Note: Descriptions are shown in the official language in which they were submitted.
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
PHARMACEUTICALLY ACCEPTABLE SALTS AND POLYMORPHIC FORMS
The present invention is concerned with new risedronate salts and new
polymorphic
forms thereof, processes of preparing the same, pharmaceutical compositions
containing the same, therapeutic uses thereof and methods of treatment
employing the
same.
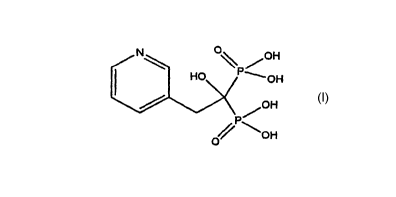
Risedronic acid is the international non-proprietary name of [1-hydroxy-2-(3-
pyridinyl)ethylidene]bisphosphonic acid. Risedronic acid has the following
structural
formula
N O \ POH
HO 'OH
OH
0 \OH
A particularly preferred salt of risedronic acid is sodium risedronate.
Bisphosphonic acids, such as risedronic acid, and pharmaceutically acceptable
salts
thereof, in particular sodium risedronate as referred to above, have been
employed in
the treatment of diseases of bone and calcium metabolism. Such diseases
include
osteoporosis, hyperparathyroidism, hypercalcemia of malignancy, ostolytic bone
metastases, myosistis ossifcans progressiva, calcinoisis universalis,
arthritis, neuritis,
bursitis, tendonitis and other inflammatory conditions.
Bisphosphonic acids, and pharmaceutically acceptable salts thereof, tend to
inhibit the
resorption of bone tissue, which is beneficial to patients suffering from
excessive
bone loss. However, in spite of certain analogies in biological activity, all
bisphosphonates do not exhibit the same degree of biological activity. Some
bisphosphonates have serious drawbacks with respect to the degree of toxicity
in
1
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
animals and the tolerability or negative side effects in humans. The salt and
hydrate
forms of bisphosphonates alter botli their solubility and their
bioavailability.
Processes of preparing risedronic acid, and salts thereof, are known in the
art, and
some examples thereof are as follows.
EP 1243592B describes a process of preparing risedronic acid by reacting 3-
pyridylacetic acid with phosphorous acid and phosphorous trichloride in a
solvent. In
the case where the solvent is chlorobenzene, the reaction is carried out at a
temperature in the range of 85-100 C. In the case where the solvent is
fluorobenzene,
the reaction is carried out at the reflux temperature of the reaction medium.
Isolation
of the risedronic acid involves separation thereof from the reaction mixture
by
treatment with alkali metal or ammonium hydroxide, bicarbonate or carbonate
and
subsequent treatment of the resulting alkali metal or ammonium risedronic acid
salt
with a strong mineral acid.
EP 04949844B also discloses a process of preparing bisphosphonic acids, but
not
risedronic acid. Bisphosphonic acids, in particular alendronic acid, of the
following
general formula are prepared according to the process of EP 0494844B
O OH 0
HO-II I II_OH
I I I
OH ( i H2)r, OH
NH2
where n is 2 to 8. The process comprises melting a mixture of the
corresponding
aminocarboxylic acid and phosphorous acid obtained by heating at 90 C in the
absence of an organic solvent, adding dropwise phosphorous trihalide under
stirring
and N2 atmosphere, adding to the reaction mixture a hydrolyzing agent selected
between water and a strong non-oxidizing acid and recovering the diphosphonic
acid
thus produced. The process is described as being characterised in that the
molar ratio
between the aminocarboxylic acid, phosphorous acid and phosphorous trihalide
in the
reaction mixture is 1:3:2 and 1:20:6.
2
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
WO 01/57052 involves use of molten phosphorous acid, an amino carboxylic acid,
phosphorous trihalide and a base in the bisphosphorylation step. The base is
employed to facilitate bisphosphorylation and can include organic and
inorganic
bases. The more preferred bases are triethylamine, trimethylamine, potassium
carbonate, pyridine and morpholine.
WO 05/063779 describes use of phosphorous oxychloride (POC13), instead of
phosphorous trihalide. More specifically, WO 05/063779 describes reaction of a
carboxylic acid with a mixture of phosphorous acid and phosphorous
oxychloride, in
the absence of solvents. Water, which is formed during bisphosphorylation,
reacts
with POC13 and consequently phosphoric acid (H3PO4) is generated. The thus
formed
phosphoric acid can influence reaction conditions and can also form as an
impurity in
final product. The scheme of the reaction is as follows
POC13 + 3H20 -* 3HC1 + H3PO4
EP 1252170B describes a process for selectively producing sodium risedronate
hemipentahydrate or monohydrate comprising the steps of (a) providing an
aqueous
solution of sodium risedronate, (b) heating the aqueous solution to a
temperature from
about 45 C to about 75 C, (c) adding a solvent to the aqueous solution,
characterised
in that the solvent is selected from the group consisting of alcohols, esters,
ethers,
ketones, amides and nitriles, and (d) optionally cooling the aqueous solution.
Various forins of sodium salts of risedronic acid are also known. For example,
WO
04/037252 discloses crystalline hydrated forms of sodium risedronate, which
contain
from 6.4 up to 22 weight % of sodium based on the anhydrous substance, and in
the
case where the sodium content is lower than 7.5 weight %, then 15 to 23
weiglit % of
crystalline water is present, or in the case where the sodium content is
higher than 7.5
weight %, then 4.5 to 18 weight % of crystalline water is present.
Specifically, there
is disclosed (i) the pentahydrate of the monosodium salt, which contains from
5.5 to
7.5 weight % of sodium and 20 to 23 weight % of crystalline water, (ii) the
trihydrate of the trisodium salt, which contains from 19 to 21 weight % of
sodium and
12 to 14 weight % of crystalline water and (iii) the monohydrate of the
disodium salt,
3
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
which contains from 13 to 15 weight % of sodium and 4.5 to 6.5 weight % of
crystalline water.
WO 03/086355 describes polymorph forms B, B1, BB, C, D, E, F, G and H of
sodium
risedronate and processes of preparing these various polymorphs.
According to the present invention, there is now provided, however, a
pharmaceutically acceptable tetra-(alkali metal) salt of risedronic acid.
Pharmaceutically acceptable alkali metal salts include sodium and potassium
salts.
Specifically, there is provided by the present invention tetra-sodium
risedronate.
The present invention also provides tetra-sodium risedronate as tetra-sodium
risedronate Form I, tetra-sodium risedronate Form II and tetra-sodium
risedronate
Form III.
Tetra-sodium risedronate Form I as provided by the present invention can be
characterised as having an X-ray powder diffraction pattern, or substantially
the same
X-ray powder diffraction pattern, as shown in Figure 1.
Tetra-sodium risedronate Form I according to the present invention is further
characterised as having characteristic peaks (20): 6.33, 9.76, 11.05, 12.15,
12.65,
15.13, 16.77, 17.0, 18.99, 22.23, 22.61 and 30.53 .
Tetra-sodium risedronate Form I according to the present invention is further
characterised as having an FTIR transmission spectrum, or substantially the
same
FTIR transmission spectrum, as shown in Figure 2.
Tetra-sodium risedronate Form I according to the present invention is further
characterised as having an FTNIR reflection spectrum, or substantially the
same
FTNIR reflection spectrum, as shown in Figure 3.
4
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
Tetra-sodium risedronate Form I can be still further characterised by a
typical DSC
thermograph as shown in Figure 4. Tetra-sodium risedronate Form I has a DSC
endotherm temperature onset of about 175 C.
Tetra-sodium risedronate Form II as provided by the present invention can be
characterised as having an X-ray powder diffraction pattern, or substantially
the same
X-ray powder diffraction pattern, as shown in Figure 5.
Tetra-sodium risedronate Form II according to the present invention is further
characterised as having characteristic peaks (20): 4.33, 5.03, 5.48, 6.94,
9.94, 11.06,
11.89, 12.94, 13.16, 14.14, 16.63, 21.38 and 22.20 .
Tetra-sodium risedronate Form II according to the present invention is further
characterised as having an FTIR transmission spectrum, or substantially the
same
FTIR transmission spectrum, as shown in Figure 6.
Tetra-sodium risedronate Form II according to the present invention is
furtlzer
characterised as having an FTNIR reflection spectru.m, or substantially the
same
FTNIR reflection spectrum, as shown in Figure 7.
Tetra-sodium risedronate Form II can be still fu.rther characterised by a
typical DSC
tllermograph as shown in Figure 8. Tetra-sodium risedronate Form II has a DSC
endotherm temperature onset of about 120 C. ,
Tetra-sodium risedronate Form III as provided by the present invention can be
characterised as having an X-ray powder diffraction pattern, or substantially
the same
X-ray powder diffraction pattern, as shown in Figure 9.
Tetra-sodium risedronate Form III according to the present invention is
further
characterised as having characteristic peaks (20): 5.17, 5.57 and 7.06 .
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
Tetra-sodium risedronate Form III according to the present invention is
further
characterised as having an FTIR transmission spectrum, or substantially the
same
FTIR transmission spectrum, as shown in Figure 10.
Tetra-sodium risedronate Form III according to the present invention is
further
characterised as having an FTNIR reflection spectrum, or substantially the
same
FTNIR reflection spectrum, as shown in Figure 11.
Tetra-sodium risedronate Form III can be still further characterised by a
typical DSC
thermograph as shown in Figure 12. Tetra-sodium risedronate Form III has a DSC
endotherm temperature onset of about 80 C.
The present invention also provides a process of preparing a tetra-(alkali
metal) salt of
risedronic acid according to the present invention substantially as
hereinbefore
described, which comprises contacting risedronic free acid with a source of a
pharmaceutically acceptable alkali metal and thus converting the free acid to
a tetra-
(alkali metal) salt of risedronic acid. More particularly, a process according
to the
present invention comprises contacting a suspension of risedronic free acid
with a
source of a pharmaceutically acceptable alkali metal, adjusting the pH to
about 13 to
14, preferably about 13.4, and thereby converting the risedronic free acid to
a tetra-
(alkali metal) salt of risedronic acid according to the present invention
substantially as
hereinbefore described.
Preferably the source of the pharmaceutically acceptable alkali metal is the
corresponding alkali metal hydroxide, preferably sodium hydroxide, whereby
addition
of the hydroxide achieves adjustment to the above referred to pH range of 13
to 14.
Suitably the reaction scheme can be illustrated as follows.
N \ ~OH N 0 ONa
POH P-ONa
HO + NaOH -~ I HO +H20
/OH ~ONa
/P -OH /P -ONa
O O
6
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
In a preferred embodiment of the present invention, a process as described
herein
prepares tetra-sodium risedronate as any one of Forms I, II or III as
described herein.
Preferably, for preparation of tetra-sodium risedronate Form I, a suspension
of
risedronic free acid and water is maintained at about 20 C, followed by the
addition of
sodium hydroxide to form a solution. Suitably the pH is adjusted to about
13.4.
Preferably a C1_4alcohol, such as methanol or ethanol, is added at a
temperature of up
to about 30 C, followed by crystallization of tetra-sodium risedronate Form I
according to the present invention.
Preferably, for preparation of tetra-sodium risedronate Form II, a suspension
of
risedronic free acid and water is maintained at about 20 C, followed by the
addition of
sodium hydroxide to form a solution. Suitably the pH is adjusted to about
13.4. The
solution is heated to reflux and preferably a Cl-4alcohol, such as methanol or
ethanol,
is added under reflux. Crystallization of tetra-sodium risedronate starts
under reflux
followed by cooling and filtration to obtain tetra-sodium risedronate Form II
according to the present invention.
Preferably, for preparation of tetra-sodium risedronate Form III, a suspension
of
risedronic free acid and water is maintained at about 20 C, followed by the
addition of
sodium hydroxide to form a solution. Suitably the pH is adjusted to about
13.4. The
solution is heated to reflux and preferably a Cl-4alcohol, such as methanol or
ethanol,
is added under reflux. Crystallization of tetra-sodium risedronate starts
under reflux
followed by cooling, further addition of a C1_4alcohol and filtration to
obtain tetra-
sodium risedronate Form III according to the present invention.
Suitably risedronic acid as employed in the above reactions is prepared by the
reaction of phosphorous acid with pyridylacetic acid, optionally present as a
hydrohalide salt. Suitably the reaction is also carried out in the presence of
phosphorous trihalide and phosphorous acid is formed in situ in the reaction
mixture
by the reaction of phosphorous trihalide and water. This can be represented by
the
following reaction scheme.
7
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
(1) PX3 (excess) + H20
1
(2) PX3 (excess) + H3PO3 + HX
N
CO2H=HX
(3) PX3 (excess) + H3P03 + CO2H.HX
N~ O'' Ps OH
OH
HO + (H20 + PX3 (excess)) 30 H3PO3 + HX
,OH
-OH
0
Preferably pyridylacetic acid is employed in the form of the hydrochloride
salt and the
phosphorous trihalide employed is phosphorous trichloride.
A tetra-(alkali metal) salt of risedronic acid as provided by the present
invention has
therapeutic utility in the treatment of diseases associated with bone
resorption
disorders and more specifically in the treatment of diseases of bone and
calcium
metabolism. Such diseases include osteoporosis, hyperparathyroidism,
hypercalcemia
of malignancy, ostolytic bone metastases, myosistis ossifcans progressiva,
calcinoisis
universalis, arthritis, neuritis, bursitis, tendonitis and other inflammatory
conditions.
The present invention further provides, therefore, a pharmaceutical
conlposition
comprising a therapeutically effective dose of a tetra-(alkali metal) salt of
risedronic
acid, together with a pharmaceutically acceptable carrier, diluent or
excipient therefor.
Excipients are chosen according to the pharmaceutical form and the desired
mode of
administration.
8
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
As used herein, the term "therapeutically effective amount" means an amount a
tetra-
(alkali metal) salt of risedronic acid as prepared by the present invention,
which is
capable of preventing, ameliorating or eliminating a bone resorption disorder.
By "pharmaceutically acceptable" it is meant that the carrier, diluent or
excipient is
compatible with a tetra-(alkali metal) salt of risedronic acid as prepared by
the present
invention and is not deleterious to a recipient thereof.
In the pharmaceutical compositions of the present invention for oral,
sublingual,
subcutaneous, intramuscular, intravenous, topical, intratracheal, intranasal,
transdermal or rectal administration, a tetra-(alkali metal) salt of
risedronic acid is
administered to animals and humans in unit forms of administration, mixed with
conventional pharmaceutical carriers, for the prophylaxis or treatment of the
above
disorders or diseases. The appropriate unit forms of administration include
forms for
oral administration, such as tablets, gelatin capsules, powders, granules and
solutions
or suspensions to be taken orally, forms for sublingual, buccal, intratracheal
or
intranasal administration, forms for subcutaneous, intramuscular or
intravenous
administration and forms for rectal administration. For topical application, a
tetra-
(alkali metal) salt of risedronic acid can be used in creams, ointments or
lotions.
To achieve the desired prophylactic or therapeutic effect, the dose of a tetra-
(alkali
metal) salt of risedronic acid can vary between about 0.01 and about 50 mg per
kg of
body weight per day. Each unit dose can contain from about 0.1 to about 1000
mg,
preferably about 1 to about 500 mg, of a tetra-(alkali metal) salt of
risedronic acid, in
combination with a pharmaceutical carrier. This unit dose can be administered
1 to 5
times a day so as to administer a daily dosage of about 0.5 to about 5000 mg,
preferably about 1 to about 2500 mg.
When a solid composition in the form of tablets is prepared, a tetra-(alkali
metal) salt
of risedronic acid is mixed with a pharmaceutical vehicle such as gelatin,
starch,
lactose, magnesium stearate, talc, gum arabic or the like. The tablets can be
coated
with sucrose, a cellulose derivative or other appropriate substances, or else
they can
be treated so as to have a prolonged or delayed activity and so as to release
a
9
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
predetermined amount of active principle continuously. The use of tablets is
generally preferred for administration of a tetra-(alkali metal) salt of
risedronic acid as
provided by the present invention.
A preparation in the form of gelatin capsules can be obtained by mixing a
tetra-(alkali
metal) salt of risedronic acid with a diluent and pouring the resulting
mixture into soft
or hard gelatin capsules.
A preparation in the form of a syrup or elixir or for administration in the
form of
drops can contain a tetra-(alkali metal) salt of risedronic acid, typically in
conjunction
with a sweetener, which is preferably calorie-free, optionally antiseptics
such as
methylparaben and propylparaben, as well as a flavoring and an appropriate
color.
Water-dispersible granules or powders can contain a tetra-(alkali metal) salt
of
risedronic acid mixed with dispersants or wetting agents, or suspending agents
such as
polyvinylpyrrolidone, as well as with sweeteners or taste correctors.
Rectal administration is effected using suppositories prepared with binders
which melt
at the rectal temperature, for example polyethylene glycols.
Parenteral administration is effected using aqueous suspensions, isotonic
saline
solutions or sterile and injectable solutions which contain pharmacologically
compatible dispersants and/or wetting agents, for example propylene glycol or
butylene glycol.
A tetra-(alkali metal) salt of risedronic acid can also be formulated as
microcapsules,
with one or more carriers or additives if appropriate.
There is also provided by the present invention a tetra-(alkali metal) salt of
risedronic
acid, for use in therapy.
The present invention further provides a tetra-(alkali metal) salt of
risedronic acid, for
use in the manufacture of a medicament for the treatment of a disease state
prevented,
ameliorated or eliminated by the administration of an inhibitor of bone
resorption.
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
More specifically, the present invention provides a tetra-(alkali metal) salt
of
risedronic acid, for use in the manufacture of a medicament for the treatment
of
diseases of bone and calcium metabolism, and even more specifically for the
treatment of any one of the following: osteoporosis, hyperparathyroidism,
hypercalcemia of malignancy, ostolytic bone metastases, myosistis ossifcans
progressiva, calcinoisis universalis, arthritis, neuritis, bursitis,
tendonitis and other
inflammatory conditions.
The present invention also provides a method of treating a disease state
prevented,
ameliorated or eliminated by the administration of an inhibitor of bone
resorption in a
patient in need of such treatment, which method comprises administering to the
patient a therapeutically effective amount of a tetra-(alkali metal) salt of
risedronic
acid. More specifically, the present invention provides a method of treating
diseases
of bone and calcium metabolism, such as osteoporosis, hyperparathyroidism,
hypercalcemia of malignancy, ostolytic bone metastases, myosistis ossifcans
progressiva, calcinoisis universalis, arthritis, neuritis, bursitis,
tendonitis and other
inflammatory conditions, in a patient in need of such treatment, which method
comprises administering to the patient a therapeutically effective amount of a
tetra-
(alkali metal) salt of risedronic acid.
The present invention is now further illustrated by the following Examples and
Figures, which are for the purpose of illustration of the invention only and
are not
intended in any way to limit the scope of the present invention. It will thus
be readily
apparent to one skilled in the art that varying substitutions and
modifications may be
made to the invention disclosed herein without departing from the scope and
spirit of
the invention. Thus, it should be understood that although the present
invention has
been specifically disclosed by preferred embodiments and optional features,
modification and variation of the concepts herein disclosed may be resorted to
by
those skilled in the art, and that such modifications and variations are
considered to be
falling within the scope of the invention.
Figure 1 is an XRPD pattern of tetra-sodium risedronate Form I according to
the
present invention.
I1
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
Figure 2 is an FTIR transmission spectrum of tetra-sodium risedronate Form I
according to the present invention recorded by KBr disc and resolution 4 cm 1.
Figure 3 is an FTNIR reflection spectrum of tetra-sodium risedronate Fonn I
according to the present invention, recorded with solid probe accessories and
resolution 8 cm l.
Figure 4 is a DSC thermogram of tetra-sodium risedronate Form I according to
the
present invention, recorded at a heat rate of 10 C/min (endotherm temperature
onset is
at 175 C ).
Figure 5 is an XRPD pattern of tetra-sodium risedronate Form II according to
the
present invention.
Figure 6 is an FTIR transmission spectrum of tetra-sodium risedronate Form II
according to the present invention recorded by KBr disc and resolution 4 cm 1.
Figure 7 is an FTNIR reflection spectrum of tetra-sodium risedronate Form II
according to the present invention, recorded with solid probe accessories and
resolution 8 cm l.
Figure 8 is a DSC thermogram of tetra-sodium risedronate Form II according to
the
present invention, recorded at a heat rate of 10 C/min (endotherm temperature
onset is
at 120 C ).
Figure 9 is an XRPD pattern of tetra-sodium risedronate Form III according to
the
present invention.
Figure 10 is an FTIR transmission spectrum of tetra-sodium risedronate Form
III
according to the present invention recorded by KBr disc and resolution 4 cm"1.
Figure 11 is an FTNIR reflection spectrum of tetra-sodium risedronate Form III
according to the present invention, recorded with solid probe accessories and
resolution 8 cm 1.
12
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
Figure 12 is a DSC thermogram of tetra-sodiuin risedronate Form III according
to the
present invention, recorded at a heat rate of 10 C/min (endotherm temperature
onset is
at 80 C ).
The above referenced XRPD analysis was carried out for each of polymorphs I,
II and
III with following experimental conditions:
Samples after being powdered in a mortar and
pestle are applied directly into a Phillips'
original circular sample holder (16 mm
Sample holder preparation diameter) PW1811/16, manually pressed with
the Phillips' original sample preparation kit
PW1770/10, and closed with the Phillips'
original bottom plate PW1 811/00
Instrument Philips X'Pert PRO
Goniometer PW3050/60
Generator PW3040; 45 kV, 40 mA
X-Ray tube PW3373/00; Cu anode LFF
Focus Linear
Sample stage PW3072/60 or PW3064
Scan angle ran e(20 4- 40
Scan mode Continuous absolute scan
Step size 20) 0.016
Time per step 100 seconds
X-ray radiation CuKa
Primary soller slit 0.04 rad
Primary mask 10 mm
PDS Fixed, 1/2
Secondary soller slit 0.04 rad
Filter Ni
Detector X'Celerator (2.022 20)
Control program X'Pert Data Collector
The above referenced FTIR transmission spectra for each of polymorphs I, II
and III
were obtained by using Perkin Elmer Spectrum GX FT-IR Spectrometer (Detector:
DTGS, Beam splitter: extended KBr, Spectral Range: 4000-400cm 1, Resolution:
4cm
1, 4 scans, Samples prepared as KBr pellets).
13
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
The above referenced FTNIR reflection spectra for each of polymorphs I, II and
III
were obtained by using Bruker NIR Multi Purpose Analyser (MPA). (The spectra
were recorded in a diffuse reflectance mode using integrating sphere for
collecting
reflecting beams. The measurements were carried out over the range 4000 cm 1-
12000 cm 1, with a resolution of 8 cm"1. The spectra were averaged over 32
scans. The
system was governed via the software OPUS that includes routines for
acquisition and
processing of spectra).
The above referenced DSC thermograms for each of polymorphs I, II and III were
obtained by using a TA Instruments MDSC Q1000, where the sample was scanned at
C/min in N2 atmosphere in closed Al pan.
Example 1
100m1 of water and 15g of risedronic acid were charged to a 1000m1 three
necked
flask. The suspension was maintained at room temperature (-20 C) and the pH
was
adjusted with sodium hydroxide (40%) until a pH of about 13.4 was achieved.
800m1
of methanol were slowly added under room temperature (up to 30 C).
Crystallization
of the salt started at about 30 C, when 320m1 of methanol were added. When all
400m1 of methanol were added, the suspension was maintained at the room
temperature (-24 C) for three hours. Risedronate tetra-sodium salt, 21.17 g,
was
obtained after filtration, washed with 100ml of a water / methanol solution (1
/ 4) and
dried. Analysis carried out confirmed the risedronate tetra-sodium salt thus
prepared
to be tetra-sodium risedronate form I.
Example 2
200m1 of water and 15g of risedronic acid were charged to a 2000ml three
necked
flask. The suspension was maintained at room temperature (-20 C) and the pH
was
adjusted with sodium hydroxide (40%) until a pH of about 13.4 was achieved.
The
solution was heated to reflux (-100 C) and 800m1 of methanol were slowly added
under reflux. Crystallization of the salt started at about 78 C, when 320m1 of
methanol were added. When all 800m1 of methanol were added, the suspension was
maintained at the reflux temperature (-77 C) for five minutes, and then
allowed to
14
CA 02649489 2008-10-16
WO 2007/132138 PCT/GB2007/000792
cool. The suspension was then slowly cooled to 0-5 C over the period of two
hours
and retained for one hour at this temperature. Risedronate tetra-sodium salt,
19.58 g,
was obtained after filtration, washed with 100ml of a water / methanol cold
solution
(1 / 4) and dried. Analysis carried out confirmed the risedronate tetra-sodium
salt
thus prepared to be tetra-sodium risedronate form II.
Example 3
100m1 of water and 15g of risedronic acid were charged to a 500m1 three necked
flask. The suspension was maintained at room temperature (-20 C) and the pH
was
adjusted with sodium hydroxide (40%) until a pH of about 13.4 was achieved.
The
solution was heated to reflux (-100 C) and 300m1 of methanol were slowly added
under reflux. Crystallization of the salt started at about 78 C, when 130m1 of
methanol were added. When al1300m1 of methanol were added, the suspension was
maintained at the reflux temperature (-77 C) for five minutes, and then
allowed to
cool to 60 C, followed by addition of 100m1 of methanol. The suspension was
then
slowly cooled to 0-5 C over the period of two hours and retained for one hour
at this
temperature. Risedronate tetra-sodium salt, 20.98 g, was obtained after
filtration,
washed with 50ml of a water / methanol cold solution (1 / 4) and dried.
Analysis
carried out confirmed the risedronate tetra-sodium salt thus prepared to be
tetra-
sodium risedronate form III.