Note: Descriptions are shown in the official language in which they were submitted.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-I-
FORMULATED LUBRICANTS MEETING OW
AND 5W LOW TEMPERATURE PERFORMANCE
SPECIFICATIONS MADE FROM A MIXTURE OF BASE STOCKS
OBTAINED BY DIFFERENT FINAL WAX PROCESSING ROUTES
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
[001] The present invention relates to formulated lubricant oils meeting SAE
OW-X and/or 5W-X specifications made from a base oil comprising a mixture of
base stocks.
DESCRIPTION OF THE RELATED ART
[0021 Formulated lubricants comprise a mixture of a base stock or a base oil
and at least one performance additive. Usually, the base stock is a single oil
secured from a single crude source and subjected to a single processing scheme
and meeting a particular specification. Mixtures of base stocks of different
specifications produce a base oil. Crude oil is typically subjected initially
to a
dewatering/demetalling step followed by atmospheric distillation to yield
various
fractions, the heavier fraction being subjected typically to vacuum
distillation
with the heavier fractions from such vacuum pipe still being subjected to
extraction to remove aromatics, hydrocracking, hydrofinishing and dewaxing to
produce a suitable fraction boiling in the desirable lubricating oil boiling
range.
The oil boiling in the lubricating oil (hereinafter lube oil) boiling range is
subsequently separated into various fractions of different viscosity for use
as
base stocks. The dewaxing can take the form of solvent dewaxing wherein the
waxy lube oil is subjected to cooling using various solvents such as
methylethyl
ketone/methylisobutyl ketone, (MEK/MIBK), MEK/toluene, liquefied propane
or butane, etc., to decrease the wax content of the oil and by so doing lower
the
pour point of the oil. Solvent dewaxing constitutes the physical removal of
the
wax using a solvent such as methyl ethyl ketone/methyl isobutyl ketone or an
autorefrigerative solvent resulting in the recovery of a reduced wax content
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-2-
stream and a separate wax stream known as slack wax. Dewaxing can also be
accomplished catalytically. In catalytic dewaxing the waxy feed is contacted
with a dewaxing catalyst in the presence of hydrogen at elevated temperature.
The wax content of the oil is reduced either by conversion of the wax
molecules,
which are typically long chain normal or long chain slightly branched
paraffin,
into short chain paraffin, or by the rearrangement of the atoms in the wax
molecule, i.e., conversion of n-paraffin or slightly branched paraffin into
more
heavily branched paraffin, a process known as isomerization. Catalytic
dewaxing changes the nature of the molecules present in the oil either by
cracking or rearrangement and clearly results in the production of a dewaxed
oil
which is compositionally different than that obtained by solvent dewaxing.
[003] For the sake of convenience lube oil base oil, produced by blending
different base stock, usually employ different base stocks produced in a
single
plant. Thus, a lube oil blending plant will use as its base stock slate the
base
stock secured from its associated refiriery and, therefore, all of the base
stocks or
base stock mixtures, i.e., base oils used to produce formulated oil in the
lube oil
blending plant will have been treated in generally the same manner including
crude pretreatment, distillation, hydroprocessing (if any) and dewaxing, be it
solvent dewaxing or catalytic dewaxing. Refineries rarely house two different
dewaxing schemes. Thus, a refinery which practices solvent dewaxing does not
usually also practice catalytic dewaxing, and vise versa. In general, solvent
and
catalytic dewaxed stocks of the same or of very similar viscosity have not
been
mixed to produce a blended base stock.
[004] USP 4,259,170 teaches a method for manufacturing a slate of lubricant
base stocks from a paraffin base stock or a mixed crude source. The heavy,
high
viscosity bright stock raffinate is catalytically dewaxed while the lighter
lower
viscosity neutral oil raffinates are solvent dewaxed. The combined use of
solvent and catalytic dewaxing is described as a highly efficient method of
manufacture without loss of product quality. The catalytically dewaxed bright
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-3-
stock can be used for blending automotive lubricating oils. As is apparent,
however, the catalytically dewaxed bright stock and the solvent dewaxed
lighter
neutral oils are not of the same or similar viscosity.
[0051 USP 6,773,578 is directed to lube oil base stocks made by a process that
involves obtaining feedstocks that have a 95% off point (T95) below 1150 F and
feed stocks that have a 95% off point (T95) above 1150 F. The feed stocks that
have a 95% off point below 1150 F are catalytically dewaxed and the feed
stocks that have a 95% off point above 1150 F are solvent dewaxed. The result-
ing products can optionally be blended and the base stocks can be combined
with various additives to form lube oil compositions. No examples are
presented
of any such blends, which even if they had been produced would have
constituted mixtures of base stocks or base oils of different viscosities, not
of the
same or substantially similar viscosities.
[006] U.S. published application 2005/0098476 is directed to a method for
improving the lubricating properties of a distillate base oil characterized by
a
pour point of 0 C or less and a boiling range having the 10% off point (TI o)
falling between about 625 F and about 790 F and the 90% off point (T90)
falling
between about 725 F and about 950 F, the method comprising blending with
said distillate base stock or base oil a sufficient amount of a pour point
depress-
ing base oil blending component to reduce the pour point of the resulting base
oil
blend at least 3 C below the pour point of the distillate base stock, wherein
the
pour point depressing base oil blending component is an isomerized Fischer-
Tropsch derived base stock bottoms product having a pour point that is at
least
3 C higher than the pour point of the distillate base stock.
[0071 USP 6,475,960 is directed to premium synthetic lubricants comprising a
synthetic isoparaffinic hydrocarbon base stock and an effective amount of at
least one performance additive. The base stock is derived from a waxy
paraffinic Fischer-Tropsch (hereinafter also referred to as F-T) synthesized
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-4-
hydrocarbon feed. The lubricant may also contain hydrocarbonaceous and
synthetic base stock materials such as mineral oils, mineral oil slack wax
isomerate, PAO, and mixtures thereof. No examples of mixtures of Fischer-
Tropsch wax isomerate with any mineral oil, synthetic oil or PAO are
presented.
[008] USP 5,149,452 is directed to wax isomerate oil having a reduced pour
point, by using a combination of low molecular weight and high molecular
weight polyalkymethacrylate pour point depressant. The preferred wax
isomerate is hydroisomerized slack wax.
[0091 USP 6,332,974 is directed to wide-cut synthetic isoparaffinic
lubricating
oils made by hydroisomerization and then catalytic dewaxing of a waxy F-T
synthesized hydrocarbon fraction feed. Formulated oils made by admixing the
base stock with a commercial automotive additive package meet all specifica-
tions, including low temperature properties, for multigrade internal
combustion
engine crankcase oils. The wide cut synthetic isoparaffinic lubricating oil
base
stock can be used as such or mixed with other base stocks including hydro-
carbonaceous base stock, synthetic base stock and mixtures thereof, hydro-
carbonaceous base stocks including conventional mineral oil, shale oil tar and
coal liquefaction oils, mineral oil derived slack wax, while synthetic base
stocks
include PAO, polyester types and other synthetics. No examples are given of
the
wide cut synthetic isoparaffinic lube oil mixed with any other base stock (see
also USP 6,475,960, WO 00/14187).
[010] USP 6,090,758 is directed to a method for reducing foaming in lubricat-
ing oils derived from wax isomerization. In an Example a conventional SAE
10W40 multigrade oil is formulated from an isomerized slack wax base stock
(EXXSYN base stock), 150 N base stock, an additive package, VI improver and
12500 cSt silicone oil.
[011] WO 03/064570 is directed to mixed Total Base Number (TBN)
detergent additive compositions for lubricating oils. In the text, examples
are
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-5-
given of such detergent mixtures in combination with various base stocks and
base oil mixtures of base stocks including hydrotreated base stocks mixed with
PAO and hydrocarbyl aromatics. Examples are present only of formulations
containing various combinations of hydrotreated base stock, PAO and hydro-
carbyl aromatics for the production of SAE 5W30 multi-grade engine oils.
[012] US published application 2004/0094453 is directed to a process for
producing a lubricating base oil blend which comprises (a) recovering a F-T
derived distillate fraction characterized by a kinematic viscosity (KV) at 100
C
of about 2 cSt or greater but less than 3 cSt and (b) blending the F-T derived
distillate fraction with a petroleum derived base oil selected from the group
consisting of a Group I, a Group II a Group III base stock or mixture of two
or
more thereof to produce a lube base oil blend having a KV at 100 C of about 3
cSt or greater. The Examples are directed to lOW-X and 15W-X engine oils.
[013] U.S. published application 2004/053030 is directed to functional fluids
having low Brookfield viscosity using high viscosity-index base stocks/base
oils.
[014] It is desirable to produce multi grade engine oil composition meeting
the SAE OW-X and 5W-X multi-grade lubricating oil low temperature MRV
(ASTM D4684) and CCS viscosity (ASTM D 5293) specification requirements
for such OW-X and 5W-X lube oils without the addition to such compositions of
viscosity modifiers and pour point depressants or only low concentrations of
viscosity modifiers and pour point depressants.
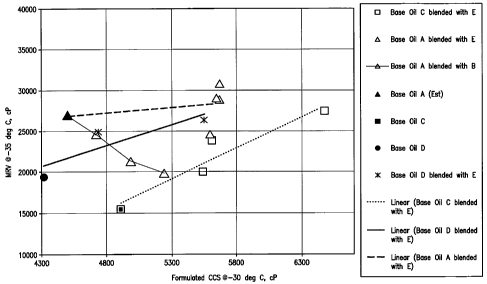
DESCRIPTION OF THE FIGURE
[015] Figure 1 presents the results in terms of Mini Rotary Viscometric Test
(ASTM D 4684) (MRV) at -35 C and Cold Crank Simulation Viscosity Text
(CCS) at -30 C (ASTM D 5293) for various oils and oil blends showing that the
combination of 2 base oils of similar viscosities but made by different final
wax
treatment process techniques (base oils A solvent dewaxed plus B catalytically
dewaxed) exhibited CCS and MRV values for the blend unexpectedly superior
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-6-
to the CCS and MRV values exhibited for blends of base oils made using the
same final wax treatment process technique (base oil A, solvent dewaxed) plus
E
(solvent dewaxed), or base oil D (solvent dewaxed) plus E or base oil C
(solvent
dewaxed) plus E).
DESCRIPTION OF THE INVENTION
[016] The present invention is directed to multi grade engine oils meeting
Society of Automotive Engineers (SAE) Surface Vehicle Standard J300, engine
oil viscosity classification for OW-X or 5W-X low temperature specifications
and Noack volatility of 15% or less, preferably 14% or less, more preferably
13% or less, still more preferably 10% or less, a OW-X specification of CCS
viscosity at -35 C of 6200 cP or less and of MRV at -40 C of 60,000 cP or
less,
preferably 40,000 cP or less, more preferably 30,000 cP or less, or a 5W-X
specification of CCS viscosity at -30 C of 6600 cP or less and MRV at -35 C of
60,000 cP or less, preferably 40,000 cP or less, more preferably 30,000 cP or
less, and a yield stress of less than 35 pascals comprising a mixture of at
least 2
base stocks or of a base stock and a base oil or of 2 base oils produced
employ-
ing different final wax removal or conversion processing routes wherein each
base stock or base oil individually has a kinematic viscosity at 100 C in the
range of about 3.5 to 7 mm2/s, and the mixture thereof without additives has a
kinematic viscosity at 100 C in the range of about 4 to about 6 mm2/s, and
wherein the pour point of each base stock and/or base oil in the mixture is
about
-30 C or higher, preferably about -25 C or higher, more preferably about -20 C
or higher provided that as compared to the temperatures at which the MRV is
measured for each engine oil grade the difference between the pour point of
the
oil mixture and the temperature of measurement of the MRV of the formulated
oil is at least about 10 C, i.e., the pour point of the oil mixture is at
least about
C higher than the temperature of measurement of the MRV of the formulated
oil, and about 0 to 0.1 wt% (as received) of a pour point depressant based on
the
total weight of the engine oil.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-7-
[017] The present invention is also directed to a method for producing a base
oil for use as the base oil in the formulation of OW-X or 5W-X multigrade
engine oils said multi grade engine oils meeting the NOACK volatility, CCS
viscosity and MRV low shear viscosity criteria previously indicated said
method
comprising mixing at least two base stocks, or base stock and base oil, or two
base oils produced by different final wax removal or conversion processing
routes, wherein each base stock or base oil individually making up the mixture
has a kinematic viscosity at 100 C in the range of about 3.5 to 7.0 mm2/s, the
mixture itself, without additive, having a kinematic viscosity at 100 C in the
range of about 4 to 6 mm2/s and wherein the pour point of each base stock or
base oil in the mixture without additives is about -30 C or higher, preferably
about -20 C or higher, more preferably about -20 C or higher provided that as
compared to the temperature at which the MRV is measured for each engine oil
grade the difference between the pour point of the oil mixture and the tempera-
ture of measurement of the MRV of the formulated oil is at least about 10 C.
[018] Very low ambient temperatures during the winter occur in most of
northern North America and parts of Europe and Asia. Adequate lubrication of
key engine parts during cold start is critical if engine damage or failure is
to be
avoided. An engine oil's viscosity at low temperature is critical for
determining
how readily the engine can be cranked, and the speed with which the oil flows
from the pan to the oil pump and from there to other parts of the engine.
[019] When an oil is used in engines operating at low ambient temperatures,
the oil must have enough low temperature fluidity to allow the engine to crank
and to lubricate the moving parts very quickly. Older vehicles relied upon a
certain cranking speed to generate air flow through the carburetor which
misted
the fuel and allowed the engine to start. If the engine could not crank
quickly
enough, then it would not start. Fuel injection engines were introduced in the
mid 1970s and by 1990, virtually all gasoline passenger car vehicles were fuel
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-8-
injected. Fuel injection coupled with computer controls will allow the engine
to
start even when the oil is too viscous to allow the engine to crank. The
situation
raised concerns that engine failure could occur when the engine started but
the
lubricant was too viscous to pump to the now-moving parts. ASTM has two
tests which measure the low temperature performance of the lubricant. The cold
cranking simulator viscosity (CCS) (ASTMD 5293) evaluates the lubricant's
capability to allow the engine to crank at low temperature. Once the engine
has
cranked and started, then the oil must be able to flow rapidly to the oil
pump.
The test for measuring low temperature pumping ability is the mini rotary
viscometer test (MRV) (ASTM D 4684). The MRV test is designed to predict
the ability of the oil to reach critical moving components under low
temperature
starting conditions. Both good low temperature cranking viscosity and good
mini rotary viscosity are required to protect engine components from damage
due to lack of lubrication during low temperature starting. The SAE viscosity
grade system is used to determine the low temperature usefulness of
multigraded
engine oils. Both the CCS viscosity and the MRV must meet the limits of the
particular SAE grade designated.
[020] The CCS viscosity and the MRV test measure different low temperature
properties of the lubricant and therefore good performance in the CCS
viscosity
test does not necessarily predict good performance in the MRV test. The MRV
performance is usually improved by the addition of low temperature flow
improver. The choice of flow improver is highly dependent upon the base stock
and can be very sensitive to changes in base stock wax structure. Situations
arise where no consistently capable low temperature flow improver is
available.
[021] High performance specifically processed API Group II+ and Group III
mineral oil base stocks set the performance standard for non-synthetic engine
oils. Key to that performance is the low temperature properties enabled by the
base stock as seen in the formulated lubricant MRV and CCS. The MRV and
CCS viscosity are measured well below the pour point of the base oils and take
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-9-
advantage of the various additive chemistries used, including pour point
depressants (PPD's).
[022] Solvent dewaxed stocks are known to generally have debits in regard to
low temperature properties and are defensive relative to stocks which have
been
catalytically dewaxed (cat dewaxed). Formulations based on cat dewaxed stocks
have lower overall formulated cost than those from solvent dewaxed stocks.
Different base stocks of the same final wax processing type from the same
plant
are generally blended to make an engine base oil and one of the base stocks
usually will have superior volatility characteristics and thus bears a price
premium. It has been found that cat dewaxed stocks of the same or
substantially
similar viscosity as the solvent dewaxed stocks (i.e., stocks having KV @ 100
C
in the range of about 3.5 to 7 mm2/s, preferably about 4 to 7 mm2/s, more
preferably about 4 to 6.5 mm2/s) can be combined with solvent dewaxed base
stock to replace part of the solvent dewaxed stock to yield a base oil which
either
itself meets the MRV and CCS viscosity target requirements for SAE OW-X
and/or 5W-X multi grade lubricating oil or which with the addition of a minor
amount of pour point depressant, i.e., zero to 0.1 wt% (as received) amounts
much lower than have heretofore been employed, can result in a formulated lube
oil meeting the SAE OW-X and/or 5W-X low temperature viscometric properties
as measured by MRV and CCS viscosity.
[023] Wax hydrodewaxate, hydroisomerate/cat (and/or solvent) dewaxate,
and GTL stocks, defined in detail below, offer yet additional choices for base
stocks made by methods which differ from either solvent dewaxing or catalytic
dewaxing. Wax hydrodewaxate or hydroisomerate/cat (and/or solvent)
dewaxate stocks and Gas-to-Liquids (GTL) stocks made from GTL materials are
base stocks characterized by the rearrangement of the carbon atoms making up
the structure of the hydrocarbon molecule. Whereas solvent dewaxing
physically removes wax from oil without changing the structure of the oil and
traditional catalytic dewaxing physically destroys the wax by converting it
from
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-10-
heavy molecules to light molecule, hydrodewaxing, or hydroisomerization/cat
(or solvent) dewaxing predominantly rearranges the carbon atoms in the waxy
molecule converting it from a normal straight-chain or slightly branch chain
structure into a branched or more branched chain structure (iso-paraffin) of
the
same or similar carbon number accompanied by minimal but selective catalytic
dewaxing (i.e., minimal cracking/fragmentation) or minimal solvent dewaxing,
producing an oil material of significantly reduced pour point.
[024] While mixtures of solvent and catalytically dewaxed stocks have been
used to produce mixed base stock useful for engine oil, such mixed stocks have
not been employed to produce SAE OW-X or 5W-X multi grade lubricating oil
compositions. In the past base oils constituting mixtures of solvent dewaxed
and
catalytically dewaxed stock have constituted mixtures of such stocks which did
not have similar kinematic viscosities at 100 C in the 3.5 to 7 mm2/s range.
[025] It has now been discovered, however, that base stocks or base oils
having KV @ 100 C in the range of about 3.5 to 7 mm2/s made by different final
wax removal (solvent or catalytic dewaxing) and/or by different final wax
molecule rearrangement (i.e., wax isomerization) techniques can be blended to
produce formulated lubricating oil compositions meeting the low temperature
viscometries and rheological property targets of SAE 0W-X and 5W-X multi-
grade engine oils without need to resort to deeply dewaxed base oils or base
oils
of very low pour point, or to viscosity modifiers or pour point depressants,
or
with the use of very low quantities of viscosity modifiers and pour point
depressants (PPD).
[026) This is different from the expectations of those who are skilled in this
art. Most low temperature properties of lubricating oils are set by the base
stocks or base oils in combination with various additives. Specific additives,
such as PPD's, are then selected to match the nature and amount of residual
wax
in order to most effectively improve low temperature properties. Different
types
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-11-
of waxes usually mandate a change in the nature or amount of PPD, increasing
cost or decreasing blending flexibility. There have been numerous examples
where a base stock changes and a formulation can no longer use the same pour
point depressant that was previously successful in adjusting low temperature
properties. This is especially true for changes in wax processing route.
[027] It has now been found that combining base stocks and/or base oils
made employing different final wax processing routes shows an unexpected
improvement in low temperature properties for lower viscosity multi-grade
engine oils in the SAE OW-X and 5W-X multi grade lubricating oil range. It is
believed that this is due to a synergistic behavior in the different residual
waxes
present in the different stocks that either eliminates the need for PPD or
greatly
improves the low temperature response to PPD permitting the use of less PPD.
[028] This improvement is seen when the pour point of the mixture of base
stocks and/or base oils used is well above the MRV temperatures of evaluation
for the fully formulated engine oils of the different SAE grades. For example,
most base oils have pour points in the range of -18 C to -30 C. The
temperature
of evaluation for 5W and OW engine oils is -30 C and -35 C respectively for
CCS viscosity high shear viscosity and -35 C and -40 C respectively for Mini
Rotary Viscometer Test (MRV) low shear viscosity.
[029] A difference between the pour point of the mixture of base stock(s) and
or base oil(s) and the temperature of MRV evaluation for the OW-X or 5W-X
formulated oil of at least 10 C is required to take advantage of the
improvement
brought about by the use of such mixed base stocks produced by different final
wax (removal and/or molecular rearrangement) processing routes in terms of the
unexpected positive effect on low temperature viscometric and rheological
properties. The stocks from different dewaxing processes will have differing
kinds, and in all probability different amounts, of residual wax. It has been
found that it is the mixing of these different residual wax oils that give the
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-12-
unexpected benefit that is the subject of the present invention, that is, the
ability
to produce base oils suitable for sue in the production of OW-X and 5W-X
lubricating oils from stocks which individually cannot be so employed. If
instead of mixing two stocks having individual pour points at last 10 C higher
than the temperature of measurement for the MRV of the different oil grades
(-35 C for 5W- or -40 C for OW-) use was made of oils having pour points
meeting the MRV measurement temperature there would be too little residual
wax remaining for the interaction seen in the present invention to occur.
There
would be too little wax remaining to make a difference. Further, the present
invention discovery of unexpected suitability of higher pour point oils to
make
lubricating oil formulations meeting the OW-X and 5W-X specification enlarges
the pool of oils useful to produce premium formulations without the need for
recourse to severe dewaxing to produce very low pour point base stock.
[030] The base stocks and/or base oils which are combined to achieve this
advantageous result are base stocks or base oils characterized each
individually
as having kinematic viscosities (KV) at 100 C (by ASTM D445) in the range of
about 3.5 to 7 mm2/s, preferably about 4 to 7 mm2/s, more preferably about 4.0
to 6.5 mm2/s, wherein each base stock or base oil in the blend is derived from
the
same or different feed stock source but processed by different final wax
process-
ing techniques or different oil synthesis techniques. The final mixture of
base
stocks and/or base oils, without additives, is characterized by a kinematic
viscosity at 100 C in the range of about 4 to 6 mm2/s.
[031] Base stocks useful as blending components in the present invention are
the API Group II, Group III, base stocks developed and defined by the American
Petroleum Institute (API Publication 1509; www.API.org). Group II base stocks
are hydrocarbon base stocks which have a viscosity index of between about 80
to 120, and contain less than or equal to 0.03 wt% sulfur and greater than or
equal to 90 wt% saturates. Group III stocks are hydrocarbon base stocks which
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-13-
have a viscosity index greater than 120 and contain less than or equal to
about
0.03 wt% sulfur and greater than 90 wt% saturates.
[032] Wax hydroisomerate/catalytic (and/or solvent) dewaxate, or
hydrodewaxate or GTL base stocks/base oils can also be used in combination
with the aforesaid dewaxed Group II and/or Group III base stocks/base oils.
[033] A characteristic which marks each oil used before blending, however, is
that each oil has a kinematic viscosity at 100 C in the range of about 3.5 to
7
mm2/s, preferably about 4 to 7 mm2/s, more preferably about 4.0 to 6.5 mm2/s,
and each base oil and the mixture of base oils has a pour point at least 10 C
higher than the temperature of measurement of the MRV specification for the
OW-X or 5W-X oil formulation (MRV specification for OW-X oil measured at
-40 C, for 5W-X oil measured at - 35 C).
[034) The oils which are blended may come from the same or different feed
sources, that is, each oil can trace their origin back to the same or to
different
crude oil or synthesis process but each oil has been subjected to different
final
wax processing procedures (i.e., solvent dewaxing, catalytic dewaxing,
hydroisomerization/catalytic (and/or solvent) dewaxing, or hydrodewaxing).
[035] Thus, a base oil pair can be derived from some particular crude oil
provided each oil has been subjected to a different final wax processing
scheme.
The first base stock or base stock mixture, for example, can be subject to
solvent
extraction, solvent dewaxing and hydrotreating while the second base stock or
base stock mixture can be, for example, subjected to solvent extraction,
catalytic
dewaxing and hydrotreating. Each base stock or base oil in a base oil pair can
further constitute a mixture of base stocks or base oil from the same or
different
feed source subjected to the same final wax processing scheme. As used herein
and in the appended claims the terms "base stock" or "base oil" is to be under-
stood as embracing a single base stock or base oil or mixture of more than one
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-14-
base stock or base oil from the same or different feed source subject to the
same
final wax processing scheme unless indicated otherwise.
[036] The Group II and/or Group III base oilslbase stocks can be combined
with non-conventional base stocks/base oils as the second oil produced by a
different wax processing procedure.
[037] Thus, the present invention embraces mixtures of base stocks or
mixtures of mixtures of base stocks having KV's @ 100 C in the range of 3.5 to
7 mm2/s, preferably 4 to 7 mm2/s, more preferably 4.0 to 6.5 mm2/s as
previously described, wherein for example:
a) one base stock or base oil or mixture of base stocks or base oils is/are
solvent
dewaxed using a single solvent dewaxing process technique and the second
base stock or base oil or mixture of base stocks or base oils is/are
catalytically dewaxed using a single catalytic dewaxing process technique;
b) one base stock or base oil or mixture of base stocks or base oils is/are
solvent
dewaxed using a single solvent dewaxing process technique or catalytically
dewaxed using a single catalytic dewaxing technique and the second base
stock or base oil or mixture of base stocks or base oils is/are GTL oil(s)
and/or hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed
oil(s) wherein the oil(s) has/have been produced using a single same
synthesis or wax hydrodewaxing, or hydroisomerization/cat (and/or solvent)
dewaxing process technique;
c) one base stock or base oil or mixture of base stocks or base oils is/are
GTL
oil(s) and/or hydrodewaxed, or hydroisomerized/cat (and/or solvent)
dewaxed, wax base stock or base oil produced using a first final wax
processing technique and the second base stock or base oil or mixture of
base stocks or base oils is/are GTL and/or hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed wax base stock or base oil
produced using a second final wax processing technique different from the
first;
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
- 15-
d) one base stock or base oil or mixture of base stocks or base oils is/are
solvent
dewaxed using a first solvent dewaxing technique and the second base stocks
or base oils or mixture of base stock or base oil is/are also solvent dewaxed
but using a second, different solvent dewaxing technique;
e) one base stock or base oil or mixture of base stocks or base oils is/are
catalytically dewaxed using a first catalytic dewaxing technique and the
second base stock or base oil or mixture of base stocks or base oils is/are
also catalytically dewaxed but using a second, different catalytic dewaxing
technique.
What is critical is that the final wax processing techniques be different for
each
oil in each oil pair or for each mixture of oils in each oil mixture in each
mixture
pair.
[038] In the present invention the amount of catalytic dewaxed oil added to
solvent dewaxed oil of the same or substantially similar viscosity ranges from
about 5 to 35 wt%, preferably about 10 to 25 wt%. For mixtures other than
mixtures of solvent dewaxed oil with catalytically dewaxed oil the weight
ratio
of first oil processed by a first wax processing technique to a second oil
processed by a second wax processing technique can be from about 10:90 to
about 90:10, preferably about 25:75 to about 75:25, more preferably about
40:60
to about 60:40.
[039] Non-conventional or unconventional base stocks and/or base oils
include one or more of a mixture of base stock(s) and/or base oil(s) derived
from
one or more Gas-to-Liquids (GTL) materials, as well as hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed base stock(s) and/or base oils
derived from natural wax or waxy feeds, mineral and or non-mineral oil waxy
feed stocks such as slack waxes, natural waxes, and waxy stocks such as gas
oils, waxy fuels hydrocracker bottoms, waxy raffinate, hydrocrackate, thermal
crackates, or other mineral, mineral oil, or even non-petroleum oil derived
waxy
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-16-
materials such as waxy materials received from coal liquefaction or shale oil,
and mixtures of such base stocks and/or base oils.
10401 As used herein, the following terms have the indicated meanings:
a) "wax" - hydrocarbonaceous material having a high pour point, typically
existing as a solid at room temperature, i.e., at a temperature in the range
from about 15 C to 25 C, and consisting predominantly of paraffinic
materials;
b) "paraffinic" material: any saturated hydrocarbons, such as alkanes.
Paraffinic materials may include linear alkanes, branched alkanes (iso-
paraffins), cycloalkanes (cycloparaffins; mono-ring and/or multi-ring), and
branched cycloalkanes;
c) "hydroprocessing": a refining process in which a feedstock is heated with
hydrogen at high temperature and under pressure, commonly in the presence
of a catalyst, to remove and/or convert less desirable components and to
produce an improved product;
d) "hydrotreating": a catalytic hydrogenation process that converts sulfur-
and/or nitrogen-containing hydrocarbons into hydrocarbon products with
reduced sulfur and/or nitrogen content, and which generates hydrogen
sulfide and/or ammonia (respectively) as byproducts; similarly, oxygen
containing hydrocarbons can also be reduced to hydrocarbons and water;
e) "catalytic dewaxing": a conventional catalytic process in which normal
paraffins (wax) and/or waxy hydrocarbons, e.g., slightly branched iso-
paraffins, are converted by cracking/fragmentation into lower molecular
weight species to insure that the final oil product (base stock or base oil)
has
the desired product pour point;
f) "solvent dewaxing": a process whereby wax is physically removed from oil
by use of a chilled solvent or an autorefrigerative solvent to solidify the
wax
which can then be removed from the oil;
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-17-
g) "hydroisomerization" (or isomerization): a catalytic process in which
normal paraffins (wax) and/or slightly branched iso-paraffins are converted
by rearrangement/isomerization into branched or more branched iso-
paraffins (the isomerate from such a process possibly requiring a subsequent
additional wax removal step to ensure that the final oil product (base stock
or base oil) has the desired product pour point);
h) "hydrocracking": a catalytic process in which hydrogenation accompanies
the cracking/fragmentation of hydrocarbons, e.g., converting heavier
hydrocarbons into lighter hydrocarbons, or converting aromatics and/or
cycloparaffins (naphthenes) into non-cyclic branched paraffins.
i) "hydrodewaxing": (e.g., ISODEWAXING of Chevron or MSDWTM of
Exxon Mobil corporation) a very selective catalytic process which in a
single step or by use of a single catalyst or catalyst mixture effects
conversion of wax by isomerization/rearrangement of the n-paraffins and
slightly branched isoparaffins into more heavily branched isoparaffins, the
resulting product not requiring a separate conventional catalytic or solvent
dewaxing step to meet the desired product pour point;
j) the terms "hydroisomerate", "isomerate", "catalytic dewaxate", and
"hydrodewaxate" refer to the products produced by the respective processes,
unless otherwise specifically indicated;
k) "base stock" is a single oil secured from a single feed stock source and
subjected to a single processing scheme and meeting a particular
specification;
1) "base oil" comprises one or more base stock(s).
[041] Thus the term "hydroisomerization/cat dewaxing" is used to refer to
catalytic processes which have the combined effect of converting normal
paraffins and/or waxy hydrocarbons by rearrangement/isomerization, into more
branched iso-paraffins, followed by (1) catalytic dewaxing to reduce the
amount
of any residual n-paraffins or slightly branched iso-paraffins present in the
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-18-
isomerate by cracking/fragmentation or by (2) hydrodewaxing to effect further
isomerization and very selective catalytic dewaxing of the isomerate, to
reduce
the product pour point. When the term "(and/or solvent)", is included in the
recitation, the process described involves hydroisomerization followed by
solvent dewaxing (or a combination of solvent dewaxing and catalytic
dewaxing) which effects the physical separation of wax from the hydroisomerate
so as to reduce the product pour point.
[042] GTL materials are materials that are derived via one or more synthesis,
combination, transformation, rearrangement, and/or degradation/deconstructive
processes from gaseous carbon-containing compounds, hydrogen-containing
compounds, and/or elements as feedstocks such as hydrogen, carbon dioxide,
carbon monoxide, water, methane, ethane, ethylene, acetylene, propane,
propylene, propyne, butane, butylenes, and butynes. GTL base stocks and/or
base oils are GTL materials of lubricating viscosity that are generally
derived
from hydrocarbons, for example waxy synthesized hydrocarbons, that are
themselves derived from simpler gaseous carbon-containing compounds,
hydrogen-containing compounds and/or elements as feedstocks. GTL base
stock(s) and/or base oil(s) include oils boiling in the lube oil boiling range
separated/fractionated from synthesized GTL materials such as for example, by
distillation and subsequently subjected to a final wax processing step which
is
either or both of the well-known catalytic dewaxing process, or solvent
dewaxing process, to produce lube oils of reduced/low pour point; synthesized
wax isomerates, comprising, for example, hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed synthesized waxy hydrocarbons;
hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed Fischer-
Tropsch (F-T) material (i.e., hydrocarbons, waxy hydrocarbons, waxes and
possible analogous oxygenates); preferably hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed F-T hydrocarbons, or
hydrodewaxed or hydroisomerized/cat (or solvent) dewaxed, F-T waxes,
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-19-
hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed synthesized
waxes, or mixtures thereof.
(043] GTL base stock(s) and/or base oil(s) derived from GTL materials,
especially, hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed F-T
material derived base stock(s) and/or base oil(s), and other hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed wax derived base stock(s) and/or
base oil(s) are characterized typically as having kinematic viscosities at 100
C of
from about 2 mmz/s to about 50 mm2/s, preferably from about 3 mm2/s to about
50 mm2/s, more preferably from about 3.5 mm2/s to about 30 mm2/s, as
exemplified by a GTL base stock derived by the hydrodewaxing or
hydroisomerization/catalytic (or solvent dewaxing) of F-T wax, which has a
kinematic viscosity of about 4 mm2/s at 100 C and a viscosity index of about
130 or greater, but the GTL base stock(s) and/or base oil(s) and or other
hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed wax derived
base stock(s) and/or base oil(s) used in the present invention have kinematic
viscosities in the range of about 3.5 mm2/s to 7 mm2/s, preferably about 4
mm2/s
to about 7 mm2/s, more preferably about 4.5 mm2/s to about 6.5 mm2/ at 100 C.
Preferably the wax treatment process is hydrodewaxing carried out in a process
using a single hydrodewaxing catalyst. Reference herein to Kinematic viscosity
refers to a measurement made by ASTM method D445.
(044] GTL base stock(s) and/or base oil(s) derived from GTL materials,
especially hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed F-T
material derived base stock(s) and/or base oil(s), and other hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed wax-derived base stock(s) and/or
base oil(s), which can be used as base stock and/or base oil components of
this
invention are further characterized typically as having pour points of about -
5 C
or lower, preferably about -10 C or lower, more preferably about -15 C or
lower, still more preferably about -20 C or lower, and under some conditions
may have advantageous pour points of about -25 C or lower, with useful pour
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-20-
points of about -30 C to about -40 C or lower. If necessary, a separate
dewaxing step may be practiced to achieve the desired pour point. In the
present
invention, however, the GTL or other hydrodewaxed or hydroisomerized/cat
(and/or solvent) dewaxed wax-derived base stock(s) and/or base oil(s) used are
those having pour points of about -30 C or higher, preferably about -25 C or
higher, more preferably about -20 C or higher. References herein to pour point
refer to measurement made by ASTM D97 and similar automated versions. If
following hydroisomerization the isomerate is subjected to subsequent
catalytic
dewaxing and/or hydrodewaxing, and/or solvent dewaxing, it is the final
dewaxing step which determines whether the base oil has been processed by
different wax processing techniques. Thus an isomerate secured from a single
hydroisomerization process technique, if divided into two fractions with one
fraction being solvent dewaxed and the second fraction being catalytically
dewaxed or hydrodewaxed, would be considered to be two fractions produced by
different final wax processing techniques. Similarly, if one fraction of wax
feed
is subjected to hydroisomerization followed by a subsequent final wax
treatment
step which is solvent dewaxing and/or catalyst dewaxing and/or hydrodewaxing
of the first isomerate and the second fraction of wax is subjected to
hydrodewaxing per se using a single catalytic such as Pt/ZSM-48, the two oils
produced from the same wax or waxy feed are considered two stocks made by
two different final wax processing techniques, provided that when the
subsequent wax treatment step to which the first isomerate is subjected is a
hydrodewaxing step, the hydrodewaxing per se practiced on the second wax
fraction uses a hydrodewaxing process or catalyst different from that
practiced
on the first isomerate. Yet further, if one fraction of wax feed is subjected
to
hydroisomerization followed by a subsequent final wax treatment step which
comprises both solvent dewaxing followed by catalytic dewaxing and the second
fraction of wax feed is subjected to hydroisomerization followed by a
subsequent final wax treatment step which comprises both catalytic dewaxing
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-21 -
followed by solvent dewaxing, the two oils produced from the same wax or
waxy feeds are considered two stocks made by two different final wax
processing techniques.
[045] The GTL base stock(s) and/or base oil(s) derived from GTL materials,
especially hydrodewaxed or hydroisomerized/cat (and/or solvent) dewaxed F-T
material derived base stock(s) and/or base oil(s), and other such wax-derived
base stock(s) and/or base oil(s) which can be used in this invention are also
characterized typically as having viscosity indices of 80 or greater,
preferably
100 or greater, and more preferably 120 or greater. Additionally, in certain
particular instances, the viscosity index of these base stocks and/or base
oil(s)
may be preferably 130 or greater, more preferably 135 or greater, and even
more
preferably 140 or greater. For example, GTL base stock(s) and/or base oil(s)
that derive from GTL materials preferably F-T materials especially F-T wax
generally have a viscosity index of 130 or greater. References herein to
viscosity index refer to ASTM method D2270.
[046] In addition, the GTL base stock(s) and/or base oil(s) are typically
highly
paraffinic (>90% saturates), and may contain mixtures of monocycloparaffins
and multicycloparaffins in combination with non-cyclic isoparaffins. The ratio
of the naphthenic (i.e., cycloparaffin) content in such combinations varies
with
the catalyst and temperature used. Further, GTL base stock(s) and/or base
oil(s)
typically have very low sulfur and nitrogen content, generally containing less
than about 10 ppm, and more typically less than about 5 ppm of each of these
elements. The sulfur and nitrogen content of GTL base stock(s) and/or base
oil(s) obtained by the hydroisomerization/isodewaxing of F-T material,
especially F-T wax, is essentially nil.
[047] In a preferred embodiment, the GTL base stock(s) and/or base oil(s)
comprises paraffinic materials that consist predominantly of non-cyclic
isoparaffins and only minor amounts of cycloparaffins. These GTL base
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-22-
stock(s) and/or base oil(s) typically comprise paraffinic materials that
consist of
greater than 60 wt% non-cyclic isoparaffins, preferably greater than 80 wt%
non-cyclic isoparaffins, more preferably greater than 85 wt% non-cyclic
isoparaffins, and most preferably greater than 90 wt% non-cyclic isoparaffins.
[048] Useful compositions of GTL base stock(s) and/or base oil(s),
hydrodewaxed or hydroisomerized/cat (and/or solvent) dewaxed F-T material
derived base stock(s), and wax-derived hydrodewaxed, or hydroisomerized/cat
(and/or solvent) dewaxed base stock(s), such as wax isomerates or
hydrodewaxates, are recited in U.S. Pat. Nos. 6,080,301; 6,090,989, and
6,165,949 for example.
[049] Base stock(s) and/or base oil(s) derived from waxy feeds, which are also
suitable for use in this invention, are paraffinic fluids of lubricating
viscosity
derived from hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed
waxy feedstocks of mineral oil, non-mineral oil, non-petroleum, or natural
source origin, e.g., feedstocks such as one or more of gas oils, slack wax,
waxy
fuels hydrocracker bottoms, hydrocarbon raffmates, natural waxes,
hyrocrackates, thermal crackates, foots oil, wax from coal liquefaction or
from
shale oil, or other suitable mineral oil, non-mineral oil, non-petroleum, or
natural
source derived waxy materials, linear or branched hydrocarbyl compounds with
carbon number of about 20 or greater, preferably about 30 or greater, and
mixtures of such isomerate/isodewaxate base stock(s) and/or base oil(s).
[050] Slack wax is the wax recovered from any waxy hydrocarbon oil
including synthetic oil such as F-T waxy oil or petroleum oils by solvent or
autorefrigerative dewaxing. Solvent dewaxing employs chilled solvent such as
methyl ethyl ketone (MEK), methyl isobutyl ketone (MIBK), mixtures of
MEK/MIBK, mixtures of MEK and toluene, while autorefrigerative dewaxing
employs pressurized, liquefied low boiling hydrocarbons such as propane or
butane.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
- 23 -
[051] Slack wax(es) secured from synthetic waxy oils such as F-T waxy oil
will usually have zero or nil sulfur and/or nitrogen containing compound
content. Slack wax(es) secured from petroleum oils, may contain sulfur and
nitrogen containing compounds. Such heteroatom compounds must be removed
by hydrotreating (and not hydrocracking), as for example by
hydrodesulfurization (HDS) and hydrodenitrogenation (HDN) so as to avoid
subsequent poisoning/deactivation of the hydroisomerization catalyst.
[0521 The term GTL base stock and/or base oil and/or wax isomerate base
stock and/or base oil as used herein and in the claims is to be understood as
embracing individual fractions of GTL base stock and/or base oil and/or of wax-
derived hydrodewaxed or hydroisomerized/cat (and/or solvent) dewaxed base
stock and/or base oil as recovered in the production process, mixtures of two
or
more GTL base stock and/or base oil fractions and/or wax-derived
hydrodewaxed, or hydroisomerized/cat (and/or solvent) dewaxed base stocks
and/or base oil fractions, as well as mixtures of one or two or more low
viscosity
GTL base stock and/or base oil fraction(s) and/or wax-derived hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed base stock and/or base oil
fraction(s) with one, two or more higher viscosity GTL base stock and/or base
oil fraction(s) and/or wax-derived hydrodewaxed, or hydroisomerized/cat
(and/or solvent) dewaxed base stock and/or base oil fraction(s) to produce a
dumbbell blend wherein the blend exhibits a kinematic viscosity within the
aforesaid recited range and meets the 15% or less Noack volatility limit and
each
such blend individually constituted one or the other, or both, of the first
and
second base stock(s) and/or base oil(s) of the present mixture.
[053] In a preferred embodiment, the GTL material, from which the GTL base
stock(s) and/or base oil(s) is/are derived is an F-T material (i.e.,
hydrocarbons,
waxy hydrocarbons, wax). A slurry F-T synthesis process may be beneficially
used for synthesizing the feed from CO and hydrogen and particularly one
employing an F-T catalyst comprising a catalytic cobalt component to provide a
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-24-
high Schultz-Flory kinetic alpha for producing the more desirable higher
molecular weight paraffins. This process is also well known to those skilled
in
the art.
[054] In an F-T synthesis process, a synthesis gas comprising a mixture of H2
and CO is catalytically converted into hydrocarbons and preferably liquid
hydrocarbons. The mole ratio of the hydrogen to the carbon monoxide may
broadly range from about 0.5 to 4, but is more typically within the range of
from
about 0.7 to 2.75 and preferably from about 0.7 to 2.5. As is well known, F-T
synthesis processes include processes in which the catalyst is in the form of
a
fixed bed, a fluidized bed or as a slurry of catalyst particles in a
hydrocarbon
slurry liquid. The stoichiometric mole ratio for a F-T synthesis reaction is
2.0,
but there are many reasons for using other than a stoichiometric ratio as
those
skilled in the art know. In cobalt slurry hydrocarbon synthesis process the
feed
mole ratio of the H2 to CO is typically about 2.1/1. The synthesis gas
comprising a mixture of H2 and CO is bubbled up into the bottom of the slurry
and reacts in the presence of the particulate F-T synthesis catalyst in the
slurry
liquid at conditions effective to form hydrocarbons, a portion of which are
liquid
at the reaction conditions and which comprise the hydrocarbon slurry liquid.
The synthesized hydrocarbon liquid is separated from the catalyst particles as
filtrate by means such as filtration, although other separation means such as
centrifugation can be used. Some of the synthesized hydrocarbons pass out the
top of the hydrocarbon synthesis reactor as vapor, along with unreacted
synthesis
gas and other gaseous reaction products. Some of these overhead hydrocarbon
vapors are typically condensed to liquid and combined with the hydrocarbon
liquid filtrate. Thus, the initial boiling point of the filtrate may vary
depending
on whether or not some of the condensed hydrocarbon vapors have been
combined with it. Slurry hydrocarbon synthesis process conditions vary
somewhat depending on the catalyst and desired products. Typical conditions
effective to form hydrocarbons comprising mostly C5+ paraffins, (e.g., Cs+-
C200)
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
- 25 -
and preferably CI o, paraffins, in a slurry hydrocarbon synthesis process
employing a catalyst comprising a supported cobalt component include, for
example, temperatures, pressures and houirly gas space velocities in the range
of
from about 320-850 F, 80-600 psi and 100-40,000 V/hr/V, expressed as standard
volumes of the gaseous CO and H2 mixture (0 C, 1 atm) per hour per volume of
catalyst, respectively. The term "C5+" is used herein to refer to hydrocarbons
with a carbon number of greater than 4, but does not imply that material with
carbon number 5 has to be present. Similarly other ranges quoted for carbon
number do not imply that hydrocarbons having the limit values of the carbon
number range have to be present, or that every carbon number in the quoted
range is present. It is preferred that the hydrocarbon synthesis reaction be
conducted under conditions in which limited or no water gas shift reaction
occurs and more preferably with no water gas shift reaction occurring during
the
hydrocarbon synthesis. It is also preferred to conduct the reaction under
conditions to achieve an alpha of at least 0.85, preferably at least 0.9 and
more
preferably at least 0.92, so as to synthesize more of the more desirable
higher
molecular weight hydrocarbons. This has been achieved in a slurry process
using a catalyst containing a catalytic cobalt component. Those skilled in the
art
know that by alpha is meant the Schultz-Flory kinetic alpha. While suitable F-
T
reaction types of catalyst comprise, for example, one or more Group VIII
catalytic metals such as Fe, Ni, Co, Ru and Re, it is preferred that the
catalyst
comprise a cobalt catalytic component. In one embodiment the catalyst
comprises catalytically effective amounts of Co and one or more of Re, Ru, Fe,
Ni, Th, Zr, Hf, U, Mg and La on a suitable inorganic support material,
preferably
one which comprises one or more refractory metal oxides. Preferred supports
for Co containing catalysts comprise Titania, particularly. Useful catalysts
and
their preparation are known and illustrative, but nonlimiting examples may be
found, for example, in U.S. Pat. Nos. 4,568,663; 4,663,305; 4,542,122;
4,621,072 and 5,545,674.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-26-
[055] As set forth above, the waxy feed from which the base stock(s) and/or
base oil(s) is/are derived is a wax or waxy feed from mineral oil, non-mineral
oil, non-petroleum, or other natural source, especially slack wax, or GTL
material, preferably F-T material, referred to as F-T wax. F-T wax preferably
has an initial boiling point in the range of from 650-750 F and preferably
continuously boils up to an end point of at least 1050 F. A narrower cut waxy
feed may also be used during the hydroisomerization. A portion of the n-
paraffin waxy feed is converted to lower boiling isoparaffinic material.
Hence,
there must be sufficient heavy n-paraffin material to yield an isoparaffin
containing isomerate boiling in the lube oil range. If catalytic dewaxing is
also
practiced after isomerization/isodewaxing, some of the isomerate/isodewaxate
will also be hydrocracked to lower boiling material during the conventional
catalytic dewaxing. Hence, it is preferred that the end boiling point of the
waxy
feed be above 1050 F (1050 F+).
[056] When a boiling range is quoted herein it defines the lower and/or upper
distillation temperature used to separate the fraction. Unless specifically
stated
(for example, by specifying that the fraction boils continuously or
constitutes the
entire range) the specification of a boiling range does not require that any
material at the specified limit has to be present, rather it excludes material
boiling outside that range.
[057] The waxy feed preferably comprises the entire 650-750 F+ fraction
formed by the hydrocarbon synthesis process, having an initial cut point
between
650 F and 750 F determined by the practitioner and an end point, preferably
above 1050 F, determined by the catalyst and process variables employed by the
practitioner for the synthesis. Such fractions are referred to herein as "650-
750 F+ fractions". By contrast, "650-750 F- fractions" refers to a fraction
with
an unspecified initial cut point and an end point somewhere between 650 F and
750 F. Waxy feeds may be processed as the entire fraction or as subsets of the
entire fraction prepared by distillation or other separation techniques. The
waxy
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-27-
feed also typically comprises more than 90%, generally more than 95% and
preferably more than 98 wt% paraffinic hydrocarbons, most of which are normal
paraffins. It has negligible amounts of sulfur and nitrogen compounds (e.g.,
less
than 1 wppm of each), with less than 2,000 wppm, preferably less than 1,000
wppm and more preferably less than 500 wppm of oxygen, in the form of
oxygenates. Waxy feeds having these properties and useful in the process of
the
invention have been made using a slurry F-T process with a catalyst having a
catalytic cobalt component, as previously indicated.
[058] The process of making the lubricant oil base stocks from waxy stocks,
e.g., slack wax or F-T wax, may be characterized as an isomerization process.
If
slack waxes are used as the feed, they may need to be subjected to a
preliminary
hydrotreating step under conditions already well known to those skilled in the
art
to reduce (to levels that would effectively avoid catalyst poisoning or
deactiva-
tion) or to remove sulfur- and nitrogen-containing compounds which would
otherwise deactivate the hydroisomerization or hydrodewaxing catalyst used in
subsequent steps. If F-T waxes are used, such preliminary treatment is not
required because, as indicated above, such waxes have only trace amounts (less
than about 10 ppm, or more typically less than about 5 ppm to nil) of sulfur
or
nitrogen compound content. However, some hydrodewaxing catalyst fed F-T
waxes may benefit from prehydrotreatment for the removal of oxygenates while
others may benefit from oxygenates treatment. The hydroisomerization or
hydrodewaxing process may be conducted over a combination of catalysts, or
over a single catalyst. Conversion temperatures range from about 150 C to
about 500 C at pressures ranging from about 500 to 20,000 kPa. This process
may be operated in the presence of hydrogen, and hydrogen partial pressures
range from about 600 to 6000 kPa. The ratio of hydrogen to the hydrocarbon
feedstock (hydrogen circulation rate) typically range from about 10 to 3500
n.1.1."1 (56 to 19,660 SCF/bbl) and the space velocity of the feedstock
typically
ranges from about 0.1 to 20 LHSV, preferably 0.1 to 10 LHSV.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-28-
[059] Following any needed hydrodenitrogenation or hydrodesulfurization,
the hydroprocessing used for the production of base stocks from such waxy
feeds may use an amorphous hydrocracking/hydroisomerization caialyst, such as
a lube hydrocracking (LHDC) catalysts, for example catalysts containing Co,
Mo, Ni, W, Mo, etc., on oxide supports, e.g., alumina, silica, silica/alumina,
or a
crystalline hydrocracking/hydroisomerization catalyst, preferably a zeolitic
catalyst.
[060] Other isomerization catalysts and processes for hydrocracking, hydro-
dewaxing, or hydroisomerizing GTL materials and/or waxy materials to base
stock or base oil are described, for example, in U.S. Pat. Nos. 2,817,693;
4,900,407; 4,937,399; 4,975,177; 4,921,594; 5,200,382; 5,516,740; 5,182,248;
5,290,426; 5,580,442; 5,976,351; 5,935,417; 5,885,438; 5,965,475; 6,190,532;
6,375,830; 6,332,974; 6,103,099; 6,025,305; 6,080,301; 6,096,940; 6,620,312;
6,676,827; 6,383,366; 6,475,960; 5,059,299; 5,977,425; 5,935,416; 4,923,588;
5,158,671; and 4,897,178; EP 0324528 (B1), EP 0532116 (B1), EP 0532118
(B1), EP 0537815 (B1), EP 0583836 (B2), EP 0666894 (B2), EP 0668342 (B1),
EP 0776959 (A3), WO 97/031693 (Al), WO 02/064710 (A2), WO 02/064711
(Al), WO 02/070627 (A2), WO 02/070629 (Al), WO 03/033320 (Al) as well
as in British Patents 1,429,494; 1,350,257; 1,440,230; 1,390,359; WO 99/45085
and WO 99/20720. Particularly favorable processes are described in European
Patent Applications 464546 and 464547. Processes using F-T wax feeds are
described in U.S. Pat. Nos. 4,594,172; 4,943,672; 6,046,940; 6,475,960;
6,103,099; 6,332,974; and 6,375,830.
[061] Hydrocarbon conversion catalysts useful in the conversion of the
n-paraffin waxy feedstocks disclosed herein to form the isoparaffinic hydro-
carbon base oil are zeolite catalysts, such as ZSM-5, ZSM-11, ZSM-23,
ZSM-35, ZSM-12, ZSM-38, ZSM-48, offretite, ferrierite, zeolite beta, zeolite
theta, and zeolite alpha, as disclosed in USP 4,906,350. These catalysts are
used
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-29-
in combination with Group VIII metals, in particular palladium or platinum.
The
Group VIII metals may be incorporated into the zeolite catalysts by
conventional
techniques, such as ion exchange.
[062] In one embodiment, conversion of the waxy feedstock may be
conducted over a combination of Pt/zeolite beta and Pt/ZSM-23 catalysts in the
presence of hydrogen. In another embodiment, the process of producing the
lubricant oil base stocks comprises hydroisomerization and dewaxing over a
single catalyst, such as Pt/ZSM-35. In yet another embodiment, the waxy feed
can be fed over a catalyst comprising Group VIII metal loaded ZSM-48,
preferably Group VIII noble metal loaded ZSM-48, more preferably PtIZSM-48
in either one stage or two stages. In any case, useful hydrocarbon base oil
products may be obtained. Catalyst ZSM-48 is described in USP 5,075,269.
The use of the Group VIII metal loaded ZSM-48 family of catalysts, e.g.,
platinum on ZSM-48, in the hydroisomerization of the waxy feedstock
eliminates the need for any subsequent, separate dewaxing step.
[063) A dewaxing step, when needed, may be accomplished using one or
more of solvent dewaxing, catalytic dewaxing or hydrodewaxing processes and
either the entire hydroisomerate or the 650-750 F+ fraction may be dewaxed,
depending on the intended use of the 650-750 F- material present, if it has
not
been separated from the higher boiling material prior to the dewaxing. In
solvent dewaxing, the hydroisomerate may be contacted with chilled solvents
such as acetone, methyl ethyl ketone (MEK), methyl isobutyl ketone (MIBK),
mixtures of MEK/MIBK, or mixtures of MEK/toluene and the like, and further
chilled to precipitate out the higher pour point material as a waxy solid
which is
then separated from the solvent-containing lube oil fraction which is the
raffinate. The raffinate is typically further chilled in scraped surface
chillers to
remove more wax solids. Autorefrigerative dewaxing using low molecular
weight hydrocarbons, such as propane, can also be used in which the
hydroisomerate is mixed with, e.g., liquid propane, a least a portion of which
is
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-30-
flashed off to chill down the hydroisomerate to precipitate out the wax. The
wax
is separated from the raffinate by filtration, membrane separation or
centrifugation. The solvent is then stripped out of the raffinate, which is
then
fractionated to produce the preferred base stocks useful in the present
invention.
Also well known is catalytic dewaxing, in which the hydroisomerate is reacted
with hydrogen in the presence of a suitable dewaxing catalyst at conditions
effective to lower the pour point of the hydroisomerate. Catalytic dewaxing
also
converts a portion of the hydroisomerate to lower boiling materials, in the
boiling range, for example, 650-750 F-, which are separated from the heavier
650-750 F+ base stock fraction and the base stock fraction fractionated into
two
or more base stocks. Separation of the lower boiling material may be
accomplished either prior to or during fractionation of the 650-750 F+
material
into the desired base stocks.
[064] Any dewaxing catalyst which will reduce the pour point of the
hydroisomerate and preferably those which provide a large yield of lube oil
base
stock from the hydroisomerate may be used. These include shape selective
molecular sieves which, when combined with at least one catalytic metal
component, have been demonstrated as useful for dewaxing petroleum oil
fractions and include, for example, ferrierite, mordenite, ZSM-5, ZSM-11,
ZSM-23, ZSM-35, ZSM-22 also known as theta one or TON, and the
silicoaluminophosphates known as SAPO's. A dewaxing catalyst which has
been found to be unexpectedly particularly effective comprises a noble metal,
preferably Pt, composited with H-mordenite. The dewaxing may be
accomplished with the catalyst in a fixed, fluid or slurry bed. Typical
dewaxing
conditions include a temperature in the range of from about 400-600 F, a
pressure of 500-900 psig, H2 treat rate of 1500-3500 SCF/B for flow-through
reactors and LHSV of 0.1-10, preferably 0.2-2Ø The dewaxing is typically
conducted to convert no more than 40 wt% and preferably no more than 30 wt%
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-31-
of the hydroisomerate having an initial boiling point in the range of 650-750
F
to material boiling below its initial boiling point.
[065] GTL base stock(s) and/or base oil(s), hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed wax-derived base stock(s) and/or
base oil(s), have a beneficial kinematic viscosity advantage over conventional
API Group II and Group III base stock(s) and/or base oil(s) , and so may be
very
advantageously used with the instant invention. Such GTL base stock(s) and/or
base oil(s) can have significantly higher kinematic viscosities, up to about
20-50
mm2/s at 100 C, whereas by comparison commercial Group II base oils can have
kinematic viscosities up to about 15 mm2/s at 100 C, and commercial Group III
base oils can have kinematic viscosities up to about 10 mm2/s at 100 C. The
higher kinematic viscosity range of GTL base stock(s) and/or base oil(s),
compared to the more limited kinematic viscosity range of Group II and Group
III base stock(s) and/or base oil(s), in combination with the instant
invention can
provide additional beneficial advantages in formulating lubricant
compositions.
[066] In the present invention mixtures of hydrodewaxate, or
hydroisomerate/cat (and/or solvent) dewaxate base stock(s) and/or base oil(s),
mixtures of the GTL base stock(s) and/or base oil(s), or mixtures thereof,
preferably mixtures of GTL base stock(s) and/or base oil(s), provided each
component in the mixture has been subjected to a different final wax
processing
technique, can constitute all or part of the base oil.
[067] The preferred base stock(s) and/or base oil(s) derived from GTL
materials and/or from waxy feeds are characterized as having predominantly
paraffinic compositions and are further characterized as having high saturates
levels, low-to-nil sulfur, low-to-nil nitrogen, low-to-nil aromatics, and are
essentially water-white in color.
[068] A preferred GTL liquid hydrocarbon composition is one comprising
paraffinic hydrocarbon components in which the extent of branching, as
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-32-
measured by the percentage of methyl hydrogens (BI), and the proximity of
branching, as measured by the percentage of recurring methylene carbons which
are four or more carbons removed from an end group or branch (CH2 > 4), are
such that: (a) BI-0.5(CH2 > 4) >15; and (b) BI+0.85 (CH2 > 4) <45 as measured
over said liquid hydrocarbon composition as a whole.
[069] The preferred GTL base stock and/or base oil can be further
characterized, if necessary, as having less than 0.1 wt% aromatic
hydrocarbons,
less than 20 wppm nitrogen containing compounds, less than 20 wppm sulfur
containing compounds, a pour point of less than -18 C, preferably less than -
30 C, a preferred BI > 25.4 and (CH2 > 4) < 22.5. They have a nominal boiling
point of 370 C+, on average they average fewer than 10 hexyl or longer
branches
per 100 carbon atoms and on average have more than 16 methyl branches per
100 carbon atoms. They also can be characterized by a combination of dynamic
viscosity, as measured by CCS at -40 C, and kinematic viscosity, as measured
at
100 C represented by the formula: DV (at -40 C) < 2900 (KV at 100 C) -
7000.
[070] The preferred GTL base stock and/or base oil is also characterized as
comprising a mixture of branched paraffins characterized in that the lubricant
base oil contains at least 90% of a mixture of branched paraffins, wherein
said
branched paraffins are paraffins having a carbon chain length of about C20 to
about C40, a molecular weight of about 280 to about 562, a boiling range of
about 650 F to about 1050 F, and wherein said branched paraffins contain up to
four alkyl branches and wherein the free carbon index of said branched
paraffins
is at least about 3.
[071] In the above the Branching Index (BI), Branching Proximity (CH2 > 4),
and Free Carbon Index (FCI) are determined as follows:
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-33-
Branching Inde
[072] A 359.88 MHz 1 H solution 1VMR spectrum is obtained on a Bruker 360
MHz AMX spectrometer using 10% solutions in CDC13. TMS is the internal
chemical shift reference. CDC13 solvent gives a peak located at 7.28. All
spectra are obtained under quantitative conditions using 90 degree pulse
(10.9 s), a pulse delay time of 30 s, which is at least five times the
longest
hydrogen spin-lattice relaxation time (T1), and 120 scans to ensure good
signal-to-noise ratios.
[073] H atom types are defined according to the following regions:
9.2-6.2 ppm hydrogens on aromatic rings;
6.2-4.0 ppm hydrogens on olefinic carbon atoms;
4.0-2.1 ppm benzylic hydrogens at the a-position to aromatic rings;
2.1-1.4 ppm paraffinic CH methine hydrogens;
1.4-1.05 ppm paraffinic CH2 methylene hydrogens;
1.05-0.5 ppm paraffinic CH3 methyl hydrogens.
[074] The branching index (BI) is calculated as the ratio in percent of non-
benzylic methyl hydrogens in the range of 0.5 to 1.05 ppm, to the total non-
benzylic aliphatic hydrogens in the range of 0.5 to 2.1 ppm.
Branching Proximity (CH2 > 4)
[075] A 90.5 MHz3CMR single pulse and 135 Distortionless Enhancement by
Polarization Transfer (DEPT) NMR spectra are obtained on a Brucker 360
MHzAMX spectrometer using 10% solutions in CDCL3. TMS is the internal
chemical shift reference. CDCL3 solvent gives a triplet located at 77.23 ppm
in
the 13C spectrum. All single pulse spectra are obtained under quantitative
conditions using 45 degree pulses (6.3 s), a pulse delay time of 60 s, which
is at
least five times the longest carbon spin-lattice relaxation time (TI), to
ensure
complete relaxation of the sample, 200 scans to ensure good signal-to-noise
ratios, and WALTZ-16 proton decoupling.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-34-
[076] The C atom types CH3, CH2, and CH are identified from the 135 DEPT
13C NMR experiment. A major CH2 resonance in all 13C NMR spectra at ;z~29.8
ppm is due to equivalent recurring methylene carbons which are four or more
removed from an end group or branch (CH2 > 4). The types of branches are
determined based primarily on the 13C chemical shifts for the methyl carbon at
the end of the branch or the methylene carbon one removed from the methyl on
the branch.
ti
[077] Free Carbon Index (FCI). The FCI is expressed in units of carbons, and
is a measure of the number of carbons in an isoparaffin that are located at
least 5
carbons from a terminal carbon and 4 carbons way from a side chain. Counting
the terminal methyl or branch carbon as "one" the carbons in the FCI are the
fifth
or greater carbons from either a straight chain terminal methyl or from a
branch
methane carbon. These carbons appear between 29.9 ppm and 29.6 ppm in the
carbon-13 spectrum. They are measured as follows:
a) calculate the average carbon number of the molecules in the sample which is
accomplished with sufficient accuracy for lubricating oil materials by simply
dividing the molecular weight of the sample oil by 14 (the formula weight of
CH2);
b) divide the total carbon- 13 integral area (chart divisions or area counts)
by the
average carbon number from step a. to obtain the integral area per carbon in
the sample;
c) measure the area between 29.9 ppm and 29.6 ppm in the sample; and
d) divide by the integral area per carbon from step b. to obtain FCI.
[078] Branching measurements can be performed using any Fourier Transform
NMR spectrometer. Preferably, the measurements are performed using a
spectrometer having a magnet of 7.OT or greater. In all cases, after
verification
by Mass Spectrometry, UV or an NMR survey that aromatic carbons were
absent, the spectral width was limited to the saturated carbon region, about 0-
80
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-35-
ppm vs. TMS (tetramethylsilane). Solutions of 15-25 percent by weight in
chloroform-dl were excited by 45 degrees pulses followed by a 0.8 sec
acquisition time. In order to minimize non-uniform intensity data, the proton
decoupler was gated off during a 10 sec delay prior to the excitation pulse
and
on during acquisition. Total experiment times ranged from 11-80 minutes. The
DEPT and APT sequences were carried out according to literature descriptions
with minor deviations described in the Varian or Bruker operating manuals.
[079] DEPT is Distortionless Enhancement by Polarization Transfer. DEPT
does not show quaternaries. The DEPT 45 sequence gives a signal for all
carbons bonded to protons. DEPT 90 shows CH carbons only. DEPT 135
shows CH and CH3 up and CH2 180 degrees out of phase (down). APT is
Attached Proton Test. It allows all carbons to be seen, but if CH and CH3 are
up,
then quaternaries and CH2 are down. The sequences are useful in that every
branch methyl should have a corresponding CH and the methyls are clearly
identified by chemical shift and phase. The branching properties of each
sample
are determined by C-13 NMR using the assumption in the calculations that the
entire sample is isoparaffinic. Corrections are not made for n-paraffins or
cyclo-
paraffins, which may be present in the oil samples in varying amounts. The
cycloparaffins content is measured using Field Ionization Mass Spectroscopy
(FIMS).
10801 GTL base stock(s) and/or base oil(s), and hydrodewaxed, or
hydroisomerized/cat (and/or solvent) dewaxed wax base stock(s) and/or base
oil(s), for example, hydrodewaxed or hydroisomerized/catalytic (and/or
solvent)
dewaxed waxy synthesized hydrocarbon, e.g., Fischer-Tropsch waxy
hydrocarbon base stock(s) and/or base oil(s) are of low or zero sulfur and
phosphorus content. There is a movement among original equipment
manufacturers and oil formulators to produce formulated oils of ever
increasingly reduced sulfated ash, phosphorus and sulfur content to meet ever
increasingly restrictive environmental regulations. Such oils, known as low
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-36-
SAPS oils, would rely on the use of base oils which themselves, inherently,
are
of low or zero initial sulfur and phosphorus content. Such oils when used as
base oils can be formulated with additives. Even if the additive or additives
included in the formulation contain sulfur and/or phosphorus the resulting
formulated lubricating oils will be lower or low SAPS oils as compared to
lubricating oils formulated using conventional mineral oil base stock(s)
and/or
base oil(s).
10811 For example, low SAPS formulated oils for vehicle engines (both spark
ignited and compression ignited) will have a sulfur content of 0.7 wt% or
less,
preferably 0.6 wt% or less, more preferably 0.5 wt% or less, most preferably
0.4
wt% or less, an ash content of 1.2 wt% or less, preferably 0.8 wt% or less,
more
preferably 0.4 wt% or less, and a phosphorus content of 0.18% or less,
preferably 0.1 wt% or less, more preferably 0.09 wt% or less, most preferably
0.08 wt% or less, and in certain instances, even preferably 0.05 wt% or less.
[082] The combination base stock is formulated with typical automotive
engine lubricating additives, but can omit or significantly reduce the amount
of
viscosity modifier and pour point depressants heretofore conventionally
utilized
to meet SAE OW-X and 5W-X multi-grade engine oil low temperature
viscometric and rheological properties.
[083] Examples of typical additives include, but are not limited to, oxidation
inhibitors, antioxidants, dispersants, detergents, corrosion inhibitors, rust
inhibitors, metal deactivators, anti-wear agents, extreme pressure additives,
anti-
seizure agents, wax modifiers, other viscosity index improvers, other
viscosity
modifiers, fluid-loss additives, seal compatibility agents, friction
modifiers,
lubricity agents, anti-staining agents, chromophoric agents, defoamants,
demulsifiers, emulsifiers, densifiers, wetting agents, gelling agents,
tackiness
agents, colorants, and others. For a review of many commonly used additives,
see Klamann in Lubricants and Related Products, Verlag Chemie, Deerfield
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-37-
Beach, FL; ISBN 0-89573-177-0. Reference is also made to "Lubricant
Additives" by M. W. Ranney, published by Noyes Data Corporation of
Parkridge, NJ (1973).
10841 Finished lubricants comprise the lubricant base stock or base oil, plus
at
least one performance additive.
[085] The types and quantities of performance additives used in combination
with the instant invention in lubricant compositions are not limited by the
examples shown herein as illustrations.
Antiwear and EP Additives
[086] Many lubricating oils require the presence of antiwear and/or extreme
pressure (EP) additives in order to provide adequate antiwear protection.
Increasingly specifications for, e.g., engine oil performance have exhibited a
trend for improved antiwear properties of the oil. Antiwear and extreme EP
additives perform this role by reducing friction and wear of metal parts.
[087] While there are many different types of antiwear additives, for several
decades the principal antiwear additive for internal combustion engine
crankcase
oils is a metal alkylthiophosphate and more particularly a metal dialkyldithio-
phosphate in which the primary metal constituent is zinc, or zinc
dialkyldithio-
phosphate (ZDDP). ZDDP compounds generally are of the formula
Zn[SP(S)(ORl)(OR2)]Z where R' and R2 are CI-C18 alkyl groups, preferably
C2-C12 alkyl groups. These alkyl groups may be straight chain or branched. The
ZDDP is typically used in amounts of from about 0.4 to 1.4 wt% of the total
lube
oil composition, although more or less can often be used advantageously.
[088] However, it is found that the phosphorus from these additives has a
deleterious effect on the catalyst in catalytic converters and also on oxygen
sensors in automobiles. One way to minimize this effect is to replace some or
all
of the ZDDP with phosphorus-free antiwear additives.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-38-
[089] A variety of non-phosphorous additives are also used as antiwear
additives. Sulfurized olefins are useful as antiwear and EP additives. Sulfur-
containing olefins can be prepared by sulfurization or various organic
materials
including aliphatic, arylaliphatic or alicyclic olefinic hydrocarbons
containing
from about 3 to 30 carbon atoms, preferably 3-20 carbon atoms. The olefinic
compounds contain at least one non-aromatic double bond. Such compounds are
defined by the formula
R3R4C=CRSR6
where each of R3-R6 are independently hydrogen or a hydrocarbon radical.
Preferred hydrocarbon radicals are alkyl or alkenyl radicals. Any two of R3-R6
may be connected so as to form a cyclic ring. Additional information concern-
ing sulfurized olefins and their preparation can be found in USP 4,941,984,
incorporated by reference herein in its entirety.
[090] The use of polysulfides of thiophosphorus acids and thiophosphorus
acid esters as lubricant additives is disclosed in U.S. Patents 2,443,264;
2,471,115; 2,526,497; and 2,591,577. Addition of phosphorothionyl disulfides
as an antiwear, antioxidant, and EP additive is disclosed in USP 3,770,854.
Use
of alkylthiocarbamoyl compounds (bis(dibutyl)thiocarbamoyl, for example) in
combination with a molybdenum compound (oxymolybdenum diisopropyl-
phosphorodithioate sulfide, for example) and a phosphorous ester (dibutyl
hydrogen phosphite, for example) as antiwear additives in lubricants is
disclosed
in USP 4,501,678. USP 4,758,362 discloses use of a carbamate additive to
provide improved antiwear and extreme pressure properties. The use of
thiocarbamate as an antiwear additive is disclosed in USP 5,693,598.
Thiocarbamate/molybdenum complexes such as moly-sulfur alkyl dithio-
carbamate trimer complex (R=Cg-CIg alkyl) are also useful antiwear agents. The
use or addition of such materials should be kept to a minimum if the object is
to
produce low SAP formulations.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-39-
[091] Esters of glycerol may be used as antiwear agents. For example, mono-,
di-, and tri-oleates, mono-palmitates and mono-myristates may be used.
[092] ZDDP is combined with other compositions that provide antiwear
properties. USP 5,034,141 discloses that a combination of a thiodixanthogen
compound (octylthiodixanthogen, for example) and a metal thiophosphate
(ZDDP, for example) can improve antiwear properties. USP 5,034,142 discloses
that use of a metal alkyoxyalkylxanthate (nickel ethoxyethylxanthate, for
example) and a dixanthogen (diethoxyethyl dixanthogen, for example) in
combination with ZDDP improves antiwear properties.
[093] Preferred antiwear additives include phosphorus and sulfur compounds
such as zinc dithiophosphates and/or sulfur, nitrogen, boron, molybdenum
phosphorodithioates, molybdenum dithiocarbamates and various organo-
molybdenum derivatives including heterocyclics, for example dimercaptothia-
diazoles, mercaptobenzothiadiazoles, triazines, and the like, alicyclics,
amines,
alcohols, esters, diols, triols, fatty amides and the like can also be used.
Such
additives may be used in an amount of about 0.01 to 6 wt%, preferably about
0.01 to 4 wt%. ZDDP-like compounds provide limited hydroperoxide
decomposition capability, significantly below that exhibited by compounds
disclosed and claimed in this patent and can therefore be eliminated from the
formulation or, if retained, kept at a minimal concentration to facilitate
production of low SAPS formulations.
Viscosity Improvers
[094] Viscosity improvers (also known as Viscosity Index modifiers, and VI
improvers) provide lubricants with high and low temperature operability. These
additives increase the viscosity of the oil composition at elevated
temperatures
which increases film thickness, while having limited effect on viscosity at
low
temperatures.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-40-
[095] Suitable viscosity improvers include high molecular weight hydro-
carbons, polyesters and viscosity index improver dispersants that function as
both a viscosity index improver and a dispersant. Typical molecular weights of
these polymers are between about 10,000 to 1,000,000, more typically about
20,000 to 500,000, and even more typically between about 50,000 and 200,000.
[096] Examples of suitable viscosity improvers are polymers and copolymers
of methacrylate, butadiene, olefins, or alkylated styrenes. Polyisobutylene is
a
commonly used viscosity index improver. Another suitable viscosity index
improver is polymethacrylate (copolymers of various chain length alkyl meth-
acrylates, for example), some formulations of which also serve as pour point
depressants. Other suitable viscosity index improvers include copolymers of
ethylene and propylene, hydrogenated block copolymers of styrene and isoprene,
and polyacrylates (copolymers of various chain length acrylates, for example).
Specific examples include styrene-isoprene or styrene-butadiene based polymers
of 50,000 to 200,000 molecular weight.
[097] The amount of viscosity modifier may range from zero to 8 wt%,
preferably zero to 4 wt%, more preferably zero to 2 wt% based on active
ingredient and depending on the specific viscosity modifier used.
Antioxidants
[098] Antioxidants retard the oxidative degradation of base oils during
service. Such degradation may result in deposits on metal surfaces, the
presence
of sludge, or a viscosity increase in the lubricant. One skilled in the art
knows a
wide variety of oxidation inhibitors that are useful in lubricating oil
composi-
tions. See, Klamann in Lubricants and Related Products, op cite, and U.S.
Patents 4,798,684 and 5,084,197, for example.
[099] Useful antioxidants include hindered phenols. These phenolic anti-
oxidants may be ashless (metal-free) phenolic compounds or neutral or basic
metal salts of certain phenolic compounds. Typical phenolic antioxidant
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-41 -
compounds are the hindered phenolics which are the ones which contain a
sterically hindered hydroxyl group, and these include those derivatives of
dihydroxy aryl compounds in which the hydroxyl groups are in the o- or
p-position to each other. Typical phenolic antioxidants include the hindered
phenols substituted with C6+ alkyl groups and the alkylene coupled derivatives
of these hindered phenols. Examples of phenolic materials of this type 2-t-
butyl-
4-heptyl phenol; 2-t-butyl-4-octyl phenol; 2-t-butyl-4-dodecyl phenol; 2,6-di-
t-
butyl-4-heptyl phenol; 2,6-di-t-butyl-4-dodecyl phenol; 2-methyl-6-t-butyl-4-
heptyl phenol; and 2-methyl-6-t-butyl-4-dodecyl phenol. Other useful hindered
mono-phenolic antioxidants may include for example hindered 2,6-di-alkyl-
phenolic proprionic ester derivatives. Bis-phenolic antioxidants may also be
advantageously used in combination with the instant invention. Examples of
ortho-coupled phenols include: 2,2'-bis(4-heptyl-6-t-butyl-phenol); 2,2'-bis(4-
octyl-6-t-butyl-phenol); and 2,2'-bis(4-dodecyl-6-t-butyl-phenol). Para-
coupled
bisphenols include for example 4,4'-bis(2,6-di-t-butyl phenol) and 4,4'-
methylene-bis(2,6-di-t-butyl phenol).
101001 Non-phenolic oxidation inhibitors which may be used include aromatic
amine antioxidants and these may be used either as such or in combination with
phenolics. Typical examples of non-phenolic antioxidants include: alkylated
and non-alkylated aromatic amines such as aromatic monoamines of the formula
RgR9R10N where R 8 is an aliphatic, aromatic or substituted aromatic group, R9
is
an aromatic or a substituted aromatic group, and R10 is H, alkyl, aryl or
R"S(O)xR 12 where R' 1 is an alkylene, alkenylene, or aralkylene group, R12 is
a
higher alkyl group, or an alkenyl, aryl, or alkaryl group, and x is 0, 1 or 2.
The
aliphatic group R8 may contain from 1 to about 20 carbon atoms, and preferably
contains from about 6 to 12 carbon atoms. The aliphatic group is a saturated
aliphatic group. Preferably, both R8 and R9 are aromatic or substituted
aromatic
groups, and the aromatic group may be a fused ring aromatic group such as
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-42-
naphthyl. Aromatic groups R 8 and R9 may be joined together with other groups
such as S.
[0101] Typical aromatic amines antioxidants have alkyl substituent groups of
at
least about 6 carbon atoms. Examples of aliphatic groups include hexyl,
heptyl,
octyl, nonyl, and decyl. Generally, the aliphatic groups will not contain more
than about 14 carbon atoms. The general types of amine antioxidants useful in
the present compositions include diphenylamines, phenyl naphthylamines,
phenothiazines, imidodibenzyls and diphenyl phenylene diamines. Mixtures of
two or more aromatic amines are also useful. Polymeric amine antioxidants can
also be used. Particular examples of aromatic amine antioxidants useful in the
present invention include: p,p'-dioctyldiphenylamine; t-octylphenyl-alpha-
naphthylamine; phenyl-alphanaphthylamine; and p-octylphenyl-alpha-
naphthylamine.
[0102] Sulfurized alkyl phenols and alkali or alkaline earth metal salts
thereof
also are useful antioxidants.
[01031 Another class of antioxidant used in lubricating oil compositions is
oil-
soluble copper compounds. Any oil-soluble suitable copper compound may be
blended into the lubricating oil. Examples of suitable copper antioxidants
include copper dihydrocarbyl thio- or dithio-phosphates and copper salts of
carboxylic acid (naturally occurring or synthetic). Other suitable copper
salts
include copper dithiacarbamates, sulphonates, phenates, and acetylacetonates.
Basic, neutral, or acidic copper Cu(I) and or Cu(II) salts derived from
alkenyl
succinic acids or anhydrides are know to be particularly useful.
[0104] Preferred antioxidants include hindered phenols, arylamines. These
antioxidants may be used individually by type or in combination with one
another. Such additives may be used in an amount of about 0.01 to 5 wt%,
preferably about 0.01 to 1.5 wt%, more preferably zero to less than 1.5 wt%,
most preferably zero.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
- 43 -
Deter,gents
[0105] Detergents are commonly used in lubricating compositions. A typical
detergent is an anionic material that contains a long chain hydrophobic
portion
of the molecule and a smaller anionic or oleophobic hydrophilic portion of the
molecule. The anionic portion of the detergent is typically derived from an
organic acid such as a sulfur acid, carboxylic acid, phosphorous acid, phenol,
or
mixtures thereof. The counterion is typically an alkaline earth or alkali
metal.
[0106] Salts that contain a substantially stochiometric amount of the metal
are
described as neutral salts and have a total base number (TBN, as measured by
ASTM D2896) of from 0 to 80. Many compositions are overbased, containing
large amounts of a metal base that is achieved by reacting an excess of a
metal
compound (a metal hydroxide or oxide, for example) with an acidic gas (such as
carbon dioxide). Useful detergents can be neutral, mildly overbased, or highly
overbased.
[0107] It is desirable for at least some detergent to be overbased. Overbased
detergents help neutralize acidic impurities produced by the combustion
process
and become entrapped in the oil. Typically, the overbased material has a ratio
of
metallic ion to anionic portion of the detergent of about 1.05:1 to 50:1 on an
equivalent basis. More preferably, the ratio is from about 4:1 to about 25:1.
The
resulting detergent is an overbased detergent that will typically have a TBN
of
about 150 or higher, often about 250 to 450 or more. Preferably, the
overbasing
cation is sodium, calcium, or magnesium. A mixture of detergents of differing
TBN can be used in the present invention.
[0108] Preferred detergents include the alkali or alkaline earth metal salts
of
sulfonates, phenates, carboxylates, phosphates, and salicylates.
[0109] Sulfonates may be prepared from sulfonic acids that are typically
obtained by sulfonation of alkyl substituted aromatic hydrocarbons. Hydro-
carbon examples include those obtained by alkylating benzene, toluene, xylene,
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-44-
naphthalene, biphenyl and their halogenated derivatives (chlorobenzene,
chlorotoluene, and chloronaphthalene, for example). The alkylating agents
typically have about 3 to 70 carbon atoms. The alkaryl sulfonates typically
contain about 9 to about 80 carbon or more carbon atoms, more typically from
about 16 to 60 carbon atoms.
[0110] Klamami in Lubricants and Related Products, op cit discloses a number
of overbased metal salts of various sulfonic acids which are useful as
detergents
and dispersants in lubricants. The book entitled "Lubricant Additives", C. V.
Smallheer and R. K. Smith, published by the Lezius-Hiles Co. of Cleveland,
Ohio (1967), similarly discloses a number of overbased sulfonates that are
useful
as dispersants/detergents.
[0111] Alkaline earth phenates are another useful class of detergent. These
detergents can be made by reacting alkaline earth metal hydroxide or oxide
(CaO, Ca(OH)2, BaO, Ba(OH)2, MgO, Mg(OH)2, for example) with an alkyl
phenol or sulfurized alkylphenol. Useful alkyl groups include straight chain
or
branched CI-C30 alkyl groups, preferably, C4-C20. Examples of suitable phenols
include isobutylphenol, 2-ethylhexylphenol, nonylphenol, dodecyl phenol, and
the like. It should be noted that starting alkylphenols may contain more than
one
alkyl substituent that are each independently straight chain or branched. When
a
non-sulfurized alkylphenol is used, the sulfurized product may be obtained by
methods well known in the art. These methods include heating a mixture of
alkylphenol and sulfurizing agent (including elemental sulfur, sulfur halides
such
as sulfur dichloride, and the like) and then reacting the sulfurized phenol
with an
alkaline earth metal base.
[0112] Metal salts of carboxylic acids are also useful as detergents. These
carboxylic acid detergents may be prepared by reacting a basic metal compound
with at least one carboxylic acid and removing free water from the reaction
product. These compounds may be overbased to produce the desired TBN level.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
- 45 -
Detergents made from salicylic acid are one preferred class of detergents
derived
from carboxylic acids. Useful salicylates include long chain alkyl
salicylates.
One useful family of compositions is of the formula
0
LorM
n(R) (
2
OH
where R is a hydrogen atom or an alkyl group having 1 to about 30 carbon
atoms, n is an integer from 1 to 4, and M is an alkaline earth metal.
Preferred R
groups are alkyl chains of at least C11, preferably C13 or greater. R may be
optionally substituted with substituents that do not interfere with the
detergent's
function. M is preferably, calcium, magnesium, or barium. More preferably, M
is calcium.
[0113] Hydrocarbyl-substituted salicylic acids may be prepared from phenols
by the Kolbe reaction. See USP 3,595,791, which is incorporated herein by
reference in its entirety, for additional information on synthesis of these
compounds. The metal salts of the hydrocarbyl-substituted salicylic acids may
be prepared by double decomposition of a metal salt in a polar solvent such as
water or alcohol.
[0114] Alkaline earth metal phosphates are also used as detergents.
[0115] Detergents may be simple detergents or what is known as hybrid or
complex detergents. The latter detergents can provide the properties of two
detergents without the need to blend separate materials. See USP 6,034,039 for
example.
[0116] Preferred detergents include calcium phenates, calcium sulfonates,
calcium salicylates, magnesium phenates, magnesium sulfonates, magnesium
salicylates and other related components (including borated detergents).
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-46-
Typically, the total detergent concentration is about 0.01 to about 6.0 wt%,
preferably, about 0.1 to 0.4 wt%.
Dispersant
[0117] During engine operation, oil-insoluble oxidation byproducts are
produced. Dispersants help keep these byproducts in solution, thus diminishing
their deposition on metal surfaces. Dispersants may be ashless or ash-forming
in
nature. Preferably, the dispersant is ashless. So called ashless dispersants
are
organic materials that form substantially no ash upon combustion. For example,
non-metal-containing or borated metal-free dispersants are considered ashless.
In contrast, metal-containing detergents discussed above form ash upon
combustion.
[0118] Suitable dispersants typically contain a polar group attached to a rela-
tively high molecular weight hydrocarbon chain. The polar group typically
contains at least one element of nitrogen, oxygen, or phosphorus. Typical
hydrocarbon chains contain 50 to 400 carbon atoms.
[0119] Chemically, many dispersants may be characterized as phenates,
sulfonates, sulfurized phenates, salicylates, naphthenates, stearates,
carbamates,
thiocarbamates, phosphorus derivatives. A particularly useful class of
dispersants are the alkenylsuccinic derivatives, typically produced by the
reaction of a long chain substituted alkenyl succinic compound, usually a
substituted succinic anhydride, with a polyhydroxy or polyamino compound.
The long chain group constituting the oleophilic portion of the molecule which
confers solubility in the oil, is normally a polyisobutylene group. Many
examples of this type of dispersant are well known commercially and in the
literature. Exemplary U.S. patents describing such dispersants are 3,172,892;
3,2145,707; 3,219,666; 3,316,177; 3,341,542; 3,444,170; 3,454,607; 3,541,012;
3,630,904; 3,632,511; 3,787,374 and 4,234,435. Other types of dispersant are
described in U.S. Pat. Nos. 3,036,003; 3,200,107; 3,254,025; 3,275,554;
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-47-
3,438,757; 3,454,555; 3,565,804; 3,413,347; 3,697,574; 3,725,277; 3,725,480;
3,726,882; 4,454,059; 3,329,658; 3,449,250; 3,519,565; 3,666,730; 3,687,849;
3,702,300; 4,100,082; 5,705,458. A further description of dispersants may be
found, for example, in European Patent Application No. 471 071, to which
reference is made for this purpose.
[0120] Hydrocarbyl-substituted succinic acid compounds are popular
dispersants. In particular, succinimide, succinate esters, or succinate ester
amides prepared by the reaction of a hydrocarbon-substituted succinic acid
compound preferably having at least 50 carbon atoms in the hydrocarbon
substituent, with at least one equivalent of an alkylene amine are
particularly
useful.
[0121] Succinimides are formed by the condensation reaction between alkenyl
succinic anhydrides and amines. Molar ratios can vary depending on the poly-
amine. For example, the molar ratio of alkenyl succinic anhydride to TEPA can
vary from about 1:1 to about 5:1. Representative examples are shown in U.S.
Patents 3,087,936; 3,172,892; 3,219,666; 3,272,746; 3,322,670; and 3,652,616,
3,948,800; and Canada Pat. No. 1,094,044.
[0122] Succinate esters are formed by the condensation reaction between
alkenyl succinic anhydrides and alcohols or polyols. Molar ratios can vary
depending on the alcohol or polyol used. For example, the condensation product
of an alkenyl succinic anhydride and pentaerythritol is a useful dispersant.
[0123] Succinate ester amides are formed by condensation reaction between
alkenyl succinic anhydrides and alkanol amines. For example, suitable alkanol
amines include ethoxylated polyalkylpolyamines, propoxylated polyalkylpoly-
amines and polyalkenylpolyamines such as polyethylene polyamines. One
example is propoxylated hexamethylenediamine. Representative examples are
shown in USP 4,426,305.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-48-
[0124] The molecular weight of the alkenyl succinic anhydrides used in the
preceding paragraphs will typically range between 800 and 2,500. The above
products can be post-reacted with various reagents such as sulfur, oxygen,
formaldehyde, carboxylic acids such as oleic acid, and boron compounds such as
borate esters or highly borated dispersants. The dispersants can be borated
with
from about 0.1 to about 5 moles of boron per mole of dispersant reaction
product.
[0125] Mannich base dispersants are made from the reaction of alkylphenols,
formaldehyde, and amines. See USP 4,767,551, which is incorporated herein by
reference. Process aids and catalysts, such as oleic acid and sulfonic acids,
can
also be part of the reaction mixture. Molecular weights of the alkylphenols
range from 800 to 2,500. Representative examples are shown in U.S. Patents
3,697,574; 3,703,536; 3,704,308; 3,751,365; 3,756,953; 3,798,165; and
3,803,039.
[0126] Typical high molecular weight aliphatic acid modified Mannich
condensation products useful in this invention can be prepared from high
molecular weight alkyl-substituted hydroxyaromatics or HN(R)2 group-
containing reactants.
[0127] Examples of high molecular weight alkyl-substituted hydroxyaromatic
compounds are polypropylphenol, polybutylphenol, and other polyalkylphenols.
These polyalkylphenols can be obtained by the alkylation, in the presence of
an
alkylating catalyst, such as BF3, of phenol with high molecular weight poly-
propylene, polybutylene, and other polyalkylene compounds to give alkyl
substituents on the benzene ring of phenol having an average 600-100,000
molecular weight.
[0128] Examples of HN(R)2 group-containing reactants are alkylene
polyamines, principally polyethylene polyamines. Other representative organic
compounds containing at least one HN(R)2 group suitable for use in the prepara-
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-49-
tion of Mannich condensation products are well known and include the mono-
and di-amino alkanes and their substituted analogs, e.g., ethylamine and
diethanol amine; aromatic diamines, e.g., phenylene diamine, diamino naphtha-
lenes; heterocyclic amines, e.g., morpholine, pyrrole, pyrrolidine, imidazole,
imidazolidine, and piperidine; melamine and their substituted analogs.
[0129] Examples of alkylene polyamide reactants include ethylenediamine,
diethylene triamine, triethylene tetraamine, tetraethylene pentaamine, penta-
ethylene hexamine, hexaethylene heptaamine, heptaethylene octaamine,
octaethylene nonaamine, nonaethylene decamine, and decaethylene undecamine
and mixture of such amines having nitrogen contents corresponding to the
alkylene polyamines, in the formula H2N-(Z-NH-)õH, mentioned before, Z is a
divalent ethylene and n is 1 to 10 of the foregoing formula. Corresponding
propylene polyamines such as propylene diamine and di-, tri-, tetra-, penta-
propylene tri-, tetra-, penta- and hexaamines are also suitable reactants. The
alkylene polyamines are usually obtained by the reaction of ammonia and dihalo
alkanes, such as dichloro alkanes. Thus the alkylene polyamines obtained from
the reaction of 2 to 11 moles of ammonia with 1 to 10 moles of dichloroalkanes
having 2 to 6 carbon atoms and the chlorines on different carbons are suitable
alkylene polyamine reactants.
[0130] Aldehyde reactants useful in the preparation of the high molecular
products useful in this invention include the aliphatic aldehydes such as
formaldehyde (also as paraformaldehyde and formalin), acetaldehyde and aldol
(0-hydroxybutyraldehyde). Formaldehyde or a formaldehyde-yielding reactant
is preferred.
[0131] Hydrocarbyl substituted amine ashless dispersant additives are well
known to one skilled in the art; see, for example, U.S. Patents 3,275,554;
3,438,757; 3,565,804; 3,755,433, 3,822,209, and 5,084,197, which are
incorporated herein in their entirety by reference.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-50-
[0132] Preferred dispersants include borated and non-borated succinimides,
including those derivatives from mono-succinimides, bis-succinimides, and/or
mixtures of mono- and bis-succinimides, wherein the hydrocarbyl succinimide is
derived from a hydrocarbylene group such as polyisobutylene having a Mn of
from about 500 to about 5000 or a mixture of such hydrocarbylene groups.
Other preferred dispersants include succinic acid-esters and amides,
alkylphenol-
polyamine-coupled Mannich adducts, their capped derivatives, and other related
components. Such additives may be used in an amount of about 0.1 to 20 wt%,
preferably about 0.1 to 8 wt%.
Optional Pour Point Depressants
101331 Conventional pour point depressants (also known as lube oil flow
improvers) may be added to the compositions of the present invention if
desired
to help meet MRV and/or yield stress targets. These pour point depressant may
be added to lubricating compositions of the present invention to lower the
minimum temperature at which the fluid will flow or can be poured. Examples
of suitable pour point depressants include alkylated naphthalenes polymeth-
acrylates, polyacrylates, polyarylamides, condensation products of
haloparaffin
waxes and aromatic compounds, vinyl carboxylate polymers, and terpolymers of
dialkylfumarates, vinyl esters of fatty acids and allyl vinyl ethers. USP Nos.
1,815,022; 2,015,748; 2,191,498; 2,387,501; 2,655, 479; 2,666,746; 2,721,877;
2.721,878; and 3,250,715 describe useful pour point depressants and/or the
preparation thereof. Such additives may be omitted totally or may be used in a
minor amount of about 0.001 to 0.1 wt% on an as-received basis.
Corrosion Inhibitors
[0134] Corrosion inhibitors are used to reduce the degradation of metallic
parts
that are in contact with the lubricating oil composition. Suitable corrosion
inhibitors include thiadiazoles. See, for example, USP Nos. 2,719,125;
2,719,126; and 3,087,932, which are incorporated herein by reference in their
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-51 -
entirety. Such additives may be used in an amount of about 0.01 to 5 wt%,
preferably about 0.01 to 1.5 wt%.
Seal Compatibility Additives
[0135] Seal compatibility agents help to swell elastomeric seals by causing a
chemical reaction in the fluid or physical change in the elastomer. Suitable
seal
compatibility agents for lubricating oils include organic phosphates, aromatic
esters, aromatic hydrocarbons, esters (butylbenzyl phthalate, for example),
and
polybutenyl succinic anhydride. Such additives may be used in an amount of
about 0.01 to 3 wt%, preferably about 0.01 to 2 wt%.
Anti-Foam Agents
[0136] Anti-foam agents may advantageously be added to lubricant composi-
tions. These agents retard the formation of stable foams. Silicones and
organic
polymers are typical anti-foam agents. For example, polysiloxanes, such as
silicon oil or polydimethyl siloxane, provide antifoam properties. Anti-foam
agents are commercially available and may be used in conventional minor
amounts along with other additives such as demulsifiers; usually the amount of
these additives combined is less than 1 percent and often less than 0.1
percent.
Inhibitors and Antirust Additives
[0137] Antirust additives (or corrosion inhibitors) are additives that protect
lubricated metal surfaces against chemical attack by water or other
contaminants. A wide variety of these are commercially available; they are
referred to in Klamann in Lubricants and Related Products, op cit.
[0138] One type of antirust additive is a polar compound that wets the metal
surface preferentially, protecting it with a film of oil. Another type of
antirust
additive absorbs water by incorporating it in a water-in-oil emulsion so that
only
the oil touches the metal surface. Yet another type of antirust additive
chemically adheres to the metal to produce a non-reactive surface. Examples of
suitable additives include zinc dithiophosphates, metal phenolates, basic
metal
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-52-
sulfonates, fatty acids and amines. Such additives may be used in an amount of
about 0.01 to 5 wt%, preferably about 0.01 to 1.5 wt%.
Friction Modifiers
[0139] A friction modifier is any material or materials that can alter the
coefficient of friction of a surface lubricated by any lubricant or fluid
containing
such material(s). Friction modifiers, also known as friction reducers, or
lubricity
agents or oiliness agents, and other such agents that change the ability of
base
oils, formulated lubricant compositions, or functional fluids, to modify the
coefficient of friction of a lubricated surface may be effectively used in
combination with the base oils or lubricant compositions of the present
invention
if desired. Friction modifiers that lower the coefficient of friction are
particularly advantageous in combination with the base oils and lube composi-
tions of this invention. Friction modifiers may include metal-containing
compounds or materials as well as ashless compounds or materials, or mixtures
thereof. Metal-containing friction modifiers may include metal salts or metal-
ligand complexes where the metals may include alkali, alkaline earth, or
transition group metals. Such metal-containing friction modifiers may also
have
low-ash characteristics. Transition metals may include Mo, Sb, Sn, Fe, Cu, Zn,
and others. Ligands may include hydrocarbyl derivative of alcohols, polyols,
glycerols, partial ester glycerols, thiols, carboxylates, carbamates,
thiocarba-
mates, dithiocarbamates, phosphates, thiophosphates, dithiophosphates, amides,
imides, amines, thiazoles, thiadiazoles, dithiazoles, diazoles, triazoles, and
other
polar molecular functional groups containing effective amounts of 0, N, S, or
P,
individually or in combination. In particular, Mo-containing compounds can be
particularly effective such as for example Mo-dithiocarbamates, Mo(DTC), Mo-
dithiophosphates, Mo(DTP), Mo-amines, Mo (Am), Mo-alcoholates, Mo-
alcohol-amides, etc. See USP 5,824,627; USP 6,232,276; USP 6,153,564;
USP 6,143,701; USP 6,110,878; USP 5,837,657; USP 6,010,987; USP
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-53-
5,906,968; USP 6,734,150; USP 6,730,638; USP 6,689,725; USP 6,569,820;
WO 99/66013; WO 99/47629; WO 98/26030.
[0140] Ashless friction modifiers may have also include lubricant materials
that
contain effective amounts of polar groups, for example, hydroxyl-containing
hydrocarbyl base oils, glycerides, partial glycerides, glyceride derivatives,
and
the like. Polar groups in friction modifiers may include hydrocarbyl groups
containing effective amounts of 0, N, S, or P, individually or in combination.
Other friction modifiers that may be particularly effective include, for
example,
salts (both ash-containing and ashless derivatives) of fatty acids, fatty
alcohols,
fatty amides, fatty esters, hydroxyl-containing carboxylates, and comparable
synthetic long-chain hydrocarbyl acids, alcohols, amides, esters, hydroxy
carboxylates, and the like. In some instances fatty organic acids, fatty
amines,
and sulfurized fatty acids may be used as suitable friction modifiers.
[0141] Useful concentrations of friction modifiers may range from about 0.01
wt% to 10-15 wt% or more, often with a preferred range of about 0.1 wt% to 5
wt%. Concentrations of molybdenum-containing materials are often described
in terms of Mo metal concentration. Advantageous concentrations of Mo may
range from about 10 ppm to 3000 ppm or more, and often with a preferred range
of about 20-2000 ppm, and in some instances a more preferred range of about
30-1000 ppm. Friction modifiers of all types may be used alone or in mixtures
with the materials of this invention. Often mixtures of two or more friction
modifiers, or mixtures of friction modifier(s) with alternate surface active
. material(s), are also desirable.
Typical Additive Amounts
[0142] When lubricating oil compositions contain one or more of the additives
discussed above, the additive(s) are blended into the composition in an amount
sufficient for it to perform its intended function. Typical amounts of such
additives useful in the present invention are shown in Table 1 below.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-54-
[0143] Note that many of the additives are shipped from the manufacturer and
used with a certain amount of base oil solvent in the formulation.
Accordingly,
the weight amounts in the table below, as well as other amounts mentioned in
this text unless otherwise indicated, are directed to the amount of active
ingredient (that is the non-solvent portion of the ingredient). The wt%
indicated
below are based on the total weight of the lubricating oil composition.
TABLE 1
Typical Amounts of Various Lubricant Oil Components
Approximate Approximate
Compound Wt% (Useful) Wt% (Preferred)
Detergent 0.01 - 6 0.01 - 4
Dispersant 0.1 - 20 0.1 - 8
Friction Reducer 0.01 - 5 0.01 - 1.5
Viscosity Improver 0.0 - 8 0.0 to 4, more
preferably 0.0 to 2
Antioxidant 0.0 - 5 0.0 - 1.5
Corrosion Inhibitor 0.01 - 5 0.01 - 1.5
Anti-wear Additive 0.01 - 6 0.01 - 4
Optional Pour Point Depressant 0 - 0.1 (as 0 - 0.05 (as received)
received)
Anti-foam Agent 0.001 - 3 0.001 - 0.15
Base Oil Balance Balance
EXAMPLES
[0144] In the following Examples, MRV was determined by ASTM D 4684,
CCS viscosity by ASTM D 5293, KV by ASTM D 445, pour point by ASTM D
97, and viscosity index by ASTM D 2270.
EXAMPLE 1
[0145] A solvent dewaxed base stock (A) was blended with varying amounts
(5 wt%, 15 wt% and 25 wt%) of a catalytically dewaxed base stock (B) and
formulated to a 5W-30 multi-grade lube oil composition using 10 wt% of a
commercially available GF-4 additive package. Base Stock A: a solvent
dewaxed base oil having a pour point of -18 C, a KV at 40 C of 20.76 mm2/s, a
KV at 100 C of 4.31 mm2/s. Base Stock B: a catalytically dewaxed base oil
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-55-
having a pour point of -18 C, a KV at 40 C of 31.99 mm2/s, a KV at 100 C of
5.58 mm2/s.
101461 The MRV at -35 C and CCS viscosity at -30 C of the formulated
5W-30 oils were measured.
[0147] For comparison a variety of solvent dewaxed base stocks of different
pour points and viscosities (base stocks A, C and D) were combined with 15
wt% of a second solvent dewaxed base stock (base stock E). The base stocks are
characterized as follows: Base Stock C: a solvent dewaxed base oil having a
pour point of -21 C, a KV at 40 C of 22.83 mm2/s, a KV at 100 C of 4.57
mm2/s; Base Stock D: a solvent dewaxed base oil characterized by a pour point
of -21 C, a KV at 40 C of 23.32 mm2/s, a KV at 100 C of 4.64 mmZ/s; Base
Stock E: a solvent dewaxed base oil having a pour point of -18 C, a KV at 40 C
of 34.87 mm2/s, a KV at 100 C of 5.91 mm2/s, all oil mixtures being formulated
with 10 wt% of the same commercially available GF-4 additive package.
[0148] Base stock B (catalytically dewaxed) and Base stock E (solvent
dewaxed) are analytically similar in terms of pour point (-18 C) and KV at 40
C
(31.9 mm2/s vs. 34.9 mm2/s) and KV at 100 C (5.58 mm2/s vs. 5.91 mm2/s).
[0149] Solvent dewaxed base stock E was added in an amount of about 15 wt%
to each of solvent dewaxed base stocks A, C and D, while 5 wt%, 15 wt% and
25 wt% of base stock B was added to Base stock A. The MRV and CCS
viscosity of all the above formulations results are plotted on Figure 1.
[0150] Only the combination of Base stock A (solvent dewaxed) plus Base
stock B (catalytically dewaxed) exhibited a superior MRV/CCS viscosity
relationship (MRV went down as CCS viscosity went up but stayed below the
5W-X CCS viscosity ceiling limit of 6600 cp @-30 C) whereas the other
combinations (solvent dewaxed base oils plus solvent dewaxed base oil E)
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-56-
exhibited significantly inferior MRV/CCS relationship (both MRV and CCS
viscosity went up).
[0151] Thus even addition of as little as 15% of catalytically dewaxed Base
stock (Base stock B) significantly and unexpectedly improved the MRV/CCS
viscosity relationship.
[0152] Some of the mixtures identified above (containing GF-4 additive
package) are reported in Table 2 which show that blending higher quantities of
catalytically dewaxed Base Stock B with solvent dewaxed Base Stock A resulted
in an increase in CCS viscosity (but still below maximum ceiling limit) but
resulted in further lowering of the MRV viscosity.
TABLE 2
Amount of Base Stock B in Base Stock 5 15 25
A (wt%)
MRV viscosity, cP at -35 C 24627 21268 19841
CCS viscosity, cP at -30 C 4720 4990 5240
[0153] Reference to Table 3 shows that when a commercial oil formulation
utilizing a single base oil having a KV at 100 C of 4.64 mm2/s is compared
against a formulated oil of the present invention made utilizing a mixture of
solvent dewaxed base oil (Base oil A) plus a catalytically dewaxed base oil
(Base oil B) blended to a KV at 100 C of 4.58 mm2/s (similar to the KV of the
commercial oil) both the MRV and the CCS viscosity are significantly
improved. While the high temperature viscometrics and VI are substantially
similar the formulated oil MRV and CCS viscosity are strikingly different.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-57-
TABLE 3
Commercial
Component (single base Invention Base stock Base stock
or base stock inspections oil * A er se B er se
KV at 40 C 23.31 22.94 20.76 28.94
KV at 100 C 4.64 4.58 4.3 5.28
VI 115 115 115 116
CCS at -25 C 1630 1550 N/A N/A
5W-30 MRV at -35 C, cP 29,075 19,841 N/A N/A
5W-30 CCS at -30 C, cP 5,650 5,240 N/A N/A
Pour point - 22 -18 - 18 - 18
Noack 15 15 16 14
* 25 wt% Base Stock B in Base Stock A (+ 10 wt% GF4 adpack)
EXAMPLE 2
(0154] In the absence of flow or pour point improver, preparing a base oil
from
a mixture of base stocks produced by different final wax processing routes or
one processed by a final wax processing route and a second produced by a
separate synthesis route (i.e., PAO) results in a decrease in MRV, while
blends
made to the same nominal high temperature viscosity targets but using two base
stocks made by the same final wax processing route failed to demonstrate this
improvement (see Table 4).
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-58-
~
N tn
cl% 00
"O 00
> N N ~ Itt
ci
> N ~
I:t N N
> N N
~c
vi o0
r-i
>
CG
ON
N
G1 00 N
,.., f~ p
N M
00 tf1 l-
l~ ~O N
pp ~ 00
-- N d ~ N
.-~ N M d
U
0
O 0 N_ y.~ N
vN vN w ~~ WN ~ ^ ~ =~ P4 UN~+
I--I ~ ~ Q (~ 0 0 00 ~ ~ 0 t!j ~ . ~" ¾ O 0 O 0 00 q
N .-~ ~ O bi) "al .~a ~a ~o N ~
--~ O aS ~ ~" o
~., v,. . Z ~ O. Z
~
U
W a. a C7 c~..~ C7 u, a
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-59-
c~
~ o
~ o
~ O N V N
kn ~z ~
`~ O
ptnp U C/]
~ O M ~O v' '~" N
i =-+ M ~ ~ i W) N Z ~--~
'G
~ pN
O O V CS
09
O N
N ~
c~
O
O v N O
~
ON
O Q1 V [- tzf:, O N
01 00 p U
~ N O
00
O O ~ ~ ~ O 110
co O N
M ON ~--~
cd
=--~ Cp
N
N Nc's O'y" N
~
cd
~O O ~O Ou r--O
qlt
O ~O M v ~ N
M N V \~o r-- V
a~
18
00 cn o ~ o
'4p l- N N
~ ~--~ M N Z
0
t8 O
~
00 kn p v
V C1 ~ =--~
M M V
~
'U U oo oo
o
N~'; v~ H ry CA F-+ U1 cn ~n H p~ rn
o ~`d o Q
+ ~.+' A M ~ y =..~" M ~ ~+ ~ .~ ~i
o a Uvo v~o ~lF-~- ~ Q
~~ ` UNi
UUMA~~'~, Z
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-60-
[0155] Table 4 shows the results for GTL base stocks and slack wax
hydrodewaxate base stocks as well as for Group III base stock (NEXBASE
which are hydrodewaxed waxy oil stock made via a process which employs a
different catalyst than that used to make the GTL base stock or slack wax
hydrodewaxate base stocks). All oils in Table 4 are formulated oils containing
substantially the same amounts of an additive package but no flow improver or
pour point depressant.
[0156] Formulated oils I-IV met the same KV at 100 C target of about 10.7
mm2/s, and exhibited similar CCS viscosities at -30 C within the limits of the
repeatability of the test.
[0157] At -35 C, in the absence of low temperature flow improver/pour point
depressant, a mixture of 2 GTL stocks each made by the same hydrodewaxing '
technique, Formulation 1, passed MRV with respect to viscosity, but failed
with
very high yield stress. Replacing the GTL 6 with Nexbase 3060 (hydrodewaxed
waxy oil made by a hydrodewaxing process which employs a different catalyst
than that used to make the GTL stocks (Formulation II)) reduced MRV at -35 C
to 22,962 cP (about 15,000 cP lower than in Formulation I), and also reduced
the
yield stress to the point of NYS (no yield stress), indicating that the
formulation
would meet the target properties for a 5W-30 oil. Replacement of GTL 6 with
Nexbase 3050 (Formulation III) also reduced the MRV at -35 C as well as
significantly reducing yield stress resulting in an oil formulation which
would
meet 5W-30 specifications with the addition of minimal pour point depressant
(to address yield stress).
[0158] At -40 C, in the absence of low temperature flow improver/pour point
depressant, Formulation 1 failed both MRV and yield stress. Replacing GTL 6
with Nexbase 3060 or 3050 (Formulations II and III) reduces the MRV by more
than half, but yield stress remains high for a OW-X grade formulation.
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-61-
[0159] Formulated oils V to IX met the same KV at 100 C target of about 10.8
mm2/s.
[0160] At -40 C, Formulation V (mixture of two hydrodewaxed slack wax
base oils produced by the same hydrodewaxing process (but of different
viscosities)) failed MRV and yield stress by such wide margins that it would
not
be expected that the formulation could be made to meet MRV or yield stress
target specification by the additive of any amount of a PPD. Replacing the
heavier hydrodewaxed slack wax base oil with Nexbase 3060 (oil made by a
hydrodewaxing process which employs a different catalyst than that used to
make the hydrodewaxed slack wax base oil) (Formulation VI) significantly
reduces MRV and yield stress which while just missing the OW target specifica-
tion for MRV and yield stress would be expected to be brought into
specification
by the addition of a minimal amount of pour point depressant. Formulation VI
would be expected to meet the less severe 5W specification for MRV and yield
stress without the addition of any pour point depressant. Substituting Nexbase
3050 for Nexbase 3060 (Formulation VII) further reduces MRV and yield stress,
yielding a formulation easily meeting OW and implicitly also the less
demanding
5W specifications.
[0161] Formulations utilizing mixtures of base oils produced by different
final
wax processing routes exhibit MRV, CCS and yield stress characteristics
approaching those exhibited by pure PAO based formulations (Formulation IX)
or those containing PAO as a component (Formulation IV and VIII).
[0162] Thus it is seen that formulated oils meeting the MRV and CCS
viscosity targets of SAE OW-X or 5W-X multi grade engine oils can be
produced by using two oils of the same or similar viscosity produced by
different final wax producing techniques (Formulations II, III, VI, VII)
without
the addition of a PPD or which came close enough to the target MRV and CCS
viscosity targets of SAE OW-X or 5W-X multi grade engine oils (evidenced by a
CA 02667224 2009-04-22
WO 2008/057250 PCT/US2007/022641
-62-
significant reduction in MRV and almost passing the yield stress target), that
the
addition of a minimal amount of PPD would bring the formulation into
specification. Such formulation approach the performance of formulations
containing PAO (pour point <-50 C) (formulations IV, VIII and IX) without
resort to such synthetic oils.
101631 These results are unexpected in view of the calculated base oil
physical
properties for the mixtures of base stocks used in the above blends but in the
absence of additives, Table 5.
TABLE 5
Blends I II III IV V VI VII VIII IX
KV at 100 C 4.82 4.34 4.25 4.45 4.83 4.49 4.44 4.57 4.46
mm2/s
CCS at-35 C 2340 2340 2320 2340 2580 2400 2530 2390 2020
Noack % 11 12 12 12 12.4 12.4 12.4 12.4 12.4