Note: Descriptions are shown in the official language in which they were submitted.
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
1
LEAD SLAG REDUCTION
This invention relates to a process for lead slag reduction, utilising top-
submerged lance injection.
Top-submerged lance (TSL) injection was developed in the early
1970's. Since then, the technology has become widely adopted for new start-
up plants for processing a range of non-ferrous, ferrous and waste materials.
The TSL technology utilises a bath smelting system in which a vertically
suspended lance has its lower tip submerged in molten slag layer of the bath
contained in a suitable furnace. Process gases, such as air or air and oxygen,
and fuel are injected through the lance into the slag. The fuel is combusted
at
the lance tip to provide heat to the furnace. The injection generates
turbulence
in, and splashing of, the slag such that the slag is thoroughly mixed.
Controlled swirling of the process gas as it flows through the lance cools the
lance outer surface sufficiently to solidify slag splashed onto the lance and
form a slag layer which protects the lance in the highly aggressive furnace
environment.
The TSL bath smelting system utilises reactions between sulphidic,
oxidic and/or metallic components in the slag or provided as feed to the bath,
and contained oxygen and ferric oxide in the bath. Critical
process
phenomena including feed material dissolution, energy transfer, reaction and
primary combustion, all take place in the slag layer. The intense agitation of
the slag resulting from the submerged injection through the lance ensures that
reactions occur rapidly and residence times are low.
The degree of process oxidation and reduction is able to be controlled
by adjusting the ratio of fuel to oxygen supplied to and through the lance,
and
by the proportion of reductant to feed. The furnace is able to be operated
through a range of conditions from strongly oxidising to strongly reducing
conditions.
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
2
TSL technology has been used to smelt lead concentrates and
secondary feeds at a temperature of from about 950 C to 1200 C, preferably
in the range of 1000 C to 1100 C, to directly produce lead bullion and a lead
rich slag, such as a slag having from 30 to 50 wt% lead. The lead bullion,
such as with about 98 wt% lead, is tapped periodically from the furnace during
smelting, for transfer to a lead refinery. The slag remaining is subjected to
a
lead reduction operation, to recover further lead and produce a discardable
low
lead slag. The smelting also produces a lead fume product which is returned
to a subsequent smelting cycle to maximise recovery of lead to bullion.
In some instances, the high lead slag produced by such use of the TSL
technology has been sent to a blast furnace for reduction and further recovery
of lead bullion. The slag from the blast furnace then has been sent to a slag
fumer for recovery of zinc as fume. In other instances, the high lead slag has
been subjected to reduction and fuming stages by further application of TSL
technology.
The TSL processing of the high lead slag resulting from smelting has
been conducted as second and third stages of reduction and fuming,
respectively, using the same reactor as used for the first stage of smelting,
in a
three stage batch process. In an alternative, the high lead slag has been
tapped periodically from a continuous TSL smelting furnace, and transferred to
a second furnace in which the slag is subjected to a reduction stage and,
optionally, to a fuming stage. In each case, the reduction stage is operated
with a lower oxygen potential and a higher temperature, such as about
1250 C, than used in the smelting stage. Lead is recovered from the slag
during the reduction stage as bullion which is tapped for downstream refining.
A lead fume product from the reduction stage is recycled to the smelting stage
to maximise recovery of lead to bullion. A resultant low lead slag, such as
with
about 5% lead, can be tapped and processed separately, or it can undergo a
TSL fuming step to clean the slag and maximise metal recovery.
The TSL slag fuming stage uses a more strongly reducing furnace
environment than the reduction stage. The intense bath turbulence and gas
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
3
dynamics of the TSL operation are used to remove much of the remaining lead
and zinc as fume, leaving a slag suitable for discard, such as with about 0.5
wt% lead and about 3 wt% zinc. When lead concentrates have been smelted,
the slag from the reduction stage will usually contain significant levels of
zinc,
in which case the fume product from the fuming stage is high in zinc oxide,
such as from about 50 to 70 wt% zinc. This fume is not suitable for recycling
to the smelting stage, but needs to be removed from the system for separate
processing to recover the zinc and lead.
The present invention provides an improved process for the reduction of
a lead containing slag. The slag may be that resulting from the TSL smelting
of a lead source material, such as at least one of lead concentrate, secondary
lead sources including lead acid batteries, and recycled lead containing
metal.
However, the invention also can be applied to a suitable lead containing slag
from another source, such as another bath smelting process. Also, while the
lead content of slag from TSL smelting of a lead concentrate may be in the
range of 40 to 50 wt%, slag with a lower or higher lead content can be used in
the process of the present invention. Indeed, at the lower end, the lead
content need only be at a level sufficient to warrant reducing the slag to
achieve a level of about 5 wt% lead in the slag.
Traditional processing of lead via pyrometallurgical routes has involved
the reduction of high lead slags by carbonaceous materials, using a range of
apparatus types. Lead blast furnaces typically utilise relatively expensive
coke
as carbonaceous reductant. Modern bath smelting technologies, such as TSL
technology, employ coal, a cheaper carbonaceous reductant. We have found
that unexpected benefits are able to be obtained from use of an alternative
reductant, instead of coal or other carbonaceous reductant, in slag reduction
using TSL technology.
According to the present invention, there is provided a process for lead
slag reduction, wherein the slag is subjected to top-submerged lance injection
of oxygen containing gas and fuel, via a vertically suspended lance having its
lower tip end submerged in the slag, to generate a combustion zone in the slag
CA 02658674 2009-10-05
4
in which fuel is combusted and to generate turbulent conditions forming a slag
coating on the lower extent of the lance which is solidified by the cooling
effect
of the injected gas. The TSL injection is conducted under reducing conditions,
using a sulphidic material as a reductant, to reduce lead in the slag to
metallic
lead and thereby form a molten lead phase which is tapped as lead bullion.
The sulphidic material may be any sulphide containing material which is
compatible with the process of lead recovery by lead slag reduction. The
sulphidic material may be a bulk concentrate, such as a bulk lead/zinc/silver
concentrate, a lead or zinc concentrate, sulphidic drosses, pyrites, or a
mixture
of two or more such materials. The sulphidic material preferably is a lead
concentrate, such as that smelted in generating the slag to be subjected to
the
lead slag reduction of the invention.
The process of the present invention obviates the need for use of
carbonaceous reductant. While a minor proportion of carbonaceous reductant
can be tolerated, this is not desirable as it partially reduces the benefits
obtainable with use of sulphidic material alone as reductant. Also, some
concentrates contain a proportion of carbonaceous material, such as graphite,
and an increase in that proportion is not desirable.
In obviating the need for carbonaceous reductant, the use of sulphidic
material as reductant reduces greenhouse gas emissions. That is, generation
of CO and CO2 is substantially avoided, although some small quantity of CO2
may result to the extent that the sulphidic material contains oxidation
product
such as lead carbonate and graphitic materials.
As indicated, the sulphidic material obviates the need for use of
carbonaceous material, and even minor proportions of carbonaceous
material are not desirable. However, it will be appreciated that
carbonaceous material can be present in combination with the sulphidic
material if the resultant carbon dioxide can be tolerated, such as if
facilities
are provided for its capture.
CA 02658674 2009-10-05
4a
The use of sulphidic material as reductant of course results in
generation of S02. However, as will be appreciated, the S02 content of
furnace off-gases are able to be recovered and utilised, such as in the
production of sulphuric acid.
The present invention, as illustrated later herein, also has further
practical benefits. The use of a sulphidic material reductant surprisingly
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
enables a better separation of lead and zinc, to give more efficient
processing.
Also, the sulphidic material reductant results in higher productivity of lead
per
smelt/reduction cycle where the sulphidic material contains lead.
5 Particularly
with the use of lead sulphide concentrate as the sulphidic
material reductant, the lead slag reduction process of the present invention
is
well suited for use as a second stage following a TSL lead concentrate first
stage smelting of a lead containing feed material. Such a second stage may
be conducted in a separate furnace rather than the same furnace used for the
first stage smelting. However, there are particular benefits in conducting the
two stages (and optionally a third stage of slag fuming) in the one furnace.
Thus, on achieving a sufficient volume of slag produced in the first stage
smelting, it is necessary only to:
(i) continue rather
than stop the feeding of lead sulphide concentrate
following termination of the first stage; and
(ii) reduce the oxygen content of the injected oxygen-containing gas
from the level used in the first stage to the level suitable for the lower
oxygen potential required in the second stage.
In the first stage (whether in the same or a different furnace used for the
second stage), oxygen is used as the driving force to convert lead sulphide
into lead metal through the direct reaction:
PbS + 02(g) ¨> Pb(l) + S02(g) (1)
with the competing reactions:
PbS + ¨3 02(g) ¨> Pb0(l) + S02(g) (2)
2
PbS ¨> PbS(g) (3)
Pb0(l)¨> Pb0(g) (4)
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
6
Pb(l) ¨> Pb(g) (5)
In this Pb-S-0 system, the lead can report to any of the metal, slag or fume
phases.
In the second, slag reduction stage, lead oxide in the slag is reduced by
sulphidic material reductant. The following equations, for use of lead
concentrate as the sulphidic material reductant, describe the reactions
involved at 1200 C:
2Pb0(siag)+ PbS ¨> 3Pb(l) + S02(g) K=26.7 (6)
2Pb0(siag)+ PbS ¨> 3PID(g)+ SO2(g) K=1.2 x 10-6 (7)
2ZnO(siag)+ PbS ¨> Pb(l) + 2Zn(l) + S02(g) K=3.4 x 10-7 (8)
2ZnO(siag)+ PbS ¨> Pb(l) + 2Zn(g) + S02(g)K=4.5 x 10-6 (9)
From equations (6) to (9) it can be determined that, by using lead sulphide
such as lead sulphide concentrate to carry out the reduction, the equilibria
favour the deportment of lead to the molten bullion phase and zinc to the slag
as zinc oxide. This enhances the separation between the lead and zinc so that
lead can be removed during the reduction stage, maximising the retention of
zinc in the slag for subsequent fuming. We have found that a key to this slag
reduction stage is the degree to which lead can be reduced from the slag by
lead sulphide before sulphur accumulates in the bullion. We have found that
lead levels in slag down to about 5% lead are achievable before the level of
sulphur dioxide in the furnace offgas begins to diminish, indicating that
reaction
(6) was substantially reduced or no longer is effective.
In order that the invention may more readily be understood, reference now is
directed to the accompanying drawings, in which:
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
7
Figure 1 is a perspective view, partly cut away, illustrating a TSL furnace
suitable for use in the present invention;
Figure 2 shows a flowsheet for a prior art three stage process for TSL
recovery
of lead from lead sulphide concentrates;
Figure 3 shows a flow chart for a three stage process according to the present
invention for TSL recovery of lead from lead sulphide concentrates;
Figure 4 is a schematic flowsheet for a multi-furnace process corresponding to
the process of Figure 3;
Figure 5 shows an alternative flowsheet to that of Figure 4.
Figure 6 shows a theoretical distribution of lead between slag, metal and fume
under equilibrium conditions for the first stage of a process as illustrated
by
each of Figures 2 to 5; and
Figure 7 illustrates a control chart for lead and zinc for a typical operation
in a
process according to the invention corresponding to the flow chart of Figure
3.
Figure 1 shows a TSL furnace 10 suitable for use in the present invention but
which is shown partly cut-away to reveal its interior. The furnace 10 has a
cylindrical lower portion 12 for containing a molten bath 14 comprising slag,
or
having an upper layer of slag. Extending from the upper extent of lower
portion 12, the furnace 10 has an asymmetrical, frusto-conical roof portion 16
and, above portion 16, an off-take flue 18. The portions 12 and 16 of furnace
10 typically have an outer shell 20 of steel which is lined with suitable
refractory 22.
A vertically suspended lance 24 extends to the furnace 10, close to the axis
of
portion 12. The lance 24 passes through the roof portion 16 and is able to be
raised or lowered by a carriage (not shown) to which the upper end of lance 24
is connected. The carriage is moveable vertically on a guide structure (also
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
8
not shown). By means of lance 24, an oxygen-containing gas and a suitable
fuel is able to be injected into the bath 14. Also, feed materials are able to
be
charged to the furnace 10, to fall into the bath 14, via inlet port 26. Means
(not
shown) are provided for substantially sealing around the opening in furnace
portion 16 through which lance 24 passes, and at port 26. Also, furnace 10 is
kept below atmospheric pressure to prevent gases from exiting from the
furnace 10 other than via flue 18.
The lance 24 in the arrangement illustrated comprises a concentric
arrangement of an outer tube 27, an intermediate tube 28 and an inner tube
29. The outer tube 27 terminates a substantial distance above the lower, tip
end of lance 24 and above the bath 14. The tubes 28 and 29 are substantially
the same length. The process gas which provides cooling for the outer surface
of intermediate tube 28 is able to be supplied via a conduit 30 to the annular
space between tubes 27 and 28. An oxygen containing gas is able to be
supplied via a conduit 31 to the annular space between tubes 28 and 29. Fuel
is able to be supplied via a conduit 32 to the bore of tube 29. While not
shown,
a swirler device is provided in the space between tubes 28 and 29 adjacent to
the lower tip end of lance 24, to impart a helical flow to the oxygen-
containing
gas and thereby enhance mixing of that gas and the fuel at the tip of lance
24.
The fuel may be entrained in a carrier gas if it is a solid such as fine
particulate
coal. However, the fuel also may be a suitable hydrocarbon gas or liquid.
On start-up with furnace 10, the lance 24 is lowered to a position in which
its
lower tip end is above the initially quiescent bath 14. With the supply of
oxygen-containing gas via conduit 31 and fuel via conduit 32, the lance 24 is
fired by igniting the resultant mixture of oxygen-containing gas and fuel
issuing
from the lower, tip end of the lance. The materials supplied through the lance
for this combustion of the fuel are supplied at a high velocity which results
in
generation of a very strong jet and splashing of the slag of bath 14. The
external surface of tube 28 below the lower end of tube 27 becomes covered
with molten slag which is solidified to form a protective coating 34 (see
enlarged insert A) by cooling of tube 28 by the oxygen-containing gas. If not
previously commenced, a flow of the cooling gas via conduit 30 is commenced,
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
9
with that gas issuing from the lower end of tube 27 to further cool the tube
28.
The lance 24 then is lowered so that its lower, tip end is submerged in the
slag, to provide ongoing submerged injection and combustion of fuel within the
slag. The top-submerged injection generates substantial turbulence in the slag
such that splashing of the slag continues, and intimate mixing of feed to the
slag is able to be achieved. The furnace then is in a condition enabling a
required pyrometallurgical process to be conducted. In the course of that
process, a cooling gas can be supplied via conduit 30 to the space between
tubes 27 and 28 so as to issue into a gas space 36 above the bath 14. The
cooling gas further assists in cooling of the outer surface of tube 28 and
maintenance of solid slag coating 34. The cooling gas may be a non-oxidising
gas such as nitrogen, or it may be an oxygen-containing gas such as air.
Turning now to Figure 2, the flowsheet shown illustrates a three stage process
for recovery of lead from lead source material by TSL technology. The
process has a SMELT stage, followed by a REDUCTION stage and finally by a
FUMING stage. In the first, SMELT stage, the lead source material such as
lead concentrate is smelted in a TSL furnace, such as a furnace 10 as shown
in Figure 1. In the TSL furnace, the source material and flux material are fed
to
a slag bath and smelted by top-submerged lance injection of an oxygen
containing gas and fuel, to generate turbulence in the slag and combustion of
a mixture of the fuel and oxygen-containing gas.
In the SMELT stage, the lead source material is fed together with lead recycle
streams. These streams include lead-rich fume from the smelting and from the
REDUCTION stage, and lead residues from the separate processing of zinc-
rich fume from the FUMING stage. However, in addition to lead concentrate,
the lead source material may include secondary lead sources including lead
acid batteries and/or recycled lead containing metal. The SMELT stage is
conducted under slightly oxidising conditions, at a temperature in the range
of
950 C to 1200 C, preferably 1000 C to 1100 C which depends on slag
chemistry and on the grade of the source material.
CA 02658674 2009-01-22
Apart from lead-rich fume, the SMELT stage produces lead bullion and a slag
having a high content of lead, as lead oxide. Thus, smelting is conducted in
the presence of slag forming fluxes. The recycle streams help to maximise the
lead content of the feed streams and so increase the amount of lead reporting
5 to the bullion.
The bullion is tapped periodically from the furnace and despatched for
refining.
The lead rich fume is collected and recycled to the SMELT stage. The high
lead slag, with for example approximately 30 to 50 wt% lead, is tapped when
10 the smelting furnace reaches capacity. However, the slag either remains
in the
furnace used for smelting to enable further recovery of lead in the
REDUCTION stage prior to tapping, or it is transferred, preferably while still
in
a molten state, to a second TSL furnace, also such as shown in Figure 1 in
which the REDUCTION stage is conducted.
The REDUCTION stage involves recovery of lead contained in the high lead
slag from the SMELT stage, by top-submerged injection. For this, a reducing
condition is maintained in the furnace by the addition of reductant coal and
control of the lance injection conditions, specifically the oxygen to fuel
ratio.
The conditions result in reduction of lead oxide to metallic lead, to produce
lead bullion, and leave a slag with a lower level of lead, for example about
5%
lead. The lower level of lead in the slag requires that the slag reduction be
carried out at a higher temperature, for example in the range 1200 C to
1250 C, due to the increasing slag liquidus temperature.
The products of the REDUCTION stage are lead bullion, a lead rich fume and
a slag containing for example approximately 5% lead and a substantially
higher level of zinc. The bullion is tapped and despatched for refining. The
lead-rich fume is collected and recycled to the SMELTING stage to maximise
direct recovery of lead to bullion. The high zinc slag, if not tapped from the
furnace, stockpiled and processed separately, is left in the REDUCTION stage
TSL furnace, or transferred to a further TSL or other furnace, for final
recovery
of metal values.
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
11
The FUMING stage recovers the last metal values to oxide and produces a
slag suitable for disposal or for use as, for example, a building material,
slag
cement or road building. To recover the metal values, the process operating
temperature is increased to about 1300 C. The supply of reductant coal is
continued from the previous REDUCTION stage and is added to the slag to
produce a more strongly reducing bath condition. The coal rate is controlled
to
generate an oxygen potential which is sufficiently low, for example about 10-8
atmospheres, to promote fuming of the volatile lead and zinc. These metals
then are oxidised above the bath and the resultant fume is collected in a gas
handling system associated with the furnace. Oxygen for oxidation of the lead
and zinc vapours may be supplied to the furnace by the lance used for top
submerged injection, such as via tube 27 in the case of a lance 24 as shown in
Figure 1. However the oxygen alternatively may be supplied above the bath
by an alternative device.
The process illustrated in Figure 3, in broad overall form, is similar to that
described with reference to Figure 2. Thus, description of Figure 3 will be
limited to matters of difference. A principal and important difference is that
the
REDUCTION stage is conducted in accordance with the present invention.
The REDUCTION stage again requires that a reducing condition is maintained
in the TSL furnace by the addition of reductant and control of lance injection
conditions, specifically the oxygen to fuel ratio. However, rather than use a
carbonaceous reductant such as coal, a sulphidic material, such as detailed
earlier herein but preferably lead sulphide concentrate is charged to the bath
and reduces the lead oxide of the slag by the reactions of equations (6) to
(9)
detailed above.
Thus, if the REDUCTION stage is in the same TSL furnace as used for a
SMELT stage for lead source material comprising lead sulphide concentrate
alone, the feeding of lead sulphide is able to be continued through these two
successive stages. The transition between the stages is not marked by the
cessation of feeding source material and the commencement of feeding of a
different reductant material to the TSL furnace (as with the commencement of
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
12
feeding reductant coal on cessation of feeding lead source material in the
process of Figure 2). Rather, that transition is marked by a continuation of
feeding source material and, without adding carbonaceous reduction, changing
conditions such that, instead of the lead sulphide source material being
oxidised to increase the lead content of the slag as in the SMELT stage, the
source material in the REDUCTION stage reduces lead oxide in the slag to
metallic lead which reports substantially to bullion. The change in conditions
is
achieved by ceasing the addition of oxygen for sulphide smelting reactions as
well as reducing the ratio of oxygen to fuel that is supplied by top-submerged
injection. At the same time the rate of addition of fuel is increased to raise
the
bath temperature from 950 C to 1200 C, preferably in the range 1000 C to
1100 C in the SMELT stage to the range of 1200 C to 1250 C in the
REDUCTION stage.
The REDUCTION stage of Figure 3, relative to that stage of Figure 2, reduces
greenhouse gas emissions by substantial avoidance of added carbonaceous
reductant, such as coal, in the REDUCTION stage and hence by less reliance
in the overall three stage process on carbonaceous reductant. Thus, carbon
dioxide generation in the REDUCTION stage is substantially reduced, and
occurs only to an extent determined by any carbonaceous content of the lead
concentrate and the combustion of fuel. Also, again in the overall process of
Figure 3, better separation of lead and zinc allows more efficient processing,
while less dilution of feeds produces a higher direct recovery of lead.
Additionally, use of lead sulphide as reductant results in the generation of
sulphur dioxide, and sulphur in the offgas can be used to monitor the progress
of the reactions of equations (6) to (9). Also, the lower tenor sulphur
dioxide
offgas produced in the REDUCTION stage according to the invention can be
treated for sulphur dioxide recovery if not required to report directly to an
acid
plant.
It is found that, in the REDUCTION stage of Figure 3, the level of lead in the
slag can be reduced to a suitable level before sulphur accumulates in the
bullion to an unacceptable level. Thus, for example, the level of lead in the
slag can be reduced at least to about 5% before the level of sulphur dioxide
in
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
13
the offgas began to diminish, indicating that the reaction of equation (6) had
ceased to be effective.
With the process of the present invention being used in the reduction stage of
a three stage process, the use of a single TSL furnace for all three stages is
suitable for small annual tonnages. However, because of the level of
recycling, such an operation requires sufficient holding capacity to
incorporate
fume produced during the slag reduction stage and the fume produced in the
slag fume stage.
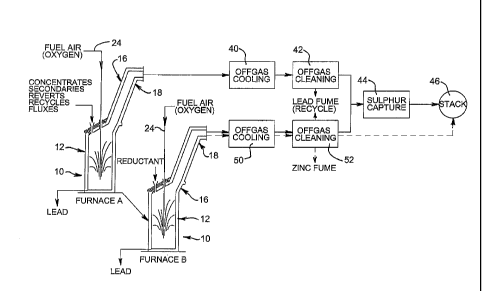
A two furnace operation is suitable for medium size installations. In the
example shown in Figure 4, TSL furnace A is used for the smelt stage, while
TSL furnace B is used for the reduction and fume stages. In this
configuration,
furnace A can be operated in a continuous smelt mode with continual removal
of lead and, once the furnace capacity has been reached, periodic tapping of
slag. The slag tapped from furnace A is transferred to furnace B where the
slag reduction stage is conducted as a batch operation. Once that batch
operation has been completed and the resultant lead bullion has been tapped,
the final slag cleaning of the fuming stage commences as the second batch
operation in furnace B.
In Figure 4, the reference numerals for each of TSL furnaces A and B indicate
the same features as for furnace 10 shown in Figure 1. For furnace B, the
"REDUCTANT" may be lead sulphide concentrate for the first batch operation
of slag reduction for lead recovery, and carbonaceous reductant (preferably
coal) for the second batch operation of slag fuming for zinc recovery.
Continuous procedures are shown by solid lines, while batch process
procedures are shown by broken lines.
Operation during the three stages with the arrangement illustrated by Figure 4
will be understood by the preceding description. However, Figure 4 also
illustrates suitable offgas processing. For furnace A, the offgases pass to a
cooling unit 40 in which heat energy is extracted. From unit 40, the gases
pass to unit 42 for cleaning for removal of particulates, to collect lead rich
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
14
fumes. After unit 42, the gases pass to unit 44 in which sulphur is captured.
Finally, the gases pass to a stack 46 for discharge. For furnace B the
processing for offgases from the slag reduction stage is shown in solid line,
while that for the fuming stage is shown in broken line. In each case, the
offgases are cooled in unit 50 and cleaned in unit 52. Lead rich fume is
recovered from unit 52 during slag reduction by lead sulphide concentrate,
while zinc rich fume is recovered from unit 52 during the slag fume stage. In
the slag reduction stage, the gases from unit 52 pass to unit 44 for sulphur
capture, and then to stack 46. However, in the fuming stage, the gases from
unit 52 pass direct to stack 46 as they are substantially free of sulphur
dioxide.
The lead rich fume recovered from units 42 and 52 is recycled to furnace A.
An alternative two furnace system is that shown in Figure 5. In this example
both the smelt and reduction stages are conducted in Furnace A with Furnace
B utilised for zinc removal in the fuming stage. The overall operation with
the
system of Figure 5 readily will be understood from the description of the
system of Figure 4. Further description therefore will be limited to matters
in
which the system of Figure 5 differs from that of Figure 4.
In Figure 5, the smelting stage and the slag reduction stage are conducted as
successive batch operations in Furnace A. The slag tapped from furnace A at
the end of the slag reduction stage is transferred to furnace B for final slag
cleaning by the fuming stage. In the smelting stage lead sulphide concentrate
(with secondaries, reverts, recycles) and fluxes are charged to furnace A, and
smelted. At the end of the smelting stage, the changes for slag reduction are
adopted. For this, the feeding of lead sulphidic concentrate and fluxes is
continued, while the secondaries, reverts and recycles are discontinued. Also
the oxygen content of the injected gas is adjusted to achieve the lower oxygen
potential required during the slag reduction stage. During each of the
smelting
and slag reduction stages, offgases pass to cooling unit 40 and then to unit
42
for cleaning and removal of particulates, to collect respective lead rich
fumes.
For each of the smelting and reduction stages, the gases then pass to unit 44
for sulphure capture, and then to stack 46. During the slag fume stage, the
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
offgases pass to unit 50 for cooling, then to unit 52 for recovery of zinc
rich
fume, and then to stack 46 without the need for sulphur capture.
The lead rich fume recovered by unit 42 during the smelting stage, as well as
5 the lead rich fume recovered by unit 42 during the slag reduction stage,
is
recycled to a further smelting stage in Furnace A.
For a large-scale installation, a three furnace configuration using three TSL
furnaces can be employed. Each of the three stages is able to be carried out
10 continuously in a respective furnace. Liquid slag flows continuously via
a weir
and launder from the smelt stage furnace to the slag reduction stage furnace,
and similarly from the latter furnace to the fume stage furnace. Lead bullion
is
removed from the smelting and slag reduction furnaces. The final discard slag
from the fume furnace may be continuously tapped through a weir for disposal.
With reference to Figure 6, there is shown a theoretical distribution of lead
in
the smelt stage of a process as illustrated in each of Figures 2 to 5, under
equilibrium conditions using HSC Chemistry for Windows 5.1 (chemical
reaction and equilibrium software available from SGE Software at
www.sge.com). The data of Figure 6 is for a typical set of conditions for a
given smelter and for one specific lead sulphide concentrate. Figure 6 shows
the weight percent distribution of lead between slag, lead bullion and fume
under those conditions, plotted against the volume of lance injected oxygen
containing gas (at a given oxygen content) expressed in normal cubic metres
per tonne of concentrate.
Figure 6 shows that the smelt stage is sensitive to the amount of lance
injected
oxygen containing gas. In the case illustrated, a difference of 50 Nm3 per
tonne of concentrate can mean the difference between 65% to 70% direct lead
metal recovery.
The equilibrium case illustrated by Figure 6 assumes substantially complete
mixing of the slag, lead source material and top-submerged injected oxygen-
containing gas. While that injection achieves a high level of turbulence in
the
CA 02658674 2009-01-22
WO 2008/014538
PCT/AU2007/001053
16
slag, the bath is not at equilibrium as predicted by the model. There are a
number of factors contributing to this, including:
(a) Injected gases contact only a small proportion of the total volume of
the slag, and oxygen transfer throughout that volume is reliant on
the Fe2+/Fe3+ redox couple to transfer oxygen from the injected
gases to molten lead source material taken up in the slag.
(b) It has been found that faster mixing is achieved above the lance tip
relative to mixing below that tip. Substantially complete mixing in
slag above the tip may be achieved in about 2 minutes for example,
whereas mixing below the tip may require up to about 20 minutes.
(c) If the lead sulphide concentrate and flux material are mixed or
pugged, as is preferred, then as the pug falls into the bath and is
heated up there is opportunity for solids to react with each other
before they are dispersed in the slag and melted. The solids are in
contact with gases, evolved from moisture and generated by
smelting reactions before contact with oxygen-containing gas. As a
consequence, the proportion of lead lost to fume may be less than
would be the case under the equilibrium condition.
(d) Lead sulphide concentrates can be quite variable, including with
respect to any graphitic carbon content. A large proportion of the
oxygen demand during smelting can be due to this carbon source.
Variations in the graphitic carbon content of the lead source material,
such as by about 4 to 10 wt%, can impact on the ability to optimise
control of the process with respect to oxygen.
However, despite these factors, Figure 6 is indicative of the general level of
distribution of lead in the smelt stage between slag, lead bullion and fume.
Direct lead recovery in that stage is able to be optimised at, for example,
about
65 to 70%. Lead recovery in the overall three stage process is able to be
stabilised at about 95%, with the use of a sulphidic material such as lead
sulphide concentrate as reductant in the reduction stage being comparable to
the prior art practice based on the use of a carbonaceous reductant such as
CA 02658674 2014-03-24
17
coal. The remaining 5% lead is accounted for by lead in zinc rich fume
produced in the
fume stage and lead losses in discard slag from that stage.
Figure 7 shows the effectiveness of, and benefits from, the use of a sulphidic
material such as lead sulphide concentrate as reductant in the reduction
stage. Figure 7
is a control chart showing the weight percentage of each of lead and zinc in
the slag
after successive time intervals during successive smelt, reduction and fume
stages of a
three stage process conducted in a single TSL furnace.
In the period of the reduction stage of the process illustrated by Figure 7,
the
level of lead in the slag can be seen to drop rapidly, with the use of a
sulphidic material,
in this case a lead sulphide concentrate, as reductant adding to the quantity
of zinc in
the slag. This difference in reporting of lead and zinc, that is recovery of
lead as bullion
while the slag substantially retains and takes up further zinc, occurs to a
significantly
greater extent than when a carbonaceous reductant is used in the reduction
stage. This
results in a better separation of lead and zinc, with both the fume produced
in the
reduction stage being richer in lead and the fume produced in the fume stage
being
richer in zinc.
Finally, it is to be understood that various alterations, modifications and/or
additions may be introduced into the constructions and arrangements of parts
previously
described without departing from the scope of the invention. An example of
such a
modification may be through the use of other sulphide bearing materials as the
reductant, such as bulk concentrates, sulphide drosses and pyrites.