Note: Descriptions are shown in the official language in which they were submitted.
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
1
TITLE
ANTIFREEZE COMPOSITION
DESCRIPTION
Field of the invention
The present invention relates to an antifreeze
composition for avoiding the production of ice, or
removing ice, from surfaces of different type, such as
roads, pavements, airstrips, vehicle windows, hinges
of freezers, industrial machines, etc.
Description of the prior art
Methods ae known and traditionally used for
avoiding the production of ice, or removing ice, from
surfaces roads, pavements, airstrips, etc. provides
salt, usually sodium chloride, calcium chloride or
magnesium chloride.
In particular, the use of sodium chloride is
preferred with respect to the use of calcium chloride,
for its low cost and its easy availability. The use of
calcium chloride is, furthermore, limited for
dissolving the ice already formed, since on the
asfalto asciutto tends to form a slippery layer.
The process through which the addition of salt
causes the water freezing point to decrease comprises,
synthetically, the dissociation of the salt molecules
into ions and their electrostatic link to the water
molecules. When the temperature drops below zero, the
water starts to form crystals of ice, which however do
not have the possibility of growing for the presence
of the ions.
However, the salts above cited have different
drawbacks. Firstly, they provide an effective
antifreeze action only above a temperature of about
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
2
-5 C, whereas they loose efficiency for lower
temperatures.
Furthermore, such substances have a considerable
environmental impact, since once dissolved they are
adsorbed by the ground and can pollute the roots of
plants, affecting the absorption of water.
Another drawback of the above described salts is
their high rate of corrosiveness due mainly to their
acidity. This can cause phenomena of corrosion, for
example for vehicles that move on the roads on which
the salt has been scattered.
Other substances commonly used as antifreeze
agents are magnesium chloride, potassium acetate,
sodium acetate, ammonium phosphate, ammonium nitrate,
alcohols having low molecular weight and urea.
However, also these substances have a
considerable environmental impact. In particular, the
alcohols with low molecular weight volatilize easily
and pollute then the surrounding atmosphere.
Summary of the invention
It is then a feature of the invention to provide
an antifreeze composition capable of providing an
effective antifreeze action also in conditions of
extremely cold temperatures.
It is another feature of the invention to
provide an antifreeze composition having low
environmental impact.
It is a further feature of the invention to
provide an antifreeze composition for avoiding the
drawbacks of the antifreeze compositions of prior art.
These and other features are accomplished with
one exemplary antifreeze composition, according to the
invention, comprising:
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
3
- at least one metal chloride;
whose main feature is to provide furthermore:
- an an aqueous solution having an alkaline pH in
which at least one of the following ions is
dissolved: Bromide (Br-), Bicarbonate (HC03-),
Borate (B033-) , Silicate (Si03-) , Fluoride (F-) ,
Iodine (I-) .
In particular, the aqueous solution may have a
pH set between 7.2 and 10.5.
Advantageously, the aqueous solution has a pH
set between 7.3 and 9.5.
Preferably, the aqueous solution has a pH set
between 7.5 and 8.5.
Advantageously, the or each ion of the an aqueous
solution having an alkaline pH has the following
concentration:
- Bromide ions (Br-) : set between 10 mg/1 and 500
mg/1;
- Bicarbonate ions (HC03-) : set between 0.1 mg/1
and 500 mg/l.
- Borate ions (B033-) : set between 10 mg/1 and 200
mg/l;
- Silicate ions (Si03-) : set between 0,01 mg/1
and 5 mg/l;
- Fluoride ions (F-) : set between 0,01 mg/1 and 5
mg/l;
- Iodine ions (I-) : set between 0,01 mg/1 and 5
mg/l.
Furthermore, the an aqueous solution having an
alkaline pH can comprise at least one of the following
ions in the concentration indicated:
- ions Cl-: set between 15000 mg/1 and 30000
mg/1;
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
4
- ions Na+: set between 5000 mg/1 and 20000 mg/l;
- ions S042-: set between 1000 mg/1 and 5000 mg/l;
- ions Mg2+: set between 500 mg/1 and 3000 mg/l;
- ions Ca2+: set between 100 mg/1 and 1000 mg/1;
- ions K+: set between 100 mg/1 and 1000 mg/l.
Advantageously, the aqueous solution is sea water.
In particular, the or each chloride can be a
metal alkaline chloride or a earth metal alkaline
chloride.
Advantageously, the or each chloride is selected
from the group comprised of:
- calcium chloride;
- magnesium chloride;
- sodium chloride;
- potassium chloride;
- lithium chloride;
- a combination thereof.
Preferably, the chloride compound is a
combination of calcium chloride and magnesium
chloride.
In particular, the antifreeze composition can
provide:
- calcium chloride: in an amount set between 5%
and 50% by weight;
- magnesium chloride: in an amount set between 5%
and 50% by weight;
- an aqueous solution having alkaline pH: in an
amount set between 50% and 95% by weight.
Advantageously, the antifreeze composition
provides:
- calcium chloride: in an amount set between 10%
and 40% by weight;
- magnesium chloride: in an amount set between 5%
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
and 40% by weight;
- an aqueous solution having alkaline pH: in an
amount set between 50% and 80% by weight.
Preferably, the antifreeze composition provides:
- calcium chloride: in an amount set between 25%
and 35% by weight;
- magnesium chloride: in an amount set between 5%
and 15% by weight;
- an aqueous solution having alkaline pH: in an
amount set between 55% and 65% by weight.
In particular, the cited metal chlorides, such as
calcium chloride and magnesium chloride, are strong
electrolytes. Therefore in the aqueous solution they
dissociate completely into metal ions, for example
Ca2+ and Cl- and Mg2+ and Cl- respectively. These ions
in the aqueous solution are surrounded by weak
electrolytes having an opposite charge ivi present,
such as bromide ions and bicarbonate ions, etc.
poducing a "shield" effect, as provided by the of
Debye-Huckel theory. The "shield" effect made by the
weak electrolytes increases, in particular, the
distance between the calcium ions (Ca2+) and the
chloride ions (Cl-) from one side, and between the
magnesium ions (Mg2+) and the chloride ions (Cl-) from
another side. Therefore, in accordance with the
Coulomb's law, the electrostatic force between the
ions is reduced and then the ions same remain in
solution also for cold temperature instead of
crystallizing.
The above described features are
diagrammatically shown in figures 1 and 2 attached.
In figure 1 is diagrammatically shown the
situation between the ions having opposite sign
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
6
present in an aqueous solution of a metal chloride
bivalent. In particular, the distance between the ions
having opposite sign, i.e. between a metal ion 10,
such as a calcium ion Ca2+, or a magnesium ion Mg2+,
and the chloride ion (Cl-) 20 corresponds to the
distance at balance where the electrostatic attraction
force is the same as the repulsive force. More in
detail, the attraction force between anions and
cations is directly proportional to the product of the
electric charge of the ions in play and is inversally
proportional to the square of their distance as
expressed by the known equation:
F=-k= qi' q2/r2 =
Under action of this force the ions approach to
one antoher, but the approaching step not will proceed
beyond a certain distance because the electronic
shields of the two ions have equal charge, i.e.
negative, is-repulsive.
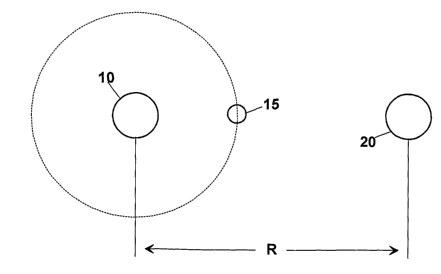
In figure 2, instead, the situation is shown of
the interaction between a metal bivalent ion 10, such
as Ca2+, or Mg2+, and a chloride ion (Cl-) 20 in an
antifreeze composition, according to the invention. As
shown in figure 2, the distance "R" between the metal
bivalent ion 10 and the chloride ion (Cl-) 20 is
higher than the distance "r" between the two ions of
the aqueous solution of figure 1. This is obtained for
the presence of at least one weak ion 15, for example
Bicarbonate (HCO3-) , Borate (B033-) , Iodine (I-),
Fluoride (F-), etc. which interact with the ions of
the bivalent metal and of the chloride ion.
Therefore, the antifreeze composition according
to the invention remains in the liquid state also for
very low temperatures, about -80 C allowing to provide
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
7
tne above advantages.
The invention will now be described in a way not
limitative, through the following examples.
Example 1
An antifreeze composition is prepared comprising
the 40% by weight of magnesium chloride (MgC12) and
the 60% by weight of sea water at pH = 8. The
composition remains in the liquid state up to -60 C.
Example 2
An antifreeze composition is prepared comprising
4% by weight of calcium chloride (CaCl2), 36% by
weight of magnesium chloride (MgC12) and 60% by weight
of sea water at pH = 8. The composition remains in the
liquid state up to -60 C.
Example 3
An antifreeze composition is prepared comprising
16% by weight of calcium chloride (CaC12), 24% by
weight of magnesium chloride (MgCl2) and 60% by weight
of sea water at pH = 8. The composition remains in the
liquid state up to -60 C.
Example 4
An antifreeze composition is prepared comprising
20% by weight of calcium chloride (CaC12), 20% by
weight of magnesium chloride (MgC12) and 60% by weight
of sea water at pH = 8. The composition remains in the
liquid state up to -70 C.
Example 5
An antifreeze composition is prepared comprising
24% by weight of calcium chloride (CaCl2), 16% by
weight of magnesium chloride (MgC12) and 60% by weight
of sea water at pH = 8. The composition remains in the
liquid state up to -80 C.
Example 6
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
8
An antifreeze composition is prepared comprising
28% by weight of calcium chloride (CaC12), 12% by
weight of magnesium chloride (MgC12) and 60% by weight
of sea water at pH = 8. The composition remains in the
liquid state up to -90 C.
Example 7
An antifreeze composition is prepared comprising
36% by weight of calcium chloride (CaC12), 4% by
weight of magnesium chloride (MgC12) and 60% by weight
of sea water at pH = 8. The composition remains in the
liquid state up to -80 C.
Example 8
An antifreeze composition is prepared comprising
40% by weight of calcium chloride (CaC12), and 60% by
weight of sea water at pH = 8. The composition remains
in the liquid state up to -80 C.
In table 1 are reported, finally, the results of
tests on some antifreeze compositions, according to
the invention.
In particular, the antifreeze compositions,
indicated in table with the Roman numerals from I to
XI, comprise a solution of sea water at pH=8, calcium
chloride (CaCl2) and magnesium chloride (MgC12) in
variable proportions. The concentration (%p/p) of each
component is expressed in grams of solute for 100
grams of composition.
The physical state of each composition at
different temperatures is indicated.
CA 02647731 2008-10-06
WO 2007/113658 PCT/IB2007/000881
9
Sea Negative temperature
CaC12 MgC12
water state of the solution
Composition
np/P %p/p %p/p - -
20 C 30 C 51 C 60 C 70 C 80 C
I 0 40 60 L L L S S S
II 4 36 60 L L L S S S
III 8 32 60 L L L S S S
IV 12 28 60 L L L S S S
V 16 24 60 L L L S S S
VI 20 20 60 L L L L S S
VII 24 16 60 L L L L L S
VIII 28 12 60 L L L L L L
IX 32 8 60 L L L L L S
X 36 4 60 L L L L L S
XI 40 0 60 L L L L L S
Table 1