Note: Descriptions are shown in the official language in which they were submitted.
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410
THERAPEUTIC SUBSTITUTED THIAZOLIDINONES, OXAZOLIDINONES, AND RELATED COMPOUNDS
by Inventor
David W. Old
DESCRIPTION OF THE INVENTION
Ocular hypotensive agents are useful in the treatment of a number of various
ocular hypertensive conditions, such as
post-surgical and post-laser trabeculectomy ocular hypertensive episodes,
glaucoma, and as presurgical adjuncts. Prostaglandin
agonists have been shown to be useful as ocular hypotensive agents.
The compounds disclosed herein are ocular hypotensive agents.
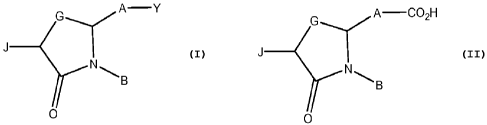
Disclosed herein is a compound having a structure
G A Y
N'11~ B
0
or a pharmaceutically acceptable salt thereof, or a prodrug thereof;
Y is an organic acid functional group, or an amide or ester thereof comprising
up to 14 carbon atoms; or Y is hydroxymethyl or an
ether thereof comprising up to 14 carbon atoms; or Y is a tetrazolyl
functional group;
A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-, wherein 1 or 2
carbon atoms may be replaced by S or 0; or A is -
(CH2)m-Ar-(CH2)o- wherein Ar is interarylene or heterointerarylene, the sum of
m and o is 1, 2, 3, or 4, and wherein one CH2 may
be replaced by S or 0;
G is 0, S, S=O, or S(=0)2;
J is H, halogen, CF3; or C,-6 alkyl; and
B is aryl or heteroaryl.
Also disclosed herein is a carboxylic acid or a bioisostere thereof, said
carboxylic acid having a structure
G A C02H
N
B
0
or a pharmaceutically acceptable salt thereof, or a prodrug thereof,;
wherein A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-, wherein 1 or
2 carbon atoms may be replaced by S or 0;
or A is -(CH2)m-Ar-(CH2)o- wherein Ar is interarylene or heterointerarylene,
the sum of m and o is 1, 2, 3, or 4, and wherein one
CH2 may be replaced by S or 0;
G is 0, S, S=O, or S(=0)2;
J is H, halogen, CF3; or C,-6 alkyl; and
B is aryl or heteroaryl.
In one embodiment, if B is unsubstituted aryl or heteroaryl and Jl is H, J2 is
not H.
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
"Bioisosteres are substituents or groups that have chemical or physical
similarities, and which produce broadly similar
biological properties." Silverman, Richard B., The Organic Chemistry of Drug
Design and Drug Action, 2nd Edition, Amsterdam:
Elsevier Academic Press, 2004, p. 29.
While not intending to be limiting, organic acid functional groups are
bioisoteres of carboxylic acids. An organic acid
functional group is an acidic functional group on an organic molecule. While
not intending to be limiting, organic acid functional
groups may comprise an oxide of carbon, sulfur, or phosphorous. Thus, while
not intending to limit the scope of the invention in
any way, in certain compounds Y is a carboxylic acid, sulfonic acid, or
phosphonic acid functional group.
Additionally, an amide or ester of one of the organic acids mentioned above
comprising up to 14 carbon atoms is also
contemplated. In an ester, a hydrocarbyl moiety replaces a hydrogen atom of an
acid such as in a carboxylic acid ester, e.g.
C02Me, C02Et, etc.
In an amide, an amine group replaces an OH of the acid. Examples of amides
include CON(R2)2, CON(0R2)R2,
CON(CH2CH2OH)2, and CONH(CH2CH2OH) where R2 is independently H, Ci-C6 alkyl,
phenyl, or biphenyl. Moieties such as
CONHS02R2 are also amides of the carboxylic acid notwithstanding the fact that
they may also be considered to be amides of
the sulfonic acid R2-SO3H. The following amides are also specifically
contemplated, CONS02-biphenyl, CONS02-phenyl,
CONSOz-heteroaryl, and CONSOz-naphthyl. The biphenyl, phenyl, heteroaryl, or
naphthyl may be substituted or unsubstituted.
Han et. al. (Biorganic & Medicinal Chemistry Letters 15 (2005) 3487-3490) has
recently shown that the groups shown
below are suitable bioisosteres for a carboxylic acid. The activity of
compounds with these groups in inhibiting HCV NS3
protease was comparable to or superior to similar compounds where the group is
replaced by C02H. Thus, Y could be any group
depicted below.
2
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Carboxylic acid bioisosteres according to Han et. al.
0
psp ~ OS 0 0 OS 0 00
~i ", pH ~ S \/kNi \ S
,H Ph N H
N-N Ph
1H N p 0 CI
N p p H~S CI 0II 0 S 0
~ ~S\ / H~ CI
~ H M e
0 0
~ 0~ 0 ~ 0\ ~0 ci
~ N"S \~ N"S N02
H CFs H p 0 0 CI
~
0 ~ N~ \
0~ 0 H
t~N~S~Ph 0 p p /
H ~ ~
~~NS Ph CO2H
0II 0 0 H
/~ 0 0 0
~ H \ \ ~S/
N
0 0 0 ~ H
H/S N\N
0 OS 0
~~H/ N02 0 0 0 00
"S
N S~NH2
H
p CI
o S~
H ~! S H / CI 0 0\O
N ~N \ I S S
H
0 H N-- /_
NnC5H11
N
0
While not intending to limit the scope of the invention in any way, Y may also
be hydroxymethyl or an ether thereof
comprising up to 14 carbon atoms. An ether is a functional group wherein a
hydrogen of an hydroxyl is replaced by carbon, e.g.,
Y is CH2OCH3, CH2OCH2CH3, etc. These groups are also bioisosteres of a
carboxylic acid.
"Up to 14 carbon atoms" means that the entire Y moiety, including the carbonyl
carbon of a carboxylic acid ester or
amide, and both carbon atoms in the -CH2O-C of an ether has 0,1, 2, 3, 4, 5,
6, 7, 8, 9, 10, 11, 12, 13, or 14 carbon atoms.
Finally, while not intending to limit the scope of the invention in any way, Y
may be a tetrazolyl functional group.
While not intending to be limiting, examples of compounds having the
identified Y are depicted below. In these
examples R is H or hydrocarbyl, subject to the constraints defined herein.
Each structure below represents a specific
embodiment which is individually contemplated, as well as pharmaceutically
acceptable salts and prodrugs of compounds which
are represented by the structures. However, other examples are possible which
may not fall within the scope of the structures
shown below.
3
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Y is tetrazolyl. A_
HN--,N M1=
J
M14\ \N N~B
N, 0
Organic Acids Esters Amides
Mi-COzH Mi-COzR Mi-COzNRz
Carboxylic Acid Carboxylic Acid Ester Carboxylic Acid Amide
Mi-P(0)(OH)2 M1-P(0)(0H)R M1-P(0)(0H)NRZ
Phosponic Acid Phosphonic Acid Ester Phosphonic Acid Amide
Mi-S03H Mi-S03R M'-SO3NR,
Sulfonic Acid Sulfonic Acid Ester Sulfonic Acid Amide
M'-CHzOH M'-CHzOR
Y is hydroxymethyl Ether
A tetrazolyl functional group is another bioisostere of a carboxylic acid. An
unsubstituted tetrazolyl functional group has
two tautomeric forms, which can rapidly interconvert in aqueous or biological
media, and are thus equivalent to one another.
These tautomers are shown below.
N NH
II
H/ N/ N
Additionally, if R2 is Cl-C6 alkyl, phenyl, or biphenyl, other isomeric forms
of the tetrazolyl functional group such as the one shown
below are also possible, unsubstituted and hydrocarbyl substituted tetrazolyl
up to C12 are considered to be within the scope of
the term "tetrazolyl."
N
~ / II
N
I
R2
While not intending to limit the scope of the invention in any way, in one
embodiment, Y is C02R2, CON(R2)2,
CON(0R2)R2, CON(CH2CH2OH)2, CONH(CH2CH2OH), CH2OH, P(0)(0H)2, CONHS02R2,
S02N(R2)2, S02NHR2,
4
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
N N
I NIN
N
N 1 \ I
1 2 N 2
R or R
wherein R2 is independently H, Ci-C6 alkyl, unsubstituted phenyl, or
unsubstituted biphenyl.
According to Silverman (p. 30), the moieties shown below are also bioisosteres
of a carboxylic acid.
Carboxylic acid bioisosteres according to Silverman
0
0`N S~N
CN
OH OH
0
H3C
_ N OH
N
~ N
\ ~
OH 0
OH
dow
dow
OH OH OH
N N
N N
F
/O H
/ N OH
N
~
F
Orlek et al. (J. Med. Chem. 1991, 34, 2726-2735) described oxadiazoles as
suitable bioisosteres for a carboxylic acid.
These ester replacements were shown to be potent muscarinic agonists having
improved metabolic stability. Oxadiazoles were
also described by Anderson et al. (Eur. J. Med. Chem. 1996, 31, 417-425) as
carboxamide replacements having improved in vivo
efficacy at the benzodiazepine receptor.
Carboxylic acid bioisosteres according to Orlek et. al.
~CH3 N~CH3 CH3
;11N "0 N
N
5
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Kohara et al. (J. Med. Chem. 1996, 39, 5228-5235) described acidic
heterocycles as suitable bioisosteres for a
tetrazole. These carboxylic acid replacements were shown to be potent
angiotensin II receptor antagonists having improved
metabolic stability.
Tetrazole bioisosteres according to Kohara et. al.
N-s `0 ~ o~ `
/N S O ~ /N 0
H H H H
Drysdale et al. (J. Med. Chem. 1992, 35, 2573-2581) have described carboxylic
acid mimics of non-peptide CCK-B
receptor antagonists. The binding affinities of many of the bioisosteres are
similar to the parent carboxylic acid.
Carboxylic acid bioisosteres according to Drysdale et. al.
HS
OH N 11
O IN ~~N\ N S N,N
H
N N-NH
S- \ N N "S-N ~~S, \ 1~1'0 H H H H 0
A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-, wherein 1 or 2
carbon atoms may be replaced by S or 0;
or A is -(CH2)m-Ar-(CH2)o- wherein Ar is interarylene or heterointerarylene,
the sum of m and o is 1, 2, 3, or 4, and wherein 1 -
CH2- may be replaced by S or 0, and 1 -CH2-CH2- may be replaced by -CH=CH- or -
C=C-.
Thus, A may be -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-.
Alternatively, A may be a group which is related to one of these three
moieties in that any carbon is replaced with S or
0. For example, A may be a moiety where S replaces one or two carbon atoms
such as one of the following or the like.
Alternatively, A may be a moiety where 0 replaces one or two carbon atoms such
as one of the following or the like.
6
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
NY ---'~/
Alternatively, A may have an 0 replacing one carbon atom and an S replacing
another carbon atom, such as one of the
following or the like.
Al" ~~s~\~# 5 -1~/
Alternatively, in certain embodiments A is -(CH2)m-Ar-(CH2)o- wherein Ar is
interarylene or heterointerarylene, the sum
of m and o is 1, 2, 3, or 4, and wherein 1 -CH2- may be replaced by S or 0,
and 1 -CH2-CH2- may be replaced by -CH=CH- or -
C=C-, In other words,
in one embodiment A comprises:
1) a) 1, 2, 3, or 4 -CH2- moieties, or
b) 0, 1 or 2 -CH2- moieties and -CH=CH- or -C=C-; and
2) Ar;
e.g. -CH2-Ar-, -(CH2)2-Ar-, -CH=CH-Ar-, -C-C-Ar-, -CH2-Ar-CH2-, -CH2Ar-(CH2)2-
, -CH2Ar-CH=CH-, -CH2Ar-C-C-, -(CH2)2-
Ar-(CH2)2-, and the like;
in another embodiment A comprises:
1) a) 0; and 0, 1, 2, or 3 -CH2- moieties; or
b) 0; and 0 or 1 -CH2- moieties and -CH=CH- or -C=C-; and
2) Ar;
e.g., -0-Ar-, -Ar-CH2-0-, -O-Ar-(CH2)2-, -OAr-CH=CH-, -0-Ar-C-C-,-0-CH2-Ar-, -
0-CH2-Ar-(CH2)2, -0-CH2Ar-CH=CH-, -
0-CH2Ar-C-C-,and the like; or
in another embodiment A comprises:
1) a) S; and 0, 1, 2, or 3 -CH2- moieties; or
b) S; and 0 or 1 -CH2- moieties and -CH=CH- or -C-C-; and
2) Ar;
e.g,, -S-Ar-, -Ar-CH2-S-, -S-Ar-(CH2)2-, -SAr-CH=CH-, -S-Ar-C=C-,-S-CH2-Ar-, -
S-CH2-Ar-(CH2)2, -S-CH2Ar-CH=CH-, -S-
CH2Ar-C-C-, and the like.
7
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
In another embodiment, the sum of m and o is 2, 3, or 4 wherein one CH2 may be
replaced with S or 0 and 1 -CH2-
CH2- may be replaced by -CH=CH- or -C--C-.
In another embodiment, the sum of m and o is 3 wherein one CH2 may be replaced
with S or 0 and 1 -CH2-CH2- may
be replaced by -CH=CH- or -C--C-.
In another embodiment, the sum of m and o is 2 wherein one CH2 may be replaced
with S or 0 or 1 -CH2-CH2- may be
replaced by -CH=CH- or -C--C-.
In another embodiment, the sum of m and o is 4 wherein one CH2 may be replaced
with S or 0 and 1 -CH2-CH2- may
be replaced by -CH=CH- or -C--C-.
Interarylene or heterointerarylene refers to an aryl ring or ring system or a
heteroaryl ring or ring system which
connects two other parts of a molecule, i.e. the two parts are bonded to the
ring in two distinct ring positions. Interarylene or
heterointerarylene may be substituted or unsubstituted. Unsubstituted
interarylene or heterointerarylene has no substituents
other than the two parts of the molecule it connects. Substituted interarylene
or heterointerarylene has substituents in addition to
the two parts of the molecule it connects.
In one embodiment, Ar is substituted or unsubstituted interphenylene,
interthienylene, interfurylene, interpyridinylene,
interoxazolylene, and interthiazolylene. In another embodiment Ar is
interphenylene (Ph). In another embodiment A is -(CH2)2-
Ph-. Substitutents of Ar each have from 0 to 4 carbon atoms, from 0 to 3
oxygen atoms, from 0 to 2 sulfur atoms, from 0 to 2
nitrogen atoms, from 0 to 3 fluorine atoms, from 0 to 1 chlorine atoms, from 0
to 1 bromine atoms, from 0 to 1 iodine atoms, and
from 0 to 10 hydrogen atoms.
In another embodiment A is -CH2-Ar-OCH2-. In another embodiment A is -CH2-Ph-
OCH2-. In another embodiment, Ph
is attached at the 1 and 3 positions, otherwise known as m-interphenylene,
such as when A has the structure shown below.
v HZC C~CH2 ~
11:)__ In another embodiment A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-
(CH2)3-, wherein 1 or 2 carbon atoms may
be replaced with S or 0; or A is -(CH2)2-Ph- wherein one -CH2- may be replaced
with S or 0.
In another embodiment A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-
, wherein 1 or 2 carbon atoms may
be replaced with S or 0; or A is -(CH2)2-Ph-.
In one embodiment, Ar is thienyl.
In other embodiments, A has one of the following structures.
8
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
1 J N
,(,p
N--
NJ ~ p S
yi-~p
p
o # S
N-- ~~;
vll~->>~ p o
`l'~~
In another embodiment A is -CH2OCH2Ar-.
In another embodiment A is -CH2SCH2Ar-.
In another embodiment A is -(CH2)3Ar-.
In another embodiment A is -CH2O(CH2)4-.
In another embodiment A is -CH2S(CH2)4-,
In another embodiment A is -(CH2)6-.
In another embodiment A is cis -CH2CH=CH-(CH2)3-.
In another embodiment A is -CH2C=C-(CH2)3-.
In another embodiment A is -S(CH2)3S(CH2)2-.
In another embodiment A is -(CH2)40CH2-.
In another embodiment A is cis -CH2CH=CH-CH2OCH2-.
In another embodiment A is -CH2CH=CH-CH20CH2-.
In another embodiment A is -(CH2)2S(CH2)3-.
In another embodiment A is -CH2-Ph-OCH2-, wherein Ph is interphenylene,.
In another embodiment A is -CH2-mPh-OCH2-, wherein mPh is m-interphenylene.
In another embodiment A is -CH2-0-(CH2)4-.
In another embodiment A is -CH2-0-CH2-Ar-, wherein Ar is 2,5-interthienylene.
In another embodiment A is -CH2-0-CH2-Ar-, wherein Ar is 2,5-interfurylene.
In another embodiment A is (3-methylphenoxy)methyl.
In another embodiment A is (4-but-2-ynyloxy)methyl.
In another embodiment A is 2-(2-ethylthio)thiazol-4-yl.
In another embodiment A is 2-(3-propyl)thiazol-5-yl.
In another embodiment A is 3-(methoxymethyl)phenyl.
In another embodiment A is 3-(3-propylphenyl).
In another embodiment A is 3-methylphenethyl.
In another embodiment A is 4-(2-ethyl)phenyl.
In another embodiment A is 4-phenethyl.
9
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
In another embodiment A is 4-methoxybutyl.
In another embodiment A is 5-(methoxymethyl)furan-2-yl .
In another embodiment A is 5-(methoxymethyl)thiophen-2-yl.
In another embodiment A is 5-(3-propyl)furan-2-yl.
In another embodiment A is 5-(3-propyl)thiophen-2-yl.
In another embodiment A is 6-hexyl.
In another embodiment A is (Z)-6-hex-4-enyl.
Interarylene or heterointerarylene refers to an aryl ring or ring system or a
heteroaryl ring or ring system which
connects two other parts of a molecule, i.e. the two parts are bonded to the
ring in two distinct ring positions. Interarylene or
heterointerarylene may be substituted or unsubstituted. Unsubstituted
interarylene or heterointerarylene has no substituents
other than the two parts of the molecule it connects. Substituted interarylene
or heterointerarylene has substituents in addition to
the two parts of the molecule it connects.
In one embodiment, Ar is substituted or unsubstituted interphenylene,
interthienylene, interfurylene, interpyridinylene,
interoxazolylene, and interthiazolylene. In another embodiment Ar is
interphenylene (Ph). In another embodiment A is -(CH2)2-
Ph-. While not intending to limit scope of the invention in any way,
substituents may have 4 or less heavy atoms, wherein the
heavy atoms are C, N, 0, S, P, F, Cl, Br, and/or I in any stable combination.
Any number of hydrogen atoms required for a
particular substituent will also be included. A substituent must be stable
enough for the compound to be useful as described
herein. In addition to the atoms listed above, a substituent may also have a
metal cation or any other stable cation having an
atom not listed above if the substituent is acidic and the salt form is
stable. For example, -OH may form an -O-Na' salt or C02H
may form a C02-K' salt. Any cation of the salt is not counted in the "4 or
less heavy atoms." Thus, the substituent may be
hydrocarbyl having up to 4 carbon atoms, including alkyl up to C4, alkenyl,
alkynyl, and the like;
hydrocarbyloxy up to C3;
organic acid such as C02H, SOaH, P(0)(OH)2, and the like, and salts thereof;
CF3;
halo, such as F, Cl, or Br;
hydroxyl;
NH2 and alkylamine functional groups up to C3;
other N or S containing substituents such as CN, N02, and the like;
and the like.
In one embodiment A is -(CH2)m-Ph-(CH2)o- wherein the sum of m and o is 1, 2,
or 3, and wherein one CH2 may be
replaced with S or 0.
In another embodiment A is -CH2-Ar-OCH2-. In another embodiment A is -CH2-Ph-
OCH2-. In another embodiment, Ph
is attached at the 1 and 3 positions, otherwise known as m-interphenylene,
such as when A has the structure shown below.
HZC O~
~ CH2
~
In another embodiment A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-
, wherein 1 or 2 carbon atoms may
be replaced with S or 0; or A is -(CH2)2-Ph- wherein one CH2 may be replaced
with S or 0.
In another embodiment A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-
, wherein 1 or 2 carbon atoms may
be replaced with S or 0; or A is -(CH2)2-Ph-.
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
In other embodiments, A has one of the following structures, where Y is
attached to the aromatic or heteroaromatic
ring.
0
HZCS HZC~O HZC\/0 11 S HZC0 fN-)/
ND N~ N~ "ZCN-IZS~S "ZCS~O "ZC~0 0 / / "ZC~0 g /
/
11 11
H2CIN. 0 ~ H2C~S ~ HZC1,_,,,O ~ HZC~~S
~ I
H2C H2C
In another embodiment A is -CH2OCH2Ar.
In another embodiment A is -CH2SCH2Ar.
In another embodiment A is -(CH2)3Ar.
In another embodiment A is -CH2O(CH2)4.
In another embodiment A is -CH2S(CH2)4.
In another embodiment A is -(CH2)6-.
In another embodiment A is cis -CH2CH=CH-(CH2)3-.
In another embodiment A is -CH2C=C-(CH2)3-.
In another embodiment A is -S(CH2)3S(CH2)2-.
In another embodiment A is -(CH2)40CH2-.
In another embodiment A is cis -CH2CH=CH-CH2OCH2-.
In another embodiment A is -CH2CH=CH-CH2OCH2-.
In another embodiment A is -(CH2)2S(CH2)3-.
In another embodiment A is -CH2-Ph-OCH2-, wherein Ph is interphenylene,.
In another embodiment A is -CH2-mPh-OCH2-, wherein mPh is m-interphenylene.
In another embodiment A is -CH2-0-(CH2)4-.
In another embodiment A is -CH2-0-CH2-Ar-, wherein Ar is 2,5-interthienylene.
In another embodiment A is -CH2-0-CH2-Ar-, wherein Ar is 2,5-interfurylene.
In another embodiment A is (3-methylphenoxy)methyl.
In another embodiment A is (4-but-2-ynyloxy)methyl.
In another embodiment A is 2-(2-ethylthio)thiazol-4-yl.
In another embodiment A is 2-(3-propyl)thiazol-5-yl.
In another embodiment A is 3-methoxymethyl)phenyl.
In another embodiment A is 3-(3-propylphenyl.
In another embodiment A is 3-methylphenethyl.
In another embodiment A is 4-(2-ethyl)phenyl.
In another embodiment A is 4-phenethyl.
In another embodiment A is 4-methoxybutyl.
In another embodiment A is 5-(methoxymethyl)furan-2-yl ,
11
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
In another embodiment A is 5-(methoxymethyl)thiophen-2-yl.
In another embodiment A is 5-(3-propyl)furan-2-yl.
In another embodiment A is 5-(3-propyl)thiophen-2-yl.
In another embodiment A is 6-hexyl.
In another embodiment A is (Z)-6-hex-4-enyl.
Compounds according to the each of the structures depicted below, and
pharmaceutically acceptable salts thereof, and
prodrugs thereof, are contemplated as individual embodiments. In other words,
each structure represents a different
embodiment.
12
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
M2=
B
M2 0 M2 Y
M2/\0 M2""'~p Y
M2/\0
I S
M2""~0 Y
M2
M2 Y
/
/ Y
M2\
M2 Y
M2~~S /N Y 0 Y
M2
S I
Y
M2
M2 0/\ Y
Y S
M2 M2 Y
G is 0, S, S=O, or S(=0)2.
13
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Thus, each of the structures below is contemplated. These structures, or
pharmaceutically acceptable salts thereof, or
prodrugs thereof, individually represent a compound which is an embodiment
contemplated herein. In other words, each
structure represents a different embodiment.
0 0
0 A-Y A-Y S A-Y ~s/ A-Y
J J S___r J __r J ___r
-<r N"'~ B Nll,, B N1-1 B NB
0 0 0 0
J is H, halogen, CF3; or C1.6 alkyl.
In one embodiment J is H.
In another embodiment J is halogen.
In another embodiment J is CF3.
In another embodiment J is C1.6 alkyl.
Thus, each of the structures below is contemplated. These structures, or
pharmaceutically acceptable salts thereof, or
prodrugs thereof, individually represent a compound which is an embodiment
contemplated herein. In other words, each
structure represents a different embodiment.
G A-Y A-Y A-Y A-Y
CI G ~ Br G ~ F3C G~
N1*11 B N1~1 B N'_1 B N'I-,' B
0 0 0 0
G A-Y G A-Y
~
CH3CH2
N~B N1~1 B
0 0
Aryl is an aromatic ring or ring system such as phenyl, naphthyl, biphenyl,
and the like.
Heteroaryl is aryl having one or more N, 0, or S atoms in the ring, i.e. one
or more ring carbons are substituted by N,
0, and/or S. While not intending to be limiting, examples of heteroaryl
include thienyl, pyridinyl, furyl, benzothienyl, benzofuryl,
imidizololyl, indolyl, and the like.
Aryl or heteroaryl may be substituted or unsubstituted. A substituent of aryl
or heteroaryl may have up to 20 non-
hydrogen atoms each in any stable combination and as many hydrogen atoms as
necessary, wherein the non-hydrogen atoms
are C, N, 0, S, P, F, Cl, Br, and/or I in any stable combination. However, the
total number of non-hydrogen atoms on all of the
substituents combined must also be 20 or less. A substituent must be
sufficiently stable for the compound, salt, or prodrug to be
useful as described herein. In addition to the atoms listed above, a
substituent may also have a metal cation or other stable
cation having an atom not listed above if the substituent is acidic and the
salt form is stable. For example, -OH may form an -0-
Na' salt or C02H may form a C02-K+ salt. Thus, while not intending to limit
the scope of the invention in any way, a substituent
may be:
14
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
hydrocarbyl, i.e. a moiety consisting of only carbon and hydrogen such as
alkyl, alkenyl, alkynyl, and the like, including linear,
branched or cyclic hydrocarbyl, and combinations thereof;
Alkvl is hydrocarbyl having no double or triple bonds;
C1-6 alkyl is alkyl having 1, 2, 3, 4, 5, or 6 carbon atoms;
hydrocarbyloxy, meaning 0-hydrocarbyl such as OCH3, OCH2CH3, 0-cyclohexyl,
etc, up to 19 carbon atoms;
alkoxy is 0-alkyl;
CI-6 alkoxy is alkoxy having 1, 2, 3, 4, 5, or 6 carbon atoms;
other ether substituents such as CH2OCH3, (CH2)20CH(CH3)2, and the like;
thioether substituents including S-hydrocarbyl and other thioether
substituents;
hydroxyhydrocarbyl, meaning hydrocarbyl-OH such as CH2OH, C(CH3)20H, etc, up
to 19 carbon atoms;
nitrogen substituents such as N02, CN, and the like, including
amino, such as NH2, NH(CH2CHaOH), NHCH3, and the like;
Co-6 amino is amino having 0, 1, 2, 3, 4, 5 or 6 carbon atoms;
carbonyl substituents, such as C02H, ester, amide, and the like;
halogen, such as chloro, fluoro, bromo, and the like
fluorocarbyl, such as CF3, CF2CF3, etc.;
phosphorous substituents, such as P032-, and the like;
sulfur substituents, including S-hydrocarbyl, SH, SOaH, S02-hydrocarbyl, S03-
hydrocarbyl, and the like.
Substituted aryl or heteroaryl may have as many substituents as the ring or
ring system will bear, and the substituents
may be the same or different. Thus, for example, an aryl ring or a heteroaryl
ring may be substituted with chloro and methyl;
methyl, OH, and F; CN, N02, and ethyl; and the like including any conceivable
substituent or combination of substituent possible
in light of this disclosure.
Subsituted aryl or substituted heteroaryl also includes a bicyclic or
polycyclic ring system wherein one or more rings are
aromatic and one or more rings are not. For example, indanonyl, indanyl,
indanolyl, tetralonyl, and the like are substituted aryl.
For this type of polycyclic ring system, an aromatic or heteroaromatic ring,
not a non-aromatic ring, must be attached to the
remainder of the molecule, i.e. the part of the molecule that is not B. In
other words, in any structure depicting -B herein, where
- is a bond, the bond is a direct bond to an aromatic ring.
In one embodiment, B is substituted aryl or heteroaryl.
In another embodiment B is substituted phenyl.
In another embodiment B has no halogen atoms.
In another embodiment B is 4-(1-hydroxy-2,2-dimethylpropyl)phenyl.
In another embodiment B is 4-(1-hydroxy-2-methylpropan-2-yl)phenyl.
In another embodiment B is 4-(1-hydroxy-2-methylpropyl)phenyl.
In another embodiment B is 4-(1-hydroxybutyl)phenyl.
In another embodiment B is 4-(1-hydroxyheptyl)phenyl.
In another embodiment B is 4-(1-hydroxyhexyl)phenyl.
In another embodiment B is 4-(1-hydroxypentyl)phenyl.
In another embodiment B is 4-(1-hydroxypropyl)phenyl,
In another embodiment B is 4-(3-hydroxy-2-methylheptan-2-yl)phenyl.
In another embodiment B is 4-(3-hydroxy-2-methyloctan-2-yl)phenyl.
In another embodiment B is 1-hydroxy-2,3-dihydro-lH-inden-5-yl.
In another embodiment B is 2,3-dihydro-1 H-inden-5-yl.
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
In another embodiment B is 3-(hydroxy(1-propylcyclobutyl)methyl)phenyl.
In another embodiment B is 4-(1-hydroxy-5,5-dimethylhexyl)phenyl.
In another embodiment B is 4-(hydroxy(1-propylcyclobutyl)methyl)phenyl.
In another embodiment B is 4-tert-butylphenyl.
In another embodiment B is 4-hexylphenyl.
In another embodiment B is 4-(1-hydroxy-2-phenylethyl)phenyl.
In another embodiment B is 4-(1-hydroxy-3-phenylpropyl)phenyl.
In another embodiment B is 4-(1-hydroxycyclobutyl)phenyl.
In another embodiment B is 4-(2-cyclohexyl-1 -hydroxyethyl)phenyl.
In another embodiment B is 4-(3-cyclohexyl-1 -hydroxypropyl)phenyl.
In another embodiment B is 4-(cyclohexyl(hydroxy)methyl)phenyl.
In another embodiment B is 4-(cyclohexylmethyl)phenyl.
In another embodiment B is 4-(hydroxy(phenyl)methyl)phenyl.
Another embodiment is a compound according to the structure
16
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
G A-Y
N
0 I ~
/ OH
or a pharmaceutical salt thereof, or a prodrug thereof,
wherein R is hydrogen or Cl_lo hydrocarbyl.
Another embodiment is a compound according to the structure
A-Y
G R
J
N
OH
0 ~
or a pharmaceutical salt thereof, or a prodrug thereof,
wherein R is hydrogen or Cl_lo hydrocarbyl.
Another embodiment is a compound according to the structure
G A-Y
J ~
N
O I ~ R
OH
or a pharmaceutical salt thereof, or a prodrug thereof,
wherein R is hydrogen or Cj_jo hydrocarbyl.
Another embodiment is a compound according to the structure
J G-_rA-Y
N OH
0 I ~ R
"C1-10 hydrocarbyl is hydrocarbyl having 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10
carbon atoms.
Hydrocarbyl is a moiety consisting of only carbon and hydrogen, and includes,
but is not limited to alkyl, alkenyl,
alkynyl, and the like, and in some cases aryl, and combinations thereof.
Alkvl is hydrocarbyl having no double or triple bonds including:
linear alkyl such as methyl, ethyl, propyl, n-butyl, n-pentyl, n-hexyl, and
the like;
branched alkvl such as isopropyl, branched butyl isomers (i.e. sec-butyl, tert-
butyl, etc), branched pentyl isomers (i.e. isopentyl,
etc), branched hexyl isomers, and higher branched alkyl fragments;
17
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
cly oalkyl. such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, etc.; and
alkyl fragments consisting of both cyclic and noncyclic components, whether
linear or branched, which may be attached to the
remainder of the molecule at any available position including terminal,
internal, or ring carbon atoms.
C1-6 alkyl is alkyl having 1, 2, 3, 4, 5, or 6 carbon atoms.
Alkenyl is hydrocarbyl having one or more double bonds including
linear alkenyl, branched alkenyl, cyclic alkenyl, and combinations thereof in
analogy to alkyl.
Alkynyl is hydrocarbyl having one or more triple bonds including linear
alkynyl, branched alkynyl, cyclic alkynyl and combinations
thereof in analogy to alkyl.
Arvl is an unsubstituted or substituted aromatic ring or ring system such as
phenyl, naphthyl, biphenyl, and the like. Aryl may or
may not be hydrocarbyl, depending upon whether it has substituents with
heteroatoms.
Arylalkyl is alkyl which is substituted with aryl. In other words alkyl
connects aryl to the remaining part of the molecule.
Examples are -CH2-Phenyl, -CH2-CH2-Phenyl, and the like. Arylalkyl may or may
not be hydrocarbyl, depending upon whether
the aryl portion has substituents with heteroatoms.
Unconjugated dienes or polyenes have one or more double bonds which are not
conjugated. They may be linear, branched, or
cyclic, or a combination thereof.
Combinations of the above are also possible.
Thus, each of the structures below is contemplated. These structures, or
pharmaceutically acceptable salts thereof, or
prodrugs thereof, individually represent a compound which is an embodiment
contemplated herein. In other words, each
structure represents a different embodiment.
18
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
M4 G I A-Y
- ~
N
0
M4 M4
OH OH
M4
OH OH
M4
M4 OH
OH
M4 4
M OH
OH
M4
M4 OH
OH
19
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Ma
I Ma \
OH
HO
Ma Ma
Ma Ma
OH
OH OH
Ma ~
M4
I
/ OH
OH
Ma
I
OH
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
M4 M4
,
OH OH
M4 ~ Ma
OH OH
M4
M4
OH
OH
M4
I OH
~ Ma \
M4 M4 M4 F F
CHF
IX y Z
OH HO CF3 HO
In the above embodiments, x is 5, 6, or 7, and y + z is 2x + 1.
In one embodiment, x is 5 and y + z is 11.
In another embodiment, x is 6 and y + z is 13.
In another embodiment, x is 7 and y + z is 15.
Hypothetical examples of useful compounds are shown below.
21
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
CO2H
I -_
0 S 0/\COz H
N
D CI
0 0 / \
OH OH 0
CO2CH3
g S
/ --r S COZH
N N ` /
0 0
OH OH
0
S CO2H
0 CO2H
N I ~
/ I ~ I
N
O
0
OH
OH
0 C02H
YI `0 0
CO2H
N S-T-1~0 \ /
O N
0
OH
OH
CO2H
S S
S~O \ / I / C02H
OH N o
N 0 0
OH
22
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
S C02H S SOgCHg
N
F
p 0
OH
HN-N
g \N/N p 0 C02H
F30 OH
N S N p
0 O
HO
P(O)(pH)2
0 C02CHZCH3
r~r S
N O
CN
S02NHCH3
0 S S C02H
F
N
0
0 11
S S S CO2H 0\/C02H
N N ~/
N
N
p
OH
23
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
0 OO2H S SOgCHg
N ~ N
p p
OH OH
HN-N
p~ 0 ~N N Br 0 S 0 CO2H
yN N
p p
OH
0
PO OH
Sp ~ ~~ )2 S S CO2CH2CH3
F3C 0 -,~r N N
OH
0 0
OH
0
S02NHCH3
S S S CO2H
N N \ \ /
p ~ 0
OH OH
S /S CO2H 0\~CO2H
\`\N_/
CI N Y // 0
N OH
0 / 0 OH
Compound examples:
The following are hypothetical examples of useful compounds:
Compound Example 1. A compound having a structure
24
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
G A Y
N~
B
0
or a pharmaceutically acceptable salt thereof, or a prodrug thereof;
Y is an organic acid functional group, or an amide or ester thereof comprising
up to 14 carbon atoms; or Y is hydroxymethyl or an
ether thereof comprising up to 14 carbon atoms; or Y is a tetrazolyl
functional group;
A is -(CH2)6-, cis -CH2CH=CH-(CH2)3-, or -CH2C=C-(CH2)3-, wherein 1 or 2
carbon atoms may be replaced by S or 0; or A is -
(CH2)m-Ar-(CH2)o- wherein Ar is interarylene or heterointerarylene, the sum of
m and o is 1, 2, 3, or 4, and wherein 1 -CH2- may
be replaced by S or 0, and 1 -CH2-CH2- may be replaced by -CH=CH- or -C=C-;
G is 0, S, S=O, or S(=0)2;
J is H, halogen, CF3; or C,_6 alkyl; and
B is aryl or heteroaryl.
Compound Example 2. The compound according to compound example 1 wherein Y is
selected from C02R2, CON(R2)2,
CON(0R2)R2, CON(CH2CH2OH)2, CONH(CH2CH2OH), CH2OH, P(O)(OH)2, CONHS02R2,
S02N(R2)2, S02NHR2,
N
/ N~
II ---< ~N
I
I R2
R2
and
wherein R2 is independently H, Ci-C6 alkyl, unsubstituted phenyl, or
unsubstituted biphenyl.
Compound Example 3. The compound according to compound example 1 or 2 wherein
B is substituted phenyl.
Compound Example 4. The compound according to compound example 1 or 2 having a
structure
G A-Y
J ~
N ~ R
0
/ OH
or a pharmaceutically acceptable salt thereof, or a prodrug thereof;
wherein R is hydrogen or C,-,o hydrocarbyl.
Compound Example 5. The compound according to compound example 4 wherein R is
alkyl.
Compound Example 6. The compound according to compound example 4 wherein R is
arylalkyl.
Compound Example 7. The compound according to compound example any one of
compound examples 1 to 6 having a
structure
G A-Y
J ~
N
0 R
OH
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
or a pharmaceutically acceptable salt thereof, or a prodrug thereof;
wherein R is hydrogen or Cl-lo hydrocarbyl.
Compound Example 8. The compound according to compound example 1 or 2 wherein
A is (3-methylphenoxy)methyl.
Compound Example 9. The compound according to compound example 1 or 2 wherein
A is (4-but-2-ynyloxy)methyl.
Compound Example 10. The compound according to compound example 1 or 2 wherein
A is 2-(2-ethylthio)thiazol-4-yl.
Compound Example 11. The compound according to compound example 1 or 2 wherein
A is 2-(3-propyl)thiazol-5-yl.
Compound Example 12. The compound according to compound example 1 or 2 wherein
A is 3-methoxymethyl)phenyl.
Compound Example 13. The compound according to compound example 1 or 2 wherein
A is 3-(3-propylphenyl.
Compound Example 14. The compound according to compound example 1 or 2 wherein
A is 3-methylphenethyl.
Compound Example 15. The compound according to compound example 1 or 2 wherein
A is 4-(2-ethyl)phenyl.
Compound Example 16. The compound according to compound example 1 or 2 wherein
A is 4-phenethyl.
Compound Example 17. The compound according to compound example 1 or 2 wherein
A is 4-methoxybutyl.
Compound Example 18. The compound according to compound example 1 or 2 wherein
A is 5-(methoxymethyl)furan-2-yl .
Compound Example 19. The compound according to compound example 1 or 2 wherein
A is 5-(methoxymethyl)thiophen-2-
yl.
Compound Example 20. The compound according to compound example 1 or 2 wherein
A is 5-(3-propyl)furan-2-yl.
Compound Example 21. The compound according to compound example 1 or 2 wherein
A is 5-(3-propyl)thiophen-2-yl.
Compound Example 22. The compound according to compound example 1 or 2 wherein
A is 6-hexyl.
Compound Example 23. The compound according to compound example 1 or 2 wherein
A is (Z)-6-hex-4-enyl.
Compound Example 24. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxy-2,2-dimethylpropyl)phenyl.
Compound Example 25. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxy-2-methylpropan-2-yl)phenyl.
Compound Example 26. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxy-2-methylpropyl)phenyl.
Compound Example 27. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxybutyl)phenyl.
Compound Example 28. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxyheptyl)phenyl.
Compound Example 29. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxyhexyl)phenyl.
Compound Example 30. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxypentyl)phenyl.
Compound Example 31. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxypropyl)phenyl.
Compound Example 32. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(3-
hyd roxy-2-meth yl hepta n-2-yl) phenyl.
Compound Example 33. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(3-
hydroxy-2-methyloctan-2-yl)phenyl.
Compound Example 34. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 1-hydroxy-
2,3-dihydro-1 H-inden-5-yl.
26
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Compound Example 35. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 2,3-
dihydro-1 H-inden-5-yl.
Compound Example 36. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 3-
(hydroxy(1-propylcyclobutyl)methyl)phenyl.
Compound Example 37. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxy-5,5-dimethylhexyl)phenyl.
Compound Example 38. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-
(hydroxy(1-propylcyclobutyl)methyl)phenyl.
Compound Example 39. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-tert-
butylphenyl.
Compound Example 40. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-
hexylphenyl.
Compound Example 41. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxy-2-phenylethyl)phenyl.
Compound Example 42. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxy-3-phenylpropyl)phenyl.
Compound Example 43. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(1-
hydroxycyclobutyl)phenyl.
Compound Example 44. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(2-
cyclohexyl-1 -hydroxyethyl)phenyl.
Compound Example 45. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-(3-
cyclohexyl-1-hydroxypropyl)phenyl.
Compound Example 46. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-
(cyclohexyl (hyd roxy) methyl) phenyl .
Compound Example 47. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-
(cyclohexylmethyl)phenyl.
Compound Example 48. The compound according to any one of compound examples 1,
2, and 8-23 wherein B is 4-
(hydroxy(phenyl)methyl)phenyl.
Compound Example 49. The compound according to any one of compound examples 1
to 48 wherein G is 0.
Compound Example 50. The compound according to any one of compound examples 1
to 48 wherein G is S.
Compound Example 51. The compound according to any one of compound examples 1
to 48 wherein G is S=O.
Compound Example 52. The compound according to any one of compound examples 1
to 48 wherein G is S(=0)2.
Compound Example 53. The compound according to any one of compound examples 1
to 48 wherein J is hydrogen.
Compound Example 54. The compound according to any one of compound examples 1
to 48 wherein J is F.
Compound Example 55. The compound according to any one of compound examples 1
to 48 wherein J is CI.
Compound Example 56. The compound according to any one of compound examples 1
to 48 wherein J is methoxy.
Compound Example 57. The compound according to any one of compound examples 1
to 48 wherein J is methyl.
Compound Example 58. The compound according to any one of compound examples 1,
and 24 to 57 wherein A is -
CH2CH2A'- or -CH2OA'-, wherein A' is linear C4H8, C3H60, or C3C6S; -CH2-Ar-; -
0-Ar-; -S-Ar-; -Ar-CH2-; -Ar-O-; -Ar-S-, or Ar;
with the proviso that A does not contain -0-0-, -S-O-, or 0-S.
The following are hypothetical examples of compositions, kits, methods, uses,
and medicaments employing the hypothetical
compound examples,
27
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Composition Example:
A composition comprising a compound according to any one of compound examples
1 to 58, wherein said composition is a liquid
which is ophthalmically acceptable.
Medicament Examples:
Use of a compound according to any one of compound examples 1 to 58 in the
manufacture of a medicament for the treatment of
glaucoma or ocular hypertension in a mammal.
Use of a compound according to any one of compound examples 1 to 58 in the
manufacture of a medicament for the treatment of
baldness in a person.
A medicament comprising a compound according to any one of compound examples 1
to 58, wherein said composition is a liquid
which is ophthalmically acceptable.
Method Example:
A method comprising administering a compound according to any one of compound
examples 1 to 58 to a mammal for the
treatment of glaucoma or ocular hypertension.
Kit Example:
A kit comprising a composition comprising compound according to any one of
compound examples 1 to 58, a container, and
instructions for administration of said composition to a mammal for the
treatment of glaucoma or ocular hypertension.
A person of ordinary skill in the art understands the meaning of the
stereochemistry associated with the hatched
wedge/solid wedge structural features. For example, an introductory organic
chemistry textbook (Francis A. Carey, Organic
Chemistry, New York: McGraw-Hill Book Company 1987, p. 63) states "a wedge
indicates a bond coming from the plane of the
paper toward the viewer" and the hatched wedge, indicated as a"dashed line",
"represents a bond receding from the viewer."
Unless otherwise indicated, a structure shown herein is intended to include
any stereoisomer or mixture thereof of the
compounds of the structure.
For the purposes of this disclosure, "treat," "treating," or "treatment" refer
to the use of a compound, composition,
therapeutically active agent, or drug in the diagnosis, cure, mitigation,
treatment, or prevention of disease or other undesirable
condition.
A "pharmaceutically acceptable salt" is any salt that retains the activity of
the parent compound and does not impart
any additional deleterious or untoward effects on the subject to which it is
administered and in the context in which it is
administered compared to the parent compound. A pharmaceutically acceptable
salt also refers to any salt which may form in
vivo as a result of administration of an acid, another salt, or a prodrug
which is converted into an acid or salt.
Pharmaceutically acceptable salts of acidic functional groups may be derived
from organic or inorganic bases. The salt
may comprise a mono or polyvalent ion. Of particular interest are the
inorganic ions lithium, sodium, potassium, calcium, and
magnesium. Organic salts may be made with amines, particularly ammonium salts
such as mono-, di- and trialkyl amines or
ethanol amines. Salts may also be formed with caffeine, tromethamine and
similar molecules. Hydrochloric acid or some other
pharmaceutically acceptable acid may form a salt with a compound that includes
a basic group, such as an amine or a pyridine
ring.
A "prodrug" is a compound which is converted to a therapeutically active
compound after administration, and the term
should be interpreted as broadly herein as is generally understood in the art.
While not intending to limit the scope of the
invention, conversion may occur by hydrolysis of an ester group or some other
biologically labile group. Generally, but not
necessarily, a prodrug is inactive or less active than the therapeutically
active compound to which it is converted. Ester prodrugs
of the compounds disclosed herein are specifically contemplated. An ester may
be derived from a carboxylic acid of Cl (i.e. the
terminal carboxylic acid of a natural prostaglandin), or an ester may be
derived from a carboxylic acid functional group on another
part of the molecule, such as on a phenyl ring. While not intending to be
limiting, an ester may be an alkyl ester, an aryl ester, or
28
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
a heteroaryl ester. The term alkyl has the meaning generally understood by
those skilled in the art and refers to linear, branched,
or cyclic alkyl moieties. CI-6 alkyl esters are particularly useful, where
alkyl part of the ester has from 1 to 6 carbon atoms and
includes, but is not limited to, methyl, ethyl, propyl, isopropyl, n-butyl,
sec-butyl, iso-butyl, t-butyl, pentyl isomers, hexyl isomers,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and combinations thereof
having from 1-6 carbon atoms, etc.
Those skilled in the art will readily understand that for administration or
the manufacture of medicaments the
compounds disclosed herein can be admixed with pharmaceutically acceptable
excipients which per se are well known in the art.
Specifically, a drug to be administered systemically, it may be confected as a
powder, pill, tablet or the like, or as a solution,
emulsion, suspension, aerosol, syrup or elixir suitable for oral or parenteral
administration or inhalation.
For solid dosage forms or medicaments, non-toxic solid carriers include, but
are not limited to, pharmaceutical grades
of mannitol, lactose, starch, magnesium stearate, sodium saccharin, the
polyalkylene glycols, talcum, cellulose, glucose, sucrose
and magnesium carbonate. The solid dosage forms may be uncoated or they may be
coated by known techniques to delay
disintegration and absorption in the gastrointestinal tract and thereby
provide a sustained action over a longer period. For
example, a time delay material such as glyceryl monostearate or glyceryl
distearate may be employed. They may also be coated
by the technique described in the U.S. Pat. Nos. 4,256,108; 4,166,452; and
4,265,874 to form osmotic therapeutic tablets for
control release. Liquid pharmaceutically administrable dosage forms can, for
example, comprise a solution or suspension of one
or more of the presently useful compounds and optional pharmaceutical
adjutants in a carrier, such as for example, water, saline,
aqueous dextrose, glycerol, ethanol and the like, to thereby form a solution
or suspension. If desired, the pharmaceutical
composition to be administered may also contain minor amounts of nontoxic
auxiliary substances such as wetting or emulsifying
agents, pH buffering agents and the like. Typical examples of such auxiliary
agents are sodium acetate, sorbitan monolaurate,
triethanolamine, sodium acetate, triethanolamine oleate, etc. Actual methods
of preparing such dosage forms are known, or will
be apparent, to those skilled in this art; for example, see Remington's
Pharmaceutical Sciences, Mack Publishing Company,
Easton, Pa., 16th Edition, 1980. The composition of the formulation to be
administered, in any event, contains a quantity of one
or more of the presently useful compounds in an amount effective to provide
the desired therapeutic effect.
Parenteral administration is generally characterized by injection, either
subcutaneously, intramuscularly or
intravenously, Injectables can be prepared in conventional forms, either as
liquid solutions or suspensions, solid forms suitable
for solution or suspension in liquid prior to injection, or as emulsions.
Suitable excipients are, for example, water, saline, dextrose,
glycerol, ethanol and the like. In addition, if desired, the injectable
pharmaceutical compositions to be administered may also
contain minor amounts of non-toxic auxiliary substances such as wetting or
emulsifying agents, pH buffering agents and the like.
The amount of the presently useful compound or compounds administered is
dependent on the therapeutic effect or
effects desired, on the specific mammal being treated, on the severity and
nature of the mammal's condition, on the manner of
administration, on the potency and pharmacodynamics of the particular compound
or compounds employed, and on the
judgment of the prescribing physician. The therapeutically effective dosage of
the presently useful compound or compounds may
be in the range of about 0.5 or about 1 to about 100 mg/kg/day.
A liquid which is ophthalmically acceptable is formulated such that it can be
administered topically to the eye. The
comfort should be maximized as much as possible, although sometimes
formulation considerations (e.g. drug stability) may
necessitate less than optimal comfort. In the case that comfort cannot be
maximized, the liquid should be formulated such that
the liquid is tolerable to the patient for topical ophthalmic use.
Additionally, an ophthalmically acceptable liquid should either be
packaged for single use, or contain a preservative to prevent contamination
over multiple uses.
For ophthalmic application, solutions or medicaments are often prepared using
a physiological saline solution as a major
vehicle. Ophthalmic solutions should preferably be maintained at a comfortable
pH with an appropriate buffer system. The
formulations may also contain conventional, pharmaceutically acceptable
preservatives, stabilizers and surfactants.
29
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Preservatives that may be used in the pharmaceutical compositions of the
present invention include, but are not limited
to, benzalkonium chloride, chlorobutanol, thimerosal, phenylmercuric acetate
and phenylmercuric nitrate. A useful surfactant is, for
example, Tween 80. Likewise, various useful vehicles may be used in the
ophthalmic preparations of the present invention. These
vehicles include, but are not limited to, polyvinyl alcohol, povidone,
hydroxypropyl methyl cellulose, poloxamers, carboxymethyl
cellulose, hydroxyethyl cellulose and purified water.
Tonicity adjustors may be added as needed or convenient. They include, but are
not limited to, salts, particularly sodium
chloride, potassium chloride, mannitol and glycerin, or any other suitable
ophthalmically acceptable tonicity adjustor.
Vanous buffers and means for adjusting pH may be used so long as the resulting
preparation is ophthalmically
acceptable. Accordingly, buffers include acetate buffers, citrate buffers,
phosphate buffers and borate buffers. Acids or bases may
be used to adjust the pH of these formulations as needed.
In a similar vein, an ophthalmically acceptable antioxidant for use in the
present invention includes, but is not limited to,
sodium metabisulfite, sodium thiosulfate, acetylcysteine, butylated
hydroxyanisole and butylated hydroxytoluene.
Other excipient components which may be included in the ophthalmic
preparations are chelating agents. A useful
chelating agent is edetate disodium, although other chelating agents may also
be used in place or in conjunction with it.
The ingredients are usually used in the following amounts:
Ingredient Amount (%w/v)
active ingredient about 0.001-5
preservative 0-0.10
vehicle 0-40
tonicity adjustor 1-10
buffer 0.01-10
pH adjustor q.s. pH 4.5-7.5
antioxidant as needed
surfactant as needed
purified water as needed to make 100%
For topical use, creams, ointments, gels, solutions or suspensions, etc.,
containing the compound disclosed herein are
employed. Topical formulations may generally be comprised of a pharmaceutical
carrier, cosolvent, emulsifier, penetration
enhancer, preservative system, and emollient.
The actual dose of the active compounds of the present invention depends on
the specific compound, and on the
condition to be treated; the selection of the appropriate dose is well within
the knowledge of the skilled artisan.
For treatment of diseases affecting the eye including glaucoma, these
compounds can be administered topically,
periocularly, intraocularly, or by any other effective means known in the art.
Synthetic Methods
Scheme 1
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
S A`Y b S A-Y
a NH ~
II A\Y SH B-X B
1 J~NH2 0 a 0
0 3 4
2
(a) 2, p-TsOH, benzene, reflux; (b) Pd or Cu catalysis, a.
While there are many ways the compounds disclosed herein, one exemplary
synthesis may begin with aldehyde 1(see
Scheme 1). Aldehydes such as 1 are commercially available or may be made
according to published literature procedures (e.g.
methyl 4-(3-oxopropyl)benzoate [commercially available, or by the procedures
of Varma and Gordon, US 4,711,900], methyl 5-(4-
oxobutyl)thiophene-2-carboxylate [Cragoe, et al. US 4,225,609], and methyl 8-
oxooctanoate [Rappoport and Volcheck J. Am.
Chem. Soc. 1956, 78, 2451 or by esterification and oxidation of commercially
available 8-hydroxyoctanoic acid]). Condensation
of 1 with mercaptoacetamide 2 provides thiazolidinone 3 employing the
procedure of Bicking et al. (J. Med. Chem. 1983, 26, 342-
348). Intermediate 3 is then arylated on nitrogen according to Buchwald's
copper-catalyzed procedure (Org. Letf. 2000, 2,1101-
1104) or palladium-catalyzed procedure (J. Am. Chem. Soc. 2002, 124, 7421-
7428) using a wide variety of substituted
bromophenyl and other bromoaryl compounds a to give compound 4. The haloarenes
a are either available commercially or may
be made according to published literature procedures. For example, United
States Patent Application No. 11 /009,298, filed on
December 10, 2004 and United States Provisional Patent Application 601742,779
filed on December 6, 2005, both of which are
expressly incorporated by reference herein, disclose methods of making a
number of useful substituted bromophenyl
compounds. These procedures may also be readily adapted to other bromoaryl
compounds such as substituted bromothienyl,
substituted bromofuryl, substituted bromopyridinyl, substituted bromonaphthyl,
substituted bromobenzothienyl, and the like.
Compound 4 may be the target compound, or may require deprotection(s) and/or
functionalization (depending on the nature of B
and Y) to arrive at the target compound.
Scheme 2
A\Y a A-Y b S~A-Y
0 + H2N-B -~ N
N,B SH B
~ J~
J 0
5 6 - oMe 4
0 7
(a) toluene, reflux; (b) 7, Et3N, toluene, reflux.
In another hypothetical route to compound 4, condensation of aldehyde 1 with
aniline 5 affords intermediate 6 (see
Scheme 2). This intermediate is not isolated, but immediately treated with
methyl thioglycolate 7 to afford product 4 by the
method of Bicking et al. Anilines such as 5 are either available commercially
or may be made according from aryl halides
according to published Buchwald-Hartwig amination reactions (for general
reviews, see Jiang and Buchwald in Metal-Catalyzed
Cross-Coupling Reactions, 2nd ed.: de Meijere, A., Diederich, F., Eds.; Wiley-
VCH: Weinheim, Germany, 2004, p 699, and
Hartwig in Handbook of Organopalladium Chemistry for Organic Synthesis;
Negishi, E. I., Ed.; Wiley-Interscience: New York,
2002; Vol. 1, p 1051; specifically for primary aniline synthesis, see Shen and
Hartwig: J. Am. Chem. Soc. 2006, 128, 10028-
10029 and references therein).
31
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Scheme 3
0
OS A`Y SA\Y b 0`S-T',Y
J-~N = a J~N~ - J~NB
B B
0 0 0
g 4 9
(a) Na104, EtOH, H20 (b) 30% H202, EtOH.
Sulfoxide and sulfone variants of compound 4 are envisioned (see scheme 3).
According to the methods of Smith, et
al. (US 4,022,794), oxidation of 4 to sulfoxide 8 is accomplished with
periodate, and oxidation of 4 (or 8) to sulfone 9 is
accomplished with hydrogen peroxide. Compounds 8 and 9 may be the target
compound, or may require deprotection(s) and/or
functionalization (depending on the nature of B and Y) to arrive at the target
compound.
Scheme 4
rA-Y a J OA'Y b J OA'Y
0 OH ~NH B-X ~N, B
1 J-~NH2 0 a 0
0 11 12
(a) 10, p-TsOH (cat), benzene, reflux; (b) Pd or Cu catalysis, a.
Oxazolidinones are also envisioned. Analogous to scheme 1, condensation of
aldehyde 1 with hydroxyacetamide 10
affords oxazolidinone 11 (see scheme 4) using the method of Campbell and
Jones, US 2,915,527. Arylation as before provides
N-aryl oxazolidinone 12. Compound 12 may be the target compound, or may
require deprotection(s) and/or functionalization
(depending on the nature of B and Y) to arrive at the target compound. An
alternative hypothetical route to compound 12 is
shown in scheme 5. Thus, condensation of intermediate aniline 5 with glycolic
acid 13 affords amido alcohol 14 using the
method of Kametani, et al., Yakugaku Zasshi 1981, 101, 336-344. Cyclization of
14 with aldehyde 1 according to the method of
Kametani et al. then provides desired compound 12.
Scheme 5
OH a OH b OA'Y
OH + H2N-B J-~N`B A~ J~N`B
0 0 II 0
0
13 5 14 1 12
L0 (a) neat, high temperature; (b) 1, p-TsOH (cat.), xylene, reflux.
The glycolate derivatives described herein (2, 7, 10 and 13) where J= H are
all available from commercial sources.
Numerous analogs of 7 and 13 where J= alkyl are also commercially available,
and are envisioned to serve as precursors to the
other glycolate starting materials by standard techniques known in the art. It
is also envisioned that analogs of compounds 4, 8,
9 and 12 where J= H may serve as precursors to compounds where J= alkyl or
halogen using techniques known in the art
32
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
(fluorination of a thiazolidinone analog, e.g., see J. Org. Chem. 1992, 57,
3755; alkylation of a thiazolidinone analog, e.g., see
Pol. J. Chem. 2001, 75, 1847-1852).
The synthetic methods described above must necessarily result in the
preparation of racemic mixtures of final products.
The individual isomers may be obtained, for instance, by a resolution
technique (e.g. see Bicking et al.), or by chiral
chromatography techniques.
Scheme 6
Br Ci
SH H S
0
a ~\/0Me a1 OM+ NH
0 b
1a 2a
3a
H
S H 1 S/ O Me S \ S~ O R
/
N / -~ N
O 0
0 OH
4a ~ 4b (R=Me)
d
4c (R=H)
(a) p-TsOH, toluene, reflux; (b) al, Pd2(dba)3, Xantphos, Cs2CO3, dioxane,
reflux;
(c) NaBH4, MeOH, CHZCIZ; (d) LiOH (aq.), THF.
Example 1
5-(3-(3-(4-(1 -hydroxyhexyl)phenyl)-4-oxo-thiazolidin-2-yl)propyl)thiophene-2-
carboxylic acid (4c)
Step 1. Condensation of 1a and 2a to give 3a
A mixture of methyl 5-(4-oxobutyl)thiophene-2-carboxylate (1 a, see Cragoe, et
al. US 4,225,609; also prepared by Swern
oxidation of the corresponding alcohol prepared as described by Shih, C., et.
al. J. Med. Chem. 1992, 35, 1109-1116; 5.3 g, 25.0
mmol) and 2-mercaptoacetamide (2a, 6.76 g, 74.2 mmol) in toluene (50 mL) was
refluxed in a flask fitted with a Dean-Stark trap.
p-Toluenesulfonic acid monohydrate (3.8 g, 20.0 mmol) was added portionwise
over several hours. After a total of 5 hours at
reflux, the mixture was cooled and the toluene layer was decanted. Additional
toluene (50 mL) was used to wash the oily
remainder and then was decanted. The combined organic phase was washed with
water (2x100 mL), saturated aqueous
NaHCOa (100 mL), water (100 mL) and brine (100 mL) then filtered through
filter paper and concentrated in vacuo to afford 1.7 g
of crude product. Purification of the residue on silica (hexane -> EtOAc,
gradient) afforded 1.08 g of thiazolidinone 3a. This
product was recrystallized from hot MeOH (3 mL) to afford 800 mg of 3a (11 %).
Step 2. Arylation of 3a with a1 to give 4a
Pd2(dba)3 (41 mg, 0.045 mmol), Xantphos (77 mg, 0.133 mmol) and Cs2C03 (428
mg, 1.31 mmol) were added sequentially to a
solution of 3a (314 mg, 1.10 mmol) and al (see Borman, et al., United States
Patent Application Publication No. 2005/0209336,
incorporated by reference herein; 255 mg, 1.00 mmol) in 1,4-dioxane (7.1 mL).
The flask was fitted with a reflux condenser,
evacuated and refilled with nitrogen (5x) then heated at reflux. After 3 d,
the reaction was cooled, diluted with EtOAc (50 mL) and
filtered through celite, washing with excess EtOAc. The EtOAc filtrate was
concentrated in vacuo. The crude residue was
purified on 40 g silica gel (hexanes -> EtOAc, gradient) to afford 56 mg (12%)
of 4a.
33
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
Step 3. Reduction of 4a to give 4b
Sodium borohydride (7 mg, 0.19 mmol) was added to a solution of 4a (55 mg,
0.12 mmol) in MeOH (0.30 mL) and CH2CI2 (0.30
mL). After 18 h at room temperature the reaction was quenched with 1 N HCI (5
mL) and extracted with EtOAc (3x20 mL). The
combined organic phase was dried (Na2SO4), filtered and concentrated in vacuo.
The crude residue was purified on 12 g silica
gel (hexanes ---> EtOAc, gradient) to afford 9 mg (16%) of 4b.
Step 4. Saponification of 4b to give 4c
Lithium hydroxide (0.10 mL of a 1.0 N solution in water, 0.10 mmol) was added
to a solution of 4b (9 mg, 0.019 mmol) in THF
(0.19 mL). The reaction mixture was heated at 40 C. After 24 h at 40 C, the
reaction mixture was cooled to room temperature
and the mixture was concentrated under a stream of nitrogen. The residue was
diluted with water (0.2 mL), acidified with 1 N
HCI (0.5 mL) and extracted with EtOAc (3x2 mL). The combined organic phase was
washed with brine (2 mL), dried (Na2SO4),
filtered and concentrated in vacuo. Purification of the crude residue by
chromatography on 4 g silica gel (CH2CI2 -> 20%
MeOH/CH2CI2, gradient) afforded 5 mg (57%) of 4c.
Scheme 7
Br ,
OH a 0 H S
OMe a1
0
0~ \ S~ OMe + NH2 NH
0 b
1 a 10a
11a
0 S~ OMe 0 S~ OR
c
N N /
0 0 ~ ~
0 OH
12a ~ 12b (R=Me)
d
12c (R=H)
(a) p-TsOH, tduene, reflux; (b) al, Pdz(dba)3, Xantphos, CsZCO3, dioxane,
reflux;
(c) NaBH4, MeOH, CH2CI2; (d) LiOH (aq.), THF.
Example 2
5-(3-(3-(4-(1 -hydroxyhexyl)phenyl)-4-oxo-oxazolidin-2-yl)propyl)thiophene-2-
carboxylic acid (12c)
Step 1. Condensation of 1a and 10a to give 11a
A mixture of 1a (2.75 g, 13.0 mmol), 2-hydroxyacetamide (10a, 2.9 g, 38.6
mmol) and p-toluenesulfonic acid monohydrate (250
mg, 1.3 mmol) in toluene (20 mL) was refluxed in a flask fitted with a Dean-
Stark trap. After 2 h, the reaction was cooled and
partitioned between water (20 mL) and EtOAc (20 mL). The organic phase was
separated and washed with water (2x50 mL) and
1 M NH4OH (50 mL), filtered through filter paper and concentrated in vacuo.
Purification of the residue on silica (hexane ~
EtOAc, gradient) afforded 300 mg of oxazolidinone 11a (9%).
Step 2. Arylation of 11 a with a1 to give 12a
Pd2(dba)3 (41 mg, 0.045 mmol), Xantphos (77 mg, 0.133 mmol) and Cs2C03 (428
mg, 1.31 mmol) were added sequentially to a
solution of 11 a (297 mg, 1.10 mmol) and al (256 mg, 1.00 mmol) in 1,4-dioxane
(7.1 mL). The flask was fitted with a reflux
condenser, evacuated and refilled with nitrogen (5x) then heated at reflux.
After 18 h, the reaction was cooled, diluted with
34
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
EtOAc (50 mL) and filtered through celite, washing with excess EtOAc. The
EtOAc filtrate was concentrated in vacuo. The crude
residue was punfied on 40 g silica gel (hexanes -> 50% EtOAc/hexanes,
gradient) to afford 363 mg (82%) of 12a as a pale
yellow solid.
Step 3. Reduction of 12a to give 12b
Sodium borohydride (22 mg, 0.58 mmol) was added to a solution of 12a (130 mg,
0.29 mmol) in MeOH (0.75 mL) and CH2CI2
(0.75 mL). After 1 h at room temperature the reaction was quenched with 1 N
HCI (5 mL) and extracted with EtOAc (3x25 mL).
The combined organic phase was dried (Na2S04), filtered and concentrated in
vacuo. The crude residue was purified on 12 g
silica gel (hexanes -~ EtOAc, gradient) to afford 130 mg (99%) of 12b.
Step 4. Saponification of 12b to give 12c
Lithium hydroxide (0.72 mL of a 1.0 N solution in water, 0.72 mmol) was added
to a solution of 12b (64 mg, 0.14 mmol) in THF
(0.72 mL). The reaction mixture was heated at 40 C. After 8 h at 40 C, the
reaction mixture was cooled to room temperature
and the mixture was concentrated under a stream of nitrogen. The residue was
diluted with water (2 mL), acidified with 1 N HCI
(2 mL) and extracted with EtOAc (3x10 mL). The combined organic phase was
dried (Na2SO4), filtered and concentrated in
vacuo. Purification of the crude residue by chromatography on 4 g silica gel
(40% EtOAc/hexanes ~ EtOAc, gradient) afforded
5 mg (8%) of 12c.
Scheme 8
Br
H S
H S
0 OMe a2 OTBS OMe b
NH \JJ/N
a O
0
11 a OTBS
12d
0 H S OMe 0 S OMe 0 S~ OR
c
~-N N N
O 0 0
OH OH OH
12e 12f 12g (R=Me)
faster eluting (HPLC) d slower eluting (HPLC)
12h (R=H)
(a) Cul, MeN(H)CH2CH2N(H)Me, K2CO3, MeCN, reflux; (b) HF-pyridine, MeCN;
(c) HPLC separation; (d) Rabbit liver esterase, pH 7.2 buffer, MeCN.
Example 3
5-(3-(3-(4-((S)-1-hydroxyhexyl)phenyl)-4-oxooxazolidin-2-yl)propyl)thiophene-2-
carboxylic acid (1 2h)
Step 1. Arylation of 11a with a2 to give 12d
Potassium carbonate (276 mg, 2.0 mmol), copper(l) iodide (19 mg, 0.10 mmol)
and N,N'-dimethylethylene diamine (21.5 uL, 0.2
mmol) were added sequentially to a solution of 11a (296 mg, 1.10 mmol) and a2
(see United States Provisional Patent
Application No. 601894,369, filed March 12, 2007, incorporated by reference
herein, 371 mg, 1.0 mmol) in MeCN (2.5 mL). The
SUBSTITUTE SHEET (RULE 26)
CA 02661827 2009-02-20
WO 2008/024765 PCT/US2007/076410 P)
reaction flask was fitted with a reflux condenser, the mixture was degassed
with nitrogen by evac/fill (5x) and then heated at
reflux. After 4 d, the mixture was cooled, diluted with EtOAc and filtered
through celite, washing with excess EtOAc. The filtrate
was concentrated in vacuo. The crude residue was purified on 40 g silica
(hexanes --> EtOAc, gradient) to afford 33 mg (6%) of
12d.
Step 2. Deprotection of 12d to give 12e
HF-pyridine (100 L) was added to a solution of 12d (33 mg, 0.059 mmol) in
MeCN (1.2 mL) at 0 C in a plastic scintillation vial.
After 45 min at 0 C, the reaction was allowed to warm to room temperature.
After 1 h at room temperature, the reaction was
quenched with saturated aqueous NaHCO3 (5 mL) and extracted with EtOAc (3x15
mL). The combined organic phase was dried
(N0O4), filtered and concentrated in vacuo. The crude residue was purified on
4 g silica (hexanes --> EtOAc, gradient) to
afford 14 mg (53%) of 12e.
Step 3. HPLC separation of 12e to give 12f and 12g
The two diastereomers of 12e (14 mg) were separated on a Waters 600 HPLC
instrument employing a Waters 2996 PDA
detector and a Phenomenex Luna 10 prep silica (2) 1 column, 50 mm x 250 mm
(p/no. 00G-4322-V0; s/no. 356757-1). Using a
flow rate of 45 mL/min and 50% EtOAcIHex as the eluent, the first diastereomer
(12f, 6 mg) eluted at 87-96 min, and the second
diastereomer (12g, 6 mg) eluted at 97-106 min.
Step 4. Saponification of 12g to give 12h
Rabbit liver esterase (5 mg) was added to a mixture of 12g (6 mg, 0.013 mmol),
MeCN (0.1 mL) and pH 7.2 buffer (2.0 mL). The
reaction mixture was stirred vigorously for 6 days at room temperature then
was concentrated in vacuo. The residue was
suspended in CH2CI2 and filtered through celite. The filtrate was concentrated
in vacuo to afford 2.5 mg (43%) of 12h.
In vitro testing
United States Patent Application Serial No. 11/553,143, filed on October 26,
2006, incorporated by reference herein,
describes the methods used to obtain the in vitro data in the table below.
EP2 data EP4 data Other Receptors (EC50 in nM)
Structure flipr cAMP Ki flipr KI hFP hEP1 hEP3A hTP hIP hDP
EC50 EC50 EC50
0
s
s
4400 515 17469 >10000 1461 NA NA 8324 NA NA NA
o
0
0
s
~ i~ \/ 8 0.06 14 NT >10000 NA NA 11 NA NA 213
o ,
The foregoing description details specific methods and compositions that can
be employed to practice the present
invention, and represents the best mode contemplated. However, it is apparent
for one of ordinary skill in the art that further
compounds with the desired pharmacological properties can be prepared in an
analogous manner, and that the disclosed
compounds can also be obtained from different starting compounds via different
chemical reactions. Similarly, different
pharmaceutical compositions may be prepared and used with substantially the
same result. Thus, however detailed the foregoing
may appear in text, it should not be construed as limiting the overall scope
hereof; rather, the ambit of the present invention is to be
governed only by the lawful construction of the claims.
36
SUBSTITUTE SHEET (RULE 26)