Note: Descriptions are shown in the official language in which they were submitted.
CA 02654754 2009-02-19
ANISOTROPIC NANOCOMPOSITE HYDROGEL
FIELD OF THE INVENTION
This invention relates to nanocomposite anisotropic hydrogels,
including methods of their preparation and uses thereof, and more particularly
this invention relates to nanocomposite anisotropic hydrogels that are
crosslinked in the presence of bacterial cellulose.
BACKGROUND OF THE INVENTION
Heart disease and stroke, which are the principal components of
cardiovascular disease, remain the leading cause of death in the western
world. One of the most common treatments for coronary artery disease is
coronary bypass graft surgery (CABG), where a suitable length of the
patient's saphenous vein or the internal thoracic or mammary arteries are
used to supply blood to the heart tissue. The number of CABG procedures in
the US was more than 600,000 (1.2 million worldwide) in the year 2000, but
these tissue grafts tend to deteriorate due to further advancement of the
patient's coronary artery disease and disruption of the normal vascularity.[1-
3]
On the other hand, total peripheral artery bypass grafting is performed to
relieve the symptoms of vascular deficiencies, where a common problem
1
CA 02654754 2009-02-19
involves the supply of autologous bypasses. The lack of nondiseased
saphenous veins as arterio-venous access fistulae for haemodialysis is a
major cause of morbidity for patients with renal failure.[4, 5]
From the composition point of view, it is important to note that
cardiovascular tissues are composite materials with elastin and collagen as
the main load bearing components. The directional manner in which collagen
fibers are arranged within the tissue creates the anisotropic behavior, with
higher tensile strength in the circumferential than in the axial direction.[6-
9].
Accordingly, the mechanical properties of soft tissues, including aortic
tissue,
are anisotropic, with a higher stiffness in the circumferential than in the
axial
direction.
For a biomaterial to be used as tissue replacement, it is important to
ensure a good match of the mechanical properties of the implanted device
and the surrounding tissues [10]. Elastic polymers have been investigated to
create compliant grafts since the mismatch of the native aorta and the
synthetic grafts, such as Dacron and ePTFE, may contribute to intimal
hyperplasia (IH) and ultimate failure. A successful replacement has been
reported as having adequate strength, kink resistance, and must allow
sutures to hold under circumferential and axial tension, as well as
circumferential and axial compliance. Difference in compliance results in
haemodynamic changes and increased shear stresses that may induce the
release of growth factors that stimulate IH.[10-12].
2
CA 02654754 2009-02-19
Even though there are several FDA approved materials for
replacement aorta, such as Dacron or e-PTFE, these materials do not posses
the same tensile properties as the tissue they are replacing, which results in
hemodynamic problems and mismatch of mechanical properties and other
problems at the implant/tissue junction. Therefore, it would be very
advantageous to be able to produce a material that provides a suitable match
with the mechanical and viscoelastic properties of biological tissues. Two
promising material systems for meeting this need are anisotropic hyrdrogels
and nanocomposite hydrogels, as described below.
Hydrogels
Hydrogels have been shown to be promising candidates for a wide
range of biocompatible tissue replacement materials. Hydrogels are
hydrophilic polymer networks produced from reactions of one or more
monomers or by association bonds between chains that can absorb from at
least 20% to up to thousands of times their dry weight in water [13, 14].
Hydrogels may be chemically stable or they may disintegrate and dissolve
with time. They are called either physical (reversible) or chemical
(permanent) hydrogels. Physical hydrogels have networks held together by
molecular entanglements and/or secondary forces such as hydrogen bonding,
van der Waals interactions, ionic or hydrophobic forces. Physical hydrogels
are not homogeneous due to regions of high crosslinking density and low
water swelling, called clusters, dispersed within low crosslinking density and
high water swelling, or hydrophobic or ionic domains that create
3
CA 02654754 2009-02-19
in homogeneities. Chemical hydrogels are covalently crosslinked networks,
but they may also be generated by crosslinking of water-soluble polymers, or
by converting hydrophobic polymers to hydrophilic polymers. Chemical
hydrogels are also not homogeneous due to clusters of molecular
entanglements. Chain loops and free chain ends also produce network
defects in both physical and chemical hydrogels, and they do not contribute to
the permanent network elasticity [13, 15].
The main areas in which hydrogels are used as biomaterials is in
contact lenses, synthetic wound coverings, drug delivery systems, organ and
tissue replacements, and permselective membranes [13, 16, 15,17-24].
An important characteristic of hydrogels is their swelling behaviour in
water, since after preparation they have to be in contact with water to yield
the final solvated network structure. Highly swollen hydrogels are those of
polyvinyl alcohol (PVA), polyethylene glycol, and poly-N-vinyl 2-pyrrolidone,
among others. PVA is a hydrophilic polymer with various characteristics
desired for biomedical applications, such as high degree of swelling,
uncomplicated chemical structure, rubbery/elastic nature, and non-toxic. PVA
can be converted into a solid hydrogel by crosslinking.
Crosslinking can be accomplished by using several methods. For
biomedical applications, physical crosslinking has the advantages of not
leaving residual amounts of the toxic crosslinking agent, and higher
mechanical strength than the PVA gels crosslinked by either chemical or
irradiative techniques.
4
CA 02654754 2009-02-19
The mechanical properties of the PVA hydrogels are similar to that of
soft tissue, including elasticity and strength, and can be controlled by
changing the number of thermal cycles, PVA concentration, thawing rate of
the thermal cycling process, and freezing holding time among other
parameters [19, 25, 26]. A PVA based bioprosthetic heart valve stent has
been fabricated. However, the mechanical strength and stiffness of these
PVA materials were weak and did not fully match the mechanical properties
displayed by the cardiovascular tissues such as arteries and heart valves.
PVA has a relatively simple chemical formula with a pendant hydroxyl
group and a crystalline nature, which allows it to form a solid hydrogel by
the
crosslinking of the PVA polymer chains. Vinyl alcohol (monomer) does not
exist in a stable form and rearranges to its tautomer, acetaldehyde. PVA is
produced by free radical polymerization of vinyl acetate to polyvinyl acetate
(PVAc), and subsequent hydrolysis of PVAc gives PVA [25].
PVA can be crosslinked using several methods, such as the use of
crosslinking chemical agents, using an electron beam or y-irradiation, or the
physical crosslinking due to crystallite formation. For biomedical
applications,
physical crosslinking has the advantages of not leaving residual amounts of
the toxic crosslinking agent, and higher mechanical strength than the PVA
gels crosslinked by either chemical or irradiative techniques [27 ,28]. In
chemical cross-linking, the chemical agents that react with the hydroxyl
groups are glutaraldehyde, ethylaldehyde, terephthalaldehyde, formaldehyde,
hydrochloric, boric or maleic acid, among others [19, 29].
5
CA 02654754 2009-02-19
Physical crosslinking forms a hydrogel with a network of semi-
crystallites of hydrogen bonds of polymer filled with solvent [30]. It has
been
shown that the mechanical properties of the hydrogels, including elasticity
and strength, can be altered by changing the PVA concentration, the number
of freeze/thaw cycles, the process thawing rate, the freezing holding time,
and
the freezing temperature [19, 29, 30]. Increasing the PVA concentration
results in hydrogels with higher crystallinity and added stability upon
swelling,
which increases its tensile strength and tear resistance. The lower the
initial
concentration of PVA, the fewer the polymer chains in solution, and there may
be less number of crystalline regions created in the cycled PVA. Increasing
the number of freeze/thaw cycles increases the strength and stiffness of the
hydrogel by reinforcing existing crystals within the structure [19, 25, 26].
Decreasing the thawing rate of frozen PVA solutions increases the tensile
strength because the solutions are kept for longer periods at temperatures
below 0 C, allowing for increasing movements of polymer chains which result
in further entanglements and increased crystallite size and numbers.
The freezing holding time also has a drastic effect, with samples frozen
up to 10 days giving the most mechanically strong PVA hydrogels [19, 26, 28,
29]. The freezing temperature has an interesting effect. The freezing
temperature controls the phase equilibria and dynamics, where the lower the
temperature of the system the lower the amount of unfrozen solvent in the
liquid regions. Therefore, the lower the temperature the less opportunity for
chain mobility in the polymer rich regions, giving less chances of crystallite
6
CA 02654754 2009-02-19
growth and formation. This explains why keeping the frozen PVA solutions at
-10 C produces somewhat more rigid hydrogels than those kept for the same
period of time at -20 or -30 C. The freezing rate was shown not to have
drastic effects on the properties of the hydrogel [19, 26, 30]. PVA hydrogels
not only have tensile strength and elongation, but also flexibility and
elasticity.
Research has proven its ability to recover to its original shape after being
deformed to strains of 50%, showing excellent persistence and repeatability
of the recovery [30].
Physical crosslinking allows the PVA hydrogels to retain their original
shape and be extended up to six times their size. This behaviour shows its
rubbery and elastic nature and the high mechanical strength [24, 31]. There
are various theories proposed in the literature to explain why thermal cycling
increases the elastic modulus of PVA. The most accepted theory describes
the physical cross-linking process as an entropic reordering phenomena.
Water is likely to bind to the polymer by hydrogen bonding. When the
solution freezes, ice crystals force the polymer chains close to each other
forming high local polymer concentration regions or nuclei. When the
material thaws, these nuclei act as crosslinking sites for polymers molecules,
which realign and form hydrogen bonds to form crystallites and polymer chain
entanglements. The crystalline regions are formed within the polymer rich
regions, with further cycling increasing both the size and number of the
crystalline regions by repeating the process [19 , 32, 27]. On a molecular
level, the crystallites of PVA can be described as layered structure, with a
7
CA 02654754 2009-02-19
double layer of molecules held together by hydroxyl bonds, while weaker van
der Waals forces operate between the double layers. This folded chain
structure leads to ordered regions (crystallites) within an unordered,
amorphous polymer matrix [25]. The mechanical properties of PVA are very
unique compared to other polymers. The stress-strain curves for the
polymeric materials are initially linear and then curve towards the strain
axis.
On the other hand, the PVA curve displays an exponential stress-strain curve
similar to the characteristics of soft biological tissues, with the curve
shifting
towards the stress axis.
PVA materials have been reported to be ideal candidates as
biomaterials, due to their high degree of swelling, uncomplicated chemical
structure, rubbery/elastic nature, non-toxic, non-carcinogenic, and
bioadhesive characteristics. Some of the biomedical applications include
tissue reconstruction and replacements, cell entrapment and drug delivery,
soft contact lens material, wound covering bandage for burn victims, quality
control phantom for MR, among other medical applications [32, 25].
Anisotropic Hydrogels
Most research PVA hydrogels has focused on materials exhibiting the
normal characteristic of isotropic mechanical behaviour, that is, the
mechanical properties of the material are the same regardless of orientation.
This is expected due to the random distribution of the polymer chains.
Most tissues, however, including cardiovascular tissues, are composite
viscoelastic biomaterials displaying mechanical properties with varying
8
CA 02654754 2009-02-19
degrees of orientation effects. This orientation effect is due to the
organization
of the structural protein components such as collagen and elastin within the
tissue. This organization gives rise to the unique exponential stress-strain
relationship exhibited by soft tissues.
Recently, an anisotropic PVA hydrogel was reported [33] that was able
to closely match the stress-strain behaviour of porcine aorta. In this study,
it
was shown that an anisotropic PVA hydrogel can be produced that displays
the exponential response of cardiovascular tissue and also displays the
anisotropic behavior of porcine aorta up to 65% strain.
Nanocomposite Hydrogels
A second material system for obtaining improved viscoelastic
properties of synthetic and biocompatible replacement tissue materials is that
of nanocomposite hydrogels.
Bacterial cellulose has many characteristics that make it valuable for
biomedical applications, including its polyfunctionality, hydrophilicity, and
biocompatibility [34]. Cellulose is a linear polymer made of glucose
molecules linked by 0 (1-4) glycosidic linkages. Its chemical formula is
(C6H10O5)r,= There are four principle sources of cellulose. The majority of
cellulose is isolated from plants. A second source is the biosynthesis of
cellulose by different microorganisms, including bacteria (acetobacter,
aerobacter, pseudomonas), algae, and fungi among others. The other two
less common sources include the enzymatic in vitro synthesis starting from
cellobiosyl fluoride, and the chemosynthesis from glucose by ring-opening
9
CA 02654754 2009-02-19
polymerization of benzylated and pivaloylated derivatives [35, 36]. Cellulose
is not uniformly crystalline, but ordered regions are extensively distributed
throughout the material, and these regions are called crystallites. The long
cellulose chains lie side by side held together by hydrogen bonds between
the hydroxyl groups. These chains are twisted into structures called
microfibrils, which are twisted into fibers [34, 35].
Bacterial cellulose is produced by strains of the bacterium Acetobacter
xylinum, which is typically found on decaying fruits, vegetables, vinegar,
fruit
juices, and alcoholic beverages. It is a Gram-negative, rod shaped and
strictly aerobic bacterium. Bacterial cellulose produced has very high purity
and contains no lignin, hemicelluloses, pectin, and waxes as plant cellulose
does. Therefore, production of bacterial cellulose has the advantage of not
requiring the harsh chemical treatment needed for plant cellulose production.
This chemical treatment also has the disadvantage of altering the natural
structural characteristics of cellulose [34, 35, 36]. Bacterial cellulose
differs
from plant cellulose with respect to its high crystallinity, ultra-fine
network
structure, high water absorption capacity, high mechanical strength in the wet
state, and availability in an initial wet state [36].
Bacterial cellulose pellicles are formed in static culture. The pellicle
has an ultra-fine network structure of ribbons 500 nm wide and 10 nm thick.
The ribbons consisted of smaller microfibrils with a width of around 3 nm and
a fiber diameter of less than 130 nm compared to the over 14 mm found in
birch [35, 36]. Bacterial cellulose including the pellicle possesses a high
CA 02654754 2009-02-19
water retention capacity. Water retention values can reach up to 1000%,
which are significantly higher than that for plant cellulose. The water
retention
is drastically decreased after air-drying the bacterial cellulose and
reswelling
in water, with values comparable to those of plant cellulose [35, 36].
Bacterial cellulose can also be prepared in shake culture in flasks and
in agitated culture in a bioreactor. These approaches are more efficient
methods for bacterial cellulose production and are preferred for large scale
production of bacterial cellulose.
Bacterial cellulose, being a hydrophilic, highly water swollen and
biocompatible natural polymer which is ideally suited to be the reinforcing
fibers in the preparation of a nanocomposite material for soft tissue
replacement devices. Such nanocomposite material can be created when it is
used in combination with PVA.
Uryu [37] reported the formation of a biodegradable polymeric material
that can be decomposed in soil. The bacterial cellulose (with ribbon shaped
micro-fibrils) that can be biologically decomposed by microbes was mixed
with a biodegradable polymeric material to produce an improved composite
with higher tensile strength. The bacterial cellulose was produced in a liquid
culture medium using different types of microbes, including Acetobacter
xylinum, collected and dried into a powdery state and mixed with the polymer
to produce the composite. Various polymers were used, including PVA. The
nanocomposites ranged from bacterial cellulose concentrations as low as 1 %
to 99%. The final composite was dried and used for high-strength cabinets
11
CA 02654754 2009-02-19
for audio/video apparatus. After the lifetime of the device is reached, the
composite material can be buried in the ground for waste disposal and it is
eventually decomposed to protect the environment.
US Patent No. 5,558,861 discloses a hydrogel formed by microbially-
produced cellulose that may be complexed with an appropriate auxiliary
material (including PVA) for the purposes of reinforcement, change of the
specific gravity, immobilization, modification of the affinity, prevention of
exudation of the liquid component and the like. This invention teaches a
hydrogel that is formed based on the crosslinking of bacterially cellulose,
whereby a concentration of PVA can be added for a number of purposes,
including reinforcing the crosslinked bacterial cellulose hydrogel.
In contrast to the hydrogel disclosed in US Patent No. 5,558,861, US
Patent Application No. 12/216809 teaches a nanocomposite hydrogel
comprising PVA and bacterial cellulose, where the hydrogel is formed by
physically crosslinking a PVA solution with a small concentration of bacterial
cellulose. This composite hydrogel, in which PVA forms the primary structure
of the hydrogel rather than a reinforcing structure, uniquely provides a
biocompatible composite hydrogel that exhibits the exponential stress-strain
behaviour that is characteristic of many biological tissues.
The nanocomposite hydrogel of US Patent Application No. 12/216809
can be further understood by considering the development of the hydrogel
during crosslinking, and the role of the bacterial cellulose in this process.
The
bacterial cellulose, which forms extensive hydrogen bonds with PVA, is
12
CA 02654754 2009-02-19
believed to act as a nucleation site for the formation of additional PVA
crystallites during crosslinking. Accordingly, the composite hydrogel can be
understood to be formed by crosslinking PVA in the presence of bacterial
cellulose nanofibers, where the bacterial cellulose promotes additional PVA
crystal growth and also contributes to the over strength and compliance
properties of the composite [38].
The Need for Additional Stiffness and Anisotropy
For cardiovascular applications, it is important to consider the full strain
range that is required in clinical applications. The physiological average
strain
between diastole and systole for porcine aorta is around 30% strain
[33,38,40]. The physiological strain range has also been reported to be
between 17 and 49% strain [40, 41]. However, in designing cardiovascular
devices, it is necessary to make allowance for higher strain conditions
(corresponding to higher systole values) to ensure the material remains
elastic at higher strains to ensure durability. Furthermore, a successful
replacement must have adequate strength, kink resistance, and must allow
sutures to hold under circumferential and axial tension, as well as
circumferential and axial compliance [39].
Accordingly, a need remains for a tissue replacement material that can
match the highly anisotropic viscoelastic properties of many different types
of
soft tissues and can also provide improved stiffness beyond typical
physiological strain conditions.
13
CA 02654754 2009-02-19
SUMMARY OF THE INVENTION
The present invention addresses this need by providing a process for
the production of an anisotropic composite hydrogel that exhibits high
anisotropy and increased stiffness at high strain. The novel anisotropic
composite hydrogel is suitable for soft tissue replacement, the controlled
release of bioagents and in the design and fabrication of medical devices.
In one aspect of the invention there is provided a process of producing
a nanocomposite hydrogel with an anisotropic stress-strain curve, comprising
the steps of preparing a solution containing a solvent, a first concentration
of
a hydrogel-forming material and a second concentration of cellulose, wherein
said cellulose comprises fibers having nanometer scale cross sectional
dimensions, crosslinking the hydrogel-forming material to obtain a
nanocomposite hydrogel, applying a tensile force to said nanocomposite
hydrogel, and thermal cycling said nanocomposite hydrogel over a
predetermined temperature range at least once while maintaining the tensile
force.
In a particular aspect of the invention, the hydrogel-forming material is
polyvinyl alcohol (PVA) and the cellulose is bacterial cellulose, the
concentrations of PVA and bacterial cellulose are about 5% to 25% and
0.05% to 1 %, respectively, and the nanocomposite hydrogel is formed by
physically crosslinking using the low temperature thermal cycling method.
The present invention also provides an anisotropic nanocomposite
hydrogel produced according to the aforementioned processes. The
14
CA 02654754 2009-02-19
anisotropic nanocomposite hydrogel preferably comprises a composite
hydrogel formed by physically crosslinking PVA in the presence of bacterial
cellulose, where the concentrations of PVA and bacterial cellulose are about
5% to 25% and 0.05% to 1 %, respectively, and the nanocomposite hydrogel
is formed by physically crosslinking using the low temperature thermal cycling
method.
Also included in the scope of the invention is the use of an anisotropic
nanocomposite hydrogel produced according to the process of the invention
for tissue replacement, tissue reconstruction, bioagent entrapment, bioagent
delivery, preparing ultrasound or radiofrequency thermal therapy transmission
pads, preparing substitutes for ice bags, as a denture base, in soft contact
lens material, wound covering bandages, dental implants, catheter covering
dressing, dialysis membranes, coatings for cardiovascular stents, coatings for
cranial stents, and membranes for tissue guided regeneration and phantoms
for medical-related uses.
A further understanding of the functional and advantageous aspects of
the invention can be realized by reference to the following detailed
description
and drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred embodiments of the invention will now be described, by way
of example only, with reference to the drawings, in which:
CA 02654754 2009-02-19
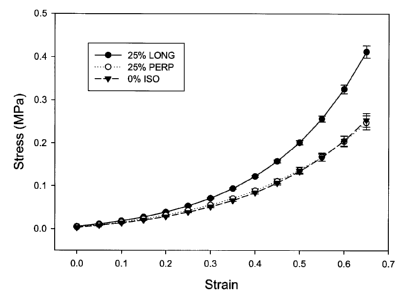
Figure 1 shows the effect of 25% initial strain on the stress-strain
curves of anisotropic 10% PVA with 0.3% bacterial cellulose, following 6
thermal cycles;
Figure 2 shows the effect of 100% initial strain on the stress-strain
curves of anisotropic 10% PVA with 0.3% bacterial cellulose, following 6
thermal cycles;
Figure 3 shows the effect of initial strain (0, 25, 50, 75, and 100%) on
the longitudinal stress-strain curves of anisotropic 10% PVA with 0.3%
bacterial cellulose, following 6 thermal cycles;
Figure 4 shows a comparison of the effect of initial strain on the ratio
of longitudinal to perpendicular stress at 65% strain (following 6 thermal
cycles) between anisotropic 10% PVA and anisotropic 10% PVA with 0.3%
bacterial cellulose nanocomposite;
Figure 5 plots the ratios of longitudinal to isotropic and perpendicular
to isotropic stress (at 65% strain) as a function of initial strain (from 0 to
100%);
Figure 6 shows the effect of 75% initial strain anisotropic 10% PVA
with 0.3% bacterial cellulose, following 2 thermal cycles;
Figure 7 shows the effect of 75% initial strain anisotropic 10% PVA
with 0.3% bacterial cellulose, following 6 thermal cycles;
Figure 8 shows the effect of number of thermal cycles (2, 4, and 6) on
the longitudinal stress-strain curves of anisotropic 10% PVA with 0.3%
bacterial cellulose (75% initial strain);
16
CA 02654754 2009-02-19
Figure 9 shows a comparison of the effect of number of thermal cycles
on the ratio of longitudinal to perpendicular stress at 65% strain (75%
initial
strain) between anisotropic 10% PVA and anisotropic 10% PVA with 0.3%
bacterial cellulose nanocomposite;
Figure 10 reveals a close match within physiological range of the
stress-strain curves of aorta (both directions) and anisotropic 10% PVA with
0.3% bacterial cellulose (75% initial strain and following 2 thermal cycles);
and
Figure 11 shows the stress relaxation response for circumferential and
axial directions of aorta and the anisotropic 10% PVA with 0.3% bacterial
cellulose (75% initial strain, following 2 thermal cycles)
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a biocompatible anisotropic
nanocomposite hydrogel material for soft tissue replacement that provides
viscoelastic properties that can be tailored to be highly anisotropic and
highly
resilient. Unlike prior hydrogel materials, this nanocomposite hydrogel can
match the highly anisotropic viscoelastic properties of many different types
of
soft tissues and can also provide improved stiffness beyond typical
physiological strain conditions. The invention is the result of
experimentation
involving nanocomposite hydrogels comprising PVA and bacterial cellulose,
where it was discovered that applying a tensile force while thermal cycling
the
nanocomposite generated a nanocomposite material with much higher
17
CA 02654754 2009-02-19
anisotropy and stiffness at large strains than a non-composite material with
PVA alone.
The figures are not to scale and some features may be exaggerated or
minimized to show details of particular elements while related elements may
have been eliminated to prevent obscuring novel aspects. Therefore, specific
structural and functional details disclosed herein are not to be interpreted
as
limiting but merely as a basis for the claims and as a representative basis
for
teaching one skilled in the art to variously employ the present invention.
As used herein, the terms "about", and "approximately" when used in
conjunction with ranges of concentrations, temperatures or other physical or
chemical properties or characteristics is meant to cover slight variations
that
may exist in the upper and lower limits of the ranges of
properties/characteristics.
As used herein, the phrase "cellulose of microbial origin" means
"microbial cellulose" in addition to other microbes (yeasts, fungi) besides
bacteria.
The anisotropic nanocomposite hydrogel of the present invention is
produced by crosslinking a nanocomposite hydrogel and subsequently
imparting anisotropy to the hydrogel nanocomposite by applying and
maintaining a tensile force during one or more thermal cycles. The
nanocomposite hydrogel is comprised of a hydrogel-forming material and
cellulose, where the cellulose has cross-sectional dimensions on the
nanometer scale.
18
CA 02654754 2009-02-19
In a preferred embodiment, the hydrogel-forming material is Polyvinyl
alcohol (PVA), which is known to produce hydrogels with exponential stress-
strain curves that are desirable for soft tissue replacement materials.
Alternatively, the hydrogel-forming material can be chosen from the list
including polyvinyl alcohol (PVA), poly(vinyl pyrrolidone) (PVP),
poly(ethylene
glycol) (PEG), poly(hydroxyethyl methacrylate) (PHEMA) and polyacrylamide.
The cellulose is preferably bacterial cellulose, which is known to
comprise fibers with typical diameters on the nanometer scale. The bacterial
cellulose may be produced in its original as-produced state and not dried but
used directly to produce the nanocomposite. The preferred bacterial cellulose
is produced using a microbial fermentation process using the bacteria
Acetobactor xylinum in either a static, shaken or agitated culture as
disclosed
in United States Patent No. 5,846,213 (which is incorporated herein by
reference).
The nanocomposite hydrogel is initially obtained by dissolving the
hydrogel-forming material and the nanoscale cellulose in a solvent, followed
by crosslinking the hydrogel-forming material to obtain a nanocomposite
hydrogel. In a preferred embodiment, the concentration of the hydrogel-
forming material may be in the range of about 5% to 25%, and the
concentration of nanoscale cellulose may be in the range of about 0.05% to
1 %. In embodiments including PVA as the hydrogel-forming material, the
PVA solution (suitably with a MW of 146,000 to 186,000, 99+% hydrolyzed)
may be prepared by heating, for example at a temperature of about 80 C to
19
CA 02654754 2009-02-19
about 100 C, suitably at about 90 C, for an amount of time to achieve the
desired solution, for example for about 2 to about 4 hours, suitably about 3
hours.
The solvent used to produce the hydrogel is preferably water, and
more preferably distilled water. In embodiments including PVA as the
hydrogel-forming material, the solvent may also be chosen from the list of
hydroxylic solvents including alcohol, ketone and aldehyde or carboxylic acid,
or any other aprotic solvent capable of forming effective hydrogen bonding to
dissolve PVA. Examples of dipolar aprotic solvents which may be used
include dimtheyl sulfoxide (DMSO), dimethyl formamide (DMF), dimethyl
acetamide (DMAc) and N-methyl pyrrolidone (NMP).
If the solvent is not water, the solvent would have to be removed by
solvent exchange with water by immersion in water before use. As described
above, the nanocomposite material can either be prepared using water as the
solvent or solvent systems consisting of combinations of water and other
solvents. The final product consists of microbial cellulose, hydrogel and the
solvent used. In the case when either water is used in combination with other
solvents or when solvent systems not containing water are used in the
fabrication process, an additional step of solvent exchange with water will be
necessary to replace the non-water solvent before the resulting product can
be used for biomedical applications
The hydrogel is preferably physically crosslinked, although the
hydrogel may also be obtained by chemical crosslinking. In embodiments
CA 02654754 2009-02-19
including PVA as the hydrogel-forming material, the hydrogel nanocomposite
is preferably produced by physically crosslinking the solution containing PVA
and nanoscale cellulose by the low temperature thermal cycling method.
As described above, anisotropy is imparted to the nanocomposite
hydrogel, following crosslinking of the nanocomposite hydrogel, by the
application of a tensile force during one or more thermal cycles. In one
embodiment, the tensile stress is applied after transferring the crosslinked
nanocomposite hydrogel into a vessel capable of applying a tensile force.
The thermal cycling preferably includes a freeze-thaw cycle, whereby
the temperature is maintained for a predetermined holding time prior the
thawing. In a preferred embodiment, the thermal cycle temperature range lies
within the range of approximately +30 C to -30 C. In another preferred
embodiment, the step of thermal cycling the nanocomposite hydrogel involves
cooling the nanocomposite hydrogel from a first temperature of about 15 C to
30 C to a second temperature of about -15 C to -30 C, maintaining said
second temperature for a holding time, and heating said nanocomposite
hydrogel back to a temperature approximately equal to said first temperature.
The rate of change of temperature during thermal cycling includes the range
of approximately 0.5 C/min to 0.05 C/min, and is preferably about 0.1 C/min.
The holding is preferably about 1 hour.
The applied tensile force preferably generates a strain in the
nanocomposite hydrogel of up to 100% of the initial length, where the length
is measured along the axis of applied force. Preferable, the applied force
21
CA 02654754 2009-02-19
produces a strain within the range of 60% to 90%, and is chosen to tailor the
resulting anisotropic stress-strain curve to the specific application.
The nanocomposite hydrogel can be thermally cycled one or more
times while applying the tensile force producing anisotropy. In a preferred
embodiment, the nanocomposite hydrogel is thermally cycled 1 to 6 times. In
another embodiment, the nanocomposite hydrogel is thermally cycled a
predetermined number of times whereby the magnitude of the resulting
anisotropy is similar to the anisotropy of a biological tissue or material.
In another embodiment of the invention, the nanocomposite hydrogel is
formed into a pre-selected shape for use in medical or therapeutic
applications, including planar sheets for wound dressing, dental implants,
vascular grafts, synthetic replacement vessels such as aorta (large diameter)
and coronary arteries (small diameter), synthetic heart valve leaflets,
synthetic cartilage, ligaments and skin catheter covering dressing, dialysis
membranes, coatings for cardiovascular stents, coatings for cranial stents,
and membranes for tissue guided regeneration.
The amount of anisotropy and ultimate mechanical properties
parameters of the anisotropic nanocomposite hydrogel can be controlled by
altering several processing parameters. These processing parameters
include, but are not limited to, the list including the concentrations of
hydrogel-
forming material and nanoscale cellulose, the temperature range and rate of
thermal cycling, the holding time during thermal cycling, the magnitude of
applied tensile force, the number of thermal cycles, and the choice of
solvent.
22
CA 02654754 2009-02-19
As described above, the anisotropic nanocomposite hydrogel produced
according to the process of the present invention provided the unexpected
results of significantly higher anisotropy and higher stiffness at large
strains
when compared to non-composite anisotropic hydrogels. Insight into these
novel properties of the nanocomposite hydrogel can be gained by considering
the role of bacterial cellulose in the formation of the nanocomposite
material.
Bacterial cellulose nanofibers are biocompatible, very hydrophilic, have
high strength, and because of the large surface area, are expected to interact
strongly by hydrogen bonding with the PVA polymer matrix [34, 36, 38, 42].
As discussed in previous studies for PVA, during the freeze/thaw cycles, ice
crystals in the amorphous regions force the polymer chains into regions of
high local polymer concentration (polymer mesh), forming crystallites [26, 30,
31, 32]. Several studies reported that after the initial cycle a few percent
of
the chain segment crystallize into 3-8 nm junctions (primary crystallites)
separated by amorphous regions of around 20-30 nm in size within the
polymer rich regions. These polymer rich regions are surrounded by polymer
poor regions (macropores) with dimensions of >100 nm [43-47]. Further
cycling augments the level of crystallinity by increasing the size of primary
crystallites, as well as forming smaller secondary crystallites in-between,
transforming the microstructure to a fibrillar network within larger pores
(polymer poor region). Bacterial cellulose is a highly crystalline and
hydrophilic nanofiber with high mechanical strength [34, 36, 42]. It is also
23
CA 02654754 2009-02-19
considered to be biocompatible and has been investigated for applications
such as blood-contacting scaffolds [36, 48, 49].
The already-formed bacterial cellulose crystallites during thermal
cycling could serve as nucleation sites for further organization of the PVA
chains, thus favoring the interaction and formation of PVA crystallites around
the bacterial cellulose nanofibers during the initial cycle [38]. When the
cycle
1 PVA- bacterial cellulose nanocomposite is stretched, the stress would
elongate the PVA polymer mesh as well as the polymer poor macropores
[42], along with the bacterial cellulose nanofibers which will tend to orient
in
the direction of the applied stress. This can be contrasted to the re-
orientation
of the bacterial cellulose fibrils within cellulose pellicles (sheets)
embedded in
a polymer matrix, in the direction of an applied stress, as reported in the
literature [50, 51]. The degree of orientation of the PVA polymer mesh and
bacterial cellulose fibers is expected to be proportional to the amount of
initial
strain. After the hydrogel is held under stress and further cycled, the
increase
in volume fraction of crystallinity is due mainly to the growth of the primary
crystallites around the bacterial cellulose fibers, as well as the formation
randomly oriented secondary crystallites, all within the polymer rich phase
[42, 45-47]. Since most of the primary crystallites and bacterial cellulose
fibers are oriented along the direction of stretch, this explains why the
ratio of
longitudinal to perpendicular stress increases as the initial strain is
increased
(see examples below). On the other hand, increasing the number of thermal
cycles at a fixed initial strain increases the volume fraction of crystallites
24
CA 02654754 2009-02-19
(primary and secondary) in the sample's polymer rich phase, regardless of
orientation. This explains why the ratio of longitudinal and the perpendicular
stress stays constant as the number of thermal cycles is increased if the
initial
strain is kept constant (see examples below).
The present invention further relates to a medical material, device or
apparatus comprising the anisotropic nanocomposite hydrogel obtained by
the following the processes described above.
The anisotropic nanocomposite hydrogel of the present invention is
particularly useful in surgical and other medical applications as an
artificial
material for replacing and reconstructing soft tissues in humans and other
animals. Soft tissue that may be replaced or reconstructed using the hydrogel
of the present invention include, but are not limited to, vascular vessels,
such
as aorta (large diameter) and coronary arteries (small diameter), heart valve
leaflets, heart valve stent, cartilage, ligaments and skin. Accordingly, the
present invention further includes an artificial material for replacing and
reconstructing soft tissues comprising the anisotropic hydrogel of the present
invention. It is an embodiment of the invention that the anisotropic
nanocomposite hydrogel is prepared using a method of the present invention.
The anisotropic nanocomposite hydrogel of the present invention can
also comprise a bioactive agent to provide the hydrogel with suitable
physiological properties for it to be used as a soft tissue replacement. The
bioactive agent can be chosen based upon the particular application planned
for the replacement, and the particular physiological properties required of
the
CA 02654754 2009-02-19
replacement in the application involved. Many such bioactive agents would be
released gradually from the hydrogel after implantation, and thereby delivered
in vivo at a controlled, gradual rate. The hydrogel can thus act as a
bioactive
agent delivery vehicle, for example, a drug delivery vehicle. Other bioactive
agents can be incorporated in to the hydrogel in order to support cellular
growth and proliferation on the surface of the material. Bioactive agents
which
can be included in the material include, for example, one or more of cell
lines,
antibodies, cytokines, thrombins, thrombin inhibitors, proteases,
anticoagulants, heparin, growth factors, collagen crosslinking inhibitors,
matrix inhibitors, glycosaminoglycans and antimicrobial agents. Heparins are
particularly suitable agents for incorporating into vascular grafts, because
of
their anticoagulant properties, and thus their ability to inhibit thrombosis
on
the surface of the hydrogel.
In order to embed bioactive agents into the hydrogel of the present
invention any of a pre-sterilized powder, aqueous solution or aqueous
suspension can be mixed into the starting solution containing the hydrogel-
forming material. After the bioactive agent is incorporated into solution, it
is
processed according to the method described herein. Bioactive agents can
also be introduced into the hydrogel by placing the hydrogel into a bath
containing an aqueous solution of the agent and allowing the agent to diffuse
into the hydrogel. Alternatively, one or more bioactive agents may be
incorporated into the anisotropic nanocomposite hydrogel after the final
thermal cycle is completed.
26
CA 02654754 2009-02-19
The concentration of the one or more bioactive agents in the mixture
may be selected for the particular application involved. For heparin
incorporation into a vascular graft, concentrations will typically range from
1
unit/ml to 1,000,000 units/ml. Lower concentrations may be employed to
inhibit coagulation on the graft surface, and higher concentrations will be
used
where local infusion of heparin into the blood is desired to inhibit
thrombosis
downstream of the graft [52].
The anisotropic nanocomposite hydrogel of the present invention can
be also be used to support the proliferation of eukaryotic cell cultures.
Vascular cells such as endothelial cells, smooth muscle cells, and fibroblasts
and other connective tissue cells, can thus be incorporated into the hydrogel.
Human aortic endothelial cells and human dermal fibroblasts are also
compatible with the hydrogels of the present invention. Hydrogels modified by
such cell lines are, in turn, especially well adapted for implantation into
the
human body, and for use as soft tissue replacement parts in the human body.
Indeed, replacement parts modified by such cell lines are better able to adapt
and adjust to changing physical and physiological conditions in the body, and
thereby to prevent any failure of the hydrogel which might otherwise occur.
These cellular lines can be incorporated into the hydrogel for example, after
it
has been produced, via standard cell culture protocol generally known in the
art. It is especially effective to culture human aortic endothelial cells and
human dermal fibroblasts using direct topical seeding and incubation in cell
culture medium.
27
CA 02654754 2009-02-19
Also included within the scope of the present invention is a use of an
anisotropic nanocomposite hydrogel of the present invention for tissue
replacement, tissue reconstruction, bioagent entrapment, bioagent delivery,
preparing ultrasound or radiofrequency thermal therapy transmission pads,
preparing substitutes for ice bags, as a denture base, in soft contact lens
material, wound covering bandages, neurological dressings (to replace for
example DuramaterTM during brain surgery) and phantoms for medical-related
uses.
The invention is further illustrated by the following non-limiting
examples.
EXAMPLES
Example 1: Sample Preparation
PVA (Sigma-Aldrich Canada) with a molecular weight (Mw) of 146,000-
186,000, 99+% hydrolyzed, was used in all solution preparations. A
suspension of 0.625 wt % bacterial cellulose in distilled water was produced
in shake flasks by fermentation using the bacteria Acetobacter xylinum, as
described by Guhados et al.[42]. PVA was added to the bacterial cellulose
suspension with additional distilled water to obtain a mixture of 10 wt % PVA
with 0.3 wt % bacterial cellulose.
The PVA-bacterial cellulose solution was transferred into seven
aluminum molds and placed in the heated/refrigerated circulator, where they
were cycled once between 20 C and -20 C to give cycle 1 samples. For a
study of the effect of initial strain, one of the samples was used as control
28
CA 02654754 2009-02-19
while the four other samples were transferred into the four custom design
molds where they were initially strained by 25, 50, 75, or 100% of the
original
length. The four stretched samples and the control were cycled 5 more times
to obtain cycle 6.
For a study of the effect of number of thermal cycles, samples were
prepared for cycles 2, 4 and 6 at a pre-defined initial strain of 75%. The two
extra cycle 1 samples were transferred and stretched at a strain of 75%, with
extra samples of non-stretched hydrogel (cycle 1) used as controls in each
mold. The molds were then cycled and a mold was removed at the end of
cycles 2 and 4.
All samples, including the controls, were cut (25 x 5 mm2) in either
longitudinal or perpendicular direction relative to the applied stress (n = 5)
for
tensile testing.
Example 2: Tensile Testing and Analysis
Testing equipment used in the studies consisted of a servo-hydraulic
material testing system (INSTRON 8872) equipped with a 1 Kg load cell.
Sample thickness was measured using a Mitutoyo thickness tester, and
testing was carried out inside a Plexiglas tank filled with distilled water
(at
37 C). All the specimens were secured onto custom designed tissue grips (10
mm grip-to-grip distance), and tensile tests were performed at a crosshead
speed of 40 mm/s to a maximum of 65% strain. Prior to the tensile tests, all
specimens were preconditioned with 10 loading and unloading cycles.
29
CA 02654754 2009-02-19
All data obtained was in the form of load-extension, which was then converted
into engineering stress-engineering strain, using the sample thickness and
the initial gauge length after preconditioning, as reported previously.
The stress-strain data for PVA-bacterial cellulose nanocomposites is
nonlinear and takes on the general shape of curving up towards the stress
axis. Therefore, the stress-strain data was fitted by Eq. (1):
a=y0+AeBe+Ce' (1)
Where 6 is stress,E is strain, and yo, A, B, C, and D are curve fitting
parameters. The elastic modulus as a function of strain was calculated as the
first derivative with respect to strain of Eq. (1).
After preconditioning and tensile testing, some samples (n = 3) were
strained to the same 65% strain used for tensile testing and held at constant
strain for 1 h, while monitoring the load. The raw data in the form of load-
time
was converted to relative stress remaining-time, relative to the initial
stress at
time zero. The time dependent properties of all samples were assessed by
stress relaxation test. The stress relaxation data was fitted to Eq. (2):
6(t) _ 6R + Ae-Bt + Ce-D' (2)
Q0 U0
where a(t) is the stress at time t, ao is the initial stress, R is the final
stress (t
= 3600 s), t is time, and A, B, C, and D are curve fitting parameters.
To facilitate data presentation, the following convention was adopted.
Samples cut in the direction of applied stress are denoted as (LONG) for
longitudinal, and samples cut perpendicular to the applied stress are denoted
CA 02654754 2009-02-19
(PERP). For the porcine aorta data, the circumferential samples are denoted
(CIRC) and the axial samples are denoted (AXIAL). Isotropic (nonstrained)
samples are denoted (ISO).
Example 3: Effect of Initial Strain
Figures 1 and 2 show the effect of 25 and 100% initial strains,
respectively, on anisotropy relative to the isotropic control. Samples in the
longitudinal direction showed higher stiffness than in the perpendicular
direction, with the results for isotropic control falling in between or close
to the
perpendicular direction. Comparing Figures 1 and 2, the longitudinal direction
displays higher stiffness at the 100% initial strain compared to 25%, as well
as a larger difference between the longitudinal and the perpendicular
directions. The data in Figure 1 does not show a statistically significant
difference at 65% strain between the perpendicular and isotropic control (p >
0.05), but both of them were statistically different from the longitudinal
samples (p < 0.05). In Figure 2, a statistically significant difference in
stress at
65% strain (p < 0.05) among all three samples was observed. Samples using
initial strains of 50, 75% were also prepared and tested, with results falling
between those of the 25 and 100% initial strains.
Figures 1 and 2 show the effect of initial strain at a level of 25 and
100% on PVA-bacterial cellulose (6 cycles). Anisotropy is observed at the low
initial strain of 25%. The difference between longitudinal and perpendicular
directions increases with increasing initial strain. The increase is mainly
due
to the increase in stiffness in the longitudinal direction, although in the
31
CA 02654754 2009-02-19
perpendicular direction, stiffness also decreases, but to a much lesser
degree. Figure 3 shows the results in the longitudinal direction as a function
of initial strain, at increments of 25% between 0 and 100%. A gradual
increase in strength in the longitudinal direction can be observed up to 75%
initial strain, with no further increase to 100% strain. This results contrast
that
of PVA alone, where the increase in stiffness of the longitudinal direction
was
observed up to 100% initial strain.
The degree of anisotropy can be more clearly quantified by calculating
the ratio of stress of longitudinal to perpendicular directions as a function
of
initial strain at a strain of 65%, as seen in Figure 4. The ratio increases
monotonically from 1 (isotropic) to around 3.7 at an initial strain of 100%.
The
effect of initial strain on strength in the longitudinal and perpendicular
directions (shown as ratios) is shown in Figure 5. It can be seen that up to
75%, the initial strain has a much larger effect in the longitudinal
direction.
Hence, the overall mechanical properties are dominated by changes in the
longitudinal direction leading to the observed leveling off of the
longitudinal to
perpendicular ratio at strains higher than 75% initial strain (statistically
the
same at 100% initial strain).
For PVA alone, the ratio leveled off at around 2 after reaching 75%
initial strain. Therefore, the addition of a small amount of bacterial
cellulose
provided the unexpected result of almost doubling the anisotropic effect
relative to that of anisotropic PVA alone.
32
CA 02654754 2009-02-19
Figure 3 shows the stress-strain curves for the longitudinal samples as
a function of initial strain. There is a clear trend of increase in stiffness
in the
longitudinal direction as the initial strain is increased from 0 to 75%, with
the
100% samples being statistically the same as the 75%. The initial strain has a
larger effect on the longitudinal direction than on the perpendicular
direction.
Results in the perpendicular direction (not shown) fell somewhat below the
isotropic samples, except the samples initially strained to 25% which were
found to be statistically the same as the isotropic control at 65% strain. In
the
longitudinal direction, a statistically significant difference of stress at
65%
strain (p < 0.05) was observed among all samples except the 75 and the
100% initial strain.
An alternate way to quantifying anisotropy is to calculate the ratio of
stress in the longitudinal and perpendicular directions as a function of
initial
strain. Figure 4 compares the effect of initial strain on the ratio of
longitudinal
to perpendicular stress at 65% strain (Cycle 6) between anisotropic 10% PVA
and anisotropic 10% PVA with 0.3% bacterial cellulose, with 0% representing
the isotropic controls. It can be seen that the stress ratio of the PVA-
bacterial
cellulose nanocomposite increases monotonically from 1 (0% isotropic
control) to around 3.7 0.4 at 100% initial strain. This is a significant
increase
from the anisotropic PVA results, where the ratio of longitudinal to
perpendicular stress increases to up to 2.1 0.2 at an initial strain of 75%
and
leveled off thereafter. When ANOVA was applied to the stress ratios of the
33
CA 02654754 2009-02-19
PVA-bacterial cellulose samples, all samples were found to be statistically
different, except the ratios at 75 and 100% initial strains (p < 0.05).
The trend of the ratio of longitudinal to perpendicular stress can be
better understood by examining the changes in stress in both the longitudinal
and perpendicular directions as a function of initial strain. The ratios of
longitudinal to isotropic and perpendicular to isotropic stress (at 65%
strain)
as a function of initial strain (from 0 to 100%) are shown in Figure 5. It can
be
seen that increasing the initial strain has a much larger effect in the
longitudinal direction for initial strains up to 75%. Initial strains larger
than
100% were not investigated since straining marks on the surface of the
hydrogel were noted for samples of PVA alone, as described elsewhere.
Example 4: Effect of Number of Thermal Cycles
Figures 6 and 7 show the effect of 75% initial strain on 10% PVA with
0.3% bacterial cellulose at cycles 2 and 6, respectively, together with the
isotropic control for the same cycle. Anisotropy was seen for all cycles,
including cycle 4 (not shown). As seen before, data for the perpendicular
samples falls lower than that for the isotropic control. In both Figures 6 and
7,
a statistically significant difference of stress at 65% strain (p < 0.05) was
observed among all three samples.
Figure 8 compares the stress-strain curves of longitudinal samples as
a function of number of thermal cycles (2, 4, and 6). The stress-strain curves
of the perpendicular and isotropic samples were removed for clarity. The
increase in stiffness with the number of cycles is clearly seen. A
statistically
34
CA 02654754 2009-02-19
significant difference of stress at 65% strain (p < 0.05) was observed among
all three samples.
Following the analysis approach used in a previous study, Figure 9
compares the effect of number of thermal cycles on the ratio of longitudinal
to
perpendicular stress at 65% strain (75% initial strain) between anisotropic
10% PVA and anisotropic 10% PVA containing 0.3% bacterial cellulose. It is
seen that the ratio of longitudinal to perpendicular stress for the PVA-
bacterial
cellulose nanocomposite is constant (3.3) regardless of number of cycles.
Thus, the stiffness in both directions increases proportionally as the number
of thermal cycles increases. As clearly seen, the anisotropic PVA ratio
showed a similar trend, with a ratio of about 1.9 under similar conditions.
When ANOVA was applied to the ratio for PVA-bacterial cellulose, all groups
were found to be statistically the same (p > 0.05). However, for each number
of thermal cycle, the ratio for PVA and PVA-bacterial cellulose were found
statistically different (p < 0.05).
Example 5: Relationship to Porcine Aorta
The mechanical response of porcine aorta in both circumferential and
axial directions was compared with the anisotropic PVA-bacterial cellulose
samples in order to assess a possible match of the mechanical properties for
tissue replacement applications. The stress-strain curves for porcine aorta in
both circumferential and axial directions had been previously shown to be
similar to a 75% initial strain anisotropic PVA (cycle 3). As seen in Figure
10,
both the circumferential and axial curves are closely matched, within
CA 02654754 2009-02-19
physiological range, by the anisotropic PVA-bacterial cellulose sample (75%
initial strain and cycle 2). It is seen that this hydrogel displays the same
tensile behavior as the aorta in both directions up to 45% strain. At higher
strains, stiffness in the longitudinal direction increases to beyond that of
the
porcine aorta. ANOVA was applied to the stress at the average strain of 30%
between systolic and diastolic cycles. The circumferential direction of aorta
and the longitudinal direction of the PVA-bacterial cellulose samples showed
no statistical difference. Similar statistical significance was also observed
in
the axial direction of aorta and the longitudinal direction of the PVA-
bacterial
cellulose samples.
To illustrate the use of this new anisotropic nanocomposite material in
soft tissue replacement applications, the properties of porcine aorta were
compared to the anisotropic PVA-bacterial cellulose nanocomposite. Aortic
tissue is anisotropic, with a ratio of circumferential to axial stress of
around
1.54 at a physiological strain of 30%. Figure 10 shows that the tensile
properties of porcine aorta within physiological range of 20-40% strain were
closely matched in both directions by the 10% PVA containing 0.3% bacterial
cellulose (cycle 2) processed at an initial strain of 75%.
Notably, the increase in stiffness at strains higher than 45% in the
longitudinal direction is an improvement, since at larger than normal strains,
as in the case of aneurisms and hypertension, the anisotropic conduit would
have larger resistance to be further stretched. The anisotropic PVA-bacterial
cellulose nanocomposite, along with the previously reported anisotropic PVA
36
CA 02654754 2009-02-19
hydrogel are the only synthetic materials known to the inventors to be able to
closely match the anisotropic response and mechanical properties displayed
by porcine aorta within the physiological range.
Example 6: Stress Relaxation
The time-dependent relaxation response of the aortic tissue and that of
10% PVA with 0.3% bacterial cellulose (75% initial strain-cycle 2) were
compared to assess the ability of the hydrogel to relax under tension as
compared to the relaxation response of the tissue it might replace. The stress
relaxation behavior was fitted using Eq. 2.
Figure 11 shows the stress relaxation response for the 10% PVA with
0.3% bacterial cellulose (75% initial strain-cycle 2) and circumferential and
axial directions of aorta. It is seen that the anisotropic PVA-bacterial
cellulose
nanocomposite relaxes to either the same or lower residual stress than aorta
and at a similar rate to aortic tissue. It is interesting to note that both
the
circumferential samples of aorta and the longitudinal samples of the PVA-
bacterial cellulose displayed a lower residual stress than the axial samples
of
aorta and the perpendicular samples of the PVA-bacterial cellulose samples,
respectively. The fact that the PVA-bacterial cellulose nanocomposite relaxes
as fast as and to either the same or lower relaxed stress than the aortic
tissue
indicate the ability of these PVA-bacterial cellulose nanocomposites to
recover as fast as the native tissue in a cardiac cycle.
As used herein, the terms "comprises", "comprising", "including" and
"includes" are to be construed as being inclusive and open ended, and not
37
CA 02654754 2009-02-19
exclusive. Specifically, when used in this specification including claims, the
terms "comprises", "comprising", "including" and "includes" and variations
thereof mean the specified features, steps or components are included.
These terms are not to be interpreted to exclude the presence of other
features, steps or components.
While the present invention has been described with reference to what
are presently considered to be the preferred examples, it is to be understood
that the invention is not limited to the disclosed examples. To the contrary,
the
invention is intended to cover various modifications and equivalent
arrangements included within the spirit and scope of the appended claims. All
publications, patents and patent applications are herein incorporated by
reference in their entirety to the same extent as if each individual
publication,
patent or patent application was specifically and individually indicated to be
incorporated by reference in its entirety. Where a term in the present
application is found to be defined differently in a document incorporated
herein by reference, the definition provided herein is to serve as the
definition
for the term.
38
CA 02654754 2009-02-19
REFERENCES
1. Kassab GS, Navia JA. Biomechanical considerations in the design of
graft: the homeostasis hypothesis. Annu. Rev. Biomed. Eng. 2006; 8: 499-
535.
2. Moustapha A, Anderson HV. Revascularization interventions for
ischemic heart disease. Curr Opin Cardiol 2000; 15: 463-471.
3. Popma JJ, Sawyer M, Selwyn AP, Kinlay S. Lipid-lowering therapy
after coronary revascularization. Am J Cardiol 2000; 86: 18H-28H.
4. Haruguchi H, Teraoka S., Intimal hyperplasia and hemodynamic
factors in arterial bypass and arteriovenous grafts: A review. J Artif Organs
2003; 6: 227-235.
5. Thomas AC, Campbell GR, Campbell JH. Advances in vascular tissue
engineering. Cardiovasc Pathol 2003; 12: 271-276.
6. Schoen FJ, Levy RJ. Founder's Award, 25th Annual Meeting of the
Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999.
Tissue heart valves: current challenges and future research perspectives.
Journal of biomedical materials research 1999;47(4):439-65.
7. Fung YC. Biomechanics: Mechanical Properties of Living Tissues. New
York: Springer-Verlag; 1993. 568 p.
8. Hayash K, Stergiopulos N, Meister J, Greenwald SE, Rachev A.
Techniques in the determination of the mechanical properties and constitutive
laws of arterial walls. Boca Raton, FL: CRC Press; 2001.
39
CA 02654754 2009-02-19
9. Abe H, Hayashi K, Sato M, editors. Data book on mechanical
properties of living cells, tissues, and organs. Tokyo: Springer-Verlag; 1996.
10. Xue L, Greisler Howard P. Biomaterials in the development and future
of vascular grafts. Journal of vascular surgery 2003;37(2):472-80.
11. Fussell G, Thomas J, Scanlon J, Lowman A, Marcolongo M. The effect
of protein-free versus protein-containing medium on the mechanical
properties and uptake of ions of poly(vinyl alcohol)/poly(vinyl pyrrolidone)
hydrogels. Journal of Biomaterials Science, Polymer Edition 2005;16(4):489-
503.
12. Chowdhury MNK, Alam AKMM, Dafader NC, Haque ME, Akhtar F,
Ahmed MU, Rashid H, Begum R. Radiation processed hydrogel of poly (vinyl
alcohol) with biodegradable polysaccharides. Bio-Medical Materials and
Engineering 2006;16(3):223-228.
13. Hoffman, A. S. "Hydrogels for Biomedical Applications." Advanced
Drug Delivery Reviews 54.1 (2002): 3-12.
14. Park, H. N., and K. Park. "Hydrogels in Bioapplications." Hydrogels and
Biodegradable Polymers for Bioapplications. Eds. R. M. Ottenbrite, S. J.
Huang, and Kinam Park. Washington, DC: ACS Symposium Series 627,
1994. 2-10.
15. Rosiak, J. M., and F. Yoshii. "Hydrogels and Their Mechanical
Applications." Nuclear Instruments and Methods in Physics Research B
151.1-4 (1999): 56-64.
CA 02654754 2009-02-19
16. Ratner, B. D., et al., eds. Biomaterials Science: An Introduction to
Materials in Medicine. San Diego: Academic Press, 1996.
17. Yannas, I. V., E. Lee, D.P. Orgill, "Synthesis and Characterization of a
Model Extracellular Matrix that Induces Partial Regeneration of Adult
Mammalian Skin." Proc. NatI. Acad. Sci. 86 (1989): 933-7.
18. Migliaresi, C., L. Nicodemo, and L. Nicolais. "Hydrogels for Artificial
Tendons." Hydrogels in Medicine and Pharmacy. Ed. N. A. Peppas. Vol. 3.
Boca Raton, FL: CRC Press, 1987. 83-94.
19. Gordon, M. J. "Controlling the Mechanical Properties of PVA Hydrogels
For Biomedical Applications." Diss. Western Ontario U., 1999.
20. Hui, A. J. "Hydrogel-Based Artificial Heart Valve Stent Material." Diss.
Western Ontario U., 1998.
21. Sefton, M. V. "Heparinized Hydrogels." Hydrogels in Medicine and
Pharmacy. Ed. N. A. Peppas. Vol. 3. Boca Raton, FL: CRC Press, 1987. 17-
52.
22. Korsmeyer, R. W., et al. "Mechanisms of Potassium Chloride Release
from Compressed, Hydrophilic, Polymeric Matrices; Effect of Entrapped Air"
Journal of Pharmaceutical Science 72 (1983): 1189-91.
23. Vyavahare, N. R., et al. "Current Progress in Anticalcification for
Bioprosthetic and Polymeric Heart Valves." Cardiovascular Pathology 6.4
(1997): 219-229.
41
CA 02654754 2009-02-19
24. Peppas, N. A. "Other Biomedical Applications of Hydrogels." Hydrogels
in Medicine and Pharmacy. Ed. N. A. Peppas. Vol. 3. Boca Raton, FL: CRC
Press, 1987. 177-86.
25. Hassan, C. M., and N. A. Peppas. "Structure and Applications of
Poly(vinyl alcohol) Hydrogels Produced by Conventional Crosslinking or by
Freezing/Thawing Methods." Advances in Polymer Science 153 (2000): 37-
65.
26. Lozinsky, V. I., and F. M. Plieva. "Poly(vinyl alcohol) Cryogels
Employed as Matrices for Cell Immobilization. 3. Overview of Recent
Research and Developments." Enzyme and Microbial Technology 23.3-4
(1998): 227-42.
27. Peppas, N. A., and C. M. Hassan. "Structure and Applications of PVA
Hydrogels Produced by Freezing/Thawing Methods." Advances in Polymer
Science 8 (2000): 37-41.
28. Cascone, M. G., et al. "Evaluation of Poly(vinyl alcohol) Hydrogels as a
Component of Hybrid Artificial Tissues." Journal of Materials in Science:
Materials in Medicine 6 (1995): 71-5.
29. Ku, D. N., L. G. Braddon, and D. M. Wootton. Poly(vinyl alcohol)
Cryogel United States Patent 5,981,826 (1999).
30. Mori, Y., H. Tokura, and M. Yoshikawa. "Properties of Hydrogels
Synthesized by Freezing and Thawing Aqueous Polyvinyl Alcohol Solutions
and their Applications." Journal of Materials Science 32 (1997): 491-6.
42
CA 02654754 2009-02-19
31. Stauffer, S. R., and N. A. Peppas. "Poly(vinyl alcohol) Hydrogels
Prepared by Freezing-Thawing Cyclic Processing." Polymer 33.18 (1992):
3932-5.
32. Chu, K. C., and B. K. Rutt. "Polyvinyl Alcohol Cryogel: An Ideal
Phantom Material for MR Studies of Arterial Flow and Elasticity." Magnetic
Resonance in Medicine 37 (1997): 314-9.
33. Wan, W. K., et al. "Anisotropic Polyvinyl Alcohol Hydrogel for
Cardiovascular Applications." Journal of Biomedical Materials Research
(Applied Biomaterials) 79 (2006): 305-311.
34. Eichhorn, S. J., et al. "Review Current International Research into
Cellulosic Fibres and Composites." Journal of Materials Science 36.9 (2001):
2107-31.
35. Joseph, G. A. "Studies of Bacterial Cellulose Production in Agitated
Culture." Diss. Western Ontario U., 2001.
36. Klemm, D., et al. "Bacterial Synthesized Cellulose - Artificial Blood
Vessel for Microsurgery." Progress in Polymer Science 26.9 (2001): 1561-
603.
37. Uryu, M., and K. Tokura. Composite Polymer Materials and Process
for Producing the Same United States Patent 6,274,652 (2001).
38. Wan, W. K., et al. "The Polyvinyl Alcohol - Bacterial Cellulose System
as a New Nanocomposite for Biomedical Applications." Journal of Biomedical
Materials Research (Applied Biomaterials) 79 (2006): 245-253.
43
CA 02654754 2009-02-19
39. Wan, W. K., et al. "Anisotropic Polyvinyl Alcohol - Bacterial Cellulose
Nanocomposite for Biomedical Applications." Journal of Biomedical Materials
Research (Applied Biomaterials) 86 (2008): 444-452.
40. Wan WK,Campbell G,Zhang ZF,Hui AJ,Boughner DR. Optimizing the
tensile properties of polyvinyl alcohol hydrogel for the construction of a
bioprosthetic heart valve stent. J Biomed Mater Res 2002; 63: 854-861.
41. Lee JM,Haberer SA,Boughner DR. The bovine pericardial xenograft. I.
Effect of fixation in aldehydes without constraint on the tensile viscoelastic
properties of bovine pericardium. J Biomed Mater Res 1989; 23: 457-475.
42. Guhados G, Wan W, Hutter JL. Measurement of the elastic
modulus of single bacterial cellulose fibers using atomic force
microscopy. Langmuir 2005;21:6642-6646.
42. Millon LE, Nieh M-P, Huffer JL, Wan W-K. SANS characterization
of an anisotropic polyvinyl alcohol hydrogel with vascular
applications. Macromolecules 2007;40:3655-3662.
44. Ricciardi R, Auriemma F, De Rosa C, Laupretre F. X-ray diffraction
analysis of poly(vinyl alcohol) hydrogels obtained by freezing and thawing
techniques. Macromolecules 2004;37: 1921-1927.
45. Ricciardi R, D'Errico G, Auriemma F, Ducouret G, Tedeschi
AM, De Rosa C, Laupretre F, Lafuma F. Short time dynamics of solvent
molecules and supramolecular organization of poly (vinyl alcohol) hydrogels
obtained by freeze/thaw techniques. Macromolecules 2005;38:6629-6639.
44
CA 02654754 2009-02-19
46. Ricciardi R, Mangiapia G, Lo Celso F, Paduano L, Triolo R, Auriemma
F, De Rosa C, Laupretre F. Structural organization of poly(vinyl alcohol)
hydrogels obtained by freezing and thawing techniques: A SANS study.
Chem Mater 2005;17: 1183-1189.
47. Willcox PJ, Howie DW Jr., Schmidt-Rohr K, Hoagland DA, Gido SP,
Pudjijanto S, Kleiner LW, Venkatraman S. Microstructure of polyvinyl alcohol)
hydrogels produced by freeze/ thaw cycling. J Polym Sci B: Polym Phys
1999; 37:3438-3454.
48. Baeckdahl H, Helenius G, Bodin A, Nannmark U, Johansson BR,
Risberg B, Gatenholm P. Mechanical properties of bacterial cellulose and
interactions with smooth muscle cells. Biomaterials 2006;27:2141-2149.
49. Helenius G, Baeckdahl H, Bodin A, Nannmark U, Gatenholm P,
Risberg B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res
A 2006;76:431-438.
50. Astley OM, Chanliaud E, Donald AM, Gidley MJ. Tensile deformation
of bacterial cellulose composites. Int J Biol Macromol 2003;32:28-35.
51. Gindl W, Keckes J. Tensile properties of cellulose acetate butyrate
composites reinforced with bacterial cellulose. Compos Sci Technol
2004;64:2407-2413
52. Chen et al. J. Vascular Surgery, 1995, 22, 237-247.