Note: Descriptions are shown in the official language in which they were submitted.
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
Medical Closure Screen Installation Systems and Methods
Technical Field
The present invention relates generally to medical closure and wound fluid
management devices, and in particular to installation systems and methods for
screen closure members and devices for closing tissue separations, such as
incisions and wounds, which closure members and devices are optionally
bioabsorbable.
Background Art
In the medical field, which is broadly defined to include dentistry,
veterinary
medicine, etc., cutaneous incisions are commonly performed in surgery to
provide
access to underlying tissue, organs, joints, skeletal structure, etc. Incision
and
closure techniques are an important part of surgery in general. They tend to
occupy
surgical teams and other resources for significant portions of many surgical
procedures.
Surgeons generally strive to minimize the traumatic and scarring effects of
surgery on their patients by both minimizing the incisions, and by employing a
variety
of closure techniques which tend to reduce postoperative swelling, bleeding,
seroma,
infection and other undesirable postoperative side effects. For example, the
fields of
endoscopic-assisted surgery, microscopic urgery, and computer-enhanced
instrumentation (e.g., the DaVinci System available from Intuitive Surgical,
Inc. of
Sunnyvale, Calif.) are generally concerned with minimally invasive surgery
("MIS")
procedures and techniques, which have proven to be increasingly popular. Such
popularity is at least partly due not only to the minimally-sized scars left
by such
techniques, but also to the minimal trauma to the fascia and muscle layers and
the
correspondingly faster recoveries this allows. However, surgeons must balance
such
considerations with providing adequate access to perform various surgical
procedures. A typical surgical procedure involves a cutting or dissecting
phase and a
closing phase. In recent years, considerable progress has been made in
minimizing
surgical cutting, dissecting and shaping. Surgical closing techniques involve
sutures,
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-2-
clips, staples and adhesives. However, suturing can be time-consuming and
tedious.
Moreover, the tissue structures to be joined may not be amenable to other
closure
techniques. MIS often restricts access to the separated tissue structures,
thus
making it more difficult to approximate and close same.
In contrast to MIS, some surgical procedures, by their nature, must include
long
incisions. Examples include cutaneous excisional procedures such as "lifts"
and
reduction procedures, flap procedures for closure of defects, and many
bariatric
procedures. Suturing in these extensive defects can be time-consuming and
tedious.
The "first intention" (primary intention healing) in surgery is to "close" the
incision. For load-bearing tissues, such as bone, fascia, and muscle, this
requires
substantial material, be it suture material, staples, or plates and screws.
For the
wound to be "closed," the epithelial layer must seal. To accomplish this, the
"load
bearing" areas of the cutaneous and subcutaneous layers (i.e., the deep dermal
elastic layer and the superficial fascia or fibrous layers of the adipose
tissue,
respectively) must also at least be held in approximation. Important
considerations
include controlling infection and bleeding, reducing scarring, eliminating the
potential
of hematoma, seroma, and "dead-space" formation and managing pain. Dead space
problems are more apt to occur in the subcutaneous closure. Relatively shallow
incisions can normally be closed with surface-applied closure techniques, such
as
sutures, staples, glues, and adhesive tape strips. However, deeper incisions
may
well require not only skin surface closure, but also time-consuming placement
of
multiple layers of sutures in the load-bearing planes. Absorbable sutures are
commonly used for this purpose and comprise an important class of surgical
sutures.
Depending on various factors, absorbable sutures typically dissolve over a
period of
a few days to a few months. Commercially available examples include
Monocryl® monofilament absorbable synthetic sutures comprising a
poliglecaprone and PDS® (palydrioxanone) and Vicryl® (polyglactin)
sutures, all available from Eth icon, Inc., of Somerville, N.J.
Surgical mesh is commonly used to span or reinforce load-bearing planes or
defects in them. When coupled with sutures or fasteners, surgical mesh
represents
another important class of surgical closure devices. Applications include
reconstruction, hernia repair, and organ repair. In such procedures, surgical
mesh
fabric prostheses are inserted into patients through either open surgery or
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-3-
endoscopic (MIS) procedures. Knitted surgical mesh for hernia repair is
disclosed in
the Agarwal et al. U.S. Pat. No. 6,287,316, which is assigned to Ethicon, Inc.
Another Ethicon, Inc. patent, Duncan U.S. Pat. No. 4,548,202, discloses mesh
tissue
fasteners including various fastening members with spaced-apart legs for
passing
through tissue portions. Another closure procedure involves the placement of
pins or
rods through skin edge or bone followed by the placement of an external clamp
or
fixator device spanning the wound and frequently incorporating a worm-screw
apparatus capable of progressive tightening over time to effect closure,
stabilization
or distraction.
Fluid management represents another important aspect of both open and
minimally invasive surgery. Postoperative fluid drainage can be accomplished
with
various combinations of tubes, sponges, and porous materials adapted for
gathering
and draining bodily fluids. The prior art includes technologies and
methodologies for
assisting drainage. For example, the Zamierowski U.S. Pat. No. 4,969,880; U.S.
Pat.
No. 5,100,396; U.S. Pat. No. 5,261,893; U.S. Pat. No. 5,527,293; and U.S. Pat.
No.
6,071,267 disclose the use of pressure gradients, i.e., vacuum and positive
pressure,
to assist with fluid drainage from wounds, including surgical incision sites.
Such
pressure gradients can be established by applying porous foam material either
internally or externally to a wound, covering same with a permeable, semi-
permeable, or impervious membrane, and connecting a suction vacuum source
thereto. Fluid drawn from the patient is collected for disposal. Such fluid
control
methodologies have been shown to achieve significant improvements in patient
healing. Another aspect of fluid management, postoperative and otherwise,
relates to
the application of fluids to wound sites for purposes of irrigation, infection
control,
pain control, growth factor application, etc. Wound drainage devices are also
used to
achieve fixation and immobility of the tissues, thus aiding healing and
closure. This
can be accomplished by both internal closed wound drainage and external vacuum
devices. Fixation of tissues in apposition can also be achieved by bolus tie-
over
dressings (Stent dressings), taping, strapping and (contact) casting.
Heretofore, there has not been available a medical closure screen assembly
with the advantages and features of the present invention, including the
combination
of same with negative pressure wound therapy ("NMI").
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-4-
Disclosure of Invention
In the practice of one aspect of the present invention, a medical closure
screen
device is provided, which includes a mesh screen comprising tubular vertical
risers,
barbed filaments therebetween and horizontal spacers. Integral or separate
sutures
can be provided. An optional perimeter member partly surrounds the screen
member
and can comprise a perimeter tube fluidically coupled with the vertical risers
to form
a tubing assembly. The tubing assembly cooperates with the vertical risers to
extract
fluid from the tissue separation in a drain mode and to introduce fluid
thereinto in an
irrigate mode.. In one embodiment of the invention the tubing assembly is
fluidically
coupled to a vacuum source to facilitate drainage. In another embodiment of
the
invention, the perimeter tube is passed through the surrounding tissue to
secure the
screen member in place. Fluid transfer elements, such as sponges, are
optionally
placed adjacent to and over an extension of the screen for fluid transfer, for
example,
in conjunction with a vacuum or pump source. Another embodiment of the
invention
includes a suture connected to the screen and adapted for securing same in a
tissue
separation. Alternative embodiment vertical risers are also disclosed, and can
provide active fluid transfer utilizing the patient's body dynamics. Yet
another
alternative embodiment of the present invention utilizes the screen barbs for
mechanical fixation in a separation for closure of same. Separation closure,
irrigation
and drainage methodologies are disclosed utilizing various combinations of
closure
screens, tubing, sutures, fluid transfer elements and gradient force sources.
The
closure screen of the present invention uses mechanical and other forces
associated
with screens and barbed strands for securing separated tissues together and
for
eliminating or reducing the formation of subcutaneous voids or pockets, which
can
potentially form hematoma and seroma effects.
Brief Description of Drawings
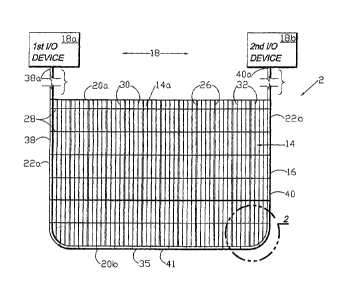
FIG. 1 is a side elevational view of a medical closure screen device embodying
the present invention.
FIG. 2 is an enlarged, fragmentary, side elevational view thereof, taken
generally within circle 2 in FIG. I.
FIG. 3 is an enlarged, fragmentary, side elevational view thereof, taken
generally along line 3-3 in FIG. 2, and particularly showing a barbed strand.
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-5-
FIGS. 4a-f show alternative perimeter tube end closures comprising: 4a)
subdermal termination; 4b) knotted end; 4c) Leur lock; 4d) transfer element
(i.e.,
sponge); 4e) vacuum source; and 4f) clamped end.
FIGS. 5a-e show a tissue separation closure procedure embodying the method
of the present invention.
FIG. 6a is an enlarged, fragmentary, cross-sectional view of the closure
screen
in a tissue separation, with skin hooks shown in hidden lines for positioning
the
separated tissue portions along the closure screen.
FIG. 6b is an enlarged, fragmentary, cross-sectional view of the closure
screen
w in a substantially closed tissue separation.
FIGS. 7a-f show a tissue separation closure procedure embodying the method
of the present invention and utilizing optional sponge or foam fluid transfer
elements
and a tubing placement tool.
FIG. 8 is a cross-sectional view of a tissue separation closure utilizing
tubing for
securing the closure screen with a fluid transfer subassembly connected to an
upper
edge of the closure screen.
FIG. 9 shows a needle mounting a length of drain tubing and adapted for
passing same through tissue.
FIG. 10 is a side elevational view of a closure screen comprising an
alternative
embodiment of the present invention, with a perimeter suture.
FIG. 11a is an enlarged, fragmentary, side elevational view thereof, taken
generally within circle 11a in FIG. 10.
FIG. 11b is an enlarged, fragmentary, side elevational view thereof, showing
modified vertical risers.
FIG. 12 is a side elevational view of a screen-only closure screen comprising
an alternative embodiment of the present invention.
FIG. 13a is an enlarged, fragmentary, side elevational view thereof, taken
generally within circle 13a in FIG. 12.
FIG. 13b is an enlarged, fragmentary, side elevational view thereof, showing
modified vertical risers.
FIGS. 14a-g show a tissue separation closure procedure utilizing the screen-
only embodiment of the closure screen.
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-6-
FIG. 15a is a side elevational view of a modified vertical riser with
flexible,
multi-tube risers forming a fluid passage.
FIG. 15b is a cross-sectional view thereof, taken generally along line 15b-15b
in
FIG. 15a.
FIG. 16a is a fragmentary; side elevational view thereof, shown in a
compressed configuration.
FIG. 16b is a cross-sectional view thereof, taken generally along line 16b-16b
in
FIG. 16a.
FIG. 17 is a cross-sectional view of another modified vertical riser
construction
with risers bundled in a different configuration, with barbs.
FIG. 18 is a cross-sectional view of a modified vertical riser or perimeter
element, comprising a fluted tube.
FIG. 19 is an enlarged, fragmentary, side elevational view of a modified
barbed
strand configuration.
FIG. 20 is an enlarged, fragmentary, side elevational view of another modified
barbed strand configuration.
FIG. 21 is an enlarged, cross-sectional view of a closure screen comprising an
alternative embodiment of the present invention, with barbs formed by cutting
off the
ends of looped filaments.
FIG. 22 is an enlarged, cross-sectional view of a closure screen comprising an
alternative embodiment of the present invention, with barbs forming hooks and
constructed by cutting looped filaments.
FIG. 23 is an enlarged, cross-sectional view of a closure screen comprising
yet
another alternative embodiment of the present invention, with barbs formed by
cutting off the ends of looped filaments, which are laid over in a common
direction or
orientation.
FIG. 24 is an enlarged, cross-sectional view of a closure screen comprising a
further alternative embodiment of the present invention, with barbs forming
hooks
and constructed by cutting looped filaments, which are laid over in a common
direction or orientation.
FIG. 25 is a perspective view of a closure screen comprising a further
alternative embodiment or aspect of the invention, comprising individual links
forming
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-7-
flexible strands and including a pre-installation enclosure assembly adapted
for
holding the screen to length and protectively covering the links.
FIG. 26 is a perspective view showing the closure screen with the pre-
installation enclosure assembly being placed in a tissue separation.
FIG. 27 is a perspective view showing a pair of the closure screens embedded
in a tissue separation, with an excess portion of one of the closure screens
being
trimmed away.
FIG. 28 is a perspective view showing a cover strip over the closure screens
and the tissue separation.
FIGS. 29-30 show a sequential procedure for approximating a tissue separation
using the closure screen and its enclosure assembly.
FIG. 31 is a perspective view of another alternative embodiment closure
screen.
FIG. 32 is exploded view thereof.
FIG. 33 is a perspective view of another alternative embodiment closure screen
system.
FIG. 34 is an exploded view thereof.
FIGS. 35-46 show approximating tissue separations using a closure screen
system embodying the present invention.
FIG. 47 is a perspective view of an alternative embodiment closure screen,
with
a partially-exposed, positioning row of prongs.
FIGS. 48a-c show another alternative embodiment closure screen including a
base clip and further show a sequential procedure for approximating separated
tissue portions.
FIG. 49 is a perspective view of another alternative embodiment closure
device,
comprising discrete closure clips individually mounted on a backing sheet.
FIG. 50 is a perspective view of yet another alternative embodiment closure
device, comprising discrete curved-prong closure clips individually mounted on
a
backing sheet.
FIG. 51 is a front view of a patient, particularly showing an abdominal
surgical
site with a flexible closure screen installed for closing an incision.
FIG. 52 is a cross-sectional view of the abdominal surgical site with the
flexible
screen installed in the abdominal cavity in the intraperitoneal position.
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-8-
Best Mode for Carrying Out the Invention
I. Introduction and Environment
As required, detailed embodiments of the present invention are disclosed
herein; however, it is to be understood that the disclosed embodiments are
merely
exemplary of the invention, which may be embodied in various forms. Therefore,
specific structural and functional details disclosed herein are not to be
interpreted as
limiting, but merely as a basis for the claims and as a representative basis
for
teaching one skilled in the art to variously employ the present invention in
virtually
any appropriately detailed structure.
Certain terminology will be used in the following description for convenience
in
reference only and will not be limiting. For example, the words "upwardly",
"downwardly", "rightwardly" and "leftwardly" will refer to directions in the
drawings to
which reference is made. The words "inwardly" and "outwardly" will refer to
directions
toward and away from, respectively, the geometric center of the embodiment
being
described and designated parts thereof The words "horizontal" and "vertical"
generally mean side-to-side and top-to-bottom, respectively. Said terminology
will
include the words specifically mentioned, derivatives thereof and words of a
similar
import.
Referring to the drawings in more detail, the reference numeral 2 generally
designates a medical closure screen device or system embodying the present
invention. Without limitation on the generality of useful applications of the
closure
screen system 2, the primary application disclosed herein is for assistance
with the
closing, draining, irrigating and healing of a separation of first and second
tissue
portions, such as a wound or incision 4. As shown in FIG. 5a, the wound 4
extends
from and is open at the dermis 6, through the deep dermal layer 7 and the
subcutaneous layer 8, and to approximately the fascia 10. The wound 4 displays
edges 12a,b, which correspond to first and second tissue portions. The closure
screen device 2 generally comprises a screen 14, a screen perimeter member 16
and an input/output (I/O) subsystem 18.
II. Screen 14
The screen 14 includes upper and lower margins 20a,b; first and second ends
22a,b; and first and second faces 24a,b. The screen 14 generally forms a grid
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-9-
configuration with vertical, hollow, perforated tubular risers 26 cross-
connected by
horizontal spacer members 28. Multiple barbed strands 30 are positioned
between
the risers 26. The risers 26, the spacers 28 and the strands 30 are preferably
joined
at their respective intersections. As shown in FIG. 3, each strand 30 includes
a
filament 32 with multiple, pointed barbs 34 extending upwardly and outwardly
on
both sides in staggered, spaced relation. The barbs 34 generally project
outwardly
from the screen faces 24a,b, for purposes which will be described in more
detail
hereinafter.
The screen or mesh 14 material can be either dissolvable (absorbable) or non-
dissolvable (non-absorbable) and can be chosen from a number of commercially-
available, biocompatible products, which are commonly used in medical
applications
for sutures, implantable meshes, and similar medical devices.
Examples of absorbable materials include, but are not limited to: aliphatic
polyesters, which include, but are not limited to: homo polymers and
copolymers of
lactide, epsilon.-caprolactone, p-dioxanone, trimethylene carbonate, alkyl
derivatives
of trimethylene carbonate, delta.-hydroxyvalerate, 1,4-dioxepan-2-one, 1,5-
dioxepan-
2-one, 6,6-dimethy1-1,4-dioxan-2-one and polymer blends thereof. Examples of
nonabsorbable materials include, but are not limited to: cotton, linen, silk,
polyamides, polyesters, fluoropolymers, polyolefins, polyethylene, metals and
combinations thereof.
III. Screen Perimeter Member 16
The optional screen perimeter member 16 can comprise, for example, a
flexible, perforated, hollow tube 35 with multiple orifices 36. As shown in
FIG. 1, the
tube 35 includes first and second legs 38, 40 extending generally along the
screen
first and second ends 22a,b, and a base leg 41 extending generally along the
screen
lower margin 20b. The tubing first and second legs 38, 40 terminate in
respective
first and second ends 38a, 40a. The tube 35 can be secured to the screen 14 by
multiple ties 42, which can comprise extensions of the horizontal spacer
members 28
and the strands 30. By providing dissolvable ties 42, the tube 35 can be
designed for
separation from the remainder of the closure screen 2 after a relatively short
period
of time. For example, the dissolvable material can dissolve into the patient's
body
after a few days, whereafter the tube 35 can be removed.
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-10-
Optionally, portions of the tube 35 can be cut away from the screen 14. For
example, the screen 14 can be separated along each screen end 22a,b, or it can
be
separated completely from the tube 35. In this manner the screen 14 and the
tube 35
can be configured to accommodate a variety of conditions and tissue separation
configurations.
The vertical risers 26 are optionally fluidically coupled to the tube 35 at
respective T intersections 44. In this configuration the tube 35 and the
vertical risers
26 cooperate to provide a manifold for fluid handling, i.e. either extraction
or
irrigation, as indicated by the fluid flow arrows 45.
IV. Input/Output (I/O) Subsystem 18
The input/output subsystem 18 is designed for extraction and/or irrigation of
the
patient's bodily fluids and/or external fluids. As shown in FIG. 1, the
input/output
subsystem 18 includes first and second I/O devices 18a,b attached to the
tubing first
and second leg ends 38a,b, which in this configuration are considered the
"port"
ends of the tube 35. One or both of the I/O devices 18a,b can comprise a
pressure
differential source, such as the NPINT device, The V.A.C.® System TM.,
available from Kinetic Concepts, Inc. of San Antonio, Tex. The use of such
units for
wound treatment and fluid management is disclosed in the Zamierowski U.S. Pat.
No. 4,969,880; U.S. Pat. No. 5,100,396; U.S. Pat. No. 5,261,893; U.S. Pat. No.
5,527,293; and U.S. Pat. No. 6,071,267, which are incorporated herein by
reference.
Alternatively, the tubing port ends 38a,b can be connected to various other
sources of pressure differential and various drainage and irrigation devices.
For
example, they can be cut short below the dermis 6 and left within the
separation 4 for
sealing by the adjacent tissue portions 12a,b. FIG. 4a shows a truncated
tubing end
38b. The tubing ends 38a/40a can be knotted (as shown at 48 in FIG. 4b),
clipped,
tied (e.g., with a suture) or otherwise closed off either above or below the
dermis 6.
FIG. 4c shows a Leur lock coupling 46 mounted on a tubing end 38a/40a. Still
further, a transfer element comprising a piece of foam or sponge 50 can be
coupled
to the tube 35 at an end 38a/40a (FIG. 4d). Examples of such foam and sponge
materials and configurations are discussed in the Zanierowski U.S. patents
identified
above. A pressure differential source, such as a vacuum source 51, can be
connected to a tube end 38a/40a and to a fluid receptacle 66, as shown in FIG.
4e. A
clamp 62 is shown in FIG. 4f and closes the tube end 38a/40a. The clamp 62 can
be
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-11-
chosen from among several suitable clamps, which are commonly used for medical
applications.
Either tube end 38a/40a can function as either an inlet port or an outlet port
with respect to the system 2. For example, suction can be applied for pulling
fluid
from the patient through the system 2 through either tube end 38a/40a. Still
further,
fluid can be pulled in both directions through the system 2 by alternately or
jointly
applying suction to the tube ends 38a/40a. For example, suction can be
simultaneously applied to both tube ends 38a/40a.
V. Operation And Closure Method
FIGS. 5a-e show an installation methodology utilizing the system 2 of the
present invention. In FIG. 5a, the closure screen 2 is placed in the
separation 4 with
the tubing base 41 located at the bottom of the separation (e.g., wound or
incision) 4
and in proximity to the fascia layer 10. As shown, the tissue portions or
wound/incision edges 12a,b are spaced apart. The screen upper margin 20a can
protrude outwardly from the dermis 6. FIG. 5b shows the tissue separation
edges 12
being pushed together as indicated by the force arrows 52. FIG. 5c shows the
separation edges 12 engaged at the dermis 6, and spaced apart somewhat within
the subcutaneous layer 8. The edges 12 can be pushed together-as indicated by
the
force arrows 52. Moreover, the screen 2 can be held or positioned inwardly in
order
to advance the barbs 34 in the separation edges 12, as indicated by the inward
or
downward force arrows 54a. FIG. 5d shows the separation edges 12a,b
substantially
closed on the screen 2. Tugging on the screen 14 in the general direction of
the
outward force arrow 54b sets the mesh barbs 34.
FIG. 5e shows the separation 4 closed on the closure screen 2, with the tubing
35 removed from the screen 14. The tubing 35 can be removed either pre-
installation
by cutting the ties 42, or post-installation by allowing the ties 42 to
dissolve,
whereafter the unsecured tubing 35 can be extracted.
FIG. 6a shows the barbs 34 compressed by engagement with the separation
edges 12a,b. As shown, the separation edges 12 can be manually closed by
pressing along the horizontal force arrows 52. The barbs 34 allow the
separation
edges 12a,b to slide upwardly or outwardly along the screen 14. This process
can be
repeated until the separation 4 is closed, as shown in FIG. 6b. Any protruding
length
of the screen 14 can be cut close to the dermis 6. In the final configuration
(FIGS. 5e
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-12-
and 6b), the barbs 34 are embedded in the tissue adjacent to the separation
edges
12a,b and thus secure the separation 4 in a closed position. The fluid
conducting
properties of the screen 14 facilitate extracting fluid. An outward or upward
force
arrow 54b indicates a force direction whereby the screen barbs 34 are set in
the
adjoining tissue. It will be appreciated that the screen 14 can be securely
set in place
with the barbs 34, yet the separation edges 12a,b will remain capable of
sliding up
on the screen 14 by disengaging the barbs 34 with lateral forces, as shown in
FIG.
6a. Skin hooks 55 can be used for engaging the tissue portions 12a,b and
tugging
same outwardly as shown in FIG. 6a. The skin hooks 55 can facilitate
positioning
and repositioning the screen 14.
VI. Alternative Embodiment Closure Screen Systems and Methodologies
FIGS. 7a-f show an alternative procedure for mounting the closure screen 2 in
a wound drainage application utilizing pressure differential. As shown in FIG.
7a, the
tubing 35 can pass through the tissue adjacent to the wound 4 and exit the
dermis 6
for termination of the tubing end 38a/40a as described above. An optional
layer of a
suitable, biocompatible adhesive 64 is shown applied to the closure screen
first face
24a for securing same to the first wound edge 12a. FIG. 7b shows the screen 14
extending upwardly from the dermis 6 with the wound edges 12a,b brought
together
in a manner similar to that described above.
The input/output subsystem 18 includes a pair of optional fluid transfer
elements comprising foam or sponge members 56a,b placed on the dermis 6 on
either side of a protruding portion 14a of the screen 14. The screen 14 is
then cut to
a level generally flush with the upper surfaces of the sponges 56a,b, as shown
in
FIG. 7c. An optional sponge bridge 58 is placed over the sponge members 56a,b
(FIG. 7d). Examples of suitable transfer element materials are discussed in
the
Zamierowski patents noted above and include open-cell, porous foam materials
(e.
g., polyurethane ester (PUE)) chosen for their hydrophobic properties and
passage
of liquids. Polyvinyl acetate (PVA) material can be used for its hydrophilic
properties.
The transfer element subassembly 59 formed by the sponge members 56a,b and 58
can be connected to a vacuum source, a fluid irrigation source, etc. Moreover,
it can
be connected to additional fluid transfer elements and covered with various
flexible
membranes and drapes, which can be semi-permeable or impervious, as indicated
for the closure and treatment of particular separations and wounds.
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-13-
FIG. 7e shows a tubing placement tool 120 with a handle 122, a shaft 124 and
a hook 126 terminating at a pointed or rounded, bullet-shaped tip 128. FIG. 7f
shows
the tool 120 passing tubing 35 through tissue in the subcutaneous layer 8 and
into
proximity with the dermis 6. The tip 128 is received in a blind end 134 of the
tubing
35 through a notch 136 formed therein. The thrust of the tool 120 causes
tenting of
the dermis 6, as shown at 138, whereat the dermis 6 can be opened with a
scalpel
140 and the tubing 35 can exit the patient for suitable termination
arrangements,
such as those shown in FIGS. 4a-f above.
FIG. 8 shows a modified embodiment closure system 202 with a pair of screens
14 positioned generally end-to-end in a separation 204. A transfer element
subassembly 59 is placed over the separation 204 and a membrane drape 205 is
placed thereover. The tube 35 is passed through tissue on either side of the
separation 204 (e.g., using the procedure and the tubing placement tool 120
described above) and exits the dermis 6 on either side of the transfer element
subassembly 59. The tube 35 lengths are knotted at 206. The tube 35 lengths
thus
function as sutures or retainers for securing the closure system 202 in the
separation
204. The tube ends 38a or 40a can be utilized for this purpose, thus leaving
the
other tubing ends available for fluid communication with one or more of the
input/output subsystems 18 described above.
The tube 35 can be secured by suitable fasteners, such as clips and the like,
located above the dermis 6. Moreover, the screens 14 can be overlapped,
abutted,
spaced slightly and otherwise configured and positioned as necessary for
particular
tissue separations. Still further, the screens 14 are adapted to be trimmed as
necessary.
FIG. 9 shows a modified embodiment tubing/suture subassembly 220 with a
Trocar instrument 222 including a sharpened, distal end 224 and a proximate
end
226 with multiple, annular ridges 226a. A length of flexible tubing 228
combines the
functions of screen perimeter member and suture. The flexible tubing 228
terminates
at an end 228a adapted for releasably mounting on the needle proximate end
226,
whereat it is retained in place by the ridges 226a. The tubing 228 is
optionally
connected to the screen 14 as described above and can include perforations
228b
for fluid drainage and/or irrigation in conjunction with input/output
subsystems 18,
also as described above. The tubing/suture subassembly 220 is adapted for
securing
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-14-
the screen 14 in place and for closing the separation 4 by passing the tubing
228
through adjacent tissue. The tubing/suture subassembly 220 and the screen 14
can
be prepackaged and presterilized for closing and treating separations, which
can
include wounds and incisions.
FIGS. 10, lla and llb show modified embodiment closure screen systems 302
with first and second suture subassemblies 304, 306 comprising the screen
perimeter member. The suture subassemblies 304, 306 include respective curved
needles 304a, 306a which are swaged or adhesively connected to opposite ends
304b, 306b of a common length of suture thread 307. The suture thread 307 can
be
absorbable or nonabsorbable. As shown in FIG. 10, the screen closure system
302
can be preassembled with the suture thread length 307 releasably secured to
the
perimeter 308a of a screen 308. Prior to installation of the screen 308, the
suture
307 can be disconnected or severed therefrom, either partly or completely. For
example, the suture 307 can be separated along the screen ends 310a, 310b
respectively, thereby leaving the suture thread lengths secured only along a
screen
lower margin 312.
In operation, the suture subassemblies 304, 306 facilitate installation of the
suture/screen closure system 302, thereby providing a preassembled device
which
incorporates the necessary components for securing same in a separation 4. For
example, the screen 308 can be secured at the bottom alone by passing the
suture
subassemblies 304, 306 through tissue portions located at the bottom of the
separation 4. Alternatively, the suture subassemblies 304, 306 can be passed
through the adjacent tissue and exit the surface of the dermis 6, whereby the
suture
subassemblies 304, 306 can be used for closing the separation 4 at the dermis
6.
Barbed strands 320 can interact with the tissue portions 12a,b as described
above,
whereby the screen 308 provides a relatively secure mechanical connection
between
the separated tissue portions 12a,b. The suture subassemblies 304, 306 can be
utilized for various purposes in the separation 4, including attachment and
tacking of
the dermis 6, the deep dermal layer 7, the subcutaneous layer 8 and the fascia
10.
Still further, all or part of the suture subassemblies 304, 306 can be
removed, and
additional suture subassemblies can be mounted on or sutured to the screen
308.
FIG. 11a shows the screen 308 attached to the suture thread 307. FIG. llb
shows an alternative construction screen 318 with hollow tubular vertical
risers 324
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-15-
located between adjacent, respective vertical strands 320, all connected by
the
spacers 322 and adapted for communicating fluid with the separation 4 through
the
open riser ends 324a and the perforations 324b, as indicated by the fluid flow
arrows
326. All or part of the screen/suture system 302 can comprise absorbable
material.
FIGS. 12, 13a and 13b show a modified embodiment screen-only closure
screen system 402 and application methodology. A screen or mesh 404, similar
to
the screen 14 with barbed strands 30 described above, is placed in a
separation 4
against the first tissue portion 12a. The second tissue portion 12b is then
placed
against the screen 404 whereby the separation 4 is closed and can be secured
by
the mechanical action of the screen 404. The screen 404 can be supplemented
with
sutures, drainage tubing, I/O devices, and other auxiliary components for
purposes
of closing the wound edges 12, draining the inside of the tissue separation 4,
fighting
infection, pain management and all other functionalities associated with the
present
invention, as discussed elsewhere herein. For example, the screen 404 can be
secured with sutures at the subcutaneous level 8. Various fluid
interconnecting
devices can be utilized as necessary, and can be designed for removal after
they
serve their initial purpose. External drainage can also be achieved at the
dermis level
6 utilizing transfer element subassemblies, such as the example designated 59
and
described above (FIG. 7d). Moreover, drainage and irrigation tubing can be
installed
within the wound 4 alongside or adjacent to the screen 404. It will be
appreciated
that a screen-only version of the invention can comprise various suitable
biocompatible absorbable and non-absorbable materials, including the materials
disclosed above.
FIG. 13a is an enlarged view of the screen 404 and particularly shows barbed
strands 406 and horizontal spacers 408, which are connected together in a grid
pattern forming the screen 404. FIG. 13b shows an alternative embodiment with
a
modified screen 410 including vertical risers 412 comprising hollow tubing,
which are
connected to and spaced by horizontal spacers 408. Fluid flows into and out of
the
vertical risers 412 through open riser ends 412a and perforations 412b, as
indicated
by the fluid flow arrows 420.
FIGS. 14a-g show the screen 404 installed in a tissue separation 4 and closing
same, utilizing the methodology of the present invention. The methodology
shown in
FIGS. 14a-g is similar to the methodology shown in FIGS. 5a-e and 6a,b. FIG.
14c
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-16-
shows a downward/inward force arrow 54a indicating a direction in which the
screen
404 is pushed or guided into the separation.
FIGS. 15a,b and 16a,b show a modified vertical riser 502 comprising bundled
tubes 504 secured together at spaced intervals by connectors 506. The normal
movement of the patient tends to alternately compress and expand the vertical
risers
502, thus providing a "pumping" action for transferring fluid from the wound
4, as
indicated by the fluid flow arrows 510. FIGS. 15a,b show a riser 502 in an
extended
configuration. Compressing the screen 14 longitudinally (i.e., end-to-end)
compresses the bundled risers 504 to the configuration shown in FIGS. 16a,b,
whereby fluid is drawn into the interstitial space 508 and pumped therefrom
when the
risers 502 extend.
FIG. 17 shows yet another configuration of a vertical riser 602 with bundled
tubes 604, which are closely bunched and define passages 606 for conveying
fluid.
Such fluid conveyance can be enhanced by a pumping action associated with
normal patient movements. Barbs 608 project outwardly from the tubes 604. It
will be
appreciated that various other bundled tube configurations, such as twisted,
braided,
etc., can be utilized.
FIG. 18 shows yet another vertical riser/perimeter member 702 alternative
embodiment configuration. The member 702 has a configuration which is commonly
referred to as a "fluted" drain and includes longitudinally-extending passages
704.
This configuration can substitute for the perimeter members described above
and
can function to communicate fluid to and from the wound 4 with the
input/output
subsystem 18.
As additional alternative embodiment configurations for the vertical risers,
they
can comprise either barbed monofilament strands, similar to strand 30 shown in
FIG.
3, or unbarbed monofilament strands. Such monofilament vertical risers can
function
as passive drains with fluid flowing alongside same. They can extend above the
dermis 6 and abut or connect to transfer elements formed in various
configurations
with suitable absorbent materials. Examples include gauze dressings and
transfer
element subassemblies, such as 59 shown in FIG. 7d.
FIG. 19 shows an alternative embodiment strand 802 constructed by twisting
and braiding multiple, individual filaments 804. Barbs 805 are formed by
respective
individual filaments 804a, which terminate at blunt ends 806. The barbs 805
project
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-17-
generally outwardly from the strand 802 and form acute angles with respect to
its
longitudinal axis. They are adapted for penetrating tissue within a separation
4, as
described above. In use, the barbs 805 would normally be oriented in
directions
generally pointing outwardly from the patient and the tissue separation 4.
FIG. 20 shows another alternative embodiment strand 902 comprising multiple
twisted and braided filaments 904. Barbs 905 are formed from individual
filaments
904a and have notches 908 and pointed ends 910. The notches 908 and the ends
910 are configured to allow the barbs 905 to easily extract from the
separation edge
tissues, whereby the screen is adapted for sliding along the separation edges
in
order to achieve the proper position.
FIG. 21 shows a further modified screen 1002 with barbs 1004 formed by
looping individual filaments 1006 and cutting same at cut locations 1010
spaced
inwardly from respective apexes 1008 of the filament loops. In operation, the
barbs
1004 slightly penetrate the tissue and are imbedded therein. It will be
appreciated
that the filaments 1006 are relatively thin in diameter, similar to
microfibers, whereby
patient comfort is optimized.
FIG. 22 shows yet another modified screen 1102 with barbs 1104 formed by
looping individual filaments 1106 and cutting same at locations 1110 spaced
inwardly from respective apexes 1108 of the filament loops whereby respective
hooks 1112 are formed. The hooks 1112 operate in a manner similar to hook-and-
loop fasteners, with the adjacent tissue forming the loop parts of the
connections. In
operation, the hooks 1112 slightly penetrate the tissue and are imbedded
therein.
The configurations of the hooks 1112 tend to retain them in the tissue
adjacent to the
separation 4 whereby the separated first and second tissue portions 12a,b can
be
closed.
FIG. 23 shows a screen 1202 with a configuration similar to the screen 1002
discussed above, with additional fiber elements or filaments 1204. The
additional
filaments 1204 tend to lay the filament barbs 1206 over whereby the screen
1202
can be directionally oriented within the wound separation 4 and operate in a
manner
similar to the screen 14 described above. The barbs 1206 are formed by cutting
the
apexes 1208 at cut locations 1210.
Similarly, FIG. 24:shows a screen 1302 with additional filaments 1304, which
engage the filament loops 1306 and orient same in a direction towards the
right as
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-18-
shown in FIG. 24. The slanted orientations of the filament loops 1306
facilitate
setting same in the tissue portions 12a,b adjacent to the separation 4 by
tugging
outwardly on the screen 1302. Repositioning the screen 1302 is also possible,
as
described above. The filament loops 1306 can be cut at cut locations 1310,
which
are spaced inwardly from filament loop apexes 1308 whereby hooks 1312 are
formed.
It will be appreciated that FIGS. 21-24 disclose screens with barbs and hooks
extending from one face thereof. The present invention also includes screens
with
barbs and hooks extending from both faces.
A closure screen comprising a further modified aspect or embodiment of the
invention is shown in FIGS. 25-30 and is generally designated by the reference
numeral 1402. The screen 1402 generally comprises a highly flexible panel
1404,
which engages and approximates adjacent tissue portions across a separation by
the semi-independent action of multiple, individual links 1406, which are
strung
together in respective strands 1408.
The screen 1402 includes a pre-installation enclosure assembly 1424
comprising front and back backing sheets 1426, 1428, which can be provided
with a
suitable releasable adhesive 1429. The backing sheets 1426, 1428 preferably
comprise paper or other material (e.g., Styrofoam® material), which is
relatively
stiff (as compared to the relatively flimsy panel 1404) for maintaining the
flat shape of
the closure screen 1402 during handling and placement in the patient and for
protection from the sharpened prong tips. An outer edge handling strip 1430 is
mounted on the upper edge of perimeter 1432 of the panel 1404 (FIG. 25) and is
adapted for grasping manually or with instruments in order to facilitate
handling,
alignment and placement.
FIG. 26 shows a closure screen 1402 being placed in a tissue separation 4 with
a suitable instrument, such as forceps 1434. FIG. 27 shows the panels 1404 of
two
closure screens 1402 in place with the backing pieces 1426 and 1428 removed
and
with the separated tissue portions 12a,b pushed together and approximated by
the
panels 1404. Selvage edges 1436 can be trimmed flush with the skin surface and
removed along with the handling strips 1430. The closed separation 4 can be
covered with a suitable cover strip 1438, which can provide tensile retaining
strength
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-19-
for securing the tissue portions 12a,b together, as well as protecting the
separation 4
during healing (FIG. 28).
FIG. 29 shows a closure screen 1502 in place in a tissue separation 4. FIG. 30
shows extracting a back backing sheet 1508, whereby the panel 1504 is placed
against the tissue portion 12b for engaging same by penetrating its prongs
into the
tissue approximately 1-2 mm or whatever length is dictated by particular
tissue
requirements.
FIGS. 31-46 show the construction and operation of another modified
embodiment or aspect of a closure screen 1502 embodying the present invention.
FIG. 31 shows the closure screen 1502 assembled, with front and back backing
sheets 1506, 1508 enclosing a tissue approximation panel 1504. FIG. 32. is an
exploded view thereof, with the tissue approximation panel 1504 adapted for
placement between the front and back backing sheets 1506, 1508. The backing
sheets 1506, 1508 have upper margins 1510, 1512 respectively, with the back
backing sheet upper margin 1512 extending higher than the front backing sheet
upper margin 1510, which facilitates gripping the back sheet 1508 at its upper
margin 1512 with forceps or some other similar instrument. The back backing
sheet
upper margin 1512 is preferably printed with a color for high visibility, to
further
facilitate grasping same with forceps or manually. FIGS. 33 and 34 show
another
embodiment closure screen 1513 with a square closure panel 1514 with a similar
construction to the rectangular panel 1504.
FIG. 35 shows the tissue separation 4 with first and second separated tissue
portions or edges 12a,b. FIG. 36 shows the placement of a closure screen 1513
with
dashed lines indicating panels 1514, which are already in place in the tissue
separation 4. As shown in FIG. 36, a tissue separation 4 can be "tiled" with
the
closure panels 1514, which can be of any suitable size and number depending
upon
the configuration of the separation 4. FIG. 37 shows the screen 1502 (FIG. 31)
in
place with its back backing sheet 1508 removed, whereby the prongs are exposed
to
and penetrate the tissue portion 12b. The opposite tissue portion 12a can be
pushed
in the direction of directional arrow 1516 to approximate closure. FIG. 38
shows the
lower (innermost) portion of the tissue separation 4 approximated, with the
front
backing sheet 1506 being removed in FIG. 39, resulting in the condition shown
in
FIG. 40 with the lower portion of the tissue separation 4 secured or
"approximated"
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-20-
against the closure panel 1504. FIGS. 41-46 show securing an upper (or
outer/distal)
portion of the tissue separation 4 with another closure screen 1502 by
essentially
repeating the procedure described above. A protruding distal selvage margin
1518 is
removed from the outermost panel 1504 as shown in FIG. 44 substantially flush
with
the skin surface. A suitable cover 1520 is placed over the tissue separation
as
shown in FIG. 45. FIG. 46 shows the prong-tissue engagement whereby the tissue
edges 12a,b are approximated, with the upwardly-and-outwardly orientation of
the
prongs 1522 tending to draw together at the tissue edges 12a,b in response to
outward tugging on the panel 1504. The prongs of the various embodiment
closure
screens and clips can have various suitable sizes and configurations,
including
curved, straight, barbed, etc. Closure and approximation of the tissue
separation 4
can be augmented with tape, adhesive, staples, sutures or other closure
devices,
including a pressure differential source, such as the NPWT device, The
V.A.C.®
System.TM., available from Kinetic Concepts, Inc. of San Antonio, Tex., which
can
be chosen to promote wound closure in conjunction with a closure screen or
screens.
FIG. 47 shows another alternative embodiment closure screen 2302 with a
backing sheet 2304 having a lower removable strip 2306, which exposes a
lowermost row 2308 of clips for initially positioning the screen 2302,
whereafter an
upper portion 2310 of the backing sheet 2304 can be removed.
FIGS. 48a-c show another embodiment closure screen 2352 with a row of
closure clips 2354, which can be mounted along a bottom edge 2356 of a panel
2358 of the closure screen 2352. Each closure clip 2354 includes first and
second
rows of laterally-projecting hooks 2360a,b, which are adapted and positioned
for
penetrating respective tissue portions 12a,b. A sequential procedure for
closing a
lower or inner portion of a tissue separation 4 can begin with the closure
clips 2354
positioned within the tissue separation 4 as shown in FIG. 48a. The first row
of hooks
2360a can be embedded in the first tissue portion 12a, for example by tilting
and
manipulating the panel 2358 (FIG. 48b). Closure can be accomplished by
embedding the second row of hooks 2360b in the second separated tissue portion
12b (FIG. 48c), for example, by tilting and manipulating the panel 2358. The
closure
screen 2352 with the closure clips 2354 can be used in conjunction with the
enclosure assembly of the closure screen 2302, whereby the lower row of links
2308
CA 02648063 2008-10-01
WO 2007/120138
PCT/US2006/014506
-21-
can be exposed for supplementing the closure clips 2354 in connection with
initially
anchoring the closure screen. The clips 2354 can be Provided in any suitable
number and spacing, including a clip 2354 at the lower end of each column of
links,
at the lower end of every other column of links, etc.
FIG. 49 shows an additional alternative embodiment closure screen 2402,
which is constructed by mounting discrete clips 2404 on a backing 2406, which
can
retain the clips 2404 by a suitable adhesive or some other means pre-
installation.
FIG. 50 shows yet another alternative embodiment closure screen 2502, which is
similar to the screen 2402 but with fewer and different clips 2504. The clips
2504
have prongs 2506, which are curved in an outwardly-convex direction with
respect to
bodies 2508 of the clips 2504. The curvature of the prongs 2506 can facilitate
penetration and closure of the separated tissue portions 12a,b. The prongs
2506 can
also be straight, barbed, etc., and can be oriented at any suitable angle with
respect
to the bodies 2508 of the clips 2504.
FIGS. 51 and 52 show a closure screen 1902 applied to an abdominal incision
1904. Such incisions are typically involved in abdominal surgery procedures
and can
result in various complications, including infection and difficulties in
closure and
healing. The abdominal tissues are exposed within the abdominal cavity to a
perimeter 1906. The screen 1902 can be provided with multiple rigid or semi-
rigid
non-collapsible members 1908 for maintaining its general shape and
facilitating its
placement in the abdominal cavity, preferably to approximately the perimeter
1906.
The screen 1902 includes prongs 1910, which can be oppositely-oriented towards
a
centerline 1916 for directing the opposite sides of the incision 1904 towards
medial
closure. The screen 1902 can be provided with a perimeter member 1912, which
is
preferably sized and configured to place and maintain the screen in proximity
to the
tissue separation perimeter 1906. Relatively extensive coverage of the
abdominal
tissue separation can thus be achieved, particularly with the positioning
effects of the
non-collapsible members 1908 and the perimeter member 1912 in combination.
With
appropriate dissection and direction of prongs, this device can also be placed
in pre-
peritoneal positions and on the musculature, in addition to placement intra-
abdominally below the musculature, as shown.