Note: Descriptions are shown in the official language in which they were submitted.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
1
WATER-SOLUBLE SUBSTRATE WITH RESISTANCE TO DISSOLUTION PRIOR TO
BEING IMMERSED IN WATER
FIELD OF THE INVENTION
This invention relates to a water-soluble substrate, and more particularly a
water-soluble
substrate which has improved resistance to dissolution prior to being immersed
in water, and
methods of making the same. This invention also relates to articles, such as
pouches, made from
the water-soluble substrate.
BACKGROUND OF THE INVENTION
Water-soluble substrates are gaining wider acceptance for use as packaging
materials.
Packaging materials include films, sheets, blown or molded hollow bodies (i.e.
sachets, pouches,
and tablets), bottles, receptacles and the like. Often, water-soluble
substrates, when used in the
preparation of certain types of these articles such as sachets and pouches,
disintegrate and/or
become sticky when exposed to small amounts of water or high humidity. This
can make them
unsuitable for usage in the packaging and storage of the compositions
contained therein.
The most common consumer complaint for water-soluble pouches is linked to
unwanted
pouch dissolution when accidentally exposed to small amounts of water, such as
when water
gets inside the outer packaging in which the pouches are sold and stored after
purchase, from
wet hands, high humidity, leaking sinks, or pipes during storage. This may
cause the water-
soluble pouches to leak prior to use and/or stick together. The second most
frequent complaint
is that of the water-soluble pouch failing to fully dissolve upon use. Thus,
there remains an
unmet need for water-soluble substrates and articles made therefrom, such as
sachets and
pouches, which have improved resistance to dissolution against exposure to
small amounts of
water yet can subsequently dissolve very quickly when immersed in an aqueous
solution, such
as rinse and/or wash water.
Various methods are known in the art to retard the dissolution of water-
soluble
substrates. These methods comprise coating the entire surface of the water-
soluble substrate with
a water-insoluble material. For example, US Patent Number 6,509,072 describes
a water-soluble
substrate comprising a barrier coating. The barrier coating is a polymeric
film which forms a
continuous film on the water-soluble substrate. Another example of a barrier
coating is
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
2
described in WO 01/23460, assigned to Kao Corporation, wherein a surface of
the water-soluble
substrate is coated with a particulate or fibrous water-insoluble material.
It has now been surprisingly found that the entire surface of the water-
soluble substrate
need not be coated in order to provide the water-soluble substrate with
improved resistance
against dissolution. As such, less coating material is required, which allows
to manufacture
water-soluble substrates with improved resistance against dissolution at lower
cost. It has also
been surprisingly found that the resistance against dissolution can be further
improved by
creating solubility gradients.
It is therefore an aspect of the present invention to provide water-soluble
substrates
which have improved resistance to dissolution prior to being immersed in
water, yet can
subsequently dissolve very quickly when immersed in an aqueous solution, such
as rinse and/or
wash water.
SUMMARY OF THE INVENTION
The present invention relates to a water-soluble substrate comprising a first
surface and a
second surface opposite to said first surface, characterized in that at least
one of said first or
second surfaces comprises first discrete zones having a water-solubility which
is less than the
water-solubility of said water-soluble substrate, said first discrete zones
representing from 10%
to 90% of the surface area of said first or second surface.
The present invention also relates to an articles comprising the water-soluble
substrate,
and to a method of making the water-soluble substrate.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 shows a cross-section of a non-coated water-soluble substrate.
Fig. 2 shows a top view of one embodiment of a water-soluble substrate
according to the
present invention.
Fig. 3 shows a top view of another embodiment of a water-soluble substrate
according to
the present invention.
Fig. 4 shows a cross-section of an article comprising a water-soluble
substrate according
to the present invention.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
3
DETAILED DESCRIPTION OF THE INVENTION
This invention relates to a water-soluble substrate, and more particularly a
water-soluble
substrate which has improved resistance to dissolution prior to being immersed
in water, and
methods of making the same. This invention also relates to articles, such as
water-soluble
pouches, made from the water-soluble substrate described herein.
Water-Soluble Substrate
FIG. 1 shows a cross-section of a water-soluble substrate 10. The water-
soluble
substrate 10 has a first surface 12, a second surface 14 opposite to the first
surface 12, and a
thickness 16 between the first surface 12 and the second surface 14. The water-
soluble substrate
10 can be in the form of a film, a sheet, or a foam, and includes woven and
nonwoven structures.
The water-soluble substrate is made of polymeric materials and has a water-
solubility of
at least 50 weight %, as measured by the method set out here after using a
glass-filter with a
maximum pore size of 20 microns. Preferably, the water-solubility of the
substrate is at least 75
weight % or even more preferably at least 95 weight %.
50 grams 0.1 gram of substrate material is added in a pre-weighed 400 ml
beaker and
245m1 1m1 of 25 C distilled water is added. This is stirred vigorously on a
magnetic stirrer set
at 600 rpm, for 30 minutes. Then, the mixture is filtered through a folded
qualitative sintered-
glass filter with a pore size as defined above (max. 20 micron). The water is
dried off from the
collected filtrate by any conventional method, and the weight of the remaining
material is
determined (which is the dissolved fraction). Then, the % solubility can be
calculated.
Typically the water-soluble substrate 10 has a basis weight of from 0.33 to
1,667 grams
per square meter, preferably from 33 to 167 grams per square meter. The
thickness of the water-
soluble substrate 10 between the first surface 12 and the second surface 14
can range from about
0.75 micrometer to about 1,250 micrometer, preferably from about 10 micrometer
to about 250
micrometer, more preferably from about 25 micrometer to about 125 micrometer.
Preferred polymers, copolymers or derivatives thereof suitable for use as
substrate
material are selected from polyvinyl alcohol (PVA), polyvinyl pyrrolidone,
polyalkylene oxides,
acrylamide, acrylic acid, cellulose, cellulose ethers, cellulose esters,
cellulose amides, polyvinyl
acetates, polycarboxylic acids and salts, polyaminoacids or peptides,
polyamides,
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
4
polyacrylamide, copolymers of maleic/acrylic acids, polysaccharides including
starch and
gelatine, natural gums such as xanthum and carragum, polyacrylates and water-
soluble acrylate
copolymers, methylcellulose, carboxymethylcellulose sodium, dextrin,
ethylcellulose,
hydroxyethyl cellulose, hydroxypropyl methylcellulose, maltodextrin,
polymethacrylates,
polyvinyl alcohol copolymers, hydroxypropyl methyl cellulose (HPMC), and
mixtures thereof.
The most preferred polymer is polyvinyl alcohol. Preferably, the level of
polymer in the
substrate is at least 60%.
An example of commercially available water-soluble films are PVA films known
under
the trade reference MonoSol M8630, as sold by MonoSol LLC of Gary, Indiana,
US, and PVA
films of corresponding solubility and deformability characteristics. Other
films suitable for use
herein include films known under the trade reference PT film or the K-series
of films supplied
by Aicello, or VF-HP film supplied by Kuraray.
Discrete zones
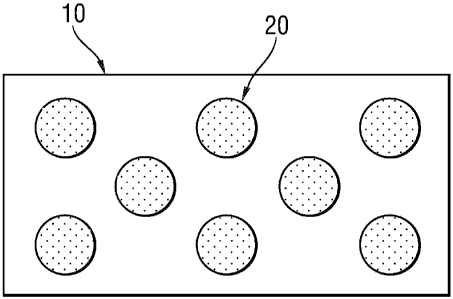
As shown in Fig. 2, at least one of the first or second surfaces 12, 14 of the
water-soluble
substrate 10 comprises first discrete zones 20 having a water-solubility which
is less than the
water-solubility of the water-soluble substrate. With "discrete zones" it is
meant zones having a
relatively small exposed surface area, preferably from 0.1 mm2 to 400 mm2,
more preferably
from 1 mm2 to 200 mm2 , even more preferably from 10 mm2 to 100 mm2,and which
do not
contact each other. As such, the first discrete zones 20 represent a pattern
which can be random
or non-random. These first discrete zones 20 combined, represent from 10%,
preferably from
20%, more preferably from 30%, even more preferably from 40% and up to 70%,
preferably up
to 80% and more preferably up to 90% of the surface area of the first or
second surface 12, 14.
The first discrete zones 20 increase the overall resistance to solubility of
the entire water-soluble
substrate. Without being bound by theory, it is believed that the resistance
to solubility does not
depend solely on the thickness or on the solubility (or resistance thereto) of
the water-soluble
substrate 10, nor solely on the thickness, solubility (or resistance thereto)
or coverage of the first
discrete zones 20. Instead, the overall resistance of the combined substrate,
that is the water-
soluble substrate 10 in combination with the first discrete zones 20, and the
time for the water to
reach the water soluble substrate 10 through the available vertical as well as
horizontal pathways
created by the discrete zones, is what matters.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
In a preferred embodiment and as shown in Fig. 3, the water-soluble substrate
10
comprises second discrete zones 21 having a water-solubility which is less
than the water-
solubility of the water-soluble substrate 10, and which is different than the
water-solubility of
the first discrete zones 20. As such, solubility gradients are created both in
a horizontal as well
5 as vertical direction, thereby further enhancing the resistance to
dissolution. The second discrete
zones 21 can be on the same surface as the first discrete zones 20, on the
opposite surface, or on
both surfaces. When the second discrete zones 21 are on the same surface of
the water-soluble
substrate than the first discrete zones 20, then the second discrete zones 21
are present in those
areas not yet covered by the first discrete zones 20. When the second discrete
zones 21 are on
the surface opposite to the surface comprising the first discrete zones 20,
then the second
discrete zones 21 are at least present in those areas of which the opposite
area is not covered by
the first discrete zones 20. The second discrete zones 21 combined, preferably
represent from
10%, preferably from 20%, more preferably from 30%, even more preferably from
40% and up
to 70%, preferably up to 80% and more preferably up to 90% of the surface area
of the first or
second surface 12, 14.
The first and second discrete zones 20, 21 can be created in various ways. In
a first
embodiment, the discrete zones 20, 21 can be created by applying a coating
material. Examples
of coating materials include, but are not limited thereto:
- a composition comprising polyvinyl alcohol having a high hydrolysis degree.
The
hydrolysis degree of the polyvinyl alcohol is preferably greater than 97%. The
composition can
be comprised substantially entirely of polyvinyl alcohol, or it can be a
mixture of polyvinyl
alcohol with other suitable water-soluble or water dispersible materials, as
described above.
- a water-insoluble material. The water-insoluble material may be a water-
insoluble
inorganic material or a water-insoluble organic material. With "water-
insoluble material", it is
meant that the solubility is less than 50 weight %, as measured according to
the previously
described method. Preferably, the water-solubility is less than 40 weight %,
more preferably less
than 30 weight %, and most preferably less than 10 weight %.
The water-insoluble inorganic material may be zeolite, bentonite, talc, mica,
kaolin,
sepiolite, silica, calcium carbonate, titanium oxide, anhydrous silicic acid,
hydroxy calcium
apatite, phthalocyanine blue, Helindone Pink, Hansa Orange, pearlescent
material, etc., while
zeolite, bentonite, talk, mica, kaolin, silica, titanium oxide, silicone, etc.
are preferred.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
6
The water-insoluble organic material may be a synthetic polymer such as
polyethylene,
polypropylene, polyamide, polyethylene terephthalate, polystyrene,
polyurethane and/or its
cross-linked product, sodium poly(meth)acrylic acid, poly(meth)acrylic acid
ester and/or its
cross-linked product, rubber such as ethylene rubber, propylene rubber,
styrene-butadiene
rubber, butadiene rubber, silicone rubber, etc. and/or its cross-linked
products, etc.; or a natural
polymer such as cellulose and/or its derivatives, seed hulls, starch and/or
their derivatives, while
polyethylene, polyamide, polystyrene, sodium poly(meth) acrylic acid,
poly(meth) acrylic acid
ester, cellulose and/or its derivatives, starch and/or their derivatives, are
preferred. Here,
poly(meth) acrylic acid means both polyacrylic acid and polymethacrylic acid.
The coating material may be in the form of particles. Preferred particles are
polymeric
particles including particles made of synthetic materials as described above.
Also preferably,
these particles have an average diameter of 500 micrometers or less, more
preferably 300
micrometers or less, even more preferably from 0.01 to 300 micrometers. In a
highly preferred
embodiment, the particles are in the nanosize-range, with average particle
diameters of from
0.01 to 1 micrometer. Coating the water-soluble substrate 10 with nano-sized
particles further
provides the benefit that the coating becomes transparent, which is
aesthetically preferred.
Suitable nanoparticles are polyethylene-, polypropylene-, wax-, silicone- or
polytetrafluoroethylene-based nanoparticles.
The coating material may be present on the surface of the water-soluble
substrate 10, or
it may be partially embedded into said substrate as will be explained later.
In a second embodiment, the discrete zones 20, 21 may be created via energetic
radiation
treatment. By applying energetic radiation to the surface of the water-soluble
substrate, portions
of the substrate material are cross-linked and as such, the properties of the
substrate is locally
modified so as to provide zones which have a different solubility than the
rest of the water-
soluble substrate. Suitable energetic radiation treatments include
ultraviolet, X-ray, gamma ray,
beta ray and high electron beam treatment.
In yet another embodiment, the discrete zones 20, 21 may be created via a
combination
of a coating material and applying energy.
The first (and where applicable, second) discrete zones create patterns which
can be
random or non-random. When the discrete zones are created with coating
materials, they may be
transparent or translucent. Alternatively, they may be colored or three-
dimensional in order to
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
7
create appealing effects such as tactile (touch) effects or visual effects,
such as graphics,
cartoons, logo's, branding, user's instructions, and the like.
When the water-soluble substrate according to the present invention is however
immersed in water (i.e. in applications for which the substrate is designed to
be used and
required to dissolve), the coating is not sufficient to resist the water
contact and ensures that the
substrate dissolves rapidly.
Optional ingredients
It may be required for certain applications that the dissolution rate (when
immersed) of
the substrate is increased. Disintegrants may be added to the coating material
(in those
embodiments where the discrete zones are formed with a coating material) in
order to speed up
the dissolution when the water-soluble substrate is immersed in water.
Preferably, the level of
disintegrant in the coating is from 0.1 to 30%, preferably from 1 to 15%, by
weight of said
coating. Alternatively, disintegrants may also be applied on the surface of
the water-soluble
substrate 10 not covered by the discrete zones, or they may be integrated into
the water-soluble
film 10, or any combination thereof. Suitable disintegrants for use herein are
corn/potato starch,
methyl cellulose/celluloses, mineral clay powders, croscarmelose (cross-linked
cellulose),
crospovidine (cross-linked polymer), sodium starch glycolate (cross-linked
starch).
The water-soluble substrate-forming composition and the water-soluble
substrate 10
formed therefrom can also comprise one or more additive or adjunct
ingredients. For example,
the water-soluble substrate-forming composition and the water-soluble
substrate 10 may
contain: plasticizers, lubricants, release agents, fillers, extenders, anti-
blocking agents, de-
tackifying agents, antifoams, or other functional ingredients. The latter may,
in the case of
articles containing compositions for washing, include, but are not limited to
functional detergent
additives to be delivered to the wash water, for example organic polymeric
dispersants, or other
detergent additives.
Suitable plasticizers include, but are not limited to: glycerol, glycerin,
diglycerin,
hydroxypropyl glycerine, sorbitol, ethylene glycol, diethylene glycol,
triethylene glycol,
tetraethylene glycol, propylene glycol, polyethylene glycols, neopentyl
glycol,
trimethylolpropane, polyether polyols, ethanolamines, and mixtures thereof.
The plasticizer can
be incorporated in the water-soluble substrate 10 in any suitable amount
including amounts in
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
8
the range of from about 5% to about 30% by weight, or in the range of from
about 12% to about
20% by weight.
Suitable surfactants may include the nonionic, cationic, anionic and
zwitterionic classes.
Suitable surfactants include, but are not limited to, polyoxyethylenated
polyoxypropylene
glycols, alcohol ethoxylates, alkylphenol ethoxylates, tertiary acetylenic
glycols and
alkanolamides (nonionics), polyoxyethylenated amines, quaternary ammonium
salts and
quaternized polyoxyethylenated amines (cationics), and amine oxides, N-
alkylbetaines and
sulfobetaines (zwitterionics). The surfactant can be incorporated in the water-
soluble substrate
in any suitable amount including amounts in the range of from about 0.01% to
about 1% by
10 weight, or in the range of from about 0.1% to about 0.6% by weight.
Suitable lubricants/release agents include, but are not limited to, fatty
acids and their
salts, fatty alcohols, fatty esters, fatty amines, fatty amine acetates and
fatty amides. The
lubricant/release agent can be incorporated in the water-soluble substrate 10
in any suitable
amount including amounts within the range of from about 0.02% to about 1.5% by
weight, or in
the range of from about 0.04% to about 0.15% by weight.
Suitable fillers, extenders, antiblocking agents, detackifying agents include,
but are not
limited to: starches, modified starches, crosslinked polyvinylpyrrolidone,
crosslinked cellulose,
microcrystalline cellulose, silica, metallic oxides, calcium carbonate, talc
and mica. The filler,
extender, antiblocking agent, detackifying agent can be present in the water-
soluble substrate 10
in any suitable amount including amounts in the range of from about 0.1% to
about 25% by
weight, or in the range of from about 1% to about 15% by weight. In the
absence of starch, it
may be desirable for the filler, extender, antiblocking agent, detackifying
agent to be present in a
range of from about 1% to about 5% by weight.
Suitable antifoams include, but are not limited to, those based on
polydimethylsiloxanes
and hydrocarbon blends. The antifoam can be present in the water-soluble
substrate 10 in any
suitable amount including amounts in the range of from about 0.001% to about
0.5%, or in the
range of from about 0.01% to about 0.1% by weight.
The water-soluble substrate-forming composition is prepared by mixing the
materials
and agitating the mixture while raising the temperature from about 70 F (about
21 C) to 195 F
(about 90 C) until solution is complete. The substrate-forming composition
may be made into
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
9
any suitable form (e.g. film or sheets) and may then be subsequently formed
into any suitable
product (e.g. single- and multiple-compartment pouches, sachets, bags, etc.).
Methods of Making a Water-Soluble Substrate
There are numerous non-limiting embodiments of the method of making the water-
soluble substrate 10 described herein.
In one embodiment, the method comprises providing a previously formed water-
soluble
substrate 10 and creating first discrete zones 20 on one or both of the
substrate's surfaces 12, 14.
Optionally, second discrete zones 21 may be created consequently.
Where the discrete zones are formed using a coating material, this material
can be
applied to the previously formed water-soluble substrate 10 in a number of
different manners.
In one non-limiting embodiment, the coating material is applied to at least
one of the surfaces
12, 14 of the previously formed water-soluble substrate 10 in the form of
particles or a powder.
Preferably, the particles or the powder are applied to the water-soluble
substrate 10 via a jet, or
electro-statically. Due to the high speed of the jet, some of the
particles/powder is embedded
into the substrate, thereby reducing, or even eliminating the need for using a
binder. Also when
the particles or powder are applied electro-statically, a binder is generally
not needed.
Nevertheless, a binder may be used. The binder may first be applied to the
water-soluble
substrate 10, before the particles or powder is applied. Or, alternatively,
the binder may be
mixed with the particles or powder, and then the mixture is added to the water-
soluble substrate
10.
In another non-limiting embodiment of the method, the coating material is
provided in
the form of a solution that is applied onto at least one of the surfaces 12,
14 of the water-soluble
substrate 10, and is allowed to dry, or undergoes a drying process. The
solution can be applied
on the film by means of any coating process, including spray, knife, rod,
kiss, slot, painting,
printing and mixtures thereof. Printing is preferred for use herein. Printing
is a well established
and economic process. Printing is usually done with inks and dyes and used to
impart patterns
and colours to substrates but in the case of the invention printing is used to
deposit the less
water-soluble material(s) onto a water-soluble substrate. Any kind of printing
method can be
used, including rotogravure, lithography, flexography, porous and screen
printing, inkjet
printing, letterpress, tampography and combinations thereof.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
These embodiments may also comprise a step of wetting at least a portion of at
least one
of the surfaces 12, 14 of the water-soluble substrate 10 prior to applying the
coating material to
the previously formed water-soluble substrate 10. The wetting of at least one
of the surfaces 12,
14 of the water-soluble substrate 10 may be used to at least partially
dissolve or solubilize an
5 outer portion of the surface 12, 14 of the substrate 10 (that is, part of
the way into the thickness
of the substrate). The water-soluble substrate 10 may be at least partially
solubilized to any
suitable depth in order to partially embed the coating into the substrate.
Suitable depths include,
but are not limited to: from about 1% to about 40% or about 45%, from about 1%
to about 30%,
from about 1% to about 20%, from about 1% to about 15%, and alternatively,
from about 1% to
10 about 10% of the overall substrate thickness 16. The coating material is
then applied to the
partially dissolved portion of at least one of the surfaces 12, 14 of the
substrate 10. This allows
the coating material to be embedded into an outer portion of the surface 12,
14 of the substrate
10, and to become a more permanent part of the substrate 10. The wetted
surface 12, 14 of the
substrate 10 with the coating material embedded into the same is then
permitted to dry. Such an
embodiment of the method may also comprise a step of removing at least some of
any loose or
excess of coating material remaining on the surface of the water-soluble
substrate 10 after it has
dried, such as by wiping or dusting the surface of the substrate 10.
In another embodiment, the coating material 20 can be added to the water-
soluble
substrate 10 after the substrate 10 is made into a product. For example, if
the water-soluble
substrate 10 is used to form a water-soluble pouch that contains a
composition, the coating
material can be added to the substrate 10 on at least a portion of the surface
of the water-soluble
pouch.
Where the first (and/or second) discrete zones are created via energetic
radiation
treatment, the radiation (as previously described) can be effected on
previously formed
substrates or on substrates which have already been made into a product.
Methods of Making a Water-Soluble Pouch
The water-soluble substrate 10 described herein can be formed into articles,
including
but not limited to those in which the water-soluble substrate 10 is used as a
packaging material.
Such articles include, but are not limited to water-soluble pouches, sachets,
and other containers.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
11
Water-soluble pouches and other such containers that incorporate the water-
soluble
substrate 10 described herein can be made in any suitable manner known in the
art. The water-
soluble substrate 10 can be provided with improved resistance to dissolution
either before or
after forming the same into the final product. In either case, in certain
embodiments it is
desirable when making such articles, that the surface 12, 14 of the substrate
10 onto which the
discrete zones are created, forms an outer surface of the product.
There are a number of processes for making water-soluble pouches. These
include, but
are not limited to processes known in the art as: vertical form-fill- sealing
processes, horizontal
form-fill sealing processes, and formation of the pouches in molds on the
surface of a circular
drum. In vertical form-fill-sealing processes, a vertical tube is formed by
folding a substrate.
The bottom end of the tube is sealed to form an open pouch. This pouch is
partially filled
allowing a head space. The top part of the open pouch is then subsequently
sealed together to
close the pouch, and to form the next open pouch. The first pouch is
subsequently cut and the
process is repeated. The pouches formed in such a way usually have pillow
shape. Horizontal
form-fill sealing processes use a die having a series of molds therein. In
horizontal form-fill
sealing processes, a substrate is placed in the die and open pouches are
formed in these molds,
which can then be filled, covered with another layer of substrate, and sealed.
In the third process
(formation of pouches in molds on the surface of a circular drum), a substrate
is circulated over
the drum and pockets are formed, which pass under a filling machine to fill
the open pockets.
The filling and sealing takes place at the highest point (top) of the circle
described by the drum,
e.g. typically, filling is done just before the rotating drum starts the
downwards circular motion,
and sealing just after the drum starts its downwards motion.
In any of the processes that involve a step of forming of open pouches, the
substrate can
initially be molded or formed into the shape of an open pouch using
thermoforming, vacuum-
forming, or both. Thermoforming involves heating the molds and/or the
substrate by applying
heat in any known way such as contacting the molds with a heating element, or
by blowing hot
air or using heating lamps to heat the molds and/or the substrate. In the case
of vacuum-
forming, vacuum assistance is employed to help drive the substrate into the
mold. In other
embodiments, the two techniques can be combined to form pouches, for example,
the substrate
can be formed into open pouches by vacuum-forming, and heat can be provided to
facilitate the
process. The open pouches are then filled with the composition to be contained
therein.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
12
The filled, open pouches are then closed, which can be done by any method. In
some
cases, such as in horizontal pouch-forming processes, the closing is done by
continuously
feeding a second material or substrate, such as a water-soluble substrate,
over and onto the web
of open pouches and then sealing the first substrate and second substrate
together. The second
material or substrate can comprise the water-soluble substrate 10 described
herein. It may be
desirable for the surface of the second substrate on which the less water-
soluble material is
distributed, to be oriented so that it forms an outer surface of the pouch.
In such a process, the first and second substrates are typically sealed in the
area between
the molds, and, thus, between the pouches that are being formed in adjacent
molds. The sealing
can be done by any method. Methods of sealing include heat sealing, solvent
welding, and
solvent or wet sealing. The sealed webs of pouches can then be cut by a
cutting device, which
cuts the pouches in the web from one another, into separate pouches. Processes
of forming
water-soluble pouches are further described in U.S. Patent Application Serial
No. 09/994,533,
Publication No. US 2002/0169092 Al, published in the name of Catlin, et al.
Articles of Manufacture
As shown in Fig. 4, the present invention may also include articles comprising
a product
composition 40 and a water-soluble substrate 10, which may be formed into a
container 30, such
as a pouch, a sachet, a capsule, a bag, etc. to hold the product composition.
The surface of the
water-soluble substrate 10 with the first discrete zones, may be used to form
an outside surface
of the container 30. The water-soluble substrate 10 may form at least a
portion of a container 30
that provides a unit dose of the product composition 40.
For simplicity, the articles of interest herein will be described in terms of
water-soluble
pouches, although it should be understood that discussion herein also applies
to other types of
containers.
The pouches 30 formed by the foregoing methods, can be of any form and shape
which
is suitable to hold the composition 40 contained therein, until it is desired
to release the
composition 40 from the water-soluble pouch 30, such as by immersion of the
water-soluble
pouch 30 in water. The pouches 30 can comprise one compartment, or two or more
compartments (that is, the pouches can be multi-compartment pouches). In one
embodiment,
the water-soluble pouch 30 may have two or more compartments that are in a
generally
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
13
superposed relationship and the pouch 30 comprises upper and lower generally
opposing outer
walls, skirt-like side walls, forming the sides of the pouch 30, and one or
more internal
partitioning walls, separating different compartments from one another. If the
composition 40
contained in the pouches 30 comprises different forms or components, the
different components
of the composition 40 may be contained in different compartments of the water-
soluble pouch
30 and may be separated from one another by a barrier of water-soluble
material.
The pouches or other containers 30 may contain a unit dose of one or more
composition
40 for use as/in laundry detergent compositions, automatic dishwashing
detergent compositions,
hard surface cleaners, stain removers, fabric enhancers and/or fabric
softeners, food and
beverage and new product forms where contact with small amounts of water could
create
premature pouch dissolution, unwanted pouch leakage and/or undesirable pouch-
to-pouch
stickiness. The compositions 40 in the pouches 30 can be in any suitable form
including, but not
limited to: liquids, liquigels, gels, pastes, creams, solids, granules,
powders, etc. The different
compartments of multi-compartment pouches 30 may be used to separate
incompatible
ingredients. For example, it may be desirable to separate bleaches and enzymes
into separate
compartments. Other forms of multi-compartment embodiments may include a
powder-
containing compartment in combination with a liquid-containing compartment.
Additional
examples of multiple compartment water-soluble pouches are disclosed in U.S.
Patent 6,670,314
B2, Smith, et al.
The water-soluble pouches 30 may be dropped into any suitable aqueous solution
(such
as hot or cold water), whereupon water-soluble substrate material 10 forming
the water-soluble
pouches 30 dissolves to release the contents of the pouches. The substrate and
the pouches
described herein may be soluble or dispersible in water, and have a water-
solubility of at least
about 50%, alternatively at least about 75%, or even at least about 95%, by
weight. The
solubility of the substrate and the pouches may be measured by a method of
adding either a
piece of substrate, or one of the pouches (including the substrate comprising
the same) to
distilled water, stirring the distilled water containing either the substrate
or pouch vigorously
using a magnetic stirrer, and filtering the water containing the substrate or
pouch using a glass-
filter with a maximum pore size of 20 microns. The dry weight of material
collected on the
filter is then compared to the weight of the initial sample, and is expressed
as a percentage.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
14
The water-soluble substrate 10 described herein can also be used for coating
products
and other articles. Non-limiting examples of such a product are laundry
detergent tablets or
automatic dishwashing detergent tablets.
Examples
A first substrate is prepared by printing an Intelimer Poly Tap 8000 (Landec
Corporation)
coating in discrete zones on one surface of a standard 3 mil soluble M8630
film supplied by
Monosol. The Intelimer coating is based on a polymer whose solubility is
temperature
activated (at 33 C) and is mixed with water (20% Intellimer , 80% water). The
final film
resulted partially coated (approx. 20% of area) with polymer particles
insoluble at 23 C water
testing conditions.
A second substrate is prepared by printing an Intelimer Poly Tap 8000 (Landec
Corporation)
coating in discrete zones on one surface of a standard 3 mil soluble M8630
film supplied by
Monosol. This time, the Intelimer coating is mixed with water to create a 50%
Intellimer /50%
water coating. The final film resulted partially coated (approx. 50% of area)
with polymer
particles insoluble at 23 C water testing conditions.
Test Methods
Droplet Test Method
To determine if a substrate is resistant to accidental water contact a Droplet
Test method has
been developed. In this test, a pouch (approx. 2"x2") is formed from the above
film in a cavity
and a droplet of 0.2 ml of room temperature water is added to the formed side
of the pouch. The
formed side is the stressed case for this test since the film is thinned
during cavity formation. A
stopwatch is started as soon as the water contacts the pouch and the time when
significant film
deformation in the body of the pouch is observed, is recorded. This time,
termed "Time to
Deform" is a precursor to film failure.
Full Solubility (full bath).
The above formed film is immersed in a agitated 23 C water bath and the time
to completely
(visually) dissolve the film is recorded.
CA 02668555 2009-05-04
WO 2008/053381 PCT/IB2007/052648
Results
Material Time to Deform Film Solubility (full bath)
Uncoated M8630 film by 15 seconds 49 seconds
Monosol, 3 mil thickness
M8630 coated with 20% 29 seconds 127 seconds
Intelimer coating
M8630 coated with 50% 29 seconds 180 seconds
Intelimer coating
At coated film exposure temperatures above the Intelimer solubility
activation temperature of
33 C, the overall coated film solubility drops as expected due to the quicker
solubilization of the
5 Intellimer polymer material.
The dimensions and values disclosed herein are not to be understood as being
strictly limited to
the exact numerical values recited. Instead, unless otherwise specified, each
such dimension is
intended to mean both the recited value and a functionally equivalent range
surrounding that
10 value. For example, a dimension disclosed as "40 mm" is intended to mean
"about 40 mm."