Note: Descriptions are shown in the official language in which they were submitted.
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
PROCESS AND REACTOR FOR UPGRADING HEAVY HYDROCARBON
OILS
FIELD OF THE INVENTION
The present invention relates to upgrading of hydrocarbons, especially heavy
hydrocarbons such as whole heavy oil, bitumen, and the like using
supercritical water.
BACKGROUND OF THE INVENTION
Oil produced from a significant number of oil reserves around the world is
simply too heavy to flow under ambierit conditions. This makes it challenging
to bring remote, heavy oil resources closer to the markets. One typrcal
example is the Namaca field in Veriezueld, In order to render sucfi heavy oils
flowab!e; one of the most commasi methods known in the art is to fedr,rce the
viscosity and density by mixing the heavy oil with a sEifficient diluent. T1le
diluent may be naphtha, or ariy other stream with a significantly higher API
gravity (i.e., much lower density) than the heavy oil.
For a case such as Hamaca, diluted crude oil is sent from the production
wellhead via pipeline to an upgrading facility. T4vo key operatiaris occur at
the
upgrading facility: (1) the diluent stream is recovered and recycled back to
the
presductinn weliliead in a separate pipeline, and (2) the heavy oil is
upgraded
with suitable technology knnwn in the art (coking, hydrocracking,
hydrotreating, etc,) to produce higher-value psnciucts for martcet. Some
typical
characteristics of these higher-valu>~ products inc.lude: lower sulfur
content,
lower r-netals content, lower total acid nucnher {TANI; lower residuum
content,
higher API gravity, and lower viscosity, Most of these desirable
characteristics are achieved by reacti.ng the heavy oil with hydrogen gas at
high temperatures arrd pressures in the presence of a catalyst, In the case of
t-iamaca, the upgraded crude is sent further to the end==users via tankers.
t
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
These diluent addition / removal processes and hydr'ogen-addition or other
r,ipgrading processes have a number of disadvar3tages;
1, The infrastructure required for the handling, recovery, and recycle of
c3iluent: coEild be expensive, espocially over long distances. Diluent
availability
is another potential issue.
2. Hydrogon-aadit#on processes such as hydrotreating or hydrocracking
requira signi#ic;ant investments iÃi capital and infrastrtÃctorp..
3. Hydrogen-artdition processes also have high operating costs, since
hydrogen produc;tion costs are highly sensitive to natural gas prices. Some
remate heavy oil reserves may not even have access to sufficient quantities of
low-cost natural gas to support a hydrogen plant. These hydrogen-actditaorÃ
processes also generally require expensive catalysts and resource intensive
catalyst handling techniques, including catalyst regenoratibr-.
4, in soÃiie cases, the refineries and/c,r upgrading facilities that are
located closest to the production site rnay have neither the capacity nor the
facilities to accept the heavy oil.
5. Coking is often used at refineries or upgrading facilities. Significant
amounts of by-proctuct solid coke are rejected during the coking process.,
leading to lower liquid hydrocarbon yielr.~, In addition, the liquid products
from
a coking plant rafteri need further hydrotreating. Further, the volume of the
product froryi the coking process is significantly less ttian the volume of
the
feeci crude oil.
A process according to the present invention overcomes these disadvantages
by using supercritical water to upgrade a heavy hydrocarbon feedstock into an
upgraded hydrocarbon product or syncrude with hiqhly desirable properties
(IOW sultur content, low metals content, lower density (higlier API), lower
viscosity, lower residUrrÃM conterit, etc.). The process nei:ther requires
exierna;
supply of hydrc:gen nor niust it tise catalysts. Further, the process in the
t,resent invention does not prcriL,ce at-i appreciable coke tsy-pÃbduc.t.
In comparison with the traditional processes for sy crride prractuctinn,
advantages that Ãyicay be obtamed by the pra4tice of the pre5ent invention
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
include a 'iigh iiquic# hydrocarbon yieid; no r-reed for extc-rnally-5upp iied
hycirogen; no need to provide catalyst; significant increases in API gravity
in
the upgraded hydrocarbon praduct, significar-it viscosity reduction in the
upgraded hydrocarbon product; and significant reduction in sulfur, metals,
nitrogen, TAN, and MCR (micro-carbon residuei in the upgraded hydrocarbon
product.
1larirris methods of treating heavy hydrocarbons using supercwrt:iz.al water
are
disclosed in the patent literature_ Examples include U.S. Patent Nc.~s.
3,948,754,
3,948 fi5, 3,960,706, 3,983,027, 3,988,238, 3,98,-~~,61t3, 4,005,005,
4,151:068;
4,55-7,820, 4,559,127, 4,594,141, 4,840;725, 555,611,915, 5;914,(}31 and
6,887.369 and EP671454.
U.S. Patent No 4.840,725 discloses a process for conversion of high boiling
Iiquid orgaÃiic materials to lower boiling materials using supercritical water
in a
tuhular, craritiriuous reactor. The wate= and tiydrocarbtaÃ) are separately
preheated and mixed in a high-}aressure feed pump just before being fed to
the reactor.
U.S, Patent No. 5,914,Ã331 discloses a three zone reactor design so that the
reactant activity, reactant solubility and phase separation of products can be
optimized separately by controlling temperature and pressiare. However, all
the examples gÃveÃf ir, the pafent were otataiÃiec.f using taat:;tt opeÃ-
ation.
U.S. Patent No, 6,887.369 discloses a super'critÃcal water pretreatment
process using hydrogen or carbon monoxide preferably carried oÃit in a deep
well reactor to hydrotreat and hydrocrack carbonaceous matersal. The deep
well reactor is adapted frcrM underground oil wells, and consists of multiple,
concentric tubes. The deop well reactor described in the patent is operated
by intrvducing feed streams ir) the core tubes and returning reactor effluent
in
the outer annular section.
iL CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
Although ttie above--rrierrtiarrecl patents disclosed and claimed var;or,is
methods and processes for heavy aii upgrading using sL,percritical Water,
such as operatirig range of temperature and pro-ssure, water to oil ratio,
etc,
none has disclased the design of the r'eactar or design related process
controls for heavy o;l upgrading tasing, supercritical water. In fact, most of
the
c-xamp{es disclosed in the patents were obtained through batch tp-sts using an
aLÃtociave. Although there are nurnerous referenep-s to reactor design for
processes inv::)lvÃrrg supercrit'ical water, most of thern are for the
appiiration of
wastta tre'-atmer}t and none of those references has addressed tht-, design of
a
reactor for both heavy oil and sLipercrltiral water, wn-eh is tLartdamentally
different from processes of waste treatment using supercritical wafer, as
discussed below.
It has long beeri known in the art. that sUpercritical water can be used for
waste treatriient, especially for treafiirrcd wastewater ccaÃitaiÃiing organic
contarninants. 1-1-ierefOre, there are Ãiurfleraus disclosures in the
literature or)
reactor design for waste treatrT3ent using supercritical water, tended to
address the following issues:
(1) Solid handling. Waste streams typically contain both organic
and inorganic rtiaferials. Although organic materials can be destroyed
quickly through 5Lrperc:riticaI water oxidation, inorganic materials are
insoluble in supercritical water, Several patents address this cot7cerrr.
For example, U.S. Patent Nos. 5;560;823 and 5,567,698 incorporated
by reference hereiri disclose a reversible flcw reactor having two
reaction zones whir:,h are alterrrately i:ised ¾csr sÃ.Ãpercritit:.al water
oxidation while the remaining r~actieti zone is flushed with, subcritical
efflueÃit from the active reactiori zOrie. U.S. Patent IdO. 6,264;844,
incorporated by refer-ence herein, discloses a tubular reactor for
sUpert;ritiGal ~J~11~~~~oXidatiC3r1. Tf=1e velocity of the recactii:>ri
ITl1xtur~.' is
sufficient to prevent settling of S6id, Inorganic salts in th~.= effiLierit
Ã7iixture: which are insoluble at corid'Ãtioris of superC.-Ãilical temperature
and pressure for water, are dissolved in a liqE? ic3 water phase durii1g
cooling dowvri of the effluent mixture at an outlet end of the reactor.
4
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
(2) Oxidizer management. tJ.S: Patent Nos. 5,384,031 and
5,558: 783, incerporated by reference herein, disclose a reactor design
for supercritical wastewater exidation. It contains a reaction zone inside
the containment vessel and apÃ.~rmeable liner arOLÃnd the reaction
zone. An oxidizer is mixed with a carrier fluid such as water. The
mixtfire is heated and pressurized to supert,ritical conditions, and then
introduced to the reaction zc+r?e qradr^Ãallv and uniformly by forcing it
rad:ally inward through the permeable liner and toward the reaction
zone. The tsernieable liner permits the continuOUs, gra'dual, untforr7i
dispersion of a reactant and therefore promotes an even and efficient
reaction. The liiier also isolates the pressure vessel from high
temperature and oxidizing conditions found in the reaction zone,
allowing a redtiction in cost of the pressure vessei, EP 1489046
discloses a deuWe-uessel design with a reaction vessel placed inside a
pressÃire vessel. Reaction takes plaw, inside the reactrr vessel at high
temperature, pr-essLire and corrosive envircno-nerits. The euter pressure
vessel will only see water.
(3) Containment of toxic material. Snrne waste streanR eontains
contaminants that are extremely harmful to humans and the
environment, therefore the possibility of releasing cf srJch harmful
ttiaterial has to t3e addressed in the reactor design. U.S. f'atent No.
6,168;771, incorporated by reference- herein, discloses a reactor design
inCludirig an autoclave inside a pressure vessel. Ttie ~.~ressi^Ire hetweeÃl
ar.rtOel~ve. and pressure vessel is essentially equal to that iriside the
autoclave, therefore el:rÃ7ir?ating possible leaking of toxic Ãnaterial inside
the autcclave.
Aftheugh heavy oil upgrading using su~,>ereritieal water Ãiiay be eOnsictesed
similar tn some respects to waste treatriient using supercritical water, and
can
be implemented using various elements of reactors designed for waste
3 5 treatrnent, there are significant differences in requirerment for reactor
design
for 1iekivy hydrcicarbr,ri upgrading frorni that for waste treatr-F-rent.
Spee.itiL,ally;
the following are among the many issues to be addressed in rJesigr>ing a
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
Ci reactor in which to conduct an effective process for heavy oil upgrading
usincw,
superesrÃtica3 water:
(1) Importance of selectivity, For waste treatment, the or31y
performance target is conversion. In other words, the reaction is rrari-
selective total oxidation and there. :s no need to wcrTy abosrrt selectivity,
which makes the reactor design r:nurh easier. For heavy oi( upgrading,
the feed is a mixture containing broad range of riiatarials; and the
reactions involved are niueh more complex. We need not only to
cansider conversion, brrrt a?so more irnportant[y to purstre high
i5 selectivity, since non-selective reactions will lead to low-value
byproducts such as solid coke or gases. Obviously, reactor design for
seiectfve reactions in acoxnple.c system is very different and much
more challenging than that for non-selective total oxidation.
(2) High concentration of feer.~. Typically the organic c:oriiparierit
concentration in the waste stream is low, artd in many situations the
c:onceritrr^ation is only in the ppm range. For oil upgrading, it is
preferable to run the reaction using the lowest possible water to oil ratio
to reduc;Ln capital and operating cost. 'l~'he oil conc,entration is typicaliy
several orciers of magnitude hRgher iri upgrading as opposed to waste
treatment.
(3) Fiigh density and viscosity, One distinguishing feature of heavy
oil is high density and viscosity. ir} fact, this is one of the primar}r
reasons that the oil has to be rapgraded. The density of heavy oil is very
close to liquid water, and viscosity can be as high as 10,000 cp. High
density and viscosity, together with higf; concentration make the
dispersion of heavy oil into supercritical water an important
consideration.
6
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
SUMMARY OF THE INVENTION
'Tlse present invention relates to a process for upgrading hydrocarbons
cornprisisig: riiixinc.~ hydrocarbons with a fluid comprising water tilat has
beetR
heated to a temperature higher than its critical temperature in a mixing zone
under conditions that disfavor therr-nal cracking and formation of coke to
form
a =riixture; passing the mixture to a reaction zone; reacting the ry'rixturp-
in the
rpat;tion zonp- having a srabstantially uniform temperature distribution and
being configLÃred to reduce the settling of solids within the reaction zone
said
reaction occurring under supercritical water conditions in the absence of
externally added hydrogen for a residetice time controlled within determined
limits to allow upgrading reactions to occur; vvithdrawing a sir3gie-phase
reaction product from the reaction zane; and separating the reaction product
into gas; effluent water, and upgraded tiydrocarbon phases.
BRIEF DESCRIPTION OF THE I:?F~AWiNGS
Fig, 1 is a process flow diagram of one embodfr3':ent of the present invention
Fig. 2 is apr-oc;ess flow diagram ot another pr7ik.}odiment of the present
invention.
Fig. 3 is a process flow diagram of another erribadirr~ent of the present
invention.
t= ig. 4 is aproeess flQw diagram of another embodiment of th~.~ present
inventiOrt.
Fig. 5 is a process flow diagraryt Gf another eMb0dirfleni L}f the t.<r-esent.
inLrenttOn,
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
ClESCfiIP'TlC)N Ot= 'T"HE PREFERRED EMBODIMENTS
Reactants
Water and hydrocarbons, preferably heavy hydrocarbons are the two
reactants employed in a process according to the present invention.
Any hydrocarbon can t3e slritahly upgraded by a prore.ss according to the
present invr`:ntinn; Preferred are heavy hydrocarbons having an API gravity of
less than 20". At1iang the preferred heavy hydrocarbons are heavy crude oil,
heavy hydrecancons extracted frem tar sands, commonly called tar sand
bitLirrsen, such as Athabasca tar sand bitLrMen nbta?ned from Canada, heavy
petrolrvzrti) crude oils such as Venezuelan Orinoco heavy oil belt crudes
Boscan heavy oil, heavy hydrocarbon fractions obtained from crude petrr;leurri
oils particularly heavy vacLrum gas oils, vacuum residuum as well as
petroleurn tar, tar sarids and coal tar. Other exarTip#es of heavy hydrocarbon
feedstocks which can be ctsed are oil shaie, shale oii, and asphaltenes.
t4'ater
Any source of water may be used in the tiLrid pompt-ising water in practicir-
ig
the present inventiorr. Sources of water include but are not limited to
drinking
water, treated or untreated wastewater, river water, lake water, seawater,
produced water or the like.
x1r.i(irfg
In accordance with the invention, the heavy },yd=ocarbon feed and a fluid
comprising water ttiat has been heateit to a ter~ipera#ure higher than its
critical
temperature are, contacted in a mixing zone prior to entering the reaction
zone. In accordance with the :nventÃcr, mixing ttlatyr he a.^.cctlipirshed in
many
ways and is preferably accomplished by a~echnique that does not employ
rTreihanical movirig parts. Stict) 1?nearrs of rnixing rinay include, hfat are
riot
limited tc, use of static mixers, spray nezzles, sonic or ultrasonic
agitation.
"t'he cil and Vv-at.er si-ictffd be heate,t andmixed so that the comt,itie'=d
sfrea:11
will readi supercritical water vcnditions in the reaction zone.
8
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
The oil aÃid wateÃ- should be f-Ãeated and rYiixect so ttiat the combined
strearri
will reach supercritical conditions in the reaction zone.
It was found that by avoiding excessive heating of ttÃe feed oil, the
formation
of byproduct 5uch as solid residues is redricec3 significantly. One aspect of
this
invention is to employ a heating sequence so ttiat the tegliperatLjre and
pressure of the hydrocarbons and water will reach supercritical reaction
conditions in a controlled manner. This will avoid excessive local heating of
oil, which will lead to solid formation and lower ttÃjality product. In order
to
achieve better perfermance, the oil should only be heated iÃp with
sÃrÃffÃeient
am0Ãint of water present and araÃ.Ãrrct the hydrocarbon molecules. This
requirement can be met by mixing aill. with water trefora heatirÃg.
1n one embodiment of the. present invention, water is heated to a temperature
higher than its t;ritÃcal temperatLÃre, and then mixed with oil. The
temperature
of heavy oil feed sh0uÃd be kept in the range of ai;oLrt 104'C to 200"C to
avoid
thermal crackÃEig but still higii eriaugh to ma(ntairi a reasonable pressure
drop.
The water strearn temperature should be high enough to make sure that after
mixing with oil, the tcrnpGrat:are of the oi!-water iiiixtrrr-e is still
higher than the
water supercritical temperattÃre, In this embodiment, the oil is actually
heated
up by water. An abÃindance of water molecules surrounding the nydrocart;on
molecules will significantly st,tapress conderisation reactions aÃld therefore
reduce formation of coke and solid product.
The- required temperature of the sripercriticai water strearr=, T,c.,,v, Can
be
estimated based on reaction teri:ÃpPratlÃre, TR, and water to oil ratio. Since
the
haat capacity of water changes significantly in the range riear its T.:ritical
conditions, for a given reaction t.emperatÃire, the required temperature for
the
supercritical water strearyi iricreases almost exponentially with decreasing
w:ater-to-oil ratio. The lower the water-to-oil ratio, the higher the T; c~v,:
`T-he
relat.ionship, howeve:~, is very riorilirlear 5iriurv higher T,~cw leads to a
lower
heat capauitR+ (far away from the critical po:nt).
9
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
U Ir) another etTibndimwnt, water is riealed up to srrrpercr"Ãtical
c;oriditÃorls. TherI
the supercrit;c:ai water mixed with heavy o=1 feed in a mixer. `T'he
temperature
of heavy oil teed should be ~ept in the range of about 100 0 to 200,,C to
avoid
thermal cracking but st>II high enough to maintain reasonable pressure drr3p.
After mixir3g with heavy oil, the temperature of the water-cail mixtcire
worild be
lower ttian critical temperatLÃre of water: therefore a second heater is
needed
to raise the temperature of the mixture stream to abave the critical
temperature of water. In this embodiment, the heavy nil is first partially
heated
up by water, and then the water-oÃI mÃxttjre is heated to sr3percrrtic,al
conditions by the second heater.
Other methods of mixing and heating sequences based on the above
teachings may be Lrsed to accomplish these objectives as will be recognized
by those skilled in the art.
.r i eaÃ: t1of? c vr-idltrvri s
After the reactants have been mixed, they are passed into a reaction zatae in
whit;h they are allowed to react under temperature and pressure conditions of
supercritÃcai water, i.e, supercritical water conditions, in the absence of
externally added hydrogen, for a residerice time sufficietit to allow
r.rpgrading
reactions to occur. The reactirari is preferably allowed to occur in the
absence
of externally added catalysts or prometers, although tiie use of such
catalysts
ar-id promoters is permissible in accordance with the present invention.
"Hydre:gerr" as used herein in the phrase, "in the absence of externally added
hydrogen" riieans hydregeti gas. This phrase is not iritended to exelude all
sources of hydrogen that are available as reactants. Other nicrlecules such as
saturated hydrecarboris may act as a hydrngeti saLrrce during the reactiot) by
ciurrating hydroger) to other unsaturated hydrocarbons. In addÃtioti; H2 rnay
be
formed in-sitif during the reaction through steam reforming of hydrocarbons
and water-gaa-siiift r'eactiori,
t4l
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
'The reaction zone preferably c=cmprises a reactor, whiah is equipped with a
means for collecting the reaction products (syncrude, water, and gases), and
a section, preferably at the bottom, where any metals or solids (,the "dreg
stream") may accumulate:
Supercritical water conditions include a temperature from 374`C: (the critical
temperature of water) to 1 0OO C, preferably from 374T to 600 G and mo5t
preferably from 374`C to 400''C, apressure from 3,205 (the r,ritiral pressure
of water) to 10,000 psia, preferably from 3,205 psia to 7,200 psia and most
preferably from 3,205 to 4,000 psia, an oil/kvater volume ratia from 1:0. 1 to
1 : 10, preferably from `i : 0.5 to 1:3 and most preferably about 1:1 to 1:2.
The reactants are allowed to react under these conditions for a suificient
time
to allow upgrading reactions to occur. Preferably, the residence time will be
selected to allow the upgrading reactions to occur selectively and to the
fullest
extent witi,Qut having undesirable side rea:,t#oris of c-oking or residue
formatiori. Reactor residence times may be from 1 minute to 6 hours,
preferably from 8 minrites to 2 hours and most preferably fror7i 20 to 40
minutes.
T'he f?e-at:ter-
A reactor desigried for heavy oil upyracliny using supercritical water in
accordance with the prtasent inventinn wÃII preferably include the following
fi~atu1'es `
The reactor wi#i have means for adequate oil-water r3iÃxing and dzspersiQn.
Contrary to the conventional thermal cracking in an uncontrolled fashion that
will lead to excPssi've formation of light hydrocarbon arid therefore lower
liquid
tiydr'ocark,en yield at the temperature and presszare i.inder supereritical
water
Gcinditions, heavy hydrocarbons will hydrothermally rrack irito lighter
corrjponents. Furthermore, hydrac,arbore radicals formed from therma[
cracking will also recombine and polymerize and eventually beccririe coke.
Wa;er r110l=11e5, especially under super{:,ritical' conditions, can quench and
11
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
stabilize hydrocarbon radicals and therefore prevent them from over cracking
aÃ>d 1?aiymÃ.arizatien: To avoid over cracking into light hydrocarbons and
coke
formation, the heavy hydrocarbon molecules are preferably sr.rrrourlded by
water molecules to the greatest practical ex#cnt. Therefore, the reactor
includes means to assure adeqLrate mixing of oil with vvatef for the ptii'pose
of
achieving a high yield of liquid hydrocarbons. Such means should be cheseÃ3
so as to be able to haÃidle heavy oil feed which has low API gravity and h'igh
viscosity at high oil to water ratio. Depending on specific applications such
rrreans car: include, among others, (a) rÃozzles; (b) static mixer; (c)
stirring
vessel; (d) micre-uharrnel device; and sonic arid ultrasonic devic:e.
The reac;tiOri zone =r accordance with the present invention will preferably:
(1) Provide an appropriate residence time to achieve high
conversion arid liquid yield. Controlling the residence time narrowly
within determined lirnits is a veÃy impertant factoÃ- #or heavy oil
upgrading usiny supercritical water. The desired products of heavy oil
upgrading are liquid hydrocarbons. IrÃsLÃfficie.nt ÃesideÃice tiriie will lead
to low conversion aÃid hence low liquid hydrocarbon yield. CJÃi the other
h~.~rid, excess ccrrversican will lead to low value by proctLÃe;ts such as
light hydrocarbon gas and coke. In order to achieve highly selective
~5
conversion to liqtÃid hydrocarbons, it is critical to maintain adequate
residence time.
(2~ Provide suffiuieÃ'rt iieat tra#3sfer rate to maintain unrforÃii
temperature distributiori. In s~:Omparing other supercritical water
applications, heavy oil is a much more complicated feed and heavy oil
upgradirrg is avenyeomplex precess, In addition, as indicated ahove,
the desired lia id hydrocarbon is ar: intermediate product from
selective, partial reaction. Therefore., it is extremely important to ccEitrol
reaction temperature to achieve high liquid hydrocarbon yield.
A>r#ettuate control of reactioÃi terriperature cati be achieved by providirng
enough heat trai-asfer area, uniform feed distrÃbution; or by quenching.
(3) Be able to handle ,dlid'. formed dLIring the reacticn. DfirirÃg the
reaction, smal: aÃYzounts of solid byproducts, primarify inoÃ-ganic
i ::`
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
15 rnaterials ,rrretals, sulfL#r: coke e.~tr;.), will be formed, and the
reactÃcrl
zone must be able to handle sLsch sc.~licl!s so they will rroi cause
operating problems and will iirat contaminate the liquid hydrocarbon
pÃodLfct.
The present rnvefitr,n also esiiploys a separation zone for product recevery.
The effluent strearra from the reaction zone c:oÃitairis liqLsid
hy:.irc:cark.on,
product, gas, water under supercritical conditions and solids. The liquid
hydrocarbons are generally separated from other cUri-iponents to achieve high
yield. The preferred way is to rc-rrÃave the solid first, and then bring the
fluid
phase containit?g hydrocarbon products, supercritica= water and gas
byprodur=ts out ef supercritical condition by laxvving temperature, pressure
or
both so that liydrocarbon product and water will condense into liquid phase.
The solids are pr'imarily inorgariic materials iorrned during the reactions
and
õant<e separ-ated from the superr,ritical f1cÃid phase using sr;:parat=ori
techniques kr3evura in the art, which could hea. disengaging zorie in the
reactor
or a separate device such as settling vessel, filter, cyclone ete;.
Another optÃon for separating the solids is to bring the prodt:ict stream out
of
supercritical regisiie by lowing temperature or pressure or bath. Then the
solid
will precipitate, A potential disadvantage of this optiori is that some of the
inorganic components in the solid may dissolve in water, which may
coritaÃi7ir3ate ttre liquid hyldroc;arbon pÃ-ottuct. It should Eae noted that
depending on the specific applications, a reactor for heavy oil upgrading
using
supercritical water it} accordance with the present iriventieri Ãiiayf have
rilere
than one of each of the three components listed above.
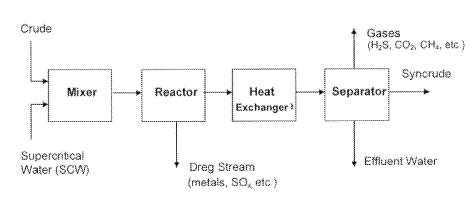
t=;gure 1shows an erTita:.}diryient of the present invention, which has been
used
in a ;aboratary. An iniirie mixer is used for r'nixing heavy oil with water.
For
th'ss specific errÃbodÃrÃier-Ãt it is a static mixer. The reaction zoÃie
compr:sesa
3 5 spiral tube react-or with large length to diarreter rat;o to attain high
velocity
iriside t.he reactor, which is he;ipful to m aintain oi:-water dispersion.
This
design also makes the fluid flow irrside the reer.tor elose to plug flow and
.!:.~
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
tl-teÃ-efoÃe acttieves narrow residence tiriie dÃstribLÃtiOÃl for selective
coÃivers-cÃ-s
to desired. Eiquid hydrocarbons. Inorganic solids in the feed and forrrred
during
the reaction will not dissolve in supercritical water. Ã-tigh velocity
insidc:~ the
reactor also prevents settling of those inorganic solids. The smai: diameter
of
the reactor body also provides large spec,ific sur-face area for heat transfer
to
maintain uniform temperature cfistrit;utien iÃiside the reactor. The length of
the
re.aetor can be designed based on residence tinie needed for specific
conversion. A second vessel is added to sett?e the salid5: The temperature
a.rÃd pressure is maintained at the same values as those in the spiral tube so
that the fluid in the second vessel is still at supercritical water
conditions. Due
to the larger cross-sectional area of the second vessel the flLÃid velocity is
riiuch iower. As a result, inorganic materials separated from the fluid will
settle
down in the vessel, and can be remaver.~ from the systerti. Thc- fluid
caritaining
hydrocarbon products, si.rpercritical water and gas t?yprodÃicta is cooled
while
maintaining at the same pressure as in the reactor, and fiydtOcarborr
procitacfs
and water are condensed in the liigti pressure separator.
A spiral tube with. a high length to diarneter, ratio, which may be frorn 50
to
10,000, preferably frcÃii 100 to 4,000 may be used as reactor body. Use of
such a reactor lias the advantages of high velocity, nLirraw residence time
;.tistribLÃtivn, and large surtace for heat transfer. The length to diamet=er
ratio is
a useful paranieter to determine preferred reactor LonttqÃar=atinr3s. The
diarYie=ter may be cteterrriiraed by velocity needed to avoid solids
ptecipitation
and then the length can be seIected to provit;t~~ the desired residence time.
Otlier reactor carrfiguratirans knnwri to those m the art can be used to
achieve
similar effects, sÃ.rch as a serpentine reactor.
i;n the errat.~odiments strow=t irà Figure 1 the separation zarir for
rernovir:q solid
and recovering hydrocarbon products is a vessel with a dip tube. Ut--rer fluid
-
solid separation devices known in tho- art can be used to achieve the
separation effect, which includes, but not limited to, cyclone, filter,
ceramic
membrane, settling tank, etc.
14
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
Iri the emk,o,iitnerit shown iri t"ic~~re- 1. as well as ir) other embodiments
described herein, the mixer, reaction and separation zones are separated.
Such arrangement is conver?ient for (ataoratory research, and is cased as an
illustrative example. It is within the scope of the prp-sent invent;ort and in
serne
applications will be beneficial to integrate these three functions into one
vessel.
As mentioned above, the reaetor may include more than one piece of each
furiction devices. Figure 2 shows an exaxmple. In order to avoid over cracking
of the feed to form uridesired byproducts such as light hydrocarbon gases and
coke, heaT/ hydrocarbvr-1 molecules are preferably surrounded by sufficient
water mOler,ules. Generally speaking, a higher water to oil ratio wil( be
helpful
to riiaiiitaiii ttie desired environment. However, high water to oil ratio
also
zrieans higti equiprr3er3t and s~peratir3g cost. The emlaadi~ient shown in
Figure
2 can achieve tiigh water to oil ratio locally withcatit iÃicreasina, overafl
water to
2~.~ feed i-:atio. Instead of mixing all the feed oil with water at reactor
inlet, this
embodiment uses multiple injections of oil to tnaititain a desired water to
oil
ratio. Such a desigri is also helpful to control reactioti ternperature. By
dÃstributirig feed oil more uniformly through the reactor length, reaction
temperature will tiot increase too much clue to the exotherrRic.nature of the
reactions.
Only two Ãr~~ections were shown in Figure 2. This is not intended as a
;ii'riitatÃon, A reactor with multiple injections may also be used, In
addition, one
or more settiiÃiq vessels can be added to a reactor witf-i a mLlitiple
injection
configÃarat:on to achieve solid se:paratioE; under supercriticai conditions.
Figure 3 shows yet another embodiment with more than one mixing and
reaction zones. A second mixer, which may or may not be the same as the
first mixer, is added beh-veer~ reaction zone to enhance the cilisupercriticai
~5 water s-iiixiria. Agairs, nnultiplG mixc-rs arid reaction zonfes cari be
used.
The upgradiriy rea ctior, is exotherrriic. A reactor with ~.~ iaraesurface
area
W'ps to mainta;n uniform temperature distribution inside the reactor,
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
Deperictirrg on feed pÃ-apertisws, heat exchange tf3rouyti the surface area
provided by the reactor may or may not be enough. Water can be used to
quench the reaction stream and thereby control the reaction temperature.
Figure 4 shows an embodiment of us:ing water to quench the reaction stream
between two reactiori zones. The amount of water used for quenching should
be enough to bring down the reaction temperature while the reaction stream
after q,LÃenching still maintain sUpercriticai conc:i#tions. NiuI ip:e
reaction zones
and water rtÃtenching may t.t-, necessary for some feerts
The quenching water can also be used to for product r=ero5.rery, as shown iÃz
Figure 5. After reaction the product stream is qLjenched by liquid water.
'f'he
solid wiil be washed out by the water, and due to the temperature reduction
caused by quenching water and the hydrocarbons will condense as liquid.
Reactior: Prodt_tcf Separatior)
After the reaction has progressed sufÃicir/ntÃy, a sitig6'e phase reactÃoti
product
is withdrawn from the reaction zone, cooled, and separated Frito gas, effluent
wat:er arid upgraded hydrocarbon phases.. I'his separation is preferably done
by cooling the stream and using one or riiore tvvo-phase separators, threa-
phase separators, or other gas-oil-vvater separation device known in the art
However, any met}iod of separation uari be used ir7 accordarice with the
;nver,tion.
Thirr comt,ositian of gaseous product obtained by treatment of the heavy
hydrocarbons ir, accordance with the process of the prasc-nt invention will
depend on feed properties and typically comprises light hydrocarbons, water
vapor, acid gas (CQ; and H28), methane and hydrogen. The effluent water
may be used: reused or dis{:arded. It may be recycled to e.g. the fQed water
tarik, the feed water treatment system or to the reaction zone.
Ttie upgraded hydrocarbon product, which is sometimes referred to as
synurÃade" herein may be Ãipgrac3ed fr,irther or processed into other
16
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
hydrocarboFi products using methods that are knoWr) Ãn the i'lydroMa;-t;rrn
proce.ssir7g art.
'T'ha process of the present invention may be, carried out either as a
continuous or semi..=ItinuO4fs process or a batch process or as acontinLious
praGess. In the continuous process th~:~ entire system operates with a feed
stream of oil and a separate feed stream of s-upere;ritica= water and reac"hes
a
steady state; whereby a(1 the flow rates, temperatrires; presswres, and
compositicÃ3 of the iiilet, outlet, and recycle streams do riot vary
appreciably
with tÃme;
While not being bound to any theory of operation, it is believed that r.a
number
of upgrading reactions are accurrina simuitapeotisly at the supercritica(
water
conditions used in the present process. In apreferrsd embodiment of the
invention the rnajor chemical/upgrading reactions are believed to be:
Thermal Crackang: CrH, --~ lighter hydrocarbons
Stear73 Refarrriing: C,H,, + 2xH,,,O = xCt.~r + t2x~y~~lHz
Water-Gas-5t1rf#: CO + H1O = CO2 + H2
ner~netaFizatiori: Cxf-iL;Ni,+ Ã~~~O/i==i~ ..-=~ NiOr'Ni(011+ + lighter
hydrocarbons
26 DesulfuriratiaÃi:: C:<Hy5, + HzOfH> H2S + lighter hydrocarbons
The exact pathway may ~eper3d on the reactor operating conditions
{temperature, prassure, OlVd volume ratica), reactor desigrt (mode of
4=cntactfmixrng, sequence of heating), and the hydrocarhan ieedstocK.
The fo;iavving Examples are i:lustr ative ntthe prc-sai#t invention, but are
not
intended to liÃtiit the invention in any way beyond what is contained ir) the
claims which foilovv.
EXAMPLE I -- Process Conditions.
Oil and superc;ritie:al water are contacted in a mixer prior tc, entering the
reactor. The reactor is equipped with an inner tube for caliactirig the
preoclucts
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
(syncrude, excess water, and gas), and a b(3ttG+rTl section where ctflsj
Ã?letclls Vr
solids comprising a ><dreg stream" of ineteterliiÃnate properties or
composition
may accumulate. The sheiE-s;de of the reactor is kept isothermal during the
reaction with a clamshell furnace and tc3'riperature oontroller: Preferred
reactor residence tirnes are 2040 miÃiutes, with preferred oil/water volume
ratios on the order of 1:3. Preferred temperatures are around :.~74'.. 440`C:
with the pressure at 3200-4000 psig: The reactor product stream :eaves as a
single phase, and is cooled and separated intc:z gas, syncrude, and effiLrent
water. The eft:fJent thfater is recycled back to the reactor. Sulfur fror-n
the
orig;raal feedstock accumulates in the dreg strearn for the riicst part, with
lesser amounts prirnariiy in the form of H,,4 fcrunrt in the gas phase and
water
phase.
As the next examples wil; show, very little gas is produced in most cases.
With suitable choice of operating conditions, it is also possible to reduce or
nearly eiiniiriato the "dreg streani." Elimination of the dreg strearii Ãneans
that
a greatei- degree of hydrocarboÃi is recovered as syncrude, but it also
rrieans
that metals and sulftsr will accumulate elsPwhore, such as in the water and
gas streams.
EXAMPLE 2 -Properties of the, Product Syncrude.
A Ha,iiaca CrLide oil was di1uted with a diluent hydrocarbon at. a ratio of
5:1
(20 vQl":'0 of diluerif). The diluted FtaÃ-Ãiaca crude oil propeit;es were
measured
before reacting it with the supercritical water process as referred to i.n
Exariiple 1 and Fig. 2. The properties of the crude were as fo;lows: 12,8 API
gravity at 60/6ir.~, 1329 CST viscosity @400C; 7.66 wt% C/H ratio; 13.04 wt%
MCRT; 3.54 ~rd"zo sulfur; 0.56 wtfio nitrogen; 3.05 mg KOH/gm acid number;
1.41 wt% water; 371 ppm tr'anadium, and 86 ppm Nrokel. The diluted Harnaca
crude oil after the s+.tper critical water treatment was converted into a
36 syncrude witxt the following properties: 24.1 API gravity at 60,160> 5.75
CST
viscosity @40 C; 7.40 wt% CfH ratio; 2.25 wl% MCRT; 2.83,wt% sulfur; 0.28
wt`!~, n,1troqean1; 1 .54 mg KOH/gÃ~n acid nur73bErt 0,96 wt% water; 24 pprtl
~~t
CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
Var3adir.uii; and ~ppr~i Nir;ieel. Suhstaritial reduct30ris ir) riletals and
resic3L#es
were observed, with s<mu,"tanear_Ãs irrtorease in the API gravity and a
significant
decrease in the viscosity of the original crude oil feedstock. There were
modest reductions in the Tntal Acid number, sulfur concentratior?, and
riitrOgeri concentration w}iic-h cOr.rld be improved with further
optinlization of
the reaction conditions.
When the c3ilErtecf Harnaca crude was sent directly to the reactor Mfl<?Or.rt
beirig
first heated with supercr'iti; al water, the procfrÃt:t syncrude had tht-!,'
following
propert:ies: 14.0 API gravity at 60/60: 188 CST viscosity @40'-G; 8.7 wt%
MCRT: 3.11 wt% sulfur, 267 ppm Vanadiurr3, and 59 ppm Nickel. This
comparison demonstrates the importance of the heating seqLjance of the
present invention.
Apart froai the occasional, sr~rall acc;umulatiOri of a dreg strearÃi, ttiere
is very
little caking or solid byproducts formed in the super.riticas water reactior).
Ttle
material balance was perfarrried for two separate experrr-neritaf runs.
In the experimental rtÃn with no dreg stream forriiÃ:d, the startingfeedstOGk
of
dilr.ited Harnae,a crude at 60 grams produced a syricrude produc.t of 59.25
grams which corresponds to a high overall recovery of 99 percerrt. It v+ras
thought that due to the absence of a dreg stream, the experimental mass
balance was impacted in the determination of the sulfur and metals. 'f'he gas
phase did not contain rnetafs species and had little sLtlfur ceÃxipOur;ds_ It
was
hypothesized that a pcrfion of the metal and sulfur may have accurnulated on
the walls of the reactor or ~ownstrearii plumbing.
In the experimer.tal rur:: with a dreg stream fOrrriert: the starting
teedstOck cf'
diluted Hamaca crude at 30 grarns produced a syncrude product of 22.73
grams. The dreg stream that was formed accounted for 5.5 grams. The
overall recovery with the dreg strea:rt, was 96.7 percerit. In Ifie c.ireg
strearrt,
sulfur accounted for 31% of the total sulfur with the remainirag sulfur in the
oil,
product, water phase; and gas phase. The mc~tals content of the dreg streaÃii
19
= CA 02667261 2009-04-22
WO 2008/055171 PCT/US2007/083038
accuLfrÃted for 82 'o of ti`{e total rrs~.~taIs with the remaining metals in
the oil
prcdÃ.rct. For commercial operations, it may be preferable to miriirÃ-Ãize
ttle
formation of a dreg strearn, since it represents a 18% reduction in syncrÃ.ade
prodtÃcf, arid generates a lower value product stream that impacts the process
in terh-is of economics and disposal concerns.
1C}
Undiluted Boscan crude oil prcperties were measured before reacting it with
thp sUperC ritical water process of the~ prese,nt invention. 'rhe fsropt~rties
of the
crude were as follows: 9 API gravity at 60/60, 1,140 CST viscosity @40 C:
8.0 vA% C/H ratie; 16 wt% MCRT; 5.8 wt% SÃi{fur; arar? 1,280 ppm Vanadium;.
The undiluted Boscan crude oil after the super critical water treatment was
converted into a syncrude with the following properties: 22 API gravity at
60160; 9 CST viscosity @40 C; 7.6 wt% C/H ratio; 2.5 wt:'u MCRT: 4.6%
sulfur; and 130 ppm Vanadium.
A simulated clFstillatio>i aÃialysis of the original crr.Ãde oil vs, the
syncrude
products from different experimental runs shows that the syncrude prepared
in accordance with the present invention clearly has superior properties than
the original crude. Specifically: the syncrudes caritain a higher fraction of
IOwer-boiiirÃg fractions. 511~,; of the diluted Hamaca crtide bo3is across a
range
of temperatures of less than 1000'F, while emplaying a process accOrdir}g to
the present invention usirig supercritical water depending on process
c;onfiguratiorss, between 79 to 94% of the syricrude boils across a range Of
ter-raperatures of less than 1000 i". 40% of the uradilr,rted Bascan crÃide
boils
across a range of ternperatures of less thar= 1000''F, while employing a
process accord:ng to the present invention iusing superÃ;riticaf water, 939/1<
of
the syncrude boils across a range of temperatures of less than 10001-.
There are riLÃmerous variations on the present invention which are possible in
light of the teach;Ã7gs arÃd sup:pGrting exarrsples described hereiÃi. It is
therefore understood that wit:hin, the scope of the following claims, the
invention may be practiced otheNvise thari as specificaify described or
exemplified here:n.
"it