Note: Descriptions are shown in the official language in which they were submitted.
=CA 02647830 2013-10-23
WO 2007/137030
PCT/US2007/068930
= CENTRAL NERVOUS SYSTEM ACTIVE
FUSED BICYCLOHETEROCYCLE SUBSTITUTED AZABICYCLIC ALKANE
DERIVATIVES
Technical Field
The invention relates to fused bicycloheterocycle substituted azabicyclic
alkane
derivatives, compositions comprising such compounds, and methods of treating
conditions and disorders using such compounds and compositions.
Description of Related Technology
Nicotinic acetylcholine receptors (nAChRs) are widely distributed throughout
the
central (CNS) and peripheral (PNS) nervous systems. Such receptors play an
important
role in regulating CNS function, particularly by modulating release of a wide
range of
neurotransmitters, including, but not necessarily limited to acetylcholine,
norepinephrine,
dopamine, serotonin and GABA. Consequently, nicotinic receptors mediate a very
wide
range of physiological effects, and have been targeted for therapeutic
treatment of
disorders relating to cognitive function, learning and memory,
neurodegeneration, pain
and inflammation, psychosis and sensory gating, mood and emotion, among
others.
Many subtypes of the nAChR exist in the CNS and periphery. Each subtype has a
different effect on regulating the overall physiological function. Typically,
nAChRs are
ion channels that are constructed from a pentameric assembly of subunit
proteins. At
least 12 subunit proteins, a2-a10 and p2-134, have been identified in neuronal
tissue.
These subunits provide for a great variety of homomeric and heteromeric
combinations
that account for the diverse receptor subtypes. For example, the predominant
receptor
that is responsible for high affinity binding of nicotine in brain tissue has
composition
(a11)2(132)3 (the a4f32 subtype), while another major population of receptors
is comprised
of homomeric (a7)5 (the a7 subtype) receptors.
Certain compounds, like the plant alkaloid nicotine, interact with all
subtypes of
-1-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
the nAChRs, accounting for the physiological effects of this compound. While
nicotine
has been demonstrated to have many biological activities, not all of the
effects mediated
by nicotine are desirable. For example, nicotine exerts gastrointestinal and
cardiovascular side effects that interfere at therapeutic doses, and its
addictive nature and
acute toxicity are well-known Ligands that are selective for interaction with
only certain
subtypes of the nACIIR offer potential for achieving beneficial therapeutic
effects with an
improved margin for safety.
The a7 and AP nAChRs have been shown to play a significant role in
enhancing cognitive function, including aspects of learning, memory and
attention
(Levin, ED, 3. Neurobia 53: 633-640, 2002). For example, a7 nAChRs have been
linked to conditions and disorders related to attention deficit disorder,
attention deficit
hyperactivity disorder (ADEID), Alzheimer's disease (AD), mild cognitive
impairment,
senile dementia, dementia associated with Lewy bodies, dementia associated
with
Down's syndrome, AIDS dementia, Pick's Disease, as well as cognitive deficits
associated with schizophrenia, among other systemic activities. The a4132
receptor
subtype is implicated in attention, cognition, schizophrenia, epilepsy, and
pain control
(Paterson and Norberg, Progress in Neurobiology 61 75-111, 2000).
The activity at both a7 and a432 nAChRs can be modified or regulated by the
administration of subtype selective nAChR ligands. The ligands can exhibit
antagonist,
agonist, or partial agonist properties. Compounds that function as positive
allosteric
modulators are also known.
Although compounds that nonselectively demonstrate activity at a range of
nicotinic receptor subtypes including the a4132 and a7 nAChRs are known, it
would be
beneficial to provide compounds that interact selectively with a7-containing
neuronal
nAChRs, a402 nAChRs, or both a7 and a4P2 nAChRs compared to other subtypes.
SUMMARY OF THE INVENTION
The invention is directed to fused bicycloheterocycle substituted azabicyclic
compounds as well as compositions comprising such compounds, and method of
using
the same.
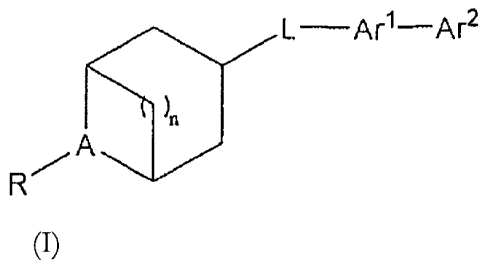
One aspect of the present invention is directed toward a compound of formula
(I)
-2-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
L¨Arl¨Ar2
FR'
(I)
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof,
wherein
n is I, 2 or 3;
A is N or N+-0-;
R is hydrogen, alkyl, cycloalkylalkyl and arylalkyl;
L is selected from the group consisting of 0, S, and --N(Ra)-;
Arl is a 6-membered aryl or 6 membered heteroaryl ring;
Ar2 is a bicyclic heteroaryl; and
Ra is selected from the group consisting of hydrogen, alkyl and alkylcarbonyl;
provided that if Ail is
/
R4 ,
then L is 0 or S.
Another aspect of the invention relates to pharmaceutical compositions
comprising compounds of the invention. Such compositions can be administered
in
accordance with a method of the invention, typically as part of a therapeutic
regimen for
treatment or prevention of conditions and disorders related to nAChR activity,
and more
particularly ca nAChR activity.
Yet another aspect of the invention relates to a method of selectively
modulating
to nAChR activity, for example ca nAChR activity. The method is useful for
treating
and/or preventing conditions and disorders related to ce7 nAChR activity
modulation in
mammals. More particularly, the method is useful for conditions and disorders
related to
attention deficit disorder, attention deficit hyperactivity disorder (AMID),
Alzheimer's
disease (AD), mild cognitive impairment, senile dementia, AIDS dementia,
Pick's
Disease, dementia associated with Lewy bodies, dementia associated with Down's
-3-
CA 02647830 2013-10-23
WO 2007/137030
PCT/1JS2007/068930
syndrome, amyotrophic lateral sclerosis, Huntington's disease, diminished CNS
function
associated with traumatic brain injury, acute pain, post-surgical pain,
chronic pain,
inflammatory pain, neuropathic pain, infertility, need for new blood vessel
growth
associated with wound healing, need for new blood vessel growth associated
with
vascularization of skin grails, and lack of circulation, more particularly
circulation
around a vascular occlusion, among other systemic activities, for example
inflammatory
response mediated by TNF.
The compounds, compositions comprising the compounds, and methods for
treating or preventing conditions and disorders by administering the compounds
are
further described herein.
DETAILED DESCRIPTION OF THE INVENTION
Definition of Terms
Certain terms as used in the specification are intended to refer to the
following
definitions, as detailed below.
The term "acyl", as used herein, means an alkyl group, as defined herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of acyl include, but are not limited to, acetyl, 1-
oxopropyl, 2,2-
dimethy1-1 -oxopropyl, 1-oxobutyl, and 1-oxopentyl.
The term "acyloxy", as used herein, means an acyl group, as defined herein,
appended to the parent molecular moiety through an oxygen atom. Representative
examples of acyloxy include, but are not limited to, acetyloxy, propionyloxy,
and
isobutyryloxy.
The term "alkenyl", as used herein, means a straight or branched chain
hydrocarbon containing from 2 to 10 carbons and containing at least one carbon-
carbon
double bond formed by the removal of two hydrogens. Representative examples of
alkenyl include, but are not limited to, ethenyl, 2-propenyl, 2-methyl-2-
propenyl, 3-
butenyl, 4-pentenyl, 5-hexenyl, 2-heptenyl, 2-methyl-l-heptenyl, and 3-
decenyl.
The term "alkoxy", as used herein, means an alkyl group as defined herein,
appended to the parent molecular moiety through an oxygen atom. Representative
examples of alkoxy include, but are not limited to, methoxy, ethoxy, propoxy,
2-propoxy,
-4-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
butoxy, tert-butoxy, pentyloxy, and hexyloxy.
The term "alkoxyalkoxy", as used herein, means an alkoxy group, as defined
herein, appended to the parent molecular moiety through another alkoxy group,
as
defined herein. Representative examples of alkoxyalkoxy include, but are not
limited to,
tert-butoxymethoxy, 2-ethoxyethoxy, 2-methoxyethoxy, and methoxymethoxy.
The term "alkoxyalkyl", as used herein, means an alkoxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of alkoxyalkyl include, but are not limited to, tert-
butoxymethyl, 2-ethoxyethyl, 2-methoxyethyl, and methoxymethyl.
The term "alkoxycarbonyl", as used herein, means an alkoxy group, as defined
herein, appended to the parent molecular moiety through a carbonyl group,
represented
by -C(0)-, as defined herein. Representative examples of alkoxycarbonyl
include, but
are not limited to, methoxycarbonyl, ethoxycarbonyl, and tert-butoxycarbonyl
The term "alkoxyimino", as used herein, means an alkoxy group, as defined
herein, appended to the parent molecular moiety through an imino group, as
defined
herein. Representative examples of alkoxyimino include, but are not limited
to,
ethoxy(imino)rnethyl and metboxy(irnino)methyl.
The term "alkoxysulfonyl", as used herein, means an alkoxy group, as defined
herein, appended to the parent molecular moiety through a sulfonyl group, as
defined
herein. Representative examples of alkoxysulfonyl include, but are not limited
to,
methoxysulfonyl, ethoxysulfonyl and propoxysulfonyl.
The term "alkyl", as used herein, means a straight OT branched chain
hydrocarbon
containing from 1 to 6 carbon atoms. Representative examples of alkyl include,
but are
not limited to, methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-
butyl, tert-butyl,
n-pentyl, isopentyl, neopentyl, and n-hexyl.
The term "alkylcarbonyl", as used herein, means an alkyl group, as defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined
herein. Representative examples of alkylcarbonyl include, but are not limited
to, acetyl,
1-oxopropyl, 2,2-dimethyl-1-oxopropyl, 1-oxobutyl, and 1-oxopentyl.
The term "alkylcarbonyloxy", as used herein, means an alkylcarbonyl group, as
defined herein, appended to the parent molecular moiety through an oxygen
atom.
Representative examples of alkylcarbonyloxy include, but are not limited to,
acetyloxy,
-5-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
ethylcarbonyloxy, and tert-butylcarbonyloxy.
The term "alkylsulfonyl", as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a sulfonyl group, as defined
herein.
Representative examples of alkylsulfonyl include, but are not limited to,
methylsulfonyl
and ethylsulfonyl.
The term "alkylthio", as used herein, means an alkyl group, as defined herein,
appended to the parent molecular moiety through a sulfur atom. Representative
examples
of alkylthio include, but are not limited, methylthio, ethylthio, tert-
butylthio, and
hexylthio.
The term "alkynyl", as used herein, means a straight or branched chain
hydrocarbon group containing from 2 to 10 carbon atoms and containing at least
one
carbon-carbon triple bond. Representative examples of alkynyl include, but are
not
limited, to acetylenyl, 1-propynyl, 2-propyrryl, 3-butynyl, 2-pentynyl, and 1-
butynyl.
The term "annido", as used herein, means an amino, alkylamino, or dialkylamino
group appended to the parent molecular moiety through a carbonyl group, as
defined
herein. Representative examples of amido include, but are not limited to,
aminocarbonyl,
methylaminocarbonyl, dimethylaminocarbonyl, and ethylmethylaminocarbonyl.
The term "aryl", as used herein, means a monocyclic or bicyclic aromatic ring
system. Representative examples of aryl include, but are not limited to,
phenyl and
naphthyl.
The aryl groups of this invention are substituted with 0, 1, 2, 3, 4, or 5
substituents independently selected from acyl, acyloxy, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyaikyl, alkoxycarbonyl, alkoxyimino, alkoxysulfonyl, alkyl,
alkylsulfonyl, alkynyl,
amino, carboxy, cyano, fonnyl, haloalkoxy, haloalkyl, halo, hydroxy,
hydroxyalkyl,
mercapto, nitro, thioalkoxy, NRgRj, (NRgRi)alkyl, (NRgRi)alkoxy,
(NRA)carbonyl, and
(NRgRi)sulfonyl, wherein Rg and R are each independently selected from the
group
consisting of hydrogen and alkyl.
The term "arylcarbonyl", as used herein, means an aryl group, as defined
herein,
or a benzyl group appended to the parent molecular moiety through a carbonyl
group,
represented by -C(0)-, as defined herein. Representative examples of
arylcarbonyl
include, but are not limited to, phenylcarbonyl and benzylcarbonyL
The term "aryloxycarbonyl", as used herein, means an aryl-O- group, wherein
the
-6-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
aryl of aryl-0- is as defined herein, or a benzyoxyl group appended to the
parent
molecular moiety through a carbonyl group, represented by -C(0)-, as defined
herein.
Representative examples of aryloxycarbonyl include, but are not limited to,
phenoxycarbonyl and benzyloxycarbonyl.
The term "arylsulfonyl", as used herein, means an aryl group, as defined
herein,
appended to the parent molecular moiety through a sulfonyl group, as defined
herein.
Representative examples of arylsulfonyl include, but are not limited to,
phenylsulfonyl,
(methylaminophenyl)sulfonyl, (dimethylaminophenyl)sulfonyl, and
(naphthyl)sulfonyl,
The term "carbonyl", as used herein, means a -C(0)- group.
The term "carboxy", as used herein, means a -CO2H group.
The term "cyano", as used herein, means a -CN group.
The term "formyl", as used herein, means a -C(0)H group.
The term "halo" or "halogen", as used herein, means -Cl, -Br, -I or -F.
The term "haloalkoxy", as used herein, means at least one halogen, as defined
herein, appended to the parent molecular moiety through an alkoxy group, as
defined
herein. Representative examples of haloalkoxy include, but are not limited to,
chloromethoxy, 2-fluoroethoxy, trifiuoromethoxy, and pentafluoroethoxy.
The term "haloalkyl", as used herein, means at least one halogen, as defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined
herein. Representative examples of haloalkyl include, but are not limited to,
chloromethyl, 2-fluoroethyl, trifluoromethyl, pentafluoroethyl, and 2-chloro-3-
fluoropentyl.
The term "heteroaryl" means an aromatic five- or six-membered ring containing
I, 2, 3, or 4 heteroatoms independently selected from group consisting of
nitrogen,
oxygen and sulfur. The heteroaryl groups are connected to the parent molecular
moiety
through a carbon or nitrogen atom. Representative examples of heteroaryl
include, but
are not limited to, furyl, imidazolyl, indazolyl, benzothiozolyl, isoxazolyl,
isothiazolyl,
oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl, pyridazinyl, pyridinyl,
pyrimidinyl, pyrrolyl,
tetrazolyl, thiadiazolyl, thiazolyl, thienyl, triazinyl, and triazolyl,
The heteroaryl groups of the invention are substituted with 0, I, 2, or 3
substituents independently selected from alkenyl, alkoxy, alkoxyalkoxy,
alkoxyalkyl,
alkoxycarbonyl, alkoxysulfonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylsulfonyl,
-7-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
alkylthio, alkynyl, carboxy, cyano, formyl, haloalkoxy, haloalkyl, halo,
hydroxy,
hydroxyalkyl, mercapto, nitro, -NRgRi, (NRgRi)alkyl, (NRaRi)alkoxy,
(NR8Ri)carbonyl,
and (NRgRi)sulfonyl, wherein Rg and Ri are each independently selected from
the group
consisting of hydrogen and alkyl.
The term "bicyclic heteroaryl" refers to fused aromatic nine- and ten-membered
bicyclic rings containing 1, 2, 3, or 4 heteroatoms independently selected
from the group
conisisting of nitrogen, oxygen and sulfur The bicyclic heteroaryl groups are
connected
to the parent molecular moiety through a carbon or nitrogen atom
Representative
examples of bicyclic heteroaryl rings include, but are not limited to,
indolyl,
benzothiazolyl, benzofuranyl, isoquinolinyl, and quinolinyl. Bicyclic
heteroaryl groups
of the invention are substituted with 0, 1, 2, or 3 substituents independently
selected from
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxysulfonyl,
alkyl,
alkylcarbonyl, alkylcarbonyloxy, alkylsulfonyl, alkylthio, alkynyl, carboxy,
cyano,
formyl, haloalkoxy, haloalkyl, halo, hydroxy, hydroxyalkyl, mercapto, nitro, -
NRgRj,
(NRgRi)alkyl, (NRgRi)alkoxy, (NRgRi)carbonyl, and (NRgRi)sulfonyl, wherein Rg
and Ri
are each independently selected from the group consisting of hydrogen and
alkyl
The term "heterocycle" or "heterocyclic" as used herein, means a monocyclic
heterocycle or a bicyclic heterocycle. The monocyclic heterocycle is a 3, 4,
5, 6 or 7
membered ring containing at least one heteroatoni independently selected from
the group
consisting of nitrogen, oxygen and sulfur, The 3 or 4 membered ring contains 1
hetero atom selected from the group consisting of nitrogen, oxygen and sulfur.
The 5
membered ring contains zero or one double bond and one, two or three
heteroatoms
selected from the group consisting of nitrogen, oxygen and sulfur. The 6 or 7
membered
ring contains zero, one or two double bonds and one, two or three heteroatoms
selected
from the group consisting of nitrogen, oxygen and sulfur. The monocyclic
heterocycle is
connected to the parent molecular moiety through any carbon atom or any
nitrogen atom
contained within the monocyclic heterocycle. Representative examples of
monocyclic
heterocycle include, but are not limited to, azetidinyl, azepanyl, aziridinyl,
diazepanyl,
1,3-dioxanyl, 1,3-dioxolanyl, 1,3-dithiolanyl, 1,3-dithianyl, imidazolinyl,
imidazolidinyl,
isothiazolinyl, isothiazolidinyl, isoxazolinyl, isoxazolidinyl, morpholinyl,
oxadiazolinyl,
oxadiazolidinyl, oxazolinyl, oxazolidinyl, piperazinyl, piperidinyl, p:yranyl,
pyrazolinyl,
pyrazolidinyl, pyrrolinyl, pyrrolidinyl, tetrahydroftiranyl,
tetrahydrothienyl,
-8-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
thiadiazolinyl, thiadiazolidinyl, thiazolinyl, thiazolidinyl, thiomorpholinyl,
1,1-
dioxidothiomorpholinyl (thiomorpholine sulfone), thiopyranyl, and trithianyl.
The
bicyclic heterocycle is a monocyclic heterocycle that is either fused to a
cycloalkyl ring, a
heteroaryl ring or another heterocyclic ring, or is formed by an alkyl chain
attached to
two non-adjacent carbons contained within the monocyclic heterocyclic ring.
The
bicyclic heterocycle is connected to the parent molecular moiety through any
carbon
atom or any nitrogen atom contained within the monocyclic heterocycle.
Representative
examples of bicyclic heterocycle include, but are not limited to,
azabicyclo[3.1.1]heptane, azabicyclo[3 2 joctane, 1,3-benzodioxolyl, 1,3-
benzodithiolyl, 2,3-dihydro-1,4-benzodioxinyl, 2,3-dihydro-1-benzofuranyl, 2,3-
dihydro-
l-benzothienyl, 2,3-dihydro-1H-indolyl, and 1,2,3,4-tetrahydroquinolinyl.
The heterocyclic groups of the invention are substituted with 0, 1, 2, or 3
substituents independently selected from alkenyl, alkoxy, alkoxyalkoxy,
alkoxyalkyl,
alkoxycarbonyl, alkoxysulfonyl, alkyl, alkylcarbonyl, alkylcarbonyloxy,
alkylsulfonyl,
alkylthio, alkynyl, carboxy, cyano, formyl, haloalkoxy, haloalkyl, halo,
hydroxy,
hydroxyalkyl, mercapto, nitro, -NRA, (NRA)alkyl, (NRA)alkoxy, (NRgRi)carbonyl,
and (NRA)sulfonyl, wherein ; and R; are each independently selected from the
group
consisting of hydrogen and alkyl.
The term "hydroxy", as used herein, means an -OH group.
The term "hydroxyalkyl", as used herein, means at least one hydroxy group, as
defined herein, is appended to the parent molecular moiety through an alkyl
group, as
defined herein. Representative examples of hydroxyalkyl include, but are not
limited to,
hydroxymethyl, 2-hydroxyethyl, 3-hydroxypropyl, 2,3-dihydroxypentyl, and 2-
ethy1-4-
hydroxyheptyl.
The term "mercapto", as used herein, means a -SH group.
The term "nitro", as used herein, means a -NO2 group
The term "-NRgRj", as used herein, means two groups, Rg and R, which are
appended to the parent molecular moiety through a nitrogen atom. Rg and Ri are
each
independently hydrogen or alkyl. Representative examples of ¨NRgRj include,
but are
not limited to, amino, methylamino, dimethylamino, and methylethylamino.
The term "(NRA)alkyl", as used herein, means a - NRgRj group, as defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined
-9-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
herein, Representative examples of (NRgRi)alkyl include, but are not limited
to,
(amino)methyl, (dimethylamino)methyl, and (ethylamino)methyl_
The term "(NRgRJ)alkoxy", as used herein, means a - NR8Rj group, as defined
herein, appended to the parent molecular moiety through an alkoxy group, as
defined
herein. Representative examples of (NRgRi)alkoxy include, but are not limited
to,
(amino)methoxy, (dimethylamino)methoxy, and (diethylamino)ethoxy.
The term "(NRgRi)carbonyl", as used herein, means a - NRA group, as defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined
herein. Representative examples of (NRgRi)carbonyl include, but are not
limited to,
aminocarbonyl, (methylamino)carbonyl, (dimethylamino)carbonyl, and
(ethylmethylamino)carbonyl.
The term "(NRgNsulfonyl", as used herein, means a -NRg11.; group, as defined
herein, appended to the parent molecular moiety through a sulfonyl group, as
defined
herein. Representative examples of (NRERi)sulfonyl include, but are not
limited to,
aminosulfonyl, (methylamino)sulfonyl, (dimethylamino)sulfonyl, and
(ethylmethylamino)sulfonyl.
The term "sulfonyl", as used herein, means a -S(0)2- group.
The term "thioalkoxy", as used herein, means an alkyl group, as defined
herein,
appended to the parent molecular moiety through a sulfur atom. Representative
examples
of thioalkoxy include, but are no limited to, methylthio, ethylthio, and
propylthio.
Although typically it may be recognized that an asterisk is used to indicate
that
the exact subunit composition of a receptor is uncertain, for example a3b4*
indicates a
receptor that contains the a3 and 134 proteins in combination with other
subunits, the term
a7 as used herein is intended to include receptors wherein the exact subunit
composition
is both certain and uncertain. For example, as used herein a7 includes
homomeric (0)5
receptors and a7* receptors, which denote a nACIIR containing at least one a7
subunit.
Compounds of the Invention
Compounds of the invention have the formula (I) as described above. More
particularly, compounds of formula (1) can include, but are not limited to,
compounds
wherein A is N, and n is 1 or 2. Certain preferred compounds exist wherein A
is N; L is
0; n is 2.
-10-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
More particularly, in compounds of formula (I) Arl is selected from:
Ri R2 R1 R2 Ri
Ri ______________________________________ R2 R2 R1
'1 111 - N¨ --K\N-7--- 1 II R3
N N--- N .
R5 R4 R4 R4 ' 1-t4 R5 R4
R1 Ri
...._ N¨ ,,,
,._ ,..c_K ' r\iõt1"C
R3 1 \ Ai R3 1
R N / , \ itR3 ___
3
,
R5 R4 R4 R5 R4 R5 R5 R4
Ri Ri Ri
¨c11'
1-c);\ R3
NN , N---- and N ,
R4 R5
wherein RI, R2, R3, R4 and R5 are independently acyl, acyloxy, alkenyl,
alkoxy,
alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxyimino, alkoxysulfonyl, alkyl,
alkylsulfonyl, alkynyl, amino, carboxy, cyano, fomryl, haloalkoxy, haloalkyl,
halo,
hydroxy, hydroxyalkyl, merca.pto, nitro, thioalkoxy, -NRgRi, (NRA)alkyl,
(NRgRi)alkoxy, (NRA)carbonyl, or (NRgRi)sulfonyl; Rg and Ri are each
independently
hydrogen or alkyl, More preferably, Ail is
RI R2
¨N
R5 .
Particularly, the invention includes, but is not limited to, compounds of
formula (I)
wherein A is N; R is methyl; L is 0; n is 2; ATI is
Ri R2
40-
-N
R5 .
Ar2 in compounds of formula (I) is selected from:
i
-11-
CA 02647830 2008-09-29
WO 2007/137030 PCT/US2007/068930
õter\ = Rb j>sr. Rb
\Z4 S )'cZel Nil
;2P,i( -\----// \N 1Z21,; /\N 1Z132, ; ;NI
Zi Zi Zi
R7 R7
R7 R7
(OP (ii), (iii), (iv),
. /i\Z4 n / 7 Rb
/ /
NZ4.,õ...0 /7\:,-Z4 Xb /
--",..-----\
II ---"Re 'Til X ,>---R6 --ir " ¨R6 1 ..,,,..,. //
N
Z2, ..7---- ¨ Z2, .""- = = Z2, ..;!="--.... Zi N
42,
Zi N Zi N Zi N Zi
(V), (V1), (Vii), (Viii), (ix), R7
/
/
X \N 4 r. \
li N
z2.z,,:
zi
(x) and (xi) ,
wherein Z1, Z2, Z3 and Z4 are each independently nitrogen or are carbon,
wherein
the carbon atom is optionally substituted with a substituent selected from the
group
consisting of hydrogen, halogen, alkyl, -ORõ -alkyl-ORõ -NRdR,, and -alkyl-
NRdRe, Rb
is selected from the group consisting of hydrogen, alkyl and alkylcarbonyl; R,
is alkyl; Rti
and R, are each independently selected from the group consisting of hydrogen
and alkyl,
R6 and R7 are each independently selected from the group consisting of
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkoxycarbonyl, alkoxyimino,
alkoxysulfonyl, alkyl, alkylcarbonyl, alkylearbonyloxy, alkylsulfonyl,
alkynyl, carboxy,
cyano, forrnyl, haloalkoxy, haloalkyl, halo, hydrogen, hydroxy, hydroxyalkyl,
mercapto,
nitro, thioalkoxy, -NRgRi, (NRgRi)alkyl, (NR8R)alkoxy, (NR8R)carbonyl, and
(NRgRi)sulfonyl; Rg and Ri are each independently selected from the group
consisting of
hydrogen and alkyl.
R is selected from hydrogen, alkyl, cycloalkylalkyl, and arylalky. Preferred
compounds are disclosed wherein R is hydrogen and alkyl. Preferably, R is
methyl and
hydrogen.
Preferred compounds are disclosed wherein Ar2 is
lir\ 7 r b siSsj, , Rb
/
\ 4-4 N /3,,,. 7 Rb
/
=:..-4 N L. "= \ -=õz.L.4 N
i)R
P R-- IP
z 6,, / z2.,.-----(N
or
R7
0), R7 R7 (iv) (ix) ,
More preferably Ar2 is
-12-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Si< 7 Rb
N-4-4 N
4,r,,_:(>--
/ R6
Z2, 1
(i) R7,
Particularly, the invention relates to compounds of formula (I) wherein A is
N; R is
selected from methyl and hydrogen; L is 0; n is 2; and Ar2 is selected from
the group of
consisting of:
Rb
'\Z4N / 7 Rb
li$S4 7 Rb
Z2
Zi 0
Zi Z2,
R7
1:17 R7
(iv) and (ix)
More particularly, the invention relates to compounds of formula (I) wherein A
is N; R is
methyl or hydrogen; L is 0; n is 2; Arl is
Ri R2
4H(14-,
R5 ;and
Ar2 is
\\,. Z4 rbN
/' Re
Zi
R7
(0,
Compounds for the method of the invention, including but not limited to those
specified in the examples or otherwise specifically named, can modulate, and
often
possess an affinity for, nAChRs, and more particularly a7 nAChRs. As a7 nAChRs
ligands, the compounds of the invention can be useful for the treatment or
prevention of a
number of a7 nAChR-mediated diseases or conditions
Specific examples of compounds that can be useful for the treatment or
prevention of a7 nAChR-mediated diseases or conditions include, but are not
limited to,
compounds described in the Compounds of the Invention and also in the
Examples, and
-13-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
also compounds such as:
5- {6-Rendo)-8-methyl-8-aza-bicyclo [3 .2. 1joct-3-yloxY]-pyr idazin-3-y1) -
1H-
indole;
(endo)-3-(6-benzo[b]thiophen-5-yl-pyridazin-3-yloxy)-8-methy1-8-aza-
bicyclo[3 .2.1 ]octane;
(endo)-346-(benzofuran-5-y1)-pyridazin-.3-y1oxy1-8-methy1-8-aza-
bicycloP 2, 1 }octane;
6- {6-Rendo)-8-methyl-8-aza-bicyclo[3.2.1 ]cot-3 -y1oxyl-pyridazin-3 -y11-1H-
indole;
5- {6-Rendo)-8-methyl-8-aza-bicyclo[3 .2.1 ioct-3-yloxyl-pyridazin-3-y1} - 1H-
indazole;
1 -methyl-5 - {6-Rendo)-8-methy1-8-aza-bicyc1o[3 .2.1 ]oct-3-yloxy}-pyridazin-
3-
y11-1 H-indole;
5- {6-[(endo)-8-methyl-8-aza-bicyclo[3.2.11oct-3-yloxy]-pyridazin-3-y1) -2-
trifluotomethy1-1 H-indole;
5- (6-[(exo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-yloxy}-pyridazin-3-y1}-1H-
indole;
5- { 5-Rendo)-8-methy1-8-aza-bicyclo[.3 .2, 1loct-3-yloxy]-pyridin-2-y1) -1H-
indole;
(endo)-3 -(6-benzo [b]thiophen-5 -yl-pyrid in-3 -yloxy)-8-rnethy1-8-aza-
bicyclo[3 .2.1 }octane;
5-15-Rexo)-8-methyl-8-aza-bicyclo{31,1]oct-3-yloxy]-pyridin-2-y1}-1H-indole;
(exo)-3-[6-(benzofuran-5-y1)-pyridin-3-yloxy]-8-methy1-8-aza-
bicyclo[3.2. 1 }octane;
5- {5-Rexo)-8-methyl-8-aza-bicycloP .2.1 loct-3-yloxy}-pyridin-2-y11-1H-
indazole;
5- {5-[(exo)-8-methy1-8-aza-bicyclo[3.2. 1 loct-3-yloxy}-pyridin-2-y1) -2-
trifluoromethyl- 1H-indole;
4- {5-{(exo)-8-methyl-8-aza-bicyclo[3 .2, 1loct-3-yloxy]-pyridin-2-y1) -1H-
indole;
5- {6-Rendo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-yloxyj-pyridin-3-y11-1H-
indole;
(endo)-3-(5-benzo [b]thiophen-5-yl-pyridin-2-yloxy)-8-rnethyl-8-aza-
bicyclo[3 .2.1 }octane;
5- { 6-[(exo)-8-methyl-8-aza-bicyclo[3 .2.1 ] -1H-
indole;
[6-(1H-indo1-5-y1)-pyridin-3-yI]-[(endo)-8-methyl-8-aza-bicyclo[3,2, 1 joct-3-
y1]-
amine;
-14-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
[6-(benzofuran-5-y1)-pyridin-3-A-Rendo)-8-rnethy1-8-aza-bicyclo[3 2, 1 ]oct-3
yli -amine;
Rendo)-8-methyl-8-aza-bieyelo[3 .2.1]oct-3-y1146-(2-trifluoromethyl-1 H-indo1-
5-
y1)-pyridin-.3-yll -amine;
[6-(1H-indazol-5-y1)-pyridin-3-y1}-[(endo)-8-methyl-8-aza-bicyclo[3 2.1 3oct-3-
y1]-amine;
[6-(1H-indo1-4-y1)-pyridin-3-y1]-Rendo)-8-methyl-8-aza-bieyelo[3 1 ]oct-3-y11-
amine;
[(enda)-8-aza-bicyclo3 .2,1 ] oct-.3-y13-[6-(1 H-indo1-5-y1)-pyridin-3-y1}-
amine ;
[4-(111-indo1-5-y1)-pheny1}-[(endo)-8-naethy1-8-aza-bicyc1o[.3 .2.1 ]oct-3-y1}-
amine;
[4-(1H-indazo1-5-y1)-pheny1]-[(endo)-8-tnethyl-8-aza-bicyclo[3 .2.1 ]oct-3-
y1:1-
amine;
Rendo)-8-m ethyl-8-aza-bicyclo[3.2, 110d-3-y1H:441-methyl-I H-indo1-5-y1)-
phenylj-amine;
(4-benzo [b]thiophen-5 -y1-phenyl)-[(endo)-8-methyl-8-aza-bicyclo [3 21]oct-3-
y11-amine;
[4-(benzofuran-5-y1)-pheny1]-[(endo)-8-methy1-8-aza-bicyclo[3 2.1] oet-3-yll-
amine;
[4-(1H-indo1-4-y1)-pheny1]-(endo)-8-methyl-8-aza-bicyc1o[3 1 ]oct-3-yri -
amine;
[3-(1H-indo1-5-y1)-pheny1]-Rendo)-8-methy1-8-aza-bicyc1o[3 , 1 loct-3-yli -
amine;
[3-( 1H-indo1-4-y1)-phenyl] -Rendo)-8-methy1-8-aza-bicyclo[3.2, 1]0d-3-y11-
amine;
5- {6-[(endo)-8-methyl-8-aza-bieyelo[3 2.1, loct-3 -yloxyl-pyridazin-3 -y1}-2-
trifluoromethyl- 1 H-indole;
4- f6-[(exo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyridazin-3-yll -1 H-
indole;
5- (6-[(exo)-8-methyl-8-aza-bicyclo[3,2,1]oct-3-yloxy}-pyridin-3-y11-1H-
indole;
5- {6-[(exo)-8-methyl-8-aza-bicyclo[3 2.1 ]oct-3-y1oxyl-pyridin-3 -yll -2-
trifluommethy1-1H-indole;
4- f 6-[(exo)-8-methyl-8-aza-bieyclo[3,2.1]oct-3-yloxy)-pyridazin-3-y1}-1H-
indole;
6- f 5-[(exo)-8-methy1-8-aza-bicyclo[32, 1 ] -1 H-
indole;
5- (5-Rendo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-y1oxyl-pyrazin-2-y1]-1H-
indole;
4- (5-Rendo)-8-methyl-8-aza-bieyelo[3 .2.1 ]oet-3-yloxy]-pyrazin-2-y1]-1H-
indole;
-15-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
6- {5-Rendo)-8-methyl-8-aza-bicyclo[3 .2.1 ]oet-3-yloxyl-pyrazin-2-y1:1-1H-
indole;
[6-(1H-indo1-6-y1)-pyridin-3-yli-Rendo)-8-methyl-8-aza-bicycloP .2.11oct-3-y1}-
amine;
5- {6-Rendo)-9-methyl-9-azabicyolo[3 3.1 ]nonan-3 -yloxyllpyridazin-3 -yll -1H-
indole;
(en do)-346-(b enzo[b]thiophen-5-yppyrid azin-.3-yloxy1-9-methyl-9-
azabicyclo[3.3,1]nonane;
5-15-[(endo)-8-methy1-8-aza-bicyc1o[3.2.1]oct-3-yloxyl-pyrazin-2-y1} -1H-
pyrrolo[2,3 -b]pyridine;
5- (5-[(exo)-8-methyl-8-aza-bicyclo [3 .2.1]oct-3-y1oxyj-pyridin-2-yll - 1H-
pyrrolo[2,3-b}pyridine;
5- {5-[(exo)-8-methyl-8-aza-bicyclo[3 .2.1 joct-3-yloxyi-pyridin-3-y1}-1H-
indole;
5- {5-1(exo)-8-methyl-8-aza-bicycloP .2.1 joet-3-yloxy]-pyrazin-2-y1}-1H-
indoie;
4- {5-1(exo)-8-methyl-8-aza-bicyclo[3.2.1ioct-.3-yloxy]-pyrazin-2-y11-1H-
indole;
6- { 5 -Rexo)-8-methyl-8-aza-bicyclo[3 .2.1]oct-3-y1oxy]-pyrazin-2-y1) -1H-
indole;
(endo)-N-(5-( I H-Indo1-5 -yl)pyridin-.3-y1)-8-methyl-8-azabicyclo[3.2 1
]oetan-3
amine;
(endo)-N-(5-(1 H-Indo1-4-Apyridin-3-y1)-8-methyl-8-azabicycloP .2,1] octan-3 -
amine;
(endo)-N-(5 -(1 H-Indo1-6-Apyridin-3 -y1)-8-methyl-8-azabicyclo[3 ,11 joctan-3-
amine;
(en do)-N- (5 [2-(tiri fluoromethyl)-1H-indo1-5-yllpyridin-3-y1}-8-Methyl-8-
azabicyclo[3 ,2.1}oetan-3-amine;
5- {5-Rendo)-8-Methyl-8-azabicyclo[3 .2.1 ]octan-3 -yloxyjpyridin-2-yll -1H-
pyrrolo[2,3 -Npyridine;
5- {5-[(endo)-8-Methyl-8-azabicyc1o[12,1 ]oetan-3-yloxy]pyridin-2-yllindolin-2-
one;
5- {5 -Rendo)-8-Azabicyclo[3.2. 1]octan-3 -yloxy]pyridin-2-yll -1H-indo1e;
(1R,3r,SS, 8s)-3 -(6-(1H4ndo1-5-Apyridin-3-yloxy)-8-methyl-8-
azabicyclo[3 l]octane 8-oxide;
(1 R, 3r, 5S, 8r)-.3 -(64 1 H-Indo1-5 -Apyridin-3-yloxy)-8-methy1-8-
azabicyclo[3 .2.1 joctane 8-oxide;
-16-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
4- {5-Rendo)-8-Azabicyclo[3 2.1] octan-3-yloxy]pyridin-2-yll -1H-indole;
5-15-[(exo)-8-Azabicyclo[3.2,1]octan-3-yloxylpridin-2-y1}-1H-indole;
5- { 5-[(endo)-8-Azabicyclo[3 .2. l]octan-3-yloxy)pyridin-2-y1) indolin-2-one;
5- {5-Rendo)-8-Azabicyclo[3.2. 1 ]octan-3-yloxyipyridin-2-y1} -1H-pyrrolo[2,3-
b]pyridine;
5- {5-[(exo)-8-Azabicyclo[3.2.1]octan-3-yloxy]pyridin-2-y1) -1H-pyrrolo[2,3-
b]pyridine,
or pharmaceutically acceptable salts, esters, amides, and prodrugs thereof.
Compound names are assigned by using AuToNom naming software, which is
provided by MDL Information Systems GmbH (formerly known as Beilstein
Infounationssysteme) of Frankfurt, Germany, and is part of the CHEMDRAW ULTRA
v. 6Ø2 software suite.
Compounds of the invention may exist as stereoisomers wherein, asymmetric or
chiral centers are present. These stereoisomers are "R" or "S" depending on
the
configuration of substituents around the chiral element. The terms "R" and "S"
used
herein are configurations as defined in IUPAC 1974 Recommendations for Section
E,
Fundamental Stereochemistry, Pure Appl. Chem., 1976, 45: 13-30.
L¨Ar1¨Ar2RA õL¨Arl¨Ar2
RA1
(la) (lb)
The attachment of L to the azabicyclic alkane may be considered to encompass
both the endo and exo geometries, such as isomer (Ia) and (lb). The
configurational
assignment of structures of foimula (Ia) are assigned endo in accordance with
that
described in Stereochemistry of Organic Compounds, EL, Eliel, S.1-1 Wilen;
John Wiley
and Sons, Inc. 1994. Structures of formula (lb) are assigned exo using the
same methods,
L¨Ar1¨Ar2 L¨Arl¨Ar2
-0' IR' Nil
R (lc) 0-(Id)
The N+-0- portion of isomer (Ic) and isomer (Id) are diastereomers. The
configurational assignment of structures of formula (Ic) are assigned (r) in
accordance
-17-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
with that described in Synthesis, 1992, 1080, Becker, D. P,; Flynn, ELL. and
as defined in
Stereochemistry of Organic Compounds, El. Eliel, S,H Wilen; John Wiley and
Sons,
Inc. 1994, In addition the configurational assignment of structures of formula
(Id) are
assigned (s) using the same methods.
The invention contemplates various stereoisomers and mixtures thereof and are
specifically included within the scope of this invention. Stereoisomers
include
enantiomers and diastereomers, and mixtures of enantiomers or diastereomers.
Individual stereoisomers of compounds of the invention may be prepared
synthetically
from commercially available starting materials which contain asymmetric or
chiral
centers or by preparation of racemic mixtures followed by resolution well-
known to those
of ordinary skill in the art, These methods of resolution are exemplified by
(1)
attachment of a mixture of enantiomers to a chiral auxiliary, separation of
the resulting
mixture of diastereorners by recrystallization or chromatography and optional
liberation
of the optically pure product from the auxiliary as described in Fumiss,
Hannaford,
Smith, and Tatchell, "Vogel's Textbook of Practical Organic Chemistry", 5th
edition
(1989), Longman Scientific & Technical, Essex CM20 2JE, England, or (2) direct
separation of the mixture of optical enantiomers on chiral chromatographic
columns or
(3) fractional recrystallization methods.
-18-
CA 02647830 2008-09-29
WO 2007/137030 PCT/US2007/068930
Methods for Preparing Compounds of the Invention
The reactions exemplified in the schemes are performed in a solvent
appropriate
to the reagents and materials employed and suitable for the transformations
being
effected. The described transformations may require modifying the order of the
synthetic
steps or selecting one particular process scheme over another in order to
obtain a desired
compound of the invention, depending on the functionality present on the
molecule.
The methods described below can entail use of various enantiomers. Where the
stereochemistry is shown in the Schemes, it is intended for illustrative
purposes only.
Scheme 1
OH
1 Ar
halo
(1) (2b) Ar2¨halo
KHMDS (6)
Me3SnSnMe3 or pd
(R0)2B-B(OR)2
'1!
OH
(4)
trSi Cul, lAru-halo Ar2¨M
Rz Cs2CO3 NIO
RZ (7)
halo
(1) (2a) (3)
Me3SnSnMe3 or Pd
(R0)2B-B(OR)2
(4)
Pd
CL-1- (6) C)1 A 2
Ar-L, m
Pd
Rz
Rz
(5) (8)
Compounds of formula (8), wherein Arl, Ar2 are as defined in formula (I), can
be
prepared as described in Scheme 1. Compounds of formula (1) when treated with
a
compound of formula (2a), wherein halo is bromide, chloride, or iodide, in the
presence
of Cul, 1,10-phenantluoline and Cs2CO3 in a solvent such as, but not limited
to, toluene
as described in Org. Lett., 2002, 4, 973, will provide compounds of formula
(3).
Compounds of formula (3) can also be prepared through the rection of compounds
of
formula (1) with compounds of formula (2b) in the presence of a base, such as,
but not
limited to, KHMDS, in a solvent such as but not limited to THF, DME and
toluene.
Compounds of formula (3) when treated with hexamethylditin or an organo-borane
-19-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
compound of formula (4), such as bis(pinacolato)diboron or
bis(catecholato)diboron,
wherein R is hydrogen, alkyl or aryl, in the presence of a palladium catalyst
will provide
the corresponding tin or boronic acid of formula (5), wherein M is ¨Sn-(Me)3
or ¨
B(OR)2, Compounds of formula (5) when treated with compounds of formula (6),
A.12-
halo, wherein Ar2 is a bicyclic heteroaryl ring and halo is bromide, chloride,
or iodide, in
the presence of a palladium catalyst to provide compounds of formula (8).
Alternatively,
compounds of formula (6) when treated with hexamethylditin or a di-borane
containing
compound of formula (4), such as bis(pinacolato)diboron and
bis(catecholato)diboron, in
the presence of a palladium catalyst will provide a corresponding tin or
boronic acid
containing compound of formula (7), wherein Ar2 is a bicyclic heteroaryl and
wherein M
is ¨Sri-(Me)3 or ¨B(OR)2. Compounds of formula (7) when treated with a
compound of
formula (3) in the presence of a palladium catalyst will provide a compound of
formula
(8).
Scheme 2
MeaSnSnMe3
or
(R0)2B-B(OR)2
OK Yi X5. 0 X5, 0 Xx5 4
(4)
= X4 )4 =( If-
= .
Rz + AL x2x.3hab Nil N. X3 hab _______
3111 X3
Rz X2 Pd Rz N' x2
(9) (10) (11) (12)
(6)
P
Pd d
N1ay, X5 x4
X3 Ar2
Rz X-
(13)
Compounds of formula (13), wherein Arl is a nitrogen-containing heteroaryl,
for
examples pyridazine, pyrimidine, pyrazine, 2-pyridyl, and Ar2 is as defined
for formula
(I), can be prepared as shown in Scheme 2, Compounds of formula (9), wherein
Rz is
alkoxyalkyl, alkyl, alkyloxycarbonyl, alkylcarbonyl, aryl,
arylalkyloxycarbonyl,
cycloalkylalkyl, arylcarbonyl and aryloxycarbonyl and K represents the
potassium, which
are prepared from treating hydroxyl containing heterocycles of similar formula
with
potassium tert-butoxide in solvents such as but not limited to THF or DMF to
provide the
-20-
CA 02647830 2008-09-29
WO 2007/137030 PCT/US2007/068930
potassium oxide containing compounds of formula (9). The compounds of formula
(9)
when treated with compounds of formula (10), wherein Y1 and halo are both
bromo,
chloro and iodo, and X2, X3' X4 and X5 are independently either carbon or
nitrogen, for
example, dichloropyridazine, will provide compounds of formula (11). Compounds
of
formula (11) when treated with hexarnethylditin or a di-borane containing
compound of
formula (4) in the presence of a palladium catalyst according to the procedure
outlined in
Scheme 1 will provide compounds of formula (12), Compounds of formula (12)
treated
with compounds of formula 6 in the presence of a palladium catalyst will
provide
compounds of fomula (13). Alternatively, the compounds of formula (11) when
treated
with organ stannane or organo boronic acid containing compounds of formula
(7), as
described in Scheme 1, in the presence of a palladium catalyst will provide a
compound
of formula (1.3),
Scheme 3
OH HO
I
3 Arl---1
N4410 Ph3P, DEAR Rz
_ N
Rz
(1) (14) THF, it, 2 days (15)
when Z3 is halo
then using
(7) and Pd
I Arl¨t Ar2
(8)
Alternatively, compounds of formula (8) may be prepared as outlined in Scheme
3. Compounds of formula (1) when treated with a compound of formula (14),
wherein Z3
is bromo, chloro or iodo or is Ar2, in the presence of diethyl
azodicarboxylate or
di(isopropyl)1 azodicarboxylate and a phosphine, such as triphenylphosphine,
will
provide compounds of formula (15). When Z3 is Ar2, compounds of formula (15)
are
representative of the present invention. When Z3 is a halogen, the further
treatment of the
compound according to conditions outlined in Schemes 1-2 outlining the Suzuki
type
coupling to provide compounds of formula (8) which are representative of the
-21-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
compounds of the present invention,
Scheme 4
I 1
(16)
-2 OH
Ar
Pd +RI N11 Cul,
(17) (1) Cs2CO3
Ar2¨M
(7)
A
Rz N r240
Ra0
Pd
Ra0
(19)
(18)
Ra Bz Pd/C, H2
HO OH
Ph3P
I Arl--t Ar2
DEAD
(20) (1)
Another method of generating compounds of formula (8) is described in Scheme
4, The activated tin or boronic acid compounds of formula (7) can be coupled
with a
variety of aryl halides that will provide a method of generating biaryl
compounds of
formula (17) and of formula (20). For example compounds of formula (7) when
treated
with diiodobenzene of formula (16) in the presence of a palladium catalyst
will provide
compounds of formula (17). Compounds of formula (17) when treated with
compounds
of formula (1) in the presence of cuprous iodide and cesium carbonate and 1,
10-
phenanthroline as described in scheme 1, will provide compounds of formula
(8).
Alternatively, compounds of formula (7) when treated with a compound of
formula (18),
wherein le is benzyl or another appropriate alcohol protecting group, in the
presence of a
palladium catalyst will provide compounds of formula (19). The deprotection of
the
alcohol protecting group, for example when Ra is benzyl the deprotection is
generally
achieved utilizing palladium on carbon and an atmosphere of hydrogen, will
provide
compounds of formula (20). Compounds of formula (20) when treated with
compounds
of formula (1) in the presence of triphenylphosphine and
diethyldiazocarboxylate or a
-22-
CA 02647830 2008-09-29
WO 2007/137030 PCT/US2007/068930
similar reagent will provide compounds of formula (8).
Scheme 5
RzN41NH2
111
(24) (2)
Pd,
Cs2CO3
Pd
Me3SnSnMe3
0
HNoaAl3cH, N( Zekse6)3 N
H21µ1., or (4)
Rz AO -halo I ;r1 -
M
4 N
Rz
Rz
(21) (22) (23) (26)
(7) d
Pd
(6)
AX,1 ¨Ar2
Rz
(25)
Compounds of formula (25), which are representative of compounds of formula
(I), wherein L is -1\111-, can be prepared as shown in Scheme 5. Compounds of
formula
(21) when treated with compounds of formula (22), wherein halo is bromide,
chloride, or
iodide, along with sodium triacetoxy borohydride and Na2SO4 in acetic acid
will provide
compounds of formula (23). Alternatively, a compound of formula (23) can be
obtained
by treating compounds of formula (24) with a compound of formula (2), wherein
Y is
bromo or iodo, in the presence of palladium catalyst, preferably in toluene.
Compounds
of formula (23) when further treated with a tin or diboron of formula (4),
such as
bis(pinacolato)diboron and bis(catecholato)diboron, under conditions described
in
Scheme 2, will provide the corresponding tin or boronic acid compounds of
formula (26)
Compounds of formula (26) when treated with a compound of formula (6) in the
presence
of a palladium catalyst, will provide the compound of formula (25).
Alternatively, the
compound of formula (23) when treated with a tin or boronic acid containing
compound
of formula (7) in the presence of a palladium catalyst will also provide
compounds of
formula (25).
-23-.
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Scheme 6
(22) +(7)
Pd
0 H2N NaBH(OAc)3
N1 IAr1-1-Ar2 1 Arl -Ar2
______________________________________________ N411011
Rz HOAc,Na2SO4 Rz
(21) (27) (25)
In addition, compounds of formula (25) can be prepared as shown in Scheme 6,
Ketone containing compounds of formula (21), when treated with compounds of
formula
(27), prepared via the coupling reaction of haloarylamine of formula (22) and
a suitable
tin or boron agent of formula (7) in the presence of a palladium catalyst,
followed by
treatment with sodium triacetate borohydride and Na2SO4 in acetic acid will
provide
compounds of formula (25) as described in Tetrahedron Lett. 1996, 37, 6045,
Scheme 7
SH Me3SnSnMe3
NaH 01 (4) + Arl¨ha/ jt
Rz 0Ms Rz Pd Rz
(28) (29) (30) (32)
Xd1/4 pd
Ar2¨halo
Ar2¨M
(7) (6)
Rz1101
I ArlfAr2
N
(31)
Compounds of formula (31), wherein L is S and Ari and Ar2 are as defined in
formula (I), can be prepared as shown in Scheme 7. Compounds of formula (29),
wherein halo is bromide, chloride, or iodide, when pretreated with sodium
hydride in a
solvent such as but not limited to DMF followed by treatment with compounds of
formula (28) will provide compounds of formula (30). Compounds of formula (30)
when
treated with a compound of formula (7) as described in Scheme 1, will provide
compounds of formula (31), which are representative of compounds of formula
(I)
-24-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
wherein L is S. Alternatively, the compound of formula (30) when treated with
a
hexamethylditin or diboron reagent of formula (4), such as
bis(pinacolato)diboron and
bis(catecholato)diboron, in the presence of a palladium catalyst will provide
a compound
of formula (32). Compounds of formula (32) when treated with compounds of
formula
(6), wherein halo is bromo, chloro or iodo, in the presence of a palladium
catalyst will
provide compounds of formula (31).
Scheme 8
lArl ____________ " I Br2, KSCN Arl-- \ NN2
Rz L NF12 1-10Ac RzN
(33) (34)
Rg-halo
I Arl¨ 1 \>¨NEIR9
IR' NIO
(35)
Compounds of formula (.35) which are representative of compounds of foimula
(I), wherein L is 0, S. or ¨N(Ra)-, Arl is as previouly defined in formula
(I), and Ar2 is an
aminosubstituted benzothiazole are prepared according to the conditions
outlined in
Scheme 8, Compounds of formula (33) which are obtained by methods described in
Schemes 1-7, wherein Ar2 is substituted with ¨N112, when treated with bromine
and
KSCN in acetic acid will provide compounds of formula (34). Compounds of
formula
(34) can be further treated with the halide of a desired Rg group, wherein Rg
is as defined
under the scope compounds of the present invention to provide compounds of
formula
(35),
-25-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Scheme 9
IR2. Nil 1_ HNO3
N-P H2SO4 N-P
(36) (37)
1) H2, Pd/C 2) deprotection
Me0H
,aNH2
I Arl---1 I
Rz 'NH2
(38)
(Et0)3CRrn
lArl ________________________________________________ I I
Rz
(39)
Compounds of formula (39), wherein L is 0, NH, or S; Arl is as previouly
defined in formula (I), Ar2 is a benzoimidazole as defined for compounds of
formula (I),
are prepared as outlined in Scheme 9. Compounds of formula (36), are obtained
by
treating compounds of formula (3.3) of Scheme 8, using conditions known to one
skilled
in the art that will incorporate a nitrogen-protecting group to the nitrogen
atom of Ar2
wherein P is tert-butyloxycarbonyl, benzyloxycarborryl, alkoxycarbonyl,
alkylcarbonyl,
arylcarbonyl or trialkylsilane. Compounds of formula (36) when treated with
nitric acid
in sulfuric acid will provide compounds of formula (37). Compounds of formula
(37)
when subjected to reducing conditions such as but not limited to treatment
with a
palladium catalyst and an atmosphere of hydrogen will reduce the nitro group
to the
corresponding amine, which is subjected to conditions known to one skilled in
the art that
will remove the nitrogen protecting group to provide compounds of formula
(38).
Compounds of formula (38) were then further subjected to treatment with an
excess of an
orthoester of formula (Et0)3Cle will provide compounds of formula (39) wherein
Rm is
alkyl or aryl.
Scheme 10
-26-
CA 02647830 2008-09-29
WO 2007/137030 PCT/US2007/068930
NOR3Sn-SnR3
NO2 L
ha 2 to¨ or (4) õõ
I halo Pd
OBn OBn N
Pd (42)
(40) (41)
NO2 1) H2, Pd/C
Me0H )1.
R2 (43) OBn 2) (Et0)3CRn Rz N
(44) 0
DMF
Benzooxazole-containing compounds of formula (44), wherein L is 0, NH, or S,
Arl is as previouly defined in formula (I), and le is alkyl hydrogen, or aryl,
can be
prepared as outlined in Schemel 0. Compounds of formula (40) can be treated
with a
ditin or diboron reagent of formula (4), such as hexamethylditin,
bis(pinacolato)diboron
and bis(catecholato)diboron, in the presence of a palladium catalyst to
provide the
corresponding tin or boronic acid of formula (41). Compounds of formula (41)
when
treated with a halogen containing compound of formula (42) in the presence of
a
palladium catalyst will provide compounds of formula (43). Compounds of
formula (43)
when treated according to conditions known to one skilled in the art that will
reduce nitro
groups to the corresponding amine group, followed by treatment with a le
substituted
ortho ester, wherein R. is hydrogen, alkyl or aryl will provide compounds of
formula
(44).
In addition, compounds of formula (I) wherein A is N can be converted to
compounds of formula (I) wherein A is N4-0- by treatment with an oxidizing
agent.
Examples of the oxidizing agent include, but not limited to, aqueous hydrogen
peroxide
and m-chloroperbenzoic acid. The reaction is generally performed in a solvent
such as,
but not limited to, acetonitrile, water, dichloromethane, acetone or mixture
thereof,
preferably a mixture of acetonitrile and water, at a temperature from about 0
C to about
80 C, for a period of about 1 hour to about 4 days.
The compounds and intermediates of the invention may be isolated and purified
by methods well known to those skilled in the art of organic synthesis.
Examples of
conventional methods for isolating and purifying compounds can include, but
are not
limited to, chromatography on solid supports such as silica gel, alumina, or
silica
derivatized with alkylsilane groups, by recrystallization at high or low
temperature with
-27-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
an optional pretreatment with activated carbon, thin-layer chromatography,
distillation at
various pressures, sublimation under vacuum, and trituration, as described for
instance in
"Vogel's Textbook of Practical Organic Chemistry", 5th edition (1989), by
Furniss,
Hannaford, Smith, and Tatchell, pub. Longman Scientific & Technical, Essex
CM20 2.1E,
England.
The compounds of the invention have at least one basic nitrogen whereby the
compound can be treated with an acid to form a desired salt. For example, a
compound
may be reacted with an acid at or above room temperature to provide the
desired salt,
which is deposited, and collected by filtration after cooling. Examples of
acids suitable
for the reaction include, but are not limited to tartaric acid, lactic acid,
succinic acid, as
well as mandelic, atrolactic, methariesulfonic, ethanesulfonic,
toluenesulfonic,
naphthalenesulfonic, carbonic, fumaric, gluconic, acetic, propionic,
salicylic,
hydrochloric, hydrobromic, phosphoric, sulfuric, citric, or hydroxybutyric
acid,
carnphorsulfonic, malic, phenylacetic, aspartic, glutamic, and the like.
Nitrogen protecting groups can be used for protecting amine groups present in
the
described compounds. Such methods, and some suitable nitrogen protecting
groups, are
described in Greene and Wuts (Protective Groups In Organic Synthesis, Wiley
and Sons,
1999), For example, suitable nitrogen protecting groups include, but are not
limited to,
tert-butoxycarbonyl (Bac), benzyloxycarbonyl (Cbz), benzyl (Bri), acetyl, and
trifluoracetyl. More particularly, the Boc protecting group may be removed by
treatment
with an acid such as trifluoroacetic acid or hydrochloric acid. The Cbz and Bn
protecting
groups may be removed by catalytic hydrogenation. The acetyl and
trifluoracetyl
protecting groups may be removed by a hydroxide ion.
The compounds and processes of the invention will be better understood by
reference to the following Examples, which are intended as an illustration of
and not a
limitation upon the scope of the invention.
Example 1
5- 6-[(endo)-8-Methyl-8-aza-bicyclo[3.2.1joct-3-yloxy]-pyridazin-3-y1) -1H-
indole
trifluoroacetate
-28-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example IA
(endo)-3-(6-chloro-pyridazin-3-yloxy)-8-methy1-8-aza-bic_yclo[3.2.11octane
A mixture of (endo)-tropine (Aldrich, 706 mg, 5,0 mmol), 3,6-
dichloropyridazine
(Aldrich, 745 mg, 5,0 mmol) and potassium t-butoxide (Aldrich, 1.12 g, 10
mmol) in
THF (anhydrous, Aldrich, 25 mL) was stirred at 60 'V under an atmosphere of
nitrogen
for 16 hours. The mixture was concentrated under reduced pressure and the
residue
purified by chromatography (150 g Si02, Et0Ac: Me0H : NH3-1120, 90:10:1, Rf,
0.20) to
provide the title compound, IH NMR (300 MHz, CD30D) 8 2.03 - 2,36 (m, 8 H),
2.45
(s, 3 H), 3.38 [s (br.), 2 H], 5.40 (t, J-5.09 Hz, 1 H), 7,20 (d, .1=9.16 Hz,
1 7.66 (d,
J=9,16 Hz, 1 H) ppm; MS (DCl/N113) m/z 254 (M+H)+,
Example 1B
5- {6-[(endo)-8-Methy1-8-aza-bicyclof3.2.1]oct-3-yloxyl-pyridazin-3-yll -1H-
indole
trifluoroacetate
The mixture of Example lA (112 mg, 0.44 mmol), 5-(4,4,5,5-tetramethyl-
[1,3,2]dioxaborolan-2-y1)-1H-indole (Aldrich, 232 mg, 0.954 mmol),
bis(triphenylphosphine)palladium(II) chloride (Aldrich, 7.02 mg, 0,01 mmol)
and
bipheny1-2-yl-dicyc1ohexyl-phosphane (Strem Chemicals, 10.5 mg, 0,03 mmol) in
dioxane/Et0H/Na2CO3 (aq,, 1 M) (v, 1/1/1, 3 mL) were heated and microwaved to
150
C and 300 watts for 15 minutes in an EniryTM Creator microwave. The solid was
filtered off with a syringe filter and the organic solution was directly
purified by
preparative HPLC (Gilson, column, Xterra 5 pun, 40 x 100 mm. Eluting Solvent,
MeCN
/ H20 containing 0,1% v, TFA (90% to 10% over 25 minutes, Flow rate of 40
mL/minute, uv detector set to 254 urn). The fractions containing the desired
product
were collected and concentrated under reduced pressure and the residue was
stirred in
ether/ethanol (v. 10/1, 5 mL) at ambient temperature for 16 hours to provide
the title
compound. 114 NMR (300 MHz, CD30D) & 231 - 2.60 (m, 8 H), 2.85 (s, 3 H), 3.97
[s
(br.), 2 1-1], 5.53 - 5,62 (m, 1 H), 6,56 (d, J=3.05 Hz, 1 H), 7,24 - 7,34 (m,
2 H), 7,51 (d,
J=8.48 Hz, 1 H), 7,74 (dd, J=8.65, L86 Hz, 1 H), 8.09 - 8.17 (m, 2 H) ppm; MS
(DCl/NH3) na/z 335 (M+H)+. Anal. Calculated for C20H22N40-1.05 CF3CO2H-0.50
C2H5OH: C, 58,14; +1,5.50; N, 11,74. Found: C, 58,07; H, 5,44; N, 11,75,
-29-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 2
Jendo)-3-(6-Benzoiblthiophen-5-yl-pyridazin-3-yloxy)-8-methyl-8-aza-
bicyclo[3.2.1loctane trifluoroacetate
The product from Example IA (121 mg, 0.48 mmol) and 2-benzo[b]thiophen-5-
y1-4,4,5,5-tetramethyl-[1,3,2jdioxaborolane (Maybridge, 219 mg, 0,84 mmol)
were
treated according to the procedure outlined in Example 1B to provide the title
compound,
1HNMR (300 MHz, CD30D) 6 2,33 - 2.58 (m, 8 H), 2,86 (a, 3 H), .3,94 - 4.02 (m,
2 H),
5,57 - 5.64 (m, 1 H), 7.34 (d, :1,-9.15 Hz, 1 H), 7.50 (d, J=5,42 Hz, I H),
7.67 (d, J=5.42
Hz, 1 H), 7,98 (dd, J=8.48, 1.70 Hz, I H), 8.06 (d, J=8.48 Hz, I H), 8.20 (d,
J-9.15 Hz, 1
H), 8.44 (d, J=1.36 Hz, 1 H) ppm. MS (DCUNH3): miz 352 (M+H)+, Anal.
Calculated for
C201-121N30S-1,10 CF3CO2H: C, 55.91; H, 4,67; N, 8,81. Found: C, 55,90; H,
4.41; N,
8.59.
Example 3
(endo)-346-(Penzofuran-5-y1)-pyridazin-3-yloxy]-8-methyl-8-aza-
bicyclo[3.2.1]octane
trifluoroacetate
The product from Example IA (131 mg, 0.52 mmol) and 1-benzofuran-5-
ylboronic acid (Apollo, 166 mg, 1,02 mol) were treated according to the
procedure
outlined in Example 1B to provide the title compound. 'I-INMR (300 MHz, CD30D)
6
2,33 - 2,64 (m, 8 H), 2,86 (s, 3 H), 3,94 - 4,02 (m, 2 H), 5.56 - 5.63 (m, 1
H), 6.96 (d,
3=1.36 Hz, 1 H), 7.32 (d, J=9,16 Hz, 1 H), 7,65 (d, 1-8,82 Hz, 1 H), 7.84 (d,
J=2,37 Hz, 1
H), 7,93 (dd, J=8,82, 2Ø3 Hz, I H), 8,15 (d, J=9,49 Hz, 1 H), 8,22 (d,
J=I.36 Hz, 1 H)
PPm; MS (iCl/N113): nth 336 (M+H)+, Anal, Calculated for C201-12iN302' 1.1
CF3CO2H:
C, 57.86; H, 4.83; N, 9.12, Found: C, 58,10; H, 4.54; N, 9.06.
-30-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
6-{6-[(endo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxvi-pridazin-3-y1)-1H-
indole
trifluoroacetate
The product of Example IA (158 mg, 0.62 mmol) was coupled with indole-6-
boronic acid (Frontier, 162 mg, 1.01 mol) were treated according to the
procedure
outlined in Example 1B to provide the title compound, 1H NMR (300 MHz, CD30D)
2,33 - 2,59 (m, 8 H), 2.85 (s, 3 H), 3,93 - 4,01 (m, 2 H), 5.58 (t, .1=3,05
Hz, 1 H), 6,51 (d,
J=3.05 Hz, 1 H), 7,29 (d, J-9.16 Hz, 1 H), 7.35 (d, J¨.3.05 Hz, 1 H), 7,58 -
7.64 (m, 1 H),
7,66 - 7,73 (m, 1 H), 8.01 (s, 1 H), 8.13 (d, J=9.49 Hz, 1 H) ppm, MS
(DC1/NH3): m./z
335 (M+H)+, Anal, Calculated for C20H2IN40-1.10 CF3CO2H: C, 57,99; H, 5.06; N,
12.18. Found: C, 58.09; H, 4.95;N, 11.97.
Example 5
5- {6-[(endo)-8-Methyl-8-aza-bicyclo[3.2.1loct-3-yloxyl-pyridazin-3-y1}-1H-
indazole
fumarate
Example 5A
5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1H-indazole
A flask containing 5-bromo-1H-indazole (Ref US 200.3199511, 9.45 g, 48 mmol)
and bis(pinacolato)diboron(Aldrich, 15.5 g, 61 mmol) in dry DMF (160 mL) was
added
KOAc (16,7 g, 170 mmol) . The mixture was degassed and purged with N2 three
times
followed by the addition of PdC12(dppi)'CH2C12 (Aldrich, 985 mg, 1.21 mmol).
The
mixture was heated to 90 C and stirred for 24 hours, The mixture was cooled to
ambient
temperature, diluted with ethyl acetate (250 rnL), washed with water (2 x 50
mL). The
organic phase was concentrated under reduced pressure and the residue was
purified by
chromatography (400 g Si02, hexane : Et0Ac 90: 10, Ri=0.6) to provide the
title
compound, 1H NMR (300 MHz, CD30D) ô 1,36 (s, 12 H), 7.51 (dt, J=8.48, 1,02 Hz,
1
H), 7.73 (dd, J=8,48, 1.02 Hz, 1 H), 8.08 (d, J=1,02 Hz, 1 H), 8,23 (t, 1=1.02
Hz, 1 H)
ppm. MS (DC1/NH3): ink 245 (M+H)+.
Example 5B
-31-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
5-16-1(endo)-8-Methy1-8-aza-bic yc1o[3.2. 1 'loct-3 -yloxyl-pyridazin-3-y1 -1H-
indazole
A mixture of Example IA (158 mg, 0,62 mmol) and the product of Example 5A
(308 mg, 1,26 mol) were treated with bis(triphenylphosphine)palladium(II)
chloride
(Aldrich, 7.02 mg, 0,01 mmol) and bipheny1-2-yl-dicyclohexyl-phosphane (Strem
Chemicals, 10,5 mg, 0,03 mmol) in dioxane/Et0H/Na2CO3 (aq,, 1 M) (v. 1/1/1, 3
mL)
were heated and microwaved to 150 C and 300 watts for 15 minutes in an Emrirm
Creator microwave reactor. The mixture was cooled to ambient temperature,
solid was
filtered off with a syringe filter and the organic solution was directly
purified by
chromatography (40 g Si02, Et0Ac:MeOH:NH3-H20, 90:10:1, Rr-0,10) to provide
the
title compound. 1H NMR (300 MHz, CD30D) 5 2,02 - 2.33 (m, 8 H), 236 (s, 3 H),
3.25
[s (hr.), 2 Hi, 5.47 (t, J=4.92 Hz, 1 H), 7.23 (d, J=9,16 Hz, 1 H), 7.67 (dt,
.1=8.82, 0,85
Hz, 1 H), 8.07 (dd, J=8,82, 1.70 Hz, 1 H), 8.10 - 8,19 (m, 2 H), 836 (dd,
J=1.53, 0.85 Hz,
1 H) ppm; MS(DCl/NH3): rn/z 3.36 (M+H)+.
Example 5C
5- 16-Rendo)-8-Methyl-8-aza-bicyclo[3.2.1loct-3-yloxy]-pyridazin-3-yll -1H-
indazole
furnarate
The product of Example 5B (128 mg, 0,38 mmol) was treated with fumaric acid
(46 mg, 0,40 mmol) in Et0Ac/Et0H (v. 1:1, 5 inL) at ambient temperature for 15
hours
The mixture was filtered to provide the titled compound. 1H NMR (300 MHz,
CD30D) 6
2.29 - 2.61 (m, 8 H), 2.86 (s, 3 H), 3,90 - 3,99 (m, 2 H), 5.59 (t, J=4.92 Hz,
1 H), 6.69 (s,
2 H), 7.32 (d, J=9.16 Hz, 1 H), 7.68 (d, J=8,82 Hz, 1 H), 8,08 (dd, J=8,82,
1,70 Hz, I H),
8.15- 821 (m, 2 H), 8,38 (dd, T=1.70, 0.68 Hz, 1 H) ppm; MS(DCl/NH3): In/z
3.36
(M+H)+, Anal Calcd, for CI9H2IN50-1,20 C4H404: C, 60,22; H, 5.48; N, 14.75.
Found:
C, 60,03; H, 5,17; N, 14,85,
Example 6
1-Methyl-5-164f endo)-8-methyl-8-az a-bicyclo[3.2.1joct-3-yloxy] -pyridazin-3-
yll -1H-
indole trifluoroacet ate
The product of Example IA (121 mg, 0,48 mmol) and N-methylindole-5-boronic
acid (Frontier, 175 mg, 1.0 mol) were treated according to the procedure
outlined in
Example 1B to provide the title compound, 1H NMR (300 MHz, CD30D) 5 2.22 -
2,70
-32-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
(m, 8 H), 2.86 (s, 3 H), 3.93 - 4,03 (m, 2 H), 5,53 - 5.62 (in, 1 H), 6.57 (d,
J=3.05 Hz, 1
H), 7,26 (d, J=3.39 Hz, 1 11), 7.37 (d, J=9.49 Hz, 1 H), 7,54 (d, J=8.82 Hz, 1
H), 7,80 (dd,
J=8,65, 1,87 Hz, 1 H), 8.16 (d, J=1,70 Hz, 1 H), 8.21 (d, J=9.16 Hz, 1 H) ppm;
MS
(DCl/NH3): miz 349 (M+H) . Anal. Calculated for C21}124N40'1,60 CF3CO2H: C,
54.75;
H, 4.86; N, 10.55. Found: C, 54,69; H, 4,80; N, 10,58.
Example 7
5- {6-[(endo)-8-Methyl-8-aza-bicyclo[3.2.1)oct-3-yloxyl-pyridazin-3-yll -2-
trifluoromethy1-1H-indole trifluoroacetate
Example 7A
5-(4,4,5,5-Tetramethy141,3,21dioxaborolan-2-y1)-2-trifluoromethyl-1H-indole
A mixture of 5-Bromo-2-trifluoromethy1-1H-indole (Ref US 2005043347, 6.05 g,
22,9 mmol), bis(pinacolato)diboron (7.74 g, 30.5 mmol), KOAc (8,05 g, 82 mmol)
and
PdC12(dP1Di)'CH2C12 (901 mg, 1.1 mmol) in anhydrous DMF (242 mL) were
processed
according to the procedure of outlined in Example 5A to provide the titled
compound, 11-1
NMR (300 MHz, CD30D) 5 1,36 (s, 12 H), 6.91 (s, 1 H), 7,43 (d, J=8,48 Hz, 1
H), 7,64
(d, J¨.8.14 Hz, 1 H), 8.11 (s, 1 H) ppm; MS (DCWNH3): 312 (M+H)+.
Example 7B
(exo)-8-Methy1-8-aza-bicyclof3.2.11oct-3-y1-4-nitro-benzoate
To a mixture of (endo)-tropine (2.82 g, 20,0 mmol), 4-nitrobenzoie acid (3.34
g,
20,0 mmol) and triphenylphosphine (5.24 g, 20.0 mmol) in dry RIF (100 mL) at
room
temperature was added diisopropyl azodicarboxylate (4,04 g, 20.0 mmol) and the
resulting mixture stirred for 40 hours. The mixture was concentrated under
reduced
pressure and the residue purified by chromatography (140 g Si02, Et0Ac : Me0H
NEl3'H20, 90:10:1, Rf=0õ30) to provide the titled compound. H NMR (300 MHz,
CD30D) 5 1.74 - 2.23 (m, 8 H), 2.38 (s, 3 H), 3.32 - 3,38 (m, 2 H), 5,23 -
5.38 (in, 1 H),
8,21 (d, J-8.82 Hz, 2 H), 8.32 (d, J-8,82 Hz, 2 H) ppm; MS (DC1/NH3): 291
(M+H)+.
Example 7C
(exo)-8-methyl-8-aza-bicyclo[3.2.11octan-3-ol
-33-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
The product of Example 7B (5.0 g, 0.017 mol) in ethanol (10 mL) was treated
with NaOH (IN, 200 mL) at room temperature for 40 hours. The mixture was
extracted
with the mixture of 10% isopropanol in chloroform (3 X 100 mL) and the
combined
extracts concentrated under reduced pressure to provide the title compound.
III NMR
(300 MHz, CD30D) 6 1.55- 1.69 (m, 4 H), 1.80 (m, 2 H), 1.99 - 2.09 (m, 2 H),
2.28 (s, 3
H), 3,14 - 3,21 (m, 2 H), 3.79 - 3.93 (m, 1 H) ppm, MS (DCl/NH3): 142 (M+H)+,
Example 7D
(exo)-3-(6-chloro-pyridazin-3-yloxy)-8-methy1-8-aza-bicyclo[3.2.1loctane
The product of Example 7C (721 mg, 5.1 mmol) and 3,6-dichlropyridazine (1,04
g, 7,0 mmol) were treated according to the procedure outlined in Example lA to
provide
the title compound. 1H NMR (300 MHz, CD30D) 5 1.87 - 2,07 (m, 4 H), 2.23 -
2,31 (m,
2 H), 2,37 (m, 2 H), 3,60 - 3.69 (m, 2 H), 5.54 (m, 1 H), 7.15 (d, J-9.16 Hz,
1 H), 7.64 (d,
J=9.16 Hz, 1 H) ppm; MS (DCl/NH3): 254 (M+H) .
Example 7E
5- {6-Rendo)-8-Methyl-8-aza-bicyclo[3.2.11oct-3-yloxy]-pyridazin-3-y1}-2-
trifluoromethyl-1H-indole trifluoroacetate
The product of Example 7D (128 mg, 0,5 mmol) and the product of Example 7A
(311 mg, 1,0 mmol) were treated according to the procedure outlined in Example
1B to
provide the title compound. 1H NMR (300 MHz, CD30D) 6 2.01 - 2.73 (m, 8 H),
2,85 (s,
3 H), 4.01 - 4,10 (m, 2 H), 5,64 - 5,80 (m, 1 H), 7,02 (s, 1 H), 7,23 (d,
J=9,15 Hz, 1 H),
7,60 (d, J=8.48 Hz, 1 H), 7,95 (dd, 3=8,48, 1.70 Hz, 1 H), 8,13 (d, J=9.49 Hz,
1 H), 8,26
(d, J=1,02 Hz, 1 H) ppm; MS (DCl/NH3): rrilz 403 (M+H)+, Anal, Calculated for
C211-121F3N40-1.55 CF3CO2H: C, 49.98; H, 3,92; N, 9.67. Found: C, 49,9.3; H,
4,09; N,
9.69.
-34-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 8
5- f (exo)-8-Methy1-8-aza-bicyclo[3 .2.1ioct-3-yloxyl-pyridazin-3-yll -IH-
indole
firmarate
The product of Example 7D (154 mg, 0.61 mmol) and 5-(4,4,5,5-tetramethyl-
[1,3,2]dioxaborolan-2-y1)-1H-indole (Aldrich, 243 mg, 1,0 mmol) were treated
with
bis(triphenylphosphine)palladiurn(11) chloride (Aldrich, 7.02 mg, 0.01 mmol)
and
biphenyl-2-yl-dicyclohexyl-phosphane (Strem Chemicals, 10.5 mg, 0,03 mmol) in
dioxane/Et0H/ ageouslM Na2CO3 (v. 1/1/1, .3 mL) were heated and microwaved to
150
C and 300 watts for 15 minutes in an EmryTm Creator microwave, The mixture was
cooled to ambient temperature, the solid was filtered off with a syringe
filter and the
organic solution was directly purified by preparative HPLC (Gilson, Xterra
column, 7
pm, 40 x 100 mm, eluting solvent, MeCN / H20 (with 0.1 M NH4HCO3/NH4OH, PH=10)
(v, 90/10 to 10/90 over 25 minutes), flow rate, 40 mL/min., uv, 254 nm) to
provide the
free base of the titled compound. The free base was treated with fumaric acid
(65 mg,
0,57 mmol) according to the procedure of Example 5C to provide the title
compound, 1H
NMR (.300 MHz, CD30D) & 2.04 - 2.50 (m, 6 H), 2.57 - 2.69 (in, 2 H), 2.85 (s,
3 H), .3.99
- 4.05 (m, 2 H), 5,63 - 5.78 (m, 1 H), 6.56 (d, J=3.05 Hz, 1 H), 6.69 (s, 2
H), 7.20 (d,
J=9,15 Hz, 1 H), 7,31 (d, J=3.39 Hz, 1 H), 7.51 (d, J=8.48 Hz, 1 H), 7.74 (dd,
1.70 Hz, I H), 8.09 (d, .1=9.49 Hz, 1 H), 8,14 (d, J=1.02 Hz, 1 H) ppm; MS
(DCFNH3):
rniz 335 (M+H)+; Anal. Calculated for C20H22N40-1.20 C4H404: C, 62.88; H,
5.70; N,
11.83. Found: C, 62.63; H, 5.70; N, 11.96.
Example 9
5- {5-gendo)-8-Methy1-8-aza-bicyclor3.2.1ioct-3-yloxy]-pyridin-2-yll -1H-
indole
bistosylate
Example 9A
(endo)-3-(6-Chloro-pyridin-3-yloxy)-8-methy1-8-aza-bicyclo[3.2.1]octane
The mixture of (endo)-tropine (Aldrich, 2.82 g, 20 mmol), 2-chloro-5-iodo-
pyridine (Aldrich, 2,39 g, 24 mmol), CuI (St-rem Chemicals, 0.19 g, 1 narnol)
and 1,10-
phenanthroline (Aldrich, 0.36 g, 2 mmol), Cs2CO3 (Aldrich, 6.52 g, 20 mmol) in
toluene
-35-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
(anhydrous, Aldrich, 25 mL) was stirred at 110 C for 40 hours. The mixture
allowed to
cool to ambient temperature and was diluted with CH2C12 (100 mL) and washed
with
water (2 x 10 mL). The organic solution was concentrated and the title
compound was
purified by chromatography (Si02, CH2C12: Me0H : NH3-H20, 90:10:1, Rf. 0, 1 0)
to
provide the titled compound, 1H NMR (300 MHz, CD30D) S L97 - 2.08 (d, J-14.5
Hzõ
2 H), 2,13 2.18 (d, J=2.37 Hz, 2 H), 2.45 (s, 3 H), 3.35 - 3.41 (m, 2 H), 4.66
(t, J=4,8
Hz, 1 H), 7,35 - 7,42 (m, 2 H), 7.96 - 8,04 (dd, J=2,3, LO Hz, 1 H) ppm. MS
DCl/NH3)
m/z 255 (M+11)+, 253 (M+H)+,
Example 91
5- {5-[(endo)-8-Methyl-8-aza-bicyclo[3.2.1loct-3-yloxy]pvridin-2-yll -1H-
indole
The mixture of the product from Example 9A (150 mg, 0.59 mmol), 5-
indolylboronic acid (Rsycor, 143.3 mg, 0,89 mmol), Pd(PPh3)4 (Aldrich, 6.8 mg,
0,006
mmol) and K2CO3 (2 M, 1 mL) was heated to 85 C in dioxane(4 mL) for 12 hours,
The
mixture was cooled to ambient, filtered and purified by preparative BPLC
[Waters
XTerra RP18 column, 30x100 mm, eluting solvents, MeCN /1120 (0.1 M aqueous
ammonium bicarbonate, adjusted to pH 10 with ammonium hydroxide) (v, 90/10 to
10/90
over 20 min.), flow rate 40 mL/min, uv, 250 nrn] to provide the titled
compound. Ili
NMR (.300 MHz, CD30D) 81.94 - 2.06 (m, 2 H), 2,06 - 2.27 (m, 6 H), 2.34 (s, 3
H), 3.21
[s(br.), 2 11], 4,67 (t, J=4.75 Hz, 1 H), 6.52 (dd, J=3,05, 1,00 Hz, 1 H),
7.26 (d, J=.3.39
Hz, 1 H), 7.40 (dd, J=8,82, 3.05 Hz, 1 H), 7,45 (dt, J=8.48, 0,7 Hz, 1 H),
7.63 (dd,
J=8.65, 1.87 Hz, 1 H), 7.77 (dd, J=8.82, 0.70 Hz, 1 H), 7.99 - 8.08 (m, 1 H),
8.18 (d,
J=3.05 Hz, 1 H) ppm. MS (DC1/NH3) m/z 334 (M+H)+,
Example 9C
5- f5-[(endo)-8-Methyl-8-aza-bicyclor.3.2.11oct-3-yloxyl-pyridin-2-y1}-1H-
indole
bistosylate
The product of Example 9B (40 mg, 0.12 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H.H20 (Aldrich, 38 mg, 0.2mmol) in a
mixture of
25 % isopropanol in iso-propylacetate (5 mL) at ambient temperature for 10
hours. The
mixture was filtered to provide the titled compound, 1H NMR (300 MHz, CD30D)
2.25-2.56 (m, 13 H), 2.77 - 2,89 (m, 411), 3,87 - 4,03 (m, 211), 4,90-2,04 (m,
111), 6,66
-36-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
(dd, J=.3.1, 0.7 Hz, 1 H), 7,19 (d, J8.10 Hz, 4H), 7,43 (d, J=3.39 Hz, 1 H),
7,55 - 7.65
(m, 2 H), 7,68 (d, J=8.14 Hz, 4 H), 8.10 - 8,17 (m, I H), 8,22 - 8.38 (m, 2
H), 8,46 (d,
1=2,03 Hz, 1 H) ppm. MS (DCFNH3): iniz 334 (M+H)+. Anal, Calculated for
C211123N30'105 C7H8S03-2.00 H20: C, 57.52; H, 6,17; N, 5.72, Found: C, 57.88;
H,
5,99; N, 5.33,
Example 10
(endo)-3-(6-Benzo[bithiophen-5-yl-pyridin-3-yloxy)-8-methy1-8-aza-
bicyclo[3.2.11octane bistosylate
Example 10A
2-Benzorblthiophen-5-y1-4,4,5,5-tetramethy141,3,2]dioxaborolane
A mixture of 5-bromo-benzo[b]thiophene (Maybridge, 4,26 g, 0,0200 mol),
bis(pinacolato)diboron (Aldrich, 6.09 g, 0.0240 mol) and potassium acetate
(Aldrich,
2.94 g, 0.0300 mol) in 1,4-dioxane (Aldrich, 50 mL) was degassed and purged
with N2
three times, [1,1 '-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)
PdC12(dPPO'CH2C12. (300 mg, 0,4 mmol, Aldrich) was and the solution was heated
to
I00 C for 20 hours. The mixture was then cooled to room temperature, diluted
with 300
rriL of Et0Ac and washed with brine (2 x 20 mL). The organic solution was
concentrated under reduced pressure and the residue was chromato graphed to
provide the
title product. tH NMR (.300 MHz, CDC13) 8 1.36 - 1.41 (S, 12 H), 7.35 (d,
J=5.50 Hz, 1
H), 7.42 (d, J=5.70 Hz, 1 H), 7.75 (d, J=8.14 Hz, 1 H), 7.89 (d, J=8.14 Hz, I
H), 8,31 (s,
1 H) ppm. MS (DC1/NH3) mlz 278 (M-FH)+
Example 10B
(endo)-3-(6-Benizo[b)thiophen-5-yl-pyridin-3-y1oxy)-8-methy1-8-aza-
bicyclo[3.2.11octane
The product from Example 9A (150 mg, 0.59 mmol) and the product of 10A
(231.6 mg, 0,89 mmol) was treated with Pd(PPh3)4 (Aldrich, 6.8 mg, 0.006 mmol)
according to the procedure of outlined in Example 9B. The title product was
purified by
preparative IIPLC [Waters XTerra RP18 column, 30x100 mm, eluting solvents,
MeCN /
H20 (0.1 M aqueous ammonium bicarbonate, adjusted to pH 10 with ammonium
-.37-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
hydroxide) (v. 90/10 to 10/90 over 20 min.), flow rate 40 mL/min, uv, 250
rim]. 'FINMR
(300 MHz, CD30D) 8 L93 - 2.07 (m, 2 H), 2.06 - 2,28 (m, 6 H), 2.34 (s, 3 H),
121
[s(br,), 2 4.70
(t, J=5,26 Hz, 1 H), 7.37 - 7.50 (m, 2 H), 7,61 (d, J=5.43 Hz, 1 H), 7,80
- 7,92 (m, 2 H), 7.94 - 8,02 (in, 1 H), 8.25 (d, J-2.71 Hz, 1 H), 8.34 (d,
J=1.36 Hz, 1
H) ppm, MS (DC1/NH3) m/z 351 (M+H)+
Example 10C
(endo)-3-(6-Benzorbjthiophen-5-yl-pyridin-3-yloxy)-8-methyl-8-aza-
bicyclo[3.2.1]octane bistosylate
The product of Example 10B (70 mg, 0,20 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H-H20 (Aldrich, 38 mg, 0.2mmol) in a
mixture of
25% isopropanol in isopropyl acetate as outlined in Exampled 9C. The mixture
was
filtered to provide the titled compound. 111 NMR (300 MHz, CD30D) 8 2.35 (s, 6
H),
2,48-2.62 (m, 8 H), 2,78 (s, .3H), 3.88 - 4.05 (m, 2 H), 5,02 (t, J=4.58 Hz, 1
H), 7.22 (d,
J=7,80 Hz, 4 H), 7.55 (d, J=5.76 Hz, 1 H), 7,70 (d, J=8,48 Hz, 4 H), 7.75 -
7.84 (m, 2 H),
8.12 - 8.22 (m, 2 H), 8.29 (d, 3=9.20 Hz, 1 H) 8.37 (d, 3=1.70 Hz, 1 H), 8.56
(d, J=3.05
Hz, 1 H) ppm. MS (DCliNH3): m/z 351 (M+H)+, Anal. Calculated for
C211123N20S'2.00
C7H8S03'1.00 H20: C, 58.97; H, 5,66; N, ,3.93, Found: C, 58.86; H, 5.61; N,
5,71,
Example 11
5- {5-gexo)-8-Methyl-8-aza-bicyclo[3.2.11oct-3-yloxyl-pyridin-2-y11-1H-indole
tosylate
Example 11A
fexo)-3-(6-Chloro-pyridin-3-y1oxy)-8-methy1-8-aza-bicyclo[3.2.1]octane
tosylate
To the mixture of (endo)-tropine (Aldrich, 2.82 g, 20 mmol), 2-chloro-5-
hydroxy-
pyridine (Aldrich, 1.29 g, 10 mmol) and Ph3P (Aldrich, 5.24 g, 20 mmol) was
added di-
isopropyl azadicarboxylate (Aldrich, 4.04 g, 20 mmol) in THF (anhydrous,
Aldrich, 100
mL) and the mixture was stirred for two days. The mixture was concentrated
under
reduced pressure and the title product was purified by chromatography (Si02,
CH2C12:
Me0H : NH3-H20, 90:10:1, Rf. 0.40) as solid (1,98 g, yield, 78,3%), IHNMR (
300
MHz, CD30D) 8 1.63- 1.92 (m, 4 H), 1.97 - 2.20 (m, 4 H), 2.33 (s, 3 H), 3,34
(s, 2 H),
4,51 - 4.75 (m, 1 H), 7.27 - 7.37 (dd,J=8,80, 0.7 Hz, 1 H), 7,37 - 7.49 (dd,
J=8.80, 3,00
-38-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Hz, 1 H), 8.01 (d, J=3,05 Hz, 1 H) ppm, MS (DCl/NH3) m/z 255 (M+H)+, 253
(M+H)+.
Example 11B
5- {54(_exo)-8-Methy1-8-aza-bicyc1o[3 .2.1loct-3-yloxyl-pyridin-2-yll -1H-
indole
The mixture of the product from Example 11A (150 mg, 0,59 mmol), 5-
Indolylboronic acid (Rsycor, 143..3 mg, 0.89 mmol) and Pd(PPh3)4 (Aldrich, 6,8
mg,
0.006 mmol) and K2CO3 (2 M, 1 mL) in dioxane(4 mL) was stirred at 85 C for 12
hours
according to the procedure of outlined in Example 9B. The title product was
purified by
preparative HPLC [Waters XTerra RP18 column, .30x100 mm, eluting solvents,
MeCN /
H20 (0,1 M aqueous ammonium bicarbonate, adjusted to pH 10 with ammonium
hydroxide) (v. 90/10 to 10/90 over 20 min.), flow rate 40 mL/min, uv, 250
rim], IHNIVIR
(300 MHz, CD30D) 8 1_61 - 1,97 (m, 4 H), 2.00 - 2,2.3 (m, 4 H), 2,35 (s, 3 H),
3,22 -
3,38 (m, 2 H), 4.56 - 4,78 (m, 1 H), 6,51 (d, J=4.07 Hz, 1 H), 7,26 (d, J=3.39
Hz, 1 H),
7,40 - 7.52 (m, 2 H), 7,62 (dd, J=8.48, 1,70 Hz, 1 H), 7,75 (d, J=8,82 Hz, 1
H), 803 (s, 1
H), 8.21 (d, J=2,37 Hz, 1 H) ppm, MS (DCITNH3) m/z, 334 (M+H)+.
Example 11C
5- {5-[(exo)-8-Methyl-8-aza-bicyclo[3.2.11oct-3-yloxy]-pyridin-2-y11-1H-indole
tosylate
The product of Example 1113 (50 mg, 0,15 mmol) was treated with p-
toluenesulfonic acid monohydrate TsOHE20 (Aldrich, 38 mg, 0,2mmol) in a
mixture of
25% isopropanol in isopropyl acetate (5 mL) at ambient temperature for 10
hours
according to the procedure of Example 9C. The mixture was filtered to provide
the titled
compound, 11-1 NMR (300 MHz, CD30D) ô 1.90- 2.13 (in, 2 H), 2,17 - 2.31 (m, 2
H),
2.33-2.42 (m, 5H), 2_44 -2.58 (m, 2 H), 2.83 (s, .3 H), 4.02 [s (br.), 2 11],
4.86 -5.03 (m,
1 H), 6,53 (dd, J=3.22, 0,85 Hz, 1 H), 7,22 (d, J=8.14 Hz, 1 H), 7.26 - 7.32
(m, 1 H), 7.47
(d, J=8.48 Hz, 1 H), 7.56 - 7.66 (in, 2H), 7.70 (dt, J=8,10, 1.80 Hz, 2 H),
7,82 (d, J=8.82
Hz, 1 II), 8.05 (d, J---1,36 Hz, 1 H), 8,28 (d, J=3.05 Hz, 1 H) ppm, MS
(DCl/NH3): m/z
.334 (M+H)+, Anal, Calculated for C211123N301,00 C7H8S03-1,00 H20: C, 64.22;
H,
6.35; N, 8.02. Found: C, 64,07; H, 6,16; N, 7.69.
Example 12
(exo)-3[6-(Benzofuran-5-y1)-pyridin-3-yloxyj-8-methyl-8-aza-
bicyclof3.2.1joctane
-39-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
bistrifluoroacetate
The product of ExamplellA (130 mg, 0,52 mmol) and 1-benzofuran-5-ylboronic
acid (Maybridge, 166 mg, 1.0 mmol) were treated according to the procedure
outlined in
Example 1B to provide the title compound, 1H NMR (300 MHz, CD30D) 8 1,98 -
2.58
(m, 8 H), 2,84 (s, 3 H), .3,98 - 4,09 (m, 2 H), 4,93 - 5.07 (m, 1 H), 6,94 (d,
J-1.36 Hz, 1
H), 7,62 (d, J=8,81 Hz, 1 H), 7_73 (dd, J=8,81, 3,05 Hz, 1 H), 7,80 - 7.86 (m,
2 H), 7.92
(d,1=8,48 Hz, 1 H), 8.13 (d, .1=1,36 Hz, 1 H), 8.38 (d, 1=2,37 Hz, 1 H) ppm;
MS
(DCl/NH3): m/z 335 (M+H)+, Anal. Calculated for C211-122N202.2,00 CF3CO2H: C,
53.39; H, 4.30; N, 4.98. Found: C, 53.28; H, 4,04; N, 4.95.
Example 13
5- {5-[(exo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyridin-2-y1}-1H-
indazole
hemifumarate
The product of Example 11A (139 mg, 0.55 mmol) and the product of Example
5A (325 mg, 1,3 mmol) were treated according to the procedure outlined in
Example 8 to
provide the title compound, 1H NMR (300 MHz, CD30D) 6 1.97 - 2,45 (m, 8 H),
2,73 (s,
3 H), 3,80 - 3.89 (m, 2 H), 4.84 - 4,96 (m, 1 H), 6.68 (s, 1 H), 7.56 (dd,
.1=8,82, 3.05 Hz,
1 H), 7,62 (d, 3=8.82 Hz, I H), 7.84 (d,1-8.82 Hz, 1 H), 7.97 (dd, J=8.82,
1.70 Hz, I H),
8,12 (d, 1=1.02 Hz, 1 H), 8,27 (dd,1-1,53, 0.85 Hz, 1 H), 8.32 (d, 1=3.05 Hz,
1 H) ppm;
MS (DC1/NH3): raiz 335 (M+H)+,
Example 14
5-[(exo)-8-Methyl-8-aza-bicyclop .2.1loct-3-yloxyi-pyridin-2-y11-2-trifluor
methyl-
1H-indole fumarate
The product of Example 11A (130 mg, 0,52 mmol) and the product of Example
7A (319 mg, 1.0 mmol) were treated according to the procedure outlined in
Example 8 to
provide the title compound, 1H NMR (300 MHz, CD30D) 6 1,99 - 2,53 (m, 8 H),
2.83 (s,
3 H), 3,96 - 4.03 (m, 2 H), 4.85 - 5,02 (m, 1 H), 6.69 (s, 2 H), 6.97 (s, 1
H), 7.50 - 7.62
(m, 2 H), 7.78 - 7.88 (m, 2 H), 8.16 (d, 1=1,36 Hz, 1 H), 8,31 (d,1=2,71 Hz, 1
H) ppm;
MS (DC1/NH3): m/z 402 (M+H)+, Anal. Calculated for C22H22F3N30-1,20 C404H4: C,
59,53; H, 5,00; N, 7,77. Found: C, 59.26; H, 5,06; N, 7.86.
-40-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 15
4- 5-[(exo)-8-Methy1-8-aza-bicyclor3.2.1joct-3-yloxv1-pridin-2-yli -1H-indole
bistrifluoroacetate
The product of Example 11A (130 mg, 0.52 mmol) and indole-4-boronic acid
(Apollo, 165 mg, 1.0 mmol) were treated according to the procedure outlined in
Example
1B to provide the title compound. IFINMR (300 MHz, CD30D) 6 2,03 - 2,64 (m, 8
H),
2.85 (s, 3 H), 4.00- 4.10 (m, 2 H), 5,02 - 5.16 (m, 1 H), 6,70 (d,1=2.37 Hz, 1
H), 7.25 -
7.40 (in, 2 H), 7.44 (d, J-3,05 Hz, 1 H), 7,59 (d, 3=7,80 Hz, 1 H), 8,01 -
8.17 (m, 2 H),
8,50 (d, J=2.71 Hz, 1 H) ppm; MS (DC1/NH3): m/z 334 (M+H)+; Anal, Calculated
for
C21H23N30'2,00 C2F302H: C, 53,48; H, 4,49; N, 7,48, Found: C, 53.29; H, 4.17;
N,
7,35,
Example 16
4:15-j(exo)-8-Methy1-8-aza-bicyclo[3.2.1loct-3-yloxy1-pyridin-2-y1}-
phenylamine
bistrifluoroacetate
Example 16A
4- {5-[(exo)-8-Methyl-8-aza-bicyclor3.2.1)oct-3-yloxyl-pyridin-2-yll -
phenylamine
The product of Example 11A (379 mg, 1.5 mmol) and 4-(4,4,5,5-tetramethyl-
[1,3,2idioxaborolan-2-y1)-phenylamine (Aldrich, 552 mg, 2.5 mmol) were
processed
according to the procedure of Example 5B, The mixture was purified by
chromatography
(140 g Si02, Et0Ac : Me0H : NH3.1120, 90:10:1) to provide the title compound.
'11
NMR (.300 MHz, CD30D) 6 1.76 - 1,91 (m, 4H), 2,08 -2.21 (m, 4H), 3,35 -3.42 js
(br.), 2 Hj, 4,62 -4.76 (m, 1 H), 6,73 - 6,81 (m, 2 H), 7.42 (dd,1=8,81, 3.05
Hz, 1 H),
7,57 - 7,68 (m, 3 H), 8,15 (d, 1,-2,37 Hz, 1 H) ppm; MS (DC1/NH3): rn/z 310
(M+H)+,
Example 16B
4- {5-j(exo)-8-Methy1-8-aza-bicyclo{3.2.11oct-3-yloxYl-pyridin-2-yll-
pheny1amine
bistrifluoroacetate
The product of Example 16A (1.35 mg, 0.44 mmol) was repurified by preparative
HPLC (Gilson, Xterra column, 5 pm, 40 x 100 mm. Eluting Solvent, MeCN / H20
(with 0,1% v, TFA) (v. 90/10 to 10/90 over 25 min.) Flow rate, 40 mL/min,, uv,
254 nm).
-41-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
The fractions of the desired product were collected and concentrated under
reduced
pressure and the residue was stirred in Ether/Ethanol (v. 10/1, 5 mL) at room
temperature
for 16 hours, The mixture was filtered to provide the bis trifluoroacetate
salt, 1H N1VIR
(300 MHz, CD30D) 3 1,99 - 2.56 (m, 8 H), 4.03 [s (br,), 2 H], 4.93 - 5,07 (m,
1 H), 6,96 -
7.07 (m, 2 H), 7.73 - 7.86 (m, 3 H), 7.88 - 7,98 (m, 1 H), 8,32 (d, J=3.05 Hz,
1 H) ppm;
MS (DC1/NH3) rn/z 310 (M+H)+; Mal, Calculated for C191123N302.30 CF3CO2H: C,
49.58; H, 4.46; N, 7,53, Found: C, 49,58; H, 4.36; N, 7,44,
Example 17
5- (6-r(endo)-8-Methyl-8-aza-bicyclof3.2.11oct-3-vloxyi-pyridin-3-yll-lH-
indole tosylate
Example 17A
(endo)-3-(5-Bromo-nyridin-2-yloxy)-8-methy1-8-aza-bicyclo[3.2.1loctane
(endo)-Tropine (Aldrich, 282 mg, 2 mmol) was treated with tBuOK (Aldrich, 224
mg, 2 mmol) in THF(20 mL) at ambient temperature for 1 hour followed by the
addition
of .3,6-dibromopyridine (Aldrich, 569 mg, 2.4 mmol). The mixture was stirred
at 60 C
for additional 10 hours and then concentrated under reduced pressure. The
residue was
dissolved in CHC13/isopropanol (10: 1, 50 mL) and washed with brine (2 x 5
mL). The
organic solution was concentrated under reduced pressure and the title
compound was
purified by chromatography (SiO2, CH2C12: Me0H : NH3.H20, 90:10:1, Ri. 0.10).
1/1
NMR (.300 MHz, CD30D) 3 1,93 (d, J=14,50 Hz, 2 H), 2.02 - 223 (m, 6 H), 2.31
(s, 3
H), 3,17 [s (In), 2 1-1], 5,16 (t, J=5.26 Hz, 1 H), 6,70 (d, J=8.82 Hz, I H),
7.77 (dd,
.1-8.81, 2.71 Hz, 1 H), 8,16 (d, J-2.71 Hz, 1 H) ppm, MS (DCTINH3): 299
(M+H)+, 297
(M+H) .
Example 171B
5- 64(end6-8-Methy1-8-aza-bicyclo3.2.1jact-3-yloxyl-pyridin-3-y1}-1H-indole
The mixture of the product from Example 17A (150 mg, 0,50 mmol), 5-
indolylboronic acid (Rsycor, 121.9 mg, 0.75 mmol), Pd(PPh3)4 (Aldrich, 6.8 mg,
0.006
mmol) and K2CO3 (2 M, 1 mL) in dioxane(4 mL) was stirred at 85 C for 12 hours
according to the procedure of outlined in Example 9B, The title product was
purified by
preparative HPLC [Waters XTerra RP18 column, 30x100 mm, eluting solvents, MeCN
/
-42-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
H20 (0.1 M aqueous ammonium bicarbonate, adjusted to pH 10 with ammonium
hydroxide) (v. 90/10 to 10/90 over 20 min,), flow rate 40 mL/min, uv, 250 nm].
11-1N1VIR
(300 MHz, CD30D) 8 2.01 (d, J=14.30 Hz, 2 H), 2,06 - 2,28 (m, 6 H), 2.34 (s, 3
H),
3,17 - 3,26 (m, 2 H), 5.19 (t, J=5.26 Hz, 1 H), 6,49 (d, J=2..37 Hz, 1 H),
6,82 (d, J=8,48
Hz, 1 H), 7.26 (d, J=3.05 Hz, 1 H), 7.31 (dd, J=8.48, 1,70 Hz, 1 H), 7,45 (d,
J-8.48 Hz, 1
H), 7,73 (s, 1 H), 7.96 (dd, J=8.65, 2,54 Hz, 1 H), 8,35 (d, J=2,03 Hz, 1
H)ppm. MS
(DCFNH3) in/z 334 (M+H)+.
Example 17C
5- f64(endo)-8-Methy1-8-aza-bicyclor3.2.11oct-3-yloxyl-pyridin-3-y11-1H-indole
bistosylate
The product of Example 11B (40 mg, 0,15 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H-H20 (Aldrich, 38 mg, 0,2mmol) in a
mixture of
25% isopropanol in isopropyl acetate (5 mL) at ambient temperature for 10
hours
according to the procedure outlined in Example 9C. The mixture was filtered to
provide
the title compound. ill NMR (300 MHz, CD30D) 2,32-2.58 (m, 14 H), 2.81 - 2.88
(s,
3 H), 3.89 - 4.01 (m, 2 H), 5,27 - 5.41 (m, 1 H), 6.52 (d, J=3.39 Hz, 1 H),
7.13 (d, J=8,48
Hz, 1 H), 7.23 (d, J=7,80 Hz, 4 H), 7,35 (dd, 5=8,48, 2.03 Hz, 1 H), 7.49 (d,
5=8.48 Hz, 1
H), 7.70 (d, 5=8,14 Hz, 4 H), 7,79 (s, 1 H), 8.24 (dd, J-8.65, 2,54 Hz, 1 H),
8.47 (d,
5=2.71 Hz, 1 H) ppm, MS (DCFNH3): m/z 334 (M+H)+. Anal, Calculated for
C211-123N30-2,20 C7H8S03-2,00 H20: C, 58.42; H, 6.01; N, 5,62, Found: C,
58,02; H,
5,84; N, 5,31,
Example 18
(endo)-3-(5-BenzoNthiophen-5-yl-pyridin-2-yloxy)-8-methy1-8-aza-
bicyclo[3.2.1]octane tosylate
Example 18A
(endo)-3-(5-Benzo[b]thiophen-5-yl-pyridin-2-yloxy)-8-methy1-8-aza-
bicyclo[3.2.1]octane
The mixture of Example 17A (150 mg, 0.50 mmol), the product of Example 10A
(197,0 mg, 0,75 mmol), Pd(PPh3)4 (Aldrich, 6.8 mg, 0,006 mmol) and K2CO3 (2 M,
1
-43-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
mL) in dioxane(4 mL) was processed according to the procedure outlined in
Example 9B,
The title product was purified by preparative HPLC [Waters XTerra RP18 column,
30x100 rum, eluting solvents, MeCN / H20 (0.1 M aqueous ammonium bicarbonate,
adjusted to pH 10 with ammonium hydroxide) (v. 90/10 to 10/90 over 20 min.),
flow rate
40 mL/min, uv, 250 rim]. 1HNMR (300 MHz, CD30D) 8 1.99 (d, J=14,50 Hz, 1 H),
2,03 - 2,28 (m, 6 H), 2,33 (s, 3 H), 114 - 3,25 (m, 2 H), 5.23 (t, J=5,26 Hz,
1 H), 6.86 (d,
J=8,48 Hz, 1 H), 7.43 (d, J=543 Hz, 1 H), 7.57 (dd, J-8.48, L70 Hz, 1 H), 7,61
(d,
J=5,43 Hz, 1 H), 7.91 - 8.09 (na, .3 H), 8.42 (d, J=1.70 Hz, 1 H) ppm, MS
(DCl/NH3) miz
351 (M+H)+,
Example 18B
(endo)-3-(5-Benzo[b]thiophen-5-yl-pyridin-2-yloxy)-8-methyl-8-aza-
bicyclo[3.2.1]octane tosylate
The product of Example 18A (60 mg, 0,17 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H+120 (Aldrich, 38 mg, 0.2 mmol) in a
mixture of
25% isopropanol in isopropyl acetate (5 mL) at ambient temperature for 10
hours
according to the procedure outlined in Example 9C, The mixture was filtered to
provide
the titled compound. 1HNMR (300 MHz, CD30D) 8 2.34-2,45 (m, 9 H), 2.48-2.55
(m, 2
H), 2,84 (s, 3 H), 3,88 - 4,00 (m, 2 H), 5.39 (t, J=4.41 Hz, 1 H), 7.06 (d, J-
8,82 Hz, 1 H),
7,23 (d, J=7,80 Hz, 2 H), 7.45 (d, J=5.43 Hz, 1 H), 7,59 (dd, J=8,48, 1.70 Hz,
1 H), 7,64
(d, J=5.76 Hz, 1 H), 7,70 (d, J=8.48 Hz, 2 H), 8.00 (d, J-8.48 Hz, 1 H), 8,08
(d,
Hz, 1 H), 8.18 (dd, J--8.82, 2,37 Hz, 1 H), 8.51 (d, J-2,0.3 Hz, 1 H) ppm MS
(DC1/NH3):
miz 351 (M+H)+. Anal. Calculated for C21H23N20S-1.10 C7H8S03'1,00 H20: C,
61.79;
H, 5.93; N, 5.02. Found: C, 61,44; H, 5.63; N, 4,68,
Example 19
exo -8-Meth 1 -111-
indolefirm_arate
Example 19A
(exo)-3-(5-Bromo-pyridin-2-yloxv)-8-methy1-8-aza-bicyclo13.2.1]octane
The product of Example 7C (721 mg, 5.1 mmol) and 2,5-dibromopyridine (1.66
-44-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
g, 7.0 mxno1) were treated according to the procedure outlined in Example 1A
to provide
the title compound, 1H NMR (300 MHz, CD30D) 6 1,88 - 2,47 (m, 8 H), 2.74 (s, 3
H),
3.82 - 3,90 (m, 2 H), 5.34 - 5.48 (rn, 1 H), 6,71 (d, J=8,82 Hz, 1 H), 7,78
(dd, J=8.82,
2,71 Hz, 1 H), 8,20 (d, J=2.37 Hz, 1 H) ppm; MS (DC1/NH3): 2997 (M+H)+, 297
(M+H)+,
Example 19B
5-16-[(exo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyridin-3-y1}-1H-indole
fumarate
The product of Example 19A (129 mg, 0,434 namol) and 5-indolylboronic acid
(165 mg, 1.02 mmol) were treated according to the procedure outlined in
Example 8 to
provide the title compound. 1H NMR (300 MHz, CD30D) 6 1,97 - 2,12 (m, 2 H),
2,20 -
2,46 (m, 4 H), 2.48 - 2,60 (m, 2 H), 2.84 (s, 3 H), 3.96 - 4.07 (m, 2 H), 5.43
- 5.60 (in, 1
H), 6,49 (d, J=3,05 Hz, 1 H), 6.70 (s, 2 H), 6,82 (d, J-8,48 Hz, 1 H), 7.23 -
7,35 (m, 2 H),
7,46 (d, J=8,14 Hz, 1 H), 7,73 (d, J=1.70 Hz, 1 H), 7,95 (dd, J=8.65, 2,54 Hz,
1 H), 8.37
(d, J=2,03 Hz, 1 H) ppm; MS DCl/NH3): rniz 334 (M+H)+; Anal, Calculated for
C211123N30* 1 .1 0C404H4"1 ,00 H20: C, 6.3.67; H, 6,18; N, 8.77. Found: C,
63.77; H,
6,26; N, 8.64.
-45..
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 20
f6-(1H-Indo1-5-y1)-pyridin-3-y1]-1(endo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-
y1}-amine
bis(hydrochloride).
Example 20A
(6-Chloro-pyridin-3-y1)-[fendo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-ylj-amine
A mixture of tropinone (Aldrich, 2,78 g, 20 mmol), 6-chloro-pyridin-3-ylamine
(Aldrich, 2.83 g, 22 mmol), Na2SO4 (anhydrous, Aldrich, 21,3 g, 150 mmol) and
NaBH(OAc)3 (Aldrich, 8.48 g, 40 mmol) in HOAc (50 mL) at ambient temperature
was
stirred for 15 hours, The mixture was filtered and the filtrate washed with
Et0H (2 x 10
mL). The organic solution was concentrated under reduced pressure and the
title
compound obtained by purified using chromatography (Si02, CH2C12: Me0H :
NH31120, 90:10:2, Rf. 010). 1H NMR (300 MHz, CD30D) 8 2,16 (d, .1=15.26 Hz, 2
H),
2,25 - 2,35 (m, 2 H), 2.37 - 2,60 (m, 4 H), 2.81 (s, 3 H), 3,65 (t, J=5,93 Hz,
1 H), 3.79 -
.3.98 (m, J=2.71 Hz, 1 H), 7,09 (dd, J=8.50, 3,00 Hz, 1 H), 7.21 (d, J=8,80Hz,
1 H), 7.73
(d, J=2.71 Hz, 1 H)ppm, MS (DC1/NH3) m/z 254 (M+H) , 252 (M+H) ,
Example 20B
6- 1H-Indo1-5- 1 -5 idin-3- 1 - endo)-8-methyl-8-aza-bicyclof3.2.1]oct-3-yll-
amine
The mixture of Example 20A (250 mg, 1,0 mmol), 5-indolylboronic acid (Rsycor,
241.0 mg, 1,50 mmol), bis(triphenylphosphine)palladium(II) chloride (Aldrich,
10,0 mg,
0,01 mmol) and bipheny1-2-yl-dicyclahexyl-phosphane (Strem Chemicals, 11.0 mg,
0Ø3
mmol) in dioxane/Et0H/ 1M aqueous Na2CO3 (v. 1/1/1 3 mL) were heated and
microwaved to 130 C and .300 watts for 15 minutes in an EmryTM Creator
microwave.
The mixture was filtered through a syringe filter and the liquid was purified
by
preparative HPLC [Waters XTerra RP18 column, 30x100 mm, eluting solvents, MeCN
/
1120 (0,1 M aqueous ammonium bicarbonate, adjusted to pH 10 with ammonium
hydroxide) (v, 90/10 to 10/90 over 20 min.), flow rate 40 mL/min, uv, 250 nm]
to provide
the titled compound. ill NMR (300 MHz, CD30D) 8 1.88 (d, J=15.20 Hz, 2 H) 2.05
-
2,18 (m, 4 H), 2.18 - 2.31 (m, 2 H), 2,37 (s, 3 H), 3,26 [s (br.), 2 HA, 3.60
(t, J=6,44 Hz,
1 H), 6,49 (d, J=3.05 Hz, 1 H), 7.05 (dd, J=8,82, 2.71 Hz, 1 H), 7,24 (d,
J=3.05 Hz, 1 H),
-46-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
7,42 (d, J=8.48 Hz, 1 H), 7.49 - 7.64 (m, 2 H), 7.95 (s, 1 H) ppm. MS
(DC1/NH3) m/z 333
(M+H)+.
Example 20C
f6-(1H-Indo1-5-y1)-pyridin-3-y11-[(endo)-8-methyl-8-aza-bicyclor3.2.1}oct-3-
y11-amine
bis(hydrochloride)
The solution of Example 20B (160 mg, 0,48 mmol) in Et0Ac (10.0 mL) at
ambient temperature was treated with 4M hydrochloric acid in dioxane (0.5 ml,õ
2.0
mmol) for 10 hours. The title compound was obtained by filtration, 11-1 NMR
(300 MHz,
CD30D) 6 2.25 (d, J=15,65 Hz, 2 H), 2.32 - 2.53 (m, 4 H), 2.54 - 2.64 (m, 2
H), 2.84 (s,
3 H), 3.83 (t, J=6,14 Hz, 1 H), 3.97 [s (br,), 2 Kb 6.63 (d, J=43.07 Hz, 1 H),
7.40-7,41 (m,
1 H), 7.54 (dd, J=8,60, 1.90Hz, 1 H), 7.62 (d, .J=8.60 Hz, 1 H), 7.83 - 7,95
(m, 2 H), 8.06
(d, J-1.53 Hz, 1 H), 8,12 (d, J=8,90 Hz, 1 H) ppm. MS (DCl/NH3): m/z 333
(M+H)+,
Anal. Calculated for C211-124N4 2,30 HC1. .3,35 H20: C, 52.92; H, 6,98; N,
11,75.
Found: C, 52.87; H, 6.78; N, 11,35.
Example 21
[6-(Benzofuran-5-y1)-pyridin-3-y1]-[(endo)-8-methy1-8-aza-bicyclo[3.2.1joct-3-
yll-amine
fumarate
The product of Example 20A (136 mg, 0.54 mmol) and 1-benzofuran-5-ylboronic
acid (Aldrich, 185 mg, 1.14 mmol) were treated according to the procedure
outlined in
Example 8 to provide the title compound. 1HNMR (300 MHz, CD30D) 6 2.14- 2.57
(m,
8 H), 2,83 (s, .3 H), 3.74 (t, J=5,93 Hz, 1 H), 3,90 [a (br.), 2 11], 6,69 (s,
2 H), 6.89 (d,
J=1.36 Hz, 1 H), 7.13 (dd, J=8.65, 2.88 Hz, 1 H), 7.54 (d, J=8.82 Hz, 1 H),
7,68 (d,
J=8.82 Hz, 1 H), 7,72 - 7.79 (m, 2 H), 7.99 - 8.07 (m, 2 H) ppm; MS DCFNH3):
rn/z 334
(M+H)+.
-47-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 22
endo -8-Meth 1-8-aza-bic clo 3.2.1 oct-3- 1 - 6- 2-trifluorometh 1-1H-indo1-5-
1 -
pyridin-3-yll-amine bistrifluoroacetate
The product of Example 20A (130 mg, 0.52 mmol) and the product of Example
7A (262 mg, 0,84 mmol) were treated according to the procedure outlined in
Example 1B
to provide the title compound. 1H NMR (300 MHz, CD30D) 8 2,17 - 2.62 (m, 8 H),
2,84
(s, 3 H), 3.82 (t, J=5.9.3 Hz, 1 H), .3.96 [s (br.), 2 1-1], 7.06 (s, 1 H),
7,6.3 - 7.80 (m, 3 H),
7,95 (d, J=2,71 Hz, 1 H), 8.06 (d, J=9.16 Hz, 1 H), 8,15 (d, I=1,.36 Hz, 1 H)
ppm; MS
DC1/NH3): m/z 401 (M+H)+; Anal. Calculated for C22H22F3N30 2.00 CF3CO2H 0.70
NH4OH: C, 47.75; H, 4,24; N, 7.92, Found: C, 47.69; H, 3.91; N, 8,14.
Example 23
[641.11-Indazol-5-y1)-pyridin-3-yll-f(endo)-8-methy1-8-aza-bicyclo[3.2.1]oct-3-
ylkamine
fumurate
The product of Example 20A (128 mg, 0.51 mmol) and the product of Example
5A (205 mg, 0,84 mmol) were treated according to the procedure outlined in
Example 8
to provide the title compound, NMR (300 MHz, CD30D) (5 2,11 - 2.55 (m, 8
H), 2,79
(s, .3 H), 3,73 (t, J=5.93 Hz, 1 H), 3.85 [s (br,), 2 H], 6,67 (s, 3 H), 7.13
(dd, J=8,65, 2.88
Hz, 1 H), 7.59 (d, J-8.82 Hz, 1 H), 7.70 (d, J=8.82 Hz, 1 H), 7.90 (dd,
.1=8.82, 1.70 Hz, 1
H), 8.04 (d, .1=2.71 Hz, 1 H), 8.09 (s, 1 H), 8,18 (s, 1 H) ppm; MS DC1/NH3):
miz 3.34
(M+H) ; Anal. Calculated for C20H23N5.1.50 C404H4. 1.00 NH4OH: C, 57,55; H,
6.32;
N, 15.49. Found: C, 57.46; H, 6,26; N, 15,55.
Example 24
16-(1H-Indo1-4-y1)-pyridin-3-yll-Rendo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-y11-
amine
fumarate
The product of Example 20A (130 mg, 0.52 namol) and indole-4-boronic acid
(Apollo, 165 mg, 1,0 mmol) were treated according to the procedure outlined in
Example
8 to provide the title compound. 111, NMR (300 MHz, CD30D) & 2.16 - 2.60 (m, 8
H),
2.84 (s, 3 H), 3.76 (t, J=5.76 Hz, 1 H), 3.88 - 3.95 [s (br.), 2 H], 6.69 (s,
2 H), 6.70 (d,
J=3,39 Hz, 1 H), 7,14 - 7,32 (m, 4 H), 7.40 (d, .1=7,80 Hz, 1 H), 7.68 (d,
J=8.48 Hz, 1 H),
-48-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
8.06 (d, J-2,71 Hz, 1 H) ppm; MS DC1/NH3): rn/z 33.3 (M+H)+; Anal, Calculated
for
C211-124N4-1.40 C404H4'0.90 H20: C, 62.50; H, 6.19; N, 10.96. Found: C, 62,40;
H,
6.17;N, 11,04,
Example 25
f(endo)-8-aza-bicyclo13.2.1loct-3-_yll-j6-(1H-indol-5-y1)-pyridin-3-y11-amine
Example 25A
fendo)-3-(6-Chloro-pyridin-3-ylamino)-8-aza-bicyclo13.2.1loctanel-8-carboxylic
acid
tert-butyl ester
The mixture of 3-oxo-8-aza-bicyclo[3.2.1]octane-8-carboxylic acid tert-butyl
ester (Fluka, 3,50 g, 15.50 mmol), 6-chloro-pyridin-.3-ylamine (Aldrich, 2.20
g, 17.1
mmol), Na2SO4 (anhydrous, Aldrich, 16,6 g, 116 mmol) and NaBH(OAc)3 (Aldrich,
6.59
g, 31.1 mmol) in HOAc (40 mL) was stirred at ambient temperature for 15 hours
according to the procedure outlined in Example 20A. The title compound was
purified
by chromatography (Si02, hexane: Et0Ac, 50 : 50, Rf. 0,40), 1HNMR (.300 MHz,
CD30D) 8 1.41- 1.56 (m, 9 H), 1,58 - 2,90 (m, 8 H) 4,13 - 4,33 (m, 1 H), 4,37 -
4.54
(m, 2 H), 7,00 (dd, J=8.81, 3_05 Hz, 0,5 H), 7.15 (d, J=8,14 Hz, 0.5 H), 7,26
(dd, J-8,30,
.3,10 Hz, 0.5 H) 7,41 (d, J=8,48 Hz, 0,5 H), 7.68 (d, J=3,05 Hz, 0,5 H) 784
(d, J=2,37 Hz,
0.5 H) ppm. MS DC1/NH3) miz 340 (M+H)+, 338 (M+H) .
Example 25B
ftendo)- 8-Aza-bicyclop.2.11oct-3-y11-(6-chloro-pyridin-3-y1)-amine
The product of Example 25A (2,92 g, 8.7 mmol) was treated with trifluoroacetic
acid (5 mL) in dichloromethane (20 mL) at ambient temperature for 4 hours. The
mixture
was concentrated under reduced pressure and the residue purified by
chromatography
(Si02, CH2C12: Me0H : NH3-H20, 90:10:2, R. 0,10) to provide the title
compound, 11-1
NMR (300 MHz, CD30D) 8 1.71. 1.94 (m, 4 H) 2,03 - 2.22(m, 4 H), 3.42- 3.64(m,
3
H), 6.98 (dd, J=8,82, 3.05 Hz, 1 H), 7,14 (d, J=8,14 Hz, 1 H), 7.65 (d, J=3.05
Hz, 1 H)
ppm. MS (DC1/NH3) rniz 238 (M+H)+, 240 (M+H)+,
Example 25C
-49-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
f(endo)-8-aza-bicyclor3.2.1joct-3-y1J46-(1H-indo1-5-y1)-pyridin-3-yli-amine
The product of Example 20A (250 mg, 1,0 mmol), 54ndolylboronic acid (Rsycor,
241.0 mg, 1.50 mmol), bis(triphenylphosphine)palladium(II) chloride (Aldrich,
10.0 mg,
0.01 mmol) and biphenyl-2-yl-dicyclohexyl-phosphane (Strem Chemicals, 11.0 mg,
0,03
mmol) in dioxane/Et0H/ 1M aqueous Na2CO3 (1/1/1 3 mL) were heated and
microwaved
to 130 C and 300 watts for 15 minutes in an Emrylm Creator microwave, The
solid was
filtered off with a syringe filter and the liquid was purified by preparative
HPLC [Waters
XTerra RP18 column, 30x100 mm, eluting solvents, MeCN / H20 (0.1 M aqueous
ammonium bicarbonate, adjusted to pH 10 with ammonium hydroxide) (v, 90/10 to
10/90
over 20 min), flow rate 40 mL/min, uv, 250 run] to provide the titled
compound. 1H
NMR (300 MHz, CD30D) 8 1.96 - 2,17 (m, 4 H), 2.20 - 2,52 (m, 4 H), 3,71 (t,
Hz, 1 H) .3.80 - 3,92 (m, 2 H), 6.49 (d, J=2.37 Hz, 1 H), 7.10 (dd, .1=8,82,
3.05 Hz, 1 H),
7,25 (d, J=3.05 Hz, 1 H), 7.42 (d, J=8.48 Hz, 1 H), 7,56 (dd,J=8.48, 1.70 Hz,
1 H), 7.63
(d, J=8,48 Hz, 1 H), 7.92 - 8.00 (s, 1 H) ppm. MS (DCITNH3) miz 319 (M+H)+
Example 26
[6-(4-Amino-3-methyl-phenyll-pyridin-3-y11-[(endo)-8-methyl-8-aza-
bicyclor3.2.1loct-3-
vfl-amine fumarate
Example 26A
[2-methy1-4-(4,4,5,5-tetramethyl-[1,3,2jdioxaboro1an-2-y1)-pheny11-
trifluoroacetamide
A mixture of N-(4-Bromo-2-methyl-phenyl)-2,2,2-trifluoro-acetamide (ref. US
2005-0043347, 4.23 g, 15,0 mmol), bis(pinacolato)diboron (Aldrich, 5.07 g, 20
mmol),
KOAc (Aldrich, 5,27 g, 53.7 mmol) and PdC1.20PPO:CH2C12 (Aldrich, 203 mg, 0,25
mmol) in anhydrous dioxane (50 mL) at 100 C for 72 hours, The mixture was
cooled to
ambient temperature, diluted with Et0Ac (150 mL), washed with water (2 x 25
mL).
The organic solution was concentrated under reduced pressure and the residue
was
purified by chromatography (140 g Si02, hexane: Et0Ac, 80:20, R. 0.6) to
provide the
titled compound, 1H NN1R (300 MHz, CDC13) 6 1,35 (s, 12 H), 2,31 (s, 3 H),
7,66 - 7.80
(m, 3 H), 7.90 (d, J=8.14 Hz, 1 H) ppm; MS (DCl/NH3): .347 (M+NH4)+,
Example 26B
-50-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
6- 4-Annno-3-rneth I- hen 1 - clo
fumarate
The product of Example 20A (130 mg, 0.52 namol) and the product of Example
26A (277 mg, 0.84 mmol) were treated according to the procedure outlined in
Example 8
to provide the title compound. '11 NMR (300 MHz, CD30D) 5 2,12 - 2.57 (m, 11
H),
2.82 (s, 3 H), 171 (t, J=6,10 Hz, 1 H), 185 - 3.94 (m, 2 H), 6.69 (s, 2 H),
6.77 (d, J=8,14
Hz, 1 H), 7.10 (dd, J=8.65, 2.88 Hz, 1 H), 7.42 (dd, J=8.14, 2,37 Hz, 1 H),
7.47 (s, 1 H),
7,53 (d, J=8,82 Hz, 1 H) ,7.92 (d, J=2.71 Hz, 1 H) ppm; MS DCl/NH3): miz 323
(M+H)+.
Example 27
f4-(1.H-Indo1-5-y1)-phenyll-[(endo)-8-methyl-8-aza-bicyclor3.2.1joct-3-v1]-
amine
fumarate
Example 27A
(4-Bromo-phenv1)-(3-endo-8-methyl-8-aza-bicyclo[3.2.1}oct-3-y1)-amine
Tropinone (Aldrich, 2.78 g, 20 mmol) and p-bromoaniline (Aldrich, 3,78 g, 22
mmol) were treated according to the procedure outlined in Example 20A to
provide the
title compound. The title compound was purified by chromatography (140 g Si02,
Et0Ac: Me0H (v. 2% NH3.H20), 50:50, Rf. 0,25), tH NMR (300 MHz, Me0H-D4) 3
1.71 - 1.82 (m, 2 H), 2.00 - 2.22 (m, 6 H), 2,29 (s, 3 H), 3.14 [s (br,), 2 1-
1], 3,46 (t, J=6,61
Hz, 1 H), 6,46 (d, J=8,81 Hz, 2 H), 7,17 (d, J=9.15 Hz, 2 H) ppm; MS
(DCl/NH3): 297
(M+H)+ 295 (M+H) .
Example 271B
[4-(1H4ndo1-5-v1)-pheny1)-r(endo)-8-methyl-8-aza-bicyclo[3.2.1joct-3-y1]-amine
fumarate
The product of Example 27A (134 mg, 0,45 mmol) and 5-(4,4,5,5-tetramethyl-
[1,3,21dioxaborolan-2-y1)-1H-indo1e (Aldrich, 198 mg, 0,81 mmol) were treated
according to the procedure outlined in Example 8 to provide the title
compound. 111
NMR (300 MHz, CD30D) 5 2.16 - 2,60 (m, 8 H), 2.82 (s, 3 H), 3,72 (t, .1=5,76
Hz, 1 H),
.3.89 [s (hr.), 2 6,44 (d, J=2.37 Hz, 1 H), 6.66 - 6,74 (m, 5..3 H), 7.21
(d, J-3.39 Hz, 1
H), 7.26 - 7.32 (m, 1 H), 7.35 - 7,41 (m, 1 H), 7.46 (d, J=8,82 Hz, 2 H), 7.67
(d, J=1.02
-51-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Hz, 1 H) ppm; MS DC1/NH3): rn/z 332 (M+H)+; Anal. Calculated for C22H25N3.1.65
C404114: C, 65.68; H, 6,09; N, 8,03, Found: C, 65.62; H, 6.40; N, 8.14.
Example 28
J4-(1H-Indazol-5-y1)-nhenyll-[(endo)-8-methyl-8-aza-bicyclor3.2.1]oct-3-y1]-
amine
fumarate
The product of Example 27A (134 mg, 0.45 mmol) and the product of Example
5A (265 mg, 1.08 mmol) were treated according to the procedure outlined in
Example 8
to provide the title compound. H NMR (300 MHz, CD30D) 6 2.14 - 2.61 (m, 8 H),
2.82
(s, 3 H), .3.72 (t, .1---5,93 Hz, I H), 3,89 [s (br,), 2 H], 6,67 - 6,77 (m, 5
H), 7.45 - 7,52 (m,
2 H), 7.52 - 7.58 (m, 1 H), 7.59 - 7.65 (m, 1 H), 7.87 (s, 1 H), 8.04 (s, 1 H)
ppm; MS
Da/N113): m/z ,333 (M+H)+; Anal. Calculated for C21H24N41.48 C404H4: C, 64,12;
H,
5.98; N, 11,11. Found: C, 64,00; H, 5.98; N, 11.22,
Example 29
endo -8-Meth 1-8-aza-bic clo 3.2.1 oct,_1(_11 -meth 1..1 1
amine fumarate
The product of Example 27A (128 mg, 0.43 mmol) and N-methylindole-5-boronic
acid (Frontier, 142 mg, 0.81 mmol) were treated according to the procedure
outlined in
Example 8 to provide the title compound, 1H NMR (300 MHz, CD30D) 6 2.12 - 2.63
(m,
8 H), 2.82 (s, 3 H), 3.72 (t, ,T=5.93 Hz, 1 H), 3.80 (s, 3 H), 3.88 [s (br,),
2 H], 6,42 (d,
..1=3.05 Hz, 1 H), 6,66 - 6.74 (m, 4 H), 7.13 (d, .1¨.3.05 Hz, 1 H), 7,36 (d,
I1.0 Hz, 2 H),
7.46 (d, J=8.48 Hz, 2 H), 7.67 (t, ,J----1.20 Hz, 1 H) ppm; MS DC1/NH3): rri/z
346 (M+H)+
Anal, Calculated for C23H27N3-1.10 C404114: C, 69.55; H, 6,69; N, 8,88. Found:
C,
69.29; H, 6,76; N, 8.85,
-52-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 30
(4-Benzo[b]thiophen-5-yl-phenyl)-[(endo)-8-methyl-8-aza-bicyclo[3.2.1]oct-3-
yll-amine
trifluoroacetate
The product of Example 27A (129 mg, 0.44 mmol) and 2-benzo[b]thiophen-5-y1-
4,4,5,5-tetramethy141,3,2]dioxaborolane (Maybridge, 189 mg, 0.73 mmol) were
treated
according to the procedure outlined in Example 1B to provide the title
compound. 1H
NMR (300 MHz, CD30D) 5 2,17 - 2,60 (m, 8 H), 2.82 (s, .3 H), 3.73 (t, J=5,76
Hz, 1 H),
3.90 [s (br.), 2 11], 6.7.3 (d, J=8,82 Hz, 2 H), 7.38 (d, J=5,76 Hz, 1 H),
7.48 - 7.59 (m, 4
H), 7.88 (d, .1=8,48 Hz, 1 H), 7.97 (d, J=1.70 Hz, 1 H) ppm; MS DCl/NH3): rn/z
.349
(M+H)+; Anal, Calculated for C22H24N2S-1,10 C2F302H: C, 61,33; H, 5.34; N,
5,91,
Found: C, 61,03; H, 5.34; N, 5,76.
Example 31
[4-(Berizofuran-5-y1)-phenyl]4(endo)-8-methyl-8-aza-bic_yclo[3.2.1loct-3-v1]-
arnine
fumarate
The product of Example 27A (135 mg, 0,46 mmol) and 5-(4,4,5,5-tetramethyl-
[1,3,2]dioxaborolan-2-y1)-benzofuran (Maybridge, 189 mg, 0,77 mmol) were
treated
according to the procedure outlined in Example 8 to provide the title
compound, 1H
NMR (300 MHz, CD30D) 5 2.15 - 2,60 (m, 8 H), 2.82 (s, 3 H), 3.72 (t, J=5.93
Hz, 1 H),
3,88 [s (br.), 2 H], 6.65 - 6,76 (m, 4 H), 6,83 (d, 1=2,71 Hz, 1 H), 7.40 -
7.52 (m, 4 H),
7,69 - 7,75 (m, 2 H) ppm; MS DCl/NH3): rri/z. 3.33 (M-1-11)+; Anal. Calculated
for
C22H24N201.15 C404H4: C, 68,57; H, 6.19;N, 6.01. Found: C, 68.42; H, 6.17;N,
6,02.
Example 32
J4-11H-Indo1-4-y1)-phenyli4(endo)-8-methyl-8-aza-bicyclof3.2.1)oct-3-y1]-amine
fumarate
The product of Example 27A (125 mg, 0,42 mmol) and indole-4-boronic acid
(Apollo, 131 mg, 0.81 mmol) were treated according to the procedure outlined
in
Example 8 to provide the title compound, 11-INMR (300 MHz, CD30D) 5 2.11 -
2,68
(m, 8 H), 2.8.3 (s, 3 H), 3.74 (t, J=8.31 Hz, 1 H), 3.89 [s (br,), 2 II] 6.58
(dd, J-3,39, 1.02
Hz, 1 H), 6,68 (s, 2 H), 6.74 (d, J=8,82 Hz, 2 H), 6.99 (dd, J=7,12, 1,02 Hz,
1 H), 7.08 -
-53-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
7.15 (m, 1 H), 7,23 (d, J=3.39 Hz, 1 H), 7,29 (d, f=8,14 Hz, 1 H), 7.51 (d,
J=8.81 Hz, 2
H) ppm; MS DC1/NH3): nrdz .3.32 (M+H)+; Anal. Calculated for C22H25N3 1.00
C404H4:
C, 69.78; H, 6,53; N, 9.39. Found: C, 70,17; H, 6.69; N, 9,58.
Example 33
f341H-Indol-5-y1)-phenyll-Rendo)-8-methy1-8-aza-bicyc1o[3.2.11oct-3-y1]-amine
fumarate
Example 33A
(3-Bromo-pheny1)-[(endo)-8-methy1-8-aza-bicyclor3.2.1]oct-3-y11-amine
Tropinone (696 mg, 5,0 amyl) and m-bromoaniline (946 mg, 5.5 mmol) were
treated according to the procedure outlined in Example 20A to provide the
title
compound. The title compound was purified by chromatography {140 g 5i02,
Et0Ac:
Me0H (v, 2% NH3.H20), 50:50, R1=0,25], 1HNMR (300 MHz, Me0H-D4) 6 1.72 - 2,23
(m, 8 H), 2.29 (s, 3 H), 3,14 [s (br,), 2 11), 3.47 (t, J-6.44 Hz, 1 H), 6,46 -
6.52 (ddd,
J=8.20, 2,00, 1,00 Hz, 1 H), 6,64 - 6,72 (m, 2 H), 6,92 - 7.02 (t, J-8,10 Hz,
1H) ppm; MS
(DCl/NH3): 297 (M+H)+, 295 (M+H)+,
Example 33B
[3-(1F1-Indol-5-y1)-pheny11-1(endo)-8-methyl-8-aza-bicyclo[3.2.1loct-3-yl]-
amine
fumarate
The product of Example 33A (128 mg, 0.43 mmol) and indole-5-boronic acid
(165 mg, 1.0 mmol) were treated according to the procedure outlined in Example
8 to
provide the title compound. IHNMR (300 MHz, CD30D) 6 2,19 -2.61 (m, 8 H), 2,81
(s,
3 H), 3.75 (t, J=5.76 Hz, 1 H), 3.83 - 3,92 (m, 2 H), 6.47 (dd, J=3.05, 0,70
Hz, 1 H), 6.55
(ddd, J=7.10, 2.60, 0,70 Hz, 1 H), 6,68 (s, 2 H), 6.89 (t, J=2.03 Hz, 1 H),
6.96 (ddd,
J=7,80, 1.70, 1.00 Hz, 1 H), 7.20 (t, J-7.80 Hz, 1 H), 7,24 (d, J= 7.10 Hz,
1H), 7.34 (dd,
J=8.50, 1.70 Hz, 1 H), 7.39 (t,1=8,40 Hz,1 H), 7,74 (dd, J=1.70, 0.70 Hz 1 H)
ppm; MS
DCIINH3): rn/z 332 (M+H)+; Anal. Calculated for C22H25N31.10 C4 H404`0.40
C4H802:
C, 68.03; H, 6.65; N, 8.50. Found: C, 67.68; H, 6.85; N, 8.78,
-54-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 34
[3-(1H-Indo1-4-y1)-phenyll-Rendo)-8-methyl-8-aza-bicyclo[3.2.11oct-3-y11-amine
trifluoroacetate
The product of Example .33A (128 mg, 0,4.3 mmol) and indole-4-boronic acid
(Apollo, 168 mg, 1.0 mmol) were treated according to the procedure outlined in
Example
1B to provide the title compound. 'H NMR (300 MHz, CD30D) 5 2,22 - 2.64 (m, 8
H),
2,81 (s, 3 H), 3.74 (t, J-5,42 Hz, 1 H), 3,87 - 3.9.3 (m, 2 H), 6,57 - 6.67
(m, 2 H), 6.92 (t,
J=2,10 Hz, 1 H), 6.99 (dt, J=7,80, 1.00 Hz, 1 H) 7,14 (t, J=7,56 Hz, 1 H),
7.21 - 7.28 (m,
2 H), 7.35 (d, J=8.14 Hz, 1 H) ppm; MS DCYNH3): rn/z 3.32 (M+H)+; Anal.
Calculated
for C2.2H25N3'1,10 CF3CO2H.0,60 Et0H: C, 62.96; H, 6,18; N, 8.67. Found: C,
62.85;
H, 5.98; N, 8,65,
Example 35
5- t6-[(endo)-8-Methyl-8-aza-bicyclol3.2.11oct-3-yloxyl-pridazin-3-y11-2-
trifluoromethyl-1H-indole trifluoroacetate
The product of Example lA (89 mg, 0,35 mmol) and the product of Example 7A
(299 mg, 0.96 mmol) were treated according to the procedure outlined in
Example 1B to
provide the title compound. 'H NMR (300 MHz, CD30D) 6 2.22 - 2.67 (m, 8 H),
2.86 (s,
3 H), 3.93 - 4,01 [s (br), 2 111, 5,55 - 5.62 (m, 1 H), 7.02 (t, J=1,02 Hz, 1
H), 7.32 (d,
1,-9.15 Hz, 1 H), 7.60 (d, J=8,81 Hz, 1 H), 7.94 (dd, J=8.82, 1,70 Hz, 1 H),
8,16 (d,
1-9.49 Hz, 1 H), 8,26 (d, .1=1.36 Hz, 1 H) ppm; MS DCl/N1-13): ink 403 (M+H)+;
Anal.
Calculated for C211-121F3N40'1,53CF3CO2H: C, 50,09; H, 3.94; N, 9,71, Found:
C,
50.07; H, 3,94; N, 9.66.
Example 36
4- {64(exo)-8-Methy1-8-aza-bicyclo[3.2.13oct-3-yloxy1-pYridazin-3-y1l-1H-
indole
fumarate
The product of Example 7D (129 mg, 0.51 mmol) and indole-4-boronic acid
(Apollo, 161 mg, 1.0 mmol) were treated according to the procedure outlined in
Example
8 to provide the title compound, Ili NMR (300 MHz, CD30D) 6 2,05 - 2.50 (m, 6
H),
2,66 (ddd, J=14.92, 5.76, 3,05 Hz, 2 H), 2,85 (s, 3 H), 4,03 (dd, J=3,73, 3.05
Hz, 2 H),
5.67 - 5.83 (m, 1 H), 6.79 (dd, 1=3,22, 0,85 Hz, 1 H), 7,22 - 7,.30 (m, 2 H),
7,37 (d,
-55-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
3=3.05 Hz, 1 H), 7.41 (dd, J=7,29, 0,85 Hz, 1 H), 7,54 (d, 3=8.14 Hz, 1 H),
8.08 (d,
J=9.15 Hz, 1 H) ppm; MS DCl/NH3): rn/z 335 (M+H)+; Anal. Calculated for
C20H22N40.1,20 C4H404: C, 62.88; H, 5.70; N, 11,83. Found: C, 62,90; H, 5,53;
N,
11.79,
Example 37
5- {6-[(exo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxy]-pyridin-3-y1}-1H-indole
fiimarate
Example 37A
texo)-3-(5-Bromo-pyridin-2-y1oxy)-8-methy1-8-aza-bicyc1o[3.2.1]octane
The product of Example 7C (721 mg, 5,1 mmol) and 2,5-dibromo-pyridine
(Aldrich, 1.66 g, 7.0 mmol) were treated according to the procedure outlined
in Example
JA to provide the title compound, 1H NMR (300 MHz, CD30D)15 1.90 - 2.46 (m, 8
H),
2.74 (s, 3 H), 3,81 - 3,90 (m, 2 H), 5,34 - 5,48 (m, 1 H), 6.71 (d, J-8,82 Hz,
1 H), 7,78
(dd, J=8,82, 2,71 Hz, 1 H), 8.20 (d, 3=2.37 Hz, 1 H) ppm; MS DC1/1\1113): nitz
299
(M+H)+ 297 (M+H)+,
Example 37B
5- { 6-[(exo)-8-Methyl-8-aza-bicyclo[3 .2.1 ]oct-3-yloxyl -1H-
indole fumarate
The product of Example 37A (129 mg, 0.43 mmol) and indole-5-boronic acid
(Ryscor Inc., 165 mg, 1,0 mmol) were treated according to the procedure
outlined in
Example 8 to provide the title compound. 1HNMR (300 MHz, CD30D) ô 1.96 - 2.62
(m, 8 H), 2,84 (s, 3 H), .3.97 - 4,04 (m, 2 H), 5.44 - 5.58 (m, 1 H), 6,49
(dd, 3=3,22, 0,85
Hz, 1 H), 6.70 (s, 2 H), 6.82 (d, J=8,48 Hz, 1 H), 7,27 (d, 3=3,05 Hz, I H),
7,30 (dd,
3=8.48, 1,70 Hz, 1 H), 7.46 (d, 3=8,14 Hz, 1 H), 7,73 (d, 3=1.70 Hz, 1 H),
7.95 (dd,
3=8.65, 2.54 Hz, I H), 8.37 (d, 3=2.03 Hz, 1 H) ppm; MS DCl/NH3): m/z 334
(M+H) ;
Anal. Calculated for C211-123N30'1.10 C4H404.1.00 H20: C, 63.67; H, 6.18; N,
8,77.
Found: C, 63,77; H, 6,26; N, 8.64,
Example 38
5- {64(exo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxy1-pvridin-3-y1}-2-
trifluoromethyl-
1H-indole bisfumarate
-56-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
The product of Example 37A (129 mg, 0.43 mmol) and 5-(4,4,5,5-tetramethyl-
[1,3,2]dioxaborolan-2-y1)-2-trifluoromethy1-1H-indole (Aldrich, 319 mg, 1,02
mmol)
were treated according to the procedure outlined in Example 8 to provide the
title
compound. 'IT NMR (300 MHz, CD30D) 5 1.92 - 2.63 (m, 8 H), 2.85 (s, 3 H), 4.02
[s
(br), 2 141, 5,46 - 5.61 (m, I H), 6.71 (s, 4 H), 6,84 (d, J=8.48 Hz, 1 H),
6.95 (s, 1 H), 7,47
- 7.59 (m, 2 H), 7,85 (s, 1 H), 7,97 (dd, J=8.65, 2,54 Hz, 2 H), 8.40 (d,
J=2.03 Hz, 1 H)
PPm; MS DCl/NH3): miz 402 (M+H)+. Anal. Calculated for C22H22F3N30'2.00C4H404:
C, 56,87; H, 4.77; N, 6,63, Found: C, 56,98; H, 5.09; N, 6.29.
Example 39
4- {64(exo)-8-Methy1-8-aza-bicyclo{3.2.1joct-3-yloxyl-pyridazin-3-yl} -1H-
indole
fumarate
The product of Example 7D (129 mg, 0.51 mmol) was coupled with indole-4-
boronic acid (Apollo, 161 mg, 1,0 mmol) to give the free base of the title
compound (150
mg, 0,45 mmol). It was then was treated with fumaric acid (52,0 mg, 0.45 mmol)
according to the procedure of Example of 5C to give the title compound as
white solid,
'H NMR (300 MHz, CD30D) 5 2.05 - 2,49 (m, 6 H) 2,60 - 2,71 (m, 2 H) 2,85 (s, 3
H)
4.01 - 4.07 (m, 2 H) 5.69 - 5.81 (m, 1 H) 6,69 (s, 2 H) 6.79 (dd, 1=3.22, 0.85
Hz, 1 H)
7,2.3 - 7.29 (m, 2 H) 7.37 (d, 1=3.05 Hz, 1 H) 7.41 (dd, J=7,29, 0.85 Hz, 1 H)
7.54 (d,
J=8,14 Hz, 1 H) 8,08 (d,1=9,15 Hz, 1 H) ppm, MS (DCIINH3): ink 335 (M+H)+.
Anal,
Calculated for C20H22N.40.1,2C404H4: C, 62.88; H, 5,70; N, 11.83, Found: C,
62,90; H,
5.53;N, 11.79.
-57-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 40
6-15-f(exo)-8-Methy1-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyridin-2-y11-1H-indole
hydrochloride
Example 40A
6- 5-[(exo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxy] -pyridin-2-y1} -1H-
indole
Under N2, the mixture of the product from Example 11A (240 mg, 0.95 mmol)
was coupled with 6-indolylboronic acid (Frontier Scientific, 229 mg, 1.42
mmol)
according to the procedure of ExamplellB to provide the title product. Ili NMR
(.300
MHz, CD30D) 5 1,72 - 1.93 (m, 4 H), 2.00 - 2,25 (m, 4 H), 2.39 (s, 3 H), 3,23-
3.35 (m,
2H), 4,56 - 4,82 (m, 1 H), 729 (d, 3=3.05 Hz, 1 H), 7,46 (d, 3=2,71 Hz, I H),
7,47 - 7,51
(m, 1 H), 7,53 (d, J-1.36 Hz, 1 H), 7,60 (d, 3=8.52 Hz, 1 H), 7,76 (d, J=8.82
Hz, 1 H),
7,88 (s, 1 H), 8.22 (d, 3=3.05 Hz, 1 H) ppm; MS (DCTINH3) raiz 334 (M+H)+,
Example 40B
6-{5-[(exo -8-Meth 1-8-aza-bicyclo[3.2.1loct-3-yloxyl-pyridin-2-y1)-1H-indole
hydrochloride
The product of Example 40A (210 mg, 0,63 mmol) was treated with HC1
(Aldrich, 4 M in dioxane, 0,5 mL, 2,0 mmol) Et0Ac (10 mL) at ambient
temperature for
hours and concentrated under reduced pressure to provide the title compound.
ill
NMR (300 MHz, CD30D) 5 2,14-2,32 (m, 2 H), 2,26 - 2,49 (m, 4 H), 2.49 - 2.65
(m, 2
H), 2,85 (s, 3 H), 3.99 - 4,18 (m, 2 H), 5.07 - 5.31 (m, 1 H), 6,60 (d, 3=4.07
Hz, 1 H),
7.46 - 7,56 (m, 2 H), 7.82 (d, 3=8.48 Hz, 1 H), 7.98 (s, 1 H), 8.34 (s, 1 H),
8,35 (d, 3=2.71
Hz, 1 H), 8,55 (d, 3=2,37 Hz, 1 H) ppm. MS (DCl/NH3): m/z 334 (M+H)+, Anal.
Calculated for C211-123N30'1.00 HC1.1,20 H20: C, 64.42; H, 6,80; N, 10,73.
Found: C,
64.54; H, 6,61; N, 10,89,
-58-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 41
5-{54(endo)-8-Methy1-8-aza-bicyc1of3.2.1]oct-3-v1oxyl-pyrazin-2-y1)-1H-indole
tosylate
Example 41A
5-Bromo-pyrazin-2-ylamine
To the solution of 2-aminopyrazine (Aldrich, 4.75 g, 50 mmol) in anhydrous
MeCN (Aldrich, 50 mL) was slowly added the solution of N-bromosuccinimide
(Aldrich,
8.90 g, 50 mmol) in MeCN (anhydrous, 50 mL) at 0-10 C. The reaction mixture
was then
stirred at ambient temperature and qunched with saturated Na2S203 (5.0 mL).
The
mixture was concentrated and the residue was extracted with Et0Ac (3 x 50 mL).
The
combined extracts were concentrated and the title compound was purified by
chromatography (Si02, Et0Ac/hexane = 1/1, v. 114=0.50), 1H NMR (300 MHz,
CDC13)
7,77 (d, J=1.36 Hz, 1 H), 8.09 (d, J=136 Hz, 1 H) ppm; miz 174 (M+H)+, 174
(M+H)+.
Example 41B
5-Bromo-2-iodopyrazine
Under N2, to the mixture of the product of Example 41A (7.50 g, 43 mmol) in
DME (anhydrous, Aldrich, 200 mL) was added CsI (Aldrich, 11.20 g, 43 mmol),
iodine
(Aldrich, 5,52 g, 21.6 mmol), CuI (Stream, 152 g, 13.2 mmol) and isoamyl
nitrite (34,8
mL, 259,2 mmol) at ambient temperature. It was then heated to 60 C and stirred
for 30
min. till no gas evolution was observed. After being cooled down to room
temperature,
the dark mixtures was poured into a flask containing Et0Ac (200 mL) and
saturated
NH4 Cl (200 mL), stirred for 10 min. The organic layer was seperated and the
aqueous
layer was extracted with Et0Ac ( 2 x 1000 mL), The combined organic solution
was
washed with 5% of Na2S203 aqueous (2 x 50 mL), brine (50 mL) and dried over
MgSO4.
The dring agents were filtered off and the organic solution was concentrated
to provide
the title compound. 1H NMR (300 MHz, CDC13) 8,50 (d,1-1,.36 Hz, 1 H), 8.62 (d,
J=1,36 Hz, 1 H) ppm; m/z 284 (M+H)+, 286 (M+H)+,
-59-
CA 02647830 2008-09-29
WO 2007/137030 PCT/US2007/068930
Example 41C
(endo)-3-(5-loclo-pyrazin-2-yloxy)-8-methyl-8-aza-bicyclo[3.2.1 octane
Under N2, the mixture of (endo)-tropine (Aldrich, 1,54 g, 11 mmol) was treated
with potassium t-butoxide (Aldrich, 0,96 g, 10 mmol) in THF (anhydrous,
Aldrich, 50
mL) at ambient temperature for 1 h. The product of Example 41B (2.85 g, 10,0
mmol)
and was added. The brown mixture was stirred at ambient temperature for 4
hours and
quenched with water (5 mL). The mixture was concentrated and the residue was
purified
by chromatography (150 g Si02, Et0Ac : Me0H : NH3.1120, 90:10:1, Rf. 0.20) to
give
the title compound. Ili NMR (300 MHz, CD30D) 8 2.16 - 2.60 (m, 8 H), 2.84 (s,
3 H),
3.78 - 4,05 (m, 2 H), 5.17 - 5.40 (m, 1 11), 8.14 (d, 3=1,36 Hz, 1 H), 8.42
(d, 3=1.36 Hz, 1
H) ppm; MS (DCFNH3) rri/z 346 (M+H)+,
Example 41D
Li5j.:(0/Lo clo 12.1õo.,ct_13.:_y_-lo2ji-
The product from Example 41C (200 mg, 0.58 mmol), was coupled with 5-
indolylboronic acid (Rsycor, 143.3 mg, 0,89 mmol) according to the procedure
of
Example 913 to provide the title product, 1HNMR (300 MHz, CD30D) 6 1.94 - 2.05
(m,
2 H), 2.07 - 2,29 (m, 6 H), 2.34 (s, 3 H), 3,15 - 3.27 (m, 2 H), 5.29 (t,
J=5.09 Hz, 1 H),
6.53 (d, J=2,37 Hz, 1 H), 7.27 (d, J=3.39 Hz, 1 H), 7.47 (d, 3=8,48 Hz, 1 H),
7,68 (dd,
J=8.48, 1.70 Hz, 1 H), 8.11 (s, 1 H), 8,17 (d, J=1.70 Hz, 1 H), 8,58 (d, 3-
1.36 Hz, 1 H)
ppm, MS (DC1/NH3) na/z 335 (M+H)+,
Example 41E
5- (.5-[(endo)-8-methyl-8-aza-bicyclo[3.2.11oct-3-yloxy]-pYrazin-2-y11-1H-
indole
tosylate
The product of Example 41D (90 mg, 0,27 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H-H20 (Aldrich, 57 mg, 0,3 mmol) in
Et0Ac/Et0H (v. 4:1, 5 mL) at ambient temperature for 10 hour. The mixture was
concentrated under reduced pressure to provide the title compound, 1HNMR (300
MHz,
CD30D) ö 2.36 (s, 3 H), 2.38 - 2.48 (m, 4 H), 2,48 - 2,61 (m, 4 H), 2,84 (s, 3
H), .3.84 -
4.05 (m, 2 H), 5.41 (t, 3=4,41 Hz, 1 H), 7.23 (d, 3=7.80 Hz, 2 H), 7,30 (s, 1
H), 7.49 (d,
3=8,48 Hz, 1 H), 7,65 - 7,77 (m, 4H), 8õ13 (d, 3=1.70 Hz, 1 H), 8.29 (s, 1 H)
ppm. MS
(DCl/NH3): rn/z 335 (M+H)+, Anal. Calculated for C201-122N4a1.38 C7H8S03-0.80
H20:
-60-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
C, 60.74; H, 5.95; N, 9.55. Found: C, 61.00; H, 5.63; N, 9,17.
Example 42
4-15-Rendo)-8-Methyl-8-aza-bicyclor3.2.1loct-3-yloxyl-pyrazin-2-yll -1H-indole
bistosylate
Example 42A
4- f 5-[(endo)-8-Methy1-8-aza-bicycloi3.2.1loct-3-vloxyl-pyrazin-2-yll -1H-
indole
The product from Example 41C (200 mg, 0.58 mmol), was coupled with 4-
indolylboronic acid (Apollo, 14.3,3 mg, 0,89 mmol) according to the procedure
of
Example 9B to provide the title compound. 'H N1VIR (300 MHz, CD30D) 8 1,97 -
2,06
(m, 2 H), 2.08 -2.30 (m, 6 H), 2,34 (s, 3 H), 3.16- 3.28 (m, 2 H), 5,33 (t,
J=5.09 Hz, 1
H), 6.82 (d, J=3.39 Hz, 1 H), 7.22 (t, J=7.50 Hz, 1 H), 7.34 (d, J=3.05 Hz, 1
H), 7,40 (d,
J=7.46 Hz, 1 H), 7.47 (d, J=8,14 Hz, 1 H), 8,27 (d, J-1,36 Hz, 1 H), 8.61 (d,
J=1,36 Hz, 1
H) ppm. MS (DCUNH3) ink 335 (M+H)*,
Example 42B
4- f 5-[(endo)-8-Methyl-8-aza-bicyclor3.2.11oct-3-yloxyi-pyrazin-2-yll -1H-
indole
bistosylate
The product of Example 42A (40 mg, 0.12 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts01-11120 (Aldrich, 27 mg, 0.15 mmol) in
Et0Ac/Et0H (v. 4:1, 5 mL) at ambient temperature for 10 hours. The mixture was
concentrated under reduced pressure to provide the title compound. 'H NMR (300
MHz,
CD30D) 8 2,36 (s, 6 H) 2,40-2,48 (m, 4 H), 2,50 - 2.64 (m, 2 H), 2.85 (s, 3
H), 3.87 -
4.04 (m, 2 H), 5.26 - 5.63 (m, 1 H), 7.19 - 7,29 (m, 6 H), 7,35 (s, 1 H), 7.42
(d, J-6,44
Hz, 1 H), 7.49 (d, J-8.14 Hz, 1 H), 7.71 (d, J=8.48 Hz, 4 H), 8.38 (d, J=1,.36
Hz, 1 H),
8.68 (d, J=1.36 Hz, 1 H) ppm. MS (DC1/NH3): rn/z 335 (M+H)+. Anal, Calculated
for
C20H22N40.2.00 C7H8S03-0.50 H20: C, 59.37; H, 5.71; N, 8.15. Found: C, 59,56;
H,
6.10; N, 8.17.
Example 43
6- f5-4(endo)-8-Methyl-8-aza-bicycloP.2.1loct-3-yloxY1-pyrazin-2-y1}-1H-indole
-61-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
tospate
Example 43A
6- f5-[(endo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyrazin-2-yll-1H-
indole
The product from Example 41C (200 mg, 0.58 mmol), was coupled with 6-
indolylboronic acid (Frontier Scientific, 143.3 mg, 0.89 mmol) according to
the
procedure of Example 9B to provide the title product, IFINMR. (300 MHz, CD30D)
8
1,93 - 2.05 (m, 2 H), 2.08 - 2.28 (m, 6 H), 2.33 (s, 3 H), 11.3 - 3.26 (m, 2
H), 5,29 (t,
J=4.92 Hz, I H), 6,47 (d, J=3,05 Hz, 1 H), 7.30 (d, J=3,39 Hz, 1 H), 7,54 -
7.68 (m, 2 H),
7.96 (s, 1 H), 8,19 (d, 3=1.36 Hz, 1 H), 8.60 (d, J=1,36 Hz, 1 H) ppm, MS
(DCl/NH3) m/z
335 (M+H)+.
Example 43B
6- t5-[(endo)-8-Methyl-8-aza-bicyclo13.2.1]oct-3-yloxvi-pyrazin-2-y11-1H-
indole
tosylate
The product of Example 43A (80 mg, 0.24 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts011.1120 (Aldrich, 57 mg, 0.30 mmol) in
Et0Ac/Et0H (v, 4:1, 5 mL) at ambient temperature for 10 hours. The mixture was
concentrated under reduced pressure to provide the title compound. 111 NMR
(300 MHz,
CD30D) 5 2,36 (s, 3 H), 2,37 - 2.48 (m, 6 H), 2.47 -2.63 (m, 2 H), 2.84 (s, .3
H), 3.83 -4M2 (m, 2 H), 5.27 - 5,50 (m, 1 H), 6.48 (d, 3=2.37 Hz, 1 H), 7,32
(t, J=1,70 Hz, 1 H),
7.53 - 7.67 (in, 2 H), 7,71 (d, 3=8,14 Hz, 2 H), 799 (s, 1 H), 8.29 (d, 3=1,36
Hz, I H),
8.64 (d, 3=1,36 Hz, 1 H) ppm, MS (DCIJNH3): ink 335 (M+14)+, Anal. Calculated
for
C20H22N40.1.15 C7H8S03Ø75 H20: C, 61.71; H, 6,04; N, 10,26. Found: C, 61,74;
H,
5,72; N, 9.87,
-62-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 44
f6-(1H-Indo1-6-y1)-pyridin-3-y1)-f(endo)-8-methyl-8-aza-bicyclor3.2.11oct-3-
y11-amine
trifluoroacetate
The product of Example 20A (139 mg, 0,55 mmol) was coupled with indole-6-
boronic acid (Frontier Scientific, 165 mg, 1,02 mmol) according to the
procedure of
Example 8 to provide the title compound, 11-1NMR (300 MHz, CD30D) 6 2.18 -
2.6.3
(m, 8 H) 2.84 (s, 3 H) 3,82 (t, .1=6.10 Hz, 1 H) .3,96 (s, 2 H)657 (dd,
.1=3,05, 0.68 Hz, 1
H) 7.41 - 7.47 (m, 2 H) 7.74- 7.93 (m, 4 H) 8.10 (d, J=9,16 Hz, 1 H) ppm, MS
(DC1/NH3): rn/z .333 (M+11)+. Anal. Calculated for C21H24N402.45 CF3CO2H: C,
5085;
H, 4,36; N, 9.16, Found: C, 50,72; H, 4,4,3; N, 9,25.
Example 45
5- 64(endo)-9-methy1-9-azabicyclo[3.3.1]nonan-3-yloxylpyridazin-3-y11-1H-
indole
trifluoroacetate
Example 45A
(endo)-9-Methyl-9-azabicyclo[3.3.1]nonan-3-ol
(endo)-9-methyl-9-azabicyclo[3.3,1}nonan-3-ol was prepared according to the
procedure as described in WO 03062235. 111 NMR (300 MHz, CD30D) 6 1,22 - 1.32
(m,
2 H), 1.35 - 1,47 (in, 3 H), 1,98 (tt, J=13.60, 5,21 Hz, 2 H), 2,30 - 2,56 (m,
6 H), 2.87 -
2.96 (m, 2 H), 4.04 - 4.15 (m, 1 H) ppm. MS (DCl/NH3): m/z 156 (M+H)+.
Example 45B
(endo)-3-(6-chloropyridazin-3-yloxy)-9-methy1-9-azabicyclor3.3.11nonane
The product of Example 45A ( 467 mg, 3.0 mmol) was coupled with 3,6-
dichloropyridazine ( 614 mg, 3.3 mmol) according to the procedure of Example
1A, 1H
N1V1R (300 MHz, CD30D) 61.59 (ddd, J=14.41, 6,27, 6.10 Hz, 1 H), 1.77 (dd,
J=14.92,
5.76 Hz, 2 H), 2.06 - 2.28 (m, 4 H), 2.52 - 182 (m, 3 H), 2,90 (s, 3 H), 3,51
(t,1=5.76 Hz,
2 H), 5,55 (tt,1=6.91, 1.74 Hz, 1 H), 7.26 (d,1=9.16 Hz, 1 H), 7.69 (d, J=9.16
Hz, 1 H)
ppm. MS (DCl/NH3): miz. 268 (M+H)+,
Example 45C
-63-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
5- {6-j(endo)-9-methyl-9-azabicyclor3.3.11nonan-3-yloxylnyridazin-3-yll -11 H-
indole
trifluoroacetate
The product of example 45B (145 mg, 0,54 mmol) was coupled with indo1e-5-
boronic acid (Ryscor, 165 mg, 1,02 mmol) according to the procedure of Example
1B to
provide the title compound. IHNMR (300 MHz, CD30D) 3 1.57 - 1.81 (m, 2 H)
1.95.
2.47 (m, 5 H) 2,67 - 2,92 (m, 3 H) 2.98 - .3,06 (m, 3 H) 3,65 (t, J=5.09 Hz, 2
H) 5.61 (t,
J=6.95 Hz, 1 H) 6,59 (d, J=3.05 Hz, 1 H) 7.34 (d, J=3.05 Hz, 1 H) 7,37 - 7,43
(m, 1 H)
7,55 (d, 7=8.48 Hz, I H) 7.74 (dd, J=8.65, 1.86 Hz, 1 H) 8.18 (d, J=1,70 Hz, 1
H) 8.20 -
8,27 (m, I H) ppm. MS (DCl/NH3): ink 349 (M+H)+, Anal, Calculated for
C211-124N40'2,10 CF3CO2H: C, 51.48; H, 4.47; N, 9,53. Found: C, 51,31; H,
4,33; N,
9,36.
Example 46
(endo)-346-(Benzo[blthiophen-5-vflpyridazin-3-yloxyl-9-methyl-9-
azabicyclo[3.3.1}nonane trifluoroacetate
The product of Example 45B (145 mg, 0.54 mmol) was coupled with the product
of 10A (280 mg, 1.02 mmol) according to the procedure of Example 1B to provide
the
title compound, 1H NMR (300 MHz, CD30D) 3 1.56 - 1.81 (m, 2 H), 1.94 - 2,48
(m, 5
H), 2.68 - 2.92 (m, 3 H), 2.98 - 3,08 (m, 3 H), 3,65 (t, J=5.09 Hz, 2 H), 5,66
(t, J=6,95
Hz, 1 H), 7.34 (d, J=9.16 Hz, 1 H), 7,50 (d, J=5.76 Hz, 1 H), 7,68 (d,1=5.76
Hz, I H),
7.96 - 8,02 (m, I H), 8.04 - 8,10 (m, 1 H), 8,20 (d, J=9,49 Hz, 1 H), 8,45 (d,
J=1.36 Hz, 1
H) ppm, MS (DCl/NH3): m/z 366 (M+H) . Anal. Calculated for C211-123N30S-1,13
CF3CO2H: C, 56,51; H, 4.92; N, 8.50. Found: C, 56.56; H, 4.75; N, 8,44,
Example 47
L{5-{(endo)-8-Methyl-8-aza-bicyclof3.2.1loct-3-yloxyl-pyrazin-2-yl}-IH-
pyrrolo[23-
blpyridine bistosylate
Example 47A
5-(4,4,5,5-Tetramethy141,3,21dioxaborolan-2-y1)-1H-pyrrolop,3-bbridine
5-Bromo-1H-pyrrolo[2,3-bjpyridine (Chemgenx, 0,90 g, 4.57 mmol) was coupled
with bis(pinacolato)diboron (Aldrich, 1.27 g, 5,0 mmol) according to the
procedure of
Example 10A. H NMR (.300 MHz, CDC13) 5 1,37 (s, 12 H) 6.52 (d, J=3,73 Hz, 1
H),
-64-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
7,38 (d, 1=3.73 Hz, 1 H), 8.34 (d, J=1.36 Hz, 1 H), 8.49 (d, 1=1.70 Hz, 1 H)
ppm; m/z
245 (M-FH)+.
Example 47B
5- { 5-[(endo)-8-Methyl-8-aza-bicyclo[3 .2.1loct-3 -yloxYl-pyrazin-2-y11-1H-
pyrrolo [2,3-
b]pyridine tosylate
The product from Example 41C (207 mg, 0,60 mmol), was coupled with the
product of Example 47A (200,0 mg, 0.82 mmol) according to the procedure of
Example
10B. 1HNMR (300 MHz, CD30D) 8 1.98 - 2,09 (m, 2 H), 2,10 - 2,32 (m, 6H),
2,40(s,
H), 5,32 (t, 1=5.09 Hz, 1 H), 6.58 (d, 1=3.39 Hz, 1 H), 7,44 (d, J=3,73 Hz, 1
H), 8,25 (d,
J=1..36 Hz, I H), 8.53 (d,1-2.03 Hz, 1 H), 8,65 (d, J=1,,36 Hz, 1 H), 8.78
(d,1=2,03 Hz, 1
H) ppm. MS (DC1/NH3) rri/z 336 (M+H)+,
Example 47C
5- {5 -{(endo)-8-Methyl-8-aza-bicyclo[3 .2.1}oct-3 -yloxy]-pyrazin-2-yll -1H-
pyrrolo [2,3-
bjpyridine bistosylate
The product of Example 47B (90 mg, 0,27 mmol) was treated with p-
toluenesulfonic acid monohydrate TsOHE20 (Aldrich, 95 mg, 0.5 mmol) in
Et0AciEt0H (v, 4:1, 10 mL) at ambient temperature for 10 hours, The mixture
was
concentrated under reduced pressure to provide the title compound. 1H NMR (300
MHz,
CD30D) 8 2.35 (s, 6H), 2.38 - 2.45 (m, 2 H), 2.45 - 2.61 (m, 6 H), 2.85 (s, 3
H), .3.85 -
4.07 (m, 2 H), 5,46 (t,1-4,75 Hz, 1 H), 6.97 (d, 1=3.39 Hz, 1 H), 7,22
(d,1=7,80 Hz, 4 H)
7,70 (d,1=8.14 Hz, 4 H), 7,76 (d, 13.73 Hz, 1 H), 8,42 (d, J=1.36 Hz, 1 H),
8,85 (d,
J=1,36 Hz, 1 H), 9,04 (d, J=1.70 Hz, 1 H), 9,27 (d, 1-1.70 Hz, 1 H) ppm. MS
(DC1/NH3):
miz 336 (M+H)+. Mal, Calculated for C1H2IN50.2.17 C7H8S03-1.00 H20: C, 56,48;
H,
5.59; N, 9,63, Found: C, 5648; H, 5,.37; N, 9.67,
-65-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 48
5- f 5-[(exo)-8-Methyl-8-aza-bicyclo[3 .2.1]oct-3-y1oxy) -pyridin-2-yll -1H-
pyrrolo[2,3-
blpyridine bistosylate
Example 48A
5- f 5-[(exo)-8-Methy1-8-aza-bicyclof 3 .2.11 oct-3-yloxyj-pyridin-2-y1 ) -1H-
pyrr olo[2,3-
b]pyridine
The product from Example I1A (152 mg, 0.60 mmol), was coupled with the
product of Example 47A (200,0 mg, 0.82 mmol) according to the procedure of
Example
913. 1H NAM (300 MHz, CD30D) 5 1,76 - 1,92 (m, 4 H), 2.06 - 2,20 (m, 4 H),
2,36 (s, 3
H), .3,18 - 3,31 (m, 2 H), 4,64 - 4.79 (m, I H), 6,57 (d,1-3,39 Hz, 1 H), 7.43
(d, J=.3.73
Hz, 1 H), 7.81 (d, 1=8.82 Hz, 1 H), 8.29 (d, .1=3.05 Hz, 1 H), 8,45 (d,1=2.03
Hz, I H),
8,72 (d, 1=2,03 Hz, 1 H) ppm, MS (DCl/NH3) rrilz 335 (M+H)+,
Example 4813
5- f54(exo)-8-Methy1-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyridin-2-yll -1H-
pyrrolo[2,3-
blpyridine bistosylate
The product of Example 48A (100 mg, 0,30 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0IIH20 (Aldrich, 95 mg, 0.5 mmol) in
Et0AciEt0H (v. 4:1, 10 mL) at ambient temperature for 10 hours, The mixture
was
concentrated under reduced pressure to provide the title compound, 1H NMR (300
MHz,
CD30D) 3 2,18 -2.47 (m, 2 H), 2.15-2,40 (m, 10H), 2.46- 2,60 (m, 2 H), 2,85
(s, 3 H),
3.99 - 4,06 (m, 2 H), 4.95 - 5.19 (m, 1 H), 6.83 (d, J=3,39 Hz, 1 H), 7.22 (d,
J=8,14 Hz, 4
H), 7,66 (d,1-3.39 Hz, 1 H), 7,70 (d, J=8.48 Hz, 4 H), 8.09 (d, 1=8.82 Hz, 1 1-
1), 8,48 (d,
1=231 Hz, 1 H), 8,87 (d,1=2.03 Hz, 1 H), 8.91 (d, J=2,03 Hz, 1 H) ppm. MS
(DC1/1\TH3):
m/z 335 (M+H)+, Anal, Calculated for C201122N40-2,14 C7H8503Ø50 H20: C,
59,01; H,
5,68; N, 7,87. Found: C, 58,88; H, 5,63; N, 7.47,
-66-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 49
5- t5-[(exo)-8-Methy1-8-aza-bicycloi3.2.11oct-3-yloxyl-pyridin-3-yri -1H-
indole
Lai hydrochloride).
Example 49A
(exo)-3-(5-Chloro-pyridin-3-y1oxy)-8-rnethy1-8-aza-bicyclo[3.2.1loctane
(endo)-Tropine (Aldrich, 0,56 g, 4,0 mmol), was coupled with 3-chloro-5-
hydroxy-pyridine (Aldrich, 0,26 g, 2,0 mmol), in the presence of D1AD (di-
isopropyl
azadicarboxylate, Aldrich, 0.81 g, 4,0 mmol) and Ph3P (Aldrich, 1,14 g, 4,0
mmol) in
THF (anhydrous, Aldrich, 20 rnL) at ambient temperature for two days. The
reaction
mixture was concentrated, The title product was purified by chromatography
(Si02,
CH2C12: Me0H : NH31120, 90:10:1, Rf. 0,45), 'H NMR ( 300 MHz, CD30D) 8 1.66 -
1.91 (m, 4H), 1.98- 2,19 (m, 4 H), 2.33 (s, 3 H), 3.22- 3,28 (m, 2 H), 4,58 -
4.79 (m, 1
H), 7.49 (dd, J2.37, 1.70 Hz, 1 H), 8,11 (d, J=1,70 Hz, 1 H), 8.15 (d, 1=2.37
Hz, 1 H)
ppm, MS (DC1/NH3) m/z 255 (M+H)+, 253 (M+H).
Example 49B
5- f 54(exo)-8-Methyl-8-aza-bicyclop.2.1loct-3-yloxyl-pyridin-3-yll -1H-indole
Under N2, the mixture of the product from Example 49A (250 mg, 1,00 mmol)
was coupled with 5-Indolylboronic acid (Rsycor, 240.0 mg, 1,50 mmol) according
to the
procedure of Example 9B. 1HNMR (300 MHz, CD30D) 8 1,71 - 1,92 (m, 4 H), 2,02 -
2.21 (m, 4 H), 2,34 (s, 3 H), 3,23 - 3,30 (m, 2 H), 4,63 - 4,80 (m, 1 H), 6,54
(d, 5=3.05
Hz, 1 H), 7,29 (d, J-3.39 Hz, 1 H), 7,38 (dd, 5=8.48, 2,03 Hz, 1 H), 7.47 -
7,53 (m, 1 H),
7,58 - 7,64 (m, 1 H), 7.83 (d, J=1.36 Hz, 1 H), 8,15 (d, 5=2,71 Hz, 1 H), 8.39
(d, 5-1.70
Hz, 1 H) ppm. MS (DCl/NH3) m/z 334 (M+1.1)+,
Example 49C
5- {5-1(exo)-8-Methy1-8-aza-bicyclo[3.2.1]oct-3-yloxYl-pyridin-3-yll -1H-
indole
tri(hydrochloride)
The product of Example 4913 (90 mg, 0,27 mmol) was treated with HC1 (Aldrich,
4 M in dioxane, 0.25 mL, 1,0 mmol) in PrOAc/iPrOH (v. 4:1, 5 mL) at ambient
-67-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
temperature for 2 hoours to provide the title compound, Ill NMR (300 MHz,
CD30D) 6
2.01 - 2.66 (m, 8 H), 2,83 (s, 3 H), .3.92 - 4,09 (m, 2 H), 4,98 - 5.15 (m, 1
H), 6.61 (d,
J=3,05 Hz, 1 H), 7.33 - 7.40 (m, 1 H), 750 - 7.63 (m, 2 H), 8.04 - 8.10 (m, 2
H), 8,44 (d,
J=1,70 Hz, 1 H), 8.80 (s, 1 H) ppm, MS (DC1/NH3): in/z 334 (M+H)+, Anal.
Calculated
for C211-123N3a3,00 HC1 4.60H20: C, 47,98; H, 6.14;N, 7.85. Found: C, 47.62;
H,
6.38; N, 7_62.
Example 50
5-15-((exo)-8-Methy1-8-aza-bicyclo[3.2.1}oct-3-yloxvkpyrazin-2-y1)-1H-indole
tosylate
Example 50A
(exo)-3-(5-Iodo-pyrazin-2-yloxy)-8-methyl-8-aza-bicyclo[3.2.1loctane
Under N2, the mixture of product from 7C (0.42 g, 3.0 mmol) was treated with
potassium t-butoxide (Aldrich, 0.32 g, 3.3 mmol) in THF (anhydrous, Aldrich,
50 mL) at
ambient temperature for 1 hours. The product of Example 41B (1.00 g, 3.5 mmol)
and
was added. The mixture was stirred at ambient temperature for 4 hours and
quenched
with water (5 mi.). The mixture was concentrated and the residue was purified
by
chromatography (150 g Si02, Et0Ac : Me0H : NH3'1120, 90:10:1, Rf, 0,40) to
provide
the title compound. 1HNMR (300 MHz, CD30D) 5 1.90 - 2.25 (m, 4 H), 2,31 - 2.60
(m,
4 H), 2.84 (s, 3 H), 3.94 - 4.11 (m, 2 H), 5.32 - 5.57 (m, 1 H), 8.06 (d, J-
1.36 Hz, 1 H),
8.42 (d, J-1.36 Hz, 1 H) ppm; MS (DC1/NH3) rrilz 346 (M+H)+.
Example 50B
5- {5-[(exo)-8-Methyl-8-aza-bicyclo[3.2.11oct-3-yloxyl-pyrazin-2-y1]-1H-indole
The product from Example 50A (200 mg, 0.58 mmol), was coupled with 5-
indolylboronic acid (Rsycor, 143,3 mg, 0.89 mmol) according to the procedure
of
Example 9B. tH NMR (300 MHz, CD30D) 5 1,95 -2.17 (m, 2 H), 2.16 - 2.31 (m, 2
H),
2,36 - 2.47 (m, 2 H), 2,48 - 2.67 (m, 2 H), 2,85 (s, 3 H), 3.90- 4.17 (m, 1
H), 5.36 - 5.69
(m, 1 H), 6,53 (d, J=3.39 Hz, 1 H), 7,29 (d, J=3.05 Hz, 1 H), 7.48 (d, J-8.48
Hz, 1 H)
7.69 (dd, J=8.48, 1,70 Hz, 1 H), 8.13 (s, 1 H) 8.20 (d, J=1.36 Hz, 1 H), 8.62
(d, J=1.36
Hz, 1 H) ppm. MS (DCl/N1-13) rrilz 335 (M+H)+,
-68-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 50C
5- f 5-[(exo)-8.Methyl-8-aza-bicyclo[3 .2.11oct-3-yloxyl-pyrazin-2-y1]-1H-
indole tosylate
The product of Example 50B (170 mg, 0.50 mrnol) was treated with p-
toluenesulfonic acid monohydrate Ts0H.H20 (Aldrich, 100 mg, 0.51 mmol) in
Et0Ac/Et0H (v. 4:1, 5 mL) at ambient temperature for 10 hours. The mixture was
concentrated under reduced pressure to provide the title compound. 1H NMR (300
MHz,
CD30D) 8 2,01 - 2.15 (m, 2 H), 2.16 - 2,30 (m, 2 H), 2.36 (s, 3 H), 2.39 -
2,49 (m, 2 H),
2,52 - 2,67 (m, 2 H), 2.84 (s, 3 H), 3,96 - 4.13 (m, 2 H), 5,43 - 5,70 (m, 1
H), 7,23 (d,
J=8,14 Hz, 2 H), 7,30 (s, 1 H), 7.49 (d, J=8,48 Hz, I H), 7.62 - 7.75 (m, 4
H), 8,12 (s, 1
H), 8,22 (d, 1=1.36 Hz, 1 H), 8.68 (d, J=1.36 Hz, 1 H) ppm, MS (DCFNH3): miz
335
(M+H)+,
Example 51
4- f5-[(exo)-8-Methy1-8-aza-bicyc1o[3.2.11oct-3-yloxyl-pyrazin-2-y11-1H-indole
tosylate
Example 51A
4- {54(exo)-8-Methy1-8-aza-bicyclo[3.2.1loct-3-yloxyl-pyrazin-2-y1]-1H-indole
The product from Example 50A (200 mg, 0,58 mmol), was coupled with 4-
indolylboronic acid (Apollo, 143.3 mg, 0.89 mmol) according to the procedure
of
Example 913. 111NM1R (3001\4Hz, CD30D) 8 2.02 - 2.28 (m, 4 H), 2.34 - 2.48 (m,
2 H),
2.50 - 2,65 (m, 2 H), 2.86 (s, 3 H), 3,96 - 4.07 (m, 2 H), 5,45 - 5.68 (m, 1
H), 6.82 (d,
J=4.07 Hz, 1 H), 7.23 (t, J-7.60 Hz 1 H), 7,35 (d, 1=3.39 Hz, 1 H), 7.41 (d, 1-
6.44 Hz, 1
H), 7.48 (d, J=8,14 Hz, 1 H), 8.29 (d,1=1,36 Hz, 1 H), 8,65 (d, J=1,36 Hz, 1
H) ppm. MS
(DCFNH3) miz 335 (M+H)+,
Example 51B
4- f51(exo)-8-Methyl-8-aza-bicyclo[3.2.1}oct-3-yloxyj-pyrazin-2-y11-1H-indole
tosylate
The product of Example 51A (120 mg, 0.36 mmol) was treated with p-
toluenesulfonic acid monohydrate TsOHE20 (Aldrich, 68 mg, 0,36 mmol) in
Et0Ac/Et0H (v. 4:1, 5 mL) at ambient temperature for 10 hours. The mixture was
concentrated under reduced pressure to provide the title compound. 1H NMR (300
MHz,
CD30D) 6 2,02 - 2,17 (m, 2 H), 2.18 - 2.32 (m, 2 H), 2,36 (s, .3 H), 2,38 -
2.50 (m, 2 H),
-69-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
2.52 - 2,69 (m, 2 H), 2,85 (s, 3 H), 4.00 - 4.11 (m, 2 H), 7,17 - 7,28 (m, I
H), 7,35 (s, 1
H), 7.42 (d, J-7,12 Hz, 1 H), 7.49 (d, J=8,14 Hz, 1 H), 7.70 (d, J=8.14 Hz, 1
H), 8.30 (d,
J=1,70 Hz, I H), 8.67 (d, J=1.36 Hz, 1 H) ppm. MS (DCl/NH3): na/z 335 (M-FH)+.
Example 52
6- f5-[(exo)-8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yloxyl-pyrazin-2-y11-1H-indole
tosylate
Example 52A
6- {54(exo)-8-Methyl-8-aza-bicyc1o[3.2.1]oct-3-yloxyl-pyrazin-2-y11-1H-indoie
The product from Example 4IC (200 mg, 0.58 mmol), was coupled with 6-
indolylboronic acid (Frontier Scientific, 143,3 mg, 0.89 mmol) according to
the
procedure of Example 9B, NMR (.300 MHz, CD30D) 8 1.97 - 2,16 (m, 2 H), 2,14
-
2.26 (m, 2 H), 2.31 -2.65 (m, 4 H), 2.81 (s, 3 H), 3,84 - 4.05 (m, 2 H), 5,3,3
-5.71 (m, I
H), 6,47 (d, J=3.05 Hz, 1 H), 7,31 (d, J=.3.05 Hz, I H), 7.48 - 7.73 (m, 2 H),
7.99 (s, 1 H),
8,20 (d, J=1,36 Hz, 1 H), 8.63 (d, J=I.36 Hz, 1 H) ppm. MS (DCl/NH3) m/z 3.35
(M+H)+.
Example 52B
6-15-[(exo)-8-Methy1-8-aza-bicyclor3.2.1loct-3-yloxy]-pyrazin-2-v1)-1H-indole
tosylate
The product of Example 52A (90 mg, 0,27 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H4120 (Aldrich, 57 mg, 0.30 mmol) in
Et0Ac/Et0H (v, 4:1, 5 mL) at ambient temperature for 10 hours. The mixture was
concentrated under reduced pressure to provide the title compound. NMR (300
MHz,
CD30D) 3 2,01 -2.14 (m, 2 H), 2.16- 2,31 (m, 2 H), 2,36 (s, 3 H), 2.39- 2,51
(m, 2 H),
2,50 - 2.65 (m, 2 H), 2.84 (s, 3 H), 3,98 - 4.08 (m, 2 H), 5.41 - 5.68 (m, 1
H), 6.48 (d,
J=2.37 Hz, 1 H), 7.23 (d, .1=7.80 Hz, 2 H), 7,32 (s, 1 H), 7.55 - 7.67 (m, 2
H), 7.71 (d,
1=8.48 Hz, 2 H), 7.99 (s, 1 H), 8,22 (d, J=1.36 Hz, I H), 8,65 (d, .1=1,36 Hz,
1 H) ppm.
MS (DC1/NH3): rn/z 335 (M+H)+,
Example 53
(endo)-N-(5-0H-Indo1-5-y1)pyridin-3-y1)-8-methy1-8-azabicyclor3.2.11octan-3-
amine
Examnle 53A
-70-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
(endo)-N-(5-Bromopyridin-3-y1)-8-methy1-8-azabicyc1o[3.2.11octan-3-amine
8-Methy1-8-azabicyclo[32,1]octan-3-one (Aldrich, 695 mg, 5.0 mmol) reacted
with bromop3rridin-3-amine (950 mg, 5.5 mmol) according to the procedure of
Example
20A to give the title compound (650 mg, yield, 44%). 11-1NMR (300 MHz, CD30D)
&
1,54 - 2.25 (m, 8 H), 2,29 (s, 3 H), 3.16 [s (broad), 2 H], 3.50 (t, J=6.61
Hz, 1 H), 7.08 (t,
J=2,20 Hz, 1 H), 7,79 (d, J=1,70 Hz, 1 H), 7.85 (d, J=2.37 Hz, 1 H) ppm; MS
(DC1/NH3): m/z 298 (M+H)+, 296 (M+H) ,
Example 53B
(endo)-N-(5-(1H-Indo1-5-__Dpyridin-3-y1)-8-methyl-8-azabicyc1o[3.2.11octan-3-
amine p-
tosylate
The product of Example 53A (150 mg, 0.5 mmol) was coupled with indole-5-
boronic acid (Frontier, 150 mg, 0.93 mmol) according to the procedure of
Example 9B to
provide the free base of the title compound (82 mg, yield, 50%), which was
treated with
p-toluenesulfonic acid hydrate (Aldrich, 47 mg, 0,25 mmol) in Et0Ac/Et0H (v.
10:1, 5
mL) at room temperature for 16 hours. The precipitate was collected and dried
to give the
title compound (99.3 mg, yield, 67.2%). iH NMR (300 MHz, CD30D) 6 2,15 - 2..30
(m,
2 H), 2,30 - 2,42 (m, 5.5 H), 2,42 - 2,63 (m, 4 H), 2,82 (s, 3 H), 3,81 (t,
J=5.9 Hz, 1 H),
3,93 [s (broad), 2 H), 6.54 (d, J=2,4 Hz, 1 H), 7.21 (d, J=8.1 Hz, .3 H), 7.32
(d, J-3.1 Hz,
1 H), 7.39 (dd, J=8.4, 1.7 Hz, 1 H), 7.47 - 7,59 (m, 2 H), 7.70 (d, J=8.5 Hz,
3 H), 7.86 (d,
J=1.7 Hz, 1 H), 7.90 (d, J=2,4 Hz, 1 H), 8,23 (d, J=1.7 Hz, 1 H) ppm, MS
DC1/NH3): nilz
333 (M+H) . Anal. Calculated for C211-124N4-1,50C7H803S.1.20H20: C, 61,78; H,
6.32;
N, 9.15, Found: C, 61.78; H, 6,19; N, 8.99.
-71-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 54
(endo)-N-(5-(1H-Indo1-4-y1)pyridin-3-y1)-8-methyl-8-azabicyclo[3.2.11octan-3-
amine p-
tosylate
Example 54A
(endo)-N-(5-(1H-Indo1-4-yl)pyridin-3-y1)-8-methyl-8-azabicyclop.2.1loctan-3-
amine
The product of Example 53A (150 mg, 0.5 mmol) was coupled with indole-4-
boronic acid (Frontier, 150 mg, 0,93 mmol) according to the procedure of
Example 913 to
provide the title compound (80 mg, yield, 48%), IFINMR (300 MHz, CD30D) 8 1.78
-
1,96 (m, 2 H), 2.05- 2,16 (m, 4 H), 2.17- 2.30 (m, 2 H), 2.33 (s, 3 H), 3.21
{s (broad), 2
H}, 3.63 (t, J-- 6,8 Hz,! H), 657 (d, J=3,4 Hz, 1 H), 7,08 (d, J=7.1 Hz, 1 H),
7.15 -7.26
(m, 2 H), 7.31 (d, J=3,1 Hz, 1 H), 7.42 (d, J-7.8 Hz, 1 H), 7.89 (d, J=2,7 Hz,
1 H), 8,05
(d, J=1.7 Hz, 1 H) ppm; MS DCl/NH3): m/z 333 (M+H)+,
Example 54B
(endol-N-(5-(1H-Indo1-4-yl)pyridin-3-y1)-8-methyl-8-azabicyclo[3.2.1loctan-3-
amine p-
to sylate
The product of Example 54A (80 mg, 0,24 mmol) was treated with p-
toluenesulfonic acid hydrate (Aldrich, 47 mg, 0,25 mmol) in Et0Ac/Et0H (v.
10:1, 5
mL) at room temperature for 16 hours. The precipitate was collected and dried
to give the
title compound (85.3 mg, yield, 58,5%). NMR (300 MHz, CD30D) 6 2.17 - 2.31
(m,
2 H), 2.31 - 2,41 (m, 5.8 H), 2,41 - 2,60 (m, 4 H), 2,82 (s, 3 H), 3.79 (t,
J=5,9 Hz, 1 H),
3.93 [s (broad), 2 H), 7,16 (dd, J=7,5, 1.0 Hz, I H), 7,21-7.27 (m, 5,2 H),
7,37 (d, J=3,1
Hz, I H), 7,50 (d, J=8,1 Hz, 1,0 H), 7.62 - 7.66 (m, 1,0 H), 7.70 (d, J=8.1
Hz, 3.2 H),
7,99 (d, J=2.4 Hz, 1 H), 8.24 (d, J=1,4 Hz, 1 H) ppm, MS DC1/NH3): m/z 333
(M+H)+.
Anal, Calculated for C211-124N4-1,60C7H803S1.20H20: C, 61,43; H, 6.28; N,
8.90,
Found: C, 61,72; H, 6,26; N, 8.64.
-72-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 55
(endo)-N-(5-(1H-Indo1-6-Apyridin-3-y1)-8-methyl-8-azabicyc1o[3.2.1]octan-3-
amine p-
tosylate
Example 55A
(endo)-N-(5-(1H-Indo1-6-yl)pyridin-3-y1)-8-methyl-8-azabicyclo[3.2.1loctan-3-
amine
The product of Example 53A (150 mg, 0.5 mmol) was coupled with indole-6-
boronic acid (Frontier, 150 mg, 0.93 mmol) according to the procedure of
Example 9B to
provide the free base of the title compound (102 mg, yield, 60%). 1H NMR (300
MHz,
CD30D) 8 1.80 - 1.98 (m, 2 H), 2.06 - 2.19 (m, 4 H), 2.19-2.32 (m, 2 H), 2.35
(s, .3 H),
3.24 [s (broad), 2 H), 3,64 (t, J-6.8 Hz,1 H), 6.47 (d, J=3,4 Hz, 1 H), 7.16 -
7.21 (m, 1
H), 7,22 - 7,34 (m, 2 H), 7,57 - 7.67 (m, 2 H), 7,83 (d, J=2.7 Hz, I H), 8.06
(d, J=2.0 Hz,
I H) ppm; MS DCl/NH3): m/z 333 (M+H)+.
Example 55B
(endo)-N-(5-(1H-Indo1-6-yl)pyridin-3-y1)-8-methyl-8-azabicyclo[3.2.1]octan-3-
aminep-
tosylate
The product of Example 55A (102 mg, 0.3 narnol) was treated with p-
toluenesulfonic acid hydrate (Aldrich, 57 mg, 0,30 mmol) in Et0Ac/Et0H (v.
10:1, 5
mL) at room temperature for 16 hours. The precipitate was collected and dried
to give the
title compound (137.2mg, yield, 59.4%). 1H NMR (300 MHz, CD30D) 2.16 - 2,64
(m,
12,2 H), 2,82 (s, .3 H), 3,78 (t, J=6.3 Hz, 1 H), 3.92 [s (broad), 2 H), 6,48
(d, J=4.1 Hz, 1
H), 7,22 (d, J=7,8 Hz, 2.8 H), 7.27 (dd, J=8,1, 1.7 Hz, 1 H), 7,30 (d, J=3.1
Hz, 1 H), 7.31
- 7.34 (m, 1 H), 7.61 - 7,66 (m, 2 H), 7.70 (d, J=8.1 Hz, 2,8 H), 7.88 (d,
J=2,4 Hz, 1 H),
8,17 (d, J=1.7 Hz, 1 1-1) ppm. MS DCl/NH3): rn/z 333 (M+H)+, Anal. Calculated
for
C211-124N4.1.40C7H8030.70H20: C, 63.11; H, 6.29; N, 9.56. Found: C, 63.17; H,
6.61;
N, 9.43.
Example 56
(endo)-N- {542-(trifluoromethyl)-1H-indol-5-yllpyridin-3-y11-8-Methyl-8-
azabicyclo[3.2.1]octan-3-amine fumarate
The product of Example 9A (110 mg, 0.4 mmol) was coupled with the product of
Example 7A (300 mg, 0.97 mrnol) according to the procedure described in
Example 9B
-73-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
to provide the free base of the title compound (38 mg, yield, 22.5%), which
was (38
mg,0,09 mmol) was then treated with fumaric acid (12 mg, 0.1 mmol) in
Et0Ac/Et0H
(v. 10:1, 5 mL ) at room temperature for 16 hours. The precipitate was
filtered and dried
to give the title compound (50,4 mg, yield, 99%). 1H NMR (300 MHz, CD30D) 8
2,28 ¨
237 (m, 4H), 2,42- 2.57 (m, 4 H), 2.84 (s, 3 H), 3.02 [s(broad), 2 H], 4.80-
4.90 (m, 1 H)
6,72 (s, 2,6 H), 6.97 (s, 1 H), 7,47 - 7.57 (m, 2 H), 7.85 (d, J=8.8 Hz, 2 H),
8,17 (s, 1 H)
830 (s, 1 H) ppm. MS DCFNH3): ink 402 (M+H)+, Anal. Calculated for
C22H22F3N4a130C404H4: C, 59.15; H, 4.96; N, 7,61, Found: C, 59.29; H, 5.07; N,
737
Example 57
5- {5-Rendo)-8-Methy1-8-azabicyclo[3.2.1 ]octan-3-yloxylpyridin-2-y11-3H-
pyrrolo[2,3-
blpyridine tosylate
Example 57A
5- {54(endo)-8-Methy1-8-aza-bicyclof3.2.1loct-3-yloxy]-pyridin-2-y1) -1H-
pyrrolo[2,3-
blpyridine
The product of Example 9A (200 mg, 0.80 mmol), was coupled with the product
of Example 47A (244.0 mg, 1,0 mmol) according to the procedure of Example 9B,
to
provide the title compound (190 mg, yield, 71%), 114 NMR (300 MHz, CD30D) 8
1.93 -
2,27 (m, 8 H), 2.33 (s, 3 H), 3.20 [s (broad,), 2 H], 4.69 (t, J=5,1 Hz, 1 H),
6.57 (d, J=3,4
Hz, 1 H), 7.39 - 7.48 (m, 2 H), 7.83 (d, J=8.5 Hz, 1 H), 8,25 (d, J=2.7 Hz, I
H), 8.46 (d,
J=2.0 Hz, 1 H), 8,7.3 (d, J-2.4 Hz, I H) ppm, MS (DC1/NH3) miz, 3.35 (M+H).
Example 57B
5- {5-[(endo)-8-Methyl-8-aza-bicyclop .2.1 loct-3-yloxyl-pyridin-2-yl -1H-
pyrrolo[2,3-
bipyridine tosylate
The product of Example 48A (80 mg, 0.24 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H.H20 (Aldrich, 57 mg, 0..3 mmol) in
Et0Ac/Et0H (v, 4:1, 10 mL) at ambient temperature for 10 hours. The
precipitated solid
was filtered and dried to provide the title compound (100 mg, yield, 79.6%).
1H NMR
(300 MHz, CD30D) 6 2.27 - 2.69 (m, 11 H), 2.84 (s, 3 H), .3.84 - 4,08 (m, 2
H), 4,84 -
-74-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
4.94 (m, 1 H), 6.62 (d, J=3.4 Hz, 1 H), 7.23 (d, J=8,1 Hz, 2 H), 7,47 (d,
J=3.7 Hz, 1 H),
7,56 (dd, J=8.8, 3,1 Hz, 1 H), 7.71 (d, j=8.1 Hz, 2 H), 7.92 (d, J=8.8 Hz, 1
H), 8,36 (d,
J=2,7 Hz, 1 H), 8,56 (d, J=2.0 Hz, 1 H), 8,77 (d, j=2.0 Hz, 1 H) ppm, MS
(DC1/NH3):
rn/z 335 (M+H)+, Anal. Calculated for C20F122N40'1 AO C7H8S00.80 H20: C,
61.81; H,
6.07; N, 10.41, Found: C, 62,15; H, 5.92; N, 10.05.
Example 58
5- f5-[(endo)-8-Methyl-8-azabicyclo[3.2.1loctan-3-yloxylpyridin-27y1) indolin-
2-one
his(hydrochloric acid)
Example 58A
4,4,5,5-Tetramethy1-1,3,2-dioxaborolan-2-yflindolin-2-one
Under N2,5-Bromoindolin-2-one (Aldrich, 2,11 g, 10.0 mmol) was coupled with
bis(pinacolato)dibon (Frontier Scientific, .3,05 g, 12 mmol) in the presence
of KOAc
(Aldrich, 1.50 g, 15,0 mmol) under the catalysis of PdC12013PD-CH2C12
(Aldrich, 163
mg, 0.2 mmol) in anhydrous dioxane (Aldrich, 50 mL) at 85 C for 15 hours,
After the
reaction was completed, it was cooled down to ambient temperature and diluted
with
Et0Ac (100 mL). The mixture was then washed with brine (2 x 10 mL) and
concentrated.
The residue was purified with chromatography on silica gel (Et0Ac/hexanes, v.
1 : 1,
R1=0,5) to provide the title compound (2.43 g, yield, 93,8%). 1H NMR (300 MHz,
CD30D) 8 1.24 (s, 12 H), 3.51 (s, 2 H), 6.88 (d, J=8,5 Hz, 1 H), 7.52 - 7.75
(m, 2 H)
ppm. MS (DC1/NH3): rniz 260 (M+H)+.
Example 5813
5-15-[(endo)-8-Methy1-8-azabicyc1or3.2.11octan-3-yloxylpyridin-2-yll indolin-2-
one
The product of Example 9A (200 mg, 0.80 mmol), was coupled with the product
of Example 58A (260 mg, 1.0 mmol) according to the procedure of Example 9B. to
provide the title compound (130 mg, yield, 46.4%). 1H NMR (300 MHz, CD30D) 8
1,9.3
- 2.04 (m, 2 H), 2.06 - 2.15 (m, J=2,4 Hz, 4 H), 2,14 - 2,25 (m, 2 H), 2.33
(s, 3 H), 3.20 [s
(broad), 2 H], 4.67 (t, J=5,1 Hz, 1 H), 6.96 (d, J=8,1 Hz, 1 H), 7,38 (dd,
J=8.8, 3,1 Hz, 1
H), 7.69 - 7.80 (m, .3 H), 8.18 (d, J=3,1 Hz, 1 H) ppm, MS (DC1/NH3) rri/z 350
(M+H)+.
-75-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Example 58C
5- {5-f(endo)-8-Methy1-8-azabicyclo{3 .2.1}octan-3-yloxylpyridin-2-y1) indolin-
2-one
bis(hvdrochloric acid)
The product of Example 48A (80 mg, 0.24 mmol) was treated with p-
toluenesulfonic acid monohydrate Ts0H.H20 (Aldrich, 57 mg, 03 mmol) in
Et0Ac/Et0H (v. 4:1, 10 mL) at ambient temperature for 10 hours, The
precipitated solid
was filtered and dried to provide the title compound (100 mg, yield, 79.6%).
1HNMR
(300 MHz, CD30D) 62,31 - 2,67 (m, 8 H), 2.85 (s, 3 H), 3.68 (s, 2 H), 3,90 -
4,08 (m, 2
H), 5.03 (t, J=4.6 Hz, 1 H), 7.14 (d, J=9.2 Hz, 1 H), 7.73 - 7.82 (m, 2 H),
8.22 - 834 (m,
2 H), 8.53 (d, J=-2,4 Hz, 1 H)ppm, MS (DCIINH3) miz 350 (M+H)+, Anal.
Calculated for
C211123N302'2.00 HC13,0 H20: C, 52,95; H, 6.56; N, 8.82. Found: C, 52.67; H,
6.47; N,
8.62_
Example 59
5- (5-[(endo)-8-Azabicyclo[3.2.1]octan-3-yloxyjpyridin-2-yll -1H-indole
bis(hydrochloric acidl
Example 59A
(endo)-3-(6-Chloropyridin-3-y1oxy)-8-azabicyclo13.2.1joetane
To a solution of the product of Example 9A (25.3 mg, 1,0 mmol) in anhydrous
1,2-dichloroethane (Aldrich, 10 mL) was added 1-chloroethyl carbonochloridate
(Aldrich, 286 mg, 2.0 mmol). The mixture was heated to reflux for 15 hours. It
was then
concentrated, the residue was diluted with 5 mL of methanol. The soltuion was
stirred at
65 C for 1 h. and then concentrated. The residue was purified with
chromatography on
silica gel (CH2C12:MeOH:NH3-H20, v. 9010:2, Rf=0.1) to give the title compound
(180
mg, yield, 75%). 111 NMR (300 MHz, CD30D) 6 2.03 - 2_62 (m, 8 H), 4.01 - 4,14
(m, 2
H), 4.75 -4.82 (m, 1 H), 7.37 - 7,42 (m, 1 H), 7.44 (d, J=3.1 Hz, 1 H), 8.03 -
8.13 (m, 1
H) ppm. MS (DCl/NH3) m/z 241 (M+H)+, 239 (M+H) .
Example 59B
5- (5-1(endo)-8-Azabicyclo1.3.2.1joctan-3-yloxy1pyridin-2-y11-1H-indole
The product of Example 59A (180 mg, 0.75 =nal), was coupled with 1H-indo1-
5-ylboronic acid (160 mg, 1,0 mmol) according to the procedure of Example 9B,
to
-76-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
provide the title compound (120 mg, yield, 50.1%), 1H NNIR (300 MHz, CD30D) 8
L77
- L94 (m, 2 H), 1.96 - 2.07 (m, 2 H), 2,07 - 2.30 (m, 4 H), 3.46 - .3.59 (m, 2
H), 4,73 (t,
J=4.9 Hz, 1 H), 6,51 (d, J=4.1 Hz, 1 H), 7,26 (d, J=3,1 Hz, 1 H), 7.39 (dd,
J=8,8, 3.1 Hz,
1 H), 7.45 (d, J=8,5 Hz, 1 H), 7.62 (dd, J=8.5, 1.7 Hz, 1 H), 7,77 (d, j=8.8
Hz, 1 H), 8,03
(d, J=1.7 Hz, 1 H), 8.17 (d, J=2.7 Hz, 1 H) ppm. MS (DCl/NH3) m/z 320 (M+H)+,
Example 59C
5- f 5-f(endo)-8-Azabicyclof 3.2.1] octan-3-yloxylpyridin-2-y1) -1H-indole
bis(hydrochloric acid)
The product of Example 598 (120 mg, 0.38 mmol) was treated with HCI (4 M, in
dioxane, 0.2 mL, 0,8 mmol) in Et0Ac (5.0 rnL) at ambient temperature for 10
hours. The
precipitated solid was filtered and dried to provide the title compound (130
mg, yield,
79.6%). 1H NMR (300 MHz, CD30D) 8 2.09 - 2,26 (in, 2 H), 2,28 - 2,4.3 (m, 2
H), 2.40 -
2.59 (m, 4 H), 4.02 - 4.23 (m, 2 H), 5.02 (t, J=4,4 Hz, 1 H), 6.65 (d, J=3.1
Hz, 1 H), 7.42
(d, J=3.4 Hz, 1 H), 7.57 - 7.71 (m, 2 H), 8.15 (s, 1 H), 8.19 (dd, J=9.1, 2,7
Hz, 1 H), 8,29
(d, J=9.1 Hz,1 H), 8.44 (d, J=2.7 Hz, 1 H) ppm. MS (DCl/NH3) m/z 320 (M+H)+,
Anal.
Calculated for C20H2IN30.2.00 HC1.L18 H20: C, 58.08; H, 6.18; N, 10.16, Found:
C,
57,73; H, 6,37; N, 9.95,
Example 60
(1 R,3r,5S, 8s)-34641H-Indo1-5-y1')pyridin-3-y1oxy)-8-methy1-8-
azabicyclo[3.2.1loctane
8-oxide
3-Chlorobenzoperoxoic acid (Aldrich, 70-75%, 240 mg, 1.0 mmol) was added to
a solution of product of example 913 (333 mg, 1.0 mmol) in Me0H (10 mL) It was
then
stirred at ambient temperature for 4 hours. The solution was directly purified
by
preparative FfPLC [Gilson, column, Xterra 5 pm, 40 x 100 mm, Eluting Solvent,
MeCN / H20 (0.1 M aqueous ammonium bicarbonate, adjusted to pH 10 with
ammonium
hydroxide) (v.10/90 to 75/25 over 20 minutes, Flow rate of 40 mL/minute, uv
detector set
to 250 nml. The fractions with lower rention time were collected and
concentrated under
reduced pressure to provide the title compound (130 mg, yield, 37.2%). 1H NMR
(300
MHz, CD30D) 6 2.19 - 2.42 (m, 4 H), 2.45 - 2,74 (m, 4H), 3.34 (s, .3 H), .3.57
- 3.70 (m,
2 H), 4,72 (t, J=5.3 Hz, 1 H), 6.52 (d, J=2,4 Hz, 1 H), 7.27 (d, J=3,1 Hz, 1
H), 7.40 - 7.52
-77-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
(m, 2 H), 7.64 (dd, J=8.5, L7 Hz, 1 H), 7,80 (d, J=8.8 Hz, 1 H), 8,05 (d,
J=2,0 Hz, 1 H),
8.23 (d, J-3.1 Hz, 1 H) ppm; MS (DCIINH3) miz 350 (M+H)+.
Example 61
( I R,3r, 5S, 8r1-3-(6-(1H-Indo1-5-yl)pyridin-3-yloxy)-8-methyl-8-
azabicyclo[3.2.11oetane
8-oxide
3-Chlorobenzoperoxoic acid (Aldrich, 70-75%, 240 mg, 1,0 nunol) was added to
a solution of product of example 9B (333 mg, 1.0 mmol) in Me0H (10 mL). It was
then
stirred at ambient temperature for 4 hours. The solution was directly purified
by
preparative HPLC [Gilson, column, Xterra 5 Am, 40 x 100 mm. Eluting Solvent,
MeCN / H20 (0.1 M aqueous ammonium bicarbonate, adjusted to pH 10 with
ammonium
hydroxide) (v,10/90 to 75/25 over 20 minutes, Flow rate of 40 mL/minute, uv
detector set
to 250 nm], The fractions with higher rention time were collected and
concentrated under
reduced pressure to provide the title compound (110 mg, yield, 31.5%), '1-1NMR
(300
MHz, CD30D) 6 1.96 - 2,07 (m, 2 H), 2.19 - 2.37 (m, 2 H), 2.44 - 2.59 (m, 2
H), 3.06 (dt,
J=15.3, 4.2 Hz, 2 H), 3,24 (s, 3 H), 3,47 - 3.59 (m, 2 H), 4,71 - 4,81 (m, 1
H), 6,52 (d,
J-3.1 Hz, 1 H), 7.27 (d, J=3,4 Hz, 1 H), 7,42 - 7.50 (m, 2 H), 7.64 (dd,
J=8.5, 1.7 Hz, 1
H), 7.80 (d, J=8.8 Hz, 1 H), 8.05 (d, J=1,7 Hz, 1 H), 8.24 (d, J=3.1 Hz, 1 H)
ppm; MS
(DCVNH3) m/z 350 (M+H)+,
Example 62
4- {5-[(endo)-8-Azabicyclo[3.2.1]octan-3-yloxylpyridin-2-yll -1H-indole
trifluoroacetate
The product of Example 59A (120 mg, 0.50 mmol), was coupled with 1H-indo1-
4-ylboronic acid (Frontier, 121 mg, 0.75 mmol) according to the procedure of
Example
9B. The crude mixture was purified with preparative HPLC (Gilson, column,
Xterra 5
Mm, 40 x 100 mm. Eluting Solvent, MeCN / H20 containing 0.1% v. TFA (90% to
10%
over 25 minutes, Flow rate of 40 mL/minute, uv detector set to 254 nm). The
fractions
containing the desired product were collected and concentrated under reduced
pressure
and the residue was stirred in ether/ethanol (v. 10/1, 5 mL) at ambient
temperature for 16
hours to provide the title compound. (80 mg, yield, 29.2%). 11-1 NMR (300 MHz,
CD30D) 8 2.06 - 2,24 (m, 2 H), 2,25 - 2.60 (m, 6 H) ,4.00 - 4.3.3 (m, 2 H),
4.90 - 5.02
(m, I H), 6.72 (dd, J=.3.39, 1.02 Hz, I H), 7.25 - 7.32 (in, 1 H), 7.34 - 7.39
(m, 1 H), 7,43
-78-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
(d, J=3.05 Hz, 1 H), 7.58 (dt, J=7.80, 1.02 Hz, 1 H), 7.93 (dd, J=8.99, 2,88
Hz, 1 H), 8,11
(d, J=8.82 Hz, 1 H), 8.46 (d, J=2,71 Hz, 1 H) ppm. MS (DCl/NH3) m/z 320
(M+Hr.Anal, Calc, for C20H21N30-2.00CF3CO2H-0,50H20: C, 51.80; H, 4,35; N,
7.55.
Found: C, 51,84; H, 4.28; N, 7,30.
Example 63
5- {5-[(exo)-8-Azabicyclof3.2.1]octan-3-yloxylpyridin-2-yl -1H-indole
bis(hydrochloric
ggich
Example 63A
(exo)-3-(6-Ch1oropyridin-3-yloxy)-8-azabicyclor3.2.1}octane
To a solution of the product of Example 11A (2,52 g, 9.97 mmol) in 1,2-
dichloroethane (25 ml) (anhydrous) was added 1-chloroethyl carbonochloridate
(5.54 ml,
49.9 mmol), The mixture was then heated to 100 C for 50h. It was then cooled
down to
ambient temperature, 25 mL of Me0H was added. The mixtue was then heated to
reflux
for I hour. It is concentrated and the crude was purified with chromatography
on silica
gel (CH2C12:MeOH:NH3.H20, v, 90:10:2, R to give
the title compound (180 mg,
yield, 75%). 1H NMR (300 MHz, CD30D) 8 1,56 - 1.71 (m, 2 H), 1.74 - 1,94 (m, 4
H),
2,01 - 2.26 (m, 2 H), 3.46 - 3.73 (m, 2 H), 4,58 - 4.76 (m, 1 H), 7.32 (d,
J=8.1 Hz, 1 1-1),
7,43 (dd, J=8,8, 3.0 Hz, 1 H), 8,01 (d, J=2,7 Hz, 1 H) ppm, MS (DCl/NH3) m/z
241
(M+H)+, 239 (M+H)+,
Example 6313
5- 5-{(exo)-8-Azabicyclo[3.2.1joctan-3-yloxylpyridin-2-yll -1H-indole
The procduct of Example 63A (0.24 g, 1.0 mmol) was coupled with 1H-indo1-5-
ylboronic acid (Frontier, 0.241 g, 1.50 mmol) accoding to the procedure of
Example 913
to provide the title compound (0,25 g, yield, 79 %). 1H NMR (300 MHz, CD30D)
8 1.69 - 1,83 (m, 2 H), 1,86 - 1.99 (m, 4 H), 2.18 - 2.32 (m, 2 H), 3.67 -
3,87 (m, 2H),
4.69 - 4,82 (m, 1 H), 6.52 (d, J=2,37 Hz, 1 H), 7.27 (d, J=3.05 Hz, I H), 7.45
(dt, J=8,48,
0.85 Hz, 1 H), 7.49 (dd, J=8.82, 3,05 Hz, 2 H), 7.62 (dd, J=8.48, 1,70 Hz, 2
H), 7,76 (d,
J=8.14 Hz, 2 H), 8.03 (d, J=1.36 Hz, 2 H), 8,22 (d, J=2,37 Hz, 1 H) ppm; MS
(DCl/NH3)
ink 320 (M+H)+.
Example 63C
-79-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
5- {5 -t(exo)-8-Azabic_yclo[3.2.1]octan-3-yloxyjpyridin-2-yll -1H-indole
bis(hydrochloric
gAd)
The product of Example 6.3B (0,25 g, 0,79 mmol) was treated with HC1 (Aldrich,
4 M in dioxane, 0.5 mL, 2.0 mmol) in Et0Ac/Et0H (v. 10/1, 10 mL). The
precipitated
solid was filtered and dried to give the title compound (0.20 g, yield,
64,9%), 'H NMR
(300 MHz, CD30D) 81,94 - 2,13 (m, 2 H), 2.12 - 2,35 (m, 4 H), 2.42 - 2.68 (m,
2 H),
4,09 - 4.37 (m, 2 H), 5.05 - 5.28 (m, 1 H), 6,67 (d, J=3 .39 Hz, 1 H), 7.43
(d, J=3.05 Hz,
1 H), 7.57 - 7.72 (m, 2 H), 8.16 (s, 1 H), 8.27- 839 (m, 2 H), 8,52 (d, J=2.37
Hz, 1 H)
ppm; MS (DCIINH3) m/z 320 (M+H) .Anal. Cale. for C20H21N30 2,00HC1-0,90H20: C,
58,80; H, 6.12; N, 10.29. Found: C, 58,50; H, 5,86; N, 10.08
Example 64
5- {5 -[(endo)-8-A2abicyclo{3 .2.1loctan-3-ylox
The product of Example 59A (119 mg, 0,50 mmol), was coupled with the product
of Example 58A (194 mg, 0.75 mmol) according to the procedure of Example 9B.
to
provide the title compound (150 mg, yield, 89.0%), 1H NMR (300 MHz, DMSO-D6) 8
1.86 - 2.42 (m, 8 H), .3.54 (s, 2 H), 3.89 - 4,06 (m, 2 H), 4,83 (t, J=4.07
Hz, 1 H), 6.88 (d,
J=7.80 Hz, 1 H), 7.47 (dd, J=8,82, 3.05 Hz, 1 H), 7.78 - 7,94 (m, 3 H), 8,32
(d, J=2.71
Hz, 1 H), 10.50 (s, 1 H) ppm; MS (DC1/1\TH3) m/z 336 (M-FH)+.
Example 65
5- {5-[(endo)-8-Azabicyclo13.2.1}octan-3-yloxylp_yridin-2-y1.1-1H-pyrrolo[2,3-
blpyridine
The product of Example 59A (200 mg, 0.80 mmol), was coupled with the product
of Example 47A (183 mg, 0,75 mmol) according to the procedure of Example 9B.
to
provide the title compound (80 mg, yield, 49,9%), 1H NMR (300 MHz, DMSO-D6) 5
1.89 - 2.16 (m, 4 H), 2,17 - 2.40 (m, 4 H), 3.78 - 4.26 (m, 2 H), 4.86 (t,
J=4.24 Hz, 1 H),
6,51 (dd, J=3.39, 1.70 Hz, 1 H), 7.46 - 7,58 (m, 2 H), 7,97 (d, J=8.82 Hz, 1
H), 8.39 (d,
J=2,71 Hz, 1 H), 8,52 (d, J=2,03 Hz, 1 H), 8.88 (d, J=2Ø3 Hz, 1 H), 11.70
(s, 1 H) ppm;
MS (DCl/NH3) rrilz 321 (M+H)+,
Example 66
-80-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
5- f5-[(exo)-8-Azabicyclo[3.2.1loctan-3-yloxYlpyridin-2-y1) -1H-pyrrolo[2,3-
bipyridine
The product of Example 63A (200 mg, 0.80 mmol) was coupled with the product
of Example 47A (183 mg, 0.75 mmol) according to the procedure of Example 98.
to
provide the title compound (120 mg, yield, 74.9%). 1HNMR (300 MHz, DMSO-D6) 8
1.82- 2.18 (m, 6 H), 2.18.- 2,40 (m, 2 H), .3,91 - 430 (m, 2 H), 4.71 - 5.30
(m, I H), 6.51
(dd, J3.39, 1.70 Hz, 1 H), 7,47 - 7.55 (m, 1 H), 7,61 (dd, J8.82, 3.05 Hz, 1
H), 7,94 (d,
J---.8.82 Hz, 1 H), 8.42 (d, J=2.71 Hz, 1 H), 8.52 (d, j=-2.03 Hz, 1 H), 8,88
(d, J---2.03 Hz,
1 H), 11.71 (s, 1 H) ppm; MS (DCl/NH3) m/z 321 (M+H)+,
Compositions of the Invention
The invention also provides pharmaceutical compositions comprising a
therapeutically effective amount of a compound of formula (1) in combination
with a
pharmaceutically acceptable carrier, The compositions comprise compounds of
the
invention formulated together with one or more non-toxic pharmaceutically
acceptable
caniers. The pharmaceutical compositions can be formulated for oral
administration in
solid or liquid form, for parenteral injection or for rectal administration.
The term "pharmaceutically acceptable carrier," as used herein, means a non-
toxic, inert solid, semi-solid or liquid filler, diluent, encapsulating
material or formulation
auxiliary of any type. Some examples of materials which can serve as
pharmaceutically
acceptable carriers are sugars such as lactose, glucose and sucrose; starches
such as corn
starch and potato starch; cellulose and its derivatives such as sodium
carboxymethyl
cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt;
gelatin; talc;
cocoa butter and suppository waxes; oils such as peanut oil, cottonseed oil,
safflower oil,
sesame oil, olive oil, corn oil and soybean oil; glycols; such a propylene
glycol; esters
such as ethyl oleate and ethyl laurate; agar; buffering agents such as
magnesium
hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic
saline;
Ringer's solution; ethyl alcohol, and phosphate buffer solutions, as well as
other non-
toxic compatible lubricants such as sodium lauryl sulfate and magnesium
stearate, as well
as coloring agents, releasing agents, coating agents, sweetening, flavoring
and perfuming
agents, preservatives and antioxidants can also be present in the composition,
according
to the judgment of one skilled in the art of formulations.
The pharmaceutical compositions of this invention can be administered to
humans
-81-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
and other mammals orally, rectally, parenterally, intracisternally,
intravaginally,
intraperitoneally, topically (as by powders, ointments or drops), bucally or
as an oral or
nasal spray. The term "parenterally," as used herein, refers to modes of
administration,
including intravenous, intramuscular, intraperitoneal, intrasternal,
subcutaneous,
intraarticular injection and infusion,
Pharmaceutical compositions for parenteral injection comprise pharmaceutically
acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions
or
emulsions and sterile powders for reconstitution into sterile injectable
solutions or
dispersions. Examples of suitable aqueous and nonaqueous carriers, diluents,
solvents or
vehicles include water, ethanol, polyols (propylene glycol, polyethylene
glycol, glycerol,
and the like, and suitable mixtures thereof), vegetable oils (such as olive
oil) and
injectable organic esters such as ethyl oleate, or suitable mixtures thereof.
Suitable
fluidity of the composition may be maintained, for example, by the use of a
coating such
as lecithin, by the maintenance of the required particle size in the case of
dispersions, and
by the use of surfactants.
These compositions can also contain adjuvants such as preservative agents,
wetting agents, emulsifying agents, and dispersing agents. Prevention of the
action of
microorganisms can be ensured by various antibacterial and antifungal agents,
for
example, parabens, chlorobutanol, phenol, sorbic acid, and the like. It also
can be
desirable to include isotonic agents, for example, sugars, sodium chloride and
the like.
Prolonged absorption of the injectable pharmaceutical form can be brought
about by the
use of agents delaying absorption, for example, aluminum mono stearate and
gelatin.
In some cases, in order to prolong the effect of a drug, it is often desirable
to slow
the absorption of the drug from subcutaneous or intramuscular injection. This
can be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with
poor water solubility. The rate of absorption of the drug can depend upon its
rate of
dissolution, which, in turn, may depend upon crystal size and crystalline
form.
Alternatively, dissolving or suspending the drug in an oil vehicle can
administer a
parenterally administered drug form.
Suspensions, in addition to the active compounds, can contain suspending
agents,
for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan
esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-
agar,
-82-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
tragacanth, and mixtures thereof.
If desired, and for more effective distribution, the compounds of the
invention can
be incorporated into slow-release or targeted-delivery systems such as polymer
matrices,
liposomes, and micro spheres. They may be sterilized, for example, by
filtration through
a bacteria-retaining filter or by incorporation of sterilizing agents in the
form of sterile
solid compositions, which may be dissolved in sterile water or some other
sterile
injectable medium immediately before use.
Injectable depot forms are made by forming microencapsulated matrices of the
drug in biodegradable polymers such as polylactide-polyglycolide. Depending
upon the
ratio of drug to polymer and the nature of the particular polymer employed,
the rate of
drug release can be controlled. Examples of other biodegradable polymers
include
poly(orthoesters) and poly(anhydrides) Depot injectable formulations also are
prepared
by entrapping the drug in liposomes or microemulsions which are compatible
with body
tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile
injectable medium just prior to use.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions can be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents, The sterile injectable preparation also
can be a
sterile injectable solution, suspension or emulsion in a nontoxic,
parenterally acceptable
diluent or solvent such as a solution in 1,3-butanediol, Among the acceptable
vehicles
and solvents that can be employed are water, Ringer's solution, U.S,P. and
isotonic
sodium chloride solution. In addition, sterile, fixed oils are conventionally
employed as a
solvent or suspending medium. For this purpose any bland fixed oil can be
employed
including synthetic mono- or diglycerides. In addition, fatty acids such as
oleic acid are
used in the preparation of injectables.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders, and granules. In such solid dosage forms, one or more compounds of
the
invention is mixed with at least one inert pharmaceutically acceptable carrier
such as
sodium citrate or dicalcium phosphate and/or a) fillers or extenders such as
starches,
-8,3-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
lactose, sucrose, glucose, mannitol, and salicylic acid; b) binders such as
carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose,
and acacia;
c) humectants such as glycerol; d) disintegrating agents such as agar-agar,
calcium
carbonate, potato or tapioca starch, alginic acid, certain silicates, and
sodium carbonate;
e) solution retarding agents such as paraffin; f) absorption accelerators such
as quaternary
ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol
monostearate; h) absorbents such as kaolin and bentonite clay; and i)
lubricants such as
talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium
lauryl
sulfate, and mixtures thereof, In the case of capsules, tablets and pills, the
dosage form
may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and
hard-filled gelatin capsules using lactose or milk sugar as well as high
molecular weight
polyethylene glycols.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well-known
in the pharmaceutical formulating art. They can optionally contain pacifying
agents and
can also be of a composition that they release the active ingredient(s) only,
or
preferentially, in a certain part of the intestinal tract in a delayed manner.
Examples of
materials useful for delaying release of the active agent can include
polymeric substances
and waxes.
Compositions for rectal or vaginal administration are preferably suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-
irritating carriers such as cocoa butter, polyethylene glycol or a suppository
wax which
are solid at ambient temperature but liquid at body temperature and therefore
melt in the
rectum or vaginal cavity and release the active compound.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions, solutions, suspensions, syrups and elixirs, In
addition to the
active compounds, the liquid dosage forms may contain inert diluents commonly
used in
the art such as, for example, water or other solvents, solubilizing agents and
emulsifiers
such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate,
benzyl alcohol,
benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide,
oils (in
particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame
oils), glycerol,
-84-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of
sorbitan, and
mixtures thereof.
Besides inert diluents, the oral compositions can also include adjuvants such
as
wetting agents, emulsifying and suspending agents, sweetening, flavoring, and
perfuming
agentsõ
Dosage forms for topical or transdermal administration of a compound of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. A desired compound of the invention is admixed under
sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives or
buffers as may be required. Ophthalmic formulation, eardrops, eye ointments,
powders
and solutions are also contemplated as being within the scope of this
invention.
The ointments, pastes, creams and gels may contain, in addition to an active
compound of this invention, animal and vegetable fats, oils, waxes, paraffins,
starch,
tragacanth, cellulose derivatives, polyethylene glycols, silicones,
bentonites, silicic acid,
talc and zinc oxide, or mixtures thereof,
Powders and sprays can contain, in addition to the compounds of this
invention,
lactose, tale, silicic acid, aluminum hydroxide, calcium silicates and
polyamide powder,
or mixtures of these substances. Sprays can additionally contain customary
propellants
such as chlorofluorohydrocarbons.
Compounds of the invention also can be administered in the form of liposomes.
As is known in the art, liposomes are generally derived from phospholipids or
other lipid
substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid
crystals
that are dispersed in an aqueous medium. Any non-toxic, physiologically
acceptable and
metabolizable lipid capable of forming liposomes may be used. The present
compositions in liposome form may contain, in addition to the compounds of the
invention, stabilizers, preservatives, and the like. The preferred lipids are
the natural and
synthetic phospholipids and phosphatidylcholines (lecithins) used separately
or together.
Methods to form liposomes are known in the art. See, for example, Prescott, EC
Methods in Cell Biology, Volume XIV, Academic Press, New York, N. Y., (1976),
p 33
et seq.
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants, The active compound is mixed under
sterile
-85-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
conditions with a pharmaceutically acceptable carrier and any needed
preservatives,
buffers or propellants. Ophthalmic formulations, eye ointments, powders and
solutions
are also contemplated as being within the scope of this invention. Aqueous
liquid
compositions of the invention also are particularly usefitl,
The compounds of the invention can be used in the form of pharmaceutically
acceptable salts, esters, or amides derived from inorganic or organic acids.
The term
"pharmaceutically acceptable salts, esters and amides," as used herein,
include salts,
zwitterions, esters and amides of compounds of formula (I) which are, within
the scope of
sound medical judgment, suitable for use in contact with the tissues of humans
and lower
animals without undue toxicity, irritation, allergic response, and the like,
are
commensurate with a reasonable benefit/risk ratio, and are effective for their
intended
use
The term "pharmaceutically acceptable salt" refers to those salts which are,
within
the scope of sound medical judgment, suitable for use in contact with the
tissues of
humans and lower animals without undue toxicity, irritation, allergic
response, and the
like, and are commensurate with a reasonable benefithisk ratio,
Pharmaceutically
acceptable salts are well-known in the art. The salts can be prepared in situ
during the
final isolation and purification of the compounds of the invention or
separately by
reacting a free base function with a suitable organic acid.
Representative acid addition salts include, but are not limited to acetate,
adipate,
alginate, citrate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate,
camphorate,
camphorsulfonate, digluconate, fumarate, glycerophosphate, hemisulfate,
heptanoate,
hexanoate, fumarate, hydrochloride, hydrobromide, hydroiodide, 2-
hydroxyethansulfonate (isethionate), lactate, maleate, methanesulfonate,
nicotinate, 2-
naphthalenesulfonate, oxalate, pamoate, pectinate, persulfate, 3-
phenylpropionate,
picrate, pivalate, propionate, succinate, tartrate, thiocyanate, phosphate,
glutamate,
bicarbonate, p-toluenesulfonate and undecanoate.
Also, the basic nitrogen-containing groups can be quaternized with such agents
as
lower alkyl halides such as methyl, ethyl, propyl, and butyl chlorides,
bromides and
iodides; dialkyl sulfates such as dimethyl, diethyl, dibutyl and diamyl
sulfates; long chain
halides such as decyl, lauryl, myristyl and stearyl chlorides, bromides and
iodides;
arylalkyl halides such as benzyl and phenethyl bromides and others. Water or
oil-soluble
-86-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
or dispersible products are thereby obtained.
Examples of acids which can be employed to form pharmaceutically acceptable
acid addition salts include such inorganic acids as hydrochloric acid,
hydrobromic acid,
sulphuric acid and phosphoric acid and such organic acids as oxalic acid,
maleic acid,
succinic acid, and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification of compounds of this invention by reacting a carboxylic acid-
containing
moiety with a suitable base such as the hydroxide, carbonate or bicarbonate of
a
pharmaceutically acceptable metal cation or with ammonia or an organic
primary,
secondary or tertiary amine. Pharmaceutically acceptable salts include, but
are not
limited to, cations based on alkali metals or alkaline earth metals such as
lithium, sodium,
potassium, calcium, magnesium, and aluminum salts, and the like, and nontoxic
quaternary ammonia and amine cations including ammonium, tetramethylanamonium,
tetraethylarnmonium, methylamine, dimethylamine, trimethylamine,
triethylamine,
diethylamine, ethylarnine and the such as, Other representative organic amines
useful for
the formation of base addition salts include ethylenediamine, ethanolamine,
diethanolamine, piperidine, and piperazine.
The term "pharmaceutically acceptable ester," as used herein, refers to esters
of
compounds of the invention which hydrolyze in vivo and include those that
break down
readily in the human body to leave the parent compound or a salt thereof.
Examples of
pharmaceutically acceptable, non-toxic esters of the invention include C1-to-
05 alkyl
esters and C5-to-C7 cycloalkyl esters, although Ci-to-C4 alkyl esters are
preferred. Esters
of the compounds of formula (I) can be prepared according to conventional
methods.
Pharmaceutically acceptable esters can be appended onto hydroxy groups by
reaction of
the compound that contains the hydroxy group with acid and an alkylcarboxylic
acid such
as acetic acid, or with acid and an arylcarboxylic acid such as benzoic acid.
In the case of
compounds containing carboxylic acid groups, the pharmaceutically acceptable
esters are
prepared from compounds containing the carboxylic acid groups by reaction of
the
compound with base such as triethylamine and an alkyl halide, alkyl trifilate,
for example
with methyl iodide, benzyl iodide, cyclopentyl iodide. They also can be
prepared by
reaction of the compound with an acid such as hydrochloric acid and an
alkylcarboxylic
acid such as acetic acid, or with acid and an arylcarboxylic acid such as
benzoic acid.
-87-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
The term "pharmaceutically acceptable amide," as used herein, refers to non-
toxic
amides of the invention derived from ammonia, primary C1-to-C6 alkyl amines
and
secondary C1-to-C6 dialkyl amines, In the case of secondary amines, the amine
can also
be in the form of a 5- or 6-membered heterocycle containing one nitrogen atom.
Amides
derived from ammonia, C1-to-C3 alkyl primary amides and C1-to-C2 dialkyl
secondary
amides are preferred. Amides of the compounds of formula (I) can be prepared
according to conventional methods. Pharmaceutically acceptable amides can be
prepared
from compounds containing primary or secondary amine groups by reaction of the
compound that contains the amino group with an alkyl anhydride, aryl
anhydride, acyl
halide, or aroyl halide. In the case of compounds containing carboxylic acid
groups, the
pharmaceutically acceptable esters are prepared from compounds containing the
carboxylic acid groups by reaction of the compound with base such as
triethylamine, a
dehydrating agent such as dicyclohexyl carbodiimide or carbonyl diimidazole,
and an
alkyl amine, dialkylamine, for example with methylamine, diethylamine,
piperidine.
They also can be prepared by reaction of the compound with an acid such as
sulfuric acid
and an alkylcarboxylic acid such as acetic acid, or with acid and an
arylcarboxylic acid
such as benzoic acid under dehydrating conditions as with molecular sieves
added. The
composition can contain a compound of the invention in the form of a
pharmaceutically
acceptable prodrug
The term "pharmaceutically acceptable prodrug" or "prodrug," as used herein,
represents those prodrugs of the compounds of the invention which are, within
the scope
of sound medical judgment, suitable for use in contact with the tissues of
humans and
lower animals without undue toxicity, irritation, allergic response, and the
like,
commensurate with a reasonable benefithisk ratio, and effective for their
intended use.
Prodrugs of the invention can be rapidly transformed in vivo to a parent
compound of
formula (I), for example, by hydrolysis in blood. A thorough discussion is
provided in T.
Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, V. 14 of the
A.C.S.
Symposium Series, and in Edward B. Roche, ed., Bioreversible Carriers in Drug
Design,
American Pharmaceutical Association and Pergamon Press (1987).
The invention contemplates pharmaceutically active compounds either chemically
synthesized or formed by in vivo biotransformation to compounds of formula
(I).
-88-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Determination of Biological Activity
To determine the effectiveness of representative compounds of this invention
as
a7 nAChRs, the compounds of the invention were evaluated according to the
[311:1-
DPPB binding or the [311]-methyllycaconitine (MLA) binding assay (both
measures of a7
NNR binding) and considering the [3111-cytisine binding assay (measure of
a41.32
interactions), which were performed as described below.
CH1-Cytisine binding
Binding conditions were modified from the procedures described in Pabreza LA,
Dhawan, S, Kellar KJ, [31-13-Cytisine Binding to Nicotinic Cholinergic
Receptors in
Brain, Mol, Phann, 39: 9-12, 1991. Membrane enriched fractions from rat brain
minus
cerebellum (ABS Inc., Wilmington, DE) were slowly thawed at 4 'V, washed and
resuspended in 30 volumes of BSS-Tris buffer (120 mM NaC1/5 m/VI KC1/2 mM
CaC12/2
mM MgC12/50 mM Tris-C1, pH 7.4, 4 C), Samples containing 100-200 p.g of
protein
and 0.75 nM [311]-cytisine (30 Ci/mmol; Perkin Elmer/NEN Life Science
Products,
Boston, MA) were incubated in a final volume of 500 gL for 75 minutes at 4 'C.
Seven
log-dilution concentrations of each compound were tested in duplicate. Non-
specific
binding was determined in the presence of 10 tM (-)-nicotine, Bound
radioactivity was
isolated by vacuum filtration onto prewetted glass fiber filter plates
(Millipore, Bedford,
MA) using a 96-well filtration apparatus (Packard Instruments, Meriden, CT)
and were
then rapidly rinsed with 2 naL of ice-cold BSS buffer (120 mM NaC1/5 mM KC1/2
mM
CaC12/2 mM MgC12). Packard MicroScint-20 scintillation cocktail (40 AL) was
added
to each well and radioactivity determined using a Packard TopCount
instrument, The
IC50 values were determined by nonlinear regression in Microsoft Excel
software, K1
values were calculated from the IC50s using the Cheng-Prusoff equation, where
Ki =
IC50/1+[Ligandj/Ka
f3H1-Methyllycaconitine (MLA) binding
Binding conditions were similar to those for [31-1]-cytisine binding, Membrane
enriched fractions from rat brain minus cerebellum (ABS Inc., Wilmington, DE)
were
slowly thawed at 4 C, washed and resuspended in 30 volumes of BSS-Tris buffer
(120
mM NaC1, 5 mM KCI, 2 rriM CaCl2, 2 mM MgC12, and 50 mM Iris-Cl, pH 7.4, 22
C).
-89-
CA 02647830 2013-10-23
WO 2007/137030
PCT/US2007/068930
.10
Samples containing 100-200 pg of protein, 5 nMf.311]-MLA (25 Ci/mmol; Perkin
Elmer/MEN Life Science Products, Boston, MA) and 0.1% bovine serum albumin
(BSA,
Millipore, Bedford, MA) were incubated in a final volume 01 500 id, for 60
minutes at 22
C. Seven log-dilution concentrations of each compound were tested in
duplicate. Non-
specific binding was determined in the presence of 10 pM MLA. Bound
radioactivity
was isolated by vacuum filtration onto glass fiber filter plates prewetted
with 2% BSA
using a 96-well filtration apparatus (Packard Instruments, Meriden, CT) and
were then
rapidly rinsed with 2 mL of ice-cold BSS. Packard MicroScint-20e scintillation
cocktail
(40 AL) was added to each well and radioactivity was determined using a
Packard
TopCount instrument. The IC50 values were determined by nonlinear regression
in
Microsoft Excel software. Ki values were calculated from the IC50s using the
Chong-
Prusoff equation, where Ki = IC50/1+[LigantIVIC0).
[3H1-DPPB binding
[3111-DPPB, (3H)-(S,S)-2,2-dimethyl-5-(6-phenyl-pyridazin-3-y1)-5-aza-2-azonia-
bicyclo[2.2.1Theptane iodide, binding to the al nAChR subtype was determined
using
membrane enriched fractions from rat brain minus cerebellum or human cortex
(ABS
Inc., Wilmington, DE). Pellets were thawed at 4 C, washed and resuspended with
a
TM
Polytron at a setting of 7 in 30 volumes of BSS-Tris buffer (120 mM NaC1, 5 mM
KC1, 2
InM CaCl2, 2 inM MgC12, and 50 rnM Tris-C1, pH 7.4, 4 C). Seven log-dilution
concentrations of test compounds containing 100-200 pg of protein, and 0.5 nM
[31-1j-
DPPB (62,8 Ci/mmol; R46V, Abbott Labs) were incubated in a final volume of 500
gl
for 75 minutes at 4 C in duplicate. Non-specific binding was determined in the
presence
of 10 pM methyllyeaconitine. Bound radioactivity was collected on Millipore
MultiScreen harvest plates FB presoaked with 0.3% PEI using a Packard cell
harvester,
washed with 2.5 ml ice-cold buffer, and radioactivity was determined using a
Packard
TopCount Microplate beta counter. IC50 values were determined by nonlinear
regression
in Microsoft Excel or Assay Explorer. Ki values were calculated from the
IC50s using
the Cheng-Prusoff equation, where KJ = IC50/1-f[Ligand]/K0]. [31i1-DPPB was
obtained
according to the preparation procedures described below.
-90-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
fMethy1-311]2,2-Dirnethyl-5-(6-phenyl-pwidazin-3-y1)-5-aza-2-azonia-
bieyclo[2.2.11heptane; iodide Preparation
[Methy1-31-112,2-dimethyl-5-(6-phenyl-pyridazin-3-y1)-5-aza-2-azonia-
bicyclo[2.2 l]heptane; iodide used in the {31-11-DPPB binding assay above was
prepared
according to the following procedures.
Step 1: Preparation of t-Butyl (S,S)-5-(6-Phenyl-pyridazin-3-y1)-2,5-diaza-
bicyclof2.2.1Theptane-2-carboxylate
Triethylamine (20 mL) was added to a suspension of t-butyl (S,S)-2,5-
diazabicyclo[2,2,1}heptane-2-carboxylate (143 g, 17,3 mmol, Aldrich Chemical
Company) and 3-chloro-6-phenylpyridazine (3,30 g, 17.3 mmol, Aldrich Chemical
Company) in toluene (50 mL) and the mixture was heated under nitrogen at 100
C for 7
days. The dark mixture was cooled to room temperature, and the resulting
precipitate
was isolated by filtration, washed with toluene (15 mL) and dried under vacuum
to
provide the title compound as an off-white solid (100 g), The filtrate was
concentrated
and the residue wa purified by column chromatography on silica gel, eluting
with ethyl
acetate, to provide additional product (0.41 g, total yield 141 g, 56%): MS
(DCl/NH3)
m/z 353 (M+H)+,
Step 2: Preparation of (S,S)-2-Methyl 5-(6-pheny1-pyridazin-3-v1)-2õ5-diaza-
bicyclo[2.2.11heptane
The product obtained from Step 1 (3,41 g, 9,7 mmol) was dissolved in formic
acid
(20 mL) and treated with formalin (37% by weight, 1,0 g, 12.3 mmol). The
mixture was
heated at 100 C for lh, and the brown solution was cooled to room temperature
and
concentrated under vacuum. The residue was purified by column chromatography
on
silica gel, eluting with C112C12 CH3OH NH4OH (95:5:1) to provide the title
compound as an off-white solid (2.50 g, 96%): MS (DCl/NH3) ink 267 (M+H)+.
Step 3: Preparation ofr3H1-(S,S)-2,2-Dimethy1-5-(6-phenyl-pyridazin-3-v1)-5-
aza-2-
azonia-bicyclo[2.2.11heptane iodide ([31-11-DPPB)
[3H]Methyl iodide in toluene (250 mCi in 0,1 mL, 850/n1mo', American
Radiolabeled Chemicals, Inc.) was combined with a solution of the product
obtained
-91-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
from Step 2 in dichloromethane (0388 mg, 2,96 rrnnole in 0.45 mL), The vial
was
capped and the mixture was allowed to react overnight at room temperature.
Methanol
was added and the solvents were evaporated to give 42 mCi. The product was
taken up
in methanol for HPLC purification,
Step 4: Purification by High Performance Liquid Chromatography (HPLC)
About 7 mCi of [311}-DPPB was evaporated to dryness and the residue was
dissolved in total about 4,5 ml acetonitrile:waterTFA (15:85:0,1).
Approximately 0,9
mL per injection were made onto a Phenomenex Luna C18(2) column (5 micron, 250
mm x 4,6 mm ID) using an Agilent HPLC system. [31-11-DPPB was eluted by a
gradient
mobile phase from .10% B to 20% B in 20 min where Mobile Phase A= 0.1%
trifluoroacetic acid in water and Mobile Phase B= 0,1% trifluoroacetic acid in
acetonitrile
at a flow rate of approximately 1 mL/min. Peak detection and chromatograms
were
obtained with an Agilent variable wavelength UV detector set at 275 rim, The
fractions
containing [31-1]-DPPB were collected at approximately 14 minutes using an
Agilent
fraction collector. The fractions were combined and the solvents were
evaporated in
vacuo. The residue was dissolved in 200 proof ethanol (2 mL) to give 0.7 mCi,
Step 5: Determination of Purity and Specific Activity
[31-I]-DPPB was assayed using an Agilent 1100 series HPLC system consisting of
a quaternary pump, an autosampler, and a photodiode array UV detector. A
Packard
Radiomatic A 500 radioactivity detector was connected to the HPLC system. For
radiodetection, a 500 mi., flow cell and a 3:1 ratio of Ultima-Flo M
scintillation cocktail
to HPLC mobile phase were used. The analyses were performed using a Phenomenex
Luna C18(2) column (5 microns, 250 mm x 4,6 mm ID), The mobile phase consisted
of a
gradient starting with 10% B and ramping to 20% B in 20 minutes followed by
ramping
to 90% B in 1 minute and hold at 90% B for 9 minutes, where Mobile Phase A =
0.1%
trifluoroacetic acid in water and Mobile Phase B= 0,1% trifluoroacetic acid in
acetonitrile. The flow rate was set at approximately 1 mL/min and the UV
detection was
set at 275 nm,
The radiochemical purity of [31-1]-DPPB was found to be >98%. The specific
activity was determined to be 62.78 Ci/mmol by mass spectroscopy.
-92-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
Compounds of the invention had Ki values of from about 1 nanomolar to about 10
micromolar when tested by the [31-1]-MLA assay, many having a Ki of less than
1
micromolar. [31-1]-Cytisine binding values of compounds of the invention
ranged from
about 1 nanomolar to at least 100 micromolar, Alternatively, the K1 value as
measured by
[311}-DPPB assay can be used in place of the Ki MLA,
Methods of the Invention
Compounds and compositions of the invention are useful for modulating the
effects of nAChRs, and more particularly a7 nAChRs. In particular, the
compounds and
compositions of the invention can be used for treating and preventing
disorders
modulated by a7 nAChRs. Typically, such disorders can be ameliorated by
selectively
modulating the a7 nAChRs in a mammal, preferably by administering a compound
or
composition of the invention, either alone or in combination with another
active agent,
for example, as part of a therapeutic regimen. Also, some compounds of the
invention
possess affinity at the a4P2 nAChRs in addition to a7 nAChRs, and selective
compounds
with dual affinities at both receptor subtypes also are expected to have
beneficial effects.
The compounds of the invention, including but not limited to those specified
in
the examples, possess an affinity for nAChRs, and more particularly a7 nAChRs.
As a7
nAChRs ligands, the compounds of the invention can be useful for the treatment
and
prevention of a number of al nAChR-mediated diseases Or conditions.
For example, a7 nAChRs have been shown to play a significant role in enhancing
cognitive function, including aspects of learning, memory and attention
(Levin, ED., I.
Neurobiol. 53: 633-640, 2002). As such, a7 ligands are suitable for the
treatment of
cognitive disorders including, for example, attention deficit disorder,
attention deficit
hyperactivity disorder (ADHD), Alzheimer's disease (AD), mild cognitive
impairment,
senile dementia, AIDS dementia, Pick's Disease, dementia associated with Lewy
bodies,
and dementia associated with Down's syndrome, as well as cognitive deficits
associated
with schizophrenia.
In addition, al-containing nAChRs have been shown to be involved in the
neuroprotective effects of nicotine both in vitro (,Tonnala, R. B. and
Buccafusco, JõJ., J.
Neurosci, Res. 66: 565-572, 2001) and in vivo (Shimohama, S. et al., Brain
Res. 779:
-93-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
359-363, 1998), More particularly, neurodegeneration underlies several
progressive CNS
disorders, including, but not limited to, Alzheimer's disease, Parkinson's
disease,
amyotrophic lateral sclerosis, Huntington's disease, dementia with Lewy
bodies, as well
as diminished CNS function resulting from traumatic brain injury. For example,
the
impaired function of a7 nAChRs by P-amyloid peptides linked to Alzheimer's
disease
has been implicated as a key factor in development of the cognitive deficits
associated
with the disease (Liu, Q.-S., Kawai, H,, Berg, D. K., PNAS 98: 4734-4739,
2001), The
activation of ca nAChRs has been shown to block this neurotoxicity (Kihara, T.
et al., J,
Biol. Chem. 276: 13541-13546, 2001), As such, selective ligands that enhance
ca
activity can counter the deficits of Alzheimer's and other neurodegenerative
diseases.
Schizophrenia is a complex disease that is characterized by abnormalities in
perception, cognition, and emotions. Significant evidence implicates the
involvement of
a7 nAChRs in this disease, including a measured deficit of these receptors in
post-
mortem patients (Leonard, S. Eur. J. Pharmacol. 393: 237-242, 2000). Deficits
in
sensory processing (gating) are one of the hallmarks of schizophrenia These
deficits can
be normalized by nicotinic ligands that operate at the a7 nAChR (Adler L. E.
et aL,
Schizophrenia Bull, 24: 189-202, 1998; Stevens, K. E. et al.,
Psychopharmacology 1.36:
320-327, 1998). Thus, a7 ligands demonstrate potential in the treatment
schizophrenia.
Angiogenesis, a process involved in the growth of new blood vessels, is
important
in beneficial systemic functions, such as wound healing, vascularization of
skin grafts,
and enhancement of circulation, for example, increased circulation around a
vascular
occlusion. Non-selective nAChR agonists like nicotine have been shown to
stimulate
angiogenesis (Heeschen, C. et al., Nature Medicine 7: 833-8.39, 2001).
Improved
angiogenesis has been shown to involve activation of the a7 nAChR (Heeschen,
C. et al,
J. Clin, Invest, 110: 527-536, 2002), Therefore, nAChR ligands that are
selective for the
a7 subtype offer improved potential for stimulating angiogenesis with an
improved side
effect profile.
A population of ca nAChRs in the spinal cord modulate serotonergic
transmission that have been associated with the pain-relieving effects of
nicotinic
compounds (Cordero-Erausquin, M. and Changeux, J.P. PNAS 98:280.3-2807, 2001),
The a7 nAChR ligands demonstrate therapeutic potential for the treatment of
pain states,
-94-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
including acute pain, post-surgical pain, as well as chronic pain states
including
inflammatory pain and neuropathic pain. Moreover, a7 nAChRs are expressed on
the
surface of primary macrophages that are involved in the inflammation response,
and that
activation of the a7 receptor inhibits release of TNF and other cytokines that
trigger the
inflammation response (Wang, H. et al Nature 421: 384-388, 2003), Therefore,
selective
a7 ligands demonstrate potential for treating conditions involving TNF'-
mediated
diseases, for example, rheumatoid arthritis, Crohn's disease, ulcerative
colitis,
inflammatory bowel disease, organ transplant rejection, acute immune disease
associated
with organ transplantation, chronic immune disease associated with organ
transplantation, septic shock, toxic shock syndrome, sepsis syndrome,
depression, and
rheumatoid spondylitis.
The mammalian sperm acrosome reaction is an exocytosis process important in
fertilization of the ovum by sperm. Activation of an al nAChR on the sperm
cell has
been shown to be essential for the acrosome reaction (Son, J.-11. and Meizel,
S. BioL
Reproduct. 68: 1348-1353 2003). Consequently, selective a7 agents demonstrate
utility
for treating fertility disorders.
Compounds of the invention are particularly useful for treating and preventing
a
condition or disorder affecting cognition, neurodegeneration, and
schizophrenia.
Cognitive impairment associated with schizophrenia often limits the ability of
patients to function normally, a symptom not adequately treated by commonly
available
treatments, for example, treatment with an atypical antipsychotic. (Rowley, M.
et aL, J.
Med. Chem. 44: 477-501, 2001), Such cognitive deficit has been linked to
dysfunction of
the nicotinic cholinergic system, in particular with decreased activity at a7
receptors.
(Friedman, J. I. et al., Biol Psychiatry, 51: 349-357, 2002). Thus, activators
of a7
receptors can provide useful treatment for enhancing cognitive function in
schizophrenic
patients who are being treated with atypical antipsychotics. Accordingly, the
combination of an a7 nAChR ligand and an atypical antipsychotic would offer
improved
therapeutic utility. Specific examples of suitable atypical antipsychotics
include, but are
not limited to, clozapine, risperidone, olanzapine, quietapine, ziprasidone,
zotepine,
iloperidone, and the like.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of
-95-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
this invention can be varied so as to obtain an amount of the active
compound(s) that is
effective to achieve the desired therapeutic response for a particular
patient, compositions
and mode of administration. The selected dosage level will depend upon the
activity of
the particular compound, the route of administration, the severity of the
condition being
treated and the condition and prior medical history of the patient being
treated. However,
it is within the skill of the art to start doses of the compound at levels
lower than required
to achieve the desired therapeutic effect and to gradually increase the dosage
until the
desired effect is achieved.
When used in the above or other treatments, a therapeutically effective amount
of
one of the compounds of the invention can be employed in pure form or, where
such
forms exist, in pharmaceutically acceptable salt, ester, amide or prodrug
form.
Alternatively, the compound can be administered as a pharmaceutical
composition
containing the compound of interest in combination with one or more
pharmaceutically
acceptable carriers. The phrase "therapeutically effective amount" of the
compound of
the invention means a sufficient amount of the compound to treat disorders, at
a
reasonable benefit/risk ratio applicable to any medical treatment. It will be
understood,
however, that the total daily usage of the compounds and compositions of the
invention
will be decided by the attending physician within the scope of sound medical
judgment.
The specific therapeutically effective dose level for any particular patient
will depend
upon a variety of factors including the disorder being treated and the
severity of the
disorder; activity of the specific compound employed; the specific composition
employed; the age, body weight, general health, sex and diet of the patient;
the time of
administration, route of administration, and rate of excretion of the specific
compound
employed; the duration of the treatment; drugs used in combination or
coincidental with
the specific compound employed; and like factors well-known in the medical
arts. For
example, it is well within the skill of the art to start doses of the compound
at levels
lower than required to achieve the desired therapeutic effect and to gradually
increase the
dosage until the desired effect is achieved.
The total daily dose of the compounds of this invention administered to a
human
or lower animal range from about 0,010 mg/kg body weight to about 1 g/kg body
weight.
More preferable doses can be in the range of from about 0,010 mg/kg body
weight to
about 100 mg/kg body weight. If desired, the effective daily dose can be
divided into
-96-
CA 02647830 2008-09-29
WO 2007/137030
PCT/US2007/068930
multiple doses for purposes of administration. Consequently, single dose
compositions
may contain such amounts or submultiples thereof to make up the daily dose.
Compounds of the invention are ca nAChRs ligands that modulate function of a7
nAChRs by altering the activity of the receptor or signaling. The compounds
can be
inverse agonists that inhibit the basal activity of the receptor or
antagonists that
completely block the action of receptor-activating agonists The compounds also
can be
partial agonists that partially block or partially activate the a7 nAChR
receptor or
agonists that activate the receptor. Binding to a7 receptor also trigger key
signaling
processes involving various kinases and phosphatases and protein-protein
interactions
that are important to effects on memory, cytoprotection, gene transcription
and disease
modification. Therefore, the administration of a therapeutically effective
amount of a
compound of formula (I) to a mammal provides a method of selectively
modulating the
effects of a4132, a7, or both a4132 and a7 nicotinic acetylcholine receptors.
Furthermore, the administration of a therapeutically effective amount of a
compound of formula (I) toa mammal provides a method of treating or preventing
a
condition or disorder selected from the group consisting of attention deficit
disorder,
attention deficit hyperactivity disorder (ADHD), Alzheimer's disease (Al)),
mild
cognitive impairment, senile dementia, AIDS dementia, Pick's Disease, dementia
associated with Lewy bodies, dementia associated with Down's syndrome,
amyotrophic
lateral sclerosis, Huntington's disease, diminished CNS function associated
with
traumatic brain injury, acute pain, post-surgical pain, chronic pain,
inflammatory pain,
neuropathic pain, infertility, need for new blood vessel growth associated
with wound
healing, need for new blood vessel growth associated with vascularization of
skin grafts,
and lack of circulation, more particularly circulation around a vascular
occlusion,
rheumatoid arthritis, Crohn's disease, ulcerative colitis, inflammatory bowel
disease,
organ transplant rejection, acute immune disease associated with organ
transplantation,
chronic immune disease associated with organ transplantation, septic shock,
toxic shock
syndrome, sepsis syndrome, depression, and rheumatoid spondylitis. More
prefered, the
administration of a therapeutically effective amount of a compound of formula
(I) toa
mammal provides a method of treating cognitive disorders, neurodegeneration,
and
schizophrenia. Furthermore, compounds of formula (I) may also be administered
in
combination with an atypical antipsychotic,
-97-
CA 02647830 2013-10-23
WO 2007/137030
PCT/US2007/068930
The scope of the claims should not be limited by the preferred embodiments
set forth in the examples, but should be given the broadest interpretation
consistent
with the description as a whole.
-98-