Note: Descriptions are shown in the official language in which they were submitted.
CA 02663448 2009-04-21
BACKGROUND
The pharmaceutical industry, contract research organizations, academia, and
government entities routinely test the efficacy and safety of new chemical
entities
using intravenous (usually) infusion in lab animals including, for example,
rats, dogs
and nonhuman primates. While some acute infusion studies may be performed in a
small number of lab animals (e.g., <10) over several minutes or hours, large-
scale
"toxicology" infusion studies of, for example, several hundred rats or, for
example,
10's of larger animals such as dogs or nonhuman primates for periods lasting,
for
example, from 30-90 days may also be performed.
Medical infusion pumps (e.g., electromechanical medical infusion pumps)
may be used during these studies (as well as in other veterinary and/or human
medical
applications). There are numerous types of electromechanical medical infusion
pumps including syringe, peristaltic, diaphragm, large volume, stationary
("pole
mount"), and portable ("ambulatory"). These pumps may be used to deliver a
substance (such as a drug) at a controlled delivery rate to, for example, a
laboratory
test animal. Lab animal infusion and human-use infusion may share similar pump
technology. The methods of use in each field may differ in that human-use
infusion
(e.g., in a healthcare application) may be tailored to a single patient's
needs while lab
animal infusion (e.g., in an industrial application) may apply common
parameters to
multiple animals.
Animals may be connected to a medical infusion pump (for example, a syringe
pump, though other pumping mechanisms may also be used) through a catheter,
tubing, tether, fluid swivel, etc. Usually, one pump is used per animal and
operators
may program and monitor each pump manually. Operators may manually enter a
delivery rate into a pump, load a substance-filled syringe for the pump, and
then
activate the pump (e.g., by pressing a start button). Operators may also
interact with
numerous medical and monitoring devices involved in the study. The process of
loading, starting, and stopping the pump, recording data from medical and
monitoring
devices, and, for example, responding to pump alarms may be manually
documented
by the operator (e.g., on a clipboard). Because studies often involve large
numbers of
animals, manually setting up numerous pumps may be time consuming and tedious.
1
CA 02663448 2009-04-21
In addition, Good Laboratory Practices (GLP's) (including documentation of
processes, data collection, and study results) are required by regulatory
agencies such
as the Food and Drug Administration (FDA). Manually documenting the processes,
data collection, and study results may also be time consuming, tedious and
subject to
human error.
SUMMARY
In various embodiments, a pump may receive a controlled delivery rate (e.g.,
from a computer system) to be used to deliver a substance to an animal (e.g.,
to study
the effects of the substance on the respective animal). In some embodiments,
multiple
pumps may communicate with the computer system and may be used to deliver
substances at respective received controlled delivery rates to respective
animals (e.g.,
one animal per pump). In some embodiments, the computer system may also
send/receive other information to/from the pumps (e.g., to control various
aspects of
the pumps and/or store information associated with the pumps). In some
embodiments, the computer system may determine respective controlled delivery
rates for the pumps based in part on a weight of a respective animal receiving
the
substance from the respective pump and/or for example, a study group the
animal is
in. For example, a study may involve testing one group of animals with a high
dose
of a substance, one group with a mid dose of the substance, one group with a
low dose
of the substance, and one group with a control substance (other study
configurations
are also contemplated). In some embodiments, the computer system may calculate
and then send the determined controlled delivery rates to the respective pumps
in
response to a global command (e.g., received from an operator). The pumps may
use
the received determined controlled delivery rates to control the rate of
substance
delivery to a respective animal that is receiving the substance from the
respective
pump (e.g., through an intravenous (IV) connection to a syringe with the
substance
being controlled by the pump). In some embodiments, the computer system may
display respective graphical profiles of the controlled delivery rates over
time for the
respective pumps. The graphical profiles may also include indicators marking
the
graphical profile at the current time point in the study.
2
CA 02663448 2009-04-21
In some embodiments, pumps and other equipment (e.g., medical or
monitoring devices) may communicate with the computer system through wired
and/or wireless connections. For example, the connections may form a mesh
network
allowing the computer system to send and receive information to the pumps and
other
equipment. In some embodiments, the computer system may communicate with the
pumps and other equipment through a data hub. In some embodiments, the pumps
and other equipment may be coupled to a box operable to send/receive
communications to/from the network. The boxes may also include memory for
storing information such as instructions (e.g., for the pump), a controlled
delivery
rate, a start time, a stop time, a duration, a target volume, etc. to allow
the box to
provide the instructions, etc. in the event of a computer system failure
and/or to allow
the box to be placed on a different pump if the original pump should fail (or
for some
other reason need to be disconnected from the study).
In some embodiments, the computer system may receive information such as
weights (e.g., from a weight scale, file, or remote computer), sensor data
(e.g., from
monitoring sensors either implanted in the animals or coupled to cages holding
the
animals), documentation (e.g., including user identifiers and documentation
identifiers
for respective events occurring in the network such as pump starting, pump
stopping,
alarm, alarm cleared, how alarm was cleared, etc). User identifiers (e.g.,
personal
identification numbers (PINs)) may be used to authenticate an operator prior
to
allowing the operator to perform an action on the pump (or other equipment).
The
user identifier may also be stored with a received documentation identifier to
indicate
which operator performed the respective action. In some embodiments, user
identifiers and documentation indicators (e.g., when clearing an alarm) may be
required prior to continued system access and/or prior to restarting pump
operation
(e.g., if stopped after an alarm).
In some embodiments, the computer system may communicate with the
pumps and/or weight scales associated with the pumps for in process pump
validation.
For example, an operator may weigh a syringe before a pump pumps a substance
and
after the pump pumps the substance according to a received controlled delivery
rate.
The weights (and, for example, start and stop times) may be used to validate
the pump
(e.g., determine if the expected delivery rate is within an acceptable range
of the
3
CA 02663448 2009-04-21
actual delivery rate (output volumes may also be used in the validation)). The
computer system may also track calibration dates for the pumps and may warn an
oper=ator (or, for example, inhibit pump operation) of pumps that have gone
past their
calibration intervals (or will go past their calibration intervals during the
study).
In some embodiments, the computer system may communicate with a filling
pump (either coupled or not coupled to an animal) to fill syringes with an
amount of
substance needed for a next phase of a study. For example, after determining a
controlled delivery rate for a pump (and a duration of pumping at the
determined
controlled delivery rate), the computer system may determine and communicate
an
amount of substance needed in a respective syringe (or, for example, a syringe
plunger displacement indication, etc.) to a filling pump and the filling pump
may fill
the respective syringe with the indicated amount of substance (the syringe and
a vat of
the substance to be used to fill the syringe may be coupled to the filling
pump by an
operator). An indicator (e.g., printed directly on the syringe or on a label
to be
coupled to the syringe) may be placed on the syringe to assist the operator in
placing
the syringe on the respective pump (in some embodiments, the same pump may
fill
the syringe and deliver the substance to the respective animal). In some
embodiments, the computer system may calculate several syringe amounts and may
display (or, for example, print) the list for an operator to use in preparing
syringes for
future phases of the study (e.g., the list may include entries with a pump
indicator, a
time indicator, an amount indicator, an animal indicator, etc. along with the
substance
amount to fill the respective syringe with). In some embodiments, when a
syringe is
placed into a pump, the pump (e.g., using information stored in the box and/or
received from the computer system) may check a diameter of the received
syringe to
make sure the received syringe diameter corresponds to the expected syringe
diameter
(different sized syringes may be used at different times in the study). In
some
embodiments, the pump may indicate an error and/or not pump the syringe if the
diameters do not match.
Brief Description of the Drawinlls
A better understanding of the present invention may be obtained when the
following detailed description is considered in conjunction with the following
drawings, in which:
4
CA 02663448 2009-04-21
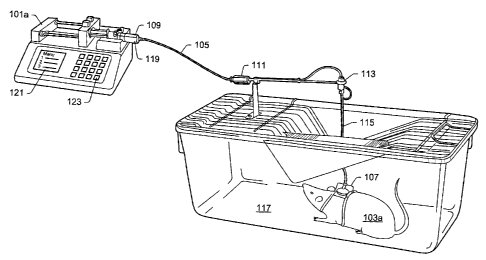
FIG. 1 illustrates a pump and an animal cage, according to an embodiment.
FIG. 2a illustrates multiple pumps communicating with a computer system,
according to an embodiment.
FIG. 2b illustrates multiple pumps communicating with a computer system
through respective boxes, according to an embodiment.
FIG. 3 illustrates a box, according to an embodiment.
FIG. 4 illustrates a food consumption monitoring device, according to an
embodiment.
FIG. 5 illustrates an embodiment of monitoring devices for monitoring the
micro-environments of multiple animal cages in a rack and cage system.
FIG. 6 illustrates a data hub communication arrangement including a pump
and medical and monitoring devices wired to an external stand-alone data hub,
according to an embodiment.
FIG. 7 illustrates a rack hub communication arrangement with multiple pumps
and medical and monitoring devices in a rack wired to an external stand-alone
data
hub, according to an embodiment.
FIG. 8 illustrates a box communication arrangement with a pump and medical
and monitoring devices respectively coupled to a removable piece of wireless
communications hardware, according to an embodiment.
FIG. 9 illustrates a set-up screen for a study, according to an embodiment.
FIG. 10 illustrates a security set-up screen, according to an embodiment.
FIG. 11 illustrates a communications port set-up screen, according to an
embodiment.
FIG. 12 illustrates a user set-up screen, according to an embodiment.
FIG. 13a illustrates a graphical user interface for pump/animal assignment,
according to an embodiment.
FIG. 13b illustrates graphical user interface for equipment access, according
to
an embodiment.
FIG. 14 illustrates a pump set-up screen, according to an embodiment.
FIG. 15a illustrates a graphical profile for a substance delivery, according
to
an embodiment.
FIG. 15b illustrates a listing of future syringes, according to an embodiment.
FIG. 16 illustrates an electronic log, according to an embodiment.
5
CA 02663448 2009-04-21
FIG. 17 illustrates a flowchart of a method for controlled delivery rate
determination and global command rate distribution, according to an
embodiment.
FIG. 18 illustrates a flowchart of a method for pump validation, according to
an embodiment.
FIG. 19 illustrates a flowchart of a method for automated syringe filling,
according to an embodiment.
FIG. 20 illustrates a flowchart of an embodiment for study documentation.
FIG. 21 illustrates an embodiment of a wide area network (WAN) and a local
area network (LAN).
FIG. 22 illustrates an embodiment of computer system that may be suitable for
implementing various embodiments of a system and method for substance delivery
and monitoring.
While the invention is susceptible to various modifications and alternative
forms, specific embodiments thereof are shown by way of example in the
drawings
and will herein be described in detail. It should be understood, however, that
the
drawings and detailed description thereto are not intended to limit the
invention to the
particular form disclosed, but on the contrary, the intention is to cover all
modifications, equivalents, and alternatives falling within the spirit and
scope of the
present invention as defined by the appended claims. Note, the headings are
for
organizational purposes only and are not meant to be used to limit or
interpret the
description or claims. Furthermore, note that the word "may" is used
throughout this
application in a permissive sense (i.e., having the potential to, being able
to), not a
mandatory sense (i.e., must). The term "include", and derivations thereof,
mean
"including, but not limited to". The term "coupled" means "directly or
indirectly
connected".
Detailed Description of the Embodiments
FIG. 1 illustrates an embodiment of pump 101a (e.g., a medical infusion
pump) and a laboratory animal cage 117 for animal 103a. In various
embodiments,
pump 101 ("pump 101" used generally herein to refer to pumps lOla, 101b, IOIc,
etc.) may be used to deliver substance 119 to animal 103 ("animal 103" used
generally herein to refer to animals 103a, 103b, 103c, etc.) at a controlled
delivery
6
CA 02663448 2009-04-21
rate (e.g., to study the effects of substance 119 on respective animal 103).
In some
embodiments, the controlled delivery rate may be calculated, for example, by
computer system 201 (e.g., see FIG. 2) and communicated to pump 101 for use in
delivering substance 119 to animal 103. As discussed herein, other information
may
also be communicated between computer system 201, pumps 101, and other
equipment in an animal drug study. While embodiments described herein include
animal applications (e.g., laboratory/veterinary research applications), other
applications are also contemplated (e.g., human study applications).
In some embodiments, pump 101 may include a stepper motor to push a
plunger on syringe 109 to deliver substance 119 in syringe 109 at the
controlled
delivery rate (or pull the plunger to load substance 119 into syringe 109).
While
syringe 109 is used throughout, other delivery containers (e.g., a holding
tank) are
also contemplated. Other pump types are also contemplated (e.g., peristaltic,
diaphragm, large volume, stationary ("pole mount"), and portable
("ambulatory")).
Animals 103 may include rodents, pigs, rabbits, dogs, cats, nonhuman primates,
etc.
Substances 119 may include a saline solution, a drug solution, or a control
solution
(which may be a saline solution). Other substances 119 are also contemplated.
In
some embodiments, substance 119 may be a liquid delivered through tube 105 on
animal 103 which may deliver substance 119 intravenously (through a catheter
107)
to animal 103. Other routes of administration are also contemplated. For
example,
substance 119 may be an airborne particle that is pumped into an animal's
breathing
space or a solid/liquid substance that is pumped into the animal's digestive
system.
Substance 119 may also be applied to the animal's eyes, ears, skin, etc.
(e.g., by a
spray pump). In some embodiments, counter balance 111, swivel 113, and spring
tether 115 may be used to guide and stabilize tube 105 transporting substance
119 to
animal 103 in animal cage 117. Other configurations are also contemplated.
FIG. 2a illustrates multiple pumps 101 communicating with computer system
201, according to an embodiment. In some embodiments, multiple pumps 101
(e.g.,
pumps 101 b, 101 c, 101 d, and 101 e) may be used to deliver substances 119 to
multiple
respective animals 103 (e.g., animals 103b, 103c, 103d, and 103e). For
example, a
toxicity study may include delivering different respective amounts of a drug
to
different animals (e.g., one animal 103 per pump 101) to determine the toxic
effects
7
CA 02663448 2009-04-21
(if any) of the drug and to determine ideal drug amount / body weight ratios.
Other
study types and study characteristics (e.g., effects of the drug on different
genders,
age groups, etc.) are also contemplated. Studies may require testing tens,
hundreds, or
thousands of animals over a few hours, days, weeks, etc. Animal studies may be
preliminary to human studies (e.g., for obtaining FDA approval). For example,
animal studies may be used in researching new formulations for drugs to treat
diseases (e.g., heart disease, diabetes, etc.)
In some embodiments, pumps 101 may communicate with computer system
201 through network 203 (e.g., through wired and/or wireless communications).
Computer system 201 may be a personal computer (such as a desktop or laptop),
mainframe, etc. Other computer system types are also contemplated. In some
embodiments, computer system 201 may include several computer systems
communicatively coupled together. In some embodiments, computer system 201 may
send/receive information to/from pumps 101 and other equipment involved in the
study (e.g., medical or monitoring devices such as weight scale 217). For
example,
computer system 201 may receive weight data from weight scales 217 to
determine,
for a respective pump 101, a respective controlled delivery rate for
delivering
substance 119 to animal 103. Computer system 101 may then send the determined
controlled delivery rate to the respective pump 101. Each animal 103 may have
an
individual weight scale 217 (e.g., incorporated in respective animal cage 117)
or
multiple animal cages 117 may share a weight scale 217. In some embodiments,
weight scale 217 may communicate (e.g., measured animal weights) with computer
system 201 through network 203.
In some embodiments, computer system 201 may provide an interface for
operator 401 (e.g., see FIG. 4) to automate control of pumps 101 and the other
equipment involved in the study. Information may also be received at computer
system 201 from pumps 101 and other equipment (e.g., other medical or
monitoring
devices) communicatively coupled to computer system 201. For example,
information may be entered at pump 101 through an operator interface 123
(e.g., an
alpha/numerical keypad, a full Qwerty keyboard, etc). In some embodiments,
information may also be displayed on pump display 121 (e.g., see menu
displayed on
display 121 in FIG. 1). Other pump configurations are also contemplated.
Computer
8
CA 02663448 2009-04-21
system 201, pumps 101, and/or other medical or monitoring devices may also be
operable to communicate (e.g., send and receive data and instructions) with
personal
digital assistants (PDAs), cell phones, smart cards, etc. For example,
operator 401
may send information to computer system 201 through a PDA (e.g., an animal
weight,
documentation of a study event, etc). As another example, operator 401 may
send
information to pump 101 by entering the information into a PDA; the PDA
sending
the information to computer system 201, and the information being transmitted
to
pump 101 from computer system 201 over network 203. As another example,
operator 401 may send information to computer system 201 by entering the
information into a PDA; the PDA sending the information to pump 101, and the
information being transmitted to computer system 201 from pump 101 over
network
203. Computer system 201 may be used by operator 401 to set-up a study (e.g.,
by
calculating respective controlled delivery rates) and automate documentation
for the
study (e.g., associated with pumps 101 and the other equipment involved in the
study). Automating control may save substantial time over manual pump set-ups.
In
addition, automating documentation may result in more accurate and complete
study
documentation (often required by the FDA and other regulatory bodies) and may
force operators 401, etc. to enter documentation at the appropriate times
(e.g., during
a pump alarm).
In some embodiments, computer system 201 may determine respective
controlled delivery rates for substance delivery for pumps 101 (e.g., based in
part on a
weight of animal 103 receiving substance 119 from respective pump 101) and
send
the determined controlled delivery rates to respective pumps 101. In some
embodiments, controlled delivery rates may include [dose/time]/animal weight
([ml/hr]/kg) where dose may indicate a substance concentration. Other
controlled
delivery rates are also contemplated (e.g., non-weight based controlled
delivery rates
may include dose/time (ml/hr)). Pumps 101 may use the received determined
controlled delivery rate to control the rate of substance delivery to animal
103 that is
receiving substance 119 from respective pump 101.
In some embodiments, studies may involve testing groups of animals with
different levels of drug doses. For example, a study may involve testing one
group of
animals with a high dose of substance 119, one group with a mid dose of
substance
9
CA 02663448 2009-04-21
119, one group with a low dose of substance 119, and one group with a control
(other
study configurations are also contemplated). In some embodiments, computer
system
201 may also use the study group criteria in determining the respective
controlled
delivery rate for pump 101 (e.g., in addition to the determined animal
weight). Pumps
101 in the high dose group may be provided a controlled delivery rate with an
increased dose of the drug per unit of body weight than the mid or low dose
group
pumps 101. In some embodiments, study ratios (of substance amount per unit
body
weight) may be provided to computer system 201 (e.g., by operator 401) for
each
group along with a number of animals 103 to test in each dose group (or a
respective
percentage of the total number of animals to include in each group). For
example,
operator 401 may provide a spreadsheet with the ratios (and, for example,
other test
parameters such as animal type, gender, age, etc.) to computer system 201.
Other
information may also be received (e.g., time periods for administering the
drugs).
Other sources of the study information are also contemplated (e.g., downloaded
from
a remote computer). Computer system 201 may use this information to set up
which
pumps 101 will provide which dose levels. The respective weights of the
animals
may also be received by computer system 201 (e.g., on a spreadsheet, through
manual
entry on a pump interface 123, through a weight received from weight scale 217
associated with pump 101, etc). Computer system 201 may arrange pump groupings
(e.g., by assigning pumps 101 to respective groups), pump controlled delivery
rates,
etc. and communicate the resulting respective controlled delivery rates to
respective
pumps 101 throughout the study.
In some embodiments, pumps 101 and/or other medical or monitoring devices
may communicate over network 203 with computer system 201 through wired and/or
wireless communications. For example, pumps 101 (e.g., pumps 101 f, 101 g, 101
h,
and 101 i) and/or other medical or monitoring devices may include and/or be
coupled
to wireless communication devices such as Wireless Fidelity (IEEE 802.11 b
wireless
networking) (Wi-Fi) transmitter/receiver, Bluetooth transmitter/receiver),
etc. for
communication with computer system 201. In some embodiments, pumps 101 and/or
other medical or monitoring devices (e.g., as seen in FIG. 2b) may communicate
with
computer system 201 through boxes 205 (e.g., see boxes 205a, 205b, 205c, and
205d
(referred to generally herein as boxes 205)). In some embodiments, box 205
attached
to a communication port of pump 101 and/or other medical or monitoring devices
CA 02663448 2009-04-21
(e.g., through communication port 307 as seen in FIG. 3) may send/receive
information to/from pump 101 (and/or other medical or monitoring devices) and
computer system 201 (e.g., wirelessly through wireless transmitter/receiver
309 or
through a wired connection through communication port 311). In some
embodiments,
box 205 may not be physically attached to pump 101 and/or other medical or
monitoring devices, but may communicate with pump 101 and/or other medical or
monitoring devices through wireless transmitter/receiver 309 (which may
include a
separate transmitter and receiver or a transceiver). Other communication
configurations are also contemplated. As seen in FIG. 2b, pumps 101 f, 101 g,
101 h,
and 101 i may use respective controlled delivery rates received from computer
system
201 to pump the determined respective amounts of substance 119 into animals
103f,
103g, 103h, and 103i.
In some embodiments, pumps 101 and/or other medical or monitoring devices
may also be coupled to computer system 201 through wired connections (in some
embodiments, boxes 205 may provide wired and/or wireless connections). In some
embodiments, pumps 101 and/or other medical or monitoring devices may have
communication ports (e.g., serial RS-232, Universal Serial Bus (USB),
Ethernet, other
communications (COM) port, etc). Connections may be made through the
communication ports directly to computer system 201 (e.g., through a wired
connection) or indirectly to computer system 201 (e.g., box 205 may be coupled
to the
communication port and may send/receive communications to/from computer system
201 through a wired and/or wireless connection). Other connections are also
contemplated.
In some embodiments, network 203 may be a mesh network. Through the
mesh network, pumps 101 (and, for example, other medical or monitoring
devices) in
network 203 may communicate directly with each other and/or communicate with
each other via computer system 201. For example, computer system 201, boxes
205,
etc. may use a ZigBeeTM wireless protocol for peer-to-peer communication
(which
may provide alternate communication paths in the network 203 if a direct path
is not
available). In some embodiments, computer system 201, boxes 205, etc. may
communicate with each other through a router. In some embodiments, the router
may
11
CA 02663448 2009-04-21
be external or internal to computer system 201. Other network configurations
and
protocols are also contemplated.
In some embodiments, pump 101 may access memory 305. Memory 305 may
be internal to pump 101 or may be external to pump 101 (e.g., memory 305 may
be in
box 205 communicatively coupled to pump 101). Memory 305 may include a non-
volatile memory (e.g., flash memory) or volatile memory (e.g., Random Access
Memory (RAM)). Other memory types are also contemplated. In some
embodiments, memory 305 may store information such as instructions (e.g., for
pump
101), a controlled delivery rate, a start time, a stop time, a duration, a
target volume,
etc. for pump 101 from computer system 201. For example, memory 305 may store
the received controlled delivery rate, a start time, and a duration from
computer
system 201 for pump 101 to use in pumping substance 119 to animal 103. Other
combinations are also contemplated (e.g., memory 305 may store controlled
delivery
rate and target volume or controlled delivery rate and a start and stop time).
Memory
305 may also include program instructions (e.g., received from computer system
201)
to control pump 101. For example, the programming instructions may be stored
as
firmware on memory 305. Because instructions for pump 101 may be stored on
memory 305, if computer system 201 fails (or, for example, is restarted,
disconnected,
etc.), pumps 101 may continue operation per the instructions stored on memory
305.
In some embodiments, programming instructions for determining the controlled
delivery rate for pump 101 may be stored in memory 305. The controlled
delivery
rate may be determined based on information collected at pump 101 and
corresponding information may be sent to computer system 201 for storage
(e.g., the
animal's weight, the controlled delivery rate, etc). In some embodiments,
computer
system 201 may communicate information needed for the calculation to pump 101
and/or box 205 (e.g., a dose ratio assigned to respective pump 101) to be used
with
the programming instructions on memory 305 and/or other data in memory 305 for
the calculation. Memory 305 may also include, for example, alarm codes, menu
options for indicating how alarms were solved, etc. Memory 305 may also store
information sent to and received from computer system 201 (e.g., as serve as a
back-
up for computer system 201). In some embodiments, memory 305 may be accessible
to other medical or monitoring devices (e.g., internal to the devices or
externally
accessible to the devices) for storing information (e.g., information
sent/received
12
CA 02663448 2009-04-21
to/from computer system 201) and/or instructions for these devices. For
example, box
205 with memory 305 may be coupled to a medical or monitoring device's
communications port. In addition to memory 305, box 205 may include processor
303 to access memory 305, electronic clock 313, and communications circuitry
301.
In some embodiments, the memory 305 and wireless transmitter/receiver 309 may
be
on the same printed circuit board (PCB). Other configurations are also
contemplated.
In some embodiments, memory 305 may be included in a router (e.g., external to
computer system 201) to allow continued operation of pumps 101, medical and
monitoring devices, network 203, etc. if computer system 201 fails (or, for
example,
is restarted, disconnected, etc).
In some embodiments, box 205 may be replaced on pump 101 (and/or other
medical or monitoring device) (e.g., if box 205 fails, is not functioning
properly, is
being updated, etc). For example, an external box 205 may be replaced without
replacing or repairing pump 101 (and/or other medical or monitoring device).
If the
memory 305 and/or communications circuitry 301 is on box 205 instead of an
interior
of pump 101, the memory 305 and communications circuitry 301 may be easier to
repair/replace by replacing box 205 (as opposed to accessing the interior of
pump
101). In some embodiments, if pump 101 (or other medical or monitoring device)
fails, is not functioning properly or, for example, is being updated, box 205
may be
placed on a different pump 101 (or other medical or monitoring device). In
some
embodiments, box 205 may not need to be reprogrammed after the switch (e.g.,
box
205 may interact with the new pump to perform the functionality expected of
the
previous pump (e.g., controlled delivery rate, delivery schedule, etc)). In
some
embodiments, box 205 may be configured to interface with different types of
pumps
101 (and/or other medical or monitoring device). Box 205 may include dedicated
programming instructions specific to the pump style (or style of other medical
or
monitoring device). In some embodiments, the pump 101 (and/or other medical or
monitoring device) may include programming instructions to be compatible with
box
205. In some embodiments, box 205 may be internal to pump 101 (and/or medical
or
monitoring device) and pump 101 (and/or medical or monitoring device) may be
repaired or replaced if the internal box 205 is not functioning properly (or,
for
example, to update box 205). In some embodiments, box 205 may include a
wireless
communications device with one or more communication port connectors (e.g.,
serial
13
CA 02663448 2009-04-21
RS-232, USB, Ethernet, etc) to configure box 205 to communicate with a
specific
pump 101. In some embodiments, communications circuitry 301 (and, for example,
wireless transmitter/receiver 309, communication ports 307/311) processor 303,
memory 305, and/or electronic clock 313 may be internal to pump 101 (and/or
medical or monitoring device). Other placements are also contemplated.
In some embodiments, other medical or monitoring devices (e.g., used to treat
or monitor humans or animals 103) may communicate with computer system 201.
For example, the medical or monitoring devices (e.g., sensors) may monitor
physiologic parameters (e.g., animal temperature, activity, pulse oxymetry,
heart rate,
blood pressure, metabolic function, etc) and animal cage conditions (e.g., a
micro-
environment monitoring apparatus may measure animal cage temperature,
humidity,
ammonia level, etc)). As seen in FIG. 4, a monitoring device may include a
food
and/or water consumption monitoring device 403 (e.g., for one animal cage 117
of a
collection of animal cages). In some embodiments, network 203 may include
individual laboratory animal cages 117 with respective devices for monitoring
the
weight of feed dispensed (and, in some embodiments, consumed) (e.g., food
consumption monitoring device 403) by animal 103 (e.g., a rat) in the
respective
animal cages 117 (e.g., separate monitoring devices for each of the respective
animal
cages 117). FIG. 5 illustrates an embodiment of monitoring devices for
monitoring
the micro-environments of multiple animal cages 117 in a rack and cage system
405.
The medical or monitoring device may include a rack and cage system 405
including
multiple laboratory animal cages 117 and micro-environment monitoring devices
attached to respective animal cages 117 to measure conditions within each
animal
cage 117 (e.g., temperature, humidity, etc). This micro-environment data may
be
transmitted to computer system 201 (e.g., wirelessly through communications
circuitry in the monitoring devices or box 205 coupled to the monitoring
devices).
In some embodiments, medical or monitoring devices may include weight
scale 217 used to determine a weight of animal 103, cage 117, etc. Other
weight
determinations are also contemplated (e.g., the weight of a syringe for pump
101 may
be weighed in weight scale 217 for transmission to computer system 201). In
some
embodiments, computer system 201, weight scale 217 (and/or other medical or
monitoring devices), and pump 101 may form a closed information loop. Other
14
CA 02663448 2009-04-21
information arrangements are also contemplated. Other medical or monitoring
devices are also contemplated (e.g., a Wireless Information Device (WID)
reader for
animal identification based on an implanted, external, and/or wearable Radio
Frequency Identification (RFID) chips) may be used to identify specific
animals
associated with a specific animal cage 117 (e.g., with the reader). Medical or
monitoring devices may thus include monitoring sensors either implanted in
animals
103 or coupled to cages 117 holding animals 103. Medical or monitoring devices
may transmit and receive information to/from computer system 201 (e.g.,
through
wired and/or wireless communications). In some embodiments, pumps 101 (and/or
medical or monitoring devices) in network 203 may have unique addresses (e.g.,
unique Internet protocol (IP) addresses). Other unique address types are also
contemplated (e.g., Media Access Control (MAC) addresses). In some
embodiments,
computer system 201 may use the unique addresses to send/receive information
to/from pumps 101 (and/or medical or monitoring devices) to control, monitor,
and/or
store information associated with pumps 101 (and/or medical or monitoring
devices).
In some embodiments, computer system 201, pumps 101 (and/or other
medical or monitoring devices) may communicate with other computers (e.g., via
an
intranet or Internet 211). For example, information from computer system 201
may
be sent to server 207 in communication with remote personal computers 209
(e.g.,
computers 209a, 209b, and 209c) over Internet 211. In some embodiments, a
network
of remote computers may communicate with computer system 201 for remote access
to data in computer system 201 (e.g., remote computers 209 may communicate
with
computer system 201 via Internet 211 and/or via server 207 coupled to and/or
including computer system 201). In some embodiments, other remote computers
215
(e.g., computers 215a, 215b, and 215c) may access computer system 201 through
server 207. Remote access may allow operators 401 (e.g., remote operators) to
monitor and/or control equipment in the study, access documentation, etc.
Other uses
for remote access are also contemplated. In some embodiments, computer system
201
may notify an entity (e.g., operator 401) of the status (e.g., normal or
abnormal) of
pumps 101 and/or medical or monitoring devices and may allow the entity to
control
pumps 101 and/or medical or monitoring devices communicating through network
203. In some embodiments, computer system 201 may notify operator 401 via
electronic mail messages, text messages, paging, voice messaging, etc. of a
status and,
CA 02663448 2009-04-21
for example, may receive control instructions through operator mobile device
213
(e.g., a phone, PDA, etc).
In some embodiments, computer system 201 may communicate through
wired, wireless, or a combination of wired and wireless network hardware to
pumps
101 and/or medical or monitoring devices to program, monitor, and collect data
from
the pump 101 and/or medical or monitoring devices. The network combinations
may
include, for example, a data hub communication arrangement (e.g., see FIG. 6),
a rack
hub communication arrangement (e.g., see FIG. 7), a box communication
arrangement (e.g., see FIG. 8), or various subsets and/or combinations of
these
communication arrangements (other network configurations are also
contemplated).
FIG. 6 illustrates an embodiment of the data hub communication arrangement
including pump 101 and/or medical or monitoring devices wired (or wirelessly
connected) to data hub 601 (e.g., an external stand-alone data hub). FIG. 6
illustrates
an embodiment including rack 405 with multiple cages 117, integrated direct
current
(DC) power ports, and a universal, removable power supply (other
configurations are
also contemplated). FIG. 7 illustrates an embodiment of a rack hub
communication
arrangement with multiple pumps 101 and/or medical or monitoring devices in
rack
405 wired or wirelessly connected to data hub 601 (e.g., an external stand-
alone data
hub mounted to rack 405). FIG. 7 illustrates an embodiment of rack 405 with
multiple cages 117 and a mounted data hub 601 operable to handle the infusion
groups within the single rack 405 (other configurations are also
contemplated). In
some embodiments, cage rack 405 may also include integrated washable DC power
ports and a Universal, removable power supply. Other data hub types and
placements
are also contemplated. The data hub hardware may include embedded programming
instructions operable to allow data input to/from multiple devices (e.g., pump
101
and/or medical or monitoring devices (such as sensors and weight scales),
etc.) and
to/from computer system 201. Data hub 601 (e.g., a universal data hub) may be
placed on, in or proximate to animal cage 117, pump 101, and/or medical or
monitoring device (e.g., one data hub 601 per animal cage 117, one data hub
601 per
pump 101, one data hub 601 supporting multiple animal cages 117 in rack 405,
etc).
In some embodiments, a single data hub 601 may be located at each of one or
more
animal cages 117. In some embodiments, a single data hub 601 may be coupled to
16
CA 02663448 2009-04-21
multiple animal cages 117 (e.g., coupled to rack 405). Other configurations
are also
contemplated. In some embodiments, pump 101 and/or medical or monitoring
devices dedicated to animal cage 117 may communicate bi-directionally with
data
hub 601 and to computer system 201 (e.g., through data hub 601).
In some embodiments, data hub 601 may accommodate multiple wired and/or
wireless data platforms and protocols used in pumps 101, and/or medical or
monitoring devices (e.g., Ethernet, RS232, USB, Wi-Fi, Bluetooth, etc). For
example, data hub 601 may pass through (and/or convert) communications to/from
pumps 101 and/or medical or monitoring devices to/from computer system 201. In
some embodiments, data hub 601 may integrate multiple data sources from pumps
101 and/or medical or monitoring devices into a data stream for transmission
to
computer system 201 (e.g., wirelessly). In some embodiments, data hub 601 may
multiplex various communications from pump 101 and/or medical or monitoring
devices to computer system 201. Computer system 201 may separate the data
streams
(e.g., using a pre-arranged template shared with data hub 601 and/or a
demultiplexer).
Other communication formats are also contemplated (e.g., data to/from pump 101
and/or medical or monitoring devices may be transmitted/received as single
serial
streams). Computer system 201 may transmit information intended for pump 101
and/or medical or monitoring devices to data hub 601 for delivery to the
intended
pump 101 and/or medical or monitoring devices (these streams may also be
combined/multiplexed streams or separate streams). In some embodiments, data
hub
601 may support a generic platform to transmit and receive data to/from
several
different types of platforms (e.g., different pump types, different computer
systems,
etc). In some embodiments, data hub 601 may include programming instructions
to
convert data in one platform to another platform prior to sending the data to
an
intended device.
In some embodiments, data hub 601 may transmit bi-directional data for a
single animal cage 117 to computer system 201 (e.g., via wired or wireless
hardware)
or data hub 601 may transmit bi-directional data for animal cages 117 in rack
405 to
computer system 201 (e.g., via wired or wireless hardware). In various
embodiments,
a lab animal cage rack 405 (other rack types are also contemplated) may hold
multiple
animal cages 117 (e.g., 10, 100, 1000, etc). The cage rack 405 may include
power
17
CA 02663448 2009-04-21
sources 603 (which may be integrated in the cage rack 405) and wires as well
as data
communication devices and wires for pumps 101 and/or medical or monitoring
devices on animal cages 117. In some embodiments, power sources 603, wires,
communication devices, etc. may be removable and/or replaceable (in some
embodiments, one or more of these devices may be permanently affixed to animal
cage 117). Removable and replaceable power and data components may allow for
racks 405 to integrate with pumps 101 and/or medical or monitoring devices
while,
when removed, allowing for cleaning and, when replaced, reuse of racks 405 and
the
power and data communication components. Data hubs 601 may reduce workspace
clutter (wired and/or wireless) and may reduce the risk of data transmission
interference between various devices.
FIG. 8 illustrates an embodiment of a box communication arrangement with
pump 101 and/or medical or monitoring devices respectively connected (e.g.,
directly
connected or connected through a separate piece of hardware) to a removable
piece of
wireless communications hardware (e.g., box 205) allowing for wireless bi-
directional
communication between pump 101 and/or medical or monitoring devices on animal
cages 117 and computer system 201. In some embodiments, boxes 205 (e.g., boxes
205e, 205f, 205g, and 205h) may be distributed to several devices. In some
embodiments, one or more boxes 205 may be shared by multiple devices. In some
embodiments, rack 405 may include multiple cages 117 with integrated DC power
ports and a universal, removable power supply (other configurations are also
contemplated).
In some embodiments, a graphical user interface (GUI) (e.g., a browser-based
GUI) may be used to allow operator 401 to configure pumps 101 and/or medical
or
monitoring equipment (e.g., see FIGs. 9-14) through computer system 201 (or,
for
example, through remote computers 209a,b,c or 215a,b,c). The GUI may also
allow
configuration of the network which may include pumps 101, communications
hardware (e.g., wireless communications hardware for networking pumps 101 to
computer system 201), computer system 201 (e.g., including programming and
data
collection software), and a network of remote computers (e.g., computers
209a,b,c)
linked to computer system 201 via lnternet 211 and, for example, a network of
remote
computers (e.g., computers 215a,b,c) linked to computer system 201 via server
207.
18
CA 02663448 2009-04-21
Other network configurations are also contemplated. As seen in FIG. 9, a GUI
may
be provided to assist operator 401 (e.g., a study director, technician, etc.)
to set up a
study. Information entered into the GUI may be used, for example, by computer
system 201 to store information about the study, control the study, etc. As
seen in
FIG. 10, operator 401 may set up a password and specify other security
parameters for
the study. As seen in FIG. 11, various pumps used in the study may be set-up
(e.g.,
communication paths may be established and/or tested between the pumps 101 and
computer system 201). As seen in FIG. 12, different operators 401 may be added
to a
study (e.g., granted access to perform actions on pumps 101 and other
equipment,
document actions performed, etc). User identifiers 1201 may also be assigned
to
respective operators 401. As seen in FIG. 13a, operator 401 may assign
respective
pumps 101 to respective animals 103 (or vice versa). For example, computer
system
201 may poll pumps 101 coupled to network 203 and pumps 101 may respond, for
example, with a pump ID (see, for example, pump IDs on the left side of FIG.
13a).
In some embodiments, computer system 201 may access respective animal IDs
(e.g.,
from a data file, from animal RF identification chips scanned from animals
103,
manually from operators 401 (e.g., reading animal tattooed IDs), etc). The
animal IDs
may also be listed (e.g., see the right side of FIG. 13a). In some
embodiments,
operator 401 may assign the animal IDs to their respective pumps 101. For
example,
the animal ID on the right side of the screen may be dragged and dropped onto
the
corresponding pump ID of respective pump 101 from which respective animal 103
is
receiving substance 119. In some embodiments, pump IDs and/or animal IDs may
be
related to each other by operator 401 (e.g., by entering respective IDs in
text boxes of
the graphical user interface). In some embodiments, RFID readers assigned to
respective cages 117 may scan RF animal ID chips and send the animal ID back
to
computer system 201 along with the respective pump ID for respective pump 101
providing substance 119 to cage 117 with animal 103 having the respective
animal
ID. Other assignment processes are also contemplated. As seen in FIGs. 13b-14,
operator 401 may navigate the GUI to check on a status of pumps 101 and other
equipment in the study, send instructions to pumps 101 and other equipment in
the
study, etc.
FIG. 17 illustrates a flowchart of a method for controlled delivery rate
determination and global command rate distribution, according to an
embodiment. It
19
CA 02663448 2009-04-21
should be noted that in various embodiments of the methods described below,
one or
more of the elements described may be performed concurrently, in a different
order
than shown, or may be omitted entirely. Other additional elements may also be
performed as desired.
At 1701, animal weight data may be received (e.g., by computer system 201,
box 205, etc). In some embodiments, weight data may be received from weight
scale
217. Weight scale 217 may be integrated into animal cage 117 (e.g., coupled to
animal cage 117 or to tether 115 for passive automatic weight data collection)
or may
be external (e.g., animal cage 117 may be placed on top of (or hung from)
weight
scale 217 by operator 401). In some embodiments, multiple pumps 101 may be
associated with a specific weight scale 217 (e.g., 10 pumps 101 assigned to
one
weight scale 217 physically located nearby). For example, operator 401 may
place
each animal 103 (e.g., in turn) associated with the pumps 101 on the weight
scale 217
for measurement (or may place respective animal 103 from pump 101 on weight
scale
217). In some embodiments, weight data from weight scale 217 may be
communicated to computer system 201. For example, computer system 201 may
receive weight data from weight scale 217 through data hub 601 and/or box 205
coupled to weight scale 217. As another example, weight scale 217 may be
coupled
to pump 101 and weight data from weight scale 217 may be sent to pump 101 (or
box
205 coupled to pump 101) for communication to computer system 201. In some
embodiments, the weight data may be automatically communicated to computer
system 201 and stored in a database (e.g., an operator's project software
database). In
some embodiments, the weight data may be sent to computer system 201 when an
instruction is received by weight scale 217 or pump 101 (e.g., from operator
401). As
another example, in some embodiments, the weight data may be sent in response
to a
query from computer system 201. Other weight data sources are also
contemplated.
For example, animal weight data may be received from a customer database on a
server, from a database in a computer hosting infusion system, etc. Computer
system
201 may query a database for the weight data to be imported into computer
system
201. In some embodiments, operators 401 may load the data directly into
computer
system 201 (e.g., by inserting a Compact Disc (CD) with the weight data,
manually
entering the weight data, etc). In some embodiments, new weight data may be
received as new animal weights are determined. For example, animals 103 may be
CA 02663448 2009-04-21
weighed continuously or at intervals (e.g., animal 103 may be weighed daily,
weekly,
monthly, etc). In some embodiments, animal weights and respective animal
weights
may not be determined (e.g., if the controlled delivery rates are not weight
based).
At 1703, the weights for respective animals 103 may be determined. Animals
103 may be associated with specific pumps 101 and computer system 201 may
associate weight data with respective pumps 101. For example, if weight scale
217 is
coupled to or assigned to one respective pump 101, the weight data received
from that
weight scale 217 may be associated (e.g., in a database) with animal 103 at
that
respective pump 101. In some embodiments, (e.g., if multiple pumps 101/cages
117
are assigned to weight scale 217) identifiers (e.g., entered by operator 401
into weight
scale 217, scanned by an RFID scanner when animal 103 with an embedded RFID
chip containing the identifier is placed on weight scale 217, etc.) may be
sent with the
weight data to computer system 201 as the animals 103 (or cages 117, etc.) are
weighed to associate the received weight data with the respective animal
103/pump
101. In some embodiments, identifiers may be stored in the database with the
weight
data to associate the weight data with respective animals 103 and/or pumps 101
(respectively assigned to animals 103). In some embodiments, identifiers may
not be
used. For example, weight data may be associated with respective animals 103
according to an order the weights were entered (which may correspond to a
predetermined order of pumps 101 in relationship to the weight scale 217). For
example, 10 pumps 101 may be assigned to a weight scale at the end of the row
of
pumps 101. When the animals 103/cages 117 are weighed, operator 401 may always
start with the cage farthest from weight scale 217 and proceed down the line
of cages
117 to the cage nearest weight scale 217 (computer system 201 may be aware of
the
order of cages 117 and may assign the weights to respective animals 103
according to
the order the weights were received. Other weight associations are also
contemplated.
The animal 103 may be weighed on weight scale 217 directly or, for example,
cage
117 and be weighed and the animal's weight may be derived (e.g., by
subtracting a
predetermined weight of the empty cage). Other weight data sources are also
contemplated (e.g., the weight data may be imported from a separate software
program or database, manually entered, etc).
21
CA 02663448 2009-04-21
At 1705, controlled delivery rate group determinations may be made for the
respective animals 103. In some embodiments, animals 103 may be assigned to
different study groups (e.g., high dose group, mid-dose group, low dose group,
and
control, etc). Group assignments may be downloaded to computer system 201
(e.g.,
from an external computer), manually entered (e.g., by operator 401), or
determined
according to criteria (e.g., entered by operator 401). For example, operator
401 may
specify 1000 cages will be used in the study and 25% are to be assigned to a
high dose
group, 25% to a mid dose group, 25% to a low dose group and 25% to a control
group. This criteria may also be downloaded from an external source. Computer
system 201 may have access to (or may determine) which pumps 101 are currently
communicatively coupled to computer system 201 (e.g., through a broadcast
query
and subsequent pump responses) and the pumps 101 may be initially assigned to
different respective groups (e.g., computer system 201 may determine and store
assignments in a database for later access). In some embodiments, respective
controlled delivery rates (e.g., [dose/time]/kg X animal weight ([ml/hr]/kg X
kg of
animal weight)) may be associated with respective groups of animals. For
example,
the respective controlled delivery rates may be downloaded from an external
source,
manually entered by operator 401, etc. Additional study parameters may also be
received andlor determined. For example, an amount of time to deliver the
respective
doses may also be received (e.g., downloaded from an external source, manually
entered by operator 401, etc). For example, computer system 201 may receive
and
store an indication that the specified controlled delivery rates are to be
delivered for
one hour a day. Computer system 201 may also receive the total trial length
(e.g., 30
days). In some embodiments, complex profiles may be received (e.g., controlled
delivery rate for one hour per day for 15 days and 2 hours per day for 15
days). Other
profiles are also contemplated. Computer system 201 may store controlled
delivery
rates, time periods, profiles, etc. to be used in determining controlled
delivery rate for
respective animals 103 in the study.
At 1707, the controlled delivery rate for animal 103 may be determined based,
for example, on the animal's weight and the controlled delivery rate group
determination (e.g., the controlled delivery rate assigned to the animal's
group). For
example, for a specific animal 103 in a high dose group, a predetermined
controlled
delivery rate of [100 ml/hr]/kg X kg of body weight may be assigned (e.g., by
22
CA 02663448 2009-04-21
computer system 201 based on received data). In this example, if the weight
data for
the specific animal 103 indicates the specific animal 103 weighs 0.7 kg, the
controlled
delivery rate for a pump 101 pumping substance 119 to the specific animal 103
is
[100 ml/hr]/kg * 0.7 kg = 70 ml/hr. Computer system 201 may also use the
received
time periods to determine a dose per time period of delivery. For example,
study
parameters may specify the high dose group should receive the specified
controlled
delivery rate for 1 hour a day. In the above example, computer system 201 may
then
prepare a profile with instructions for respective pump 101 to deliver 70 ml
of
substance 119 to respective animal 103 for one hour every 24 hours. Study
parameters may also specify the animals 103 are to receive saline solution
during the
hours animals 103 are not receiving substance 119 in order that the positive
saline
flow reduces the risk of catheter clotting. Other controlled delivery rate
calculations
are also contemplated for the other groups (e.g., mid dose, low dose, etc).
Other time
periods may also be used (e.g., 2 hrs/day, 2 min/day, 1 hour every 3 days,
etc). In
some embodiments, computer system 201 may determine multiple respective
profiles
with instructions for respective animals in the study according to their
respective
weights and their respective dose groups.
At 1709, the profiles for respective animals 103 may be delivered to the
respective pumps 101. In some embodiments, the profiles may include respective
controlled delivery rates, relevant time periods for delivery (e.g.,
indicating number of
hours every 24 hours for delivery and total study period), start/stop times,
etc. In
some embodiments, a global command may instruct computer system 201 to send
the
multiple profiles to their respective pumps 101 (e.g., in some embodiments,
all of the
pumps 101 in the study may receive their specific profile from computer system
101).
In some embodiments, a subset of pumps 101 may be sent their respective
profiles in
response to the global command (e.g., the global command may instruct computer
system 201 to send profiles to pumps 101 in the high dose group). As another
example, the global command may instruct computer system 201 to send profiles
to
pumps 101 with animals in a certain weight group (e.g., with animals 103
having
weights between 0.5 kg and 0.6 kg) or to animals of a certain gender (e.g.,
all male
animals). Other groups are also contemplated. In some embodiments, multiple
groups may be specified (e.g., profiles may be sent to the low dose group and
the
placebo group in response to receiving the global command). In some
embodiments,
23
CA 02663448 2009-04-21
multiple profiles may be pushed to their respective pumps 101 after performing
a
sequence of calculations (e.g., by computer system 201) to generate the
multiple
profiles. In some embodiments, operator 401 may indicate when to send the
profiles
(e.g., by pressing a button (or by some other input) on computer system 201
(e.g., to
select an on screen menu item), sending a command to computer system 201 from
a
remote device, etc). As part of the global command, operator 401 may also
specify
which groups (or, for example, all of the pumps 101) to send profiles. In some
embodiments, computer system 201 may deliver infusion rate commands (e.g.,
including controlled delivery rates based on the animals weight and determined
group
weight-based controlled infusion rates) to pumps 101 individually instead of
in
groups.
Other global commands are also contemplated. For example, a global
command may instruct computer system 201 to send other instructions to
multiple
pumps 101 and/or medical or monitoring devices on the network. For example,
the
global command may cause computer system 201 to send other instructions to
pumps
101 instead of or in addition to inputting commands (e.g., by operator 401) to
pumps
101 on a one-by-one basis. In some embodiments, the global command may
instruct
computer system 201 to send inquiries to pump 101, a group of pumps 101, or
all of
pumps 101 in the study. For example, upon receiving an indication from
operator
401, computer system 201 may request information from a grotip of pumps 101
(such
as current amount of delivery time remaining, last calibration date, etc). In
some
embodiments, the global command may reduce the manpower needed to perform and
send the calculations, reduce manual calculation errors, and reduce manual
data input
errors. In some embodiments, the global command may be used to automate
scheduling to reduce scheduling errors by including start/stop times with the
profiles
delivered to respective pumps.
In some embodiments, the instructions for determining a controlled delivery
rate may be included in box 205 (or, for example, internally to pump 101).
Pumps
101 may determine their respective controlled delivery rate based on the
stored
instructions, the animal weight (e.g., received at pump 101 from weight scale
217),
and other information (e.g., the dose/body weight for animal 103 associated
with
respective pump 101, times for delivery, etc). In some embodiments, pumps 101
may
24
CA 02663448 2009-04-21
perform the calculations to determine their own controlled delivery rates
(e.g.,
computer system 201 may send a global command to pumps 101 to calculate their
controlled delivery rates). In some embodiments, the calculated controlled
delivery
rates (and, for example, animal weight data) may be sent by pumps 101 to
computer
system 201 (e.g., for storage and/or validation). Other locations for
controlled
delivery rate determination are also contemplated.
FIG. 18 illustrates a flowchart of a method for pump validation, according to
an embodiment. It should be noted that in various embodiments of the methods
described below, one or more of the elements described may be performed
concurrently, in a different order than shown, or may be omitted entirely.
Other
additional elements may also be performed as desired.
At 1801, computer system 201 may receive an indication of an acceptable
validation deviation. For example, operator 401 may indicate that an
acceptable
validation deviation of +/-1% of actual syringe weight difference (before and
after
substance delivery) compared to calculated syringe weight difference (based on
pump
determined substance delivery and substance density) is acceptable. In some
embodiments, the acceptable validation deviation may be received from other
sources
(e.g., downloaded from a remote computer). Acceptable validation deviations
may
also be specified in other terms. For example, an acceptable validation
deviation may
include +/- X% of actual controlled delivery rate (e.g., determined using the
difference in syringe weights, density of substance 119, and start/stop times
from
pump 101) compared to provided/calculated controlled delivery rate (e.g., the
controlled delivery rate provided to pump 101). Other acceptable validation
deviations are also contemplated. Acceptable validation deviations may be
provided
in non-percent indicators. For example, operator 401 may be prompted to enter
an
acceptable validation deviation as a difference in weight (e.g., +/- X ml)
between the
actual volume output and the provided/calculated volume output (e.g., X=
actual
volume - volume provided to pump 101 in profile instructions). Other sources
of
acceptable validation deviations are also contemplated. In some embodiments, a
range of acceptable validation deviations may be received.
CA 02663448 2009-04-21
At 1803, computer system 201 may receive a beginning syringe weight. In
some embodiments, operator 401 may place syringe 109 on weight scale 217 prior
to
delivering substance 119. For example, operator 401 may place syringe 109 for
pump
101 on a shared weight scale 217 (e.g., shared with other pumps 101). In some
embodiments, the weight (and, for example, a pump identifier) may be sent to
computer system 201 by weight scale 217. In some embodiments, weight scale 217
may be built into pump 101 to weigh syringe 109 without syringe 109 having to
be
removed from pump 101 (the weights (and/or weight difference) may be sent to
computer system 201 by pump 101).
In some embodiments, operator 401 may be prompted to enter a beginning
syringe weight. For example, operator 401 may enter the weight into computer
system 201 or into pump 101 (e.g., for delivery to computer system 201). For
example, operator 401 may place syringe 109 on weight scale 217, see weight of
syringe 109 (e.g., on a display of weight scale 217), and may enter the weight
in, for
example, pump 101 associated with animal 103 or computer system 201. Other
sources of the beginning syringe weight are also contemplated. In some
embodiments, weight scale 217 on, in, or proximate to animal cage 117 or pump
101
(e.g., one weight scale 217 per pump 101 or animal cage 117 or one weight
scale 217
per a group of pumps 101 or animal cages 117) may communicate weights of
syringe
109 to computer system 201 (e.g., through box 205 coupled to the weight scale
217).
For example, weight scale 217 may determine a weight of syringe 109 prior to
delivering substance 119 to animal 103.
At 1805, computer system 201 may receive an ending syringe weight. In
some embodiments, weight scale 217 may determine a weight of syringe 109 after
delivering substance 119 to animal 103 (e.g., operator 401 may place syringe
109 on
weight scale 217 after the delivery time period or weight scale 217 may be
built into
pump 101). In some embodiments, operator 401 may be prompted to enter ending
syringe weight. For example, operator 401 may place syringe 109 on weight
scale
217, see weight of syringe 109 (e.g., on a display of weight scale 217), and
may enter
the weight in, for example, pump 101 associated with animal 103 or computer
system
201. Other sources of the ending syringe weight are also contemplated.
26
CA 02663448 2009-04-21
At 1807, computer system 201 may compare the actual volume output
(determined using the substance density and the difference in the beginning
syringe
weight and the ending syringe weight) to a nominal volume output (e.g., an
expected
volume output based on the calculated controlled delivery rate delivered to
pump 101
by computer system 201 prior to delivery).
At 1809, computer system 201 may compare the actual controlled delivery
rate (e.g., using substance density, difference in the beginning syringe
weight and the
ending syringe weight and a received actual start time and end time from pump
101)
to a nominal controlled delivery rate (e.g., based on the calculated
controlled delivery
rate delivered to pump 101 by computer system 201). In some embodiments,
computer system 201 may receive a start and stop time (or, for example, a
total time
of delivery) to use with the received weights to calculate the pump's actual
controlled
delivery rate. In some embodiments, computer system 201 may compare an actual
controlled delivery rate (e.g., ((beginning syringe weight - ending syringe
weight)/substance density / (stop time - start time)) to a calculated/provided
delivery
controlled delivery rate (e.g., calculated by computer system 201 prior to
substance
delivery and provided to pump 101 as the respective controlled delivery rate
for
respective animal 103) to determine an accuracy of pump 101. Other information
may also be sent to computer system 201 (e.g., a controlled delivery rate
determined
locally by pump 101). Other controlled delivery rate determination
calculations are
also contemplated. For example, computer system 201 or pump 101 may use a
displacement volume and delivery time to determine an actual controlled
delivery
rate. The displacement volume may be determined using dimensions of syringe
109
(e.g., radius of a cylindrical syringe) and, for example, the amount of
plunger
displacement (e.g., indicated by a sensor on pump 101) (where displaced volume
may
equal the amount of displacement * internal area (e.g., 7E * radius2). The
actual
controlled delivery rate may be represented by the displaced volume over time
of
displacement (e.g., as determined by start and stop times). In some
embodiments,
information such as the dimensions of syringe 109 may be received by computer
system 201 (e.g., from pump 101 detecting a diameter of syringe 109, operator
401, or
other external source).
27
CA 02663448 2009-04-21
At 1811, computer system 201 may determine if the comparisons of the actual
volume output to the nominal volume output and/or the comparisons of the
actual
controlled delivery rate to the nominal controlled delivery rate fall within
the
acceptable validation deviation (e.g., as determined/received at 1801). For
example,
the actual controlled delivery rate may be compared to the nominal controlled
delivery rate (e.g., the controlled delivery rate provided to pump 101 by
computer
system 201 for the corresponding time period (or, for example, the controlled
delivery
rate calculated by pump 101 for the corresponding time period)). In some
embodiments, comparison may include subtracting the actual volume output from
the
nominal volume output (or vice versa) and comparing the difference to an
acceptable
validation deviation (which may include a range of acceptable differences
between
the actual volume output and the nominal volume output). In some embodiments,
comparison may include subtracting the actual controlled delivery rate from
the
nominal controlled delivery rate (or vice versa) and comparing the difference
to an
acceptable validation deviation (which may include a range of acceptable
differences
between the actual controlled delivery rate and the nominal controlled
delivery rate).
Other statistical comparisons are also contemplated. As another example, the
weight
(or, for example, volume) of actual substance 119 delivered (collected
infusate) may
be plotted versus time along with a plot of the weight (or, for example,
volume) of
substance 119 that would be delivered versus time according to the nominal
controlled delivery rate. In some embodiments, operator 401 may review the
plots for
semi-automatic validation. In some embodiments, accuracy may be provided as a
+/-
X % accuracy (e.g., representative of the difference between the actual
controlled
delivery rate and the nominal controlled delivery rate). In some embodiments,
the
validation may be fully automatic (e.g., computer system 201 may compare
statistics
of the validation against acceptable validation ranges). In some embodiments,
indications of the success or failure of validation may be presented to
operator 401.
For example, accuracies falling out of the acceptable ranges may be reported
(e.g., to
operator 401) as pump 101 failing validation. Validation may be performed
prior to
(e.g., with a dummy substance 119), during (e.g., with the actual substance
119
delivered to animal 103), and/or after a lab animal infusion study. In some
embodiments, each pump 101 may be validated or a sampling of pumps 101 may be
validated. In some embodiments, if pump 101 fails validation, pump 101 may not
be
used until successfully validated. In some embodiments, automated validation
may
28
CA 02663448 2009-04-21
reduce the manpower needed to perform and send the calculations, reduce manual
calculation errors, and reduce manual data input errors. In some embodiments,
the
validations may be performed according to an automated schedule to reduce
scheduling errors. In addition, automated validations may allow for an
increased
validation frequency (e.g., pumps 101 may be validated before a study, one or
more
times during the study, and after the study).
In some embodiments, pumps 101 may be calibrated (e.g., on a regular basis
such as once a year). Calibration may include testing controlled delivery rate
accuracy over a period of time (e.g., comparing actual pump controlled
delivery rate
to instructed pump controlled delivery rate). Calibration may further include
comprehensive periodic checks to confirm proper pump functioning (e.g.,
several
aspects of pump 101 may be checked with sensors, etc. to insure proper
functioning).
In some embodiments, information related to the next calibration may be
stored, for
example, on computer system 201, pump 101, box 205, etc. Calibration
information
may include a date pump 101 was last calibrated, a next date pump 101 should
be
calibrated by, etc. Calibration information may be stored, for example, in
firmware in
pump 101 (or, for example, coupled to pump 101 (such as in memory 305)).
Calibration information may also be included on an outside of pump 101 (e.g.,
written
on a pump label). Computer system 201 (or executable instructions on box 205,
etc.)
may check the calibration information (e.g., prior to the beginning of a
study) and
may indicate (e.g., to operator 401) pumps 101 that have surpassed their
calibration
interval (or will surpass their calibration interval during the study). For
example, if
the calibration dates are stored at pumps 101, computer system 201 may poll
pumps
101 in the network for their calibration dates to determine if any of pumps
101 are
outside of their calibration period or will be outside the calibration period
at any time
during the next study. In some embodiments, computer system 201 (or, for
example,
box 205) may prevent use of pump 101 until pump 101 is calibrated and the
information stored for pump 101 indicates that the calibration is current. In
some
embodiments, a calibration database may include pump identifiers and
respective
calibration dates for pumps 101 (e.g., the calibration dates may not be stored
in the
pumps 101). In some embodiments, operators 401 may read calibration
information
on pump 101 (e.g., on an outer label) and may enter the calibration
information into
an interface on pump 101 and/or computer system 201 to be stored. Computer
system
29
CA 02663448 2009-04-21
201 may poll pumps 101 to determine pump identifiers (indicating which pumps
101
are currently coupled to the network) and compare this list of pumps 101 to
the
calibration database to determine if the current pumps 101 have current
calibration
dates. Computer system 201 may alert operator 401 as to which pumps 101 have
calibration problems to allow operator 401 to replace and/or calibrate the
problem
pumps 101. In some embodiments, computer system 201 (or, for example, box 205)
may calibrate pump 101 (e.g., using techniques described above). Other
calibration
techniques are also contemplated. Automating the calibration check may save
time,
assure compliance with documentation requirements, and reduce the risk of
human
error.
FIG. 19 illustrates a flowchart of a method for automated syringe filling,
according to an embodiment. It should be noted that in various embodiments of
the
methods described below, one or more of the elements described may be
performed
concurrently, in a different order than shown, or may be omitted entirely.
Other
additional elements may also be performed as desired.
At 1901, syringe 109 to be filled may be loaded onto pump 101. For example,
computer system 201 may instruct operator 401 to load syringe 109 onto a
filling
pump (which may be a pump 101). In some embodiments, computer system 201 may
instruct operator 401 to attach a vat holding substance 119 to be loaded into
syringe
109 to pump 101 (or the vat may already be attached to syringe 109 on pump
101). In
some embodiments, pumps 101 at animal cages 117 may fill syringe 109 (e.g.,
operator 401 may carry the vessel from pump 101 to pump 101 and the filling
instructions may be sent by computer system 201 to respective pump 101). For
example, pump 101 may be a bi-directional pump 101 capable of pulling the
appropriate fluid volume into syringe 109 (e.g., by pulling plunger of syringe
109 to
fill syringe 109). In some embodiments, pump 101 for filling syringe 109 may
be
located next to respective animal cage 117 or may be a separate pump 101
(e.g.,
communicatively coupled to computer system 201 but not necessarily at animal
cage
117).
At 1903, computer system 201 may determine an amount of substance 119 to
be filled into syringe 109. For example, computer system 201 may determine an
CA 02663448 2009-04-21
amount of substance 119 needed for a next round of delivery for a respective
animal
103 (e.g., based on a controlled delivery rate assigned to animal 103). In
some
embodiments, computer system 201 may determine an amount of substance 119 to
be
delivered by pump 101 during a next phase of the study and the amount may be
communicated to pump 101.
At 1905, filling pump 101 may fill syringe 109 with the amount of substance
119 directed by computer system 201. For example, pump 101 may pull the
syringe
plunger backward to aspirate fluid (e.g., substance 119) from a vessel into
syringe 109
until the directed amount is in syringe 109. In some embodiments, the filling
pump
101 may operate in a reverse direction of pumps 101 delivering substance 119
to
animal 103 (e.g., at the animal cages 117). Pump 101 may aspirate an
appropriate
volume of substance 119 on an animal-by-animal (pump-by-pump) basis (e.g., for
different syringes 109). In some embodiments, operator 401 may instruct pump
101
(e.g., at animal cage 117) to enter a filling mode and pump 101 may receive
data from
computer system 201 for the proper fill amount. In some embodiments, pump 101
may be controlled by computer system 201 (or, for example, box 205 coupled to
pump 101) to load syringe 109 with a predetermined amount of substance 119.
Pump
101 and/or computer system 201 may also specify to operator 401 what type of
substance 119 to load into syringe 109 (and operator 401 may attach the
appropriate
vat of substance 119). In some embodiments, operator 401 may receive an
indicator
such as "Vat A" instead of or in addition to the specific type of substance
119 to load
into syringe 109 (e.g., in a blind study). In some embodiments, syringe 109
may be
loaded several times a day.
At 1907, an indicator may be provided on syringe 109. For example, operator
401 may write the animal identification (ID) (e.g., of the respective animal
to receive
the substance) and sequence of use data on syringe 109. As another example, an
attached printhead may apply the data onto syringe 109 (e.g., automatically
and/or by
operator 401) (which may be printed directly on the syringe 109 or on a label
to be
coupled to the syringe 109). In some embodiments, operator 401 may apply a
label
generated by an attached label printer. In some embodiments, a printer (e.g.,
coupled
to computer system 201, pump 101, etc.) may print a label for syringe 109
(e.g., with
a pump identifier, the substance type, amount, animal identifier, etc.) Other
31
CA 02663448 2009-04-21
information may also be printed onto the label. The label may be attached to
syringe
109 (e.g., by operator 401). In some embodiments, a separate pump 101 may be
used
to fill syringes 109 (e.g., at a dedicated filling station (which may also
have a
printer)). Other filling techniques are also contemplated. Automating filling
the
syringe may decrease manpower needed to fill the syringe, reduce manual
calculation
errors and reduce manual data input errors.
In some embodiments, computer system 201 may display and/or print out a
list (e.g., list 1505 in FIG. 15b) of dosages for future syringes 109. For
example,
computer system 201 may determine a dosage amount needed for multiple syringes
109 based on the respective animal weights, dosage ratios, etc. The dosage
(e.g., a
substance volume) for each syringe 109 may be displayed and/or printed with an
identifier for pump ID, animal ID 103, dosage, approximate time/day for next
syringe
change, syringe type (e.g., syringe volume), etc. The displayed or printed
list 1505
may allow operator 401 to pre-load syringes 109 in advance (e.g., without
performing
additional calculations). In some embodiments, animals 103 may be reweighed
weekly (or other time interval) and the future syringes 109 for a week may be
displayed (beyond a week, computer system 201 may need a new weight for animal
103 and therefore, may not be able to provide a listing past the current
week). Other
weigh in times (e.g., continuous, once a day, once a month, etc.) are also
contemplated. The future syringe print outs may reduce manpower needed to
perform
the calculations, reduce manual calculation errors, and reduce manual data
input
errors.
In some embodiments, pump 101 may measure a size of syringe 109 (e.g.,
may detect a diameter of syringe 109). Pumps 101 may include a mechanism for
determining a diameter of a loaded syringe 109 (e.g., a lever arm coupled to a
gear to
measure the diameter of syringe 109). In the lever arm example, the gear may
detect
a displacement of the lever arm when syringe 109 is placed between the lever
arm and
pump 101. Other diameter detections are also contemplated. A study may use a
syringe of saline solution in an intermittent infusion profile (or a KVO (Keep
Vein
Open) solution to prevent catheter clotting) and a different sized syringe for
a test
article (TA) solution (e.g., the new chemical entity to be tested). Syringe
109 with the
KVO solution may have a larger diameter than syringe 109 for the test
solution. For
32
CA 02663448 2009-04-21
example, the KVO solution syringe may be a 20 cubic centimeter (cc) syringe
used to
deliver saline solution to animal 103 for 23 hours and the test solution
syringe may be
a 5 cc syringe used to deliver a test solution to animal 103 for one hour.
Other sizes
and times are also contemplated. In some embodiments, pump 101 may detect the
size (e.g., diameter and/or length) of syringe 109 in pump 101 and, if syringe
109 size
does not correspond to syringe 109 that pump 101 is assigned to be pumping
(e.g., as
noted by instructions from computer system 201 stored, for example, in the box
memory), pump 101 may give operator 401 an indicator, sound an alarm, and/or
not
pump syringe 109. Pump 101 may reduce human loading error to insure compliance
with the provided infusion profile. In some embodiments, operator 401 may
input
information about syringe 109 (e.g., type of syringe, brand of syringe, size
of syringe,
syringe identifier, etc.) into pump 101 and/or computer system 201. The
information
may be stored and/or used to verify that the correct syringe 109 has been
loaded.
FIG. 20 illustrates a flowchart of an embodiment for study documentation.
Computer system 201 may communicate with pumps 101 and/or other medical or
monitoring devices involved in the study to document events occurring in the
study
(e.g., start times, stop times, alarms, how alarms were cleared, animal
weights,
amount of feed/water consumed, etc). These events may also be stored with
respective user identifiers 1201 to identify operators 401 associated with the
events
(e.g., to identify operator 401 who cleared an alarm). The documentation may
be
used to support the validity of the study. It should be noted that in various
embodiments of the methods described below, one or more of the elements
described
may be performed concurrently, in a different order than shown, or may be
omitted
entirely. Other additional elements may also be performed as desired.
At 2001, user identifier 1201 may be received at computer system 201. In
some embodiments, operator 401 may enter user identifier 1201 (e.g., an
identifier
such as a PIN code or, for example, a pre-assigned (by computer system 201)
alpha
numeric user code unique to operator 401) into pump 101 and/or medical or
monitoring device. Other user identifiers 1201 are also contemplated (e.g.,
operator
401 may enter their name as user identifier 1201, scan a bar code (e.g., on
the
operator's uniform), swipe a magnetic card with user identifier 1201,
biometric scan
(e.g., scanning an user's thumbprint or retina), Radio Frequency
Identification (e.g.,
33
CA 02663448 2009-04-21
transmitted from a PDA, etc)). User identifier 1201 may be sent to computer
system
201 for storage relative to the actions performed by (or other documentation
submitted by) operator 401. For example, in responding to an alarm, operator
401
may enter user identifier 1201 (e.g., into the pump interface or into computer
system
201) assigned to that operator 401 prior to taking action to correct the
alarm. The
alarm may be indicated on computer system 201 and/or a communication (such as
an
email, short message service (SMS), etc.) may be sent to operator 401. The
communication may include a pump identifier and an alarm type indicator (e.g.,
indicating why the alarm sounded). For example, if a pressure transducer on
pump
101 detects an occlusion in the delivery tube, pump 101 may indicate an alarm
and
send a communication.
At 2003, operator 401 may be authenticated based on the received user
identifier 1201. In some embodiments, user identifier 1201 may be used by pump
101
(or, for example, computer system 201, etc.) to authenticate operator 401
prior to
allowing operator 401 to take action on pump 101. User identifier 1201 may
thus act
as user stamp/e-signature for the actions taken by operator 401. In some
embodiments, operator 401 may be authenticated prior to taking action on other
devices (e.g., computer system 201, medical devices, monitoring devices,
animal cage
117, etc). In some embodiments, authentication may include comparing the
received
user identifier 1201 to user identifiers 1201 stored in an authentication
database.
Other authentication is also contemplated. In some embodiments, user
identifiers
1201 may be changed for each study (e.g., by a study administrator who may set
up
which operators 401 are authorized to interact with the study equipment).
At 2005, computer system 201 may receive a documentation indicator
associated with pump 101 (or other equipment). For example, documentation
indicators (e.g., see documentation indicator 1601 in FIG. 16) may correspond
to
events (such as starting pump 101, responding to an alarm, stopping pump 101,
etc.)
and actions taken by operators 401 in response to the events. Documentation
indicators 1601 may also correspond to information related to general and/or
specific
observations by operator 401 (e.g., animal 103 is sick) which may or may not
be event
specific. Documentation indicators 1601 may include a cause of an alarm.
Alarms
(e.g., as discussed above) may occur when equipment (e.g., pump 101) or other
34
CA 02663448 2009-04-21
variables (e.g., health conditions of animal 103) in the study encounter a
problem.
For example, pump 101 may encounter a problem such as occlusion in delivery
tube
105, low battery, no power, empty syringe, etc. When problems occur, an alarm
may
sound (or in some way be indicated to operator 401). Actions taken to clear an
alarm
may be entered (e.g., by operator 401 into a graphical interface on pump 101
or
computer system 201) and a corresponding documentation indicator 1601 may be
assigned. In some embodiments, operator 401 may be presented with menu (e.g.,
a
drop down menu) and other options at computer system 201 and/or pump 101
(operator may have flexibility to document the event and/or enter other
information
(e.g., observations and/or non-event related information) at pump 101 or
computer
system 201). The menu may be specific to the type of alarm encountered. For
example, if an alarm is triggered because of a kinked delivery tube, the alarm
menu
provided to operator 401 may include options for how the kinked delivery tube
was
fixed (e.g., "1: Tube unkinked"; "2: Tube replaced"; "3: Other"). In some
embodiments, pump 101 may determine what caused the alarm, the actions taken
by
operator 401 to fix the alarm, etc. and may transmit appropriate documentation
indicators 1601 to computer system 201. In some embodiments, operators 401 may
enter documentation indicators 1601 indicative of what caused the alarm, the
actions
taken to clear the alarm, etc. into a pump interface (and/or computer system
interface).
Other interfaces are also contemplated (e.g., operators 401 may enter
documentation
indicators into a PDA which may transmit the documentation indicators 1601 to
computer system 201 and/or pump 101 (e.g., to be transmitted to computer
system
201)). Documentation indicators 1601 may also be stored relative to events not
corresponding to an operator's actions (e.g., documentation indicator 1601 may
be
stored to indicate the occurrence of the alarm). Documentation indicators 1601
may
be textual descriptions (e.g., "Alarm cleared by refilling syringe").
Documentation
indicators 1601 may also be numerical or alpha-numerical (e.g., numbers or
alpha
numeric entries linked to textual description, for example, through a look-up
table).
Other documentation indicators 1601 are also contemplated. In some
embodiments,
operators 401 may define menus and menu selections for receiving documentation
indicators. For example, operators 401 may define a menu for a specific type
of
alarm and the menu may be provided to pump 101 for presentation the next time
that
alarm is triggered. Operator 401 may respond to the alarm by entering
appropriate
CA 02663448 2009-04-21
menu selections and the information may be stored in computer system 201 as
documentation indicators (e.g., along with the respective user identifiers
1201).
At 2007, computer system 201 may store user identifier 1201 and
documentation indicator 1601. In some embodiments, computer system 201 may
store
corresponding documentation indicators 1601 for the operator's actions.
Operator
401 may respond to the alarm and indicate on pump 101 (e.g., using a pump
keypad
and menu options presented on the pump display) the cause of the problem
and/or
how the problem was fixed. Information about the alarm, the technician
identification
(e.g., user identifier 1201), how the alarm was fixed, etc. may be entered
into
computer system 201 by operator 401 or may be entered into pump 101 and
relayed to
computer system 201 to be stored (e.g., in an electronic log) (see, for
example, FIG.
16). Computer system 201 may store user identifiers 1201 with the
corresponding
documentation indicators 1601 (and, for example, a pump identifier or other
device
identifier).
At 2009, computer system 201 (and/or pump 101 or other equipment) may
require a separate user identifier 1201 for separate documentation indicators
1601 to
be stored with the separate documentation indicators 1601. In some
embodiments,
operator 401 may enter their user identifier 1201 prior to each action
operator 401
takes on pump 101 (or in relationship to animal cage 117, medical, and/or
monitoring
device). In some embodiments, operator 401 may be required to enter user
identifier
1201 prior to any intervention with pump 101 (or other equipment). For
example, if
user identifier 1201 for operator 401 is "231" and operator 401 starts and
stops pump
101, operator 401 may be required to enter "231" prior to pressing a button to
start
pump 101 and enter "231" again prior to stopping pump 101. Computer system 201
may log documentation indicators 1601 with user identifiers 1201 (e.g., "231
start
pump; 231 stop pump"). In some embodiments, computer system 201 may store a
time and/or date with documentation indicators 1601. In some embodiments,
computer system 201 may prompt operator 401 for additional documentation at
computer system 201. For example, in clearing an alarm, operator 401 may
indicate
at pump 101 "other" for how alarm was cleared (e.g., using menu options
provided at
pump 101). Computer system 201 may then blink a screen of computer system 201,
provide an alert indicator, or in some other fashion request additional
description from
36
CA 02663448 2009-04-21
the operator 401 as to how the pump alarm was cleared (or for other prior pump
or
equipment interactions). Operator 401 may enter one or more phrases,
sentences, etc.
in a text box that may be saved with log information for the respective pump
101.
Other documentation may also be required of operator 401 (e.g., documentation
may
be requested for why pump 101 was stopped, why animal 103 was removed from
animal cage 117, etc). In some embodiments, pump 101 may require operator 401
to
enter information about the alarm (e.g., cause of problem, how the problem was
fixed,
etc.) prior to allowing operator 401 to continue pump operations (e.g.,
restart pump
101). This may force documentation of the alarm and the solution. In some
embodiments, operator 401 may select "Other" in the menu options of the alarm.
Operator 401 may then be prompted (e.g., at computer system 201) to enter
additional
information (e.g., a written statement of the problem solution) at computer
system
201. In some embodiments, operator 401 may be required to enter the additional
documentation before the pump 101 will be allowed to resume. In some
embodiments, computer system 201 may prevent operator's future access to
computer
system 201 or pump 101 until the required documentation is entered. This may
improve documentation by reducing human error (intentional and inadvertent)
and
enforcing compliance with protocols for documentation including documentation
requirements.
In some embodiments, a graphical profile of a substance delivery for a
respective pump 101 may be displayed by computer system 201. For example, as
seen in FIG. 15, the amount of the substance delivered (Y axis) over time (X
axis)
may be plotted as graphical profile line 1501. The Y axis may also be
substance
volume/body weight and the graphical profile may represent substance volume
per
weight per time unit. The graphical profile may make it easier for operator
401 to see
when the syringe changes occur, what types of syringes are being exchanged
(e.g.,
size of syringes being exchanged), etc. The profile may present a preview
(e.g.,
which may be printed out) for one or more pumps for the study. The graphical
profile
may assist operator 401 in confirming proper infusion profile input and better
visualize a sequence of future pump activities.
In some embodiments, indicator 1503 may be displayed on the graphical
profile to indicate a current status of the substance delivery (e.g., where in
the profile
37
CA 02663448 2009-04-21
the current pump 101 is in the study (e.g., see line 1503)). Line 1503 may be
in a
different color (e.g., red) than graphical profile line 1501. Other graphical
indicators
1503 are also contemplated (e.g., asterisk, arrow, etc). In some embodiments,
by
viewing indicator 1503, operator 401 may be able to graphically determine a
current
controlled delivery rate and substance type being delivered by the selected
pump 101
(operator 401 may also select other respective pumps 101 to view their
respective
profiles). In some embodiments, indicator 1503 may assist operator 401 in
determining what point in the infusion profile pump 101 is current operating.
For
example, operator 401, upon viewing indicator 1503, may determine whether pump
101 is at a point in the infusion profile for a KVO syringe or a TA syringe.
Fig. 21 illustrates an embodiment of a WAN 2102 and a LAN 2104. WAN 2102
may be a network that spans a relatively large geographical area. Internet 211
is an
example of a WAN 2102. WAN 2102 typically includes a plurality of computer
systems
that may be interconnected through one or more networks. Although one
particular
configuration is shown in Fig. 21, WAN 2102 may include a variety of
heterogeneous
computer systems and networks that may be interconnected in a variety of ways
and that
may run a variety of software applications.
One or more LANs 2104 may be coupled to WAN 2102. LAN 2104 may be a
network that spans a relatively small area. Typically, LAN 2104 may be
confined to a
single building or group of buildings. Each node (i.e., individual computer
system or
device) on LAN 2104 may have its own Central Processing Unit (CPU) with which
it
may execute programs. Each node may also be able to access data and devices
anywhere on LAN 2104. LAN 2104, thus, may allow many users to share devices
(e.g., printers) and data stored on file servers. LAN 2104 may be
characterized by a
variety of types of topology (i.e., the geometric arrangement of devices on
the
network), of protocols (i.e., the rules and encoding specifications for
sending data,
and whether the network uses a peer-to-peer or client/server architecture),
and of
media (e.g., twisted-pair wire, coaxial cables, fiber optic cables, and/or
radio waves).
Each LAN 2104 may include a plurality of interconnected computer systems
(e.g., computers 201, 215a, 215b, 215c, etc.) and optionally one or more other
devices. For example, LAN 2104 may include one or more workstations 2110a, one
38
CA 02663448 2009-04-21
or more personal computers 2112a, one or more laptop or notebook computer
systems
2114, one or more server computer systems 2116 (e.g., server 207), and one or
more
network printers 2118. As illustrated in Fig. 21, an example LAN 2104 may
include
one of each computer systems 2110a, 2112a, 2114, and 2116, and one printer
2118.
LAN 2104 may be coupled to other computer systems and/or other devices and/or
other LANs through WAN 2102.
One or more mainframe computer systems 2120 may be coupled to WAN
2102. As shown, mainframe 2120 may be coupled to a storage device or file
server
2124 and mainframe terminals 2122a, 2122b, and 2122c. Mainframe terminals
2122a, 2122b, and 2122c may access data stored in the storage device or file
server
2124 coupled to or included in mainframe computer system 2120.
WAN 2102 may also include computer systems connected to WAN 2102
individually and not through LAN 2104. For example, workstation 2110b and
personal computer 2112b may be connected to WAN 2102. For example, WAN 2102
may include computer systems that may be geographically remote and connected
to
each other through the Internet.
Fig. 22 illustrates an embodiment of computer system 201 that may be suitable
for implementing various embodiments of a system and method for test animal
substance delivery and monitoring. Each computer system 201 typically includes
components such as CPU 2252 with an associated memory medium such as Compact
Disc Read Only Memories (CD-ROMs) 2260. The memory medium may store
program instructions for computer programs. The program instructions may be
executable by CPU 2252. Computer system 201 may further include a display
device
such as monitor 2254, an alphanumeric input device such as keyboard 2256, and
a
directional input device such as mouse 2258. Computer system 201 may be
operable
to execute the computer programs to implement computer-implemented systems and
methods for test animal substance delivery and monitoring.
Computer system 201 may include a memory medium on which computer
programs according to various embodiments may be stored. The term "memory
medium" is intended to include an installation medium, e.g., floppy disks or
Compact
39
CA 02663448 2009-04-21
Disc Read Only Memories (CD-ROMs) 2260, a computer system memory such as
Dynamic Random Access Memory (DRAM), Static Random Access Memory
(SRAM), Extended Data Out Random Access Memory (EDO RAM), Double Data
Rate Random Access Memory (DDR RAM), Rambus Random Access Memory
(RAM), etc., or a non-volatile memory such as a magnetic media, e.g., a hard
drive or
optical storage. The memory medium may also include other types of memory or
combinations thereof. In addition, the memory medium may be located in a first
computer, which executes the programs or may be located in a second different
computer, which connects to the first computer over a network. In the latter
instance, the
second computer may provide the program instructions to the first computer for
execution. Computer system 201 may take various forms such as a personal
computer
system, mainframe computer system, workstation, network appliance, Internet
appliance,
PDA, television system or other device. In general, the tenn "computer system"
may
refer to any device having a processor that executes instructions from a
memory
medium.
The memory medium may store a software program or programs operable to
implement a method for test animal substance delivery and monitoring. The
software
program(s) may be implemented in various ways, including, but not limited to,
procedure-based techniques, component-based techniques, and/or object-oriented
techniques, among others. For example, the software programs may be
implemented
using ActiveX controls, C++ objects, JavaBeans, Microsoft Foundation Classes
(MFC),
browser-based applications (e.g., Java applets), traditional programs, or
other
technologies or methodologies, as desired. A CPU such as host CPU 2252
executing
code and data from the memory medium may include a means for creating and
executing the software program or programs according to the embodiments
described
herein.
Various embodiments may also include receiving or storing instructions
and/or data implemented in accordance with the foregoing description upon a
carrier
medium. Suitable carrier media may include storage media or memory media such
as
magnetic or optical media, e.g., disk or CD-ROM, as well as signals such as
electrical,
electromagnetic, or digital signals, may be conveyed via a communication
medium
such as a network and/or a wireless link.
CA 02663448 2009-04-21
Embodiments of a subset or all (and portions or all) of the above may be
implemented by program instructions stored in a memory medium or carrier
medium
and executed by a processor. A memory medium may include any of various types
of
memory devices or storage devices. The term "memory medium" is intended to
include an installation medium, e.g., a Compact Disc Read Only Memory (CD-
ROM),
floppy disks, or tape device; a computer system memory or random access memory
such as Dynamic Random Access Memory (DRAM), Double Data Rate Random
Access Memory (DDR RAM), Static Random Access Memory (SRAM), Extended
Data Out Random Access Memory (EDO RAM), Rambus Random Access Memory
(RAM), etc.; or a non-volatile memory such as a magnetic media, e.g., a hard
drive, or
optical storage. The memory medium may comprise other types of memory as well,
or combinations thereof. In addition, the memory medium may be located in a
first
computer in which the programs are executed, or may be located in a second
different
computer that connects to the first computer over a network, such as the
Internet. In
the latter instance, the second computer may provide program instructions to
the first
computer for execution. The term "memory medium" may include two or more
memory mediums that may reside in different locations, e.g., in different
computers
that are connected over a network.
In some embodiments, a computer system at a respective participant location
may include a memory medium(s) on which one or more computer programs or
software components according to one embodiment of the present invention may
be
stored. For example, the memory medium may store one or more programs that are
executable to perform the methods described herein. The memory medium may also
store operating system software, as well as other software for operation of
the
computer system.
In this patent, certain U.S. patents, U.S. patent applications, and other
materials (e.g., articles) have been incorporated by reference. The text of
such U.S.
patents, U.S. patent applications, and other materials is, however, only
incorporated
by reference to the extent that no conflict exists between such text and the
other
statements and drawings set forth herein. In the event of such conflict, then
any such
conflicting text in such incorporated by reference U.S. patents, U.S. patent
41
CA 02663448 2009-04-21
applications, and other materials is specifically not incorporated by
reference in this
patent.
Further modifications and alternative embodiments of various aspects of the
invention may be apparent to those skilled in the art in view of this
description.
Accordingly, this description is to be construed as illustrative only and is
for the
purpose of teaching those skilled in the art the general manner of carrying
out the
invention. It is to be understood that the forms of the invention shown and
described
herein are to be taken as embodiments. Elements and materials may be
substituted for
those illustrated and described herein, parts and processes may be reversed,
and
certain features of the invention may be utilized independently, all as would
be
apparent to one skilled in the art after having the benefit of this
description of the
invention. Changes may be made in the elements described herein without
departing
from the spirit and scope of the invention as described in the following
claims.
42