Note: Descriptions are shown in the official language in which they were submitted.
CA 02661157 2013-09-19
75704-283
IMPROVED MALEIC ANHYDRIDE CATALYST
AND METHOD FOR ITS PREPARATION
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] Not applicable.
STATEMENT REGARDING FEDERALLY SPONSORED
RESEARCH OR DEVELOPMENT
[0002] Not applicable.
BACKGROUND OF THE INVENTION
Field of the Invention
[0003] The present invention is directed to a vanadium/phosphorus oxide
catalyst,
method of preparation of such catalyst, and the use of the catalyst in the
production of
maleic anhydride.
Background of the Invention
[0004] Maleic anhydride may be used as a raw material in the production of
many
products, such as synthetic resins, and may generally be prepared by the
catalytic oxidation
of n-butane. The catalyst of choice for this oxidation is typically a catalyst
containing
vanadium, phosphorus, oxygen (VPO), and optionally a promoter component.
[0005] These VPO catalysts are generally prepared by contacting vanadium-
containing
compounds with phophorus-containing compounds and optionally promoter
component
containing-compounds under conditions suitable to reduce the pentavalent
vanadium to the
tetravalent state to thereby form a catalyst precursor containing vanadyl
hydrogen
phosphate and optionally the promoter component. The catalyst precursor may
then be
recovered and typically formed into a shaped body, such as a tablet or pellet,
by
compression in a die. A lubricant is ordinarily incorporated as well to aid in
the tableting or
pelleting process. The pellet or tablet may then be subjected to calcination
to transform the
catalyst precursor into an active catalyst containing vanadyl pyrophosphate.
[0006] In addition to promoter components, VPO catalysts may be prepared by
further
adding high vapor pressure additives to control the pore structure of the
catalyst as
disclosed in U.S. Pat. No. 5,275,996.
Additives disclosed in this reference include polyethylene oxide, adipic acid,
citric
CA 02661157 2013-09-19
75704-283
acid, oxalic acid, stearic acid, palmitic acid, lauric acid, myristic acid,
esters of such acids,
naphthalene, polyethylene glycol, polyvinyl alcohol, polyacrylic acid,
cellulosic materials,
monosaccharides, polysaccharides, hydrogenated vegetable oils, waxes, and
gelatin. One
drawback to the use of these additives is that the additives are removed from
the catalyst by
heat treatment at elevated temperatures, typically with the use of a stripping
gas, which can
deactivate the catalyst, cause overstripping or dehydration and reduce
productivity of the
catalyst due to increased processing time and rigorous control needed in
handling a
flammable material at elevated temperatures.
[0007] Thus, efforts are continually being made to define new and improved VP0
catalysts and methods and processes of making new and old VPO catalysts in
order to
reduce cost and/or upgrade the activity, selectivity, and productivity of such
catalysts.
BRIEF SUMMARY OF SOME OF THE PREFERRED EMBODIMENTS
[0008] These and other needs in the art are addressed in one embodiment by a
process for
producing vanadium/phosphorus oxide catalysts, which are useful for the
oxidation of
nonaromatic hydrocarbons to produce maleic anhydride. The process includes the
steps of
preparing a catalyst precursor powder by mixing a vanadium compound with a
phosphorus
compound in a medium comprising alcohol and drying the mixture, forming the
catalyst
precursor powder into catalyst precursor slugs under compression, converting
the catalyst
precursor slugs into an activated catalyst by heat treatment, and forming the
activated
catalyst into a predetermined shape under compression to produce the
vanadium/phosphorus oxide catalyst. The activated catalyst may also be treated
with a
solvent-removable pore building agent prior to forming into the predetermined
shape to
provide a vanadium/phosphorus oxide catalyst containing a high concentration
of pores
therein for rapid internal and external diffusion of product and reactant
gases within the
oxide catalyst.
2
CA 02661157 2014-07-08
75704-283
[0008a] In one process aspect, the present invention relates to a process for
the preparation
of a vanadium/phosphorus oxide catalyst comprising: (a) preparing a catalyst
precursor
powder by mixing a vanadium compound with a phosphorus compound in a medium
comprising alcohol and drying the mixture; (b) forming the catalyst precursor
powder into
catalyst precursor slugs under compression; (c) converting the catalyst
precursor slugs into an
activated catalyst by heating the catalyst precursor slugs in three controlled
stages; (d)
forming the activated catalyst into a predetermined shape under compression to
produce the
vanadium/phosphorus oxide catalyst; and (e) treating the activated catalyst
with a solvent,
wherein prior to step (d), the activated catalyst is granulated and then mixed
with a solvent-
removable pore building agent in proportions sufficient to provide a pore
building agent
concentration of between about 6% and about 16% by weight based on the total
weight of the
activated catalyst, and wherein the step of treating the activated catalyst
with a solvent
comprises removing the solvent-removable pore building agent.
[0008b] In another process aspect, the present invention relates to a process
for the oxidation
of a hydrocarbon comprising contacting a hydrocarbon with a
vanadium/phosphorus oxide
catalyst at a temperature between about 300 C and about 600 C, wherein the
vanadium/phosphorus oxide catalyst is prepared by the above process.
[0009] The foregoing has outlined rather broadly the features and technical
advantages of
the present invention in order that the detailed description of the invention
that follows may be
better understood. Additional features and advantages of the invention will be
described
hereinafter that form the subject of the claims of the invention. It should be
appreciated by
those skilled in the art that the conception and the specific embodiments
disclosed may be
readily utilized as a basis for modifying or designing other structures for
carrying out the same
purposes of the present invention. It should also be realized by those skilled
in the art
2a
CA 02661157 2013-09-19
75704-283
that such equivalent constructions do not depart from the scope of the
invention
as set forth in the appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] For a detailed description of the preferred embodiments of the
invention,
reference will now be made to the accompanying drawings in which:
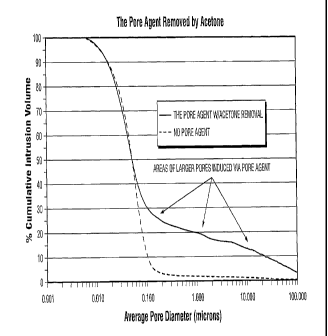
[0011] Figure 1 illustrates percent cumulative intrusion volume against pore
diameter for
no pore agent and use of a pore agent;
[0012] Figure 2 illustrates cumulative intrusion volume against pore diameter
for no pore
agent and use of a pore agent; and
[0013] Figure 3 illustrates log differential intrusion against pore diameter
for no pore
agent and use of a pore agent.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0014] An embodiment includes a process for preparing a vanadium/phosphorus
oxide
catalyst effective in the catalytic oxidation of hydrocarbons, more
particularly, the catalytic
oxidation of C4 hydrocarbons to maleic anhydride. It has been surprisingly
discovered that
enhanced activity and productivity in the conversion of n-butane or other
hydrocarbons to
maieic anhydride may be achieved when using the catalyst prepared by the
process. In
addition, the vanadium/phosphorous oxide catalyst may exhibit better
uniformity in its final
chemical properties as compared to catalysts produced by traditional methods.
[0015] The vanadium/phosphorus oxide catalyst comprises shaped bodies having a
B.E.T. surface area of at least about 15 m2/g, an average vanadium oxidation
state from
about 4.0 to about 4.5, a total pore volume of at least about 0.15 cm3/g, a
normalized
apparent shaped body density of between about 1.0 and 'about 2.0 g/cm, a crush
strength of
at least about 4 pounds, and a phosphorous:vanadium atomic ratio from about
1.0 to about
1.2. The catalysts may be prepared by a process that includes activating a
catalyst precursor
to produce an activated catalyst, optionally treating the activated catalyst
with a solvent-
removable pore building agent, forming the activated catalyst into a
predetermined shape,
removing the optional pore building agent from the shaped catalyst with the
appropriate
solvent and drying at moderate temperatures to produce the vanadium/phosphorus
oxide
catalyst.
3
CA 02661157 2013-09-19
75704-283
[0016] For purposes of this invention, the term "yield" means the ratio of the
moles of
maleic anhydride obtained to the moles of hydrocarbon feedstock introduced
into the
reactor multiplied by 100 with the term expressed as mole percent.
[0017] The term "selectivity" means the ratio of the moles of maleic anhydride
obtained
to the moles of hydrocarbon feedstock reacted or converted multiplied by 100
with the term
expressed as mole percent.
[0018] The term "conversion" means the ratio of the moles of hydrocarbon
feedstock
reacted to the moles of hydrocarbon feedstock introduced into the reactor
multiplied by 100
with the term expressed as mole percent.
[0019] The term "weight/weight productivity" means the weight of maleic
anhydride
produced per unit of catalyst per hour.
[0020] The term "weight/area productive" means the weight of maleic anhydride
produced per unit B.E.T. developed surface area of catalyst per hour.
[0021] The term "space velocity" or "gas hourly space velocity" or "GHSV"
means the
volumetric flow rate of gaseous feed expressed in standard (273K, 14.7 psig)
cubic
centimeters per hour divided by the bulk catalyst volume expressed in cubic
centimeters
with the term expressed as cm3/cm3/hour or hr'.
[0022] The term "gas flow volume to catalyst weight ratio" means the ratio of
the
volumetric flow rate of gas containing a hydrocarbon and air or oxygen to the
weight of a
catalyst bed through which the gas is flowing with the term expressed in g/cc-
min.
[0023] Catalyst precursors suitable for use may be prepared according to those
described
in U.S. Pat. Nos. 5,137,860 and 5,364,824.
In general, the catalyst precursors are represented by the formula:
VO(M)1liPa4.aH20.b(P2jc0).n(organics)
where M is at least one promoter element selected from the group consisting of
elements
from Groups IA, IB, IIA, IIB, IIIA, IIIB, [VA, IVB, VA, VB, VIA, VIB, and
VIIIA of the
Periodic Table of the Elements, and mixtures thereof; m is a number from zero
(0) to
about 0.2; a is a number of at least about 0.5; b is a number taken to provide
a PN atom
ratio from about 0.9 to about 1.3; c is a number representing the oxidation
number of
phosphorus and has a value of 5; and n is a number taken to represent the
weight % of
intercalated or occluded organics component.
4
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
[0024] The catalyst precursor may be prepared by introducing a substantially
pentavalent
vanadium-containing compound and a pentavalent phosphorus-containing compound
into
an alcohol medium to form a catalyst precursor slurry. The vanadium and
phosphorus-
containing compounds may be added simultaneously, or one after the other, in
any
convenient manner to the alcohol medium. After the vanadium and phosphorus-
containing
compounds are introduced into the alcohol medium to form the catalyst
precursor slurry,
reduction of at least a portion of the vanadium to a valence state of +4 is
effected,
preferably by heating the mixture, with stirring, if desired, until a blue
solution or slurry is
obtained. In general, heating the slurry at the reflux temperature for a
period of time
ranging from about four (4) hours to about twenty (20) hours is sufficient.
[0025] The pentavalent vanadium-containing compounds that may be used as a
source of
vanadium in the vanadium/phosphorous oxide catalysts include vanadium
pentoxide or
vanadium salts, such as ammonium metavanadate, vanadium oxytrihalides, and
vanadium
alkylcarboxylates. Among these compounds, vanadium pentoxide is preferred.
[0026] The pentavalent phosphorus-containing compounds useful as a source of
phosphorus in the vanadium/phosphorous oxide catalysts include phosphoric
acid,
phosphorus pentoxide, or phosphorus perhalides such as phosphorus
pentachloride. Of
these phosphorus compounds, phosphoric acid and phosphorus pentoxide are
preferred.
[0027] The alcohols employed in the preparation of the catalyst precursor are
preferably
anhydrous and, in some embodiments, capable of reducing at least a portion of
the
vanadium to a +4 valence state, either upon addition of the vanadium compound
or upon
mixing and heating. In addition, the alcohol may be a solvent for and
relatively unreactive
toward the phosphorus compound. Preferably, the alcohol is not a solvent for
the catalyst
precursor. In those instances where the catalyst precursor is soluble in the
alcohol medium,
precursor precipitation may be easily induced by removal of a portion of the
alcohol.
Suitable alcohols include primary and secondary alcohols, such as methanol,
ethanol, 1-
propanol, 2-propanol, 1-butanol, 2-methyl-1 -propanol (isobutyl alcohol), 2-
butanol, 3-
methy1-2-butanol, 2,2-dimethyl-1-propanol, 4-methyl-2-pentanol, and 1,2-
ethanediol
(ethylene glycol). Of these alcohols, isobutyl alcohol (IBA) is preferred.
[0028] If desired, optional promoter elements may be added as solids,
suspension of
solids, or solutions to the catalyst precursor slurry. Promoter compounds that
may serve as
sources of the promoter elements include metal halides, metal alkoxides, and
metal
carboxylates. Of these compounds, metal carboxylates are preferred. Suitable
carboxylates
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
for metal salts include formate, acetate, propionate, butyrate, isobutyrate,
pentanoate,
hexanoate, heptanoate, octanoate, nonanoate, decanoate, and 2-ethylhexanoate.
Of these
carboxylates, 2-ethylhexanoate is preferred. In an embodiment, the promoter
elements
comprise Mo, Nb, Cr, Fe, or combination thereof.
[0029] The promoter elements may be added to the catalyst precursor slurry as
metal 2-
ethylhexanoates in solutions of alcohols, esters, aromatics, and alkanes. Of
these solvents,
isobutyl alcohol, isobutyl isobutyrate, decane, and mineral spirits constitute
preferred but
not limiting solvents of choice. In an embodiment, the metal 2-ethylhexanoates
are
dissolved in suitable solvents in amounts of 20 percent by weight or less
before they are
added to the slurry.
[0030] The promoter metal 2-ethylhexanoates may be added to the vanadium-
phosphorus
oxide catalyst precursor slurry before, during, or after the reflux period at
slurry
temperatures ranging from ambient to the reflux temperature of the catalyst
precursor slurry
mixture. Of these times of addition, during the reflux period is preferred and
at a slurry
temperature of less than 40 C. Because the promoter source is generally
reactive with the
phosphorus compound, it is preferably withheld from the reaction system until
the
vanadium compound has been substantially consumed by reaction to a VP0
compound.
Otherwise, in some embodiments, the PN ratio may be increased to above the
optimum.
Without being limited by theory, such increase is for the purpose of driving
the reaction of
the vanadium compound to completion. One method of preparation, therefore, is
referred
to as the "post" method, in which the vanadium compound is first reacted at
elevated
temperature with a modest excess of phosphorus compound, for example, at a PN
ratio of
1.05 to 1.20, until the vanadium compound is substantially exhausted; and
thereafter the
promoter source compound is reacted with the residual phosphorus compound to
incorporate the promoter in the catalyst precursor. The reaction between the
vanadium and
phosphorus compounds may be carried out at any suitable temperature. In an
embodiment,
the reaction may be carried out at a temperature in the range of between about
90 and
about 120 C, conveniently at atmospheric reflux temperature. The reaction
mixture may
then be cooled to below 40 C for addition of the promoter source.
[0031] In another embodiment, the vanadium compound and phosphorus compound
are
reacted at a temperature in the range of between about 90 C and about 120 C,
again using a
PN ratio of 1.05 to 1.15; the reaction mixture is cooled below 40 C for
addition of the
promoter source, and optionally a further increment of phosphoric acid; and
then the
6
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
reaction system is again heated to a temperature in the range of between about
90 C and
about 120 C for incorporation of the promoter compound into the precursor
structure.
[0032] During the course of carrying out the vanadium reduction, the catalyst
precursor
foims and precipitates from the alcohol medium as a finely divided precipitate
that contains
the optional promoter elements. The catalyst precursor precipitate may be
recovered after
cooling to below about 50 C by conventional techniques well known to those
skilled in the
art, including filtration, centrifugation, and decantation. The resulting
catalyst precursor
powder after drying has a powdery, free-flowing consistency in contrast to a
caked residue
nonnally obtained when the catalyst precursor is recovered by heating the
solution to
dryness.
[0033] To avoid reaction of the alcohol with catalytically active vanadium
sites, the
drying may be performed in an atmosphere of low or no oxygen content such as
dry
nitrogen. The catalyst precursor precipitate may be dried at a relatively
modest temperature
of, for example, about 110 C to about 150 C, and then subjected to "post dry"
treatment
(roasting) at a temperature in the range of about 200 C to about 275 C. In one
embodiment, the post dry treatment is carried out by fluidizing the catalyst
precursor
powder in an inert gas in the post dry temperature range. Once the bed reaches
the desired
temperature, it may be held at that temperature for a suitable period, for
example 30
minutes to two hours, and thereafter an air/steam mixture is introduced,
preferably on an
incremental schedule to a maximum of about 10 to about 30% oxygen, after which
the bed
is cooled in an inert atmosphere to room temperature.
[0034] Although the catalyst precursor powder that is obtained may be directly
converted
to an activated catalyst by the gas and thermal treatments described below, a
preferred
embodiment includes that the catalyst precursor powder first be compressed in
a press or
die to produce a catalyst precursor slug. The slug may be compressed into any
desired
shape or faun, such as a cylinder, cube, or sphere, to a measured density of
between about
1.20 g/cm3 to about 1.70 g/cm3, preferably between about 1.40 g/cm3 to about
1.60 g/cm3.
The catalyst precursor slug may have a minimum principal dimension of at least
about 1/16
inch to about 1/8 inch, preferably at least about 5/32 inch to about 1/2 inch.
Binding and/or
lubricating agents may be added if desired at amounts ranging from about 2 to
about 6 %,
alternatively from about 0 to about 10 %, and alternatively from about 2 to
about 6 % by
weight based on the total weight of the precursor slug and may include starch,
calcium
stearate, stearic acid and graphite.
7
CA 02661157 2013-09-19
75704-283
[0035] The catalyst precursor powder or slugs may then be converted into an
activated
catalyst by a series of steps in a controlled manner using a sequence of gas
and thermal
treatments, sometimes referred to as calcination. Without being limited by
theory, such
conversion is preferred for the preparation of superior catalysts. This
conversion may be
accomplished in three controlled stages: (1) an initial heat-up stage, (2) a
rapid heat-up
stage, and (3) a maintenance/finishing stage.
[0036] In one embodiment, the catalyst precursor slug is heat treated in the
three stages to
produce the activated catalyst as described in U.S. Pat. No. 5,137,860.
The activated catalyst produced by activation
corresponds to a composition represented by the formula:
(V0)2(M)mP2.07-b(P2/,0)
where M is at least one promoter element selected from among elements of
Groups IA,
IB, IIA, JIB, IIIA, IIIB, IVA, IVB, VA, VB, VIA, VIB, and VIIIA of the
Periodic Table
of the Elements, and mixtures thereof; m is a number from zero (0) to about
0.2; b is a
number taken to provide a PN atom ratio from about 1.0 to about 1.3; and c is
a number
representing the oxidation number of phosphorus and has a value of 5. The
oxidation
state of the vanadium is between about 4.0 and about 4.5, preferably between
about 4.06
and about 4.30.
[0037] Although the activated catalyst, as represented by the above fonnula,
is indicated
as having a phosphorus-to-vanadium (phosphorus/vanadium or PA') atom ratio
from about
1.0 to about 1.3, preferably from about 1.0 to about 1.2, most preferably from
about 1.05 to
about 1.15, the actual PN atom ratio may range from a value as low as about
0.9 up to the
stated value of about 1.3. The total atom ratio of promoter element-to-
vanadium (promoter
element/vanadium or M/V), when a promoter element is present as a component of
the
activated catalyst, may be in the range from about 0.0001 to about 0.2,
preferably from
about 0.0005 to about 0.1, most preferably from about 0.001 to about 0.05. The
activated
catalyst may exhibit enhanced catalyst activity and excellent selectivities to
and yields of
maleic anhydride. Further enhancement of activity may be provided by the use
of a
solvent-removable pore modification agent to produce high fractions of pores
within the
catalyst as described below.
[0038] In the initial heat-up stage, the catalyst precursor slug may be heated
in an
atmosphere selected from among air, steam, inert gas, and mixtures thereof; at
any
8
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
convenient heat-up rate. In an embodiment, the catalyst precursor slug may be
heated to a
temperature not to exceed the phase transformation initiation temperature,
which may be
about 300 C. In general, suitable temperatures for the initial heat-up stage
range from
about 200 to about 300 C, alternatively a temperature from about 250 to
about 275 C.
[0039] After the desired temperature has been achieved in the initial heat-up
stage, the
initially selected atmosphere (in the event it does not contain molecular
oxygen and steam
and/or has a different composition than that which is desired for the rapid
heat-up stage)
may be replaced by a molecular oxygen/steam-containing atmosphere, while
maintaining
the catalyst precursor at the temperature achieved in the initial heat-up
stage. Such
atmosphere optionally may contain an inert gas and, as such, may be
conveniently
represented by the formula:
(02)x(H20)y(IG),
where IG is an inert gas and x, y, and z represent mole % (or volume %) of the
02, H20,
and IG components, respectively, in the molecular oxygen/steam-containing
atmosphere;
with x having a value greater than zero (0) mol %, but less than 100 mol %; y
having a
value greater than zero (0) mol %, but less than 100 mol %; and z having a
value
representing the balance of the molecular oxygen/steam-containing atmosphere.
In an
embodiment, the atmosphere may contain at least a portion of molecular oxygen
and
water (as steam). The presence of the inert gas in such atmosphere, as
indicated by the
formula, is optional. Nonlimiting examples of inert gases suitable for use in
the
molecular oxygen/steam-containing atmosphere include (molecular) nitrogen,
helium,
argon, and the like, with nitrogen generally being preferred.
[0040] Once the molecular oxygen/steam-containing atmosphere is provided, the
catalyst
precursor slug may be subjected to the rapid heat-up stage. In the rapid heat-
up stage, the
initial heat-up stage temperature is increased at a programmed rate of from
about 2 C per
minute ( C /min) to about 12 C /min, preferably from about 4 C /min to about 8
C /min, to
a value effective to eliminate or remove the water of hydration from the
catalyst precursor
slug. In general, a temperature from about 340 C to about 450 C, alternatively
at least
about 350 C, alternatively from about 375 C to about 425 C is suitable.
[0041] Following the rapid heat-up stage, the catalyst precursor may be
subjected to the
maintenance/finishing stage. In the maintenance/finishing stage, while the
molecular
oxygen/steam-containing atmosphere, is maintained, the temperature may be
adjusted to a
value greater than 350 C but less than 550 C, preferably from about 375 C to
about 450 C,
9
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
most preferably from about 400 C to about 425 C. The adjusted temperature is
then
maintained, first in the molecular oxygen/steam-containing atmosphere for a
time effective
to provide a vanadium oxidation state of from about +4.0 to about +4.5 or
simply from
about 4.0 to about 4.5, and thereafter in a nonoxidizing, steam-containing
atmosphere for a
time effective to complete the catalyst precursor-to-active catalyst
transformation to yield
the activated catalyst. In a manner similar to the molecular oxygen/steam-
containing
atmosphere, the nonoxidizing, steam-containing atmosphere may also optionally
contain an
inert gas, with nitrogen generally being the preferred inert gas.
[0042] It is to be understood that the nonoxidizing, steam-containing
atmosphere need
not necessarily be completely free of molecular oxygen. However, in an
embodiment, such
atmosphere preferably is substantially free of molecular oxygen. Accordingly,
molecular
oxygen may be present in an amount that is not effective to cause further
oxidation of the
vanadium beyond the desired oxidation state of about +4.0 to about +4.5, more
particularly,
not beyond the maximum desired oxidation state of about +4.5. In general,
molecular
oxygen may be present in amounts that do not exceed about 0.5 mol % of the
nonoxidizing,
steam-containing atmosphere.
[0043] The period of time during which the adjusted temperature is maintained
in the
molecular oxygen/steam-containing atmosphere in order to provide the desired
vanadium
oxidation state of from about +4.0 to about +4.5 may depend to some extent
upon the
vanadium oxidation state achieved during the rapid heat-up stage, which, in
turn, may
depend to some extent upon the period of time during which the catalyst
precursor is
exposed to the molecular oxygen/steam-containing atmosphere at the stated
rapid heat-up
stage temperatures. In an embodiment, a period of time of from about 0.25
hours to about 2
hours is suitable, with a period of time of from about 0.5 hour to about 1
hour being
preferred.
[0044] A suitable period of time during which the adjusted temperature is
maintained in
the nonoxidizing, steam-containing atmosphere is at least 1 hour, although
longer periods of
time up to 24 hours, or longer, may be employed, if desired, with a period of
time of from
about 3 hours to about 10 hours being preferred, and a period of about 6 hours
being most
preferred.
[0045] The activated catalyst may then be granulated, optionally treated with
a solvent-
removable pore agent, and further compressed in a press or die into a
predetermined shape
to produce the shaped body catalyst. Granulation of the activated catalyst
into granules
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
may be carried out by the mechanical action of mill knives operated in
conjunction with a
retaining screen having holes which pass the desired the size granule. In one
embodiment,
the granules are on the order of 200 gm to 1 mm in size, as produced by
passage through a
screen having 1/16" to 1/8" holes.
[0046] The granules may then be optionally mixed with a solvent-removable pore
building agent. A substantial volume of pores within the catalyst may be
obtained through
the use of the solvent-removable pore building agent. In addition, since mild
conditions are
employed in generating the pores, the desired pore size distribution may be
realized without
adversely affecting the activity at the active internal surfaces of the
catalyst.
[0047] The solvent-removable pore building agent may be added to the granules
so that
the mixture contains between about 6% and about 16%, preferably between about
8% and
about 12% by weight of pore building agent based on the total weight of the
granules.
[0048] The solvent-removable pore building agent that may be added includes a
carboxylic acid, anhydride, ester, alcohols, polyols, carbohydrates, ketones,
waxes,
aromatic hydrocarbons (e.g., naphthalene), polymers (e.g., polystyrene,
polyvinyl alcohol
(PVA)), or combination thereof. In an embodiment, the pore agent is a solid at
the
temperatures typically found during compaction and tableting and does not
negatively
chemically interact with the vanadium phosphate catalyst.
[0049] In one embodiment, the solvent-removable pore building agent is 1,1,1-
tris(hydroxymethypethane, trimethyolpropane, maleic anhydride, polyethylene
oxide, or
combination thereof. In an embodiment, the solvent-removable pore building
agent is
1,1,1-tris(hydroxymethyl)ethane.
[0050] Addition of the solvent-removable pore building agent allows for rapid
internal
diffusion of product and reactant gases within the final shaped body catalyst.
Without
being limited by theory, the larger pores constitute flow arteries for
distribution of these
gases, thereby providing access for reaction gases to the active surfaces of
the catalyst and
egress of the product gases from finer pores of the catalyst. Further, without
being limited
by theory, this rapid exchange of gases allows for maximum effective use of
more of the
internal surface of the catalyst in the catalytic oxidation of C4 hydrocarbons
to maleic
anhydride. The catalyst may therefore be formed into large tablets or pellets,
resulting in
low pressure drop through the catalyst bed without sacrificing productivity.
Figures 1-3
illustrate the advantages of a pore building agent in relation to no pore
building agent.
11
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
[0051] The catalysts may also exhibit crush strengths satisfactory for use in
commercial
reactors. Gravity and other compaction forces tend to crush porous catalyst
bodies to a
powder form, which may result in high pressure drop through the catalyst bed.
Without
being limited by theory, inadequate crush strength is generally associated
with low apparent
density of the catalyst bodies. Despite their high total pore volume and large
proportion of
macropores, the vanadium/phosphorous oxide catalysts have been found to
exhibit a
substantial normalized apparent shaped body density, in the range of between
about 1.0 and
about 2.0 g/cm3 and a crush strength of at least about 4 pounds, alternatively
at least about 6
pounds to about 10 pounds. It is to be understood that normalized apparent
shaped body
density is the same as measured apparent density where the solid phase of the
catalyst is
entirely constituted of vanadium/phosphorus oxide catalyst. Where the solid
phase contains
a foreign material, the normalized apparent density may be determined by
adjusting the
measured apparent density for the weight fraction of VPO in the catalyst body.
Thus if:
an = the normalized apparent body density;
a. = the measured apparent body density;
x = the weight fraction VPO in the catalyst body; then
an = ainx
Where no foreign material is present, the normalized (and measured) apparent
body
density is between about 1.25 g/cm3 and about 2.0 g/cm3.
[0052] The granules and optional solvent-removable pore building agent mixture
may
then be compressed into a predetermined shape, such as pellets, tablets,
spheres, cubes or
other shaped body, in a press or die. The pellet or shaped body contains a
structure of
mixed particulate vanadium/phosphorus oxide structure and optional solvent-
removable
pore building agent having a principal dimension of at least about 1/8 inch,
at least about
5/32 inch to Y2 inch and therefore a volume per shaped body catalyst of at
least about 0.02
cm3, preferably at least about 0.03 cm3, more preferably at least about 0.05
cm3.
[0053] In an embodiment, the solvent-removable pore building agent may be
removed
from the shaped body catalyst by applying the appropriate solvent to the
shaped body
catalyst. For example, the solvent-removable pore building agent may be
removed from the
catalyst, such as by soaking and/or washing the catalyst at least once in the
appropriate
solvent for a certain period of time. The soaking period may depend on the
particular
solvent-removable pore building agent/solvent combination but may generally
range from
about 2 hours to about 24 hours, alternatively from about 6 hours to about 8
hours, and
12
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
alternatively from about 6 hours to about 24 hours. The amount of solvent that
may be
used may range from about a 1:2 to 2:1 weight ratio of solvent to shaped body
catalyst.
The solvent-removable pore building agent may also be removed from the shaped
body
catalyst by washing the catalyst in a continuous stream of solvent. Again, the
amount of
washing may depend on the particular solvent-removable pore building
agent/solvent
combination.
[0054] Any solvent that may solubilize the solvent-removable pore building
agent may
be used. For example, the solvent may include one or more low molecular weight
alcohols
such as methanol or ethanol, a ketone such as acetone or methyl ethyl ketone,
a
supercritical CO2, and/or esters such as ethyl acetate. For instance, an
embodiment includes
the solvent comprising ethanol, methanol, methyl ethyl ketone, ethyl acetate,
acetone,
supercritical CO2 or combination thereof.
[0055] After the solvent-removable pore building agent is removed, no
substantial
residue of carbon, ash or adsorbed organic species may remain at the internal
surfaces of
the catalyst, which allows for the highest feasible catalyst activity.
Furthermore, the
solvent-removable pore building agent may be recovered from the solvent by any
suitable
means such as distillation or extraction.
[0056] The pellet or other shaped body catalyst may then be dried at a
temperature range
from about 45 C to about 75 C for a period of time ranging from about 1 hour
to about 24
hours to produce the vanadium/phosphorus oxide catalyst.
[0057] The vanadium/phosphorus oxide catalysts prepared may be useful in a
variety of
reactors to convert non-aromatic hydrocarbons to maleic anhydride. The
catalysts may be
used in a fixed-bed reactor in the foim of tablets, pellets or the like, or in
a fluid-bed or
transport-bed reactor using comminuted catalyst particles having a particle
size of less than
about 300 microns.
[0058] In one embodiment, the vanadium/phosphorus oxide catalysts are used in
fixed-
bed (tubular), heat exchanger-type reactors. The tubes of such reactors may be
constructed
of iron, stainless steel, carbon steel, nickel, and/or glass and may vary in
diameter from
about 0.635 cm (0.25 inch) to about 3.81 cm (1.5 inches) and in length from
about 15.24
cm (6 inches) to about 609.6 cm (20 feet) or more. It is desirable to have the
surfaces of the
reactors at relatively constant temperatures, and some medium to conduct heat
from the
reactors. Without being limited by theory, such medium aids in temperature
control. Non-
limiting examples of such media include Woods metal, molten sulfur, mercury,
molten
13
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
lead, and eutectic salt baths. A metal block reactor whereby the metal
surrounding the tube
acts as a temperature regulating body may also be used.
[0059] The reaction to convert non-aromatic hydrocarbons to maleic anhydride
may
include only contacting the hydrocarbons having at least four (4) carbons in a
straight chain
(or in a cyclic structure) admixed with a molecular oxygen-containing gas
(including
molecular oxygen), such as air or molecular oxygen-enriched air, with the
vanadium/phosphorus oxide catalyst at elevated temperatures. In addition to
the
hydrocarbon and molecular oxygen, other gases such as nitrogen and steam may
be present
or added to the reactant feed stream. In an embodiment, the hydrocarbon may be
admixed
with the molecular oxygen-containing gas, preferably air, at a concentration
of from about
one (1) mole percent to about ten (10) mole percent hydrocarbon and contacted
with the
vanadium/phosphorus oxide catalyst at a space velocity of about 100 hr4 to
about 4,000 hr'
at a temperature between about 300 C and about 600 C, preferably 1,500 Id' and
about
325 C to about 425 C, to provide an excellent yield and selectivity to maleic
anhydride.
[0060] The reaction may be conducted at atmospheric, super atmospheric, or
subatmospheric pressure. In an embodiment, the reaction may be conducted at or
near
atmospheric pressure. Generally, pressures of from about 1.013 x 10-2 kPa-
gauge (14.7
psig, 1 atmosphere) to about 3.45 x 10-2 kPa-gauge (50 psig) may be
conveniently
employed.
[0061] A large number of nonaromatic hydrocarbons having from four to ten
carbon
atoms may be converted to maleic anhydride using the catalysts prepared by the
process of
the instant invention. In an embodiment, the hydrocarbon may contain not less
than four
carbon atoms in a straight chain or in a cyclic ring. As an example, the
saturated
hydrocarbon n-butane is satisfactory, but isobutane (2-methylpropane) is not
satisfactory
for conversion to maleic anhydride although its presence is not haimful. In
addition to n-
butane, other suitable saturated hydrocarbons include the pentanes, the
hexanes, the
heptanes, the octanes, the nonanes, the decanes, and mixtures of any of these,
with or
without n-butane, so long as a hydrocarbon chain having at least four carbon
atoms in a
straight chain is present in the saturated hydrocarbon molecule.
[0062] Unsaturated hydrocarbons are also suitable for conversion to maleic
anhydride
using the shaped catalyst structures of the instant invention. Suitable
unsaturated
hydrocarbons include the butenes (1-butene and 2-butene), 1,3-butadiene, the
pentenes, the
hexenes, the heptenes, the octenes, the nonenes, the decenes, and mixtures of
any of these,
14
CA 02661157 2013-09-19
75704-283
with or without the butenes, again, so long as the requisite hydrocarbon chain
having at
least four carbon atoms in a straight chain is present in the molecule.
[0063] Cyclic compounds such as cyclopentane and cyclopentene also are
satisfactory
feed materials for conversion to maleic anhydride using the
vanadium/phosphorous oxide
catalysts.
[0064] Of the aforementioned feedstocks, n-butane is the preferred saturated
hydrocarbon, and the butenes are the preferred unsaturated hydrocarbons, with
n-butane
being most preferred of all feedstocks. It will be noted that the
aforementioned feedstocks
may not be pure substances but may be technical grade hydrocarbons.
[0065] In an embodiment, the principal product from the oxidation of the
aforementioned
suitable feedstock is maleic anhydride, although small amounts of citraconic
anhydride
(methyl maleic anhydride) may also be produced when the feedstock is a
hydrocarbon
containing more than four carbon atoms. The maleic anhydride produced by using
the
vanadium/phosphorus oxide catalysts may be recovered by any suitable means.
For
example, maleic anhydride may be recovered by direct condensation or by
absorption in
suitable media with subsequent separation and purification of the maleic
anhydride.
[0066] The following specific examples illustrating the best currently-known
method of
practicing this invention are described in detail in order to facilitate a
clear understanding of
the invention. It should be understood, however, that the detailed expositions
of the
application of the invention, while indicating preferred embodiments, are
given by way of
illustration only and are not to be construed as limiting the invention since
various changes
and modifications within the claimed scope of the invention will become
apparent to those skilled
in the art from this detailed description.
EXAMPLE 1
[0067] A catalyst precursor was prepared in accordance with Example 1 of U.S.
Pat.
No. 5,137,860. In a 12-liter, round
bottom flask, fitted with a paddle stirrer, a thermometer, a heating mantle,
and a reflux
condenser, was charged 9,000 mL of isobutyl alcohol, 378.3 g (4.20 mol) of
oxalic acid
(C2H204), and 848.4 g (4.66 mol) of vanadium pentoxide (V205). To this stirred
mixture
was added 997.6 g (10.76 mol) of phosphoric acid (I-131)04, 105.7% by weight).
The
resultant mixture was refluxed for about 16 hours to give a bright blue
mixture. After
stripping off approximately 25% (2.2 L) of the isobutyl alcohol over a 1-hour
period, the
mixture was cooled and approximately 50% of the remaining isobutyl alcohol
removed by
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
decantation. The resultant concentrated slurry was then quantitatively
transferred to a flat
porcelain dish and dried for 24 hours at 110 -150 C in nitrogen. The dried
material was
thereafter heated in air at 250 -260 C for approximately 5 hours to yield a
grey-black
catalyst precursor powder.
[0068] The catalyst precursor powder was blended to contain approximately
three (3.0)
weight % graphite and compressed on a Stokes 512 Rotary Tableting machine
equipped
with appropriate dies and punches to produce catalyst precursor cylindrical
slugs having a
1/2 inch diameter, a 0.10-0.12 thickness, and a density of about 1.50 g/cm3.
[0069] The catalyst precursor slugs were then activated in accordance as
taught in U.S.
Pat. No. 5,137,860. The catalyst precursor slugs were placed onto a 30.48 cm x
30.48 cm x
2.54 cm tray fonned from stainless steel mesh screen having approximately 40%
open area
stainless steel, which was placed in a box oven. The slugs were then heated in
the initial
heat-up stage from room temperature (approximately 25 C) to 275 C in air with
no control
of the heat-up rate. The temperature was thereafter increased in the rapid
heat-up stage to
425 C at a programmed rate of 4 C/min in an atmosphere of 50 mol % air/50 mol
% steam.
The temperature was maintained at 425 C in the maintenance/finishing stage,
first in the
rapid heat-up stage atmosphere for a period of 1 hour, and thereafter in an
atmosphere of 50
mol % nitrogen/50 mol % steam for a period of 6 hours.
[0070] The activated catalyst was then granulated to pass through an 18 mesh
screen,
blended with stearic acid to produce a mixture containing 10% stearic acid by
weight, and
compressed to produce 1/4 inch trilobe pellets having three equidistant
grooves etched in the
longitudinal surface. The stearic acid was then removed from the trilobe
pellets by placing
the pellets into a box oven purged with nitrogen gas and heating the pellets
approximately
240 C for one hour. The atmosphere in the oven was then changed to 50 volume
percent
nitrogen and 50 volume percent steam, and air was incremented in three steps
over
approximately 60 minutes to give a gas composition of [25:25:50] volume per
cent
[air:nitrogen:steam]. The temperature was maintained at 240 C in this
atmosphere for
approximately 60 minutes.
[0071] The vanadium/phosphorus oxide catalyst pellets were perfonnance tested
at a
standardized set of reaction conditions of 2.0 0.2 mol % n-butane in
synthetic air (21 mol
% oxygen/71 mol % helium), 1.034 x 10-2 kPa-gauge (15.0 psig) inlet pressure,
and 1,500
GHSV. The catalyst pellets (12.0 g) were charged to a 1.092 cm inside diameter
x 30.48
cm long (0.43 in. inside diameter x 1 ft long) reactor to provide a catalyst
bed of
16
CA 02661157 2009-02-19
WO 2008/030714 PCT/US2007/076747
approximately 15.24 cm (6 in.) in length. The catalyst pellets were then run
at 85 2 mol
% n-butane conversion for about 100 hours and generated a maleic anhydride
yield of
57.9%.
COMPARATIVE EXAMPLE 1
[0072] A catalyst precursor powder was prepared according to the method
described in
EXAMPLE 1. The catalyst precursor powder was then blended to contain
approximately
ten (10) weight % stearic acid and compressed on a Stokes 512 Rotary Tableting
machine
equipped with appropriate dies and punches to produce catalyst precursor slugs
having a V2
inch diameter, a 0.10-0.12 thickness, and a density of about 1.30 ¨ 1.40
g/cm3. The slugs
were then granulated to less than 1 mm particles, which were then tableted to
produce 1/4
inch trilobe pellets having three equidistant grooves etched in the
longitudinal surface.
[0073] The stearic acid was then removed from the trilobe pellets by placing
the pellets in
a box oven purged with nitrogen gas and heating the pellets at approximately
240 C for one
hour. The atmosphere in the oven was then changed to 50 volume percent
nitrogen and 50
volume percent steam, and air was incremented in three steps over
approximately 60
minutes to give a gas composition of [25:25:50] volume per cent
[air:nitrogen:steam]. The
temperature was maintained at 240 C in this atmosphere for approximately 60
minutes.
[0074] The 1/4 inch trilobe pellets were then activated similar to the
activation procedure
of EXAMPLE 1. After cooling, the trilobe pellets were performance tested as
described in
EXAMPLE 1 and generated a maleic anhydride yield of 56.8%.
EXAMPLE 2
[0075] A catalyst precursor powder was prepared according to the procedure
described in
EXAMPLE 1. The catalyst precursor powder was then blended to contain three (3)
weight
percent graphite and compressed on a Stokes 512 Rotary Tableting machine to
produce
catalyst precursor cylindrical slugs having a 1/2 inch diameter, a 0.10-0.12
thickness, and a
density of about 1.50 g/cm3.
[0076] The catalyst precursor slugs were activated by the heat treatment
described in
EXAMPLE 1. The activated catalyst was then granulated to pass through an 18
mesh
screen and then blended with 1,1,1-tris(hydroxymethylethane) to provide a
mixture
containing ten (10) weight percent 1,1,1-tris(hydroxymethylethane). This
mixture was then
compressed to produce 1/4 inch diameter trilobe pellets. The 1,1,1-
tris(hydroxymethylethane) was then removed from the trilobe pellets by
submersing the
17
CA 02661157 2013-09-19
75704-283
pellets in an acetone bath for 22.5 hours at a 1:1.5 pellet to acetone ratio.
The pellets were
removed, further washed with acetone, and then dried at 60 C for 18 hours.
[0077] The trilobe pellets were then performance tested according to the
method
described in EXAMPLE 1 and generated a maleic anhydride yield of 61.9%.
COMPARATIVE EXAMPLE 2
[0078] A catalyst precursor powder was prepared according to the method
described in
EXAMPLE 1. The catalyst precursor powder was then blended to contain
approximately
ten (10) weight percent 1,1,1-tris(hydroxymethylethane) and compressed on a
Stokes 512
Rotary Tableting machine equipped with appropriate dies and punches to produce
catalyst
precursor slugs having a 1/2 inch diameter, a 0.10-0.12 thickness, and a
density of about 1.30
¨ 1.40 g/cm3. The slugs were then granulated to less than 1 mm particles which
were then
tableted to produce 1/4 inch trilobe pellets having three equidistant grooves
etched in the
longitudinal surface.
[0079] The 1,1,1-tris(hydroxymethylethane) was then removed from the trilobe
pellets by
placing the pellets in acetone for 22.5 hours at a 1:1.5 pellet to acetone
ratio. The pellets
were then removed, further washed with acetone, then dried at 60 C for 18
hours.
[0080] The trilobe pellets were then activated similar to the activation
procedure of
EXAMPLE 1. After cooling, the trilobe pellets were performance tested as
described in
EXAMPLE 1 and generated a maleic anhydride yield of 58.8%.
[0081] From the results above, it can be seen that activating the catalyst
precursor slug
prior to tableting improves the performance of the vanadium/phosphorus oxide
catalyst.
The catalyst's performance may be further improved by treating the activated
catalyst
precursor slug with a solvent-removable pore building agent prior to
tableting. It is
believed that activating the catalyst precursor slug, which is less dense than
the tablet, prior
to tableting allows the slug to be uniformly gas and thermally treated and
subsequently
provides a vanadium/phosphorus oxide catalyst exhibiting uniform chemical
properties, and
thus, improved selectivity.
[0082] Although making and using various embodiments of the present invention
have
been described in detail above, it should be appreciated that the present
invention provides
many applicable inventive concepts that can be embodied in a wide variety of
specific
contexts. The specific embodiments discussed herein are merely illustrative of
specific
ways to make and use the invention, and do not delimit the scope of the
invention, which is set
out by the appended claims.
18