-7H-pyrrolo[2,3-
d]pyrimidine
- 3-cyano-4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-
buten-l-yl]amino } -7-ethoxy-quinoline
- 4-{[3-chloro-4-(3-fluoro-benzyloxy)-phenyl]amino}-6-(5-{[(2-methanesulphonyl-
ethyl)amino]methyl } -furan-2-yl)quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6- { [4-((R)-6-methyl-2-oxo-morpholin-4-yl)-1-
oxo-2-
buten-l-yl]amino} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]-
amino}-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline
CA 02646337 2008-09-17
WO 2007/110402 17 PCT/EP2007/052854
- 4-[(3-chloro-4-fluorophenyl)amino]-6-( {4-[N,N-bis-(2-methoxy-ethyl)-amino]-
1-oxo-
2-buten-1-yl } amino)-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6- { [4-(5.5-dimethyl-2-oxo-morpholin-4-yl)-1-
oxo-2-
buten-1-yl] amino } -quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2.2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy]-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2.2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy]-7-[(R)-(tetrahydrofuran-2-yl)methoxy] -quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-7-[2-(2.2-dimethyl-6-oxo-morpholin-4-yl)-
1o ethoxy]-6-[(S)-(tetrahydrofuran-2-yl)methoxy]-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{2-[4-(2-oxo-morpholin-4-yl)-piperidin-
l-yl]-
ethoxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(tert.-butyloxycarbonyl)-piperidin-
4-yloxy]-
7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexan-1-yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methanesulphonylamino-
cyclohexan-
1-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-3-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(morpholin-4-yl)carbonyl]-
piperidin-4-yl-
oxy} -7-methoxy-quinazoline
- 4-[(3 -chloro-4-fluoro-phenyl)amino] -6- { 1-[(methoxymethyl)carbonyl]-
piperidin-4-yl-
oxy} -7-methoxy-quinazol ine
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-3-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(2-acetylamino-ethyl)-piperidin-4-
yloxy]-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-ethoxy-
quinazoline
CA 02646337 2008-09-17
WO 2007/110402 18 PCT/EP2007/052854
- 4-[(3 -chloro-4-fluoro-phenyl) amino] -6-((S)-tetrahydrofuran- 3 -yloxy)-7-
hydroxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-methoxy-
ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {trans-4-
[(dimethylamino)sulphonylamino]-
cyclohexan-l-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {trans-4-[(morpholin-4-
yl)carbonylamino]-
cyclohexan-l-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {trans-4-[(morpholin-4-
yl)sulphonylamino]-
cyclohexan-l-yloxy}-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
acetylamino-
ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
methanesulphonylamino-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(piperidin-l-yl)carbonyl]-
piperidin-4-
yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-aminocarbonylmethyl-piperidin-4-
yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(tetrahydropyran-4-
yl)carbonyl]-N-
methyl-amino}-cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-[(morpholin-4-yl)carbonyl]-
N-
methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-[(morpholin-4-yl)sulphonyl]-
N-
methyl-amino } -cyclohexan-1-yloxy)-7-methoxy- quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-ethansulphonylamino-
cyclohexan-l-
yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-
ethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-(2-
methoxy-ethoxy)-quinazoline
CA 02646337 2008-09-17
WO 2007/110402 19 PCT/EP2007/052854
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-piperidin-4-
yloxy]-7-(2-
methoxy-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-acetylamino-cyclohexan-1-yloxy)-
7-
methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-[ 1-(tert.-butyloxycarbonyl)-piperidin-4-
yloxy]-7-
methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(tetrahydropyran-4-yloxy]-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-[(piperidin-l-yl)carbonyl]-
N-methyl-
amino}-cyclohexan-l-yloxy)-7-methoxy-quinazoline
lo - 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(4-methyl-piperazin-l-
yl)carbonyl]-
N-methyl-amino}-cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- {cis-4-[(morpholin-4-
yl)carbonylamino]-
cyclohexan-l-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[2-(2-oxopyrrolidin-1-yl)ethyl]-
piperidin-4-
yloxy} -7-methoxy-quinazoline
4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(morpholin-4-yl)carbonyl]-
piperidin-4-
yloxy} -7-(2-methoxy-ethoxy)-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(1-acetyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7(2-
methoxy-
ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-isopropyloxycarbonyl-piperidin-4-
yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-methylamino-cyclohexan-l-yloxy)-
7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{cis-4-[N-(2-methoxy-acetyl)-N-methyl-
amino]-cyclohexan-l-yloxy} -7-methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-quinazoline
CA 02646337 2008-09-17
WO 2007/110402 20 PCT/EP2007/052854
- 4-[(3-ethynyl-phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-piperidin-4-yloxy]-7-
methoxy-
quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-{1-[(morpholin-4-yl)carbonyl]-piperidin-4-
yloxy}-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(cis-2,6-dimethyl-morpholin-4-
yl)carbonyl] -piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(2-methyl-morpholin-4-
yl)carbonyl]-
piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(S,S)-(2-oxa-5-aza-bicyclo[2,2,1
]hept-5-
yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(N-methyl-N-2-methoxyethyl-
amino)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-ethyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(2-methoxyethyl)carbonyl]-
piperidin-4-
yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6- { 1-[(3-methoxypropyl-amino)-
carbonyl]-
piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-methanesulphonyl-N-methyl-
amino)-
cyclohexan-l-yloxy]-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-acetyl-N-methyl-amino)-
cyclohexan-
1-yloxy]-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methylamino-cyclohexan-l-
yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-(N-methanesulphonyl-N-methyl-
amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-dimethylamino-cyclohexan-l-
yloxy)-
7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4- {N-[(morpholin-4-
yl)carbonyl]-N-
methyl-amino}-cyclohexan-l-yloxy)-7-methoxy-quinazoline
CA 02646337 2008-09-17
WO 2007/110402 21 PCT/EP2007/052854
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2.2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy]-7-[(S)-(tetrahydrofuran-2-yl)methoxy]-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-methoxy-
quinazoline
optionally in the form of the racemates, enantiomers, diastereomers thereof
and optionally
in the form of the pharmacologically acceptable acid addition salts, solvates
or hydrates
thereof. According to the invention these acid addition salts are preferably
selected from
among the hydrochloride, hydrobromide, hydriodide, hydrosulphate,
hydrophosphate,
hydromethanesulphonate, hydronitrate, hydromaleate, hydroacetate,
hydrocitrate,
hydrofumarate, hydrotartrate, hydroxalate, hydrosuccinate, hydrobenzoate and
hydro-p-
toluenesulphonate.
The dopamine agonists used are preferably compounds selected from among
bromocriptin,
cabergoline, alpha-dihydroergocryptine, lisuride, pergolide, pramipexol,
roxindol,
ropinirol, talipexol, tergurid and viozan, optionally in the form of the
racemates,
enantiomers, diastereomers thereof and optionally in the form of the
pharmacologically
acceptable acid addition salts, solvates or hydrates thereof. According to the
invention
these acid addition salts are preferably selected from among the
hydrochloride,
hydrobromide, hydriodide, hydrosulphate, hydrophosphate,
hydromethanesulphonate,
hydronitrate, hydromaleate, hydroacetate, hydrocitrate, hydrofumarate,
hydrotartrate,
hydrooxalate, hydrosuccinate, hydrobenzoate and hydro-p-toluenesulphonate.
H 1-Antihistamines which may be used are preferably compounds selected from
among
epinastine, cetirizine, azelastine, fexofenadine, levocabastine, loratadine,
mizolastine,
ketotifen, emedastine, dimetindene, clemastine, bamipine, cexchlorpheniramine,
pheniramine, doxylamine, chlorophenoxamine, dimenhydrinate, diphenhydramine,
promethazine, ebastine, desloratidine and meclozine, optionally in the form of
the
racemates, enantiomers, diastereomers thereof and optionally in the form of
the
pharmacologically acceptable acid addition salts, solvates or hydrates
thereof. According
CA 02646337 2008-09-17
WO 2007/110402 22 PCT/EP2007/052854
to the invention these acid addition salts are preferably selected from among
the
hydrochloride, hydrobromide, hydriodide, hydrosulphate, hydrophosphate,
hydromethanesulphonate, hydronitrate, hydromaleate, hydroacetate,
hydrocitrate,
hydrofumarate, hydrotartrate, hydroxalate, hydrosuccinate, hydrobenzoate and
hydro-p-
toluenesulphonate.
It is also possible to use inhalable macromolecules, as disclosed in EP 1 003
478.
In addition, the compounds may come from the groups of ergot alkaloid
derivatives, the
triptans, the CGRP-inhibitors, the phosphodiesterase-V inhibitors, optionally
in the form of
the racemates, enantiomers or diastereomers thereof, optionally in the form of
the
pharmacologically acceptable acid addition salts, the solvates and/or hydrates
thereof.
Examples of ergot alkaloid derivatives are dihydroergotamine and ergotamine.
For inhalation, suitable substances include pharmaceutical compositions,
pharmaceutical
formulations and mixtures with the above-mentioned active substances, as well
as the salts
and esters thereof and combinations of these active substances, salts and
esters.
CA 02646337 2008-09-17
WO 2007/110402 23 PCT/EP2007/052854
Description of the Figures:
The Figures show, by way of example, the different shapes of a capsule or of
an individual
well of a blister (known over all as packaging means) and the corresponding
piercing
positions.
The Figures serve to illustrate the invention without restricting its scope.
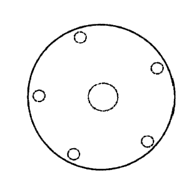
Fig 1: sphere or hemisphere, 5 hole-shaped inlet openings, I outlet
Fig 2: sphere or hemisphere, 4 hole-shaped inlet openings, 1 outlet
Fig 3: oval shape, 6 hole-shaped inlet openings, I outlet
Fig 4: oval shape, 5 hole-shaped inlet openings, asymmetrical, I outlet
Fig 5: oval shape, 3 hole-shaped inlet openings, asymmetrical, 1 outlet
Fig 6: oval shape, 2 hole-shaped inlet openings
Fig 7: bone-shaped, 2x3 hole-shaped inlet openings, I outlet
Fig 8: bone-shaped, 2x2 hole-shaped inlet openings, asymmetrical, I outlet
Fig 9: figure-of-eight shape, 2x3 hole-shaped inlet openings, 1 outlet
Fig 10: figure-of-eight shape, 2x2 hole-shaped inlet openings, asymmetrical, I
outlet
Fig 11: teardrop-shape, 3 hole-shaped inlet openings, I outlet
Fig 12: teardrop-shape, 4 hole-shaped inlet openings, 1 outlet
Fig 13: teardrop-shape, crescent-shaped inlet, 1 outlet
Example:
The emptying properties were determined using a standard powder (glass beads)
by
measuring the time taken for emptying a given constant flow by volume
(corresponding to
10 L/min of air). Table 1 shows the of the different shapes of capsules or
blisters. The inlet
openings are shown in the drawings as small circles while the outlet openings
are shown as
large circles.
CA 02646337 2008-09-17
WO 2007/110402 24 PCT/EP2007/052854
Table 1
type name emptying time (s)
1 sphere or hemisphere, 5 inlets > 100
2 sphere or hemisphere, 4 inlets > 100
3 oval shape, 6 inlets 57
4 oval shape, 5 inlets, asymmetrical 10
oval shape, 3 inlets, asymmetrical 2.2
6 oval shape, 2 holes > 100
7 bone, 2x3 inlets 38
8 bone, 2x2 inlets, asymmetrical 2.7
9 figure-of-eight, 2x3 inlets > 100
figure-of-eight, 2x2 inlets, asymmetrical > 100
11 teardrop, 3 inlets 2.9
12 teardrop, 4 inlets 2.4
13 teardrop, crescent-shaped inlet 1.7
The results in Table 1 show that the emptying properties of a package depend
to a
5 considerable extent on its shape and opening or openings.
CA 02646337 2008-09-17
WO 2007/110402 25 PCT/EP2007/052854
Table 2
residue after emptying (%) emptying time (seconds)
Name micronised fenoterol tiotropium micronised fenoterol tiotropium
fenoterol powder powder fenoterol powder powder
mixture mixture mixture mixture
1 88.63 84.02 46.896 8.46 7.91 6.8
2 87.04 87.58 45.832 9.35 7.42 6.44
3 52.95 39.28 13.936 4.29 3.13 3.46
4 29.93 23.49 5.744 1.19 1.27 1.66
32.88 16.37 11.408 1.2 1.31 1.03
6 80.25 71.81 33.816 6.95 6.53 5.63
7 51.37 33.93 20.56 3.95 3.18 2.92
8 38.39 19.33 13.216 1.71 1.41 0.86
9 71.6 57.52 31.56 5.7 4.76 4.23
68.69 54.36 28.608 5.85 4.03 5.24
11 38.07 22.15 8.304 2.21 1.82 0.93
12 37.73 9.78 6.984 1.85 1.27 0.3
13 34.36 10.81 5.12 0.69 0.35 0.6
The flow rate of the air through the package of standard commercial size: 10
litres per
5 minutes
The active substance in micronised form has a particle size of 1- 5 m. As
well as the
active substance the powder mixture also contains lactose 200 m.
10 The results of Table 2 show, for the active substances fenoterol and
tiotropium
a) the residue left in the well, and
b) the emptying time of the well.
CA 02646337 2008-09-17
WO 2007/110402 26 PCT/EP2007/052854
A well of a package which has an optimum air flow by reason of its shape and
opening
patter has significantly enhanced emptying properties. The following formulae
applies to
the ratio V (sum of the inflow surfaces divided by the sum of the outflow
surfaces): 0.5 <
V < 2. Preferably the sum of the inflow surfaces should be equal to the sum of
the outflow
surfaces.