Note: Descriptions are shown in the official language in which they were submitted.
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
CGRP RECEPTOR AN.TAGONISTS
TECHNICAL FIELD OF THE INVENTION
[0001] The present invention relates to CGRP receptor antagonists,
pharmaceutical
compositions thereof, and methods therewith for treating CGRP receptor-
mediated diseases
and conditions.
BACKGROUND OF THE INVENTION
[0002] CGRP (Calcitonin Gene-Related Peptide) is a naturally occurring 37-
amino acid
peptide that is generated by tissue-specific alternate processing of
calcitonin messenger RNA
and is widely distributed in the central and peripheral nervous system. CGRP
is localized
predominantly in sensory afferent and central neurons and mediates several
biological
actions, including vasodilation. CGRP is expressed in alpha- and beta-forms
that vary by one
and three amino acids in the rat and human, respectively. CGRP-alpha and CGRP-
beta
display similar biological properties. When released from the cell, CGRP
initiates its
biological responses by binding to specific cell surface receptors that are
predominantly
coupled to the activation of adenylyl cyclase. CGRP receptors have been
identified and
pharmacologically evaluated in several tissues and cells, including those of
brain,
cardiovascular, endothelial, and smooth inuscle origin.
[0003) CGRP is a potent vasodilator that has been implicated in the pathology
of
cerebrovascular disorders such as migraine and cluster headache. In clinical
studies, elevated
levels of CGRP in the jugular vein were found to occur during migraine attacks
(Goadsby et
al., Ann. Neurol., 1990, 28, 183-187). CORP activates receptors on the smooth
muscle of
intracranial vessels, leading to increased vasodilation, which is thought to
be the major source
of headache pain during migraine attacks (Lance, Headache Pathogenesis:
Monoamines,
Neuropeptides, Purines and Nitric Oxide, Lippincott-Raven Publishers, 1997, 3-
9). The
middle meningeal artery, the principle artery in the dura inater, is
innervated by sensory
fibers from the trigeminal ganglion which contain several neuropeptides,
including CGRP.
Trigeminal ganglion stimulation in the cat resulted in increased levels of
CGRP, and in
humans, activation of the trigeminal system caused facial flushing and
increased levels of
CGRP in the external jugular vein (Goadsby et al., Ann. Neurol., 1988, 23, 193-
196).
Page 1 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Electrical stimulation of the dura mater in rats increased the diameter of the
middle
meningeal artery, an effect that was blocked by prior administration of CGRP
(8-37), a
peptide CGRP antagonist (Williamson et al., Cephalalgia, 1997, 17, 525-531).
Trigeminal
ganglion stimulation increased facial blood flow in the rat, which was
inhibited by CGRP (8-
37) (Escott et al., Brain Res. 1995, 669, 93-99). Electrical stimulation of
the trigeminal
ganglion in marmoset produced an increase in facial blood flow that could be
blocked by the
non-peptide CGRP antagonist BIBN4096BS (Doods et al., Br. J. Pharmacol., 2000,
129, 420-
423). Thus the vascular effects of CGRP may be attenuated, prevented or
reversed by a
CGRP antagonist. In recently reported clinical trials, the CGRP receptor
antagonist BIBN
4096 BS was reported to be effective in treating acute attacks of migraine
(Olesen et al., N.
Engl. J. Med. 2004, 350:1104-1110).
[00041 CGRP-mediated vasodilation of rat middle meningeal artery was shown to
sensitize
neurons of the trigeminal nucleus caudalis (Williamson-et al., The CGRP
Family: Calcitonin
Gene-Related Peptide (CGRP), Amylin, and Adrenomedullin, Landes Bioscience,
2000, 245-
247). Similarly, distention of dural blood vessels during migraine headache
may sensitize
trigeminal neurons. Some of the associated symptoms of migraine, including
extra-cranial
pain and facial allodynia, maybe the result of sensitized trigeminal neurons
(Burstein et al.,
Ann. Neurol. 2000, 47, 614-624). A CGRP antagonist may be beneficial in
attenuating,
preventing or reversing the effects of neuronal sensitization.
[00051 The ability of the compounds of the present invention to act as CGRP
antagonists
makes them useful pharmacological agents for disorders that involve CGRP in
humans and
animals, but particularly in humans. Such disorders include migraiine and
cluster headache
(Doods, Curr. Opin. Inves. Drugs, 2001, 2 (9), 1261-1268; Edvinsson et al.,
Cephalalgia,
1994, 14, 320-327); chronic tension type headache (Ashina et al., Neurology,
2000, 14, 1335-
1340); pain (Yu et al., Eur. J. Pharm., 1998, 347, 275-282); chronic pain
(Hulsebosch et al.,
Pain, 2000, 86, 163-175); neurogenic inflammation and inflammatory pain
(Holzer,
Neurosci., 1988, 24, 739-768; Delay-Goyet et al., Acta Physiol. Scanda. 1992,
146, 537-538;
Salmon et al., Nature Neurosci., 2001, 4(4), 357-358); eye pain (May et al.
Cephalalgia,
2002, 22, 195-196), tooth pain (Awawdeh et al., Int. Endocrin. J., 2002, 35,
30-36), non-
insulin dependent diabetes mellitus (Molina et al., Diabetes, 1990, 39, 260-
265); vascular
disorders; inflammation (Zhang et al., Pain, 2001, 89, 265), arthritis,
bronchial
hyperreactivity, asthma, (Foster et al., Ann. NY Acad. Sci., 1992, 657, 397-
404; Schini et al.,
Am. J. Physiol., 1994, 267, H2483-H2490; Zheng et al., J. Virol., 1993, 67,
5786-5791);
shock, sepsis (Beer et al., Crit. Care Med., 2002, 30 (8), 1794-1798); opiate
withdrawal
Page 2 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
syndrome (Salmon et al., Nature Neurosci., 2001, 4(4), 357-358) morphine
tolerance
(Menard et al., J. Neurosci., 1996, 16 (7), 2342-2351); hot flashes in men and
women (Chen
et al., Lancet, 1993, 342, 49; Spetz et al., J. Urology, 2001, 166, 1720-
1723); allergic
dermatitis (Wallengren, Contact Dermatitis, 2000, 43 (3), 137-143); psoriasis;
encephalitis,
brain trauma, ischaemia, stroke, epilepsy, and neurodegenerative diseases
(Rohrenbeck et al.,
Neurobiol. of Disease 1999, 6, 15-34); skin diseases (Geppetti and Holzer,
Eds., Neurogenic
Inflammation, 1996, CRC Press, Boca Raton, Fla.), neurogenic cutaneous
redness, skin
rosaceousness and erythema; tinnitus (Herzog et al., J. Membrane Biology,
2002, 189(3),
225); inflammatory bowel disease, irritable bowel syndrome, (Hoffinan et al.
Scandinavian
Journal of Gastroenterology, 2002, 37(4) 4I4-422) and cystitis. Of particular
importance is
the acute or prophylactic treatment of headache, including migraine and
cluster headache.
[00061 The present invention relates to compounds that are useful as ligands
for CGRP
receptors, in particular antagonists of CGRP receptors, pharmaceutical
compositions thereof,
and uses therewith.
SUMMARY OF THE INVENTION
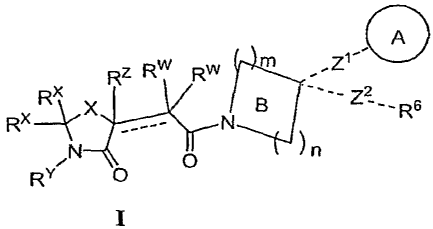
[0007] The present invention provides compounds of formula I:
Rz Rw RW m
, Z1
Rx x g R6
RX~ N
/N O )n
RY
or a pharmaceutically acceptable salt thereof.
These compounds are useful as antagonists of CGRP receptors and thus treating
CGRP-
mediated conditions. The present invention also provides pharmaceutical
compositions
thereof and uses therewith.
DETAILED DESCRIPTION OF THE INVENTION
1000812. Compounds and Definitions:
[0009] Compounds of this invention include those described generally above,
and are further
illustrated by the classes, subclasses, and species disclosed herein. As used
herein, the
following definitions shall apply unless otherwise indicated.
Page 3 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00101 For purposes of this invention, the chemical elements are identified in
accordance
with the Periodic Table of the Elements, CAS version, Handbook of Chemistry
and Physics,
75`h Ed. Additionally, general principles of organic chemistry are described
in "Organic
Chemistry", Thomas Sorrell, University Science Books, Sausalito: 1999, and
"March's
Advanced Organic Chemistry", 5h Ed., Ed.: Smith, M.B. and March, J., John
Wiley & Sons,
New York: 2001, the entire contents of which are hereby incorporated by
reference.
[0011] As described herein, compounds of the invention may optionally be
substituted with
one or more substituents, such as are illustrated generally above, or as
exemplified by
particular classes, subclasses, and species of the invention. It will be
appreciated that the
phrase "optionally substituted" is used interchangeably with the phrase
"substituted or
unsubstituted." In general, the term "substituted", whether preceded by the
term "optionally"
or not, refers to the replacement of hydrogen radicals in a given structure
with the radical of a
specified substituent. Unless otherwise indicated, an optionally substituted
group may have a
substituent at each substitutable position of the group, and when more than
one position in
any given structure may be substituted with more than one substituent selected
from a
specified group, the substituent may be either the same or different at every
position.
Combinations of substituents envisioned by this invention are preferably those
that result in
the formation of stable or chemically feasible compounds. The term "stable",
as used herein,
refers to compounds that are not substantially altered when subjected to
conditions to allow
for their production, detection, and preferably their recovery, purification,
and use for one or
more of the purposes disclosed herein. In some embodiments, a stable compound
or
chemically feasible compound is one that is not substantially altered when
kept at a
temperature of 40 C or less, in the absence of moisture or other che2nically
reactive
conditions, for at least a week.
[00121 The term "aliphatic" or "aliphatic group", as used herein, means a
straight-chain (i.e.,
unbranched) or branched, substituted or unsubstituted hydrocarbon chain that
is completely
saturated or that contains one or more units of unsaturation, or a monocyclic
hydrocarbon or
bicyclic hydrocarbon that is completely saturated or that contains one or more
units of
unsaturation, but which is not aromatic (also referred to herein as
"carbocycle"
"cycloaliphatic" or "cycloalkyl"), that has a single point of attachment to
the rest of the
molecule. Unless otherwise specified, aliphatic groups contain 1-20 aliphatic
carbon atoms.
In some embodiments, aliphatic groups contain 1-10 aliphatic carbon atoms. In
other
embodiments, aliphatic groups contain 1-8 aliphatic carbon atoms. In still
other
Page 4 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
embodiments, aliphatic groups contain 1-6 aliphatic carbon atoms, and in yet
other
embodiments aliphatic groups contain 1-4 aliphatic carbon atoms. In some
embodiments,
"eycloaliphatic" (or "carbocycle" or "cycloalkyl") refers to a monocyclic C3-
C8 hydrocarbon
or bicyclic or tricyclic C8-C14 hydrocarbon that is completely saturated or
that contains one or
more units of unsaturation, but which is not aromatic, that has a single point
of attachment to
the rest of the molecule wherein any individual ring in said bicyclic ring
system has 3-7
members. Suitable aliphatic groups include, but are not limited to, linear or
branched,
substituted or unsubstituted alkyl, alkenyl, alkynyl groups and hybrids
thereof such as
(cycloalkyl)alkyl, (cycloalkenyl)alkyl or (cycloalkyl)alkenyl. Suitable
cycloaliphatic groups
include cycloalkyl, bicyclic cycloalkyl (e.g., decalin), bridged bicycloalkyl
such as norbornyl
or [2.2.2]bicyclo-octyl, or bridged tricyclic such as adamantyl.
[0013] The term "heteroaliphatic", as used herein, means aliphatic groups
wherein one or two
carbon atoms are independently replaced by one or more of oxygen, sulfur,
nitrogen,
phosphorus, or silicon. Heteroaliphatic groups may be substituted or
unsubstituted, branched
or unbranched, cyclic or acyclic, and include "heterocycle", "heterocyclyl",
"heterocycloaliphatic", or "heterocyclic" groups.
[0014] The tenn "heterocycle", "heterocyclyl", "heterocycloaliphatic", or
"heterocyclic" as
used herein means non-aromatic, monocyclic, bicyclic, or tricyclic ring
systems in which one
or more ring atom is an independently selected heteroatom. In some
embodiments, the
"heterocycle", "heterocyclyl", "heterocycloaliphatic", or "heterocyclic" group
has three to
fourteen ring members in which one or more ring members is a heteroatom
independently
selected from oxygen, sulfur, nitrogen, or phosphorus, and each ring in the
system contains 3
to 7 ring members.
[0015] The term "heteroatom" means one or more of oxygen, sulfur, or nitrogen
(including,
any oxidized forms thereof, e.g., S=O, SO2, etc.; the quatemized form of any
basic nitrogen
or; a substitutable nitrogen of a heterocyclic ring, for example N (as in 3,4-
dihydro-2H-
pyrrolyl), NH (as in pyrrolidinyl) or NR-' (as in N-substituted
pyrrolidinyl)).
[0016] The terms "haloaliphatic" and "haloalkoxy" means aliphatic or alkoxy,
as the case
may be, subsfituted with one or more halo atoms. The term "halogen" or "halo"
means F, Cl,
Br, or I. Examples of haloaliphatic include -CHF2, -CH2F, -CF3, -CF2-, or
perhaloalkyl, such
as, -CF2CF3.
[0017] The terny "aryl" used alone or as part of a larger moiety as in
"aralkyl", "aralkoxy", or
"aryloxyalkyl", refers to monocyclic, bicyclic, and tricyclic ring systems
having a total of
five to fourteen ring members, wherein at least one ring in the system is
aromatic and
Page 5 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
wherein each ring in the system contains 3 to 7 ring members. The term "aryl"
may be used
interchangeably with the term "aryl ring". The term "aryl" also refers to
heteroaryl ring
systems as defined hereinbelow.
[0018] The term "heteroaryl", used alone or as part of a larger moiety as in
"heteroaralkyl" or
"heteroarylalkoxy", refers to monocyclic, bicyclic, and tricyclic ring systems
having a total of
five to fourteen ring members, wherein at least one ring in the system is
aromatic, at Ieast one
ring in the system contains one or more heteroatoms, and wherein each ring in
the system
contains 3 to 7 ring members. The term "heteroaryl" may be used
interchangeably with the
term "heteroaryl ring" or the term "heteroaromatic".
[00191 An aryl (including aralkyl, aralkoxy, aryloxyalkyl and the like) or
heteroaryl
(including heteroaralkyl and heteroarylalkoxy and the like) group may contain
one or more
substituents. Suitable substituents on the unsaturated carbon atom of an aryl
or heteroaryl
group are selected from halo; -R ; -OR ; -SR ; 1,2-methylene-dioxy; 1,2-
ethylenedioxy;
phenyl (Ph) optionally substituted with R ; -O(Ph) optionally substituted with
R ; -(CH2)1_
2(Ph), optionally substituted with R ; -CH=CH(Ph), optionally substituted with
R ; -NO2; -
CN; -N(R )2; -NR C(0)R ; -NR C(O)N(R )2; -NR C02R ; -NR NR C(O)R ; -
NR NR C(O)N(R )2; -NR NR CO2R ; -C(O)C(O)R ; -C(O)CH2C(O)R ; -C02R ; -C(O)R ;
-C(O)N(R )2i -OC(0)N(R )2i -S(0)2R ; -SO2N(R )2i -S(O)R ; -NR SO2N(R )2; -NR
S02R ;
-C(=S)N(R )a; -C(=NH)-N(R )2; or -(CH2)0_2NHC(0)R wherein each independent
occurrence ofR is selected from hydrogen, optionally substituted C1_6
aliphatic, an
unsubstituted 5-6 membered heteroaryl or heterocyclic ring, phenyl, -O(Ph), or
-CH2(Ph), or,
notwithstanding the definition above, two independent occurrences of R , on
the same
substituent or different substituents, taken together with the atom(s) to
which each R group
is bound, form a 3-8-membered cycloalkyl, heterocyclyl, aryl, or heteroaryl
ring having 0-3
heteroatoms independently selected from nitrogen, oxygen, or sulfur. Optional
substituents
on the aliphatic group of R are selected from NH2, NH(C1_4aliphatic),
N(C1_4aliphatic)2, halo,
CI-4aliphatic, OH, O(Ci-4aliphatic), NO2a CN, COaH, CO2(Q_4aliphatic),
O(haloCi4
aliphatic), or haloC .aliphatic, wherein each of the foregoing Ci-4aliphatic
groups of R is
unsubstituted.
[0020] An aliphatic or heteroaliphatic group, or a non-aromatic heterocyclic
ring may coritain
one or more substituents. Suitable substituents on the saturated carbon of an
aliphatic or
heteroaliphatic group, or of a non-aromatic heterocyclic ring are selected
from those listed
above for the unsaturated carbon of an aryl or heteroaryl group and
additionally include the
Page 6 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
following: =0, =S, =NNHR*, =NN(R*)a, =NNHC(O)R*, =NNI-iCO2(alkyl),
=NNHSO2(alkyl), or =NR", where each R* is independently selected from hydrogen
or an
optionally substituted C1_6 aliphatic. Optional substituents on the aliphatic
group of R* are
selected from NH2, NH(CI-4 aliphatic), N(C14 aliphatic)2, halo, C14 aliphatic,
OH, O(Ci4
aliphatic), NO2, CN, CO2H, CO2(C14 aliphatic), O(halo CI4 aliphatic), or
halo(C[4 aliphatic),
wherein each of the foregoing CI.4aliphatic groups of R* is unsubstituted.
[0021] Optional substituents on the nitrogen of a non-aromatic heterocyclic
ring are selected
from -R+, -NW)a, -C(O)R+, -COaR+, -C(O)C(O)R, -C(O)CH2C(O)R+, -SOZR+, -
SO2N(R+)2,
-C(=S)N(R})2, -C(=NH)-N(R+)2, or -NR+SOZR+; wherein R+ is hydrogen, an
optionally
substituted C1.6 aliphatic, optionally substituted phenyl, optionally
substituted -O(Ph),
optionally substituted -CHZ(Ph), optionally substituted -(CH2)i_2(Ph);
optionally substituted -
CH=CH(Ph); or an unsubstituted 5-6 membered heteroaryl or heterocyclic ring
having one to
four heteroatoms independently selected from oxygen, nitrogen, or sulfur, or,
notwithstanding
the definition above, two independent occurrences of R+, on the sarne
substituent or different
substituents, taken together with the atom(s) to which each R+ group is bound,
form a 3-8-
membered cycloalkyl, heterocyclyl, aryl, or heteroaryl ring having 0-3
heteroatoms
independently selected from nitrogen, oxygen, or sulfur. Optional substituents
on the
aliphatic group or the phenyl ring of R+ are selected from NH2, NH(C1 -4
aliphatic), N(CI-4
aliphatic)z, halo, CI-4 aliphatic, OH, O(Ct4 aliphatic), NO2, CN, CO2H,
C02(C14 aliphatic),
O(halo C14 aliphatic), or halo(CI-4 aliphatic), wherein each of the foregoing
Ci-4aliphatic
groups of R+ is unsubstituted.
[0022] The term "spirocyclic ring'system" refers to a moiety comprising two or
more rings,
wherein at least one ring has two points of attachment to another ring through
a common
carbon ring atom.
[0023] As detailed above, in some embodiments, two independent occurrences of
R (or R+,
or aiiy other variable similarly defined herein), are taken together with the
atom(s) to which
each variable is bound to form a 3-8-membered cycloalkyl, heterocyclyl, aryl,
or heteroaryl
ring having 0-3 heteroatoms independently selected from nitrogen, oxygen, or
sulfur.
Exemplary rings that are formed when two independent occurrences of R (or R+,
or any
other variable similarly defined herein) are taken together with the atom(s)
to which each
variable is bound include, but are not limited to the following: a) two
independent
occurrences of R (or R+, or any other variable similarly defined herein) that
are bound to the
same atom and are taken together with that atom to form a ring, for example,
N(R )2, where
both occurrences of R are taken together with the nitrogen atom to form a
piperidin-l-yl,
Page 7 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
piperazin-l-yl, or morpholin-4-yl group; and b) two independent occurrences of
R (or R, or
any other variable similarly defined herein) that are bound to different atoms
and are taken
together with both of those atoms to form a ring, for example where a phenyl
group is
~ OR
OR
substituted with two occurrences of ORo I ~ ~- , these two occurrences of R
are
taken together with the oxygen atoms to which they are bound to form a fused 6-
membered
" 1 o)
oxygen containing ring: d. It will be appreciated that a variety of other
rings can
be formed when two independent occurrences of R (or R+, or any other variable
similarly
defined herein) are taken together with the atom(s) to which each variable is
bound and that
the examples detailed above are not intended to be limiting.
[0024] Unless otherwise stated, structures depicted herein are also meant to
include all
isomeric (e.g., enantiomeric, diastereorneric, and geometric (or
conformational)) forms of the
structure; for example, the R and S configurations for each asymmetric center,
(Z) and (E)
double bond isomers, and (Z) and (E) conformational isomers. Therefore, single
stereochemical isomers as well as enantiomeric, diastereomeric, and geometric
(or
conformational) mixtures of the present compounds are within the scope of the
invention.
Unless otherwise stated, all tautomeric forms of the compounds of the
invention are within
the scope of the invention. Additionally, unless otherwise stated, structures
depicted herein
are also meant to include compounds that differ only in the presence of one or
more
isotopically enriched atoms. For example, compounds having the present
structures except
for the replacement of hydrogen by deuterium or tritium, or the replacement of
a carbon by a
13C- or 14C-enriched carbon are within the scope of this invention. Such
compounds are
useful, for example, as analytical tools or probes in biological assays.
[0025] The term "aryl-Cl-C6 aliphatic-" and similar such terms mean that the
aryl group is
linked to the core molecule by a CI to C6 aliphatic linker. For instance, the
term "aryl-C2--
alkyl- means a-CH2CHaPh group or a phenylethyl group is attached to the core
molecule.
[0026] In one embodiment, the present invention provides compounds of formula
1:
Page 8 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
A
RW
Rz Rw _.. Z1
Rx X B R6
RX--V N
/N 0 )n
RY
wherein:
X is S, SO, or SO2;
Z' is a bond or NR7,0, S, CH2, C(O), orNR7 C(O)NR7, wherein R7 is hydrogen, C1-
C4 aliphatic or C(O)C1-C4 aliphatic;
Z2 is a bond', 0, CHaO, or C(O);
ring A is phenyl or a 4-7 membered heterocyclic or heteroaryl ring or a 10-14
membered bicyclic heteroaryl or heterocyclic ring, wherein said heterocyclic
or heteroaryl
ring has 1-4 heteroatoms selected from 0, N, or S; wherein ring A is
optionally substituted
with up to 5 R' substituents;
wherein:
Z2 is a bond, Z' is a bond, NR', 0, S, CH2, C(O), or NR7C(O)NR7; or
wherein:
Z', Z2, and R6 are absent, ring A is not aromatic, and ring A together with
ring B
form a spirocyclic ring system;
R6 is hydrogen or C1-C4 aliphatic;
m is 1-3;
n is 1-3; provided that m+n is S4;
RY is aryl, heteroaryl, cycloaliphatic, Cl -C6 aliphatic, aryl-C1-C6 aliphatic-
,
heteroaryl-Ci-C6 aliphatic-, heterocyclyl-C1-C6 aliphatic- or cycloaliphatic-
Cl -C6
aliphatic-; wherein RY is optionally substituted with up to 5 R2 substituents;
Rx is hydrogen, aryl, heteroaryl, C l-C6 aliphatic, aryl-C 1 -C6 aliphatic-,
heteroaryl-Cl-C6 aliphatic-, wherein Rx is optionally substituted with up to 5
R3 substituents;
or two Rx, taken together with the carbon atom that they are attached to, form
a 3-9
membered monocyclic, a 9-14 membered bicyclic, or a 12-14 membered tricyclic
aryl,
heteroaryl or heterocyclic ring system wherein each heteroaryl or heterocyclic
ring has up to
3 heteroatoms selected from 0, S, and N; wherein said ring system formed by
two Rx is
optionally substituted with up to 5 R4 substituents;
Page 9 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
RZ is absent, hydrogen, CN, C1-C6 aliphatic, halo-C1-C6 aliphatic, O-Cl-C6
aliphatic, O-(halo-C1-C6 aliphatic), halo, aryl-C1-C6 aliphatic, orheteroaryl-
Cl-C6
aliphatic;
-= is a single or a double bond; provided that when it is a double bond, then
Rz
and one of RW is absent;
each RW is independently absent, hydrogen, halo, oxo, C1-C6 aliphatic, halo-C1-
C6
aliphatic, -O-Cl-C6 aliphatic, -O-(halo-Cl-C6 aliphatic), aryl, aryl-C1-C6
aliphatic-, C3-C7
cycloaliphatic; or
two Rw taken together form an optionally substituted C3-C7 cycloaliphatic or
heterocyclic ring, wherein said heterocyclic ring has up to 3 heteroatoms
selected from 0, S,
and N; wherein said ring formed by two Rti'' is optionally substituted with up
to 5 R5
substituents;
wherein each occurrence of R', R2, R3, R4, and RS is independently Q-R"';
wherein Q is a bond or is a C1-C6 aliphatic chain wherein up to two non-
adjacent
methylene units of Q are optionally and independently replaced by CO, C02,
COCO, CONR,
OCONR, NRNR, NRNRCO, NRCO, NRCO2, NRCONR, SO, SOa, NRSOa, SOzNR,
NRSO2NR, 0, S, or NR;
wherein each occurrence of RM is independently selected from R', halogen, NO2,
CN,
OR', SR', N(R')2, NR'C(O)R', NR'C(O)N(R')Z, NR'CO2R', C(O)R', CO2R', OC(O)R',
C(O)N(R')z, OC(O)N(R')2, SOR', SO2R', SO2N(R')2, NR'SOaR', NR'SO2N(R')2a
C(O)C(O)R', or C(O)CH2C(O)R';
wherein each occurrence of R is independently selected from hydrogen or a CI_6
aliphatic group optionally substituted with 0-5 occurrences of RK ; and each
occurrence of RK
is independently selected from -RV, halogen, -NO2, -CN, -ORv, -SRV, -N(RV)2, -
NRvCORV,
-NRYCON(Rv)2, -NR'COaRv, -CORV, -CO2RV, -OCORV, -CON(RV)Z, -C(=N-CN),
-OCON(Rv)2, -SORv, -SOaRv, -SO2N(Rv)2, -NRvSOaRv, -NRVSO2N(Rv)2, -COCORV,
-COCH2CORv, -OP(O)(ORV)2, -P(O)(ORV)a, -OP(O)2ORV, -P(O)20Rv, -PO(RV)2, or
-OPO(Rv)Z, wherein Rv is hydrogen or unsubstituted C1_6 aliphatic; and
wherein each occurrence of R' is independently hydrogen, a C1_6 aliphatic
group optionally
substituted with 0-5 occurrences of R"' 1; and each occurrence of RM 1 is
independently
selected from -RT, halogen, -NO2, -CN, -ORT, -SRT, --N(RT)2, -NRTCORT, -
NRTCON(RT)2,
-NRTCOaRT, -CORT, -CO2RT, -OCORT, -CON(RT)a, -C(=N-CN), -OCON(RT)2, -SORT,
-SO2RT, -SOzN(RT)a, -NRTSO2RT, -NRTSO2N(RT)2, -COCORT, -COCH2CORT,
-OP(O)(ORT)a, -P(O)(ORT)2, -OP(O)aORT, -P(O)2ORT, -PO(RT)2, or -OPO(RT)2,
wherein RT
Page 10 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
is hydrogen or unsubstituted C1_6 aliphatic; or R' is a 3-8-membered
saturated, partially
unsaturated, or fully unsaturated monocyclic ring having 0-3 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur, or an 5-12 membered saturated,
partially
unsaturated, or fully unsaturated bicyclic ring system having 0-5 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur wherein said monocyclic or bicyclic
ring is
optionally substituted with 0-5 occurrences of RU; and each occurrence of Ru
is
independently selected from a: 3-8-membered saturated, partially unsaturated,
or fully
unsaturated monocyclic ring optionally substituted with 0-3 occurrences of -
RQ' and having
0-3 heteroatoms independently selected from nitrogen, oxygen, or sulfur, or e
is -RQ,
halogen, =0, =NRQ, -N02, -CN, -ORQ, -SRQ, -N(RQ)2, -NRQCORQ, -NReCON(RQ)a,
-NRQC02RQ, -CORQ, -COzRQ, -OCORQ, -CON(RQ)Z, -C(=N-CN), -OCON(RQ)2, -SORQ,
-SO2RQ, -SO2N(RQ)2, -NRQSOzRe, -NRQSOzN(RQ)z, -COCORQ, -COCHaCORQ,
-OP(O)(ORQ)Z, -P(O)(ORQ)2, -OP(0)20RQ, -P(O)2ORQ, -PO(RQ)Z, or -OPO(RQ)Z,
wherein
RQ and RQ 1 are hydrogen or unsubstituted C1_6 aliphatic; or R and R', two
occurrences of R,
or two occurrences of R, are taken together with the atom(s) to which they are
bound to fonn
a 3-12 membered saturated, partially unsaturated, or fully unsaturated
monocyclic or bicyclic
ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur wherein
said monocyclic or bicyclic ring is optionally substituted with 0-5
occurrences of RT'; and
each occurrence ofRT' is independently selected from -Rs, halogen, =0, =NRs, -
NOz, -CN, -
ORS, -SRs, -N(RS)a, -NRSCORs, -NR5CON(Rs)a, -NRsCOzRs, -CORs, -C02Rs, -OCORs
,
-CON(RS)2a -C(=N-CN), -OCON(RS)a, -SORS, -SOZRs, -SO2N(Rs)z, -NRsSO2Rs,
-NRSSOzN(Rs)2, -COCORs, -COCHZCORs, -OP(O)(ORS)2, -P(O)(ORs)2, -OP(O)2ORs,
-P(O)20RS, -PO(Rs)2, or -OPO(Rs)2, wherein Rs is hydrogen or unsubstituted
C1_6 aliphatic.
[0027] In one embodiment of formula 1, Z2 is a bond, R6 is hydrogen, and Z' is
a bond.
[0028] In another embodiment of formula I, Z2 is a bond, R6 is hydrogen, and
Z' is NR7,0,
S, CH2, C(O), or NR7C(O)NR'.
[0029] In one embodiment of formula I, ZZ-Rb is other than hydrogen and Z, is
a bond.
[0030] In one embodiment of formula I, Z2-R6 is other than hydrogen and Z, is
NR', O, S,
CH2, C(O), or NR7C(O)NR'.
[0031] In one embodiment of forcnula I, is a single bond.
[0032] In one embodiment of formula I, is a single bond and both of Rw are
hydrogen.
[0033] In one embodiment of formula 1, Rz, if present, is C1-C6 alkyl, halo-CI-
C6 alkyl- or -
O-C 1-C6 alkyl.
Page I 1 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[0034] In one embodiment of formula I, Rz, if present, is fluoro, methyl,
ethyl, n-propyl,
CF3, CHF2, OMe or OEt.
[0035] In one embodiment of formula 1, at least one Rw is C1-C6 alkyl, halo-Cl-
C6 alkyl or
-O-C l -C6 alkyl.
[0036] In one embodiment of formula I, at least one RW is fluoro, methyl,
ethyl, n-propyl,
CF3, CHF2, OMe or OEt.
[0037] In one embodiment of formula I, one Rw is hydrogen and the other Rw is
C1-C6
alkyl, halo-Cl-C6 alkyl- or -O-C1-C6 alkyl.
[0038] In one embodiment of formula I, one of RW is hydrogen and the other Rw
is fluoro,
methyl, ethyl, n-propyl, CF3, CHF2, OMe or OEt.
[0039] In one embodiment of formula I, RY is C1-C6 aliphatic optionally
substituted with
one or more halo, OH, -Cl-C4 alkoxy, -Cl-C4 alkoxy carbonyl, or di-(Cl-C4
alkyl) amino-.
[0040] In one embodiment of formula I, RY is methyl, ethyl, propyl, isopropyl,
butyl, t-butyl,
3,3-dimethyl-butyl, 3-methyl-butyl, 2-methyl-propyl, 2-methoxy-ethyl, 3-
ethoxypropyl, 1-
(methoxy carbonyl)-3-methyl-butyl, 1-(hydroxy methyl)-3-methyl-butyl, allyl,
acetenyl, 2-
(diethylamino)ethyl, 1-methyl-2-methoxy-ethyl, 3-hydroxy-2,2-dimethyl-propyl,
2,2,2-
trifluoroethyl, 3,3,3-trifluoro-propyl, or 2,2,3,3,3-pentafluoro-propyl.
j0041] In one embodiment of formula I, RY is methyl, ethyl, propyl, isopropyl,
butyl, t-butyl,
3,3-dimethyl-butyl, 3-methyl-butyl or 2-methyl-propyl.
[0042] In one embodiment of formula 1, RY is C3-C8 cycloaliphatic or a C3-C8
cycloaliphatic substituted Cl-C6 aliphatic-.
100431 In one embodiment of formula I, RY is C3-C6 cycloalkyl or a C3-C6
cycloalkyl
substituted C1-C6 alkyl-.
[0044] In one embodiment of formula I, RY is cyclopropyl, cyclohexyl,
cyclohexylmethyl-,
cyclopropylmethyl-, or cyclohexylethyl-.
[0045] In one embodiment of formula I, RY is pyridyl (C1-C6)- alkyl-,
tetrahydrofuranyl
(C1-C6 alkyl)-, orN-(C1-C4 alkyl)-pyrrolidinyl-(C1-C6 alkyl)-.
[0046] In one embodiment of formula I, tetrahydrofuran-2-yl-methyl-, pyridin-3-
yl-methyl-,
pyridin-4-yl-ethyl-, pyridin-2-yl-ethyl-, pyridin-4-yl-methyl-, I H-indazol-5-
yl, or 2-(N-
m ethyl)-pyrroli din-2-yl-ethyl -.
100471 In one embodiment of formula I, RY is phenyl or (phenyl)-substituted C1-
C6
aliphatic- each optionally substituted with up to 5 R2 substituents
independently selected from
halogen or a 5-6 membered heterocyclyl ring having 1-3 heteroatoms selected
from N, 0, or
S.
Page 12 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[0048] In one embodiment of formula I, RY is phenyl, 2,6-difluorophenyl,
benzyl, 4-
fluorophenylmethyl-, 4-morpholinophenyl-, 2-piperidinylphenyl- or phenylethyl-
.
[0049] In one embodiment of formula I, one Rx is hydrogen and the other Rx is
an aryl or
heteroaryl ring optionally substituted with up to 5 R3 substituents
independently selected
from C1-C6 aliphatic, phenyl, halogen, C3-C6 cycloaliphatic or a 4-7 membered
heterocyclic
ring wherein said heterocyclic ring is optionally substituted with up to 3 Ru
substituents
wherein said heteroaryl or heterocyclic ring has up to three heteroatoms
selected from N, 0,
or S.
[0050] In one embodiment of formula I, one Rx is hydrogen and the other RX is
phenyl or
pyridyl with up to 2 R3 substituents independently selected from halogen or a
4-7 membered
heterocyclic ring wherein said heterocyclic ring is optionally substituted
with up to 2 Ru
substituents wherein said heterocyclic ring has up to three heteroatoms
selected from N, 0, or
S.
[0051] In one embodiment of formula I, one Rx is hydrogen and the other Rx is
phenyl
substituted with a 4-7 membered heterocyclic ring in the 2 position and a
halogen in the 3
position.
[0052] In one embodiment of formula I, one Rx is hydrogen and the other Rx is
phenyl, or
phenyl substituted with piperazine, 4-methyl-piperazin-1-yl, 4-ethyl-piperazin-
lyl, 4-propyl-
piperazin-1 yl, 4-butyl-piperazin-1 yl, 4-isopropyi-piperazin-l yl, 4-t-
butylpiperazin-1 yl, 4-
cyclopropylpiperazin-l-yl, 4-t-butoxycarbonyl-piperazin-l-yl, 4-hydroxy-
piperidinyl, 4-
ethoxycarbonyl-piperidin-l-yI, morpholin-4-yl, 1-H-pyrazol-l-yl, irnidazol-l-
yl, pyrrolidin-
1-yl, 3-dimethylamino-pyrrolidin-l-yl, 4-(piperidin-l-yl)piperidine, pyridyl
(1 -methylpiperidin-4-yl)piperazin- 1 -yl, or 1-(2,2,2-
trifluoroethyl)piperazin-l-yl.
[0053] In one embodiment of formula I, one Rx is hydrogen and the other Rx is
pyridyl, or
pyridyl substituted with piperazine, 4-methyl-piperazin-1-yl, 4-ethyl-
piperazin-lyl, 4-propyl-
piperazin-lyl, 4-butyl-piperazin-lyl, 4-isopropyl-piperazin-lyl, 4-t-
butylpiperazin-lyl, 4-
cyclopropylpiperazin-l-yl, 4-t-butoxycarbonyl-piperazin-l-y1, 4-hydroxy-
piperidinyl, 4-
ethoxycarbonyl-piperidin-1-yl, morpholin-4-yl, 1-HHpyrazol-1-yl, imidazol-1-
yl, pyrrolidin-
1-yl, 3-dimethylamino-pyrrolidin-1-yl, 4-(piperidin-l-yl)piperi dine, pyridyl
(1-methylpiperidin-4-y1)piperazin-1-yl, or 1-(2,2,2-trifluoroethyl)piperazin-l-
yl.
[0054] In one embodiment of formula I, one RX is hydrogen and theother Rx is
phenyl or
heteroaryl optionally substituted with one or more substituents independently
selected from
Cl-C6 aliphatic, cyano, halo, halo-C1-C6 aliphatic-, aryl-C1-C6 aliphatic-,
heteroaryl-C1-C6
Page 13 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
aliphatic-, aralkyloxy, di(Cl-C6 aliphatic)amino-, -0-Cl-C6 aliphatic, -S(O)-
C1-C6
aliphatic, or -S(O)a-C 1-C6 aliphatic.
[0055] In one embodiment of formula I, one Rx is hydrogen and the other Rx is
a C3-C7
cycloaliphatic or a heterocycloaliphatic ring optionally substituted with up
to five R3
substituents and having up to three heteroatoms selected from 0, N, or S,
wherein said ring is
optionally fused to one or more phenyl or heteroaryl rings.
[0056] In one embodiment of formula I, said Rx is selected from cyclopentyl,
cyclohexyl,
cyclohexenyl, cycloheptyl, tetrahydro-2H-pyranyl, tetrahydro-2H-thiopyranyl,
9H-fluoren-9-'
yl or piperidinyl.
[00571 In one embodiment of formula I, two RX, taken together with the carbon
atom that
they are attached to, form a 3-9 membered monocyclic, a 9-14 membered
bicyclic, or a 12-14
membered tricyclic aryl, heteroaryl or heterocyclic ring system wherein each
heteroaryl or
heterocyclic ring has up to 3 heteroatoms selected from 0, S, and N; wherein
said ring system
formed by two Rx is optionally substituted with up to 5 R4 substituents.
[0058] In one embodiment of formula i, said ring system is selected from 9H-
fluroen-9-yl,
tetrahydro-2H-pyran-4-yl, tetrahydro-2H-thiopyran-4-yl, cyclobutyl,
cyclopentyl, cyclohexyl,
cycloheptyl, cyclohexenyl, piperidinyl, or 1-benzyl-piperidin-4-yl.
[0059] In another embodiment of formula I, said compound is of formula I-A:
O
1 N.R-
RZ Rw Rw CaA
RX
RX--~x N~
RY N O O
I-A;
wherein:
ring A is a 4-7 membered heterocyclic ring that forms a spirocyclic ring
system with
said piperidine ring through carbon atom CA, wherein ring A is optionally
fused with a
phenyl or heteroaryl ring that is optionally substituted with up to 5 R1
substituents;
wherein said ring A, in addition to the nitrogen ring atom, has up to two
additional
ring heteroatoms selected from 0, N, or S;
wherein ring A, in addition to the oxo group, is optionally substituted with
up to 5 R,
substituents;
R', Rx, RY, RZ, R"", and X are as defined herein.
Page -14 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[0060] In one embodiment of formula I-A, is a single bond and Rz, if present,
is
hydrogen.
[0061] In one embodiment of formula I-A, is a single bond and Rz is CI-C6
alkyl, halo-
Cl-C6 alkyl-, or -O-Cl-C6 alkyl.
[0062] In one embodiment of formula I-A, Rz, if present, is fluoro, methyl,
ethyl, n-propyl,
CF3, CHF2, OMe or OEt.
[00631- In one embodiment of formula I-A, at least one RW is C1-C6 alkyl, halo-
C1-C6 alkyl-
or -O-C1-C6 alkyl.
[00641 In one embodiment of formula I-A, at least one Rw is fluoro, methyl,
ethyl, n-propyl,
CF3, CHF2, OMe or OEt.
[00651 In one embodiment of formula I-A, - is a single bond, one RW is
hydrogen and the
other Rw is C1-C6 alkyl, halo-CI-C6 alkyl or -O-C1-C6 alkyl.
10066] In one embodiment of forinula I-A, one R`N is hydrogen and the other Rw
is fluoro,
methyl, ethyl, n-propyl, CF3, CHF2, OMe or OEt.
[00671 In one embodiment of formula I-A, - is a single bond and each RW is
hydrogen.
[00681 In one embodiment of formula I-A, RY is Cl-C6 aliphatic optionally
substituted with
one or more halo, OH, C 1-C4 alkoxy, C 1-C4 alkoxy carbonyl, or di-(C 1-C4
alkyl) amino-.
[00691 In one embodiment of formula I-A, RY is methyl, ethyl, propyl,
isopropyl, butyl, t-
butyl, 3,3-dimethyl-butyl, 3-methyl-butyl, 2-methyl-propyl, 2-methoxy-ethyl, 3-
ethoxypropyl, 1 -(methoxy carbonyl)-3 -methyl-butyl, 1-(hydroxy methyl)-3-
methyl-butyl,
allyl, acetenyl, 2-(diethylamino)ethyl, l-methyl-2-methoxy-ethyl, 3-hydroxy-
2,2-dimethyl-
propyl, 2,2,2-trifluoroethyl, 3,3,3-trifluoro-propyl, or 2,2,3,3,3-pentafluoro-
propyl.
[0070] In one embodiment of formula I-A, RY is methyl, ethyl, propyl,
isopropyl, butyl, t-
butyl, 3,3-dimethyl-butyl, 3-methyl-butyl or 2-methyl-propyl.
[0071] In one embodiment of formula I-A, RY is C3-C8 cycloaliphatic or a C3-C8
cycloaliphatic substituted C1-C6 aliphatic-.
[0072] In one embodiment of formula I-A, RY is C3-C6 cycloalkyl or a C3-C6
cycloalkyl
substituted C 1-C6 alkyl-.
[00731 In one embodiment of formula I-A, RY is cyclopropyl, cyclohexyl,
cyclohexylmethyl-, cyclopropylmethyl-, or cyclohexylethyl-.
[0074J In one embodiment of forrnula I-A, RY is pyridyl (Cl -C6) alkyl-,
tetrahydrofuranyl
(C1-C6 alkyl)-, N-(C1-C4 alkyl)-pyrrolidinyl-(Cl -C6 alkyl)-.
Page15of153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[0075] In one embodiment of formula I-A, RY is tetrahydrofuran-2-yl-methyl-,
pyridin-3-yl-
methyl-, pyridin-4-yl-ethyl-, pyridin-2-yl-ethyl-, pyridin-4-yl-methyl-, 1H-
indazol-5-yl, or 2-
(N-methyl)-pyrrolidin-2-yl-ethyl-.
[0076] In one embodiment of formula I-A, R'r is phenyl or (phenyl)-substituted
C1-C6
aliphatic- optionally substituted with up to 5 R2 substituents independently
selected from
halogen or a 5-6 membered heterocyclyl ring having 1-3 heteroatoms selected
from N, 0, or
S.
[0077] In one embodiment of fornula I-A, Ry is phenyl, 2,6-difluorophenyl,
benzyl, 4-
fluorophenylmethyl-, 4-morpholinophenyl-, 2-piperidinylphenyl- or phenylethyl-
.
[0078] In one embodiment of formula I-A, - is a single bond, one RX is
hydrogen and the
other Rx is an aryl or heteroaryl ring optionally substituted with up to 5 R3
substituents
independently selected from C1-C6 aliphatic, phenyl, halogen, C3-C6
cycloaliphatic or a 4-7
membered heterocyclic ring with up to 3 RU substituents wherein said
heteroaryl or
heterocyclic ring has up to three heteroatoms selected from N, 0, or S.
[0079] In one embodiment of formula I-A, one Rx is hydrogen and the other RX
is phenyl or
pyridyl with up to 2 R5 substituents independently selected from halogen or a
4-7 membered
heterocyclic ring with up to 2 RU substituents wherein said heterocyclic ring
has up to three
heteroatoms selected from N, 0, or S.
[0080] In one embodiment of formula I-A, one Rx is bydrogen and the other Rx
is phenyl
substituted with a 4-7 membered heterocyclic ring in the 2 position and a
halogen in the 3
position.
[0081] In one embodiment of formula I-A, one Rx is hydrogen and the other Rx
is phenyl, or
phenyl substituted with piperazine, 4-methyl-piperazin-l-yl, 4-ethyl-piperazin-
lyl, 4-propyl-
piperazin-lyl, 4-butyl-piperazin-lyl, 4-isopropyl-piperazin-lyl, 4-t-
butylpiperazin-lyl, 4-
cyclopropylpiperazin-l-yl,. 4-t-butoxycarbonyl-piperazin-l-yl, 4-hydroxy-
piperidinyl, 4-
ethoxycarbonyl-piperidin-1-yl, morpholin-4-yl, l-HHpyrazol-l-yl, imidazol-l-
yl, pyrrolidin-
1-yl, 3-dimethylamino-pyrrolidin-1-yl, 4-(piperidin-l-yl)piperidine, pyridyl
(1-methylpiperidin-4-yl)piperazin-l-yl, or 1-(2,2,2-trifluoroethyl)piperazin-l-
yl.
[0082] In one embodiment of formula I-A, one Rx is hydrogen and the other Rx
is pyridyl, or
pyridyl substituted with piperazine, 4-methyl-piperazin-l-yl, 4-ethyl-
piperazin-1 yl, 4-propyl-
piperazin-lyl, 4-butyl-piperazin-lyl, 4-isopropyl-piperazin-lyl, 4-t-
butylpiperazin-lyl, 4-
cyclopropylpiperazin-l-yl, 4-t-butoxycarbonyl-piperazin-l-yl, 4-hydroxy-
piperidinyl, 4-
etlioxyearbonyl-piperidin-1-yl, morpholin-4-yl, l-HHpyrazol-l-yl, imidazol-l-
yl, pyrrolidin-
Page 16 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
1-yl, 3-dimethylamino-pyrroiidin-1-yl, 4-(piperidin-1-yl)piperidine, pyridyl
(1-methylpiperidin-4-yl)piperazin-l-yl, 1-(2,2,2-trifluoroethyl)piperazin-1-
yl.
[0083] In one embodiment of formula I-A, one Rx is hydrogen and the other Rx
is phenyl or
heteroaryl optionally substituted with one or more substituents independently
selected from
C1-C6 aliphatic, cyano, halo, halo-C1-C6 aliphatic-, aryl-Cl-C6 aliphatic-,
heteroaryl-Cl-C6
aliphatic-, aralkyloxy, di(C1-C6 aliphatic)amino-, -O-C1-C6 aliphatic, -S(O)-
C1-C6
aliphatic, or -S(O)2-C1-C6 aliphatic.
[0084] In one embodiment of formula I-A, at least one Rx is hydrogen and the
other Rx is a
C3-C7 cycloaliphatic or a heterocycloaliphatic ring optionally substituted
with up to five R3
substituents and having up to three heteroatoms selected from 0, N, or S,
wherein said ring is
optionally fused to one or more phenyl or heteroaryl rings.
[0085] In one embodiment of formula I-A, said Rx is selected from cyclopentyl,
cyclohexyl,
cyclohexenyl, cycloheptyl, tetrahydro-2H-pyranyl, tetrahydro-2H-thiopyranyl,
9H-fluoren-9-
yl or piperidinyl.
[0086] In one embodiment of formula I-A, - is a single bond, two RX, taken
together with
the carbon atom that they are attached to, form a 3-9 membered monocyclic, a 9-
14
membered bicyclic, or a 12-14 membered tricyclic aryl, heteroaryl or
heterocyclic ring
system wherein each heteroaryl or heterocyclic ring has up to 3 heteroatoms
selected from 0,
S, and N; wherein said ring system formed by two Rx is optionally substituted
with up to 5 R4
substituents.
[0087] In one embodiment of formula I-A, said ring system is selected from 9H-
fluroen-9-yl,
tetrahydro-2H-pyran-4-yl, tetrahydro-2H-thiopyran-4-yl, cyclobutyl,
cyclopentyl, cyclohexyl,
cycloheptyl, cyclohexenyl, piperidinyl, or 1-benzyl-piperidin-4-yl.
[00881 In one embodiment of formula I or I-A, ring A is selected from:
A A
D
SWA ~ ~ WAP
WE (WB) q (WB~q
``, ,~ ~
W~-N O N O
' I I
Ri Ri
A-i A-ii;
Page 17 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
.r~ i'z k i'"
A
(,V1/A P { WA P /a
W~ (W B )(WB) ~"F___TrN / Q B1N/ q
,Ri ,R'
0 0
A-iii or A-iv;
wherein:
p is 0-2;
q is 0-2; provided that p+q <2;
each of WA and WB is independently selected from NR', 0, S, SO, SOZ, C(R')Z,
or
=CR' (when p or q is 2);
WE is -C(R')2, =C(R')-, N-, or -N(R' )-;
WF is absent or is selected from -C(R')2, =C(R')-, =N-, or -N(R')-; provided
that both
of WE and Wr are not simultaneously =N- or -N(R')-;
ring B I is a phenyl or 5-6 membered heteroaryl ring optionally substituted
with up to
R' substituents; and
R' is as defined herein.
[0089] In another embodiment of formula I or I-A, ring A has formula A-i.
[0090] In one embodiment of formula I or I-A, ring A has formula A-ii.
[0091] In one embodiment of formula I or I-A, ring A has formula A-iii.
[0092] In one embodiment of formula I or I-A, ring A has formula A-iv.
10093] In one embodiment of formula I or I-A, both, WE and WF are =C(R').
[0094] In one embodiment of formula I or I-A, WE is =C(R')- and WF is =N-.
(0095] In one embodiment of formula I or I-A, p is 0 and q is 0.
[0096] In one embodiment of formula I or I-A, p is 1 and q is 0.
[0097] In one embodiment of formula I or I-A, p is 0 and q is 2.
[0098] In one embodiment of formula I or I-A, WA is NR'.
[0099] In one embodiment of formula I or I-A, WA is O.
[00100] In one embodiment of formula I or I-A, WA is C(R')2.
[00101] In one embodiment of formula I or I-A, WA is C(R')2 and R' is
hydrogen.
[00102] In one embodiment of formula I or I-A, WB is NR'.
[00103] In one embodiment of formula I or I-A, Wg is O.
1001041 In one embodiment of formula I or I-A, WB is C(R')2.
Page 18 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00105] In one embodiment of formula I or I-A, WB is C(R')2 and R' is
hydrogen.
[00106] In one embodiment of formula I or I=A, p is 2 and WA is C(RI)2-C(RI)2
or -
CR1=CRt-.
[00107] In one embodiment of formula I or I-A, q is 2 and WB is C(RI)Z-C(RI)2
or -
CR'=CR'-.
[00108] In one embodiment of formula I or I-A, ring A is selected from:
~ `'~.
CCA ~ ~ / CA
, C~ ~ A
I O ~ NH cLo Ii~ ~
O n1 O N ~O N ~~O
H H H
A-i-a A-i-b A-i-c A-i-d A-i-h
~ ~ ~ ~ ,, / ~`
,.~` i/~.. : . .~.~ ;'',/~
CA ~ , , ~ ,
~ NH HN O
H ~ NH O
N~O H
A-i-i A-i-j A-i-k or A-i-I;
wherein said ring is optionally substituted with up to 4 R' substituents.
[00109] In one embodiment of forniula I or I-A, ring A is selected from:
~ H
CO
/ /
'-, CN
C y
v ~~` A
H~O H ~ H~o
A-i-e A-i-f or A-i-g;
wherein said ring is optionally substituted with up to 4 R' substituents.
[00110] In one embodiment of formula I or I-A, ring A is selected from:
X 1/11/1 'k, 1/7-11 1)113 1/h,"
NH CA
CA C__O CA
N O N"~O \ N~O ~ N~O
H H H H
A-ii-a A-ii-b A-ii-c or A-ii-d;
wherein said ring system is optionally substituted with up to 4 R'
substituents.
[001111 In one embodiment of formula I or I-A, ring A is selected from:
Page 19 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
O N V CA ~
\ ~ CAs`s' C(N- CA CA ~ ~ I ~O
N0 O N~sO'` N
H H H H
A-ii-e A-ii-f A-ii-g A-ii-h
O
~ 'k ~ ~
N N O N N 'tO
H H
A-ii-i or A-ii-j;
wherein said ring system is optionally substituted with up to 4 R'
substituents.
[00112] In another embodiment of formula I or I-A, the compound is of formula
I-B:
O
(LRN"
A
R-__,'X N
~N_~~` 0
r{Y O
I-B;
wherein ring A is a 4-7 membered heterocyclic ring optionally fused with an
phenyl
or heteroaryl ring that is optionally substituted with up to 5 R'
substituents;
wherein said ring A, in addition to the nitrogen ring atom, contains up to two
additional ring heteroatoms selected from 0, N, or S;
wherein ring A, in addition to the oxo group, is optionally substituted with
up to 5 R'
substituents;
R', Rx, RY, and X are as defined herein.
[00113] In one embodiment of formula I-B, RY is C1-C6 aliphatic optionally
substituted
with one or more halo, OH, C1-C4 alkoxy, C 1-C4 alkoxy carbonyl, or di-(C 1-C4
alkyl)
amino-.
[00114] In one embodiment of formula I-B, RY is inethyl, ethyl, propyl,
isopropyl, butyl, t-
butyl, 3,3 -dimethyl -butyl, 3-methyl-butyl, 2-methyl-propyl, 2-1nethoxy-
ethyl, 3-_
ethoxypropyl, 1-(methoxy carbonyl)-3 -m ethyl -butyl, 1-(hydroxy methyl)-3-
methyl-butyl,
Page 20 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
allyl, acetenyl, 2-(diethylamino)ethyl, 1-methyl-2-methoxy-ethyl, 3-hydroxy-
2,2-dimethyl-
propyl, 2,2,2-trifluoroethyl, 3,3,3-trifluoro-propyl, or 2,2,3,3,3-pentafluoro-
propyl.
[00115) In one embodiment of formula I-B, RY is methyl, ethyl, propyl,
isopropyl, butyl, t-
butyl, 3,3-dimethyl-butyl, 3-methyl-butyl or 2-methyl-propyl.
[001161 In one embodiment of formula I-B, RY is C3-C8 cycloaliphatic or a C3-
C8
cycloaliphatic substituted C1-C6 aliphatic-.
[00117] In one embodiment of formula I-B, RY is C3-C6 cycloalkyl or C3-C6
cycloalkyl
substituted C1-C6 alkyl-.
[001181 In one embodiment of formula I-B, RY is cyclopropyl, cyclohexyl,
cyclohexylmethyl-, cyclopropylmethyl-, or cyclohexylethyl-.
[00119] In one embodiment of formula I-B, RY is pyridyl (C1-C6) alkyl-,
tetrahydrofuranyl (C1-C6 alkyl)-, N-(C1-C4 alkyl)-pyrrolidinyl-(C1-C6 alkyl)-.
[001201 In one embodiment of formula I-B, RY is tetrahydrofuran-2-yl-methyl-,
pyridin-3-
yl-methyl-, pyridin-4-yl-ethyl-, pyridin-2-yl-ethyl-, pyridin-4-yl-methyl-, 1H-
indazol-5-yl, or
2-(N-methyl)-pyrrolidin-2-yl-ethyl-.
[001211 In one embodiment of formula I-B, RY is phenyl or (phenyl)-substituted
C1-C6
aliphatic each optionally substituted with up to 5 R2 substituents
independently selected from
halogen or a 5-6 membered heterocyclyl ring having 1-3 heteroatoms selected
from N, 0, or
S.
[00122] In one embodiment of formula I-B, RY is phenyl, 2,6-difluorophenyl,
benzyl, 4-
fluorophenylmethyl-, 4-morpholinophenyl-, 2-piperidinylphenyl- or phenylethyl-
.
[001231 In one embodiment of formula I-B, Rx is an aryl or heteroaryl ring
optionally
substituted witli up to 5 R3 substituents independently selected from C1-C6
aliphatic, phenyl,
halogen, C3-C6 cycloaliphatic or a 4-7 membered heterocyclic ring with up to 3
Ru
substituents wherein said heteroaryl or heterocyclic ring has up to three
heteroatoms selected
from N, 0, or S.
[00124] In one embodiment of formula I-B, Rx is phenyl or pyridyl with up to 2
R3
substituents independently selected from halogen or a 4-7 membered
heterocyclic ring
wherein said heterocyclic ring is optionally substituted with up to 2 Ru
substituents wherein
said heterocyclic ring has up to three heteroatoms selected from N, 0, or S.
1001251 In one embodiment of formula I-B, Rx is phenyl substituted with a 4-7
membered
heterocyclic ring in the 2 position and a halogen in the 3 position.
[001261 In one embodiment of formula I-B, RX is pyridyl, phenyl, or phenyl
substituted
with piperazine, 4-methyl-piperazin-l--yl, 4-ethyl-piperazin- I yl, 4-propyl-
piperazin-1yl, 4-
Page 21 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
butyl-piperazin-lyl, 4-isopropyl-piperazin-1yl, 4-t-butylpiperazin-1y1, 4-
cyclopropylpiperazin-l-yl, 4-t-butoxycarbonyl-piperazin-1-yl, 4-hydroxy-
piperidinyl, 4-
ethoxycarbonyl-piperidin-l-yl, morpholin-4-yl, 1-HHpyrazol-1-yl, imidazol-1-
yl, pyrrolidin-
1-yl, 3-dimethylamino-pyrrolidin-l-yl, 4-(piperidin-l-yl)piperidine, pyridyl
(1-methylpiperidin-4-yl)piperazin- I -yi, or 1-(2,2,2-trifluoroethyl)piperazin-
l-yl.
[00127] In one embodiment of formula I-B, Rx is phenyl or heteroaryl
optionally
substituted with one or more substituents independently selected from Cl -C6
aliphatic,
cyano, halo, halo-C1-C6 aliphatic-, aryl-Cl-C6 aliphatic-, heteroaryl-C1-C6
aliphatic-,
aralkyloxy, di(C1-C6 aliphatic)amino-, -0-C1-C6 aliphatic, -S(O)-C1-C6
aliphatic, or
-S(0)2-C1-C6 aliphatic.
[001281 In one embodiment of formula I-B, RX is a C3-C7 cycloaliphatic or a
heterocycloaliphatic ring optionally substituted with up to five R3
substituents and having up
to three heteroatoms selected from 0, N, or S, wherein said ring is optionally
fused to one or
more phenyl or heteroaryl rings.
[00129] In one embodiment of formula I-B, said fused ring is selected from
cyclopentyl,
cyclohexyl, cyclohexenyl, cycloheptyl, tetrahydro-2H-pyranyl, tetrahydro-2H-
thiopyranyl,
9H-fluoren-9-yl or piperidinyl.
[00130] In one embodiment of formula I-B, ring A is selected from:
c
WE~W W ~! ~ r ~z
WpF
(1 /
N
WN/Y
R1 R1
A-v or A-vi;
wherein:
Wc is -C(Rl)2, C(O), or =CRl-;
r is 0-2;
WD is N or =C-;
WE is -C(R')2, =C(R')-, =N-, or -N(R')-;
WF is absent or is selected from -C(R')2, =C(R')-, =N-, or -N(R)-; provided
that=both
of WE and WF are not siinultaneously =N- or -N(Rl)-;
Y is C(O), S(O), or S(O)2;
ring B 1 is a phenyl or 5-6 membered heteroaryl ring optionally substituted
with up to
Ri substituents; and =- is a single or a double bond;
Page 22 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
R' is as defined herein. ..
[001311 In one embodiment of formula I-B, W' is -C(R')2.
[00132] In another embodiment of formula I-B, Wc is =CR'-.
[00133] In one embodiment of formula I-B, Wc is C(O).
.[00134] In one embodiment of formula I-B, r is 0.
[00135) In one embodiment of formula I-B, r is 1.
[00136] In one embodiment of formula I-B, r is 2.
[00137] In one embodiment of formula I-B, WD is N.
[001381 In one embodiment of formula I-B, WD is -C-.
[00139] In one embodiment of formula I-B, Y is C(O).
[001401 In one embodiment of formula I-B, Y is S(O).
[00141] In one embodiment of formula I-B, Y is S(O)2.
[00142] In one embodiment of formula I-B, ring A is selected from:
O
Ni~z a NA Ni~
C CS 2 ~
H O H NO H H O
A-v-a A-v-b A-v-c A-v-d
N~O c ~NO ~NO ;>=
A-v-e A-v-f A-v-g A-v-h
N/~ N/~
' \H O H~O N ~O
N
H
A-v-i A-v-j or A-v-k;
wherein said ring is optionally substituted with up to 4 R' substituents.
[00143] In one embodiment of formula I-B, ring A is selected from:
Page 23 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
O
~x
N N N
~ s0
H 0 H 0 \ H 2 H
A-vi-a A-vi-b A-vi-c A-vi-d
~1,.
~ r~
3>= 1j_-zo
H O N H
A-vi-e A-vi-f A-vi-g A-vi-h
C H
or A-vi-I;
wherein said ring is optionally substituted with up to 4 R' substituents.
[00144] In one embodiment of formula I-B, ring A is optionally substituted
with up to 5
substituents selected from C1-C6 aliphatic, Cl-C6 aliphatic-oxy, Cl-C6
haloaliphatic, CN,
halo, oxo, optionally substituted C3-C7 cycloaliphatic, or an optionally
substituted ring
selected from phenyl, furanyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imadazolyl,
imidazolinyl,
imidazolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, pyridyl, pyrimidinyl,
piperidinyl,
piperazinyl, or morpholinyl.
[00145] In one embodiment of formula I-B, in R', Q is a bond.
[00146] In one embodiment of formula I-B, in R', Q-R"' is Q-R'.
[00147] In one embodiment of formula I-B, Q is present and R is hydrogen.
[00148] In one embodiment of formula I-B, Q is present and R is Cl-C6
aliphatic.
[00149] In one embodiment of formula I-B, R is methyl, ethyl, propyl, or
butyl.
(00150] In one embodiment of formula I-B, R' is hydrogen.
[00151] In one embodiment of formula I-B, R' is a C1-C8 aliphatic group,
optionally
substituted with up to 3 substituents selected from halo, CN, CF3, CHF2, OCF3,
or OCHF2,
wherein up to two methylene units of said C1-C8 aliphatic is optionally
replaced with -CO-,
-CONH(C 1-C4 alkyl)-, -COz-, -OCO-, -N(C l-C4 alkyl)C02-, -0-, -N(C 1-C4
alkyl)CON(C 1-
C4 alkyl)-, -OCON(C1-C4 alkyl)-, -N(Cl-C4 alkyl)CO-, -S-, -N(C1-C4 alkyl)-, -
SOaN(C1-
C4 alkyl)-, N(C 1-C4 alkyl)SOz.-, or -N(C I -C4 alkyl)SO2N(C1-C4 alkyl)-.
Page 24 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00152] In one embodiment of formula I-B, R' is a 3-8 membered saturated,
partially
unsaturated, or fully unsaturated monocyclic ring having 0-3 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur, wherein R' is optionally
substituted with up to 3
substituents selected from halo, CN, CF3, CHF2, OCF3, OCHF2, or C1-C6 alkyl,
wherein up
to two methylene units of said C1-C6 alkyl is optionally replaced with -CO-, -
CONH(C1-C4
alkyl)-, -C02-, -OCO-, -N(C1-C4 alkyl)C02-, -0-, -N(C1-C4 alkyl)CON(C1-C4
alkyl)-,
-OCON(C1-C4 alkyl)-, -N(C1-C4 alkyl)CO-, -S-, -N(C1-C4 alkyl)-, -SO2N(Cl-C4
alkyl)-,
N(C1-C4 alkyl)S02-, or -N(C1-C4 alkyl)SO2N(C1-C4 alkyl)-.
[00153) In one embodiment of formula I-B, R' is an 8-12 membered saturated,
partially
unsaturated, or fully unsaturated bicyclic ring system having 0-5 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur; wherein R' is optionally
substituted with up to 3
substituents selected from halo, CN, CF3, CHF2, OCF3, OCHF2, or C1-C6 alkyl,
wherein up
to two methylene units of said Cl-C6 alkyl is optionally replaced with -CO-, -
CONH(CI-C4
alkyl)-, -C02-, -OCO-, -N(C1-C4 alkyl)C02-, -0-, -N(C1-C4 alkyl)CON(C1-C4
alkyl)-,
-OCON(C1-C4 alkyl)-, -N(C1-C4 alkyl)CO-, -S-, -N(Cl-C4 alkyl)-, -SO2N(C1-C4
alkyl)-,
N(C1-C4 alkyl)SOZ-, or -N(C1-C4 alkyl)SO2N(C1-C4 alkyl)-.
[00154] In one embodiment of formula I-B, two occurrences of R' are taken
together with
the atom(s) to which they are bound to form an optionally substituted 3-12
membered
saturated, partially unsaturated, or fully unsaturated monocyclic or bicyclic
ring having 0-4
heteroatoms independently selected from nitrogen, oxygen, or sulfur, wherein
R' is optionally
substituted with up to 3 substituents selected from halo, CN, CF3, CHF2, OCF3,
OCHF2, or
C1-C6 alkyl, wherein up to two methylene units of said Cl-C6 alkyl is
optionally replaced
with -CO-, -CONH(C1-C4 alkyl)-, -C02-, -OCO-, -N(CI-C4 alkyl)C02-, -0-, -N(C1-
C4
alkyl)CON(C'1-C4 alkyl)-, -OCON(C1-C4 alkyl)-, -N(C1-C4 alkyl)CO-, -S-, -N(C1-
C4
alkyl)-, -SO2N(C1-C4 alkyl)-, N(C1-C4 alkyl)S02-, or -N(C1-C4 alkyl)SO2N(C1-C4
alkyl)-.
[001551 In one embodiment, compounds of the present invention include those in
Table I
and Table 1 A.
[001561 In another embodiment, compounds of the present invention include
those in
Table 1.
[00157] In another embodiment, compounds of the present invention include
those in
Table lA.
[00158] In another embodiment, compounds of the present invention include
those in
Table IA and Table I except for compound numbers 85, 97, and 105.
Page 25 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00159] In another embodiment, compounds of the present invention include
those in
Table 1 except for compound numbers 85, 97, and 105.
[001601 In one embodiment, the present invention provides compounds of formula
I':
"'C~A.
Rz Rw W m "Z"
R
Rx B
RX-~X N
/N O n
RY
I, .
wherein:
X is S, SO, or SO2;
Z is present or absent;
wherein:
when Z is present, then ring A is attached to ring B through a single bond;
when Z is absent, then ring A together with ring B forms a spirocyclic ring
system;
ring A is a 4-7 membered heterocyclic or heteroaryl ring or a 10-14 membered
bicyclic heterocyclic ring, wherein ring A has 1-4 heteroatoms selected from
0, N, or S;
wherein ring A is optionally substituted with up to 5 R' substituents;
m is 1-3;
n is 1-3; provided that m+n is --54;
RY is aryl, heteroaryl, cycloaliphatic, CI-C6 aliphatic, aryl-aliphatic, or
cycloaliphatic-aliphatic; wherein RY is optionally substituted with up to 5 R2
substituents;
Rx is hydrogen, halo, aryl, heteroaryl, CI-C6 aliphatic, aryl-C1-C6 aliphatic,
heteroaryl-C1-C6 aliphatic, whereiti Rx is optionally substituted with up to 5
R3 substituents;
or two RX, taken together with the carbon atom that they are attached to, form
a 3-9
membered cycloaliphatic or heterocyclic ring, wherein said heterocyclic ring
has up to 3
heteroatoms selected from 0, S, and N; wherein said ring is optionally
substituted with up to
3 R3 substituents;
wherein said ring formed by two RX is optionally substituted with up to 5 R4
substituents;
Page 26 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
RZ is absent, hydrogen, CN, C 1-C6 aliphatic, halo-C 1-C6 aliphatic, O-C 1-C6
aliphatic, O-(halo-C 1-C6 aliphatic), halo, aryl-Cl -C6 aliphatic, or
heteroaryl-C 1-C6
aliphatic;
- is a single or a double bond; provided that when it is a double bond, then
RZ
and one of RW is absent;
Rw is independently hydrogen, halo, oxo, C1-C6 aliphatic, halo-C1-C6
aliphatic, 0-
C1-C6 aliphatic, O-(halo-Cl-C6 aliphatic), aryl, aryl-Cl-C6 aliphatic, C3-C7
cycloaliphatic;
or
two Rw taken together form an optionally substituted C3-C7 cycloaliphatic or
heterocyclic ring, wherein said heterocyclic ring has up to 3 heteroatoms
selected from 0, S,
and N; wherein said ring formed by two RW is optionally substituted with up to
5 R5
substituents;
wherein each occurrence of R', R2, R3, R4, and RS is independently Q-Rm;
wherein Q is a bond or is a CI-C6 aliphatic chain wherein up to two non-
adjacent
methylene units of Q are optionally replaced by CO, C02, COCO, CONR, OCONR,
NRNR,
NRNRCO, NRCO, NRCO2, NRCONR, SO, SO2, NRSO2, SO2NR, NRSO2NR, 0, S, or NR;
wherein each occurrence of RM is independently selected from R', halogen, NOz,
CN,
OR', SR', N(R')2, NR'C(O)R', NR'C(O)N(R')Z, NR'COaR', C(O)R', COZR', OC(O)R',
C(O)N(R')2, OC(O)N(R')2, SOR', SOZR', SOzN(R')Z, NR'SOZR', NR'SO2N(R')2,
C(O)C(O)R', or C(O)CH2C(O)R', wherein each occurrence of R is independently
selected
from hydrogen or an optionally substituted CI_6 aliphatic group;
wherein each occurrence of R' is independently selected from hydrogen or an
optionally substituted group selected from CI_$ aliphatic, C6_1o aryl, a
heteroaryl ring having
5-10 ring atoms, or a heterocyclyl ring having 3-10 ring atoms, or wherein R
and R' taken
together with the atom(s) to which they are bound, or two occurrences of R'
taken together
with the atom(s) to which they are bound, form a 5-8 membered cycloalkyl,
heterocyclyl,
aryl, or heteroaryl ring having 0-3 heteroatoms independently selected from
nitrogen, oxygen,
or sulfur.
[00161] In one embodiment, - is a double bond and Rz and one of RW is absent;
[00162] In another embodiment, - is a single bond. In another embodiment, one
or
RW is hydrogen and the other is not. In another embodiment, both of Rw are
hydrogen.
[00163] In one embodiment, m is I and n is 1. In another embodiment, m is I
and n is
2. Or,mis2andnisl. Or,mis2andnis2.
Page 27 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00164] In another embodiment, Rz.is C1-C6 alkyl or halo.-C1-C6 alkyl. Or, RZ
is -O-
C1-C6 alkyl. Exemplary Rz include fluoro, methyl, ethyl, n-propyl, CF3, CHF2,
OMe, OEt,
etc.
[00165] In another embodiment, Rw is Cl-C6 alkyl or halo-Cl-C6 alkyl. Or, Rw
is -
O-C1-C6 alkyl. Exemplary Rw include fluoro, methyl, ethyl, n-propyl, CF3,
CHF2, OMe,
OEt, etc.
[00166] In another embodiment, two Rw, taken together with the carbon atom
they are
attached to, form an optionally substituted C3-C9 cycloalkyl or a 3-9 membered
heterocyclyl
ring. Exemplary such rings include cyclopropyl, cyclopentyl, or cyclohexyl.
[00167] In one embodiment, RY is C 1-C6 aliphatic optionally substituted with
one or
more halo, OH, C1-C4 alkoxy, C1-C4 alkoxy carbonyl, or di-(Cl-C4 alkyl) amino.
Exemplary embodiments include methyl, ethyl, propyl, isopropyl, butyl, t-
butyl, 3,3-
dimethyl-butyl, 3-methyl-butyl, 2-methyl-propyl, 2-methoxy-ethyl, 3-
ethoxypropyl, 1-
(methoxy carbonyl)-3-rnethyl-butyl, 1 -(hydroxy methyl)-3 -methyl-butyl,
allyl, acetenyl, 2-
(diethylamino)ethyl, 1-methyl-2-methoxy-ethyl, 3-hydroxy-2,2-dimethyl-propyl,
2,2,2-
trifluoroethyl, 3,3,3-trifluoro-propyl, or 2,2,3,3,3-pentafluoro-propyl.
[00168] In another embodiment, RY is C3-C8 cycloaliphatic or C3-C8
cycloaliphatic
substituted C1-C6 aliphatic. In one embodiment, Ry is C3-C6 cycloalkyl or C3-
C6
cycloalkyl substituted Cl-C6 alkyl. Exemplary embodiments include cyclopropyl,
cyclohexyl, cyclohexylmethyl, cyclopropylmethyl, or cyclohexylethyl.
[00169] In another embodiment, RY is pyridyl (C1-C6) alkyl, tetrahydrofuranyl
(C1-C6
alkyl), N-(C1-C4 alkyl)-pyrrolidinyl-(C1-C6 alkyl). Exemplary embodiments
include
tetrahydrof-uran-2-ylmethyl, pyridin-3-yl-methyl, pyridin-4-yl-ethyl, pyridin-
2-yl-ethyl,
pyridin-4-yl-methyl, I H-indazol-5-yl, or 2-(N-methyl)-pyrrolidin-2-yl-ethyl.
[00170] In another embodiment, RY is optionally substituted phenyl or
(optionally
substituted phenyl)-substituted C1-C6 aliphatic. Exemplary embodiments include
phenyl,
2,6-difluorophenyl, benzyl, 4-fluorophenyhnethyl, or phenylethyl.
[00171] In one embodiment, both Rx are hydrogen.
[00172] In one embodiment, Rx is a phenyl or a heteroaryl, such as pyridyl,
wherein
said phenyl or heteroaryl is optionally substituted with an optionally
substituted 3-7
membered heterocyclic or heteroaryl ring having up to three heteroatoms
selected from 0, S,
or N. Exemplary Rx include phenyl, pyridyl, or phenyl substituted with
piperazine, 4-
methyl-piperazin-1-yl, 4-t-butoxycarbonyl-piperazin-1-yl, 4-hydroxy-
piperidinyl, 4-
ethoxycarbonyl-piperidin-l-yl, morpholin-4-yl, 1-HHpyrazol-l-yl, imidazol-I-yl
or pyridyl.
Page 28 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00173] In another embodiment, Rx is phenyl or heteroaryl optionally
substituted with
one or more substituents independently selected from C1-C6 aliphatic, cyano,
halo, halo-Cl-
C6 aliphatic, aryl-Cl-C6 aliphatic, heteroaryl-C1-C6 aliphatic, aralkyloxy, di
(C1-C6
aliphatic)amino, O-Cl-C6 aliphatic, S(O)-Cl-C6 aliphatic, or S(O)2-C1-C6
aliphatic.
[00174] In another embodiment, Rx is an optionally substituted C3-C7
cycloaliphatic
or a heterocycloaliphatic ring having up to three heteroatoms selected from 0,
N, or S,
wherein said ring is optionally fused to one or more phenyl or heteroaryl
ring. Exemplary
rings include cyclopentyl, cyclohexyl, cyclohexenyl, cycloheptyl, tetrahydro-
2F1 pyranyl,
tetrahydro-2H-thiopyranyl, 9H-fluoren-9-yl, piperidinyl, etc.
[001751 In another embodiment, two RX, taken together with the carbon atom
that they
are attached to, form an optionally substituted 3-9 membered cycloaliphatic or
heterocyclic,
monocyclic, bicyclic, or tricyclic ring. Exemplary embodiments include 9H-
fluroen-9-yl,
tetrahydro-2H-pyran-4-yl, tetrahydro-2H-thiopyran-4-yl, cyclobutyl,
cyclopentyl, cyclohexyl,
cycloheptyl, cyclohexenyl, piperidinyl, or 1-benzyl-piperidin-4-yl.
[001761 In another embodiment, the present invention provides compounds of
formula
O
(LNR
RZ Rw Rw A
RRX C,a
X --~-X N,,)
= _--
RY N O
wherein:
ring A is a 4-7 membered heterocyclic ring that forms a spirocyclic ring
system with
said piperidine ring through carbon atom CA, wherein said heterocyclic ring is
optionally
fused with an optionally substituted phenyl or heteroaryl ring;
wherein said ring A, in addition to the nitrogen ring atom, up to two
additional ring
heteroatoms selected from 0, N, or S;
wherein ring A, in addition to the oxo group, is optionally substituted with
up to 5 R~
substituents;
R~, RX, RY, Rz, Rw, and X are as defined above.
[00177] In one embodiment, - is a double bond and RZ and one of Rw is absent;
[00178] In another embodiment, - is a single bond.
Page29of153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00179] In another embodiment, RZ is C1-C6 alkyl or halo-Cl-C6 alkyl. Or, Rz
is -0-
Cl-C6 alkyl. Exemplary Rz include methyl, ethyl, n-propyl, CF3, CHF2, OMe,
OEt, etc.
[00180] In another embodiment, Rw is C1-C6 alkyl or halo-Cl-C6 alkyl. Or, RW
is -
O-Cl-C6 alkyl. Exemplary Rw include methyl, ethyl, n-propyl, CF3, CHF2, OMe,
OEt, etc.
[00181] In another embodiment, two Rw, taken together with the carbon atom
they are
attached to, form an optionally substituted C3-C9 cycloalkyl or a 3-9 membered
heterocyclyl
ring. Exemplary such rings include cyclopropyl, cyclopentyl, or cyclohexyl.
[00182] In one embodiment, ring A is selected from:
i ~. k :4-
~ A= / -- A
~WA P CC B)
a l WA p C( B) a
~
T'_4B1
F-N O N O
I I
R' R1
A-i A-ii;
~
Ii''+iz-
A A
(A)-' ('N ~'"C
~ C 8) A p w
V1/E ~
q \ /= q
B 1
WF--_ir N, R' N`R'
0
O
A-iii or A-iv;
wherein:
p is 0-2;
q is 0-2; provided that p+q <?;
each of WA and WB is independently selected from NR', 0, S, SO, S02, C(R')Z,
or
=CR' (when p or q is 2);-
WE is -C(R')2, =C(R')-, =N-, or -N(R')-;
WF is absent or is selected from -C(R')2, =C(R')-, =N-, or -N(R')-; provided
that both
of WE and WF are not simultaneously =N- or -N(R')-;
ring BI is an optionally substituted phenyl or 5-6 membered heteroaryl ring;
R' is as defined above.
[00183] In one embodiment, ring A has formula A-i. In another embodiment, ring
A
has formula A-ii. Or, ring A has formula A-iii. Or, ring A has formula A-iv.
Page 30 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00184] In one embodiment, both, WE and WF are =C(R'). In another embodiment,
WE.1S =C(Rl)- and WF is =N-.
[00185] In one embodiment, p is 0 and q is 0. In another embodiment, p is 1
and q is
0. In another embodiment, p is 0 and q is 1. In yet another embodiment, both p
and q are 1.
Or, p is 0 and q is 2. Or,pis2 andqis0.
[00186] In one embodiment, WA is NR1. In another embodiment, WA is O. Or, WA
is
C(R')2. In one einbodiment Rl is hydrogen.
[00187] In one embodiment, W~ is NR': In another embodiment, WB is O. Or,-WB
is .. .
C(R])2. In one embodiment R' is hydrogen.
[00188] In another embodiment, p is 2 and WA is C(R')2-C(R')2 or -CR'=CR'-.
[00189] In another embodiment, q is 2 and WB is C(RI)2-C(R1)2 or -CR1=CR!-.
[001901. In one embodiment, ring A is selected from:
(CA CA CA
O NH cc ~O O C~H H H H H
A-i-a A-i-b A-i-c A-i-d A-i-h
~ ~^. i'1s
~ ~ i ~
N_ A A
CA ~NH CNH HN y O
H
\ N ~O
H ~ ~ 0
A-i-i . A-i-j A-i-k or A-i-l;
wherein said ring is optionally substituted with up to 4 Ri substituents.
[00191] In another embodiment, ring A is selected from:
O : L H
~ NA~-
I CA~~ I CA C C
-~ -K ~
H ~ H H. O
A-i-e A-i-f or A-i-g;
wherein said ring is optionally substituted with up to 4 R' substituents.
1001921 In another embodiinent, ring A is selected from:
Page 31 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
~ i ~ i
~
C'A CA C'4
I \O \NH CA
N O N'k-O N--tz'--O N~O
H H H H
A-ii-a A-ii-b A-ii-c or A-ii-d;
wherein said ring system is optionally substituted with up to 4 Rl
substituents.
[00193] In another embodiment, ring A is selected from:
0 CA
N
NO N N N/}c
OCNLO CA~` CA/ ~ I \ 0
H H H H
A-ii-e A-ii-f A-ii-g A-ii-h
~.. ~i `~
OCA ~ rN C A N N~O N ~O
H H
A-ii-i or A-ii-j;
wherein said ring system is optionally substituted with up to 4 R'
substituents.
[001941 In another embodiment, the compounds of the present invention have
formula
O
R'
RXX N B 4A)
N_Z
0
O .
1'-B;
wherein said ring A, in addition to the nitrogen ring atom, contains up to two
additional ring heteroatoms selected from 0, N, or S;
wherein ring A, in addition to the oxo group, is optionally substituted with
up to 5 R,
substituents;
R', Rx, RY, and X are as defined above.
[00195] In one embodiment, ring A is selected from:
Page 32 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
(_WC .
WE W W~
wD.
F B1 '
WN__-Y N--Y
A-v or A-vi;
wherein:
W'- is -C(R')Z, C(O), or =CR'-;
r is 0-2;
W is N or =C-;
WE is -C(R')z, =C(R')-, N-, or -N(R')-;
WF is absent or is selected from -C(R')2, =C(R')-, =N-, or -N(R')-; provided
that both
of WE and WF are not simultaneously. N- or -N(R')-;
Y is C(O), S(O), or S(O)2;
ring BI is an optionally substituted phenyl or a heteroaryl ring;
--- is a single or a double bond;
R' is as defined above.
[00196] In one embodiment, Wc is -C(R')2. Or, WC is =CR'-. Or, Wc is C(O).
[00197] In one embodiment, r is 0. Or, r is 1. Or, r is 2.
[00198] In another embodiment, W is N. Or, W is =C-_
[00199] ln one embodiment, Y is C(O). Or, Y is S(O). Or, Y is S(O)2.
[00200j In one einbodiinent, ring A is selected from:
O
N~~ N~x.
I
C aO ft02
N~O N
N~O
H H H H
A-v-a A-v-b A-v-c A-v-d
N~O CN ~N~O O
H H H 0 TH
A-v-e A-v-f A-v-g A-v-h
Page 33 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
~ ciN ~ a ~ o . vO
A-v-i A-v-j or A-v-k;
wherein said ring is optionally substituted with up to 4 R' substituents.
[00201] In one embodiment, ring A is selected from:
O
N~~4 / I \ . N~~ N
SO
! ~ \ I ~
H O \ H O Hi 2 H
A-vi-a A-vi-b A-vi-c A-vi-d
N oc., N N~O
N ~HH~O N
H
A-vi-e A-vi-f A-vi-g A-vi-h or
aNN" O
H
A-vi-I;
wherein said ring is optionally substituted with up to 4 R' substituents.
[00202] In one embodiment, ring A is optionally substituted with up to 5
substituents
selected from Cl-C6 aliphatic, Cl-C6 aliphatic-oxy, Cl-C6 haloaliphatic, CN,
halo, oxo,
optionally substituted C3-C7 cycloaliphatic, or an optionally substituted ring
selected from
phenyl, furanyl, pyrrolyl, pyrrolinyl, pyrrolidinyl, imadazolyl, imidazolinyl,
imidazolidinyl,
pyrazolyl, pyrazolinyl, pyrazolidinyl, pyridyl, pyrimidinyl, piperidinyl,
piperazinyl, or
morpholinyl.
[00203] In one embodiment Q is absent. In another embodiment, Q-RM is R'.
[00204] In one embodiment, R is hydrogen. Or, R is C1-C6 aliphatic. Exemplary
R
includes CI-C6 alkyl, e.g., methyl, ethyl, propyl, or butyl.
[00205] In one embodiment, R' is hydrogen.
[00206] In one embodiment, R' is a Cl-C8 aliphatic group,
optionally'substituted with up
to 3 substituents selected from halo, CN, CF3, CHF2, OCF3, or OCHF2, wherein
up to two
Page 34 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
methylene units of said Cl-C8 aliphatic is optionally replaced with -CO-, -
CONH(C1-C4
alkyl)-, -C02-, -OCO-, -N(C1-C4 alkyl)CO2-, -0-, -N(C1-C4 alkyl)CON(C1-C4
alkyl)-,
-OCON(Cl-C4 alkyl)-, -N(C1-C4 alkyl)CO-, -S-, -N(Cl-C4 alkyl)-, -SO2N(Cl-C4
alkyl)-,
N(Cl-C4 alkyl)SO2-, or -N(C1-C4 alkyl)SO2N(Cl-C4 alkyl)-.
[00207] In one embodiment, R' is a 3-8 membered saturated, partially
unsaturated, or fully
unsaturated monocyclic ring having 0-3 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur, wherein R' is optionally substituted with up to 3
substituents selected from
halo, CN, CF3, CHF2, OCF3, OCHF2, or C1-C6 alkyl, wherein up to two methylene
units of
said Cl-C6 alkyl is optionally replaced with -CO-, -CONH(Cl-C4 alkyl)-, -CO2-,
-OCO-,
-N(C1-C4 alkyl)C02-, -0-, -N(C1-C4 alkyl)CON(Cl-C4 alkyl)-, -OCON(C1-C4 alkyl)-
,
-N(C1-C4 alkyl)CO-, -S-, -N(Cl-C4 alkyl)-, -SO2N(C1-C4 alkyl)-, N(C1-C4
alkyl)S02-, or -
N(C1-C4 alkyl)SO2N(Cl-C4 alkyl)-.
[00208] In one embodiment, R' is an 8-12 membered saturated, partially
unsaturated, or
fully unsaturated bicyclic ring system having 0-5 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur; wherein R' is optionally substituted with up to 3
substituents
selected from halo, CN, CF3, CHF2, OCF3, OCHF2, or Cl-C6 alkyl, wherein up to
two
methylene units of said Cl-C6 alkyl is optionally replaced with -CO-, -CONH(C1-
C4
alkyl)-, -C02-, -OCO-, -N(C1-C4 alkyl)C02-, -0-, -N(C1-C4 alkyl)CON(C1-C4
alkyl)-,
-OCON(C 1-C4 alkyl)-, -N(C 1-C4 alkyl)CO-, -S-, -N(C 1-C4 alkyl)-, -SO2N(C 1-
C4 alkyl)-,
N(C1-C4 alkyl)SOa-, or -N(Cl-C4 alkyl)SO2N(C1-C4 alkyl)-.
[002091 In one embodiment, two occurrences of R' are taken together with the
atom(s) to
which they are bound to form an optionally substituted 3-12 membered
saturated, partially
unsaturated, or fully unsaturated monocyclic or bicyclic ring having 0-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, wherein R' is
optionally substituted
with up to 3 substituents selected from halo, CN, CF3, CHF2, OCF3, OCHF2, or C
1-C6 alkyl,
wherein up to two methylene units of said Cl -C6 alkyl is optionally replaced
with -CO-, -
CONH(C 1-C4 alkyl)-, -COa-, -OCO-, -N(C 1-C4 alkyl)C02-, -0-, -N(C 1-C4
alkyl)CON(C 1-
C4 alkyl)-, -OCON(C1-C4 alkyl)-, -N(C1-C4 alkyl)CO-, -S-, -N(C1-C4 alkyl)-, -
SO2N(CI-
C4 alkyl)-, N(Cl-C4 alkyl)S02-, or-N(C1-C4 alkyl)SO2N(C1-C4 alkyl)-.
[00210] Exemplary compounds of the present invention are shown in Table I and
Table
lA below.
Table 1:
Page 35 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
1 2 3
ICN
N p S N
tJ
~ U10
S N
Q N~ d ~
O
p
H '
4 5 6
p 5
l?~p
N,,~,~o,, .,
N O
VO
N S
N S
O
N N O N
0 ~
o:`N
H
7 8 9
1 ~
i ~
~J s rJ s N~
0
0 ~cl)o O NaN N
~O ~O N
~ r'y-i ,~ P}i p~=,N ~ .~
H
11 12
p Nl N^~'~
/ S ~
pp UO ~ S .
N
Q O
N-r- O N~--O tJ I
NH pJ, N
H
Page 36 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
13 14 15
F
F t r ~ ,~ ~ \ f
S s N
O
!-~ N
o a S. )(
0 ~J ~s
N i tJ
~O HN~N N
16 17 18
q Fr0F eoo ~ A. `~N` F ~\s
N O N ~"U ^
a "
}FO N N
O--~'N I~ OPL=N
H H
19 20 21
~
tJ o S-'J ~ g N
Q Ns ~O ~O
~~ $ a
'N N
O O
~ ~ . =h~t ~ ~
~ 0+
22 .23 24
F
F
F
N H ~. t S rl ~ 5 tJ ~
O 5 j~N 1 ~a UO
LNJ
~-N` t ~ C )
(> ~/] -a 0
CN
H~O
Page 37 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
25 26 27
0
F
, rN S 0
N O
0 N Y O
N S
~10 N
28 29 30
N \
N S
4
R
t,00 N EJ S Q p S O O N
N'F~,O N +
d=H -
32 33 ~
Ci
o rJ o ~
s~s ~s N s
O EJ O O O ON
NxrJH aNANH N
ON
34 35 36
~ ~ F F F
~'Nt `~ F F
rN/ s rJ~ F F
a E=J
o S
o m o
N"p- o
'}i
H
H
Page 38 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
737 38 39
S g N N.= t g N
~010 ~1O ~100
N
O NFlO NIiO
40 41 42
i~~ S PJ~ ~ C3 S N
O y
~NJ~S O UO
yil O ti
fl ma
!~ N;=p
0%N' NH
N
43 44 46
F . N ~
N_ 1~ 0 1
~ ./ .
O S
`O
O O S
S N
O t14,'3'~yI`N
N v;, N
H &'Ilfo ~ O
46 47 48
F
~ / T'H S ~J
S O $ R ~
N
N N F:;O
N ItH
Q H N N~o
H
Page 39 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
49 50 51
S NN O
6N
ks
~ " OlN
~ O N
~Nit,, O N
ON
H H
52 53 54
F
~ N` ^ ~
~N f S N `~ \ ~~O S 'N~
'r
~Ioo O
r'; NP~ s n~i o ri} 'FZ-'o
e12
b'N' N-I
55 56 57
Q
1 ~ c N O S O~s
s
0 I N o r~' 1 ;+' 0
N .,. ~,J=,N ~ `..,~N~r~
O~~H
H
58 59 60
ra
X 14 ~J
~s
N r~ s
~ N
N rl ~
O rd
N
~=o
N H H
Page 40 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
61 62 63
F
tv Nt~FO
S'
~tJ~
0
~N
O
O aN
P! N ~ O ! \ ~ oi~ pN
H
64 65 66
/ = i ~ F
N.ko ttF
"Q
to S'
N
N N
d ~ o tJ
g4 N~ ~N
N
NH H
67 68 69.
N`( ~
CI ~
~S S
N N p~~
N N
~ O N
H ~=o
H N rH
70 71 72
q-,
O...J~
a J S C t ~
o
O N
Q s
4
tJ S L~
PJ
N
~Q 0~1
H
Page 41 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
73 74 75
s N 0
to 1S
O N Q Q N~
O ~.
. O H
76 77 78
S ri^..~
C~ S s N
~
~100
O.Q N~ N O O N,
I O
O N I'~
79 80 81
F
i1 ~rt_
ci
U10 tJ UO
N N
~ O Ni~ Y
Ny-O N'Ir- 0
Ni NH
H 82 83 84
t
S tJ `~i ~1 N 0
RS }1O
--.N N (}..O HN
O Q N~ Q ~
O
O tJApiti
O
Page 42 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
87
d IsU
\-ps
O N ?~
~N 1 $ N~~H g~yd
..*~ ~ d
9
oi
y~! ~ ~ Gi H
S r~ g r~~.~=, ~
UO H O
N
t~i tJ O N IP, O O
92 93
F L f \ 1
aml'~ d p i t~ ~ O^1J 94 96 ~,
N $ N ~S
O
0 N
N NF=o
N"
Page 43 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
97 8 99
N1 ~
N ~ ~!/ 3 !~
U = ~ ~ ~-t~
o
0 0
$ H 14
~-,O
-'" 102
\ 1 =' 1 / ~t~.~
s ~s
s
0 N
ox ~ O N
O~ti I ~ ~ = ~~~
,
103 't(}5
ON
Q
. ,~ ~s a
r~ S -i s .~/~,
v 4~=N^~ ~ L~,N
I [ t! ~' ~
0tiN
3!N H
106 107 4 ~
N s _ N S ~
ti
Q
0 r=J i =
~/'N = ~tF = ~~
td )=O
H
Page 44 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
109 110 111
F F ~ ~ tJH
~F O N~p O
\I s ~ N
0
O N~ p
p
N I g O
O ~%H N~ ~ ~.
112 113 114
97-
ko ~ ~ ~J
$ s
6
N
Q rJ ~NO C7"
O O j~
g N `./ `N I "
O~r,
f ~ ~ O~" 11
CI
115 116 117
ri
rJ,~ Hpc
s s H p
N ~rJ
~ s
N H o N o N
V `N ~N aN -
4N O%' N f"H
H H
118 119 120
C1 N rJt
S
Hoc
~J~ ~p
p
N
O p N O N ~
N N~O
~. it J
Q H p H `i
Page 45 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
121 122 123
fi
\/O S N
N N S O Q
O
-QQ FJ ~O O N
N ~
~FJ
O PJ' 0~,~
FI ttll
124 125 126
N,
N / \
O~ S N
~O F N S
O
Q d O O N
M N
)=O N
NH
O.H r ~ i O N
127 128 129
i=
O i /
~ F S
S
N
N~ N
"
N S O '=N Q OIN
~
I .~ O tJ
`/`~N
O~ ~N
O~õ
F
130 131 132
0
F / N
F ~
F S FJ'
O O
0 O
/N O!
~f S O
~Q N O
Page 46 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
133 134 11,15
F
-- ~N
aN" ~ p N~ .
a
O~
F
'F ~ r \
&' F s N
~ = ~ ~' ~~~
rJ~
o0 IN aQ ti
a =- ~ -e
139 140 141
+y. ~
O \ ~ ~rt S 40
`'~
p
0
O IN $ 0
rJ ~. M~p N
~~H ' ' ~ ~ =P~ r ,
........
IL
142 143 144
M,,~ ,,~= HO= " 0
O
~,j ~ ~ O P1
IJ S~d
'F;p td
Page 47 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
145 146 147
H
N
qs S N
dN ~
N
O
-~
0 W
N ?:-- o
N I ~ ~N
H
N1
148 149 150
q S
, a
SH O ~N4O `O ~100
N~p N)r-"p
p IN
H
151 152 153
~ !i t
ry s N,~~ 1 / f r~
s o s
N
'o
o o N ~ .~~ O N
N NH &)--o
o: ~
154 155 156
F
F~= ~
D ~ N 0
tJ ~ N S
0
o l-~ ~
rJ 0 ~--j Q o
tJ ~tJH N~O
N ll~~
0
H
Page 48 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
157 158 159
O--r- cl
N _
~ \ r
Q~ O O TJ~
ri'1
S o 1~,=~ N~
160 9:61 162
h QQth
QCO ~ H.
N N S. N N S
O~
0 N I O
v 'N \ N v`N \
Jl (
H I ~d OtN
H
163 164 165
N
N F
S N~
q
s N
d .~*~
~, 7
0 r7 N~O I~N~
NH H
0i ~"`~ /
166 167 168
/
Qci
F S -ti
H ~ S
N
O
~NO O N O ~N00
S
N I `~ p~ v "N ' ~=
H `=%' ~ J H
Page 49 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
169 170 171
;
FIP 1l
. ~O
S N
F 5 S M
~100
Y
~00~ N 00 N
N)--O
O~,N
H i
172 173 174
p tN O
N~( N S~
l__/ 'S
~ \ /
O~N p N p O
~N 114I-l N r'`kNH
i
H `t \ r
175 176 177
1 1
Nc S N \ fN RS
~/ HS O N H ~N
tJ ~
~ O v VJ p O N
t~
~O ~N
H
178 179 180
H ~
11..~0 g
HN 0 S 10
g }~
O ~O
t~tJ O ~I+N
H
Page 50 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
181 182 183
S N S
q ~
O
~1100
N ~N to
0 O 1 N
N)ZO `-,='=N I tJ,,:O
{ ~i p pi.'~,./
H
184 - 185 186
No s
s Q O N ~100 N\`t H
ON ~
N
~ 0
{ ~ ~O 0
Ol" NH
nn i
187 188 189
N 4
0 N
N N S N~Q
O td O O
N~~ NN O S
N
H~~=~~
'Ft'^' ~
19Q 191 192
F F `
Fp `.~
S ~S
S F N N
O~M
O N
aO aN
~ti N 0~ H
D~.
Page 51 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
193'^
~/ ~N-~ ~ F FS N.~ p
O
N N U00
N
0
O ri
a
196 187 1
o s S ~ra~
a- N o= a o ta
oJIM
1 8 200 201
1 f N
-..~
N~ ~ N S
00 N N p ~ ~N
~N *Jd
H
203 204
F. F
F-q , F e y
S =0
~O O N ~~ Ot 1 a O M
~~ci
Page 52 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
205 206 207
o~
O s
S
HN O O O
S N ~10 N O
O N 2 ~
N PO )--O
0JM U1
H
208 209 210
0
F
F
N N$ S
O O~'N ON ON
I~(~
F ~I ~/ ~N"
o~`
H ''~i o~H~.!~
211 212 213
Q4( HN
, qO 8 F s ~NoN ~r s
~r~
O O N
N ONy ~.
0~~, N 4~
p~.F{ H
H p~r
H
214 215 216
~~ r t F
F
g
O ~100 S
OlN
N~o j_0
r'i
H
Page 53 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
217 218 219
F
F F
Ftii.F ~N F F
S N~ S F
~0 F 5
~010 N
~ N \r- J
O N~
N~O r\JT~ 10/LN-,,,
1 ~=o
H
H
220 221 222
Oli
F c~
O S N U 5
~ ~ 5 N
O N O J
tJ N
O
OJ'H I " ; ~ O~H /
223 224 225
H
~
O - ~
\ f NJ O N
r7 g 1:0
O ^-~
O S O N Q
N tJ ~ ~ N-r- O
226 227 228
.
F 0
F N O O / ~
S rJ~ tJ ; S
O O
o N
0
0 r~'1
`,=L1J
H
I ~.
H O~N i
H
Page 54 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
229 230 231
N ~
b
~
S ~oo
~10 ~N
b b N ( )
~ \ Y
N1=-! ~ 1 N~O
~ ~b O H Ni
~
232 233 234
F F.~ F
F F 1 I ti b
(O ~ \ = N
O F $
g }}}~~~ D
N
~ ~Y? -V O
~ p O"N O N
N
O~ri O N ~N H
~ ~ 6ko
H
235 236 237
N
S (
N ra' ~ o~ N p\~o _e'
~ p N l~ rJ S
L
O H . ~
F
238 239 240
F
F F
HO qtj,-,k F O
4 S
O
~p
N
O N Y ~NON
~O ~/`N H 6I~i p~H
Page 55 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
241 242 243
1 k O~
0
g tJ ~S
p ~ O
tilINH
N I `' ~p
p%~N
244 245 246
N
F,r
$ NN~~
~100 g J ~ N
r~ N O
l~
(~-~ Q 00, M,
~ip N~p
N I `
Nd p
247 248 249
F
F IF
NH
F p
~ p \ /
Sfff...N 5
S
~O p N
O ~
N
~ p (YI p O tJ
N'rp ~ ~
1r p H /
250 251 252
p `_
d ~
=='C
' F
_ fJ p N
0 J N ~ ~N
Lv ~
p f~'~` AI ~F! ~ `= N ~=
p~N ~ = ~ p H O P! '~
H H
Page 56 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
253 254 255
N a
RS
F-~--~( g F S ~N
O N
O N
r' O O N ~
NxNH f\/I`
N f ~
O~H O
H
256 257 258
0
~ tJ S N ~
F (( a
O
N ON
Fg
0 O ;i
I
F F
O N
N =,N=^1 S N rJ~a
o ~. ~ ~N ~ Nr~
~ `r
259 260 261
~
a~N \ N ` t
N .i ~. S N J,
O O N N
N ~010 Q p p
)::,
0
262 263 264
~
a S N~~,.
~ \ 1
S
to 100 ~ ~ O O N
N~o Nr o ~/ `r! I
~ N o~
~i H
Page 57 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
265 266 267
e\ =
C
N~ N S N S N S
ON ~
268 269 270
~-s q,,,
/ ~ Br S S
~ N ~-N o
ril
0 N DN N
~PfJ ~ ~= v N ~ ~
o~` C~ O
H H
271 272 273
Qr
0
RS b
S
O Na
O N~ O N
C
O PJ
o %'~~ N ' ~ N `+ D~
/ H
H O H
274 275 276
r~ ~ .= ` l ~s
O S " ~,i 0
C~ [[[~.~~ N ~ S
N
O N
N
14
~=p
N H p~.H
N H
Page 58 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
277 -` 78 ~ 279
9~ ` ~-.
s N'~-k , iz N
1a tloo
N
j;ts
N ..~
aI
lr H (i
280 28'1 282
'. .""
F N-ri
~
g
a n ~
~1.1 'N
a
N
r{t ` ~
F
283 284 2$5'i
_
0 9-N
f4is
~ ~ ~010 Fd
ri N
O
r~ i ~= .. r~j o~`~
H
H
28Ei 287
~i= H
rN
taV=0 ~.
O O t~
0
54 $
, ~ 0
-o
Table 1A:
Page 59 of 153 .
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
288 289 290
t H l
O p N z>~)
O p N N N
~ ~ H
O~v ~=O U
291 292 Fi 293
N `t ~ `- 1
N M o ~
N`
pY~
Q Q Q
aN )= )=Q %i=p
H H NH
294 295 296
`4
-<--, -, -lc-\
N ~ ~1 H
~5s C~ S NNl pO
~ Q
NO ~ /rN~ ~5oH
aN\ p \ I N,,. O
O
H H
297 298 299
N O O
p S N N O leN`
~ ! ~'`/J
N N
\~= )C=
Page 60 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
300 301 302
o Q-1 o \1
H
Q~S pS N Q~ CNJ
Q
N N
P H
\ ~ N O O
H
li 303 304 li 305
N N._
N S
~ O S N N1 -S
QN
N OH (~
Q N '
Y
N
=U.I~
306 307 308
_ ~-N "
~ \ s N~ S N
N S ScO vo Q
N
~ N
N
o
ON ~ ?-~
HN)~-N
309 310 311
op
S N~ S N---~ S N
O Q
_~, ~ ,_..N (010
O
)~..'_'/~ 0
HN N O ON NP
Page 61 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
312 313 314
qm-" N
~ I s N v\ SN
4O O
-KNO N 0 N p
O N~
`~`N ~ N N Np~o .~ + ~ ~i
~s
3'15 316 317
s N g N Q HN/`
~
O~-N
O b
O ~041
N N
O ~ O O
O
HN H Nt g
318 319 320
1 ~
~N O ~ B N~ / /N-..
S S
N
~N
bN
O O`r" N O ~<OYOIN
~ ~
0 N O~N ' N11 H
321 322 323
F
\ /H ~N cH-/ ~
S
H N H
o O~ ~ O ~~ ~
N ~ N O N
a I~
N ~/'~N
O~N ~ i' O~N ~.-
H H H
Page 62 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
324 325 326
r N-~
nt..1 s H1t . ~
a
Q ~N ~
N ON ~Q
4
~O \N~ NN~ S N
L..N ~õ~N
327 328 329
O Q' iJH
O O O
N N N
O O
O S~ ~ ) O t) S( ~ ~`S , N
N N/ N N~ N N~
330 331 332
O
NH 0 O
0 O O
N N N
p i p ~
p N
~N
A1 N~ N~ N t3
\ / ~ \ t / ``\ I
333 334 335
14 (<,o ~p H
N SH
~
O N `~Q N O
~ 1~., O N
O ~;1 N ~ ~
~ ~
H
Page 63 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
336 337 338
QO-
S N H 0s ~ S
<
N N !! !
0 (N
N~o SN
H N H
339 340 341
ti
N
s N VNo N N F
O
a
N
~
N
N f ~. ~=o
N
)==o
342 343 344
F
ck&o
A:,
S N qs
~l N
N
N ~ ~ O N
OF ~Qh K
345 346 347
vN~ S N" HN H1V
UO O~-N O O~-}ON
1- Y
,~~ ~
N
~ ~
-. N S N~ N S N~
~-Fy ~ N F
-~ ~ I .~~I
Page 64 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
348 3349 350
F
F
p {N~ 4
\~ L.N N
N } N ~ ,-Nt 1
N N
O
j 1 O ~fl ~
` ` N}{ 'N ~ N ti
351 352 353
F
~.
'1
N~. JN N 1
"
~100 ~ C~
~N YC
O trt Q N S
o
354 r r-_ 355 356
F Ct Ci
f} 1 f N N- N - ~! õ S
O.< N eo
N
'a (Y'}
N N ~
357 358 39 ~
O
N } ON}~
~~ '.,p~ sYN t)"'"=J
N
CI ` %
-f I 0 N ' ~ 1 `=O L . ............
Page 65 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
360 361 362
F F
~ ~
EICN N S N ~ {
UO O
N S p (~`1
" J
t
~ N
O Nv`
l~ ~3'
~ `Fd
N
ON ~H I / (io
363 364 tl 365
H 1
1 ~ N.~fl "
N ~! ~/N ~~,N~ s N
s O
N N ~co
~ N
ST-YN-f Q
LI N N nj=p
~j ~
NH
=^
H
366. 367 368
-1c, -- r--~ _ -,
N NJ S N~
~.
p S N N S /, ~(>
N
N ~ 0
N
N
H ~~ -~,
N p
~ ~ ~}CO ~ r
N
369 370 371
S ~'\
'p r.,.',
p N S
0 N
0' N ~ ) 0 U N
Y ~
`-p N
O~`~
H
Page 66 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
372 373 374
t
qxCNI,
` , f N
J S N~--f H~--~
~S
U10
~N O H NH
N
IN O
N
l~
v `N
I i
O, N
H
H
375 376 377
Q1 N N/ ~N `i ~,,. O~ N
~, Sl~, ! \ N /1 5 N`~''\
~O n O ~O
N ~ N
N
%- O N ~--O
1r 1~ -
379 379 380
.r-
~ 1 t
!"` N N ~ I
rN H 1dJ S N"l
O p...
O
(-~ N
~`GJO O N U
Ni Ni th
381 382 383
o N~ O N~ S
N g N ~O
N~ ~ A-I N
N.r N '-O
N N NH
Page 67 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
384 385 386
1 1
Nw
N N S N
NN S 1 S N
~ ~100 ~00 O NIN~
~-N ~N
~/ ~,~ 1
~ FO Ny~.O (}- ')
N
N O
H
387 388 389
`1 F `1
4
, / S / S
N N N N NN"
P-24 N O Voo
~clo0
~ ) N ~ Y
N.r- O
N
N N0
ti
390 391 392
F
t,-~1 F `1
~ ` 1 N~T,^
N~F ~1~,.~
O a O~S O 0
~~ ,-N
N
~ -r- 0
N O
N Fi
393 .394 395
F F '
H
H..`~ 4 N N N
N
1. ~ H~ v\ N
0
OS O C
"~~HS
N
N F N
N
N 'F~=.F N
Nf O N
~O
Page 68 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
396 397 398
F .~ F
N H N
r .~,~ r. S
N ~ `.-FO O H = ~ H."O
~O O H ~c0
N C ,
Y N N N
Y
,F:O Q )--O
i % ' ~ O Mi
N Fi
399 400 401
s_ F~ 1 F _ 1
`
41 H N f^N~tN ~N H N ~ 'i
iN-f ~N/ ~~ ~N/ 'v \
N O N O N O
N.F~O N.--O N.~--O
(5N, 402 403 404
O N UO
~ 5 `~'~
N N H N--
Nr-O ~
N O
H~ ~ I ~
405 .406 Fi 407
4
N"~JN g NH"V
~010 O g N O
O ~O
N }~ ~ r N
N ~
)FO Q N'FO
N
N
Page 69 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
408 409 410
0
[V, RNN '''\ N S N O=rl
N
s 'N '--~N ~- U O ~100
N
Q ~ ~ Y Q
/ ~ N,~0 N~O
,
O
FI r ~ '
N
411 412 413
N~
16~N -k,
F S O S N~ N~N
~ dO
N
Q
N
1F0 N.r-O
&Nki
L ~ ~ ~ ~ ~ O
N H
414 415 416
RN~ 1 0 N
O O S N
~ O O
rN) ~N?
~ YH ~Y
1r0 Y-- O
JNf i I .;.-O 4{
N r
417 418 419
vl
O N N~ -N S N
S O N
O ~100
M
N ~
p N,
N '- O
N
)=O =~ ~ O t~i
N NH
Page 70 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
420 421 422
O
O N O
O
%S O N~ ~
N ~O O
~) N N
Y
N'T-- O N)--O N~pO
423 424 425
O
~N N
/
N O ~ S N
O ~ o~O
~O
0 N N
N O P
Q
N~ ~O N~ N O
i N
1-I H
426 427 428
o rDo 0-~
O=
~N
N f~ 5 N
s N ~ OO
S N
O
Np N, N
~ N"`TT~~Y" ~
N
~=O `' H Q
429 430 431
N N
pl N NF O N NF O N NF
jO / \ illo ~y./ \ 0
n
2-
'PO NNP~
Page 71 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
432 433 434
~ C N cf
õ N~ N J 5 N" N /)
O N
p ~p
~ p
);::O Q ~IF- -I0
..
435 436 437
-l ~ 1 ~ {V ~ y~ 1 1
r F
N F N
t3 ~N~ a N ( ~ t~, (x
~ ~ -A-
No N
)==O ~=O
438 439 440
F ~ 1 F dL
o -1c, C, H ~OO
`` ~HS ~ 0 ~ O
7 tV
~ ~ ~
Q 0 Y-0
.~ N Nd
N
441 442 443
.- A-t
y Nhl ~ 1 F N~~N
0 `~tHi' O H O }1 O N
N a
N
~ j=o f I '.~o ! )1=0
N 3'1 NN L N
Page 72 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
....,~, .~~,_._.,.... ~
F F
rd td
g
t; ~ 0 ~I ~ a
~1
1
ra
447 448 449
__ .. ....~. ~. ~..... -.................Y~. _._~___.. ~,.~ _. ,,,._..
H ~.,,.N Ct ! \ N F ~ \ .
O ~ .
H N
fl HNI
~ ) `~~~ ~/' `~H~ S N
14 ./ / ` / '
450 451 452
.__..~....._..,.....
~
Q
O
O ~ l. N
O o! t,t
~
O ~
i ~' ~.~- ~`'N
v N F ~.
45Z 455
F
HO HO X'I H F
o~
hT N O H
~,
0 ~
~
\/ \~ F M~ ~Q
Page 73 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
450 458
Br
1 f \ ~1
H,, H ooo4~~~F
O N
N
O li -~ N M
11~Q Pi H
r0xt
K
r
450 4M 464
Q ~ fl
O }- H
o
d S N
N
v,-
~ ,~
F
462 14
ti
s
H
d ~UH ~Q O
~ ~
~
r ~ ~Q y ~ "~i Q~N ~ =,,,.
=" -F~ ~ H
4U5 466 467
i H F H -
\ ~
H fi~H S t H
o H a ~H
H 0
o- =^1
= - ~a ~J-N ~ ~
ti
H ~ -~~~ ` F~ ~~~ /
Page74of153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
468 469 470
H
o
X-~ tt ~ t F
H~( H
qs Q~
~ N O H S
__ /
H N H
N oH N~--^ H N
O Q
/ O N FI
f N
N` ~o d-H I - N H
~O
471 472 473
F
h'I `N~N qH~ N~ XHH O HS N
f
~O N H 0~
,-N o N N H H
~N
Ni d~'~f
N N=O
H
474 475 476
F
F~_ = i, ' r 1
yV ~N N ~. F N
~-- Q N
N o '~.--~ c ~~H.N O
Y p Q
N-y-O N 'r-4
)~=O
ti
477
S.
O N~
N I \
fl~N 'r'
H
Page 75 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00211) Compounds of the present invention may be readily prepared by methods
well
known in the art. Synthetic schemes for preparing the compounds of the present
invention
are shown below for illustrative purposes.
[00212] Scheme 1: Preparation of compounds of formula I:
Rx
x X~Rx
B NH X~Rx 0 N_RY
, a
Z + O N-RY N Rz O
HO z O . Z' Rw Rw
R
Rw Rw
Amine Core Thiazolidinone Acid I'
Core
a) HATU, D`PEA, DMF, RT, 16 h.
[00213] Compounds of formula I are prepared as shown in Scheme 1 above,
wherein
an axnine core, containing the ring A, and the thiazolidinone acid core are
combined under
suitable conditions to provide compounds of formula I.
[00214] Scheme lA: Preparation of compounds of formula I:
Rx
6 X~ Rx
RZz NH R~Rx R6 0 N-RY
X
+ O N-RY a ,z2,~ NI ~,'' Rz O
Rw w
HO Z
z 0
Rw w R
Amine Core Thiazolidinone Acid (I)
Core
[00215] Scheme 2: Preparation of thiazolidinone core acid (Acid-I):
Rx -
O ~Rx
Rx ~ R" + RY-NHz HO" a, b O S N_RY
0 (Acid-I)
a) DMF or Toluene or benzene, 4A molecular sieves, 80 C, 1-2 h
b) Mercaptosuccinic acid, 80 C, 16 h
(00216] Scheme 3: Preparation of thiazolidinone core acid (Acid-II):
Page 76 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
O Sj~,Rx a O S~Rx b O S~Rx
N-RY -'" N-RY N-(~v
HO ~~0~~ ~~O~U~
O O RW O
ic
Rx
Rx
O S
N-RY
HO
Rw 0 (Acid-
II)
a) EtOH / H2SO4, 80 C, 24 h
b) LiHMDS, THF, 15 min, then RW-LG, 0 C to RT, 16 h
NaOH (aq.), MeOH; wherein LG is a suitable leaving group.
[00217] Scheme 4: Preparation of thiazolidinone core acid (Acid-III):
x x Rx
~ x ~Rx R
R X b S:" NR RY a N-RY Rz s N-RY
HO'~~ HO_ t~ HO~~\
0 O O
lc
Rx
Rx
O Rz S N`RY
HO~
0 (Acid-
III)
a) BOP, D'PEA, THF, 6 h, then NaBH4, RT
b) LiCI, LiHMDS, RZ-LG, -78 C C to -40 C; wherein LG is a suitable leaving
group
c) Jones oxidation, 0 C
[00218] Scheme 5: Preparation of thiazolidinone core acid (Acid-IV):
Page 77 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
O Rx ~ Rx Rx Rx
a S
R'~~Rx + Rv-NH2 N-Rv b
O/S N"Rv
~~0J~
~ = ~
O O
1 c
Rx
Rx
O S
N`Rv
HO ~
0 (Acid-
IV)
a) THF / trimethoxyorthoformate, thioacetic acid, 80 C, 16 h or DMF, 2 h, 80
C, then
thioacetic acid, 80 C, 16 h.
b) LDA, -78 C C to RT, then ethyl glyoxalate, RT, 16 h
c) NaOH (aq.), MeOH.
[00219] Scheme 6: Preparation of thiazolidinone core acid (Acid V):
O S~Rx a O O~S-~ Rx O ~ S~(~x
HO N-Ry HO N-Rv + HO J~N-Rv
~~~
O O O
(Acid-V-a) (Acid-V-b)
a) mCPBA, CHC13i 0 C to RT, 16 h
[00220] Scheme 7: Preparation of Amine core (C-A-i-d):
-O N
)
O a N b,c
~ ---
H O
H
(A-i-d) & (A-i-h);
a) 4-Methoxybenzylchloride, TEA, DMF
b) tert-butyl bis(2-chloroethyl)carbamate, LDA, THF
c) TFA / DCM
[00221] Amine core C-A-i-e, wherein ring A is A-i-e (see, supra) can be
prepared
using the method of Scheme 7.
[00222] Scheme 8: Preparation of Amine core C-A-ii-c:
Page78of153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Bz
O N H
a b
-- -~.
N NH I~ NH
Bz H --QO N --~-O
H (C-A-ii-c)
a) Polyphosphoric acid, 100 C, then, phenylurea, 150 C
b) MeOH, HCI, Pd / C, H2
[00223] Scheme 9: Preparation of Amine core C-A-ii-d:
Boc H
Boc N N
O:N~=O + CI f NICI a O b O
H N N
H H (C-A-ii-d)
a) LiHMDS
b) TFA / DCM
1002241 Scheme 10: Preparation of Amine core C-A-ii-e:
Boc, N Br
+ a Boc'N NO2
O-
-,,-
0 NOa O0
O
1 b
HN Boc-N
O H O H
(C-A-ii-e)
a) NaHMDS
b) H2, Pd/C
c) HCI
Page 79 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[002251 Scheme 11: Preparation of Amine core C-A-v-e:
Boc
H
Boc N N
+ HzN NHz a b, c
C J N
HN N
~ YD/
H2N HN
(C-A-v-e)
a) NaBH4CN
b) CDI or SOC12 or 1,1'-Sulfonyldiimidazole
c) TFA / DCM
[00226] Amine cores C-A-v-a, C-A-v-c, and C-A-v-f, containing ring A
embodiments, A-v-a, A-v-c, and A-v-f, respectively, can be readily prepared
using the
method of Scheme 11.
[002271 Scheme 12: Alternative Preparation of Amine core C-A-v-e:
Boc Boc Boc
B ac N N
a b c
-- -- -r
N ~~ ~!N~O
N H2 C ~
p NH NH (C-A-v-e)
a) COC12
b) Aminoacetaldehyde dimethylacetal
c) TFA / DCM
[00228] Scheme 13: Preparation of Amine Core C-A-vi-a:
Boc j --N-Boc NH
c~0 ~ Br + a H ~
zNOZ H O
NH2 (C-A-vi-a)
a) TEA, DCM, RT, 16 h
b) CDI; THF / DCM, 16 h.
c) TFA / DCM
[00229] Scheme 14: Preparation of Amne core C-A-vi-c:
Page 80 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Boc "Boc NH
Br
+ --- ~ '
~ a ~ H bc ~\ N
~S\ O
2 NOZ H O
NH2 (C-A-
vi-c)
a) TEA, DCM, RT, 16 h
b) 1, i'-Sulfonyldiirnidazole, THF / DCM.
c) TFA / DCM
[00230] Scheme 15: Preparation of Amine Core C-A-vi-f:
Boc
OH Br NHZ
d NH a (/ - ~ / -- ~ /
N02 NOZ 02 NO2
le
i Boc
N
N--CINH 9 NN-Boc f ~ NH
H.,.~O N~~JJ (~'
H \`O NHz
(C-A-vi-f)
a) PPh3, CBr4, DCM, 0 C to RT, overnight
b) NaN3, H20, CH3CN
c) 1) PPh3, toluene, RT, 16 hours;
2) Acetic acid / 48% HBr in Acetic acid, 100 C 1h.
d) tert-butyl 4-oxopiperidine-l-carboxylate, NaBH(OAc)3, AcOH, DMF
e) CDI, THF
t) TFA / DCM
[002311 Scheme 16: Preparation of Amine Core A-v-e:
Page 81 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Boc
N ~N.Boc N ~NH
/ + a b N c
Br J
O NH2 HN'"~Q HN~O
(A-v-
e)
a) DCM, D'PEA
b) NaOCN, AcOH,
c) TFA / DCM
[00232] Scheme 17: Preparation of Amine Core A-v-f:
JJH NH
N a N
HN--,~O HN-'~O
(A-v-f)
a) H2, Pd/C, MeOH
[00233] Scheme 18: Preparation of Amine Core A-v-g:
Fmoc Fmoc Fmoc s O
N t
a~ N b,c + e N~Oi~
H
0 HN,
N, NHBoc NH2
d
JD H N,Fmoc
N'N e N,N
t-iN--~
O HN--~O
(A-v-g)
a) H2NNHBoc, EtOH
b) Pt02, AcOH, H2
c) TFA
d) D`PEA, THF
e) Et2NH, THF
[00234] Scheme 19: Preparation of Amine Core A-vi-h:
Page 82 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Boc H
O N N
CCNH2
+ a b,c
--- N
N~ NH2 CN NH
Boc ~ ~, al~ N
N NHz N H (A-vi-h)
a) NaBH(OAc)3, DCE
b) CDI, CH3CN
c) HCI, Et20
1002351 Scheme 20: Preparation of Amine Core A-vi-i:
\ CN I~ CN NH2
~~ -- N H N N H a N C! Dao / ~ ~
i O O
Boc ~
N 0,Boc
NH ~ N~' \./ .. N N 0
r--- ccrl H
N N \
N H O I\
H ~,
O O
O
I I
(A-vi-i)
a) 2,4-Dimethoxybenzylamine, DMA, TEA
b) LiAIH3, THF
c) tert-butyl 4-oxopiperi dine-l-carboxylate, NaBH(OAc)3, AcOH, DCE
d) CDI, DMF
e) TFA / DCM
[00236] Scheme 21: Preparation of Amine Core A-ii-h:
Page 83 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
HO
OH
a, c O a' e` O f- ~
N H N N N N (~ O
SEM H N H
9, h
yH
N
N N
H
h)
a) NaH, SEM-Cl, DMF.
b) Pyridinehydrobromide perbromide, dioxane
c) Zn, AcOH
d) cis- 1, 4-dichl orobut-2-ene, Cs2CO3, DMF
e) TFA / DCM
f) Os04, Me3N-O, DCM
g) Na104, EtOH, H20
h) NH4OH, H2, Pd/C
[00237] Scheme 22: Preparation of Amine Core A-ii-i:
Cbz H
N N
nc,_ a ajC~ b CINH CI N8oc O O
CI N H O N H
(A-
a) NaHMDS, BOC2O, THF
b) nBuLi, TMEDA, benzyl 4-oxopiperidine-l-carboxylate, THF
c) H2, Pd/C
d) EtOH
[00238] Scheme 23: Preparation of Amine Core A-i-h:
Page 84 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Npc Noc Boc Boc
a b N c N
-' i'= O
O O ~S
O f O i O i N
O O O O
i d
HN e Boc, N
\ / \ /
O NH O NH
A-i-h
a) KHMDS, allylbromide, THF
b) 03, MeOH, DCM, then Me2S
c) `BuSONH2, CuSO4, DCE
d) PhLi, EtzO
e) HCl, MeOH
[00239] Scheme 24: Preparation of Amine Core A-ii-j:
O O
O O Cbz.N O N ~
a b c ~
-- / N ~
N H Br
Cbz N N
Cbz Cbz
Cbz
H I N N
N''
f,g e Cbz,N ~O/~ N ~ ,
l~.%
~
N N 0 N N O SEM Br
H SEM
A-ii-j
a) (2-Methoxy-2-oxoethyl)(triphenyl)phosphonium chloride, benzene
b) DBU, DMF
c) 3-Bromo-2-aminopyridine, A1Me3, DCE
d) NaH, SEM-Cl, THF
e) Pd(`Bu3)2, dicyclohexylmethylamine, dioxane
f j TFA
g) H2, Pd/C
[00240] Scheme 25: Preparation of Amine Core A-i-x:
Page 85 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Boc, N a Boc, N 0 Boc.N
COOH b - VNH2
NHCbz NHCL~H2 lc
NH N,goc
o-~ o H 0
A-i-i
a) EDC, HOBt, NH3, TEA, DMF
b) H2, Pd/C, EtOH
c) 1-(Trimethoxymethyl)benzene, toluene
d) TFA / DCM
[002411 Scheme 26: Preparation of Amine Core A-i-j:
Bn
Bn.N~ 0
.N O a N b
CN Bn-N (::)eNH2
~~ Bn, -0
HN N
d,e ~ ~ 0
NH N
N_O N1-O
H H
A-i-j
a) 2,4-Dimethoxybenzylamine, TMSCN
b) H2, Rh/alumina
c) CDI
d) TFA / DCM
e) H2, Pd/C
[00242] Scheme 27: Preparation of Amine Core A-i-k:
Page 86 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
O / O . O / O
Bn.N a Bn b Bn`N
~N NH
O NH
CN O NH2
n .
I I
O o
Bn, O' o/ o
N
e Bn`N NH d Bn~N
N NH
O O
OH
f
HN
NH
O~O
A-i-k
a) 2,4-Dimethoxybenzylamine, TMSCN
b) H2SO4
c) 1) KOH
2) HZSO4: KOH
d) LiAIH4
e) CDI
fl 1)TFA/DCM
2) H2, Pd/C
Page 87 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[002431 Scheme 28: Preparation of Amine Core A-i-I:
Bn.N a b Bn,
- Bn.N TMS
O ``^--~r6 _C OH
CN NH2
c
Bn,N
HCLO d O)=
O
N O H
H
A-i-I
a) TMSCN
b) LiA1H4
c) COC12
d) 1)TFA/DCM
2) H2, Pd/C
[00244] Amine core A-i-a may be prepared according to the method disclosed in
W02005097795. Amine core A-ii-a may be prepared according to the method
disclosed in
US2006293281. Amine core A-ii-a wherein the fused 6-membered ring is pyridyl
may be
prepared according to the method disclosed in W02007016087. Amine core A-v-b
may be
prepared according to the method disclosed in W02006044504. Amine core A-v-i
may be
prepared according to the method disclosed in W02006044504. Amine core A-vi-b
as the
HCI salt may be prepared according to the method disclosed in W02005056550.
Amine core
A-vi-d may be prepared according to the method disclosed in Chem. Pharm.
Bull., 34(5), pp.
1907-1916 (1986). Amine core A-vi-e is commercially available. Amine core A-v-
h may be
prepared according to the method disclosed in W02007016087. Other amine cores
not
described in the schemes, experimentals, or referenced herein, can be prepared
by methods
known to one of skill in the art.
[00245] It will also be appreciated that certain of the compounds of present
invention
can exist in free form for treatment, or where appropriate, as a
pharmaceutically acceptable
derivative thereof. According to the present ilivention, a pharmaceutically
acceptable
derivative includes, but is not limited to, pharmaceutically acceptable salts,
esters, salts of
such esters, or any other adduct or derivative which upon administration to a
patient in need
Page 88 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
is capable of providing, directly or indirectly, a compound as otherwise
described herein, or a
metabolite or residue thereof.
[002461 As used herein, the term "pharmaceutically acceptable salt" refers to
those
salts wliich are, within the scope of sound medical judgment, suitable for use
in contact with
the tissues of humans and lower animals without undue toxicity, irritation,
allergic response
and the like, and are commensurate with a reasonable benefit/risk ratio. A
"pharmaceutically
acceptable salt" means any non-toxic salt or salt of an ester of a compound of
this invention
that, upon administration to a recipient, is capable of providing, either
directly or indirectly, a
compound of this invention or an active metabolite or residue thereof. As used
herein, the
term "active metabolite or residue thereof' ineans that a metabolite or
residue thereof is also
an antagonist of CGRP.
[002471 Pharmaceutically acceptable salts are well known in the art. For
example, S.
M. Berge, et al. describe pharmaceutically acceptable salts in detail in J.
Pharmaceutical
Sciences, 1977, 66, 1-19, incorporated herein by reference. Pharmaceutically
acceptable salts
of the compounds of this invention include those derived from suitable
inorganic and organic
acids and bases. Examples of pharmaceutically acceptable, nontoxic acid
addition salts are
salts of an amino group formed with inorganic acids such as hydrochloric acid,
hydrobromic
acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids
such as acetic
acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or
malonic acid or by
using other methods used in the art such as ion exchange. Other
pharmaceutically acceptable
salts include adipate, alginate, ascorbate, aspartate, benzenesulfonate,
benzoate, bisulfate,
borate, butyrate, camphorate, camphorsulfonate, citrate,
cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate,
glycerophosphate,
gluconate, hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-
ethanesulfonate,
lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate,
methanesulfonate, 2-
naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate,
pamoate, pectinate,
persulfate, 3-phenylpropionate, phosphate, picrate, pivalate, propionate,
stearate, succinate,
sulfate, tartrate, thiocyanate, p-toluenesulfonate, undecanoate, valerate
salts, and the like.
Salts derived from appropriate bases include alkali metal, alkaline earth
metal, ammonium
and N+(CI-yalkyl)4 salts. This invention also envisions the quatemization of
any basic
nitrogen-containing groups of the compounds disclosed herein. Water or oil-
soluble or
dispersable products may be obtained by such quateniization. Representative
alkali or
alkaline earth metal salts include sodium, lithium, potassium, calcium,
magnesium, and the
like. Further pharmaceutically acceptable salts include, when appropriate,
nontoxic
Page 89 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
axnmonium, quaternary ammonium, and amine cations formed using counterions
such as
halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, loweralkyl
sulfonate and aryl
sulfonate.
[00248] As described above, the pharmaceutically acceptable compositions of
the
present invention additionally comprise a pharmaceutically acceptable carrier,
adjuvant, or
vehicle, which, as used herein, includes, any and all solvents, diluents, or
other liquid vehicle,
dispersion or suspension aids, surface active agents, isotonic agents,
thickening or
emulsifying agents, preservatives, solid binders, lubricants and the like, as
suited to the
particular dosage form desired. Reinington's Pharmaceutical Sciences,
Sixteenth Edition, E.
W. Martin (Mack Publishing Co., Easton, Pa., 1980) discloses various carriers
used in
formulating pharmaceutically acceptable compositions and known techniques for
the
preparation thereof. Except insofar as any conventional carrier medium is
incompatible with
the compounds of the invention, such as by producing any undesirable
biological effect or
otherwise interacting in a deleterious manner with any other component(s) of
the
pharmaceutically acceptable composition, its use is contemplated to be within
the scope of
this invention. Some examples of materials which can serve as pharmaceutically
acceptable
carriers include, but are not limited to, ion exchangers, alumina, aluminum
stearate, lecithin,
serum proteins, such as human serum albumin, buffer substances such as
phosphates, glycine,
sorbic acid, or potassium sorbate, partial glyceride mixtures of saturated
vegetable fatty acids,
water, salts or electrolytes, such as protamine sulfate, disodium hydrogen
phosphate,
potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica,
magnesium
trisilicate, polyvinyl pyrrolidone, polyacrylates, waxes, polyethylene-
polyoxypropylene-
block polymers, wool fat, sugars such as lactose, glucose and sucrose;
starches such as corn
starch and potato starch; cellulose and its derivatives such as sodium
carboxymethyl
cellulose, ethyl cellulose and cellulose acetate; powdered tragacanth; malt;
gelatin; talc;
excipients such as cocoa butter and suppository waxes; oils such as peanut
oil, cottonseed oil;
safflower oil; sesame oil; olive oil; corn oil and soybean oil; glycols; such
a propylene glycol
or polyethylene glycol; esters such as ethyl oleate and ethyl laurate; agar;
buffering agents
such as magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free
water;
isotonic saline; Ringer's solution; ethyl alcohol, and phosphate buffer
solutions, as well as
other non-toxic compatible lubricants such as sodium lauryl sulfate and
magnesium stearate,
as well as coloring agents, releasing agents, coating agents, sweetening,
flavoring and
perfuming agents, preservatives and antioxidants can also be present in the
composition,
according to the judgment of the formulator.
Page 90 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00249] The pharmaceutically acceptable compositions of this invention can be
administered to humans and other animals orally, rectally, parenterally,
intracisterrrnally,
intravaginally, intraperitoneally, topically (as by powders, ointments, or
drops), bucally, as an
oral or nasal spray, or the like, depending on the severity of the infection
being treated. In
certain embodiments, the compounds of the invention may be administered orally
or
parenterally at dosage levels of about 0.01 rng/kg to about 50 mg/kg and
preferably from
about 1 mg/kg to about 25 mg/kg, of subject body weight per day, one or more
times a day, to
obtain the desired therapeutic effect.
[00250] Liquid dosage fonns for oral administfation include, but are not
limited to,
pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions,
syrups and
elixirs. In addition to the active compounds, the liquid dosage forms may
contain inert
diluents commonly used in the art such as, for example, water or other
solvents, solubilizing
agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl
carbonate, ethyl acetate,
benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol,
dimethylformamide,
oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and
sesame oils),
glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid
esters of sorbitan,
and mixtures thereof. Besides inert diluents, the oral compositions can also
include adjuvants
such as wetting agents, emulsifying and suspending agents, sweetening,
flavoring, and
perfuming agents.
[002511 Injectable preparations, for example, sterile injectable aqueous or
oleaginous
suspensions may be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation may
also be a sterile
injectable solution, suspension or emulsion in a nontoxic parenterally
acceptable diluent or
solvent, for example, as a solution in 1,3-butanediol. Among the acceptable
vehicles and
solvents that may be employed are water, Ringer's solution, U.S.P. and
isotonic sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent or
suspending medium. For this purpose any bland fixed oil can be employed
including
synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid
are used in the
preparation of injectables.
[00252] The injectable formulations can be sterilized, for example, by
filtration
through a bacterial-retaining filter, or by incorporating sterilizing agents
in the form of sterile
solid compositions which can be dissolved or dispersed in sterile water or
other sterile
injectable medium prior to use.
Page 91 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
(00253] In order to prolong the effect of a compound of the present invention,
it is
often desirable to slow'the absorption of the compound from subcutaneous or
intramuscular
injection. This may be accomplished by the use of a liquid suspension of
crystalline or
amorphous material with poor water solubility. The rate of absorption of the
compound then
depends upon its rate of dissolution that, in turn, may depend upon crystal
size and crystalline
form. Alternatively, delayed absorption of a parenterally administered
compound form is
accomplished by dissolving or suspending the compound in an oil vehicle.
Injectable depot
forms are made by forming microencapsule matrices of the compound in
biodegradable
polymers such as polylactide-polyglycolide. Depending upon the ratio of
compound to
polymer and the nature of the particular polymer employed, the rate of
compound release can
be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the
compound in liposomes or microemulsions that are compatible with body tissues.
[00254] Compositions for rectal or vaginal administration are preferably
suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-irritating
excipients or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which
are solid at ambient temperature but liquid at body temperature and therefore
melt in the
rectum or vaginal cavity and release the active compound.
(00255] Solid dosage forms for oral administration include capsules, tablets,
pills,
powders, and granules. In such solid dosage forms, the active compound is
mixed with at
least one inert, pharnzaceutically acceptable excipient or carrier such
as.sodium citrate or
dicalcium phosphate and/or a) fillers or extenders such as starches, lactose,
sucrose, glucose,
mannitol, and silicic acid, b) binders such as, for exainple,
carboxymethylcellulose, alginates,
gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as
glycerol, d)
disintegrating agents such as agar--agar, calcium carbonate, potato or tapioca
starch, alginic
acid, certain silicates, and sodium carbonate, e) solution retarding agents
such as paraffin, f)
absorption accelerators such as quaternary ammonium compounds, g) wetting
agents such as,
for example, cetyl alcohol and glycerol monostearate, h) absorbents such as
kaolin and
bentonite clay, and i) lubricants such as talc, calcium stearate, magnesium
stearate, solid
polyethylene glycols, sodium lauryl sulfate, and mixtures thereof. In the case
of capsules,
tablets and pills, the dosage form may also comprise buffering agents.
[00256] Solid corripositions of a similar type may also be employed as fillers
in soft
and hard-filled gelatin capsules using such excipients as lactose or milk
sugar as well as high
molecular weight polyethylene glycols and the like. The solid dosage forms of
tablets,
Page 92 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
dragees, capsules, pills, and granules can be prepared with coatings and
shells such as enteric
coatings and other coatings well known in the pharmaceutical formulating art.
They may
optionally contain opacifying agents and can also be of a composition that
they release the.
active ingredient(s) only, or preferentially, in a certain part of the
intestinal tract, optionally,
in a delayed manner. Examples of embedding compositions that can be used
include
polymeric substances and waxes. Solid compositions of a similar type may also
be employed
as fillers in soft and hard-filled gelatin capsules using such excipients as
lactose or milk sugar
as well as high molecular weight polethylene glycols and the like.
[00257] The active compounds can also be in microencapsulated form with one or
more excipients as noted above. The solid dosage forms of tablets, dragees,
capsules, pills,
and granules can be prepared with coatings and shells such as enteric
coatings, release
controlling coatings and other coatings well known in the pharmaceutical
formulating art. In
such solid dosage forms the active compound may be admixed with at least one
inert diluent
such as sucrose, lactose or starch. Such dosage forms may also comprise, as is
normal
practice, additional substances other than inert diluents, e.g., tableting
lubricants and other
tableting aids such a magnesium stearate and microcrystalline cellulose. In
the case of
capsules, tablets and pills, the dosage forms may also comprise buffering
agents. They may
optionally contain opacifying agents and can also be of a composition that
they release the
active ingredient(s) only, or preferentially, in a certain part of the
intestinal tract, optionally,
in a delayed manner. Examples of embedding compositions that can be used
include
polymeric substances and waxes.
[00258] Dosage forms for topical or transdermal administration of a compound
of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
required. Ophthalmic formulation, eardrops, and eye drops are also
contemplated as being
within the scope of this invention. Additionally, the present invention
contemplates the use of
transdermal patches, which have the added advantage of providing controlled
delivery of a
compound to the body. Such dosage forms are prepared by dissolving or
dispensing the
compound in the proper medium. Absorption enhancers can also be used to
increase the flux
of the compound across the skin. The rate can be controlled by either
providing a rate
controlling membrane or by dispersing the compound in a polymer matrix or gel.
[00259] It will also be appreciated that the compounds and pharmaceutically
acceptable compositions of the present invention can be employed in
combination therapies,
Page 93 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
that is, the compounds and pharmaceutically acceptable compositions can be
administered
concurrently with, prior to, or subsequent to, one=or more other desired
therapeutics or
medical procedures. The particular combination of therapies (therapeutics or
procedures) to
employ in a coinbination regimen will take into account compatibility of the
desired
therapeutics and/or procedures and the desired therapeutic effect to be
achieved. It will also
be appreciated that the therapies employed may achieve a desired effect for
the same disorder
(for example, an inventive compound may be administered concurrently with
another agent
used to treat the same disorder), or they may achieve different effects (e.g.,
control of any
adverse effects). As used herein, additional therapeutic agents that are
normally administered
to treat or prevent a particular disease, or condition, are known as ';
appropriate for the
disease, or condition, being treated". For example, exemplary additional
therapeutic agents
include, but are not limited to: nonopioid analgesics= (indoles such as
Etodolac,
Indomethacin, Sulindac, Tolmetin; naphthylalkanones such sa Nabumetone;
oxicams such as
Piroxicam; para-aminophenol derivatives, such as Acetaminophen; propionic
acids such as
Fenoprofen, Flurbiprofen, Ibuprofen, Ketoprofen, Naproxen, Naproxen sodium,
Oxaprozin;
salicylates such as Asprin, Choline magnesium trisalicylate, Diflunisal;
fenamates such as
meclofenamic acid, Mefenamic acid; and pyrazoles such as Phenylbutazone); or
opioid
(narcotic) agonists (such as Codeine, Fentanyl, Hydromorphone, Levorphanol,
Meperidine,
Methadone, Morphine, Oxycodone, Oxymorphone, Propoxyphene, Buprenorphine,
Butorphanol, Dezocine, Nalbuphine, and Pentazocine). Additionally, nondrug
analgesic
approaches may be utilized in conjunction with administration of one or more
compounds of
the invention. For example, anesthesiologic (intraspinal infusion, neural
blocade),
neurosurgical (neurolysis of CNS pathways), neurostimulatory (transcutaneous
electrical
nerve stimulation, dorsal column stimulation), physiatric (physical therapy,
orthotic devices,
diathermy), or psychologic (cognitive methods-hypnosis, biofeedback, or
behavioral
methods) approaches may also be utilized. Additional appropriate therapeutic
agents or
approaches are described generally in The Merck Manual, Seventeenth Edition,
Ed. Mark H.
Beers and Robert Berkow, Merck Research Laboratories, 1999, and the Food and
Drug
Administration website, www.fda.~ov, the entire contents of which are hereby
incorporated
by reference.
[002601 The amount of additional therapeutic agent present in the compositions
of this
invention will be no more than the amount that would normally be administered
in a
composition comprising that therapeutic agent as the only active agent.
Preferably the
amount of additional therapeutic agent in the presently disclosed compositions
will range
Page 94 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
from about 50% to 100% of the amount normally present in a composition
comprising that
agent as the only therapeutically active agent.
[00261] The compounds of this invention or pharmaceutically acceptable
compositions
thereof may also be incorporated into compositions for coating an implantable
medical
device, such as prostheses, artificial valves, vascular grafts, stents and
catheters. =-
Accordingly, the present inveiition, in another aspect, includes a composition
for coating an
implantable device comprising a compound of the present invention as described
generally
above, and in classes and subclasses herein, and a carrier suitable for
coating said implantable
device. In still another aspect, the present invention includes-an implantable
device coated
with a composition comprising a compound of the present invention as described
generally
above, and in classes and subclasses herein, and a carrier suitable for
coating said implantable
device. Suitable coatings and the general preparation of coated implantable
devices are
described in US Patents 6,099,562; 5,886,026; and 5,304,121. The coatings are
typically
biocompatible polymeric materials such as a hydrogel polymer,
polymethyldisiloxane,
polycaprolactone, polyethylene glycol, polylactic acid, ethylene vinyl
acetate, and mixtures
thereof. The coatings may optionally be further covered by-a suitable topcoat
of
fluorosilicone, polysaccarides, polyethylene glycol, phospholipids or
combinations thereof to
impart controlled release characteristics in the composition.
[00262] The compounds of the present invention are useful in a method of
antagonism
of CGRP receptors in a patient such as a mainmal iri need of such antagonism
comprising the
administration of an effective amount of the compound. The present invention
is directed to
the use of the compounds disclosed herein as antagonists of CGRP receptors. In
addition to
primates, especially humans, a variety of other mammals can be treated
according to the
method of the present invention.
[00263] Another embodiment of the present invention is directed to a method
for the
treatment, control, amelioration, or reduction of risk of a disease or
disorder in which the
CGRP receptor is involved in a patient that comprises administering to the
patient a
therapeutically effective amount of a compound that is an antagonist of CGRP
receptors.
[00264] The present invention is further directed to a method for the
manufacture of a
medicament for antagonism of CGRP receptors activity in humans and animals
comprising
combining a compound of the preserit invention with a phannaceutical carrier
or diluent.
[00265] The subject treated in the present methods is generally a mammal, for
example
a human being, male or female, in whom antagonism of CGRP receptor activity is
desired.
The term "therapeutically effective amount" means the amount of the subject
compound that
Page 95 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
will elicit the biological or medical response of a tissue, system, animal or
human that is
being sought by the researcher, veterinarian, medical doctor or other
clinician. As used
herein, the term "treatment" refers both to the treatment and to the
prevention or prophylactic
therapy of the mentioned conditions, particularly in a patient who is
predisposed to such
disease or disorder. -
[00266] The ability of the compounds of the present invention to act as CGRP
antagonists makes them useful pharmacological agents for disorders that
involve CGRP in
humans and animals, but particularly in humans.
[00267] The compounds of the present invention have utility in treating,
preventing,
ameliorating, controlling or reducing the risk of one or more of the following
conditions or
diseases: headache; migraine; cluster headache; chronic tension type headache;
pain; chronic
pain; neurogenic inflammation and inflammatory pain; neuropathic pain; eye
pain; tooth
pain; diabetes; non-insulin dependent diabetes mellitus; vascular disorders;
inflammation;
arthritis; bronchial hyperreactivity, asthma; shock; sepsis; opiate withdrawal
syndrome;
morphine tolerance; hot flashes in men and women; allergic dermatitis;
encephalitis; brain
trauma; epilepsy; neurodegenerative diseases; skin diseases; neurogenic
cutaneous redness,
skin rosaceousness and erythema; tinnitus; infla.mmatory bowel disease,
irritable bowel
syndrome, cystitis; and other conditions that may be treated or prevented by
antagonism of
CGRP receptors. Of particular importance is the acute or prophylactic
treatment of headache,
including migraine and cluster headache.
[00268] The coinpounds of the present invention are further useful in a method
for the
prevention, treatment, control, amelioration, or reduction of risk of the
diseases, disorders and. .
conditions noted herein.
[00269] The compounds of the present invention are further useful in a method
for the
prevention, treatment, control, amelioration, or reduction of risk of the
aforementioned
diseases, disorders and conditions in combination with other agents.
[00270] The compounds of the present invention may be used in combination with
one
or more other drugs in the treatment, prevention, control, amelioration, or
reduction of risk of
diseases or conditions for which compounds of Formula I or the other drugs may
have utility,
where the combination of the drugs together are safer or more effective than
either drug
alone. Such other drug(s) may be administered, by a route and in an amount
commonly used
therefor, contemporaneously or sequentially with a compound of Formula I. When
a -
compound of Formula I is used contemporaneously with one or more other drugs,
a
pharmaceutical composition in unit dosage form containing such other drugs and
the
Page 96 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
compound of Formula I is preferred. However, the combination therapy may also
include
therapies in which the compound of Formula I and one or more other drugs are
administered
on different overlapping schedules. It is also contemplated that when used in
combination
with one or more other active ingredients, the compounds of the present
invention and the
other active ingredients may be used in lower doses than when each is used
singly.
Accordingly, the pharmaceutical compositions of the present invention include
those that
contain one or more other active ingredients, in addition to a compound of
Formula I.
[00271] For example, the present compounds may be used in conjunction with an
anti-
inflammatory or analgesic agent or -an anti-migraine agent, such as an
ergotamine or 5-
HTl agonists, especially a 5-HT1B/ID agonist, for example
sumatriptan,
naratriptan, zolmitriptan, eletriptan, almotriptan, frovatriptan, donitriptan,
and rizatriptan; a
cyclooxygenase inhibitor, such as a selective cyclooxygenase-2 inhibitor, for
example
rofecoxib, etoricoxib, celecoxib, valdecoxib or paracoxib; a non-steroidal
anti-inflammatory
agent or a cytokine-suppressing anti-inflammatory agent, for example with a
compound such
as aspirin, ibuprofen, ketoprofen, fenoprofen, naproxen, indomethacin,
sulindac, meloxicam,
piroxicam, tenoxicam, lornoxicam, ketorolac, etodolac, mefenamic acid,
meclofenamic acid,
flufenamic acid, tolfenamic acid, diclofenac, oxaprozin, apazone, nimesulide,
nabumetone,
tenidap, etanereept, tolmetin, phenylbutazone, oxyphenbutazone, diflunisal,
salsalate,
olsalazine or sulfasalazine and the like; or a steroidal analgesic. Similarly,
the instant
compounds may be administered with a pain reliever such as acetaminophen,
phenacetin,
codeine, fentanyl, sufentanil, methadone, acetyl methadol, buprenorphine or
morphine.
[00272] Additionally, the present compounds may be used in conjunction with an
interleukin inhibitor, such as an interleukin-1 inhibitor; an NK-1 receptor
antagonist, for
example-aprepitant; an NMDA antagonist; an NR2B antagonist; a bradykinin- 1
receptor
antagonist; an adenosine A 1 receptor agonist; a sodium channel blocker, for
example
lamotrigine; an opiate agonist such as levomethadyl acetate or methadyl
acetate; a
lipoxygenase inhibitor, such as an inhibitor of 5-lipoxygenase; an alpha
receptor antagonist,
for example indoramin; an alpha receptor agonist; a vanilloid receptor
antagonist; an mGluR5
agonist, antagonist or potentiator; a GABA A receptor modulator, for example
acamprosate
calcium; nicotinic antagonists or agonists including nicotine; muscarinic
agonists or
antagonists; a selective serotonin reuptake inhibitor, for example fluoxetine,
paroxetine,
sertraline, duloxetine, escitalopram, or citalopram; a tricyclic
antidepressant, for example
amitriptyline, doxepin, protriptyline, desipramine, trimipramine, or
imipramine; a leukotriene
Page 97 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
antagonist, for example montelukast or zafirlukast; an inhibitor of nitric
oxide or an inhibitor
of the synthesis of nitric oxide.
[00273] Also, the present compounds may be used in conjunction with ergot
alkaloids,
for example ergotamine, ergonovine, ergonovine, methylergonovine, metergoline,
ergoloid
mesylates, dihydroergotamine, dihydroergocornine, dihydroergocristine,
dihydroergocryptine, dihydro-I-ergocryptine, dihydro-.theta.-ergocryptine,
ergotoxine,
ergocornine, ergocristine, ergocryptine, 1-ergocryptine, .theta.-ergocryptine,
ergosine,
ergostane, bromocriptine, or methysergide.
[00274] Additionally, the present compounds may be used in conjunction with a
beta-
adrenergic antagonist such as timolol, propanolol, atenolol, or nadolol, and
the like; a MAO
inhibitor, for example phenelzine; a calcium channel blocker, for example
flunarizine,
nimodipine, lomerizine, verapamil, nifedipine, prochlorperazine or gabapentin;
neuroleptics
such as olanzapine and quetiapine; an anticonvulsant such as topiramate,
zonisamide,
tonabersat, carabersat or divalproex sodium; an angiotensin II antagonist, for
example
losartan and candesartan cilexetil; an angiotensin converting enzyme inhibitor
such as
lisinopril; or botulinum toxin type A.
[00275] The present compounds may be used in conjunction with a potentiator
such as
caffeine, an H2-antagonist, simethicone, aluminum or magn.esium hydroxide; a
decongestant
such as phenylephrine, phenylpropanolamine, pseudoephedrine, oxymetazoline,
epinephrine,
naphazoline, xylometazoline, propylhexedrine, or levo-desoxy-ephedrine; an
antitussive such
as codeine, hydrocodone, caramiphen, carbetapentane, or dextromethorphan; a
diuretic; a
prokinetic agent such as metoclopramide or domperidone, and a sedating or non-
sedating
antihistamine.
In a particularly preferred embodiment the present compounds are used in
conjunction with
an anti-migraine agent, such as: an ergotamine; a 5-HTl agonist,
especially a 5-
HT1B/1D agonist, in particular, sumatriptan, naratriptan, zolmitriptan,
eletriptan,
almotriptan, frovatriptan, donitriptan and rizatriptan; and a cyclooxygenase
inhibitor, such as
a selective cyclooxygenase-2 inhibitor, in particular, rofecoxib, etoricoxib,
celecoxib,
meloxicam, valdecoxib or paracoxib.
[00276] The above combinations include combinations of a compound of the
present
invention not only with one other active compound, but also with two or more
other active
compounds. Likewise, compounds of the present invention may be used in
combination with
other drugs that are used in the prevention, treatment, control, amelioration,
or reduction of
Page 98 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
risk of the diseases or conditions for which compounds of the present
invention are useful. '
Such other drugs may be administered, by a route and in an amount commonly
used therefor,
contemporaneously or sequentially with a compound of the present invention.
When a
compound of the present invention is used contemporaneously with one or more
other drugs,
a pharmaceutical composition containing such other drugs in addition to the
compound of the
present invention is preferred. Accordingly, the pharmaceutical compositions
of the present
invention include those that also contain one or more other active
ingredients, in addition to a
compound of the present invention.
[00277] The weight ratio of the compound of the compound of the present
invention to
the other active ingredient(s) may be varied and will depend upon the
effective dose of each
ingredient. Generally, an effective dose of each will be used. Thus, for
example, when a
compound of the present invention is combined with another agent, the weight
ratio of the
compound of the present invention to the other agent will generally range from
about 1000:1
to about 1:1000, or from about 200:1 to about 1:200. Combinations of a
compound of the
present invention and other active ingredients will generally also be within
the
aforementioned range, but in each case, an effective dose of each active
ingredient should be
used.
[00278] In such combinations the compound of the present invention and other
active
agents may be administered separately or in conjunction. In addition, the
administration of
one element may be prior to, concurrent to, or subsequent to the
administration of other
agent(s), and via the same or different routes of administration.
[00279] The compounds of the present invention may be administered by oral,
parenteral (e.g., intramuscular, intraperitoneal, intravenous; ICV,
intracisternal injection or
infusion, subcutaneous injection, or implant), by inhalation spray, nasal,
vaginal, rectal,
sublingual, or topical routes of administration and may be formulated, alone
or together, in
suitable dosage unit formulations containing conventional non-toxic
pharmaceutically
acceptable carriers, adjuvants and vehicles appropriate for each route of
administration. In
addition to the treatment of warm-blooded animals the compounds of the
invention are
effective for use in humans.
[00280] In order that the invention described herein may be more fully
understood, the
following examples are set forth. It should be understood that these examples
are for
illustrative purposes only and are not to be construed as limiting this
invention in any manner.
Page 99 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
EXAMPLES
[00281] General .LC/MS Methods
[00282] LC/MS data.were acquired using a PESciex API-1'50-EX LC/MS, Shimadzu
LC-8A pumps, Gilson 215 autosampler, Gilson 819 injection module, 3.0 mL/min
flow rate,
10-99% CH3CN (0.035 % TFA) / H20 (0.05 % TFA) gradient, Phenomenex Luna 5u C18
column (50 x 4.60 mm), Shimadzu SPD-10A UV/Vis detector, Cedex 75 ELSD
detector.
[00283] Mass Spec Method for separating diasteromeric mixtures:
[00284] A Semi-Prep Gilson HPLC was used to purify various diastereomeric
mixtures
in the present invention using Gilson 322 pumps, a Gilson 215 liquid handler,
a Gilson 819
injection module. Flow rate was 15.0 mL/min using a gradient of 20-70% CH3CN
(0.1 Oo
TFA) / H20 (0.1 % TFA) on an Agilent Zorbax, SB-C18 column (21.2 x 100 mm,
5um)
monitoring with a Gilson 156 UV/Vis detector.
[002851 tert-Buty14-(1,2-dihydro-2-oxo-5-phenylimidazol-3-yl)piperidine-l-
carboxylate
.Boc
[00286] HN__~\o
[002871 tert-Butyl 4-(1,2-dihydro-2-oxo-5-phenylimidazol-3-yl)piperidine-l-
carboxylate
was synthesised as described in J. Med. Chem., 2005, 48, 5921. A solution of 2-
bromo-l-
phenylethanone (5 g, 25 mmol) in DCM (10 ml) was added dropwise to a stirred
solution of
tert-butyl 4-aminopiperidine-l-carboxylate.(6 g, 30 mmol) and D'PEA (9.84 ml,
57.5 ml) in
DCM (50 ml)'over I hour, the reaction mixture was then stirred at room
temperature for 16
hours. Sodium cyanate (3_41 g, 52.5 nimol) was added, the reaction mixture was
then cooled
to 0 C, the pH was brought to pH 4 with acetic acid and the reaction mixtures
was stirred
from 0 C to RT over 16 hours. The reaction mixture was poured into water and
extracted
with DCM (3x). Organics combined, washed with water (3x), brine, dried (MgSO4)
and
evaporated to dryness. The residue was triturated with ether, filtered and the
solid was
washed with ether to give a pale yellow solid (4.04 g, 47%). LC/MS (10% to
99%): M/Z
(M+H)+ (obs) = 344 ; tR = 3.01.
[002881 5-Phenyl-3-(piperidin-4-yl)-1H-imidazol-2(3H)-one
~NH
N
[00289] HN~O
Page 100 of 153
1
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
1002901 To a solution of tert-buty.l 4-(1,2-dihydro-2-oxo-5-phenylimidazol-3-
yl)piperidine- 1 -carboxylate (4 g) in DCM (20 ml) was added TFA (4 ml) and
the reaction
mixture was stirred at RT for 4 hours. Evaporation gave the TFA salt of the
desired product
(Quant.). LC/MS (10% to 99%): MIZ (M+H)+ (obs) = 244 ; tR = 1.06.
[00291] tert-Butyl4-(2-nitrobenzylamino)piperidine-l-carboxylate
Boc, N
=
NH
[00292] No2
[002931 A solution of 1-(bromomethyl)-2-nitrobenzene (13.2 g, 61 mmol) in DCM
(60 ml)
was added dropwise to a solution of tert-butyl 4-aminopiperi dine-l-
carboxylate (14.6 g, 73
mmol) and TEA (13.4 ml, 91 mmol) in DCM (100 ml), followed by stirring the
reaction
mixture for a further 16 hours. The reaction mixture was then poured into
water, and the
layers separated. The aqueous layer was then extracted with DCM (2x). The
organic layers
were combined, washed with water (2x), brine, dried (MgSO4) and evaporated to
dryness.
The residue was taken up in EtOAc and filtered through a large plug of silica.
= The silica was
washed with EtOAc until TLC analysis show no further material was eluting.
Evaporation
gave the product as an orange oil (24g, 74%). LC/MS (10% to 99%): M/Z (M+H)+
(obs) _
336;tR=2.23.
[002941 tert-Butyl4-(2-aminobenzylamino)piperidine-l-carboxylate
N,Boc
I~ H
[00295] NH2
[00296] A solution of tert-Buty14-(2-nitrobenzylamino)piperidine-l-carboxylate
(24g,
71.6 mmol) in MeOH (150 ml) was stirred under an atmosphere of hydrogen for 24
hours.
The reaction mixture was filtered and evaporated to give the crude amine,
which was used
without further purification.
[002971 tert-Buty14-(1,2-dihydro-2-oxoquinazolin-3(4H)-yl)piperidine-l-
carboxylate
N,Boc
\
N ---O
[00298] H
Page 101 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00299] To a solution of tert-butyl 4-(2-nitrobenzylamino)piperidine-l-
carboxylate (13.2g,
43.2 mmol) in THF (400 ml) was added a solution of CDI (7.7g, 47.5 mmol) in 1:
1 DCM :
THF (100 ml) dropwise over 1 hour followed by stirring the reaction mixture
for a further 16
hours. The reaction mixture was evaporated to give an oil that, when treated
with EtOAc,
precipitated the desired product. The precipitate was washed with cold EtOAc
and dried to
give a yellow solid (3.5g). LC/MS (10% to 99%):. M/Z (MtH)+ (obs) = 332 ;. tR
=..3..01.
[00300] 3,4-Dihydro-3-(piperidin-4-yl)quinazolin-2(1H)-one
OH
c~x0
[00301] H
[00302) To a solution of tert-Butyl 4-(1,2-dihydro-2-oxoquinazolin-3(4H)-
yl)piperidine-1-
carboxylate (3.5 g,-10.6 mmol) in DCM (20 ml) was added TFA (15 ml) and the
reaction
mixture was stirred at RT for 2 h. The reaction mixture was evaporated, then
co-evaporated
with EtOH (2x), to give the TFA salt of the desired product (Quant.). LC/MS
(10% to 99%):
M/Z (M+H)} (obs) = 232 ; tR = 0.38.
[00303] 1-(2-Bromoethyl)-2-nitrobenzene
Br [00304] (:) N02
[00305] To a solution of 1-(2-hydroxyethyl)-2-nitrobenzene (21 ml, 150 mmol)
and
triphenylphosphine (39.2 g, 150 mmol) in DCM (400 -ml) at 0 C was add CBr4
(49.5 g, 150
mmol) in portions and the reaction mixture was stirred from 0 C to RT
overnight. The
reaction mixture was quenched with sat. aq. Na2CO3, the layers were separated
and the
organic layer was washed with brine, dried (MgSO4) and evaporated to dryness.
The residue
was treated with EtOAc and the precipitated Ph30 was filtered and the solvent
removed. This
was repeated twice more. Purification by column chromatography (0% to 10%
EtOAc in Hx)
gave an oil that solidified on standing.
[00306] 2-(2-Nitrophenyl)ethanamine
~ NH2
( /
[00307] NO2
[00308] To a solution of l-(2-Bromoethyl)-2-nitrobenzene (6.96 g, 30.5 mmol)
in CH3CN
was added a solution of NaN3 (6 g, 91.6 mmol) in water (20 ml) and the
reaction mixture was
refluxed for 20 hours. The solution was cooled and extracted with DCM (3x).
The organics
Page 102 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
were combined, washed with brine, dried (MgSO4) and evaporated to dryness. The
residue
was taken up in toluene (160 ml) and to this was added PPh3 (8 g, 30.5 mmol)
and the
reaction mixture was stirred at =RT for 16 hours. The solvent was evaporated
to dryness and
the residue was treated with acetic acid (30 ml) and 48% HBr in acetic acid
(30 ml) at 100 C
for 1 h. The reaction mixture was cooled, concentrated =and extracted with
DCM. The
aqueous was brought to pH -10 with NaOH (aq.) and extracted with EtOAc (3x).
The
organics were combined, washed with brine, dried (MgSO4) and evaporated to
dryness (4.2
g)=
[00309] tert-Butyl 4-(2-nitrophenethylamino)piperidine-l.-carboxylate
Boc
~~ NH
[003101 NO~
[00311] A stirred solution of 2-(2-nitrophenyl)ethanamine (4 g, 24 mmol) and
tert-butyl 4-
oxopiperidine-l-carboxylate (4.8 g, 24 mmol) in MeOH (48 ml) was brought to pH
5 by the
addition of acetic acid. NaBH3CN (2.3 g, 36 mmol) was added and the reaction
mixture was
stirred at RT for 3 hours. The solvent was evaporated and the residue was
taken up in EtOAc
and sat. aq. Na2CO3. The layers were separated and the organic layer was
washed with brine,
dried (Na2SO4) and evaporated to dryness. Purification by column
chromatography (0% to
7% MeOH in DCM) gave the desired product. LC/MS (10% to 99%): M/Z (M+H)+ (obs)
=
350;tR=2.22.
[00312] tert-Buty14-(2-aminophenethylamino)piperidine-l-carboxylate
pyBoc
[00313] NH2 H
[00314] To a solution of tert-butyl 4-(2-nitrophenethylamino)piperidine-l-
carboxylate
(10.5 g) in EtOH (180 ml) was added 10% Pd/C (1.05 g) and the reaction mixture
was stirred
at RT under an atmosphere of H2 overnight. The reaction mixture was filtered
and the
resulting solution was evaporated to dryness giving the desired product (9.6
g). LC/MS (10%
to 99%): Ivl/Z (M+H)+ (obs) = 320 ; tR = 2.06.
[00315] tert-Butyl 4-(1,2,4,5-tetrahydro-2-oxobenzo[d] [1,3]diazepin-3-
yl)piperidine-
1-carboxylate
Page 103 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
N~N-Boc
[00316] H O
[00317] To a solution of tert-butyl4-(2-aminophenethylamino)piperidine-l-
carboxylate
(6.9 g, 30 mmol) in DMF (110 ml) was added CDI (4.86 g, 30 mmol) in portions
followed by
stirring the reaction mixture at RT for 2 h. The reaction mixture was diluted
with water and
extracted with EtOAc. The organics were combined, washed with water, brine,
and
evaporated to dryness to give the desired product. LC/MS (10% to 99%): M/Z
(M+H)+ (obs)
=346; tR=3.24.
[00318] 4,5-Dihydro-3-(piperidin-4-yl)-1H-benzo[d][1,3]diazepin-2(3H)-one
~NH
C~D) N
[00319] H O
[00320] To a solution of tert-butyl 4-(1,2,4,5-tetrahydro-2-
oxobenzo[d][1,3]diazepin-3-
yl)piperidine-l-carboxylate (10 g, 2.89 mmol) in DCM (5 ml) was added TFA (5
ml) and the
reaction mixture was stirred at RT for 1 h. The reaction mixture was
evaporated, then co-
evaporated with EtOH (2x), to give the TFA salt of the desired product
(Quant.). LClMS
(10% to 99%): M/Z (M+H)+ (obs) = 246 ; tR = 1.75.
[00321] tert-Butyl4-(2-atninopyridin-3-ylamino)piperidine-l-carboxylate
Boc
NH = =
[00322] N NH2
[00323] To a solution of 2,3 -di aminopyri dine (3.0 g, 27.5 inmol) in DCE (45
ml) was
added tert-butyl 4-oxopiperidine-l-carboxylate (5.75 g, 28.8 mmol) and the
reaction mixture
stirred for 5 min at RT before the portion-wise addition of NaBH(Oac)3 (8.7 g,
41.7 nunol)
and continued stirring at RT until the reaction judged complete by LCMS. The
reaction was
quenched with 5% NaOH, the layers separated and the organic layer was dried
over Na2SO4.
Evaporation gave the desired product as a brown solid (4.96 g). LC/MS (10% to
99%): M/Z
(M+H)+ (obs) = 293 ; tR = 2.31.
[00324] ' tert-Butyl 4-(2,3-dihydro-2-oxoimidazo [4,5-b] pyridin-1-
yl)piperidine-l-
carboxylate
Page 104 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Boc
~
I i N
>=
[00325] N H =
[00326] To a solution of tert-Butyl 4-(2-aminopyridin-3-ylamino)piperi dine- 1
-carboxyl ate
(3.0 g, 10.3 mmol) in CH3CN (206 ml) at RT was added CDI (4.2 g, 25.7 mmol) in
portions
and the reaction mixture was stirred at RT for 16 hours. The reaction mixture
was evaporated
to dryness and the residue was take up in DCM and water. The layers were
separated and the
organic layer was washed with brine, dried (Na2SO4) and evaporated to dryness.
Purification
by column chromatography (1-10% MeOH in DCM) gave the desired solid as a beige
solid
(3.55 g). LC/MS (10% to 99%): M/Z (M+H)+ (obs) = 319 ; tR = 2_31.
[00327] 1-(Piperidin-4-yl)-1H-irnidazo[4,5-b]pyridin-2(3H)-one
H
N
N>==O
[00328] N H
[003291 A solution of tert-butyl 4-(2,3-dihydro-2-oxoimidazo[4,5-b]pyridin-l-
yl)piperidine-l-carboxylate (3.39 g, 10.7 mmol) in 2N HCI in Et20 (20 ml) was
stirred from
0 C to RT over 2 h. The solvent was evaporated and the residue triturated with
Et20, filtered
washed with -Et20 and dried to give the bis-HCI Salt of the desired product
(2.62 g). LC/MS
(10% to 99%): M/Z (M+H)+ (obs) = 219 ; tp, = 0.36.
[00330] 2-(2,4-Dimethoxybenzylaniina)pyridine-3-carbonitrile
CN
N H ~
~
O ~ O
[00331] 1
[00332] To a solution of 2-chloro-3-cyanopyridine (4.0 g, 28.9 mmol) in DMA
(58 ml)
was added 2,4-dimethoxybenzealdehyde (5.2 mi, 34.6 mmol) and TEA (4.8 ml (34.6
mmol)
and the reaction mixture stirred at 80 C for 4 hours. The reaction mixture
was poured into
water and extracted with Et20. The organics were combined, dried (NaySO4) and
evaporated
to dryness. Column chromatography (0.5% to 5% EtOAc (with 0.1% TEA) in DCM)
gave
the desired product. LC/MS (10% to 99%): M/Z (M+H)+ (obs) = 270; tR = 3.05.
Page 105 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00333] N-(2,4-Dimethoxybenzyl)-3-(aminomethyl)pyridin-2-annine
I ~ NH2
~
N H 0 I/ Oi
[00334] 1
[00335] A solution of 2-(2,4-Dimethoxybenzylamino)pyridine-3-carbonitrile
(0.55 g, 2.04
mmol) and LiAlH4 (2.2 ml of 1N, 4.4 mmol) was stirred at RT until the reaction
was judged
complete by LCMS. The reaction was quenched with sat. aq. Na2CO3 and the
layers were
separated. The organic layer was dried (Na2SO4) and the solvents removed under
reduced
pressure giving the desired product which was used without further
purification. LC/MS
(10% to 99%): M/Z (M+H)a- (obs) = 274 ; tR = 0.28.
[00336] tert-Butyl-4-((2-(2,4-dimethoxybenzylamino)pyridin-3-
yl)methylamino)piperidine-l-carboxylate
N N,Boc -
"~/
N ~ - ) O Oi
a H
[00337] 1
[00338] To a stirred solution of N-(2,4-Dimethoxybenzyl)-3-
(arninomethyl)pyridin-2-
amine (2.04 mmol) and tert-butyl 4-oxopiperidine-1 -carboxylate (0.41 g, 2.04
mmol) in DCE
(8 ml) and AcOH (115 L, 2.04 mmol) was added NaBH(OAc)3 (0.43 g, 2.04 mmol)
and the
reaction stirred at RT until judged complete by LCMS. The reaction mixture was
diluted
with DCM and sat. aq. Na2CO3, the layers were separated and the organic layer
was dried
(Na2SO3) and evaporated to dryness. Purificaion by column chromatography
(MeOH/DCM)
gave the desired product (0.64g, 69%). LC/MS (10% to 99%): M/Z (M+H) + (obs) =
457 ; tR
= 2.19.
[00339] tert-Butyl 4-(1-(2,4-dimethoxybenzyl)-1,2-dihydro-2-oxopyrido[2,3-
d] pyrimidin-3 (4H)-yl)piperidine-l-carboxylate
QCX9
N O
~-O
O [00340] I I
Page 106 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00341] To a solution of tert-butyl 4-((2-(2,4-dimethoxybenzylamino)pyridin-3-
yl)methylamino)piperidine-l-carboxylate (2.89 g, 6.33 mmol) in DMF (42 ml) was
added
CDI (1.23 g, 7.6 mmol) in portions and the reaction mixture was stirred at 120
C for 2 hours.
A further portion of CDI was added (0.82 g) was added and the reaction mixture
stirred at
130 C for 6 hours, followed by stirring at RT for 16 hours. The reaction was
diluted with
water and extracfed with DCM. The organics were combined, dried (NaSO4) and
evaporated
to dryness. Purification by col.umn chromatography (10 to 80% EtOAc in Hx)
gave the
desired product (1.17 g). LC/MS (10% to 99%): M/Z (M+H)+(obs) = 483 ; tR =
3.58.
[00342] 3,4-Dihydro-3-(piperidin-4-yl)pyrido[2,3-d]pyrimidin-2(IH)-one
~NH
N N O
[00343] H
[00344]
[00345] 2-(4-Oxo-2-phenyl-3-((pyridin-4-yl)methyl)thiazolidin-5-yl) acetic
acid
P
O S
HO N
O ~ ~
1003461 N
[00347] A solution of benzaldehyde (0.75 mmol, 79.6 n7g) and 2-(pyridin-4-
yl)ethanamine
(97.3 mg, 0.9 mmol) in DMF (0.5 ml) with 4A molecular sieves was heated at 80
C for 2
hours. A solution of mercaptosuccinic acid (1.13 mmol, 168 mg) in DMF (0.2 ml)
was added
and the reaction was heated at 80 C for an additional 16 hours. The reaction
mixture was
diluted with water and extracted with EtOAc. The organic layer was washed with
IN HCI,
water and evaporated to dryness to give the desired product which was used
without further
purification. LC/MS (10% to 99%): M/Z (M+H)+ (obs) = 329 ; tR = 1.95.
[00348] 1-(1-(2-(4-Oxo-2-phenyl-3-((pyridin-4-yl)methyl)thiazolidin-5-
yI)acetyl)piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one (Compound # 45)
Page 107 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
~ ~
`
~ S N
O
N N
[00349] H
[00350] To a solution of 2-(4-oxo-2-phenyl-3-((pyridin-4-yl)methyl)thiazolidin-
5-yl)acetic
acid (0.15 mmol, 49 mg), 1-(piperidin-4-yl)-1 H-benzo[d]imidazol-2(3H)-one
(0.15 mmol, 33
mg) and D'PEA (0.375 mmol, 65.3 }rl) in 4:1 CH3CN:DMF (0.5 ml) was added HATU
(0.18
mmol, 68 mg) and the reaction mixture was stirred at room temperature for 16
h. Purification
by preparative reverse phase HPLC using 10%-99% CH3CN (0.035% TFA)/H20 (0.05%
TFA) gave the title compound. LC/MS (10% to 99%): 1VI/Z (M+H)} (obs) = 528.1 ;
tR = 2.28.
H NMR (400 MHz, CDC13) 8 9.00 (s, 1H), 8.60 (d, J = 6.3 Hz, 2H), 7.40 - 7.38
(in, 2H),
7.33 - 7.29 (m, 5H), 7.06 - 6.92 (m, 4H), 5.55 - 5.53 (m, IH), 4.55 (d, J =
4.4 Hz, 2H), 4.45 -
4.42 (m, 3H), 4.07 (d, m, 2H), 3.42 - 3.41 (m, 1 H), 3.20 - 3.15 (m, 1H), 3.01
- 2.90 (m, 1 H),
2.66 (m, 2H), 1.88 (m, 2H) ppm.
[00351] 2-(3-Methyl-4-oxo-2-phenylthiazolidin-5-yi)acetic acid
~ ~
O S
N--
HO
[00352] O
[00353) A solution of benzaldehyde (0.75 mmol, 79.6 mg) and methylamine
hydrochloride
(60.8 mg, 0.9 mmol) in DMF (0.5 ml) with 4A molecular sieves was heated at 80
C for 2
hours. A solution of inercaptosuccinic acid (1.13 mmol, 168 mg) in DMF (0.2
ml) was added
and the reaction was heated at 80 C for an additional 16 hours. The reaction-
mixture was
diluted with water and extracted with EtOAc. The organic layer was washed with
1N HCI,
water and evaporated to dryness to give the desired product which was used
without further
purification.
[003541 3,4-Dihydro-3-(I-(2-(3-methyl-4-oxo-2-ph enylthiazolidin-5-
yl)acetyl)piperidin-4-yl)quinazolin-2(1H)-one (Compound # 273)
Page 108 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
0
(a: ~N
O N
1.003551 0
[003561 To a solution of 2-(3-methyl-4-oxo-2-phenylthiazolidin-5-yl)acetic
acid (0.2
mmol, 50 mg), 3,4-dihydro-3-(piperidin-4-yl)quinazolin-2(1H)-one TFA salt
(0.15 mmol, 49
mg) and D'PEA (0.375 mmol, 65.3 p,l) in 4:1 CH3CN:DMF (0.5 ml) was added HATU
(0.18
mmol, 68 mg) and the reaction mixture was stirred at room temperature for 16
h. Purification
by preparative reverse phase HPLC using 10 Jo-99 Jo CH3CN (0.035% TFA)/H20
(0.05%
TFA) gave the title compound. LC/MS (10% to 99%): M/Z (M+H)+ (obs) = 465.5 ;
eR = 2.18.
'H NMR (400 MHz, CDC13) S 7.34 - 7.22 (m, 5H), 7.12 (t, J = 7.5 Hz, 1H), 7.00
(d, J = 7.5
Hz, 2H), 6.95 - 6.89 (in, 2H),6.61 (d, J = 7.8 Hz, 2H), 5.46 - 5.41 (m, 1 H),
4.70 (m, 1 H), 4.56
(m, 1H), 4.26 (m, 3H), 3.86 (m, 1H), 3.50 (m, 1H), 3.32 (m, iH), 3.12 - 3.08
(m, 1H), 2.89 -
2.73 (m, 114), 1.69 (m, 3H) ppm.
[003571 2-(3-Isopropyl-4-oxo-2-phenylthiazolidin-5-yl)acetic acid
O S !
P
N--f\
HO
[00358] 0
[003591 A solution of benzaldehyde (0.75 mmol, 79.6 mg) and isopropylamine
(53.1 mg,
0.9 mmol) in DMF (0.5 ml) with 4A molecular sieves was heated at 80 C for 2
hours. A
solution of inercaptosuccinic acid (1.13 mmol, 168 mg) in DMF (0.2 ml) was
added and the
reaction was heated at 80 C for an additional 16 hours. The reaction mixture
was diluted
with water and extracted with EtOAc. The organic layer was washed with IN HCI,
water and
evaporated to dryness to give the desired product which was used without
further
purification.
[003601 3,4-Dihydro-3-(1-(2-(3-isopropyl-4-oxo-2-ph enylthiazolidin-5-
yl)acetyl)piperidin-4-yl)quinazolin-2(1H)-one (Compound #255)
Page 109 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
O S ~
N
N~Jj~
(DO"
N O
[00361] H
[00362] To a solution of 2-(3-isopropyl-4-oxo-2-phenylthiazolidin-5-yl)acetic
acid (0.2
mmol, 56 mg), 1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one (0.15 mmol, 33
mg) and
D'PEA (0.375 mmol, 65.3 l) in 4:1 CH3CN:DMF (0.5 ml) was added HATU (0.18
inmol, 68
mg) and the reaction mixture was stirred at room temperature for 16 h.
Purification by
preparative reverse phase HPLC using 10%-99% CH3CN (0.035% TFA)/H20 (0.05%
TFA)
gave the title compound. LC/MS (10% to 99%): M/Z (M+H)+ (obs) = 493.5 ; tR =
3.1. H
NMR (400 MHz. CDC13) S 7.31 - 7.25 (m, 5H), 7.14 - 7.10 (m, 1H), 7.05 (s, 1H),
7.00 (m,
1 H), 6.93 - 6.89 (m, 1 H), 6.62 (d, J = 7.8 Hz, IH), 5.56 (m, I H), 4.72 (m,
1 H), 4.47 - 4.41 (m,
2H), 4.27 - 4.19 (m, 2H), 4.02 - 3.96 (m, 1 H), 3.87 (m, 1 H), 3.36 - 3.29 (m,
IH), 3.13 - 3.10
(m, 1H), 2.70 (m, 2H), 1.70 - 1.60 (m, 3H), 1.20 (dd, J = 2.0, 6.9 Hz, 3H),
0.94 (m, 3H).
[003631 2-(3 Isopentyl-4-oxo-2-phenylthiazolidin-5-yl)acetic acid
0 S N
HO~~ " ~~~
[00364]
1003651 A solution of benzealdehyde (5.06 ml, 50 mmol) and isopentylamine
(5.82 ml, 50
mmol) was stirred at 80 C for 2 hours before the addition of
inercaptosuccinic acid (7.51 g,
50 mmol) and a further 16 hours of stirring at 80 C. The reaction mixture was
poured into
water and extracted with EtOAc. The organics combined, dried and evaporated to
dryness.
Purification by column chromatography (EtOAc / Hx) gave the desired product as
a yellow
oil (11.3 g).
[00366] Ethy12-(3-isopentyl-4-oxo-2-phez+ylthiazolidin-5-yl)acetate
~ ~
.--
O S
/'- " N-\--~
[00367] 0
Page 110 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00368] A solution of 2-(3-lsopentyl-4-oxo-2-phenylthiazolidin-5-yl)acetic
acid (2.2 g, 7.2
mmot) in EtOH (20 ml) and H2SO4 (1 ml) was refluxed for 16 hours. The solution
was
evaporated to dryness and the residue was taken up in EtOAc and washed with
sat. aq.
Na2CO3 (3x), brine and evaporated to give the desired product as an oil.
[00369] Ethyl2-(3-isopentyl-4-oxo-2-phenylthiazolidin-5-yl)propanoate
0 S N
[00370] O No
[00371] To a stirred solution of ethyl 2-(3-isopentyl-4-oxo-2-
phenylthiazolidin-5-
yl)acetate (84 mg; 0.25 mmol) in THF at 0 C was added LiHMDS (0.28 ml of I N,
0.28
mmol) dropwise and the reaction mixture was stirred from 0 C to RT over 16
hours. The
reaction mixture was poured in to 1 N HCl and extreacted with EtOAc (4x). The
organics
were combined, dried (MgSO4) and evaporated to dryness. Purification by
preparative TLC
(7 : 1; Hx : EtOAc) gave the the desired product as an oil (12 ing).
[00372] 2-(3-Isopentyl-4-oxo-2-phenylthiazolidin-5-yl)propanoic acid
~ \
_-
0 S N
HO ~~
[00373]
[00374] A solution 2-(3-isopentyl-4-oxo-2-phenylthiazolidin-5-yl)propanoic
acid (12 mg,
0.034 mmol) and NaOH aq. (0.068 ml of 1N, 0.068 mmol) in MeOH (0.2 ml) was
stirred at
60 C for 16 hours. The solution was neutralized with 1 N-HCi (0.068 ml of I
N), the
solvents removed and the crude product used with out further purification.
[00375] 3-(1-(2-(3-Isopentyl-4-oxo-2-phenylthiazolidin-5-
yl)propanoyl)piperidin-4-y1)-
3,4-dihydroquinazolin-2(1H)-one (Compound #156)
,/~~ p ~ 1 .
N--( ,N S
N--~ t-- / N
O
[00376] O
[00377] To a solution of 2-(3-Isopentyl-4-oxo-2-phenylthiazolidin-5-
yl)propanoic acid (11
mg, 0.034 mmol), 3,4-Dihydro-3-(piperidin-4-yl)quinazolin-2(1H)-one'TFA (17
mg, 0.051
mmol) and D'PEA (24 ul, 0.14 mmol) in DMF (0.2 ml) was added HATU (17 mg,
0.044
Page 111 of 1 S3
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
mmol) and the reaction mixture was stirred at RT for 16 hours. Purification by
preparative
reverse phase HPLC using 10%-99% CH3CN (0.035% TFA)/Ha0 (0.05% TFA) gave the
title compound.
[00378] 3-Isopentyl-2-phenylthiazolidin-4-one
~ ~ ~
.---
S
.[003791 0
[00380] A solution of isopentylamine (0.58 ml, 5 mmol), benzealdehyde (1 ml,
10 mmol)
and mercaptoacetic acid (1.05 ml g, 15 mmol) in THF (7 ml) and
trimethoxyorthoformate (2
ml) was stirred at 75 C for 16 hours. The RM was poured in to water and
extracted with
EtOAc (3x). The organics were combined, washed with IN HCl (2x), brine, dried
(MgSO4)
and evaporated to dryness. Purification by column chromatography (10 - 25 1o
EtOAc in Hx)
gave the desired product as an oil (1.07 g, 86%).
[003811 Ethyl 2-(3-isopentyl-4-oxo-2-phenylthiazolidin-5-ylidene)acetate
O S N
[00382] O
[003831 To a stirred solution of 3-isopentyl-2-phenylthiazolidin-4-one (0.25
g, I mmol) in
THF was added LDA (1.1 ml of - I M in THF; freshly prepared from nBuLi and
Diisopropylamine) at -78 C and the reaction mixture was allowed to warm to
room
temperature. Ethyl glyoxalate (0.24 ml of --50% w / v in toluene, 1.2 mmol)
was added and
the reaction mixture was stirred at room temperature for'16 hours. The
reaction mixture was
poured into I N HC1 and extracted with EtOAc (3x). The organics were combined,
washed
with brine, dried (MgSO4) and evaporated to dryness. Purification by column
chromatography (5 to 15% EtOAc in Hx) gave the desired product as an oil.
[00384] 2-(3-Isopentyl-4-oxo-2-phenylthiazolidin-5-ylidene)acetic acid
.--
O S N
00385l HO
0 [
Page 112 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00386] To a solution of ethyl 2-(3-isopentyl-4-oxo-2-phenylthiazolidin-5-
ylidene)acetate
(0.031 g, 0.1 mmol) and aq. NaOH (0.3 ml of 1 N) in MeOH was stirred at 40 C
for 2 hours.
HCI (0.5 ml of 1 N) was added and the MeOH was evaporated. Water and EtOAc was
added
and the layers separated. The aqueous layer was extracted with EtOAc (2x), all
organic
layers were combined, dried (MgSO4) and evaporated to dryness to give the
desired product
as an orange oil (11 mg, 36%).
[00387] 3,4-Dihydro-3-(1-(2-(3-isopentyl-4-oxo-2-phenylthiazolidin-5-
ylidene)acetyl)piperid.in-4-yl)quinazolin-2 (1H)-one
O
HN--<\ ~-/ - N
(00388] o
[00389] To a solution of 2-(3-isopentyl-4-oxo-2-phenylthiazolidin-5-
ylidene)acetic acid
(11 mg, 0.036 mmol), 3,4-Dihydro-3-(piperidin-4-yl)quinazolin-2(1H)-one'TFA
(18 mg,
0.054 mmol) and D`PEA (22 ul, 0.14 mmol) in DMF (0.2 ml) was added HATU (16
mg,
0.043 mmol) and the reaction mixture was stirred at RT for 16 hours.
Purification by
preparative reverse phase HPLC using 10%-99% CH3CN (0.035% TFA)/H20 (0.05%
TFA)
gave the title compound.
[00390] Preparation A: Synthesis of 1'H-spiro[piperidine-4,4'-quinolin]-
2'(3'H)-one
N c N N Cbz
HCI/MeOH CbzCI
/ I ---' /
\ HCf
O O O
15a 15b 95c
Cbz
CIN` GI Cbz H
Y N iN
NH2OH.t-ICI C' HN Pd/C
N"OH DMF H O ~ \ I N O
H
15d
15e 15f
[00391] The mixture of tert-butyl 3-oxo-2,3-dihydrospiro[indene-1,4'-
piperidine]-1'-
carboxylate (20 g, 66.4 mmol) and MeOH/HCl (2.5 mol/L, 100 mL) were stirred
overnight_
Page 113 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
After evaporation the residue was washed by petroleum ether to provide
spiro[indene-1,4'-
piperidin]-3(2H)-one hydrochloride (15.4 g, 97.6%).
100392] To a solution spiro[indene-1,4'-piperidin]-3(2H)-one hydrochloride
(5.0 g,
24.84 mmol) and Et3N (7.54 g, 74.53 mol) in CH2CI2 (50 mL) was added drop-wise
Cbz-Cl
(4.66 g, 27.33 mmol) at 0 C. The reaction was allowed to warm to room
temperature and
stirred, overnight. The precipitate was filtered, washed with Et20 and dried
to fizrnish benzyl
3-oxo-2,3-dihydrospiro[indene-1;4'-piperi dine]-1'-carboxylate (6.1 g, yield
99%).
[00393] A solution of benzyl 3-oxo-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-
carboxylate (3 g, 10.3 mmol) in EtOH (30 mL) containing NHzOH.HCI (1.43 g,
20.6 inmol)
and NaOAc (1.52 g, 18.53 mmol) was heated under reflux for 1.5 h. The solvent
was
removed by evaporation and the residue was partitioned between CHZCl2 and
water. The
organic phase was washed with brine, dried over Na2SO4, and concentrated to
provide benzyl
3-(hydroxyimino)-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-carboxylate (3.14
g, yield
99%), which was used= directly in the next step.
[00394] 2,4,6-trichloro-[1,3,5]-triazine (1.32 g, 7.16 mmol) was added to DMF
(9.6
mL) maintained at 25 C. The reaction was monitored by TLC until TCT was
consumed.
Then benzyl3-(hydroxyimino)-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-
carboxylate (1.6
g, 4.77 mmol) in DMF (17 mL) was added. After the addition, the mixture was
stirred at
room temperature overnight. Water was added. The mixture was extracted with
EtOAc. The
combined organic layers were washed with sat. NaaCO3, followed by 1N HCI and
brine,
dried over Na2SO4 and concentrated. The residue was purified by prep HPLC to
obtain
benzyl 2'-oxo-2',3'-dihydro-1'H-spiro[piperidine-4,4'-quinoline]-1-carboxylate
(260 mg, yield
16%).
(00395] The mixture of benzyl 2'-oxo-2',3'-dihydro-1'H-spiro[piperidine-4,4'-
quinoline]-I-carboxylate (1.2 g, 3.4 mmol) and Pd/C (200 mg) in MeOH (20 mL)
was
hydrogenated under atmosphere pressure at room temperature for 3 h. The
catalyst was
filtered and the filtrate was concentrated under reduced pressure. The residue
was purified by
preparative HPLC twice to give 1'H-spiro[piperidine-4,4'-quinolin]-2'(3'H)-one
(110 mg, 11
%) as a TFA salt. 'H NMR (CDC13) S 7.65 (d, J=7.5 Hz,1 H), 7.29-7.45 (m, 3 H),
3.45 (d, J
= 12.3 Hz, 2 H), 3.20 (t, J= 12.3 Hz, 2 H), 2.96 (s, 2 H), 2.10-2.21 (m, 2 H),
1.70 (d, J= 14.1
Hz, 2 H). MS (ESI) m/z 217.06 [M+H]'.
[00396] Preparation B: Synthesis of spiro[4H-3,1-benzoxazine-4,4'-piperidin]-
2(1FI)-
one
Page 114 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Boc H
Boc N
HN"Boc
I\ + N 2.3 eq tBuLi I\ O TFA O
700C to RT CH2CI2 --__O THF F { H O
[003971 N-Boc-aniiine (16.12 g, 83.4 mmol) was dissolved in anhydrous
tetrahydrofuran (120 mL) and cooled to -70 C. To this solution was added
dropwise, under
nitrogen, a 1.7 M solution of tert-butyllithium in pentane (110 mL, 187 mmol)
at -70 C.
After 30 min at -70 C, the solution was warmed to -20 C and maintained at
that temperature
for 2 h. The solution was again cooled to -70 C and treated dropwise with a
solution of N-
Boc-4-piperidone (15.98 g, 80.2 mmol) in anhydrous tetrahydrofuran (50 mL).
The solution
was slowly warmed to room temperature, treated with potassium tert-butoxide
(25 mg) and
stirred at room temperature overnight under nitrogen. The solution was diluted
with diethyl
ether (300 mL), cooled in an ice-H20 bath and adjusted to pH 7 with 1.0 N HCl
(aq). The
layers were separated and the aqueous layer extracted once with diethyl ether
(100 mL). The
pooled organic layers were washed with H20 and saturated brine, then dried
over Na2SO4 and
filtered. The filtrate was concentrated under reduced pressure to afford 39.09
g crude product
as a viscous pale yellow oil. The crude product was purified via silica gel
flash
chromatography (25-50% ethyl acetate in hexanes) to afford tert-butyl 2-oxo-
1,2-
dihydrospiro[benzo[d][1,3]oxazine-4,4'-piperidine]-1'-carboxylate as a pale
yellow solid
(8.687 g, 34% yield). LC/MS m/z 319.0 [M+H]+, retention time 2.72 min (RP-
C18,10-99%
CH3CN/0.05% TFA); 'H-NMR (400 MHz, CDC13) & 9.06 (br s, 1 H), 7.28 (m, 1 H),
7.12 (m,
2H), 6.91 (d, J= 8.5 Hz, 1 H), 4.12 (br d, J= 9.9 Hz, 2H), 3.36 (br t, J= 12.4
Hz, 2H), 2.13
(br d, J= 13.1 Hz, 2H), 1.98 (m, 2H), 1.51 (s, 9H).
[003981 tert-Butyl 2-oxo-1,2-dihydrospiro[benzo[d] [ 1,3]oxazine-4,4'-
piperidineJ-1'-
carboxylate (6.71 g, 21.1 mmol) was dissolved in dichloromethane (50 inL),
treated with
trifluoroacetic acid (20 mL) and stirred at room temperature for 45 min. The
reaction was
concentrated under reduced pressure, re-dissolved in acetonitrile and re-
concentrated under
reduced pressure. The crude TFA salt was cooled in an ice-H20 bath, dissolved
in ice-cold
saturated brine (20 mL) and H20 (50 mL) and basified with ice-cold 35%
NaOH'(aq). A
small amount of product (obtained from extraction with 50 mL ethyl acetate)
was added to
the aqueous layer to initiate crystallization. The suspension obtained was
cooled in an ice-
H20 bath, filtered, rinsed with ice-cold H20 and dried to afford 3.071 g
Page 115 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
spiro[benzo[d][1,3]oxazine-4,4'-piperidin]-2(1H)-one free base as a white
crystalline solid.
An additional 800 mg free base was obtained via extraction of the mother
liquor with ethyl
acetate (10 x 50 mL) and subsequent trituration of the crude free base with
acetonitrile
(overall yield = 84%). LC/MS m/z 219.2 [M+H]+, retention time 0.58 min (RP-
C18,10-99%
CH3CN/0.05% TFA); 'H-NMR (400 MHz, DMSO-d6) S 10.17 (br s, 1H), 7.23 (m, 2H),
7.02
(m, 1 H), 6.87 (dd, J= 8.2, 1.2 Hz, IH), 2.89 (m, 2H), 2.82 (m, 2H), 1.84 (m,
4H).
1003991 1-B enzyl-4- (2-chloro qu inolin-3-yl) piperidin-4-ol
Bn, N
OH
CI N
To a solution of LDA (3.4 ml of 2 M in Hept / THF) at -78 C in THF (5 ml) was
added a
solution of 2-chloroquinoline (1.0 g. 6.11 mmol) in THF (10 ml) dropwise, and
the reaction
mixture stirred at -78 C for 1 hour before a solution of 1-benzylpiperidin-4-
one (1.22 g, 6.22
mmol) in THF (2 ml) was added dropwise. The reaction mixture was stirred from -
78 C to
RT over two hours, cooled to -20 C, quenched with water and extracted with
EtOAc. The
organics combined, dried (Na2SO4) and evaporated to dryness. Purification by
column
chromatography (1 to 15% MeOH in DCM) gave the desired product. LC/MS (10% to
99%):
M/Z (M+H)+ (obs) = 353; tR = 2.24.
[00400] 3-(1-Benzyl-1,2,3,6-tetrahydropyridin-4-yl)quinolin-2(1H)-one
Bn, N
O N !-!
X~O
A solution of 1-Benzyl-4-(2-chloroquinolin-3-yl)piperidin-4-ol (1 g, 2.84
mmol) in 6 N HCI
(9 ml) was heated at 100 C for 8 h. The reaction mixture was cooled, water
was added and
the precipitated product was filtered and dried (0.27 g). LC/MS (10% to 99%):
M/Z (M+H)+
(obs) = 317; tR = 2.18.
[00401] 3-(Piperidin-4-yl)quinolin-2(1H)-one
Page 116 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
HN
r)O
O N H
A solution of 3-(1-Benzyl-1,2,3,6-tetrahydropyridin-4-y1)quinolin-2(IH)-one
(0.25 g. 0.29
mmol) and 10% Pd/C (130 mg) in MeOH (20 ml) was stirred at 40 C for 6 hours.
The
catalysis was filtered and solvent evaporated affording the desired product.
LC/MS (10% to
99%): M/Z (M+H)" (obs) = 229; tR = 1.27.
[00402] Analytical data for certain compounds of the present invention are
shown
below in Table 2.
[004031 Table 2
Page 117 of 153
7
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
.: .~.R . =
LC1MS :.:LC/RT'=, , =LC/MS .' LC/RT ;
Grnpd.# tiM+~'` ` ~~n,' mpd~#;;`..=~:M*1 rtii
1 533.5 3.36 45 542.5 2.13
2 633. 1.45 46 542.5 2.39
3 603.5 3.35 .. 47 620.5 1.27
4 551.5 3.22 48 585.3 3.82
599.5 3.07 49 634.5 1.74
6 549.5 1.98 50 632.7 1.49
7 632.7 2.34 51 563.7 2.08
8 549.5 3.63 52 661.7 1.47
9 507.5 3.24 53 568.5 2.22
620.5 1.89 54 585.3 3.84
11 563.5 3.77 55 619.5 3.68
12 521. 3.31 56 612.5 1.52
13 621.5 3.77 57 555.5 2.13
14 595.5 3.8 58 555.3 3.37
559.3 3.72 59 606.4 2.39
16 597.5 3.62 60 601.5 1.37
17 633. 1_47 61 563.5 3.34
18 569.5 3.37 62 573.4 3.06
19 593.5 3.17 63 546.5 3.27
615.5 3.23 64 563.5 3.36
21 577.7 3.94 65 641.3 3.89
22 619.7 1.39 66 573.4 3.05
23 621.5 3.8 67 539.5 3.45
24 507.3 3_25 68 667.5 1.42
571.5 3.72 69 508.6 3.1
26 567.5 3.72 70 634.5 1.93
27 562.4 3.28 71 543.5 2.31
28 615.7 1.41 72 690.5 2.16
29 506.4 2.96 73 563.5 3.82
509.7 2.91 74 557.5 4.15
31 545.7 3.64 75 501.5 3.5
32 527.3 1_98 76 589.5 1.98
33 555.3 3_55 77 562.5 2.41
34 647. 1.46 78 535.5 3.51
529.3 3.12 79 587.3 3.79
36 583.3 1.86 80 551.5 3.47
37 541.7 3.8 81 665.7 . 1.46
38 507.5 3.25 82 550.5 2.42
39 626.7 1.53 83 549.5 3.12
633.5 1.42 84 531. 1.78
41 535.5 3.54 85 517.5 1.21
42 704.7 2.23 86 513.3 3.53
43 536.3 2.24 87 525.5 3.5
44 571.3 3.57 88 615.5 1.88
Page 118 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
LC/MS.. LGI~iT :LGlMS LG'LRT
;Crnpd '#
iriin
89 603.5 3.77 133 551.5 3.22
90 551.5 3.22 134 549.5 1.98
91 541.5 2.05 135 597.5 3.65
92 583.5 3.6 136 607.3 1.96
93 535.5 3.51 137 583.5 3.58
94 574.5 2. 138 519.5 3.28
95 527.3 1.96 139 551.5 3.44
96 505.3 3.2 140 626.5 1.58
97 487.5 1.41 141 493.1 3.76
98 549.7 3.63 142 681.7 1.47
99 733.7 2.26 143 538.7 3.52
100 553.5 2.08 144 620.7 1.68
101 549.5 1.89 145 601.5 3.15
102 619.5 2.58 146 469.5 2.56
103 579.5 3.64 147 561.5 3.7
104 507. 3.22 148 522. 1.52
105 487.5 1.37 ' 149 553.5 3.57
106 604.7 2.21 150 648.7 1.79
107 507.5 3.27 151 515.7 1.96
108 528.1 2.28 152 578.5 1.89
109 533.3 3.11 153 612.5 1.46
110 533. 3.28 154 541.7 2.06
111 492.5 3.43 155 571.5 3.6
112 567.5 3.53 156 535. 3.52
113 535.5 2.95 157 534.4 2.77
114 587.5 1.32 158 555.3 3.6
115 601.5 1.84 159 537.5 3.06
116 489.5 3. 160 620.5 1.89
117 551.5 3.12 161 619.7 1.38
118 537.4 2.95 162 619.7 1.36
119 633. 1.41 163 633.5 1.37
120 634.5 1.74 164 579.5 3.62
121 564.7 1.84 165 571.5 3.75
122 553,6 3.16 166 573.5 1.91
123 563.7 3.79 167 477.3 4.08
124 564.7 2.91 168 574.5 2.05
125 621.5 3.22 169 605.5 3.61
126 565.5 3.55 170 539.5 3.38
127 569.5 3.44 171 549.5 3.62
128 556.5 2.39 172 535.5 3,45
129 651.5 1.39 173 618.7 1.41
130 588.5 2.13 174 531.5 3.37
131 588.4 3.22 175 586.5 3.47
132 479.3 3.02 176 522. 1.52
Page 119 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
~ -:LG/M.S LCXRT: ~L~C%MS.. ILCIRT'=-:
Cmpd # Cmpd # .
__-~ = `M 1 ' .'min '' M+1 min
177 479.5 3. 221 587.5 3.7
178 579.5 3.39 222 549.5 1.98
179 589.5 3.3 223 508.2 2.53
180 563.7 3.67 224 587.5 1.78
181 567.5 3.95 225 593.5 3,53
182 522.5 2.54 226 513.3 3.29
183 601.7 1.83 227 546.5 3.28
184 606.5 1.83 228 581.3 3.27
185 556.5 2.48 229 647.7 1.42
186 521.6 2.67 230 491.3 2.97
187 527.3 3.19 231 569.5 3.5
188 513.5 3.52 232 592.5 3.38
189 559.3 3.13 233 597.3 1.91
190 547.3 3.15 234 571.5 3.77
191 547.5 1.74 235 618.7 2.28
192 487.5 1.82 236 556.5 2.2
193 719.7 2.21 237 569.5 3.43
194 621.5 3.79 238 551.5 3.13
195 525.5 3.41 239 543.5 3.3
196 515.7 3.07 240 637.4 3.61
197 577.7 3.84 241 539.5 3.57
198 578.5 1.64 242 601.3 3.64
199 647.7 1.41 243 633.5 3.8
200 510.8 2.76 244 567.5 3.79
201 529.5 1.56 245 550.5 2.53
202 557.5 3.44 246 627.5 2.06
203 589.4 3.21 247 651.5 3.69
204 549.5 3.58 248 587.5 3.02
205 579.5 3.39 249 518.2 3.49
206 589.7 3.15 250 523.5 3.04
207 529.5 3.79 251 507.5 3.24
208 559.5 3.38 252 553.3 3.72
209 529.5 1.61 253 581.3 3.6
210 583.5 3.53 254 491.3 3.06
211 578.5 1.92 255 493.5 3.1
212 557.5 3.44 256 607.5 1.98
213 578.4 2.95 257 645.7 1.38
214 565.5 3.22 258 543.5 3.25
215 618.7 2.28 259 519. 3.07
216 589.5 3.57 260 601.5 1.34
217 585.3 3.77 261 446.5 3.03
218 471.3 2.79 262 563.7 3.75
219 607.5 1.99 263 592.7 1.98
220 537.5 3. 264 647.5 3.57
Page 120 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
:_- _ _... ..
C/M:S LC/RT:, LCl:M.S: LGZRT
;Cmp'd #'; .jyM+1 !min ':Cmpd # . M+1 min
265 572.7 3.4 309 609.5 2.18
266 522.5 2.96 310 623.7 2.23
267 553.5 1.91 311 595.5 2.12
268 601.3 3.61 312 549.7 2
269 493.3 3.13 313 539.5 1.32
270 506.4 2.77 314 539.6 4.31
271 505.5 3.14 315 575.7 2.08
272 551.5 3.41 316 561.5 2.02
273 465.5 2.88 317 561.5 2.04
274 620.7 1.24 318 575.5 2.09
275 485.5 3.31 319 620.4 1.61
276 515.7 1.98 320 634.4 1.68
277 588.7 2.05 321 633.7 1.47
278 542.5 2.35 322 633.7 1.5
279 597.5 3.65 323 651.5 1.42
280 561.5 1.78 324 665.5 1.48
281 499.1 3.98 325 663.7 1.42
282 575.5 3.05 326 663.7 1.45
283 569.5 3.45 327 620,5 1.32
284 493.5 3.13 328 634.7 1.41
285 607.5 1.54 329 620.5 1.3
286 563.5 3.32 330 634.5 1.35
287 567.5 2.85 331 605.5 1.48
288 570.5 1.33 332 619.7 1.56
289 573.5 1.27 333 647.7 1.41
290 606.5 1.62 334 647.7 1.49
291 573.5 1.73 335 633.5 1.45
292 590.7 2.16 336 633.5 1.47
293 550.5 = 1.79 337 638.5 1.26
294 592.7 1.79 338 637.5 1.39
295 587.5 1.34 339 679.7 1.49
296 620.7 1.71 340 693.7 1.52
297 587.5 1.79 341 666.5 1.34
298 604.5 2.23 342 691.5 1.51
299 564.5 1.88 343 665.7 1.41
300 606.5 1.86 344 665.5 1.42
301 620.7 1.78 ' 345 679.7 1.46
302 619 1.42 346 677.7 1.44
303 630 1.49 347 663.7 1.39
304 606.5 1.71 348 637.7 1.34
305 525.5 1.24 349 652.5 1.26
306 525.5 1.25 350 638.5 1.18
307 649.7 1.41 351 651.5 1.46
308 589.5 2.13 352 665.5 1.49
Page 121 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
.~Cm `d' .LC%MS T ;LCLMS ;<LC1R7:
=p;#~ -,.. ,Cmp~,~' .~ ..,..::.. .
M+1` min M*1 in353 638.5 1.26 397 638.5 1.24
354 637.7 1.42 398 651.7 1.47
355 667.5 1.47 399 651.7 1.47
356 681.7 1.52 400 707.7 1.58
357 654.7 1.29 401 707.7 1.53
358 679.7 1.46 402 710.9 1.5
359 653.7 1.41 403 737.7 1.68
360 651.5 1.45 404 634.5 1.32
361 665.5 1.5 405 661.7 1.49
362 638.5 1.29 406 634.5 1.3
363 663.7 1.44 407 661.5 1.49
364 675.7 1.56 408 710.7 1.51
365 689.5 1,63 409 737.7 1.69
366 662.5 1.38 410 633.5 1.4
367 695.7 2.02 411 680.7 2.29
368 709 2.01 412 647.7 1.5
369 647.7 1.52 413 620.5 1.27
370 661.7 1.55 414 630 1.5
371 663.7 1.45 415 621.6 1.59
372 677.7 1.5 416 535.5 1.92
373 633 1.42 417 508.6 1.62
374 633 1.42 418 648.7 1.3
375 661.6 1.53 419 675.7 1.52
376 707 1.56 420 549.6 1.51
377 661.6 1.61 421 632.4 1.88
378 634.6 1.26 422 634.6 1.44
379 730.9 1.31 423 620.6 1.83
380 634.6 1.37 424 520.4 1.65
381 703.9 1.16 425 605.4 1.52
382 636.7 1.33 426 607.4 1.12
383 649:7 1.48 427 593.4 1.52
384 715.7 1.61 428 591.6 1.7
385 663.7 1.54 429 707.4 1.77
386 622.7 1.28 430 707.4 1.91
387 688.7 1.43 431 707.4 1.77
388 647.7 1.43 432 707.4 1.87
389 691.7 1.53 433 709 1.49
390 664.7 1.32 434 709 1.49
391 651.7 1.47 435 623 1.21
392 733.7 2.07 436 680 1.29
393 706.7 1.82 437 680 1.29
394 638.5 0.95 438 682 1-3
395 693.6 1.63 439 733.6 1.68
396 693.6 1.7 440 733.6 1.7
Page 122 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
.. . .
. ., . .
Q%Ftl' '~ :.Y,:= .- . LC/MS 'LC/.RT
CInpd. # = {. . :=
M+1':i: min~s,
. . . . . . ., ,.
441 706.6 1.45 460 695.2 2.3
442 706.4 1.5 461 681.3 2.45
443 649.7 1.21 462 625.4 2.87
444 676.5 1.44 463 652.4 3.31
445 680 1.48 464 637.3 3.29
446 680 1.33 465 624.5 1.91
447 645.5 1.98 466 651.2 2.13
448 672.6 2.42 467 637.5 1.97
449 669.5 3.2 468 625.4 2.94
450 693.6 2.75 469 638.5 3.2
451 664.5 2.98 470 624.5 1.98
452 694.7 3.12 471 651.2 2.21
453 657 3.05 472 637.2 2.13
454 657.5 . 3.1 473 652.5 1.94
455 652.5 1.98 474 679.5 2.13
456 652.5 1.98 475 652.5 1.98
457 717.1 2.68 476 679.5 2.16
458 638.5 1.94 477 605 1.36
459 638.5 2
100404J Measuring CGRI' functional antagonism using SK-N-MC-BLA (4C10):
[004051 CGRP functional antagonism was characterized in a cell based
transcriptional
assay using a recombinant SK-N-MC line. To introduce the transcriptional
reporter system, SK-
N-MC cell line was transduced with a retroviral vector containing P-lactamase
gene downstream
of cAMP responsive promoter. The expression of P-lactamase is triggered by
cAMP increase
that is a downstream event of activation of endogenous CGRP receptor. Single
clones were
separated using Fluorescent Activated Cell Sorting (FACS) based on CGRP
induced P-lactamase
activity. (3-lactamase activity was measured using a fluorescence energy
transfer (FRET) dye,
CCF4. CCF4 is a substrate of P-lactama.se (Zlokarnik G, et al., Science, 279
(5347): 84-88,
1998) and cleaved into a product with different fluorescent signal from that
of the parent. .4C10
clone was selected for dose dependent (3-lactamase expression to different
concentrations of
CORP and consistent pharmacology with previously published values. To evaluate
functional
antagonist activity of compounds in SK-N-MC (4C10) line, compounds were
evaluated for their
inhibition of (3-lactamase expression in the presence of CGRP.
[004061 SK-N-MC (4C10) was cultured in Minimal Essential Media (MEM)
(Invitrogen)
supplemented with 1mM non-essential amino acids solution (Invitrogen),100
units/ml Penicillin-
Page 123 of 153
, = .
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
Streptomycin (Invitrogen), ImM sodiuin pyruvate (Invitrogen) and 10% fetal
bovine serum. For
the (3-lactamase assay, low serum, 1% FBS in MEM was used. 30,000 cells were
plated into
each wells of poly-D-lysine coated 384-well plate (Becton Dickinson) a day
prior to the assay.
SK-N-MC (4C 10) was preinciubated with compounds for 30 min before the
addition of 200 pM
CGRP. The assay was incubated for 3 hours at 37 C to allow P-lactamase
expression. CCF4
dye was added and incubated for 2 hours at room temperature. The fluorescent
signals were read
using a fluorescence plate reader, Topology Compensatory Plate Reader (tcPR)
at excitation
wavelength, 400 nm and emission wavelengths, 460 nm for the product and 535 nm
for the
parent. The ratio of values at 460 to 535 nm was used to calculate percent of
activation. Curve
fitting and ICSO calculation were carried about using MOD3.
[004071 I125-CGRP binding displacement assay to calculate K; of compounds.
[004081 Purified SK-N-MC membrane was purchased from Perkin Elmer. The
membrane
was thawed quickly and placed on ice. The compounds were diluted with CGRP
binding
solution (25 mM Tris-HCI, pH7.4, 5 mM MgC12, 0.1% BSA and 0.05% Tween). The
membrane
was diluted 1:20 with the binding solution and homogenized with Tissue Matster-
50
Homogenizer (Omni International) for 30 sec. The homogenized membrane was
added to
compounds in the binding solution. After 10 minutes incubation at room
temperature, the final
concentration of 46 pM, 1125-iodotyrosyl-Calcitonin-Gene-Related Peptide (GE
healthcare) was
added to the membrane and compounds. After 2 hour incubation at room
temperature, the
reaction was stopped by rapid filtration through 0.5% PEI treated GF/C filter
plate (Perkin
Elmer) and the filter plate was washed with ice-cold washing solution (50 mM
Tris HC1, pH7.4,
mM MgC12 and 0.1 %BSA) using cell harvestor (Tomtec). The radioactivity of the
filter plates
were read on Topcount (Packard). The nonspecific binding was determined in the
control
reaction where I uM unlabelled CGRP was preincubated with the membrane prior
to 1125-
CGRP addition. The total binding was determined in the control reaction of the
membrane and
1125-CGRP in the absence of compound. The percent displacement of 1125-CGRP
with
compounds was calculated using nonspecific and total binding controls. The
curve fitting was
carried out using MOD3. Ki of compound was calculated by the equation of Cheng
and Prusoff
(Cheng Y., Prusoff W. H., Biochem. Pharmacol. 22: 3099-3108, 1973) using Kd of
CGRP for
the membrane and the amount of I125-CGRP used for the assay.
Page 124 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
[00409] Exemplary compounds of the present invention in Table 1 were found to
be
antagonists of CGRP in the I125-CGRP binding assay and in the CGRP functional
antagonism
assay described above.
[00410] IC5o and Ki data for selected compounds of the present invention are
shown
below in Table 3. Tn Table 3, for both the IC5o column and the Ki column, the
symbols have the
following meaning: "A" means <1 M; "B" means between l M and 5 M; "C" means
>5 M
and "ND" means no data.
[00411] Table 3
IFC t`~' ~ ~~ r .r .
G~ mpcl~ ~ r.
_~_,~ ~Crn d #~~ r'a `,#~ ~I;C'50A K~
.,.....:, P...~.. e~ },..._._,._;......;r Se:.. Sy
1 A A 40 ND ND 79 A A
2 A A 41 A A 80 A A
3 A A 42 ND ND 81 ND ND
4 A ND 43 B A 82 B ND
B ND 44 A A 83 A ND
6 A ND 45 C ND 84 A A
7 A ND 46 B ND 85 C ND
8 A A 47 A ND 86 B ND
9 A A 48 A A 87 B B
A A 49 A A 88 A A
11 A ND . 50 ND ND 89 A ND
12 A A 51 A ND 90 B ND
13 A ND 52 ND ND 91 A ND
14 A A 53 C C 92 A ND
A A 54 A A 93 A A
16 A A 55 B 'ND 94 C ND
17 ND A 56 ND ND 95 A ND
18 B ND 57 A ND 96 B ND
19 C C 58 A A 97 C ND
A A 59 A ND 98 B ND
21 A ND 60 A A 99 ND ND
22 B ND 61 B C 100 A ND
23 A ND 62 A A 101 C ND
24 B ND 63 A A 102 A A
A ND 64 B A 103 C ND
26 A ND 65 A ND 104 C C
27 C ND 66 A A 105 C C
28 A A 67 A A 106 A A
29 C ND 68 ND ND 107 A A
B A 69 A ND 108 C ND
31 A ND 70 A A 109 B A
32 A ND 71 C ND 110 C C
33 A A 72 ND ND 111 C ND
34 A ND 73 A ND 112 C ND
B ND 74 B ND 113 A A
36 . A ND 75 B- ND 114 A A
37 A A 76 A A 115 A A
38 A ND 77 C ND 116 EB A
39 ND ND 78 A A 117 B ND
Page 125 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
~Gm ;d=,~~ ~IC'50r~ ~Ki~~ ~CmpdH#~ i k I ,C5U~ ~~~KiN , IC;50~
118 B A 170 A A 222 ~A~ ND
119 ND A 171 A A 223 C ND
120 A A 172 A A 224 A A
121 A ND 173 ND ND 225 A A
122 C A 174 A A 226 B A
123 A N D 175 A A 227 A A
124 B ND 176 B A 228 B ND
125 A ND 177 B ND 229 ND ND
126 A ND 178 B ND 230 C B
127 B C 179 A ND 231 A ND
128 B A 180 B ND 232 B ND
129 ND ND 181 A ND 233 A ND
130 B ND 182. A A 234 A ND
131 C ND 183 A A 235 B ND
132 B ND 184 A A 236 B A
133 C ND 185 A ND 237 A C
134 A ND 186 C ND 238 B ND
135 A ND 187 B A 239 A ND
136 A ND 188 A A 240 B A
137 A ND 189 C ND 241 B ND
138 A ND 190 C B 242 B ND
139 B ND 191 A ND 243 B ND
140 ND ND 192 A ND 244 A A
141 C ND 193 ND ND 245 C ND
142 ND ND 194 A A 246 A ND
143 C ND 195 B C 247 B ND
144 A A 196 B ND 248 A C
145 A A 197 C ND 249 A ND
146 C ND 198 A A 250 C ND
147 A A 199 ND ND 251 C B
148 B A 200 C ND 252 A ND
149 A A 201 B ND 253 B ND
150 A A 202 A A 254 B ND
151 A ND 203 B A 255 B A
152 A ND 204 B B 256 A A
153 ND ND 205 B ND 257 A ND
154 A ND 206 A ND 258 - B ND
155 A ND 207 A ND 259 A A
156 A ND 208 A A 260 A A
157 C ND 209 A ND 261 C ND
158 B ND 210 A ND 262 B ND
159 A A 211 B A 263 A A
160 A A 212 A A 264 A A
161 A ND 213 B ND 265 A A
162 ND ND 214 B ND 266 A A
163 ND ND 215 A ND 267 A ND
164 B ND 216 ND ND 268 A A
165 A ND 217 A ND 269 B ND
166 A ND 218 C ND 270 C ND
167 C ND 219 B ND 271 A A
168 B ND 220 ND ND 272 B ND
169 B A 221 A ND 273 B ND
Page 126 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
,.. .~~ .
274 A ND 326 ND ND 378 A ND
275 C ND 327 ND ND 379 A ND
276 B ND 328 A A 380 A A
277 A ND 329 ND ND 381 C ND
278 A A 330 B A 382 A ND
279 A A 331 A A 383 B ND
280 A ND 332 C ND 384 C ND
281 C ND 333 B ND 385 B ND
282 A A 334 A ND 386 ND ND
283 B A 335 A A 387 A ND
284 B ND 336 A A 388 C ND
285 A ND 337 ND ND 389 A A
286 B C 338 A ND 390 C ND
287 B B 339 ND ND 391 C C
288 A A 340 C C 392 C ND
289 A A 341 A A 393 A ND
290 A A 342 B ND 394 A A
291 A ND 343 ND ND 395 C ND
292 B ND 344 A ND 396 B ND
293 A ND 345 A A 397 C C
294 A ND 346 A ND 398 C ND
295 A A 347 A A 399 C ND
296 A A 348 B C 400 A A
297 A A 349 A A 401 A A
298 A ND 350 A A 402 A A
299 A A 351 B A 403 B A
300 A ND 352 A ND 404 B ND
301 A A 353 A A 405 B A
302 A A 354 A A 406 ND A
303 A A 355 ND ND 407 A A
304 ND A 356 A ND 408 A ND
305 B ND 357 A A 409 C A
306 C C 358 C ND 410 A ND
307 A A 359 ND ND 411 B ND
308 A ND 360 A ND 412 A ND
309 B ND 361 B ND 413 A ND
310 A ND 362 B ND 414 B C
311 B ND 363 A A 415 B A
312 A ND 364 C ND 416 ND ND
313 A ND 365 A A 417 B ND
314 C ND 366 A ND 418 C ND
315 A A 367 A A 419 B ND
316 C ND 368 ND ND 420 C ND
317 B A 369 B ND 421 A ND
318 A ND 370 A ND 422 A ND
319 A ND 371 A A 423 A ND
320 A ND 372 C ND 424 A ND
321 A ND 373 B ND 425 A ND
322 B ND 374 B B 426 B ND
323 A ND 375 A A 427 ND ND
324 A A 376 A ND 428 C ND
325 A ND 377 B ND 429 ND ND
Page 127 of 153
CA 02655085 2008-12-11
WO 2007/146349 PCT/US2007/013896
~C ~ 4 ~' FrCm "dE#e~ 1:C:50~ FKii~s~ ~Gmpd~#?~ ~IC50 ' a Ki
mpd~~ s~I,C.;50.~ ~Ki,~ ~ P.,. ~. _..
~.'' _
430 C ND 446 A A 462 A A
431 A A 447 A A 463 B A
432 A A 448 A ND 464 B ND
433 C ND 449 ND ND 465 B ND
434 A A 450 ND ND 466 A ND
435 B A 451 B ND 467 B ND
436 A A 452 A ND 468 A ND
437 A A 453 A ND 469 A A
438 = A ND 454 C ND 470 A A
439 A ND 455 B ND 471 A A
440 ND ND 456 B A 472 A ND
441 A ND 457 A A 473 C ND
442 A N D. 458 A A 474 B A
443 A ND 459 A A 475 A A
444 C ND 460 ND ND 476 C ND
445 B ND 461 A A 477 C A
Page 128 of 153