Note: Descriptions are shown in the official language in which they were submitted.
CA 02666247 2013-01-21
Method And System For Tracking Medical Products
This application is a U.S. national stage of PCT/FR2007/001675, filed on Oct.
12, 2007,
which claims priority to French application no. 0608939 filed on Oct. 12,
2006.
The present invention concerns a method and a system for interactive and
continuous tracking
of medical products, enabling total traceability thereof, notably for
insurance and after-sales
service purposes, whilst guaranteeing that medical confidentiality is
preserved.
It finds a particularly important, although not exclusive, application in the
field of internal
prostheses, such as those of the knee, hip, etc., which are liable to wear
aird/or to change over
time, leading to the necessity for a replacement or for further surgery.
It is notably of particular benefit when using innovative surgical implants or
implants that
have become the norm or generic.
By minimizing costs, it contributes to the savings that have become imperative
by virtue of
the evolving market linked to ageing of the population and the necessity for
ever greater
medical cover.
It also finds an important application in the field of tracking medications,
notably by
providing virtually instantaneous access to products that represent a risk,
have been sold to
individual persons and suddenly need to be withdrawn and/or recalled.
Methods and devices are already known for storing information to enable
automatic
restocking of products that have been sold, based on entry and exit of
products to and from a
warehouse. Such systems (which generally use barcodes) cannot track products
beyond the
warehouse, in particular in the home of the actual end user.
Systems for overall management of data concerning a patient are also known, in
particular for
CA 02666247 2013-01-21
tracking patients in hospital.
Such systems do not take account of the intervention of external services not
bound by
professional confidentiality and employing more ordinary industrial practices.
Also known (WO 2004/008387) is a system for monitoring medications or
prescriptions using
microchips or labels transmitting and receiving radio frequencies.
In particular, such systems cannot track information reliably and
unambiguously.
The invention aims to provide a method and a system for tracking medical
products, such as
surgical implants in particular, which method and system represent a better
response to
practical requirements than previously, notably by providing traceability and
reliable and
uninterrupted tracking of products, from the manufacturer to the patient, with
automated and
considerably simplified logistics. The product is intended to be used by
and/or implanted in a
named patient, whose medical confidentiality is nevertheless preserved, in
compliance with
statutory standards in respect of traceability that until now have not been
complied with, in
the absence of satisfactory methods for their implementation, at the same time
as minimizing
costs.
The invention now enables professionals to comply with a legal requirement
whose
implementation has been attempted for many years without success.
Moreover, using the invention, not much manual intervention is required of the
hospital,
paramedical and/or medical staff, which improves safety by minimizing the risk
of human
error, at the same time as, if necessary, providing a filter to ensure that
medical confidentiality
is preserved.
Immediate identification of patients affected by a problem or potential
problem in respect of a
2
CA 02666247 2013-01-21
type of implant or medication also becomes possible, which is a considerable
advantage, with
the potential for saving human lives.
The invention also greatly facilitates management for medical bodies such as
hospitals,
pharmacies or doctor's surgeries, for suppliers of medications and/or
implants, and for
doctors, thanks to permanent and immediate access to some or all of the
databases concerning
the products concerned via any kind of data sorting or analysis means.
With this aim, the invention essentially proposes a method of tracking medical
products in
which the product and/or its packaging is marked and identified by the
supplier of the product
with a first reference that is stored in a first file and the packaged product
is shipped to a client
for storage by the client for subsequent use by or implantation in a patient
listed in a second
file,
characterized in that
the references of the product are detected automatically and remotely when it
is shipped
arid stored in a third, or stock control, file of the client,
the removal of the product from stock is detected automatically,
the references of the product are inserted in the second file corresponding to
the patient of
the client for which it is used or is to be used,
a fourth file is constructed comprising partial references coming from the
file of the
patient and the references of the product that have been determined for
tracking purposes, and
this fourth file is transmitted automatically or semi-automatically to the
supplier of the
product.
Advantageous embodiments use one or more of the following features:
the medical product is a surgical implant;
the medical product is a medication;
the simultaneous or substantially simultaneous removal from stock of the
references of at
least two products is detected automatically and the references of the product
used for the
3
CA 02666247 2013-01-21
patient are inserted automatically in the second file by automatic detection
and then
subtraction of the product or products re-entered into stock after the
operation, with the
products removed; the products and/or their packaging are marked and detected
by optical
recognition means;
the products and/or their packaging are marked and detected by radio-frequency
recognition means;
the product corresponding to the fourth file is re-ordered automatically for
the client
concerned;
an Intranet or Internet computer network is used to interrogate and to
dialogue with the
remote data storage server of the supplier to order and transmit the fourth
file and update the
first file of products, if necessary;
the various files are sorted and analysed to identify and mark automatically
or semi-
automatically products and/or patients affected by a potential or existing
problem linked to
said product and/or to the type of patient.
The invention also proposes a system implementing the method as described
above.
It also proposes a system for tracking medical products comprising means for
marking and
identification of products and/or their packaging with a first reference,
means for storage of
said references in a first file, means for storage following transport of at
least one of the
packaged products by a client with a view to subsequent use thereof for a
patient listed in a
second file of said client,
characterized in that it further includes
means for automatic and remote detection of the references of the product when
it is
shipped,
means for storing said references in a third, or stock control, file of the
client,
means for automatic detection of removal and/or entry of one or more products
from/into
said stock,
4
CA 02666247 2013-01-21
means for insertion of the references of the product into the second file
corresponding to
the patient of the client in which it is or will be implanted,
means for forming a fourth file comprising partial references coming from the
file of the
patient and the references determined of the product used, for tracking
purposes, and
means for automatic or semi-automatic remote transmission of this fourth file
to the
supplier of the product.
It advantageously includes means for marking and detecting products and/or
their packaging
by optical recognition.
The product being placed in packaging comprising a transparent face or a
transparent box, it
is equally advantageous if the marking means are glued or stuck to the inside
of said
transparent face of the packaging containing the product.
Although such a label is more difficult to apply, it has the particular
advantage of improved
protection.
One advantageous embodiment includes means for marking and detection of the
products
and/or their packaging by radio-frequency recognition.
An RFId (Radio Frequency Identification) label is advantageously used for this
purpose,
notably fixed to the interior of the packaging.
For example, a surface carrying no information (internal face of the packaging
ancVor of a flap
of the packaging) is used, which enables the selection for each size of box of
the maximum
size RFId chip so that reading of the chip is optimized (transmit-receive
distance).
This also improves protection of the RFId microchip against damage caused by
manipulation
during handling, transportation and storage.
5
CA 02666247 2013-01-21
This avoids coexistence with the external labelling of the box, which would
make it
obligatory to reduce the size of the RFId microchip (or that of the
labelling), to the detriment
of optical and radio legibility, or to choose an RFId microchip serving also
as a label (paper
face carrying legally required information such as: designation, size,
reference, etc.).
In another advantageous embodiment, the label is stuck on so that its bottom
right-hand
corner is as close as possible to the bottom right-hand corner of the
packaging and/or its flap.
Such positioning of the RFId chip optimizes its detection and reading by any
portable radio
wave transmission-reception means. It has been observed that hospital
personnel have a
natural tendency to align the reading means with the right-hand side of boxes.
Another advantageous embodiment includes means adapted to re-order
automatically the
product corresponding to the fourth file for the client concerned.
Equally advantageously, it includes means adapted to use an Intranet or
Internet computer
network to interrogate and dialogue with the remote data storage server of the
supplier to
order and to transmit the fourth file and update the first file of products,
if necessary.
Even more advantageously, it includes means for sorting and analysing the
various files to
identify and mark automatically or semi-automatically products and/or patients
affected by a
potential or existing problem linked to said products and/or to the type of
patient.
In one advantageous embodiment, the product marking and identification means
also
comprise at least one card attached to an RFId label contained in the product
packaging.
This credit card format card initially contains no identifier and the RFId
microchip that is
integrated into it contains only information for the product contained in the
packaging.
6
CA 02666247 2013-01-21
Following the operation, the card contains the partial identity of the patient
and the date and
type of operation carried out.
It then receives, via radio, all the data concerning the implant used
(designation, reference
code, batch number) stored in the RFId microchip. The card can then be
returned to the
patient.
Accordingly, during post-operative follow-up consultations, it can be used to
store additional
information on post-operative action.
During consultations with a view to potential further surgery, it enables
immediate recovery
of all the patient/product information, simply by reading the microchip.
It also has an individual serial number, and in the event of planning further
surgery in the
same hospital, this enables automatic recovery of all the data for the
previous operation, either
directly by reading a file contained in the microchip or by automatically
calling up the patient
file.
In one embodiment, the individual serial number of the card provides access to
the partial file
of the patient via the Internet site of the manufacturer of the device. This
function is used if
the hospital in which the consultation takes place is not (or no longer)
equipped with the
system.
The patient card can advantageously be further equipped with a detachable
portion (electronic
microchip) that can be inserted into a memory card reader to consult patient
information.
The partial files referred to hereinabove are advantageously created
automatically when
information from the RFId microchip of the implant is entered into the patient
file that is the
7
CA 02666247 2013-01-21
property of the hospital.
The partial files, which enable the manufacturer to obtain a great quantity of
information from
which personal information relating to the patient is excluded, therefore
provide absolute
protection of medical confidentiality.
What is more, the partial files of the various implants can be cross-
referenced so that it is
possible to tell the precise number of implants of each category that have
been implanted,
with their actual implantation dates, and the pathologies for which they were
used.
This data can also be used to draw up automatically implant survival curves
and therefore to
obtain an ongoing overview of the real service life of each type of implant.
Finally, the patient card described above is useful for tracking the company's
implants as it
can decrement the number of implants each time that a revision (withdrawal of
a prosthesis) is
effected.
This constitutes an exceptional result because, unless extremely costly
studies are carried out,
no implant manufacturer is at present capable of this level of traceability in
the context of
medical devices vigilance at the same time as preserving medical
confidentiality.
The advantage of this use of partial files and patient cards for the post-
market tracking
required by international quality standards is also clear.
Thus although the company cannot tell the identity of patients, an immediate
match can be
established by the hospital between the partial files contained in the
information system of the
company and the patient files contained in its own database.
This makes it possible to identify almost instantaneously the patients to be
contacted in the
8
CA 02666247 2013-01-21
context of medical devices vigilance (product recall).
The invention will be better understood on reading the following description
of embodiments
provided by way of nonlimiting example. The description refers to the
accompanying
drawings, in which:
FIG. 1 is a general diagram showing the principle of the method of the
invention.
FIG. 2 shows diagrammatically a system and its operation in the embodiment of
the invention
more particularly described here, applied to surgical implants.
FIG. 3 is a block diagram showing the sequence of steps executed in the method
of the
invention more particularly described here.
FIG. 1 is a diagram 1 showing the principle of the method of the invention.
Products manufactured by an industrial company 2 are shipped (arrow 3) to the
product
supplier 4, where they are packaged and marked with a first reference stored
in a first file.
The products are then ordered by the client 6 (a hospital, a pharmacy, a
doctor, etc.) via the
Internet 5, by telephone, or by any other means, and then shipped (arrow 7).
On arrival, they
are detected automatically and their references are stored in a third file.
At the time of use, they are removed from stock (arrow 8) and transmitted for
use at 9, where
they are detected automatically.
The chosen product is then taken by or implanted in the patient, the products
not used being
returned to stock (arrow 10) and detected again.
9
CA 02666247 2013-01-21
Information corresponding to patients and implants is taken into account to
form the fourth
file, which is then forwarded via the Internet 5 for tracking the product as
necessary to comply
with statutory provisions, etc., and/or for re-ordering by the supplier 4.
Hereinafter, the same numbers will be used to identify the same references or
identical or
similar references.
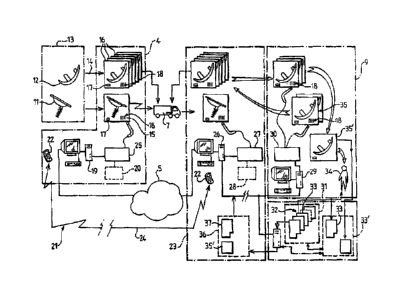
FIG. 2 shows in more detail the embodiment of the invention more particularly
described
here.
Implants 11, 12 manufactured by a first manufacturer 13 are ordered by the
supplier 4 (arrow
14), who packages the implants, for example by placing them in sachets 15, 16.
These sachets are here marked by numbers 17, colour codes, microchips (not
shown) and/or
barcodes 18 in a manner that is known in itself, to form first references
establishing a one-to-
one relationship between an identifier and an implant. The references of the
implants and/or
their sealed packaging are then entered into a computer 19 to constitute a
file 20 designated
"FILE N 1" of implants available from and listed by the supplier.
"FILE N"1"
PRODUCT NAME Format Width Example
Denomination Alpha 20 Right
internal
condyle
Product family Alpha 8 TARTAR
Type Alpha 10 P0950D01
Kind 999
Size 99 2 45*
Materials Alpha 10 Titanium
PRODUCT REFERENCE
Manufacturer 999 3
Supplier 999 3
Batch number Alpha Num 8 02P114
Sire Alpha Num 3 HOB
STERILIZATION
Sterilization method 99 2 ¨
Sterilization expiry YY Him 4 06 07
Sterilization batch 99 2
.Supplicr internal code
A surgery clinic 23 places an order (arrow 24) via the Internet 5 and/or other
means 21, such
as by telephone 22; the order is entered into the computer 19, which comprises
calculation,
CA 02666247 2013-01-21
analysis, printing, etc. means for data corresponding to the order and to the
types of implants
ordered.
This order authorizes the removal from stock of the implants, which are
automatically logged
by reading their bar code (device 25) or by other means (not shown), such as
remote reading
of a microchip integrated into the packaging and/or the implant.
The data concerning implants ordered and removed from stock for shipping are
also
transmitted for confirmation purposes to the computer 26 of the clinic 23 via
the Internet, for
example.
The products ordered are then shipped to the clinic (delivery van 7).
On arrival, they are detected automatically and remotely by means 27 located
on the client
premises to constitute a file 28 designated "FILE N 3", for example of the
following type.
"FILE N*3"
STORAGE
ESTABLISHMENT Format Width Example
Department
Pharmacy Alpha Num 8 Block 2
Surgical department Alpha 15 Orthopaedics
Operating theatre Alpha Num 8 Theatre3
Date
Creation of form dd ram yy 6 15 01 06
Movements 99 2 03
Last movement dd mm yy 6 ¨**
Product name
Designation Alpha 20 Right
internal
condyle
Product family Alpha 8 TARTAR
TYP. Alpha Num 10 P09501301
Kind 999 3 ¨*
Size 99 2 45*
Materials Alpha 10 Titanium
Product reference
Mauldin:finer 999 3 ¨*
Supplier 999 3 ¨*
Batch number Alpha Num 3 02P114
Size Alpha Num 3 1108
Sterilization
Sterilization method 99 2 ¨*
Sterilization expiry yy BIM 4 06 07
Sterilization batch 99 2 ¨*
*Supplier internal code
...Hospital internal code
11
CA 02666247 2013-01-21
If a surgeon is to operate in operating theatre 9, similar means programmed
accordingly, for
example a computer or a PDA 29 and a bar code and/or microchip card reader 30,
detect the
references 18 of the implants necessary a priori.
There are generally several implants of different sizes, the size to be used
often being chosen
by the surgeon at the last moment.
At the same time, and from the file 31 designated "FILE N . 2" containing the
medical records
32 of the patients of the department, the medical record 33 of the patient 34
to be operated on
is obtained from the computer 29, for example.
"EL& MT
PATIENT NAME Format Width Example
Name Alpha 20 ¨
Forename Alpha 20
Date of birth dd mm yy 6 ¨
Place of birth Alpha 10 ¨
Social security 9999999999 10 ¨
number
Address Alpha Num 20 ¨
Telephone 9999999999 10 ¨
E-mail Alpha 40 ¨
Previous swirly Alpha 40 Appendicitis
Risk factors Alpha 30 Haemophilia
SURGERY
Establishment Alpha. Num 40 NECKER-
Department Alpha Nom 15 Orthopaedics
Surgeon Alpha 20 ¨
Type of surgery 999 3 _11,1
Location of surgery Alpha 20 Right knee
"Hospital ioternsl sods
The surgeon operates and then returns the unused implants 35, which are
automatically
and remotely detected by the device 30, the difference giving the references
of the implant 35'
25 that has been used.
The bar code or the identifier of the latter implant can also be read directly
rather than
obtained from the difference.
12
CA 02666247 2013-01-21
A hybrid record 33' is then assembled and used on the one hand to update the
file 33 of the
patient (whose medical history from now on includes the operation) and to
produce the record
36 designated "FILE N 4" containing partial references 37 enabling the
medical
confidentiality of the patient operated on to be preserved and the references
of the implant 35'
that have been determined.
"FILE NM"
PATIENT Format Width Example
Name Alpha 20 ¨
Forename Alpha 20 ¨
Date of birth dd mm yy 6 ¨
Place of birth Alpha 10 ¨
Social security 9999999999 10 ¨
number
Address Alpha Num 20 ¨
Telephone 9999999999 10 ¨
E-mail Alpha 40 ¨
"FILE N*4"
PATIENT Format Width Example
SURGERY
Establishment Alpha Num 40 NECKER
Department Alpha Num 15 Orthopaedics
Surgeon Alpha 20 ¨
Type of surgery
Description Alpha 30 ¨
DRG
Location of surgery Alpha 20 ¨
Limb Alpha 20 Leg
Joint Alpha 20 Knee
Right/left Alpha 1 R
Implant fitted 999 3 ___**
RAS 999 3 ___)1,*
Problems 9 1 5*5
*.11ospitel code
The fourth file is then transmitted automatically or semi-automatically, for
example on
validation by medical staff pressing a key, via the networked computer 26, to
the supplier 4
via the Internet 5.
The information received is then processed (19) by data processing means to
track the implant
in accordance with legal requirements and for other operations such as re-
ordering, for
example.
The operations of the invention in the embodiment more particularly described
here with
reference to the FIG. 3 functional flowchart are described and represented
hereinafter.
13
CA 02666247 2013-01-21
After manufacture 40 by the manufacturer of the implants concerned, there
follows a step 41
of ordering by the supplier. The product having been shipped, it is then
stored by the supplier
(step 42) who identifies and marks it in a manner known in itself (step 43).
At the same time, the supplier constructs FILE N 1, as described above.
There follows a step 44 of ordering by the hospital 46, followed by shipping
(step 45). On
completion of shipping, FILE N 3 is constructed by remote scanning and
storage (step 46) of
implants introduced into the hospital stores, for example by a bar code reader
and/or a
contactless microchip card reader.
On use of an implant following a request from a surgeon, a step 47 of removing
the implants
necessary for the operation from the hospital stores is effected with
automatic detection 48 of
said implants by identical means.
In step 49 the operation is effected by the surgeon (the medical records of
the patient
concerned are obtained beforehand from the hospital FILE N 2).
After the operation has been carried out, the implants that have not been used
are returned to
the hospital stores and are detected automatically (50).
There follows a step 51 of constructing FILE N 4, expurgated to protect the
medical
confidentiality of the patient operated on.
In other words, FILE N 4 therefore contains non-confidential information on
the patient and
information on the implant that has been implanted in the patient.
A step 52 of transmitting the elements of FILE N 4 to the supplier enables
complete tracking
14
CA 02666247 2013-01-21
of the implant via the operation on the patient.
A step 53 of verification and storage of the files is followed by updating of
the implants file
(FILE N 1) by the supplier (step 54), possibly followed by a step 55 for
automatic re-
ordering of new implants as a function of that to be used in the operation,
and possibly a final
step 56 of producing and analysing statistics linked to the implants and/or to
the types of
operation linked to a particular type of patient.
It goes without saying, and also follows from the foregoing description, that
the present
invention is not limited to the embodiments more particularly described. To
the contrary, it
encompasses all variants and in particular those in which the product is a
medication or a
series of medications intended for complex and/or vital treatments, such as
treatment for
AIDS, for example, or for degenerative neural diseases such as Alzheimer's or
Parkinson's
disease.