Note: Descriptions are shown in the official language in which they were submitted.
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
U.S. Patent Application
for
Compositions Comprising Nanoparticulate
Meloxicam and Controlled Release Hydrocodone
By
John Devane
Paul Stark
Niall M. M. Fanning
Gurvinder Singh Rekhi
Scott A. Jenkins
Gary G. Liversidge
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of provisional Application No. 60/815,884,
filed June
23, 2006, and this application is a continuation-in-part of Application No.
11/372,857, filed March
10, 2006, is a continuation-in-part of Application No. 10/827,689, filed April
19, 2004, which is a
continuation of Application No. 10/354,483, filed January 30, 2003, now U.S.
Pat. No. 6,793,936,
which is a continuation of Application No. 10/331,754, filed December 30,
2002, now U.S. Pat.
No. 6,902,742, which is a continuation of Application No. 09/850,425, filed
May 7, 2001, now
U.S. Pat. No. 6,730,325, which is a continuation of Application No.
09/566,636, filed May 8,
2000, now U.S. Pat. No. 6,228,398, which is a continuation of Application No.
PCT/US99/25632,
filed November 1, 1999, which claims the benefit of provisional Application
No. 60/106,726, filed
November 2, 1998.
FIELD OF INVENTION
The present invention relates to compositions comprising nanoparticulate
meloxicam, or a
salt or derivative thereof, in combination with a multiparticulate modified
release composition
comprising hydrocodone, or a salt or derivative thereof. In particular the
present invention relates
to compositions comprising nanoparticulate meloxicam, or a salt or derivative
thereof, in
combination with a multiparticulate modified release composition that in
operation delivers
hydrocodone, or a salt or derivative thereof, in a bimodal or multimodal
manner. The present
invention further relates to solid oral dosage forms comprising such
multiparticulate controlled
release compositions as well as methods for delivering such compositions to a
patient in need
thereof.
BACKGROUND OF INVENTION
A. Background Regarding Oral Controlled Release Compositions
The effectiveness of pharmaceutical compounds in the prevention and treatment
of disease
states depends on a variety of factors including the rate and duration of
delivery of the compound
from the dosage form to the patient. The combination of delivery rate and
duration exhibited by a
given dosage form in a patient can be described as its in vivo release profile
and, depending on the
pharmaceutical compound administered, will be associated with a concentration
and duration of
the pharmaceutical compound in the blood plasma, referred to as a plasma
profile. As
pharmaceutical compounds vary in their pharmacokinetic properties such as
bioavailability, and
rates of absorption and elimination, the release profile and the resultant
plasma profile become
important elements to consider in designing effective drug therapies.
2
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
The release profiles of dosage forms may exhibit different rates and durations
of release
and may be continuous or pulsatile. Continuous release profiles include
release profiles in which
one or more pharmaceutical compounds are released continuously, either at a
constant or variable
rate, and pulsatile release profiles include release profiles in which at
least two discrete quantities
of one or more pharmaceutical compounds are released at different rates and/or
over different time
frames. For any given pharmaceutical compound or combination of such
compounds, the release
profile for a given dosage form gives rise to an associated plasma profile in
a patient. Similar to
the variables applicable to the release profile, the associated plasma profile
in a patient may
exhibit constant or variable blood plasma concentration levels of the
pharmaceutical compounds
in the dosage form over the duration of action and may be continuous or
pulsatile. Continuous
plasma profiles include plasma profiles of all rates and duration which
exhibit a single plasma
concentration maximum. Pulsatile plasma profiles include plasma profiles in
which at least two
higher blood plasma concentration levels of pharmaceutical compound are
separated by a lower
blood plasma concentration level. Pulsatile plasma profiles exhibiting two
peaks may be described
as "bimodal."
When two or more components of a dosage form have different release profiles,
the release
profile of the dosage form as a whole is a combination of the individual
release profiles. The
release profile of a two-component dosage form in which each component has a
different release
profile may described as "bimodal." For dosage forms of more than two
components in which
each component has a different release profile, the resultant release profile
of the dosage form
may be described as "multimodal." Depending on, at least in part, the
pharmacokinetics of the
pharmaceutical compounds that are used as well as the specific release
profiles of the components
of the dosage form, a bimodal or multimodal release profile may result in
either a continuous or a
pulsatile plasma profile in a patient. Conventional frequent dosage regimes in
which an immediate
release (IR) dosage form is administered at periodic intervals typically gives
rise to a pulsatile
plasma profile. In such cases, a peak in the plasma drug concentration is
observed after
administration of each JR dose with troughs (regions of low drug
concentration) developing
between consecutive administration time points. Such dosage regimes (and their
resultant pulsatile
plasma profiles) can have particular pharmacological and therapeutic effects
associated with them
that are beneficial for certain drug therapies. For example, the wash out
period provided by the fall
off of the plasma concentration of the active ingredient between peaks has
been thought to be a
contributing factor in reducing or preventing patient tolerance to various
types of drugs.
Many controlled release drug formulations are aimed at producing a zero-order
release of
the drug compound. Indeed, it is often a specific object of these formulations
to minimize the
peak-to-trough variation in plasma concentration levels associated with
conventional frequent
dosage regimes. For certain drugs, however, some of the therapeutic and
pharmacological effects
intrinsic in a pulsatile system may be lost or diminished as a result of the
constant or nearly
3
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
constant plasma concentration levels achieved by zero-order release drug
delivery systems. Thus,
modified release compositions or formulations which substantially mimic the
release of frequent
JR dosage regimes, while reducing the need for
frequent dosing, is desirable. Similarly, modified release compositions or
formulations which
combine the benefits of at least two different release profiles to achieve a
resultant plasma profile
exhibiting pharmacokinetic values within therapeutically effective parameters
is also desirable.
Shah et al., J Cont. Rel. (1989) 9:169-175 purports to disclose that certain
types of
hydroxypropyl methylcellulose ethers compressed into a solid dosage form with
a therapeutic
agent may produce a bimodal release profile. However, it is noted that while
polymers from one
supplier yielded a bimodal profile, the same polymers with almost identical
product specifications
obtained from a different source gave non-bimodal release profiles.
Giunchedi et al., Int. J. Pharm (1991) 77:177-181 discloses the use of a
hydrophilic matrix
multiple-unit formulation for the pulsed release of ketoprofen. Giunchedi et
al. teach that
ketoprofen is rapidly eliminated from the blood after dosing (plasma half-life
1-3 hours) and
consecutive pulses of drug may be more beneficial than constant release for
some treatments. The
multiple-unit formulation disclosed comprises four identical hydrophilic
matrix tablets placed in a
gelatin capsule. Although the in vivo studies show two peaks in the plasma
profile there is no well
defined wash out period and the variation between the peak and trough plasma
levels is small.
Conte et al., Drug Dev. md. Pharm, (1989) 15:2583-2596 and EP 0 274 734
(Pharmidea
Srl) teach the use of a three layer tablet for delivery of ibuprofen in
consecutive pulses. The three
layer tablet is made up of a first layer containing the active ingredient, a
barrier layer (the second
layer) of semi-permeable material which is interposed between the first layer
and a third layer
containing an additional amount of active ingredient. The barrier layer and
the third layer are
housed in an impermeable casing. The first layer dissolves upon contact with a
dissolving fluid
while the third layer is only available after dissolution or rupture of the
barrier layer. In such a
tablet the first portion of active ingredient must be released instantly. This
approach also requires
the provision of a semi-permeable layer between the first and third layers in
order to control the
relative rates of delivery of the two portions of active ingredient.
Additionally, rupture of the
semi-permeable layer leads to uncontrolled dumping of the second portion of
the active ingredient
which may not be desirable.
U.S. Pat. No. 5,158,777 (E. R. Squibb & Sons Inc.) discloses a formulation
comprising
captopril within an enteric or delayed release coated pH stable core combined
with additional
captopril which is available for immediate release following administration.
In order to form the
pH stable core, chelating agents such as disodium edetate or surfactants
such as polysorbate 80 are used either alone or in combination with a
buffering agent. The
compositions have an amount of captopril available for immediate release
following oral
4
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
administration and an additional amount of pH stabilized captopril available
for release in the
colon.
U.S. Pat. Nos. 4,728,512, 4,794,001 and 4,904,476 (American Home Products
Corp.)
relate to preparations providing three distinct releases. The preparation
contains three groups of
spheroids containing an active medicinal substance: the first group of
spheroids is uncoated and
rapidly disintegrates upon ingestion to release an initial dose of medicinal
substance; the second
group of spheroids is coated with a pH sensitive coat to provide a second
dose; and the third group
of spheroids is coated with a pH independent coat to provide to third dose.
The preparation is
designed to provide repeated release of medicinal substances which are
extensively metabolized
presystemically or have relatively short elimination half-lives.
U.S. Pat. No. 5,837,284 (Mehta et al) discloses a methylphenidate dosage form
having immediate
release and delayed release particles. The delayed release is provided by the
use of ammonio
methacrylate pH independent polymers combined with certain fillers.
B. Background Regarding Hydrocodone
A typical example of a drug which may produce tolerance in patients is
hydrocodone.
Hydrocodone or dihydrocodeinone (marketed as Vicodin0, Anexsia0, Dicodid0,
Hycodan0, Hycomine0, Lorcet0, LortabO, Norco , Hydroco0, Tussionex0, and
Vicoprofen0), also known as 4,5a-Epoxy-3 -methoxy- 1 7-methylmorphinan-6-one
tartrate
(1:1) hydrate (2:5), is an opiod derived from either of the naturally
occurring opiates codeine
or thebaine. The compound has the following structure:
~a o
Hydrocodone has the chemical formula C18H21N03, a molecular weight of 299.368,
and a
half life of 4-8 hours.
Hydrocodone is an orally active narcotic analgesic and antitussive. Sales and
production
of this drug have increased significantly in recent years, as have diversion
and illicit use.
Hydrocodone is commonly available in tablet, capsule and syrup form.
As a narcotic, hydrocodone relieves pain by binding to opioid receptors in the
brain and
spinal cord, It may be taken with or without food. When taken with alcohol, it
can intensify
drowsiness It may interact with monoamine oxidase inhibitors, as well as other
drugs that cause
5
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
drowsiness. Common side effects include dizziness, lightheadedness, nausea,
drowsiness,
euphoria, vomiting, and constipation Some less common side effects are
allergic reaction, blood
disorders, changes in mood, mental fogginess, anxiety, lethargy, difficulty
urinating, spasm of the
ureter, irregular or depressed respiration and rash.
Hydrocodone can be habit-forming, and can lead to physical and psychological
addiction. In the
U.S., pure hydrocodone and forms containing more than 15 mg per dosage unit
are considered
Schedule II drugs. Those containing less than or equal to 15 mg per dosage
unit in combination
with acetaminophen or another non-controlled drug are called Hydrocodone
Compounds and are
considered Schedule III drugs Hydrocodone can be found in combination with
other drugs such as
paracetamol (acetaminophen), aspirin, ibuprofen and homatropine methylbromide.
The presence of acetaminophen in hydrocodone-containing products deters many
drug
users from taking excessive amounts. However, some users will get around this
by extracting a
portion of the acetaminophen using hot/cold water, taking advantage of the
water-soluble element
of the drug It is not uncommon for addicts to have liver problems from
consuming excessive
amounts of acetaminophen over a long period of time, taking 10,000 to 15,000
milligrams of
acetaminophen in a period of 24 hours typically results in severe
hepatoxicity, and doses in the
range of 15,000-20,0000 milligrams a day have been reported as fatal It is
this factor that leads
many addicts to use only single entity opiates such as Oxycontin.
Daily consumption of hydrocodone should not exceed 40 milligrams in patients
not
tolerant to opiates However, it clearly states in the 2006 PDR (Physicians
Desk Reference) that
Norco 10, containing 10 milligrams of hydrocodone and 325 milligrams of Apap,
can be taken at
a dosage of up to twelve tablets per day (120 milligrams of hydrocodone) Such
high amounts of
hydrocodone are only intended for opiate tolerant patients, and titration to
such levels must be
monitored very carefully This restriction is only limited by the fact that
twelve tablets, each
containing 325 milligrams of Apap, puts the patient right below the 24 hour
FDA maximum of
4,000 mg of Apap. Some specially compounded products are routinely given to
chronic pain
patients in doses of up to 180 mg of hydrocodone per day. Tolerance to this
drug can increase very
rapidly if abused. Because of this, addicts often overdose from taking
handfuls of pills, in pursuit
of the high they experienced very early on in their hydrocodone use. Symptoms
of hydrocodone
overdosagc include respiratory depression, extreme somnolence, coma, stupor,
cold and/or
clammy skin, sometimes bradycardia, and hypotension. A severe overdose may
involve
circulatory collapse, cardiac arrest and/or death.
C. Background Regarding Meloxicam
Meloxicam, also known as 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2-H- 1,2-
benzothiazine-3-carboxamide 1,1-dioxide, is a member of the enolic acid group
of nonsteroidal
6
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
anti-inflammatory drugs (NSAIDs). Meloxicam is an oxicam derivative with the
following
cheniical structure: ~ 0 OH
SN
H
Meloxicam has an empirical formula of C14H13N304S2 and a molecular weight of
351.41. See The
Physicians' Desk Reference, 56th Ed., pp. 1054 (2002); and The Merck Index,
Ed., pp. 1040-1041
(Merck & Co. 2001). Meloxicam is practically insoluble in water with higher
solubility observed
in strong acids and bases. It is very slightly soluble in methanol. The
Physicians' Desk Reference,
56th Ed., pp. 1054.
4-hydroxy-2H- 1,2-benzothiazine-3 -carboxamide- 1,1-dioxides and salts
thereof, as well as
methods of preparing these compounds, pharmaceutical compositions containing
them as active
ingredients, and methods of using them as antiphlogistics, are discussed in
U.S. Patent No.
4,233,299. See also German Patent No. 2,756,113. The pharmacology of meloxicam
in horses is
discussed in Lees et al., Brit. Vet. J., 147: 97 (1991); veterinary trials in
dogs are discussed in
Henderson et al., Prakt. Tierarzt., 75:179 (1994); the physiochemical
properties of meloxicam are
discussed in Tsai et al., Helv. Chim. Acta, 76:842 (1993); the pharmacology,
mechanism of action,
and clinical efficacy are discussed in Brit. J. Rheumatol., 35(Suppl. 1):1-77
(1996); and clinical
trials of gastrointestinal tolerability in arthritis is discussed in Hawkey et
al., Brit. I Rheumatol.,
37:937 (1998), and Dequeker et al., Brit. J. Rheumatol., 37:946 (1998).
Meloxicam exhibits anti-inflammatory, analgesic, and antifebrile activities.
Like other
NSAIDs, the primary mechanism of action of meloxicam is via inhibition of the
cyclooxygenase
(COX) enzyme system resulting in decreased prostaglandin synthesis. See The
Physicians' Desk
Reference, 56th Ed., pp. 1054 (2002). The COX enzyme system is comprised of at
least two
isoforms of COX. COX-1 is constitutively expressed in the gastrointestinal
tract and the kidneys
and is involved in the production of prostaglandins required for gastric
mucosal production and
proper renal blood flow. See Vane et al., Proc. Natl. Acad. Sci. USA, 91:2046-
2050 (1994);
Oulette et al., Proc. Natl. Acad. Sci., 98:14583- 14588 (2001); and Seibert et
al., Proc. Natl. Acad.
Sci., 91:12013-12017 (1994). In contrast, COX-2 is not present in healthy
tissue and its expression
is induced in certain inflammatory states. Id.
The pathological production of prostaglandins by COX-2 is implicated in a
number of
human disease states, including rheumatoid arthritis, osteoarthritis, pyrexia,
asthma, bone
resorption, cardiovascular diseases, nephrotoxicity, atherosclerosis, and
hypotension. Id. Elevated
levels of prostaglandins enhance or prolong pro-inflammatory signals which
cause the pain,
7
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
stiffness, and inflammation associated with these conditions. See Smith et
al., Proc. Natl. Acad.
Sci., 95:13313-13318 (1998).
Meloxicam is superior to traditional non-selective NSAIDs because it
selectively inhibits
COX-2, thus causing fewer gastrointestinal problems such as bleeding,
heartburn, reflux, diarrhea,
nausea, and abdominal pain. Meloxicam preferentially inhibits COX-2 with a COX-
2/COX-1
inhibition ratio of 0.09. It is desirable to selectively inhibit COX-2 and the
pathological production
of prostaglandins for which that enzyme is responsible because the therapeutic
analgesic/anti-
inflammatory properties of NSAIDs occur by inhibition of inducible COX-2 at
the site of
inflammation. Conversely, the majority of adverse drug reactions to NSAIDs,
including
gastrointestinal ulcers and renal failure, result from inhibition of the
constitutive COX- 1 enzymes.
This is because as a result of such COX-1 inhibition, prostaglandins necessary
for gastric mucosal
production and renal blood circulation are not produced. See Vane et al.,
Proc. Natl. Acad. Sci.
USA, 91:2046 (1994); Oulette et al., Proc. Natl. Acad. Sci., 98:14583 (2001);
and Seibert et al.,
Proc. Natl. Acad. Sc!., 91:12013 (1994). Compounds that selectively inhibit
the biosynthesis of
prostaglandins by inhibiting the activity of the inducible enzyme, COX-2,
exert anti-inflammatory
effects without the adverse side effects associated with COX- 1 inhibition.
Some of the trade names under which meloxicam has or is marketed include MOBIC
,
MOBEC , MOBICOX , MOVALIS , and MOVATEC . Meloxicam has been shown to be
useful in the symptomatic treatment of painful osteoarthritis (arthrosis,
degenerative joint disease),
symptomatic treatment of rheumatoid arthritis, symptomatic treatment of
ankylosing spondylitis,
and symptomatic treatment of the signs and symptoms of osteoarthritis,
including pain, stiffness,
and inflammation.
The form of meloxicam currently marketed in the United States is MOBIC
(Boehringer
Ingelheim Pharmaceuticals, Inc., Ridgefield, CT), provided in 7.5 and 15 mg
tablets. The
bioavailability of a single 30 mg oral dose is 89% as compared to a 30 mg
intravenous bolus
injection. The pharmacokinetics of a single intravenous dose of meloxicam is
dose-proportional in
the range of 5 to 60 mg. See The Physicians' Desk Reference, 56th Ed., pp.
1054 (2002). After
administration of multiple oral doses of meloxicam, the pharmacokinetics is
dose-proportional in
the range of 7.5 to 15 mg. The rate or extent of absorption is not affected by
multiple dose
administration. Under fasted steady state conditions, the mean Cmax is
achieved within four to
five hours, with a second meloxicam concentration peak occurring at
approximately twelve to
fourteen hours post- dose, which suggests gastrointestinal recirculation.
Under steady state fed
conditions in healthy adult males, the 7.5 mg tablets have a mean Cmax of 1.05
g/mL, a T,,,aR of
4.9 hrs, and a tiiz of 20.1 hours. Under steady state fed conditions in
elderly males and females,
the 15 mg tablets have a C,,,,,x of 2.3 and 3.2 g/ml, respectively, a T,,,aR
of 5 and 6 hrs,
8
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
respectively, and a tiiz of 21 and 24 hrs, respectively. See The Physicians'
Desk Reference, 56th
Ed., pp. 1054 (2002).
Although meloxicam has been tested and approved by the FDA only for relief of
the signs
and symptoms of osteoarthritis, it may be useful in relieving the signs and
symptoms of
rheumatoid arthritis, lower back pain, and acute pain, e.g. treatment of post
surgical pain,
treatment of pain resulting from battle field wounds, and migraine headaches.
Meloxicam may be
especially effective for treatment of all types of pain associated with
inflammation.
NSAIDs, like meloxicam, are useful in pain management because NSAIDs provide
an
analgesic effect without the sedation and addictive properties of narcotic
analgesics. Furthermore,
the long tiiz of meloxicam makes it useful for long-lasting relief which is
not provided by narcotic
analgesics. However, due to their typically long onset of action, conventional
NSAIDs, including
conventional meloxicam, are frequently inappropriate for management of acute
pain.
Because meloxicam is practically insoluble in water, attaining sufficient
bioavailability of
this drug is problematic. Prior art methods of increasing the bioavailability
of meloxicam include
increasing its solubility by forming a cyclodextrin complex of the drug (see
U.S. Pat. No.
6.284,269) or by forming a salt of meloxicam with an inorganic or organic base
(U.S. Pat. Appln.
Pub. No. US 2002/0035107 Al).
Published U.S. Patent Application No. 20020035264, for "Ophthalmic Formulation
of a
Selective Cyclooxygenase-2 Inhibitory Drug," describes pharmaceutical
compositions suitable for
topical administration to an eye which contain a selective COX-2 inhibitory
drug, or nanoparticles
of a drug of low water solubility, in a concentration effective for treatment
and/or prophylaxis of a
disorder in the eye, and one or more ophthalmically acceptable excipients that
reduce rate of
removal from the eye such that the composition has an effective residence time
of about 2 to about
24 hours. Examples of such ophthalmically acceptable excipients given in the
published
application include cross-linked carboxyl-containing polymers which form in
situ gellable
aqueous solution, suspension or solution/suspension. Such excipients, which
are described in U.S.
Pat. No. 5,192,535, can be undesirable. Moreover, this disclosure, which is
limited to ocular
formulations, does not address a need for oral fast onset meloxicam
formulations for treating
migraine.
Published U.S. Patent Application No. 20020077328, for "Selective
Cyclooxygenase2
Inhibitors and Vasomodulator Compounds for Generalized Pain and Headache
Pain," refers to a
therapeutic combination useful in the treatment, amelioration, prevention, or
delay of pain
comprising a high energy form of a selective cyclooxygenase-2 inhibitor, a
vasomodulator, and a
pharmaceutically acceptable excipient, carrier, or diluent. The cyclooxygenase-
2 inhibitor and
vasomodulator are each being present in an amount effective to contribute to
the treatment,
9
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
prevention, ameloriation or delay of pain. Disclosed vasomodulators include
vasoconstrictors,
vasodilators, bronchodilation agents, and bronchoconstriction agents, such as
rennin-angiotensin
system antagonists, nitrovasodilators, direct vasodilators, calcium channel
blocking drugs,
phosphodiesterase inhibitors, sympathomimetics, sympatholytics, and nitric
oxide synthase
inhibitors. Such additional pharmaceutical agents can be undesirable, as they
can cause unwanted
side-effects.
D. Background Regarding Nanoparticulate Active Agent Compositions
Nanoparticulate active agent compositions, first described in U.S. Patent No.
5,145,684
("the `684 patent"), are particles consisting of a poorly soluble therapeutic
or
diagnostic agent having adsorbed onto or associated with the surface thereof a
noncrosslinked
surface stabilizer.
Methods of making nanoparticulate active agent compositions are described in,
for
example, U.S. Patent Nos. 5,518,187 and 5,862,999, both for "Method of
Grinding
Pharmaceutical Substances;" U.S. Patent No. 5,718,388, for "Continuous Method
of Grinding
Pharmaceutical Substances;" and U.S. Patent No. 5,510,118 for "Process of
Preparing
Therapeutic Compositions Containing Nanoparticles."
Nanoparticulate active agent compositions are also described, for example, in
U.S. Patent
Nos. 5,298,262 for "Use of Ionic Cloud Point Modifiers to Prevent Particle
Aggregation During
Sterilization;" 5,302,401 for "Method to Reduce Particle Size Growth During
Lyophilization;"
5,318,767 for "X-Ray Contrast Compositions Useful in Medical Imaging;"
5,326,552 for "Novel
Formulation For Nanoparticulate X-Ray Blood Pool Contrast Agents Using High
Molecular
Weight Non-ionic Surfactants;" 5,328,404 for "Method of X-Ray Imaging Using
lodinated
Aromatic Propanedioates;" 5,336,507 for "Use of Charged Phospholipids to
Reduce Nanoparticle
Aggregation;" 5,340,564 for "Formulations Comprising Olin 10-G to Prevent
Particle
Aggregation and Increase Stability;" 5,346,702 for "Use of Non-Ionic Cloud
Point Modifiers to
Minimize Nanoparticulate Aggregation During Sterilization;" 5,349,957 for
"Preparation and
Magnetic Properties of Very Small Magnetic-Dextran Particles;" 5,352,459 for
"Use of Purified
Surface Modifiers to Prevent Particle Aggregation During Sterilization;"
5,399,363 and 5,494,683,
both for "Surface Modified Anticancer Nanoparticles;" 5,401,492 for "Water
Insoluble Non-
Magnetic Manganese Particles as Magnetic Resonance Enhancement Agents;"
5,429,824 for "Use
of Tyloxapol as a Nanoparticulate Stabilizer;" 5,447,710 for "Method for
Making Nanoparticulate
X-Ray Blood Pool Contrast Agents Using High Molecular Weight Non-ionic
Surfactants;"
5,451,393 for "X-Ray Contrast Compositions Useful in Medical Imaging;"
5,466,440 for
"Formulations of Oral Gastrointestinal Diagnostic X-Ray Contrast Agents in
Combination with
Pharmaceutically Acceptable Clays;" 5,470,583 for "Method of Preparing
Nanoparticle
Compositions Containing Charged Phospholipids to Reduce Aggregation;"
5,472,683 for
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
"Nanoparticulate Diagnostic Mixed Carbamic Anhydrides as X-Ray Contrast Agents
for Blood
Pool and Lymphatic System Imaging;" 5,500,204 for "Nanoparticulate Diagnostic
Dimers as X-
Ray Contrast Agents for Blood Pool and Lymphatic System Imaging;" 5,518,738
for
"Nanoparticulate NSAID Formulations;" 5,521,218 for "Nanoparticulate
lododipamide
Derivatives for Use as X-Ray Contrast Agents;" 5,525,328 for "Nanoparticulate
Diagnostic
Diatrizoxy Ester X-Ray Contrast Agents for Blood Pool and Lymphatic System
Imaging;"
5,543,133 for "Process of Preparing X-Ray Contrast Compositions Containing
Nanoparticles;"
5,552,160 for "Surface Modified NSAID Nanoparticles;" 5,560,931 for
"Formulations of
Compounds as Nanoparticulate Dispersions in Digestible Oils or Fatty Acids;"
5,565,188 for
"Polyalkylene Block Copolymers as Surface Modifiers for Nanoparticles;"
5,569,448 for
"Sulfated Non-ionic Block Copolymer Surfactant as Stabilizer Coatings for
Nanoparticle
Compositions;" 5,571,536 for "Formulations of Compounds as Nanoparticulate
Dispersions in
Digestible Oils or Fatty
Acids;" 5,573,749 for "Nanoparticulate Diagnostic Mixed Carboxylic Anydrides
as X-Ray
Contrast Agents for Blood Pool and Lymphatic System Imaging;" 5,573,750 for
"Diagnostic
Imaging X-Ray Contrast Agents;" 5,573,783 for "Redispersible Nanoparticulate
Film
Matrices With Protective Overcoats;" 5,580,579 for "Site-specific Adhesion
Within the GI
Tract Using Nanoparticles Stabilized by High Molecular Weight, Linear
Poly(ethylene
Oxide) Polymers;" 5,585,108 for "Formulations of Oral Gastrointestinal
Therapeutic Agents
in Combination with Pharmaceutically Acceptable Clays;" 5,587,143 for
"Butylene Oxide-
Ethylene Oxide Block Copolymers Surfactants as Stabilizer Coatings for
Nanoparticulate
Compositions;" 5,591,456 for "Milled Naproxen with Hydroxypropyl Cellulose as
Dispersion Stabilizer;" 5,593,657 for "Novel Barium Salt Formulations
Stabilized by Non-
ionic and Anionic Stabilizers;" 5,622,938 for "Sugar Based Surfactant for
Nanocrystals;"
5,628,981 for "Improved Formulations of Oral Gastrointestinal Diagnostic X-Ray
Contrast
Agents and Oral Gastrointestinal Therapeutic Agents;" 5,643,552 for
"Nanoparticulate
Diagnostic Mixed Carbonic Anhydrides as X-Ray Contrast Agents for Blood Pool
and
Lymphatic System Imaging;" 5,718,388 for "Continuous Method of Grinding
Pharmaceutical
Substances;" 5,718,919 for "Nanoparticles Containing the R(-) Enantiomer of
Ibuprofen;"
5,747,001 for "Aerosols Containing Beclomethasone Nanoparticle Dispersions;"
5,834,025
for "Reduction of Intravenously Administered Nanoparticulate Formulation
Induced Adverse
Physiological Reactions;" 6,045,829 "Nanocrystalline Formulations of Human
Immunodeficiency Virus (HIV) Protease Inhibitors Using Cellulosic Surface
Stabilizers;"
6,068,858 for "Methods of Making Nanocrystalline Formulations of Human
Immunodeficiency Virus (HIV) Protease Inhibitors Using Cellulosic Surface
Stabilizers;"
6,153,225 for "Injectable Formulations of Nanoparticulate Naproxen;" 6,165,506
for "New
Solid Dose Form of Nanoparticulate Naproxen;" 6,221,400 for "Methods of
Treating
Mammals Using Nanocrystalline Formulations of Human Immunodeficiency Virus
(HIV)
11
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Protease Inhibitors;" 6,264,922 for "Nebulized Aerosols Containing
Nanoparticle
Dispersions;" 6,267,989 for "Methods for Preventing Crystal Growth and
Particle Aggregation in
Nanoparticle Compositions;" 6,270,806 for "Use of PEG-Derivatized Lipids as
Surface Stabilizers
for Nanoparticulate Compositions;" 6,316,029 for "Rapidly Disintegrating Solid
Oral Dosage
Form," 6,375,986 for "Solid Dose Nanoparticulate Compositions Comprising a
Synergistic
Combination of a Polymeric Surface Stabilizer and Dioctyl Sodium
Sulfosuccinate;" 6,428,814
for "Bioadhesive Nanoparticulate Compositions Having Cationic Surface
Stabilizers;" 6,431,478
for "Small Scale Mill;" 6,432,381 for "Methods for Targeting Drug Delivery to
the Upper and/or
Lower Gastrointestinal Tract," 6,592,903 for "Nanoparticulate Dispersions
Comprising a
Synergistic Combination of a Polymeric Surface Stabilizer and Dioctyl Sodium
Sulfosuccinate,"
6,582,285 for "Apparatus for sanitary wet milling;" 6,656,504 for
"Nanoparticulate Compositions
Comprising Amorphous Cyclosporine;" 6,742,734 for "System and Method for
Milling
Materials;" 6,745,962 for "Small Scale Mill and Method Thereof;" 6,811,767 for
"Liquid droplet
aerosols of nanoparticulate drugs;" 6,908,626 for "Compositions having a
combination of
immediate release and controlled release characteristics;" 6,969,529 for
"Nanoparticulate
compositions comprising copolymers of vinyl pyrrolidone and vinyl acetate as
surface
stabilizers;" and 6,976,647 for "System and Method for Milling Materials," all
of which are
specifically incorporated by reference. In addition, U.S. Patent Publication
No. 20020012675 Al,
for "Controlled Release Nanoparticulate Compositions;" U.S. Patent Publication
No.
20050276974 for "Nanoparticulate Fibrate Formulations;" U.S. Patent
Publication No.
20050238725 for "Nanoparticulate compositions having a peptide as a surface
stabilizer;" U.S.
Patent Publication No. 20050233001 for "Nanoparticulate megestrol
formulations;" U.S. Patent
Publication No. 20050147664 for "Compositions comprising antibodies and
methods of using the
same for targeting nanoparticulate active agent delivery;" U.S. Patent
Publication No.
20050063913 for "Novel metaxalone compositions;" U.S. Patent Publication No.
20050042177
for "Novel compositions of sildenafil free base;" U.S. Patent Publication No.
20050031691 for
"Gel stabilized nanoparticulate active agent compositions;" U.S. Patent
Publication No.
20050019412 for "Novel glipizide compositions;" U.S. Patent Publication No.
20050004049 for
"Novel griseofulvin compositions;" U.S. Patent Publication No. 20040258758 for
"Nanoparticulate topiramate formulations;" U.S. Patent Publication No.
20040258757 for" Liquid
dosage compositions of stable nanoparticulate active agents;" U.S. Patent
Publication No.
20040229038 for "Nanoparticulate meloxicam formulations;" U.S. Patent
Publication No.
20040208833 for "Novel fluticasone formulations;" U.S. Patent Publication No.
20040195413
for" Compositions and method for milling materials;" U.S. Patent Publication
No. 20040156895
for "Solid dosage forms comprising pullulan;" U.S. Patent Publication No. U.S.
Patent Publication
No. U.S. Patent Publication No. 20040156872 for "Novel nimesulide
compositions;" U.S. Patent
Publication No. 20040141925 for "Novel triamcinolone compositions;" U.S.
Patent Publication
No. 20040115134 for "Novel nifedipine compositions;" U.S. Patent Publication
No. 20040105889
12
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
for "Low viscosity liquid dosage forms;" U.S. Patent Publication No.
20040105778 for "Gamma
irradiation of solid nanoparticulate active agents;" U.S. Patent Publication
No. 20040101566 for
"Novel benzoyl peroxide compositions;" U.S. Patent Publication No. 20040057905
for
"Nanoparticulate beclomethasone dipropionate compositions;" U.S. Patent
Publication No.
20040033267 for "Nanoparticulate compositions of angiogenesis inhibitors;"
U.S. Patent
Publication No. 20040033202 for "Nanoparticulate sterol formulations and novel
sterol
combinations;" U.S. Patent Publication No. 20040018242 for "Nanoparticulate
nystatin
formulations;" U.S. Patent Publication No. 20040015134 for "Drug delivery
systems and
methods;" U.S. Patent Publication No. 20030232796 for "Nanoparticulate
polycosanol
formulations & novel polycosanol combinations;" U.S. Patent Publication No.
20030215502 for
"Fast dissolving dosage forms having reduced friability;" U.S. Patent
Publication No.
20030185869 for "Nanoparticulate compositions having lysozyme as a surface
stabilizer;" U.S.
Patent Publication No. 20030181411 for "Nanoparticulate compositions of
mitogenactivated
protein (MAP) kinase inhibitors;" U.S. Patent Publication No. 20030137067 for
"Compositions
having a combination of immediate release and controlled release
characteristics;" U.S. Patent
Publication No. 20030108616 for "Nanoparticulate compositions comprising
copolymers of vinyl
pyrrolidone and vinyl acetate as surface stabilizers;" U.S. Patent Publication
No. 20030095928 for
"Nanoparticulate insulin;" U.S. Patent Publication No. 20030087308 for "Method
for high
through put screening using a small scale mill or microfluidics;" U.S. Patent
Publication No.
20030023203 for "Drug delivery systems & methods;" U.S. Patent Publication No.
20020179758
for "System and method for milling materials; and U.S. Patent Publication No.
20010053664 for
"Apparatus for sanitary wet milling," describe nanoparticulate active agent
compositions and are
specifically incorporated by reference.
In particular, U.S. Patent Publication No. 20040229038 for "Nanoparticulate
meloxicam
formulations," describes nanoparticulate meloxicam formulations and is
incorporated by
reference.
Amorphous small particle compositions are described, for example, in U.S.
Patent Nos.
4,783,484 for "Particulate Composition and Use Thereof as Antimicrobial
Agent;" 4,826,689 for
"Method for Making Uniformly Sized Particles from Water-Insoluble Organic
Compounds;" 4,997,454 for "Method for Making Uniformly-Sized Particles From
Insoluble
Compounds;" 5,741,522 for "Ultrasmall, Non-aggregated Porous Particles of
Uniform Size for
Entrapping Gas Bubbles Within and Methods;" and 5,776,496, for "Ultrasmall
Porous Particles
for Enhancing Ultrasound Back Scatter." All of the aforementioned patents are
hereby
incorporated by reference.
The problem with conventional hydrocodone formulations is that they can be
habit
forming. There is a need in the art for controlled release formulations that
may alleviate such side
13
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
effects. Moreover, there is a need in the art for new combination compositions
of controlled
release hydrocodone that provide alternatives to the existing combination
compositions. The
present invention satisfies these needs.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a
multiparticulate modified release
composition comprising at least two populations of hydrocodone-comprising
particles which,
upon administration to a patient, exhibit a bimodal or multimodal release
profile.
Another object of the invention is to provide a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a controlled
release hydrocodone
composition in which a first portion of the composition, i.e., a hydrocodone
or a salt or derivative
thereof, is released immediately upon administration and a second portion of
the hydrocodone, or
a salt or derivative thereof, is released rapidly after an initial delay
period in a bimodal manner.
It is another object of the invention to provide a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a
multiparticulate modified release
composition comprising at least two populations of hydrocodone-comprising
particles which,
upon administration to a patient, exhibits a bimodal or multimodal release
profile that results in a
plasma profile within therapeutically effective pharmacokinetic parameters.
It is a further object of the invention to provide a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a
multiparticulate modified release
composition comprising at least two populations of
hydrocodone-comprising particles which, upon administration to a patient,
exhibits a pulsatile
release profile, and/or a pulsatile plasma profile.
It is still another object of the invention to provide a composition
comprising
nanoparticulate meloxicam, or a salt or derivative thereof, in combination
with a multiparticulate
modified release composition comprising at least two populations of
hydrocodone-comprising
particles which, upon administration to a patient, (1) produces a plasma
profile substantially
similar to the plasma profile produced by the administration of two or more JR
dosage forms
given sequentially, and/or (2) substantially mimics the pharmacological and
therapeutic effects
produced by the administration of two or more JR dosage forms given
sequentially.
Conventional frequent dosage regimes in which an immediate release (IR) dosage
form is
administered at periodic intervals typically gives rise to a pulsatile plasma
profile. In this case, a
peak in the plasma drug concentration is observed after administration of each
JR dose with
troughs (regions of low drug concentration) developing between consecutive
administration time
14
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
points. Such dosage regimes (and their resultant pulsatile plasma profiles)
have particular
pharmacological and therapeutic effects associated with them. For example, the
wash out period
provided by the fall off of the plasma concentration of the active between
peaks has been thought
to be a contributing factor in reducing or preventing patient tolerance to
various types of drugs.
The present invention further relates to a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a controlled
release composition
comprising hydrocodone, or a salt or derivative thereof, which in operation
produced a
hydrocodone plasma profile that eliminates the "peaks" and "troughs" produced
by the
administration of two or more JR dosage forms given sequentially if such a
profile is beneficial.
This type of profile can be obtained using a controlled release mechanism that
allows for "zero-
order" delivery. Thus, it is a further object of the invention to provide a
composition comprising
nanoparticulate meloxicam, or a salt or derivative thereof, in combination
with a controlled release
hydrocodone composition which in operation delivers hydrocodone, or a salt or
derivative thereof,
in a pulsatile manner or a zero-order manner.
Multiparticulate modified controlled release compositions similar to those
disclosed herein
are disclosed and claimed in the United States Patent Nos. 6,228,398 and
6,730,325 to Devane et
al; both of which are incorporated by reference herein. All of the relevant
prior art in this field
may also be found therein.
Another object of the invention is to provide a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a controlled
release composition
which substantially reduces or eliminates the development of patient tolerance
to hydrocodone, or
a salt or derivative thereof.
Another object of the invention is to formulate the dosage in the form of
erodable
formulations, diffusion controlled formulations, or osmotic controlled
formulations.
Another object of the invention is to provide a controlled release composition
capable of releasing
a hydrocodone or a nanoparticulate meloxicam in a bimodal or multi-modal
manner in which a
first portion of the active is released either immediately or after a delay
time to provide a pulse of
drug release and one or more additional portions of the hydrocodone or a
nanoparticulate
meloxicam is released, after a respective lag time, to provide additional
pulses of drug release
during a period of up to twenty-four hours.
It is still a further object of the invention to provide a composition
comprising
nanoparticulate meloxicam, or a salt or derivative thereof, in combination
with a multiparticulate
modified release composition comprising at least two populations of
hydrocodone-comprising
particles in which the amount of the one or more active ingredients in the
first population of
particles is a minor portion of the amount of the one or more active
ingredients in the composition,
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
and the amount of the one or more active ingredients in the one or more
additional population of
particles is a major portion of the amount of the one or more active
ingredients in the composition.
It is yet a further object of the invention to provide a solid dosage form
comprising the
composition comprising nanoparticulate meloxicam, or a salt or derivative
thereof, in combination
with the multiparticulate modified release composition of the present
invention. A preferred
dosage form of the invention is a solid oral dosage form, although any
pharmaceutically
acceptable dosage form can be utilized.
Another aspect of the invention is directed to pharmaceutical compositions
comprising a
composition according to the invention and a pharmaceutically acceptable
carrier, as well as any
one or more of a number of desired excipients.
One embodiment of the invention encompasses a composition comprising
nanoparticulate
meloxicam, or a salt or derivative thereof, in combination with a
multiparticulate modified release
composition comprising at least two populations of hydrocodone-comprising
particles, wherein
the pharmacokinetic profile of the
nanoparticulate meloxicam, or a salt or derivative thereof, is not affected by
the fed or fasted state
of a subject ingesting the meloxicam composition.
In yet another embodiment, the invention encompasses a composition comprising
nanoparticulate meloxicam, or a salt or derivative thereof, in combination
with a multiparticulate
modified release composition comprising at least two populations of
hydrocodone-comprising
particles, wherein administration of the nanoparticulate meloxicam to a
subject in a fasted state is
bioequivalent to administration of the nanoparticulate meloxicam to a subject
in a fed state.
In all of the embodiments above, the nanoparticulate meloxicam particles have
an effective
average particle size of less than about 2000 nm, and preferable also comprise
at least one surface
stabilizer adsorbed on or are associated with the surface of the meloxicam
particles.
This invention further discloses a method of making the nanoparticulate
meloxicam
compositions. Such a method comprises contacting the meloxicam particles with
at least one
surface stabilizer for a time and under conditions to reduce the effective
average particle size of
the meloxicam particles to less than about 2000 nm.
The present invention is also directed to methods of treatment including but
not limited to,
the treatment of pain, comprising administering a dosage form comprising a
therapeutically
effective amount of the composition of the invention to provide bimodal or
multimodal release of
the hydrocodone comprised therein.
16
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Other objects of the invention include provision of a once daily dosage form
of a
hydrocodone and a nanoparticulate meloxicam which, in operation, produces a
plasma profile
substantially similar to the plasma profile produced by the administration of
two immediate
release hydrocodone dosage forms given sequentially and a method for
prevention and treatment
of pain conditions based on the administration of such a dosage form.
The above objects are realized by a composition comprising a nanoparticulate
meloxicam,
or a salt or derivative thereof, in combination with a controlled release
composition having a first
component comprising a first population of hydrocodone particles, and a second
component or
formulation comprising a second population of hydrocodone particles. The
hydrocodone-
comprising particles of the second component further comprise a modified
release constituent
comprising a release coating or release matrix material, or both. Following
oral delivery, the
composition in operation delivers a hydrocodone in a pulsatile or zero order
manner. In one
embodiment of the invention, the compositions of the invention delivers a
hydrocodone in a
pulsatile or zero order manner during a period of up to twenty- four hours.
The present invention utilizes controlled release delivery of hydrocodone, or
a salt or
derivative thereof, from a solid oral dosage formulation to allow dosage less
frequently than
before, and preferably once-a-day administration, increasing patient
convenience and compliance.
The mechanism of controlled release would preferably utilize, but not be
limited to, erodable
formulations, diffusion controlled formulations and osmotic controlled
formulations. A portion of
the total dose may be released immediately to allow for rapid onset of effect.
The invention would
be useful in improving compliance and, therefore, therapeutic outcome for all
treatments requiring
a hydrocodone, including but not limited to, the treatment of pain conditions.
Preferred controlled release formulations are erodable formulations, diffusion
controlled
formulations and osmotic controlled formulations. According to the invention,
a portion of the
total dose may be released immediately to allow for rapid onset, of effect,
with the remaining
portion of the total dose released over an extended time period. The invention
would be useful in
improving compliance and, therefore, therapeutic outcome for all treatments
requiring a
hydrocodone.
Both the foregoing general description and the following brief description of
the figures
and the detailed description are exemplary and explanatory and are intended to
provide further
explanation of the invention as claimed. Other objects, advantages, and novel
features will be
readily apparent to those skilled in the art from the following detailed
description of the invention.
17
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
DESCRIPTION OF THE DRAWINGS
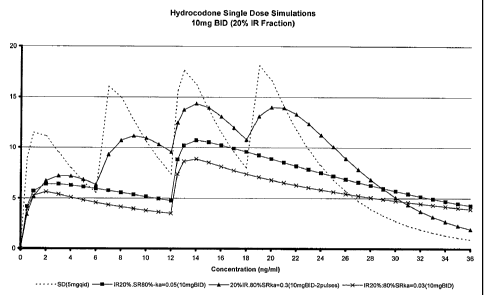
FIG. 1 shows single dose simulations of 10 mg hydrocodone formulations in
which 20% of
the hydrocodone is contained in the IR component.
FIG. 2 shows single dose simulations of 10 mg hydrocodone formulations in
which 20% of
the hydrocodone is contained in the JR component.
FIG. 3 shows steady state simulations of 10 mg hydrocodone formulations in
which 20%
of the hydrocodone is contained in the JR component.
FIG. 4 shows steady state simulations of 10 mg hydrocodone formulations in
which 20%
of the hydrocodone is contained in the JR component.
FIG. 5 shows single dose simulations of 10 mg hydrocodone formulations in
which 50% of
the hydrocodone is contained in the JR component.
FIG. 6 shows single dose simulations of 10 mg hydrocodone formulations in
which 50% of
the hydrocodone is contained in the JR component.
FIG. 7 shows steady state simulations of 10 mg hydrocodone formulations in
which 50%
of the hydrocodone is contained in the JR component.
FIG. 8 shows steady state simulations of 10 mg hydrocodone formulations in
which 50%
of the hydrocodone is contained in the IR component.
FIG. 9 shows single dose simulations of 20-160 mg/day hydrocodone formulations
(Option 1) in which 20% of the hydrococlone is contained in the IR component.
FIG. 10 shows steady state simulations of 20-160 mg/day hydrocodone
formulations
(Option 1) in which 20% of the hydrocodone is contained in the JR component.
FIG. 11 shows single dose simulations of 20-80 mg BID hydrocodone formulations
(Option 3) in which 20% of the hydrocodone is contained in the IR component.
FIG. 12 shows steady state simulations of 20-80 mg BID hydrocodone
formulations
(Option 3) in which 20% of the hydrocodone is contained in the JR component.
FIG. 13 shows single dose simulations of 20-160 mg/day hydrocodone
formulations
(Option 1) in which 50% of the hydrocodone is contained in the JR component.
FIG. 14 shows steady state simulations of 20-160 mg/day hydrocodone
formulations
(Option 1) in which 50% of the hydrocodone is contained in the JR component.
18
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
FIG. 15 shows single dose simulations of 20-160 mg/day hydrocodone
formulations
(Option 3) in which 50% of the hydrocodone is contained in the JR component.
FIG. 16 shows steady state simulations of 20-160 mg/day hydrocodone
formulations
(Option 3) in which 50% of the hydrocodone is contained in the JR component.
DETAILED DESCRIPTION OF THE INVENTION
The compositions of the invention comprise (a) a composition comprising a
nanoparticulate meloxicam, or a salt or derivative thereof, and at least one
surface stabilizer; and
(b) an oral controlled release composition of a hydrocodone, or a salt or
derivative thereof.
The purpose of the non-controlled release nanoparticulate meloxicam
compositoin in
combination with the controlled release hydrocodone is at least twofold: (1)
to provide increased
analgesia via drug synergy; and (2) To limit the intake of hydrocodone by
causing unpleasant and
often unsafe side effects at higher than prescribed doses.
The present invention provides a method of treating a patient needing pain
relief utilizing a
composition according to the invention. The method comprises administering a
therapeutically
effective amount of a dosage form, such as a solid oral dosage form,
comprising a nanoparticulate
meloxicam composition in combination with a controlled release hydrocodone
composition, to
provide a pulsed or bimodal or zero order delivery of the hydrocodone.
Advantages of the present
invention include reducing the dosing frequency required by conventional
multiple IR dosage
regimes while still maintaining the benefits derived from a pulsatile plasma
profile or eliminating
or minimizing the "peak" to "trough" ratio. This reduced dosing frequency is
advantageous in
terms of patient compliance to have a formulation which may be administered at
reduced
frequency. The reduction in dosage frequency made possible by utilizing the
present invention
would contribute to reducing health care costs by reducing the amount of time
spent by health care
workers on the
administration of drugs.
In one embodiment of the invention, the nanoparticulate meloxicam composition,
in
accordance with standard pharmacokinetic practice, has a bioavailability that
is about 100%
greater, about 90% greater, about 80% greater, about 70% greater, about 60%
greater, about 50%
greater, about 40% greater, about 30% greater, about 20% greater, or about 10%
greater than a
cnventional non-nanoparticulate meloxicam dosage form.
The compositions of the invention can be administered to a subject via any
conventional
means including, but not limited to, orally, rectally, ocularly, parenterally
(e.g., intravenous,
intramuscular, or subcutaneous), intracisternally, pulmonary, intravaginally,
intraperitoneally,
locally (e.g., powders, ointments or drops), or as a buccal or nasal spray. As
used herein, the term
19
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
"subject" is used to mean an animal, preferably a mammal, including a human or
non-human. The
terms patient and subject may be used interchangeably.
Compositions suitable for parenteral injection may comprise physiologically
acceptable
sterile aqueous or nonaqueous solutions, dispersions, suspensions or
emulsions, and sterile
powders for reconstitution into sterile injectable solutions or dispersions.
Examples of suitable
aqueous and nonaqueous carriers, diluents, solvents, or vehicles including
water, ethanol, polyols
(propyleneglycol, polyethylene-glycol, glycerol, and the like), suitable
mixtures thereof, vegetable
oils (such as olive oil) and injectable organic esters such as ethyl oleate.
Proper fluidity can be
maintained, for example, by the use of a coating such as lecithin, by the
maintenance of the
required particle size in the case of dispersions, and by the use of
surfactants.
The compositions may also contain adjuvants such as preserving, wetting,
emulsifying,
and dispensing agents. Prevention of the growth of microorganisms can be
ensured by various
antibacterial and antifungal agents, such as parabens, chiorobutanol, phenol,
sorbic acid, and the
like. It may also be desirable to include isotonic agents, such as sugars,
sodium chloride, and the
like. Prolonged absorption of the injectable pharmaceutical form can be
brought about by the use
of agents delaying absorption, such as aluminum monostearate and gelatin.
Solid dosage forms for oral administration include, but are not limited to,
capsules, tablets,
pills, powders, and granules. In such solid dosage forms, the active agents
are admixed with at
least one of the following: (a) one or more inert excipients (or carriers),
such as sodium citrate or
dicalcium phosphate; (b) fillers or extenders, such as starches, lactose,
sucrose, glucose, mannitol,
and silicic acid; (c) binders, such as carboxymethylcellulose, alignates,
gelatin,
polyvinylpyrrolidone, sucrose, and acacia; (d) humectants, such as glycerol;
(e) disintegrating
agents, such as agar-agar, calcium carbonate, potato or tapioca starch,
alginic acid, certain
complex silicates, and sodium carbonate; (f) solution retarders, such as
paraffin; (g) absorption
accelerators, such as quaternary ammonium compounds; (h) wetting agents, such
as cetyl alcohol
and glycerol monostearate; (i) adsorbents, such as kaolin and bentonite; and
(j) lubricants, such as
talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium
lauryl sulfate, or
mixtures thereof. For capsules, tablets, and pills, the dosage forms may also
comprise buffering
agents.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups, and elixirs. In addition to
hydrocodone and meloxicam,
the liquid dosage forms may comprise inert diluents commonly used in the art,
such as water or
other solvents, solubilizing agents, and emulsifiers. Exemplary emulsifiers
are ethyl alcohol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl
benzoate,
propyleneglycol,1,3-butyleneglycol, dimethylformamide, oils, such as
cottonseed oil, groundnut
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
oil, corn germ oil, olive oil, castor oil, and sesame oil, glycerol,
tetrahydrofurfuryl alcohol,
polyethyleneglycols, fatty acid esters of sorbitan, or mixtures of these
substances, and the like.
Besides such inert diluents, the composition can also include adjuvants, such
as wetting
agents, emulsifying and suspending agents, sweetening, flavoring, and
perfuming agents.
"Therapeutically effective amount" as used herein with respect to a meloxicam
and
hydrocodone dosage shall mean that dosage that provides the specific
pharmacological response
for which a hydrocodone or a meloxicam is administered in a significant number
of subjects in
need of such treatment. It is emphasized that "therapeutically effective
amount,"
administered to a particular subject in a particular instance will not always
be effective in treating
the conditions described herein, even though such dosage is deemed a
"therapeutically effective
amount" by those skilled in the art. It is to be further understood that
meloxicam and hydrocodone
dosages are, in particular instances, measured as oral dosages, or with
reference to drug levels as
measured in blood.
One of ordinary skill will appreciate that effective amounts of a meloxicam
and a
hydrocodone can be determined empirically and can be employed in pure form or,
where such
forms exist, in pharmaceutically acceptable salt, ester, or prodrug form.
Actual dosage levels of a
meloxicam and a hydrocodone in the compositions of the invention may be varied
to obtain an
amount of a meloxicam and a hydrocodone that is effective to obtain a desired
therapeutic
response for a particular composition and method of administration. The
selected dosage level
therefore depends upon the desired therapeutic effect, the route of
administration, the potency of
the administered meloxicam, the desired duration of treatment, and other
factors.
Dosage unit compositions may contain such amounts of such submultiples thereof
as may
be used to make up the daily dose. It will be understood, however, that the
specific dose level for
any particular patient will depend upon a variety of factors: the type and
degree of the cellular or
physiological response to be achieved; activity of the specific agent or
composition employed; the
specific agents or composition employed; the age, body weight, general health,
sex, and diet of the
patient; the time of administration, route of administration, and rate of
excretion of the agent; the
duration of the treatment; drugs used in combination or coincidental with the
specific agent; and
like factors well known in the medical arts.
I. Controlled Release Hydrocodone
Component of the Compositions of the Invention
A. Definitions
As used herein, the term "enhancer" refers to a compound which is capable of
enhancing
the absorption and/or bioavailability of an active ingredient by promoting net
transport across the
21
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
gastrointestinal tract (GIT) in an animal, such as a human. Enhancers include
but are not limited to
medium chain fatty acids and salts, esters, ethers and derivatives thereof,
including glycerides and
triglycerides; non-ionic surfactants such as those that can be prepared by
reacting ethylene oxide
with a fatty acid, a fatty alcohol, an alkyiphenol or a sorbitan or glycerol
fatty acid ester;
cytochrome P450 inhibitors, P-glycoprotein inhibitors and the like; and
mixtures thereof.
The term "particulate" as used herein refers to a state of matter which is
characterized by
the presence of discrete particles, pellets, beads or granules irrespective of
their size, shape or
morphology. The term "multiparticulate" as used herein means a plurality of
discrete or
aggregated particles, pellets, beads, granules or mixture thereof,
irrespective of their size, shape or
morphology.
The term "modified release" as used herein with respect to the coating or
coating material
or used in any other context, means release which is not immediate release and
is taken to
encompass controlled release, sustained release and delayed release.
The term "time delay" as used herein refers to the duration of time between
administration of the
composition and the release of the hydrocodone from a particular component.
The term "lag time" as used herein refers to the time between delivery of the
hydrocodone from
one component and the subsequent delivery hydrocodone from another component.
The term "erodable" as used herein refers to formulations which may be worn
away, diminished,
or deteriorated by the action of substances within the body.
The term "diffusion controlled" as used herein refers to formulations which
may spread as
the result of their spontaneous movement, for example, from a region of higher
to one of lower
concentration.
The term "osmotic controlled" as used herein refers to formulations which may
spread as
the result of their movement through a semipermeable membrane into a solution
of higher
concentration that tends to equalize the concentrations of the formulation on
the two sides of the
membrane.
B. Exemplary Embodiments
The composition according to the invention comprises at least two populations
of
hydrocodone, or a salt or derivative thereof, comprising particles which have
different in vitro
dissolution profiles.
The multiparticulate modified release composition and dosage forms made
therefrom
comprise at least two hydrocodone-comprising components. In one embodiment,
the release of the
hydrocodone, or a salt or derivative thereof, from the second and subsequent
components, if any,
is modified such that there is a lag time between the release of hydrocodone,
or a salt or derivative
22
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
thereof, from the first component and each subsequent component. The number of
pulses in the
release profile arising from such a composition in operation will depend on
the number of
hydrocodone-comprising components in the composition. For example, a
composition comprising
two hydrocodone-comprising components will give rise to two pulses in the
release profile, and a
composition comprising three hydrocodone-comprising components will give rise
to up to three
pulses in the release profile. In another embodiment, the release of the
active ingredients from
subsequent components is modified such that the release of hydrocodone from
the first component
and each subsequent component begins substantially upon administration but
over different
periods of time and/or at different rates.
For example, a controlled release composition can have a first component
comprising a
first population of a hydrocodone, or a salt or derivative thereof, and a
second component
comprising a second population of a hydrocodone, or a salt or derivative
thereof. The
hydrocodone-comprising particles of the second component are coated with a
modified release
coating. Alternatively or additionally, the second population of
hydrocodonecomprising particles
further comprises a modified release matrix material. Following oral delivery,
the composition in
operation delivers the hydrocodone, or a salt or derivative thereof, in a
pulsatile or zero order
manner.
In one embodiment of the invention, the controlled release composition
comprising
hydrocodone, or a salt or derivative thereof, in operation delivers the
hydrocodone in a bimodal or
pulsatile or zero order manner. Such a composition in operation produces a
plasma profile which
substantially mimics that obtained by the sequential administration of two JR
hydrocodone doses.
The present invention further relates to a controlled release composition
comprising a
hydrocodone, or a salt or derivative thereof, which in operation produced a
plasma profile that
eliminates or minimizes the "peaks" and "troughs" produced by the
administration of two or more
JR dosage forms given sequentially if such a profile is beneficial. This type
of profile can be
obtained using a controlled release mechanism that allows for "zero-order"
delivery.
Any suitable dosage form can be used for the compositions of in the invention.
In one
embodiment, the invention provides solid oral dosage forms comprising a
composition according
to the invention.
The time release characteristics for the delivery of the hydrocodone, or a
salt or derivative
thereof, from each of the components may be varied by modifying the
composition of each
component, including modifying any of the excipients or coatings which may be
present. In
particular, the release of the hydrocodone, or a salt or derivative thereof,
may be controlled by
changing the composition and/or the amount of the modified release coating on
the particles, if
such a coating is present. If more than one modified release component is
present, the modified
23
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
release coating for each of these components may be the same or different.
Similarly, when
modified release is facilitated by the inclusion of a modified release matrix
material, release of the
hydrocodone, or a salt or derivative thereof, may be controlled by the choice
and amount of
modified release matrix material utilized. The modified release coating may be
present, in each
component, in any amount that is sufficient to yield the desired delay time
for each particular
component. The modified release coating may be preset, in each component, in
any amount that is
sufficient to yield the desired time lag between components.
The lag time or delay time for the release of the hydrocodone, or a salt or
derivative
thereof, from each component may also be varied by modifying the composition
of each of the
components, including modifying any excipients and coatings which may be
present. For
example, the first component may be an immediate release component wherein the
hydrocodone,
or a salt or derivative thereof, is released immediately upon administration.
Alternatively, the first
component may be, for example, a time-delayed immediate release component in
which the
hydrocodone, or a salt or derivative thereof, is released substantially in its
entirety immediately
after a time delay. The second component may be, for example, a time-delayed
immediate release
component as just described or, alternatively, a time-delayed sustained
release or extended release
component in which the hydrocodone, or a salt or derivative thereof, is
released in a controlled
fashion over an extended period of time.
It will be understood that suitable hydrocodones also include all
pharmaceutically
acceptable salts, acids, esters, complexes or other derivatives of
hydrocodone, and may be
present either in the form of one enantiomer or as a mixture, racemic or
otherwise, of enantiomers.
The hydrocodone in each component may be the same or different. In one
embodiment,
the first component comprises a first hydrocodone, or a salt or derivative
thereof, and the second
component comprises a second hydrocodone, or a salt or derivative thereof. In
another
embodiment, two or more hydrocodones may be incorporated into one or more
components.
Further, a hydrocodone present in one component of the composition may be
accompanied by, for
example, an enhancer compound or a sensitizer compound in another component of
the
composition, to modify the bioavailability or therapeutic effect of the
hydrocodone.
The amount of the hydrocodone comprised in the composition and in dosage forms
made
therefrom may be allocated evenly or unevenly across the different particle
populations
comprising the components of the composition and comprised in the dosage forms
made
therefrom. In one embodiment, the hydrocodone, or a salt or derivative
thereof, comprised in the
particles of the first component comprises a minor portion of the total amount
of hydrocodone, or
a salt or derivative thereof, in the composition or dosage form, and the
amount of the
hydrocodone, or a salt or derivative thereof, in the other components
comprises a major portion of
the total amount of hydrocodone, or a salt or derivative thereof, in the
composition or dosage
24
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
form. In one such embodiment comprising two components, about 20% of the total
amount of the
hydrocodone, or a salt or derivative thereof, is comprised in the particles of
the first component,
and about 80% of the total amount of the hydrocodone, or a salt or derivative
thereof, is comprised
in the particles of the second component.
The hydrocodone, or a salt or derivative thereof, is preferably present in the
composition and in
dosage forms made therefrom in an amount of from about 0.1 to about 1000 mg,
from about 1 to
about 160 mg, or from about 5 to about 80 mg. Depending at least in part on
the particular
hydrocodone, or a salt or derivative thereof, that are included in the
composition and dosage
forms, the hydrocodone, or a salt or derivative thereof, is present in an
amount of from about 5 to
about 80 mg, about 5 to about 60 mg, about 5 to about 40 mg, about 5 to about
20 mg, about 5 to
about 10 mg, about 10 to about 80 mg, about 10 to about 60 mg, about 10 to
about 40 mg, about
10 to about 20 mg, about 20 to about 80 mg, about 20 to about 60 mg, about 20
to about 40 mg,
about 40 to about 80 mg, about 40 to about 60 mg, or about 60 to about 80 mg.
When the active ingredient is hydrocodone, it is preferably present in the
composition and
in dosage forms made therefrom in an amount of from about 5 to about 160 mg;
more preferably
the active ingredient is present in the first component in an amount of from
about 10 to about 80
mg.
The profile for the release of the hydrocodone, or a salt or derivative
thereof, from each
component of the composition may be varied by modifying the composition of
each component,
including modifying any of the excipients or coatings which may be present. In
particular the
release of the hydrocodone, or a salt or derivative thereof, may be controlled
by the choice and
amount of the modified release coating applied to the particles where such a
coating is present. If
more than one modified release component is present, the modified release
coating for each of
these components may be the same or different. Similarly, when the modified
release is
accomplished by means of a modified release matrix material, release of the
hydrocodone, or a
salt or derivative thereof, may be controlled by the choice and amount of
modified release matrix
material utilized.
For example, the modified release coating applied to the second population of
a hydroco
done, or a salt or derivative thereof, causes a lag time between the release
of active from the first
population of active hydroco done-comprising particles and the release of
active from the second
population of hydrocodone-comprising particles. Similarly, the presence of a
modified release
matrix material in the second population of hydrocodone-comprising particles
causes a lag time
between the release of hydrocodone from the first population of hydrocodone-
comprising particles
and the release of hydrocodone, or a salt or derivative thereof, from the
second population of
hydrocodone-comprising particles. The duration of the lag time may be varied
by altering the
composition and/or the amount of the modified release coating and/or altering
the composition
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
and/or amount of modified release matrix material utilized. Thus, the duration
of the lag time can
be designed to mimic a desired plasma profile.
In one embodiment, the first component may be an immediate release component
wherein the
hydrocodone, or a salt or derivative thereof, comprised therein is released
substantially
immediately upon administration. In another embodiment, the first component
may be a delayed
release component in which the hydrocodone, or a salt or derivative thereof,
is released
substantially immediately after a time delay. In either of such embodiments,
the second
component may be a modified release component in which the hydrocodone, or a
salt or
derivative thereof, is released over a period of time or substantially
immediately after a time delay.
In another embodiment, the controlled release composition comprise an
immediate release
component and at least one modified release component, the immediate release
component
comprising a first population of hydrocodone-comprising particles and the
modified release
components comprising second and subsequent populations of hydrocodone-
comprising particles.
The second and subsequent modified release components may comprise a
controlled release
coating. Additionally or alternatively, the second and subsequent modified
release components
may comprise a modified release matrix material. In operation, administration
of such a multi-
particulate modified release composition having, for example, a single
modified release
component results in characteristic pulsatile plasma concentration levels of
the hydrocodone in
which the immediate release component of the composition gives rise to a first
peak in the plasma
profile and the modified release component gives rise to a second peak in the
plasma profile.
Embodiments of the invention comprising more than one modified release
component give rise to
further peaks in the plasma profile.
As will be appreciated by those skilled in the art, the exact nature of the
plasma profile will
be influenced by the combination of all of the factors described above. Thus
by variation of the
composition of each component thereof, including the amount and nature of the
hydrocodone, or a
salt or derivative thereof, and the modified release coating or modified
matrix material, if any,
numerous plasma profiles may result therefrom upon administration to a
patient. Depending on
the release profile of each component, the plasma profile resulting therefrom
may be bimodal or
multimodal, and may define well separated and clearly defined peaks associated
with each
component (e.g., when the lag time between immediate release and delayed
release components is
long) or superimposed peaks associated with each component (e.g., in when the
lag time is short).
For example, administration of a multiparticulate modified release composition
having an
immediate release component and a single modified release component can result
in a plasma
profile in which the immediate release component of the composition gives rise
to a first peak in
the plasma profile and the modified release component gives rise to a second
peak in the plasma
profile. Embodiments of the invention comprising more than one modified
release component
may give rise to further peaks in the plasma profile. Alternatively,
administration of a
26
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
multiparticulate modified release composition having an immediate release
component and one or
more modified release components can result in a bimodal or multimodal release
profile but a
plasma profile having a single peak or peaks fewer in number than the number
of components
contained in the composition.
The plasma profile produced from the administration of a single dosage unit of
the present
invention is advantageous when it is desirable to deliver two or more portions
of hydrocodone, or
a salt or derivative thereof, without the need for administration of two or
more dosage units.
Additionally, in the case of some disorders it is particularly useful to have
such a bimodal plasma
profile. In embodiments which include drug compounds used for pain management,
such as for
example hydrocodone, the compositions and dosage forms of the present
invention may provide
continuous analgesia for up to 24 hours by providing minimum peak to trough
fluctuations in
plasma levels and reduce or eliminate side effects associated with such drug
compounds.
Any coating material which modifies the release of the hydrocodone, or a salt
or derivative
thereof, in the desired manner may be used in the practice of the present
invention. In particular,
coating materials suitable for use in the practice of the invention include
but are not limited to
polymer coating materials, such as cellulose acetate phthalate, cellulose
acetate trimaletate,
hydroxy propyl methylcellulose phthalate, polyvinyl acetate phthalate,
ammonio. methacrylate
copolymers such as those sold under the Trade Mark Eudragit RS and RL, poly
acrylic acid and
poly acrylate and methacrylate copolymers such as those sold under the
trademark Eudragit S
and L, polyvinyl acetaldiethylamino acetate, hydroxypropyl methylcellulose
acetate succinate,
shellac; hydrogels and gel-forming materials, such as carboxyvinyl polymers,
sodium alginate,
sodium carmellose, calcium carmellose, sodium carboxymethyl starch, poly vinyl
alcohol,
hydroxyethyl cellulose, methyl cellulose, gelatin, starch, and cellulose based
cross-linked
polymers in which the degree of crosslinking is low so as to facilitate
adsorption of water and
expansion of the polymer matrix, hydroxypropyl cellulose, hydroxypropyl
methylcellulose,
polyvinylpyrrolidone, crosslinked starch, microcrystalline cellulose, chitin,
aminoacryl-
methacrylate copolymer (Eudragit RS-PM, Rohm & Haas), pullulan, collagen,
casein, agar, gum
arabic, sodium carboxymethyl cellulose, (swellable hydrophilic polymers)
poly(hydroxyalkyl
methacrylate) (m. wt. -5k-5,000k), polyvinylpyrrolidone (m. wt. -lOk-360k),
anionic and cationic
hydrogels, polyvinyl alcohol having a low acetate residual, a swellable
mixture of agar and
carboxymethyl cellulose, copolymers of maleic anhydride and styrene, ethylene,
propylene or
isobutylene, pectin (m. wt. -30k-300k), polysaccharides such as agar, acacia,
karaya, tragacanth,
algins and guar, polyacrylamides, Polyox polyethylene oxides (m. wt. -100k-
5,000k),
AquaKeep acrylate polymers, diesters of polyglucan, crosslinked polyvinyl
alcohol and poly N-
vinyl-2-pyrrolidone, sodium starch glycolate (e.g., Explotab ; Edward Mandell
C. Ltd.);
hydrophilic polymers such as polysaccharides, methyl cellulose, sodium or
calcium
carboxymethyl cellulose, hydroxypropyl methyl cellulose, hydroxypropyl
cellulose, hydroxyethyl
27
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
cellulose, nitro cellulose, carboxymethyl cellulose, cellulose ethers,
polyethylene oxides (e.g.
Polyox , Union Carbide), methyl ethyl cellulose, ethylhydroxy ethylcellulose,
cellulose acetate,
cellulose butyrate, cellulose propionate, gelatin, collagen, starch,
maltodextrin, pullulan, polyvinyl
pyrrolidone, polyvinyl alcohol, polyvinyl acetate, glycerol fatty acid esters,
polyacrylamide,
polyacrylic acid, copolymers of methacrylic acid or methacrylic acid (e.g.,
Eudragit , Rohm and
Haas), other acrylic acid derivatives, sorbitan esters, natural gums,
lecithins, pectin, alginates,
ammonia alginate, sodium, calcium, potassium alginates, propylene glycol
alginate, agar, and
gums such as arabic, karaya, locust bean, tragacanth, carrageens, guar,
xanthan, scleroglucan and
mixtures and blends thereof.
Excipients such as plasticisers, lubricants, solvents and the like may be
added to the
coating. Suitable plasticisers include for example acetylated monoglycerides;
butyl phthalyl butyl
glycolate; dibutyl tartrate; diethyl phthalate; dimethyl phthalate; ethyl
phthalyl ethyl glycolate;
glycerin; propylene glycol; triacetin; citrate; tripropioin; diacetin; dibutyl
phthalate; acetyl
monoglyceride; polyethylene glycols; castor oil; triethyl citrate; polyhydric
alcohols, glycerol,
acetate esters, gylcerol triacetate, acetyl triethyl citrate, dibenzyl
phthalate, dihexyl phthalate,
butyl octyl phthalate, diisononyl phthalate, butyl octyl phthalate, dioctyl
azelate, epoxidized
tallate, triisoctyl trimellitate, diethylhexyl phthalate, di-n-octyl
phthalate, di-i-octyl phthalate, di-i-
decyl phthalate, di-n-undecyl phthalate, di-n-tridecyl phthalate, tri-2-
ethylhexyl trimellitate, di-2-
ethylhexyl adipate, di-2-ethylhexyl sebacate, di-2-ethylhexyl azelate, dibutyl
sebacate.
When the modified release component comprises a modified release matrix
material, any
suitable modified release matrix material or suitable combination of modified
release matrix
materials may be used. Such materials are known to those skilled in the art.
The term "modified
release matrix material" as used herein includes hydrophilic polymers,
hydrophobic polymers and
mixtures thereof which are capable of modifying the release of a hydrocodone,
or a salt or
derivative thereof, dispersed therein in vitro or in vivo. Modified release
matrix materials suitable
for the practice of the present invention include but are not limited to
microcrystalline cellulose,
sodium carboxymethylcellulose, hydroxyalkylcelluloses such as
hydroxypropylmethyl-cellulose
and hydroxypropylcellulose, polyethylene oxide, alkylcelluloses such as
methylcellulose and
ethylcellulose, polyethylene glycol, polyvinylpyrrolidone, cellulose acetate,
cellulose acetate
butyrate, cellulose acetate phthalate, cellulose acetate trimellitate,
polyvinylacetate phthalate,
polyalkylmethacrylates, polyvinyl acetate and mixture thereof.
A multiparticulate modified release composition according to the present
invention may be
incorporated into any suitable dosage form which facilitates release of the
hydrocodone, or a salt
or derivative thereof, in a bimodal or multimodal manner. Typically, the
dosage form may be a
blend of the different populations of a hydrocodone, or a salt or derivative
thereof, comprising
particles which make up the immediate release and the modified release
components, the blend
28
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
being filled into suitable capsules, such as hard or soft gelatin capsules.
Alternatively, the different
individual populations of a hydrocodone, or a salt or derivative thereof,
comprising particles may
be compressed (optionally with additional excipients) into mini-tablets which
may be
subsequently filled into capsules in the appropriate proportions. Another
suitable dosage form is
that of a multilayer tablet. In such dosage forms, the first component of the
multiparticulate
modified release composition may be compressed into one layer with the second
component being
subsequently added as a second layer of the multilayer tablet. The populations
of hydrocodone, or
a salt or derivative thereof, comprising particles comprising the composition
of the invention may
further be included in rapidly dissolving dosage forms such as an effervescent
dosage form or a
fast-melt dosage form.
In one embodiment, the composition of the invention and the dosage forms made
therefrom release the hydrocodone, or a salt or derivative thereof, such that
substantially all
hydrocodone, or a salt or derivative thereof, comprised in the first component
is released prior to
release of a hydrocodone, or a salt or derivative thereof, from the second
component. For example,
when the first component comprises an IR component, release of the
hydrocodone, or a salt or
derivative thereof, from the second component may be delayed until
substantially all the
hydrocodone, or a salt or derivative thereof, in the JR component has been
released. Release of the
hydrocodone, or a salt or derivative thereof, from the second component may be
delayed as
detailed above by the use of a modified release coating andlor a modified
release matrix material.
Because the plasma profile produced by the controlled release hydrocodone
composition
upon administration can be substantially similar to the plasma profile
produced by the
administration of two or more JR hydrocodone dosage forms given sequentially,
the controlled
release composition of the present invention is particularly useful for
administering a
hydrocodone, or a salt or derivative thereof, for which patient tolerance may
be problematical.
This controlled release composition is therefore advantageous for reducing or
minimizing the
development of patient tolerance to the hydrocodone, or a salt or derivative
thereof, in the
composition.
When it is desirable to minimize patient tolerance by providing a dosage
regime which
facilitates wash-out of a first dose of hydrocodone, or a salt or derivative
thereof, from a patient's
system, release of the hydrocodone, or a salt or derivative thereof, from the
second component is
delayed until substantially all of the hydrocodone, or a salt or derivative
thereof, comprised in the
first component has been released, and further delayed until at least a
portion of the hydrocodone,
or a salt or derivative thereof, released from the first component has been
cleared from the
patient's system. In one embodiment, release of the hydrocodone, or a salt or
derivative thereof,
from the second component of the composition is substantially, if not
completely, delayed for a
period of at least about two hours after administration of the composition.
29
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
In another embodiment, the composition of the invention and the dosage forms
made
therefrom release the hydrocodone, or a salt or derivative thereof, such that
the hydrocodone, or a
salt or derivative thereof, comprised in the first component is released
during the release of
hydrocodone, or a salt or derivative thereof, from the second component. In
one such
embodiment, release of the hydrocodone, or a salt or derivative thereof, from
the second
component of the composition occurs during and beyond the release of the
hydrocodone, or a salt
or derivative thereof, from the first component.
C. Other Types of Controlled Release Hydrocodone Compositions
As described herein, the invention includes various types of controlled
release systems by
which the hydrocodone, or a salt or derivative thereof, may be delivered in a
pulsatile or zero
order manner. These systems include, but are not limited to: films with the
hydrocodone, or a salt
or derivative thereof, in a polymer matrix (monolithic devices); the
hydrocodone, or a salt or
derivative thereof, contained by the polymer (reservoir devices); polymeric
colloidal particles or
microencapsulates (microparticles, microspheres or nanoparticles) in the form
of reservoir and
matrix devices; hydrocodone, or a salt or derivative thereof, contained by a
polymer containing a
hydrophilic and/or leachable additive e.g., a second polymer, surfactant or
plasticiser, etc. to give
a porous device, or a device in which the hydrocodone, or a salt or derivative
thereof, release may
be osmotically `controlled' (both reservoir and matrix devices); enteric
coatings (ionise and
dissolve at a suitable pH); (soluble) polymers with (covalently) attached
`pendant' hydrocodone,
or a salt or derivative thereof, molecules; devices where release rate is
controlled dynamically:
e.g., the osmotic pump.
The delivery mechanism of the invention will control the rate of release of
the drug. While
some mechanisms will release the hydrocodone, or a salt or derivative thereof,
at a constant rate
(zero order), others will vary as a function of time depending on factors such
as changing
concentration gradients or additive leaching leading to porosity, etc.
Polymers used in sustained
release coatings are necessarily biocompatible, and ideally biodegradable.
Examples of both
naturally occurring polymers such as Aquacoat (FMC Corporation, Food &
Pharmaceutical
Products Division, Philadelphia, USA) (ethylcellulose mechanically spheronised
to sub-micron
sized, aqueous based, pseudo-latex dispersions), and also synthetic polymers
such as the
Eudragit (Rohni Pharma, Weiterstadt.) range of poly(acrylate, methacrylate)
copolymers are
known in the art.
1. Reservoir Devices
A typical approach to controlled release is to encapsulate or contain the
hydrocodone, or a
salt or derivative thereof, entirely (e.g., as a core), within a polymer film
or coat (i.e., micro
capsules or spray/pan coated cores).
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
The various factors that can affect the diffusion process may readily be
applied to reservoir
devices (e.g., the effects of additives, polymer functionality {and, hence,
sink-solution pH}
porosity, film casting conditions, etc.) and, hence, the choice of polymer
must be an important
consideration in the development of reservoir devices. Modeling the release
characteristics of
reservoir devices (and monolithic devices) in which the transport of the
hydrocodone, or a salt or
derivative thereof, is by a solution-diffusion mechanism therefore typically
involves a solution to
Ficks second law (unsteady-state conditions; concentration dependent flux) for
the relevant
boundary conditions. When the device contains dissolved hydrocodone, or a salt
or derivative
thereof,, the rate of release decreases exponentially with time as the
concentration (activity) of the
agent (i.e., the driving force for release) within the device decreases (i.e.,
first order release). If,
however, the hydrocodone, or a salt or derivative thereof, is in a saturated
suspension, then the
driving force for release is kept constant (zero order) until the device is no
longer saturated.
Alternatively the release-rate kinetics may be desorption controlled, and a
function of the square
root of time.
Transport properties of coated tablets, may be enhanced compared to
freepolymer films,
due to the enclosed nature of the tablet core (permeant) which may enable the
internal build-up of
an osmotic pressure which will then act to force the permeant out of the
tablet.
The effect of deionised water on salt containing tablets coated in
poly(ethylene glycol)
(PEG)-containing silicone elastomer, and also the effects of water on free
films has been
investigated. The release of salt from the tablets was found to be a mixture
of diffusion through
water filled pores, formed by hydration of the coating, and osmotic pumping.
KC1 transport
through films containing just 10% PEG was negligible, despite extensive
swelling observed in
similar free films, indicating that porosity was necessary for the release of
the KC1 which then
occurred by `trans-pore diffusion.' Coated salt tablets, shaped as disks, were
found to swell in
deionised water and change shape to an oblate spheroid as a result of the
build-up of internal
hydrostatic pressure: the change in shape providing a means to measure the
`force' generated. As
might be expected, the osmotic force decreased with increasing levels of PEG
content. The lower
PEG levels allowed water to be imbibed through the hydrated polymer; whilst
the porosity
resulting from the coating dissolving at higher levels of PEG content (20 to
40%) allowed the
pressure to be relieved by the flow of KC1.
Methods and equations have been developed, which by monitoring (independently)
the
release of two different salts (e.g., KC1 and NaC1) allowed the calculation of
the relative
magnitudes that both osmotic pumping and trans-pore diffusion contributed to
the release of salt
from the tablet. At low PEG levels, osmotic flow was increased to a greater
extent than was trans-
pore diffusion due to the generation of only a low pore number density: at a
loading of 20%, both
mechanisms contributed approximately equally to the release. The build-up of
hydrostatic
31
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
pressure, however, decreased the osmotic inflow, and osmotic pumping. At
higher loadings of
PEG, the hydrated film was more porous and less resistant to outflow of salt.
Hence, although the
osmotic pumping increased (compared to the lower loading), trans-pore
diffusion was the
dominant release mechanism. An osmotic release mechanism has also been
reported for
microcapsules containing a water soluble core.
2. Monolithic Devices (Matrix Devices)
Monolithic (matrix) devices are possibly the most common of the devices for
controlling
the release of drugs. This is possibly because they are relatively easy to
fabricate, compared to
reservoir devices, and there is not the danger of an accidental high dosage
that could result from
the rupture of the membrane of a reservoir device. In such a device the
hydrocodone, or a salt or
derivative thereof, is present as a dispersion within the polymer matrix, and
they are typically
formed by the compression of a polymer/drug mixture or by dissolution or
melting. The dosage
release properties of monolithic devices may be dependent upon the solubility
of the hydrocodone,
or a salt or derivative thereof, in the polymer matrix or, in the case of
porous matrixes, the
solubility in the sink solution within the particle's pore network, and also
the tortuosity of the
network (to a greater extent than the permeability of the film), dependent on
whether the
hydrocodone, or a salt or derivative thereof, is dispersed in the polymer or
dissolved in the
polymer. For low loadings of drug, (0 to 5% W/V) the hydrocodone, or a salt or
derivative thereof,
will be released by a solution-diffusion mechanism (in the absence of pores).
At higher loadings
(5 to 10% W/V), the release mechanism will be complicated by the presence of
cavities formed
near the surface of the device as the hydrocodone, or a salt or derivative
thereof, is lost: such
cavities fill with fluid from the environment increasing the rate of release
of the drug.
It is common to add a plasticiser (e.g., a poly(ethylene glycol)), or
surfactant, or adjuvant
(i.e., an ingredient which increases effectiveness), to matrix devices (and
reservoir devices) as a
means to enhance the permeability (although, in contrast, plasticiser may be
fugitive, and simply
serve to aid film formation and, hence, decrease permeability - a property
normally more desirable
in polymer paint coatings). It was noted that the leaching of PEG acted to
increase the
permeability of (ethyl cellulose) films linearly as a function of PEG loading
by increasing the
porosity, however, the films retained their barrier properties, not permitting
the transport of
electrolyte. It was deduced that the enhancement of their permeability was as
a result of the
effective decrease in thickness caused by the PEG leaching. This was evinced
from plots of the
cumulative permeant flux per unit area as a function of time and film
reciprocal thickness at a
PEG loading of 50% W/V: plots showing a linear relationship between the rate
of permeation and
reciprocal film thickness, as expected for a (Fickian) solution-diffusion type
transport mechanism
in a homogeneous membrane. Extrapolation of the linear regions of the graphs
to the time axis
gave positive intercepts on the time axis: the magnitude of which decreased
towards zero with
decreasing film thickness. These changing lag times were attributed to the
occurrence of two
32
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
diffusional flows during the early stages of the experiment (the flow of the
`drug' and also the
flow of the PEG), and also to the more usual lag time during which the
concentration of permeant
in the film is building-up. Caffeine, when used as a permeant, showed negative
lag times. No
explanation of this was forthcoming, but it was noted that caffeine exhibited
a low partition
coefficient in the system, and that this was also a feature of aniline
permeation through
polyethylene films which showed a similar negative time lag.
The effects of added surfactants on (hydrophobic) matrix devices has been
investigated. It
was thought that surfactant may increase the hydrocodone, or a salt or
derivative thereof, release
rate by three possible mechanisms: (i) increased solubilisation, (ii) improved
`wettability' to the
dissolution media, and (iii) pore formation as a result of surfactant
leaching. For the system
studied (Eudragit RL 100 and RS 100 plasticised by sorbitol, Flurbiprofen as
the drug, and a
range of surfactants) it was concluded that improved wetting of the tablet led
to only a partial
improvement in drug release (implying that the release was diffusion, rather
than dissolution,
controlled), although the effect was greater for Eudragit RS than Eudragit
RL, whilst the
greatest influence on release was by those surfactants that were more soluble
due to the formation
of `disruptions' in the matrix allowing the dissolution medium access to
within the matrix. This is
of obvious relevance to a study of latex films which might be suitable for
pharmaceutical coatings,
due to the ease with which a polymer latex may be prepared with surfactant as
opposed to
surfactant-free. Differences were found between the two polymers - with only
the Eudragit RS
showing interactions between the anionic/cationic surfactant and drug. This
was ascribed to the
differing levels of quaternary ammonium ions on the polymer.
Composite devices consisting of a polymer/drug matrix coated in a polymer
containing no
drug also exist. Such a device was constructed from aqueous Eudragit latices,
and was found to
give zero order release by diffusion of the drug from the core through the
shell. Similarly, a
polymer core containing the drug has been produced, but coated this with a
shell that was eroded
by the gastric fluid. The rate of release of the drug was found to be
relatively linear (a function of
the rate limiting diffusion process through the shell) and inversely
proportional to the shell
thickness, whereas the release from the core alone was found to decrease with
time.
3. Microspheres
Methods for the preparation of hollow microspheres ('microballoons') with the
drug
dispersed in the sphere's shell, and also highly porous matrix-type
microspheres (`microsponges')
have been described. The microsponges were prepared by dissolving the drug and
polymer in
ethanol. On addition to water, the ethanol diffused from the emulsion droplets
to leave a highly
porous particle.
33
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
The hollow microspheres were formed by preparing a solution of
ethanol/dichloro-
methane containing the drug and polymer. On pouring into water, this formed an
emulsion
containing the dispersed polymer/drug/solvent particles, by a coacervation-
type process, from
which the ethanol (a good solvent for the polymer) rapidly diffused
precipitating polymer at the
surface of the droplet to give a hard- shelled particle enclosing the drug,
dissolved in the
dichloromethane. At this point, a gas phase of dichioromethane was generated
within the particle
which, after diffusing through the shell, was observed to bubble to the
surface of the aqueous
phase. The hollow sphere, at reduced pressure, then filled with water, which
could be removed by
a period of drying. (No drug was found in the water.) A suggested use of the
microspheres was as floating drug delivery devices for use in the stomach.
4. Pendent devices
A means of attaching a range of drugs such as analgesics and antidepressants,
etc., by
means of an ester linkage to poly(acrylate) ester latex particles prepared by
aqueous emulsion
polymerization has been developed. These latices when passed through an ion
exchange resin
such that the polymer end groups were converted to their strong acid form
could `self-catalyse' the
release of the drug by hydrolysis of the ester link.
Drugs have been attached to polymers, and also monomers have been synthesized
with a
pendent drug attached. The research group have also prepared their own dosage
forms in which
the drug is bound to a biocompatible polymer by a labile chemical bond e.g.,
polyanhydrides
prepared from a substituted anhydride (itself prepared by reacting an acid
chloride with the drug:
methacryloyl chloride and the sodium salt of methoxy benzoic acid) were used
to form a matrix
with a second polymer (Eudragit RL) which released the drug on hydrolysis in
gastric fluid. The
use of polymeric Schiff bases suitable for use as carriers of pharmaceutical
amines has also been
described.
5. Enteric films
Enteric coatings consist of pH sensitive polymers. Typically the polymers are
carboxylated
and interact (swell) very little with water at low pH, whilst at high pH the
polymers ionise causing
swelling, or dissolving of the polymer. Coatings can therefore be designed to
remain intact in the
acidic environment of the stomach (protecting either the drug from this
environment or the
stomach from the drug), but to dissolve in the more alkaline environment of
the intestine.
6. Osmotically controlled devices
The osmotic pump is similar to a reservoir device but contains an osmotic
agent (e.g., the
active agent in salt form) which acts to imbibe water from the surrounding
medium via a semi-
permeable membrane. Such a device, called the `elementary osmotic pump', has
been described.
34
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Pressure is generated within the device which forces the active agent out of
the device via an
orifice (of a size designed to minimise solute diffusion, whilst preventing
the build-up of a
hydrostatic pressure head which has the effect of decreasing the osmotic
pressure and changing
the dimensions {volume} of the device). Whilst the internal volume of the
device remains
constant, and there is an excess of solid (saturated solution) in the device,
then the release rate
remains constant delivering a volume equal to the volume of solvent uptake.
7. Electrically stimulated release devices
Monolithic devices have been prepared using polyelectrolyte gels which
swelled when, for example, an external electrical stimulus was applied,
causing a change in pH.
The release could be modulated, by the current, giving a pulsatile release
profile.
8. Hydrogels
Hydrogels find a use in a number of biomedical applications, in addition to
their use in
drug matrices (e.g., soft contact lenses, and various `soft' implants, etc.).
II. Nanoparticulate Meloxicam Component of the Compositions of the Invention
The compositions of the invention comprise a nanoparticulate meloxicam
composition.
The nanoparticulate meloxicam composition comprises particles of meloxicam
having an effective
average particle size of less than about 2000 nm and preferably at least one
surface stabilizer
adsorbed on or associated with the surface of the drug.
Advantages of the nanoparticulate meloxicam compositions of the invention as
compared
to conventional, non-nanoparticulate or solubilized dosage forms of meloxicam
include, but are
not limited to: (1) smaller tablet or other solid dosage form size; (2)
smaller doses of drug required
to obtain the same pharmacological effect; (3) increased bioavailability; (4)
substantially similar
pharmacokinetic profiles of the meloxicam compositions when administered in
the fed versus the
fasted state; (5) bioequivalency of the meloxicam compositions when
administered in the fed
versus the fasted state; (6) an increased rate of dissolution for the
meloxicam compositions; and
(7) the meloxicam compositions can be used in conjunction with other active
agents useful in the
prevention and treatment of infective conditions.
The present invention also includes nanoparticulate meloxicam compositions
together with
one or more non-toxic physiologically acceptable carriers, adjuvants, or
vehicles, collectively
referred to as carriers. The compositions can be formulated for parental
injection (e.g.,
intravenous, intramuscular, or subcutaneous), oral administration in solid,
liquid, or aerosol form,
vaginal, nasal, rectal, ocular, local (powders, ointments, or drops), buccal,
intracisternal,
intraperitoneal, or topical administrations, and the like.
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
A preferred dosage form of the invention is a solid dosage form, although any
pharmaceutically acceptable dosage form can be utilized. Exemplary solid
dosage forms include,
but are not limited to, tablets, capsules, sachets, lozenges, powders, pills,
or granules, and the solid
dosage form can be, for example, a fast melt dosage form, controlled release
dosage form,
lyophilized dosage form, delayed release dosage form, extended release dosage
form, pulsatile
release dosage form, mixed immediate release and controlled release dosage
form, or a
combination thereof. A solid dose tablet formulation is preferred.
The present invention is described herein using several definitions, as set
forth below and
throughout the application.
The term "effective average particle size," as used herein, means that at
least about 50% of
the nanoparticulate meloxicam particles have a size of less than about 2000
nm, by weight or by
other suitable measurement technique (e.g., such as by volume, number, etc.),
when measured by,
for example, sedimentation flow fractionation, photon correlation
spectroscopy, light scattering,
disk centrifugation, and other techniques known to those of skill in the art.
As used herein, "about" will be understood by persons of ordinary skill in the
art and will
vary to some extent depending upon the context in which it is used. If there
are uses of the term
which are not clear to persons of ordinary skill in the art given the context
in which it is used,
"about" will mean up to plus or minus 10% of the particular term.
As used herein with reference to stable meloxicam particles, "stable" means
that the
particles do not appreciably flocculate or agglomerate due to interparticle
attractive forces or
otherwise increase in particle size. "Stable" connotes, but is not limited to
one or more of the
following parameters: (1) the particles do not appreciably flocculate or
agglomerate due to
interparticle attractive forces or otherwise significantly increase in
particle size over time; (2) the
physical structure of the particles is not altered over time, such as by
conversion from an
amorphous phase to a crystalline phase; (3) the particles are chemically
stable; and/or (4) where
the meloxicam or a salt or derivative thereof has not been subject to a
heating step at or above the
melting point of the meloxicam particles in the preparation of the
nanoparticles of the present
invention.
The term "conventional" or "non-nanoparticulate active agent" shall mean an
active agent
which is solubilized or which has an effective average particle size of
greater than about 2000 nm.
Nanoparticulate active agents as defined herein have an effective average
particle size of less than
about 2000 nm.
36
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
The phrase "poorly water soluble drugs" as used herein refers to drugs having
a solubility
in water of less than about 30 mg/mi, less than about 20 mg/mi, less than
about 10 mg/mi, or less
than about 1 mg/ml.
As used herein, the phrase "therapeuticaily effective amount" shaii mean that
drug dosage
that provides the specific pharmacological response for which the drug is
administered in a
significant number of subjects in need of such treatment. It is emphasized
that a therapeutically
effective amount of a drug that is administered to a particular subject in a
particular instance will
not always be effective in treating the conditions/diseases described herein,
even though such
dosage is deemed to be a therapeutically effective amount by those of skiil in
the art.
A. Preferred Characteristics of the Nanoparticulate Meloxicam
Compositions of the Invention
1. Increased Bioavailability
The nanoparticulate meloxicam formulations of the invention exhibit increased
bioavailability, and require smaller doses as compared to prior conventional,
nonnanoparticulate
meloxicam formulations.
2. Improved Pharmacokinetic Profiles
The invention also provides nanoparticulate meloxicam, or a salt or derivative
thereof,
compositions having a desirable pharmacokinetic profile when administered to
mammalian
subjects. The desirable pharmacokinetic profile of the compositions comprising
meloxicam
includes but is not limited to: (1) a Cmax for meloxicam, when assayed in the
plasma of a
mammalian subject following administration, that is preferably greater than
the C,,,aR for a non-
nanoparticulate formulation of the same meloxicam, administered at the same
dosage; andlor (2)
an AUC for meloxicam, when assayed in the plasma of a mammalian subject
following
administration, that is preferably greater than the AUC for a non-
nanoparticulate formulation of
the same meloxicam, administered at the same dosage; and/or (3) a T,,,aR for
meloxicam, when
assayed in the plasma of a mammalian subject following administration, that is
preferably less
than the T,,,aR for a non-nanoparticulate formulation of the same meloxicam,
administered at the
same dosage. The desirable pharmacokinetic profile, as used herein, is the
pharmacokinetic profile
measured after the initial dose of meloxicam or a salt or derivative thereof.
In one embodiment, a composition comprising a nanoparticulate meloxicam
exhibits in
comparative pharmacokinetic testing with a non-nanoparticulate formulation of
the same
meloxicam, administered at the same dosage, a T,,,aR not greater than about
90%, not greater than
about 80%, not greater than about 70%, not greater than about 60%, not greater
than about 50%,
not greater than about 40%, not greater than about 30%, not greater than about
25%, not greater
37
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
than about 20%, not greater than about 15%, not greater than about 10%, or not
greater than about
5% of the T,,,aR exhibited by the non-nanoparticulate meloxicam formulation.
In another embodiment, the composition comprising a nanoparticulate meloxicam
exhibits
in comparative pharmacokinetic testing with a non-nanoparticulate formulation
of the same
meloxicam, administered at the same dosage, a C,,,,,x which is at least about
50%, at least about
100%, at least about 200%, at least about 300%, at least about 400%, at least
about 500%, at least
about 600%, at least about 700%, at least about 800%, at least about 900%, at
least about 1000%,
at least about 1100%, at least about 1200%, at least about 1300%, at least
about 1400%, at least
about 1500%, at least about 1600%, at least about 1700%, at least about 1800%,
or at least about
1900% greater than the Cmax exhibited by the non-nanoparticulate meloxicam
formulation.
In yet another embodiment, the composition comprising a nanoparticulate
meloxicam
exhibits in comparative pharmacokinetic testing with a non-nanoparticulate
formulation of the
same meloxicam, administered at the same dosage, an AUC which is at least
about 25%, at least
about 50%, at least about 75%, at least about 100%, at least about 125%, at
least about 150%, at
least about 175%, at least about 200%, at least about 225%, at least about
250%, at least about
275%, at least about 300%, at least about 350%, at least about 400%, at least
about 450%, at least
about 500%, at least about 550%, at least about 600%, at least about 650%, at
least about 700%, at
least about 750%, at least about 800%, at least about 850%, at least about
900%, at least about
950%, at least about 1000%, at least about 1050%, at least about 1100 Io, at
least about 1150%, or
at least about 1200% greater than the AUC exhibited by the non-nanoparticulate
meloxicam
formulation.
In one embodiment of the invention, the Tmax of meloxicam, when assayed in the
plasma
of the mammalian subject, is less than about 6 to about 8 hours. In other
embodiments of the
invention, the Tmax of meloxicam is less than about 6 hours, less than about 5
hours, less than
about 4 hours, less than about 3 hours, less than about 2 hours, less than
about 1 hour, or less than
about 30 minutes after administration.
The desirable pharmacokinetic profile, as used herein, is the pharmacokinetic
profile
measured after the initial dose of meloxicam or a salt or derivative thereof.
The compositions can
be formulated in any way as described herein and as known to those of skill in
the art.
3. The Pharmacokinetic Profiles of the Meloxicam Compositions of the
Invention are not Affected by the Fed or Fasted State of the Subject
Ingesting the Compositions
The invention encompasses meloxicam compositions wherein the pharmacokinetic
profile
of meloxicam is not substantially affected by the fed or fasted state of a
subject ingesting the
composition. This means that there is no substantial difference in the
quantity of drug absorbed or
38
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
the rate of drug absorption when the nanoparticulate meloxicam compositions
are administered in
the fed versus the fasted state.
Benefits of a dosage form, which substantially eliminates the effect of food,
include an
increase in subject convenience, thereby increasing subject compliance, as the
subject does not
need to ensure that they are taking a dose either with or without food. This
is significant, as with
poor subject compliance an increase in the medical condition for which the
drug is being
prescribed may be observed.
4. Bioequivalency of Meloxicam Compositions of the
Invention When Administered in the Fed Versus the Fasted State
The invention also encompasses provides a nanoparticulate meloxicam
composition in
which administration of the composition to a subject in a fasted state is
bioequivalent to
administration of the composition to a subject in a fed state.
The difference in absorption (AUC) or Cmax of the nanoparticulate meloxicam
compositions of the invention, when administered in the fed versus the fasted
state, preferably is
less than about 60%, less than about 55%, less than about 50%, less than about
45%, less than
about 40%, less than about 35%, less than about 30%, less than about 25%, less
than about 20%,
less than about 15%, less than about 10%, less than about 5%, or less than
about 3%.
In one embodiment of the invention, the invention encompasses compositions
comprising
a nanoparticulate meloxicam, wherein administration of the composition to a
subject in a fasted
state is bioequivalent to administration of the composition to a subject in a
fed state, in particular
as defined by Cmax and AUC guidelines given by the U.S. Food and Drug
Administration and the
corresponding European regulatory agency (EMEA). Under U.S. FDA guidelines,
two products or
methods are bioequivalent if the 90% Confidence Intervals (CI) for AUC and
C,,,aR are between
0.80 to 1.25 (T,,,,,x measurements are not relevant to bioequivalence for
regulatory purposes). To
show bioequivalency between two compounds or administration conditions
pursuant to Europe's
EMEA guidelines, the 90% CI for AUC must be between 0.80 to 1.25 and the 90%
CI for C,,aR
must between 0.70 to 1.43.
5. Dissolution Profiles of the Meloxicam Compositions of the Invention
The nanoparticulate meloxicam compositions of the invention are proposed to
have
unexpectedly dramatic dissolution profiles. Rapid dissolution of an
administered active agent is
preferable, as faster dissolution generally leads to faster onset of action
and greater
bioavailability. To improve the dissolution profile and bioavailability of the
meloxicam, it would
be useful to increase the drug's dissolution sO that it could attain a level
close to 100%.
The meloxicam compositions of the invention preferably have a dissolution
profile in which
39
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
within about 5 minutes at least about 20% of the composition is dissolved. In
other embodiments
of the invention, at least about 30% or at least about 40% of meloxicam
composition is dissolved
within about 5 minutes. In yet other embodiments of the invention, preferably
at least about 40%,
at least about 50%, at least about 60%, at least about 70%, or at least about
80% of the meloxicam
composition is dissolved within about 10 minutes. Finally, in another
embodiment of the
invention, preferably at least about 70%, at least about 80%, at least about
90%, or at least about
100% of the meloxicam composition is dissolved within about 20 minutes.
Dissolution is preferably measured in a medium which is discriminating. Such a
dissolution medium will produce two very different dissolution curves for two
products having
very different dissolution profiles in gastric juices; i.e., the dissolution
medium is predictive of in
vivo dissolution of a composition. An exemplary dissolution medium is an
aqueous medium
containing the surfactant sodium lauryl sulfate at 0.025 M. Determination of
the amount dissolved
can be carried out by spectrophotometry. The rotating blade method (European
Pharmacopoeia)
can be used to measure dissolution.
6. Redispersibility Profiles of the Meloxicam Compositions of the
Invention
An additional feature of the meloxicam compositions of the invention is that
the
compositions redisperse such that the effective average particle size of the
redispersed meloxicam
particles is less than about 2 microns. This is significant, as if upon
administration the meloxicam
compositions of the invention did not redisperse to a substantially
nanoparticulate particle size,
then the dosage form may lose the benefits afforded by formulating the
meloxicam into a
nanoparticulate particle size.
This is because nanoparticulate active agent compositions benefit from the
small particle
size of the active agent; if the active agent does not redisperse into the
small particle sizes upon
administration, then "clumps" or agglomerated active agent particles are
formed, owing to the
extremely high surface free energy of the nanoparticulate system and the
thermodynamic driving
force to achieve an overall reduction in free energy. With the formation of
such agglomerated
particles, the bioavailability of the dosage form may fall well below that
observed with the liquid
dispersion form of the nanoparticulate active agent.
Moreover, the nanoparticulate meloxicam or a salt or derivative thereof
compositions of
the invention exhibit dramatic redispersion of the nanoparticulate meloxicam
particles upon
administration to a mammal, such as a human or animal, as demonstrated by
reconstitution/redispersion in a biorelevant aqueous media such that the
effective average particle
size of the redispersed meloxicam particles is less than about 2 microns. Such
biorelevant aqueous
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
media can be any aqueous media that exhibit the desired ionic strength and pH,
which form the
basis for the biorelevance of the media. The desired pH and ionic strength are
those that are
representative of physiological conditions found in the human body. Such
biorelevant aqueous
media can be, for example, aqueous electrolyte solutions or aqueous solutions
of any salt, acid, or
base, or a combination thereof, which exhibit the desired pH and ionic
strength.
Biorelevant pH is well known in the art. For example, in the stomach, the pH
ranges from
slightly less than 2 (but typically greater than 1) up to 4 or 5. In the small
intestine the pH can
range from 4 to 6, and in the colon it can range from 6 to 8. Biorelevant
ionic strength is also well
known in the art. Fasted state gastric fluid has an ionic strength of about
0.1M while fasted state
intestinal fluid has an ionic strength of about 0.14. See e.g., Lindahi et
al., "Characterization of
Fluids from the Stomach and Proximal Jejunum in Men and Women," Pharm. Res.,
14 (4): 497-
502 (1997).
It is believed that the pH and ionic strength of the test solution is more
critical than the
specific chemical content. Accordingly, appropriate pH and ionic strength
values can be obtained
through numerous combinations of strong acids, strong bases, salts, single or
multiple conjugate
acid-base pairs (i.e., weak acids and corresponding salts of that acid),
monoprotic and polyprotic
electrolytes, etc.
Representative electrolyte solutions can be, but are not limited to, HC1
solutions, ranging
in concentration from about 0.00 1 to about 0.1 M, and NaC1 solutions, ranging
in concentration
from about 0.00 1 to about 0.1 M, and mixtures thereof. For example,
electrolyte solutions can be,
but are not limited to, about 0.1 M HC1 or less, about 0.01 M HC1 or less,
about 0.00 1 M HC1 or
less, about 0.1 M NaC1 or less, about 0.01 M NaC1 or less, about 0.00 1 M NaC1
or less, and
mixtures thereof. Of these electrolyte solutions, 0.01 M HC1 and/or 0.1 M
NaC1, are most
representative of fasted human physiological conditions, owing to the pH and
ionic strength
conditions of the proximal gastrointestinal tract.
Electrolyte concentrations of 0.001 M HC1, 0.01 M HC1, and 0.1 M HCI
correspond to
pH 3, pH 2, and pH 1, respectively. Thus, a 0.01 M HC1 solution simulates
typical acidic
conditions found in the stomach. A solution of 0.1 M NaC1 provides a
reasonable approximation
of the ionic strength conditions found throughout the body, including the
gastrointestinal fluids,
although concentrations higher than 0.1 M may be employed to simulate fed
conditions within the
human GI tract.
Exemplary solutions of salts, acids, bases or combinations thereof, which
exhibit the
desired pH and ionic strength, include but are not limited to phosphoric
acid/phosphate salts +
sodium, potassium and calcium salts of chloride, acetic acid/acetate salts +
sodium, potassium and
41
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
calcium salts of chloride, carbonic acid/bicarbonate salts + sodium, potassium
and calcium salts of
chloride, and citric acid/citrate salts + sodium, potassium and calcium salts
of chloride.
In other embodiments of the invention, the redispersed meloxicam particles of
the
invention (redispersed in an aqueous, biorelevant, or any other suitable
media) have an effective
average particle size of less than about less than about 1900 nm, less than
about 1800 nm, less
than about 1700 nm, less than about 1600 nm, less than about 1500 nrn, less
than about 1400 nm,
less than about 1300 nm, less than about 1200 nm, less than about 1100 nm,
less than about 1000
nm, less than about 900 nm, less than about 800 nm, less than about 700 nm,
less than about 600
nm, less than about 500 nm, less than about 400 nm, less than about 300 nm,
less than about 250
nm, less than about 200 nm, less than about 150 nm, less than about 100 nm,
less than about 75
nm, or less than about 50 nm, as measured by light- scattering methods,
microscopy, or other
appropriate methods.
B. Nanoparticulate Meloxicam Compositions
The invention provides compositions comprising meloxicarn particles and at
least one
surface stabilizer. The surface stabilizers preferably are adsorbed on, or
associated with, the
surface of the meloxicam particles. Surface stabilizers especially useful
herein preferably
physically adhere on, or associate with, the surface of the nanoparticulate
meloxicam particles, but
do not chemically react with the meloxicam particles or itself. Individually
adsorbed molecules of
the surface stabilizer are essentially free of intermolecular crosslinkages.
The invention also includes meloxicam compositions together with one or more
nontoxic
physiologically acceptable carriers, adjuvants, or vehicles, collectively
referred to as carriers. The
compositions can be formulated for parenteral injection (e.g., intravenous,
intramuscular, or
subcutaneous), oral administration in solid, liquid, or aerosol form, vaginal,
nasal, rectal, ocular, local (powders, ointments or drops), buccal,
intracistemal, ntraperitoneal, or
topical administration, and the like.
1. Meloxicam Particles
The compositions of the invention comprise particles of meloxicam or a salt or
derivative
thereof. The particles can be in a crystalline phase, semi-crystalline phase,
amorphous phase,
semi-amorphous phase, or a combination thereof.
2. Surface Stabilizers
Combinations of more than one surface stabilizers can be used in the
invention. Useful
surface stabilizers which can be employed in the invention include, but are
not limited to, known
organic and inorganic pharmaceutical excipients. Such excipients include
various polymers, low
42
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
molecular weight oligomers, natural products, and surfactants. Exemplary
surface stabilizers
include nonionic, ionic anionic, cationic, and zwitterionic surfactants or
compounds.
Representative examples of surface stabilizers include hydroxypropyl
methylcellulose
(now known as hypromellose), hydroxypropylcellulose, polyvinylpyrrolidone,
sodium lauryl
sulfate, dioctylsulfosuccinate, gelatin, casein, lecithin (phosphatides),
dextran, gum acacia,
cholesterol, tragacanth, stearic acid, benzalkonium chloride, calcium
stearate, glycerol
monostearate, cetostearyl alcohol, cetomacrogol emulsifying wax, sorbitan
esters,
polyoxyethylene alkyl ethers (e.g., macrogol ethers such as cetomacrogol
1000), polyoxyethylene
castor oil derivatives, polyoxyethylene sorbitan fatty acid esters (e.g., the
commercially available
Tweens such as e.g., Tween 20 and Tween 80 (ICI Speciality Chemicals));
polyethylene
glycols (e.g., Carbowaxs 3550 and 934 (Union Carbide)), polyoxyethylene
stearates, colloidal
silicon dioxide, phosphates, carboxymethylcellulose calcium,
carboxymethylcellulose sodium,
methylcellulose, hydroxyethylcellulose, hypromellose phthalate, noncrystalline
cellulose,
magnesium aluminium silicate, triethanolamine, polyvinyl alcohol (PVA), 4-
(1,1,3,3-
tetramethylbutyl) -phenol polymer with ethylene oxide and formaldehyde (also
known as
tyloxapol, superione, and triton), poloxamers (e.g., Pluronics F68 and F108 ,
which are block
copolymers of ethylene oxide and propylene oxide); poloxamines (e.g., Tetronic
908 , also
known as Poloxamine 908 , which is a tetrafunctional block copolymer derived
from sequential
addition of propylene oxide and ethylene oxide to ethylenediamine (BASF
Wyandotte
Corporation, Parsippany, N.J.)); Tetronic 1508 (T-1508) (BASF Wyandotte
Corporation),
Tritons X-200 , which is an alkyl aryl polyether sulfonate (Rohm and Haas);
Crodestas F-110 ,
which is a mixture of sucrose stearate and sucrose distearate (Croda Inc.); p-
isononylphenoxypoly-(glycidol), also known as Olin-lOG or Surfactant 10-G
(Olin
Chemicals, Stamford, CT); Crodestas SL-40 (Croda, Inc.); and SA9OHCO, which
is
C18H37CH2(CON(CH3)H2(CHOH) 4(CH2OH) z(Eastman Kodak Co.); decanoyl-.N-
methylglucamide; n-decyl (3-D-glucopyranoside; n-decyl (3-maltopyranoside; n-
dodecyl (3-D-
glucopyranoside; n-dodecyl (3-D-maltoside; heptanoylN-methylglucamide; n-
heptyl- (3-D-
glucopyranoside; n-heptyl (3-D-thioglucoside; n-hexyl 0- D-glucopyranoside;
nonanoyl-N-
methylglucamide; n-noyl (3-D-glucopyranoside; octanoyl-Nmethylgiucamide; n-
octyl- (3-D-
glucopyranoside; octyl -D-thioglucopyranoside; PEGphospholipid, PEG-
cholesterol, PEG-
cholesterol derivative, PEG-vitamin A, PEG-vitamin E, lysozyme, random
copolymers of vinyl
pyrrolidone and vinyl acetate, and the like.
Examples of useful cationic surface stabilizers include, but are not limited
to, polymers,
biopolymers, polysaccharides, cellulosics, alginates, phospholipids, and
nonpolymeric
compounds, such as zwitterionic stabilizers, poly-n-methylpyridinium, anthryul
pyridinium
chloride, cationic phospholipids, chitosan, polylysine, polyvinylimidazole,
polybrene,
polymethylmethacrylate trimethylammoniumbromide bromide (PMMTMABr),
43
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
hexyldesyltrimethylammonium bromide (1-IDMAB), and polyvinylpyrrolidone-2-
dimethylaminoethyl methacrylate dimethyl sulfate. Other useful cationic
stabilizers include, but
are not limited to, cationic lipids, sulfonium, phosphonium, and quartemary
ammonium
compounds, such as stearyltrimethylammonium chloride, benzyl-di(2-
chloroethyl)ethylammonium bromide, coconut trimethyl ammonium chloride or
bromide, coconut
methyl dihydroxyethyl ammonium chloride or bromide, decyl triethyl ammonium
chloride, decyl
dimethyl hydroxyethyl ammonium chloride or bromide, C12_15dimethyl
hydroxyethyl ammonium
chloride or bromide, coconut dimethyl hydroxyethyl ammonium chloride or
bromide, myristyl
trimethyl ammonium methyl sulphate, lauryl dimethyl benzyl ammonium chloride
or bromide,
lauryl dimethyl (ethenoxy) 4 ammonium chloride or bromide, N-alkyl
(C12_18)dimethylbenzyl
ammonium chloride, N-alkyl (C14_ls)dimethyl-benzyl ammonium chloride, N-
tetradecylidmethylbenzyl ammonium chloride monohydrate, dimethyl didecyl
ammonium
chloride, N-alkyl and (C12_14) dimethyl i-napthylmethyl ammonium chloride,
trimethylammonium
halide, alkyl-trimethylammonium salts and dialkyl-dimethylammonium salts,
lauryl trimethyl
ammonium chloride, ethoxylated alkyamidoalkyldialkylammonium salt and/or an
ethoxylated
trialkyl ammonium salt, dialkylbenzene dialkylammonium chloride, N-
didecyldimethyl
ammonium chloride, N-tetradecyldimethylbenzyl ammonium, chloride monohydrate,
N-alkyl(C12_
14) dimethyl l-naphthylmethyl ammonium chloride and dodecyldimethylbenzyl
ammonium
chloride, dialkyl benzenealkyl ammonium chloride, lauryl trimethyl ammonium
chloride,
alkylbenzyl methyl ammonium chloride, alkyl benzyl dimethyl ammonium bromide,
C12, C15, C17
trimethyl ammonium bromides, dodecylbenzyl triethyl ammonium chloride, poly-
diallyldimethylammonium chloride (DADMAC), dimethyl ammonium chlorides,
alkyldimethylammonium halogenides, tricetyl methyl ammonium chloride,
decyltrimethylammonium bromide, dodecyltriethylarnmonium bromide,
tetradecyltrimethylammonium bromide, methyl trioctylammonium chloride (ALIQUAT
336Tm),
POLYQUAT 10 Tm, tetrabutylammonium bromide, benzyl trimethylammonium bromide,
choline
esters (such as choline esters of fatty acids), benzalkonium chloride,
stearalkonium chloride
compounds (such as stearyltrimonium chloride and Distearyldimonium chloride),
cetyl pyridinium
bromide or chloride, halide salts of quaternized polyoxyethylalkylamines,
MIRAPOLTM and
ALKAQUAY Tm (Alkaril Chemical Company), alkyl pyridinium salts; amines, such
as
alkylamines, dialkylamines, alkanolamines, polyethylenepolyamines, N,N-
dialkylaminoalkyl
acrylates, and vinyl pyridine, amine salts, such as lauryl amine acetate,
stearyl amine acetate,
alkylpyridinium salt, and
alkylimidazolium salt, and amine oxides; imide azolinium salts; protonated
quatemary
acrylamides; methylated quatemary polymers, such as poly[diallyl
dimethylammonium chloride]
and poly-[N-methyl vinyl pyridinium chloride]; and cationic guar.
Such exemplary cationic surface stabilizers and other useful cationic surface
stabilizers are described in J. Cross and E. Singer, Cationic Surfactants:
Analytical and
44
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Biological Evaluation (Marcel Dekker, 1994); P. and D. Rubingh (Editor),
Cationic
Surfactants: Physical Chemistry (Marcel Dekker, 1991); and J. Richmond,
Cationic
Surfactants: Organic Chemistry, (Marcel Dekker, 1990).
Nonpolymeric surface stabilizers are any nonpolymeric compound, such
benzalkonium
chloride, a carbonium compound, a phosphonium compound, an oxonium compound, a
halonium
compound, a cationic organometallic compound, a quartemary phosphorous
compound, a
pyridinium compound, an anilinium compound, an ammonium compound, a
hydroxylammonium
compound, a primary ammonium compound, a secondary ammonium compound, a
tertiary
ammonium compound, and quartemary ammonium compounds of the formula NRIR2R3RI.
For
compounds of the formula NRiR2R3R4 (+):
(i) none of Ri-R4 are CH3;
(ii) one of Ri-R4 is CH3;
(iii) three of Ri-R4 are CR3;
(iv) all of Ri-R4 are CH3;
(v) two of Ri-R4 are CH3, one of Ri-R4 is C6H5CH2, and one of Ri-R4 is an
alkyl
chain of seven carbon atoms or less;
(vi) two of Ri-R4 are CH3, one of Ri-R4 is C6H5CH2, and one of Ri-R4 is an
alkyl
chain of nineteen carbon atoms or more;
(vii) two of Ri-R4 are CH3 and one of Ri-R4 is the group C6H5(CH2), where n>1;
(viii) two of Ri-R4 are CH3, one of Ri-R4 is C6H5CH2, and one of Ri-R4
comprises at
least one heteroatom;
(ix) two of Ri-R4 are CH3, one of Ri-R4 is C6H5CH2, and one of Ri-R4 comprises
at
least one halogen;
(x) two of Ri-R4 are CH3, one of Ri-R4 is C6H5CH2, and one of Ri-R4 comprises
at
least one cyclic fragment;
(xi) two of Ri-R4 are CH3 and one of Ri-R4 is a phenyl ring; or
(xii) two of Ri-R4 are CH3 and two of Ri-R4 are purely aliphatic fragments.
Such compounds include, but are not limited to, behenalkonium chloride,
benzethonium chloride, cetylpyridinium chloride, behentrimonium chloride,
lauralkonium
chloride, cetalkonium chloride, cetrimonium bromide, cetrimonium chloride,
cethylamine
hydrofluoride, chlorallylmethenamine chloride (Quatemium- 15),
distearyldimonium chloride
(Quatemium-5), dodecyl dimethyl ethylbenzyl ammonium chloride(Quaternium- 14),
Quaternium-
22, Quaternium-26, Quatemium- 18 hectorite, dimethylaminoethylchloride
hydrochloride,
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
cysteine hydrochloride, diethanolammonium POE (10) oletyl ether phosphate,
diethanolammonium POE (3)oleyl ether phosphate, tallow alkonium chloride,
dimethyl
dioctadecylammoniumbentonite, stearalkonium chloride, domiphen bromide,
denatonium
benzoate, myristalkonium chloride, laurtrimonium chloride, ethylenediamine
dihydrochloride, guanidine hydrochloride, pyridoxine HC1, iofetamine
hydrochloride, meglumine
hydrochloride, methylbenzethonium chloride, myrtrimonium bromide,
oleyltrimonium chloride,
polyquatemium- 1, procainehydrochloride, cocobetaine, stearalkonium bentonite,
stearalkoniumhectonite, stearyl trihydroxyethyl propylenediamine
dihydrofluoride,
tallowtrimonium chloride, and hexadecyltrimethyl ammonium bromide.
The surface stabilizers are commercially available and/or can be prepared by
techniques known in
the art. Most of these surface stabilizers are known pharmaceutical excipients
and are described in
detail in the Handbook of Pharmaceutical Excipients, published jointly by the
American
Pharmaceutical Association and The Pharmaceutical Society of Great Britain
(The Pharmaceutical
Press, 2000), specifically incorporated by reference.
3. Other Pharmaceutical Excipients
Pharmaceutical compositions according to the invention may also comprise one
or more
binding agents, filling agents, lubricating agents, suspending agents,
sweeteners, flavoring agents,
preservatives, buffers, wetting agents, disintegrants, effervescent agents,
and other excipients.
Such excipients are known in the art.
Examples of filling agents are lactose monohydrate, lactose anhydrous, and
various
starches; examples of binding agents are various celluloses and cross-linked
polyvinylpyrrolidone, microcrystalline cellulose, such as Avicel PH101 and
Avicel PH102,
microcrystalline cellulose, and silicified microcrystalline cellulose (ProSolv
SMCCTM).
Suitable lubricants, including agents that act on the flowability of the
powder to be
compressed, are colloidal silicon dioxide, such as Aerosil 200, talc, stearic
acid, magnesium
stearate, calcium stearate, and silica gel.
Examples of sweeteners are any natural or artificial sweetener, such as
sucrose, xylitol,
sodium saccharin, cyclamate, aspartame, and acsulfame. Examples of flavoring
agents are
Magnasweet (trademark of MAFCO), bubble gum flavor, and fruit flavors, and
the like.
Examples of preservatives are potassium sorbate, methylparaben, propylparaben,
benzoic
acid and its salts, other esters of parahydroxybenzoic acid such as
butylparaben, alcohols such as
ethyl or benzyl alcohol, phenolic compounds such as phenol, or quarternary
compounds such as
benzalkonium chloride.
46
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Suitable diluents include pharmaceutically acceptable inert fillers, such as
microcrystalline
cellulose, lactose, dibasic calcium phosphate, saccharides, and/or mixtures of
any of the foregoing.
Examples of diluents include microcrystalline cellulose, such as Avicel PH101
and Avicel
PH102; lactose such as lactose monohydrate, lactose anhydrous, and Pharmatose
DCL21;
dibasic calcium phosphate such as Emcompress ; mannitol; starch; sorbitol;
sucrose; and glucose.
Suitable disintegrants include lightly crosslinked polyvinyl pyrrolidone, corn
starch, potato
starch, maize starch, and modified starches, croscarmellose sodium, cross-
povidone, sodium
starch glycolate, and mixtures thereof.
Examples of effervescent agents are effervescent couples such as an organic
acid and a
carbonate or bicarbonate. Suitable organic acids include, for example, citric,
tartaric, malic,
fumaric, adipic, succinic, and alginic acids and anhydrides and acid salts.
Suitable carbonates and
bicarbonates include, for example, sodium carbonate, sodium bicarbonate,
potassium carbonate,
potassium bicarbonate, magnesium carbonate, sodium glycine carbonate, L-lysine
carbonate, and
arginine carbonate. Alternatively, only the sodium bicarbonate component of
the effervescent
couple may be present.
4. Nanoparticulate Meloxicam Particle Size
The compositions of the invention comprise meloxicam particles which have an
effective
average particle size of less than about 2000 nm (i.e., 2 microns), less than
about 1900 nm, less
than about 1800 nm, less than about 1700 nm, less than about 1600 nm, less
than about 1500 nm,
less than about 1400 nm, less than about 1300 nm, less than about 1200 nm,
less than about 1100
nm, less than about 1000 nm, less than about 900 nm, less than about 800 nm,
less than about 700
nm, less than about 600 nm, less than about 500 nm, less than about 400 nm,
less than about 300
nm, less than about 250 nm, less than about 200 nm, less than about 150 nm,
less than about 100
nm, less than about 75 nm, or less than about 50 nm, as measured by light-
scattering methods,
microscopy, or other appropriate methods.
By "an effective average particle size of less than about 2000 nm" it is meant
that at least
50% of the meloxicam particles have a particle size of less than the effective
average, by weight
or by another suitable measurement technique (e.g., volume, number, etc.),
i.e., less than about
2000 nm, 1900 nm, 1800 nm, etc., when measured by the above-noted techniques.
In other
embodiments of the invention, at least about 60%, at least about 70%, at least
about 80%, at least
about 90%, at least about 95%, or at least about 99% of the meloxicam
particles have a particle
size of less than the effective average, i.e., less than about 2000 nm, 1900
nm,1800nm, 1700 nm,
etc.
47
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
In the present invention, the value for D50 of a nanoparticulate meloxicam
composition is
the particle size below which 50% of the meloxicam particles fall, by weight.
Similarly, D90 is the
particle size below which 90% of the meloxicam particles fall, by weight.
5. Concentration of Meloxicam and Surface Stabilizers
The relative amounts of meloxicam and one or more surface stabilizers can vary
widely. The optimal amount of the individual components can depend, for
example, upon the
particular meloxicam selected, the hydrophilic lipophilic balance (HLB),
melting point, and the
surface tension of water solutions of the stabilizer, etc.
The concentration of meloxicam can vary from about 99.5% to about 0.001 Io,
from about
95% to about 0.1 Io, or from about 90% to about 0.5%, by weight, based on the
total combined dry
weight of meloxicam and at least one surface stabilizer, not including other
excipients.
The concentration of the at least one surface stabilizer can vary from about
0.5% to about
99.999%, from about 5.0% to about 99.9%, or from about 10% to about 99.5%, by
weight, based
on the total combined dry weight of meloxicam and at least one surface
stabilizer, not including
other excipients.
C. Methods of Making Nanoparticulate Meloxicam Compositions
The nanoparticulate meloxicam compositions can be made using, for example,
milling,
homogenization, precipitation, freezing, or template emulsion techniques.
Exemplary methods of
making nanoparticulate compositions are described in the `684 patent. Methods
of making
nanoparticulate compositions are also described in U.S. Patent No. 5,518,187
for "Method of
Grinding Pharmaceutical Substances;" U.S. Patent No. 5,718,388 for "Continuous
Method of
Grinding Pharmaceutical Substances;" U.S. Patent No. 5,862,999 for "Method of
Grinding
Pharmaceutical Substances;" U.S. Patent No. 5,665,331 for
"CoMicroprecipitation of
Nanoparticulate Pharmaceutical Agents with Crystal Growth Modifiers;" U.S.
Patent No.
5,662,883 for "Co-Microprecipitation of Nanoparticulate Pharmaceutical Agents
with Crystal
Growth Modifiers;" U.S. Patent No. 5,560,932 for "Microprecipitation of
Nanoparticulate
Pharmaceutical Agents;" U.S. Patent No. 5,543,133 for "Process of Preparing X-
Ray Contrast
Compositions Containing Nanoparticles;" U.S. Patent No. 5,534,270 for "Method
of Preparing
Stable Drug Nanoparticles;" U.S. Patent No. 5,510,118 for "Process of
Preparing Therapeutic
Compositions Containing Nanoparticles;" and U.S. Patent No. 5,470,583 for
"Method of
Preparing Nanoparticle Compositions Containing Charged Phospholipids to Reduce
Aggregation," all of which are specifically incorporated by reference.
The resultant nanoparticulate meloxicam compositions or dispersions can be
utilized in
solid or liquid dosage formulations, such as liquid dispersions, gels,
aerosols, ointments, creams,
controlled release formulations, fast melt formulations, lyophilized
formulations, tablets, capsules,
48
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
delayed release formulations, extended release formulations, pulsatile release
formulations, mixed
immediate release and controlled release formulations, etc.
1. Milling to Obtain Nanoparticulate Meloxicam Dispersions
Milling a meloxicam to obtain a nanoparticulate dispersion comprises
dispersing the
meloxicam particles in a liquid dispersion medium in which the meloxicam is
poorly soluble,
followed by applying mechanical means in the presence of grinding media to
reduce the particle
size of the meloxicam to the desired effective average particle size. The
dispersion medium can
be, for example, water, safflower oil, ethanol, t-butanol, glycerin,
polyethylene glycol (PEG),
hexane, or glycol. A preferred dispersion medium is water.
The meloxicam particles can be reduced in size in the presence of at least one
surface
stabilizer. Alternatively, meloxicam particles can be contacted with one or
more surface stabilizers
after attrition. Other compounds, such as a diluent, can be added to the
meloxicam/surface
stabilizer composition during the size reduction process. Dispersions can be
manufactured
continuously or in a batch mode.
2. Precipitation to Obtain Nanoparticulate Meloxicam Compositions
Another method of forming the desired nanoparticulate meloxicam composition is
by
microprecipitation. This is a method of preparing stable dispersions of poorly
soluble active
agents in the presence of one or more surface stabilizers and one or more
colloid stability
enhancing surface active agents free of any trace toxic solvents or
solubilized heavy metal
impurities. Such a method comprises, for example: (1) dissolving the meloxicam
in a suitable
solvent; (2) adding the formulation from step (1) to a solution comprising at
least one surface
stabilizer; and (3) precipitating the formulation from step (2) using an
appropriate non-solvent.
The method can be followed by removal of any formed salt, if present, by
dialysis or diafiltration
and concentration of the dispersion by conventional means.
3. Homogenization to Obtain Nanoparticulate Meloxicam Compositions
Exemplary homogenization methods of preparing active agent nanoparticulate
compositions are described in U.S. Patent No. 5,510,118, for "Process of
Preparing Therapeutic
Compositions Containing Nanoparticles." Such a method comprises dispersing
particles of a
meloxicam in a liquid dispersion medium, followed by subjecting the dispersion
to
homogenization to reduce the particle size of a meloxicam to the desired
effective average particle
size. The meloxicam particles can be reduced in size in the presence of at
least one surface
stabilizer. Alternatively, the meloxicam particles can be contacted with one
or more surface
stabilizers either before or after attrition. Other compounds, such as a
diluent, can be added to the
49
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
meloxicam/surface stabilizer composition, either before, during, or after the
size reduction
process. Dispersions can be manufactured continuously or in a batch mode.
4. Cryogenic Methodologies to Obtain
Nanoparticulate Meloxicam Compositions
Another method of forming the desired nanoparticulate meloxicam composition is
by
spray freezing into liquid (SFL). This technology comprises an organic or
organoaqueous solution
of meloxicarn with stabilizers, which is injected into a cryogenic liquid,
such as liquid nitrogen.
The droplets of the meloxicam solution freeze at a rate sufficient to minimize
crystallization and
particle growth, thus formulating nanostructured meloxicam particles.
Depending on the choice of
solvent system and processing conditions, the nanoparticulate meloxicam
particles can have
varying particle morphology. In the isolation step, the nitrogen and solvent
are removed under
conditions that avoid agglomeration or ripening of the meloxicam particles.
As a complementary technology to SFL, ultra rapid freezing (URF) may also be
used to.
created equivalent nanostructured meloxicam particles with greatly enhanced
surface area. URF
comprises an organic or organoaqueous solution of meloxicam with stabilizers
onto a cryogenic
substrate.
5. Emulsion Methodologies to Obtain
Nanoparticulate Meloxicam Compositions
Another method of forming the desired nanoparticulate meloxicam composition is
by
template emulsion. Template emulsion creates nanostructured meloxicam
particles with controlled
particle size distribution and rapid dissolution performance. The method
comprises an oil-in-water
emulsion that is prepared, then swelled with a non-aqueous solution comprising
the meloxicam
and stabilizers. The particle size distribution of the meloxicam particles is
a direct result of the
size of the emulsion droplets prior to loading with the meloxicam a property
which can be
controlled and optimized in this process. Furthermore, through selected use of
solvents and
stabilizers, emulsion stability is achieved with no or suppressed Ostwald
ripening. Subsequently,
the solvent and water are removed, and the stabilized nanostructured meloxicam
particles are
recovered. Various meloxicam particles morphologies can be achieved by
appropriate control of
processing conditions.
***
In the following examples all percentages are weight by weight unless
otherwise stated.
The term purified water" as used throughout the Examples refers to water that
has been purified
by passing it through a water filtration system. It is to be understood that
the examples are for
illustrative purposes only, and should not be interpreted as restricting the
spirit and scope of the
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
invention, as defined by the scope of the claims that follow. All references
identified herein,
including U.S. patents, are hereby expressly incorporated by reference.
EXAMPLE 1
The purpose of this example is to describe preparation of a multiparticulate
modified
release composition comprising a hydrocodone that can be used in the
combination compositions
of the invention.
Multiparticulate modified release hydrocodone compositions according to the
present
invention having an immediate release component and a modified release
component having
a modified release coating are prepared according to the formulations shown in
Tables 1 and
2.
TABLE 1
Immediate Release Component Hydrocodone Solutions
Ingredient Amount, 0% (w/w)
(i) (ii) (iii) (iv) (v) (vi)
Hydrocodone Bitartrate 6.0 6.0 6.0 6.0 6.0 6.0
HPMC 2910 1.0 2.0 2.0 - - 1.5
Polyethylene Gy1co16000 - - - 0.5 - -
Povidone K3 - - - - 5.0 -
Fumaric Acid - 6.0 - - - -
Citric Acid - - 6.0 - - -
Dilicon Dioxide 1.5 1.0 1.0 - - 2.0
Talc 1.5 - - - - -
Purified Water 90.0 85.0 85.0 93.5 89.0 90.5
TABLE 2
Modified Release Component Hydrocodone Solutions
Ingredient Amount, 0% (w/w)
(i) (ii) (iii) (iv) (v) (vi) (vii)
Eudragit RS 100 4.1 4.9 5.5 4.4 - 5.5 7.5
Eudragit RL 100 - 0.5 - 1.1 - - -
Eudragit L 100 1.4 - - - - - -
Ethocel - - - - 3.0 - -
Triethyl Citrate 1.5 1.6 - 1.1 - - 1.5
Dibutyl Sebacate - - - - 0.6 1.0 -
Silicon Dioxide 1.0 1.0 1.0 - 2.0 1.0 -
Talc 2.5 2.5 1.0 2.8 - 1.0 2.5
Acetone 34.0 34.0 15.0 35.6 - 14.0 33.5
Isopropyl Alcohol 50.0 50. 72.5 50. 94.4 72.5 50.0
Purified Water 5.5 5.5 5.0 5.0 - 5.0 5.0
51
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
In these exemplary hydrocodone formulations, the sugar spheres (30/35 mesh)
are
provided as inert cores that act as a carrier for the active ingredient and
other excipients present in
the formulation. The quality and size selected reflect the requirement to
produce multiparticulates
with a mean diameter in the size range 0.5-0.6 mm to facilitate the subsequent
coating and
encapsulation process. Hydroxypropylmethylcellulose (2910) (Methocal E6
Premium LV) is used
to prepare the immediate-release coating solution that is coated onto the
sugar spheres to produce
the JR beads and acts as a binding agent. Silicon Dioxide (Syloid 244FP) is an
anti-adherent that
is used in the preparation of the IR coating solution (Table 1) and the
modified release coating
suspension (Table 2).
Ammonio methyacrylate copolymer Type B (Eudragit RS 100) is a rate-controlling
polymer that imparts the controlled release properties to the formulation and
exhibits pH
independent release properties. Talc (Altaic 200) is used as an anti-adherent
in the modifiedrelease
coating process to manufacture the modified release beads. Acetone and
isopropyl alcohol are the
two solvents in which the rate-controlling polymer is dissolved to produce the
coating suspension
that is applied to the IR beads to form the modified release beads. The
resultant coating
suspension is applied to the IR beads to form the modified release beads.
Modified release beads
are dried in an oven for 10-20 hours at 40-500C / 30-60% RH to remove residual
solvents and to
obtain a moisture content of about 3 -6 %. Suitable processing procedures are
further detailed in
U.S. Patent No. 6,066,339 which is incorporated herein by reference in its
entirety.
Table 3 shows the dissolution profiles for two multiparticulate modified
release
formulations prepared in accordance with Tables 1 and 2. These results
indicate that about 20% of
the hydrocodone was released in the first hour and about 80% of the
hydrocodone was released
over a period of about 11 hours.
TABLE 3
Dissolution Data for Compositions Containing an
IR Component and a Modified Release Component
Formulation
Time (hr) Fumaric Acid Non-Fumaric Acid
0 0 0
1 22 26
2 33 31
4 54 54
6 68 64
8 77 73
12 93 86
52
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
In vivo Study
A randomized, single-dose, parallel-group, placebo-controlled, active-
comparator study was
performed to evaluate the safety, efficacy, and PK of hydrocodone formulations
in subjects
immediately following bunionectomy study. The study treatments were 10, 20,
30, 40 mg of
hydrocodone bitartarate, matching active comparator (10 mg hydrocodone/APAP)
or matching
placebo. During the 24-hour confinement periods, blood was collected at
baseline and at up to 17
additional time points, from 115 subjects (approx. 17 to 21 subjects per
group), to determine the
concentrations in plasma of hydrocodone. The following PK parameters were
calculated and are
presented in Tables 4-6.
Table 4
HC ER 10 mg HC ER 20 mg HC ER 30 mg NC ER 40 mg HC/APAP P1aCeb0
Parameter Stati Sti CS N = 21 N=19 N = 19 N = 17 N = 18 N = 21
Cmax (ng/mL) n 21 19 19 17 18 21
Mean 8.9 17.9 31.7 37.5 19.5 0.1
5td. Dev. 2.11 5.85 8.50 8.82 8.69 0.17
Median 9.1 16.3 30.1 34.1 20.2 0.0
MintMax 5/15 10/27 16/46 28/62 9/45 0/1
Tmax (hr) n 21 19 19 17 18 3
Mean 6.3 6.0 6.3 6.1 2.7 8.2
5td. 0ev. 1.46 1.80 1.88 1.62 1.65 13.70
Median 6.1 5.2 6.1 6.0 2.1 0.6
MintMax 4/9 4/12 4/10 4/10 1/7 0/24
Kel (1/hr) n 21 19 19 17 18 NC (a)
Mean 0.090 0.095 0.086 0.079 0.138 NC
5td. 0ev. 0.0276 0.0289 0.0229 0.021 0.0297 NC
Median 0.092 0.089 0.083 0.079 0.147 NC
MintMax 0.02/0.13 0.05/0.16 0.05/0.13 0.05/0.13 0.06/0.18 NC
(a) NC = Not CalCulated
53
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Table 5
HC ER 10 mg HC ER 20 mg NC ER 30 mg HC ER 40 mg HG/APAP P1aCeb0
Parameter StatistiCs N 21 N^ 19 N = 19 N"17 N=18 N = 21
NC
T1/2 (hr) n 21 19 19 17 18
Mean 9.5 7.9 8.6 9.4 5.3 NC
Std. 0ev. 8.25 2.44 2.32 2.40 1.64 NC
Median 7.6 7.8 8.4 8.8 4.7 NC
Min/Max 5/45 4/15 5/13 5/14 4/11 NC
AUClast (ng hr/mL) n 21 19 19 17 18 21
Mean 109.0 212.9 392.5 464.6 131.2 0.1
Std. Dev. 27.25 73.19 117.74 124.01 36.80 0.19
Median 104.2 196,2 367.0 471.0 129.9 0.0
Min/Max 73/179 130/377 177/671 321/712 80/182 0/1
AUCinf (ng*hr/mL) n 21 19 19 17 18 NC
Mean 136.9 255.6 480.7 596.2 137.6 NC
Std. Dev. 39.48 85.66 138.70 172.73 39.99 NC
Median 128.1 252.7 459.5 578.0 135.4 NC
Min/Max 80/217 151/468 226/756 375/992 83/189 NC
Table 6
HC ER 10 mg HC ER 20 mg HC ER 30 mg HC ER 40 mg HGIAPAP Placebo
Ratio using AUClast Statistics N = 21 No 19 N a19 N M17 N 18 N = 21
Hydromorphone/ N 21 19 19 17 18 3
Hydrocodone Mean 0.000 0.001 0.002 0.003 0.001 0.000
Std.0ev. 0.0009 0.0038 0.0027 0.0050 0.0012 0.0000
Median 0.000 0.000 0.001 0.002 0.000 0,000
Min/Max 0.00/0.00 0.00/0.02 0.00/0.01 0.00/0.02 0.00/0.00 0.00/0.00
Hydromorphone/ N 21 19 19 17 18 3
Hydrocodone Mean 0.366 0.360 0.327 0.362 0.448 0.000
Std.0ev. 0.1189 0.1215 0.1243 0.1310 0.2144 0.0000
Median 0.368 0.324 0.297 0.334 0.400 0,000
Min/Max 0.11/0.61 0.17/0.58 0.20/0.76 0.23/0.74 0.22/0.84 0.00/0.00
Hydrocodone Simulations
Studies of hydrocodone formulations of the present invention were conducted to
simulate the profiles associated with twice-daily administration hydrocodone
for both single
dose and steady state. The target doses were 10, 20, 40 and 80 mg, and the
targeted minimum
concentration was 5-10 ng/ml. The formulations of the study were two-component
dosage
forms comprising an immediate release component and a modified release
component in
which the hydrocodone was allocated evenly (50/50) or unevenly (20/80) across
the two
components. Non-compartmental parameters were used to find estimates of the
unit input
response and a one-compartment model was assumed for all simulations.
Non-compartmental parameters following a 10 mg oral dose of hydrocodone
administered to five adult males are reported as shown in Table 7 below.
54
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
Table 7 - Non-Compartmental Parameters
Cmax 23.6 5.2 ng/ml
Tmax 1.3 0.3 hours
Tnaif 3.8 0.3 hours
K10 and V/f were estimated to be 0.18 and 334.29L respectively. For the
absorption rate
constant k01, several profiles were simulated using different estimates of
k01. The secondary
parameters estimates were compared to identify an appropriate ka as set forth
in Table 8
below.
Table 8 - Comparison of Absorption Rate Constant (ka).
ka=1 AUC 166.
ka=1 K01-HL 0.6
ka=1 K10-HL 3.8
ka=1 CL/F 60.1
ka=1 Tmax 2.0
ka=1 Cmax 20.5
ka=2 AUC 166.
ka=2 K01-HL 0.3
ka=2 K10-HL 3.8
ka=2 CL/F 60.1
ka=2 Tmax 1.3
ka=2 Cmax 23.5
ka=6 AUC 166.
ka=6 K01-HL 0.1
ka=6 K10-HL 3.8
ka=6 CL/F 60.1
ka=6 Tmax 0.6
ka=6 Cmax 26.8
ka=2 appeared to be the best estimate of the absorption rate of the instant
release
hydrocodone given that the maximum concentration observed and the time to
maximum
concentration were comparable to previous data set forth above.
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
In conducting these simulations, three options were identified. Options 1 and
2 assumed a
first order release and option 3 a zero-order release. Plots of the plasma
concentrations of these
simulations are shown in Figs. 1 to 16.
EXAMPLE 2
The purpose of this example was to prepare nanoparticulate dispersions of
meloxicam
stabilized with various surface stabilizers.
Aqueous dispersions of 5 wt. % meloxicam (Unichem Laboratories, Ltd.) and 1
wt. %
stabilizer (see Table 9, below) were charged into a NanoMill milling system
(Elan Drug
Delivery, Inc., King of Prussia, PA; see e.g., U.S. Patent No. 6,431,478 for
"Small Scale Mill")
equipped with a 10cc batch chamber.
The following NanoMill milling system parameters were used for all of the
formulations:
mill speed = 5500 rpm; total milling time = 1 hour; polymeric milling media
type =
Po1yMi11TM200 (The Dow Chemical Co.); and a media load sufficient to process.
Particle size analysis of the resultant milled dispersions was performed using
a Horiba LA-
9 10 particle size analyzer ((Horiba Instruments, Irvine, CA). The results are
shown below in
Table 9. In the table below, the value for D50 is the particle size below
which 50% of the active
agent particles fall. Similarly, D90 is the particle size below which 90% of
the active agent
particles fall.
TABLE 9
Stabilizer Stabilizer Mean D50 D90 Optical Microscopy*
Manufacturer (nm) (nm) (nm)
Pluronic F68 BASF 133 110 226 Stable
(poloxamer 188)
Pluronic F108 BASF 129 108 219 Stable
(poloxamer 388)
Kollidon 12 PF BASF 98 90 125 Stable
Kollidon 17 PF BASF 98 95 135 Stable
Polysorbate 80 Spectrum 227 227 322 Stable
Sodium Prodotti Chimici 119 101 198 Stable
Deoxycholate
Lecithin Penta 190 89 271 Mild aggregation at initial
Lysozyme Fordras 95 89 117 Moderate aggregation at
initial; Stable at 24 hours
*All formulations were taken at initial time except for Lethicin and Lysozyme,
wherein the
initial particle size was measured at 24 hr.
56
CA 02660650 2008-12-01
WO 2007/150075 PCT/US2007/072054
The results demonstrate that meloxicam can be formulated into stable
nanoparticulate
compositions with each of the surface stabilizers shown in Table 9, as all of
the formulations have
a D50 particle size of less than about 2000 nm. Nanoparticulate meloxicam
compositions shown
in Table 9 had mean particles sizes ranging from 95 to 227 nm, with D50 and
D90 sizes ranging
from 89 nm to 227 nm and 117 nm to 322 nm, respectively.
It will be apparent to those skilled in the art that various modifications and
variations can
be made in the methods and compositions of the present inventions without
departing from the
spirit or scope of the invention. Thus, it is intended that the present
invention cover the
modification and variations of the invention provided they come within the
scope of the appended
claims and their equivalents.
57