Note: Descriptions are shown in the official language in which they were submitted.
CA 02657986 2009-03-10
TITLE: SUBSTITUTED PIPERAZINE COMPOUNDS
1. Field of the Invention
The present invention is concerned with substituted piperazine compounds,
therapeutic
dosage forms including one or more of the compounds, and methods for treating
diseases in
mammals, and in particular, in a human in a therapy selected from the group
including
protecting skeletal muscles against damage resulting from trauma, protecting
skeletal muscles
subsequent to muscle or systemic diseases such as intermittent claudication,
to treat shock
conditions, to preserve donor tissue and organs used in transplants, and to
treat cardiovascular
diseases including atrial and ventricular arrhythmias, Prinzmetal's (variant)
angina, stable
angina, and exercise induced angina, congestive heart disease, and myocardial
infarction.
2. Description of the Art
U.S Patent No. 4,567,264,
discloses a class of substituted piperazine compounds that includes a compound
known as ranolazine, ( )-N- (2,6-dimethylphenyl)-4-[2-hydroxy-3- (2-
methoxyphenoxy)-
propyl]-1-piperazineacetamide, and its pharmaceutically acceptable salts, and
their use in the
treatment of cardiovascular diseases, including arrhythmias, variant and
exercise-induced
angina, and myocardial infarction.
U.S. Patent No. 5,506,229, discloses the use
of ranolazine and its pharmaceutically acceptable salts and esters for the
treatment of tissues
experiencing a physical or chemical insult, including cardioplegia, hypoxic or
reperfusion
injury to cardiac or skeletal muscle or brain tissue, and for use in
transplants. In particular,
ranolazine is particularly useful for treating arrhythmias, variant and
exercise-induced angina,
and myocardial infarction by partially inhibiting cardiac fatty acid
oxidation. Conventional
oral and parenteral ranolazine formulations are disclosed, including
controlled release
formulations. In particular, Example 7D of U.S. Patent No. 5,506,229 describes
a controlled
1
CA 02657986 2009-03-10
release formulation in capsule form comprising microspheres of ranolazine and
microcrystalline cellulose coated with release controlling polymers.
Despite the important discovery that ranolazine is a very useful cardiac
therapeutic
agent, there remains a need for compounds that are partial fatty acid
oxidation inhibitors that
have a half-life greater than ranolazine and that have activities as least
similar to ranolazine.
SUMMARY OF THE INVENTION
This invention includes novel substituted piperazine compounds that are
partial fatty
acid oxidation inhibitors with good therapeutic half-lives.
This invention also includes novel substituted piperazine compounds that can
be
administered to a mammal to protect skeletal muscles against damage resulting
from trauma,
to protecting skeletal muscles subsequent to muscle or systemic diseases such
as intermittent
claudication, to treat shock conditions, to preserve donor tissue and organs
used in transplants,
and to treat cardiovascular diseases including atrial and ventricular
arrhythmias, Prinzmetal's
(variant) angina, stable angina, and exercise induced angina, congestive heart
disease, and
myocardial infarction.
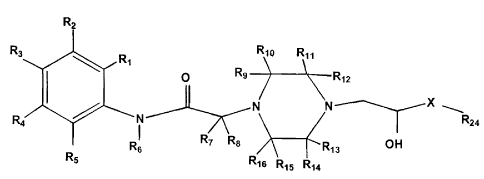
This invention includes a class of substituted piperazine compounds having the
following formula:
R
R3 R, Rlo 11
O Ra _'R12
I X
N N ~.
4 N --J~ Rza
RI R7 Rs R13 OH
6 4
R5
Rie Ris RU
wherein X is selected from the group consisting of:
m and m O
2
CA 02657986 2009-03-10
wherein m = 1 or 2 or 3;
Rõ Rz, Rõ R4 and RS are each independently selected from the group consisting
of
hydrogen, halo, NO2, CF31 CN, OR23, SR23, N(RZ3)2, S(O)R22, SOzRZZ,
SO2N(R23)Z,
NR23CO2R72, NR23CON(R23)2, COR23, CO2R23, CON(R23)2, NR23S02R22, C,-,s alkyl,
C2-,s
alkenyl, C2_15 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituent are optionally substituted with 1 substituent selected from the
group consisting of
halo, NOZ, CF31 CN, OR23, SR23, N(R23)2, S(O)R22, and S02R22 , wherein R2 and
R3 may join
together to form a fused ring system having from three to four carbon atoms,
and wherein R4
and R may join together to form -CH=CH-CH=CH-;
R6i R, and Re are each independently selected from the group consisting of
hydrogen
and C,_,S alkyl;
R9, R,o, R,,, R12, R13, R14, R,5 and R6 are each independently selected from
the group
consisting of hydrogen, COZR23, CON(R23)Z, C,.q alkyl, and aryl wherein the
alkyl and aryl
substituents are optionally substituted with 1 substituent selected from the
group consisting of
halo, CF31 CN, OR2,, N(R2.,)2, C02R23i CON(R23)2 and aryl, wherein R, and R,o
may together
form a carbonyl, or Rõ and R,Z may together form a carbonyl, or Rõ and R14 may
together
form a carbonyl, or R15 and R16 may together form a carbonyl wherein Rõ and Rõ
or R9 and
R15 or R9 and Rõ or Rõ and R15 or F. and R13 may join together to form a
bridging ring system
having from 1 to 4 carbon atoms and wherein R and R,o or Rõ and R12 or R13 and
R14 or R,S
2o and R16 may join to form a bridging ring system having from 1 to 5 carbon
atoms.
R,z is selected from the group consisting of C1_,S alkyl, aryl, and
heteroaryl, wherein the
alkyl and aryl substituents are optionally substituted with I substituent
selected from the
group consisting of halo, alkyl, monoalkylamino, dialkylamino, alkyl amide,
aryl arnide,
heteroaryl amide, CN, O-C,, alkyl, CF3, and heteroaryl;
R23 is selected from the group consisting of H, C1_15 alkyl, aryl, and
heteroaryl, wherein
the alkyl and aryl substituents are optionally substituted with I substituent
selected from the
group consisting of halo, alkyl, monoalkylamino, dialkylamino, alkyl-CN, -O-C1-
4 allcyl, and
CF3; and
R24 is selected from the group consisting of alkyl, cycloalkyl, and fused
phenylcycloalkyl wherein the point of attachment is on the cycloalkyl, wherein
the alkyl,
cycloalkyl, and fused phenylcycloalkyl are optionally substituted with from 1
to three
substituents selected from the group consisting of halo, CF3, CN, OR23, SR23,
S(O)R22,
S02R22i SO2N(R23)2, NR23CO2R22, C,_2 alkyl, and aryl~wherein the optional aryl
substituent is
3
CA 02657986 2009-03-10
optionally substituted with from 1 to 3 substituents selected from the group
consisting of halo,
phenyl, CF31CN, OR23, and C,, alkyl, and
Ria
R,? R
Rp
Rp ~
wherein R17, RtB, R19, R20, and R21 are each independently selected from the
group consisting
of hydrogen, halo, NOz, CF3, CN, ORZ,, SR23, N(R23)2, S(O)R22, SOZR,2,
SOZN(R23)2,
NR23COZR22, NR23CON(Rz3)z, COR,_3, C02R23, CON(Rz3)2, NR23SO2R22, C,-,s alkyl,
C2-,5
alkenyl, C2_15 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituent are optionally substituted with 1 substituent selected from the
group consisting of
1 o halo, NO2, CF31CN, OR23, SRZ,, N(R23)2, S(O)R22, and SOZRZZ.
In yet another embodiment, this invention is a method for administering one or
more
composition of this invention to a mammal in a treatment selected from the
group consisting
of protecting skeletal muscles against damage resulting from trauma,
protecting skeletal
muscles subsequent to muscle or systemic diseases such as intermittent
claudication, to treat
shock conditions, to preserve donor tissue and organs used in transplants, and
to treat
cardiovascular diseases including atrial and ventricular arrhythmias,
Prinzmetal's (variant)
angina, stable angina, and exercise induced angina, congestive heart disease,
and myocardial
infarction.
4
CA 02657986 2009-03-10
DETAILED DESCRIPTION OF THE INVENTION
This invention includes a class of substituted piperazine compounds having the
following formula:
R
R3 R, R, Rt
O Re R12
N N ---~ X
R4 N Rsa
RI R7 Rs R13 OH
RS Rjs Ris R14
I
wherein X is selected from the group consisting of:
and O
m
wherein m = 1 or 2 or 3;
R,, R2, R3, R4 and RS are each independently selected from the group
consisting of
hydrogen, halo, NO2, CF3, CN, OR23, SR23, N(R23)2, S(O)R22, S02R22,
SO2N(R23)2,
NR23C02R221 NR23CON(R23)21 COR23, CO2R23, CON(R23)2, NR23SO2R22, C1-15 allCyl,
C2-15
alkenyl, C2_15 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituent are optionally substituted with 1 substituent selected from the
group consisting of
halo, NO2, CF31 CN, OR23, SR23, N(R23)2, S(O)R22, and S02R22 , wherein R2 and
R3 may join
together to form a fused ring system having from three to four carbon atoms,
and wherein R4
and RS may join together to form -CH=CH-CH=CH-;
R6, R7 and R. are each independently selected from the group consisting of
hydrogen
and C1.15 alkyl;
R9, R,a, R,,, R12, R13, R10 R,5 and R,6 are each independently selected from
the group
consisting of hydrogen, C02R23, CON(R23)2, C,-4 alkyl, and aryl wherein the
alkyl and aryl
substituents are optionally substituted with 1 substituent selected from the
group consisting of
halo, CF31 CN, OR23, N(R23)2, C02R23, CON(R23)2 and aryl, wherein R9 and R,o
may together
form a carbonyl, or Rõ and R12 may together form a carbonyl, or R13 and R14
may together
form a carbonyl, or R15 and R16 may together form a carbonyl wherein Rõ and
R13 or R9 and
R15 or R9 and Rõ or Rõ and R15 or R9 and R13 may join together to form a
bridging ring system
5
CA 02657986 2009-03-10
.iaving from 1 to 4 carbon atoms and wherein R9 and R,o or Rõ and R,Z or Rõ
and Rõ or R,s
and R16 may join to form a bridging ring system having from 1 to 5 carbon
atoms;
R22 is selected from the group consisting of C1-15 alkyl, aryl, and
heteroaryl, wherein the
alkyl and aryl substituents are optionally substituted with 1 substituent
selected from the
group consisting of halo, alkyl, monoalkylamino, diallcylamino, alkyl amide,
aryl amide,
heteroaryl amide, CN, O-C,, alkyl, CF3, and heteroaryl;
RZ, is selected from the group consisting of H, C1-15 alkyl, aryl, and
heteroaryl, wherein
the alkyl and aryl substituents are optionally substituted with 1 substituent
selected from the
group consisting of halo, alkyl, monoalkylamino, dialkylamino, alkyl-CN, -O-C1
-,, alkyl, and
CF3; and
R24 is selected from the group consisting of alkyl, cycloalkyl, and fused
phenylcycloalkyl wherein the point of attachment is on the cycloalkyl wherein
the alkyl,
cycloalkyl, and fused phenylcycloalkyl are optionally substituted with from 1
to three
substituents selected from the group consisting of halo, CF3, CN, OR23, SR23,
S(O)R22,
S02R22, SO2N(R23)2, NR23CO2RZ2, C,-Z alkyl, and aryl wherein the optional aryl
substituent is
optionally substituted with from 1 to 3 substituents selected from the group
consisting of halo,
phenyl, CF31 CN, OR23, and C,, alkyk and
Rta
Rt7 Rts
Rzo
R21
wherein R,,, R18, R,9, R20, and R21 are each independently selected from the
group consisting
of hydrogen, halo, NOZ, CF3, CN, OR23, SR23, N(R23)2, S(O)RZZ, S02R22,
SOZN(R23)Z,
NR23CO2R22, NR,3CON(R23)2, COR23, COZR,,, CON(RA, NR23SO2R22, C,_,s alkyl,
CZ_,5
alkenyl, C245 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituents are optionally substituted with 1 substituent selected from the
group consisting of
halo, NO2, CF31 CN, OR23, SRZõ N(R23)2, S(O)Ru, and SO2R,2.
This invention also includes a subset of the class of substituted piperazine
compounds
identified in Formula I above having the following Formula (IA):
6
CA 02657986 2009-03-10
90 11
R 1 R9 R12 R17 R18
~~
R3 \ R m 0 R19
` /
Rs RT Re OH
R
R4 R 5 R15 R14 13
R5 R21 R20
IA
wherein m = 1, 2;
R', RZ, R3, R 4 and RS are each independently selected from the group
consisting of
hydrogen, halo, CF3, ORz3 and Ci-4alkyl and wherein R23 is a C1_3 alkyl;
R6, R' and Ra each independently selected from the group consisting_of
hydrogen and
C,_, alkyl;
R9, R'o, R", R12, R13, R'a, R`s and R16 are each independently selected from
the group
consisting of hydrogen and C,., alkyl, or R4 and R1 may together form a
carbonyl, or R" and
R'2 may together form a carbonyl, or R" and R14 may together form a carbonyl,
or R15 and R16
may together form a carbonyl wherein Rõ and Rõ or R9 and R15 or R9 and Rõ or
Rõ and R15 or
R9 and Rõ may join together to form a bridging ring system wherein the two R
groups
together comprise of from 1 to 4 carbon atoms with the proviso that R9, R10,
R", R'2, R", R14,
Rls and R16 are not all simultaneously hydrogen, when RI7, R18, R19, R20 and
R21 are all hydrogen.
R", R18, R19, R20 and RZ' are each independently selected from the group
consisting of
hydrogen, halo, CF3, CN, OR23, S(O)R22, S02R22, SO2N(R23)2, CON(R23)2, Ct-4
alkyl wherein
R23 is C,_, alkyl, or R" and R'B may together form -CH=CH-CH=CH-, or R18 and
R19 may
together form -OCH2O-.
In more preferred compounds of Formula IA, R', R; R'-, R4 and RS are each
selected
from the group consisting of hydrogen, halo, CF31 OR23 and Ci-4alkyl where R23
is a CI_3
alkyl; R6 is selected from hydrogen and methyl; R', Ra, R9, R10, R", R'2, R",
R'4, R'S and R'6
are each independently selected from hydrogen and methyl or R9 and R10 may
together form a
carbonyl, or R" and R14 may together form a carbonyl with the proviso that R',
R10, R", R'2,
R13, R14, R15 and R16 are not all simultaneously hydrogen ; R", R'$, R19, R20
and W' are each
independently selected from the group consisting of hydrogen, halo, CF3, OR23
and
C,., alkyl, or R" and R18 may together form -CH=CH-CH=CH-, or R" and R19
may together form -OCHZO-.
In still more preferred compounds of Formula IA, R', R2, R', R4, R5, R6, R'
and Rg are
each independently selected from the group consisting of methyl and hydrogen;
R9, R10, R",
7
CA 02657986 2009-03-10
'Z, R13, R", R15 and R'6 are each independently selected from hydrogen and
methyl or R9 and
R10 may together form a carbonyl, or R" and R'4 may together form a carbonyl
with the
proviso that R9, R10, R", R'z, R", R", R'S and R'6 are not all sunultaneously
hydrogen; R",
R18, R19, R20 and Rz1 are each independently selected from the group
consisting of hydrogen,
halo, CF31 ORZ3 wherein R23 is methyl, or R" and R'a may together form -CH=CH-
CH=CH-, or R' 8 and R19 may together form -OCHzO-
In an even more preferred compounds of Fornnula IA, R' and RS are each methyl;
R2,
R3, R4, R6, R', R8 are each hydrogen; R9, RtO, R", R1z, R", R14, R15 and R16
are each
independently selected from hydrogen and methyl or R9 and R10 may together
form a
carbonyl, or R13 and R14 may together form a carbonyl with the proviso that
R9, R10, R", R'z,
R", R14, R's and R16 are not all simultaneously hydrogen; R", R'g, R19, R20
and Rz1 are each
independently selected from the group consisting of hydrogen, halo, methyl,
OR23 wherein R23
is methyl, or R" and R1e may together form -CH=CH-CH=CH-, or R18 and R19 may
together
form -OCH2O-.
In still more preferred compounds of Formula IA, R' and RS are each methyl;
RZ, R3,
R', R6, R', R8 are each hydrogen; R9, R10 are selected from hydrogen, methyl,
or may together
form a carbonyl; R" and R12 are selected from hydrogen and methyl; R" and R14
are selected
from hydrogen and methyl or may together form a carbonyl; R15 and R16 are
hydrogen with
the proviso that R9, R10, R", R'Z, R", R'", R'S and R'6 are not all
simultaneously hydrogen; R"
is selected from the group consisting of hydrogen, chloro, fluoro or methoxy;
R1e and R19 are
each selected from the group consisting of hydrogen or methoxy, or R'$ and R19
may together
form -OCH2O-1 or R" and R'g may together form -CH=CH-CH=CH-, R20 is hydrogen;
and RZ'
is selected from hydrogen or chloro.
Most preferably, the substituted piperazine compounds of Formula IA are
selected
from the group consisting of N-(2,6-dimethylphenyl)-2- {4-[2-hydroxy-3-(2-
methoxyphenoxy)propyl]-3-oxopiperazinyl} acetamide, N-(2,6-dimethylphenyl)-2-
{4-[2-
hydroxy-3-(2-methoxyphenoxy)propyl]-3,5-dimethylpiperazinyl} acetamide, 2-
{(5S,2R)-4-[2-
hydroxy-3-(2-methoxyphenoxy)propyl]-2,5-dimethylpiperazinyl} -N-(2,6-
dimethylphenyl)acetamide, 2- {2,5 -di aza-5-[2-hydroxy-3-(2-
methoxyphenoxy)propyl]bicyclo[4.4.0]dec-2-yl}-N-(2,6-dimethylphenyl)acetamide,
N-(2,6-
dimethylphenyl)-2- {4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-3-
oxopiperazinyl } acetamide, N-(2,6-dimethylphenyl)-2- {4-[2-hydroxy-3 -(2-
methoxyphenoxy)propyl]-3,3-dimethylpiperazinyl} acetamide, 2-{5-[(2S)-2-
hydroxy-3-(2-
methoxyphenoxy)propyl](1 S,4S)-2,5-diazabicyclo[2.2.1 ]hept-2-yl} -N-(2,6-
8
CA 02657986 2009-03-10
dimethylphenyl)acetamide, N-(2,6-dimethylphenyl)-2- {4-[2-hydroxy-4-(2-
methoxyphenoxy)butyl]- piperazinyl} acetamide, N-(2,6-dimethylphenyl)-2- {4-[4-
(4-
fluorophenoxy)-2-hydroxybutyl]- piperazinyl} acetamide, 2-(4- {4-[4-(tert-
butyl)phenoxy]-2-
hydroxybutyl}piperazinyl)-N-(2,6-dimethylphenyl) acetamide, N-(2,6-
dimethylphenyl)-2- {4-
[2-hydroxy-4-(4-phenylphenoxy)butyl] piperazinyl} acetamide, N-(2,6-
dimethylphenyl)-2- {4-
[2-hydroxy-4-(4-methoxyphenoxy)butyl]- piperazinyl) acetamide, 2- {(3S)-4-
[(2S)-3-(2-
fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl} -N-(2,6-
dimethylphenyl)acetamide,
2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl } -
N-(2, 6-
dichlorophenyl) acetamide, 2- {(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-3-
methylpiperazinyl}-N-(4-sulfamoylphenyl) acetamide, 2-{(3S)-4-[(2S)-3-(2-
fluorophenoxy)-
2-hydroxypropyl]-3-methylpiperazinyl} -N-(5-methoxy-3-(trifluoromethyl)phenyl]
acetamide,
2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl} -N-
indan-5-
ylacetamide, 2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-
methylpiperazinyl} -
N-naphthylacetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-
methylpiperazinyl}-N-(4-chloronaphthyl) acetamide, 2-{(3S)-4-[(2S)-3-(2-
fluorophenoxy)-2-
hydroxypropyl]-3-methylpiperazinyl}-N-(2-pyrrolylphenyl) acetamide, 2-{(3S)-4-
[(2S)-3-(2-
fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl} -N-phenylacetamide, 2-
{(3S)-4-[(2S)-
3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-(2-chlorophenyl)
acetamide,
2- {(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-
(2-chloro-4-
2o methylphenyl)acetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-3-
methylpiperazinyl}-N-[2-(1-methylvinyl)phenyl] acetamide, 2-{(3S)-4-[(2S)-3-(2-
fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-(2-methylphenyl)
acetamide, 2-
{ (3 S)-4-[(2S)-3 -(2-fluorophenoxy)-2-hydroxypropyl] -3-methylpiperazinyl } -
N-[6-methyl-2-
(methylethyl)phenyl] acetamide, 2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-3-
methylpiperazinyl}-N-(3-methylthiophenyl) acetamide, 2-{(3S)-4-[(2S)-3-(2-
fluorophenoxy)-
2-hydroxypropyl]-3-methylpiperazinyl}-N-(4-chloro-2-methoxy-5-methylphenyl)
acetamide,
2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl } -
N-[4-
(dimethylamino) phenyl] acetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-
3-methylpiperazinyl} -N-(2,4-dimethoxyphenyl) acetamide, 2- {(3S)=4-[(2S)-3-(2-
fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-(3,4-dichlorophenyl)
acetamide,
2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl } -
N-(4-
chlorophenyl) acetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-
3-
methylpiperazinyl} -N-(3-chlorophenyl) acetamide, 2- {(3 S)-4-[(2S)-3-(2-
fluorophenoxy)-2-
hydroxypropyl]-3-methylpiperazinyl}-N-(3,5-dichlorophenyl) acetamide, 2-{(3S)-
4-[(2S)-3-
9
CA 02657986 2009-03-10
(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-(4-methoxyphenyl)
acetamide,
2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-
(4-
methylphenyl) acetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-
3-
methylpiperazinyl} -N-(3-methylphenyl) acetamide, 2- {(3S)-4-[(2S)-3-(2-
fluorophenoxy)-2-
hydroxypropyl]-3-methylpiperazinyl}-N-(4-fluorophenyl) acetamide, 2-{(3S)-4-
[(2S)-3-(2-
fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl}-N-(4-cyanophenyl)
acetamide, 2-
{ (3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl } -N-
(4-
acetylphenyl) acetamide, 2- {(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-
3-
methylpiperazinyl}-N-(2-methoxyphenyl) acetamide, 2- {(3S)-4-[(2S)-3-(2-
fluorophenoxy)-2-
hydroxypropyl]-3-methylpiperazinyl}-N-[4-(trifluoromethyl)phenyl] acetamide, 2-
{(3S)-4-
[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl} -N-[4-chloro-3-
(trifluoromethyl)phenyl] acetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-
3-methylpiperazinyl}-N-(3,5-dimethoxyphenyl) acetamide, 2-{(3S)-4-[(2S)-3-(2-
fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl } -N-(4-morpholin-4-
ylphenyl)
acetamide, 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-
methylpiperazinyl}-N-
(3-fluoro-4-methoxyphenyl) acetamide, 2- {(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-3-methylpiperazinyl}-N-(3,4,5-trimethoxyphenyl) acetamide, 2-
{(3S)-4-
[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-methylpiperazinyl} -N-(3,4-
dimethoxyphenyl)
acetamide, 2- {(3 S)-4-[(2S)-3-(2-fluorophenoxy)-2-hydroxypropyl]-3-
methylpiperazinyl} -N-
(4-chloro-2-fluorophenyl) acetamide, and 2-{(3S)-4-[(2S)-3-(2-fluorophenoxy)-2-
hydroxypropyl]-3-methylpiperazinyl}-N-[2-(hydroxymethyl-6-methylphenyl]
acetamide.
This invention includes a subset of substituted piperazine compounds of
formula I
having the following formula IB:
2 R~ R Rlo R11 1~
R12 R~$
R N R19
Rs R7 s OH
R4 R5 R 6 R15R14R13 R21 R20
IB
wherein m 0, 1 or 2 or 3;
CA 02657986 2009-03-10
R', RZ, R3, R and RS are each independently selected from the group
consisting of
hydrogen, halo, NOZ, CF31 CN, OR23, SRZ3, N(R23)2, S(O)R22, SO,RZ2,
SOzN(Rz3)z,
NR23C0ZR22, NR23CON(R23)2, COR23 CO2R23, CON(R23)21 NR23SOzR22, Ci-t5 aIkY" C2-
15
alkenyl, C2-15 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituent are optionally substituted with 1 substituent selected from the
group consisting of
halo, NOZ, CFõ CN, OR23, SR,3, N(RZ,)z, S(O)R22, and SO2RZ2;
R6, R' and Rg are each independently selected from the group consisting of
hydrogen an,'
C,_,s alkyl;
R9, R10, R", R'z, R`3, R,a, R's and R`6 are each independently selected from
the group
1 o consisting of hydrogen, C02R23, CON(R23)Z, C,, alkyl, and aryl wherein the
alkyl and aryl
substituents are optionally substituted with 1 substituent selected from the
group consisting of
halo, CF3, CN, OR23, N(RZ,)Z, COZR23, CON(R23)2 and aryl, wherein R9 and R10
may together
form a carbonyl, or R" and R'Z may together form a carbonyl, or R13 and R14
may together
form a carbonyl, or R'S and R16 may together form a carbonyl wherein R" and
R13 or R9 and
R15 or R9 and R" or R" and R15 or R9 and R13 may join together to form a
bridging ring system
wherein the two R groups together comprise of from 1 to 4 carbon atoms and
wherein R9 and
R10 or R" and R'Z or R'3 and R'4 or R'S and R'6 may join to form a spiro ring
system wherein
the two R groups together comprise of from 1 to 5 carbon atoms;
R", R'a, R", R20, and RZ' are each independently selected from the group
consisting of
hydrogen, halo, NO2, CF3, CN, OR23 SR23, N(R2,)2, S(O)R22, S02R22, SO2N(R23)2,
NR23CO2R22, NR23CON(R2,)2, COR23, CO2RZ3, CON(R23)2, NR23S02R22I C,-is alkyl,
C245
alkenyl, C2_15 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituent are optionally substituted with I substituent selected from the
group consisting of
halo, NO2, CF31 CN, OR23, SR23, N(R23)21 S(O)R221 and SO2R22 or wherein R" and
R'8 may join
together may join together to form -CH=CH-CH=CH- or wherein R" and R1e or R18
and R19
or RL9 and R20 or RZ0 and RZ' may combine to form a saturated ring including
from 3 to 6
carbon atoms wherein from 0 to 2 carbon atoms may be substituted with an
oxygen atom and
wherein the ring may be optionally substituted with from I to 3 substituents
selected from the
group consisting of hydrogen, halo, NO2, CF3, CN, OR23, SR23, N(R")Z, S(O)R
22, S02R22,
SOZN(R'3)2, NR23CO2 R22, NRZ'CON(R23)2, COR23, CO2R23, CON(RZ')2, NR23SOZR23,
Cl_ls
alkyl, C2.15 alkenyl, CZ.15 alkynyl, heterocyclyl, aryl, or heteroaryl,
wherein the alkyl and aryl
substituents are optionally substituted with 1 substituent selected from the
group consisting of
halo, NO2, CFõ CN, OR23, SR23, N(R23)2, S(O)R22, or S02R22;
11
CA 02657986 2009-03-10
R21 is selected from the group consisting of C,_,5 alkyl, aryl, or heteroaryl,
wherein the
alkyl and aryl substituents are optionally substituted with 1 substituent
selected from the
group consisting of halo, alkyl, monoalkylamino, dialkylamino, allcyl amide,
aryl amide,
heteroaryl amide, CN, O-C,-6 alkyl, CF3, and heteroaryl; and
R23 is selected from the group consisting of H, C1_15 alkyl, aryl, or
heteroaryl, wherein
the allcyl and aryl substituents are optionally substituted with I substituent
selected from the
group consisting of halo, alkyl, mono- or dialkylamino, alkyl-CN, -O-C1_6
alkyl, or CF3.
In preferred compositions of this invention, m= 0, 1 or 2 or 3; R', R2, R', R4
and RS
are each independently selected from the group consisting of hydrogen, halo,
CF3, OR2,3 and
C,4 alkyl; R6, R' and Re each independently selected from the group consisting
of hydrogen
and C,_3 alkyl; R9, R10, R", R'Z, R", R'4, R15 and R'6 are each independently
selected from the
group consisting of hydrogen and C,.4 alkyl, or R9 and R10 may together form a
carbonyl, or
R" and R'Z may together form a carbonyl, or R" and R14 may together form a
carbonyl, or R's
and R16 may together form a carbonyl, or wherein R" and R13 or R9 and R15 or
R9 and R" or
R" and R15 or R9 and R" may join together to form a ring including from I to 4
carbon atoms
wherein R9, R10, R", R'2, R'3, R'4, R'5 and R'6 are not all hydrogen; and R",
R'e, R'9, R20 and
RZ' are each independently selected from the group consisting of hydrogen,
halo, CF31 CN,
OR23, S(O)R22, S02R22, SOZN(R23)Z, CON(RZ3)Z,C, , alkyl or R" and R18 may
together form -
CH=CH-CH=CH-, and phenyl.
In other preferred compounds, R', RZ, and RS are each independently selected
from the group consisting of hydrogen, halo, CF31 OR23 and C1_2 alkyl wherein
R23 is a Ct_3
alkyl; R6, R' and Ra are each independently selected from the group consisting
of hydrogen
and methyl; R9, R10, R", R'Z, R'3, R'4, R'S and R`6 are each independently
selected from the
group consisting of hydrogen and C,_Z alkyl, or R9 and R10 may together form a
carbonyl, or
R15 and R16 may together form a carbonyl with the proviso that R9, R10, R",
R'2, R'3, R'4, R'5
and R" are not all simultaneously hydrogen and wherein R" and R" or R9 and R15
or R9 and
R" or R" and R'S or R' and R13 may join to form a ring including from 1 to 4
carbon atoms
and R", R'g, R19, R20 and RZ' are each independently selected from the group
consisting of
hydrogen, halo, CF3, CN, OR23, and C alkyl and wherein R" and
3o R" may together form a substituent selected from the group consisting of -
CH=CH-CH=CH-
and phenyl.
In still other preferred compounds, m= I or 2; R', RZ, R', R4 and RS are each
independently selected from the group consisting of hydrogen, halo, CF3, OR23
and Ci.a alkyl
where R23 is a C1.3 alkyl; R6, R', R', R9, R10, R", R`Z, R'3, R'4, R'S and R'6
are each
12
CA 02657986 2009-03-10
independently selected from hydrogen and methyl; R", R18, R19, R20 and RZ' are
each
independently selected from the group consisting of hydrogen, halo, CFõ OR22,
C_, alkyl
where R22 is methyl, or R" and R18 may together form -CH=CH-CH=CH-, or R1e and
R19 nlay
together form -OCH2O-.
In more preferred compounds, m = 1 or 2; R', RZ, R', R4, R5, R6, R' and Rfl
are each
independently selected from methyl and hydrogen; R9, R10, R", R'z, R", R'4,
R'5 and R'6 are
each hydrogen; and R", R18, R19, R20 and RZ' are each independently selected
from the group
consisting of hydrogen, halo, CF3, and OR23 wherein R23 is methyl, or R" and
R18 may together
form -CT-I=CH-CH=CH-, or R18 and R19 may together form -OCHzO-.
In yet other preferred compounds, m = 1 or 2; R' and RS are methyl; R2, R', R4
R6, R7,
R8,R9, R10, R", R'2, R'3, R'4, R'5 and R'6 are hydrogen; R", R'B, R'9, R20 and
R21 are each
independently selected from the group consisting of hydrogen, halo, and OR23
wherein R23 is
methyl, or R" and R1e may together form -CH=CH-CH=CH-, or R1e and R19 may
together
form -OCHZO-.
In still other preferred compounds, R' and RS are methyl; R2, R3, R4, R6; R',
R8,R9, R10,
R", R1z, R13, Rt4, R15 and R16 are hydrogen; R" is selected from the group
consisting of
hydrogen, chloro, fluoro and methoxy; R18 is selected fiom hydrogen and
methoxy; R19 is
selected from hydrogen and methoxy; R20 is hydrogen; R21 is selected from
hydrogen and
chloro, or R" and R18 may together form -CH=CH-CH=CH-, or R'a and R'9 may
together
form -OCH2O-.
Most preferably, the substituted piperazine compounds of this invention are
selected
from N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-4-
phenylbutyl)piperazinyl]acetamide; N-(2,6-
dimethylphenyl)-2- {4-[2-hydroxy-3-(2-methoxyphenyl)propyl]piperazinyl}
acetamide; 2-[4-
(3-(2H-benzo[d] 1,3-dioxolen-5-yl)-2-hydroxypropyl)piperazinyl]-N-(2,6-
dimethylphenyl)acetamide; N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(4-
methoxyphenyl)propyl]piperazinyl} acetamide; N-(2,6-dimethylphenyl)-2- {4-[2-
hydroxy-3-
phenylpropyl]piperazinyl} acetamide, N-(2,6-dimethylphenyl)-2- {4-[4-(4-
methoxyphenyl)-2-
hydroxybutyl]piperazinyl} acetamide, 2- {4-[4-(2,6-difluorophenyl)-2-
hydroxybutyl]piperazinyl} -N-(2,6-dimethylphenyl)acetamide, N-(2,6-
dimethylphenyl)-2- {4-
[4-(2-chlorophenyl)-2-hydroxybutyl]piperazinyl} acetamide, 2-(4- {4-[4-(tert-
butyl)phenyl]-2-
hydroxybutyl } piperazinyl)-N-(2,6-dimethylphenyl)acetamide, N-(2,6-
dimethylphenyl)-2- {4-
[4-(2-fluorophenyl)-2-hydroxybutyl]piperazinyl} acetamide, N-(2,6-
dimethylphenyl)-2-(4- {2-
hydroxy-4-[4-(trifluoromethyl)phenyl]butyl}piperazinyl)acetamide, 2-[4-(3-(2H-
benzo[d] 1,3-
dioxolen-5-yl)-2-hydroxypropyl)piperazinyl]-N-(2,6-dimethylphenyl)-2-
methylpropanamide,
13
CA 02657986 2009-03-10
1-I-(2,6-dimethylphenyl)-2-[4-(2-hyd.roxy-3-phenylpropyl)piperazinyl]-2-
methylpropanamide,
N-(2,6-dimethylphenyl)-2- {4-[2-hydroxy-3-(3,4,5-
trimethoxyphenyl)propyl]piperazinyl} -2-
metliylpropanamide,
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-5-phenylpentyl)piperazinyl]acetamide, N-
(2,6-
dimethylphenyl)-2- f 4-[5-(2-flucropl:enyl)- 2-hydroxy-pentyl]piperazinyl}
acetamide, and N-
(2,6-dimethylphenyl)-2- {4-[5-(2-chlorophenyl)- 2-hydroxy-pentyl]piperazinyl}
acetamide.
This invention further includes a subset of compounds of Formula I above
having the
following Formula IC:
Ft2
~3 , ~10 ~11
1
_._ ~2
~9
N N m O~ ft2a
I O
$ 3 OFi
R16 R15 R14
IC
wherein m 1, 2, or 3;
R', R2, R3, R4 and RS are each independently selected from the group
consisting of
hydrogen, halo, NOZ, CF3, CN, OR20, SRZO, N(R20)2, S(O)R22, S02R22,
SO2N(R20)Z,
NR20C02R22, NR20CON(R.Z)2, COR20, C02R2o, CON(RZ0)Z, NRZ0S02RZ2, C,-õ alkyl,
Czas
alkenyl, C2-4 5 alkynyl, heterocyclyl, aryl, and heteroaryl, wherein the alkyl
and aryl
substituent are optionally substituted with 1 substituent selected from the
group consisting of
halo, NO2, CF3, CN, OR20, SR20, N(RZ0)2, S(O)R22, and SO2R22 ;
R6, R' and R8 are each independently selected from the group consisting of
hydrogen and C1-3 alkyl;
R', R10, R", R'Z, R", R'4, R15 and R'6 are each independently selected from
the group
consisting of hydrogen, COZR20, CON(R20)2, C,-4 alkyl, and aryl wherein the
alkyl and aryl
substituents are optionally substituted with 1 substituent selected from the
group consisting of
halo, CF3, CN, OR20, N(R20)2, . COZR20, CON(RZ0)2 or aryl, wherein R' and R10
may together
form a carbonyl, or R" and R'Z may together form a carbonyl, or R" and R14 may
together
form a carbonyl, or R15 and Rt6 may together form a carbonyl, wherein R" and
R13 or R9 and R15 or R9 and R" or R" and R15 or R9 and R" may join together to
form a ring
including from I to 3 carbon atoms;
R24 is selected from the group consisting of alkyl, eyeloalkyl, and fused
phenylcycloalkyl wherein the point of attachment is on the cycloalkyl wherein
the alkyl,
14
CA 02657986 2009-03-10
:,ycloalkyl, and fused phenylcycloalkyl are optionally substituted with from I
to three
substituents selected from the group consisting of halo, CF3, CN, OR20, SRzO,
S(O)RZZ, SOZRZ',
SOZN(R20)z, NR20CO2R21, C,_Z alkyl, and aryl wtierein the optional aryl
substituent is
optionally substituted with from I to 3 substituents selected from the group
consisting of halo,
phenyl, CF3, CN, OR20, and C, , alkyl;
Rz is selected from the group consisting of H, C,_,S alkyl, aryl,and
heteroaryl,wherein
the alkyl and aryl substituents are optionally substituted with I substituent
selected from the
group consisting of halo, alkyl, mono- or dialkylamiiio, alkyl-CN, -O-C1_6
alkyl, and CF3; and
RZ' is selected from the group consisting of C1_15 alkyl, aryl, and
heteroaryl, wherein the
alkyl and aryl substituents are optionally substituted with I substituent
selected from the
group consisting of halo, alkyl, monoalkylanuno, dialkylamino, alkyl amide,
aryl amide,
heteroaryl amide, CN, O-C,-, alkyl, CF3, and heteroaryl.
In Formula IC, it is preferred that m = 1 or 2 and most preferred when m = 1.
In preferred compositions of Fonnula IC, R', R2, R', R4 and RS are each
independently
selected from the group consisting of hydrogen, halo, CF3, OR22 and C, alkyl
and wherein
R22 is C,_, alkyl. In other preferred compositions, R', RZ, R', R' and RS are
each
independently selected from the group consisting of hydrogen, CFõ OR20, and
C1_2 alkyl. More
preferably R', R2, R', R4 and RS are each independently selected from the
group consisting of
hydrogen, and methyl with RZ, R', and R' as hydrogen and R' and RS as methyl
being preferred.
In other preferred compositions of Formula IC, R6, R' and R8 each
independently
selected from the group consisting of hydrogen and C,_, alkyl with hydrogen or
methyl being
preferred and hydrogen being most preferred.
In yet other preferred compositions of Formula IC, R9, R10, R", R'Z, R", R'4,
R'S and
R16 are each independently selected from the group consisting of hydrogen,
CON(R2)2, C1
I
alkyl, and aryl wherein the alkyl and aryl substituents are each optionally
substituted with I
substituent selected from the group consisting of halo, CFõ OR20, N(RZ0)2,
CON(R2)2 and aryl
wherein R9 and R10 may together form a carbonyl, or R" and R'2 may.together
form a
carbonyl, or R13 and R14 may together fonn a carbonyl, or R15 and R16 may
together form a
carbonyl, wherein R' 1 and R 13 or R9 and R15 or R9 and R" 1 or R" and RIS or
R9 and R13 may join
together to form a ring having from 1 to 3 carbon atoms. In alternative
preferred compositions,
R9, Rio~ Rtt~ Rt2~ R13~ Rta, Rts and R16 are each independently selected from
the group consisting
of hydrogen and C1_4 alkyl, or R9 and R10 together foi-m a carbonyl, or R" and
R12 together form
a carbonyl, or R13 and R14 together form a carbonyl, or R15 and R16 together
form a carbonyl,
R10 and R' 1 together form -CH2CH2CH2CH2-. In another embodiment, R9, R1O, Rl
1, R12, R13,
CA 02657986 2009-03-10
R14, R15 and R" are each independently selected from the group consisting of
hydrogen, and CI_2
alkyl, wherein the alkyl substituent is optionally substituted with 1
substituent selected from
the group consisting of N(R20)z.and arylor wherein R9 and R10 may together
form a carbonyl.
More preferably, R9, R10, R", R'Z, R", R'4, R'5 and R'6 are each independently
selected from
the group consisting of hydrogen and C,_z alkyl, or wherein R9 and R10 may
together form a
carbonyl. In another embodiment, R" and R15 are each selected from the group
consisting of
hydrogen or methyl, R9, R10, R'z, R'3, R'-0 and R'6 are each hydrogen and R9
and R'0 may
together form a carbonyl, or, R9, R10, R", R'2, R", R'4, R'S and R'6 may each
be hydrogen.
In compounds of Formula IC, R24 may be selected from the group consisting of
alkyl,
cycloalkyl, and fused phenylcycloalkyl wherein the point of attachment is on
the cycloalkyl
wherein the alkyl, cycloalkyl, and fused phenylcycloalkyl are optionally
substituted with from
I to three substituents selected from the group consisting of halo, CF3, CN,
OR20, SRZO,
S(O)R22, S02R 22, SOZN(RZ)Z, NR20C02 R22, C1_2 alkyl, and aryl wherein the
optional aryl
substituent is optionally substituted with from I to 3 substituents selected
from the group
consisting of halo, phenyl, CF3, CN, OR20, and C, alkyl. In certain preferred
compounds of
Formula IC, R24 is selected from the group consisting of alkyl , cycloalkyl,
and fused
phenylcycloalkyl wherein the point of attachment is on the cycloalkyl wherein
the alkyl,
cycloalkyl, and fused phenylcycloalkyl are optionally substituted with from 1
to two
substituents selected from the group consisting of halo, CFõ CN, OR20, SRZO,
S(O)R22,
S02 R22, C1_2 alkyl, and aryl wherein the optional aryl substituent is
optionally substituted with
from 1 to 3 substituents selected from the group consisting of halo, phenyl,
CF3, CN, OR20,
and C,., alkkyl. In other preferred compounds of Formula IC, R24 is selected
from the group
consisting of alkyl, cycloalkyl, and fused phenylcycloalkyl wherein. the point
of attachment is
on the cycloalkyl wherein the alkyl, cycloalkyl, and fused phenylcycloalkyl
are optionally
substituted with from 1 to two substituents selected from the group consisting
of halo, CF3,
OR20, and aryl wherein the optional aryl substituent is optionally substituted
with from 1 to 3
substituents selected from the group consisting of halo, phenyl, CF31 CN,
OR20, and C,, alkyl.
In still other preferred compounds of Formula IC, R24 is selected from the
group consisting of
alkyl having from I to 6 carbon atoms, cycloalkyl having from 4 to 6 carbon
atoms, fused
phenylcycloalkylwith a phenyl that is optionally substituted with from 1 to 2
substituents
selected from the group consisting of halo, CF31 OH, methyl, and aryl, and
aryl that is
optionally substituted with from I to 2 substituents selected from the group
consisting of halo,
CFõ OH, C1_2 alkyl, and aryl. In still other preferred compounds of Formula
IC, R24 is alkyl
having from I to 6 carbon atoms and cycloalkyl or R24 is a fused
phenylcycloalkyl that is
16
CA 02657986 2009-03-10
optionally substituted with from 1 to 2 substituents selected from the group
consisting of halo,
CF31 OR20, C,_Z alkyl, and aryl or R24 is phenylmethyl that is optionally
substituted with from I
to 2 substituents selected from the group consisting of halo, CF31 OR20, C,,
alkyl, and aryl.
In the compounds of Formula IC, R20 is selected from the group consisting of
H, C1_3
alkyl, or aryl, wherein the alkyl and aryl substituents are optionally
substituted with 1
substituent individually selected from the group consisting of halo, -OMe, and
CF3. More
preferably, R20 is selected from the group consisting of H or C1_3 alkyl and
most preferably, RZ0
is methyl or H.
Most preferably, the substituted piperazine compounds of Formula IC are
selected
from the group consisting of 2-( {2-[4-(3-isopropoxy-2-
hydroxypropyl)piperazinyl]- N-( {2,6-
dimethylphenyl)acetamide; N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3-indan-2-
yloxypropyl)piperazinyl]acetamide; N-(2,6-dimethylphenyl)-2- {4-[2-hydroxy-3-
(phenylmethoxy)propyl]piperazinyl}acetamide, 2-[4-(3-{[4-(tert-
butyl)phenyl]methoxy}-2-
hydroxypropyl)piperazinyl]-N-(2,6-dimethylphenyl)acetamide, N-(2,6-
dimethylphenyl)-2-(4-
{3-[(2-fluorophenyl)methoxy]-2-hydroxypropyl}piperazinyl)acetamide, 2-(4- {3-
[(2,4-
difluorophenyl)methoxy]-2-hydroxypropyl} piperazinyl)-N-(2,6-
dimethylphenyl)acetamide,
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3- { [4-
(trifluoromethyl)phenyl]methoxy}propyl)piperazinyl]acetamide, N-(2,6-
dimethylphenyl)-2-
(4- {2-hydxoxy-3-[(2-methoxyphenyl)methoxy]propyl}piperazinyl)acetamide, 2-(4-
{3-[(2,4-
dimethoxyphenyl)methoxy]-2-hydroxypropyl}piperazinyl)-N-(2,6-
dimethylphenyl)acetamide,
N-(2,6-dimethylphenyl)-2-(4- {2-hydroxy-3-[(4-
rnethoxyphenyl)methoxy]propyl}piperazinyl)acetamide, N-(2,6-dimethylphenyl)-2-
(4-{3-[(4-
fluorophenyl)methoxy]-2-hydroxypropyl}piperazinyl)acetamide, N-(2,6-
dimethylphenyl)-2-
(4-{2-hydroxy-3-[(4-methylphenyl)methoxy]propyl}piperazinyl)acetamide, N-(2,6-
dimethylphenyl)-2-(4- { 2-hydroxy-3 - [(4-
phenylphenyl)methoxy]propyl } piperazinyl)acetamide,
N-(2,6-dimethylphenyl)-2-(4- {3-[(4-butylphenyl)methoxy]-2-
hydroxypropyl} piperazinyl)acetamide, N-(2,6-dimethylphenyl)-2- {4-[2-hydoxy-3-
(2-
naphthylmethoxy)propyl]piperazinyl} acetamide, N-(2,6-dimethylphenyl)-2- {4-[3-
(cyclohexylmethoxy)-2-hydroxypropyl]piperazinyl} acetamide, and N-(2,6-
dimethylphenyl)-
2-(4- {3-[(4-fluorophenyl)methoxy]-2-hydroxypropyl} -3,3-
dimethylpiperazinyl)acetamide.
The following definitions apply to terms as used herein.
"Halo" or "Halogen" - alone or in combination means all halogens, that is,
chloro (Cl),
fluoro (F), bromo (Br), iodo (I).
17
CA 02657986 2009-03-10
"Hydroxyl" refers to the group -OH.
"Thiol" or "mercapto" refers to the group -SH.
"Alkyl" - alone or in combination means an alkane-derived radical containing
from I
to 20, preferably 1 to 15, carbon atoms (unless specifically defined). It is a
straight chain
alkyl, branched alkyl or cycloalkyl. Preferably, straight or branched alkyl
groups containing
from 1-15, more preferably I to 8, even more preferably 1-6, yet more
preferably 1-4 and
most preferably 1-2, carbon atoms, such as methyl, ethyl, propyl, isopropyl,
butyl, t-butyl and
the like. The term "lower alkyl" is used herein to describe the straight chain
alkyl groups
described immediately above. Preferably, cycloalkyl groups are monocyclic,
bicyclic or
tricyclic ring systems of 3-8, more preferably 3-6, ring members per ring,
such as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, adamantyl and the like. Alkyl also
includes a straight
chain or branched alkyl group that contains or is interrupted by a cycloalkyl
portion. The
straight chain or branched alkyl group is attached at any available point to
produce a stable
compound. Examples of this include, but are not limited to, 4-(isopropyl)-
cyclohexylethyl or
2-methyl-cyclopropylpentyl. A substituted alkyl is a straight chain alkyl,
branched alkyl, or
cycloalkyl group defined previously, independently substituted with 1 to 3
groups or
substituents of halo, hydroxy, alkoxy, alkylthio, alkylsulfinyl,
alkylsulfonyl, acyloxy, aryloxy,
heteroaryloxy, amino optionally mono- or di-substituted with alkyl, aryl or
heteroaryl groups,
amidino, urea optionally substituted with alkyl, aryl, heteroaryl or
heterocyclyl groups,
aminosulfonyl optionally N-mono- or N,N-di-substituted with alkyl, aryl or
heteroaryl groups,
alkylsulfonylamino, arylsulfonylamino, heteroarylsulfonylamino,
alkylcarbonylamino,
arylcarbonylamino, heteroarylcarbonylamino, or the like.
"Alkenyl" - alone or in combination means a straight, branched, or cyclic
hydrocarbon
containing 2-20, preferably 2-17, more preferably 2-10, even more preferably 2-
8, most
preferably 2 to 4 carbon atoms with at least one, preferably 1-3, more
preferably 1-2, and most
preferably one, carbon to carbon double bond. In the case of a cycloalkyl
group, conjugation
of more than one carbon to carbon double bond is not such as to confer
aromaticity to the ring.
Carbon to carbon double bonds may be either contained within a cycloalkyl
portion, with the
exception of cyclopropyl, or within a straight chain or branched portion.
Examples of alkenyl
groups include ethenyl, propenyl, isopropenyl, butenyl, cyclohexenyl,
cyclohexenylalkyl and
the like. A substituted alkenyl is the straight chain alkenyl, branched
alkenyl or cycloalkenyl
group defined previously, independently substituted with 1 to 3 groups or
substituents of halo,
hydroxy, alkoxy, alkylthio, alkylsulfinyl, alkylsulfonyl, acyloxy, aryloxy,
heteroaryloxy,
amino optionally mono- or di-substituted with alkyl, aryl or heteroaryl
groups, amidino, urea
18
CA 02657986 2009-03-10
optionally substituted with alkyl, aryl, heteroaryl or heterocyclyl groups,
aminosulfonyl
optionally N-mono- or N,N-di-substituted with alkyl, aryl or heteroaryl
groups,
alkylsulfonylamino, arylsulfonylamino, heteroarylsulfonylamino,
alkylcarbonylamino,
arylcarbonylamino, heteroarylcarbonylamino, carboxy, alkoxycarbonyl,
aryloxycarbonyl,
heteroaryloxycarbonyl, or the like attached at any available point to produce
a stable
compound.
"Alkynyl" - alone or in combination means a straight or branched hydrocarbon
containing 2-20, preferably 2-17, more preferably 2-10, even more preferably 2-
8, most
preferably 2-4, carbon atoms containing at least one, preferably one, carbon
to carbon triple
bond. Examples of alkynyl groups include ethynyl, propynyl, butynyl and the
like. A
substituted alkynyl refers to the straight chain alkynyl or branched alkynyl
defined previously,
independently substituted with 1 to 3 groups or substituents of halo, hydroxy,
alkoxy,
alkylthio, alkylsulfmyl, alkylsulfonyl, acyloxy, aryloxy, heteroaryloxy, amino
optionally
mono- or di-substituted with alkyl, aryl or heteroaryl groups, amidino, urea
optionally
substituted with alkyl, aryl, heteroaryl or heterocyclyl groups, aminosulfonyl
optionally N-
mono- or N,N-di-substituted with alkyl, aryl or heteroaryl groups,
alkylsulfonylamino,
arylsulfonylamino, heteroarylsulfonylamino, alkylcarbonylamino,
arylcarbonylamino,
heteroarylcarbonylamino, or the like attached at any available point to
produce a stable
compound.
"Alkyl alkenyl" refers to a group -R-CR'=CR"' R"", where R is lower alkyl, or
substituted lower alkyl, R', R`, R"" may independently be hydrogen, halogen,
lower alkyl,
substituted lower alkyl, acyl, aryl, substituted aryl, hetaryl, or substituted
hetaryl as defined
below.
"Alkyl alkynyl" refers to a groups -RC=CR' where R is lower alkyl or
substituted
lower alkyl, R' is hydrogen, lower alkyl, substituted lower alkyl, acyl, aryl,
substituted aryl,
hetaryl, or substituted hetaryl as defmed below.
"Alkoxy" denotes the group -OR, where R is lower alkyl, substituted lower
alkyl, acyl,
aryl, substituted aryl, aralkyl, substituted aralkyl, heteroalkyl,
heteroarylalkyl, cycloallcyl,
substituted cycloalkyl, cycloheteroalkyl, or substituted cycloheteroalkyl as
defined.
"Alkylthio" denotes the group -SR, -S(O)n_1_2-R, where R is lower alkyl,
substituted
lower alkyl, aryl, substituted aryl, aralkyl or substituted aralkyl as defined
herein.
"Acyl" denotes groups -C(O)R, where R is hydrogen, lower alkyl substituted
lower
alkyl, aryl, substituted aryl and the like as defined herein.
19
CA 02657986 2009-03-10
"Aryloxy" denotes groups -OAr, where Ar is an aryl, substituted aryl,
heteroaryl, or
substituted heteroaryl group as defined herein.
"Amino" denotes the group NRR', where R and R' may independently by hydrogen,
lower alkyl, substituted lower alkyl, aryl, substituted aryl, hetaryl, or
substituted hetaryl as
defined herein or acyl.
"Amido" denotes the group -C(O)NRR', where R and R' may independently by
hydrogen, lower alkyl, substituted lower alkyl, aryl, substituted aryl,
hetaryl, substituted
hetaryl as defined herein.
"Carboxyl" denotes the group -C(O)OR, where R is hydrogen, lower alkyl,
substituted
lower alkyl, aryl, substituted aryl, hetaryl, and substituted hetaryl as
defined herein.
"Aryl" - alone or in combination means phenyl or naphthyl optionally
carbocyclic
fused with a cycloalkyl of preferably 5-7, more preferably 5-6, ring members
and/or
optionally substituted with I to 3 groups or substituents of halo, hydroxy,
alkoxy, alkylthio,
alkylsulfinyl, alkylsulfonyl, acyloxy, aryloxy, heteroaryloxy, amino
optionally mono- or di-
substituted with alkyl, aryl or heteroaryl groups, amidino, urea optionally
substituted with
alkyl, aryl, heteroaryl or heterocyclyl groups, aminosulfonyl optionally N-
mono- or N,N-di-
substituted with alkyl, aryl or heteroaryl groups, alkylsulfonylamino,
arylsulfonylamino,
heteroarylsulfonylamino, alkylcarbonylamino, arylcarbonylamino,
heteroarylcarbonylamino,
or the like.
"Substituted aryl" refers to aryl optionally substituted with one or more
functional
groups, e.g., halogen, lower alkyl, lower alkoxy, alkylthio, acetylene, amino,
amido,
carboxyl, hydroxyl, aryl, aryloxy, heterocycle, hetaryl, substituted hetaryl,
nitro, cyano, thiol,
sulfamido and the like.
"Heterocycle" refers to a saturated, unsaturated, or aromatic carbocyclic
group having
a single ring (e.g., morpholino, pyridyl or furyl) or multiple condensed rings
(e.g.,
naphthpyridyl, quinoxalyl, quinolinyl, indolizinyl or benzo[b]thienyl) and
having at least one
hetero atom, such as N, 0 or S, within the ring, which can optionally be
unsubstituted or
substituted with, e.g., halogen, lower alkyl, lower alkoxy, alkylthio,
acetylene, amino, amido,
carboxyl, hydroxyl, aryl, aryloxy, heterocycle, hetaryl, substituted hetaryl,
nitro, cyano, thiol,
sulfamido and the like.
"Heteroaryl" - alone or in combination means a monocyclic aromatic ring
structure
containing 5 or 6 ring atoms, or a bicyclic aromatic group having 8 to 10
atoms, containing
one or more, preferably 1-4, more preferably 1-3, even more preferably 1-2,
heteroatoms
independently selected from the group 0, S, and N, and optionally substituted
with 1 to 3
CA 02657986 2009-03-10
groups or substituents of halo, hydroxy, alkoxy, alkylthio, alkylsulfinyl,
atkylsulfonyl,
acyloxy, aryloxy, heteroaryloxy, amino optionally mono- or di-substituted with
alkyl, aryl or
heteroaryl groups, amidino, urea optionally substituted with alkyl, aryl,
heteroaryl or
heterocyclyl groups, aminosulfonyl optionally N-mono- or N,N-di-substituted
with alkyl, aryl
or heteroaryl groups, alkylsulfonylamino, arylsulfonylamino,
heteroarylsulfonylamino,
alkylcarbonylamino, arylcarbonylamino, heteroarylcarbonylamino, or the like.
Heteroaryl is
also intended to include oxidized S or N, such as sulfinyl, sulfonyl and N-
oxide of a tertiary
ring nitrogen. A carbon or nitrogen atom is the point of attachment of the
heteroaryl ring
structure such that a stable aromatic ring is retained. Examples of heteroaryl
groups are
pyridinyl, pyridazinyl, pyrazinyl, quinazolinyl, purinyl, quinolinyl,
isoquinolinyl, pyrimidinyl,
pyrrolyl, oxazolyl, thiazolyl, thienyl, isoxazolyl, oxathiadiazolyl,
isothiazolyl, tetrazolyl,
imidazolyl, triazinyl, furanyl, benzofuryl, indolyl, benzothiazolyl,
benzoxazolyl, and the like.
A substituted heteroaryl contains a substituent attached at an available
carbon or nitrogen to
produce a stable compound.
"Heterocyclyl" - alone or in combination means a non-aromatic cycloalkyl group
having from 5 to 10 atoms in which from 1 to 3 carbon atoms in the ring are
replaced by
heteroatoms of 0, S or N, and are optionally benzo fused or fused heteroaryl
of 5-6 ring
members and/or are optionally substituted as in the case of cycloalkyl.
Heterocycyl is also
intended to include oxidized S or N, such as sulfinyl, sulfonyl and N-oxide of
a tertiary ring
nitrogen. The point of attachment is at a carbon or nitrogen atom. Examples of
heterocyclyl
groups are tetrahydrofuranyl, dihydropyridinyl, piperidinyl, pyrrolidinyl,
piperazinyl,
dihydrobenzofuryl, dihydroindolyl, and the like. A substituted hetercyclyl
contains a
substituent nitrogen attached at an available carbon or nitrogen to produce a
stable compound.
"Substituted heteroaryl" refers to a heterocycle optionally mono or poly
substituted
with one or more functional groups, e.g., halogen, lower alkyl, lower alkoxy,
alkylthio,
acetylene, amino, amido, carboxyl, hydroxyl, aryl, aryloxy, heterocycle,
substituted
heterocycle, hetaryl, substituted hetaryl, nitro, cyano, thiol, sulfamido and
the like.
"Aralkyl" refers to the group -R-Ar where Ar is an aryl group and R is lower
alkyl or
substituted lower alkyl group. Aryl groups can optionally be unsubstituted or
substituted
with, e.g., halogen, lower alkyl, alkoxy, alkylthio, acetylene, amino, amido,
carboxyl,
hydroxyl, aryl, aryloxy, heterocycle, substituted heterocycle, hetaryl,
substituted hetaryl, nitro,
cyano, thiol, sulfamido and the like.
"Heteroarylalkyl" refers to the group -R-HetAr where HetAr is an heteroaryl
group
and R lower alkyl or substituted lower alkyl. Heteroarylalkyl groups can
optionally be
21
CA 02657986 2009-03-10
unsubstituted or substituted with, e.g., halogen, lower alkyl, substituted
lower alkyl, alkoxy,
alkylthio, acetylene, aryl, aryloxy, heterocycle, substituted heterocycle,
hetaryl, substituted
hetaryl, nitro, cyano, thiol, sulfamido and the like.
"Cycloalkyl" refers to a divalent cyclic or polycyclic alkyl group containing
3 to 15
carbon atoms.
"Substituted cycloalkyl" refers to a cycloalkyl group comprising one or more
substituents with, e.g., halogen, lower alkyl, substituted lower alkyl,
alkoxy, alkylthio,
acetylene, aryl, aryloxy, heterocycle, substituted heterocycle, hetaryl,
substituted hetaryl,
nitro, cyano, thiol, sulfamido and the like.
"Alkyl cycloalkyl" denotes the group -R-cycloalkyl where cycloalkyl is a
cycloalkyl
group and R is a lower alkyl or substituted lower alkyl. Cycloalkyl groups can
optionally be
unsubstituted or substituted with e.g. halogen, lower alkyl, lower alkoxy,
alkylthio, acetylene,
amino, amido, carboxyl, hydroxyl, aryl, aryloxy, heterocycle, substituted
heterocycle, hetaryl,
substituted hetaryl, nitro, cyano, thiol, sulfamido and the like.
"Optional" and "optionally" mean that the subsequently described event or
circumstance may or may not occur, and that the description includes instances
where the
event or circumstance occurs and instances in which it does not. For example,
"optional
pharmaceutical excipients" indicates that a formulation so described may or
may not include
pharmaceutical excipients other than those specifically stated to be present,
and that the
formulation so described includes instances in which the optional excipients
are present and
instances in which they are not.
"Treating" and "treatment" refer to any treatment of a disease in a mammal,
particularly a human, and include:
(i) preventing the disease from occurring in a subject which may be
predisposed
to the disease but has not yet been diagnosed as having it;
(ii) inhibiting the disease, i.e., arresting its development; or
(iii) relieving the disease, i.e., causing regression of the disease.
The compositions of this invention are useful for treating mammals in a
therapy
selected from the group consisting of protecting skeletal muscles against
damage resulting
from trauma, protecting skeletal muscles subsequent to muscle or systemic
diseases such as
intermittent claudication, to treat shock conditions, to preserve donor tissue
and organs used
in transplants, and to treat cardiovascular diseases including atrial and
ventricular arrhythmias,
Prinzmetal's (variant) angina, stable angina, and exercise induced angina,
congestive heart
disease, and myocardial infarction. The treatment is accomplished using a
therapeutically
22
CA 02657986 2009-03-10
:ffective amount of at least one compound of this invention and/or a
pharmaceutically
acceptable acid addition salt thereof in admixture with a pharmaceutically
acceptable
excipient.
Compounds falling within the scope of this invention include the optical
isomers (+)
and (-) and R- and S- isomers of the above-identified compounds and mixtures
thereof. This
invention includes the individual isomers and all possible mixtures thereof.
All of the aforementioned embodiments include the pharmaceutically acceptable
acid
addition salts thereof, particularly the mono- and dihydrochlorides, and
mixtures thereof
The compounds having the general Formula I and IA can be prepared as outlined
in
Schemes IA-7A. A general synthesis of the compounds of this invention is
outlined in
Scheme 1A. Compound IV can be prepared by N-acylation of substituted aniline
II with 2-
substituted chloroacetylchloride M. Compound II is available commercially or
readily
prepared through reduction of the corresponding nitrobenzene derivative
(acid/SnC12 or
catalytic hydrogenation, see Advanced Organic Chemistry, Ed. J. March, (1992)
A. Wiley-
Interscience). Some examples of commercially available substituted anilines
corresponding to
general structure H include 2,6-dimethylaniline, 2,3-dimethylaniline, 2-
methylaniline,
, 4-methylaniline, 2,4-dichloroaniline, 3,4-dichloroaniline, 2,5-
dichloroaniline,
2,4-dichloroaniline, 2-chloroaniline, 3-chloroaniline, 2,6-difluoroaniline,
2,5-difluoroaniline,
3,4-difluoroaniline, 2-fluoroaniline, 4-fluoroaniline, 3-fluoroaniline, 2-
fluoro-6-chloroaniline,
4-fluoro-3-chloroaniline.
23
CA 02657986 2009-03-10
SCHEME 1A
0
R2 Rt cl C' R2 Rt
O
III /
R NH R7 R8 R / \ N CI
- RB _ Re R7 R8
R, RS R, R5
u tv
R1o R11
Ra~1 I/Riz
H-N/7-~\N-P R2 R, ReRto Rt1R
tz
R1eRt5R14Ru R N N N-P
V
loom RB R7 Rej~
P BOC,CBZaBmzyl R4 R5 R1e R15Rt4Rt3
vi
R9R1a R11Rtz
R Rt
O
Dc{avtoa R N N - H )MP I Y
R6 R7 Re
R4 R5 Rts R15R14R13
VII
R18 R17 O
R / \ O~''6~
t m
- R1o Rtt
R Rt O R4\I ~R12 R17 R1E
R~ R21 'T- (`
VIII R / ~ i N N~O R79
RB R R-1Y OIH m
R4 Rg R16 R15RuRts R21 R
I
5
Compound VI can be obtained by reacting compound IV with N-protected
substituted
piperazine V through warming in an appropriate solvent (e.g. DMF, EtOH).
Protection of the
nitrogen of compound V is only required when it is useful to control the
regiochemistry of the
addition of Compound V with compound IV. In some
10 cases, compound V can be obtained from commercial resources. Examples of
commercially
available compounds corresponding to general structure V include 2-methyl
piperazine, 2,5-
24
CA 02657986 2009-03-10
dimethyl piprazine and 2,6-dimethyl piperazine. Deprotection of compound VI
can be
accomplished using the standard conditions (e.g. for Boc group use TFA, for
CBZ and benzyl
use hydrogenation). Compound I can be prepared by reacting compound VII with
epoxide
VIII through warming in an appropriate solvent (ethanol, DMF).
SCHEME 2A
Rie R17 R18 R17 O
X acetone, K SCO3
O
R19 OH + R~e m
X
R20 R21 R20 R21
X = C1 or Br
ix VIII
Epoxide VIII (where m = I or 2) can be prepared as outlined in Scheme 2.
Heating
substituted phenol IX with epichlorohydrin, epibromohydrin, or 4-bromo-1,2-
epoxybutane
and potassium carbonate in acetone can afford epoxide VIII. Compound IX can be
obtained
from commercial resources. Example of commercially available compounds of
compounds
IX include 2-chlorophenol, 2-fluorophenol, 2-methoxyphenol, 2-methylphenol,
sesamol, 2,6-
dichlorophenol, 3,5-dichlorophenol, 2,6-difluorophenol, 2,4-difluorophenol5-
indanol, 3-
chloro-4-fluorophenol, 2,chloro-4-fluorophenol and 5,6,7,8-tetrahydro-2-
naphthol. In some
cases compound VIlI can be obtained from commercial sources. Examples of
commercially
available compounds corresponding to general structure VIII include benzyl
glycidyl ether,
glycidyl 2-methylphenyl ether, glycidyl 4-methoxyphenyl ether, glycidyl 4-
chlorophenyl
ether, glycidyl 2-chlorophenyl ether, glycidyl 2-methoxyphenyl ether, glycidyl
4-
methylphenyl ether, glycidyl 3,4-dichlorophenyl ether and glycidyl 4-
fluorophenyl ether.
CA 02657986 2009-03-10
SCHEME 3A
O
Rio Re
TFA
_ t-BuLi , R 9,IOBr DeQrotecho^
Bn-N BOC Bn-N BOC
XI I X7I1
R ' R 9 O R Rto
Diborane
Bn-N flH ~ Bn-N NH
\~_XIv// \t_J/
v
Compound V can be prepared as described in Scheme 3. Alkylation of compound
XII
with alkyl halides using t-BuLi as base can afford compound XIH as described
by Pohlman et.
al. (J. Org. Chem, (1997), 62, 1016-1022). Reduction of XIV using diborane can
afford N-
benzyl protected version of compound V after N-Boc deprotection with
trifluoroacetic acid
(TFA) [ for the diborane reduction see Jacobson et. al, J. Med. Chem, (1999),
42, 1123-1144].
1 o SCHEME 4A
toR9 o
e R10 O R13 O R`~4
R14~~4 l.coupling H-N N-Bn
+ i~R1s
Boc--NH OH
Bn~NH OR Z, T'FA, ring dosure O R14
XVI XVII XVIII
It=MeorEt
R~~Rs
diborane ~
H-N N-Bn
\-~ Rts
xlx R 14
Compound V can also be prepared through standard coupling (eg. EDC or PyBroP)
of D or
L amino acids and standard deprotection as outlined in Scheme 4 [For
preparations of
diketopiperazines see - P. Cledera et al. Tetrahedron, (1998) p. 12349-12360
and R. A. Smith
26
CA 02657986 2009-03-10
et al Bioorg. Med. Chem. Lett. (1998) p. 2369-2374]. Reduction of the
diketopiperazine with
diborane can afford compound XIX the N-benzyl protected version of compound V.
Compound V also includes the bicyclic homologs of piperazine (1S,4S)-(+)-2,5-
diazabicyclo[2.2.1]heptane 83, 3,8-diazabicyclo[3.2.1] octane 84, and 2,5-
diazabicyclo[2.2.2]
octane 85.
HN NH HN NH H NH
83 84 85
Commercially available bicyclic analogs include (1 S,4S)-(+)-2,5-
diazabicyclo[2.2.1]heptane 83. Compounds 84, 85, and the (1R,4R) isomer of 83
can be
prepared by published procedures (for 84 and 85- see Sturm, P. A et al, J.
Med. Chem. 1974,
17, 481-487; for 83 see- Barish, T. F. and Fox, D. E. J. Org. Chem.,1990, SS,
1684-1687).
A specific example of the preparation of a compound of Formula IA is disclosed
in
Schemes 5A, 6A and 7A to further illustrate how to prepare the compounds of
this invention.
In particular, 2,6-dichloroaniline was acylated with 2-chloroacetyl chloride 2
using saturated
bicarbonate and ether (1:1) as base and co-solvent, respectively to afford the
chloroacetamide
derivative 3. Further reaction of compound 3 with 2,6-dimethyl piperazine
afforded
compound 5 through warming in ethanol. Reaction of compound 5 with epoxide 6
by
warming both components in ethanol at reflux afforded 2,6-dimethyl piperazine
derivative 7.
Compound 6 in turn was prepared by warming epichlorohydrin with 2-
methoxyphenol in
acetone in the presence of K2C03 as described in Scheme 6.
27
CA 02657986 2009-03-10
SCHEME 5A
0 HN NH
C Ci
NHZ NC1
410-
sat NaHCO 3/ Et ZO (1:1) H EtOH DrPEA refluz 24 hrs.
0 RT
1 3
CO
O
O
HLb
O /,--< 6
N NH
N
H EtOI-); Rcflux
H~C
N NO
H
OH
7
5
SCHEME 6A
CHs O
OH O
CI acetone, K2C03 8
9 OCH3
6
A specific synthesis of compound 14 is described in Scheme 7. Compound 11 was
prepared by opening of epoxide 6 with Boc-ethylenediamine through warming in
EtOH.
Acylation of compound 11 was accomplished using chloroacetyl chloride in
dichioromethane
using diisopropylethyl amine as a base. Removal of the Boc group using TFA
followed by
ring closure through warming in EtOH afforded compound 13. Reaction of
compound 13
with 3 as described above afforded compound 14.
28
CA 02657986 2009-03-10
~ ~c H OCH,
HN. J~IHz + O H~ O
5,~
v HN- fl
v
6 il
H 1.TFA
chbroacetyl chloride Yoc T-~f O
HN~ O OCH,
DCM, DIPEA jw- 2. EtOH, DIPEA
12
H
OCH, 3 I - OCH,
O \~ N~ O
HN \ j ' n
I'
EtOA,DIPEA 0
O 13 14 O
The compounds having the general formula I and IB can be prepared as outlined
in
5 Schemes 1B-7B. A general synthesis of the compounds of this invention is
outlined in
Scheme 1B. Compound IV can be prepared by N-acylation of substituted anilines
of general
structure II with' 2-substituted chloroacetylchloride III. Compound II is
available
commercially or readily prepared through reduction of the corresponding
nitrobenzene
derivative (acid/SnCl2 or catalytic hydrogenation, see Advanced Organic
Chemistry, Ed. J.
lo March, (1992) A. Wiley-Interscience). Some examples of commercially
available substituted
anilines of general structure II include 2,6-dimethylaniline, 2,3-
dimethylaniline, 2-
methylaniline, 4-methylaniline, 2,4-dichloroaniline, 3,4-dichloroaniline, 2,5-
dichloroaniline,
2,4-dichloroaniline,, 2-chloroaniline, 3-chloroaniline, 2,6-difluoroaniline,
2,5-difluoroaniline,
3,4-difluoroaniline, 2-fluoroaniline, 4-fluoroaniline, 3-fluoroaniline, 2-
fluoro-6-chloroaniline,
4-fluoro-3-chloroaniline.
29
CA 02657986 2009-03-10
2 R' C IQR~ Ra CI *2,W
O
Ra ~ ` i Ra H R N RR RRR5
II IV
Rio Rtt
Ro~' 4 J _R' 2
H,^N/ \N_P RsRto R" i2
RZ Rt O )L__~R
R
18~ 15R1 ,4Ria Ra N N-P
R
V Ra R~ Ra
P=BOC,CBZorBenzy R4 5 Rta RISR14Rt9
~
RgRto Rtt 12
R2 Rt I/R
Deprotect R N NN-H
Ra R7 Re
Ra R5 R1e RuRt4 Rta
VII
Rte Rt7
Rt ~ `
n
- O 2 R ReRto RttR12 Rt7 Rie
R2o R2t
VIII R3 N N Rta
~
' Ra Rt Re OH
R4 6 Rta Rt5RtaRt3 ~1 R2o
R
I
CA 02657986 2009-03-10
Compound VI can be obtained by reacting compound IV with a N-protected
substituted
piperazine V through warming in an appropriate solvent (e.g. DMF, EtOIT).
Protection of the
nitrogen of compound V is only required when it is useful to control the
regiochemistry of the
addition of Compound V with compound IV. In some cases, compound V can be
obtained
from commercial sources. Examples of commercially available compounds of
general
structure V include 2-methyl piperazine, 2,5-dimethyl piperazine and 2,6-
dimethyl piperazine.
Deprotection of compound VI can be accomplished using the standard conditions
(e.g. for
Boc group use TFA, for CBZ and benzyl use hydrogenation). Compound I can be
prepared
by reacting compound VII with epoxide VIII through warming in an appropriate
solvent
(ethanol, DMF).
SCHEME 2B
R17 R17
18
R \ CHO R18
I ~ \ CI
R1 ~ R21 R1 ~ R21
R2D
IX X R20
~/MgBr
PhgPCH2Br, NaH, THF
Diethylether
R17 R17
Ri8 R1a 0
\ mCPBA,DCM
Rtg I~ R~ R1 R21
R20
XI Vlll
Epoxide VIII can be prepared as outlined in Scheme 2B. Epoxidation of
substituted
allylbenzene XI using mCPBA or hydrogen peroxide can afford epoxide VIII (G.
Majetich,
R. Hicks, G. Sun and P. McGill, (1998), 63, 2564-2573). Compound XI in turn
can be
prepared by reacting aldehyde IX with methylenetriphenylphosphorane under
Wittig
conditions or Homer Emmons conditions [Advanced Organic Chemistry, Eds. J.
March,
31
CA 02657986 2009-03-10
(1992), Wiley-Interscience publication and S. Pine, G. Shen and H. Hoang,
Synthesis,
(1991), 1]. The compound XI can also be conveniently prepared by coupling a
halide with the
general formula X with allyl magnesium bromide. In some cases compound XI can
be
obtained from commercial sources. Examples of commercially available compounds
corresponding to the general structure XI include (where m = 0) 3-
fluorostyrene, 4-
fluorostyrene, 2-chlorostyrene, 3-chlorostyrene, 4-chiorostyrene, 2,6-
dichlorostyrene, 3,4-
dichlorostyreneand 3,4-dimethoxystyrene. Other examples of commercially
available
compounds with the general structure XI include (where m = 1) 4-
methoxyallylbenzene, 2-
hydroxyallylbenzene, 4,5-dimethoxyallylbenzene, 2-methylallylbenzene safrole
and 1-
allylnaphthalene.
SCHEME 3B
R10Ra O
n-SuLi, Rg,ipBr ~ Deprotection
Bn-N~-2-BOC Bn-N_ JJ--BOC
XII XIvII
Ri oR9 O R' R9
~4 ~
BH3 Bn~N_ J~H lo= Bn--N_ rIH
jav \~~, J/
Compound V can be prepared as described in Scheme 3B. Alkylation of compound
XII with alkyl halides using t-BuLi as base can afford compound XIII as
described by
Pohiman et. al. (J. Org. Chem, (1997), 62, 1016-1022). Reduction of XIII using
diborane
can afford N-benzyl protected version of compound V after N-Boc deprotection
with
trifluoroacetic acid (TFA , for the diborane reduction see Jacobson et. al, J.
Med. Chem,
(1999), 42, 1123-1144).
32
CA 02657986 2009-03-10
SCHEME 4B
R10R9 0
8 R10 O R13 0
R14~\~/ // 1.coupling H-N N-~Bn
+ ~.~((` lob ~R13
Boa--=-NH OH
Bn~ NH OR Z TFp, ringdosure O 14
XVIII R
XVI XVII
IZ=MeorEt
RloRs
diborane ~
H-N N-Bn
~R13
XIX R14
Compound V can also be prepared through standard coupling (eg. EDC or PyBroP)
of
D or L amino acids and standard deprotection (e.g., Boc removal by TFA
treatment) as
outlined in Scheme 4 [For preparations of diketopiperazines see - P. Cledera
et al.
Tetrahedron, (1998) p. 12349-12360 and R. A. Smith et al Bioorg. Med. Chem.
Lett. (1998) p.
2369-2374]. Reduction of the diketopiperazine with diborane can afford the N-
benzyl
protected version of compound V.
Compound V also includes the bicyclic homologs of piperazine (1S,4S)-(+)-2,5-
diazabicyclo[2.2.1]heptane 83, 3,8-diazabicyclo[3.2.1] octane 84, and 2,5-
diazabicyclo[2.2.2]
octane 85.
\
HN H H -\H H H
83 84 85
Commercially available bicyclic analogs include (1S,4S)-(+)-2,5-
diazabicyclo[2.2.1]heptane 83. Compounds 84, 85, and the (1R,4R) isomer of 83
can be
prepared by published procedures (for 84 and 85- see Sturm, P. A. et al, J.
Med. Chem. 1974,
17, 481-487; for 83 see- Barish, T. F. and Fox, D. E. J. Org. Chem., 1990, 55,
1684-1687).
A specific example of the preparation of a compound from this invention is
disclosed
in Scheme 5B to further illustrate how to prepare the compounds of this
invention. In
33
CA 02657986 2009-03-10
particular, 2,6-dichloroaniline was acylated with 2-chloroacetyl chloride 2
using saturated
bicarbonate and ether (1:1) as base and co-solvent, respectively to afford the
chloroacetamide
derivative 3. Further reaction of compound 3 with piperazine afforded compound
5 through
warming in ethanol. Reaction of compound 5 with epoxide 6 by warming both
components in
ethanol at reflux afforded piperazine derivative 7.
SCHEME 5B
o /-\
-j~ HN- NH
v
cl cl
(NH2 Z fLci 4 sat NaHCOS1 Et2O (175 EtOH, DIPEA, reflux 24 hrs.
0 ---> RT
J
__ O
O
O
'~` H
N
H EtOH, Reflux
O
O^1
NN N
H OH
7
Compound 8 is commercially available and was epoxidized using 3-
chloroperoxybenzioc acid in dichloromethane as illustrated in Scheme 6B.
Scheme 6B
CH3
OH Q....o Nl~ + C acetone, K=C0= m
y 6a.
m=1
8 OCH3 6b. m= 2
6
34
CA 02657986 2009-03-10
Four carbon epoxide 15 can be prepared by coupling commercially available 4-
methoxybenzyl chloride with allylmagnesium bromide followed by oxidation with
mCPBA
as illustrated in Scheme 7B.
SCHEME 7B
CI MgBr
I f - I~
H3CO Ethylether OCH3
14
mCPBA, DCM 1--
0)~ OCH3
The compounds having the general Formula I and IC can be prepared as outlined
in
Schemes 1C-6C. A general synthesis of the compounds of this invention is
outlined in
Scheme 1C.
CA 02657986 2009-03-10
SCHEIVIE 1C
U
R Rj CI Ci R 0
III
R3 / ` NH R,Rs R 0",
N CI _ RB Re R7 RB
R4 RS R4 R
s
II IV
Rio Rit
R"~Rtz
H-N --P ReRto RttRIz
R R~ O
RtsRisR14R~a R3 N N p
V
P= BOC, CBZ or Benz R4 R5 RB R7 RA
~e R~sRt~ u
VI
R Rt O Ip---E' ' l R9Rto RttRtz
Deprotect R3 N N ~ H
I JY
s R7 R
R4 Rs R A 16 RuRt4Rta
VII
~ Rto Rt~R1z
Rt7 R R' o R9`
V 111 ~/R'T
R oH
R6 R7 Rts Rta
R4 Rs RtsR14
I
Compound IV can be prepared by N-acylation of substituted aniline II with 2-
substituted
chloroacetylchloride III. Compound II is available commercially or readily
prepared through
reduction of the corresponding nitrobenzene derivative (acid/SnC12 or
catalytic hydrogenation,
see Advanced Organic Chemistry, Ed. J. March, (1992) A. Wiley-Interscience).
Some
examples of commercially available substituted aniline II include 2,6-
dimethylaniline, 2,3-
dimethylaniline, 2-methylaniline, 4-methylaniline, 2,4-dichloroaniline, 3,4-
dichloroaniline, 2,5-dichloroaniline, 2,4-dichloroaniline, 2-chloroaniline, 3-
chloroaniline, 2,6-
36
CA 02657986 2009-03-10
difluoroaniline, 2,5-difluoroaniline, 3,4-difluoroaniline, 2-fluoroaniline, 4-
fluoroaniline, 3-
fluoroaniline, 2-fluoro-6-chloroaniline, 4-fluoro-3-chloroaniline.
Compound VI can be obtained by reacting compound IV with N-protected
substituted
piperazine V through warming in an appropriate solvent (e.g. DMF, EtOH).
Protection of the
nitrogen of compound V is only required when it is useful to control the
regiochemistry of the
addition of Compound V with compound IV. In some cases, compound V can be
obtained
from commercial sources. Examples of commercially available compound
corresponding to
the general structure V include 2-methyl piperazine, 2,5-dimethyl piperazine,
2,6-dimethyl
piperazine and 4-benzyloxycarbonylpiperazin-2-one. Deprotection of compound VI
can be
accomplished using the standard conditions (e.g. for Boc group use TFA, for
CBZ and benzyl
use hydrogenation). Compound I can be prepared by reacting compound VII with
epoxide
VIII through warming in an appropriate solvent (ethanol, DMF).
SCHEME 2C
X NaH, DMF O
R~7
~-OH +
X
IX
X=ClorBr VIII
Epoxide VIII can be prepared as outlined in Scheme 2C. Heating alkyl alcohol
IX
with epichlorohydrin or epibromohydrin and sodium hydride in DMF can afford
epoxide
VIII. In some cases compound VIII can be obtained from commercial resources.
Examples
of commercially available compounds of general structure VIII include glycidyl
isopropyl
2o ether, N butyl glycidyl ether, T butyl glycidyl ether and iso-butyl
glycidyl ether.
Compound V can be prepared as described in Scheme 3C. Alkylation of compound
XII with alkyl halides using t-BuLi as base can afford compound XIII as
described by
Pohlmanet. al. (J. Org. Chem, (1997), 62, 1016-1022). Reduction of XIV using
diborane can
afford N-benzyl protected version of compound V after N-Boc deprotection with
trifluoroacetic acid (TFA , for the diborane reduction see Jacobson et. al, J.
Med. Chem,
(1999), 42, 1123-1144).
37
CA 02657986 2009-03-10
SCHEME 3C
O RioRe O
I~ n-BuLi. R9103r /~ TFA
Bn-N N-BOC Bn-NN-BOC )OW
XI XII
R, oRa O R Ry
Diborane
l~~
,~
Bn-N NH 00 Bn- N NH
\xiII
v
Compound V can also be prepared through standard coupling (eg. EDC or PyBroP)
of D or L
amino acids as outlined in Scheme 4C [For preparations of diketopiperazines
see - P. Cledera
et al. Tetrahedron, (1998) p. 12349-12360 and R. A. Smith et al Bioorg. Med.
Chem. Lett.
(1998) p. 2369-2374]. Reduction of the diketopiperazine with diborane can
afford the N-
benzyl protected version of compound V.
1 o SCHEME 4C
R10R9 O
s R'O O R1s O ~
R74 i.coupung H-N N-Bn
+ ~ Rt3
Boo-NH OIH
B~NH OR
2. TFA, ring dos nre 0 R14
xiv xv xvi
R=Me or Flt
oR9
RR1
diborane
H-NN-Bn
Rta
R14
A specific example of the preparation of a compound from this invention is
disclosed
in Schemes 5C and 6C to further illustrate how to prepare the compounds of
this invention.
38
CA 02657986 2009-03-10
HN H
y C CI
1(NH2 (Lc N
set NeHC03/ Et20 (1:1) H EtOH, DIPEA, rcflux 24 hro.
0 ---> RT
1 3
QocO
N NH
:enux
N EtOH, H
s
OH
7
In particular, 2,6-dichloroaniline was acylated with 2-chloroacetyl chloride 2
using
saturated bicarbonate and ether (1:1) as base and co-solvent, respectively to
afford the
chloroacetamide derivative 3. Further reaction of compound 3 with piperazine
afforded
compound 5 through warming in ethanol. Reaction of compound 5 with epoxide 6
by
warming both components in ethanol at reflux afforded piperazine derivative 7.
Compound 6
in turn was prepared by warming epibromohydrin with 2-indanol in DMF in
presence of NaH
as described in Scheme 6C.
SCHEME 6C
-oH
NaH, DMF
$ 6
39
CA 02657986 2009-03-10
The acid addition salts of the compounds of this invention may be converted to
the
corresponding free base by treating with a suitable base, such as potassium
carbonate or
sodium hydroxide, typically in the presence of aqueous solvent, and at a
temperature of
between about 0 degrees C and 100 degrees C. The free base form is isolated by
conventional
means, such as extraction with an organic solvent.
Salts of the compounds of this invention may be interchanged by taking
advantage of
differential solubilities and volatilities, or by treating with the
appropriately loaded ion
exchange resin. This conversion is carried out at a temperature between about
0 C and the
boiling point of the solvent being used as the medium for the procedure.
Administration of the active compounds and salts described herein can be via
any of the
accepted modes of administration for therapeutic agents. These methods include
oral,
parenteral, transdermal, subcutaneous and other systemic modes. The preferred
method of
administration is oral, except in those cases where the subject is unable to
ingest, by himself,
any medication. In those instances it may be necessary to administer the
composition
parentarally.
Depending on the intended mode, the compositions may be in the form of solid,
semi-
solid or liquid dosage forms, such as, for example, tablets, suppositories,
pills, capsules,
powders, liquids, suspensions, or the like, preferably in unit dosage forms
suitable for single
administration of precise dosages. The compositions may include one or more
conventional
pharmaceutical excipients and at least one active compound of this invention
or the
pharmaceutically acceptable salts thereof and, in addition, may include other
medicinal
agents, pharmaceutical agents, carriers, adjuvants, diluents, etc.
The amount of active compound administered will, of course, be dependent on
the
subject being treated, the subject's weight, the severity of the affliction,
the manner of
administration and the judgment of the prescribing physician. The effective
amount ranges
from about 0.01 to about 100 mg/kg weight of the mammal. However, an effective
dosage is
in the range of 0.1-30 mg/kg/day, preferably 0.5-20 mg/kg/day. For an average
70 kg human,
this would amount to 7-2100 mg per day, or preferably 35-1400 mg/day. Since
many of the
effects of the compounds herein (protect skeletal muscles against damage
resulting from
trauma; protect skeletal muscles subsequent to muscle or systemic diseases
such as
intermittent claudication; treat shock conditions; preserve donor tissue and
organs used in
transplants; and treat cardiovascular diseases including atrial and
ventricular arrhythmias,
Prinzmetal's (variant) angina, stable angina, exercise induced angina,
congestive heart
disease, and myocardial infarction) are achieved through a similar mechanism
(partial
CA 02657986 2009-03-10
fatty acid oxidation inhibition) dosages (and forms of administration) are all
generally within
the same general and preferred ranges for all these utilities.
For solid compositions, conventional non-toxic solid include, for example,
pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium
saccharin,
talcum, cellulose, glucose, sucrose, magnesium carbonate, and the like may be
used. The
active compound as defined above may be formulated as suppositories using, for
example,
polyalkylene glycols, for example, propylene glycol, as the carrier. Liquid
pharmaceutically
administrable compositions can, for example, be prepared by dissolving,
dispersing, etc. an
active compound as defined above and optional pharmaceutical adjuvants in a
excipient, such
as, for example, water, saline, aqueous dextrose, glycerol, ethanol, and the
like, to thereby
form a solution or suspension. If desired, the pharmaceutical composition to
be administered
may also contain minor amounts of nontoxic auxiliary substances such as
wetting or
emulsifying agents, pH buffering agents and the 1ike, for example, sodium
acetate, sorbitan
monolaurate, triethanolamine sodium acetate, triethanolamine oleate, etc.
Actual methods of
preparing such dosage forms are known, or will be apparent, to those skilled
in this art; for
example, see Remington's Pharmaceutical Sciences, Mack Publishing Company,
Easton,
Pennsylvania, 15th Edition, 1975. The composition or formulation to be
administered will, in
any event, contain a quantity of the active compound(s), a therapeutically
effective amount,
i.e. in an amount effective to alleviate the symptoms of the subject being
treated. For oral
administration, a pharmaceutically acceptable non-toxic composition is formed
by the
incorporation of any of the normally employed excipients, such as, for example
pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium
saccharin,
talcum, cellulose, glucose, sucrose, magnesium, carbonate, and the like. Such
compositions
take the form of solutions, suspensions, tablets, pills, capsules, powders,
sustained release
formulations and the like. Such compositions may contain 10%-95% active
ingredient,
preferably 1-70%.
Parenteral adniinistration is generally characterized by injection, either
subcutaneously, intramuscularly or intravenously. Injectables can be prepared
in conventional
forms, either as liquid solutions or suspensions, solid forms suitable for
solution or suspension
in liquid prior to injection, or as emulsions. Suitable excipients are, for
example, water, saline,
dextrose, glycerol, ethanol or the like. In addition, if desired, the
pharmaceutical compositions
to be administered may also contain minor amounts of non-toxic auxiliary
substances such as
wetting or emulsifying agents, pH buffering agents and the like, such as for
example, sodium
acetate, sorbitan monolaurate, triethanolamine oleate, etc.
41
CA 02657986 2009-03-10
A more recently devised approach for parenteral administration employs the
implantation of a slow-release or sustained-release system, such that a
constant level of
dosage is maintained. See, e.g., U.S. Pat. No. 3,710,795.
In another recent approach, the compositions of this invention can be
administered
orally in a sustained release dosage form.
It is within the scope of this invention to administer one or more compounds
of this
invention to a mammal, and preferably to a human by other known routes of
pharmaceutical
dosage form administration including, but not limited to by bolus,
intravenously,
transdermally, through inhalation, sub-cutaneously, or any other therapeutic
agent
administration method or route know to one sldlled in the art.
The following Examples are representative of the invention, but are not to be
construed as limiting the scope of the claims.
42
CA 02657986 2009-03-10
Example 1
N-(2,6-dimethylphenyl)-2-{4-(2-hydroxy-3-(2-meth oxy)propyl] -3,5-
dimethylpiperazinyl}acetamide (7).
H3CO
O
~ ~ N~/N N p
OH
7
Part A.
Synthesis of N-(2,6-dimethylphenyl)-2-chloroacetamide (3).
2,6-dimethylaniline (9.8 g, 81.2 mmol) was dissolved in ether (100 mL) and
saturated
aqueous NaHCO3 (100 mL) and the reaction mixture was cooled in an ice/water
bath. To the
cold solution was added chloroacetyl chloride 2 (9.17 g, 81.2 mmol) dropwise
over a period
of 2 h. The mixture was allowed to warm to RT over 14 h. The mixture was
extracted with
EtOAc (3 X 50). The combined organic layers were dried over MgSO4, filtered
and
concentrated. The residue was triturated in ether and filtered to afford
compound 3 as a white
solid.
Part B.
Synthesis of N-(2,6-dimethylphenyl)-2-(3,5-dimethylpiperazinyl)acetamide (5).
To a solution of compound 3 (5 g, 25.2 mmol) in ethanol (100 mL) was added 2,6-
dimethylpiperazine 4 (2.1 g, 25.0 mmol) and N,N-diisopropylamine (3.2 g, 25.2
mmol). The
reaction mixture was refluxed for 24 h. The mixture was concentrated in vacuo
and
the residue was purified by column chromatography (10:1, DCM: MeOH) to afford
compound 5.
43
CA 02657986 2009-03-10
=
=
NH
N OH OCH3
O N O
`
Part C.
Synthesis of glycidyl 4-methoxyphenyl ether (6).
2-methoxyphenol (1.0 g, 8.0 mmol) and epichlorohydrin (3.7 g, 40.0 mmol) were
dissolved in
acetone (20 mL). KZC03 (2.2 g, 16.0 mmol) was added and the mixture was heated
at 70 C
for 24 h. The reaction mixture was concentrated in vacuo. The residue was
dissolved 100 mL
of EtOAc , washed with 100 mL water, dried over MgSOq and filtered. The
mixture was
evaporated to dryness and the residue was purified using column chromatography
(2:1,
hexane: ethyl acetate) to afford compound 6.
Part D.
Synthesis of N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxy)propyl]-3,5-
dimethylpiperazinyl}acetamide (7).
To a solution of compound 5 in 10 mL EtOH (0.4 g, 1.4 mmol) was added compound
6 (0.27
g, 1.5 mmol). The reaction mixture was refluxed for 24 h. The mixture was
concentrated in
vacuo and the residue was purified by using Prep. TLC (10:1, DCM:MeOH) to
afford
compound 7.
0
NH
IrN OH OCH3
O N O
2-{(5S,2R)-4-[2-hydroxy-3-(2-meth oxyphen oxy)propyl]-2,5-dimethylpiperazinyl}-
N-(2,6-
dimethylphenyl)acetamide (15)
44
CA 02657986 2009-03-10
Compound 15 was prepared in the manner of compound 7 substituting (2R, 5S)-
dimethylpiperazine for 2,6-dimethylpiperazine 4 in part B to afford compound
15: Mass
spectrum (M+l) = 456_4_
&":" H
~N OH OCH 3
O N O
`
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-2-
oxopiperazinyl}acetamide (16)
Compound 16 was prepared substituting 4-benzyloxycarbonyl-2-oxo-piperazine for
2,6-
dimethylpiperazine 4 in part B of compound 7 that was carried on to the final
target in the
manner of compound 7 after removal of the CBZ protecting group (hydrogenation -
20 psi,
10% palladium on carbon) to afford compound 16: Mass spectrum (M+1) = 442.41.
NH
*'r' N "") OH OCH3
O N O
O
2,5-diaza-5-[2-hydroxy-3-(2-methoxyphenoxy)propyl]bicyclo [4.4.0]dec-2-yl}-N-
(2,6-
dimethylphenyl)acetamide (17)
Compound 17 was prepared in the manner of compound 7 substituting
perhydroquinoxaline
for 2,6-dimethylpiperazine 4 in part B to afford compound 17: Mass spectrum
(M+1) = 482.4.
CA 02657986 2009-03-10
H3C
O edrO
000"~ OH
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl)-3,3-
dimethylpiperazinyl}acetamide (18)
Compound 18 was prepared in the manner of compound 7 substituting 2,2-
dimethylpiperazine
for 2,6-dimethylpiperazine 4 in part B to afford compound 18: Mass spectrum
(M+1) _
456.51
H3C0
Q~H ~^
N
O ` /
HN r
N 0
2-{5-[(2S)-2-hydroxy-3-(2-methoxyphenoxy)propyl](1 S,4S)-2,5-diazabicyclo
[2.2.1 ] hept-
2-yl}-N-(2,6-dimethylphenyl)acetamide (19)
Compound 19 was prepared in the manner of compound 7 substituting (IS,4S)-(+)-
2,5-
Diazabicyclo[2.2.1]heptane for 2,6-dimethylpiperazine 4 in part B to afford
compound 19:
Mass spectrum (M+1) = 481.5
H3CO
O O Z
N JL-.-~ N N
OH
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-4-(2-methoxyphenoxy)butyll-
piperazinyl}acetamide (20)
46
CA 02657986 2009-03-10
Compound 20 was prepared in the manner of compound 7 substituting 4-bromo-1,2-
epoxybutane 6b for epichlorohydrin 6a in part B to afford compound 20: Mass
spectrum
(M+1) = 442.37
F
O
N NOH
c N
N-(2,6-dimethylphenyl)-2-{4-[4-(4-flu orophenoxy)-2-hydroxybu tyl] -
piperazinyl}acetamide (21) Compound 21 was prepared in the manner of compound
7
substituting 4-bromo-1,2-epoxybutane 6b for epichlorohydrin 6a in part B to
afford
compound 21: Mass spectrum (M+1) = 430.35
o N J"'., N N
4)4
H
OH
2-(4-{4-[4-(tert-butyl)phenoxy]-2-hydroxybutyl}piperazinyl)-N-(2,6-
dimethylphenyl)
acetamide (22)
Compound 22 was prepared in the manner of compound 7 substituting 4-bromo-1,2-
epoxybutane 6b for epichlorohydrin 6a in part B to afford compound 22: Mass
spectrum
(M+1) = 468.32
..~*
O Z
Z
CS\ NJL~= N ~N
OH
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-4-(4-phenylphenoxy)butyl]
piperazinyl}acetamide (23)
Compound 23 was prepared in the manner of compound 7 substituting 4-bromo-1,2-
epoxybutane 6b for epichlorohydrin 6a in part B to afford compound 23: Mass
spectrum
(1VI+1)=488.41
47
CA 02657986 2009-03-10
JL,,., N N
N
H OH
N-(2,6-dimethylphenyl)-2-{4- [2-hydroxy-4-(4-meth oxyphenoxy)butyl]-
piperazinyl}acetamide (24)
Compound 24 was prepared in the manner of compound 7 substituting 4-bromo-1,2-
epoxybutane 6b for epichlorohydrin 6a in part B to afford compound 24: Mass
spectrum
(M+1) = 442.37
48
CA 02657986 2009-03-10
Example 2
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-3-
oxopiperazinyl}acetamide (14)
Part E.
Synthesis of (tert-butoxy)-N-(2-{[2-hydroxy-3-(2- methoxyphenoxy)propyl]amino)
ethyl)carboxamide (11).
Epoxide 6 (1.0 g, 5.5 mmol) and Boc-ethylenediamine (0.88 g, 5.5 mmol) were
dissolved in 20 mL EtOH and the mixture was heated at reflux for 24 h. The
solvent was
evaporated and the residue was purified using column chromatography (1:1,
Hex:EtOAc) to
afford compound 11.
Synthesis of N-{2-[(tert-butoxy)carbonylamino]ethyl}-2-chloro-N[2-hydroxy-3-(2-
methoxyphenoxy)propyl]acetamide (12)
Compound 11 (1.0 g, 3.0 mmol) was dissolved in 20 mL DCM and treated witll
diisopropylethyl amine (0.76 g, 4.5 mmol). The mixture was cooled in an ice
water bath. To the
cold mixture was added dropwise chloroacetyl chloride in 5 mL DCM. The
reaction mixture was
allowed to stir at RT for 24 h. The mixture was diluted with 50 mL DCM and
washed with 50
mL of water and 10% citric acid. The organic layer was dried over MgSO4 and
filtered. The
solvent was evaporated under reduced pressure and the residue was crystallized
from
ethylether to afford compound 12.
Synthesis of 1-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-2-one (13).
Compound 12 (0.5 g, 1.5 mmol) was dissolved in 10 mL TFA. The mixture was
allowed to
stir at RT for 2 h. TFA was removed under reduced pressure. The residue was
dissolved in. 20
mL EtOH and treated with diisopropylethyl amine (0.76 g, 4.5 mmol). The
mixture was
heated at reflux for 24 h. The solvent was removed under reduced pressure to
afford
compound 13 which was used without further purification.
Part F.
Synthesis of N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-
methoxyphenoxy)propyl]-3-oxopiperazinyl}acetatnide (14)
To a solution of compound 13 in 10 mL EtOH (0.1 g, 0.30 mmol) was added
compound 3
(0.7 g, 0.36 mmol) and diisopropylethyl amine (0.76 g, 0.36 mmol). The
reaction mixture was
heated at reflux for 24 h. The mixture was concentrated in vacuo and the
residue was purified
by using Prep. TLC (10:1, DCM:MeOH) to afford compound 14: Mass spectrum (M+1)
442.34
49
CA 02657986 2009-03-10
Example 3
S .,,.=~` F
H N S
R..- y 0*141~ N N O
O OH
The compounds listed in Table 1, below were made in the manner of compound 14
of
Example 2.
Table 1
R NIIT~
25 2,6-dimeth 1 hen l 430.3
26 2,6-dichloro hen l 471
27 4-aminosulfon 1 hen l 481.2
28 3-trifluorometh 1-5methox hen 1 500.2
29 5-indanyl 442.2
30 1-na hth 1 452.3
31 1 - 4-chlorona hth 1 486.3
32 2-N- ol l- hen l 467.3
33 Phenyl 402.2
34 2-chloro hen 1 436.2
35 2-chloro-4-meth 1 hen 1 450.2
36 2- 1-meth lethen 1 hen l 442.3
37 2-meth 1 hen 1 416.2
38 2-iso ro 1-6-meth 1 hen l 458.4
39 3-meth lthio hen 1 448.2
40 2-methox -4-chloro-5-meth 1 hen 1 480.2
41 4-dimeth lamino hen 1 445.3
42 2,4-dimethox hen 1 462.3
43 3,4-dichloro hen l 471.1
44 4-chloro hen 1 436.3
45 3-chloro hen 1 436.2
46 3,5-dichloro hen 1 471.1
47 4-methox hen l 432.3
48 4-meth 1 hen l 416.2
49 3-meth l hen l 416.2
50 4-fluoro hen 1 420.2
51 4-c ano hen 1 427.3
52 4-ace 1 hen 1 444
53 2-methox hen 1 432.4
54 4-trifluorometh i hen 1 470.2
55 3-trifluorometh l-4-chloro hen 1 504.1
56 3,5-dimethox hen 1 462.3
57 4-N-mo holin 1 hen 1 487.4
58 3-fluoro-4-methox hen 1 450.2
CA 02657986 2009-03-10
59 3,4,5-trimethox hen 1 492.3
60 3,4-dimethox hen 1 490
61 2-fluoro-4-chloro hen 1 454.2
62 2-h drox eth 1-6-meth 1 hen 1 446
51
CA 02657986 2009-03-10
Example 4
2-[4-(3-(2H-benzo [d] 1,3-dioxolen-5-yl)-2-hydroxypropyl)piperazinylJ-N-(2,6-
dimethylphenyl)acetamide (7B).
NH
~,
N OH I
O N /
\~/ O
Part A.
Synthesis of N-(2,6-dimethylphenyl)-2-chloroacetamide (3B).
2,6-dimethylaniline (9.8 g, 81.2 mmol) was dissolved in ether (100 mL) and
saturated
aqueous NaHCO3 (100 mL) and the reaction mixture was cooled in an ice/water
bath. To the
cold solution was added chloroacetyl chloride 2B (9.17 g, 81.2 mmol) dropwise
over a
period of 2 h. The mixture was allowed to warm to RT over 14 h. The mixture
was extracted
with EtOAc (3 X 50). The combined organic layers were dried over MgSO4,
filtered and
concentrated. The residue was triturated in ether and filtered to afford
compound 3B as a
white solid.
Part B.
Synthesis of N-(2,6-dimethylphenyl)-2-piperazinylacetamide (5B).
To a solution of compound 3 (5 g, 25.2 mmol) in ethanol (100 mL) was added
compound 4B
(2.1 g, 25.0 mmol) and N,N-diisopropylamine (3.2 g, 25.2 mmol). The reaction
mixture was
refluxed for 24 h. The mixture was concentrated in vacuo and the residue was
purified by
colunm chromatography (10:1 dichloromethane: methanol) to afford compound 5B.
Part C.
Synthesis of 5-(oxiran-2-ylmethyl)-2H-benzo[dj1,3-dioxane (6B).
To an ice cold solution of 8 (1.0 g, 6.17 mmol) in dichioromethane was added
dropwise a
solution of 3-chloroperoxybenzoic acid (1.8 g, 10.43 mmol) in 20 mL
dichioromethane over a
period of 1 h. The reaction mixture was allowed to stir at RT for 12 h. The
reaction mixture
was filtered to remove any solids and concentrated in vacuo. To the residue
was added
diethyl ether (200m1), and it was washed with saturated sodium bicarbonate
(3x100ml). The
organic layer was dried over MgSO4 , and concentrated in vacuo . The residue
was purified
using Prep. TLC (2:1 hexane: ethyl acetate) to yield 6B.
Part D.
52
CA 02657986 2009-03-10
2-[4-(3-(2H-benzo[d] 1,3-dioxolen-5-yl)-2-hydroxypropyl)piperazinyl]-N-(2,6-
dimethylphenyl)acetamide (7B)
To a solution of compound 5B (0.4 g, 1.64 mmol) in ethanol (100 mL) was added
compound
6B (0.38 g, 2.14 mmol) in 10 mL EtOH. The reaction mixture was refluxed for 24
h. The
mixture was concentrated in vacuo, and the residue was purified by using Prep.
TLC (10:1
dichioromethane: methanol) to afford compound 7B: Mass spectrum (MH+1) =
426.34.
NH
'\ N OH
01,10 O N
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-4-phenylbutyl)piperazinyl]acetamide
(9B).
Compound 9B was prepared in the manner of compound 7B substituting 4-phenyl-
butene for
3-(3,4-methylendioxyphenyl)-1-propene in part C to afford compound 9B: Mass
spectrum
(MH+1) = 396.32.
NH O
I ~ N 100 OH
/ O N
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenyl)-
propyl]piperazinyl}acetamide (10B)
Compound lOB was prepared in the manner of compound 7B substituting 3-(2-
methoxyphenyl)-1-propene for 3-(3,4-methylendioxyphenyl)-1-propene in part C
to afford
compound 10B: Mass spectrum (MH+1) = 412.35.
53
CA 02657986 2009-03-10 , *) NH N OH OCH3
( \ ( \
O N /
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(4-
methoxyphenyl)propyl]piperazinyl}acetamide (11B).
Compound 11B was prepared in the manner of compound 7B substituting 3-(4-
methoxyphenyl)-1-propene for 3-(3,4-methylendioxyphenyl)-1-propene in part C
to afford
compound 11B: Mass spectrum (MIi+l) = 412.35.
NH
\
Jro'o~ N OH
/ O N
N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-phenylpropyl]piperazinyl}acetamide
(12B)
Compound 12B was prepared in the manner of compound 7B substituting 3-phenyl-l-
propene
for 3-(3,4-methylendioxyphenyl)-1-propene in part C to afford compound 12B:
Mass spectrum (MH+1) = 382.
NH
X 00~ N -100 OH
~ o- N
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3-naphthylpropyl)piperazinyl]acetamide
(13B).
Compound 13B was prepared in the manner of compound 7B substituting 3-(1-
naphthyl)-1-
propene for 3-(3,4-methylendioxyphenyl)-1-propene in part C to afford compound
13:Mass
spectrum (MH+l) = 432.55.
54
CA 02657986 2009-03-10
EXAMPLE 5
Part A
Intermediate (14B): To a solution of 4-methoxybenzyl chloride (2-mmol) in
anhydrous ether
(10 niL), was added allylmagnesium bromide ( 4 mL, 1M solution in THF) and the
reaction
mixture was allowed to stir for 16h at room temperature. Sat. ammonium
chloride solution
9lmL) was added and the ether layer was separated, washed with water and
dried.
Evaporation of ether under reduced pressure afforded olefin 14B as an oil. It
was used in the
next reaction without purification.
Part B
Intermediate (15B): To an ice cold solution of 15B (2 mmol) in dichloromethane
was added
dropwise a solution of 3-chloroperoxybenzoic acid (4 mmol) in 20 mL
dichloromethane over
a period of 1 h. The reaction mixture was allowed to stir at RT for 12 h. The
reaction
mixture was filtered to remove any solids and concentrated in vacuo. To the
residue was
added diethyl ether (200m1), and it was washed with saturated sodium
bicarbonate (3x100ml).
The organic layer was dried over MgSO4, and concentrated in vacuo. The residue
was
purified using Prep. TLC (2:1 hexane: ethyl acetate) to yield 15B.
Part C
Synthesis of N-(2,6-dimethylphenyl)-2-{4-[4-(4-methoxyphenyl)-2-
hydroxybntyl]piperazinyl}acetamide(16B)
To a solution of compound 5B (0.4 g, 1.64 mmol) in ethanol (100 mL) was added
compound
15B (2.14 mmol) in 10 mL EtOH. The reaction mixture was refluxed for 24 h. The
mixture
was concentrated in vacuo, and the residue was purified by using Prep. TLC
(10:1
dichloromethane: methanol) to afford compound 16. (M+1) = 426.3
H
~ H
N ON
O 17
F
CA 02657986 2009-03-10
2-{4-[4-(2,6-difluorophenyl)-2-hydroxybutyl] piperazinyl}-N-(2,6-
dimethylphenyl)acetamide(17B)
Compound 17B was prepared in a manner similar to that of compound 16B
substituting 2,6-
difluorobenzyl chloride for 4-methoxybenzyl chloride. (M+1) 432.2
H
N
N OH ~
O N
\ ~
18
C
N-(2,6-dimethylphenyl)-2-{4-[4-(2-chlorophenyl)-2-
hydroxybutyl]piperazinyl}acetamide(18B)
Compound 18B was prepared in a manner similar to that of compound 16B
substituting 2-
chlorobenzyl chloride for 4-methoxybenzyl chloride. (M+1) = 430.2
H
N H
O N
19
2-(4-{4-[4-(tert-butyl)phenyl)-2-hydroxybutyl}piperazinyl)-N-(2,6-
dimethylphenyl)acetamide(19B)
Compound 19B was prepared in a manner similar to that of compound 16B
substituting 4-t-
butylbenzyl chloride for 4-methoxybenzyl chloride. (M + 1) = 452.3
H
N ~ N H
O N
F
56
CA 02657986 2009-03-10
N-(2,6-dimethylphenyl)-2-{4-[4-(2-fluorophenyl)-2-
hydroxybutyl] piperazinyl} acetamide(20B)
Compound 20B was prepared in a manner similar to that of compound 16B
substituting 2-
fluorobenzy] chloride for 4-methoxybenzyl chloride. (M + 1) = 414.2
H
\ N N H ~
0 N CF3
21
N-(2,6-dimethylphenyl)-2-(4-{2-hydroxy-4-[4-
(trifluoromethyl)phenyl] butyl}piperazinyl)acetamide(21 B)
Compound 21B was prepared in a manner similar to that of compound 16B
substituting 4-
trifluoromethylbenzyl chloride for 4-methoxybenzyl chloride. (M + 1) = 464.2
O-\
H
H
22
2-[4-(3-(2H-benzo[d] 1,3-dioxolen-5-yl)-2-hydroxypropyl)piperazinyl]-N-(2,6-
dimethylphenyl)-2-methylpropanamide (22B)
This compound was prepared in a manner similar to that of 7B, substituting 2-
chloro-2-
methylpropionyl chloride for chloroacetyl chloride in part A. (M+1) = 454.54
q1i7coO
23
57
CA 02657986 2009-03-10
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3-phenylpropyl)piperazinylJ-2-
methylpropanamide (23B)
This compound was prepared in a manner similar to that of 7B, substituting 2-
cbloro-2-
methylpropionyl chloride for chloroacetyl chloride in part A and allylbenzene
for 8B. (M+1)
= 410.34.
cLr\j:,HaCH:
~ OCH3
H
24
N-(2,6-dimethylphen yl)-2- {4-[2-hydroxy-3-(3,4,5-
trimethoxyphenyl)propylJpiperazinyl}-2-methylpropanamide (24B)
This compound was prepared in a manner similar to that of 7B, substituting 2-
chloro-2-
methylpropionyl chloride for chioroacetyl chloride in part A and 3,4,5-
trimethoxy
alkybenzene for 8B. (M+1) = 472.54
H
NY' C~JH~ 25
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-5-phenylpentyl)piperazinyl]acetamide
(25B)
This compound was prepared in a manner similar to that of 16B, substituting
phenethyl
chloride for 4-methoxybenzyl chloride in part A. (M+1) = 410.4.
H
H
O
26
N-(2,6-dimethylphenyl)-2-{4-[5-(2-fluorophenyl)- 2-hydroxy-
pentyl] piperazinyl} acetamide(26B)
This compound was prepared in a manner similar to that of 16B, substituting 2-
fluorophenethyl chloride for 4-methoxybenzyl chloride in part A. (M+1) =
428.1.
58
CA 02657986 2009-03-10
H
' N)rN H
O N
CI
27
N-(2,6-dimethylphenyl)-2-{4-[5-(2-chlorophenyt)- 2-hydroxy-
pentyl) piperazinyl} acetamide(27B)
This compound was prepared in a manner similar to that of 16B, substituting 2-
chlorophenethyl chloride for 4-methoxybenzyl chloride in part A. (M+1) = 444.3
59
CA 02657986 2009-03-10
Example 6
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3-indan-2-yloxypropyl)piperazinyl]
acetamide
(7C)
Part A.
Synthesis of N-(2,6-dimethylphenyl)-2-chloroacetamide (3C).
2,6-dimethylaniline (9.8g, 81.2 mmol) was dissolved in ether (100 mL) and
saturated aqueous
NaHCO3 (100 mL) and the reaction mixture was cooled in an ice/water bath. To
the cold
solution was added chloroacetyl chloride 2C (9.17 g, 81.2 mmol) dropwise over
a period of
2h. The mixture was allowed to warm to RT over 14 h. The mixture was diluted
with 100
mL ether and the organic layer was dried over MgSOõ filtered and concentrated
to afford
compound 3C as a white solid.
Part B.
Synthesis of N-(2,6-dimethylphenyl)-2-piperazinylacetamide (5C).
To a solution of compound 3C in 100 mL EtOH (5 g, 25.2 mmol) was added
compound 4C
(2.1 g, 25.0 mmol) and N,N-diisopropylethylamine (3.2 g, 25.2 mmol). The
reaction mixture
was refluxed for 24 h. The mixture was concentrated in vacuo and the residue
was purified
by column chromatography ( 10:1, DCM:MeOH) to afford compound 5C.
Part C.
Synthesis of 2-(oxiran-2-ylmethoxy) propane (6C)
To a solution of 60% NaH (0.18g, 4.5mmol) in DMF (l Oml) cooled to 0 degrees
was added
2-propanol (0.5g, 3,73mmol) in DMF (2ml) dropwise. After stirring for
30minutes
epibromohydrin (1.l lg, 8.18mmol) in DMF (1m1) was added dropwise. The
reaction was
allowed to warm to room temperature and stirred for 48 h. The solvent was
removed in vacuo
and the residue was purified using Prep TLC (30:1, DCM:MeOH) to afford
compound 6C.
Part D
Synthesis of N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3-indan-2-
yloxypropyl)piperazinyl] acetamide (7C)
To a solution of 6C (0.43g, 2.3mmol) in ethanol(4m1) was added 5C (0.405g,
1.64mmo1).
The solution was heated to reflux and stirred for 24 h. Upon completion the
solution was
concentrated in vacuo and purified using Prep TLC (10:1, DCM:MeOH) to yield
7C. Mass
Spectrum (M+1) = 438.36.
CA 02657986 2009-03-10
Q ~
N N O
H
OH
2-({2-[4-(3-isopropoxy-2-hydroxypropyl)piperazinyl]- N-({2,6-
dimethylphenyl)acetamide
(lOC)
Compound 10C was prepared in a similar manner to compound 7C, substituting the
commercially available glycidyl isopropyl ether for 2-(oxiran-2-
ylmethoxy)indane in part D to
afford 10C : Mass spectrum MS (MH+) = 364.37.
NH
N .01 OH '
N O ~
~ O
(4-,~ %~
N-(2,6-dimethylphenyl)-2-{4- [2-hydroxy-3
(phenylmethoxy)propyl] piperazinyl} acetamide (11 C)
Compound 11C was prepared in a similar manner to compound 7C, substituting the
commercially available benzyl glycidyl ether for 2-(oxiran-2-ylmethoxy)indane
in part D to
afford 11C. Mass Spectrum (M+1) = 412.36.
O
N ~=~'_= O
N
H
l.~
OH
2-({2-[4-(3-cyclopentyloxy-2-hydroxypropyl)piperazinyl]- N-(12,6-
dimethylphenyl)acetamide (12C)
61
CA 02657986 2009-03-10
Compound 12C was prepared in a similar manner to compound 7C, substituting the
commercially available cyclopentanol for 2-indanol in part C to afford 12C: MS
(1VIH+) _
390.
2-({2-[4-(3-cyclohexyloxy-2-hydroxypropyl)piperazinyl]- N-({2,6-
dimethylphenyl)acetamide (13C)
Compound 13C was prepared in a similar manner to compound 7C, substituting the
commercially available cyclohexanol for 2-indanol in part C to afford 13C - MS
(MH+) _
404.
H
14
2-[4-(3-{ [4-(tert-butyl)phenyl] methoxy}-2-hydroxypropyl)piperazinyl]-N-(2,6-
dimethylphenyl)acetamide (14C): Compound 14C was prepared in a similar manner
to
compound 7C, substituting the commercially available 4-t-bu-benzylalcohol for
2-propanol in
part C. MS (M+1) = 468.44
H
NO N
J!~QDO
N-(2,6-dimethylphenyl)-2-(4-{3- [(2-f luorophenyl)meth oxy]-2-
hydroxypropyl}piperazinyl)acetamide(15C): Compound 15C was prepared in a
similar
manner to compound 7C, substituting the commercially available 2-
fluorobenzylalcohol for
2-propanol in part C. MS (M+1) = 430.39
H
NH F
O
16
62
CA 02657986 2009-03-10
2-(4-{3- [(2,4-diiluorophenyl)methoxy]-2-hydroxypropyl} piperazinyl)-N-(2,6-
dimethylphenyl)acetamide(16C): Compound 16C was prepared in a similar manner
to
compound 7, substituting the commercially available 2,4-difluorobenzylalcohol
for 2-
propanol in part C. MS (M+1) = 448.38
H
Nj_--~N H / F
O
17
N-(2,6-dimethylphenyl)-2-[4-(2-hydroxy-3-{ [4-
(trifluoromethyl)phenyl]methoxy}propyl)piperazinyl]acetamide (17C): Compound
17C
was prepared in a similar manner to compound 7C, substituting the commercially
available 4-
trifluoromethyl-benzylalcohol for 2-propanol in part C. MS (M+1) = 480.37
H
O
O ~ \ I
18
N-(2,6-dimethylphenyl)-2-(4-{2-hyd roxy-3- [(2-
methoxyphenyl)methoxy]propyl}piperazinyl)acetamide (18C): Compound 18C was
prepared in a similar manner to compound 7C, substituting the commercially
available 2-
methoxy-benzylalcohol for 2-propanol in part C. MS (M+1) = 442.41
H O/
, H / I \
O ~I
19
2-(4-{3-[(2,4-dimethoxyphenyl)methoxy]-2-hydroxypropyl}piperazinyl)-N-(2,6-
dimethylphenyl)acetamide (19C): Compound 19C was prepared in a similar manner
to
63
CA 02657986 2009-03-10
compound 7C, substituting the commercially available 2,4-dimethoxy-
benzylalcohol for 2-
propanol in part C. MS (M+1) = 472.42
H
N --C
O
ON,~,
N-(2,6-dimethylphenyl)-2-(4-{2-hydroxy-3-[(4-
5 methoxyphenyl)methoxy]propyl}piperazinyl)acetamide(20C): Compound 20C was
prepared in a similar manner to compound 7C, substituting the commercially
available 4-
methoxy-benzylalcohol for 2-propanol in part C. MS (M+1) = 442.42
H
N~ ~N^ H
O ~N" 0 / IF
~
21
N-(2,6-dimethylp h enyl)-2-(4- {3- [(4-flu oroph enyl)meth oxy] -2-
hydroxypropyl}piperazinyl)acetamide (21C) Compound 21C was prepared in a
similar
10 manner to compound 7C, substituting the commercially available 4-fluoro-
benzylalcohol for
2-propanol in part C. MS (M+1) = 430.40
O ~N-'I _
NJ OH
H 22
N-(2,6-dimethylphenyl)-2-(4-{2-hydroxy-3-[(4-
15 methylphenyl)methoxy]propyl}piperazinyl)acetamide (22C): Compound 22C was
prepared in a similar manner to compound 7C, substituting the commercially
available 4-
methyl-benzylalcohol for 2-propanol in part C. MS (M+1) = 426.41
O rJ O
N OH
H 23
64
CA 02657986 2009-03-10
N-(2,6-dimethylphenyl)-2-(4-{2-hydroxy-3-[(4-
phenylphenyl)methoxy]propyl}piperazinyl)acetamide (23C) Compound 23C was
prepared
in a similar manner to compound 7C, substituting the commercially available 4-
phenyl-
benzylalcohol for 2-propanol in part C. MS
(M+1) = 488.42
N-"~O
NK~N J OH
H
24
N-(2,6-dimethylphenyl)-2-(4-{3-[(4-butylphenyl)methoxy]-2-
hydroxypropyl}piperazinyl)acetamide (24C): Compound 24C was prepared in a
similar
manner to compound 7C, substituting the commercially available 4-n-bu-
benzylalcohol for 2-
propanol in part C. MS (M+l) = 468.45
cxLCrC0iX
H 25
N-(2,6-dimethylphenyl)-2-{4- [2-hydroxy-3-(2-
naphthylmethoxy)propyl]piperazinyl}acetamide (25C) Compound 25C was prepared
in a
similar manner to compound 7C, substituting the commercially available 2-
naphthylmethanol
for 2-propanol in part C. MS (M+1) = 462.41
H
NH
O "0
26
CA 02657986 2009-03-10
1V -(2,6-dimethylp henyl)-2- {4- [3-(cyclohexylmeth oxy)-2-
hydroxypropyl]piperazinyl}acetamide (26C) Compound 26C was prepared in a
similar
manner to compound 7C, substituting the commercially available
cyclohexylmethanol for 2-
propanol in part C. MS (M+1) = 418.55
H
I \ ~N H
/ O N O
27
N-(2,6-dimethylphenyl)-2-(4-{3-[(4-fluorophenyl)methoxy]-2-hydroxypropyl}-3,3-
dimethylpiperazinyl)acetamide (27C) Compound 27C was prepared in a similar
manner to
compound 7C, substituting the commercially available 4-fluorobenzylalcohol for
2-propanol
in part C and 2,2-dimethylpiperazine for compound 4 part B. MS (M+1) = 458.5
66
CA 02657986 2009-03-10
Example 7
Mitochondrial Assays
Rat heart mitochondria were isolated by the method of Nedergard and Cannon
(Methods in Enzymol. 55, 3, 1979).
Palmitoyl CoA oxidation - The Palmityl CoA oxidation was carried out in a
total
volume of 100 micro liters containing the following agents: 110 mM KCI, 33 mM
Tris buffer
at pH 8, 2 mM KPi, 2 mM MgCIZ1 0.1 mM EDTA, 14.7 microM defatted BSA, 0.5 mM
malic
acid, 13 mM carnitine, 1 mM ADP, 52 micrograms of mitochondrial protein, and
16 microM
1-C14 palmitoyl CoA (Sp. Activity 60 mCi/mmole; 20 microCi/ml, using 5
microliters per
assay). The compounds of this invention were added in a DMSO solution at the
following
concentrations: 100 microM, 30 microM, and 3 microM. In each assay, a DMSO
control was
used. After 15 min at 30 oC, the enzymatic reaction was centrifuged (20,000 g
for 1 min),
and 70 microliters of the supernatant was added to an activated reverse phase
silicic acid
column (approximately 0.5 ml of silicic acid). The column was eluted with 2 ml
of water,
and 0.5 ml of the eluent was used for scintillation counting to determine the
amount of C14
trapped as C14 bicarbonate ion.
Table 1
Inhibition of mitochondrial fatty acid oxidation using palmitoyl CoA as
substrate -% of
Control at 3 concentrations.
Compound # 100 M 30 M 3 M
Ranolazine 75% 90% --
14 -- -- --
7 85% 98% 107%
15 78% 97% 103%
17 89% 98% 100%
16 100% 96% --
18 17%
19 -
22 25%
23 -
9B 84% 84% --
l OB -- -- --
7B -- -- --
11B 83% 92% --
12B 42% 95%
136 -- -- --
16B 37%
17B 78%
18B 78%
19B 35%
67
CA 02657986 2009-03-10
20B 56%
21B 56%
23B 70%
24B 72%
lOC 100% 97% --
7C 68% -- --
11 C 79% -- --
12C 41%
-- --
13C 30% -- --
14C 21% - -
15C 100% - -
16C 97% - -
17C 35% - -
18C 96% - -
19C 97% - -
20C 100% - -
21C 87% - -
22C 45% - -
23C 12% - -
24C 15% - -
25C 38% - -
26C 70% - -
27C 73% - -
Example 8
Palmitoyl Carnitine Oxidation
The Palmitoyl carnitine oxidation was carried out in a total volume of 100
microliters
containing the following agents: 110 mM KCI, 33 mM Tris buffer at pH 8, 2 mM
KPi, 2 mM
MgCIZ1 0.1 mM EDTA, 0.1 mg/ml of defatted BSA, 0.5 mM malic acid, 3 mM ADP, 52
micrograms of mitochondrial protein, and 43 microM 1-C14 palmitoyl camitine
(Sp. Activity
60 mCi/mmole; 20 microCi/ml, using 5 microliters per assay). The compounds of
this
invention were added in a DMSO solution at the following concentrations: 100
microM, 30
microM, and 3 microM. In each assay, a DMSO control was used. After 15 min at
30 C, the
enzymatic reaction was centrifuged (20,000 g for 1 min), and 70 microliters of
the supematant
was added to an activated reverse phase silicic acid column (approximately 0.5
ml of silicic
acid). The column was eluted with 2 ml of water, and 0.5 ml of the eluent was
used for
scintillation counting to determine the amount of C14 trapped as C14
bicarbonate ion. The data
are presented as % activity of control.
68
CA 02657986 2009-03-10
Table 2
Inhibition of mitochondrial fatty acid oxidation using palmitoyl carnitine as
substrate -
% of Control At 3 concentrations.
Compound # 100 M 30 12M 3 M
Ranolazine 63% 98% --
14 -- -- --
7 95% 102% 109%
15 82% 98% 106%
17 80% 88% 103%
16 64% 8 -- --
9B -- -- --
10B -- -- --
7B -- -- --
11 B -- -- --
12B 56% -- --
13 B -- -- --
lOC 80% -- --
7C -- -- --
11 C -- -- --
12C -- -- --
13 C -- -- --
69
CA 02657986 2009-03-10
Example 9
Metabolic Stability: As a measure of metabolic stability the compounds of this
invention
were incubated with human liver S-9 microsomal fractions. After, 30 minutes at
37 C, the
amount of parent drug remaining was determined using LC-mass spec. The
response factors
for each compound was determined by establishing a standard curve and using an
internal
standard during the analysis of the samples. An average of five experiments
for percentage of
ranolazine remaining at the 30 minute time point is 57%. The compounds of this
invention
were assayed as described in the protocol below and the percentage of parent
remaining was
divided by the average % of ranolazine remaining (57%) affording a metabolic
stability factor.
A compound with a stability number greater than 1.2 has a better stability
than ranolazine in
the liver S-9 assay. A compound with a stability number between 1.2 and 0.8
has an
equivalent stability in the liver S-9 assay. A compound with a stability
number less than 0.8
is less stable than ranolazine in the liver S-9 assay.
The purpose of this experiment is to compare the percentages remaining for
compounds of this invention with the percentage remaining for ranolazine after
30 minutes of
incubation with human liver S9 fractions.
Reagents:
The following reagents were used; Potassium phosphate, 0.5M pH 7.4 (incubation
buffer), kept at room temperature; 0.05M MgCl2 kept at 4 C; P-Nicotinamide
adenine
dinucleotide phosphate, tetrasodium salt, reduced form (NADPH), 0.02M solution
in water
(-16.6mg/mL) from Sigma Lot # 79H7044 prepared on day of use. 1mM of
ranolazine or
Compounds 43, 45, 47, 52, 70, 74, 76, 78, and 80 in ACN further diluted to
obtain 100 M in
10% ACN; Human S9 stock: 20mgImL from Gentest.
Procedure:
Incubation mixtures were prepared as follows:
CA 02657986 2009-03-10
Table 3
Component Volume per 0.25mL of Incubation Final
Mixture concentration
M CVT 25 L 10 M
compounds
MgC12 25 L 0.005 M
NADPH 25 L 0.002 M
S9 25 L 2 mg/mL
Incubation Buffer 25 L 0.05 M
Water 125 L ----
* 1% organic solvent (acetonitrile) was used in incubation mixture. Generally,
30 incubates
were prepared at a time by pre-mixing 0.75 niL of MgC121 0.75 mL of incubation
buffer, 0.75
5 mL of NADPH, 3.75 mL of water. Then pipette 200 L/incubate, add 25 L of
compound
being tested, mix, and initiate reaction by addition of S-9.
Combine all components with incubation buffer and re-pipette 200 L/tube +
25 L of the compound being tested along with 25 L of S-9.
10 After 5 min of pre-incubation at 37 C, at 0 and 30min after starting the
reaction, a 50
l aliquot of the incubation mixture was removed and added to 100 L of 9:1
acetonitrile:
methanol containing the internal standard.
The mixture was centrifuged and a 100 L aliquot of the supernatant was
diluted in
lmL of solvent C (0.1% Formic Acid in water). Then samples were analyzed for
change
between the ratio of compound to internal standard at time zero and 30 minutes
by LClMS
(injected 10 L).
Analytical and Data Calculations:
Samples were analyzed for the starting compounds and potential metabolite/s by
LC/MS using an internal standard and an ODS-C18 column with a flow rate of
0.25 mi/min.
Following the above procedure resulted in the following relative stability
factors as compared
to ranolazine for the compounds of this invention as illustrated in Table 4.
If a compound is
more stable than ranolazine in the liver S9 assay, than the stability factor
will be greater than
1Ø If a compound is less stable than ranolazine, than the stability factor
will be less than 1Ø
71
CA 02657986 2009-03-10
Table 4
Compound # Liver S9 Stabili Factor
Ranolazine 1.0
0.45
7 1.51
1.20
16 0.15
17 0.45
9B 1.18
lOB 1.03
7B 1.46
11B 1.33
12B 1.38
13B 0.10
16B 0.99
17B 0.71
18B 0.68
19B -
20B -
21B -
22B 1.49
23B 0.5
24B 1.05
25B -
26B -
27B -
21C --
22C 0.61
23C 0.05
24C 0.02
25C 0.01
26C --
27C --
72