Note: Descriptions are shown in the official language in which they were submitted.
CA 02668749 2011-03-04
AN APPARATUS FOR EXTRACORPOREAL BLOOD TREATMENT
The present invention relates to an apparatus for extracorporeal blood
treatment.
Specifically, though not exclusively, the invention can be usefully applied in
an
apparatus for treating kidney failure (hemodialysis and/or
hemo(dia)filtration).
Such an apparatus is already disclosed in US 4348280, which describes a
dialysis machine provided with means for continuous preparation of dialyzing
liquid by mixing water with dialysis concentrate. The dialysis machine
includes a
device for removing and minimizing gas in the dialysis liquid, as well as
means
io for controlling pressure and flow rate of the dialysing liquid in the
dialyzer. The
means for controlling comprise a first flow rate limiter which is located
upstream
of the dialyzer and a second flow limiter located downstream of the dialyzer.
The
degassing device comprises a degassing reservoir which is continuously under
a negative pressure supplied by two pumps: the first pump removing gas from
the tank and the second removing degassed water from the tank. The pressure
in the degassing reservoir is therefore unrelated to the dialysing liquid
rate. The
first gas pump is a constant speed pump producing a negative pressure in the
degassing reservoir which is not affected by the flow rate of liquid to be
degassed. The first gas pump is arranged on the drainage line connecting the
dialyzer outlet to the discharge. The first pump is therefore useful both for
applying a negative pressure on the degassing reservoir and for aspirating the
used dialysis liquid from the dialyzer. The above mentioned prior art machine
has the drawback of being unable to permit either a precise control over the
gas
quantity which has been removed from the dialysis liquid, or a regulation of
the
percentage of gas contained in the dialysis liquid operating inside the
dialyzer. A
further drawback is that it cannot estimate variation in the fluid flow rate
through
the vent line of the degassing reservoir.
CA 02668749 2011-03-04
-2-
SUMMARY OF THE INVENTION
A purpose of the invention is to provide an apparatus for extracorporeal blood
treatment provided with a system for fluid aspiration, which apparatus is
independent of the operative conditions (particularly pressure and treatment
fluid
flow rate) in the blood treatment device.
A further purpose of the invention is to provide a device which is
constructionally
simple and economical.
An advantage of the invention is that it provides an apparatus whose operating
conditions are not affected by the situation (especially by the pressure
level) at
io the discharge of the used treatment fluid.
The invention also has the advantage of providing, in a blood treatment
apparatus, a fluid aspiration system which is highly versatile, reliable,
effective,
easily adaptable to many possible uses, among which the evacuation of the
liquid used for the tangential flushing of the ultrafilters, or the evacuation
of fluids
from various parts of the apparatus (such as gas evacuation from the degassing
system upstream and/or downstream of the blood treatment device, or liquid and
gas evacuation from the extracorporeal circuit during the priming stage).
A further advantage of the invention is that it provides a device which is
equipped with a protection system for the hydraulic circuit against any
possible
pressure change in the used treatment fluid discharge.
According to one aspect of the present invention, there is provided an
apparatus
for extracorporeal blood treatment comprising:
CA 02668749 2011-03-04
2a -
a blood treatment device having a blood chamber, a fluid chamber, and a
semipermeable membrane separating the blood chamber from the fluid
chamber;
a used fluid line configured to connect the fluid chamber to a used fluid
discharge;
a first actuator predisposed in the used fluid line, the first actuator being
configured to control a pressure and/or a fluid flow rate in the fluid
chamber;
a second actuator arranged in the used fluid line between the first actuator
and the used fluid discharge, the second actuator being configured to control
a
io pressure in an intermediate tract of the used fluid line, said intermediate
tract
being comprised between the first actuator and the second actuator;
characterised in that it further comprises:
a first pressure sensor configured to emit a pressure signal indicating a
pressure in said intermediate tract of the used fluid line;
a control unit programmed for controlling the second actuator as a function
of said pressure signal.
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-3-
[0011] Further characteristics and advantages of the present invention
will better emerge from the detailed description that follows, of at least one
preferred but not exclusive embodiment of the invention, which is
described by way of non-limiting example in the appended figures of the
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] In the following description reference is made to the appended
drawings, which are provided by way of example and therefore have no
limiting purpose and in which figure 1 schematically shows the apparatus
io of the invention.
DETAILED DESCRIPTION
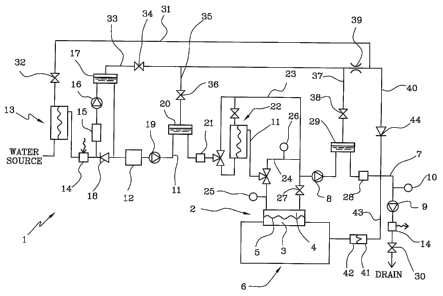
[0013] In figure 1, 1 denotes in its entirety an apparatus for
extracorporeal blood treatment. In this specific case the apparatus is an
apparatus for treating kidney failure, more particularly a hemodialysis
apparatus. The invention is usable in any kind of hemodialysis and/or
hemo(dia)filtration apparatus. In this specific case, in order to provide a
clear and simple explanation, not all the components of a hemodialysis or
hemo(dia)filtration apparatus, will be explicitly described, such as the
disinfection system, the blood loss detection system, the monitoring and
control system for the various parameters of the apparatus (such as
temperature, conductivity, pH, pressure etc. in the dialysis fluid), the
substitute fluid infusion system in case of hemo(dia)filtration etc.
[0014] The apparatus 1 comprises a blood treatment device 2 (dialyzer
or hemo(dia)filter) having a blood chamber 3, a fluid chamber 4 and a
semipermeable membrane 5 which separates the blood chamber 3 from the
fluid chamber 4.
[0015] The apparatus 1 comprises a prior art extracorporeal blood circuit
which is known and denoted in its entirety by 6, for connecting the blood
chamber 3 to a patient's vascular access (not shown) during treatment. The
3o extracorporeal circuit 6 comprises any extracorporeal circuit usable for
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-4-
blood circulation in a hemodialysis and/or hemo(dia)filtration apparatus. In
particular the extracorporeal blood circuit comprises an arterial blood line
for removing blood to be treated from the vascular access and sending it to
chamber 3, and a venous blood line to return the treated blood from blood
chamber 3 to the vascular access. Each blood line (arterial or venous)
comprises a device end with a connection to blood chamber 3 and a patient
end with a connection to the vascular access. Each blood line (arterial and
venous) also has the various elements (expansion chambers, clamps,
service lines, syringe accesses, etc.) which a blood line is normally
io provided with; in this description these elements are not described in
order
to provide a short and clear exposition. Figure 1 shows the blood
extracorporeal circuit 6 in a priming configuration.
[0016] The apparatus 1 comprises a used fluid line 7 for connecting the
fluid chamber 4 to a used fluid discharge. A first actuator is predisposed in
the used fluid line. The first actuator is in particular a first fluid
circulation
pump 8, which, when operating, is able to change pressure and flow rate of
the fluid crossing the fluid chamber 4. This first pump 8 is a positive
displacement pump (for instance a gear pump).
[0017] A second actuator is arranged in the used fluid line 7 downstream
of the first actuator. The second actuator is in this case a second fluid
circulating pump 9, in particular a positive displacement pump (for instance
a gear pump). The second actuator, which is arranged in series with the first
actuator along line 7, is capable, in particular, of varying a pressure in an
intermediate tract of the used fluid line 7, i. e. in the tract between the
first
and the second actuator.
10018] The apparatus 1 comprises a first pressure sensor 10 designed to
emit a pressure signal indicating pressure in the above-mentioned
intermediate tract of the used fluid line 7. In the specific case the pressure
sensor 10 is arranged along the used fluid line 7 upstream of the second
pump 9.
[0019] The apparatus 1 comprises a control unit (not shown)
programmed to control the second pump 9 in accordance with the above-
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-5-
described pressure signal. In particular the control unit controls the second
pump 9 in feedback so as to keep pressure upstream of the second pump 9
at a desired value; the desired value can change according to need, situation
or operating mode of the apparatus; besides, the desired pressure value can
be constant or can be changed according to predetermined criteria.
[0020] The apparatus comprises a fresh fluid line 11 for connecting a
source of a treatment fluid to the fluid chamber 4 and/or to the
extracorporeal blood circuit 6 (depending on whether the treatment is
hemodialysis or hemo(dia)filtration). In this particular case the fresh fluid
io line 11 is connected to a device for preparing a treatment fluid, which is
schematically shown and globally referred to as 12. The preparation device
can be any device for preparing a treatment fluid (such as a dialysis or a
substitute fluid). In particular the preparation device 12 is specially
designed to prepare a treatment fluid by mixing water with solid and/or
liquid concentrates. The fresh fluid line 11 in this case has an initial tract
connecting the preparation device 12 to a water source. The initial tract is
equipped with a first ultrafilter 13 having a retentate chamber (upstream
chamber) connected to a source of a fluid to be ultrafiltrated (water), a
filtrate chamber (downstream chamber) connected to the fluid chamber 4
through the preparation device 12, and a semipermeable membrane, for
instance of the hollow fibre bundle type which separates the retentate
chamber from the filtrate chamber. The apparatus 1 comprises a pressure
regulator (not shown) predisposed in the fresh fluid line 11 before the first
ultrafilter 13. The pressure regulator controls the downstream pressure at a
predefined pressure. The pressure regulator maintains the. downstream
pressure at the predefined pressure irrespective of the upstream pressure
(i.e. the pressure at the water source). The pressure regulator may control
the downstream pressure by restricting the flow. The pressure regulator
may be manually adjusted at the predefined downstream pressure. The
3o apparatus 1 further comprises an inlet valve (not shown) arranged in the
fresh fluid line 11 between the pressure regulator and the first ultrafilter
13.
The inlet valve is normally closed and is opened to allow water supply.
[0021] The apparatus 1 also comprises a heat exchanger 14 predisposed
after the first ultrafilter 13 in order to recover heat from the used
treatment
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-6-
fluid. After the heat exchanger 14 the fresh fluid line 11 has a degassing
(and heating) loop of the fluid (water) including a heater 15, a degassing
pump 16, and a first liquid-gas separator 17 (degassing chamber). A
temperature sensor (not shown) controls the heater 15. A degassing choke
(not shown) causes a lowering of the water pressure and a subsequently
easier water degassing in the separator 17. On the main line (fresh fluid line
11) a check valve 18 prevents the fluid flow from a first branch point
(removal of water to be degassed) of the degassing loop to a second branch
point (degassed water return) of the same loop placed upstream of the first
io branch. The fresh fluid line 11 is further provided with a supply pump 19
(for example of the gear pump type) to circulate fluid in the same line to
the fluid chamber 4. A second liquid-gas separator 20 (degassing chamber)
is arranged on line 11 downstream of the preparation device 12 to degas the
treatment fluid. A first flow sensor 21 is placed downstream the second
separator 20 to emit a signal indicating the flow rate in the fresh fluid line
11. A second ultrafilter 22 is arranged before the fluid chamber 4 in order
to ultrafilter the treatment fluid. A first by-pass line 23 is arranged
upstream of the second ultrafilter 22, while a second by-pass line 24 is
arranged downstream of the second ultrafilter 22. Each by-pass line 23 and
24 by-passes the fluid chamber 4 and puts the fresh fluid line 11 in
communication with the used fluid line 7. Each by-pass line 23, 24 is
provided with a by-pass valve (in this particular case a three-way valve in.
order to selectively open or shut the by-pass line and the main line). A
pressure sensor upstream 25 and a pressure sensor downstream 26 are pre-
arranged to measure pressure respectively on the inlet and outlet of the
fluid chamber 4. An on-off valve 27 can shut the outlet of the fluid
chamber 4 on command of the control unit.
[0022] A second flow sensor 28 is predisposed to send to the control
unit a signal indicating the fluid flow rate in the used fluid line 7. The
control unit operates the first pump 8 according to the flow rate signal
emitted by the second sensor 28 in order to achieve a desired fluid balance
in the treatment device 2. The fluid balance depends, as is known, on the
fluid flow rate through the membrane 5 (ultrafiltration rate), which rate
results from the difference between the flow rates measured by the now
sensors 28 and 21. In order to get the desired fluid balance, the rate signal
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-7-
emitted by the first sensor 21 can be used, as known, to command the
supply pump 19 or the first pump 8. A third liquid-gas separator 29
(degassing chamber) is arranged on the used fluid line 7 upstream of the
second flow sensor 28. The used fluid line 7 is also provided with an on-off
valve 30 located downstream of the second pump 9.
[0023] The apparatus 1 comprises a fluid balance control device or fluid
balance system for controlling the ultrafiltration rate through the membrane
5. The fluid balance system includes in this case the first flowmeter 21, the
second flowmeter 28, and the first pump 8. In other embodiments (not
io shown) the fluid balance system may be of different (known) type, e.g. a
system comprising an equalizing device (with equalizing volumetric
chambers, or with equalizing flowmeters, or of another type) and an
ultrafiltration line bypassing the equalizing device.
[0024] The intermediate tract of line 7 between the first pump 8 and the
second pump 9 is kept at a desired pressure by controlling the second pump
9 in accordance with the signal emitted by the pressure sensor 10. The
intermediate tract is connected or designed to be connected to various
elements of the apparatus 1. Firstly the above mentioned intermediate tract
is connected to an outlet of the retentate chamber of the first ultrafilter 13
through a tangential flushing line 31. The flushing line 31 is used for the
tangential washing of the first ultrafilter 13. A flushing valve 32, arranged
on the flushing line 31, is periodically opened in order to carry out the
tangential washing. Secondly, the intermediate tract is connected to a gas
outlet (vent) of the first separator 17 through a first vent line 33 provided
with a first vent valve 34. The intermediate tract is further connected to a
gas outlet (vent) of the second separator 20 through a second vent line 35,
provided with a second vent valve 36. The above-mentioned intermediate
tract is also connected to a gas outlet (vent) of the third separator 29
through a third vent line 37 provided with a third vent valve 38. The
various vent lines 33, 35, 37 are connected to the intermediate tract through
a single restrictor choke 39, which serves all lines. The choke 39 is formed
by a fixed pre-calibrated section restriction. The various vent lines 33, 35,
37 unite to the flushing line 31 to form a unique main branch line 40 which
branches from a branch point of the used fluid line 7, the branch point of
CA 02668749 2011-03-04
-8-
used fluid line 7 being located between the second flow sensor 28 and the
second pump 9. Each vent valve 34, 36, 38 is opened and shut according to a
predetermined rule (for instance periodically, or depending on the liquid
level or
on the pressure level in the corresponding separator 17, 20, 29). The branch
line
40 is provided with a check valve 44 to prevent return flow to the separators
and
to the ultrafilter.
The intermediate tract between pump 8 and pump 9 is further connected to a
connection port 41 suitable for connection to an access port 42 to the
extracorporeal circuit 6. The connection between port 41 and port 42 is a seal
io connection. The connection between port 41 and port 42 is removable. The
connection between port 41 and port 42 includes the connection disclosed in US
Pat. No. 5,041,215 between drain 32 and, respectively, priming cap 44
(reference numbers as cited by US Pat. No. 5,041,215). (The connection
between port 41 and port 42 may include, in other embodiments not shown, a
screw connection, particularly of the luer type, or other known removable
connections). The connection port 41 is arranged on the external panel of the
hemodialysis or hemo(dia)filtration apparatus. The access port 42 comprises,
in
this particular case, a connection placed on a patient end of one of the blood
lines of the extracorporeal circuit 6, for instance the arterial line or the
venous
line. Alternatively the access port 42 can be a connection arranged in a
branch-
off relationship with the arterial or venous blood line. The access port 42
can be,
for example, a connection arranged on a service line connected to the arterial
line or to the venous line; in particular the service line can be connected to
an
arterial or venous blood expansion chamber. The configuration in which ports
41
and 42 are connected to each other (as in figure 1) is used particularly in
the
priming stage of the extracorporeal circuit in order to perform fluid
evacuation
(air and a part of the priming liquid used) through the discharge of the
treatment
apparatus 1. In this case the second pump 9 is used for the aspiration of both
the air and the used priming liquid. The connection port 41 is connected to a
3o discharge line 43 branching out from a branch point of the used fluid line
7, the
branching point being located between the second flow sensor 28 and the
second pump 9. The priming liquid can be sourced by connecting the
extracorporeal circuit to a priming liquid container (for
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-9-
example a saline solution bag), or by back-filtrating a sterile liquid (for
example dialysis fluid) from the fluid chamber 4 to the blood chamber 3. In
case of backfiltration a difference in pressure between the fluid chamber 4
and the blood chamber 3 is generated for instance by aspiration via the
second pump 9 and/or by aspiration using a blood pump associated to the
extracorporeal circuit 6.
[0026] The second fluid circulation pump 9 is arranged along the main
pathway of the used treatment fluid. The second pump 9 is arranged in
series with the first pump 8. The inlet of the second pump 9 is designed to
io receive the used fluid (the entire amount or essentially the entire amount
of
the used fluid, eventually minus the weight loss) coming from the outlet of
the first pump 8. The second pump 9 does not control the ultrafiltration
through the membrane 5. The second pump 9 is not responsible for the
fluid balance. The second pump 9 is not part of the fluid balance system of
the apparatus. The second pump 9 is placed downstream the first pump 8.
The second pump 9 is placed between the used fluid discharge and the first
pump 8. The second pump 9 is used to protect the hydraulic circuit of
apparatus 1 from any possible pressure change in the used fluid discharge.
In particular the second pump 9 has a protective role of the fluid balance
system, the circuit gas evacuation system, the flushing system of an
ultrafilter, and the used priming liquid evacuation system. In short the
second pump 9 acts to uncouple the discharge of the used fluid line 7
(whose conditions are sometimes uncontrollable, unpredictable,
considerably changeable from one clinic to another) from the various
operative elements of the apparatus 1, so that any pressure change at the
discharge does not perturb the rest of the apparatus 1. For example, the
second pump 9 can keep the pressure measured by the sensor 10 at a
constant level (for instance about 0 mmHg during treatment), or can act so
that pressure change occurs according to a predetermined sequence (in
particular with negative values, i.e. values lower than 0 mmHg, in the
priming stage of the extracorporeal circuit 6). The second pump 9 operates
in order to keep a constant pressure, particularly at a predetermined value
(for instance about 0 mmHg), at the branch point of the fluid line/s
communicating with one or more operative elements of the apparatus,
where the operative elements can comprise a gas-liquid separator of the
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-10-
treatment fluid circuit, a blood line or a service line of the extracorporeal
circuit, an ultrafilter of the treatment fluid circuit, etc.
[0027] = In a further embodiment of the invention (not shown), the heat
exchanger 14 is also arranged upstream of the second pump 9, so that it is
uncoupled from and therefore not perturbed by what happens in the
discharge.
[0028] The second pump 9 facilitates the venting of the separators 17,
20, 29. This keeps pressure at a relatively low level in the entire hydraulic
circuit of the apparatus. In particular this keeps pressure at a relatively
low
io level at the inlet of the fresh fluid line 11 (which is located upstream of
the
first ultrafilter 13, after the inlet connection connected to the municipal
water supply providing water at a relatively high pressure). The inlet
pressure is set by a pressure regulator (as a rule a known pressure reducer,
not shown in figure 1) at a constant pre-set value which is high enough to
enable the above mentioned separators to breathe. The aspirating action of
the second pump 9 makes it possible to pre-set a relatively low value in the
pressure regulator.
[0029] In a further embodiment of the invention (not shown), the supply
pump 19 is arranged in the fresh fluid line 11 downstream the second gas-
liquid separator 20. The supply pump 19 is arranged between the separator
20 and the first by-pass line 23, or between the separator 20 and the first
flow sensor 21, or between the separator 20 and the fluid balance system,
or between the first flow sensor 21 (or the fluid balance system) and the
first by-pass line 23, or between the fluid balance system and the first by-
pass line 23.
[0030] Legend:
1 extracorporeal blood treatment apparatus
2 blood treatment device
3 blood chamber
4 fluid chamber
5 semipermeable membrane
6 extracorporeal blood circuit
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-11-
7 used fluid line
8 first fluid circulation pump
9 second fluid circulation pump
first pressure sensor
5 11 fresh fluid line
12 treatment fluid preparation device
13 first ultrafilter
14 heat exchanger
heater
10 16 degassing pump
17 first gas-liquid separator
18 check valve
19 supply pump
second gas-liquid separator
15 21 first flow sensor
22 second ultrafilter
23 first by-pass line
24 second by-pass line
upstream pressure sensor
20 26 downstream pressure sensor
27 on-off valve
28 second flow sensor
29 third gas-liquid separator
on-off valve
25 31 tangential flushing line
32 flushing valve
33 first vent line
34 first vent valve
second vent line
30 36 second vent valve
37 third vent line
38 third vent valve
39 restrictor choke
branch line
35 41 connection port
42 access port
CA 02668749 2009-05-06
WO 2008/059305 PCT/IB2006/003215
-12-
43 discharge line
44 check valve