Note: Descriptions are shown in the official language in which they were submitted.
CA 02653422 2008-11-25
WO 2007/141203 PCTIEP2007/055383
PCT P01-2031
Atomizer
Description
The invention relates to an atomizer for delivering a specific amount of a
fluid, particularly
one that contains a medicament, as an aerosol through a nozzle from a
pressurised store,
wherein a mechanical pressure generator acts upon the measured amount of fluid
in the
pressurised store which is to be released abruptly for atomization.
Zo It is known to administer a medicament in the form of a spray through the
nose or mouth in
order to absorb it through the mucosa in the nasal cavity or through the
lungs.
In addition, EP 0 521 061 B1 discloses a metering device for delivering a
measured
amount of a liquid as a spray with droplets of a size suitable for inhalation
into the lungs by
releasing the measured amount of liquid through an atomizing means comprising
a cham-
ber for holding the measured amount of liquid, an energy store and means for
delivering a
predetermined amount of energy to the energy store. Moreover, means are
provided for
releasing the predetermined amount of energy from the energy store to the
chamber in or-
der to expose the liquid contained therein to a predetermined rise in pressure
from a low
pressure to a higher pressure and initiate the release of liquid from the
chamber. Atomis-
ing means serve to atomize the measured amount of the liquid placed under
pressure.
The compounds listed below may be used in the device according to the
invention on their
own or in combination. In the compounds mentioned below, W is a
pharmacologically
active substance and is selected (for exainple) from among the betamimetics,
anticholiner-
gics, corticosteroids, PDE4-inhibitors, LTD4-antagonists, EGFR-inhibitors,
dopamine ago-
nists, H1-antihistamines, PAF-antagonists and P13-kinase inhibitors. Moreover,
double or
triple combinations of W may be combined and used in the device according to
the inven-
tion. Combinations of W might be, for example:
- W denotes a betamimetic, combined with an anticholinergic, corticosteroid,
PDE4-
inhibitor, EGFR-inhibitor or LTD4-antagonist,
- W denotes an anticholinergic, combined with a betamimetic, corticosteroid,
PDE4-
iiihibitor, EGFR-inhibitor or LTD4-antagonist,
CA 02653422 2008-11-25
PCT P0 ] -2031 2
- W denotes a corticosteroid, combined with a PDE4-inhibitor, EGFR-inhibitor
or LTD4-
antagonist
- W denotes a PDE4-inhibitor, combined with an EGFR-inhibitor or LTD4-
antagonist
- W denotes an EGFR-inhibitor, combined with an LTD4-antagonist.
The compounds used as betamimetics are preferably compounds selected from
among al-
buterol, arformoterol, bambuterol, bitolterol, broxaterol, carbuterol,
clenbuterol, fenoterol,
formoterol, hexoprenaline, ibuterol, isoetharine, isoprenaline,
levosalbutamol, mabuterol,
meluadrine, metaproterenol, orciprenaline, pirbuterol, procaterol, reproterol,
rimiterol, ri-
todrine, salmefamol, salmeterol, soterenol, sulphonterol, terbutaline,
tiaramide, tolubuterol,
zinterol, CHF-1035, HOKU-81, KUL-1248 and
- 3-(4-{6-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
hexyloxy}-
butyl)-benzyl-sulphonamide
- 5-[2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-lH-quinolin-2-
one
- 4-hydroxy-7-[2-{[2-{[3-(2-phenylethoxy)propyl]sulphonyl}ethyl]-amino}ethyl]-
2(3H)-benzothiazolone
- 1-(2-fluoro-4-hydroxyphenyl)-2-[4-(1-benzimidazolyl)-2-methyl-2-
butylamino]ethanol
- 1-[3-(4-methoxybenzyl-amino)-4-hydroxyphenyl]-2-[4-(1-benzimidazolyl)-2-
methyl-
2-butylamino] ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-[3-(4-N,N-
dimethylaminophenyl)-
2-methyl -2 -propylamino] ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-[3-(4-methoxyphenyl)-2-
methyl-2-
propylamino]ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-[3-(4-n-butyloxyphenyl)-2-
methyl-
2-propylamino] ethanol
- 1-[2H-5-hydroxy-3-oxo-4H-1,4-benzoxazin-8-yl]-2-{4-[3-(4-methoxyphenyl)-
1,2,4-
triazol-3-yl]-2-methyl-2-butylamino } ethanol
- 5-hydroxy-8-(1-hydroxy-2-isopropylaminobutyl)-2H-1,4-benzoxazin-3-(4H)-one
- 1-(4-amino-3-chloro-5-trifluoromethylphenyl)-2-tert.-butylamino)ethanol
- 6-hydroxy-8- { 1-hydroxy-2-[2-(4-methoxy-phenyl)-1,1-dimethyl-ethylamino]-
ethyl } -
4H-benzo[ 1,4]oxazin-3-one
- 6-hydroxy-8-{ 1-hydroxy-2-[2-( ethyl 4-phenoxy-acetate)-1,1-dimethyl-
ethylamino]-
ethyl} -4H-benzo [ 1,4] oxazin-3 -one
- 6-hydroxy-8-{ 1-hydroxy-2-[2-(4-phenoxy-acetic acid)-1,1-dimethyl-
ethylamino]-
ethyi}-4H-benzo[1,4]oxazin-3-one
- 8-{2-[1,1-dimethyl-2-(2,4,6-trimethylphenyl)-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-benzo [ 1,4] oxazin-3 -one
CA 02653422 2008-11-25
PCT P01-2037
~
~
- 6-hydroxy-8-{ 1-hydroxy-2-[2-(4-hydroxy-phenyl)-1,1-dimethyl-ethylamino]-
ethyl}-
4H-benzo [ 1,4] oxazin- 3 -one
- 6-hydroxy-8- { 1-hydroxy-2-[2-(4-isopropyl-phenyl)-1. i dimethyl-ethylamino]
-ethyl } -
4H-benzo[ 1,4] oxazin-3-one
- 8-{2-[2-(4-ethyl-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-hydroxy-
4H-
benzo[1,4]oxazin-3-one
- 8-{2-[2-(4-ethoxy-phenyl)-1,1-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-4H-
benzo[1,4]oxazin-3-one
- 4-(4-{2-[2-hydroxy-2-(6-hydroxy-3-oxo-3,4-dihydro-2H-benzo[1,4]oxazin-8-yl)-
lo ethylamino]-2-methyl-propyl}-phenoxy)-butyric acid
- 8-{2-[2-(3.4-difluoro-phenyl)-l,l-dimethyl-ethylamino]-1-hydroxy-ethyl}-6-
hydroxy-
4H-benzo[1,4]oxazin-3-one
- 1-(4-ethoxy-carbonylamino-3-cyano-5-fluorophenyl)-2-(tert-butylamino)ethanol
- 2-hydroxy-5-(1-hydroxy-2-{2-[4-(2-hydroxy-2-phenyl-ethylamino)-phenyl]-
ethylamino } -ethyl)-benzaldehyde
- N-[2-hydroxy-5-(1-hydroxy-2-{2-[4-(2-hydroxy-2-phenyl-ethylamino)-phenyl]-
ethylamino }-ethyl)-phenyl]-formamide
- 8-hydroxy-5-(1-hydroxy-2-{2-[4-(6-methoxy-biphenyl-3-ylamino)-phenyl]-
ethylamino } -ethyl)-1 H-quinolin-2-one
- 8 -hydroxy-5- [1-hydroxy-2-(6-phenethylamino-hexylamino)-ethyl]-1 H-quinolin-
2-one
- 5-[2-(2-{4-[4-(2-amino-2-methyl-propoxy)-phenylamino]-phenyl}-ethylamino)-1-
hydroxy-ethyl]-8-hydroxy-1 H-quinolin-2-one
- [3-(4-{6-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
hexyloxy}-
butyl)-5 -m ethyl-phenyl] -urea
- 4-(2- { 6-[2-(2,6-dichloro-benzyloxy)-ethoxy]-hexylamino } -1-hydroxy-ethyl)-
2-
hydroxymethyl-phenol
- 3-(4-{6-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
hexyloxy}-
butyl)-benzylsulphonamide
- 3 -(3-{ 7-[2-hydroxy-2-(4-hydroxy-3-hydroxymethyl-phenyl)-ethylamino]-
heptyloxy}-
3o propyl)-benzylsulphonamide
- 4-(2-{6-[4-(3-cyclopentanesulphonyl-phenyl)-butoxy]-hexylamino}-1-hydroxy-
ethyl)-
2-hydroxymethyl-phenol
- N-Adamantan-2-yl-2-(3 - { 2-[2-hydroxy-2-(4-hydroxy-3 -hydroxymethyl-phenyl)-
ethylamino]-propyi } -phenyl)-acetamide
optionally in the form of the racemates, enantiomers, diastereomers thereof
and optionally
in the form of the pharmacologically acceptable acid addition salts, solvates
or hydrates
thereof. According to the invention the acid addition salts of the
betamimetics are prefera-
CA 02653422 2008-11-25 pC,h p01-20' ) 1
4
bly selected from among the hydrochloride, hydrobromide, hydriodide,
hydrosulphate, hy-
drophosphate, hydromethanesulphonate, hydronitrate, hydromaleate,
hydroacetate, hy-
drocitrate, hydrofumarate, hydrotartrate, hydroxalate, hydrosuccinate,
hydrobenzoate and
hydro-p-toluenesulphonate.
The anticholinergics used are preferably compounds selected from among the
tiotropium
salts, preferably the bromide salt, oxitropium salts, preferably the bromide
salt, flutropium
salts, preferably the bromide salt, ipratropium salts, preferably the bromide
salt, glycopyr-
ronium salts, preferably the bromide salt, trospium salts, preferably the
chloride salt,
tolterodine. In the above-mentioned salts the cations are the
pharmacologically active con-
stituents. As anions the above-mentioned salts may preferably contain the
chloride, bro-
mide, iodide, sulphate, phosphate, methanesulphonate, nitrate, maleate,
acetate, citrate,
fumarate, tartrate, oxalate, succinate, benzoate or p-toluenesulphonate, while
chloride,
bromide, iodide, sulphate, methanesulphonate or p-toluenesulphonate are
preferred as
counter-ions. Of all the salts the chlorides, bromides, iodides and
methanesulphonates are
particularly preferred.
Other preferred anticholinergics are selected from among the salts of formula
AC-1
0N o
p
0
X- HO s
s
AC-i
wherein X - denotes an anion with a single negative charge, preferably an
anion selected
from among the fluoride, chloride, bromide, iodide, sulphate, phosphate,
methanesuipho-
nate, nitrate, maleate, acetate, citrate, fumarate, tartrate, oxalate,
succinate, benzoate and
p-toluenesulphonate, preferably an anion with a single negative charge,
particularly pref-
erably an anion selected from among the fluoride, chloride, bromide,
methanesulphonate
and p-toluenesulphonate, particularly preferably bromide, optionally in the
form of the ra-
cemates, enantiomers or hydrates thereof. Of particular importance are those
pharmaceuti-
cal combinations which contain the enantiomers of formula AC-1-en
CA 02653422 2008-11-25
PCT PO l -203 l
O O
0
X- HO
s
s
AC-1-en
wherein X may have the above-mentioned meanings. Other preferred
anticholinergics
are selected from the salts of formula AC-2
5
OH
N\
R X
AC-2
wherein R denotes either methyl or ethyl and wherein X may have the above-
mentioned
meanings. In an alternative embodiment the compound of formula AC-2 may also
be pre-
1 o sent in the form of the free base AC-2-base.
OH
NJ-"
y
AC-2-base
Other specified compounds are:
i5 - tropenol 2,2-diphenylpropionate methobromide,
- scopine 2,2-diphenylpropionate methobromide,
- scopine 2-fluoro-2,2-diphenylacetate methobromide,
- tropenol 2-fluoro-2,2-diphenylacetate methobromide;
- tropenol 3,3',4,4'-tetrafluorobenzilate methobromide,
20 - scopine 3,3',4,4'-tetrafluorobenzilate methobromide,
- tropenol 4,4'-difluorobenzilate methobromide,
- scopine 4,4'-difluorobenzilate methobromide,
- tropenol 3,3'-difluorobenzilate methobromide,
- scopine 3,3'- difluorobenzilate methobromide;
CA 02653422 2008-11-25 PC'f PO 1-2031
6
tropenol 9-hydroxy-fluorene-9-carboxylate methobromide;
tropenol9-fluoro-fluorene-9-carboxylate methobromide;
- scopine 9-hydroxy-fluorene-9-carboxylate methobromide=,
- scopine 9-fluoro-fluorene-9-carboxylate methobromide;
tropenol 9-methyl-fluorene-9-carboxylate methobromide;
- scopine 9-methyl-fluorene-9-carboxylate methobromide;
- cyclopropyltropine benzilate methobromide;
- cyclopropyltropine 2,2-diphenylpropionate methobromide;
- cyclopropyltropine 9-hydroxy-xanthene-9-carboxylate methobromide;
- cyclopropyltropine 9-methyl-fluorene-9-carboxylate methobromide;
- cyclopropyltropine 9-methyl-xanthene-9-carboxylate methobromide;
- cyclopropyltropine 9-hydroxy-fluorene-9-carboxylate methobromide;
- cyclopropyltropine methy14,4'-difluorobenzilate methobromide.
- tropenol 9-hydroxy-xanthene-9-carboxylate methobromide;
- scopine 9-hydroxy-xanthene-9-carboxylate methobromide;
- tropenol 9-methyl-xanthene-9-carboxylate methobromide;
- scopine 9-methyl-xanthene-9-carboxylate methobromide;
- tropenol 9-ethyl-xanthene-9-carboxylate methobromide;
- tropenol 9-difluoromethyl-xanthene-9-carboxylate methobromide;
- scopine 9-hydroxymethyl-xanthene-9-carboxylate methobromide,
The above-mentioned compounds may also be used as salts within the scope of
the present
invention, wherein instead of the methobromide the salts metho-X are used,
wherein X
may have the meanings given hereinbefore for X.
As corticosteroids it is preferable to use compounds selected from among
beclomethasone,
betamethasone, budesonide, butixocort, ciclesonide, deflazacort,
dexamethasone, etipred-
nol, flunisolide, fluticasone, loteprednol, mometasone, prednisolone,
prednisone, roflepon-
ide, triamcinolone, RPR-4 06541, NS-126, ST-26 and
- (S)-fluoromethyl 6,9-difluoro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-l6-
methyl-3-
oxo-androsta-1,4-diene-17-carbothi onate
- (S)-(2-oxo-tetrahydro-furan-3S-yl)6,9-difluoro-ll-hydroxy-l6-methyl-3-oxo-17-
propionyloxy-androsta-1,4-diene-17-carbothionate,
- cyanomethyl 6a,9a.-difluoro-11 [3-hydroxy-16a-methyl-3-oxo-17a-(2,2,3,3-
tertamethylcyclopropylcarbonyl)oxy-androsta-l,4-diene-17(3-carboxylate
optionally in the form of the racemates, enantiomers or diastereomers thereof
and option-
ally in the form of the salts and derivatives thereof, the solvates and/or
hydrates thereof.
Any reference to steroids includes a reference to any salts or derivatives,
hydrates or sol-
CA 02653422 2008-11-25 PC']' PO 1-2031
7
vates thereof which may exist. Examples of possible salts and derivatives of
the steroids
may be: alkali metal salts, such as for example sodium or potassium salts,
sulphobenzoates,
phosphates, isonicotinates, acetates, dichloroacetates, propionates,
dihydrogen phosphates,
palmitates, pivalates or furoates.
PDE4-inhibitors which may be used are preferably compounds selected from among
en-
profyllin, theophyllin, roflumilast, ariflo (cilomilast), tofimilast,
pumafentrin, lirimilast,
arofyllin, atizoram, D-4418, Bay-198004, BY343, CP-325.366, D-4396 (Sch-
351591),
AWD-12-281 (GW-842470), NCS-613, CDP-840, D-4418, PD-168787, T-440, T-2585,
V-11294A, C1-1018, CDC-801, CDC-3052, D-22888, YM-58997, Z-15370 and
- N-(3,5-dichloro-l-oxo-pyridin-4-yl)-4-difluoromethoxy-3-
cyclopropylmethoxybenzamide
- (-)p-[(4aR*,10bS*)-9-ethoxy-1,2,3,4,4a,1 Ob-hexahydro-8-methoxy-2-
methylbenzo [s] [ 1,6]naphthyridin-6-yl]-N,N-diisopropylbenzamide
- (R)-(+)-1-(4-bromobenzyl)-4-[(3-cyclopentyloxy)-4-methoxyphenyl]-2-
pyrrolidone
- 3-(cyclopentyloxy-4-methoxyphenyl)-1-(4-N'-[N-2-cyano-S-methyl-
isothioureido]benzyl)-2-pyrrolidone
- cis[4-cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)cyclohexane-l-carboxylic
acid]
- 2-carbomethoxy-4-cyano-4-(3-cyclopropylmethoxy-4-difluoromethoxy-
2 fl phenyl)cyclohexan-l-one
- cis[4-cyano-4-(3-cyclopropylmethoxy-4-difluoromethoxyphenyl)cyclohexan-l-ol]
- (R)-(+)-ethyl[4-(3-cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-
ylidene]acetate
- (S)-(-)-ethyl [4-(3 -cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-ylidene]
acetate
- 9-cyclopentyl-5,6-dihydro-7-ethyl-3-(2-thienyl)-9H-pyrazolo[3,4-c]-1,2,4-
triazolo[4,3-
2 5 a]pyridine
- 9-cyclopentyl-5,6-dihydro-7-ethyl-3-(tert-butyl)-9H-pyrazolo[3,4-c]-1,2,4-
triazolo[4,3-
a]pyridine
optionally in the form of the racemates, enantiomers or diastereomers thereof
and option-
ally in the form of the pharmacologically acceptable acid addition salts
thereof, the sol-
30 vates and/or hydrates thereof. According to the invention the acid addition
salts of the
PDE4 inhibitors are preferably selected from among the hydrochloride,
hydrobromide, hy-
driodide, hydrosulphate, hydrophosphate, hydromethanesulphonate, hydronitrate,
hydro-
maleate, hydroacetate, hydrocitrate, hydrofumarate, hydrotartrate,
hydroxalate, hydrosuc-
cinate, hydrobenzoate and hydro-p-toluenesulphonate.
The LTD4-antagonists used are preferably compounds selected from among
montelukast,
pranlukast, zafirlukast, MCC-847 (ZD-3523), MN-001, MEN-91507 (LM-1507), VUF-
5078.. VUF-K-8707, L-733321 and
CA 02653422 2008-11-25 pCT PO 1-20' ) 1
g
- 1-(((R)-(3-(2-(6,7-difluoro-2-auinolinyl)ethenyl)phenyl)-3-(2-(2- hydroxy-2-
propyl)phenyl)thio)methylcyclopropane-acetic acid,
- 1-(((1(R)-3(3-(2-(2,3-dichlorothieno[3,2-b]pyridin-5-yl)-(E)-ethenyl)phenyl)-
3-(2-(1-
hydroxy-l-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid
- [2-[[2-(4-tert-butyl-2-thiazolyl)-5-benzofuranyl]oxymethyl]phenyl]acetic
acid
optionally in the form of the racemates, enantiomers or diastereomers thereof
and option-
ally in the form of the pharmacologically acceptable acid addition salts,
solvates and/or
hydrates thereof. According to the invention these acid addition salts are
preferably se-
lected from among the hydrochloride, hydrobromide, hydroiodide, hydrosulphate,
hydro-
phosphate, hydromethanesulphonate, hydronitrate, hydromaleate, hydroacetate,
hydrocit-
rate, hydrofumarate, hydrotartrate, hydroxalate, hydrosuccinate, hydrobenzoate
and hydro-
p-toluenesulphonate. By salts or derivatives which the LTD4-antagonists may
optionally
be capable of forming are meant, for example: alkali metal salts, such as for
example so-
dium or potassium salts, alkaline earth metal salts, sulphobenzoates,
phosphates, isonicoti-
nates, acetates, propionates, dihydrogen phosphates, palmitates, pivalates or
furoates.
EGFR-inhibitors which may be used are preferably compounds selected from among
cetuximab, trastuzumab, ABX-EGF, Mab ICR-62 and
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]-
2 o amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{ [4-(N,N-diethylamino)-1-oxo-2-buten-l-
yl]-
amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-l-oxo-2-buten-l-
yl] amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-{[4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]amino}-7-
cyclopentyloxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ [4-((R)-6-methyl-2-oxo-morpholin-4-
yl)-l-
oxo-2-buten-l-yl] amino }-7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{[4-((R)-6-methyl-2-oxo-morpholin-4-
yl)-l-
oxo-2-buten-l-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]-quinazoline
- 4- [(3 -chloro-4-fluoro-phenyl)amino]-6- { [4-((R)-2-methoxymethyl-6-oxo-
morpholin-4-
yl)-1-oxo-2-buten- l -yl] amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-((S)-6-methyl-2-oxo-moapholin-4-yl)-
ethoxy]-7-methoxy-quinazo line
- 4-[(3-chloro-4-fluorophenyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-
amino]-1-
oxo-2-buten-l-yl} amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N;N-dimethylamino)-1-oxo-2-buten-l-
yl] amino } -7-cyclopentyloxy-quinazoline
CA 02653422 2008-11-25 PCT pO 1-2.031
9
- 4-[(R)-(1-phenyl-ethyl)amino]-6-{[4-(N,N-to-(2-methoxy-ethyl)-amino)-1-oxo-2-
buten-l-yl] amino } -7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-( { 4-[N-(2-methoxy-ethyl)-N-ethyl-amino]-1-
oxo-2-
buten-l-yl } amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-({4-[N-(2-methoxy-ethyl)-N-methyl-amino]-l-
oxo-2-
buten-l-yl } amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-({4-[N-(tetrahydropyran-4-yl)-N-methyl-
amino]-1-
oxo-2-buten-l-yl } amino)-7-cyclopropylmethoxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-
yl]amino}-7-((R)-tetrahydrofuran-3-yloxy)-quinazoline
- 4- [(3 -chloro-4-fluorophenyl)amino]-6- { [4-(N,N-dimethylamino)-1-oxo-2-
buten-l-
yl] amino } -7-((S)-tetrahydrofuran-3-yloxy)-quinazoline
- 4-[(3 -chioro-4-fluorophenyl)amino]-6-( {4-[N-(2-methoxy-ethyl)-N-methyl-
amino]-1-
oxo-2-buten-l-yl } amino)-7-cyclopentyloxy-quinazoline
- 4- [(3-chloro-4-fluorophenyl)amino] -6- { [4-(N-cyclopropyl-N-methyl-amino)-
1-oxo-2-
buten-l-yl] amino } -7-cyclopentyloxy-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6- { [4-(N,N-dimethylamino)-1-oxo-2-buten-
l-
yl]amino } -7-[(R)-(tetrahydrofuran-2-yl)methoxy] -quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-l-
yl]amino } -7-[(S)-(tetrahydrofuran-2-yl)methoxy]-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6.7-to-(2-methoxy-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)-propyloxy]-6-
[(vinyl-
carbonyl)amino]-quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-(4-hydroxy-phenyl)-7H-pyrrolo[2,3-
d]pyrimidine
- 3-cyano-4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-
buten-l-yl] amino } -7-ethoxy-quinoline
- 4-{[3-chloro-4-(3-fluoro-benzyloxy)-phenyl]amino}-6-(5-{[(2-methanesulphonyl-
ethyl)amino]methyl } -furan-2-yl)quinazoline
- 4-[(R)-(1-phenyl-ethyl)amino]-6-{ [4-((R)-6-methyl-2-oxo-morpholin-4-yl)-1-
oxo-2-
3 o buten-l-yl] amino } -7-methoxy-quinazoline
- 4- [(3-chloro-4-fluorophenyl)amino]-6-{ [4-(morpholin-4-yl)-1-oxo-2-buten-l-
yl]-
amino } -7 - [ (tetrahy drofuran-2-yl )methoxy] -quinazoline
- 4-[(3-chloro-4-fluorophenyl)amino]-6-({4-[N,N-to-(2-methoxy-ethyl)-amino]-1-
oxo-2-
buten-l-yl } amino)-7-[(tetrahydrofuran-2-yl)methoxy]-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-{[4-(5,5-dimethyl-2-oxo-morpholin-4-yl)-1-oxo-
2-
buten-l-yl] amino } -quinazo line
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy]-7-methoxy-quinazoline
CA 02653422 2008-11-25 pCT pO 1-2031
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy] -7- [(R)-(tetrahydrofuran-2-yl)methoxy] -quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-7-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy] -6- [(S)-(tetrahydrofuran-2-yl)methoxy] -quinazoline
5 - 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{2-[4-(2-oxo-morpholin-4-yl)-
piperidin-l-yl]-
ethoxy } -7-methoxy-quinazo line
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(tert.-butyloxycarbonyl)-piperidin-
4-yloxy]-
7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexan-1-yloxy)-7-
1 o methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methanesulphonylamino-
cyclohexan-
1-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-3-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(morpholin-4-yl)carbonyl]-
piperidin-4-yl-
oxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(methoxymethyl)carbonyl]-
piperidin-4-yl-
2 o oxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-3-yloxy)-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-acetylamino-ethyl)-piperidin-4-
yloxy]-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-ethoxy-
2 5 quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-
hydroxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-methoxy-
ethoxy)-quinazoline
30 - 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-
[(dimethylamino)sulphonylamino]-
cyclohexan-l-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)aznino]-6-{trans-4-[(morpholin-4-
yl)carbonylamino]-
cyclohexan-l-yloxy } -7-rnethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(morpholin-4-
yl)sulphonylamino]-
3 5 cyclohexan-l-vloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
acetylainino-
ethoxy)-quinazoline
CA 02653422 2008-11-25 pC`j' p01-203 1
ll
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
methanesulphonyl amino-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(piperidin-1-yl)carbonyl]-
piperidin-4-
yloxy } -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-aminocarbonylmethyl-piperidin-4-
yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4- {N-[(tetrahydropyran-4-
yl)carbonyl]-N-
methyl-amino } -cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(morpholin-4-yl)carbonyl]-N-
1 o methyl-amino } -cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(morpholin-4-yl)sulphonyl]-
N-
methyl-amino } -cyclohexan-l-yloxy)-7-methoxy- quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-ethanesulphonylamino-
cyclohexan-l-
yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-
ethoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-(2-
methoxy-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(2-methoxy-acetyl)-piperidin-4-
yloxy]-7-(2-
2 o methoxy-ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-acetylamino-cyclohexan-1-yloxy)-
7-
methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-[ 1-(tert.-butyloxycarbonyl)-piperidin-4-
yloxy]-7-
methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(tetrahydropyran-4-yloxy]-7-methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(piperidin-1-yl)carbonyl]-N-
methyl-
amino } -cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(4-methyl-piperazin-1-
y1)carbonyl]-
N-methyl-amino } -cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{cis-4-[(morpholin-4-yl)carbonylamino]-
cyclohexan-l-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[2-(2-oxopyrrolidin-l-yl)ethyl]-
piperidin-4-
yloxy}-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(morpholin-4-yl)carbonyl]-
piperidin-4-
yloxy}-7-(2-methoxy-ethoxy)-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(1-acetyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4-[(3 -ethynyl-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
CA 02653422 2008-11-25 j>C'j' PO 1 -2203 ) 1
12
- 4-[(3-ethynyl-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-7-
methoxy-
quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7(2-
methoxy-
ethoxy)-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-isopropyloxycarbonyl-piperidin-4-
yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-methylamino-cyclohexan-l-yloxy)-
7-
methoxy-quinazoline
- 4- [(3 -chloro-4-fluoro-phenyl) amino] -6- {cis-4-[N-(2-methoxy-acetyl)-N-
methyl-
amino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-[1-(2-methoxy-acetyl)-piperidin-4-yloxy]-7-
methoxy-
quinazoline
- 4-[(3-ethynyl-phenyl)amino]-6-{ 1-[(morpholin-4-yl)carbonyl]-piperidin-4-
yloxy}-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(cis-2,6-dimethyl-morpholin-4-
yl)carbonyl] -piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(2-methyl-morpholin-4-
yl)carbonyl]-
piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{ 1-[(S,S)-(2-oxa-5-aza-
bicyclo[2,2,1]hept-5-
yl)carbonyl]-piperidin-4-yloxy} -7-methoxy-quinazoline
- 4- [(3 -chloro-4-fluoro-phenyl) amino] -6- { 1-[(N-methyl-N-2-methoxyethyl-
amino)carbonyl] -piperidin-4-yloxy } -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-ethyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
- 4- [(3 -chloro-4-fluoro-phenyl)amino] -6- {1- [(2-methoxyethyl)carbonyl] -
piperidin-4-
yloxy} -7-methoxy-quinazoline
- 4- [(3 -chloro-4-fl uoro-phenyl)amino] -6- { 1-[(3-methoxypropyl-amino)-
carbonyl]-
piperidin-4-yloxy} -7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-znethanesulphonyl-N-methyl-
amino)-
cyclohexan-l-yloxy]-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-acetyl-N-methyl-amino)-
cyclohexan-
1-yloxy] -7-methoxy-quinazoline
- 4- [(3 -chloro-4-fluoro-phenyl)amino] -6-(trans-4-methylamino-cyclohexan- 1 -
yloxy)-7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-(N-methanesulphonyl-N-methyl-
amino)-cyclohexan-l-yloxy]-7-methoxy-quinazoline
CA 02653422 2008-11-25 pC'r'Po]-2031
13
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-dimethylamino-cyclohexan-l-
yloxy)-
7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4- {N-[(morpholin-4-
yl)carbonyl]-N-
methyl-amino } -cyclohexan-l-yloxy)-7-methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[2-(2,2-dimethyl-6-oxo-morpholin-4-yl)-
ethoxy] -7- [(S)-(tetrahydrofuran-2-yl)methoxy]-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-yloxy)-
7-
methoxy-quinazoline
- 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-methoxy-
quinazoline
optionally in the form of the racemates, enantiomers, diastereomers thereof
and optionally
in the form of the pharmacologically acceptable acid addition salts, solvates
or hydrates
thereof. According to the invention these acid addition salts are preferably
selected from
among the hydrochloride, hydrobromide, hydriodide, hydrosulphate,
hydrophosphate, hy-
dromethanesulphonate, hydronitrate, hydromaleate, hydroacetate, hydrocitrate,
hydrofu-
marate, hydrotartrate, hydroxalate, hydrosuccinate, hydrobenzoate and hydro-p-
toluenesulphonate.
The dopamine agonists used are preferably compounds selected from among
bromocriptin,
cabergoline, alpha-dihydroergocryptine, lisuride, pergolide, pramipexol,
roxindol, ropini-
rol, talipexol, tergurid and viozan, optionally in the form of the racemates,
enantiomers,
diastereomers thereof and optionally in the form of the pharmacologically
acceptable acid
addition salts, solvates or hydrates thereof. According to the invention these
acid addition
salts are preferably selected from among the hydrochloride, hydrobromide,
hydriodide,
hydrosulphate, hydrophosphate, hydrometlianesulphonate, hydronitrate,
hydromaleate, hy-
droacetate, hydrocitrate, hydrofumarate, hydrotartrate, hydroxalate,
hydrosuccinate, hydro-
benzoate and hydro-p-toluenesulphonate.
H1-Antihistamines which may be used are preferably compounds selected from
among
epinastine, cetirizine, azelastine, fexofenadine, levocabastine, loratadine,
mizolastine, ke-
totifen, emedastine, dimetindene, clemastine, bamipine, cexchloipheniramine,
phenirarnine, doxylamine, chlorophenoxamine, dimenhydrinate, diphenhydramine,
pro-
methazine, ebastine, desloratidine and meclozine, optionally in the form of
the racemates,
enantiomers, diastereomers thereof and optionally in the form of the
pharmacologically
acceptable acid addition salts, solvates or hydrates thereof. According to the
invention
these acid addition salts are preferably selected from among the
hydrochloride, hydrobro-
mide, hydriodide, hydrosulphate, hydrophosphate, hydromethanesulphonate,
hydronitrate,
hydromaleate, hydroacetate, hydrocitrate, hydrofumarate, hydrotartrate,
hydroxalate, hy-
CA 02653422 2008-11-25 PCT P01-2031
14
drosuccinate, hydrobenzoate and hydro-p-toluenesulphonate.
Any inhalable compounds, including also inhalable macromolecules as disclosed
in EP 1
003 478, may be used as pharmaceutically effective substances, formulations or
mixtures
of substances. Preferably, substances, formulations or mixtures of substances
administered
by inhalation may be used for treating respiratory complaints.
In addition, the compound may come from the groups of ergot alkaloid
derivatives, the
triptans, the CGRP-inhibitors, the phosphodiesterase-V inhibitors, optionally
in the form of
the racemates, enantiomers or diastereomers thereof, optionally in the form of
the pharma-
cologically acceptable acid addition salts, the solvates and/or hydrates
thereof.
Examples of ergot alkaloid derivatives are dihydroergotamine and ergotamine.
An aim of the invention is to provide an atomizer of the kind mentioned
hereinbefore
which uniformly and reproducibly nebulises a fluid for absorption through the
mucosa in
the nasal cavity, without the use of propellant.
According to the invention the aim is achieved by associating a nose piece
with the nozzle.
The nose piece can be inserted in at least one of the nostrils and using the
atomizer the
fluid is uniformly and reproducibly nebulised for absorption through the
mucosa. Through
the nosepiece inserted in the nostril, the finely divided fluid in the form of
fine particles
reaches the nasal mucosa, from where, by diffusion, the active substance
either reaches its
site of activity directly or enters the bloodstream and in this way reaches
its target area.
High bioavailability is ensured by distribution over a large surface area of
the nasal mu-
cosa. Obviously, it is possible to construct the nose piece such that it is
designed for inser-
tion in both nostrils at the same time or for insertion in one nostril.
To enable the nosepiece to be replaced and/or cleaned as necessary, the nose
piece is ad-
vantageously attached in removable manner.
According to one feature, the nozzle is arranged in a mouthpiece of the
atomizer which
carries the nosepiece in sealed manner. Accordingiy, the atomizer is to be
used with the
mouthpiece in known manner as an inhaler for administering an, in particular,
lung-bound
CA 02653422 2008-11-25 PCT PO 1 -20' ) j
fluid for treatment of the airways and with the nosepiece fitted onto the
mouthpiece, which
is also referred to as the nasal tube, for the nasal administration of
solutions, suspensions
and so-called solutions, namely a mixture of solution and suspension. A known
atomizer
is sold under the brand name "Respimat" by Boehringer Ingelheim KG in the form
of an
5 inhaler and is described in WO 91/14468 Al and WO 97/12687 Al.
To avoid undesirable turbulence or loss of the fluid during nasal application,
the nose piece
preferably has a geometry that is congruent with the mouthpiece in the
attachment region
associated with the mouthpiece. As a result of this feature, there is no need
to provide a
10 seal between the attaclunent region of the nose piece and the mouthpiece.
Rather, an inter-
lockingly engaging seal resulting from the contour is obtained after the
attachment of the
nose piece.
As users find it pleasant to inhale through a mouthpiece adapted to the shape
of the mouth,
15 the attachment region of the nose piece is usefully designed to be
elliptical as a result of
the congruent geometries.
In order to adapt the nose piece to the shape of a human nostril, the nasal
insertion region
of the nose piece is preferably elliptical in shape. The opening in the nasal
insertion region
is smaller than the opening in the attachment region. According to a further
feature, the
cross-section of the nasal section narrows continuously from the attachment
region to the
nasal insertion region.
Preferably, the pressure generator comprising a piston comprises a holder for
the storage
container, an associated drive spring with a release button and a conveying
tube, while ax-
ial tensioning of the drive spring moves the holder with the storage container
and the con-
veying tube in the opposite direction to the nose piece and sucks fluid out of
a storage con-
tainer into the pressure chan-iber. According to a further feature the
specified amount is
adjustable.
Preferably, actuation of the release button causes relaxation of the tensioned
drive spring,
which moves the conveying tube in the direction of the nose piece and applies
pressure to
the fluid to expel it through the nozzle. In order to prevent liquid from
flowing back out of
CA 02653422 2008-11-25 pCT PO 1_2031
16 the pressure chamber into the storage container during the atomization, a
non-return valve
is associated with the conveying tube.
For using the atomizer for a number of applications that use medicaments, a
storage con-
tainer for the fluid is replaceably mounted inside a housing. Advantageously,
the nozzle
comprises a filter system associated with nozzle channels for producing two
spray jets that
meet to form a spray mist. The nozzle channels are constructed such that two
spray jets are
formed that meet one another substantially at right angles. The filter system
associated
with the nozzle channels ensures that solid particles are retained and
prevents the nozzle
1 o channels from becoming blocked. Preferably, the nozzle comprises a filter
system and at
least two nozzle channels for producing at least two spray jets meeting one
another in order
to produce a spray mist.
It will be appreciated that the features mentioned hereinbefore and those
still to be de-
scribed hereinafter may be used not only in the particular combination
specified but also in
other combinations. The scope of the invention is defined only by the claims.
The invention is explained in more detail hereinafter by an illustrative
embodiment with
reference to the associated drawing. The single Figure of the drawings shows:
Fig. 1 a sectional view of an atomizer according to the invention and
Fig. 2 a schematic plan view of a nose piece of the atomizer according to Fig.
1.
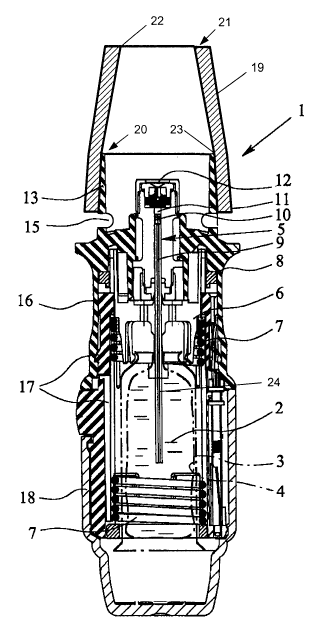
The atomizer 1 is used to atomize a fluid 2, particularly a highly effective
medicainent, and
is in the form of a portable inhaler that operates without propellant gas.
During the atomi-
zation of the fluid 2, preferably a liquid, an aerosol is formed which can be
breathed in by a
user (not shown).
3o The atomizer 1 has a replaceable storage container 3 containing the fluid
2, which is of
substantially cylindrical construction and can be inserted in the opened
atomizer 1 from
below. In the rigid storage container 3 is a bag 4 that holds the fluid 2. For
atomizing the
fluid 2 in a predetermined adjustable amount, the atomizer 1 has a pressure
generator 5
CA 02653422 2008-11-25 PCT p01_2031
17
comprising a piston 24 with a holder 6 for the container 3, a drive spring 7
with a release
button 8 which is to be operated manually to release the tension, a conveying
tube 9 with a
non-return valve 10 inserted therein, a pressure chamber 11 and a nozzle 12
with an asso-
ciated mouthpiece 13.
When the drive spring 7 is axially tensioned by rotating a lower housing part
18 with an
inner part 17 releasably attached thereto, relative to an upper housing part
16 formed on the
mouthpiece 13, the holder 6 with the storage container 3 and the conveying
tube 9 is
moved downwards and fluid is sucked out of the container 3 through the non-
return valve
10 into the pressure chamber i 1 associated with the piston 24 of the pressure
generator 5.
During the subsequent abrupt relaxation of the drive spring 7 by the actuation
of the re-
lease button 8, the fluid 2 in the pressure chamber 11 is put under pressure
by the drive
spring 7 moving the conveying tube 9 upwards and is expelled through the
nozzle 12,
whereupon atomization takes place. The atomization results, for example, in
particles in
the micron size range, preferably particles about 20 m in size, which form a
mist or jet of
aerosol. A user can inhale the aerosol, while supply air can be taken in
through supply air
openings 15 in the mouthpiece 13.
In order to administer fluid 2 as an aerosol through the nose so that it is
absorbed through
the mucosa in the nasal cavity, a nose piece 19 is removably fitted onto the
mouthpiece 13.
The nose piece 19 has a geometry that is congruent with the mouthpiece 13 in
the attach-
ment region 20 associated with the mouthpiece 13 and is substantially
elliptical in cross-
section, both in the attachment region 20 and in the nasal insertion region
21. The opening
22 in the nasal insertion region 21 of the nose piece 19 is smaller in size
than the opening
23 in the attachment region 20 and broadens out continuously up to the
attachment region
20 abutting in sealed manner on the mouthpiece 13. The dimensions of the nose
piece 19
are, of course, adapted to the dimensions of a human nostril, the largest and
smallest exter-
nal diameters being for example 16 mm and 10 mm in size.