Note: Descriptions are shown in the official language in which they were submitted.
CA 02664039 2009-04-24
Medicine Dosing Compliance System
INDEX TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional
Patent Application No. 61/047,461, filed April 24, 2008,
the disclosure of which is incorporated herein by
reference in its entirety.
BACKGROUND OF THE INVENTION
Compliance when taking any product that is to be
administered over a period of time is always a major
concern. When the compliance relates to a patient
compliance of a medical dosing regimen, the concerns for
compliance are heightened. The present invention
addresses the need for an effective article and method
for assisting patient compliance.
BRIEF SUMMARY OF THE INVENTION
The present invention is a label configured to be secured
to a prescription bottle or other container in which
dosing and compliance are important. The label has
indicia for specific days and times in which the medicine
should be taken.
1
CA 02664039 2009-04-24
By way of example, if a patient has a prescription filled
on Monday January 14 with instructions for the medicine
to be taken three times a day for five days, the label
may read as follows:
a.m. p.m. night
Monday, January 14, 2008
Tuesday, January 15, 2008
Wednesday, January 16, 2008
Thursday, January 17, 2008
Friday, January 18, 2008
The area after each day would be for the patient to
indicate or mark the dosage for that day and/or the time
the dosage had been taken.
The indicia would change based on the dosing regimen
prescribed and dates, but would be automatically
generated for a label based upon the dosing regimen
prescribed.
In a preferred embodiment the present invention is an
article for recording dosing compliance comprising:
2
CA 02664039 2009-04-24
a. compliance recordation indicia printed on a
compliance recordation article; and
b. attaching the article to a container.
The dosing regimen may be a prescription or non-
prescription medicinal product, a nutraceutical, a
nutritional supplement and the like.
The dosing regimen may be a schedule of taking a
prescription or non-prescription medicinal product, a
nutraceutical, a nutritional supplement and the like
under the direction of a physician, health care provider,
or as directed by a distributor or manufacturer of a
product.
The article indicia are customizable to a dosing regimen.
The customization may include specific days and times.
The indicia are configured for marking dose compliance.
The indicia are marked contemporaneously with the
administration of a dose by a marking letter "x", a
circle, a square, and the like, other markings, and/or
combinations thereof.
In one embodiment, the article is in a unitary
configuration with a container label. That is, the label
3
CA 02664039 2009-04-24
is affixed to the container and the article is a
contiguous extension of the label.
Alternatively, the article may have a mechanism for
attaching to a container which may be an adhesive that
secures the article, in a permanent or substantially
permanent manner to the container or to another label
attached to the container.
In one embodiment, the article has a releasable adhesive
on one portion of the article such that the article may
be closed and conceal the recordation indicia.
Preferably, the closing will be along a predefined fold
line.
The present invention also comprises a method for
recording dosing compliance comprising the steps of:
a. providing a compliance recordation article with
compliance recordation indicia;
b. attaching the article to a container;
c. dispensing a dose from the container pursuant to a
dosing regimen;
d. marking the compliance recordation article with a
mark indicating a time a dose was taken.
The present invention also includes a method of
producing a compliance recordation article having the
4
CA 02664039 2009-04-24
steps of:
a. providing a customizable computer readable
medium unique for a patient and a
medication;
b. operatively associating at least one user
computer with said computer readable medium;
c. inputting dosage and medication information
unique to a patient and into said user
computer;
d. providing an output that produces a dosage
compliance article;
e. affixing said dosage compliance article to a
medicinal container;
f. providing said container to a patient.
BRIEF DESCRIPTION OF THE DRAWINGS
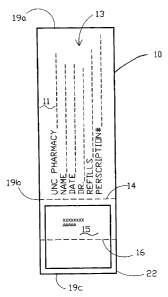
FIG. 1 is a front view of a prescription label with a
compliance calendar label.
FIG. 2 is a rear view of a prescription label with a
compliance calendar label.
FIG. 3 is perspective view of the front of a compliance
label affixed to a container.
FIG. 4 is a cross section view of the prescription label
of Figs. 1 and 2 with a fold line 16.
FIG. 5 is a cross section view along line A-A from Fig.
5
CA 02664039 2009-04-24
3.
FIG. 6 is a cross section view of a compliance label on a
container with an open tab.
FIG. 7 is a cross section view of a compliance label with
the tab open.
FIG. 8 is a front view of a generic compliance label.
FIG. 9 is a front view of an alternate generic compliance
label.
FIG. 10 is a front view of another alternate generic
compliance label with a user marking.
FIG. 11 is a front view of a compliance label with dates
and times.
FIG. 12 is a front view of an alternate compliance label
with days and times.
FIG. 13 is a front view of an alternate compliance label
with days and times with user markings.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is a compliance calendar label for
monitoring patient compliance and a dosing regimen.
Pharmaceutical label 10 is formed from a label sheet or
roll as known in the art and has a front face 11. Label
10 is an adhesive label, having a layer of adhesive 17
generally behind said label 10. A silicon liner 20
6
CA 02664039 2009-04-24
provides a protective cover over said adhesive 17.
Silicon liner 20 has a rear label face 12.
Front face 11 includes printed thereon a prescription in
a prescription area 13 and a first compliance indicia
area 15. Rear face 12 includes printed thereon a second
compliance indicia area 24. Label 10 has a score line
14. Said score line is also on liner 20. In one
embodiment, areas 15 and 24 have a fold line 16.
Label 10 is affixed to a container 21 by removal of a
portion of liner 20, the portion 24a at score line 14,
and exposing adhesive 17 for affixing reverse side of
label 10 circumferentially around container 21. Said
container 21 having a top rim 29 for receiving a closure
cap not shown. Label 10 is constructed and arranged to
be wrapped circumferentially around the perimeter of a
desired container 21 starting at first end 19a of label
10 to second end 19b of label 10. An informational tab
22 is formed approximately from score line 14 to the end
of label 10 at tab end 19c. Patient compliance indicia
can be printed on one or both sides of tab 22 on
compliance indicia areas 15 and 24. Understandably,
label 10 could be rearranged with the compliance indicia
areas in different locations on label 10.
7
CA 02664039 2009-04-24
The rear of label 10 includes a liner 24 that is
typically scored at one or more locations. In at least a
first location at the base of tab 22 at score line 14 and
at a fold line 16 of tab 22. Score 14 provides a fold
line for face 11 and a tear line for face 12 to easily
detach liner portion 24a. Label 10, as known in the art,
and manufactured by Rx Technology Corporation, Joplin, MD
64801, may be taken either from a label sheet that is
passed through a printer and then removed or label 10 may
be on a roll of labels attached end to end that are
thermally printed and the label is then detached from the
roll.
Tab 22 can be folded back onto itself as shown in Figs. 6
and 7. Tab 22 folds in the directions - back and forth -
depicted by arrow B such that a hinge is created at fold
line 16 such that compliance indicia 24a folds back on
itself. Edge portion of adhesive 17 releasably secures a
first portion 24b of indicia area 24 against a second
portion 24c of indicia area 24 one to the other.
In the compliance label embodiments of Figs. 9, 10 and 11
compliance indicia area 24 is surrounded at least on 2
side edges with adhesive 17 exposed such that compliance
indicia area 24 when folded along fold line 16 as seen in
Figs. 6 and 7 will fold against itself and is released by
8
CA 02664039 2009-04-24
lifting tab end 19c at release points 33.
The present invention provides for compliance indicia to
be disposed on label 10 affixed to container 21 at the
front face 11 and/or rear face 12. Preferably, the
compliance indicia are a compliance calendar disposed on
tab 22 that extends outward from the perimeter of
container 21 to which an incorporated label 10 is formed
a part thereof or is attached. Compliance calendar
labels in indicia areas 15 and/or 24 of the present
invention are marked manually by a user. The user
typically being the patient taking the medication or a
person administering the medication for the patient. The
marking does not require any electronics to keep track of
dosing. The manual marking is by pencil, pen, hole
puncher, combinations thereof, or any other appropriate
marking mechanism. The compliance calendar to be marked
is at the site of dispensing the medicine to the patient
either by the patient directly or by a person
administering it to the patient.
The compliance calendar is presented as either a generic
listing of days and dosing times, as is shown in Figs. 8,
9, 10 and 12 or may be presented to a particular patient
with days and times specific to a patient dosing regimen,
as in Figs. 11 and 13.
9
CA 02664039 2009-04-24
By way of example, and not limiting thereby, a patient is
directed to take a particular medication twice a day,
that has a prescription dispensed at a pharmacy beginning
on April 1, 2009 at 6 p.m. The patient would not have a
morning dose but only an evening dose. As seen below,
the morning dose on April 1, 2009 has been marked
indicating no morning dose. A personal compliance
calendar that begins:
April 1, 2009 XXX
p.m.
April 2, 2009
a.m. p.m.
The calendar would continue with subsequent dates until
dosing regimen is completed.
The compliance calendar of the present invention will
have indicia for particular start dates 26 and end dates
27 as applicable to a particular patient and medication,
see Fig. 12.
In the above example, the personal compliance calendar
indicates only one dosage to be taken on April 1, 2009
CA 02664039 2009-04-24
with subsequent days if applicable (only April 2nd shown)
to be taken morning and evening. The a.m. dosage of April
1, 2009 is blocked out, thus indicating to the patient
that no dosage be taken on that day and time. Similarly
the calendars in Figs. 10 and 13 have been customized for
the patient's prescribed dosing regimen. In Fig. 10, the
Day 1 "a.m." space has been blocked out indicating no
dosage to be taken on that time. The first dosage called
for at Day 1 "lunch." In Fig. 13, the days and dates
have been customized to a start date of noon on Tuesday
March 31st (3/31). In the top row of the compliance
calendar the days March 29 and 30 have been blocked out
for the times a.m., noon, p.m. and bed when the
compliance label was printed. Thus indicating the start
time and day of noon March 31St. Also shown in Fig. 13,
are the markings by the user on March 31 for noon, p.m.,
bed and April 1 a.m. Said user markings indicate the
compliance of the user in taking the "medicine use each
time" taken.
In an example of ongoing dosing, such as, but not limited
to blood pressure medication that is taken continually
every day, the personal compliance calendar is presented
to the patient with date and time indicia indicative of
either an initial prescription, or date and time indicia
indicative of when a previous prescription supply is
11
CA 02664039 2009-04-24
exhausted and a new prescription supply begins.
By way of example only, a patient dispensed an initial 30
day supply of blood pressure medication on May 1, 2009
will receive a compliance calendar indicative of dosing
starting on May 1, 2009. This patient calls a pharmacy
for refill and typically requests a refill before the
current supply is depleted. A patient may call in a
refill on May 25, 2009, and the personal compliance
calendar of the refill will have indicia beginning on May
31, 2009 i.e. the day the refill begins to replace the
initial 30 day supply.
A particular dosing regimen, for example, a regimen given
by a physician for a medicine, is indicated e.g. printed
on compliance indicia areas 15 and/or 24. In the
embodiment of Fig. 10, as an example, the patient is to
take a particular dosage three times a day (t.i.d - a
medical abbreviation from the Latin phrase ter in die
meaning a thrice-daily dosage) for five days. The
patient is instructed to mark the label at the time of
taking each individual dose. The present invention
provides an easy way for the patient to mark the dosage
taken on a particular day and time. Later, if a patient
is unsure if they completed a particular dose, they need
only look at label 10 on the specific container in
12
CA 02664039 2009-04-24
question. As seen in Fig. 10, Day 1 "a.m." has been
marked with an x.
After review of the marked doses of label 10, a patient
will be able to proceed accordingly. A dose will be
taken at a regimen specified time if there is no
indication it had been taken. Conversely, the dose will
be skipped if label 10 indicates the dose had been taken.
Although the figures show a particular embodiments with
the indicia placed on one region of label 10, the
compliance information may be printed on the entire
compliance indicia area 15 and/or 24 of label 10 in
various manners and formats. Various manners and formats
are shown in Figs. 8 to 13.
The indicia would be specific for the dosing regimen.
For example, if the dosing regime called for taking a
particular dose two times the first day, and once a day
for six more days, the label may appear as follows noting
that the afternoons after day 1 are "marked out"
indicating no dosage for that period:
13
CA 02664039 2009-04-24
a.m. p.m
Start
Day 1
Day 2 X
Day 3 X
Day 4 X
Day 5 X
Day 6 X
Day 7 X
End
As seen in Fig. 12, label 10 has indicia to accommodate
many doses dispensed from a single container. Label 10
of Fig. 12 has indicia for four doses a day, seven days a
week, for five weeks. This compliance calendar provides
indicia for
recordation of 140 individual doses of a medication from
a single container.
The compliance calendars shown in Figs. 8 to 13 may be in
either compliance indicia area 15 or 24 in any
combination and where only a compliance calendar is
placed in either 15 or 24 then the other area 15 or 24
without a compliance calendar may contain any other
information.
14
CA 02664039 2009-04-24
While the invention has been described in its preferred
form or embodiment with some degree of particularity, it
is understood that this description has been given only
by way of example and that numerous changes in the
details of construction, fabrication, and use, including
the combination and arrangement of parts, may be made
without departing from the spirit and scope of the
invention.
i5