Note: Descriptions are shown in the official language in which they were submitted.
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
INDUCING CELLULAR IMMUNE RESPONSES TO INFLUENZA VIRUS USING
PEPTIDE AND NUCLEIC ACID COMPOSITIONS
BACKGROUND OF THE INVENTION
[0001] The present invention relates to influenza virus vaccine compositions
and
methods of treating or preventing influenza infection and disease in mammals.
Influenza
is caused by an RNA virus of the myxovirus group. Influenza viruses can be
classified
into three types (A, B and C), based on antigenic differences in the
nucleoprotein and the
matrix protein. Type A, which includes several subtypes, causes widespread
epidemics
and global pandemics. Type B causes regional epidemics. Influenza C is less
severe and
has been isolated from humans and pigs. Type C causes sporadic cases and
minor, local
outbreaks. Influenza A viruses can be further classified based on the viral
surface
proteins hemagglutinin (HA or H) and neuraminidase (NA or N). There are
sixteen
known H subtypes and nine known N subtypes of Type A viruses; while there is
only
one known H subtype and one N subtype of Type B viruses. Typical nomenclature
identifies an influenza virus by both proteins, e.g., H3N2.
[0002] Type A and B influenza viruses each contain 8 RNA segments, while type
C only
has 7 RNA segments. Influenza A is most important and is very pathogenic for
man, as
well as for animals, for example pigs and horses. Type B influenza causes
disease in
humans. These virus types are distinguished in part on the basis of
differences in two
structural proteins, the nucleoprotein, found in the center of the virus, and
the matrix
protein, which forms the viral shell. The virus is transmitted through the
air, mainly in
droplets expelled during coughing and sneezing. The influenza viruses cause an
infection
of the respiratory tract, which is usually accompanied with coughing, high
fever and
myalgia.
[0003] Although an influenza infection does not often lead to the death of the
infected
individual, the morbidity can be severe. As a consequence thereof influenza
epidemics
may lead to substantial economic loss. Furthermore, influenza infection can be
more
dangerous for certain groups of individuals, such as those having suffered
from a heart
attack, CARA patients or the elderly. A vaccine against influenza is therefore
highly
desirable.
1
CA 02658559 2009-01-20
WO 2008/039267 2 PCT/US2007/016529
Influenza Epidemiology and Virology
[0004] Pandemics of influenza A viruses continue to occur at sporadic
intervals in
human populations. Three have occurred in the twentieth century alone in 1918,
1957
and 19686-8. These worldwide pandemics are noted for their high mortality with
rates
approaching 30-50%9. For example, it is estimated that 20-40 million people
died in the
1918 pandemic and at least 1.5 million people in the 1957 and 1968 outbreaks
combined10. Whether a pandemic occurs from an act of nature or from the
deliberate
release of a novel influenza strain with pandemic potential, the extent of
world travel will
ensure the rapid global spread of the pandemic agent. Such an event could
result in
world-wide deaths totaling in the millions and severely impact health care
systems such
that economies and governments of smaller countries could collapse9'1 1.
[0005] The capacity of the influenza virus to cause disease in a recurring
manner is due
to a complex set of factors that include: 1) the presence of an established
reservoir of
influenza A viruses of different subtypes in shorebirds and waterfowl; 2) the
ability of
avian influenza viruses to recombine with influenza viruses of other animals,
most
notably swine12, a process termed `antigenic shift'; 3) accumulation of
mutations in viral
gene products caused by a lack of proofreading activity of the viral RNA
polymerase, a
process termed `antigenic drift'. These reassortment and mutation events
combine to
cause the well-characterized antigenic variability in the two surface
glycoproteins of the
virus, hemagglutinin (HA) and neuraminidase (NA)13-" which provides the virus
a
mechanism for escaping immune responses, particularly neutralizing antibodies,
induced
as the result of previous infections or vaccinations. Antigenic shift, which
occurs only
among influenza A viruses, results in major antigenic change introducing
viruses with a
new gene segment(s). Antigenic shift can occur when an animal influenza A
virus is
transmitted directly to humans, such as the transmission of the H1N1 from
swine-to-
human16 or the transmission of the H5N1, H7N7 or H9N2 variants from avian to
human17'18. Alternatively, a virus may acquire a new gene segment(s) as a
result of
genetic reassortment between animal and human influenza A viruses, the cause
of the
1957 H2N2 and 1968 H3N2 pandemics19
[0006] Since 1997, several novel avian subtypes have crossed the so-called
species
barrier from domestic poultry to humans and have caused a spectrum of mild to
severe
and even fatal human disease. In 1997, 18 cases of human infection with highly
pathogenic avian H5N1 influenza viruses, including 6 deaths were documented in
Hong
Kong following outbreaks of disease in domestic poultry. Avian H5N1 viruses
CA 02658559 2009-01-20
WO 2008/039267 3 PCT/US2007/016529
reemerged in Hong Kong and from December 30, 2003 to March 17, 2004, there
were 12
human cases of confirmed H5N1 influenza in Thailand and 23 in Vietnam,
including 23
deaths. As of May 2006, approximately 115 deaths have been attributed to H5N1
infection. The H5N1 strain does not jump easily from birds to humans or
between
humans. However since the human virus, H3N2, can coexist with avian influenza
viruses and is widespread in pigs from southeast China, reassortment has the
potential to
occur with a highly pathogenic human-to-human transmissible H5N1 being the
result.
Although these wholly avian viruses were associated with only limited human-to-
human
transmission, their repeated emergence in humans highlights the potential for
the
generation of an avian-human reassortant virus with the potential for spread
in the human
population. Thus, the development of effective vaccines against these avian
subtypes is
of the highest public health priority.
[0007] Vaccine production must rely on surveillance programs to predict the
influenza
subtypes likely to have global impact on human health. The time required to
produce
subtype-matched vaccines, composed of inactivated or `split' virions,
typically requires a
minimum of 6-8 months. In the face of a serious influenza virus pandemic
caused by a
viral subtype, this lag time could allow for national or international spread
with
excessive morbidity and mortality.
Virus Structures
[0008] An influenza virus is roughly spherical, but it can also be elongated
or irregularly
shaped. Inside the virus, eight segments of single-stranded RNA contain the
genetic
instructions for making the virus. The most striking feature of the virus is a
layer of
spikes projecting outward over its surface. There are two different types of
spikes: one is
composed of the molecule hemagglutinin (HA), the other of neuraminidase (NA).
The
HA molecule allows the virus to "stick" to a cell, initiating infection. The
NA molecule
allows newly formed viruses to exit their host cell without sticking to the
cell surface or
to each other. The viral capsid is comprised of viral ribonucleic acid and
several so
called "internal" proteins (polymerase proteins (PB1, PB2, and PA), matrix
protein (Ml)
and nucleoprotein (NP)). Because antibodies against HA and NA have
traditionally
proved the most effective in fighting infection, much research has focused on
the
structure, function, and genetic variation of those molecules. Researchers are
also
interested in two non-structural proteins M2 and NS1; both molecules play
important
roles in viral infection.
CA 02658559 2009-01-20
WO 2008/039267 4 PCT/US2007/016529
[0009] Type A subtypes are described by a nomenclature system that includes
the
geographic site of discovery, a lab identification number, the year of
discovery, and in
parentheses the type of HA and NA it possesses, for example, A/Hong
Kong/156/97
(H5N1). If the virus infects non-humans, the host species is included before
the
geographical site, as in A/Chicken/Hong Kong/G9/97 (H9N2).
100101 Virions contain 7 segments (influenza C virus) to 8 segments (influenza
A and B
virus) of linear negative-sense single stranded RNA. Most of the segments of
the virus
genome code for a single protein. For many influenza viruses, the whole genome
is now
known. Genetic reassortment of the virus results from intermixing of the
parental gene
segments in the progeny of the viruses when a cell is co-infected by two
different viruses
of a given type. This phenomenon is facilitated by the segmental nature of the
genome
of influenza virus. Genetic reassortment is manifested as sudden changes in
the viral
surface antigens.
[0011] Antigenic changes in HA and NA allow the influenza virus to have
tremendous
variability. Antigenic drift is the term used to indicate minor antigenic
variations in HA
and NA of the influenza virus from the original parent virus, while major
changes in HA
and NA which make the new virions significantly different, are called
antigenic shift.
The difference between the two phenomena is a matter of degree.
100121 Antigenic drift (minor changes) occurs due to accumulation of point
mutations in
the gene which results in changes in the amino acids in the proteins. Changes
which are
extreme, and drastic (too drastic to be explained by mutation alone) result in
antigenic
shift of the virus. The segmented genomes of the influenza viruses reassort
readily in
double infected cells. Genetic reassortment between human and non-human
influenza
virus has been suggested as a mechanism for antigenic shift. Influenza is a
zoonotic
disease, and an important pathogen in a number of animal species, including
swine,
horses, and birds, both wild and domestic. Influenza viruses are transferred
to humans
from other species.
[0013] Because of antigenic shift and antigenic drift, immunity to an
influenza virus
carrying a particular HA and/or NA protein does not necessarily confer
protective
immunity against influenza.virus strains carrying variant, or different HA
and/or NA
proteins. Because antibodies against HA and NA have traditionally proved the
most
effective in fighting influenza virus infection, much research has focused on
the
structure, function and genetic variation of those molecules.
CA 02658559 2009-01-20
WO 2008/039267 5 PCT/US2007/016529
Role of Cellular Immune Responses in Protection Against Influenza
[0014] Cellular immune responses are known to contribute to the control of
viral
replication in vivo and to mediate viral clearance. In murine models,
influenza-specific
CD8+ cytotoxic T-lymphocytes (CTL) limit virus replication and protect against
lethal
virus challenge2o"27 . Recovery from infection correlated with virus-specific
CD8+ CTL
activity22 and lack of CD8+ CTL activity was associated with delayed viral
clearance and
increased mortality28. Studies completed by Ulmer and Okuda using a DNA
vaccine
encoding the viral nucleoprotein and M gene proteins, respectively are
particularly
relevant. These vaccines induced influenza-specific CD8+ CTL that provided
cross-
strain protection2' 29 3o The contribution of CTL and Helper T-lymphocytes
(HTL) was
definitively demonstrated by adoptive transfer of CD8+ and CD4+ T-
lymphocytes31.
Similarly, Epstein and colleagues demonstrated that either CD8+ or CD4+ T-
lymphocytes
promoted survival in mice immunized with an experimental DNA vaccine encoding
internal viral proteins32. Finally, virus-specific HTL augment the generation
of CTL and
size of the CTL memory pool, an effect known to be associated with long term
protection33. Cellular immune responses clearly contribute to the control and
clearance
of infection and reduce pathogenesis.
[0015] The exposure to an influenza virus of one subtype often induces immune
responses that protect against infection or disease with another subtype, a
phenomena
referred to as Heterosubtypic Immunity (HSI)34-37 The mechanisms of
heterosubtypic
immunity appears to involve functional activity of both CD8+ and CD4+ T-
lymphocytes23,26'38-a1 , although more recently antibody responses have also
been
implicated4z. HSI is not only observed using the murine models; influenza
virus-specific
CTL appear to provide partial protection against multiple influenza A virus
strains in
humans. Early human studies demonstrated that cellular immune responses play a
role
in controlling influenza infectiona3,aa McMichael and colleagues inoculated 63
volunteers intranasally with live unattenuated influenza A/Munich/1/79 virus
and
evaluated the protective effects of serum antibody and cytotoxic T-cell
immunity against
influenza.43 It was found that all subjects with demonstrable T-cell responses
cleared
virus effectively. Sonoguchi and colleagues found that students previously
infected with
H3N2 virus were partially protected against subsequent infection with H1N1
subtype
virus suggesting cross-subtype protection in humans during sequential
epidemics. Thus,
the use of vaccines to induce cellular responses against pandemic influenza
virus is
logical and the development of suitable vaccine technologies is warranted.
CA 02658559 2009-01-20
WO 2008/039267 6 PCT/US2007/016529
[0016] Immune system-mediated selection pressure on influenza virus can lead
to CTL
viral escape mutantsa5-a7 While this phenomena clearly documents the
importance of
virus-specific CTL it also reveals a potential limitation for vaccines
designed to induce
CTL responses. However, the use of carefully selected epitopes in the design
of a
vaccine provides a means to address this problem. Selection of epitopes that
are highly
conserved amongst multiple viral strains is the first step and the selection
of those
epitopes predicted to be capable of inducing CTL responses to the majority of
related
epitopes is the second step.
Role of Humoral Immune Responses in Protection Against Influenza
[00171 Influenza vaccines are formulated to include human influenza strains
predicted to
pose the greatest risk for infectious spread. This vaccine development process
requires
approximately 6-8 months using conventional strains. Neutralizing antibodies
induced
primarily to the surface hemagglutinin protein by the conventional vaccines
are highly
protective. However, due to antigenic drift of the virus, the vaccines must be
reformulated on a yearly basis. The danger persists that a "new" strain will
emerge by
antigenic shift for which the human population has little or no pre-existing
immunity.
Also, since vaccine production relies on embryonated chicken eggs or
potentially cells in
tissue culture, there are no assurances that sufficient new virus can be
produced even
within the 6-8 month time frame especially if the new influenza strain is
lethal to birds.
Pandemic influenza vaccine development would benefit by inclusion of conserved
B cell
epitopes capable of inducing protective immune responses. To this end, it has
been
reported that the external domain of the transmembrane viral M2 protein is
highly
conserved and that antibodies directed to this epitope are protective in
mice48-54. The M2
protein is an integral membrane protein of influenza A virus that is expressed
at the
plasma membrane in virus-infected cells. Due to the low abundance of the
protein in the
virus, the mechanism of protection of the antibody response directed against
this epitope
is not mediated via viral neutralization but rather by antibody-dependent,
cell-mediated
cytotoxicitys ~ .
[0018] Conserved CTL, HTL and B-cell epitopes can be used as the basis for a
vaccine
designed to augment and improve prototype pandemic vaccine candidates that may
be
poorly immunogenic or a sub-optimal match against a pandemic strain that
emerges.
The advantages to using defined epitopes in vaccines are many but one
advantage is that
many epitopes can be incorporated into a vaccine to induce a broadly specific
immune
CA 02658559 2009-01-20
WO 2008/039267 7 PCT/US2007/016529
response targeting numerous viral gene products. Data from natural infection
studies
wherein human memory CTL specific to influenza A virus were restricted by
multiple
HLA Class I alleles have shown that responses within a given individual were
broadly
directed to epitopes within the NP, NA, HA, M1, NS1 and M2 viral proteins.
Design and Testing of Vaccines to Induce Cellular and Humoral Immune
Responses:
[0019] The use of recombinant DNA technology to produce influenza vaccines
offers
several advantages: a recombinant DNA influenza vaccine can be produced under
safer
and more stringently controlled conditions; propagation with infectious
influenza in eggs
is not required; recombinant protein can be more highly purified, virtually
eliminating
side effects due to contaminating proteins; purification procedures for
recombinant
protein does not have to include virus inactivation or organic extraction of
viral
membrane components, therefore avoiding denaturation of antigens and
additional safety
concerns due to residual chemicals in the vaccine. Production of protein via
recombinant
DNA technology provides an opportunity to avoid the genetic heterogeneity
which
occurs during adaptation and passage through eggs, which should make it
possible to
better match vaccine strains with influenza epidemic strains, resulting in
improved
efficacy; and a recombinant approach may also allow for strain selection later
in the year,
thereby allowing time for selections based on more reliable epidemiological
data.
[0020] A major obstacle to the development of vaccines that induce immune
responses is
the selection of a suitable delivery format. DNA plasmid vaccines and viral
vectors,
used either alone or together, and recombinant protein or peptides are logical
vaccine
delivery formats; however, each format has advantages and disadvantages. For
example,
DNA vaccines are readily produced and safe to administer but potency has been
lacking,
especially in clinical trials, requiring the administration of large
(milligram) doses59 65
Studies completed in small animals have indicated increased vaccine potency66-
69.
Polymer formulation technology based on polyvinylpyrrolidone (PVP) can also be
utilized. PVP is a nontoxic formulation excipient used to enhance DNA plasmid
uptake
by muscle cells70-'3. Such vaccine design parameters can correct for at least
some of the
limitations of naked-DNA vaccine technology.
[0021] The use of viral vectors to deliver vaccines has raised concerns,
usually related to
safety and pre-existing immunity to the vector. However, A1phaVax replicons
are
reported to be safe, non-transmissible and there is a general lack of pre-
existing
immunity to the vector. Another delivery vehicle that is being evaluated is
peptides in
CA 02658559 2009-01-20
WO 2008/039267 8 PCT/US2007/016529
adjuvant. Generally, peptides in adjuvant have shown to be immunogenic and
efficacious in humans. However, there are concerns regarding vaccine
formulation
wherein high numbers of peptides will need to be delivered.
[0022] Several adjuvants have been developed for the administration of
influenza virus
vaccines, including alum based compounds, emulsions (e.g. MF59), (lipophilic
immune
stimulating complexes ISCOMS) containing Quil A adjuvant) and liposomes. A
development of the liposomal technique has been the use of immunopotentiating
reconstituted influenza virosomes (IRIVs) as antigen delivery systems. See
Mischler, R.
and Metcalfe, I.C., Vaccine 20: B17-B23 (2002). The IRIV vaccine delivery
system is
comprised of spherical unilamellar vesicles comprising naturally occurring
phospholipids (PL) and phosphatidylcholine (PC) and envelope phospholipids
originating from influenza virus used to provide influenza virus NA and HA
glycoproteins. See id. The fusion mechanism of IRIVs enables stimulation of
the MHC
Class I or Class II pathway, depending upon how antigens are presented to the
APCs.
Virosomes are able to induce either a B- or T-cell response. See id.
[0023] The use of antigenic epitopes in vaccines has several advantages over
current
vaccines, particularly when compared to the use of whole antigens in vaccine
compositions. There is evidence that the immune response to whole antigens is
directed
largely toward variable regions of the antigen, allowing for immune escape due
to
mutations. The epitopes for inclusion in an epitope-based vaccine may be
selected from
conserved regions of influenza antigens, which thereby reduces the likelihood
of escape
mutants. Furthermore, immunosuppressive epitopes that may be present in whole
antigens can be avoided with the use of epitope-based vaccines.
An additional advantage of an epitope-based vaccine approach is the ability to
combine
selected epitopes (e.g., multiple CTL and/or HTL epitopes), and further, to
modify the
composition of the epitopes, achieving, for example, enhanced immunogenicity.
Accordingly, the immune response can be modulated, as appropriate, for the
target
disease. Similar engineering of the response is not possible with traditional
approaches.
[0024] Several groups have established the mouse model as a tool for
evaluating the
efficacy of influenza vaccines26"31'74'75 The testing of vaccines comprised of
epitopes
restricted by HLA is a unique challenge, requiring the appropriate restriction
elements.
Specifically cell-surface expressed HLA Class I molecules for CTL epitopes and
HLA
Class II molecules for HTL epitopes on antigen presenting cells are required.
With
respect to CTL epitopes, HLA-A*0201, -A*1101 and -B*0702 transgenic mice have
CA 02658559 2009-01-20
WO 2008/039267 9 PCT/US2007/016529
been developed as representative of the HLA-A2, -A3 and -B7 supertype
families,
respectively76-7g. The utility of these HLA transgenic mice for testing DNA,
viral,
protein and peptide vaccines have been validated 68'79. Three other transgenic
lines
(HLA-A*0101, HLA-A*2402 and HLA-B*4002, representing HLA-A1, -A24 and B44
supertype families, respectively) are being developed and can be utilized to
evaluate the
efficacy of vaccines using the established murine challenge models. With
regard to
evaluating Class II-restricted responses, HLA-DR4 mice are available
commercially.
Most HTL epitopes restricted to HLA Class II can bind murine H-2 IAb molecules
and
initiate a response80.
100251 Virus-specific, human leukocyte antigen (HLA) class I-restricted
cytotoxic T
lymphocytes (CTL) are known to play a major role in the prevention and
clearance of
virus infections in vivo (Oldstone, et al., Nature 321:239, 1989; Jamieson, et
al., J. Virol.
61:3930, 1987; Yap, et al., Nature 273:238, 1978; Lukacher, et al., J. Exp.
Med.
160:814, 1994; McMichael, et al., N. Engl. J Med. 309:13, 1983; Sethi, et al.,
J. Gen.
Virol. 64:443, 1983; Watari, et al., J. Exp. Med. 165:459, 1987; Yasukawa, et
al., J.
Immunol. 143:2051, 1989; Tigges, et al., J. Virol. 66:1622, 1993; Reddenhase,
et al., J
Virol. 55:263, 1985; Quinnan, et al., N. Engl. J. Med. 307:6, 1982). HLA class
I
molecules are expressed on the surface of almost all nucleated cells.
Following
intracellular processing of antigens, epitopes from the antigens are presented
as a
complex with the HLA class I molecules on the surface of such cells. CTL
recognize the
peptide-HLA class I complex, which then results in the destruction of the cell
bearing the
HLA-peptide complex directly by the CTL and/or via the activation of non-
destructive
mechanisms e.g., the production of interferon, that inhibit viral replication.
[0026] Virus-specific T helper lymphocytes are also known to be critical for
maintaining.
effective immunity in chronic viral infections. Historically, HTL responses
were viewed
as primarily supporting the expansion of specific CTL and B cell populations;
however,
more recent data indicate that HTL may directly contribute to the control of
virus
replication. For example, a decline in CD4+ T cells and a corresponding loss
in HTL
function characterize infection with HIV (Lane, et al., N. Engl. J Med.
313:79, 1985).
Furthermore, studies in HIV infected patients have also shown that there is an
inverse
relationship between virus-specific HTL responses and viral load, suggesting
that HTL
plays a role in controlling viremia (see, e.g., Rosenberg, et al., Science
278:1447, 1997).
[0027] The epitope approach, as we describe herein, allows the incorporation
of various
antibody, CTL and HTL epitopes, from various proteins, in a single vaccine
CA 02658559 2009-01-20
WO 2008/039267 10 PCT/US2007/016529
composition. Such a composition may simultaneously target multiple dominant
and
subdominant epitopes and thereby be used to achieve effective immunization in
a diverse
population.
[0028] The technology relevant to multi-epitope ("minigene") vaccines is
developing.
Several independent studies have established that induction of simultaneous
immune
responses against multiple epitopes can be achieved. For example, responses
against a
large number of T cell specificities can be induced and detected. In natural
situations,
Doolan, et al. (Immunity, Vol. 7(1):97-112 (1997)) simultaneously detected
recall T cell
responses, against as many as 17 different P. falciparum epitopes using PBMC
from a
single donor. Similarly, Bertoni and colleagues (J. Clin. Invest., 100(3):503-
13 (1997))
detected simultaneous CTL responses against 12 different HBV-derived epitopes
in a
single donor. In terms of immunization with multi-epitope nucleic acid
vaccines, several
examples have been reported where multiple T cell responses were induced. For
example, minigene vaccines composed of approximately ten MHC Class I epitopes
in
which all epitopes were immunogenic and/or antigenic have been reported.
Specifically,
minigene vaccines composed of 9 EBV (Thomson, et al., Proc. Natl. Acad. Sci.
USA,
92(13):5845-49 (1995)), 7 HIV (Woodberry, et al., J. Virol., 73(7):5320-25
(1999)), 10
murine (Thomson, et al., J. Immunol., 160(4):1717-23 (1998)) and 10 tumor-
derived
(Mateo, et al., J. Immunol., 163(7):4058-63 (1999)) epitopes have been shown
to be
active. It has also been shown that a multi-epitope DNA plasmid encoding nine
different
HLA-A2.1- and All-restricted epitopes derived from HBV and HIV induced CTL
against all epitopes (Ishioka, et al., J. Immunol., 162(7):3915-25 (1999)).
[0029] Recently, several multi-epitope DNA plasmid vaccines specific for HIV
have
entered clinical trials (Nanke, et al., Nature Med., 6:951-55 (2000); Wilson,
C.C., et al.,
J. Immunol. 17](10):5611-23 (2003).
[0030] Thus, vaccines containing multiple MHC Class I (i.e., CTL) and Class II
(i.e.,
HTL) epitopes can be designed, and presentation and recognition can be
obtained for all
epitopes. However, the immunogenicity of such multi-epitope constructs appears
to be
strongly influenced by a number of variables, a number of which have
heretofore been
unknown. For example, the immunogenicity (or antigenicity) of the same epitope
expressed in the context of different vaccine constructs can vary over several
orders of
magnitude. Thus, there exists a need to identify strategies to optimize such
multi-epitope
containing vaccine constructs. Such optimization is important in terms of
induction of
potent immune responses and ultimately, for clinical efficacy. Accordingly,
the present
CA 02658559 2009-01-20
WO 2008/039267 11 PCT/US2007/016529
invention provides strategies to optimize antigenicity and immunogenicity of
multi-
epitope vaccines encompassing a certain number of epitopes. The present
invention also
provides optimized multi-epitope containing vaccines, particularly minigene
vaccines,
generated in accordance with these strategies. [0031] The following paragraphs
provide a brief review of some of the main variables
potentially influencing minigene immunogenicity, epitope processing, and
presentation
on antigen presenting cells (APCs) in association with Class I and Class II
MHC
molecules.
Immunodominance
[0032] Of the many thousand possible peptides that are encoded by a complex
foreign
pathogen, only a small fraction ends up in a peptide form capable of binding
to MHC
Class I antigens and thus of being recognized by T cells. This phenomenon, of
obvious
potential impact on the development of a multi-epitope vaccine, is known as
immunodominance (Yewdell et al., Annu Rev Immunol, 17:51-88 (1999)). Several
major
variables contribute to immunodominance. Herein, we describe variables
affecting the
generation of the appropriate peptides, both in qualitative and quantitative
terms, as a
result of intracellular processing.
Junctional Epitopes
[0033] A junctional epitope is defined as an epitope created due to the
juxtaposition of
two other epitopes. The new epitope is composed of a C-terminal section
derived from a
first epitope, and an N-terminal section derived from a second epitope.
Creation of
junctional epitopes is a potential problem in the design of multi-epitope
minigene
vaccines, for both Class I and Class II restricted epitopes for the following
reasons.
Firstly, when developing a minigene composed of, or containing, human
epitopes, which
are typically tested for immunogenicity in HLA transgenic laboratory animals,
the
creation of murine epitopes could create undesired immunodominance effects.
Secondly,
the creation of new, unintended epitopes for human HLA Class I or Class II
molecules
could elicit in vaccine recipients, new T cell specificities that are not
expressed by
infected cells or tumors that are the targets-induced T cell responses. These
responses
are by definition irrelevant and ineffective and could even be
counterproductive, by
creating undesired immunodominance effects.
CA 02658559 2009-01-20
WO 2008/039267 12 PCT/US2007/016529
[0034] The existence of junctional epitopes has been documented in a variety
of different
experimental situations. Gefter and collaborators first demonstrated the
effect in a
system in which two different Class II restricted epitopes were juxtaposed and
colinearly
synthesized (Perkins et al., J Immunol, 146(7):2137-44 (1991)). The effect was
so
marked that the immune system recognition of the epitopes could be completely
"silenced" by these new junctional epitopes (Wang et al., Cell Immunol,
143(2):284-97
(1992)). Helper T cells directed against junctional epitopes were also
observed in
humans as a result of immunization with a synthetic lipopeptide, which was
composed of
an HLA-A2-restricted HBV-derived immunodominant CTL epitope, and a universal
Tetanus Toxoid-derived HTL epitope (Livingston et al., J Immunol, 159(3):1383-
92
(1997)). Thus, the creation of junctional epitopes is a major consideration in
the design
of multi-epitope constructs.
[0035] The present invention provides methods of addressing this problem and
avoiding
or minimizing the occurrence of junctional epitopes.
Flanking Regions
[0036] Class I restricted epitopes are generated by a complex process (Yewdell
et al.,
Annu Rev Immunol, 17:51-88 (1999)). Limited proteolysis involving
endoproteases and
potential trimming by exoproteases is followed by translocation across the
endoplasmic
reticulum (ER) membrane by transporters associated with antigen processing
(TAP)
molecules. The major cytosolic protease complex involved in generation of
antigenic
peptides, and their precursors, is the proteosome (Niedermann et al.,
Immunity, 2(3):289-
99 (1995)), although ER trimming of CTL precursors has also been demonstrated
(Paz et
al., Immunity 11(2):241-51 (1999)). It has long been debated whether or not
the residues
immediately flanking the C and N terminus of the epitope, have an influence on
the
efficiency of epitope generation.
[0037] The yield and availability of processed epitope has been implicated as
a major
variable in determining immunogenicity and could thus clearly have a major
impact on
overall minigene potency in that the magnitude of immune response can be
directly
proportional to the amount of epitope bound by MHC and displayed for T cell
recognition. Several studies have provided evidence that this is indeed the
case. For
example, induction of virus-specific CTL that is essentially proportional to
epitope
density (Wherry et al., Jlmmunol, 163(7):3735-45 (1999)) has been observed.
Further,
recombinant minigenes, which encode a preprocessed optimal epitope, have been
used to
CA 02658559 2009-01-20
WO 2008/039267 13 PCT/US2007/016529
induce higher levels of epitope expression than naturally observed with full-
length
protein (Anton et al., Jlmmunol, 158(6):2535-42 (1997)). In general, minigene
priming
has been shown to be more effective than priming with the whole antigen
(Restifo et al.,
J Immunol, 154(9):4414-22 (1995); Ishioka et al., J Immunol, Vol. 162(7):3915-
25
(1999)), even though some exceptions have been noted (Iwasaki et al., Vaccine,
17(15-
16):2081-8 (1999)).
[0038] Early studies concluded that residues within the epitope (Hahn et al.,
JExp Med,
176(5):1335-41 (1992)) primarily regulate immunogenicity. Similar conclusions
were
reached by other studies, mostly based on grafting an epitope in an unrelated
gene, or in
the same gene, but in a different location (Chimini et al., J Exp Med,
169(1):297-302
(1989); Hahn et al., JExp Med, 174(3):733-6 (1991)). Other experiments however
(Del
Val et al., Cell, 66(6):1145-53 (1991); Hahn et al., J. Exp Med, 176(5):1335-
41 (1992)),
suggested that residues localized directly adjacent to the CTL epitope can
directly
influence recognition (Couillin et al., J Exp Med, 180(3):1129-34 (1994);
Bergmann et
al., J Virol. 68(8):5306-10 (1994)). In the context of minigene vaccines, the
controversy
has been renewed. Shastri and coworkers (Shastri et al., J Immunol,
155(9):4339-46
(1995)) found that T cell responses were not significantly affected by varying
the N-
terminal flanking residue but were inhibited by the addition of a single C-
terminal
flanking residue. The most dramatic inhibition was observed with isoleucine,
leucine,
cysteine, and proline as the C-terminal flanking residues. In contrast,
Gileadi (Gileadi et
al., Eur Jlmmunol, 29(7):2213-22 (1999)) reported profound effects as a
function of the
residues located at the N terminus of mouse influenza virus epitopes. Bergmann
and
coworkers found that aromatic, basic and alanine residues supported efficient
epitope
recognition, while G and P residues were strongly inhibitory (Bergmann et al.,
J
Immunol, 157(8):3242-9 (1996)). In contrast, Lippolis (Lippolis et al., J
Virol, Vol.
69(5):3134-46 (1995)) concluded that substituting flanking residues did not
affect
recognition. However, only rather conservative substitutions which are
unlikely to affect
proteosome specificity, were tested.
[0039] It appears that the specificity of these effects, and in general of
natural epitopes,
roughly correlates with proteosome specificity. For example, proteosome
specificity is
partly trypsin-like (Niedermann et al., Immunity, 2(3):289-99 (1995)), with
cleavage
following basic amino acids. Nevertheless, efficient cleavage of the carboxyl
side of
hydrophobic and acidic residues is also possible. Consistent with these
specificities are
the studies of Sherman and collaborators, which found that an R to H mutation
at the
CA 02658559 2009-01-20
WO 2008/039267 14 PCT/US2007/016529
position following the C-terminus of a p53 epitope affects proteosome-mediated
processing of the protein (Theobald et al., J Exp Med, 188(6):1017-28 (1998)).
Several
other studies (Hanke et al., J Gen Virol, 79 (Pt 1):83-90 (1998); Thomson et
al., Proc
Natl Acad Sci USA, 92(13):5845-9 (1995)) indicated that minigenes can be
constructed
utilizing minimal epitopes, and that these flanking sequences appear not be
required,
although the potential for further optimization by the use of flanking regions
was also
acknowledged.
[0040] HLA Class II peptide complexes are also generated as a result of a
complex
series of events that is generally distinct from HLA Class I processing. The
processing
pathway involves association with Invariant chain (Ii), its transport to
specialized
compartments, the degradation of Ii to CLIP, and HLA-DM catalyzed removal of
CLIP
(see Blum et al., Crit Rev Immunol, 17(5-6):411-7 (1997); Arndt et al.,
Immunol Res,
16(3):261-72 (1997)) for review. Moreover, there is potentially crucial role
of various
cathepsins in general, and cathepsin S and L in particular, in Ii degradation
(Nakagawa et
al., Immunity, 10(2):207-17 (1999)). In terms of generation of functional
epitopes
however, the process appears to be somewhat less selective (Chapman H. A.,
Curr Opin
Immunol, 10(1):93-102 (1998)), and peptides of many sizes can bind to MHC
Class I/II
(Hunt et al., Science, 256(5065):1817-20 (1992)). Most or all of the possible
peptides
appear to be generated (Moudgil et al., Jlmmunol, 159(6):2574-9 (1997); and
Thomson
et al., J Virol, 72(3):2246-52 (1998)). Thus, as compared to the issue of
flanking
regions, the creation of junctional epitopes can be a more serious concern in
particular
embodiments.
[0041] One of the most formidable obstacles to the development of broadly
efficacious
epitope-based immunotherapeutics, however, has been the extreme polymorphism
of
HLA molecules. To date, effective non-genetically biased coverage of a
population has
been a task of considerable complexity; such coverage has required that
epitopes be used
that are specific for HLA molecules corresponding to each individual HLA
allele.
Impractically large numbers of epitopes would therefore have to be used in
order to
cover ethnically diverse populations. Thus, there has existed a need for
peptide epitopes
that are bound by multiple HLA antigen molecules for use in epitope-based
vaccines.
The greater the number of HLA antigen molecules bound, the greater the breadth
of
population coverage by the vaccine.
[0042] Furthermore, as described herein in greater detail, a need has existed
to modulate
influenza virus peptide binding properties, e.g., so that influenza virus
peptides that are
CA 02658559 2009-01-20
WO 2008/039267 15 PCT/US2007/016529
able to bind to multiple HLA antigens do so with an affinity that will
stimulate an
immune response. Identification of influenza virus epitopes restricted by more
than one
HLA allele at an affinity that correlates with immunogenicity is important to
provide
thorough population coverage, and to allow the elicitation of responses of
sufficient
vigor to prevent or clear an infection in a diverse segment of the population.
Such a
response can also target a broad array of epitopes. In certain embodiments,
the
technology disclosed herein provides for such favored immune responses.
Accordingly,
the present invention provides multi-epitope vaccine constructs optimized for
immunogenicity and antigenicity, and methods of designing such constructs.
[0043] The unpredictable emergence of novel subtypes of influenza virus
exposes us to
variants against which we possess little or no preexisting immunity. The
resulting
morbidity, mortality and economic strain on the world can be devastating. As a
result of
global surveillance, the human influenza strains that pose the greatest risk
for infectious
spread during yearly epidemics can be predicted and the annual influenza
vaccines can
be reformulated accordingly. The vaccine development process requires
approximately
6-8 months and despite this effort, an average of 36,000 people still die
annually from
complications of influenza infection in the United States alonel. In the event
of a natural
influenza pandemic, or an intentional introduction of a highly pathogenic
subtype, it is
likely that significantly higher numbers of deaths will occur. The Spanish
influenza
pandemic of 1918 killed 675,000 people in the USA and 20-40 million
individuals
worldwide2. Epidemiological modeling suggests that the next pandemic will
cause
between 2-7.4 million deaths worldwide3. The increasing emergence of H5N1
viruses in
the Far East and potential adaptation of these agents to human systems is just
one
example of potential influenza strains that could initiate the next pandemic.
To address
such a catastrophe a vaccine product capable of inducing significant levels of
efficacy
rapidly upon administration must be designed and manufactured prior to the
beginning of
a pandemic.
100441 The present invention is thus directed to the design and production of
vaccines
that are capable of inducing immune responses specific for regions within
viral gene
products, or epitopes, that are conserved amongst the most divergent of
influenza
subtypes. Vaccines according to the invention are based on conserved cytotoxic
T-
lymphocyte (CTL), helper T-lymphocyte (HTL) and B-lymphocyte influenza-
specific
epitopes which can be designed and effective at rapidly inducing immune
responses
upon administration. While vaccine induction of cellular immunity alone will
not
CA 02658559 2009-01-20
WO 2008/039267 16 PCT/US2007/016529
provide optimal levels of protection, cellular immune responses may contribute
to the
initial control of viral replication and thus reduce disease progression in
individuals and
slow viral spread within a vaccinated population. A vaccine designed to induce
conserved cellular and humoral responses is used to supplement conventional
influenza
vaccines which are designed to induce protective neutralizing antibodies.
Several
prototype candidate strains for conventional vaccines against a number of
novel avian
influenza subtypes are currently under preparation, but may be a suboptimal
antigenic
match when an actual pandemic strain emerges. Recent clinical trials have also
demonstrated that such avian HA-based vaccines may be poorly immunogenic and
additional strategies to optimize immunogenicity of these vaccines are
needed4'5. The
induction of CTL and HTL responses using selected and highly immunogenic
epitopes
should augment the immunogenicity of such protein-based vaccines.
BRIEF SUMMARY OF THE INVENTION
[0045] The present invention is directed to the identification of CTL and HTL
epitopes
from viral gene sequences that are restricted by multiple HLA types with
predictable
levels of immunogenicity. The selection of epitopes restricted to multiple
related HLA
types, a phenomenon referred to as supertype restriction, provides a mechanism
to
achieve non-ethnically biased population coverage. The present invention is
also
directed to the development of a vaccine encompassing CTL, HTL and B-cell
epitopes
derived from influenza viral isolates from avian, porcine and human sources
which are
potential components of a pandemic influenza virus.
[0046] In certain embodiments, the present invention is directed to the
identification of
conserved HLA Class I and II-restricted peptides derived from influenza
subtypes that
have the potential to initiate pandemic influenza disease using established
motif search
algorithms and HLA-peptide binding assays. In further embodiments, the
invention
relates to the identification of epitopes that are naturally processed and
presented to the
immune system using peptides identified as high affinity binders to HLA
molecules and
peripheral blood mononuclear cells (PBMC) from normal human donors and HLA
transgenic mice.
CA 02658559 2009-01-20
WO 2008/039267 17 PCT/US2007/016529
[0047] In other embodiments, the present invention is directed to the design
and
optimization of an influenza virus vaccine for immunogenicity using nucleic
acids or
peptides, including, e.g., DNA plasmids, AlphaVax replicons, liposomes,
virosomes and
peptide vaccines.
[0048] In further embodiments, the present invention is directed to evaluating
the
efficacy of the experimental vaccines alone and in combination with
recombinant HA
protein using HLA transgenic mice and infectious challenges and the
identification of an
effective rapid vaccination schedule.
[0049] The present invention is also directed to the development of a single
epitope-
based vaccine, delivered using a DNA plasmid, viral vector and/or peptides
suitable for
preclinical development, e.g., as liposomes, virosomes, or other
pharmaceutically
.acceptable carriers. In certain embodiments, this vaccine product will not
induce
neutralizing antibody responses and may therefore be designed for use in
combination
with protein or inactivated viral vaccines. The unique advantage to this
approach is that
an epitope-based vaccine can be produced prospectively for administration to
at-risk
populations while the more conventional vaccines are being produced.
[0050] The present invention is further directed to enhancing the immune
response of a
vertebrate in need of protection against influenza virus infection by
administering in
vivo, into a tissue of the vertebrate, at least one multi-epitope construct,
wherein the
multi-epitope construct comprises an influenza virus CTL and/or HTL epitope,
and
wherein the multi-epitope construct is capable of eliciting an inunune
response.
[0051] In certain embodiments, the invention is directed to a polynucleotide
selected
from the group consisting of:
(a) a multi-epitope construct comprising between five and fifty nucleic acids,
each
encoding an influenza virus cytotoxic T lymphocyte (CTL) epitope, wherein the
CTL
epitope is any one of the epitopes listed in Tables 1-17, and wherein the
nucleic acids are
directly or indirectly joined to one another in the same reading frame;
(b) a multi-epitope construct comprising between five and fifty nucleic acids,
each
encoding an influenza virus cytotoxic T lymphocyte (CTL) epitope, wherein the
CTL
epitope is any one of the epitopes listed in Tables 3, 6, 8, 11, 14 and 17,
and wherein the
nucleic acids are directly or indirectly joined to one another in the same
reading frame;
(c) a multi-epitope construct comprising between five and fifty nucleic acids,
each
encoding an influenza virus helper T lymphocyte (HTL) epitope wherein the HTL
CA 02658559 2009-01-20
WO 2008/039267 18 PCT/US2007/016529
epitope is any one of the epitopes listed in Tables 18-49; and wherein the
nucleic acids
are directly or indirectly joined to one another in the same reading frame;
(d) a multi-epitope construct comprising between five and fifty nucleic acids,
each
encoding an influenza virus helper T lymphocyte (HTL) epitope wherein the HTL
epitope is any one of the epitopes listed in Tables 20, 22, 24, 26, 28, 30,
32, 35, 37, 39,
41, 44, 46, and 49, and wherein the nucleic acids are directly or indirectly
joined to one
another in the same reading frame;
(e) a multi-epitope construct comprising between five and fifty nucleic acids,
each
encoding an influenza virus helper T lymphocyte (HTL) epitope wherein the HTL
epitope is any one of the epitopes listed in Tables 18, 33, 42 and 47, and
wherein the
nucleic acids are directly or indirectly joined to one another in the same
reading frame;
(f) the multi-epitope construct of (a) or (b), further comprising any of said
nucleic
acids of (c), (d), or (e), directly or indirectly joined in the same reading
frame to said
CTL epitope nucleic acids of (a) or (b); ,
(g) the multi-epitope construct of (c), (d), or (e), further comprising any of
said
nucleic acids of (a) or (b), directly or indirectly joined in the same reading
frame to said
HTL epitope nucleic acids of (c), (d) or (e);
(h) the multi-epitope construct of (a) or (b) or (c) or (d) or (e) or (f) or
(g), further
comprising one or more spacer nucleic acids, directly or indirectly joined in
the same
reading frame to said CTL and/or HTL epitope nucleic acids;
(i) the multi-epitope construct of (h), wherein said one or more spacer
nucleic acids
are positioned between the CTL epitope nucleic acids of (a) or (b), between
the HTL
epitope nucleic acids of (c) or (d) or (e), or between the CTL and/or HTL
epitope nucleic
acids of (f) or (g);
(j) the multi-epitope construct of (h) or (i), wherein said one or more spacer
nucleic
acids each encode 1 to 8 amino acids;
(k) the multi-epitope construct of any one of (h) to (j), wherein one or more
of said
spacer amino acid residues are selected from the group consisting of : K, R,
N, Q, G, A,
S, C, and T at a C+1 position of one of said CTL epitopes;
(1) the multi-epitope construct of any of (h) to (k), wherein two or more of
said
spacer nucleic acids encode different (i.e., non- identical) amino acid
sequences;
(m) the multi-epitope construct of any of (h) to (1), wherein two or more of
said
spacer nucleic acids encode an amino acid sequence different from an amino
acid
sequence encoded by one or more other spacer nucleic acids;
CA 02658559 2009-01-20
WO 2008/039267 19 PCT/US2007/016529
(n) the multi-epitope construct of any of (h) to (m), wherein two or more of
the
spacer nucleic acids encodes the identical amino acid sequence;
(o) the multi-epitope construct of any of (h) to (n), wherein one or more of
said
spacer nucleic acids encode an amino acid sequence comprising or consisting of
three
consecutive alanine (Ala) residues;
(p) the multi-epitope construct of (h) to (o), wherein one or more of said
spacer
nucleic acid encodes an amino acid sequence selected from the group consisting
of: an
amino acid sequence comprising or consisting of GPGPG (SEQ ID NO:_), an amino
acid sequence comprising or consisting of PGPGP (SEQ ID NO:_), an amino acid
sequence comprising or consisting of (GP)n, an amino acid sequence comprising
or
consisting of (PG)n, an amino acid sequence comprising or consisting of
(GP)nG, and an
amino acid sequence comprising or consisting of (PG)nP, where n is an integer
between
zero and eleven;
(q) the multi-epitope construct of any of (a) to (p), further comprising one
or more
nucleic acids encoding one or more HTL epitopes, directly or indirectly joined
in the
same reading frame to said CTL and/or HTL epitope nucleic acids and/or said
spacer
nucleic acids;
(r) the multi-epitope construct of (q), wherein said one or more HTL epitopes
comprises a pan-DR binding epitope;
(s) the multi-epitope construct of any of (a) to (r), further comprising one
or more
MHC Class I and/or MHC Class II targeting nucleic acids;
(t) the multi-epitope construct of (s), wherein said one or more targeting
nucleic
acids encode one or more targeting sequences selected from the group
consisting of : an
Ig kappa signal sequence, a tissue plasminogen activator signal sequence, an
insulin
signal sequence, an endoplasmic reticulum signal sequence, a LAMP-1 lysosomal
targeting sequence, a LAMP-2 lysosomal targeting sequence, an HLA-DM lysosomal
targeting sequence, an HLA-DM-association sequence of HLA-DO, an Ig-a
cytoplasmic
domain, Ig-ss cytoplasmic domain, a li protein, an influenza matrix protein,
an HCV
antigen, and a yeast Ty protein;
(u) the multi-epitope construct of any of (a) to (t), which is optimized for
CTL and/or
HTL epitope processing;
(v) the multi-epitope construct of any of (a) to (u), wherein said CTL and/or
HTL
nucleic acids are sorted to minimize the number of CTL and/or HTL junctional
epitopes
encoded therein;
CA 02658559 2009-01-20
WO 2008/039267 20 PCT/US2007/016529
(w) the multi-epitope construct of any of (a)-(v), wherein said influenza
virus CTL
and/or HTL epitopes are selected from the epitopes listed in Table 50; and
(x) the multi-epitope construct of any of (c), (d) or (e)-(v) wherein said
influenza
virus CTL and/or HTL epitopes are directly or indirectly joined in the order
shown in
Figure 6.
[0052] In certain embodiments, the multi-epitope construct comprises between
10 and 50
nucleic acids, each encoding influenza virus CTL and/or HTL epitopes.
[0053] In certain other embodiments, the polynucleotide or peptide of the
present
invention comprises a CTL epitope, where the CTL epitope is from about 8 to
about 13
amino acids in length. In further embodiments, the CTL epitope is from about 8
to about
11 amino acids in length, about 9 to about 11 amino acids in length, or about
9 to about
amino acids in length.
[0054] In certain other embodiments, the polynucleotide or peptide of the
present
invention comprises an HTL epitope, where the HTL epitope is from about 6 to
about 30
amino acids in length. In further embodiments, the HTL epitope is from about 8
to about
amino acids in length, or from about 10 to about 18 amino acids in length.
[0055] In other embodiments, the influenza virus CTL and/or HTL epitope is
from a
polypeptide at least 90% identical to an influenza virus hemagglutinin (HA),
neuraminidase (NA), nucleoprotein (NP), RNA polymerase subunit PA, RNA
polymerase basic protein 1(PB 1), RNA polymerase basic protein 2 (PB2),
nonstructural
gene 1(NS1), nonstructural gene 2 (NS2), matrix protein 1(M1) or matrix
protein 2
(M2) polypeptide.
[0056] In additional embodiments, the influenza virus CTL and/or HTL epitope
is from
an influenza strain selected from the group consisting of: Human A/Viet
Nam/1203/2004
(H5N1), Human A/Hong Kong/156/97 (H5N1), Human A/Hong Kong/483/97 (H5N1),
Human A/Hong Kong/1073/99 (H9N2), Avian A/Chicken/HK/G9/97 (H9N2), Swine
A/Swine/Hong Kong/10/98 (H9N2), Avian A/FPV/Rostock/34 (H7N1), Avian
A/Turkey/Italy/4620/99 (H7N1), Avian A/FPV/Weybridge/34 (H7N7 ), Human A/New
Caledonia/20/99 (H1N1), Human A/Hong Kong/1/68 (H3N2), Human A/Shiga/25/97
(H3N2), Human A/Singapore/1/57 (H2N2), Human A/Leningrad/134/57 (H2N2),
Human A/Ann Arbor/6/60 (H2N2), Human A/Brevig Mission/1/18 (H1N1), Swine
A/Swine/Wisconsin/464/98 (H1N1), Human A/Netherlands/219/03 (H7N7).
[0057] In certain embodiments, the CTL epitope comprises a Class I HLA motif
selected
from the group consisting of HLA-A1, HLA-A2, HLA-A3, HLA-A24, HLA-B7 and
CA 02658559 2009-01-20
WO 2008/039267 21 PCT/US2007/016529
HLA-B44. In further embodiments, the polynucleotide of the present invention
comprises at least one HLA-A1 epitope, at least one HLA-A2 epitope, at least
one HLA-
A3/A11 epitope, at least one HLA-A24 epitope, at least one HLA-B7 epitope, or
at least
one HLA-B44 epitope; or any combinations thereof.
[0058] In certain embodiments, the CTL epitope is any one of the HLA-A3
epitopes
listed in Tables 1-3, any one of the HLA-A24 epitopes listed in Tables 4-6,
any one of
the HLA-B7 epitopes listed in Tables 7-8, any one of the HLA-B44 epitopes
listed in
Tables 9-11, any one of the HLA-A1 epitopes listed in Tables 12-14, or any one
of the
HLA-A2 epitopes listed in Tables 15-17.
100591 In other embodiments, the HTL epitope comprises a Class II HLA motif
selected
from the group consisting of HLA-DR1 and HLA-DR3.
[0060] , In certain embodiments, the HTL epitope is any of the DR epitopes
listed in
Tables 48-49.
100611 In other embodiments, the HTL epitope is any one of the DR1 epitopes
listed in
Tables 18-39. In further embodiments, the HTL DRl epitope is from an influenza
virus
protein NA, NP, NS1, NS2, PA, PB1, PB2, HA, Ml, or M2 sequence. For example,
the
HTL epitope is any one of the NA DR1 epitopes listed in Table 18, any one of
the NP
DR1 epitopes listed in Tables 19-20, any one of the NSl DR1 epitopes listed in
Tables
21-22, any one of the NS2 DR1 epitopes listed in Tables 23-24, any one of the
PA DR1
epitopes listed in Tables 25-26, any one of the PBl DR1 epitopes listed in
Tables 27-28,
any one of the PB2 DR1 epitopes listed in Tables 29-30, any one of the HA DR1
epitopes listed in Tables 31-33, any one of the M1 DRl epitopes listed in
Tables 34-35,
any one of the M2 DR1 epitopes listed in Tables 36-37, or any one of the NA
DR1
epitopes listed in Tables 38-39.
[0062] In other embodiments, the HTL epitope is any one of the DR3 epitopes
listed in
Tables 40-47. In further embodiments, the HTL DR3 epitope is from an influenza
virus
protein NA, NP, NS1, NS2, PA, PB1, PB2, HA, Ml or M2 sequence. For example,
the
HTL epitope any one of the NA DR3 epitopes listed in Tables 40-42, or any one
of the
HA DR3 epitopes listed in Tables 45-47.
100631 In certain embodiments, the polynucleotide of the invention further
comprises a
polynucleotide encoding a polypeptide at least 90% identical to an influenza
virus NA,
NP, NS1, NS2, PA, PB1, PB2, Ml, M2 or HA sequence, or fragment, variant, or
derivative thereof. The HA sequence can be a wild-type HA sequence from any of
the
CA 02658559 2009-01-20
WO 2008/039267 22 PCT/US2007/016529
influenza virus strains set forth above. The M2 sequence can be an M2e
sequence,
where the M2e sequence is selected from the sequences listed in Table 51.
[0064] In certain embodiments, the polynucleotide of the present invention
comprises a
nucleic acid sequence encoding a pan-DR binding epitope, where the pan-DR
binding
epitope comprises the amino acid sequence AFKVAAWTLKAAA (SEQ ID NO:_J.
[0065] In additional embodiments, the polynucleotide of the invention further
comprises
one or more regulatory sequences, where the one or more regulatory sequences
comprises an IRES element or a promoter.
[0066] The present invention is also directed to a polypeptide encoded by a
polynucleotide of the present invention, or a synthetic polypeptide. In
further
embodiments, the polypeptide further comprises a pan-DR binding epitope. In
further
embodiments, the pan-DR binding epitope comprises the amino acid sequence
a,KXVAAWTLKAAa2, where "X" is selected from the group consisting of
cyclohexylalanine, phenylalanine, and tyrosine; and "al" is either D-alanine
or L-alanine;
and "a2" is either D-alanine or L-alanine; or if encoded by a nucleic acid the
pan-DR
binding epitope comprises the amino acid sequence AFKVAAWTLKAAA. In further
embodiments, the polypeptide of the present invention is from about 10 to
about 2000
amino acids in length.
[0067] The present is also directed to a vector comprising the polynucleotide
of the
present invention. In further embodiments, the vector is an expression vector.
[0068] The present invention is also directed to a composition comprising the
polynucleotide of the present invention, a polypeptide of the present
invention, or the
vector of the present invention. In further embodiments, the composition of
the present
invention further comprises an influenza HA or NA polypeptide, wherein said HA
polypeptide is encoded by a sequence 90% identical to a wild-type HA sequence
from an
influenza strain selected from the group consisting of: Human A/Viet
Nam/1203/2004
(H5N1), Human A/Hong Kong/156/97 (H5N1), Human A/Hong Kong/483/97 (H5N1),
Human A/Hong Kong/1073/99 (H9N2), Avian A/Chicken/HK/G9/97 (H9N2), Swine
A/Swine/Hong Kong/10/98 (H9N2), Avian A/FPV/Rostock/34 (H7N1), Avian
A/Turkey/Italy/4620/99 (H7N1), Avian A/FPV/Weybridge/34 (H7N7 ), Human A/New
Caledonia/20/99 (H1N1), Human A/Hong Kong/1/68 (H3N2), Human A/Shiga/25/97
(H3N2), Human A/Singapore/1/57 (H2N2), Human A/Leningrad/134/57 (H2N2),
Human A/Ann Arbor/6/60 (H2N2), Human A/Brevig Mission/1/18 (H1N1), Swine
A/Swine/Wisconsin/464/98 (H1N1), Human A/Netherlands/219/03 (H7N7).
CA 02658559 2009-01-20
WO 2008/039267 23 PCT/US2007/016529
[0069] In certain embodiments, the polynucleotide further comprises a nucleic
acid
encoding a targeting sequence located at the N-terminus of said construct. In
further
embodiments, the targeting sequence is selected from the group consisting of:
an Ig
kappa signal sequence, a tissue plasminogen activator signal sequence, an
insulin signal
sequence, an endoplasmic reticulum signal sequence, a LAMP-1 lysosomal
targeting
sequence, a LAMP-2 lysosomal targeting sequence, an HLA-DM lysosomal targeting
sequence, an HLA-DM-association sequence of HLA-DO, an Ig-a cytoplasmic
domain,
Ig-ss cytoplasmic domain, a li protein, an influenza matrix protein, an HCV
antigen, and
a yeast Ty protein, a baculovirus signal sequence, or a prokaryotic signal
sequence.
[0070] Polypeptides of the present invention, for example an influenza virus
HA or M2e
polypeptide, can be altered from their native state in one or more of the
following ways.
An influenza virus polypeptide can be mutated so as to, for example, remove
from the
encoded polypeptide non-desired protein motifs present in the encoded
polypeptide or
virulence factors associated with the encoded polypeptide. For example, the
polypeptide
sequence could be mutated so as not to encode a membrane anchoring region that
would
prevent release of the polypeptide from the cell as with, e.g., M2e. Upon
delivery, the
polynucleotide of the invention is incorporated into the cells of the
vertebrate in vivo, and
a prophylactically or therapeutically effective amount of an immunologic
epitope of an
influenza virus is produced in vivo. Additionally, epitopes may be modified
(to create
analogs thereof) to increase their immunogenicity as compared to native
epitopes.
[0071] The present invention further provides polypeptides encoded by the
polynucleotides described above, a vector comprising the polynucleotides
described
above as well as immunogenic compositions comprising the polynucleotides
and/or
polypeptides described above. In certain other embodiments, the present
invention is
directed to a cell comprising polynucleotides, polypeptides, or immunogenic
compositions as described above. In certain other embodiments, a composition
comprises two or more polypeptides as described above, where the polypeptides
are
different from each other.
[0072] In certain embodiments, immunogenic compositions can further comprise,
for
example, carriers, excipients, transfection facilitating agents, lipids,
liposomes and/or
adjuvants as described herein. In certain other embodiments, immunogenic
compositions can further comprise a virosome. In further embodiments, the
virosome is
an immunopotentiating reconstituted influenza virosome (IRIV).
CA 02658559 2009-01-20
WO 2008/039267 24 PCT/US2007/016529
[0073] The compositions of the invention can be univalent, bivalent, trivalent
or
multivalent. A univalent composition will comprise only one polynucleotide of
the
present invention, or a polypeptide encoding the polynucleotide of the present
invention,
where the polynucleotide comprises between 10 and 100 nucleic acids encoding
an
influenza virus CTL and/or HTL multi-epitope and a second influenza virus
polypeptide
or a fragment, variant, or derivative thereof. A bivalent composition will
comprise,
either in polynucleotide or polypeptide form, two different influenza virus,
polypeptides,
each capable of eliciting an immune response. The polynucleotide(s) of the
composition
can encode two influenza virus polypeptides or alternatively, the
polynucleotide can
encode only one influenza virus polypeptide and the second influenza virus
polypeptide
would be provided by an isolated influenza virus polypeptide of the invention
as in, for
example, a single formulation heterologous prime-boost vaccine composition. In
the
case where both influenza virus polypeptides of a bivalent composition are
delivered in
polynucleotide form, the nucleic acid operably encoding those influenza virus
multi-
epitope constructs need not be on the same polynucleotide, but can be on two
different
polynucleotides. A trivalent or further multivalent composition will comprise
three
influenza virus polypeptides or fragments, variants or derivatives thereof,
either in
isolated form or encoded by one or more polynucleotides of the invention.
[0074] In one embodiment, a multivalent composition comprises a single multi-
epitope
polynucleotide construct, e.g., plasmid, comprising one or more CTL and/or HTL
influenza virus epitopes. Reducing the number of polynucleotides, e.g.,
plasmids, in the
compositions of the invention can have significant impacts on the manufacture
and
release of product, thereby reducing the costs associated with manufacturing
the
compositions. There are a number of approaches to include more than one
expressed
antigen coding sequence on a single plasmid. These include, for example, the
use of
Internal Ribosome Entry Site (IRES) sequences, dual promoters/expression
cassettes,
and fusion proteins.
[0075] The present invention is further directed to enhancing the immune
response of a
vertebrate in need of protection against influenza virus infection by
administering, in
vivo, into a tissue of the vertebrate, a polynucleotide, a polypeptide, or a
composition as
described above. The isolated polypeptide can be, for example, a purified
subunit, a
recombinant protein, a viral vector expressing an isolated influenza virus
polypeptide, or
can be an inactivated or attenuated influenza virus, such as those present in
conventional
influenza virus vaccines. According to either method, the polynucleotide is
incorporated
CA 02658559 2009-01-20
WO 2008/039267 25 PCT/US2007/016529
into the cells of the vertebrate in vivo, and an immunologically effective
amount of an
immunogenic influenza virus multi-epitope construct is produced in vivo. When
utilized,
an isolated influenza virus polypeptide or a fragment, variant, or derivative
thereof is
also administered in an immunologically effective amount.
BRIEF DESCRIPTION OF THE DRAWINGS
[0076] Figures 1A-D. Influenza peptide specific responses obtained from Human
Donors. Peripheral blood samples from 3 HLA A2.1 positive healthy volunteers
were
obtained by leukopheresis and PBMC isolated by Ficoll gradient separation. HLA
typing was performed by Terasaki First HLA-ABC well Tray analysis.
Cryopreserved
PBMC were thawed, resuspended in 1 ml of 5% HS RPMI and plated at 4 x 106
cells per
well in 24-well plates. The PBMC were stimulated with a pool (9-10) peptides
at a final
concentration of 2 g/ml of each peptide. Cell cultures were supplemented on
days 1, 3
and 6 with a final concentration of 10 U/ml IL2. After 7 days in culture, CD8+
cells were
purified using MACS Miltenyi Microbeads for use in IFN-y ELISPOT assays.
Membrane-based 96-well Millipore plates were coated overnight at 4 C with the
Mabtech murine mAb specific for human IFN- y. In triplicate wells, two
concentrations
of PBMC (25,000 and 5,000), A2.1 transfected .221 target cells (10,000) and
peptide at a
final concentration of 10 g/ml were added. The assay plates were incubated at
37 C for
20 h, after which they were washed with PBS + 0.05% Tween 20. To each well,
100 l
of biotinylated mAb specific for human IFN-y (Mabtech) at the concentration of
2 g/ml
was added and plates were incubated at 37 C for 2 h. The plates were again
washed,
avidin-peroxidase complex (Vectastain) was added to each well, and the plates
were
incubated at room temperature for 1 h. The plates were developed using 3-amino-
9-
ethyl-carbazole (Sigma), washed and dried. Spots were counted using an AID
ELISPOT
reader. Triplicate well experimental values are expressed as the mean net
spots/106
CD8+ lymphocytes SEM for each peptide. Responses were determined for
positive
(EBV bmlfl 259, Flu Ml 58, CMV pp65 495) and negative (HBVenv 183, HBVcore 18,
Plasmodium falciparum (Pf) exp 1 83, Pf exp 1 2, Pf exp 1 91) control
peptides. All three
donors exhibited responses in the range of 200-10,000 SFC specific for the
positive
control peptides. Responses to the negative control peptides were generally <
10 SFC
except for Donor 638 which responded to 4 of 7 peptides in the range of 40-
2,000 SFC.
CA 02658559 2009-01-20
WO 2008/039267 26 PCT/US2007/016529
[0077] Figure 2. Broadly specific CTL responses induced by vaccination with
the
multi-peptide epitope vaccine. CTL responses were measured against each
vaccine
epitope from weeks 9 and 18 peripheral blood samples from colon cancer patient
#604.
The two post-vaccination samples were collected after the patient had received
3 and all
6 treatments with vaccine, respectively. Post-vaccination samples were tested
in separate
experiments together with a pre-vaccination sample. CTL responses were
measured
using an IFN- 7 ELISPOT assay following short-term in vitro expansion of PBMCs
with
each vaccine peptide. Effector cells (5 x 104 and 1.25 x 104 cells/well) and
peptide-
treated irradiated autologous PBMCs as APCs (1 x 105/well) were plated into
nitrocellulose wells pre-coated with an a-human IFN-y mAb. An irrelevant HLA-
A2-
binding HBV core antigen peptide was used as a negative control. Eighteen
hours later,
assay wells were developed to detect spot-forming cells which were counted by
a
computer-assisted ELISPOT reader (Carl Zeiss Inc.). Data are expressed as net
spot-
forming cells (SFC) per 5 x 104 effector cells induces by the vaccine peptide
after
subtracting background (SFC induced by the irrelevant HBV core antigen
peptide). A
positive vaccine response must meet 3 criteria: 1) The net spots of post-
vaccination
sample (after subtracting background spots induced by irrelevant peptide) must
be >5; 2)
The response must exceed the background and account for its variability such
that net
spots, post-vaccination> background spots + (2 x SD); 3) The response must
exceed the
pre-vaccination response and account for its variability such that net spots,
specific
peptide, post-vaccination > (2 x net spots, specific peptide, pre-vaccination)
+ (2 x SD).
[0078] Figures 3A-D. Broadly specific CTL responses induced by vaccination
with a
DNA plasmid vaccine encoding SIV-derived CTL and HTL epitopes. Peripheral
blood samples from 8 Mamu A*01 positive rhesus macaques were obtained by
venipuncture and PBMC isolated by Ficoll gradient separation following an
overnight
shipment of blood. Results are depicted from PBMC obtained 2 weeks prior, 2
weeks
post and 14 weeks post SIV infection. The 6 immunized macaques received 4 DNA
immunizations (4 mg/animal formulated in polyvinylpyrollidone, PVP) on a
monthly
basis. Following a 5 month rest period, 3 animals (1D, 2D, 3D, depicted as
grey bars)
received 2 additional DNA immunizations on a monthly basis. The remaining 3
animals
(4DP, 5DP, 6DP, depicted as black bars) received 2 polyepitope, 100 g/animal
formulated in Al (OH)3 (same order of epitopes and spacers as DNA vaccine)
immunizations on a monthly basis. The polyepitope protein vaccine was obtained
from a
baculovirus system. Two animals (7N, 8N, depicted as white bars) were naive or
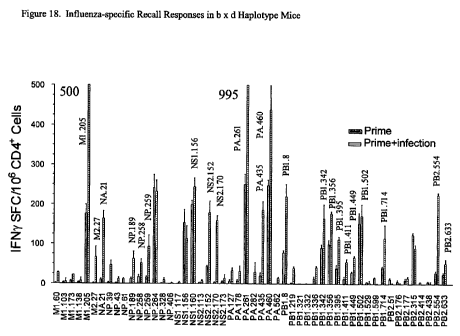
non-
CA 02658559 2009-01-20
WO 2008/039267 27 PCT/US2007/016529
immunized animals. The PBMCs were depleted of CD4+ cells by Dynal Microbeads
for
use in IFN-7 ELISPOT assays. Membrane-based 96-well Millipore plates were
coated
overnight at 4 C with the Mabtech murine mAb specific for monkey IFN-y. In
triplicate
wells, CD4+ depleted PBMC (200,000/well), and peptide at a final concentration
of 10
g/ml were added. The assay plates were incubated at 37 C for 20 h, after which
they
were washed with PBS + 0.05% Tween 20. To each well, 100 gl of biotinylated
mAb
specific for monkey IFN-7 (Mabtech) at the concentration of 2 g/ml was added
and
plates were incubated at room temperature for 2 h. The plates were again
washed,
avidin-peroxidase complex (Vectastain) was added to each well, and the plates
were
incubated at room temperature for 1 h. The plates were developed using 3-amino-
9-
ethyl-carbazole (Sigma), washed and dried. Spots were counted using an AID
ELISPOT
reader. Triplicate well experimental values are expressed as the mean net
spots/106
CD8+ lymphocytes SEM for each peptide. To determine the level of
significance, a
Student's t test was performed in which p< 0.05 using the mean of triplicate
values of
immunized animals (peptide response-no peptide response) versus non-immunized
animals (peptide response-no peptide response). Only responses with values of
p< 0.05
are shown. Plasma viral loads were determined following SIV infection.
Macaques
were challenged intravenously 8 months following the last immunization with
SIVmac239. Quantitation of virion-associated RNA in plasma was performed by
real
time PCR.
[0079] Figures 4A-D. Induction of CTL epitope-specific responses following
virus
infection was similar in humans and mice. Groups of 10 HLA-A2 transgenic mice
were infected by intranasal route using 600 and 1,200 HA units of virus. After
1 week of
infection, the group receiving the higher dose exhibited signs of illness
(weight loss,
immobilization) and were sacrificed to obtain splenocytes. The lower dose
group
showed no signs of illness and the mice were sacrificed at 2 weeks following
infection to
obtain splenocytes. The CD8+ cells were purified using MACS Miltenyi
Microbeads for
use in IFN- 7 ELISPOT assays. Membrane-based 96-well Millipore plates were
coated
overnight at 4 C with the Mabtech mAb specific for mouse IFN-y. In triplicate
wells,
CD8+ cells (200,000/well), A2.1/Kb transfected Jurkat target cells
(100,000/well) and
peptide at a final concentration of 10 g/ml were added. The assay plates were
incubated
at 37 C for 20 h, after which they were washed with PBS + 0.05% Tween 20. To
each
well, 100 l of biotinylated mAb specific for mouse IFN-y (Mabtech) at the
concentration of 2 gg/ml was added and plates were incubated at 37 C for 4 h.
The
CA 02658559 2009-01-20
WO 2008/039267 28 PCT/US2007/016529
plates were again washed, avidin-peroxidase complex (Vectastain) was added to
each
well, and the plates were incubated at room temperature for 1 h. The plates
were
developed using 3-amino-9-ethyl-carbazole (Sigma), washed and dried. Spots
were
counted using an AID ELISPOT reader. Triplicate well experimental values are
expressed as the mean net spots/106 CD8+ lymphocytes SEM for each peptide.
To
determine the level of significance, a Student's t test was performed in which
p< 0.05
using the mean of triplicate values of immunized mice (relevant peptide
response-
irrelevant peptide response) versus non-immunized mice (relevant peptide
response-
irrelevant peptide response). Only responses with values of p< 0.05 are shown.
[0080] Figures 5A-B. HTL Human Recall Responses in Donor X753. Immune
responses in Donor X753 using a panel of negative control HTL epitope-
containing
peptides and a panel of HTL epitope-containing peptides derived from internal
flu
proteins, NS 1, NS2, PB 1, PB2, PA, NP, M1 and M2. The sequences of the HTL
epitopes used in the experiment correspond to the nomenclature of the
influenza HTL
candidates in Tables 48 and 49.
[0081] Figure 6. Influenza virus multi-epitope construct. An influenza virus
multi-
epitope construct is illustrated showing influenza virus CTL and HTL epitopes
linked or
joined by spacer sequences. The sequences of the CTL and HTL epitopes shown
correspond to the nomenclature of the influenza candidate CTL and HTL epitopes
shown
in Table 50. Table 50 also provides binding data for each of the epitopes
within the
multi-epitope construct.
[0082] Figures 7A-D. HLA-A1 Influenza-Specific Recall Responses for Humans.
Immune responses in Donors X6018, X1257, X757 and X716 using a panel of
influenza-
derived HLA-A1 peptides and a panel of HLA-A1 and -A24 negative control
peptides.
The sequences of the influenza derived HLA-AI peptides used in the experiment
correspond to the nomenclature of the influenza peptides in Tables 12 and 13.
[0083] Figures 8A-D. HLA-A2 Influenza-Specific Recall Responses for Humans.
Immune responses in Donors X121 1, X716, AC08 and AC04 using a panel of
influenza-
derived HLA-A2 peptides and a panel of HLA-A2 negative control peptides. The
sequences of the influenza derived HLA-A2 peptides used in the experiment
correspond
to the nomenclature of the influenza peptides in Table 16.
100841 Figure 9. HLA-A2 Influenza-Specific Recall Responses for Mice. Immune
responses in HLA-A2 transgenic mice using a panel of influenza-derived HLA-A2
CA 02658559 2009-01-20
WO 2008/039267 29 PCT/US2007/016529
peptides. The sequences of the influenza derived HLA-A2 peptides used in the
experiment correspond to the nomenclature of the influenza peptides in Table
16.
[0085] Figures 1OA-D. HLA-A3/A11 Influenza-Specific Recall Responses for
Humans. Immune responses in Donors X709 and X3501 using a panel of influenza-
derived HLA-A3/A11 peptides and a panel of HLA-A3/All negative control
peptides.
The sequences of the influenza derived HLA-A3/All peptides used in the
experiment
correspond to the nomenclature of the influenza peptides in Table 2.
[0086] Figures 11A-C. HLA- A3/A11 Influenza-Specific Recall Responses for
Mice.
Immune responses in HLA-A11 transgenic mice using a panel of influenza-derived
HLA-A3/A11 peptides. The sequences of the influenza derived HLA-A3/A11
peptides
used in the experiment correspond to the nomenclature of the influenza
peptides in Table
2.
[0087] Figures 12A-D. HLA-A24 Influenza-Specific Recall Responses for Humans.
Inunune responses in Donors 1257, X759, X716 and XBB24 using a panel of
influenza-
derived HLA-A24 peptides and a panel of HLA-A24 negative control peptides. The
sequences of the influenza derived HLA-A24 peptides used in the experiment
correspond
to the nomenclature of the influenza peptides in Table 5.
[0088] Figures 13A-B. HLA-A24 Influenza-Specific Recall Responses for Mice.
Immune responses in HLA-A11 transgenic mice using a panel of influenza-derived
HLA-A24 peptides. The sequences of the influenza derived HLA-A24 peptides used
in
the experiment correspond to the nomenclature of the influenza peptides in
Table 5.
[0089] Figures 14A-C. HLA-B7 Influenza-Specific Recall Responses for Humans.
Immune responses in Donors X685, 7357, and X3501 using a panel of influenza-
derived
HLA-B7 peptides and a panel of HLA-B7 negative control peptides. The sequences
of
the influenza derived HLA-B7 peptides used in the experiment correspond to the
nomenclature of the influenza peptides in Table 7.
[0090] Figure 15. HLA-B7 Influenza-Specific Recall Responses for Mice. Immune
responses in HLA-B7 transgenic mice using a panel of influenza-derived HLA-B7
peptides. The sequences of the influenza derived HLA-B7 peptides used in the
experiment correspond to the nomenclature of the influenza peptides in Table
7.
[0091] Figures 16A-H. HLA-DR Influenza-Specific Recall Responses for Humans.
Immune responses in Donors X753, X6018,X3501 and X709 using a panel of
influenza-
derived HLA-DR peptides and a panel of negative control peptides. The
sequences of
CA 02658559 2009-01-20
WO 2008/039267 30 PCT/US2007/016529
the influenza derived HLA-DR peptides used in the experiment correspond to the
nomenclature of the influenza peptides in Tables 19, 21, 23, 25, 27, 29, 35,
37, and 38.
[0092] Figure 17. HLA-DR Influenza-Specific Recall Responses for DR4
Transgenic Mice. Immune responses in DR4 transgenic mice using a panel of
influenza-derived HLA-DR peptides. The sequences of the influenza derived HLA-
DR
peptides used in the experiment correspond to the nomenclature of the
influenza peptides
in Tables 19, 21, 23, 25, 27, 29, 35, 37, and 38.
[0093] Figure 18. HLA-DR Influenza-Specific Recall Responses for b x d
Haplotype Mice. Immune responses in b x d haplotype mice using a panel of
influenza-
derived HLA-DR peptides. The sequences of the influenza derived HLA-DR
peptides
used in the experiment correspond to the nomenclature of the influenza
peptides in
Tables 19, 21, 23, 25, 27, 29, 35, 37, and 38.
DETAILED DESCRIPTION OF THE INVENTION
[0094] The present invention is directed to compositions and methods for
enhancing the
immune response of a vertebrate in need of protection against influenza virus
infection
by administering in vivo, into a tissue of a vertebrate, at least one multi-
epitope
polynucleotide construct, at least one polypeptide encoded by such a multi-
epitope
polynucleotide construct, or at least one synthetic peptide, where the multi-
epitope
polynucleotide construct, polypeptide, or synthetic peptide comprises one or
more CTL
andlor HTL epitopes, where each CTL and/or HTL epitope is identified from an
influenza virus polypeptide, in cells of the vertebrate in need of protection.
The
polynucleotide or polypeptide can also comprise a nucleic acid sequence
encoding a pan-
DR binding epitope (e.g. PADRE ) or the peptide encoded therein.
[0095] The invention also relates to a method of designing and constructing a
multi-
epitope influenza virus vaccine construct with optimized immunogenicity and
comprising influenza virus CTL and/or HTL epitopes. A multi-epitope influenza
virus
vaccine construct in accordance with the invention allows for significant, non-
ethnically
biased population coverage, and can preferably focus on epitopes conserved
amongst
different viral or other antigenic isolates. Through the method and system
disclosed
herein, a multi-epitope influenza virus vaccine construct can be optimized
with regard to
the magnitude and breadth of responses, and can allow for the simplest epitope
configuration.
CA 02658559 2009-01-20
WO 2008/039267 31 PCT/US2007/016529
[0096] In one aspect, the present invention provides for simultaneous
induction of
responses against specific influenza virus CTL and/or HTL epitopes, using
single
promoter multi-epitope constructs. Such constructs can contain many different
epitopes,
between 5 and 50, preferably greater than 10, often greater than 20, 25, 30,
25, 40, or 45.
[0097] Non-limiting examples of influenza virus CTL and/or HTL epitopes within
the
scope of the invention include, but are not limited to, CTL and/or HTL
epitopes from the
influenza HA, NA, NP, PA, PB1, PB2, NS1, NS2, M1 or M2 polypeptides, and
fragments, e.g., M2e derivatives, and variants thereof. Nucleotide and amino
acid
sequences of influenza polypeptides from a wide variety of influenza types and
subtypes
are known in the art.
[0098] Epitopes for inclusion in the multi-epitope constructs typically bear
HLA Class I
or Class II binding motifs as described, for example, in PCT applications
PCT/US00/27766, or PCT/US00/19774. Multi-epitope constructs can be prepared
according to the methods set forth in Ishioka, et al., Jlmmunol 162(7):3915-
3925 (1999),
for example, the disclosure of which is herein incorporated by reference.
[0099] Multiple HLA class II or class I epitopes present in a multi-epitope
construct can
be derived from the same antigen, or from different antigens. For example, a
multi-
epitope construct can contain one or more HLA epitopes that can be derived
from two
different antigens of the same virus or from two different antigens of
different viruses.
Epitopes for inclusion in a multi-epitope construct can be selected by one of
skill in the
art, e.g., by using a computer to select epitopes that contain HLA allele-
specific motifs or
supermotifs. The multi-epitope constructs of the invention also encode one or
more
broadly cross-reactive binding, or universal, HLA class II epitopes, i.e., pan-
DR binding
epitopes, e.g., PADRE . (Epimmune, San Diego, Calif.), (described, for
example, in
U.S. Pat. No. 5,736,142) or a PADRE family molecule.
[00100] Universal HLA Class II epitopes can be advantageously combined with
other
HLA Class I and Class II epitopes to increase the number of cells that are
activated in
response to a given antigen and provide broader population coverage of HLA-
reactive
alleles. Thus, the multi-epitope constructs of the invention can include HLA
epitopes
specific for an antigen, universal HLA class II epitopes, or a combination of
specific
HLA epitopes and at least one universal HLA class II epitope.
[00101] HLA Class I epitopes, referred to as "CTL epitopes" are peptides of
defined
length that can be from about 8 to about 13 amino acids in length, from about
8 to about
11 amino acids in length, from about 9 to about 11 amino acids in length, or
about 9 to
CA 02658559 2009-01-20
WO 2008/039267 32 PCT/US2007/016529
about 10 amino acids in length. HLA Class II epitopes, referred to as "HTL
epitopes" as
peptides of defined length that can be from about 6 to about 30 amino acids in
length,
from about 8 to about 30 amino acids in length, from about 10 to about 30
amino acids,
from about 12 to about 30 amino acids in length, from about 6 to about 25
amino acids in
length, from about 8 to about 25 amino acids in length, from about 10 to about
25 amino
acids, from about 12 to about 25 amino acids in length, from about 6 to about
18 amino
acids in length, from about 8 to about 18 amino acids in length, from about 10
to about
18 amino acids, or from about 12 to about 18 amino acids in length, which is
recognized
by a particular HLA molecule. An HLA Class I or II epitope can be derived from
any
desired antigen of interest. The antigen of interest can be any protein from
an influenza
virus for which an immune response is desired. Epitopes can be selected based
on their
ability to bind one or multiple HLA alleles. Epitopes that are analogs of
naturally
occurring sequences can also be included in the multi-epitope constructs
described
herein. Such analog peptides are described, for example, in PCT applications
PCT/US97/03778, PCT/US00/19774, and co-pending U.S. Ser. No. 09/260,714 filed
Mar. 1, 1999.
[00102] Initially, influenza CTL epitopes of the present invention were
obtained from the
influenza virus HA, NA, NP, PA, PB1, PB2, NS1, NS2, M1 or M2 protein sequences
which were scanned for HLA-A3, -A24, -B7, B44, -Al and -A2 motifs using
computer
algorithm analysis as previously described. Approximately 450 sequences
bearing the
appropriate motifs were identified. In order to select potential epitopes that
would be
cross-reactive amongst a variety of influenza strains, these sequences were
compared to
other viral strains, typically I 1 to 20, and conserved sequences were
selected for peptide
synthesis. Peptide binding assays were performed using peptide and purified
HLA
molecules.
[00103] As used herein, the phrases "sequence conservancy", "strain
conservancy", or
"strain sequence conservancy" reflect the results of an amino acid sequence
comparison
among a plurality of influenza strains to determine the degree of homology
between
amino acid sequences of the same protein of various strains. Typically, the
following
influenza strains were compared to determine the percentage sequence
conservancy:
Virus Host Virus
Subtype Origin Strain
H5N1 Human A/Hong Kong/156/97
H5N1 Human A/Hong Kong/483/97
H9N2 Human A/Hong Kong/1073/99
CA 02658559 2009-01-20
WO 2008/039267 33 PCT/US2007/016529
H9N2 Avian A/Chicken/HK/G9/97
H9N2 Swine A/Swine/Hong Kong/10/98
H7N1 Avian A/FPV/Ro.stock/34
H7N1 Avian A/Turkey/Italy/4620/99
H7N7 Avian A/FPV/Weybridge/34
H1N1 Human A/New Caledonia/20/99
H3N2 Human A/Hong Kong/1/68
H3N2 Human A/Shiga/25/97
H2N2 Human A/Singapore/1 /57
H2N2 Human A/Leningrad/134/57
H2N2 Human A/Ann Arbor/6/60
H1N1 Human A/Brevig Mission/1/18
H1 N1 Swine A/Swine/Wisconsin/464/98
H7N7 Human A/Netherlands/219/03
[00104) Binding analyses of 119 conserved HLA-A3 peptides are provided in
Table 1. In
order to select epitopes that would be cross-reactive amongst various humans
to obtain
maximal population coverage, the number of vaccine candidate peptides was.
subsequently reduced to 77 by selecting only degenerate binding peptides
demonstrating
binding at < 500 nM to at least A*0301 or A*1101, and a strain sequence
conservancy
equal to or greater than 30%, provided in Table 2. These 77 candidate peptides
were
again reduced to 25 peptides demonstrating binding at < 500 nM to at least
A*0301 or
A* 1101, and a strain sequence conservancy equal to or greater than 38%,
provided in
Table 3. The most preferred HLA-A3 candidate epitopes demonstrate binding at <
500
nM to at least A*0301 or A* 1101, have a strain sequence conservancy equal to
or greater
than 30% and are positive in human and/or mouse influenza recall responses.
These
most preferred HLA-A3 candidate epitopes are listed in Table 52.
[00105] Binding analyses of 50 conserved HLA-A24 peptides are provided in
Table 4.
These candidate peptides were reduced to 32 peptides demonstrating binding at
< 500
nM to A*2402, and a strain sequence conservancy equal to or greater than 30%,
provided in Table 5. These candidate peptides were again reduced to 20
peptides by
limiting to 3 peptides at most per influenza virus protein, provided in Table
6. The most
preferred HLA-A24 candidate epitopes demonstrate binding at < 500 nM to
A*2402,
have a strain sequence conservancy equal to or greater than 30% and are
positive in
human and/or mouse influenza recall responses. These most preferred HLA-A3
candidate epitopes are listed in Table 53.
CA 02658559 2009-01-20
WO 2008/039267 34 PCT/US2007/016529
[00106] Binding analyses of 30 conserved HLA-B7 peptides are provided in Table
7.
These candidate peptides were reduced to 16 peptides demonstrating binding at
< 500
nM to B*0702 and a strain sequence conservancy equal to or greater than 38%,
and a
total limit of 3 peptides at most per influenza virus protein, provided in
Table 8. The
most preferred HLA-B07 candidate epitopes demonstrate binding at < 500 nM to
at least
A*B702 or B*3501, have a strain sequence conservancy equal to or greater than
30%
and are positive in human and/or mouse influenza recall responses. These most
preferred
HLA-B07 candidate epitopes are listed in Table 54.
[00107] Binding analyses of 131 conserved HLA-B44 peptides are provided in
Table 9.
These candidate peptides were reduced to 36 peptides demonstrating binding at
< 500
nM to at least two of B*4001, B*4402 or B*4403, and a strain sequence
conservancy
equal to or greater than 30%, provided in Table 10. These candidate peptides
were again
reduced to 24 peptides by limiting to 3 peptides at most per influenza virus
protein,
provided in Table 11.
[00108] Binding analyses of 46 conserved HLA-A1 peptides are provided in Table
12.
These candidate peptides were reduced to 33 peptides demonstrating binding at
< 500
nM to at least A*0101 or A*3002, and a strain sequence conservancy equal to or
greater
than 38%, provided in Table 13. These candidate peptides were again reduced to
20
peptides by limiting to 3 peptides at most per influenza virus protein,
provided in Table
14. The most preferred HLA-A01 candidate epitopes demonstrate binding at < 500
nM
to at least A*0101 or A*3002, have a strain sequence conservancy equal to or
greater
than 38% and are positive in human and/or mouse influenza recall responses.
These
most preferred HLA-Al candidate epitopes are listed in Table 55.
[00109] Binding analyses of 68 conserved HLA-A2 peptides are provided in Table
15.
These candidate peptides were reduced to 40 peptides demonstrating binding at
< 500
nM to A*0201 and 2 additional alleles, and a strain sequence conservancy equal
to or
greater than 30%, provided in Table 16. These candidate peptides were again
reduced to
26 peptides by limiting to 3 peptides at most per influenza virus protein,
provided in
Table 17. The most preferred HLA-A2 candidate epitopes demonstrate binding at
< 500
nM to A*0201 and two additional alleles, have a strain sequence conservancy
equal to or
greater than 30% and are positive in human and/or mouse influenza recall
responses.
These most preferred HLA-A2 candidate epitopes are listed in Table 56.
[00110] Influenza HTL epitopes of the present invention were obtained from the
H5N1
(AF036362) and H2N2 (M25924) viral protein sequences which were scanned for
HLA-
CA 02658559 2009-01-20
WO 2008/039267 35 PCT/US2007/016529
DR1 and -DR3 motifs using computer algorithm analysis as described above.
Approximately 1,500 sequences bearing the appropriate motifs were identified.
Conserved sequences were selected and peptide binding assays were performed as
described above. Binding analyses of 157 conserved DR peptides are provided in
Table
48. In order to select epitopes that would be cross-reactive amongst various
humans to
obtain maximal population coverage, the number of vaccine candidate peptides
was
subsequently reduced to 53 by selecting only degenerate binding peptides
demonstrating
at least high to intermediate binding and high strain conservancy, provided in
Table 49.
Binding analyses of 163 conserved DR3 peptides are provided in Table 43. These
163
candidate DR3 peptides was subsequently reduced to 67 peptides demonstrating
binding
at < 1100 nM to DRB 1*0301, and a strain sequence conservancy of equal to or
greater
than 30% provided in Table 44.
[00111] Analyses of 133 conserved NP HLA-DR1 peptides are provided in Table
19.
These candidate peptides were reduced to 40 peptides predicted to bind to at
least
DRB1*0101 at < 100 nM, and a strain sequence conservancy >_35%, provided in
Table
20.
[00112] Analyses of 72 conserved NS 1 HLA-DRI peptides are provided in Table
21.
These candidate peptides were reduced to 25 peptides predicted to bind to at
least
DRB1*0101 at < 100 nM, and a strain sequence conservancy >-30%, provided in
Table
22.
[00113] Analyses of 27 conserved NS2 HLA-DRI peptides are provided in Table
23.
These candidate peptides were reduced to 15 peptides predicted to bind to at
least
DRB1*0101 at < 200 nM, and a strain sequence conservancy >-35%, provided in
Table
24.
[00114] Analyses of 185 conserved PA HLA-DR1 peptides are provided in Table
25.
These candidate peptides were reduced to 58 peptides predicted to bind to at
least
DRB1*0101 at < 100 nM, and a-strain sequence conservancy >_3 5 %, provided in
Table
26.
[00115] Analyses of 239 conserved PBI HLA-DR1 peptides are provided in Table
27.
These candidate peptides were reduced to 81 peptides predicted to bind to at
least
DRB1*0101 at < 100 nM, and a strain sequence conservancy >_35%, provided in
Table
28.
[00116] Analyses of 223 conserved PB2 HLA-DR1 peptides are provided in Table
29.
These candidate peptides were reduced to 78 peptides predicted to bind to at
least
CA 02658559 2009-01-20
WO 2008/039267 36 PCT/US2007/016529
DRB1*0101 at < 100 nM, and a strain sequence conservancy >_35%, provided in
Table
30.
1001171 Analyses of 118 conserved HA HLA-DRl peptides are provided in Table
31.
These candidate peptides were reduced to 59 peptides predicted to bind to at
least
DRB1*0101 at < 100 nM, and a strain sequence conservancy >_35%, provided in
Table
32.
[00118] Analyses of 80 conserved M1 HLA-DR1 peptides are provided in Table 34.
These candidate peptides were reduced to 33 peptides predicted to bind to at
least
DRB1*0101 at < 100 nM, and a strain sequence conservancy >_35%, provided in
Table
35.
[00119] Analyses of 23 conserved M2 HLA-DR1 peptides are provided in Table 36.
These candidate peptides were reduced to 7 peptides predicted to bind to at
least
DRB1*0101 at < 300 nM, and a strain sequence conservancy >_35%, provided in
Table
37.
[00120] Analyses of 145 conserved NA HLA-DR1 peptides are provided in Table
38.
These candidate peptides were reduced to 79 peptides predicted to bind to at
least
DRB 1*0101 at < 100 nM, and a strain sequence conservancy >_35%, provided in
Table
39.
[00121] Analyses of 39 conserved NA HLA-DR3 peptides are provided in Table 40.
These candidate peptides were reduced to 29 peptides demonstrating anchor
conservancy
_40%, provided in Table 41.
[00122] Analyses of 28 conserved HA HLA-DR3 peptides are provided in Table 45.
These candidate peptides were reduced to 19 peptides demonstrating anchor
conservancy
>_30%, provided in Table 46.
1001231 Preferred HLA-DR candidate epitopes are listed in Table 57. The most
preferred
HLA-DR candidate epitopes demonstrate binding to at least five of the thirteen
common
DR alleles listed and are positive in human and/or mouse influenza recall
responses.
These most preferred HLA-A3 candidate epitopes are listed in Table 58.
[00124] The influenza hemagglutinin (HA) and neuraminidase (NA) are highly
variable
sequences. Because of the high variability of these sequences, subsets of HLA-
DR1 and
HLA-DR3 epitopes from the HA and NA sequences specific to influenza strain
Human
A/Viet Nam/1203/2004 (H5N1) were identified and provided in Tables 18, 33, 42
and
47.
CA 02658559 2009-01-20
WO 2008/039267 37 PCT/US2007/016529
[00125] The number of candidate HTL epitopes with increased binding
characteristics
and/or having an increased percentage of conservancy are again reduced to 1-10
HTL
peptides for inclusion in an influenza virus vaccine. The selection of these 1-
10 HTL
peptides is based on obtaining positive immune responses in human and mouse
recall
assays. A preference is also given for inclusion of peptides representing each
of the 10
influenza proteins.
[00126] The amino acid sequence of a representative influenza virus multi-
epitope
construct has the following sequence, referred to herein as SEQ ID NO: -.
AKFVAAWTLKAAAKAAAGEISPLPSLKMPAHGPAKSMKAAAMEVASQARQMNAPIEHIA
SMNRLFFKCIYRGAANMDRAVKLYNAAAFYRYGFVANFGAAALPFERATIMKAAAMQAL
QLLLEVGAAAILGFVFTLTVNAMLASIDLKYGAAALMEWLKTRGAAAGLFGAIAGFINA
AAFYIQMCTELKFAAICTHLNAAAFEDLRVSSFKAAASYINRTGTFKAAAMVLSAFDER
NARMGTVTTEVNLTIGECPKYNAAMGTVTTEVALGLVCAGPGPGFEQITFMQALQLLLE
GPGPGIRPLLVEGTASLSPGGPGPGVGTMVMELIRMIKRGGPGPGLRHFQKDAKVLFQN
WGPGPGEYIMKGVYINTALLNGPGPGLIFLARSALILRGSVGPGPGIRWLIEEVRHRLR
ITGPGPGISSMVEAMVSRARIDGPGPGNPLIRHENRMVLASTGPGPGDLIFLARSALIL
RGSGPGPGARILTSESQLTITKEGPGPGDFALIVNAPNHEGIQGPGPGITFMQALQLLL
EVEQGPGPGLFTIRQEMASRGLWDGPGPGQNSITIERMVLSAFDGPGPGPTLLFLKVPA
QNAIST
A diagram of the multi-epitope construct shown above is illustrated in Figure
6.
Binding data of the individual CTL and HTL epitopes of the multi-epitope
construct
shown above is presented in Table 50.
[00127] In certain embodiments, the composition according to the invention
comprises a
multi-epitope polynucleotide influenza virus vaccine construct or a
polypeptide encoded
by such a polynucleotide, and further comprises an influenza virus
polypeptide. The
influenza virus polypeptide can be an HA, NA, NP, PA, PB1, PB2, NS1, NS2, M1
or M2
polypeptide, or fragment, variant, or derivative thereof. For example, the
influenza virus
polypeptide can correspond to the mature HA protein of Influenza
A/Vietnam/1203/2004
(H5N1) which is available in GenBank (Accession Number AAT73274), and has the
following sequence, referred to herein as SEQ ID NO: _'
DQICIGYHANNSTEQVDTIMEKNVTVTHAQDILEKKHNGKLCDLDGVKPLILRDCSVAG
WLLGNPMCDEFINVPEWSYIVEKANPVNDLCYPGDFNDYEELKHLLSRINHFEKIQIIP
KSSWSSHEASLGVSSACPYQGKSSFFRNVVWLIKKNSTYPTIKRSYNNTNQEDLLVLWG
IHHPNDAAEQTKLYQNPTTYISVGTSTLNQRLVPRIATRSKVNGQSGRMEFFWTILKPN
CA 02658559 2009-01-20
WO 2008/039267 38 PCT/US2007/016529
DAINFESNGNFIAPEYAYKIVKKGDSTIMKSELEYGNCNTKCQTPMGAINSSMPFHNIH
PLTIGECPKYVKSNRLVLATGLRNSPQRERRRKKRGLFGAIAGFIEGGWQGMVDGWYGY
HHSNEQGSGYAADKESTQKAIDGVTNKVNSIIDKMNTQFEAVGREFNNLERRIENLNKK
MEDGFLDVWTYNAELLVLMENERTLDFHDSNVKNLYDKVRLQLRDNAKELGNGCFEFYH
KCDNECMESVRNGTYDYPQYSEEARLKREEISGVKLESIGIYQILSIYSTVASSLALAI
MVAGLSLWMCSNGSLQCR
[00128] Additional HA sequences of the present invention correspond to
isolated wild-
type HA sequences from influenza A and influenza B strains as are known in the
art. For
example, wild-type HA sequences from influenza strains can also be found at
http://www.flu.lanl.gov/search/index.html?form_page=search.
[00129] The influenza virus polypeptide can correspond to an M2e sequence.
Examples
of M2e sequences are set forth in Table 51.
[00130] Multi-epitope constructs can be generated using methodology well known
in the
art. For example, polypeptides comprising the multi-epitope constructs can be
synthesized and linked. Typically, multi-epitope constructs are constructed
using
recombinant DNA technology.
[00131] The present invention is also directed to administering in vivo, into
a tissue of the
vertebrate the above described polynucleotide and/or at least one isolated
influenza
polypeptide, or a fragment, variant, or derivative thereof. The isolated
influenza
polypeptide or fragment, variant, or derivative thereof can be, for example, a
recombinant protein, a purified subunit protein, a protein expressed and
carried by a
heterologous live or inactivated or attenuated viral vector expressing the
protein, or can
be an inactivated influenza, such as those present in conventional,
commercially
available, inactivated influenza vaccines. According to either method, the
polynucleotide is incorporated into the cells of the vertebrate in vivo, and
an
immunologically effective amount of the influenza protein, or fragment or
variant
encoded by the polynucleotide is produced in vivo. The isolated protein or
fragment,
variant, or derivative thereof is also administered in an immunologically
effective
amount. The polynucleotide can be administered to the vertebrate in need
thereof either
prior to, at the same time (simultaneously), or subsequent to the
administration of the
isolated influenza polypeptide or fragment, variant, or derivative thereof.
Expression Vectors and Construction of a Multi-Epitope Constructs
CA 02658559 2009-01-20
WO 2008/039267 39 PCT/US2007/016529
[00132] The multi-epitope constructs of the invention are typically provided
as an
expression vector comprising a nucleic acid encoding the multi-epitope
polypeptide.
Construction of such expression vectors is described, for example in
PCT/US99/10646,
the disclosure of which is herein incorporated by reference. The expression
vectors
contain at least one promoter element that is capable of expressing a
transcription unit
encoding the nucleic acid in the appropriate cells of an organism so that the
antigen is
expressed and targeted to the appropriate HLA molecule. For example, for
administration to a human, a promoter element that functions in a human cell
is
incorporated into the expression vector.
[00133] In preferred embodiments, the invention utilizes routine techniques in
the field of
recombinant genetics. Basic texts disclosing the general methods of use in
this invention
include Sambrook et al., Molecular Cloning, A Laboratory Manual (2nd ed.
1989);
Kriegler, Gene Transfer and Expression: A Laboratory Manual (1990); and
Current
Protocols in Molecular Biology (Ausubel et al., eds., 1994); Oligonucleotide
Synthesis.
A Practical Approach (Gait, ed., 1984); Kuijpers, Nucleic Acids Research
18(17):5197
(1994); Dueholm, J. Org. Chem. 59:5767-5773 (1994); Methods in Molecular
Biology,
volume 20 (Agrawal, ed.); and Tijssen, Laboratory Techniques in Biochemistry
and
Molecular Biology--Hybridization with Nucleic Acid Probes, e.g., Part I,
chapter 2
"Overview of principles of hybridization and the strategy of nucleic acid
probe assays"
(1993)).
[00134] The nucleic acids encoding the epitopes are assembled in a construct
according to
standard techniques. In general, the nucleic acid sequences encoding multi-
epitope
polypeptides are isolated using amplification techniques with oligonucleotide
primers, or
are chemically synthesized. Recombinant cloning techniques can also be used
when
appropriate. Oligonucleotide sequences are selected which either amplify (when
using
PCR to assemble the construct) or encode (when using synthetic
oligonucleotides to
assemble the construct) the desired epitopes.
[00135] Amplification techniques using primers are typically used to amplify
and isolate
sequences encoding the epitopes of choice from DNA or RNA (see U.S. Pat. Nos.
4,683,195 and 4,683,202; PCR Protocols: A Guide to Methods and Applications
(Innis et
al., eds, 1990)). Methods such as polymerase chain reaction (PCR) and ligase
chain
reaction (LCR) can be used to amplify epitope nucleic acid sequences directly
from
mRNA, from cDNA, from genomic libraries or cDNA libraries. Restriction
endonuclease sites can be incorporated into the primers. Multi-epitope
constructs
CA 02658559 2009-01-20
WO 2008/039267 40 PCT/US2007/016529
amplified by the PCR reaction can be purified from agarose gels and cloned
into an
appropriate vector.
[00136] Synthetic oligonucleotides can also be used to construct multi-epitope
constructs.
This method is performed using a series of overlapping oligonucleotides,
representing
both the sense and non-sense strands of the gene. These DNA fragments are then
annealed, ligated and cloned. Oligonucleotides that are not commercially
available can
be chemically synthesized according to the solid phase phosphoramidite
triester method
first described by Beaucage & Caruthers, Tetrahedron Letts. 22:1859-1862
(1981), using
an automated synthesizer, as described in Van Devanter et al., Nucleic Acids
Res.,
12:6159-6168 (1984). Purification of oligonucleotides is by either native
acrylamide gel
electrophoresis or by anion-exchange HPLC as described in Pearson & Reanier,
J.
Chrom. 255:137-149 (1983).
[00137] The epitopes of the multi-epitope constructs are typically subcloned
into an
expression vector that contains a strong promoter to direct transcription, as
well as other
regulatory sequences such as enhancers and polyadenylation sites. Suitable
promoters are
well known in the art and described, e.g., in Sambrook et al. and Ausubel et
al.
Eukaryotic expression systems for mammalian cells are well known in the art
and are
commercially available. Such promoter elements include, for example,
cytomegalovirus
(CMV), Rous sarcoma virus LTR and SV40.
[00138] The expression vector typically contains a transcription unit or
expression
cassette that contains all the additional elements requiired for the
expression of the multi-
epitope construct in host cells. A typical expression cassette thus contains a
promoter
operably linked to the multi-epitope construct and signals required for
efficient
polyadenylation of the transcript. Additional elements of the cassette may
include
enhancers and introns with functional splice donor and acceptor sites.
[00139] In addition to a promoter sequence, the expression cassette can also
contain a
transcription termination region downstream of the structural gene to provide
for
efficient termination. The termination region may be obtained from the same
gene as the
promoter sequence or may be obtained from different genes.
[00140] The particular expression vector used to transport the genetic
information into the
cell is not particularly critical. Any of the conventional vectors used for
expression in
eukaryotic cells may be used. Expression vectors containing regulatory
elements from
eukaryotic viruses are typically used in eukaryotic expression vectors, e.g.,
SV40
CA 02658559 2009-01-20
WO 2008/039267 41 PCT/US2007/016529
vectors, CMV vectors, papilloma virus vectors, and vectors derived from
Epstein Bar
virus.
[00141] The multi-epitope constructs of the invention can be expressed from a
variety of
vectors including plasmid vectors as well as viral or bacterial vectors.
Examples of viral
expression vectors include attenuated viral hosts, such as vaccinia or
fowlpox. As an
example of this approach, vaccinia virus is used as a vector to express
nucleotide
sequences that encode the peptides of the invention. Upon introduction into a
host
bearing a tumor, the recombinant vaccinia virus expresses the immunogenic
peptide, and
thereby elicits a host CTL and/or HTL response. Vaccinia vectors and methods
useful in
immunization protocols are described in, e.g., U.S. Pat. No. 4,722,848.
[00142] A wide variety of other vectors useful for therapeutic administration
or
immunization, e.g. adeno and adeno-associated virus vectors, retroviral
vectors, non-
viral vectors such as BCG (Bacille Calmette Guerin), Salmonella typhi vectors,
detoxified anthrax toxin vectors, and the like, will be apparent to those
skilled in the art.
[00143] Immunogenicity and antigenicity of the multi-epitope constructs are
evaluated as
described herein.
Targeting Sequences
[00144] The expression vectors of the invention may encode one or more MHC
epitopes
operably linked to a MHC targeting sequence, and are referred to herein as
"targeting
nucleic acids" or "targeting sequences." The use of a MHC targeting sequence
enhances
the immune response to an antigen, relative to delivery of antigen alone, by
directing the
peptide epitope to the site of MHC molecule assembly and transport to the cell
surface,
thereby providing an increased number of MHC molecule-peptide epitope
complexes
available for binding to and activation of T cells.
[00145] MHC Class I targeting sequences can be used in the present invention,
e.g., those
sequences that target an MHC Class I epitope peptide to a cytosolic pathway or
to the
endoplasmic reticulum (see, e.g., Rammensee et al., Inmunogenetics 41:178-228
(1995)).
For example, the cytosolic pathway processes endogenous antigens that are
expressed
inside the cell. Although not wishing to be bound by any particular theory,
cytosolic
proteins are thought to be at least partially degraded by an endopeptidase
activity of a
proteosome and then transported to the endoplasmic reticulum by the TAP
molecule
(transporter associated with processing). In the endoplasmic reticulum, the
antigen binds
to MHC Class I molecules. Endoplasmic reticulum signal sequences bypass the
cytosolic
CA 02658559 2009-01-20
WO 2008/039267 42 PCT/US2007/016529
processing pathway and directly target endogenous antigens to the endoplasmic
reticulum, where proteolytic degradation into peptide fragments occurs. Such
MHC
Class I targeting sequences are well known in the art, and include, e.g.,
signal sequences
such as those from Ig kappa, tissue plasminogen activator or insulin. A
preferred signal
peptide is the human. Ig kappa chain sequence. Endoplasmic reticulum signal
sequences
can also be used to target MHC Class II epitopes to the endoplasmic reticulum,
the site
of MHC Class I molecule assembly. MHC Class II targeting sequences can also be
used
in the invention, e.g., those that target a peptide to the endocytic pathway.
These
targeting sequences typically direct extracellular antigens to enter the
endocytic pathway,
which results in the antigen being transferred to the lysosomal compartment
where the
antigen is proteolytically cleaved into antigen peptides for binding to MHC
Class II
molecules. As with the normal processing of exogenous antigen, a sequence that
directs a
MHC Class II epitope to the endosomes of the endocytic pathway and/or
subsequently to
lysosomes, where the MHC Class II epitope can bind to a MHC Class II molecule,
is a
MHC Class II targeting sequence. For example, group of MHC Class II targeting
sequences useful in the invention are lysosomal targeting sequences, which
localize
polypeptides to lysosomes. Since MHC Class II molecules typically bind to
antigen
peptides derived from proteolytic processing of endocytosed antigens in
lysosomes, a
lysosomal targeting sequence can function as a MHC Class II targeting
sequence.
Lysosomal targeting sequences are well known in the art and include sequences
found in
the lysosomal proteins LAMP-1 and LAMP-2 as described by August et al. U.S.
Pat. No.
5,633,234, issued May 27, 1997), which is incorporated herein by reference.
[00146] Other lysosomal proteins that contain lysosomal targeting sequences
include
HLA-DM. HLA-DM is an endosomal/lysosomal protein that functions in
facilitating
binding of antigen peptides to MHC Class II molecules. Since it is located in
the
lysosome, HLA-DM has a lysosomal targeting sequence that can function as a MHC
Class II molecule targeting sequence (Copier et al., J. Immunol. 157:1017-1027
(1996),
which is incorporated herein by reference).
[00147] The resident lysosomal protein HLA-DO can also function as a lysosomal
targeting sequence. In contrast to the above described resident lysosomal
proteins
LAMP-1 and HLA-DM, which encode specific Tyr-containing motifs that target
proteins
to lysosomes, HLA-DO is targeted to lysosomes by association with HLA-DM
(Liljedahl
et al., EMBO J., 15:4817-4824 (1996)), which is incorporated herein by
reference.
Therefore, the sequences of HLA-DO that cause association with HLA-DM and,
CA 02658559 2009-01-20
WO 2008/039267 43 PCT/US2007/016529
consequently, translocation of HLA-DO to lysosomes can be used as MHC Class II
targeting sequences. Similarly, the murine homolog of HLA-DO, H2-DO, can be
used to
derive a MHC Class H targeting sequence. A MHC Class II epitope can be fused
to
HLA-DO or H2-DO and targeted to lysosomes.
[00148] In another example, the cytoplasmic domains of B cell receptor
subunits Ig-a and
Ig-(3 mediate antigen internalization and increase the efficiency of antigen
presentation as
described in, for example, Bonnerot et al., Immunity, 3:335-347 (1995).
Therefore, the
cytoplasmic domains of the Ig-a and Ig-P proteins can function as MHC Class II
targeting sequences that target a MHC Class II epitope to the endocytic
pathway for
processing and binding to MHC Class H molecules.
[00149] Another example of a MHC Class II targeting sequence that directs MHC
Class II
epitopes to the endocytic pathway is a sequence that directs polypeptides to
be secreted,
where the polypeptide can enter the endosomal pathway. These MHC Class II
targeting
sequences that direct polypeptides to be secreted mimic the normal pathway by
which
exogenous, extracellular antigens are processed into peptides that bind to MHC
Class II
molecules. Any signal sequence that functions to direct a polypeptide through
the
endoplasmic reticulum and ultimately to be secreted can function as a MHC
Class II
targeting sequence so long as the secreted polypeptide can enter the
endosomal/lysosomal pathway and be cleaved into peptides that can bind to MHC
Class
II molecules.
1001501 In another example, the Ii protein binds to MHC Class H molecules in
the
endoplasmic reticulum, where it functions to prevent peptides present in the
endoplasmic
reticulum from binding to the MHC Class II molecules. Therefore, fusion of a
MHC
Class II epitope to the Ii protein targets the MHC Class II epitope to the
endoplasmic
reticulum and a MHC Class II molecule. For example, the CLIP sequence of the
Ii
protein can be removed and replaced with a MHC Class II epitope sequence so
that the
MHC Class H epitope is directed to the endoplasmic reticulum, where the
epitope binds
to a MHC Class II molecule.
[00151] In some cases, antigens themselves can serve as MHC Class II or I
targeting
sequences and can be fused to a universal MHC Class II epitope to stimulate an
immune
response. Although cytoplasmic viral antigens are generally processed and
presented as
complexes with MHC Class I molecules, long-lived cytoplasmic proteins such as
the
influenza matrix protein can enter the MHC Class MHC Class II molecule
processing
pathway as described in, for example, Gueguen & Long, Proc. Natl. Acad. Sci.
USA,
CA 02658559 2009-01-20
WO 2008/039267 44 PCT/US2007/016529
93:14692-14697 (1996). Therefore, long-lived cytoplasmic proteins can function
as a
MHC Class MHC Class II targeting sequence. For example, an expression vector
encoding influenza matrix protein fused to a universal MHC Class I/MHC Class
II
epitope can be advantageously used to target influenza antigen and the
universal MHC
Class I/MHC Class II epitope to the MHC Class I/MHC Class II pathway for
stimulating
an immune response to influenza.
[00152] Other examples of antigens functioning as MHC Class I/MHC Class II
targeting
sequences include polypeptides that spontaneously form particles. The
polypeptides are
secreted from the cell that produces them and spontaneously form particles,
which are
taken up into an antigen-presenting cell by endocytosis such as receptor-
mediated
endocytosis or are engulfed by phagocytosis. The particles are proteolytically
cleaved
into antigen peptides after entering the endosomal/lysosomal pathway.
[00153] One such polypeptide that spontaneously forms particles is HBV surface
antigen
(HBV-S) as described in, for example, Diminsky et al., Vaccine 15:637-647
(1997) or Le
Borgne et al., Virology, 240:304-315 (1998). Another polypeptide that
spontaneously
forms particles is HBV core antigen as described in, for example, Kuhrober et
al.,
International Immunol., 9:1203-1212 (1997). Still another polypeptide that
spontaneously forms particles is the yeast Ty protein as described in, for
example, Weber
et al., Vaccine, 13:831-834 (1995). For example, an expression vector
containing HBV-S
antigen fused to a universal MHC Class II epitope can be advantageously used
to target
HBV-S antigen and the universal MHC Class II epitope to the MHC Class II
pathway for
stimulating an immune response to HBV.
[00154] Methods of designing and.selecting CTL and/or HTL epitopes having an
HLA-
DR binding motif according to the present invention are described in Rammensee
et al.,
"MHC ligands and peptide motifs: first listing," Immunogenetics 41:178-228
(1995) and
Sette et al., "Prediction of major histocompatibility complex binding regions
of protein
antigens by sequence pattern analysis," Proc. Natl. Acad. Sci. 86: 3296-3300
(1989), the
disclosure of each which is incorporated herein by reference in its entirety.
[00155] Methods of designing and generating a multi-epitope construct
comprising an
influenza virus CTL and/or HTL epitope are performed according to methods of
designing and using multi-epitope constructs as described in WO 01/47541, WO
02/083714 and US 2004/0248113 Al, the disclosure of each which is incorporated
herein by reference in its entirety. The minimization of junctional motifs,
the influence
of flanking regions on CTL and HTL epitope immunogenicity within a multi-
epitope
CA 02658559 2009-01-20
WO 2008/039267 45 PCT/US2007/016529
!
construct, and the correlation between epitope immunogenicity and levels of
epitope
presentation in transfected cell lines are also described in WO 01/47541, WO
02/083714
and US 2004/0248 1 1 3 Al, the disclosure of each which is incorporated herein
by
reference in its entirety.
[00156] The present invention also provides vaccine compositions and methods
for
delivery of influenza virus multi-epitope constructs to a vertebrate with
optimal
expression and safety. These vaccine compositions are prepared and
administered in such
a manner that the encoded gene products are optimally expressed in the
vertebrate of
interest. As a result, these compositions and methods are useful in
stimulating an
immune response against influenza virus infection. Also included in the
invention are
expression systems and delivery systems.
[00157] It is to be noted that the term "a" or "an" entity refers to one or
more of that
entity; for example, "a polynucleotide," is understood to represent one or
more
polynucleotides. As such, the terms "a" (or "an"), "one or more," and "at
least one" can
be used interchangeably herein.
[00158] The term "polynucleotide" is intended to encompass a singular nucleic
acid or
nucleic acid fragment as well as plural nucleic acids or nucleic acid
fragments, and refers
to an isolated molecule or construct, e.g., a virus genome (e.g., a non-
infectious viral
genome), messenger RNA (mRNA), plasmid DNA (pDNA), or derivatives of pDNA
(e.g., minicircles as described in (Darquet, A-M et al., Gene Therapy 4:1341-
1349
(1997)) comprising a polynucleotide. A polynucleotide may comprise a
conventional
phosphodiester bond or a non-conventional bond (e.g., an amide bond, such as
found in
peptide nucleic acids (PNA)).
[00159] The terms "nucleic acid" or "nucleic acid fragment" refer to any one
or more
nucleic acid segments, e.g., DNA or RNA fragments, present in a polynucleotide
or
construct. A nucleic acid or fragment thereof may be provided in linear (e.g.,
mRNA) or
circular (e.g., plasmid) form as well as double-stranded or single-stranded
forms. By
"isolated" nucleic acid or polynucleotide is intended a nucleic acid molecule,
DNA or
RNA, which has been removed from its native environment. For example, a
recombinant polynucleotide contained in a vector is considered isolated for
the purposes
of the present invention. Further examples of an isolated polynucleotide
include
recombinant polynucleotides maintained in heterologous host cells or purified
(partially
or substantially) polynucleotides in solution. Isolated RNA molecules include
in vivo or
in vitro RNA transcripts of the polynucleotides of the present invention.
Isolated
CA 02658559 2009-01-20
WO 2008/039267 46 PCT/US2007/016529
polynucleotides or nucleic acids according to the present invention further
include such
molecules produced synthetically.
[00160] As used herein, a "coding region" is a portion of nucleic acid which
consists of
codons translated into amino acids. Although a "stop codon" (TAG, TGA, or TAA)
is
not translated into an amino acid, it may be considered to be part of a coding
region, but
any f[anking sequences, for example promoters, ribosome binding sites,
transcriptional
terminators, and the like, are not part of a coding region. Two or more
nucleic acids or
nucleic acid fragments of the present invention can be present in a single
polynucleotide
construct, e.g., on a single plasmid, or in separate polynucleotide
constructs, e.g., on
separate (different) plasmids. Furthermore, any nucleic acid or nucleic acid
fragment
may encode a single influenza polypeptide or fragment, derivative, or variant
thereof,
e.g., or may encode more than one polypeptide, e.g., a nucleic acid may encode
two or
more polypeptides. In addition, a nucleic acid may include a regulatory
element such as
a promoter, ribosome binding site, or a transcription terminator, or may
encode
heterologous coding regions fused to the influenza coding region, e.g.,
specialized
elements or motifs, such as a secretory signal peptide or a heterologous
functional
domain.
[00161] The terms "fragment," "variant," "derivative" and "analog" when
referring to
influenza virus polypeptides of the present invention include any polypeptides
which
retain at least some of the immunogenicity or antigenicity of the
corresponding native
polypeptide. Fragments of influenza virus polypeptides of the present
invention include
proteolytic fragments, deletion fragments and in particular, fragments of
influenza virus
polypeptides which exhibit increased secretion from the cell or higher
immunogenicity
or reduced pathogenicity when delivered to an animal, such as deletion of
signal
sequences or one or more domains. Polypeptide fragments further include any
portion of
the polypeptide which comprises an antigenic or immunogenic epitope of the
native
polypeptide, including linear as well as three-dimensional epitopes. Variants
of influenza
virus polypeptides of the present invention include fragments as described
above, and
also polypeptides with altered amino acid sequences due to amino acid
substitutions,
deletions, or insertions. Variants may occur naturally, such as an allelic
variant. By an
"allelic variant" is intended alternate forms of a gene occupying a given
locus on a
chromosome or genome of an organism or virus. Genes II, Lewin, B., ed., John
Wiley &
Sons, New York (1985), which is incorporated herein by reference. For example,
as
used herein, variations in a given gene product. When referring to influenza
virus NA or
CA 02658559 2009-01-20
WO 2008/039267 47 PCT/US2007/016529
HA proteins, each such protein is a "variant," in that native influenza virus
strains are
distinguished by the type of NA and HA proteins encoded by the virus. However,
within
a single HA or NA variant type, further naturally or non-naturally occurring
variations
such as amino acid deletions, insertions or substitutions may occur. Non-
naturally
occurring variants may be produced using art-known mutagenesis techniques.
Variant
polypeptides may comprise conservative or non-conservative amino acid
substitutions,
deletions or additions. Derivatives of influenza virus polypeptides of the
present
invention are polypeptides which have been altered so as to exhibit additional
features
not found on the native polypeptide. Examples include fusion proteins. An
analog is
another form of an influenza virus polypeptide of the present invention. An
example is a
proprotein which can be activated by cleavage of the proprotein to produce an
active
mature polypeptide.
[00162] The terms "infectious polynucleotide" or "infectious nucleic acid" are
intended to
encompass isolated viral polynucleotides and/or nucleic acids which are solely
sufficient
to mediate the synthesis of complete infectious virus particles upon uptake by
permissive
cells. Thus, "infectious nucleic acids" do not require pre-synthesized copies
of any of
the polypeptides it encodes, e.g., viral replicases, in order to initiate its
replication cycle
in a permissive host cell.
[00163] The terms "non-infectious polynucleotide" or "non-infectious nucleic
acid" as
defined herein are polynucleotides or nucleic acids which canriot, without
additional
added materials, e.g, polypeptides, mediate the synthesis of complete
infectious virus
particles upon uptake by permissive cells. An infectious polynucleotide or
nucleic acid
is not made "non-infectious" simply because it is taken up by a non-permissive
cell. For
example, an infectious viral polynucleotide from a virus with limited host
range is
infectious if it is capable of mediating the synthesis of complete infectious
virus particles
when taken up by cells derived from a permissive host (i.e., a host permissive
for the
virus itself). The fact that uptake by cells derived from a non-permissive
host does not
result in the synthesis of complete infectious virus particles does not make
the nucleic
acid "non-infectious." In other words, the term is not qualified by the nature
of the host
cell, the tissue type, or the species taking up the polynucleotide or nucleic
acid fragment.
[00164] In some cases, an isolated infectious polynucleotide or nucleic acid
may produce
fully-infectious virus particles in a host cell population which lacks
receptors for the
virus particles, i.e., is non-permissive for virus entry. Thus viruses
produced will not
CA 02658559 2009-01-20
WO 2008/039267 48 PCT/US2007/016529
infect surrounding cells. However, if the supernatant containing the virus
particles is
transferred to cells which are permissive for the virus, infection will take
place.
[00165] The terms "replicating polynucleotide" or "replicating nucleic acid"
are meant to
encompass those polynucleotides and/or nucleic acids which, upon being taken
up by a
permissive host cell, are capable of producing multiple, e.g., one or more
copies of the
same polynucleotide or nucleic acid. Infectious polynucleotides and nucleic
acids are a
subset of replicating polynucleotides and nucleic acids; the terms are not
synonymous.
For example, a defective virus genome lacking the genes for virus coat
proteins may
replicate, e.g., produce multiple copies of itself, but is NOT infectious
because it is
incapable of mediating the synthesis of complete infectious virus particles
unless the coat
proteins, or another nucleic acid encoding the coat proteins, are exogenously
provided.
[00166] In certain embodiments, the polynucleotide, nucleic acid, or nucleic
acid
fragment is DNA. In the case of DNA, a polynucleotide comprising a nucleic
acid which
encodes a polypeptide normally also comprises a promoter and/or other
transcription or
translation control elements operably associated with the polypeptide-encoding
nucleic
acid fragment. An operable association is when a nucleic acid fragment
encoding a gene
product, e.g., a polypeptide, is associated with one or more regulatory
sequences in such
a way as to place expression of the gene product under the influence or
control of the
regulatory sequence(s). Two DNA fragments (such as a polypeptide-encoding
nucleic
acid fragment and a promoter associated with the 5' end of the nucleic acid
fragment) are
"operably associated" if induction of promoter function results in the
transcription of
mRNA encoding the desired gene product and if the nature of the linkage
between the
two DNA fragments does not (1) result in the introduction of a frame-shift
mutation, (2)
interfere with the ability of the expression regulatory sequences to direct
the expression
of the gene product, or (3) interfere with the ability of the DNA template to
be
transcribed. Thus, a promoter region would be operably associated with a
nucleic acid
fragment encoding a polypeptide if the promoter was capable of effecting
transcription of
that nucleic acid fragment. The promoter may be a cell-specific promoter that
directs
substantial transcription of the DNA only in predetermined cells. Other
transcription
control elements, besides a promoter, for example enhancers, operators,
repressors, and
transcription termination signals, can be operably associated with the
polynucleotide to
direct cell-specific transcription. Suitable promoters and other transcription
control
regions are disclosed herein.
CA 02658559 2009-01-20
WO 2008/039267 49 PCT/US2007/016529
[00167] A variety of transcription control regions are known to those skilled
in the art.
These include, without limitation, transcription control regions which
function in
vertebrate cells, such as, but not limited to, promoter and enhancer segments
from
cytomegaloviruses (the immediate early promoter, in conjunction with intron-
A), simian
virus 40 (the early promoter), and retroviruses (such as Rous sarcoma virus).
Other
transcription control regions include those derived from vertebrate genes such
as actin,
heat shock protein, bovine growth hormone and rabbit 13-globin, as well as
other
sequences capable of controlling gene expression in eukaryotic cells.
Additional suitable
transcription control regions include tissue-specific promoters and enhancers
as well as
lymphokine-inducible promoters (e.g., promoters inducible by interferons or
interleukins).
[00168] Similarly, a variety of trainslation control elements are known to
those of ordinary
skill in the art. These include, but are not limited to ribosome binding
sites, translation
initiation and termination codons, elements from picornaviruses (particularly
an internal
ribosome entry site, or IRES, also referred to as a CITE sequence).
[00169] A DNA polynucleotide of the present invention may be a circular or
linearized
plasmid or vector, or other linear DNA which may also be non-infectious and
nonintegrating (i.e., does not integrate into the genome of vertebrate cells).
A linearized
plasmid is a plasmid that was previously circular but has been linearized, for
example, by
digestion with a restriction endonuclease. Linear DNA may be advantageous in
certain
situations as discussed, e.g., in Cherng, J.Y., et al., J. Control. Release
60:343-53 (1999),
and Chen, Z.Y., et al. Mol. Ther. 3:403-10 (2001), both of which are
incorporated herein
by reference. As used herein, the terms plasmid and vector can be used
interchangeably.
[00170] Alternatively, DNA virus genomes may be used to administer DNA
polynucleotides into vertebrate cells. In certain embodiments, a DNA virus
genome of
the present invention is nonreplicative, noninfectious, and/or nonintegrating.
Suitable
DNA virus genomes include without limitation, herpesvirus genomes, adenovirus
genomes, adeno-associated virus genomes, and poxvirus genomes. References
citing
methods for the in vivo introduction of non-infectious virus genomes to
vertebrate
tissues are well known to those of ordinary skill in the art, and are cited
supra.
[00171] In other embodiments, a polynucleotide of the present invention is
RNA, for
example, in the form of messenger RNA (mRNA). Methods for introducing RNA
sequences into vertebrate cells are described in U.S. Patent No. 5,580,859,
the disclosure
of which is incorporated herein by reference in its entirety.
CA 02658559 2009-01-20
WO 2008/039267 50 PCT/US2007/016529
[00172] Polynucleotides, nucleic acids, and nucleic acid fragments of the
present
invention may be associated with additional nucleic acids which encode
secretory or
signal peptides, which direct the secretion of a polypeptide encoded by a
nucleic acid
fragment or polynucleotide of the present invention. According to the signal
hypothesis,
proteins secreted by mammalian cells have a signal peptide or secretory leader
sequence
which is cleaved from the mature protein once export of the growing protein
chain across
the rough endoplasmic reticulum has been initiated. Those of ordinary skill in
the art are
aware that polypeptides secreted by vertebrate cells generally have,a signal
peptide fused
to the N-terminus of the polypeptide, which is cleaved from the complete or
"full length"
polypeptide to produce a secreted or "mature" form of the polypeptide. In
certain
embodiments, the native leader sequence is used, or a functional derivative of
that
sequence that retains the ability to direct the secretion of the polypeptide
that is operably
associated with it. Alternatively, a heterologous mammalian leader sequence,
or a
functional derivative thereof, may be used. For example, the wild-type leader
sequence
may be substituted with the leader sequence of human tissue plasminogen
activator
(TPA) or mouse 13-glucuronidase.
[00173] As used herein, the term "plasmid" refers to a construct made up of
genetic
material (i.e., nucleic acids). Typically a plasmid contains an origin of
replication which
is functional in bacterial host cells, e.g., Escherichia coli, and selectable
markers for
detecting bacterial host cells comprising the plasmid. Plasmids of the present
invention
may include genetic elements as described herein arranged such that an
inserted coding
sequence can be transcribed and translated in eukaryotic cells. Also, the
plasmid may
include a sequence from a viral nucleic acid. However, such viral sequences
normally are
not sufficient to direct or allow the incorporation of the plasmid into a
viral particle, and
the plasmid is therefore a non-viral vector. In certain embodiments described
herein, a
plasmid is a closed circular DNA molecule.
[00174] The term "expression" refers to the biological production of a product
encoded by
a coding sequence. In most cases a DNA sequence, including the coding
sequence, is
transcribed to form a messenger-RNA (mRNA). The messenger-RNA is then
translated
to form a polypeptide product which has a relevant biological activity. Also,
the process
of expression may involve further processing steps to the RNA product of
transcription,
such as splicing to remove introns, and/or post-translational processing of a
polypeptide
product.
CA 02658559 2009-01-20
WO 2008/039267 51 PCT/US2007/016529
[00175] As used herein, the term "polypeptide" is intended to encompass a
singular
"polypeptide" as well as plural "polypeptides," and comprises any chain or
chains of two
or more amino acids. Thus, as used herein, terms including, but not limited to
"peptide,"
"dipeptide," "tripeptide," "protein," "amino acid chain," or any other term
used to refer to
a chain or chains of two or more amino acids, are included in the definition
of a
"polypeptide," and the term "polypeptide" can be used instead of, or
interchangeably
with any of these terms. The term further includes 'polypeptides which have
undergone
post-translational modifications, for example, glycosylation, acetylation,
phosphorylation, amidation, derivatization by known protecting/blocking
groups,
proteolytic cleavage, or modification by non-naturally occurring amino acids.
[00176] Also included as polypeptides of the present invention are fragments,
derivatives,
analogs, or variants of the foregoing polypeptides, and any combination
thereof.
Polypeptides, and fragments, derivatives, analogs, or variants thereof of the
present
invention can be antigenic and immunogenic polypeptides related to influenza
virus
polypeptides, which are used to prevent or treat, i.e., cure, ameliorate,
lessen the severity
of, or prevent or reduce contagion of infectious disease caused by the
influenza virus.
[00177] As used herein, an "antigenic polypeptide" or an "immunogenic
polypeptide" is a
polypeptide which, when introduced into a vertebrate, interacts with the
vertebrate's
immune system molecules, i.e., is antigenic, and/or induces an immune response
in the
vertebrate, i.e., is immunogenic.
[00178] An affinity threshold associated with immunogenicity in the context of
HLA
class II DR molecules has been delineated (see, e.g., Southwood et al. J.
Immunology
160:3363-3373,1998, and U.S.S.N. 60/087192 filed 5/29/98). In order to define
a
biologically significant threshold of DR binding affinity, a database of the
binding
affinities of 32 DR-restricted epitopes for their restricting element (i.e.,
the HLA
molecule that binds the motif) was compiled. In approximately half of the
cases (15 of
32 epitopes), DR restriction was associated with high binding affinities, i.e.
binding
affinity values of 100 nM or less. In the other half of the cases (16 of 32),
DR restriction
was associated with intermediate affinity (binding affinity values in the 100-
1000 nM
range). In only one of 32 cases was DR restriction associated with an IC50 of
1000 nM
or greater. Thus, 1000 nM can be defined as an affinity threshold associated
with
immunogenicity in the context of DR molecules.
[00179] By an "isolated" influenza virus polypeptide or a fragment, variant,
or derivative
thereof is intended an influenza virus polypeptide or protein that is not in
its natural
CA 02658559 2009-01-20
WO 2008/039267 52 PCT/US2007/016529
form. No particular level of purification is required. For example, an
isolated influenza
virus polypeptide can be removed from its native or natural environment.
Recombinantly produced influenza virus polypeptides and proteins expressed in
host
cells are considered isolated for purposes of the invention, as are native or
recombinant
influenza virus polypeptides which have been separated, fractionated, or
partially or
substantially purified by any suitable technique, including the separation of
influenza
virus virions from eggs or culture cells in which they have been propagated.
In addition,
an isolated influenza virus polypeptide or protein can be provided as a live
or inactivated
viral vector expressing an isolated influenza virus polypeptide and can
include those
found in inactivated influenza virus vaccine compositions. Thus, isolated
influenza virus
polypeptides and proteins can be provided as, for example, recombinant
influenza virus
polypeptides, a purified subunit of influenza virus, a viral vector expressing
an isolated
influenza virus polypeptide, or in the form of an inactivated or attenuated
influenza virus
vaccine.
[00180] The term "immunogenic carrier" as used herein refers to a first
polypeptide or
fragment, variant, or derivative thereof which enhances the immunogenicity of
a second
polypeptide or fragment, variant, or derivative thereof. Typically, an
"immunogenic
carrier" is fused to or conjugated to the desired polypeptide or fragment
thereof. An
example of an "immunogenic carrier" is a recombinant hepatitis B core antigen
expressing, as a surface epitope, an immunogenic epitope of interest. See,
e.g., European
Patent No. EP 0385610 B1, which is incorporated herein by reference in its
entirety.
[00181] In the present invention, antigenic epitopes preferably contain a
sequence of at
least 8, at least 9, at least 10, at least 15, at least 20, at least 25, or
between about 8 to
about 30 amino acids contained within the amino acid sequence of an influenza
virus
polypeptide of the invention, e.g., an NP polypeptide, an M1 polypeptide or an
M2
polypeptide. Certain peptides comprising immunogenic or antigenic epitopes are
at least
8, 9, 10, 11, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90,
95, or 100
amino acid residues in length. Antigenic as well as immunogenic epitopes may
be
linear, i.e., be comprised of contiguous amino acids in a polypeptide, or may
be three
dimensional or conformational, i.e., where an epitope is comprised of non-
contiguous
amino acids which come together due to the secondary or tertiary structure of
the
polypeptide, thereby forming an epitope.
[00182] As to the selection of peptides or polypeptides bearing an antigenic
epitope (e.g.,
that contain a region of a protein molecule to which an antibody or T cell
receptor can
CA 02658559 2009-01-20
WO 2008/039267 53 PCT/US2007/016529
bind), it is well known in that art that relatively short synthetic peptides
that mimic part
of a protein sequence are routinely capable of eliciting an antiserum that
reacts with the
partially mimicked protein. See, e.g., Sutcliffe, J. G., et al., Science
219:660-666 (1983),
which is herein incorporated by reference.
[00183] Peptides capable of eliciting an immunogenic response are frequently
represented
in the primary sequence of a protein, can be characterized by a set of simple
chemical
rules, and are confined neither to immunodominant regions of intact proteins
nor to the
amino or carboxyl terminals. Peptides that are extremely hydrophobic and those
of six
or fewer residues generally are ineffective at inducing antibodies that bind
to the
mimicked protein; longer peptides, especially those containing proline
residues, usually
are effective. Sutcliffe et al., supra, at 661. For instance, 18 of 20
peptides designed
according to these guidelines, containing 8-39 residues covering 75% of the
sequence of
the influenza virus hemagglutinin HA1 polypeptide chain, induced antibodies
that
reacted with the HA1 protein or intact virus; and 12/12 peptides from the MuLV
polymerase and 18/18 from the rabies glycoprotein induced antibodies that
precipitated
the respective proteins.
[00184] Throughout this disclosure, "binding data" results are often expressed
in terms of
"IC50." IC50 is the concentration of peptide in a binding assay at which 50%
inhibition of
binding of a reference peptide is observed. Given the conditions in which the
assays are
run (i.e., limiting HLA proteins and labeled peptide concentrations), these
values
approximate KD values. Assays for determining binding are described in detail,
e.g., in
PCT publications WO 94/20127 and WO 94/03205, the disclosure of each which is
herein incorporated by reference. It should be noted that IC50 values can
change, often
dramatically, if the assay conditions are varied, and depending on the
particular reagents
used (e.g., HLA preparation, etc.). For example, excessive concentrations of
HLA
molecules will increase the apparent measured IC50 of a given ligand.
Alternatively,
binding is expressed relative to a reference peptide. Although as a particular
assay
~ becomes more, or less, sensitive, the IC50's of the peptides tested may
change somewhat,
the binding relative to the reference peptide will not significantly change.
For example,
in an assay run under conditions such that the IC50 of the reference peptide
increases 10-
fold, the IC50 values of the test peptides will also shift approximately 10-
fold. Therefore,
to avoid ambiguities, the assessment of whether a peptide is a good,
intermediate, weak,
or negative binder is generally based on its IC50, relative to the IC50 of a
standard
peptide. Binding may also be determined using other assay systems including
those
CA 02658559 2009-01-20
WO 2008/039267 54 PCT/US2007/016529
using: live cells (e.g., Ceppellini et al., Nature 339:392, 1989; Christnick
et al.; Nature
352:67, 1991; Busch et al., Int. Immunol. 2:443, 19990; Hill et al., J.
Immunol. 147:189,
1991; del Guercio et al., J. Immunol. 154:685, 1995), cell free systems using
detergent
lysates (e.g., Cerundolo et al., J. Immunol. 21:2069, 1991), immobilized
purified MHC
(e.g., Hill et al., J Immunol. 152, 2890, 1994; Marshall et al., J. Immunol.
152:4946,
1994), ELISA systems (e.g., Reay et al., EMBO J. 11:2829, 1992), surface
plasmon
resonance (e.g., Khilko et al., J. Biol. Chem. 268:15425, 1993); high flux
soluble phase
assays (Hammer et al., J. Exp. Med. 180:2353, 1994), and measurement of class
I MHC
stabilization or assembly (e.g., Ljunggren et al., Nature 346:476, 1990;
Schumacher et
al., Cell 62:563, 1990; Townsend et al., Cell 62:285, 1990; Parker et al., J.
Immunol.
149:1896, 1992).
[00185] The designation of a residue position in an epitope as the "carboxyl
terminus" or
the "carboxyl terminal position" refers to the residue position at the end of
the epitope
that is nearest to the carboxyl terminus of a peptide, which is designated
using
conventional nomenclature as defined below. "C+1" refers to the residue or
position
immediately following the C-terminal residue of the epitope, i.e., refers to
the residue
flanking the C-terminus of the epitope. The "carboxyl terminal position" of
the epitope
occurring at the carboxyl end of the multi-epitope construct may or may not
actually
correspond to the carboxyl terminal end of polypeptide. In preferred
embodiments, the
epitopes employed in the optimized multi-epitope constructs are motif-bearing
epitopes
and the carboxyl terminus of the epitope is defined with respect to primary
anchor
residues corresponding to a particular motif.
[00186] The designation of a residue position in an epitope as "amino
terminus" or
"amino-terminal position" refers to the residue position at the end of the
epitope which is
nearest to the amino terminus of a peptide, which is designated using
conventional
nomenclature as defined below. "N-1" refers to the residue or position
immediately
adjacent to the epitope at the amino terminal end (position number 1) of an
epitope. The
"amino terminal position" of the epitope occurring at the amino terminal end
of the
multi-epitope construct may or may not actually correspond to the amino
terminal end of
the polypeptide. In preferred embodiments, the epitopes employed in the
optimized
multi-epitope constructs are motif-bearing epitopes and the amino terminus of
the
epitope is defined with respect to primary anchor residues corresponding to a
particular
motif.
CA 02658559 2009-01-20
WO 2008/039267 55 PCT/US2007/016529
[00187] A "construct" as used herein generally denotes a composition that does
not occur
in nature. A construct can be produced by synthetic technologies, e.g.,
recombinant
DNA preparation and expression or chemical synthetic techniques for nucleic or
amino
acids. A construct can also be produced by the addition or affiliation'of one
material
with another such that the result is not found in nature in that form. A
"multi-epitope
construct" can be used interchangeably with the term "minigene" or "multi-
epitope
nucleic acid vaccine," and comprises multiple epitope nucleic acids that
encode peptide
epitopes of any length that can bind to a molecule functioning in the immune
system,
preferably a class I HLA and a T-cell receptor or a class II HLA and a T-cell
receptor.
All of the epitope nucleic acids in a multi-epitope construct can encode class
I HLA
epitopes or class II HLA epitopes. Class I HLA-encoding epitope nucleic acids
are
referred to as CTL epitope nucleic acids, and class II HLA-encoding epitope
nucleic
acids are referred to as HTL epitope nucleic acids. Some multi-epitope
constructs can
have a subset of the multi-epitope nucleic acids encoding class I HLA epitopes
and
another subset of the multi-epitope nucleic acids encoding class II HLA
epitopes. The
CTL epitope nucleic acids preferably encode an epitope peptide of about eight
to about
thirteen amino acids in length, more preferably about eight to about eleven
amino acids
in length, and most preferably about nine amino acids in length. The HTL
epitope
nucleic acids can encode an epitope peptide of about six to about thirty,
preferably seven
to about twenty three, preferably about seven to about seventeen, and even
more
preferably about eleven to about fifteen, and most preferably about thirteen
amino acids
in length. The multi-epitope constructs described herein preferably include
five or more,
ten or more, fifteen or more, twenty or more, or twenty-five or more epitope
nucleic
acids. All of the epitope nucleic acids in a multi-epitope construct may be
from one
organism (e.g., the nucleotide sequence of every epitope nucleic acid may be
present in
HIV strains), or the multi-epitope construct may include epitope nucleic acids
present in
two or more different organisms (e.g., some epitopes from HIV and some from
HCV).
As described hereafter, one or more epitope nucleic acids in the multi-epitope
construct
may be flanked by a spacer nucleic acid.
[00188] A "multi-epitope vaccine," which is synonymous with a "polyepitopic
vaccine,"
or a "multi-epitope construct" or "minigene" is a vaccine comprising multiple
epitopes.
[00189] "Cross-reactive binding" indicates that a peptide is bound by more
than one HLA
molecule; a synonym is "degenerate binding."
CA 02658559 2009-01-20
WO 2008/039267 56 PCT/US2007/016529
[00190] A "cryptic epitope" elicits a response by immunization with an
isolated peptide,
but the response is not cross-reactive in vitro when intact whole protein that
comprises
the epitope is used as an antigen.
[00191] A "dominant epitope" is an epitope that induces an immune response
upon
immunization with a whole native antigen (see, e.g., Sercarz, et al., Annu.
Rev. Immunol.
11:729-766, 1993). Such a response is cross-reactive in vitro with an isolated
peptide
epitope.
[00192] A "subdominant epitope" is an epitope which evokes little or no
response upon
immunization with whole antigens which comprise the epitope, but for which a
response
can be obtained by immunization with an isolated epitope, and this response
(unlike the
case of cryptic epitopes) is detected when whole protein is used to recall the
response in
vitro or in vivo.
[00193] With regard to a particular amino acid sequence, an "epitope" is a set
of amino
acid residues which is involved in recognition by a particular immunoglobulin,
or in the
context of T cells, those residues necessary for recognition by T cell
receptor proteins
and/or Major Histocompatibility Complex (MHC) receptors. In an immune system
setting, in vitro or in vivo, an epitope is the collective features of a
molecule, such as
primary, secondary and tertiary peptide structure, and charge, that together
form a site
recognized by an immunoglobulin, T cell receptor or HLA molecule. Throughout
this
disclosure epitope and peptide are often used interchangeably. It is to be
appreciated,
however, that isolated or purified protein or peptide molecules larger than
and
comprising an epitope of the invention are still within the bounds of the
invention.
[00194] A "flanking residue" is a residue that is positioned next to an
epitope. A flanking
residue can be introduced or inserted at a position adjacent to the N-terminus
or the C-
terminus of an epitope.
[00195] An "immunogenic peptide" or "peptide epitope" or "epitope" is a
peptide that
comprises an allele-specific motif or supermotif such that the peptide will
bind an HLA
molecule and induce a CTL and/or HTL response. Thus, immunogenic peptides of
the
invention are capable of binding to an appropriate HLA molecule and thereafter
inducing
a cytotoxic T cell response, or a helper T cell response, to the antigen from
which the
immunogenic peptide is derived.
[00196] "Heteroclitic analogs" are defined herein as a peptide with increased
potency for
a specific T cell, as measured by increased responses to a given dose, or by a
requirement
of lesser amounts to achieve the same response. Advantages of heteroclitic
analogs
CA 02658559 2009-01-20
WO 2008/039267 57 PCT/US2007/016529
ti
include that the epitopes can be more potent, or more economical (since a
lower amount
is required to achieve the same effect). In addition, modified epitopes might
overcome
antigen-specific T cell unresponsiveness (T cell tolerance).
[00197] "Human Leukocyte Antigen" or "HLA" is a human class I or class II
Major
Histocompatibility =Complex (MHC) protein (see, e.g., Stites, et al.,
Immunology, 8th
ed., Lange Publishing, Los Altos, Calif. (1994)).
[00198] An "HLA supertype or HLA family," as used herein, describes sets of
HLA
molecules grouped based on shared peptide-binding specificities. HLA class I
molecules
that share similar binding affinity for peptides bearing certain amino acid
motifs are
grouped into such HLA supertypes. The terms HLA superfamily, HLA supertype
family, HLA family, and HLA xx-like molecules (where xx denotes a particular
HLA
type), are synonyms. HLA types, include, for example, HLA-A1, -A2, A3/A11, -
A24, -
B7, B44.
[00199] As used herein, "high affinity" with respect to HLA class I molecules
is defined
as binding with an IC50, or KD value, of 50 nM or less; "intermediate
affinity" with
respect to HLA class I molecules is defined as binding with an IC50 or KD
value of
between about 50 and about 500 nM. "High affinity" with respect to binding to
HLA
class. II molecules is defined as binding with an IC50 or KD value of 100 nM
or less;
"intermediate affinity" with respect to binding to HLA class II molecules is
defined as
binding with an IC50 or KD value of between about 100 and about 1000 nM.
[00200] An " IC50" is the concentration bf peptide in a binding assay at which
50%
inhibition of binding of a reference peptide is observed. Depending on the
conditions in
which the assays are run (i.e., limiting HLA proteins and labeled peptide
concentrations),
these values may approximate KD values.
[00201] The terms "identical" or percent "identity," in the coritext of two or
more peptide
sequences, refer to two or more sequences or subsequences that are the same or
have a
specified percentage of amino acid residues that are the same, when compared
and
aligned for maximum correspondence over a comparison window, as measured using
a
sequence comparison algorithm or by manual alignment and visual inspection.
[00202] "Introducing" an amino acid residue at a particular position in a
multi-epitope
construct, e.g., adjacent, at the C-terminal side, to the C-terminus of the
epitope,
encompasses configuring multiple epitopes such that a desired residue is at a
particular
position, e.g., adjacent to the epitope, or such that a deleterious residue is
not adjacent to
the C-terminus of the epitope. The term also includes inserting an amino acid
residue,
CA 02658559 2009-01-20
WO 2008/039267 58 PCT/US2007/016529
preferably a preferred or intermediate amino acid residue, at a particular
position. An
amino acid residue can also be introduced into a sequence by substituting one
amino acid
residue for another. Preferably, such a substitution is made in accordance
with analoging
principles set forth, e.g., in PCT application number PCT/USOO/19774.
[00203] The phrases "isolated" or "biologically pure" refer to material that
is substantially
or essentially free from components which normally accompany the material as
it is
found in its native state. Thus, isolated peptides in accordance with the
invention
preferably do not contain materials normally associated with the peptides in
their in situ
environment.
[00204] "Link" or "join" refers to any method known in the art for
functionally
connecting peptides, including, without limitation, recombinant fusion,
covalent
bonding, disulfide bonding, ionic bonding, hydrogen bonding, and electrostatic
bonding.
[00205] The term "directly joined" refers to being joined without anything
intervening.
For example, in the case of two peptides being directly joined, one peptide
would be
joined or bonded to another peptide, as described above, without any sequence,
molecule, spacer, linker, etc. intervening between the two peptides.
[00206] The term "indirectly joined" refers to being joined with something
intervening.
For example, in the case of two peptides being indirectly joined, one peptide
would be
joined or bonded to another peptide, as described above, with a sequence,
molecule,
spacer, linker, etc. intervening between the two peptides.
[00207] "Major Histocompatibility Complex" or "MHC" is a cluster of genes that
plays a
role in control of the cellular interactions responsible for physiologic
immune responses.
In humans, the MHC complex is also known as the HLA complex. For a detailed
description of the MHC and HLA complexes, see, Paul, Fundamental Immunology,
3rd
ed., Raven Press, New York, 1993.
[00208] As used herein, "middle of the peptide" is a position in a peptide
that is neither an
amino nor a carboxyl terminus.
[00209] A "minimal number of junctional epitopes" as used herein refers to a
number of
junctional epitopes that is lower than what would be created using random
selection
criteria.
[00210] The term "motif' refers to the pattern of residues in a peptide of
defined length,
usually a peptide of from about 8 to about 13 amino acids for a class I HLA
motif and
from about 6 to about 25 amino acids for a class II HLA motif, which is
recognized by a
particular HLA molecule. Peptide motifs are typically different for each
protein encoded
CA 02658559 2009-01-20
WO 2008/039267 59 PCT/US2007/016529
by each human HLA allele and differ in the pattern of the primary and
secondary anchor
residues.
[00211] A "supermotif' is an amino acid sequence for a peptide that provides
binding
specificity shared by HLA.molecules encoded by two or more HLA alleles.
Preferably,
a supermotif-bearing peptide is recognized with high or intermediate affinity
(as defined
herein) by two or more HLA antigens.
[00212] The term "peptide" is used interchangeably with "oligopeptide" in the
present
specification to designate a series of residues, typically L-amino acids,
connected one to
the other, typically by peptide bonds between the a-amino and carboxyl groups
of
adjacent amino acids. The preferred CTL-inducing peptides of the invention are
13
residues or less in length and usually consist of between about 8 and about 11
residues,
preferably 9 or 10 residues. The preferred HTL-inducing peptides are less than
about 50
residues in length and usually consist of between about 6 and about 30
residues, more
usually between about 12 and 25, and often between about 15 and 20 residues.
[00213] The term "CTL epitope" refer to a peptide of defined length that can
be from
about 8 to about 13 amino acids in length, from about 9 to about 11 amino
acids in
length, or from about 9 to about 10 amino acids in length, which is recognized
by a
particular HLA class I molecule.
[00214] The term "HTL epitope" refers to a peptide of defined length that can
be from
about 6 to about 30 amino acids in length, from about 8 to about 30 amino
acids in
length, from about 10 to about 30 amino acids, from about 12 to about 30 amino
acids in
length, from about 6 to about 25 amino acids in length, from about 8 to about
25 amino
acids in length, from about 10 to about 25 amino acids, from about 12 to about
25 amino
acids in length, from about 6 to about 18 amino acids in length, from about 8
to about 18
amino acids in length, from about 10 to about 18 amino acids, or from about 12
to about
18 amino acids in length, which is recognized by a particular HLA class II
molecule.
[00215] A "PanDR binding peptide or pan-DR binding epitope" is a member of a
family
of molecules that binds more than one HLA class II DR molecule. The pattern
that
defines this family of molecules can be thought of as an HLA Class II
supermotif. For
example, PADRE binds to most HLA-DR molecules and stimulates in vitro and in
vivo
human helper T lymphocyte (HTL) responses.
[00216] A "negative binding residue" or "deleterious residue" is an amino acid
which, if
present at certain positions (typically not primary anchor positions) in a
peptide epitope,
CA 02658559 2009-01-20
WO 2008/039267 60 PCT/US2007/016529
results in decreased binding affinity of the peptide for the peptide's
corresponding HLA
molecule.
[00217] "Optimizing" refers to increasing the immunogenicity or antigenicity
of a multi-
epitope construct having at least one epitope pair by sorting epitopes to
minimize the
occurrence of junctional epitopes, inserting flanking residues that flank the
C-terminus or
N-terminus of an epitope, and inserting spacer residue to further prevent the
occurrence
of junctional epitopes or to provide a flanking residue. An increase in
immunogenicity
or antigenicity of an optimized multi-epitope construct is measured relative
to a multi-
epitope construct that has not been constructed based on the optimization
parameters and
is using assays known to those of skill in the art, e.g., assessment of
immunogenicity in
HLA transgenic mice, ELISPOT, inteferon-gamma release assays, tetramer
staining,
chromium release assays, and presentation on dendritic cells.
[00218] "Pathogenic virus strain" is used herein to refer to any virus strain
that is capable
of causing disease; preferably, the virus is on the current World Health
Organization
(WHO), Centers for Disease Control and Prevention (CDC), Food and Drug
Administration (FDA) or other public health authority list of likely
circulating viruses;
more preferably, the virus has been indicated as one of the three annual viral
strains for
inclusion in an influenza annual vaccine (i.e., "seasonal strains"). This
information is
readily available from these agencies, e.g., at
http://www.fda.gov/cber/flu/flu.htm or at
http://www.who.int/csr/disease/influenza/vaccinerecommendations 1
/en/index.html.
[00219] "Pharmaceutically acceptable" refers to a generally non-toxic, inert,
and/or
physiologically compatible composition.
[00220] "Presented to an HLA Class I processing pathway" means that the multi-
epitope
constructs are introduced into a cell such that they are largely processed by
an HLA
Class I processing pathway. Typically, multi-epitope constructs are introduced
into the
cells using expression vectors that encode the multi-epitope constructs. HLA
Class II
epitopes that are encoded by such a multi-epitope construct are also presented
on Class II
molecules, although the mechanism of entry of the epitopes into the Class II
processing
pathway is not defined.
[00221] A "primary anchor residue" or a "primary MHC anchor" is an amino acid
at a
specific position along a peptide sequence that is understood to provide a
contact point
between the immunogenic peptide and the HLA molecule. One to three, usually
two,
primary anchor residues within a peptide of defined length generally defines a
"motif'
for an immunogenic peptide. These residues are understood to fit in close
contact with
CA 02658559 2009-01-20
WO 2008/039267 61 PCT/US2007/016529
peptide binding grooves of an HLA molecule, with their side chains buried in
specific
pockets of the binding grooves themselves. In one embodiment, for example, the
primary anchor residues of an HLA class I epitope are located at position 2
(from the
amino terminal position) and at the carboxyl terminal position of a 9-residue
peptide
epitope in accordance with the invention. The primary anchor positions for
each motif
and supermotif are described, for example, in Tables I and III of
PCT/US00/27766, or
PCT/US00/19774, the disclosure of each which is herein incorporated by
reference.
Preferred amino acids that can serve as in the anchors for most Class II
epitopes consist
of M and F in position one and V, M, S, T, A and C in position six. Tolerated
amino
acids that can occupy these positions for most Class II epitopes consist of L,
I, V, W, and
Y in position one and P, L and I in position six. The presence of these amino
acids in
positions one and six in Class 11 epitopes defines the HLA-DR1, 4, 7
supermotif. The
HLA-DR3 binding motif is defined by preferred amino acids from the group of L,
I, V,
M, F, Y and A in position one and D, E, N, Q, S and T in position four and K,
R and H in
position six. Other amino acids may be tolerated in these positions but they
are not
preferred.
[00222] Furthermore, analog peptides can be created by altering the presence
or absence
of particular residues in these primary anchor positions. Such analogs are
used to
modulate the binding affinity of a peptide comprising a particular motif or
supermotif
[00223] "Promiscuous recognition" occurs where a distinct peptide is
recognized by the
same T cell clone in the context of various HLA molecules. Promiscuous
recognition or
binding is synonymous with cross-reactive binding.
[00224] A "protective immune response" refers to a CTL and/or an HTL response
to an
antigen derived from an infectious agent, which in some way prevents or at
least partially
arrests disease symptoms, side effects or progression, and clears the
infectious agent.
The immune response may also include an antibody response that has been
facilitated by
the stimulation of helper T cells.
[00225] The term "residue" refers to an amino acid or amino acid mimetic
incorporated
into a peptide or protein by an amide bond or amide bond mimetic.
[00226] A "secondary anchor residue" is an amino acid at a position other than
a primary
anchor position in a peptide that may influence peptide binding. A secondary
anchor
residue occurs at a significantly higher frequency amongst bound peptides than
would be
expected by random distribution of amino acids at one position. The secondary
anchor
residues are said to occur at "secondary anchor positions." A secondary anchor
residue
CA 02658559 2009-01-20
WO 2008/039267 62 PCT/US2007/016529
can be identified as a residue which is present at a higher frequency among
high or
intermediate affinity binding peptides, or a residue otherwise associated with
high or
intermediate affinity binding. For example, analog peptides can be created by
altering
the presence or absence of particular residues in these secondary anchor
positions. Such
analogs are used to finely modulate the binding affinity of a peptide
comprising a
particular motif or supermotif. The terminology "fixed peptide" is sometimes
used to
refer to an analog peptide.
[00227] "Sorting epitopes" refers to determining or designing an order of the
epitopes in a
multi-epitope construct.
[00228] A "spacer" refers to a sequence that is inserted between two epitopes
in a multi-
epitope construct to prevent the occurrence of junctional epitopes and/or to
increase the
efficiency of processing. A multi-epitope construct may have one or more
spacer nucleic
acids. A spacer nucleic acid may flank each epitope nucleic acid in a
construct, or the
spacer nucleic acid to epitope nucleic acid ratio may be about 2 to 10, about
5 to 10,
about 6 to 10, about 7 to 10, about 8 to 10, or about 9 to 10, where a ratio
of about 8 to
has been determined to yield favorable results for some constructs.
[00229] The spacer nucleic acid may encode one or more amino acids. A spacer
nucleic
acid flanking a class I HLA epitope in a multi-epitope construct is preferably
between
one and about eight amino acids in length, between two and eight amino acids
in length,
between three and eight amino acids in length, between four and eight amino
acids in
length, between five and eight amino acids in length, between six and eight
amino acids
in length, or between seven and eight amino acids in length. A spacer nucleic
acid
flanking a class II HLA epitope in a multi-epitope construct is preferably
greater than
five, six, seven, or more amino acids in length, and more preferably five or
six amino
acids in length.
[00230] The number of spacers in a construct, the number of amino acids in a
spacer, and
the amino acid composition of a spacer can be selected to optimize epitope
processing
and/or minimize junctional epitopes. It is preferred that spacers are selected
by
concomitantly optimizing epitope processing and junctional motifs. Suitable
amino
acids for optimizing epitope processing are described herein. Also, suitable
amino acid
spacing for minimizing the number of junctional epitopes in a construct are
described
herein for class I and class II HLAs. For example, spacers flanking class II
HLA
epitopes preferably include G, P, and/or N residues as these are not generally
known to
be primary anchor residues (see, e.g., PCT/USOO/19774). A particularly
preferred spacer
CA 02658559 2009-01-20
WO 2008/039267 63 PCT/US2007/016529
for flanking a class II HLA epitope includes alternating G and P residues, for
example,
(GP)n, (PG),,, (GP).,G, (PG)õP, and so forth, where n is an integer between
one and ten,
preferably two or about two, and where a specific example of such a spacer is
GPGPG or
PGPGP. A preferred spacer, particularly for class I HLA epitopes, comprises
one, two,
three or more consecutive alanine (A) residues, optionally preceded by K, N or
G.
[00231] In some multi-epitope constructs, it is sufficient that each spacer
nucleic acid
encodes the same amino acid sequence. In multi-epitope constructs, having two
spacer
nucleic acids encoding the same amino acid sequence, the spacer nucleic acids
encoding
those spacers may have the same or different nucleotide sequences, where
different
nucleotide sequences may be preferred to decrease the likelihood of unintended
recombination events when the multi-epitope construct is inserted into cells.
[00232] In other multi-epitope constructs, one or more of the spacer nucleic
acids may
encode different amino' acid sequences. While many of the spacer nucleic acids
may
encode the same amino acid sequence in a multi-epitope construct, one, two,
three, four,
five or more spacer nucleic acids may encode different amino acid sequences,
and it is
possible that all of the spacer nucleic acids in a multi-epitope construct
encode different
amino acid sequences. Spacer nucleic acids may be optimized with respect to
the
epitope nucleic acids they flank by determining whether a spacer sequence will
maximize epitope processing and/or minimize junctional epitopes, as described
herein.
[00233] Multi-epitope constructs may be distinguished from one another
according to
whether the spacers in one construct optimize epitope processing or minimize
junctional
epitopes over another construct, and preferably, constructs may be
distinguished where
one construct is concomitantly optimized for epitope processing and junctional
epitopes
over the other. Computer assisted methods and in vitro and in vivo laboratory
methods
for determining whether a construct is optimized for epitope processing and
junctional
motifs are described herein.
[00234] "Synthetic peptide" refers to a peptide that is not naturally
occurring, but is man-
made using such methods as chemical synthesis or recombinant DNA technology.
[00235] A "TCR contact residue" or "T cell receptor contact residue" is an
amino acid
residue in an epitope that is understood to be bound by a T cell receptor;
these are
defined herein as not being any primary MHC anchor. T cell receptor contact
residues
are defined as the position/positions in the peptide where all analogs tested
induce T-cell
recognition relative to that induced with a wild type peptide.
CA 02658559 2009-01-20
WO 2008/039267 64 PCT/US2007/016529
[00236] The term "homology," as used herein, refers to a degree of
complementarity
between two nucleotide sequences. The word "identity" may substitute for the
word
"homology" when a nucleic acid has the same nucleotide sequence as another
nucleic
acid. Sequence homology and sequence identity can also be determined by
hybridization
studies under high stringency and/or low stringency, and disclosed herein are
nucleic
acids that hybridize to the multi-epitope constructs under low stringency or
under high
stringency. Also, sequence homology and sequence identity can be determined by
analyzing sequences using algorithms and computer programs known in the art.
Such
methods may be used to assess whether a nucleic acid is identical or
homologous to the
multi-epitope constructs disclosed herein. The invention pertains in part to
nucleotide
sequences having 80% or more, 85% or more, 90% or more, 95% or more, 97% or
more,
98% or more, or 99% or more identity to the nucleotide sequence of a multi-
epitope
construct disclosed herein.
[00237] As used herein, the term "stringent conditions" refers to conditions
which permit
hybridization between nucleotide sequences and the nucleotide sequences of the
disclosed multi-epitope constructs. Suitable stringent conditions can be
defined by, for
example, the concentrations of salt or formamide in the prehybridization and
hybridization solutions, or by the hybridization temperature, and are well
known in the
art. In particular, stringency can be increased by reducing the concentration
of salt,
increasing the concentration of formamide, or raising the hybridization
temperature. For
example, hybridization under high stringency conditions could occur in about
50%
formamide at about 37 C to 42 C. In particular, hybridization could occur
under high
stringency conditions at 42 C in 50% formamide, 5 X SSPE, 0.3% SDS, and 200
g/ml
sheared and denatured salmon sperm DNA or at 42 C in a solution comprising 50%
formamide, 5 X SSC (750 mM NaCI, 75 mM trisodium citrate), 50 mM sodium
phosphate (pH 7.6), 5 X Denhardt's solution, 10% dextran sulfate, and 20 g/ml
denatured, sheared salmon sperm DNA, followed by washing the filters in 0.1 X
SSC at
about 65 C. Hybridization could occur under reduced stringency conditions in
about
35% to 25% formamide at about 30 C to 35 C. For example, reduced stringency
conditions could occur at 35 C in 35% formamide, 5 X SSPE, 0.3% SDS, and 200
g/ml
sheared and denatured salmon sperm DNA. The temperature range corresponding to
a
particular level of stringency can be further narrowed by calculating the
purine to
pyrimidine ratio of the nucleic acid of interest and adjusting the temperature
accordingly.
Variations on the above ranges and conditions are well known in the art.
CA 02658559 2009-01-20
WO 2008/039267 65 PCT/US2007/016529
[00238] In addition -to utilizing hybridization studies to assess sequence
identity or
sequence homology, known computer programs may be used to determine whether a
particular nucleic acid is homologous to a multi-epitope construct disclosed
herein. An
example of such a program is the Bestfit program (Wisconsin Sequence Analysis
Package, Version 8 for Unix, Genetics Computer Group, University Research
Park, 575
Science Drive, Madison, Wis. 53711), and other sequence alignment programs are
known in the art and may be utilized for determining whether two or more
nucleotide
sequences are homologous. Bestfit uses the local homology algorithm of Smith
and
Waterman, Advances in Applied Mathematics 2: 482-489 (1981), to find the best
segment of homology between two sequences. When using Bestfit or any other
sequence alignment program to determine whether a particular sequence is, for
instance,
95% identical to a reference sequence, the parameters may be set such that the
percentage of identity is calculated over the full length of the reference
nucleotide
sequence and that gaps in homology of up to 5% of the total number of
nucleotides in the
reference sequence are allowed.
1002391 The nomenclature used to describe peptide compounds follows the
conventional
practice wherein the amino group is presented to the left (the N-terminus) and
the
carboxyl group to the right (the C-terminus) of each amino acid residue. When
amino
acid residue positions are referred to in an epitope, they are numbered in an
amino to
carboxyl direction with position one being the position closest to the amino
terminal end
of the epitope, or the peptide or protein of which it may be a part. In the
formulae
representing selected specific embodiments of the present invention, the amino-
and
carboxyl-terminal groups, although not specifically shown, are in the forrn
they would
assume at physiologic pH values, unless otherwise specified. In the amino acid
structure
formulae, each residue is generally represented by standard three-letter or
single-letter
designations. The L-form of an amino acid residue is represented by a capital
single
letter or a capital first letter of a three-letter symbol, and the D-form for
those amino
acids having D-forms is represented by a lower case single letter or a lower
case three
letter symbol. Glycine has no asymmetric carbon atom and is simply referred to
as
"Gly" or G.
[00240] Symbols for the amino acids are shown below.
CA 02658559 2009-01-20
WO 2008/039267 66 PCT/US2007/016529
Single Letter Symbol Three Letter Symbol Amino Acids
A Ala Alanine
C Cys Cysteine
D Asp Aspartic Acid
E Glu Glutamic Acid
F Phe Phenylalanine
G Gly Glycine
H His Histidine
I Ile Isoleucine
K Lys Lysine
L Leu Leucine
M Met Methionine
N Asn Asparagine
P Pro Proline
Q Gln Glutamine
R Arg Arginine
S Ser Serine
T Thr Threonine
V Val Valine
W Trp Tryptophan
Y Tyr Tyrosine
[00241] Amino acid "chemical characteristics" are defined as: Aromatic (F, W,
Y);
Aliphatic-hydrophobic (L, I, V, M); Small polar (S, T, C); Large polar (Q, N);
Acidic (D,
E); Basic (R, H, K); Proline; Alanine; and Glycine.
[00242] Acronyms used herein are as follows:
APC: Antigen presenting cell
CD3: Pan T cell marker
CD4: Helper T lymphocyte marker
CD8: Cytotoxic T lymphocyte marker
CFA: Complete Freund's Adjuvant
CTL: Cytotoxic T lymphocytes
DC: Dendritic cells. DC functioned as potent antigen presenting cells by
stimulating
cytokine release from CTL lines that were specific for a model peptide derived
from
hepatitis B virus (HBV). In vitro experiments using DC pulsed ex vivo with an
HBV
peptide epitope have stimulated CTL immune responses in vitro following
delivery to
naive mice.
DMSO: Dimethylsulfoxide
ELISA: Enzyme-linked immunosorbant assay
E:T: Effector:target ratio
FCS: Fetal calf serum
CA 02658559 2009-01-20
WO 2008/039267 67 PCT/US2007/016529
G-CSF: Granulocyte colony-stimulating factor
GM-CSF: Granulocyte-macrophage (monocyte)-colony stimulating factor
HBV: Hepatitis B virus
HLA: Human leukocyte antigen
HLA-DR: Human leukocyte antigen class II
HPLC: High Performance Liquid Chromatography
HTC: Helper T cells
HTL: Helper T Lymphocyte
ID: Identity
IFA: Incomplete Freund's Adjuvant
IFNy: Interferon gamma
IL-4: Interleukin-4 cytokine
IV: Intravenous
LU30%: Cytotoxic activity required to achieve 30% lysis at a 100:1 (E:T) ratio
MAb: Monoclonal antibody
MLR: Mixed lymphocyte reaction
MNC: Mononuclear cells
PB: Peripheral blood
PBMC: Peripheral blood mononuclear cell
SC: Subcutaneous
S.E.M.: Standard error of the inean
QD: Once a day dosing
TCR: T cell receptor
WBC: White blood cells
[00243] In particular embodiments to prevent HTL junctional epitopes, a spacer
composed of amino acid residues that do not correspond to any known HLA Class
II
anchor residue, are used, e.g, alternating G and P residues (a GP spacer) is
included
between two HTL epitopes.
[00244] Another aspect of the invention, (consideration (ii) above) involves
the
introduction or substitution of particular amino acid residues at positions
that flank
epitopes, e.g., a position immediately adjacent to the C-terminus of the
epitope, thereby
generating multi-epitope constructs with enhanced antigenicity and
immunogenicity
compared to constructs that do not contain the particular residue introduced
or
substituted at that site, i.e., non-optimized multi-epitope constructs. The
methods of
CA 02658559 2009-01-20
WO 2008/039267 68 PCT/US2007/016529
optimizing multi-epitope constructs comprise a step of introducing a flanking
residue,
preferably K, N, G, R, or A at the C+1 position of the epitope, i.e., the
position
immediately adjacent to the C-terminus of the epitope. In an alternative
embodiment,
residues that contribute to decreased immunogenicity, i.e., negatively charged
residues,
e.g., D, aliphatic residues (I, L, M, V) or aromatic non-tryptophan residues,
are replaced.
The flanking residue can be introduced by positioning appropriate epitopes to
provide
the favorable flanking residue, or by inserting a specific residue.
Eliminating Class I and Class II Junctional Epitopes and Testing for Class II
Restricted
Responses In Vivo
[00245] As a further element in eliminating junctional epitopes, spacer
sequences can be
inserted between two epitopes that create a junctional epitope when
juxtaposed.
[00246] In one embodiment, to correct the problem of junctional epitopes for
HTL
epitopes, a spacer of, for example, five amino acids in length is inserted
between the two
epitopes. The amino acid residues incorporated into such a spacer afe
preferably those
amino acid residues that are not known to be primary anchor residues for any
of the HLA
Class II binding motifs. Such residues include G, P, and N. In a preferred
embodiment,
a spacer with the sequence GPGPG is inserted between two epitopes. Previous
work has
demonstrated that the GP spacer is particularly effective in disrupting Class
II binding
interactions (Sette et al., J. Immunol., 143:1268-73 (1989)). All known human
Class II
binding motifs and the mouse IAb (the Class II expressed by HLA transgenic
mice) do
not tolerate either G or P at these main anchor positions, which are spaced
four residues
apart. This approach virtually guarantees that no Class II restricted epitopes
can be
formed as junctional epitopes.
[00247] Polypeptides are synthesized incorporating influenza-derived HTL
epitopes.
These epitopes are broadly cross-reactive HLA DR binding epitopes. These
epitopes
will also efficiently bind the murine IAb Class II molecule.
[00248] Responses against multiple influenza-derived Class II epitopes can be
simultaneously induced, and IAb/DR crossreactivity can be utilized to
investigate the
immunogenicity of various constructs incorporating HTL epitope candidates.
Finally,
appropriate spacers can be employed to effectively disrupt Class II junctional
epitopes
that would otherwise interfere with effective vaccine immunogenicity.
[00249] In the case of Class I restricted responses, one case of a naturally
occurring
junctional epitope and the consequent inhibition of epitope specific responses
has been
CA 02658559 2009-01-20
WO 2008/039267 69 PCT/US2007/016529
presented by McMichael and coworkers (Tussey et al., Immunity, 3(l):65-77
(1995)).
To address the problem of junctional epitopes for Class I, similar analyses
can be
performed. For example, a specific computer program is employed to identify
potential
Class I restricted junctional epitopes, by screening for selected murine
motifs and for the
most common human Class I HLA A and B motifs.
[00250] Spacer sequences can also similarly be employed to prevent CTL
junctional
epitopes. Often, very small residues such as A or G are preferred spacer
residues. G also
occurs relatively infrequently as a preferred primary anchor residue (see,
e.g.,
PCT/US00/24802) of an HLA Class I binding motif. These spacers can vary in
length,
e.g., spacer sequences can typically be 1, 2, 3, 4, 5, 6, 7, or 8 amino acid
residues in
length and are sometimes longer. Smaller lengths are often preferred because
of physical
constraints in producing the multi-epitope construct.
Sorting and Optimization of Multi-Epitope Constructs
[00251] To develop multi-epitope constructs using the invention, the epitopes
for
inclusion in the multi-epitope construct are sorted and optimized using the
parameters
defined herein. Sorting and optimization can be performed using a computer or,
for
fewer numbers of epitopes, not using a computer. Methods of sorting and
optimization
and disclosed in WO 02/083714, the disclosure of which is herein incorporated
by
reference.
[00252] Multi-epitope constructs can also be optimized by determining the
structure of
each construct to be considered. Macromolecular structures such as polypeptide
structures can be described in terms of various levels of organization. For a
general
discussion of this organization, see, e.g., Alberts et al., Molecular Biology
of the Cell (3a
ed., 1994) and Cantor and Schimmel, Biophysical Chemistry Part I: The
Conformation
of Biological Macromolecules (1980). "Primary structure" refers to the amino
acid
sequence of a particular peptide. "Secondary structure" refers to locally
ordered, three
dimensional structures within a polypeptide. These structures are commonly
known as
domains. Domains are portions of a polypeptide that form a compact unit of the
polypeptide. Typical domains are made up of sections of lesser organization
such as
stretches of (3-sheet and a-helices. "Tertiary structure" refers to the
complete three
dimensional structure of a polypeptide monomer. "Quaternary structure" refers
to the
three dimensional structure formed by the noncovalent association of
independent
tertiary units.
CA 02658559 2009-01-20
WO 2008/039267 70 PCT/US2007/016529
[00253] Structural predictions such as charge distribution,
hydrophobic/hydrophilic
region analysis, or folding predictions can be performed using sequence
analysis
programs known to those of skill in the art, for example, hydrophobic and
hydrophilic
domains can be identified (see, e.g., Kyte & Doolittle, J. Mol. Biol. 157:105-
132 (1982)
and Stryer, Biochemistry (3`d ed. 1988); see also any of a number of Internet
based
sequence analysis programs, such as those found at dot.imgen.bcm.tmc.edu.
[00254] A three-dimensional structural model of a multi-epitope construct can
also be
generated. This is generally performed by entering amino acid sequence to be
analyzed
into the computer system. The amino acid sequence represents the primary
sequence or
subsequence of the protein, which encodes the structural information of the
protein. The
three-dimensional structural model of the protein is then generated by the
interaction of
the computer system, using software known to those of skill in the art.
[00255] The amino acid sequence represents a primary structure that encodes
the
information necessary to form the secondary, tertiary and quaternary structure
of the
protein of interest. The software looks at certain parameters encoded by the
primary
sequence to generate the structural model. These parameters are referred to as
"energy
terms," and primarily include electrostatic potentials, hydrophobic
potentials, solvent
accessible surfaces, and hydrogen bonding. Secondary energy terms include van
der
Waals potentials. Biological molecules form the structures that minimize the
energy
terms in a cumulative fashion. The computer program is therefore using these
terms
encoded by the primary structure or amino acid sequence to create the
secondary
structural model. The tertiary structure of the protein encoded by the
secondary structure
is then formed on the basis of the energy terms of the secondary structure.
The user can
enter additional variables such as whether the protein is membrane bound or
soluble, its
location in the body, and its cellular location, e.g., cytoplasmic, surface,
or nuclear.
These variables along with the energy terms of the secondary structure are
used to form
the model of the tertiary structure. In modeling the tertiary structure, the
computer
program matches hydrophobic faces of secondary structure with like, and
hydrophilic
faces of secondary structure with like. Those multi-epitope constructs that
are most
readily accessible to the HLA processing apparatus are then selected.
Assessment of Immunogenicity of Multi-Epitope Vaccines
[00256] The development of multi-epitope constructs represents a unique
challenge,
because the species-specificity of the peptide binding to MHC. Different MHC
types
CA 02658559 2009-01-20
WO 2008/039267 71 PCT/US2007/016529
from different species tend to bind different sets of peptides (Rammensee et
al.,
Immunogenetics, 41(4):178-228 (1995)). As a result, it is not possible to test
in regular
laboratory animals a construct composed of human epitopes. Alternatives to
overcome
this limitation are generally available. They include: 1) testing analogous
constructs
incorporating epitopes restricted by non-human MHC; 2) reliance on control
epitopes
restricted by non human MHC; 3) reliance on crossreactivity between human and
non-
human MHC; 4) the use of HLA transgenic animals; and 5) antigenicity assays
utilizing
human cells in vivo. The following is a brief overview of the' development of
the
technology for analyzing antigenicity and immunogenicity.
Class I HLA Transgenics
[00257] The utility of HLA transgenic mice for the purpose of epitope
identification
(Sette et al., J Immunol, 153(12):5586-92 (1994); Wentworth et al., Int
Immunol,
8(5):651-9 (1996); Engelhard et al., Jlmmunol, 146(4):1226-32 (1991); Man et
al., Int
Immunol, 7(4):597-605 (1995); Shirai et al., J Immunol, 154(6):2733-42
(1995)), and
vaccine development (Ishioka et al., J Immunol, 162(7):3915-25 (1999)) has
been
established. Most of the published reports have investigated the use of HLA
A2.lKb
mice but it should be noted that B*27, and B*3501 mice are also available.
Furthermore,
HLA A*11/Kb mice (Alexander et al., J Immunol, 159(10):4753-61 (1997)), HLA
A24/Kb, HLA B7/Kb and HLA A1/Kb mice have also been generated.
[00258] Data from 38 different potential epitopes was analyzed to determine
the level of
overlap between the A2.1-restricted CTL repertoire of A2.1/Kb-transgenic mice
and
A2.l+humans (Wentworth et al., Eur Jlmmunol, 26(1):97-101 (1996)). In both
humans
and mice, an MHC peptide binding affinity threshold of approximately 500 nM
correlates with the capacity of a peptide to elicit a CTL response in vivo. A
high level of
concordance between the human data in vivo and mouse data in vivo was observed
for
85% of the high-binding peptides, 58% of the intermediate binders, and 83% of
the
low/negative binders. Similar results were also obtained with HLA A11 and HLA
B7
transgenic mice (Alexander et al., J Immunol, 159(10):4753-61 (1997)). Thus,
because
of the extensive overlap that exists between T cell receptor repertoires of
HLA transgenic
mouse and human CTLs, transgenic mice are valuable for assessing
immunogenicity of
the multi-epitope constructs described herein.
[00259] The different specificities of TAP transport as it relates to HLA A11
mice does
not prevent the use of HLA-A11 transgenic mice of evaluation of
immunogenicity.
CA 02658559 2009-01-20
WO 2008/039267 72 PCT/US2007/016529
While both murine and human TAP efficiently transport peptides with an
hydrophobic
end, only human TAP has been reported to efficiently transport peptides with
positively
charged C terminal ends, such as the ones bound by A3, All and other members
of the
A3 supertype. This concern does not apply to A2, Al or B7 because both murine
and
human TAP should be equally capable of transporting peptides bound by A2, B7
or Al .
Consistent with this understanding, Vitiello (Vitiello et al., J Exp Med,
173(4):1007-15
(1991)) and Rotzschke (Rotzschke 0, Falk K., Curr Opin Immunol, 6(1):45-51
(1994))
suggested that processing is similar in mouse and human cells, while Cerundolo
(Rotzschke 0, Falk K., Curr Opin Immunol, 6(1):45-51 (1994)) suggested
differences in
murine versus human cells, both expressing HLA A3 molecules. However, using
HLA
A11 transgenics, expression of HLA molecules on T and B cells in vivo has been
observed, suggesting that the reported unfavorable specificity of murine TAP
did not
prevent stabilization and transport of A11/Kb molecules in vivo (Alexander et
al., J
Immunol, 159(10):4753-61 (1997)). These data are in agreement with the
previous
observation that peptides with charged C termini could be eluted from murine
cells
transfected with All molecules (Maier et al., Immunogenetics; 40(4):306-8
(1994)).
Responses in HLA A11 mice to complex antigens, such as influenza, and most
importantly to All restricted epitopes encoded by multi-epitope constructs
(Ishioka et
al., J Immunol, 162(7):3915-25 (1999)) has also been detected. Thus, the TAP
issue
appears to be of minor concern with transgenic mice.
[00260] Another issue of potential relevance in the use of HLA transgenic mice
is the
possible influence of (32 microglobulin on HLA expression and binding
specificity. It is
well known that human (32 binds both human and mouse MHC with higher affinity
and
stability than mouse (32 microglobulin (Shields et al., Mol Immunol, 35(14-
15):919-28
(1998)). It is also well known that more stable complexes of MHC heavy chain
and P2
facilitate exogenous loading of MHC Class I (Vitiello et al., Science,
250(4986):1423-6
(1990)). We have examined the potential effect of this variable by generating
mice that
are double transgenics for HLA/Kb and humanP2. Expression of human (32 was
beneficial in the case HLA B7/Kb mice, and was beneficial to achieve good
expression
levels in the case of HLA Al transgenic mice. Accordingly, HLA/Kb and (32
double
transgenic mice are currently and routinely bred and utilized by the present
inventors.
Thus, HLA transgenic mice can be used to model HLA-restricted recognition of
five
major HLA specificities (namely A2, A11, B7, A24 and Al) and transgenic mice
for
CA 02658559 2009-01-20
WO 2008/039267 73 PCT/US2007/016529
other HLA specificities can be developed as suitable models for evaluation of
immunogenicity.
Antigenicity Testing for Class I Epitopes
[00261] Several independent lines of experimentation indicate that the density
of Class
Upeptide complexes on the cell surface may correlate with the level of T cell
priming.
Thus, measuring the levels at which an epitope is generated and presented on
an APC's
surface provides an avenue to indirectly evaluate the potency of multi-epitope
nucleic
acid vaccines in human cells in vitro. As a complement to the use of HLA Class
I
transgenic mice, this approach has the advantage of examining processing in
human
cells. (Ishioka et al., Jlmmunol,162(7):3915-25 (1999)).
[00262] Several possible approaches to experimentally quantitate processed
peptides are
available. The amount of peptide on the cell surface can be quantitated by
measuring the
amount of peptide eluted from the APC surface (Sijts et al., J Immunol,
156(2):683-92
(1996); Demotz et al., Nature, 342(6250):682-4 (1989)). Alternatively, the
number of
peptide-MHC complexes can be estimated by measuring the amount of lysis or
lymphokine release induced by infected or transfected target cells, and then
determining
the concentration of peptide necessary to obtain equivalent levels of lysis or
lymphokine
release (Kageyama et al., Jlmmunol, 154(2):567-76 (1995)).
[00263] A similar approach has also been used to measure epitope presentation
in multi-
epitope nucleic acid-transfected cell lines. Specifically, multi-epitope
constructs that are
immunogenic in HLA transgenic mice are also processed into optimal epitopes by
human cells transfected with the same constructs, and the magnitude of the
response
observed in transgenic mice correlates with the antigenicity observed with the
transfected
human target cells (Ishioka et al., Jlmmunol, 162(7):3915-25 (1999)).
[00264] Using antigenicity assays, a number of related constructs differing in
epitope
order or flanking residues can be transfected into APCs, and the impact of the
aforementioned variables on epitope presentation can be evaluated. This can be
a
preferred system for testing where a relatively large number of different
constructs need
to be evaluated. Although it requires large numbers of epitope-specific CTLs,
protocols
that allow for the generation of highly sensitive CTL lines (Alexander-Miller
et al., Proc
Natl Acad Sci USA, 93(9):4102-7 (1996)) and also for their expansion to large
numbers
(Greenberg P. D., Riddell S. R., Science, 285(5427):546-51 (1999)) have been
developed
to address this potential problem.
CA 02658559 2009-01-20
WO 2008/039267 74 PCT/US2007/016529
[00265] It should also be kept in mind that, if the cell selected for the
transfection is not
reflective of the cell performing APC function in vivo, misleading results
could be
obtained. Cells of the B cell lineage, which are known "professional" APCs,
are typically
employed as transfection recipients. The use of transfected B cells of this
type is an
accepted practice in the field. Furthermore, a good correlation has already
been noted
between in vitro data utilizing transfected human B cells and in vivo results
utilizing
HLA transgenic mice, as described in more detail herein.
Measuring HTL Responses
[00266] In preferred embodiments, vaccine constructs are optimized to induce
Class II
restricted immune responses. One method of evaluating multi-epitope constructs
including Class II epitopes, is to use HLA-DR transgenic mice. Several groups
have
produced and characterized HLA-DR transgenic mice (Taneja V., David C. S.,
Immunol
Rev, 169:67-79 (1999)).
[00267] An alternative also exists which relies on crossreactivity between
certain human
MHC molecules and particular MHC molecules expressed by laboratory animals.
Bertoni and colleagues (Bertoni et al., J Immunol, 161(8):4447-55 (1998)) have
noted
that appreciable crossreactivity can be demonstrated between certain HLA Class
I
supertypes and certain PATR molecules expressed by chimpanzees.
Crossreactivity
between human and macaques at the level of Class II (Geluk et al., J Exp Med,
177(4):979-87 (1993)) and Class I molecules (Dzuris, et al., J. Immunol., July
1999) has
also been noted. Finally, it can also be noted that the motif recognized by
human HLA
B7 supertype is essentially the same as the one recognized by the murine Class
I Ld
(Rammensee et al., Immunogenetics, 41(4):178-228 (1995)). Of relevance to
testing
HLA DR restricted epitopes in mice, it has been shown by Wall et al. (Wall et
al., J.
Immunol., 152:4526-36 (1994)) that similarities exist in the motif of DR1 and
IAb. We
routinely breed our transgenic mice to take advantage of this fortuitous
similarity.
Furthermore, we have also shown that most of our peptides bind to IAb, so that
we use
these mice for the study of CTL and HTL immunogenicity.
Measuring and Quantitating Immune Responses from Clinical Samples
[00268] A crucial element to assess vaccine performance is to evaluate its
capacity to
induce immune responses in vivo. Analyses of CTL and HTL responses against the
immunogen, as well as against common recall antigens are commonly used and are
CA 02658559 2009-01-20
WO 2008/039267 75 PCT/US2007/016529
known in the art. Assays employed included chromium release, lymphokine
secretion
and lymphoproliferation assays.
[00269] More sensitive techniques such as the ELISPOT assay, intracellular
cytokine
staining, and tetramer staining have become available in the art. It is
estimated that these
newer methods are 10- to 100-fold more sensitive than the common CTL and HTL
assays (Murali-Krishna et al., Immunity, 8(2): 177-87 (1998)), because the
traditional
methods measure only the subset of T cells that can proliferate in vitro, and
may, in fact,
be representative of only a fraction of the memory T cell compartment (Ogg G.
S.,
McMichael A. J., Curr Opin Immunol, 10(4):393-6 (1998)). Specifically in the
case of
HIV, these techniques have been used to measure antigen-specific CTL responses
from
patients that would have been undetectable with previous techniques (Ogg et
al., Science,
279(5359):2103-6 (1998); Gray et al., J Immunol, 162(3):1780-8 (1999); Ogg et
al., J
Virol, 73(11):9153-60 (1999); Kalams et al., J Viro; 73(8):6721-8 (1999);
Larsson et al.,
AIDS, 13(7):767-77 (1999); Come et al., JAcquir Immune Defic Syndr Hum
Retrovirol,
20(5):442-7 (1999)).
[00270] With relatively few exceptions, direct activity of freshly isolated
cells has been
difficult to demonstrate by the means of traditional assays (Ogg G. S.,
McMichael A. J.,
Curr Opin Immunol, 10(4):393-6 (1998)). However, the increased sensitivity of
the
newer techniques has allowed investigators to detect responses from cells
freshly isolated
from infected humans or experimental animals (Murali-Krishna et al., Immunity,
8(2):177-87 (1998); Ogg G. S., McMichael A. J., Curr Opin Immunol, 10(4):393-6
(1998)). The availability of these sensitive assays, which do not depend on an
in vitro
restimulation step, has greatly facilitated the study of CTL function in
natural infection
and cancer. In contrast, assays utilized as an endpoint to judge effectiveness
of
experimental vaccines are usually performed in conjunction with one or more in
vitro
restimulation steps (Ogg G. S., McMichael A. J., Curr Opin Immunol, 10(4):393-
6
(1998)). In fact, with few exceptions (Hanke et al., Vaccine, 16(4):426-35
(1998)),
freshly isolated Class I-restricted CD8+ T cells have been difficult to
demonstrate in
response to immunization with experimental vaccines designed to elicit CTL
responses.
The use of sensitive assays, such as ELISPOT or in situ IFNy ELISA, has been
combined
with a restimulation step to achieve maximum sensitivity; MHC tetramers are
also used
for this purpose.
[00271] MHC tetramers were first described in 1996 by Altman and colleagues.
They
produced soluble HLA-A2 Class I molecules which were folded with HIV-specific
CA 02658559 2009-01-20
WO 2008/039267 76 PCT/US2007/016529
peptides containing a CTL epitope complexed together into tetramers tagged
with
fluorescent markers. These are used to label populations of T cells from HIV-
infected
individuals that recognize the epitope (Ogg G. S., McMichael A. J., Curr Opin
Immunol,
10(4):393-6 (1998)). These cells were then quantified by flow cytometry,
providing a
frequency measurement for the T cells that are specific for the epitope. This
technique
has become very popular in HN research as well as in other infectious diseases
(Ogg G.
S., McMichael A. J., Curr Opin Immunol, 10(4):393-6 (1998); Ogg et al.,
Science,
279(5359):2103-6 (1998); Gray et al., J Immunol, 162(3):1780-8 (1999); Ogg et
al., J
Virol, 73(11):9153-60 (1999); Kalams et al., J Virol, 73(8):6721-8 (1999)).
However,
HLA polymorphism can limit the general applicability of this technique, in
that the
tetramer technology relies on defined HLA/peptide combinations. However, it
has been
shown that a variety of peptides, including HIV-derived peptides, are
recognized by
peptide-specific CTL lines in the context of different members of the A2, A3
and B7
supertypes (Threlkeld et al., J Immunol, 159(4):1648-57 (1997); Bertoni et
al., J Clin
Invest, 100(3):503-13 (1997)). Taken together these observations demonstrate
that a T
cell receptor (TCR) for a given MHC/peptide combination can have detectable
affinity
for the same peptide presented by a different MHC molecule from the same
supertype.
[00272] In circumstances in which efficacy of a prophylactic vaccine is
primarily
correlated with the induction of a long-lasting memory response, restimulation
assays
can be the most appropriate and sensitive measures to monitor vaccine-induced
immunological responses. Conversely, in the case of therapeutic vaccines, the
main
immunological correlate of activity can be the induction of effector T cell
function, most
aptly measured by primary assays. Thus, the use of sensitive assays allows for
the most
appropriate testing strategy for immunological monitoring of vaccine efficacy.
Antigenicity of Multi-Epitope Constructs in Transfected Human APCs
[00273] Antigenicity assays are performed to evaluate epitope processing and
presentation in human cells. An episomal vector to efficiently transfect human
target
cells with multi-epitope nucleic acid vaccines is used to perform such an
analysis.
1002741 For example, 221 A2Kb target cells were transfected with an HIV multi-
epitope
vaccine. The 221 A2Kb target cell expresses the A2Kb gene that is expressed in
HLA
transgenic mice, but expresses no endogenous Class I (Shimizu Y, DeMars R., J
Immunol, 142(9):3320-8 (1989)). These transfected cells are assayed for their
capacity to
present antigen to CTL lines derived from HLA transgenic mice and specific for
various
CA 02658559 2009-01-20
WO 2008/039267 77 PCT/US2007/016529
HIV-derived CTL epitopes. To correct for differences in antigen sensitivity of
different
CTL lines, peptide dose titrations, using untransfected cells as APC, are run
in parallel.
[00275] These data have several important implications. First, they suggest
that different
epitopes contained within a given construct may be processed and presented
with
differential efficiency. Second, they suggest that immunogenicity is
proportional to the
amount of processed epitope generated. Finally, these results provide an
important
validation of the use of transgenic mice for the purpose of optimization of
multi-epitope
vaccines destined for human use.
Methods of Administration
[00276] The invention also relates to pharmaceutical compositions comprising a
pharmaceutically acceptable carrier and an expression vector of the invention
or a
polypeptide derived therefrom. Pharmaceutically acceptable carriers are well
known in
the art and include aqueous or non-aqueous solutions, suspensions and
emulsions,
including physiologically buffered saline, alcohol/aqueous solutions or other
solvents or
vehicles such as glycols, glycerol, oils such as olive oil or injectable
organic esters, lipids
or liposomes.
[00277] A pharmaceutically acceptable carrier can contain physiologically
acceptable
compounds that act, for example, to stabilize the expression vector or
increase the
absorption of the expression vector. Such physiologically acceptable compounds
include,
for example, carbohydrates, such as glucose, sucrose or dextrans, antioxidants
such as
ascorbic acid or glutathione, chelating agents, low molecular weight
polypeptides,
antimicrobial agents, inert gases or other stabilizers or excipients.
Expression vectors can
additionally be complexed with other components such as peptides, polypeptides
and
carbohydrates. Expression vectors can also be complexed to particles or beads
that can
be administered to an individual, for example, using a vaccine gun. One
skilled in the art
would know that the choice of a pharmaceutically acceptable carrier, including
a
physiologically acceptable compound, depends, for example, on the route of
administration of the expression vector.
[00278] The invention further relates to methods of administering a
pharmaceutical
composition comprising an expression vector of the invention or a polypeptide
derived
therefrom to stimulate an immune response. The expression vectors are
administered by
methods well known in the art as described in, for example, Donnelly et al.
(Ann. Rev.
Immunol., 15:617-648 (1997)); Felgner et al. (U.S. Pat. No. 5,580,859, issued
Dec. 3,
CA 02658559 2009-01-20
WO 2008/039267 78 PCT/US2007/016529
1996); Felgner (U.S. Pat. No. 5,703,055, issued Dec. 30, 1997); and Carson et
al. (U.S.
Pat. No. 5,679,647, issued Oct. 21, 1997). In one embodiment, the multi-
epitope
construct is administered as naked nucleic acid.
[00279] A pharmaceutical composition comprising an expression vector of the
invention
or a polypeptide derived therefrom can be administered to stimulate an immune
response
in a subject by various routes including, for example, orally, intravaginally,
rectally, or
parenterally, such as intravenously, intramuscularly, subcutaneously,
intraorbitally,
intracapsularly, intraperitoneally, intracistemally or by passive or
facilitated absorption
through the skin using, for example, a skin patch or transdermal
iontophoresis,
respectively. Furthermore, the composition can be administered by injection,
intubation
or topically, the latter of which can be passive, for example, by direct
application of an
ointment or powder, or active, for example, using a nasal spray or inhalant.
An
expression vector also can be administered as a topical spray, in which case
one
component of the composition is an appropriate propellant. The pharmaceutical
composition also can be incorporated, if desired, into liposomes, microspheres
or other
polymer matrices as described in, for example, Felgner et al., U.S. Pat. No.
5,703,055;
Gregoriadis, Liposome Technology, Vols. I to III (2nd ed. 1993). Liposomes,
for
example, which consist of phospholipids or other lipids, are nontoxic,
physiologically
acceptable and metabolizable carriers that are relatively simple to make and
administer.
[00280] The expression vectors of the invention or a polypeptide. derived
therefrom can
be delivered to the interstitial spaces of tissues of an animal body as
described in, for
example, Felgner et al., U.S. Pat. Nos. 5,580,859 and 5,703,055.
Administration of
expression vectors of the invention to muscle is a particularly effective
method of
administration, including intradermal and subcutaneous injections and
transdermal
administration. Transdermal administration, such as by iontophoresis, is also
an effective
method to deliver expression vectors of the invention to muscle. Epidermal
administration of expression vectors of the invention can also be employed.
Epidermal
administration involves mechanically or chemically irritating the outermost
layer of
epidermis to stimulate an immune response to the irritant (Carson et al., U.S.
Pat. No.
5,679,647).
[00281] Other effective methods of administering an expression vector of the
invention or
a polypeptide derived therefrom to stimulate an immune response include
mucosal
administration as described in, for example, Carson et al., U.S. Pat. No.
5,679,647. For
mucosal administration, the most effective method of administration includes
intranasal
CA 02658559 2009-01-20
WO 2008/039267 79 PCT/US2007/016529
administration of an appropriate aerosol containing the expression vector and
a
pharmaceutical composition. Suppositories and topical preparations are also
effective for
delivery of expression vectors to mucosal tissues of genital, vaginal and
ocular sites.
Additionally, expression vectors can be complexed to particles and
administered by a
vaccine gun.
[00282] The dosage to be administered is dependent on the method of
administration and
will generally be between about 0.1 g up to about 200 g. For example, the
dosage can
be from about 0.05 g/kg to about 50 mg/kg, in particular about 0.005-5 mg/kg.
An
effective dose can be determined, for example, by measuring the immune
response after
administration of an expression vector. For example, the production of
antibodies
specific for the MHC Class II epitopes or MHC Class I epitopes encoded by the
expression vector can be measured by methods well known in the art, including
ELISA
or other immunological assays. In addition, the activation of T helper cells
or a CTL
response can be measured by methods well known in the art including, for
example, the
uptake of 3H-thymidine to measure T cell activation and the release of 51 Cr
to measure
CTL activity.
[00283] The pharmaceutical compositions comprising an expression vector of the
invention or a polypeptide derived therefrom can be administered to mammals,
particularly humans, for prophylactic or therapeutic purposes. Diseases
related to
influenza virus infection can be treated or prevented using the expression
vectors of the
invention.
[00284] In therapeutic applications, the expression vectors of the invention
or a
polypeptide derived therefrom are administered to an individual already
suffering from
influenza virus infection or a related disease. Those in the incubation phase
or acute
phase of the disease can be treated with expression vectors of the invention,
including
those expressing all universal MHC Class II epitopes, separately or in
conjunction with
other treatments, as appropriate.
[00285] In therapeutic and prophylactic applications, pharmaceutical
compositions
comprising expression vectors of the invention or a polypeptide derived
therefrom are
administered to a patient in an amount sufficient to elicit an effective
immune response
to an antigen and to ameliorate the signs or symptoms of a disease. The amount
of
expression vector to administer that is sufficient to ameliorate the signs or
symptoms of a
disease is termed a therapeutically effective dose. The amount of expression
vector
sufficient to achieve a therapeutically effective dose will depend on the
pharmaceutical
CA 02658559 2009-01-20
WO 2008/039267 80 PCT/US2007/016529
composition comprising an expression vector of the invention, the manner of
administration, the state and severity of the disease being treated, the
weight and general
state of health of the patient and the judgment of the prescribing physician.
[00286] The present invention also provides methods for delivering an
influenza
polypeptide or a fragment, variant, or derivative thereof to a human, which
comprise
administering to a human one or more of the compositions described herein;
such that
upon administration of compositions such as those described herein, an
influenza
polypeptide, or fragment, variant, or derivative thereof is expressed in human
cells, in an
amount sufficient to generate an immune response to the influenza virus or
administering
the influenza virus polypeptide or a fragment, variant, or derivative thereof
itself to the
human in an amount sufficient to generate an immune response.
[00287] The present invention further provides methods for delivering an
influenza virus
polypeptide or a fragment, variant, or derivative thereof to a human, which
comprise
administering to a vertebrate one or more of the compositions described
herein; such that
upon administration of compositions such as those described herein, an immune
response
is generated in the vertebrate.
[00288] The term "vertebrate" is intended to encompass a singular "vertebrate"
as well as
plural "vertebrates" and comprises mammals and birds, as well as fish,
reptiles, and
amphibians.
[00289] The term "mammal" is intended to encompass a singular "mammal" and
plural
"mammals," and includes, but is not limited to humans; primates such as apes,
monkeys
(e.g., owl, squirrel, cebus, rhesus, African green, patas, cynomolgus, and
cercopithecus),
orangutans, baboons, gibbons, and chimpanzees; canids such as dogs and wolves;
felids
such as cats, lions, and tigers; equines such as horses, donkeys, and zebras,
food animals
such as cows, pigs, and sheep; ungulates such as deer and giraffes; ursids
such as bears;
and others such as rabbits, mice, ferrets, seals, whales. In particular, the
mammal can be
a human subject, a food animal or a companion animal.
[00290] The term "bird" is intended to encompass a singular "bird" and plural
"birds," and
includes, but is not limited to feral water birds such as ducks, geese, terns,
shearwaters,
and gulls; as well as domestic avian species such as turkeys, chickens, quail,
pheasants,
geese, and ducks. The term "bird" also encompasses passerine birds such as
starlings
and budgerigars.
[00291] The present invention further provides a method for generating,
enhancing or
modulating an immune response to an influenza virus comprising administering
to a
CA 02658559 2009-01-20
WO 2008/039267 81 PCT/US2007/016529
vertebrate one or more of the compositions described herein. In this method,
the
compositions may include one or more isolated polynucleotides comprising at
least one
nucleic acid fragment where the nucleic acid fragment is optionally a fragment
of a
coding region encoding an influenza virus polypeptide, or a fragment, variant,
or
derivative thereof. In another embodiment, the compositions may include both a
polynucleotide as described above, and also an isolated influenza virus
polypeptide, or a
fragment, variant, or derivative thereof, wherein the protein is provided as a
recombinant
protein, in particular, a fusion protein, a purified subunit, a chemically
synthesized
peptide, viral vector expressing the protein, or in the form of an inactivated
influenza
virus vaccine. Thus, the latter compositions include both a polynucleotide
encoding an
influenza virus polypeptide or a fragment, variant, or derivative thereof and
an isolated
influenza virus polypeptide or a fragment, variant, or derivative thereof. The
influenza
virus polypeptide or a fragment, variant, or derivative thereof encoded by the
polynucleotide of the compositions need not be the same as the isolated
influenza virus
polypeptide or a fragment, variant, or derivative thereof of the compositions.
Compositions to be used according to this method may be univalent, bivalent,
trivalent or
multivalent.
[00292] The polynucleotides of the compositions may comprise a fragment of a
human
(or other vertebrate) coding region encoding a protein of the influenza virus,
or a
fragment, variant, or derivative thereof. The polynucleotides are incorporated
into the
cells of the vertebrate in vivo, and an antigenic amount of the influenza
virus
polypeptide, or fragment, variant, or derivative thereof, is produced in vivo.
Upon
administration of the composition according to this method, the influenza
virus
polypeptide or a fragment, variant, or derivative thereof is expressed in the
vertebrate in
an amount sufficient to elicit an immune response. Such an immune response
might be
used, for example, to generate antibodies to the influenza virus for use in
diagnostic
assays or as laboratory reagents, or as therapeutic or preventative vaccines
as described
herein.
[00293] The present invention further provides a method for generating,
enhancing, or
modulating a protective and/or therapeutic immune response to influenza virus
in a
vertebrate, comprising administering to a vertebrate in need of therapeutic
and/or
preventative immunity one or more of the compositions described herein. In
this method,
the compositions include one or more polynucleotides comprising at least one
nucleic
acid fragment, where the nucleic acid fragment is optionally a fragment of a
coding
CA 02658559 2009-01-20
WO 2008/039267 82 PCT/US2007/016529
region encoding an influenza virus polypeptide, or a fragment, variant, or
derivative
thereof. In a further embodiment, the composition used in this method includes
both an
isolated polynucleotide comprising at least one nucleic acid fragment, where
the nucleic
acid fragment is optionally a fragment of a coding region encoding an
influenza virus
polypeptide, or a fragment, variant, or derivative thereof; and at least one
isolated
influenza virus polypeptide, or a fragment, variant, or derivative thereof.
Thus, the latter
composition includes both an isolated polynucleotide encoding an influenza
virus
polypeptide or a fragment, variant, or derivative thereof and an isolated
influenza virus
polypeptide or a fragment, variant, or derivative thereof, for example, a
recombinant
protein, a purified subunit, viral vector expressing the protein, or an
inactivated virus
vaccine. Upon administration of the composition according to this method, the
influenza
virus polypeptide or a fragment, variant, or derivative thereof is expressed
in the human
in a therapeutically or prophylactically effective amount.
[00294] As used herein, an "immune response" refers to the ability of a
vertebrate to elicit
an immune reaction to a composition delivered to that vertebrate. Examples of
immune
responses include, an antibody response or a cellular, e.g., cytotoxic T-cell,
response.
One or more compositions of the present invention may be used to prevent
influenza
infection in vertebrates, e.g., as a prophylactic vaccine, to establish or
enhance immunity
to influenza virus in a healthy individual prior to exposure to influenza or
contraction of
influenza disease, thus preventing the disease or reducing the severity of
disease
symptoms.
[00295] As mentioned above, compositions of the present invention can be used
to
prevent influenza virus infection. The term "prevention" refers to the use of
one or more
compositions of the present invention to generate immunity in a vertebrate
which has not
yet been exposed to a particular strain of influenza virus, thereby preventing
or reducing
disease symptoms and death if the vertebrate is later exposed to the
particular strain of
influenza virus. The methods of the present invention therefore may be
referred to as a
preventative or prophylactic vaccination. It is not required that any
composition of the
present invention provide total immunity to influenza or totally cure or
eliminate all
influenza disease symptoms. As used herein, a "vertebrate in need of
preventative
immunity" refers to an individual for whom it is desirable to treat, i.e., to
prevent, cure,
retard, or reduce the severity of influenza disease symptoms, and/or result in
no
worsening of influenza disease over a specified period of time. Vertebrates to
treat
and/or vaccinate include humans, apes, monkeys (e.g., owl, squirrel, cebus,
rhesus,
CA 02658559 2009-01-20
WO 2008/039267 83 PCT/US2007/016529
African green, patas, cynomolgus, and cercopithecus), orangutans, baboons,
gibbons,
and chimpanzees, dogs, wolves, cats, lions, and tigers, horses, donkeys,
zebras, cows,
pigs, sheep, deer, giraffes, bears, rabbits, mice, ferrets, seals, whales,
ducks, geese, tems,
shearwaters, gulls, turkeys, chickens, quail, pheasants, geese, starlings and
budgerigars.
[00296] One or more compositions of the present invention are utilized in a
"prime boost"
regimen. An example of a"prime boost" regimen may be found in Yang, Z. et al.
J.
Virol. 77:799-803 (2002), which is incorporated herein by reference in its
entirety. In
these embodiments, one or more polynucleotide vaccine compositions of the
present
invention are delivered to a vertebrate, thereby priming the immune response
of the
vertebrate to. an influenza virus, and then a second immunogenic composition
is utilized
as a boost vaccination. One or more compositions of the present invention are
used to
prime immunity, and then a second immunogenic composition, e.g., a recombinant
viral
vaccine or vaccines, a different polynucleotide vaccine, or one or more
purified subunit
isolated influenza virus polypeptides or fragments, variants or derivatives
thereof is used
to boost the anti-influenza virus immune response.
[00297] In one embodiment, a priming composition and a boosting composition
are
combined in a single composition or single formulation. For example, a single
composition may comprise an isolated influenza virus polypeptide or a
fragment, variant,
or derivative thereof as the priming component and a polynucleotide encoding
an
influenza protein as the boosting component. In this embodiment, the
compositions may
be contained in a single vial where the priming component and boosting
component are
mixed together. In general, because the peak levels of expression of protein
from the
polynucleotide does not occur until later (e.g., 7-10 days) after
administration, the
polynucleotide component may provide a boost to the isolated protein
component.
Compositions comprising both a priming component and a boosting component are
referred to herein as "combinatorial vaccine compositions" or "single
formulation
heterologous prime-boost vaccine compositions." In addition, the priming
composition
may be administered before the boosting composition, or even after the
boosting
composition, if the boosting composition is expected to take longer to act.
[00298] In another embodiment, the priming composition may be administered
simultaneously with the boosting composition, but in separate formulations
where the
priming component and the boosting component are separated.
[00299] The terms "priming" or "primary" and "boost" or "boosting" as used
herein may
refer to the initial and subsequent immunizations, respectively, i.e., in
accordance with
CA 02658559 2009-01-20
WO 2008/039267 84 PCT/US2007/016529
the definitions these terms normally have in immunology. However, in certain
embodiments, e.g., where the priming component and boosting component are in a
single
formulation, initial and subsequent immunizations may not be necessary as both
the
"prime" and the "boost" compositions are administered simultaneously.
[00300] In certain embodiments, one or more compositions of the present
invention are
delivered to a vertebrate by methods described herein, thereby achieving an
effective
therapeutic and/or an effective preventative immune response. More
specifically, the
compositions of the present invention may be administered to any tissue of a
vertebrate,
including, but not limited to, muscle, skin, brain tissue, lung tissue, liver
tissue, spleen
tissue, bone marrow tissue, thymus tissue, heart tissue, e.g., myocardium,
endocardium,
and pericardium, lymph tissue, blood tissue, bone tissue, pancreas tissue,
kidney tissue,
gall bladder tissue, stomach tissue, intestinal tissue, testicular tissue,
ovarian tissue,
uterine tissue, vaginal tissue, rectal tissue, nervous system tissue, eye
tissue, glandular
tissue, tongue tissue, and connective tissue, e.g., cartilage.
[00301] Furthermore, the compositions of the present invention may be
administered to
any internal cavity of a vertebrate, including, but not limited to, the lungs,
the mouth, the
nasal cavity, the stomach, the peritoneal cavity, the intestine, any heart
chamber, veins,
arteries, capillaries, lymphatic cavities, the uterine cavity, the vaginal
cavity, the rectal
cavity, joint cavities, ventricles in brain, spinal canal in spinal cord, the
ocular cavities,
the lumen of a duct of a salivary gland or a liver. When the compositions of
the present
invention is administered to the lumen of a duct of a salivary gland or liver,
the desired
polypeptide is expressed in the salivary gland and the liver such that the
polypeptide is
delivered into the blood stream of the vertebrate from each of the salivary
gland or the
liver. Certain modes for administration to secretory organs of a
gastrointestinal system
using the salivary gland, liver and pancreas to release a desired polypeptide
into the
bloodstream is disclosed in U.S. Patent Nos. 5,837,693 and 6,004,944, both of
which are
incorporated herein by reference in their entireties.
[00302] In certain embodiments, the compositions are administered into
embryonated
chicken eggs or by intra-muscular injection into the defeathered breast area
of chicks as
described in Kodihalli S. et al., Vaccine 18:2592-9 (2000), which is
incorpQrated herein
by reference in its entirety.
[00303] In certain embodiments, the compositions are administered to muscle,
either
skeletal muscle or cardiac muscle, or to lung tissue. Specific, but non-
limiting modes for
CA 02658559 2009-01-20
WO 2008/039267 85 PCT/US2007/016529
administration to lung tissue are disclosed in Wheeler, C.J., et al., Proc.
Natl. Acad. Sci.
USA 93:11454-11459 (1996), which is incorporated herein by reference in its
entirety.
[00304] According to the disclosed methods, compositions of the present
invention can be
administered by intramuscular (i.m.), subcutaneous (s.c.), or intrapulmonary
routes.
Other suitable routes of administration include, but are not limited to
intratracheal,
transdermal, intraocular, intranasal, inhalation, intracavity, intravenous
(i.v.), intraductal
(e.g., into the pancreas) and intraparenchymal (i.e., into any tissue)
administration.
Transdermal delivery includes, but not limited to intradermal (e.g., into the
dermis or
epidermis), transdermal (e.g., percutaneous) and transmucosal administration
(i.e., into
or through skin or mucosal tissue). Intracavity administration includes, but
not limited to
administration into oral, vaginal, rectal, nasal, peritoneal, or intestinal
cavities as well as,
intrathecal (i.e., into spinal canal), intraventricular (i.e., into the brain
ventricles or the
heart ventricles), inraatrial (i.e., into the heart atrium) and sub arachnoid
(i.e., into the
sub arachnoid spaces of the brain) administration.
[00305] Any mode of administration can be used so long as the mode results in
the
expression of the desired peptide or protein, in the desired tissue, in an
amount sufficient
to generate an immune response to influenza virus and/or to generate a
prophylactically
or therapeutically effective immune response to influenza virus in a human in
need of
such response. Administration means of the present invention include needle
injection,
catheter infusion, biolistic injectors, particle accelerators (e.g., "gene
guns" or pneumatic
"needleless" injectors) Med-E-Jet (Vahlsing, H., et al., J. Immunol. Methods
171:11-22
(1994)), Pigjet (Schrijver, R., et al., Vaccine 15: 1908-1916 (1997)),
Biojector (Davis,
H., et al., Vaccine 12: 1503-1509 (1994); Gramzinski, R., et al., Mol. Med. 4:
109-118
(1998)), AdvantaJet (Linmayer, I., et al., Diabetes Care 9:294-297 (1986)),
Medi-jector
(Martins, J., and Roedl, E. J. Occup. Med. 21:821-824 (1979)), gelfoam sponge
depots,
other commercially available depot materials (e.g., hydrogels), osmotic pumps
(e.g.,
Alza minipumps), oral or suppositorial solid (tablet or pill) pharmaceutical
formulations,
topical skin creams, and decanting, use of polynucleotide coated suture (Qin,
Y., et al.,
Life Sciences 65: 2193-2203 (1999)) or topical applications during surgery.
Certain
modes of administration are intramuscular needle-based injection and pulmonary
application via catheter infusion. Energy-assisted plasmid delivery (EAPD)
methods
may also be employed to administer the compositions of the invention. One such
method involves the application of brief electrical pulses to injected
tissues, a procedure
commonly known as electroporation. See generally Mir, L.M. et al., Proc. Natl.
Acad.
CA 02658559 2009-01-20
WO 2008/039267 86 PCT/US2007/016529
Sci USA 96:4262-7 (1999); Hartikka, J. et al., Mol. Ther. 4:407-15 (2001);
Mathiesen, I.,
Gene Ther. 6:508-14(1999); Rizzuto G. et al., Hum. Gen. Ther. 11:1891-900
(2000).
Each of the references cited in this paragraph is incorporated herein by
reference in its
entirety.
[00306] Determining an effective amount of one or more compositions of the
present
invention depends upon a number of factors including, for example, the antigen
being
expressed or administered directly, e.g., HA, NA, NP, M1 or M2, or fragments,
e.g.,
M2e, variants, or derivatives thereof, the age and weight of the subject, the
precise
condition requiring treatment and its severity, and the route of
administration. Based on
the above factors, determining the precise amount, number of doses, and timing
of doses
are within the ordinary skill in the art and will be readily determined by the
attending
physician or veterinarian.
[00307] Compositions of the present invention may include various salts,
excipients,
delivery vehicles and/or auxiliary agents as are disclosed, e.g., in U.S.
Patent Application
Publication No. 2002/0019358, published February 14, 2002, which is
incorporated
herein by reference in its entirety.
[00308] Furthermore, compositions of the present invention may include one or
more
transfection facilitating compounds that facilitate delivery of
polynucleotides to the
interior of a cell, and/or to a desired location within a cell. As used
herein, the terms
"transfection facilitating compound," "transfection facilitating agent," and
"transfection
facilitating material" are synonymous, and may be used interchangeably. It
should be
noted that certain transfection facilitating compounds may also be "adjuvants"
as
described infra, i.e., in addition to facilitating delivery of polynucleotides
to the interior
of a cell, the compound acts to alter or increase the immune response to the
antigen
encoded by that polynucleotide. Examples of the transfection facilitating
compounds
include, but are not limited to inorganic materials such as calcium phosphate,
alum
(aluminum sulfate), and gold particles (e.g., "powder" type delivery
vehicles); peptides
that are, for example, cationic, intercell targeting (for selective delivery
to certain cell
types), intracell targeting (for nuclear localization or endosomal escape),
and ampipathic
(helix forming or pore forming); proteins that are, for example, basic (e.g.,
positively
charged) such as histones, targeting (e.g., asialoprotein), viral (e.g.,
Sendai virus coat
protein), and pore-forming; lipids that are, for example, cationic (e.g.,
DMRIE, DOSPA,
DC-Chol), basic (e.g., steryl amine), neutral (e.g., cholesterol), anionic
(e.g.,
phosphatidyl serine), and zwitterionic (e.g., DOPE, DOPC); and polymers such
as
CA 02658559 2009-01-20
WO 2008/039267 87 PCT/US2007/016529
dendrimers, star-polymers, "homogenous" poly-amino acids (e.g., poly-lysine,
poly-
arginine), "heterogeneous" poly-amino acids (e.g., mixtures of lysine &
glycine), co-
polymers, polyvinylpyrrolidinone (PVP), poloxamers (e.g. CRL 1005) and
polyethylene
glycol (PEG). A transfection facilitating material can be used alone or in
combination
with one or more other transfection facilitating materials. Two or more
transfection
facilitating materials can be combined by chemical bonding (e.g., covalent and
ionic
such as in lipidated polylysine, PEGylated polylysine) (Toncheva, et al.,
Biochim.
Biophys. Acta 1380(3):354-368 (1988)), mechanical mixing (e.g., free moving
materials
in liquid or solid phase such as "polylysine + cationic lipids") (Gao and
Huang,
Biochemistry 35:1027-1036 (1996); Trubetskoy, et al., Biochem. Biophys. Acta
1131:311-313 (1992)), and aggregation (e.g., co-precipitation, gel forming
such as in
cationic lipids + poly-lactide, and polylysine + gelatin). Each of the
references cited in
this paragraph is incorporated herein by reference in its entirety.
EXAMPLES
Materials and Methods
[00309] The following materials and methods apply generally to all the
examples
disclosed herein. Specific materials and methods are disclosed in each
example, as
necessary.
[00310] The practice of the present invention will employ, unless otherwise
indicated,
conventional techniques of cell biology, cell culture, molecular biology
(including PCR),
vaccinology, microbiology, recombinant DNA, and immunology, which are within
the
skill of the art. Such techniques are explained fully in the literature. See,
for example,
Molecular Cloning A Laboratory Manual, 2nd Ed., Sambrook et al., ed., Cold
Spring
Harbor Laboratory Press: (1989); DNA Cloning, Volumes I and II (D. N. Glover
ed.,
1985); Oligonucleotide Synthesis (M. J. Gait ed., 1984); Mullis et al. U.S.
Pat. No:
4,683,195; Nucleic Acid Hybridization (B. D. Hames & S. J. Higgins eds. 1984);
Transcription And Translation (B. D. Hames & S. J. Higgins eds. 1984); Culture
Of
Animal Cells (R. I. Freshney, Alan R. Liss, Inc., 1987); Immobilized Cells And
Enzymes
(IRL Press, 1986); B. Perbal, A Practical Guide To Molecular Cloning (1984);
the
treatise, Methods In Enzymology (Academic Press, Inc., N.Y.); Gene Transfer
Vectors
CA 02658559 2009-01-20
WO 2008/039267 88 PCT/US2007/016529
For Mammalian Cells (J. H. Miller and M. P. Calos eds., 1987, Cold Spring
Harbor
Laboratory); Methods In Enzymology, Vols. 154 and 155 (Wu et al. eds.),
Immunochemical Methods In Cell And Molecular Biology (Mayer and Walker, eds.,
Academic Press, London, 1987); and in Ausubel et al., Current Protocols in
Molecular
Biology, John Wiley and Sons, Baltimore, Maryland (1989). Each of the
references cited
in this paragraph is incorporated herein by reference in its entirety.
In situ IFNy ELISA
[00311] An in situ IFNy ELISA assay has been developed and optimized for both
freshly
isolated and peptide-restimulated splenocytes (see, e.g., McKinney et al., J.
Immunol.
Meth. 237 (1-2):105-117 (2000)). This assay is based on the ELISPOT assay, but
utilizes
a soluble chromagen, making it readily adaptable to high-throughput analysis.
In both the
primary and restimulation assays, this technique is more sensitive than either
a traditional
supematant ELISA or the 51cr-release assay, in that responses are observed in
the in situ
ELISA that are not detectable in these other assays. On a per cell basis, the
sensitivity of
the in situ ELISA is approximately one IFNy secreting cell/104 plated cells.
[00312] 96-well ELISA plates are coated with anti-IFNy (rat anti-mouse IFN-a-
MAb,
Clone R4-6A2, Pharmingen) overnight at 4 C., and then blocked for 2 hours at
room
temperature with 10% FBS in PBS. Serially diluted primary splenocytes or CTLs
are
cultured for 20 hours with peptide and 105 Jurkat A2.1/Kb cells/well at 37 C
with 5%
C02. The following day, the cells are washed out and the amount of IFNy that
had been
secreted into the wells is detected in a sandwich ELISA, using biotinylated a-
IFNy (rat
anti-mouse IFNy mAb, Clone XMG1.2, Pharmingen) to detect the secreted IFNy.
HRP-
coupled streptavidin (Zymed) and TMB (ImmunoPureTM TMB Substrate Kit, Pierce)
are
used according to the manufacturer's directions for color development. The
absorbance
is read at 450 nm on a Labsystems Multiskan RC ELISA plate reader. In situ
IFNy
ELISA data is evaluated in secretory units (SU), based on the number of cells
that
secrete 100 pg of IFNy in response to a particular peptide, corrected for the
background
amount of IFN in the absence of peptide.
ELISPOT assay
[00313] The ELISPOT assay quantifies the frequency of T cells specific for a
given
peptide by measuring the capacity of individual cells to be induced to produce
and
release specific lymphokines, usually IFNy. The increased sensitivity of the
ELISPOT
CA 02658559 2009-01-20
WO 2008/039267 89 PCT/US2007/016529
assay has allowed investigators to detect responses from cells freshly
isolated from
infected humans or experimental animals (Murali-Krishna et al., Immunity,
8(2):177-87
(1998); Ogg et al., Science, 279(5359):2103-6 (1998)). The ELISPOT assays are
conducted as described above for the IFNy ELISA until the final steps, where
ExtrAvidin-AP (Sigma, 1:500. dilution) is used in place of HRP-streptavidin.
Color is
developed using the substrate 5-BCIP (BioRad) according to the manufacturer's
directions. Spots are counted using a phase contrast microscope.
Alternatively, spots are
counted utilizing the Zeiss KS ELISPOT reader. In this case the BCIP/NBT
substrate is
used.
[00314] The ELISPOT assay is routinely utilized to quantitate immune
responses. The
spots can be manually counted, however, in a preferred mode, a KS ELISPOT
reader
from Zeiss, a microscope-based system with software specifically designed to
recognize
and count spots is used.
Hemagglutination Inhibition (HAI) Assays
[00315] Preimmune and postimmune mouse sera were treated with receptor-
destroying
enzyme (RDE). HAI antibodies were measured against influenza
rgA/Vietnam/1203/2004 x A/PR/8/34 influenza (H5N1) vaccine virus. Four HA
units of
virus were incubated with serial dilutions of RDE-treated mouse sera for at
least 30
minutes at room temperature followed by a 30 minute incubation with 0.5% horse
erythrocytes. The HAI titer was recorded as the reciprocal of the highest
dilution of
antisera which inhibits the agglutination of horse erythrocytes.
Viral Micro Neutralization Assays
1003161 Influenza vaccine virus rgA/Vietnam/1203/2004 x A/PR/8/34 (H5N1) and
diluted RDE-treated mouse sera were incubated together at room temperature for
1 hour.
The mixture was titrated on monolayers of Madin-Darby canine kidney (MDCK)
cells
grown in 96-well tissue culture plates. Plates were incubated for 3 days at 37
C in 5%
COz. At the end of 3 days, the. preserice of cytopathic effects on cell
monolayers was
evaluated. Neutralization titers were expressed as the reciprocal of the
antibody dilution
that completely inhibited virus infectivity in 50% of quadruplicate cultures
CA 02658559 2009-01-20
WO 2008/039267 90 PCT/US2007/016529
Mice, Immunizations and Cell Cultures
[00317] The derivation of the HLA-A2.1/Kb (Vitiello et al., J Exp Med,
173(4):1007-15
(1991)) and A11Kb (Alexander et al., J Immunol, 159(10):4753-61 (1997))
transgenic
mice used in this study has been described. HLA B7 Kb, HLA Al/Kb and HLA
A24/Kb
transgenic mice are available. HLA DR4 transgenic mice are obtained from C.
David
(Mayo Clinic) or purchased from Taconic. Non-transgenic H-2b mice are
purchased from
Charles River Laboratories or other commercial vendors. Immunizations are
performed
as described in (Ishioka et al., Jlmmunol, 162(i):3915-25 (1999)). All cells
are grown in
culture medium consisting of RPMI 1640 medium with HEPES (Gibco Life
Technologies) supplemented with 10% FBS, 4 mM L-glutamine, 50 M 2-ME, 0.5 mM
sodium pyruvate, 100 g/mi streptomycin and 100 U/ml penicillin.
[00318] The natural crossreactivity between HLA-DR and IAb can also be
exploited to
test HTL responses. This evaluation provides an assessment of the antigenicity
and
immunogenicity of multi-epitope constructs.
Example 1: Identification of conserved HLA Class I- and Class II- restricted
peptides derived from influenza subtypes using established motif search
algorithms and
HLA-peptide binding assays
[00319] To identify epitopes useful for vaccine design, a multidisciplinary
approach was
used based initially on amino acid motif searching of viral sequences to
identify potential
HLA Class I and Class II motifs (see Tables 1-49). This was followed by high
throughput synthetic peptide binding assays using purified HLA molecules to
determine
affinity and breadth of epitope peptide binding.
[00320] Selection of influenza virus strains with potential to initiate
pandemics: Influenza
virus strains for this study were selected on the basis of host diversity
(avian, swine,
human), agents of past pandemics (H1N1, H2N2, H3N2) and capacity to cause
zoonotic
influenza infections of man (H5N1, H1N1, H7N7, H9N2). Examples of selected
strains
are shown below.
[00321] Algorithm motif searches: Motif search algorithms were validated for
the most
common HLA Class I alleles and HLA Class II alleles and were focused on the
HLA-
Al, -A2, -A3/1 1, -A24, -B7, -B44, -DR1 and -DR3 supertypes in order to attain
virtually
100% population coverage. The selected influenza viral sequences were scanned
for
motif positive amino acid sequences using the motif definitions. The peptides
specific
CA 02658559 2009-01-20
WO 2008/039267 91 PCT/US2007/016529
for HLA-A1, -A2, -A3/1 1, -A24, -B7, -B44, -DR1 and -DR3 supertypes are
produced as
synthetic peptides.
Selected viral strains with potential to initiate pandemics are as follows:
Virus Host Virus Availability of Gene Sequencesa
Subtype Origin Strain PB2 PB1 PA HA NP NA M NS
77777 Human A/Hong Kong/156/97 ^ ^ ^ ^ ^ ^ ^ ^
H5N1 Human A/Hong Kong/483/97 ^ ^ ^ ^ m ^ ^ ^
H9N2 Human A/Hong Kong/1073/99 ^ ^ ^ ^ ^ ^ ^ ^
H9N2 Avian A/Chicken/HK/G9/97 ^ ^ ^ ^ ^ ^ ^ ^
H9N2 Swine A/Swine/Hong Kong/10/98 ^ ^ ^ ^ ^ ^ ^ ^
H7N1 Avian A/FPV/Rostock/34 ^ ^ ^ ^ ^ ^
H7N1 Avian A/Turkey/Itaty/4620/99 ^
H7N7 Avian A/FPV/Weybridge/34 ^ ^ ^ ^
H1N1 Human A/NewCaledonia/20/99 ^ ^ ^ ^ ^
H3N2 c) Human A/Hong Kong/1/68 ^ ^ ^ ^ ^ ^ ^ ^
H3N2 Human A/Shiga/25/97 ^ ^ ^ ^ ^ ^ ^ ^
H2N2 d) Human A/Singapore/1/57 ^ ^ ^ ^ ^ ^ ^ ^
H2N2 Human A/Leningrad/134/57 ^ ^ ^ ^ ^ ^ ^ ^
H2N2 Human A/Ann Arbor/6/60 ^ ^ ^ ^ ^ ^ ^ ^
H1N1 Human A/Brevig Mission/1/18 ^ ^ ^ ^
H1N1 e) Swine A/Swine/Wisconsin/464/98 ^ ^ ^ ^ ^ ^ ^ ^
H7N7 f) Human A/Netherlands/219/03 ^ ^ ^ ^ ^ ^ ^ ^
(a) Presence of this symbol (^) indicates that the gene sequence is available;
(b)
numerous cases of avian-to-human transmission and fatalities caused by H5N1;
(c) The
1968 pandemic was due to a H3N2 virus; (d) The 1957 pandemic was due to H2N2
virus; (e) Classical swine H1N1 virus strain; (f) Isolated from a fatal human
case.
[00322] Peptide synthesis: The Class I and Class II peptides were synthesized
initially as
crude peptides from Mimotopes (Minneapolis, MN/Clayton, Victoria, Australia)
or
Pepscan Systems B.V. (Lelystad, Netherlands). These peptides were supplied in
small
amounts and were typically only 50-70% pure. Larger quantities of selected
peptides
were synthesized, when needed, using an Applied Biosystems (Foster City, CA)
430A
peptide synthesizer and fluronylmethyloxy carbonyl (F-moc) solid phase
methods.
Peptides synthesized were typically purified to >95% homogeneity by reverse
phase
HPLC.
[00323] In vitro HLA-peptide epitope binding assays: High affinity binding of
epitope
peptides to HLA molecules is required for immune recognition and has proved to
be one
of the most highly predictive approaches for identifying epitopes. Capture
assays based
on the use of the TopCount benchtop microplate scintillation counter (Packard
Instruments) allow the high throughput, sensitivity and compatibility with
data
automation platforms.
CA 02658559 2009-01-20
WO 2008/039267 92 PCT/US2007/016529
[00324] HLA Class I and II purification: The binding assay requires the use of
purified
HLA Class I and II molecules. A large number of different types of cells are
available
including EBV-transformed homozygous human B cell lines, mouse B cell
lymphomas
or mastocytomas, transfected fibroblasts or single MHC allele transfected
721.221 lines.
HLA molecules were purified from cell lysates using monoclonal antibody-based
affinity
chromatography.
[00325] Measurement of peptide binding to HLA molecules and data analysis: The
binding assay utilized is a competitive system that is based on the use of
known 1251
radiolabeled peptide ligands112. To determine the IC50 of peptide binding, the
concentration of test peptide yielding 50% inhibition of the binding of the
radiolabeled
peptide was calculated. Typical test concentrations ranged from 120 g/m1-120
pg/ml.
Under the conditions utilized, the measured IC50 values were reasonable
approximations
of the Kd values.
[00326] - Epitopes that are naturally processed and presented to the immune
system using
peptides were identified as high affinity binders to HLA molecules and
peripheral blood
mononuclear cells (PBMC) from normal human donors and HLA transgenic mice. It
was necessary to address epitope immunogenicity because not all motif positive
peptides
are immunogenic nor is it likely that all epitopes are generated equally
during infection.
Two methods to document epitope immunogenicity and utility were used; 1) in
vitro
assays using PBMC from normal donors and 2) immunization studies with HLA
transgenic mice. Recognition of epitope peptides by human PBMC in a recall
assay was
the most direct method to verify the authenticity of an epitope because
responses
demonstrated that the epitope was generated as the course of natural infection
and that
the needed T-cell receptor (TCR) repertoire exists. Finally, the HLA
transgenic mouse is
well suited for testing vaccine constructs because the proteosome processing
preferences
and TCR repertoires of mice overlap significantly with humans.
[00327] Assay for recall memory influenza responses using human PBMC: Based on
preliminary data presented, past studies44, and those of others 42,43,45,
responses to
multiple epitopes were expected because the selection process was for
immunologically
conserved epitopes. The assays detecting IFN-y were performed as described for
Figure
1. Since CTL contribute to influenza viral clearance by releasing perforin and
granzymes
from cytoplasmic granules, measurement of these factors may also be performed
by
ELISPOT analysis. Manufacture's (Mabtech) instructions are followed to perform
these
CA 02658559 2009-01-20
WO 2008/039267 93 PCT/US2007/016529
additional assays. The assays are specifically based on detection of perforin
and
granzymes from human PBMC.
[00328] It has been demonstrated that CD4+ cells can promote survival to a
lethal dose of
influenza infection. The mechanisms that may be involved are several including
their
classic contribution as helpers during the generation of flu-specific CD8+ CTL
and
antibody producing B cells. Potentially, CD4+ cells following influenza
infection may
have an effector function and directly mediate viral clearance by IFN-y-
dependent
mechanisms and/or by direct cytolytic effects on infected cells. Accordingly,
HTL
activity was measured as a function of IFN-y secretion by CD4+ T-lymphocytes,
again
using an ELISPOT assay as described. Depending on the results obtained using
IFN-y as
a readout, IL-2 or TNF-a may also be assayed using an ELISPOT format.
[00329] A collection of positive and control peptides for each supertype was
required to
ensure the specificity of the influenza-specific responses. Defined epitopes
from various
pathogens, generally HIV, HBV, HCV and Plasmodium falciparum were used as
negative controls when the donors had not been exposed. Positive control
peptides were
usually derived from HCMV, EBV, and influenza. Negative and positive control
peptides for each supertype were identified from previous studies and the
literature.
[00330] Immunogenicity testing of CTL, HTL and B cell epitopes in HLA
transgenic and
non transgenic mice: Large colonies of HLA-A2, A11 and B7 transgenic mice have
been established and HLA-A24 and Al mice have been generated76"78. HLA-DR4
transgenic mice from Taconic, a commercial source were also utilized.
Additionally,
mice of the b haplotype, e.g., C57BU6 were utilized to evaluate the
immunogenicity of
HLA-DR-restricted peptides67'139 The rationale for using b haplotype mice was
based
on the observation that the motifs recognized by DR alleles often cross-react
on murine
class II alleles. Immunogenicity of test epitopes were generally accomplished
by
immunizing mice with pools of peptides (5-10) emulsified in IFA (for CTL) and
CFA
(for HTL) followed by in vitro testing of splenocytes 14 days later for
epitope-specific T
lymphocyte responses.
Example 2: Identification of CTL and HTL epitopes for influenza virus vaccine
inclusion
[00331] A clear understanding of how T-lymphocytes recognize antigen has
emerged
over the past decade. It is now well established that small fragments of
protein antigens
are generated, defined as peptide epitopes, which bind to MHC molecules
expressed on
CA 02658559 2009-01-20
WO 2008/039267 94 PCT/US2007/016529
the cell surface. These epitope-MHC complexes represent the ligands recognized
by T-
lymphocytes through the function of T-cell receptors (TCR)81'82. The main
anchor
residues of peptides that bind to HLA Class I molecules typically occur at
position two
and the carboxyl terminus of peptides 8-11 amino acids in length8z-93. Amino
acids at
other positions can contribute to peptide-MHC binding affinity; these sites
are commonly
referred to as secondary anchors94. The analysis of data on both primary and
secondary
anchors led to the definition of statistically based algorithms, generally
referred to as
polynomial algorithms, for estimating the likelihood that peptides can bind to
HLA Class
I molecules. This approach is referred to as a matrix-based method. In
addition to the
matrix-based method, other computer-based approaches for predicting epitopes
have
been developed. These include variations of the motif scanning and matrix
approaches9s-
98 neural networks99 and threading algorithms'oo All of these methods function
comparably'oi,ie2 and regardless of the method used to predict epitopes,
laboratory
studies to document epitope peptide binding to HLA molecules and
immunogenicity
studies are needed to document the authenticity of predicted epitopes.
[00332] Motifs for different HLA molecules were found to be similar and this
lead to the
definition of HLA supertypes103. The biological effect of this supertype
relationship was
first demonstrated for HIV-1 epitopes in a study where the HLA-A3 and -Al 1
repertoires
were demonstrated to be overlapping, not only with each other but also with
HLA-A3 1, -
A33 and -A*6801104,1e5 This biriding specificity was defined as the HLA-A3
supertype.
A significant overlap in peptide binding repertoires was also demonstrated
amongst
several serologically distant HLA-B alleles'06'107, and multiple HLA-A2
alleles'08,109~
resulting in the definition of the HLA-B7 and HLA-A2 supertype families. A
large
fraction of HLA Class I molecules can be classified into a relatively few
supertypes, each
characterized by largely overlapping peptide binding repertoires and consensus
structures
of the main peptide binding pockets. Recognition of epitopes by CTL in
supertype
manner has been demonstrated to occur naturally in infectious diseases and
cancerl 04,1io-
"s Each of the known HLA Class I supertypes includes a relatively common set
of
HLA alleles and due to this, the use of these supertype relationships when
identifying
epitopes allows for the selection of those most likely to be targets for the
immune system
in a genetically diverse population.
[00333] A similar approach is utilized to identify potential HTL epitopes,
focusing on the
identification of peptides that bind to Class II MHC molecules. There exists a
sigriificantly higher level of variation in the motif definition and peptide
length, since
CA 02658559 2009-01-20
WO 2008/039267 95 PCT/US2007/016529
binding to Class II molecules is generally more promiscuous. However, highly
predictive peptide binding motifs for the major supertypes, HLA-DR1 and HLA-
DR3,
which include most of the common HLA-DR types have been identified116 These
motifs have been used to identify HLA-DR-restricted epitopes from several
viruses.
[00334] Epitope predictions are useful but a significant number of motif-
positive peptides
identified using predictive algorithms will fail to bind with high affinity to
MHC
molecules. Thus, it is logical to increase the accuracy of the identification
process using
laboratory assays to directly measure the affinity of the binding between
peptides and
MHC molecules 83'106 Epitope peptide binding assays are based on the use of
purified
HLA-A, -B or -DR molecules and radio-labeled peptides with known binding
affinity. A
library of >100 different HLA specificities, in the purified form, has been
developed and
can be utilized. To determine the binding affinity of an unknown peptide,
increasing
concentrations are allowed to compete with the known radio-labeled peptide for
binding
to the HLA molecule. The assay endpoint is based on the ratio of bound and
unbound
radio-labeled peptide and actual binding affinities can be calculated. Binding
affinity
threshold values,. based on IC50 values, of < 500 nM for CTL epitopes and <1
gM for
HTL epitopes are routinely utilized, for initial selection of epitopes, since
peptides
binding with these affinity levels are most likely to be immunogenic 66'117
[00335] The goal of epitope identification is to assemble sufficient numbers
of epitopes
for vaccine development based on a need for sufficient population coverage.
Potential
population coverage was calculated using gene frequencies for HLA-A and -B
alleles.
Total potential supertype and/or population coverage was calculated from the
sum gene
frequencies of corresponding alleles and subsequently converted to phenotypic
frequencies. In summary, population coverage is estimated using the
distribution data on
all HLA types (generally 5 for each supertype) in the world. HLA-A2, -A3/A11,
and -
B7 are very common so using these supertypes dictates high population coverage
of >
90%. 100% population coverage can be approximated by adding HLA-A1 and -A24
restricted epitopes. In the case of class II, > 95% population coverage can be
achieved
by considering epitopes representative of the DR1, and DR3 supermotifs.
[00336] Epitopes from several disease pathogens including Plasmodium
falciparium,
HIV, HBV, HCV and HPV for the goal of vaccine development have been
identified.
Epitope identification is presented herewith for the influenza virus. Viral
protein amino
acid sequences were scanned using algorithm-based computer programs for the
presence
of class I-restricted HLA-A1, -A2 -A3/A11, -A24, -B7 -B44 and class II-
restricted DR1
CA 02658559 2009-01-20
WO 2008/039267 96 PCT/US2007/016529
and DR3 supermotif viral conserved peptide supertype sequences. Critical
parameters
used to identify the peptide sequences were conservancy of peptide sequence
amongst
divergent influenza subtypes and a predicted binding affinity IC50nM of <100
to the
prototypic MHC allele representing the supertype, i.e., HLA-A*0101 (Al), HLA-
A*0201 (A2), HLA-A*1101 (A3/A11), HLA-A*2402 (A24), HLA-B*0702 (B7) and
HLA-B*4002 (B44). For example, in the case of peptides restricted by the A2
supertype, 68 motif positive peptides were identified according to the methods
described
in Example 1(see Table 15). However, only about 40 peptides were predicted to
have
strain sequence conservancy and to bind with high affinity (see Table 16).
This number
of peptides was further reduced by limiting to 3 peptides at most per
influenza virus
protein. (see Table 17).
[00337] Peptides restricted by the other Class I supertypes HLA-A3/A11, -A24, -
B7, -
B44, and -Al, identified according to methods described above, are listed in
Tables 1, 4,
7, 9, and 12, respectively. HLA-A3/A11, -A24, -B7, -B44, and -A1 peptides
predicted
to be conserved and to bind with high affinity are listed in Tables 2, 5, 8,
10, and 13,
respectively. In the case of HLA-A3/A11, -A24, -B44, and -Al, further
restrictions
limiting to a specific number of peptides at most per influenza virus protein,
reduced the
number of peptides to those listed in Tables, 3, 6, '11 and 14. The number of
peptides
identified for each supertype and protein are shown below.
[00338] PIC analysis of conserved influenza sequences:
Iden6fied Peptides From HLA-A and -B Supertypes HLA-A, -B DR Supertypes HLA-DR
Proteins Al A2 A3/A11 A24 B7 B44 Total/Proteina) DR1 DR3 Total/Proteina)
HA 2 2 1 0 1 2 8 5 3 8
M1 1 4 2 2 0 10 19 18 11 29
M2 1 1 3 3 0 1 9 7 6 13
NA 6 2 11 6 5 5 35 7 6 13
NP 7 3 15 10 3 19 57 26 20 46
NS1 1 4 4 1 1 6 17 7 12 19
NS2 1 2 5 3 0 10 21 9 6 15
PA 14 6 22 12 2 27 83 40 37 77
PB1 11 11 25 8 10 21 86 70 39 109
PB2 9 4 31 7 8 21 80 33 32 65
Total 53 39 119 52 30 122 415 222 172 394
a Peptides are identified based on sequence conservancy ?33% and predicted
IC50 5100nM
[00339] A total of about 450 Class I-restricted peptide sequences were
identified with a
relatively high number selected from NP, PA, PB 1 and PB2 proteins. An
intermediate
CA 02658559 2009-01-20
WO 2008/039267 97 PCT/US2007/016529
number of sequences were identified from Ml, NS1, NS2 and the least number of
sequences were identified from the HA and M2 proteins.
[00340] A total of about 1500 Class II-restricted peptide sequences were
identified that
were specific for the DR1 and DR3 supertypes (see Tables 18-49). The DR1 and
DR3
peptides in Tables 18-49 are organized based on the influenza virus proteins
from which
each peptide is derived. Tables 20, 22, 24, 26, 28, 30, 32, 35, 37, 39, 41,
and 46 list
those DR1 and DR3 peptides predicted to be conserved and to bind with high
affinity
from influenza virus proteins NP, NS1, NS2, PA, PB1, PB2, HA, Ml, M2, NA and
NA,
respectively. There are two main contributors to the number of peptides
identified, size
of the protein and amino acid mutations occurring within the protein. As might
be
expected, numerous DR1 supertype peptide sequences were identified from the HA
and
NA proteins that were subtype specific. However, relatively very little
sequence
conservancy was observed amongst the subtypes. DR1 peptides from the NA and HA
protein specific to influenza strain A/Viet Nam/1203/04 were identified and
are listed in
Tables 18 and 33, respectively. DR3 peptides from the NA and HA protein
specific to
influenza strain A/Viet Nam/1203/04 were identified and are listed in Tables
42 and 47,
respectively. Finally, a set of DR3 peptides was identified and is listed in
Table 43 with
the corresponding preferred subset listed in Table 44. Additionally a set of
DR peptides
is listed in Table 48, with the corresponding preferred subset of these
peptides listed in
Table 49.
[00341] 40 HLA-A2-restricted peptide sequences were synthesized and were
evaluated
for their binding capacity to purified MHC molecules. The 40 peptides were
highly
degenerate with exhibited binding of high (<50 IC50nM) or intermediate (50-500
IC50nM) affinity to multiple MHC alleles within the supertype. As expected,
the 2
identified HA- and NA-derived peptides had relatively lower sequence
conservancy
amongst influenza subtypes, in the range of 38-50%. However, the majority of
the other
identified peptide sequences were highly conserved.
[00342] The 40 peptides exhibiting high binding capacity and sequence
conservancy were
next evaluated for their capacity to induce influenza-specific recall
responses. Human
donor PBMCs were cultured in the presence of a pool of peptides, generally 9-
10
peptides per pool. Following expansion of the T cells for 1 week, CD8+ cells
were
purified and influenza peptide-specific responses were measured using peptide
coated
HLA-A2.1 transfected target cells and an IFN-y ELISPOT assay. As shown in
Figure 1,
19 of the 40 peptides induced measurable responses in the range of 10-6,000
spot
CA 02658559 2009-01-20
WO 2008/039267 98 PCT/US2007/016529
forming cells (SFC) per 1 x 106 CD8+ cells. Two of the peptides, NS2 173 and
PB2 193,
were relatively less immunogenic inducing SFC of only 10. However, the
remaining 17
peptides induced responses greater than 100 SFC. Four of the peptides (M1 3,
NP 458,
PB1 87, and PB2 446 version 1-histidine at position 8) were active in 3
donors, 9
peptides (NA 360, HA 447, Ml 58, NSl 14, PA 283, PB1 83, PBl 413, PB1 501, and
PB2 446 version 2-proline at position 8) were active in 2 donors and the
remaining 7
peptides were recognized by 1 donor (NA 128, NP 275, NS2 173, PA70, PA 335,
PB2
193, and PB2 630). Control peptides were also used to validate the specificity
of
influenza responses (data not shown). Three positive control peptides were
used in each
assay, EBV bmlfl 259, CMV pp65 495 and influenza Ml 58 which induced recall
responses in all cases in the range of 200-10,000 SFC118. Known HLA-A2.1-
restricted
epitopes were used as negative control peptides, HBVenv 183, HBVcore 18,
HIVenv
134, Plasmodium falciparium (Pf)expl 83, Pfexp12 and Pfexpl 91. It was assumed
that
there would be absence of recall responses specific for the Hepatitis, HIV and
malaria-
derived peptides. However, it was observed that Donor 638 responded to 4 of
the 7
peptides with responses in the range of 40-100 SFC for 3 peptides. Therefore,
additional
donors are included in the analysis to be confident that the final selection
of vaccine
candidate epitopes is based on a sufficient number of donors. At least 5
donors are being
utilized to identify epitopes that are immunogenic. It should be noted that
two of the 19
immunogenic influenza peptides have been previously described to induce recall
responses in humans, M1 58 and PB1 413.
[00343] The 19 epitope sequences were aligned with a collection, 12 to 16, of
various
influenza strains which have the potential to initiate pandemics. Eight
epitopes were a
perfect match, HA 360, Ml 3, PA 283, PB1 83, PB1 87, PB1 413, PB1 501, and
therefore would be considered as potential vaccine epitope candidates. Seven
epitopes,
HA 447, M1 58, NP 458, PA 70, PA 335, PB2 630, PB2 446, have conservative
amino
acid changes at either MHC anchor positions or at TCR contact positions. These
epitopes are also considered to be potential vaccine candidates. However,
addition
evaluation would be required to determine whether an immune responses specific
for the
vaccine epitope would also recognize target cells presenting the variant
sequence. Four
epitopes, NA 128, NP 275, PB2 193, have mutations considered to diminish
binding to
the MHC molecule and are not viable vaccine candidate epitopes. Examples of
epitope
sequence alignments are shown below. The PB2 630 epitope is considered a good
vaccine candidate since its sequence is altered in only 1 of 13 virus strains,
CA 02658559 2009-01-20
WO 2008/039267 99 PCT/US2007/016529
A/Swine/Wisconsin/464/98, with a conservative threonine to alanine
substitution at a
non-MHC anchor position. Similarly, PB2 446 has substitutions at position 8, a
non-
MHC anchor, of either serine or histidine for proline. In the PB2 446 case,
epitopes with
proline and histidine at position 8 were evaluated for the capacity to induce
a recall
response. As shown in Figure 1, both versions induced a significant recall
response
suggesting that PB2 446 would be a good vaccine candidate. However, it should
be
noted that the virus strain A/Chicken/Hong Kong/G9/97 has a non-preferred
lysine at a
primary MHC anchor position. An immune response would not be generated against
this
strain. In the case of PB2 193, lysine or arginine at position 2 would
abrogate peptide
binding to MHC and render this vaccine epitope ineffective against 5 of the 13
strains
depicted. Therefore, PB2 193 would not be considered a vaccine candidate.
[00344] In summary, 15 HLA-A2-restricted epitopes, based on the studies
described
above, have been identified for potential vaccine inclusion.
[00345] The same process is applied to the collection of the additional Class
I and II-
restricted peptides
Virus PB2 630 PB2 446 PB2193
Virus Strain Subtype E ito e Sequence E ito e Sequence E ito e Sequence
Vaccine Epitope R M Q F S S L T V F Q N W G I E P I L Q D C K I A P L
A/SwinelWisconsin/464/98 H1N1 ------- A - ------- S - --E --- S - -
A/Singapore/1/57 H2N2 ---------- ------ H - ------ S - -
A/Ann Arbor 6160 H2N2 - - - - - - - - - - - - - - - - H - - - - - - - S - -
AlLeningrad/134/57 H2N2 ---------,: ------- H - ------ S - -
A/HongKongl1l68 H3N2 - - - - - - - - -. - - - - - - - H - - - - - - - S - -
AlShigal25/97 H3N2 - - - - - - - - - ------- H - -R -------
AlHongKongl156197 H5N1 - - - - - - - - - - - - - - - - - - -K N -N -S --
AlHongKong/483197 H5N1 - - - - - - - - - ., - - - - - - - - - - K N - N - - - -
A/FPVIWeybridgel34 H7N7 - - - - - - - - - - - - - - - - - - ---------
A/Netherlands1218103 H7N7 - - - - - - - - - - - - - - - - - - ---------
A/Swine/HongKong/10198 H9N2 ---------- ---------- ---------
AlChicken/HongKonglG9l97 H9N2 - - - - - - - - - - K - - - - - - - - K N - N - -
- -
A/HongKong/1073199 H9N2 - - - - - - - - - - - - - - - - - - - K - - N - - - -
[00346] Following candidate epitope selection, the design of multi-epitope
constructs is
undertaken. A representative multi-epitope construct is shown in Figure 6. The
order of
epitopes and amino acid spacers used between epitopes was determined to
promote
optimum processing and presentation of the vaccine epitopes. The peptide
binding data
of each of the epitopes within this representative construct is shown in Table
50.
Example 3: Design and development of multi-epitope vaccines
1003471 Immunogenicity of multi-peptide epitope in adjuvant vaccines: Several
vaccine
delivery methods amenable for use with epitopes. Synthetic peptides
representing CTL
CA 02658559 2009-01-20
WO 2008/039267 100 PCT/US2007/016529
or HTL epitopes derived from HIV-1 have been tested in clinical trials
delivered in the
. Phase I
high quality Incomplete Freund's Adjuvant119'120 or as lipidated peptides121
cancer clinical trial with 16 patients suffering from non-small cell lung
carcinoma (stage
IIb/IIIa) and colon (stage III) cancer have been initiated. The cancer vaccine
is based on
9 CTL epitopes derived from carcinoembryonic antigen (CEA), MAGE 2/3, p53 and
HER-2/neu tumor-associated antigens (TAA). All 9 epitopes displayed high HLA-
A2
supertype binding affinity and immunogenicity in human primary in vitro
induction
assay and in in vivo HLA-A2 transgenic mice. The CTL epitopes together with a
previously described universal HTL epitope, PADRE, were emulsified in
Montanide
ISA51 adjuvant. Patients received 6 vaccine treatments at 3 week intervals, at
a dose of
0.5 mg/epitope. CTL responses in the peripheral blood of patients were
measured using
a validated IFN-y ELISPOT assay. Fifty percent of the patients treated with
the vaccine
demonstrated CTL responses to at least 5 of the vaccine epitopes at the week 9
and/or 18
week time-points. As an example, shown in Figure 2 patient 604, significant
responses
were induced in the range of 50-100 SFC/5 x 104 cells for 6 of the 9 vaccine
CTL
epitopes at the 9 week time-point. These responses were subsequently boosted
with
responses measured in the range of 200-300 SFC at the 18 week time-point. No
responses specific for the vaccine were measured pre-vaccination.
[00348] The influenza virus multi-epitope vaccine is formulated in various
test adjuvants
as described above. Other vaccine delivery formats are also utilized including
DNA,
AlphaVax viral vaccines and virosomes, and in particular IRIVs.
[00349] Immunogenicity of multi-epitope based DNA vaccines: Efficient delivery
of
multiple CTL and HTL epitopes encoded in a DNA plasmid or viral vector cannot
be
accomplished by simply aligning epitopes in a`string-of-beads' format. At
least three
factors contribute to significant variation of the cellular immune responses
induced using
epitope-based vaccines: 1) the efficiency with which an epitope is generated
through
intracellular processing and then presented bound to MHC molecules; 2) the
binding
affinity of the epitope to MHC molecules and 3) the existence of a suitable
TCR
repertoire. ~
[00350] The influence that amino acids flanking CTL epitopes have on the
efficiency of
processing and presentation can be significant, particularly for C-terminal
flanking
amino acids12z'1z3 Immunogenicity data obtained from HLA-A2, -A11 and -B7
transgenic mice immunized with a number of unrelated experimental multi-
epitope DNA
vaccine constructs have been analyzed. A total of 94 different
epitope/flanking residue
CA 02658559 2009-01-20
WO 2008/039267 101 PCT/US2007/016529
combinations were analyzed. Significant effects of the C-terminus flanking
amino acids,
the Cl residue, were identified. Positively charged amino acids, such as K or
R , were
most frequently associated with optimal CTL responses and amino acids, such as
N and
Q, or small amino acids, such as C, G, A, T, and S were also associated with
moderate
epitope immunogenicity.
1003511 The design process and evaluation of HTL epitope-based vaccines
includes
different features to address the properties of HTL epitopes, including the
highly
promiscuous manner in which they bind to MHC Class II molecules and the
properties of
antigen processing pathways most commonly utilized. To address both of these
issues,
universal spacers, such as one consisting of GPGPG are utilized. Neither G or
P in the
GPGPG spacer are routinely used as primary anchors, at positions one or six in
the core
region of an HTL peptide epitope, by any know murine or human MHC Class II
molecule. The gap of five amino acids introduced by this spacer separates
adjacent
epitopes so the amino acids of two epitopes cannot physically serve as anchors
in the 1
and 6 positions67. This type of spacer is also predicted to introduce a(3-
turn, which
should enhance processing between epitopes124.
[00352] In summary, the use of appropriate spacers to promote efficient CTL
and HTL
epitope processing is an important strategy to use in vaccine design.
[00353] Epitope-based vaccines optimized for antigen processing also addresses
the
question of competition or immunological dominance between CTL epitopes, which
would effectively reduce the breath of the total response induced by
vaccination.
Approximately 20 CTL and HTL DNA plasmid constructs for HBV, HIV, HPV, and
malaria indications have been generated and tested. The HBV-derived DNA
vaccine
based on CTL and HTL epitopes is currently in Phase I clinical testing and the
HIV
DNA vaccine construct is slated to begin clinical trials within a year. As an
example, as
shown in Figure 3, the results obtained following immunization of rhesus
macaques with
a DNA construct encoding 12 Mamu A*01-restricted SIV-derived CTL epitopes, 4
Mamu class II-restricted SIV-derived epitopes and the universal HTL epitope
PADRE is
given. The epitope order and spacers used between epitopes were chosen to
maximize
epitope processing and presentation. The model also provides an opportunity to
evaluate
the efficacy of the vaccine by performing a viral challenge. Six macaques were
immunized on a monthly basis for 4 months with the DNA encoding the CTL and
HTL
epitopes formulated in polyvinylpyrollidone (PVP). PVP is thought to protect
against
degradation and promote distribute of the DNA following intramtiscular
injection. Three
CA 02658559 2009-01-20
WO 2008/039267 102 PCT/US2007/016529
of the macaques received an additional 2 DNA immunizations following a rest
period of
months. The other 3 monkeys, following 4 DNA immunizations, received 2
immunizations with a polyepitope protein containing the same epitopes order
and spacers
used in the DNA construct. Vaccine induced immunogenicity was measured 2 weeks
prior, 2 weeks post and 14 weeks post-SIVmac239 infection. Significant vaccine
induced IFN-y responses were observed for all 12 CTL epitopes following
immunizations.
[00354] The response pattern with regard to consistency and magnitude fell
into 2 groups.
Responses specific for Tat 28, Gag 181, Env 235 and Env 622 following
immunizations
and prior to SIV infection were in the 50-300 SFC/106 CD8+ range. Responses
induced
specific for the other 8 epitopes, Gag 340, Gag 372, Vpx 39, Pol 359, Pol 143,
Vif 144,
Pol 147 and Pol 588 were typically more variable and in the 10-50 SFC range.
Responses specific for Pol 359, Pol 143, Vif 144, Pol 147 and Pol 588 are not
depicted in
the graphs. Following SIV infection, the CTL responses were dramatically
increased to
values generally in the 200-3,000 SFC range. The epitopes Tat 28 and Gag 181
have
been previously described as inducing dominant responses following viral
infection. In
this study, responses in the 200-1,000 SFC range were measured using PBMC from
the
non-immunized animals confirming their dominant role. Responses to epitopes
Env 235
and Env 622 were not observed 2 weeks following infection in non-immunized
animals
and are considered subdominant epitopes. Responses have persisted out to the
14 week
post-infection time point but are typically reduced in magnitude, 204,000 SFC.
[00355] The immunogenicity observed in this study suggests two important
considerations for vaccine development. First, multiple CTL responses can be
induced
following DNA immunization specific for dominant and subdominant epitopes
using an
epitope-based vaccine. Second, these responses were boosted following
infection
suggesting that vaccine immunization resulted in priming for these responses.
To
establish whether vaccine induced immunity had an influence on viral levels,
plasma
virus loads were measured following infection. As shown in Figure 3, virus
numbers
peaked at 2 weeks following infection with levels measured in the 5 x 105 to 1
x
107 range. Five of the six immunized animals controlled infection with an
average
decrease in viral load of 2.0 logs at the 8 week time point. Control of viral
infection is
still evident although decreases to an average of 1.4 log reduction of viral
load of the 6
immunized animals versus un-immunized animals. These results indicate that the
vaccine induced immune response can initially control viral infection. In the
case of
CA 02658559 2009-01-20
WO 2008/039267 103 PCT/US2007/016529
influenza infection, it is anticipated that cellular immunity would be
required to control
infection for a much shorter time relative to control of persistent viral
infections such as
HBV and HIV.
[00356] SIV-specific HTL responses were also measured following immunization
and
post-infection, data not shown. Responses were induced specific for the 4 SN-
derived
HTL epitopes, Rev 9, Rev 40, Nef 210, Gag 260 and the universal helper epitope
PADRE in the 20-200 SFC range prior to infection. By week 14 following
infection,
responses were maintained in the immunized animals.
[00357] Identification, characterization and use of CTL and HTL epitopes in
DNA
vaccines are in Phase 1 clinical testing for HIV in both infected and
uninfected
volunteers.
[00358] Delivery of epitope-based vaccines using AlphaVax vector technolog:
The
utility of self-replicating RNA (replicon) vector technology to induce
protective antiviral,
antibacterial and antitumor cellular and humoral immune responses in several
animal
models including guinea pig, mouse, Cynomolgus Monkey and Rhesus macaques has
been established'zs-i3o Based on these studies, a clade C HIV vaccine based on
the
A1phaVax replicon vector is being tested in a dose-escalation, placebo-
controlled trial
under the NIH VTN at 4 sites in the U.S. and 2 sites in South Africa.
Specifically, the
AlphaVax vector system is genetically derived from an avirulent form of
Venezuelan
equine encephalitis virus (VEE) virus. Alphaviruses such as VEE are positive-
strand
RNA viruses that can mediate efficient cytoplasmic gene expression in
mammalian cells.
Since an RNA virus vector cannot integrate into chromosomal DNA, concerns
about cell
transformation are reduced. At least two immunological mechanisms may explain
the
enhanced immunogenicity of this vector; 1) the spike glycoproteins target the
vector to
dendritic cells in the draining lymph node and 2) cells transfected with the
vector activate
the innate pathways via double-stranded RNA recognition and interferon
action131-13z
Several characteristics of A1phaVax replicon make it competitive relative to
other viral
vector systems such as vaccinia and adenovirus. The vector has been proven to
be safe
and non-transmissible with the potential for multiple delivery routes, nasal,
mucosal,
subcutaneous and intramuscular. In contrast to the vaccinia and Adenovirus
vectors,
there is a lack of pre-existing immunity to the replicon vector.
[00359] With regard to an influenza virus vaccine, the replicon vector
containing the VEE
nonstructural genes with the structural genes of the virus may be replaced by
the
minigene encoding the influenza-derived CTL, HTL and B cell epitopes. The
replicon
CA 02658559 2009-01-20
WO 2008/039267 104 PCT/US2007/016529
RNA is packaged into VEE replicon particles by supplying the structural genes
in trans
via split structural protein gene helpers. The AlphaVax replicon delivers
multiple
influenza-derived CTL and HTL epitopes.
[00360] A crucial step in the process of evaluating immunogenicity of epitope-
based
vaccines delivered by DNA plasmid or viral vector is use of the HLA transgenic
mouse.
Development of a successful vaccine would require that the epitopes encoded by
the
vaccine are correctly processed and presented to the immune system following
immunization. To establish whether antigen processing and T cell repertoire
are similar
in man and mouse, HLA-A2 transgenic mice were infected with the influenza
A/Puerto
Rico/8/34 (PR8) strain and IFN-y responses specific for the HLA-A2-restricted
peptides
were evaluated. As shown in Figure 4, significant responses were measured for
a
majority of the peptides tested inducing responses specific for a majority of
the peptides
in the 20-100 SFC range. The most dominate response in the mouse, specific for
M1 3,
was also the most dominant response observed in humans. Of the 19 epitopes
that
generated recall responses in humans, 18 induced responses in mice following
infection.
A specific response for PB 1 501 was not generated in HLA-A2 transgenic mice
following infection although a recall response specific for this peptide was
demonstrated
in humans. These results show that the HLA transgenic mouse is a tool for
evaluating
epitope processing and presentation from DNA or viral epitope-based vaccines.
These
attributes also suggest that the mouse model can be used in influenza
challenge studies
following vaccination.
Example 4: Design and optimization of genetic DNA plasmid and viral vectored
vaccines
[00361] Constructs are designed based on computer programs to optimize
proteosomal
processing and minimize junctional epitopes: Strategies have been developed to
optimize
epitope processing efficiency from multi-epitope genetic constructs and to
minimize the
generation of neo-epitopes generated at the junction of epitopes which may
divert the
immune responses from the specified desired epitopes67'69 The incorporation of
preferred flanking amino acids to optimize proteosomal processing and a motif
searching
function is performed using a computer program.
[00362] DNA Vaccine production: DNA vaccine production is performed using
routine
methods based on primer extension with overlapping oligonucleotide PCR
primers,
averaging 70 nucleotides in length with 15 nucleotide overlaps58. The
synthetic gene
CA 02658559 2009-01-20
WO 2008/039267 105 PCT/US2007/016529
encoding the epitopes is cloned into the clinically accepted pMB75.6 vaccine
backbonet4s
[00363] Assessment of vaccine immunogenicity: Immunogenicity testing is
performed
primarily using the HLA-DR4 transgenic mice from Taconic and CB6F1 (b x d
haplotype) mice to measure responses specific for the influenza-derived HTL
epitopes
and HA-specific antibodies. Immunogenicity evaluation in mice is a useful tool
to assess
efficient antigen processing and epitope presentation specifically for the
vaccine
construct. The spacers adjacent to epitopes that are found to be suboptimally
immunogenic in a vaccine construct can be modified, through site-directed
mutagenesis,
in one or more cycles of secondary optimization.
[00364] Immunization of mice: HLA transgenic or normal inbred strains of mice,
in
groups of 10, will be injected with 1-100 g of DNA vaccine using the tibialis
anterior
muscle as the injection site. When the AlphaVax replicon is used, mice will be
immunized with 1 x 104- 5 x 106 infectious units of the virus, s.c. Ten to 14
days later,
the mice are sacrificed, a single-cell suspension of splenocytes prepared for
ELISPOT
assay purposes. When heterologous prime:boost experiments are run, DNA vaccine
immunization will precede the AlphaVax replicon and peptides in adjuvant
immunizations by 2-4 weeks. Alternative vaccine immunization schedules are
also
evaluated. For example, repeat administration of DNA vaccines daily or twice
weekly
are evaluated as a way to better prime CTL and HTL responses prior to A1phaVax
boost.
[00365] In the case of delivery of DNA and peptides in adjuvant, novel
adjuvant systems
are evaluated for ease of formulation and immunogenicity of the formulated
vaccine.
IC31 is mixed with the antigen and delivered by either a s.c. or i.m. route.
Specifically,
the peptide solution (KLK) and oligodeoxynucleotide solution (ODNIa) are
prepared
and sterile filtered separately before mixing. The optimal concentration of
IC31 is
evaluated for each antigen system using a dose range of 100-1,000 mmol KLK/ml
+ 4-40
nmol/ml ODN1a/ml). A dose range of peptide (0.1, 1, 10 g) of each peptide per
mouse
is typically used. In the case of the TLR7/8 agonists, a dose range of the
adjuvant (1, 10,
100 g/mouse) is evaluated to determine optimal dose. DNA in adjuvant is
administered
i.m. and peptides in adjuvant s.c. Initially, a single immunization is
evaluated followed
by administration of booster immunizations.
[00366] Murine CTL and HTL assays: An IFN-y-based ELISPOT assay is utilized to
measure CTL and HTL activity. The assay is performed essentially as described
in the
legend to Figure 4. The ELISPOT is performed using an 18 hour culture step
with the
CA 02658559 2009-01-20
WO 2008/039267 106 PCT/US2007/016529
peptide epitope (10 g/ml), A2.1/Kb transfected .221 target cells (or other
supertype
transfected target cells) and purified CD8+ and CD4+ T lymphocytes
(200,000/well).
[00367] Aumentation of HA-derived HTL and antibody responses using DNA
vaccines
followed by HA protein immunization: Prior immunization with conserved
influenza
virus HTL epitopes will augment HTL and antibody responses induced using
protein-
based or inactivated virus-based vaccines. HLA transgenic mice are initially
immunized
separately or in a prime-boost format using the DNA, and peptides in adjuvant
vaccines.
These immunizations are followed by inoculation with various HA proteins (0.1,
1, 10
g/mouse). The HTL and antibody responses are measured (as described above) and
directly compared to mice receiving only the conventional HA vaccines.
Purified
baculovirus-expressed recombinant HA proteins (Protein Sciences, Inc, Meriden,
CT)
corresponding to A/Hong Kong/156/97 (H5) and A/Hong/Kong/1073/99 (H9) are
used.
The rationale for using H5 and H9 proteins is due to their pandemic potential
as observed
by transmission of these variants from avian to human 18,146
Example 5. Evaluation of efficacy of the experimental vaccines alone and in
combination with recombinant HA protein using HLA transgenic mice and
infectious
challenges
[00368] The efficacy of vaccines composed of conserved influenza HTL and B
cell
epitopes are evaluated in an influenza viral challenge mouse model. For
example,
peptides are formulated in various adjuvants and tested for immunogenicity. If
a
particular adjuvant is superior in augmenting cellular and humoral responses
then this
adjuvant is used in the challenge studies. Initially, protection against
various divergent
influenza subtypes is determined by immunizing mice separately with selected
DNA,
peptides in adjuvant, HA proteins, inactivated and live attenuated vaccines.
Doses and
immunization schedules are determined according to the immunogenicity studies
described above. The capacity of the influenza HTL and B cell epitope-based
vaccines
to afford protection is compared to the HA protein, inactivated and live
attenuated
vaccines. Finally, the HA protein combined with the DNA, and peptides in
adjuvant
vaccines using heterologous prime boost immunization schemes are evaluated for
protection. Additionally, emphasis is placed on validating an immunization
strategy that
induces a protective immune response in the shortest amount of time which is
likely an
important factor to consider in the event of a pandemic influenza occurrence.
CA 02658559 2009-01-20
WO 2008/039267 107 PCT/US2007/016529
[00369] Murine influenza challenge models: Viral challenge studies are
performed as
previously described 75,147,148 Initially, mice are immunized with selected
vaccines or
combinations using doses and immunization schedules that are most immunogenic.
To
determine the level of protection afforded by the various immunization
strategies,
immunized mice are challenged with various subtypes of influenza viruses that
differ in
virulence for mice including human viruses as well as avian and viruses with
pandemic
potential. Using a number of different subtypes the level of protective
broadly cross-
reactive immunity induced by immunization of mice with the various vaccines
expressing conserved HTL epitopes are evaluated. The following are examples of
subtypes for challenge studies: mouse adapted A/Taiwan/l/86 (H1N1); mouse-
adapted
A/Ann Arbor/6/60 (H2N2); mouse-adapted A/Philippines/1/82 (H3N2); highly
pathogenic avian A/Hong Kong/483 (H5N1); a recent human isolate A/Hong
Kong/213/03 (H5N1); A/Hong Kong/1073/99 (H9N2); and an H7N7 strain.
[00370] The 50% mouse infectious dose (MID50) and 50% lethal dose (LD50)
titers are
determined for the C57B1/6 mouse strain. Groups of 10-20 mice are lightly
anesthetized
and infected intranasally (i.n.) with approximately 100-1,000 MID50 of virus.
Three and
six days post-infection, 5 mice per group are sacrificed and multiple organs
including
nasal turbinates, lungs and brains are collected and titered in embryonated
eggs or
MDCK cells for the presence of infectious virus. For viruses that cause lethal
disease,
and additional group of ten mice are monitored for weight loss and survival
over a period
of 14 days post-infection.
[00371] The use of conserved HTL epitopes delivered by peptides in adjuvant
and DNA
viral vehicles are used to generate a protective vaccine against influenza.
Example 6: Human Recall Responses in Donor X753
[00372] Primary interferon-gamma (IFN-y) ELISPOT (enzyme linked immunospot)
assay
was used to identify candidate vaccine epitopes. Peripheral blood mononuclear
cells
(PBMCs) were collected by leukapheresis from healthy human donors. The PBMCs
were purified using standard Ficoll-Paque (Amersham) density gradient
centrifugation
and subsequently frozen at 5x 107 cells per ml. PBMCs were thawed and were
either
rested for 5 days (no peptide) or stimulated for 7 days with the appropriate
peptides at
37 C in media at 2.5 x 106 cells per mL. Elispot plates (Millipore IP plate)
were coated
with anti-human IFN-,y antibody clone 1-D1K (Mabtech, Cat# 3420-3, 1 mg/mL)
and
incubated overnight at 4 C. The following day, PBMCs were depleted of CD8+
cells
CA 02658559 2009-01-20
WO 2008/039267 108 PCT/US2007/016529
using human DYNAbeads (DYNAL Biotec Cat# 111.47, OSLO, Norway). The depleted
PBMCs with enriched CD4+ cells were then plated onto ELISPOT plates previously
blocked with RPMI 1640 containing 10% FCS. Irradiated PBMCs coated with
peptide
were added to the plated PBMCs and the plates were incubated at 37 C for 20
hours.
The next day the plates were incubated with biotinylated mouse anti-human IFN-
y
antibody and developed with Vectastain Elite Vector Cat# PK-6100 according to
manufacturer's instructions. The spots were counted on an ELISPOT counter
(AID).
Donors were considered positive for a peptide if the number of spots was over
3 times
background as determined by responses to irrelevant peptides (non influenza).
Representative results are shown in Figure 5. Five peptides induced
significant immune
responses, PB2.438, PB1.94, M1.173, NP.189 and PA.178
Example 7: Influenza-Specific Recall Responses for Humans
[00373] Primary interferon-gamma (IFN-y) ELISPOT (enzyme linked immunospot)
assay
was performed to identify candidate HLA-A1, HLA-A2, HLA-A3/All, HLA-A24, HLA-
B7 and HLA-DR vaccine epitopes. Representative results are shown in Figures 7,
8, 10,
12, 14 and 16 respectively. Assays to identify CTL epitopes were performed
essentially
as described in Example 2 and the legend to Figure 1. Assays to identify HTL
epitopes
were performed essentially as described in Example 6.
Example 8: Influenza-Specific Recall Responses for Mice
[00374] Primary interferon-gamma (IFN-y) ELISPOT (enzyme linked immunospot)
assay
was performed using mice carrying an HLA-A2, HLA-A11, HLA-A24, HLA-B7 or
HLA-DR4 transgene, and results are shown in Figures 9, 11, 13, 15 and 17
respectively.
In addition, HLA-DR4 candidate vaccine epitopes were tested in b x d haplotype
mice,
and results are shown in Figure 18. Assays were performed essentially as
described in
Example 4 and the legend to Figure 4.
CA 02658559 2009-01-20
WO 2008/039267 109 PCT/US2007/016529
Literature Cited
1. Thompson,W.W., D.K. Shay, E.Weintraub, L. Brammer, N. Cox, L.J.
Anderson, and K. Fukuda, 2003, "Mortality associated with influenza and
respiratory
syncytial virus in the United States", JAMA 289:179-186.
2. Nguyen-Van-Tam, J.S., and A.W.Hampson, 2003, "The epidemiology
and clinical impact of pandemic influenza", Vaccine. 21:1762-1768.
3. "The Lancet Infectious Diseases", 4:595, 2004.
4. Nicholson, K.G., A.E. Colegate, A. Podda, I. Stephenson, J. Wood, E.
Ypma, and M.C. Zambon, 2001, "Safety and antigenicity of non-adjuvanted and
MF59-
adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of
two
potential vaccines against H5N1 influenza", Lancet 357:1937-1943.
5. Treanor, J.J., B.E. Wilkinson, F. Masseoud, J. Hu-Primmer, R. Battaglia,
D. O'Brien, M. Wolff, G. Rabinovich, W. Blackwelder, and J.M. Katz, 2001,
"Safety
and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in
humans", Vaccine 19:1732-1737.
6. Potter, C.W., 2001, "A history of influenza", J Appl. Microbiol, 91:572-
579.
7. Hilleman, M.R., 2002, "Realities and enigmas of human viral influenza:
pathogenesis, epidemiology and control", Vaccine 20:3068-3087.
8. Beveridge, W.I., 1991, "The chronicle of influenza epidemics", Hist.
Philos. Life Sci. 13:223-234.
9. Gust, I.D., A.W. Hampson, and D. Lavanchy, 2001, "Planning for the
next pandemic of influenza" Rev. Med. Virol. 11:59-70.
10. Oxford, J.S., 2000, "Influenza A pandemics of the 20th century with
special reference to 1918: virology, pathology and epidemiology", Rev.Med.
Virol.
10:119-133.
11. Laver, G. and E. Garman, 2002, "Pandemic influenza: its origin -and
control" Microbes.Infect. 4:1309-1316.
12. Tollis, M. and L. Di Trani, 2002, "Recent developments in avian
influenza research: epidemiology and immunoprophylaxis", Vet. J. 164:202-215.
13. Palese, P. and J.F. Young, 1982, "Variation of influenza A, B, and C
viruses", Science 215:1468-1474.
CA 02658559 2009-01-20
WO 2008/039267 110 PCT/US2007/016529
14. Gorman, O.T., W.J. Bean, and R.G. Webster, 1992, "Evolutionary
processes in influenza viruses: divergence, rapid evolution, and stasis",
Curr. Top.
Microbiol. Immunol 176:75-97.
15. Yewdell, J.W., R.G.Webster, and W.U. Gerhard, 1979, "Antigenic
variation in three distinct determinants of an influenza type A haemagglutinin
molecule",
Nature 279:246-248.
16. Top, F.H., Jr. and P.K. Russell, 1977, "Swine influenza A at Fort Dix,
New Jersey (January-February 1976). IV, Summary and speculation. J. Infect.
Dis. 136
Suppl:S376-S380.
17. Kurtz, J., R.J. Manvell, and J.Banks, 1996, "Avian influenza virus
isolated
from a woman with conjunctivitis", Lancet 348:901-902.
18. Peiris, M., K.Y. Yuen, C.W. Leung, K.H. Chan, P.L. Ip, R.W. Lai, W.K.
Orr, and K.F. Shortridge, 1999, "Human infection with influenza H9N2", Lancet
354:916-917.
19. Scholtissek, C., W. Rohde, H. Von, V, and R. Rott, 1978, "On the origin
of the human influenza virus subtypes H2N2 and H3N2", Virology 87:13-20.
20. Wells, M.A., F.A. Ennis, and P. Albrecht, 1981, "Recovery from a viral
respiratory infection, II. Passive transfer of immune spleen cells to mice
with influenza
pneumonia", J. Immunol. 126:1042-1046.
21. Yap, K.L. and G.L. Ada, 1978, "Cytotoxic T cells in the lungs of mice
infected with an influenza A virus", Scand. J. Immunol. 7:73-80.
22. Mackenzie, C.D., P.M. Taylor, and B.A. Askonas, 1989, "Rapid recovery
of lung histology correlates with clearance of influenza virus by specific
CD8+ cytotoxic
T cells", Immunology 67:375-381.
23. Taylor, P.M. and B.A. Askonas, 1986, "Influenza nucleoprotein-specific
cytotoxic T-cell clones are protective in vivo", Immunology 58:417-420.
24. Kuwano, K., M. Scott, J.F. Young, and F.A.Ennis, 1988, "HA2 subunit of
influenza A H1 and H2 subtype viruses induces a protective cross-reactive
cytotoxic T
lymphocyte response", J. Immunol. 140:1264-1268.
25. Kuwano, K., M. Tamura, and F.A. Ennis, 1990, "Cross-reactive
protection against influenza A virus infections by an NSl-specific CTL clone",
Virology
178:174-179.
26. Sambhara, S., A. Kurichh, R. Miranda, T. Tumpey, T. Rowe, M.
Renshaw, R. Arpino, A. Tamane, A. Kandil, O. James, B. Underdown, M. Klein, J.
Katz,
CA 02658559 2009-01-20
WO 2008/039267 111 PCT/US2007/016529
and D. Burt, 2001, "Heterosubtypic immunity against human influenza A viruses,
including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM
vaccine
in mice requires both cytotoxic T-lymphocyte and macrophage function", Cell
Immunol.
211:143-153.
27. Okuda, K., A. Ihata, S. Watabe, E. Okada, T. Yamakawa, K. Hamajima,
J. Yang, N. Ishii, M. Nakazawa, K. Okuda, K. Ohnari, K. Nakajima, and K.Q.
Xin, 2001,
"Protective immunity against influenza A virus induced by immunization with
DNA
plasmid containing influenza M gene", Vaccine 19:3681-3691.
28. Bender, B.S., T. Croghan, L. Zhang, and P.A. Small, Jr:, 1992,
"Transgenic mice lacking class I major histocompatibility complex-restricted T
cells
have delayed viral clearance and increased mortality after influenza virus
challenge", J.
Exp. Med. 175:1143-1145.
29. Ulmer, J.B., J.J. Donnelly, S.E. Parker, G.H. Rhodes, P.L. Felgner, V.J.
Dwarki, S.H. Gromkowski, R.R. Deck, C.M. DeWitt, A. Friedman, et al., 1993,
"Heterologous protection against influenza by injection of DNA encoding a
viral
protein", Science 259:1745-1749.
30. Fu, T.M., L. Guan, A. Friedman, T.L. Schofield, J.B. Ulmer, M.A. Liu,
and J.J. Donnelly, 1999, "Dose dependence of CTL precursor frequency induced
by a
DNA vaccine and correlation with protective immunity against influenza virus
challenge", J. Irnmunol. 162:4163-4170.
31. Ulmer, J.B., T.M. Fu, R.R. Deck, A. Friedman, L. Guan, C. DeWitt, X.
Liu, S. Wang, M.A. Liu, J.J. Donnelly, and M.J. Caulfield, 1998, "Protective
CD4+ and
CD8+ T cells against influenza virus induced by vaccination with nucleoprotein
DNA",
J. Virol. 72:5648-5653.
32. Epstein, S.L., A. Stack, J.A. Misplon, C.Y. Lo, H. Mostowski, J. Bennink,
and K. Subbarao,. 2000, "Vaccination with DNA encoding internal proteins of
influenza
virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+)
T
cells can promote survival and recovery after challenge", Int. Immunol 12:91-
101.
33. Langlade-Demoyen, P., F. Garcia-Pons, P. Castiglioni, Z. Garcia, S.
Cardinaud, S. Xiong, M. Gerloni, and M. Zanetti, 2003, "Role of T cell help
and
endoplasmic reticulum targeting in protective CTL response against influenza
virus",
Eur. J. Immunol. 33:720-728.
CA 02658559 2009-01-20
WO 2008/039267 112 PCT/US2007/016529
34. Anker, W.J., A. K. Bakker, and N. Masurel. 1978, "Cross-protection in
mice after immunization with H2N2, H3N2, and H2N2 influenza virus strains".
Infect.
Immun. 21:96-101.
35. Schulman, J.L. and E.D. Kilboume, 1965, "Induction of partial specific
heterotypic immunity in mice by a single infection with influenza A virus", J.
Bacteriol.
89:170-174.
36. Benton, K.A., J.A. Misplon, C.Y. Lo, R.R. Brutkiewicz, S.A. Prasad, and
S.L. Epstein, 2001, "Heterosubtypic immunity to influenza A virus in mice
lacking IgA,
all Ig, NKT cells, or gamma delta T cells", J. Immunol. 166:7437-7445.
37. O'Neill, E., S.L. Krauss, J.M. Riberdy, R.G. Webster, and D.L.
Woodland, 2000, "Heterologous protection against lethal A/HongKong/156/97
(H5N1)
influenza virus infection in C57BL/6 mice", J. Gen. Virol. 81:2689-2696.
38. Epstein, S.L., T.M. Tumpey, J.A. Misplon, C.Y. Lo, L.A. Cooper, K.
Subbarao, M. Renshaw, S. Sambhara, and J.M. Katz, 2002, "DNA vaccine
expressing
conserved influenza virus proteins protective against H5N1 challenge infection
in mice",
Emerg. Infect. Dis. 8:796-801.
39. Nguyen, H.H., Z. Moldoveanu, M.J. Novak, F.W. Van Ginkel, E. Ban, H.
Kiyono, J.R. McGhee, and J. Mestecky, 1999, "Heterosubtypic immunity to lethal
influenza A virus infection is associated with virus-specific CD8(+) cytotoxic
T
lymphocyte responses induced in mucosa-associated tissues", Virology 254:50-
60.
40. Lukacher, A.E., V.L. Braciale, and T.J. Braciale, 1984, "In vivo effector
function of influenza virus-specific cytotoxic T lymphocyte clones is highly
specific", J.
Exp. Med. 160:814-826.
41. Liang, S., K. Mozdzanowska, G. Palladino, and W. Gerhard, 1994,
"Heterosubtypic immunity to influenza type A virus in mice. Effector
mechanisms and
their longevity", J. Immunol. 152:1653-1661.
42. Tumpey, T.M., M. Renshaw, J.D. Clements, and J.M. Katz, 2001,
"Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent
heterosubtypic cross-protection against lethal influenza A H5N1 virus
infection", J.
Virol. 75:5141-5150.
43. McMichael, A.J., F.M. Gotch, G.R. Noble, and P.A.S. Beare, 1983,
"Cytotoxic T-cell immunity to influenza", N. Engl. J. Med. 309:13-17.
CA 02658559 2009-01-20
WO 2008/039267 113 PCT/US2007/016529
44. Sonoguchi, T., H. Naito, M. Hara, Y. Takeuchi, and H. Fukumi, 1985,
"Cross-subtype protection in humans during sequential, overlapping, and/or
concurrent
epidemics caused by H3N2 and HINI influenza viruses", J. Infect. Dis. 151:81-
88.
45. Voeten, J.T., T.M. Bestebroer, N.J. Nieuwkoop, R.A. Fouchier, A.D.
Osterhaus, and G.F. Rimmelzwaan, 2000, "Antigenic drift in the influenza A
virus
(H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes",
J.
Virol. 74:6800-6807.
46. Kashiwagi, T., N. Hamada, J. Iwahashi, K. Hara, T. Ueda, H. Noguchi,
and T. Toyoda, 2000, "Emergence of new influenza A viruses which carry an
escape
mutation of the HLA-B27-restricted CTL epitope of NP in Japan", Microbiol.
Immunol
44:867-870.
47. Boon, A.C., G.de Mutsert, Y.M. Graus, R.A. Fouchier, K. Sintnicolaas,
A.D. Osterhaus, and G.F. Rimmelzwaan, 2002, "Sequence variation in a newly
identified
HLA-B35-restricted epitope in the influenza A virus nucleoprotein associated
with
escape from cytotoxic T lymphocytes", J. Virol. 76:2567-2572.
48. Treanor, J.J., E.L. Tierney, S.L. Zebedee, R.A. Lamb, and B.R. Murphy,
1990, "Passively transferred monoclonal antibody to the M2 protein inhibits
influenza A
virus replication in mice", J. Virol. 64:1375-1377.
49. Frace, A.M., A.I. Klimov, T. Rowe, R.A. Black, and J.M. Katz, 1999,
"Modified M2 proteins produce heterotypic immunity against influenza A virus",
Vaccine 17:2237-2244.
50. Neirynck, S., T. Deroo, X. Saelens, P. Vanlandschoot, W.M. Jou, and W.
Fiers, 1999, "A universal influenza A vaccine based on the extracellular
domain of the
M2 protein", Nat. Med. 5:1157-1163.
51. Jegerlehner, A., N. Schmitz T. Storni, and M.F. Bachmann, 2004,
"Influenza A vaccine based on the extracellular domain of M2: Weak protection
mediated via antibody-dependent NK cell activity", J Immunol. 172:5598-5605.
52. Fiers, W., M. DeFilette, A. Birkett, S. Neirynck, and W.M. Jou, 2004, "A
"universal' human influenza A vaccine", Virus Res. 103:173-176.
53. Wanli, L., P. Aou, Y.-H. Chen, 2004, "Monoclonal antibodies recognizing
EVETPIRN epitope of influenza A virus M2 protein could protect mice from
lethal
influenza A virus challenge", Immunol. Lett. 93:131-136.
54. Fan, J., X. Liang, M.S. Horton, H.C. Perry, M.P. Citron, G.J. Heidecker,
T.M. Fu, J. Joyce, C.T. Przysiecki, P.M. Keller, V.M. Garsky, R. lonescu, Y.
Rippeon,
CA 02658559 2009-01-20
WO 2008/039267 114 PCT/US2007/016529
L. Shi, M.A. Chastain, J.H. Condra, M.E. Davies, J. Liao, E.A. Emini, and J.W.
Shiver,
2004, "Preclinical study of influenza virus A M2 peptide conjugate vaccines in
mice,
ferrets, and rhesus monkeys", Vaccine 22:2993-3003.
55. Jameson, J., J. Cruz, M. Terajima, and F.A. Ennis, 1999, "Human CD8+
and CD4+ T lymphocyte memory to influenza A viruses of swine and avian
species", J.
Immunol. 162:7578-7583.
56. Jameson, J., J. Cruz, and F.A. Ennis, 1998, "Human cytotoxic T-
lymphocyte repertoire to influenza A viruses", J. Virol. 72:8682-8689.
57. Gianfrani, C., C. Oseroff, J. Sidney, R.W. Chesnut, and A. Sette, 2000,
"Human memory CTL response specific for influenza A virus is broad and
multispecific", Hum. Immunol. 61:438-452.
58. Boon, A.C., G.de Mutsert, Y.M. Graus, R.A. Fouchier, K. Sintnicolaas,
A.D. Osterhaus, and G.F. Rimmelzwaan, 2002, "The magnitude and specificity of
influenza A virus-specific cytotoxic T-lymphocyte responses in humans is
related to
HLA-A and -B phenotype", J. Virol. 76:582-590.
59. Liu, M.A, 2003, "DNA vaccines: a review", J. Intern. Med. 253:402-410.
60. Gurunathan, S., D.M. Klinman, and R.A. Seder, 2000, "DNA vaccines:
immunology, application, and optimization", Annu. Rev. Immunol 18:927-974.
61. Calarota, S.A., A.C. Leandersson, G. Bratt, J. Hinkula, D.M. Klinman,
K.J. Weinhold, E. Sandstrom, and B. Wahren, 1999, "Immune responses in -
asymptomatic HIV- 1 -infected patients after HIV-DNA immunization followed by
highly
active antiretroviral treatment", J. Immunol. 163:2330-2338.
62. Roy, M.J., M.S. Wu, L.J. Barr, J.T. Fuller, L.G. Tussey, S. Speller, J.
Culp, J.K. Burkholder, W.F. Swain, R.M. Dixon, G. Widera, R. Vessey, A. King,
G.
Ogg, A. Gallimore, J.R. Haynes, and F.D. Heydenburg, 2000, "Induction of
antigen-
specific CD8+ T cells, T helper cells, and protective levels of antibody in
humans by
particle-mediated administration of a hepatitis B virus DNA vaccine", Vaccine
19:764-
778.
63. Ugen, K.E., S.B. Nyland, J.D. Boyer, C. Vidal, L. Lera, S. Rasheid, M.
Chattergoon, M.L. Bagarazzi, R. Ciccarelli, T. Higgins, Y. Baine, R. Ginsberg,
R.R.
MacGregor, and D.B. Weiner, 1998, "DNA vaccination with HIV-1 expressing
constructs elicits immune responses in humans", Vaccine 16:1818-1821.
CA 02658559 2009-01-20
WO 2008/039267 115 PCT/US2007/016529
64. Calarota, S.A., A. Kjerrstrom, K.B. Islam, and B. Wahren, 2001, "Gene
combination raises broad human immunodeficiency virus-specific cytotoxicity",
Hum.
Gene Ther. 12:1623-1637.
65. Wang, R., J. Epstein, F.M. Baraceros, E.J. Gorak, Y. Charoenvit, D.J.
Carucci, R.C. Hedstrom, N. Rahardjo, T. Gay, P. Hobart, R. Stout, T.R. Jones,
T.L.
Richie, S.E. Parker, D.L. Doolan, J. Norman, and S.L. Hoffinan, 2001,
"Induction of
CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA
vaccine", Proc. Natl. Acad. Sci. USA 98:10817-10822.
66. Sette, A., M. Newman, B. Livingston, D. McKinney, J. Sidney, G.
Ishioka, S. Tangri, J. Alexander, J. Fikes, and R. Chesnut, 2002, "Optimizing
vaccine
design for cellular processing, MHC binding and TCR recognition", Tissue
Antigens
59:443-451.
67. Livingston, B., C. Crimi, M. Newman, Y. Higashimoto, E. Appella, J.
Sidney, and A. Sette, 2002, "A Rational Strategy to Design Multiepitope
Immunogenes
Based on Multiple TH Lymphocyte Epitopes", J. Immunol. 168:5499-5506.
68. Ishioka, G.Y., J. Fikes, G. Hermanson, B. Livingston, C. Crimi, M. Qin,
M.F. del Guercio, C. Oseroff, C. Dahlberg, J. Alexander, R.W. Chesnut, and A.
Sette,
1999, "Utilization of MHC class I transgenic mice for development of minigene
DNA
vaccines encoding multiple HLA-restricted CTL epitopes", J. Immunol. 162:3915-
3925.
69. Livingston, B.D., M. Newman, C. Crimi, D. McKinney, R. Chesnut, and
A. Sette, 2001, "Optimization of epitope processing enhances immunogenicity of
multiepitope DNA vaccines", Vaccine 19:4652-4660.
70. Alila, H., M.E. Coleman, H. Nitta, M. French, K. Anwer, Q. Liu, T.
Meyer, J. Wang, R.J. Mumper, D. Oubari, S.D. Long, J.L. Nordstrom, and A.P.
Rolland,
1997, "Expression of biologically active human insulin-like growth factor-I
following
intramuscular injection of a formulated plasmid in rats", Human Gene Therapy
8:1785-
1795.
71. Anwer, K., K.A. Earle, M. Shi, J. Wang, R.J. Mumper, B. Proctor, K.
Jansa, H.C. Ledebur, S.S. Davis, W. Eaglstein, and A.P. Rolland, 1999,
"Synergistic
Effect of Formulated Plasmid and Needle-Free Injection for Genetic Vaccines",
Pharm.
Res. 16:889-95.
72. Mumper, R.J., J.G. Duguid, K. Anwer, M.K. Barron, H. Nitta, and A.P.
Rolland, 1996, "Polyvinyl derivatives as novel interactive polymers for
controlled gene
delivery to muscle", Pharm. Res. 13:701-709.
CA 02658559 2009-01-20
WO 2008/039267 116 PCT/US2007/016529
73. Mumper, R.J., J. Wang, S.L. Klakamp, H. Nitta, K. Anwer, F. Tagliaferri,
and A.P. Rolland, 1998, "Protective interactive noncondensing (PINC) polymers
for
enhanced plasmid distribution and expression in rat skeletal muscle", J.
Contr. Rel.
52:191-203.
74. Epstein, S.L., A. Stack, J.A. Misplon, C.Y. Lo, H. Mostowski, J. Bennink,
and K. Subbarao, 2000, "Vaccination with DNA encoding internal proteins of
influenza
virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+)
T
cells can promote survival and recovery after challenge", Int. Immunol 12:91-
101.
75. Epstein, S.L., T.M. Tumpey, J.A. Misplon, C.Y. Lo, L.A. Cooper, K.
Subbarao, M. Renshaw, S. Sambhara, and J.M. Katz, 2002, "DNA vaccine
expressing
conserved influenza virus proteins protective against H5N1 challenge infection
in mice",
Emerg. Infect. Dis. 8:796-801.
76. Vitiello, A., D. Marchesini, J. Furze, L.A. Sherman, and R.C. Chesnut,
1991, "Analysis of the HLA-restricted influenza-specific cytotoxic T
lymphocyte
response in transgenic mice carrying a chimeric human-mouse class I major
histocompatibility complex", J. Exp. Med. 173:1007-15.
77. Alexander, J., C. Oseroff, J. Sidney, P. Wentworth, E. Keogh, G.
Hermanson, F.V. Chisari, R.T. Kubo, H.M. Grey, and A. Sette, 1997, "Derivation
of
HLA-A11/Kb transgenic mice: functional CTL repertoire and recognition of human
A11-
restricted CTL epitopes", J. Immunol. 159:4753-4761.
78. Alexander, J., C. Oseroff, J. Sidney, and A. Sette, 2003, "Derivation of
HLA-B*0702 transgenic mice: functional CTL repertoire and recognition of human
B*0702-restricted CTL epitopes", Hum. Immuno164:211-223.
79. Alexander, J., C. Oseroff, C. Dahlberg, M. Qin, G. Ishioka, M. Beebe, J.
Fikes, M. Newman, R.W. Chesnut, P.A. Morton, K. Fok, E. Appella, and A. Sette,
2002,
"A decaepitope polypeptide primes for multiple CD8+ IFN-gamma and Th
lymphocyte
responses: evaluation of multiepitope polypeptides as a mode for vaccine
delivery", J.
Immunol. 168:6189-6198.
80. Wall, K.A., J.Y. Hu, P. Currier, S. Southwood, A. Sette, and A.J. Infante,
1994, "A disease-related epitope of Torpedo acetylcholine receptor. Residues
involved in
I-Ab binding, self-nonself discrimination, and TCR antagonism", J. Immunol.
152:4526-
4536.
81. Townsend, A. and H. Bodmer, 1989, "Antigen recognition by class I-
restricted T lymphocytes", Annu. Rev. Immunol. 7:601-624.
CA 02658559 2009-01-20
WO 2008/039267 117 PCT/US2007/016529
82. Germain, R.N. and D.H. Margulies, 1993, "The biochemistry and cell
biology of antigen processing and presentation", Annu. Rev. Immunol. 11:403-
450.
83. Sette, A. and H.M. Grey, 1992, "Chemistry of peptide interactions with
MHC proteins", Current Opinion in Imunology 4:79-86.
84. Sinigaglia, F. and J. Hammer, 1994, "Defining rules for the peptide-MHC
class II interaction", Current Opinion in Imunology. 6:52-56.
85. Engelhard,V.H., 1994, "Structure of peptides associated with MHC class I
molecules", Current Opinion in Imunology. 6:13-23.
86. Brown, K., T.S. Jardetzky, J.C. Gorga, L.J. Stern, J.L. Strominger, and
D.C. Wiley, 1993, "Three-dimensional structure of the human class II
histocompatibility
antigen HLA-DRI ", Nature 364:33-39.
87. Guo, H.C., D.R. Madden, M.L. Silver, T.S. Jardetzky, J.C. Gorga, J.L.
Strominger, and D.C. Wiley, 1993, "Comparison of the P2 specificity pocket in
three
human histocompatibility antigens: HLA-A*6801, HLA-A*0201, and HLA-B*2705",
Proc. Nat. Acad. Sci. USA 90:8053-8057.
88. Guo, H.C., T.S. Jardetzky, T.P. Garrett, W.S. Lane, J.L. Strominger, and
D.C. Wiley, 1992, "Different length peptides bind to HLA-Aw68 similarly at
their ends
but bulge out in the middle", Nature 360:364-366.
89. Silver, M.L., H.C. Guo, J.L. Strominger, and D.C. Wiley, 1992, "Atomic
structure of a human MHC molecule presenting an influenza virus peptide",
Nature
360:367-369.
90. Matsumura, M., D.H. Fremont, P.A. Peterson, and I.A. Wilson, 1992,
"Emerging principles for the recognition of peptide antigens by MHC class I
molecules".
Science 257:927-934.
91. Madden, D.R., J.C. Gorga, J.L. Strominger, and D.C. Wiley, 1995, "The
three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general
mechanism for tight peptide binding to MHC" Cell 70:1035-1048.
92. Fremont, D.H., M. Matsumura, E.A. Stura, P.A. Peterson, and I.A.
Wilson, 1992, "Crystal structures of two viral peptides in complex with murine
MHC
class I H-2Kb", Science 257:919-927.
93. Sapp, M., P.J. Bjorkman, and D.C. Wiley, 1991, "Refined structure of the
human histocompatibility antigen HLA-A2 at 2.6 A resolution", J. Mol. Biol.
219:277-
319.
CA 02658559 2009-01-20
WO 2008/039267 118 PCT/US2007/016529
94. Ruppert, J., J. Sidney, E. Celis, R.T. Kubo, H.M. Grey, and A. Sette,
1993, "Prominent role of secondary anchor residues in peptide binding to HLA-
A2.1
molecules", Cell 74:929-37.
95. Parker, K.C., M.A. Bednarek, and J.E. Coligan, 1994, "Scheme for
ranking potential HLA-A2 binding peptides based on independent binding of
individual
peptide side-chains", J. Immunol. 152:163-175.
96. De Groot, A.S., A. Bosma, N. Chinai, J. Frost, B.M. Jesdale, M.A.
Gonzalez, W. Martin, and C. Saint-Aubin, 2001, Vaccine 19:4385-4395.
97. Schueler-Furman, 0., Y. Altuvia, A. Sette, and H. Margalit, 2000,
"Structure-based prediction of binding peptices to MHC class I molecules:
Application to
a broad range of MHC alleles", Protein Science 9:1838-1846.
98. Meister, G.E., C.G.P. Roberts, J.A. Berzofsky, and A.S. DeGroot, 1995,
"Two novel T cell epitope prediction algorithms based on MHC-binding motifs;
comparison of predicted and published epitopes from Mycobacterium tuberculosis
and
HIV protein sequences", Vaccine 13:581-591.
99. Bhasin, M., and G.P.S. Raghava, 2004, "Prediction of CTL epitopes using
QM, SVM and ANN techniques", Vaccine 22:3195-3204.
100. Altuvia, Y., O. Schueler, and H. Margalit, 1995, "Ranking potential
binding peptides to MHC molecules by a computational threading approach", J.
Mol.
Biol. 249:244-250.
101. Methods for prediction of peptide binding to MHCmolecules: a
comparative study, Molecular Medicine 8:137-148.
102. Gulukota, K., J. Sidney, and A. Sette, 1997, "Two complementary
methods for predicting peptide binding major histocompatibility complex
molecules", J.
Mol. Biol. 267:1258-1267.
103. Kubo, R.T., A. Sette, H.M. Grey, E. Appella, K. Sakaguchi, N.Z. Zhu, D.
Arnott, N. Sherman, J. Shabanowitz, and H. Michel, 1994, "Definition of
specific
peptide motifs for four major HLA-A alleles", J. Immun. 152:3913-24.
104. Threlkeld, S.C., P.A. Wentworth, S.A. Kalams, B.M. Wilkes, D.J. Ruhl,
E. Keogh, J. Sidney, S. Southwood, B.D. Walker, and A. Sette, 1997,
"Degenerate and
promiscuous recognition by CTL of peptides presented by the MHC class I A3-
like
superfamily - Implications for vaccine development", J. Immun. 159:1648-1657.
105. Sidney, J., H.M. Grey, S. Southwood, E. Celis, P.A. Wentworth, M.F. Del
Guercio, R.T. Kubo, R.C. Chesnut, and A. Sette, 1996, "Definition of an HLA-A3-
like
CA 02658559 2009-01-20
WO 2008/039267 119 PCT/US2007/016529
supermotif demonstrates the overlapping peptide-binding repertoires of common
HLA
molecules", Hum. Immunol. 45:79-93.
106. Sette, A. and Sidney, J., "Nine major HLA class I supertypes account for
the vast preponderance of HLA-A and -B polymorphism", Immunogenetics 50(3-4),
201-212, 1999.
107. Sidney, J., S. Southwood, M.F. Del Guercio, H.M. Grey, R.C. Chesnut,
R.T. Kubo, and A. Sette, 1996, "Specificity and degeneracy in peptide binding
to HLA-
B7-like class I molecules", J. Immunol. 157:3480-3490.
108. Del Guercio, M.F., J. Sidney, G. Hermanson, C. Perez, H.M. Grey, R.T.
Kubo, and A. Sette, 1995, "Binding of a peptide antigen to multiple HLA
alleles allows
definition of an A2-like supertype" J. Immunol. 54:685-693.
109. Fruci, D., P. Rovero, G. Falasca, A. Chersi, R. Sorrentino, R. Butler, N.
Tanigaki, and R. Tosi, 1993, "Anchor residue motifs of HLA class-I-binding
peptides
analyzed by the direct binding of synthetic peptides to HLA class I alpha
chains", Hum.
Immunol. 38:187-192.
110. Bertoni, R., J. Sidney, P. Fowler, R.W. Chesnut, F.V. Chisari, and A.
Sette, 1997, "Human histocompatibility leukocyte antigen-binding supermotifs
predict
broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute
hepatitis", J. Clin. Invest. 100:503-513.
111. Khanna, R., S.R. Burrows, A. Neisig, J. Neefjes, D.J. Moss, and S.L.
Silins, 1997, "Hierarchy of Epstein-Barr virus-specific cytotoxic T-cell
responses in
individuals carrying different subtypes of an HLA allele: Implications for
epitope-based
antiviral vaccines", J. Virol. 71:7429-7435.
112. Bertoletti, A., S. Southwood, R. Chesnut, A. Sette, M. Falco, G.B.
Ferrara, A. Penna, C. Boni, F. Fiaccadori, and C. Ferrari, 1997, "Molecular
features of
the hepatitis B virus nucleocapsid T-cell epitope 18-27: interaction with HLA
and T-cell
receptor", Hepatology 26:1027-1034.
113. Fleischhauer, K., S. Tanzarella, H.J. Wallny, C. Bordignon, and C.
Traversari, 1996, "Multiple HLA-A alleles can present an immunodominant
peptide of
the human melanoma antigen Melan-A/MART-1 to a peptide-specific HLA-A*0201+
cytotoxic T cell line", J. Immunol. 157:787-297.
114. Kawashima, I., S.J. Hudson, V. Tsai, S. Southwood, K. Takesako, E.
Appella, A. Sette, and E. Celis, 1998, "The multi-epitope approach for
immunotherapy
CA 02658559 2009-01-20
WO 2008/039267 120 PCT/US2007/016529
for cancer: Identification of several CTL epitopes from various tumor-
associated
antigens expressed on solid epithelial tumors", Hum. Immunol. 59:1-14.
115. Wang, R.F., S.L. Johnston, S. Southwood, A. Sette, and S.A. Rosenberg,
1998, "Recognition of an antigenic peptide derived from tyrosinase-related
protein-2 by
CTL in the context of HLA-A31 and -A33", J. Immunol. 160:890-897.
116. Southwood, S., J. Sidney, A. Kondo, M.F. Del Guercio, E. Appella, S.
Hoffman, R.T. Kubo, R.W. Chesnut, H.M. Grey, and A. Sette, 1998, "Several
Common
HLA-DR Types Share Largely Overlapping Peptide Binding Repertoires", J.
Immunol.
160:3363-3373.
117. Schaeffer, E.B., A. Sette, D.L. Johnson, M.C. Bekoff, J.A. Smith, H.M.
Grey, and S. Buus, 2000, "Relative contribution of "determinant selection" and
"holes in
the T-cellrepertoire" to T-cell responses", Proc. Nat. Acad. Sci. USA 86:4649-
4653.
118. Currier, J.R., E.G. Kuta, E. Turk, B. L.B. Earhart, L. Loomis-Price, S.
Janetzki, G. Ferrari, D.L. Birx, and J.H. Cox, 2002, "A panel of MHC class I
restricted
viral peptides for use as a quality control for vaccine trial ELISPOT assays",
J.
Immunological Meth. 260:157-172.
119. Bartlett, J.A., S.S. Wasserman, C.B. Hicks, R.T. Dodge, K.J. Weinhold,
C.O. Tacket, N. Ketter, A.E. Wittek, T.J. Palker, and B.F. Haynes, 1998,
"Safety and
immunogenicity of an HLA-based HIV envelope polyvalent synthetic peptide
immunogen DATRI 010 study group. Division of AIDS Treatment Research
Inititive",
AIDS 12:1291-1300.
120. Pinto, L.A., J.A. Berzofsky, K.R. Fowke, R.F. Little, F. Merced-Galindez,
R. Humphrey, J. Ahlers, N. Dunlop, R.B. Cohen, S.M. Steinberg, P. Nara, G.M.
Shearer,
and R. Yarchoan, 1999, "HIV-specific immunity following immunization with HIV
synthetic envelope peptides in asymptomatic HIV-infected patients", AIDS
13:2003-
2012.
121. Gahery-Segard, H., G. Pialoux, B. Charmeteau, S. Sermet, H. Poncelet,
M. Raux, A. Tartar, J.P. Levy, H. Gras-Masse, and J.G. Guillet, 2000,
"Multiepitopic B-
and T-cell responses induced in humans by a human immunodeficiency virus type
1
lipopeptide vaccine", J. Virol. 74:1694-1703.
122. Serwold, T. and N. Shastri, 1999, "Specific Proteolytic Cleavages Limit
the Diversity of the Pool of Peptides Available to MHC Class I Molecules in
Living
Cells", J. Irnmunol. 162:4712-4719.
CA 02658559 2009-01-20
WO 2008/039267 121 PCT/US2007/016529
123. Shastri, N., T. Serwold, and F. Gonzalez, 1995, "Presentation of
endogenous peptide/MHC class I complexes is profoundly influenced by specific
C-
terminal flanking residues", J. Immunol. 155:4339-4346.
124. Chou, K.C., 2000, "Prediction of tight turns and their types in
proteins",
Anal. Biochem. 286:1-16.
125. Davis, N.L., A. West, E. Reap, G. MacDonald, M. Collier, S. Dryga, MM
Maughan, M. Connell, C. Walker, K. McGrath, C. Cecil, L.H. Ping, J. Frelinger,
R.
Olmstged, P. Keith, R. Swanstrom, C. Williamson, P. Johnson, D. Montefiori,
and R.E.
Johnston, 2002, "Alphavirus replicon particles as candidate HIV Vaccines",
IUBMB Life
53:209-211.
126. Lee, J.S., B.K. Dyas, S.S. Nystrom, C.M. Lind, J.F. Smith, and R.G.
Ulrich, 2002, "Immune protection against Staphylococcal enterotoxin-induced
toxic
shock by vaccination with a Venezuelan Equine Encephalitis virus replicon", J.
Infec.
Dis. 185:1192-1196.
127. Hevey, M., D. Negley, L. VanderZanden, R.F. Tammariello, J. Geisbert,
C. Schmaljohn, J.F. Smith, P.B. Jahrling, and A.L. Schmaljohn, 2002, "Marburg
virus
vacciness: comparing classical and new approaches", Vaccine 20:586-593.
128. Pratt, W.D., N.L. Davis, R.E. Johnston, J.F. Smith, 2003, "Genetically
engineered, live attenuated vaccines for Venezuelan equine encephalitis:
testing in
animal models. 2003", Vaccine 21:3854-3862.
129. Gipson, C.L., N.L. Davis, R.E. Johnston, and A.M. deSilva, 2003,
"Evaluation of Venezuelan Equine Encephalitis (VEE) replicon-based outer
surface
protein A (OspA) vaccines in a tick challenge mouse model of Lyme disease",
Vaccine
21:3875-3884.
130. Nelson, E.L., D. Prieto, T.G. Alexander, P. Pushko, L.A. Lofts, J.O.
Rayner, K.I. Kamrud, B. Fralish, and J.F. Smith, 2003, "Venezuelan equine
encephalitis
replicon immunization overcomes intrinsic tolerance and elicits effective anti-
tumor
immujnity to the `self tumor-associated antigen, neu in a rat mammary tumor
model",
Breast Cancer Research and Treatment 82:169-183.
131. Leitner, W.W., L.N. Hwang, M.J. DeVeer, A. Zhou, R.H. Silverman,
B.R.G. Williams, T.W. Dubensky, H. Ying, and N.P. Restifo, 2003, "Alphavirus-
based
DNA vaccine breaks immunological tolerance by activating innate antiviral
pathways",
Nat. Med. 9:33-39.
CA 02658559 2009-01-20
WO 2008/039267 122 PCT/US2007/016529
132. Leitner, W.W., L.N. Hwang, E.S. Bergmann-Leitner, S.E. Finkelstein, S.
Frank, and N.P. Restifo, 2004, "Apoptosis is essential for the increased
efficacy of
alphaviral replicase-based DNA vaccines", Vaccine 22:1537-1544.
133. Garland, S.M., 2003, "Imiquimod", Curr. Opin. Infect. Dis. 16:85-89.
134. Jurk, M., F. Heil, J. Vollmer, C. Schetter, A.M. Krieg, H. Wagner, F.
Lipford, and S. Bauer, 2002, "Human TLR7 or TLR8 independently confer
responsiveness to the antiviral compound R-848", Nat. Immunol. 3:499.
135. Tyring, S.T., IArany, I., M.A. Stanley, M.A. Tomai, R.L. Miller, M.H.
Smith, D.J. McDermott and H.B. Slade, 1998, "A randomized, controlled,
molecular
study of condlomata acuminate clearance during treatment with imiquimod", J.
Infect.
Dis. 178:551-555.
136. Fritz, J.H., S. Brunner, M.L. Bimstiel, M. Buschle, A.V. Gabain, F.
Mattner, and W. Zauner, 2004, "The artificial antimicrobial peptide
KLKLLLLLKLK
induces predominantly a TH2-type immune response to co-injected antigens",
Vaccine
22:3274-3284.
137. Alexander, J., J. Sidney, S. Southwood, J. Ruppert, C. Oseroff, A.
Maewal, K. Snoke, H.M. Serra, R.T. Kubo, A. Sette and 1994, "Development of
high
potency universal DR-restricted helper epitopes by modification of high
affinity DR-
blocking peptides", Immunity. 1:751-761.
138. del Guercio, M.F., J. Alexander, R.T. Kubo, T. Arrhenius, A. Maewal, E.
Appella, S.L. Hoffman, T. Jones, D. Valmori, K. Sakaguchi, H.M. Grey, and A.
Sette,
1997, "Potent immunogenic short linear peptide constructs composed of B cell
epitopes
and Pan DR T helper epitopes (PADRE ) for antibody responses in vivo", Vaccine
15:441-448.
139. Alexander, J., M.F. delGuercio, B. Frame, A. Maewal, A. Sette, M.H.
Nahm, and M.J. Newman, 2004, "Development of experimental carbohydrate-
conjugate
vaccines composed of Streptococcus pneumoniae capsular polysaccharides and the
universal helper T-lyphocyte epitope (PADRE )", Vaccine 22:2362-2367.
140. Rosa, D.S., F. Tzelepis, M.G. Cunha, I.S. Soares, and M.M. Redriques,
2004, "The pan HLA DR-binding epitope improves adjuvant-assisted immunization
with
a recombinant protein containing a malaria vaccine candidate", Immunol. Lett.
92:259-
268.
141. Vichier-Guerre, S., R. Lo-Man, L. BenMohamed, E. Deriaud, S. Kovats,
C. Leclerc, and S. Bay, 2003, "Induction of carbohydrate-specific antibodies
in HLA-DR
CA 02658559 2009-01-20
WO 2008/039267 123 PCT/US2007/016529
transgenic mice by a synthetic glycopeptide: a potential anti cancer vaccine
for human
use", J. Peptide Res. 62:117-124.
142. Pamonsinlapatham, P., N. Decroix, L. Mihaila-Amrouche, A. Bouvet, and
J.P.
Bouvet, 2004, "Idnuction of a mucosal immune response to the Streptococcal M
protein
by intramuscular administration of a PADRE-ASREAK peptide.
143. Ressing, M.E., W.J. vanDriel, R.M.P. Brandt, G.G. Kenter, J.H. deJong, T.
Bauknecht, G.J. Fleuren, P. Hoogerhout, R. Offringa, A. Sette, E. Celis, H.
Grey, B.J.
Trimbos, W.M. Kast, and C.J.M. Melief, 2000, "Detection of T helper
respoonses, but
not a f human papillomavirus-specific cytotoxic T lymphocyte responses, after
peptide
vaccination of patients with cervical carcinoma", J. Immunother. 23:255-266.
144. Crotty, S., R.D. Aubert, J. Glidewell, and R. Ahmed, 2004, "Tracking
human
antigen-specific memory B cells: a sensitive and generalized ELISPOT system",
J.
Immunological Meth. 286:111-122.
145. Wilson, C., D.M. McKinney, M. Anders, S. MaWhinney, J. Forster, C. Crimi,
S.
Southwood, A. Sette, R. Chesnut, M. Newman, and B. Livingston, 2003,
"Development
of a DNA Vaccine Designed to Induce Cytotoxic T Lymphocyte Responses to
Multiple
Conserved Epitopes in HIV-1", J. Immunol. 171:5611-5623.
146. Claas, E.C., A.D. Osterhaus, R. Van Beek, J.C. De Jong, G.F. Rimmelzwaan,
D.A. Senne, S. Krauss, K.F. Shortridge, and R.G. Webster, 1998, "Human
influenza A
H5N1 virus related to a highly pathogenic avian influenza virus" Lancet
351:472-477.
147. Katz, J.M., X. Lu, A.M. Frace, T. Morken, S.R. Zaki, and T.M. Tumpey,
2000,
"Pathogenesis and immunity to avian influenza A H5 viruses", Biomed.
Pharmacother.
54:178-187.
148. Lu, X., T.M. Tumpey, T. Morken, S.R. Zaki, N.J. Cox, and J.M. Katz, 1999,
"A
mouse model for the evaluation of pathogenesis and immunity to influenza
A(H5N1)
viruses isolated from humans", J. Virol. 73:5903-5911.
149. Nicholson, K.G., A.E. Colegate, A. Podda, I. Stephenson, J. Wood, E.
Ypma, and
M.C. Zambon, 2001, "Safety and antigenicity of non-adjuvanted and MF59-
adjuvanted
influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two
potential
vaccines against H5N1 influenza", Lancet 357:1937.
150. Hehme, N., H. Engelmann, W. Kuenzel, E. Neumeier, and R. Saenger, 2004,
"Immunogenicity of. a monovalent, aluminum-adjuvanted influenza whole virus
vaccine
for pandemic use", Virus Res. 103:163-171.
CA 02658559 2009-01-20
WO 2008/039267 124 PCT/US2007/016529
151. Hehme, N., H. Engelmann, W. Kunzel, E. Neumeier, and R. Sanger, 2002,
"Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines",
Med.
Microbiol. Immunol. 191:203 -208.
152. Stephenson, I., K.G. Nicholson R. Gluck, R. Mischler, R.W. Newman, A.M.
Palache, N.Q. Verlander, F. Warburton, J.M. Wood, and M.C. Zambon, 2003,
"Safety
and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99
(H9N2)
vaccine in healthy adults: phase I randomised trial", Lancet 362:1959-1966.
153. Stephenson, I., K.G. Nicholson, J.M. Wood, M.C. Zambon, and J.M .Katz,
2004,
"Confronting the avian influenza threat: vaccine development for a potential
pandemic",
The Lancet Infectious Diseases 4:499-509.
154. Wood, J.M.,and J.S. Robertson, 2004, "From lethal virus to life-saving
vaccine:
developing inactivated vaccines for pandemic influenza", Nat. Rev. Microbiol.
2:842-
847.
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
EO
a~
v ~ o
~~ yl r-i t11 dq Lf1 lfl dl N O M M rl O N N O ri N N di N v N M ;v r-I
m o
.Q o
O U
z v
~
oOD ~ v H (V 00 (1) N O N l0 lfl %0 l- Ln l0 r-I lfl L- ~ N OD N
w N l0 01 r-1 00 01 N N r-I I ~ l~ I- l!1 N O rl U1 N %O O W
4 00 M M ~ r H [- I- ~ m M ~ M
1=1
C
F O r"i O tf1 oD O~ L~ O N 00 01 01 01 m 10 N H U1
O M Ol l0 Lfl O1 O1 m I, N ri Lfl 01 m ko l0 ri
Ol O 00 M ~ M ~ M I ~ kO l(1 [, ~ rl O N m H lfl
~ rl d' N l~ .-1 r 10 ~ 00 [- l0 m H H r lf1 ~ H
O1 N
.~
~
N + 0 [- O m o L!1 M o v o0 lf1 0 N %D N 0) W
r-I N r-1 l- l- O r-I N M 01 Ul [~ DO l.(1 rl Ul d4
CL M l~ M [~ d' 00 ri M Ul Lfl d~ M M 14 d~ N r-I N M 00
f=1 O m rl r-I ~ N H r-I ri
N "
ro ~
N %O H U1 01 ri
0 0) N N [- Nv N Ln l0 ri II1 OD 01 01 0 N lf1 OD tO
N ,n r-I M 01 01 l0 = = M ~N L~ ~ M , ,
~y H * M Lfl M N N QO ~ Ll) l0 C~l N 01 ri M lfl H H H rl M M
U1 tO
~
44 H
O QO 10 r-1 M O) l0 O) M r-i m H 0
O l0 l0 W L- OD kO
~ H M L~ , r1 = M N l~ Ln N 00 O O l11 lll r-I v ~ 00 O m N r d~ l0
O N U1 1- H ~-- O N r-1 lfl O [- aD Ln 00
H O~ a~ ~O ~- r-i 00 m l~ 10 N H m d~ 00 H ~ rl
W F<
0
~
U
N
Oo rl O m [, l- Oo Oo oD Oo Oo Do 00 oD o0 Oo Oo m fn M m m lo m f"1
b a) M 00 r-I l- 14 di m m m m m m M m m m M O1 O1 (YN 0) O1 OD m 01
t3l G
r. 0
ri U) [- H Lf1 Ln N %D m Ln kD tD r r N m 0 N m r ko N lfl L- L- O1 O
O N d~ N V OD 01 01 (A m M OJ l!1 l, Ln Lfl Ln %D v l0 w 00 00 O
.~ Q+ r-I H r-i H N N M W H r-I rl rl rl N
U
~ ~4
;J F:
O ,a
cf) ~ r-1 r-I N N N F:~ a LL a a a LL a a
41
0 2: z z z z z z z z z z z z z z z z z
~ pp o
a ~
~ Q) O 0 0 0 0 0 0 0 N
..a rl rn H Orn H m m H m m m m 01 H m m H m m m H rn m H m A
M
O 4-)
x rx a a a a x vxi x~4 x x x w>4 >q H x x x a x x H a .~
H~>4 >4 xm ~H m m A A 0 H xx 0 w w a a a Hx :3". w
U .^b H H H,+ H fs4 G*+ Z T~ L] ~4 >1 fX4 Ul H Q >-+ P W ~-l f1~' G4 4-+
LL ~-l ~] U U H FC W H a x G4 Z UI P H 44 Ei En ~4 W H
3 0 x x U 3 % C FC H U H a f j . 0 H r . 4 4 . C7 P
~ W W X Cu f3 pG >+ 0 Cu 3 FC A A
~7 z U w[*a w w~n C7 C7 c7 0 u~i x w Z a ~ 0ri) W
n H u c a a a a H C~ a oi w >7 i z~~ 0
~ CH7 ~
A
m
(1)
~
U
W ri
J ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
a~
~ C o
'-A ;j VI M rl d' M W r-I N O N r-I (n M O M (+1 r-1 d4N rl r=I I;r O r=I N r-
I
ro S~ o
O U
z v
O N M N rl OD l~
' 00 0~ l- d~ 01 d~ 10 r-i M M M V- M N 10
I I I I I I
1D lfl Ul OD 00 H r-I 01 M 1- lf1 N N l- r-I rl H O ~ N Lll r=I 01 r=I
%O
~
~
~ H d~ I~ O
OD N
Fi p QO 01 rl 01 r-I Op, Lfl rl 01 O L-
~- 1 I Op I ~ I I O I I I
O d~ I- d4 01 M r-I 01
M O1 O 10
[- 00 m (V kD I M I O I ~O Ln I fV -44 O I H O I I I
O1 N ~ I- 01 rl 01 M ~ Ln O 0) 01 ri 00 rl r- d~
r I r i r=1
=,=i
w
.i
b
(D ~-I O 10 01 N Lfl kD ~O 10 r-i
> :j r-I M DO r-I N O H l- 10 rl 00 01
~ O I ~ LN!1 M ~
,~ O+ M N l- N N H ~ ri ~ N Lfl N O [~ ~ N d~ = M = r1
~I O ~ M N d' H l!1 N ri r-i
~ 41
ro ~ ~ 00
(a p L11 . M M l, M
N V 'i O O '~ M rl ~ N N M ~ M d~ ri 00 ~ ~ O N M ~ %0 [, O~
~ M
H d~ M H ~ M M I~ N r1 d~ Lll r-i N d~ N O N
~ M c~ H O M N N
~ H N
~
44 N I1) O M %O
N 0 O O O 0\ M ~ N l~ I a0 ~ M ~ N M 1 M LIl [- O ri Lll ~ l,
H d' M d~
~ M tf1 M 01 0% M l0 N k0 N U1 d4 aD d~
O . N .~ . O ' 'n ~ Lfl O d~ Lfl O I M lIl r1 I l!1 d~ [\ M
r'~ ~ % 0 kr-I M (V l- N ~ H N H ~-1 H N H
44 r-I
0
~
U
4J C
ro
k0 M O M l0 l0 M 10 M m ri M M N N lfl O M O N N O N O N
00 01 O 01 o0 o0 01 Qo V' 01 l- M M 01 01 l- U1 00 O O~ 01 O 01 O On
N ri r-I r-1 r-I
0) G
f+y 0
,rl U
rci
~ N L- a0 M d4 N O N N (V W l0 r-1 rn W t!1 w QO 01 l- r-I M
~ 0 00 l- O O r-I ri l0 H kD O N L, Ol O O M 0) O O N N v v OD O
,A v W N M W W d~ d~ d~ H ri rl ~-1 r-I r-I r-I r-I r-I r-I N N M
U
~ ~4
O .,~
co a)
a a a a a a a N N N N N 4 ~
1~ '!~ U) U] U) U) U] U] Ul CA (n ~
~ 0 7 'i 'i 'i 'i 'Zi '.7. z'7-z `T-z 'Z+ 7 .7z 'i ', $z. a a a a a a a a a p
N p, p
a p
m (1) O O O O O O O O O O O O O N
a r=i rn r-I rn r-I m rn ~-I rn rn r-I 01 r-I 01 r-1 rn rl r-I Om 01 r-I r-I
ri rn r-I n
M ~
O y~
~' fk fx fx ~ x x f~ x x x x `.ry x =~
' w>+ a fx w fa x x x0 tx U~ x>+ a w>+ x x p FG x~ ~
~i f=+ f11 > a G+ w w a a H i-] ~ 4 ~ ~ ~ ~ U 4-4
x ~ ~ a w c~n a w uQi a a a Hz H a w 4 0 /H a ~ w w W ca H 4-4
~ C7 a H[~ z a a[~+ w`~ fa a rX4 cn H A~-l >1 ~-l w x~9
~ O m w a H fx ~ w U> ~4 A a H a w~ z a A H? C7 u] a A ~
a a a a [i+ fj. C] E-1 44 U a a x ~+ E i ~ a 7+ ,r
~- En m ~ c m> ai a 01 c>7 0+ a 3 C u~ i a~ a a u H i ~ ra w x vi a 01 x a ro
~
.~,
A
N
N
a~
~ ro
U
W ri
J ~
m . =~'
H '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~
o
VI M (N H N .44 H (N lf1 M M O r1 H lzr M M N M rl 114 lzr N N
ro A
0
z
0 0 Ln o) O OD M M O r l0 rl N 0) m 10 m C'
a0 l0 [, OD , N 10 d~ M ~,,~ ~ N 01 rl N O 00 d~ H
O O OD m kO lfl r-I d' O~ L- M = H r-I Ul ~0
01 M M N M U1 N 01 ri l- ri M r-I rl H
14
0
O I M Ln r Ln I O fD o O I I W co I I
O ~ M I r-~ ~ M H N 41 I O r M M M I I O I I
m O~ Ll) M kO L- O l!1 l0 d~
Lf1 00 Lfl L(1
Ol N
"I
b ~1{ H O
~ ?4 O 01 M O
01 N 01 rl I I N 10 O 01 H kD OD
~ '-I L(1 ri N Lfl 10 ~O ~O O1
rl Lf1 N r-I N d~ = I 1 0 d~ t!1 lf1 N = .1
M lfl 10 H Lfl O ri N r-I [- OD
lfl rl rl r~ rl ko ~0 r U1 [- ul N H Ln
~ o
ro ~ ~
b o o ao ao in
N N r-I 10 ~ 0 lfl Lf1 H 01 00 Oo O d~ 0~1 O ~ tN+1 r-I ~ Ul 00 ~ rl 0D ~
U H d~ N M rl rl 1I1 00 N H ~"1 O m [~ N l, N r,
~++ H * m r-I po N H H M m r-I M N Lfl M
~
44 N
O OD 10 N 10 00 00 O [- " [- r kD [- N l-
M 0 O M r-I 01 m 01 0 N , Lf) , 01 ri [,
H O 01 00 lf1 lfl N N Lfl t- r-I d' N 10 H rl r-1
* d~ H r-1 L, H H ri N ~O 40 40 H lf1
0
~
(~ U
4.)
ro
(O y.-. O O
N N N N N N m N N M OD O 0 0 0 0 0 0 O 0 0 0 0
ra dP ~ ~ m o-i O O ~ ~ o) m Ln O 0 0 0 0 0 0 0 0 0 0 0
W`-' r1 r-I r-I ri rl r-I r-I r-I r-I r-I r-I rl r-1 r-I
d) C
ci 0
,ri U
0 N N M 01 O l~ O~ 10 ~-1 L~ L11 d~ O~ 01 ~O l~ N 01 O~ r-I l~ 00 00 01 r~ ~-1
rl t!1 l~ 0~ 01 01 U1 O O M ~O l~ l~ N N d' 01 a1 N tf1 r-I M lf1 l~ l~
0
A U a M M . . . . U1 W W k0 k0 \0 k0 H r'I rl H H N N r'1 M It
U
~ ~4
:J
~y U~ r-I rl .-i r-I ri r-I r-I ri r-i r-I r-I r-I
1.) y~ ~ ~ ~ ~ ~ W PO P~1 P~1 W Pq LQ W W W PQ PO ~
0 a a a a a a a a a a a a a a a a a a a a a a a a
N p, 0
c
O O O O O 0 0 0 0 O O O O
i-7 r-I rl .-i m r1 r~ m rn rn m r-~ rn rn ri rn r-I m ri rn rn rl rl H m r-I
n
cr1 ~
O 4-J
~ x x x a a x a a~ ~ ~
~ a x u~ x H~~+ x x x ~H ~H z x w x x x Hxzx ~x+ x =~
W H ~n H x a H z~C a a a z a 0 0 H w H M w cn cn W
~ x~~ H k+ [~+ w a a 0 0 E-1 a w H H~ U~ w a 4-4
3 a2: 0 HE w a> w 0 3 F H Ea E4 E-i H Ea 2: ro
P: 0 [-4 H >4 LT+ a fX4 E4 H a a C7 3: E-, a' a Zi U) H H H H
H G4 a Ea a > > Ei H H a E+ a H a
n P vi C7 c7 rx a~ 44 ~n H~~
a A w x z a
~
= N
4)
4.)
~ ro
U
w =.~
J Cr.
m =`~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
v
~
o
r-i ::5 yi di d' N rl M r-1 i-I di N N N -w H N N r-I dq O rl U1 W lf1 m lf1 H
(o A
p U
z
~ o Ln
p O Ln l!1 (n ON H O L- OD 00 M I 00 (1) ~
a0 O 0) CD 01 l- d4 10 lD L- N 0 01 0 %0 O
lo O = ~ O N O ~ l0 O 01 [- Qo rl 0 O l0 M rl N M ~ ri .
* H O rl '~ rl rl N rl r i-I ~ L!1 ~ O1
~
p H l- O l, Ol O U1 N r-1 10 'n m 10
rl h N OD 00 U1 O O
O M r-1 M M L(1 ~ U1 co N
~{ .'~'. t+l d~ ~ m 00 r-1 ~ \p ~ N ~ l0 M M [-
Cl N Ul Oo N 00 '44 M H N %0 Ol Ol
.~
44
1 r-I
> ~$ H N 0 r-I O ON 41 M H l, lIl H l- N ~ H ~ r-I r-I w M 00 l- m m
C1~ M ~ rl 10 O r1 ri r1 rl N ' rl rl O ri ri ri = lf1
'rI N [~ H l~ H ~ M r-I M M ~0 [~ rl M ~ O L!1
1-I 0
H
ro o p ri M O O l- O 00 H lfl r-I Lfl [- 00
N N ri N rl rI O rl = O OD 01 rl Oo l0 O rl L(1 . lll . . . p
H N rl d~ r-I OD 0 ;v N lfl m 0 rl
~'i H .~ r=1 N M H 10 M N N M r.{ d4 a0 l0
44 ,.~ 00 lfl r- 0
l~
00 p O O It ~ l0 O1% OD l- 01 m 00 H 0 m
CN H M 10 00 N ~ O 0~ rl N M N 00 O~ d' lll m [- r-I O d~ H M lfl
0 ri H M 01 01 0I l, 00 [, H N M I,
ko -14 ko H H m ~-I M N Q, d~ ~O N
44 r-I r-I ri ri
0
~
0 0 O 0 0 0 0 0 0 M l, 0 0 0 0 0 [~ M m r- kO w m m m
aW 0 0 0 0 0 0 0 0 0 M ~ 0 0 0 0 0
m a%
N`-' r-1 ri ri r-1 r-I H r-I r-I r-I ri r-1 ~-=I r-I ri
0
~ U
0 N QO 01 d' [~ 40 OD [- H rl 01 01 m m M d~ d~ N N p1 tf1 [- 00 c+1 00 H
.rl 0 OD 00 d~ 'IV W l0 N w l0 00 00 m 01 0 0 0 H N 00
r~ v a+ d' d4 Ul Ul Lfl Lfl 10' 10 ko 10 k0 10 10 N N N 10 L~ O~ H H H H H H
U
~ ~4
O
M
v r-I ri r-I r-I r-I r-I r=I r-I r-I rl r-I rl r-I N N N N N N N N N N N N
4J 11 pq pq pq pq pq PA CO PO W W PO GO W Ln PO PO W G0 f11 LQ W W 00 PO PO ~
0 a a a a a a a a a w a a a a a a a a a a a a p
N p~', 0
a p
0 0 0 0 O 0 0 0 0 0 0 0 C y
i-7 rl rl ri H m r-I rn rn rl rl rt rn rl rt rn H 01 01 01 01 r-I Orn m Om 01
A
M ~
O y
~ x x a x x x a x x x '~
w>, H~ a x x~ w w a a H x x a x x x x a x ~
1-~ U U) Ga ~4 44 UO H H ~q ~-7 M UI H H a 3: >+ G+ ~
H a 3 a
F cn 0 H x v) > 3 3 F Z cn ~n 9
fj4 F~-l f=+ fx P4 M M U U cn M pC ~~~ F F~~ a F a H
w w a a H z H H x w w~ xM a uFi w a ~
~ m -4 H 9 ~ a H a x H a w a H in ~ a
. .,
A
m
a)
a~
U
w H '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
'""i :j VI N l!1 lfl Lfl d4 N rl H M N W N M M w rl M M H
ro 0
.~ o
0 U
z
,..4
0 00 N O M 00 O 0 ~ ' l~ N Oo Ul m ~O M Lf1
~O ~ kO 00 ~ 10 ' N N N N M m H d4 0D
* OD r-I LIl r-I l0
~
C O
OF -I M l- l11 H H OD M U1 01 l~ 0 : : O ' ' N L (1 ~ l0 00 r-I M l- Ol l0 W r-
I O I
01 N 0) U) d d4 N rl N cq
~y =-1
-ri H Ln
~ ~ OD ~ M N O W ~ O O O ~ ON Lfl N 0
C~ M rl ~' ri r1 OD H M l1l d, \O M N N 00 k0
O U1 ri ri ri 0)
~ 41
~ o ~ N l~ ~ H M lfl ~ r-I ~ 00 H l- r ~ O ~ 0 ~-~j l0 lfl W N O ~ m ~ OD ~ N
H r U) ~ H ~
H
.~
Lf1 0
w O k0 01 O M p% W l0 H Lfl H '-I oo Ln Ul
M lzM w N Ul DO Lfl kD tO lfl N N O)
H 0 M L- 0~ (+1 M 01 N 10 I- M lfl r-I r-I .
~ Lf1 N r-1 m 0) ri d4 r-I 40 N Do o) N
44 rl rl
0
~
(Q U
41 P:
O O 0 0 0 O 0 0 0 M M kO V O O O 10 10
S1 oW m 0 0 0 0 0 0 0 0 0 M M M W M M O O co
N H H H H H H H H r-I r-I
G
(~+ 0
~ U
W rl N M M d4 d~ N ("1 N d~ U1 l11 dD 0~ N Lfl O 01 [-
O OD N N N N d~ l- O m m m l0 O 0 H lf1 O1 0 N
.A a rl M M M M M M d~ V' d~ d~ ul ~ lO \0 ~0 ~O [~ [~
U
~ ~4
:j r.
0 ,i
U) v N N N N N N N N N N N N N N N N N N N
+-) 41 m oo m oo m ao ao w m oo ao 00 0o ro o0 0o w m w ~
~ 0 a a a a a a a a a a a a a a w a a a
N p~4
w ~
m
O O 0 O 0 0 O O O O O O O N
ri r-I m rl 01 rl 01 r-i Om H 01 ri .-i H rl Orn H H H A
4J
DL4 x fx x x oG x x x x x x x ='~
' DG ~ a Ri H x a a H H a ~.~~1
a O W H w w P fx X a w a H H a H a a w
O x w H H w a U w a w a a a H z 4-+
E-4 C7 w w P a A W. A a' 2: E4 > a co
O H0 0 CD w z0 W Lx H A H H E+ 44 4
w 0 0 0 0 a a a M H a A A 0 w A 01
u] w w 0 Ea a u] a M A w w a z 0
~
~w a~~M F14 H w a a H E+ a w H a ~
.,
.~
~
v
4J
U
w J c
m =~'
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
v
o
~--~ :j yi r-I L!1 4~ ll1 ll1 W M M N N N 14 d4 N M d4 M d4 M t N N M fn
r0 O
A o
0 V
z v
~
0 Q1 d4 H N a0 M N %D lfl %0 ri l- m d~ m m ~O H M
w N 10 m H QO 01 N H l- OD ri Lfl 10 ~ ~ l!1 OD O ri m U) [-
OD M M l- M M r-I N
14
r{ rl
0 O H O Lfl OD 01 N 00 0) 01 r m 10 N rl O rl OD ri I I I I
M 01 10 01 01 N Ul M 10 10 01
M 01 O It QO M = M l0 ~ l- m O N M r-I r 00 O~ N 1 1 1 1
~ rl N l0 ~0 O l~ m H r-I l~ L!1 l0 r r~ 01 M
Ol dp ~ /C r-~
O -rl
ro M W
~ Al ~4 O r oo Ln (A d~ 1 N
> :3 =~ I~ l~ ~ l0 M 01 L(1 N QO lfl r-I M m r-1 N O l-
r~ ~
=~ Q) 0' N O M 0 U) M m [~ = = = = = L11 t11 Q1 M M d~ ri N N N lf1
14 y d~ M [- d, d~ 00 H H H N M N r-I t11
G) 1+ O
ro ~
0 c O O~ d~ N N N l- H L!1 O L(1 dD l0 10 Lf1 Ol [, [- Lfl ri N m m OD
ri = = i- =
H * M lll M N ON 0, ri lf1 r- M r-1 m = O m m H m [- N Lfl
m N DO ~0 M 11 r-1 l0 l!1 N H 44 ,-I N lfl
C) ry O O O w 10 ~v [, l0 Ol ~'~1 r-I r-I N O O~ M l- pp N
M O M (, 0o lf1 00 m N U1 01 01 l0 N
~ H O 0 N r-I = M N N [~ N ~ lfl l- OD r Ln . . N O tfl ~ l!1
m N
~õ~ 41 OD ~0 rl H (+1 r~ Lf1 r-I N l~ N 10 H
Vl ~= r-I rl
44 0
ro ~
4-) ro
(d ~ 00 rl 0 m t, [- OD 00 OD 00 m m m m ~D m %0 0 M 10 m m H N
`. fn a0 H I- d4 v m m M m 01 01 O% 01 oD 01 00 m 00 m 144 [- 01
Li 0
0
U
b O
(y M
.~ 0 UI l, r-I Ln lf1 lf) W W 10 M M ~ kD lf1 [- l- (A d' [- 00 m N N N d'
# H N l~ N 1*4 0) m lfl Lfl Ln w l0 %O 00 dD 03 0 0 r-I l0 ko N O
=A A~' U m r-I N d4 rl H H H N d' d' d' d~ rl H
~4
O ~J Aco z z z ~
o
a b 0
o
11 ~ O rn O rn O rn m rn O O m m O m m 0 0 0 ' 0 % m O ~N a a H N
o >.
~, a~ x a a x~+ a x a a a x c
~ U a a a a x x w H a a a a a H w a a w x x a x ~
N ~H ~H a U] U] H 0 w w a a H 2: 44 > (Di G4 w a H
x H H H ~+ Lc4 LL x U) H Q E-~ W a a Q ~ > aL Q H H UI W
Fj a a ] U U H W H G Ei Ei f=, vn , 7 w H,+ 44 U) ,a u2 a w
a v U 3, 0 x x U4 4 H c~ H~ c~ H a c~ H w z a w~ w
w wE w w x w 3 x U cn cn a cn w~n a H w> Q a o,
v ~ H cn a a w H H v~ a a a H ~ a> > a H H rl
~ E-~ ~ rC a a ~x 4 H H r~ ~ H ~ Ul ~ a~ U .'~ rC ~ ~
.~,
A
a)
u
N ro
U
W rl
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
a~
v d o
O yi M w N C' N m N N d, v N U1 M M v (n w M N M 44 v N N
A o
0
z
~
0 00 r O i 0) O 00 M OD 0) M ~t'
00 M M %O a0 %O d~ M r-i N d4 OD 144 H
kO r-1 r-1 l!1 H O ~ M m N Lf1 H d~ N r r-i d' M M ~ ri rl L11 10
M M Lf) r-1 ri rl
14
~ r O r
p 0 O r O N , lf1 r ko Ln ao 0 00 i i
~..~ M 01 M O) 01 d4 m r-I N O1 r M O r
M ~ 00 . 0 ~ ~ 01 Lf) m 10 r 0 L(1 ~ ~ I kD ~, I I
O1 l11 a0 lf) rl 01 lfl
dp O r-I r-I H
O =r=I
P'1 W
N ^l -~ ~
O ~O 1D 01 O O~ O r ~ r r-I l0 OD
> 'd N
-1 l0 O M Lfl r-I lfl 01 H N ~ rl rl 10
r ~
~, lp %p 1p p~
yõ1 G) 0' N ~"~ r' Lf1 ~ ~ rl rl ~ '~ rl ~0 r N H ~ N N H
t!1
o N K4
N U on ~ O N %D %O ~ Lfl Lf1 ~ d~ 00 OD O O N r-i N Ln '~ H ap N
~ r-i N N ~ V~ M m r-i ~ W df H N M M m r M N ~ N r M
c0
r--1
44
~
M H
~ 0 ~ M lfl d~ 0 00 10 ~ 00 O r 01 O N lf1 N ri r r V~
0 0 O M lf1 Ln r m qw 00 N lf1 N r-i m
r '-1 d' N r-1 r-i r1
w N r-1 N N W r-i r-1 .1 ri (N M 00 OD l(1 0 \14
fO U
4.) ro
o O o O O o O o O O o 0
O M N N N N N M N N
d N 01 Lf) 00 01 O O O 01 00 01 01 0 0 0 0 0 0 0 0 0 0
V' 01 01 H H '-I r=I r-I r=I r1 r-I r-I r-I r-I r-I
m
Ci 0 O
.~ U
b O
(y M
O W 10 d, 00 H N M O r 01 H r tf1 d~ r N O1 01 H r OD O~ H H
0 0 m O N m rl ln OD 01 01 O O M w N 1w 01 01 N m m Lf1 r r
N a r-I rl ~-i N M M c;r qw W w 10 10 w H H H H N N M d~ d' d~
11
0 r~i
~ A ~ ; ~ a a 4 ~C 4 ~C 4 4 4 4 4 4 4 H H H .~ ,~
~ ~
a a a a a a a a a a a
~ o z a a a a a w a a w
=.~ c
4.) o o 0 0 0 rn 0 0 i 0 0 rn rn m 0 m 0 m 0 m 0) 0 0 0
0
N
4)
ch p, ^
~. v x x x x >4 x x rx a a x x a x ~
~ O ~+ w >+ x a x x H C7 x x ,y~ R: 4+ x x x 'Zi >+ x x ~
r1 ~ > A W ~ H H Z Z > 0 0 E-~ w u] f~ v] C~ =~
~-7 c4 O ~ 0 Y ~ x H fs, HE W,a a a W H HX U Ga f1; 44
~ m H q ax 0 a~H w xW w P H S a a Z can H~~~
w czi a~H a w a a ~ u a A a a H ~0~~ ~
v I x~ a~ 1 z m~~ i ~
a
.~
~
a,
N ro
W ~
J 'd
m =~
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
v
n o
=~ 0 yi -w -,v N m w N N N w N N v lfl w l11 m Lfl L(1 lfl Ul w N M w
0
A o
0
z
~ 0
0 a0 0 ~ O ~ 01 L- 144 l0 10 I, N O d~ 0 10 Oo M O M L(1 O i l!1
0 10 O 01 l~ QO rl O l0 M ri N ~ p` ~ d~ r-1 O ' N
m H N r-i rl
r-I
p 0 r-i m l- ko, oN O l11 N i ~ OD ~ M N l.f) O
~.=~ M r-I M ~ ri I- N O OD N N d' tf1 0 lfl H H OD M 0 a%
x * lw H d~ ao r-i H V' N O ~0 M M OD r-i M L- 01 00 N
lfl 00 m H 10 01 Lfl v
~ a1p ~ r-I
O ='=I
M W
Q) A] '~4 ri
> ro N 0 01 r-1 l, lfl r-I l, l- ~ H H 10 M OD l~ (3) f"1 N 0 O OD
= ~
* d4 l~ H = rl M rril r M l N M 1D
=~ ~ O N ~ r-I ~ rl rl r-I O H ~--1 r-I OD ~ v lp
1)
0 0 r-I M O l~ [- O OD Ul H L(1 [~ l~ ~ r-i m ln
N U n rl N ~-1 N 00 10 rl lf1 . . . . tO 00 rl
H * d' N 144 O 00 01 O V' N M r-i O ON M C10
N H M N N M H d' W l0 l0 L(1 di N
44 ~ ro
r
~
N
o OD r Ol QO M r-I
M H O rl %O l~ O OD O r-I N m N 00 d4 m H 0 d1 r-1 m IV 0) 0 %0 N 00 %O
~-- O O r.{ I r-1 M 01 m 01 . 00 =~ [- rl N M I- 0) m N [,
w U1 w rl m i-i N N r-1 M H
0 vi
rl ~
C
rq
=k o 0 0 0 0 0 M r o 0 o o 0 0 0 0 0
'd rC ~i aa o 0 0 0 0 0 0 0 o 0 0 0 0 0 0
~ L(1 OD 00 01 01 ~--I ri r-I r-I '-1 H ~
r-1 r-i r-i r-i r-i r-i M r-i ri r-i
N
Li 0 O
0 OJ 00 01 v OD H r-i 01 m N Lf1 l, Oo m O N M M -o d4 N Lfl
0 Oo OD d~ d~ 10 10 00 OD O~ N N ~ O O O H N N N N N d~ M O~
'A p' L(1 Lfl l0 w 1D w l0 H H H H H M M M M f~1 V~ -w
(D 'd ~=I 13 z
ri .I
,f] N H H H H H H H H H N N N N N N N N N N N N N N N
+1 W Oq fll CO GQ GU LQ W PO P0 Lq L0 CQ W PO n1 PO L10 Pq !A pq pq pq pq ~
0 a a a a a a a a a a a a a a a a a a a a a a a a
N N o
4.) z o 0 0 0 0 0 0 0 0 0
O) H 01 O~ O% H 01 01 ON O~ H 0) H 0) 01 N
m ~ a H r-I r-I ~~ ri H H
w A
p
a) >+ a x ~ x x x x x ~ x ~
r-i U Cz. ,+ H a x w w a H x a x x a L=+ x p: E~ a x a a ~
~ w r~ w~ a a w w >+ H H w a w r~ E Gv Ga Y, a
1 a ro v Ul 44 a A H H ~4 a (A W } G+ Lv E E-1 C=. a Lt+ w ,W
`.4 3 3 E' z U] H >+ C7 Ga G4 E+ a x R:
w F~ H tp
H ~n cn u U ~n x ~+ a a 0 0 0 w z a ca
v [rX4-4 fW a Oa p-~ H O+ a f~ ~-~+ H E+ H[~z m C~1) C0*.~ F' Ol ~ b
E-1 F< a~C >1 >1 x p F a EA u] C7 v) w 44 U)
A
N
N
J-1
N b
u
W
J 'd
m -=~~
F- ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
a~
o
q
:~Q yi N ~+1 d' ~'~1 ~'~1
A o
0 U
z
~
CO N U1 0) ~ N (~1 ~ 10 M
r l
m rI
11
4)
,--~ O
03
N
ON dP r-i
o -~
b M w
~ =I Ln
~
O N O1 0
r1 a~ lfl 01
a M . N O a0 l0
14
(L) U
0 0
N.' ,-.i r m w oko
ri to
44 ro
M
ul
0 ~
M OD u1
H 0 rn w in N
0 0 Ul r=1 pN N
w
Ln
0 vl
rl
~ O U
~
4.)
ri ro
'd da r~ w o 0 oN w Ln m
ul
0 O
=~{ U
ro o
m Ln ao ao O rn
A k 0 %D 0 H m 0
a a~ a
a~ b
0
A ~ ~ ~ PNO PO N[Q oq
m 0 a a a a a
N N
L~ 't7 0' o
N ~- o O o o O 04 H H rl r-1 rl O
41
M a A
O
u xz r=i aCD f< G
ro ~ a a a H z =~
w
[ [ r
91 2: - > - ay a ri) 4-4
m
co A Q a 0 z
v a F-I a uHi v
G
.~,
A
N
4)
N
W u
J 'd
m ~
Fa-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
W
a)
r1
~ ~ O
r-I ;j yl L!1 d LI1 d~ Ifl M N N d d d N M M M d d~ Ul d d d Lfl
N 0
A o
0
z
~
W H N M OD l0 Ul r-I [- O I rl M M M O W ~ d' O ~O
~O M m H ON 00 1-1 ~ Lfl l0 ~ N [, r-I ri M H r~ N
~=1
r~ H rI
O ~7 M O lf1 %D 01 OD ~ N ~ [~ M rl ri O d4 N l, 0 r-I W
= M ~ Ol ri N O
x M O ~D N w ri O 10 01 r- U1 r- Lf1 w d~ l0
[, rl QO lfl Lfl
H
O W
M H
Al rl
~ ~4 0 O M O M L.fl N w 0) do Lfl l~ N N Ol 10 r~ N
> f3+ M = = = = = ~ Ol m ~ N l0 H ~ ~ Lf1 rl ~ d~ N d~
M Lll M r-I ll N N
= M [~ QO 0 H Ol N
14 0
ro ~
O 0 N d' vl t0 m N rl rl r~
N H N N [- O Lf1 rõ~ l0 , M M OD O t0 tfl OD O .
Li H H lfl m N 00 N m H Lfl H H ~ [, N Lfl H N rl d4 m Lfl
ro
w ~
N 0
H m O l0 W l- d~ 00 U1 Oo M d' O N N Lf1 d' m O m rl kO
0 r- 0 I N M l- N 1- Lf1 [- Lf1 N lf1 ~ 00 Lf) Lll N l, r-I rl H
O O) 00 10 rl r--1 r-I M N kD rl
w Vi
0
rl
~ r~ C
~ r=I ~
" rl O M l, l- aD Qo OD m M m m rl N N N 0 M 0 0 0 p 0o O C- d' d' M M M 01
01 O) l~ 0N 01 Ol O O O O O O
r-~1 = ri r-I r-I r-I
0 r:
ri L". U
'd o N
O N rl Ln l0 Lf1 1D M M U1 01 N N d~ l0 00 l- [~ I~ QO 00 40
=~ .k w 0 N 0) t11 l.fl kD kO QO w N O O N 01 O N M aD O
,~ ~= a aJ a r-I N d~ r-i r-I H H H H w H m lzv ri
U
~4
~~1 q N 0 ~
a ~
r-1 r-I N N Na a am m cn m ~~~ uq al aq m ~
y~ ~ o ~ ~ x z z z z z a a a
z z z z a a a a
LL d H p 0
=,1 4.) ~ a v 0 0 O O o o 0 O O O O O (.~
a rn~ m rn~ rn~~ rn~~ m~+ rn~~~ rn m~~ rn A
O M 41
o x a >4 x x x a x x x~ .''
~ a.) rx a a rx x W H a a H x a >+ H x z w x C:
H /' >1 1% >4 m H 0 W a2: H a !-~ H z v) w w
1-~ (0 'd ~ x H H >i H G4 x (!] Q [-1 Ri H H UO G4 W a L4 U) 4-1
V a a U H U H f=+ E+ 44 U1 H R W i 7 W U 44 F P ~4 ~tl
ri 11 r 3 C7 x U x a H U a 0 pq W fs~ W `-l J+ 4~ 3 H 44 a
^ .i Ei & X U G4 N N C7 >+ N t-l X W H X [U-HI N >+ z A 4 G4 q
a) . R a O~ H H O~ H E-~ E4 E+ H[~ b
U) q Ri H U1
~- a x~C H x 2: o+ En 0 H gi
. .r.,
A
m
a)
41
ro
U
W -.i
J ro
m =''
F- '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N
~
rl
O
ri :j vi lfl L(1
t6 0
A o
0
z
~
0
0o M O
kD ~D
m K4
$4
r-I
O m o u1
m m 00
rn aro,
.~
0o W
M
'14 H
y nl s4 p w
'y CL ri r H
.~4 O O
b O
p q 1
O o r r
N U "
kD 1~
N
ro
w
CV)
H o M M w
0 o M r
-' Ln
4
0 vI
43 ri a *
'd FC ~ aa o
~ O
f+ U
ro o ;
43 O do N
v a rNi
a
U ri a
4J A m o w w
N N 'Od p~' o
0
-.l aJ o
0 N
a) a) m r-i n
a >,
o M õ
=~
o
v rx
a o `` w W
FZ4 O
v la ~ =rl
b
C/
Fi
m
41
41
U
W -=~
W C
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v
~j V I N O M N N N M r1 N N N N O O M O N O N H rl 0 N M N
N O
A o
O U
m z
N 0 N dD
Fi O H 01 Lfl N ~ I I I rl 01 14 O% ~ rl a0 ~ ~ U)
~ 0 l0 r-I O H ~ m w i~ i i
r-1 ~ 0 O ~ M d4 M 01 d' 01
N ~ N ri ~ M rl H d' ~ N
O1
N r N
~ (1) 0 rl m -~ ~ o ~ L!1 ~ m ~ ~ ' M 10 0
~ M
> =ri N i i 10 l- i i ~ 01 ~ i r ~ i N . m O~ = i
d' N H N O H 00 [, ar1 ~ N
~4
0 N ~
4.1 O M I Q1 lIl lfl L!1 01 Lfl 01 l0 r-I O H OD lll 00 Ln M OD ~ l- M [,
N N d~ = N r=I = 01 00 rl N N ~ O 01 O = N d4 ~ I [~ N ~ ~0
N M l0 H d~ l0 l, V~ H N ~ N lIl l.fl H M H Lf1 N N
ri
~ U
H H 00
W kO i M 01 00 V' O O d~ M M l- 0) 0 M O 01 w l0 aD m
0 L!1 ~ = N N ri = Ul lfl N M = (3) rl O M , l~ 00 = l~
r-i H .K ri Lfl H H M Ul H H M O O 01 00 M N N H14 rl N lfl H 01 d'
(C) w r-i
M 0
c- ~
(d ~
j
1-) (13
3-1 oW O O O l- l- 00 0o 40 OD OD d, M M O m m m m 0 tD rl ~0 0 N
'b N`-' ri r=I W M M m M m U1 01 d' lf) 01 01 01 01 dD l- 00 d~
N
ty) r.
Li 0
-ri
N ~O -V~ d~ l0 ~0 ~O Lfl 00 l- l!1 O r-I 00 IIl N
~ o d' 01 O [~ H H m 0 d' 10 ~D O~ ~D 1D m H Lf1 01 m r-I 00 00 " l,
A U a d' 01 VW v lfl H H m Nr m 01 01 H N N N M d~ d~ ri r-I
U
O
U r-I rl N N N a a a a a a a a LL a N N
4-) o~~~~~ z z z z z z z z z z z z z z z z
14 z z z
~,
y a
a
~ v O O O 0 0 0
a rn 01 a1 01 01 01 Orn Orn H H r-I 01 01 01 01 01 01 H Om H 01 Om Q1 rn rn O
0
0
N
H G4 Ga ~ 7 H a 4 'J L4 a ~-l H L4 LL LL ~+ 3 H 44 4 n
a) LL =~=i
1 a W f~' 'N O. ~ OI H H H 0 0 CA '=i FG A a w
~ 0 ~r x 7 ~ x w z ~n u A x u cn v~ ~~ CL u '3 a ~-7 a EH u
~ H ca H w a a w z0 0 ~p a~~ x a z H H i
a U x H H U) cn A A a x x H rx r) > ca ~ w~ r~ W
^ ~+ a a H X a > a > H a a H fx a E W a u) m 0 a w a r~ `N
3 fX4 ~H G*a G. 3 3 3 3 7+ ~H y+ Z Cra >4 G4 44 [*a Cs4 2: fs+ 0
Cn W ~l 4 44 H > [X4 W ul (1] LV H H H U' S E-+ C24 u] ."!. 4~ D4 0)
~
G
=~
A
N
u)
a~
U
ri
w
J ~
'i
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
~
~
O'bo
'"i :1 VI m N ri O N N H N N r-i N N N M N N N M N O N O N N O
(a A
O
z
Q) N r-i 10 l-
0 0 %O I 1 O N ~ co Oo i r-i r
O Lfl 1 I ~ ~ I I I ~ I O r M m I O ~ ~ I I
~=-i * [~ N ~ 00 M ~ N ri
ts ~ rl rl. r-I
O1
N Ol m lll
o O aD r-I ri r-i 00 00 11 N
N U) N r
=~i N ~ ~ i ~ d , U1 k r r+ i ~ H
N 0
'rI .,wi * C~1 rl lfl O O L(1 O rl r-i H
$=I 1=1 r-I r-I ri
G) ~
a
~
ro 0 O 10 O 10 rl tf1 H ll1 01 p U1 ~
lf1 N O ~ r~ OD ~ 0 l~ w , O , l- l0 M OD U1 O O rl
~ C * d4 M M l0 M ~ Ol r-I w ~0 M M M N r-i 00 N N ~ M M r-1 O
~ U
H '..~
OD M O N 0 O l, d~ rl 00 O r-4 l- H N m l!1 N l0 m ~ w I O O% I
F..~ * N M ~ ~ N M l0 01 10 ~ r-I H r-i N r1 N ~-I 0N ~ N ~ N l0 I
~
M 44
0
ro
4-) ro
(d 0 N N N N ~ ~ l- N N N ~ N N ~ ~ l- M p m M m 0 m ~0
. ro O`. H O% d4 d4 01 H H ~0 O) O1 01 H 01 01 H l0 M 01 Oh 01 O% 00
N
Li u
.rl
Li cA Ln O f'7 ,v [- I- 01 r-i Ol Oo lD [- O N l0 Oo 00 OD O O L- H r-i I-
=r~ 0 m r-i r-i m lf1 r-I H n4 O% H r-i M 00 m ON Oo oo r-i H m [- 0% L!1
A O a H M M M T Ul l0 10 l0 N M di di W Lf1 l0 l0 r-i rl qN U1 lf1 l0
u
O
C1l a) rl ri r-i r-I ri ri r-I .-1 N N N N N N N
~ ~ a a a 00 m m 0 p 0 P P
~ a a a a a a a a a a a a a a a a a a a a a
Q)
a) 0 0 0 0 O O 0
a rn rn ri rn rn m m m ri rn m m m m ri m rn rn rn rn r-1 m ri ri m 0
N 0
N
44 3 ~-3: 4 H Lra z~ H Cra G4 H >+ >i a a 1x7 ^
4 4 H z H C'0
a v w x w a r~ w w ap H~ cE7 ~ a z H a w w w~ x
u ca rx w a c~ w cn a z H H N rJ4 u u w
cn fx >E x a 0 LY. u u fJ4
a a 2: (1) H -~
z u) H x >I H ~4 ~4 E E bG 0 4+
a rZ4 u x H> w a ap H x (Y a a a a u) 4-4
(1) w ~H w w >+ w w ~H w >+ >4 >-+ >+ >+ x ~H >4 w >4 ro
U) H w w z w a a v) a CO w 4 :3: x x x a a z 01
a
.r,
~
N
N
L
'a ro
O
W "'
J q
m =~'
H '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v
~ ~ o
r-I :j yI N m N N M N N N N M N N N c`1 N M N N N (N N N N
ro A
0
z V
N O N
O 14 r-i 'n N I I I r-i .41 Ch I I Ln 1p I I I I I
O w r-i r-i N I I I M w w I I kD lfl I I I I I I I
M r-i . O ~ M M 01 0%
N m H ~ ri H N
W N N ON m
U) 0 I r-i I O I 114 l11 `-i CC) m M r M I I H H H
~ rq N I ~ lp I l H ~ - ~ ~ r ~ Ol N O = I ~ I ~ ~
W
H 0o I- ~0 N lfl O O
1"I r-I r-I r-I
¾+
O1 0 N
11 0 m m Ul lf1 T 01 l0 H 0~ 00 m m l- 10 ll) r-i L(1
='d N 'd~ = d~ r-i = OJ r-i N N Oll = d' N rl ko m l0 O %O = O =
~ N m rl kO rN H N N lfl m N N H M M M
>
r=~ uo
U
~ H H
'ci O 10 lf1 00 O lw M 01 m l0 00 01
1 dp M ~ = N H = ~ N M = = L~ ~ = r 00 M I~ rl 00 rl [~ rl
~ N M 0o d~ N M M 01 l0 ~-1 H rl
r-i L11 r-i M l!1 ri 40 M N H r-1 01 d4
M N nl
44 4) ro
O O [- OD 00 QO aD d4 M M M O O N O O N O L- N N O N
H m ~.1 oW O op d~ m M M m Ul O) 01 01 O O O O% ~ l0 ON O% 01
O r.
44 V 0
0 v
t'+
W l0 d~ k0 %O l0 U1 l- O tIl N O l- H 01 ~O I- O
~ U d~ O r-1 H t+1 44 %0 10 ~ m U1 m d4 l- ~ m l!1 ri d~ H H M
d~ lll t-I H d' d' V~ M r-i N M H H M r-1 " l0 l0 N M I;v
b O ~4
y~ =ri
~ o
ro r-i N N~c ~c a a~c a a a a N N rC 9 rC 1:C 9 4
o o x z z z z z z z z z z z a a a a a a a a a
N a
'A a rn m rn rn rn0 0 0 rn m m m rn m rn rn rn rn rn r i rn rn m o
o
.i
y.) .4 N
}4 G4 CX+ Ga G+ A
a Lt+ a H a ,7 a Zi a a H 44 H 44 4 w H $ U' 4 H $ ~
a ,0 H Lu U' Cra H H H W',7 a a]C pC x H W ?4 ~ a ~ H zz H m ~ a a H w ~ w a
H~ ~
~ a a a a x w 0 t!~ FC U pC U) u) fx a U A~ C7 ~ a H E+ q
a ro E~ Ca fj+ a a z (7 C7 X 3 x H H u) x a H
M x0 a H E-+ m QQ a U w w Z U] F i X W
V a a H f~ > a E a a H w a X a H W rC a ~7 E+
N 3 3 ~ [~.~ [*.~ 3 3 3 >+ [r-i G.i G4 f~ >+ >+ Gv >+ [~.~ ?1 ro
w a H a w3: m cn w x H x rX4 x U~H w w a U)
a
.H
a
A
N
iJ
~ ro
U
W =,
J q
F- '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U) v
~ rl o
r-I ~ ~I M N N N M N N N N
` . Q o
0
z ~
N
O O N O O r w
O O r O M
N d W M N rl
H m N Lfl
$4 O co N r N
C =rl N ~ . I ~ ~- H ~
4-4
FFii
N O ~ r=~ r-1
0 y4
r=1
,,8 a
a1 0 N
O
I- %O M tD ~d4 tf1 Ln O
~
~ * N ~-I 00 N N M M r-I
}4 U
(D H
r(i ~ OM N M U) N kD ~ %O 0 ~
~ O N ~ rl N H 01 r-1 O N 10
M N Al .d
r'I O
w ro
N 0 O [~ M O m O M
H ~ ~1 oW m 0 0 10 c.,l 0 O~ 0
01
O
44 0
0 b ~
~
UI N ~O 00 OO 00 ~ 0 H rl
0 ao m 01 aD aD ri l- m
tO ~ v a w v~ in ~o ~ d ~-+ in in
'd o u
~4
o
vI O 0 r
~ r-1 rl i-I rl rl N N N N
=rl N
b 0 a w w w a w a a LL
W
N
a) m rn rn rn rn o O o ~ r-i
0
N ~
0
o
y-) A ro
14 W W W W
Pr4 N ~ 4 u u w ~
m a U) W
N >-, J U f~ 4 ~ ~. ~. 3 . ~ Oa ro
a
.,~
b
~
~
A
Ul
4)
41
U) ro
U
W rl
m ='~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
a~
~
r-i :j Vl N rn N M N N N m N N M M N N N M N N N N
cd O
O V
Z
C N O N ~"~ ~0 [~
Fi O -w '-i Ln N I I ~-1 w I ~p I I I O N r-
0 p M ~ H 0 ~ I I M M I I L11 I I I 0 ~
N 00 r-i U1 M rl ~ N d~ N r-I
ri r~ ri ri r=I
Ol
rd ,O N N 01 U1
~ N O I H I 0 Lfl rl OD M l- M : rl 00 N r N l0
> =ri N I l0 I L- ,O 00 m lfl l- 01 ~ = ~ I ~ r rl ri O d~
,r'~ H N ~ H OD l- ~ N ~ ~
~ ~ i-I r-i
~ ~
04
rd dP O N
M J-~ 0 M 01 Lfl 41 \0 H OD 00 M 0 Ul N OD I- "' Ul O
N * -;v = d1 = rl N 01 = N 'O
N L11 H d' M 10 N rl N M rl
a N M ri l0 rl N N N
w %O l11 00 O N M M 01 M ~0 00 00 M L- r-i M %0
=
H ~ N . M m dp = pp = N M M r=I = r=I pp 03
0 K ~- Lf1 r~ Lf1 a0 M N ri 01 N H %D
44 O FC
~
0
u
(d O O [~ QO 00 d~ M M M ~ N N 0 N N 0 0 M M
0 O.~ H 00 M M 111 01 O~ 01 H H 01 H 01 01 H H 0) O1
O U]
vi 0
~ q
ro N
~
0 10 1J W d' %0 l0 l!1 [- L!1 N O l- 0 N l0 O r~
.~ N 0 U O rl M 10 l0 M ~ m l11 d' I- M u1 H OD 01 ri 01
A w U a d' Lf1 H ri N H H r-I d~ N d~ d~ r-i lf1
~i a U
~ ro ~+ a
=~ w ''
U ~-I N N fy' Q,' 9 a a a H r-1 r=1 N N N
4.) A ~ o z z z z z z zz a a a a a a a a a
14 ~4
4) N ro a
'r~l 1'
4.) v o 0 0 O O ~
O
Orn Om 01 01 r-i r-i o1 01 01 01 Orn Orn Orn 01 o) o) '=-1 T r-i H
N cn o
N
H }I a
rl 1J t-4 Cu -7 a a ~Zi .l ra H ~r.~ [T4 H H ~.] G4 ~ A
~i r-i H G~ U H w 7 a a H ~"
~ ~ a H A W ~ ~ ~ m
H a vAi ~ R'~ U
~ =~
~ V ~ a) x a a H`~ A a w wx H z>I W
a H a a a a H E 4 w a X a H a H a a
v 3 3 >+ [J4 3 3 N [X4 [X4 E 44 ?I >+ ~H >+ J+ >+ ~H (a
a Ul w a H f14 U) U] fs4 x H SG fs4 U > w M U] G+ 3 x a ~
a
b
~
..,
EF)
a,
cfl
W
J q
m ='
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
a)
o
r-i :j V, Ul ri N M r-i r-i N V~ Ul N d' N H r-I N v H N d~ ~--I d~ ri H N H
lll ,v
(0 0 o
z H
r-i 00 Ln
p pp OD lp [, l- 0 M N ~O %0 N
~ I ~p O I p I I
Ul 01 N O L- 01 tf1 r-1 Lfl QO N lf1 01
U1 H . lfl d~ N lfl d, N O r-I d1
~ lf1 01 N M 10 N QO N rl l~ 14
a1 H ri r-i
,-i O N l0 01
p Ll1 N d' N rl O~ 1p O O OD 0) l0 OD r1 OJ
I
I Nv
m ,~,= M O N Lf) I ~ l ~ O [- rl 00 Lfl 14 ~ m QO L!1 ~ O d4
~ Lfl d' r-I Q1 r r-1 Ul OD [- m 01
00 OD l- r-I Lf1 M H rl O N H
r N r-I r-I r-I r-I
~ -,wi N QO
~4 O N [- 01 00 ri Q lfl L!1 d~ O
O l- DO i i l0 N O O %O U1 M l0
::$ ri W M 0 [- L!1 m rl 00 L- l- 00 l- O
01 Oa U1 N O L(1 0 = O~ N O m ~ 01 l- "
r N H r-I r-i m N ~ l, f+1 m r-i r-I ~ m HN V~ N N
0
~ M
p 00 M 01 O I O I ri M l~ r- 1 I- 144 r O Ul v pp M
N ~ v ~ I M r = ~ Lfl M N Lfl M 00 O ~-{ OD 10 r-i
u+ r=1 = r-i l0 M O 00 0N O% Ul Ul r-i
~ H * ~- ~0 ln M H rl r-1 ~- H 10 ~ d~ m H ~O N
H
~ N
N 0 r 10 OD l0 m O l- 0) CD H O r-I l- lf1 o w m H 40 H w
0)
N N d' N N N N r-I d~ r-I N H H N M ri ri M H '~ N 0~ N
r'1
4-1
H ~
rt _
0
44 00 00 00 DO 0.7 00 m 0 0 N N 0 0 O 0 0 0 0 0 0 0 M M 0 m
dp
.~ M M M M M M (3)Ul mLf1 01 Ol O~--I r-OI ~-Oi Ln rOl r-O1 rOl r-OI Or-I Or-I
Or-I T ONOH 01
-~ 0 U]
0
41 U
b 0] 00 O QO o M L- N l0 OD m N d4 0~ O OD d1 0 10 l, O L, r-I H C~D O~
0 rl O ~O [- O N I- [- H Q m L!1 H N d' l0 0 d4 d4 10 O O N H m l0 Ol
N a' m H rl r-1 m rl N d4 4 l0 m M M M di Ul ~0 1D l- l- r-i r-i r-1 r-i
V, U
~~
ra U) rl r-I ri H H H .-i H H H r1 N N N N N
'~ " x z z z z .7. 'azi a7-z 'a7-~z EO Gi a PU Po OU W W PO W
W
A 0 z a a a a a a a a a a a a a a a
a
r. a 0) 0) rn 0 om 0 0 m m 0) rn m m rn m rn m rn m 0) 0) o rn
4J
N
N
a H LL U] 'Zi H x H p.' ~+ O~
~ a U] RS W O~ a
Z cn w z C7 H a w z ~C c7 rC ~n x u~ Ea
0 a cn aX a a rx ~H M
p U T x ~+ ~ V] 3 UI W fJ4 G 44 FC C7 cn H [*a >
pq U) m a rz~ x U w cn W a 3x W E~ x U) 0 w
N 44 c n U a r J + FC H O W H ~C C7 rC rC U) > z al
a a w a w a a a a a w a a w a a a a a a w a a w a wCP4
cn a a a a a>~C z 0 9 H cn ~7 ~ H w a z~H 0 w a)
x a~ ~
r-I N M W lfl 10 l- a0 Ol O ri N M W L!1 k0 l- W (3) O r-I N M zr l11 10
~ ~ O O O Q O O O O O H r-I H H H H r-i r-i r-i r-i N N N N N N N ~]
~V ~ H w V 4 4 4 W 4 4 d' 4 4 4 144 4 4 4 4 4 " 't " zv d, K" d'
% p
u1 u1 u1 u1 ul u1 t11 u1 u1 u1 u1 u1 m tn ul u1 lf1 u1 ul u1 U1 u1 u1 ul u1 u1
l0 W l0 W l0 W %0 kO 10 k0 k0 l0 l0 10 l0 l0 l0 l0 10 1D k0 kO kO l0 l0 %D
H H r-1 r-I r-i r-i ri r-i r-1 r-i r-i H H r-i r-i r-I r-I H r-1 ri H H H r-i
r-I ri trJ
~
Ca ~
rl N m r-i N [M m qzv lfl Lfl kO [- 00 Ol 0 ri N M w " [- l(1 w l- QO O
v 00 00 00 O O 00 O O DO 0 00 00 OO 00 01 0\ 01 01 O 01 O 0) 0) 0) ON rl
b O O O O O O O O O O O O O O O O O O O O O O O O O O m
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 aD
~ v N N N N N N N N N N N N N N N N N N N N N N N N N N u?,i'(y N N N N N N N
(N N N N N N N N N N N N N N N N N N N
0 0 0 0 0 0 0 0 0 0 0 0 (D 0 0 0 0 0 O 0 0 0 0 0 0 O
0% 0) 0) m 0) 01 0) 0% m 01 01 01 01 01 6% 01 01 01 01 01 01 01 0\ 0% m 01
.~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
vl
(0 A o
0
z
~
0 Ln
00
N
r bu, w
O w
ri "i
~' C)
ts
O~ ~, uH
i 0 1
O Gq M
y
G) U + N
b H (~q
(d
N ~ to o) r
to
r-I
44 ry U
H
rt
w
O ... u1 di 01
cy 0 m
0
J.1
'd m d W v1
O N N O
d1 N p' ~O kO [-
U
Sa
'b O -.0I
V) v N N N
o w a a
A ~+
p~ o 0
a
43
N
U a
a a
04 04 H
Cll CJ~ ~
p a1 a a Cl] 0
~
w
al
0'
P4 P4 Ca'7 A
x v >1
G DO 01 O
N N M ~
N ~ H a d w
w~,
,
W u1 Ln Ln
%D %O
r-i
G
.~
C] b
H
ao rn 0 ~ o 0 0
O O r-I U)
i O O O O
JJ yJ
04 N N N U
a O O O ~
01 01 0N
~o
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
v
~ O
r-i ~s VI Lf1 rl m H (V d, Lfl N W N N q4 d' N Ul di
t0 O
A o
0
z
~ Ln
p co lo w O l- l- N N
d~ Ul 01 O l- 01 Ul r-I O d~ L11 01
i!1 M H U1 144 N U1 v N di d,
$4 Lfl N N W
0 kD
M O N ~ H ~ O O l~ ~ 00 ~ M Ul 0
t}j u1 0o M 01
Ol OD 40 l~ rl M N H
v m r+
w
> H o r QO M i 10 N O o ~ r-I 0 ~D l11 m l0
t2i tfl = N L11 lw 0 = a% l- O1 O 0) d' 01 L- 'r
}4 'r kO N H H M N M H M v (N N
a) 0 pq
ro co
N Al Ul O ~d4 O M : H M l- r Lfl M M ~ ~ l0 H
m r-i l0 M O 00 ON L!1 rl
~ rd H -~' M rl r-I r-I
fYl
44 4) N
m v 00 M O l- 00 r-I O L!1 rl rl
. . . . m .
H ~ 0 rl H N N N O rl N r-~ N M rl r-i ON N
w U pq
0
U
~ b ro ro
OD Co 01) OD M O M O N N O O p M 0 0 M
OFFii ~M M M M 01 Lfl 01 lfl 01 Ol r-i r-i r-i r-i
~
O U)
Ln 0
vl 0
=~ pD a0 O o [- N w aD m N O ~0 O ri a0 O1
n ~ 0 ri o r N [- l, H m lfl d~ O rl ~O 01
=~'1 ~ a M H H d' H N d~ ~O m l0 r H H H
A a~ p, u
~4
a
w
Uj r-I rl rl rl N N N
C+ ~
=~ p' " z z z Z az z a Qi Qm 1 a a w a a
41 A m 0
14 a) a
~ -ri 4.)
i.) W v o 0 0 0 0 0 0
(]W v O) O1 r-1 01 H r-i O\ r-i O) Ol 01 H r-i 01 r-1 m
~ a a
O M O
~ r-i iJ a H cn H C7 FC E AX FC H 0
r-i a M W a a H p m m ~4 a ~H rx c
a ro b v z w w~C c~ H a~C w 4 rx H~C ~+ N
0 cn a Y
=~ aJ cn cn cz a x w 44 c7 U) >
rl w x w cn w ax u)
M rl (~' ~ Ga G4 U R; G4 a' H a H U] z a
r+ v a a a a w w w w a a w w a a a w
a ~n a~C cn x a a a>~C cn ~C z w~H w M
W
w
ro
C.a ri N H d4 m W lfl Ul w L- O lD l- l- O 01 (3)
H aW 00 O 00 O O OD 0 QO 00 01 O O m H 01 =rl
O O O O O O O O O O O O O O O O
,d O O O O O O O O O O O O O O O O H
=~ N N N N N N N N N N N N N N N N
a N N N N N N N N N N N N N N N N N
v O O O O O O O O O O O O O O O O ~
rn om m orn m rn m om rn m rn rn m m m m
00 u
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
a)
d o
r-i :3 yi N fn di rl N N O N N d' %O lfl r-i N N N O am r-1 N r-i M ;v m ;v
ro. n o
0
z
~
O O~ ll1 l.(1 ~ H r M Lf1 N r m 'n H r Ul
M d~ 00 00
LI1 l0 r-1 N 00 r d~ H 00 H t0 H m
H d~ O O rl 1-1 O m H lf1 r r L(1 M M H M O N M V- N w r
* rl Ln ri Lfl rl 'n N 00 rl aD ai H M H W
P~l
M 00 N
O I M r %D M di t!1 M r l!1 r r r O m 01 m m O lfl O N
N d4 ~ O OD Lf1 w 01 M ~ O m di ~ O H r N N
M N ~ OD l0 r 0 iD H H If v N Lfl N N 0 m r-1 r-I N
W ri r-I
{=I ,~ N tl1 H N OD 0)
w O 00 m 0 O N m r Ln 00 ~ m 01 O N O U1 r r Ln
Fi ,r=1 Vl N H a0 00 %0 N d~ M ~ N lw l!1 Ln m 01 N H M r r-I O
~0 i r tfl 00 = = l0
44 v L!1 r 00 N M d
H H %0 r r'~ O N N r1 r O= lfl ri M N N d~ H
LS LQ . rl rl r-i
a
N r M
0
'b 1~ O Ul Lf1 w 00 tfl N N 'v N M
~ O l0 0 ~ M M N d~ r , N DO aD Lf1 d1
r.{ I I 01
r-i N H d~ ~0 40 N ~ r ~ ~,.~ 10 OH v U1 m r{ m
=~(~ r-I r-I
F1 N
4) U
~{ H
H L!1 r
V 0 O% M lfl O 00 N l0 W H m 0 lll
I O M I O 0~ ~-I I I ~O u1 o v m r-i r ~ oo l1 rn m ao H w ao w
(d M d' O ~ r ~ r ' ~ ~O d~ rl l0 M OD . ~ I I M 00
N H 41 ri r-i r 01 N m r. ri l!1 ri N r-I ~ rl r-I
H r
O N H ko 14, m 144 U1 O O `-' 0) N r-I I N
44 aD r-i d~ r-I N O d, 00 rl 00 00 a0 r N tN
l0 M 0 00 i i O I M 00
(~D d~ 00 N
r-I O 01 d~ l0 01 N ~0 01 m
H ln H r-+ H ~o rl
r+ rt ao rl r '~={ N N
w ~
44
O
U
(d ro
4.) 00 lll p d' 0 OD O O d' 0 W O M O O OD OD OJ QO aD -w 0D 0D OD w
(d N - m N 0) 01 O O O ~ O m m OD N m m m m m Ul m M m o0
ra r-I r-1 r-i r-I r1
N
r.
01 U
b U] O r-1 OD M M 0 O 'n kD M d~ 40 QO O 01 m l0 41 0\ M 00 r
ry 0 r lD lIl r N ~ r d4 r Ol O r r r Lf) N N w r N r r r
.r={ a N v H r-i r-i N H r-i rl r1 N N N M
4
N(~' O 0
O H H rl H rl rl r-1 ri r-I rl N N N 0
E x z z z z z z z z z z
14 N
~ A
Ea 0 0 O O O O O O O O 0 O O O
rn rn H orn r+ rn H H H H H H H H rn rnH rnH H H m rn rn m
a
dl w
w W
H a w9 H~~ c~ ~C a 0~C
' ~C a fn H~ H H p U V] w a 3 m~ ~ x E~ a U~ ~~~+ C7 ~
> w a H co a tr 4 1 a wH
Q 04 Ga a
1-a v a m C7 fx a a a FC a w U DL+ Cu Cn () a a a F -4
z H~~~c H H a t~ ~ a HUl w a a a~ ~ ca ~c
O Ri EA Cx4 .~C Y f 7 L4 Ul H H H E"i J U
0
N w w > a a 0 a r~ a x a rJ4 x 0 w ~n ~n H a A
v] Z> E+ A 3 a z a F>+ a c~ a U a cn m U Ux a
Q, w w w w w w w w w w w w w w w w w w w w w w w m
cn w a H> ax x u~ H x cn x > w~ H a a z w (Y w w rX4 0 0)
O
W
J ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v
r-i
0 o
r1 ~ Vl N N O N N r-I :v O M M rl L!1 O m v U1 -,v N rl N dl zr rl N dl
(0 A
O V
z
~ co
O
O d~ w lzr M p 0) H 00 m O OD m O l0 0
lfl ri lfl d4 N 0 m Ul 00 l- M l(1 01 Oo ri 0) N OD
d' Lf1 M L(1 O N r-1 O N m OD M L- M H r-I O 01 O Oo 'õ~ [-
* M d~ ri N l- 10 00 ri W 1-4 f-0 H
M
O 00 1p U1 Ul 0N l0 lp LIl H M OD O [- M L!1 0)
I
M d~ O rl M d~ 0) L, 1 01 1p O m Lf1 40
O U1 ~ O r-I M
lfl Q1 l- 1
.-I N aD OD rl . ri tO M QO 01
~ r M 0~ rl M 01 l- N ~ N ri L!1 ~--1 rl r-1 H rl
fll r-I r-I
m
N 01 l0
~ a) l~ 40 N ~ ~ r-I N O O [, d4 m N
Ff .,~ V~ L!1 I- l, Lfl M OD M l!1 Lfl 00 I~ M O l~ ~0 l~ N M
0 44 11 M rl 00 p OD ~ N 01 dD N Ul 01 lfl 01 k0 -,~ M l0 OD M N 00 N d~ H
M
La r-I r-I
o~ a
N
0
4J O l~ d~ 0) ;v m ri I d4 Lf1 lf1 l0 [, l0 N 01 l- ~
~ O H ~N , m 00 M ,
l, r-I U1 l~ r-I ~ N l~ r-I N
> M M ~ N N r"I ON l!1 I~ M 00 Lfl M Nv r
=~(. P~I
M N
~ U
0
O 01 ~ O %D l- N 01 ri m [- M 'n H N r-I O M 01 r-I N
O o Lf1 L!1 01 I~ 144 L(1 0) O N
(O cp = ~ . ~ k0 M 0 I M O~ N = ~ d' N N ~ O pN O ri Ul l-
N * Ul p O H N '-I r-I
l1) 01 01 H N d~ rl H l- rl rl r-I r-I
~
O
yõ~ O 1 1 O tf1 N L!1 N M 'n L(1 O r-I M 0h O OD d4 N OD
lp I i O N N N O
r-1 d~ rl rl d~ 01 L- ~ U1 N O
H * 00 10 U1 d' L- N M O M M rl r-I rl
44
O ~
U
4J M V M M 0 0 M M f=n M M lD W M L- [- l0 M H %O M l0 M W W
(d a) - 01 10 m 01 L(1 U1 01 (n O) 01 O% Oo o0 01 lf1 Lf1 m 0) l- M 01 Oo ON
OD OD
b C
U
=~
ra N M N M ~ m ON M r-I rl r-1 M Oo 00 01 O O N M ~ ~ v Ln ko rl r-I
VI 0 N l11 10 r-I O O d' U1 01 01 m m m l, (N N Nzv L(1 N Lfl l- 01 01 d~ d~
.~ LL H N N N N N N N M M M v v 144
~4
:j 0
a a a a a a a a a a a w a a a a a a 0
~~T11 p z z z z z z z z z z z z z z z z z z z z z z z z z 0
a 0
cN
N A
~
O O 0 0 0 O O 0 0 0 0 0
a ~~ m~ rn~ rn m m~-+ rn rn~-+ ~ rn~ rn~ m.-~ m~ rn rn~ +
C
44
W
H H Ga Lt+ ro
LL a H H FI,' a > H G4 44 4 0 Ri a a 01
0 z~C a a a w a acn u) 3 W0 w W H ~ w
~7 u > (Di m 3 E~ FPq C7 H u) u] U) Un >4 2: a a> H C] 3 a a =, ~d
a5 a~~m w w w~ a a a~ F H~ w~~ H H f.Yi H Zi Zi CA H a a 0 (a Ga ~l ~~ H Ca ~7
C~ ~ W 2: H H
N W W W W W W Lc~ W W W W W W W W W W W W W W W W W ~n
m H ~+ H W C7 C7 a FC 44 44 fx Ga fLI .a G4 [*a r~ CL O a A 0 W ~l 4
4J
~ ro
U
W =ri
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U)
o
'"i :J VI w M N N O Lf1 dl r-I l!1 r-I dl dl M H N N r=1 O dl Ln H N M 1-1 dl
ro 0
A o
0
z
1 Ln Ln 0
O r- d~ O [~ Lf1 ~ M r 40 N r-U1 d~ 00 N N m %0 H N
Ul N OD lfl N O N H 1 N ~N M M d~ ri
N d~ Lfl r-I ~
M d4 00 r-I -W
dl 00 Ll1 00 M , O ri . 10 0)
* 01 U1 M dl dl r-i dl ~+1 ~-1 N O H dl N N
fY1 r-i H ri
U1 I;r N
O %0 IW 00 N OD 1 (N 0 -W 00 %0 OC) r-I ~ N Lf1 di M
L!1 r-1 kO O N Ol [- 10 N N H M N N M l- r-I N
d~ dl lfl H m r-i m 0 1 [~ w w ~ H L!1 ~ l- O ` ~ O m
l- %0 t!1 01 rl QO U1 00 d4 r O N N 01 lfl rl
P~l r=I ri H
N N l0 k0
01 O di N O kO m rl O O H [~ dl ~ N 1 U1 V1 t-I ~
O lfl 00 Lf1 Ul O r k0 ~ N ll1 O Lfl N l0 O r-1 O N OD 1 O O lfl H
a 44 -;r dl N l- dl rl Lf1 -1 rl c+1 dl [, dl N U1 Ul N 10 rl ` Lf1 k0 ~
.,i Lfl M N 0~ d' k0 M N '-I aD N M M
ca ~$ r-I r-I r-1
O1 Q+
N
~ p U1 l11 O~ 1 00 N 1p '~ O 10 M W ri ~ N O l- N N
O . M . . ~ 00 , 00 Lll O r,=~ a0 , 00 N l0 O N , N O
1-i N r-I
V~ 01 lD 00 dl O
l- 01 r-I (1) 01 01 l- N 01
* l11 [~ [~ H 01 H Q1 ~ H d~ lfl
>
=~ W
11
~ U
ra H N M
O ko M I dl rl '~ 1 O Ln O l~ l~ M QO dl ~ M
0 rl lfl N %O M dl ~ M M [- O) OD r-i N
nS V = 0 M 0 M O N I- 10 l0 O Ul Lfl l0 H N W kD . 01
N * ~ H H ri r-I ~ Ol ~ H r-I c='1 ~ L!1 rl M M N
~a !Y1 r-I ri
(0 ~
r-I cc) w 0 00 1 lfl 1 L!1 l~ 01 0) N k0 01 N ri 1 1 dl Lf1
dl N 1 01 1 rl ~ 1 VI 1 ~ = I ~ ~ I I O. 1 I rl l~
H * r-I l0 01 lf1 H rl ~ OD d' m rl N l~ N
f~0 H
44
O
1J %0 dl N N 0 0 N 0 0 0 N N N O N lll Lfl Ul L- 0 M M N Ul
dP O \10 0) m O 0 r- 0 0 0 0) ON IqH O v r r r- w OC) O 0) O r,
H ri ri ri ri r-I r-I rl
b EO
G
U
.rl
b N 00 Lfl OD 01 l- dl W M [, l- O dl N O 00 M M 00 O [- ri L11 01 r-1 OD
~y 0 L(1 m 0) r-1 -W U1 lf) %D %0 W 00 00 N M Lf1 r-I r=1 -1 dl 01 O O 0N r,
01
.rl (L) W rl H H '-1 r-1 i-i rl ri ri H ri H H H H H N N N M M
A
s4
N o
r-I r-I N N N N N N N N N N 9 4 ~ 4 4 9 9 9 9 p
4J m m m m m m m m m m m m a a a a a a a a a Q+ a a a p
4, o z z z z z z z z z z z z o
a N
~ A
a
0 0 0 0 0 0 0 0 0 4-)
='i
a rn rn m H H rn rl rn m ri o, rl rn o, rn rn H H 01 01 01 H Om Om H
a
w
`I-1
~
~ a 3 N A W H H H rC F Ol H H 0 C7 0 W[4 44 0 a r.C W 3 ~
H (7 w x x H a a CL' G+ x r~ m H H a H fa p a q cA
u a G. H a a aX X H M a FC ~ W W CL x W H 2: 1 w tA d
x a a c~ a a rx a W w w W w ca w H H E-1 H w w E-1 a a a p
,-.
a A z a a W H ~0-1 H m w w ~ C7 ~ a
U) H w w a a H 0 `N4 x H U] 0 H fXl C7 C~ 0+ ~
v W W W W W W W w W W W W x
U
W
J q
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v
~
r-I ~~ N N Ul N d~ H N 10 L!1 N d~ di m d4 O ~-1 d~ M O O M N N lf1 0
0 A
O
z v
~ m in
O -W O %O Ln I- Lfl r-i r-i r-i ~v 01 lfl O M
Ln ~ l, 01) M r N l0 O r-i 0 " N OD %O O L- r-i l0 01 Ln m k0 d' 10
O 0 10 OD 00 N r-i M M M 10 r-i l~ O
rl
~ H rl 0) m r-i r-i O l: ~ r-i
LQ r-I r-I
M ~
O O r-I M di Lf1 LI) I i O O~ r O ~
d~ 10 O 01 N 0~ N ON l0 r-i 0 kO ~11
r-{ r={ ~ Qp I I %O Lf) I ri I
m u1 r-i N N ~ N [~ ~ ~ r-1 00 M N W l- N
* r-I 01 [- L!1 H N ri rl
W ri
rjl m
1-I N N
',1 0 00 01 ~ lf1 d~ [~ M 01 ~ o ll1 O ri l~ ~ aD l- QO ~ N ~
~y.~ d~ M 00 OD l- l- d~ 00 N m 01 %0 O 00 Ll1
Lfl 10 N M l!1 d~ Ol m H
~
LS ::l
Ol ¾+
0 Ln
b J.~ 0 ln M N N N OD (lq N 01 DO (3) O N lf1 [- M
O M O O OD N O N r-i O DO
Q) 'cr N N ' O l0 %D = = r-1 . ri N U1 N O M r H OD r-i %D ko N M
> =~ * N rl 41 r-I 01 m rl U1 m N N r-i N r-i L!1 O)
=r'I PQ r-1
11
Q) U
ro H ~ M O
O IIl w l11 I M ~' I d~ l0 Lfl m ~ m N 10 41 ~ N ~
O lfl L~ M L!1 rl l0 H r-i L- l- r- N lfl d' 00
(d W r-i N M ' lf1 d~ ' = = r- N (l rl N 0) 0 O ~[, 'n U1 0N T-
[~
(~7 * M N ~ l- r-i ~ N rl r-i ~ ~- 01 ~ r N
L(1 0) 4-I ~ m ~ i Lf1 [- 01 H ~ N 01 d~ N I ~ ~ 1D O M O
I 0p N I = I 1p OD O ~0 k0 0) = ~1 I M ri
MH * M Ol r-1 N r-I m H H 0) N ~ Lfl ri r-I N 10 O.
W r-i r-i r-i ri H
44
0 ~
U
Q5 O O O O O O O O O O O O
~ 00 N N N lfl [- N N [, N l- O N
oV O O O O O O O O O O O O
N=.~ ~ d' ~' m r-I ri r-1 ri r-I ~ w~ mw ri m ~ r-I r-1 r=I 1O LnW
b N
~
b y 01 O1 01 O~ ~O l0 N M [~ Ol N 0 0 l, H 00 m w 0 0 l- 0 0 01 0
ry 0 O O O N lf) lfl m N M O rl l0 W OD H tfl w m H m Ul l- l- aD 01
=~ ~ W . . . W Lf) Lf1 10 l0 w w w H H N N M M M M M M W
A ~
4
Q, CO r-I r-I ~- rl ri r-i r-I ri r-1 r=I r-I O
,, ~C FC ~~ FC FC ~C ~C FC ~C ~C ~C FC rC W w a1 al 00 m oU a1 m pv w o
4-) 0 a a a a a a a a a a a a a a a a a a a a w w w a a o
N a N
N A
N U 0 0 0 0 0 0 0 0 0 0 +1
a rn rn r-1 r-i m r-1 rn H m H rl m r-I m rn rn rn m rn r-i r-i rn rn m r-i
=''1
a
w
W
F"~ w H H 'J ~s+ a 0 L+ !
a A a 4 a H
a a a H.7". ~ a H ~
O u u u H H a x H a a cai H A H a a A A a W b
O r~ a x x x E+ > Z w a w O~ a a H m z a G4
x ~ x r~ rx a u w m w (DI rx wa w z U) x cn ~
w w w A.~+ H w x wf z c an can C u~i a z 3 cxn ~~H w Q
~ c n 'wZ c w n U w i 0 H F 3.Yi fW FL Fti ~ Z U Ew-i z W fW H W ~
0) fo
U
w =~'
J a
m =~'
F- '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
w
~
0
H :J vi H 0 N N l0 m N m H N H H m H H v M N N 0 0 0 H N
0
A o
O
z
~ w ao 0
O M I 1 I ~ lfl N w 01 N m lf1 :
m lo H lo 1 d, m ul r=I ~ O N l- H O O M ul d4 ~ %O N O Lf1 1 O m N N N U1 N H
H O H 00 Oo l0 0o . N r=I .
.-1
* cn rn H ul H Ul N t!1 d4 lfl m
r 1
W H H
m [- 10 H N Oh ~v l0
p N 00 01 d~ W O m r-I O M ~ M
01 N OD L(1 l- Ul O N 01 ~1'
m lf1 ~ 1 ~ I H 1 ~ I I- I- O 1 1 1 O
* d' ll1 N 00 O~ [- d~ k0 M rl rl ri r
rl r=I ri ri r-I ri ri
M :31
N N rl 10 rl d~
O 1 lfl ~ d~ lll N H d~ 0 rl N m V~ I 1 N
l- r-I OD OD 41 M M I I N
FI -~ d' 1 OD 01 ~0 O 01 l- lfl
y..~ . 01 Oo H 0 = = M 0 . l- W 0 H
=,i O~ ~ O O I, lD l0 lzr M M r=I v
r=I ri r~ t-I r=I
Ol ¾+
..y O N O1
-V 11 p M r=I ;v Lfl r 40 %O v ON OD Ol 1 Ln 01 00
~ O N Lfl w [, w l11 L!1 N kO O [- Oo
M ~ H H N L- ~ l11 N d~ l- H
N ~ M N r=~ M M N M N H aD O ~ M c~ H r=I
r{ CO r=I
$1
Q) U
.a %O OD l~ %O
N l, Oo O Lfl O l- 00 i m H O 00 ll1 Oo , Ol M d~ L!1 l.fl ~ H O ~O , 01 10
r=I 1 1 lD 1 l~
d' . O OD N rl r=1 0) 0 N ~ m
N *~ m N ~ %p r=I '~ H rl M c ~ m
00 a
ON
0 QO 00 -W ri v v dD l0 r- 01
w N U1 O O M 1 [- Lfl l- ON Qo N1 H Oo qzr '- N H (1 w Lfl
V r-I lf1 0) L(1 w Ln ' N 1 10 v H N N m M O tf1 r CO , 'IV
H 00 H 01 00 H M M OD H di N N L!1
(~1 r I r=I
44
0
0 0 0 0 0 0 M M m 0 0 0 0 0 0 0 0 M 0 0 0 0 0 0
(d Sa oW O O O O O O O O O O O O O O O O O O O O
H O O O 0)
U`-' r-i r r=I r-I r-I r=I r=I ri rl r=i r=1 r=I r-i r-1
ra r-I r=I r=I r=I r=I r=I
tn
V4 U
.rl
b UI I~ d~ M Lf1 [~ [~ l0 00 r-I r-I ~ ~ d~ dl O 01 01 0~ O l~ lf1 L11 l0 l~
[~
0 0 0 l- rl m m m m m 14 111 H M ~0 ~0 l- O O N V' Ul Oh Ol a0 m m
.~ U p+ l!1 lf) l0 l0 %O lD 10 [- [- l- rl rl r=I N N N M M M M v lll Lll
~
N o
a ~ v rl rl rl rl r=I r-1 H rl rl H N N N N N N N N (N N N N N N N 0
~yy 11 CQ PO (Y1 (.10 LQ [Q LQ (~0 (Y1 LQ f0 GQ PO 00 W LQ PO W Q~ PO PO (~
(~0 LQ LQ 0
4.) 0 a a a a a a a a a a a a a a a a a a a a a a a o
{=~1 a N
G) ^
0 >1
o 0 0 0 0 0 0 0 0 0 0 0 0 a ~+ ~1 ~-+ rn~~ m rn~ rn m rn~ m m~ rn~ m m~1 ~ rn~
~
.r.,
w
W
H U a a 54
a ro
m a a FC u~ H E-1 P > E > H fz, f=+ 0 zE > > 0)
> w~+ H x w w a rx rx a~~~ w w
~
u u z z H~Y H H H
~ w ~ ~H-l w W C~ a w ~ tH-1 H uM ~Tz i ' '~T ; j
& a la 14 HW Ul UJ .Yi L4 'J ra a 'J W (7 (-~ ~i H H
-- a ax x Z a x H x x ~C ca 3 3 ~
~ cn x 44 3 ~G H H G W FC ~C W W W W W W W W W W a W W W W m
CY)
U
W
J G
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w
~
'"i V I Ln N c+1 N t0 r-i
t0 O
A N
O V
z ~-!
14 m
0 ` 10
Ln 10
~ ~ ~ rl M N 01
W r-I n r-i r-i l0
M Ln
V N ~ N l0
~ O
m
$d N 14 U1 dD
%D (V ~ N
0 l- 10 01 Ll1
W V~ = = 114
'~ Ol lfl d'
~ ~4 ri r~ r-I
C1 Q
N
~ 0
O 114 O l- 01 aD
M
> Lfl H M if1 N
=~ PO
4) U
H . rl 10 (14
O ~ ~
~ 0 N U1 ~ M
-ZV r-I Ln M
N ~y # ~ v
~ ~
0
Ol 00 ~ I [~ I
w c0
H 0 rn 0 ~ =
'..I PQ r-I N N r-1
w
0 ~
U
4.) O 0 O 0 0 ko (d ~ dP 0 0 0 0 0
O
ro N`-' H H H H H
N
G
~ 0
=r{
tn u1 u1 ~0 0~ 0~ 0~
W U1 U1 kp 10 W f-
=rl N
A 0
~4
u) o c
U) a) PO ~ ~ P0 P0 PO o 0
41 o a a a w w a o
5=1 a (N
0) A
a) O o 0 w
W
AS
a a 44 0)
rC ~a C7
~ a a a >1
N
~ W W FC ~G `~4 ,q
0)
U
W =l
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w
a~
~
ri yi -w v 10 lfl rn d' M w 14 Ul t lf1 di q4 W l0 M Lf1 L(1 d' W d' Ill
ro
0 V
z
~ 00
ul ~ r-I OD ~ 00 N 00 ~ d4 L(1 M ~ 40 0 aD N M 00 O 0 N N N ~
N rl l!1 d' M 10 O N M O~ O [, 00 01 M 114 r- 'Im r-I 01
ri rl rl N OD lzr r-1 d' N
dP CO ri
O
M M
A
0 l, U1 [- O ll1 O N l0 Ol l0 f~1 L!1 d~ N N [-
b 01 M [- N ~ N I, r O Ul O rl M L!1 r-I M ~ [, l0 r N
N N ri r-I rl rl ~ N kO ~ O~ N H rl r-I ~ U1 ~ ~,,,I 10 d' N OD
w pq
~
N
O O W 00 U1 N ~ Lf1 [- [- OLf) o ~ U1 Oo l~ ~0 I~ M O Lfl N 01 ~ O [- N
ri V .,~ ~ N ~ r N N ~D ~ N 00 N 01 l0 M d~ N O ~ =d~ l- ~ OD r-i
Lb b ~ ~
a% ro a
N
0 O L(1 d- U1 I I M r-I lf1 l~ Lf1 10 0 %O
1J O L~ d~ r-I d~ M N M OD l0 O
~ * v p~ N = = I I 01 M = = l- rl N N 01 10 O rl N
r-I 01 L~ r-I N 01 Ln 01 [~ 01
r-I O
}4 o
~ v V O M 01 H ~ ~ 10 L11 01 M N r-i N M %D d~ O ~ d~
O M L- = M 00 O N [- l11 01 N r-I M M d' [-
M * d~ N O r-I [- O N [- r-I
M lw r-I r-I N 01 v r-i r-1 01 %D r-I
~
N ~
N *
O 10 0 N %O N 0) 0 O N 0 Oo Ln l, m 01 w H
00 d4 00 N d~ N M lfl 0 0 00 OD O) t!1
Ln M 00 N l0 M 00 N O d4 d~ N N [- . O d~ N r-I lfl = O
w N H 01 N H N l~ M M rl ri ko U1 ri ~ ~ lfl H N
ry O
H d'
w roy~
0 LI v lw 1w O 00 OD OD 10 M l0 l- I- M 10 10 l0 14 0 O N N [, O
~ (N 01 ~ 01 M M m oo 0~ 00 U1 Lf) (n oD 0D OD 10 0 0 01 0) 1p 0
~O O U ~
ro * U
U1 t(1 k0 M 01 01 Oo M O O O rl Oo L(1 -w [, O v O [-
Ul l, 01 O [, [, [, d4 f'1 N N ~ ~ d' U1 01 lfl 10 00 00 d' (Y1
~+ ~q V a ri r-I N N N (+1 N M V T H H H H H ri r-1 r-1 H
~
r-I
4)
rl rl rl r-I rl N N N N
.,~ ,~ ca ~H ~+ ~+ w~ w w~ w cn ~n ro + E E~~ z z z
b T
z z z z z z z z z z z z z z a a
,~ 4 a , ~ H H m rn rn rn m m rn rn rn rn m m rn rn rn
y,) 0 0
a N ~ cn uHi W O+ >+ >+ 0 H [T
~C ~C a a 3 H ~C H Ol [i4 fj4 N
q a~ r~ A a z w (1) H 3 0: cn aC a a 44 H P:
v ~ FC FC a a a E-4 0 u) E fa a a P4 a H cn x w
~
a >4 ~H A ~ a '~ H H rx a a rx rx W w W H w
H ul U u 3 z H H cn H a a H a H x
~ ,J ~ w Nc ~~C ul m H a~ a~~~~ a v~ a~ H w a H H
V V x a U~ Q w H F-I [-4 a > --I .,i w .~
~ > a
w w w w w w w w w w w w w w w w w w w w w w w w
E4 x E w w GL+ C7 ca [z+ [z+ [z+ A a a 0 M w[3+ a a cn 0 w
ro
1=~ 'd t-. ri 10 l~ O lI1 ~M ~O l~ 00 O rl N M lfl 10 d' Lf1 l0 r DO r-I O r-I
01
.
x =rl ~ Q 0 0 0 0 0 0 0 0 0 H rl N ~-I N N H H H H H f~1 (N N
4.) .,.4
. . . . . . . . . . . . . . . . . . . . . . .
~ tf1 L(1 l!1 tf1 1O 10 l0 10 l0 W ~0 U1 10 Ln tf1 10 W l0 10 10 ~(1 ~O 10 ro
a rl rl H rl U1 U1 U1 Ul l11 Lf1 U1 rl l!1 H r-I lf1 l11 U1 L(1 L(1 r-1 l11 U1
-=~-I
v a %D ko %D %D W %D %o %o kD %o kO ko kO ko kO W W ko W W A
U)
o '
~ ro
U
W =,I
J =d
H
H-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
v
~
0
r-+ vi vi Ln w in w M w v4 Ln %o v in to
ro A
0
z !7!
H
ifl rl O 0 ri N H 00 [- d' 0 rl ~
N 00 aD M %D
dP [q
O
f~1
A M
o M
v N N 01 N ~ ~ i- 00 o [-
* r~1 ~0 l~ l- H ~ m N 10 M N l0
ri
x w
N
d~ ~ DO [- M O lf1 O 1D [- 01 r-1 m
O 0 y..w d~ H OD 104 00 01 01 w [- N O kD O
==~ U -.-1 ~- M r-I
O+ ro a
N
0 0 N M N 00 N O N OD ~ VW O N1 01
~ r
~ l!1 01 01 01 N Lf) N N N N Ul
.~ O Cq
}4 Ln
~ V
U
0 N M d' k0 l0 rl H L- 00 O f+) 0~
* 01 , W 4 N O1 r-1 0 m M H d'
(d O N H r-I
N ~
0)
O U1 l0 Lfl [- O d4 m %D N
lt) O l, N 10 00 L- ko 10 M w di 01
W N * N 01 r-1 01 ~ 01 N d~ M r-I
~+ O r-I ri
H d'
w ~q ro ~
0 Ln 0 o O o o O
N O O N N r o o o o O o
lld~ H H Ol 01 ~ r-I r~ r-I r-I ri r-I
4J p 0
,d * U
vi m 00 Ol M [~ o o r M O1 r 01 L11 m
0 01 O N ("1 ~0 l0 OD ~O 00 M O t- 01
~ u C4 M d~ lfl lf1 ~0 10 l0 N f~1 l0 N lt1 10
N }4
~
It a a a a a a a ~ M po pq M
a a a a a a
N M q ~
w a o 0 0 0) m o ON 0) m m 0 m o
1) 0 o
y~
N o
b U ~ a G: H H H H a H CV a N
w a w a a 4 0 a x U) a A
A a a a u m aF< H a 0
vai 4 U w w a
,J a z ~n ~+ a x H w
En a w ca
~ W W ~] H
a u Wi ~ 3~C ~H cWi H H a X: w
44
ro
Lf1 %O 01 d' lf1 N l0 l- 01 O V l11 rl ~
~'~1
~ c"1 M N N d~ N N N f~l lf1 M 10
,l
~ (11 lfl L11 lf1 l0 1D Lfl 10 10 1O %D lf1 10 ll1 ~
N N Q H r-1 r-i l11 Lf1 rl L11 Ln Lf1 Lf1 H Ln r-I .,.~
GL W ~O ~o t~ ~p ~O 1p lp l0 ~0 ~0 10 ~O ~O ,A
`~ r-I r-I rl ~i r-I ri r-I ~i ri r-I r-1 ri r-I
W
CD a)
J-~
v- ro
W =lu
J 10
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
~
~
o
r-1 ;j yi d~ l0 ul M di m d4 t lfl d 144 l0 l!1 Lfl d, lfl l0 Lfl d lfl k0 d
lfl
r0 0
A o
0
z
~
0 a, r in ~ [- ~ m ~o 0 O to r r
U1 00 N 00 It lf1 OD H N 0 N O rl l- v 0 H
v O Lfl '- 1tv N 10 '- N M 01 O Do 00 -t [- H 00 Oo m l- N H
* Ln N r-1 M r-I co N H m lzr N m r'1
fll
M
O [- L, cr O U1 0 N k0 I- M l11 Ll1 N N l- N N m H %O 00
O
lzr m 14 [, N O N I- tD O r-i 114 m L- l0 00 lD L- M H t0 r,
N H ri r-i r N ko H H W H N m
!~1
~ ~.
N
C N 0 O Lf1 l- l, Ln %0 N 41 N 10 l, 01 N rl
Fi -14 00 N L~ r-I 00 ~ L!1 k0 L- O O 'n QO ~ M l, N O ~ 0
d~ 00 d~ l- M l0 N 01 l0 d~ d~ Oo 1D
~ PQ tD N N d' H 144 ri r-I r-I M H H m H
LS
0 N
W 0 Ul 41 Lfl I I M H 111 l-
o~ M Lfl l0 O o N 00 00 ~ d~ 00 M ro~ i m l~ ri m N 01 N Lfl ~ 0~ l~ N 01 01 N
N
* N Oo [-
.rl W
14
41 U
H ~
IV ~ M H I I l0 Lfl O~ M N ~ M d~ M O r d~ ~0 ~ ~ O M
m = I I m 00 O = = M
N * r-I d' ri r-I N 01 0 ~ W rI N O~ ~' si' d~ 0 0)
04
to ~
V-I OD kD 00 N -44 N N M O 00 O lll l- ~ H tf1 [- di M r 01
ry r-1 m N l0 N 00 N N d' d~ r rl l!1 ~-1 N ~ m r- ~ ~ m O
~..~ * rl I~ M r-~ d'
4-1
0 ~
U
N OD 40 l0 m w m 10 10 O 0 N 0 0 0 0 0 0 0 0
ro oN O M O O O O O O O O O O
M M 00 O~ N O~ a0 OD al
`-' 01 01 r-I r-l r-I ri rl '-1 r-I r-I r-I r-I r-I
W
G
U
.,~
b 0) m l0 M Ol O1 W m OD oo d' [- o l, M r M 01 I- 01 lfl
J~ 0 ul 01 O I- [- [, ~ dI M ~ ~ lfl lll l0 00 Ol N M W 00 M O l-
.~ ~ a rl N N N m N M r1 rl ri r-i r'i lIl L(1 N m l0 N lll
a
a~ H o
a N ri) rl H '-1 N N N rl r- H N N 0
',y v ~ r-i a a a o
a " ~~~ z z z z z z`~ `~ c' `~ m`~ a a a w w w w o0 0
41 0 z z z z z z a a a a a .
$-1 m a c ~
N b ^
a r+
~ y ,~a ~ m m rn rn rn rn rn m rn m rn rnH m m rn rn H m ~
a W
4-4
ro
JOl m w a~H ~H U H ~C C~+ ~C a a H Q P G4 a H a H w a ~
n a z w v] i-+ 3 cn ~G CM fx a a w 0 a Ga cn
~ ~~ a a a H C~ co Ea A a a~ H w A a U) a rC H a b
u a~H ~+ L] a > rx a a W W W E- > 00 a fs+ ~
~ w uui oi H a fx a w H Oa ~ w G+ m `.~ ~G 3HG in a L~+
w
> a~ u u~ a cn A
M rl v W W W W W W w W W W W W W W W W w W W W W W W ~n
~ v r] cn E+ u] W W Ga C7 fx fs+ A 4 0 W W ~q C7 ~ 3 U H H f~ ~ 41
ro
U
W
m
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U)
~
o
rto"l t0
A o
0
z
~
o kD
Ln N
'r H
fY1
M
0
w r
d' 10
CO
m
p
Q)
O%
Q W d d
1 ~ y4
Gl ¾+
0 o rn
>
-~
ny H ~
c~
N *
~
4~,d oco
H
44
O
U
~ roy
4J ar oo
~
G
U
H
~ N ~n 01
4J 0 0)
.rl 0 ~ a io
A a
N -+ o
f]~ N Q) N O
R' o a
m f4 o
14 4) a N
4) b A
~
a
w
1 M ro
o
~ rn
4J
~ a
U
W
J ~
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
~
b o
vl M N M r-1 N rl r-i N r1 r-i H r-1 O N N O N rl O N H H N r1 r-i H
r6 O
o
N 0 U
14 z v
w
M d'
03 N
~ O 01 O O 01 0 O O r 01 N r 0 m
O O 01 O c+l ~w N OD Lfl d' M [M kO t, rl 01 N 01 M r-1
rl = i i d' i
. rt N W k0 O N d' I, Lfl rl ~ ri N N ~ r-1 V~
N r-I M Lfl O N
C1 r-I r-I
b x
~ ~ N m L(1 OD
N N m l0 0) i r OD w ~ 10 N r-i Ul 00 CO r-i N r-i
-,i ON r-i (- N ~ 00 %D M 10 00 ON (N l0 r-I H [, dD l- m
'rI 44 N N CO m m N (3) N CO ~ (- O) N M N m
}=I ,i H '-i 01 l(1 r, r-i 10 M rl [- ~ ON
~4
a
O N Lfl N
(d '~
N t0 10 lf1 a0 U1 l- c0 0)
N 1J w ~ i M N 0 w Ol H CV ~ '~ l.fl oN OD ~ ~ O CO L~ kO
N N i V' L(1 N 0 ~--I M w tO 01
N m r-I -1 N r CO H M d' Ln N M r-i kO
Hd H ri [N rI
O l0
I- M L- 00 H 01 ri Lf1 l- 01 O N ri Lfl Ul O c+l lf1
~--~ r-i 0 l0 41 CO M r N l0 cH l0 i l- U) Ln w OD H M O) 0 r-1 N
O cr U1 M r-i O O O O N
44 r-I N N 10 [~ M H m ~-i m r-1 rl M N N ~ H
r-I
Ln 0
J-) q
fd ~
CO 00 O M N Co CO OD 00 CD M d' M m M 10 0 N 0 0 N 00 N N O O
M M O [- w M M M M m 01 10 m m 01 CO Lfl 01 O O m lfl 144 01 O O
N r-I r-I r-I r-I r-I
01 N
~'+ 0
-ri U
b
-r{ W 10 Lfl N O 01 CO LIl %0 r-i M -q- O O M 00 O L11 kD 4M N di N I- 01 0
O N [- d- l0 O O O N N d' I- OD O N N M c+l C~ LIl
~ QJ p+ M M ~ ~ r-I N M M d~ cr ~ r ri N N O r-I 1-4 M ri N N d' C' d'
~ U
O .,~
1) cn U r~ ~C r-1 N a a a a a a '-1 N
14 o ~~ z Z Z z z Z Z Z z Z Z z z z a a a a a a a a
4) ~4
Q+ Q'
N v o 0 o 0 0 0 o C
~ a m rn m rn rn rn rl m rl rn rn m rn m rn m rl rl rn r-I rn Orn H 01 H Orn 0
0
O o
>4 >-4 ~H >4 ~H N
w a z a Q Cn w m Q rx c7 a a~ w w H~ z 7~ H w
u>-4 5 a a~ a z Q a x a a Q a a m ~ w w a~d ,~
z A a H Z x a z w> w~ w Z wX -+
^ H Q Q w> w a Q u~ w Q z u AM oa xw x a a~~ cxn x ~
4-4
> m
cn ~ m E r~ cn ~n >+ H H E=4 ~n
> > ~n
r~ v E X a H
"-'
`-' cn -l C7 Z > 0 3 > H ~G FC U rC ]C ~.' C7 E+ H U u] ]C w >
C+
~
C'.
A
N
v
N +-)
U
W
J ~
m -='~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N q
~
r-I ;j VI O O V rl N N H M d' M ("1 N N O rl O ri O N d'
(d 0
p
z H
q N
c~ ~ d' Ul r-I Ol O d' Ul Ul d~ kO [- Ln 0) L1) N M Lfl O lw 0D
kO Lf) r-I CO Lfl 00 'n '~ N kO ~ M l00 , ~ ~ r1 m
Oh
.d x
N N M
N O i M 10 '- OD M 0 0~ N 'n r- O i kO o
>
~ ~,~ N N 00 O ~ N ~ . . . [- N %O i ~ 00 i d' N 44 }-I r=i (1) ~ r-I r-i l0
kO d' ~ r-I H ON Ln M N
~ }4 r-i
~J
04
r{ O d
0
4-) O ~ OD O M ~ O O H 10 (D Ln kD I ~ I Lf)
N kp lp O Lfl M O M Lfl kO rl
N = = ~ N ~ N = d' '~ M Lfl Lfl ~ ~ ~ kO N
(1) YO1 r-I d' 01 d' ri
V
U
. 4e-1I H H kO 0 U) 0 01
0 O I 01 01 O ~ d4 w IZV l0 M I I I I OD I r
~='~ a r-i m N lw i OD 0 N ~ Ul N ~-I ~
'K rl '~ ri d~ CO rl N [~ O ('~1 Lf)
LO O
~ ai r-I r-I r-I r-I r-I
1-) C.'
0 0 0 0 N 0 0 0 0 0 0 0 w
b 1~-i oW M M 0 0 ~ 0 O 0 O O M 0 0 0 0 O M
CO 00 r-I r-I ~ r-i '-1 ~ r-I r-I i-1 ri `-I 00 Ol
t3)
Q
rl U
'd
=~ U) H N O H Lfl w (- N N m O L- l, m m O M N M 1;44
0 M d' V' H d' [- I- 00 O~ O O~ H l- 00 N d' kO ~O
'A a l11 1p M N M m M M d' L(1 H H N d' lfl Ul Lfl U)
U
~ U
14
O
v '-I r-I rl r-I r-I r-I r-I H r-1 N N N N N N N NN
4-) W
4-) ~ ~ W fYl W f~q fYl 00 W Cp fYl 00 CQ 00 Ln Cn (ll W pq 00
~ a a a a a aa a a a aa a, a a a a a a a a a a
a+ '
N v o 0 o 0 0 0
~ a li m rn rn rn rn m rn rn rnH rn mH H rn rn rnH rn
0
O 0
o
~ a >+ w ~ ~ ~
W400 F C7 x x w z U) w E+
I=~ U ~'3 u~ H x C*a H x a 4 CJ4 a u]
z 0 p C7 C7 Ll fa cn 0 a E~ C7 E~ ~ a
v x w H 04 a~ a H H H 0 ~+ a c7 c7 a w a ~
a> 0 H a~ z M w A a c7 ~ a Q c
a w w v~ ~n ~C ~n w~n cn a
~- c~n~ X~ q4 H a ~ Ha a~ H a Oa 3 '
ro
rn
~
.~,
~
.~,
A
N
4)
!V ~
U
W
m ='~
Q
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w
a, q
w ro o
'-I a yi M N M N N rl H H ri N N N 1-1 N ri rl N ri H W N N rl M W M M N N H
rl N 'd'
O4
z
m ~^
~ N N
O' 0 o o 01 N r o M rl dr l11 U1 d~ ul 01 d'
O O O M V' M ~O l~ N M CO \O [~ N t11 W
O rl r1 CD ~O ~ N N V~ lfl rl r-I N N N r-1 d~ rl 01 t11 aD C l!1 l11 ry M 01
q N lf) 00 N M
O N dr 1f1 \O N rl ul OD LD rl N H I, aD T N Ul m
b V ~ p~ ~p ~O M O O~ ~O O
. . . N OD N
Or H , M IO fD O% N %O rr =-1 l- OD I~ M O O rl U) r
N N N ON N 00 I~ T N (1) N = M 1I1 N 'O d' N
ri M N
b =.1 = rl O M ri Ol V1 !~ rl ~O M H t- ON O~ m
.~ y.~ Q ri .~ rl r=i
$4
r11 d~
ro O v N ~ M ~ ~ ~ r1 N rl m aD r C ~ ~ OD r-1 O O N
~O W O~ t0 N
r-I lf1 (9 O 1 W ~D O vl M O VI M M tO r-I
O V~ r O1 = = I
V 1
N N N 0 . r t- N M M ~O Ol v N . N m V~ ~ w 1f1 ID N
N CD H ID M M O) rl
[.] r-I rl M N
GI N
01 r r
~ 0 U H V~ lfl 0
M r l0 M [, ON 1-1 1f1 [, 01 O N Ln 1f1 O UI O d~ l0 ~ W r q'
W rl ~O a0 N ~O C l11 C M 01 o N V= aD V~ (D 1f1
O C 10 I~ Ifl ri I O~ N l!1 1 1 I H N
a' O r-I W (V Ul M r-I O M r1 M O O N N = r ~ r-1 ~ N : m r-1 r rl
~==~ Q N M M rl '-I OD rl r N
N
W 0
O ,-i u
ro b
K) 1J 0 m W N cD c0 cD M w M M o N N oD N N O O r O O N O M V= ~O
M
op O O O O O O O O O O
~ ~ RC ~`-' M M ~ tO M M M Ol ~O O~ Ol Ifl 01 ~ O1 Ul C Ol w rl r-~ ~ lfl Ol
t0 (9 (3)
C.
q q 0
=ri A
b ro w kO 1f1 O% 10 H M o M Ifl N d~ N r o l!1 ~O r N N ON o [- l- ON M M d~
0 N [- dr 0 0 N N w V' h m O ~ N N M M ul m W H d' l- l- CD On O Ot l- N w w
,H L U P. M M rl M V' C r'r N rl rl N N v 'r N M M M M dr ul r-i r-i C ul l!1
l!1
A q w
1 a
m m o .,
O) fA 01 Q rl ~ a a LL W W rl N Q Q ~ 4 4 4 rl ri rl rl rl rl rl r1 N N N N N
N
u V1 w PO W W CC GQ f0 fU 01 Rl W W W W
~ aC ~ z z z z z z z z Z Z 0 a a a a a a a a a a a a a a a W a a a a
a
N to
4) ro
"i y 0 0 0 0 0 0 0 0
ry ~ a a m m m m m r - i m m m m H m rn~ m rn m m m m m m m=-+ m o, IH mi am
,a a
~
a (A w U ) q N ~ a Q w Hx~ Z w ~ H~~ J~4 w z> Q Q F E a
pC ~p a.~ ~ c 3~>4 Ena' Z 3Q Ua cn `qt ~ a ai 9 9W w w a E. tF7 [7 ~~y x A O
w w a H a[-~
=a ~ A J4 O H H F 0.] a t~A W a fk w 0 m u ~ a ~7 "~ W 8 a~C ~ Oa A
... A>wau`~wzuaaxaa~~xxaw~C w~,>wwwwa
F cn ~-+ cn cn > F 9 F 3 cn
,'E w F E. u1 > m u~ ul a1 - F F W
.] 0 Z 0 > ~-+ J4 Q U 5C JG F , U > u] >+ dq > >+ C9 A~44 F .] m ~G GS C7
a 3
rl N M ul OD m O H N v U1 I, W 0 rl N M W w O1 H N M 111 c} w [, 01 0 N Vr w
l,
,~ O O O O O O rl rl rl '-I rl .-1 r-1 N N N N N N N M M M M M M M M d' d= V'
=0' V'
~ M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M
r-i H r-i H ~ ~ ~ ri ri r-i 14 H H H H .-4 H r-i H H
w q 1D lD ~D ~O ~O l0 ~O 10 10 10 1D w w w ~O 1D lD lD \D 10 10 w w w l0 l0 w
tO w w w l0 l0
H 4 H H H H H H H ,~ ~ H r4 H H H .-+ H ,-4 H H H o
0
0
N
A
~
L
.~
C.
W
ro
rn
.,~
U
W d
J e
m
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U)
~
~ d 0
r-I d~
v~ m N M N N '-1 r-I N N N H N H r-i W d4 m H
~ A
N dp Z
OD
cn
AI
O 'C O O O 0 OM O d4 m %0 I- O~ N O r-1 W l0 N LIl Oo
0 r-i r-i r-I ~ ~0 N N d~ U1 H r- N r-I ri 4 Lf1 M 0 01
11==II
01 O
m x m
'd O N in ao
0 p N l0 N rl Lf1 00 ~ N 10 M O ~ O O
> =,~ O) rl m %0 ~ m N l0 H H [- p = OD N N
44 N OD ~,.~ N 01 00 ~ [, 01 N ~' d~ ~O ~ ~ N
S-I ',~ * rl rl U1 l' rl l0 m 01 H t11
ro :J
ol
1 O H lll
(~ Fpi L O W N ~ M ~ r-I O Oo ~ O OD rl
N o ~0 d~ i w i m N r- oo i D = = O i i H
U1 * r-i N 0 l- ~ r-1 M Lll ~ d~ 01 d' N
vi
.~ o
N
W p H ~ rn
M O r ~0 M l~ 01 l- M O N Lfl 'n w I OD d4
H o O %O 00 N D l- U1 ~ v ri M ~ 01 N ~ ~ ~-I N
~ d' N lfl m r-i ~ M O O N N H N : rl rl
~ N M m r-I r-I N r
W
O O
~
~ o G
~ ~
0 "' Oo 00 O N oo 00 M M r~1 O N O N N 0 O O O O
~S k ~ M M ~O M M Oh 01 01 lfl O~ O m ~ O N O O O ~
~~ r-I r-i r-I r-i r-I r-i
01
'd .A a~
4'' y lp Lf) 0) kO H 0 M Lfl N l- N O% Ol M d4
A ~ a O W 0 N [- 1 O O N 'd~ l- 0 O ~ N M O L- ~ [- N ~O
r d1 M d~ r-1 N O rl M r'1 d~ M M d~ d~ lfl Ul
M M
U
N N 1+ ~4
m O
~ LL v] =a
0
o z z z z z z z a a a a w w a a a
N m b a
a a) .,~
=y~,~j ~ (1) 0 O O O 0
rl Ila R' a rn rn rn m rn~ m rn rn~-+ ~ m rn m rn mH m m
o a M
0 ~+ a ~ ~+ >H >H aI ~H ~H
r-1 a A u) ul H a C*4 H C7 DG C-4 q H a
x m d (1) u 3 a z A q a ~~ L7 u~i C~7 [- [-1 o
v w C7 a H z a> w w w2: H H Ea o
A H u a a M w a x cn u(n w~ a~ A 0
E ~+ u a v Zi uU) HE+ ' H a H ai a w~ H a N
M a ~ H~ A
`=' a cn a C7 C7 H FC x x E H u ~ x ~ ~ p od 0 3 A
2^
.F
~
rn
~
c
cn
W
J c~
H '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~.
v
+c
r-1 :3 iy~ U1 d~ m N N r-1 L!1 M d~ d~ m V~ ~i C UI d~ d~ d~ d~ l71 M C U1 d~
d~ d~ tf1 d~ V~ d~ m V~ d~ V~
~tlO
o u
z
N lp r \O M
H H r M ui r r+ m r n'+ m r w r H r n r
m m V' r-I d~ 01 01 M Il1 O M m m N M r m r-I O p N V~ r IO ~ l0
i0 M 0 lfl O~ sM IO I m m m Ul 01 . Ifl r 0 0 d~ m N M rl N H M v
H m OD rl m OD l0 H rl N tfl N M ~ H aD M M m dp m
m o r
Ln m w H H tD m 01 ~ U1 m V~ r M m r H m r N H m d~ N OD N H O~ f'1
N N r m N O r r W N M M . (`=1 . . o p . . . H . r r H m O
0 1f1 O ri . = U1 lll U1 O% ri M O d~ M m
'O Q O H N H H H O H N IO l0 01 d~ %0 01 H r1 M H 10 -W l0 m '=I ei N
~ O
~ i
.=~ M
ro W
~ N N df CO r d~ r O' N m N V~ O r W l!1 O m tl1 M M H H m H m o O% ~ 1I1 O m
M UI O%
CL O = = = m CO r ~ N 10 = o m ri = r N = = = ~ II1 = N = r-1 = .-1 rl ri M =
~
.~ i N U) ~ H m O= m p ri rl N N rl N N M V~ r M M H
.m
V N
(d N N m ry O l0 M r, l(1 ~ O M O~ N. r m N O M ~O ~ ~ M 1I1 1f1 ~ N rl r=~ V~
H r 0
N = = ao ao w , . . . . . . . . . w . ,1
H 0 m ri lfl H M r w ry H Ln N H r. ri N N o HM O N M m ri M N r-1
~ Q
~--~
aD W H
ln V. r ~. ~^i r m M m m o O~ d~ ~ ~ 1I1 m r l0 r
N r O m O W O rl N H m m ri m m 1I1 m r ~U
~ H O H H 01 m l0 = M .-1 = C = ry r = = ~M r-1 N d~ N = = M = ri r-1 d~ M =
V~
M H m r-1 r ri l11 N d~ ~D 1f1 O Ifl lIl OD l11 r r-1
W
0
~
~ u
ro
(d 01 M M O% m 01 01 O1 Oi m 0 v m m m m m m M m m m d' p O d~ r rl lfl lfl M
m V1 CO
ro U:p rl rV r-I r-I r-I rl H H ri ri Ul d' rl H rl ri rl ei H rV rl H 01 0 0
O1 00 m H rl N (+1 H m
0)
O
O r Ol M M N N N r m r-1 N T m Ol Ifl
~-1 M aM d' d' d' 10 10 ~O ~O m 01 H tD N N d~
O IO d' m d~ d, V~ ul U1 lf1 U1 \0 %O M M N 1f1 M
A O ~y rl rl H H .-1 .-I r-I Ol ol O~ M d' Ul Il1 1f1 1f1 Ul lfl l11 Ul U1 Il1
Ul tll H m H N N N rl rl N
U
Cl) ~4
y) fO =4 r~ ~G ri r1 r~i r-I 4F~ , R, R,
$I O N7 7 !.7 7 7 7r
~ ~4
W a
Gl ~ 0 p p p p O p p
a m m rn m m~ m m m m~ m rn m.-~ m m~.-+ m m m m m~ m m m~~ m m m m
(V
O
~i r~ r1 > c7 >E+ >
~ > E. w H w~ m u~i v~i ~~ ai vFi a~7 ~.~7 w a a.z7 a Fr!~~ w>~.7]
O W w w ~ H ~ a H ~i H c fw) a a ~ ~r~ u wi w w > W F 4 U r.~ 4 i-+ F CL U
7 C7 u m . Q u u
x v 4 H a a i-a a
CQ7 H H H H .' -l w H y N w a Z q
H O
a a H H > H a H a a a Q ~ a a a H H u aHl ~ H a a 4 4
p1 E. H H H a H ~H w w w w U1 >+ H 4 F4 H U1 F4 a V= H w w a x C,~ u C:
0
N
A
~
U
Ci
W
W
ro
rn
.,,
v
a
.,,
A
w
N
U
W b
J q
m
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w ~
w
r1 ~ y, -w U1 -w d' d' Ln U1 U1 m d' Ifl U1 -w l11 tf1 m V1 w v d' d' Il1 aM
Il1 U1 v In
ro A o
O V
z
M U1 m
O M H O 01 v l, ON ri m 10 rl O1 Q1
M I O N d' ~ M N ~='~ H = I i r ~ ~ m M m [' a1 r-1 [, h O V~ r-I d~ ~O U1 M
7D Ol M U1 M r~l OD ~0 M l~
= = t
~O N 10 O I- lll N r-I m r r-I d' ri m ~M oD lD In i a0 M M O T d~ 0
t r -I
N lfl m r-1 ~ r-I ~ r-I ~ M ri Ul -1 1l1 U1
a)C ~
fi ~ O U1 l= O n O l~ O r-I 01 O ~D 01 GD l!1 a0 l~ M 1f1 M M O~ rl rl d~
Q N 01 Ol d~ Ol l = 01 = m l~ M = M m = d' M r-1 O~~ l~ . . pp . O . . . . .
I= = M
H x o = r ,- C C r-1
H '.{ .-~ r O N r 10 r-1 ~ M r-1 O~ M H ul M N M d~ d~ H N M
cn
=~ M
b w
a) ~4 N 0 00 N l' l' GD N ~ N N M M 10 W N f.,~ M [, O aD 0 l!1 '=1 H \D ~ O N
O 10 N
a O = O ri ~ ri O = N O .-1 ' ' ~ . N . m pp . . . . H . H rl
.~ lfl N H f=1 N N d~ H ~O N M H N d~ H N lD l~ .-~ N N N N l!1 H N
{a L =C
..m~
I~ 4= N
b 0 H a0 01 r-1 W l17 ~O O1 l~ M r O~ r-1 M V~ M r-1 n ~ o ~ r N N d~ rl . . ,
. . d~ , (='1 O O1 . . l0 M '"I IO 1f1 H , M M
O ~ M M ri ~O M M 01 r-1 O 0 v O ~O Ul H / H O N d~ M O lf1 O U1 ID M H -0
~ Q
~ 44 rl
l() (y
N Ul d' OD 0 v M Ul OD N Lll 0 v V1 10 OD O~ aO r=I 10 Ul ~D lD ~==I . . OD l~
V~ M OD %O UI OD
00 , . . N N
H 0 r-1 l, N = N N H 0 ' N OD = d~ H M N N N IO aD 0 H r
F V~ H M V' 1 N W M d' M I~ U1 l~ M d' CD CD ri m H
w
0
~
ro
+.) ro
111 !fl U1 O M M 10 k0 kO M o O o N 0 0 N N 0 0 0 1 1 0 1 U1 0 0 0 0 d' w l~
>
yj de O O O O O O O O O O O O O O
~,,,, ri rl .--1 Vl 01 OI CO W M O, ~-i rl .==I rl r-I m m rl r~l r-1 ~O l0
rõI ~D N rl r-1 r=1 rl ~O m Ill
0) O
~ u
W a0 w V' U1 l' a0 O 00 N M lD O 0 M 1f1 d~ M r N 1, rl ID Ill V1 M .-1 'd~ m
m 10 ~0 O
m v N NH I~ lH W M 'D O O k0 IW l11 ri O% Ol r-1 O -W N 01 -0 W M
A O O l(1 V~ Ul [~ O lf1 O~ H H
a V~ M d~ H N M v H N fV m M M -w lf1 L(1 w rl -w -w w
U
m ~4
O G
~ ri r=I '-1 r-I N N r-1 ri ri ei r~ r-I rl ri ri ri rl rl N N N N
; ~ m m ro w w w m m w w ao aa ao ro ao
y~ o z z z z z z w z 0 z z z cn z m m w
z a a a a a a a a a a a a a a a a a a a a a a
N w
W
0~1 v o 0 0 0 0
a m m m m m m .-1 m m m m r=1 m m m r-1 m m Om 01 m (h o, rl 01 Om Om Om 01 .-
1 Ol 01 01 Om
N
O
~ a H ',1 a a a 7 .~ ~J a w H H H fH!] a a .7 =J H 4 =/ a H w 4 H H
v~ a~r m w~ x O ~n a a w w ~n a z r~ w 9 w~~ m cn w w a a x a
~ ~ a 7 ~ a w i- ~-l w d 1-1 a SG ~ a a ~ w H a F4i H H (aJl
x ,c o ~n u a u u w~~~~ u w w a~!A w a a aG c~ ~ u)
~ z~ u z~~~ a a aca~ a s z~~Z H~ Q a~ w Q Q a Z
o
w u ~ wl a w H W w w a ~ w !j a a w w ~
0
N
A
~
U
~=
.~
W
W
ro
L'
.~
~
.~
A
N
N
N
~ ro
u
W =v''
J C
co
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v C
v 'd o
="'~ yi M V' L!1 d' V' v L11 d' d' zN d- d' w Ul Ul LIl m V' Lfl lf) -w lfl
lfl M lfl
f0 O
A o
O
~ Z V
0
M N kO f~1 M L(1 M
N 0 H ~ 0 N W I- f=i Lfl l- r-I O 01 01 OD N r N O V' l- CYN M
OD M d' d' H [- 01 10 t- 01 f- O d' 10 00
lfl M 01
ro 10 ~ 00 N M '=i N 'd' t, Lfl r-I l~ I~ M r-I V
~
Ul H OD M ~ M M r-i v N 1-1 ~ r-i ~ r-i r-I M d'
>
0 N N M Lfl l~ N H 01 v N rl M N [~ O O r M I~ M O ri 01 r ~ 00
rl 0 v 0 Ln Ln M ~ w ~ ~o rri ~ oo rn ,~ r ~n M M %o H ~
U =rl O
W
~4 M
ro ro ~ N ~ O W A M 0 O~ ~ M 01 N N l- I, OD N N N M M 10 w N
Q+ 0 O = N = ~ = H M ~0 d' H C. ' N O rl w n N '
V~ N
> ~+ O r-1 N M H ri ('~1 N N V~ ~O N M H
=rl (~ FCC
O
N
0) O
LO x N M 01 M 00 lf1 l11 ~ N r-I O 01 H dD U1 l0 m
.. r M w w m r ~O
r-i
(~ U O O ri M M c~ M ~-I M H 10 M M m Ln r1 M O V O kO u) H
N O ~==~
r I
4) O M d~ w 01 L(1 OD l~ M L!1 OD L(1 00 O~ 00 H
Q~ rl N H W Ifl tO 01 OD N tfl , d~ , w ,
Ul lfl M lll 00 r41 ~ [- N ~ M N N H tf) d~ N N OD M
rl
W 4
q
~ H
b
0
W ~o V~ V~ O O d' l- ~-I OD 00 O M ("1 l0 1p ~p M O O O N O O N N
O N lfl -W m ~ ~ 01 00 M M M lf1 01 O~ Op pp M p~ O O O m O O ro ~ ~
rt ro 0
1-) b
b N N O l~ CO 0) r-I ~O 00 l!1 Lf1 !- OD O v OD N M W O kD N1 Lfl V'
0 w d' M M N m L, O Ul 01 N l- I- DO (`1 w
[: O1 LL M Il) l!1 M H r-i N (N M d' 1-1 ~ ~-i r-I rl O~ I, OD N M v
O p -.i
N m ,dj rl H rl rl N a' 4 4 a a W(n
0 z z z z z z z z z z z z z a a a a a a
a
R, p a~i rn rn m rn m rn rn rn m rn m rn m m rn rn rn O N rn rn
?+ .=~ a
,yJ A
14
N
~ b C C4 H a H 'J H H a '~ a a a > > > a w H F-I H (H!] /si ~'i f~.'
C7 z H a w a r] R+ E+ 0 u] w 3 x Ul a 7 Ct+ w CA r7
41 v a 0
F Cu F [-~ x a f~ G. ~ ~ fy a a X a a ~ -l Cr. x a 0
a 6 H H w> w H a U H U
41 v FC S > CI. > > f i a a Vl U a 0 U Cu rC a a L] o
o w A H a~ P7 H uHi w z a z~ n~ aaa
rj GG a~ N xa 3~ z N
a a a H I--1 a a H a a a a H ,'~ a ra rJ H ^
Ch [Tl a C7 ~+ c0 FC x U U a Cr C=. a fs. H [r4
41
H
f0 C~j d~ r-i N M lfl V' lfl l0 l- W 01 O r-i 10 N M V' lfl [, ,O I- CO CO 01
O
y 7pp Q M O O O M O O O O O O r-I ri f~ rl rl r-I rl M H H H M r.1 N W
01 01 O~ O~ O~ 01 01 O~ 0~ 01 01 O; O~ O~ O~ 01 O~ 01 O~ 01 O; W
rl w O 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 O 0 0 O (o
W o w w w w w w w w w w ~ w w w io w w to w %D w w w to
01
b
O
.H
A
N
U)
~ 41
ro
U
W ri
J q
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
i 0
v d 0
w a ;p ui v in w u) w 4- v q, w a
ro 0
Q o
o
~ z
0
M N
N O ~ ri (n N w r=1 -i !- 1 i m 0) i
aD M t!1 M H w . I I M r,
$I ~ K4 ~ ~ ul ~-i ~ f'~1 ~ V~ lvfl lOfl
41 +
~ H rl
N Ol OD Lfl CO O L, lfl M M 01 v
00
~ U a) 0 01 M ri L() v M -;v r-i N M
01 W
~ M
N M M l- O 00 l11 l!1 H %O ~ O N O w N
oo
> 0 N %D l~ r=I H N H N N lfl rl N
O
~
~ If1 N 0 ri H ~ M d' r+l ~-i M r O M ~ t11 M
b V~ M M = . . , H , M
0
ri '~ N d~ M O L!1 O U1 ~ M rl O V~
ro ~ H
N ri N l0 O lfl l0 w H 00 d' M CO 10 Lfl 00 N N
= N = = ~0 OD 0
.~ ro 0 M ~ l, Ln N ~ l, -W OD OD rl M r=I r-I L~
~
W F:4
~ ~==~ {..~r N
O
y4 .,~ w o 0 o r r 0 ~ 0 0 0 o d kD r
4J U O O O ~ w O w O O O O w M ~
0 .ri = r-I ri r-I ~-i r=I r-I r-I r-I i-I
0
ro U
~ ro
b tA N l~ H w l!1 M H V M m W l0 O
O M r 00 l0 d' U1 H 01 H O d~ N O~ M
ro a OD r-I N N M M d' Ln Lfl ~O rl d' d~ ~0 U
=,~ r-i :3 U ql
r-1 r-I r-i N N N N
Li N ~ rl ri r-I ri '-I ri rl H
pq ! Cl LO W W Ln Cn CQ f 0 P~l W [ 0 CO W CY1
rl 0 0 a a a a a a a a a a a a a a a
A ,y
a m orn rn rn m rn orn om orn m rn rn rn
r+ a
y-) A
N m
U > > > a H C>ci a H H > (.,'
.+ G w r24 w a H M 0 w rJ4 a a x a H o
1, yC[- H a' x a z4 w w a 0
N ~ ~ CaL W ~ ~ (/] a a H H H m 0
N a ~ C] w A 0 Cs. A m~ Z U 3 w N
~ a~~ a`~ ~ a a a a a A
u a a r~ a w~C x a w r~ E
a fd ~ r-I N M V~ lfl O1 10 [~ 00 Ol O O rl N M
~y ~y ~ Q N N N N N M N N N N a' M M M M W
(A m O) m ON O) O) O) 01 ON Ol ON 4-4
O O O O O O O O O O O O O O O ro
N Q' ko w w w w w w w w w w w ~ w w
.~,
rc$
r:
.~,
~a
N
N
~ 41
T ro
J G
m ='~
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N
N p
.--I
O y~ m tfl V~ d' Ln d~ dp a~ d' w w Ln L(1 m a~ ln Ln Ln l!1 W tn d~ V~ V~
O U
z
O m (+1 ul
O1
m O rl Ifl [, 1-4 O 01
01 00 d~ [~ N M ri [~
Ll1 10 [,
aD M O rl [- O~ ~D L- O) [, O H d' m
lll OD N N H r m ~==I N ~ d' I, Ul ~ M ~-1 l0 0 0
ww N m r-I m d~ M M r-I V' N '~ rl N r-i rl lll
14 Q r-I r=I r-I
QC)
Iq %D
e=I 0 ~ N M U) l~ N r-I cM r- OD Ln t~ O O M l~ M O M r 00 lJ1 M r ri M
V x 0 N lfl m r-I VO 10 M r-I O m r-1 l~ ll1 L~ M 10 d~ '~ M M d~ N M
01 ,d ~ rf
b Q) W
'1i M
> ~4 0 N ~ CO ~ M 01 O1 m ~0 d~ N O l~ N M ~ N N 1M [~
p0 a O O rl N N M d' H ri r-I ~ M N N 0 -1 kD N rl 'r r1 r-i N N r-I N
ro VI JJ
q ~
N
[.] 4) o N 01 M CO lfl ~ aD ri 0 O~ ri CO U1 10 r 10 01 l0 M M
Li N U ~ 0 [~ 0 rl m C; N m r-i M rl 10 M M Ln r=1 M 0 O 10 0 lfl r1 0
(d
w 14
N
(p co O M d' ko H LI N 00 m lfl CO 10 [- 01 d' U1 W lfl , OD t!1 CO 01 ll1 W M
l!1
rl d~ . . . . . N N
0 O ~ . . . . . m cM r N = N rl L!1 O O
d~ (N CO l- W CO m
ri lll O= l!1 LIl CD ~--I r-I M d,
F4
0
fd 'C7
1-) b [.
f0 'd
N O v w O O r ~..i ~ op O m M 10 tp M O O O O O O O O r~ O
b op O O O O O O O O O O O
l!1 d' 01 Ca m m m 0 01 Ol f0 N O~ H
rl ~==1 r-I ~--I r=1 ri r-I ~ rl
W
s"+ g C~.
0
O y U
0 Ol O I~ CO Ul l!1 I- CD CO N m M N m H m kO 0
LI 0 10 d' M OD 01 r-I "0 N M L, O lfl O~ N L, I- CO l0 r-I O 01 d' M
(1~ ~ a M d' U1 L71 m H
H N N m d~ H r-1 rl 1=1 O N rl -ze ll1 r=I dl 1O
~ b U N
a r-I r-1 rl N N H rl ~==I N N N
A m " r1 ~~~ N r~ rC r~ a a a m u) v) m v~ m w a1 a1 w w
~~~ ~ x~ E~ E z z z z z z z z z z z a a a a a a a a a
~ b +'
y 4+ a
P.
0 N a a ~ rn m m~ rn m rn rn m 0) rn rn 0) rn rn rn~ am rn rn m rn m
o a M p
'J
JOJ w R~ a H F-I a a a a a w H I==1 CHlI a a H
ri ~4 0 w~ Vm w m a a w CO a C7 w a a p
~ w w w a u H u a u W a a Q ~ w "~i 0 A
=ri 1) .~ > r'~ ~"'~ ~4 Ri fn Fl', a z
=.pl ~ ~ C~7i4 A F~~7 w 0i CHp W Z W z 1340 U~.] O~ FC ~ Q~] RC A~ Ol ~
v }." -.i N a a a H ~'=~ ~ x V V S a a a H w ~ w a Ra~. ~ a a
U) C7 w a~ u~ a w ~
w
w
rn
C
ro
a
A
N
Ol
~ 41
ro
W v
m
CA 02658559 2009-01-20
163
WO 2008/039267 PCT/US2007/016529
TABLE 18
DR1
1/20/2006 Strain PIC+
Protein A/Viet Nam/1203/04 Pos Sequence Len Motif DR1 PIC (<100nM)
NA H5N1 16 VTGIVSLMLQIGNMI 15 DRp16 24 1
NA H5N1 62 SVKLAGNSSLCPING 15 DRp16 4.4 1
NA H5N 1 71 LCPING WAVYSKDNS 15 DRp 16 22 1
NA H5N1 126 NGTVKDRSPHRTLMS 15 DRp16 53 1
NA H5N1 135 HRTLMSCPVGEAPSP 15 DRp16 94 1
NA H5N1 140 SCPVGEAPSPYNSRF 15 DRp16 13 1
NA H5NI 167 GTSWLTIGISGPDNG 15 DRp16 8.1 1
NA H5N1 196 IKSWRNNILRTQESE 15 DRp16 19 1
NA H5N1 201 NNILRTQESECACVN 15 DRp16 17 1
NA H5N1 289 NLEYQIGYICSGVFG 15 DRp16 24 1
NA H5N1 315 CGPVSSNGAYGVKGF 15 DRpl6 1.0 1
NA H5N1 334 GNGVWIGRTKSTNSR 15 DRp16 7.7 1
NA H5N1 379 WSGYSGSFVQHPELT 15 DRp16 38 1
NA H5N1 384 GSFVQHPELTGLDCI 15 DRp16 51 1
NA H5NI 389 HPELTGLDCIRPCFW 15 DRp16 19 1
NA H5NI 403 WVELIRGRPKESTIW 15 DRpl6 44 1
NA H5N1 413 ESTIWTSGSSISFCG 15 DRp16 84 1
NA H5N1 422 SISFCGVNSDTVGWS 15 DRp16 57 1
NA H5N1 4 NQKIITIGSICMVTGI 16 DRp16 1
NA H5N1 12 SICMVTGIVSLMLQIG 16 DRpl6 1
NA H5NI 26 IGNMISIWVSHSIHTG 16 DRp16 1
NA H5NI 92 GDVFVIREPFISCSHL 16 DRp16 1
NA H5N1 110 RTFFLTQGALLNDKHS 16 DRp16 1
NA H5N1 21 SLMLQIGNMISIWVSHS 17 DRp16 I
NA H5N1 183 VAVLKYNGIITDTIKSW 17 DRp16 1
CA 02658559 2009-01-20
TABLEW~OJ2008/039267 164 PCT/US2007/016529
(1) NP DRI Supertype Sequence
Protein Strain Acc. No. Pos Sequence Core sequence DRI PIC String red
Conservancy
NP H5N1 AF036359 1 ***MASQGTKRSYEQ MASQGTKRS 0.13 M????T??? 0 8/11
NP H2N2 M63752 1 ***MASQGTKRSYEQ MASQGTKRS 0.13 M????T??? 1 8/11
NP H5N 1 AF036359 10 KRSYEQMETGGERQN YEQMETGGE 201 Y????T??? 0
NP H2N2 M63752 10 KRSYEQMETDGERQN YEQMETDGE 494 Y????T??? 0
NP H5NI AF036359 36 VGGIGRFYIQMCTEL IGRFYIQMC 619 I????I??? 0
NP H2N2 M63752 36 IDGIGRFYIQMCTEL IGRFYIQMC 619 I????I??? 1
NP H5N1 AF036359 39 IGRFYIQMCTELKLS FYIQMCTEL 37.1 F????C??? 0 10/11
NP H2N2 M63752 39 IGRFYIQMCTELKLS FYIQMCTEL 37.1 F????C??? 1 10/11
NP H5N1 AF036359 40 GRFYIQMCTELKLSD YIQMCTELK 55.0 Y????T??? 0 10/11
NP H2N2 M63752 40 GRFYIQMCTELKLSD YIQMCTELK 55.0 Y????T??? 1 10/11
NP H2N2 M63752 52 LSDYEGRLIQNSLTI YEGRLIQNS 102 Y????I??? 0
NP H5N1 AF036359 56 EGRLIQNSITIERMV LIQNSITIE 42.7 L????I??? 0 8/11
NP H2N2 M63752 56 EGRLIQNSLTIERMV LIQNSLTIE 37.1 L????L??? 0 1/11
NP H5N 1 AF036359 57 GRLIQNSITIERMVL IQNSITIER 15.5 I????T??? 0 8/11
NP H2N2 M63752 57 GRLIQNSLTIERMVL IQNSLTIER 7.67 I????T??? 0 1/11
NP H5N1 AF036359 61 QNSITIERMVLSAFD ITIERMVLS 3.90 I????M??? 0 8/11
NP H2N2 M63752 61 QNSLTIERMVLSAFD LTIERMVLS 4.02 L????M??? 0 1/11
NP H5N1 AF036359 63 SITIERMVLSAFDER IERMVLSAF 11.1 I????L??? 0 8/11
NP H2N2 M63752 63 SLTIERMVLSAFDER IERMVLSAF 11.1 I????L??? 1 1/11
NP H5N1 AF036359 78 RNRYLEEHPSAGKDP YLEEHPSAG 238 Y????P??? 0
NP H2N2 M63752 78 RNKYLEEHPSAGKDP YLEEHPSAG 238 Y????P??? 1
NP H5N1 AF036359 79 NRYLEEHPSAGKDPK LEEHPSAGK 156 L????S??? 0
NP H2N2 M63752 79 NKYLEEHPSAGKDPK LEEHPSAGK 156 L????S??? 1
NP H2N2 M63752 100 YKRVNGKWMRELVLY VNGKWMREL 315 V????M??? 0
NP H5N1 AF036359 104 DGKWVRELILYDKEE WVRELILYD 480 W????I??? 0
NP H2N2 M63752 104 NGKWMRELVLYDKEE WMRELVLYD 971 W????V??? 0
NP H5N1 AF036359 105 GKWVRELILYDKEEI VRELILYDK 1704 V????L??? 0
NP H2N2 M63752 105 GKWMRELVLYDKEEI MRELVLYDK 201 M????L??? 0
NP H5NI AF036359 111 LILYDKEEIRRIWRQ YDKEEIRRI 30.5 Y????I??? 0 6/11
NP H2N2 M63752 111 LVLYDKEEIRRIWRQ YDKEEIRRI 30.5 Y????I??? 1 3/11
NP H5N1 AF036359 133 TAGLTHMMIWHSNLN LTHMMIWHS 139 L????I??? 0
NP H2N2 M63752 133 TAGLTHMMIWHSNLN LTHMMIWHS 139 L????I??? 1
NP H5N1 AF036359 136 LTHMMIWHSNLNDAT MMIWHSNLN 1.20 M????S??? 0 3/8
NP H2N2 M63752 136 LTHMMIWHSNLNDTT MMIWHSNLN 1.20 M????S??? 1 3/8
NP H5N1 AF036359 138 HMMIWHSNLNDATYQ IWHSNLNDA 15308 I????L??? 0
NP H2N2 M63752 138 HMMIWHSNLNDTTYQ IWHSNLNDT 30078 I????L??? 0
NP H5N1 AF036359 148 DATYQRTRALVRTGM YQRTRALVR 266 Y????A??? 0
NP H2N2 M63752 148 DTTYQRTRALVRTGM YQRTRALVR 266 Y????A??? 1
NP H5N1 AF036359 154 TRALVRTGMDPRMCS LVRTGMDPR 124 L????M??? 0
NP H2N2 M63752 154 TRALVRTGMDPRMCS LVRTGMDPR 124 L????M??? 1
NP H5N1 AF036359 159 RTGMDPRMCSLMQGS MDPRMCSLM 14.2 M????C??? 0 11/11
NP H2N2 M63752 159 RTGMDPRMCSLMQGS MDPRMCSLM 14.2 M????C??? 1 11/11
NP H5N1 AF036359 166 MCSLMQGSTLPRRSG LMQGSTLPR 3.39 L????T??? 0 11/11
NP H2N2 M63752 166 MCSLMQGSTLPRRSG LMQGSTLPR 3.39 L????T??? 1 11/11
NP H5N1 AF036359 167 CSLMQGSTLPRRSGA MQGSTLPRR 1.30 M????L??? 0 11/11
NP H2N2 M63752 167 CSLMQGSTLPRRSGA MQGSTLPRR 1.30 M????L??? 1 11/11
NP H5N1 AF036359 183 GAAIKGVGTMVMELI IKGVGTMVM 0.028 I????T??? 0 3/11
NP H2N2 M63752 183 GAAVKGVGTMVMELI VKGVGTMVM 0.041 V????T??? 0 4/11
NP H5N1 AF036359 186 IKGVGTMVMELIRMI VGTMVMELI 0.21 V????M??? 0 3/11
NP H2N2 M63752 186 VKGVGTMVMELIRMI VGTMVMELI 0.21 V????M??? 1 4/11
NP H5N1 AF036359 189 VGTMVMELIRMIKRG MVMELIRMI 0.39 M????I??? 0 6/11
CA 02658559 2009-01-20
TABLEW y2008/039267 165 PCT/US2007/016529
NP H2N2 M63752 189 VGTMVMELIRMIKRG MVMELIRMI 0.39 M????I??? 1 6/11
NP H5N1 AF036359 191 TMVMELIRMIKRGIN MELIRMIKR 5.03 M????M??? 0 8/11
NP H2N2 M63752 191 TMVMELIRMIKRGIN MELIRMIKR 5.03 M????M??? 1 8/11
NP H5N1 AF036359 196 LIRMIKRGINDRNFW MIKRGINDR 23.6 M????I??? 0 10/11
NP H2N2 M63752 196 LIRMIKRGINDRNFW MIKRGINDR 23.6 M????I??? 1 10/11
NP H5N1 AF036359 217 RTRIAYERMCNILKG IAYERMCNI 152 I????M??? 0
NP H2N2 M63752 217 KTRIAYERMCNILKG IAYERMCNI 152 I????M??? I
NP H5N1 AF036359 257 EDLIFLARSALILRG IFLARSALI 1.38 I????S??? 0 11/11
NP H2N2 M63752 257 EDLIFLARSALILRG IFLARSALI 1.38 I????S??? 1 11/11
NP H5N1 AF036359 258 DLIFLARSALILRGS FLARSALIL 4.49 F????A??? 0 11/11
NP H2N2 M63752 258 DLIFLARSALILRGS FLARSALIL 4.49 F????A??? 1 11/11
NP H5N1 AF036359 259 LIFLARSALILRGSV LARSALILR 5.79 L????L??? 0 11/11
NP H2N2 M63752 259 LIFLARSALILRGSV LARSALILR 5.79 L????L??? 1 11/11
NP H5N1 AF036359 264 RSALILRGSVAHKSC LILRGSVAH 8.35 L????S??? 0 11/11
NP H2N2 M63752 264 RSALILRGSVAHKSC LILRGSVAH 8.35 L????S??? 1 11/11
NP H5N1 AF036359 265 SALILRGSVAHKSCL ILRGSVAHK 34.1 I????V??? 0 11/11
NP H2N2 M63752 265 SALILRGSVAHKSCL ILRGSVAHK 34.1 I????V??? 1 11/11
NP H5N1 AF036359 266 ALILRGSVAHKSCLP LRGSVAHKS 0.81 L????A??? 0 11/11
NP H2N2 M63752 266 ALILRGSVAHKSCLP LRGSVAHKS 0.81 L????A??? 1 11/11
NP H5N1 AF036359 270 RGSVAHKSCLPACVY VAHKSCLPA 538 V????C??? 0
NP H2N2 M63752 270 RGSVAHKSCLPACVY VAHKSCLPA 538 V????C??? I
NP H5N1 AF036359 280 PACVYGPAVASGYDF VYGPAVASG 1.10 V????V??? 0 3/11
NP H2N2 M63752 280 PACVYGPAVASGYDF VYGPAVASG 1.10 V????V??? 1 3/11
NP H5N1 AF036359 281 ACVYGPAVASGYDFE YGPAVASGY 1.07 Y????A??? 0 3/11
NP H2N2 M63752 281 ACVYGPAVASGYDFE YGPAVASGY 1.07 Y????A??? 1 3/11
NP H5N1 AF036359 296 REGYSLVGIDPFRLL YSLVGIDPF 673 Y????I??? 0
NP H2N2 M63752 296 KEGYSLVGIDPFKLL YSLVGIDPF 673 Y????I??? 1
NP H5N1 AF036359 298 GYSLVGIDPFRLLQN LVGIDPFRL 15.9 L????P??? 0 6/11
NP H2N2 M63752 298 GYSLVGIDPFKLLQN LVGIDPFKL 9.34 L????P??? 0 5/11
NP H5N1 AF036359 301 LVGIDPFRLLQNSQV IDPFRLLQN 77.1 I????L??? 0 6/11
NP H2N2 M63752 301 LVGIDPFKLLQNSQV IDPFKLLQN 37.1 I????L??? 0 5/11
NP H5N1 AF036359 307 FRLLQNSQVFSLIRP LQNSQVFSL 25.0 L????V??? 0 4/11
NP H2N2 M63752 307 FKLLQNSQVYSLIRP LQNSQVYSL 22.4 L????V??? 0 3/11
NP H5N1 AF036359 313 SQVFS,LIRPKENPAH FSLIRPKEN 231 F????P??? 0
NP H2N2 M63752 313 SQVYSLIRPNENPAH YSLIRPNEN 508 Y????P??? 0
NP H5N1 AF036359 328 KSQLVWMACHSAAFE LVWMACHSA 0.39 L????C??? 0 9/11
NP H2N2 M63752 328 KSQLVWMACNSAAFE LVWMACNSA 0.66 L????C??? 0 2/11
NP H5N1 AF036359 330 QLVWMACHSAAFEDL WMACHSAAF 21.1 W????S??? 0 9/11
NP H2N2 M63752 330 QLVWMACNSAAFEDL WMACNSAAF 52.0 W????S??? 0 2/11
NP H5N1 AF036359 331 LVWMACHSAAFEDLR MACHSAAFE 93.9 M????A??? 0 9/11
NP H2N2 M63752 331 LVWMACNSAAFEDLR MACNSAAFE 46.5 M????A??? 0 2/11
NP H5N1 AF036359 338 SAAFEDLRVSSFIRG FEDLRVSSF 190 F????V??? 0
NP H2N2 M63752 338 SAAFEDLRVSSFIRG FEDLRVSSF 190 F????V??? 1
NP H5N1 AF036359 347 SSFIRGTRVIPRGQL IRGTRVIPR 14.7 I????V??? 0 5/11
NP H2N2 M63752 347 SSFIRGTKVIPRGKL IRGTKVIPR 7.05 I????V??? 0 1/11
NP H5N1 AF036359 353 TRVIPRGQLSTRGVQ IPRGQLSTR 31.3 I????L??? 0 5/11
NP H2N2 M63752 353 TKVIPRGKLSTRGVQ IPRGKLSTR 88.8 I????L??? 0 1/11
NP H5N1 AF036359 358 RGQLSTRGVQIASNE LSTRGVQIA 4.37 L????V??? 0 6/11
NP H2N2 M63752 358 RGKLSTRGVQIASNE LSTRGVQIA 4.37 L????V??? 1 5/11
NP H5N1 AF036359 371 NENVEAMDSTTLELR VEAMDSTTL 10.5 V????S??? 0 1/11
NP H2N2 M63752 371 NENMDTMESSTLELR MDTMESSTL 0.54 M????S??? 0 1/11
NP H5N1 AF036359 374 VEAMDSTTLELRSRY MDSTTLELR 206 M????L??? 0
NP H2N2 M63752 374 MDTMESSTLELRSRY MESSTLELR 637 M????L??? 0
NP H5N1 AF036359 385 RSRYWAIRTRSGGNT YWAIRTRSG 50.6 Y????T??? 0 10/11
NP H2N2 M63752 385 RSRYWAIRTRSGGNT YWAIRTRSG 50.6 Y????T??? 1 10/11
NP H5N1 AF036359 406 AGQISVQPTFSVQRN ISVQPTFSV 0.064 I????T??? 0 8/11
CA 02658559 2009-01-20
166
TABLEW y2008/039267 PCT/US2007/016529
NP H2N2 M63752 406 AGQISVQPTFSVQRN ISVQPTFSV 0.064 I????T??? 1 8/11
NP H5N1 AF036359 408 QISVQPTFSVQRNLP VQPTFSVQR 42.7 V????S??? 0 8/11
NP H2N2 M63752 408 QISVQPTFSVQRNLP VQPTFSVQR 42.7 V????S??? 1 8/11
NP H5NI AF036359 414 TFSVQRNLPFERVTI VQRNLPFER 96.6 V????P??? 0 1/11
NP H2N2 M63752 414 TFSVQRNLPFDKTTI VQRNLPFDK 2135 V????P??? 0
NP H5N1 AF036359 418 QRNLPFERVTIMAAF LPFERVTIM 165 L????V??? 0.
NP H2N2 M63752 418 QRNLPFDKTTIMAAF LPFDKTTIM 274 L????T??? 0
NP H5N1 AF036359 420 NLPFERVTIMAAFKG FERVTIMAA 2.10 F????I??? 0 1/11
NP H2N2 M63752 420 NLPFDKTTIMAAFTG FDKTTIMAA 0.30 F????I??? 0 1/11
NP H5N1 AF036359 423 FERVTIMAAFKGNTE VTIMAAFKG 0.79 V????A??? 0 1/11
NP H2N2 M63752 425 KTTIMAAFTGNAEGR IMAAFTGNA 6.86 I????T??? 0 1/11
NP H5N1 AF036359 440 TSDMRTEIIRMMESA MRTEIIRMM 1.16 M????I??? 0 4/11
NP H2N2 M63752 440 TSDMRAEIIRMMEGA MRAEIIRMM 0.61 M????I??? 0 3/11
NP H5N1 AF036359 445 TEIIRMMESARPEDV IRMMESARP 0.45 I????S??? 0 4/11
NP H5N1 AF036359 448 IRMMESARPEDVSFQ MESARPEDV 1855 M????P??? 0
NP H2N2 M63752 448 IRMMEGAKPEEVSFQ MEGAKPEEV 8.12 M????P??? 0 1/11
NP H5N1 AF036359 458 DVSFQGRGVFELSDE FQGRGVFEL 9.08 F????V??? 0 6/11
NP H2N2 M63752 458 EVSFQGRGVFELSDE FQGRGVFEL 9.08 F????V??? 1 1/11
NP H5N1 AF036359 466 VFELSDEKATNPIVP LSDEKATNP 152 L????A??? 0
NP H2N2 M63752 466 VFELSDEKATNPIVP LSDEKATNP 152 L????A??? 1
NP H5N1 AF036359 476 NPIVPSFDMSNEGSY VPSFDMSNE 47185 V????M??? 0
NP H2N2 M63752 476 NPIVPSFDMSNEGSY VPSFDMSNE 47185 V????M??? I
NP H5N1 AF036359 481 SFDMSNEGSYFFGDN MSNEGSYFF 13.1 M????S??? 0 10/11
NP H2N2 M63752 481 SFDMSNEGSYFFGDN MSNEGSYFF 13.1 M????S??? 1 10/11
NP H5N1 AF036359 488 GSYFFGDNAEEYDN* FFGDNAEEY 306 F????A??? 0
NP H2N2 M63752 488 GSYFFGDNAEEYDN* FFGDNAEEY 306 F????A??? 1
CA 02658559 2009-01-20
TABLFW p2008/039267 167 PCT/US2007/016529
(2) NP DR1 Supertype
Minimally, peptides predicted to bind DR1 s100nM and sequence conservancy z35%
Sequence
Protein Pos Sequence Core sequence DR1 PIC String Conservancy
NP 1 ***MASQGTKRSYEQ MASQGTKRS 0.13 M????T??? 8/11
NP 39 IGRFYIQMCTELKLS FYIQMCTEL 37.1 F????C??? 10/11
NP 40 GRFYIQMCTELKLSD YIQMCTELK 55.0 Y????T??? 10/11
NP 56 EGRLIQNSITIERMV LIQNSITIE 42.7 L????I??? 8/11
NP 57 GRLIQNSITIERMVL IQNSITIER 15.5 1????T??? 8/11
NP 61 QNSITIERMVLSAFD ITIERMVLS 3.90 I????M??? 8/11
NP 63 SITIERMVLSAFDER IERMVLSAF 11.1 I????L??? 8/11
NP 111 LILYDKEEIRRIWRQ YDKEEIRRI 30.5 Y????I??? 6/11
NP 136 LTHMMIWHSNLNDAT MMIWHSNLN 1.20 M????S??? 3/8
NP 136 LTHMMIWHSNLNDTT MMIWHSNLN 1.20 M????S??? 3/8
NP 159 RTGMDPRMCSLMQGS MDPRMCSLM 14.2 M????C??? 11/11
NP 166 MCSLMQGSTLPRRSG LMQGSTLPR 3.39 L????T??? 11/11
NP 167 CSLMQGSTLPRRSGA MQGSTLPRR 1.30 M????L??? 11/11
NP 183 GAAVKGVGTMVMELI VKGVGTMVM 0.041 V????T??? 4/11
NP 186 VKGVGTMVMELIRMI VGTMVMELI 0.21 V????M??? 4/11
NP 189 VGTMVMELIRMIKRG MVMELIRMI 0.39 M????I??? 6/11
NP 191 TMVMELIRMIKRGIN MELIRMIKR 5.03 M????M??? 8/11
NP 196 LIRMIKRGINDRNFW MIKRGINDR 23.6 M????I??? 10/11
NP 257 EDLIFLARSALILRG IFLARSALI 1.38 I????S??? 11/11
NP 258 DLIFLARSALILRGS FLARSALIL 4.49 F????A??? 11/11
NP 259 LIFLARSALILRGSV LARSALILR 5.79 L????L??? 11/11
NP 264 RSALILRGSVAHKSC LILRGSVAH 8.35 L????S??? 11/11
NP 265 SALILRGSVAHKSCL ILRGSVAHK 34.1 I????V??? 11/11
NP 266 ALILRGSVAHKSCLP LRGSVAHKS 0.81 L????A??? 11/11
NP 298 GYSLVGIDPFRLLQN LVGIDPFRL 15.9 L????P??? 6/11
NP 301 LVGIDPFRLLQNSQV IDPFRLLQN 77.1 I????L??? 6/11
NP 307 FRLLQNSQVFSLIRP LQNSQVFSL 25.0 L????V??? 4/11
NP 328 KSQLVWMACHSAAFE LVWMACHSA 0.39 L????C??? 9/11
NP 330 QLVWMACHSAAFEDL WMACHSAAF 21.1 W????S??? 9/11
NP 331 LVWMACHSAAFEDLR MACHSAAFE 93.9 M????A??? 9/11
NP 347 SSFIRGTRVIPRGQL IRGTRVIPR 14.7 I????V??? 5/11
NP 353 TRVIPRGQLSTRGVQ IPRGQLSTR 31.3 I????L??? 5/11
NP 358 RGQLSTRGVQIASNE LSTRGVQIA 4.37 L????V??? 6/11
NP 385 RSRYWAIRTRSGGNT YWAIRTRSG 50.6 Y????T??? 10/11
NP 406 AGQISVQPTFSVQRN ISVQPTFSV 0.064 I????T??? 8/11
NP 408 QISVQPTFSVQRNLP VQPTFSVQR 42.7 V????S??? 8/11
NP 440 TSDMRTEIIRMMESA MRTEIIRMM 1.16 M????I??? 4/11
NP 445 TEIIRMMESARPEDV IRMMESARP 0.45 I????S??? 4/11
NP 458 DVSFQGRGVFELSDE FQGRGVFEL 9.08 F????V??? 6/11
NP 481 SFDMSNEGSYFFGDN MSNEGSYFF 13.1 M????S??? 10/11
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~ U
CO(O CO(flC0(OCO CO (D(OCO(flCOCDtfl(D(O(D COCO(fl(flCOtO
~ ,~õ~ ~ ~~- ~ ~ ~ ~ ~-- ~ ~~- ~- ~-- ~ ~ ~ ~ ~ c-= ~- ~ ~ ~ e- ~
M (0 0~0 ~~It d' C~O in q- iq qT t N f ~IT N IT t~A in in
C/] 0
U
C-= C, C., C., C., c- . ~, ~, C`= Cl= C`= c., C, C: ~; Cl= Cl= Cl=
Qacjavv.QQ¾Q>c¾FF.ac ~pp
="= ~ ~= ~
`~=`-: ~~~~~~~ c.~~~:~:c:~:~:~ ~ ~~c..~~~~¾~. C''~'~=~'~ ~: ~: ~. ~
~
C,~ w> aaC'i~>~~:~~~~ :~>>a~~~>3
U
0-4
Qy v1 0~~ l- 01 0~ vl v1 0 ~O ~!1 [~ M M~= N 00 ~O O~ 0 00 O\ p, Vl ~O o~=-' o
M
"0 WQ O O ~~ 't)~-~ C v i 00 V~ 00 O
OG M~~~ N O M N M~ O O M C[~ N V'1 v1 M M M
OC)
~ ~ ww~ WwQQC7C7 ¾abHHH qAQQCA7CA7HH~a>4~W Wu.~ y
>ww~7c7wwA aa
FAWqa~w~~
o Q~~a AQ~c~".7rGw~~ww¾~
v~ F F- E- v~ a
t~ ¾ CL c~ c~ ~ Z C7 W W W E- ~ Q a a
v w>aaa> aa
AA QQww`"`" p, wW~~w~a~aWQ~v~ic~
~ aa~ w w ¾ ¾ Aa ~ o ~ ~ A Q a a a a ~ ~ j. ~ ~ `~' 3 a > x
~ ~~QAAAa~a>WwwWr~0~p~
a~ v~~nAA¾aaa~v~iF"F">wxAAQ¾>v¾iQa¾.E"F"a~~~ aaa
~HHaa (D/ a~~HHaaawwHH~ a¾~~~FwW3a~
~
Za~" AAV.~c~w ¾
W ww F QNF- wA -
Q ~c~rxA2 ¾~a F-E.E- Hv~ w
AA >aaaa>~~aaaa
H H av~ 0~ x O
zz aaHH¾o o ao¾¾
* * v~ a a a
~O "0 00 00 N N M M N N O d~ 00 c0 l~ [~ O~ O -- -- O~ O~ O O M M~ O O
a ~-+ N N d' ~ v1 W) "G "0 "O 1.0 %0 t- l- l- l- o0 00 00 00 O, O~ O, 64 ON .~
....
z p o O O o O O O O o o O o 0 o O o
M N M N M N M N M N M N N M N M N M N M N M N M N M N M N C1 C*1 N
Q= ~ I- p I- ~p I- "p l- "p l- ~p I- t- ~p l- 1%p l- I'p [- ~p h ~p [- I'p l-
~p I~
() M kr) M kf) M W) M V) m kn M t1r) tr) M te) M kn M W) M kt) f!1 W) M W) M
W) M W) M m kf)
U O""' O^ O^' O'""' O^~ O""' '""' O^" O'"' O"" O'"' O^" O'~ O"' O O O
~ ~
a~ w ~ w~ ~ ~~ w~ w~ ~ ~ ~ ~ w ~
~2 :RV
=--~ N--+ N N=--N N N N~ N N N=--~ N N N=--N=--~ N--~ N
~ z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z
x~xxxxx~xxxxxxxxxxxax~x~xxxxxx~
N
w .~ $Oz z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z
m " a
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
cflcflcocDCOCD cOCOCflcpcflCOCOCOCDcflCOCOCpcOCflcO cOCD(D cOCDCO(Ocfl(OcO
N~ N N M NLO N CO N CO N CO LO (O 0 CO (fl (O (D (0 LO a0 N d' O) O)
C= ~= C` C~
C~ ~. C~ ~. C. C. C~ C~ ~. C. C. C. C~ C~ C: ~- C-. C~ ~. ~- C: ~~
C= ~' ' C' C ~l= . ~~ C C C~ C'
C= C= ~= ~. C.. C-= C~= C'. C. ~= C C. ~. C. C. ~= ~. C= C. C= C= ~. ~, ~= c-
C= C C= ~. C~ C~
>c-=CU-.VQQ~~c.c'-C-~ C-l v~~na QF- ¾¾¾Q~ c' P.fs.~ c'ar.a>>
~. C~ C~ C ~. ~ C C~. ~.. C. ~.. C= ~~ C= C= C= ~= ~= ~= ~= ~= C/~ C/~ C. C.
V] V] C'= C'= C. C.
C`. Cl= ~. - C'= C~= C' C~= C'`. C`. C~ C~= ~. ~. C~= C~= C. ~, C= C= CC`= C=
C= C= c. C= ~= ~= C= C'= Cl. C. C'= C~. C, C,
~= C- ~. ~= - ~= C ~ ~= ~= ~~ = = ~= ~=
~ ~~~~ aa wwa~aa~:iww C ;c- ~c-, 33
O_ ~n M M 00 o0 N l~ M=--~ 00 O O M'-0 00 v) 1~0 (D NW) ~ Nkn Ntn tn %,O 1~0
zt q v) W)
=-: t o0 00 "0 N 00 cG N"o oo N N N 0 0 N N cC cG
.~ C=~p O~ O~ M l~ O 0 v1 W) N N^=-= kn v)
0)
>
(0 ¾Ea~~a~r~~Awww>>aaC7C7~~~ a'
~~~~~ A A a~..a >> w w a a a a w w A w~~ a a a w a a. w >>
>UU¾¾~~~~~~cnv~ ¾[~ ¾¾[-E- ¾¾G~.a.aGy ~ww.~F-~F-~
wwAA z~ c`"k" ww~~wwwwC7 ~~_ v) cn
ac oaa p a A Q
O z~ ~ x¾ H a a c~ c~ O w w w w w a
a 7 x
> > > zw pw .w ~ .w ~ a a a w w > ? > > > > 3 3
Uaza'w,~ ww¾ AwQQ>>~~aaC7C7w ZZW~
~C7~~AA~~z zz~~aa~~kF"Fc~., wwQ¾~vrai~~a~'"axxAA>>
t~: A a=~ ~¾ A w~ C7 a a~n v~ C7 C7
F<paa ~~QH~~ lxHHww~?,a.~aaaaww
> vU¾Q~~ aw wwa.aaawwAw¾¾aa.aawa [-E-~
Az~~v~v~ a¾H ~a¾ HH ~~aav~v~a~
~O'vwiaaa - QQa~~~Zww~xww jac~~~'u"Wwww~~aaC~c7ZZ
a a H¾~~ A~~ A p z z~" Q~~ w w? d~~ A C w7 c w7 vQ i~ a n' a A A
wa~C~CUU~~~QQ~..~._aw_v~v~ .~~~QF- ~awc~ C7C7
aacn a U U A AaaaA Z Z Z r>i~ ~ wmw w w Q[-+ w~ w w 0 0 >> < Q 0 ~
O'O 'O cC oO 'D 0 O 'C 'O [- l- 0 0 O O I- l-
O O=-~ ^~ N N N N M M M M M ~~n ~n Vl ~~/1 ~'G O CO CO CO CO
.-. ,--~ .~ --~ .--~ .--i .-. .~ '.. .~ .~ --~ .-~ .-,
O O O O O O O 0 O O O O O O O O O O
~10 ~.O ~.O I'O "O ~p 1.0 "D ~O 110 %p ~10
Mcq r4 MN MC14 Mr4 MN Mcq MN Mcq M~ M~ M~ M~ M~ M~ M~ M~ M~
1(~ ~ l/') ~ Vl ~ V~ ~ ~ ~ ,n ~ Vl ~ ~ ~ ~l ~ `o ~ ~ ~ ~ \o Vl ~ V'1 ~ Vl Vl
M M_ M M M M M M M M Vl M M ~ M Wl _ M M M M M
~ w w w ~ r ~ w = w m rw ~ w ~ w ~
¾122Q~Q2 Q>E Q2 Q2 ¾
N N-- N=--~ N N-- N N --~ N=--~ N=--~ N - N - N=--~
zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
zxxxW)xxxxW) xxxx~xxxxxxx~xxxxxxxxx~ xx
N - - - - - - - - - - r. .... - - r. r. - - .-.. - - - - r. r. - - - - - ....
.-. - r. r. r.
Z Z Z 2 Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z 2 Z Z Z Z Z Z Z Z Z Z Z Z Z
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
cfl (fl cn
N LO N
C= C-= C., ~=
C= C`= C= C,
C'= C=
C C= cn En C\= C= C.= C.=
= = ~.= ~
C\ C =
C= C - C C. > > w w
~ O M Op N M
M N
O
ti
zz
aa'oz
>
> > w w
z
aa az
wxcn~
zza~
Qoaa
N N
O~ CO O
--= - N N
O
0
~ n ~ r-
W) M W)
O ^ O ^'
Q ~E Q
- N - N
vzi N ~ N
xxxx
~
N
- - - -
J zzzz
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U
(DCOCD(OCOCOCOC8C0C0C0(flCOCOtflCOCO~DCO(O~(0C0(O
~
o~0 (O 0 i i ) 0 (D 0 0 0 i(O 0 Cfl 0 ~ 0~0 ~ ~) i
~ ~
0
U
=
C- . ~. ~ ~ C= C '. ~. ~.. C= C=. C.. C. ~. C= C'= C.= Cl. C= C= C
C. C = C'. Cl= C' C' C C C= C C'. C. C, C, ~, Cl Cl C' C- C` C' C Cl
C~= C= C= C~= ~'`' C= C~= C~=
~ ~ c~ F. a a~~ c~ ¾~ c c Q¾¾ Q~j p= c c
w a : : ~ ~ > > ~ ~ ~ a > w w ~i ci a ~ w w ~ > > >
0
U
Hy
p, ~n o ~, r=, d~- o M ~.- `~ 00 v, ~o 0 N~n v~ Nn ~o ~ o
nl
~ m Z d' V' vj 00 O N O o0 ~y 00 W) N v~ O N (,i N C N vi
u pp =--~ m N M M M=--l~ MW) O~ =='" G O~ I~ V'1 M 0 V'1
+~=~õI--I
G)
~ u-aQ;aa~a
.aWww¾QaaaE-
~ a~ "wQzzw ww~-Jcf)
~ AQ~~wAQQ~~~~a¾H~aAwCw7cw.7
~
v > ~wwaa iiww? >
c7
>~~`'a' ¾¾ Caw¾¾~~ac7 Z ¾
vooi QaaA~a>~ z~ "~aar~wr~ F"WC7wwaaQk7a
~ o d a Q .~a ~ Q Q a ~ f~ p4 ~ w > >
HH.~ -aa
a)v~av) Q a ~>w.~aawww¾¾aawH
z > w o - ¾Haw¾ 0 (Aaaa~
F" p~ W W~ a GL " w W 0
zAc7wa~~~~p x~~~~¾H~aQwED~~
> > ~ A w w a a w w ? > > >
~ww~a¾ ~zQwwa~a<¾o
z
a(D >U)vAazzv~v~k.waawawC7¾
~b
^"t t O O~D l- O N
O.-==N d. ~Z a, O O=--N N M M M M d' ~' ~~ vl Vl W1 V1 00 O~
N n ~n
J azzzzzzzzzzzzzzzzzzzzzzzzz
m
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
y U
- r T r\\\\ - - r r ~.'r l~ r r r r r~ r r r' r
\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \
rn rn\N\ 0) O) 030) m m CO CO f- I- CO N00 CO o0 (0
C/) O
U
o~oooO - oo
Cl. C= C; C; C; C. C= ~.. ~. C~= C~= C; C. ~: C, C: ~: C, C~= C`= ~.. ~.. C.
C.
C C ~= C= ~== C= C. ~; C= C= C. C; C; C~, C. C. C. c.. c'. C-= C` C C. c-. C.
c-.
. v;~~ ~ c~~;;;aaaa~~ ~ `~"`~"ccU' c=~=aa
~= c= ~= c ¾¾ C: c:
= C. C, = = ~= C~ C'` C'~ ~ C~ C~
c~= c= c~= c~= c= r= c`= c c`. c. C,= C
CI~ C'. C-= C-. = ~. c`. ~ C C, C~. Cl. ~ C= ~, ~ ~ .. ~ C C
.. c.. C.. C' C' C~= C~= C~= ~.. C. C~' C~=
~' ~~ C= ~ ~= ~= C Cl= ~. ~. C= C= c= ~= C~ C= C~ C~ C~ C` C~ C` C~ ~.. ~. C~
C~
aa~~wwaaaa~~~~333~C-.
U
0--(
O d O~ ~~ M M~D ~O N N~n ~n O O l~ ~ O~ O~ l~ I~ tn ~O c+~ N N O~O
.~ N~p o0 0o M~.,~ vl ~n W) v1 "O "O N N o0 00 M M M "t N N~ ~t
N ri O O~O ~O N N ^^" "~p ~p N N~ C O O oo ^" M O O O C
I-
~
c~c~aazzw ww
u~ ao o A A w w L L¾¾~ CA V) a a 04~ a a
aa~~r~.~r~.~cw7Cw7 0 0a aa~~
aaww 0 azwwaa~
w
o cn cn aaaaA 7 Co
v ~a~'~ww.~"aaaa~~~~3`3~~3~
aaz
v~QwZZxx~~c7c7~~A A z a pr~~w wwa
~aaaa aa w~a~awww
c7c7aa w
zz ~~aaw
ac~.7 v Q A w w~~~~ w w x~ cy aaa w
e,x aav,v,aaaa vc~ aa~'~v~~~aaaa
~ ~~ ~c~7c~7~Fww~~~~cQ7Qaad a0
aaa
0aa~2ww~~AA ~ WW~~¾
~ ~ ~ ~
a¾
Aaa~~v~v~zz~~v~v~ ww zwwww~~
O~~N N O O O O O OM m w W N N NW v~j v~ O Om m
~ p O O O O O O O O O O O O O
~o ~o ~o ~o ~o 110
N N N M N M N M N M N M N M N N N M M N N N
r ~ ~ r ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ r ~ ~ ~ ~ ~ ~ ~ ~ ~
~ M M kt) M W) M tn ~o
M tn M tf) (+1 W) M W'l (+1 M W) M kf) N) vi
. w w w w w 00 w 00 w 00
N N
N N N N=--~ N N=--~ N N N N =--~ N
~ N N~ NW) N~ N~ N~ N~ N~ N~ N~ z~zz N~ N
Q~,xxxxxxxxxxxxxxxxxxx~xx~xxxx
N Z+2~~~~~~~~~~~~~~~~o~~~ov~o~~~
W OzzzzZZZZ?-.ZZZZZZZZZZ -7,z7-.7-.ZZzz
m
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
O U
N C)
m m m CO r-- CO 00 0 00 CO
C/~ O
U
c: c`. c-= c`= C`. c= r= c`. C`.
t~ bA C= ~= C= c`= cl= C' C`= C= C`= L`' ~" C. C= ~=
C. ~.. C" C' C'C. C'. C-= Cl. C'`. CC. C-.
~ ,+-~' ~~. C Cl. = C C C~.. C ~ C . C ~ C
n C: C =
~ C. C. C= P= = P= PC= C.. C. C'-. C. C. P= P=
~ ~~~ i~ ~
c U
co
~ a'ct O~ ~ M~D N~ l~ O~ l~ ~1 N N O~D O N~D o0 ~,~ v~ W) "p 00 ~ M M N N ~
O
C t+i O~D NN~ O oo O C 0
C0.)
N y
c z ww
~ o'~ aooaa
~ ~ 0
~, vi~~Qx~1a¾a0~
.a >~ aoI
co a o-
` o
Zwa~~ ~:5
tn _
o~ a w a
~ o PJHd
~,~aaa
~ O>(Daw¾daa
aaao
~~ ~~~ C7O~~ cQ7p4ww~¾¾
CY
> aa
c~ Q
AA~~cn ZE"ww>ZwwHH
y N vl 00 00 M V=1 OO O O M M
Q a a W)o ooooo~ooor,- - - - - -
N y C y N N N N N N N N N N N N N N N
z ''J' ~-+ V] ] (n 0 0 C/) V] Vl V] V] V] (n Cn 0 V]
m ~= zzzzzzzzzzzzzzz
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
VI " Vl ~==~ M~ O~ QN N MW1 M~ N(~ l- 00 00 00 00 =-r l!1
V~ 0
U
~ o =~ooooo o~-o.- o000 -o o~oooo- o- o oo
c` C CC` C,, C-= C-= C= C= C-= C= C' C- C~ C= C.' C C` =
C~= C~= C- . Cl= ~, C= G~= Cl= C ~ C= C-= C-= C-= C~= C~= ~.. c.= . CC~= C~=
C,= C~= C-~= = C
C~ C~ = ~`= = ~' C~=
~ = = c.. C. ~ C~= ~' C~' C`= C`= C' C~= C~= ~.
C C~ C
. ~.. C`= C~= C~= C~ ~.~, = C= C~= C= C, C. ~. C, ~ C`= ~= ~`= ~= ~= C~' C~'
~`= C= ' C' C~= LC~~=
> > ~=
Q Q Fr ~=+ Cn C/J a C.. C.. C-= ~ Cl= U U > > ~. , ~.,
> > C~l r a ¾ ¾ ~=
' . ~. F F
~ C"= C= CC. C. C,= C= Cl= Cl= Cl= C- = C~' C' C' C~' C= ~.. ~. C C= C'= C'
C~. ~= ~. C',. C. C. C. C.. c..
~= C. C. C= ~. ~= C. C= ~. C. C. C. C, C. C.
C. C C' . C. C. C. L. . CC~= ~= C~
C= C= F F
~= ~= ' ' C'
C= C ~. ~= ~, C, = C ~, C, ~.. ~. ~ C.. -
C, C~ ~.. ~= C, C~ C~. ~. ~. C. C.. C= C~= C-
w w w a w w a a > > > > w 3 3 > > w w > > > > c-,
U
a 00 00 00 M N ON a, O-, M m 1,0 ID O N ON O~ O~ p, M M=--, ~p ~p N N~O ~D O O
O~ =--~
~ "O '.O M M OC 00 00 00 O O"T ==r "O "O 00 00 .-r .-. ~~. M O\ Q1 ~O ~O OC 00
M l~
O O O O~~O ~ 'n ~.+ ~ N M M<~ N M M -- N .. tn W) N N Oll t-
~~l `~ A 1=1 Q Q Q~ U U F F 9 y~" 4 ~2~"
> > ¾ Q ,~. x x Q Q Q > > Cn U U F > > a47 `~w4 .Y E' F
~ww~~~U~vAa33> >>
cn A `~~~wwww
a a_wwq4wwW ~~Fxx~~Wv~v~
U zw w~~~a¾ww¾a,w> A~~33 w >?~~x
FF~c~Aw
AUUxx UUHH>Q>x~~ax~~~~~
~ a > wWAAww a.~~5 n~5 UUFF- >J~"~~~~xxxx
Z z~ a~~ x x W w z Z-..
> >¾¾~~FF~~~~~~. z > UU>F"F
~" ~" ~1a44Fx-Ex-
awF UUww aazFF ~~~> u~r~ ~} a4a4W~
~UU~~AAa3 o>~a~a~ ~"wwww
W wwc7AA ¾F F w jC7 ~~.a.av~v~
..~ wUUv~.a
¾¾ A 33 .
w aa ww ~
U U p p w W
ZZ xww ~~~~33 `~0 0xr~ xxz
a!t~;w??F-E-UUx~v~~n?AA¾F¾¾wwzwwww ¾
~ N N~O ~O In v'i N N't 1*1 M MW) ~O 110 CG o0 O Ow N N l~ l- O O 00 00
- - N N N N M M M
a =- M M T d' 4 ,1 ~D ,C ,O 00 00 00 00 O, O) .~ .-+ ..r .--i .--i .-. .-r .-r
c~ z ~c o =n \o ~o =n \o ~ \ o "c ~ o "o ~ "o ~o
(,~ M M M ~ M ~ ~ M ~ ~ ~ M ~ ~ ~ M ~ ~ ~ M ~ ~ M ~ ~ M ~ ~ ~ M ~ ~ ~ M ~ M ~
M M
~ ~ o t- I'D r- %0 ~
.~,= U M O M O M O M O M O M 0 M O O M 0 M 0 M O M O O M 0 M O M O M O
+ V O ~ O ~ 0 ~ 0 ~ O `D O ~ O ~ ~ O ~ O ~ O ~ O \0 ~.o O %0 O %0 O "0 (=>
~
~ Q w N N[sõ N fjõi w N N(S,i N N N NNN N w (y N(s,~ N(,~ N
¾~¾~¾~~¾~¾~¾~Q~~¾
N N=--N=--~ N^=~ N N N N= N N-- N~+ N N~ N N N-- N
~~zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
tj kn NW) Nkn NW) Nkn Nkn NV) Nr~ NV) Nkn N kfl Nrr~kn N Nr~+t/) N vi N v'1 N
vl N
^ (n r~i .~. x x x rTi x x.Ti. x x x x`r~i x x x x x x r+i x~i r~i ~i ~i '+i x
x x x x
N F-a-I~Q¾Q¾¾¾¾¾¾QQ¾¾¾Q¾¾¾¾¾¾¾¾¾¾¾¾¾¾¾a¾
W ,_~ o a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a
m " w
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N N N N N N N N N N N N ~~~~~~ ~ N
N N N N
^. .=-~ 00 00 "0 O [- d' M Vl I- T l- M ~ =-r .--~ .-=. ~O M
O O-- O=--~ O - O- O O O - O O O O O- O O O- O- O- O- O- O- O O
C`. Cl- C`= C,= C, C, c.. c.. ~. ~. C~= C~= C~= C~= C~. C'= c.. c.. C.. C.. C=
C= C,= C-= C: : C-= C~= c.. c.. . .
~. C= C'= C. C= C`= C~. C, C-
= ~= ~= c.. C. C- = C`= C, = C, = ~ C=
C. ~= ~, C, C, C, ~ . . ~ . . C. C. ~ . . ~ . . C ~ C' C'. Cl C~ ~.. C. C=
C''= ~= C=
c.~,, ~..~..~.c.~,.~..c~ c ~ ~ ~ c, r c ~.~.~ ~ ~ r ~=cl c ~ c= ~=
c
~~QQc FFaacn(AQQaaU~ ~~aaa.a.QQaa~ c aaQ¾ U U
Cl= C-= = ~= C`= C~ C' C' C' C' C' C' C' C' C' C' C' C' C' ~= C' C~= C~= ~= C-
C~. C. ~.. ~.
C~= C= C ~= C'= C'= ~= C' C' '
~. C. C= C= ~.. C. C. C. C .' c" ~.' ' ~' C' C ~. ~.
C= Cl=
. ~= C. C.
C= C= C. ~. C`= ~, ~' C' C' C' ~' C' G ~' C' C' C~'.. C. ~.' ~.' CC~= C. . ~.
C= C= C= C= C= C' C= ~' C' C' ~'
C= G~=
a a a C~= C~ = C~= C~= C' C' C' C C`= C~' C~ C~'
C-= C`= C~= Cl= C= C' Cl' Cl' C' C`' C'' C-= C~= C'= C= C-= C'= C'' Cl= Cl= C=
C- C=
ww~~~~wwaaa w~~~wwaawwaa~~aa~~~~
N N kn tn m m N N =--~ ,~ I- M M N M M O~ v1 =--~ ~~ y~ ^~ .--~ ~G ~O ~D ~C
~~~p ~p O~O
~ ^, M 00 00 O~ 01 ~ ~ ~ p p vl ~A
M M 6 pvi V-1 l- 00 ~O Vl V'1 V~ ~ p p ~ M M 1n V'1
V~ ~ M M~~~~ N N M.-r M M M M M O N N l~ ^" '~ O O~n N l~ [~ .-. r. M M.-. ~j
ln
~
~
HH(D ~ 0 u En cn
ce 9 ~9 aa w~jA~oc~~v)v~o, o~~WWwwUU~~
aaaazz~ o
~~ a, a U d~ a a a a¾¾~ Q~~~~ U U
~~C7c7FFv~v~ ~~xw~~ FFaaAA aaQQ~x
wWww~c+"õ~~'"nHE-~=a ZZwwv~zC7~~FFC7c7~~AA¾QAA~~QQ
C~c7p ~~AAaazz wc7c7xxAAaa~~AA=,~aa
F F~~~~ w w¾¾ a a w w A A~~ w w a a a a a a a a v~v~ a a A A
w w~.. w w a a a a a w~., w w a a w w a a~~
Q¾A33C4xA¾nn U,xx~wc~c~~W~> >AAaawwx ~ww
a. a w ~ Q ~ a a ~ ~ U U
~ `~' v~ A Q c~ ~7 H H
FF ~~ a aa z oz d
~'aaa~x nv~~
v~Uo,U~'o ~a~aUUQ¾aa aa¾QUU
W~~C7C7FFv~v~v~¾QaGw~~v~~aa.~ a= AQ¾~~
rxC40 wwwxwwz FFp"A~~~A aaAAAA~x
wwwwv~v~E.,FAAaaww C7c7a, w~c~7F E Qc7aa~~¾¾ww>4? ~.~
aQQ
c7c7(Y Q wwQ¾a zAAx~ xaC A AAv~v~)
aaww w~ W Waa "a r~~=,`c=a.,a-laa~~ A - AA
ww~ Hww~~~~~UU~w~ww~~~4~4Wr~ a a~~C7C7.~.~~
~' w w Oaaw w a v) ¾ ¾ A ~Q~ ~ a4 a a a a w w
a a c4 aG w w~~ A A w w r~4 > z z W~ a a u: a! v~ v~ w w Q¾ C7 C7 ~"
O O oO c0 N N~n ~n O O~ \O ~O M M~O N N O O O O N N M M v1 kn O O N Ntn ~n
v'~ ~n [~ [~ 00 00 O O=-~ =--~ --~ =--~ N N M M M~~ ct' ~O ~O l~ [~ 00 00 OO
00 OO 00 O, O, O p O O
N N N N N N N N N N N N N N N N N N N N N N N M M M M
M00 00 00 00 00 00 Mco M00 00 00 00 00 00 00 00 00 00 00
lp l- ~p [- ~p n~p l- ~p l- ~Q l- ~p I- "p n~p [~ ~p I~ ~p l~ ~p r ~p l~ ~p
n~p l~ ~p r~p l~ ~p I~
p O O O O pO M O M O O OO O O O O O M O
"0 \.D ~ ~o Oklo ~o O~o OlZ O1.0 O~ O~ O~( O\0 O~D O1.0 ~o O\~o Oko
Nr N w w N~==~ N Nr N~+ N Nr w (r~ w N w r~ w N~r Nr N~=-~ Nr N N
N=--~ N N=--~ N N=--~ N =--~ N~ N N=--~ N =--~ N-- N=~ N-- N-~ N=- N-- N N
z z z z zZz z z z z z z z z z z z z z z z z z z z z z z z z z z z z z
vi Nkn NW) NW) NW) Ntn N v) NW) tn Ntn W) Nkn Ntn N v1 NW) W) R v1 N W) N
xxxxxxxxxxxxxxx~xxx~xxxxxxxxx~xxxxx
LO
N Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q¾ Q Q Q Q Q¾ Q
W aaaaaaa.aaa,a aaaaaaaa.aaaaaaawaaaaaaaaa
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N N N N N N N N N N N N N N N N
rr
~ \ \ ~ ~ ~ ~ ~ ~ ~ ~ \O \O ~ ~ \ =-\~
M M N M N vl M vl 'IT t!1 M N 00 N =~ õ~ ^,,,~ ^
O O O O O O O O O O O O O O O O O O O O O O O~ O O-- O O O O O
C. C` C~ ~' C' C' C= C' C' C' ~= ~= ~' C' C~= C~= C' C' C'= C= C`: C`= C* C=
c`. c`. ~. C
c= c= ~, , ~. ~,. c~= c~ c, c~. ~,. C. c~. c~. C, C, C- = cI= C. C, cl. cl.
c,. c.. C,. C.. C. c,, C= c~= `= ~= C~. ,.
~ .
c= C= C= C= cl~= ~= C-. ~= c= c~= ~= ~=
c~= >
= ~= ~ ~= c~ Q ¾ c C~ c ~ ~ c C ~ c
aa~c. ~= ~.c.QQ~ > .c > .UUv~v~a.a.v~v~~=c.. ~~Q¾
c. c. ~,. ~,. a ~.. r ~,. ~,. ~.. ~.= ~. ~, c, ~, r. ~ c c .-~ ~ ¾ Q v~ v~ ~.
r c. ~. ~= ~.
P= P= P P= C' P= P= c.. C. C= P= c.. c.. P= P= ~'`. C `. c. ~. P. P. P= P=
C, C, C~' C= C~. C= Cl= C'= C. C. Cl= Cl- C.. C~= C~. C~= C= C= C= C= L
. ~. C C C. C.
P= P= C~= P= C' C' P= P= C' C' ~= ~= C' C'' C' C' C' C`' C C'
C= Cl= C. C~. C~' ~' C`' . C`. C' ~. C. C. C= C= C= C~=
= C`= C' ~.' C~= c- ~.= ~'
~= C= c.= C= C`= C' C-
P= C= P= P- C C= C= C' ' ~'
~~ww33~-aa a..a~. . . >~ ww
00 t-
00 O~ ~ W) N N N -- MON C" N N M pp ro- N00
M N~ 00 0~0 M M p N
00 O~ ~ l~ ~Y '~' ~="~ l!1 M N 01 O~ QN N M M O O"O ,O 4 M 01 O',
N v'i =--' M et O --~ =--~ t+l (7~ W) tn V1:T 00 ~ I- N %,O M~,n ~A N N o0 00
O, -,T~=--~
CA
~
vv~>a, ~33¾¾awwwwA`"`z"ww~~o ww UU
x rxx~~
wwwz?~~aa > > ¾¾aaAA~i~AAWri 3~AwAWa ~~UUww
~~a'a~ aap UUv~v~p a ~~C7C7¾~~ vi~
ax WAAxAwwQ0WW v~v~ww
zzaawA~~AA,~aAAAA¾~c~¾wwUU
w ww wwp cn F- F- C7Qxx
uU0~~ aa¾~~~~~~~AAxxxAc~r~wwCw7cw7wwww~Hv~v~
~ww ~~aw aa
xaxWO>aA~~?jUUaaxC4 ¾v~wwpõa ¾
~3a, xwx WwAAxxAAAA r~r~
c7C7x>xa,~~~3¾wa wwwAQ0aa ~ ;a4zz>>xx~~UU
w~wT.Z>>¾v~a~W~jQr''AA wwaaa AAww xxUUww
> a a ~> a¾~~~ U U A A a a~ 3 Cw~7 w ¾~¾ ~ vxi E--
xx ww v~ ~ v~v~ww~a~
aag>> W zwwQQ~AAAQ~ww
¾¾c~¾wwUU
zz O' waaww v~ ~n C7
~a~aAAAA~~F~E- ¾xx
UU w 3x v a ~v~a wwxx
x~ w ~~.Q Aj Aj a x~x ~A , a a w w c 7 C 7 w w>4~14 >>
¾¾wwvvC7C7wa¾v~3QZZx~AOO ww33wwaa ~~ww
AAHHww~~~~ ~ ~ 3wwww ~a~aav~~,AA¾¾ zc7Q
~~xxwwwwzzwa~xt7c7aaQv)aawwv~v~wa ~c~HH
00 00 v'1 v'1 t- I- O O~D "D o0 0o O O V~ O~ O~ O O M M O O M M o0 00 v1 v1 v1
v1 "0 ~D O O
O O='-~ ="^ ="~ =~ m M M M M M l~ (~ l~ l~ l~ l~ O~ O~ Q~ O~ O O N N N N M
M'cl' R~ h vl
K1 en M M M M M M M M M M M M M M M M M M M M'cl' ~~~' ~~~~~'ch ~'h ~h ~=~
.-~ =-r .-+ .--~ .-~
M00 M00 M00 00 M00 00 00 M00 M 00 00 00 M00 M00 00 M00 M00 M00
M00
110 [- "p r- ~p (- ~p r- 10 r- ~p l- ~p ~ I'p l- "p I- ~p [- ~p l- %O l- ~.p I-
~p I- ~p I- "p l- "p l-
M O M (D C+1 O M O M 0 f+l O KI ~ M O M O M O M ~ M 0 M O M O K1 ~ C~l ~ M ~ M
O
O"0 O~ O~ ~ O~ ~ O~
w w N wc~ w N wc~ wc~ wc~ w, N wc~ wc~ w, N wc~ w N N N
¾~ Q~¾ z Q~ Q z¾ z Q z¾~¾ z Q 2¾~ Q z Q~¾ z¾~¾~ Q~ Q
N N N N N N N N N=--~ N N N=--N N N~ N N N
z z z z z Z z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
v'1 N v1 N~1 ~/'1 N U1 N~!1 N v1 N~/1 N vl v1 N v'1 V) ~!1 ~ ~1 Nt/1 Vl N vl N
xxxxx~xxxxxxxxxxz~xxxxxx~xxxx
~
N Q Q Q Q¾¾ Q Q¾ Q¾ Q ¾ Q Q Q¾¾ Q¾¾ ¾ Q Q Q Q ¾¾¾
W a a a a a. a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a
J
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N N N N N N N N N N N N N N N N N N N NN N N N=--~ N N
N N
-- O O O O O O O O -- ^ ^ ^ ^" ^" ^" " "
,~ ~ ~ ..-~ r. ~ .-. .-. =--~ ,--~ ~ .-+ .--~ .-.
O=-=~ 0 O - (D O O O O O-- O-- O-- O-
C~= C'= Cl= C~= C.. ~, C.= ~; ~; C, C= C' ~'
C, C, C, ~, C`= C"~. C, ~. ~. C~= C~= ~., .. ~.. ~. Cl. Cl=
Cl= Cl, C, , C, = G, = ~. C. C-= Cl= ~, ~.. C= C~= C~' Cl' C'' C' ~.' ~' C= C'
c., ~' C= C'= Cl= Cl=
C, ~, C, C, C= C= C, C, C, C. c.. C. C~= C~= C. C'= c`, C== C'= C. C., C, C~
C` ~, = C= C= C'` C,
c~= ~= C' ~' ~' c`' c= c'= c= C= C.= C.= C.=
~=~= wa cn v~
cn
~., ~.. c~= C= ~. ~.. c, ~.= a.] CU CU Q Q c.. ~. ~~~~ >~" ~ c- v~ . c`. F. F.
c. c. c". ~. a a
C= C= ~, ~, C= C. ~= C C, ~, Cl Cl Cl C'. C.. C. C- C-. ~ C C.= ~, C C= C C..
~.. C. C ~. C. , . C C' C C-
= ~= C, ~, ~. C ~= ~= C= ', C C' Cl C- C= ~. C' CC\ ~= Cl C C~= C~, C.= C, C.
= ~.= ~.= ~. C' hC C l= C`
C= C= C, C, C. C= C= ~= C~ C, C- C- C- C'. C.. C. C' C- Cl C` C' C' C, ~, c..
C= ~, C= C= C, C~ C` C-
~ C C= P P P= P= C' ~'`= C.' C.'
C- C~= C~= C~' Cl' Cl'
-aw~.~a ~ ww
O OI'O \G o0 0o N NW) kn O, O\ zr l- l- 00 N~O '7 W) tn M M\O 10 M M 00 00
v1 ~n 0 l ~ t- vl w) o0 00 .-= =-= pp pp ~,O ,q l~ (~ N N pp pp pp 0\ F V~ N
N~,N M-..-. ~t q
N N O C O N N O O M M'n ri M O O=--~ --~ N N N~ O O[, t~ ^ 00 00 m O0 =--~ .~
ti
~
zz¾¾v~v~~~p~aax~~~~v~~~~wwww~Qaaww
azzQQ4~
aa-iazz UUvi~
QQaw wuvUiUa ~x>?~0 v~v~ww,~"a~AA
C7C7HH¾Q U
.a ~ wwaw ~~~ww rs:ww wv~v~
UU C7 ~ wwaa
C7C7~~ Q¾ .~.a C7c7 c7 ww
2 :E~x>~~~F F"~.ZazzApaaaa~aC~
~? ~~wwa.a~~wwa ~ ~~ww
wwzzUU¾Q¾¾ OAdw~xF- E- a F- HF- AA~~ aaxx
QQaa¾¾~~U¾Q AAAv~v'cs:r~~~~~Nxpz~~~~a~A~A~wwxx
~ a¾¾ vUi U~~ p A~~ U U H H x ~ w w w w w w~>-E- [- a.azz¾¾ x~4UVC7C7 Q~~aa
4Q¾ ,.a -aUUp ~ ~
aazz~~ HliH
C7C7 ~ a~ZZAAaaa.axa4~~x~ 7>>~~viv~iWw~rai~
> ~ ~ ~ w w
w w~~ C7 > ~~ Q¾..a a A A p ..~ ~ w w A
H ¾ Q w w~~ w w
A A k., w~~ zz
AA
QQww~ZaCaCC7C7zzF- UU~~AAa."~"a:~~~AAF- F->>wwv) cn
00 00 O O M MIt ~T vi kn ON O\ O O l- [- O O N N~O \,D O O~t qtT [- t' cO c0 ~
M M v1 v1
~ tn ~10 \.D ~p \.p I'p \.p ~p \~p ~p ~p [- l- (- l- o0 00 00 00 00 00 O O O
O~=--~ =--~ =--~ N N N N N N
,t !t ,T ,I- d' qt It d' It It I~t 'T 111- It 'IT V'1 W) ln V1 V'1 ~A Vl V~ V1
N Vl V'1 tn l/l
~ ..
M M00 M00 M00 M00 M 00 M00 M 00 M00 M~
M00 M00 M00 M00 M00 M00 M00 M00 ~
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ r- "0 r- 10 r-
M 0 M O f+l O M 0 M O M 0 M CD M ~ M (D M C) M O M 0 M O M C> M O M O M O M 0
O O O O O%0 OIC O~o O O\C O"C OIC O\C O%0 O~o O1%0 0 "0 O%0 (D ~o
w w w w w N w N w N N N N w c~ w N N w c~ w N w c~ w N
¾ z Q~ Q~¾~ Q~ Q~ Q~ Q~ Q z Q~¾~ Q 2 Q~ Q z Q~¾
N=--~ N~ N=--~ N=--~ N~ N=--~ N^~ N=--~ N=-~ N-+ N~ N~ N~ N=--~ N=--~ N=--~ N=-
-~ N
zzzzzzzzzzzzzzzzzzzzz,zzz-,zzzzzzzzzzzz-.
~n Nkn NW) NW) V) NW) Ntn Nkn Nkn NW) tn Ntn NW) N vl N ul vl NW) N v1 N
xxxxxxx~xxxxxxxxxxx~xxxxxxxxx~xxxxxx
~
N ¾ Q¾ Q Q Q¾ Q Q¾ Q¾ Q Q¾¾ Q Q Q¾ Q¾ Q¾ Q¾ Q Q¾ Q Q¾
W a a a a a a a a a a a a a a a a a a a a a a a a a a a a w a a a a a a a
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N N N N N N N N N Nr*4 =_, ,...
,~ =--~ =--~
~ \ \ \ \ \ \ - \_, \ \ \ ,_\_, ~ \ \ \ ~
NIt N M N- M N,_=, O, O, l- l- t- [- cn
O-= O-- O O O O O O O O O O O=--~ O~ O O O O O O O O O O O O O O O O O O
Cl= Ch= C.= C, C'= Cl= , ~, C= C'= C'= C'= C. C~= C~= ~= c .= c'. C, ~.= C. C.
C'-. C. C~= C~= ~.. ~.. c... C..= ~, C= C~= ~. C, Cl= C`= C, C, = C', = . .
C~= C., , C,= C~= C.. C, ~, ~= C~= (~= C~= C~=
C= C`= C= Cl- C.. ~
= C.= C. ~, ~, C'`= Cl= C= C. C~= C~= C= C, C~= C~= C. C. C~= ~.= ~, C'`= Cl=
C~= Cl=
~ ~., C, ~. C`, ~., C= C,
a a c' c= C`' ~`=
' c' c= ~= c-= ~= c= c= c'' U U v~
aac.c..~ c~ca=ac.~.~~F- ~ c' r.c..~.~.~ . ~v]c v -, - a>aQ¾Q
= C= C~=
Cl= Cl= C. ~. C~= C~= C~= C= ~'`' C`= C~= C- = C~= C~= C' ~`= C ~= C
C~= ~= G~= C~. C= C~ ~. ~., C~. C. C= Cl ~, ~., C, C, C-= C'= C C C C= C= C=
C'= c. C. C~' G~' C= C~. C~= C. C~= C'~=
~., C, ~, C, C~= C~= ~, C, C`= C~= Cl= C~ .. ~. ~.. ~. C'= C'`= CC C CC= C= ~'
C~= C.. ~.. Cl= C~= ~. ~. C~= C.. C= C=
C= C= ~= ~= C. C= ~.. C'= C'= CC, C, ~.= C. C.. C~. Cl CCl C c.. Cl= C`= ~..
Cl= Cl= C. C. C.= C= C= C~=
aa33>~aa~~~~aaww>>~>w3 aw~aaa
v1 00 N O N O G~ O'% M M O\ vl l- 00 ~~O ~C o0 00 v1 a~-
00 00 00 00 ;,o N O o0 - M t r ; t- [- lO l~ 'r; 00 vy v'1 O O=--~ 00 t'~ r:
O~ m
N ~ N ,O O O O =--~ N N w1 ~n . ~ v1 ^-+ N ON ~D ~n O N ~ ~
~Ot N
00
N
xxww~~x04d~o v~xa~aaEnwa4 3o >cn w~ ~a
wwaawOaaH ?~7aa v~v~aap >~HH~x z~W~ a
w>> c7 C7 a a a a~7 o~~ H H U U o >~ W a. ;¾ a¾¾ Q
UUW W~~a E-r/~a¾~~C7C7~~aaWWv~~~Wv~av~~. r/~
UU~¾w z~c7F"xaaw
L1A ~.. aacs:n;? ~ ~ wQW~w~wa:¾~~'c7> >
>
[-. ~ w w U U >
w w v Q a a a a a a w w w w w U a d w a
a a 3 3 ~ ~- a a -. ~ ~ a a 4.4. > > ~5 >E > w ~ > > a ~w a a a
a
W~laa O' c~w'x~a~Lxxx~~ v~ ~w~w~' Q~wawa
v~
a¾¾ v~ v~ v~
33C7C7a F~õc7c7 v,v~~~ pa w~f~w~ z
xxwWc1;¾¾o,> ~aa'õzaõ~¾ a4c~~ ~ U ~ z
vv~~?a~ uUaao-> ~"wa~¾~~wW
w w v~~, > d
aa U U w w ~ ~ a a ¾ ¾ ¾ ¾ ~ g ~ v
xx aawAaaH~~v~ zz UUwww Zcw 7 w ¾
Ha~~aw
t7 ? ~ ~ >
aaaCaC~a~a Q
QQ~~~uww~vaa~aaaxx~~~~ w_Q w~W ~ >
x ~ j a a~ a
a a x a a~~~~ >> ~ >
v~v~x w wwQ~~ c7 o >
wwxx~ UUaa wLl ~~33~~ v,v,a~~wx ~
wwaa 3? ~vc~c7~a~aww~xc7c7aWwwHv~aU~CZ
[~ [~ I~ l~ O O M M~/1 v~ 00 CO O~ O~ N N v'i vl O~ O~ --~ ~ O~ v1 IC N=--~ 0~
CO \O 0 10 O~~O I'O
N N M M d V~~~ d"0 IC 1.0 I'D l- l- 00 cC oG QN 0~ (D - N cn ~ItT v) v) ~D ~.O
Vl Vl V1 v) V) h tn v'1 W) v) kn tn W1 W) v) W) v'1 V) W) Vl kr) vi v'f V'1
v'1 "G ~D ~D 10 1.0 ~O \C ~O \~O \~O ~O
0 0 O
.-
M ~ Kf ~ M ~ M ~ M 0 ~ M ~ M ~ M ~ M w w w w ~ w
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 1.0 r- r- r- r- t- r-
m O m O m O m O ivy O m O m O m O m O~ Or- O O O O O O O O O O O O O~ O
o \.D O ~p O ~O O ~O O \C O ~O O ~O O ~D O ~O Z `O Z \40 .O \.D `O \O `D \C ~O
\D 'O `D ~-O \O Z `D
N N N w N V. N w w N w l, l- N N N N N N N N N N
dQ:E dQ~d~Q~¾~d"~x~x~~~~~~~~~~
N- N~ N - N N~ N N~ N~ N l~ N l~ N N N N N N N N N N N N N[~ N
zzzzzzzzzzzzzzzzzzzzzzzzzzz.zzzzzzzzz
v) N v1 Nkn NW) W) Nkn v1 Ntn N v) l- N I- N N N N N N N N N N N N[-N
=xxxxxx~zxx~x=xxx~xxx~xxxxxxxxxxxxxx
LO
N d Q Q d Q d Q Q¾ d Q Q d d Q Q Q d Q d Q d Q Q Q d d d Q ¾ d d Q d
W a a a a a a. a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a
J
m
E-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
r. .~ .~
r- rn o~ o~ o~
rn o~ ,o Z
~
0 0 0 0 0 0 0 0 0
C= C.
P= P= P= C P. P= ~. C`=
G~= C~= C~= C~= ~.. C~= C~ C= C:
C' h F.
C~. C. c. ~--~ > Cc.. C. G-=
C`. C= C.= ~= ' c. ~= C`.
C~. Cl. C-. C. ~= G~. C~= Cl
=
~=`=`'c~:3>w
OO M[- N
p 00 M N 00
[~ M ~.y N N "C kn
~
~
z Z <a<
<
> a V ~ v)
>izwW~~>~Zw
~`aw>
zza3z3z~a
2~oaQ
<~~~zQu~.a
a>AWA~a~~
...=1 w ~
a a E-~ Q~..] o. t/~
UL~aQon
wzM zQ
~r-.~otno, o"cr-
~p ~O o0 O~ O~ ON o 0 0
0
ooooo0 00 00 00 00 00
~ o 0 0 0 0 0 0 0
z"oc "o
r- N N N N N N N N
I- N N N N N N N N
zzzzzzzzz
I- N N N N N
xxxxx~x~
LO
N Q Q Q Q Q Q Q Q Q
W a a a a a a a w a
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~ ~ N C,I Cq Cq c"I N cq N N ~ c-'l C',
N N \ \ \ \ \ O O =-\-~ =-\-~ ^ O
tPl V) O, [- l- 00 00 Vl ~ 00 'o l- W) l- (- =-r rr .-. I'p tn W1 W) 00 =-r .-
. =-. ..r ~ .--i
U
O c on C C: ~ C~~~: ~: C. ~~ ~~: : ~: c~ : C; ~: ~
co M ~¾~ ~:c c>-.c ~ Qc Qc c`j ~=c~` c?A=Qc=~ ~, ~¾Q~c ~?~ Q
N ~ C; C' C; : C~; C; C= C~= C= ~= ~: C== C. C: c. C=. C' C; C: c; C. C; C;
c.. c.. C-= C-=
~.. ~. ~= ~. c: r= ~= c: ~. c; ~= ~` ; ~. ~. c= c' c; C= c= c~= ~' ~'
~ w >>~..w~w.a? ..w3w~~-w?~~>
U
*-.4 _
Q) ~, 00 00 O, O, M ~O ~C \,O O~ N~n N Q~ tn - - ~D ~O O N-- O~ N M v~ ~n ~D ~
O
(n M 00 "q =~ M\~O 00 l-~ M 0 ,O N N N N
U O O=--~ cM t+M ~ N N l- ~n M~ N O N=--~ O~1 l~ =--~ =--~ tM ~~7 00 -==~ N O
N Q
U y
x3¾CY 4z
wwx~zzcyp~-aa5'4a>¾ (n x>
> Q>VE- Q¾~U p >
~ ¾
E->F"> >
cu o az > ¾
c U > > w >
0
0
~~¾x ~~ >w1a~ ~~o A~
o w`nH>>~~x~Ca7~aZõ~da~u
>¾H~~~~~z~~¾2~>~>
>H~ ~ w>c7
wv >www~v~~~F~qaxzxoW¾¾wa~>
~,wwHAwo E" a
Z > w- x
>~awQw~4 >
n w:F~> >> - Hrx ~=~a ~~ ~a`~3aw y
p aZxzz~"'~~a-a~>~3¾
~a¾w
~ oG E- AQwZ>za: cn w~"wZ>
Q
a) M
N vl ~O O~~~ O 00 O 00 vl ~ N O N Mt/'~ ~/=~ O ~O 00 O~n vi "G o0 O rM
O O~ =~ N M M~ l~ O \O 00 00 OO O M M M l- M~' h~O ~
M~~=--~=--= =--=--=--~ =--N N N N N N N N M M t+=f M M:T~~~t 'tt
f--1
(0
=~ Q Q Q Q Q Q Q Q Q Q ¾ Q Q Q Q Q ¾ Q ¾ Q Q Q Q Q ¾ Q ¾ Q Q Q Q
~c o a aaaaa.aaaaa.aaaaaaaaa.aaaaaaaaaaa
w
m C-4~
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N N N N N N N N N N N N r. ~-. r. ~. r, r. ^"
O O O -~- ~- ~ N ~ ~p =-. .-. ~ -. -r -r .-. -. ~ O, C% "O IO
.-~ . C= ~, C C C; ~; C; C; C C;C C; ~ C; ~; ~: ~; C C;
= ; C
C :
~~Hc C~ ~~>Qc Qa~¾ Cc?~
~ ~c=U~ vi~~
C~. c.. . C.. Cl. C= C c.= . ~. C-. C, ~. c.. C~. C= C Cl= ~ C'-= C=
~. ~; C: ~.C; ~; C'. c-. C c-. c;
C~ C' C; C C. C. C; C= ~= C= C; C~`. C`= C-= ~; c-.
~~a~waa~ >~: aw>-aa :3>3w
~C o0 N O~ ~t [~ N~tn t+i
00 D .--N oo ~O l, t cl ^-+ 00 M N 00
N O M M O~~ O l- 00 v'1 =--' "O N N O~ "O v1 O N Nk!1 N N'D ,t vl
azQa~~xF a>>a
Qa'nda~w~wwWW>~~Uc~W Q`~aQz`n
zHaQOa~~a~C>v~awwr~dW ¾~'¾a>~aa
>
<
ucnHW aHa~x~~'a~'f~ ¾~
~U Uz wH W x~aav~zaQC~zax
Qv~~~ H q ~a~õQ pw Zz3
zQzo ac~~q
~aU >QWaac~-aQ~naH
Qav~da~w~w W u:C7 w~'~~'p¾azvwiua
~~aW¾>aaaa
> w > o w
Q~¾ wcn~~H>a aaa In
a v G c. Z U~ 0 A>~a w~ C~7 d W w U~ Z w Z Q Z¾
v1 ON l- O N O ~t l- N v1 O~ ~ O~ v1 'D O~O O~ t, ~ O~ O"C l-
O D ~O [~ 00 00 O O- N 'D ~D [ -00 00 O~ O~ It v) D D O~ ON O O O
v1 v) Vl W) tn v') W) v~ W) W) v') l-
to
N Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q
W a a a a a a a a a a a a a a a a a a a a a a a a a a a
J
m
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~ U
N M M M M M M M M M M M M M M M M M
=--~ ~ ~ ~ ~ c- ~ ~~- ~~- ~ ~~--
~
4) (y N N N N N N N N CO CY) M M O O v T"
~~-
N
C/] O
U
~ O -~~00.~0 - O~O=-O~o -O- O - 0~0~00000 O
~: ~: c.~= c C= = C~= = C~`= = cC.. , c, ~= c-. c; c; ~= c,= c; C-. ~: ~: ~:
~: c: c: C: c: C: ; ~: ~: ~: ; ~:
C- ~. C= C= c- c- C. C. C' C' C~= C= C'' C' C C~ C- C~ C~
` ~.. C~= C~=
aa¾¾QQ~'c'aa ~'=c' UUQQaaU`-'wa>
c~= c~= c'= c'= c.. c.. c. c. c. ~. r= ~= C. G4 F. F. ~. C. P. 0. ~= ~= ~. cl.
C.. ~. c= U C. ~,. ~.
r= ~= c. c. ~., ~. ~, c, c.= ~= ~. C.= r= t= ~= ~. ~. c'. c= ~= c. r= c,. c,=
c, c. c. cl= ~. ~, c.
V~ C= ~ C- ~~= C' c' C, ~. C= C= C= C= C= C= C= C= ~= Cc`= C= C= C, C, C, C~=
C; ~; C,
C-= C~= c.. c-. c.. c.. c.. c..
;E .a a a a w c-, - c--' > ? ~ ~ c-, : .a w a ~ w w a
U
00
a N NW00 O N W) tn M M _ ~ N N Cp Gp N N O, p~ ~ ~ 0 -0 ~~ vi ~~ N
l~ tn
N V-~4
O
co
.5, x2 aazzQQHH xzz~a~~wwH a
0 ,..a QQaazzv~v~C7C7v~~n AAu.a~wwF"F"
w w a a. Q¾ aCY `" " E- F- >4 V. >
aE"aaaaQQQ¾~~a a wwUU >
aU w-l a
pE".
> a a z z w w P. O A A a C) Qm~ U) z vUi U
o aa~~~~~? aa aaawww~.wa
~
>4 >4
> x " ''Qwwa~~ xaaa~~wwzz>w>>
Uww~~aazzQQ~~Q xx~~wwwwwwww~~wwa
¾ Q aaz z~~ E- H v'v' Z~ Q A w w ~~" w w~~ >
o.aQ¾oo¾Qy~ wwUU
~~p..-aaww>
H H aa¾¾ Q p,aaHha a vUaa
a, M `i'UU
a~ a aHH~x aaZZww ~aAA QQ ¾v~i
U' a a a >> v H H A A~~ a ' aaw w w w z
~zSaaww..~~aaa~_~_HHHH~~QQaawwwwWzW
Ew=H."~a~wawa> x¾¾aac~7C~7p.a~~UUwwCa7C7~~U
z z w w H H 2 z v ~ v ~ A A ~ a C7 c7 o
zzaaF~E-wwaaHHC~~aaaa.E-F~ x~aaz
N N o0 oO ,t th oo ao O~ O\ N N cO oo v~ tn N N M M o0
O - M M[~ t~ 00 00 C~ O~ .. .,_, r.. N N M M~O ~0 00 00 00 00 O~ O~ O O O O O
~ p N N N N N N N N N N N N N N N N
~ ~ ~ ~ ~ ~ ~ \.O \0 10 ~o \0 ~
M~~ M M M M M~ M~ M M M~ M~ MI'* M~ M~
~p N N\4p ~10 N~p NID N 10 N~p N~p N%p Nkp Nsp N\0 N1p N~p N%0 N~p
M M~ Mas Mc~ MCN MCYN M(ZN a\ M~ M~ M
(õ) 0\ al M Mc\ ~
(D OV) OVI) O'n Okt) O'n Otn O'f) O~ O~ O'n O~ O
r~. w N N w w N N N N w w N~r w N w N~+ w N~+ N N w w N w
N N -- N-- N N- N-- N
04 im-+ vzi N Z vZi vz1 Z z Z v~i N~ Z vZi N vz'~ Z v~i Z~ Z vzi N vzi Z vz) Z
vzi z vzi N vz'~
~,xx~xx~x~xxx~xxx~x~x~xxx~~x~xxx
~_
W a Y ao aaaaoamwoaaaoaaaaaaaaamaaaaoamaacnaacoaawaaaaaaaawoaca
I-N 0 a a a a a a a a a a a a a a a a a a a a a a a a a a a a a P. m " a
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
M M M C~) M Ce) M C~) M M M M M M M M
M M M M M M M M M M M M M
r ~~ r r r r r r[~ r r r l'r r T l~ l~ ~ r[~ r l'r r{'r ~.,\ \ \ \ \ \ \ \ \ \
\ \ \ \ \ \
M M \\ \\\\\ N N N N N N \\\\ O O M M M M M M\
pp ~ 00 N N (O ~
O O O O=--~ O O O O O O O O OO OOOO- O O0 O -- O~ O
c"' Cl. C-= Cl= C: C: C-= C= C" C' C, = C`= C= C= Cl Cl C' C' C.. c.. C~.
= C== C, C-= C-= C.C C-. ~. Cl= ~.. ~.. ~. C'` C'` C. C-= C' C' G'= C`= C-= C-
= Cl. C= ~. C
. C'. C-. C= C-= C. c.. C'l C~ C..
~= c~= ~= ~= >> F' E' ~~.~ a H H C ~~' c¾
~ ~>aav;v;c ~~~ ~~ c.c.~:~:~:~~~:~~~:~:c:~:~;~:~ ~:c:~ ~ ~~~:
~= C.' C- C' C' C. C' C' Cl. C' Cl' C' C' C'' C'' c.' ~ C`= Cl= Cl= Cl= C.' C.
C.' C' C= C, C'= C'= C. c..
> ~ i ~i ~ ~ C.. c`. C ~'' C'' C'
wa w w~ ?J aa.c.
aaaa~~>
w
M M O~ O\ v1 v'1 00 00 00 00 00 00 O
I_~ p~ N[~ l~ N p~ N O~ N=--M~ O O O O M M00 00
~=--~ =--~ vl O~m C! M O O M M 00 00 O O~~ ~D "O N N O O O O d: =--=--~j
p~ . ~ N^ N^' M v~ N N N~O ~O ~~ O ~~~ M M O O 0~ O~ ~D ~0 O O l~
cM
W4ww aa
zz
A F C7 w~~ E- F E- F- C7 C7 F- F- a a ¾ ¾ O O
aazz
~ wwwu~wu~~~ xx~ E aaHH z z
p~ p~ v~ aa~ ~~w
wc~'"r.ZZ¾~~xxzz~~ddr~"r~"~~¾¾~~zz aa>
ww ~~ w
w ddz z W W~~ H~" a a A A~~ H >> d0a~ a~~~~ H H ¾ a x
>~aa~~wwa~ww~~ ~~aa~~aaaa~~>
> dda z z(D v~ w W w O a~ ~ p 0 a~"O F- E~C ~ 'CY >> U
aAA~aa¾~WF~~aaaazzaa
>>aazzHH~~,ww~W~HH HH~~HHaazzHH¾Q
ao~~a~ xHHaaHHaa~~~~~~
wzzz¾ zz
aa~~aaHazz aa>
Fa~zz cn
~¾Ha~a~A~~~H~~H~a~~ aa FFx
>aaawwxx~" aa A ~~~~HH ¾¾
~~~~wwa~ww~~ ~~
Z~~OAQQWwvzivzgw~c~E-
~~It M ~It O~ O~ O~ O~ O~ O~ O Or-õr-~ 00 0_o C_~ O~ N N M M V' vNi
O
=--.--.---~ ~= ==-~ --= =--=--=-.,. .~ --. ... =--~ =--=--=--=-.,. r. N N N N
N N N N N N N N N N N
N N N N N N N N N N N N N N N N N N
~ ~ ~ ~ ~ ~ ~ ~ ~ ~o ~o ~o "0 "0 "0 1%0 %D %D
M~ M M M M MM M M M M M M M M MIt M1* M
N N~p N%~p N N"0 N ~p N~p N~p N%0 N ~p N~ N~p N ~p N~p N~p N~p N~p N
as M aN M ON M a% (~1 ON M c~ M ~ M c~ M c~ M cs M ON M CN M ON M a~ M a, M
(7\ M ON M c~ M
'n Otn OW)
(=>W~ Otn OW) Otn O'n O'n Otn O
~r Lr. L:. Lz+ fi. N w w N Lz. L4 N~+ N~r N~r N N N LL N Lir N
N~+ N N N
rG Q ~4 Q ~ Q ~ !-. /-. Q ~ Q :541 Q ¾ Q
N N N N N N N N N N N N N=--N~ N N~ N N
zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
xx~xN xxxN x" W) xxxxxxxx~x~xxxxx~xxx~x~x
~
- - - - - ,-. r. r. r, - - - - - .-. ... r.. - - r.. - - - - - - - ,-.. r. r.
r. .-. - '. r, -
N
W a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a m a
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
M M M M M M M M M M M M M M M M M M M
M M c) CY) O O N N N N
00 NT 00 ~-- ~ ~-- CO 1~ CO O O O O O O
O O O O O=--~ O~ O=-+ O=--~ O O O O O O O r+ O=-~ O~ O=--~ O~ O=--~ O~ O=--~
O=--~
~= ~=
c~ = C~= C-= C= C'= C`= C
~.. = G'= C"= C. C'.
C. C= C~ C= ~- C= ~= C= C. ~. C` ~= ~. C. C`= C'. C'= C= C= C'= C`= C`= c..
C`= C' C' ~= ~= ~.. ~.. C= C'= C C.. C..
Q¾u u ~aw~~HHHHc ~=~ HHc ~ ~ ~;c c ~~aa~~~~¾
~: ~: ~; C; ~: C; C: ~: ~: C: C: ~: ~: ~; ~: ~ ~= ~ c: ~: C= C= ~= ~= C: c; c:
~: ~~ ~~ ~: ~: ~' C' C: C:
Cl= C= Cl- C~= Cl= Cl. Cl= G~= C`= Cl= C= C= C. C. Cl, C. C.. Cl= C= C~ C~ C-
~: C: C~= C~= C~= C~= ~, ', C-= C~= C` C'
C= C= C. C. ~ ~. C= C. C- C. ~. C. C C= ~= C.. C. C'l= C. ~. C. C. C.= C`. C.
~. C' C'. C.. ~.
wwaw.~a a> :: c-, - ;33ww wa~~ww
p O~ N l~ 'n ~'n N N tn tn -tt "t O, O, 'O 'O O O'n on rM M v~ ~n
~~ 00 00 N nj W) 00 00 c'? O O r vj W) p~ p p-. r. .. -, O O
11 O N M M~~ M M O O N O N N M
=--~ N
O
x~ AQ~~3~ 33~"U)
z Q,o
w a a U U > z
ww¾H~ ~~¾¾Z Z
>wwx ~v~v~wwvZivZ
> w w a a >>>>.~'~ > >> a. a~~ w w
HHww p c~c~~>
uxx0 zzaaAQ33z ~z33zz~~ww xx~c~
7 v~ v~ O ' z z W w
U U U a 07 0 O
U) U) z~~~ c x7 c x7
~Qaa
¾
Ha¾¾wwaa FC~H~H~>H_~a~wwwacn cn~~~~
> ~ Q a a x
w H a w ~ v~ ~ A Z ~ ¾ ~ ~ ~ ~ dz dz > > >
w>wwxao wwc7A¾a~¾E- E- ~c4z ~¾¾~~
> v~v~HC7aw¾w~~-cd~4 ac~a.av~v~w ~r~
¾ww
i)
aa ww
>x~QHa~a~zz ww~ ~~a~w a¾~~ww~~zz
a~' c w7 w w>> U Uj a Q Q Q A vw io Z Z aP. w w~~ d d a a w w o G a; Q Q a a
N M14, It 00 00 ~D "0 W) W) 110 ~O o0 0o N N l- l- c0 c0 -- - vi in N N M M ~O
~O [~ l~ M M~~
lry tP) Vl V'1 V1 t!1 "p "p 00 00 00 00 O~ ON O O=--~ ="~ ="~ ^~ N N N N M M M
M M M M M d' ~~'
N N N N N N N N N N N N N N M M M M M M M M M M M M M M M M M M M M M M
N N N N N N N N N N N N N N N N N
%O \C \G \C \.D 110 \1O ~p b ~O \~O ~O "G kO \C \O ~O
K1 f'n M ~t M I'll M M ~= M 'IT M It M 11, M IT M It m M CK1 ~ M M
01 O, \D 0\ ~ ON ~o O\ ~ ON ~o 01 110 CN ID O~ \0 ON 0~ (71 c~ MON Ma% MON MON
MCN Mq~ cf) 'n kn O 'n O 'n O `~ O 'n O kr) O W) (=> V) O 'n O tr) O 'n O kt)
O 'n O tr) O V) O V) O 'n
a 4 N N N=--~ N N N N N N N N N N-- N N N N-- N~ N
zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
xxxC14 xxxxxxxxxxxxxxxinxxxxxRxxtnxx~
ti
N - - .-. - .-. .-. .-. - - .. ,-. - .-, .-. - - - - .-. - .-. - - - - .-. - -
- - .-.
J aaaaaaaaaaaa.aa.aa.aaaaaaaaa~aaaa~.aaa~.aaaa
W
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
M M M M M M M M M M M M M M M CM M M M M M M M M M M M M M M M M
M M M Cr) M Ce) C`') m
00 00 00 00 00 QO tn ~ ln ~ ~ ~ ~- I~ ~ N ~ N~- N~ N0) N~
O'-~ O~ O=--~ O-- O O O O O-- O O O O O O O O O O O-- O~ O O-- O~ O~+
P= P= C,-~= ~ C~= ~ C= C C= C; C; 1- C C' C= C. P P ~= ~=
C ~= ~= ~= C C; C' C' ~, c= C, C'C' ~= C= C' ~= C' C' ~= C
~ ~ ~ ~ ~ ~ ' C~ C~ = ~ C~= C~= ~= ~= C= C= C~= C~=
c=~=~ ~ c=c = ~ ~ ~ ~c.~=~=~=~ ~~ c C~ Cv~ v~
C' C' c~ aa ~ ~
~~~~~ a a C C Ca Ca > -C- ~ ~. C ~>. C~. C/i ~~.. ~. c. ~. F E C~= C'= C.. ~.
a a
~= ~= ~. ~. C= C= ~= C= C= C. C. ~. ~= ~= C= C= C= C= ~= C~ C~ C- Cl. C~G~= C.
C C. C.
C. C= C== Cl= ~.= ~.. ~.= C= C~. C~= ~.= ~.. C- C Cl C- Cl C- ~.. C.
C= C= C'= C~= C ~= C= C= C~. C.. C. ~. C.. C. C= C.. Cl= C~= ~. C. C. C. C~=
C~. ~. C C Cl C' C= ~= C C` C. C..
aa~~wwaa~~aa~~~~aaa>~a;~ ;~ ww2
M M=--~ v~ in 00 00 N vl p, ,O ,O o O, rM v'1 l- -,i- cM o O OC', -,t ~ M M~p
~p N N
00 00 N Nm m Nc~ 6 00 t.j n V~O wi O tM M O~ c~ O O~VI 10 O O O~ O~
O 0 M M tn v1 O O M~ N , N N N-- v1 ^~ ~D O~ O O N M 0 O O O O O O~~ M M
LO
~
HH~a~~~ac7v~~~~ aa
> > ~ c 7 a Z
~ ~
¾ ¾ ~ > a a a v c 7 Q ` ~' ~a ~
~~~~~aa¾~=aaaviviaa>C7H~ , , >v~z ~~ vv
Env~ pp~~QAw aw0c7~H~c~7~~~~aaHH
~>
Q ~
waa¾¾~~ww~
aa wwwaa-aa~>~~~~aaaa>aa~~~ zw~~
vippzw Inv~~aC >>OWZZ~4~4
aC x o4 c4 H H Q Q a a w w w w~" H a a v(D 0~~~~~ H H~ >> a a a a
cn cn ~~ a a A -= C7 0 cn w~ a a C7 v~ v~ v v
~p aC7C7a-aHH 0 0
aa ao
ww~ a ~~~A~w>~~~wA¾> v~ v~
wwzv~ E-~~ ~aaav~v~ ~ C~c7zz
jaHHa~wQH>>~~~~ zwa ~~>ad
vvcn v)a~xaa~~AAww>>~ HHv~cn
w~7c7~HHC7c7~7c7w
xa~wwv,v~HHwwzv2
c7c~~~wrwxr~;¾~.aaQ~~ywWW~~aA>CaWQgL, a4~~~~zwzw~~a~
>:-5 0 0 > H
>E :FHHwwaa~C ww~a~a~~aaQ.> ~Ec~c~~zwv~v~
v) 0rxQQAAw~.-.~.~~ aF- 0 0 -= ~D "G I~ l~ It qzr 00 co M M O (D ON O~ N NW)
v) I'G 1~0 [- I- cM cn O~ O~
v~ ~V1 v1 kn W) "O ~D ~O ~10 t- l- 00 00 00 00 O, QN ON O1 O, O1 O, O, O O O O
=--~
M M M M M M M K1 M M M M M M M M M M M M M M M M~~~~~' V' ~~~' ~~~
N N N N N N N N N N N N N N N N N N
"C kO \~D "O \O 110 \C \G \.O
~ N~ N~ N~ N~ N~ N~ N~ N N~ N~ N~ N~ N~ N~ N~ N~ N~ N
MCS MCN M0\ MON MCN Ma\ Mas Mc\ Mc\ a-, MC\ M(71 Ma, Ma-, Mc~ MCN Mc\ Mc\
O'r) O'n O'f) OW) Okn (0 W~ Ovi O'f) (D V) O't) (D 'n OW') O'n O'f) (D W) O'f)
O'n O'n
w c~ w N w c~ w N N N N N N w w N N N
NN.--~ N N=--~ N=--N=--+ N=--~ N =--~ N N N=-'+ N=--~ N~ N --~ N=--~ N N=--~ N
z z z z z z z z z z z z z z z z zzzzz zZz z zzzz z z zzz z z
W) N u1 NW) NW) N v1 NW) NW) Nkn NW) NW) N VI1 Ntn NW) NV) kn N kn tn Ntn
xxxxxxxxxxxxxxxxxxxxxxxxxxxa~xxx~xxx
ti
r. r. - ... r.. r. r. r.. r, r, r. .r - r. - r. - r. - ..-. r. - r. r. r. - .-
, r.. - - r. r. - .--. r. r.
N
W a Qa a a a a a a a a a a a a aM a a a a a a a a a a a a a a a a a a a a
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
M M M M M M M M M M M M M M M M M M M M M M M M M M M
00 M 0~0 M N N~- ~ ~ ~ ~ ~ N N M M M M M M M M N N N N N N
O^-O=--~ O~ O=--~ O=--~ O=--~ O=--O~ O=--~ O~ O=--~ O=--~ O~ O~ O=--~ O~ O=--~
O~
cc-
C~= C= Cl= C`= C`= C`= = C~=
C C. ~.. C~= C~=
C= ~. C' C, C, C, C= C= ~C= C= ~= C= "= C= ,= "= C= C ~ C= ~= C= C= C, C, C~=
C~= C~
~~a~aa~ ~ia: waa~~v~v~v~v~~ ~ vi vi 2 ~a a c E-' C. c.~.
... C. ~
c = C, = ~ = ~ = c,' cl' ~ c = c' ~,' ~ . c . c , , ~ . ~ . ; ; F" E c`' c.' c
= ~ = F.
~' c. = ~ = = C = C= " = c . c= c . .
C= C ~; C; C; Cl= C= C`= C-= C C; C; CC`; C, C. ~. ~, C, C. C; ~= ~= C; C; C;
C; C; C= ~=
~= C= C`, C, C, C~, C~ C` C. C.. C-= C-= C. C. C-= C. CC C C` C, C= C. ~. C.=
~. ~= ~, C. c.. ~. C.= C~ C~
aa 33.a.a? ~.aa 2 :~~~w wwawww~~
a, C, c~ a,[~ I~ M M M M V) Vl O O ON ON O O="~ - M M- - N(y W) tn M O~ Q~
[~ O O V1 h N N~j ~j 00 00 \D "O N i/1 V=1 t(1 v1 V1 M M=-= =-r ="'~ M M~~ M
M M~~ 00 00 O O=--~ N N~'n 0 O~O ~D N N N N vi kn O O o0 oO
(0
co
Z Z W a~ a~~~~ v~~n x Z Z
wwaa~~ww~ ~ww
~
ZZC7C7~~v~v~zz
a.aaa a > >
~
~.a c7C7
UU~~ C7C7~Zww wC7C7v~v~
- - 0 0Zj~E-~F C7C7~~ z
C7 C~ v~ v~ 3 3 A ~ x > > C7 C7 ci! a~ > > ¾ ¾ v~ v~ a a C7 C7 ~`" C7 C7
aw ~~~ ~~ww wwaaww~~~~,
~ pp¾¾ v) cnJ
aa v~v~~~ ww v~v~wwv~v~
? J~AAww~ ,~~ cnw 0aa0 cn wwAA~~~~
~v~v~AAC7C7 r~c~: v~v>
dd~ aaA A w w~ v x w w w w W w a a c7 c7 aC x
ww
U'~'aa~nv~xx~~~~J~~wwQ Q ~ v~~ ~
a a a a 2 En En cn w w w w > 7 C > 7 c ~ 7 C~7 > > > >
cn Za~33A~Az~~FH¾¾z
>7 > 2~~>;
:~v~3333 ..~xx c7c7v~cn ~nv~aac7C7
w
w~a ~~ aw? wwaa ~~C7C7
0 0 wc7C~ cr. P.
HH33¾awc~-,
F>- E>- .a a F- F- y~ w w a4 x U U~~~~~n v~ kw uw ~~ C7 C7 Q¾~~ a a¾ Q~~
~nv>>>aC~4E-F- AAAAE- UUC7C7~C~Cv) oa:Y4>o oawv) cnAA
~p ~D o0 00 O~ O~ [- l- N N m M[- I~ ~[~ l- ^ N N~n O" C, N N M M~n v1
N N M M M MT ~~O ~O l- [~ t- [- l- [- 00 00 Q~ O~ O O O O O O O O=--~ =--N N N
N
t7 ~ ~ ~ ~ ~ t ~ ~ ~ "T t ~ ~ It Vl kn Vl Vl tn Vl tn V) t~ ~!1 kn v1 W) W)
N N N N N N N N N N N N N N N N N N
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~o 1.0 \0
C1 M~= M MIt M11 MIt Mq:l- M"t MIt MIt M"T M'It Mllzr M1:T MIt M MI'* M
~p N~p N%p N1~0 N "0 N ~p N~o N~p N~p N\p N'p N~p N\~p N~p N~10 N ~p N I'p N
"p N
(71 Mall Ma, Ma, MCN M~ MON MCN MON M(7\ M c~ Ma, Mcl, M CN Ma, M~ M ~ M~
O~ O'~ O'~ ~ O'n O'n O'~ O~ O~ O'~ O~ O'~ O~ O'n O'~ O'n OW) O'n
w c~ w N w N N w c~ w N N N w N w c~ w N w N N N N N
N N N=--N=--= N N N N N N N N=--~ N=--~ N N N N~ N
zzzzzzzzzzzzzzzzzzzzzzz+zz zzzzzzzzzzz
xxxxxaxxxxxxxxxxxxxxaxxxx~xcq xxx~xxxx
ti
N - .-. .-. - - .. r.. - r.. r, - - - - .. - ... =-, .-. ... - - - .... .-, - -
- r. - - .-. .-. ..,
W aaaaaaaaaaaaaaa~.aaaaaaa ~.aaaaaaaaaaaaaa
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
M M M M M M M M M M M M M M M M M M M
e-
N N N N M M N ~~ ~rl- 0) 0) O~ O=--~ O=--~ O~ O~ O=--~ O~ O~ O~ O=--~ O O O O
O~ O-+ O=--~ O O O=--~ O=--~
C'' C. Cl= C~= C. Cl= C= C~= C= C~= C= C~= C-. C-= C,= C= C`. c. c C C. C..
~.. C. ~.
~= C.= C: C: C' G' C: C= C. C. C: ~= C= C; C, ~. ~. ~= C. ~. ~. ~. C. ~. ~. ~.
C. C. C. ~: ~= CC`= ~= C=
c.. C`=
~ ~ ~ c c ¾ ¾ ~ ~ c= i--i = ~> > a a ~ cU~ cV ~ ~ ~ ~ ~ C a a
~.. ~. ~. ~.. ~r . c.. C. ~. C.. c..
= ~= C' C` c.. C. C' C' ~. ~. ~.. C.. C'= C'=
C. C. Cl. C. C'= C'= C' C= cl. C, c= Cl= C'= C'= c-. c.. c-. C. C-= Cl= C C-
c'
C`. C`. C: C: C'' C~= C. C= : C; C. ~. C= C= C~. Cl. C`. C`. C~ C~ ~. C. C: ~:
C. C. C`. C`. C. C. C~= Cl= Cl= C'= ~'
w33 ~...- waw..,
~
00M MW)W) N N N N N N N N v1 0O m=--' Gm~~~ O~i C~O ~~ n O O
O O~t rt M M O O M M r. ~~~ l~ l~ O O M N ~y ~ N N N N N N N rn O O-= -=
.-r
00
> aa~~
~ AA¾¾¾Q a~~~-~ qo1
K~ ac7a aaa 3 z W w a a 3 3 ~
~~
ww~~QQxx
aa 33vva.axx
a C x x ~ a
20, ' w w 3 > > a > >
OwO~ v~v)aa~~ awAAaaaav~~Q¾~~ww
xx o U2~
AAaW¾Q~¾~~F- ~~~aaAA~a>~ ~Qxv~v~
AA aa WWC7U W WW QaCx
~a ~W~~.a.aan. ~3~AAZZ`nv~~~¾¾
a c~ aaw
w 3
~Z UUv,vx,> W>>Ca7Ca7
zzz ¾¾gg55HH~~7~- 7~~awwc7Uw ww~~¾¾¾¾
~0xx
aC~Z ~~A A F- F- aW ~ ~AA. a 3~ a a
aW.x~ OZZv~v~I
aaww~xa
a~~~ww aa
E-F- zz AA a v~
xxc7UAA a a,wwv>
C7 C7 >> a~~ Z Z A A r~ w5~cW.7 Ca7 a a~~~~ Z Z w w A A U U~ Z> W Q Q
ON Ol 0 Oqt It Ol~ O~ 00 oO qt qt 0 O O', ON ,t qt ~O ~'O =-~ =--~ O 0 o0 00 N
N 0 OID ~D
~ N ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ N N b N ~ ~ ~ ~ ~ ~
N N N N N N N N N N N N N N N N N N
"C %C %G %O 1.0 ~O "O "O \~O 1.0 "O ~.O 110 "O 110 110 ~C "O
MqT M M Mqt M~t MItt M Mqt MIt MIt MIt MI'll Mqi Mqzr MIt M'IT Mqt M~
~p N%0 N "p N~p N~p N ~p N \~p N110 N\p N ~p N\p N%.p N\~p N~p N~p N~p N%p N%p
N
M0~ MC71, M Ma', MON MCN MON MON MCN Moll Ma,\ Mall MG~ Ma\ M0\ MON MCN K1 c\
Okn O tn O tn O In O W) OW) O W) OW) O W) Okn 0 tt) 0 tn OW) O tn O W*l O tn O
W) OW)
cli~~~N¾~~N
N=--~ N=--~ N =--= N =--~ N =--~ N=--~ N=--~ N N --~ N =--~ N =--N=-~ N=^ N=--
~ N ~ N=--~ N ~ N
zzzzzzzzzzzzzzzzzzzzzzzzzzzzz+zzz.zzzz
tncN tn xxxxxW) xxxxxx~xxxxxxx~xxx~xxxxx~xx
ti
r. - .-. - - r. r. r. r. ..-. r. r. r. r. .- - .... - - - .., r. .-.. r. r. r.
- - - r. - - - - -
N
W a a a a a a a a a a a a a M a a a a a a a a a a a a a a a a a a a a a a
J
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
c7 MCV) CV) CV) Ce) M M m M m O O
~ ~
O) O) O O ~qT O
O-~ O~ O-- O-~ O O O O--O O O O O O O O-- O~ O O O O
C; C; ~; C. ~. c= CC`= ~; C~= C~= C= C~= Cl= G, C C` C. C; C~= C'~. ~; C; C;
C; ~;
C'= C.
H H v~ C'`=
CArr ~ u F.c, c- c- c- C-=C-=C-=c'=~
~: ~: ~. C: ~: C C C= C= C a a h h C C. c. C. C. C; C; C; c c C= C
Cl= Cl= C.. c. C.. C.. C; C; C`= C'= c.. C; ~; ~: ~; C- C~. C= C.= c`= ~. C.
C. C; ~; Cl. C~= ~; C'
.aa~~~wwww >ww ::E
0~0 0`00 \O \O N N M M~ M N N~O 00
l~ 00 00 " O O
N N~ ON N ry W) kn n n~~ O 0 qt 4 ~ M O
O
O
~ HH~xa.ap~p~aaw Q¾~w~¾xx
>~'¾ wwwW
¾¾HH33~~~z ~
F- UU v~v~~ > >v~v~AA~~FF-
¾¾¾¾xxaaVUUa.Ci.>>~~~~v~v~E
¾¾HHwwaaUwr~.,wu.,~~v~ivcniww¾jQ¾ww~~UUV
wWAAQQAAw~ y ~W W~~"r.taC7C7~>wwv~v~W
~ > > > > a w ~ ~ >- w w w w > > > > > w w a ~ .a
330. 0.
HZ ~v~
~ AA¾Qc~ u
>
Fx- Ex- v3i~ UUwwx~~ ~~~a~~AAwwc7~a:
aa~x w ~aa~wwa c~~
HH~~aau ~H'n>> cnA
¾¾Qaxx~~u u uaa, ~~ ~>> H
ax U w w vi o vi U
A(~ >> a
¾Q~Hwwaaa~w~v~~~Q¾~a v~~v
~~QQ AL1~~dx aa - - ww v~v~-x
ww wwwww~~~> wwa~x~
cn 4. > >
~ w A Q cw~7 ~ aaaH z ~~ v~ ~¾¾ A A¾¾ w w
>oGwAAwUUww ~~cn cnww~a~awwQQ
v i ~n l~ l- O O M M o0 00 O~ ID "O O~ O~ ON O1 't :T tn tn (7) O O M M~~
v1 ~n ~n ~n ~O ~O o0 00 00 00 0o ON ON O~ ON O O _ =~ =..~ =--M M~'
~ ~ ~ ~ ~ r ~ ~ ~ ~ ~ r- n r-
N N N N N N N N N N N N N N N
~O 1.0 ~O ~O ~10 ~O IC ~O
M~' M~ M~ M M~ M~ M~ Mqtt MI:T M M~ M~
N~p N~p N ~p N ~p N N ~p N`p N "p N~p N ~p N~p N %p N~ N
MON MCN 0~ a, 0~ M Moll MON MCN MON MCN M~ M (7N
LOL N w w w w N ~
N N N w N w N w N N N N N w N w N
~5 Q>E>-,>-~2>EQ~¾2~5>~ Q2:E Q:~ Q2
N=--N=--N=--~ N=-+ N ^-~ ^N=--N~ N --~N =--~N ^-~N ^-~N-~N ~ N
Z Z z z Z z Z z Z z Z Z Z z Z Z Z Z Z Z z z z Z Z Z 7 7, -7,
kn N~n N tn NW) Ntn NW) W) Ntn NW) kn v1 NW) NW) N'n N ~1 N
xxxxxxxxxxxxxxxx~x~xxxxxxxxxx
ti
N - -
W a a a a a a a a a a a a a a a a a a a a a a a a a a a a a
J
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~
y U
V ~ N M M M M M M M M M M M M M M M M M M M M M
~ -. -. M MM -M. -r -.M~r, r ~M -r -r~M"" ~MM.
O ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ^'" ~ ~ ~ ^" _ ~ ~ ~ ~ "" ~ ~ ~ ~ ~
v' y N N~ N N M M~~ O~O M~ O~O r N+ ~ N-= ~O ~O ~ M.M. ~ 0~0 O~O _M_ ~ O O~~
O~ O~ ~~
N , ~ ~ 'r ~ ^i 'r ' .-.
Cn O
U
C~' C'~= ~ C'~. C~= ~.. C~= C'~ C~= C. C, C, C~= C, C. ~= C= ~.. ~.. C~ C= C.
~.. C= C== C~= C~' C, C= C~= C= C ~= C\ ~i
rn c~' c~= c: ~= r' ~= r c= ~= c= ~. ~.. c= ~`= ~= c=~= ~= c`= c= r' ~= ~= H.
a ~= C' ~:
r ~ C ~ a Q¾ p: ~ y Q c~~~., ~~ ~ ~ c~~. ~~1 ~ C~ ~¾~ ~~~~. C; ~: `~"= a ~ C:
~ C: C: C: C; ~. ~. C. ~= C. C: C: ~: C: C: C: ~; C; C= C: C: C: ~: ~; C= C:
~: C: C; C: C; C: C: C: C: ~;
C~= C~= C= ~' C`=
o ~w~w>~>-~>a.aww~>~~~ ~~a.a~'> w ~w~3w>~a~
~,
M C~)
~
~ C N o~ c%, o~ aM, o~~ o o o o`~'o o~ N o o v O0 v o~o N~ o ~ M~ o ~_n M_
~ ~ O~ N O O oo -- O~ l~ O-- ~n N~O ^-~ M O ~ O~ ~O O~ N M O M O -- O N O~
~ Q
N
~
G
,~~, ~ a a~ z ~ ~ ~7 z .w.l dH ¾ ~ W ~ ~ z H ~ ~ ~ x `~ ~ ~ d~, H a~ a. w ~
~
~ ~Ha"aQa¾arQw>~Ha~~F->HEa- aZ~Qa j¾ ~~~"GGa~~~~
~ ~, aza.Ho,~wa~>w~ ~aHaH ~H~ ~~~~>aa a~
O- õa"~>Q q.a a¾~-~H C7 Qa
a~ ~~j~~>aQ a.Q~~zA~z(~ Hf~ az~¾wF- ~w~~ p~7 ¾w
~' O w.a~C¾C7~~wH v~aGpc~F">~~~H c~wj> QaH a~~
'c U~ a w.a a H H ~ w z aC .~
~ aaw>~~Q>a...~ww~>~~>-a~.~a.~~w ~r.r,~ w>,a~
¾ w d~ ~ H w
>~~nHa.vac~c~i~> ~c7~x>H~CH~C~W~ww~~,A~~z3~~x~x
vi ~¾~v~wF"xaw~"¾~ H r~x~F" ¾ Uwv~a w z C7
'b a~aZ¾ _ H~HwwHHwq~H aaCH~H ~~ ""U v~F"C~aa.
~ ~waz¾HxR;z~a¾z - ~HC7zH~~Q'~~ ""~~Z~=F"a¾¾c~c.~~
a ~aQaZ~~n~Qwa¾QWw >=~ azH H a!v~ ~zv~~ w
o ~~>aa¾aHw¾>a~~wH~ ~~ha~¾~a¾~~~Ha~~'~~
~' ~ ~ ~ w x > a ~ ~ a w H ~ z > A ~(~ ~ a ~ a~ ~ a H > w ~ ~ a ¾ H ~ >z ~ ¾ ~
~~a Hc7 ~wzv~~ f~ a~w ~ w>> ~~~. c~~v~k
~ ~ ~ a wa w > ~ ~ a v U H w w ~ >c~ z ~ ~~ ~ a ~ ¾¾ > ? z > ~ w Q w 3 >Z > ~
~ ~ * aHFa- ~awHzLlv~3>Qwr~ ~ v~~ ~C7r~ w>Qza~~aww'z a
~ b * zaHwHC7 aHz~1Wa~~C~H~rxv~~oGaC7waQzawaawaGQ
",,~'~ ~ fn 00 M O~D ~}' =-~ V1 01 l~ 00 O~ N~O '=~+ M~ V=f ~D l~ 00 .-r N M
~O l~ M
a a.--~ l~ 00 O1 N~~~~ O M Vl ~D (~ Q~ O~ O~ ^' "" N M~ Vl ~1 00 OO ^' ^' N M
M M M~
^ =--~ N M~O OO ,_, ^, ,_, ,_, ,~ ,_=, ,_, .~ N N N N N N N N N N t+1 M M M M
r1 C1 M
1-i ~
00 '-
.-~ ,~ ,~ r. .~ r. r. .-~ .~ r. ,~ ..=. .~ r, r. .-~ .-. ,-~ r. ,~
N ~ ~
W a. aa~.aaa~.aaaaaaaaaaaaaaaaw.aaaaaaaaaaaaaa
J ~~ a
m
Q
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M
.~ .~ .--i .--i .-. .--i ,--i .-. r. r. .-.-i .--~ =--.--i .--.-. .~ =--=--~ ~
~ ."' "" ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
~ W 00 N ~ _ r- (7, M M M 00 00 N =^ N M M M ~ - - ~ ~ ~ ~
~ ~; C~. ~= ~.= C`= C. c. C. C~. C= c C~= c.. C~' ~= C,= C. C= C'= C`. C'= L'=
C. C. Cl= C-= Cl= ~. C' ~. C. C`= C. C= C. C. C~.
~ ¾ ~ ~ a c~c- p"= ~ c ~ ~.. ' c~ a: ~~~c . ~.c. ' ~ a: c ~ c¾ a' ~ ~= c ~ ~ a
cl. C. C~= C; C`= G~= C; C'~`; C~= C C; C~= C ~; C C; C; ~; ~; C; C= C; C= C=
~; ~ C; C= C= C; ; C; C= c; ~= ~=
w a ~ i a ~ ~ a > > > w w ~ > > C., > >_:
Vl M00 N~O 01 N M N="~ l- M Mkn O O~ M=--V) M O~ I~ 00 O m l- O" 00 - c 1) OD
O 00 N M N C~ ~ O'0 0~ O N0~ "0 V1 !1 !1 ~ M M~ M~. - N Nr ^- 01 O 00
[- O M vi O M N=--O O O O cM M,Y ^" 00 O Ntn O.O Ntn O 00 O M O~ l- O N N ,D
>,a C7x ZwaC7v~~Z~~Aa~~~+>x~a¾H
>aa0~>v~ c7oZxwa
~~aaHa~zH aawv~~a,~Hx~~aQa ~v ¾
~õ=~v~~zQ H > wwac7Z AH~~xw0 QQA
v 0z>>z~ ~a¾.O aa
dww- ~c7> >
v~C7~wapQ~Wv~~~ =aC7v~..r~
C7v'n;¾v~C7a'c7 a
wa wa -.~ a~ zw>a>a~a>~?w>ww~~> >
¾awH~z> >>x~> ~FcnrC7z.vi~~~aC7ddC~U~4 -akp"..Ca7Zx
a~~QC~7aF>>azW Qa>~ ~aQQ
~ ~xw z a0 >~õ~> c70z w
z z~a.
~v, QQ a Za Z0 v~v~ v~~a. F x ~a¾axa.UU >
' wk z AH~a~> >
z ~> C7 ~nC7H~~~~~ - aw~¾aQ+~
zQw05 c>7> >~x A ~x
>>a>,~ >~~ >~>~~za>
~Cw7Qa~Q~>[->rZZaaZ>44
~CC7rAc~A HC7~~ av>>AAUC7a:>cn AC7>xC7aa~AWQQ
~D l- tt 00 O O% Ma~=--N c0 O~ =--O~ t- N M l- l- N~n N Mtn O~ O~ O~ ON O O~O
~ v'~n W1 ~O \O o0 00 O O ---~ N d' ~O [`- l- l- ON O O O=--~ N N N rf M M Qi
ON O O N~ d' kn
M M M M M M M M V' ~ ~ ~ Vl Vl ~(1 Vl Vl Vl V) 1n V1 V'1 !1 w1 \O "0 %0 %0 "0
~0
co
r. r. r. - - .... - r. r. r. r. .-- - - r. r. r. r.. .-- - r. - r. r. r. - . -
r-. - - - r. r. .-.. - r. r. -
N
W a a a a. a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a
a
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~ .-M=, M-'==" M M-i M
~ ~ ~ CN
c- c- c'= cc~: CCZ: c=
C. c: c' C= c'' c-'
O) F- `-= > V~
v~
c= ~= ~~] c'= c; t-=
Cl= C= C. c.. C= C~=
C-= C`= . C'. C.. C`. C; C.
Cl= Cl= C. c.. C`. C`=
5"' > R. > >
\O N M O~ M 11
N ~ O~ t~ 0 =-=
> > a
x:~ >
v cn w
Q~Wap c7>w
> a w > >
CIO >
~a W~
x>cf)
A¾ aa~'wW
>Wk">>>
C,
vi?xw c)cn
l- O M OO~ N_
v1 ~p o0 OO--~O D ~D ~O l~ l~ l~
co
N - .,
W aaaaaaoFQ
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~
a~ ~
~ v a' v =~ v ~ v v ~ v ~ ~ v v ~ ~ ~ ~ v v v ~ v ~ ~ ~ ~ v ~
n ~ C \ \ \ ~ C ~ ~ ~ ~ ~ ~ . .. ~ ~ ~ ~ . r , ~ . . , ~ ~ C ~ ~ ~
C tU/I ~ I~ ~ I~ M M.-M. .-M=. ~~~~ O\O V~l O~0 V~1 ~~~~ l ~O M N~~~ M M M
N ~ . - -. -. -. -- . . r __
v~ o
U
~ o oor-o=-=o=-o-~o-~o -o -~o ~o ~O.~ooOO--O~o=-=~
w
bA C~. C`= C; ~. ~. C. ~' ~' C~= CC= C~= C~= C~= C~= C= C~= C~= C~' C~`= C.
C~= C~= C~= C~= C~= C. C= C`' C~= ~.= ~`.
C~= C`= ~. C= ~.. ~.. C; ~; C~= C~= C~. ~.. ~.. ~.. C= C= C= C' C~= C= C= G~=
~.. C. C= C. ~.. C' ~' C~= G~=
~~' ~' F- E- r ~. r= ~= r r ~. c= ~~ Q Q a' a" E-~ E-~ c~= ~= v~ a a Q Q>>
~ v~ v] ~`= ~= E- E- >> ~ ~ E- F- v~ v~ ~= c`= ~= ~= ~`= ~= 0+ ~. r c~. c~. ~=
~= ~. c~.
CI~ C~= C`. ~; C. C; ~; C~. C~: C; C; ~; C; ~; C. C~' C~' C= ~. C~. C~. C~=
C~. C. ~. C= C. C. C`. C~= C. ~.
c. c.c'c'~:c:~.c.~=~=c:c;~'c aa~~~~~~~~~ ~~
a a ~ ~ .-~ a a ..~.-~ .-~
U
~ _~
a v~ ~n r1 M~~ M M v'~ v~ ~O ~0 O~ O~ "~~ O O ~ O~ N O~ O~ N ~y N N
,...~ N~~~~~~~ O O O O o0 oO ~~ O O O O M M M O op ~~ O O N N
a/ '""' '"' '~ O O O O ~~ O O
' Q
~ ~
~ ~ ~~x~ >~H3333
~ x x~.. ~~a~a~ aa~ ~~Q¾x~~ x
~ aa~~A~QO~~~~'~~~ ~ aaA~ ~A>Q>¾ HH
.~ ~ ~~~~~"HH~~~"~"HH ~ a~~Gi,a'-'~"Q~~aaQ¾
~. a a~~ ~"HH ~"~"~~xx3 ~~~~~~A ~~aa
U ~ ~~~adaa ~~~~~a~~~~~~~~~ ~ ~~
aaaa~a~~H~ > `~`~~~
~~~~ 33~~
~ `~HH~~~~HHww~~~~~" ~A ~ a>33
~ ~ ~~a~~~~~~~~aa~~~~aa~AaWA HH33
~ aa~~ AOA~~~~N~~3 ~~x~~"F~~~¾¾ HH
~ a a Q Q
~ y~ H g a a-. a¾ a a a a¾ Q
~ ~ ~v,v~HHHHZ ? ~HHxx~~~~~~~- pv~v~a.aaa
v'~~aaxxHHx ~~~~~~~3~~~x~W ~~~~~~
~~HHxxoA~~
a a H H ~ ~
a a~~~~~aHH~~~~~¾~~ ~~~~~~~,~~ ~~
~~~~hH~ax~xx~~zz¾~~~~~oA3AAAQ~~
~ =--~ =--~ O~ O~ O O N V'1 O O~=--~ vl ~A l~ l~ ^-' ^--' M M M M=~=-~ Q~ O~ O
O=--~
O l~ l~ _ N N N N M M M M d ~~~ Vl V'~ tn V~ ~O ~0 00 00 00 0~ O~ O~ 0~
a
U ~ M M M M M M M M M M M M M M M
~ ~ ~O _ ~O ~p _ ~p ~O _ ~p ~O _ ~p _ ~p ~p _ ~D ~p ~O _ ~p ~p
~ M M'""' l+1 M'""' M M"'"~ M M M^~ M (+1 "'~ K1 '""~ '"'~ M M M
~p N~ N ~p N~p N~p N~p N~p N ~0 N ~0 N~0 N~p N~p N N~ N~p N~p N
M ~ M~ M~ M~ M~ M~ M~ M~ K1 ~ M~ M~ M~~ M~ K1 ~ M~
U U O M O M O M O M O M O M O M O M O M O M O M O M M O M O M O M
w ~ w ~ w ~ ~ w ~ w ~ w ~ w ~ w ~ ~ w ~ w ~ ~ w ~ w ~ ~
~~¾~Q~Q~~~¾~¾~¾~Q~Q~~~¾~¾~~¾~¾~~~
~ ~ N~ N=-=~ N=--' N~=+ N=--~ N=--~ N~ N=--~ N~ N=--~ N=--~ N N=--~ N=--~ N=--
~ N
~=~zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
~ ~ ~n N ~n N ~n N ~n N ~n N ~n N ~n N ~n N ~n ~n N ~n ~n N N ~n N ~n N ~n N
q~,xxxxxxxxxxxxxxxxx~xxx~xxxxxxxxx
N _
N a =~ N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
0~ fl~ GG 0~ LYl 0.1 GO G~ fA GC~ f~ 0.1 ~A a1 al fA 0~ LY7 W fA ~ LY~ CQ ~ CU
W CO CU GQ f~ CO
J ,-. o a aaaaaaaaaaaaaaaaaaaaaaao,aaaaaa
00 " Q"
Q
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~r~rvvvvv~~v~~vvvvvvv vvvvvv~~ vvvvvv
--~ ~_ ~_ ~ ~ C ~ ~ C .-. .-. .-. =-. ,~ r. .~ ~ ~ C C C ~_ C_ ~ ~
1.0 lzr "0 M M l~ - M O W, 00 00 00 00 M M M M N N'. .-. ^. .-.
O O O 0 0 0 0 O~--~ O =--~ O~ O'-'+ O -- O O O- 0 r O-- OO OO~ O -
I = C= C= '= C= '= c.. c.. C C~ C C' C C c. ~. ~= ~= ~= C= C= C C= ~= C~ C~ C=
C~= ~ C~
Cl= C= Cl= C'= C' C'= C, C, C= C= C= C= CC~= C= C. C. ~. ~. ~= C= C' C' = c`=
C~= ~.'= c.' ~.= ~. ~. C. C. C. C ~= ~=
prpr> ¾~ QQ c a aaC C ~~c'~'~~aaaa V]C/~aa~.C..
C Cl C= C= ~. C. C= C. C~ C~ C: ~; C: C-. C. ~. C? C. ~. C' ~' C' C= C. ~. C=
C'C`= C= C=
Cl= Cl= C== ~.= = C~= C~=
C C=
C~= C= ~.= ~.= Cl= : C= C. C= C.= C~= C'= C.= C.= C.= C.= ~: C: c.. ~.= C: ~=
C= C`= C`= C~= C~= ~= C= Cl= Cl= Cl= C`= C. ~.
33 ~ aa ~-aa~~aa~~~~aa ~~
W) tn M M M M M=--~ ~tn W)~ p~ O O O~ C~ N N O O O~ O~ W) W1 N N M M
p~ h\O Nv1 vl 0~ 0~ N Nzr M M~ pp M M vl r 00 OC N N 00 00 N N N N=-= =-=
^' =~ ="~ Nq:rN N O (D V~^' ^' M M o0 00 .-=~ N M M 0 OW) kn V'~ W) - - I- l-
N N1.0 ~D
M
0) W W
A Q a a > W H H -z~ w~ W W~~ H H ~,Ha
a a>~¾ aw k, z 0 O
a C7 (7 F .a a~~¾¾~~ a a > >
a
p~ ~~~ x x H H
c7c7 x ¾¾ ~õ~õ aao'oz~ HHaa
~~aaC7 w ww~~ww~~¾Q~ p;waa(7C7
aa¾ w U U
¾~~~Aaa ¾Qww~~c~~W Wa.a
z xx p v¾i~~~wwwwC7C7HH aaa~~ > awwQ¾~
~~aa~~aa~~~~aa
>- H ~' cwz >>>>wwaa>>~~aa>>HHUU
ww~~ aa aa aaHH`~~aww~~
HHHHa~ zz~
aa~xAQaawwzz~~~~aa~~¾aaaaaaa~~~~x
~aa
c7c7>>"~ a aaaa~~. ~
wwww v~v~ aa aa a
a~a~aaav`~~ww zz~u
-5 50W W
aQQ~~ aa¾Q zCa > ¾Q w w C7 C~ ..,..,
~~aa aa ~~ ~~aa¾¾~~ww
33HHAA~AAQ~~ waa~r>n>>
HHcn cn ~¾aa wwzQ¾W v~~~aa¾¾~ aav~v)
C7 C7 z A..~ v~ cn >> Q Q w w F. H o o
>> H H a: a: x Ll
Q Q A a a
a, [- t- 7 N NIt V' l- [- N N"O ~10 M M o0 00 N N ~O "0 t- [~ O O l- I~ ON O~
~~ O O tn W) %~C ~O ~c ~10 110 "G t- [- l- t- O~ O\ O~ O~ O O O O O O O O N N
N N N N
.-. .--~ - . + - -- -. . ~ . -- r .-~ .-. =--+ ~ N N N N N N N N N N N N N N
M M M en M M M M M M m M M M M M M M
"0 ~ b %0 "0 ~O ~ ~ ~ ~ "0 ~ ~ ~ ~ ~ ~ ~
M M N M N M - M N M - M M - M N ~
~ M N M N M M N M N M M N M N ~ M N
~ ~ ~ ~ ~ ~ ~O "0 -0 "D ~O
M M M W) M M tn M M W) M M VI) M W) M ~ M M N M M M M ~ M M M M M M M M ~ M
f~1 M(,1 ~ M~'~1 ~
M
O~ O~ O~ O~ O M ~ O M ~ O ~ O ~ O ~ O M ~ O ~ O ~ O M ~ O O~ O ~
N --= N N N N N N N N N N N N=--~ N N'--~ N N
zzzzzzzzzzzzzzzzzzzzzzzzzzzzzz-=zzzzzz.
x xxxxN xxxxxxxxxxxxxxkn~xxx~xxxxxxxxx
v~
N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
J aaaaaaan.aaaaaaa.aaaaaaaaaao.aa,aaaaaa.a
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v v'~ ~ v v~ v v~ v v~ v~ v v v v v v v~ v
- r. r, r. - - r. r. r. - r. r. ... r. - - r. - -
.-. ... .--~ .-. l~ ~,O ~,O 1,0 ~,O 1.0 1,0 ~,O W) kn v't W) kn 00 W) I- ON ,I-
O~ "T O~ 't
O- O=--~ O O O O O - OO O O 0 O O O O -~ O O O O 0 O O=--~ O
c-, c-, C~ ~., -, C`. C= C~= C'`= ~.. . C~. C-. C~= C'. C= C= C~. C C= C~. C~=
C~=
C, C, C~ C~ ~. C. C-= C-= C C C~. ~.. C= - ~:c
Uc ~;c;~ c ~~~' ~ ]v~v~~~~ G.A.QQ~~~ c'=~c?c
I- Q . c~. c. c~= . ~.. c'. c.
c= cl. c, c~. C: ~: c: ~: c: ~; ~: ~: ~; ~; ~; ~; c L: ~: ~: ~: ~: ~: c: ~: ~:
~: ~ ~. ~= = c c; r= t= c:
C' C' C'= C'= C'= C`= C~= C~= C: C; C= C; C~= C~= C~= C~= C C-= C; ~; C'' C;
C; C'= C= C= C~= C. C, C' C= C' ~.. C; C; .
aa3 ~...~~~ >- ... ~
. v ~ W) kn 'n ~O ~O -- ~D ~~ O O O O t~ O~ 00 O N~O l~ l~ O~ O~ N O ~p ~O ~~
M M N N~~=--~ =--~ N N~~'? N N N M M~n M N N~ l~ t+i
N NMM N N M M O O N N~ ~O ~O N~^ ~^~~f M I~ N N N~O N N N
O)
~ ~
aaaaQ ¾¾AA~~aaQA www~~~~ww w
~1 aa v~ ww v~v~wwt7C7c7
3HF AA¾ ~n
>>?E- E-ww In
U U Q O >> P
~= a j E" > > a a x x~~
F.AA~~~ ~
AAu u >H>aazz~~~ww
aaOoa¾¾ ~~aQ~c~c~x `"- cn
> > > > > > > > > > > a a a > > U u a
, ~..~ >HapH AA~~w cn
~~ww.aa >>aa...~wwwc7C7xc+;aQa ,aa~'~~~wc' ~ww>
(De acn v)~ E- A~aa ~o
0 a a ~aaO0~ C7C7 2 f-. (= C7C7www
33~' a"AA~ v~`~¾av~v~ppAA~wwww~~v~~ncn cn~C7C7
> > w cn¾QE- E-->>,,.aF- v~a'wwC7C7~
UU~~ >>.aav)v~AZZa
HH~~AQ~~ aaxx
aaaa aa~ A A U U> > aaZ Z U U~~ a a~n w w~
E-H ~~AAa U'a'wwC~7c~7~~~a~Aj~~
~ v v
a.a33 ¾
>A..a C7C7
~a ~ c7
c7 A
x x U U w w A A~~ Z Z~z~z H¾ ~¾ a w aa¾¾AA
¾ a x~~~tw.7Ca7w
c~7c~7C~7C~7~i ~a¾r¾xwaww~~
O ¾ ¾
z z
~,T O O O OW) tn - ~O ~D t~ l~ N N-- ~ O~ O~ N l~ l~ o0 00 00 0o O O v~ v1 [~
l~ O~ C~ o0
M M V~tn V'1 ~n Vl \G ~O "O "O 'O 'O [- I- 00 CC 00 00 O~ 0~ ON C1 Q\ 0 O -= ~
-- -= ^= =-= N
N N N N N N N N N N N N N N N N N N N N N N N N N rM rM M M M M M M M cn
M K1 M M M M M M M M M M M en M M M M M
_ ~ _ ~ _ _ _ ~ M~ r4 _
M M M M M M M M~ M""' M M'""~ M M
M'""~ M M^~ M M^"
~p N~p N ~p N"0 N~p N ~p N~ N~ N~p N_~ N~p ~p N ~p N~p \p N~p N~0 NI'p N
M
MW) MW) MW) Mtn MW) MV') MW) MW) Mkn Mwi K1 MW) MW) Mwi en MW) Mkn MW)
p M p M p M p e'M p M p M p M Orn O M O M O O M O M O M O M O M O M Oen O
w~ w~ w~ w~ w~ w~ w~ w~ w~ w~ w w~ w~ w~ w~ w~ w~ w~ w
N N N N=--~ N--~ N N N N N N N N=-=~ N=--~ N=--~ N N~
zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
kn Cq xC14 xxxCq xxxxx~xxW) xxxkn xxxN xxxxR! x
~
N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
W a a a a~. a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a
J
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v~~~ v~ v v~' v~~ v v v v z v~ v v ~~ v v v v v
v1 =--~ vl 00 I- v'1 1,0 v1 ~D W1 M =--~ N =-~ .--~ .-=. .-. =-. "O qtt "O 'IT
l- It \C 1.0
O O O O O O O O O O O O O O O O~ O-- O-~ O-~ O-- O O O O O O O O O O O
C= P= P= P= P= C. el. P= C-. P= Cl. C`. C'=
Cc-C c=~=~ c,Fc-~E~-~~.~. > c > cc=c= r= c~= cc > = c~ c C v,:":~~~~~ ~>aQ~=c~
H ~~ Q < C= C= = c= c- U U~ ~ c"" l. c-= c. ~.
C~= C. C'~. C~= C~= ~. C. C'`. C~= C. ~. h h~. C= ~ C~= C C~= ~= C"C`. C' F.
C' F. C' C' C~= C~= C~= ~ C~= C= C.
~, c== C. C. C= C. c.. CC= ~= C.. C. C-= C`. C C.. C'C = C~= CC= C~. CGl' = .
C. C~. C~= ~.. ~.. ~.. C. C. C. C. C. C. CC = . C; C,. '.
w>~a~i> > ~~ ~~~:~~:~~~~ wwww>a
~ l- -,t O~ O, M l~ N~D =-+ N N O~ O~ 00 00 ~ vl l~ I~ N~O ~p M=--~ l~ ~O N O
OO ~
~=-r r- N~p a,=~ M M=--~ ~f .r .-. "'~ ^" M M O~ O~ O~ 01 p~ ~p O[~ 00 (71 `1:
6 00 Vl
N vj ~ l- O O l- =~ W) N O O N N p p O O r' ~ cM ~n Nm O O O^+ N N c
U)
0)
aaAQ>>aaF~F~~
ad~ > ~ >> F F H H~~~~ rx aG Q Q w w dd~ W A A> A A a Fa'- a
z2~~ FF> ?;
z QQC7C7a~C7C7~~~~F
~ 4 33ww >
FFFFwwC7c7 paa~Q¾ ~ ¾~~ooZz
F.~ aaww
04 w Q¾~~aa c~~~~awa
aa wwww33~~~~d>
>~aw?? > >
xxo a aooo- - zzo o
>`""~~ aww A P. ~,aC~ww xw..a - w wwUU AA~ FFww
aox~ ~HHH~~2¾¾aaaaU UWWAAa>QaaF
zz~~~~ FF>>vi AAC7C7a~C7C7a>
C7 v W a w w ~ ~ ~ j >
> ; w w 3 3 w w ~ ~ A
wFFFFWWC7C7 ~o a ¾¾Q¾ aazz
FawwwD a aww v~
a a 33~~~~a~~ja,
Wa?~ dd-~.Op¾¾zz~¾Qww~~
>2 >
~~¾¾ wazz
~wwzzvv~~
wwwHH~xwwwwa¾F~~~aaw¾¾¾a¾¾x~aaQA
00 d' '~ O~ O~ O O v'~ ~n ~O ~O M M=--~ =--~ N N~~ 00 00 O O cf ~~O ~C O~ Q~
OO 00 O O M M~f
N~ ~t ~~' ~O ~D ~G ,D ~O ,D l- l~ 00 00 00 00 O~ O~ O, O~ O O O O tY ~i= ~~tn
v~ ~O ~O "O ~G ~O
M M M M M M M M M M M M M M M M M M M M Mlqr It}= ~f ~~I'll
M M M M M K1 M M KI M M M M Kf M M M M
k-C _ ~p ~O _ "0 _ ~O ~O ~O ~O _ ~O _ "p "O
M M=""' M M M N=""' M MM=""' M=""' M M M"~ M=""' M="" M M
N ~p N~p N~p N ~p N~p ~p N~p N%p N ~p N~p N~p N~p N~p N ~p N%p N %p N%p N ~p
~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ M~ K1
M p M p M p M p M p M p M p M p M p M p M p M p K1 p M p M p M p f+1 O M O
N N N=--~ N N N N N N N N=--~ N =--N N N N N N=--~
z~-,zzzzzzzzzzzzzzzzzzzz+zzzzzzzzzzzzzz
xkn~xxxxkn~xxx~x~xxx~xxxx~~xxxRx~xxx~x
o)
N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
J aaaaM.aM.aaaaaaaaaaaaaM.aaaaaaaaaaaaaaaaaa
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N 't 4 V
~O M [~
~ O O O O O~ O~ O O O~ O O- O~ O~ O~ O - O~ O-- O O O O O~ O --~
r c- c- c' ~ r c ~. c. C. ,. ~..
> > ~= c`. c. ~.
c. > c c`= ~= ~= ~.. `. c~. c. c. C'. c= c= ~. `l. ~= ~= c= ~= c-= ~= c c c c
c
~~aa. c. ~= ~=. c.
2 v~ v~ - `' ~= c= ~
~: F. C'>`= ~= ~: C= C= C= ~.= C= ~ C CC~; C; C: CCA = C~~= CC`: C: C: C: C`n=
~ h C~~= C: ~: v, ~õ
C~ C: ~: C: C: C: ~
a a~ ~ a a a c c~~ 3 3 ~ ~ ~ ~ c c c~ ~ : c' C.. C= ~= c c:
332 ww
00 TIn w) vl tn NM M l- 00 00 ~D ~O M M O O M M [~ O Q~ ^~ ~\O ~O 00 00
V'1 M O~ l~ r; ci ~D "G "O 00 00 --~ =-= M M~O O~O p
~O M v1 ~ C M M v'1 v1 ,~ .--i o0 00 ~D O~ Q~ 00 00 p N N
(0
0)
T cn cn
> ~Q>
> QAWQww~"~~~~3~~~ ~x33wz~aWWaa
awaaaa
ww
>>>> ~~v~v)~~ a~~F- vi~
F- c7 v rx ~" c7 c7 v~ v> > > v) v) v, v~ ~ c7 C7 3 3~ a
~ ww wz zaa
>~,~, ~ ~ ww ~~.~.v,v)
>C7 ~~wwww~CF- E- v~v~v'v~~~aa ~~ooaa~? ww
.~aa~.~"aaF F" >
A Q >
cn>,H¾>> w zz cn cna a oa
cn v>>>~H~~3
~ c~c~~ 3 wc~c~a~ a 33 a o o
~ >ww~r~a a 0 2
> v'v'a' ' wwaa
w>~~>0QOwwHH~~~ 33~33w a z a ~aWw
H r~~, H¾ww v~ ww
vH~ > v~cn v~ cnv~0 2 2 P. P. ~~> Faacnv~~~ ~
>>~~c7c7>>wwr ya'`~"~' ~~ww a~x33aa~~
> ~i rz~ 4 r'' a
~ aawzzaa
v>>~ www~w~xE F ~õa, wC7c7 '"Od0' aa
cl)~QQ >
> v~ rn tWw
~~~
>>
>wwv~v~vc~a:u:vvwwc7~a~HH~"~"~~ OOF Hww
2 v i r / ~ w w 5 0 z z Ew ~ H H v~i ~ a a 3 z
~ M M v1 v'1 00 00 O O~n ~qtt O l- l-
[- l- l- 00 OO OO 00 N N N N N M M K1 M M Mtn V1 V'1 N%O ~.O ~.D ~.O l- [- l~
l-
"T ~t It t t I}' q:T It d' qi q:T V) Wl lf1 l/') V) tn ln V) tPl ln l^ 1f1 l/)
ln tn ln 1P1 Vl ln ln V1 V=1 W1 l/i 1^
M M M M en Kf M M M M M M K1 M M M M M
%1O r4 ~O ~O ~O ~O 74 ~D "O ~O %p ""'
M M'"" M~" M."' M"" M M MMM M""' M""' M'""' M'.r r4 M""' Mr4 M
N tp ~p N~ N %p N%p N~p ~p %0 N%0 N~p N~p N~p N~p N~p N~p 'p (V 'p %p kn Mtn
MW) MW) K1 VI) MW) MW) M MW) MW) Mwi MW) MW) MW) MIn K1 W) MIn MW) Cr1 kn
M p M p M p M p c+1 p M p M p O M O M O M O M Or') O M O M O M O M O M O M
¾¾¾
N~--~ N - N --~ N-~N--~ N--~ N~--~ ~ N~--~N--~ N--~ N ~ N~ N N --~ N-" N--~ N--
~ N
Z Zi N~ Z vZ=i N vZi z vz i N vZi N~~ N vZ' z v zi Z vZ=~ N~ Z vZ1 z vz1 z~ Z
vZi N vzl Z~ N
v
~xxx~xxx~xxxxxxxx~xRxxxRx~x~xRxxxRxx
oD
N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
Ln (3~ 0~ C] f~ 00 Oa 0~ CQ (~ 0~ G~ G~ CG L~ W W G~ CQ C~ Ln G~ G~ OU W W CU
Q~ Q~ CO CA f~ GO GO W C~
J aaaa.aaaaa.aaaaaaaaaaaaaaaaan.aaaao.aaaa
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v v n~10 n i n i i n in 00 n o n o i v v i v
O O O O O o o o O O O r -o- OOOOO~o ~o--oooOO- OO(D -
~= C.. ~. ~= r= ~- ~= cl= ~ "' c~' c-= c-= ~= c-= Z ~= ~= cl. cl. ~= ~=
r~= ; c ~. = c- ~: ~; C= C= ~; c: ~; c: ~: ; ~; ~; c; c c. ~= c= c; c; ~; ~=
~= c`. ~= ~: ~; c'r = ~~= ~= c= c: ~:
c. ~= ~' c' ~= c~= r ~= ' c' ~~ r ~= = C.. C..
aac.~.vav~~ ~~aaFF w.~
aaQ FF ~ Q¾QQ
Qc:c:
~:c:~ ~: ~~.~~~.~~ca a ~. ~. ~~~~~: ~ c:~:~ ~ c;c;c;c:~:~:~:~:~;c:¾cC:
Cl= C= C= C' C`= C= C= C= C= ~. C~, C., g . C.. C.. C. C.= C`. ('. C~. C= C~=
~. ~.. C. C. C' ~' C. ~, C' C' G.. C~. C~, ~,
C`= C`= Cl= C= C= C= C`= c.= C-. C' C. c.' c.= - ~.' c`. C-. C' C .. C..
w w w ~ a a w >c" c" w a a w w w~~ w >>>>..a >> C': c_
--~ V M[~ ~O ~ O O~n ~n ~~ cM M O~ O~ 00 ~~ q O Q p~ ~~~n ~ N O~ O O N N~~
~1 O~O M_,~ C~ O~ M ~ ~ O O~n 00 N p N N~j N~~ M M N O~ ct v1 M MIn O,^ ~n
O N vi O-- N O N o0 O O v1 v1 00 4 G O^= =- -- = O~ O~ -. -. ~~-- 00 \O "0 "^
N N
~
O)
oa>-~ aaw~~v'r4~~aa¾>~zw~~¾HC7cw7
aa~ ¾ a' a aa aa daF F C7 C7 a a > F F~~ nC r~
a!C7>F
- cnFF~~www a-aa~~`~a'~~v~v~aaFF¾¾..a..a
> a~4o qOa~~~ a~~HN~~~~Z~zz~¾a
a U. d ~
v~v~avFFF - ,~ aaww~vwi~zz~~~~~~ ~'
a a a a ~ a ~F mw A d ~ M a ~ ~ w w ~ ~ w w > > a a > > > >
a
cn cnrs! C arx~~ a~r~cG FE ww a
~ ~ ~ ~ ~ F f~ w l
p4 ? ~= x .-, ~. a ~ ~ a a aa~ ~ ~ a >
>aa w aax~o~~
w a>¾
>aaa F-f- C7C7 aa FF rxr~
~~,FF~~.aww Q "w~ ~~~Q>¾¾w.a
a oAZ Z
zz 2 2¾¾
v
iaacc 7 FF~~C c~"w ~~W:cG~~ C7C7w~
aa 7 a ; w w z
w w >>
a=a a ~ww~~OO wa~~
a cn c/) F F F aa a a F F z > F F~ C7
C'C'~GQ c7C7L1F~~ .a~~
w >
~H
wwaa!wawFF- = . - a
w w a
O O M M OO l- [- O_ O M V1 V~ O_O 00 G\ O, ~--" =--' ^' M M 00 00 O O 00 OO O~
O~ ~O ,O \O
00 00 00 OO OO o O - ^+ --~ - N N M M M M M M~~' t/1 \O ~O 00 00
V1 V1 V'1 1P1 V~ ~O \G ~O ~O ~O ~C ~O ~O ~O ~O ~O 1.0 ~D "G "O ~O \O
M M M M M M C+1 M M M M M M M M M M M
M M"" '""i M M'""' M M,""' M'""i M'"'" M".' M'".' M'""i M M"'"~ M'""i M'"='i
M'""i M'""' M'""'
~p N~p N N ~p N~p N~p ~p N~p N ~p N~p N~p N~p N~p N ~p N ~p N ~p N~ N~p N~p N
Mtn Mkf) vi MW) MW) K1 Mkn Mkf) Mvi Mkf) Mkt) MW) Mkf) M~ M~ M M~ M~ M~
O ~ry O ~'~ M O M O M O O m O M o M O M o M O M o M p M O M p M p M O M O M
wr- w~ wr- wr- r- t- r- r- ~
¾¾Q¾¾¾'Q<¾2 ¾:~ ¾¾¾
N N N -- N '- N N -- N N =-~ N =-~ N =--~ N - N ~-" N N N -- N -- N -- N
zzzzzzzzzzzzz~-,zzzzzzzzzzzzzzzzzzzzz
x N xN xxN xxxxxxxx~xxxxx~xxxxxxx~xx~
o)
nl N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
OU0.1OafAQ~CaLnGU0.10~Q~00W W Wf~Q~0.lalCOQ~CI~CAO~f~Oq0.1Q1(~0.1GQf~CQGGf~CG
w
a a a a a a a a a a a a a a a a a a a a a a a a a a a. a a a a a a a a a
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
o-- o- O~ O- o 0 0 -
Cl= C= c== C. c.. C.. C= C, Cl. C,=
C`. C. C~= Cl= C'. C'= C'= Cl= c.= C. C'
= C~=
C= C`= Cl= C'' C'l. C-= C-= Cl= C.. c., C'= C'l.
C= FC-+ C/1 Gn
. C. ~, , C. C. C= ~,
> >
C C.. C.
~. C. C. C. C`. C= C. ~. C= C~= C.. C,
.
= C. C. C'' Cl' ~= C=
C= C= ~. C. ~=~= ~
> > ~
O~ O~ 00 00 tn v)
~D ~O l~ N N N N N~n
N N
00
O)
~ ~~r~xwwara.=~raF. F"
zz aa
aa?~~~o cn E-~E-
ww,~,.a..~awalaLlO'
a a
o Q
aa
oQ2c4a~ox~~
oQWw
a>
zz
¾¾
w w ~~
~~QAca.7ca7Zz~~
OOi ~ O~ O~ O O O O N N~~
"0 "0 110 ~o t- r ~ ~ r- r r- r-
M M M M M M
M~ M~ M M tn Mtn
wp ow
zzzzzzzzzzzz
xNxxx~xxxxxx
~
N N N N N N N N N N N N N
CO 0~ W 0~ 00 L1~ W 0~ C0 0~ CO OU
J aaaaaaa.aaaaa
m
H-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U
U
N .-.= .= ~ ~ ... .... ,~ r. =--i .--^~ ~ ... ... .-.~ ~ ~ ~
w ~o B "0 r- ~ M O ~ M M N N M r- Z
C=" V] =--,--i ,-r .-. ..-+ .--.-. .--i .-r .r rr .=.i .--~ .~y
0
U
C C, ' C= C'. C= C =~. c .~= CC= ~
~`= C= ~.. C: C="= C= C= C= ~: C= ~= C: C. C`= C~= C~= C~= C
C: . C= C= C=
r' c. ~= C Q Gc. ~~j Q~ 0~ c' ~. ~ ~j ~: ~ ~ uj c~ c: c=
o~ c ~~ c ~~~ ~:~:~: ~~:~:~:c:~: ¾¾ c: :c ~~ ~c~c~c :c:~ :~
0) o ~= C: C= ~.. C-. C. C= C: ~: C'= C.. C= C. C; c.. C= ~., C= C C'= C'= c.=
C. C: ~: ~. C`= C~= C= C: C~= C~= G=
~ ~ ~.. C. C~. C~= C~= ~., C~= C~. C~. C~. C-= ~., h. C'= C-= C= C., C, C. C.
C`= C~. C~ C=
, a~a>~~>>3>>>~~a~~a >~~=
M
Al U
U an cn t+M tn ~O Os m O=--: c" N~~=--r- cM l- =--tn O ON (N O O% W) N cM tn
"O =--m O~ O oc O O M MD O t- kn N~ M Mn ~ N~ N N~ N-- N o0
oo O~ f- - - ~ C) M M~ 0 N"' ^==-N O~ M o0 M (D W) vl =-=+ I- N~O l- N m O
N /1
C F-1
0
N U
a~~Qx ~33cn>z>w
HA~>H3~~ aAw~,H~,aw >
>Ha Ap a wzF aQ >-~ > a
>
N õ cn v) > > p a v) E= ~>
> Hv~3 ? ~p A >c7~¾¾ wa>>" >F"aa~"a
>~ww~¾~"r~ W-ac7w
c ~ ' zav~F W v,
c~ ~~~nz xa~~>~Haa.> >wW¾j> ~aQQ
o a~a>45>C/)a~~a >¾>
O
Wx~H~4 z~4 oH>¾a >>wz~
~ z > p H~ r~ a E" ~ w D FV- c7 rx > v ~~a~c~'"7oaG aQ>H3~x aAaw~H~.w~~oaa~
>
a~ ~P w< ¾>>A>aHa¾`'a~bIhil
>¾H~n3 x a a ~ ~>Q >
a~ a au.~aaE¾ ~Q a:>~3HAAA~r~x~a>>?U
~ Q
r~-7 aa3~~Ax>Hv>>~o, >zQva, av>>HA
xi~a!x ZQ~ v) A>F- AC7A~v>>Q[~,E- v~C7zv~Q
" C/i N ~O l~ O l~ O~ O tn ~D
kf) cq N~ M~ ~t n 'n "O 00 Q` Q` O, O~n ~O ~O n~ O, N N N N N N N N N N N
Q ~ ,-. ... .-.
N
i0
M A=+ =~ +~' ~ 0.N1 GNG ~ f~A 0.N1 C~A C~A N N C14 N GNG N N N fNA N NW fNA N
N fNA N CNA N r,4 CNA GNG N fNA NW N
w ...g o aaaaaaaaaaaaaaaaaaaaaaaaa.awo.aa.aa
m C,4 2
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~t Zt "I'~vvvv~~~vc
o~ n o n oi o c oi r- o 0 o
C`= C~= C~= C~' C~' Cl= C~= C`=
C' ~= ~.. C~= C' ~= C~' C~= C~' ~.' C~' C, C, ~.' C= C. ~`= C'= C'= C, C' C'
C= ~.' C, C~. C.. C'= C.' C~= C~= C= C-= C= C~' C. ~.' G-=
> C/~ C' F-i Q '~"' Cn C~' ' ¾ E-+ ~.= ~L~.', C~. >>>> 0 ~.. ~. h
~= c' E.y ~-~-'i C Q Q CS, >~
p c c= c. ~. ~. c c-= r= r ~. .. >~= C`= E-~ C ~= cl' c-' c~~ ~.. ~, ~.. c. ~
~. r=
p Cl = C= C. ~; ~; C. Gl= C. C~, C. Gl= ~; C`= C-' C-' C, C; C C; C; C; ~; C;
C. ~; C; C; C; C~ C; C; C; C; C; C; C; C; C
N C= C= ~; C; C= ~. C~= C; ~' C= C. C~= C~= C~= C; ~; C C' C C; Cl= C' C= C~=
C= C- C; C; C; C~= C~= ; C`= C= C~= Cl= C`= C.' C,'
-~>->a>..aa>aa~~ ~ww awww>w>
V O O[~ O~D l~ ~ N~~7 O~ ~ M O~ M N0~~ O~=- ~ v~ M 00 O N M O~
N N v~ v1 ^ N [~ N O~ ~ O~ O~ M M~D O
N ~D N l~ ~O V M Nkn O O N~O M --~ kn oo CN c0 kn" O tn~
¾C~/]oa0w~'~ak-Hxc.~7¾'~~U'aH>Q~3>x0'
~~ ~a>
v~ >w >>~"¾ ~ wv~~ >
> w.. Ca a a > 0 >
C7C7aF" FC7~~> o
E-ax~za~ =:i3~ a~~a>~w~v~ww da (Dv
z
U >~a
U~ -aFV- Fw- w~~¾¾z.~~xaF~v~a3F ~aw.w ~c7a:C7
~>.wlaQ~~>dw > >W ~>v~-1~~"~~C7Ll~wa~v~>
C7.~ U ww~.awww>a>
¾a> o >H~~ Q~H
.awc7xQ> w ~Zv a'
~aQa >aaAaaa`'>
Q w C7 >F- v~ >~_ ad >
> ~ n O a. Z vwi
H a x ~ aZ a H ~ 3 > W ?
Ud >
ZU~.~c7ww wE v~c7 Ew->w,~ r~i~c7v2
z
;
adw> >d v~c~ cnE-.~ ~a~ x ~a¾w >a!
H¾Q>>o>
~z a
> A a¾ a~ w H~ w w a¾ x A>- 7 c> Z H x~ ar~ H~ a
l- N=--~ O4% t- o0 0o O~n O~ O~n "D ~ c0 ~O oo O M~qt MW) O M t- 0~ (7, V[~ O_
W) o_O O_\ --~ M 0 o0 O~,
%D l~ 00 00 (2N O~ O=--~ --It ~O ~D ~D ON QS d' kn ~O ~O ~C t- [- 00 --~ N N M
v) O = N M d' ~t
N N N N N N C~1 M M M. M M M M M MIt~~h W1 k!1 V'1 k!1 ~O kO %G %G "C
~
M N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
N
J aaaaaaaaaaaaaaaaaaaaaaamaaaaaa~aaaaaaaa
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v~vvd'
- --
i o o
C-= ~= . Cl=
c'= c-= c-= cl.
~~~.~C"
P= P= C= P' > C.. C- c.. C'= Cl=
N ~. C. ~= C: cC`.
> >
G~ 00 tn
N =-+ t~ O
>
>
¾ww~
Wa~ca7~
>>
w Q w
w >
>
aa~a>
QCw7waQ
> > ~ ~ C7
Qv¾iQx>
HwcnAz
~oo.~o
00 ON O, o N
~ ~ "0 r- r-
~
M N N N N N
J a a a a a
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U
ir
O O O O 0 0 0 O O O
kn ~ M M O O
VUi ^--N =--N =--=--L".
O
U
c: c; c: ~~ c: ~: c: ~: ~~ c c: ~~ ~~ c; c; ~: c: ~= ~: ~= ~: ~'= c: c: ~: ~:
c: c:
aa`'¾` :c-c'Hddaa c- UU~'>~ac'a p"UU~
c.c ~.~;~:~:~:~ ~ ~ c:c:c:c:~:~:~ ~:c:~c:~:~~c c:~~~:~~~~c
~= ~. = ~. ~. C. ~. ~. ~= ~.
~=~. c. ~=~.c,~.~.~=c=c.~.c;c;~.~;~;c:~.~=~'~=~'~'~
~= ~= ~~ ~ ~' ~=
~~a~>~ aa>~aa~aa>~3a3 ~= ~=
U
O
a~n O tt p~ O~ oo -+ tn M=--M v~ ^' O oo M l~ N N~O oo ^' ~ oo N M O, W)
VD N^ 06 0~0 n Oo, i N N~D ~~ ~ N M N ~ N 00
r ~O o~0 ~ M
N Q
O
N
dWFx x=~ 3 P. a,AwAw
>~"E" Hp~~aUUQVV3zzUAUw a
A
> r~ v H H~ > O a W~ >~ d¾0 00 U W A
H oG U v~ v~ E F- a., v> > Q a P. P.
U
N .~aEd- ¾>pzZZ z>~x`~a~aUpp~v~aZ~Z~ww
~ a~ w w Q ¾ ¾ W~ i~ 0 C7 aa, a a: ~ U Up 0~ 3 a~ a Z Z
a~a aa:xx wwwHHz2xaaw~wC7Q~~a~c7c7U
>~~~~da aa>a~a~aa>¾ a aa~
v o~ U ~ Q a o x w w" U~ a~ au A o
>>~~~ a a~ Q ~~~3 ~ w A w~ w a 3
> Ap O2 ~ ".a U Q~7 C7 ZaUAAw~w
aH~~vUFFw-F > O'WO>¾QC7C7o ~UGUApa
>>r~- v~v>>>dC~~ ~aaC7Uv) ~QQazap. ww
0 Zaaaw
~?w¾¾QZaZ¾~Z>ZF" ~~" -aaoGAUVU65
~a_a~w~xx~Wwaw~>>" zZ~aa~ar~ p¾~aa~aczcZ7pU
a a a > > > > ~ a W a ~ ~ > Q -~ a ~
z ~a, ~3 3 ~a ~a
C~7 c~7 A A ~ pU U Z Z x a' a i.wa U U¾ 0 0 3 3 z
wUU>A>Awwaaa>a~aaAA>Qavvc7
d= kn ~C t- [- 00 N N~kn I- 00 00 C. Ot w) l- o0 0o OO M
.--N N M M en M~ l~ I- l- l- l- [- 00 00
N 0 ~D ~O ~O O O D ~O ~O O O O ~O ~O ~
1!1 V) V'1 V'1 ~ W) vl Vl V'1 V'1 t!1 f) V'1
N N N N N N M N M M N N M N M N M N en N en M ey M N en M N M N M
"o %0 %D
,~ M,.~ M M=~ ,~ M,.r M,~ M,~ M,..M M,.ti M,~ M M,...M,~ M
N~ C,Uj ~,._, ,_, ~, .~ =~ O=~ O O... .~ O=~ O O.~ O._. O O.~ O=~ O O O O
r...~.~w.~ww,~~w.~ .~w.~ wwr.w,~ww.~ =~w
Q a~aaaaQaQQaaQa`¾~aQa`~aQQaQaQQa aQ
N N N N N N --= N
'-' =cd ZZZZZZZZZZZ 7ZZ 7ZZZZ -7.ZZZZZZZZZZZ
Q~,xx~x~xxxxxNxx~x~xxxxxxx~x~xx~x~x
M ^" Q ¾ ¾ ¾ ¾¾ ¾ ¾¾¾ ¾ a ¾ ¾
J ;-; o ~Cx~~~~x~C~xxx~Cxx~x~~xxxxx~xxxx~~
m ...a
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
0 0 0 o p o v, p o o p o o p o 0 0 0 0 0 0 0 0 0 0
N N N N N N N N N N N N N N N N N
~ ~ ~ ~ ~ ~ ~
M,~ ~O ~O o0 00 M N O p 00 N p p ~~~ - - .-. - O O v1
.-. r. .-. .-. ,-. r. N N ._., -
c~ ' C~= ~ C. ~.' ~.. C' "; C-= C`= C'= C-= C`= C. c.. C C: ~; C; C~= ~; C; C;
c C\
C C; C; C; ~; ~; C;
. ~~ C. C= L'C`; C' C' C; ~; C' C' C. C. C; "; C. C; ~; C~= L'= ~' C C~' C~
C~= C~= C`= C-= C~= C= C= ~.
~= C~= C~= C~= ~. C.. C ..
~ ¾~ a a 0 v~ rr) ~~ ¾ ~ v~ v~ vi E- c ~n a a >
= ~.. ~.
Cn C. C/) C-. Cl= C== C'= C`= ,~' C= F" Cl. C.. C C. 0 C-= C-= C= C,
C~= C'= G-= C'= C; C; ~; C; C; C; ~; C,= C`= Cl= ~; C; ~= ~= ~= C= ~ CC= C'
C~' C' C' C= C`' C' C' C' C~= C~=
= ~. C C. ~.=
~' C= C' ~' C= ~ = C= C~= ~ C. C= ~. C. C~
C~= C, C`= C~= C' ~ C~ ; CC. CC~. ~.. ~.. G'. C~= Cl= CC' = ~~' = CC' = C. ~.
~= C= ~= ~= Cl= C~= ~= CC. = ~C. . C.. C.. C'' CC~= C,`. = C~= CC. ~= C~.`= .
C`.
~~>aaaww~33>~>wwaaaa33a~~>c-, >aa
d I~ p~ pp O O~ O~ tn 00 l- d' M O O M~n ~O O~ N N l~ oo M v i [~ ~ Q~
- M M~D v~ <y p--; - ~O N~ 00 M
N'~ .-. M N N O M[~ M^-' M vi M v1 O~ D
t''=~ O =--~ N N o0 in t ~ l- ~O - .-. p N - N - - -- ~= ~p =--~ l- N ~
cM
O
N
>zQ~La x x v~iC~7~a~a;~ra~axA >C7 ZaO~Wq
=.~a¾¾~~~>~~F- aww ~ a" xxZE ~,~Ha~nHaa
"'a;aav~v~~3AHa~~>>¾z Z > z z
>
z >
c~H
aa~a>~xx¾ z>~zx30 x z>~ ~
>zc~~azzH>>w >HHa
>wwU v)Hv~av~w~H>~ a a 0 > v~ c7 C7 Z
aa xawwwwwwwaaaa33a~~>~~~ >a
U~ w w w w v~ a: a, >> z~~¾ Q A~O C7 E- v H r~ `i' a F a: r~
4L1aUV ww A¾v~a~~>~ wE-> >C~>~na~
a4
>¾~~x~~~~~zza~~W¾
> ~ Z a ~ cF7 > H ~ c~7 H aa >
c"zav.a~o'> v~ ~AxUz>~ w3~x~z>> vF~ cn
w>w¾ ~, ~xxo'¾~~z
a cn z~~õa3~xazz~">v~>Ha~
cnC~L~ o 0 ~w0
VZ
a'~>ww~~Wwa3~ > wa3v aaz ~ H >~a -
w3~~~wwx ~A~U~a>>Qa>~a~Ha~C~4 >
0w3v) vi w~? >x~ v~¾¾c4 z>~wwaaoaHC4 ~- a.H>c7~- ~-Ht-~
00 qt v'i ~D 00 l- O~ 0 NW) ~,O l- 00 O~ O~ - q:l- ~O
N N N
00 O, O, O, O, p~ ~ N N M M~ V~n v~ ~O ~O ~D ~D Q~ ON O~ O, O- - - - =-, - - -
~ .- r. -+ . - - - N N N N N N N N N N N N
~O ~10 ~10 I'O ~10 "G
W) tn W1 V1 v) W) U1 vl Wf v) V) Vl vl vl v1 Vl vl tn tn V1
M M M M Cy N M N M N M M N M M N M N M N M N M Cy M M~y ~y M M N N M N M N
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ \.D
M M M M r.. =~ M,... M,-. M M,-. M M,..~ M.~ M~ M,~ M.~ M M,~ ,~ M M,-r rr M..
M,~
O O O O... O^.. O O=~ O O... O=~ O O.~ O._.. O O=~ O O,~ O=_.~ O r_.
wwww....~w..-. ww.~.~ww.~..w._w.~
¾¾¾¾aa¾a~¾~¾¾a¾¾a¾a¾a¾a¾a¾¾aa¾¾aa¾a
N N=--~ N N-- ^" N-- -~ N-- N=--~ N- N N~ N N~ N N~ N=--~ N
z z z z z z z z zzz z z z z z z z zzz z z z z z z z z z z z z z z z
kn xxxCq xxxx~xW)xxxxxCq xx~xxW)x~xxxx
J x x x x x x~ x x x x x x x x x~ x x x x x~ x x x x x x x x x x
x
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
O O N p N p N ~ N O ~ en ~en
00 CN CN C14 l- a,
~= C'= ~= C' ~' C= C. C' C= C'' C= C= C= ~`= C. ~; C'~`= C. C`. C`= C= C~= Cl=
C~. C: C= C: C' C, C`' C' C; C:
C' C C' C, C= C= C. a E-r ~.. C.
¾F-v~c ~~ ~'v~~ c~ ~~ ~ ~~vc v v~~.c- ~. v>>~..c'= C`' c= ~: c: ~~= `' ~= c''
~ ~ a ~ a ~= ~ ~= ~= ~= ¾ ~= ~ ~= ~'' ~: ~= ~= c' ~: c: ~= c: ~ Cl. C, ~.. ~.
C~= C= C C` ~= ~.. ~.. G= C-= c.. ~.. C~= ~.. C= C~= ~. C~= C~= C~= ~.= C~.
C~= C~= C.. C'= C~= C.. c.. C= ~.. C`.
a w 3 a t~ C-- w > a > a ~ C-- - > >
f+1 ~p l": kn M~ 00 p p M~p 01 "T.-r MM 00 I- M\O -. 00 00 00 ~~ N~ 00
~~ v1 ~= OO ~~ M~ O M p N O p~ ~ M~~ t/~ Vl I~ CO oC N M'~ ~ CC Op M~r
O\ OO"7 v1 O N - 00 ~~1 O n O
O
N
E-+
zwx>QzAza
F- rz v~ E- E- A~ A C7 vxi F- F w > a C7 a a a C7 v~ F a F a F- v> >
¾~ ~33aq F-. c~w a xFU~C7~~x ww3 a~Az
w¾awv~Ha3v~xc7wv~a~>aww~~¾x u~ >~wpli
>
awvzww3H~rwc~ zQ~w ac7~n¾p~?~XAq
za
z 3 QHwH z a~ ~v ~w~z
w~~UHxx~wxHac¾7~~0'¾~3w¾>xZ>
xW~Q~Q~~C7~zCYaw>U>wlwqww> a-lau,xa-5>~
z wua aHwx ~7wzwa >q zCY (D
~a~~~aq3H ~c7ax x >~ o'z q a~¾aa~Zx v~
¾Hv~HHa~ ~FE How,> t?v a~¾xHcnk a~"v>>
~¾a33~q3v,~~vw a~xHwa~c~w.~x w~3~wWqxz>
ww~ wv~ a w~4U >aC7~¾oQJ~~q>HQZc1n) >
c7 w
w3 ww HzQHw
>azwwwE...0~~7~,~wc7 a~xHa~aawvwc7wA3~wAxqz
>> >
O~x v~ pwx~Ha¾HaZa~ zc7 >Q~c7q aHAa~w
vv~~wE acr,C7C7HC7Haxx~~c~a:~~w Aa3>zv)
zacn 0 w33¾vooaazHw>awav~Azzww>awH~4
00 ON Okn l- O~ -~ O M l- O N M~O O N c0 l- M M M[ - [- v i O" Ntn M O~ O O~
N N~~~ ~t ~1 ~n ~D t~ l~ oo O~ O O - N M~n l~ O M M~' W1 tn ~O t- =--N
N N N N N N N N N N N N N M M M M M M M M M M M~~~~~ CP N1 r1 ~kn
W) vi N
r1 M N M N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
10 't q* ~ ~ ~ _ ~ V ~ 4 qt '1' ~ ~ ~ .4 "t qt 4 V
M M -r M .--i .-r .r .-r .--=-. =-r .~ .-r =--i .--i =--=-r .r .-.-+ .r .r .--
=-. .-,.i .-. .--i .-r =-r .-. r+ .-+
O O .,. O .=-.--, .-. ,~ .--.-. .--.--=--,--.~ ,--r..i .-. ,~ .--~ ...--.--=-.
..-^' .-. ^' .-. .--^' .--wwr.w.~ .~=~.~
¾¾aQaaa-1.~aaa-1 ~.aa~a.:aaaa~aaa.a .4 .3 -1a~a~aaa
N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N N
zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz
xx~xx~xxxxxxxxxxx~" ~xx~~~xx~" ~ xxxx~x
x~ x x x~~ x x x x~~~ x x x x x x x~
J x x~ x x x x~ x x x 'm
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N NC-4 N
00 cG ~ ~ ~
C- c= C. C'= C` C- C= C- ~, C-= ~.. C-= C- C-=
~=r C`' ~ c.. ~. C`= c`. C' ~ ~ P' (n C C C. ¾ Cl. C¾ C¾ 0 C C; C c;
C. C; C= C= C= C; C ~= ~; ~; C= C' C=
> > > a a c, O V O ~O -- C~i o0 ~a, m O
M
M~ O O ~1 ~=--~ O=--~ N O~ N
U')
O
N
>a¾¾~¾~a~~~3
>;~
> U
z
a¾¾¾> ~3
>aa>~
IaaQ>¾u cna
>¾¾¾¾c~~a¾z~
>> ~
~2a¾>N
~>a ¾~'¾aa~~ wu
>>
>adHcncn
> ~
>¾ ~ ~
> cn
[- oo Olzt oo O N M t-
N N M M M M MIt -* 11tnkn vl V'1 \D
Vl W) tn t/'1 Vl W) V'1 W1 Vl W1 kn V'1 W1 vl
N N N N N N N N N N N N N N N
~' ~ d' ~t' ~ ~ d' d= .~ r. .-r
a a..~ ~.:a a~ a.:a a a a a~:a a a
N N N N N N N N N N N N N N N
zzzzzzzzzzzzzzz
~x~xxx~~xxxxxxx
J x x x x~ x x x x x x x x x x
F-
CA 02658559 2009-01-20
206
TABLEWO 2008/039267 PCT/US2007/016529
42
(2) HA DRI Supertype
Minimally, peptides predicted to bind DR1 5100nM and anchor conservancy 2:35%
Anchor
Protein Pos Sequence Core sequence DR1 PIC String Conservancy
HA 7 MAIIYLILLFTAVRG IYLILLFTA 4.25 I????L??? 15/17
HA 9 IIYLILLFTAVRGDQ LILLFTAVR 6.30 L????T??? 11/17
HA 10 IYLILLFTAVRGDQI ILLFTAVRG 22.4 I????A??? 11/17
HA 24 CIGYHANNSTEQVDT YHANNSTEQ 18.9 Y????S??? 13/20
HA 25 CIGYHANNSTEKVDT YHANNSTEK 88.8 Y????S??? 13/20
HA 36 VDTIMEKNVTVTHAQ IMEKNVTVT 77.1 I????V??? 20/20
HA 37 DTIMEKNVTVTHAQD MEKNVTVTH 0.85 M????T??? 11/20
HA 37 VDTILERNVTVTHAK ILERNVTVT 79.3 I????V??? 20/20
HA 38 DTILERNVTVTHAKD LERNVTVTH 21.1 L????T??? 11/20
HA 61 LCDLNGVKPLILRDC LNGVKPLIL 36.1 L????P??? 11/20
HA 62 LCKLNGIPPLELGDC LNGIPPLEL 6.30 L????P??? 11/20
HA 74 DCSVAGWLLGNPMCD VAGWLLGNP 36.1 V????L??? 17/20
HA 75 DCSIAGWLLGNPECD IAGWLLGNP 25.0 I????L??? 17/20
HA 94 EWSYIVEKASPANDL YIVEKASPA 0.54 Y????A??? 13/20
HA 117 YEELKHLLSRINHFE LKHLLSRIN 20.0 L????S??? 16/20
HA 119 YEELKHLLSSVKHFE LKHLLSSVK 83.9 L????S??? 16/20
HA 127 INHFEKIQIIPKTSS FEKIQIIPK 5.79 F????I??? 18/20
HA 129 VKHFEKVKILPKTDR FEKVKILPK 46.5 F????I??? 18/20
HA 132 KIQIIPKSSWSNHDA IIPKSSWSN 47.8 I????S??? 13/15
HA 160 RSSFFRNVVWLIKKN FFRNVVWLI .1.54 F????V??? 20/20
HA 162 NPSFFRNMVWLTEKG FFRNMVWLT 1.93 F????V??? 20/20
HA 167 VVWLIKKNSAYPTIK LIKKNSAYP 13.1 L????S??? 8/20
HA 192 EDLLVLWGIHHPNDA LVLWGIHHP 4.37 L????I??? 20/20
HA .194 EQMLIIWGVHHPNDE LIIWGVHHP 0.61 L????V??? 20/20
HA 209 QTKLYQNPTTYISVG LYQNPTTYI 20.5 L????T??? 12/20
HA 216 PTTYISVGTSTLNQR YISVGTSTL 4.62 Y????T??? 15/20
HA 217 TTYISVGTSTLNQRL ISVGTSTLN 1.27 I????S??? 15/20
HA 218 VGTYVSVGTSTLNKR YVSVGTSTL 6.48 Y????T??? 15/20
HA 219 GTYVSVGTSTLNKRS VSVGTSTLN 1.83 V????S??? 15/20
HA 219 YISVGTSTLNQRLVP VGTSTLNQR 7.05 V????L??? 10/20
HA 221 YVSVGTSTLNKRSTP VGTSTLNKR 12.7 V????L??? 10/20
HA 224 TSTLNQRLVPEIATR LNQRLVPEI 23.6 L????V??? 15/20
HA 228 NQRLVPEIATRPKVN LVPEIATRP 0.93 L????A??? 8/20
HA 254 WTLLDMWDTINFEST LDMWDTINF 88.8 L????T??? 20/20
HA 277 GFKISKRGSSGIMKT ISKRGSSGI 9.34 I????S??? 9/20
HA 286 SGIMKTEGTLENCET MKTEGTLEN 0.53 M????T??? 11/20
HA 306 LGAINTTLPFHNVHP INTTLPFHN 40.4 I????P??? 20/20
HA 312 TLPFHNVHPLTIGEC FHNVHPLTI 5.03 F????P??? 15/20
HA 327 PKYVKSEKLVLATGL VKSEKLVLA 1.38 V????L??? 20/20
HA 333 EKLVLATGLRNVPQI VLATGLRNV 5.47 V????L??? 9/20
HA 353 SRGLFGAIAGFIEGG LFGAIAGFI 0.56 L????A??? 15/15
HA 407 NSVIEKMNTQFEAVG IEKMNTQFE 17.8 1????T??? 9/15
HA 417 FEAVGKEFSNLERRL VGKEFSNLE 88.8 V????S??? 7/13
HA 445 VWTYNAELLVLMENE YNAELLVLM 0.11 Y????L??? 13/13
HA 470 KNLYDKVRMQLRDNV YDKVRMQLR 5.32 Y????M??? 7/13
HA 519 ESKLNRNEIKGVKLS LNRNEIKGV 12.4 L????I??? 9/13
HA 524 RNEIKGVKLSSMGVY IKGVKLSSM 0.58 1????L??? 13/13
HA 529 GVKLSSMGVYQILAI LSSMGVYQI 0.42 L????V??? 9/13
HA 532 LSSMGVYQILAIYAT MGVYQILAI 3.30 M????I??? 6/13
HA 535 MGVYQILAIYATVAG YQILAIYAT 0.66 Y????I??? 9/13
HA 537 VYQILAIYATVAGSL ILAIYATVA 0.13 1????A??? 12/13
HA 538 YQILAIYATVAGSLS LAIYATVAG 5.96 L????T??? 9/13
HA 540 ILAIYATVAGSLSLA IYATVAGSL 47.8 I????A??? 13/13
HA 544 YATVAGSLSLAIMMA VAGSLSLAI 1.83 V????S??? 8/12
HA 548 AGSLSLAIMMAGISF LSLAIMMAG 0.39 L????M??? 11/12
HA 550 SLSLAIMMAGISFWM LAIMMAGIS 1.99 L????A??? 8/12
HA 552 SLAIMMAGISFWMCS IMMAGISFW 2.10 I????I??? 7/12
HA 553 LAIMMAGISFWMCSN MMAGISFWM 0.41 M????S??? 7/12
HA 561 SFWMCSNGSLQCRIC MCSNGSLQC 23.0 M????S??? 7/12
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U o r, - ._ - .... - .., ._.. r. - r. - - - - - - r. - - - - ... - - - - ...
... - r. -
v
U
C M a, Vl "O O a? M M N~' "l' "0 ,t N~~ v~ M N l~ M~O
N~ N M N v~ W) kn tn N O~ l~ r!
c,~, "o Io 10 %o ~O ~ ~o "o ~o 10 ~o ~c 'C ~O ~ ~c ~o %o ~O ~o '4c ~c ~o ~ "O
~D ~o ~c
Y aaaao.aaaaaaaaaaaaaa - a- fl.a.a - aaaaaa - a. aQ -
.a
N A 0 A Q A A A A Q A Q Q A Q Q A Q Q A Q A A Q A O Q A Q A Q Q Q Q
M V'1 V1 1!1 Vl W1 kn V'1 v1 V1 W1 W) 1!1 V1 kn V1 V1 V') V1 V) vl Vl W) Vl Vl
V) V1 ~O \D 1.0 %D ~O ~D
.-.. - .-. .r .--, ..r .--i .--i - .-. - - - .--i - - - - ..r r. .-. .-r - ,--
i - - - .-. - .-r - .--i
xQ"U ¾>>~Q~~~
c a>o z x~ x a~ azp., a, ~ w a > w ~ >
~v~idaW~¾>~
a~c~v)>>¾-Q>Zt7QzE- >zo,H?"
~~>4~3~a~x>Q ~ z uz~ Q ¾ o > >zzo a
u¾~c~ Haa3
x~ ww~ Ac7~¾Q~~d~
xAQ=~~wA pw>z>¾
.a ~~wa>~a
>
QAH >
' N M ct ~t O, O'D 'D M 00 N O k/'i O~ v1 vl vl ~ O~ N~O ~' ~O M M O~O
O tn O[~ O r. N N v1 n o0 N~~ ~O l~ 00 O~ N dD O~ ~O -- N M~ N~O O~ N
f~. tn NW) r-N N N N N N N N N M M M M~Y W) Wl v1 M--N Nt/')
O
N_
- - - - - - - - - - - - - - - - - - - - - - - - - - -
cd lz z z z z z z z z z z z zzz z z z z z z z z z z z Z z z z Z z z
~ z ~~-4 ` , r4 H+i W W ~ W `~r" `F+i `W r+w W `W `~ W `~r W W W W W W W `W
~`Ni W W
N
C1.
CII)
M
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
OC) aass aa
N A 0 Q A A A
z
cnW3'n
¾ , x .a U
¾ >
> ~ ¾ x >
cn¾w` ~C7
~~> 0.
a'zaa
C~ N N O It
N M O V
ln V1 M Vl V1
zzzzzz
-
xxxxxx
W x x x~ x x
J
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U
~
cn co 00 00 co co ~00 t- 00 t- 00 t- 00 w, 'n
.- r. - - ~ r, r.. - - - - - - r, - - -
C/] 0
U
0--~0000000000000~0~0~-Or-O~O 0-- O O~O
~. ~ C= C= C CC= : C ~ C= = = C~= C~. ~.= C= C= C= C, C= C' C.' C~= C-= C..
C.. ~.. ~.. C-. C.. ~. C. C..
P= = ~= ~. C. C. ~. C'
> a= a= fC' /) V~]' C C~ ~. C. Q Q~. Q Q C C'= C'`= C~= a~~ C C F" F" H
c. c ~= ~= r= r= c= r= ". ~: a Cl- ~ ~ ~; ~; ~; ~; ~; "; ~: c: ~; ~: ~ ~ c c
~' c. c' c'
~ ~: ~: ~ ~ ~ ~ c ~ ~: C: C' ~~ ~ ~: ~: C-- C: C: ~: ~' ~' C' ~' ~ ~ C ~= ~:
~: c: ~: ~:
>>>> a w~; ~~> a a w a w w~~ 3 3 a ~~ w a a w w
U
A+ N_ N_ N'""' N--= OO ~i; ^~ N ~n ~ ~~=--' ^" M M N N M M M M l~ l~ O O o0
M = 00 -'Zl- 00 l-~ M 01 00 r- N N r-: ~O ~O ~ 00 00 ~!1 v 00 00 r
v1 W) ~ O O O O n v1 r1 M --~
CF) U
N :::s > > gi. -J QJa .a . a a a w >,
Q¾Q~~~aaJa~~ 1~~
d d
,U >>~ H
a,">o,~Ca7a~wW ~x a a w w
ww~Faa~~> =-~=-~C7C7
,~~,v~ v~ ¾AAC7c7 3~~F-~E-~aa~xwww
U E.~-.ww wav~v~a>?~a c7C7>
>-~ .aa~.~.. .a a. ~aawaw
~.> QA~~aaE-
n va a a~~ w¾~¾ Q a a Q A~ z w w~ a a a a a cw7 C7 r~~ w c, a w
> >
~nv~~a`i',,C7vaaw>
;3 a,,
a
HH...~aa>a,a ~wwZZx~~a~a x~~aFw- Ew- H
>>> > = .>p"0. c7aaww>>~aC~aF- ~ar~aa~~wwa
> C~ ~, a aC ~C F- F- c7 C~
v~ wwHHawv~v~~~v, C7 AAC7c7 3 xx ~c~~ ww
aa >-~ a v~ ?a a"~~ w w ¾ w ¾ w~ w w 23~E-~E-~c7c7>
HHww awaw
F- } v) v~> aw v~ v~ w
wwE-4 a
wwHH aa0aAwAwww~aa~~~~aax~a
O O- N N~ d~n vi O O cC o0 N N M M v'
~n l IO ~O -- -- ~n ~n O O N
.... tn v1 ~D "O \0
O 00 00 00 00 00 00 00 00 00 00 00 00 00 00 00 00 00
~ V'1 V~
Zt!1 Vl V1 vl l!1 kn v') 1n kn t!1 V1 tr)
M M M M M M M M M en M M M M M M M M M M C!1 M M M M M M M M M M M M
ON GN "0 01 \~O O, "o O, \,O 01 ZD Q\ \,D 01 %0 Q1 %0 Q~ \O Q~ ~O O~ ~O Q~ ~D
M O M O M O M O M O M O M O M O M O M O M O M O M O M O M O M O M
O 00 O o0 O oo O o0 O o0 O oo O oo O o0 O o0 O o0 O o0 O oo O o0 O o0 O o0 O
o0 O
ax¾x¾xaxax¾~¾x¾xax~x¾x¾x¾x¾x~ax¾~¾
=--N =--~ N =--~ N =--~ N =--= N ~ N ~ N -+ N =--~ N =--~ N ~ N =--~ N ^~ N ~
N ~
zzzzzzzzzzzzzzzz~ zzzzzzzzzzzzzzzzz
~n N kn N ~n ~n N ~n N ~n N ~n ~n N tn N W1 N kn N ~n N W) N W~ N W) ~n
~~,xxxxxxx~xxxxxxxxxxxxxxxxxxxxxx x
-. .-. - .-. r.
M ~) - .
LU ~ o
00 ,~ a.=
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~ .-~r .-~-i .-~. .-~= ~D "D W) ~tn 00 N :T 00 O) V=1 M \O O~ M a - - .--i .--
i - .-r
- O O-- O O O O O O O O O O O O O O O O O O O O O O O-- O O O--
C-= C= C~= C- C- C C ~.' ~.' C. C- C- C~= C= CC'= C= CC= G~= ~.. C~= C~= C'=
CC~C~ ~= C~= CC~= C,= C.. C.. C.. C. C~= C' C' C- C'~~
.. ..
c.= ~.. C~. c.= ~. C`= C= C~= C~= C~= ~. ~.= C, C= ~=. ~= C~= L~ ~. C C~ C ~
C~ c.= ~.. ~.. C. ~. C L
r ~' c,' ~ c= ~, c= ~.= ~.= < ~~= ~~~ c- ~~¾ E E d d c~ c= c'= c~= ~= ~- ~
c`. ~'
~= ~=
~ > C ~ ~ ~ e- c,¾¾c.c~- ~. c= c= ~. ~=c_ c.~.~.c,c.~:c:¾¾~ c ac.~.c c
C= C'= C'= C'`= C-= C-= C'`= C`= ~.. ~., ~.. ~, C=. ~., C, ~, ~. ~, C= C~= C
. ~. ~.. ~. . C, w C,= C'
w C c` C` w ~ C w ~ w cl P. c coi ` ~.-.' r.=.= > > . ~ . ~ ~ >
cC "O ~D ~~T t^--~ ~'O 00 N O\ v) N ON (=> co O, W) Mkn O N00 "o 1%O ~O
l~ 't "T N l- M~O O 00 M~~.-r M M
~tt O O[~ tn ~ cM ~ N vi N O N N l~ M vi M N^^ N^" ^ O O
O
N cnWWrxC;0 0 0>u~= >~¾~vUivUHa¾¾~~aa
jaazZwx`~~~F C7QQ¾C7C7F>- a>HH~wUUFFww.aEF~EF~¾Q
>>A..a¾Q~H~d¾~ ~j,QQ~~C7C7FF>>> aUUFFU- az`n`nFFEF-
>- >zZW >¾W WC7C7 QQE-
wFF ~ ~aar~5wxx a ,a~ ¾ FFaw,.a,.,~UU QQ
>wwFF aC FwwdaQcnv~ C7C~FFddC7C7 aa
> > w w w w a a 2 ~ . > > > > > > a w w a > > >
aa v~ ~, v~aawwddaaQ¾aa zwW~~
waaaA a~w ~ ~a~ ¾
~v~aac7c7¾x~~aaQa~~~~
> > a vu aa v~
w waw
CR 44 v 0 z Z v d>¾¾ d w¾~~ F F¾ Q a F F w w¾¾~
~wõwwG~xC7c7xc7w >u ~F¾FF>>0 0
¾QUUaa~dQ
av~v~wwZZwx`~C wE- C7Q¾¾C7C7>>Ww~k C,UFFww~FFQd
>aav~v~~aFwQ~~~Qcn.~ QF"F¾Q>>QdUUA~FFFF
>> a a Q¾~ H v Q Q~, -- Q d~~ ~7 j>> a U U F F~ Q d~~
a~FIE>- [>- ZZ xc7C7v~~~C7c7Z~~~F ¾F F C7C7 QdF~aQQ
FFF~~aa wxx~ ~ ~+ UU
w ~F~ F Q¾¾vFivQi.~ni >> VC7 j
>
w w F F > >
> >
w>z.w W~W~~ ~~~~ awaa
H~wd~~
~C7c7ww ~~ r~ x~4~~ a 5
I Id¾ d d U U 0 0 w w¾R w w Z
N M M~~ O~ G~ M M l, l- n`n W) W) O~ O~ O O N NW) V) w w N N"I- ,t ~O "G ~O
(or, 0, O O
~ ~ ~ ~ ~ ~ O O O O O =^'-~ ~ ^~ ^' M M M M M M M M ~ ~ ~ Cf ~ ~ "0 [- l- 00
00
.-. .-r .-+ .--i .-. .-r .-. ... .-r .-. ... .-. .--+ .-r .r .=r .--i .--i .-r
- - .--+
O ~
00 00 00 00 00 Q+ 00 G~ o0 00 00 00 00 00 00 00 00 00
W) kn W) vy v) v~ W) Wl W) kn vl W) v) kn kn
M M M M M M M M M M M~ M M~ M M M M M M M M M M M M M M M M M M M M M
o~ ~o o, " 0 o1 ~o o, 110 rn~O c, r-~ o, p~o rn~o rn c o, "o oN %o rn~o rn"o
o~ \~c rn o\ ~D rn~ Q,
O M O M O M O M O M O M O z M O M O M O M O M O M O M O M O O M O M O
00 O 00 O 00 O cG O CC O cG ,Z O o0 O o0 O o0 O o0 O 00 O 00 O 00 O 00 O o0 00
O 00 O oG
O w O w O O O O r- O w O w O w O V. O O w O O V. 0 O O V. 0
XQ>GQ>C¾>CQXQXxd>G Q>GQ>Gd>C¾>Gd>CQ~GQ>CQ>C>CQ>Cd~C
N
zzzzzzzzzzzzzzzzz.zzzzzzzzzzzzzzzzzzz
xxxxxxxxxxxxxxxx~xxxxx~xxxxxxxxx
_____
M - - .-. ,-. .-. .-, - .-. ... - - - ..... - .-. - - - - - - - - - - - - - .-
. - ...
W 2 ~2 2 2 2 2 ~~ 2 2
J
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
oo 110
o o o 0 0 0 0 0 0
c
~ ~= = = c= c= . ~=
~= ~= ~= = ~ C' ~=
. ~.. ~.. cl'
P. P= C= C' P. P= ~. C..
Q Q F ¾ E-~ C= ~ a Cl= Cl= ¾
~: C: C. C= C= ~. C. ~. C: C= :
C.. C.. C/~ ¾
C. C~=
C'~= C,= C'' C'' C= C' C' C= C= C~ C~=
r~ > > i-C-=i r.~ ~ H
N N O O M N t- ~~ O~
fV O O O G O~ M N N
N Cn z w a C
cn
¾¾o'o'04Ww
wa~~~¾x~,~ vC~
o~-a¾O
a~z0
a- a¾ Q
cn¾¾H xoA~a
>0>0C4W
0
00 00 ===~ =--~ N N Mlt
M '
~ N N N N N N N N N
00 00 00 00 cC 00
W) tn kr) kf) tn W)
M M M M M M C1 M M M M
I'O O~ 1~0 0\ O ~D O\ 1%O O~
M M O M O M[+1 O M O
00 O cG O cG O O CC O o0
w o w o w o w P. o w o
¾xQxQx¾¾xQx
=--~ N=-~ NN ~ ~ NN =-- ~ ~ ~ -=+ NN ~ =--~ NN
'i N ~
v
xxxxxxxxxxx
M ______
W
J
m
~
CA 02658559 2009-01-20
212
TABLEWS2008/039267 PCT/US2007/016529
(2) M1 DRI Supertype
Minimally, peptides predicted to bind DR1 :5100nM and sequence conservancy
>_35%
Sequence
Protein Pos Sequence Core sequence DRl PIC String Conservancy
M1 7 LTEVETYVLSIVPSG VETYVLSIV 21.1 V????L??? 8/16
M1 10 VETYVLSIVPSGPLK YVLSIVPSG 34.1 Y????V??? 8/16
M1 11 ETYVLSIVPSGPLKA VLSIVPSGP 17.4 V????P??? 8/16
M1 12 TYVLSIVPSGPLKAE LSIVPSGPL 49.2 L????S??? 8/16
M1 14 VLSIVPSGPLKAEIA IVPSGPLKA 0.85 I????P??? 8/16
M1 15 LSIIPSGPLKAEIAQ IPSGPLKAE 77 I????L??? 7/16
M1 20 SGPLKAEIAQRLEDV LKAEIAQRL 0.61 L????A??? 15/16
M1 32 EDVFAGKNTDLEALM FAGKNTDLE 77.1 F????T??? 15/16
M1 43 EALMEWLKTRPILSP MEWLKTRPI 5.63 M????T??? 14/16
M1 45 LMEWLKTRPILSPLT WLKTRPILS 45.2 W????P??? 15/16
M1 46 MEWLKTRPILSPLTK LKTRPILSP 0.83 L????I??? 16/17
M1 51 TRPILSPLTKGILGF ILSPLTKGI 0.53 I????T??? 15/17
M1 55 LSPLTKGILGFVFTL LTKGILGFV 5.47 L????L??? 16/17
M1 60 KGILGFVFTLTVPSE LGFVFTLTV 3.80 L????T??? 16/17
M1 62 ILGFVFTLTVPSERG FVFTLTVPS 1.78 F????T??? 16/17
M1 63 LGFVFTLTVPSERGL VFTLTVPSE 1.46 V????V??? 17/17
M1 79 RRRFVQNALNGNGDP FVQNALNGN 0.74 F????L??? 17/17
M1 103 YRKLKREITFHGAKE LKREITFHG 50.6 L????T??? 10/17
M1 107 KREMTFHGAKEVALS MTFHGAKEV 1.78 M????A??? 6/17
MI 115 AKEVALSYSTGALAS VALSYSTGA 4.49 V????S??? 8/17
M1 119 ALSYSAGALASCMGL YSAGALASC 2.79 Y????L??? 9/17
M1 130 CMGLIYNRMGTVTTE LIYNRMGTV 1.20 L????M??? 12/17
M1 132 GLIYNRMGTVTTEVA YNRMGTVTT 0.21 Y????T??? 12/17
M1 135 YNRMGTVTTEVALGL MGTVTTEVA 4.49 M????T??? 6/17
M1 138 MGTVTTEVALGLVCA VTTEVALGL 2.63 V????A??? 6/17
M1 142 TTEVAFGLVCATCEQ VAFGLVCAT 5.32 V????V??? 9/17
M1 179 ENRMVLASTTAKAME MVLASTTAK 1.16 M????T??? 17/17
M1 180 NRMVLASTTAKAMEQ VLASTTAKA 0.36 V????T??? 17/17
M1 181 RMVLASTTAKAMEQM LASTTAKAM 2.29 L????A??? 17/17
MI 213 RQMVQAMRTIGTHPS VQAMRTIGT 0.053 V????T??? 7/17
M1 216 VQAMRTIGTHPSSSA MRTIGTHPS 0.36 M????T??? 7/17
M1 219 MRTIGTHPSSSAGLK IGTHPSSSA 0.79 I????S??? 7/17
M1 234 DDLIENLQAYQKRMG IENLQAYQK 2.04 I????A??? 8/17
CA 02658559 2009-01-20
TABLEW b2008/039267 213 PCT/US2007/016529
(1) M2 DR1 Supertype Sequence
Protein Strain Acc. No. Pos Sequence Core sequence DR1 PIC String red
Conservancy
M2 H5N1 AF036358 4 MSLLTEVETLTRNGW LTEVETLTR 75.0 L????T??? 0 2/13
M2 H2N2 X08093 4 MSLLTEVETPIRNEW LTEVETPIR 281 L????T??? 0 4/13
M2 H5N1 AF036358 15 RNGWGCRCSDSSDPL WGCRCSDSS 1000000 W????S??? 0
M2 H5N1 AF036358 26 SDPLVVAASIIGILH LVVAASIIG 0.96 L????S??? 0 8/13
M2 H2N2 X08093 26 SDPLVVAASIIGILH LVVAASIIG 0.96 L????S??? 1 8/13
M2 H5N1 AF036358 27 DPLVVAASIIGILHL VVAASIIGI 0.098 V????I??? 0 8/13
M2 H2N2 X08093 27 DPLVVAASIIGILHL VVAASIIGI 0.098 V????I??? 1 8/13
M2 H5N1 AF036358 28 PLVVAASIIGILHLI VAASIIGIL 185 V????I??? 0
M2 H2N2 X08093 28 PLVVAASIIGILHLI VAASIIGIL 185 V????I??? 1
M2 H5N1 AF036358 33 ASIIGILHLILWILD IGILHLILW 0.22 I????L??? 0 11/14
M2 H2N2 X08093 33 ASIIGILHLILWILD IGILHLILW 0.22 I????L??? 1 11/14
M2 H5N1 AF036358 35 IIGILHLILWILDRL ILHLILWIL 259 I????L??? 0 10/14
M2 H2N2 X08093 35 IIGILHLILWILDRL ILHLILWIL 259 I????L??? 1 10/14
M2 H5N1 AF036358 38 ILHLILWILDRLFFK LILWILDRL 195 L????L??? 0 10/14
M2 H2N2 X08093 38 ILHLILWILDRLFFK LILWILDRL 195 L????L??? 1 10/14
M2 H5N1 AF036358 41 LILWILDRLFFKCIY WILDRLFFK 4078 W????L??? 0
M2 H2N2 X08093 41 LILWILDRLFFKCIY WILDRLFFK 4078 W????L??? 1
M2 H5N1 AF036358 46 LDRLFFKCIYRRFKY LFFKCIYRR 2323 L????I??? 0
M2 H2N2 X08093 46 LDRLFFKCIYRFFKH LFFKCIYRF 2457 L????I??? 0
M2 H2N2 X08093 54 IYRFFKHGLKRGPST FFKHGLKRG 3.03 F????L??? 0 2/13
M2 H5N1 AF036358 59 KYGLKRGPSTEGVPE LKRGPSTEG 12.7 L????S??? 0 8/13
M2 H2N2 X08093 59 KHGLKRGPSTEGVPE LKRGPSTEG 12.7 L????S??? 1 2/13
M2 H2N2 X08093 84 QSAVDADDSHFVSIE VDADDSHFV 45.2 V????S??? 0 3/13
CA 02658559 2009-01-20
TABLEWO 2008/039267 214 PCT/US2007/016529
(2) M2 DR1 Supertype
Minimally, peptides predicted to bind DR1 <_300nM and sequence conservancy
?35%
Sequence
Protein Pos Sequence Core sequence DR1 PIC String Conservancy
M2 26 SDPLVVAASIIGILH LVVAASIIG 0.96 L????S??? 8/13
M2 27 DPLVVAASIIGILHL VVAASIIGI 0.10 V????I??? 8/13
M2 28 PLVVAASIIGILHLI VAASIIGIL 185 V????I??? 8/13
M2 33 ASIIGILHLILWILD IGILHLILW 0.22 I????L??? 11/14
M2 35 IIGILHLILWILDRL ILHLILWIL 259 I????L??? 10/14
M2 38 ILHLILWILDRLFFK LILWILDRL 195 L????L??? 10/14
M2 59 KYGLKRGPSTEGVPE LKRGPSTEG 12.7 L????S??? 8/13
CA 02658559 2009-01-20
215
TABLEWO 2008/039267 PCT/US2007/016529
(1) NA DRI Supertype Anchor
Protein Strain Acc. No. Pos Sequence Core sequence DR1 PIC String Conservancy
NA H5N1 AF036357 13 NQKIITIGSICMVVG IITIGSICM 0.14 I????S??? 13/16
NA H2N2 L37329 13 NQKIITIGSVSLTIA IITIGSVSL 0.37 I????S??? 13/16
NA H5N1 AF036357 14 QKIITIGSICMVVGI ITIGSICMV 15.5 I????I??? 16/16
NA H2N2 L37329 14 QKIITIGSVSLTIAT ITIGSVSLT 1.68 I????V??? 16/16
NA H5N1 AF036357 16 IITIGSICMVVGIIS IGSICMVVG 602 I????M???
NA H2N2 L37329 16 IITIGSVSLTIATAC IGSVSLTIA 28.0 I????L??? 16/16
NA H2N2 L37329 19 IGSVSLTIATACSLM VSLTIATAC 139 V????A???
NA H5N1 AF036357 21 SICMVVGIISLMLQI MVVGIISLM 0.030 M????I??? 16/16
NA H2N2 L37329 21 SVSLTIATACSLMQI LTIATACSL 4.13 L????A??? 16/16
NA H5N1 AF036357 22 ICMVVGIISLMLQIG VVGIISLML 1.13 V????S??? 4/16
NA H5NI AF036357 23 CMVVGIISLMLQIGN VGIISLMLQ 0.47 V????L??? 11/16
NA H2N2 L37329 23 SLTIATACSLMQIAI IATACSLMQ 9.08 I????S??? 11/16
NA H5N1 AF036357 25 VVGIISLMLQIGNII IISLMLQIG 17.4 I????L??? 8/16
NA H2N2 L37329 29 ACSLMQIAILATTVT LMQIAILAT 1.59 L????I??? 8/16
NA H5N1 AF036357 30 SLMLQIGNIISVWVS LQIGNIISV 0.16 L????I??? 16/16
NA H2N2 L37329 30 CSLMQIAILATTVTL MQIAILATT 0.038 M????L??? 16/16
NA H5N1 AF036357 32 MLQIGNIISVWVSHI IGNIISVWV 1.38 I????S??? 16/16
NA H2N2 L37329 32 LMQIAILATTVTLHF IAILATTVT 0.33 I????T??? 16/16
NA H2N2 L37329 34 QIAILATTVTLHFKQ ILATTVTLH 3.03 I????V??? 8/16
NA H5N1 AF036357 35 IGNIISVWVSHIIQT IISVWVSHI 86.3 I????V??? 16/16
NA H2N2 L37329 35 IAILATTVTLHFKQH LATTVTLHF 3.21 L????T??? 16/16
NA H5N1 AF036357 36 GNIISVWVSHIIQTW ISVWVSHII 21.1 I????S??? 7/16
NA H5N1 AF036357 38 IISVWVSHIIQTWHP VWVSHIIQT 3.30 V????I??? 8/16
NA H5N1 AF036357 39 ISVWVSHIIQTWHPN WVSHIIQTW 118 W????I???
NA H2N2 L37329 43 TLHFKQHECDSPASN FKQHECDSP 867 F????C???
NA H5N1 AF036357 44 SHIIQTWHPNQPEP- IQTWHPNQP 1.59 I????P??? 2/16
NA H5N1 AF036357 47 IQTWHPNQPEPCNQS WHPNQPEPC 4694 W????P???
NA H2N2 L37329 56 SNQVMPCEPIIIERN VMPCEPIII 88.8 V????P??? 7/16
NA H2N2 L37329 57 NQVMPCEPIIIERNI MPCEPIIIE 324 M????I???
NA H5N1 AF036357 62 SINFYTEQAAASVTL FYTEQAAAS 1.83 F????A??? 2/16
NA H5N1 AF036357 63 INFYTEQAAASVTLA YTEQAAASV 0.11 Y????A??? 2/16
NA H2N2 L37329 63 EPIIIERNITEIVYL IIERNITEI 68.9 1????I??? 7/13
NA H2N2 L37329 64 PIIIERNITEIVYLN IERNITEIV 201 I????T???
NA H2N2 L37329 72 TEIVYLNNTTIE-KE VYLNNTTIE 619 V????T???
NA H2N2 L37329 73 EIVYLNNTTIEKEIC YLNNTTIEK 7.46 Y????T??? 11/14
NA H2N2 L37329 74 IVYLNNTTIEKEICP LNNTTIEKE 1908 L????I???
NA H2N2 L37329 79 NTTIEKEICPEVVEY IEKEICPEV 70.9 I????C??? 8/14
NA H2N2 L37329 84 *KEICPEVVEYRNWS ICPEVVEYR 8242 I????V???
NA H5N1 AF036357 92 SVTLAGNSSLCPISG LAGNSSLCP 4.37 L????S??? 8/16
NA H2N2 L37329 94 YRNWSKPQCQITGFA WSKPQCQIT 20.0 W????C??? 11/16
NA H5N1 AF036357 101 LCPISGWAIYSKDNS ISGWAIYSK 25.0 I????I??? 16/16
NA H2N2 L37329 101 QCQITGFAPFSKDNS ITGFAPFSK 190 I????P???
NA H5N1 AF036357 107 WAIYSKDNSIRIGSK YSKDNSIRI 19.4 Y????S??? 15/16
NA H2N2 L37329 107 FAPFSKDNSIRLSAG FSKDNSIRL 17.8 F????S??? 15/16
NA H5N1 AF036357 122 GDVFVIREPFISCSH FVIREPFIS 42.7 F????P??? 16/16
NA H2N2 L37329 122 GDIWVTREPYVSCDP WVTREPYVS 244 W????P???
NA H5N1 AF036357 124 VFVIREPFISCSHLE IREPFISCS 118 I????I???
NA H2N2 L37329 128 REPYVSCDPGKCYQF YVSCDPGKC 4828 Y????P???
NA H5N1 AF036357 129 EPFISCSHLECRTFF ISCSHLECR 28432 I????L???
NA H5N1 AF036357 140 RTFFLTQGALLNDKH FLTQGALLN 0.008 F????A??? 5/16
NA H5N1 AF036357 141 TFFLTQGALLNDKHS LTQGALLND 42.7 L????L??? 16/16
CA 02658559 2009-01-20
TABLEW32008/039267 216 PCT/US2007/016529
NA H2N2 L37329 141 QFALGQGTTLDNKHS LGQGTTLDN 20.5 L????T??? 16/16
NA H5N1 AF036357 147 GALLNDKHSNGTVKI) LNDKHSNGT 68.9 L????S??? 16/16
NA H2N2 L37329 147 GTTLDNKHSNGTIHD LDNKHSNGT 7.46 L????S??? 16/16
NA H5NI AF036357 156 NGTVKDRSPYRTLMS VKDRSPYRT 111 V????P???
NA H2N2 L37329 156 NGTIHDRIPHRTLLM IHDRIPHRT 102 I????P???
NA H2N2 L37329 160 HDRIPHRTLLMNELG IPHRTLLMN 775 I????L???
NA H5N1 AF036357 162 RSPYRTLMSCPVGEA YRTLMSCPV 1.73 Y????S??? 9/16
NA H5N1 AF036357 165 YRTLMSCPVGEAPSP LMSCPVGEA 93.9 L????V??? 16/16
NA H2N2 L37329 165 HRTLLMNELGVPFHL LLMNELGVP 99.3 L????L??? 16/16
NA H2N2 L37329 167 TLLMNELGVPFHLG- MNELGVPFH 4.37 M????V??? 8/16
NA H5NI AF036357 170 SCPVGEAPSPYNSRF VGEAPSPYN 12.7 V????S??? 8/16
NA H2N2 L37329 176 PFHLGTKQVCVAWSS LGTKQVCVA 14.2 L????V??? 11/16
NA H2N2 L37329 182 TKQVCVAWSSSSCHD VCVAWSSSS 454 V????S???
NA H5N1 AF036357 184 FESVAWSASACHDGI VAWSASACH 231 V????S???
NA H2N2 L37329 184 QVCVAWSSSSCHDGK VAWSSSSCH 1657 V????S???
NA H5N1 AF036357 186 SVAWSASACHDGISW WSASACHDG 20283 W????C???
NA H2N2 L37329 186 CVAWSSSSCHDGKAW WSSSSCHDG 809560 W????C???
NA H5N1 AF036357 195 HDGISWLTIGISGPD ISWLTIGIS 96.6 I????I??? 8/16
NA H5N1 AF036357 197 GISWLTIGISGPDNG WLTIGISGP 8.12 W????I??? 13/16
NA H2N2 L37329 197 GKAWLHVCVTGDDRN WLHVCVTGD 48532 W????V???
NA H5N1 AF036357 198 ISWLTIGISGPDNGA LTIGISGPD 135 L????S???
NA H2N2 L37329 198 KAWLHVCVTGDDRNA LHVCVTGDD 18641 L????T???
NA H5NI AF036357 200 WLTIGISGPDNGAVA IGISGPDNG 4694 I????P???
NA H5N1 AF036357 213 VAVLKYNGIITDTIK LKYNGIITD 1.30 L????I??? 8/16
NA H2N2 L37329 214 ASFIYDGRLVDSIGS IYDGRLVDS 2389 I????L???
NA H5N1 AF036357 215 VLKYNGIITDTIKSW YNGIITDTI 33.1 Y????T??? 16/16
NA H2N2 L37329 215 SFIYDGRLVDSIGSW YDGRLVDSI 58.2 Y????V??? 16/16
NA H5N1 AF036357 218 YNGIITDTIKSWRNN IITDTIKSW 13.1 I????I??? 8/16
NA H2N2 L37329 220 GRLVDSIGSWSQNIL VDSIGSWSQ 63.3 V????S??? 6/16
NA H5N1 AF036357 226 IKSWRNNILRTQESE WRNNILRTQ 19.4 W????L??? 16/16
NA H2N2 L37329 226 IGSWSQNILRTQESE WSQNILRTQ 20.5 W????L??? 16/16
NA H5N1 AF036357 231 NNILRTQESECACVN LRTQESECA 17.4 L????S??? 16/16
NA H2N2 L37329 231 QNILRTQESECVCIN LRTQESECV 11.1 L????S??? 16/16
NA H2N2 L37329 239 SECVCINGTCTVVMT VCINGTCTV 10.8 V????T??? 13/16
NA H2N2 L37329 241 CVCINGTCTVVMTDG INGTCTVVM 99.3 I????T??? 10/16
NA H5N1 AF036357 248 CFTVMTDGPSNEQAS VMTDGPSNE 1251 V????P???
NA H2N2 L37329 248 CTVVMTDGSASGRAD VMTDGSASG 72.9 V????S??? 16/16
NA H5NI AF036357 249 FTVMTDGPSNEQASY MTDGPSNEQ 12.0 M????S??? 16/16
NA H2N2 L37329 249 TVVMTDGSASGRADT MTDGSASGR 11.4 M????A??? 16/16
NA H5N1 AF036357 260 QASYKIFKIEKGRVV YKIFKIEKG 754 Y????I???
NA H5N1 AF036357 265 IFKIEKGRVVKSVEL IEKGRVVKS 4.25 I????V??? 16/16
NA H2N2 L37329 265 ILFIKEGKIVHISPL IKEGKIVHI 2.95 I????I??? 16/16
NA H2N2 L37329 270 EGKIVHISPLSGSAQ IVHISPLSG 281 I????P???
NA H5N1 AF036357 271 GRVVKSVELNAPNYH VKSVELNAP 35.1 V????L??? 13/16
NA H2N2 L37329 271 GKIVHISPLSGSAQH VHISPLSGS 40.4 V????L??? 13/16
NA H5N1 AF036357 274 VKSVELNAPNYHYEE VELNAPNYH 1962 V????P???
NA H5N1 AF036357 281 APNYHYEECSCYPDA YHYEECSCY 16656 Y????C???
NA H5N1 AF036357 283 NYHYEECSCYPDAGE YEECSCYPD 28432 Y????C???
NA H2N2 L37329 283 AQHIEECSCYPRYPD IEECSCYPR 10037 I????C???
NA H2N2 L37329 292 YPRYPDVRCICRDNW YPDVRCICR 13299 Y????C???
NA H2N2 L37329 310 NRPVIDINMEDYSID VIDINMEDY 6961 V????M???
NA H2N2 L37329 315 DINMEDYSIDSSYVC MEDYSIDSS 13678 M????I???
NA H2N2 L37329 318 MEDYSIDSSYVCSGL YSIDSSYVC 3256 Y????S???
NA H5N1 AF036357 320 *LEYQIGYICSGVFG YQIGYICSG 23.6 Y????I??? 13/16
NA H2N2 L37329 320 DYSIDSSYVCSGLVG IDSSYVCSG 944 I????V???
NA H5N1 AF036357 322 EYQIGYICSGVFGDS IGYICSGVF 10037 I????S???
CA 02658559 2009-01-20
217
TABLE s~2008/039267 PCT/US2007/016529
NA H5NI AF036357 324 QIGYICSGVFGDSPR YICSGVFGD 201 Y????V???
NA H2N2 L37329 324 DSSYVCSGLVGDTPR YVCSGLVGD 102 Y????L???
NA H2N2 L37329 325 SSYVCSGLVGDTPRN VCSGLVGDT 3349 V????V???
NA H5NI AF036357 329 CSGVFGDSPRPNDGT VFGDSPRPN 40.4 V????P??? 16/16
NA H2N2 L37329 329 CSGLVGDTPRNDDSS LVGDTPRND 99.3 L????P??? 16/16
NA H5N1 AF036357 346 CGPVSLNGAYGVKGF VSLNGAYGV 0.72 V????A??? 5/16
NA H5N1 AF036357 369 GNGVWIGRTKSTSSR VWIGRTKST 7.67 V????T??? 13/16
NA H2N2 L37329 369 GDDVWMGRTISKDSR VWMGRTISK 9.88 V????T??? 13/16
NA H2N2 L37329 370 DDVWMGRTISKDSRS WMGRTISKD 61.6 W????I??? 11/16
NA H5N1 AF036357 371 GVWIGRTKSTSSRSG IGRTKSTSS 212 I????S???
NA H2N2 L37329 371 DVWMGRTISKDSRSG MGRTISKDS 892 M????S???
NA H2N2 L37329 383 RSGYETFKVIGGWST YETFKVIGG 156 Y????V???
NA H2N2 L37329 388 TFKVIGGWSTPNSKS VIGGWSTPN 3.12 V????S??? 11/16
NA H2N2 L37329 389 FKVIGGWSTPNSKSQ IGGWSTPNS 18.4 I????T??? 7/16
NA H2N2 L37329 392 IGGWSTPNSKSQVNR WSTPNSKSQ 4.62 W????S??? 13/16
NA H5N1 AF036357 392 PNGWTETDSSFSLKQ WTETDSSFS 25405 W????S???
NA H2N2 L37329 401 KSQVNRQVIVDNNNW VNRQVIVDN 46.5 V????I??? 9/16
NA H5N1 AF036357 402 *FSLKQDIIAITDWS LKQDIIAIT 12.0 L????I??? 5/16
NA H5N1 AF036357 415 WSGYSGSFIQHPELT YSGSFIQHP 65.1 Y????I??? 16/16
NA H2N2 L37329 415 WSGYSGIFSVEGKSC YSGIFSVEG 53.5 Y????S??? 16/16
NA H5NI AF036357 420 GSFIQHPELTGLNCM IQHPELTGL 35.1 I????L??? 5/16
NA H5N1 AF036357 425 HPELTGLNCMRPCFW LTGLNCMRP 1.63 L????C??? 13/16
NA H2N2 L37329 425 ***VEGKSCINRCFY VEGKSCINR 1440 V????C???
NA H5NI AF036357 428 LTGLNCMRPCFWVEL LNCMRPCFW 2258 L????P???
NA H5N1 AF036357 435 RPCFWVELIRGRPKE FWVELIRGR 16.9 F????I??? 16/16
NA H2N2 L37329 435 NRCFYVELIRGRPQE FYVELIRGR 21.7 F????I??? 16/16
NA H5NI AF036357 439 WVELIRGRPKEKTIW LIRGRPKEK 128 L????P???
NA H2N2 L37329 439 YVELIRGRPQETRVW LIRGRPQET 21.1 L????P??? 14/16
NA H5N1 AF036357 451 EKTIWTSGSSISFCG IWTSGSSIS 83.9 I????S??? 5/16
NA H2N2 L37329 451 TRVWWTSNSIVVFCG WWTSNSIVV 35.1 W????S??? 16/16
NA H5N1 AF036357 452 KTIWTSGSSISFCGV WTSGSSISF 143 W????S???
NA H2N2 L37329 452 RVWWTSNSIVVFCGT WTSNSIVVF 944 W????I???
NA H5N1 AF036357 458 GSSISFCGVNSDTVG ISFCGVNSD 152 I????V???
NA H2N2 L37329 458 NSIVVFCGTSGTYGT VVFCGTSGT 4.49 V????T??? 11/16
NA H2N2 L37329 459 SIVVFCGTSGTYGTG VFCGTSGTY 494 V????S???
NA H5N1 AF036357 460 SISFCGVNSDTVGWS FCGVNSDTV 56.6 F????S??? 7/16
NA H5N1 AF036357 463 FCGVNSDTVGWSWPD VNSDTVGWS 11883 V????V???
NA H5N1 AF036357 468 SDTVGWSWPDDAELP VGWSWPDDA 209692 V????P???
CA 02658559 2009-01-20
WO 2008/039267 218 PCT/US2007/016529
TABLE 39
(2) NA DR1 Supertype
Minimally, peptides predicted to bind DR1 5100nM and anchor conservancy >_35%
Anchor
Protein Pos Sequence Core sequence DRl PIC String Conservancy
NA 13 NQKIITIGSICMVVG IITIGSICM 0.14 I????S??? 13/16
NA 13 NQKIITIGSVSLTIA IITIGSVSL 0.37 I????S??? 13/16
NA 14 QKIITIGSICMVVGI ITIGSICMV 15.5 I????I??? 16/16
NA 14 QKIITIGSVSLTIAT ITIGSVSLT 1.68 I????V??? 16/16
NA 16 IITIGSVSLTIATAC IGSVSLTIA 28.0 I????L??? 16/16
NA 21 SICMVVGIISLMLQI MVVGIISLM 0.030 M????I??? 16/16
NA 21 SVSLTIATACSLMQI LTIATACSL 4.13 L????A??? 16/16
NA 23 CMVVGIISLMLQIGN VGIISLMLQ 0.47 V????L??? 11/16
NA 23 SLTIATACSLMQIAI IATACSLMQ 9.08 I????S??? 11/16
NA 25 VVGIISLMLQIGNII IISLMLQIG 17.4 I????L??? 8/16
NA 29 ACSLMQIAILATTVT LMQIAILAT 1.59 L????I??? 8/16
NA 30 SLMLQIGNIISVWVS LQIGNIISV 0.16 L????I??? 16/16
NA 30 CSLMQIAILATTVTL MQIAILATT 0.038 M????L??? 16/16
NA 32 MLQIGNIISVWVSHI IGNIISVWV 1.38 I????S??? 16/16
NA 32 LMQIAILATTVTLHF IAILATTVT 0.33 I????T??? 16/16
NA 34 QIAILATTVTLHFKQ ILATTVTLH 3.03 I????V??? 8/16
NA 35 IGNIISVWVSHIIQT IISVWVSHI 86.3 I????V??? 16/16
NA 35 IAILATTVTLHFKQH LATTVTLHF 3.21 L????T??? 16/16
NA 36 GNIISVWVSHIIQTW ISVWVSHII 21.1 I????S??? 7/16
NA 38 IISVWVSHIIQTWHP VWVSHIIQT 3.30 V????I??? 8/16
NA 56 SNQVMPCEPIIIERN VMPCEPIII 88.8 V????P??? 7/16
NA 63 EPIIIERNITEIVYL IIERNITEI 68.9 I????I??? 7/13
NA 73 EIVYLNNTTIEKEIC YLNNTTIEK 7.46 Y????T??? 11/14
NA 79 NTTIEKEICPEVVEY IEKEICPEV 70.9 I????C??? 8/14
NA 92 SVTLAGNSSLCPISG LAGNSSLCP 4.37 L????S??? 8/16
NA 94 YRNWSKPQCQITGFA WSKPQCQIT 20.0 W????C??? 11/16
NA 101 LCPISGWAIYSKDNS ISGWAIYSK 25.0 I????I??? 16/16
NA 107 WAIYSKDNSIRIGSK YSKDNSIRI 19.4 Y????S??? 15/16
NA 107 FAPFSKDNSIRLSAG FSKDNSIRL 17.8 F????S??? 15/16
NA 122 GDVFVIREPFISCSH FVIREPFIS 42.7 F????P??? 16/16
NA 141 TFFLTQGALLNDKHS LTQGALLND 42.7 L????L??? 16/16
NA 141 QFALGQGTTLDNKHS LGQGTTLDN 20.5 L????T??? 16/16
NA 147 GALLNDKHSNGTVKD LNDKHSNGT 68.9 L????S??? 16/16
NA 147 GTTLDNKHSNGTIHD LDNKHSNGT 7.46 L????S??? 16/16
NA 162 RSPYRTLMSCPVGEA YRTLMSCPV 1.73 Y????S???' 9/16
NA 165 YRTLMSCPVGEAPSP LMSCPVGEA 93.9 L????V??? 16/16
NA 165 HRTLLMNELGVPFHL LLMNELGVP 99.3 L????L??? 16/16
NA 167 TLLMNELGVPFHLG- MNELGVPFH 4.37 M????V??? 8/16
NA 170 SCPVGEAPSPYNSRF VGEAPSPYN 12.7 V????S??? 8/16
NA 176 PFHLGTKQVCVAWSS LGTKQVCVA 14.2 L????V??? 11/16
NA 195 HDGISWLTIGISGPD ISWLTIGIS 96.6 I????I??? 8/16
NA 197 GISWLTIGISGPDNG WLTIGISGP 8.12 W????I??? 13/16
NA 213 VAVLKYNGIITDTIK LKYNGIITD 1.30 L????I??? 8/16
NA 215 VLKYNGIITDTIKSW YNGIITDTI 33.1 Y????T??? 16/16
NA 215 SFIYDGRLVDSIGSW YDGRLVDSI 58.2 Y????V??? 16/16
NA 218 YNGIITDTIKSWRNN IITDTIKSW 13.1 I????I??? 8/16
NA 220 GRLVDSIGSWSQNIL VDSIGSWSQ 63.3 V????S??? 6/16
NA 226 IKSWRNNILRTQESE WRNNILRTQ 19.4 W????L??? 16/16
NA 226 IGSWSQNILRTQESE WSQNILRTQ 20.5 W????L??? 16/16
NA 231 NNILRTQESECACVN LRTQESECA 17.4 L????S??? 16/16
NA 231 QNILRTQESECVCIN LRTQESECV 11.1 L????S??? 16/16
NA 239 SECVCINGTCTVVMT VCINGTCTV 10.8 V????T??? 13/16
NA 241 CVCINGTCTVVMTDG INGTCTVVM 99.3 I????T??? 10/16
NA 248 CTVVMTDGSASGRAD VMTDGSASG 72.9 V????S??? 16/16
CA 02658559 2009-01-20
219
WO 2008/039267 PCT/US2007/016529
TABLE 39
NA 249 FTVMTDGPSNEQASY MTDGPSNEQ 12.0 M????S??? 16/16
NA 249 TVVMTDGSASGRADT MTDGSASGR 11.4 M????A??? 16/16
NA 265 IFKIEKGRVVKSVEL IEKGRVVKS 4.25 I????V??? 16/16
NA 265 ILFIKEGKIVHISPL IKEGKIVHI 2.95 I????I??? 16/16
NA 271 GRVVKSVELNAPNYH VKSVELNAP 35.1 V????L??? 13/16
NA 271 GKIVHISPLSGSAQH VHISPLSGS 40.4 V????L??? 13/16
NA 320 *LEYQIGYICSGVFG YQIGYICSG 23.6 Y????I??? 13/16
NA 329 CSGVFGDSPRPNDGT VFGDSPRPN 40.4 V????P??? 16/16
NA 329 CSGLVGDTPRNDDSS LVGDTPRND 99.3 L????P??? 16/16
NA 369 GNGVWIGRTKSTSSR VWIGRTKST 7.67 V????T??? 13/16
NA 369 GDDVWMGRTISKDSR VWMGRTISK 9.88 V????T??? 13/16
NA 370 DDVWMGRTISKDSRS WMGRTISKD 61.6 W????I??? 11/16
NA 388 TFKVIGGWSTPNSKS VIGGWSTPN 3.12 V????S??? 11/16
NA 389 FKVIGGWSTPNSKSQ IGGWSTPNS 18.4 I????T??? 7/16
NA 392 IGGWSTPNSKSQVNR WSTPNSKSQ 4.62 W????S??? 13/16
NA 401 KSQVNRQVIVDNNNW VNRQVIVDN 46.5 V????I??? 9/16
NA 415 WSGYSGSFIQHPELT YSGSFIQHP 65.1 Y????I??? 16/16
NA 415 WSGYSGIFSVEGKSC YSGIFSVEG 53.5 Y????S??? 16/16
NA 425 HPELTGLNCMRPCFW LTGLNCMRP 1.63 L????C??? 13/16
NA 435 RPCFWVELIRGRPKE FWVELIRGR 16.9 F????I??? 16/16
NA 435 NRCFYVELIRGRPQE FYVELIRGR 21.7 F????I??? 16/16
NA 439 YVELIRGRPQETRVW LIRGRPQET 21.1 L????P??? 14/16
NA 451 TRVWWTSNSIVVFCG WWTSNSIVV 35.1 W????S??? 16/16
NA 458 NSIVVFCGTSGTYGT VVFCGTSGT 4.49 V????T??? 11/16
NA 460 SISFCGVNSDTVGWS FCGVNSDTV 56.6 F????S??? 7/16
CA 02658559 2009-01-20
220
TABLEW4u2008/039267 PCT/US2007/016529
(1) NA DR3 Supertype Anchor
Protein Strain Acc. No. Pos Sequence Core sequence String Motif Conservancy
NA H5NI AF036357 7 ***MNPNQKIITIGS MNPNQKIIT M??N?K??? DR3b 16/16
NA H2N2 L37329 7 ***MNPNQKIITIGS MNPNQKIIT M??N?K??? DR3b 16/16
NA H5N1 AF036357 43 VSHIIQTWHPNQPEP IIQTWHPNQ I??T?H??? DR3b 2/16
NA H2N2 L37329 57 NQVMPCEPIIIERNI MPCEPIIIE M??E????? DR3a 8/16
NA H2N2 L37329 62 CEPIIIERNITEIVY IIIERNITE I??E????? DR3a 7/14
NA H2N2 L37329 79 NTTIEKEICPEVVEY IEKEICPEV I??E????? DR3a 8/14
NA H2N2 L37329 84 *KEICPEVVEYRNWS ICPEVVEYR I??E????? DR3a 3/14
NA H2N2 L37329 107 FAPFSKDNSIRLSAG FSKDNSIRL F??D????? DR3a 16/16
NA H5N1 AF036357 107 WAIYSKDNSIRIGSK YSKDNSIRI Y??D????? DR3a 16/16
NA H5N1 AF036357 123 DVFVIREPFISCSHL VIREPFISC V??E????? DR3a 16/16
NA H2N2 L37329 123 DIWVTREPYVSCDPG VTREPYVSC V??E????? DR3a 16/16
NA H2N2 L37329 129 EPYVSCDPGKCYQFA VSCDPGKCY V??D????? DR3a 8/16
NA H5N1 AF036357 145 TQGALLNDKHSNGTV ALLNDKHSN A??N?K??? DR3b 5/16
NA H5N1 AF036357 146 QGALLNDKHSNGTVK LLNDKHSNG L??D?H??? DR3b 5/16
NA H2N2 L37329 166 RTLLMNELGVPFHLG LMNELGVPF L??E????? DR3a 8/16
NA H2N2 L37329 202 HVCVTGDDRNATASF VTGDDRNAT V??D?R??? DR3b 7/16
NA H2N2 L37329 213 TASFIYDGRLVDSIG FIYDGRLVD F??D?R??? DR3b 11/16
NA H5N1 AF036357 218 YNGIITDTIKSWRNN IITDTIKSW I??D????? DR3a 9/16
NA H5NI AF036357 219 NGIITDTIKSWRNNI ITDTIKSWR I??T?K??? DR3b 8/16
NA H5N1 AF036357 248 CFTVMTDGPSNEQAS VMTDGPSNE V??D????? DR3a 16/16
NA H2N2 L37329 248 CTVVMTDGSASGRAD VMTDGSASG V??D????? DR3a 16/16
NA H5N1 AF036357 263 YKIFKIEKGRVVKSV FKIEKGRVV F??E????? DR3a 7/16
NA H2N2 L37329 264 RILFIKEGKIVHISP FIKEGKIVH F??E?K??? DR3b 8/16
NA H5N1 AF036357 281 APNYHYEECSCYPDA YHYEECSCY Y??E????? DR3a 5/16
NA H2N2 L37329 298 VRCICRDNWKGSNRP ICRDNWKGS 1??D????? DR3a 16/16
NA H5N1 AF036357 298 ITCVCRDNWHGSNRP VCRDNWHGS V??D????? DR3a 16/16
NA H2N2 L37329 313 VIDINMEDYSIDSSY INMEDYSID I??E????? DR3a 4/16
NA H2N2 L37329 318 MEDYSIDSSYVCSGL YSIDSSYVC Y??D????? DR3a 10/16
NA H2N2 L37329 329 CSGLVGDTPRNDDSS LVGDTPRND L??D????? DR3a 16/16
NA H5N1 AF036357 329 CSGVFGDSPRPNDGT VFGDSPRPN V??D????? DR3a 16/16
NA H5N1 AF036357 330 SGVFGDSPRPNDGTG FGDSPRPND F??S?R??? DR3b 16/16
NA H2N2 L37329 330 SGLVGDTPRNDDSSS VGDTPRNDD V??T?R??? DR3b 16/16
NA H2N2 L37329 375 GRTISKDSRSGYETF ISKDSRSGY I??D?R??? DR3b 7/16
NA H5N1 AF036357 385 GFEMIWDPNGWTETD MIWDPNGWT M??D????? DR3a 5/16
NA H5N1 AF036357 402 *FSLKQDIIAITDWS LKQDIIAIT L??D????? DR3a 5/16
NA H2N2 L37329 405 NRQVIVDNNNWSGYS VIVDNNNWS V??D????? DR3a 8/16
NA H5NI AF036357 435 RPCFWVELIRGRPK- FWVELIRGR F??E????? DR3a 16/16
NA H2N2 L37329 435 NRCFYVELIRGRPQ- FYVELIRGR F??E????? DR3a 16/16
NA H5NI AF036357 463 FCGVNSDTVGWSWPD VNSDTVGWS V??D????? DR3a 5/16
CA 02658559 2009-01-20
WO 2008/039267 221 PCT/US2007/016529
TABLE 41
(2) NA DR3 Supertype
Minimally, anchor conservancy >40% Anchor
Protein Pos Sequence Core sequence String Motif Conservancy
NA 7 ***MNPNQKIITIGS MNPNQKIIT M??N?K??? DR3b 16/16
NA 57 NQVMPCEPIIIERNI MPCEPIIIE M??E????? DR3a 8/16
NA 62 CEPIIIERNITEIVY IIIERNITE I??E????? DR3a 7/14
NA 79 NTTIEKEICPEVVEY IEKEICPEV I??E????? DR3a 8/14
NA 107 FAPFSKDNSIRLSAG FSKDNSIRL F??D????? DR3a 16/16
NA 107 WAIYSKDNSIRIGSK YSKDNSIRI Y??D????? DR3a 16/16
NA 123 DVFVIREPFISCSHL VIREPFISC V??E????? DR3a 16/16
NA 123 DIWVTREPYVSCDPG VTREPYVSC V??E????? DR3a 16/16
NA 129 EPYVSCDPGKCYQFA VSCDPGKCY V??D????? DR3a 8/16
NA 166 RTLLMNELGVPFHLG LMNELGVPF L??E????? DR3a 8/16
NA 202 HVCVTGDDRNATASF VTGDDRNAT V??D?R??? DR3b 7/16
NA 213 TASFIYDGRLVDSIG FIYDGRLVD F??D?R??? DR3b 11/16
NA 218 YNGIITDTIKSWRNN IITDTIKSW I??D????? DR3a 9/16
NA 219 NGIITDTIKSWRNNI ITDTIKSWR I??T?K??? DR3b 8/16
NA 248 CFTVMTDGPSNEQAS VMTDGPSNE V??D????? DR3a 16/16
NA 248 CTVVMTDGSASGRAD VMTDGSASG V??D????? DR3a 16/16
NA 263 YKIFKIEKGRVVKSV FKIEKGRVV F??E????? DR3a 7/16
NA 264 RILFIKEGKIVHISP FIKEGKIVH F??E?K??? DR3b 8/16
NA 298 VRCICRDNWKGSNRP ICRDNWKGS I??D????? DR3a 16/16
NA 298 ITCVCRDNWHGSNRP VCRDNWHGS V??D????? DR3a 16/16
NA 318 MEDYSIDSSYVCSGL YSIDSSYVC Y??D????? DR3a 10/16
NA 329 CSGLVGDTPRNDDSS LVGDTPRND L??D????? DR3a 16/16
NA 329 CSGVFGDSPRPNDGT VFGDSPRPN V??D????? DR3a 16/16
NA 330 SGVFGDSPRPNDGTG FGDSPRPND F??S?R??? DR3b 16/16
NA 330 SGLVGDTPRNDDSSS VGDTPRNDD V??T?R??? DR3b 16/16
NA 375 GRTISKDSRSGYETF ISKDSRSGY I??D?R??? DR3b 7/16
NA 405 NRQVIVDNNNWSGYS VIVDNNNWS V??D????? DR3a 8/16
NA 435 RPCFWVELIRGRPK- FWVELIRGR F??E????? DR3a 16/16
NA 435 NRCFYVELIRGRPQ- FYVELIRGR F??E????? DR3a 16/16
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U ~ - - - - - - - - - -
v
..
U o 0 0--~O
L~r o 0 o N vl 00 oN
0 0 0"o ~o 00
ooor- "o~
ooo-w
+ cc c~ co cd c~ c~ c~ ca ctl co m m co .a
o~ A(~CaCaA~1~1CaCaCaA~1CaC~
N
N
N ~O ~O O ~D ~O ~D D D ~O ~D
N ~_O ~O ~D ~O
4.. cO cd ,.~ - .-. r+ .~ - .-~
o~~k
Qcl A o A Q A A o A Q o A A Q
NW) v) tn v1 tn v) in v1 ~O ~o "o l- l- l~
~ - - - - - - - - - .-. r. - - - - -
L4
~, A W Q Q'~ ~ Q V] ~ Q~ H V]
zao>z aAC~xz
~ ~ x~ A~0 0 0 zz>
~>z >
z
cnQ~
z0"AwAZ>>- oQ~wx
a~3Wx~~a?~a>>
H a
>W~z0 >~u >><~
z v v a~
--V) \O O~ 00 00 00 ~ M
O.--~D ~~ v'1 N~~O
0. N M N N~~ M M~~ N N N N
M
0
N_ -
z.zzzzzzzzz-.z+zzzzz
kn v') tn v1 tn vl tn kn v1 tn vl W) tn V1 vl tn kn
C/~ `[. x x x x x.rr x x`rr. x~i x x x x x x
N
J ~ .3 Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q
m Moozzzzzzzzzzzzzzzzz
H ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
rn
a)b
r1 C O
Z ri O H O O M O O Lfl ri O L11 O Lf1 tfl O O O O O O N l.(1 H O O H N
-iA~- o
rt ~
vi
0 0
N
r Ln 01 r~ 1
lll lfl a, l- M
a r=I
G]
~ C 0 0D rq %D r-i
b 0 w r ~ ~ o
41 po r-i ,-+ `n ` w w ~
~
Q+
rci 0
~ ~ ~ N
N =,4){ %O M kO ~O N ~ ~ k0
> 4-4 ~ M 01 OD N ~ N
.~ Q
$1 ~4
N a
b 0 0
kD w co r-i
~ ~ ~
N
r r
a ~ ~o ui ~o ~ ~ N ao c
U Q
r'I H
w o CC) Ln
o 00 ao ao Lf) oN O rl
H N H kD (N %D ~ r.
~ r ~
w a ~ ~
ch 0 Q
N
N
4-) o t!1 01
rn Ln 0h 0) L, O ~D 00 [- O
(d o O O~ d~ N a0 i ~ ~
U1 r [~ lfl O lf1 rl l11 ~ m M l11 O Lf1 m m dI ~p ~ 01 N
,..~ l- ~ l, d4 ~ ~ [- M ~ H 01 r-I Ln l0 U1 N
fA DO L(1 d' Ol r-i ri OD r-I r=I r-I
fl.' r=I r-I
b ~
o~ ~ r ~ r r ~ ~
rtf 10 l~ M M M M M M H H H rl H rl rl
r'~ r+ rl r-I r=I ~-I ri r-I ~-i r-I ri
w
rw-1 rNl ri r0-1 rH-i r0-1 rr-I rr-1 w 00 d4 M Lfl W di L(1 [- 00 W 14 0~ l0
10
o
0
.ri W m c0 14 111 m N lf1 0% 01 r1
0 m O O M L(1 Lfl L, OD O N m H ~ N ~0 d~ m ~ O OD lfl O H
.r-l a) N k0 r-1 ri H '-i H rl N N H l0 r r 00 d4 l0 [- l- OD rl r-I
~ U
Sa ~
~d 0 H 0
0
ro ~ ~ rl ri H H rl H H ri H rl H N N N N N N a a a a a a LL O
O ~ ~ ~ ~ ~ ~ z 2: 2: 2: z z Z Z Z z z z Z 0
~4
N
A
O +J
M =H
O ~
> w m w~C w~ ca w a H:R: a w
~ ui w a~~ a~a a wzZ a a"~~ a ary"a 3 t
a a 0 a x a a w a ~H a A c/> >+ C7 C7 a H tn
a a x 0 rA x z H x w w cn x~C H rx a
H H x a H U a a a 0 a a w a ~
w H rx H> a x z ~H a A a a a x a
> w w w Cn a w H a A~a w cn w w w w w w
u H a H~ ~ uHi H a a a u H w a~ a E,
a a ~'
co ~ a a w w~ aH a w a a m w a u u9 ~
m0 a 04 0 w a a u) H w w w z H z a w H ro
W -- cn X: m H~~ u w z ~a cn ~ w H a a a~H a x x a -1
J G
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w b o ~
o
r-1 O H O M 0 N O O O O O O O O O N O M rl N M rl N l0 m d4 0
z .-I A o
'~ vl
o O
~ ~ O ~ I I ri ~p N
00
r-I Ln Ln kD I O N ~v
O M O M d~ O O
W m O~ Ul lfl r-1
A r-i
m Ln
er~~ ~ 00 L- m l- 00
0 Ol 0 lfl I 1 rl Ol ri r-i
k0 N l- l- N O) ~
~ lf) QO r-i N
L~ x o N
b ~ p r-I N I r-i Lf1 N N ~ 0 O 0~
W 00 I 01 lfl l- N d' rl .-1
r-i L11 l- Lf1 Ul O Ln 1-1 M M
.~ H
rl
~4 Q P4
~ 0 0 N O,
~ 1? -0 0 l~ %D 01 d4 ~--I N
0 ~ N d~ W 01 ~ 0~ ~ M Ifl
d
N ,..I O Ol M M kD N M
ry f0 N ~-1 rl r-I O N rl
r-i
~ [L' r-i
U Q
r-I ~+
w o t~
p 10 H I ~ ~ M d4 01
H In I m ' ~-O-I o ~ `r nni Ul
~ M O
w ~ L(1 10 L!1 N
0 Q
N
CV
~ o O ~0
r'1 ~ l0 0 I N l0 0~ N OD m M ~ d~ L(1 10 (3) O rl m r
(O O O N I d~ ' r1 [- I l- N U1 fn M M M LI1 di l- M M 0)
b ,~ r N rl N ~O d~ r-I rl r-I r-I O M ~O '-I M r-I rl ~
Pq rl O N r-i kD ri V r-i
r-I
r-i
=~ ~
ro
U~ ~ rl rl rl H rl L!1 l0 kO 10 l0 l0 10 l0 l0 ~0 ~0
r-i H r-i r-i r-I rir1 rl yd r-I r-i ri 01 H r-i
'-1 r-I Hr-1 r-I r-1 ri r-I r-I
\ \ \ \ \ \ \ \ \ \ \ \ \
~ U \ \ \ \ \ \ ~ ~ ~ ~ 0 0 I, N H
~ ~ M d~ l!1 N N ~0 00
U~ l0 10 N 10 l0 ~0 ri ri r-I ri
~
ro 0
4~r ~
~
.rl W Ol l- 0D M rl 01 l, OD O %0 W rl OD l0 0D OD M f'1 00 [- V l0 0
4") 0 O r-i m OD Ol Ol m r-1 d4 tfl kD 00 00 r-4 N M lfl
=rl LL rl N N N N N M ~t' d~ d~ d4 d' m l0 ~O OD 01 01 r-i
b ~4
ro o
U) Q) ri rl r-I r-1 r-I r-1 r-i ri r-I
,b õ a a a a a a a a a a a a a ri r-I m m m m w~~m In co
V4 0 z z z z z z z z z z z z z z z z z z z z z z z z o
(d (14
a n
o ~
O =~
* cx7 ~7 c~ c7 A U) x w 4 cn a z ~7 w w a~ a H M M w
a H H vi ~ a rX4i w W a W E w A H w7 rn
z z m~ a a cn > w w:3: a w w w rx 0 2: H~
rx u w 0 m a> H x 0 H>4 a cn A Q H m z H a w H -,i
H2: w>+ L*a a' > H P4 Ul rC [14 ~4 M Ul E+ X 0 9 I"I 7 'd
a rx a 0 a a rx H0 a z u a w wE w a a H a ~
a v w w a w w ca A w w a w w ca 44~ A
w H X ~ W ~ ~ a ~ ~ ~ ~ W a a H H ~ ~ W v
ch ~ 9 H x 0 ca a z~ A 0 w ca ~ m a a x a ca w U H v,
0 H m >+ ~n u~ ~n w [v f~ uo cn fa w w v~ E- w i ~C Cz ro
W v 1i > rx a rC c7 >4]C a p a> un c7 > a 5> ItC a a m a z a ~
J ~
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
a) b
z~ O H 0 O rn lfl lfl rl r-I N zv O O O f"1 O O O O O O H V1 O O Lfl O
H ~ O
ro ~I
o ko
~ r w ~~ v rn M
~
M l- M 10 r-I N 00
Q ri H
m Ln
0 M co %0 00 lIl 0 0 b 0 r-I r-1 H 10 C~1 rl d~ N ~ 01 ~
=~ ,~ LI1 O 00 rl ~ N 0 ,~ pq [~ N 00 t!1 d~ ri 0) c`1
(D
¾+ x w
O
a) o 'n w vi o o i O kD
N in rn r-i ~ w
> W [Q m N N N ri M w 00 00 10
=ri Q
14
~J
b a
' 0 a~' N 01 N N 1D O
(O O H O O1 U1 ~-1 'n
M N Ol m
Ln M N 00 N l- N
c~ ~ 00 ri r-I
~ U Q
~..~ H
4-1 o Ul H
~ df H
H 0 0 ~ r 00 M 0) ~ aD QO N l~0
H co r-i , r- ' io ri
w a `" H r-4 ~
0
N
N
4-)= M rl 01 O w OD O 01 l0 M N O% M H
M ~--I O O O~ d' M l, rl ~ M M 01 d~ w L!1 ~ d~ d~ Lfl
ro co rl d~ l- lfl l- 01 m rl CC) O\ . N = ~ = ~ m 01 M r O
W N l, l, ON H N rl N kO O l0 cM W
Q r-1 r-I ri ri r-I
=~ ~
ry V~ %0 1D dt cw d' zv ;v H H H H H H rl r1 H H N N N N N N N
H
r-1 ri rl r-I r-I r-I r-1 r-I r-I ri r-I r-1 ri r-I rl r-I r-I rl r-I ~-1 H ~-
i rl
A v {~' \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \
M r-I 00 N 10 OD %0 Ul L, Lfl L!1 N 00 r- 01 l!1 rl rl rl 00 01 H co lfl
~ r-~ rl r-I r=I ri r-I
~ ca
O
0
y 10 tD m N m 0 N N r M H lfl 0 0 QO 0 H C'1 W r- H l- Ol
0 Lf) r-I tf1 l~ Op 00 O N M M lf1 ~O l0 L~ a0 O~ O d~
a) fl+ rl M rl r-I rl ri rl rl N d' Ul 10 rl ri r-I rl rl rl r-1 ~-1 rl ri N N
b ~4
0 H 0
cl) a) v~i c~n vi vi ai cNn vi o
~
0 z z z z z z z a a a a a a a a a a a a a a a o
(l3 ~4 N
a A
O +1
M
O ~
a a F w w a w a rx >1 w U) >-I~ A w w U) rJ4 '"
~ a H H ~n a a4 a~ z~ u) w H H a c~ H a a '"
a
U) U' R; Qi L~ w ~ r~~-=~ w H F~ [~ H ,. H N7 .. ~] w
(1~ a a a H [-1 m H t-+ A F='~ z .Y.~ F"i x H C~ w w F7;
a a w a w P. Lz+ x w H cn 04 >1~ x H E-i paa rx m w a r~ a
a ~n x a H H H w w A A w uo ~n a c7 rx r~ H
m
' ~ w w a ~4 x 0 Ca a H~ w a a fx > a H a a Ga 04 a a P: ~i w w Uo GV w ~
w A A w w w w w A A w w ca H w w w A w w Ul cn H =4
a rx w w> a> w W A> a> a U) 0 a w a ca a0
a>+ H a w w H 0 ?4 H H a 0 >+ ~C H P fa a 3 a E+ U)
M ^ ~ C a 3 a a w a w x v w H~ v a i F F 0 c~n w a ~
V ~ ~7 Q a x a w z u w w a H w ~+ w a ro
a wX > A 0 w~~a H~ A a ~n ~ a w ~n
u~ a ~
W ~ w a H a
m ='~
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
~
v v o ~
0 N:j V O lfl lfl O O r-I O O H O r-I r1 M v ;v Lfl O O O M ;r O O O U1 z ~ A
~ o
ro H
vl
o M
,.M.~ ri ri kp O~ %D l0 01
~ H
~p I I 01 O lzv O
~ m t m N N m
Q rl
~ ~
~ 0 co
l!1 rl l~ m 10 -zr
p Ln I 1 N (A ID 0) r-i
V~ ~ I 0 I 1 [- %O 10 O N
4J pq r-I Ul l- M
Q: ~
a x ~õ
0
'a N N 00 N M
N 0 ~ N O O V~ O H ~ M Ul M ~
~ w 10 O kO l- %0 N U) ~ l~
.~ ~ ~ U) ~ oo r
$/-~ Q
W QI
0 0
41 ~ ~ 00 ~ lf1 OA N L(1 O M O ri ~ Lf1
Lll N m 0 114 O w kD 0)
M ~ ,O ~ N N OD M
U Q
rl H
W o u1 w
ry r-1 , N d' N ~ k0 01
~..~ ~ Lf1 v kD N l- H Ol N -W tf1
W l- M ID d, lfl rl m N
w f~A l~ M [~ l0
PS H r-I N r-I
co 0 CV
N
o OD 00 m 00 L- Lll
p OD m ~0 N v m N 01 M M 01 ri [- ~ I H 00 ~ ~ O
r l0 lfl W Ol m Lfl k0 00 d' m O 00 01 ~--I 40 ~ ~ ~ 10 rl ~ v 0 Ol
~ H N O rl N d' 10 ~ ~ Lfl OD rl 00 01 l0 kD N L~ M
Q r-i ri rl r-I r-I r-I
b
U~ N N N N N N N N N N N N N N r, H H r-I m rn rn M
qid r-I ~ r-i H rl r-1 rl r-1 r-i r-I ri rl rl r-I r-I r-I rl r-I r-I rl ri r-
i
r-I r' N r- r'i M M O
~ M L!1 H l0 N m rl rl r-I rl LIl lll p 10 T-I ri ri rl r
O
0
.rl U1 r-1 M O ri OD d4 01 m L- M W k0 r-I r-I w dD I- ri 00
4.) 0 l0 00 O~ d' W t- l- O) O N N rl N Ol O% O N U1 01 d~ QO f~1
'r'I O W N N N M M M M m d~ d~ W d~ lfl lll l11 lf) lp kp ~p kp N M [~ Ol
b ~4
o
.d `n ; FC FC rC FC rC ~C FC ~C FC FC FC FC FC ~C FC FC FC ~C o~o G0 00 0
0 a a a a a a a a a a a a a a a a a a a a a a a a o
a A
o ~
cn -~
o ~
~ ca ca 0 w ~y a~ a m N a w W aX x H u) u) a >-I a z W
a w w w .Y C) C] a r~ i"i f a > a' xi pw w p.7 CL'a 9 + L+
w* ro
a1 a H 0 z H A ~n v~ w x w A 7 ~ w a a x H c~ H rn
A a m w w rx w H a ca w ~]
a x H a W z a w H u H z > U] m U' q
a U) A H rx w~ C7 aa ac a J W w C7
a a a x u a z H FC ~n A m w
cn ~4 x a a w x ~
~ a~ ca a w 5~ A w w w> H w UM) > w u) a a zU) ~
x H~H~azaw~zAwww~ww
~ w w O~ A~~4 a 3 3 w~ x w~ w a 0 U a C7 w~
eh 41'
U) w ai ~a w a a~ w A w w~w H a w H~ A ~
~ ,
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
a~b o~
0 N::j U O lf1 O Lf1 O N O O O lJ1 U1 o O O O LI) O O N O M N O O N
z 0H
p = o
vI
N
O
0 0) 0% [~ Ul l~ lfl lf)
OO Lfl 00 = = 0 0 l- 0 0
r-i I;r N M Lfl M l- d' l- r-1
m Ln
~ ~ O
p N Idi I 1 Ln 00 I I %D I
b M a0 l0 M
=r{ M U1 N 01
4) r-i N N W [~
fQY r-I
LL x ~, .
p
ro d' N l0 N r-1 O
N o M r 01 r ~ L(1 OD m 01 01
.H ~ ON l- r 0~ r-1 10 l!1 00 H d4
> 00 aD ~ H m
01 r-I d~ m
N
b ~
~ 0 0
4-) o [~ M M r r r r O rN-I ~
~ m ~ m rn
co m N ~ M rl 44 ~ N N
~ a
U Q
rl H
w o H
ry l~ N U1 N 0
0 ui ~ m N o ~ ~o 00 0 N
Ol m ~ 0 01 H
w pp ~ rl H c m kO 00
QM
rl- 0
N
N
4-) O 01 m lfl
01 114 0 w 01 01 M [- r- OD r Ln l- O r,
~ o rn ~ H
kD O N ri %D M OD Lfl d~ M l(1 M d' I H L- U) N di r- O
b ,..~ M 00 H N N 0 ~ N N H L(1 01 OD O N ~ l!1 r-1 N m r-I
CQ m r-i N 00 N L(1 m M r-I N m lfl
r-I r-I
tn r-i
ry Vu
0 M m m M m m m M m m M m M M m m m m m m M m m m
rd H rl r-I H H H r-i r-1 r-' rI H rl r-i r-I H H H H H H H H H H
O O M M O O r-I r-I ri 0 M N M M 0 M
~ rl r-I O r-i r-I r-I O1 r~ ~'O r-I r-I H ~ ri r-i rl ri r-1 r-i r-i r-i
~
0
0
~ W m L, 10 v 01 r- m N N U1 OD 14 O r-i tf1 10 l0 l!1 Lf1 Ul rl u'1 01 O
~ 0 r-i Lf) l0 [- l- N l- O ;v Lfl w L- W o0 Ol M l0 l- O m Ul L!1 l- 01
=rI O a r-i r-i H r-i r-i N N M M M M M M M M d4 W :v Lfl l!1 ln Ul Ul L!1
~4 ~
0 0
Cll O r-I r-i H r-1 r-i r-1 r-i r-i ri r-i H r-I r-i r-i H H H rl r-1 H H H H
r-i 0
J-) W PQ W W Ltil W I0 Lq pq LYl Cq W Pq PO W Pq m W (rl W W W W Pq c
o a a a a a a a a a a a a a a a a a a a a a a a a o
a A
o
O
44
~ aa a`~4 a~ rC z 0 a a m w x a A~ z~n U A a Hy 4-4
A ~ a F ~ a ~_l
a w w a~vi ~ p4 ~ Oa x cA a~ U~ w ~
' a a 2: x W x H x a E- a H a~ a 0 a a a 0
d
E-40 w F rC w z z~ a u] H C7 C7 U x r 7 a~~ H C7 ~
a rn N r~ z A ~n w A w ~n w A E+ ~n w H a A
a w~ H x t~ 0 w w~C z w> 3 1:4 2:~ ~+ w cn
H a e w F C7 E+ X X a [z+ Z a 3>+ tn H a 3> y
N ,7 L*a Z 2: 2 ,7 H H 1 H 44 ~4 >4 GL Ga Cct ~H 4 a)
M~ ~ w Ea A M w H a H a a a a [-~ E POa x~ Ca7 b
v a H w z a w ~C x HX a
W Ei C7 a ~ ~ a C7 u] H C7 a w A a H x a ~ a H a rC u
cn
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U)
vb o~
z~ O O O O m tf1 O Lf1 M N r-I O O d~ N O N O O O f) r-i di O O N
~ q = o
vl
r1 00 0) U1 10 0 r-I N 00
m tD H 00 01 Ifl 00 ~ M ri kO 0)
. OD L- = = H l0 M ~ O
W l~ M O L~ rl N N
r-I r-I
r-i r-i
Ln
0
OD -w ul 01 0) OD 00
p 1 N N O 00 H 00 ~ m [- M
=r1 ' M ~ ~ N O 10 ~ 0 [- Ill
a ~ M M m kO N U1
a x o %O Lf1
e~ a t!1 00 m 0 00 lD l- Oi aD
M O kO U1 H 0 00 [, N 00
~ W ~ 01 N N . r N 01 01 1f1
a l- '-' N U1
=~ ri
~ ~4 Ga
~ a
' 0
4-) ~ N l, r1 N M %0
0 kO ~0 M M l- O) Ol O l0
O N N LIl l- d4 0) m N 14
' N N U) lfl d1 0) r-i N U Q
ri H
44 o
0 p O% M 01 M I
H o N M m ~
01 l11 01 0)
W r-i N
fQY
cW 0
N
N
4-3 0
rn ri OD 10 r-I lzr l- M N 00 OD 00 00 M M 01 00 00 01 M
1 l~
O r, 00 d~ M r-i 00 OD ri
lfl di U1 k0 L- rl lfl 01 l0 N 01 l- 01 W
b ,..~ N N r-i M (3) 01 O 4 [- O
%O r-I ~ 00 N M N l d- ll) N 40 N M N M
~ Q
=~ ~
M M m M M 4 di d4 14 dl d' W d' d'
ro V d
r+ U~ M M M M M M d~ d~ d~ d~
=r'~ ~-I rl rl rl ri r-I . ri r-I r-I rl r-1 r-I ~-1 ri rl rl rl r-I ~ r-I r-I
rl rl
r-i m m N r-I M H m 0 m m H d~
~ OD 0o rn rt ~ ~ ~n r ~o ~ 1O
0
U
0
W i-1 l!1 l0 L!1 01 U1 N M N 00 Lf1 O [~ ~O L11 N N DO l- LIl L!1 l- l- O
4-) 0 ~ ~ r-i ul Ul l- 00 a0 N N M m tf) %0 k0 [, a0 O O N L11
=r~ ~ a 10 kD l0 l0 l0 10 10 l0 l- l- l- rl lfl l0 rl r-i r-i ri H r-i N N N N
ro U
0 ~ O
UT O r-i r-i H r-i r-1 r-i H H r-1 r-1 r-i N N N N N N N N N N N N N 0
'd PO W GO PD W W PO W Pq PO PO PU fY1 PO PO !Y1 Gl fY1 W clq R1 W 00 PU O
q 0 a a a a a a a a a a a a a a a a a a a a a a a a o
~4
N
A
o 41
o ~
u z u3i Q U u mw x a H CL4 H > > H A W 0 H w
X a a' A w a H a c~: a a ro
A a a Ex-+ a 4 Oa x G w u X H(~ w w 0 a Z H H a p Ei u]
A Oa 0 04 (1) U H ~ a A a FC H i- x w H~ G a . a w `~ x a ~+
a> x a a~ c~ w rx a z a~ a~ ~ a~ w~
a A>+ r.4 H a w a rC m w u] ~L R: rC ~~~`=G a a A ~
w w A Hcn ca w A w a w a v~ w w w w w w
a H a H A~ a~~ a ~ w a H~ a~~ v
CV) w W ~n A n a 0 0 a a 0
w u] w w LYi a R: > n U) a."~. H a r4 w F1 a~ FC f+ C7 O
J b
m ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w
a)b o~
0 N : j U O O O U1 N lfl lfl M O vO O O O O O O H N W
zH~o H
ro vl
N
0
M (y r- r-I M N H H r 00 00
k0 ~ d~ 01 ~ = 0 H H
H 01 l0 r-I 00 H m
pq Ol r-I ri r-I
P.'
Ga
o O
~ d' 0 00 O m H N
0 ~ 00 ~ OJ 00 LIl H N
r-i 14 O
4õ) 10 ri ~ N 0 m
p+ x
O
0 ~ l0 O l- N i 0 H 00
GI .,~ M d' d' i O i 01
> y.., m 114 m lfl r-I N %0
-~ A
$1 p
0
a
~
~ -W 01 N 01
~ O 10 L- O N rl ' `-I d' m m
N ~pq m M ~ r m ' H N r-I r
H
U a
~..~ H
4-I O a0 a1
ry ~ [- d4 M m OD %0
m O m'ODD i i ~o ~ ao d
ri . 01 H OD M ~
w tO r-i OD O
rn 0
N
N
4J o Lf) t!1
m OD lfl l- H l11 l11 O 00 01 ' 44 t11 Lf1
~ l d~ L!1 m N l- r-1 Lfl O Ul 14 OJ d' ~ \O
b ,..~ OD r d4O 10 I 10 I l- 00 O [- m m
r- r-i r-I H d4 m ri N H 144 N [- lfl
Q ri ri
b V
r'~ q~ ri r-I ~=-I r-I r-I H d~ d' rõ~ ~, r-I ~-1 r-I r-I r=I r-I r-I r-I H
,A ~ la \ \ \ H m \ \ \ \ \ r1 m M \ \ \ \ N
1p m k0 r-1 r-I CN 10 m m m N ~-i rl rl rl ~N qzv 10 m
~ En
0
U
~
W N aD d' -v di DO M m l0 M lfl Lfl m tO W QO lfl [- M
0 l, M N O H M l0 QO O1 H m L- 81 m Lfl 10 r~ (n v
'rl Q) a N M M di d' d' w dl di U1 Lf1 Lf1 lfl l0 10 10 10 lN [~
~4
(d o ~ O
(!l Q) N N N N N N N N N N N N N N N N N N N 0
4J PO o0 PU Oq W W PO a1 W W PO PU W o0 W W P~1 GO PO
a a a a a a a a a a a a a a a a a a
a
A
O +J
~ =~
~ W z > (Y 0 ~ H fx A > fx w
a C7 u] a w w ~ a u) > C~ z w a `NG
a H a [*+ E+ H E w a a 0 Ei p F 0 H
cn a w H am m rx w a cn > m a 0 y+ 4
~C
>AZw au~aU~C7 aAt7 ~ a Oa b
a w rx u z~ ~+ w > w rx Q
z
a w C7 A a r~ w x w H a2: H~C a A u]
x ~ a > a0 a A a w a w Q
H cn a a 0 cn Cn 2: w ts+ 0 H C7 a ~
eh ~ > m Oa .~'T'. (~ ~ a U~i H vai z H !!] 1~.~
~ H a M ~n vn ~==~ Cn w H~ a vn (o
w
W a ~J -~i ~ f!) W > a rX4 A ..~1
m =~'
H '
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
m
w v o ~
O N ~ V O m lll H Ul Ul t!1 N lIl r-I H N M N M rl N N d' m Ul H H N
G~ O
4v
ro ~
vI
o O d
m N M N l0 O L(1 N l- lfl N
,..~ N H L- M rl -W O r-I lfl l0 H a0 l- r ~ ri I-
M I H Lfl 01 rl O1 ri O m 0 O O ~ OD
i
~ M Lfl Lfl r~ 0)
L- M m Lfl Ul r-I rl M M 10
a ,-i . ~
A
N U)
N 0 ~ r-i
ri rl ~0 rl OD l~ M 00 l0 aD l11
ra l, O 40 N
1 : I I
kO OD m 0 O 01 L(1 ri H rl k0 m H
=rl i i oo r i 0N
,..~ tf1 01 M lfl 44 1D l- Ul O 00 r-I ~ Q ri r-I d' l, d4 r-I H l- N Oo Lf1
d~
H r-I
a
p
GJ N p ~ 10 ~ r-I lf1 N rl i rl lf1 N ~ 01 U1 o ~ ~ OD
l0 ~ M 10 10 N r kO CO I O1 U1 [- H N L11 H ~ 01
=~ y..~ H m 01 OD N H N rl l11 l- Lfl m 01 N N H m
LI
(D Q A
ro 0 O -W N 0 LI1 N
JJ d' lp lf1 Oo r-I ri r-1 O L- l0 (Y1 m N N 0) N N
i 0 r-I ~ N l- N r-I ~ lfl OD 01 01 lll M M N 01 0
N =~ 01 rl d~ 01 M M ; M L!1 00
N rl 10 Ln l0 l- [- N 00 c~ c ~ H
rl ri O rl N ri r-I O
o Q
~ U
w H ~
p O CO L(1 L!1
Li o ao ~ oo ao in 01 0'~ to i ~ i 1 M rn o
H O 10 N lfl [, OJ 00 ri d4 00 01
~ = = ~ ~ ~o ~n ~ M ui o
W n~ k0 00 N lIl l- v N l~ O l- M ~ Lf1 L!1 H O M rl
p 0 ri rl r-1
CY)
N Ia U
4J ~ rl
p
(I U
N m O 01 d~ H H r-1 N v ~ m N rl 01 01 M O M M N M N N
0 l- U1 O r-I M M l0 01 M M l- M r-I O 01 d~
H H OD v 01 r-1 d~ w L- N l- r-I r-I r-1 r-I O ri 44 I- l11
U a
Cl
.~ .
=~ U b 10 l~ l~ r l~ ~=~-I H ri H ri ri 01 ~ %O 10 l0 ~0 ~ d' d' d~ d'
~ r-i
A ~+ N~ r=i r-I r-I ri ri ri ~ ~ ~
O 1- H H
[~ H
O N lfl ~ H l- 10 OD l~ 00 1- ~0 1-1 l0 W ~ ~0 ~ H Lfl lfl lf1 10 H H 00 %O 00
%O
~'=~ r-~ N. H rl ri r-I
b H O
L"i Vi U
0
.,~
41 M N O 00 di M lll01 m rl O 01 rl 01 00 l0 OD OD OD 1D %D c+1 OD O N
.r{ p 0 M U1 l- O N O r-i OD OD M l0 l0 M ri l- O aD
r~ .K N a N r-I r-I ri N N rl r-I H ~ 01 rl M rl H i-i rl
H U ~
Cd pp
`~ r-+ r-+ r-+ r-+ ~r-I a a a a a a a z z z z z z z z z z o 0
o E X z z z z z z z
a N
"
r=I
o u,
m a)
o 'o 0
~ ~, A a uHi w A w H z aa a a H vi a W
a) w> a a>+ a rx 3~C A 0 0 a w 44 ro
q a Pa'. aU Ux] a Q ~ a' H H ~ P W W F"~ a a ~--~ E~ Ul ~
z rn rx H rx X w a w w a H a W w rx w
a a a w z w a a [r~ cA Q Ca E W Cn 0 0 H H
5. FC pC 104 .Ny H~ fx W H H rC ~4 cA U) 4 .a AC 4 w w fx z3
Cll z (.~ a a a a' SG W 4 Z Ol W W 3: Ri CY U) 'J Ol H q
` H H A>+ W 0 x w w x x w> a A
E u H w a ~ r~ rx a a ~n rx >+ a w w N
=rl N a H ',~ H 4 H ~ 44 .l H H > a a >
N v 0 H a a w a u~ cn a a H~ rx rx rx w cn a a a w
0 w a H z a w H cn
Q w w w 0 a a w
b
~- ~ U, v~ z u z~a >q a> a a 0 a> a z a cn a a
m =~'
F-
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
= ~ C ~ ~
'. O ri U1 Lfl lf1 ri ri m d~ di lf1 m W U1 l!1 N Lf1 Ln N m N N m Lf1
H ~ O
ro VI
o %O kD
M ON r l0 m rl m I l0 0) N r- 10 m W 01 01 l- lfl l- Lfl Lfl OD 01
H rl M l0 I r i m 0 r-i %D -v O Lfl OD = = O l- O O M l0
CN N 00 v M L(1 44 M N N M d~ N M L(1 [- d' l- ri M
Q r=I r1
Ln
O O 00
V= 0 m L(1 r, m 10 dt qT
0 d~ 01 'l1 N 01 d~ l0 01 rl OD ~ ~0
N l0 0 l, k0 10 OD . N lfl 01 M
p~q H m rl lfl [- m W l- N N L-
Q ri r=I
0
o o o ~O N 0 ~ o ri 00 M ~ M l0 r ~ r Ul ('N~1 H Op1 ~
==a 10 00 r %D N tD u1 r-I p ' ~ l, m H Ul a0 r-I d, O~
y,.~ k0 dD %0 Lf1 00 I- N ~ r-I L~ 00 p ri M 01 ri d~ M l~ H
==~ a
a
0 0
o 0) ~ L(1 ~ p ~ N N 0 M O rl ~ Ll1 M M r r~ ~ rn p io
N l- N Lfl N O d~ N N d~ 00 N m N ~ m r-I l- ~ 01 01 N
(q rl [, r-I r-I lfl N N
, n A
U
H
ri
-4 ri N H %0 0) l, N Lfl N O
O 0 Ln N N m N ~
d~ l~ [- rl 01 N d~ Lf1 O~ N O ~O 00 O N N
M ~ = %O r k0 N ~ Lfl ri M N ~ m 0) m 0 0) H O N
/V H r M %O r. w 0 M k0 OD N
H N H H
M
N
o
m o dt 00 M M M M M 01 H [- rl 00 0 o O~ l0 l~ [~ tfl r ~O rl
.('., + d~ 00 d~ OD lf) p O OD 01 rl 00 l0 ri 01 N ~O ~ m l- N 111 0 U1 r
0 m Ol [- N p lf1 OD H a0 01 w l0 m H N N rl N lll r-1 H %O ri
U a
Ca
C. ~
H1 M M M m M M M M M
N N N N N N M M
N rl rl
"~" U~ HI N N
r-I H r-I r-I H r-I r-I r1 r-i H H r-I H r-I r-I ~==i H r-I r-I rl r-1 H r-I
N ~ \ \ \ \ \ \ \ \ \ \ \ \ \ \
p U \ \ \ \ \ \ \ \ \ \
rl N r-i H N H H O 0 0 0 N m m
p
H ~ '~ r-i p p r-I ~ r-I H H H '~ r=1 r ~--I r-I ~--1 O ri H r-I r-I p Ol
rl 0
VI U
~
M N N M 00 [, M M 00 l0 H H lD OD Tv 10 %D 01 N L(1 l!1 If1 ri O l0 lfl
p 0 l0 [- O 00 01 N d' H N 01 Lfl 41 m l0 [- W L(1 l- M lfl 01 r-I Lll
=k v rl rl N N M d' d' Ul lfl Lf1 lD %D r-1 H M M W Ul lf1 lf) l0 %O
U
P0 ~4
0
A Vl (1) r-I ri r-I H rl ri ri H r-1 r-I ri 0
A-) 9 FC FC FC FC 9 FC FC rC rC 9 ~C o
W PO P0 00 00 00 04 PO Gq PO G4
~ o a a a a a a a a (34 a a a a a a a a a a a a a a a o
H CL N
A
En 41
Q) =.~
.,~ =~
4-) a A cn AU) 4 w cn a AU) W D4 0 PFG z O U Z z ai 4-4
r~ x ~+ w
p, H H Ca7 FC uHi Pd W R: W A W ral .zl IW-I fx a Q? ~ 7 ~-U7 H ro
[r~ x 0 u] OI x (1) ~C F Z fk F m
U) W ~ F f~, ~ W ~ W H 7 C A X 0 ~ 0 a 4 ~
~ W W w F A A A Cc~ W Cr~qW q F u) W tn A 24 A =~
c0 ~ W O~ C7 ~ u) W FC FC ~C z W`~4 H Cs, G. cn >4
E q A fx F a 0 F u] w m H a a wx 2 H a A w
=ri N ~] H H 4 H Lc4 > H fsa a L14 54 X H ~ H H Ga 4 X 2 O
~ ~ a F F W w w wE 0 U A w a~~ a a a M
z~ w w A v~ ~~n w w H r~ ~C ~ 5~ a c7 w
cn f=+ A a a t/~ a a a w a w a H C7 a a r~ 3 v
J ~
m =''
H
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
-Ni C o ~
O N5 U O lfl M N H :v N N Ill H zr N Ul N t11 Ul m O zv r-1 v
z H O .H.. O
Vl
O m N H
M U1 lp O rl N 00 l- N rl l-
* H 00 O~ Lfl ~ 40 ~ M r-I lD 01 O~ ~ m dI Lf) ~ 00 ~
. ..-I l0 ~ O d~ H Lfl C~D 0
W O L~ V~ L!1 M OD H N N ~ Ol H r-~ r-i rl m
Q r-I ri ~-1
o kO 00
OD 4 t11 01 01 00 00 O 00 0 m H N
O N 0 dN rl 00 M [- ~ m ~ O OD H OO OD l11 H N
r 4 0 , O
~ O N O %0 O L- ~ Lfl l- d
pq lfl M M 01 10 N lfl M k0 Hr-i N N O m
Q r=I ri r-1
o l0 lf1 N
O W f~l 0 O OD 10 [- 01 OD %O L- N 1 O 'i 00
N l0 U1 H OD [- N 00 1D O M
N 01 lll M d~ d~ 0 01
4-4 p~q v M O~ M H N Lfl ~ d' ~ M H lIl rl ~t N k0
==~ fY ri ri ri
(L
ri
0 o M
.U o H N l- H N M kO ,O N H N M
~ x N l0 M M l- Ol 01 O %D kO w l, O M H I ~=-I v l-
H cy1 N Ul
N ~ M H ~
Lfl L- V, ON M N d. H
pq N l!1 l!1 d~ ON r-I N M c
~ n fCa~
U
H
H
0-0 O O ~
M 0 M ~ r-I M l0 10 I- M O M lM0 ~
~ ~ c~1 01 H ~ 0) ~ H ~ H 00 O
Al pq '~ ri N 10 r-I ri r-I 0
N Q ri rl
M N
N ~>I
~
4) M
W o I~ M 00 OD 01 W 00 L- OD Lfl l- U1
G,' x lll M %0 l- O lfl N Ol l- ~M rl d4 L!1 M N [~ M ~0
0 H a0 M ~ N 01 d~ [- N 00 m O [- r-I rl O %0 r M
U
A
v q M M fn M cr It W d' W d~ d~
o w ~, \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ ~ \ \ \
O (D M M 0) N r- ko M O %D M It kD r-I M (N M ~ N
m ri rl rl r-I H rl r-i ri rl H ~0 Ol H rl
'I co O
VI U
~
fv) UI Lfl N M N O r l!1 l- lfl lfl 0 df czV [r 00 M M l0 lfl m
O 0 l, OD 00 N H U) M l- 00 O Lf) 00 O rl M %O OD 01 l, lw
* 4) LL kO kO kO l- H r-i rl N N M V 'IV W d' d' V' t0 l,
O 0
A U~ v rl H H rl N N N N N N N N N N N N N N N N 0
0 nl W fA PO nl PO W CQ 00 Pa PO PU W PO PO Ln PO f~q PO c~
~,i a a a a a CL a w w a a a a a a a a a a a 0
.H a N
A
En >1
=~
b -a
.,~
41 w u z t~ N a P~ ~ A H H H~ 9 ~ a ' ~ H W
N w`~UC U w t-i x E+ aacn a ~~C '~
H H H ~,
a a a~ w w w a H a cn a w
a G1 a a F w a w H U) u~ fk C7 FC
(ry x >4 H H H H a~ a~ A fs+ GL cn ~4 w p H
~ a a2: x X a w U z H~H w A a
.-+ a w a U) > cn x rx ca c~ A a 2: w a a U) ~
u] q w C] a a w W w cJ] w A A A f~ cd H w Q H cm ~C z cn ~4 a 5 a 0 a H w H
E q z a w E E+ fx E-+ H2: HU) a a 0 U] H a
-rl a1 H a a H Lc4 a H a w 44 H N
n a n ~ H a w
~ a H ui ro
~ a a a > a a a a z> a4 A U
J
m +
H ~
CA 02658559 2009-01-20
TABL~ 45 008/039267 233 PCT/US2007/016529
(1) HA DR3 Supertype Anchor
Protein Strain Acc. No. Pos Sequence Core sequence String Motif Conservancy
HA H2N2 L11142 16 FTAVRGDQICIGYHA VRGDQICIG V??D????? DR3a 6/15
HA H5N1 AF036356 68 KPLILRDCSVAGWLL ILRDCSVAG I??D????? DR3a 3/16
HA H5N1 AF036356 83 GNPMCDEFINPEWSY MCDEFINPE M??E????? DR3a 3/16
HA H5N1 AF036356 88 DEFINPEWSYIVEKA INPEWSYIV I??E????? DR3a 1/16
HA H5NI AF036356 94 EWSYIVEKASPANDL YIVEKASPA Y??E????? DR3a 16/16
HA H2N2 L11142 96 EWSYIMEKENPRDGL YIMEKENPR Y??E????? DR3a 16/16
HA H2N2 L11142 98 SYIMEKENPRDGLCY MEKENPRDG M??E????? DR3a 3/16
HA H2N2 L11142 122 LKIILLSSVKHFEKVK LLSSVKHFE L??S?K??? DR3b 4/16
HA H2N2 L11142 135 VKILPKDRWTQHTTT LPKDRWTQH L??D????? DR3a 3/15
HA H2N2 L11142 167 RNMVWLTEKGSNYPV VWLTEKGSN V??T?K??? DR3b 6/16
HA H5N1 AF036356 175 KNSAYPTIKRSYNNT AYPTIKRSY A??T?K??? DR3b 1/16
HA H5N1 AF036356 203 PNDAAEQTKLYQNPT AAEQTKLYQ A??Q?K??? DR3b 3/16
HA H5N1 AF036356 228 NQRLVPEIATRPKVN LVPEIATRP L??E????? DR3a 5/16
HA H2N2 L11142 260 WDTINFESTGNLIAP INFESTGNL I??E????? DR3a 9/16
HA H2N2 L11142 269 GNLIAPEYGFKISKR IAPEYGFKI I??E????? DR3a 8/16
HA H2N2 L11142 275 EYGFKISKRGSSGIM FKISKRGSS F??S?R??? DR3b 3/16
HA H2N2 L11142 286 SGIMKTEGTLENCET MKTEGTLEN M??E????? DR3a 11/16
HA H2N2 L11142 326 CPKYVKSEKLVLATG YVKSEKLVL Y??S?K??? DR3b 8/15
iIA H2N2 L11142 327 PKYVKSEKLVLATGL VKSEKLVLA V??E????? DR3a 10/15
HA H2N2 L11142 385 GSGYAADKESTQKAF YAADKESTQ Y??D????? DR3a 8/12
HA H2N2 L11142 417 FEAVGKEFSNLERRL VGKEFSNLE V??E????? DR3a 11/12
HA H2N2 L11142 445 VWTYNAELLVLMENE YNAELLVLM Y??E????? DR3a 11/11
HA H2N2 L11142 451 ELLVLMENERTLDFH VLMENERTL V??E????? DR3a 11/11
HA H2N2 L11142 452 LLVLMENERTLDFHD LMENERTLD L??N?R??? DR3b 11/11
HA H2N2 L11142 453 LVLMENERTLDFHDS MENERTLDF M??E????? DR3a 5/11
HA H2N2 L11142 473 YDKVRMQLRDNVKEL VRMQLRDNV V??Q?R??? DR3b 8/11
HA H2N2 L11142 477 RMQLRDNVKELGNGC LRDNVKELG L??N?K??? DR3b 5/11
HA H2N2 L11142 513 YPKYEEESKLNRNEI YEEESKLNR Y??E?K??? DR3b 11/11
CA 02658559 2009-01-20
WO 2008/039267 234 PCT/US2007/016529
TABLB 46
(2) HA DR3 Supertype
Minimally, anchor conservancy 2:30% Anchor
Protein Pos Sequence Core sequence String Motif Conservancy
HA 16 FTAVRGDQICIGYHA VRGDQICIG V??D????? DR3a 6/15
HA 94 EWSYIVEKASPANDL YIVEKASPA Y??E????? DR3a 16/16
HA 96 EWSYIMEKENPRDGL YIMEKENPR Y??E????? DR3a 16/16
HA 167 RNMVWLTEKGSNYPV VWLTEKGSN V??T?K??? DR3b 6/16
HA 228 NQRLVPEIATRPKVN LVPEIATRP L??E????? DR3a 5/16
HA 260 WDTINFESTGNLIAP INFESTGNL I??E????? DR3a 9/16
HA 269 GNLIAPEYGFKISKR IAPEYGFKI I??E????? DR3a 8/16
HA 286 SGIMKTEGTLENCET MKTEGTLEN M??E????? DR3a 11/16
HA 326 CPKYVKSEKLVLATG YVKSEKLVL Y??S?K??? DR3b 8/15
HA 327 PKYVKSEKLVLATGL VKSEKLVLA V??E????? DR3a 10/15
HA 385 GSGYAADKESTQKAF YAADKESTQ Y??D????? DR3a 8/12
HA 417 FEAVGKEFSNLERRL VGKEFSNLE V??E????? DR3a 11/12
HA 445 VWTYNAELLVLMENE YNAELLVLM Y??E????? DR3a 11/11
HA 451 ELLVLMENERTLDFH VLMENERTL V??E????? DR3a 11/11
HA 452 LLVLMENERTLDFHD LMENERTLD L??N?R??? DR3b 11/11
HA 453 LVLMENERTLDFHDS MENERTLDF M??E????? DR3a 5/11
HA 473 YDKVRMQLRDNVKEL VRMQLRDNV V??Q?R??? DR3b 8/11
HA 477 RMQLRDNVKELGNGC LRDNVKELG L??N?K??? DR3b 5/11
HA 513 YPKYEEESKLNRNEI YEEESKLNR Y??E?K??? DR3b 11/11
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
U o - - ~ -. - ~
y o
v
U O o O 0 O 0 0 0 0 0 0
O O O O O O N C. M
. O O O O O O O O OC\ Nkn
O O O O O O O O O os Nr-~
O O O O O O O O O N0,
A O O O O O O O O
w
cd co m co m co co cd ct m
0
~ O A A Q Q 2 Q Q A A
N
w cd cz co ta td ~ ~ ~ .O ~D D ~D O ~O ~O ~O ~D ~O ~O
N 0 R ~R R o~04 0404
A A A A A A A A A A A A A A A A A A A
v, W) W) V) tn vi tn W) W) V) vi vi W) v) kn ~o t- r t-
a r. r. - - - - r. r. r. r. - - - - r. r. - -
>~ cn
_ aU w `aU
w¾ Z aC ~ Z z w x a~l 3~ w w¾ A q a
U 3w ~ ~
~ Q z Z ~w Z > ~ > > > z
~ Z~>Ex- ~~¾Ov'~a W W Z Z
cl)~ a Z w Q U A w a ~~ w w
>
U
~~~.
U a~ c7 ¾ A x a> >
AH~wzAaacnawzz3¾a3a~
A Z cn (7 w a~ 5- > c7 c7 > w U w w
It N cC [- O~ [- v) ON 11 - ~*- (7, ~O M~ ~.= t~ =~--~ oo M O~
0 v) I- l- O ON "O "D O '
G+ -- N N M~ =d' ~ ~1 ~~ N cY ~ en ~ ~'
"T v)
KI
O
N_
- - .. .--i .-- - - .-+ - .
ct3 zzzzzzzzzzzzzzzzzzz
C~ z
N
ti
m ~
a
2 cli
~ A
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
o ~
Q N p
J N p
2 y~ ~~~ a M~7 M N V sl tD ~ V ~ N M~ M~ N~ n ~ 0) CO M M O O M C~) C') M
O N p
O ~
Z U
m o o ~ ~ I
Q Q N M n
CO O(O N ~ (D (O N a0 M OO M~ O O N M a~ f0 O p O lf) ~ fM0 ~ n~ a~
Q_' fD C') (O CO f0 M ~ ~ 1n M ~ n~ O ~
Q Q N O p~ M N n N 00 O n n fp n n aD N p~
m O O~ N O O N N CND O(O ~ N n p O O O M N pp O Q) ~ n N m
~~ n~ OMO 01 (O ~ p O N M N p~ N ~ ~ ~ ~ N ~ N~ N M ~ CO ~~
Q i ~ N ~
N asO~ ~A n V M O_) a0 O~ N M ~~ N O~ C~O{
~~ 00 ~ N ~~ O(~O M~ n O V ~~ M M O N O I n I (O ~ O~ ~ O ~ M 00
Q v M n ~ ~ rn
n_ ~
m o
~ ~'? c=Ni v ~
g Q; ~ ~
_ ~ N
N ma0p0
C Q F
7
d
O
~ m~ N N N O
C ~ O O) N ~ ~
0
U
~
~p~ ~ o I ~ ~ I
Q O p ~ N
N ~ V On0 ~ ~ ~p (nD
Q~ O. M M ~ W
CO O N_M V 01 O~ O O~ OD ~ O ~ O_~ OD ~p O) ~~ n O N N p N
~ O n~~ N ~~ N ~ 000 ~ V M OD M O~~ ~ ~(j N~ I n N~ ap N ~ O) ~ ~ M
m~,O' `' p M~ ~ O CO ~ p M~ N N
Q O O N (D O M GOO ~ OMp O N N
m O ~ ~ ~p ~ f00 N N~ lOf'1 M~ N U'/ 00 ~ N N 0p n OD ~ ~ ~ ~ (p V O Qnj O
~ p N ~'C ~ p N O~ ~ p fD O) ~~ N fD tn ~ p (+) p ~~~
~ oi ~ co n n n n
'f` CO C ~ \ ~ ~ r- ~_ ~ ~
ln U> ~ ~~ ~~ ~
~
c
~ Z Z Z Z Z Z Z Z Z Z 2 Z Z Z Z Z Z Z Z Z Z Z
tA ~~~~~ ln 1n t(') ~ h 1n 47 ~~ N N ~A U~ ~ ln tf)
cn 2 I 2 2 2 I 2 2 2 2 2 2 2 2 2 I 2 2= 2 2 2
tD M M O_ (D OD tn N) ~ O) O) N _ f~) OD M 1n O) _
_ _p CO O ~ st N~~ N M O M fD O O M~ n O N n N (D n N
~ N N N N M M~~ l[) O~ N[Y ~~ CO ~-- ~ ~~-- N N N n N N 1[~ n O)
$ Q ¾ Q Q Q Q Q Q Q Q Q Q Q Q Q _ _ _ _ _. . . . N N Q Q Q Q Q
~= 2 2 2 2 2 S== 2 2 2 2 2=~~~~~~~~~~~~~ z z z z z
p 7 7 J 7 7 7 7 7 ~ 7 7 7 7 ~ 7 7 7 7 7 7 3 ~ 7 7 7 7 7 7 7 ~ 7 7 7
~ LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL
LL LL LL LL LL LL LL LL
J ~ N p
Y Q Y y C~ ~ Q Z~ d= 2 tn ~_> d Y W W V~ H~}Q ccp ~(7 ~ Y J o
~} J~ fn Z Z LUjJ ~~~ 0 J(n ~ W J a~ d Q J= g~ C~ 2 d~_~(_~ U CV
~_ U ~ F- Y Z J ~ ~ ~ ~ Q g ~ ~ _J U ~ _ ~J Q' ~ q z J Y C~ _ ~ ~ ~ ~
Q ~~~~ J Q~ U Z W~~ U~~ d~ J LL Q=~~Q W~~ g l4 s~ Z d C
~ Q Z Y Z U Z U H W Z~ CA Q W J~ J~ I1J W d Z CJ Q U W Z~ i Q W `t
~ J fn ~ Y C7 ~~ d Y W W}~ F- n~~ ~ W UJ Q~ W U_` F Y~ ~
a~i > Y W ~} tn tL Y ~~~ Y~(A ~~ J> J U Y Q~ Q? Y ~ C7 ~ J U c
Q Y J (~ Y ~ W J ~ J J J ~ J r ~ ~> d J Y~ C~ J W U ~ (/~ ~ ~ Z ~ 1 ~
00 al W ln Cn I- F~ d~ J ~ C~ Ci (A U` Q W~(7 ~ U W d Q d W J Z H Q Q ~
~ (A ~> Y C'J d~~ U Z W J~> ~(n LL] ~ H Y}~ U z~ CA Q d l4 ~ z~ U y
J ~
m ~
~ c
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
o c
~ y O
N p
_a) ~! C) M Nv v v V Mv v u') v f- N O N V V i1) V U) N M N C') ~ N h M h U)
O
O Lr)
z 'n O
mo
CO N f'N7 04
m O M (D O f0 M O ~ M M
N n 0) 1, 0 N M O') CO h N CO 0 00 I ~ O O ,a ~v sf ~
N 00 N~7 M N ~ M Q) M N 1- 00 V tn
(O h l[)
M W C+ON N~ M ~~ M N Lf) O N n ~ N NV pj M QMf M
Q i M N (D
N N O
~ CO ~ O O 00 O t_ C.~j tn O cl) O O~j N O I, f0 M faD U) v
C') ~ a p M~ O~ ~l) O N N M a t t f7 N N
e-
O
M
LC) co
N
N m ap0
a
g m o ~ o rn 'N o
c ~ o ,~ m co
'r) v rn r- CN
M v M n m
~ CO GO tp!) Op) ct (O 1n Nr.- ~ ONO O~) ~
N tn h ~2 I- L[f GO N
N
M m V O) p M N I N h N O a0 1~ 0 ~
<V Q O ~ v ~ N ~ O~ tn p~ N ~
~~ ) ~ CY) rn~ c^o n n~ aN0 N O I a p~ tD a 0~ CO a(`N') I~ t p~j M M 00
0 O N pp 1~ M ~ N~l) a O M f- (~j ~ OD M ~ Mv a N O(O p N N~ st 04
f- Lf) Ln OD aO 00 01 N(O 0 O M LO (O O O O N
O p ~ ln 1n (D N N~ ln N ~ M O M( ~ O ~~ O
CD
V
CO O O I 00 N n~~ ~ 0 tN p N
[) tn p O N p 0 N 04 p t O OD O I v O N P~
C) r0 LC'1 M ~ e- M R 00 O ~ O~(O tn N N~
N C Ln
C
in U> rn n ~ ~.- ~ rn rn a
m Z Z Z Z Z Z Z Z Z Z Z Z Z Z ZZZ Z z Z
U) U) tn U) tf) 0 0 1f) 1f) ll) N U) 0 Lf) U) N N N W) 1f)
t n 2 2 2 2 2 2 2 2 2 2 2 2 2 2 212 2= _
O~f) CO M 00 M ~1 O) O O OD CO Ov CO fD 00 f0 00 M M 00 V
M tn
a0 aD O M V t0 O) M(O O_O a0 M u') Ll) (O N O a0 M (D 00 O O~ N (O (D
N N N N N Mlo: U~(O h N N N N M V ~
g Q Q d Q Q¾ Q Q Q n: a n. n. n. a a a a a~ a a a in in in in u~ in a~ in in
z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z z
p 7 7 7 7 7 7 7 > > j > > > > > > j > j > j > > 7 7 > > j > j 7 7 7
N LL LL LL LL LL LL LL LL LL LL LL LL LL LL U. LL LL LL LL LL LL LL LL LL LL
LL 0= LL LL LL LL LL LL
~ Y Z V Y} I i ~ > > ~ ~ C1' ' Z (~ U W Z~ Y l~ LL~Y Ct J a ~
J a Y L~ J J cli
Z~Q ~ Y Q} ~ ~ lp Y 0 }
x
} C7 J 0 Q (/~ ~ ~ W S u W J Z J d
J2= ~
J U kz m w cn W_ J z~ JxQ W v)~WW~
_ 2M_ ~t-- J
Q Y(a Y~ C~ d U Y~> x ww Q ~> U~ Z Y~~~ J w d c
o c~ wYQy J<n~~ Wa ~a O 236SL)
d z z} p w~~~ z W p w w tp ~ (7 mU ~ w O w~ p ~ p w
Q LL; C
y J Q Y J H~ ; d}2~ Q J CI ~~~~Q' J~(> LL Q J J F- 0
W U~Q Q _
~ N ~ Cy CD Q Z Z Y Y J LL~ d C7 z J W J~ w Q C7 C7 }w w 0 Cn
(q lY Z Z S> Z W C'J J~ J> C7 C1 ~ Y Q U ~> Q J J tn C'J Z w ~
N
W 2
J
H t
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
cr-
o
g y O
p
2 N -.t V n M nW) V NV fn n GO C~! - - - - M n-t V L[) LO Cn V
M Vp
o u)
z U
LO co
M N ~ N ~ (O ~ co c6 O ln
LO
co
O N rMj v
~ ~ co M N
O
0 00 N ~ M n fD l0 ~ CO
co p co aD p N M to tn n OO 0 N n O M fD LO 1f)
~~ M~ Cn~) ~ n e~- a N~ tnA n 00 O N
~ N
N Q fh O n N(O O ~ N O O~V p
N O p M W O ~ O
~p+)O N O N) N CO _ CO n M(O OD
o OV 00 ~ O M N vi ~ N ~ C7 ~ V f~) ~ N N M
m O O ~ (O ~ O) ~ O O CNl) ~ M ~j p
~=' ~ n rn v n N pp ~ p v
O
= N
~ CO O ~ ~ ~ ~ ~ ~ ~
C o r
a
ao
mo
~ ~ N cl)
cM,) Lf) N ~ O LOv ~ M
U
- U)
cc v _ o0
co O p~ f0 _M 00) ~ tD r~ p ~ ~OC) N N ~ ~ M N(D pOj ~
~ y0 ~ M 00 ov N O n ~ ~ ~ lA v ^v n~04 M m O O~ O O 00 ~ p M Nq O O~ O n p nV
M tn
N O O OD (D M~ QO crj O O OD (O n V' f0 f0 N~tp N CO
M N ~ n
a0 n
Lf7 N 04 m O n M 00 ~ O~ N~ O Q1 ~ M Oc") N M ~~ N C') ~ C~O O C~) M O O_ O
~ O n~vV n n O M~ N V co ~~ ln n~ O~ O N N n f00 co m O N V co GO M MLO 't (M
~ fh p O r- 00
Op
ar (h Ov n O O~ V n I 00 N~ n fM ~~ O~ co ~ O CD I p f0 ~
n ~ n co O N 7M N tn co M ~ co
p CD f0 (O 04 O 'R ('7 ~~ M N co OMj CO CD a ~ n ~ I !~ ~T ~ N NW O~ O N v O
N ~ N M v MC N ~ M( N~ c~o in M N Cn
'~p C C t1') M M M M '- ~- p N
0
u) U > r- co r- co co ao rn rn ao
C N N N N N N N N N
Lo Z Z Z z Z z Z 2 z z z Z Z Z z
NLo N p N tC> N N NLO N pLC) N N
2 2 2 = 2 2 2 2 2= 2 2= 2 2
O ~2 tn N O CM 00 O N
CO n N~ n n n OO a0 _ n C') CO n N CO C') C~) OD 0 f0 O e- N f0 OD v
a0 ~ ~ ~ ~ ~ N N(D n O(O CO O~ N N F) V (O N (0 00 O~ ~ O~ 00
N N N N N N N N N ~ p ~ ~ N N N~ M st ~~~~~C1 ~A ~ U) (O (p
~ !n <n cA fn fn cA l4 fn l/) cn Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q m
:3 z z z z z z z z z z a a a a a a a a a a a a a a a a a n. a a a
O 7 7 7 7 7 7 7 7 7 7 7 =i 7 7 7 7 7 7 7 7 7 7 7 7 =i 7 7 7 =i 7 7
V) U. U. LL U. U. LL U. U. U. lL U. lL U. LL LL U. LL L LL U. L L L U. U. U.
U. LL L U. U. U. U.
U~~ W C'J LL LL J Y J Y~~ C' a W Z J a Q} Z~ d QT p
= U 1 Z J> a LL LL ~ QZ Y J o J fn ~ 2 Z9 J Z ~ I-~ J Y J Z Z N
W Y J J
a(~ ~~ CJ J ~_ LL W Z J Y~ a g~ Y~ J O W~ U Q~ F C~ ~ A
U Y(A J~ J UW W~ o ~ Q a a Q X a U Q~ fA Z_ o(n H ~
cn o o W~ d w d gW W~ a~ w p w ¾ ~ o w a>1-~ W w o~c
~f
a~i (n ~ } U W_ LL ~ J w W ~ ~]= O C7 U Y J w ~ W= QFL~ U~(n JZ LL
Q ~ Y a' J~J W fA J~ W LL Y o(''J W a. }_~2 W~ LL J J a
00 N U J ~~ W J J LL Z W w E LL a fn ~ Wo Q Z} lA Z a2 !/) W W H ~
(n > o(n >_ LL Q CI J LL (~ a o Jx W a J J(q J~ a W a > a (~ W J W a y
W
J
m v
Q c
H I
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
o
~ y p
',R - o lf) N(O ~~ ~ ~ O~ O~ N 00 (O I~ OD M N N CO M(O O~fl M N(O c0
p
O LO
z
U
~ n
~
p M~~~ p r~. N N I I fr p j ~ NC) N ~
M
p O
~
~~+j v V ~Mf,j ~ f'n' OV NNn~~N O ~ ~ O
M 00 ~ O O N
~ r I~ n~ p N ~ M V' N aD O) a) N Q)
o i p
O ,, O QI N O ln OD p h ln 0 00 QO M ~~ 00 (D
M '" CO 'Aa00N N GO O) 1, h CO (O -T O O M ~ I- O Ov M p M O~
o i h (V h~ 1~ ~ N p~ V r, ln
CO O O tn fD O p CO p O M p~ M O O M O)
a M Q
O M N ~ ~~ LL) ~ ~ ~ l) ~ QI
~ N
N N f0
Q o
p l[) fo) CO N h (0 N N N N M
C or m
a
o rn
~ ~ O aVo (D p' ~ M p ~ ol~o cfDO N M ~ n rn ~ v
o r M p CO c0 O) p N .n- N
C)
m O O r~7 a~0 N O n O N O M N N ~ n N COe) I I ~ I N N pp a0 0
O000
~ ~ N R N M'7 p 00 00 ~~ f0 ln v n N (O ~ I, ~,Ny fo T M~
o w
M O M f, ~ N~ v (O - O M~ p O W QI p M ~ O M p p M M N O O N OO 00 ~(D CO N p0
fD M p M M MVM p I~ 01 fD ~0~
m O GO V V M N (D V)~ M M h h O I~ V' N O10 ~~ M N t0 04 Cr) N M N) n
Q O (O M Nl V ~~.. N~ M~ a e- O N N'- d. ~ N N~ O Lo '7
01 _
Co o ~ o M LO f~ O n O h N h tD Of n p M aD
M O N(D - M N~ O ~ U-) O ln V N tD h
N~ CO O GO O N O
a p
fO O m V) ~~~ M 1~ ~ CO O~ n 1~ ~(O 0~0 O N CO O O v M ~~ N p_M
M O ~ (O M N~ O~ N tn M M(O CO O N N p~ - O u-)(O
C N U N N N N N N (V N N N N N N N N
,t= \
N C C
O
U> a) 00 00
C N
ZN Z Z N
Z Z Z Z Z Z Z Z Z Z Z
N Lf) 1f7 LO L() LO LO 1n ln l() ln N LO N
U) _ _ _ _ _ _ = _ _ _
M(O O Q) Q_f N fD N f0 ~ 7 O0 N h N_ O~A 0 O f0 M p~ N
(D 1~ O N C~) C') aLOm v h 0 O N M ~7 0 O 0 L[) 0p 0p O N 0
W N M M M M M M~ ln tn 0 Lq NU~ N f0 fD (O t0 (O h h 1~
04
m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m
a a a a a a a a a a a a a a a a a a a a n. a a a a a a a a a a a a
c > > > ? > > ? > > > > > > > > > > > > ? 2 > > > > > ? > > > > > >
~ LL LL LL ~ LL LL ~ LL LL LL LL LL LL LL LL LL LL LL LL LL LL U. LL LL LL U.
U. LL U. LL LL LL LL
w Y~~ o~~ Y 0 > ~ LL u~ C~ ~ I Z Z~~ U Z ln o H o
a J o Y Y Y W a Z a Z t~ >~ Q U J a o Y 0 2Q~~ J o
a Z cD 2 Y ul w Z ~ a~_ JV C}J 0w A
_~ L` Z cn J Z _ = >
cn J Z ~ Y Q Z a 2 Ja CJ 0 } > w Z w w Z z zw> z Z 0~w ~ Q d w~~ d
~ Y W W - lUi W ~ Z (A (~ Y > Z W~ w U uu>77 rn
> > J > Z J J J (/7 o J J a
0o a~i o w Y r g a~ a~x~ u¾- ~ Z a z d z W> Y}~ a a vi ~
~ Cn < H J Y (1) LL d LL Z J Q a Q W x dx w> J N
w
J
m v
H I
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
Q
g y O
= (D ~ h N V N M 1- I- V N ~2 CO N OD h Cn O V U-) N tf) ~
0 CU O
O O
z U
U,j o
O CO M
~ n V N I~ M
Or
CO O ~ ~ O) OD V O) Q1 tA O O) N ~ 000
0 N .- ~ O tn O M rl- ~ f0
O 00 M (O O 1, 1- N N
O N CO ~ O ~v O N N~ M M
Q
N
m o ao rMi o rn ~ rn a o rn ~ v~n =- o m ano ~ co ao
M w~ M M
co M(O N N N V O N N
m o a0 O 1~ pp N V OD n M
O co I M vLNC'1 ) N r' o'~ n v
g o:
_
v 0 o M ~ I I ~ ~~ o
a ~ G 00
n
rn co ~
m o c_o ~~ o ~ co
v I.- ~n
c Q Q CO M M N M V) N M N I-
.9
LO u7 O CO a0
O M O
Q rp aM0 ~(D ~ ~ ~ vv fnD 1o N N p O ~ lw CO')
O v n co N O aN0 N O m fOD ~ O rn~~M M=n- N st N O I C) co
Q P N V N
fD O M 1, t0 O) M N 0 N O O N M
M O O O CO ~p t~ M h U) O~ M~ N M a0 n
Q O ~ O N 0 N~ 00 M f- V M ~.. v M N M O n
M !. O 00 OD h 0_D M N MLlf
COD ~ (O ~ O~ 1~ V' OM O m O
O
(D M M M
Q O Op O N 00 M ~ L[) I" ~ d. O f~ LO
Cll O LO O N tn IT 00 O M M N V O_O
Q O N O) v~ O) C7 ~~ n COO q O) co co C a)i Cj` ~ ~ a ~ ~ a 'ct a
05 V> 2i ~-5 25
C N N
L z z z z z z z z z z z z
O O N ln Ll) LO O lO tn O N 1n
ln 2 2 == 2 S = 2 2 2 2 2
LO v (D 1- 0 lO OLO_ ~~ 00 M M(D st M 47 O h f0 m
f~ O_M fD n_ n_ 0_0 O t('1 ~ 0 M(D OD m 1n f~') I' O) O N~
1C) ln O N N M M~~ ~T ~t ~f 'cf O CO tD (O
~ N N N N N N N N N N N N N N N N N N N N N N N N N
m m m m m m m m m m m m m m m m m m m m m m m m m
a a a a a a a a a a a a a a a a E. a a a a a n. a a
o > > > > > > > > > > > > > > > > > > > > > > > > >
~ LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL LL U.
Z Qcr g Zco~ Cj > Y o
Q W p Y J li > Q~i U` Q
W J H W J (A (n Q' Q' < > N
Z C7 J W > 0 Ji> ¾
Jw~
$ Q o can z~ uWi w uYa Wz ~ c1 o > o cJn o C~ o~~
j ~ 2 J J ~ LL a ~ LL
J 1 C7 ~ ~
_
~ ~ Z a> x > ¾ w ~ > C7 ~ ~Q = Y d
r1 Y Q a Q cD ¾ C7 cD ¾ ~>~ c
¾w LL w ~
W (D
J ~
m v
Q c
H I
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
Q' y O
x a~ tn ~n tn v t- rn oo M tn kn r- rn v v kn e w) tn v c- c- kn oo
o o
Z ~
oo
~ W ~ N M N ~G b
t- M
N ~o C. v~i ~ 7 o0 M O N ~ M 'cY i O ~ ~ M
Q o0 t, t~ l- O, r- o0 vi V 10
(Y.1 O l, O, M N oo O V V ~O F o0 l- M n ON n oo p, p
N N M M M N v~'~ m N N V M M N v^'l "
Q x N
oo
O ~ N O O ~ M O N ~O O O <f N_ O
~O 00 N r? M 'n 1- O m N ~O tn O
00 N N N 00 O, M N
0 O N T
M fN~l V o0 N M
= vl v 1- W
N
o ~ ~
O O~
w Q o
a
o .
LI O 00 'If ~D vl O 1- ^
M
O ~ N ~ M Opo ~ ~ W N M vl N
C Q N V1 M
y M
r O O oo N ~ GMO ~ O~1 Vl ~ oc~ M
'~ Q = .-. N vi ~n M ^
N
0
LG M 01 oo 00 o~
Q O M .. V C~ M M N
O, eq t- C. V7 N ^ G\ o0 oo ^ M M M ^ M
00 O~ N N 1- N N o0 1- Q\ p ~O O c~ ~ M O O M t~ ~O ~n ~n
Q O w) M r- M ~n M t+l V O,
M oo M N w1 M ^
~ =
vi o0 n oo l~ ^ ~o t N " oP O N oo w~ -,t r r'? N V t- ^
In N ~O v'f ^ \0 M ~O M ~ N N op M ~ o0 N M N ^
[~ =
=~ C U y .r .--i .r ~ ~ ~ .-. .r
!"J
V)
(, >tl
~ x x x
O~ N M wM " ~ O ~ N O M
N t+1 O O M I~ O 1~ ~+ 0\ ol It w lD
w~ V'~ 14D N N N ~ M oo ~n 'n ~O N O N_ t~ ~O o0
a, ¾¾ ^^ N Q a a a a N a a a ~n v `~'i ri v 'i vNi ¾¾¾¾
z z z z z z z z z z z z z z z z a a a a
> > ~ > > > > > > > > > > > o ~ o d > > > > > > > > >
v ~ w w w w w w w w w w w w w w w w w w w rs. n w w w w u. w u
j
> h(i,
a a> a _N a a xd x > a ~ ~ a a a~ ~e
> a [~-^- ¾ >5 > v '" a a a Q a w ~
¾ a a w a N N a~ Q
J o F ? W a dw - a~ w w a~
c a `~' > ; a rr [y- a dr a a x >
m w r] p R H p w
a C7 a a z C7 a w v~ v) ~ a
C7 C7 w
`n ~>"' z 0 ~? rr > 0 c ~G ¾ w> v) c:. a w w
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
n ^ a~ rn ~ o0 00 00 0o r r n2! oo n o 0
~o 00 O 0~ ~n N M O N N ~ O M M ~D W,
00i N N.
M M ~ n ~O M V1 00 ~ O~ ~õ O~ N
~O M ^ r t+1 t'1 ~O n N.
Vl O, ~ r
M N M M
O
T O\ ... 'n N N
r- oM0 N ri N. rr-I ~O N .... M V N 00 Q,
o 00 n oo N. N. ~ n o o Q ~00 o en rn ~ ~~
N N. M 00 O" N. N. 00 ~O M P! N N 00
_
~ t- pp 7 N 00 yl 00 'O C1 N N qzr W) M ~ M N
N
en ~ N 2 ~O Vl N n M C\ M 2 M : n M
N N M oO
t- cs
O M r M ~O o0 00 O, N M N M pp V1 N M
00 W~ O N T C. V O~ ~ 'O V1 r r O. O ^ N O N. 00
N O vl N O N. O Q` O M N N M M N. N 00
~ N. 7 N M a O" 00 00 n ~O ~ N GMC v~l ~ ^-~
N
n n ~n ~ en O O ~ O N ~n N N r- 00 ~O
~G oG ~O N pC ~ ~O M p c0
(ff' r N N O vi r~ M f'? N. M 7 O ~ O~ en N. ~ O ~ r~ tnr M O~ MM
, ^' ~O Q, ^ _. . 00 ~ ~ i o~0 W r ~ ^ M N N
^ M O V CO W) 00 vl
w' N. N
M N N. n N. M p, Ul ~p M (.j ~ "{ M ~O V Vl M Irl-
_ N N N N N N N V' V ^ z N ^ N ~ 2.1
^ O ^ ^ O ^ ~ -N O V' O O ~ M
Q~ C\ O~ O~ 00 00 .--~ .=== pp
O~ N ~O N ~O vl _ O~ N O, T V ~O r ~n ~ 00 7 t+l
vi O N --N M M 7 ~n O~ ^ V O N O,
M ~O ~O 00 N M M f+l M M M v1 V1 Vl n V1 M ~ ql: N ~O
~ ~ M N N N N N N N N
Q Q Q (b CQ Q] (~ (]] (]~ C~ LY1 Qa (]] (~ Oa Lt~ Ga Ln Ln CO CQ Q~ (]a p] Q]
.
a n a a a a. . . . . . . . . . ~ n. a a a a a n. n. a. a a a a a a a a. a
. . . . . . . . . . . . . .
o o o > > > > o 0 0 > > > > o > > o o o
w w r~. w w w w u. w w P. w w LL. L.I. W. w u. w w w w w w w
w O > "
z V) >
F CF7 0~ >
Z x J- F- w
W > z w > z,
~e z a Q 3
a ~Q Nj u~ [~ > Q W F~ vWi a O v~
m W w rx F 3 > ~ 2-.JC7 > > j 2 t > w p
Fa- 0. U. a u Q
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
d~ m m r- m v v n~n v n rn m n n
ro "d
U
N m m N
~p]
0_ ~ ~ ~ N f'~) V ~ 07N ~ 0
m o 100 m 0 m~a o p~ rni v a o a~
O o '[1 m ~ n N a M v
a' M V csj N r N O N O N(") N O 1 N N
0 i
N a g
~ f` ') f~0 ~ ~n., PN1 N( O N~ M lD l~ ~ Gl C
^ i n m mNN N 01 M N M n ~ N rl
O
_ roo i
g n N N a n A o
m O
~ ~ {~N) n 00 ~0 N ~ ~ z N f.l d' d' 111
=
~
Ip
N N
7 m O H W [,
cl)
O
00
O 0~ N~ t~ rl rl m'~
N ` b
N C n af N o ~.a rn
~ m N m ) N Q O 0 r (r~ N N m tm0 O~1 0 N O U1 H Nl
O O 01 N m V m 0 m N N ln N 10 d'
o d
m y
N~ N fV n N N M p N
m O ~ M~ m 0
umi m omi ~ m~ m v`~ rn H d H w
q ~+ a a
o ro
a 0
s~ ~ O
~ O M~ v! ~ " cO M O o N ~ ~ ro o ~ nl rl ~
cl) R7 q v = o
ro Q
$4
d o .+
m o O~ n~ tn m n M~ n m M fV f~ ro N ry rl a' 111 d'
('NI n ~n o M~~ r n u~ o r~ rn D ~ 1 vi a
O N w
Q
N m
ro
mv V Omf r N N 1 ~ ~ ~mN 1(1 '1 0
p O c~ N v> M m hl ro O1
r
W O
O L
.H
IC b U
C
m O m~ N 1A ^ P9 1n N 1n n N c) ro ro d) m \o N N
O 0 N N N~tp 1N N N N ri(7 v ln N g r-/ rl rl
N
b1 w
c .~1 ro
a
O O C f~l
m('7 0
Of m O) N 0 C) m 0 N O) ~ n m
C) n m if) U) v) n n n m O) n t~7 .r1 N O ~ ~ ~ r
W m N H H
a fV N V m C7 4 n ~1 Aa
N
~
w a ~ b 0
O a a a a vNi 0 ( ¾¾ m m m m m m O v M N N ~
n. z z z z z z z a a a a a a a a ~ a a c~ E2: z a
w w pib ~
0
~ Uyo~ Fwc7~zu~ic9o~wz a ~
o a u m
-~~Jw~QZaN,w~Fa U.
(I~ a J~ J Q_= J Q'' a, J J~ F-- V~ = N C
g a wzWVia>~cy ~ a~Qvaio
w U = w wU) w~ a wU C7 > Z 0 YG V x.~ N w F Qa :{p=
m J r~(n I lL J 2 J J Q J= J
~ _ z d> O J LL ~ J w dI Cn Q J (U ~'' (~ ~+ R~G C
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v
,r o
N 7
o
p e
g g U u1
ti p ~ o Z
. :roqu , N c
~ y
A vl H p vI
b b .~
o ~.
m m o u" in in o in c w p r
.= 'i ~ :' 0 z z = '"
~cm
r=1 7
H 7 VI
Vi ~
~o a o tr N o
z H N N t'1 N z H ~'1 t=1 d' ~O rl U1 N v v O
0
IO N ~
N o l0 I r ~+1 o l0 t +f ~ a V'
~ N N ~ 111 V' d' y rl rl N r ~ N =d
Q .d Q t~ Q t~ LA 0 .~j q1
N N N G) 'i rA ^
N =y H N
mr ~ 1Q ~ T rl Hf ~ W ~ O O N d W rl N (71 o N
H v N '^ ' m H m m a 14 N ri w H p
m
w Q ~ ~ a 't ~ p~ ~ N u
N
p .N O =~+ O 0 ~71 0 r{ r{ y 7 r~ u ON u r{ Q
a N Is Is ~ .y N IS u ~ .1 0
01 p p d, ID O~ r 01 ~ 0 ~ ~ ei 01 N r ~ n ~ r nf ~.~ o
ro L N N N N rl ~p Y N ~ ~ ro~ o 111
d a d o ~ a d r ~ Q ,"" m d w
~ >
H N ~ >
N H~ N H~ d H o
N .+ d W .y d ~ N G) Ln
0 0 0 0'o V o
. m ~j r Ol , , m m
b dv r
~ aLn 'i p ~ N N b m m Ln ~ o o Nm ~,=, ~w
N~ Q N p Q N 0 Q N al N m
N N N vl d u r~i d m
p ro^ o ro ro 44 p u
~,.~,r [0
44
44 44
Hp o
W N t nf N 0 FI N p
H m w" m.i m.+ H M~ ui ~..~ ~ v m r m 44 w m rn~ H
r 0
C V
44 44 0 q y'
44
~ u u ~ o 0 V~ ~ 10 0
0
' 0 C y a G b c y u
m C-4 H N ro k w ro v v m ~+ N ~ ~
N
o m Q w Q 9 M w~ b i w
~ q 0 r. ~ w .i = ~ .~ q =.i m
N Rl A 'd ~ =.1 R7 m
0
~ N a pl M C V' ~ d CL m O, nl w V' d~ w A Opi V' -0 w w N
A A a+ A dp~ A.~
m Q w dp~ d 'v a) w
~ . a Q w w 1~i ~ w ~~ ~ .= N a H N a 4 w w 0 1) .0 [L q 2 a a W +J a a ~ a
a) .O w E 7 W 1 'T. a a 41 O a E o
N y m N m ~ UH1 N o
ai d w
b P. a u n
a ab o ~
~=~ ~a a.~
m a m a m a o m A
m y d d w ai v ~
N [ T c .0 m q ~
v Q Q v Q v aa 7 v
HP4 w ~C w Hx u' Q m a
w u u F E~ aS'. ~6 ~ V~ an x~ m a V w . N HQ rQ ~ paC bi m~ ~
m
=.1 ~r C7 =.i W ti =./ 3 ~C Q N tk C7 L (!1
a a~ z r a 6 0 mQ q W w cn
m N.1 H H a N.1 .. p ~7 N~ a a H a a a N a W ~
~ ~ w ~ cn w ~- uvi a
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
lfl fV
m m
r in
ko a
LO
N lf1 o
N V
N 1~
m If1
rl t~l
o+ a
` o
M
~
ID %D
m m
N N
rl ri
m m
r=1 N
M rl
C
z z o
d
N
A
C
N
~
C
V
p u' a
U) Ef) a
~ y
J 'x a 3
W ~
ED
CA 02658559 2009-01-20
246
WO 2008/039267 PCT/US2007/016529
Table 51, M2e sequences from representative subtype isolates
Conserved Human 8e uence S L L 7 E V E T P R N W C 0 o S s 01 A/Witson-
Smflhl33(H1N1) _
A/NewCaledonla/20/99(H1N1) 7 ? ? ? ? ? ? ?
A/3wlne Korea/S1012004 (H1N1) - - - - .. .. ._ .. ..
AlJapan/305/57 (H2N2) -
AtAnn Abor/e/80 (H2N2) - -- - =- -- == .= - - _ _ _. _
A/CanadafJ20/65 (H2N2) - - - -- =- -- - - . _ ..
ARiong Kongl1/88 (H3N2) - - -- == -- -= - - - .. ..
A/Charlottesville/03/2004 (H3N2) - =- - -- -= -- .. .. _ _ .. _ .. .. _ _
NCanterbury/129/2005 (H3N2) - - - - -= - - - _ _ _ _
AfBrevig Mlsslon/1/1918 (H1N1) - - _ - - - 2
A/Puerlo Rlco/8/34/Mount Slnal (H1N1) - - -- - - - -- - - - - _ O .. .. 3
A/F lan/411/02=I(ke (H3N2) - N_ a
A/Swlne/Saskatchewan/18789/02 (H1N1) - - - -= -- - - -- - - .- -. _ _ _ _ 5
A/mallard/Alber1a/130l2003 (H 1 N 1) -
.. .. .. _ _. _ _ _ .. _ _ _ _ _=
A/mallardMY/6750U8 (H2N2) - -
A/mallard/Potsdaml177-4/83 (H2N2) - - - - -- -- - _ - - - -- .. _ _
A/ducWHokkaido/9512001 (H2N2) 7 ? 7 ? ? ? ? ? - - - - - _- ~ -
A/Ouck/Korea/89/2003 (H3N2) - - -= - - -- - - -
A/swlne/Shandong/2/03 (H5N1) - - - - - - - -= - - _ _ .= _
A/Chicken/Cali(omia/013912001 (H6N2)
- -- - -- -- -= - - - - - - - _ _ _ ._
A!GulllemotlSweden/3/2000 (H6N2) 7 ? ? - --v--
A/GooselHong Kong/W217/97 (H6N9) - - - - r - -- - - - _ _ __
A/Chlcken/Br18sh ColumbiaJ04 (H7N3) - - - - - - - - - - - -:
A/Shorebird/Delaware/9/96 (H9N2) - - - - - -ro - - - - - - -
A/Duck/Hong Kong/S'439/97 (H9N2) - - - - - - - - - -
A/TealMong Kong/WS12197 (H6N1) - - - - - - - - L - - - - - - 6
A/swine/KorealS452/2004 (H9N2) - - - - - - - - - = - - - -- _ E 7
A/Hong Korfg/1073/99 (H9N2) - =- -- - - - - - L 8
A/OckerVNetherlandsll12003 (H7N7) - - - - - - - - - - - - - - - - 9
A/Nefherlands/219l03 (H7N7 - - - - - - - - - ~ - - -= - - _ _ _
A!Swlnefrexas/4199-2198 (H3N2) - - - - - - - - - - - - - - 10
A/diAcey/Ohiof313053/2004 (H3N2) - - - - - - - - - - - - - - - -
A/Turlcey/Norih Carofina/12344/03.(H3N2) - - - - - - - - - - $ - - - . - 11
A/Gcose/Guangdong/1/90 H5N1 - - - K - - 12
A/FPV/Dobson/27 (H7N7) - - - - - - - - - - - - - - -13
A%hloken/FPVANe rSd e (H7N7)
/Vma9ard/AlbeHd/20ot (H1N1) = ' 7.4 7 ? 7 ? ? ? - - - - - -- = - 14
AtOuckMunan/114N5 (H5N1) - - - - - - - - - - - - - - -
A/Slvlne/Cotes dAmior/1492/99 (HINI) V = - - - - - = - - - - Y - - N -15
A/Wne/Betzlgl'1J200t (H1N1) ? ? 7 ? ? ? ? ? - =- - Y O - - - 16
Altud:ey/(talyr220158/2002 (H7N3) - - - - - - - - - - - - L - - 17
AIHKl2108/2003 (149N2) ' - - - - -- - - - L
- O - - -18
A/FPV1R6etocW34 7N1
AMeI Nanil1203l2004 (H5N1) - - - - - - - - - - - - - - - 19
A7lllet Nam10T-03612005 (H5N1) - - - -- - - - - -
NgrebA/Ncvosibirek/29/2005 (H5N1) - - - - - - - - - - - - - - - -
A/Ber-headed (36oseMkngofla/1108 (H5NI) - - - - - - - -
A/caV1'hallandACU-02/04 (H5N1) - - - - -- - - - - - - -
AlHong Kaig/213103 (H5N1) - - - - - - - - H - - - - - 20
A1ohidcen/0uangdbng1174J04 5N1 Y - 21
AMK/1¾8l97 (H5N1) . - - - - - L - - - - - - 22
MQuaNHong KongR31197 (F19N2) - - - - - - - - L - - - - - -
A/Duok/Hong Ko'nglY28o197 (H9N2) - - - - - - - - H - - - - 23
A/ohlbken HK/F1F23eD3 (H9N2) - - - - - - - - H - - - - -
A/Ch(dcen HK/0919,7 (H9N2) - - - - - O - 24
AKurKe nnan 1 (H1N1 - -+ - - - - - - j =-+ Y - 26
~ = ' =
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
v b p
'"i ~j VI zr Ln Ln q, m v v -czv M -,v v N N M M N T :r v N di dl NT
(a 0
dp
O 0 V
M z
Al
b ~
Q) ao '-i N Oo m l0 r-1 [, 01 d, 01 lD M l' O '0 O ~ O ll)
M H 00 O~ r-I ln ~0 lfl 40 r-~1 a1 N r-I N ~ r r=I ~ ~ l0
W
L~.
0
U
r-i ri H I I I r-I I H M I
p Lfl Oo 01 O l~ M O ~ r
N ~ M ~D O1 M 01 M d~ l~ [~ rl
U M OD M . ~ p H r O N ~ I I N lfl ln d~ ~ d~ = ~
N w r-i H k0 r- r-i m 0) OD 00 Lf1
Q) b ~. r-I r-I
.
H ~
N ~ m o ll1 M ~ Ln Oo U1 m H 't l0 kO O 0) l0 l0 00 N O r=I
04 M = = = = Ln IV N l0 m N 0 ~ r-i L11 t- d' H d' = H d' = H
N N ( ) N H lfl N H [- di lIl N l- M
O
p o O 41 N Lfl %O Ol I- r=1 N ln r-i M L~
Ln N ri N [- O) m = M `o ~ O I~ H N M M O N ll1 O ri ~
V I H m N N O m H r-1 ~ N M ~ m [- N H N H m N M H N M
r=I
O
H r{ N Lf)
rI
p r-1 O Ol l- m 0 ~O d~ ~` 00 Oo m d4 LIl 01 l0 ~ N Lf1 ~ 0) 01 d
r O
4 lo r-i
~y N OJ l0 r-I rl [- U1 I- lf1 0 M lf1 00 N Lf1 l~ ~ r-i ri ~ ri
rr~ l c~ H k0
0 (1)
O U)
N rl o O ?.
Q~ o N N
=K ~4 '~ O m [- l- 00 M M m ko O l0 M M rl N M O O O O O O O
~ oW O [- W d~ M O~ 01 01 QO C) dD 01 44 [- 01 OD 0 O O O 0 0 O
a=-~ 'Lj r-I N
0
'o .q N U
. r-i ~4
ro U) W lfl lf1 0~ W [- m N N 1o W l- [- 0) r-1 00 01 H
N (0 0 N L11 Lf1 l0 10 10 1o 00 OD O r-i l0 N N O O 01 N lfl r- Oo (io 10
~ 'CS t`7~., a r-{ d' 0) 1o r=1 r-i N 144 -IV d~ ~ r=i ri H ~V H ;V V' W 44 %O
U " )
04 :J 4
a
04 ~ o
r U)
a) r-i N N N a a a a a a a ~ ~ ~ H ~ ~ ~ H H
o z z z z z z z z z z a a a a a a
U ~ ~n a
U
E~ E o rn 0 rn rn rn 0 0 0 0 0 ` ` 0 0 0 0 ` 0 0 0 0 0) 0
>
0
~4
x O
~ o
(Y) ~ ~ a x x x x x a x x x x x ~ N
RS RS a X a' a H w a w x x a ~H ~H H ,+ w >+ x
o~ ~+ ~+ a cn w~4 r~ > 44 w a H ~~ a a ~ v a i f~ H ~
=t ~ H H H 7+ [~ A H Ri C] cn a A H H -~
0 ~ a U U H H fs Ul H J+ G4 ~l U] (Y z a a C~ H E~ U] 3
Q) C~ x x U FC FC C7 ~] 0 E-+ a w A>+ 3 44 E=1 (n
x N~ ~ w w x cn a w uo a w D w a a w w w w
cV (d U) a a a F~+ 5 can a a C7 0 ~ x a H 0 C~-~ ~ ~
~ A a
0 a) -H
v =ri 'J N N M M k0 m l- Oo 01 O r-1 U1 N M l!1 [- Lll [- M U1 kO zV rc$
1J =rl b O O O O O H O O O r-1 r-i N r=1 r-i r-i N M M M M M d~
v r i õ N W r~ ko kD ~o r~ r~ r~ t~ t rnko r r~ r~ r~ to ~ ~ t~ r1~
~ r-i ul p, o O o p p p O O o o p m p p o O O O p O O o o N
N 0 U1 W W W 10 l0 W W W 10 W W N k0 W l0 %D %O %O l0 %0 W W l0
E-i U] a H r-i H H H H r-I r-I H H r-I r-i r-i H H r1 r-i r-i ri H r-i r-i r-i
1-J
N
U
.1
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
N
M
O
r-i ~:j VI N N N d' Ul Lfl Lfl tI) d' M fn M
rt 0
d'O A N
0 0 V
m z
Al
b
~ 0 O a0 ao M M U1 H
00 I- l0 N O O 00 U1 10 m
w O [- O r-1 1 l0 00 m r-i d4
~
0
U 1 ~M 0
o rn tn Ln rl ~D ~
U) M r- N N O L11 H r-1 QO N H U x M r-i M aD ri m l, O 00 ~
~ b r-I H H ri
Q) N
~ -
41 -,w~
N -i
73 ~ l- rl ~ %O [- 01 m N O r~ lfl 01
f34 N ~ rl = rl = = = N 00 10
m r-i r-i OD ri 01
O
O
0 o O [, OD ~ rl m O
O 01 ~ ~ = . l0 ~ Ol w
Vi H * N M N H w l0 ~ lfl '0 H ~N H
r-1
O
rI H
r-I
O 00 m M
H 01 O aD v1
04 N O M 0~ ~ O c+1 l- N r-I 0) Ln Ol N
. ~ ~ H M
p ~-l a)
OO 41 O ~
V* = H H 0 >+
U
N 04 o 04
N ro
O r O M O O 0 0 ~ 0 ~
1-1 cW O O O O O O O
~ r-I ri r-1 r-I ~ r-I O
0
'd A u~) ~
.rH S4
ro ~~ H m ~ O N m m W 00 0 Q1
O lO 00 O N N N N N O 01 0
~ N 1p lp N H H M M M M 10 kO [-
U p, ~ ~4
a~ o C
14
r3 cn
v H H N N N N N N N N N N
~ 4-1 pq W PO LQ LQ PQ CO m PQ LQ Gq CQ
o w a a a a aa a a a a a a
U ~ N a
~ E C O o ` 0 rn 0 rn 0 O1 o O O ~
q a
~4
2: 0
0
O~. ~ W G4 H L~ E'~ CG4 ~ ~
i
~-l ~ m u ~ ~ ~ w a w a 0.
x (L) x f~ U ~ i C0x4 f~ CJ GA a z w
N ~ ~ E-+ f:+ U] tA fz, F a H rt
Lr) 0 M 44 G*4 M C7 > M
~
0 (L)
=ri > O rl 10 %0 M OD L(1 m 10 lfl l, m 'b
U1 1M u1 1! u1 ln ul ul ~
U 11 ~
Q) =r-I -41 l [- %o [- %0 l- %O [- ~0 l- ll~ l~
ri N [l4 0 0 O O 0 0 0 O 0 0 0 0
E~ c ) n a, a ~ W W ~ W W W W W W W W y
m
U
-rl
b
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w
M 0
A C O
ro U vi N M N N M N N N N M N N N N N M N N M
A o
H
d z
0 M
Al N O N t--~
b 0 zv r-i N N 1 I I ~--~ -0- I I 1 O N ~ CO
0 ~ ~ r- Ln 1 I 1 M M I I I O r 0 m
a0 H U1 r-i N d' 00
M
r-i ri
H
U)
C
0 N 01 M lll
Gl 0 1 1-1 1 0 lzr lfl H p m H H O N 1 O
~ 4-4 N 1 10 1 l- kO 0) lll I, 01 lfl %O L- 'õi 1 ~
U -,q * r-I N O r-I Co l, O O lll p H
~ > 4 RC ~ 1-4 H
W
a
N
4D JJ 0 m 01 lfl lf) 01 o) 10 r-i Co CO lf1 rl l!1 01 ~O
N w dl = d, H =[O H N 01 = N = O = = r M =
* N M H dl l0 dl r-1 N N N lfl M M M M N H N Ol
N
~ H -1
ln c0 dl O dl M 01 m
N ~0
~ p W M N N H ~ N m , p m r-i l- H M N
~ t'N r-i lf1 r-i m lf) rl M O CO M N M rl rl rl H ri
J1 NI 0 <
~ o P.
04 N N U
s4 (0
IT O O l- f0 CO Co Co W m m m N N O N N 0 [- M
N a) rr-I -i y oW O aD d' m M M m U1 01 01 m m (n 0 m m 0 10 m
U U)
r
0
`~ A ,. ~ U
ro b N N dl O rH 'o d' l0 10 10 ~ l!1 L- O 10 l, O N w c0 W
I O m d~ ~O 10 f'~1 L!1 m r-1 rl m [O O~ aD a0
~ ~~ U 04 dl V' ~ r1 H H dl dl v M rl N ri N M cN -IV d' w w
U N ~ ~4
CL 4-4 ~p
N N R' R' A'i ~ a a a r.~ '~ '~ ''i '~ '~ '~ '~
v =ri U H
0 E~~ z z z z z z z z a a a a w w a
~ r-i w
U ~ ~n a
rt~
U =.~i ~ N m m 01 01 01 0 0 0 01 01 01 01 Ol Ol Ol O% O O) O)
r-i
A
x 0
ro
z 7=~ 7 z
N 0 a a0 a H H H H m ~ x=!~y H Ha U E`qt
v fC4- A C4 Rw O z 0 V ~xS ~~4 H a 0 U u
x u, v, x a rz H H m Q A a w x z a4 x x
a H a a H ~ a a E-1 H R; a a
10 3 3 ~H r-4 r:. ~ 3 3 ? G1 >+ [v >+ >+ ~+ >+ >+ ~
M w ~7 H ',~ C4 VI Ul Ga .'T'. H Cq P: U] G4 E 0
Ul p Cl o
O N H
=ri 'J v rl M lf1 kO l~ 01 ri O N U1 [- t- 10 l- OD ON O N M N
~ 1J =ri d O O O O O O r-i r-i r-i r-i r-i N M M m M-Izr v -W A
,A N-rl 4-1 VI dl W W W V~ d~ d~ d~ d~ d~ d~ d~ dt d~ d~ d~ d~ d~ ~.
0 v l0 %O IO 10 10 w lD w w w lD w w w 10 w w w 10 'rI
H u~ c~, ~ ~ ~ ~ ~=-i ~ ~ ,-=~ ~ ~ ~ ~ ~ ,1 ~ ~ ~ ~ ,-i ~
w
w
ro
m
a
.,
ro
a
.,
N
41
Ll
m
U
-./
b
F'.
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
W
a)
"i
O
~ ~ ~ O
U r-1 :~ V
l l!1 H N M rl H N U1 N r=I r-i
N A N
z H
~
co Ln
N cM d, Lfl 01 N O [- 0,
t!1 M ri . Ul d~ ~ ~ lf1 01 N '4 M
.~.CQ r-I ri
L".
O 0
lfl x M O N Lf1 %D r=1 O O I ~
Vi lll 144 r=1 0)
r-i OD l-
b ~ r-I ri
r~ Q)
Ln 4-4~ ,..~ M
~4 O O l- N OD : N O a0 L(1
0 rI 00 M M O
=K CL ul kO N
0 I ~ ri M N =
*co r r1 r rn
~ H
~4 N ~
O N O 00 m 01 O I O i M L- I
Ln M I r t!1 i
Q U * '-i %D r=I M
O r-I l0 u1 l
M H H
r, ~ H co
U) QQ
W* N
i^-+-1 V l- kO 00 lo M O 00 l- OD
~ 0 r1 N N d~ N N r-I r-~ r-i
co
.r-i ~ N
C> 04
=
ro
00 00 00 00 00 W M M O 0 0
M M M M M M Ol O~ L11 H H
rI
fn W
`o 04 o 0
=r-I 4) ~
ro 04 L-
r 0 OD 0 00 0 M l, N a0 lw Ol l-
,r! 0 rl O ~0 L- O N l- H N k0
'~y C ~ a M ri rl ~-I M d~ r-i d' O M 10
U ,-a CU ~
r-i C
N
(0 N ri ri r-I
rl L ~ `l~ Z z ~T+ z a a
~4
U
U ~ c a
rn~ -1 0) ~ m r-i m rn
~ 0
0
0
~ O M N .
H 44 C~ll Z ~" aN
N ccv ~ a z uai rW*~ Z w E+ a cHi~ A
(1' ~ p ~ ~
x A ch `~ w r vai y+ ~ u a rw ~ w~ ~
ni a a a a a a a a a (11
a
44
H ro
~ o,~ a~C ~C U) a x a a> (Y w
.,~
~ 1-) ~-I r-1 N M W Lf1 kO l- 01 O d~ O 0)
~
O O O O O O O O H H N .,={
N
0 w w w w to 10 w w w w w A
E-i CO U H H H H H H H H H H H
~
a~
4J
b
U
b
G
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
rn
a~
(U 'b
'"'I :j V I H N N N r-I -~V N M N r-I
it A
. ~
Z
0
o M l0 oo l- ` uNi =
m U N d' l11 H Lfl
N N
p l0 N r-1 H l0 01 N O
~ Or=~ .,~ O) Ol N %O M O O r N
N 00 . r m L~l 114
rA .~ ~ lfl ~ H m ~
^ O
,-I 0 '-{ Ln
4-) r-i N r-i ON m w Ln
O U ~ N ~ N ~ ~ I ~ 1
OD w Ol
O 4)
LO ~4
V l H --1 lll
(12 RS O lll [- m 0 N 01 O m I ao
N N w [- L(1 Ln N rn " Lf) i H
04 O N M ~-i M 0 ri ; 00 H
O M
=k r1 ~
4--I
_ Q-I c~. ~
lf) ~ 0 rl t c+1 m O O O O `~ O
N ~... k0 01 01 Lfl ~- ri ~ r-I tD r-I
rn
'o o 0 ~
- r-I k
rc5 (!1 -44 O M O O O lfl 0 I- m
0 O 'IV [, O ul m d4 01 m N
rl O1-- (1) LL r-I N N lfl r-1 lf1
41 ro ~4
U
bl t d =
a a a~' ~' ~~+ N N
o z z z z a a a a a a
r I r, E C,,
U
U
0 0
y ^ O a rn m rn r-i rn rn m r+ rn rn
r=1
.,~
o
>1
0
~ O H a W 0 Z 1.1 ~ o
a~
a Z a ~ P w a N
N 4 F P~G CU7 7 H A
x ~^ 1 a a a En u u H ca a~
A r~ w z ug x x a 'j) ri) w
~ N E+ CA U] H U] CIl 2: > H E-4 ==i
~'L~ m U pC `~C H > >+ 0 ~-7 ~G C7
Ll1 O r~ w
-~ I td ~y
N W Lfl [- 10 01 r-i l- o -1'
r-i U^ ~ ri ri rl rl N N M (n V~ V' Ql
0 \ 0 M M M M M M M M ~
T-f r-i 0o 4-)
M 04 C] 10 l0 k0 l0 l0 lD 10 l0 l0 10 0
~ Ul Al a H ri ri r-i r-i r-i ri, rl r-i r-i H -'i
Q
y
N
J-1
~
O
rl
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
R
~ b O
rI
yI m Ln d' d~ l(1 d' d' d' l!1 U1 Ul L(1 lfl d'
N f0 0
o
o N z
o
N
U) N kO M
V I 0~ 0 H O N d~ r ~-1 r-I O~ O~ 00 N N
00 OD N v V r r r-I r r '-I ~
N U) M = H N r e-i O
N H
H M lfl --1 00 M M N ~--I r-I r-I
U }4
ri %D
H N (1) Lfl r N rl O~ d' N ~ 0 ~ O r M O ~(1 r
U N 0 N N M rl ~O a' 10 M ~ r 0 lfl M M
N
=.~
}4 W
O N CL O Oo M 0~ N r r QO N N N N
d' O O . . N . ~ r
p
O O r-I N N M r-i V' V' p M N N d' ~O N N
b H N
4-4 C'" N M m M OD Ln U1 OD 01 00 U1 kO 01 O r
~ . . . . . . . . .
~_ N ;r~r-I ('~l M N (~'1 l0 M M O1 M Ul M
O=~1
a-=l G ~-1
= r'~ N r-1 M V' O% N Ul 0~ ~ M lll CD N d' l!1 d' lll
pp
04 O 0 rl L(1 l.fl M lfl OD r '-i M N N tfl d' -W M
v N \
04
N
O V' V' O O d' r r-i O m l0 ~0 l0 O O O
r~ =~ N L(1 O) 0 0 O) 00 M Ul 01 OD OD M O O O ko
o
`o U
rl > N O r Lll CO M M M
U 41 =H 0 M m m ~to Ln rn 0 v rw ~-+ m
¾+ N a M lfl lfl M rl N r-I r-I r-I d' r=I
.,~
04a
0
r-i
U ~ z z z z z z a a a
E ~ a
~ G
a~ v ~ rn rn rn m rn m m rn rn rn rn rn m
o
N
nI
v
a0 (1) U~ H a w a a uy w>~.S+ a a > a ~
> [-4 44 P E-4 0 :4
v }=I H W u W ~-9
T3 ~4 > 44 u rZ4 4 ~ a W H ~
r-4 >~ H a U~ U w a A o
N N cn C7 H W 0 Cc C7 H 9 P: p U~ r3 (!l V1 U
tl1 .ra 0 a Q a H a a a a a wCY N
U U' 44 ~l 0 H Ul U CL CN 44 2: CL A
0 v v ~
,-I =r+ U ~ -w ~ N M in ~r ui tD mr-i w N M rtD r o
LJ ( Q M O O O M O O O O r1 M rI r1 M r1 N M =~
U N C
(Ti N 0~ O; 01 0) 0~ o~ rn O~ 0~ 01 O~ O~ O~ O~ o~ o~ m =`~
~ 'a W0000000000000000 44 m (1) W o w w w w w w w w w w w w w w w w '
C1~ N rl ri ri r1 ri rl rl rl .-1 ~-i ri ri ri rl ri rl rl ro
rn
~
.~,
rd
z
.~
A
N
N
i.t
ro
U
.~
T5
R
.~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
w
0 0 ai ``> ~n r o 0 0 0~
o ~, r~ co co co rn rn co -
z= 76 vj
~
m O O M O O) ~ aO 0 1 tA U) 0 M M N p~ O (O ~ N) N CO r 0~0
Q p N (^O M~ N h aO N M - (O M(O c,.~ N t0 LO
V
C~ O ~ 00 000 Opi ~ M co Op) O o V' N ap0 M~ an0 ~ O ~ p M N M
Q p M ~ 0) M N M V' ~ (D N M
m O t- I- N pp _O ~ N sh 00 O~ O N M aO O~ I- f- O N I-
t[) N N V' 00 I- O) O) (`') U7 O 00 O M M - N M N ln 00 I~ GO N p N M N .- O ~
N tA I~
m N p
~ O O) - tA O O CD p~ a0 0 N M N O~ N_ 0
~ (O (p 00 (~O N tn `~ N ~~~ Opo pMj O N ~ M N I~
m O f- 00 N O O M M~ N N (D a0 O I O co t0 N Q) 0 M 04
M N M aD 00 O a0~ M cM N c0 O I- I- p co
N
0 i
m M N M tA M ~ (O M GOD p M p N 0 0 Q I- G N p ! M O
0 tp M V p o p ip Op0 M O N tn ~ 1n N^~~ N ap N - ^ N
~ -
04
N Q ~ M f- I 0 O O) M I p ~ ~ ~ N ~ GO M M
M tt ~7 O tn ~ M 0~ QO N ~ N N p p~~(~O eM-
~ O O 0
( O v C ~ ~ M~(O U) M N O M N O f~ a0 (O M^ M
~. ^ I~ N O N M N M O O) I- I- O 1- I O 1- M N N M tn N 3 O
O 0 O M M N~ v LO a0 V' =- NU')U) I- 00 M ..-
LO
M m O - 0~0 M O I- O) f~ t[) M M M: O 00 O~A
~ ~1') I l0 M 00 ~ N O M ONO M N 1n M N (O M N
N N a O 0) ~ N(O v N q ~ M tA ~A .- M N tf) M M N t~ It
N Lo MU) N M CO ~ 000 W~ r ~ n m~ ~(00 N aD M O.~- '7 t~D
N p
M N
M
N N _ _ ln
p N ~ ONi ONi M p a0 (OD~1 Q O r~ M M M M O
m
p M I- O M Q (O N - I- N p 0 <,.) <p LA p ~~~
~ o t
~wt
,v m p p M p p M i ~ N ( 0 0 N tC N M p M dD ~l (D CM tC) N ~ N .~-
M N V V a0 V Co M N 00 aO f0 I M ~ I~ ~ N M =- M
~=/~ m p tn O 00 00 Co M N N CO M M tn N N M M sT ~ N
0 Q~ N .- O I- V M p CV (D ~ - M N - N `- M N
4- f- (O 0 cl) N 0 M
0 (D Ce) p ) 0 0 C r O I~ O) C") 0 0 0 f~ ~( O N O- U) p - U) ~ ~ NW N M 0
(D aO (~
iA L (O ~"' ~"' N N N M N N N M ; N N N N
Y/ N Q d d a. n: a a C L a a ( n ( n u ) ( / ) f n u ) ( n Q¾ Q Q¾ Q
0 2 2 2 2 2 z z z z z z z z z z z z z z z z z a a a a (L a
U) 7 3 7 7 7 7 7 7 7 7 7 7 7 7 7 7 7 3 7 7 7 7 7 7 7 7 7 7 7
U) E FL E: a: FL FL u CE rL UL a: FL FL FL CL EL 6: ~ E FL FL FL 6=
W 0 M M M M'7 N7 ~ ~ ~(O 0 (~O 0 (00 ~ IZ ~ aD ONO ~ OpD 0~0 OOD ~~ N N O O
z
~
W 0
>
W Y U~~ W~ fn > LL w5a0~ W d X> d W~ z o LLJ d Q g=~ Q Y 0 0 YW > 2>~ J> z- J
z ~
~ O = 0 > 0 w vOi w w0 _= cn aa W Y~¾ w
< a ~ ~ w a Q ?
m m - W Z w ~ Z V J w ~
Q (7 } O ~
(j _ o¾> c1 ~ w ~ , c¾i~ > g ~ a ~ w > u} Tw> D~ > a! iy W -p
W fn 1- Q(~J 0 Q W Y C7 W LL Y a _
U) Y ~ z Q N ~ C'1 > w Y ¾ <n W ~ N ~ Wi ~ w E W ~ > W
~ ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
,no
O O S2 oo N - ~! sN- p~ p p) 00 O) CO 00 00 0D O 00
Z vj
U
m 0 CO 0 O pp O 00 O O N N (p
Q p cD r~i c o ~ rM) ^ v c V ~ C'J N`~ n v v
m O O o M M V f- CD ('N~) fN0 0 ~ ~ co O O N
Q 0 O tp cvj .-- M M N M~ LA O c'rM
m O (M M No 00 O O ~ N N 00 OIT .- N N
Q' ~ a0 Nr,: I- cJ (O N M v N pp p) O- ~ N N O
~ i
N
Q.' M q O
0~) a 0 O) r r O ~ M e
0 i O t0 If 0) I- (p CV ~ N o0
lC) ln O 1-
O
~ N N M O N t~j M ~
N O N M CO
~ ~ OD M O I- O CD O 1- O
00 (O (n0 O a0 00 00 N~ ~ M t~ n N M
LO
04 N Q ~ N tn (p tn N I- O~ ~~~ c,Oj p CM I I (00 O M~(+M')
o p 0 CO N I- N (D N N M U) LO 00
.N o m 00 LO O O~ O tA M N ~~ M ^ ~(o 1~ p) 00 O
M (O 0000 O~ CO O N M N c"~ j N ('7
~
~- tn 0 N O 0 Ict O co Lf) I~ f- O
LC) ~.j N N O ~ V 00
m~ O tn N O I- O I O M N N ~.- I M Nu 00
N N Q' N M V 00 00 (O V N O U-) V' ~A O e- O 00
0 'q 0 M - O~ M ~ (D O Oo N ~ M~
~ Q O (O OD N 00 - - 00 .-
~ m O M N 0 O v M m M 1- O M M N h 0 0 O M
Q p CO C`> v p) O a0 v M
t~ O M
n CN rNi
o I I rn I rn v u~ ' M I N~ m o N
p v co rn Ln vtn rn
!n rn M
~lj ~ 1~ ~ M pj ~ 0 M C'j M (D OV LA M N LO
V ~
~ O_f ~ N(O N(4 ~ _ O N O~ O _(O I~ ~_A OD M
O N N N M M ~ Q) V' O N O) t~ I~ M ~ M
` ~ 00 N M M N) M(~') CM ~ ln 0 ln co
.~ ~ Q m m m m m[fl C~ m m m m m m C~ m C~ m m m m m m
o a a a a a acL n. a a a a a a a a n. a a a a a a
~ (~ > > > > > > > > > > > > > > > > > > > > >
W C~ O O ~t O N M LO co r- co 0 M I~ tA OD M 00 N fD 1~
L W Z O N N N N N N N N M M M V V' U7 ~A CO (O CO
~ - - - - - - .-- - - - - -
LL (~J cn O
= U Y~ O~~ ~ C7 I~i U 0 O1 W~ Z~ p
Y W d Z _j - w J Z > J >
~ C7 ¾ a LL Z W
~ z Q Z Z JQ J z~ Z~ a CJ (n Z c:
~ Z Q z w w z Z Z z~ Q ai o Z rn
J > Y } 2 2 J 2 > ~ CJ C
~ a - r g - 0- m
L- x cn 0 Q z ¾ aU) Q
~ .~
~ ~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
C , ~ c
,n 0
O 0 ~ oo 00 M M tn 00 N- O 0 O) 00 t~ O
p~_ - - - - - -
Z
-
0
N U) m O O~ (8) O~ a0 O 1 0 0 ~~~ Q) 0 c~) N 7
~ Q N 64 (~O M^ N f~ 00 N M~ CO M N t0
M f~ ~~ co 0 M~ N M~ ~N
N N
N
C
co O h I~ N pp O_ N~ - 00 O N C) O O ~) 0 h O I-
~ ~ ~ CN') ~ N M~ ~~ 000 Nc6 N O M N 00 M 1- IT
N tf) ~
~ N
C m O O i O - Lr) O O N(D 0 N OV N ~
(O 00 cF O M 0 O - N
I- O ~ `- CO N~ U) a0 M M"t N
~ 0 m O ~ 00 ~ N O M~ M N N~ OO O O ~ M
p. ~ p+ M N M~ 00 aO ~ M O h O 00 ~
N
'd m O N M tn M co M
O N 0~0 ~ N M
p ~ N M N O) V CO M N N O(D m N O N
~ ~
U ~ m O a0 N O N v , fO N I- M
M ~ i ~ v 0 0 0 c~N) ~ a~0 N (nD 0~) OM
Q" O ~ (O
= O p ~
M ~ M
a0 ~ M~ 0 0 M N 0 N , 0 I-
O~) QN) N ~N ~ a00 0 ~ N~ ~ N a~0 N M~~
~ ~ p O 00 M M M O a0 CY) ~ m ~ t0 ln i ~(O 0 0 ~ N M M M p 0) lA ~ ~
O
0 ~ (D
~ C p (7) N M M
0
W)
N E
m O O~ (~D q OD M~ ~t M~ N
E Q 0 N M M ~ N t0 pp O M N
O
U
M N N N O N~~ M~
~ Q 0 ~ ~N ~~ t!) O M m O M,'V N~ N O t~
~
y.+
~- m p 0 M a0 O cri ~ N o0 a0 h~ M t j
N , tM V
o O ( CM V a 0 M N 0 0 ~ - c0 M I- ~ M
L(~ ~ D
f0 m~ o O 00 f- 1* W M N N N M O N N M~ tA h
C Q O N tn .-- v M 00 N C`~ M N <, )
CC
VIP C p 0 O CO M N
a) E m CO (") U') Q) 00 00 Ufl tf) (O ~ - 0 N~ ~
(O Cn
p_ 0 0 c~) I- O~- 0 C ) 0 ~--~ a0 0 N 0 - ~- - - -
N N MV M(O M14: N N cF
O C ~ ~ Q a a a o_ a n Q. am w m m m Q Q Q Q
2 2 2 2 Z Z Z Z Z Z Z Z Z Z Z Z Z Z a a a a
Q (A > > > zi > > > > > > > > > > > > > > =i > > > >
U) v~
(~ p
(U ~ 0 0
~ Q Y ~ } W
U ¾ (n fn 0 p W 0! Z d d~ f_ p> O
W
U Y a ~ J W J LL x f/) J > Y J d
~ ~ ~ ~ < J ~ Q Y U~ > a ZQ Z ~
0 O
J a = Y a w =-
0 W W
C 0 > Q ~ U U Q a a Q (1)
V -a (A >w W w ~ aw a W gw~ C1 W a w p w~ w ~
N N ~ w ~ L}i li F- W 2 U N
> ~ C CO 2 d~~W Z C~ J V) C~ CO U U J~ ~ a Q U
C_
p Y Z Q U ) } ~ OJ > p Y Q W W c n -j W
Q 0 41) n
~
oo Q O ~ O O O(MD O O O O V~ N Cv) O O O O0 O ~ - O
~ i0
C U
c ui 6 ~ O O O O O O N N a0 ~.-- N.-. N
0 0 co O~ CO ~ 0 0 ~ tn M t1') ~ l0 (O O l0 (O O ~
~ T ~U/~ O a CO (O f0 Cfl CD 0 C0 C0 CO CD (O t0 (O (O (0 O (0 (0 O UO UO O C
(p + v J ~ ~ ~ - a- N - - --- -- e- .- a-- =- - N - - N
~
CA 02658559 2009-01-20
WO 2008/039267 PCT/US2007/016529
00 O o
p N~ - e- - - O O co
Z = m
LO - O N
Lr)Lr) M N tO
[p O i t O h cp M O) ~~ Q)
M LO O
m O OLO Lo oD (O I- -7 O N
NN~ N N
0
m O O o00 1~ O p~ 0 V O op
Q~ m aO d p~ N N N op
m O M Q~ a00 M M O~`_ 00 N
~ 0# Nv N~ ~ I~ M M I-
m O O m cM 00 ; Ncl)
I= 0) 04 tp N~ Lc) ~ N
=
`
N CY)
L0
0 cl ~ =- lo N V v U)
~
M
O vO C~~) ~ 0~0 (00 v O N
~o ~O ap M ~
U m p LO N O 1- N O T
(O O 04
~= 04 U) 04 I- U) -
~
LO
O 04 op
m OCO~ O
m O N. NM I- 0 1, LO M Q)
Cp MV V hV V
m O tOC) p p) ~ N N
~ O
w M M ~ O) LM f) - v
m O N CO ~ f~ ^ CO ^ O M
0! N I- t~ N V Mcli LO
=
O) NLf) It a0 V
COO 00 N ct tCr:)) CM V CT LUB)
- N N N N
CD 00 CO m m 00 m m CO
0 a. a a (L a a a a a
V)
Z o
U) U)0 U' 0
~ H C7 = w U) > J Y cs
(n ln lL > A
Z} a Z-i f_n Z Y w
C
V~ H Q L L LLl
U J u- Z Z J U Y Z (0
Q d
lL 2 > W rn
W ( L f}/) L L C) pp ~ t[) a0 OLC') (O 11- 00 m v O)
LO +r Q O O 7 7 .- -: N~- N
Q"
U~ O O O O O O O C~ O (0 O
~ a 0 O O O 0 O O tD (0 O
f0 N N N N N N N - - N
~