Note: Descriptions are shown in the official language in which they were submitted.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
1
APPARATUS AND METHOD FOR MAKING A PEROXYCARBOXYLIC ACID
Field of the Invention
The present invention relates to apparatus and methods for making a
peroxycarboxylic acid. The apparatus includes a reaction catalyst and a
pretreatment
column for pretreating one or more reagents, which can increase the life,
activity, and/or
safety of the reaction catalyst. The peroxycarboxylic acid compositions made
by the
method and apparatus can include one or more peroxycarboxylic acids.
Background of the Invention
Present methods for making peroxycarboxylic acids include mixing a carboxylic
acid or anhydride with an oxidizing agent, such as hydrogen peroxide, in water
and
waiting. At ambient conditions, the reaction can take a week or more to reach
desirable
concentrations of peroxycarboxylic acid at equilibrium. In addition,
regulations
regarding and practices in shipping of ingredients such as hydrogen peroxide
and acetic
acid can limit the concentration, stability, content, or purity of these
reagents and,
therefore, of the resulting peroxycarboxylic acid. For example, acetic acid
inevitably
contains metal due to common shipping and handling practices. Conventional
peroxycarboxylic acid compositions typically include short chain
peroxycarboxylic
acids or mixtures of short chain peroxycarboxylic acids and medium chain
peroxycarboxylic acids (see, e.g., U.S. Patent Nos. 5,200,189, 5,314,687,
5,409,713,
5,437,868, 5,489,434, 6,674,538, 6,010,729, 6,111,963, and 6,514,556).
Ongoing research efforts have strived for improved peroxycarboxylic acid
compositions and methods of making them. In particular, these efforts have
strived for
methods that can more rapidly make purer and/or more stable peroxycarboxylic
compositions even at a point of use.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
2
Summary of the Invention
The present invention relates to apparatus and methods for making a
peroxycarboxylic acid. The apparatus includes a reaction catalyst and a
pretreatment
column for pretreating one or more reagents, which can increase the life,
activity, and/or
safety of the reaction catalyst. The peroxycarboxylic acid compositions made
by the
method and apparatus can include one or more peroxycarboxylic acids.
The present invention includes an apparatus for making peroxycarboxylic acid.
In an embodiment, this apparatus can include a first pretreatment column, a
first
reaction catalyst column, a first and a second reagent vessel, a plurality of
conduits, and
a safety system. The first and second reagent vessels are in fluid
communication with
the first pretreatment column. The first pretreatment column is in fluid
communication
with the first reaction catalyst column. The first reaction catalyst column
can be in fluid
communication with a site of storage or use of the peroxycarboxylic acid
composition.
The first reagent vessel can be configured for containing a liquid hydrogen
peroxide
composition and the second reagent vessel can be configured for containing a
liquid
carboxylic acid composition. The safety system can be configured to measure
temperature, pressure, metal content, or combination thereof of the hydrogen
peroxide
and carboxylic acid composition in, at, or before entry to the pretreatment
column.
The present method includes a method for making a peroxycarboxylic acid. In
an embodiment the method includes pretreating a liquid composition of a
carboxylic
acid, hydrogen peroxide, or both with a pretreatment column. The method can
optionally include mixing the pretreated liquid composition with a liquid
composition
of carboxylic acid, hydrogen peroxide or both to yield a composition
comprising
carboxylic acid and hydrogen peroxide. The method then includes reacting the
composition comprising carboxylic acid and hydrogen peroxide in the presence
of a
reaction catalyst to produce a peroxycarboxylic acid composition and
recovering the
peroxycarboxylic acid composition. The method includes monitoring temperature,
pressure, or metal content of the carboxylic acid, hydrogen peroxide, or both
before
pretreating, during pretreating, or both. If the temperature, difference in
temperatures,
pressure, difference in pressures, metal content, or difference in metal
content exceeds a
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
3
predetermined value, the method includes actuating a pressure release valve,
stopping
flow of one or more reagents, causing water to flow into the apparatus,
causing
carboxylic acid composition to flow into the apparatus, shutting down the
method, or a
combination thereof.
Brief Description of the Drawings
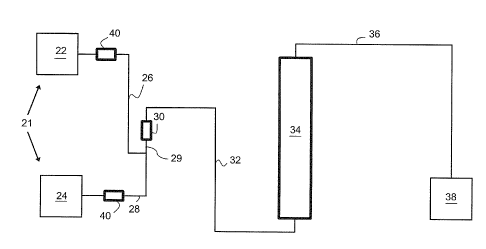
Figures 1-5 schematically represent embodiments of apparatus that generates
peroxycarboxylic acid including embodiments of pretreatment column and
reaction
catalyst.
Figure 6 schematically represents embodiments of the safety system and
pretreatment column.
Figures 7-10 schematically represent embodiments of apparatus that generates
peroxycarboxylic acid including embodiments of pretreatment column, reaction
catalyst, and safety system.
Figure 11 schematically represents an embodiment of apparatus that generates
peroxycarboxylic acid in fluid communication with an embodiment of aseptic
packaging system.
Figures 12 and 13 schematically represent embodiments of apparatus that
generates peroxycarboxylic acid including embodiments of pretreatment column
and
reaction catalyst.
Figure 14 schematically represents embodiments of the safety system and
reaction catalyst.
Figure 15 schematically represents embodiments of apparatus that generates
peroxycarboxylic acid including an embodiment of storage system.
Figure 16 is a flow chart illustrating an embodiment of a process by which the
controller monitors and/or regulates the concentrations of peroxycarboxylic
acid and/or
of hydrogen peroxide in the use composition.
Figure 17 is a flowchart illustrating an embodiment of a "generator check"
process by which the controller monitors and regulates operation of
peroxycarboxylic
acid generator.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
4
Figure 18 is a diagram of a beverage plant, including a cold aseptic filling
plant,
in which either carbonated or non-carbonated beverages can be prepared and
bottled.
Various embodiments of the present invention will be described in detail with
reference to the drawings, wherein like reference numerals represent like
parts
throughout the several views. Reference to various embodiments does not limit
the
scope of the invention, which is limited only by the scope of the claims.
Detailed Description of the Invention
Definitions
As used herein, the phrase "medium chain carboxylic acid" refers to a
carboxylic acid that: 1) has reduced or is lacking odor compared to the bad,
pungent, or
acrid odor associated with an equal concentration of small chain carboxylic
acid, and 2)
has a critical micellar concentration greater than 1 mM in aqueous buffers at
neutral pH.
Medium chain carboxylic acids exclude carboxylic acids that are infinitely
soluble in or
miscible with water at 20 C. Medium chain carboxylic acids include carboxylic
acids
with boiling points (at 760 mm Hg pressure) of 180 to 300 C. In an
embodiment,
medium chain carboxylic acids include carboxylic acids with boiling points (at
760 mm
Hg pressure) of 200 to 300 C. In an embodiment, medium chain carboxylic acids
include those with solubility in water of less than 1 g/L at 25 C. Examples
of medium
chain carboxylic acids include pentanoic acid, hexanoic acid, heptanoic acid,
octanoic
acid, nonanoic acid, decanoic acid, undecanoic acid, and dodecanoic acid.
As used herein, the phrase "medium chain peroxycarboxylic acid" refers to the
peroxycarboxylic acid form of a medium chain carboxylic acid.
As used herein, the phrase "short chain carboxylic acid" refers to a
carboxylic
acid that: 1) has characteristic bad, pungent, or acrid odor, and 2) is
infinitely soluble in
or miscible with water at 20 C. Examples of short chain carboxylic acids
include
formic acid, acetic acid, propionic acid, and butyric acid.
As used herein, the phrase "short chain peroxycarboxylic acid" refers to the
peroxycarboxylic acid form of a short chain carboxylic acid.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
As used herein the term "inert metal cations" refers to those metal cations
which
are substantially unreactive (e.g., do not undergo an undesirable level of
reaction) or
unreactive with hydrogen peroxide or a peroxycarboxylic acid (i.e., peroxygen
species).
For example, sodium and potassium are inert metal cations, whereas iron and
copper are
5 not.
As used herein, the term "insoluble" is used to describe a substance that does
not
dissolve to give more than a negligible concentration (e.g., < 0.1 mg/mL) in a
carrier or
solvent employed for the carboxylic acid, oxidizing agent, peroxycarboxylic
acid, or
combination thereof to give a reasonable concentration.
As used herein, a composition or combination "consisting essentially" of
certain
ingredients refers to a composition including those ingredients and lacking
any
ingredient that materially affects the basic and novel characteristics of the
composition
or method. The phrase "consisting essentially of" excludes from the claimed
compositions and methods a sequestrant, builder, chelating agent, or
stabilizing agent;
unless such a process or ingredient is specifically listed after the phrase.
As used herein, a composition or combination "substantially free of' one or
more ingredients refers to a composition that includes none of that ingredient
or that
includes only trace or incidental amounts of that ingredient. Trace or
incidental
amounts can include the amount of the ingredient found in another ingredient
as an
impurity or stabilizer or that is generated in a minor side reaction during
formation or
degradation of the peroxycarboxylic acid. For example, commercially available
hydrogen peroxide often contains minor amounts of a stabilizer such as a tin
compound
or in some cases trace amounts of HEDP.
As used herein, the phrases "objectionable odor", "offensive odor", or
"malodor" refer to a sharp, pungent, or acrid odor or atmospheric environment
from
which a typical person withdraws if they are able to. Hedonic tone provides a
measure
of the degree to which an odor is pleasant or unpleasant. An "objectionable
odor",
"offensive odor", or "malodor" has an hedonic tone rating it as unpleasant as
or more
unpleasant than a solution of 5 wt-% acetic acid, propionic acid, butyric
acid, or
mixtures thereof.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
6
As used herein, the term "microorganism" refers to any noncellular or
unicellular (including colonial) organism. Microorganisms include all
prokaryotes.
Microorganisms include bacteria (including cyanobacteria), lichens, fungi,
protozoa,
virinos, viroids, viruses, phages, and some algae. As used herein, the term
"microbe" is
synonymous with microorganism.
As used herein, the term "object" refers to a something material that can be
perceived by the senses, directly and/or indirectly. Objects include a
surface, including
a hard surface (such as glass, ceramics, metal, natural and synthetic rock,
wood, and
polymeric), an elastomer or plastic, woven and non-woven substrates, a food
processing
surface, a health care surface, and the like. Objects also include a food
product (and its
surfaces); a body or stream of water or a gas (e.g., an air stream); and
surfaces and
articles employed in hospitality and industrial sectors. Objects also include
the body or
part of the body of a living creature, e.g., a hand.
As used herein, the phrase "food product" includes any food substance that
might require treatment with an antimicrobial agent or composition and that is
edible
with or without further preparation. Food products include meat (e.g. red meat
and
pork), seafood, poultry, fruits and vegetables, eggs, living eggs, egg
products, ready to
eat food, wheat, seeds, roots, tubers, leafs, stems, corms, flowers, sprouts,
seasonings,
or a combination thereof. The term "produce" refers to food products such as
fruits and
vegetables and plants or plant-derived materials that are typically sold
uncooked and,
often, unpackaged, and that can sometimes be eaten raw.
As used herein, the phrase "plant product" includes any plant substance or
plant-
derived substance that might require treatment with an antimicrobial agent or
composition. Plant products include seeds, nuts, nut meats, cut flowers,
plants or crops
grown or stored in a greenhouse, house plants, and the like. Plant products
include
many animal feeds.
As used herein, a processed fruit or vegetable refers to a fruit or vegetable
that
has been cut, chopped, sliced, peeled, ground, milled, irradiated, frozen,
cooked (e.g.,
blanched, pasteurized), or homogenized. As used herein a fruit or vegetable
that has
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
7
been washed, colored, waxed, hydro-cooled, refrigerated, shelled, or had
leaves, stems
or husks removed is not processed.
As used herein, the phrase "meat product" refers to all forms of animal flesh,
including the carcass, muscle, fat, organs, skin, bones and body fluids and
like
components that form the animal. Animal flesh includes the flesh of mammals,
birds,
fishes, reptiles, amphibians, snails, clams, crustaceans, other edible species
such as
lobster, crab, etc., or other forms of seafood. The forms of animal flesh
include, for
example, the whole or part of animal flesh, alone or in combination with other
ingredients. Typical forms include, for example, processed meats such as cured
meats,
sectioned and formed products, minced products, finely chopped products,
ground meat
and products including ground meat, whole products, and the like.
As used herein the term "poultry" refers to all forms of any bird kept,
harvested,
or domesticated for meat or eggs, and including chicken, turkey, ostrich, game
hen,
squab, guinea fowl, pheasant, quail, duck, goose, emu, or the like and the
eggs of these
birds. Poultry includes whole, sectioned, processed, cooked or raw poultry,
and
encompasses all forms of poultry flesh, by-products, and side products. The
flesh of
poultry includes muscle, fat, organs, skin, bones and body fluids and like
components
that form the animal. Forms of animal flesh include, for example, the whole or
part of
animal flesh, alone or in combination with other ingredients. Typical forms
include, for
example, processed poultry meat, such as cured poultry meat, sectioned and
formed
products, minced products, finely chopped products and whole products.
As used herein, the phrase "poultry debris" refers to any debris, residue,
material, dirt, offal, poultry part, poultry waste, poultry viscera, poultry
organ,
fragments or combinations of such materials, and the like removed from a
poultry
carcass or portion during processing and that enters a waste stream.
As used herein, the phrase "food processing surface" refers to a surface of a
tool,
a machine, equipment, a structure, a building, or the like that is employed as
part of a
food processing, preparation, or storage activity. Examples of food processing
surfaces
include surfaces of food processing or preparation equipment (e.g., slicing,
canning, or
transport equipment, including flumes), of food processing wares (e.g.,
utensils,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
8
dishware, wash ware, and bar glasses), and of floors, walls, or fixtures of
structures in
which food processing occurs. Food processing surfaces are found and employed
in
food anti-spoilage air circulation systems, aseptic packaging sanitizing, food
refrigeration and cooler cleaners and sanitizers, ware washing sanitizing,
blancher
cleaning and sanitizing, food packaging materials, cutting board additives,
third-sink
sanitizing, beverage chillers and warmers, meat chilling or scalding waters,
autodish
sanitizers, sanitizing gels, cooling towers, food processing antimicrobial
garment
sprays, and non-to-low-aqueous food preparation lubricants, oils, and rinse
additives.
As used herein, the phrase "air streams" includes food anti-spoilage air
circulation systems. Air streams also include air streams typically
encountered in
hospital, surgical, infirmity, birthing, mortuary, and clinical diagnosis
rooms.
As used herein, the term "waters" includes food process or transport waters.
Food process or transport waters include produce transport waters (e.g., as
found in
flumes, pipe transports, cutters, slicers, blanchers, retort systems, washers,
and the like),
belt sprays for food transport lines, boot and hand-wash dip-pans, third-sink
rinse
waters, and the like. Waters also include domestic and recreational waters
such as pools,
spas, recreational flumes and water slides, fountains, and the like.
As used herein, the phrase "health care surface" refers to a surface of an
instrument, a device, a cart, a cage, furniture, a structure, a building, or
the like that is
employed as part of a health care activity. Examples of health care surfaces
include
surfaces of medical or dental instruments, of medical or dental devices, of
electronic
apparatus employed for monitoring patient health, and of floors, walls, or
fixtures of
structures in which health care occurs. Health care surfaces are found in
hospital,
surgical, infirmity, birthing, mortuary, and clinical diagnosis rooms. These
surfaces can
be those typified as "hard surfaces" (such as walls, floors, bed-pans, etc.,),
or fabric
surfaces, e.g., knit, woven, and non-woven surfaces (such as surgical
garments,
draperies, bed linens, bandages, etc.,), or patient-care equipment (such as
respirators,
diagnostic equipment, shunts, body scopes, wheel chairs, beds, etc.,), or
surgical and
diagnostic equipment. Health care surfaces include articles and surfaces
employed in
animal health care.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
9
As used herein, the term "instrument" refers to the various medical or dental
instruments or devices that can benefit from cleaning with a stabilized
composition
according to the present invention.
As used herein, the phrases "medical instrument", "dental instrument",
"medical
device", "dental device", "medical equipment", or "dental equipment" refer to
instruments, devices, tools, appliances, apparatus, and equipment used in
medicine or
dentistry. Such instruments, devices, and equipment can be cold sterilized,
soaked or
washed and then heat sterilized, or otherwise benefit from cleaning in a
composition of
the present invention. These various instruments, devices and equipment
include, but
are not limited to: diagnostic instruments, trays, pans, holders, racks,
forceps, scissors,
shears, saws (e.g. bone saws and their blades), hemostats, knives, chisels,
rongeurs,
files, nippers, drills, drill bits, rasps, burrs, spreaders, breakers,
elevators, clamps,
needle holders, carriers, clips, hooks, gouges, curettes, retractors,
straightener, punches,
extractors, scoops, keratomes, spatulas, expressors, trocars, dilators, cages,
glassware,
tubing, catheters, cannulas, plugs, stents, scopes (e.g., endoscopes,
stethoscopes, and
arthoscopes) and related equipment, and the like, or combinations thereof.
As used herein, "agricultural" or "veterinary" objects or surfaces include
animal
feeds, animal watering stations and enclosures, animal quarters, animal
veterinarian
clinics (e.g. surgical or treatment areas), animal surgical areas, and the
like.
As used herein, "residential" or "institutional" objects or surfaces include
those
found in structures inhabited by humans. Such objects or surfaces include
bathroom
surfaces, drains, drain surfaces, kitchen surfaces, and the like.
As used herein, the phrase "densified fluid" refers to a fluid in a critical,
subcritical, near critical, or supercritical state. The fluid is generally a
gas at standard
conditions of one atmosphere pressure and 0 C. As used herein, the phrase
"supercritical fluid" refers to a dense gas that is maintained above its
critical
temperature, the temperature above which it cannot be liquefied by pressure.
Supercritical fluids are typically less viscous and diffuse more readily than
liquids. In
an embodiment, a densified fluid is at, above, or slightly below its critical
point. As
used herein, the phrase "critical point" is the transition point at which the
liquid and
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
gaseous states of a substance merge into each other and represents the
combination of
the critical temperature and critical pressure for a substance. The critical
pressure is a
pressure just sufficient to cause the appearance of two phases at the critical
temperature.
Critical temperatures and pressures have been reported for numerous organic
and
5 inorganic compounds and several elements.
As used herein, the terms "near critical" fluid or "subcritical" fluid refer
to a
fluid material that is typically below the critical temperature of a
supercritical fluid, but
remains in a fluid state and denser than a typical gas due to the effects of
pressure on the
fluid. In an embodiment, a subcritical or near critical fluid is at a
temperature and/or
10 pressure just below its critical point. For example, a subcritical or
near critical fluid can
be below its critical temperature but above its critical pressure, below its
critical
pressure but above its critical temperature, or below both its critical
temperature and
pressure. The terms near critical and subcritical do not refer to materials in
their
ordinary gaseous or liquid state.
As used herein, weight percent (wt-%), percent by weight, % by weight, and the
like are synonyms that refer to the concentration of a substance as the weight
of that
substance divided by the weight of the composition and multiplied by 100.
Unless
otherwise specified, the quantity of an ingredient refers to the quantity of
active
ingredient.
As used herein, the terms "mixed" or "mixture" when used relating to
"peroxycarboxylic acid composition" or "peroxycarboxylic acids" refer to a
composition or mixture including more than one peroxycarboxylic acid, such as
a
composition or mixture including peroxyacetic acid and peroxyoctanoic acid.
As used herein, the term "about" modifying the quantity of an ingredient in
the
compositions of the invention or employed in the methods of the invention
refers to
variation in the numerical quantity that can occur, for example, through
typical
measuring and liquid handling procedures used for making concentrates or use
solutions
in the real world; through inadvertent error in these procedures; through
differences in
the manufacture, source, or purity of the ingredients employed to make the
compositions or carry out the methods; and the like. The term about also
encompasses
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
11
amounts that differ due to different equilibrium conditions for a composition
resulting
from a particular initial mixture. Whether or not modified by the term
"about", the
claims include equivalents to the quantities.
For the purpose of this patent application, successful microbial reduction is
achieved when the microbial populations are reduced by at least about 50%, or
by
significantly more than is achieved by a wash with water. Larger reductions in
microbial population provide greater levels of protection.
As used herein, the term "sanitizer" refers to an agent that reduces the
number of
bacterial contaminants to safe levels as judged by public health requirements.
In an
embodiment, sanitizers for use in this invention will provide at least a
99.999%
reduction (5-log order reduction). These reductions can be evaluated using a
procedure
set out in Germicidal and Detergent Sanitizing Action of Disinfectants,
Official
Methods of Analysis of the Association of Official Analytical Chemists,
paragraph
960.09 and applicable sections, 15th Edition, 1990 (EPA Guideline 91-2).
According to
this reference a sanitizer should provide a 99.999% reduction (5-log order
reduction)
within 30 seconds at room temperature, 25 2 C, against several test organisms.
As used herein, the term "disinfectant" refers to an agent that kills all
vegetative
cells including most recognized pathogenic microorganisms, using the procedure
described in A.O.A.C. Use Dilution Methods, Official Methods of Analysis of
the
Association of Official Analytical Chemists, paragraph 955.14 and applicable
sections,
15th Edition, 1990 (EPA Guideline 91-2).
As used in this invention, the term "sporicide" refers to a physical or
chemical
agent or process having the ability to cause greater than a 90% reduction (1-
log order
reduction) in the population of spores of Bacillus cereus or Bacillus subtilis
within 10
seconds at 60 C. In certain embodiments, the sporicidal compositions of the
invention
provide greater than a 99% reduction (2-log order reduction), greater than a
99.99%
reduction (4-log order reduction), or greater than a 99.999% reduction (5-log
order
reduction) in such population within 10 seconds at 60 C.
Differentiation of antimicrobial "-cidal" or "-static" activity, the
definitions
which describe the degree of efficacy, and the official laboratory protocols
for
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
12
measuring this efficacy are considerations for understanding the relevance of
antimicrobial agents and compositions. Antimicrobial compositions can effect
two
kinds of microbial cell damage. The first is a lethal, irreversible action
resulting in
complete microbial cell destruction or incapacitation. The second type of cell
damage
is reversible, such that if the organism is rendered free of the agent, it can
again
multiply. The former is termed microbiocidal and the later, microbistatic. A
sanitizer
and a disinfectant are, by definition, agents which provide antimicrobial or
microbiocidal activity. In contrast, a preservative is generally described as
an inhibitor
or microbistatic composition.
Apparatus for Making Peroxycarboxylic Acid
The present invention relates to an apparatus for making a peroxycarboxylic
acid and to methods employing the apparatus. The apparatus includes a reaction
catalyst and a pretreatment column. The pretreatment column pretreats one or
more of
the reagents employed in making the peroxycarboxylic acid. For example, a
cation
exchanger in acid form or inert metal (e.g., Na + or K ) form can remove
positively
charged contaminants, such as metal ion, from hydrogen peroxide, carboxylic
acid, or a
mixture of hydrogen peroxide and carboxylic acid. The reaction catalyst
catalyzes the
reaction of carboxylic acid (or suitable precursor) with an oxidizing agent
(e.g., a
peroxide, a peroxide donor, such as a hydrogen peroxide donor) to form a
peroxycarboxylic acid. For example, an reaction catalyst that is a strong acid
(e.g., a
polystyrene sulfonic acid) can catalyze reaction of hydrogen peroxide with
carboxylic
acid to form peroxycarboxylic acid. The pretreatment column can increase the
life,
activity, and/or safety of the reaction catalyst.
The apparatus can also include a safety system. The safety system can monitor
and/or regulate one or more conditions of the pretreatment column and/or the
reaction
catalyst. For example, the safety system can monitor and/or regulate the
pressure,
temperature, metal content, and/or presence of gas resulting from decay of
peroxide
(e.g., oxygen). The safety system can measure one or more of these parameters
at or in
the pretreatment column, at or in the reaction catalyst, for one or more of
the reagents
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
13
before, in, or after a pretreatment column, for the reaction mixture before,
in, or after a
pretreatment column, for the reaction mixture before, in, or after the
reaction catalyst, or
more than one of these (a combination thereof). The safety system can measure
a
difference in one or more of these parameters between any two points in the
apparatus,
for example, between any two of the listed locations.
In an embodiment, the present apparatus includes one or more reagent vessels,
each of which can contain hydrogen peroxide or carboxylic acid(s). These
vessels can
be in fluid communication with a pretreatment column, with mixing of the
reagents
either before or in that column. The pretreatment column can be in fluid
communication with the reaction catalyst (typically in a column). The
resulting
peroxycarboxylic acid emerges from the reaction catalyst and can be either
used or
stored, for example, in a day tank.
Reaction of the carboxylic acid with the peroxide occurs in the presence of
the
reaction catalyst as the reagents are contacted with (e.g., move through
and/or around)
the reaction catalyst at a controlled and predetermined flow rate. The size of
the
pretreatment column, the size of the bed of reaction catalyst, and the
residence time in
each of these are predetermined and controlled to provide the desired amount
(often as
much as possible) conversion of carboxylic acid to peroxycarboxylic acid. The
size of
the column, bed, or bag of reaction catalyst and the residence time in it are
predetermined and controlled to provide the desired amount (often as much as
possible)
conversion of carboxylic acid to peroxycarboxylic acid. System parameters,
such as the
amount of reaction catalyst, size of the column, bed, or bag of reaction
catalyst, and
reagent flow rate, for the apparatus can be selected to provide sufficient
residence time
of the reaction mixture on the reaction catalyst for conversion into the
desired
peroxycarboxylic acid composition. The reaction catalyst can produce
peroxycarboxylic acid at concentrations as high as, for example, about 35 wt-
%, for
example, about 5 (e.g., 5.3), about 10, about 15, about 20 (e.g., 19), about
25 wt-%,
about 30 wt-%, or about 35 wt-%.
The apparatus can also include additional useful or desired systems such as
fittings, valves, pumps, mixing chambers, water or additive supply
connections,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
14
commonly employed for operation of systems including beds or columns of
catalyst or
cation exchanger.
The present apparatus can employ reagents that include only volatile
components or only insignificant amounts of non-volatile components.
Insignificant
amounts of non-volatile compounds include an amount acceptable on a food or
beverage container (e.g., an aseptic package) after washing and drying. For
example,
the present apparatus can employ reagents that do not include or are
substantially free of
stabilizer or chelating agent (e.g., HEDP). By way of further example, the
present
apparatus can employ reagents that are phosphate-free.
Accordingly, the present apparatus can produce peroxycarboxylic acid
compositions that include only volatile components or only insignificant
amounts of
non-volatile components. For example, the present apparatus can produce
peroxycarboxylic acid compositions that do not include or are substantially
free of
stabilizer or chelating agent (e.g., HEDP). By way of further example, the
present
apparatus can produce peroxycarboxylic acid compositions that are phosphate-
free.
Pretreatment Column
In an embodiment, the apparatus includes one or more pretreatment columns
each in fluid communication with a conduit from a single reagent vessel. The
pretreatment column can be coupled directly to the bed, bag, or column of
reaction
catalyst. Alternatively, the pretreatment column can be in fluid communication
with
second pretreatment column that is also in fluid communication with a source
of a
second reagent. The pretreatment column can be in fluid communication with a
conduit
for the second reagent (pretreated or not) in which the reagents mix before
entry into the
second pretreatment column. The size of the pretreatment column and the
residence
time in the pretreatment column are predetermined and controlled to provide
the desired
amount of contaminant removal from the pretreated composition.
In an embodiment, the apparatus includes a plurality of (e.g., two)
pretreatment
columns coupled in parallel between a plurality of reagent vessels and the
reaction
catalyst. Reagent flow through conduits can be controlled by valve systems.
Flow can
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
be directed through a pretreatment column until that pretreatment column has
received
sufficient use or is in a condition that indicates it is no longer fit for
use. During use of
the first pretreatment column, the second pretreatment column can remain ready
for use.
The valve system can then direct flow through the second pretreatment column
when
5 the first is no longer to be used. The column that is not being used can
be replaced,
maintained, washed, or the like. For ease of replacement, the pretreatment
column can
be a cartridge that is quickly and easily removed from and placed into the
apparatus. A
pretreatment column can be washed with, for example, a dilute strong mineral
acid,
such as sulfuric acid.
10 Alternatively, the pretreatment column or system can be configured
as a
pretreatment bed or pretreatment bag. The pretreatment bed or bag can be
employed in
place of the pretreatment column in the embodiments described herein.
Use of the apparatus can continue while one of the pretreatment columns is
being maintained or replaced. Changing columns can be done according to a
15 predetermined schedule. Alternatively, the condition of the pretreatment
column that is
in use can be measured by the safety system, which can also control the valve
system.
In an embodiment, the pretreatment column is a cartridge or segment that
precedes the reaction catalyst and that can be within the column, bag, or bed
that
contains the reaction catalyst. Such a cartridge can be exchanged into and out
of the
column, bag, or bed of reaction catalyst. In an embodiment, the pretreatment
column
can be a portion of cation exchanger at the entry to or beginning of the
column, bag, or
bed of reaction catalyst. This portion is configured to be removed and
replaced when,
for example, the safety system so indicates or after a certain amount of use.
Reaction Catalyst
The reaction catalyst can be in one or more beds, bags, or columns. The beds,
bags, or columns can be coupled in series, in parallel, or with some in series
and some
in parallel. In an embodiment, the apparatus includes four columns containing
reaction
catalyst and connected in series. In other embodiments, the apparatus includes
up to
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
16
about 10 columns of reaction catalyst, for example one to ten columns, for
example,
two, three, four, or five columns.
Reagent flow through beds, bags, or columns of reaction catalyst can be
controlled by a valve system. Flow can be directed through a bed, bag, or
column until
that bed, bag, or column has received sufficient use or is in a condition that
indicates it
is no longer fit for use. During use of a first bed, bag, or column, a second
bed, bag, or
column can remain ready for use. The valve system can then direct flow through
the
second bed, bag, or column when the first is no longer to be used. During use
of a first
set of beds, bags, or columns, a second set of beds, bags, or columns can
remain ready
for use. The valve system can then direct flow through the second set of beds,
bags, or
columns when the first set is no longer to be used. The bed, bag, or column
(or set
thereof) that is not being used can be replaced, maintained, washed, or the
like. Use of
the apparatus can continue while one of the (sets of) beds, bags, or columns
is being
maintained or replaced. The condition of the bed, bag, or column that is in
use can be
measured by the safety system, which can also control the valve system.
Safety System
The apparatus can include a safety system that can measure one or more
properties of the pretreatment column, of the reaction catalyst, or both. For
example,
the safety system can measure pressure (e.g., increased pressure), temperature
(e.g.,
increased temperature), or both. An increase in temperature or pressure from a
nominal
value for a pretreatment column can indicate, for example, unwanted active
metal ion
catalyzed decomposition of hydrogen peroxide. For example, the safety system
can
measure a difference in temperature between two points in or around (e.g.,
before and
after or before and in) the pretreatment column. An increase in the difference
in
temperature or difference in pressure from a nominal value for two points in
or around a
pretreatment column can indicate, for example, unwanted active metal ion
catalyzed
decomposition of hydrogen peroxide. The point or points at which temperature
or
pressure is measured can be selected to provide the desired sensitivity to
contamination
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
17
or decomposition. The safety system can include a manometric sensor for
measurements of values or changes in values.
The safety system can measure pressure, temperature, difference in pressure,
difference in temperature, or a combination thereof and provide a perceptible
signal if
one or more of these increases above a predetermined level. Pressure,
temperature,
difference in pressure, difference in temperature, or a combination thereof
above a
certain level can indicate danger from the reaction of peroxide with metals.
The level of
pressure, temperature, difference in pressure, difference in temperature, or a
combination thereof at which safety system provides a perceptible signal can
be
selected to allow intervention to avoid undesirable or unsafe conditions.
The safety system, upon detecting pressure, temperature, difference in
pressure,
difference in temperature, or a combination thereof above the preselected
level, can
provide a perceptible signal that alerts the operator to interrupt operation
of the
apparatus by, for example, actuating a pressure release valve, stopping flow
of one or
more reagents, causing water to flow into the apparatus, causing carboxylic
acid
composition to flow into the apparatus, shutting down the apparatus, or a
combination
thereof. The safety system, upon detecting pressure, temperature, difference
in
pressure, difference in temperature, or a combination thereof above the
preselected
level, can provide a perceptible signal that alerts the operator to switch to
another
pretreatment column or bed or column of reaction catalyst.
The safety system can provide a signal to a controller (e.g., a controllable
logic
controller) and the controller can actuate a pressure release valve, stop flow
of one or
more reagents, cause water to flow into the apparatus, cause carboxylic acid
composition to flow into the apparatus, shut down the apparatus, or a
combination
thereof. The safety system, upon detecting pressure, temperature, difference
in
pressure, difference in temperature, or a combination thereof above the
preselected
level, can provide a signal to a controller to switch to another pretreatment
column or
bed or column of reaction catalyst.
The safety system can measure conditions at an inlet or outlet of a
pretreatment
column, within that column (e.g., near the entrance of the column, in the
interior of the
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
18
column, or near the exit from the column), or in a conduit entering or leaving
the
pretreatment column. Another embodiment of the safety system can quantify the
amount of metal that enters or has entered the pretreatment column or reaction
catalyst.
In an embodiment, the safety system is configured to measure temperature at
the
entrance to the pretreatment column and in the first 25% of the pretreatment
column.
Although not limiting to the present invention, it is believed that measuring
this
difference can be desirable because contamination of the pretreatment column
can occur
in an exponential gradient and the reaction between the hydrogen peroxide and
the
contamination (e.g., metal ions, such as Fe2+ or Cu2 ) on the column is
exothermic.
In an embodiment, the safety system can include a processor and two condition
sensors (e.g., temperature sensor, pressure sensor, metal sensor, or the
like). The
processor can, for example, perform calculations on input received from the
condition
sensors and provide a signal that can be received and/or perceived by an
operator of the
apparatus or one or more actuators. In an embodiment, the actuator can signal,
activate,
or operate a valve, pump, switch, or other system for actuating a pressure
release valve,
stopping flow of one or more reagents, causing water to flow into the
apparatus, causing
carboxylic acid composition to flow into the apparatus, shutting down the
apparatus, or
a combination thereof.
The safety system can be configured to measure conditions at an inlet or
outlet
of a column, bed, or bag of reaction catalyst, within that column, bed, or bag
(e.g., near
the entrance, in the interior, or near the exit), or in a conduit entering or
leaving the
reaction catalyst. In an embodiment, the safety system is configured to
measure
temperature at the entrance to the reaction catalyst and in the first 25% of
the reaction
catalyst.
Additional Systems
The apparatus can also include systems for storing, handling, diluting, and
formulating the composition made by the apparatus. For example, the resulting
peroxycarboxylic acid that emerges from the reaction catalyst can be either
used or
stored, for example, in a day tank. The storage system can be a vessel such as
a day
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
19
tank or another vessel suitable for containing a peroxycarboxylic acid
composition
between synthesis and use. Alternatively, a conduit from the apparatus can
lead directly
to a dilution apparatus or point of use.
The apparatus can include dilution and/or formulation systems for diluting
and/or formulating the composition from the apparatus or the day tank. The
apparatus
can produce a concentrate, which can be diluted before use. The concentration
of
peroxycarboxylic acid in the use solution can be, for example, about 2 to
about 5000
ppm or about 750 ppm to about 3600 ppm. Additional suitable use dilutions and
compositions are described hereinbelow. The dilution apparatus can add and/or
mix
into the peroxycarboxylic acid a diluent or carrier, such as water, to achieve
a diluted
composition containing, for example, a desired use concentration of
peroxycarboxylic
acid. In an embodiment, the dilution system can include a pump that takes in
both
carboxylic acid composition and diluent and puts them out in one or more
conduits in a
desired proportion. The dilution system can provide the diluted composition
directly to
the site of use, to the day tank, or to a diluted composition storage system.
In an
embodiment in which the dilution system applies the diluted composition
directly to the
site of use, this system can include an applicator nozzle. The applicator
nozzle can be
configured to heat the composition while applying it.
In an embodiment, the apparatus and/or the diluting system can be configured
to
add another ingredient to the peroxycarboxylic acid composition. A variety of
such
ingredients are described hereinbelow. For example, the diluting system can
add a
diluent that contains an added ingredient. A formulating system can dispense a
desired
amount of an added ingredient into the composition or diluted composition.
Such a
system is useful for adding an ingredient that is not compatible with
synthesis or storage
of peroxycarboxylic acid, such as a quaternary ammonium chloride.
The storage system can include a storage monitor configured to measure the
content of peroxycarboxylic acid, carboxylic acid, and/or hydrogen peroxide in
the
composition, for example, in a stored use composition. In an embodiment, the
diluted
composition storage system includes an replenishing system. The replenishing
system
can monitor the content of the use composition. If, for example, the
concentration of
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
peroxycarboxylic acid decreases below a predetermined level or the
concentration of
carboxylic acid increases above a predetermined level, the replenishing system
can add
more concentrated peroxycarboxylic acid composition to the use composition or
empty
the vessel of the spent use composition. The replenishing system can include,
for
5 example, flow meters and a sensor that detects the concentration of
peroxycarboxylic
acid.
The apparatus can also include a reagent flow control system. The reagent flow
system can monitor the peroxycarboxylic acid composition after the reaction
catalyst,
for example, at an outlet from the last reaction catalyst column. This system
can
10 determine whether the composition includes the desired concentration of
peroxycarboxylic acid (e.g., the equilibrium concentration). If the
composition includes
less than the desired concentration, the system can slow the flow rate of the
reaction
mixture through the reaction catalyst to a flow rate that results in the
desired
concentration. The system can calculate the change in flow rate employing
factors
15 including the temperature of the composition and the concentration of
peroxycarboxylic
acid. The desired concentration of peroxycarboxylic acid can be a lower limit
and the
desired concentration can be any achievable concentration above that lower
limit.
In an embodiment, the present apparatus can include a middle vessel configured
to receive one or more reagents after the reagent(s) passes through the
pretreatment
20 column. The middle vessel can be in fluid communication with the
pretreatment
column and the reaction catalyst. The middle vessel can be configured to
receive
pretreated reagent(s) and to contain them. The middle vessel can be
simultaneously in
fluid communication with a pretreatment column and reaction catalyst. In an
embodiment, the middle vessel can be in fluid communication with a
pretreatment
column and with the reaction catalyst at different times or at overlapping
times. In an
embodiment, the middle vessel can in be in a first position for receiving
reagent(s) from
the pretreatment column and transported to a second position to provide
reagents to the
reaction catalyst.
In an embodiment, the present apparatus can include a purification system that
removes non-volatile components from one of more of the reagents, e.g., one or
more of
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
21
carboxylic acid and peroxide. In an embodiment, the purification system is
configured
as a column, bag, or bed of anion exchanger in fluid communication with the
source of
hydrogen peroxide and the pretreatment column.
The present apparatus can be in fluid communication with an aseptic packaging
system and configured to provide peroxycarboxylic acid composition to the
aseptic
packaging system. The peroxycarboxylic acid composition can be ready to use or
can
require dilution before use in the aseptic packaging system. In an embodiment,
the
present apparatus can provide ready to use peroxycarboxylic acid composition,
for
example, to a bottle rinse vessel and/or to a cap rinse vessel tank. In an
embodiment,
the present apparatus can supply a concentrate, which can be mixed with water
or
another diluent in or by the aseptic packaging system. Such a packaging system
can
include a water vessel or be coupled to a source of purified water. The
aseptic
packaging system can include a chamber in which bottles are rinsed and a
chamber in
which caps are contacted with the diluted or ready to use peroxycarboxylic
acid
composition. The aseptic packaging system can include a recycling system that
recovers peroxycarboxylic acid composition that has been applied to bottle
and/or cap
and returns it to the appropriate vessel for reuse or that reapplies the
composition to
additional bottles and/or caps.
Embodiments of the Apparatus
In an embodiment, the present apparatus can include two or three reagent
vessels, one containing hydrogen peroxide, one containing short chain
carboxylic acid
(e.g., acetic acid), and, optionally, a third vessel containing medium chain
carboxylic
acid (e.g., octanoic acid). The short chain carboxylic acid (e.g., acetic
acid) vessel can
be coupled by a conduit to a short chain carboxylic acid (e.g., acetic acid)
pretreatment
column, with mixing of reagents occurring after this column. The short chain
carboxylic acid (e.g., acetic acid) pretreatment column can include a cation
exchanger in
acid form or in inert metal (e.g., Na + or lc') form, which can remove
positively charged
contaminants, such as metal ion (e.g. non-inert metal ion, e.g., iron (Fe2+
and/or Fe3 ) or
copper (Cu2 ) ion), from the acetic acid. The inert metal cation can be
selected to be
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
22
only weakly bound by the cation exchanger. Pretreatment columns dedicated to
the
hydrogen peroxide and/or medium chain carboxylic acid are optional.
Embodiments for Producing a Peroxycarboxylic Acid
In an embodiment, only the short chain carboxylic acid (e.g., acetic acid) has
a
dedicated pretreatment column and medium chain carboxylic acid is not
employed. In
this embodiment, the conduits for short chain carboxylic acid (e.g., acetic
acid) and for
hydrogen peroxide join and these reagents mix before a main pretreatment
column. The
main pretreatment column can include a cation exchanger in acid form or in
inert metal
(e.g., Na + or K ) form, which can remove positively charged contaminants,
such as
metal ion, from the mixture of hydrogen peroxide and carboxylic acid. The
conduit of
the mixture of acetic acid and hydrogen peroxide couples to the main
pretreatment
column and provides these mixed reagents to the column. This embodiment
includes
four columns of reaction catalyst. These four columns are in series and are
coupled by
a conduit to the main pretreatment column. At the other end, the four columns
feed
short chain peroxycarboxylic acid (e.g., peroxyacetic acid) into a conduit
that leads to
either a storage vessel or to a point of use for this composition.
This embodiment can also include a safety system. The safety system can
include sensors that monitor temperature, for example, in the conduit after
mixing of
hydrogen peroxide and short chain carboxylic acid (e.g., acetic acid) and/or
at the inlet
to the main pretreatment column. The safety system can also include a sensor
that
monitors temperature within the main pretreatment column, e.g., within the
first 25% of
the pretreatment column. The safety system can provide a perceptible signal
when
temperature difference between the sensor before the main pretreatment column
and in
the sensor in the main pretreatment column increases above a predetermined
level, for
example, about 10 C. In this embodiment, the safety system, provides a
perceptible
signal to an operator and/or provides a perceptible signal to a controller.
Upon receipt
of the signal, the operator or controller stops flow of reagents into the main
guard
column and/or flushes the conduits and main guard column with water or short
chain
carboxylic acid (e.g., acetic acid).
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
23
This embodiment of the apparatus can also include additional useful or desired
systems such as fittings, valves, pumps, mixing chambers, and water or
additive supply
connections useful or advantageous in this apparatus. This embodiment can also
include one or more of the systems for storing, handling, diluting, and
formulating the
composition made by the apparatus that are described above.
Embodiments for Producing Mixed Peroxycarboxylic Acids
In an embodiment, the present apparatus can include three reagent vessels, one
containing hydrogen peroxide, one containing short chain carboxylic acid
(e.g., acetic
acid), and, a third vessel containing a medium chain carboxylic acid, such as
octanoic
acid. The short chain carboxylic acid (e.g., acetic acid) vessel can be
coupled by a
conduit to an short chain carboxylic acid (e.g., acetic acid) pretreatment
column, with
mixing of reagents occurring after this column. The short chain carboxylic
acid (e.g.,
acetic acid) pretreatment column can be and operate as described for the
embodiment
above. Pretreatment columns dedicated to the hydrogen peroxide and/or medium
chain
carboxylic acid (e.g., octanoic acid) are optional.
In this embodiment, the conduits for short chain carboxylic acid (e.g., acetic
acid) and for hydrogen peroxide join and these reagents mix before a first
main
pretreatment column. The main pretreatment column can be and operate as
described
for the embodiment above. The conduit of the mixture of short chain carboxylic
acid
(e.g., acetic acid) and hydrogen peroxide couples to the first main
pretreatment column
and provides these mixed reagents to the column. This embodiment includes
(e.g.,
four) columns of reaction catalyst dedicated to producing short chain
peroxycarboxylic
acid (e.g., peroxyacetic acid). These columns are in series and are coupled by
a conduit
to the first main pretreatment column. At the other end, these columns feed
short chain
peroxycarboxylic acid (e.g., peroxyacetic acid) into a conduit.
This embodiment also includes conduits for medium chain carboxylic acid and
hydrogen peroxide that join and mix these reagents before a second main
pretreatment
column. The second main pretreatment column can be and operate as described
for the
embodiment above. The conduit of mixed medium chain carboxylic acid and
hydrogen
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
24
peroxide couples to the second main pretreatment column and provides these
mixed
reagents to the column. This embodiment includes (e.g., four) columns of
reaction
catalyst dedicated to producing medium chain peroxycarboxylic acid. These
columns
are in series and are coupled by a conduit to the second main pretreatment
column. At
the other end, these columns feed medium chain peroxycarboxylic acid into a
conduit.
The short chain peroxycarboxylic acid (e.g., peroxyacetic acid) conduit and
the
medium chain peroxycarboxylic acid (e.g., octanoic acid) conduit can send
these
peracids into a storage and/or mixing vessel to produce a mixed
peroxycarboxylic acid
composition. Alternatively, these conduits can join to produce a mixed
peroxycarboxylic acid composition.
This embodiment can also include a safety system. The safety system can
include sensors that monitor temperature before and in each pretreatment
column and
that responds to an increased temperature difference for either pretreatment
column.
This embodiment of the apparatus can also include additional useful or desired
systems
such as fittings, valves, pumps, mixing chambers, and water or additive supply
connections useful or advantageous in this apparatus. This embodiment can also
include one or more of the systems for storing, handling, diluting, and
formulating the
composition made by the apparatus that are described above.
Components of the Apparatus
Pretreatment Column
The pretreatment column can include any of a variety of cation exchangers,
such
as strong cation exchangers. Suitable cation exchangers for the pretreatment
column
include polystyrene sulfonic acid resins, such as those sold under the
tradenames
Dowex M31, Dowex DR-2030, Dowex Monosphere M-31, Dowex Monosphere DR-
2030, Dowex Marathon 545C, Dowex 50W X8-H, Dowex 545C, Dowex G26,
Amberlyst 15Wet, Amberlyst 15Dry, Amberlyst 31Wet, Amberlyst 131Wet, Amberlyst
CH10, Purolite C-100H, Purolite C-150H, Lewatit MonoPlus S 100 H, Lewatit
MonoPlus SP 112 H, and the like. Additional cation exchangers suitable for the
pretreatment column include sulfonated tetrafluoroethylene copolymers such as
those
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
sold under the tradenames Nafion NR50 (beads), Nafion SAC-13 (granules), and
Nafion 117 (film), and the like. Other cation exchangers suitable for the
pretreatment
column include those sold under the trade names Dowex 545C, Dowex G26, which
have high ionic capacity. In an embodiment, the pretreatment column includes
an alkali
5 metal (e.g., sodium) form of the ion exchanger.
Although not limiting to the present invention, it is believed that, all other
things
being equal, polystyrene sulfonic acid resins with minimal crosslinking (via
divinylbenzene) show an improved selectivity for exchanging early alkali metal
(e.g.,
sodium and potassium) ions for the problematic transition or heavy metal
(e.g., iron and
10 copper) ions.
A suitable pretreatment column can be dimensioned for sufficient flow and
binding capacity to support the volume demanded of the apparatus. For example,
a
pretreatment column in an apparatus that employs 4 columns of reaction
catalyst, each
having a volume of about 10 L, can employ a pretreatment column having a
volume of
15 about 5 L. For example, a pretreatment column in an apparatus that
produces about 11
(e.g., 10.7) liters per hour of peracid composition can employ a pretreatment
column
including about 4 (e.g., 3.9) L of resin. For example, a pretreatment column
in an
apparatus that produces about 20 (e.g., 21.3) liters per hour of peracid
composition can
employ a pretreatment column including about 8 (e.g., 7.8) mL of resin. For
example, a
20 pretreatment column in an apparatus that produces about 45 (e.g., 42.6)
liters per hour
of peracid composition can employ a pretreatment column including about 15
(e.g.,
about 15.5) L of resin.
A pretreatment column can be configured for advantageous ease of cleaning the
resin or exchanging the column. For example, the pretreatment column can be in
fluid
25 communication with the inlet and outlet conduits with quick connect
couplings.
Suitable quick connect couplings include Parkers Indi-Lok (Stratoflex) or
Slide-Lok
coupling or Cole-Parmers, EW-31306-16 couplings, the materials of construction
being
preferably polypropylene, polyethylene or polyfluorocarbon. Quick connect
couplings
are also used for the acid backflush, inlets and outlets which can be operated
by the
controller or operated manually. The pretreatment column can be a cartridge
that can
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
26
be exchanged in and out of the apparatus. Suitable cartridges can be machined
from
Schedule 40 Polypropylene, or high density polyethylene tubing and are fitted
with
manometric and or temperature sensors which can be coupled to the controller.
The apparatus can be configured to accept only cartridges suitable for use in
the
apparatus. For example, the apparatus and/or cartridge can include a fitting,
radio
frequency identification circuit, or other electronic device (e.g., a logic
chip or bar code
and reader) to indicate to the apparatus that the cartridge is suitable for
use in the
apparatus. For example, the cartridge can include a programmable device that
stores
the number of times the cartridge has been rinsed. The cartridge and/or
apparatus can
indicate after a predetermined number of washes that the cartridge is no
longer suitable
for use. The indication can result in the cartridge being locked out of the
apparatus.
Similarly, the apparatus can be configured to lock out a cartridge that is not
appropriate
for use in the apparatus.
For example, a transponder programmed with an identifier can be positioned on
the pretreatment column and/or reaction catalyst. This will allow
identification of the
pretreatment column and/or reaction catalyst as suitable for the present
apparatus. For
example, the transponder can be placed on or molded into the pretreatment
column
and/or reaction catalyst. A small injectable transponder (1/16" x 1/2") would
work best
on a pretreatment column and/or reaction catalyst, in part because of its ease
of
placement. Also, while it would be possible to mold the transponder into the
rack at the
time the rack is manufactured, being able to retrofit existing racks may be
desirable. In
alternative embodiments, other sizes of transponders are acceptable.
The transponder can be placed in any suitable location on or in the
pretreatment
column and/or reaction catalyst. In an embodiment, a particular orientation of
the
pretreatment column and/or reaction catalyst can be enforced by off-setting
the
transponder on one side or end of the pretreatment column and/or reaction
catalyst and
off-setting the transponder antenna appropriately.
The transponder can be pre-programmed with unique identifying information,
such as an identifier value indicating the type of pretreatment column and/or
reaction
catalyst being used. An example of a transponder that may be used is
Destron/IDI
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
27
Injectable Transponder Model TX1400L. The Injectable Transponder is a passive
radio-frequency identification tag, designed to work in conjunction with a
compatible
radio-frequency ID reading system.
In an alternative embodiment, image identification could also be used, wherein
each pretreatment column and/or reaction catalyst could be identified before
it is
received in the present apparatus visually. An example of visual
identification would be
where the machine operator could have a choice of several different icons on a
computer screen which will match the pretreatment column and/or reaction
catalyst
placed in the apparatus.
Identification of the pretreatment column and/or reaction catalyst could be
done,
for example, by use of specifically designed pretreatment column and/or
reaction
catalyst; by use of optical recognition; by use of bar codes; by color of the
pretreatment
column and/or reaction catalyst; or by use of a proximity sensor.
An embodiment of the present apparatus includes a transceiver, which is able
to
detect the type of pretreatment column and/or reaction catalyst from the
identifier, and
communicate that identifying information to a processor. The transceiver
generally
includes a transponder antenna which can located on the outer edge of the
apparatus
adjacent to the pretreatment column and/or reaction catalyst and its
transponder. The
transponder antenna could also be located within the apparatus. The
transceiver also
includes a transponder interface, which is coupled to the processor in order
for the
identifying information to be received by the processor, and subsequently in
order to be
looked up in the storage device.
For the detector, a barcode scanner similar to the type used in a supermarket
could also be utilized in an embodiment. An infrared scanner or proximity
sensor could
be used. Examples of scanners that may be used are Destron-Fearing
Corporation's (of
South St. Paul, Minn.) Pocket Reader and Pocket Reader EX Scanners.
Corresponding
bar codes are affixed to the rack for detection by the bar code scanner.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
28
Reaction Catalyst
The reaction catalyst can include any of a variety of cation exchangers, such
as
strong cation exchangers. In an embodiment, the reaction catalyst is the
protonated
form of the cation exchanger. Suitable cation exchangers as the reaction
catalyst
include polystyrene sulfonic acid resins, such as those sold under the
tradenames
Dowex M31, Dowex DR-2030, Dowex Monosphere M-31, Dowex Monosphere DR-
2030, Dowex Marathon 545C, Dowex 50W X8-H, Dowex 545C, Dowex G26,
Amberlyst 15Wet, Amberlyst 15Dry, Amberlyst 31Wet, Amberlyst 131Wet, Amberlyst
CH10, Purolite C-100H, Purolite C-150H, Lewatit MonoPlus S 100 H, Lewatit
MonoPlus SP 112 H, and the like. Additional cation exchangers suitable as the
reaction
catalyst include sulfonated tetrafluoroethylene copolymers such as those sold
under the
tradenames Nafion NR50 (beads), Nafion SAC-13 (granules), and Nafion 117
(film),
and the like. Other cation exchangers suitable as the reaction catalyst
include those sold
under the trade names Dowex 545C, Dowex G26, which have high ionic capacity.
Additional suitable reaction catalysts include an inorganic compound that is
or
includes an insoluble strong acid, in certain embodiments, with a high surface
area/weight ratio. Such inorganic catalysts include those sold under generic
names such
as "Sulfated Zirconia", "Silica Stabilized Tetragonal Zirconia" and
"Tungstated
Zirconia" (from Saint-Gobain Norpro). Suitable inorganic catalysts also
include
zirconia oxides sold as generic "Zr02" (MET Chemicals). A zirconia oxide can
be
treated with sulfuric acid followed by calcination at ¨ 700 deg C to produce a
"sulfated
Zirconia." Other suitable inorganic catalysts include sulfated silicas or
silicon oxides,
sulfated or acidified zeolites, sulfated or acidified aluminum oxides, and
phosphonic
acid derivatized silicon oxides (e.g., those sold under the tradename "Si-
P0H2" and an
alkylphosphonic acid modified silica from Phosphononics Ltd.)
A suitable column, bag, or bed of reaction catalyst can be dimensioned for
sufficient flow and catalyst capacity to support the volumes demanded of the
apparatus.
For example, a column, bag, or bed of reaction catalyst in an apparatus that
produces
about 40 (e.g., 41) liters per hour of peracid composition can employ four
columns of
reaction catalyst each with volume of about 30 (e.g., 31) L and being 1 meter
in length
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
29
and 20 cm in diameter. A bed, bag, or column of reaction catalyst can be
dimensioned
in any suitable dimension for achieving the desired flow. Suitable dimensions
include
about 0.1 (e.g., 0.13) to about 15 (e.g., 13) meters in, for example, length
by about 10 to
about 100 cm in, for example, diameter. Suitable columns include those
dimensioned
about 15 (e.g., 13) meters in length by about 10 cm in diameter. Suitable
columns
include those dimensioned about 0.5 (e.g., 0.4) meters in length by about 20
cm in
diameter. Suitable columns include those dimensioned about 0.15 (e.g., 0.13)
meters in
length by about 100 cm in diameter. A column can be in any of a variety of
configurations. For example, a column can be a normal cylindrical tube or a
coiled
tube. Suitable coiled tubes include a tube about 60 (e.g., 62) meters long by
about 5 cm
in diameter in a coil about 1 meter in diameter with about 20 turns. The
reaction
catalyst can be configured to provide about 30 to about 300 minutes of contact
time of
the catalyst and the reaction mixture.
A column, bag, or bed of reaction catalyst can be configured for advantageous
ease of cleaning, regenerating, or backflushing the bed, bag, or column.
Advantageous
features of the column, bag, or bed of reaction catalyst include a plurality
of ports and
valves between segments of catalyst that allow for selective backflushing of
isolated
segments of the overall catalytic bed as well as venting of the accumulated
gases to
facilitate pumping and circulating of cleaning or backflushing agents.
Reagents
Suitable reagents include hydrogen peroxide at about 5 to about 70 wt-%, about
5 to about 50 wt-%, or about 35 to about 50 wt-% in water; e.g., hydrogen
peroxide at
about 35 wt-%, about 45 wt-%, about 50 wt-%, or about 70 wt-% in water.
Suitable
reagents include acetic acid at about 5 to about 100 wt-% (remainder water) or
at about
80 to about 98 wt-%; for example, acetic acid at about 80 wt-%, about 98 wt-%,
or
about 100 wt-%. Glacial acetic acid is a suitable form of acetic acid.
Suitable reagents
include octanoic acid at about 1 to about 10 wt-% in glacial acetic acid.
Additional suitable hydrogen peroxide reagents include urea-hydrogen peroxide
or any of a variety of other non-ionic hydrogen peroxide complexes. Additional
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
suitable oxidizing reagents include caros acid, acidified sodium persulfate,
or other
peroxy species which equilibrate to form hydrogen peroxide in water.
Additional suitable acetic acid reagents include acetic anhydride, acetyl
chloride, polyvinyl acetate, and mono, di and triacetyl glycerine. Additional
suitable
5 octanoic acid reagents include about 1 to about 10 wt-% octanoic acid in
propylene
glycol; about 1 to about 10 wt-% octanoic acid in water with a hydrotrope
coupling
agent, such as sodium octane sulfonate, or acidic forms of xylene sulfonate,
toluene
sulfonate, dioctyl sulfosuccinate, or other alkyl or aryl sulfonates. Other
suitable
hydrotropes include fatty alcohol ethoxylate phosphate esters, such as
Ecolab's PE 362,
10 Emphos PS-236 or Gafac RA-600. Additional suitable carboxylic acid
reagents include
C1 and C20 alkanoic acids; polyprotic acids including glycolic, succinic,
glutaric, adipic,
citric, malic, or lactic acid; alpha and omega dicarboxylic acids such as
succinic, adipic,
pimelic, suberic, azelaic, or sebacic acid. Additional suitable peracid
precursors include
alcohol ethoxylate carboxylates and amido or imidocarboxylic acids.
15 The reagent compositions employed in the present apparatus need not
include,
and in embodiments, lack or are substantially free of stabilizer or chelating
agent (e.g.,
HEDP). The reagent compositions employed in the present apparatus can include
only
volatile compounds. The reagent compositions including only volatile compounds
can
be phosphate-free.
20 In certain embodiments, the composition applied to the reaction
catalyst
includes about 55 (e.g., 56.5) wt-% carboxylic acid and about 30 (e.g., 30.5)
wt-%
hydrogen peroxide; about 45 (e.g., 43.6) wt-% carboxylic acid and about 20
(e.g., 20.5)
wt-% hydrogen peroxide; about 20 wt-% carboxylic acid and about 30 (e.g., 28)
wt-%
hydrogen peroxide; about 80 (e.g., 78) wt-% carboxylic acid and about 10
(e.g., 7.7) wt-
25 % hydrogen peroxide; or about 5 wt-% carboxylic acid and about 5 wt-%
hydrogen
peroxide.
In certain embodiments, the composition applied to the reaction catalyst
includes about 55 (e.g., 56.5) wt-% short chain carboxylic acid and about 30
(e.g., 30.5)
wt-% hydrogen peroxide; about 45 (e.g., 43.6) wt-% short chain carboxylic acid
and
30 about 20 (e.g., 20.5) wt-% hydrogen peroxide; about 20 wt-% short chain
carboxylic
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
31
acid and about 30 (e.g., 28) wt-% hydrogen peroxide; about 80 (e.g., 78) wt-%
short
chain carboxylic acid and about 10 (e.g., 7.7) wt-% hydrogen peroxide; or
about 5 wt-%
short chain carboxylic acid and about 5 wt-% hydrogen peroxide.
In certain embodiments, the composition applied to the reaction catalyst
includes about 20 wt-% medium chain carboxylic acid and about 30 wt-% hydrogen
peroxide; about 10 wt-% medium chain carboxylic acid and about 20 wt-%
hydrogen
peroxide; about 5 wt-% medium chain carboxylic acid and about 20 wt-% hydrogen
peroxide; or about 3 wt-% medium chain carboxylic acid and about 20 to about
25 (e.g.,
22.5) wt-% hydrogen peroxide.
In certain embodiments, the composition applied to the reaction catalyst
includes about 50 (e.g., 48) wt-% short chain carboxylic acid, about 20 wt-%
medium
chain carboxylic acid, and about 10 wt-% hydrogen peroxide; about 55 (e.g.,
56) wt-%
short chain carboxylic acid, about 10 (e.g., 8) wt-% medium chain carboxylic
acid, and
about 12 wt-% hydrogen peroxide; about 60 wt-% short chain carboxylic acid,
about 2
wt-% medium chain carboxylic acid, and about 15 (e.g., 13) wt-% hydrogen
peroxide;
or about 45 (e.g., 44) wt-% short chain carboxylic acid, about 1 wt-% medium
chain
carboxylic acid, and about 20 (e.g., 21) wt-% hydrogen peroxide.
In certain embodiments, the present composition includes peroxycarboxylic acid
and hydrogen peroxide in a ratio of about 0.3:1 to about 7:1, about 1:1 to
about 3:1, or
about 2:1 to about 3:1. Certain embodiments include peroxycarboxylic acid and
hydrogen peroxide in a ratio of about 2:1 to about 3:1, for example, 2.4:1;
peroxycarboxylic acid and hydrogen peroxide in a ratio of about 1:1 to about
2:1, for
example 1.4:1; peroxycarboxylic acid and hydrogen peroxide in a ratio of about
0.3:1 to
about 1:1, for example 0.4:1; or peroxycarboxylic acid and hydrogen peroxide
in a ratio
of about 7:1, for example 7.1:1.
In certain embodiments, the reagents used in the present apparatus can include
impurities, such as metal ions, at levels up to 100 ppm, up to 10 ppm, up to 1
ppm, or
up to 0.1 ppm. Such impurities can include Fe, Cu, Mn, Ni, Ti, Co or any of
the
transition metal ions.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
32
Illustrated Embodiments
Figure 1 illustrates an embodiment of the present apparatus in the form of
peroxycarboxylic acid generator 20. In Figure 1, one or more reagent supply
vessels
21, for example, first reagent supply vessel 22 containing hydrogen peroxide
and
second reagent supply vessel 24 containing one or more carboxylic acids are
coupled by
first and second lines 26 and 28, respectively, to guard column 30. Hydrogen
peroxide
and carboxylic acid are delivered individually from first and second reagent
supply
vessels 22 and 24 via first and second lines 26 and 28, respectively, into a
mix line 29
leading into guard column 30. In mix line 29 the reagents combine into a
reaction
mixture, although combining may also occur in guard column 30. Guard column 30
contains a cation exchanger (not shown) that removes metal ions from the
reaction
mixture. The reaction mixture then proceeds to one or more reactor columns 34
via
third line 32.
Reactor column 34 is packed with strong acid catalyst (not shown). Inside
reactor column 34, the reaction mixture of hydrogen peroxide and carboxylic
acid react
as they move through the strong acid catalyst at a predetermined, controlled
flow rate.
System parameters, such as column size and reagent flow rate, for the
peroxycarboxylic
acid generator 20 are selected and/or controlled to provide sufficient
residence time of
the reaction mixture on the strong acid catalyst for conversion into the
desired
peroxycarboxylic acid composition. Generator design and process control are
described
in more detail herein below. The peroxycarboxylic acid composition is
discharged from
a reaction column 34 via third line 36, for example, into a holding tank 38.
In an embodiment, a peroxycarboxylic acid generator 20 can also include one or
more additional structural components, such as fittings, valves, pumps, mixing
chambers, water or additive supply connections, commonly employed for
operation of
systems including packed columns. For example, flow from each reagent supply
vessel
can be individually controlled by providing a valve and a pump proximal to
each
reagent supply vessel.
Additional representative configurations for peroxycarboxylic acid generators
20 of the present invention are provided below. Aspects of the various
configurations
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
33
shown below may be combined or separated to present still further
configurations of
peroxycarboxylic acid generators. As in Figure 1, basic components such as
control
valves, fittings and pumps which may be present are omitted from the schematic
representation for clarity.
In an embodiment, peroxycarboxylic acid generator 20 includes one or more
guard columns 30 in the form of a reagent guard column 40, which is positioned
to
receive material from one of first reagent supply vessel 22 or second reagent
supply
vessel 24. The output from reagent guard column 40 can go directly to a
reactor
column 34. The reagent guard column 40 can be positioned in the fluid flow
between
the first reagent supply vessel 22 or second reagent supply vessel 24 and the
reactor
column 34.
Figure 2 illustrates an embodiment of the present peroxycarboxylic acid
generator 20 including two reagent guard columns 40. In the embodiment shown
in
Figure 2, a reagent guard column 40 is positioned in first line 26 connecting
the
hydrogen peroxide supply vessel 22 with the guard column 30 and another
reagent
guard column 40 is placed in second line 28 connecting the carboxylic acid
supply
vessel 24 with the guard column 30. Other embodiments can include only one
(either
one) of these reagent guard columns 40 and/or can omit the guard column 30.
Reagent
guard column 40 can be configured as a cartridge that can be readily removed
and
replaced in the peroxycarboxylic acid generator 20. The other components in
Figure 2
are as described above for Figure 1.
In another embodiment, peroxycarboxylic acid generator 20 includes a plurality
of guard columns 30. Figure 3 schematically illustrates an embodiment
including two
guard columns 30, the second in the form of second guard column 130. As
illustrated,
guard column 30 and second guard column 130 are positioned in parallel between
the
first and second reagent supply vessels 22 and 24 and reactor column 34.
Reagent flow
through first and second lines 26 and 28 into one or both of guard column 30
and
second guard column 130 under the control of valves 54. With valves 54
directing flow
through guard column 30, that column becomes full of contaminants, but second
guard
column 130 remains ready for use. When guard column 30 is no longer suitable
for use,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
34
valves 54 can be set to direct flow through second guard column 130. The
column that
is not receiving flow can be washed, maintained, or replaced. In this fashion,
operation
of this embodiment of peroxycarboxylic acid generator 20 can continue while
either
first or second guard column 30 or 130 is being maintained or replaced. The
condition
of first and/or second guard column 30 or 130 can be determined by the
measuring
device (below), which can also control the setting of valves 54.
In another embodiment, peroxycarboxylic acid generator 20 includes a plurality
of reactor columns 34. A plurality of reactor columns can be connected either
in series,
parallel, or both. Figure 4 illustrates an embodiment including two reactor
columns 34
connected in series. Fifth line 42 couples the two reactor columns. In various
embodiments, the peroxycarboxylic acid generator 20 can include up to about
ten
reactor columns 34, for example one to ten reactor columns 34, for example,
two, three,
four, or five reactor columns 34, for example 4 reactor columns 34 coupled in
series.
In an embodiment, the present apparatus includes a plurality of reactor
columns
34 connected in parallel. Such an embodiment can include a plurality of
reactor
columns 34 coupled in series and also a plurality of reactor columns 34
coupled in
parallel. Figure 5 schematically illustrates such a system. In this
illustration, the
reaction mixture flows from guard column 30 to a first pair of reactor columns
34 in
series that are coupled by fifth line 42. The guard column 30 is also coupled
to a
second pair of reactor columns 134 in series that are coupled by line 142. The
first pair
of reactor columns 34 and second pair of reactor columns 134 are connected in
parallel.
The reaction mixture flows from the first pair of reactor columns 34 to
holding tank 38
through third line 36. The reaction mixture flows from the second pair of
reactor
columns to holding tank 38 through sixth line 136. Reactor valves 44 can
direct the
flow of reaction mixture through either first pair of reactor columns 34 or
the second
pair of reactor columns 134.
With the reactor valves 44 set to direct flow through first pair of reactor
columns
34, those columns are subject to wear and can be consumed or fail, but the
second pair
of reactor columns 134 remains ready for use. When the first pair of reactor
columns
34 is no longer suitable for use, reactor valves 44 can be set to direct flow
through the
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
second pair of reactor columns 134. The pair of columns that is not receiving
flow can
be washed, maintained, or replaced. In this fashion, operation of this
embodiment of
peroxycarboxylic acid generator 20 can continue while either first or second
pair of
reactor columns 34 or 134 is being maintained or replaced. The condition of
first and/or
5 second reactor columns 34 or 134 can be determined by the measuring
device (below),
which can also control the setting of reactor valves 44.
Monitoring Device
In an embodiment, peroxycarboxylic acid generator 20 can include apparatus for
10 measuring one or more properties of the cation exchanger, reagents on
the cation
exchanger, the cation exchanger column assembly as a whole, the catalyst, the
reagents
on the catalyst, or the catalyst column assembly as a whole. For example, such
a device
can monitor pressure (e.g., increased pressure), temperature (e.g., increased
temperature), or both. An increase in temperature or pressure from a nominal
value can
15 indicate unwanted active metal ion catalyzed decomposition of hydrogen
peroxide.
For example, the monitoring device 46 can measure a difference in temperature
between two points in or around (e.g., before and after or before and in) the
guard
column 30. An increase in the difference in temperature or difference in
pressure from
a nominal value for two points in or around a guard column 30 can indicate,
for
20 example, unwanted active metal ion catalyzed decomposition of hydrogen
peroxide.
The point or points at which temperature or pressure is measured can be
selected to
provide the desired sensitivity to contamination or decomposition.
Figure 6 illustrates an embodiment of guard column 30 and monitoring device
46, which is an embodiment of the safety system. Monitoring device 46 includes
25 controller 48 and first and second sensors 50 and 52, respectively, and
lead 54. Lead 54
couples first and second sensors 50 and 52 to controller 48. In the
illustrated
embodiment, first sensor 50 monitors the condition (e.g., temperature or
pressure) of the
reaction mixture in mixing line 29 and second sensor 52 monitors the condition
within
guard column 30. In an embodiment, second sensor can be positioned into guard
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
36
column 30 about 10% to about 25% of the distance along the axis of guard
column 30.
This same configuration can be employed with reagent guard column 40.
Monitoring device 46 can measure a difference in conditions (e.g., temperature
or pressure) between first sensor 50 and second sensor 52. First sensor can be
positioned before guard column 30 (or reagent guard column 40) in, for
example, first
line 26, second line 28, or mixing line 29. Second sensor 52 can be positioned
at the
entrance of, in, or after guard column 30 (or reagent guard column 40). For
example,
second sensor 52 can be positioned at the inlet 60 to guard column 30, within
62 guard
column 30 but before the cation exchanger, within 64 the cation exchanger of
guard
column 30 (near the entrance of the column, in the interior of the column, or
near the
exit from the column), within guard column 30 between the cation exchanger and
the
exit 66 from the guard column 30, or at the outlet 68 from the guard column
30. The
second sensor can be in the same positions in reagent guard column 40. A
difference or
an increase in the difference in temperature or pressure from a nominal value
between
first sensor 50 and second sensor 52 can indicate, for example, unwanted
active metal
ion catalyzed decomposition of hydrogen peroxide.
The device illustrated in Figure 6 is monitored guard column 56. Any of the
embodiments illustrated in Figures 1-5 can employ monitored guard column 56 in
place
of guard column 30 or reagent guard column 40. For example, Figure 7
schematically
illustrates the embodiment of Figure 2 modified to include monitored guard
column 56
in place of guard column 30. In an embodiment, one or more of the reagent
guard
columns 40 can be monitored guard column 56. For a reagent guard column
receiving
carboxylic acid, the sensors can measure metal ion.
Upon measuring a difference in temperature or pressure above a preselected
level, monitoring device 46 can provide a detectible signal that alerts the
operator to
interrupt operation of the apparatus. For example, the operator can actuate a
pressure
release valve 58, stop flow of one or more reagents, cause water to flow into
the guard
column 30 and/or reactor columns 34, cause carboxylic acid to flow into the
guard
column 30 and/or reactor columns 34, shut down the peroxycarboxylic acid
generator
20, or a combination thereof. In an embodiment, the monitoring device 46 can
provide
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
37
a signal to the controller 48, which can be a controllable logic controller,
and the
controller 48 can actuate a pressure release valve 58, stop flow of one or
more reagents,
cause water to flow into the guard column 30 and/or reactor columns 34, cause
carboxylic acid to flow into the guard column 30 and/or reactor columns 34,
shut down
the peroxycarboxylic acid generator 20, or a combination thereof.
Upon measuring a difference in temperature or pressure above a preselected
level, monitoring device 46 can provide a detectible signal that alerts the
operator or
that signals the controller 48 to switch to another guard column. For example,
Figure 8
schematically illustrates the embodiment of Figure 3 modified to include first
and
second monitored guard columns 56 and 156 in place of first and second guard
columns
30 and 130. In an embodiment, the operator can actuate valves 54 to send the
reagent
flow through a second monitored guard column 156. In an embodiment, the
monitoring
device 46 can provide a signal to a controller 48 (e.g., a controllable logic
controller)
and the controller 48 can actuate valves 54 to send the reagent flow through
second
monitored guard column 156.
Another embodiment of the measuring device can quantify the amount of metal
that enters or has entered the column. For example, metal monitoring device 68
can be
positioned at any of the positions described for monitoring device 46 and can
provide
the detectable signal when the amount of metal in the flow through the system
exceeds
a predetermined level. Alternatively, the metal monitoring device 68 can
provide the
detectable signal when a predetermined amount of metal has passed the position
of the
device. The detectable signal can be directed to an operator or controller for
the
purposes and responses described above.
Figure 9 schematically illustrates an embodiment of peroxycarboxylic acid
generator 20 including first and second reagent vessels 22 and 24. In this
embodiment,
first reagent vessel 22 can contain a short chain carboxylic acid, such acetic
acid (e.g.,
98% acetic acid). Second reagent vessel 24 can contain oxidizing agent, such
as
hydrogen peroxide (e.g., 35-45% hydrogen peroxide). This embodiment includes
one
reagent guard column 40, optional second reagent guard column 140, monitored
guard
column 56, four reactor columns 34 connected in series, and five pressure
release valves
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
38
58. The monitored guard column 56 can be of the configuration shown in Figure
6
(e.g., with sensors before the guard column 30 and in the cation exchanger).
The
reagent guard column 40 can include a cation exchanger in acid form or in
inert metal
(e.g., Na + or K ) form.
Figure 10 schematically illustrates an embodiment of peroxycarboxylic acid
generator 20 including first peracid generator 70 and second peracid generator
72. First
peracid generator 70 is configured schematically illustrated in Figure 9 and
described
above.
Second peracid generator 72 in Figure 10 has components generally configured
according to Figure 9 and as described above. Second peracid generator 72 is,
however,
configured for producing medium chain peroxycarboxylic acid. In this
embodiment,
third reagent vessel 23 is configured to contain and supply a medium chain
carboxylic
acid, such as octanoic acid (e.g., 5 wt-% octanoic acid in propylene glycol).
Second
reagent vessel 124 is configured to contain and supply oxidizing agent, such
as
hydrogen peroxide (e.g., 35-45% hydrogen peroxide). This embodiment includes
one
reagent guard column 240, optional second reagent guard column 340, second
monitored guard column 156, four reactor columns 134 connected in series, and
five
pressure release valves 158.
The second monitored guard column 156 can be of the configuration shown in
Figure 6 (e.g., with sensors before the guard column 30 and in the cation
exchanger).
The reagent guard column 240 can include a cation exchanger in acid form or in
inert
metal (e.g., Na + or K ) form.
Hydrogen peroxide and medium chain carboxylic acid are delivered individually
from second and third reagent supply vessels 124 and 23 via first and second
lines 126
and 128, respectively, into a mix line 129 leading into guard column 30. In
mix line
129 the hydrogen peroxide and medium chain carboxylic acid reagents combine
into a
medium chain reaction mixture, although combining may also occur in second
monitored guard column 156.
In the embodiment schematically illustrated in Figure 10, holding tank 38 and
second holding tank 138 are optional. Holding tank 38 may be employed to
collect
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
39
short chain peroxycarboxylic acid composition. Second holding tank 138 may be
employed to collect medium chain peroxycarboxylic acid composition. The
peroxycarboxylic acid compositions can then be supplied (e.g., pumped) from
these
tanks in the desired proportions into mixed peracid holding tank 70.
Alternatively, the
holding tanks 38 and 138 can be omitted and the peroxycarboxylic acid
compositions
can be supplied directly from the reactor columns 34 and 134 in the desired
proportions.
In another embodiment, the generator includes one holding tank 38 or 138 and
the
mixed peracid holding tank 70. In this embodiment, holding tank 38 or 138
collects one
excess peracid composition and then supplies that to the mixed peracid holding
tank in
the desired proportion. One peracid generator 70 or 72 then supplies peracid
composition directly to mixed peracid holding tank 70.
Additional Components and Configurations
Figure 11 schematically illustrates a system including the peroxycarboxylic
acid
generator 20 and an aseptic packaging line 74. The peroxycarboxylic acid
generator 20
is configured to provide peroxycarboxylic acid composition to the aseptic
packaging
line 74. Peroxycarboxylic acid generator 20 can be any of the embodiments
illustrated
or described herein.
In this embodiment, the peroxycarboxylic acid composition can be ready to use
or can require dilution before use in aseptic packaging. The peroxycarboxylic
acid
generator 20 can provide ready to use peroxycarboxylic acid composition
directly to
bottle rinse tank 78 and/or cap rinse tank 80. When the peroxycarboxylic acid
composition in supplied as a concentrate, aseptic packaging line 74 can
include optional
water source 76 to supply water for diluting the peroxycarboxylic acid
composition.
Water and peroxycarboxylic acid composition can mix in bottle rinse tank 78
and cap
rinse tank 80. Water can be supplied to the rinse tanks through optional first
and second
water conduits 86 and 88. Peroxycarboxylic acid composition can be supplied to
the
rinse tanks through peracid conduit 90.
The diluted or ready to use composition can be applied to bottles and caps at
bottle rinse station 82 and cap rinse station 84. The mixing tanks are in
fluid
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
communication with the rinsing stations through station conduits 90. Used
composition
can be recirculated through first and second recirculation conduits 92 and 94.
The
capped bottles can be removed from the system, for example, by a conveyor (not
shown).
5 Figure 12 schematically illustrates an embodiment, of the present
peroxycarboxylic acid generator 20 in which guard column 30 is a cartridge or
segment
in reactor column 34. In Figure 12, one or more reagent supply vessels 21, for
example,
first reagent supply vessel 22 containing hydrogen peroxide and second reagent
supply
vessel 24 containing one or more carboxylic acids are coupled by first and
second lines
10 26 and 28 and mixing line 29 to guard column 30. Guard column 30
contains a cation
exchanger (not shown) that removes metal ions from the reaction mixture. The
reaction
mixture then proceeds to the one or more reactor columns 34. Reactor column 34
is
packed with strong acid catalyst (not shown). The peroxycarboxylic acid
composition
is discharged from a reaction column 34 via third line 36, for example, into a
holding
15 tank 38. In this embodiment, the guard column 30 and/or cation exchanger
can be
exchanged into and out of the reaction column 34, for example, when the safety
system
so indicates or after a certain amount of use. The guard column can make up
the first
about the first 1 vol-% to about the first 50 vol-%, for example about 10 to
about 15
vol-%, of the combined guard and reaction columns 30 and 34. Such a guard
column
20 30 can be employed in any of the illustrated embodiments.
Figure 13 illustrates an embodiment of the present peroxycarboxylic acid
generator 20 including middle tank 96. In Figure 13, one or more reagent
supply
vessels 21, for example, first reagent supply vessel 22 containing hydrogen
peroxide
and second reagent supply vessel 24 containing one or more carboxylic acids
are
25 coupled by first and second lines 26 and 28 and mixing line 29 to guard
column 30. The
reaction mixture proceeds through guard column 30 and third line 32 to middle
tank 96.
The reagent or mixed reagents can accumulate in middle tank 96. In an
embodiment
including a reagent guard column 40 or 140, middle tank 96 can be positioned
after the
reagent guard column 40 or 140 and/or after guard column 30. Middle tank 96 is
30 coupled to reactor column 34 by middle line 98. Reactor column 34 is
packed with
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
41
strong acid catalyst (not shown). The peroxycarboxylic acid composition is
discharged
from a reaction column 34 via third line 36, for example, into a holding tank
38.
In an embodiment, middle tank 96 can be configured to receive one or more
reagents from guard column 30 and/or reagent guard column 40 and to contain
the
reagent(s). The generator 20 can be configured so that middle tank 96 is
simultaneously
in fluid communication with guard column 30 and/or reagent guard column 40 and
with
reactor column 34. In an embodiment, the generator 20 is configured so that
the middle
tank 96 is in fluid communication with guard column 30 and/or reagent guard
column
40 and with reactor column 34 at different times or at overlapping times. In
an
embodiment, the generator 20 is configured so that the middle tank 96 can in
be in a
first position for receiving reagent(s) from guard column 30 and/or reagent
guard
column 40 and transported to a second position to provide reagents to the
reactor
column 34. That is, in such an embodiment, generator 20 can be configured into
two
separate sets of equipment. The first set of equipment can include all of the
components
upstream (in the direction of guard column 30 and/or reagent guard column 40)
from
middle tank 96 and the second set of equipment can include all of the
components
downstream (in the direction of reactor column 34) from middle tank 96.
Any of the embodiments illustrated in Figures 1-13 can include a middle tank
96
and/or can be configured as first and second sets of components.
Figure 14 illustrates an embodiment of reactor column 34 and monitoring device
46, which is an embodiment of the safety system. Monitoring device 46 includes
controller 48 and first and second sensors 50 and 52, respectively, and lead
54. Lead 54
couples first and second sensors 50 and 52 to controller 48. In the
illustrated
embodiment, first sensor 50 monitors the condition (e.g., temperature or
pressure) of the
reaction mixture in third line 32 and second sensor 52 monitors the condition
within
reactor column 34. In an embodiment, second sensor can be positioned into
reactor
column 34 about 10 to about 25 % of the distance along the axis of reactor
column 34.
Alternatively, the sensors can be positioned as described above for
positioning sensors
in guard column 30.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
42
In an embodiment such as that illustrated in Figure 14, the safety system can
measure conditions at an inlet or outlet of a reactor column 34, within
reactor column
34 (e.g., near the entrance of the column, in the interior of the column, or
near the exit
from the column), or in a conduit entering or leaving the reactor column 34.
Another
embodiment of the safety system can quantify the amount of metal that enters
or has
entered the reactor column 34. In an embodiment, the safety system is
configured to
measure temperature at the entrance to the reactor column 34 and in the first
25% of the
reactor column 34.
Monitoring and Control of Use Compositions
Figure 15 is a schematic diagram illustrating an embodiment of a
peroxycarboxylic acid generator 20, a controller 48, a POAA holding tank 38, a
diluent
holding tank 16 and a use composition vessel 166. Controller 48 may manage
several
functions with respect to peroxycarboxylic acid generator 20. For example,
controller
48 may control various safety system functions as described above with respect
to FIG.
6. Controller 48 may also manage dilution of the concentrate composition
generated by
peroxycarboxylic acid generator 20 to form a use composition.
In addition, controller 48 receives concentration data concerning the
concentrations of peroxycarboxylic acid and hydrogen peroxide in the use
composition
via line 180. Based on the concentration data, controller 48 may monitor the
concentration of peroxycarboxylic acid and/or hydrogen peroxide in the use
composition and replenish the use composition when these concentrations do not
satisfy
predetermined criteria. In addition, controller 48 may, based on the
concentration data,
regulate various operating parameters of peroxycarboxylic acid generator 20 to
affect
the concentration of peroxycarboxylic acid in the peroxycarboxylic acid
concentrate
composition output on line 36.
The concentrations of peroxycarboxylic acid and/or of hydrogen peroxide in the
use composition may be determined in any number of ways. An example apparatus
that
may be used to determine the concentrations of peroxycarboxylic acid and/or of
hydrogen peroxide in the use composition is the Oxycheck System, available
from
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
43
Ecolab Inc. of St. Paul, Minnesota. The concentration data may also be
determined
manually. For example, concentration could be obtained by any number of
conventional techniques such as titration, potentiometric or ampermetic
techniques.
However, it shall be understood that the invention is not limited in this
respect, and that
the concentration data may be determined in any number of ways without
departing
from the scope of the present invention.
To manage dilution of the concentrate composition, controller 48 may add
and/or mix into the peroxycarboxylic acid concentrate stored in POAA holding
tank 38
a diluent, such as water, stored in diluent holding tank 164. In one
embodiment,
controller 48 may regulate one or more valves or pumps that control the flow
of the
carboxylic acid concentrate composition from POAA holding tank 38 and diluent
from
diluent holding tank 164. Controller 48 may regulate the pump or pumps such
that the
carboxylic acid composition and diluent flow into use composition vessel 166
in a
desired proportion to achieve a use composition containing, for example, a
target
concentration of peroxycarboxylic acid.
Controller 48 may replenish the use composition when the concentrations of
peroxycarboxylic acid and/or hydrogen peroxide do not satisfy predetermine
criteria.
For example, based on the concentration data, controller 48 may regulate
addition of
peroxycarboxylic acid concentrate from POAA holding tank 38 or diluent 164 to
the
use composition 166 to ensure that the concentrations of peroxycarboxylic acid
and/or
hydrogen peroxide in the use composition satisfy the predetermined criteria.
If, for
example, the concentration of peroxycarboxylic acid in the use composition is
too low,
controller 48 may manage addition of additional peroxycarboxylic acid
concentrate may
to the use composition until a target concentration of peroxycarboxylic acid
in the use
composition is met. If the concentration of peroxycarboxylic acid in the use
composition is too high, controller 48 may manage addition of additional
diluent to the
use composition until the target concentration of peroxycarboxylic acid in the
use
composition is met. The target concentration may include a specific
concentration or
may include a range of acceptable concentrations. As another example, if the
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
44
concentration of hydrogen peroxide is too high, controller 48 may manage the
emptying
of the use composition vessel and production of a new use composition.
Figure 16 is a flow chart illustrating the process (200) by which controller
48
monitors and/or regulates the concentrations of peroxycarboxylic acid and/or
of
hydrogen peroxide in the use composition. Once controller 48 receives the
concentration data (202), controller 48 compares the received hydrogen
peroxide
concentration with predetermined H202 target criteria (204). If the received
hydrogen
peroxide concentration does not satisfy the H202 target criteria, controller
48 may
manage the emptying of the use composition vessel to the spent use composition
(206).
In other words, controller 48 may generate a control signal or sequence of
control
signals that cause the use composition vessel to be emptied of the spent use
composition. Controller 48 then manages production of a new use composition by
managing the flow of peroxycarboxylic acid and diluent into use composition
vessel
166 (208).
If the hydrogen peroxide concentration satisfies the predetermined H202 target
criteria (204), controller 48 compares the peroxycarboxylic acid concentration
in the use
composition with predetermined POAA target criteria (210). If the
peroxycarboxylic
acid concentration in the use composition does not satisfy the POAA target
criteria,
controller 48 may manage replenishing of the use composition. That is,
controller 48
may adjust the peroxycarboxylic acid concentration in the use composition
until it
satisfies the POAA target criteria (212). To do this, controller 48 may
control valves or
pumps on POAA concentrate holding tank 38 and/or diluent holding tank 164 such
that
a given amount of peroxycarboxylic acid and/or diluent is added to the use
composition
in use composition vessel 166, causing a resultant increase or decrease in the
concentration of peroxycarboxylic acid in the use composition.
In one embodiment, controller 48 may compute the amount of peroxycarboxylic
acid concentrate or diluent to be added to the use composition, for example,
based on
the known concentration of peroxycarboxylic acid in the use composition and
the
known or expected concentration of peroxycarboxylic acid in the concentrate
holding
tank 38. In another embodiment, controller 48 may iteratively add incremental
amounts
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
of peroxycarboxylic acid and/or diluent to the use composition until the
target
concentration of peroxycarboxylic acid in the use composition is reached.
After a new composition is produced (208) or the POAA concentration in the
use composition is replenished/adjusted (212), controller 48 may record the
information
5 concerning the timing, received concentration data, amount of use
composition made or
the relative amounts of concentrate or diluent required to bring use
composition into
satisfactory compliance with the POAA and/or H202 target criteria. Controller
48 may
also analyze the data and generate various alarms, alerts, or reports based on
the stored
information and the results of the analysis. The alarms, alerts or reports may
be
10 communicated to a user via audio alarms such as beepers, buzzers or
recorded scripts
and/or visual indicators such as LEDS, numerical, graphical or interactive
displays on
peroxycarboxylic acid generator 20. The alarms, alerts or reports may also be
sent,
either by request or at periodic intervals, to a remote monitoring site via a
telephone
network, wireless network, e-mail, local area network, wide area network or
the
15 internet. Further, the alarms, alerts or reports may be obtained on site
or remotely via a
portable device such as a laptop computer, tablet PC, personal digital
assistant or other
handheld or portable devices. Controller 48 then waits for initiation of the
next
monitoring period (214), at which point controller 48 will receive the most
recently
measured concentrations of peroxycarboxylic acid and/or hydrogen peroxide. The
next
20 monitoring period may be initiated by a user, either locally or
remotely, or controller 48
may be programmed to periodically monitor and/or regulate the peroxycarboxylic
acid
and/or hydrogen peroxide concentrations in the use composition.
The H202 target criteria and POAA target criteria may vary depending upon the
application to which the use solution is directed. For example, the H202
target criteria
25 and POAA target criteria may vary depending upon the degree of efficacy
required for
the particular application to which the use solution is directed. In one
embodiment, the
POAA target criteria may be a minimum or a maximum POAA target concentration
(e.g., the measured POAA concentration in the use solution must remain above a
minimum POAA concentration or below a maximum POAA concentration). In another
30 embodiment, the POAA target criteria may be a range of acceptable POAA
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
46
concentrations (e.g., the measured POAA concentration in the use solution must
remain
above a minimum POAA concentration and below a maximum POAA concentration).
Likewise, in one embodiment, the H202 target criteria may be a minimum or a
maximum H202 target concentration (e.g., the measured H202 concentration in
the use
solution must remain above a minimum H202 concentration or below a maximum
H202
concentration). In another embodiment, the H202 target criteria may be a range
of
acceptable H202 concentrations (e.g., the measured H202 concentration in the
use
solution must remain above a minimum H202 concentration and below a maximum
H202 concentration).
Another function of controller 48 may be to monitor the overall performance of
peroxycarboxylic acid generator 20. Controller 48 may analyze the
concentration data
concerning the concentrations of peroxycarboxylic acid and hydrogen peroxide
in the
use composition to infer information concerning operation of the
peroxycarboxylic acid
generator 20. For example, peroxycarboxylic acid generator 20 is designed to
generate
a peroxycarboxylic acid concentrate composition having a known, controllable
peroxycarboxylic acid concentration. From this known concentration, the
concentrate
composition in POAA holding tank 38 is mixed with a known volume of diluent to
arrive at a corresponding expected and predictable POAA concentration in the
use
composition stored in use composition vessel 166. Concentration data
indicating a
lower than expected POAA concentration in the use composition may suggest that
the
concentration of peroxycarboxylic acid in the POAA concentrate is not at the
expected
level. This in turn may suggest that peroxycarboxylic acid generator 20 is not
performing according to specifications.
Controller 48 may take any of several courses of action when concentration
data
indicating a lower than expected POAA concentration in the use composition,
and thus
a lower than expected POAA concentration in the concentrate composition, is
received.
For example, controller 48 may compensate for a lower or higher than expected
POAA
concentration in the use composition by adjusting certain operating parameters
of
peroxycarboxylic acid generator 20 such that the resulting concentration of
peroxycarboxylic acid in the POAA concentrate composition output on line 36 is
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
47
increased or decreased. This may be an iterative process which is repeated
until a
desired concentration of peroxycarboxylic acid in the POAA concentrate output
on line
36 is achieved. For example, controller 48 may control operation of pumps 162A
and/or 162B to adjust the amount of reagent flowing out of reagent supply
vessels 22
and/or 24 and into peroxycarboxylic acid generator 20 to cause a corresponding
increase or decrease in the concentration of the POAA concentrate generated.
Alternatively or in addition to compensating for lower/higher than expected
concentrations, controller 48 may generate alarms, alerts or reports directed
to a user
that maintenance of certain components of peroxycarboxylic acid generator 20
may be
required. For example, one or both of reagent supply vessels 22 or 24 may need
to be
replenished or pump or valve parameters may require adjustment. Analysis and
reports
of the data may also be generated. For example, statistical trending of the
acetic acid
and hydrogen peroxide pump rates versus the concentrations of POAA and
hydrogen
peroxide within vessel 38 can be used to predict conversion efficiency.
Figure 17 is a flowchart illustrating an example "generator check" process
(220)
by which controller 48 monitors and regulates operation of peroxycarboxylic
acid
generator 20. Once the concentration data concerning the concentrations of
peroxycarboxylic acid and/or hydrogen peroxide in the use composition are
received
(222), controller 48 compares the peroxycarboxylic acid concentration to an
expected
POAA concentration (224). If the concentration of peroxycarboxylic acid does
not
meet the expected POAA concentration (224), controller 48 may adjust certain
operating parameters of peroxycarboxylic acid generator 20 to cause a
resultant change
in the peroxycarboxylic acid concentration in the POAA concentrate (228).
For example, controller 48 may control operation of pumps 162A and/or 162B
to adjust the amount of reagent flowing out of reagent supply vessels 22
and/or 24 and
into peroxycarboxylic acid generator 20 to cause a corresponding increase or
decrease
in the concentration of the POAA concentrate composition output on line 36.
Controller 48 may also increase or slow the flow rate of the reaction mixture
through
the reaction catalyst to a flow rate that results in the desired
concentration. Controller
48 may calculate the change in flow rate employing factors including the
temperature of
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
48
the composition and the concentration of peroxycarboxylic acid. Based on the
equilibration reaction kinetics and thermodynamics, contact time to the
reaction catalyst
will determine the end concentration of POAA, just as the concentration of the
reactant
species. In this way, the concentration of peroxycarboxylic acid in the use
composition
may be maintained within an expected range, even when generator 20 is not
operating
entirely up to specifications.
After the POAA concentration has been checked and adjusted, if necessary (224,
228), controller 48 may record and store the information concerning the
timing,
received concentration data, specific adjustments made to the various
operating
parameters of generator 20 (i.e., specific adjustments to parameters such as
pump and/or
valve speed and timing, the amounts of additional POAA or hydrogen peroxide
added
from reagent vessels 22 and/or 24 to get the system back up to specifications,
etc.).
This information may be useful to service personnel in performing diagnostic
and
maintenance tasks on generator 20, and in monitoring efficiency of
peroxycarboxylic
acid generator 20. For example, if the system continuously generates a product
identified as low in POAA content, this indicates the system will require
service by
changing the reaction catalyst.
Controller 48 may also analyze the information and generate various alarms,
alerts, or reports based on this stored information. The alarms, alerts or
reports may be
communicated to a user via audio alarms such as beepers, buzzers or recorded
scripts
and/or visual indicators such as LEDS, numerical, graphical or interactive
displays on
peroxycarboxylic acid generator 20. The alarms, alerts or reports may also be
sent,
either by request or at periodic intervals, to a remote monitoring site via a
telephone
network, wireless network, e-mail, local area network, wide area network or
the
internet. Further, the alarms, alerts or reports may be obtained on site or
remotely via a
portable device such as a laptop computer, tablet PC, personal digital
assistant or other
handheld or portable devices.
Controller 48 then waits for initiation of the next generator check (230), at
which point controller 48 will receive the most recent concentration data. The
next
generator check may be initiated by a user, either locally or remotely, or
controller 48
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
49
may be programmed to perform generator checks at predetermined periodic
intervals.
For example, controller 48 may repeat the process shown in Figure 17
periodically to
ensure that generator 20 is performing in accordance with specifications and
to ensure
that the desired level of peroxycarboxylic acid in the use composition is
maintained.
Generator checks may be performed, for example, on a daily, weekly or monthly
basis.
Methods of Making Peroxycarboxylic Acids
The present invention includes a method for making a peroxycarboxylic acid.
The method includes contacting a reagent with a pretreatment column and a
reaction
mixture with a reaction catalyst. Contacting can include contacting one or
more
reagents employed in making the peroxycarboxylic acid with, for example, a
cation
exchanger in acid form or inert metal (e.g., Na + or K ) form. The reagent can
include
hydrogen peroxide, carboxylic acid, or a mixture of hydrogen peroxide and
carboxylic
acid. Contacting with the reaction catalyst can include contacting the
catalyst with
carboxylic acid (or suitable precursor) and oxidizing agent (e.g., a peroxide)
to form a
peroxycarboxylic acid. The reaction catalyst can be a strong acid (e.g., a
polystyrene
sulfonic acid) to catalyze reaction of hydrogen peroxide with carboxylic acid
to form
peroxycarboxylic acid. Pretreating one or more reagents can increase the life,
activity,
and/or safety of the reaction catalyst.
The method can also include monitoring the safety of the method and the
apparatus carrying it out. Monitoring safety can include monitoring and/or
regulating
one or more conditions of the pretreatment column and/or the reaction
catalyst.
Monitoring can include monitoring and/or regulating pressure, temperature,
metal
content, and/or presence of gas resulting from decay of peroxide (e.g.,
oxygen).
Measuring of one or more of these parameters can take place at or in the
pretreatment
column, at or in the reaction catalyst, for one or more of the reagents
before, in, or after
a pretreatment column, for the reaction mixture before, in, or after a
pretreatment
column, for the reaction mixture before, in, or after the reaction catalyst,
or more than
one of these (a combination thereof). Measuring can include determining a
difference
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
in one or more of these parameters between any two points, for example,
between any
two of the listed locations.
The method can include providing one or more reagents (e.g., hydrogen
peroxide or carboxylic acid(s)) in one or more reagent vessels. Mixing of the
reagents
5 can take place before or after one or more of the reagents contact a
pretreatment
column. Reacting the pretreated reaction mixture or mixture of pretreated
reactant with
untreated reactant then occurs by contacting with the reaction catalyst.
Reacting can
include contacting the reaction mixture with the reaction catalyst at a
controlled and
predetermined flow rate and/or for a predetermined time. Reacting produces
10 peroxycarboxylic acid. The method can also include using or storing the
peroxycarboxylic acid.
In an embodiment, the method includes pretreating one or more reagents
independently of the others. Mixing of the one or more pretreated reagents
with an
untreated reagent can then occur before pretreating the mixed reagents.
Alternatively,
15 each reagent can be pretreated independently and then mixed and
contacted with the
reaction catalyst. Each pretreatment takes place for a predetermined time to
provide the
desired amount of contaminant removal from the pretreated composition.
Pretreating can employ a plurality of (e.g., two) pretreatment columns coupled
in parallel. One column can be idle while the other column is pretreating. The
method
20 can include switching flow from a used pretreatment column to a
pretreatment column
that is ready for use. The method can include replacing, maintaining, washing,
or the
like the pretreatment column that is not being used. Washing can include
washing with,
for example, a dilute strong mineral acid, such as sulfuric acid. Washing can
include
back flushing the pretreatment column. The method can continue while one of
the
25 pretreatment columns is being maintained or replaced. Changing columns
can be done
according to a predetermined schedule. Alternatively, the method can include
monitoring the safety of the pretreatment column and replacing it when the
monitoring
finds a predetermined condition.
Contacting the reaction mixture with the reaction catalyst can occur in one or
30 more beds, bags, or columns, which can be coupled in series, in
parallel, or with some
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
51
in series and some in parallel. The method can employ contacting with four
columns
containing reaction catalyst and connected in series. Reacting can employ a
bed, bag,
or column until that bed, bag, or column has received sufficient use or is in
a condition
that indicates it is no longer fit for use. During reacting on a first bed,
bag, or column, a
second bed, bag, or column can remain ready for use. The method can include
switching flow from the first bed, bag, or column to the second bed, bag, or
column
when the first is no longer to be used.
The condition of the bed, bag, or column that is in use can be measured by the
safety system, which can also control the valve system. Changing reaction
catalysts can
be done according to a predetermined schedule. Alternatively, the method can
include
monitoring the safety of the reaction catalyst and replacing it when the
monitoring finds
a predetermined condition. The method can include washing the reaction
catalyst.
Washing can include washing with, for example, a dilute strong mineral acid,
such as
sulfuric acid. Washing can include back flushing a bed, bag, or column of the
reagent
catalyst.
Monitoring safety can include measuring one or more properties of the
pretreatment column, of the reaction catalyst, or both. Monitoring safety can
include
measuring, for example, pressure (e.g., increased pressure), temperature
(e.g., increased
temperature), or both. In an embodiment, measuring can include measuring a
difference in temperature between two points in or around (e.g., before and
after or
before and in) the pretreatment column. Measuring an increase in the
difference in
temperature or difference in pressure for two points in or around a
pretreatment column
can result in the system providing a perceptible signal if the increase is
above a
predetermined level. Measuring a change above the predetermined level can
trigger
(manual or automated) actuating a pressure release valve, stopping flow of one
or more
reagents, causing water to flow into the pretreatment column, causing
carboxylic acid
composition to flow into the pretreatment column, shutting down the method, or
a
combination thereof. Triggering can also result in switching to another
pretreatment
column or bed or column of reaction catalyst.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
52
Monitoring safety can include measuring conditions at an inlet or outlet of a
pretreatment column, within that column (e.g., near the entrance of the
column, in the
interior of the column, or near the exit from the column), or in a conduit
entering or
leaving the pretreatment column. Monitoring can include measuring temperature
at the
entrance to the pretreatment column and in the first 25% of the pretreatment
column.
The method can also include storing, handling, diluting, and formulating the
composition made by the method. For example, the method can include using or
storing the peroxycarboxylic acid. The method can include diluting and/or
formulating
the composition from the reaction catalyst or storage. The method can include
diluting
a concentrate fore use. Diluting can add and/or mix a diluent or carrier, such
as water,
into the peroxycarboxylic acid to achieve a diluted composition containing,
for
example, a desired use concentration of peroxycarboxylic acid. The desired
concentration can be, for example, about 2 to about 5000 ppm. Diluting can
include
adding another ingredient to the peroxycarboxylic acid composition.
Formulating can
include dispensing a desired amount of an added ingredient into the
composition or
diluted composition.
Storing can include monitoring the condition of the composition during
storage.
Monitoring can measure the content of peroxycarboxylic acid, carboxylic acid,
and/or
hydrogen peroxide in the composition, for example, in a stored use
composition. In an
embodiment, the method includes replenishing system a stored use composition.
Replenishing can include monitoring the content of the use composition. If,
for
example, the concentration of peroxycarboxylic acid decreases below a
predetermined
level or the concentration of carboxylic acid increases above a predetermined
level,
replenishing then includes adding more concentrated peroxycarboxylic acid
composition to the use composition or emptying the vessel of the spent use
composition.
The method can also include controlling reagent flow. Controlling reagent flow
can include monitoring the peroxycarboxylic acid composition after the
reaction
catalyst, for example, at an outlet from the last reaction catalyst column.
Monitoring
can determine whether the composition includes the desired concentration of
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
53
peroxycarboxylic acid (e.g., the equilibrium concentration). If the
composition includes
less than the desired concentration, the controlling can include slowing the
flow rate of
the reaction mixture through the reaction catalyst to a flow rate that results
in the
desired concentration. Controlling can include calculating the change in flow
rate
employing factors including the temperature of the composition and the
concentration
of peroxycarboxylic acid.
The present invention includes a method for making a composition including
one peroxycarboxylic acid. The method includes contacting a carboxylic acid
with a
pretreatment column, mixing the pretreated carboxylic acid with hydrogen
peroxide,
and contacting the reaction mixture with a reaction catalyst to produce the
peroxycarboxylic acid. The peroxycarboxylic acid can be a short chain
peroxycarboxylic acid (e.g., peroxyacetic acid) or a medium chain
peroxycarboxylic
acid (e.g., peroxyoctanoic acid).
The present invention includes a method for making a composition of mixed
peroxycarboxylic acids. The method includes contacting a short chain
carboxylic acid
with a first pretreatment column, mixing the pretreated short chain carboxylic
acid with
hydrogen peroxide, and contacting the first reaction mixture with a first
reaction
catalyst to produce the short chain peroxycarboxylic acid. The method includes
contacting a medium chain carboxylic acid with a second pretreatment column,
mixing
the pretreated medium chain carboxylic acid with hydrogen peroxide, and
contacting
the second reaction mixture with a second reaction catalyst to produce the
medium
chain peroxycarboxylic acid. Mixing the short chain peroxycarboxylic acid and
the
medium chain peroxycarboxylic acid produces the mixed peroxycarboxylic acid
composition.
Methods Conducted at the Site of Use
The present invention also relates to methods of making a peroxycarboxylic
acid
at the site of its use. For example, the method of making peroxycarboxylic
acid
described above can be conducted at a plant, e.g. a beverage plant, where the
peroxycarboxylic acid will be used. The site of use can be any of a variety of
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
54
production facilities where a peroxycarboxylic acid might be used. Sites of
use include
a beverage plant, a food processing plant, a disassembly plant, a meat
processing plant,
or the like. At the site of use, the peroxycarboxylic acid composition can be
applied to
objects including equipment, containers, and food products. Food products
include, for
example, plant product, product, meat, meat product, poultry, and the like. In
an
embodiment, the method can include applying the present peroxycarboxylic acid
composition to a beverage container, e.g., a plastic bottle or a can.
For example, the method of making peroxycarboxylic acid described above can
be conducted at a wood pulp producing or paper plant where the
peroxycarboxylic acid
will be used. By way of further example, the method of making peroxycarboxylic
acid
described above can be conducted at a waste treatment plant where the
peroxycarboxylic acid will be used. Sites of use include any of a variety of
plants that
process, use or handle (e.g., bleach) pulp or make paper, plants that handle
waste, such
as industrial waste, food production waste, waste from a beverage plant, waste
from a
food processing plant, waste from a disassembly plant, waste from a meat
processing
plant, or the like. At the site of use, the peroxycarboxylic acid composition
can be
applied to objects including equipment, pulp, waste, plant surfaces and
buildings, other
objects in the plant or facility, or the like. In an embodiment, the method
can include
applying the present peroxycarboxylic acid composition to pulp, to waste, to a
waste
treatment facility, or to waste treatment equipment.
The method can include providing carboxylic acid (e.g., acetic acid and/or
octanoic acid) and/or oxidizing agent (e.g., hydrogen peroxide) at the site of
use (e.g., a
beverage plant, a pulp processing plant, or a waste treatment plant) and
conducting the
present method with those reagents at the site of use. The method can include
shipping
the carboxylic acid (e.g., acetic acid and/or octanoic acid) and/or oxidizing
agent (e.g.,
hydrogen peroxide) to the site of use (e.g., a beverage plant, a pulp
processing plant, or
a waste treatment plant) for conducting the present method with those reagents
at the
site of use. The method can include plant or plant organization personnel
requesting or
ordering the carboxylic acid (e.g., acetic acid and/or octanoic acid) and/or
oxidizing
agent (e.g., hydrogen peroxide) for delivery to the site of use (e.g., a
beverage plant, a
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
pulp processing plant, or a waste treatment plant) for conducting the present
method
with those reagents at the site of use.
An Embodiment of the Method
5 In an embodiment, the method includes contacting a reaction mixture
with a
reaction catalyst and monitoring the safety of the method and the apparatus
carrying it
out. The reaction mixture can include a mixture of hydrogen peroxide and
carboxylic
acid. Contacting with the reaction catalyst can include contacting the
catalyst with
carboxylic acid (or suitable precursor) and oxidizing agent (e.g., a peroxide)
to form a
10 peroxycarboxylic acid. The reaction catalyst can be a strong acid (e.g.,
a polystyrene
sulfonic acid) to catalyze reaction of hydrogen peroxide with carboxylic acid
to form
peroxycarboxylic acid.
Monitoring safety can include monitoring and/or regulating one or more
conditions of the reaction catalyst. Monitoring can include monitoring and/or
15 regulating pressure, temperature, metal content, and/or presence of gas
resulting from
decay of peroxide (e.g., oxygen). Measuring of one or more of these parameters
can
take place at or in the reaction catalyst, for example, for the reaction
mixture before, in,
or after the reaction catalyst, or more than one of these (a combination
thereof).
Measuring can include determining a difference in one or more of these
parameters
20 between any two points, for example, between any two of the listed
locations.
Monitoring safety can include measuring one or more properties of the reaction
catalyst. Monitoring safety can include measuring, for example, pressure
(e.g.,
increased pressure), temperature (e.g., increased temperature), or both. In an
embodiment, measuring can include measuring a difference in temperature
between two
25 points in or around (e.g., before and after or before and in) the
reaction catalyst.
Measuring an increase in the difference in temperature or difference in
pressure for two
points in or around the reaction catalyst can result in the system providing a
perceptible
signal if the increase is above a predetermined level. Measuring a change
above the
predetermined level can trigger (manual or automated) actuating a pressure
release
30 valve, stopping flow of one or more reagents, causing water to flow into
the reaction
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
56
catalyst, causing carboxylic acid composition to flow into the reaction
catalyst, shutting
down the method, or a combination thereof. Triggering can also result in
switching to
another bed or column of reaction catalyst.
Monitoring safety can include measuring conditions at an inlet or outlet of a
column, bed, or bag of reaction catalyst, within the column, bed, or bag of
reaction
catalyst (e.g., near the entrance, in the interior, or near the exit), or in a
conduit entering
or leaving the reaction catalyst. Monitoring can include measuring temperature
at the
entrance to the reaction catalyst and in the first 25% of the reaction
catalyst.
This embodiment need not include pretreating the reagents in with material
outside the column, bed, or bag of reaction catalyst.
Peroxycarboxylic Acid Compositions
The present method and apparatus can be employed to make any of a variety of
peroxycarboxylic acid compositions. In an embodiment, the present method
includes a
peroxycarboxylic acid composition made by the method and/or apparatus
described
hereinabove. A peroxycarboxylic acid composition according to the present
invention
can have advantageous stability, which can be due to a low level of metal ion
(e.g., less
than about 10 ppm or less than about 10 ppb metal ion). The low level of metal
ion can
be achieved and maintained in the present compositions without added
stabilizer or
chelating agent. Accordingly, the present invention relates to a stable
peroxycarboxylic
acid composition lacking or substantially free of stabilizer or chelating
agent. The
present invention also includes a stable peroxycarboxylic acid composition
that includes
only volatile compounds. The present invention also includes a
peroxycarboxylic acid
composition that includes only volatile compounds.
The term "stable" as applied herein to a peroxycarboxylic acid composition
means a composition that retains about 90% of the peroxycarboxylic acid for at
least
about 6 months, that retains about 90% of the peroxycarboxylic acid for at
least about 7
days, or that retains about 90% of the peroxycarboxylic acid for at least
about 1 day.
The stable composition can be one that retains about 95% of the
peroxycarboxylic acid
for at least about 14 days, that retains about 95% of the peroxycarboxylic
acid for at
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
57
least about 7 days, or that retains about 95% of the peroxycarboxylic acid for
at least
about 3 days. Being depleted of trace metals by the generator, the "90%"
stability
threshold is generally speaking a function of the equilibrium percarboxylic
acid
concentration. The higher concentrations tending to decompose more rapidly.
In certain embodiments, the present peroxycarboxylic acid composition includes
metal ion at a level less than about 10 ppm, less than about 1 ppm, less than
about 100
ppb, less than about 10 ppb, or less than about 1 ppb ppm. Such metal ion can
include
Fe, Cu, Mn, Ni, Ti, Co, a mixture thereof, or any of the transition metal
ions.
In certain embodiments, the composition at equilibrium includes about 35 wt-%
peroxycarboxylic acid and about 15 wt-% hydrogen peroxide; about 15 (e.g., 17)
wt-%
peroxycarboxylic acid and about 15 (e.g., 13) wt-% hydrogen peroxide; about 10
(e.g.,
9.7) wt-% peroxycarboxylic acid and about 25 (e.g., 24) wt-% hydrogen
peroxide; about
(e.g., 13) wt-% peroxycarboxylic acid and about 2 (e.g., 1.9) wt-% hydrogen
peroxide, or about 0.5 wt-% peroxycarboxylic acid and about 5 (e.g., 4.8) wt-%
15 hydrogen peroxide.
In certain embodiments, the composition at equilibrium includes about 35 wt-%
short chain peroxycarboxylic acid and about 15 wt-% hydrogen peroxide; about
15
(e.g., 17) wt-% short chain peroxycarboxylic acid and about 15 (e.g., 13) wt-%
hydrogen peroxide; about 10 (e.g., 9.7) wt-% short chain peroxycarboxylic acid
and
about 25 (e.g., 24) wt-% hydrogen peroxide; about 15 (e.g., 13) wt-% short
chain
peroxycarboxylic acid and about 2 (e.g., 1.9) wt-% hydrogen peroxide, or about
0.5 wt-
% short chain peroxycarboxylic acid and about 5 (e.g., 4.8) wt-% hydrogen
peroxide.
In certain embodiments, the composition at equilibrium includes about 20
(e.g.,
19) wt-% medium chain peroxycarboxylic acid and about 30 (e.g., 32) wt-%
hydrogen
peroxide; about 5 (e.g., 6.8) wt-% medium chain peroxycarboxylic acid and
about 20
wt-% hydrogen peroxide; about 2 (e.g., 2.1) wt-% medium chain peroxycarboxylic
acid
and about 20 (e.g., 21) wt-% hydrogen peroxide; or about 1 (e.g., 1.2) wt-%
medium
chain peroxycarboxylic acid and about 20 (e.g., 22) wt-% hydrogen peroxide.
In certain embodiments, the composition at equilibrium includes about 15
(e.g.,
14) wt-% short chain peroxycarboxylic acid, about 5 (e.g., 5.7) wt-% medium
chain
CA 02663953 2014-10-30
58
peroxycarboxylic acid, and about 3 (e.g., 2.8) wt-% hydrogen peroxide; about
20 (e.g.,
19) wt-% short chain peroxycarboxylic acid, about 3 (e.g., 2.7) wt-% medium
chain
peroxycarboxylic acid, and about 4 wt-% hydrogen peroxide; about 20 (e.g., 22)
wt-%
short chain peroxycarboxylic acid, about 1 (e.g., 0.7) wt-% medium chain
peroxycarboxylic acid, and about 5 (e.g., 4.6) wt-% hydrogen peroxide; about
15 (e.g.,
17.4) wt-% short chain peroxycarboxylic acid, about 0.4 wt-% medium chain
peroxycarboxylic acid, and about 15 (e.g., 13) wt-% hydrogen peroxide.
In certain embodiments, the present composition includes peroxycarboxylic acid
and hydrogen peroxide in a ratio of about 2:1 (e.g., 2.4:1); peroxycarboxylic
acid and
hydrogen peroxide in a ratio of about 1.4:1; peroxycarboxylic acid and
hydrogen
peroxide in a ratio of 0.5:1 (e.g., 0.4:1); or peroxycarboxylic acid and
hydrogen
peroxide in a ratio of about 7:1.
The present apparatus and method can be employed to make any of a variety of
peroxycarboxylic acid compositions. Compositions that can he made by the
present
apparatus and method (which can include adding materials such as adjuvant,
stabilizing
agent, chelating agent, or the like after forming the peroxycarboxylic acid)
include
compositions disclosed in U.S. Patent Nos. 5,200,189, 5,314,687, 5,718,910,
and
6,183,807 and in pending U.S. Application Nos. 09/614,631, filed July 12,
2000,
10/754.426. filed January 9,2004, and 11/030,641, filed January 4,2005.
Embodiments of the Invention
Embodiments of the invention include, but are not limited to:
In an embodiment, the present invention includes an apparatus for making
peroxycarboxylic acid. This embodiment of the apparatus can include a first
pretreatment column, a first reaction catalyst column, a first and a second
reagent
vessel, a safety system, a reagent conduit, a reaction mixture conduit, and a
peracid
conduit. The first and second reagent vessels can be in fluid communication
through
the reagent conduit with the first pretreatment column, The first reagent
vessel can be
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
59
configured for containing a liquid oxidizing agent composition and the second
reagent
vessel can be configured for containing a liquid carboxylic acid composition.
The
reagent conduit can define mixing chamber for the reagents.
The first pretreatment column can be in fluid communication through the
reaction mixture conduit with the first reaction catalyst column. The first
pretreatment
column can be configured for removing metal ion from a mixture of the
carboxylic acid
composition and the oxidizing agent composition. The first reaction catalyst
column
can be configured for catalyzing a reaction of the carboxylic acid and the
oxidizing
agent to produce peroxycarboxylic acid. The first reaction catalyst column can
be in
fluid communication through the peracid conduit with a site of storage or use
of a
peroxycarboxylic acid composition. The safety system including a processor, a
first
condition sensor, and a second condition sensor. The first condition sensor
can be
disposed in or on the mixing chamber and can be configured for measuring a
condition
of the reagents. The second condition sensor can be disposed at or in the
first
pretreatment column or in the reaction mixture conduit proximal an exit from
the first
pretreatment column and can be configured for measuring the condition of the
reagents.
The processor can be configured for determining a difference between the
condition
measured by the first condition sensor and the condition measured by the
second
condition sensor and providing a detectable signal if the difference meets or
exceeds a
predetermined value.
In an embodiment, the first pretreatment column includes a strong cation
exchanger in acid form or in inert metal form.
The apparatus can also include a second pretreatment column. The second
pretreatment column can be in fluid communication through the reagent conduit
with
the second reagent vessel and the first pretreatment column. The second
pretreatment
column can be configured for removing metal ion from the carboxylic acid
composition. In an embodiment, the second pretreatment column can include a
strong
cation exchanger in acid form or in inert metal form.
The apparatus can also include a third pretreatment column. The third
pretreatment column can be in fluid communication through the reagent conduit
with
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
the first reagent vessel and the first pretreatment column. The third
pretreatment
column can be configured for removing metal ion from the oxidizing agent
composition. In an embodiment, the third pretreatment column can include a
strong
cation exchanger in acid form or in inert metal form.
5 The apparatus can also include a second, a third, and a fourth
reaction catalyst
column. The first, second, third, and fourth reaction catalyst columns can be
coupled in
series and can be in fluid communication through the peracid conduit with the
site of
storage or use of the peroxycarboxylic acid composition.
In an embodiment, the reaction catalyst includes a strong acid catalyst that
can
10 be physically removed from the reaction mixture. In an embodiment, the
reaction
catalyst includes a strong cation exchanger in acid form. In an embodiment,
the
reaction catalyst includes an inorganic compound including an insoluble strong
acid.
The first and second condition sensors can be configured to measure
temperature, pressure, metal content, or combination thereof. For example, the
first and
15 second condition sensors are configured to measure temperature.
In an embodiment, the safety system is configured to provide a detectable
signal
if the temperature difference is greater than 10 C, equal to 10 C, or
greater than or
equal to 10 C.
The detectable signal can actuates interruption of operation of the apparatus.
20 For example, the detectable signal can actuate interruption of operation
of the apparatus
by: actuating a pressure release valve to release pressure in the first
pretreatment
column; stopping flow of one or more reagents into the columns; causing water
to flow
through the reagent conduit, the first pretreatment column, and the reaction
mixture
conduit; causing carboxylic acid composition to flow through the reagent
conduit, the
25 first pretreatment column, and the reaction mixture conduit; shutting
down the
apparatus; or a combination thereof.
The apparatus can also include a peracid vessel, a dilution system, a dilute
tank,
a replenishing system, and an output conduit. The peracid vessel can be in
fluid
communication with the peracid conduit and can be configured to receive and
contain
30 the peroxycarboxylic acid composition. The peracid vessel can be in
fluid
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
61
communication through the output conduit with the dilution system. The
dilution
system can be configured to mix the peroxycarboxylic acid composition and a
predetermined amount of carrier to form a diluted composition of a
predetermined
concentration of peroxycarboxylic acid in the dilute tank. The replenishing
system can
be configured to monitor a concentration of peroxycarboxylic acid, carboxylic
acid,
oxidizing agent, or combination thereof in the diluted composition and to add
peroxycarboxylic acid composition to the diluted composition if the
concentration of
peroxycarboxylic acid, carboxylic acid, oxidizing agent, or combination
thereof is less
than a predetermined value, equal to a predetermined value, or less than or
equal to a
predetermined value.
In yet another embodiment, the apparatus can also include a fourth
pretreatment
column, a fifth reaction catalyst column, a third and a fourth reagent vessel,
a medium
reagent conduit, a medium reaction mixture conduit, and a medium peracid
conduit.
The third and fourth reagent vessels can be in fluid communication through the
medium
reagent conduit with the fourth pretreatment column. The third reagent vessel
can be
configured for containing a liquid composition of oxidizing agent, the fourth
reagent
vessel can be configured for containing a liquid composition of medium chain
carboxylic acid. The medium reagent conduit can define medium mixing chamber
for
the medium reagents. The fourth pretreatment column can be in fluid
communication
through the medium reaction mixture conduit with the fifth reaction catalyst
column.
The fourth pretreatment column can be configured for removing metal ion from a
mixture of the liquid composition of medium chain carboxylic acid and the
oxidizing
agent composition. The fifth reaction catalyst column can be configured for
catalyzing
a reaction of the medium chain carboxylic acid and the oxidizing agent to
produce
medium chain peroxycarboxylic acid. The fifth reaction catalyst column can be
in fluid
communication through the medium peracid conduit with a site of storage or use
of a
medium chain peroxycarboxylic acid composition. The fourth pretreatment column
can
include a strong cation exchanger in acid form or in inert metal form.
In this or another embodiment, the safety system can also include a third
condition sensor and a fourth condition sensor. The third condition sensor can
be
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
62
disposed in or on the medium mixing chamber and can be configured for
measuring a
condition of the medium reagents. The fourth condition sensor can be disposed
at or in
the fourth pretreatment column or in the medium reaction mixture conduit
proximal an
exit from the fourth pretreatment column and can be configured for measuring
the
condition of the medium reagents. The processor can be configured for
determining a
difference between the condition measured by the third condition sensor and
the
condition measured by the fourth condition sensor and providing a detectable
signal if
the difference meets or exceeds a predetermined value.
In this or another embodiment, the second reagent vessel is configured
for containing a liquid composition of a short chain carboxylic acid. The
first
pretreatment column is configured for removing metal ion from a mixture of the
short chain carboxylic acid composition and the oxidizing agent composition.
The first reaction catalyst column is configured for catalyzing a reaction of
the
short chain carboxylic acid and the oxidizing agent to produce short chain
peroxycarboxylic acid.
In this embodiment, the third and fourth condition sensors can be configured
to
measure temperature, pressure, metal content, or combination thereof. For
example, the
third and fourth condition sensors can be configured to measure temperature.
This or another embodiment can also include a fifth pretreatment column. The
fifth pretreatment column can be in fluid communication through the medium
reagent
conduit with the fourth reagent vessel and the fourth pretreatment column. The
fifth
pretreatment column can be configured for removing metal ion from the liquid
composition of medium chain carboxylic acid. The fifth pretreatment column can
include a strong cation exchanger in acid form or in inert metal form.
This or another embodiment can also include a sixth pretreatment column. The
sixth pretreatment column can be in fluid communication through the medium
reagent
conduit with the third reagent vessel and the fourth pretreatment column. The
sixth
pretreatment column can be configured for removing metal ion from the liquid
composition of oxidizing agent. The sixth pretreatment column can include a
strong
cation exchanger in acid form or in inert metal form.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
63
In this or another embodiment, the reaction catalyst can include a strong acid
catalyst that can be physically removed from the reaction mixture; a strong
cation
exchanger in acid form; or an inorganic compound including an insoluble strong
acid.
This embodiment of the apparatus can also include a sixth, a seventh, and an
eighth reaction catalyst column. The fifth, sixth, seventh, and eighth
reaction catalyst
columns can be coupled in series and can be in fluid communication through the
medium peracid conduit with the site of storage or use of the medium chain
peroxycarboxylic acid composition.
In this embodiment, the peracid vessel can be in fluid communication with the
medium peracid conduit and can be configured to receive and contain the medium
chain
peroxycarboxylic acid composition.
This or another embodiment can also include a second processor. The
second processor can be configured for determining a difference between the
condition measured by the third condition sensor and the condition measured by
the fourth condition sensor and providing a detectable signal if the
difference
meets or exceeds a predetermined value.
In an embodiment, the first reaction catalyst column has a volume of about 9.6
L. In certain embodiments, each reaction catalyst column has a volume of about
9.6 L.
In an embodiment, the fifth reaction catalyst column has a volume of about 9.6
L.
In an embodiment, the first pretreatment column has a volume of about 4.6 L.
In an embodiment, the second pretreatment column has a volume of about 4.6 L.
In an
embodiment, the third pretreatment column has a volume of about 4.6 L. In an
embodiment, the fourth pretreatment column has a volume of about 4.6 L. In an
embodiment, the fifth pretreatment column has a volume of about 4.6 L. In an
embodiment, the sixth pretreatment column has a volume of about 4.6 L.
In an embodiment, the first reagent vessel contains about 35 to about 45 wt-%
hydrogen peroxide. In an embodiment, the second reagent vessel contains about
80 to
about 98 wt-% acetic acid. In an embodiment, the third reagent vessel contains
about
to about 45 wt-% hydrogen peroxide. In an embodiment, the second reagent
vessel
30 contains about 1 to about 10 wt-% octanoic acid.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
64
The present apparatus can also include a third reagent vessel configured to
contain a liquid medium chain carboxylic acid composition and in fluid
communication
through the reagent conduit with the first pretreatment column. Such an
embodiment
can also include a fourth pretreatment column. The fourth pretreatment column
can be
in fluid communication through the reagent conduit with the third reagent
vessel and the
first pretreatment column. The third reagent vessel can contains about 1 to
about 10 wt-
% octanoic acid.
The present invention also includes a method for making a peroxycarboxylic
acid. This method can include: providing a liquid composition of a carboxylic
acid and
an oxidizing agent; pretreating the liquid composition with a pretreatment
column to
remove metal ion from the mixed composition; measuring a condition of the
liquid
composition i) before pretreating and ii) at site of pretreating during
pretreating;
determining a difference between i) and ii); providing a detectable signal if
the
difference meets or exceeds a predetermined value; reacting the pretreated
composition
in the presence of a reaction catalyst that can be physically removed from
reaction
mixture to produce a peroxycarboxylic acid composition; and recovering the
peroxycarboxylic acid composition.
In an embodiment, pretreating includes contacting the mixed composition and a
strong cation exchanger in acid form or in inert metal form.
This method can also include: pretreating a liquid composition of carboxylic
acid to remove metal ion from the liquid composition of carboxylic acid; and
mixing the
pretreated liquid composition of carboxylic acid and oxidizing agent to form
the liquid
composition of a carboxylic acid and an oxidizing agent. In this embodiment,
pretreating can include contacting the liquid composition of carboxylic acid
and a
strong cation exchanger in acid form or in inert metal form.
This method can also include: pretreating a liquid composition of oxidizing
agent to remove metal ion from the liquid composition of oxidizing agent; and
mixing
the pretreated liquid composition of oxidizing agent and carboxylic acid to
form the
liquid composition of a carboxylic acid and an oxidizing agent. In this
embodiment,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
pretreating can include contacting the liquid composition of oxidizing agent
and a
strong cation exchanger in acid form or in inert metal form.
The method can include reacting in a column of insoluble reaction catalyst.
This
embodiment can also include reacting in a second, a third, and a fourth column
of
5 insoluble reaction catalyst. The first, second, third, and fourth
reaction catalyst columns
can be coupled in series.
In the method, reacting can include contacting the pretreated composition and
an
insoluble strong acid catalyst. In an embodiment, reacting can include
contacting the
pretreated composition and a strong cation exchanger in acid form. In an
embodiment,
10 reacting can include contacting the pretreated composition and an
inorganic compound
including an insoluble strong acid.
The method can include measuring temperature, pressure, metal content, or
combination thereof of the mixed composition. In an embodiment, the method
includes
measuring temperature of the mixed composition.
15 The method can include providing a detectable signal if the
temperature
difference is greater than 10 C, equal to 10 C, or greater than or equal to
10 C.
The method can also include, if the difference meets or exceeds a
predetermined
value, interrupting of operation of the apparatus by: actuating a pressure
release valve
to release pressure in an apparatus carrying out the method; stopping flow of
one or
20 more reagents into the apparatus; causing water to flow into the site of
pretreating;
causing carboxylic acid composition into the site of pretreating; shutting
down the
apparatus; or a combination thereof.
The method can also include mixing the peroxycarboxylic acid composition and
a predetermined amount of carrier to form a diluted composition of a
predetermined
25 concentration of peroxycarboxylic acid; storing the diluted composition;
monitoring
concentration of peroxycarboxylic acid, carboxylic acid, oxidizing agent, or
combination thereof in the diluted composition. If the concentration of
peroxycarboxylic acid, carboxylic acid, oxidizing agent, or combination
thereof is less
than a predetermined value, equal to a predetermined value, or less than or
equal to a
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
66
predetermined value, the method can include adding peroxycarboxylic acid
composition
to the diluted composition.
The method can also include mixing liquid composition of carboxylic acid and
oxidizing agent to form the liquid composition of a carboxylic acid and an
oxidizing
agent. This can form a liquid composition of carboxylic acid that includes
about 80 to
about 98 wt-% acetic acid. In an embodiment, the oxidizing agent includes
about 35 to
about 45 wt-% hydrogen peroxide. In an embodiment, the liquid composition of
carboxylic acid includes about 1 to about 20 wt-% octanoic acid.
The method can include providing a liquid composition of a plurality of
carboxylic acids and an oxidizing agent. In an embodiment, the method can also
include mixing a first liquid composition of carboxylic acid, a second liquid
composition of carboxylic acid, and oxidizing agent to form the liquid
composition of a
plurality of carboxylic acids and an oxidizing agent. In an embodiment, the
first liquid
composition of carboxylic acid includes about 80 to about 98 wt-% acetic acid.
In an
embodiment, the oxidizing agent includes about 35 to about 45 wt-% hydrogen
peroxide. In an embodiment, the second liquid composition of carboxylic acid
includes
about 1 to about 20 wt-% octanoic acid.
This or another embodiment of the method can also include pretreating a first
liquid composition of carboxylic acid to remove metal ion from the first
liquid
composition of carboxylic acid; and including the pretreated first liquid
composition of
carboxylic acid in the liquid composition of a plurality of carboxylic acids
and an
oxidizing agent.
This or another embodiment of the method can also include pretreating a liquid
composition of oxidizing agent to remove metal ion from the liquid composition
of
oxidizing agent; and including the pretreated liquid composition of oxidizing
agent in
the liquid composition of a plurality of carboxylic acids and an oxidizing
agent.
This or another embodiment of the method can also include pretreating a second
liquid composition of carboxylic acid to remove metal ion from the second
liquid
composition of carboxylic acid; and including the pretreated second liquid
composition
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
67
of carboxylic acid in the liquid composition of a plurality of carboxylic
acids and an
oxidizing agent.
In an embodiment, the liquid composition of a carboxylic acid and an oxidizing
agent includes about 40 to about 50 wt-% acetic acid and about 15 to about 25
wt-%
hydrogen peroxide. In an embodiment, the liquid composition of a carboxylic
acid and
an oxidizing agent includes about 25 to about 35 wt-% acetic acid, about 10 to
about 20
wt-% hydrogen peroxide, and about 2 to about 4 wt-% octanoic acid.
The method can include carrying out providing, pretreating, measuring,
determining, providing, reacting, and recovering at a site at which the
peroxycarboxylic
acid composition will be used to reduce the population of a microbe on an
object. This
embodiment of the method can also include delivering carboxylic acid and
oxidizing
agent to the site. In an embodiment, the method includes delivering a
plurality of
carboxylic acids to the site. In an embodiment, the method also includes
requesting
delivery of the carboxylic acid and the oxidizing agent from the site.
In an embodiment, the method also includes applying the peroxycarboxylic acid
composition to a beverage container at a beverage plant.
The invention also includes a method for making a peroxycarboxylic acid,
including. This method includes delivering carboxylic acid and oxidizing agent
to a site
at which a peroxycarboxylic acid composition will be made and used; providing
a liquid
composition of the carboxylic acid and oxidizing agent; pretreating the liquid
composition with a pretreatment column to remove metal ion from the mixed
composition; reacting the pretreated composition in the presence of a reaction
catalyst
that can be physically removed from reaction mixture to produce the
peroxycarboxylic
acid composition; recovering the peroxycarboxylic acid composition; and
applying the
peroxycarboxylic acid composition to an object to reduce the population of
microbe on
the object.
In an embodiment, the method includes delivering a plurality of carboxylic
acids
to the site. In an embodiment, the method also includes requesting delivery of
the
carboxylic acid and the oxidizing agent from the site.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
68
In an embodiment, the method also includes applying the peroxycarboxylic acid
composition to a beverage container at a beverage plant.
The present invention also includes a method for making a mixed
peroxycarboxylic acid composition. The method includes providing a liquid
composition of a short chain carboxylic acid and an oxidizing agent;
pretreating the
mixed short chain composition with a pretreatment column to remove metal ion
from
the short chain mixed composition; reacting the pretreated short chain
composition in
the presence of an insoluble reaction catalyst to produce a short chain
peroxycarboxylic
acid composition; providing a liquid composition of a medium chain carboxylic
acid
and an oxidizing agent; pretreating the mixed medium chain composition with a
pretreatment column to remove metal ion from the mixed medium chain
composition;
reacting the pretreated medium chain composition in the presence of an
insoluble
reaction catalyst to produce a medium peroxycarboxylic acid composition;
mixing the
short chain peroxycarboxylic acid composition and the medium chain
peroxycarboxylic
acid composition to produce a mixed peroxycarboxylic acid composition;
measuring a
condition of the short chain composition i) before pretreating and ii) at site
of
pretreating during pretreating; determining a difference between i) and ii);
and
providing a detectable signal if the difference between i) and ii) meets or
exceeds a
predetermined value; measuring a condition of the mixed medium chain
composition
iii) before pretreating and iv) at site of pretreating during pretreating;
determining a
difference between iii) and iv); and providing a detectable signal if the
difference
between iii) and iv), or both differences meets or exceeds a predetermined
value.
The present invention also includes a peroxycarboxylic acid composition made
by a method according to the invention. The method can include providing a
liquid
composition of a carboxylic acid and an oxidizing agent; pretreating the
liquid
composition with a pretreatment column to remove metal ion from the mixed
composition; measuring a condition of the liquid composition i) before
pretreating and
ii) at site of pretreating during pretreating; determining a difference
between i) and ii);
providing a detectable signal if the difference meets or exceeds a
predetermined value;
reacting the pretreated composition in the presence of an reaction catalyst
that can be
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
69
physically removed from reaction mixture to produce a peroxycarboxylic acid
composition; and recovering the peroxycarboxylic acid composition.
The invention includes a peroxycarboxylic acid composition. The composition
can include about 1 to about 35 wt-% peroxycarboxylic acid; about 5 to about
30 wt-%
hydrogen peroxide; and less than about 10 ppb metal. In an embodiment, the
composition retains 85 % of the peroxycarboxylic acid for at least about 13
days at 140
F. In an embodiment, the composition retains 95 % of the peroxycarboxylic acid
for at
least about 7 days at 140 F. In an embodiment, the composition includes about
0.5 to
about 35 wt-% short chain peroxycarboxylic acid. In an embodiment, the
composition
includes about 0.5 to about 20 wt-% medium chain peroxycarboxylic acid. In an
embodiment, the composition includes about 0.5 to about 35 wt-% short chain
peroxycarboxylic acid; and about 0.5 to about 20 wt-% medium chain
peroxycarboxylic
acid. In an embodiment, the composition includes peroxycarboxylic acid and
hydrogen
peroxide in a ratio of about 0.5:1 to about 7:1. In an embodiment, the
composition
includes only volatile compounds.
The present invention also includes a system. The system can include a
peroxycarboxylic acid generator that outputs a peroxycarboxylic acid
concentrate; a use
composition vessel that stores a use composition included of diluted
peroxycarboxylic
acid concentrate; and a controller that receives concentration data concerning
the
concentrations of peroxycarboxylic acid and hydrogen peroxide in the use
composition
and manages replenishing of the use composition when these concentrations do
not
satisfy predetermined criteria.
In an embodiment, the controller compares the concentration of
peroxycarboxylic acid to predetermined POAA target criteria and manages
addition of
peroxycarboxylic acid concentrate to the use composition when the
concentration data
indicates that the peroxycarboxylic acid concentration in the use composition
is too low.
In an embodiment, the controller compares the concentration of
peroxycarboxylic acid to predetermined POAA target criteria and manages
addition of
diluent to the use composition when the concentration data indicates that the
peroxycarboxylic acid concentration in the use composition is too high.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
In an embodiment, the controller compares the concentration of hydrogen
peroxide to predetermined H202 target criteria and manages emptying of the use
composition vessel and production of a new use composition when the
concentration
data indicates that the hydrogen peroxide concentration in the use composition
is too
5 high.
In an embodiment, the controller compares the concentration of
peroxycarboxylic acid to an expected POAA target concentration and regulates
operating parameters of the peroxycarboxylic acid generator to affect the
concentration
of peroxycarboxylic acid in the peroxycarboxylic acid concentrate output by
the
10 peroxycarboxylic acid generator.
The present invention also includes a method. The method can include
receiving concentration data concerning the concentrations of peroxycarboxylic
acid
and hydrogen peroxide in a use composition; comparing the concentration of
peroxycarboxylic acid with predetermined POAA target criteria; and
automatically
15 replenishing the use composition when the peroxycarboxylic acid
concentration does
not satisfy the predetermined POAA target criteria.
In an embodiment of this method, automatically replenishing the use
composition also includes automatically adding peroxycarboxylic acid
concentrate to
the use composition when the concentration data indicates that the
peroxycarboxylic
20 acid concentration in the use composition is too low.
In an embodiment of this method, automatically replenishing the use
composition also includes automatically adding diluent to the use composition
when the
concentration data indicates that the peroxycarboxylic acid concentration in
the use
composition is too high.
25 In an embodiment of this method, automatically replenishing the use
composition also includes automatically emptying the use composition vessel
and
producing a new use composition when the concentration data indicates that the
hydrogen peroxide concentration in the use composition is too high.
In an embodiment, the method also includes comparing the concentration of
30 peroxycarboxylic acid to an expected POAA target concentration and
regulating
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
71
operating parameters of the peroxycarboxylic acid generator to affect the
peroxycarboxylic acid concentration in the peroxycarboxylic acid concentrate
output by
a peroxycarboxylic acid generator.
Carboxylic Acids, Peroxycarboxylic Acids, and Additional Ingredients
Peroxycarboxylic (or percarboxylic) acids generally have the formula
R(CO3H)11, where, for example, R is an alkyl, arylalkyl, cycloalkyl, aromatic,
or
heterocyclic group, and n is one, two, or three, and named by prefixing the
parent acid
with peroxy. The R group can be saturated or unsaturated as well as
substituted or
unsubstituted. Peroxy forms of carboxylic acids with more than one carboxylate
moiety
can have one or more of the carboxyl moieties present as peroxycarboxyl
moieties.
The methods of the invention can employ peroxycarboxylic acids containing, for
example, 2 to 12 carbon atoms. For example, peroxycarboxylic (or
percarboxylic) acids
can have the formula R(CO3H)11, where R is a C1-C11 alkyl group, a C1-C11
cycloalkyl, a
Ci-Cii arylalkyl group, Ci-Cii aryl group, or a Ci-Cii heterocyclic group; and
n is one,
two, or three. The methods of the invention can employ a medium chain
peroxycarboxylic acid containing, for example, 6 to 12 carbon atoms. For
example,
medium chain peroxycarboxylic (or percarboxylic) acids can have the formula
R(CO3H)11, where R is a C5-C11 alkyl group, a C5-C11 cycloalkyl, a C5-C11
arylalkyl
group, C5-C11 aryl group, or a C5-C11 heterocyclic group; and n is one, two,
or three.
The methods of the invention can employ a short chain peroxycarboxylic acid
containing, for example, 1 to 4 carbon atoms. For example, short chain
peroxycarboxylic (or percarboxylic) acids can have the formula R(CO3H)11,
where R is
H, a Ci-C3 alkyl group, or a C3 cycloalkyl and n is one or two. The mixed
peroxycarboxylic acid composition employed in the present invention can
include one
or more short chain peroxycarboxylic acids and one or more medium chain
peroxycarboxylic acids.
Peroxycarboxylic acids can be made by the direct action of an oxidizing agent
on a carboxylic acid, by autoxidation of aldehydes, or from acid chlorides,
and
hydrides, or carboxylic anhydrides with hydrogen or sodium peroxide. In an
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
72
embodiment, percarboxylic acid can be made by the direct, acid catalyzed
equilibrium
action of hydrogen peroxide on the carboxylic acid. Scheme 1 illustrates an
equilibrium
between carboxylic acid and oxidizing agent (Ox) on one side and
peroxycarboxylic
acid and reduced oxidizing agent (Oxred) on the other:
RCOOH + Ox = RCOOOH + Ox,d (1)
Scheme 2 illustrates an embodiment of the equilibrium of scheme 1 in which the
oxidizing agent is hydrogen peroxide on one side and peroxycarboxylic acid and
water
on the other:
RCOOH + H202 = RCOOOH + H20 (2)
In conventional mixed peroxycarboxylic acid compositions it is believed that
the
equilibrium constant for the reaction illustrated in scheme 2 is about 2.7,
which may
reflect the equilibrium for acetic acid.
Peroxycarboxylic acids useful in the methods of the present invention include
peroxyformic, peroxyacetic, peroxypropionic, peroxybutanoic, peroxypentanoic,
peroxyhexanoic, peroxyheptanoic, peroxyoctanoic, peroxynonanoic,
peroxydecanoic,
peroxyundecanoic, peroxydodecanoic, peroxylactic, peroxymaleic,
peroxyascorbic,
peroxyhydroxyacetic, peroxyoxalic, peroxymalonic, peroxysuccinic,
peroxyglutaric,
peroxyadipic, peroxypimelic, peroxysubric acid, or mixtures thereof. Medium
chain
peroxycarboxylic acids useful in the compositions and methods of the present
invention
include peroxypentanoic, peroxyhexanoic, peroxyheptanoic, peroxyoctanoic,
peroxynonanoic, peroxydecanoic, peroxyundecanoic, peroxydodecanoic,
peroxyascorbic, peroxyadipic, peroxycitric, peroxypimelic, or peroxysuberic
acid,
mixtures thereof, or the like. Short chain peroxycarboxylic acids useful in
the
compositions and methods of the present invention include peroxyformic,
peroxyacetic,
peroxypropionic, peroxybutanoic, peroxyoxalic, peroxymalonic, peroxysuccinic
acid,
mixtures thereof, or the like. The alkyl backbones of these peroxycarboxylic
acids can
be straight chain, branched, or a mixture thereof. Peroxy forms of carboxylic
acids with
more than one carboxylate moiety can have one or more (e.g., at least one) of
the
carboxyl moieties present as peroxycarboxyl moieties.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
73
In an embodiment, the methods of the present invention employ peroxyacetic
acid. Peroxyacetic (or peracetic) acid is a peroxycarboxylic acid having the
formula:
CH3C000H. Generally, peroxyacetic acid is a liquid having an acrid odor at
higher
concentrations and is freely soluble in water, alcohol, ether, and sulfuric
acid. A 50%
solution of peroxyacetic acid can be obtained by combining acetic anhydride,
hydrogen
peroxide and sulfuric acid.
Peroxyoctanoic (or peroctanoic) acid is a peroxycarboxylic acid having the
formula, for example, of n-peroxyoctanoic acid: CH3(CH2)6C000H. Peroxyoctanoic
acid can be an acid with a straight chain alkyl moiety, an acid with a
branched alkyl
moiety, or a mixture thereof. Peroxyoctanoic acid is surface active and can
assist in
wetting hydrophobic surfaces, such as those of an arthropod.
In an embodiment, the method of the invention utilizes a combination of
several
different peroxycarboxylic acids. Such a combination can include one or more
short
chain, e.g., C2-C4, peroxycarboxylic acids and one or more medium chain, e.g.,
C7-C9,
peroxycarboxylic acids. For example, the short chain peroxycarboxylic acid can
be
peroxyacetic acid and the medium chain peroxycarboxylic acid can be
peroxyoctanoic
acid. In an embodiment, the methods of the present invention employ a
composition
including peroxyoctanoic acid, peroxynonanoic acid, or peroxyheptanoic acid,
e.g.,
peroxyoctanoic acid. In an embodiment, the present method employs a
composition
including acetic acid, octanoic acid, peroxyacetic acid, and peroxyoctanoic
acid. Such a
composition can also include a chelating agent.
The present compositions and methods can include a medium chain
peroxycarboxylic acid. The medium chain peroxycarboxylic acid can include or
be a
C6 to C12 peroxycarboxylic acid. The C6 to C12 peroxycarboxylic acid can
include or
be peroxyhexanoic acid, peroxyheptanoic acid, peroxyoctanoic acid,
peroxynonanoic
acid, peroxydecanoic acid, peroxyundecanoic acid, peroxydodecanoic acid, or
mixture
thereof. The medium chain peroxycarboxylic acid can include or be a C7 to C12
peroxycarboxylic acid. The C7 to C12 peroxycarboxylic acid can include or be
peroxyheptanoic acid, peroxyoctanoic acid, peroxynonanoic acid, peroxydecanoic
acid,
peroxyundecanoic acid, peroxydodecanoic acid, or mixture thereof. The medium
chain
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
74
peroxycarboxylic acid can include or be a C6 to C10 peroxycarboxylic acid. The
C6 to
C10 peroxycarboxylic acid can include or be peroxyhexanoic acid,
peroxyheptanoic
acid, peroxyoctanoic acid, peroxynonanoic acid, peroxydecanoic acid, or
mixture
thereof. The medium chain peroxycarboxylic acid can include or be a C8 to C10
peroxycarboxylic acid. The C8 to C10 peroxycarboxylic acid can include or be
peroxyoctanoic acid, peroxynonanoic acid, peroxydecanoic acid, or mixture
thereof. In
certain embodiments, the medium chain peroxyoctanoic acid includes or is
peroxyoctanoic acid, peroxydecanoic acid, or mixture thereof. In an
embodiment, the
medium chain peroxycarboxylic acid includes or is peroxyoctanoic acid.
The composition of the present invention can include a carboxylic acid.
Generally, carboxylic acids have the formula R-COOH wherein the R can
represent any
number of different groups including aliphatic groups, alicyclic groups,
aromatic
groups, heterocyclic groups, all of which can be saturated or unsaturated as
well as
substituted or unsubstituted. Carboxylic acids can have one, two, three, or
more
carboxyl groups. The composition and methods of the invention can employ
carboxylic
acids containing as many as 18 carbon atoms.
Suitable carboxylic acids include those having one or two carboxyl groups
where the R group is a primary alkyl chain having a length of C2 to C12. The
primary
alkyl chain is that carbon chain of the molecule having the greatest length of
carbon
atoms and directly appending carboxyl functional groups. For example,
carboxylic
acids can have the formula R-COOH in which R can be a Cl-C12 alkyl group, a Ci-
Cii
cycloalkyl group, a Ci-C12 arylalkyl group, Ci-Cii aryl group, or a Ci-Cii
heterocyclic
group. The methods of the invention can employ medium chain carboxylic acids
containing, for example, 6 to 12 carbon atoms. For example, medium chain
carboxylic
acids can have the formula R-COOH in which R can be a C5-C11 alkyl group, a C5-
C11
cycloalkyl group, a C5-C11 arylalkyl group, C5-C11 aryl group, or a C5-C11
heterocyclic
group. For example, short chain carboxylic acids can have the formula R-COOH
in
which R is H, a C1-C3 alkyl group, or a C3 cycloalkyl and n is one or two.
Suitable carboxylic acids include formic, acetic, propionic, butanoic,
pentanoic,
hexanoic, heptanoic, octanoic, nonanoic, decanoic, undecanoic, dodecanoic,
lactic,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
maleic, ascorbic, citric, hydroxyacetic, neopentanoic, neoheptanoic,
neodecanoic,
oxalic, malonic, succinic, glutaric, adipic, pimelic, subric acid, mixtures
thereof, or the
like. Suitable medium chain carboxylic acids include pentanoic, hexanoic,
heptanoic,
octanoic, nonanoic, decanoic, undecanoic, dodecanoic, ascorbic, citric,
adipic, pimelic,
5 suberic acid, mixtures thereof, or the like. Suitable short chain
carboxylic acids include
formic, acetic, propionic, butanoic, hydroxyacetic, oxalic, malonic, succinic
acid,
mixtures thereof, or the like. The alkyl backbones of these carboxylic acids
can be
straight chain, branched, or a mixture thereof. Carboxylic acids which are
generally
useful are those having one or two carboxyl groups where the R group is a
primary
10 alkyl chain having a length of C4 to C11. The primary alkyl chain is
that carbon chain of
the molecule having the greatest length of carbon atoms and directly appending
carboxyl functional groups.
In an embodiment, the present compositions and methods include a medium
chain carboxylic acid. The medium chain carboxylic acid can include or be a C6
to C12
15 carboxylic acid. The C6 to C12 carboxylic acid can include or be
hexanoic acid,
heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, undecanoic acid,
dodecanoic acid, or mixture thereof. The medium chain carboxylic acid can
include or
be a C7 to C12 carboxylic acid. The C7 to C12 carboxylic acid can include or
be
heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, undecanoic acid,
20 dodecanoic acid, or mixture thereof. The medium chain peroxycarboxylic
acid can
include or be a C6 to C10 carboxylic acid. The C6 to C10 carboxylic acid can
include
or be hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic
acid, or
mixture thereof. The medium chain carboxylic acid can include or be a C8 to
C10
carboxylic acid. The C8 to C10 carboxylic acid can include or be octanoic
acid,
25 nonanoic acid, decanoic acid, or mixture thereof. In certain
embodiments, the medium
chain carboxylic acid includes or is octanoic acid, decanoic acid, or mixture
thereof. In
an embodiment, the medium chain carboxylic acid includes or is octanoic acid.
In an embodiment, the compositions and methods include mixed
peroxycarboxylic acids and the corresponding mixed carboxylic acids.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
76
In an embodiment, the present composition includes an amount of mixed
peroxycarboxylic acid effective for killing one or more (e.g., at least one)
of the food-
borne pathogenic bacteria associated with a food product, such as Salmonella
typhimurium, Salmonella javiana, Campylobacter jejuni, Listeria monocytogenes,
and
Escherichia coli 0157:H7, yeast, mold, and the like. In an embodiment, the
present
composition includes an amount of mixed peroxycarboxylic acid effective for
killing
one or more (e.g., at least one) of the pathogenic bacteria associated with a
health care
surfaces and environments, such as Salmonella typhimurium, Staphylococcus
aureus,
Salmonella choleraesurus, Pseudomonas aeruginosa, Escherichia coli,
mycobacteria,
yeast, mold, and the like. The compositions and methods of the present
invention have
activity against a wide variety of microorganisms such as Gram positive (for
example,
Listeria monocyto genes or Staphylococcus aureus) and Gram negative (for
example,
Escherichia coli or Pseudomonas aeruginosa) bacteria, yeast, molds, bacterial
spores,
viruses, etc. The compositions and methods of the present invention, as
described
above, have activity against a wide variety of human pathogens. The present
compositions and methods can kill a wide variety of microorganisms on a food
processing surface, on the surface of a food product, in water used for
washing or
processing of food product, on a health care surface, or in a health care
environment.
Carrier
The composition of the invention can also include a carrier. The carrier
provides a medium which dissolves, suspends, or carries the other components
of the
composition. For example, the carrier can provide a medium for solubilization,
suspension, or production of peroxycarboxylic acid and for forming an
equilibrium
mixture. The carrier can also function to deliver and wet the antimicrobial
composition
of the invention on an object. To this end, the carrier can contain any
component or
components that can facilitate these functions.
Generally, the carrier includes primarily water which can promote solubility
and
work as a medium for reaction and equilibrium. The carrier can include or be
primarily
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
77
an organic solvent, such as simple alkyl alcohols, e.g., ethanol, isopropanol,
n-propanol,
and the like. Polyols are also useful carriers, including glycerol, sorbitol,
and the like.
Suitable carriers include glycol ethers. Suitable glycol ethers include
diethylene
glycol n-butyl ether, diethylene glycol n-propyl ether, diethylene glycol
ethyl ether,
diethylene glycol methyl ether, diethylene glycol t-butyl ether, dipropylene
glycol n-
butyl ether, dipropylene glycol methyl ether, dipropylene glycol ethyl ether,
dipropylene glycol propyl ether, dipropylene glycol tert-butyl ether, ethylene
glycol
butyl ether, ethylene glycol propyl ether, ethylene glycol ethyl ether,
ethylene glycol
methyl ether, ethylene glycol methyl ether acetate, propylene glycol n-butyl
ether,
propylene glycol ethyl ether, propylene glycol methyl ether, propylene glycol
n-propyl
ether, tripropylene glycol methyl ether and tripropylene glycol n-butyl ether,
ethylene
glycol phenyl ether (commercially available as DOWANOL EPHTM from Dow
Chemical Co.), propylene glycol phenyl ether (commercially available as
DOWANOL
PPHTM from Dow Chemical Co.), and the like, or mixtures thereof. Additional
suitable
commercially available glycol ethers (all of which are available from Union
Carbide
Corp.) include Butoxyethyl PROPASOLTM, Butyl CARBITOLTm acetate, Butyl
CARBITOLTm, Butyl CELLOSOLVETM acetate, Butyl CELLOSOLVETM, Butyl
DIPROPASOLTM, Butyl PROPASOLTM, CARBITOLTm PM-600, CARBITOLTm Low
Gravity, CELLOSOLVETM acetate, CELLOSOLVETM, Ester EEPTM, FILMER IBTTm,
Hexyl CARBITOLTm, Hexyl CELLOSOLVETM, Methyl CARBITOLTm, Methyl
CELLOSOLVETM acetate, Methyl CELLOSOLVETM, Methyl DIPROPASOLTM, Methyl
PROPASOLTM acetate, Methyl PROPASOLTM, Propyl CARBITOLTm, Propyl
CELLOSOLVETM, Propyl DIPROPASOLTM and Propyl PROPASOLTM.
Generally, the carrier makes up a large portion of the composition of the
invention and may be the balance of the composition apart from the active
antimicrobial
components, solubilizer, oxidizing agent, adjuvants, and the like. Here again,
the
carrier concentration and type will depend upon the nature of the composition
as a
whole, the environmental storage, and method of application including
concentration of
the peroxycarboxylic acid, among other factors. Notably the carrier should be
chosen
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
78
and used at a concentration which does not inhibit the antimicrobial efficacy
of the
peroxycarboxylic acid in the composition of the invention.
In certain embodiments, the present composition includes about 0 to about 98
wt-% carrier, about 0.001 to about 99.99 wt-% carrier, about 0.2 to about 60
wt-%
carrier, about 1 to about 98 wt-% carrier, about 5 to about 99.99 wt-%
carrier, about 5 to
about 97 wt-% carrier, about 5 to about 90 wt-% carrier, about 5 to about 70
wt-%
carrier, about 5 to about 20 wt-% carrier, about 10 to about 90 wt-% carrier,
about 10 to
about 80 wt-% carrier, about 10 to about 50 wt-% carrier, about 10 to about 20
wt-%
carrier, about 15 to about 70 wt-% carrier, about 15 to about 80 wt-% carrier,
about 20
to about 70 wt-% carrier, about 20 to about 50 wt-% carrier, about 20 to about
40 wt-%
carrier, about 20 to about 30 wt-% carrier, about 30 to about 75 wt-% carrier,
about 30
to about 70 wt-% carrier, about 40 to about 99.99 wt-% carrier, about 40 to
about 90 wt-
% carrier, or about 60 to about 70 wt-% carrier. The composition can include
any of
these ranges or amounts not modified by about.
Oxidizing Agent
The present compositions and methods can include any of a variety of oxidizing
agents. The oxidizing agent can be used for maintaining or generating
peroxycarboxylic acids.
Examples of inorganic oxidizing agents include the following types of
compounds or sources of these compounds, or alkali metal salts including these
types of
compounds, or forming an adduct therewith:
hydrogen peroxide;
group 1 (IA) oxidizing agents, for example lithium peroxide, sodium peroxide,
and the like;
group 2 (IA) oxidizing agents, for example magnesium peroxide, calcium
peroxide, strontium peroxide, barium peroxide, and the like;
group 12 (JIB) oxidizing agents, for example zinc peroxide, and the like;
group 13 (IIIA) oxidizing agents, for example boron compounds, such as
perborates, for example sodium perborate hexahydrate of the formula
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
79
Na2[Br2(02)2(OH)41 = 6H20 (also called sodium perborate tetrahydrate and
formerly
written as NaB03=4H20); sodium peroxyborate tetrahydrate of the formula
Na2Br2(02)2[(011)4]. 4H20 (also called sodium perborate trihydrate, and
formerly
written as NaB03=3H20); sodium peroxyborate of the formula Na2[B2(02)2(OH)41
(also
called sodium perborate monohydrate and formerly written as NaB03=H20); and
the
like; in an embodiment, perborate;
group 14 (IVA) oxidizing agents, for example persilicates and
peroxycarbonates, which are also called percarbonates, such as persilicates or
peroxycarbonates of alkali metals; and the like; in an embodiment,
percarbonate; in an
embodiment, persilicate;
group 15 (VA) oxidizing agents, for example peroxynitrous acid and its salts;
peroxyphosphoric acids and their salts, for example, perphosphates; and the
like; in an
embodiment, perphosphate;
group 16 (VIA) oxidizing agents, for example peroxy sulfuric acids and their
salts, such as peroxymono sulfuric and peroxydisulfuric acids, and their
salts, such as
persulfates, for example, sodium persulfate; and the like; in an embodiment,
persulfate;
group VIIa oxidizing agents such as sodium periodate, potassium perchlorate
and the like.
Other active inorganic oxygen compounds can include transition metal
peroxides; and other such peroxygen compounds, and mixtures thereof.
In an embodiment, the compositions and methods of the present invention
employ one or more of the inorganic oxidizing agents listed above. Suitable
inorganic
oxidizing agents include ozone, hydrogen peroxide, hydrogen peroxide adduct,
group
IIIA oxidizing agent, group VIA oxidizing agent, group VA oxidizing agent,
group
VIIA oxidizing agent, or mixtures thereof. Suitable examples of such inorganic
oxidizing agents include percarbonate, perborate, persulfate, perphosphate,
persilicate,
or mixtures thereof.
Hydrogen peroxide presents one suitable example of an inorganic oxidizing
agent. Hydrogen peroxide can be provided as a mixture of hydrogen peroxide and
water, e.g., as liquid hydrogen peroxide in an aqueous solution. Hydrogen
peroxide is
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
commercially available at concentrations of 35%, 70%, and 90% in water. For
safety,
the 35% is commonly used. The present compositions can include, for example,
about
2 to about 30 wt-% or about 5 to about 20 wt-% hydrogen peroxide.
In an embodiment, the inorganic oxidizing agent includes hydrogen peroxide
5 adduct. For example, the inorganic oxidizing agent can include hydrogen
peroxide,
hydrogen peroxide adduct, or mixtures thereof. Any of a variety of hydrogen
peroxide
adducts are suitable for use in the present compositions and methods. For
example,
suitable hydrogen peroxide adducts include percarbonate salt, urea peroxide,
peracetyl
borate, an adduct of H202 and polyvinyl pyrrolidone, sodium percarbonate,
potassium
10 percarbonate, mixtures thereof, or the like. Suitable hydrogen peroxide
adducts include
percarbonate salt, urea peroxide, peracetyl borate, an adduct of H202 and
polyvinyl
pyrrolidone, or mixtures thereof. Suitable hydrogen peroxide adducts include
sodium
percarbonate, potassium percarbonate, or mixtures thereof, for example sodium
percarbonate.
15 In an embodiment, the present compositions and methods can include
hydrogen
peroxide as oxidizing agent. Hydrogen peroxide in combination with the
percarboxylic
acid can provide certain antimicrobial action against microorganisms.
Additionally,
hydrogen peroxide can provide an effervescent action which can irrigate any
surface to
which it is applied. Hydrogen peroxide can work with a mechanical flushing
action
20 once applied which further cleans the surface of an object. An
additional advantage of
hydrogen peroxide is the food compatibility of this composition upon use and
decomposition.
In certain embodiments, the present composition includes about 0.001 to about
30 wt-% oxidizing agent, about 0.001 to about 10 wt-% oxidizing agent, 0.002
to about
25 10 wt-% oxidizing agent, about 2 to about 30 wt-% oxidizing agent, about
2 to about 25
wt-% oxidizing agent, about 2 to about 20 wt-% oxidizing agent, about 4 to
about 20
wt-% oxidizing agent, about 5 to about 10 wt-% oxidizing agent, or about 6 to
about 10
wt-% oxidizing agent. The composition can include any of these ranges or
amounts not
modified by about.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
81
Optional Ingredients
Acidulant
In an embodiment, the present composition can include an acidulant. The
acidulant can act as a catalyst for conversion of carboxylic acid to
peroxycarboxylic
acid. The acidulant can be effective to form a concentrate composition with pH
of
about 1 or less. The acidulant can be effective to form a use composition with
pH of
about 5, about 5 or less, about 4, about 4 or less, about 3, about 3 or less,
about 2, about
2 or less, or the like. In an embodiment, the acidulant includes an inorganic
acid.
Suitable inorganic acids include sulfuric acid, phosphoric acid, nitric acid,
hydrochloric
acid, methane sulfonic acid, ethane sulfonic acid, propane sulfonic acid,
butane sulfonic
acid, xylene sulfonic acid, benzene sulfonic acid, mixtures thereof, or the
like.
In an embodiment, the acidulant includes a carboxylic acid with pKa less than
4.
Suitable carboxylic acids with pKa less than 4 include hydroxyacetic acid,
hydroxypropionic acid, other hydroxycarboxylic acids, mixtures thereof, or the
like.
Such an acidulant is present at a concentration where it does not act as a
solubilizer.
In certain embodiments, the present composition includes about 0.001 to about
50 wt-% acidulant, about 0.001 to about 30 wt-% acidulant, about 1 to about 50
wt-%
acidulant, about 1 to about 30 wt-% acidulant, about 2 to about 40 wt-%
acidulant,
about 2 to about 10 wt-% acidulant, about 3 to about 40 wt-% acidulant, about
5 to
about 40 wt-% acidulant, about 5 to about 25 wt-% acidulant, about 10 to about
40 wt-
% acidulant, about 10 to about 30 wt-% acidulant, about 15 to about 35 wt-%
acidulant,
about 15 to about 30 wt-% acidulant, or about 40 to about 60 wt-% acidulant.
The
composition can include any of these ranges or amounts not modified by about.
Stabilizing Agent
One or more stabilizing agents can be added to the composition of the
invention,
for example, to stabilize the peracid and hydrogen peroxide and prevent the
premature
oxidation of this constituent within the composition of the invention.
Suitable stabilizing agents include chelating agents or sequestrants. Suitable
sequestrants include organic chelating compounds that sequester metal ions in
solution,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
82
particularly transition metal ions. Such sequestrants include organic amino-
or
hydroxy-polyphosphonic acid complexing agents (either in acid or soluble salt
forms),
carboxylic acids (e.g., polymeric polycarboxylate), hydroxycarboxylic acids,
or
aminocarboxylic acids.
The sequestrant can be or include phosphonic acid or phosphonate salt.
Suitable
phosphonic acids and phosphonate salts include 1-hydroxy ethylidene-1,1-
diphosphonic
acid (CH3C(P03H2)20H) (HEDP); ethylenediamine tetrakis methylenephosphonic
acid
(EDTMP); diethylenetriamine pentakis methylenephosphonic acid (DTPMP);
cyclohexane-1,2-tetramethylene phosphonic acid; amino[tri(methylene phosphonic
acid)]; (ethylene diamine[tetra methylene-phosphonic acid)]; 2-phosphene
butane-1,2,4-
tricarboxylic acid; or salts thereof, such as the alkali metal salts, ammonium
salts, or
alkyloyl amine salts, such as mono, di, or tetra-ethanolamine salts; or
mixtures thereof.
Suitable organic phosphonates include HEDP.
Commercially available food additive chelating agents include phosphonates
sold under the trade name DEQUEST including, for example, 1-hydroxyethylidene-
1,1-diphosphonic acid, available from Monsanto Industrial Chemicals Co., St.
Louis,
MO, as DEQUEST 2010; amino(tri(methylenephosphonic acid)), (N[CH2P03H2]3),
available from Monsanto as DEQUEST 2000;
ethylenediamine[tetra(methylenephosphonic acid)] available from Monsanto as
DEQUEST 2041; and 2-phosphonobutane-1,2,4-tricarboxylic acid available from
Mobay Chemical Corporation, Inorganic Chemicals Division, Pittsburgh, PA, as
Bayhibit AM.
The sequestrant can be or include aminocarboxylic acid type sequestrant.
Suitable aminocarboxylic acid type sequestrants include the acids or alkali
metal salts
thereof, e.g., amino acetates and salts thereof. Suitable aminocarboxylates
include N-
hydroxyethylaminodiacetic acid; hydroxyethylenediaminetetraacetic acid,
nitrilotriacetic acid (NTA); ethylenediaminetetraacetic acid (EDTA); N-
hydroxyethyl-
ethylenediaminetriacetic acid (HEDTA); diethylenetriaminepentaacetic acid
(DTPA);
and
alanine-N,N-diacetic acid; and the like; and mixtures thereof.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
83
The sequestrant can be or include a polycarboxylate. Suitable polycarboxylates
include, for example, polyacrylic acid, maleic/olefin copolymer,
acrylic/maleic
copolymer, polymethacrylic acid, acrylic acid-methacrylic acid copolymers,
hydrolyzed
polyacrylamide, hydrolyzed polymethacrylamide, hydrolyzed polyamide-
methacrylamide copolymers, hydrolyzed polyacrylonitrile, hydrolyzed
polymethacrylonitrile, hydrolyzed acrylonitrile-methacrylonitrile copolymers,
polymaleic acid, polyfumaric acid, copolymers of acrylic and itaconic acid,
phosphino
polycarboxylate, acid or salt forms thereof, mixtures thereof, and the like.
In certain embodiments, the present composition includes about 0.5 to about 50
wt-% sequestrant, about 1 to about 50 wt-% sequestrant, about 1 to about 30 wt-
%
sequestrant, about 1 to about 15 wt-% sequestrant, about 1 to about 5 wt-%
sequestrant,
about 1 to about 4 wt-% sequestrant, about 2 to about 10 wt-% sequestrant,
about 2 to
about 5 wt-% sequestrant, or about 5 to about 15 wt-% sequestrant. The
composition
can include any of these ranges or amounts not modified by about.
In certain embodiments, the present composition includes about 0.001 to about
50 wt-% stabilizing agent, about 0.001 to about 5 wt-% stabilizing agent,
about 0.5 to
about 50 wt-% stabilizing agent, about 1 to about 50 wt-% stabilizing agent,
about 1 to
about 30 wt-% stabilizing agent, about 1 to about 10 wt-% stabilizing agent,
about 1 to
about 5 wt-% stabilizing agent, about 1 to about 3 wt-% stabilizing agent,
about 2 to
about 10 wt-% stabilizing agent, about 2 to about 5 wt-% stabilizing agent, or
about 5 to
about 15 wt-% stabilizing agent. The composition can include any of these
ranges or
amounts not modified by about.
Surfactants
Nonionic Surfactants
Suitable nonionic surfactants for use as solvents include alkoxylated
surfactants.
Suitable alkoxylated surfactants include EO/PO copolymers, capped EO/PO
copolymers, alcohol alkoxylates, capped alcohol alkoxylates, mixtures thereof,
or the
like. Suitable alkoxylated surfactants for use as solvents include EO/PO block
copolymers, such as the Pluronic and reverse Pluronic surfactants; alcohol
alkoxylates,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
84
such as Dehypon LS-54 (R-(E0)5(P0)4) and Dehypon LS-36 (R-(E0)3(P0)6); and
capped alcohol alkoxylates, such as Plurafac LF221 and Tegoten EC11; mixtures
thereof, or the like. When employed as a solvent a surfactant, such as a
nonionic
surfactant, can be at concentrations higher than those conventionally employed
as
surfactant.
Semi-Polar Nonionic Surfactants
The semi-polar type of nonionic surface active agents are another class of
nonionic surfactant useful in compositions of the present invention. Semi-
polar
nonionic surfactants include the amine oxides, phosphine oxides, sulfoxides
and their
alkoxylated derivatives.
Amine oxides are tertiary amine oxides corresponding to the general formula:
R2
1
R1¨ (0 R 4 ) n¨ N ¨11.- 0
1
R3
wherein the arrow is a conventional representation of a semi-polar bond; and,
Rl, R2,
and R3 maybe aliphatic, aromatic, heterocyclic, alicyclic, or combinations
thereof.
Generally, for amine oxides of detergent interest, Rl is an alkyl radical of
from about 8
to about 24 carbon atoms; R2 and R3 are alkyl or hydroxyalkyl of 1-3 carbon
atoms or a
mixture thereof; R2 and R3 can be attached to each other, e.g. through an
oxygen or
nitrogen atom, to form a ring structure; R4 is an alkylene or a
hydroxyalkylene group
containing 2 to 3 carbon atoms; and n ranges from 0 to about 20. An amine
oxide can
be generated from the corresponding amine and an oxidizing agent, such as
hydrogen
peroxide.
Useful water soluble amine oxide surfactants are selected from the octyl,
decyl,
dodecyl, isododecyl, coconut, or tallow alkyl di-(lower alkyl) amine oxides,
specific
examples of which are octyldimethylamine oxide, nonyldimethylamine oxide,
decyldimethylamine oxide, undecyldimethylamine oxide, dodecyldimethylamine
oxide,
iso-dodecyldimethyl amine oxide, tridecyldimethylamine oxide,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
tetradecyldimethylamine oxide, pentadecyldimethylamine oxide,
hexadecyldimethylamine oxide, heptadecyldimethylamine oxide,
octadecyldimethylaine
oxide, dodecyldipropylamine oxide, tetradecyldipropylamine oxide,
hexadecyldipropylamine oxide, tetradecyldibutylamine oxide,
octadecyldibutylamine
5 oxide, bis(2-hydroxyethyl)dodecylamine oxide, bis(2-hydroxyethyl)-3-
dodecoxy-1-
hydroxypropylamine oxide, dimethyl-(2-hydroxydodecyl)amine oxide, 3,6,9-
trioctadecyldimethylamine oxide and 3-dodecoxy-2-hydroxypropyldi-(2-
hydroxyethyl)amine oxide.
10 Anionic Surfactants
The present composition can include an anionic surfactant as solubilizer.
Suitable anionic surfactants include organic sulfonate surfactant, organic
sulfate
surfactant, phosphate ester surfactant, carboxylate surfactant, mixtures
thereof, or the
like. In an embodiment, the anionic surfactant includes alkyl sulfonate,
alkylaryl
15 sulfonate, alkylated diphenyl oxide disulfonate, alkylated naphthalene
sulfonate, alcohol
alkoxylate carboxylate, sarcosinate, taurate, acyl amino acid, alkanoic ester,
phosphate
ester, sulfuric acid ester, salt or acid form thereof, or mixture thereof. The
particular
salts will be suitably selected depending upon the particular formulation and
the needs
therein.
20 Suitable anionic surfactants include sulfonic acids (and salts),
such as
isethionates (e.g. acyl isethionates), alkylaryl sulfonic acids and salts
thereof, alkyl
sulfonates, and the like.
Examples of suitable synthetic, water soluble anionic detergent compounds
include the ammonium and substituted ammonium (such as mono-, di- and
25 triethanolamine) and alkali metal (such as sodium, lithium and
potassium) salts of the
alkyl mononuclear aromatic sulfonates such as the alkyl benzene sulfonates
containing
from about 5 to about 18 carbon atoms in the alkyl group in a straight or
branched
chain, e.g., the salts of alkyl benzene sulfonates or of alkyl toluene,
xylene, cumene and
phenol sulfonates; alkyl naphthalene sulfonate, diamyl naphthalene sulfonate,
and
30 dinonyl naphthalene sulfonate and alkoxylated derivatives or their free
acids. Suitable
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
86
sulfonates include olefin sulfonates, such as long chain alkene sulfonates,
long chain
hydroxyalkane sulfonates or mixtures of alkenesulfonates and hydroxyalkane-
sulfonates.
In certain embodiments, the present compositions including an anionic
surfactant, such as a normal C8 sulfonate, can be non-foam or low foam
compositions.
Such compositions can be advantageous for applications such as clean in place,
machine warewashing, destaining, and sanitizing, laundry washing, destaining,
and
sanitizing, etc.
For applications in which foaming is desirable, a foaming agent can be added
as
part of the present composition or separately. In a two-step offering, a
foaming agent
can be combined with a dilution of the non-foam or low foam composition to
form a
foaming use solution. In a one-step offering, the foaming agent can be
incorporated
into the concentrated composition. One suitable foaming agent is LAS acid. LAS
acid
can form a microemulsion in the present compositions. LAS acid can form a
viscoelastic gel or liquid in the present compositions.
Anionic sulfate surfactants suitable for use in the present compositions
include
alkyl ether sulfates, alkyl sulfates, the linear and branched primary and
secondary alkyl
sulfates, alkyl ethoxysulfates, fatty oleyl glycerol sulfates, alkyl phenol
ethylene oxide
ether sulfates, the C5 -C17 acyl-N-(Ci -C4 alkyl) and -N-(C1 -C2 hydroxyalkyl)
glucamine sulfates, and sulfates of alkylpolysaccharides such as the sulfates
of
alkylpolyglucoside, and the like. Also included are the alkyl sulfates, alkyl
poly(ethyleneoxy) ether sulfates and aromatic poly(ethyleneoxy) sulfates such
as the
sulfates or condensation products of ethylene oxide and nonyl phenol (usually
having 1
to 6 oxyethylene groups per molecule).
Anionic carboxylate surfactants suitable for use in the present compositions
include carboxylic acids (and salts), such as alkanoic acids (and alkanoates),
ester
carboxylic acids (e.g. alkyl succinates), ether carboxylic acids, and the
like. Such
carboxylates include alkyl ethoxy carboxylates, alkyl aryl ethoxy
carboxylates, alkyl
polyethoxy polycarboxylate surfactants and soaps (e.g. alkyl carboxyls).
Secondary
carboxylates useful in the present compositions include those which contain a
carboxyl
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
87
unit connected to a secondary carbon. The secondary carbon can be in a ring
structure,
e.g. as in p-octyl benzoic acid, or as in alkyl-substituted cyclohexyl
carboxylates. The
secondary carboxylate surfactants typically contain no ether linkages, no
ester linkages
and no hydroxyl groups. Further, they typically lack nitrogen atoms in the
head-group
(amphiphilic portion). Suitable secondary soap surfactants typically contain
11-13 total
carbon atoms, although more carbons atoms (e.g., up to 16) can be present.
Suitable
carboxylates also include acylamino acids (and salts), such as acylgluamates,
acyl
peptides, sarcosinates (e.g. N-acyl sarcosinates), taurates (e.g. N-acyl
taurates and fatty
acid amides of methyl tauride), and the like.
Suitable anionic surfactants include alkyl or alkylaryl ethoxy carboxylates of
Formula 3:
R - 0 - (CH2CH20).(CH2)m - CO2X (3)
Ri
in which R is a C8 to C22 alkyl group or , in
which R1 is a C4-C16 alkyl
group; n is an integer of 1-20; m is an integer of 1-3; and X is a counter
ion, such as
hydrogen, sodium, potassium, lithium, ammonium, or an amine salt such as
monoethanolamine, diethanolamine or triethanolamine. In an embodiment, in
Formula
3, n is an integer of 4 to 10 and m is 1. In an embodiment, in Formula 3, R is
a C8-C16
alkyl group. In an embodiment, in Formula 3, R is a C12-C14 alkyl group, n is
4, and m
is 1.
Ri
In an embodiment, in Formula 3, R is and
R1 is a C6-C12 alkyl
group. In an embodiment, in Formula 3, Rl is a C9 alkyl group, n is 10 and m
is 1.
Such alkyl and alkylaryl ethoxy carboxylates are commercially available. These
ethoxy
carboxylates are typically available as the acid forms, which can be readily
converted to
the anionic or salt form. Commercially available carboxylates include, Neodox
23-4, a
C12-13 alkyl polyethoxy (4) carboxylic acid (Shell Chemical), and Emcol CNP-
110, a C9
alkylaryl polyethoxy (10) carboxylic acid (Witco Chemical). Carboxylates are
also
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
88
available from Clariant, e.g. the product Sandopan DTC, a C13 alkyl
polyethoxy (7)
carboxylic acid.
Amphoteric Surfactants
Amphoteric, or ampholytic, surfactants contain both a basic and an acidic
hydrophilic group and an organic hydrophobic group. These ionic entities may
be any
of anionic or cationic groups described herein for other types of surfactants.
A basic
nitrogen and an acidic carboxylate group are the typical functional groups
employed as
the basic and acidic hydrophilic groups. In a few surfactants, sulfonate,
sulfate,
phosphonate or phosphate provide the negative charge.
Amphoteric surfactants can be broadly described as derivatives of aliphatic
secondary and tertiary amines, in which the aliphatic radical may be straight
chain or
branched and wherein one of the aliphatic sub stituents contains from about 8
to 18
carbon atoms and one contains an anionic water solubilizing group, e.g.,
carboxy, sulfo,
sulfato, phosphato, or phosphono. Amphoteric surfactants are subdivided into
two
major classes known to those of skill in the art and described in "Surfactant
Encyclopedia" Cosmetics & Toiletries, Vol. 104 (2) 69-71 (1989). The first
class
includes acyl/dialkyl ethylenediamine derivatives (e.g. 2-alkyl hydroxyethyl
imidazoline derivatives) and their salts. The second class includes N-
alkylamino acids
and their salts. Some amphoteric surfactants can be envisioned as fitting into
both
classes.
Amphoteric surfactants can be synthesized by methods known to those of skill
in the art. For example, 2-alkyl hydroxyethyl imidazoline is synthesized by
condensation and ring closure of a long chain carboxylic acid (or a
derivative) with
dialkyl ethylenediamine. Commercial amphoteric surfactants are derivatized by
subsequent hydrolysis and ring-opening of the imidazoline ring by alkylation --
for
example with chloroacetic acid or ethyl acetate. During alkylation, one or two
carboxy-
alkyl groups react to form a tertiary amine and an ether linkage with
differing alkylating
agents yielding different tertiary amines.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
89
Long chain imidazole derivatives having application in the present invention
generally have the general formula:
(MONO)ACETATE (DI)PROPIONATE AMPHOTERIC
SULFONATE
CH2C000 CH2CH2C00 OH
I ,
RCONHCH2CH2Nc-71-1 RCONHCH2CH2M"CH2CH2COOH
CH2CHCH2SO3NP
H2CH2OH CH2CH2OH RCONHCH2CH2N
CH2CH2OH
Neutral pH - Zwitterion
wherein R is an acyclic hydrophobic group containing from about 8 to 18 carbon
atoms
and M is a cation to neutralize the charge of the anion, generally sodium.
Commercially prominent imidazoline-derived amphoterics that can be employed in
the
present compositions include for example: Cocoamphopropionate,
Cocoamphocarboxy-propionate, Cocoamphoglycinate, Cocoamphocarboxy-glycinate,
Cocoamphopropyl-sulfonate, and Cocoamphocarboxy-propionic acid.
Amphocarboxylic acids can be produced from fatty imidazolines in which the
dicarboxylic acid functionality of the amphodicarboxylic acid is diacetic acid
and/or
dipropionic acid.
The carboxymethylated compounds (glycinates) described herein above
frequently are called betaines. Betaines are a special class of amphoteric
discussed
herein below in the section entitled, Zwitterion Surfactants.
Long chain N-alkylamino acids are readily prepared by reaction RNH2, in which
R=C8-C18 straight or branched chain alkyl, fatty amines with halogenated
carboxylic
acids. Alkylation of the primary amino groups of an amino acid leads to
secondary and
tertiary amines. Alkyl substituents may have additional amino groups that
provide
more than one reactive nitrogen center. Most commercial N-alkylamine acids are
alkyl
derivatives of beta-alanine or beta-N(2-carboxyethyl) alanine. Examples of
commercial
N-alkylamino acid ampholytes having application in this invention include
alkyl beta-
amino dipropionates, RN(C2H4COOM)2 and RNHC2H4COOM. In an embodiment, R
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
can be an acyclic hydrophobic group containing from about 8 to about 18 carbon
atoms,
and M is a cation to neutralize the charge of the anion.
Suitable amphoteric surfactants include those derived from coconut products
such as coconut oil or coconut fatty acid. Additional suitable coconut derived
5 surfactants include as part of their structure an ethylenediamine moiety,
an
alkanolamide moiety, an amino acid moiety, e.g., glycine, or a combination
thereof; and
an aliphatic substituent of from about 8 to 18 (e.g., 12) carbon atoms. Such a
surfactant
can also be considered an alkyl amphodicarboxylic acid. These amphoteric
surfactants
can include chemical structures represented as: C12-alkyl-C(0)-NH-CH2-CH2-N
(CH2-
10 CH2-CO2Na)2-CH2-CH2-0H or C12-alkyl-C(0)-N(H)-CH2-CH2-N (CH2-CO2Na)2-CH2-
CH2-0H. Disodium cocoampho dipropionate is one suitable amphoteric surfactant
and
is commercially available under the tradename MiranolTM FBS from Rhodia Inc.,
Cranbury, N.J. Another suitable coconut derived amphoteric surfactant with the
chemical name disodium cocoampho diacetate is sold under the tradename
MirataineTM
15 JCHA, also from Rhodia Inc., Cranbury, N.J.
A typical listing of amphoteric classes, and species of these surfactants, is
given
in U.S. Pat. No. 3,929,678 issued to Laughlin and Heuring on Dec. 30, 1975.
Further
examples are given in "Surface Active Agents and Detergents" (Vol. I and II by
Schwartz, Perry and Berch).
Zwitterionic Surfactants
Zwitterionic surfactants can be thought of as a subset of the amphoteric
surfactants and can include an anionic charge. Zwitterionic surfactants can be
broadly
described as derivatives of secondary and tertiary amines, derivatives of
heterocyclic
secondary and tertiary amines, or derivatives of quaternary ammonium,
quaternary
phosphonium or tertiary sulfonium compounds. Typically, a zwitterionic
surfactant
includes a positive charged quaternary ammonium or, in some cases, a sulfonium
or
phosphonium ion; a negative charged carboxyl group; and an alkyl group.
Zwitterionics generally contain cationic and anionic groups which ionize to a
nearly
equal degree in the isoelectric region of the molecule and which can develop
strong"
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
91
inner-salt" attraction between positive-negative charge centers. Examples of
such
zwitterionic synthetic surfactants include derivatives of aliphatic quaternary
ammonium, phosphonium, and sulfonium compounds, in which the aliphatic
radicals
can be straight chain or branched, and wherein one of the aliphatic
substituents contains
from 8 to 18 carbon atoms and one contains an anionic water solubilizing
group, e.g.,
carboxy, sulfonate, sulfate, phosphate, or phosphonate. Betaine and sultaine
surfactants
are exemplary zwitterionic surfactants for use herein.
A general formula for these compounds is:
1Z2)x
1 1 + 3 -
R¨Y¨CH2¨R¨Z
wherein Rl contains an alkyl, alkenyl, or hydroxyalkyl radical of from 8 to 18
carbon
atoms having from 0 to 10 ethylene oxide moieties and from 0 to 1 glyceryl
moiety; Y
is selected from the group consisting of nitrogen, phosphorus, and sulfur
atoms; R2 is an
alkyl or monohydroxy alkyl group containing 1 to 3 carbon atoms; x is 1 when Y
is a
sulfur atom and 2 when Y is a nitrogen or phosphorus atom, R3 is an alkylene
or
hydroxy alkylene or hydroxy alkylene of from 1 to 4 carbon atoms and Z is a
radical
selected from the group consisting of carboxylate, sulfonate, sulfate,
phosphonate, and
phosphate groups.
Examples of zwitterionic surfactants having the structures listed above
include:
4- [N,N-di(2-hydroxyethyl)-N-octadecylammonio] -butane-l-carboxylate; 5- [ S-3-
hydroxypropyl-S-hexadecylsulfonio] -3-hydroxypentane-l-sulfate; 3- [P,P-
diethyl-P-
3,6,9-trioxatetraco sanepho sphonio] -2-hydroxypropane-l-pho sphate ; 3- [N,N-
dipropyl-
N-3-dodecoxy-2-hydroxypropyl-ammonio] -propane-l-phosphonate; 3- (N,N-dimethyl-
N-hexadecylammonio)-propane-l-sulfonate; 3- (N,N-dimethyl-N-hexadecylammonio)-
2-hydroxy-propane- 1- sulfonate; 4-[N,N-di(2(2-hydroxyethyl)-N(2-
hydroxydodecyl)ammonio] -butane-l-carboxylate; 3- [S-ethyl-S- (3-dodecoxy-2-
hydroxypropyl)sulfonio] -propane-l-phosphate; 3- [P,P-dimethyl-P-dodecylpho
sphonio] -
propane-l-phosphonate; and S[N,N-di(3-hydroxypropy1)-N-hexadecylammonio]-2-
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
92
hydroxy-pentane- 1-sulfate. The alkyl groups contained in said detergent
surfactants can
be straight or branched and saturated or unsaturated.
The zwitterionic surfactant suitable for use in the present compositions
includes
a betaine of the general structure:
R" R R
, 1
RI CH2¨0O2 R¨S¨CH2¨0O2 R,-13-CH2¨0O2
1 ,,, 1
R R
These surfactant betaines typically do not exhibit strong cationic or anionic
characters at
pH extremes nor do they show reduced water solubility in their isoelectric
range.
Unlike "external" quaternary ammonium salts, betaines are compatible with
anionics.
Examples of suitable betaines include coconut acylamidopropyldimethyl betaine;
hexadecyl dimethyl betaine; C12-14 acylamidopropylbetaine; C8-14
acylamidohexyldiethyl betaine; 4-C1416 acylmethylamidodiethylammonio-l-
carboxybutane; C16-18 acylamidodimethylbetaine; C12-16
acylamidopentanediethylbetaine; and C1246 acylmethylamidodimethylbetaine.
Sultaines useful in the present invention include those compounds having the
formula (R(R1)2N+ R2S03-, in which R is a C6 -C18 hydrocarbyl group, each Rl
is
typically independently C1-C3 alkyl, e.g. methyl, and R2 is a C1-C6
hydrocarbyl group,
e.g. a C1-C3 alkylene or hydroxyalkylene group.
A typical listing of zwitterionic classes, and species of these surfactants,
is given
in U.S. Pat. No. 3,929,678 issued to Laughlin and Heuring on Dec. 30, 1975.
Further
examples are given in "Surface Active Agents and Detergents" (Vol. I and II by
Schwartz, Perry and Berch).
In an embodiment, the composition of the present invention includes a betaine.
For example, the composition can include cocoamidopropyl betaine.
Adjuvants
The antimicrobial composition of the invention can also include any number of
adjuvants. Specifically, the composition of the invention can include
antimicrobial
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
93
solvent, antimicrobial agent, wetting agent, defoaming agent, thickener, a
surfactant,
foaming agent, solidification agent, aesthetic enhancing agent (i.e., colorant
(e.g.,
pigment), odorant, or perfume), stabilizing agent (e.g., HEDP) among any
number of
constituents which can be added to the composition. Such adjuvants can be
preformulated with the antimicrobial composition of the invention or added to
the
system simultaneously, or even after, the addition of the antimicrobial
composition.
The composition of the invention can also contain any number of other
constituents as
necessitated by the application, which are known and which can facilitate the
activity of
the present invention. The adjuvant(s) can be added to the present carboxylic
acid
composition in the day tank, in a line or conduit after the reaction catalyst,
or in the
apparatus or system using the peroxycarboxylic acid composition.
Antimicrobial Solvent
Any of a variety of solvents can be useful as antimicrobial solvents in the
present compositions. Antimicrobial solvent can be added to use compositions
before
use. Suitable antimicrobial solvents include acetamidophenol; acetanilide;
acetophenone; 2-acetyl-1-methylpyrrole; benzyl acetate; benzyl alcohol; benzyl
benzoate; benzyloxyethanol; essential oils (e.g., benzaldehyde, pinenes,
terpineols,
terpinenes, carvone, cinnamealdehyde, borneol and its esters, citrals,
ionenes, jasmine
oil, limonene, dipentene, linalool and its esters); diester dicarboxylates
(e.g., dibasic
esters) such as dimethyl adipate, dimethyl succinate, dimethyl glutarate
(including
products available under the trade designations DBE, DBE-3, DBE-4, DBE-5, DBE-
6,
DBE-9, DBE-IB, and DBE-ME from DuPont Nylon), dimethyl malonate, diethyl
adipate, diethyl succinate, diethyl glutarate, dibutyl succinate, and dibutyl
glutarate;
dimethyl sebacate, dimethyl pimelate, dimethyl suberate; dialkyl carbonates
such as
dimethyl carbonate, diethyl carbonate, dipropyl carbonate, diisopropyl
carbonate, and
dibutyl carbonate; organo-nitriles such as acetonitrile and benzonitrile; and
phthalate
esters such as dibutyl phthalate, diethylhexyl phthalate, and diethyl
phthalate. Mixtures
of antimicrobial solvents can be used if desired.
CA 02663953 2009-03-17
WO 2008/047263
PCT/1B2007/053809
94
The antimicrobial solvent can be selected based upon the characteristics of
the
surface and microbes to which the antimicrobial composition will be applied
and upon
the nature of any coating, soil or other material that will be contacted by
the
antimicrobial composition and optionally removed from the surface. Polar
solvents,
and solvents that are capable of hydrogen bonding typically will perform well
on a
variety of surfaces and microbes and thus, for such applications, can be
selected. In
certain applications, the antimicrobial solvent can be selected for a high
flashpoint (e.g.,
greater than about 30 C, greater than about 50 C, or greater than about 100
C), low
odor, and low human and animal toxicity.
In an embodiment, the antimicrobial solvent is compatible as an indirect or
direct food additive or substance; especially those described in the Code of
Federal
Regulations (CFR), Title 21--Food and Drugs, parts 170 to 186. The
compositions of
the invention should contain sufficient antimicrobial solvent to provide the
desired rate
and type of microbial reduction.
The present composition can include an effective amount of antimicrobial
solvent, such as about 0.01 wt-% to about 60 wt-% antimicrobial solvent, about
0.05 wt-
% to about 15 wt-% antimicrobial solvent, or about 0.08 wt-% to about 5 wt-%
antimicrobial solvent.
Additional Antimicrobial Agent
The antimicrobial compositions of the invention can contain an additional
antimicrobial agent. Additional antimicrobial agent can be added to use
compositions
before use. Suitable antimicrobial agents include carboxylic esters (e.g., p-
hydroxy
alkyl benzoates and alkyl cinnamates), sulfonic acids (e.g., dodecylbenzene
sulfonic
acid), iodo-compounds or active halogen compounds (e.g., elemental halogens,
halogen
oxides (e.g., Na0C1, HOC1, HOBr, C102), iodine, interhalides (e.g., iodine
monochloride, iodine dichloride, iodine trichloride, iodine tetrachloride,
bromine
chloride, iodine monobromide, or iodine dibromide), polyhalides, hypochlorite
salts,
hypochlorous acid, hypobromite salts, hypobromous acid, chloro- and bromo-
hydantoins, chlorine dioxide, and sodium chlorite), organic peroxides
including benzoyl
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
peroxide, alkyl benzoyl peroxides, ozone, singlet oxygen generators, and
mixtures
thereof, phenolic derivatives (e.g., o-phenyl phenol, o-benzyl-p-chlorophenol,
tert-amyl
phenol and C1-C6 alkyl hydroxy benzoates), quaternary ammonium compounds
(e.g.,
alkyldimethylbenzyl ammonium chloride, dialkyldimethyl ammonium chloride and
5 mixtures thereof), and mixtures of such antimicrobial agents, in an
amount sufficient to
provide the desired degree of microbial protection.
In an embodiment, the present composition can include added peroxycarboxylic
acid and/or hydrogen peroxide.
The present composition can include an effective amount of antimicrobial
agent,
10 such as about 0.001 wt-% to about 60 wt-% antimicrobial agent, about
0.01 wt-% to
about 15 wt-% antimicrobial agent, or about 0.08 wt-% to about 2.5 wt-%
antimicrobial
agent.
Hydrotrope
15 The composition employed in the methods of the invention may also
include a
hydrotrope coupler or solubilizer. Such materials can be used to ensure that
the
composition remains phase stable and in a single highly active aqueous form.
Such
hydrotrope solubilizers or couplers can be used at compositions which maintain
phase
stability but do not result in unwanted compositional interaction.
20 Representative classes of hydrotrope solubilizers or coupling agents
include an
anionic surfactant such as an alkyl sulfate, an alkyl or alkane sulfonate, a
linear alkyl
benzene or naphthalene sulfonate, a secondary alkane sulfonate, alkyl ether
sulfate or
sulfonate, an alkyl phosphate or phosphonate, dialkyl sulfosuccinic acid
ester, sugar
esters (e.g., sorbitan esters) and a C8-10 alkyl glucoside.
25 Preferred coupling agents for use in the methods of the invention
include n-
octane sulfonate and aromatic sulfonates such as an alkyl aryl sulfonate
(e.g., sodium
xylene sulfonate or naphthalene sulfonate). Many hydrotrope solubilizers
independently exhibit some degree of antimicrobial activity at low pH. Such
action
adds to the efficacy of the invention but is not a primary criterion used in
selecting an
30 appropriate solubilizing agent. Since the presence of the
peroxycarboxylic acid material
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
96
in the protonated neutral state provides beneficial biocidal or antimicrobial
activity, the
coupling agent should be selected not for its independent antimicrobial
activity but for
its ability to provide effective single phase composition stability in the
presence of
substantially insoluble peroxycarboxylic acid materials and the more soluble
compositions of the invention. Generally, any number of surfactants may be
used
consistent with the purpose of this constituent.
Anionic surfactants useful with the invention include alkyl carboxylates,
linear
alkylbenzene sulfonates, paraffin sulfonates and secondary n-alkane
sulfonates,
sulfosuccinate esters and sulfated linear alcohols.
Zwitterionic or amphoteric surfactants useful with the invention include .8,-N-
alkylaminopropionic acids, n-alkyl-8,-iminodipropionic acids, imidazoline
carboxylates,
n-alky-Iletaines, amine oxides, sulfobetaines and sultaines.
Nonionic surfactants useful in the context of this invention are generally
polyether (also known as polyalkylene oxide, polyoxyalkylene or polyalkylene
glycol)
compounds. More particularly, the polyether compounds are generally
polyoxypropylene or polyoxyethylene glycol compounds. Typically, the
surfactants
useful in the context of this invention are synthetic organic polyoxypropylene
(P0)-
polyoxyethylene (E0) block copolymers. These surfactants have a diblock
polymer
including an E0 block and a PO block, a center block of polyoxypropylene units
(PO),
and having blocks of polyoxyethylene grated onto the polyoxypropylene unit or
a center
block of E0 with attached PO blocks. Further, this surfactant can have further
blocks
of either polyoxyethylene or polyoxypropylene in the molecule. The average
molecular
weight of useful surfactants ranges from about 1000 to about 40,000 and the
weight
percent content of ethylene oxide ranges from about 10-80% by weight.
Also useful in the context of this invention are surfactants including alcohol
alkoxylates having E0, PO and BO blocks. Straight chain primary aliphatic
alcohol
alkoxylates can be particularly useful as sheeting agents. Such alkoxylates
are also
available from several sources including BASF Wyandotte where they are known
as
"Plurafac" surfactants. A particular group of alcohol alkoxylates found to be
useful are
those having the general formula R-(E0)m--(P0)11 wherein m is an integer of
about 2-10
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
97
and n is an integer from about 2-20. R can be any suitable radical such as a
straight
chain alkyl group having from about 6-20 carbon atoms.
Other useful nonionic surfactants of the invention include capped aliphatic
alcohol alkoxylates. These end caps include but are not limited to methyl,
ethyl, propyl,
butyl, benzyl and chlorine. Useful alcohol alkoxylated include ethylene
diamine
ethylene oxides, ethylene diamine propylene oxides, mixtures thereof, and
ethylene
diamine EO-PO compounds, including those sold under the tradename Tetronic.
Preferably, such surfactants have a molecular weight of about 400 to 10,000.
Capping
improves the compatibility between the nonionic and the oxidizers hydrogen
peroxide
and peroxycarboxylic acid, when formulated into a single composition. Other
useful
nonionic surfactants are alkylpolyglycosides.
Another useful nonionic surfactant of the invention is a fatty acid alkoxylate
wherein the surfactant includes a fatty acid moiety with an ester group
including a block
of EO, a block of PO or a mixed block or heteric group. The molecular weights
of such
surfactants range from about 400 to about 10,000, a preferred surfactant has
an EO
content of about 30 to 50 wt-% and wherein the fatty acid moiety contains from
about 8
to about 18 carbon atoms.
Similarly, alkyl phenol alkoxylates have also been found useful in the
invention.
Such surfactants can be made from an alkyl phenol moiety having an alkyl group
with 4
to about 18 carbon atoms, can contain an ethylene oxide block, a propylene
oxide block
or a mixed ethylene oxide, propylene oxide block or heteric polymer moiety.
Preferably such surfactants have a molecular weight of about 400 to about
10,000 and
have from about 5 to about 20 units of ethylene oxide, propylene oxide or
mixtures
thereof.
The concentration of hydrotrope useful in the present invention generally
ranges
from about 0.1 to about 20 wt-%, preferably from about 0.5 to about 10 wt-%,
most
preferably from about 1 to about 4 wt-%.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
98
Wetting or Defoaming Agents
Also useful in the composition of the invention are wetting and defoaming
agents. Wetting agents function to increase the surface contact or penetration
activity of
the antimicrobial composition of the invention. Wetting agents which can be
used in
the composition of the invention include any of those constituents known
within the art
to raise the surface activity of the composition of the invention.
Generally, defoamers which can be used in accordance with the invention
include silica and silicones; aliphatic acids or esters; alcohols; sulfates or
sulfonates;
amines or amides; halogenated compounds such as fluorochlorohydrocarbons;
vegetable oils, waxes, mineral oils as well as their sulfated derivatives;
fatty acid soaps
such as alkali, alkaline earth metal soaps; and phosphates and phosphate
esters such as
alkyl and alkaline diphosphates, and tributyl phosphates among others; and
mixtures
thereof.
In an embodiment, the present compositions can include antifoaming agents or
defoamers which are of food grade quality given the application of the method
of the
invention. To this end, one of the more effective antifoaming agents includes
silicones.
Silicones such as dimethyl silicone, glycol polysiloxane, methylphenol
polysiloxane,
trialkyl or tetralkyl silanes, hydrophobic silica defoamers and mixtures
thereof can all
be used in defoaming applications. Commercial defoamers commonly available
include
silicones such as Ardefoam0 from Armour Industrial Chemical Company which is a
silicone bound in an organic emulsion; Foam Kill or Kresseo0 available from
Krusable Chemical Company which are silicone and non-silicone type defoamers
as
well as silicone esters; and Anti-Foam A and DC-200 from Dow Corning
Corporation
which are both food grade type silicones among others. These defoamers can be
present at a concentration range from about 0.01 wt-% to 5 wt-%, from about
0.01 wt-%
to 2 wt-%, or from about 0.01 wt-% to about 1 wt-%.
Thickening or Gelling Agents
The present compositions can include any of a variety of known thickeners.
Suitable thickeners include natural gums such as xanthan gum, guar gum, or
other gums
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
99
from plant mucilage; polysaccharide based thickeners, such as alginates,
starches, and
cellulosic polymers (e.g., carboxymethyl cellulose); polyacrylates thickeners;
and
hydrocolloid thickeners, such as pectin. In an embodiment, the thickener does
not leave
contaminating residue on the surface of an object. For example, the thickeners
or
gelling agents can be compatible with food or other sensitive products in
contact areas.
Generally, the concentration of thickener employed in the present compositions
or
methods will be dictated by the desired viscosity within the final
composition.
However, as a general guideline, the viscosity of thickener within the present
composition ranges from about 0.1 wt-% to about 1.5 wt-%, from about 0.1 wt-%
to
about 1.0 wt-%, or from about 0.1 wt-% to about 0.5 wt-%.
Bleaching Agent
The present composition can include a known bleaching agent, such as an active
halogen compound. Suitable bleaching agents include any of the well known
bleaching
agents capable of removing stains from such substrates as dishes, flatware,
pots and
pans, textiles, countertops, appliances, flooring, etc. without significantly
damaging the
substrate. A nonlimiting list of bleaches includes hypochlorites, chlorides,
chlorinated
phosphates, chloroisocyanates, chloramines, etc.; and peroxide compounds such
as
hydrogen peroxide, perborates, percarbonates, etc. Generally, if the
application requires
a color sensitive active agent, bleaches such as peroxide compounds are
generally
preferred. However, if the application does not require color sensitivity,
halogen
bleaches may be used.
Suitable bleaching agents include those that liberate an active halogen
species
such as chlorine, bromine, hypochlorite ion, hypobromide ion, under conditions
normally encountered in typical cleaning processes. The active halogen
compound can,
for example, be a source of a free elemental halogen or --0X-- wherein X is Cl
or Br,
under conditions normally used in detergent-bleaching cleaning processes. In
an
embodiment, the active halogen compound releases chlorine or bromine species.
In an
embodiment, the active halogen compound releases chlorine.
CA 02663953 2014-10-30
100
Chlorine releasing compounds include potassium dichloroisocyanurate, sodium
dichloroisocyanurate, chlorinated trisodiumphosphate, calcium hypochlorite,
lithium
hypochlorite, monochloramine, dichloroamine, [(monotrichloro)-tetra
(monopotassium
dichloro)lpentaisocyanurate, paratoluene sulfondichloro-amide,
trichloromelamine, N-
chlorammeline, N-chlorosuccinimide, N,N'-dichloroazodicarbonamide, N-chloro-
acetyl-urea, N,N'-dichlorobiuret, chlorinated dicyandiamide, trichlorocyanuric
acid,
dichloroglycoluril, 1,3-dichloro-5,5-dimethyl hydantoin, 1-3-dichloro-5-ethyl-
5-methyl
hydantoin, 1-choro-3-bromo-5-ethy1-5-methyl hydantoin, dichlorohydantoin,
trichloromelamine, sulfondichloroamide, trichlorocyanuric acid,salts or
hydrates
thereof, and mixtures thereof. In an embodiment, an chlorine releasing
compound
includes sodium dichloroisocyanurate. In an embodiment, an organic chlorine
releasing
compound can be sufficiently soluble in water to have a hydrolysis constant
(K) of
about 10-4 or greater.
Encapsulated chlorine sources may also be used to enhance the stability of the
chlorine source in the composition (see, for example, U.S. Pat. Nos. 4,618,914
and
4,830,773).
A bleaching agent may also include an agent containing or acting as a source
of
active oxygen. The active oxygen compound acts to provide a source of active
oxygen,
for example, may release active oxygen in aqueous solutions. An active oxygen
compound can be inorganic or organic, or can be a mixture thereof. Some
examples of
active oxygen compound include peroxygen compounds, or peroxygen compound
adducts. Some examples of active oxygen compounds or sources include hydrogen
peroxide, perborates, sodium carbonate peroxyhydrate, phosphate
peroxyhydrates,
potassium permonosulfate, and sodium perborate mono and tetrahydrate, with and
without activators such as tetraacetylethylene diamine, and the like.
In an embodiment the bleach is an alkali metal salt of a chloroisocyanurate, a
hydrate thereof, or a mixture thereof. Dichloroisocyanurate dihydrate, a
suitable
chlorine releasing compound, is commercially available. This compound can be
represented by the formula:
NaC12C1N1032H2O.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
101
The composition can also include an effective amount of a known bleach
activator, such as tetraacetylethylene diamine or a metal, such as manganese.
The composition can include bleaching agent at about 0.5 to 20 wt-%, about 1
to
wt-%, or about 2 to 8 wt-% of the composition. The composition can include up
to
5 about 10 wt-% bleaching agent, and in some embodiments, about 0.1 to
about 6 wt-%.
Use Compositions
The present compositions include concentrate compositions and use
compositions. For example, a concentrate composition can be diluted, for
example with
10 water, to form a use composition. In an embodiment, a concentrate
composition can be
diluted to a use solution before to application to an object. For reasons of
economics,
the concentrate can be marketed and an end user can dilute the concentrate
with water
or an aqueous diluent to a use solution.
The level of active components in the concentrate composition is dependent on
the intended dilution factor and the desired activity of the peroxycarboxylic
acid
compound. Generally, a dilution of about 1 fluid ounce to about 20 gallons of
water to
about 5 fluid ounces to about 1 gallon of water is used for aqueous
antimicrobial
compositions. Higher use dilutions can be employed if elevated use temperature
(greater than 25 C) or extended exposure time (greater than 30 seconds) can
be
employed. In the typical use locus, the concentrate is diluted with a major
proportion of
water using commonly available tap or service water mixing the materials at a
dilution
ratio of about 3 to about 20 ounces of concentrate per 100 gallons of water.
For example, a use composition can include about 0.01 to about 4 wt-% of a
concentrate composition and about 96 to about 99.99 wt-% diluent; about 0.5 to
about 4
wt-% of a concentrate composition and about 96 to about 99.5 wt-% diluent;
about 0.5,
about 1, about 1.5, about 2, about 2.5, about 3, about 3.5, or about 4 wt-% of
a
concentrate composition; about 0.01 to about 0.1 wt-% of a concentrate
composition; or
about 0.01, about 0.02, about 0.03, about 0.04, about 0.05, about 0.06, about
0.07, about
0.08, about 0.09, or about 0.1 wt-% of a concentrate composition. Amounts of
an
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
102
ingredient in a use composition can be calculated from the amounts listed
above for
concentrate compositions and these dilution factors.
The present methods can employ peroxycarboxylic acid at a concentration
effective for reducing the population of one or more microorganisms. Such
effective
concentrations include about 2 to about 500 ppm medium chain peroxycarboxylic
acid,
about 2 to about 300 ppm peroxycarboxylic acid, about 5 to about 100 ppm
peroxycarboxylic acid, about 5 to about 60 ppm peroxycarboxylic acid, about 5
to about
45 ppm peroxycarboxylic acid, about 5 to about 35 ppm peroxycarboxylic acid,
about 5
to about 25 ppm peroxycarboxylic acid, about 8 to about 50 ppm
peroxycarboxylic acid,
about 10 to about 500 ppm peroxycarboxylic acid, about 10 to about 50 ppm
peroxycarboxylic acid, about 40 to about 140 ppm peroxycarboxylic acid, about
100 to
about 250 ppm peroxycarboxylic acid, or about 200 to about 300 ppm
peroxycarboxylic
acid. In an embodiment, the use composition can include about 2 to about 500
ppm
peroxycarboxylic acid, about 5 to about 2000 ppm carboxylic acid, about 95 to
about
99.99 wt-% carrier and/or diluent (e.g., water); and about 2 to about 23,000
ppm
polyalkylene oxide, capped polyalkylene oxide, alkoxylated surfactant, anionic
surfactant, or mixture thereof.
The level of reactive species, such as peroxycarboxylic acids and/or hydrogen
peroxide, in a use composition can be affected, typically diminished, by
organic matter
that is found in or added to the use composition. For example, when the use
composition is a bath or spray used for washing an object, soil on the object
can
consume peroxy acid and peroxide. Thus, the present amounts of ingredients in
the use
compositions refer to the composition before or early in use, with the
understanding that
the amounts will diminish as organic matter is added to the use composition.
In an embodiment, the present use composition can be made more acidic by
passing the concentrate through an acidifying column, or by adding additional
acidulant
to the use composition.
Other Fluid Compositions
The present and compositions can include a critical, near critical, or
supercritical
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
103
(densified) fluid and an antimicrobial agent or a gaseous composition of an
antimicrobial agent. The densified fluid can be a near critical, critical,
supercritical
fluid, or another type of fluid with properties of a supercritical fluid.
Fluids suitable for
densification include carbon dioxide, nitrous oxide, ammonia, xenon, krypton,
methane,
ethane, ethylene, propane, certain fluoroalkanes (e.g., chlorotrifluoromethane
and
monofluoromethane), and the like, or mixtures thereof. Suitable fluids include
carbon
dioxide.
In an embodiment, the present compositions or methods include densified
carbon dioxide, peroxycarboxylic acid, and carboxylic acid. Such a composition
can be
referred to as a densified fluid peroxycarboxylic acid composition. In another
embodiment, the antimicrobial composition includes the fluid, an antimicrobial
agent,
and any of the optional or added ingredients, but is in the form of a gas.
Densified fluid antimicrobial compositions can be applied by any of several
methods known to those of skill in the art. Such methods include venting at an
object a
vessel containing densified fluid and antimicrobial agent. The aqueous phase,
which
includes hydrogen peroxide, is advantageously retained in the device. The
vented gas
includes an effective amount of antimicrobial agent making the densified fluid
peroxycarboxylic acid compositions effective antimicrobial agents.
Because of the high pressure nature of the densified fluid compositions of the
invention, these compositions are typically applied by venting a vessel
containing the
composition through a pressure relief device that is designed to promote rapid
efficient
coverage of an object. Devices including such a pressure relief device include
sprayers,
foggers, foamers, foam pad applicators, brush applicators or any other device
that can
permit the expansion of the fluid materials from high pressure to ambient
pressure while
applying the material to an object. The densified fluid peroxycarboxylic acid
composition can also be applied to an object by any of a variety of methods
known for
applying gaseous agents to an object.
Densified fluid antimicrobial compositions can be made by reacting an
oxidizable substrate with an oxidizing agent in a medium comprising a
densified fluid
to form an antimicrobial composition. This reaction is typically carried out
in a vessel
,
CA 02663953 2014-10-30
104
suitable for containing a densified fluid. Reacting can include adding to the
vessel the
oxidizable substrate and the oxidizing agent, and adding fluid to the vessel
to form the
densified fluid. In an embodiment, the reaction is between a carboxylic acid
and
hydrogen peroxide to form the corresponding peroxycarboxylic acid. The
hydrogen
peroxide is commonly supplied in the form of an aqueous solution of hydrogen
peroxide.
Supercritical, subcritical, near supercritical, and other dense fluids and
solvents
that can be employed with such fluids are disclosed in U.S. Patent No.
5,306,350, issued
April 26, 1994 to Hoy et al.
Supercritical and other dense forms of carbon dioxide, and cosolvents, co-
surfactants, and other additives that can be employed with these forms of
carbon
dioxide are disclosed in U.S. Patent No. 5,866,005, issued February 2, 1999 to
DeSimone et al.
Methods Employing the Peroxycarboxylic Acid Compositions
The present invention includes methods employing the present peroxycarboxylic
acid compositions. Typically, these methods employ the antimicrobial or
bleaching
activity of the peroxycarboxylic acid. For example, the invention includes a
method for
reducing a microbial population, a method for reducing the population of a
microorganism on skin, a method for treating a disease of skin, a method for
reducing
an odor, or a method for bleaching. These methods can operate on an object,
surface, in
a body or stream of water or a gas, or the like, by contacting the object,
surface, body,
or stream with a stabilized ester peroxycarboxylic acid composition of the
invention.
Contacting can include any of numerous methods for applying a composition,
such as
spraying the composition, immersing the object in the composition, foam or gel
treating
the object with the composition, or a combination thereof.
The compositions of the invention can be used for a variety of domestic or
industrial applications, e.g., to reduce microbial or viral populations on a
surface or
object or in a body or stream of water. The compositions can be applied in a
variety of
areas including kitchens, bathrooms, factories, hospitals, dental offices and
food plants,
, õ
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
105
and can be applied to a variety of hard or soft surfaces having smooth,
irregular or
porous topography. Suitable hard surfaces include, for example, architectural
surfaces
(e.g., floors, walls, windows, sinks, tables, counters and signs); eating
utensils; hard-
surface medical or surgical instruments and devices; and hard-surface
packaging. Such
hard surfaces can be made from a variety of materials including, for example,
ceramic,
metal, glass, wood or hard plastic. Suitable soft surfaces include, for
example paper;
filter media, hospital and surgical linens and garments; soft-surface medical
or surgical
instruments and devices; and soft-surface packaging. Such soft surfaces can be
made
from a variety of materials including, for example, paper, fiber, woven or
nonwoven
fabric, soft plastics and elastomers. The compositions of the invention can
also be
applied to soft surfaces such as food and skin (e.g., a hand). The present
compositions
can be employed as a foaming or nonfoaming environmental sanitizer or
disinfectant.
The antimicrobial compositions of the invention can be included in products
such as sterilants, sanitizers, disinfectants, preservatives, deodorizers,
antiseptics,
fungicides, germicides, sporicides, virucides, detergents, bleaches, hard
surface
cleaners, hand soaps, waterless hand sanitizers, and pre- or post-surgical
scrubs.
The antimicrobial compositions can also be used in veterinary products such as
mammalian skin treatments or in products for sanitizing or disinfecting animal
enclosures, pens, watering stations, and veterinary treatment areas such as
inspection
tables and operation rooms. The present compositions can be employed in an
antimicrobial foot bath for livestock or people.
The present compositions can be employed for reducing the population of
pathogenic microorganisms, such as pathogens of humans, animals, and the like.
The
compositions can exhibit activity against pathogens including fungi, molds,
bacteria,
spores, and viruses, for example, S. aureus, E. coli, Streptococci,
Legionella,
Pseudomonas aeruginosa, mycobacteria, tuberculosis, phages, or the like. Such
pathogens can cause a varieties of diseases and disorders, including Mastitis
or other
mammalian milking diseases, tuberculosis, and the like. The compositions of
the
present invention can reduce the population of microorganisms on skin or other
external
or mucosal surfaces of an animal. In addition, the present compositions can
kill
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
106
pathogenic microorganisms that spread through transfer by water, air, or a
surface
substrate. The composition need only be applied to the skin, other external or
mucosal
surfaces of an animal water, air, or surface.
The antimicrobial compositions can also be used on foods and plant species to
reduce surface microbial populations; used at manufacturing or processing
sites
handling such foods and plant species; or used to treat process waters around
such sites.
For example, the compositions can be used on food transport lines (e.g., as
belt sprays);
boot and hand-wash dip-pans; food storage facilities; anti-spoilage air
circulation
systems; refrigeration and cooler equipment; beverage chillers and warmers,
blanchers,
cutting boards, third sink areas, and meat chillers or scalding devices. The
compositions of the invention can be used to treat produce transport waters
such as
those found in flumes, pipe transports, cutters, slicers, blanchers, retort
systems,
washers, and the like. Particular foodstuffs that can be treated with
compositions of the
invention include eggs, meats, seeds, leaves, fruits and vegetables.
Particular plant
surfaces include both harvested and growing leaves, roots, seeds, skins or
shells, stems,
stalks, tubers, corms, fruit, and the like. The compositions may also be used
to treat
animal carcasses to reduce both pathogenic and non-pathogenic microbial
levels.
The present composition is useful in the cleaning or sanitizing of containers,
processing facilities, or equipment in the food service or food processing
industries.
The antimicrobial compositions have particular value for use on food packaging
materials and equipment, and especially for cold or hot aseptic packaging.
Examples of
process facilities in which the composition of the invention can be employed
include a
milk line dairy, a continuous brewing system, food processing lines such as
pumpable
food systems and beverage lines, etc. Food service wares can be disinfected
with the
composition of the invention. For example, the compositions can also be used
on or in
ware wash machines, dishware, bottle washers, bottle chillers, warmers, third
sink
washers, cutting areas (e.g., water knives, slicers, cutters and saws) and egg
washers.
Particular treatable surfaces include packaging such as cartons, bottles,
films and resins;
dish ware such as glasses, plates, utensils, pots and pans; ware wash
machines; exposed
food preparation area surfaces such as sinks, counters, tables, floors and
walls;
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
107
processing equipment such as tanks, vats, lines, pumps and hoses (e.g., dairy
processing
equipment for processing milk, cheese, ice cream and other dairy products);
and
transportation vehicles. Containers include glass bottles, PVC or polyolefin
film sacks,
cans, polyester, PEN or PET bottles of various volumes (100 ml to 2 liter,
etc.), one
gallon milk containers, paper board juice or milk containers, etc.
The antimicrobial compositions can also be used on or in other industrial
equipment and in other industrial process streams such as heaters, cooling
towers,
boilers, retort waters, rinse waters, aseptic packaging wash waters, and the
like. The
compositions can be used to treat microbes and odors in recreational waters
such as in
pools, spas, recreational flumes and water slides, fountains, and the like.
A filter containing the composition can reduce the population of
microorganisms
in air and liquids. Such a filter can remove water and air-born pathogens such
as
Legionella.
The present compositions can be employed for reducing the population of
microbes, fruit flies, or other insect larva on a drain or other surface.
The composition may also be employed by dipping food processing equipment
into the use solution, soaking the equipment for a time sufficient to sanitize
the
equipmentõ and wiping or draining excess solution off the equipment, The
composition
may be further employed by spraying or wiping food processing surfaces with
the use
solution, keeping the surfaces wet for a time sufficient to sanitize the
surfaces, and
removing excess solution by wiping, draining vertically, vacuuming, etc.
The composition of the invention may also be used in a method of sanitizing
hard surfaces such as institutional type equipment, utensils, dishes, health
care
equipment or tools, and other hard surfaces. The composition may also be
employed in
sanitizing clothing items or fabric which have become contaminated. The use
solution
is contacted with any of the above contaminated surfaces or items at use
temperatures in
the range of about 4 C to 60 C, for a period of time effective to sanitize,
disinfect, or
sterilize the surface or item. For example, the concentrate composition can be
injected
into the wash or rinse water of a laundry machine and contacted with
contaminated
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
108
fabric for a time sufficient to sanitize the fabric. Excess solution can then
be removed
by rinsing or centrifuging the fabric.
The antimicrobial compositions can be applied to microbes or to soiled or
cleaned surfaces using a variety of methods. These methods can operate on an
object,
surface, in a body or stream of water or a gas, or the like, by contacting the
object,
surface, body, or stream with a composition of the invention. Contacting can
include
any of numerous methods for applying a composition, such as spraying the
composition, immersing the object in the composition, foam or gel treating the
object
with the composition, or a combination thereof.
A concentrate or use concentration of a composition of the present invention
can
be applied to or brought into contact with an object by any conventional
method or
apparatus for applying an antimicrobial or cleaning composition to an object.
For
example, the object can be wiped with, sprayed with, foamed on, and/or
immersed in
the composition, or a use solution made from the composition. The composition
can be
sprayed, foamed, or wiped onto a surface; the composition can be caused to
flow over
the surface, or the surface can be dipped into the composition. Contacting can
be
manual or by machine. Food processing surfaces, food products, food processing
or
transport waters, and the like can be treated with liquid, foam, gel, aerosol,
gas, wax,
solid, or powdered stabilized compositions according to the invention, or
solutions
containing these compositions.
The composition can be employed for bleaching pulp. Such a method includes
contacting the pulp with a peroxycarboxylic acid composition according to the
present
invention. Such a peroxycarboxylic acid composition can include added
bleaching
agent.
The compositions can be employed for waste treatment. Such a method
includes contacting the waste with a peroxycarboxylic acid composition
according to
the present invention. Such a peroxycarboxylic acid composition can include
added
bleaching agent.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
109
Clean in Place
Other hard surface cleaning applications for the antimicrobial compositions of
the invention include clean-in-place systems (CIP), clean-out-of-place systems
(COP),
washer-decontaminators, sterilizers, textile laundry machines, ultra and nano-
filtration
systems and indoor air filters. COP systems can include readily accessible
systems
including wash tanks, soaking vessels, mop buckets, holding tanks, scrub
sinks, vehicle
parts washers, non-continuous batch washers and systems, and the like.
Generally, the actual cleaning of the in-place system or other surface (i.e.,
removal of unwanted offal therein) is accomplished with a different material
such as a
formulated detergent which is introduced with heated water. After this
cleaning step,
the instant composition would be applied or introduced into the system at a
use solution
concentration in unheated, ambient temperature water. CIP typically employ
flow rates
on the order of about 40 to about 600 liters per minute, temperatures from
ambient up to
about 70 C, and contact times of at least about 10 seconds, for example, about
30 to
about 120 seconds. The present composition can remain in solution in cold
(e.g.,
40 F./4 C.) water and heated (e.g., 140 F./60 C.) water. Although it is not
normally
necessary to heat the aqueous use solution of the present composition, under
some
circumstances heating may be desirable to further enhance its antimicrobial
activity.
These materials are useful at any conceivable temperatures.
A method of sanitizing substantially fixed in-place process facilities
includes the
following steps. The use solution of the invention is introduced into the
process
facilities at a temperature in the range of about 4 C to 60 C. After
introduction of the
use solution, the solution is held in a container or circulated throughout the
system for a
time sufficient to sanitize the process facilities (i.e., to kill undesirable
microorganisms).
After the surfaces have been sanitized by means of the present composition,
the use
solution is drained. Upon completion of the sanitizing step, the system
optionally may
be rinsed with other materials such as potable water. The composition can be
circulated
through the process facilities for 10 minutes or less.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
110
The present method can include delivering the present composition via air
delivery to the clean-in-place or other surfaces such as those inside pipes
and tanks.
This method of air delivery can reduce the volume of solution required.
Contacting a Food Product with the Peroxycarboxylic Acid Composition
The present method and system provide for contacting a food product with a
peroxycarboxylic acid composition employing any method or apparatus suitable
for
applying such a composition. For example, the method and system of the
invention can
contact the food product with a spray of the composition, by immersion in the
composition, by foam or gel treating with the composition, or the like.
Contact with a
spray, a foam, a gel, or by immersion can be accomplished by a variety of
methods
known to those of skill in the art for applying antimicrobial agents to food.
Contacting
the food product can occur in any location in which the food product might be
found,
such as field, processing site or plant, vehicle, warehouse, store,
restaurant, or home.
These same methods can also be adapted to apply the stabilized compositions of
the
invention to other objects.
The present methods require a certain minimal contact time of the composition
with food product for occurrence of significant antimicrobial effect. The
contact time
can vary with concentration of the use composition, method of applying the use
composition, temperature of the use composition, amount of soil on the food
product,
number of microorganisms on the food product, type of antimicrobial agent, or
the like.
The exposure time can be at least about 5 to about 15 seconds.
In an embodiment, the method for washing food product employs a pressure
spray including the composition. During application of the spray solution on
the food
product, the surface of the food product can be moved with mechanical action,
e.g.,
agitated, rubbed, brushed, etc. Agitation can be by physical scrubbing of the
food
product, through the action of the spray solution under pressure, through
sonication, or
by other methods. Agitation increases the efficacy of the spray solution in
killing
micro-organisms, perhaps due to better exposure of the solution into the
crevasses or
small colonies containing the micro-organisms. The spray solution, before
application,
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
111
can also be heated to a temperature of about 15 to 20 C, for example, about
20 to 60 C
to increase efficacy. The spray stabilized composition can be left on the food
product
for a sufficient amount of time to suitably reduce the population of
microorganisms, and
then rinsed, drained, or evaporated off the food product.
Application of the material by spray can be accomplished using a manual spray
wand application, an automatic spray of food product moving along a production
line
using multiple spray heads to ensure complete contact, or other spray
apparatus. One
automatic spray application involves the use of a spray booth. The spray booth
substantially confines the sprayed composition to within the booth. The
production line
moves the food product through the entryway into the spray booth in which the
food
product is sprayed on all its exterior surfaces with sprays within the booth.
After a
complete coverage of the material and drainage of the material from the food
product
within the booth, the food product can then exit the booth. The spray booth
can include
steam jets that can be used to apply the stabilized compositions of the
invention. These
steam jets can be used in combination with cooling water to ensure that the
treatment
reaching the food product surface is less than 65 C, e.g., less than 60 C. The
temperature of the spray on the food product is important to ensure that the
food
product is not substantially altered (cooked) by the temperature of the spray.
The spray
pattern can be virtually any useful spray pattern.
Immersing a food product in a liquid stabilized composition can be
accomplished by any of a variety of methods known to those of skill in the
art. For
example, the food product can be placed into a tank or bath containing the
stabilized
composition. Alternatively, the food product can be transported or processed
in a flume
of the stabilized composition. The washing solution can be agitated to
increase the
efficacy of the solution and the speed at which the solution reduces micro-
organisms
accompanying the food product. Agitation can be obtained by conventional
methods,
including ultrasonics, aeration by bubbling air through the solution, by
mechanical
methods, such as strainers, paddles, brushes, pump driven liquid jets, or by
combinations of these methods. The washing solution can be heated to increase
the
efficacy of the solution in killing micro-organisms. After the food product
has been
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
112
immersed for a time sufficient for the desired antimicrobial effect, the food
product can
be removed from the bath or flume and the stabilized composition can be
rinsed,
drained, or evaporated off the food product.
In another alternative embodiment of the present invention, the food product
can
be treated with a foaming version of the composition. The foam can be prepared
by
mixing foaming surfactants with the washing solution at time of use. The
foaming
surfactants can be nonionic, anionic or cationic in nature. Examples of useful
surfactant
types include, but are not limited to the following: alcohol ethoxylates,
alcohol
ethoxylate carboxylate, amine oxides, alkyl sulfates, alkyl ether sulfate,
sulfonates,
quaternary ammonium compounds, alkyl sarcosines, betaines and alkyl amides.
The
foaming surfactant is typically mixed at time of use with the washing
solution. Use
solution levels of the foaming agents is from about 50 ppm to about 2.0 wt-%.
At time
of use, compressed air can be injected into the mixture, then applied to the
food product
surface through a foam application device such as a tank foamer or an
aspirated wall
mounted foamer.
In another alternative embodiment of the present invention, the food product
can
be treated with a thickened or gelled version of the composition. In the
thickened or
gelled state the washing solution remains in contact with the food product
surface for
longer periods of time, thus increasing the antimicrobial efficacy. The
thickened or
gelled solution will also adhere to vertical surfaces. The composition or the
washing
solution can be thickened or gelled using existing technologies such as:
xanthan gum,
polymeric thickeners, cellulose thickeners, or the like. Rod micelle forming
systems
such as amine oxides and anionic counter ions could also be used. The
thickeners or gel
forming agents can be used either in the concentrated product or mixing with
the
washing solution, at time of use. Typical use levels of thickeners or gel
agents range
from about 100 ppm to about 10 wt-%.
Aseptic Packaging
In the method of the present invention, aseptic packaging includes contacting
the
container with a composition according to the present invention. Such
contacting can
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
113
be accomplished using a spray device or soaking tank or vessel to intimately
contact the
inside of the container with the composition for sufficient period of time to
clean or
reduce the microbial population in the container. The container is then
emptied of the
amount of the present composition used. After emptying, the container can then
be
rinsed with potable water or sterilized water (which can include a rinse
additive) and
again emptied. After rinsing, the container can be filled with the liquid
beverage. The
container is then sealed, capped or closed and then packed for shipment for
ultimate
sale.
Figure 18 shows a schematic for an embodiment of a bottle spraying/bottling
operation using a composition according to the present invention. The
operation can be
a cold aseptic operation. Figure 18 shows a plant 100 that can contact
beverage bottles
with a medium chain peroxycarboxylic acid composition for a sanitizing regime.
In
Figure 18, bottles 110 are passed through a sterilizing tunnel 102. The
sanitized bottles
110a then pass through a rinsing tunnel 103 and emerge as sanitized rinsed
bottles 110b.
In the process, bulk medium chain peroxycarboxylic acid composition is added
to a holding tank 101. Commonly, the materials are maintained at a temperature
of
about 22 C in tank 101. To obtain the effective use concentration of the
medium chain
peroxycarboxylic acid composition, make-up water 105 is combined with the
concentrated medium chain peroxycarboxylic acid composition into the tank 101.
The
medium chain peroxycarboxylic acid use composition is passed through a heater
108 to
reach a temperature of about 45-50 C. The heated medium chain
peroxycarboxylic
acid use composition is sprayed within sterilizing tunnel 102 into and onto
all surfaces
of the bottle 110. An intimate contact between the medium chain
peroxycarboxylic acid
composition and the bottle 110 is essential for reducing microbial populations
to a
sanitizing level.
After contact with the medium chain peroxycarboxylic acid use composition and
after dumping any excess composition from the bottles, the sanitized bottles
110 are
then passed to a fresh water rinse tunnel 103. Fresh water 108 is provided
from a fresh
water make-up into a spray rinsing tunnel 103. The fresh water can include a
rinse
additive. Excess spray drains from rinsing tunnel 103 to drain 106. Within the
tunnel
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
114
103, sanitized bottles 110a are thoroughly rinsed with fresh water. The
complete
removal of the medium chain peroxycarboxylic acid composition from the bottles
110a
is important for maintaining high quality of the beverage product. The rinsed
and
sanitized bottles 110b are then removed from the rinsing tunnel.
The day tank 101, the sterilizing tunnel 102 and the rinsing tunnel 103 are
all
respectively vended to wet scrubber or vent 111a, 111b or 111c to remove vapor
or
fumes from the system components. The sanitizer material that has been sprayed
and
drained from the bottles 110a accumulate in the bottom of the spray tunnel 102
and is
then recycled through recycle line and heater 107 into the day tank 101.
The contact between the bottles and the medium chain peroxycarboxylic acid
antimicrobial composition can be at a temperature of greater than about 0 C ,
greater
than 25 C, or greater than about 40 C. Temperatures between about 40 C and
90 C
can be used. In certain embodiments, contact at 40 C to 60 C for at least 5
sec, for
example at least about 10 sec, contact time is employed.
In the cold aseptic filling of 16 ounce polyethylene terephthalate (PET
bottle), or
other polymeric, beverage containers, a process has been adopted using a
medium chain
peroxycarboxylic acid composition. The medium chain peroxycarboxylic acid
composition can be diluted to a use concentration of about 0.1 to about 10 wt%
and
maintained at an effective elevated temperature of about 25 C to about 70 C,
e.g., about
40 C to about 60 C. The spray or flood of the bottle with the material ensures
contact
between the bottle and the sanitizer material for at least 5, e.g., about 10,
seconds. After
flooding is complete, the bottle can be drained of all contents for a minimum
of 2
seconds and optionally followed by a 5 second water rinse with sterilized
water using
about 200 milliliters of water at 38 C (100 F). If optionally filled with the
rinse water,
the bottle is then drained of the sterilized water rinse for at least 2
seconds and is
immediately filled with liquid beverage. The rinse water can include a rinse
additive.
After the rinse is complete, the bottles usually maintain less than 10, e.g.,
3, milliliters
of rinse water after draining.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
115
Textile Cleaning
The present invention includes methods and compositions for removing soil
from textiles. The composition of the invention can be used with typical
commercial
textile cleaning or laundering processes and machines. The present method can
include
contacting a laundry item in a laundry machine with a penetrant composition in
the
form of an aqueous presoak, preflush, prewash, or other step prior to the
cleaning step.
A suitable laundry process employs a washer/extractor. Laundry cleaning
processes can
include processes such as flushing, sudsing, draining, bleaching, rinsing,
extracting,
repetitions thereof, or combinations thereof. The bleaching composition can
include a
composition according to the present invention.
Flushing can include contacting the laundry item with a flushing composition.
In an embodiment, flushing is the initial wetting step in the machine that
carries out the
washing procedure. A method of cleaning laundry can include flushing one, two,
or
more times. Conventional flushing compositions are water (e.g., soft or tap
water). In
conventional systems, flushing can separate loose soil from and wet a laundry
item, but
little more. Flushing can also be referred to as presoaking, preflushing, or
prewashing.
Sudsing can include cleaning the laundry item with a sudsing cleaning
composition. The sudsing cleaning composition typically includes surfactants
and other
cleaners, and can include a bleach. Sudsing can follow flushing.
Draining includes removing a cleaning, flushing, or other composition from the
laundry item, for example, by gravity and/or centrifugal force. Draining can
follow
sudsing. Draining can occur between repeats of flushing. In an embodiment, the
textile
is cleaned with a textile cleaning composition including a built detergent and
chlorine
bleach in a suds/bleach combination or in two separate wash steps, i.e. suds
steps with
built detergent followed by bleach step with chlorine.
Bleaching can include cleaning the laundry item with a bleach composition.
Bleaching can follow draining and/or sudsing. The bleaching composition can
include
a composition according to the present invention.
Rinsing can include contacting the laundry item with a rinse composition
suitable for removing remaining cleaning (sudsing and/or bleach) composition.
The
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
116
rinse composition can, for example, be water (e.g., soft or tap water), a sour
rinse, or a
rinse including softener. A method of cleaning laundry can include one, two,
three, or
more rinses. Rinsing can follow bleaching and/or sudsing.
Extracting can include removing a rinse composition from the laundry item,
typically with centrifugal force. Extracting can follow one or more rinsings.
The present method and composition can be employed on any of a variety of
textiles. Suitable textiles include cotton, cotton/polyester blend, polyester,
and the like.
The present invention may be better understood with reference to the following
examples. These examples are intended to be representative of specific
embodiments of
the invention, and are not intended as limiting the scope of the invention.
EXAMPLES
Example 1 -- Making Peroxyacetic Acid with an Apparatus Including a
Pretreatment Column and A Reaction catalyst
The present apparatus and method were employed to make the compositions on
Tables 1 through 4 below. All equilibrium values were calculated values from
the
reported Keq of 2.70 for peroxyacetic acid. In the case of mixed peracids
etc., the Keq
was assumed to be 2.70.
The stability of certain of the test compositions was monitored with and
without
stabilizer (HEDP) added.
CA 02663953 2009-03-17
WO 2008/047263 PCT/1B2007/053809
117
Table 1
Initial wt-% Wt-% at
Equilibrium
Carboxylic Hydrogen
Peroxy Hydrogen
Test Water Total
Acid Peroxide Acid
Peroxide
I 56.5 30.5 13.0 100.0 35.3 14.6
II 43.6 20.5 35.9 100.0 17.3 12.7
III 20.0 28.0 52.0 100.0 9.7 23.6
IV 78.0 7.7 14.3 100.0 13.1 1.9
V 5.0 5.0 90.0 100.0 0.5 4.8
Table 2
Initial wt-% Wt-% at
Equilibrium
Short Chain Hydrogen
Peroxy Hydrogen
Test Water Total
Carboxylic Acid Peroxide Acid
Peroxide
VI 56.5 30.5 13.0 100.0 35.3
14.6
VII 43.6 20.5 35.9 100.0 17.3
12.7
VIII 20.0 28.0 52.0 100.0 9.7 23.6
IX 78.0 7.7 14.3 100.0 13.1
1.9
X 5.0 5.0 90.0 100.0 0.5 4.8
Table 3
Initial wt-% Wt-% at
Equilibrium
0
Medium
w
o
Chain Hydrogen Peroxy
Hydrogen c'
Go
Test Hydrotrope Water Total
Carboxylic Peroxide Peroxide Acid
Peroxide .6.
-4
Acid w
o
(...)
XI 20.0 30.0 25 25.0 100.0
18.5 31.7
XII 10.0 20.0 15 55.0 100.0
6.8 20.4
XIII 5.0 20.0 10 65.0 100.0 2.1 21.3
XIV 3.0 22.5 10 64.5 100.0
1.2 21.6
0
0
I.)
c7,
c7,
UJ
Table 4
,-, =
co
Initial wt-% Wt-% at
oe I.)
0
Equilibrium
0
1
Short Medium
o
Short Medium Hydr UJ
Chain Chain
I
Hydrogen Chain
Chain ogen H
Test Carbo Carbox Hydrotrope Water Total
-1
Peroxide Peroxy
Peroxy Pero
xylic ylic
Acid Acid xide
Acid Acid
XV 48.0 20.0 10.0 12 10.0 100 13.6 5.7 2.8
XVI 56.0 8.0 12.0 12 12.0 100 18.8 2.7 4.0
IV
XVII 60.0 2.0 13.0 12 13.0 100 22.1 0.7 4.6
n
1-i
xvii
5
43.6 1.0 20.5 0 34.9 100 17.4 0.4 12.6
I
w
o
o
-4
o
(...)
ce
o
Table 5
o
Initial wt-%
=
o
oe
Measured
C-5
.6.
Carboxylic Hydrogen Peroxy Output of Predicted % of
--.1
n.)
Test Water Total
cA
Acid Peroxide Acid Percarboxylic Peracid Pred.
w
Acid (wt-%)
XIX Acetic acid 11 30 59 100 5.3 5.6 95
XX Acetic acid 20 28 52 100 9.2 9.7 95
XXI Acetic acid 44 18 38 100 14.3 15.3 93
XXII Acetic acid 78 7 15 100 13.0 12.6
103 0
0
XXIV Acetic acid 41 17 42 100 12.0 13.4
90 1.)
c7,
c7,
XXV Acetic acid 50 20 30 100 20.0 19.9
101 u.)
q3.
1-,
u,
XXVI Acetic acid 60 20 20 100 25.0 25.2 99
1.)
0
XXVII Acetic acid 5 5 90 100 0.4 0.5
78 0
q3.
1
Glycolic
0
XXVIII 55 8 38 100 1.2 8.3 14
u.)
1
acid
H
-..3
Succinic
XXIX 7 3 90 100 0.2 0.4 51
acid
Octanoic
XXX 50 18 32 100 0.54 16.0 3
acid
XXXI Acetic acid 46 19 35 100 17.0 17.2 99
IV
n
XXXII Acetic acid 50 20 30 100 20.2 19.9
102 1-3
=
=
-.,
=
u,
c,
=
,.,
CA 02663953 2016-05-25
120
Table 6 - Stability of Peracetic Acid Products from Dowex M31 Catalyst at 70
F
_______________________ Measured Concentration of Peracetic Acid (wt-%)
Time (days) XXV (no HEDP) XXV (HEDP added)
0.0 18.3 18.9
7.0 19.0 19.1
13.0 19.1 19.0
Table 7 - Stability of Peracetic Acid Products From Dowex M31 Catalyst at 140
F
Measured Concentration of Peracetic Acid (wt-%)
Time (days) XXVI (no HEDP) XXV (HEDP added)
0.0 18.3 18.9
7.0 15.8 15.4
13.0 15.8 14.3
Table 8 - Stability of Peracetic Acid Products From Dowex M31 Catalyst at 70
F
Measured Concentration of Peracetic Acid (wt-%)
Time (days) XXVI (no HEDP) XXVI (HEDP added)
0.0 25.7 25.7
3.0 23.6 22.8
It should be noted that, as used in this specification and the appended
claims, the
singular forms "a," "an," and "the" include plural referents unless the
content clearly
dictates otherwise. Thus, for example, reference to a composition containing
"a
compound" includes a mixture of two or more compounds. It should also be noted
that
the term "or" is generally employed in its sense including "and/or" unless the
content
clearly dictates otherwise.
All publications and patent applications in this specification are indicative
of the
level of ordinary skill in the art to which this invention pertains.
The scope of the claims should not be limited by the preferred embodiments
set forth in the examples but should be given the broadest interpretation
consistent
with the description as a whole.