Note: Descriptions are shown in the official language in which they were submitted.
CA 02656063 2014-08-01
TARGETING COMPLEMENT FACTOR H FOR TREATMENT OF
DISEASES
TECHNICAL FIELD
[0002] This application pertains to compositions and methods of treating
diseases in which the alternative complement pathway is implicated.
Specifically, the
application pertains to a CR2-FH molecule and uses thereof for treating
diseases in
which the alternative complement pathway is implicated.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
[0003] This invention was made with Government support under Grant
(Contract) Nos.: AI47469, AI31 105, and EY13520 awarded by the National
Institutes
of Health.
BACKGROUND
[00041 Complement is the collective term for a series of blood proteins
and is
a major effector mechanism of the immune system. Complement plays an important
role in the pathology of many autoimmune, inflammatory, and ischemic diseases,
and
is also responsible for many disease states associated with
bioincompatibility.
Inappropriate complement activation and its deposition on host cells can lead
to
complement-mediated cell Iysis of target structures, as well as tissue
destruction due
to the generation of powerful mediators of inflammation.
[00051 Complement can be activated by one of the three pathways, the
classical, lectin, and alternative pathways. The classical pathway is
activated through
the binding of the complement system protein Clq to antigen-antibody
complexes, ,
pentraxins, or apoptotic cells. The pentraxins include C-reactive protein and
serum
arayloid P component. The lectin pathway is initiated by microbial saccharides
via
the mannose-binding lectin. The alternative pathway is activated on surfaces
of
1
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
pathogens that have neutral or positive charge characteristics and do not
express or
contain complement inhibitors. This is due to the process termed "tickover" of
C3
that occurs spontaneously, involving the interaction of conforrnationally
altered C3
with factor B, and results in the fixation of active C3b on pathogens or other
surfaces.
The alternative pathway can also be initiated when certain antibodies block.
endogenous regulatory mechanisms, by IgA-containing immune complexes, or when
expression of complement regulatory proteins is decreased. In addition, the
alternative pathway is activated by a mechanism called the "amplification
loop" when
C3b that is deposited onto targets via the classical or lectin pathway then
binds factor
B. Muller-Eberhard, 1988, Ann. Rev. Biochem. 57:321. For example, Holers and
collaborators have shown that the alternative pathway is amplified at sites of
local
injury when inflammatory cells are recruited following initial complement
activation.
Girardi et al., J Clin. Invest. 2003, 112:1644. Dramatic complement
amplification
through the alternative pathway then occurs through a mechanism that involves
either
the additional generation of injured cells that fix complement, local
synthesis of
alternative pathway components, or more likely because these infiltrating
inflammatory cells that carry preformed C3 and properdin greatly increase
activation
specifically at that site.
[0006] Alternative pathway activation is initiated when circulating
factor B
binds to activated C3. This complex is then cleaved by circulating factor D to
yield
an enzymatically active fragment, C3bBb. C3bBb cleaves C3 generating C3b,
which
drives inflammation and also further amplifies the activation process,
generating a
positive feedback loop. Factor H (FH) is a key regulator (inhibitor) of the
alternative
complement pathway. It functions by competing with factor B for binding to
C3b.
Binding of C3b to Factor H also leads to degradation of C3b by factor Ito the
inactive
form C3bi (also designated iC3b), thus exerting a further check on complement
activation. The actual plasma concentration of factor H is approximately 500
g/rril,
providing complement regulation in the fluid phase, but its binding to cells
is a
regulated phenomenon that is enhanced by the presence of a negatively charged
surface as well as fixed C3b, C3bi, or C3d. Jozsi et al., Histopathol (2004)
19:251-
258.
[0007] The down-regulation of complement activation has been demonstrated
to be effective in treating several disease indications in animal models and
in ex vivo
studies, e.g. systemic lupus erythematosus and glomerulonephritis (Y. Wang et
al.,
2
CA 02656063 2014-08-01
Proc. Natl. Acad. Sci.; 1996, 93: 8563-8568), rheumatoid arthritis (Y. Wang et
al.,
Proc. Natl. Acad. Sci., 1995; 92: 8955-8959), cardiopulmonary bypass and
hemodialysis (C. S. Rinder, I Clin. Invest., 1995; 96: 1564-1572), hypercute
rejection
in organ transplantation (T. J. Kroshus et al., Transplantation, 1995; 60:
1194-1202),
myocardial infarction (I. W. Homeister et al., J. Immunol, 1993; 150: 1055-
1064; H.
F. Weisman et al., Science, 1990; 249: 146-151), reperfusion injury (E. A.
Amsterdam
et al., Am. J. Physiol., 1995; 268: H448-H457), and adult respiratory distress
syndrome (R. Rabinoviei et al., J. Immunol., 1992; 149: 1744-1750). In
addition,
other inflammatory conditions and autoimmune/immune complex diseases are also
closely associated with complement activation (B. P. Morgan. Eur. J. Clin.
Invest.,
1994: 24: 219-228), including thermal injury, severe asthma, anaphylactic
shock,
bowel inflammation, urticaria, angioedema, vaseulitis, multiple sclerosis,
myasthenia
gravis, mernbranoproliferative glomerulonephritis, and Sjogren's syndrome.
Complement inhibitors and uses thereof are also disclosed in W004/045520 and
U.S.
Pat. No. 6,521,450.
BRIEF SUMMARY OF THE INVENTION
[0009] The invention
in one aspect provides a CR2-FH molecule comprising:
a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a FH portion
comprising a FH or a fragment thereof. In some embodiments, there is provided
a
CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or a fragment
thereof, and b) a FH portion comprising a FR or a fragment thereof, wherein
the CR2-
FH molecule is capable of binding to a CR2 ligand and wherein the CR2-FH
molecule
is capable of inhibiting complement activation of the alternative pathway. In
some
embodiments, there is provided an isolated CR2-FH molecule. In some
embodiments,
there is provided a composition (such as a pharmaceutical composition)
comprising a
CR2-FH molecule. In some embodiments, the CR2 portion and the FR portion are
directly or indirectly fused to each other in the form of a fusion protein. In
some
embodiments, the CR2 portion and the FH portion are linked via a chemical
crosslinker. In some embodiments, the CR2 portion and the FH portion are non-
covalently linked.
3
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[00101 In some embodiments, there is provided a CR2-FH fusion protein
comprising: a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a
FH
portion comprising a FH or a fragment thereof. In some embodiments, there is
provided a CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or a
fragment thereof, and b) a FH portion comprising a FH or a fragment thereof,
wherein
the CR2-FH molecule is capable of binding to a CR2 ligand and wherein the CR2-
FH
molecule is capable of inhibiting complement activation of the alternative
pathway.
In some embodiments, the CR2 portion and the FH portion are directly fused
(i.e.,
linked) to each other. In some embodiments, the CR2 portion and the FH portion
are
linked via an amino acid linker sequence. In some embodiments, the C-terminus
of
the CR2 portion is linked (directly or indirectly) to the N-terminus of the FH
portion.
In some embodiments, the N-terminus of the CR2 portion is linked (directly or
indirectly) to the C-terminus of the FH portion.
[0011] In some embodiments, the CR2-FH molecule comprises two or more
(such as any of two, three, four, five, or more) CR2 portions. These CR2
portions
may be the same or different, for example in terms of amino acid sequences,
structures, and/or functions. For example, in some embodiments, the CR2-FH
molecule (such as a CR2-FH fusion protein) comprises: 1) two or more CR2
portions
comprising a CR2 or a fragment thereof, and 2) an FH portion comprising a FH
or a
fragment thereof. In some embodiments, the CR2-FH molecule (such as a CR2-FH
fusion protein) comprises: 1) two or more CR2 portions comprising a CR2 or a
fragment thereof, and 2) an FH portion comprising a FH or a fragment thereof,
wherein the CR2-FH molecule is capable of binding to a CR2 ligand and wherein
the
CR2-FH molecule is capable of inhibiting complement activation of the
alternative
pathway.
[0012] In some embodiments, the CR2-FH molecule comprises two or more
(such as any of two, three, four, five, or more) FH portions. These FH
portions may
be the same or different, for example in terms of amino acid sequences,
structures,
and/or functions. For example, in some embodiments, the CR2-FH molecule (such
as
a CR2-FH fusion protein) comprises: 1) a CR2 portion comprising a CR2 or a
fragment thereof, and 2) two or more FH portions comprising a FH or a fragment
thereof. In some embodiments, the CR2-FH molecule (such as a CR2-FH fusion
protein) comprises: 1) a CR2 portion comprising a CR2 or a fragment thereof,
and 2)
two or more (such as two) FH portions comprising a FH or a fragment thereof,
4
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
wherein the CR2-FH molecule is capable of binding to a CR2 ligand and wherein
the
CR2-FH molecule is capable of inhibiting complement activation of the
alternative
pathway.
[0013] In some embodiments, the CR2-FH molecule (such as a CR2-FH
fusion protein) comprises: 1) two or more CR2 portions comprising a CR2 or a
fragment thereof, and 2) two or more FH portions comprising a FH or a fragment
thereof. In some embodiments, the CR2-FH molecule (such as a CR2-FH fusion
protein) comprises: 1) two or more CR2 portions comprising a CR2 or a fragment
thereof, and 2) two or more (such as two) FH portions comprising a FH or a
fragment
thereof, wherein the CR2-FR molecule is capable of binding to a CR2 ligand and
wherein the CR2-FH molecule is capable of inhibiting complement activation of
the
alternative pathway.
[0014] In some embodiments, the CR2-FH molecule (such as a CR2-FH
fusion protein) comprises: 1) full length CR2; and 2) a FH portion comprising
a FH or
a fragment thereof. In some embodiments, the CR2-FH molecule (such as a CR2-FH
fusion protein) comprises: 1) a fragment of CR2, and 2) a FH portion
comprising a
FR or a fragment thereof. In some embodiments, the CR2-FH molecule (such as a
CR2-FH fusion protein) comprises: 1) a CR2 portion comprising at least the
first two
N-terminal SCR domains of CR2, and b) a FH portion comprising a FH or a
fragment
thereof. In some embodiments, the CR2-FH molecule (such as a CR2-FH fusion
protein) comprises: 1) a CR2 portion comprising at least the first four N-
terminal SCR
domains of CR2, and b) a FH portion comprising a FH or a fragment thereof. In
some
embodiments, the CR2-FH molecule is capable of binding to a CR2 ligand and
inhibiting complement activation of the alternative pathway. In some
embodiments,
the CR2-FH molecule comprises two or more FH portions. In some embodiments,
the FH portion comprises a full length FH. In some embodiments, the FH portion
comprises a fragment of FH. In some embodiments, the FH portion comprises at
least
the first four N-terminal SCR domains of FH. In some embodiments, the FH
portion
comprises at least the first five N-terminal SCR domains of FH. In some
embodiments, the FH portion lacks a heparin binding site. In some embodiments,
the
FR portion comprises a FH or a fragment thereof having a polymorphism that is
protective against age-related macular degeneration.
[0015] In some embodiments, there is provided a CR2-FH molecule (such as
a CR2-FH fusion protein) comprising: a) CR2 portion comprising a ligand
binding
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
site that is any of (and in some embodiments selected from the group
consisting of)
(1) a site on strand B and the B-C loop of CR2 SCR comprising the segment G98-
G99-Y100-K1014102-R103-G104-S105-T106-P107-Y108 with respect to SEQ ID
NO: 1, (2) a site on the B strand of CR2 SCR2 comprising position K119 with
respect to SEQ ID NO:1, (3) a segment comprising V149-F150-P151-L152 with
respect to SEQ ID NO:1, and (4) a segment of CR2 SCR2 comprising T120-N121-
F122 with respect to SEQ ID NO:1; and (b) a FH portion comprising a FH or a
fragment thereof. In some embodiments, the CR2-FH molecule is capable of
binding
to a CR2 ligand and inhibiting complement activation of the alternative
pathway. In
some embodiments, the CR2 portion further comprises sequences required to
maintain the three dimensional structure of the ligand binding site. In some
embodiments, the CR2-FH molecule comprises two or more FH portions. In some
embodiments, the FH portion comprises a full length FH. In some embodiments,
the
FH portion comprises a fragment of FH. In some embodiments, the FH portion
comprises at least the first four N-terminal SCR domains of FH. In some
embodiments, the FH portion comprises at least the first five N-terminal SCR
domains of FH. In some embodiments, the FH portion lacks a heparin binding
site.
In some embodiments, the FH portion comprises a FH or a fragment thereof
having a
polymorphism that is protective against age-related macular degeneration.
[0016] In some
embodiments, there is provided a CR2-FH molecule (such as a
CR2-FH fusion protein) comprising: a) a CR2 portion comprising at least the
first two
N-terminal SCR domains of CR2, and b) a FH portion comprising at least the
first
four N-terminal SCR domains of FH. In some embodiments, the CR2-FH molecule is
capable of binding to a CR2 ligand and inhibiting complement activation of the
alternative pathway. In some embodiments, the CR2 portion comprises at least
the
first 3, 4, 5, 6, 7, or more N-terminal SCR domains of CR2. In some
embodiments,
the FH portion comprises at least the first 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
or more N-
terminal SCR domains of FH. In some embodiments, the CR2-FH molecule (such as
a CR2-FH fusion protein) comprises (and in some embodiments consists of or
consists essentially of): a) a CR2 portion comprising the first four N-
terminal SCR
domains of CR2, and b) a FH portion comprising the first five N-terminal SCR
domains of FH. In some embodiments, the CR2-FH molecule (such as a CR2-FH
fusion protein) comprises (and in some embodiments consists of or consists
essentially of): a) a CR2 portion comprising the first four N-terminal SCR
domains of
6
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
CR2, and b) two or more (such as two) FH portions comprising the first five N-
terminal SCR domains of FH. In some embodiments, the CR2-FH molecule
comprises (and in some embodiments consists of or consists essentially of): a)
a CR2
portion comprising amino acids 23 to 271 of SEQ ID NO:1, and b) a FH portion
comprising amino acids 21 to 320 of SEQ ID NO:2. In some embodiments, the CR2-
FH molecule comprises (and in some embodiments consists of or consists
essentially
of): a) a CR2 portion comprising amino acids 23 to 271 of SEQ ID NO:1, and b)
two
or more (such as two) FH portions comprising amino acids 21 to 320 of SEQ ID
NO:2.
[0017] In some
embodiments, the CR2-FH is a fusion protein having an amino
acid sequence of any of SEQ ID NO:3, SEQ ID NO:21, and SEQ ID NO:23. In some
embodiments, the CR2-FH molecule is a fusion protein having amino acid
sequence
that is at least about any of 50%, 60%, 70%, 80%, 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, 98%, or 99% identical to that of any of SEQ ID NO:3, SEQ ID NO:21,
and SEQ ID NO:23. In some embodiments, the CR2-FH is a fusion protein
comprising at least about 400, 450, 500, 550, or more contiguous amino acids
of any
of SEQ ID NO:3, SEQ ID NO:21, and SEQ ID NO:23. In some embodiments, the
CR2-FH molecule is a fusion protein encoded by a polynucleotide having nucleic
acid
sequence of any of SEQ ID NO:4, SEQ ID NO:22, and SEQ ID NO:24. In some
embodiments, the CR2-F1-1 molecule is a fusion protein encoded by a
polynucleotide
having a nucleic acid sequence that is at least about any of 50%, 60%, 70%,
80%,
90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to that of any
of
SEQ ID NO:4, SEQ ID NO:22, and SEQ ID NO:24. Also encompassed herein are
polynucleotides encoding a CR2-FH fusion protein described herein. For
example, in
some embodiments, there is provided a polynucleotide encoding a fusion protein
comprising a CR2 portion comprising CR2 or a fragment thereof and a FH portion
comprising a FH or a fragment thereof. In some embodiments, the polynucleotide
also comprises a sequence encoding a signal peptide operably linked at the 5'
end of
the sequence encoding the CR2-FH fusion protein. In some embodiments, a linker
sequence is used for linking the CR2 portion and the FH portion. In some
embodiments, the polynucleotide encodes a CR2-FH fusion protein having an
amino
acid sequence of any of SEQ ID NO:3, SEQ ID NO:21, and SEQ ID NO:23. In some
embodiments, the polynucleotide encodes a CR2-FH fusion protein having an
amino
acid sequence that is at least about any of 75%, 76%, 77%, 78%, 79%, 80%, 81%,
7
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%,
96%, 97%, 98%, or 99% identical to the nucleic acid sequence of any of SEQ ID
NO:3, SEQ ID NO:22, and SEQ ID NO:24. Also provided are vectors comprising a
polynucleotide encoding a CR2-FH fusion protein, host cells comprising the
polynucleotide, and methods of producing a CR2-FH fusion protein comprising
culturing the host cells under suitable conditions to express the fusion
protein and
recovering the fusion protein from the host cell culture.
[0018] In another aspect, there is provided a pharmaceutical composition
comprising a CR2-FH molecule and a pharmaceutically acceptable carrier. In
some
embodiments, the pharmaceutical composition comprises a CR2-FH molecule and a
pharmaceutically acceptable carrier suitable for administration to a human. In
some
embodiments, the pharmaceutical composition comprises a CR2-FH molecule and a
pharmaceutically acceptable carrier suitable for intraocular injection. In
some
embodiments, the pharmaceutical composition comprises a CR2-FH molecule and a
pharmaceutically acceptable carrier suitable for topical application to the
eye. In
some embodiments, the pharmaceutical composition comprises a CR2-FH molecule
and a pharmaceutically acceptable carrier suitable for intravenous injection.
In some
embodiments, the pharmaceutical composition comprises a CR2-Fli molecule and a
pharmaceutically acceptable carrier suitable for injection into the arteries
(such as
renal arteries), liver, or kidney.
[0019] In some embodiments, the pharmaceutical composition comprises a
CR2-FH molecule (such as a CR2 fusion protein) comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof; and a pharmaceutically acceptable carrier. In some
embodiments,
the CR2-FH molecule is capable of binding to a CR2 ligand and inhibiting
complement activation of the alternative pathway. In some embodiments, the
pharmaceutical composition comprises a CR2-FH molecule comprising: a CR2-FH
molecule (such as a CR2-FIT fusion protein) comprising: a) a CR2 portion
comprising
at least the first two N-terminal SCR domains of CR2, and b) a FH portion
comprising
at least the first four N-terminal SCR domains of FH, and a pharmaceutically
acceptable carrier. In some embodiments, the pharmaceutical composition
comprises
a CR2-FH molecule (such as a CR2-FH fusion protein) comprising (and in some
embodiments consists of or consists essentially of): a) a CR2 portion
comprising the
first four N-terminal SCR domains of CR2, and b) a FH portion comprising the
first
8
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
five N-terminal SCR domains of FH, and a pharmaceutically acceptable carrier.
In
some embodiments, the pharmaceutical composition comprises a CR2-FH molecule
(such as a CR2-FH fusion protein) comprising (and in some embodiments consists
of
or consists essentially of): a) a CR2 portion comprising amino acids 23 to 271
of SEQ
ID NO:1, and b) a FH portion comprising amino acids 21 to 320 of SEQ ID NO:2,
and a pharmaceutically acceptable carrier. In some embodiments, the
pharmaceutical
composition comprises a CR2-FH fusion protein having an amino acid sequence
that
is at least about 50%, 60%, 70%, 80%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%, or 99% identical to that of any of SEQ ID NO:3, SEQ ID NO:21, and SEQ ID
NO:23, and a pharmaceutically acceptable carrier. In some embodiments, the
pharmaceutical composition is suitable for delivery to the eye (for example by
intraocular injection or by topical delivery to the eye). In some embodiments,
the
pharmaceutical composition is suitable for intravenous injection. In some
embodiments, the pharmaceutical composition is suitable for injection into
arteries
(such as renal arteries), liver, or kidney. In some embodiments, the
composition is
suitable for intraocular, intravenous, intraarterial, sub-cutaneous,
intratracheal, or
inhalational administration.
[00201 In
another aspect, the invention provides a method of treating a disease
in which the alternative complement pathway is implicated in an individual,
comprising administering to the individual an effective amount of a
composition
(such as a pharmaceutical composition) described herein. In some embodiments,
the
method comprises administering to the individual an effective amount of a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, the method comprises administering to
the
individual an effective amount of a composition comprising a CR2-FH molecule
comprising: a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a
FH
portion comprising a FH or a fragment thereof, wherein the CR2-FH molecule is
capable of binding to a CR2 ligand and wherein the CR2-FH molecule is capable
of
inhibiting complement activation of the alternative pathway. In some
embodiments,
the disease to be treated is a disease that involves local inflammation. In
some
embodiments, the disease to be treated is a disease that is associated with FH
deficiencies (including for example decrease in level of FH, decrease in
activity of
FH, or lacking wildtype or protective FH). In some embodiments, the disease to
be
9
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
treated is not a disease that is associated with FH deficiencies. In some
embodiments,
the disease to be treated is a drusen-associated disease. In some embodiments,
the
disease to be treated does not involve the classical complement pathway.
[00211 In some embodiments, there is provided a method of treating
macular
degeneration (such as age-related macular degeneration or AMD) in an
individual,
comprising administering to the individual an effective amount of a
composition
comprising a CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or
a
fragment thereof, and b) a FH portion comprising a FH or a fragment thereof.
In
some embodiments, the disease to be treated is a dry form of AMD. In some
embodiments, the disease to be treated is a wet form of AMD. In some
embodiments,
the CR2-FH molecule is administered by intravenous administration. In some
embodiments, the CR2-FH molecule is administered by intraocular injection. In
some
embodiments, the CR2-FH molecule is administered by topical administration to
the
eye (for example in the form of eye drops).
[0022] In some embodiments, one or more aspects of AMD are treated by
methods of the present invention. For example, in some embodiments, there is
provided a method of treating (such as reducing, delaying, eliminating, or
preventing)
formation of drusen in the eye of an individual, comprising administering to
the
individual an effective amount of a composition comprising a CR2-FH molecule
comprising: a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a
FH
portion comprising a FH or a fragment thereof. In some embodiments, there is
provided a method of treating (such as reducing, delaying, eliminating, or
preventing)
inflammation in the eye of an individual, comprising administering to the
individual
an effective amount of a composition comprising a CR2-FH molecule comprising:
a)
a CR2 portion comprising a CR2 or a fragment thereof, and b) a FH portion
comprising a FH or a fragment thereof. In some embodiments, there is provided
a
method of treating (such as reducing, delaying, eliminating, or preventing)
loss of
photoreceptors cells in an individual, comprising administering to the
individual an
effective amount of a composition comprising a CR2-FH molecule comprising: a)
a
CR2 portion comprising a CR2 or a fragment thereof, and b) a FH portion
comprising
a FH or a fragment thereof. In some embodiments, there is provided a method of
improving (including for example decreasing, delaying, or blocking loss of)
visual
acuity or visual field in the eye of an individual, comprising administering
to the
individual an effective amount of a composition comprising a CR2-FH molecule
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
comprising: a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a
RI
portion comprising a FH or a fragment thereof. In some embodiments, there is
provided a method of treating neovascularization (such as choroidal
neovascularization or CNV), comprising administering to the individual an
effective
amount of a composition comprising a CR2-FH molecule comprising: a) a CR2
portion comprising a CR2 or a fragment thereof, and b) a FH portion comprising
a FH
or a fragment thereof. Treatments of other aspects of A.MD are also
contemplated.
[0023] The methods described herein are also useful for treatment of
certain
renal diseases. For example, in some embodiments, there is provided a method
of
treating membranoproliferative glomerulonephritis type II (MPGN II),
comprising
administering to the individual an effective amount of a composition
comprising a
CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or a fragment
thereof, and b) a FH portion comprising a FH or a fragment thereof. In some
embodiments, there is provided a method of treating hemolytic-uremic syndrome
(HUS), comprising administering to the individual an effective amount of a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there is provided a method of treating
lupus
nephritis, comprising administering to the individual an effective amount of a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof.
[0024] In some embodiments, there is provided a method of treating
ischernia
reperfusion (including for example renal ischemia reperfusion and intestinal
ischemia
reperfusion), comprising administering to the individual an effective amount
of a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof.
[0025] Also provided are methods of treating organ transplant rejections.
For
example, in some embodiments, there is provided a method of delaying onset of
acute
vascular rejection (such as antibody-mediated rejection of heart transplant)
in an
individual, comprising administering to the individual an effective amount of
a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
11
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof.
[0026] In some embodiments, there is provided a method of improving organ
transplant survival in an individual, comprising administering to the
individual an
effective amount of a composition comprising a CR2-FH molecule comprising: a)
a
CR2 portion comprising a CR2 or a fragment thereof, and b) a FH portion
comprising
a FH or a fragment thereof. In some embodiments, there is provided a method of
improving organ transplant survival in an individual, the method comprises
perfusing
the organ to be transplanted to an individual with a composition comprising a
CR2-
FH molecule comprising: a) a CR2 portion comprising a CR2 or a fragment
thereof,
and b) a FH portion comprising a FH or a fragment thereof. In some
embodiments,
there is provided a method of improving survival of an organ transplant donor,
comprising administering to the organ transplant donor an effective amount of
a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof.
[0027] In some embodiments, there is provided a method of treating
rheumatoid arthritis, comprising administering to the individual an effective
amount
of a composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
fragment thereof.
[0028] Also provided are unit dosage forms, kits, and articles of
manufacture
that are useful for methods described herein.
[0029] It is to be understood that one, some, or all of the properties of
the
various embodiments described herein may be combined to form other embodiments
of the present invention.
BRIEF DESCRIPTION OF THE FIGURES
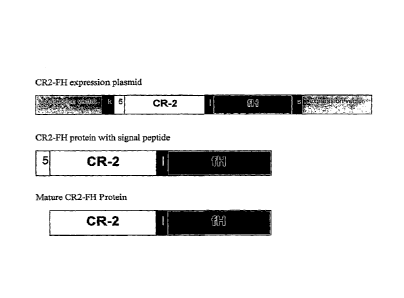
[0030] Figure 1 provides schematic diagrams of an exemplary CR2-FH
expression plasmid and CR2-FH proteins. For the CR2-FH expression plasmid, k
refers to Kozak sequence, 5 refers to CD5 signal peptide, I refers to an
optional linker,
s refers to stop codon and polyA signal. For the CR2-FH proteins (with or
without
signal peptide), 5 refers to the CD5 signal peptide, 1 refers to an optional
linker.
12
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0031] Figure 2 provides the amino acid sequence of human CR2 (SEQ
ID
NO:1) and the amino acid sequence of human FH (SEQ ID NO:2).
[0032] Figure 3 provides the amino acid sequence of an exemplary
human
CR2-FH fusion protein (SEQ ID NO: 3) and an exemplary polynucleotide sequence
encoding a human CR2-FH fusion protein (SEQ ID NO:4).
[0033] Figures 4-6 provide exemplary amino acid sequences of CR2-
FH
molecules described herein (SEQ ID NOs: 5-10). "mm" represents an optional
linker.
[0034] Figure 7 provides exemplary amino acid sequences of
signaling
peptides described herein (SEQ ID NOs: 11, 13, and 25) and exemplary
polynucleotide sequences encoding the signaling peptides (SEQ ID NOs:12, 14,
and
26).
= 100351 Figure 8 provides amino acid sequence of mouse CR2 (SEQ ID
NO:15) and amino acid sequence of mouse FH (SEQ ID NO:16).
[0036] Figure 9 provides amino acid sequence of an exemplary mouse
CR2-
FH fusion protein (SEQ ID NO:17) and an exemplary polynucleotide sequence that
encodes a mouse CR2-FR plus the signal peptide (SEQ ID NO:18).
[0037] Figure 10 provides an exemplary DNA sequence of CR2NLFHFH,
a
mouse CR2-FH fusion protein containing a CR2 portion and two FH portions
without
a linker sequence (SEQ ID NO:19).
[0038] Figure 11 provides an exemplary DNA sequence of CR2LFIAFH,
a
mouse CR2-FH fusion protein containing a CR2 portion linked to two FH portions
via
a linker sequence (SEQ ID NO:20).
[0039] Figure 12A provides a graphic representation of data
obtained in an in
vitro zymosan complement assay using a mouse CR2-FH fusion protein (CR2-fH)
and factor H alone (a1). Figure 12B provides a graphic representation of data
obtained in an in vitro zymosan complement assay using the first five SCR
domains
of FH (FH 15) and the first four domains of CR2 (CR2).
[0040] Figure 13 provides a graphic representation of data
obtained in an in
vitro zymosan complement assay using mouse CR2-FH fusion protein with linker
(CR2LFH), CR2-FH fusion protein without linker (CR2NLFH), CR2-FH-FH with
linker (CR2LFHFH), and CR2-Crry.
[0041] Figures 14A and 14B provide graphic representations of data
obtained
in an animal model of intestine ischemia and reperfusion injury using mouse
CR2-FH
fusion protein having one FH portion (CR2-fH) or two FH portions (CR2-fHH).
13
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0042] Figure 15A provides a graphic representation of effects of CR2-fH
on
kidney function as measured by serum urea nitrogen (SUN). Figure 15B provides
a
graphic representation of effects of CR2-fH on renal morphology. Figure 15C
and
15D provide immunofluorescence staining results of control mouse (15C) and CR2-
fH treated mouse (15D) kidney sections incubated with FTIC-conjugated antibody
to
mouse C3.
[0043] Figure 16 provides a- and b-wave retinal response results in mice
treated with or without CR2-fH.
[0044] Figures 17A and 17B provides isolectin-b staining of lesions of
mouse
retina from control mouse (17A) and mouse treated with CR2-fH by intravenous
injection (17B). Figure 17C show quantification of lesion sizes based on the
isolectin-b staining of Figures 17A and 17B.
[0045] Figures 18A and 18B provides isolectin-b staining of lesions of
mouse
retina from control mouse (18A) and mouse treated with CR2-fH by intraoptical
injection (18B). Figure 18C provides quantification of lesion sizes based on
the
isolectin-b staining of Figures 18A and 1813.
[0046] Figure 19 provides a survival curve of mouse heart transplant
recipient
treated with single dose of CR2-fH (CR2-fH), multiple doses of CR2-fH (CR2-fH
(m)), and control buffer (PBS).
[0047] Figure 20 provides amino acid sequence of an exemplary human CR2-
FH fusion protein (designated as human CR2-fH or CR2fH) (SEQ ID NO:21) and an
exemplary polynucleotide sequence that encodes a human CR2-fH plus the signal
peptide (SEQ ID NO:22). Sequence encoding the signal peptide is underlined.
[0048] Figure 21 provides an exemplary amino acid sequence of a human
CR2-FH fusion protein containing two FH portions (designated as human CR2-FH2
or human CR2fH2) (SEQ ID NO:23) and an exemplary polynucleotide sequence that
encodes a human CR2-FH2 plus the signal peptide (SEQ ID NO :24). Sequence
encoding the signal peptide is underlined.
[0049] Figure 22A shows inhibition of human CR2fH and CR2fH2 on
alternative pathway specific C3b deposition onto zymosan particles. Figure 22B
shows inhibition of alternative pathway-mediated erythrocyte lysis by human
CR2fH
and human CR2fH2.
14
CA 02656063 2008-12-15
WO 2007/149567 PCT/US2007/014602
100501 Figure 23 shows the effects of mouse CR2-FH on C3 activation
induced by immune-complexes of collagen-anti-collage antibodies. The Y-axis
shows mean OD values.
=
[0051] Figure 24 shows titration of mouse CR2-FH in calcium sufficient
buffer using serum from C4-1C4- knockout mouse. The Y-axis shows mean OD
values.
DETAILED DESCRIPTION OF THE INVENTION
[0052] The present invention provides a CR2-FH molecule, compositions
(such as pharmaceutical compositions) comprising a CR2-FH molecule, and
methods
of treating a disease in which the alternative complement pathway is
implicated by
administering the composition. The CR2-FH molecule comprises a CR2 portion and
a FH portion. The CR2 portion is responsible for targeted delivery of the
molecule to
the sites of complement activation, and the FH portion is responsible for
specifically
inhibiting complement activation of the alternative pathway. Preliminary
studies have
shown that a CR2-FH molecule, specifically, a CR2-FH fusion protein containing
the
first four N-terminal SCR domains of the CR2 protein and the first five N-
terminal
SCR domains the factor H protein, has both targeting activity and complement
inhibitory activity in vitro. This molecule is significantly more effective
than a factor
H molecule lacking the CR2 portion, suggesting that targeting FH to complement
activation sites will be an effective therapeutic tool in treating disease in
which the
alternative complement pathway is implicated, such as macular degeneration
(for
example age-related macular degeneration). This observation is surprising
because of
the relatively high concentration of FH in the plasma and the long-held belief
that
cells which are in direct contact with plasma are already completely covered
with FH.
Jozsi et al., Histopathol. (2004) 19:251-258.
[0053] Accordingly, in one aspect, there is provided a CR2-FH molecule
comprising: a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a
FH
portion comprising a FH or a fragment thereof. In some embodiments, there is
provided an isolated CR2-FH molecule. In some embodiments, there is provided a
composition (such as a pharmaceutical composition) comprising a CR2-FH
molecule.
For example, in some embodiments, there is provided a pharmaceutical
composition
comprising a CR2-FH molecule and a pharmaceutically acceptable carrier
suitable for
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
administration to an individual systemically (such as intravenous injection),
or locally
(such as intraocular injection or injection into arteries including renal
arteries).
[0054] In another aspect, there is provided a method of treating a
disease in
which the alternative complement pathway is implicated in an individual,
comprising
administering to the individual an effective amount of a composition
comprising a
CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or a fragment
thereof, and b) a FH portion comprising a FH or a fragment thereof. Suitable
diseases that can be treated by methods of the present invention include, for
example,
macular degeneration (such as age-related macular degeneration), rheumatoid
arthritis, ischemia reperfusion, organ transplant rejection, and renal
diseases such as
MPGN II, HUS, and lupus nephritis.
[0055] Also provided are unit dosage forms, kits, and articles of
manufacture
that are useful for methods described herein.
[0056] General reference to "the composition" or "compositions" includes
and
is applicable to compositions of the invention.
[0057] As used herein, the singular form "a", "an", and "the" includes
plural
references unless indicated otherwise. For example, "a" FH portion includes
one or
more FH portions.
[0058] Reference to "about" a value or parameter herein includes (and
describes) embodiments that are directed to that value or parameter per se.
For
example, description referring to "about X" includes description of "X".
[0059] It is understood that aspects and embodiments of the invention
described herein include "consisting" and/or "consisting essentially of'
aspects and
embodiments.
CR2-FH molecules and compositions comprising a CR2-FH molecule
[0060] Provided herein are CR2-FH molecules and compositions (such as
pharmaceutical compositions) comprising a CR2-FH molecule.
[0061] "CR2-FH molecule" used herein refers to a non-naturally occurring
molecule comprising a CR2 or a fragment thereof (the "CR2 portion") and a FH
or a
fragment thereof (the "F1-1 portion"). The CR2 portion is capable of binding
to one or
more natural ligands of CR2 and is thus responsible for targeted delivery of
the
molecule to the sites of complement activation. The FH portion is responsible
for
specifically inhibiting complement activation of the alternative complement
pathway.
16
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
The CR2 portion and the FH portion of the CR2-FH molecule can be linked
together
by any methods known in the art, as long as the desired functionalities of the
two
portions are maintained.
[0062] The CR2-FH molecule described herein thus generally has the dual
functions of binding to a CR2 ligand and inhibiting complement activation of
the
alternative pathway. "CR2 ligand" refers to any molecule that binds to a
naturally
occurring CR2 protein, which include, but are not limited to, C3d, iC3b, C3dg,
C3d,
and cell-bound fragments of C3b that bind to the two N-terminal SCR domains of
CR2. The CR2-FH molecule may, for example, bind to a CR2 ligand with a binding
affinity that is about any of 10%, 20%, 30%, 40%; 50%, 60%, 70%, 80%, 90%, or
100% of the CR2 protein. Binding affinity can be determined by any method
known
in the art, including for example, surface plasmon resonance, calorimetry
titration,
ELISA, and flow cytometry. In some embodiments, the CR2-FH molecule has one or
more of the following properties of CR2: (1) binding to C3d, (2) binding to
iC3b, (3)
binding to C3dg, (4) binding to C3d, and (5) binding to cell-bound fragment(s)
of C3b
that bind to the two N-terminal SCR domains of CR2.
[0063] The CR2-FH molecule described herein is generally capable of
inhibiting complement activation of the alternative pathway. The CR2-FH
molecule
may be a more potent complement inhibitor than the naturally occurring FH
protein.
For example, in some embodiments, the CR2-FH molecule has a complement
inhibitory activity that is about any of 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8,
9, 10, 12, 14, 16,
18, 20, 25, 30, 40, or more fold of that of the FH protein. In some
embodiments, the
CR2-FH molecule has an EC50 of less than about any of 100 nM, 90 nM, 80 nM, 70
nM, 60 nM, 50 nM, 40 nM, 30 nM, 20 nM, or 10 nM. In some embodiments, the
CR2-FH molecule has an EC50 of about 5-60 nM, including for example any of 8-
50
nM, 8-20 nM, 10-40 nM, and 20-30 nM. In some embodiments, the CR2-FH
molecule has complement inhibitory activity that is about any of 50%, 60%,
70%,
80%, 90%, or 100% of that of the FH protein.
[0064] Complement inhibition can be evaluated based on any methods known
in the art, including for example, in vitro zymosan assays, assays for lysis
of
erythrocytes, immune complex activation assays, and marman activation assays.
In
some embodiments, the CR2-FH has one or more of the following properties of
FH:
(1) binding to C-reactive protein (CRP), (2) binding to C3b, (3) binding to
heparin,
(4) binding to sialic acid, (5) binding to endothelial cell surfaces, (6)
binding to
17
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
cellular integrin receptor, (7) binding to pathogens, (8) C3b co-factor
activity, (9) C3b
decay-acceleration activity, and (10) inhibiting the alternative complement
pathway.
[0065] In some embodiments, the CR2-FH molecule is a fusion protein.
"Fusion protein" used herein refers to two or more peptides, polypeptides, or
proteins
operably linked to each other. In some embodiments, the CR2 portion and the FH
portion are directly fused to each other. In some embodiments, the CR2 portion
and
the FH portion are linked by an amino acid linker sequence. Examples of linker
sequences are known in. the art, and include, for example, (Gly4Ser),
(GlY4Ser)2,
(Gly4.Ser)3, (GlY3Ser)4, (SerGly4), (SerGly4)2, (SerGly4)3, and (SerGly4)4.
Linking
sequences can also comprise "natural" linking sequences found between
different
domains of complement factors. For example, VSVFPLE, the linking sequence
between the first two N-terminal short consensus repeat domains of human CR2,
can
be used. In some embodiments, the linking sequence between the fourth and the
fifth
N-terminal short consensus repeat domains of human CR2 (EEIF) is used. The
order
of CR2 portion and FH portion in the fusion protein can vary. For example, in
some
embodiments, the C-terminus of the CR2 portion is fused (directly or
indirectly) to the
N-terminus of the FH portion of the molecule. In some embodiments, the N-
terminus
of the CR2 portion is fused (directly or indirectly) to the C-terminus of the
FH portion
of the molecule.
[0066] In some embodiments, the CR2-FH molecule is a CR2-FH fusion
protein having an amino acid sequence of any of SEQ ID NO:3, SEQ ID NO:21, and
SEQ ID NO:23. In some embodiments, the CR2-FH molecule is a fusion protein
having an amino acid sequence that is at least about 50%, 60%, 70%, 80%, 90%,
91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to that of any of SEQ
ID NO:3, SEQ ID NO:21, or SEQ ID NO:23. In some embodiments, the CR2-FH
molecule comprises at least about 400, 450, 500, 550, or more contiguous amino
acids
of any of SEQ ID NO:3, SEQ ID NO:21, and SEQ ID NO:23.
[0067] In some embodiments, the CR2-FH molecule is a CR2-FH fusion
protein having an amino acid sequence of any of SEQ ID NOs:5-10. In some
embodiments, the CR2-FH molecule is a fusion protein having an amino acid
sequence that is at least about 50%, 60%, 70%, 80%, 90%, 91%, 92%, 93%, 94%,
95%, 96%, 97%, 98%, or 99% identical to that of any of SEQ ID NOs:5-10. In
some
embodiments, the CR2-FH molecule comprises at least about 400, 450, 500, 550,
or
more contiguous amino acids any of SEQ ID NOs:5-10.
18
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0068] In some embodiments, the CR2-FH molecule is encoded by a
polynucleotide having nucleic acid sequence of any of SEQ ID NO:4, SEQ ID
NO:22,
and SEQ ID NO:24. In some embodiments, the CR2-FH molecule is encoded by a
polynucleotide having a nucleic acid sequence that is at least about 50%, 60%,
70%,
80%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to that of
any of SEQ ID NO:4, SEQ ID NO:22, and SEQ ID NO:24.
[0069] In some embodiments, the CR2-FH molecule comprises a CR2 portion
and a FR portion linked via a chemical cross-linker. Linking of the two
portions can
occur on reactive groups located on the two portions. Reactive groups that can
be
targeted using a crosslinker include primary amines, sulfhydryls, carbonyls,
carbohydrates, and carboxylic acids, or active groups that can be added to
proteins.
Examples of chemical linkers are well known in the art and include, but are
not
limited to, bismaleimidohexane, maleimidobenzoyl-N-hydroxysuccinimide ester,
NHS-Esters-Maleimide Crosslinkers such as SPDP, carbodiimide, glutaraldehyde,
MBS, Sulfo-MBS, SMPB, sulfo-SMPB, GMBS, Sulfo-GMBS, EMCS, Sulfo-EMCS,
imidoester crosslinkers such as DMA, DMP, DMS, DTBP, EDC and DTME.
[0070] In some embodiments, the CR2 portion and the FH portion are non-
covalently linked. For example, the two portions may be brought together by
two
interacting bridging proteins (such as biotin and streptavidin), each linked
to a CR2
portion or a FR portion.
[0071] In some embodiments, the CR2-FH molecule comprises two or more
(same or different) CR2 portions described herein. In some embodiments, the
CR2-
FH molecule comprises two or more (same or different) FR portions described
herein.
These two or more CR2 (or FR) portions may be tandemly linked (such as fused)
to
each other. In some embodiments, the CR2-FH molecule (such a CR2-F11 fusion
protein) comprises a CR2 portion and two or more (such as three, four, five,
or more)
FH portions. In some embodiments, the CR2-FR molecule (such a CR2-FH fusion
protein) comprises a FH portion and two or more (such as three, four, five, or
more)
CR2 portions. In some embodiments, the CR2-FR molecule (such a CR2-FR fusion
protein) comprises two or more CR2 portions and two or more FR portions.
[0072] In some embodiments, there is provided an isolated CR2-FR
molecule.
In some embodiments, the CR2-FR molecules form dimers or multimers.
[0073] The CR2 portion and the FR portion in the molecule can be from the
same species (such as human or mouse), or from different species.
19
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
CR2 portion
[0074] The CR2 portion described herein comprises a CR2 or a fragment
thereof. CR2 is a transmembrane protein expressed predominantly on mature B
cells
and follicular dendritic cells. CR2 is a member of the C3 binding protein
family.
Natural ligands for CR2 include, for example, iC3b, C3dg, and C3d, and cell-
bound
breakdown fragments of C3b that bind to the two N-terminal SCR domains of CR2.
Cleavage of C3 results initially in the generation of C3b and the covalent
attachment
of this C3b to the activating cell surface. The C3b fragment is involved in
the
generation of enzymatic complexes that amplify the complement cascade. On a
cell
surface, C3b is rapidly converted to inactive iC3b, particularly when
deposited on a
host surface containing regulators of complement activation (i.e., most host
tissue).
Even in absence of membrane bound complement regulators, substantial levels of
iC3b are formed. iC3b is subsequently digested to the membrane bound fragments
C3dg and then C3d by serum proteases, but this process is relatively slow.
Thus, the
C3 ligands for CR2 are relatively long lived once they are generated and will
be
present in high concentrations at sites of complement activation. CR2
therefore can
serve as a potent targeting vehicle for bringing molecules to the site of
complement
activation.
[0075] CR2 contains an extracellular portion having 15 or 16 repeating
units
known as short consensus repeats (SCR domains). The SCR domains have a typical
framework of highly conserved residues including four cysteines, two prolines,
one
tryptophane and several other partially conserved glycines and hydrophobic
residues.
SEQ ID NO:1 represents the full-length human CR2 protein sequence. Amino acids
1-20 comprise the leader peptide, amino acids 23-82 comprise SCR1, amino acids
91-
146 comprise SCR2, amino acids 154-210 comprise SCR3, amino acids 215-271
comprise SCR4. The active site (C3d binding site) is located in SCR1-2 (the
first two
N-terminal SCR domains). These SCR domains are separated by short sequences of
variable length that serve as spacers. The full-length mouse CR2 protein
sequence is
represented herein by SEQ ID NO:15. The SCR1 and SCR2 domains of the mouse
CR2 protein are located with the mouse CR2 amino sequence at positions 14-73
of
SEQ ID NO:15 (SCR1) and positions 82-138 of SEQ ID NO:15 (SCR2). Human and
mouse CR2 are approximately 66% identical over the full length amino acid
sequences represented by SEQ ID NO:1 and SEQ ID NO:15, and approximately 61%
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
identical over the SCR1-SCR2 regions of SEQ ID NO:1 and SEQ ID NO:15. Both
mouse and human CR2 bind to C3 (in the C3d region). It is understood that
species
and strain variations exist for the disclosed peptides, polypeptides, and
proteins, and
that the CR2 or a fragment thereof described herein encompasses all species
and
strain variations.
[0076] The CR2 portion disclosed herein refers to a polypeptide that
contains
some or all of the ligand binding sites of the CR2 protein, and includes, but
is not
limited to, full-length CR2 proteins (such as human CR2 as shown in SEQ ID
NO:1
or mouse CR2 as shown in SEQ ID NO:15), soluble CR2 proteins (such as a CR2
fragment comprising the extracellular domain of CR2), other biologically
active
fragments of CR2, a CR2 fragment comprising SCR1 and SCR2, or any homologue
of a naturally occurring CR2 or fragment thereof, as described in detail
below. In
some embodiments, the CR2 portion has one of the following properties or CR2:
(1)
binding to C3d, (2) binding to iC3b, (3) binding to C3dg, (4) binding to C3d,
and (5)
binding to cell-bound fragment(s) of C3b that bind to the two N-terminal SCR
domains of CR2.
[0077] In some embodiments, the CR2 portion comprises the first two N-
terminal SCR domains of CR2. In some embodiments, the CR2 portion comprises
the
first three N-terminal SCR domains of CR2. In some embodiments, the CR2
portion
comprises the first four N-terminal SCR domains of CR2. In some embodiments,
the
CR2 portion comprises (and in some embodiments consists of or consists
essentially
of) at least the first two N-terminal SCR domains of CR2, including for
example at
least any of the first 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 SCR
domains of
CR2.
[0078] A homologue of a CR2 protein or a fragment thereof includes
proteins
which differ from a naturally occurring CR2 (or CR2 fragment) in that at least
one or
a few amino acids have been deleted (e.g., a truncated version of the protein,
such as a
peptide or fragment), inserted, inverted, substituted and/or derivatized
(e.g., by
glycosylation, phosphorylation, acetylation, myristoylation, prenylation,
palmitation,
amidation and/or addition of glycosylphosphatidyl inositol). In some
embodiments, a
CR2 homologue has an amino acid sequence that is at least about 70% identical
to the
amino acid sequence of a naturally occurring CR2 (e.g., SEQ ID NO:1, or SEQ ID
NO:15), for example at least about any of 75%, 76%, 77%, 78%, 79%, 80%, 81%,
82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%,
21
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
96%, 97%, 98%, or 99% identical to the amino acid sequence of a naturally
occurring
CR2 (e.g., SEQ ID NO:1, or SEQ ID NO:15). A CR2 homologue or a fragment
thereof preferably retains the ability to bind to a naturally occurring ligand
of CR2
(e.g., C3d or other C3 fragments with CR2-binding ability). For example, the
CR2
homologue (or fragment thereof) may have a binding affinity for C3d that is at
least
about 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%,
88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% of that of CR2
(or a fragment thereof).
[0079] In some embodiments, the CR2 portion comprises at least the first
two
N-terminal SCR domains of a human CR2, such as a CR2 portion having an amino
acid sequence containing at least amino acids 23 through 146 of the human CR2
(SEQ
ID NO:1). In some embodiments, the CR2 portion comprises at least the first
two
SCR domains of human CR2 having an amino acid sequence that is at least about
any
of 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%,
89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% identical to amino
acids 23 through 146 of the human CR2 (SEQ ID NO:1).
[0080] In some embodiments, the CR2 portion comprises at least the first
four
N-terminal SCR domains of a human CR2, such as a CR2 portion having an amino
acid sequence containing at least amino acids 23 through 271 of the human CR2
(SEQ
ID NO:1). In some embodiments, the CR2 portion comprises at least the first
four
SCR domains of human CR2 having an amino acid sequence that is at least about
any
of 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%,
89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% identical to amino
acids 23 through 271 of the human CR2 (SEQ ID NO:1).
[0081] An amino acid sequence that is at least about, for example, 95%
identical to a reference sequence (such as SEQ ID NO:1) is intended that the
amino
acid sequence is identical to the reference sequence except that the amino
acid
sequence may include up to five point alterations per each 100 amino acids of
the
reference sequence. These up to five point alterations may be deletions,
substitutions,
additions, and may occur anywhere in the sequence, interspersed either
individually
among amino acids in the reference sequence or in one or more continuous
groups
within the reference sequence.
[0082] In some embodiments, the CR2 portion comprises part or all of the
ligand binding sites of the CR2 protein. In some embodiments, the CR2 portion
22
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
further comprises sequences required to maintain the three dimensional
structure of
the binding site. Ligand binding sites of CR2 can be readily determined based
on the
crystal structures of CR2, such as the human and mouse CR2 crystal structures
disclosed in U.S. Patent Application Publication No. 2004/0005538. For
example, in
some embodiments, the CR2 portion comprises the B strand and B-C loop of SCR2
of
CR2. In some embodiments, the CR2 portion comprises a site on strand B and the
B-
C loop of CR2 SCR comprising the segment G98-G99-Y100-K101-I102-R103-G104-
S105-T106-P107-Y108 with respect to SEQ ID NO: 1. In some embodiments, the
CR2 portion comprises a site on the B strand of CR2 SCR2 comprising position
K119
with respect to SEQ ID NO:1 . In some embodiments, the CR2 portion comprises a
segment comprising V149-F150-P151-L152, with respect to SEQ ID NO:l. In some
embodiments, the CR2 portion comprises a segment of CR2 SCR2 comprising T120-
N121-F122. In some embodiments, the CR2-FH molecule has two or more of these
sites. For example, in some embodiments, the CR2 portion comprises a portion
comprising G98-G99-Y100-K101-4102-R103-G104-S105-T106-P107-Y108 and
K119 with respect to SEQ ID NO: 1. Other combinations of these sites are also
contemplated.
Factor H portion
[00831 The FH portion of the CR2-FH molecule described herein comprises a
FH or a fragment thereof.
[0084] Complement factor H (FH) is a single polypeptide chain plasma
glycoprotein. The protein is composed of 20 repetitive SCR domains of
approximately 60 amino acids, arranged in a continuous fashion like a string
of 20
beads. Factor H binds to C3b, accelerates the decay of the alternative pathway
C3-
convertase (C3Bb), and acts as a cofactor for the proteolytic inactivation of
C3b. In
the presence of factor H, C3b proteolysis results in the cleavage of C3b.
Factor H has
at least three distinct binding domains for C3b, which are located within SCR
1-4,
SCR 5-8, and SCR 19-20. Each site of factor H binds to a distinct region
within the
C3b protein: the N-terminal sites bind to native C3b; the second site, located
in the
middle region of factor H, binds to the C3c fragment and the sited located
within
SCR19 and 20 binds to the C3d region. In addition, factor H also contains
binding
sites for heparin, which are located within SCR 7, SCR 5-12, and SCR20 of
factor H
and overlap with that of the C3b binding site. Structural and functional
analyses have
23
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
shown that the domains for the complement inhibitory activity of FH are
located
within the first four N-terminal SCR. domains.
[0085] SEQ ID NO:2 represents the full-length human FH protein sequence.
Amino acids 1-18 correspond to the leader peptide, amino acids 21-80
correspond to
SCR1, amino acids 85-141 correspond to SCR2, amino acids 146-205 correspond to
SCR3, amino acids 210-262 correspond to SCR4, amino acids 267-320 correspond
to
SCRS. The full-length mouse FH protein sequence is represented herein by SEQ
ID
NO:16. The SCR1 and SCR2 domains of the mouse FH protein are located with the
mouse FH amino sequence at positions 21-27 of SEQ ID NO:16 (SCR1) and
positions
82-138 of SEQ ID NO:16 (SCR2). Human and mouse FH are approximately 61%
identical over the full length amino acid sequences represented by SEQ ID NO:2
and
SEQ ID NO:16. It is understood that species and strain variations exist for
the
disclosed peptides, polypeptides, and proteins, and that the FH or a fragment
thereof
encompasses all species and strain variations.
[0086] The FH portion described herein refers to any portion of a FH
protein
having some or all the complement inhibitory activity of the FH protein, and
includes,
but is not limited to, full-length FH proteins, biologically active fragments
of FH
proteins, a FR fragment comprising SCR1-4, or any homologue of a naturally
occurring FH or fragment thereof, as described in detail below. In some
embodiments, the FR portion has one or more of the following properties: (1)
binding
to C-reactive protein (CRP), (2) binding to C3b, (3) binding to heparin, (4)
binding to
sialic acid, (5) binding to endothelial cell surfaces, (6) binding to cellular
integrin
receptor, (7) binding to pathogens, (8) C3b co-factor activity, (9) C3b decay-
acceleration activity, and (10) inhibiting the alternative complement pathway.
[0087] In some embodiments, the FH portion comprises the first four N-
terminal SCR domains of FH. In some embodiments, the construct comprises the
first
five N-terminal SCR domains of FH. In some embodiments, the construct
comprises
the first six N-terminal SCR domains of FH. In some embodiments, the FH
portion
comprises (and in some embodiments consists of or consisting essentially of)
at least
the first four N-terminal SCR domains of FH, including for example, at least
any of
the first 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or more N-terminal SCR domains of
FH.
[00881 In some embodiments, the FH is a wildtype FH. In some
embodiments, the FH is a protective variant of FH.
24
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0089] In some embodiments, the FH portion lacks a heparin binding site.
This can be achieved, for example, by mutation of the heparin binding site on
FH, or
by selecting FH fragments that do not contain a heparin binding site. In some
embodiments, the FH portion comprises a FH or a fragment thereof having a
polymorphism that is protective to age-related macular degeneration. Hageman
et al.,
Proc. Nail Acad. Sci. USA 102(20):7227. One example of a CR2-FH molecule
comprising such a sequence is provided in Figure 4 (SEQ ID NO:6).
[0090] A homologue of a FH protein or a fragment thereof includes
proteins
which differ from a naturally occurring FH (or FH fragment) in that at least
one or a
few, but not limited to one or a few, amino acids have been deleted (e.g., a
truncated
version of the protein, such as a peptide or fragment), inserted, inverted,
substituted
and/or derivatized (e.g., by glycosylation, phosphorylation, acetylation,
myristoylation, prenylation, palmitation, amidation and/or addition of
glycosylphosphatidyl inositol). For example, a FH homologue may have an amino
acid sequence that is at least about 70% identical to the amino acid sequence
of a
naturally occurring FH (e.g., SEQ ID NO:2, or SEQ ID NO:16), for example at
least
about any of 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%,
87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical
to the amino acid sequence of a naturally occurring FH (e.g., SEQ ID NO:2, or
SEQ
ID NO:16). In some embodiment, a homologue of FH (or a fragment thereof)
retains
all the complement inhibition activity of FH (or a fragment thereof). In some
embodiments, the homologue of FH (or a fragment thereof) retains at least
about
50%, for example, at least about any of 60%, 70%, 80%, 90%, or 95% of the
complement inhibition activity of FH (or a fragment thereof).
[0091] In some embodiments, the FH portion comprises at least the first
four
N-terminal SCR domains of a human FH, such as a FH portion having an amino
acid
sequence containing at least amino acids 21 through 262 of the human FH (SEQ
ID
NO:2). In some embodiments, the FH portion comprises at least the first four N-
terminal SCR domains of human FH having an amino acid sequence that is at
least
about any of 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%,
87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% identical to
amino acids 21 through 262 of the human FH (SEQ ID NO:2).
[0092] In some embodiments, the FH portion comprises at least the first
five
N-terminal SCR domains of a human FH, such as a FH portion having an amino
acid
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
sequence containing at least amino acids 21 through 320 of the human FH (SEQ
ID
NO:2). In some embodiments, the FH portion comprises at least the first five N-
terminal SCR domains of human FH having an amino acid sequence that is at
least
about any of 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%,
87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% identical to
amino acids 21 through 320 of the human FH (SEQ ID NO:2).
[0093] In some embodiments, the FH portion comprises a full length or a
fragment of factor-H like 1 molecule (FHL-1), a protein encoded by an
alternatively
spliced transcript of the factor H gene. The mature FHL-1 contains 431 amino
acids.
The first 427 amino acids organize seven SCR domains and are identical to the
N-
terminal SCR domains of FH. The remaining four amino acid residues Ser-Phe-Thr-
Leu (SFTL) at the C-terminus are specific to FHL-1. FHL-1 has been
characterized
functionally and shown to have factor H complement regulatory activity. The
term
"FH portion" also encompasses full length or fragments of factor H related
molecules,
including, but are not limited to, proteins encoded by the FHR1, FHR2, FHR3,
FFIR4,
FHR5 genes. These factor H related proteins are disclosed, for example, in de
Cordoba et al., Molecular Immunology 2004, 41:355-367.
Variants of CR2-FH molecules
[0094] Also encompassed are variants of the CR2-FH molecules (such as the
CR2-FH fusion proteins). A variant of the CR2-FH molecule described herein may
be: (i) one in which one or more of the amino acid residues of the CR2 portion
and/or
the FH portion are substituted with a conserved or non-conserved amino acid
residue
(preferably a conserved amino acid residue) and such substituted amino acid
residue
may or may not be one encoded by the genetic code; or (ii) one in which one or
more
of the amino acid residues in the CR2 portion and/or FH portion includes a
substituent
group, or (iii) one in which the CR2-FH molecule (such as the CR2-FH fusion
protein) is fused with another compound, such as a compound to increase the
half-life
of the CR2-FH molecule (for example, polyethylene glycol), or (iv) one in
which
additional amino acids are fused to the CR2-FH molecule (such as the CR2-FH
fusion
protein), such as a leader or secretory sequence or a sequence which is
employed for
purification of the CR2-FH molecule (such as the CR2-FH fusion protein), or
(v) one
in which the CR2-FH molecule (such as the CR2-FH fusion protein) is fused with
a
larger polypeptide, i.e., human albumin, an antibody or Fe, for increased
duration of
26
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
effect. Such variants are deemed to be within the scope of those skilled in
the art
from the teachings herein.
[0095] In some embodiments, the variant of the CR2-FH molecule contains
conservative amino acid substitutions (defined further below) made at one or
more
predicted, preferably nonessential amino acid residues. A "nonessential" amino
acid
residue is a residue that can be altered from the wild-type sequence of a
protein
without altering the biological activity, whereas an "essential" amino acid
residue is
required for biological activity. A "conservative amino acid substitution" is
one in
which the amino acid residue is replaced with an amino acid residue having a
similar
side chain. Families of amino acid residues having similar side chains have
been
defined in the art. These families include amino acids with basic side chains
(e.g.,
lysine, arginine, histidine), acidic side chains (e.g., aspartic acid,
glutamic acid),
uncharged polar side chains (e.g., glycine, asparagine, glutamine, serine,
threonine,
tyrosine, cysteine), nonpolar side chains (e.g., alanine, valine, leucine,
isoleucine,
proline, phenylalanine, methionine, tryptophan), beta-branched side chains
(e.g.,
threonine, valine, isroleucine) and aromatic side chains (e.g., tyrosine,
phenylalanine,
tryptophan, histidine).
(00961 Amino acid substitutions in the CR2 or FH portions of the CR2-FH
molecule can be introduced to improve the functionality of the molecule. For
example, amino acid substitutions can be introduced into the CR2 portion of
the
molecule to increase binding affinity of the CR2 portion to its ligand(s),
increase
binding specificity of the CR2 portion to its ligand(s), improve targeting of
the CR2-
FH molecule to desired sites, increase dimerization or multimerization of CR2-
FH
molecules, and improve pharmacokinetics of the CR2-FH molecule. Similarly,
amino
acid substitutions can be introduced into the FH portion of the molecule to
increase
the functionality of the CR2-FH molecule and improve phannacokinetics of the
CR2-
FH molecule.
[0097] In some embodiments, the CR2-FH molecule (such as the CR2-FH
fusion protein) is fused with another compound, such as a compound to increase
the
half-life of the polypeptide and/or to reduce potential immunogenicity of the
polypeptide (for example, polyethylene glycol, "PEG"). The PEG can be used to
impart water solubility, size, slow rate of kidney clearance, and reduced
immunogenicity to the fusion protein. See e.g., U.S. Pat. No. 6,214,966. In
the case
of PEGylations, the fusion of the CR2-FH molecule (such as the CR2-FH fusion
27
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
protein) to PEG can be accomplished by any means known to one skilled in the
art.
For example, PEGylation can be accomplished by first introducing a cysteine
mutation into the CR2-FH fusion protein, followed by site-specific
derivatization with
PEG-maleimide. The cysteine can be added to the C-terminus of the CR2-FH
fusion
protein. See, e.g., Tsutsumi et al. (2000) Proc. Natl. Acad. ScL USA
97(15):8548-
8553. Another modification which can be made to the CR2-FH molecule (such as
the
CR2-FH fusion protein) involves biotinylation. In certain instances, it may be
useful
to have the CR2-FH molecule (such as the CR2-FH fusion protein) biotinylated
so
that it can readily react with streptavidin. Methods for biotinylation of
proteins are
well known in the art. Additionally, chondroitin sulfate can be linked with
the CR2-
FH molecule (such as the CR2-FH fusion protein).
[0098] In some embodiments, the CR2-FH molecule is fused to another
targeting molecule or targeting moiety which further increases the targeting
efficiency
of the CR2-FH molecule. For example, the CR2-FH molecule can be fused to a
ligand (such as an amino acid sequence) that has the capability to bind or
otherwise
attach to an endothelial cell of a blood vessel (referred to as "vascular
endothelial
targeting amino acid ligand"). Exemplary vascular endothelial targeting
ligands
include, but are not limited to, VEGF, FGF, integrin, fibronectin, I-CAM,
PDGF, or
an antibody to a molecule expressed on the surface of a vascular endothelial
cell.
[0099] In some embodiments, the CR2-FH molecule is conjugated (such as
fused) to a ligand for intercellular adhesion molecules. For example, the CR2-
FH
molecule can be conjugated to one or more carbohydrate moieties that bind to
an
intercellular adhesion molecule. The carbohydrate moiety facilitates
localization of
the CR2-FH molecule to the site of injury. The carbohydrate moiety can be
attached
to the CR2-FH molecule by means of an extracellular event such as a chemical
or
enzymatic attachment, or can be the result of an intracellular processing
event
achieved by the expression of appropriate enzymes. In some embodiments, the
carbohydrate moiety binds to a particular class of adhesion molecules such as
integrins or selectins, including E-selectin, L-selectin or P-selectin. In
some
embodiments, the carbohydrate moiety comprises an N-linked carbohydrate, for
example the complex type, including fucosylated and sialylated carbohydrates.
In
some embodiments, the carbohydrate moiety is related to the Lewis X antigen,
for
example the sialylated Lewis X antigen.
28
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0100] For treatment of eye diseases such as AMD, the CR2-FIT can be
conjugated (such as fused) to an antibody that recognizes a neoepitope of the
clrusen.
Other targeting molecules such as small targeting peptide can also be used.
Other
modifications of the CR2-FH molecule include, for example, glycosylation,
= acetylation, phosphorylation, amidation, derivatization by known
protecting/blocking
groups, and the like.
[0101] The CR2-FH molecule may include the addition of an
immunologically active domain, such as an antibody epitope or other tag, to
facilitate
targeting or purification of the polypeptide. The use of 6xHis and GST
(glutathione S
transferase) as tags is well known. Inclusion of a cleavage site at or near
the fusion
junction will facilitate removal of the extraneous polypeptide after
purification. Other
amino acid sequences that may be included in the CR2-FH molecule include
functional domains, such as active sites from enzymes such as a hydrolase,
glycosylation domains, and cellular targeting signals.
[0102] Variants of the CR2-FH molecule (such as the CR2-FH fusion
protein)
include polypeptides having an amino acid sequence sufficiently similar to the
amino
acid sequence of the CR2-FH molecule. The term "sufficiently similar" means a
first
amino acid sequence that contains a sufficient or minimum number of identical
or
equivalent amino acid residues relative to a second amino acid sequence such
that the
first and second amino acid sequences have a common structural domain and/or
common functional activity. For example, amino acid sequences that contain a
common structural domain that is at least about 45%, preferably about 75%
through
98%, identical are defined herein as sufficiently similar. Variants include
variants of
fusion proteins encoded by a polynucleotide that hybridizes to a
polynucleotide of this
invention or a complement thereof under stringent conditions. Such variants
generally retain the functional activity of the fusion proteins of this
invention.
Libraries of fragments of the polynucleotides can be used to generate a
variegated
population of fragments for screening and subsequent selection. For example, a
library of fragments can be generated by treating a double-stranded PCR
fragment of
a polynucleotide with a nuclease under conditions wherein nicking occurs only
about
once per molecule, denaturing the double-stranded DNA, renaturing the DNA to
form
double-stranded DNA which can include sense/antisense pairs from different
nicked
products, removing single-stranded portions from reformed duplexes by
treatment
with Si nuclease, and ligating the resulting fragment library into an
expression vector.
29
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
By this method, one can derive an expression library that encodes N-terminal
and
internal fragments of various sizes of the fusion proteins of this invention.
[0103] Variants include fusion proteins that differ in amino acid
sequence due
to mutagenesis. In addition, bioequivalent analogs of the CR2-FH molecule
(such as
fusion protein) may also be constructed by making various substitutions on
residues -
or sequences in the CR2 portion andJor the FH portion.
[0104] In some embodiments, the CR2-F1-1 molecule, particularly the CR2-
FH
fusion protein, is fused at its N-terminus a signal peptide. Such signal
peptides are
useful for the secretion of the CR2-FH molecule. Suitable signal peptides
include, for
example, the signal peptide of the CD5 protein (such as signal peptide of the
human
CD5 protein MPMGSLQPLATLYLLGMLVAS, SEQ ID NO:11). In some
embodiments, the signal peptide of the CR2 protein is used. For example, in
some
embodiments, the signal peptide of the human CR2 protein
(MGAAGLLGVFLALVAPG, SEQ ID NO:13 or MGAAGLLGVFLALVAPGVLG,
SEQ ID NO:25) is used.
Preparation of CR2-FH molecules
[0105] The CR2-FH molecules (or the two portions of the CR2-FH molecules)
described herein may be made by chemical synthesis methods, or by linkage of a
polynucleotide encoding the CR2 portion and a polynucleotide encoding the FH
portion (with or without a linker sequence), and introducing the resulting
polynucleotide molecule in a vector for transfecting host cells that are
capable of
expressing the molecule. Chemical synthesis, especially solid phase synthesis,
is
preferred for short peptides or those containing unnatural or unusual amino
acids such
as D-Tyr, Ornithine, and the like. Recombinant procedures are preferred for
longer
polypeptides. The CR2-FH molecule can be isolated in vitro by protein
purification
methods. The CR2-FH molecule can also be provided "in situ" by introduction of
a
gene therapy system to the tissue of interest which then expresses the CR2-FH
fusion.
[0106] Recombinant DNA techniques for making a CR2-FH fusion protein
involves, in simplified form, taking the a CR2-FH encoding polynucleotide,
inserting
it into an appropriate vector, inserting the vector into an appropriate host
cell, and
recovering or isolating the fusion protein produced thereby.
[0107] Provided herein are polynucleotides that encode a CR2-FH molecule
(i.e., a CR2-FH fusion protein). Such polynucleotide may also be used for
delivery
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
and expression of CR2-FH. For example, in some embodiments, there is provided
a
polynucleotide encoding a fusion protein coMprising a CR2 portion comprising a
CR2
or a fragment thereof and a FH portion comprising a FH or a fragment thereof.
In
some embodiments, the polynucleotide also comprises a sequence encoding a
signal
peptide operably linked at the 5' end of the sequence encoding the CR2-FH
fusion
protein. Exemplary nucleotide sequences of signal peptides are provided in
Figure 7
(SEQ ID NO:12, 14, and 25). In some embodiments, a linker sequence is used for
linking the CR2 portion and the FH portion. In some embodiments, the
polynucleotide encodes a CR2-FH fusion protein having an amino acid sequence
of
SEQ ID NO:3. In some embodiments, the polynucleotide encodes a CR2-FH fusion
protein having an amino acid sequence that is at least about any of 75%, 76%,
77%,
78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%,
92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to the amino acid sequence
of any of SEQ ID NO:3, SEQ ID NO:21, and SEQ ID NO:23. In some embodiments,
the polynucleotide encodes a CR2-FH molecule comprising at least about any of
400,
450, 500, 550, or more contiguous nucleotides of any of SEQ ID NO:4, SEQ ID
NO:22, and SEQ ID NO:24. In some embodiments, the polynucleotide comprises a
sequence any of SEQ ID NO:4, SEQ ID NO:22, and SEQ ID NO:24. In some
embodiments, the polynucleotide comprises a sequence that is at least about
any of
75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%,
89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to the
nucleic acid sequence any of SEQ ID NO:4, SEQ ID NO:22, and SEQ ID NO:24. In
some embodiments, the polynucleotide comprises least about any of 1200, 1300,
1400, 1500, 1600, or more contiguous nucleotides any of SEQ ID NO:4, SEQ ID
NO:22, and SEQ ID NO:24. The polynucleotide may further include a sequence
encoding a secretory signal sequence to secret the fusion protein into a
medium. The
polynucleotide encoding a secretory signal sequence include, for example, a
polynucleotide encoding the signal sequence of CD5 or a polynucleotide
sequence
encoding the signal sequence of CR2.
[01081 Also provided are expression vectors comprising a polynucleotide
described herein for expression of the CR2-FH fusion protein. The expression
vector
can be used to direct expression of a CR2-FH fusion protein in vitro or in
vivo. The
vector may include any element to establish a conventional function of a
vector, for
example, promoter, terminator, selection marker, and origin of replication.
The
31
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
promoter can be constitutive or regulative, and is selected from, for example,
promoters of genes for galactokinase (GAL1), uridylyltransferase (GAL7),
epimerase
(GAL10), phosphoglycerate kinase (PGK), glyceraldehydes-3-phosphate
dehydrogenase (GPD), alcohol dehydrogenase (ADH), and the like.
101091 Many expression vectors are known to those of skill in the art.
For
example, E. coli may be transformed using pBR322, a plasmid derived from an E.
coli
species (Mandel et al., J. Mol. Biol.,53:154(1970)). Plasmid pBR322 contains
genes
for ampicillin and tetracycline resistance, and thus provides easy means for
selection.
Other vectors include different features such as different promoters, which
are often
important in expression. For example, plamids pICK223-3 (Pharmacia Fine
Chemicals, Uppsala, Sweden), pKK233-2 (Clontech, Palo Alto, Calif, USA), and
pGEM1 (Promega Biotech, Madison, Wis., USA), are all commercially available.
Other vectors that can be used in the present invention include, but are not
limited to,
pET2la (Studier etal., Methods Enzymol., 185: 60-89 (1990)), pR1T5, and pR1T2T
(Pharmacia Biotechnology), and pB0475 (Cunningham et al., Science, 243: 1330-
1336 (1989); U.S. Pat. No. 5,580,723). Mammalian expression vectors may
contain
non-transcribed elements such as an origin of replication, promoter and
enhancer, and
or 3' nontranslated sequences such as ribosome binding sites, a
polyadenylation
site, acceptor site and splice donor, and transcriptional termination
sequences.
Promoters for use in mammalian expression vectors usually are for example
viral
promoters such as Polyoma, Adenovirus, HTLV, Simian Virus 40 (SV 40), and
human cytomegalovirus (CMV). Vectors can also be constructed using standard
techniques by combining the relevant traits of the vectors described above.
(01101 Also provided are host cells (such as isolated cells, transient
cell lines,
and stable cell lines) for expressing a CR2-FH fusion protein. The host cell
may be
prokaryotic or eukaryotes. Exemplary prokaryote host cells include E. coli K12
strain
294 (ATCC No. 31446), E. coli B, E. coli X1776 (ATCC No. 31537), E. coli W3110
(F-, ganmma-, prototrophic/ATCC No. 27325), bacilli such as Bacillus subtilis,
and
other enterobacteriaceae such as Salmonella typhimurium or Serratia marcesans,
and
various Pseudomonas species. One suitable prokaryotic host cell is E. coli
BL21
(Stratagene), which is deficient in the OmpT and Lon proteases, which may
interfere
with isolation of intact recombinant proteins, and useful with T7 promoter-
driven
vectors, such as the pET vectors. Another suitable prokaryote is E. coli W3110
(ATCC No. 27325). When expressed by prokaryotes the peptides typically contain
an
32
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
N-terminal methionine or a formyl methionine and are not glycosylated. In the
case of
fusion proteins, the N-terminal methionine or formyl methionine resides on the
amino
terminus of the fusion protein or the signal sequence of the fusion protein.
These
examples are, of course, intended to be illustrative rather than limiting.
[01111 In addition to prokaryotes, eukaryotic microbes such as
filamentous
fungi or yeast are suitable cloning or expression hosts for fusion-protein-
encoding
vectors. Saccharomyces cerevisiae is a commonly used lower eukaryotic host
microorganism. Others include Schizosaccharomyces pombe (Beach and Nurse,
Nature, 290: 140 (1981); EP 139,383 published 2 May 1985); Kluyveromyces hosts
(U.S. Pat. No. 4,943,529; Fleer et al., Bio/Technology, 9:968-975 (1991)) such
as,
e.g., K. lactis (MW98-8C, CBS683, CBS4574; Louvencourt et al., J. Bacteriol.,
154(2):737-742 (1983)), K. fragilis (ATCC 12,424), K. bulgaricus (ATCC No.
16,045), K. wickeramii (ATCC No. 24,178), K. waltii (ATCC No. 56,500), K.
drosophilarurn (ATCC No. 36,906; Van den Berg et al., Bio/Technology, 8:135
(1990)), K. thermotolerans, and K. marxianus; yarrowia (EP 402,226); Pichia
pastoris
(EP 183,070; Sreekrishna et al., J. Basic Microbiol., 28:265-278 (1988));
Candida;
Trichoderma reesia (EP 244,234); Neurospora crassa (Case et al., Proc. Natl.
Acad.
Sci. USA, 76:5259-5263 (1979)); Schwarmiomyces such as Schwanniomyces
occidentalis (EP 394,538 published 31 Oct. 1990); and filamentous fungi such
as, e.g.,
Neurospora, Penicillium, Tolypocladium (WO 91/00357 published 10 Jan. 1991),
and
Aspergillus hosts such as A. nidulans (Ballance et al., Biochem. Biophys. Res.
Commun., 112:284-289 (1983); Tilburn et al., Gene, 26:205-221 (1983); Yelton
et al.,
Proc. Natl. Acad. Sci. USA, 81: 1470-1474 (1984)) and A. niger (Kelly and
Hynes,
EMBO J., 4:475-479 (1985)). Methylotropic yeasts are suitable herein and
include,
but are not limited to, yeast capable of growth on methanol selected from the
genera
consisting of Hansenula, Candida, Kloeckera, Pichia, Saccharomyces,
Torulopsis, and
Rhodotorula. A list of specific species that are exemplary of this class of
yeasts may
be found in C. Anthony, The Biochemistry of Methylotrophs, 269 (1982). Host
cells
also include insect cells such as Drosophila S2 and Spodoptera Sf9, as well as
plant
cells.
[0112] Examples of useful mammalian host cell lines include, but are not
limited to, HeLa, Chinese hamster ovary (CHO), COS-7, L cells, C127, 3T3, BHK,
CHL-1, NSO, HE1C293, WI38, BHK, C127 or MDCK cell lines. Another exemplary
mammalian cell line is CHL-1. When CHL-1 is used hygromycin is included as a
33
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
eukaryotic selection marker. CHL-1 cells are derived from RPMI 7032 melanoma
cells, a readily available human cell line. Cells suitable for use in this
invention are
commercially available from the ATCC.
[0113] In some embodiments, the host cell is a non-human host cell. In
some
embodiment, the host cell is a CHO cell. In some embodiments, the host cell is
a 293
cell.
[0114] The CR2-FH molecules can be isolated by a variety of methods known
in the art. In some embodiments, when the CR2-FH molecule is a fusion protein
secreted into the growth media, the molecule can be purified directly from the
media.
If the fusion protein is not secreted, it is isolated from cell lysates. Cell
disruption can
be done by any conventional method, including freeze-thaw cycling, sonication,
mechanical disruption, or use of cell lysing agents. The CR2-FH molecules can
be
obtained by various methods. These include, but are not limited to,
immunoaffinity
chromatography, reverse phase chromatography, cation exchange chromatography,
anion exchange chromatography, hydrophobic interaction chromatography, gel
filtration chromatography, and HPLC. For example, the CR2-FH molecule can be
purified by inununoaffinity chromatography using an antibody that recognizes
the
CR2 portion or an antibody that recognizes the FH portion, or both. In some
embodiments, an antibody recognizing the first two N-terminal SCR domains of
CR2
is used for purifying the CR2-FH molecule. In some embodiments, the CR2-F1-1
molecule is purified by ion change chromatography.
[01151 The peptide may or may not be properly folded when expressed as a
fusion protein. These factors determine whether the fusion protein must be
denatured
and refolded, and if so, whether these procedures are employed before or after
cleavage. When denaturing and refolding are needed, typically the peptide is
treated
with a chaotrope, such a guanidine HC1, and is then treated with a redox
buffer,
containing, for example, reduced and oxidized dithiothreitol or glutathione at
the
appropriate ratios, pH, and temperature, such that the peptide is refolded to
its native
structure.
[0116] The CR2-FH molecules described herein may also contain a tag (such
as a cleavable tag) for purification. This tag can be fused to the C-terminus
or N-
terminus of the CR2 portion or the FH portion, and can be used to facilitate
protein
purification.
34
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[01171 In some embodiments, the CR2-FH molecule could be synthesized de
novo in whole or in part, using chemical methods well known in the art. For
example,
the component amino acid sequences can be synthesized by solid phase
techniques,
cleaved from the resin, and purified by preparative high performance liquid
chromatography followed by chemical linkage to form a desired polypeptide. The
composition of the synthetic peptides may be confirmed by amino acid analysis
or
sequencing.
[0118J The CR2-FH molecules can be assayed for their desired properties
using in vitro or in vivo assays. For example, binding of CR2-FH to CR2 ligand
can
be determined by surface plasmon resonance method. By way of example, kinetic
analysis of the interaction of the CR2-FH with C3dg-biotin can be performed
using
surface plasmon. resonance (SPR) measurements made on a BIAcore 3000
instrument
(Biacore AB, Uppsala, Sweden). Human C3dg-biotin can be bound to the surface
of
BIAcore streptavidin sensor chips by injecting C3dg-biotin over the surface of
one
flow cell of the chip. Binding can be evaluated over a range of CR2-FH
concentrations. Association of CR2-FH molecule with the ligand can be
monitored
for a certain period of time (such as 120 seconds), after which the complex is
allowed
to dissociate in the presence of buffer only for an additional period of time
(such as
120 seconds). Binding of CR2 fusion protein fragments to C3dg-immobilized flow
cells can be corrected for binding to control flow cells. Binding data can be
fitted to a
1:1 Langrnuir binding model using BIAevaluation Version 3.1 software (BIAcore)
and evaluated for best fit. The kinetic dissociation profiles obtained can be
used to
calculate on and off rates (ka and kd) and affinity constants (KD) using the
BIAevaluation Version 3.1 program. Other assay methods for ligand binding are
known in the art and can also be used.
[0119] In vitro zymosan complement assay can be used to determine
complement inhibitory activity of CR2-FH molecules. Lysis of rabbit
erythrocytes by
serum in Mg-EGTA is another measure of activity that can be used. Lysis in Mg-
EGTA of human or sheep erythrocytes that have had sialic acid removed provides
for
additional measures of activity.
Pharmaceutical compositions
[01201 Also provided herein are pharmaceutical compositions comprising a
CR2-FH molecule and a pharmaceutically acceptable carrier. The pharmaceutical
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
compositions may be suitable for a variety of modes of administration
described
herein, including for example systemic or localized administration. The
pharmaceutical compositions can be in the form of eye drops, injectable
solutions, or
in a form suitable for inhalation (either through the mouth or the nose) or
oral
administration. The pharmaceutical compositions described herein can be
packaged in
single unit dosages or in multidosage forms.
101211 In some embodiments, the pharmaceutical compositions comprise a
CR2-FH molecule and a pharmaceutically acceptable carrier suitable for
administration to human. In some embodiments, the pharmaceutical compositions
comprise a CR2-FH molecule and a pharmaceutically acceptable carrier suitable
for
intraocular injection. In some embodiments, the pharmaceutical compositions
comprise a CR2-FH molecule and a pharmaceutically acceptable carrier suitable
for
topical application to the eye. In some embodiments, the pharmaceutical
compositions comprise a CR2-FH molecule and a pharmaceutically acceptable
carrier
suitable for intravenous injection. In some embodiments, the pharmaceutical
compositions comprise a CR2-FH molecule and a pharmaceutically acceptable
carrier
suitable for injection into the arteries (such as renal arteries).
[0122] The compositions are generally formulated as sterile,
substantially
isotonic, and in full compliance with all Good Manufacturing Practice (GMP) -
regulations of the U.S. Food and Drug Administration. In some embodiments, the
composition is free of pathogen. For injection, the pharmaceutical composition
can
be in the form of liquid solutions, for example in physiologically compatible
buffers
such as Hank's solution or Ringer's solution. In addition, the CR2-FH
pharmaceutical
composition can be in a solid form and redissolved or suspended immediately
prior to
use. Lyophilized compositions are also included.
[0123] For oral administration, the pharmaceutical compositions can take
the
form of, for example, tablets or capsules prepared by conventional means with
pharmaceutically acceptable excipients such as binding agents (e.g.,
pregelatinized
maize starch, polyvinylpyrrolidone or hydroxypropyl methyleellulose); fillers
(e.g.,
lactose, microcrystalline cellulose or calcium hydrogen phosphate); lubricants
(e.g.,
magnesium stearate, talc or silica); disintegrants (e.g., potato starch or
sodium starch
glycolate); or wetting agents (e.g., sodium lauryl sulfate). Liquid
preparations for oral
administration can take the form of, for example, solutions, syrups or
suspensions, or
they can be presented as a dry product for constitution with water or other
suitable
36
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
vehicle before use. Such liquid preparations can be prepared by conventional
means
with pharmaceutically acceptable additives such as suspending agents (e.g.,
sorbitol
syrup, cellulose derivatives or hydrogenated edible fats); emulsifying agents
(e.g.,
lecithin or acacia); non-aqueous vehicles (e.g., ationd oil, oily esters,
ethyl alcohol or
fractionated vegetable oils); and preservatives (e.g., methyl or propyl-p-
hydroxybenzoates or sorbic acid). The preparations can also contain buffer
salts,
flavoring, coloring and sweetening agents as appropriate.
[0124] The present invention in some embodiments provides compositions
comprising a CR2-FH molecule and a pharmaceutically acceptable carrier
suitable for
administration to the eye. Such pharmaceutical carriers can be sterile
liquids, such as
water and oil, including those of petroleum, animal, vegetable or synthetic
origin,
such as peanut oil, soybean oil, mineral oil, and the like. Saline solutions
and aqueous
dextrose, polyethylene glycol (PEG) and glycerol solutions can also be
employed as
liquid carriers, particularly for injectable solutions. Suitable
pharmaceutical
excipients include starch, glucose, lactose, sucrose, gelatin, malt, rice,
sodium state,
glycerol monostearate, glycerol, propylene, water, and the like. The
pharmaceutical
composition, if desired, can also contain minor amounts of wetting or
emulsifying
agents, or pH buffering agents. The CR2-FH molecule and other components of
the
composition may be encased in polymers or fibrin glues to provide controlled
release
of the molecule. These compositions can take the form of solutions,
suspensions,
emulsions, ointment, gel, or other solid or semisolid compositions, and the
like. The
compositions typically have a pH in the range of 4.5 to 8Ø The compositions
must
also be formulated to have osmotic values that are compatible with the aqueous
humor of the eye and ophthalmic tissues. Such osmotic values will generally be
in the
range of from about 200 to about 400 milliosrnoles per kilogram of water
("mOsm/kg"), but will preferably be about 300 mOsm/kg.
[0125] In some embodiment, the composition is formulated in accordance
with routine procedures as a pharmaceutical composition adapted for injection
intravenously, introperitoneally, or intravitreally. Typically, compositions
for
injection are solutions in sterile isotonic aqueous buffer. Where necessary,
the
composition may also include a solubilizing agent and a local anesthetic such
as
lignocaine to ease pain at the site of the injection. Generally, the
ingredients are
supplied either separately or mixed together in unit dosage form, for example,
as a dry
lyophilized powder or water free concentrate in a hermetically sealed
container such
37
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
as an ampoule or sachette indicating the quantity of active agent. Where the
composition is to be administered by infusion, it can be dispensed with an
infusion
bottle containing sterile pharmaceutical grade water or saline. Where the
composition
is administered by injection, an ampoule of sterile water for injection or
saline can be
provided so that the ingredients may be mixed prior to administration.
[0126] The compositions may further comprise additional ingredients, for
example preservatives, buffers, tonicity agents, antioxidants and stabilizers,
nonionic
wetting or clarifying agents, viscosity-increasing agents, and the like.
[0127] Suitable preservatives for use in a solution include
polyquaternium-1,
benzalkonitun chloride, thimerosal, chlorobutanol, methyl paraben, propyl
paraben,
phenylethyl alcohol, edetate disodium, sorbic acid, benzethonium chloride, and
the
like. Typically (but not necessarily) such preservatives are employed at a
level of
from 0.001% to 1.0% by weight.
[0128] Suitable buffers include boric acid, sodium and potassium
bicarbonate,
sodium and potassium borates, sodium and potassium carbonate, sodium acetate,
sodium biphosphate and the like, in amounts sufficient to maintain the pH at
between
about pH 6 and pH 8, and preferably, between about pH 7 and pH 7.5.
[0129] Suitable tonicity agents are dextran 40, dextran 70, dextrose,
glycerin,
potassium chloride, propylene glycol, sodium chloride, and the like, such that
the
sodium chloride equivalent of the ophthalmic 'solution is in the range 0.9
plus or
minus 0.2%.
[0130] Suitable antioxidants and stabilizers include sodium bisulfite,
sodium
metabisulfite, sodium thiosulfite, thiourea and the like. Suitable wetting and
clarifying
agents include polysorbate 80, polysorbate 20, poloxamer 282 and tyloxapol.
Suitable
viscosity-increasing agents include dextran 40, dextran 70, gelatin, glycerin,
hydroxyethylcellulose, hydroxmethylpropylcellulose, lanolin, methylcellulose,
petrolatum, polyethylene glycol, polyvinyl alcohol, polyvinylpyrrolidone,
carboxymethylcellulose and the like.
[0131] The use of viscosity enhancing agents to provide topical
compositions
with viscosities greater than the viscosity of simple aqueous solutions may be
desirable to increase ocular absorption of the active compounds by the target
tissues
or increase the retention time in the eye. Such viscosity building agents
include, for
example, polyvinyl alcohol, polyvinyl pyrmlidone, methyl cellulose, hydroxy
propyl
methylcell-ulose, hydroxyethyl cellulose, carboxymethyl cellulose, hydroxy
propyl
38
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
cellulose or other agents know to those skilled in the art. Such agents are
typically
employed at a level of from 0.01% to 2% by weight.
[0132] In some embodiments, there is provided a pharmaceutical
composition
for delivery of a nucleotide encoding a CR2-FH molecule. The pharmaceutical
composition for gene therapy can be in an acceptable diluent, or can comprise
a slow
release matrix in which the gene delivery vehicle or compound is imbedded.
Alternatively, where the complete gene delivery system can be produced intact
from
recombinant cells, e.g., retroviral vectors, the pharmaceutical composition
can
comprise one or more cells which produce the gene delivery system.
[0133] In clinical settings, a gene delivery system for a gene
therapeutic can
be introduced into a subject by any of a number of methods. For instance, a
pharmaceutical composition of the gene delivery system can be introduced
systemically, e.g., by intravenous injection, and specific transduction of the
protein in
the target cells occurs predominantly from specificity of transfection
provided by the
gene delivery vehicle, cell-type or tissue-type expression due to the
transcriptional
regulatory sequences controlling expression of the receptor gene, or a
combination
thereof. In other embodiments, initial delivery of the recombinant gene is
more
limited with introduction into the animal being quite localized. For example,
the gene
delivery vehicle can be introduced by catheter, See U.S. Pat. 5,328,470, or by
stereotactic injection, Chen et al. (1994), Proc. Natl. Acad. Sci., USA 91:
3054-3057.
A polynucleotide encoding a CR2-FH molecule can be delivered in a gene therapy
construct by electroporation using techniques described, Dev et al. (1994),
Cancer
Treat. Rev_ 20:105-115.
[0134] In some embodiments, there is provided a pharmaceutical
composition
for gene delivery to the eye. Ophthalmic solutions useful for storing and/or
delivering
expression vectors have been disclosed, for example, in W003077796A2.
Uses of CR2-FH molecules and compositions thereof
[0135] The CR2-FH molecules described herein can function to specifically
inhibit in vivo complement activation in the alternative complement pathway
and
inflammatory manifestations that accompany it, such as recruitment and
activation of
macrophages, neutrophils, platelets, and mast cells, edema, tissue damage, and
direct
activation of local and endogenous cells. Compositions comprising these
molecules
can therefore be used for treatment of diseases or conditions that are
mediated by
39
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
excessive or uncontrolled activation of the complement system, particularly
diseases
or conditions mediated by excessive or uncontrolled activation of the
alternative
complement pathway. In some embodiments, there are provided methods of
treating
diseases involving local inflammation process. In some embodiments, there are
provided methods of treating diseases associated with FH deficiencies (for
example a
decrease in FH level, decrease in FH activity, or lack of wild type or
protective FH),
including, for example, age-related macular degeneration,
membranoproliferative
glomerulonephritis, proteineuric disease, hemolytic-uremic syndrome, recurrent
microbial infection, ischemia reperfusion (such as renal ischemia reperfusion
or
intestinal ischemia reperfusion), organ transplant rejection, and chronic
inflammation
such as rheumatoid arthritis.
[01361 In some embodiments, there is provided a method of treating a
disease
in which the alternative complement pathway is implicated (such as macular
degeneration, for example AIVID) in an individual, comprising administering to
the
individual an effective amount of a composition comprising a CR2-FH molecule
comprising: a) a CR2 portion comprising a CR2 or a fragment thereof, and b) a
FH
portion comprising a FH or a fragment thereof. In some embodiments, there is
provided a method of inhibiting complement activation in an individual having
a
disease in which the alternative complement pathway is implicated (such as
macular
degeneration, for example AMD), comprising administering to the individual an
effective amount of a composition comprising a CR2-FH molecule comprising: a)
a
CR2 portion comprising a CR2 or a fragment thereof, and b) a FH portion
comprising
a FH or a fragment thereof. In some embodiments, there is provided a method of
inhibiting inflammation in an individual having a disease in which the
alternative
pathway is implicated (such as macular degeneration, for example A.MD),
comprising
administering to the individual an effective amount of a composition
comprising a
CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or a fragment
thereof, and b) a FH portion comprising a FH or a fragment thereof.
[0137] "Treating" or "to treat" a disease is defined as administering one
or
more CR2-FH molecules, with or without other therapeutic agents, in order to
palliate, ameliorate, stabilize, reverse, slow, delay, prevent, reduce, or
eliminate either
the disease or a symptom of the disease, or to retard or stop the progression
of the
disease or a symptom of the disease. An "effective amount" is an amount
sufficient to
treat a disease, as defined above.
CA 02656063 2014-08-01
10138] An "individual" is a vertebrate, preferably a mammal, more
preferably
a human. Mammals include, but are not limited to, farm animals, sport animals,
pets,
primates, mice and rats. In some embodiments, the individual is human. In some
embodiments, the individual is an individual other than human. In some
embodiments, the individual is an animal model for the study of a disease in
which
the alternative complement pathway is implicated. Individuals amenable to
treatment
include those who are presently asymptomatic but who are at risk of developing
a
symptomatic macular degeneration-related disorder at a later time. For
example,
human individuals include those having relatives who have experienced such a
disease, and those whose risk is determined by analysis of genetic or
biochemical
markers, by biochemical methods, or by other assays such as T cell
proliferation
assay. In some embodiments, the individual is a human having a mutation or
polymorph in its FH gene that indicates an increased susceptibility to develop
a
disease in which alternative complement pathway is implicated (such as age-
related
macular degeneration). In some embodiments, the individual has a vvildtype or
protective haplotype of FH. Different polymorphs of FH have been disclosed in
US
Patent No. 7,745,389.
[01391 The compositions described herein are particularly useful for
treating
macular degeneration, such as age-related macular degeneration (AMD). AMD is
clinically characterized by progressive loss of central vision which occurs as
a result
of damage to the photoreceptor cells in an area of the retina called the
macula. AMD
has been broadly classified into two clinical states: a wet form and a dry
form, with
the dry form making up to 80-90% of total cases. The dry form is characterized
clinically by the presence of macular drusen, which are localized deposits
between the
retinal pigment epithelium (RPE) and the Bruch's membrane, and by geographic
atrophy characterized by RPE cell death with overlying photoreceptor atrophy.
Wet
AMD, which accounts for approximately 90% of serious vision loss, is
associated
with neovascularization in the area of the macular and leakage of these new
vessels.
The accumulation of blood and fluid can cause retina detachment followed by
rapid
photoreceptor degeneration and loss of vision. It is generally accepted that
the wet
form of AMD is preceded by and arises from the dry form.
[0140] Analysis of the contents of drusen in AMD patients has shown a
large
number of inflammatory proteins including amyloid proteins, coagulation
factors, and
a large number of proteins of the complement pathway. A genetic variation in
the
41
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
complement factor H substantially raises the risk of age-related macular
degeneration
(AMD), suggesting that uncontrolled complement activation underlies the
pathogenesis of AMD. Edward et al., Science 2005, 308:421; Haines et al.,
Science
2005, 308:419; Klein et al., Science 308:385-389; Hageman et al., Proc. Natl.
Acad.
Sci. USA 2005, 102:7227.
[0141] The present invention provides methods of treating AMD (such as
wet
or dry forms of AMD) by administering an effective amount of a composition
comprising a CR2-FH molecule. In some embodiments, the invention provides
methods of treating or preventing one or more aspects or symptoms of AMD,
including, but not limited to, formation of ocular drusen, inflammation in the
eye or
eye tissue, loss of photoreceptor cells, loss of vision (including for example
visual
acuity and visual field), neovascularization (such as choroidal
neovascularization or
CNV), and retinal detachment. Other related aspects, such as photoreceptor
degeneration, RPE degeneration, retinal degeneration, chorioretinal
degeneration,
cone degeneration, retinal dysfunction, retinal damage in response to light
exposure
(such as constant light exposure), damage of the Bruch's membrane, loss of RPE
function, loss of integrity of the histoarchitecture of the cells and/or
extracellular
matrix of the normal macular, loss of function of the cells in the macula,
photoreceptor dystrophy, mucopolysaccharidoses, rod-cone dystrophies, cone-rod
dystrophies, anterior and posterior uvitis, and diabetic neuropathy, are also
included.
[0142] In some embodiments, there are provided methods of treating
macular
degeneration (such as age-related macular degeneration or AMD) in an
individual,
comprising administering to the individual an effective amount of a
composition
comprising a CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or
a
fragment thereof, and b) a FH portion comprising a FH or a fragment thereof.
In
some embodiments, the disease to be treated is a dry form of AMD. In some
embodiments, the disease to be treated is a wet form of MM.
10143] In. some embodiments, there are provided methods of treating (such
as
reducing, delaying, eliminating, or preventing) formation of drusen in the eye
of an
individual, comprising administering to the individual an effective amount of
a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there are provided methods of treating
(such
as reducing, delaying, eliminating, or preventing) inflammation in the eye of
an
42
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
individual, comprising administering to the individual an effective amount of
a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there are provided methods of treating
(such
as reducing, delaying, eliminating, or preventing) loss of photoreceptors
cells in an
individual, comprising administering to the individual an effective amount of
a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there are provided methods of treating
(such
as reducing, delaying, eliminating, or preventing) loss of photoreceptors
cells in an
individual, comprising administering to the individual an effective amount of
a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there are provided methods of treating
(such
as reducing, delaying, eliminating, or preventing) neovascularization
associated with
AMD, comprising administering to the individual an effective amount of a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there are provided methods of treating
(such
as reducing, delaying, eliminating, or preventing) retinal detachment
associated with
AMD, comprising administering to the individual an effective amount of a
composition comprising a CR2-FH molecule comprising: a) a CR2 portion
comprising a CR2 or a fragment thereof, and b) a FH portion comprising a FH or
a
fragment thereof. In some embodiments, there are provided methods of improving
(including for example decreasing, delaying, or blocking loss of) visual
acuity or
visual field in the eye of an individual, comprising administering to the
individual an
effective amount of a composition comprising a CR2-FH molecule comprising: a)
a
CR2 portion comprising a CR2 or a fragment thereof, and b) a FH portion
comprising
a FH or a fragment thereof.
[0144] In addition to macular degeneration, other eye diseases that can
be
treated by methods of the present invention include, for example, retinitis
pigmentosa,
diabetic retinopathy, and other eye diseases that involve a local inflammatory
process.
In some embodiments, the eye disease is diabetic retinopathy. In some
embodiments,
the eye disease is retinitis pigmentosa.
43
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0145] The methods described herein can also be useful for treatment of
certain renal diseases. In some embodiments, there are provided methods of
treating
membranoproliferative glomerulonephritis type II (MPGN II). MPGN II is a rare
kidney disease leading to persisting proteinuria, hematuria, and nephritic
syndrome.
FH deficiency and dysfunction in MPGN II have been reported in several cases.
For
example, mutations in FH have been found in human patients with MPGN IL Pigs
of
the Norwegian Yorkshire breed have FH defects that are inherited in a
recessive
pattern. These animals develop MPGN II and show massive complement deposits in
the renal glomeruli and die at an early age because of the renal failure.
Furthermore,
an autoantibody that recognizes FH has been described in a patient with
hypocomplementemic MPGN IL. Targeting FH to complement activation sites thus
will have therapeutic effects on an individual having MPGN IL Accordingly, in
some
embodiments, there are provided methods of treating MPGN II in an individual,
comprising administering to the individual a composition comprising a CR2-FH
molecule comprising: a) a CR2 portion comprising a CR2 or a fragment thereof,
and
b) a FH portion comprising a FH or a fragment thereof. In some embodiments,
there
are provided methods of treating proteinuria associated with MPGN II. In some
embodiments, there are provided methods of treating hematuria associated with
MPGN II. In some embodiments, there is provided a method of treating nephritic
syndrome associated with MPGN IL
[0146] In some embodiments, there are provided methods of treating
hemolytic-uremic syndrome (HUS). HUS is a disease consisting of
microangiopathic
hemolytic anemia, thrombocytopenia, and acute renal failure, caused by
continuous
platelet degradation in the periphery and platelet thrombin in the
microcirculation of
the kidney. Zipfel, Seminars in Thrombosis Hemostasis, 2001, 27(3):191-199.
There
is now considerable evidence that the nondiarrheal form of HUS (D-HUS) is
associated with alternations and mutations of FH. In addition, autoantibodies
to FH
have been reported in HUS patients. Targeting FH to complement activation
sites
thus will have therapeutic effects on an individual having HUS. Accordingly,
in some
embodiments, there are provided methods of treating HUS in an individual,
comprising administering to the individual an effective amount of a
composition
comprising a CR2-FH molecule comprising: a) a CR2 portion comprising a CR2 or
a
fragment thereof, and b) a FH portion comprising a FH or a fragment thereof.
In
some embodiments, there are provided methods of treating microangiopathic
44
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
hemolytic anemia associated with HUS. In some embodiments, there is provided a
method of treating thrombocytopenia associated with HUS. In some embodiments,
there are provided methods of treating acute renal failure associated with
HUS.
[0147] In some embodiments, the disease to be treated is systemic lupus
erythematosus, such as lupus nephritis. Systemic lupus erythernatosus (SLE) is
the
prototypic autoinunune disease resulting in multiorgan involvement. This anti-
self
response is characterized by autoantibodies directed against a variety of
nuclear and
cytoplasmic cellular components. These.autoantibodies bind to their respective
antigens, forming immune complexes which circulate and eventually deposit in
tissues. This immune complex deposition causes chronic inflammation and tissue
damage. Complement pathways (including the alternative complement pathway) are
implicated in the pathology of SLE, and the methods provided herein are thus
useful
for treating SLE (such as lupus nephritis).
[0148] In some embodiments, the disease to be treated is rheumatoid
arthritis.
Rheumatoid arthritis is a chronic disease which can exhibit a variety of
systemic
manifestations. This disease has an unknown etiology and characteristically
exhibits a
persistent inflammatory synovitis which usually involves peripheral joints in
a
symmetric distribution. Complement-mediated inflammation which causes
cartilage
destruction, bone erosions and, ultimately, joint deformities is the most
important
feature of this disease. Methods provided herein are thus useful for treatment
of
rheumatoid arthritis.
[0149] In some embodiments, the disease to be treated is ischemia
reperfusion. Ischemia reperfusion (FR) injury refers to inflammatory injury to
the
endothelium and underlying parenchymal tissues following reperfusion of
hypoxic
tissues. It is a general syndrome that is responsible for both acute and
chronic injury
to various tissues including, for example, myocardium, central nervous system,
hind
limb and intestine. Ischemia reperfusion injury can result in necrosis and
irreversible
cell injury. The complement pathway (including the alternative complement
pathway) is a major mediator of I/R injury. Methods provided herein are thus
useful
for treatment of ischemia reperfusion that occurs in any organ or tissues,
including,
but not limited to, intestinal ischemia-reperfiision injury, renal ischemia-
reperfusion
injury, cardiac ischemia-reperfusion injury, ischemia-reperfusion injury of
other
internal organs such as the lung or liver, central nervous system ischemia-
reperfusion
injury, ischemia-reperfusion injury of the limbs or digits, trauma-induced
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
hypovolemia, or ischemia-reperfusion injury of any transplanted organ or
tissue.
Ischemia-reperfusion injury can also occur in conjunction with a variety of
other
conditions including, but not limited to, stroke, spinal cord injury, trauma-
induced
hypovolemic shock, and autoimmune diseases such as rheumatoid arthritis (e.g.,
which can be greatly worsened by ischemic injury of the synovium) or a variety
of
other inflammatory diseases (diseases mediated by inflammation or wherein
inflammation is a symptom that may result in or be associated with ischemic
events
and reperfusion). Other conditions and diseases in which ischemia-reperfusion
injury
occurs will be known to those of skill in the art.
[0150] In some embodiments, there are provided methods of treating a
drusen-
associated disease. The term "drusen-associated disease" refers to any disease
in
which formation of drusen or drusen-like extracellular disease plaque takes
place, and
for which drusen or drusen-like extracellular disease plaque causes or
contributes to
thereto or represents a sign thereof. For example, AlvID, characterized by the
formation of macular drusen, is considered as a drusen-associated disease. Non-
ocular drusen-related disease include, but are not limited to, amyloidosis,
elastosis,
dense deposit disease, and/or atherosclerosis. The term "drusen-related
disease" also
includes glomerulonephritis (such as MPGN II).
[0151] Other diseases in which the alternative complement pathway is
implicated that can be treated by methods of the present invention include,
for
example: (1) tissue damage due to ischemia-reperfusion following acute
myocardial
infarction, aneurysm, stroke, hemorrhagic shock, crush injury, multiple organ
failure,
hypovolemic shock intestinal ischemia, spinal cord injury, and traumatic brain
injury;
(2) inflammatory disorders, e.g., bums, endotoxemia and septic shock, adult
respiratory distress syndrome, cardiopulmonary bypass, hemodialysis;
anaphylactic
shock, severe asthma, angioedema, Crohn's disease, sickle cell anemia,
poststreptococcal glomerulonephritis, membraneous nephritis, and pancreatitis;
(3)
transplant rejection, e.g., hyperacute xenograft rejection; (4) pregnancy
related
diseases such as recurrent fetal loss and pre-eclampsia, and (5) adverse drug
reactions,
e.g., drug allergy, IL-2 induced vascular leakage syndrome and radiographic
contrast
media allergy. Autoimmune disorders including, but not limited to, myasthenia
gravis,
Alzheimer's disease, multiple sclerosis, emphysema, obesity, rheumatoid
arthritis,
systemic lupus erythematosus, multiple sclerosis, myasthenia gravis, insulin-
dependent diabetes mellitus, acute disseminated encephalomyelitis, Addison's
disease,
46
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
antiphospholipid antibody syndrome, autoimmune hepatitis, Crolm's disease,
Goodpasture's syndrome, Graves' disease, Guillain-Barre syndrome, Hashimoto's
disease, idiopathic thrombocytopenic purpura, pemphigus, Sjogren's syndrome,
and
Takayasu's arteritis, may also be treated with the inhibitors of the
invention.
[0152] In some embodiments, the disease to be treated is any of the
following:
post cardiopulmonary bypass complications; myocardial infarction;
ischemia/reperfusion injury; stroke; acute respiratory distress syndrome
(ARDS);
sepsis; burn injury; inflammation associated with cardiopulmonary bypass and
hemodialysis; plasmapheresis; plateletpheresis; leukophereses; extracorporeal;
membrane oxygenation (ECM0); heparin-induced extracorporeal LDL precipitation
(HELP); radiographic contrast media induced allergic response; transplant
rejection;
and other inflammatory conditions and autoimmune/immune complex diseases such
as multiple sclerosis, myasthemia gravis, pancreatitis, rheumatoid arthritis,
Alzheimer's disease, asthma, thermal injury, anaphylactic shock, bowel
inflammation,
urticaria, angioedema, vasculitis, glomerularnephritis, and Sjogren's
syndrome, lupus
erythromatosus, and glomerular nephritis.
[0153] The compositions described herein can be administered to an
individual via any route, including, but not limited to, intravenous (e.g., by
infusion
pumps), intraperitoneal, intraocular, intra-arterial, intrapulmonary, oral,
inhalation,
intravesicular, intramuscular, intra-tracheal, subcutaneous, intraocular,
intrathecal,
transdermal, transpleural, intraarterial, topical, inhalational (e.g., as
mists of sprays),
mucosal (such as via nasal mucosa), subcutaneous, transdermal,
gastrointestinal,
intraarticular, intracistemal, intraventricular, rectal (i.e., via
suppository), vaginal (i.e.,
via pessary), intracranial, intrauxethral, intrahepatic, and intratumoral. In
some
embodiments, the compositions are administered systemically (for example by
intravenous injection). In some embodiments, the compositions are administered
locally (for example by intraarterial or intraocular injection).
[0154] In some embodiments, the compositions are administered directly to
the eye or the eye tissue. In some embodiments, the compositions are
administered
topically to the eye, for example, in eye drops. In some embodiments, the
compositions are administered by injection to the eye (intraocular injection)
or to the
tissues associated with the eye. The compositions can be administered, for
example,
by intraocular injection, periocular injection, subretinal injection,
intravitreal
injection, trans-septal injection, subscleral injection, intrachoroidal
injection,
47
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
intracameral injection, subconjectval injection, subconjuntival injection, sub-
Tenon's
injection, retrobulbar injection, peribulbar injection, or posterior
juxtascleral delivery.
These methods are known in the art. For example, for a description of
exemplary
periocular routes for retinal drug delivery, see Periocular routes for retinal
drug
delivery, Raghava et al. (2004), Expert Opin. Drug Deily. 1(1):99-114. The
compositions may be administered, for example, to the vitreous, aqueous humor,
sclera, conjunctiva, the area between the sclera and conjunctiva, the retina
choroids
tissues, macula, or other area in or proximate to the eye of an individual.
The
compositions can also be administered to the individual as an implant.
Preferred
implants are biocompatible and/or biodegradable sustained release formulations
which gradually release the compounds over a period of time. Ocular implants
for
drug delivery are well-known in the art. See, e.g., U.S. Pat. No. 5,501,856,
5,476,511,
and 6,331,313. The compositions can also be administered to the individual
using
iontophoresis, including, but are not limited to, the ionophoretic methods
described in
U.S. Pat. No. 4,454,151 and U.S. Pat. App. Pub. No. 2003/0181531 and
2004/0058313.
[0155] In some embodiments, the compositions are administered
intravascularly, such as intravenously (IV) or intraarterially. In some
embodiments
(for example for the treatment of renal diseases), the compositions are
administered
directly into arteries (such as renal arteries).
[0156] The optimal effective amount of the compositions can be determined
empirically and will depend on the type and severity of the disease, route of
administration, disease progression and health, mass and body area of the
individual.
Such determinations are within the skill of one in the art. The effective
amount can
also be determined based on in vitro complement activation assays. Examples of
dosages of CR2-FH molecules which can be used for methods described herein
include, but are not limited to, an effective amount within the dosage range
of any of
about 0.01 gig/kg to about 300 mg/kg, or within about 0.1 g/kg to about 40
mg/kg, or
with about 1 gig/kg to about 20 mg/kg, or within about 1 gig/kg to about 10
mg/kg.
For example, when administered intraocularly, the composition may be
administered
at low microgram ranges, including for example about 0.1 gig/kg or less, about
0.05
gig/kg or less, or 0.01 gig/kg or less. In some embodiments, the amount of CR2-
FH
administered to an individual is about 10 gig to about 500 mg per dose,
including for
example any of about 10 gig to about 50 g, about 50 gig to about 100 gig,
about 100
48
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
lig to about 200 n, about 20011g to about 300 gg, about 300 fig to about 500
g,
about 500 lig to about 1 mg, about 1 mg to about 10 mg, about 10 mg to about
50 mg,
about 50 mg to about 100 mg, about 100 mg to about 200 mg, about 200 mg to
about
300 mg, about 300 mg to about 400 mg, or about 400 mg to about 500 mg per
dose.
[0157] The CR2-FH compositions may be administered in a single daily
dose,
or the total daily dose may be administered in divided dosages of two, three,
or four
times daily. The compositions can also be administered less frequently than
daily, for
example, six times a week, five times a week, four times a week, three times a
week,
twice a week, once a week, once every two weeks, once every three weeks, once
a
month, once every two months, once every three months, or once every six
months.
The compositions may also be administered in a sustained release formulation,
such
as in an implant which gradually releases the composition for use over a
period of
time, and which allows for the composition to be administered less frequently,
such as
once a month, once every 2-6 months, once every year, or even a single
administration. The sustained release devices (such as pellets, nanoparticles,
microparticles, nanospheres, microspheres, and the like) may be administered
by
injection or surgical implanted in various locations in the eye or tissue
associated with
the eye, such as intraocular, intravitreal, subretinal, periocular,
subconjunctival, or
sub-Tenons.
[0158] The pharmaceutical compositions can be administered alone or in
combination with other molecules known to have a beneficial effect on retinal
attachment or damaged retinal tissue, including molecules capable of tissue
repair and
regeneration and/or inhibiting inflammation. Examples of useful cofactors
include
anti-VEGF agents (such as an antibody against VEGF), basic fibroblast growth
factor
(bFGF), ciliary neurotrophic factor (CNTF), axokine (a mutein of CNTF),
leukemia
inhibitory factor (LIF), neutrotrophin 3 (NT-3), neurotrophin-4 (NT-4), nerve
growth
factor (NGF), insulin-like growt.h factor II, prostaglandin E2, 30 kt)
survival factor,
taurine, and vitamin A. Other useful cofactors include symptom-alleviating
cofactors,
including antiseptics, antibiotics, antiviral and antifimgal agents and
analgesics and
anesthetics.
Gene Therapy
[0159] The CR2-FH molecules can also be delivered by expression of the
CR2-FH fusion protein in vivo, which is often referred to as "gene therapy".
For
49
CA 02656063 2014-08-01
example, cells may be engineered with a polynucleotide (DNA or RNA) encoding
for
the fusion protein ex vivo, the engineered cells are then provided to an
individual to be
treated with the fusion protein. Such methods are well-known in the art. For
example,
cells may be engineered by procedures known in the art by use of a retroviral
particle
containing RNA encoding for the fusion protein of the present invention.
[0160] Local delivery of the fusion proteins of the present invention using
gene therapy may provide the therapeutic agent to the target area, for example
to the
eye or the eye tissue.
[0161] Methods of gene delivery are known in the art. These methods
include, but are not limited to, direct DNA transfer, see, e.g., Wolff et al.
(1990)
Science 247: 1465-1468; 2) Liposome-mediated DNA transfer, see, e.g., Caplen
et al.
(1995) Nature Med. 3:39-46; Crystal (1995) Nature Med. 1:15-17; Gao and Huang
(1991) Biochem. Biophys. Res. Comm. 179:280-285; 3) Retrovirus-mediated DNA
transfer, see, e.g., Kay et al. (1993) Science 262:117-119; Anderson (1992)
Science
256:808-813; 4) DNA Virus-mediated DNA transfer. Such DNA viruses include
adenoviruses (preferably Ad2 or Ad5 based vectors), herpes viruses (preferably
herpes simplex virus based vectors), and parvoviruses (preferably "defective"
or non-
autonomous parvovirus based vectors, more preferably adeno-associated virus
based
vectors, most preferably AAV-2 based vectors). See, e.g., Ali et al. (1994)
Gene
Therapy 1:367-384; U.S. Pat. No. 4,797,368 and U.S. Pat. No. 5,139,941.
[0162] Retroviruses from which the retroviral plasmid vectors hereinabove
mentioned may be derived include, but are not limited to, Moloney Mouse
Leukemia
Virus, spleen necrosis virus, retroviruses such as Rous Sarcoma Virus, Harvey
Sarcoma Virus, avian leukosis virus, gibbon ape leukemia virus, human
immunodeficiency virus, adenovirus, Myeloproliferative Sarcoma Virus, and
mammary tumor virus. In one embodiment, the retroviral plasmid vector is
derived
from Moloney Mouse Leukemia Virus.
[0163] Adenoviruses have the advantage that they have a broad host range,
can infect quiescent or terminally differentiated cells, such as neurons or
hepatocytes,
and appear essentially non-oncogenic. See, e.g., Ali et al. (1994), supra, p.
367.
Adenoviruses do not appear to integrate into the host genome. Because they
exist
extrachromosomally, the risk of insertional mutagenesis is greatly reduced.
Ali et al.
(1994), supra, p. 373.
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[01641 Adeno-associated viruses exhibit similar advantages as adenoviral-
based vectors. However, AAVs exhibit site-specific integration on human
chromosome 19 (Ali et al. (1994), supra, p. 377).
[01651 The gene therapy vectors include one or more promoters. In some
embodiments, the vector has a promoter that drives expression in multiple cell
types.
In some embodiments, the vector has a promoter that drives expression in
specific cell
types (such as cells of retina or cells in the kidney). Suitable promoters
which may be
employed include, but are not limited to, the retroviral LTR; the SV40
promoter; and
the human cytomegalovirus (CVM) promoter described in Miller etal. (1989)
Biotechniques 7(9):980-990, or any other promoter (e.g., cellular promoters
such as
eukaryotic cellular promoters including, but not limited to, the histone, pol
III, and
.beta.-actin promoters). Other viral promoters which may be employed include,
but
are not limited to, adenovirus promoters, thymidine kinase (TK) promoters, and
B19
parvovirus promoters. The selection of a suitable promoter will be apparent to
those
skilled in the art from the teachings contained herein.
[0166] The nucleic acid sequence encoding a CR2-FH fusion protein is
under
the control of a suitable promoter. Suitable promoters which may be employed
include, but are not limited to, adenoviral promoters, such as the adenoviral
major late
promoter; or heterologous promoters, such as the cytomegalovirus (CMV)
promoter;
the respiratory syncytial virus (RSV) promoter; inducible promoters, such as
the
MMT promoter, the metallothionein promoter; heat shock promoters; the albumin
promoter; the ApoAl promoter; human globin promoters; viral thymidine kinase
promoters, such as the Herpes Simplex thymidine kinase promoter; retroviral
LTRs
(including the modified retroviral LTRs hereinabove described); the f3-actin
promoter;
and human growth hormone promoter.
[0167] Retroviral plasmid vectors can be employed to transduce packaging
cell lines to form producer cell lines. Examples of packaging cells which
maybe
transfected are described in Miller (1990) Human Gene Therapy 1:5-14. The
vectors
may transduce the packaging cells through any means known in the art. Such
means
include, but are not limited to, electroporation, the use of liposomes, and
CaPO4
precipitation. In one alternative, the retroviral plasmid vector may be
encapsulated
into a liposorne, or coupled to a lipid, and then administered to a host. The
producer
cell line generates infectious retroviral vector particles which include the
nucleic acid
sequence(s) encoding the polypeptides. Such retroviral vector particles then
may be
51
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
employed, to transduce eukaryotic cells, either in vitro or in vivo. The
transduced
eukaryotic cells will express the nucleic acid sequence(s) encoding the
polypeptide.
Eukaryotic cells which may be transduced include, but are not limited to,
embryonic
stem cells, embryonic carcinoma cells, as well as hematopoietic stem cells,
hepatocytes, fibroblasts, myoblasts, keratinocytes, endothelial cells, and
bronchial
epithelial cells.
[01681 In some embodiments, gene delivery vectors which direct expression
of CR2-FH in the eye are used. Vectors for gene delivery to the eye are known
in the
art, and have been disclosed, for example, in U.S. Patent No. 6,943,153, and
U.S.
Patent Application Publication Nos. US20020194630, US20030129164,
US200600627165.
101691 In some embodiments, the complement activation is inhibited by
contacting a body fluid with a composition comprising a CR2-FH molecule ex
vivo
under conditions that permit the CR2-FH molecule to function to inhibit
complement
activation. Suitable body fluids include those that can be returned to the
individual,
such as blood, plasma, or lymph. Affinity adsorption apheresis is described
generally
in Nilsson et al. (1988) Blood 58(1):38-44; Christie et al. (1993) Transfusion
33:234-
242; Richter et al. (1997) ASAIO J. 43(1):53-59; Suzuki et al. (1994)
Autoinununity
19: 105-112; U.S. Pat. No. 5,733,254; Richter et al. (1993) Metabol. Clin.
Exp.
42:888-894; and Wallukat et al. (1996) Int? J. Card. 54:1910195.
[01701 Accordingly, the invention include methods of treating one or more
diseases described herein in an individual comprising treating the
individual's blood
extracoporeally (i.e., outside the body or ex viva) with a composition
comprising a
CR2-FIT molecule under conditions that permit the molecule to function to
inhibit
complement activation, and returning the blood to the individual.
Unit dosages, articles of manufacture, and kits
[0171] Also provided are unit dosage forms of CR2-FIT molecule
compositions, each dosage containing from about 0.01 mg to about 50 mg,
including
for example any of about 0.1 mg to about 50 mg, about 1 mg to about 50 mg,
about 5
mg to about 40 mg, about 10 mg to about 20 mg, or about 15 mg of the CR2-FH
molecule. In some embodiments, the unit dosage forms of CR2-FH molecule
composition comprises about any of 0.01 mg-0.1rng, 0.1 mg-0.2 mg, 0.2 mg-0.25
mg,
0.25 mg-0.3 mg, 0.3 mg-0.35 mg, 0.35 mg-0.4 mg, 0.4 mg-0.5 mg, 0.5 mg-1.0 mg,
10
52
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
mg-20 mg, 20rng -50 tug, 50 mg-80 mg, 80 mg-100 mg, 100 mg-150 mg, 150 mg-200
mg, 200 mg-250 mg, 250 mg-300 mg, 300 mg-400 mg, or 400 mg-500 mg CR2-FH
molecule. In some embodiments, the unit dosage form comprises about 0.25 mg
CH2-FH molecule. The term "unit dosage form" refers to a physically discrete
unit
suitable as unitatry dosages for an individual, each unit containing a
predetermined
quantity of active material calculated to produce the desired therapeutic
effect, in
association with a suitable pharmaceutical carrier, diluent, or excipient.
These unit
dosage forms can be stored in a suitable packaging in single or multiple unit
dosages
and may also be further sterilized and sealed.
[0172] Also provided are articles of manufacture comprising the
compositions
described herein in suitable packaging. Suitable packaging for compositions
(such as
ophthalmic compositions) described herein are known in the art, and include,
for
example, vials (such as sealed vials), vessels, ampules, bottles, jars,
flexible
packaging (e.g., sealed Mylar or plastic bags), and the like. These articles
of
manufacture may further be sterilized and/or sealed.
[0173] The present invention also provides kits comprising compositions
(or
unit dosages forms and/or articles of manufacture) described herein and may
further
comprise instruction(s) on methods of using the composition, such as uses
described
herein. The kits described herein may further include other materials
desirable from a
commercial and user standpoint, including other buffers, diluents, filters,
needles,
syringes, and package inserts with instructions for performing any methods
described
herein.
EXAMPLES
Example 1. Exemplary sequences of CR2-FH molecules and signal peptides
[0174] Figures 4-6 provide exemplary amino acid sequences of CR2-FH
molecules described herein (SEQ ID NOs: 5-10). "min" represents an optional
linker.
[0175] . Figure 7 provides exemplary amino acid sequences of signaling
peptides described herein (SEQ ID NOs: 11 and 13) and polynucleotides encoding
the signaling peptides (SEQ ID NOs:12 and 14).
[0176] Figure 9 provides amino acid sequence of a mouse CR2-FH fusion
protein (designated as CR2-fH or CR2NLFH) (SEQ ID NO:17) and a polynucleotide
that encodes a mouse CR2-FH plus the signal peptide (SEQ ID NO:18).
53
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
[0177] Figure 10 provides the DNA sequence of CR2NLFHFH, a mouse
CR2-FH fusion protein containing a CR2 portion and two FH portions without a
linker sequence (SEQ ID NO:19).
[0178] Figure 11 provides the DNA sequence of CR2LFHFH, a mouseCR2-
FH fusion protein containing a CR2 portion linked to two FH portions via a
linker
sequence (SEQ ID NO:20).
[0179] Figure 20 provides amino acid sequence of a human CR2-FH fusion
protein (designated as human CR2-fH or CR2fH) (SEQ ID NO:21) and a
polynucleotide that encodes a human CR2-fH plus the signal peptide (SEQ ID
NO:22).
[0180] Figure 21 provides amino acid sequence of a human CR2-FH fusion
protein containing two FH portions (designated as human CR2-FH2 or CR2fH2 or
human CR2fH2) (SEQ ID NO:23) and a polynucleotide that encodes a human CR2-
FH2 plus the signal peptide (SEQ ID NO:24).
Example 2. In vitro inhibition of alternative pathway by CR2-FH
[0181] Mouse fusion proteins containing the first four SCR domains of CR2
and the first five SCR domains of FH (with linker (CR2LFH) or without linker
(CR2NLFH or CR2-ill)) were made by recombinant DNA cloning and gene
expression method. The sequence for one of the CR2-FH fusion proteins is
provided
in Figure 9. SEQ ID NO:17 is the polypeptide sequence of the CR2-F11 fusion
protein. SED ID NO:18 is the nucleotide used to encode the fusion protein, as
well as
a signal peptide at the N-terminus of the signal peptide.
[0182] A mouse CR2-FH fusion protein (designated as CR2LFHFH, CR2-fH2
or CR2-fHH) containing the first four SCR domains of CR2 and two tandemly
linked
FH portions (each containing the first five SCR domains of FH) was also made.
The
CR2 portion and the first FH portion was linked by a linker sequence. The DNA
sequence (including the DNA encoding the signal peptide) of CR2LFHFH is
provided
in Figure 11 (SEQ ID NO:20).
[0183] In vitro assays for activation of the alternative pathway were
conducted
as essentially described in Quigg et al., J. Immuna 1998, I60(9):4553-60.
Factor H
(ill) or CR2-Crry were used as controls in the experiment. Specifically, 50 mg
of
zyrnosan beads in 10 ml of 0.15M NaC1 were activated by boiling for 60
minutes, and
washed twice in PBS. In each reaction mixture add: 1) 10 m.M EGTA and 5MM
54
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
MgCl2 (final concentration); 2) 1x107 beads; 3) 10 m.M EDTA (negative control
1) or
HIC serum (negative control 2) or increasing concentration of one of the CR2-
FH
fusion proteins or control proteins; 4) 10 1 of serum; and 5) PBS to bring
the total
volume to 100 pl. The mixtures were incubated at 37 C for 20 minutes, and the
reactions were stopped by addition of 10 mM EDTA (final concentration). The
beads
were washed twice with cold PBSB (PBS with 1% BSA), and incubated with FTIC-
conjugated goat-anti-C3 antibody for one hour on ice. The sample were then
washed
twice in PBSB, resuspended with 1% paraforrnaldehyde and analyzed under flow
cytometry.
[0184] Figure 12A provides a graphic representation of data obtained in
an in
vitro zymosan complement assay using mouse CR2-FR fusion proteins (CR2-f11)
and
factor H alone (fH). As shown in the figure, CR2-fH was significantly more
effective
than FR in inhibiting complement activation. Figure 12B provides a graphic
representation of data obtained in an in vitro zymosan complement assay using
the
first five SCR domains of mouse FR (FR 15) and the first four domain's of
mouse
CR2 (CR2). The first five SCR domains of mouse FR had an EC50 of 250 nM,
which approximately equal to the amount of FH in serum. The molecule having
the
first four domains of CR2 has no inhibitory effect at all. These data
demonstrate that
the effect seen with CR2-FH is due to the combined effects of the two portions
of the
molecule, rather than the independent function of each portion.
[0185] Figure 13 provides a graphic representation of data obtained in an
in
vitro zymosan complement assay using mouse CR2-FH fusion protein with linker
(CR2LFH), CR2-FH fusion protein without linker (CR2NLFH), CR2-FH-FH with
linker (CR2LFHFH), and CR2-Crry. As shown in the figure, CR2-F1-1 was more
effective than CR2-Crry in inhibiting complement activation of the alternative
pathway. CR2LFH and CR2NLFH were equally effective in inhibiting complement
activation of the alternative pathway. CR2LFHFH is much more effective than
CR2LFH and CR2NLFH.
Example 3. Treatment of intestinal ischemia and reperfusion injury by CR2-FR
[0186] This experiment shows treatment of intestinal ischemia and
reperfusion
injury in a mouse model.
[0187] Intestinal isehemia reperfusion injury. Three adult male mice aged
8
weeks and weighing 20-25 g were anesthetized with 10 mg/kg ketamine and 6
mg/kg
=
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
xylazine by i.p. injection. Animals were breathing spontaneously and body
temperature was maintained using a heat mat for the entire experiment. A
medial
laparotomy was preformed and the intestines were carefully moved allowing
access to
the superior mesenteric artery. The superior mesenteric artery was clamped
using a
microsurgical clamp (Fine Instruments, USA). Ischemia was confirmed by palor
of
the small intestine. Sham treated mice underwent laparotomy without clamping
of
superior mesenteric artery. After 30 min. ischemia the arterial clamp was
removed
allowing reperfusion of the mesenteric vasculature. Animals were sutured using
6.0
ethicon suture and allowed to reperfuse for 2 hours. 0.1 mg or 0.05 mg CR2-fH,
or
control (PBS) were administered i.v. 30 minutes post reperfusion and animals
were
sacrificed 90 minutes later following a total of 2 hours of reperfusion.
[0188) Histology. Tissue samples for histological staining were taken
from the
intestine and either fixed in 10% formalin at 4 C overnight and subsequently
processed to paraffin, or frozen in liquid nitrogen for irnmunofluorescence
analysis.
Sections of intestine from each animal were stained with hematoxylin and eosin
and
scored for mucosal damage and villi height as previously described (46).
Briefly, a
score of 0 was assigned to a normal villus; villi with tip distortion were
scored as 1;
villi lacking goblet cells and containing Gugenheims' spaces were scored 2;
villi with
patchy disruption of the epithelial cells were scored 3; villi exposed but
intact lamina
propria and epithelial cell sloughing were assigned 4; villi in which lamina
propria
were exuding were scored as 5, and finally, villi displaying hemorrhage or
denuded
villi were scored as 6. All histological evaluations were carried out in a
blinded
fashion.
[01891 The results of the experiment are shown in Figure 14A. As shown in
the figure, both 0.1 mg and 0.05 mg of CR2-fH showed protective effect in the
animal
model compared to the control animals even though the control animals had
normal
levels of circulating endogenous factor H (about 0.5 mg/ml) in excess of the
amounts
of CR2-fH administered.
Example 3.1. Treatment of intestinal isehemia and reperfusion injury by mouse
CR2-FH
[0190] The experiment was carried out essentially as disclosed in Example
3.
[0191] Briefly, 0.05 mg, 0.1 mg, or 0.2 mg of mouse CR2-fH or mouse CR2-
f112 (CR2-fHH) were administered i.v. 30 minutes post reperfusion and animals
were
56
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
sacrificed 90 minutes later for histology analysis. The results of the
experiment are
shown in Figure 14B. As shown in Figure 14B, both mouse CR24H and mouse
CR241-1H protected the intestine from complement-mediated ischemia reperfusion
injury.
Example 3.2. Treatment ofVestinal ischemia and reperfusion injury by mouse
CR2-FH
[0192] This experiment shows the effects of mouse CR24H and CR241-12 on
alternative complement pathway and intestinal ischemia reperfusion. The
experiments are carried out essentially as described above.
[0193] In vitro assays demonstrated that mouse CR24H was significantly
more effective in inhibiting the alternative pathway of complement than CR2-
Crry,
and that mouse CR2-fH2 was about 2-fold more effective than mouse CR2-fH. The
complement inhibitory activity of mouse CR241-1 was dependent on CR2-mediated
targeting as demonstrated by anti-CR2 antibody blocking experiments.
Furthermore,
purified mouse factor H had only minimal complement inhibitory activities in
the in
vitro assays.
[0194] Mouse CR2-fH and mouse CR2-fH2 targeted to sites of local and
remote (lung) complement activation following intestinal ischemia and
reperfusion
injury, and both proteins protected the intestinal mucosa and the lung
parenchyma
from injury at a low dose and in a dose dependent manner. Although mouse CR2-
fH2
was a More potent inhibitor of the alternative complement pathway than mouse
CR2-
= f1-1 in vitro, there was no difference in the protective effect of the
two proteins in the
in vivo model. Compared to CR2-Crry, an approximate 2-fold higher dose of
mouse
CR24H was required to provide equivalent protection from local injury.
Example 4. Treatment of renal ischemia reperfusion by mouse CR2-FH
[0195] This example shows the effect of CR2-FH on renal ischemia
reperfusion.
[0196] Protocol for induction of ischemic ARF. Mice weighing 20-25
grams
were anesthetized with 300 1 of 2,2,2-Tribromoethanol (Sigma-Aldrich)
injected
intra-peritoneally. After the mice were anesthetized, they were placed on a
heating
pad to maintain their body temperature during surgery. Laparotornies were then
performed, and the renal pedicles were located and isolated by blunt
dissection. The
57
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
pedicles were clamped with surgical clips (Miltex Instrument Company, Inc.),
and
occlusion of blood flow was confirmed by visual inspection of the kidneys. The
clamps were left in place for 24 minutes and then released. The time of
ischemia was
chosen to obtain a reversible model of ischemic ARF with a minimum of vascular
thrombosis, and to avoid animal mortality. The kidneys were observed for
approximately one minute to ensure blood re-flow. After 15 minutes of
reperfusion
the mice received 0.25 mg of the mouse CR24H (CR2NLFH) intraperitoneally.
Fascia and skin were sutured with 4-0 silk (United States Surgical). The mice
were
volume resuscitated with 0.5 ml of normal saline and kept in an incubator at
290C to
maintain body temperature.
101971 After 24 hours of reperfusion the mice were anesthetized, and
blood
was obtained by cardiac puncture. Laparotomy was performed and the kidneys
were
harvested. The study protocol was approved by the University of Colorado
Health
Sciences Center Animal Care and Use Committee.
[0198] Serum Urea Nitrogen Measurements. Serum urea nitrogen was
determined for each mouse using a Beckman Autoanalyzer (Beckman). The result
of
is shown in Figure 15A. As shown in the figure, serum urea nitrogen was
reduced in
mouse CR2411 treated animals, indicating preservation of kidney function.
[0199] Renal morphology. After the kidneys were removed from the mice,
sagittal sections were fixed in 4% paraformaldehyde. After being embedded in
paraffin, four gm sections were cut and stained with periodic acid Schiff. The
sections
were evaluated by a renal pathologist in a blinded fashion. The cortex and
outer stripe
of the outer medulla were assessed for epithelial necrosis, loss of brush
border,
tubular dilatation and cast formation. At least ten fields (400x) were
reviewed for each
slide, and the percentage of tubules displaying these findings was determined.
The
kidney sections were scored as follows based on the percentage of affected
tubules: 0,
none; 1, <10%, 2, 11-25%, 3, 26-45%, 4, 46-75%, 5, >75%. The result of the
experiment is shown in Figure 15B. As shown in the figure, CR2-fH showed
protective effect in the animal model compared to the control animal.
[0200] Immunofluorescence: For immunofluorescence, sagittal sections of
the
kidneys were snap frozen in OCT compound (Sakura Finetek). Four gm sections
were
cut with a cryostat and stored at -70 C. The slides were later fixed with
acetone and
incubated with the FITC conjugated antibody to mouse C3 (Cappel). After
hybridization with the antibody for one hour at room temperature, the slides
were
=
58
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
counterstained with hematoxylin (Vector Laboratories, Inc.). The results of
the
experiment are shown in Figures 15C and 15D. As shown in the figure, more C3
was
deposited into kidneys of sham treated mice (15C) relative to mouse CR24H-
fireated
mice (15D).
Example 5. Treatment of age-related macular degeneration by CR2-FH
10201] Constant light exposed albino rats are used as animal models for
age-
related macular degeneration (dry AMD). Five to eight animals are injected
intraocularly under anesthesia every other day with a CR2-FH fusion protein (1
pl of
4.3 mg/ml stock solution), starting with the first injection the day prior to
the onset of
continuous light exposure (days -1, 1, 3, 5, 7). One eye serves as the
experimental,
while the other eye serves as the PBS-injected control eye. Animals are tested
with
ERG on day 8 and then euthanized for histology and PCR analysis. Number of
rows
of photoreceptors in eyes injected with CR2-FH are compared with those of the
PBS
control eyes.
[0202] The effect of CR2-FH are measured using three parameters:
functional
activity (ERG and DC potentials, i.e., photoreceptor and RPE responses),
histology
and measures of inflammation (e.g., gene expression by RT-PCR and protein
expression by immunohistochemistry.
[0203] In a second animal model (wet AMD), we test whether eliminating
complement activators reduces choroidal neovascularization (CNV). CNV is
produced in five to eight rats with a Krypton laser (200 mW, 50 pm, 0.05 sec)
and
documented in choroidal flatrnounts after fluorescein injections.
[0204] The effect of CR2-FH are measured using four parameters:
functional
activity (ERG and DC potentials, i.e., photoreceptor and RPE responses),
histology,
vascular integrity (choroidal flatmounts after fluorescein injections) and
measures of
inflammation (e.g., gene expression by RT-PCR and protein expression by
immunohistochemistry).
Example 6. Reduction in CNV volume by mouse CR2-FH
[0205] For generation of CNV, 3-month-old animals were anesthetized using
xylazine and ketarnin.e (20 and 80 mg/kg, respectively) and pupils dilated
with a drop
of phenylephrine HC1 (2.5%) and atropine sulfate (1%). Argon laser
photocoagulation
(532 nm, 50 p.m spot size, 0.05 s duration, 250 mW) was used to generate four
laser
59
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
spots in each eye surrounding the optic nerve, using a handheld coverslip as a
contact
lens. A bubble formed at a laser spot indicated the rupture of Bruch's
membrane.
Nozaki et al., Proc. Natl. Acad. Sci. 2006, 103(7):2328-33.
[0206] For assessment of CNV lesions, CNV size was determined in flat-
mount preparations of RPE/choroids stained with isolectin B (which binds to
terminal
13-D-galactose residues on the surface of endothelial cells and selectively
labels the
mouse vasculature). Fluorescence measurements taken in 2 fim sections using
confocal microscopy were used for size determination. In short, a Z-stack of
images
through the CNV lesion was obtained, using the same laser intensity setting
for all
experiments. For each slice the overall fluorescence was determined and
plotted
=
against depth.
[0207] For electroretinography, animals were anesthetized using xylazine
(20
mg/kg bodyweight) and ketamine (80 mg/kg bodyweight). Pupils were dilated with
a
drop of phenylephrine HC1 (2.5%) and tropicamide (1%). Body temperature was
stabilized via a DC-powered heating pad held at 37 C. The ERG setup used was
previously described by Rohrer et al., J. Neurosci., 1999, 19(20): 8919-30 and
was
built according to Lyubarsky and Pugh Lyubarsky et al., J. Neurosci., 1996,
16(2):563-571. Stimulus light intensity was controlled using neutral density
filters.
Stimulus paradigms. Animals were dark-adapted overnight and ERGs will be
recorded. Rods were analyzed in response to single-flash stimuli of increasing
light
intensity. The single-flash responses were an average of at least 3 flashes
with an
inter-stimulus interval (IS I) of 15 s to 2 min (lowest intensity to highest,
respectively).
The different ISIs ensured that ERG amplitudes at a given intensity were
identical
between the first and the last flash. Data analysis. For all ERG recordings, a-
wave
amplitude were measured from baseline to trough; b-wave amplitude were
measured
from a-wave trough or baseline to peak of b-wave, and implicit times were
measured
from onset of stimulus to a-wave trough or b-wave peak.
[0208] In one experiment, mice were treated with intravenous mouse CR2-fH
(2501.1g) 30 minutes post laser burn, 48 hours post laser burn, and 6 hours
post laser
burn. 6 days post later bum, retinal function was assessed, then mice were
sacrificed
for histology.
[0209] Figure 16 shows a- and b-wave retinal responses in mice treated
with
or without CR2411. As shown in Figure 16, both a- and b-waves of retinal
response
were protected by CR2411 treatment relative to PBS treatment. Figures 17A and
17B
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
show isolectin-b staining of lesions 6 days post laser burn. Figures 17C shows
quantification of lesion sizes based on the isolectin-b staining. As shown in
Figures
17A-C, mice treated with CR2-fH show significant reduction in lesion size as
compared to animals treated with PBS.
[0210] In a separate experiment, lgg mouse CR2-fH was administered
intraoptically immediately after laser burn, 48 hours post burn, and 96 hours
post
burn. Eyes were collected at day 6 for histology. Lesions were visualized by
isolectin-b staining. The results are shown in Figure 18. Figure 18A and 18B
show
isolectin-b staining of lesions 6 days post laser burn. Figure 18C shows
quantification
of lesion size based on the isolectin-b staining. As shown in Figures 18A-C,
CR2-fH
delivered directly to the eye reduces spread of the lesion.
Example 7. Delay of onset of antibody-mediated rejection in a mouse
heterotropic heart transplant model by mouse CR2-FH
[0211] In this experiment, hearts were heterotopically transplanted from
C3H
donor mice into Balt* recipient mice. This strain combination promotes a TH2
immune phenotype which promotes acute vascular rejection, and is characterized
by
anti-graft antibody production and graft deposition of complement activation
fragments.
[0212] Recipient mice were treated with 1) PBS, i.v., 2) a single 0.25 mg
dose
of mouse CR2-fH, i.v. 30 minutes post reperfusion, and 3) multiple doses of
0.25 mg
mouse CR2-111 i.v. starting 30 minutes post reperfusion and then every three
days
thereafter.
[0213] Hearts were harvested 24 hours post reperfusion for analysis.
Mouse
CR2-ill treated animals were protected from ischemia and reperfusion injury as
assessed by histology, the absence of C3, a reduction in neutrophil
infiltration, and a
reduction in inflammatory cytolcines.
[0214] The effects of mouse CR2-fH on acute vascular rejection are shown
in
Figure 21. As shown in the figure, control heart transplant recipients
survived 7.1 1
days, compared to 11.1 1.6 days (single dose group) and 10.7 1.3 days
(multiple
dose group). There is a significant improvement in survival in mice treated
with
mouse CR2-fH when compared to controls (p=0.02).
[0215] At the time of harvest there were no obvious differences in
pathological rejection profiles or in the levels of anti-donor antibodies
between any of
61
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
the groups. Interestingly, there appears to be no significant improvement in
survival
associated with the administration of multiple doses of mouse CR2-fH when
compared to the single dose group (p<0.05).
Example 8. Inhibition of alternative complement pathway by human CR2-FH
[0216] The protein sequences of human CR2-FH (SEQ ID NO:21, also
designated as CR2fEI) and human CR2-FH2 (SEQ ID NO:23, also designated as
CR2fH2), not including signal peptides, are shown in Figure 20 and 21,
respectively.
The nucleic acid sequences of human CR2-FH (SEQ ID NO: 22) and human CR2-
F112 (SEQ ID NO:24), including nucleotide sequences for signal peptides, are
shown
in Figure 20 and 21, respectively.
[0217] Human CR2-FH and human CR2-FH2 were purified from transfected
293 cell supernatants by affinity chromatography using HB5-separose, which
contains
= anti-human CR2 monoclonal antibody HB5 (ATCC catalog # HB-135) linked to
CNBr-activated sepharose (Amershan Biosciences). Crude CR2-FH or CR2-FH2
supernatants were passed over the matrix, washed with PBS, and eluted in 0.1M
glycine-HC1, pH 3Ø The eluted fraction was immediately neutralized by the
addition
of 1M Tris-C1, pH 9.0 followed by exchange into PBS using centricon columns
(Millipore). 300 ng of nonreduced, purified CR2-FH and CR2-FH2 were resolved
on
SDS-PAGE and visualized by Commassie staining. CR2-FH was present as two
distinct proteins, as determined by mass spectrometry (Alphalyse, Palo Alto,
CA) of
64.0 and 65.3 kDa which resolved into a single band following deglycosylation,
while
CR2-FH2 was a single species of 99.2 kDa. The inherent secondary structure of
these
molecules makes them run smaller than their actual molecular weight under
nonreducing conditions.
[0218] The effects of human CR2-FH and human CR2-FH2 on alternative
pathway specific C3b deposition onto zymosan particles are shown in Figure
22A.
Briefly, Zymosan particles were incubated in PBS containing 5 mM Mg2+, 10 mM
EGTA, 10% human serum, and increasing concentrations of CR2-FH and CR2-FH2
for 30 minutes at room temperature with FITC conjugated goat anti-human C3
antibody. Zymosan was pelleted and washed, followed by FACS analysis. As shown
in Figure 24A, both CR2-FH and CR2-FH2 inhibited activation of the alternative
complement pathway. Similar results were obtained by incubating with mouse
serum
followed by detection with FITC conjugated goat anti-mouse C3 antibody.
62
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
Significantly, there was 200-400 nM FH present in the assay system. The CR2-FH
had an EC50 of 8-22nM, which was 20-fold lower than the amount of FH present
in
the assay, demonstrating a clear benefit of targeted FH over endogenous FH.
[0219] The effects of human CR2-FH and human CR2-FH2 on alternative
pathway-mediated erythrocyte lysis are shown in Figure 22B. Briefly, rabbit
erythrocytes (1 x 108) were incubated with varying concentrations of CR2-FH or
CR2-FH2 in 1 x GVB++ (Boston BioProducts) and 17% human serum for 30 minutes
at 37 C. The reaction was stopped with the addition of one tenth volume cold
PBS
followed by centrifugation to pellet unlysed erythrocytes. Hemolysis was
quantified
by measuring Gatisnm. As shown in Figure 24B, both CR2-FH and CR2-F112
significantly inhibited activation of the alternative complement pathway.
Significantly, there was 340-680 nMFH present in the assay. The CR2-FH had an
EC50 of 20-30nM, which was 15-20 fold lower than the amount of FH present in
the
assay, demonstrating a clear benefit of targeted FH over endogenous FH.
Example 9. Inhibition of the alternative complement pathway by mouse CR2-
FH
[0220] This example shows inhibition of the alternative complement
pathway
by mouse CR2-FH using serum for mice deficient in the classical pathway.
[0221] ELISA assay with immune complexes of collagen-anti-collagen
antibodies on the plates were used. C3 deposition/activation was measured by
using
anti-C3b antibody in the presence of serum from wildtype or from C4-/C4- mice.
Different amounts of full length mouse FH (21410 1), the first four SCR
domains of
mouse CR2 (2pg/100), and mouse CR2-FH (2n/10111) were added to the serum.
The result of the in vitro study is shown in Figure 23. As shown in the
figure, mouse
CR2-FH had little effect on C3b deposition using serum from wildtype mice. By
contrast, mouse CR2-FH almost completely prevented C3b deposition in serum
from
classical pathway deficient mice. Mouse FH or mouse CR2, on the other hand,
had
little effects in both assay systems. This experiment demonstrates a clear
advantage
of using CR2-FH to inhibit alternative complement pathway, particularly when
the
classical complement pathway is not involved.
[0222] To
further demonstrate that the inhibition of C3b deposition observed
with CR2-FH was due to inhibition of the alternative pathway, we studied the
effects
of CR2-FH on C3b deposition in the absence of the classical pathway (C4-/C4-
mice).
63
CA 02656063 2008-12-15
WO 2007/149567
PCT/US2007/014602
Calcium inhibits the lectin complement pathway. Figure 24 shows a titration
curve
of mouse CR2-FH in calcium sufficient buffer using serum from C4-/C4- knockout
mice. As shown in the figure, CR2-FH significantly inhibits C3b deposition at
the
concentration of 0.5 1.1g/111.
10223) Although the foregoing invention has been described in some detail
by
way of illustration and example for purposes of clarity of understanding, it
is apparent
to those skilled in the art that certain minor changes and modifications will
be
practiced. Therefore, the description and examples should not be construed as
limiting the scope of the invention.
=
64