Note: Descriptions are shown in the official language in which they were submitted.
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
METHODS AND COMPOSITIONS RELATING TO ZPA POLYPEPTIDES
FIELD OF THE INVENTION
The present invention is directed to ZPA polypeptides, antibodies, nucleic
acid
molecules, antagonists, agonists, and compositions relating to ZPA
polypeptides, and
methods of making and using the same, including methods for diagnosing and
treating of
apoptosis-related disorders in mammals. The present invention is also directed
to model
systems for the intrinsic apoptotic pathway.
BACKGROUND OF THE INVENTION
Uncontrolled cell growth is the cause of many illnesses in a variety of cell
types. For
example, cancer occurs when there is an increase in the number of abnormal, or
neoplastic,
cells derived from a normal tissue that proliferate to form a tumor mass. The
tumor cells
often invade the adjacent tissues and can spread via the blood or lymphatic
system to regional
lymph nodes and to distant sites via a process called metastasis. In a
cancerous growth, a cell
proliferates under conditions in which normal cells would not grow. Cancer
manifests itself
in a wide variety of forms, characterized by different degrees of invasiveness
and
aggressiveness. Malignant tumors (cancers) are the second leading cause of
death in the
United States, after heart disease (Boring et al., CA Cancel J. Clin. 43:7
(1993)).
Much research has been devoted to discovering new treatments for cell
proliferative
disorders, such as cancer. Despite recent advances, there is a great need to
identify and
understand the role of new cellular targets for modulating cell proliferation
and to develop
alternative or more effective methods of treatment and therapeutic and
diagnostic agents.
There is also a need to develop alternative therapeutics and methods for
treating specific cell
types and for treating illnesses caused by or associated with abnormal cell
proliferation, such
as cancers. One approach to developing anti-cancer therapeutics is to study
the mechanisms
of apoptosis, also known as programmed cell death.
Robust control of the apoptotic mechanisms that determine cell fate is
required for
organism development, homeostasis, and cellular damage response. Dysregulation
of such
pathways often leads to serious diseases. For example, many cancers
selectively inhibit pro-
apoptotic pathways and/or enhance pro-survival pathways in order to evade host
responses
intended to regulate growth (Kirkin et al., Biochim Biophys Acta 1644 (2-3):
229-249
1
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
(2004); LeBlanc et al., Nature Med. 8:2 274-281 (2002); Cory and Adams, Trends
Biochem.
Sci. 26: 61-66 (2001)). Therefore, understanding and treating a variety of
diseases, including
cancer, autoimmune diseases, and degenerative disorders necessitates an
understanding of
apoptosis (Strasser, Nat. Rev. Immunol. 5: 189-200 (2005); Strasser et al.,
Biochim Biophys
Acta 1333: F151-178 (1997)).
Two apoptosis signaling pathways have been described in mammals (Anderson et
al.,
Nat. Rev. Drug Discov. 4(5): 399-409 (2005)): the extrinsic pathway, typically
initiated by
the death-inducing ligands of the TNF family, and the intrinsic pathway,
primarily
responding to intracellular stimuli mediated by the Bcl-2 family (Strasser et
al., Nat. Rev.
Imm. 5:189-200 (2005); Borner, Mol. Immunol. 39: 615-647 (2003)), but also
activated by
components of the extrinsic pathway.
The Bcl-2 proteins are characterized by four distinct alpha-helical sequence
motifs
known as the Bcl-2 homology (BH) domains BH1 to BH4. In some cases, Bcl-2
proteins
also have a C-terminal transmembrane region that localizes them to the
cytoplasmic face of
the outer mitochondrial membrane, nuclear envelope, or endoplasmic reticulum
(Borner,
Mol. Immunol. 39: 615-647 (2003)). Bcl-2 protein family members may be divided
into
three groups: (i) pro-survival Bcl-21ike proteins (e.g., Bcl-2, Bcl-xL, Bcl-w,
Mcl-1, A1/Bfl-1,
NR-13, BHRF1, LMW5-HL, ORF16, v-Bcl-2(KSHV), E1B-19K, CED-9, Boo/DIVA/Bc12-
L-10, and Bcl-B); (ii) pro-apoptotic multidomain proteins (e.g., Bax, Bak,
Bok/Mtd, and Bcl-
xs); and (iii) BH3-only pro-apoptotic proteins (e.g., Bik/Nbk, Blk, Hrk/DP5,
BNip3,
BimL/Bod, Bad, Bid, EGL-1, Noxa, Puma/Bbc3, and Bmf) (id.). Most pro-survival
members
contain all four BH domains while most multi-domain pro-apoptotic proteins
lack a BH4
domain.
In unstimulated cells, interactions between pro-survival and pro-apoptotic
multidomain family members prevent the Bax-like proteins from oligomerizing at
the
mitochondrial membrane and initiating the apoptotic program. Upon stimulation,
BH3 -only
proteins relieve the inhibition of Bax-like proteins by dimerizing with the
pro-survival
proteins, freeing the pro-apoptotic multidomain proteins to compromise
mitochondrial
membrane potential and initiate apoptosis. Previous experiments have
demonstrated that
several members of the Bcl-2 family are critical for normal development
(Lindsten et al.,
Mol. Cell. 6: 1389-1399 (2000); Motoyama et al., Science 267: 1506-1510
(1995); Veis et al.,
Ce1175: 229-240 (1993); Rinkenberger et al., Genes Dev. 14: 23-27 (2000)).
However, the
function of this protein family during development is largely unknown: even in
Bcl-2-related
2
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
gene knockouts with significant developmental effects, neither the initiating
apoptotic signal
nor the BH3-only proteins activated in response to the signal are known.
A number of novel Bc1-2 family members have been identified in vertebrates by
sequence similarity to Bcl-2, the eponymous member of the family named for its
role in B
cell lymphoma (Tsujimoto et al., Science 228: 1440-1443 (1985)). However,
because Bcl-2
family members are critical to development and regulation, aberrations in or
deletions of one
or more members of this family of proteins often cause pathologies which
prevent a
characterization of their functional importance, or result in nonviable
animals in the first
instance. A model system in which developmental and regulatory changes could
be
monitored from the earliest stages of growth would provide a crucial tool for
addressing
questions regarding the roles of the Bcl-2 family of genes in apoptosis.
Zebrafish (Danio rerio) have served as a useful model system for a variety of
biological pathways. Zebrafish can serve as an exceptional model for studying
apoptosis not
only because development in the fish is rapid, and zebrafish embryos remain
transparent
throughout most of embryogenesis, but also because of the availability of
mutant zebrafish
lines displaying abnormal apoptosis (see, e.g., Cole and Ross, Devel. Biol.
240: 123-142
(2001)). Apoptosis patterns have been examined in zebrafish, so detection of
apoptotic cells
and the general dynamics of apoptosis are known in that organism (id.).
However, the
biochemical pathways responsible for those apoptotic patterns in zebrafish
have not been
characterized. It remains an open question whether the intrinsic apoptotic
pathway functions
in the zebrafish.
A prerequisite to establishing zebrafish as a model for apoptotic signaling
through the
intrinsic pathway is a demonstration that the major members of the Bcl-2
family are present
in the zebrafish. Several studies have tried to identify Bcl-2 family members
in zebrafish.
Inohara and Nunez found many zebrafish genes homologous to mammalian and avian
extrinsic pathway members such as the caspases, but only identified eight
zebrafish genes
putatively related to only six members of the intrinsic pathway Bcl-2 family
(Bcl-xL, Mcl-1,
NR-13, Bax, BNIP3, and Bad) (Inohara and Nunez, Cell Death Diff. 7: 509-510
(2000)).
Coultas et al. exhaustively searched the zebrafish non-redundant and EST
Genbank databases
by tblastn and identified only three further BH3 -only Bcl-2 family members:
Bid, Noxa, and
Bmf (Cell Death Differ. 9: 1163-1166 (2002)). In fact, that group particularly
commented on
the failure to identify Bik, Bim, and Puma in zebrafish using translated BLAST
searching
(id.). This observation was recently confirmed by Aouacheria et al. after
exhaustively
3
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
searching Ensembl and GenBank nucleotide and protein sequences using PSI-BLAST
and
tblastn (Mol. Biol. Evol. 22(12): 2395-416 (2005)).
SUMMARY OF THE INVENTION
The present invention provides new model systems for investigating apoptosis
in vivo
and in vitro, and provides methods for identifying agents that modulate
apoptosis. The
present invention also provides new therapeutic agents, diagnostic agents, and
methods for
treating or preventing apoptosis-related disease, including cancer, by
targeting apoptosis,
particularly the intrinsic apoptotic pathway.
In certain embodiments, the invention provides zebrafish pro-apoptosis ("ZPA")
polypeptides and polynucleotides. In one embodiment, a polypeptide having an
amino acid
sequence selected from SEQ ID NOs: 1, 5, 7, and 9 is provided, wherein the
polypeptide is a
zebrafish Bcl-2-related ("B2R") pro-apoptotic polypeptide. In another
embodiment, a
polypeptide having an amino acid sequence of SEQ ID NO: 1 is provided, wherein
the
polypeptide is a zebrafish B2R multidomain pro-apoptotic polypeptide. In
another
embodiment, a polypeptide having an amino acid selected from SEQ ID NOs: 5, 7,
and 9 is
provided, wherein the polypeptide is a zebrafish B2R BH3-only pro-apoptotic
polypeptide.
In another embodiment, a polynucleotide having a nucleotide sequence selected
from SEQ ID
NOs: 2, 6, 8, and 10 is provided, wherein the polynucleotide encodes a
zebrafish B2R pro-
apoptotic polypeptide. In another embodiment, a polynucleotide having a
nucleotide
sequence of SEQ ID NO: 1 is provided, wherein the polynucleotide encodes a
zebrafish B2R
multidomain pro-apoptotic polypeptide. In another embodiment, a polynucleotide
having a
nucleotide sequence selected from SEQ ID NOs: 6, 8, and 10 is provided,
wherein the
polynucleotide encodes a zebrafish B2R BH3-only pro-apoptotic polypeptide.
In other embodiments, the invention provides zebrafish transgenic for one or
more
apoptosis-related proteins. In one embodiment, a transgenic zebrafish is
provided, wherein
one or more polynucleotides selected from SEQ ID NOs: 2, 6, 8, and 10 is
deleted. In
another embodiment, a transgenic zebrafish is provided, wherein the expression
of one or
more polynucleotides selected from SEQ ID NOs: 2, 6, 8, and 10 is modulated
relative to the
expression of the one or more polynucleotides in a wild-type zebrafish. In one
aspect, the
expression is increased. In another aspect, the expression is decreased. In
another
embodiment, a transgenic zebrafish is provided, wherein one or more
polypeptides selected
from SEQ ID NOs: 1, 5, 7, and 9 are not expressed. In another embodiment, a
transgenic
4
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zebrafish is provided, wherein the expression of one or more polypeptides
selected from SEQ
ID NOs: 1, 5, 7, and 9 is modulated relative to the expression of the one or
more polypeptides
in a wild-type zebrafish. In one aspect, the expression is increased. In
another aspect, the
expression is decreased.
In another embodiment, a transgenic zebrafish is provided, wherein one or more
endogenous B2R genes are replaced with a B2R gene counterpart from another
organism. In
one aspect, the counterpart is mammalian. In another aspect, the counterpart
is human. In
another aspect, all of the endogenous B2R genes are replaced with B2R gene
counterparts
from another organism. In one aspect, the counterpart is mammalian. In another
aspect, the
counterpart is human. In another embodiment, a transgenic zebrafish is
provided, wherein
one or more endogenous intrinsic apoptotic pathway genes are replaced with an
intrinsic
apoptotic pathway gene counterpart from another organism. In one aspect, the
counterpart is
mammalian. In another aspect, the counterpart is human. In another aspect, the
one or more
endogenous intrinsic apoptotic pathway genes are selected from SEQ ID NOs: 2,
6, 8, and 10.
In another embodiment, a transgenic zebrafish is provided, wherein all of the
endogenous
intrinsic apoptotic pathway genes are replaced with intrinsic apoptotic
pathway gene
counterparts from another organism. In one aspect, the counterpart is
mammalian. In
another aspect, the counterpart is human. In another aspect, the endogenous
intrinsic
apoptotic pathway genes include SEQ ID NOs: 2, 6, 8, and 10.
In certain embodiments, the invention provides model systems for apoptosis. In
one
embodiment, a model system for apoptosis is provided comprising a zebrafish as
described in
any of the previous embodiments. In one aspect, the model system is a model
system for the
intrinsic apoptotic pathway. In another embodiment, an in vitro model system
for apoptosis
is provided comprising at least one polypeptide encoded by an amino acid
sequence selected
from SEQ ID NOs: 1, 5, 7, and 9. In one aspect, the model system is a model
system for the
intrinsic apoptotic pathway. In another aspect, the model system is a model
system for the
extrinsic apoptotic pathway. In another embodiment, an in vitro model system
for apoptosis
is provided comprising at least one polynucleotide encoded by a nucleotide
sequence selected
from SEQ ID NOs: 2, 6, 8, and 10. In one aspect, the model system is a model
system for the
intrinsic apoptotic pathway. In another aspect, the model system is a model
system for the
extrinsic apoptotic pathway.
In certain embodiments, the invention provides methods of identifying a
compound
that binds to a ZPA polypeptide, comprising contacting a ZPA polypeptide with
a compound
5
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
and determining whether the compound binds to the ZPA polypeptide. In certain
embodiments, the invention provides methods for identifying a compound which
modulates
the activity of a ZPA polypeptide, comprising contacting a ZPA polypeptide
with a
compound and determining whether the compound modulates the activity of the
ZPA
polypeptide.
In certain embodiments, the invention provides methods for identifying agents
that
modulate apoptosis. In one embodiment, a method for identifying an agent for
reducing or
preventing apoptosis is provided, comprising administering at least one agent
to a zebrafish
and determining whether apoptosis is reduced or prevented. In one aspect, the
method further
comprises determining the presence or amount of apoptosis in the zebrafish
prior to
administering the at least one agent. In another aspect, the method further
comprises
stimulating apoptosis in the zebrafish prior to administering the at least one
agent. In another
aspect, the agent reduces or prevents apoptosis through the intrinsic
apoptotic pathway. In
another aspect, the agent reduces or prevents apoptosis through the extrinsic
apoptotic
pathway. In another aspect, the expression and/or activity of one or more B2R
proteins in the
zebrafish is increased relative to the expression or activity of the one or
more B2R proteins in
a wild-type zebrafish. In another aspect, one or more B2R proteins is not
expressed in the
zebrafish. In another aspect, the expression and/or activity of one or more
B2R proteins is
reduced in the zebrafish relative to the expression and/or activity of the one
or more B2R
proteins in a wild-type zebrafish. In another aspect, the agent is selected
from an antibody,
an antigen-binding antibody fragment, an aptamer, and a small molecule. In
another aspect,
the zebrafish is a larval zebrafish. In another aspect, the determining step
comprises
microscopic examination of cell viability. In another aspect, the determining
step comprises
determining caspase activation.
In another embodiment, a method for identifying an agent for initiating and/or
stimulating apoptosis is provided, comprising administering at least one agent
to a zebrafish
and determining whether apoptosis is initiated or increased. In one aspect,
the method further
comprises determining the presence or amount of apoptosis in the zebrafish
prior to
administering the at least one agent. In another aspect, the method further
comprises
preventing and/or decreasing apoptosis in the zebrafish prior to administering
the at least one
agent. In another aspect, the agent initiates and/or stimulates apoptosis
through the intrinsic
apoptotic pathway. In another aspect, the agent initiates and/or stimulates
apoptosis through
the extrinsic apoptotic pathway. In another aspect, the expression and/or
activity of one or
6
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
more B2R proteins in the zebrafish is increased relative to the expression or
activity of the
one or more B2R proteins in a wild-type zebrafish. In another aspect, one or
more B2R
proteins is not expressed in the zebrafish. In another aspect, the expression
and/or activity of
one or more B2R proteins is reduced in the zebrafish relative to the
expression and/or activity
of the one or more B2R proteins in a wild-type zebrafish. In another aspect,
the agent is
selected from an antibody, an antigen-binding antibody fragment, an aptamer,
and a small
molecule. In another aspect, the zebrafish is a larval zebrafish. In another
aspect, the
determining step comprises microscopic examination of cell viability. In
another aspect, the
determining step comprises determining caspase activation.
In certain embodiments, the invention provides further methods for identifying
agents
for modulating apoptosis. In one embodiment, a method for identifying an agent
for
preventing or decreasing apoptosis is provided, comprising contacting at least
one
polypeptide encoded by an amino acid sequence selected from SEQ ID NOs: 1, 5,
7, and 9
with the agent and determining the ability of the agent to block or decrease
activity of the at
least one polypeptide. In another embodiment, a method for identifying an
agent for
preventing or decreasing apoptosis is provided, comprising contacting a cell
comprising at
least one polynucleotide encoded by a nucleotide sequence selected from SEQ ID
NOs: 2, 6,
8, and 10 with the agent and determining the ability of the agent to prevent
or decrease
expression of the at least one polynucleotide.
In another embodiment, a method for identifying an agent for initiating or
stimulating
apoptosis is provided, comprising contacting at least one polypeptide encoded
by an amino
acid sequence selected fromSEQ ID NOs: 1, 5, 7, and 9 with the agent and
determining the
ability of the agent to stimulate or increase activity of the at least one
polypeptide. In one
embodiment, a method for identifying an agent for initiating or stimulating
apoptosis,
comprising contacting a cell comprising at least one polynucleotide encoded by
a nucleotide
sequence selected from SEQ ID NOs: 2, 6, 8, and 10 with the agent and
determining the
ability of the agent to stimulate or increase expression of the at least one
polynucleotide.
In certain embodiments, the invention provides methods of treatment. In one
embodiment, a method of treating an apoptosis-related disorder is provided,
comprising
administering to a patient at least one polypeptide encoded by an amino acid
sequence
selected from SEQ ID NOs: 1, 5, 7, and 9. In another embodiment, a method of
treating an
apoptosis-related disorder is provided, comprising administering to a patient
in need of such
treatment an effective amount of at least one polypeptide encoded by an amino
acid sequence
7
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
selected from SEQ ID NOs: 1, 5, 7, and 9, whereby the apoptosis-related
disorder is treated in
the patient. In another embodiment, a method of treating an apoptosis-related
disorder is
provided, comprising administering to a patient an agonist of at least one
polypeptide
encoded by an amino acid sequence selected from SEQ ID NOs: 1, 5, 7, and 9. In
another
embodiment, a method of treating an apoptosis-related disorder is provided,
comprising
administering to a patient in need of such treatment an effective amount of an
agonist of at
least one polypeptide encoded by an amino acid sequence selected from SEQ ID
NOs: 1, 5, 7,
and 9, whereby the apoptosis-related disorder is treated in the patient. In
another
embodiment, a method of treating an apoptosis-related disorder is provided,
comprising
administering to a patient an antagonist of at least one polypeptide encoded
by an amino acid
sequence selected from SEQ ID NOs: 1, 5, 7, and 9. In another embodiment, a
method of
treating an apoptosis-related disorder is provided, comprising administering
to a patient in
need of such treatment an effective amount of an antagonist of at least one
polypeptide
encoded by an amino acid sequence selected from SEQ ID NOs: 1, 5, 7, and 9,
whereby the
apoptosis-related disorder is treated in the patient. In one aspect, the
antagonist is selected
from an aptamer, an antibody, an antigen-binding antibody fragment, and a
small molecule.
In another aspect, the apoptosis-related disorder is selected from a cell
proliferative disorder,
a viral apoptosis disorder, an autoimmune disorder, a hematologic disorder,
and a
neurological disorder. In one aspect, the apoptosis-related disorder is
cancer. In another
embodiment, a method of treating an apoptosis-related disorder is provided,
comprising
administering to a patient at least one polypeptide selected from the group of
polypeptides
encoded by the polynucleotide sequences of SEQ ID NOs: 2, 6, 8, and 10. In one
aspect, the
apoptosis-related disorder is selected from a cell proliferative disorder, a
viral apoptosis
disorder, an autoimmune disorder, a hematologic disorder, and a neurological
disorder. In
one aspect, the apoptosis-related disorder is cancer.
In certain embodiments, the invention provides compositions for modulating
apoptosis. In one embodiment, a composition for increasing apoptosis is
provided,
comprising a polypeptide encoded by an amino acid sequence selected from SEQ
ID NOs: 1,
5, 7, and 9. In one aspect, the composition further comprises a
pharmaceutically-acceptable
carrier. In another embodiment, a composition for increasing apoptosis is
provided,
comprising an agonist of a polypeptide encoded by an amino acid sequence
selected from
SEQ ID NOs: 1, 5, 7, and 9. In another embodiment, a composition for reducing
or
preventing apoptosis is provided, comprising an antagonist of one or more of
SEQ ID NOs:
8
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
1, 5, 7, and 9. In one aspect, the antagonist is selected from an antibody, an
antigen-binding
antibody fragment, an aptamer, and a small molecule. In another aspect, the
composition
further comprises a pharmaceutically-acceptable carrier. In another
embodiment, a
composition for reducing or preventing apoptosis is provided, comprising an
agent that
reduces or inhibits expression of one or more of SEQ ID NOs: 2, 6, 8, and 10.
In one aspect,
the composition further comprises a pharmaceutically-acceptable carrier.
In certain embodiments, the invention provides methods of treating an
apoptosis-
related disorder in a subject in need of treatment, comprising administering
at least one of the
compositions of the invention. In certain embodiments, the invention provides
methods of
treating an apoptosis-related disorder in a subject in need of treatment,
comprising
administering an effective amount of at least one of the compositions of the
invention,
whereby the apoptosis-related disorder is treated in the patient. In certain
aspects, the
apoptosis-related disorder is selected from a cell proliferative disorder, a
viral apoptosis
disorder, an autoimmune disorder, a hematologic disorder, and a neurological
disorder.
In certain embodiments, the invention provides methods of detecting the
presence,
severity, and/or predisposition to an apoptosis-related disorder in a subject.
In one
embodiment, the presence of an apoptosis-related disorder is detected by
detecting the
presence or amount of a ZPA polypeptide in cells from the subject. In another
embodiment, a
predisposition to an apoptosis-related disorder is detected by detecting the
presence or
amount of a ZPA polypeptide in cells from the subject. In another embodiment,
the severity
of an apoptosis-related disorder is detected by detecting the presence or
amount of a ZPA
polypeptide in cells from the subject. In another embodiment, the presence of
an apoptosis-
related disorder is detected by detecting the presence or amount of a ZPA
polypeptide
homolog in cells from the subject. In another embodiment, a predisposition to
an apoptosis-
related disorder is detected by detecting the presence or amount of a ZPA
polypeptide
homolog in cells from the subject. In another embodiment, the severity of an
apoptosis-
related disorder is detected by detecting the presence or amount of a ZPA
polypeptide
homolog in cells from the subject. In certain aspects, the apoptosis-related
disorder is
selected from a cell proliferative disorder, a viral apoptosis disorder, an
autoimmune disorder,
a hematologic disorder, and a neurological disorder.
In another embodiment, the presence of an apoptosis-related disorder is
detected by
detecting the presence or amount of expression of a ZPA polynucleotide in
cells from the
subject. In another embodiment, a predisposition to an apoptosis-related
disorder is detected
9
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
by detecting the presence or amount of expression of a ZPA polynucleotide in
cells from the
subject. In another embodiment, the severity of an apoptosis-related disorder
is detected by
detecting the presence or amount of expression of a ZPA polynucleotide in
cells from the
subject. In another embodiment, the presence of an apoptosis-related disorder
is detected by
detecting the presence or amount of expression of a ZPA polynucleotide homolog
in cells
from the subject. In another embodiment, a predisposition to an apoptosis-
related disorder is
detected by detecting the presence or amount of expression of a ZPA
polynucleotide homolog
in cells from the subject. In another embodiment, the severity of an apoptosis-
related
disorder is detected by detecting the presence or amount of expression of a
ZPA
polynucleotide homolog in cells from the subject. In certain aspects, the
apoptosis-related
disorder is selected from a cell proliferative disorder, a viral apoptosis
disorder, an
autoimmune disorder, a hematologic disorder, and a neurological disorder.
In certain embodiments, the invention also provides kits and articles of
manufacture
for the compounds and compositions described herein, in any useful
combination. In one
embodiment, a kit is provided comprising one or more of the compositions of
the invention
and instructions for use. In one aspect the use is a therapeutic use. In
another aspect the use
is a diagnostic use. In another aspect the use is a research use. In another
embodiment, a kit
is provided comprising an in vitro intrinsic apoptotic pathway model system
and instructions
for its use. In one aspect the use is a diagnostic use. In another aspect the
use is a research
use. In another embodiment, a kit is provided comprising a zebrafish intrinsic
apoptotic
pathway model system and instructions for its use. In one aspect the use is a
diagnostic use.
In another aspect the use is a research use. In another embodiment, the
invention provides an
article of manufacture comprising: (a) a composition comprising one or more
ZPA
polypeptides, agonists, and antagonists, (b) a container containing said
composition; and (c) a
label affixed to the container, or a package insert included in the container
referring to the use
of the composition in the treatment of an apoptosis-related disorder.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows precision-recall plots for Hidden Markov Models (HMM)
constructed
from PROSITE patterns and matrices, as described in Example 1(b). The
precision and recall
of PROSITE patterns are indicated by diamonds; those for pattern-derived HMMs
at various
scores are plotted as lines; and hourglasses denote the precision and recall
at the HMM score
thresholds used herein. Figure 2 shows an alignment of the BH3 domains of
known and
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
candidate Bcl-2-related ("B2R") proteins, as described in Example 2. Amino
acids with
similar physicochemical properties are shaded similarly, in accordance with
standard
ClustalX color patterns.
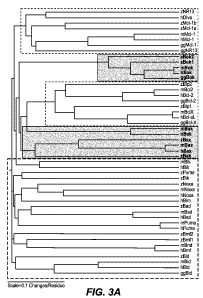
Figure 3A depicts an alignment of known and candidate zebrafish B2R proteins
with
human (h), mouse (m), and chicken (gg) counterparts, as described in Example
2(g). Pro-
survival proteins appear as the topmost and middle unshaded sections; BH3 -
only proteins
appear as the bottom-most unshaded section; and the remaining shaded sections
are
multidomain pro-apoptotic proteins. Figure 3B shows an alignment of the BH3
domains of
human, mouse, and zebrafish BH3 -only proteins grouped according to gene, as
discussed in
Example 2. Amino acids with similar physicochemical properties are shaded
similarly.
Figures 3C and 3D depict the results of experiments described in Example 3(a).
Figure 3C
depicts the electrophoretic results of stage-specific RT-PCR, showing that
most zebrafish
Bcl-2 family members were expressed at consistent levels from the maternal
contribution
unti172 hours post fertilization (hpf). Figure 3D depicts the electrophoretic
results of tissue-
specific RT-PCR, showing expression of many zebrafish Bcl-2 family members in
a variety
of adult zebrafish tissues.
Figures 4A and 4B depict graphs showing the results of ectopic zebrafish B2R
protein
expression in vivo, as described in Example 3(b). With the exception of zBad,
zBokl, and
zBok2, ectopic expression of each pro-apoptotic zBcl-2 family member induced
death in a
dose-dependent manner. Figure 4C shows brightfield microscopic images (left
panels) and
immunofluorescent staining for activated caspase-3 (right panels) in zebrafish
embryos
injected with synthetic zebrafish B2R proteins or a green fluorescent protein
(GFP) control,
as described in Example 3(b). Figure 4D shows a graphical depiction of the
data obtained
from experiments described in Example 3(c). The percent of surviving embryos
is plotted for
the indicated combinations of ectopically expressed zebrafish B2R proteins.
The top of the
graph shows the total number of embryos examined for each combination.
Figures 5A-5F depict the results of experiments described in Example 3(d).
Figure
5A shows immunostaining for caspase-3 activity in untreated (left panels) or
gamma-
irradiated (right panels) zebrafish embryos ectopically expressing one of the
zebrafish pro-
survival B2R proteins (zBlpl, zMcl-la, zMcl-lb, or zBlp2) or a control (WT (no
injection) or
GFP). Figure 5B shows immunostaining for caspase-3 activity in untreated (left
panels) or
gamma-irradiated (right panels) zebrafish injected with a morpholino to p53 or
a control
morpholino alone or in combination with morpholinos to zBax or zBak. Figure 5C
shows a
11
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
graph quantifying the fluorescence from zBax and zBak single and double
knockdowns.
Figure 5D shows immunostaining for caspase-3 activity in untreated (left
panels) or gamma-
irradiated (right panels) zebrafish embryos injected with a control morpholino
or with a
morpholino to a zebrafish BH3-only B2R protein (zBid, zBadl, zBmfl, zNoxa,
zPuma, or
zBik). Figure 5E shows immunostaining for caspase-3 activity in untreated
(left panels) or
gamma-irradiated (right panels) zebrafish embryos uninjected or injected with
a control
morpholino or a morpholino against p53. Figure 5F graphically depicts the
results of
quantitative PCR analysis of the increase in zPuma or zNoxa transcription in
gamma-
irradiated zebrafish embryos untreated or treated with a control or p53
morpholino.
Figure 6A depicts the results of experiments described in Example 3(e). The
figure
shows a graph depicting the percent survival of zebrafish embryos subjected to
morpholino
knockdown of zMcl-la, zMcl-lb, and/or B1p2. Figures 6B and 6C depict the
results of
experiments described in Example 3(f). The figures show graphs depicting the
percent
survival of zebrafish embryos subjected to morpholino knockdown of zMcl-la
and/or zMcl-
lb and Apo2L-induced apoptosis (either with zebrafish Apo2L ortholog DL1b, or
with
another Apo2L pathway-related molecule such as zDLl a, zDL2, zDL3, zTNF 1,
zTNF2, or
zFasL).
DETAILED DESCRIPTION OF THE INVENTION
Applicants, using customized searching techniques, have identified five
zebrafish
genes previously unknown to be related to the Bcl-2 family of proteins, four
of which
represent Bcl-2 family members not previously identified in the zebrafish:
Bak, Bik, Puma,
and Bim. Applicants also herein characterize for the first time the functional
activities of
certain zebrafish Bcl-2-related ("B2R") proteins, and demonstrate the
existence and function
of the intrinsic apoptotic pathway in zebrafish, and the utility of the
zebrafish as a model
system for the intrinsic apoptotic pathway. Applicants' invention permits the
identification
of new agents and therapeutics to prevent, decrease, initiate and/or stimulate
apoptosis and
new methods of studying the role of the Bcl-2 genes and/or the intrinsic
apoptotic pathway in
apoptosis-related disorders. Applicants' invention also provides new
therapeutics for and
methods of treating diseases or disorders associated with or caused by
aberrant apoptosis.
As described herein, SEQ ID NOs: 1, 3, 5, 7, and 9 (encoded by, respectively,
SEQ
ID NOs: 2, 4, 6, 8, and 10) are homologous to certain human members of the Bcl-
2 family of
proteins involved in the intrinsic apoptotic pathway. SEQ ID NO: 1 is a
zebrafish protein
12
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
with sequence identity to human Bak, a multidomain pro-apoptotic protein. SEQ
ID NO: 3 is
a zebrafish protein with sequence identity to human Bad, a BH3-only pro-
apoptotic protein.
SEQ ID NO: 5 is a zebrafish protein with sequence identity to human Bik, a BH3-
only pro-
apoptotic protein. SEQ ID NO: 7 is a zebrafish protein with sequence identity
to human
Puma, a BH3 -only pro-apoptotic protein. SEQ ID NO: 9 is a zebrafish protein
with sequence
identity to Bmf, a BH3 -only pro-apoptotic protein. Applicants have also
identified a
zebrafish homolog of human Bim, a BH3 -only pro-apoptotic protein, but, as
described in
Example 2(d), the gene could not be cloned due to an apparent error in the
current
construction of the zebrafish genome.
SEQ ID NOs: 2, 6, 8, and 10 (encoding the proteins of SEQ ID NOs: 1, 5, 7, and
9)
were previously identified as part of the zebrafish genome project, but until
Applicants' work
had not been (1) identified as encoding homologs of human Bcl-2 family
members, or (2)
implicated as encoding members of one or more apoptosis pathways. Applicants
identified
SEQ ID NOs: 1, 5, 7, and 9 as zebrafish homologs of human Bak, Bik, Puma, and
Bmf,
respectively, as described herein, by both sequence identity/similarity and by
functional
analysis.
The invention therefore provides in one embodiment proteins selected from SEQ
ID
NOs: 1, 3, 5, 7, and 9 which are zebrafish B2R multidomain or BH3-only pro-
apoptotic
proteins, compositions containing them, and methods of using the proteins and
compositions.
The invention also provides in another embodiment polynucleotides selected
from SEQ ID
NOs: 2, 4, 6, 8, and 10 which encode zebrafish B2R multidomain or BH3-only pro-
apoptotic
proteins, compositions containing them, and methods of using the
polynucleotides and
compositions. In another embodiment, variant proteins are provided comprising
one or more
amino acid additions, deletions, or mutations from a sequence selected from
SEQ ID NOs: 1,
3, 5, 7, and 9. In another embodiment, variant polynucleotides are provided
comprising one
or more nucleotide additions, deletions, or mutations from a sequence selected
from SEQ ID
NOs: 2, 4, 6, 8, and 10.
The proteins, variant proteins, nucleic acids, and variant nucleic acids of
the invention
may be used for therapeutic purposes. For example, one or more of the ZPA
("zebrafish pro-
apoptosis") proteins of the invention or variants thereof may be used as a
therapeutic to treat
an apoptosis-related disorder in which increased apoptosis is desirable (e.g.,
a cellular
proliferation disorder). The invention also provides compositions comprising
one or more
ZPA proteins of the invention and a pharmaceutically acceptable carrier,
optionally including
13
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
one or more additional therapeutic agents. In another embodiment, one or more
of the ZPA
nucleic acids of the invention or variants thereof may be used as a
therapeutic to treat an
apoptosis-related disorder in which increased apoptosis is desirable, e.g., by
expressing the
nucleic acid in a subject in need of such treatment such that one or more ZPA
proteins is
expressed in the patient's cells. Zebrafish proteins and nucleic acids may be
preferred for use
as a therapeutic over any mammalian homologs, e.g., because of a lesser risk
of triggering
anti-self reactions.
The ZPA proteins of the invention also find utility in methods of identifying
agents to
initiate, stimulate, inhibit, or block apoptosis. Agonists for one or more ZPA
proteins can be
identified by their ability to initiate or stimulate the activity of the one
or more ZPA proteins
in the intrinsic apoptotic pathway. Such stimulation may be, e.g., by
activating the ZPA
protein or by interfering with one or more molecules that normally inhibit ZPA
protein
activity, and suitable agonists include, but are not limited to, antibodies
and small molecules.
Conversely, antagonists for one or more ZPA proteins can be identified by
their ability to
block or inhibit the activity of the one or more ZPA proteins in the intrinsic
apoptotic
pathway. Such inhibition may be, e.g., by prevention of the ZPA protein
binding to one or
more ligands or targets, or by prevention of the activity of the ZPA protein
itself, and suitable
antagonists include antibodies and antigen-binding fragments thereof,
aptamers, and small
molecules. Certain appropriate assays to measure ZPA protein activity in the
intrinsic
apoptosis pathway are described herein. The ZPA protein agonists may be used
as
therapeutics to treat an apoptosis-related disorder in which increased
apoptosis is desirable,
and the ZPA protein antagonists may be used as therapeutics to treat an
apoptosis-related
disorder in which decreased apoptosis is desirable.
The intrinsic apoptotic pathway responds to intracellular signals directing
programmed cell death. Dysregulation of this pathway can lead to inappropriate
apoptosis or
an inappropriate lack of apoptosis, either of which may result in disorders
such as cancer.
Thus, a greater understanding is needed of the apoptotic pathway and model
systems in
which the expression and/or activity of one or more pathway components can be
perturbed
and the repercussions readily examined. In addition to the identification and
analysis of the
ZPA proteins described herein, Applicants also have demonstrated that an
intrinsic apoptotic
pathway exists in zebrafish similar to the intrinsic apoptotic pathway
previously characterized
in mammals.
14
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Thus, the invention also provides methods of using the zebrafish as a model
system
for studying apoptosis. In some embodiments, transgenic zebrafish are
provided, in which
the expression and/or activity of one or more ZPA proteins is modulated
relative to a wild-
type zebrafish. Such transgenic zebrafish may serve to elucidate the normal
operation of
zebrafish apoptosis pathways, and also provide a tool for use in screening for
agents having
agonistic or antagonistic apoptotic activity. In other embodiments, the
invention provides
transgenic zebrafish in which one or more ZPA proteins are replaced with their
counterparts
from other organisms, thereby creating a model system to assess whether and to
what degree
cofactors, environmental factors, or modifications in sequence and structure
impact the
functioning of a particular apoptotic pathway component. In some embodiments,
all of the
zebrafish intrinsic apoptotic pathway proteins (i.e., all of the B2R proteins)
are genetically
replaced by intrinsic apoptotic pathway components from another organism
(i.e., mammalian
or human). Such transgenic zebrafish provide a tool for studying the intrinsic
apoptotic
pathway that can be examined and manipulated far more readily than it could in
the other
organism.
In some embodiments, it may be useful to examine the biochemical interactions
between intrinsic apoptotic pathway members in the absence of other pathways
or stimuli that
might interfere with the analysis. Thus, the invention also provides in vitro
model systems,
whereby the zebrafish intrinsic apoptotic pathway is reconstituted in vitro,
optionally with
one or more cofactors, reagents, inhibitors, and/or stimulators. In one
aspect, the in vitro
model system comprises one or more ZPA proteins modified in activity or
amount. In
another aspect, the in vitro model system comprises one or more B2R proteins
modified in
activity or amount. In another aspect, the in vitro model system comprises one
or more ZPA
protein variants. In another aspect, the in vitro model system comprises one
or more B2R
protein variants. In another aspect, the in vitro model system lacks at least
one ZPA protein.
In another aspect, the in vitro model system lacks at least one B2R protein.
In another aspect,
at least one ZPA protein is replaced with a counterpart protein from another
organism. In
another aspect, at least one B2R protein is replaced with a counterpart
protein from another
organism.
The ZPA proteins and nucleic acids described herein also find use in detecting
an
apoptosis-related disorder in a subject. In one embodiment, the presence of an
apoptosis-
related disorder is detected by detecting the presence or amount of a ZPA
polypeptide or a
ZPA polypeptide homolog in cells from the subject. In another embodiment, a
predisposition
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
to an apoptosis-related disorder is detected by detecting the presence or
amount of a ZPA
polypeptide or a ZPA polypeptide homolog in cells from the subject. In another
embodiment,
the severity of an apoptosis-related disorder is detected by detecting the
presence or amount
of a ZPA polypeptide or a ZPA polypeptide homolog in cells from the subject.
In another embodiment, the presence of an apoptosis-related disorder is
detected by
detecting the presence or amount of expression of a ZPA polynucleotide or a
ZPA
polynucleotide homolog in cells from the subject. In another embodiment, a
predisposition to
an apoptosis-related disorder is detected by detecting the presence or amount
of expression of
a ZPA polynucleotide or a ZPA polynucleotide homolog in cells from the
subject. In another
embodiment, the severity of an apoptosis-related disorder is detected by
detecting the
presence or amount of expression of a ZPA polynucleotide or a ZPA
polynucleotide homolog
in cells from the subject.
The invention also provides kits and articles of manufacture for the compounds
and
compositions described herein, in any useful combination. For example, a kit
is provided
comprising one or more of the compositions of the invention and instructions
for use, e.g.,
therapeutic, diagnostic, and/or research use. In another example, a kit is
provided comprising
an in vitro or zebrafish intrinsic apoptotic pathway model system and
instructions for its use
in research or screening for agents to modulate apoptosis.
Details of these methods, compositions, model systems, kits, and articles of
manufacture are provided herein.
Definitions
The terms "Bcl-2-related protein", "Bcl-2-related polypeptide" and "B2R
protein" as
used herein include native sequence polypeptides, polypeptide variants and
fragments of
native sequence polypeptides and polypeptide variants (which are further
defined herein),
unless specified otherwise. B2R proteins can be obtained from various species,
e.g., humans,
by using antibodies according to this invention or by recombinant or synthetic
methods,
including using deposited nucleic acid molecules. In certain embodiments, B2R
proteins are
obtained from zebrafish. When obtained from zebrafish, B2R proteins are
designated as
"zB2R proteins." B2R proteins include, but are not limited to, Bcl-2-like
survival factors
(including, but not limited to, Bc12, Bcl-xL, Bcl-w, Mcl-1, A1/Bfl-1, NR-13,
BHRF1,
LMW5-HL, ORF16, v-Bcl-2(KSHV), E1B-19K, CED-9, Boo/DIVA/Bc12-L-10, Bcl-B); to
pro-apoptotic multidomain factors (including, but not limited to, Bax, BpR,
Bak, Bok/Mtd,
16
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Bcl-Rambo, Bcl-xs, and Bcl-G); and to pro-apoptotic BH3-only factors
(including, but not
limited to, Bik/Nbk, Blk, Hrk/DP5, BNIP3, BimL/Bod, Bad, Bid, EGL-1, Noxa,
PUMA/Bbc3, Bmf, Bnipl, Bnip2, and Bnip3). zB2R proteins include, but are not
limited to,
pro-survival factors (including, but not limited to, zBlpl, zBlp2, zMcl-la,
zMcl-lb, and
zNR13); to pro-apoptotic multidomain factors (including, but not limited to,
zBak, zBax,
zBokl, and zBok2); and to pro-apoptotic BH3 -only factors (including, but not
limited to,
zBadl, zBad2, zBid, zBik, zBmfl, aBmf2, zNoxa, zPuma, and zBim).
The terms "zebrafish pro-apoptosis protein", "zebrafish pro-apoptosis
polypeptide",
"zebrafish pro-apoptotic protein", "zebrafish pro-apoptotic polypeptide", "ZPA
polypeptide"
and "ZPA protein" are used interchangeably herein, and include native sequence
polypeptides, polypeptide variants and fragments of native sequence
polypeptides and
polypeptide variants (which are further defined herein), unless specified
otherwise. ZPA
proteins can be obtained from zebrafish by using antibodies according to this
invention or by
recombinant or synthetic methods, including using deposited nucleic acid
molecules. ZPA
proteins include the zebrafish proteins identified herein, e.g., zBak (SEQ ID
NO: 1), zBik
(SEQ ID NO: 5), zBim, zPuma (SEQ ID NO: 7), and zBmf2 (SEQ ID NO: 9).
The terms "intrinsic apoptotic pathway", "intrinsic apoptosis pathway" or
"intrinsic
pathway" are used interchangeably herein, and refer to a cellular biochemical
pathway
resulting in apoptosis of the cell which is initiated intracellularly.
The terms "extrinsic apoptotic pathway", "extrinsic apoptosis pathway" and
"extrinsic
pathway" are used interchangeably herein, and refer to a cellular biochemical
pathway
resulting in apoptosis of the cell which is initiated extracellularly.
As used herein, the term "zebrafish" refers to any fish or strain of fish that
is
considered to be of the genus and species Danio rerio.
A "native sequence" polypeptide or "native" polypeptide is one which has the
same
amino acid sequence as a polypeptide (e.g., antibody) derived from nature. A
"native
sequence" polypeptide is one which has the same amino acid sequence as a
polypeptide (e.g.,
antibody) derived from nature. Such native sequence polypeptides can be
isolated from
nature or can be produced by recombinant or synthetic means. Thus, a native
sequence
polypeptide can have the amino acid sequence of a naturally occurring human
polypeptide,
zebrafish polypeptide, or polypeptide from any other species. A "native
sequence" ZPA
polypeptide or a "native" ZPA polypeptide comprises a polypeptide having the
same amino
acid sequence as the corresponding ZPA polypeptide derived from nature. For
example, in
17
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
one embodiment, the nucleic acid sequence encoding a native sequence of the
zebrafish ZPA
protein zPuma can be found in SEQ ID NO: 8 and Example 2(e).
Such ZPA polypeptides can be isolated from nature or can be produced by
recombinant or synthetic means. The term "native sequence" or "native" ZPA
polypeptide or
protein specifically encompasses naturally-occurring truncated or secreted
forms of the ZPA
protein, naturally-occurring variant forms (e.g., alternatively spliced forms)
and naturally-
occurring allelic variants of the polypeptide. In certain embodiments of the
invention, the
native sequence ZPA polypeptides disclosed herein are mature or full-length
native sequence
polypeptides comprising the full-length amino acid sequences set forth herein.
The approximate location of the "signal peptides" of the various ZPA
polypeptides
disclosed herein can be seen in the present specification and/or the
accompanying figures. It
is also recognized that, in some cases, cleavage of a signal sequence from a
secreted
polypeptide is not entirely uniform, resulting in more than one secreted
species. These
mature polypeptides, where the signal peptide is cleaved within no more than
about 5 amino
acids on either side of the C-terminal boundary of the signal peptide as
identified herein, and
the polynucleotides encoding them, are contemplated by the present invention.
A "ZPA polypeptide variant" or "ZPA protein variant" means a ZPA polypeptide
having at least about 80% amino acid sequence identity with a full-length
native sequence
ZPA polypeptide sequence as disclosed herein, or any fragment of a full-length
ZPA
polypeptide sequence as disclosed herein (such as those encoded by a nucleic
acid that
represents only a portion of the complete coding sequence for a full-length
ZPA polypeptide).
Such ZPA polypeptide variants include, for instance, ZPA polypeptides wherein
one or more
amino acid residues are added, or deleted, at the N- or C-terminus of the full-
length native
amino acid sequence. Ordinarily, a ZPA polypeptide variant will have at least
about 80%
amino acid sequence identity, alternatively at least about 81%, 82%, 83%, 84%,
85%, 86%,
87%,88%,89%,90%,91%,92%,93%,94%,95%,96%,97%,98%, or 99% amino acid
sequence identity, to a full-length native sequence ZPA polypeptide sequence
as disclosed
herein, or any specifically defined fragment of a full-length ZPA polypeptide
sequence as
disclosed herein. Ordinarily, ZPA variant polypeptides are at least about 10
amino acids in
length, alternatively at least about 20, 30, 40, 50, 60, 70, 80, 90, 100, 110,
120, 130, 140, 150,
160, 170, 180, 190, 200, 210 amino acids in length, or more. Optionally, ZPA
variant
polypeptides will have no more than one conservative amino acid substitution
as compared to
18
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
the native ZPA polypeptide sequence, alternatively no more than 2, 3, 4, 5, 6,
7, 8, 9, or 10
conservative amino acid substitution as compared to the native ZPA polypeptide
sequence.
"Percent (%) amino acid sequence identity" with respect to the ZPA polypeptide
sequences identified herein is defined as the percentage of amino acid
residues in a candidate
sequence that are identical with the amino acid residues in the specific ZPA
polypeptide
sequence, after aligning the sequences and introducing gaps, if necessary, to
achieve the
maximum percent sequence identity, and not considering any conservative
substitutions as
part of the sequence identity. Alignment for purposes of determining percent
amino acid
sequence identity can be achieved in various ways that are within the skill in
the art, for
instance, using publicly available computer software such as BLAST, BLAST-2,
ALIGN or
Megalign (DNASTAR) software. Those skilled in the art can determine
appropriate
parameters for measuring alignment, including any algorithms needed to achieve
maximal
alignment over the full length of the sequences being compared. For purposes
herein,
however, % amino acid sequence identity values are generated using the
sequence
comparison computer program ALIGN-2. The ALIGN-2 sequence comparison computer
program was authored by Genentech, Inc. and the source code has been filed
with user
documentation in the U.S. Copyright Office, Washington D.C., 20559, where it
is registered
under U.S. Copyright Registration No. TXU510087. The ALIGN-2 program is
publicly
available through Genentech, Inc., South San Francisco, California or can be
compiled from
the publicly available source code. The ALIGN-2 program should be compiled for
use on a
UNIX operating system, e.g., digital UNIX V4.OD. All sequence comparison
parameters are
set by the ALIGN-2 program and do not vary.
In situations where ALIGN-2 is employed for amino acid sequence comparisons,
the
% amino acid sequence identity of a given amino acid sequence A to, with, or
against a given
amino acid sequence B (which can alternatively be phrased as a given amino
acid sequence A
that has or comprises a certain % amino acid sequence identity to, with, or
against a given
amino acid sequence B) is calculated as follows:
100 times the fraction X/Y
where X is the number of amino acid residues scored as identical matches by
the sequence
alignment program ALIGN-2 in that program's alignment of A and B, and where Y
is the
total number of amino acid residues in B. It will be appreciated that where
the length of
19
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
amino acid sequence A is not equal to the length of amino acid sequence B, the
% amino acid
sequence identity of A to B will not equal the % amino acid sequence identity
of B to A.
Unless specifically stated otherwise, all % amino acid sequence identity
values used herein
are obtained as described in the immediately preceding paragraph using the
ALIGN-2
computer program.
As used herein, "conserved synteny" refers to evidence that the human locus
evolved
from the zebrafish locus, e.g., similar neighboring genes on one or both sides
of a ZPA gene
and a human gene to which the ZPA gene is believed to be homologous.
" ZPA variant polynucleotide" or "ZPA variant nucleic acid sequence" means a
nucleic acid molecule which encodes a ZPA polypeptide, preferably an active
ZPA
polypeptide, as defined herein and which has at least about 80% nucleic acid
sequence
identity with a nucleotide acid sequence encoding a full-length native
sequence ZPA
polypeptide sequence as disclosed herein, or any fragment of a full-length ZPA
polypeptide
sequence as disclosed herein. Ordinarily, a ZPA variant polynucleotide will
have at least
about 80% nucleic acid sequence identity, alternatively at least about 81%,
82%, 83%, 84%,
85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
nucleic acid sequence identity with a nucleic acid sequence encoding a full-
length native
sequence ZPA polypeptide sequence as disclosed herein, or any fragment of a
full-length
ZPA polypeptide sequence as disclosed herein. Variants do not encompass the
native
nucleotide sequence.
Ordinarily, ZPA variant polynucleotides are at least about 5 nucleotides in
length,
alternatively at least about 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18,
19, 20, 21, 22, 23, 24,
25, 26, 27, 28, 29, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95,
100, 105, 110, 115,
120, 125, 130, 135, 140, 145, 150, 155, 160, 165, 170, 175, 180, 185, 190,
195, 200, 210,
220, 230, 240, 250, 260, 270, 280, 290, 300, 310, 320, 330, 340, 350, 360,
370, 380, 390,
400, 410, 420, 430, 440, 450, 460, 470, 480, 490, 500, 510, 520, 530, 540,
550, 560, 570,
580, 590, 600, 610, 620, or 625 nucleotides in length, wherein in this context
the term
"about" means the referenced nucleotide sequence length plus or minus 10% of
that
referenced length.
"Percent (%) nucleic acid sequence identity" with respect to ZPA-encoding
nucleic
acid sequences identified herein is defined as the percentage of nucleotides
in a candidate
sequence that are identical with the nucleotides in the ZPA nucleic acid
sequence of interest,
after aligning the sequences and introducing gaps, if necessary, to achieve
the maximum
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
percent sequence identity. Alignment for purposes of determining percent
nucleic acid
sequence identity can be achieved in various ways that are within the skill in
the art, for
instance, using publicly available computer software such as BLAST, BLAST-2,
ALIGN or
Megalign (DNASTAR) software. For purposes herein, however, % nucleic acid
sequence
identity values are generated using the sequence comparison computer program
ALIGN-2.
The ALIGN-2 sequence comparison computer program was authored by Genentech,
Inc. and
the source code has been filed with user documentation in the U.S. Copyright
Office,
Washington D.C., 20559, where it is registered under U.S. Copyright
Registration No.
TXU510087. The ALIGN-2 program is publicly available through Genentech, Inc.,
South
San Francisco, California or can be compiled from the publicly available
source code. The
ALIGN-2 program should be compiled for use on a UNIX operating system, e.g.,
digital
UNIX V4.OD. All sequence comparison parameters are set by the ALIGN-2 program
and do
not vary.
In situations where ALIGN-2 is employed for nucleic acid sequence comparisons,
the
% nucleic acid sequence identity of a given nucleic acid sequence C to, with,
or against a
given nucleic acid sequence D (which can alternatively be phrased as a given
nucleic acid
sequence C that has or comprises a certain % nucleic acid sequence identity
to, with, or
against a given nucleic acid sequence D) is calculated as follows:
100 times the fraction W/Z
where W is the number of nucleotides scored as identical matches by the
sequence alignment
program ALIGN-2 in that program's alignment of C and D, and where Z is the
total number
of nucleotides in D. It will be appreciated that where the length of nucleic
acid sequence C is
not equal to the length of nucleic acid sequence D, the % nucleic acid
sequence identity of C
to D will not equal the % nucleic acid sequence identity of D to C. Unless
specifically stated
otherwise, all % nucleic acid sequence identity values used herein are
obtained as described
in the immediately preceding paragraph using the ALIGN-2 computer program.
In other embodiments, ZPA variant polynucleotides are nucleic acid molecules
that
encode a ZPA polypeptide and which are capable of hybridizing, e.g., under
stringent
hybridization and wash conditions, to nucleotide sequences encoding a full-
length ZPA
polypeptide as disclosed herein. ZPA variant polypeptides can be those that
are encoded by a
ZPA variant polynucleotide.
21
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
The term "full-length coding region" when used in reference to a nucleic acid
encoding a ZPA polypeptide refers to the sequence of nucleotides which encode
the full-
length ZPA polypeptide of the invention (which is herein often shown between
start and stop
codons, inclusive thereof).
"Isolated," when used to describe the various ZPA polypeptides disclosed
herein,
means polypeptide that has been identified and separated and/or recovered from
a component
of its natural environment. Contaminant components of its natural environment
are materials
that would typically interfere with diagnostic or therapeutic uses for the
polypeptide, and can
include enzymes, hormones, and other proteinaceous or non-proteinaceous
solutes. In certain
embodiments, the polypeptide will be purified (1) to a degree sufficient to
obtain at least 15
residues of N-terminal or internal amino acid sequence by use of a spinning
cup sequenator,
or (2) to homogeneity by SDS-PAGE under non-reducing or reducing conditions
using
Coomassie blue and/or silver stain. Isolated polypeptide includes polypeptide
in situ within
recombinant cells, since at least one component of the ZPA polypeptide natural
environment
will not be present. Ordinarily, however, isolated polypeptide will be
prepared by at least one
purification step.
An "isolated" ZPA polypeptide-encoding nucleic acid or other polypeptide-
encoding
nucleic acid is a nucleic acid molecule that is identified and separated from
at least one
contaminant nucleic acid molecule with which it is ordinarily associated in
the natural source
of the polypeptide-encoding nucleic acid. An isolated polypeptide-encoding
nucleic acid
molecule is other than in the form or setting in which it is found in nature.
Isolated
polypeptide-encoding nucleic acid molecules therefore are distinguished from
the specific
polypeptide-encoding nucleic acid molecule as it exists in natural cells.
However, an isolated
polypeptide-encoding nucleic acid molecule includes polypeptide-encoding
nucleic acid
molecules contained in cells that ordinarily express the polypeptide where,
for example, the
nucleic acid molecule is in a chromosomal location different from that of
natural cells.
The term "control sequences" refers to DNA sequences necessary for the
expression
of an operably linked coding sequence in a particular host organism. The
control sequences
that are suitable for prokaryotes, for example, include a promoter, optionally
an operator
sequence, and a ribosome binding site. Eukaryotic cells are known to utilize
promoters,
polyadenylation signals, and enhancers.
Nucleic acid is "operably linked" when it is placed into a functional
relationship with
another nucleic acid sequence. For example, DNA for a presequence or secretory
leader is
22
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
operably linked to DNA for a polypeptide if it is expressed as a preprotein
that participates in
the secretion of the polypeptide; a promoter or enhancer is operably linked to
a coding
sequence if it affects the transcription of the sequence; or a ribosome
binding site is operably
linked to a coding sequence if it is positioned so as to facilitate
translation. Generally,
"operably linked" means that the DNA sequences being linked are contiguous,
and, in the
case of a secretory leader, contiguous and in reading phase. However,
enhancers do not have
to be contiguous. Linking is accomplished by ligation at convenient
restriction sites. If such
sites do not exist, the synthetic oligonucleotide adaptors or linkers are used
in accordance
with conventional practice.
"Stringency" of hybridization reactions is readily determinable by one of
ordinary
skill in the art, and generally is an empirical calculation dependent upon
probe length,
washing temperature, and salt concentration. In general, longer probes require
higher
temperatures for proper annealing, while shorter probes need lower
temperatures.
Hybridization generally depends on the ability of denatured DNA to reanneal
when
complementary strands are present in an environment below their melting
temperature. The
higher the degree of desired homology between the probe and hybridizable
sequence, the
higher the relative temperature which can be used. As a result, it follows
that higher relative
temperatures would tend to make the reaction conditions more stringent, while
lower
temperatures less so. For additional details and explanation of stringency of
hybridization
reactions, see Ausubel et al., Current Protocols in Molecular Biology, Wiley
Interscience
Publishers, (1995).
"Stringent conditions" or "high stringency conditions", as defined herein, can
be
identified by those that: (1) employ low ionic strength and high temperature
for washing, for
example 0.015 M sodium chloride/0.0015 M sodium citrate/0.1% sodium dodecyl
sulfate at
50 C; (2) employ during hybridization a denaturing agent, such as formamide,
for example,
50% (v/v) formamide with 0.1% bovine serum albumin/0. 1% Ficoll/0.1%
polyvinylpyrrolidone/50mM sodium phosphate buffer at pH 6.5 with 750 mM sodium
chloride, 75 mM sodium citrate at 42 C; or (3) overnight hybridization in a
solution that
employs 50% formamide, 5 x SSC (0.75 M NaC1, 0.075 M sodium citrate), 50 mM
sodium
phosphate (pH 6.8), 0.1% sodium pyrophosphate, 5 x Denhardt's solution,
sonicated salmon
sperm DNA (50 g/ml), 0.1% SDS, and 10% dextran sulfate at 42 C, with a 10
minute wash
at 42 C in 0.2 x SSC (sodium chloride/sodium citrate) followed by a 10 minute
high-
stringency wash consisting of 0.1 x SSC containing EDTA at 55 C.
23
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
"Moderately stringent conditions" can be identified as described by Sambrook
et al.,
Molecular Cloning: A Laboratory Manual, New York: Cold Spring Harbor Press,
1989, and
include the use of washing solution and hybridization conditions (e.g.,
temperature, ionic
strength and %SDS) less stringent that those described above. An example of
moderately
stringent conditions is overnight incubation at 37 C in a solution comprising:
20%
formamide, 5 x SSC (150 mM NaC1, 15 mM trisodium citrate), 50 mM sodium
phosphate
(pH 7.6), 5 x Denhardt's solution, 10% dextran sulfate, and 20 mg/ml denatured
sheared
salmon sperm DNA, followed by washing the filters in 1 x SSC at about 37-50 C.
The
skilled artisan will recognize how to adjust the temperature, ionic strength,
etc. as necessary
to accommodate factors such as probe length and the like.
The term "epitope tagged" when used herein refers to a chimeric polypeptide
comprising a ZPA polypeptide or anti- ZPA antibody fused to a "tag
polypeptide". The tag
polypeptide has enough residues to provide an epitope against which an
antibody can be
made, yet is short enough such that it does not interfere with activity of the
polypeptide to
which it is fused. In certain embodiments, the tag polypeptide also is fairly
unique so that the
antibody does not substantially cross-react with other epitopes. Suitable tag
polypeptides
generally have at least six amino acid residues and usually between about 8
and 50 amino
acid residues (in certain embodiments, between about 10 and 20 amino acid
residues).
Polypeptides and antibodies of this invention that are epitope-tagged are
contemplated.
"Biologically active" and "biological activity" and "biological
characteristics" with
respect to a ZPA polypeptide means (1) having the ability to initiate or
stimulate apoptosis in
vivo or ex vivo; (2) having the ability to specifically bind to an upstream
and/or downstream
member of the intrinsic apoptotic pathway; and/or (3) having the ability to
otherwise
modulate ZPA signaling or ZPA activity, except where specified otherwise.
"Biologically active" and "biological activity" and "biological
characteristics" with
respect to a modified ZPA polypeptide means (1) having the ability to initiate
or stimulate
apoptosis in vivo or ex vivo; (2) having the ability to specifically bind to
an upstream and/or
downstream member of the intrinsic apoptotic pathway; and/or (3) having the
ability to
otherwise modulate ZPA signaling or ZPA activity, except where specified
otherwise.
"Biologically active" and "biological activity" and "biological
characteristics" with
respect to an anti-ZPA antibody of this invention means (1) having the ability
to partially or
fully block, inhibit or neutralize a biological activity of a native ZPA
polypeptide (either in an
antagonistic or blocking manner); (2) having the ability to specifically bind
a ZPA
24
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
polypeptide; and/or (3) having the ability to modulate ZPA signaling or ZPA
activity, except
where specified otherwise. In one embodiment, an antibody of this invention
binds to a ZPA
protein with an affinity of at least luM or less, 100 nm or less, 50 nm or
less, 10 nm or less, 5
nM or less, 1 nm or less. As used herein, "antibody variable domain" refers to
the portions of
the light and heavy chains of antibody molecules that include amino acid
sequences of
Complementary Determining Regions (CDRs; ie., CDR1, CDR2, and CDR3), and
Framework Regions (FRs). VH refers to the variable domain of the heavy chain.
VL refers to
the variable domain of the light chain. According to the methods used in this
invention, the
amino acid positions assigned to CDRs and FRs are defined according to Kabat
(Sequences
of Proteins of Immunological Interest (National Institutes of Health,
Bethesda, Md., 1987 and
1991)). Amino acid numbering of antibodies or antigen binding fragments is
also according
to that of Kabat.
As used herein, "codon set" refers to a set of different nucleotide triplet
sequences
used to encode desired variant amino acids. A set of oligonucleotides can be
synthesized, for
example, by solid phase synthesis, containing sequences that represent all
possible
combinations of nucleotide triplets provided by the codon set and that will
encode the desired
group of amino acids. A standard form of codon designation is that of the IUB
code, which is
known in the art and described herein.
"Heterologous DNA" is any DNA that is introduced into a host cell. The DNA can
be
derived from a variety of sources including genomic DNA, cDNA, synthetic DNA
and
fusions or combinations of these. The DNA can include DNA from the same cell
or cell type
as the host or recipient cell or DNA from a different cell type, for example,
from a mammal
or plant. The DNA can, optionally, include marker or selection genes, for
example, antibiotic
resistance genes, temperature resistance genes, etc. Host cells encoding
heterologous DNAs
comprising the polypeptides and antibodies of this invention are contemplated
as well as their
use.
As used herein, "library" refers to a plurality of polypeptides (for example,
antibody
or antibody fragment sequences), or the nucleic acids that encode these
sequences, the
sequences being different in the combination of variant amino acids that are
introduced into
these sequences according to the methods of the invention.
"Phage display" is a technique by which variant polypeptides are displayed as
fusion
proteins to a coat protein on the surface of phage, e.g., filamentous phage,
particles. A utility
of phage display lies in the fact that large libraries of randomized protein
variants can be
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
rapidly and efficiently sorted for those sequences that bind to a target
molecule with high
affinity. Display of peptide and protein libraries on phage has been used for
screening
millions of polypeptides for ones with specific binding properties. Polyvalent
phage display
methods have been used for displaying small random peptides and small proteins
through
fusions to either gene III or gene VIII of filamentous phage. Wells and
Lowman, Curr. Opin.
Struct. Biol., 3:355-362 (1992), and references cited therein. In monovalent
phage display, a
protein or peptide library is fused to a gene III or a portion thereof, and
expressed at low
levels in the presence of wild type gene III protein so that phage particles
display one copy or
none of the fusion proteins. Avidity effects are reduced relative to
polyvalent phage so that
sorting is on the basis of intrinsic ligand affinity, and phagemid vectors are
used, which
simplify DNA manipulations. Lowman and Wells, Methods: A companion to Methods
in
Enzymology, 3:205-0216 (1991).
A "phagemid" is a plasmid vector having a bacterial origin of replication,
e.g., Co1E1,
and a copy of an intergenic region of a bacteriophage. The phagemid can be
used on any
known bacteriophage, including filamentous bacteriophage and lambdoid
bacteriophage. The
plasmid will also generally contain a selectable marker for antibiotic
resistance. Segments of
DNA cloned into these vectors can be propagated as plasmids. When cells
harboring these
vectors are provided with all genes necessary for the production of phage
particles, the mode
of replication of the plasmid changes to rolling circle replication to
generate copies of one
strand of the plasmid DNA and package phage particles. The phagemid can form
infectious
or non-infectious phage particles. This term includes phagemids which contain
a phage coat
protein gene or fragment thereof linked to a heterologous polypeptide gene as
a gene fusion
such that the heterologous polypeptide is displayed on the surface of the
phage particle.
The term "phage vector" means a double stranded replicative form of a
bacteriophage
containing a heterologous gene and capable of replication. The phage vector
has a phage
origin of replication allowing phage replication and phage particle formation.
The phage can
be a filamentous bacteriophage, such as an M13, fl, fd, Pf3 phage or a
derivative thereof, or a
lambdoid phage, such as lambda, 21, phi80, phi8l, 82, 424, 434, etc., or a
derivative thereof.
The term "proteoglycan" refers to a molecule where at least one
glycosaminoglycan
side chain is covalently attached to the protein core of the molecule. A
proteoglycan
synthesis deficient cell line according to this invention includes a cell line
that is deficient in
galactosyltransferase I. According to one embodiment, the cell line is a CHO-
psbg cell line.
26
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
The term "antagonist" is any molecule that partially or fully blocks,
inhibits, or
neutralizes a biological activity of a native ZPA polypeptide and that
specifically binds to a
native ZPA polypeptide. According to one embodiment, the antagonist is a
polypeptide.
According to another embodiment, an antibody of the invention can inhibit the
binding of the
antagonist to the native ZPA polypeptide.
The term "small molecule antagonist" refers to any molecule wherein the
molecular
weight is 1500 daltons or less and is an antagonist according to this
invention. According to
one embodiment the small molecule antagonist is below about 500 Daltons.
According to one embodiment, the antagonist blocks, inhibits, decreases, or
neutralizes apoptosis in cells expressing at least one native ZPA polypeptide.
Suitable
antagonists include antibodies, antigen-binding antibody fragments, amino acid
sequence
variants of native ZPA polypeptides, peptides of this invention, aptamers,
etc. Methods for
identifying antagonists of a ZPA polypeptide can comprise contacting a ZPA
polypeptide
with a candidate antagonist molecule and measuring a detectable change in one
or more
biological activities associated with the ZPA polypeptide.
The term "aptamer" refers to a nucleic acid molecule that is capable of
binding to a target
molecule, such as a ZPA polypeptide. The generation and therapeutic use of
aptamers are well
established in the art. See, e.g., US Pat. No. 5,475,096, and the therapeutic
efficacy of Macugen
(Eyetech, New York) for treating age-related macular degeneration.
The terms "potentiator" and "agonist" refer to any molecule that enhances a
biological
activity of a native ZPA polypeptide, wherein the potentiator initiates and/or
stimulates
apoptosis. In one embodiment, an agonist specifically binds to a native ZPA
polypeptide and
enhances a biological activity of that native ZPA polypeptide. In another
embodiment, an
agonist stimulates the transcription and/or translation of a polynucleotide
encoding a native
ZPA polypeptide such that the expression of the native ZPA polypeptide is
increased. In
another embodiment, an agonist inhibits the normal functioning of an inhibitor
of a native
ZPA polypeptide. It is understood that the foregoing embodiments are not
mutually
exclusive, such that an agonist may, e.g., specifically bind to a native ZPA
polypeptide and
enhance a biological activity of that native ZPA polypeptide while also
inhibiting the normal
functioning of an inhibitor of a native ZPA polypeptide. Methods for
identifying agonists of
a ZPA polypeptide can comprise contacting a molecule that binds ZPA with a ZPA
polypeptide and the candidate agonist and measuring a detectable change in one
or more
27
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
biological activities associated with the ZPA polypeptide (e.g., increased
caspase activation
or increased rate or amount of apoptosis).
"Treating" or "treatment" or "alleviation" refers to both therapeutic
treatment and
prophylactic or preventative measures, wherein the object is to prevent or
slow down (lessen)
the targeted pathologic condition or disorder. Those in need of treatment
include those
already with the disorder as well as those prone to have the disorder or those
in whom the
disorder is to be prevented. These terms indicate the therapeutic and
prophylactic uses herein
are successful if they ameliorate, lessen or decrease the symptoms,
complications or other
problems associated with a disease or ameliorate, lessen or decrease the
chance of onset or
frequency of the symptoms, complications or other problems associated with a
disease.
A subject or mammal is successfully "treated" for a cancer if, after receiving
a
therapeutic amount of an antagonist according to the methods of the present
invention, the
patient shows observable and/or measurable reduction in or absence of one or
more of the
following: reduction in the number of cancer cells or absence of the cancer
cells; reduction in
the tumor size; inhibition (i.e., slow to some extent and preferably stop) of
cancer cell
infiltration into peripheral organs including the spread of cancer into soft
tissue and bone;
inhibition (i.e., slow to some extent and preferably stop) of tumor
metastasis; inhibition, to
some extent, of tumor growth; and/or relief to some extent, one or more of the
symptoms
associated with the specific cancer; reduced morbidity and mortality, and
improvement in
quality of life issues. To the extent an anti-ZPA antibody or ZPA-binding
oligopeptide can
prevent growth and/or kill existing cancer cells, it can be cytostatic and/or
cytotoxic.
Reduction of these signs or symptoms can also be felt by the patient.
A subject or mammal is successfully "treated" for an apoptosis-related
disorder if,
after receiving a therapeutic amount of an antagonist or agonist according to
the methods of
the present invention, the patient shows observable and/or measurable
modulation of
apoptosis, and/or relief to some extent, of one or more of the symptoms
associated with the
aberrant apoptosis; and improvement in quality of life issues.
The above parameters for assessing successful treatment and improvement in the
disease are readily measurable by procedures familiar to a physician. For
cancer therapy,
efficacy can be measured, for example, by assessing the time to disease
progression (TTP)
and/or determining the response rate (RR). Metastasis can be determined by
staging tests and
by bone scan and tests for calcium level and other enzymes to determine spread
to the bone.
CT scans can also be done to look for spread to the pelvis and lymph nodes in
the area. Chest
28
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
X-rays and measurement of liver enzyme levels by known methods are used to
look for
metastasis to the lungs and liver, respectively. Other known methods for
monitoring the
disease include transrectal ultrasonography (TRUS) and transrectal needle
biopsy (TRNB),
among other methods well known in the art.
"Chronic" administration refers to administration of the agent(s) in a
continuous mode
as opposed to an acute mode, so as to maintain the initial therapeutic effect
(activity) for an
extended period of time. "Intermittent" administration is treatment that is
not consecutively
done without interruption, but rather is cyclic in nature.
"Mammal" for purposes of the treatment of, alleviating the symptoms of or
diagnosis
of a cancer refers to any animal classified as a mammal (aka "patient"),
including humans,
domestic and farm animals, and zoo, sports, or pet animals, such as dogs,
cats, cattle, horses,
sheep, pigs, goats, rabbits, etc. In certain embodiments, the mammal is human.
Administration "in combination with" one or more further therapeutic agents
includes
simultaneous (concurrent) and consecutive administration in any order.
"Carriers" as used herein include pharmaceutically acceptable carriers,
excipients, or
stabilizers which are nontoxic to the cell or mammal being exposed thereto at
the dosages and
concentrations employed. Often the physiologically acceptable carrier is an
aqueous pH
buffered solution. Examples of physiologically acceptable carriers include
buffers such as
phosphate, citrate, and other organic acids; antioxidants including ascorbic
acid; low
molecular weight (less than about 10 residues) polypeptide; proteins, such as
serum albumin,
gelatin, or immunoglobulins; hydrophilic polymers such as
polyvinylpyrrolidone; amino
acids such as glycine, glutamine, asparagine, arginine or lysine;
monosaccharides,
disaccharides, and other carbohydrates including glucose, mannose, or
dextrins; chelating
agents such as EDTA; sugar alcohols such as mannitol or sorbitol; salt-forming
counterions
such as sodium; and/or nonionic surfactants such as TWEEN , polyethylene
glycol (PEG),
and PLURONICS .
By "solid phase" or "solid support" is meant a non-aqueous matrix to which an
antibody, an antagonist or a polypeptide of the present invention can adhere
or attach.
Examples of solid phases encompassed herein include those formed partially or
entirely of
glass (e.g., controlled pore glass), polysaccharides (e.g., agarose),
polyacrylamides,
polystyrene, polyvinyl alcohol and silicones. In certain embodiments,
depending on the
context, the solid phase can comprise the well of an assay plate; in others it
is a purification
29
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
column (e.g., an affinity chromatography column). This term also includes a
discontinuous
solid phase of discrete particles, such as those described in U.S. Patent No.
4,275,149.
As used herein, the term "immunoadhesin" designates antibody-like molecules
that
combine the binding specificity of a heterologous protein (an "adhesin") with
the effector
functions of immunoglobulin constant domains. Structurally, the immunoadhesins
comprise
a fusion of an amino acid sequence with the desired binding specificity that
is other than the
antigen recognition and binding site of an antibody (i.e., is "heterologous"),
and an
immunoglobulin constant domain sequence. The adhesin part of an immunoadhesin
molecule typically is a contiguous amino acid sequence comprising at least the
binding site of
a receptor or a ligand - such as a portion of a native ZPA protein. The
immunoglobulin
constant domain sequence in the immunoadhesin can be obtained from any
immunoglobulin,
such as IgG-1, IgG-2, IgG-3, or IgG-4 subtypes, IgA (including IgA-1 and IgA-
2), IgE, IgD,
or IgM.
A "liposome" is a small vesicle composed of various types of lipids,
phospholipids
and/or surfactant which is useful for delivery of a drug (such as a ZPA
polypeptide, an
antibody thereto or a ZPA-binding oligopeptide) to a mammal. The components of
the
liposome are commonly arranged in a bilayer formation, similar to the lipid
arrangement of
biological membranes.
An "effective amount" of a polypeptide, antibody, antagonist or composition
as disclosed herein is an amount sufficient to carry out a specifically stated
purpose. An
"effective amount" can be determined empirically and by known methods relating
to the
stated purpose.
The term "therapeutically effective amount" refers to an amount of an
antibody,
polypeptide or antagonist of this invention effective to "treat" a disease or
disorder in a
mammal (aka patient). In the case of cancer, the therapeutically effective
amount of the drug
can reduce the number of cancer cells; reduce the tumor size; inhibit (i.e.,
slow to some
extent and preferably stop) cancer cell infiltration into peripheral organs;
inhibit (i.e., slow to
some extent and preferably stop) tumor metastasis; inhibit, to some extent,
tumor growth;
and/or relieve to some extent one or more of the symptoms associated with the
cancer. See
the definition herein of "treating". To the extent the drug can prevent growth
and/or kill
existing cancer cells, it can be cytostatic and/or cytotoxic.
A "cytotoxic amount" of a polypeptide, antibody, antagonist or composition of
this
invention is an amount capable of causing the destruction of a cell,
especially tumor, e.g.,
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
cancer cell, either in vitro or in vivo. A "cytotoxic amount" of a
polypeptide, antibody,
antagonist or composition of this invention for purposes of inhibiting, e.g.,
neoplastic cell
growth, can be determined empirically and by methods known in the art.
The term "antibody" is used in the broadest sense and specifically covers, for
example, single anti-ZPA polypeptide monoclonal antibodies (including agonist,
antagonist,
and neutralizing antibodies), anti-ZPA polypeptide antibody compositions with
polyepitopic
specificity, polyclonal antibodies, single chain anti-ZPA polypeptide
antibodies, and
fragments of anti-ZPA polypeptide antibodies (see below) as long as they
specifically bind a
native ZPA polypeptide and/or exhibit a biological activity or immunological
activity of this
invention. The phrase "functional fragment or analog" of an antibody is a
compound having
a qualitative biological activity in common with an antibody to which it is
being referred.
For example, a functional fragment or analog of an anti- ZPA polypeptide
antibody can be
one which can specifically bind to a ZPA molecule. In one embodiment, the
antibody can
prevent or substantially reduce the ability of a ZPA molecule to induce
apoptosis. The term
"immunoglobulin" (Ig) is used interchangeably with "antibody" herein.
An "isolated antibody" is one which has been identified and separated and/or
recovered from a component of its natural environment. Contaminant components
of its
natural environment are materials which would interfere with diagnostic or
therapeutic uses
for the antibody, and can include enzymes, hormones, and other proteinaceous
or
nonproteinaceous solutes. In certain embodiments, the antibody will be
purified (1) to
greater than 95% by weight of antibody as determined by the Lowry method, and
most
preferably more than 99% by weight, (2) to a degree sufficient to obtain at
least 15 residues
of N-terminal or internal amino acid sequence by use of a spinning cup
sequenator, or (3) to
homogeneity by SDS-PAGE under reducing or nonreducing conditions using
Coomassie blue
or, preferably, silver stain. Isolated antibody includes the antibody in situ
within recombinant
cells since at least one component of the antibody's natural environment will
not be present.
Ordinarily, however, isolated antibody will be prepared by at least one
purification step.
The basic 4-chain antibody unit is a heterotetrameric glycoprotein composed of
two
identical light (L) chains and two identical heavy (H) chains (an IgM antibody
consists of 5
of the basic heterotetramer unit along with an additional polypeptide called J
chain, and
therefore contain 10 antigen binding sites, while secreted IgA antibodies can
polymerize to
form polyvalent assemblages comprising 2-5 of the basic 4-chain units along
with J chain).
In the case of IgGs, the 4-chain unit is generally about 150,000 daltons. Each
L chain is
31
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
linked to an H chain by one covalent disulfide bond, while the two H chains
are linked to
each other by one or more disulfide bonds depending on the H chain isotype.
Each H and L
chain also has regularly spaced intrachain disulfide bridges. Each H chain has
at the N-
terminus, a variable domain (VH) followed by three constant domains (CH) for
each of the a
and y chains and four CH domains for and s isotypes. Each L chain has at the
N-terminus, a
variable domain (VL) followed by a constant domain (CL) at its other end. The
VL is aligned
with the VH and the CL is aligned with the first constant domain of the heavy
chain (CH1).
Particular amino acid residues are believed to form an interface between the
light chain and
heavy chain variable domains. The pairing of a VH and VL together forms a
single antigen-
binding site. For the structure and properties of the different classes of
antibodies, see, e.g.,
Basic and Clinical Immunolo~y, 8th edition, Daniel P. Stites, Abba I. Terr and
Tristram G.
Parslow (eds.), Appleton & Lange, Norwalk, CT, 1994, page 71 and Chapter 6.
The L chain from any vertebrate species can be assigned to one of two clearly
distinct
types, called kappa and lambda, based on the amino acid sequences of their
constant
domains. Depending on the amino acid sequence of the constant domain of their
heavy
chains (CH), immunoglobulins can be assigned to different classes or isotypes.
There are five
classes of immunoglobulins: IgA, IgD, IgE, IgG, and IgM, having heavy chains
designated a,
8, s, y, and , respectively. The y and a classes are further divided into
subclasses on the
basis of relatively minor differences in CH sequence and function, e.g.,
humans express the
following subclasses: IgG1, IgG2, IgG3, IgG4, IgAl, and IgA2.
The term "variable" refers to the fact that certain segments of the variable
domains
differ extensively in sequence among antibodies. The V domain mediates antigen
binding
and define specificity of a particular antibody for its particular antigen.
However, the
variability is not evenly distributed across the 110-amino acid span of the
variable domains.
Instead, the V regions consist of relatively invariant stretches called
framework regions (FRs)
of 15-30 amino acids separated by shorter regions of extreme variability
called
"hypervariable regions" that are each 9-12 amino acids long. The variable
domains of native
heavy and light chains each comprise four FRs, largely adopting a(3-sheet
configuration,
connected by three hypervariable regions, which form loops connecting, and in
some cases
forming part of, the (3-sheet structure. The hypervariable regions in each
chain are held
together in close proximity by the FRs and, with the hypervariable regions
from the other
chain, contribute to the formation of the antigen-binding site of antibodies
(see Kabat et al.,
Sequences of Proteins of Immunological Interest, 5th Ed. Public Health
Service, National
32
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Institutes of Health, Bethesda, MD. (1991)). The constant domains are not
involved directly
in binding an antibody to an antigen, but exhibit various effector functions,
such as
participation of the antibody in antibody dependent cellular cytotoxicity
(ADCC).
The term "hypervariable region" when used herein refers to the amino acid
residues
of an antibody which are responsible for antigen-binding. The hypervariable
region generally
comprises amino acid residues from a "complementarity determining region" or
"CDR" (e.g.
around about residues 24-34 (Ll), 50-56 (L2) and 89-97 (L3) in the VL, and
around about 1-
35 (H1), 50-65 (H2) and 95-102 (H3) in the VH (in one embodiment, H1 is around
about 31-
35); Kabat et al., Sequences of Proteins of Immunological Interest, 5th Ed.
Public Health
Service, National Institutes of Health, Bethesda, MD. (1991)) and/or those
residues from a
"hypervariable loop" (e.g. residues 26-32 (Ll), 50-52 (L2) and 91-96 (L3) in
the VL, and 26-
32 (H1), 53-55 (H2) and 96-101 (H3) in the VH; Chothia and Lesk J. Mol. Biol.
196:901-917
(1987)).
The term "monoclonal antibody" as used herein refers to an antibody obtained
from a
population of substantially homogeneous antibodies, i.e., the individual
antibodies
comprising the population are identical except for possible naturally
occurring mutations that
can be present in minor amounts. Monoclonal antibodies are highly specific,
being directed
against a single antigenic site. Furthermore, in contrast to polyclonal
antibody preparations
which include different antibodies directed against different determinants
(epitopes), each
monoclonal antibody is directed against a single determinant on the antigen.
In addition to
their specificity, the monoclonal antibodies are advantageous in that they can
be synthesized
uncontaminated by other antibodies. The modifier "monoclonal" is not to be
construed as
requiring production of the antibody by any particular method. For example,
the monoclonal
antibodies useful in the present invention can be prepared by the hybridoma
methodology
first described by Kohler et al., Nature, 256:495 (1975), or can be made using
recombinant
DNA methods in bacterial, eukaryotic animal or plant cells (see, e.g., U.S.
Patent No.
4,816,567). The "monoclonal antibodies" can also be isolated from phage
antibody libraries
using the techniques described in Clackson et al., Nature, 352:624-628 (1991),
Marks et al., J.
Mol. Biol., 222:581-597 (1991), and the Examples below, for example.
The monoclonal antibodies herein include "chimeric" antibodies in which a
portion of
the heavy and/or light chain is identical with or homologous to corresponding
sequences in
antibodies derived from a particular species or belonging to a particular
antibody class or
subclass, while the remainder of the chain(s) is identical with or homologous
to
33
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
corresponding sequences in antibodies derived from another species or
belonging to another
antibody class or subclass, as well as fragments of such antibodies, so long
as they exhibit a
biological activity of this invention (see U.S. Patent No. 4,816,567; and
Morrison et al., Proc.
Natl. Acad. Sci. USA, 81:6851-6855 (1984)). Chimeric antibodies of interest
herein include
"primatized" antibodies comprising variable domain antigen-binding sequences
derived from
a non-human primate (e.g. Old World Monkey, Ape etc), and human constant
region
sequences.
An "intact" antibody is one which comprises an antigen-binding site as well as
a CL
and at least heavy chain constant domains, CH1, CH2 and CH3. The constant
domains can be
native sequence constant domains (e.g. human native sequence constant domains)
or amino
acid sequence variant thereof. In certain embodiments, the intact antibody has
one or more
effector functions.
"Antibody fragments" comprise a portion of an intact antibody, preferably the
antigen
binding or variable region of the intact antibody. Examples of antibody
fragments include
Fab, Fab', F(ab')2, and Fv fragments; diabodies; linear antibodies (see U.S.
Patent No.
5,641,870, Example 2; Zapata et al., Protein Eng. 8(10): 1057-1062 [1995]);
single-chain
antibody molecules; and multispecific antibodies formed from antibody
fragments.
The expression "linear antibodies" generally refers to the antibodies
described in Zapata et
al., Protein Eng., 8(10):1057-1062 (1995). Briefly, these antibodies comprise
a pair of
tandem Fd segments (VH-CHI-VH-CH1) which, together with complementary light
chain
polypeptides, form a pair of antigen binding regions. Linear antibodies can be
bispecific or
monospecific.
Papain digestion of antibodies produces two identical antigen-binding
fragments,
called "Fab" fragments, and a residual "Fc" fragment, a designation reflecting
the ability to
crystallize readily. The Fab fragment consists of an entire L chain along with
the variable
region domain of the H chain (VH), and the first constant domain of one heavy
chain (CH1).
Each Fab fragment is monovalent with respect to antigen binding, i.e., it has
a single antigen-
binding site. Pepsin treatment of an antibody yields a single large F(ab')2
fragment which
roughly corresponds to two disulfide linked Fab fragments having divalent
antigen-binding
activity and is still capable of cross-linking antigen. Fab' fragments differ
from Fab
fragments by having additional few residues at the carboxy terminus of the CH1
domain
including one or more cysteines from the antibody hinge region. Fab'-SH is the
designation
herein for Fab' in which the cysteine residue(s) of the constant domains bear
a free thiol
34
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
group. F(ab')2 antibody fragments originally were produced as pairs of Fab'
fragments which
have hinge cysteines between them. Other chemical couplings of antibody
fragments are also
known.
The Fc fragment comprises the carboxy-terminal portions of both H chains held
together by disulfides. The effector functions of antibodies are determined by
sequences in
the Fc region, which region is also the part recognized by Fc receptors (FcR)
found on certain
types of cells.
"Fv" is the minimum antibody fragment which contains a complete antigen-
recognition and -binding site. This fragment consists of a dimer of one heavy-
and one light-
chain variable region domain in tight, non-covalent association. From the
folding of these
two domains emanate six hypervariable loops (3 loops each from the H and L
chain) that
contribute the amino acid residues for antigen binding and confer antigen
binding specificity
to the antibody. However, even a single variable domain (or half of an Fv
comprising only
three CDRs specific for an antigen) has the ability to recognize and bind
antigen, although at
a lower affinity than the entire binding site.
"Single-chain Fv" also abbreviated as "sFv" or "scFv" are antibody fragments
that
comprise the VH and VL antibody domains connected into a single polypeptide
chain. In
certain embodiments, the sFv polypeptide further comprises a polypeptide
linker between the
VH and VL domains which enables the sFv to form the desired structure for
antigen binding.
For a review of sFv, see Pluckthun in The PharmacoloPy of Monoclonal
Antibodies, vol. 113,
Rosenburg and Moore eds., Springer-Verlag, New York, pp. 269-315 (1994);
Borrebaeck
1995, infra.
The term "diabodies" refers to small antibody fragments prepared by
constructing sFv
fragments (see preceding paragraph) with short linkers (about 5-10 residues)
between the VH
and VL domains such that inter-chain but not intra-chain pairing of the V
domains is
achieved, resulting in a bivalent fragment, i.e., fragment having two antigen-
binding sites.
Bispecific diabodies are heterodimers of two "crossover" sFv fragments in
which the VH and
VL domains of the two antibodies are present on different polypeptide chains.
Diabodies are
described more fully in, for example, EP 404,097; WO 93/11161; and Hollinger
et al., Proc.
Natl. Acad. Sci. USA, 90:6444-6448 (1993).
"Humanized" forms of non-human (e.g., rodent) antibodies are chimeric
antibodies
that contain minimal sequence derived from the non-human antibody. For the
most part,
humanized antibodies are human immunoglobulins (recipient antibody) in which
residues
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
from a hypervariable region of the recipient are replaced by residues from a
hypervariable
region of a non-human species (donor antibody) such as mouse, rat, rabbit or
non-human
primate having the desired antibody specificity, affinity, and capability. In
some instances,
framework region (FR) residues of the human immunoglobulin are replaced by
corresponding non-human residues. Furthermore, humanized antibodies can
comprise
residues that are not found in the recipient antibody or in the donor
antibody. These
modifications are made to further refine antibody performance. In general, the
humanized
antibody will comprise substantially all of at least one, and typically two,
variable domains,
in which all or substantially all of the hypervariable loops correspond to
those of a non-
human immunoglobulin and all or substantially all of the FRs are those of a
human
immunoglobulin sequence. The humanized antibody optionally also will comprise
at least a
portion of an immunoglobulin constant region (Fc), typically that of a human
immunoglobulin. For further details, see Jones et al., Nature 321:522-525
(1986);
Riechmann et al., Nature 332:323-329 (1988); and Presta, Curr. Op. Struct.
Biol. 2:593-596
(1992).
A "species-dependent antibody," e.g., a mammalian anti-human IgE antibody, is
an
antibody which has a stronger binding affinity for an antigen from a first
mammalian species
than it has for a homologue of that antigen from a second mammalian species.
Normally, the
species-dependent antibody "bind specifically" to a human antigen (i.e., has a
binding affinity
(Kd) value of no more than about 1 x 10-7 M, no more than about 1 x 10-8, and
no more than
about 1 x 10-9 M) but has a binding affinity for a homologue of the antigen
from a second
non-human mammalian species which is at least about 50 fold, or at least about
500 fold, or
at least about 1000 fold, weaker than its binding affinity for the human
antigen. The species-
dependent antibody can be of any of the various types of antibodies as defined
above, an in
certain embodiments is a humanized or human antibody.
A "ZPA-binding oligopeptide" is an oligopeptide that binds, preferably
specifically,
to a ZPA polypeptide as described herein. ZPA-binding oligopeptides can be
chemically
synthesized using known oligopeptide synthesis methodology or can be prepared
and purified
using recombinant technology. ZPA-binding oligopeptides are usually at least
about 5 amino
acids in length, alternatively at least about 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20,
21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39,
40, 41, 42, 43, 44, 45,
46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64,
65, 66, 67, 68, 69, 70,
71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89,
90, 91, 92, 93, 94, 95,
36
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
96, 97, 98, 99, or 100 amino acids in length or more, wherein such
oligopeptides that are
capable of binding, preferably specifically, to a ZPA polypeptide as described
herein. ZPA-
binding oligopeptides can be identified without undue experimentation using
known
techniques. In this regard, it is noted that techniques for screening
oligopeptide libraries for
oligopeptides that are capable of specifically binding to a polypeptide target
are known in the
art (see, e.g., U.S. Patent Nos. 5,556,762, 5,750,373, 4,708,871, 4,833,092,
5,223,409,
5,403,484, 5,571,689, 5,663,143; PCT Publication Nos. WO 84/03506 and
W084/03564;
Geysen et al., Proc. Natl. Acad. Sci. U.S.A., 81:3998-4002 (1984); Geysen et
al., Proc. Natl.
Acad. Sci. U.S.A., 82:178-182 (1985); Geysen et al., in Synthetic Peptides as
Antigens, 130-
149 (1986); Geysen et al., J. Immunol. Meth., 102:259-274 (1987); Schoofs et
al., J.
Immunol., 140:611-616 (1988), Cwirla, S. E. et al. (1990) Proc. Natl. Acad.
Sci. USA,
87:6378; Lowman, H.B. et al. (1991) Biochemistry, 30:10832; Clackson, T. et
al. (1991)
Nature, 352: 624; Marks, J. D. et al. (1991), J. Mol. Biol., 222:581; Kang,
A.S. et al. (1991)
Proc. Natl. Acad. Sci. USA, 88:8363, and Smith, G. P. (1991) Current Opin.
Biotechnol.,
2:668).
A polypeptide, antibody, antagonist or composition of this invention "which
binds" an
antigen of interest, e.g. a ZPA polypeptide, is one that binds the antigen
with sufficient
affinity such that a polypeptide, antibody, antagonist or composition is
useful as a diagnostic
and/or therapeutic agent in targeting a cell or tissue expressing the antigen,
and does not
significantly cross-react with other proteins. In such embodiments, the extent
of binding of
the polypeptide, antibody, antagonist or composition to a "non-target" protein
will be less
than about 10% of the binding of the polypeptide, antibody, antagonist or
composition to its
particular target protein as determined by fluorescence activated cell sorting
(FACS) analysis
or radioimmunoprecipitation (RIA). With regard to the binding of a
polypeptide, antibody,
antagonist or composition to a target molecule, the term "specific binding" or
"specifically
binds to" or is "specific for" a particular polypeptide or an epitope on a
particular polypeptide
target means binding that is measurably different from a non-specific
interaction. Specific
binding can be measured, for example, by determining binding of a molecule
compared to
binding of a control molecule, which generally is a molecule of similar
structure that does not
have binding activity. For example, specific binding can be determined by
competition with
a control molecule that is similar to the target, for example, an excess of
non-labeled target.
In this case, specific binding is indicated if the binding of the labeled
target to a probe is
competitively inhibited by excess unlabeled target. The term "specific
binding" or
37
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
"specifically binds to" or is "specific for" a particular polypeptide or an
epitope on a
particular polypeptide target as used herein can be exhibited, for example, by
a molecule
having a Kd for the target of at least about 10-4 M, alternatively at least
about 10-5 M,
alternatively at least about 10-6 M, alternatively at least about 10-7 M,
alternatively at least
about 10-8 M, alternatively at least about 10-9 M, alternatively at least
about 10-10 M,
alternatively at least about 10-11 M, alternatively at least about 10-12 M, or
greater. In one
embodiment, the term "specific binding" refers to binding where a molecule
binds to a
particular polypeptide or epitope on a particular polypeptide without
substantially binding to
any other polypeptide or polypeptide epitope (e.g., a non-ZPA protein). It is
understood that
an antibody that specifically binds to a zebrafish native ZPA polypeptide may
also bind a
non-zebrafish polypeptide homologous to the ZPA polypeptide.
A polypeptide, antibody, antagonist or composition that "inhibits the growth"
of
tumor cells or a "growth inhibitory" polypeptide, antibody, antagonist or
composition is one
which results in measurable growth inhibition of cancer cells. In certain
embodiments,
growth inhibitory polypeptides, antibodies, antagonists or compositions
inhibit growth of
tumor cells by greater than 20%, from about 20% to about 50%, and by greater
than 50%
(e.g., from about 50% to about 100%) as compared to the appropriate control,
the control
typically being tumor cells not treated with the polypeptide, antibody,
antagonist or
composition being tested. In one embodiment, growth inhibition can be measured
at an
antibody concentration of about 0.1 to 30 g/ml or about 0.5 nM to 200 nM in
cell culture,
where the growth inhibition is determined 1-10 days after exposure of the
tumor cells to the
antibody. Growth inhibition of tumor cells in vivo can be determined in
various ways such as
is described in the Experimental Examples section below. The antibody is
growth inhibitory
in vivo if administration of the anti-ZPA antibody at about 1 g/kg to about
100 mg/kg body
weight results in reduction in tumor size or tumor cell proliferation within
about 5 days to 3
months from the first administration of the antibody, for example within about
5 to 30 days.
Antibody "effector functions" refer to those biological activities
attributable to the Fc
region (a native sequence Fc region or amino acid sequence variant Fc region)
of an antibody,
and vary with the antibody isotype. Examples of antibody effector functions
include: Clq
binding and complement dependent cytotoxicity; Fc receptor binding; antibody-
dependent
cell-mediated cytotoxicity (ADCC); phagocytosis; down regulation of cell
surface receptors
(e.g., B cell receptor); and B cell activation.
38
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
"Antibody-dependent cell-mediated cytotoxicity" or "ADCC" refers to a form of
cytotoxicity in which secreted Ig bound onto Fc receptors (FcRs) present on
certain cytotoxic
cells (e.g., Natural Killer (NK) cells, neutrophils, and macrophages) enable
these cytotoxic
effector cells to bind specifically to an antigen-bearing target cell and
subsequently kill the
target cell with cytotoxins. The antibodies "arm" the cytotoxic cells and are
absolutely
required for such killing. The primary cells for mediating ADCC, NK cells,
express FcyRIII
only, whereas monocytes express FcyRI, FcyRII and FcyRIII. FcR expression on
hematopoietic cells is summarized in Table 3 on page 464 of Ravetch and Kinet,
Annu. Rev.
Immunol. 9:457-92 (1991). To assess ADCC activity of a molecule of interest,
an in vitro
ADCC assay, such as that described in US Patent No. 5,500,362 or 5,821,337 can
be
performed. Useful effector cells for such assays include peripheral blood
mononuclear cells
(PBMC) and Natural Killer (NK) cells. Alternatively, or additionally, ADCC
activity of the
molecule of interest can be assessed in vivo, e.g., in a animal model such as
that disclosed in
Clynes et al. (USA) 95:652-656 (1998).
"Fc receptor" or "FcR" describes a receptor that binds to the Fc region of an
antibody.
In certain embodiments, the FcR is a native sequence human FcR. Moreover, the
FcR can be
an FcR which binds an IgG antibody (a gamma receptor) and includes receptors
of the FcyRI,
FcyRII and FcyRIII subclasses, including allelic variants and alternatively
spliced forms of
these receptors. FcyRII receptors include FcyRIIA (an "activating receptor")
and FcyRIIB
(an "inhibiting receptor"), which have similar amino acid sequences that
differ primarily in
the cytoplasmic domains thereof. Activating receptor FcyRIIA contains an
immunoreceptor
tyrosine-based activation motif (ITAM) in its cytoplasmic domain. Inhibiting
receptor
FcyRIIB contains an immunoreceptor tyrosine-based inhibition motif (ITIM) in
its
cytoplasmic domain. (see review M. in Daeron, Annu. Rev. Immunol. 15:203-234
(1997)).
FcRs are reviewed in Ravetch and Kinet, Annu. Rev. Immunol. 9:457-492 (1991);
Capel et
al., Immunomethods 4:25-34 (1994); and de Haas et al., J. Lab. Clin. Med.
126:330-41
(1995). Other FcRs, including those to be identified in the future, are
encompassed by the
term "FcR" herein. The term also includes the neonatal receptor, FcRn, which
is responsible
for the transfer of maternal IgGs to the fetus (Guyer et al., J. Immunol.
117:587 (1976) and
Kim et al., J. Immunol. 24:249 (1994)).
"Human effector cells" are leukocytes which express one or more FcRs and
perform
effector functions. In certain embodiments, the cells express at least FcyRIII
and perform
ADCC effector function. Examples of human leukocytes which mediate ADCC
include
39
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
peripheral blood mononuclear cells (PBMC), natural killer (NK) cells,
monocytes, cytotoxic
T cells and neutrophils; with PBMCs and NK cells being preferred. The effector
cells can be
isolated from a native source, e.g., from blood.
"Complement dependent cytotoxicity" or "CDC" refers to the lysis of a target
cell in
the presence of complement. Activation of the classical complement pathway is
initiated by
the binding of the first component of the complement system (Clq) to
antibodies (of the
appropriate subclass) which are bound to their cognate antigen. To assess
complement
activation, a CDC assay, (e.g., as described in Gazzano-Santoro et al., J.
Immunol. Methods
202:163 (1996)), can be performed.
An "apoptosis-related disorder" refers to a physiological condition or disease
state
caused by, prolonged by, or which is characterized by aberrant or misregulated
apoptosis.
Apoptosis-related disorders include, but are not limited to, cell
proliferative disorders, viral
apoptosis disorders, autoimmune disorders, hematologic disorders, neurological
disorders,
and other disorders characterized by an undesirably high or low rate of
apoptosis.
The terms "cell proliferative disorder" and "proliferative disorder" refer to
disorders
that are associated with some degree of abnormal cell proliferation. In one
embodiment, the
cell proliferative disorder is cancer. Aberrant apoptosis is one cause of
abnormal cell
proliferation. A number of cancers have been linked to inactivation of one or
more pro-
apoptotic proteins (e.g., p53 and fas) or overproduction or dysregulation of
pro-survival
proteins (e.g., Bcl-2).
The terms "cancer" and "cancerous" refer to or describe the physiological
condition
that is typically characterized by unregulated cell growth. Examples of cancer
include, but
are not limited to, carcinoma, lymphoma, blastoma, sarcoma, and leukemia or
lymphoid
malignancies. More particular examples of such cancers include squamous cell
cancer (e.g.,
epithelial squamous cell cancer), lung cancer including small-cell lung
cancer, non-small cell
lung cancer, adenocarcinoma of the lung and squamous carcinoma of the lung,
cancer of the
peritoneum, hepatocellular cancer, gastric or stomach cancer including
gastrointestinal
cancer, pancreatic cancer, glioblastoma, cervical cancer, ovarian cancer,
liver cancer, bladder
cancer, cancer of the urinary tract, hepatoma, breast cancer, colon cancer,
rectal cancer,
colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma,
kidney or
renal cancer, prostate cancer, vulval cancer, thyroid cancer, hepatic
carcinoma, anal
carcinoma, penile carcinoma, melanoma, multiple myeloma and B-cell lymphoma,
brain, as
well as head and neck cancer, and associated metastases.
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
"Tumor", as used herein, refers to all neoplastic cell growth and
proliferation, whether
malignant or benign, and all pre-cancerous and cancerous cells and tissues.
The term "viral apoptosis disorder" refers to or describes aberrant apoptosis
in a
patient caused by or as a result of viral infection. The term includes both
aberrant apoptosis
(e.g., decreased or blocked apoptosis) directly caused by an infecting virus,
as well as
aberrant apoptosis (e.g., increased cell death) caused by excessive,
uncontrolled, or
mistargeted immune system function in response to a viral infection or by the
virus itself.
Examples of aberrant (decreased) apoptosis directly caused by an infecting
virus include, but
are not limited to, production of Bcl-2-like proteins (pro-survival B2R
polypeptides) and
stimulators of Bcl-2 production by the Epstein-Barr virus such that infected
cells do not
undergo apoptosis; inactivation or degradation of p53 (a pro-apoptotic
polypeptide) by
papillomavirus such that infected cells do not undergo apoptosis; and
production of an
inhibitor of the pro-apoptotic ICE-like proteases by cowpox virus, such that
infected cells do
not undergo apoptosis. An example of aberrant (increased) apoptosis caused by
viral
infection is inappropriate expression of fas at the surface of infected helper
T cells, which
causes those cells to undergo premature apoptosis, thereby eliminating an
important
component of the immune system. Examples of aberrant apoptosis caused by
excessive,
uncontrolled, or mistargeted immune system function in response to a viral
infection includes
the inadvertent killing of uninfected cells neighboring infected cells because
the neighboring
cells may also have been induced to express fas at the cell surface, and are
thus targeted for
destruction by apoptosis pathway activation by circulating cytotoxic T
lymphocytes.
The term "autoimmune disorder", refers to a non-malignant disease or disorder
arising
from and directed against an individual's own tissues. Autoimmune disorders
are typically
characterized by the failure of autoreactive immune cells to be destroyed by
the immune
system; autoreactive lymphocytes have been identified that overexpress or
otherwise have
increased activity of pro-survival apoptotic factors or have reduced
expression or activity of
pro-apoptotic factors. The autoimmune diseases herein specifically exclude
malignant or
cancerous diseases or conditions, especially excluding B cell lymphoma, acute
lymphoblastic
leukemia (ALL), chronic lymphocytic leukemia (CLL), Hairy cell leukemia and
chronic
myeloblastic leukemia. Examples of autoimmune diseases or disorders include,
but are not
limited to, inflammatory responses such as inflammatory skin diseases
including psoriasis
and dermatitis (e.g. atopic dermatitis); systemic scleroderma and sclerosis;
responses
associated with inflammatory bowel disease (such as Crohn's disease and
ulcerative colitis);
41
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
respiratory distress syndrome (including adult respiratory distress syndrome;
ARDS);
dermatitis; meningitis; encephalitis; uveitis; colitis; glomerulonephritis;
allergic conditions
such as eczema and asthma and other conditions involving infiltration of T
cells and chronic
inflammatory responses; atherosclerosis; leukocyte adhesion deficiency;
rheumatoid arthritis;
systemic lupus erythematosus (SLE) (including but not limited to lupus
nephritis, cutaneous
lupus); diabetes mellitus (e.g. Type I diabetes mellitus or insulin dependent
diabetes
mellitus); multiple sclerosis; Reynaud's syndrome; autoimmune thyroiditis;
Hashimoto's
thyroiditis; allergic encephalomyelitis; Sjogren's syndrome; juvenile onset
diabetes; and
immune responses associated with acute and delayed hypersensitivity mediated
by cytokines
and T-lymphocytes typically found in tuberculosis, sarcoidosis, polymyositis,
granulomatosis
and vasculitis; pernicious anemia (Addison's disease); diseases involving
leukocyte
diapedesis; central nervous system (CNS) inflammatory disorder; multiple organ
injury
syndrome; hemolytic anemia (including, but not limited to cryoglobinemia or
Coombs
positive anemia); myasthenia gravis; antigen-antibody complex mediated
diseases; anti-
glomerular basement membrane disease; antiphospholipid syndrome; allergic
neuritis;
Graves' disease; Lambert-Eaton myasthenic syndrome; pemphigoid bullous;
pemphigus;
autoimmune polyendocrinopathies; Reiter's disease; stiff-man syndrome; Behcet
disease;
giant cell arteritis; immune complex nephritis; IgA nephropathy; IgM
polyneuropathies;
immune thrombocytopenic purpura (ITP) or autoimmune thrombocytopenia, etc.
The term "hematologic disorder" refers to or describes a disease or disorder
characterized by aberrant production of blood cells, or by inappropriate blood
flow.
Hematologic disorders include, but are not limited to, anemia associated with
chronic disease,
aplastic anemia, chronic neutropenia, and the myelodysplastic syndromes,
myocardial
infarction, and stroke.
The terms "neurological disorder" or "neurological disease" refer to or
describe a
disease or disorder of the central and/or peripheral nervous system and/or
specific neurons
that is typically characterized by deterioration of nervous tissue or
deterioration of
communication between cells in nervous tissue. Examples of neurological
disorders include,
but are not limited to, neurodegenerative diseases (including, but not limited
to, Lewy body
disease, postpoliomyelitis syndrome, Shy-Draeger syndrome, retinitis
pigmentosum
olivopontocerebellar atrophy, Parkinson's disease, spinal muscular atrophy,
multiple system
atrophy, amyotrophic lateral sclerosis, striatonigral degeneration,
tauopathies (including, but
not limited to, Alzheimer disease and supranuclear palsy), prion diseases
(including, but not
42
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
limited to, bovine spongiform encephalopathy, scrapie, Creutzfeldt-Jakob
syndrome, kuru,
Gerstmann-Straussler-Scheinker disease, chronic wasting disease, and fatal
familial
insomnia), bulbar palsy, motor neuron disease, and nervous system
heterodegenerative
disorders (including, but not limited to, Canavan disease, Huntington's
disease, neuronal
ceroid-lipofuscinosis, Alexander's disease, Tourette's syndrome, Menkes kinky
hair
syndrome, Cockayne syndrome, Halervorden-Spatz syndrome, lafora disease, Rett
syndrome,
hepatolenticular degeneration, Lesch-Nyhan syndrome, and Unverricht-Lundborg
syndrome),
dementia (including, but not limited to, Pick's disease), spinocerebellar
ataxia, ischemic and
hypoxic brain injury, and traumatic and excitotoxic brain damage.
A polypeptide, antibody, antagonist or composition of this invention which
"induces
cell death" or "induces apoptosis" is one which causes a viable cell to become
nonviable. In
certain embodiments, the cell is a cancer cell, e.g., a breast, ovarian,
stomach, endometrial,
salivary gland, lung, kidney, colon, thyroid, pancreatic or bladder cell. Cell
death in vitro can
be determined in the absence of complement and immune effector cells to
distinguish cell
death induced by antibody-dependent cell-mediated cytotoxicity (ADCC) or
complement
dependent cytotoxicity (CDC). Thus, the assay for cell death can be performed
using heat
inactivated serum (i.e., in the absence of complement) and in the absence of
immune effector
cells. To determine whether a polypeptide, antibody, antagonist or composition
of this
invention is able to induce cell death, loss of membrane integrity as
evaluated by uptake of
propidium iodide (PI), trypan blue (see Moore et al. Cytotechnology 17:1-11
(1995)) or
7AAD can be assessed relative to untreated cells.
A "ZPA-expres sing cell" is a cell which expresses an endogenous or
transfected ZPA
polypeptide. A "ZPA-expressing cancer" is a cancer comprising cells that
produce a ZPA
polypeptide. A cancer which "overexpresses" a ZPA polypeptide or a homolog
thereof is one
which has significantly higher levels of ZPA polypeptide or a homolog thereof
compared to a
noncancerous cell of the same tissue type. Such overexpression can be caused
by gene
amplification or by increased transcription or translation. ZPA polypeptide or
ZPA
polypeptide homolog overexpression can be determined in a diagnostic or
prognostic assay
by evaluating increased levels of the ZPA protein or ZPA protein homolog
present in the cell
(e.g., via an immunohistochemistry assay, etc.). Alternatively, or
additionally, one can
measure levels of ZPA polypeptide-encoding or ZPA polypeptide homolog-encoding
nucleic
acid or mRNA in the cell, e.g., via fluorescent in situ hybridization using a
nucleic acid based
probe corresponding to a ZPA-encoding nucleic acid or the complement thereof;
(FISH; see
43
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
W098/45479 published October, 1998), Southern blotting, Northern blotting, or
polymerase
chain reaction (PCR) techniques, such as real time quantitative PCR (RT-PCR).
Aside from
the above assays, various in vivo assays are available to the skilled
practitioner. For example,
one can expose cells within the body of the mammal to an internalizing
antibody which is
optionally labeled with a detectable label, e.g., a radioactive isotope, and
binding of the
antibody to a ZPA polypeptide or a ZPA polypeptide homolog in the mammal can
be
evaluated, e.g., by external scanning for radioactivity or by analyzing a
biopsy taken from a
mammal previously exposed to the antibody.
The word "label" when used herein refers to a detectable compound or
composition
which is conjugated directly or indirectly to the polypeptide, antibody,
antagonist or
composition so as to generate a "labeled" a polypeptide, antibody, antagonist
or composition.
The label can be detectable by itself (e.g. radioisotope labels or fluorescent
labels) or, in the
case of an enzymatic label, can catalyze chemical alteration of a substrate
compound or
composition which is detectable.
The term "cytotoxic agent" as used herein refers to a substance that inhibits
or
prevents the function of cells and/or causes destruction of cells. The term is
intended to
211 I131 Ii2s ~,9o Re186 Rei88 Smi53 Bi2i2 P32 and
include radioactive isotopes (e.g., At ,
, , , , , , ,
radioactive isotopes of Lu), chemotherapeutic agents e.g. methotrexate,
adriamicin, vinca
alkaloids (vincristine, vinblastine, etoposide), doxorubicin, melphalan,
mitomycin C,
chlorambucil, daunorubicin or other intercalating agents, enzymes and
fragments thereof such
as nucleolytic enzymes, antibiotics, and toxins such as small molecule toxins
or
enzymatically active toxins of bacterial, fungal, plant or animal origin,
including fragments
and/or variants thereof, and the various antitumor or anticancer agents
disclosed below.
Other cytotoxic agents are described below. A tumoricidal agent causes
destruction of tumor
cells.
A "chemotherapeutic agent" is a chemical compound useful in the treatment of
cancer. Examples of chemotherapeutic agents include alkylating agents such as
thiotepa and
CYTOXAN cyclosphosphamide; alkyl sulfonates such as busulfan, improsulfan and
piposulfan; aziridines such as benzodopa, carboquone, meturedopa, and uredopa;
ethylenimines and methylamelamines including altretamine, triethylenemelamine,
trietylenephosphoramide, triethiylenethiophosphoramide and
trimethylolomelamine;
acetogenins (especially bullatacin and bullatacinone); a camptothecin
(including the synthetic
analogue topotecan); bryostatin; callystatin; CC-1065 (including its
adozelesin, carzelesin
44
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
and bizelesin synthetic analogues); cryptophycins (particularly cryptophycin 1
and
cryptophycin 8); dolastatin; duocarmycin (including the synthetic analogues,
KW-2189 and
CB1-TM1); eleutherobin; pancratistatin; a sarcodictyin; spongistatin; nitrogen
mustards such
as chlorambucil, chlornaphazine, cholophosphamide, estramustine, ifosfamide,
mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembichin,
phenesterine, prednimustine, trofosfamide, uracil mustard; nitrosureas such as
carmustine,
chlorozotocin, fotemustine, lomustine, nimustine, and ranimnustine;
antibiotics such as the
enediyne antibiotics (e. g., calicheamicin, especially calicheamicin gammall
and
calicheamicin omegall (see, e.g., Agnew, Chem Intl. Ed. Engl., 33: 183-186
(1994));
dynemicin, including dynemicin A; bisphosphonates, such as clodronate; an
esperamicin; as
well as neocarzinostatin chromophore and related chromoprotein enediyne
antiobiotic
chromophores), aclacinomysins, actinomycin, authramycin, azaserine,
bleomycins,
cactinomycin, carabicin, carminomycin, carzinophilin, chromomycinis,
dactinomycin,
daunorubicin, detorubicin, 6-diazo-5-oxo-L-norleucine, ADRIAMYCIN doxorubicin
(including morpholino-doxorubicin, cyanomorpholino-doxorubicin, 2-pyrrolino-
doxorubicin
and deoxydoxorubicin), epirubicin, esorubicin, idarubicin, marcellomycin,
mitomycins such
as mitomycin C, mycophenolic acid, nogalamycin, olivomycins, peplomycin,
potfiromycin,
puromycin, quelamycin, rodorubicin, streptonigrin, streptozocin, tubercidin,
ubenimex,
zinostatin, zorubicin; anti-metabolites such as methotrexate and 5-
fluorouracil (5-FU); folic
acid analogues such as denopterin, methotrexate, pteropterin, trimetrexate;
purine analogs
such as fludarabine, 6-mercaptopurine, thiamiprine, thioguanine; pyrimidine
analogs such as
ancitabine, azacitidine, 6-azauridine, carmofur, cytarabine, dideoxyuridine,
doxifluridine,
enocitabine, floxuridine; androgens such as calusterone, dromostanolone
propionate,
epitiostanol, mepitiostane, testolactone; anti- adrenals such as
aminoglutethimide, mitotane,
trilostane; folic acid replenisher such as frolinic acid; aceglatone;
aldophosphamide
glycoside; aminolevulinic acid; eniluracil; amsacrine; bestrabucil;
bisantrene; edatraxate;
defofamine; demecolcine; diaziquone; elfornithine; elliptinium acetate; an
epothilone;
etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidainine; maytansinoids
such as
maytansine and ansamitocins; mitoguazone; mitoxantrone; mopidanmol;
nitraerine;
pentostatin; phenamet; pirarubicin; losoxantrone; podophyllinic acid; 2-
ethylhydrazide;
procarbazine; PSK polysaccharide complex (JHS Natural Products, Eugene, OR);
razoxane;
rhizoxin; sizofiran; spirogermanium; tenuazonic acid; triaziquone; 2,2',2"-
trichlorotriethylamine; trichothecenes (especially T-2 toxin, verracurin A,
roridin A and
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
anguidine); urethan; vindesine; dacarbazine; mannomustine; mitobronitol;
mitolactol;
pipobroman; gacytosine; arabinoside ("Ara-C"); cyclophosphamide; thiotepa;
taxoids, e.g.,
TAXOL paclitaxel (Bristol- Myers Squibb Oncology, Princeton, N.J.),
ABRAXANETM
Cremophor-free, albumin-engineered nanoparticle formulation of paclitaxel
(American
Pharmaceutical Partners, Schaumberg, Illinois), and TAXOTERE doxetaxel (Rh6ne-
Poulenc Rorer, Antony, France); chloranbucil; GEMZAR gemcitabine; 6-
thioguanine;
mercaptopurine; methotrexate; platinum analogs such as cisplatin and
carboplatin;
vinblastine; platinum; etoposide (VP-16); ifosfamide; mitoxantrone;
vincristine;
NAVELBINE vinorelbine; novantrone; teniposide; edatrexate; daunomycin;
aminopterin;
xeloda; ibandronate; CPT-11; topoisomerase inhibitor RFS 2000;
difluorometlhylornithine
(DMFO); retinoids such as retinoic acid; capecitabine; and pharmaceutically
acceptable salts,
acids or derivatives of any of the above.
Also included in this definition are anti-hormonal agents that act to regulate
or inhibit
hormone action on tumors such as anti-estrogens and selective estrogen
receptor modulators
(SERMs), including, for example, tamoxifen (including NOLVADEX tamoxifen),
raloxifene, droloxifene, 4-hydroxytamoxifen, trioxifene, keoxifene, LY117018,
onapristone,
and FARESTON= toremifene; aromatase inhibitors that inhibit the enzyme
aromatase, which
regulates estrogen production in the adrenal glands, such as, for example,
4(5)-imidazoles,
aminoglutethimide, MEGASE megestrol acetate, AROMASIN exemestane,
formestanie,
fadrozole, RIVISOR vorozole, FEMARA letrozole, and ARIMIDEX anastrozole;
and
anti-androgens such as flutamide, nilutamide, bicalutamide, leuprolide, and
goserelin; as well
as troxacitabine (a 1,3-dioxolane nucleoside cytosine analog); antisense
oligonucleotides,
particularly those which inhibit expression of genes in signaling pathways
implicated in
aberrant cell proliferation, such as, for example, PKC-alpha, Ralf and H-Ras;
ribozymes such
as a VEGF expression inhibitor (e.g., ANGIOZYME ribozyme) and a HER2
expression
inhibitor; vaccines such as gene therapy vaccines, for example, ALLOVECTIN
vaccine,
LEUVECTIN vaccine, and VAXID vaccine; PROLEUKIN rIL-2; LURTOTECAN
topoisomerase 1 inhibitor; ABARELIX rmRH; and pharmaceutically acceptable
salts, acids
or derivatives of any of the above.
A "growth inhibitory agent" when used herein refers to a compound or
composition
which inhibits growth of a cell, especially a cancer cell, either in vitro or
in vivo. Thus, the
growth inhibitory agent can be one which significantly reduces the percentage
of cells in S
phase. Examples of growth inhibitory agents include agents that block cell
cycle progression
46
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
(at a place other than S phase), such as agents that induce G1 arrest and M-
phase arrest.
Classical M-phase blockers include the vincas (vincristine and vinblastine),
taxanes, and
topoisomerase II inhibitors such as doxorubicin, epirubicin, daunorubicin,
etoposide, and
bleomycin. Those agents that arrest G1 also spill over into S-phase arrest,
for example, DNA
alkylating agents such as tamoxifen, prednisone, dacarbazine, mechlorethamine,
cisplatin,
methotrexate, 5-fluorouracil, and ara-C. Further information can be found in
The Molecular
Basis of Cancer, Mendelsohn and Israel, eds., Chapter 1, entitled "Cell cycle
regulation,
oncogenes, and antineoplastic drugs" by Murakami et al. (WB Saunders:
Philadelphia, 1995),
especially p. 13. The taxanes (paclitaxel and docetaxel) are anticancer drugs
both derived
from the yew tree. Docetaxel (TAXOTERE , Rhone-Poulenc Rorer), derived from
the
European yew, is a semisynthetic analogue of paclitaxel (TAXOL , Bristol-Myers
Squibb).
Paclitaxel and docetaxel promote the assembly of microtubules from tubulin
dimers and
stabilize microtubules by preventing depolymerization, which results in the
inhibition of
mitosis in cells.
"Doxorubicin" is an anthracycline antibiotic. The full chemical name of
doxorubicin
is (8S-cis)-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexapyranosyl)oxy]-7,8,9,10-
tetrahydro-
6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione.
The term "package insert" is used to refer to instructions customarily
included in
commercial packages of therapeutic products, that contain information about
the indications,
usage, dosage, administration, contraindications and/or warnings concerning
the use of such
therapeutic products.
Compositions and Methods of the Invention
ZPA Polypeptide Variants
In addition to the full-length native sequence ZPA polypeptides described
herein, it is
contemplated that ZPA polypeptide variants can be prepared. ZPA polypeptide
variants can
be prepared by introducing appropriate nucleotide changes into the ZPA nucleic
acid, and/or
by synthesis of the desired ZPA polypeptide. Those skilled in the art will
appreciate that
amino acid changes can alter post-translational processes of the ZPA
polypeptide such as
changing the number or position of glycosylation sites or altering membrane
anchoring
characteristics.
Variations in a native full-length sequence ZPA polypeptide or in various
domains of
a ZPA polypeptide described herein can be made, for example, using any of the
techniques
47
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
and guidelines for conservative and non-conservative mutations set forth, for
instance, in U.S.
Patent No. 5,364,934. Variations can be a substitution, deletion or insertion
of one or more
codons encoding a ZPA polypeptide that results in a change in the amino acid
sequence of the
ZPA polypeptide as compared with the native sequence ZPA polypeptide.
Optionally the
variation is by substitution of at least one amino acid with any other amino
acid in one or
more of the domains of the ZPA polypeptide. Guidance in determining which
amino acid
residue can be inserted, substituted or deleted without adversely affecting
the desired activity
can be found by comparing the sequence of a ZPA polypeptide with that of
homologous
known protein molecules and minimizing the number of amino acid sequence
changes made
in regions of high homology. Amino acid substitutions can be the result of
replacing one
amino acid with another amino acid having similar structural and/or chemical
properties,
such as the replacement of a leucine with a serine, i.e., conservative amino
acid replacements.
Insertions or deletions can optionally be in the range of about 1 to 5 amino
acids. The
variation allowed can be determined by systematically making insertions,
deletions or
substitutions of amino acids in the sequence and testing the resulting
variants for activity
exhibited by the full-length or mature native sequence.
In particular embodiments, conservative substitutions of interest are shown in
Table 1
under the heading of preferred substitutions. If such substitutions result in
a change in
biological activity, then more substantial changes, denominated exemplary
substitutions in
Table 1, or as further described below in reference to amino acid classes, are
introduced and
the products screened.
Table 1
Original Exemplary Preferred
Residue Substitutions Substitutions
Ala (A) val; leu; ile val
Arg (R) lys; gln; asn lys
Asn (N) gln; his; lys; arg gln
Asp (D) glu glu
Cys (C) ser ser
Gln (Q) asn asn
Glu (E) asp asp
Gly (G) pro; ala ala
His (H) asn; gln; lys; arg arg
48
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Ile (I) leu; val; met; ala; phe;
norleucine leu
Leu (L) norleucine; ile; val;
met; ala; phe ile
Lys (K) arg; gln; asn arg
Met (M) leu; phe; ile leu
Phe (F) leu; val; ile; ala; tyr leu
Pro (P) ala ala
Ser(S) thr thr
Thr (T) ser ser
Trp (W) tyr; phe tyr
Tyr (Y) trp; phe; thr; ser phe
Val (V) ile; leu; met; phe;
ala; norleucine leu
Substantial modiflcations in function or immunological identity of a ZPA
polypeptide
are accomplished by selecting substitutions that differ significantly in their
effect on
maintaining (a) the structure of the polypeptide backbone in the area of the
substitution, for
example, as a sheet or helical conformation, (b) the charge or hydrophobicity
of the molecule
at the target site, or (c) the bulk of the side chain. Naturally occurring
residues are divided
into groups based on common side-chain properties:
(1) hydrophobic: norleucine, met, ala, val, leu, ile;
(2) neutral hydrophilic: cys, ser, thr;
(3) acidic: asp, glu;
(4) basic: asn, gln, his, lys, arg;
(5) residues that influence chain orientation: gly, pro; and
(6) aromatic: trp, tyr, phe.
Non-conservative substitutions will entail exchanging a member of one of these
classes for another class. Such substituted residues also can be introduced
into the
conservative substitution sites or into the remaining (non-conserved) sites.
Variations can be made using methods known in the art such as oligonucleotide-
mediated (site-directed) mutagenesis, alanine scanning, and PCR mutagenesis.
Site-directed
mutagenesis [Carter et al., Nucl. Acids Res., 13:4331 (1986); Zoller et al.,
Nucl. Acids Res.,
49
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
10:6487 (1987)], cassette mutagenesis [Wells et al., Gene, 34:315 (1985)],
restriction
selection mutagenesis [Wells et al., Philos. Trans. R. Soc. London SerA,
317:415 (1986)] or
other known techniques can be performed on the cloned DNA to produce a ZPA
variant
DNA.
Scanning amino acid analysis can also be employed to identify one or more
amino
acids along a contiguous sequence. In certain embodiments, scanning amino
acids are
relatively small, neutral amino acids. Such amino acids include alanine,
glycine, serine, and
cysteine. Alanine is typically a preferred scanning amino acid among this
group because it
eliminates the side-chain beyond the beta-carbon and is less likely to alter
the main-chain
conformation of the variant [Cunningham and Wells, Science, 244: 1081-1085
(1989)].
Alanine is also typically preferred because it is the most common amino acid.
Further, it is
frequently found in both buried and exposed positions [Creighton, The
Proteins, (W.H.
Freeman & Co., N.Y.); Chothia, J. Mol. Biol., 150:1 (1976)]. If alanine
substitution does not
yield adequate amounts of variant, an isoteric amino acid can be used.
Modifications of ZPA polypeptides
Covalent modifications of ZPA polypeptides are included within the scope of
this
invention. One type of covalent modification includes reacting targeted amino
acid residues
of a ZPA polypeptide with an organic derivatizing agent that is capable of
reacting with
selected side chains or the N- or C- terminal residues of the ZPA polypeptide.
Derivatization
with bifunctional agents is useful, for instance, for crosslinking the ZPA
polypeptide to a
water-insoluble support matrix or surface for use in the method for purifying
anti- ZPA
antibodies, and vice-versa. Commonly used crosslinking agents include, e.g.,
1,1-bis(diazo-
acetyl)-2-phenylethane, glutaraldehyde, N-hydroxysuccinimide esters, for
example, esters
with 4-azidosalicylic acid, homobifunctional imidoesters, including
disuccinimidyl esters
such as 3,3'-dithiobis(succinimidylpropionate), bifunctional maleimides such
as bis-N-
maleimido- 1, 8 -octane and agents such as methyl-3-[(p-
azidophenyl)dithio]propioimidate.
Other modifications include deamidation of glutaminyl and asparaginyl residues
to
the corresponding glutamyl and aspartyl residues, respectively, hydroxylation
of proline and
lysine, phosphorylation of hydroxyl groups of seryl or threonyl residues,
methylation of the
a-amino groups of lysine, arginine, and histidine side chains [T.E. Creighton,
Proteins:
Structure and Molecular Properties, W.H. Freeman & Co., San Francisco, pp. 79-
86 (1983)],
acetylation of the N-terminal amine, and amidation of any C-terminal carboxyl
group.
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Another type of covalent modification of a ZPA polypeptide included within the
scope of this invention comprises altering the native glycosylation pattern of
the polypeptide.
"Altering the native glycosylation pattern" is intended for purposes herein to
mean deleting
one or more carbohydrate moieties found in the native sequence ZPA polypeptide
(either by
removing the underlying glycosylation site or by deleting the glycosylation by
chemical
and/or enzymatic means), and/or adding one or more glycosylation sites that
are not present
in the native sequence ZPA polypeptide. In addition, the phrase includes
qualitative changes
in the glycosylation of the native proteins, involving a change in the nature
and proportions of
the various carbohydrate moieties present.
Addition of glycosylation sites to a ZPA polypeptide can be accomplished by
altering
the amino acid sequence. The alteration can be made, for example, by the
addition of, or
substitution by, one or more serine or threonine residues to the native
sequence ZPA
polypeptide (for 0-linked glycosylation sites). The ZPA amino acid sequence
can optionally
be altered through changes at the DNA level, particularly by mutating the DNA
encoding the
ZPA polypeptide at preselected bases such that codons are generated that will
translate into
the desired amino acids.
Another means of increasing the number of carbohydrate moieties on a ZPA
polypeptide is by chemical or enzymatic coupling of glycosides to the
polypeptide. Such
methods are described in the art, e.g., in WO 87/05330 published 11 September
1987, and in
Aplin and Wriston, CRC Crit. Rev. Biochem., pp. 259-306 (1981).
Removal of carbohydrate moieties present on a ZPA polypeptide can be
accomplished
chemically or enzymatically or by mutational substitution of codons encoding
for amino acid
residues that serve as targets for glycosylation. Chemical deglycosylation
techniques are
known in the art and described, for instance, by Hakimuddin, et al., Arch.
Biochem.
Biophys., 259:52 (1987) and by Edge et al., Anal. Biochem., 118:131 (1981).
Enzymatic
cleavage of carbohydrate moieties on polypeptides can be achieved by the use
of a variety of
endo- and exo-glycosidases as described by Thotakura et al., Meth. Enzymol.,
138:350
(1987).
Another type of covalent modification of a ZPA polypeptide comprises linking
the
ZPA polypeptide to one of a variety of nonproteinaceous polymers, e.g.,
polyethylene glycol
(PEG), polypropylene glycol, or polyoxyalkylenes, in the manner set forth in
U.S. Patent
Nos. 4,640,835; 4,496,689; 4,301,144; 4,670,417; 4,791,192 or 4,179,337.
51
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
A ZPA polypeptide of the present invention can also be modified in a way to
form a
chimeric molecule comprising a ZPA polypeptide fused to another, heterologous
polypeptide
or amino acid sequence.
In one embodiment, such a chimeric molecule comprises a fusion of a ZPA
polypeptide with a protein transduction domain which targets the ZPA
polypeptide for
delivery to various tissues. In one aspect, the ZPA polypeptide is targeted to
the brain across
the brain blood barrier, using, for example, the protein transduction domain
of human
immunodeficiency virus TAT protein (Schwarze et al., 1999, Science 285: 1569-
72).
In another embodiment, such a chimeric molecule comprises a fusion of a ZPA
polypeptide with a tag polypeptide which provides an epitope to which an anti-
tag antibody
can selectively bind. The epitope tag is generally placed at the amino- or
carboxyl- terminus
of the ZPA polypeptide. The presence of such epitope-tagged forms of the ZPA
polypeptide
can be detected using an antibody against the tag polypeptide. Also, provision
of the epitope
tag enables the ZPA polypeptide to be readily purified by affinity
purification using an anti-
tag antibody or another type of affinity matrix that binds to the epitope tag.
Various tag
polypeptides and their respective antibodies are known in the art. Examples
include poly-
histidine (poly-His) or poly-histidine-glycine (poly-His-gly) tags; the flu HA
tag polypeptide
and its antibody 12CA5 [Field et al., Mol. Cell. Biol., 8:2159-2165 (1988)];
the c-myc tag
and the 8F9, 3C7, 6E10, G4, B7 and 9E10 antibodies thereto [Evan et al.,
Molecular and
Cellular Biolo", 5:3610-3616 (1985)]; and the Herpes Simplex virus
glycoprotein D (gD)
tag and its antibody [Paborsky et al., Protein En 'n~ eering, 3 6:547-553
(1990)]. Other tag
polypeptides include the Flag-peptide [Hopp et al., BioTechnology, 6:1204-1210
(1988)]; the
KT3 epitope peptide [Martin et al., Science, 255:192-194 (1992)]; an a-tubulin
epitope
peptide [Skinner et al., J. Biol. Chem., 266:15163-15166 (1991)]; and the T7
gene 10 protein
peptide tag [Lutz-Freyermuth et al., Proc. Natl. Acad. Sci. USA, 87:6393-6397
(1990)].
In an alternative embodiment, the chimeric molecule can comprise a fusion of a
ZPA
polypeptide with an immunoglobulin or a particular region of an
immunoglobulin. For a
bivalent form of the chimeric molecule (also referred to as an
"immunoadhesin"), such a
fusion could be to the Fc region of an IgG molecule. In one embodiment, the
immunoglobulin fusion includes the hinge, CH2 and CH3, or the hinge, CH1, CH2
and CH3
regions of an IgGI molecule. For the production of immunoglobulin fusions see
also, U.S.
Patent No. 5,428,130.
52
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Preparation of a ZPA polypeptide
The description below relates primarily to production of ZPA polypeptides by
culturing cells transformed or transfected with a vector containing nucleic
acid encoding ZPA
polypeptides. It is, of course, contemplated that alternative methods that are
known in the art
can be employed to prepare a ZPA polypeptide. For instance, a ZPA polypeptide
sequence,
or portions thereof, can be produced by direct peptide synthesis using solid-
phase techniques.
See, e.g., Stewart et al., Solid-Phase Peptide Synthesis (W.H. Freeman Co.:
San Francisco,
CA, 1969); Merrifield, J. Am. Chem. Soc., 85: 2149-2154 (1963). In vitro
protein synthesis
can be performed using manual techniques or by automation. Automated synthesis
can be
accomplished, for instance, with an Applied Biosystems Peptide Synthesizer
(Foster City,
CA) using manufacturer's instructions. Various portions of a ZPA polypeptide
can be
chemically synthesized separately and combined using chemical or enzymatic
methods to
produce the full-length ZPA polypeptide.
Selection and Transformation of Host Cells
Host cells are transfected or transformed with expression or cloning vectors
described
herein for ZPA polypeptide production and cultured in conventional nutrient
media modified
as appropriate for inducing promoters, selecting transformants, or amplifying
the genes
encoding the desired sequences. The culture conditions, such as media,
temperature, pH, and
the like, can be selected by the skilled artisan without undue
experimentation. In general,
principles, protocols, and practical techniques for maximizing the
productivity of cell cultures
can be found in Mammalian Cell Biotechnology: A Practical Approach, M. Butler,
ed. (IRL
Press, 1991) and Sambrook et al., supra.
Methods of transfection are known to the ordinarily skilled artisan, for
example,
CaPO4 treatment and electroporation. Depending on the host cell used,
transformation is
performed using standard techniques appropriate to such cells. The calcium
treatment
employing calcium chloride, as described in Sambrook et al., supra, or
electroporation is
generally used for prokaryotes or other cells that contain substantial cell-
wall barriers.
Infection with Agrobacterium tumefaciens is used for transformation of certain
plant cells, as
described by Shaw et al., Gene, 23: 315 (1983) and WO 89/05859 published 29
June 1989.
For mammalian cells without such cell walls, the calcium phosphate
precipitation method of
Graham and van der Eb, Virology, 52:456-457 (1978) can be employed. General
aspects of
mammalian cell host system transformations have been described in U.S. Patent
No.
53
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
4,399,216. Transformations into yeast are typically carried out according to
the method of
Van Solingen et al., J. Bact., 130: 946 (1977) and Hsiao et al., Proc. Natl.
Acad. Sci. (USA),
76: 3829 (1979). However, other methods for introducing DNA into cells, such
as by nuclear
microinjection, electroporation, bacterial protoplast fusion with intact
cells, or polycations,
e.g., polybrene or polyornithine, can also be used. For various techniques for
transforming
mammalian cells, see, Keown et al., Methods in Enzymolo~y, 185: 527-537 (1990)
and
Mansour et al., Nature, 336: 348-352 (1988).
Suitable host cells for cloning or expressing the DNA in the vectors herein
include
prokaryote, yeast, or higher eukaryote cells. Suitable prokaryotes include,
but are not limited
to, eubacteria, such as Gram-negative or Gram-positive organisms, for example,
Enterobacteriaceae such as E. coli. Various E. coli strains are publicly
available, such as E.
coli K12 strain MM294 (ATCC 31,446); E. coli X1776 (ATCC 31,537); E. coli
strain W3110
(ATCC 27,325); and K5 772 (ATCC 53,635). Other suitable prokaryotic host cells
include
Enterobacteriaceae such as Escherichia, e.g., E. coli, Enterobacter, Erwinia,
Klebsiella,
Proteus, Salmonella, e.g., Salmonella typhimurium, Serratia, e.g., Serratia
marcescans, and
Shigella, as well as Bacilli such as B. subtilis and B. licheniformis (e.g.,
B. licheniformis 41P
disclosed in DD 266,710 published 12 April 1989), Pseudomonas such as P.
aeruginosa, and
Streptomyces. These examples are illustrative rather than limiting. In one
embodiment,
strain W3110 is the host or parent host because it is a common host strain for
recombinant
DNA product fermentations. In certain embodiments, the host cell secretes
minimal amounts
of proteolytic enzymes. For example, strain W3110 can be modified to effect a
genetic
mutation in the genes encoding proteins endogenous to the host, with examples
of such hosts
including E. coli W3110 strain 1A2, which has the complete genotype tonA ; E.
coli W3110
strain 9E4, which has the complete genotype tonA ptr3; E. coli W3110 strain
27C7 (ATCC
55,244), which has the complete genotype tonA ptr3 phoA E15 (argF-lac)169 degP
ompT
kanr; E. coli W3110 strain 37D6, which has the complete genotype tonA ptr3
phoA E15
(argF-lac) 169 degP ompT rbs7 ilvG kanr; E. coli W3110 strain 40B4, which is
strain 37D6
with a non-kanamycin resistant degP deletion mutation; and an E. coli strain
having mutant
periplasmic protease disclosed in U.S. Patent No. 4,946,783 issued 7 August
1990.
Alternatively, in vitro methods of cloning, e.g., PCR or other nucleic acid
polymerase
reactions, are suitable.
In addition to prokaryotes, eukaryotic microbes such as filamentous fungi or
yeast are
suitable cloning or expression hosts for vectors encoding a ZPA polypeptide.
Saccharomyces
54
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
cerevisiae is a commonly used lower eukaryotic host microorganism. Others
include
Schizosaccharomycespombe (Beach and Nurse, Nature, 290: 140 [1981]; EP 139,383
published 2 May 1985); Kluyveromyces hosts (U.S. Patent No. 4,943,529; Fleer
et al.,
Bio/Technolo9: 968-975 (1991)) such as, e.g., K. lactis (MW98-8C, CBS683,
CBS4574;
Louvencourt et al., J. Bacteriol., 737 [1983]), K. fragilis (ATCC 12,424), K
bulgaricus
(ATCC 16,045), K. wickeramii (ATCC 24,178), K. waltii (ATCC 56,500), K.
drosophilarum
(ATCC 36,906; Van den Berg et al., Bio/Technolo8: 135 (1990)), K.
thermotolerans, and
K. marxianus; yarrowia (EP 402,226); Pichia pastoris (EP 183,070; Sreekrishna
et al., J.
Basic Microbiol., 28: 265-278 [1988]); Candida; Trichoderma reesia (EP
244,234);
Neurospora crassa (Case et al., Proc. Natl. Acad. Sci. USA, 76: 5259-5263
[1979]);
Schwanniomyces such as Schwanniomyces occidentalis (EP 394,538 published 31
October
1990); and filamentous fungi such as, e.g., Neurospora, Penicillium,
Tolypocladium (WO
91/00357 published 10 January 1991), and Aspergillus hosts such as A. nidulans
(Ballance et
al., Biochem. Biophys. Res. Commun., 112: 284-289 [1983]; Tilburn et al.,
Gene, 26: 205-
221 [1983]; Yelton et al., Proc. Natl. Acad. Sci. USA, 81: 1470-1474 [1984])
and A. niger
(Kelly and Hynes, EMBO J., 4: 475-479 [1985]). Methylotropic yeasts are
suitable herein
and include, but are not limited to, yeast capable of growth on methanol
selected from the
genera consisting of Hansenula, Candida, Kloeckera, Pichia, Saccharomyces,
Torulopsis,
and Rhodotorula. A list of specific species that are exemplary of this class
of yeasts can be
found in C. Anthony, The Biochemistry of Methylotrophs, 269 (1982).
Suitable host cells for the expression of nucleic acid encoding a ZPA
polypeptide are
derived from multicellular organisms. Examples of invertebrate cells include
insect cells
such as Drosophila S2 and Spodoptera Sf9, as well as plant cells. Examples of
useful
mammalian host cell lines include Chinese hamster ovary (CHO) and COS cells.
More
specific examples include monkey kidney CV 1 line transformed by SV40 (COS-7,
ATCC
CRL 1651); human embryonic kidney line (293 or 293 cells subcloned for growth
in
suspension culture, Graham et al., J. Gen. Virol., 36: 59 (1977)); Chinese
hamster ovary
cells/-DHFR (CHO, Urlaub and Chasin, Proc. Natl. Acad. Sci. USA, 77:4216
(1980)); mouse
sertoli cells (TM4, Mather, Biol. Reprod., 23:243-251 (1980)); human lung
cells (W138,
ATCC CCL 75); human liver cells (Hep G2, HB 8065); and mouse mammary tumor
(MMT
060562, ATCC CCL51). The selection of the appropriate host cell is deemed to
be within the
skill in the art.
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Selection and Use of a Replicable Vector
The nucleic acid (e.g., cDNA or genomic DNA) encoding a polypeptide or
antibody
of this invention can be inserted into a replicable vector for cloning
(amplification of the
DNA) or for expression. Various vectors are publicly available. The vector
can, for
example, be in the form of a plasmid, cosmid, viral particle, or phage. The
appropriate
nucleic acid sequence can be inserted into the vector by a variety of
procedures. In general,
DNA is inserted into an appropriate restriction endonuclease site(s) using
techniques known
in the art. Vector components generally include, but are not limited to, one
or more of a
signal sequence if the sequence is to be secreted, an origin of replication,
one or more marker
genes, an enhancer element, a promoter, and a transcription termination
sequence.
Construction of suitable vectors containing one or more of these components
employs
standard ligation techniques that are known to the skilled artisan.
The polypeptide or antibody of this invention can be produced recombinantly
not only
directly, but also as a fusion polypeptide with a heterologous polypeptide,
which can be a
signal sequence or other polypeptide having a specific cleavage site at the N-
terminus of the
mature protein or polypeptide. In general, the signal sequence can be a
component of the
vector, or it can be a part of the DNA encoding the polypeptide or antibody
that is inserted
into the vector. The signal sequence can be a prokaryotic signal sequence
selected, for
example, from the group of the alkaline phosphatase, penicillinase, lpp, or
heat-stable
enterotoxin II leaders. For yeast secretion the signal sequence can be, e.g.,
the yeast invertase
leader, alpha factor leader (including Saccharomyces and Kluyveromyces a-
factor leaders, the
latter described in U.S. Patent No. 5,010,182), or acid phosphatase leader,
the C. albicans
glucoamylase leader (EP 362,179 published 4 April 1990), or the signal
described in WO
90/13646 published 15 November 1990. In mammalian cell expression, mammalian
signal
sequences can be used to direct secretion of the protein, such as signal
sequences from
secreted polypeptides of the same or related species, as well as viral
secretory leaders.
Both expression and cloning vectors contain a nucleic acid sequence that
enables the
vector to replicate in one or more selected host cells. Such sequences are
known for a variety
of bacteria, yeast, and viruses. The origin of replication from the plasmid
pBR322 is suitable
for most Gram-negative bacteria, the 2q plasmid origin is suitable for yeast,
and various viral
origins (SV40, polyoma, adenovirus, VSV, or BPV) are useful for cloning
vectors in
mammalian cells.
56
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Expression and cloning vectors will typically contain a selection gene, also
termed a
selectable marker. Typical selection genes encode proteins that (a) confer
resistance to
antibiotics or other toxins, e.g., ampicillin, neomycin, methotrexate, or
tetracycline, (b)
complement auxotrophic deficiencies, or (c) supply critical nutrients not
available from
complex media, e.g., the gene encoding D-alanine racemase for Bacilli.
An example of suitable selectable markers for mammalian cells are those that
enable
the identification of cells competent to take up the nucleic acid encoding the
polypeptide or
antibody such as DHFR or thymidine kinase. An appropriate host cell when wild-
type DHFR
is employed is the CHO cell line deficient in DHFR activity, prepared and
propagated as
described by Urlaub et al., Proc. Natl. Acad. Sci. USA, 77: 4216 (1980). A
suitable selection
gene for use in yeast is the trp 1 gene present in the yeast plasmid YRp7.
Stinchcomb et al.,
Nature, 282: 39 (1979); Kingsman et al., Gene, 7: 141 (1979); Tschemper et
al., Gene, 10:
157 (1980). The trpl gene provides a selection marker for a mutant strain of
yeast lacking
the ability to grow in tryptophan, for example, ATCC No. 44076 or PEP4-1.
Jones, Genetics,
85: 12 (1977).
Expression and cloning vectors usually contain a promoter operably linked to
the
nucleic acid sequence encoding the polypeptide or antibody of this invention
to direct mRNA
synthesis. Promoters recognized by a variety of potential host cells are
known. Promoters
suitable for use with prokaryotic hosts include the (3-lactamase and lactose
promoter systems
(Chang et al., Nature, 275: 615 (1978); Goeddel et al., Nature, 281: 544
(1979)), alkaline
phosphatase, a tryptophan (trp) promoter system (Goeddel, Nucleic Acids Res.,
8: 4057
(1980); EP 36,776), and hybrid promoters such as the tac promoter (deBoer et
al., Proc. Natl.
Acad. Sci. USA, 80: 21-25 (1983)). Promoters for use in bacterial systems also
will contain a
Shine-Dalgarno (S.D.) sequence operably linked to the DNA encoding the
polypeptide or
antibody of this invention.
Examples of suitable promoting sequences for use with yeast hosts include the
promoters for 3-phosphoglycerate kinase (Hitzeman et al., J. Biol. Chem., 255:
2073 (1980))
or other glycolytic enzymes (Hess et al., J. Adv. Enzyme Reg., 7: 149 (1968);
Holland,
Biochemistry, 17: 4900 (1978)), such as enolase, glyceraldehyde-3 -phosphate
dehydrogenase, hexokinase, pyruvate decarboxylase, phosphofructokinase,
glucose-6-
phosphate isomerase, 3-phosphoglycerate mutase, pyruvate kinase,
triosephosphate
isomerase, phosphoglucose isomerase, and glucokinase.
57
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Other yeast promoters that are inducible promoters having the additional
advantage of
transcription controlled by growth conditions are the promoter regions for
alcohol
dehydrogenase 2, isocytochrome C, acid phosphatase, degradative enzymes
associated with
nitrogen metabolism, metallothionein, glyceraldehyde-3 -phosphate
dehydrogenase, and
enzymes responsible for maltose and galactose utilization. Suitable vectors
and promoters
for use in yeast expression are further described in EP 73,657.
Nucleic acid transcription from vectors in mammalian host cells is controlled,
for
example, by promoters obtained from the genomes of viruses such as polyoma
virus, fowlpox
virus (UK 2,211,504 published 5 July 1989), adenovirus (such as Adenovirus 2),
bovine
papilloma virus, avian sarcoma virus, cytomegalovirus, a retrovirus, hepatitis-
B virus, and
Simian Virus 40 (SV40); by heterologous mammalian promoters, e.g., the actin
promoter or
an immunoglobulin promoter; and by heat-shock promoters, provided such
promoters are
compatible with the host cell systems.
Transcription of a DNA encoding a polypeptide or antibody of this invention by
higher eukaryotes can be increased by inserting an enhancer sequence into the
vector.
Enhancers are cis-acting elements of DNA, usually about from 10 to 300 bp,
that act on a
promoter to increase its transcription. Many enhancer sequences are now known
from
mammalian genes (globin, elastase, albumin, a-fetoprotein, and insulin).
Typically, however,
one will use an enhancer from a eukaryotic cell virus. Examples include the
SV40 enhancer
on the late side of the replication origin (bp 100-270), the cytomegalovirus
early promoter
enhancer, the polyoma enhancer on the late side of the replication origin, and
adenovirus
enhancers. The enhancer can be spliced into the vector at a position 5' or 3'
to the sequence
coding for a polypeptide or antibody of this invention, but is preferably
located at a site 5'
from the promoter. Expression vectors used in eukaryotic host cells (yeast,
fungi, insect,
plant, animal, human, or nucleated cells from other multicellular organisms)
will also contain
sequences necessary for the termination of transcription and for stabilizing
the mRNA. Such
sequences are commonly available from the 5' and, occasionally 3',
untranslated regions of
eukaryotic or viral DNAs or cDNAs. These regions contain nucleotide segments
transcribed
as polyadenylated fragments in the untranslated portion of the mRNA encoding
polypeptide
or antibody.
Still other methods, vectors, and host cells suitable for adaptation to the
synthesis of
the polypeptide or antibody of this invention in recombinant vertebrate cell
culture are
58
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
described in Gething et al., Nature, 293: 620-625 (1981); Mantei et al.,
Nature, 281: 40-46
(1979); EP 117,060; and EP 117,058.
Detecting Gene Amplification/Expression
Gene amplification and/or expression can be measured in a sample directly, for
example, by conventional Southern blotting, Northern blotting to quantitate
the transcription
of mRNA (Thomas, Proc. Natl. Acad. Sci. USA, 77:5201-5205 (1980)), dot
blotting (DNA
analysis), or in situ hybridization, using an appropriately labeled probe,
based on the
sequences provided herein. Alternatively, antibodies can be employed that can
recognize
specific duplexes, including DNA duplexes, RNA duplexes, and DNA-RNA hybrid
duplexes
or DNA-protein duplexes. The antibodies in turn can be labeled and the assay
can be carried
out where the duplex is bound to a surface, so that upon the formation of
duplex on the
surface, the presence of antibody bound to the duplex can be detected.
Gene expression, alternatively, can be measured by immunological methods, such
as
immunohistochemical staining of cells or tissue sections and assay of cell
culture or body
fluids, to quantitate directly the expression of gene product. Antibodies
useful for
immunohistochemical staining and/or assay of sample fluids can be either
monoclonal or
polyclonal, and can be prepared in any mammal or can be synthesized (e.g., the
monoclonal
antibodies of this invention). Conveniently, the antibodies can be prepared
against a native-
sequence ZPA polypeptide or against a synthetic peptide based on the DNA
sequences
provided herein or against exogenous sequence fused to DNA encoding a ZPA
polypeptide
and encoding a specific antibody epitope.
Purification of ZPA polypeptides
Forms of ZPA polypeptides can be recovered from culture medium or from host
cell
lysates. If membrane-bound, it can be released from the membrane using a
suitable detergent
solution (e.g., TRITON-XTM 100) or by enzymatic cleavage. Cells employed in
expression
of nucleic acid encoding a ZPA polypeptide can be disrupted by various
physical or chemical
means, such as freeze-thaw cycling, sonication, mechanical disruption, or cell-
lysing agents.
According to one embodiment, it is desirable that a ZPA polypeptide is
purified from
recombinant cell proteins or polypeptides. The following procedures are
exemplary of
suitable purification procedures: by fractionation on an ion-exchange column;
ethanol
precipitation; reverse phase HPLC; chromatography on silica or on a cation-
exchange resin
59
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
such as DEAE; chromatofocusing; SDS-PAGE; ammonium sulfate precipitation; gel
filtration using, for example, Sephadex G-75; protein A Sepharose columns to
remove
contaminants such as IgG; and metal chelating columns to bind epitope-tagged
forms of the
ZPA polypeptide. Various methods of protein purification can be employed and
such
methods are known in the art and described, for example, in Deutscher, Methods
in
Enzymology, 182 (1990); Scopes, Protein Purification: Principles and Practice
(Springer-
Verlag: New York, 1982). The purification step(s) selected will depend, for
example, on the
nature of the production process used and the particular ZPA polypeptide
produced.
According to one embodiment, a ZPA polypeptide is purified by affinity
chromatography
using an antibody of this invention.
Assaying inhibition of cell proliferation
The inhibitory activity of one or more ZPA polypeptides and/or agonists of
this
invention on cell growth and proliferation can be measured using the assays
described herein
and other assays known in the art.
Animal models of tumors and cancers (e.g., breast cancer, colon cancer,
prostate
cancer, lung cancer, etc.) include both non-recombinant and recombinant
(transgenic)
animals. Non-recombinant animal models include, for example, rodent, e.g.,
murine models.
Such models can be generated by introducing tumor cells into syngeneic mice
using standard
techniques, e.g., subcutaneous injection, tail vein injection, spleen
implantation,
intraperitoneal implantation, implantation under the renal capsule, or
orthopin implantation,
e.g., colon cancer cells implanted in colonic tissue. See, e.g., PCT
publication No. WO
97/33551, published September 18, 1997. Probably the most often used animal
species in
oncological studies are immunodeficient mice and, in particular, nude mice.
The observation
that the nude mouse with thymic hypo/aplasia could successfully act as a host
for human
tumor xenografts has led to its widespread use for this purpose. The autosomal
recessive nu
gene has been introduced into a very large number of distinct congenic strains
of nude mouse,
including, for example, ASW, A/He, AKR, BALB/c, B10.LP, C17, C3H, C57BL, C57,
CBA,
DBA, DDD, I/st, NC, NFR, NFS, NFS/N, NZB, NZC, NZW, P, RIII, and SJL. In
addition, a
wide variety of other animals with inherited immunological defects other than
the nude
mouse have been bred and used as recipients of tumor xenografts. For further
details see,
e.g., The Nude Mouse in Oncology Research, E. Boven and B. Winograd, eds. (CRC
Press,
Inc., 1991).
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
The cells introduced into such animals can be derived from known tumor/cancer
cell
lines, such as any of the above-listed tumor cell lines, and, for example, the
B104-1-1 cell
line (stable NIH-3T3 cell line transfected with the neu protooncogene); ras-
transfected NIH-
3T3 cells; Caco-2 (ATCC HTB-37); or a moderately well-differentiated grade II
human colon
adenocarcinoma cell line, HT-29 (ATCC HTB-38); or from tumors and cancers.
Samples of
tumor or cancer cells can be obtained from patients undergoing surgery, using
standard
conditions involving freezing and storing in liquid nitrogen. Karmali et al.,
Br. J. Cancer, 48:
689-696 (1983).
Tumor cells can be introduced into animals such as nude mice by a variety of
procedures. The subcutaneous (s.c.) space in mice is very suitable for tumor
implantation.
Tumors can be transplanted s.c. as solid blocks, as needle biopsies by use of
a trochar, or as
cell suspensions. For solid-block or trochar implantation, tumor tissue
fragments of suitable
size are introduced into the s.c. space. Cell suspensions are freshly prepared
from primary
tumors or stable tumor cell lines, and injected subcutaneously. Tumor cells
can also be
injected as subdermal implants. In this location, the inoculum is deposited
between the lower
part of the dermal connective tissue and the s.c. tissue.
Animal models of breast cancer can be generated, for example, by implanting
rat
neuroblastoma cells (from which the neu oncogene was initially isolated), or
neu-transformed
NIH-3T3 cells into nude mice, essentially as described by Drebin et al. Proc.
Nat. Acad. Sci.
USA, 83: 9129-9133 (1986).
Similarly, animal models of colon cancer can be generated by passaging colon
cancer
cells in animals, e.g., nude mice, leading to the appearance of tumors in
these animals. An
orthotopic transplant model of human colon cancer in nude mice has been
described, for
example, by Wang et al., Cancer Research, 54: 4726-4728 (1994) and Too et al.,
Cancer
Research, 55: 681-684 (1995). This model is based on the so-called
"METAMOUSETM"
sold by AntiCancer, Inc., (San Diego, California).
Tumors that arise in animals can be removed and cultured in vitro. Cells from
the in
vitro cultures can then be passaged to animals. Such tumors can serve as
targets for further
testing or drug screening. Alternatively, the tumors resulting from the
passage can be
isolated and RNA from pre-passage cells and cells isolated after one or more
rounds of
passage analyzed for differential expression of genes of interest. Such
passaging techniques
can be performed with any known tumor or cancer cell lines.
61
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
For example, Meth A, CMS4, CMS5, CMS21, and WEHI-164 are chemically induced
fibrosarcomas of BALB/c female mice (DeLeo et al., J. Exp. Med., 146: 720
(1977)), which
provide a highly controllable model system for studying the anti-tumor
activities of various
agents. Palladino et al., J. Immunol., 138: 4023-4032 (1987). Briefly, tumor
cells are
propagated in vitro in cell culture. Prior to injection into the animals, the
cell lines are
washed and suspended in buffer, at a cell density of about 10x106 to 10x10'
cells/ml. The
animals are then infected subcutaneously with 10 to 100 l of the cell
suspension, allowing
one to three weeks for a tumor to appear.
In addition, the Lewis lung (3LL) carcinoma of mice, which is one of the most
thoroughly studied experimental tumors, can be used as an investigational
tumor model.
Efficacy in this tumor model has been correlated with beneficial effects in
the treatment of
human patients diagnosed with small-cell carcinoma of the lung (SCCL). This
tumor can be
introduced in normal mice upon injection of tumor fragments from an affected
mouse or of
cells maintained in culture. Zupi et al., Br. J. Cancer, 41: suppl. 4, 30
(1980). Evidence
indicates that tumors can be started from injection of even a single cell and
that a very high
proportion of infected tumor cells survive. For further information about this
tumor model
see, Zacharski, Haemostasis, 16: 300-320 (1986).
One way of evaluating the efficacy of a test compound in an animal model with
an
implanted tumor is to measure the size of the tumor before and after
treatment. Traditionally,
the size of implanted tumors has been measured with a slide caliper in two or
three
dimensions. The measure limited to two dimensions does not accurately reflect
the size of
the tumor; therefore, it is usually converted into the corresponding volume by
using a
mathematical formula. However, the measurement of tumor size is very
inaccurate. The
therapeutic effects of a drug candidate can be better described as treatment-
induced growth
delay and specific growth delay. Another important variable in the description
of tumor
growth is the tumor volume doubling time. Computer programs for the
calculation and
description of tumor growth are also available, such as the program reported
by Rygaard and
Spang-Thomsen, Proc. 6th Int. Workshop on Immune-Deficient Animals, Wu and
Sheng eds.
(Basel, 1989), p. 301. It is noted, however, that necrosis and inflammatory
responses
following treatment can actually result in an increase in tumor size, at least
initially.
Therefore, these changes need to be carefully monitored, by a combination of a
morphometric method and flow cytometric analysis.
62
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Further, recombinant (transgenic) animal models can be engineered by
introducing
the coding portion of a ZPA gene identified herein into the genome of animals
of interest,
using standard techniques for producing transgenic animals. Animals that can
serve as a
target for transgenic manipulation include, without limitation, mice, rats,
rabbits, guinea pigs,
sheep, goats, pigs, zebrafish, and non-human primates, e.g., baboons,
chimpanzees and
monkeys. Techniques known in the art to introduce a transgene into such
animals include
pronucleic microinjection (U.S. Patent No. 4,873,191); retrovirus-mediated
gene transfer into
germ lines (e.g., Van der Putten et al., Proc. Natl. Acad. Sci. USA, 82: 6148-
615 (1985));
gene targeting in embryonic stem cells (Thompson et al., Cell, 56: 313-321
(1989));
electroporation of embryos (Lo, Mol. Cell. Biol., 3: 1803-1814 (1983)); and
sperm-mediated
gene transfer. Lavitrano et al., Cell, 57: 717-73 (1989). For a review, see
for example, U.S.
Patent No. 4,736,866.
For the purpose of the present invention, transgenic animals include those
that carry
the transgene only in part of their cells ("mosaic animals"). The transgene
can be integrated
either as a single transgene, or in concatamers, e.g., head-to-head or head-to-
tail tandems.
Selective introduction of a transgene into a particular cell type is also
possible by following,
for example, the technique of Lasko et al., Proc. Natl. Acad. Sci. USA, 89:
6232-636 (1992).
The expression of the transgene in transgenic animals can be monitored by
standard
techniques. For example, Southern blot analysis or PCR amplification can be
used to verify
the integration of the transgene. The level of mRNA expression can then be
analyzed using
techniques such as in situ hybridization, Northern blot analysis, PCR, or
immunocytochemistry. The animals are further examined for signs of tumor or
cancer
development.
Alternatively, "knock-out" animals, e.g., zebrafish, can be constructed that
have a
defective or altered gene encoding a ZPA polypeptide identified herein, as a
result of
homologous recombination between an endogenous gene encoding a ZPA polypeptide
and
altered genomic DNA encoding the same polypeptide introduced into an embryonic
cell of
the animal. For example, cDNA encoding a particular ZPA polypeptide can be
used to clone
genomic DNA encoding that polypeptide in accordance with established
techniques. A
portion of the genomic DNA encoding a particular ZPA polypeptide can be
deleted or
replaced with another gene, such as a gene encoding a selectable marker that
can be used to
monitor integration. Similarly, knock-out animals other than zebrafish can be
constructed
that have a defective or altered gene encoding an endogenous homolog of a ZPA
polypeptide,
63
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
as a result of homologous recombination between an endogenous gene encoding a
ZPA
homolog and altered genomic DNA encoding the homologous ZPA polypeptide
introduced
into an embryonic cell of the animal. Typically, several kilobases of
unaltered flanking DNA
(both at the 5' and 3' ends) are included in the vector. See, e.g., Thomas and
Capecchi, Cell,
51: 503 (1987) for a description of homologous recombination vectors. The
vector is
introduced into an embryonic stem cell line (e.g., by electroporation) and
cells in which the
introduced DNA has homologously recombined with the endogenous DNA are
selected. See,
e.g., Li et al., Cell, 69: 915 (1992). The selected cells are then injected
into a blastocyst of an
animal (e.g., a mouse or rat) to form aggregation chimeras. See, e.g.,
Bradley, in
Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, E. J.
Robertson, ed.
(IRL: Oxford, 1987), pp. 113-152. A chimeric embryo can then be implanted into
a suitable
pseudopregnant female foster animal and the embryo brought to term to create a
"knock-out"
animal. Progeny harboring the homologously recombined DNA in their germ cells
can be
identified by standard techniques and used to breed animals in which all cells
of the animal
contain the homologously recombined DNA. Knockout animals can be
characterized, for
instance, by their ability to defend against certain pathological conditions
and by their
development of pathological conditions due to absence of one or more ZPA
polypeptides.
"Knock-down" animals (e.g., zebrafish), can be constructed in which the gene
encoding a ZPA polypeptide is selectively prevented from being transcribed
and/or
translated. For example, silencing RNA or morpholinos may be used to block
translation of
one or more ZPA polypeptides. In such animals, the gene encoding a ZPA
polypeptide
remains intact, but the protein encoded by that gene is not produced.
The efficacy of antibodies specifically binding a ZPA polypeptide identified
herein,
and other drug candidates, can be tested also in the treatment of spontaneous
animal tumors.
A suitable target for such studies is the feline oral squamous cell carcinoma
(SCC). Feline
oral SCC is a highly invasive, malignant tumor that is the most common oral
malignancy of
cats, accounting for over 60% of the oral tumors reported in this species. It
rarely
metastasizes to distant sites, although this low incidence of metastasis can
merely be a
reflection of the short survival times for cats with this tumor. These tumors
are usually not
amenable to surgery, primarily because of the anatomy of the feline oral
cavity. At present,
there is no effective treatment for this tumor. Prior to entry into the study,
each cat undergoes
complete clinical examination and biopsy, and is scanned by computed
tomography (CT).
Cats diagnosed with sublingual oral squamous cell tumors are excluded from the
study. The
64
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
tongue can become paralyzed as a result of such tumor, and even if the
treatment kills the
tumor, the animals may not be able to feed themselves. Each cat is treated
repeatedly, over a
longer period of time. Photographs of the tumors will be taken daily during
the treatment
period, and at each subsequent recheck. After treatment, each cat undergoes
another CT
scan. CT scans and thoracic radiograms are evaluated every 8 weeks thereafter.
The data are
evaluated for differences in survival, response, and toxicity as compared to
control groups.
Positive response may require evidence of tumor regression, preferably with
improvement of
quality of life and/or increased life span.
In addition, other spontaneous animal tumors, such as fibrosarcoma,
adenocarcinoma,
lymphoma, chondroma, or leiomyosarcoma of dogs, cats, and baboons can also be
tested. Of
these, mammary adenocarcinoma in dogs and cats is a preferred model as its
appearance and
behavior are very similar to those in humans. However, the use of this model
is limited by
the rare occurrence of this type of tumor in animals.
Construction of Transgenic Zebrafish
Transgenic zebrafish may be constructed as described herein, or as well known
in the
art (see, e.g., Westerfield, The Zebrafish Book. A guide for the laboratory
use of zebrafish
(Danio rerio). 4th ed., Univ. of Oregon Press: Eugene (2000)). Transgenic
constructs can be
introduced into zebrafish cells (for example, at the 1-4 cell stage of
development), and the
injected embryos then be allowed to develop until such time as appropriate to
examine the
effects of the transgene. Transgenic constructs can be linear or circular
polynucleotides, and
optionally may include regulatory sequences as described elsewhere herein.
Methods of
introducing the transgenic construct into embryonic zebrafish cells include,
but are not
limited to, microinjection, electroporation, particle gun bombardment, viral
infection, and via
liposomes. A reporter molecule can be included in the transgenic construct for
ease of
determining the presence of the transgene in the adult zebrafish (e.g., GFP or
some other
readily identifiable label); in situations where no reporter was included in
the construct, the
zebrafish nucleic acid (e.g., isolated from a tail cutting from an adult
zebrafish) can be
examined for the presence of the transgene by well-known genetic methods such
as PCR or
southern blotting.
Any strain and/or variety of laboratory or commercially available zebrafish
may be
used in the methodologies described herein. In some embodiments, zebrafish are
from inbred
lines (including, but not limited to, SJD, C32, and WIK). In some embodiments,
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
visualization of transgene activity is facilitated by the use of zebrafish
having other
mutations, for example "non-pigmented" mutant zebrafish substantially devoid
of
melanophore deposition (e.g. albino mutants) or irridiphore deposition (e.g.
roy orbison,
transparent mutants).
Assays for Evaluating Apoptotic Activity
Assays that are useful for measuring the pro-apoptotic or anti-apoptotic
activity of the
agonists, progenitors, and antagonists of this invention include the assays of
Example 3 or
other suitable assays known in the art such as those included below.
Assays for apoptotic activity include, for example, cytotoxicity assays (e.g.,
radiometric or non-radiometric assays measuring increased membrane
permeability or
colorimetric assays measuring reduction in the metabolic activity of
mitochondria); assays
measuring DNA fragmentation (including, but not limited to, in situ nick
translation (ISNT)
and TdT-mediated X-dUTP nick end labeling (TUNEL) (Cole and Ross, Devel. Biol.
240:
123-142 (2001))); assays measuring changes in cellular organization and
packaging which
are precursors to cell death (e.g., alterations in membrane asymmetry
(including, but not
limited to, translocation of phosphatidylserine (Nicoletti et al., "Common
Methods for
Measuring Apoptotic Cell Death by Flow Cytometry," The Purdue Cytometry CD-ROM
Volume 3, J. Parker, C. Stewart, Guest Eds., J.Paul Robinson, Publisher.
Purdue University,
West Lafayette, 1997, ISBN 1-890473-02-2), and release of cytochrome C or AIF
from the
mitochondria into the cytoplasm); and assays measuring activation of one or
more
biochemical cascades resulting in apoptosis (including, but not limited to,
caspase activation
(see, e.g., Example 3), and cleavage of poly-ADP-ribose polymerase (PARP)).
Assays for
apoptotic activity can be performed on single cells and/or on cellular
populations.
Antibody Binding Studies
Antibody binding studies can be carried out using known assay methods, such as
competitive binding assays, direct and indirect sandwich assays, and
immunoprecipitation
assays. Zola, Monoclonal Antibodies: A Manual of Technidues (CRC Press, Inc.,
1987),
pp.147-158.
Competitive binding assays rely on the ability of a labeled standard to
compete with
the test sample analyte for binding with a limited amount of antibody. The
amount of target
protein in the test sample is inversely proportional to the amount of standard
that becomes
66
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
bound to the antibodies. To facilitate determining the amount of standard that
becomes
bound, the antibodies can be insolubilized before or after the competition, so
that the standard
and analyte that are bound to the antibodies can conveniently be separated
from the standard
and analyte that remain unbound.
Sandwich assays involve the use of two antibodies, each capable of binding to
a
different immunogenic portion, or epitope, of the protein to be detected. In a
sandwich assay,
the test sample analyte is bound by a first antibody that is immobilized on a
solid support, and
thereafter a second antibody binds to the analyte, thus forming an insoluble
three-part
complex. See, e.g., U.S. Pat. No. 4,376,110. The second antibody can itself be
labeled with
a detectable moiety (direct sandwich assays) or can be measured using an anti-
immunoglobulin antibody that is labeled with a detectable moiety (indirect
sandwich assay).
For example, one type of sandwich assay is an ELISA assay, in which case the
detectable
moiety is an enzyme.
Competitive ELISA assays can be performed to screen polypeptides, agonists or
antagonists for those that specifically bind to a ZPA polypeptide, which
binding can be
inhibited by a monoclonal antibody of this invention.
In one example, a competitive ELISA assay can be conducted following methods
known in the art. A full length or truncated form of a native ZPA protein
(2ug/ml in PBS)
can be coated on a microtiter plate at 4 C overnight or at room temperature
for 2 hours. The
wells can be blocked by adding 65 1 1% BSA for 30 minutes followed by 40u1 1%
Tween 20
for another 30 minutes. Next, the wells can be washed with PBS - 0.05% Tween
20 5 times.
Various concentrations of antibody (in ELISA buffer) can be incubated in the
wells for 30
minutes at room temperature. Then, polypeptides or antibodies to be tested can
be added to
different wells for 10 minutes at a concentration that would normally produce
90% binding
capacity in the absence of the antibody. Next, the wells can be washed with
PBS - 0.05%
Tween 20 5 times. Binding can be quantified by methods known in the art.
For immunohistochemistry, the tissue sample can be fresh or frozen or can be
embedded in paraffin and fixed with a preservative such as formalin, for
example.
Cell-Based Tumor Assays
Cell-based assays and animal models for proliferative disorders, such as
tumors, can
be used to verify the inhibitory activity of the antagonists of this
invention. Appropriate
assays are known in the art. For example, cells of a cell type known to be
involved in a
67
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
proliferative disorder can be transfected with one or more ZPA cDNAs herein,
and the ability
of these cDNAs to inhibit growth is analyzed in the presence or absence of an
antagonist. If
the proliferative disorder is cancer, suitable tumor cells include, for
example, stable tumor
cell lines such as the B104-1-1 cell line (stable NIH-3T3 cell line
transfected with the neu
protooncogene) and ras-transfected NIH-3T3 cells, which can be transfected
with a ZPA
sequence and monitored for tumorigenic growth. Such transfected cell lines can
then be used
to test the ability of poly- or monoclonal antibodies or antibody compositions
to inhibit
tumorigenic cell growth by exerting cytostatic or cytotoxic activity on the
growth of the
transformed cells, or by mediating antibody-dependent cellular cytotoxicity
(ADCC).
In addition, primary cultures derived from tumors in transgenic animals (as
described
above) can be used in the cell-based assays herein, although stable cell lines
are preferred.
Techniques to derive continuous cell lines from transgenic animals are known
in the art. See,
e.g., Small et al., Mol. Cell. Biol., 5: 642-648 (1985).
Gene Therapy
Described below are methods and compositions whereby disease symptoms can be
ameliorated. The ZPA polypeptides (including ZPA polypeptide variants)
described herein,
and antagonists and antibodies of this invention can be employed in accordance
with the
present invention by expression of each in vivo, which is often referred to as
gene therapy.
For example, ZPA polypeptide variants can be expressed in cells using these
methods.
According to one embodiment, the methods or the vectors used to express the
ZPA
polypeptides (including variants) involve the use of a targeting agent to
direct the vehicle
containing the ZPA polypeptide or nucleic acid molecule to a desired tissue.
There are two major approaches to getting the nucleic acid (optionally
contained in a
vector) into the mammal's cells: in vivo and ex vivo. For in vivo delivery the
nucleic acid is
injected directly into the mammal, usually at the sites where the ZPA
polypeptide is required,
i.e., the site of synthesis of the ZPA polypeptide, if known, and the site
(e.g., wound) where
biological activity of the ZPA polypeptide is needed. For ex vivo treatment,
the mammal's
cells are removed, the nucleic acid is introduced into these isolated cells,
and the modified
cells are administered to the mammal either directly or, for example,
encapsulated within
porous membranes that are implanted into the mammal (see, e.g., U.S. Pat. Nos.
4,892,538
and 5,283,187). There are a variety of techniques available for introducing
nucleic acids into
viable cells. The techniques vary depending upon whether the nucleic acid is
transferred into
68
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
cultured cells in vitro, or transferred in vivo in the cells of the intended
host. Techniques
suitable for the transfer of nucleic acid into mammalian cells in vitro
include the use of
liposomes, electroporation, microinjection, transduction, cell fusion, DEAE-
dextran, the
calcium phosphate precipitation method, etc. Transduction involves the
association of a
replication-defective, recombinant viral (including, but not limited to,
retroviral) particle with
a cellular receptor, followed by introduction of the nucleic acids contained
by the particle into
the cell. A commonly used vector for ex vivo delivery of the gene is a
retrovirus.
Commonly used in vivo nucleic acid transfer techniques include transfection
with
viral or non-viral vectors (such as adenovirus, lentivirus, Herpes simplex I
virus, or adeno-
associated virus (AAV)) and lipid-based systems (useful lipids for lipid-
mediated transfer of
the gene are, for example, DOTMA, DOPE, and DC-Chol; see, e.g., Tonkinson et
al., Cancer
Investigation, 14 1: 54-65 (1996)). Such vectors are used to synthesize virus
that can be
used as vehicles for delivering agents, such as antagonists and nucleic acid
molecules of this
invention. The most commonly used vectors for use in gene therapy are viruses,
e.g.,
adenoviruses, AAV, lentiviruses, or retroviruses. A viral vector such as a
retroviral vector
includes at least one transcriptional promoter/enhancer or locus-defining
element(s), or other
elements that control gene expression by other means such as alternate
splicing, nuclear RNA
export, or post-translational modification of messenger. In addition, a viral
vector such as a
retroviral vector includes a nucleic acid molecule that, when transcribed in
the presence of a
gene encoding a ZPA polypeptide, is operably linked thereto and acts as a
translation
initiation sequence. Such vector constructs also include a packaging signal,
long terminal
repeats (LTRs) or portions thereof, and positive and negative strand primer
binding sites
appropriate to the virus used (if these are not already present in the viral
vector). In addition,
such vector typically includes a signal sequence for secretion of the ZPA
polypeptide from a
host cell in which it is placed. In certain embodiments, the signal sequence
for this purpose is
a mammalian signal sequence, including, but not limited to, the native signal
sequence for the
ZPA polypeptide. Optionally, the vector construct can also include a signal
that directs
polyadenylation, as well as one or more restriction sites and a translation
termination
sequence. By way of example, such vectors will typically include a 5' LTR, a
tRNA binding
site, a packaging signal, an origin of second-strand DNA synthesis, and a 3'
LTR or a portion
thereof. Other vectors can be used that are non-viral, such as cationic
lipids, polylysine, and
dendrimers.
69
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
In some situations, it is desirable to provide the nucleic acid source with an
agent that
targets the target cells, such as an antibody specific for a cell-surface
membrane protein or the
target cell, a ligand for a receptor on the target cell, etc. Where liposomes
are employed,
proteins that bind to a cell-surface membrane protein associated with
endocytosis can be used
for targeting and/or to facilitate uptake, e.g., capsid proteins or fragments
thereof tropic for a
particular cell type, antibodies for proteins that undergo internalization in
cycling, and
proteins that target intracellular localization and enhance intracellular half-
life. The
technique of receptor-mediated endocytosis is described, for example, by Wu et
al., J. Biol.
Chem., 262: 4429-4432 (1987); and Wagner et al., Proc. Natl. Acad. Sci. USA,
87: 3410-
3414 (1990). For a review of the currently known gene marking and gene therapy
protocols,
see, Anderson et al., Science, 256: 808-813 (1992). See also WO 93/25673 and
the
references cited therein.
Suitable gene therapy and methods for making retroviral particles and
structural
proteins can be found in, e.g., U.S. Pat. No. 5,681,746.
Detecting ZPA mutations
This invention is also related to the use of the gene encoding a ZPA
polypeptide as a
diagnostic. Detection of a mutated form of a ZPA polypeptide can be indicative
of a
proclivity for developing an apoptosis-related disorder. Detection of levels
of the ZPA
polypeptide in the tissue of a zebrafish over the levels of the same tissue in
a normal
zebrafish can also be indicative of a proclivity for developing an apoptosis-
related disorder.
Similarly, detection of a mutated form of a homolog of a ZPA polypeptide in an
organism
other than zebrafish can be indicative of a proclivity for developing an
apoptosis-related
disorder. Detection of levels of a homolog of a ZPA polypeptide in the tissue
of an organism
(e.g., a mammal), over the levels of the same tissue in a normal organism can
also be
indicative of a proclivity for developing an apoptosis-related disorder.
Individuals carrying mutations in the genes encoding a human homolog of a ZPA
polypeptide can be detected at the DNA level by a variety of techniques.
Nucleic acids for
diagnosis can be obtained from a mammal's cells, such as from blood, urine,
saliva, tissue
biopsy, and autopsy material. The genomic DNA can be used directly for
detection or can be
amplified enzymatically by using PCR (Saiki et al., Nature, 324: 163-166
(1986)) prior to
analysis. RNA or cDNA can also be used for the same purpose. As an example,
PCR
primers complementary to the nucleic acid encoding a ZPA polypeptide can be
used to
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
identify and analyze mutations in the human homolog of a ZPA polypeptide. For
example,
deletions and insertions can be detected by a change in size of the amplified
product in
comparison to the normal genotype. Point mutations can be identified by
hybridizing
amplified DNA to radiolabeled RNA encoding a ZPA polypeptide, or
alternatively,
radiolabeled antisense DNA sequences encoding a ZPA polypeptide. Perfectly
matched
sequences can be distinguished from mismatched duplexes by RNase A digestion
or by
differences in melting temperatures.
Genetic testing based on DNA sequence differences can be achieved by detection
of
alteration in electrophoretic mobility of DNA fragments in gels with or
without denaturing
agents. Small sequence deletions and insertions can be visualized by high
resolution gel
electrophoresis. DNA fragments of different sequences can be distinguished on
denaturing
formamidine gradient gels in which the mobilities of different DNA fragments
are retarded in
the gel at different positions according to their specific melting or partial
melting
temperatures. See, e.g., Myers et al., Science, 230: 1242 (1985).
Sequence changes at specific locations may also be revealed by nuclease
protection
assays, such as RNase and S1 protection or the chemical cleavage method, for
example,
Cotton et al., Proc. Natl. Acad. Sci. USA, 85: 4397-4401 (1985).
Thus, the detection of a specific DNA sequence can be achieved by methods such
as
hybridization, RNase protection, chemical cleavage, direct DNA sequencing, or
the use of
restriction enzymes, e.g., restriction fragment length polymorphisms (RFLP),
and Southern
blotting of genomic DNA.
In addition to more conventional gel-electrophoresis and DNA sequencing,
mutations
in a ZPA polypeptide can also be detected by in situ analysis.
Detecting ZPA Polypeptide or Nucleic Acid Levels
Levels of ZPA polypeptide or nucleic acid molecules can be detected, e.g.,
using the
reagents disclosed herein in combination with methods known in the art, such
as in situ
hybridzation, RT-PCR, northern blots, western blots, or by using the Examples
and reagents
provided herein. Similarly, levels of polypeptides or nucleic acid molecules
homologous to a
ZPA polypeptide or nucleic acid molecule can be detected, e.g., using the
reagents disclosed
herein in combination with methods known in the art, such as in situ
hybridization, RT-PCR,
northern blots, western blots, or by using the Examples and reagents provided
herein.
71
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
A competition assay can be employed wherein antibodies specific to a ZPA
polypeptide are attached to a solid support and a labeled ZPA polypeptide and
a sample
derived from the host comprising at least one polypeptide homologous to one or
more ZPA
polypeptides are passed over the solid support and the amount of label
detected attached to
the solid support can be correlated to a quantity of the at least one
polypeptide homologous to
one or more ZPA polypeptides in the sample. In one embodiment, antibodies that
specifically bind a ZPA polypeptide as described herein are used to monitor
ZPA polypeptide
levels or levels of a ZPA polypeptide homolog.
Screening Assays for Drug Candidates
This invention encompasses methods of screening compounds to identify those
that
mimic a ZPA polypeptide activity (agonists) or prevent the effect of a ZPA
polypeptide
(antagonists). Generally, a ZPA polypeptide is exposed to the drug candidate
by incubation
or contact under various conditions. Screening assays for antagonist drug
candidates are
designed to identify compounds that specifically bind or complex with a native
ZPA
polypeptide. Such screening assays will include assays amenable to high-
throughput
screening of chemical libraries, making them particularly suitable for
identifying small
molecule drug candidates.
The assays can be performed in a variety of formats, including protein-protein
binding
assays, biochemical screening assays, immunoassays, and cell-based assays in
combination
with a ZPA polypeptide, fragments thereof, or cells expressing a ZPA
polypeptide or
fragments thereof.
All assays for antagonists are common in that they call for contacting the
drug
candidate with a ZPA polypeptide encoded by a nucleic acid identified herein
under
conditions and for a time sufficient to allow these two components to
interact.
In binding assays, the interaction is binding and the complex formed can be
isolated
or detected in the reaction mixture. For example, binding of a ZPA polypeptide
to a
polypeptide with which it normally interacts in one or more biochemical
pathways in the
absence or presence of the candidate antagonist can be performed to evaluate
whether the
antagonist blocked binding of the ZPA polypeptide to polypeptide with which it
normally
interacts. In another embodiment, a ZPA polypeptide encoded by a gene
identified herein or
the drug candidate is immobilized on a solid phase, e.g., on a microtiter
plate, by covalent or
non-covalent attachments. Non-covalent attachment generally is accomplished by
coating
72
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
the solid surface with a solution of the ZPA polypeptide and drying.
Alternatively, an
immobilized antibody, e.g., a monoclonal antibody, specific for a ZPA
polypeptide to be
immobilized can be used to anchor it to a solid surface. The assay is
performed by adding the
non-immobilized component, which can be labeled by a detectable label, to the
immobilized
component, e.g., the coated surface containing the anchored component. When
the reaction
is complete, the non-reacted components are removed, e.g., by washing, and
complexes
anchored on the solid surface are detected. When the originally non-
immobilized component
carries a detectable label, the detection of label immobilized on the surface
indicates that
complexing occurred. Where the originally non-immobilized component does not
carry a
label, complexing can be detected, for example, by using a labeled antibody
specifically
binding the immobilized complex.
If the candidate compound interacts with but does not bind to a particular ZPA
polypeptide, its interaction with that polypeptide can be assayed by methods
known for
detecting protein-protein interactions. Such assays include traditional
approaches, such as,
e.g., cross-linking, co-immunoprecipitation, and co-purification through
gradients or
chromatographic columns. In addition, protein-protein interactions can be
monitored by
using a yeast-based genetic system described by Fields and co-workers (Fields
and Song,
Nature (London), 340: 245-246 (1989); Chien et al., Proc. Natl. Acad. Sci.
USA, 88: 9578-
9582 (1991)) as disclosed by Chevray and Nathans, Proc. Natl. Acad. Sci. USA,
89: 5789-
5793 (1991). Many transcriptional activators, such as yeast GAL4, consist of
two physically
discrete modular domains, one acting as the DNA-binding domain, the other one
functioning
as the transcription-activation domain. The yeast expression system described
in the
foregoing publications (generally referred to as the "two-hybrid system")
takes advantage of
this property, and employs two hybrid proteins, one in which the target
protein is fused to the
DNA-binding domain of GAL4, and another, in which candidate activating
proteins are fused
to the activation domain. The expression of a GALl-lacZ reporter gene under
control of a
GAL4-activated promoter depends on reconstitution of GAL4 activity via protein-
protein
interaction. Colonies containing interacting polypeptides are detected with a
chromogenic
substrate for (3-galactosidase. A complete kit (MATCHMAKERTM) for identifying
protein-
protein interactions between two specific proteins using the two-hybrid
technique is
commercially available from Clontech. This system can also be extended to map
protein
domains involved in specific protein interactions as well as to pinpoint amino
acid residues
that are crucial for these interactions.
73
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Compounds that interfere with binding between a ZPA polypeptide and another
protein, including another ZPA polypeptide, can be tested as follows: usually
a reaction
mixture is prepared containing the ZPA polypeptide and the other protein under
conditions
and for a time allowing for the interaction and binding of the two proteins.
To test the ability
of a candidate compound to inhibit binding, the reaction is run in the absence
and in the
presence of the test compound. In addition, a placebo can be added to a third
reaction
mixture, to serve as positive control. The binding (complex formation) between
the test
compound and the other polypeptide present in the mixture is monitored as
described
hereinabove. The formation of a complex in the control reaction(s) but not in
the reaction
mixture containing the test compound indicates that the test compound
interferes with the
interaction of the ZPA polypeptide and the other polypeptide.
According to one embodiment, assays described herein are performed to test
antagonists of this invention. Alternatively, antagonists can be detected by
combining a ZPA
polypeptide and a potential antagonist with unlabeled ZPA polypeptide under
appropriate
conditions for a competitive inhibition assay. The ZPA polypeptide can be
labeled, such as
by radioactivity or a colorimetric method, such that the number of ZPA
polypeptide
molecules bound can be used to determine the effectiveness of the potential
antagonist. The
ZPA polypeptide can be labeled by a variety of means including iodination or
inclusion of a
recognition site for a site-specific protein kinase. Following fixation and
incubation, the
slides are subjected to autoradiographic analysis
Drug candidates include anti-ZPA antibodies including, without limitation,
poly- and
monoclonal antibodies and antibody fragments, single-chain antibodies, anti-
idiotypic
antibodies, and chimeric or humanized versions of such antibodies or
fragments, as well as
human antibodies and antibody fragments. Alternatively, a drug candidate can
be a closely
related protein, for example, a mutated form of a ZPA polypeptide that
competitively inhibits
the action of the endogenous ZPA polypeptide or endogenous ZPA polypeptide
homolog.
Administration Protocols, Schedules, Doses, and Formulations
The molecules herein and antagonists thereto are pharmaceutically useful as a
prophylactic and therapeutic agents for various disorders and diseases as set
forth above.
Therapeutic compositions of the polypeptides, agonists or antagonists of this
invention are prepared for storage by mixing the desired molecule having the
appropriate
degree of purity with optional pharmaceutically acceptable carriers,
excipients, or stabilizers
74
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
(Remington's Pharmaceutical Sciences, 16th edition, Osol, A. ed. (1980)), in
the form of
lyophilized formulations or aqueous solutions. Acceptable carriers,
excipients, or stabilizers
are nontoxic to recipients at the dosages and concentrations employed, and
include buffers
such as phosphate, citrate, and other organic acids; antioxidants including
ascorbic acid and
methionine; preservatives (such as octadecyldimethylbenzyl ammonium chloride;
hexamethonium chloride; benzalkonium chloride, benzethonium chloride; phenol,
butyl or
benzyl alcohol; alkyl parabens such as methyl or propyl paraben; catechol;
resorcinol;
cyclohexanol; 3-pentanol; and m-cresol); low molecular weight (less than about
10 residues)
polypeptides; proteins, such as serum albumin, gelatin, or immunoglobulins;
hydrophilic
polymers such as polyvinylpyrrolidone; amino acids such as glycine, glutamine,
asparagine,
histidine, arginine, or lysine; monosaccharides, disaccharides, and other
carbohydrates
including glucose, mannose, or dextrins; chelating agents such as EDTA; sugars
such as
sucrose, mannitol, trehalose or sorbitol; salt-forming counter-ions such as
sodium; metal
complexes (e.g., Zn-protein complexes); and/or non-ionic surfactants such as
TWEENTM
PLURONICSTM or polyethylene glycol (PEG).
Additional examples of such carriers include ion exchangers, alumina, aluminum
stearate, lecithin, serum proteins, such as human serum albumin, buffer
substances such as
phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride
mixtures of saturated
vegetable fatty acids, water, salts, or electrolytes such as protamine
sulfate, disodium
hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts,
colloidal
silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based
substances, and
polyethylene glycol. Carriers for topical or gel-based forms of agonist or
antagonist include
polysaccharides such as sodium carboxymethylcellulose or methylcellulose,
polyvinylpyrrolidone, polyacrylates, polyoxyethylene-polyoxypropylene-block
polymers,
polyethylene glycol, and wood wax alcohols. For all administrations,
conventional depot
forms are suitably used. Such forms include, for example, microcapsules, nano-
capsules,
liposomes, plasters, inhalation forms, nose sprays, sublingual tablets, and
sustained-release
preparations. The ZPA polypeptides or agonists or antagonists will typically
be formulated in
such vehicles at a concentration of about 0.1 mg/ml to 100 mg/ml.
Another formulation comprises incorporating a ZPA polypeptide or agonist or
antagonist thereof into formed articles. Such articles can be used in
modulating endothelial
cell growth and angiogenesis. In addition, tumor invasion and metastasis can
be modulated
with these articles.
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
ZPA polypeptides or agonists or antagonists to be used for in vivo
administration must
be sterile. This is readily accomplished by filtration through sterile
filtration membranes,
prior to or following lyophilization and reconstitution. ZPA polypeptides
ordinarily will be
stored in lyophilized form or in solution if administered systemically. If in
lyophilized form,
a ZPA polypeptide or agonist or antagonist thereto is typically formulated in
combination
with other ingredients for reconstitution with an appropriate diluent at the
time for use. An
example of a liquid formulation of a ZPA polypeptide or agonist or antagonist
is a sterile,
clear, colorless, unpreserved solution filled in a single-dose vial for
subcutaneous injection.
Preserved pharmaceutical compositions suitable for repeated use can contain,
for example,
depending mainly on the indication and type of polypeptide:
a. a ZPA polypeptide or agonist or antagonist thereto;
b. a buffer capable of maintaining the pH in a range of maximum stability of
the polypeptide or other molecule in solution, e.g., about pH 4-8;
c. a detergent/surfactant primarily to stabilize the polypeptide or molecule
against agitation-induced aggregation;
d. an isotonifier;
e. a preservative selected from the group of phenol, benzyl alcohol and a
benzethonium halide, e.g., chloride; and
f. water.
If the detergent employed is non-ionic, it can, for example, be polysorbates
(e.g.,
POLYSORBATETM (TWEENTM) 20, 80, etc.) or poloxamers (e.g., POLOXAMERTM 188).
The use of non-ionic surfactants permits the formulation to be exposed to
shear surface
stresses without causing denaturation of the polypeptide. Further, such
surfactant-containing
formulations can be employed in aerosol devices such as those used in a
pulmonary dosing,
and needleless jet injector guns (see, e.g., EP 257,956).
An isotonifier can be present to ensure isotonicity of a liquid composition of
a ZPA
polypeptide or agonist or antagonist thereto, and includes polyhydric sugar
alcohols, e.g.,
trihydric or higher sugar alcohols, such as glycerin, erythritol, arabitol,
xylitol, sorbitol, and
mannitol. These sugar alcohols can be used alone or in combination.
Alternatively, sodium
chloride or other appropriate inorganic salts can be used to render the
solutions isotonic.
The buffer can, for example, be an acetate, citrate, succinate, or phosphate
buffer
depending on the pH desired. The pH of one type of liquid formulation of this
invention is
buffered in the range of about 4 to 8, preferably about physiological pH.
76
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
The preservatives phenol, benzyl alcohol and benzethonium halides, e.g.,
chloride, are
known antimicrobial agents that can be employed.
Therapeutic ZPA polypeptide, agonist, and/or antagonist compositions generally
are
placed into a container having a sterile access port, for example, an
intravenous solution bag
or vial having a stopper pierceable by a hypodermic injection needle. In
certain
embodiments, the formulations can be administered as repeated intravenous
(i.v.),
subcutaneous (s.c.), or intramuscular (i.m.) injections, or as aerosol
formulations suitable for
intranasal or intrapulmonary delivery (for intrapulmonary delivery see, e.g.,
EP 257,956).
ZPA polypeptides, agonists and/or antagonists thereto can also be administered
in the
form of sustained-released preparations. Suitable examples of sustained-
release preparations
include semipermeable matrices of solid hydrophobic polymers containing the
protein, which
matrices are in the form of shaped articles, e.g., films, or microcapsules.
Examples of
sustained-release matrices include polyesters, hydrogels (e.g., poly(2-
hydroxyethyl-
methacrylate) as described by Langer et al., J. Biomed. Mater. Res., 15: 167-
277 (1981) and
Langer, Chem. Tech., 12: 98-105 (1982) or poly(vinylalcohol)), polylactides
(U.S. Patent No.
3,773,919, EP 58,481), copolymers of L-glutamic acid and gamma ethyl-L-
glutamate
(Sidman et al., Biopolymers, 22: 547-556 (1983)), non-degradable ethylene-
vinyl acetate
(Langer et al., supra), degradable lactic acid-glycolic acid copolymers such
as the Lupron
DepotTM (injectable microspheres composed of lactic acid-glycolic acid
copolymer and
leuprolide acetate), and poly-D-(-)-3-hydroxybutyric acid (EP 133,988).
While polymers such as ethylene-vinyl acetate and lactic acid-glycolic acid
enable
release of molecules for over 100 days, certain hydrogels release proteins for
shorter time
periods. When encapsulated proteins remain in the body for a long time, they
can denature or
aggregate as a result of exposure to moisture at 37 C, resulting in a loss of
biological activity
and possible changes in immunogenicity. Rational strategies can be devised for
protein
stabilization depending on the mechanism involved. For example, if the
aggregation
mechanism is discovered to be intermolecular S-S bond formation through thio-
disulfide
interchange, stabilization can be achieved by modifying sulfhydryl residues,
lyophilizing
from acidic solutions, controlling moisture content, using appropriate
additives, and
developing specific polymer matrix compositions.
Sustained-release ZPA polypeptide, agonist, and/or antagonist compositions
also
include liposomally entrapped ZPA polypeptides, agonists, and/or antagonists.
Liposomes
containing a ZPA polypeptide, antibody, agonist, and/or antagonist are
prepared by methods
77
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
known per se: DE 3,218,121; Epstein et al., Proc. Natl. Acad. Sci. USA, 82:
3688-3692
(1985); Hwang et al., Proc. Natl. Acad. Sci. USA, 77: 4030-4034 (1980); EP
52,322; EP
36,676; EP 88,046; EP 143,949; EP 142,641; Japanese patent application 83-
118008; U.S.
Patent Nos. 4,485,045 and 4,544,545; and EP 102,324. Ordinarily the liposomes
are of the
small (about 200-800 Angstroms) unilamellar type in which the lipid content is
greater than
about 30 mol. % cholesterol, the selected proportion being adjusted for the
optimal therapy.
The therapeutically effective dose of a ZPA polypeptide, agonist, and/or
antagonist
thereto will, of course, vary depending on such factors as the disorder to be
treated (including
prevention), the method of administration, the type of compound being used for
treatment,
any co-therapy involved, the patient's age, weight, general medical condition,
medical
history, etc., and its determination is well within the skill of a practicing
physician.
Accordingly, it will be necessary for the therapist to titer the dosage and
modify the route of
administration as required to obtain the maximal therapeutic effect. The
clinician will
administer a ZPA polypeptide, antibody, agonist and/or antagonist thereto
until a dosage is
reached that achieves the desired effect for treatment of the condition in
question. For
example, if the objective is the treatment of cancer, the amount would be one
that inhibits the
growth of the cancer.
With the above guidelines, the effective dose generally is within the range of
from
about 0.001 to about 1.0 mg/kg, about 0.01-1.0 mg/kg, and/or about 0.01-0.1
mg/kg.
For non-oral use in treating apoptotic-related disorders, it is advantageous
to
administer the ZPA polypeptide, agonist and/or antagonist thereto in the form
of an injection
at about 0.01 to 50 mg, about 0.05 to 20 mg, and/or about 1 to 20 mg per kg
body weight, 1
to 3 times daily by intravenous injection. For oral administration, in certain
embodiments, a
molecule based on a ZPA polypeptide, agonist, and/or antagonist is
administered at about 5
mg to 1 g, and/or about 10 to 100 mg per kg body weight, 1 to 3 times daily.
It should be
appreciated that endotoxin contamination should be kept minimally at a safe
level, for
example, less than 0.5 ng/mg protein. Moreover, for human administration, the
formulations
preferably meet sterility, pyrogenicity, general safety, and purity as
required by FDA Office
and Biologics standards.
The dosage regimen of a pharmaceutical composition containing a ZPA
polypeptide,
agonist, and/or antagonist to be used in tissue regeneration will be
determined by the
attending physician considering various factors that modify the action of the
polypeptides,
e.g., amount of tissue weight desired to be formed, the site of damage, the
condition of the
78
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
damaged tissue, the size of a wound, type of damaged tissue (e.g., bone), the
patient's age,
sex, and diet, the severity of any infection, time of administration, and
other clinical factors.
The dosage can vary with the type of matrix used in the reconstitution and
with inclusion of
other proteins in the pharmaceutical composition. For example, the addition of
other known
growth factors, such as IGF-I, to the final composition can also affect the
dosage. Progress
can be monitored by periodic assessment of tissue/bone growth and/or repair,
for example, X-
rays, histomorphometric determinations, and tetracycline labeling.
The route of ZPA polypeptide, antagonist and/or agonist administration is in
accord
with known methods, e.g., by injection or infusion by intravenous,
intramuscular,
intracerebral, intraperitoneal, intracerobrospinal, subcutaneous, intraocular,
intraarticular,
intrasynovial, intrathecal, oral, topical, or inhalation routes, or by
sustained-release systems
as noted below. A ZPA polypeptide, agonist and/or antagonist thereof also are
suitably
administered by intratumoral, peritumoral, intralesional, or perilesional
routes, to exert local
as well as systemic therapeutic effects.
If a peptide or small molecule is employed as an antagonist or agonist, it is
preferably
administered orally or non-orally in the form of a liquid or solid to mammals.
Examples of pharmacologically acceptable salts of molecules that form salts
and are
useful hereunder include alkali metal salts (e.g., sodium salt, potassium
salt), alkaline earth
metal salts (e.g., calcium salt, magnesium salt), ammonium salts, organic base
salts (e.g.,
pyridine salt, triethylamine salt), inorganic acid salts (e.g., hydrochloride,
sulfate, nitrate),
and salts of organic acid (e.g., acetate, oxalate, p-toluenesulfonate).
The location of the desired action of a ZPA polypeptide, agonist, and/or
antagonist
thereto of the invention may be taken into consideration in preparation and
administration of
the polypeptide, agonist, and/or antagonist. When the desired action is
located intracellularly,
certain embodiments of the invention provide for the polypeptide, agonist,
and/or antagonist
to be introduced into the cell. In one embodiment, intracellular expression of
a polypeptide,
proteinaceous agonist, and/or proteinaceous antagonist is effected by
introducing a nucleic
acid encoding the polypeptide, proteinaceous agonist, and/or proteinaceous
antagonist
(lacking the wild-type leader sequence and secretory signals normally
associated with the
gene encoding such molecules) into a target cell. Any standard method of
introducing
nucleic acids into a cell may be used, including, but not limited to,
microinjection, ballistic
injection, electroporation, calcium phosphate precipitation, liposomes, and
transfection with
79
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
retroviral, adenoviral, adeno-associated viral and vaccinia vectors carrying
the nucleic acid of
interest.
In another embodiment, internalizing molecules are provided. Polypeptides can
possess certain characteristics that enhance their delivery into cells, or can
be modified to
possess such characteristics. Techniques for achieving this are known in the
art. For
example, cationization, lipofections or liposomes can be used to deliver the
antibody into
cells. Translocation of molecules into cells can also be facilitated by
conjugating a pH (low)
insertion peptide ("pHLIP") to the molecule to be translocated (e.g., by
disulfide bonds, see,
for example, Reshetnyak et al., Proc. Natl. Acad. Sci. 103(17): 6460-6465
(2006)) and
lowering the extracellular pH. Where fragments are used, the smallest fragment
that
performs the desired function is generally advantageous. For example, a ZPA
polypeptide
lacking a membrane anchor region while retaining anti-apoptotic activity may
be
advantageous for intracellular introduction over the wild-type polypeptide.
Such peptides can
be synthesized chemically and/or produced by recombinant DNA technology.
Entry of modulator polypeptides, agonists, and antagonists into target cells
can be
enhanced by methods known in the art. For example, certain sequences, such as
those
derived from HIV Tat or the Antennapedia homeodomain protein are able to
direct efficient
uptake of heterologous proteins across cell membranes. See, e.g., Chen et al.,
Proc. Natl.
Acad. Sci. USA (1999), 96:4325-4329.
When the location of desired activity of a ZPA polypeptide, agonist, and/or
antagonist
is in the brain, certain embodiments of the invention provide for the ZPA
polypeptide,
agonist, and/or antagonist to traverse the blood-brain barrier. Certain
neurodegenerative
diseases are associated with an increase in permeability of the blood-brain
barrier, such that
the antibody or antigen-binding fragment can be readily introduced to the
brain. When the
blood-brain barrier remains intact, several art-known approaches exist for
transporting
molecules across it, including, but not limited to, physical methods, lipid-
based methods, and
receptor and channel-based methods.
Physical methods of transporting the ZPA polypeptide, agonist, and/or
antagonist
across the blood-brain barrier include, but are not limited to, circumventing
the blood-brain
barrier entirely, or by creating openings in the blood-brain barrier.
Circumvention methods
include, but are not limited to, direct injection into the brain (see, e.g.,
Papanastassiou et al.,
Gene Therapy 9: 398-406 (2002)), interstitial infusion/convection-enhanced
delivery (see,
e.g., Bobo et al., Proc. Natl. Acad. Sci. USA 91: 2076-2080 (1994)), and
implanting a
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
delivery device in the brain (see, e.g., Gill et al., Nature Med. 9: 589-595
(2003); and Gliadel
WafersTM, Guildford Pharmaceutical). Methods of creating openings in the
barrier include,
but are not limited to, ultrasound (see, e.g., U.S. Patent Publication No.
2002/0038086),
osmotic pressure (e.g., by administration of hypertonic mannitol (Neuwelt, E.
A., Implication
of the Blood-Brain Barrier and its Manipulation, Vols 1& 2, Plenum Press, N.Y.
(1989))),
permeabilization by, e.g., bradykinin or permeabilizer A-7 (see, e.g., U.S.
Patent Nos.
5,112,596, 5,268,164, 5,506,206, and 5,686,416), and transfection of neurons
that straddle the
blood-brain barrier with vectors containing genes encoding the ZPA
polypeptide,
proteinaceous agonist, and/or proteinaceous antagonist (see, e.g., U.S. Patent
Publication No.
2003/0083299).
Lipid-based methods of transporting a ZPA polypeptide, agonist, and/or
antagonist
across the blood-brain barrier include, but are not limited to, encapsulating
the ZPA
polypeptide, agonist, and/or antagonist in liposomes that are coupled to
antibody binding
fragments that bind to receptors on the vascular endothelium of the blood-
brain barrier (see,
e.g., U.S. Patent Application Publication No. 20020025313), and coating the
ZPA
polypeptide, agonist, and/or antagonist in low-density lipoprotein particles
(see, e.g., U.S.
Patent Application Publication No. 20040204354) or apolipoprotein E (see,
e.g., U.S. Patent
Application Publication No. 20040131692).
Receptor and channel-based methods of transporting the ZPA polypeptide,
agonist,
and/or antagonist across the blood-brain barrier include, but are not limited
to, using
glucocorticoid blockers to increase permeability of the blood-brain barrier
(see, e.g., U.S.
Patent Application Publication Nos. 2002/0065259, 2003/0162695, and
2005/0124533);
activating potassium channels (see, e.g., U.S. Patent Application Publication
No.
2005/0089473), inhibiting ABC drug transporters (see, e.g., U.S. Patent
Application
Publication No. 2003/0073713); coating the molecules with a transferrin and
modulating
activity of the one or more transferrin receptors (see, e.g., U.S. Patent
Application Publication
No. 2003/0129186), and cationizing the molecules (see, e.g., U.S. Patent No.
5,004,697).
Combination Therapies
The effectiveness of a ZPA polypeptide or an agonist or antagonist thereof in
preventing or treating an apoptosis-related disorder can be improved by
administering the
active agent serially or in combination with another agent that is effective
for those purposes,
either in the same composition or as separate compositions.
81
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
For example, for treatment of cell proliferative disorders, ZPA polypeptide
and/or
ZPA polypeptide agonist therapy can be combined with the administration of
other inhibitors
of cell proliferation, such as cytotoxic agents.
In addition, ZPA polypeptides and/or agonists used to treat cancer can be
combined
with cytotoxic, chemotherapeutic, or growth-inhibitory agents as identified
above. Also, for
cancer treatment, a ZPA polypeptide and/or agonist thereof is suitably
administered serially
or in combination with radiological treatments, whether involving irradiation
or
administration of radioactive substances.
If the treatment is for cancer, it may be desirable also to administer
antibodies against
tumor-associated antigens, such as antibodies that bind to one or more of the
ErbB2, EGFR,
ErbB31 ErbB4, or VEGF receptor(s). Alternatively, or in addition, two or more
antibodies
binding the same or two or more different antigens disclosed herein may be co-
administered
to the patient. Sometimes, it may be beneficial also to administer one or more
cytokines to
the patient. In one embodiment, a ZPA polypeptide and/or agonist thereof
described herein
are co-administered with a growth-inhibitory agent. For example, the growth-
inhibitory
agent may be administered first, followed by a ZPA polypeptide and/or agonist
thereof of the
present invention. However, simultaneous administration or administration of a
ZPA
polypeptide and/or agonist thereof of the present invention first is also
contemplated.
Suitable dosages for the growth-inhibitory agent are those presently used and
may be lowered
due to the combined action (synergy) of the growth-inhibitory agent and the
polypeptides and
agonists described herein.
In one embodiment, vascularization of tumors is attacked in combination
therapy. A
ZPA polypeptide and/or agonist thereof of this invention and an antibody
(e.g., anti-VEGF)
are administered to tumor-bearing patients at therapeutically effective doses
as determined,
for example, by observing necrosis of the tumor or its metastatic foci, if
any. This therapy is
continued until such time as no further beneficial effect is observed or
clinical examination
shows no trace of the tumor or any metastatic foci. Then TNF is administered,
alone or in
combination with an auxiliary agent such as alpha-, beta-, or gamma-
interferon, anti-HER2
antibody, heregulin, anti-heregulin antibody, D-factor, interleukin-1 (IL-1),
interleukin-2 (IL-
2), granulocyte-macrophage colony stimulating factor (GM-CSF), or agents that
promote
microvascular coagulation in tumors, such as anti-protein C antibody, anti-
protein S antibody,
or C4b binding protein (see, WO 91/01753, published 21 February 1991), or heat
or
radiation.
82
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
The effective amounts of the therapeutic agents administered in combination
with a
ZPA polypeptide or antagonist thereof will be at the physician's or
veterinarian's discretion.
Dosage administration and adjustment is done to achieve maximal management of
the
conditions to be treated. The dose will additionally depend on such factors as
the type of the
therapeutic agent to be used and the specific patient being treated.
Typically, the amount
employed will be the same dose as that used, if the given therapeutic agent is
administered
without a ZPA polypeptide.
Articles of Manufacture
An article of manufacture such as a kit containing one or more ZPA
polypeptides or
agonists or antagonists thereof useful for the diagnosis or treatment of the
disorders described
above comprises at least a container and a label. Suitable containers include,
for example,
bottles, vials, syringes, and test tubes. The containers can be formed from a
variety of
materials such as glass or plastic. The container holds a composition that is
effective for
diagnosing or treating the condition and can have a sterile access port (for
example, the
container can be an intravenous solution bag or a vial having a stopper
pierceable by a
hypodermic injection needle). The active agent in the composition is one or
more ZPA
polypeptides or an agonist or antagonist thereto. The label on, or associated
with, the
container indicates that the composition is used for diagnosing or treating
the condition of
choice. The article of manufacture can further comprise a second container
comprising a
pharmaceutically-acceptable buffer, such as phosphate-buffered saline,
Ringer's solution, and
dextrose solution. It can further include other materials desirable from a
commercial and user
standpoint, including other buffers, diluents, filters, needles, syringes, and
package inserts
with instructions for use. The article of manufacture can also comprise a
second or third
container with another active agent as described above.
Polyclonal Antibodies
Methods of preparing polyclonal antibodies are known to the ordinarily skilled
artisan. Polyclonal antibodies can be raised in a mammal, for example, by one
or more
injections of an immunizing agent and, if desired, an adjuvant. Typically, the
immunizing
agent and/or adjuvant will be injected in the mammal by multiple subcutaneous
or
intraperitoneal injections. The immunizing agent can include a ZPA polypeptide
or a fusion
protein thereof. It can be useful to conjugate the immunizing agent to a
protein known to be
83
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
immunogenic in the mammal being immunized. Examples of such immunogenic
proteins
include, but are not limited to, keyhole limpet hemocyanin, serum albumin,
bovine
thyroglobulin, and soybean trypsin inhibitor. Examples of adjuvants that can
be employed
include Freund's complete adjuvant and MPL-TDM adjuvant (monophosphoryl Lipid
A or
synthetic trehalose dicorynomycolate). The immunization protocol can be
selected by one
skilled in the art without undue experimentation.
Monoclonal Antibodies
Anti-ZPA antibodies can be monoclonal antibodies. Monoclonal antibodies can be
prepared, e.g., using hybridoma methods, such as those described by Kohler and
Milstein,
Nature, 256:495 (1975) or can be made by recombinant DNA methods (US Patent
No.
4,816,567) or can be produced by the methods described herein in the Example
section. In a
hybridoma method, a mouse, hamster, or other appropriate host animal is
typically
immunized with an immunizing agent to elicit lymphocytes that produce or are
capable of
producing antibodies that will specifically bind to the immunizing agent.
Alternatively, the
lymphocytes can be immunized in vitro.
The immunizing agent will typically include a ZPA polypeptide or a fusion
protein
thereof. Generally, either peripheral blood lymphocytes ("PBLs") are used if
cells of human
origin are desired, or spleen cells or lymph node cells are used if non-human
mammalian
sources are desired. The lymphocytes are then fused with an immortalized cell
line using a
suitable fusing agent, such as polyethylene glycol, to form a hybridoma cell.
Goding,
Monoclonal Antibodies: Principles and Practice (New York: Academic Press,
1986), pp. 59-
103. Immortalized cell lines are usually transformed mammalian cells,
particularly myeloma
cells of rodent, bovine, and human origin. Usually, rat or mouse myeloma cell
lines are
employed. The hybridoma cells can be cultured in a suitable culture medium
that contains
one or more substances that inhibit the growth or survival of the unfused,
immortalized cells.
For example, if the parental cells lack the enzyme hypoxanthine guanine
phosphoribosyl
transferase (HGPRT or HPRT), the culture medium for the hybridomas typically
will include
hypoxanthine, aminopterin, and thymidine ("HAT medium"), which substances
prevent the
growth of HGPRT-deficient cells.
Exemplary immortalized cell lines are those that fuse efficiently, support
stable high-
level expression of antibody by the selected antibody-producing cells, and are
sensitive to a
medium such as HAT medium. In certain embodiments, immortalized cell lines are
murine
84
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
myeloma lines, which can be obtained, for instance, from the Salk Institute
Cell Distribution
Center, San Diego, California and the American Type Culture Collection,
Manassas,
Virginia. Human myeloma and mouse-human heteromyeloma cell lines also have
been
described for the production of human monoclonal antibodies. Kozbor, J.
Immunol.,
133:3001 (1984); Brodeur et al., Monoclonal Antibody Production Techniques and
Applications (Marcel Dekker, Inc.: New York, 1987) pp. 51-63.
The culture medium in which the hybridoma cells are cultured can then be
assayed for
the presence of monoclonal antibodies directed against a ZPA polypeptide. In
certain
embodiments, the binding specificity of monoclonal antibodies produced by the
hybridoma
cells is determined by immunoprecipitation or by an in vitro binding assay,
such as
radioimmunoassay (RIA) or enzyme-linked immunoabsorbent assay (ELISA). Such
techniques and assays are known in the art. The binding affinity of the
monoclonal antibody
can, for example, be determined by the Scatchard analysis of Munson and
Pollard, Anal.
Biochem., 107:220 (1980).
After the desired hybridoma cells are identified, the clones can be subcloned
by
limiting dilution procedures and grown by standard methods. Goding, supra.
Suitable culture
media for this purpose include, for example, Dulbecco's Modified Eagle's
Medium and
RPMI-1640 medium. Alternatively, the hybridoma cells can be grown in vivo as
ascites in a
mammal.
The monoclonal antibodies secreted by the subclones can be isolated or
purified from
the culture medium or ascites fluid by conventional immunoglobulin
purification procedures
such as, for example, protein A-Sepharose, hydroxylapatite chromatography, gel
electrophoresis, dialysis, or affinity chromatography.
The monoclonal antibodies can also be made by recombinant DNA methods, such as
those described in U.S. Patent No. 4,816,567. DNA encoding the monoclonal
antibodies of
the invention can be readily isolated and sequenced using conventional
procedures (e.g., by
using oligonucleotide probes that are capable of binding specifically to genes
encoding the
heavy and light chains of murine antibodies). The hybridoma cells of the
invention can serve
as a source of such DNA. Once isolated, the DNA can be placed into expression
vectors,
which are then transfected into host cells such as simian COS cells, Chinese
hamster ovary
(CHO) cells, or myeloma cells that do not otherwise produce immunoglobulin
protein, to
obtain the synthesis of monoclonal antibodies in the recombinant host cells.
The DNA also
can be modified, for example, by substituting the coding sequence for human
heavy- and
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
light-chain constant domains in place of the homologous murine sequences (U.S.
Patent No.
4,816,567; Morrison et al., supra) or by covalentlyjoining to the
immunoglobulin coding
sequence all or part of the coding sequence for a non-immunoglobulin
polypeptide. Such a
non-immunoglobulin polypeptide can be substituted for the constant domains of
an antibody
of the invention, or can be substituted for the variable domains of one
antigen-combining site
of an antibody of the invention to create a chimeric bivalent antibody.
The antibodies can be monovalent antibodies. Methods for preparing monovalent
antibodies are known in the art. For example, one method involves recombinant
expression
of immunoglobulin light chain and modified heavy chain. The heavy chain is
truncated
generally at any point in the Fc region so as to prevent heavy-chain
crosslinking.
Alternatively, the relevant cysteine residues are substituted with another
amino acid residue
or are deleted so as to prevent crosslinking.
In vitro methods are also suitable for preparing monovalent antibodies.
Digestion of
antibodies to produce fragments thereof, particularly Fab fragments, can be
accomplished
using techniques known in the art.
Human and Humanized Antibodies
The anti-ZPA antibodies can further comprise humanized antibodies or human
antibodies. Humanized forms of non-human (e.g., murine) antibodies are
chimeric
immunoglobulins, immunoglobulin chains, or fragments thereof (such as Fv, Fab,
Fab',
F(ab')z, or other antigen-binding subsequences of antibodies) that contain
minimal sequence
derived from non-human immunoglobulin. Humanized antibodies include human
immunoglobulins (recipient antibody) in which residues from a CDR of the
recipient are
replaced by residues from a CDR of a non-human species (donor antibody) such
as mouse,
rat, or rabbit having the desired specificity, affinity, and capacity. In some
instances, Fv
framework residues of the human immunoglobulin are replaced by corresponding
non-human
residues. Humanized antibodies can also comprise residues that are found
neither in the
recipient antibody nor in the imported CDR or framework sequences. In general,
the
humanized antibody will comprise substantially all of at least one, and
typically two, variable
domains, in which all or substantially all of the CDR regions correspond to
those of a non-
human immunoglobulin, and all or substantially all of the FR regions are those
of a human
immunoglobulin consensus sequence. The humanized antibody can also comprise at
least a
portion of an immunoglobulin constant region (Fc), typically that of a human
86
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
immunoglobulin. Jones et al., Nature, 321: 522-525 (1986); Riechmann et al.,
Nature, 332:
323-329 (1988); Presta, Curr. Op. Struct. Biol., 2:593-596 (1992).
Methods for humanizing non-human antibodies are known in the art. Generally, a
humanized antibody has one or more amino acid residues introduced into it from
a source
that is non-human. These non-human amino acid residues are often referred to
as "import"
residues, which are typically taken from an "import" variable domain.
Humanization can be
essentially performed following the method of Winter and co-workers (Jones et
al., Nature,
321: 522-525 (1986); Riechmann et al., Nature, 332: 323-327 (1988); Verhoeyen
et al.,
Science, 239: 1534-1536 (1988)), by substituting rodent CDRs or CDR sequences
for the
corresponding sequences of a human antibody. Accordingly, such "humanized"
antibodies
are chimeric antibodies (U.S. Patent No. 4,816,567), wherein substantially
less than an intact
human variable domain has been substituted by the corresponding sequence from
a non-
human species. In practice, humanized antibodies are typically human
antibodies in which
some CDR residues and possibly some FR residues are substituted by residues
from
analogous sites in rodent antibodies.
As an alternative to humanization, human antibodies can be generated. For
example,
it is now possible to produce transgenic animals (e.g., mice) that are
capable, upon
immunization, of producing a full repertoire of human antibodies in the
absence of
endogenous immunoglobulin production. For example, it has been described that
the
homozygous deletion of the antibody heavy-chain joining region (JH) gene in
chimeric and
germ-line mutant mice results in complete inhibition of endogenous antibody
production.
Transfer of the human germ-line immunoglobulin gene array into such germ-line
mutant
mice will result in the production of human antibodies upon antigen challenge.
See, e.g.,
Jakobovits et al., Proc. Natl. Acad. Sci. USA, 90:2551 (1993); Jakobovits et
al., Nature,
362:255-258 (1993); Bruggemann et al., Year in Immuno., 7:33 (1993); U.S.
Patent Nos.
5,545,806, 5,569,825, 5,591,669 (all of GenPharm); 5,545,807; and WO 97/17852.
Alternatively, human antibodies can be made by introducing human
immunoglobulin loci
into transgenic animals, e.g., mice in which the endogenous immunoglobulin
genes have been
partially or completely inactivated. Upon challenge, human antibody production
is observed
that closely resembles that seen in humans in all respects, including gene
rearrangement,
assembly, and antibody repertoire. This approach is described, for example, in
U.S. Patent
Nos. 5,545,807; 5,545,806; 5,569,825; 5,625,126; 5,633,425; and 5,661,016, and
in the
following scientific publications: Marks et al., Bio/Technology, 10: 779-783
(1992);
87
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Lonberg et al., Nature, 368: 856-859 (1994); Morrison, Nature, 368: 812-813
(1994);
Fishwild et al., Nature Biotechnolo14: 845-851 (1996); Neuberger, Nature
Biotechnolo14: 826 (1996); Lonberg and Huszar, Intern. Rev. Immunol., 13: 65-
93
(1995).
Alternatively, phage display technology (McCafferty et al., Nature 348:552-553
[1990]) can be used to produce human antibodies and antibody fragments in
vitro, from
immunoglobulin variable (V) domain gene repertoires from unimmunized donors.
According
to this technique, antibody V domain genes are cloned in-frame into either a
major or minor
coat protein gene of a filamentous bacteriophage, such as M13 or fd, and
displayed as
functional antibody fragments on the surface of the phage particle. Because
the filamentous
particle contains a single-stranded DNA copy of the phage genome, selections
based on the
functional properties of the antibody also result in selection of the gene
encoding the antibody
exhibiting those properties. Thus, the phage mimics some of the properties of
the B-cell.
Phage display can be performed in a variety of formats, reviewed in, e.g.,
Johnson, Kevin S.
and Chiswell, David J., Current Opinion in Structural Biology 3:564-571
(1993). Several
sources of V-gene segments can be used for phage display. Clackson et al.,
Nature, 352:624-
628 (1991) isolated a diverse array of anti-oxazolone antibodies from a small
random
combinatorial library of V genes derived from the spleens of immunized mice. A
repertoire
of V genes from unimmunized human donors can be constructed and antibodies to
a diverse
array of antigens (including self-antigens) can be isolated essentially
following the techniques
described by Marks et al., J. Mol. Biol. 222:581-597 (1991), or Griffith et
al., EMBO J.
12:725-734 (1993). See, also, U.S. Patent Nos. 5,565,332 and 5,573,905.
As discussed above, human antibodies may also be generated by in vitro
activated B
cells (see U.S. Patents 5,567,610 and 5,229,275).
Human antibodies can also be produced using various techniques known in the
art,
including phage display libraries. Hoogenboom and Winter, J. Mol. Biol., 227:
381 (1991);
Marks et al., J. Mol. Biol., 222: 581 (1991). The techniques of Cole et al.
and Boerner et al.
are also available for the preparation of human monoclonal antibodies. Cole et
al.,
Monoclonal Antibodies and Cancer Therapy, Alan R. Liss, p. 77 (1985) and
Boerner et al., J.
Immunol., 147 1: 86-95 (1991).
88
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Bispecific anti-ZPA Antibodies
Bispecific antibodies are monoclonal, optionally human or humanized,
antibodies that
have binding specificities for at least two different antigens. In the present
case, one of the
binding specificities is for a ZPA polypeptide, the other one is for any other
antigen.
Methods for making bispecific antibodies are known in the art. Traditionally,
the
recombinant production of bispecific antibodies is based on the co-expression
of two
immunoglobulin heavy-chain/light-chain pairs, where the two heavy chains have
different
specificities. Milstein and Cuello, Nature, 305: 537-539 (1983). Because of
the random
assortment of immunoglobulin heavy and light chains, these hybridomas
(quadromas)
produce a potential mixture of ten different antibody molecules, of which only
one has the
correct bispecific structure. The purification of the correct molecule is
usually accomplished
by affinity chromatography steps. Similar procedures are disclosed in WO
93/08829,
published 13 May 1993, and in Traunecker et al., EMBO J., 10: 3655-3659
(1991).
Antibody variable domains with the desired binding specificities (antibody-
antigen
combining sites) can be fused to immunoglobulin constant-domain sequences. In
certain
embodiments, the fusion is with an immunoglobulin heavy-chain constant domain,
comprising at least part of the hinge, CH2, and CH3 regions. In certain
embodiments, the
first heavy-chain constant region (CH1) containing the site necessary for
light-chain binding
is present in at least one of the fusions. DNAs encoding the immunoglobulin
heavy-chain
fusions and, if desired, the immunoglobulin light chain, are inserted into
separate expression
vectors, and are co-transfected into a suitable host organism. For further
details of generating
bispecific antibodies, see, for example, Suresh et al., Methods in Enzymology,
121: 210
(1986).
Various techniques for making and isolating bispecific antibody fragments
directly
from recombinant cell culture have also been described. For example,
bispecific antibodies
have been produced using leucine zippers. Kostelny et al., J. Immunol.,
148(5):1547-1553
(1992). The leucine zipper peptides from the Fos and Jun proteins were linked
to the Fab'
portions of two different antibodies by gene fusion. The antibody homodimers
were reduced
at the hinge region to form monomers and then re-oxidized to form the antibody
heterodimers. This method can also be utilized for the production of antibody
homodimers.
The "diabody" technology described by Hollinger et al., Proc. Natl. Acad. Sci.
USA,
90:6444-6448 (1993) has provided an alternative mechanism for making
bispecific antibody
fragments. The fragments comprise a VH connected to a VL by a linker which is
too short to
89
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
allow pairing between the two domains on the same chain. Accordingly, the VH
and VL
domains of one fragment are forced to pair with the complementary VL and VH
domains of
another fragment, thereby forming two antigen-binding sites. Another strategy
for making
bispecific antibody fragments by the use of single-chain Fv (sFv) dimers has
also been
reported. See Gruber et al., J. Immunol., 152:5368 (1994).
Antibodies with more than two valencies are contemplated. For example,
trispecific
antibodies can be prepared. Tutt et al. J. Immunol. 147: 60 (1991)
Heteroconjugate Antibodies
Heteroconjugate antibodies are composed of two covalently joined antibodies.
Such
antibodies have, for example, been proposed to target immune-system cells to
unwanted cells
(U.S. Patent No. 4,676,980), and for treatment of HIV infection. WO 91/00360;
WO
92/200373; EP 03089. It is contemplated that the antibodies can be prepared in
vitro using
known methods in synthetic protein chemistry, including those involving
crosslinking agents.
For example, immunotoxins can be constructed using a disulfide-exchange
reaction or by
forming a thioether bond. Examples of suitable reagents for this purpose
include
iminothiolate and methyl-4-mercaptobutyrimidate and those disclosed, for
example, in U.S.
Patent No. 4,676,980.
Effector Function Engineering
It can be desirable to modify the antibody of the invention with respect to
effector
function, so as to enhance, e.g., the effectiveness of the antibody in
treating cancer. For
example, cysteine residue(s) can be introduced into the Fc region, thereby
allowing interchain
disulfide bond formation in this region. The homodimeric antibody thus
generated can have
improved internalization capability and/or increased complement-mediated cell
killing and
antibody-dependent cellular cytotoxicity (ADCC). See, Caron et al., J. Exp.
Med., 176:
1191-1195 (1992) and Shopes, J. Immunol., 148: 2918-2922 (1992). Homodimeric
antibodies with enhanced anti-tumor activity can also be prepared using
heterobifunctional
cross-linkers as described in Wolff et al., Cancer Research, 53: 2560-2565
(1993).
Alternatively, an antibody can be engineered that has dual Fc regions and can
thereby have
enhanced complement lysis and ADCC capabilities. See, Stevenson et al., Anti-
Cancer Drug
Desi~m 3: 219-230 (1989).
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Immunoconjugates
The invention also pertains to immunoconjugates comprising an antibody
conjugated
to a cytotoxic agent such as a chemotherapeutic agent, toxin (e.g., an
enzymatically active
toxin of bacterial, fungal, plant, or animal origin, or fragments thereof), or
a radioactive
isotope (i.e., a radioconjugate).
Chemotherapeutic agents useful in the generation of such immunoconjugates have
been described above. Enzymatically active toxins and fragments thereof that
can be used
include diphtheria A chain, nonbinding active fragments of diphtheria toxin,
exotoxin A
chain (from Pseudomonas aeruginosa), ricin A chain, abrin A chain, modeccin A
chain,
alpha-sarcin, Aleuritesfordii proteins, dianthin proteins, Phytolaca americana
proteins
(PAPI, PAPII, and PAP-S), momordica charantia inhibitor, curcin, crotin,
sapaonaria
officinalis inhibitor, gelonin, mitogellin, restrictocin, phenomycin,
enomycin, and the
tricothecenes. A variety of radionuclides are available for the production of
radioconjugated
antibodies. Examples include 212 Bi, 1311, 131In 90Y and 186Re.
Conjugates of the antibody and cytotoxic agent are made using a variety of
bifunctional protein-coupling agents such as N-succinimidyl-3-(2-
pyridyldithiol) propionate
(SPDP), iminothiolane (IT), bifunctional derivatives of imidoesters (such as
dimethyl
adipimidate HC1), active esters (such as disuccinimidyl suberate), aldehydes
(such as
glutaraldehyde), bis-azido compounds (such as bis (p-azidobenzoyl)
hexanediamine), bis-
diazonium derivatives (such as bis-(p-diazoniumbenzoyl)-ethylenediamine),
diisocyanates
(such as tolyene 2,6-diisocyanate), and bis-active fluorine compounds (such as
1,5-difluoro-
2,4-dinitrobenzene). For example, a ricin immunotoxin can be prepared as
described in
Vitetta et al., Science, 238: 1098 (1987). Carbon-14-labeled 1-
isothiocyanatobenzyl-3-
methyldiethylene triaminepentaacetic acid (MX-DTPA) is an exemplary chelating
agent for
conjugation of radionucleotide to the antibody. See, W094/11026.
In another embodiment, the antibody can be conjugated to a "receptor" (such as
streptavidin) for utilization in tumor pretargeting wherein the antibody-
receptor conjugate is
administered to the patient, followed by removal of unbound conjugate from the
circulation
using a clearing agent and then administration of a "ligand" (e.g., avidin)
that is conjugated to
a cytotoxic agent (e.g., a radionucleotide).
91
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Immunoliposomes
The antibodies disclosed herein can also be formulated as immunoliposomes.
Liposomes containing the antibody are prepared by methods known in the art,
such as
described in Epstein et al., Proc. Natl. Acad. Sci. USA, 82: 3688 (1985);
Hwang et al., Proc.
Natl. Acad. Sci. USA, 77: 4030 (1980); and U.S. Pat. Nos. 4,485,045 and
4,544,545.
Liposomes with enhanced circulation time are disclosed in U.S. Patent No.
5,013,556.
Particularly useful liposomes can be generated by the reverse-phase
evaporation
method with a lipid composition comprising phosphatidylcholine, cholesterol,
and PEG-
derivatized phosphatidylethanolamine (PEG-PE). Liposomes are extruded through
filters of
defined pore size to yield liposomes with the desired diameter. Fab' fragments
of the
antibody of the present invention can be conjugated to the liposomes as
described in Martin
et al., J. Biol. Chem., 257: 286-288 (1982) via a disulfide-interchange
reaction. A
chemotherapeutic agent (such as Doxorubicin) is optionally contained within
the liposome.
See, Gabizon et al., J. National Cancer Inst., 81 19 : 1484 (1989).
Pharmaceutical Compositions of Antibodies
Antibodies specifically binding a ZPA polypeptide identified herein, as well
as other
molecules identified by the screening assays disclosed hereinbefore, can be
administered for
the treatment of various disorders as noted above and below in the form of
pharmaceutical
compositions.
Lipofectins or liposomes can be used to deliver the polypeptides, nucleic acid
molecules, antibodies, antagonists or composition of this invention into
cells. Where
antibody fragments are used, the smallest inhibitory fragment that
specifically binds to the
binding domain of the target protein can be used. For example, based upon the
variable-
region sequences of an antibody, peptide molecules can be designed that retain
the ability to
bind the target protein sequence. Such peptides can be synthesized chemically
and/or
produced by recombinant DNA technology. See, e.g., Marasco et al., Proc. Natl.
Acad. Sci.
USA, 90: 7889-7893 (1993).
The formulation herein can also contain more than one active compound as
necessary
for the particular indication being treated, preferably those with
complementary activities that
do not adversely affect each other. Alternatively, or in addition, the
composition can
comprise an agent that enhances its function, such as, for example, a
cytotoxic agent,
92
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
chemotherapeutic agent, or growth-inhibitory agent. Such molecules are
suitably present in
combination in amounts that are effective for the purpose intended.
The active ingredients can also be entrapped in microcapsules prepared, for
example,
by coacervation techniques or by interfacial polymerization, for example,
hydroxymethylcellulose or gelatin-microcapsules and poly-(methylmethacylate)
microcapsules, respectively, in colloidal drug delivery systems (for example,
liposomes,
albumin microspheres, microemulsions, nano-particles, and nanocapsules) or in
macroemulsions. Such techniques are disclosed in Remington's Pharmaceutical
Sciences,
supra.
The formulations to be used for in vivo administration must be sterile. This
is readily
accomplished by filtration through sterile filtration membranes.
Sustained-release preparations can be prepared. Suitable examples of sustained-
release preparations include semipermeable matrices of solid hydrophobic
polymers
containing the antibody, which matrices are in the form of shaped articles,
e.g., films, or
microcapsules. Examples of sustained-release matrices include polyesters,
hydrogels (for
example, poly(2-hydroxyethyl-methacrylate), or poly(vinylalcohol)),
polylactides (U.S. Pat.
No. 3,773,919), copolymers of L-glutamic acid and y ethyl-L-glutamate, non-
degradable
ethylene-vinyl acetate, degradable lactic acid-glycolic acid copolymers such
as the LUPRON
DEPOT TM (injectable microspheres composed of lactic acid-glycolic acid
copolymer and
leuprolide acetate), and poly-D-(-)-3-hydroxybutyric acid. While polymers such
as ethylene-
vinyl acetate and lactic acid-glycolic acid enable release of molecules for
over 100 days,
certain hydrogels release proteins for shorter time periods. When encapsulated
antibodies
remain in the body for a long time, they can denature or aggregate as a result
of exposure to
moisture at 37 C, resulting in a loss of biological activity and possible
changes in
immunogenicity. Rational strategies can be devised for stabilization depending
on the
mechanism involved. For example, if the aggregation mechanism is discovered to
be
intermolecular S-S bond formation through thio-disulfide interchange,
stabilization can be
achieved by modifying sulfhydryl residues, lyophilizing from acidic solutions,
controlling
moisture content, using appropriate additives, and developing specific polymer
matrix
compositions.
93
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Methods of Treatment using an anti-ZPA Antibody or Fragment Thereof
It is contemplated that the antibodies to a ZPA polypeptide can be used to
treat
various apoptosis-related disorders as noted above. It will be appreciated
that antigen-
binding fragments of an anti-ZPA polypeptide antibody can also be used in the
following
methods.
The antibodies are administered to a mammal, e.g. a human, in accord with
known
methods, such as intravenous administration as a bolus or by continuous
infusion over a
period of time, by intramuscular, intraperitoneal, intracerobrospinal,
intravenous,
subcutaneous, intra-articular, intrasynovial, intrathecal, oral, topical, or
inhalation routes.
The location of the binding target of an antibody of the invention may be
taken into
consideration in preparation and administration of the antibody. When the
binding target is
an intracellular molecule, certain embodiments of the invention provide for
the antibody or
antigen-binding fragment thereof to be introduced into the cell where the
binding target is
located. In one embodiment, an antibody of the invention can be expressed
intracellularly as
an intrabody. The term "intrabody," as used herein, refers to an antibody or
antigen-binding
portion thereof that is expressed intracellularly and that is capable of
selectively binding to a
target molecule, as described in Marasco, Gene Therapy 4: 11-15 (1997);
Kontermann,
Methods 34: 163-170 (2004); U.S. Patent Nos. 6,004,940 and 6,329,173; U.S.
Patent
Application Publication No. 2003/0104402, and PCT Publication No.
W02003/077945.
Intracellular expression of an intrabody is effected by introducing a nucleic
acid encoding the
desired antibody or antigen-binding portion thereof (lacking the wild-type
leader sequence
and secretory signals normally associated with the gene encoding that antibody
or antigen-
binding fragment) into a target cell. Any standard method of introducing
nucleic acids into a
cell may be used, including, but not limited to, microinjection, ballistic
injection,
electroporation, calcium phosphate precipitation, liposomes, and transfection
with retroviral,
adenoviral, adeno-associated viral and vaccinia vectors carrying the nucleic
acid of interest.
One or more nucleic acids encoding all or a portion of an anti-ZPA antibody of
the invention
can be delivered to a target cell, such that one or more intrabodies are
expressed which are
capable of intracellular binding to a ZPA protein and modulation of one or
more ZPA-
mediated cellular pathways.
In another embodiment, internalizing antibodies are provided. Antibodies can
possess
certain characteristics that enhance delivery of antibodies into cells, or can
be modified to
possess such characteristics. Techniques for achieving this are known in the
art. For
94
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
example, cationization of an antibody is known to facilitate its uptake into
cells (see, e.g.,
U.S. Patent No. 6,703,019). Lipofections or liposomes can also be used to
deliver the
antibody into cells. Where antibody fragments are used, the smallest
inhibitory fragment that
specifically binds to the binding domain of the target protein is generally
advantageous. For
example, based upon the variable-region sequences of an antibody, peptide
molecules can be
designed that retain the ability to bind the target protein sequence. Such
peptides can be
synthesized chemically and/or produced by recombinant DNA technology. See,
e.g.,
Marasco et al., Proc. Natl. Acad. Sci. USA, 90: 7889-7893 (1993).
Entry of antibodies into target cells can be enhanced by methods known in the
art.
For example, certain sequences, such as those derived from HIV Tat or the
Antennapedia
homeodomain protein are able to direct efficient uptake of heterologous
proteins across cell
membranes. See, e.g., Chen et al., Proc. Natl. Acad. Sci. USA (1999), 96:4325-
4329.
When the binding target is located in the brain, certain embodiments of the
invention
provide for the antibody or antigen-binding fragment thereof to traverse the
blood-brain
barrier. Certain neurodegenerative diseases are associated with an increase in
permeability of
the blood-brain barrier, such that the antibody or antigen-binding fragment
can be readily
introduced to the brain. When the blood-brain barrier remains intact, several
art-known
approaches exist for transporting molecules across it, including, but not
limited to, physical
methods, lipid-based methods, and receptor and channel-based methods.
Physical methods of transporting the antibody or antigen-binding fragment
across the
blood-brain barrier include, but are not limited to, circumventing the blood-
brain barrier
entirely, or by creating openings in the blood-brain barrier. Circumvention
methods include,
but are not limited to, direct injection into the brain (see, e.g.,
Papanastassiou et al., Gene
Therapy 9: 398-406 (2002)), interstitial infusion/convection-enhanced delivery
(see, e.g.,
Bobo et al., Proc. Natl. Acad. Sci. USA 91: 2076-2080 (1994)), and implanting
a delivery
device in the brain (see, e.g., Gill et al., Nature Med. 9: 589-595 (2003);
and Gliadel
WafersTM, Guildford Pharmaceutical). Methods of creating openings in the
barrier include,
but are not limited to, ultrasound (see, e.g., U.S. Patent Publication No.
2002/0038086),
osmotic pressure (e.g., by administration of hypertonic mannitol (Neuwelt, E.
A., Implication
of the Blood-Brain Barrier and its Manipulation, Vols 1& 2, Plenum Press, N.Y.
(1989))),
permeabilization by, e.g., bradykinin or permeabilizer A-7 (see, e.g., U.S.
Patent Nos.
5,112,596, 5,268,164, 5,506,206, and 5,686,416), and transfection of neurons
that straddle the
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
blood-brain barrier with vectors containing genes encoding the antibody or
antigen-binding
fragment (see, e.g., U.S. Patent Publication No. 2003/0083299).
Lipid-based methods of transporting the antibody or antigen-binding fragment
across
the blood-brain barrier include, but are not limited to, encapsulating the
antibody or antigen-
binding fragment in liposomes that are coupled to antibody binding fragments
that bind to
receptors on the vascular endothelium of the blood-brain barrier (see, e.g.,
U.S. Patent
Application Publication No. 20020025313), and coating the antibody or antigen-
binding
fragment in low-density lipoprotein particles (see, e.g., U.S. Patent
Application Publication
No. 20040204354) or apolipoprotein E (see, e.g., U.S. Patent Application
Publication No.
20040131692).
Receptor and channel-based methods of transporting the antibody or antigen-
binding
fragment across the blood-brain barrier include, but are not limited to, using
glucocorticoid
blockers to increase permeability of the blood-brain barrier (see, e.g., U.S.
Patent Application
Publication Nos. 2002/0065259, 2003/0162695, and 2005/0124533); activating
potassium
channels (see, e.g., U.S. Patent Application Publication No. 2005/0089473),
inhibiting ABC
drug transporters (see, e.g., U.S. Patent Application Publication No.
2003/0073713); coating
antibodies with a transferrin and modulating activity of the one or more
transferrin receptors
(see, e.g., U.S. Patent Application Publication No. 2003/0129186), and
cationizing the
antibodies (see, e.g., U.S. Patent No. 5,004,697).
Other therapeutic regimens can be combined with the administration of the
antibodies
of the instant invention as noted above. For example, if the antibodies are to
treat cancer, the
patient to be treated with such antibodies can also receive radiation therapy.
Alternatively, or
in addition, a chemotherapeutic agent can be administered to the patient.
Preparation and
dosing schedules for such chemotherapeutic agents can be used according to
manufacturers'
instructions or as determined empirically by the skilled practitioner.
Preparation and dosing
schedules for such chemotherapy are also described in Chemotherapy Service,
Ed., M.C.
Perry (Williams & Wilkins: Baltimore, MD, 1992). The chemotherapeutic agent
can
precede, or follow administration of the antibody, or can be given
simultaneously therewith.
The antibody can be combined with an anti-estrogen compound such as tamoxifen
or
EVISTATM or an anti-progesterone such as onapristone (see, EP 616812) in
dosages known
for such molecules.
If the antibodies are used for treating cancer, they can be, optionally,
administered
with antibodies against one or more tumor-associated antigens, such as
antibodies that bind to
96
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
one or more of the ErbB2, EGFR, ErbB3, ErbB4, or VEGF receptor(s). These also
include
the agents set forth above. Also, the antibody is suitably administered
serially or in
combination with radiological treatments, whether involving irradiation or
administration of
radioactive substances. Alternatively, or in addition, two or more antibodies
binding the same
or two or more different antigens disclosed herein can be co-administered to
the patient. In
one embodiment, the antibodies herein are co-administered with a growth-
inhibitory agent.
For example, the growth-inhibitory agent can be administered first, followed
by an antibody
of the present invention. However, simultaneous administration or
administration of the
antibody of the present invention first is also contemplated. Suitable dosages
for the growth-
inhibitory agent are those presently used and can be lowered due to the
combined action
(synergy) of the growth-inhibitory agent and the antibody herein.
In one embodiment, vascularization of tumors is attacked in combination
therapy. An
anti-ZPA polypeptide antibody and another antibody (e.g., anti-VEGF) are
administered to
tumor-bearing patients at therapeutically effective doses as determined, for
example, by
observing necrosis of the tumor or its metastatic foci, if any. This therapy
is continued until
such time as no further beneficial effect is observed or clinical examination
shows no trace of
the tumor or any metastatic foci. Then TNF is administered, alone or in
combination with an
auxiliary agent such as alpha-, beta-, or gamma-interferon, anti-HER2
antibody, heregulin,
anti-heregulin antibody, D-factor, interleukin-1 (IL-1), interleukin-2 (IL-2),
granulocyte-
macrophage colony stimulating factor (GM-CSF), or agents that promote
microvascular
coagulation in tumors, (such as anti-protein C antibody, anti-protein S
antibody, or C4b
binding protein, see, WO 91/01753, published 21 February 1991), or heat or
radiation.
Since the auxiliary agents will vary in their effectiveness, it can be
desirable to
compare their impact on the tumor by matrix screening in conventional fashion.
The
administration of an anti-ZPA polypeptide antibody and TNF is repeated until
the desired
clinical effect is achieved. Alternatively, an anti-ZPA polypeptide antibody
is administered
together with TNF and, optionally, auxiliary agent(s). In instances where
solid tumors are
found in the limbs or in other locations susceptible to isolation from the
general circulation,
the therapeutic agents described herein are administered to the isolated tumor
or organ. In
other embodiments, a FGF or PDGF antagonist, such as an anti-FGF or an anti-
PDGF
neutralizing antibody, is administered to the patient in conjunction with an
anti-ZPA
polypeptide antibody.
97
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
For the prevention or treatment of an apoptosis-related disorder, the
appropriate
dosage of an antibody herein will depend on the type of disorder to be
treated, as defined
above, the severity and course of the disease, whether the antibody is
administered for
preventive or therapeutic purposes, previous therapy, the patient's clinical
history and
response to the antibody, and the discretion of the attending physician. The
antibody is
suitably administered to the patient at one time or over a series of
treatments.
For example, depending on the type and severity of the disorder, about 1 g/kg
to 50
mg/kg (e.g., 0.1-20 mg/kg) of antibody is an initial candidate dosage for
administration to the
patient, whether, for example, by one or more separate administrations, or by
continuous
infusion. A typical daily or weekly dosage might range from about 1 g/kg to
100 mg/kg or
more, depending on the factors mentioned above. For repeated administrations
over several
days or longer, depending on the condition, the treatment is repeated or
sustained until a
desired suppression of disorder symptoms occurs. However, other dosage
regimens can be
useful. The progress of this therapy is easily monitored by conventional
techniques and
assays, including, for example, radiographic tumor imaging.
Articles of Manufacture with Antibodies
An article of manufacture comprising a container with the antibody and a label
is also
provided. Such articles are described above, wherein the active agent is an
anti-ZPA
antibody.
Diagnosis and Prognosis of Apoptosis-Related Disorders Using Antibodies
Antibodies directed against one or more ZPA polypeptides can be used to
diagnose
and/or determine the prognosis of an apoptosis-related disorder. For example,
antibodies
directed against one or more ZPA polypeptides can be used as tumor diagnostics
or
prognostics.
For example, antibodies, including antigen-binding antibody fragments, can be
used
qualitatively or quantitatively to detect the expression of genes including
the gene encoding
the ZPA polypeptide, either in intact cells or in cell lysates. In certain
embodiments, the
antibody is equipped with a detectable label, e.g., a fluorescent label, and
binding can be
monitored by microscopy, flow cytometry, fluorimetry, or other techniques
known in the art.
Such binding assays are performed essentially as described above.
98
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
All publications (including patents and patent applications) cited herein are
hereby
incorporated in their entirety by reference.
Commercially available reagents referred to in the Examples were used
according to
manufacturer's instructions unless otherwise indicated. The source of those
cells identified in
the following Examples, and throughout the specification, by ATCC accession
numbers is the
American Type Culture Collection, Manassas, VA. Unless otherwise noted, the
present
invention uses standard procedures of recombinant DNA technology, such as
those described
hereinabove and in the following textbooks: Sambrook et al., supra; Ausubel et
al., Current
Protocols in Molecular Biolo~y (Green Publishing Associates and Wiley
Interscience, N.Y.,
1989); Innis et al., PCR Protocols: A Guide to Methods and Applications
(Academic Press,
Inc.: N.Y., 1990); Harlow et al., Antibodies: A Laboratory Manual (Cold Spring
Harbor
Press: Cold Spring Harbor, 1988); Gait, Oligonucleotide Synthesis (IRL Press:
Oxford,
1984); Freshney, Animal Cell Culture, 1987; Coligan et al., Current Protocols
in
Immunolo~y, 1991.
Throughout this specification and claims, the word "comprise," or variations
such as
"comprises" or "comprising," will be understood to imply the inclusion of a
stated
integer or group of integers but not the exclusion of any other integer or
group of integers.
The foregoing written description is considered to be sufficient to enable one
skilled
in the art to practice the invention. The following Examples are offered for
illustrative
purposes only, and are not intended to limit the scope of the present
invention in any way.
Indeed, various modifications of the invention in addition to those shown and
described
herein will become apparent to those skilled in the art from the foregoing
description and fall
within the scope of the appended claims.
EXAMPLES
Example 1: Identification of Putative Zebrafish Bcl-2 Family Members
Homologs of certain members of the human Bcl-2 family had previously been
identified in zebrafish using traditional sequence searching methodologies
(Inohara and
Nunez, Cell Death Diff. 7: 509-510 (2000); Coultas et al., Cell Death Diff. 9:
1163-1166
(2002); Aouacheria et al., Mol. Biol. Evol. 22(12): 2395-2416 (2005)). Despite
significant
effort by several groups, equivalents to many proteins important in the
mammalian intrinsic
apoptotic pathway had not been found in zebrafish (id.). Applicants suspected
the existence
99
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
of novel and divergent Bcl-2 genes in zebrafish that would not appear in the
most common
sequence databases and that would not be readily discoverable by traditional
sequence-based
searching. A comprehensive search for these sequences using both traditional
BLAST and
PROSITE searching in a customized sequence database and feature-based database
mining
with customized Hidden Markov Models in conjunction with the Unison database
(http://unison-db.org) was therefore undertaken.
a. BLAST/PROSITE Database Mining
Previous BLAST and PROSITE searching by other groups had failed to identify
zebrafish homologs for several Bcl-2 family members, including Bak, Bik, Bim,
and PUMA
(Inohara and Nunez, Cell Death Diff. 7: 509-510 (2000); Coultas et al., Cell
Death Diff. 9:
1163-1166 (2002); Aouacheria et al., Mol. Biol. Evol. 22(12): 2395-2416
(2005)). To
investigate whether the database used for those prior studies had been
insufficiently broad, a
custom database containing 136,655 zebrafish amino acid sequences was
constructed. The
custom database was queried by standard BLAST (version 2.2.10) and PROSITE
(release 18)
searching techniques using default arguments and known human, mouse, and
chicken
members of the Bcl-2 family as queries.
This search identified many sequences, two of which had not previously been
identified in similar searches for Bcl-2-related sequences in zebrafish. The
first sequence
(Ensembl:ENSDARP00000040899 (SEQ ID NO: 1)) was 33% identical to human Bax
over
51% of the sequence with an e-value of 1e-14. None of the PROSITE BH patterns
aligned to
ENSDARP00000040899, and the Ensembl database (available at www.ensembl.org;
Birney
et al., Nucleic Acids Res. 34: D556-561 (2006)) annotates that gene as
hypothetical. The
second sequence (XP_693331 (SEQ ID NO: 3)) was 33% identical to human Bad over
68%
of the sequence with an e-value of 1.3.
Searching with PROSITE patterns did not identify any of the known zebrafish
Bcl-2
family members and no new zebrafish sequences were identified using PROSITE
patterns
alone.
b. Feature-Based Database Mining
Even using a customized sequence database tailored to zebrafish sequences
(described
in Example 1(a)), only a single new putative Bcl-2 family member was
identified. Because
standard BLAST and PROSITE database searching were apparently unable to
identify further
zebrafish Bcl-2 members, the possibility that family members may have
significantly
different overall base sequences in zebrafish and humans, yet still share
function was
100
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
considered. To address this possibility, feature-based sequence mining was
employed.
Feature-based mining identifies sequences that match a set of specified
features.
(1) Feature-Based Mining Sequence Sources
Feature-based mining was performed in the Unison database. Briefly, Unison
comprises a non-redundant compendium of sequences from many source databases
and
extensive precomputed proteomic predictions within a relational database
(http://unison-
db.org). The Unison schema, non-proprietary data and predictions, tools, and
Internet
interface have been released under the Academic Free License and are available
for use or
download at http://unison-db.org/. At the time of the study, Unison included
6.5 million
distinct sequences, including 136,655 distinct zebrafish sequences from the
RefSeq database
(Wheeler et al., Nucl. Acid Res. 34: D173-180 (2006)), the UniProt/Swiss-Pro
and
UniProt/TrEMBL databases (Wu, Nucl. Acids Res. 34: D187-191 (2006)), and
Ensembl
Release 35 (Birney et al., Nucleic Acids Res. 34: D556-561 (2006)).
(2) Construction of Custom Hidden Markov Models for Bcl-2 and BH
Domains
Multiple sequence alignments were constructed from PROSITE patterns and
matrices
(Sigrist et al., Brief Bioinform. 3: 265-274 (2002)). For patterns PS01080
(BH1), PS01258
(BH2), PS01259 (BH3) and PS01260 (BH4), and for the matrix PS50063 (BH4), the
false
negative sequences were manually incorporated into the alignment of true
positive sequences
obtained from the PROSITE website (http://us.expasy.org/cgi-
bin/nicedoc.po?pdoc00829).
For the PS50062 (Bcl-2) matrix, manual alignment of the three false negatives
was not
obvious and only the true positives were used. The alignments were used to
build "global"
hidden Markov models (HMM)s using the hmmbuild and hmmcalibrate programs from
HMMER v.23.2 (Eddy, Bioinformatics 14:755-763 (1998)) incorporating the
default
arguments. In order to assess recall and precision, and to determine
appropriate score
thresholds, the six constructed HMMs were aligned to the UniProt/Swiss-Prot
sequence
database (Figure 1). The results demonstrated that the constructed HMMs had
improved
recall without loss of precision at suitable score thresholds. From the
results HMM
alignment score cutoffs were selected as follows: BH1: 21; BH2: 13; BH3: 12;
BH4: 22; BH4
matrix: 20; and Bc12 matrix: 20.
Unison's cluster-based update framework was used to run hmmsearch results for
all
136,655 zebrafish sequences. HMM alignments for all zebrafish sequences were
computed
and loaded into an in-house copy of Unison using Unison's automated update
facility. A
101
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
transmembrane domain analysis was also performed using TMHMM v. 2.Oc (Krogh et
al., J.
Mol. Biol. 305: 567-580 (2001)) with the default options. Once the results
were loaded,
feature-based mining involved framing SQL queries to represent appropriate
conjunctions of
precomputed proteomic predictions. A query was created to identify all
zebrafish sequences
which aligned to any of the HMMs using the score criteria defined above. The
query
identified each sequence that overlapped genomically with a known Bcl-2 family
member.
The four most promising candidate genes not previously identified as zebrafish
Bcl-2 family
members were analyzed further.
Example 2: Analysis of Identified Sequences
Each of the putative Bcl-2 related ("B2R") zebrafish genes (ENSDARP0000040899
and XP693331 identified from the BLAST searching described in Example 1(a) and
ENSDARP00000066976, FGENESH00000065416, FGENESH00000065416, and
FGENESH00000082230 identified from the feature-based mining described in
Example
1(b)) were further analyzed to assign a specific identity as a particular
member of the Bcl-2
family. Each sequence was subj ected to BLAST searching, and aligned with the
most
homologous identified sequence, resulting in an e-value, a score, a percent
identity, and a
percent coverage (see Table 2). The presence or absence of a putative
transmembrane
domain within the encoded protein was determined. The neighboring genes within
the
zebrafish genome were also examined so that any conserved synteny could be
assessed (see
Table 2).
a. zBak (ENSDARP00000040899)
BLAST searching (described in Example 1(a)) identified the sequence
ENSDARP00000040899 as being 33% identical to human Bax over 51% of the
sequence
with an e-value of le-14 (see Table 2). The amino acid sequence of
ENSDARP00000040899
and its encoding nucleotide sequence are shown below.
ENSDARP00000040899:
MACEASQDDQIGEALLIGVVRQELMEVMEVTEGNAAPPALPEAKPISNSQDQILVQQ
LANTIKVIGDKLDQDQAFNDMIDGLVKVADKSSFWKLVEKVFTDGQINWGRIIVLFY
SVGKLSAKMVVARLPRIVSDILSLSLDYFKRNLLQWIRTVGGWMNSIPALACFSVDQ
FSGSSMRKYSPYVGVVFAFTGGLLLGGFIVSRFQKT (SEQ ID NO: 1)
ENSDARG00000030881:
ATGGCTTGTGAAGCCTCACAGGATGATCAGATTGGAGAGGCACTCTTAATAGGG
GTAGTAAGGCAGGAGCTAATGGAGGTGATGGAGGTGACTGAAGGAAATGCAGCT
CCTCCAGCTCTTCCTGAAGCTAAACCAATAAGCAACAGCCAGGACCAGATTCTGG
TTCAGCAGCTGGCGAACACAATCAAAGTGATCGGTGACAAACTCGACCAGGATC
AAGCATTTAACGACATGATCGATGGCTTAGTAAAGGTAGCTGATAAAAGCAGTT
102
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
TCTGGAAACTTGTGGAAAAGGTGTTCACAGATGGCCAGATCAACTGGGGCAGAA
TTATCGTGCTGTTTTATTCTGTTGGAAAACTGTCAGCCAAGATGGTCGTCGCTCGC
CTACCCAGAATTGTTTCAGATATTTTATCATTAAGTCTTGATTACTTCAAAAGGAA
TCTGTTGCAGTGGATTCGCACAGTAGGAGGATGGATGAACAGTATCCCTGCACTG
GCCTGTTTCTCTGTTGACCAATTTTCTGGTTCTTCAATGAGAAAATATTCTCCTTA
CGTTGGAGTTGTGTTTGCCTTCACTGGTGGCCTACTGCTGGGTGGCTTCATCGTCT
CGAGATTTCAGAAAACCTGA (SEQ ID NO:2)
Ensembl annotated ENSDARP00000040899 as hypothetical, with no ascribed
identity. None of the PROSITE BH patterns aligned to the sequence. Of the HMMs
constructed in Example 1(b), the Bcl-2, BH1, BH2, and BH3 HMMs each aligned to
the
sequence with scores of 33, 14, 6.1, and 2.4, respectively, and e-values of
7.5e-7, 0.03 1, 110,
and 1300, respectively. An alignment of the sequence was also made with the
BH3 domains
of several known or proposed Bcl-2 family members, and examined using Jalview
2.05
(Clamp et al., Bioinformatics 12: 426-427 (2004)) (Figure 2). Despite the poor
score of the
BH3 HMM alignment to ENSDAR00000040899, the sequence showed qualitatively good
similarity with the BH3 domains of other Bcl-2 family members and plausible
amino acid
substitutions at all positions which were not conserved (see Figure 2). TMHMM
analysis
also predicted that the sequence contained a transmembrane domain from about
amino acids
180-202.
The presence of a conserved syntenic relationship was examined for
ENSDAR00000040899 and all other sequences described herein by comparing
flanking
genes for the zebrafish gene to flanking genes for the human functional
homologue using the
publicly available ENSEMBL database (available at www.ensembl.org; Birney et
al., Nucleic
Acids Res. 34: D556-561 (2006)). The zebrafish gene was designated to share a
conserved
syntenic relationship with the human gene if more than a single flanking gene
on either or
both sides of the zebrafish gene had a human equivalent that also flanked the
human gene.
ENSDAR00000040899 was not in a conserved syntenic relationship with human Bak,
but
was to human Bax.
ENSDAR00000040899 was also included in an alignment of candidate zebrafish Bcl-
2 family members against human Bcl-2 sequences, and vice versa, using BLAST to
identify
reciprocal best BLAST alignments, pairwise identity, and alignment coverage
(Table 2).
Although alignment of the candidate sequence to human Bax resulted in the best
e-value, the
best overall sequence coverage was obtained by an alignment to human Bak.
Although the
BH2 and BH3 HMM alignments did not score significantly, the presence of those
domains in
the context of significant scores for Bcl-2 and BH1, the absence of a
predicted BH4 domain,
103
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
the similarity of the BH3 domain with those of other Bcl-2 members, the
prediction of a TM
domain, and the reciprocal BLAST analysis combined to suggest that
ENSDAR00000040899
was a pro-apoptotic member of the Bcl-2 family, most likely functionally
homologous to
Bak.
b. zBad2 (XP_693331)
As described in Example 1(a), XP_693331 was also identified by a BLAST search
in
a customized zebrafish sequence database. The amino acid sequence of XP_693331
and the
mRNA sequence encoding it are set forth below.
XP693331:
MENTSHDHQDDSSTLDEKERSHLKGTIKNHGQHQDRTSANISPQGRVRLYSESQVYT
VSRWQDTETQDGASVEENGDGLPFRGRSQSAPAALWKAKKYGRQLRRMSDEFDTW
LDKGEVKRANSQKQTYRGWFSFLWSPKEEEGRE (SEQ ID NO: 3)
XM_688239 (nucleotides 367-804):
ATGGAGAACACCTCGCATGACCATCAAGATGATTCCAGCACCTTGGATGAAAAA
GAGAGATCACATCTGAAAGGGACAATCAAGAACCATGGACAACATCAGGATCGA
ACATCGGCCAACATTTCTCCTCAAGGGCGTGTGCGGCTCTATTCGGAATCTCAAG
TGTATACAGTCAGCCGCTGGCAGGACACAGAGACCCAGGATGGAGCATCGGTGG
AGGAGAACGGAGATGGACTTCCATTCAGGGGTCGTTCTCAATCAGCACCTGCTGC
ACTGTGGAAAGCAAAAAAGTATGGCCGTCAGTTGAGGAGAATGAGCGATGAATT
CGACACATGGCTCGATAAAGGGGAGGTCAAGAGAGCGAACAGCCAGAAACAGA
CCTACCGAGGATGGTTTTCGTTCCTCTGGAGTCCCAAAGAAGAAGAGGGCAGAG
AATGA (SEQ ID NO: 4)
The GenBank annotation for XP693331 predicted that the sequence was similar to
the BH3-
only proapoptotic protein, Bad. An alignment of XP693331 with human Bad showed
that
the two proteins have 33% identity over 72% of the sequence. The HMMer score
versus the
BH3 domain was 12.6, with an e-value of 1.3. A TMHMM analysis of XP_693331
showed
no predicted transmembrane domains in the sequence, similar to human Bad. A
clone of
XP693331 was obtained (Open Biosystems), and the surrounding sequences within
the
zebrafish genome were identified.
The genes adjacent to XP_693331 differed from those adjacent to human Bad,
thus
XP693331 was not in a conserved syntenic relationship with human Bad. However,
another
zebrafish gene (A1332008) had previously been identified as having significant
homology to
human Bad and was syntenic to human bad (Inohara and Nunez, Cell Death Diff.
7: 509-5 10
(2000); Coultas et al., Cell Death Diff. 9: 1163-1166 (2002)). XP_693331 was
approximately 32% identical to that previously identified sequence. Thus, the
above data
suggested that XP_693331 was a Bcl-2 family member related to human Bad.
Because a
104
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zebrafish gene with sequence similarity to and conserved synteny to human Bad
was
previously known, XP693331 was named "zBad2" as the second zebrafish gene with
identity to human Bad.
c. zBik (ENSDARP00000066976)
The zebrafish sequence ENSDARP00000066976 was identified by feature-based
database mining as described in Example 1(b) as a B2R protein. The amino acid
sequence of
ENSDARP00000066976 and the nucleotide sequence encoding it are set forth
below.
ENSDARP00000066976:
MVEETRQQKNATTLQAGPAEVDHSNLYAFNMRVTQTIGRQLAQIGDEMDNKWRQE
PPVPWQNLNFGIYPYVLSRRVFSGRILANLWGSKIMPIFRTSWLLPQLQNGCQEARK
WAAWVSNLHVSDWSRSTTYTLASALLLVTVSIFLVNWNEYEG (SEQ ID NO: 5)
ENSDARG00000045549:
ATGGTGGAAGAAACTAGACAGCAGAAAAACGCCACAACCCTGCAGGCTGGACCT
GCTGAGGTTGACCACAGTAATCTCTATGCATTCAATATGAGAGTCACCCAGACTA
TCGGACGACAGCTGGCTCAAATAGGGGACGAAATGGACAATAAATGGCGCCAAG
AACCGCCTGTCCCATGGCAGAACCTGAATTTCGGGATTTATCCTTATGTCCTAAG
TAGGAGAGTGTTCTCTGGAAGAATCCTCGCTAATCTTTGGGGGTCTAAGATTATG
CCGATATTCAGGACGTCCTGGTTGCTTCCACAGCTTCAAAATGGCTGTCAGGAGG
CTAGAAAGTGGGCAGCTTGGGTGTCCAACTTGCATGTTTCTGACTGGTCTCGCAG
CACTACATACACCCTGGCATCTGCTTTACTACTGGTCACTGTGTCTATCTTCCTTG
TAAACTGGAATGAGTATGAAGGCTGA (SEQ ID NO: 6)
BLAST searching in the customized zebrafish sequence database (as described in
Example 1(a)) did not identify ENSDARP00000066976. TMHMM analysis of the
sequence
predicted the presence of a transmembrane domain from about amino acids 130-
149.
ENSDARP00000066976 did not match to the BH1, BH2, and BH4 HMMs. The sequence
did match to the BH3 HMM with score of 18 and an e-value of 0.023. The
presence of a
BH3-related region and a transmembrane domain and an absence of BH1, BH2, and
BH4-
related regions suggested that ENSDARP00000066976 might be a member of the pro-
apoptotic BH3-only subfamily of Bcl-2 proteins.
The ENSDARP00000066976 sequence in the zebrafish genome shared a conserved
syntenic relationship with human Bik. The ENSDARP00000066976 putative BH3
region
was compared with previously known human zebrafish Bcl-2 genes (see Figure 2).
The BH3
domain of ENSDARP00000066976 was most similar to that of human Bid despite the
synteny and BLAST similarity with human Bik (Figure 3B). However, based on a
comparison of the two sequences, ENSDARP000000669761acked a putative caspase
cleavage site, while human Bid contains such a site. The presence of the BH3
domain, the
105
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
absence of other BH domains, the transmembrane domain prediction, overall
sequence
similarity, and syntenic relationship analyses all suggested that
ENSDARP00000066976 was
a zebrafish pro-apoptotic BH3-only Bcl-2 subfamily member orthologous to human
Bik.
d. zBim (FGENESH00000065416)
The zebrafish sequence FGENESH00000065416 was identified by feature-based
database mining as described in Example 1(b) as a B2R protein. BLAST searching
in the
customized zebrafish sequence database (as described in Example 1(a)) did not
identify
FGENESH00000065416. FGENESH00000065416 did not match to the BH1, BH2, and BH4
HMMs. The sequence did match to the BH3 HMM with score of 18 and an e-value of
0.023.
The presence of a BH3-related region and a transmembrane domain and an absence
of BH1,
BH2, and BH4-related regions suggested that FGENESH00000065416 might be a
member of
the pro-apoptotic BH3-only subfamily of Bcl-2 proteins.
BLAST alignments with the FGENESH00000065416 sequence show that the N-
terminal half of the sequence was 32% identical to human Bim over 47% of human
Bim with
an e-value of 9e-7, further supporting the assignment of FGENESH00000065416 as
a
member of the pro-apoptotic BH3-only subfamily of Bcl-2 proteins. However, the
C-
terminal half of FGENESH00000065416 was most similar (32% identical) to
developmentally-regulated RNA-binding protein 1, a mouse and rat RNA binding
protein
with putative involvement in neural development.
The juxtaposition of Bim homology and homology to an unrelated
protein/function in
the same contiguous region may be attributable to an assembly error within the
zebrafish
genome. In fact, no RNA product could be amplified from either the entire
sequence or from
the Bim-like fragment alone. Despite the apparent assembly error, the presence
of the BH3
domain, the absence of other BH domains, and sequence similarity suggested
that at least the
N-terminal portion of FGENESH00000065416 was homologous to human Bim.
e. zPuma (FGENESH00000078270)
The zebrafish sequence FGENESH00000078270 was identified by feature-based
database mining as described in Example 1(b) as a B2R protein. The amino acid
sequence of
FGENESH00000078270 and the nucleotide sequence encoding it (accession number
CN323956) are set forth below.
FGENESH00000078270:
MTLCFLNTSAALADEEGDPLPTALINSLDLAVNQPVSGSGFCKLKLANEQTVVTLQQ
LATREPMGDEEEVQGFQSTDPHGTTVCGMARPEMESRVDEHNSGTPNSCRMEVLRQ
DAWPNGSIIQPCHRRRTIATQTSTLSAPLPHIPSHDAFSLDSVQQQDSLLRDNSGTEQE
106
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
VSRPLPLPDLLADNQSSSEESTSSSSSTAEEDPTLEEQAVERVAVQLRTIGDEMNAVFL
QRNAVPHWQNWRGLYRGLMALVSDTINALYQHGLR (SEQ ID NO: 7)
FGENESH00000078270:
ATGACTTTGTGCTTTTTAAACACAAGCGCAGCGCTCGCTGATGAAGAGGGCGATC
CTCTGCCCACTGCTCTGATAAACAGTCTTGACCTAGCAGTGAATCAGCCGGTGTC
AGGTTCTGGCTTTTGTAAACTCAAACTGGCCAATGAGCAAACTGTTGTGACTCTC
CAACAATTAGCAACAAGAGAACCCATGGGGGATGAAGAGGAGGTGCAGGGCTTT
CAGAGCACAGACCCACACGGGACAACTGTATGTGGAATGGCCCGACCAGAGATG
GAAAGCAGAGTGGACGAACATAACTCTGGCACGCCGAACAGCTGCAGGATGGA
GGTGCTGCGTCAGGACGCCTGGCCAAATGGCAGCATCATCCAGCCCTGCCATCG
ACGCCGAACCATTGCCACTCAAACCAGCACTCTCTCTGCACCACTGCCCCACATC
CCCTCACATGATGCCTTCAGCTTGGACAGCGTCCAGCAGCAGGACAGTCTACTCA
GGGACAATTCAGGAACAGAACAGGAAGTGTCCAGGCCTCTTCCTCTGCCAGATC
TGCTAGCAGACAACCAGAGCTCCTCAGAGGAGTCCACGTCCAGCAGCAGCTCGA
CCGCTGAGGAGGACCCCACACTGGAGGAGCAGGCTGTGGAGAGGGTGGCCGTAC
AACTGAGGACAATTGGGGACGAGATGAACGCTGTCTTCCTTCAGAGGAATGCCG
TCCCGCACTGGCAGAACTGGAGAGGCCTGTACCGCGGGCTCATGGCGCTGGTCTC
GGACACCATCAATGCCCTCTACCAGCACGGCCTCAGATGA (SEQ ID NO:8)
BLAST searching in the customized zebrafish sequence database (as described in
Example
1(a)) did not identify FGENESH00000078270. TMHMM analysis did not predict a
transmembrane domain for this sequence. FGENESH00000078270 did not match to
the
BH1, BH2, and BH4 HMMs. The sequence did match to the BH3 HMM with score of 13
and an e-value of 0.77. FGENESH00000078270 was similar to human Puma based on
a
BLAST alignment showing a 25% identity with an e-value of 2.1e1.
Identification of the
genes surrounding FGENESH00000078270 in the zebrafish genome indicated that
FGENESH00000078270 shared a conserved syntenic relationship with human Puma.
The presence of a BH3-related region and an absence of BH1, BH2, and BH4-
related
regions and a transmembrane domain, combined with the sequence similarity and
conserved
synteny suggested strongly that FGENESH00000078270 was a member of the pro-
apoptotic
BH3 -only subfamily of Bcl-2 proteins, and more specifically was orthologous
to human
Puma.
f. zBmf2 (FGENESH00000082230)
The zebrafish sequence FGENESH00000082230 was identified by feature-based
database mining as described in Example 1(b) as a B2R protein. The amino acid
sequence of
FGENESH00000082230 and the nucleotide sequence encoding it are set forth
below.
FGENESH00000082230:
MDDEEDEQLPRCCETPLRNKRSEKRDAHGGDVGQTAHRHASTQTAGSVLNSARDA
DMAPFQGSQREASSLCRVVGARTAFRAPCGTGGLVSLTMGPGARGGPRALFHGNAG
107
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
FRAHFPALFEPALDGLQNAEQREQDEGRPEEKEEDRDAGISVEVQIGRKLREMGDQF
QQEHLQLIILDETRYRI (SEQ ID NO: 9)
FGENESH00000082230:
ATGGATGATGAGGAGGATGAACAGCTTCCTCGCTGCTGTGAAACGCCGCTAAGA
AACAAGCGCTCAGAGAAGAGAGACGCCCACGGTGGAGACGTGGGACAAACAGC
TCACAGACACGCATCCACACAGACTGCTGGCTCTGTCCTCAATTCAGCGAGAGAC
GCAGATATGGCACCATTCCAGGGCTCACAGAGAGAAGCATCATCTCTTTGCAGA
GTTGTGGGTGCTAGGACTGCATTCAGGGCTCCCTGTGGAACTGGGGGCCTTGTGT
CACTTACTATGGGCCCCGGAGCCCGGGGTGGGCCAAGAGCACTTTTTCATGGAA
ACGCTGGATTTCGTGCACACTTCCCTGCGCTGTTCGAACCCGCCCTGGATGGCTT
ACAAAACGCCGAACAGAGAGAGCAGGACGAAGGCAGACCAGAAGAAAAAGAA
GAGGATCGCGACGCAGGGATTAGCGTGGAGGTTCAGATTGGACGTAAATTACGT
GAAATGGGGGATCAGTTTCAGCAAGAGCATCTTCAGCTGATTATATTAGATGAA
ACCAGATACAGGATTTAA (SEQ ID NO: 10)
BLAST searching in the customized zebrafish sequence database (as described in
Example
1(a)) did not identify FGENESH00000082230. TMHMM analysis did not predict a
transmembrane domain for this sequence. FGENESH00000082230 did not match to
the
BH1, BH2, and BH4 HMMs. The sequence did match to the BH3 HMM with score of
18.6
and an e-value of 0.02. FGENESH00000082230 was similar to human Bmf based on
BLAST alignment (41% identity over 42% of the molecule with an e-value of
2.1e1). The
alignment also showed that FGENESH00000082230 contained a putative dynein
light chain
binding domain, similar to human Bmf (Day et al., Biochem J. 377:597-605
(2004)).
Identification of the genes surrounding FGENESH00000082230 in the zebrafish
genome
indicated that FGENESH00000082230 shared a conserved syntenic relationship
with human
Bmf as well. The presence of a BH3-related region and an absence of BH1, BH2,
and BH4-
related regions and a transmembrane domain, combined with the sequence
similarity and
conserved synteny suggested strongly that FGENESH00000082230 was a member of
the
pro-apoptotic BH3-only subfamily of Bcl-2 proteins, and more specifically was
orthologous
to human Bmf.
Another zebrafish gene homologous to human Bmf had already been identified in
zebrafish (Coultas et al., Cell Death Diff. 9: 1163-1166 (2002); accession
number BI891121).
That gene did not share conserved synteny with human Bmf, though, unlike
FGENESH00000082230. The similarity between FGENESH00000082230 and BI891121
was 43% over 27% of the molecule. Accordingly, FGENESH00000082230 was
designated
zBmf2 to differentiate it from that earlier-identified gene.
108
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
TABLE 2: Characteristics of putative Bcl-2 family members in zebrafish
Assignment Protein accession number e- Scor % % Human Syn-
value e identity coverage gene tenic?
zBim FGENESH00000065416 9e-7 55 32 47 Bim Y
zBak ENSDARP00000040899 4e-5 50 25 86 Bak N
le-14 81 33 51 Bax Y
zBax ENSDARP00000023687 2e-6 54 29 68 Bak N
2e-47 189 51 98 Bax N
zBik ENSDARP00000066976 1.41e4 20 47 12 Bik Y
zPuma FGENESH00000078270 2.1e1 30 25 49 Puma Y
zBmf2 FGENESH00000082230 0.011 42 41 42 Bmf Y
zBad2 XP 693331 8e-12 64 33 72 Bad N
g. General Observations Regarding Zebrafish B2R Genes
Overall, the previously known and herein identified zebrafish homologs of the
Bcl-2
family genes strongly resembled their human counterparts (Table 3), with the
multi-domain
Bcl-2 family members having the highest degree of similarity (Figure 3A). The
zebrafish
BH3 -only proteins were the most divergent from the human genes (Figure 3A),
ranging from
27% (zPuma) to 46% (zBad and zBmf2) overall similarity (see Table 3). However,
the BH3-
only proteins are known to be the most divergent of the vertebrate Bcl-2
subfamilies. A
much higher degree of similarity was observed within the BH3 domain itself,
ranging from
66-79% (Table 3; Figure 3B).
Table 3: Comparison of zebrafish and human B2R proteins
Zebrafish Mam- % Accession no. Fish Human Syntenic?
gene malian homol- chromo- chromo-
homolog ogy some some
Pro-survival
zBlpl Bcl-xL 67% AF317837 5 20q11.21 Yes
zBlp2 Bcl-2 57% NM_001030253.1 24 18q21.33 No
zMcl-la Mcl-1 43% NM131599 19 1q21.2 Yes
zMcl-lb Mcl-1 38% NM194394.2 16 1q21.2 Yes
zNR13 Boo/ 30% AF441285 18 15q21.2 Yes
DIVA
Pro-apoptotic BH3-only
zBadl Bad 46% BC097099.1 7 11q13.1 Yes
(79%)
zBad2 Bad 35% XP693331 21 11q13.1 Yes
(66%)
zBid Bid 30% XM698543.1 18 22q11.21 No
(66%)
zBik Bik 38% ENSDARP0000006697 4 22q13.2 Yes
(66%)
109
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zBmfl Bmf 46% XM695596.1 23 15q15.1 No
(72%)
zBmf2 Bmf 38% FGENESH00000082230 20 15q15.1 Yes
(66%)
zNoxa Noxa 27% DV595521.1 (EST) 19 18q21.32 No
(66%)
zPuma Puma 86% FGENESH00000078270 16 19q13.32 Yes
zBim Bim ND FGENESH00000065416 13 2q13 ND
Pro-a o totic multidomain
zBak Bak 41% ENSDARP00000040899 3 6p21.31 to hBax
zBax Bax 73% ENSDARP00000023687 ND 19q13.33 No
zBokl Bok 81% NM001003612.1 ND 2q37.3 ND
zBok2 Bok 74% BC053218.1 2 2q37.3 Yes
ND indicates not determined. The parenthetical percentages indicate the %
homology within
the BH3 domain only.
Example 3: Experimental Validation of Bcl-2 Candidate Genes
The studies in Examples 1 and 2 demonstrated that the above-identified
proteins
shared homology with certain Bcl-2 family members and were likely members of
the intrinsic
apoptotic pathway. To confirm those assignments, the function of each protein
was assessed
in zebrafish.
a. Expression Patterns of Zebrafish B2R Genes
To assess the normal expression of the previously known and herein identified
zebrafish B2R proteins, the mRNA encoding each protein was analyzed by RT-PCR
at
specific developmental stages and in specific adult tissues (Figures 3C and
3D). Adult
Tubingen long-fin fish were obtained from the Zebrafish International Resource
Center. Fish
were maintained according to the Zebrafish Book (Westerfield, The Zebrafish
Book. A guide
for the laboratory use of zebrafish (Danio rerio). 4th ed., Univ. of Oregon
Press: Eugene
(2000)). RNA was isolated from dechorionated wildtype zebrafish embryos (100
ng) at the
indicated time points using QiaShredder (Qiagen) for 30 cycles followed by
purification with
the RNAeasy Mini kit (Qiagen) using DNasel digestion according to the
manufacturer's
instructions. Adult tissues were isolated from an adult female zebrafish, and
RNA (50 ng)
was extracted from those tissues in a manner similar to the extraction from
the embryonic
tissue. GAPDH was included as a control for the amount of input RNA. The
resulting
products were resolved on 0.8% e-gels (Invitrogen) and photographed.
With the exception of zBik and zBok, all of the examined zebrafish B2R genes
were
expressed maternally (at the 1000 cell stage) (Figure 3 C). After initiation
of zygotic
transcription (approximately 5 hours post fertilization), zBadl, zBik, zBad,
zBmfl, zNoxa,
110
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zBax, zMcl-la, zMcl-lb, zBlpl, and zNR13 were expressed at fairly consistent
levels (Figure
3C). Conversely, the transcription of zBak, zBmf2, and zBlp2 decreased
dramatically before
increasing again later in development (Figure 3C). zBok2 was strongly
expressed maternally
but was largely absent later in embryonic development (Figure 3C).
Of the seven adult zebrafish tissues examined, the ovary displayed significant
transcription of each of the genes except zBmf2 (Figure 3D). In contrast, the
liver displayed
only weak expression of zBid, zMcl-la, and zMcl-lb (Figure 3D). Several of the
zebrafish
BH3-only genes were expressed in only a few tissues (Figure 3D).
b. Apoptotic Effect of Putative Zebrafish B2R Proteins In Vivo
The ability of each of the previously known and herein identified zebrafish
B2R genes
to activate the intrinsic apoptotic pathway was assessed. Zebrafish cDNAs were
directionally
cloned into the expression vector pCS108 (Fletcher et al., Gene Expr. Patterns
5(2):225-30
(2004); http://tropicalis.berkeley.edu/home/genomic_resources/Ests/
vectors/cs108.pdf.,
dated August 13, 2001) using standard protocols. The primers used for the
cloning were as
follows (a115' to 3'):
zBak forward: GGGGGTTGTTGTAAAGTACAGTGG (SEQ ID NO: 65)
zBak reverse: TCAGGTTTTCTGAAATCTCGAGACG (SEQ ID NO: 66)
zBik forward: ACTGTACTGAGACACATCACAGCAACA (SEQ ID NO: 67)
zBik reverse: TCAGCCTTCATACTCATTCCAG (SEQ ID NO: 68)
zPuma forward: CTAACTGAGTACTCATCTAATGAATTAACACCGCT (SEQ ID NO:
69)
zPuma reverse: TCATCTGAGGCCGTGCTGGTAGAG (SEQ ID NO: 70)
zBmfl forward: ATGGATGAGGACGAGGATGAT (SEQ ID NO: 71)
zBmfl reverse: TCACCTGCGGTTCTCTCTG (SEQ ID NO: 72)
zBmf2 forward: ATGGATGATGAGGAGGATGAAC (SEQ ID NO: 73)
zBmf2 reverse: TTAAATCCTGTATCTGGTTTCATCTA (SEQ ID NO: 74)
zBadl forward: CTTCCACAACACTTCCACTGGATAC (SEQ ID NO: 75)
zBadl reverse: TTCACTGTCAGTCACTCTGCGGGGC (SEQ ID NO: 76)
zBlp 1 forward: GCCGAATTCCACCATGTCTTACTATAACCGAGAACTG (SEQ ID NO:
77)
zBlp 1 reverse: GGCCTCGAGTCACAGGCGTTTCTGTGCAATGAGTCC (SEQ ID NO:
78)
zBlp2 forward: ATGGCTAACGAAATTAGC (SEQ ID NO: 79)
111
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zBlp2 reverse: TCACTTCTGAGCAAAAAAGGC (SEQ ID NO: 80)
zBokl forward: ATGGAGATGTTGCGCCGCT (SEQ ID NO: 81)
zBokl reverse: TCATTTCTCTCTCAGCAGGTAGAAAAC (SEQ ID NO: 82)
zBok2 forward: ATGAACGTGTTCGCGCGCT (SEQ ID NO: 83)
zBok2 reverse: GCGCAAGTCTACTTCGTCAGGTA (SEQ ID NO: 84)
zMcl-la forward: AAGGGGCTGCAGCTGGACG (SEQ ID NO: 85)
zMcl-la reverse: AGCGGGGCTCAGGTCAGTACAG (SEQ ID NO: 86)
zMcl-lb forward: CATCTTCGATTGATTTGATT (SEQ ID NO: 87)
zMcl-lb reverse: ATAGTTTGAAAGTTCTGAATCC (SEQ ID NO: 88)
zNR13 forward: ATGTCCTGTTGGTTGAGGG (SEQ ID NO: 89)
zNR13 reverse: TCAGCGTACTAAGAGGAAGGT (SEQ ID NO: 90)
The clone for zBad2 was obtained from Open Biosystems.
Capped synthetic mRNA for zBid, zBadl, zBmfl, zBmf2, zPuma, zBik, zNoxa,
zBak, and zBax was generated by Ambion mMessage mMachineTM (Ambion) according
to
the manufacturer's directions, and purified over NucAway spin columns
(Ambion). The
resulting messenger RNA was diluted to the appropriate concentration (ranging
from 0.11
mg/mL to 3.5 x 10-5 mg/mL) in lx Danieu's solution including 0.2% phenol red.
One to
four-cell stage embryos were injected with 4.6 nL of the diluted mRNA solution
using a
Nanoliter 2000 (World Precision Instruments) microinjector. The faint pink
cast to the
blastomeres was residual phenol red from the injection solution.
Injection of synthetic mRNA encoding most BH3-only proapoptotic proteins or
multidomain pro-apoptotic Bcl-2 proteins resulted in dose-dependent apoptosis,
characterized
by disintegration of the blastomeres and yolk cell (Figures 4A and 4B). zBad,
zBokl, and
zBok2 did not induce significant apoptosis in early embryos injected with up
to 500 pg of
those synthetic mRNAs.
To confirm that ectopic expression of the zebrafish pro-apoptotic B2R genes
killed
embryos by engaging the apoptotic pathway, embryos were injected with a low
dose of each
synthetic mRNA, and the resulting degree of activation of the apoptosis
effector caspase-3
was monitored by immunohistochemistry.
Zebrafish embryos were injected with 500 pg GFP, 500 pg zBad, 100 pg zBid, 20
pg
zBik, 0.8 pg zBmfl, 4 pg of zBmf2, 20 pg zNoxa, 20 pg zPuma, 20 pg zBak, or 20
pg zBax
at the 1-4 cell stage. Injected embryos were fixed at 10 hours post-
fertilization in 4%
paraformaldehyde in PBS for approximately 4 hours at room temperature or
overnight at 4
112
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
C. Embryos were subsequently dehydrated in methanol for a minimum of two
hours. After
rehydration, embryos were washed with water, permeabilized in acetone for 7
minutes at -20
C, and washed again in water. Embryos were washed several additional times
with PBS
containing 0.5% Tween (PBST). The washed embryos were blocked for two hours at
room
temperature in 5% fetal bovine serum, 2 mg/mL BSA in PBST. Embryos were
incubated
with rabbit anti-activated caspase-3 antibody (Pharmingen) diluted 1:500 in
blocking solution
overnight at 4 C. Embryos were then washed several times in PBST before
incubation with
the secondary antibody, goat anti-rabbit Cy3 (Jackson Immunology) diluted
1:500 in
blocking solution, at room temperature for two hours. Embryos were washed
again with
PBST before visualization with a Leica MZFL3 fluorescence microscope.
Compared to wildtype and mock-injected embryos, ectopic expression of the pro-
apoptotic B2R proteins resulted in a dramatic increase in caspase-3 activity,
indicating that
ectopic expression of Bax-like and BH3-only B2R proteins initiated the
apoptotic program in
zebrafish embryos (Figure 4C). The only exceptions were zBad, zBokl and zBok2,
which
also did not initiate apoptosis when ectopically expressed (Figures 4A and
4C).
c. Rescue of Zebrafish B2R Protein-Induced Apoptosis
If ectopic expression of zebrafish pro-apoptotic B2R proteins in zebrafish
embryos
induced apoptosis via a mechanism similar to the mammalian apoptotic pathway,
it should be
possible to prevent lethality by co-expressing pro-survival B2R proteins. zMcl-
la, zMcl-lb,
zBlp 1, and zBlp2 were each cloned using the primers and protocol set forth in
Example 3(b).
Synthetic mRNA was created for each of zMcl-la, zMcl-lb, zBlpl, and zBlp2 and
co-
injected into zebrafish embryos also according to the protocol described in
Example 3(b),
with the following B2R protein mRNA injection amounts: 500 pg of each pro-
survival
mRNA, 500 pg of zBid, 20 pg of zBmfl, 100 pg of zBmf2, 50 pg of zNoxa, 100 pg
of zBik,
100 pg of zPuma, 100 pg of zBax, and 100 pg of zBak. The results are shown in
Figure 4D.
Apoptosis induced by ectopic expression of zBid, zBik, zBmfl, zBmf2, zNoxa,
zPuma, or
zBax were rescued by co-expression with zMcl-la, zMcl-lb, zBlpl, or zBlp2
(Figure 4D).
Ectopic expression of zBak, however, was rescued by co-expression with zMcl-
la, zMcl-lb,
or zBlpl, but not by co-expression with zBlp2 (Figure 4D), suggesting that
zBak did not
interact with zBlp2. Human B1p2 is a known homolog of Bcl-2. Previous studies
had shown
that human Bak does not interact with human Bcl-2. Thus, the inability of
zBlp2 to rescue
zBak-induced apoptosis further supported the designation of ENSDARP00000040899
as the
zebrafish ortholog of human zBak.
113
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
d. Zebrafish B2R Gene Response to Gamma Radiation In Vivo
To investigate the role of the zebrafish B2R genes in response to an exogenous
apoptotic stimulus, embryos were subjected to gamma irradiation. Gamma
irradiation was
known to trigger apoptosis in mammalian cells via the intrinsic pathway,
resulting in an
increase in caspase-3 activity in a p53-dependent manner (Gong et al., Cell
Growth Differ.
10(7): 491-502 (1999))The ability of the zebrafish pro-survival molecules to
shield zebrafish
embryos from gamma radiation-mediated apoptosis was therefore examined.
Embryos were irradiated at approximately 7 hours post fertilization in 1 mL of
embryo media with a 50 Gy gamma irradiation dose. Embryos were subsequently
moved to a
tissue culture dish in a greater volume of embryo media and incubated at 28.5
C until further
analysis. Ecotopic expression of each of the zebrafish pro-survival B2R genes
(zBlpl, zMcl-
la, zMcl-lb, and zBlp2), as described in Example 3(b) (modified to 500 pg
injections)
protected embryos from gamma irradiation-induced apoptosis (Figure 5A). Thus
gamma
radiation triggered apoptosis in zebrafish embryos via the intrinsic pathway,
similar to its
effects in mammalian cells.
To determine which zebrafish B2R genes were responsible for mediating the
apoptotic effects of gamma radiation on the zebrafish embryos, translational
knockdowns of
the pro-apoptotic B2R zebrafish genes were made using a morpholino approach.
Morpholinos to each pro-survival B2R gene were selected based on their ability
to abrogate
cognate mRNA-mediated rescue of ectopically expressed zNoxa. Morpholinos for
each pro-
apoptotic B2R gene were selected based on their ability to rescue ectopic
expression of the
cognate mRNA. As morpholino efficacy could not be verified for zBokl, zBok2,
or zBad
(due to the fact that those genes did not induce apoptosis when ectopically
expressed, see
Figures 4A and 4C), those genes were not included in the knockdown analyses.
Morpholinos were designed around the translational start site of each
transcript
(Nasevicius and Ekker, Nat. Genet. 26: 216-220 (2000)), and obtained from
GeneTools.
Morpholino ("MO") sequences were as follows (a115' to 3'):
zMcl-la MO: GCCTAAAATCCAAACTCAGAGCCAT (SEQ ID NO: 91)
zMcl-lb MO: TGTCGTTGTTTCTTCCAGCGAACAT (SEQ ID NO: 92)
zBlpl MO: AGGTTGTTGCTCGTTCTCCGATGTC (SEQ ID NO: 93)
zBlp2 MO: GTCATAGCTAATTTCGTTAGCCATG (SEQ ID NO: 94)
zBax MO: TGAAAATAAGCGAACTGAAGAAGAC (SEQ ID NO: 95)
zBak MO: ATTTTTCGGCTAAAACGTGTATGGG (SEQ ID NO: 96)
114
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zBid MO: GGTCAAAGTTCCTGTTGAAGTCCAT (SEQ ID NO: 97)
zBmf MO: ACACATCATCCTCGTCCTCATCCAT (SEQ ID NO: 98)
zNoxa MO: CTTTCTTCGCCATTTCAGCAAGTTT (SEQ ID NO: 99)
zPuma MO: TGCTTTCCATCTCTGGTCGGGCCAT (SEQ ID NO: 100)
zBik MO: CTACAAACAAGGACACAATGGTGGA (SEQ ID NO: 101)
p53 MO: GCGCCATTGCTTTGCAAGAATTG (SEQ ID NO: 102)
control MO: CCTCTTACCTCAGTTACAATTTATA (SEQ ID NO: 103)
The control morpholino was designed to restore normal human beta globin mRNA
sequence
messages containing the mutant beta thalassemia splice site, and was expected
to have no
effect in zebrafish embryos. The p53 morpholino was according to Langheinrich
et al., Curr.
Biol. 12: 2023-2028 (2002).
Morpholinos were diluted to 1 mg/mL in 1X Danieu's solution + 0.2% phenol red.
A
total of either 4.6 ng or 9.2 ng of morpholino was injected. In experiments
where a
combination of two morpholinos was used, 4.6 ng of each morpholino was
injected. An
additiona14.6 ng of control morpholino was added to single morpholino
injections when the
experiment included comparison to a dual morpholino injection sample, such
that each
sample was injected with 9.2 ng of morpholino. 1-4 cell stage embryos were
injected with
4.6 nL of diluted morpholino using a Nanoliter 2000 (World Precision
Instruments)
microinj ector.
In most mammalian cell types, either Bax or Bak is required to transduce most
apoptotic stimuli (Wei et al., Science 292: 727-730 (2001)). To determine
whether zBax and
zBak were functionally redundant in zebrafish, morpholinos directed against
zBax and zBak
were injected singly or pairwise into embryos, and the embryos were
subsequently subjected
to gamma irradiation. Translational knockdown of zBax was sufficient to
protect the
embryos from the effects of gamma radiation (Figures 5B and 5C). In a small
percentage of
clutches, knockdown of both zBax and zBak was required to abrogate gamma
irradiation-
induced Caspase-3 activation. Some caspase-3 activity remained in the
irradiated embryos
injected with zBax and zBak morpholinos (Figures 5B and 5C), possibly due to
incomplete
knockdown or the function of maternal proteins present in the embryo. zBax
appeared to be
primarily responsible for executing the apoptotic program in response to gamma
irradiation.
Knockdown of translation of several BH3 -only proapoptotic genes revealed that
when
zPuma translation was impaired, caspase-3 activation was dramatically reduced
in response
to gamma irradiation (Figure 5D). zNoxa impairment also greatly reduced
caspase-3
115
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
activation, but not as completely as zPuma inactivation. This effect mirrored
the protective
effect of p53 knockdown (Figure 5E), and was supported by previous studies in
mammalian
cells indicating that Puma is the primary mediator of gamma irradiation-
induced apoptosis
(Erlacher et al., Blood 106: 4131-4138 (2005); Jeffers et al., Cancer Ce114:
321-328 (2003)).
A quantitative PCR analysis was undertaken to better understand the actions of
zNoxa
and zPuma during gamma irradiation. Taqman analyses were performed using the
7500 Real
Time PCR System (Applied Biosystems) according to the manufacturer's
instructions. The
primer and probe sequences for each gene are shown below (5' to 3'):
zGAPDH forward: TGCGTTCGTCTCTGTAGATGT (SEQ ID NO: 104)
zGAPDH reverse: GCCTGTGGAGTGACACTGA (SEQ ID NO: 105)
zGAPDH probe: TGTGTGTGTGTGTTAGTTTCTTTTGACAGTATTTG (SEQ ID
NO: 106)
zNoxa forward: CGAACCTGTGACAGAAACTTG (SEQ ID NO: 107)
zNoxa reverse: CTGCGCGCACTCTACTACA (SEQ ID NO: 108)
zNoxa probe: CGGTTTGCTCTTTCTTCGCCATTTC (SEQ ID NO: 109)
zPuma forward: GAACACACGGGTTACAAAAGAC (SEQ ID NO: 110)
zPuma reverse: GAAAATTCCCAGAGTCTGTAAGTG (SEQ ID NO: 111)
zPuma probe: ACGAGTGCAGGCGCTCTCCTT (SEQ ID NO: 112)
Embryos were collected approximately 30 minutes post-fertilization and
maintained in
embryo media for further analysis. Coding regions for each B2R gene were
amplified by RT-
PCR using the OneStep PT-PCR kit (Qiagen) according to the manufacturer's
directions.
Multiple PT-PCR products were sequenced to verify the correct coding sequence.
Each
amplified sequence was cloned by Topo TA (Invitrogen) cloning into the plasmid
pCRII
(Invitrogen). Embryos were irradiated with 50 Gy at seven hours post
fertilization, and the
RNA was collected at 10 hours post fertilization.
The quantitative PCR analysis revealed that while zNoxa transcription was
upregulated 3-4 fold, zPuma transcription was upregulated almost 100-fold in
response to
gamma irradiation (Figure 5F). None of the other zebrafish BH3 -only genes
showed an
increase in transcription in response to gamma irradiation. Upregulation of
zPuma was p53-
dependent (Figure 5F), such that knockdown of p53 decreased zPuma
upregulation. This
suggested that in the zebrafish gamma irradiation induced p53 activity, which
in turn
transcriptionally upregulated zPuma. Puma upregulation was known in mammalian
systems
to activate Bax and Bak (Liu et al., Biochem. Biophys. Res. Commun. 310(3):
956-62
116
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
(2003)), correlating with the above data suggesting that zBax is critical to
induction of
apoptosis in response to gamma irradiation. Thus, gamma irradiation of
zebrafish embryos
triggered the intrinsic apoptotic pathway, mediated in particular by p53,
zPuma, and zBax.
e. Knockdown of Zebrafish Pro-Survival B2R Proteins During Normal
Development
Having established that the intrinsic apoptotic pathway was present and
functional in
zebrafish, the zebrafish intrinsic pathway was compared to the known mammalian
intrinsic
pathway system. Zebrafish pro-survival B2R genes were subjected to morpholino
knockdown, and the resulting effects on the developing embryo were monitored.
Morpholinos were directed against the translational start site of zMcl-la,
zMcl-lb, and zBlp2
according to the methodology described in Example 3(d).
Knockdown of zBlp2 had no obvious effect on early zebrafish development
(Figure
6A). The gross morphology of the zBlp2 knockdown fish was normal. There was no
increase in caspase-3 activity as a result of zBlp2 knockdown. Similarly,
knockdown of
either zMcl-la or zMcl-lb had no apparent effect on survival of fish embryos.
However,
knocking down both zMcl-la and zMcl-lb resulted in a variable but significant
decrease in
viability by 8 hours post fertilization (Figure 6A). Pairwise knockdowns of
either of the zMcl
genes in combination with zBlp2 had no impact on survival (Figure 6A). Thus,
only
impairing transcription of both copies of zMcl-1 significantly affected the
survival of injected
zebrafish embryos. Notably, previous studies had demonstrated that knockouts
of
mammalian Mcl-1 in mice are pre-implantation lethal (Rinkenberger et al.,
Genes Dev. 14:
23-27 (2000)).
f. Zebrafish Pro-Survival B2R Proteins and Apo2L Signaling
Zebrafish embryos injected with both zMcl-la and zMcl-lb morpholinos displayed
a
significant range of viability (Example 3(e)). This range of viability
appeared to vary with
the overall "health" of the clutch. One possible explanation for the
variability was that zMcl-
la/b might protect early zebrafish embryos from endogenous or environmental
apoptotic
stimuli. Mcl-1 had previously been implicated in mediating sensitivity to
Apo2L-induced
apoptosis in several cell lines (Henson et al., J. Cell Biochem. 89:1177-1192
(2003); Taniai et
al., Cancer Res. 64: 3517-3524 (2004); Wirth et al., Cancer Res. 65: 7393-7402
(2005);
Kobayashi et al., Gastroenterology 128: 2054-2065 (2005)). Thus, the effect of
zMcl-la and
zMcl-lb knockdown on the Apo2L-induced extrinsic apoptotic pathway was
investigated.
117
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
Knockdowns of zMcl-la and zMcl-lb were performed as described in Example 3(e).
Zebrafish Apo2L homolog zDLlb and other TNF-related genes were cloned as
described in
Example 3(b) using the following primer sequences:
zDLla forward: ACCATGATGGTCCCGGCGAACAGCCGC (SEQ ID NO: 113)
zDLla reverse: ACTTTACAGATCCAATCGGAAAGCTCC (SEQ ID NO: 114)
zDLlb forward: ATCATGGTGCAGCCTAAAAATCGT (SEQ ID NO: 115)
zDLlb reverse: CCCTCAGAGGTCAAACAGGAAGGC (SEQ ID NO: 116)
zDL2 forward: CACGCGATGGTCAGCATGACAAGC (SEQ ID NO: 117)
zDL2 reverse: TACTGACTAGCTCACCAGAAATGC (SEQ ID NO: 118)
zDL3 forward: ACCATGACATCCAACCTTCCTATCGGT (SEQ ID NO: 119)
zDL3 reverse: AGTTTATTTAATCATGAATGCCCCAAA (SEQ ID NO: 120)
zFasL forward: ATGAGTGCTAACTTCGGCCACTCG (SEQ ID NO: 121)
zFasL reverse: TCAGTGGATCTTAAAGAGGCCGAA (SEQ ID NO: 122)
zTNF1 forward: GCAACCATGAAGCTTGAGAGTCGGGCGTTT (SEQ ID NO: 123)
zTNF1 reverse: TTTCGTTCACAAACCAAACACCCCAAAGAA (SEQ ID NO: 124)
zTNF2 forward: ATGGTGAGATACGAAACAACATTA (SEQ ID NO: 125)
zTNF2 reverse: ATTAAATCACAACGCGAACACCCCGAAGAA (SEQ ID NO: 126)
. Synthetic zDLlb mRNA was produced and injected into zebrafish embryos as
described in
Example 3(b).
In wildtype embryos, ectopic expression of zebrafish Apo2L ortholog zDLlb had
minimal effect on early embryonic viability (Figure 6B). However, when
knockdown of
zMcl-la and zMcl-lb was combined with ectopic expression of zDLlb, embryos
rapidly
underwent massive apoptotic death (Figure 6B). The effect was specific to zMcl-
la and
zMcl-lb, because knockdown of either zMcl-la or zMcl-lb in conjunction with
zBlp2 did not
increase the sensitivity to zDLlb-induced apoptosis (Figure 6B).
Differing effects were obtained with other Apo2L/TNF-related molecules aside
from
zDLlb (see Figure 6C). For example, ectopic expression of zDLla in conjunction
with the
dual translational knockdown of zMcl-la and zMcl-lb resulted in nearly as
great a reduction
in survival of zebrafish embryos as with zDLlb (Figure 6C). The dual knockdown
also
markedly decreased survival when zDL3 was ectopically expressed (Figure 6C).
Significantly less reduction in percent survival was obtained when zDL2,
zTNF1, or zTNF2
expression was paired with the dual zMcl-la/b translational knockdowns (Figure
6C). zFasL
did not induce apoptosis, either alone or in combination with the knockdown of
zMcl-1a and
118
CA 02651199 2008-10-28
WO 2007/131133 PCT/US2007/068180
zMcl-lb. The expression of receptors for zTNF1, zTNF2, and zFasL on embryonic
cells has
not yet been established.
Thus, zMcl-la and zMcl-lb together protected zebrafish embryos from zDLla and
zDLlb (Apo2L)-induced apoptosis, and had a lesser, but still significant
protective effect
from zDL3-induced apoptosis.
119