Note: Descriptions are shown in the official language in which they were submitted.
CA 02667692 2014-02-24
CATALYTIC SYSTEM FOR CONVERTING LIQUID FUELS INTO SYNGAS
BACKGROUND OF THE INVENTION
Field of the Invention
[0002] The present invention is directed to a method and system for the
process of
reactively converting a liquid fuel into a gasified stream. More particularly,
the method and
system of the present invention provide a novel means for converting the
liquid fuel into a
gas by partial oxidation and steam reforming. In addition, when fuels
containing sulfur are
used, a device according the present invention can be employed to provide de-
sulfurization.
Brief Description of the Related Art
[0003] Gasification of liquid fuels typically comprises use of a vaporizer.
Vaporization
of liquid fuels (e.g., alcohols, hydrocarbons) typically is achieved by
indirectly supplying
heat into a stream of liquid fuel via heat exchange with a hot wall. One
disadvantage of this
method is that the rate of vaporization is limited by the rate of heat
transfer such that
relatively large surface area is required for fuel vaporization. Another
disadvantage of this
method, especially for vaporizing long chain hydrocarbons, is that heating the
fuel stream to
the vaporization temperature tends to cause fuel decomposition and formation
of deposits.
1
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
More specifically, coke formation is problematic. Moreover, preventing
deposits from
forming within the fuel passages in the nozzle during steady state operation
due to heat-up of
the nozzle from the downstream hot zone is challenging.
[0004] Another known method for gasification of a fuel stream comprises
mixing
atomized fuel with a hot gas such as superheated steam that supplies the heat
required for
fuel vaporization and prevents coke formation. However, the large amounts of
superheated
steam required in this method result in a large heat load for steam
production.
[0005] Spray methods for atomization of liquid fuels known in the art
include air-blast or
pressure atomizers, ultrasonic and electrospray atomizers. These spray systems
are capable
of providing a uniform distribution of atomized fuel across the entrance of
the catalyst bed.
Such atomizers may include a co-flow of air that allows mixing of the fuel and
oxidizer.
However, very fine and uniform droplet size along with homogeneous fuel-air
distribution,
required to avoid coke formation and obtain temperature/mixture uniformity in
the reactor, is
difficult to achieve in practical systems.
[0006] Ignition devices, such as a spark or glow plugs, are widely used to
ignite fuel-
oxidizer mixtures at startup. These devices often are subject to failure due
to the high
operating temperatures by virtue of their location required for ignition.
[0007] Monoliths are commonly used catalyst substrates for the gasification
of liquid
fuel. Fuel oxidizer mixture inhomogeneities are usually detrimental to these
substrates as
they lead to localized lean or rich zones respectively causing hot spots or
carbon precipitation
regions. Since there is no opportunity for these zones to re-mix within the
long, separated
channels of a monolith, these substrates are particularly vulnerable. In
addition, carbon
2
CA 02667692 2014-02-24
precipitation is favored in monoliths due to the boundary layers that develop
in these
substrates.
[0008] Combustion of liquid fuels in fuel cell or internal combustion
engine systems
poses significant problems, especially for fuels with high aromatic content
and wide boiling
point distribution. This can be attributed to the propensity of the heavier
aromatic
compounds in the fuel to form deposits or coke when vaporized at high
temperatures.
[0009] Liquid hydrocarbon fuels such as gasoline, kerosene or diesel may be
used with
high temperature solid oxide fuel cells ("SOFC") to directly produce electric
power. For
these fuel cells, the choice of fuel is not limited to pure hydrogen as is the
case for low
temperature proton exchange membrane ("PEM") fuel cells. Conversion of the
hydrocarbon
fuel into gaseous mixture containing syngas, though, is required before the
fuel may be fed to
the SOFC. Furthermore, removal of sulfur normally contained in the fuel prior
to feeding to
the SOFC is needed.
100101 These and other known methods and systems for gasification of liquid
fuels,
together with their associated disadvantages, are described further in U.S.
2005/0028445.
[0011] Gasification and pre-reforming of liquid fuel would resolve many of
the issues
noted above with respect to the prior art. Accordingly, there is a need for a
pre-reforming
reactor capable of operating with a range of liquid fuels. It is therefore an
object of the
present invention to provide a pre-reforming reactor for partially oxidizing
and cracking the
heavy components of the fuel. The pre-reformed fuel subsequently can be
further reformed
3
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
or combusted to power fuel cell systems, internal combustion engines, burners,
and other
known devices.
[00121 It is therefore another object of the current invention to provide a
catalyst
substrate that facilitates mixing of the stream flowing therethrough, for
example a substrate
having plurality of voids in random order and short channels extending in the
downstream
direction the length of which is similar to the channel diameter. Such a
configuration results
in a comparatively high conversion rate of the reactants to the desired
products and
minimizes break through of unreacted fuel.
[00131 It also is an object of the current invention to provide a catalytic
reactor for the
gasification of liquid fuels comprising a catalyst that yields partial
oxidation products, such
as CO and H2. This results in a higher level of fuel conversion for the same
amount of added
air and produces hydrogen-rich gas directly from the gasifier reactor. It is a
further object of
the current invention to provide a method whereby steam or atomized water
and/or CO2 may
be added to the fuel/air stream to adjust the amount of hydrogen in the
product stream. It
also is a further object of the current invention to provide a method whereby
no external pre-
heating of either air or fuel is required.
[0014] Lastly, it is a further object of the present invention to provide
de-sulfurization of
the fuel in the liquid form when required by a particular application of the
gasification
system taught herein.
4
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
DESCRIPTION OF THE INVENTION
[0015] The system of the present invention eliminates the need for a liquid
fuel vaporizer
in a typical gasification system. By eliminating the vaporizer, an essential
and critical part of
the reforming systems known in the art, the entire system is less complex and
more robust.
Vaporizing hydrocarbon fuels is difficult because of low decomposition
temperature of these
fuels which leads to coke deposits and clogging of the fuel delivery lines.
Spraying cold fuel
directly into the catalyst bed eliminates this problem.
[0016] In one embodiment of this invention, a heat exchanger is positioned
downstream
of the catalyst bed thereby utilizing the heat generated in the reforming
reaction to produce
steam required for the system while cooling the reformate stream to the
temperature required
by the downstream components, i.e. de-sulfurization bed.
[0017] In yet another embodiment of this invention, a de-sulfiirization bed
is positioned
downstream of the catalyst bed. In other systems known in the art, de-
sulfurization is
achieved by de-sulfurization of the fuel in the liquid form in a hydro-de-
sulfurization
("HDS") process prior to the reforming process.
[0018] In summary, the present invention is a system for converting liquid
fuels into gas
mixture containing CO and H2 (syngas). The system is comprised of: (i) a
nozzle; (ii) a
catalyst bed in fluid communication with the nozzle exhaust stream; (iii) a
heat source for
igniting the catalyst; and (iv) a heat exchanger. The hot side of the heat
exchange is in fluid
communication with the catalyst bed and the cold side of the heat exchanger in
fluid
communication with the nozzle.
CA 02667692 2014-02-24
[0019] Atomized liquid fuel is exits the nozzle in a stream comprising an
oxidizer and
optional steam. In most applications, oxygen as a constituent of air is a
preferred oxidizer.
The ratio of the fuel stream to the oxidizer stream should be such that there
is insufficient
amount of oxidizer to completely oxidize all fuel into CO2 and H20, i.e. the
ratio should be
fuel rich.
[0020] The fuel entering the nozzle is cold (i.e., below the temperature at
which the fuel
starts to decompose creating coke deposits). The nozzle design is such that
the liquid fuel
remains cold before exiting the nozzle. This is an important point
distinguishing this
invention from previous methods, for example US Patent No. 4,381,187.
[00211 The nozzle may be of any type (i.e., based on pressure atomization,
air blast,
ultrasonic atomization, electrospray, or other type known in the art). The
nozzle provides
fine atomization of cold liquid fuel and uniform distribution of the atomized
fuel within the
inlet air or optionally steam containing inlet air. Appropriate nozzles in
which reaction air
and/or steam flow and/or fuel flow are used to atomize liquid fuel.
[0022] The catalyst bed comprises catalyst suitable for supporting partial
oxidation and
reforming reactions. Preferably the catalyst is one of the metals of group
VIII of the periodic
system of elements, preferably, rhodium. The substrate on which the catalyst
is supported
preferably provides good mixing for the fuel/oxidizer mixture passing
therethrough. To
provide good mixing capabilities, the substrate preferably comprises a
multiplicity of void
volumes in random order. This may be best achieved by using porous metal or
ceramic
substrates or by using multiple ceramic or metal screens or foams.
[0023] The preferred catalyst bed geometry provides a decreasing mass flux
of the
reactive mixture flow through the catalyst bed as disclosed in WO 2004/060546.
6
CA 02667692 2014-02-24
As an example of such geometry is a coil of MicrolithTM short-contact-time,
ultra-short-
channel-length substrate and catalyst where the reactive mixture is introduced
in the ID
plenum and the reformed gas exits at the OD of the coil. Prefen-ed catalyst
formulations
could be used on different parts of the coil. MicrolithTM short-contact-time,
ultra-short-
channel-length substrate is available from Precision Combustion, Inc., 410
Sackett Point
Road, North Haven, Connecticut.
[0024] The nozzle and the catalyst bed are arranged in such a way that the
stream of
atomized fuel mixed with air and steam provided by the nozzle is uniformly
distributed
across the entry face of the catalyst bed. It is preferred that the rate of
flow of the reacting
mixture through the catalyst bed is sufficiently high such that significant
amounts of partial
oxidation products (i.e., CO and H2) are formed. When partial oxidation
products are
formed, less heat is released thereby resulting in lower temperatures of the
catalyst bed.
[0025] The heat/ignition source is placed in closed proximity with the
catalyst bed. It is
required for the initial pre-heat of the catalyst to the temperature where the
oxidation reaction
between the fuel and the oxidant would ignite. The heat source may be of any
type known in
the art. An electrically heated glow plug is a preferred heat source.
[0026] The heat exchanger downstream of the catalyst bed can be any type of
a heat
exchanger known in the art. The heat exchanger should be placed such that the
gaseous
reformate flow exiting the catalyst bed passes on the hot side the heat
exchanger. The heat
exchanger then cools the reformate flow to the required temperature. The heat
exchanger
vaporizes cold liquid water to produce steam which is fed to the nozzle
outlet.
7
CA 02667692 2014-12-30
[0027] In some embodiments of the invention, a sulfur removal bed may be
placed in
fluid communication with the heat exchanger. In this embodiments, the cooled
reformate
stream containing sulfur in the form of H2S exiting the heat exchanger passes
through the
sulfur removal bed to provide a sulfur free reformate stream.
100281 The method and system of the present invention provide gasification of
liquid fuel
without a requirement for supplying external heat or steam to the gasifier.
Fuel and air may
be supplied to the gasifier at ambient temperatures. This allows a smaller
mixing volume,
since the catalytic bed tolerates partial unmixedness, and a simpler fuel and
air delivery
system design. This also allows a means for start up and operation in the
absence of initial
heat at the reactor inlet. More importantly, the method and system of the
present invention
provide a means for the gasification of a liquid fuel without the use of an
external vaporizer.
10028a1 In accordance with an aspect of the present invention, there is
provided a process
for gasification of a liquid fuel comprising: (a) providing a supply of the
liquid fuel; (b)
providing a supply of an oxidant; (c) providing a supply of liquid water; (d)
atomizing the
liquid fuel through a nozzle into a mixer such that the fuel entering the
nozzle and before
exiting the nozzle is maintained at a temperature below the coking temperature
of the fuel;
(e) feeding the oxidant into the mixer and mixing the atomized fuel with the
oxidant; (f)
catalytically reacting the fuel-oxidant mixture in the presence of steam in a
catalytic reactor
thereby producing gaseous reformate; wherein the catalytic reactor comprises a
substrate in
a coiled configuration having an inner diameter and an outer diameter and a
radial flow
path, and having supported thereon one or more Group VIII metals; (g)
initiating the
catalytic reaction of step (f) with an ignition source positioned inside the
inner diameter of
the coiled substrate; (h) contacting the gaseous reformate with a heat
exchanger positioned
downstream of and in fluid communication with the catalytic reactor, such that
a hot side of
8
CA 02667692 2014-02-24
the heat exchanger contacts the gaseous reformate and a cold side of the heat
exchanger
contacts the supply of water; and further such that the heat exchanger is
displaced radially
with respect to a center axis of the catalytic reactor and wherein the gaseous
reformate exits
through the radial flow path of the catalytic reactor and contacts the heat
exchanger in a
crossflow direction; (i) transferring heat from the gaseous reformate via the
heat exchanger
to the liquid water to produce steam; and (j) providing the steam to the
fuel-oxidant
mixture in the catalytic reaction of step (f).
[0028b] In accordance with a further aspect of the present invention, there is
provided a
system for converting a liquid fuel into a gasified stream, the system
comprising: (a) a
nozzle having an outlet for feeding a fuel and an oxidant into contact with a
reforming
catalyst; (b) a fuel inlet for feeding the fuel into the nozzle; (c) an
oxidant inlet for feeding
the oxidant into the nozzle; (d) the reforming catalyst positioned downstream
from the outlet
of the nozzle, the catalyst being supported on a substrate comprising a metal
screen in a
coiled configuration having an inner diameter and an outer diameter and a
radial flow path;
(e) an ignition source located inside the inner diameter of the metal screen
for igniting the
catalyst; (f) a heat exchanger placed within the system downstream of the
catalyst such that
a hot side of the heat exchanger is in fluid communication with the catalyst
and a cold side
of the heat exchanger is in fluid communication with a supply of water so as
to produce
steam; and further such that the heat exchanger is displaced radially with
respect to a center
axis of the coiled metal screen and the heat exchanger has a flow path
positioned in
crossflow direction with respect to the radial flow path of the coiled metal
screen; (g) a
means for feeding steam into contact with the catalyst.
Brief Description of the Drawings
8a
CA 02667692 2014-02-24
[0029] Fig. 1 depicts a schematic representation of an embodiment of a
gasification
system.
[0030] Fig. 2 depicts a schematic representation of another embodiment of a
gasification
system according.
[0031] Fig. 3 depicts a diagrammatic representation of a detailed design of
a gasification
system according to the present invention.
[0032] Fig. 4a and 4b provide a three-dimensional rendering of a detailed
design of a
gasification system according to the present invention.
8b
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
[0033] Fig. 5 provides a graphical representation of lightoff temperature
versus time in a
gasification system according to the present invention.
[0034] Fig. 6 provides a graphical representation of the dependence of fuel
conversion on
the air-to-fuel ratio in a gasification system according to the present
invention.
[0035] Fig. 7 provides a schematic flow diagram of a gasification system
according to the
present invention.
Detailed Description of the Invention
[0036] As depicted schematically in Fig. 1, a typical gasification system
(10) comprises a
path (12) defining a flow of air (14). Fuel stream (16) is introduced into
injector (18), which
atomizes fuel stream (16). Atomized fuel (20) and air (14) enter catalyst bed
(22) where fuel
(20) is additionally mixed, vaporized and partially reformed. Gasified fuel
stream (24)
leaves the catalyst bed (22). Ignition source (26), in close proximity with
catalyst bed (22), is
used to initiate the process.
[0037] Fig. 2 schematically depicts alternative gasification system (110).
Functional
elements corresponding to those depicted in Fig. 1 are referenced by
corresponding 100-
series reference numbers. In this embodiment, catalyst bed (122) defines a
cylindrical shape
and comprises a wound catalytically coated, short-contact-time, ultra-short-
channel-length
substrate. Atomized fuel (120) and airflow (114) enter into the inner diameter
(128) of
catalyst bed (122) and flow out radially (130) through catalyst bed (122). The
igniter (126)
in this embodiment comprises an electric glow plug (132) placed inside inner
diameter (128)
of catalyst bed (122). Glow plug (132) may be coated with catalyst (134) to
further assist the
9
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
start up process. Electric current initially is supplied to glow plug (132) to
preheat catalyst
bed (122) to the start up temperature. Fuel stream (116) is introduced into
injector (118), and
air (114) is then mixed with atomized fuel (120) causing catalyst bed (122) to
heat up to the
operating temperature at which point the electric current to the glow plug
(132) is stopped.
Gasified fuel stream (124) exits the system (110).
[0038] Fig. 3 depicts a diagrammatic representation of a design of a
gasification system
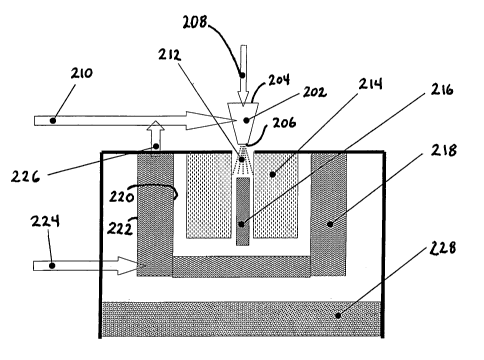
(200) according to the present invention for converting liquid fuels into gas
mixture
containing CO and H2 (syngas). The system (200) is comprised of a nozzle (202)
having an
inlet (204) and an outlet (206). A cold fuel liquid stream (208) and an inlet
oxidizer stream
(210) are introduced into the inlet (204) (which inlet may have more than one
orifice). A
nozzle exhaust stream (212) comprising atomized liquid fuel mixed with
oxidizer and, if
desired, steam exits nozzle (202) at outlet (206). Catalyst bed (214) is in
fluid
conununication with the nozzle exhaust stream (212). The system (200) further
comprises a
heat source (216) for igniting the catalyst bed (214). A heat exchanger (218)
is in fluid
communication with, or placed in close proximity with, the catalyst bed (214)
and the nozzle
(202).
[0039] Atomized liquid fuel exits the nozzle (202) in exhaust stream (212)
which further
comprises an oxidizer and optional steam. In most applications, oxygen as a
constituent of
air is a preferred oxidizer. The ratio of the fuel stream to the oxidizer
stream should be such
that there is insufficient amount of oxidizer to completely oxidize all fuel
into CO2 and H20,
(i.e., the ratio should be fuel rich).
[0040] Exhaust stream (212) is uniformly distributed across the entry face
of the catalyst
bed (214). Preferably, the reactive mixture comprising exhaust stream (212) is
introduced in
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
the ID plenum of the catalyst bed (214) and the reformed gas exits at the OD
of the catalyst
bed (214) coil.
[0041] The heat/ignition source (216) is placed in closed proximity with
the catalyst bed
(214) in order to pre-heat the catalyst bed (214) to the temperature where the
oxidation
reaction between the fuel and the oxidant ignite. Gaseous reformate flow exits
the catalyst
bed (214) in fluid communication with a first side (220) of heat exchanger
(218). The
gaseous reformate is then cooled to the required temperature by passing
through, or in close
proximity with, heat exchanger (218). The second side (222) of heat exchanger
(218)
vaporizes cold liquid water stream (224) to produce steam (226), which is fed
to the inlet
(204) of nozzle (202).
[00421 If desired, a sulfur removal bed (228) may be placed in fluid
communication with
the first side (220) of heat exchanger (218). In this embodiment, the cooled
reformate stream
containing sulfur in the form of H2S exiting the heat exchanger (218) passes
through the
sulfur removal bed (228) to provide a sulfur free reformate stream.
[0043] Fig. 4a and 4b provide a three-dimensional rendering of detailed
design of a
gasification system (300) according to the present invention. An Auto Thermal
Reforming
(ATR) reactor comprised a coiled catalyst bed, a fuel atomization nozzle and a
start up glow
plug. The reactor comprises the core of the reforming system, which system is
further
incorporated into a system comprising a heat exchanger/steam generator, ZnO de-
sulfurization bed and fuel, air and water pump. The ATR was enclosed in a
quartz housing
to enable visual observation of the catalyst temperature uniformity. The
reactor was also
equipped with eight thermocouples for studying temperature distribution within
the catalyst
bed. Gasification system (300) is an embodiment of gasification system (200)
described
11
CA 02667692 2014-02-24
hereinabove and some of the features are called out using similar
characteristics numbers for
descriptive and illustrative purposes.
[0044] Fig. 4a depicts the system (300), catalyst bed (314), heat/ignition
source (316),
and heat exchanger (318). Fig. 4a also depicts, among other features described
hereinabove
with reference to Fig. 3, cold fuel liquid stream inlet (308), oxidizer stream
inlet (310), and
liquid water stream inlet (324).
[0045] The glow plug permits the reactor to lightoff at ambient conditions.
In order to
start the reactor, 12 V DC potential is applied to the glow plug providing
heat directly to the
catalyst. This results in catalyst temperature increasing to above lightoff
temperature in
about 30 seconds. Fuel flow is then started resulting in the reactor lightoff
and transition to
operational state in about 1 minute. As the catalyst lights off, the glow plug
is shut off and
steam flow to the reactor is started. This causes temperature decrease on the
front of the
catalyst bed, such that more air can be added to the reactor and complete fuel
conversion
achieved. Reactor temperatures during the lightoff process are shown in Figure
5.
[0046] The reformate gas was analyzed by a GC at each 0:C setting to
measure the gas
composition and the reactor performance. The inlet temperature and the S:C
ratio were then
varied and the 0:C scan repeated to measure the dependence of the ATR
performance on
inlet temperature, air-to-fuel ratio (0:C) and steam-to-fuel ratio (S:C). The
results are
provided in Figure 6. It was found that the reactor could be operated with low
water addition
(S:C 1). It was also found that increasing the inlet temperature improved
reactor
performance. At 400 C and S:C = 1.1, the JP-8 reforming efficiency (LHV based)
was
¨65%. Note that due to equipment tolerances analytical considerations resulted
in a
12
CA 02667692 2009-04-27
WO 2008/057335 PCT/US2007/022891
maximum 95% material balance. The flattening of the conversion curve indicates
that this
corresponds in actuality to essentially complete fuel conversion.
[0047] ASPEN modeling was used to examine and determine the system layout
including sensitivities to water addition/recycle/recapture and their
associated impacts. The
system configuration is illustrated in Figure 7. The system operates at
approximately 1 ¨ 2
atm. The ATR feed water is delivered to a heat exchanger where it is vaporized
prior to
mixing with air supplied at the same pressure. The steam/air mix is combined
with
hydrocarbon fuel (represented by dodecane) through a nozzle, prior to delivery
to the ATR.
The ATR is represented as an adiabatic reactor yielding an equilibrium product
distribution.
The hot ATR product serves as the heat source for vaporizing the feed water.
This is a
benefit because it allows low temperature valving to be used to control the
reformate flows.
[0048] Typically, the feed 0/C ratio was fixed and two convergence criteria
were
imposed on the simulation. The first required that the temperature of the
steam exiting the
heat exchanger was sufficient to achieve a specified ATR mixed feed
temperature (300 C ¨
400 C). The second required that the temperature of the cooled ATR product
leaving the heat
exchanger be compatible with effective sulfur removal in a downstream ZnO bed
(typically
300 ¨ 350 C). This was controlled by regulating the water feed rate. Thus, for
a given 0/C,
the S/C ratio was that value which simultaneouslysatisfied these two
requirements. Case
studies showed that there was a preferred 0/C range (-1.1 ¨ 1.2) below which
target ATR
feed temperatures could not be achieved and above which system efficiencies
declined
excessively. As an example, at a fixed 0/C of 1.2, acceptable operation for a
400 C target
ATR feed temperature and a 300 C ATR product temperature exiting the heat
exchanger,
could be obtained at a feed S/C of 2.16. The resultant LHV based thermal
efficiency for
13
CA 02667692 2014-02-24
these conditions was ¨75% for the reforming system (including BOP parasitics
but not
including fuel-cell efficiency). Without a fuel cell, heat integration was
relaxed in the system
prototype. Integration of heat and water-recovery from the downstream SOFC
will be
required when operating with the stack.
[0049] Although the invention has been described in considerable detail
with respect to
reactively converting a liquid fuel into a gasified stream by partial
oxidation and steam
reforming, it will be apparent that the invention is capable of numerous
modifications and
variations, apparent to those skilled in the art, without departing from the
scope of the
invention.
14