Note: Descriptions are shown in the official language in which they were submitted.
CA 02670049 2010-06-30
ROCKS AND AGGREGATE, AND METHODS OF
MAKING AND USING THE SAME
BACKGROUND
Carbon dioxide (C02) emissions have been identified as a major contributor to
the phenomenon of
global warming and ocean acidification. CO2 is a by-product of combustion and
it creates operational,
economic, and environmental problems. It is expected that elevated atmospheric
concentrations of (:O2 and
25 other greenhouse gases will facilitate greater storage of heat within the
atmosphere leading to enhanced
surface temperatures and rapid climate change. The impact of climate change
will likely he economically
expensive and environmentally hazardous. Reducing potential risks of climate
change will require
sequestration of atmospheric CO2.
30 SUMMARY
In one aspect the invention provides compositions. In some embodiments, the
invention provides
an aggregate containing a C02-sequestering component. The C02-sequestering
component may contain
one or more carbonate compounds; in some embodiments carbonate compounds make
up at least 50% w/w
of the aggregate, or at least 90% w/w of the aggregate, or at least 98% w/w of
the aggregate; optionally the
35 aggregate may also contain sulfate and/or sulfite, e.g. where the
sulfate/sulfite combined comprise at least
0.1% w/w of the aggregate. In some embodiments the carbonate compounds
comprise magnesium
carbonate, calcium carbonate, magnesium calcium carbonate, or a combination
thereof; in some of these
embodiments the molar ratio of calcium to magnesium in the aggregate is from
1/1 Ca/Mg to 1/10 Ca/Mg,
or from 150/1 Ca/Mg to 10/1 Ca/Mg, or from 2/1 Ca/Mg to 1/2 Ca/Mg. In some
embodiments the
40 invention provides aggregate containing a C02-sequestering component where
the aggregate has a carbon
1
CA 02670049 2009-07-02
isotopic fractionation (S13C) value more negative than (less than) -IO%o, or
more negative than -20%o. In
some embodiments the invention provides aggregate containing a C02-
sequestering component where the
aggregate has a bulk density of between 75 lb/ft3 and 125 lb/ lb/ft3, or
between 90 lb/ft3 and 115 lb/ lb/ft3.
In some embodiments the invention provides a structure containing aggregate
containing a C02-
sequestering component, e.g., one of the aggregates described in this
paragraph. Some exemplary
structures of the invention include a building, a roadway, or a dam. In some
embodiments, the structure is a
roadway, for example a roadway that sequesters at least 1 ton of CO2 per lane
mile of roadway, or a
roadway that sequesters at least 100 tons of CO2 per lane mile of roadway, or
a roadway that sequesters at
least 1000 tons of CO2 per lane mile of roadway.
22. In some embodiments the invention provides an aggregate containing carbon
wherein the carbon
has a carbon isotopic fractionation (S13C) value more negative than (less
than) -10%o, or more negative than
-20%o, or more negative than -30%o. In some of these embodiments, the
aggregate contains carbonate, for
example, at least 10% w/w carbonate, or at least 50% w/w carbonate; the
aggregate may optionally further
contain a sulfate and/or a sulfite, such as a calcium or magnesium sulfate or
sulfite, and in some cases the
combined sulfate and sulfite comprise at least 0.1% w/w of the aggregate. In
some embodiments
containing carbonate the carbonate includes calcium carbonate, magnesium
carbonate, calcium magnesium
carbonate, or a combination thereof; for example, the calcium and magnesium
may be present in a
calcium:magnesium molar ratio between 200:1 and 1:2. In some embodiments the
invention provides an
aggregate containing carbon wherein the carbon has a carbon isotopic
fractionation (S1$C) value more
negative than (less than) -10%o, or more negative than -20%o, or more negative
than -30%o where the
aggregate has a bulk density of between 75 lb/ft 3 and 125 lb/ lb/ft3, for
example, between 90 lb/ft3 and 115
lb/ lb/ft3. In some embodiments the invention provides a structure containing
an aggregate containing
carbon wherein the carbon has a carbon isotopic fractionation (S1$C) value
more negative than (less than) -
10%o, or more negative than -20%o, or more negative than -30%o; in some
embodiments the structure is a
building, a roadway, or a dam. In some embodiments the structure is a roadway.
In some embodiments, the invention provides an aggregate containing 90-99.9%
carbonate, 0.1 to
10% sulfate and/or sulfite, in some embodiments the aggregate further contains
0.00000001 to 0.000001%
mercury or mercury-containing compound. In some embodiments the aggregate has
a carbon isotopic
fractionation (S13C) value more negative than -1 O%o. In some embodiments the
aggregate has a bulk
density of between 75 lb/ft3 and 125 lb/ lb/ft3, e.g., between 90 lb/ft3 and
115 lb/ lb/ft3. In some
embodiments the invention provides a structure containing an aggregate
containing 90-99.9% carbonate,
0.1 to 10% sulfate and/or sulfite, in some embodiments the aggregate further
contains 0.00000001 to
0.000001% mercury or mercury-containing compound; exemplary structures include
a building, a roadway,
or a dam. In some embodiments the structure is a roadway.
In another aspect the invention provides methods. In some embodiments the
invention provides
method of sequestering CO2 comprising (i) precipitating a C02-sequestering
carbonate compound
composition from a divalent cation-containing water to form a precipitate; and
(ii) producing aggregate
containing the C02-sequestering carbonate compound composition; in some
embodiments the method
further includes contacting the divalent cation-containing water with CO2 from
an industrial waste gas
stream, such as flue gas from a power plant or a cement plant, e.g., flue gas
from a coal-fired power plant;
2
CA 02670049 2009-07-02
in some embodiments the method comprises contacting the divalent cation-
containing water with CO2 from
the combustion of a fossil fuel. In some embodiments the producing of the
aggregate comprises subjecting
the precipitate to elevated temperature, elevated pressure, or a combination
thereof, such as elevated
temperature, elevated pressure, or combination thereof produced by an
extruder. In some embodiments the
divalent cations of the divalent cation-containing water come at least
partially from a saltwater, such as
seawater or brine, e.g., seawater. In some embodiments the producing of the
aggregate includes producing
aggregate of a predetermined size and shape.
In some embodiments the invention provides a method of manufacturing aggregate
by a process
that includes precipitating a carbonate compound from a divalent cation-
containing water and processing
the precipitate to produce an aggregate; in some embodiments the method
further includes contacting
contacting the divalent cation-containing water with CO2 from an industrial
waste gas stream, such as flue
gas from a power plant or a cement plant, e.g. flue gas from a coal-fired
power plant. In some
embodiments the method includes contacting contacting the divalent cation-
containing water with CO2
from the combustion of a fossil fuel such as natural gas or coal, e.g., coal.
In some embodiments the
processing of the precipitate includes treating the precipitate with elevated
temperature, elevated pressure,
or a combination thereof. In some embodiments the processing of the
precipitate comprises combining the
precipitate with a cementitious material and water, allowing the combination
to set to provide a solidified
material, and may further include breaking up the solidified material.
In some embodiments the invention provides a system for producing an aggregate
that includes (i)
an input for a divalent cation-containing water; (ii) a carbonate compound
precipitation station that subjects
the water to carbonate compound precipitation conditions and produces a
precipitated carbonate compound
composition; and (iii) an aggregate producer for producing aggregate from the
precipitated carbonate
compound composition.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 provides a flow diagram of a precipitation process according to an
embodiment of the
invention.
Figure 2 provides a schematic of a system according to one embodiment of the
invention.
Figures 3 illustrates exemplary aggregate structures and aggregate mixtures
according to aspects of
the present invention 3A: cylinders; 3B: triangular prism; 3C: mixture of
spheres and bridges; 3D: gap-
graded spheres; 3E: mixture of prisms; 3F-3H: hollow aggregate with tubular
void space; 31-3L: aggregate
mixtures with different combinations of aggregates.
Fig. 4 provides an X-ray diffraction (XRD) spectrum for the precipitation
material produced in
Example 1.
Fig. 5 provides a thermogravimetic analysis (TGA) of wet precipitation
material produced in
Example 1.
Fig. 6 provides a TGA of dry precipitation material produced in Example 1.
Fig. 7 provides a Fourier transform-infrarad (FT-IR) spectrum for the
precipitation material
produced in Example 1.
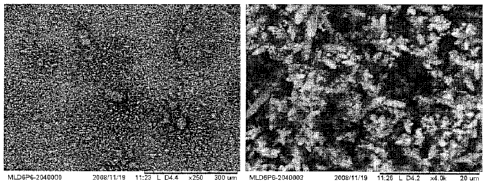
Fig. 8 provides scanning electron microscope (SEM) images for precipitation
material produced in
3
CA 02670049 2009-07-02
Example 1.
Fig. 9 provides an XRD spectrum for the aggregate produced in Example 2.
Fig. 10 provides an FT-IR spectrum for for the aggregate produced in Example
2.
Fig. 11 provides a TGA for the aggregate produced in Example 2.
Fig. 12 provides SEM images for the aggregate produced in Example 2.
Fig. 13 provides XRD spectra for the aggregate and related materials in
Example 3.
Fig. 14 provides a TGA for the aggregate produced in Example 3.
Fig. 15 provides SEM images for the aggregate and related materials in Example
3.
Fig. 16 provides XRD spectra for the aggregate and related materials in
Example 4.
Fig. 17 provides a TGA for the aggregate and related materials in Example 4.
Fig. 18 provides SEM images for the aggregate of Example 4.
Fig. 19 provides an XRD spectrum for the precipitation material produced in
Example 6.
Fig. 20 provides a TGA for the precipitation material produced in Example 6.
Fig. 21 provides an FT-IR spectrum for the precipitation material produced in
Example 6.
Fig. 22 provides SEM images for the precipitation material produced in Example
6.
Fig. 23 provides XRD spectra for the aggregate and a related material in
Example 6.
Fig. 24 provides an FT-IR spectra for the aggregate and a related material in
Example 6.
Fig. 25 provides a TGA for the aggregate and a related material in Example 6.
Fig. 26 provides SEM images for the aggregate of Example 6.
Fig. 27 presents a graphical illustration of the procedure for preparing a
sample and measuring
carbon isotope values in the sample
DETAILED DESCRIPTION
I. Introduction
II. Compositions
A. Synthetic Rock and Aggregates
1. Aggregate and rock compositions
2. Making compositions of the invention
B. Settable Compositions
C. Structures
1. Roadways
III. Methods
A. Method of manufacturing aggregate
B. Other methods
IV. Systems
V. Utility
1. Introduction
4
CA 02670049 2010-06-30
The invention provides compositions comprising synthetic rock, aggregates, and
other materials,
as well as structures, and other materials found in the manmade environment
and methods of making and
using synthetic rocks, aggregates, structures, and other manmade materials; in
addition the invention
provides systems and methods of doing business.
Before the present invention is described in greater detail, it is to be
understood that this invention
is not limited to particular embodiments described, as such may, of course,
vary. It is also to he understood
that the terminology used herein is for the purpose of describing particular
embodiments only, and is not
intended to he limiting, since the scope of the present invention will be
limited only by the appended
claims.
Where a range of values is provided, it is understood that each intervening
value, to the tenth of
the unit of the lower limit unless the context clearly dictates otherwise,
between the upper and lower limit
of that range and any other stated or intervening value in that stated range,
is encompassed within the
invention. The upper and lower limits of these smaller ranges may
independently be included in the smaller
ranges and are also encompassed within the invention, subject to any
specifically excluded limit in the
stated range. Where the stated range includes one or both of the limits,
ranges excluding either or both of
those included limits are also included in the invention.
Certain ranges are presented herein with numerical values being preceded by
the term "about." The
term "about" is used herein to provide literal support for the exact number
that it precedes, as well as a
number that is near to or approximately the number that the term precedes. In
determining whether a
number is near to or approximately a specifically recited number, the near or
approximating unrecited
number may be a number which, in the context in which it is presented,
provides the substantial equivalent
of the specifically recited number.
Unless defined otherwise, all technical and scientific terms used herein have
the same meaning as
commonly understood by one of ordinary skill in the art to which this
invention belongs. Unless otherwise
indicated or apparent from context, percentages given herein are w/w. Although
any methods and materials
similar or equivalent to those described herein can also be used in the
practice or testing of the present
invention, representative illustrative methods and materials are now
described.
The citation of any publication is for its disclosure prior to
the filing date and should not be construed as an admission that the present
invention is not entitled to
antedate such publication by virtue of prior invention. Further, the dates of
publication provided may be
different from the actual publication dates which may need to be independently
confirmed.
It is noted that, as used herein and in the appended claims, the singular
forms "a," "an," and "the"
include plural references unless the context clearly dictates otherwise. It is
further noted that the claims
may be drafted to exclude any optional element. As such, this statement is
intended to serve as antecedent
basis for use of such exclusive terminology as "solely," "only" and the like
in connection with the recitation
of claim elements, or use of a "negative" limitation.
5
CA 02670049 2009-07-02
As will be apparent to those of skill in the art upon reading this disclosure,
each of the individual
embodiments described and illustrated herein has discrete components and
features which may be readily
separated from or combined with the features of any of the other several
embodiments without departing
from the scope or spirit of the present invention. Any recited method can be
carried out in the order of
events recited or in any other order which is logically possible.
H. Compositions
A. Synthetic Rock and Aggregates
In some embodiments the invention provides a synthetic rock that is made
without chemical
binders. In some embodiments, the invention provides aggregates, e.g.,
aggregate that contains CO2
sequestered from a gaseous industrial waste stream, and/or aggregates of a
certain composition, such as
aggregates containing carbonate and/or bicarbonate minerals, aggregates of a
certain isotopic composition
(often indicative of a fossil fuel origin), aggregates of a certain chemical
composition, aggregates
containing novel minerals, aggregates with certain fracture characteristics,
lightweight aggregates, and
customized aggregate sets. The invention further provides settable
compositions, and structures, such as
roadways, buildings, dams, and other manmade structures, containing the
synthetic rock or aggregates of
the invention.
The term aggregate is used herein in its art-accepted manner to include a
particulate composition
that finds use in concretes, mortars and other materials, e.g., roadbeds,
asphalts, and other structures and is
suitable for use in such structures. Aggregates of the invention are
particulate compositions that may in
some embodiments be classified as fine or coarse. Fine aggregates according to
embodiments of the
invention are particulate compositions that almost entirely pass through a
Number 4 sieve (ASTM C 125
and ASTM C 33). Fine aggregate compositions according to embodiments of the
invention have an average
particle size ranging from 0.00 1 inch (in) to 0.25 in, such as 0.05 in to
0.125 in and including 0.01 in to
0.08 in. Coarse aggregates of the invention are compositions that are
predominantly retained on a Number 4
sieve (ASTM C 125 and ASTM C 33). Coarse aggregate compositions according to
embodiments of the
invention are compositions that have an average particle size ranging from
0.125 in to 6 in, such as 0.187 in
to 3.0 in and including 0.25 in to 1.0 in. As used herein, "aggregate" may
also in some embodiments
encompass larger sizes, such as 3 in to 12 in or even 3 in to 24 in, or
larger, such as 12 in to 48 in, or larger
than 48 in, e.g., such as sizes used in riprap and the like. In some
embodiments, such as producing wave-
resistant structures for the ocean, the sizes may be even larger, such as over
48 in, e.g., over 60 in, or over
72 in.
1. Aggregate and Rock Compositions
Compositions of the invention may be made by synthetic methods, described
herein, that allow for
great control over the properties of the compositions. Significant properties
of the compositions may
include one or more of hardness, abrasion resistance, density, porosity,
chemical composition, mineral
composition, isotopic composition, size, shape, acid resistance, alkaline
resistance, leachable chloride
content, retention of C02, reactivity (or lack thereof), as will be described
more fully herein. In some
6
CA 02670049 2009-07-02
embodiments one or more of these properties, such as two or more, three or
more, or even four or more or
five or more, may be specifically engineered into a composition of the
invention, e.g., an aggregate.
Aggregates of the invention have a density that may vary so long as the
aggregate provides the
desired properties for the use for which it will be employed, e.g., for the
building material in which it is
employed. In certain instances, the density of the aggregate particles ranges
from 1.1 to 5 gm/cc, such as 1.3
gm/cc to 3.15 gm/cc, and including 1.8 gm/cc to 2.7 gm/cc. Other particle
densities in embodiments of the
invention, e.g., for lightweight aggregates, may range from 1.1 to 2.2 gm/cc,
e.g, 1.2 to 2.0 g/cc or 1.4 to
1.8 g/cc. In some embodiments the invention provides aggregates that range in
bulk density (unit weight)
from 50 lb/ft3 to 200 lb/ft3, or 75 lb/ft3 to 175 lb/ft3, or 501b/ft3 to 100
lb/ft3, or 75 lb/ft3 to 125 lb/ft3, or 90
lb/ft3 to 115 lb/ft3, or 100 lb/ft3 to 200 lb/ft3, or 125 lb/ft3 to 175
lb/ft3, or 1401b/ft3 to 160 lb/ft3, or 50 lb/ft3
to 200 lb/ft3. Some embodiments of the invention provide lightweight
aggregate, e.g., aggregate that has a
bulk density (unit weight) of 75 lb/ft3 to 125 lb/ft3. Some embodiments of the
invention provide
lightweight aggregate, e.g., aggregate that has a bulk density (unit weight)
of 901b/ft3 to 115 lb/ft3.
The hardness of the aggregate particles making up the aggregate compositions
of the invention
may also vary, and in certain instances the hardness, expressed on the Mohs
scale, ranges from 1.0 to 9,
such as 1 to 7, including 1 to 6 or 1 to 5. In some embodiments, the Mohr's
hardness of aggregates of the
invention ranges from 2-5, or 2-4. In some embodiments, the Mohs hardness
ranges from 2-6. Other
hardness scales may also be used to characterize the aggregate, such as the
Rockwell, Vickers, or Brinell
scales, and equivalent values to those of the Mohs scale may be used to
characterize the aggregates of the
invention; e.g., a Vickers hardness rating of 250 corresponds to a Mohs rating
of 3; conversions between
the scales are known in the art.
The abrasion resistance of an aggregate may also be important, e.g., for use
in a roadway surface,
where aggregates of high abrasion resistance are useful to keep surfaces from
polishing. Abrasion
resistance is related to hardness but is not the same. Aggregates of the
invention include aggregates that
have an abrasion resistance similar to that of natural limestone, or
aggregates that have an abrasion
resistance superior to natural limestone, as well as aggregates having an
abrasion resistance lower than
natural limestone, as measured by art accepted methods, such as ASTM C131-03.
In some embodiments
aggregates of the invention have an abrasion resistance of less than 50%, or
less than 40%, or less than
35%, or less than 30%, or less than 25%, or less than 20%, or less than 15%,
or less than 10%, when
measured by ASTM C131-03.
Aggregates of the invention may also have a porosity within a particular
ranges. As will be
appreciated by those of skill in the art, in some cases a highly porous
aggregate is desired, in others an
aggregate of moderate porosity is desired, while in other cases aggregates of
low porosity, or no porosity,
are desired. Porosities of aggregates of some embodiments of the invention, as
measured by water uptake
after oven drying followed by full immersion for 60 minutes, expressed as %
dry weight, can be in the
range of 1-40%, such as 2-20%, or 2-15%, including 2-10% or even 3-9%.
The chemical, mineral, and/or isotopic composition of aggregates of the
invention varies
depending on methods of manufacturing, raw materials, and the like. In some
embodiments, some or all of
the carbonate compounds are metastable carbonate compounds that are
precipitated from a water, such as a
7
CA 02670049 2009-07-02
salt-water, as described in greater detail below; in some embodiments these
metastable compounds are
further processed to provide stable compounds in the aggregates of the
invention.
The carbonate compounds in embodiments of the invention include precipitated
crystalline and/or
amorphous carbonate compounds and in some embodiments bicarbonate compounds.
Specific carbonate
minerals of interest include, but are not limited to: calcium carbonate
minerals, magnesium carbonate
minerals and calcium magnesium carbonate minerals. Calcium carbonate minerals
of interest include, but
are not limited to: calcite (CaCO3), aragonite (CaCO3), vaterite (CaCO3),
ikaite (CaCO3=6H2O), and
amorphous calcium carbonate(CaCO3=nH2O). Magnesium carbonate minerals of
interest include, but are
not limited to: dypingite (Mg5(CO3)4(OH)2.5(H2O); the term dypingite is used
herein to include dypingite
minerals of this formula), magnesite (MgCO3), barringtonite (MgCO3=2H2O),
nesquehonite
(MgCO3.3H2O), lanfordite (MgCO3=5H2O) and amorphous magnesium calcium
carbonate
(MgCO3=nH2O). Calcium magnesium carbonate minerals of interest include, but
are not limited to dolomite
(CaMgCO3), huntitte (CaMg(C03)4) and sergeevite (Ca2Mg11(CO3)13=H2O). In
certain embodiments, non-
carbonate compounds like brucite Mg(OH)2 may also form in combination with the
minerals listed above.
As indicated above, the compounds of the carbonate compounds may be metastable
carbonate compounds
(and may include one or more metastable hydroxide compounds) that are more
stable in saltwater than in
freshwater, such that upon contact with fresh water, they dissolve and re-
precipitate into other fresh water
stable compounds, e.g., minerals such as low-Mg calcite.
In some embodiments, aggregates of the invention are formed, in whole or in
part, from metastable
compounds as described herein that have been exposed to freshwater and allowed
to harden into stable
compounds, which may then be further processed, if necessary, to form
particles as appropriate to the type
of aggregate desired. In some embodiments, aggregates of the invention are
formed from metastable
compounds exposed to conditions of temperature and/or pressure that convert
them into stable compounds.
In some embodiments, silica minerals may co-occur with the carbonate
compounds, forming
carbonate silicate compounds. These compounds may be amorphous in nature or
crystalline. In certain
embodiments, the silica may be in the form of opal-A, amorphous silica,
typically found in chert rocks.
Calcium magnesium carbonate silicate amorphous compounds may form, within
crystalline regions of the
carbonate minerals listed above. Non-carbonate, silicate minerals may also
form. Sepiolite is a clay mineral,
a complex magnesium silicate, a typical formula for which is
Mg4SiO15(OH)2.6H20. It can be present in
fibrous, fine-particulate, and solid forms. Silcate carbonate minerals may
also form. Carletonite,
KNa4Ca4(CO3)4Si8O18 (F, OH) - H2O, Hydrated potassium sodium calcium carbonate
silicate, can form
under these conditions. Like any member of the phyllosilicates subclass,
carletonite's structure is layered
with alternating silicate sheets and the potassium, sodium and calcium layers.
Unlike other phyllosilicates,
carletonite's silicate sheets are composed of interconnected four and eight-
member rings. The sheets can be
thought of as being like chicken wire with alternating octagon and square
shaped holes. Both octagons and
squares have a four fold symmetry and this is what gives carletonite its
tetragonal symmetry; 4/m 2/m 2/m.
Only carletonite and other members of the apophyllite group have this unique
interconnected four and
eight-member ring structure.
The carbonate and/or bicarbonate compounds of aggregates of the invention
generally are derived
from, e.g., precipitated from, an aqueous solution of divalent cations (as
described in greater detail below).
8
CA 02670049 2009-07-02
As the carbonate and/or bicarbonate compound compositions of the aggregates
are precipitated from the
aqueous solution of divalent cations, they will include one or more components
that are present in the
solution from which they are derived. For example, where the aqueous solution
of divalent cations is salt
water, the carbonate and/or bicarbonate compounds and aggregates that include
the same can include one or
more compounds found in the aqueous cation solution source. These compounds
can be correlated to
components that originate at the aqueous cation solution source, where these
identifying components and
the amounts thereof are collectively referred to herein as a cation solution
source identifier. For example, if
the cation solution source is sea water, identifying compounds that may be
present in the precipitated
mineral compositions include, but are not limited to: chloride, sodium,
sulfur, potassium, bromide, silicon,
strontium and the like. Any such, source-identifying or "marker" elements are
generally present in small
amounts, e.g., in amounts of 20,000 parts per million (ppm) or less, such as
amounts of 2000 ppm or less.
In certain embodiments, the "marker" compound is strontium, which may be
present in the precipitated
composition comprising carbonates and/or bicarbonates. Strontium may be
incorporated into the aragonite
(calcium carbonate) lattice, and contribute 10,000 ppm or less, ranging in
certain embodiments from 3 to
10,000 ppm, such as from 5 to 5000 ppm, including 5 to 1000 ppm, e.g., 5 to
500 ppm, including 5 to 100
ppm. Another "marker" compound is magnesium, which may be present in amounts
of up to 20% mole
substitution for calcium in carbonate compounds. The aqueous cation solution
source identifier of the
compositions may vary depending on the particular aqueous cation solution
source employed to produce the
saltwater-derived precipitate composition comprising carbonates and/or
bicarbonates. In certain
embodiments, the calcium carbonate content of the aggregate is 5%, 10%, 15%,
20% or 25% w/w or
higher, such as 30 % w/w or higher, and including 40% w/w or higher, e.g., 50%
w/w or even 60% w/w or
higher, 70% w/w or higher, 80% w/w or higher, 90% w/w or higher, or 95% w/w or
higher. In certain
embodiments, the magnesium carbonate content of the aggregate is 5%, 10%, 15%,
20% or 25% w/w or
higher, such as 30 % w/w or higher, and including 40% w/w or higher, e.g., 50%
w/w or even 60% w/w or
higher, 70% w/w or higher, 80% w/w or higher, 90% w/w or higher, or 95% w/w or
higher.
The aggregate has, in certain embodiments, a calcium/magnesium ratio that is
influenced by, and
therefore reflects, the water source from which it has been precipitated,
e.g., seawater, which contains more
magnesium than calcium, or, e.g., certain brines, which often contain one-
hundred-fold the calcium content
as seawater; the calcium/magnesium ratio also reflects factors such as the
addition of calcium and/or
magnesium-containing substances during the production process, e.g., the use
of flyash, red mud, slag, or
other calcium and/or magnesium-containing industrial wastes, or the use of
calcium and/or magnesium-
containing minerals such as mafic and ultramafic minerals, such as serpentine,
olivine, and the like, as
further described herein, or wollastonite. Because of the large variation in
raw materials as well as
materials added during production, the calcium/magnesium molar ratio may vary
widely in various
embodiments of the compositions and methods of the invention, and indeed in
certain embodiment the ratio
may be adjusted according to the intended use of the aggregate. Thus, in
certain embodiments, the
calcium/magnesium molar ratio in the aggregate ranges from 200/1 Ca/Mg to
1/200 Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to
1/100 Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to 1/50
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to 1/10
Ca/Mg. In some
9
CA 02670049 2009-07-02
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to 1/5
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to 1/1
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to 5/1
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 150/1 Ca/Mg to 10/1
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 100/1 Ca/Mg to 10/1
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 1/1 Ca/Mg to 1/100
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 1/1 Ca/Mg to 1/50
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 1/1 Ca/Mg to 1/25
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 1/1 Ca/Mg to 1/10
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 1/1 Ca/Mg to 1/8
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 1/1 Ca/Mg to 1/5
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 10/1 Ca/Mg to 1/10
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 8/1 Ca/Mg to 1/8
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 6/1 Ca/Mg to 1/6
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 4/1 Ca/Mg to 1/4
Ca/Mg. In some
embodiments, the calcium magnesium molar ratio ranges from 2/1 Ca/Mg to 1/2
Ca/Mg. In some
embodiments, the calcium/magnesium molar ratio is 20/1 or greater, such as
50/1 or greater, for example
100/1 or greater, or even 150/1 or greater. In some embodiments, the
calcium/magnesium molar ratio is
1/10 or less, such as 1/25 or less, for example 1/50 or less, or even 1/100 or
less. In some embodiments,
Ca/Mg ratio ranges are 2/1 to V2, 3/2 to 2/3, or 5/4 to 4/5. In some
embodiments, Ca/Mg ratio ranges are
1/7 to 200/1, 1/15 to 12/10, 1/10 to 5/1, 1/7 to V2, or 1/9 to 2/5. In some
embodiments, Ca/Mg ratio ranges
are 1/200 to 1/7, 1/70 to 1/7, or 1/65 to 1/40. In some embodiments, Ca/Mg
ranges are 1/10 to 50/1, 1/5 to
45/1, 1/6 to 6/1, 6/5 to 45/1,'/ to 11/3, or 13/2 to 19/2. In some
embodiments, Ca/Mg ranges are 1/3 to 3/1
or ''Ato 2/1. In some embodiments, Ca/Mg ranges are 2/1 to all calcium, 3/1 to
200/1, 5/1 to 200/1, or 10/1
to 200/1.
In some embodiments, aggregates are provided where the compositions contain
carbonates and
bicarbonates, e.g., of divalent cations such as calcium or magnesium; in some
cases the aggregate contains
substantially all carbonates, or substantially all bicarbonates, or some ratio
of carbonate to bicarbonate. The
molar ratio of carbonates to bicarbonates may be any suitable ratio, such as
100/1 to 1/100, or 50/1 to 1/50,
or 25/1 to 1/25, or 10/1 to 1/10, or 2/1 to %i, or about 1/1, or substantially
all carbonate or substantially all
bicarbonate. In some embodiments the invention provides aggregate that
contains carbonates and/or
bicarbonates of calcium or magnesium or combinations thereof. In some
embodiments the invention
provides aggregate that contains only carbonates of calcium or magnesium or
combinations thereof without
containing bicarbonate, or containing only trace amounts of bicarbonate. Other
embodiments provide
aggregate that is comprised solely of bicarbonates of calcium or magnesium or
combinations thereof.
In certain embodiments, aggregate is characterized by having a carbonate to
hydroxide compound
ratio, where in certain embodiments this ratio ranges from 100 to 1, such as
10 to 1 and including 1 to 1.
Where silica is present, the ratio of calcium/magnesium to silica may range
from 100:1 to 1:1,
such as from 50:1 to 10:1.
CA 02670049 2009-07-02
In addition, aggregates of the invention may further include or exclude
substances such as
chloride. These substances are considered undesirable in some applications;
for example, chloride is
undesirable in aggregates intended for use in concrete because of its tendency
to corrode rebar. However,
in some uses, such as base course for a roadway, aggregate containing chloride
may be acceptable.
Methods of making aggregates of the invention may include one or more steps to
minimize the chloride
and/or sodium content of the aggregate, if chloride is a component of the
starting materials; in some
embodiments, such a step or steps is not necessary as the intended final use
of the aggregate is relatively
insensitive to the content of these materials. Thus, in some embodiments, the
leachable chloride content of
the aggregates of the invention is less than 5%. In some embodiments, the
leachable chloride content of the
aggregate ranges from 0.0001% to 0.05%. In some embodiments the leachable
chloride content is less than
0.05%, in some embodiments the leachable chloride content is les than 0.1%,
and in some embodiments the
leachable chloride content is less than 0.5%.
In some embodiments the aggregates of the invention are formed from CO2 and,
in some cases,
other elements or compounds, having a specific isotopic composition, e.g., an
isotopic composition
consistent with an origin in a fossil fuel, as described further herein.
The aggregate of the invention may be of any size and shape suitable for a
particular use, as
described further herein. As the aggregates are synthetic, both the size and
the shape may be almost
completely controlled, allowing for a great variety of specific aggregates as
well as aggregate mixes, as
described further. In some embodiments, the invention provides coarse
aggregate, e.g., compositions that
are predominantly retained on a Number 4 sieve (ASTM C 125 and ASTM C 33).
Coarse aggregate
compositions according to embodiments of the invention are compositions that
have an average particle
size ranging from 0.125 in to 6 in, such as 0.187 in to 3.0 in and including
0.25 in to 1.0 in. Fine aggregate
compositions according to embodiments of the invention have an average
particle size ranging from 0.001
inch (in) to 0.25 in, such as 0.05 in to 0.125 in and including 0.01 in to
0.08 in.
Aggregates of the invention may be reactive or non-reactive. Reactive
aggregate are those
aggregate particles that upon initiation by a substance (e.g., water) undergo
a reaction with constituents
(e.g., compounds) in other aggregate particles to form a reaction product. In
some instances, the reaction
product may be a matrix between aggregate particles forming a stabilizing
structure. In other instances the
matrix formed may be an expansive gel that, depending on the environment, may
act to destabilize the
mass; in some cases where there is room for the expansive gel to expand, e.g.,
in aggregate that is laid as
part of a road bed, with void spaces, a reactive aggregate of this type is
acceptable. Aggregate of the
invention may also be non-reactive.
In addition, in some instances the invention provides aggregates that are
resistant to acid, resistant
to base, or resistant to both acid and base. For example, in some instances
the invention provides
aggregates that, when exposed to a pH of 2, 3, 4, or 5, depending on the test
desired (e.g., an H2SO4
solution that has been diluted to a pH of 2, 3, 4, or 5), release less than 1,
0.1, 0.01, or 0.001 % of the CO2
contained in the aggregate in a 48 hour period, or a 1-week period, or a 5-
week period, or a 25-week period,
while remaining intact and retaining a portion or substantially all of its
hardness, abrasion resistance, and
the like. Similar results may be obtained for aggregates of the invention that
are resistant to base, e.g., when
exposed to a pH of 12, 11, 10, or 9, release less than 1, 0.1, 0.01, or 0.001%
of their CO2 in a 48 hour,
11
CA 02670049 2009-07-02
1 week, 5 week, or 25 week period, while remaining intact and retaining a
portion or substantially all of its
hardness, abrasion resistance, and the like. CO2 content of the material may
be monitored by, e.g.,
coulometry, or any other suitable method.
In some embodiments the invention provides aggregates that are stable to CO2
release as described
further below.
In some embodiments, the aggregates of the invention are aggregates that
sequester one or more
components of a human-produced waste stream, typically an industrial waste
stream that includes, though is
not limited to, gaseous components. Generally the one or more components
sequestered by the aggregates
are components for which release to the atmosphere or to the environment in
general is undesirable. For
example, for a flue gas waste stream, undesirable components include C02, CO,
sulfur oxides (SOx, such as
SO2 and SO3), nitrogen oxides (NOx, such as NO and NO2), heavy metals such as
mercury, cadmium, lead,
and/or others well-known in the art, particulates, radioactive substances,
organic compounds, and other
undesirable components, e.g., any component regulated by governmental or other
regulatory agencies.
In particular embodiments, the invention includes CO2 sequestering aggregates.
The term "CO2
sequestering aggregate" as used herein includes that the aggregate contains
carbon derived from a fuel used
by humans, e.g., carbon having a fossil fuel origin. For example, CO2
sequestering aggregate according to
embodiments of the present invention contain carbon that was released in the
form of CO2 from the
combustion of fuel. In certain embodiments, the carbon sequestered in a CO2
sequestering aggregate
contains carbonate compounds. Therefore, CO2 sequestering aggregate according
to embodiments of the
subject invention contain carbonate compounds where at least part of the
carbon in the carbonate
compounds is derived from a fuel used by humans, e.g., a fossil fuel. As such,
production of aggregate of
the invention results in the placement of CO2 into a storage stable form,
e.g., a component that can be used
in a variety of ways in the built environment, i.e., a man-made structure,
such as a building, wall, road, etc.,
or even transported to a source of fossil fuel, e.g., a coal mine, and stored
there. As such, production of the
CO2 sequestering aggregate of the invention results in the prevention of CO2
gas from entering the
atmosphere.
C02-sequestering aggregate of the invention provides for long term storage of
CO2 in a manner
such that CO2 is sequestered (i.e., fixed) in the aggregate, where the
sequestered CO2 does not become part
of the atmosphere. "Long term storage" includes that the aggregate of the
invention keeps its sequestered
CO2 fixed for extended periods of time (when the aggregate is maintained under
conditions conventional
for its intended use) without significant, if any, release of the CO2 from the
aggregate. Extended periods of
time in the context of the invention may be 1 year or longer, 5 years or
longer, 10 years or longer, 25 years
or longer, 50 years or longer, 100 years or longer, 250 years or longer, 1000
years or longer, 10,000 years
or longer, 1,000,000 years or longer, or even 100,000,000 years or longer,
depending on the particular
nature and downstream use of the aggregate. With respect to the CO2
sequestering aggregate, when
employed for their intended use and over their lifetime, the amount of
degradation, if any, as measured in
terms of CO2 gas release from the product will not exceed 10% per year, for
example, will not exceed
5%/year, and in certain embodiments will not exceed 1 %/year or even will not
exceed 0.5% per year or
even 0.1 % per year.
12
CA 02670049 2009-07-02
Tests of the aggregate can be used as surrogate markers for the long-term
storage capability of the
aggregate. Any art-accepted test may be used, or any test that reasonably
would be thought to predict long-
term storage of CO2 in a material under its intended conditions of use may be
used, e.g., any test that
reasonably would be thought to predict that the composition keeps a
significant fraction, or substantially all,
of its CO2 fixed for a certain amount of time. For example, aggregate may
considered long term storage
aggregate for sequestered CO2 if, when exposed to 50, 75, 90, 100, 120, or 150
C for 1, 2, 5, 25, 50, 100,
200, or 500 days at between 10% and 50% relative humidity, it loses less than
1%, 2%,3%,4%, 5%, 10%,
20%, 30%, or 50% of its carbon. Test conditions are chosen according to the
intended use and environment
of the material. CO2 content of the material may be monitored by any suitable
method, e.g., coulometry.
To verify that a material is a C02-sequestering material, e.g., a material
containing carbon dioxide
originating in the combustion of fossil fuel, tests such as isotope
measurements (e.g., measurement of 513C
values) and carbon coulometry may be used; any other suitable measurement may
also be used to verify,
e.g., that the composition contains carbonates and/or that carbonates are
present at a given percentage of the
composition.
Thus, in some embodiments the invention provides a composition comprising a
CO2-sequestering
aggregate. The aggregate may be precipitated from a divalent cation-containing
water, e.g., an alkaline-
earth-metal-ion containing water, such as salt water, e.g. sea water or
geologic brine, or a water derived
from sea water or geologic brine. The divalent cation-containing water may
contain CO2 derived from an
industrial process, e.g. from an industrial waste gas stream which is then
converted into a carbonate that is
contained in the aggregate. Thus in some embodiments the aggregates have a
813C value reflective of a
fossil fuel origin, as described below. The C02-sequestering aggregate may
contain a calcium carbonate,
magnesium carbonate, calcium magnesium carbonate, or any combination thereof.
In some embodiments
the aggregate contains at least about 10, 20, 30, 40, 50, 60, 70, 80, or 90%
carbonate. In some
embodiments the aggregate contains at least about 50% carbonate. The molar
Ca/Mg ratio in some
embodiments can be 1/10 to 1/3, or 1/3 to 3/1, or 10/1 to 100/1, or about 1/1.
The CO2-sequestering
aggregate may contain any of the mineral forms listed herein, e.g., calcite,
nesquehonite, aragonite,
dypingite, in the percentages given. Such aggregates may have further
properties as described herein, e.g.,
size, shape, density, reactivity, and the like. For example, in some
embodiments, such aggregates may have
a hardness of at least 2, or at least 3 on the Mohs hardness scale or
equivalent. In some embodiments such
aggregates may have a bulk density of 50 lb/ft3 to 200 lb/ft3, or 75 lb/ft3 to
175 lb/ft3, or 50 lb/ft3 to 100
lb/ft3, or 75 lb/ft3 to 125 lb/ft3, or 90 lb/ft3 to 115 lb/ft3, or 100 lb/ft3
to 200 lb/ft3, or 125 lb/ft3 to 175 lb/ft3,
or 140 lb/ft3 to 160 lb/ft3, or 50 lb/ft3 to 200 lb/ft3. In some embodiments
such aggregates are aggregate
that has a bulk density (unit weight) of 75 lb/ft3 to 125 lb/ft3. In some
embodiments such aggregates are
aggregate that has a bulk density (unit weight) of 90 lb/ft3 to 115 lb/ft3. In
some embodiments such
aggregates are coarse aggregates. In some embodiments such aggregates are fine
aggregates. Such
aggregates may also have Ca/Mg ratios, crystal structures and polymorphs,
porosity, reactivity or lack
thereof, stability to CO2 release, and/or other characteristics as described
further herein.
In certain embodiments aggregates of the invention will contain carbon from
fossil fuel; because
of its fossil fuel origin, the carbon isotopic fractionation (S13C) value of
such aggregate will be different
from that of, e.g., limestone. As is known in the art, the plants from which
fossil fuels are derived
13
CA 02670049 2009-07-02
preferentially utilize 12C over 13C, thus fractionating the carbon isotopes so
that the value of their ratio
differs from that in the atmosphere in general; this value, when compared to a
standard value (PeeDee
Belemnite, or PDB, standard), is termed the carbon isotopic fractionation
(S13C) value. 513C values for coal
are generally in the range -30 to -20%o and S13C values for methane may be as
low as -20%o to -40%o or
even -40%o to -80%o. 513C values for atmospheric CO2 are -10%o to -7%o, for
limestone aggregate +3%o to -
3%o, and for marine bicarbonate, O%o. Even if the aggregate contains some
natural limestone, or other
source of C with a less negative S13C value than fossil fuel, its S13C value
generally will still be negative
and more negative (less than) values for limestone or atmospheric CO2.
Aggregates of the invention thus
include aggregates with a 813C more negative than (less than) -10%0, such as
more negative than (less than)
-12%0, -14 o, -16%o, -18%o, -20%o, -22%0, -24%0, -26%0, -28%o, or more
negative than (less than) -30%o. In
some embodiments the invention provides an aggregate with a S13C more negative
than (less than) -10%o.
In some embodiments the invention provides an aggregate with a 613C more
negative than (less than) -14%o.
In some embodiments the invention provides an aggregate with a S13C more
negative than (less than) -18%0.
In some embodiments the invention provides an aggregate with a S13C more
negative than (less than) -
20%o. In some embodiments the invention provides an aggregate with a 513C more
negative than (less than)
-24%o. In some embodiments the invention provides an aggregate with a S13C
more negative than (less
than) -28%o. In some embodiments the invention provides an aggregate with a
S13C more negative than (less
than) -30%o. In some embodiments the invention provides an aggregate with a
S13C more negative than (less
than) -32%o. In some embodiments the invention provides an aggregate with a
613C more negative than (less
than) -34%o. Such aggregates may be carbonate-containing aggregates, as
described above, e.g., aggregate
with that contains at least 10, 20, 30, 40, 50, 60, 70, 80, or 90% carbonate,
e.g., at least 50% carbonate w/w.
Such aggregates may have further properties as described herein, e.g., size,
shape, density, reactivity, and
the like. For example, in some embodiments, such aggregates may have a
hardness of at least 2, or at least
3 on the Mohs hardness scale or equivalent. In some embodiments such
aggregates may have a bulk
density of 50 lb/ft3 to 200 lb/ft3, or 75 lb/ft3 to 175 lb/ft3, or 50 lb/ft3
to 100 lb/ft3, or 75 lb/ft3 to 125 lb/ft3,
or 90 lb/ft3 to 115 lb/ft3, or 100 lb/ft3 to 200 lb/ft3, or 125 lb/ft3 to 175
lb/ft3, or 140 lb/ft3 to 160 lb/ft3, or 50
lb/ft3 to 200 lb/ft3. In some embodiments such aggregates are aggregate that
has a bulk density (unit
weight) of 75 lb/ft3 to 125 lb/ft3. In some embodiments such aggregates are
aggregate that has a bulk
density (unit weight) of 90 lb/ft3 to 115 lb/ft3. In some embodiments such
aggregates are coarse aggregates.
In some embodiments such aggregates are fine aggregates. Such aggregates may
also have Ca/Mg ratios,
crystal structures and polymorphs, porosity, reactivity or lack thereof,
stability to CO2 release, and/or other
characteristics as described further herein.
In some embodiments the aggregate of the invention is carbon-negative
aggregate, and the
methods of production of the aggregate are carbon-negative methods. The term
"carbon negative," as it is
used herein, includes the meaning that the amount by weight of CO2 that is
sequestered (e.g., through
conversion of CO2 to carbonate) by practice of the methods or in a composition
made by a method is
greater that the amount of CO2 that is generated (e.g., through power
production, production or mining of
reactants such as base, transportation, and other parts of the manufacture of
the product that produce CO2)
14
CA 02670049 2010-06-30
to practice the methods or produce the product in final form ready for use,
which may be expressed as a
percentage as shown in the following equation:
((Amount CO, captured-amount CO, expended in capture)/Amount CO, captured)
x100 = % carbon
negative.
Thus, a product which captures carbon dioxide while expending no carbon
dioxide in the capture process is
100% carbon negative. In some instances, the products or processes of the
invention are Ito 100% carbon
negative, such as 5 to 100%, including 10 to 95%, 10 to 90%, 10 to 80%, 10 to
70%, 10 to 60%, 10 to 50%,
10 to 40%, 10 to 30%, 10 to 20%, 20 to 95%, 20 to 90%, 20 to 80%, 20 to 70%,
20 to 60%, 20 to 50%, 20
to 40%, 20 to 30%, 30 to 95%, 30 to 90%, 30 to 80%, 30 to 70%, 30 to 60%, 30
to 50%, 30 to 40%, 40 to
95%, 40 to 90%, 40 to 80%,40 to 70%, 40 to 60%, 40 to 50%, 50 to 95%, 50 to
90%, 50 to 80%, 50 to
70%, 50 to 60% , 60 to 95%, 60 to 90%, 60 to 80%, 60 to 70%, 70 to 95%, 70 to
90%, 70 to 80%, 80 to
95%, 80 to 90%, and 90 to 95% carbon negative. In some instances, the products
or processes of the
invention are at least 5% carbon negative, or at least 10% carbon negative, or
at least 20% carbon negative,
or at least 30% carbon negative, or at least 40% carbon negative, or at least
50% carbon negative, or at least
60% carbon negative, or at least 70% carbon negative, or at least 80% carbon
negative, or at least 90%
carbon negative. Carbon negative methods in general are described in more
detail in U.S. Patent
Application Publication No. 2009-0169452.
Aggregates of the invention may, in some embodiments, include further
sequestered components
found, e.g., in industrial waste gases, as described above. Accordingly, in
some embodiments, in addition
to containing carbonates, e.g., from sequestered CO2, aggregates of the
invention may include one or more
substances that are, and/or are derived from, the following compounds or
elements: CO, sulfur oxides
(SOx, such as S02 and SO3), nitrogen oxides (NOx, such as NO and N02), heavy
metals such as mercury,
cadmium, lead, and/or others well-known in the art, particulates, radioactive
substances, and organic
compounds. Thus the invention includes aggregates that, in addition to a CO,-
sequestering component
such as a carbonate, contain a SOx-derived component, such as a sulfate or a
sulfite, e.g., a calcium or
magnesium sulfate or sulfite, or a combination of calcium and magnesium
sulfate or sulfites. In some
embodiments, the invention provides aggregates containing carbonate compounds,
e.g., derived from CO2,
and sulfate and/or sulfite compounds, e.g., derived from SOx, where the molar
ratio of carbonates to
sulfates/sulfites (in combination, if both are present) is between 200:1 to
10:1, such as between 150:1 to
20:1, or 120:1 to 80:1. In some embodiments, the invention provides aggregates
containing carbonate
compounds, e. g., derived From C02, and sulfate and/or sulfite compounds,
e.g., derived from SOx, where
the carbonates make up 20%-99% of the aggregate and the sulfate/sulfite
compounds make up 0.01-5% of
the aggregate, e.g., where the carbonates make up 50%-99% of the aggregate and
the sulfate/sulfite
compounds make up 0.1-3% of the aggregate, such as where the carbonates make
up 85%-99% of the
aggregate and the sulfate/sulfite compounds make up 0.2-2% of the aggregate.
In some embodiments, the
invention provides aggregates containing carbonate compounds and sulfate
and/or sulfite compounds where
the molar ratio of carbonates to sulfates/sulfites (in combination, if both
are present) is between 200:1 to
10:1, such as between 150:1 to 20: 1, or 120:1 to 80:1. In some embodiments,
the invention includes
CA 02670049 2009-07-02
aggregates that include, in addition to carbonate compounds, e.g., derived
from, and, optionally, a sulfate or
sulfite, e.g., derived from SOx, further includes a heavy metal, e.g.,
mercury, or a heavy-metal derived
compound. In such embodiments, the aggregate may contain carbonate and mercury
compounds in a molar
ratio of carbonate to mercury compounds of 5 X 109 :1 to 5 X 108:1, such as
2X109: 1 to 5X108:1. In some
embodiments the aggregates of the invention include a C02-derived component, a
SOx-derived component,
and a mercury-derived component, optionally also including a NOx-derived
component.
In some embodiments, an aggregate of the invention contains at least one of. a
calcium carbonate
compound, a magnesium carbonate compound and a calcium magnesium carbonate
compound. The molar
ratio of the calcium to magnesium for the aggregate may be any of the ratios
given herein, e.g., in a
magnesium:calcium range of 7:1 to 2:1, 2:1 to 1:2, or 1:10 to 1:200, depending
on starting materials,
manufacturing conditions, and the like. In some embodiments, the one or more
carbonate compounds make
up at least 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99% by weight of the
aggregate, for example, at least 50%,
including at least 80%, such as at least 90%. The one or more carbonate
compounds may include a
precipitate from an divalent cation-containing water, for example a divalent
cation-containing water that
contains CO2 derived from a gaseous industrial waste stream. Industrial
gaseous waste streams may be as
described herein, e.g., from a power plant, foundry, cement plant, refinery,
or smelter. In some
embodiments, the aggregate contains specific minerals that are produced by the
manufacturing conditions,
as described elsewhere herein. In some specific embodiments, the aggregate
contains dypingite at a
percentage w/w of at least 0.1%, or at least 0.5%, or at least 1%, or at least
2%, or at least 5%, or at least
10%. In some embodiments the aggregate contains dypingite as well as
nesquehonite. In some specific
embodiments, the aggregate contains dypingite at a percentage w/w of at least
0.1%, or at least 0.5%, or at
least I%, or at least 2%, or at least 5%, or at least 10% and nesquehonite at
a percentage w/w of at least
0.1%, or at least 0.5%, or at least 1%, or at least 2%, or at least 5%, or at
least 10%. In some embodiments
the aggregate contains calcite at a percentage w/w of at least 0.1%, or at
least 0.5%, or at least 1%, or at
least 2%, or at least 5%, or at least 10%, or at least 20%, or at least 30%.
In some embodiments the
aggregate contains dolomite at a percentage w/w of at least 0.1%, or at least
0.5%, or at least 1%, or at least
2%, or at least 5%, or at least 10%, or at least 20%, or at least 30%.
In some embodiments the invention provides a synthetic rock that does not
contain binders, i.e., a
self-cementing synthetic rock. The methods of the invention allow for
production of a hard, durable rock
through processes that involve physical reactions without the need for
extrinsic or intrinsic binders, as
described more fully elsewhere herein. Thus, in some embodiments the invention
provides synthetic rock
that contains less than 10, 5, 2, 1, 0.5, 0.2, 0.1, 0.05, 0.02, 0.01, 0.005,
0.001, 0.0005, 0.0001% w/w of
binder, where "binder," as that term is used herein, includes compounds or
substances that are added to a
synthetic rock system in order to cause or promote chemical reactions that
cause components of the
synthetic rock to bind together during a synthetic process. Typical binders
are described elsewhere herein.
In some embodiments, the synthetic rock of the invention includes
substantially no binder. Such synthetic
rock can be artificially lithified in processes that mimic geologic processes
in which physical, rather than
chemical, processes are the processes by which rocks are formed, e.g.,
dissolution and reprecipitation of
compounds in new forms that serve to bind the composition together. Such
synthetic rocks in certain
embodiments contain one or more carbonate compounds, e.g., carbonate compounds
derived from a fossil
16
CA 02670049 2009-07-02
fuel source. The synthetic rock may in some embodiments have a carbon isotopic
fractionation (S13C)
value more negative than (less than) -10%0 or -12%0, or -14%0 or -180.60, or -
22%0, or -26%o or -30%0, or -
32%o, or -36%0. The synthetic rock may in some embodiments have a carbon
isotopic fractionation (S13C)
value between -10%o and -40%o.
In some embodiments, the synthetic rock with low or no binder content includes
at least one of: a
calcium carbonate compound, a magnesium carbonate compound and a calcium
magnesium carbonate
compound. The molar ratio of the calcium to magnesium for the synthetic rock
may be any of the ratios
given herein, e.g., in a magnesium:calcium range of 7:1 to 2:1, 2:1 to 1:2, or
1:10 to 1:200, depending on
starting materials, manufacturing conditions, and the like. In some
embodiments, the one or more
carbonate compounds make up at least 10, 20, 30, 40, 50, 60, 70, 80, 90, 95,
99% by weight of the synthetic
rock, for example, at least 50%, including at least 80%, such as at least 90%.
The one or more carbonate
compounds may include a precipitate from an divalent cation-containing water,
for example a divalent
cation-containing water that contains CO2 derived from a gaseous industrial
waste stream. Industrial
gaseous waste streams may be as described herein, e.g., from a power plant,
foundry, cement plant,
refinery, or smelter. The artificial rock may be produced in a process in
which metastable components,
such as metastable carbonates, are converted to more stable components. For
example, in some
emodiments the synthetic rock is produced in a process where aragonite is
converted to calcite, and/or
vaterite is converted to aragaonite and/or calcite, and/or protodolomite is
converted to dolomite.
In some embodiments the invention provides a lightweight aggregate, e.g., an
aggregate with a
bulk density of 75-125 lb/ft3, or 90-115 lb/ft3. In some embodiments the
lightweight aggregate is a C02-
sequestering aggregate, which may be an aggregate containing carbonates, e.g.,
at least 10, 20, 30, 40, 50,
60, 70, 80, or 90% carbonates derived from a fossil fuel. In some embodiments
the aggregate has a carbon
isotopic fractionation (S13C) value more negative than (less than) -10%0, or -
12%0, or -14%o, or -18%o, or -
22%o, or -26%0 or -30%0, or -32%0, or -36%0. The lightweight aggregate may in
some embodiments have a
carbon isotopic fractionation (S13C) value between -10%o and -40%o. The
lightweight aggregate may in
some embodiments have a carbon isotopic fractionation (S13C) value more
negative than (less than) -20%0.
The lightweight aggregate may in some embodiments have a carbon isotopic
fractionation (S13C) value
more negative than (less than) -30%0. The lightweight aggregate in some
embodiments contains carbonate
and sulfate or sulfite, or a combination of sulfate and sulfite. In some
embodiments the molar ration of
carbonate to sulfate and/or sulfite is 1000:1 to 10:1, or 500:1 to 50:1, or
300:1 to 75:1. In some of these
embodiments the aggregate further contains mercury or a mercury compound,
which may be of fossil fuel
origin. In some embodiments the aggregate contains dypingite.
In some embodiments the invention provides a customized set of aggregates,
e.g., a set of
aggregates with a plurality of characteristics that is chosen to match a
predetermined set of characteristics,
such as at least two, three, four, or five of size, shape, surface texture,
hardness, abrasion resistance,
density, porosity, acid stability, base stability, CO2 release stability, and
color. In some embodiments the
invention provides a set of aggregates with a plurality of characteristics
that are chosen to match a
predetermined set of characteristics, where the characteristics include size,
shape, and hardness. In some
embodiments the invention provides a set of aggregates with a plurality of
characteristics that are chosen to
match a predetermined set of characteristics, where the characteristics
include size, shape, hardness, and
17
CA 02670049 2009-07-02
surface texture. In some embodiments the invention provides a set of
aggregates with a plurality of
characteristics that are chosen to match a predetermined set of
characteristics, where the characteristics
include size, shape, hardness, and density. In some embodiments the invention
provides a set of aggregates
with a plurality of characteristics that are chosen to match a predetermined
set of characteristics, where the
characteristics include size, shape, and density.
In some embodiments, the invention provides an aggregate comprising a
synthetic carbonate. The
synthetic carbonate may contain sequestered CO2, such as a carbonate that is a
precipitate from divalent
cation containing water, e.g., an alkaline-earth-metal-ion containing water,
such as salt water as described
further herein, for example, sea water. The divalent cation containing-water,
e.g., alkaline-earth-metal-ion
containing water, may contain CO2 derived from an industrial waste stream,
wherein at least part of the CO2
derived from the industrial waste stream is present in the synthetic carbonate
as sequestered CO2. The
industrial gaseous waste stream may be any waste stream as described herein,
e.g, from a power plant,
foundry, cement plant, refinery, or smelter. The synthetic carbonate can
contain at least one of. a calcium
carbonate compound, a magnesium carbonate compound and a calcium magnesium
carbonate compound,
in any ratio as described more fully herein, for example, where the weight
ratio of magnesium to calcium
ranges from 10/1 to 1/10. The calcium carbonate compounds, if present, may
include one or more any of
the polymorphs described herein, for example, calcite, aragonite, vaterite,
ikaite or amorphous calcium
carbonate. The magnesium carbonate compounds, if present, may include one or
more of the any of the
polymorphs described herein, for example, dypingite, magnesite, barringtonite,
nesquehonite, lansfordite,
hydromagnesite or amorphous magnesium carbonate, such as dypingite in an
amount of at least 1% w/w, or
in an amount at least 5% w/w; embodiments including dypingite may, in some
cases, further include
nesquehonite, hydromagnesite, or a combination thereof. The calcium magnesium
carbonate compounds, if
present, may include one or more of any of the polymorphs described herein,
for example dolomite, huntite
or sergeevite. The aggregate may comprise strontium in an amount as described
herein. The aggregate
may be reactive or may not be reactive, also as described further herein. In
some embodiments, the
synthetic carbonate comprises from 1% to 99% of the aggregate. The aggreagate
may be coarse aggregate,
.g., having an average particle size that ranges between 0.125 inches to 6
inches, or fine aggregate, e.g.,
having an average particle size that ranges between 0.001 inches to 0.25
inches, or a combination of coarse
and fine. The aggregate may have particle shapes selected from the group
consisting of rounded, irregular,
flaky, angular, elongated, flaky-elongated, subangular, subrounded, well
rounded and any mixtures thereof;
in some cases the aggregate further has particle surface textures that are
selected from the group consisting
of. glassy, smooth, granular, rough, crystalline, honeycombed and mixtures
thereof. In some
embodiments, the aggregate has particle shapes selected from the group
consisting of polygonal,
cylindrical, spherical, triangular, curved shapes, annulus, ellipsoidal, oval,
star shaped, prisms or any
mixtures thereof; and in some cases may further have particle surface textures
that are selected from the
group consisting of glassy, smooth, granular, rough, crystalline, honeycombed
and mixtures thereof. The
aggregate may have a Mohs hardness that ranges from about 1.5 to 9, such as
about 2.5 to 6, or equivalent
hardness on the Rockwell, Vickers, or Brinell scales. Any of the above
aggregates may further include one
or more of: Portland cement, fly ash, lime and a binder, for example, Portland
cement, such as where the
18
CA 02670049 2009-07-02
weight ratio of the synthetic carbonate and Portland cement ranges from 0.1/1
to 5/1. The aggregate has a
unit density of between 100 to 150 lb/ft3, such as between 75-125 lb/ft3.
In some embodiments the invention provides a method of producing an aggregate
comprising a
synthetic carbonate, the method comprising: obtaining a synthetic carbonate;
and producing an aggregate
comprising the synthetic carbonate. In some embodiments the synthetic
carbonate comprises sequestered
CO2. In some embodiments the obtaining step comprises precipitating the
synthetic carbonate from a
divalent cation-containg water, e.g., an alkaline-earth-metal-ion containing
water such as salt water, e.g.,
sea water. The obtaining step may further comprise contacting the divalent
cation-containing water, e.g.,
alkaline-earth-metal-ion containing water, to an industrial gaseous waste
stream comprising CO2 prior to,
and/or during, the precipitating step. The industrial gaseous waste stream may
be any stream as described
herein, such as from a power plant, foundry, cement plant, refinery, or
smelter, e.g. a flue gas. In some
embodiments the obtaining step further comprises raising the pH of the
alkaline-earth-metal-ion containing
water to 10 or higher prior to or during the precipitating step. The producing
step may further include
generating a settable composition comprising the synthetic carbonate; and
allowing the settable
composition to form a solid product, such as by mixing the synthetic carbonate
with one or more of. water,
Portland cement, fly ash, lime and a binder, and optionally mechanically
refining the solid product, such as
by molding, extruding, pelletizing or
crushing. The producing step may include contacting the synethetic carbonate
with fresh water to convert
the synthetic carbonate to a freshwater stable product; in one embodiment this
is done by spreading the
synthetic carbonate in an open area; and contacting the spread synthetic
carbonate with fresh water.
In some embodiments, the invention provides an aggregate suitable for use in a
building material
wherein the aggregate has a unit density of less than 115 lb/cu ft and is a
carbon negative aggregate.
In some embodiments the invention provides a composition that includes a
hydraulic cement; and
an aggregate containing a synthetic carbonate, such as any of the synthetic
carbonates described above.
The composition may further include water, and the composition is a settable
composition such as a
concrete, mortar, or a soil stabilizer. The composition may further contain at
least one admixture. The
hydraulic cement may contain a second synthetic carbonate, e.g, where the
second synthetic carbonate
comprises sequestered C02-
The invention also provides a method that includes obtaining a composition
comprising a
hydraulic cement and an aggregate comprising a synthetic carbonate, such as
any of the synthetic
carbonates described above, e.g., a C02-sequestering carbonate, i.e., a
carbonate that contains sequestered
C02; and producing a settable composition comprising the obtained composition.
The method may further
include allowing the settable composition to set into a solid product, such as
a structural product, e.g. part
of a road, such as asphalt, or a building foundation.
In some embodiments the invention provides road base comprising aggregate
comprising a
synthetic carbonate, such as any of the synthetic carbonates described above.
In some embodiments the
invention provides an asphalt comprising aggregate comprising a synthetic
carbonate, such as any of the
synthetic carbonates described above.
The invention also provides a system for producing an aggregate containing a
synthetic carbonate,
the system comprising: an input for an alkaline-earth-metal-containing water;
carbonate compound
19
CA 02670049 2009-07-02
precipitation station that subjects the water to carbonate compound
precipitation conditions and produces a
synthetic carbonate; and an aggregate producer for producing aggregate
comprising the synthetic carbonate.
In some embodiments, the aggregate producer comprises a refining station to
mechanically refine the
aggregate comprising the synthetic carbonate.
In some embodiments the invention provides a method of sequestering C02, that
includes
contacting an alkaline-earth-metal-ion containing water to a gaseous
industrial waste stream comprising
C02;
precipitating a synthetic carbonate from the alkaline-earth-metal-ion
containing water, wherein the
synthetic carbonate comprises CO2 derived from the gaseous industrial waste
stream; and producing
aggregate comprising the synthetic carbonate.
In some embodiments, the invention provides a concoidally-fracturing
aggregate.
2. Making the compositions of the invention
Aggregates of the invention can be produced by any suitable method. For
example, aggregates of
the invention can be produced by precipitating a precursor calcium and/or
magnesium carbonate
composition from a water and then processing the resultant precipitate to
produce an aggregate. The
carbonate compound compositions that make up the aggregates of the invention
can be metastable
carbonate compounds, or derived from such compounds, that are precipitated
from a water, such as a salt-
water, as described in greater detail below. The carbonate compound
compositions of the invention
included precipitated crystalline and/or amorphous carbonate compounds.
As reviewed above, the aggregates of the invention include a carbonate
compound composition,
e.g., a composition precipitated from a divalent cation-containing water, such
as an alkaline-earth-metal-
containing water, such as a saltwater-derived carbonate compound composition.
As such, the carbonate
compound composition of the aggregates is one that is made up of one or more
different carbonate
compounds, which may be amorphous or crystalline. As reviewed above, the
carbonate compound
compositions of the cements may include one or more hydroxide compounds.
Exemplary methods for preparation of compositions of the invention include
methods that may be
divided into 1) preparation of a precipitate, and 2) preparation of aggregate
from the precipitate.
1) Preparation of precipitate.
The precipitates for use in aggregates of the invention may be prepared from
divalent cations, e.g.,
magnesium and/or calcium ions and CO2, e.g., from an industrial waste gas
source. The precipitates are
generally carbonates and/or bicarbonates, and in order to prepare the
precipitate it is necessary to remove
protons from the solution, e.g., by use of a base, by use of electrochemical
methods, or a combination.
Divalent cations Divalent cations (e.g., cations of alkaline earth metals such
as Ca 2+ and Mgt+),
are used to produce aggregate using systems and methods of the invention.
Divalent cations may come
from any of a number of different divalent cation sources depending upon
availability at a particular
location. Such sources include industrial wastes, seawater, brines, hard
waters, minerals, and any other
suitable source.
CA 02670049 2009-07-02
In some locations, industrial waste streams from various industrial processes
provide for
convenient sources of divalent cations (as well as in some cases other
materials useful in the process, e.g.,
metal hydroxide). Such waste streams include, but are not limited to, mining
wastes; fossil fuel burning ash
(e.g., flyash); slag (e.g. iron slag, phosphorous slag); cement kiln waste;
oil refinery/petrochemical refinery
waste (e.g. oil field and methane seam brines); coal seam wastes (e.g. gas
production brines and coal seam
brine); paper processing waste; water softening waste brine (e.g., ion
exchange effluent); silicon processing
wastes; agricultural waste; metal finishing waste; high pH textile waste; and
caustic sludge.
In some locations, a convenient source of divalent cations for use in systems
and methods of the
invention is water (e.g., an aqueous solution comprising divalent cations such
as seawater or surface brine),
which may vary depending upon the particular location at which the invention
is practiced. Suitable
aqueous solutions of divalent cations that may be used include solutions
comprising one or more divalent
cations, e.g., alkaline earth metals (e.g., calcium, magnesium). In some
embodiments, the aqueous source of
divalent cations comprises alkaline earth metal cations. In some embodiments,
the alkaline earth metal
cations include calcium, magnesium, or a mixture thereof. In some embodiments,
the aqueous solution of
divalent cations comprises calcium in amounts ranging from 50 to 50,000 ppm,
50 to 40,000 ppm, 50 to
20,000 ppm, 100 to 10,000 ppm, 200 to 5000 ppm, or 400 to 1000 ppm. In some
embodiments, the
aqueous solution of divalent cations comprises magnesium in amounts ranging
from 50 to 40,000 ppm, 50
to 20,000 ppm, 100 to 10,000 ppm, 200 to 10,000 ppm, 500 to 5000 ppm, or 500
to 2500 ppm. In some
embodiments, where Ca2+ and Mg2+ are both present, the ratio of Ca2+/Mg2+ in
the aqueous solution of
divalent cations is 1 to 1000; 1 to 800; 1 to 500; 1 to 250; 1 to 200; 1 to
150; 1 to 100; 1 to 50; and 1 to 25.
The aqueous solution of divalent cations may comprise divalent cations derived
from freshwater,
brackish water, seawater, or brine (e.g., naturally occurring brines or
anthropogenic brines such as
geothermal plant wastewaters, desalination plant waste waters), as well as
other salines having a salinity
that is greater than that of freshwater. Brackish water is water that is
saltier than freshwater, but not as salty
as seawater. Brackish water has a salinity ranging from about 0.5 to about 35
ppt (parts per thousand).
Seawater is water from a sea, an ocean, or any other saline body of water that
has a salinity ranging from
about 35 to about 50 ppt. Brine is water saturated or nearly saturated with
salt. Brine has a salinity that is
about 50 ppt or greater. In some embodiments, the saltwater source from which
divalent cations are derived
is a naturally occurring source selected from a sea, an ocean, a lake, a
swamp, an estuary, a lagoon, a
surface brine, a deep brine, an alkaline lake, an inland sea, or the like. In
some embodiments, the saltwater
source from which the divalent cations are derived is a anthropogenic brine
selected from a geothermal
plant wastewater or a desalination wastewater.
Freshwater is often a convenient source of divalent cations (e.g., cations of
alkaline earth metals
such as Ca2+ and Mg2) . Any of a number of suitable freshwater sources may be
used, including freshwater
sources ranging from sources relatively free of minerals to sources relatively
rich in minerals. Mineral-rich
freshwater sources may be naturally occurring, including any of a number of
hard water sources, lakes, or
inland seas. Some mineral-rich freshwater sources such as alkaline lakes or
inland seas (e.g., Lake Van in
Turkey) also provide a source of pH-modifying agents. Mineral-rich freshwater
sources may also be
anthropogenic. For example, a mineral-poor (soft) water may be contacted with
a source of divalent cations
such as alkaline earth metal cations (e.g., calcium or magnesium) to produce a
mineral-rich water that is
21
CA 02670049 2009-07-02
suitable for systems and methods for producing aggregate according to the
invention. Divalent cations or
precursors thereof (e.g. salts, minerals) may be added to freshwater (or any
other water described herein)
using any convenient protocol (e.g., addition of solids, suspensions, or
solutions). In some embodiments,
divalent cations selected from calcium and magnesium are added to freshwater.
In some embodiments,
monovalent cations selected from sodium and potassium are added to freshwater.
In some embodiments,
freshwater comprising calcium is combined with magnesium silicates (e.g.,
olivine or serpentine), or
products or processed forms thereof, yielding a solution comprising calcium
and magnesium cations.
Many minerals provide sources of divalent cations and, in addition, some
minerals are sources of
base. Mafic and ultramafic minerals such as olivine, serpentine, and any other
suitable mineral may be
dissolved using any convenient protocol. Dissolution may be accelerated by
increasing surface area, such
as by milling by conventional means or by, e.g., jet milling, as well as by
use of, e.g., ultrasonic techniques.
In addition, mineral dissolution may be accelerated by exposure to acid or
base. Metal silicates (e.g.,
magnesium silicates) and other minerals comprising cations of interest may be
dissolved, e.g., in acid (e.g.,
HCl such as HCl from an electrochemical process) to produce, for example,
magnesium and other metal
cations for use in precipitation material, and, subsequently, aggregate or
other compositions of the
invention. In some embodiments, magnesium silicates and other minerals may be
digested or dissolved in
an aqueous solution that has become acidic due to the addition of carbon
dioxide and other components of
waste gas (e.g., combustion gas). Alternatively, other metal species such as
metal hydroxide(e.g.,
Mg(OH)2, Ca(OH)2) may be made available for use in aggregate by dissolution of
one or more metal
silicates (e.g., olivine and serpentine) with aqueous alkali hydroxide (e.g.,
NaOH) or any other suitable
caustic material. Any suitable concentration of aqueous alkali hydroxide or
other caustic material may be
used to decompose metal silicates, including highly concentrated and very
dilute solutions. The
concentration (by weight) of an alkali hydroxide (e.g., NaOH) in solution may
be, for example, from 30%
to 80% and from 70% to 20% water. Advantageously, metal silicates and the like
digested with aqueous
alkali hydroxide may be used directly to produce precipitation material, and,
subsequently, aggregate from
a waste gas stream. In addition, base value from the precipitation reaction
mixture may be recovered and
reused to digest additional metal silicates and the like.
In some embodiments, an aqueous solution of divalent cations may be obtained
from an industrial
plant that is also providing a combustion gas stream. For example, in water-
cooled industrial plants, such as
seawater-cooled industrial plants, water that has been used by an industrial
plant for cooling may then be
used as water for producing precipitation material, and, subsequently,
aggregate in a system or method of
the invention. If desired, the water may be cooled prior to entering the
precipitation system. Such
approaches may be employed, for example, with once-through cooling systems.
For example, a city or
agricultural water supply may be employed as a once-through cooling system for
an industrial plant. Water
from the industrial plant may then be employed for producing precipitation
material, which may
subsequently be used to produce aggregate in a system or method of the
invention, and wherein output
water has a reduced hardness and greater purity. If desired, such systems may
be modified to include
security measures (e.g., to detect tampering such as addition of poisons) and
coordinated with governmental
agencies (e.g., Homeland Security or other agencies). Additional tampering or
attack safeguards may be
employed in such embodiments.
22
CA 02670049 2009-07-02
CO2 s ources Although in some embodiments there is sufficient carbon dioxide
in the water
source to precipitate significant amounts of carbonates (e.g., from seawater),
additional carbon dioxide is
generally used-for C02-sequestering aggregates it will be apparent that this
is generally the case. Thus, in
certain embodiments, the methods further include contacting the volume of
aqueous solution, e.g., an
aqueous solution of divalent cations that is to be subjected to mineral
precipitation conditions, with a source
of CO2. The source of CO2 that is contacted with the aqueous solution, e.g.,
of divalent cations may be any
convenient CO2 source. The CO2 source may be a gas, a liquid, a solid (e.g.,
dry ice), a supercritical fluid,
or CO2 dissolved in a liquid. In certain embodiments, the CO2 source is a
gaseous CO2 source. This
gaseous CO2 source is, in certain instances, a waste feed (i.e., a byproduct
of an active process of the
industrial plant) from an industrial plant. The nature of the industrial plant
may vary in these embodiments,
where industrial plants of interest include power plants, chemical processing
plants, mechanical processing
plants, refineries, cement plants, steel plants, and other industrial plants
that produce CO2 as a byproduct of
fuel combustion or another processing step (such as calcination by a cement
plant). For C02-sequestering
aggregate these waste streams, in some embodiments, provide the CO2 to be
sequestered. The gaseous
stream may be substantially pure CO2 or comprise multiple components that
include CO2 and one or more
additional gases and/or other substances such as ash and other particulates.
Waste gas streams comprising CO2 include both reducing (e.g., syngas, shifted
syngas, natural gas,
hydrogen and the like) and oxidizing condition streams (e.g., flue gases from
combustion). Particular waste
gas streams that may be convenient for the invention include oxygen-containing
combustion industrial plant
flue gas, turbo charged boiler product gas, coal gasification product gas,
shifted coal gasification product
gas, anaerobic digester product gas, wellhead natural gas stream, reformed
natural gas or methane hydrates,
and the like. Combustion gas from any convenient source may be used to produce
aggregate. In some
embodiments, combustion gases in post-combustion effluent stacks of industrial
plants such as power
plants, cement plants, and coal processing plants is used
Thus, the waste streams may be produced from a variety of different types of
industrial plants.
Suitable waste streams for the invention include waste streams produced by
industrial plants that combust
fossil fuels (e.g., coal, oil, natural gas) and anthropogenic fuel products of
naturally occurring organic fuel
deposits (e.g., tar sands, heavy oil, oil shale, etc.). In some embodiments,
waste streams suitable for
systems and methods of the invention are sourced from a coal-fired power
plant, such as a pulverized coal
power plant, a supercritical coal power plant, a mass burn coal power plant, a
fluidized bed coal power
plant; in some embodiments the waste stream is sourced from gas or oil-fired
boiler and steam turbine
power plants, gas or oil-fired boiler simple cycle gas turbine power plants,
or gas or oil-fired boiler
combined cycle gas turbine power plants. In some embodiments, waste streams
produced by power plants
that combust syngas (i.e., gas that is produced by the gasification of organic
matter, for example, coal,
biomass, etc.) are used. In some embodiments, waste streams from integrated
gasification combined cycle
(IGCC) plants are used. In some embodiments, waste streams produced by Heat
Recovery Steam Generator
(HRSG) plants are used to produce aggregate in accordance with systems and
methods of the invention.
Waste streams produced by cement plants are also suitable for systems and
methods of the
invention. Cement plants waste streams include waste streams from both wet
process and dry process
23
CA 02670049 2009-07-02
plants, which plants may employ shaft kilns or rotary kilns, and may include
pre-calciners. These industrial
plants may each bum a single fuel, or may burn two or more fuels sequentially
or simultaneously.
Industrial waste gas streams may contain carbon dioxide as the primary non-air
derived
component, or may, especially in the case of coal-fired power plants, contain
additional components such as
nitrogen oxides (NOx), sulfur oxides (SOx), and one or more additional gases.
Additional gases and other
components may include CO, mercury and other heavy metals, and dust particles
(e.g., from calcining and
combustion processes). Additional components in the gas stream may also
include halides such as hydrogen
chloride and hydrogen fluoride; particulate matter such as fly ash, dusts, and
metals including arsenic,
beryllium, boron, cadmium, chromium, chromium VI, cobalt, lead, manganese,
mercury, molybdenum,
selenium, strontium, thallium, and vanadium; and organics such as
hydrocarbons, dioxins, and PAH
compounds. In various embodiments, one or more of these additional components
is precipitated in
precipitation material formed by contacting the waste gas stream comprising
these additional components
with an aqueous solution comprising divalent cations (e.g., alkaline earth
metal ions such as Ca 2+ and
Mg2). For example, where SO2 is contained in the gas stream, sulfates and
sulfites of calcium and
magnesium may be precipitated in precipitation material, which precipitation
may further comprise calcium
and/or magnesium carbonates. Other components, such as heavy metals, e.g.,
mercury, may be trapped in
the precipitate or may precipitate as solid compounds.
Although industrial waste gas offers a relatively concentrated source of
combustion gases, the
methods and systems are also applicable to removing combustion gas components
from less concentrated
sources (e.g., atmospheric air), which contains a much lower concentration of
pollutants than, for example,
flue gas. Thus, in some embodiments, methods and systems encompass decreasing
the concentration of
pollutants in atmospheric air by producing a stable precipitation material,
and, subsequently, aggregate
using the procedures outlined herein. In these cases, the concentration of
pollutants, e.g., C02, in a portion
of atmospheric air may be decreased by 10% or more, 20% or more, 30% or more,
40% or more, 50% or
more, 60% or more, 70% or more, 80% or more, 90% or more, 95% or more, 99% or
more, 99.9% or more,
or 99.99%. Such decreases in atmospheric pollutants may be accomplished with
yields as described
herein, or with higher or lower yields, and may be accomplished in one
precipitation step or in a series of
precipitation steps.
A variety of different gaseous waste streams may be treated in order to
utilize various combustion
gas components. Suitable gaseous waste streams have, in some embodiments, CO2
present in amounts of
200 ppm to 1,000,000 ppm, such as 200,000 ppm to 1000 ppm, including 200,000
ppm to 2000 ppm, for
example 180,000 ppm to 2000 ppm, or 180,000 ppm to 5000 ppm, also including
180,000 ppm to 10,000
ppm. The waste streams may include one or more additional components, for
example, water, NOx
(mononitrogen oxides: NO and N02), SOx (monosulfur oxides: SO, S02 and S03),
VOC (volatile organic
compounds), heavy metals such as mercury, and particulate matter (particles of
solid or liquid suspended in
a gas). Flue gas temperature may also vary. In some embodiments, the
temperature of the flue gas is from
0 C to 2000 C, such as from 60 C to 7000 C, and including 100 C to 400 C.
A source of CO2 is contacted with an aqueous solution, e.g., an aqueous
solution of divalent
cations (e.g., alkaline earth metal cations) at some point during the method,
such as before, during, or even
after the aqueous solution of divalent cations has been subjected to
precipitation conditions. Contact of the
24
CA 02670049 2009-07-02
aqueous solution, e.g. of divalent cations such as alkaline earth metal ions,
with the source of CO2 may
occur before and/or during the time when the cation solution is subject to CO2
precipitation conditions.
Accordingly, embodiments of the invention include methods in which the volume
of aqueous solution of
divalent cations is contacted with a source of CO2 prior to subjecting the
volume of cation solution to
mineral precipitation conditions. Embodiments of the invention include methods
in which the volume of
divalent cation solution is contacted with a source of CO2 while the volume of
divalent cation solution is
being subjected to carbonate and/or bicarbonate compound precipitation
conditions. Embodiments of the
invention include methods in which the volume of aqueous solution of divalent
cations is contacted with a
source of a CO2 prior to subjecting the volume of cation solution to carbonate
and/or bicarbonate compound
precipitation conditions. Embodiments of the invention include methods in
which the volume of aqueous
solution of divalent cations is contacted with a source of a CO2 both prior to
and while subjecting the
volume of cation solution to carbonate and/or bicarbonate compound
precipitation conditions. In some
embodiments, the same divalent cation solution may be cycled more than once,
wherein a first cycle of
precipitation removes primarily calcium carbonate and magnesium carbonate
minerals, and leaves
remaining alkaline water to which other alkaline earth ion sources may be
added, that can have more carbon
dioxide cycled through it, precipitating more carbonate and/or bicarbonate
compounds. It will be
appreciated that in these cases the CO2 may be contacted with the water
before, during, and/or after divalent
cations have been added.
A gaseous waste stream may be provided from the industrial plant to the site
of precipitation in
any convenient manner that conveys the gaseous waste stream from the
industrial plant to the precipitation
plant. In some embodiments, the gaseous waste stream is provided with a gas
conveyer (e.g., a duct) that
runs from a site of the industrial plant (e.g., an industrial plant flue) to
one or more locations of the
precipitation site. The source of the gaseous waste stream may be a distal
location relative to the site of
precipitation such that the source of the gaseous waste stream is a location
that is 1 mile or more, such as 10
miles or more, including 100 miles or more, from the precipitation location.
For example, the gaseous waste
stream may have been transported to the site of precipitation from a remote
industrial plant via a CO2 gas
conveyance system (e.g., a pipeline). The industrial plant generated CO2
containing gas may or may not be
processed (e.g., remove other components) before it reaches the precipitation
site (i.e., the site in which
precipitation and/or production of aggregate takes place). In yet other
instances, the gaseous waste stream
source is proximal to the precipitation site. For example, the precipitation
site is integrated with the gaseous
waste stream source, such as a power plant that integrates a precipitation
reactor for precipitation of
precipitation material that may be used to produce aggregate.
A portion of the gaseous waste stream (i.e., not the entire gaseous waste
stream) from an industrial
plant may be used to produce precipitation material and, subsequently,
aggregate. In these embodiments,
the portion of the gaseous waste stream that is employed in precipitation of
precipitation material may be
75% or less, such as 60% or less, and including 50% and less of the gaseous
waste stream. In yet other
embodiments, substantially (e.g., 80% or more) the entire gaseous waste stream
produced by the industrial
plant is employed in precipitation of precipitation material useful for
producing aggregate of the invention.
In these embodiments, 80% or more, such as 90% or more, including 95% or more,
up to 100% of the
CA 02670049 2010-06-30
gaseous waste stream (e.g., flue gas) generated by the source may be employed
for precipitation of
precipitation material.
As indicated above, the gaseous waste stream may be one that is obtained from
a flue or analogous
structure of an industrial plant. In these embodiments, a line (e.g., duct) is
connected to the flue so that gas
leaves the flue through the line and is conveyed to the appropriate
location(s) of a precipitation system.
Depending upon the particular configuration of the precipitation system at the
point which the gaseous
waste stream is employed, the location of the source from which the gaseous
waste stream is obtained may
vary (e.g., to provide a waste stream that has the appropriate or desired
temperature). As such, in certain
embodiments, where a gaseous waste stream having a temperature ranging for 0 C
to 1800 C, such as 60 C
to 700 C, is desired, the flue gas may be obtained at the exit point of the
boiler or gas turbine, the kiln, or at
any point of the power plant or stack, that provides the desired temperature.
Where desired, the flue gas is
maintained at a temperature above the dew point (e.g., 125 C) in order to
avoid condensation and related
complications. If it is not possible to maintain the temperature above the dew
point, steps may be taken to
reduce the adverse impact of condensation (e.g., employing ducting that is
stainless steel, fluorocarbon
(such as poly(tetrafluoroethylene)) lined, diluted with water, and pH
controlled, etc.) so the duct does not
rapidly deteriorate.
The volume of water may be contacted with the CO2 source using any convenient
protocol. Where
the CO, is a gas, contact protocols of interest include, but are not limited
to: direct contacting protocols,
e.g., bubbling the gas through the volume of saltwater, concurrent contacting
means, i.e., contact between
unidirectionally flowing gaseous and liquid phase streams, countercurrent
means, i.e., contact between
oppositely flowing gaseous and liquid phase streams, and the like. Thus,
contact may be accomplished
through use of infusers, bubblers, fluidic Venturi reactor, sparger, gas
filter, spray, tray, or packed column
reactors, and the like, as may be convenient. In one embodiments, contact is
between a flat jet liquid sheet
and the gas, where the sheet and the gas may he moving in countercurrent,
cocurrent, or crosscurrent
directions, or in any other suitable manner.
In one embodiment contact is between
neutrally buoyant liquid droplets of solution, of a diameter of 5 mircometers
or less, and gas in a chamber.
In some embodiments a catalyst is used to accelerate the dissolution of carbon
dioxide into water by
accelerating the reaction toward equilibrium; the catalyst may be an inorganic
substance such as zinc
trichloride or cadmium, or an organic substance, e.g., an enzyme such as
carbonic anhydrase
Proton removal The dissolution of CO2 into aqueous solution produces carbonic
acid, which is
in equilibrium with bicarbonate and carbonate. In order to precipitate
carbonates, protons are removed
from the solution to shift the equilibrium toward carbonate. In addition,
removal of protons allows more
CO2 to go into solution. In some embodiments proton removal is used together
with CO2 contact with the
aqueous solution, e.g. containing divalent cations, to increase CO2 absorption
in one phase of the reaction,
where the pH may remain constant, increase, or even decrease, followed by a
rapid removal of protons
(e.g., by addition of a base) to cause rapid precipitation of carbonate
compounds. Protons may be removed
from the solution by any convenient approach. Approaches of interest include,
but are not limited to: use of
26
CA 02670049 2009-07-02
naturally occurring pH raising agents, use of microorganisms and fungi, use of
synthetic chemical pH
raising agents, recovery of man-made waste streams, and using electrochemical
means.
The term naturally occurring pH raising agents encompasses any means that can
be found in the
wider environment that may create or have a basic local environment. Some
embodiments provide for
naturally occurring pH raising agents including minerals that create basic
environments upon addition to
solution, e.g. dissolution. Such minerals include, but are not limited to:
lime (CaO), periclase (MgO),
volcanic ash, ultramafic rocks and minerals such as serpentine, and iron
hydroxide minerals, e.g. goethite
and limonite. Methods of dissolution of such rocks and minerals are provided
herein. Some embodiments
provide for using naturally alkaline bodies of water as naturally occurring pH
raising agents. Examples of
naturally alkaline bodies of water include, but are not limited to: surface
water sources, e.g. alkaline lakes
such as Mono Lake in California, and ground water sources, e.g. basic
aquifers. Other embodiments
provide for the use of deposits from dried alkaline bodies of water, such as
the crust along Lake Natron in
Africa's Great Rift Valley. Other embodiments provide using organisms that
excrete basic solutions or
molecules in their normal metabolism as pH raising agents. Examples of such
organisms are fungi that
produce alkaline protease, e.g. the deep-sea fungus Aspergillus ustus with an
optimal pH of 9, and bacteria
that create alkaline molecules, e.g. the cynobacteria, Lyngbya sp. found in
the Arlin wetland in Bristish
Columbia that increase pH as a byproduct of photosynthesis. In some
embodiments, organisms are used
where there are co-contaminants that are used in the metabolism that produces
pH raising molecules or
solutions, e.g. B. pasteurii that hydrolyzes urea to ammonia is used where
urea exists as a contaminant. In
some embodiments, organisms are cultured away from the process and their
alkaline excretions are used for
addition to the sequestration process.
Chemical pH raising agents generally refer to synthetic chemicals, produced in
large quantities,
that are commercially available. Some embodiments provide for use of chemicals
including: hydroxides,
organic bases, super bases, oxides, ammonia, and carbonates. Hydroxides
include molecules that contain
OH. Exemplary hydroxides are: sodium hydroxide (NaOH), potassium hydroxide
(KOH), calcium
hydroxide (Ca(OH)2), and magnesium hydroxide (Mg(OH)2). Organic bases are
carbon containing
molecules and are generally are of the form (-NR2H+). Some embodiments provide
for use of organic bases
to raise pH, including: pyridine, methyl amine, imidazole, benimidazol,
histidine, and the phophazene
bases. Some embodiments provide for removing protons pH with ammonia, NH3.
Ammonia is considered
by some to be an organic base of sorts though it lacks carbon molecules. Other
embodiments provide for
the use of super bases as pH raising chemicals, including but not limited to:
ethoxide, sodium amide
(NaNH2), sodium hydride (NaH), butyl lithium, lithium diisopropylamide,
lithium diethylamide, and
lithium bis(trimethylsilyl)amide. Oxides are other chemicals that can be used
as proton acceptors/pH
raising agents. Some embodiments provide for using oxides as pH raising
agents, including, but not limited
to: calcium oxide (CaO), magnesium oxide (MgO), strontium oxide (SrO), and
beryllium oxide (BeO).
Waste streams from various processes are other sources of agents that may be
used to react with
protons in the aqueous solution, e.g., bases. In some embodiments waste
streams are provided as bases.
Such waste streams include, but are not limited to: mining wastes; fossil fuel
burning ash; slag, e.g. iron
slag, phosphorous slag; cement kiln waste; oil refinery/petrochemical refinery
waste, e.g. oil field and
methane seam brines; coal seam wastes, e.g. gas production brines and coal
seam brine; paper processing
27
CA 02670049 2011-01-14
waste; water softening, e.g. ion exchange waste brine; silicon processing
wastes; agricultural waste; metal
finishing waste; high pH textile waste; and caustic sludge. Mining wastes
include any wastes from the
extraction of metal or another precious or useful mineral from the earth. Some
embodiments provide for
wastes from mining to be used to raise pH, including: red mud from the Bayer
aluminum extraction
process; the waste from magnesium extraction for sea water, e.g. at Moss
Landing, California; and the
wastes from other mining processes involving leaching. Ash from processes
burning fossil fuels, such as
coal fired power plants, create ash that is often rich in CaO or other metal
oxides that can create a basic
environment when in solution. In some embodiments, ashes resulting from
burning fossil fuels, e.g. coal
fired power plants, are provided as pH raising agents, including fly ash, e.g.
ash that exits out the smoke
stack, and bottom ash. Cement kiln waste is useful as a pH raising agent
because the powder remaining in
cement kilns often contains CaO, and is provided as such in some embodiments.
Agricultural waste, either
through animal waste or excessive fertilizer use, may contain potassium
hydroxide (KOH) or ammonia
(NH3) or both, and agricultural waste is provided in some embodiments of the
invention as a pH raising
agent. This agricultural waste is often collected in ponds, but it may also
percolate down into aquifers,
where it can be accessed for use in the sequestration process.
Electrochemical methods are another means to remove protons from a solution,
either by removing
protons from molecules (deprotonation) from the aqueous solution of divalent
cations , e.g., if proton
production from CO2 dissolution matches or exceeds proton removal by an
electrochemical process, or by
creating caustic molecules, e.g. hydroxides, as through the chlor-alkali
process or other electrochemical
processes. For example, electrodes (cathode and anode) may be provided in the
reactor which holds the
aqueous solution, e.g., in some embodiments, of divalent cations , where the
electrodes may be separated
by a selective barrier, such as a membrane, as desired. Where desired,
byproducts of the hydrolysis product,
e.g., H2, sodium metal, etc. may be harvested and employed for other purposes,
as desired.
In some instances, low-voltage electrochemical protocols are employed remove
protons from the
aqueous solution, e.g. while CO2 is dissolved (either directly removing
protons or indirectly by providing
base), and at the precipitation step, (again either directly or indirectly).
"Low-voltage" includes an
electrochemical protocol that operates at an average voltage of 2, 1.9, 1.8,
1.7, or 1.6 V or less, such as less
than 1.5, 1.4, 1.3, 1.2, 1.1 V or less, such as IV or less, including 0.9V or
less, 0.8V or less, 0.7V or less,
0.6V or less, 0.5V or less, 0.4V or less, 0.3V or less, 0.2V or less, or 0.1V
or less. Of interest are
electrochemical protocols that do not generate chlorine gas. Also of interest
are electrochemical protocols
that do not generate oxygen gas. Also of interest are electrochemical
protocols that do not generate
hydrogen gas. In some instances, the electrochemical protocol is one that does
not generate any gaseous by-
byproduct. In some embodiments, the electrochemical protocol generates
hydrogen gas at the cathode
which is transported to the anode where it is converted to protons. See, e.g.,
US Patent Application No.
12/344,019, filed December 24, 2008, and U.S. Patent Application No.
12/375,632, filed December 23,
2008, and PCT Application No. US08/088242, filed December 23, 2008, and PCT
Application No.
US09/32301, filed January 28, 2009.
28
CA 02670049 2009-07-02
These proton removal approaches may be used in any suitable combination. Some
embodiments
provide for combination of pH raising/proton removal methods including: use of
man made waste, e.g. fly
ash or mining waste, in combination with commercially available base, e.g.
NaOH; man made waste in
combination with electrochemical methods, e.g. deprotonation, and naturally
occurring pH raising agents,
e.g. serpentine minerals; or man made waste in combination with commercially
available base and naturally
occurring pH raising agents. Some embodiments provide for the combination of
pH raising/proton removal
such that 2-30% of the pH raising agent is fly ash, 20-80%% of the pH raising
agent is waste, e.g. from a
mining process such as red mud, or mineral, such as serpentine, or a
combination thereof, and 10-50% of
the pH raising agent is proton removal using deprotonation in an
electrochemical process.
Precipitation conditions Following CO2 dissolution in aqueous solution
containing divalent
cations, or in some embodiments during, or after dissolution, precipitation
occurs. Precipitation conditions
of interest may vary. For example, the temperature of the water may be within
a suitable range for the
precipitation of the desired mineral to occur. In some embodiments, the
temperature of the water may be in
a range from 5 to 70 C, such as from 20 to 50 C and including from 25 to 45 C.
As such, while a given set
of precipitation conditions may have a temperature ranging from 0 to 100 C,
the temperature of the water
may have to be adjusted in certain embodiments to produce the desired
precipitate.
While the pH of the aqueous solution of divalent cations may range from 5 to
14 during a given
precipitation process, in some instances the protons are removed, e.g. pH is
raised to alkaline levels, in
order to produce the desired precipitation product. In some embodiments, the
pH is raised to a level
sufficient to cause precipitation of the desired CO2 sequestering product. As
such, the pH may be raised to
9.5 or higher, such as 10 or higher, including 10.5 or higher. In some
embodiments, conditions are
adjusted so that little or no CO2 is released during the precipitaion. Using
sea water as an example, in
normal sea water, 93% of the dissolved CO2 is in the form of bicarbonate ions
(HCO3) and 6% is in the
form of carbonate ions (C03-2). When calcium carbonate precipitates from
normal sea water at ambient pH,
CO2 is released. In fresh water, at pH greater than 10.33, greater than 90% of
the carbonate is in the form
of carbonate ion, and no CO2 is released during the precipitation of calcium
carbonate. In sea water this
transition occurs at a slightly lower pH, closer to a pH of 9.7. Where
desired, the pH may be raised to a
level which minimizes if not eliminates CO2 production during precipitation.
For example, the pH may be
raised to a value of 10 or higher, such as a value of 11 or higher. In certain
embodiments, the pH is raised to
between 7 and 11, such as between 8 and 11, including between 9 and 11, for
example between 9 and 10, or
between 10 and 11. In this step, the pH may be raised to and maintained at the
desired alkaline level, such
that the pH is maintained at a constant alkaline level, or the pH may be
transitioned or cycled between two
or more different alkaline levels, as desired.
Additives other than pH elevating agents may also be introduced into the
aqueous solution of
divalent cations in order to influence the nature of the precipitate that is
produced. As such, certain
embodiments of the methods include providing an additive in the solution
before or during the time when
the cation solution is subjected to the precipitation conditions. Certain
calcium carbonate polymorphs can
be favored by trace amounts of certain additives. For example, vaterite, a
highly unstable polymorph of
CaCO3 which precipitates in a variety of different morphologies and converts
rapidly to calcite, can be
29
CA 02670049 2009-07-02
obtained at very high yields by including trace amounts of lanthanum as
lanthanum chloride in a
supersaturated solution of calcium carbonate. Other additives beside lathanum
that are of interest include,
but are not limited to transition metals and the like. For instance, the
addition of ferrous or ferric iron is
known to favor the formation of disordered dolomite (protodolomite) where it
would not form otherwise.
The nature of the precipitate can also be influenced by selection of
appropriate major ion ratios. Major ion
ratios also have considerable influence of polymorph formation. For example,
as the magnesium:calcium
ratio in the water increases, aragonite becomes the favored polymorph of
calcium carbonate over low-
magnesium calcite. At low magnesium: calcium ratios, low-magnesium calcite is
the preferred polymorph.
As such, a wide range of magnesium:calcium ratios can be employed, including,
e.g., more than 100/1,
50/1, 20/1, 10/1, 5/1, 2/1, 1/1, or less than 1/2, 1/5, 1/10, 1/20, 1/50,
1/100. In certain embodiments, the
magnesium:calcium ratio is determined by the aqueous solution of divalent
cations employed in the
precipitation process (e.g., seawater, brine, brackish water, fresh water),
whereas in other embodiments, the
magnesium:calcium ratio is adjusted to fall within a certain range, e.g., by
addition of exogenous calcium or
magnesium, for example from dissolution of a rock or mineral, such as
serpentine. In some embodiments a
high-calcium water source is used, such as a geologic or other brine, and the
mineral ratio is adjusted
toward 1:1 Ca:Mg by addition of a high-magnesium source, such as dissolved
serpentine or other rock or
mineral. Such a Ca:Mg ratio can allow the formation of protodolomite in the
precipitation stage, which
may be further converted to dolomite in the formation of aggregate or
artificial rock.
When silica is present, a number of additional minerals may be formed.
Replacement of carbonate
minerals by silica is a common feature of ancient sedimentary rocks and deep-
sea sediments. Silica can be
added in many forms. At alkaline pHs, silica dissolves and becomes available
for reaction with
precipitating carbonates. Sources of silica include diatomaceous earth, fly
ash from the burning of coal, and
silica fume. Also, Mg-carbonates are used to scavenge silica in wastewaters
indicating that dissolved
silica/carbonate mineral interactions can occur on short time scales as well.
Klein and Walter (1992)
conducted experiments to determine of the rate, time dependence, and extent of
aqueous SiO2 uptake onto
well characterized Ca-Mg carbonate at temperatures between 25 C and 50 C,
where the solutions of
aqueous SiO2 ranged from 1.5 to 3.5 mM SiO2. Three different reaction
conditions were tested: (1) silica
uptake during short term calcite overgrowth precipitation onto calcite seeds
at fixed degrees of calcite
supersaturation; (2) silica uptake near equilibrium with respect to calcite;
and (3) silica uptake during the
relatively long term (3 weeks) recrystallization of metastable carbonates
(aragonite, 18 mol% Mg-calcite).
Silica uptake onto carbonates is greatest during rapid carbonate
precipitation. Calcite precipitation kinetics,
however, are unaffected by the SiO2 interaction with carbonate surface and
similar precipitation rates are
observed at equivalent degrees of calcite supersaturation in silica-spiked and
silica-free experiments. In
near equilibrium experiments, SiO2 uptake was strongly time-dependent but
smaller in magnitude and
uptake was enhanced at higher SiO2 concentrations, lower pH, and higher
temperature. In longer term
aragonite and Mg-calcite recrystallization experiments. SiO2 uptake was
similar to near-equilibrium
experiments conducted with low Mg-calcite. One advantage of having silica
present in the carbonate
precipitates is related to their later potential to form hard, stable
aggregate particles.
Rate of precipitation also has a large effect on compound phase formation. The
most rapid
precipitation can be achieved by seeding the solution with a desired phase.
Without seeding, rapid
CA 02670049 2009-07-02
precipitation can be achieved by rapidly increasing the pH of the aqueous
solution of divalent cations,
which results in more amorphous constituents. When silica is present, the more
rapid the reaction rate, the
more silica is incorpated with the carbonate precipitate. The higher the pH
is, the more rapid the
precipitation is and the more amorphous the precipitate is.
Accordingly, a set of precipitation conditions to produce a desired
precipitate from an aqueous
solution of divalent cations include, in certain embodiments, the solution's
temperature and pH, and in some
instances the concentrations of additives and ionic species in the aqueous
solution of divalent cations.
Precipitation conditions may also include factors such as mixing rate, forms
of agitation such as ultrasonics,
and the presence of seed crystals, catalysts, membranes, or substrates. In
some embodiments, precipitation
conditions include supersaturated conditions, temperature, pH, and/or
concentration gradients, or cycling or
changing any of these parameters. The protocols employed to prepare carbonate
and/or bicarbonate
compound precipitates according to the invention may be batch or continuous
protocols. It will be
appreciated that precipitation conditions may be different to produce a given
precipitate in a continuous
flow system compared to a batch system.
Following production of the carbonate mineral precipitate from the water, the
resultant precipitated
carbonate mineral composition is separated from the mother liquor to produce
separated carbonate mineral
precipitate product, also described herein as a dewatered precipitate or water
precipitate cake. Separation of
the precipitate can be achieved using any convenient approach, including a
mechanical approach, e.g.,
where bulk excess water is drained from the precipitated, e.g., either by
gravity alone or with the addition of
vacuum, mechanical pressing, by filtering the precipitate from the mother
liquor to produce a filtrate, etc.
Separation of bulk water produces a wet, dewatered precipitate.
2) Production of aggregate or artificial rock from precipitate
The precipitate produced by the methods above is then further treated to
produce aggregates or
artificial rock of the invention.
In some embodiments, the dewatered precipitate is then dried to produce a
product. Drying can be
achieved by air drying the filtrate. Where the filtrate is air dried, air
drying may be at a temperature ranging
from -70 C to 120 C, as desired. In certain embodiments, drying is achieved by
freeze-drying (i.e.,
lyophilization), where the precipitate is frozen, the surrounding pressure is
reduced and enough heat is
added to allow the frozen water in the material to sublime directly from the
frozen precipitate phase to gas.
In yet another embodiment, the precipitate is spray dried to dry the
precipitate, where the liquid containing
the precipitate is dried by feeding it through a hot gas (such as the gaseous
waste stream from the power
plant), e.g., where the liquid feed is pumped through an atomizer into a main
drying chamber and a hot gas
is passed as a co-current or counter-current to the atomizer direction.
Depending on the particular drying
protocol of the system, the drying station may include a filtration element,
freeze drying structure, spray
drying structure, etc. In certain embodiments, waste heat from a power plant,
or similar operation, is used to
perform the drying step when appropriate.
Where desired, the precipitate may be stored in the mother liquor for a period
of time following
precipitation and prior to separation. For example, the precipitate may be
stored in the mother liquor for a
31
CA 02670049 2009-07-02
period of time ranging from 1 to 1000 days or longer (e.g., many years or a
decade or more), such as I to 10
days or longer, at a temperature ranging from 1 C to 40 C, such as 20 C to 25
C.
At the stage of the water precipitate cake, any convenient method may be used
to produce
aggregates. Several methods are described herein. In some cases, the dewatered
precipitate may be ball-
milled, in the presence of water, binders, surfactants, flocculents (which may
be present from an earlier
stage of the process), or other suitable substances. The precipitate is then
further treated; this may be as
simple as removing it from the ball mill and putting it in a container under
airflow, where it self-
consolidates into a mass that can then be further used. In some cases, the
cake may be reacted with
freshwater to produce a different set of solid precipitated compounds that are
more stable in freshwater,
then further processed to produce aggregate. In some cases, the cake may be
subjected to conditions of
temperature and pressure that cause an artificial lithification, i.e., the
artificial production of rock, which
may then be further processed; e.g., the filter cake may be pressed, or
stacked, or the filter cake may be
passed through an extruder. In some of these cases the process is performed
without the use of binders, to
produce a synthetic rock, e.g., aggregate, that is free of binders, or with a
minimal level of binders. In other
cases one or more binders are used.
Exemplary methods in which freshwater-stable re-precipitated substances are
produced include the
following: the precipitate may be combined with fresh water in a manner
sufficient to cause the precipitate
to form a solid product, where it is thought that the metastable carbonate
compounds present in the
precipitate have converted to a form that is stable in fresh water. By
controlling the water content of the wet
material, the porosity, and eventual strength and density of the final
aggregate may be controlled. Typically
a wet cake will be 40 - 60 volume % water. For denser aggregates, the wet cake
will be < 50% water, for
less dense cakes, the wet cake will be >50% water. After hardening, the
resultant solid product may then be
mechanically processed, e.g., crushed or otherwise broken up and sorted to
produce aggregate of the desired
characteristics, e.g., size, particular shape, etc. In these processes the
setting and mechanical processing
steps may be performed in a substantially continuous fashion or at separate
times.
In certain embodiments, large volumes of precipitate may be stored in the open
environment where
the precipitate is exposed to the atmosphere. The precipitate may be irrigated
in a convenient fashion with
fresh water, or allowed to be rained on or otherwise exposed to freshwater
naturally or order to produce the
aggregate product. The aggregate product may then be mechanically processed as
described above.
In an example of one embodiment of the invention, the precipitate is
mechanically spread in a
uniform manner using a belt conveyor and highway grader onto a compacted earth
surface to a depth of
interest, e.g., up to twelve inches, such as 1 to 12 inches, including 6 to 12
inches. The spread material is
then irrigated with fresh water at a convenient ratio, e.g., of one/half
gallon of water per cubic foot of
precipitate. The material is then compacted using multiple passes with a steel
roller, such as those used in
compacting asphalt. The surface is re-irrigated on a regular, e.g., weekly
basis until the material exhibits the
desired chemical and mechanical properties, at which point the material is
mechanically processed into
aggregate by crushing.
In processes involving the use of temperature and pressure, the dewatered
water precipitate cake is
generally first dried. The cake is then exposed to a combination of
rewatering, and elevated temperature
and/or pressure for a certain time. The combination of the amount of water
added back, the temperature,
32
CA 02670049 2009-07-02
the pressure, and the time of exposure, as well as the thickness of the cake,
can be varied according to
composition of the starting material and the desired results. A number of
different ways of exposing the
material to temperature and pressure are described herein; it will be
appreciated that any convenient method
may be used. An exemplary drying protocol is exposure to 40 C for 24-48
hours, but greater or lesser
temperatures and times may be used as convenient, e.g., 20-60 C for 3-96
hours or even longer. Water is
added back to the desired percentage, e.g., to 1%-50%, e.g, 1% to 10%, such as
1, 2, 3, 4, 5, 6, 7, 8, 9, or
10% w/w, such as 5% w/w, or 4-6% w/w, or 3-7% w/w. In some cases an exact
percentage of water added
back is not important, as in materials that are stored outdoors and exposed to
meteoric precipitation.
Thickness and size of the cake may be adjusted as desired; the thickness can
vary in some embodiment
from 0.05 inch to 5 inches, e.g. 0.1-2 inches, or 0.3-1 inch. In some
embodiments the cake may be 0.5 inch
to 6 feet or even thicker. The cake is then exposed to elevated temperature
and/or pressure for a given time,
by any convenient method, for example, in a platen press using heated platens.
The heat to elevate the
temperature, e.g., for the platens, may be provided, e.g., by heat from an
industrial waste gas stream such as
a flue gas stream. The temperature may be any suitable temperature; in
general, for a thicker cake a higher
temperature is desired; examples of temperature ranges are 40-150 C, e.g., 60-
120 C, such as 70-110 C,
or 80-100 C. Similarly, the pressure maybe any suitable pressure to produce
the desired results;
exemplary pressures include 1000-100,000 pounds per square inch (psi),
including 2000-50,000 psi, or
2000-25,000 psi, or 2000-20,000 psi, or 3000-5000 psi. Finally, the time that
the cake is pressed may be
any suitable time, e.g., 1-100 seconds, or 1-100 minute, or 1-50 minutes, or 2-
25 minutes, or 1-10,000 days.
The resultant hard tablet may optionally then cured, e.g., by placing outside
and storing, by placing in a
chamber wherein they are subjected to high levels of humidity and heat, etc.
These hard tablets, optionally
cured, are then used as building materials themselves or crushed to produce
aggregate.
One method of providing temperature and pressure is to stack dewatered and
dried slabs. For
example, in such a method a dewatered precipitate may be dried, e.g., with
flue gas, in a slab, e.g., 1 inch to
10 feet thick, or 1 foot to 10 feet thick. Pressure is supplied by placing
slabs on top of each other; greater
pressure is achieved by greater thicknesses of slab layers, e.g., 10-1000 feet
or even greater, such as 100-
5000 feet. At an appropriate time, which may be days, weeks, months, or even
years, depending on the
desired result, lithified slabs from a given level of the layers, e.g., from
the bottom, is removed, e.g., by
quarrying, and treated as desired to produce an aggregate or other rock
material.
Another method of providing temperature and pressure is the use of a press, as
described more
fully in the Examples. A suitable press, e.g., a platen press, may be used to
provide pressure at the desired
temperature (using heat supplied, e.g., by a flue gas or by other steps of the
process to produce a precipitate,
e.g., from an electrochemical process) for a desired time. A set of rollers
may be used in similar fashion.
Another way to expose the cake to elevated temperature and pressure is by
means of an extruder,
e.g., a screw-type extruder, also described further in the Examples. The
barrel of the extruder can be
outfitted to achieve an elevated temperature, e.g., by jacketing; this
elevated temperature can be supplied
by, e.g., flue gases or the like. Extrusion maybe used as a means of pre-
heating and drying the feedstock
prior to a pressing operation. Such pressing can be performed by means of a
compression mold, via rollers,
via rollers with shaped indentations (which can provide virtually any shape of
aggregate desired), between a
belt which provides compression as it travels, or any other convenient method.
Alternatively, the extruder
33
CA 02670049 2009-07-02
may be used to extrude material through a die, exposing the material to
pressure as it is forced through the
die, and giving any desired shape. In some embodiments, the carbonate mineral
precipitate is mixed with
fresh water and then placed into the feed section of a rotating screw
extruder. The extruder and/or the exit
die may be heated to further assist in the process. The turning of the screw
conveys the material along its
length and compresses it as the flite depth of the screw decreases. The screw
and barrel of the extruder may
further include vents in the barrel with decompression zones in the screw
coincident with the barrel vent
openings. Particularly in the case of a heated extruder, these vented areas
allow for the release of steam
from the conveyed mass, removing water from the material.
The screw conveyed material is then forced through a die section which further
compresses the
material and shapes it. Typical openings in the die can be circular, oval,
square, rectangular, trapezoidal,
etc., although any shape which the final aggregate is desired in could be made
by adjusting the shape of the
opening. The material exiting the die may be cut to any convenient length by
any convenient method, such
as by a fly knife. A typical length can be from 0.05 inches to 6 inches,
although lengths outside those
ranges are possible. Typical diameters can be be 0.05 inches to 1.0 inches,
though diameters outside of
these ranges are possible.
Use of a heated die section may further assist in the formation of the
aggregate by accelerating the
transition of the carbonate mineral to a hard, stable form. Heated dies may
also be used in the case of
binders to harden or set the binder. Temperatures of 100 C to 600 C are
commonly used in the heated die
section. Heat for the heated die may come in whole or in part from the flue
gas or other industrial gas used
in the process of producing the precipitate, where the flue gas is first
routed to the die to transfer heat from
the hot flue gas to the die.
Without being bound by theory, it is thought that the above process induces
artificial lithification,
i.e., the formation of rock, through reformulation of the compounds in the
original filter cake, into forms
that bind to each other without the need for additional binders, and which
remain together in a cohesive
mass that is resistant to fracture or crushing. Thus, in some embodiments the
invention provides methods
of making a synthetic rock, e.g., an synthetic carbonate-containing rock,
without the use of binders. The
rock may be foremed by, e.g., using methods such as the methods described
above. In some embodiments
only heat and pressure are used to form an artificial rock, where the rock has
a hardness of at least 2.5
Mohs, or at least 3 Mohs, or 3-10 Mohs, or 3-8 Mohs, or 3-6 Mohs, or 2-6 Mohs.
Binders may be added to the carbonate mineral prior to aggregate formation to
assist in holding the
powdered material together, either to provide structural stability or to act
to hold the powders in place while
further processing takes place. Typical binders include, but are not limited
to, portland cement, flyash,
silica, citric acid, gum xantham, or combinations thereof. Binders include
those which become relatively
fluid during heating and reharden when cooled. These binders provide
processing aids in extrusion as well
as binding the mineral powders together. Examples of these binders include
asphalt and thermoplastic
polymers such as polyethylene. Other binders of interest are those which react
chemically with themselves
or with the mineral feedstock to form a matrix which encapsulates and binds
the mineral feedstock.
Examples of these binders include thermosetting resins, such as epoxy,
phenolic or polyester, and reactive
inorganic materials such as portland cement, flyash and lime. When a binder is
used, any suitable
percentage of binder may be used, depending on the properties of the mineral
feedstock; in some
34
CA 02670049 2009-07-02
embodiments, from 0.05% to 50% w/w maybe used, such as 0.1% to 20%, or 0.5% to
10%, or 0.5% to 5%,
or 0.5% to 2%.
Post-forming processing may include further moisture treatment, drying,
sintering, or similar
techniques designed to accelerate and complete any chemical reactions or
morphological changes desired.
Other post-processing techniques may include particle agglomeration or
particle size reduction, such as by
jaw-crushing or grinding. Particle sizes of aggregate may be further separated
using any convenient sieve
or filtering device. In some instances, the particle sizes of the aggregate
may be uniform (i.e., relatively
similar particle sizes) and in other instances, the particle sizes may vary
greatly.
Aggregate of the invention produced by the formation techniques outlined above
may vary greatly
depending on the conditions to which it is subjected during formation. By
controlling the size, shape,
surface texture and internal cavity structure of the aggregate, desired
properties may be engineered into the
aggregate.
In some embodiments, aggregate of the invention may be processed into a shape
which possesses a
high aspect ratio, where its length is substantially longer than its width. By
"substantially longer" is meant
a range between 2 to 100 times longer, such as 5 to 50 times longer, including
5 to 10 times longer.
Aggregate with high aspect ratios may improve concrete flow properties and
aggregate interlock due to
longitudinal alignment along its long axis. In some instances, aggregate of
the invention may be in the
shape of cylinder, tube or capsule (Figure 3A). By "capsule" is meant a
cylindrical tube with rounded
edges. In other instances, aggregate of the invention are in the shape of a
prism. The term "prism" is used
in its conventional sense to mean a polyhedron made of an n-sided polygonal
base, a translated copy, and n
faces joining corresponding sides. The joining faces of the prism are
parallelograms and all cross-sections
parallel to the base faces are the same.
Figure 3B depicts an example of a triangular (i.e., n = 3) prism aggregate
provided by the present
invention. This aggregate can have high concrete flow properties while
providing excellent aggregate
interlock.
In some embodiments, aggregate of the invention may include a mixutre of
shapes and sizes.
Aggregate mixtures may have shapes that include but are not limited to prisms
(n = 3 to 15), spherical,
polygonal, cylindrical, triangular, curved shapes, annulus, ellipsoidal, oval,
star shaped, disk shaped and
any combination thereof. Depending on its intended use, the type and number of
different shapes in the
mixture may vary. The type and number of shapes in the mixture may be equally
distributed or include
some shapes at a higher percentage than others. In one embodiment, the
aggregate mixture of the invention
may be of different shapes but have particle sizes that vary only slightly. By
"vary only slightly" is meant a
deviation in particle sizes that does not exceed 0.05 inches in some
embodiments, or 0.10 inches in some
embodiments, or 0.20 inches in some embodiments. In another embodiment, the
aggregate mixtures may
be of different sizes, but possess similar or identical shapes (e.g.,
different sizes of triangular prism
aggregate). In yet another embodiment, the aggregate mixture may vary in both
shape and size. Also
provided by the invention is an aggregate mixture that contain particles of
identical shapes and sizes.
In an exemplary embodiment, aggregate mixtures of the invention comprise
aggregate of both
different shapes and different sizes. The void space between larger aggregate
may be occupied by smaller
aggregate reducing the overall space between aggregate particles. This allows
for the production of a
CA 02670049 2009-07-02
strong and durable aggregate base, reducing the amount cement content in roads
or concretes. For example,
an aggregate mixture may comprise spheres and "bridges" (Figure 3C). Aggregate
shaped as bridges can
occupy the void space between spherical aggregate particles creating a densely
packed aggregate mixture.
In another embodiment, aggregate mixtures of the invention comprise aggregate
that produces a
high level of open void space when employed in a concrete. These aggregates
generally contain particles of
similar size with shapes designed to produce open void space between the
aggregate particles, increasing
the porosity of packed aggregate beds. Figures 3D and 3E show exemplary
aggregates in this category
("gap-graded spheres" and prisms, respectively). In certain embodiments, the
open void space may be left
unfilled to provide higher levels of porosity and liquid flow through
material. In certain embodiments, the
open space may be filled with cement to create a high cement content concrete
or may also be filled with an
unreactive filler. The void space created by a mixture comprising similar
shapes of similar sizes may also
be filled with polymeric material or other structural support features.
Aggregate of the invention may also be produced to have one or more connected
open spaces
along one or more axis of the aggregate particle. In some instances, such
aggregate may be in the form of a
hollow cylinder or a polyhedral prism that contains a tubular void space
extending through the aggregate
(see Figures 3F, 3G, and 3H). Such structures may be produced by extrusion,
molding or creating the hole
from a solid aggregate particle. The open space in the aggregate may be later
filled (e.g., with cement,
polymeric fibers, etc.) or may be left unfilled.
Another embodiment provided by the present invention is hollow aggregate.
Hollow aggregate
may have any shape (e.g., spherical, disk-shaped, polyhedral prism, etc.) and
size while possessing one or
more internal cavities that are substantially empty. By "substantially empty"
is meant that the internal
cavity contains a void space in the internal cavity that, in certain
embodiments, ranges from 10% to 100%
of the total volume of the internal cavity of the aggregate. The internal
cavity of the aggregate may be
porous with pockets of void space or may have a honeycomb-like structure.
Another embodiment provided by the present invention is aggregate that possess
exterior grooves,
which may facilitate the flow of any desired liquid through the packed
aggregate bed. Exterior grooves
may be, e.g., etched into smooth faced aggregate or may be produced by molding
or extruding the
aggregate. The types of grooves may vary, where in some instances the groove
pattern may be regular (i.e.,
grooves in non-random intervals) or may be random. The grooves may also be
produced straight across the
surface of the aggregate or may have a curved pattern.
In an exemplary embodiment, the aggregate exterior grooves may form
interlocking aggregate.
Interlocking aggregate particles are shaped such that the exterior grooves of
aggregate particles fit into the
grooves of other aggregate particles. The interlock between particles may be
tight (i.e., grooves closely fit
reducing interparticle void space) or may be loose.
In an exemplary embodiment of the invention, a variety of aggregate shapes
having different types of
external grooves can be combined to make an aggregate that interlocks to form
a smooth durable surface
yet allow for passage of any desired fluid through the material. A well-graded
(i.e., uniformly covering a
wide variety of sizes) spherical aggregate having external grooves is one such
embodiment. Additional
embodiments may include aggregates having external grooves in a variety of
shapes with open connected
spaces through the center which allow fluid passage through the aggregate
particle. In certain embodiments,
36
CA 02670049 2009-07-02
one or more of the aggregate shapes include through holes (e.g., as described
above) which facilitate liquid
flow through the material. Exemplary aggregate mixtures having different
combinations of aggregate
particles are illustrated in Figures 31, 3J, 3K, and 3L.
As indicated above, aggregate compositions of the subject invention comprise
aggregate particles
that have a wide variety of shapes and surface textures which can be selected
based on the intended use of
the aggregate (e.g., the desired property of the material in which the
aggregate is used). Exemplary
aggregate shapes include, but are not limited to: rounded, irregular, flaky,
angular, elongated, flaky-
elongated, subangular, subrounded, well rounded, polygonal, cylindrical,
spherical, triangular, curved
shapes, annulus, ellipsoidal, oval, star shaped, prisms, and any mixtures
thereof. Exemplary aggregate
surface textures include, but are not limited to surface textures that are
selected from the group consisting
of. glassy, smooth, granular, rough, grooved, crystalline, honeycombed and
mixtures thereof.
Figure 1 provides a schematic flow diagram of an aggregate production process
according to an
embodiment of the invention. In the embodiment depicted in Figure 1, an
aqueous solution of divalent
cations (10) such as Ca2+ or Mg2+ is first charged with waste gas stream 30 to
produce a precipitation
reaction mixture comprising C02, which reaction mixture is then subjected to
precipitation conditions. In
some embodiments, the CO2 charging and the precipitation may occur
simultaneously, e.g., in a single
piece of equipment. As depicted in Figure 1, a waste gas stream 30 is
contacted with divalent cations 10 at
precipitation step 20. By charging an aqueous solution of divalent cations
with waste gas components,
components such as CO2 combine with water molecules to produce, for example,
carbonic acid, bicarbonate
and carbonate ion. Likewise, waste gas components such as SOx and NOx form
aqueous sulfur- and
nitrogen-containing species. As such, charging water results in an increase
in, for example, the CO2 content
of the water, manifested in the form of carbonic acid, bicarbonate and
carbonate ion, which results in a
concomitant decrease in the partial pressure of CO2 in the waste stream that
is contacted with the water. The
precipitation reaction mixture may be acidic, having a pH of 6 or less, such
as 5 or less, and including 4 or
less; however, as described in further detail herein, the precipitation
reaction mixture may be made basic
(pH of 7 or more, for example, pH 8, 9, 10, 11, or 12) prior to charging the
aqueous solution of divalent
cations to form the precipitation reaction mixture. In certain embodiments,
the concentration of CO2 in
the waste gas that is used to charge the water is 1% or higher, 2% or higher,
4% or higher, 8% or higher,
10% or higher, 11% or higher, 12% or higher, 13% or higher, 14% or higher, 15%
or higher, 20% or higher,
25 % or higher, including 50 % or higher, such as 75% or even higher. In some
embodiments, the waste gas
comprises further components, such as sulfur oxides (SOx); nitrogen oxides
(NOx); heavy metals such as
mercury, cadmium, lead, selenium, and the like; radioactive substances;
particulate matter; volatile organic
constituents, and the like. One or more of these components may also go into
solution to form an aqueous
solution. For example, SOx may go into solution as sulfate and/or sulfite; NOx
as nitrate and/or nitrite;
mercury as mercuric chloride; etc. In some embodiments contacting conditions
are adjusted so that, in
addition to C02, further components of the waste gas are moved from the gas
phase to the water phase, such
as SOx and/or mercury, to be ultimately captured in the aggregates of the
invention.
Contact protocols of interest include, but are not limited to: direct
contacting protocols, e.g.,
bubbling the gas through the volume of water, concurrent contacting means,
i.e., contact between
unidirectionally flowing gaseous and liquid phase streams, countercurrent
means, i.e., contact between
37
CA 02670049 2009-07-02
oppositely flowing gaseous and liquid phase streams, crosscurrent means, and
the like. Thus, contact may
be accomplished through use of infusers, bubblers, fluidic Venturi reactor,
sparger, gas filter, spray, tray, or
packed column reactors, and the like, as may be convenient. In one embodiment
contact is via a
crosscurrent contacter where the gas is flowed in a direction perpendicular to
a flat sheet of water or other
liquid. In one embodiment contact is between neutrally buoyant liquid droplets
of solution, of a diameter
of 5 micrometers or less, and gas in a chamber.
At precipitation step 20, carbonate and/or bicarbonate compounds are
precipitated. Precipitation
conditions of interest include those that change the physical environment of
the water to produce the
desired precipitate product. For example, the temperature of the water may be
raised to an amount suitable
for precipitation of the desired carbonate compound to occur. In such
embodiments, the temperature of the
water may be raised to a value from 5 to 70 C, such as from 20 to 50 C and
including from 25 to 45 C. As
such, while a given set of precipitation conditions may have a temperature
ranging from 0 to 100 C, the
temperature may be raised in certain embodiments to produce the desired
precipitate. In certain
embodiments, the temperature is raised using energy generated from low or zero
carbon dioxide emission
sources, e.g., solar energy source, wind energy source, hydroelectric energy
source, etc. In some
embodiments the temperature is raised by exposure to the heat of the flue gas.
While the pH of the water
may range from 7 to 14 during a given precipitation process, in certain
embodiments the pH is raised to
alkaline levels in order to drive the precipitation of carbonate mineral as
desired. In certain of these
embodiments, the pH is raised to a level which minimizes if not eliminates CO2
gas generation production
during precipitation. In these embodiments, the pH maybe raised to 10 or
higher, such as 11 or higher.
Where desired, the pH of the water is raised using any convenient approach. In
certain embodiments, a pH
raising agent may be employed, where examples of such agents include oxides,
hydroxides (e.g., sodium
hydroxide, potassium hydroxide, brucite), carbonates (e.g. sodium carbonate)
and the like. The amount of
pH elevating agent that is added to the saltwater source will depend on the
particular nature of the agent and
the volume of saltwater being modified, and will be sufficient to raise the pH
of the salt water source to the
desired value. Alternatively, the pH of the saltwater source can be raised to
the desired level by electrolysis
of the water.
CO2 charging and carbonate mineral precipitation may occur in a continuous
process or at separate
steps. As such, charging and precipitation may occur in the same reactor of a
system, e.g., as illustrated in
Figure i at step 20, according to certain embodiments of the invention. In yet
other embodiments of the
invention, these two steps may occur in separate reactors, such that the water
is first charged with CO2 in a
charging reactor and the resultant CO2 charged water is then subjected to
precipitation conditions in a
separate reactor.
Amorphous silica in the aggregate product may be desired, for example, to
improve hardness and
durability of the aggregate product. Siliceous materials may be added to the
aqueous solution of divalent
cations prior to charging the water with waste gas such as combustion gas
(e.g., gases comprising CO2). In
such embodiments, silica is added with a pH-raising agent, such as fly ash
from the burning of coal. Due to
the oxide content of fly ash(i.e., CaO), the addition of fly ash to an aqueous
solution of divalent cations will
substantially increase the pH, which will help dissolve the silica in the fly
ash. When an alkaline solution
of divalent cations with the dissolved silica is charged with waste gas
comprising carbon dioxide , the
38
CA 02670049 2010-06-30
carbon dioxide forms carbonic acid that quickly disassociates into carbonate
ions. The presence of
carbonate ions at a precipitation concentration allows carbonate compounds to
form, which may
simultaneously precipitate silica intercalated with the precipitation
material.
Following production of precipitation material from the precipitation reaction
mixture, the
precipitation material is separated from the precipitation reaction mixture to
produce separated precipitation
material, as illustrated at step 40 of Figure 1. Separation of precipitation
material from the precipitation
reaction mixture is achieved using any of a number of convenient approaches,
including draining (e.g.,
gravitational sedimentation of the precipitation product followed by
draining), decanting, filtering (e.g.,
gravity filtration, vacuum filtration, filtration using forced air),
centrifuging, pressing, or any combination thereof.
Separation of bulk water produces a wet, dewatered precipitation material.
Effluent liquid resulting from the separation
process may be sent to a tailings pond, ocean or other liquid repository 42.
The resulting dewatered precipitation material may then be optionally dried to
produce a dried
precipitation material, as illustrated at step 60 of Figure 1. Drying may be
achieved by air drying the
precipitation material. Where the precipitation material is air dried, air
drying may be at room or elevated
temperature. In certain embodiments, the elevated temperature is provided by
the industrial plant gaseous
waste stream. In such embodiments, the gaseous waste stream (e.g., flue gas)
from the power plant may be
first used in the drying step, where the gaseous waste stream may have a
temperature ranging from 30 to
700 C, such as 75 to 300 C. The gaseous waste stream may be contacted directly
with the wet precipitation
material in the drying stage, or used to indirectly heat gases (such as air)
in the drying stage. The desired
temperature may be provided in the gaseous waste stream by having the gas
conveyer (e.g., duct) from the
industrial plant originate at a suitable location, for example, at a location
a certain distance in the heat
recovery steam generator (HRSG) or up the flue, as determined based on the
specifics of the exhaust gas
and configuration of the industrial plant. In yet another embodiment, the
precipitation material is spray
dried to dry the precipitation material, wherein a slurry comprising the
precipitation material is dried by
feeding it through a hot gas (such as the gaseous waste stream from the power
plant), for example, where
the slurry is pumped through an atomizer into a main drying chamber and a hot
gas is passed as a co-current
or counter-current to the atomizer direction. In certain embodiments, drying
is achieved by freeze-drying
(i.e., lyophilization), where the precipitation material is frozen, the
surrounding pressure is reduced and
enough heat is added to allow the frozen water in the precipitation material
to sublime. Depending on the
particular drying protocol of the system, the drying station may include a
filtration element, freeze drying
structure, spray drying structure, etc. Effluent discharge air and fine
particulates 62 that cannot be used to make
aggregate may also be discharged by the dryer.
Where desired, the dewatered precipitation material from the separation
reactor 40 may be washed before drying, as
illustrated at optional step 50 of Figure 1. The precipitation material may be
washed with freshwater, for example, to
remove salts such as NaCl from the dewatered precipitation material. Used wash
water may be disposed of as
convenient, for example, by disposing of it in a tailings pond, an ocean, a
sea, a lake etc.
At step 70, the dried precipitation material is processed where necessary to
provide the desired aggregate product.
As reviewed above, this step may include contacting the precipitation material
with fresh water (with or without drying
it first) to produce a set product followed by mechanical processing of the
set product to produce the desired
aggregate 80.
39
CA 02670049 2010-06-30
In certain embodiments, a system is employed to perform the above methods,
wherein such
systems include those described below in greater detail.
B. Settable Compositions
Additional embodiments of the invention are settable compositions which
include a hydraulic
cement and '()2 sequestering aggregate of the invention; with the addition of
an aqueous fluid, e.g., water,
the composition sets and hardens, e.g., into a concrete or a mortar. The term
"hydraulic cement" includes
its conventional sense to refer to a composition which sets and hardens after
combining with water or a
solution where the solvent is water. e.g. an admixture solution. Setting and
hardening of the product
produced by combination of the cements of the invention with an aqueous liquid
results from the
production of hydrates that are formed from the cement upon reaction with
water, where the hydrates are
essentially insoluble in water.
Aggregates of the invention find use in place of conventional natural rock
aggregates used in
conventional concrete when combined with pure portland cement. Other hydraulic
cements of interest in
certain embodiments are portland cement blends. The phrase "portland cement
blend" includes a hydraualic
cement composition that includes a portland cement component and significant
amount of a non-portland
cement component. As the cements of the invention are portland cement blends,
the cements include a
portland cement component. The portland cement component may be any convenient
Portland cement. As
is known in the art, Portland cements are powder compositions produced by
grinding portland cement
clinker (more than 90%), a limited amount of calcium sulfate which controls
the set time, and up to 5%
minor constituents (as allowed by various standards). When the exhaust gases
used to provide carbon
dioxide for the reaction contain SOx, then sufficient sulphate may be present
as calcium sulfate in the
precipitated material, either as a cement or aggregate to off set the need for
additional calcium sulfate. As
defined by the European Standard FN 197.1, "portland cement clinker is a
hydraulic material which shall
consist of at least two-thirds by mass of calcium silicates (3CaO.SiO2 and
2CaO.SiO2), the remainder
consisting of aluminium- and iron-containing clinker phases and other
compounds. The ratio of CaO to
S'02 shall not he less than 2Ø The magnesium content (MgO) shall not exceed
5.0% by mass." The
concern about MgO is that later in the setting reaction, magnesium hydroxide,
brucite, may form, leading to
the deformation and weakening and cracking of the cement. In the case of
magnesium carbonate containing
cements, brucitc will not form as it may with MgO. In certain embodiments, the
Portland cement
constituent of the present invention is any Portland cement that satisfies the
ASTM Standards and
Specifications of C150 (Types I-VIII) of the American Society for Testing of
Materials (ASTM C50-
Standard Specification for Portland Cement). ASTM C150 covers eight types of
portland cement, each
possessing different properties, and used specifically for those properties.
Also of interest as hydraulic cements are carbonate containing hydraulic
cements. Such carbonate
containing hydraulic cements, methods for their manufacture and use are
described in co-pending United
States Patent Application Publication No. 2009-0020044 Al.
In certain embodiments, the hydraulic cement may be a blend of two or more
different kinds of
hydraulic cements, such as Portland cement and a carbonate containing
hydraulic cement. In certain
CA 02670049 2009-07-02
embodiments, the amount of a first cement, e.g., Portland cement in the blend
ranges from 10 to 90%
(w/w), such as 30 to 70% (w/w) and including 40 to 60% (w/w), e.g., a blend of
80% OPC and 20%
carbonate hydraulic cement.
Settable compositions of the invention, such as concretes and mortars, are
produced by combining
the hydraulic cement with an amount of aggregate (fine for mortar, e.g., sand;
coarse with or without fine
for concrete) and water, either at the same time or by pre-combining the
cement with aggregate, and then
combining the resultant dry components with water. The choice of coarse
aggregate material for concrete
mixes using cement compositions of the invention may have a minimum size of
about 3/8 inch and can vary
in size from that minimum up to one inch or larger, including in gradations
between these limits. Finely
divided aggregate is smaller than 3/8 inch in size and again may be graduated
in much finer sizes down to
200-sieve size or so. Fine aggregates may be present in both mortars and
concretes of the invention. The
weight ratio of cement to aggregate in the dry components of the cement may
vary, and in certain
embodiments ranges from 1:10 to 4:10, such as 2:10 to 5:10 and including from
55:1000 to 70:100.
The liquid phase, e.g., aqueous fluid, with which the dry component is
combined to produce the
settable composition, e.g., concrete, may vary, from pure water to water that
includes one or more solutes,
additives, co-solvents, etc., as desired. The ratio of dry component to liquid
phase that is combined in
preparing the settable composition may vary, and in certain embodiments ranges
from 2:10 to 7:10, such as
3:10 to 6:10 and including 4:10 to 6:10.
In certain embodiments, the cements may be employed with one or more
admixtures. Admixtures
are compositions added to concrete to provide it with desirable
characteristics that are not obtainable with
basic concrete mixtures or to modify properties of the concrete to make it
more readily useable or more
suitable for a particular purpose or for cost reduction. As is known in the
art, an admixture is any material
or composition, other than the hydraulic cement, aggregate and water, that is
used as a component of the
concrete or mortar to enhance some characteristic, or lower the cost, thereof.
The amount of admixture that
is employed may vary depending on the nature of the admixture. In certain
embodiments the amounts of
these components range from 1 to 50% w/w, such as 2 to 10% w/w.
Admixtures of interest include finely divided mineral admixtures such as
cementitious materials;
pozzolans; pozzolanic and cementitious materials; and nominally inert
materials. Pozzolans include
diatomaceous earth, opaline cherts, clays, shales, fly ash, silica fume,
volcanic tuffs and pumicites are some
of the known pozzolans. Certain ground granulated blast-furnace slags and high
calcium fly ashes possess
both pozzolanic and cementitious properties. Nominally inert materials can
also include finely divided raw
quartz, dolomites, limestone, marble, granite, and others. Fly ash is defined
in ASTM C618.
Other types of admixture of interest include plasticizers, accelerators,
retarders, air-entrainers,
foaming agents, water reducers, corrosion inhibitors, and pigments.
As such, admixtures of interest include, but are not limited to: set
accelerators, set retarders, air-
entraining agents, defoamers, alkali-reactivity reducers, bonding admixtures,
dispersants, coloring
admixtures, corrosion inhibitors, dampproofing admixtures, gas formers,
permeability reducers, pumping
aids, shrinkage compensation admixtures, fungicidal admixtures, germicidal
admixtures, insecticidal
admixtures, rheology modifying agents, finely divided mineral admixtures,
pozzolans, aggregates, wetting
agents, strength enhancing agents, water repellents, and any other concrete or
mortar admixture or additive.
41
CA 02670049 2010-06-30
Admixtures are well-known in the art and any suitable admixture of the above
type or any other desired
type may be used; see, e.g., U.S. Patent Application Publication No. 2009-
0020044 Al.
In certain embodiments, settable compositions of the invention include a
cement employed with
fibers, e.g., where one desires fiber-reinforced concrete. Fibers can be made
of zirconia containing
materials, steel, carbon, fiberglass, or synthetic materials, e.g.,
polypropylene, nylon, polyethylene,
polyester, rayon, high-strength aramid, (i.e. KevtarOD), or mixtures thereof.
The components of the settable composition can be combined using any
convenient protocol. Each
material may be mixed at the time of work, or part of or all of the materials
may be mixed in advance.
Alternatively, some of the materials are mixed with water with or without
admixtures, such as high-range
water-reducing admixtures, and then the remaining materials may be mixed
therewith. As a mixing
apparatus, any conventional apparatus can be used. For example, Hobart mixer,
slant cylinder mixer, Omni
Mixer, Henschel mixer, V-type mixer, and Nauta mixer can be employed.
Following the combination of the components to produce a settable composition
(e.g., concrete),
the settable composition will set after a given period of time. The setting
time may vary, and in certain
embodiments ranges from 30 minutes to 48 hours, such as 30 minutes to 24 hours
and including from 1
hour to 4 hours.
The strength of the set product may also vary. In certain embodiments, the
strength of the set
cement may range from 5 Mpa to 70 MPa, such as 10 MPa to 50 MPa and including
from 20 MPa to 40
MPa. In certain embodiments, set products produced from cements of the
invention are extremely durable.
e.g., as determined using the test method described at ASTM C1157.
Aspects of the invention further include structures produced from the
aggregates and settable
compositions of the invention. Because these structures are produced from
aggregates and/or settable
compositions of the invention, they will include markers or components that
identify them as being
obtained from a water precipitated carbonate compound composition, such as
trace amounts of various
elements present in the initial salt water source, e.g., as described above.
For example, where the mineral
component of the aggregate component of the concrete is one that has been
produced from sea water, the
set product will contain a seawater marker profile of different elements in
identifying amounts, such as
magnesium, potassium, sulfur, boron, sodium, and chloride, etc.
C. Structures
Further embodiments include manmade structures that contain the aggregates of
the invention and
methods of their manufacture. Thus in some embodiments the invention provides
a manmade structure that
includes one or more aggregates as described herein. The manmade structure may
be any structure in
which an aggregate may be used, such as a building, dam, levee, roadways or
any other manmade structure
that incoroporates an aggregate or rock. The aggregate may be a carbon dioxide
sequestering aggregate, an
aggregate with a E' 3C more negative than negative -10%o, and the like, or any
aggregate described herein.
In some embodiments, the invention provides a manmade structure, e.g. a
building, a dam, or a
roadway, that includes an aggregate that contains CO2 from a fossil fuel
source, e.g., aggregate that is at
least 10% w/w CO, from a fossil fuel source, or at least 20% CO2 from a fossil
fuel source, or at least 30%
42
CA 02670049 2009-07-02
CO2 from a fossil fuel source. In some cases the aggregate has a S13C value
more negative than -I O%o, or
more negative than -20%o. In some embodiments the invention provides a manmade
structure, e.g. a
building, a dam, or a roadway, containing aggregate, where a portion or all of
the aggregate is a lightweight
aggregate, e.g., an aggregate that has a density of 90-115 lb/ft3, and where
the aggregate contains CO2 from
a fossil fuel source, e.g., aggregate that is at least 10% w/w CO2 from a
fossil fuel source, or at least 20%
CO2 from a fossil fuel source, or at least 30% CO2 from a fossil fuel source.
In some cases the aggregate
has a 513C value more negative than -10%o, or more negative than -20%o.
In some embodiments the invention provides a method of manufacturing a
structure, comprising
providing an aggregate that contains CO2 from a fossil fuel source, e.g.,
aggregate that is at least 10% w/w
CO2 from a fossil fuel source, or at least 20% CO2 from a fossil fuel source,
or at least 30% CO2 from a
fossil fuel source. In some cases the aggregate has a 513C value more negative
than -1O%o, or more negative
than -20%o and manufacturing at least a portion of the structure using the
aggregate. In some embodiments
at least a portion of the aggregate is a lightweight aggregate, , e.g., an
aggregate that has a density of 90-115
lb/ft3.
1. Roadways
In some embodiments the invention provides a roadway that includes one or more
of the
aggregates of the invention, or a component of a roadway that includes one or
more of the aggregates of the
invention, and methods and systems for manufacturing such roadways and/or
components. In some
embodiments the invention provides a carbon dioxide-sequestering roadway, that
is, a roadway that is built
with components, which may include one or more aggregates of the invention,
whose overall manufacture
results in sequestration of carbon dioxide, e.g., from an industrial source;
in some. embodiments the
invention provides a roadway for which the amount of carbon dioxide produced
in manufacturing the
roadway is less than the amount of carbon dioxide sequestered within the
material of the roadway, which
may include aggregates of the invention, carbon-dioxide-sequestering cements,
forms, and other
components, i.e., a carbon-negative roadway.
The term "roadway" is used herein to include a general class of surfaces used
for conveyance and
recreation. It includes pavements used by motorized vehicles, animal and
pedestrian traffic, bicycles and
any other conveyance used either singly or as a group. Roadways of the
invention may include but are not
limited to roads, sidewalks, bridge surfaces, bicycle paths, paved walking
paths and the like, as described in
further detail below. Roadways include structures as simple as gravel roads,
which may be a single layer,
as well as asphalt- and concrete-paved roadways, which typically contain two
or more layers.
In some embodiments the invention provides a roadway that includes a C02-
sequestering
aggregate, such as an aggregate that contains CO2 derived from a industrial
waste gas source, such as any of
the C02-sequestering aggregates described herein. In some embodiments the
roadway includes an
aggregate that comprises a synthetic carbonate. In some embodiments, the
roadway includes an aggregate
that has a S13C value less than -15%o, or less than -20%o, or less than -25%o.
In some embodiments the
roadway includes an aggregate that contains dypinginite, nesquehonite,
magnesite, or a combination of one
or more of these.
43
CA 02670049 2009-07-02
The aggregate of the above embodiments may be used in one or more components
of the roadway,
as described in further detail below. The aggregate may make up more than 1,
2, 5, 10, 20, 30, 40, 50, 60,
70, 80, or 90% of the roadway, e.g., more than 20%, or more than 50%, by
weight.
In some embodiments, the roadway is a highway, highways system, city street,
airport runway,
sidewalk, or open-space pavement. A highway includes a main road intended for
travel by the public
between important destinations, such as cities and towns. An interconnected
set of highways can be
variously referred to as a "highway system", a "highway network" or a "highway
transportation system". A
city street includes any public thoroughfare that is a parcel of land
adjoining buildings on which people may
move about. City streets of the invention refer to those roadways that are
primarily used for vehicular
traffic but do not experience the high of volume of traffic as a highway, but
accomadate higher applied
loads than sidewalks. Another exemplary roadway structure provided by the
present invention is an airport
runway. A runway includes a strip of land on an airport, on which aircraft can
take off and land, and may
also include blast pads, which are overrun areas or stopways at the ends of a
runway as well as thresholds
which are used for airplane taxiing, takeoff and landing rollout. Sidewalk
includes the paved surface
conventionally found alongside roadways for vehicular traffic. Sidewalks of
the present invention may
include any paved roadway primarily employed for pedestrian traffic including
cobblestone pavements,
brick paved roads as well as paved walkways that run along beaches (i.e.,
beach paths), inside parks and
between residential and commercial buildings. Sidewalks of the invention may
also include bicycle paths
and other roadways designed for non-vehicular and/or animal traffic. An open-
space pavement may be a
plot of land of any size or shape which has been paved so that it may be used
for a multitude of different
purposes. For example, an open-space pavement may be a playground, a sports
recreation surface (e.g.,
basketball court, rollerskating rink), a parking lot and the like. The paved
surface may be the foundation for
temporary buildings or storage facilities. The open-space pavement may be
constructed depending upon
the applied load and the thickness of each layer may vary considerably.
The invention also provides a roadway containing material that sequesters at
least 1, 5, 10, 50, 100,
500, 1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000, 9000, or 10,000 tons of
CO2 per lane mile of
roadway. In some embodiments, the roadway is at least 10, 100, 1000 10,000
feet long, or at least 3, 5, 10,
50, or 100 miles long. The material maybe any material, e.g., the aggregates
as described herein, that is
produced in a manmade process so that CO2 from an industrial source is trapped
within the material, e.g.,
by chemical reaction to produce stable precipitates, and will remain in the
material under ordinary
conditions of use to the desired degree, or when subjected to specific tests,
such as temperature, acid, and/or
base stability, as described elsewhere herein. For example, a one-lane road 15
feet wide, with a base course
of 18 inches deep that contains aggregates, some or all of which is an
aggregate of embodiments of the
invention, with a bulk density of 100 lb/ft3 contains approximately 2250
pounds of aggregate per linear foot
of roadway, or approximately 1.1 ton per linear foot, and thus approx 5,500
tons per lane mile. If the
aggregate, overall, sequesters only I% of its weight as CO2, the roadway will
contain material sequestering
55 tons of CO2 per lane mile. If it sequesters 50% of its weight as CO2 (e.g.,
if essentially all of the
aggregate is CO2-aggregate according to some embodiments of the invention)
then the roadway will contain
material sequestering at least 2750 tons of CO2 per lane mile. A roadway with
a deeper base course would
have correspondingly more aggregate and one with a shallower base course,
less. This calculation,
44
CA 02670049 2010-06-30
assuming aggregate as the CO,-sequestering component, is merely a simple
example to illustrate the
principle. Other components of the roadway may also contain C02-sequestering
material, such as the
surface cement or asphalt, other layers of the roadway, and the like. It can
easily be calculated how much
CO2 is sequestered per lane mile of a roadway. To verify that a material is a
C02-sequestering material,
e.g., a material containing carbon dioxide originating in the combustion of
fossil fuel, tests such as isotope
measurements (e.g., measurement of S"C values) and carbon coulometry may be
used; any other suitable
measurement may also be used.
The invention also provides a carbon-negative roadway, where "carbon-negative"
has the meaning
as used herein. In some embodiments, the roadway is at least 10, 100, 1000
10,000 feet long, or at least 3,
5, 10, 50, or 100 miles long. In some instances the roadway is at least 5%
carbon negative, or at least 10%
carbon negative, or at least 20% carbon negative, or at least 30% carbon
negative, or at least 40% carbon
negative, or at least 50% carbon negative, or at least 60% carbon negative, or
at least 70% carbon negative,
or at least 80% carbon negative, or at least 90% carbon negative.
Roadways are made from various components and the invention also provides one
or more of the
components of a roadway. "Roadway component" includes any component (e.g.,
structural component)
used in the construction of a roadway. In certain embodiments, the roadway
component may be an
aggregate, a binder, a soil stabilizer, a concrete, a formed material, or
asphalt. In certain other
embodiments, the roadway component may be a settable composition such as
cement, concrete or formed
building material (e.g., brick). In some embodiments the roadway component
comprises a CO2
sequestering aggregate, such as that described for use in the aggregates of
the invention, or a carbonate with
a S"C value of less than -lS%c or -20%x. Depending on the particular type and
size of roadway structure
to be constructed and its geographical location, the amount of the carbonate
that is present in a roadway
component of the roadway may vary. In certain embodiments, the amount of the
carbonate in a roadway
component can range from I to 100% w/w, such as from 5 to 99% w/w including
from 10 to 90%, or from
15 to 50%, , or 30% to 70%, or 50% to 80%, or 60-90%, or 70-100%, or 70-99%.
In producing the
roadway component, an amount of the carbonate component is combined with water
and other additional
components, which include, but are not limited to: clay, shale, soft slate,
calcium silicate, quarried stone,
Portland cement, fly ash, slag cement, hinder, aggregate (e.g., blast furnace
slag, bottom ash, gravel,
limestone, granite, sand, etc.), silica fume, silicate and pozzolans.
Synthetic carbonate production protocols
of interest include, but are not limited to, those disclosed in U.S. Patent
Application Publication Nos. 2009-
0020044 Al; 2009-0001020 Al; and 2009-0169452 Al.
Synthetic carbonate employed in roadway components and roadways of the
invention may he produced by
precipitating a calcium and/or magnesium carbonate composition from a water,
as described elsewhere
herein.
In some embodiments, the roadway component is an asphalt product. The term
"asphalt" (i.e.,
bitumen) is used in its conventional sense to include the natural or
manufactured black or dark-colored
solid, semisolid or viscous material composed mainly of high moleculer weight
hydrocarbons derived from
a cut in petroleum distillation after naptha, gasoline, kerosene and other
fractions have been removed from
CA 02670049 2010-06-30
crude oil. Thus the invention provides an asphalt product that includes
asphalt and an aggregate as
described herein. The amount of aggregate in the roadway asphalt products of
the present invention may
vary greatly. It may range from 5 to 50%, including 10 to 40%, such as 25 to
35%. CO, sequestering
asphalt, the methods and systems for producing them are further described in
United States
Application Publication No. 2010-0111810 Al.
In other embodiments, the roadway component is a soil stabilizer. By "soil
stabilizer" is meant a
composition used to improve the stability and structural integrity (i.e.,
maintains its shape) of a soil.,-A2
In other embodiments, the roadway component is a formed building material. By
"formed" is
meant shaped, e.g., molded, cast, cut or otherwise produced, into a man-made
structure with defined
physical shape, i.e., configuration. Formed building materials are distinct
from amorphous building
15 materials (e.g., powder, paste, slurry, etc.) that do not have a defined
and stable shape, but instead conform
to the container in which they are held, e.g., a bag or other container. CO2
sequestering formed building
materials, the method and systems for producing them are further described in
United States
Application Publication No. 2010-0077691 Al.
20 As indicated above, roadways of the present invention may include one or
more roadway layer.
By way of example, a roadway, e.g., CO2 sequestering roadway or carbon
negative roadway of the
invention, may include one or more of a sub-grade layer, a sub-base layer, a
base course layer and a surface
layer (as those terms are understood by those of skill in the art; equivalent
terms may be substituted if the
meaning is substantially the same). It will he appreciated that the compostion
of these layers determines
25 the type of material that may be used in them. For example, when aggregates
of the invention are used in
one or more of the layers, if there is no rebar or other corrosion-prone
materials, the aggregate may be an
aggregate with virtually any leachable chloride content that does not detract
from the strength and durability
properties of the aggregate. Thus, aggregates of the invention that are
produced from waters containing
high amounts of chloride, such as seawater or brine, do not necessarily have
to be processed, or only
30 minimally processed, to remove chloride if the aggregate is to be used in
an appropriate layer of a roadway.
In addition, in some embodiments the invention provides one or more of the
layers as containing aggregate
in which a portion or all of the aggregate is reactive aggregate. Unlike in
conventional structures, reactive
aggregate may be an advantage in roadways because in reacting the aggregate
provides a stronger bond
between particles and thus a more durable layer. A roadway layer such as a
base course layer, in which the
35 aggregate is loose, allows for reactive aggregate that forms an expansive
gel, so long as the expansion does
not exceed the void space.
Methods for producing roadway, e.g., CO2 sequestering roadways include the
construction of any
part of one or more of these layers. As such, methods for constructing
roadway, e.g., CO2 sequestering
roadways according to aspects of the present invention include constructing a
new roadway, replacing a
40 previously-constructed roadway, or repairing/improving any portion of a
previously-constructed roadway.
46
CA 02670049 2009-07-02
In other embodiments, the roadway, e.g., CO2 sequestering roadways may be a
full depth reclamation. In
yet other embodiments, roadways of the invention may be resurfacing (i.e.,
overlay) of only the top layer.
The bottom layer of a roadway can be the subgrade layer. In preparing the
subgrade, the first step
may include a soil stabilization step. The underlying subgrade soil may also
be stabilized using the roadway
soil stabilizer component of the present invention. The subgrade soil should
be blended with the roadway
soil stabilizer component so as to give a uniform composition. Depending upon
the desired properties (e.g.,
load-bearing capacity, frost resistance), the subgrade may be further mixed
with other roadway components
described above (e.g., cementitious materials) to provide increased
stabilization. The subgrade may also be
treated with herbicides to prevent or retard the growth of vegetation which
may affect the long-term
structural integrity of the subgrade.
Following final compaction, a primecoat may be added to the surface of the
graded subgrade. In general, if
the final roadway surface is less than 100 mm in thickness, a primecoat should
be added to the subgrade
layer. An exemplary primecoat employed in present invention include an
emulsified asphalt product
comprising an amount of an aggregate of the invention, e.g., a CO2
sequestering synthetic carbonate
described above.
The second layer of a roadway can be the sub-base layer. The sub-base layer is
situated above the
subgrade and functions primarily for structural support of the overlying base
and surface layers. In some
embodiments, the sub-base may be of minimum thickness or altogether absent,
depending upon the final
desired load-bearing capacity of the roadway. Since the purpose of a stable
sub-base is to provide even
distribution of the traffic load on the underlying subgrade, suitable sub-base
materials employed are those
that are able to evenly distribute the applied load.
In some embodiments, sub-base may comprise unbound granular materials. By
"unbound granular
materials" is meant those which do not bond or adhere to each other when laid
and compacted but rely on
the natural interlocking of adjacent particles. The proportion of fine and
coarse particles in unbound
granular materials will depend upon the desired load-bearing capacity of the
roadway. Therefore, the
particle sizes of unbound granular material in the sub-base may vary greatly,
ranging from 0.05 mm to 25
mm, although they should not to exceed 37.5 mm. In some instances, unbound
granular material may be a
non-reactive aggregate comprising a CO2 sequestering synthetic carbonate. The
aggregate component may
be produced, as described above, by crushing a settable composition or may be
a molded aggregate that
possesses a shape suitable for interlocking with adjacent aggregate particles
(e.g., star shaped).
In other embodiments, the sub-base may comprise bound material. Bound
materials are those
which bond with neighboring particles by means of a binder. By "binder" is
meant a component that is able
to substantially set or adjoin adjacent particles. In some instances of the
present invention, the binder is an
asphalt product comprising a CO2 sequestering synthetic carbonate. In other
instances, the binder may be a
cement comprising a CO2 sequestering synthetic carbonate. In some embodiments,
the sub-base comprises
a reactive aggregate. By employing a reactive aggregate, a stabilized matrix
between aggregate particles is
formed which allows the sub-base to minimize the intrusion of fines from the
subgrade into the roadway
structure and minimize frost action damage. In some embodiments, water may be
added to the
composition to provide optimum moisture content and material uniformity. After
an appropriate thickness
47
CA 02670049 2009-07-02
of sub-base material is laid, the sub-base may be compacted in the same manner
as described above for the
subgrade.
In some embodiments, the sub-base may comprise a precast concrete slab. The
concrete may be
prepared by mixing and molding an amount of the CO2 sequestering synthetic
carbonate and a cementitious
component such as Portland cement in addition to other supplementary
cementitious materials as described
above. The concrete slab may also employ reinforcing materials, such as a
steel rebar structure or
aluminum wire mesh.
Another layer of a roadway, provided by the present invention is the base
course layer. The base
course is situated immediately below the surface layer and contributes
additional load distribution, drainage
and frost resistance and provides a stable platform for construction
equipment. Base course layers of the
present invention may be comprised substantially of the aggregate as described
above for the sub-base.
Aggregate comprising a CO2 sequestering synthetic carbonate is preferred
especially in instances where
sub-surface drainage problems may exist in the roadbed, in areas where the
roadbed soil is unstable, in
areas where unsuitable materials have been removed or under full depth
flexible roadways. In some
embodiments, the aggregate base course comprises a mixture of reactive
aggregate and non reactive
aggregate. The proportion of reactive aggregate in the mixture may vary,
ranging from 5 to 25%, including
5 to 15%, such as 10%. The aggregate composition may also include an amount of
a cementitious
component. The amount of cementitious component added varies depending upon
the type of roadway,
ranging from 1 to 20% by weight of the base course, including I to 10%, such
as 5%. The base course may
be further prepared by employing and mixing in a dense-graded or permeable hot
mix asphalt.
The top layer provided by the invention of roadways is the surface layer. The
surface course is the
layer situated immediately above the base course and is in contact with
traffic loads. The surface layer
should be constructed such that it provides characteristics such as friction,
smoothness, noise control and
drainage. In addition, the surface layer serves as a waterproofing layer to
the underlying base, sub-base and
subgrade. The surface layer may be constructed in two separate stages to
prepare its two layers- the
wearing course and the binder course. The wearing course is the layer in
direct contact with traffic loads.
It is meant to take the brunt of traffic wear and can be removed and replaced
as it becomes worn. The
binder course is the bulk of the surface layer structure and serves to
distribute the overlying traffic load.
In some embodiments, the surface course provided by the invention is comprised
substantially of
aggregate, of which a portion or all is aggregate of the invention, and
asphalt binder. In addition, an
amount of aggregate of the invention maybe further employed in powdered form
as a mineral filler. The
amount of asphalt binder used in the surface course may vary, ranging from 5
to 50%, including 5 to 40%,
such as 5 to 35%. The particle sizes of the aggregate used in the surface
layer may vary, ranging from 50
mm to 15 mm, including 100mm to 12.5 mm, such as 75mm to 10 mm. The surface
course layer is
prepared by mixing the aggregate and mineral filler with hot asphalt binder
until all of the aggregate and
mineral filler material is fully coated. The asphalt coated aggregate material
may then be spread onto the
surface of the base course such that it produces a smooth, uniform layer.
Additional asphalt binder may be
employed to fill in any void space or grading changes along the surface. The
surface course is then
compacted at a high temperature. By "high temperature" is meant a temperature
not lower than 125 C.
48
CA 02670049 2009-07-02
In other embodiments, the surface layer may be a rigid, formed paved concrete
surface as
described above. In instances where the surface layer is a concrete slab, the
surface may be treated with
chemical admixtures to improve frost resistance, moisture damage and stripping
damage. A rigid concrete
surface layer may be employed in roadways used primarily for pedestrian
traffic or for lighter applied loads.
Roadways components comprising a CO2 sequestering synthetic carbonate find use
in a variety of
different applications. Specific roadway structures in which the roadway
component compositions of the
invention find use include, but are not limited to: highways, sidewalks,
bicycle paths, beachfront paths,
airport runways, city streets, cobblestone roads, parking lots, rollerskating
rinks and any other paved plot of
land.
In some embodiments the invention provides a method comprising:
constructing a roadway comprising a CO2 sequestering component comprising a
synthetic carbonate. In
some embodiments the invention provides a method comprising: constructing a
roadway comprising an
aggregate where the aggregate has a 513C value more negative than -1060, or,
in some embodiments, more
negative than -20%0. The aggregate may make up more than 10, 20, 30, 40, 50,
60, 70, 80, or 90% of the
roadway.
In some embodiments the invention provides a method of producing a roadway
component, the
method comprising: obtaining a CO2 sequestering synthetic carbonate; and
producing a roadway
component comprising the CO2 sequestering synthetic carbonate. The roadway
component may be, e.g., an
aggregate, a cement, a blended cement, an asphalt, a soil stabilizer, a
concrete, a binder, a formed material
(brick, stone slab) a settable composition. In some embodiments the invention
provides a method of
producing a roadway component, the method comprising: obtaining a synthetic
carbonate where the
carbonate has a 613C value more negative than -10, or, in some embodiments,
more negative than -20; and
producing a roadway component comprising the CO2 sequestering synthetic
carbonate. The roadway
component may be, e.g., an aggregate, a cement, a blended cement,.an asphalt,
a soil stabilizer, a concrete,
a binder, a formed material (brick, stone slab) a settable composition.
In some embodiments the invention provides a system for producing a roadway
component
comprising a CO2 sequestering synthetic carbonate, the system comprising: an
input for an alkaline-earth-
metal-containing water; carbonate compound precipitation station that subjects
the water to carbonate
compound precipitation conditions and produces a CO2 sequestering synthetic
carbonate; and a roadway
component producer for producing the roadway component comprising the CO2
sequestering synthetic
carbonate. In some embodiments the invention provides a system for producing a
roadway component
comprising a synthetic carbonate where the carbonate has a 813C value more
negative than -10%o, or, in
some embodiments, more negative than -20%0, the system comprising: an input
for an alkaline-earth-metal-
containing water; carbonate compound precipitation station that subjects the
water to carbonate compound
precipitation conditions and produces a synthetic carbonate where the
carbonate has a 513C value more
negative than -10%0, or, in some embodiments, more negative than -20%0; and a
roadway component
producer for producing the roadway component comprising the synthetic
carbonate where the carbonate has
a 613C value more negative than -10%0, or, in some embodiments, more negative
than -20%0.
In some embodiments the invention provides a method of sequestering CO2, the
method
comprising: contacting an alkaline-earth-metal-ion containing water to a
gaseous industrial waste stream
49
CA 02670049 2009-07-02
comprising C02; precipitating a CO2 sequestering synthetic carbonate from the
alkaline-earth-metal-ion
containing water, wherein the synthetic carbonate comprises CO2 derived from
the gaseous industrial waste
stream; and producing a roadway component comprising the CO2 sequestering
synthetic carbonate. In some
embodiments the invention provides a method of sequestering C02, the method
comprising: contacting an
alkaline-earth-metal-ion containing water to a gaseous industrial waste stream
comprising CO2;
precipitating a synthetic carbonate where the carbonate has a 613C value more
negative than -10%o, or, in
some embodiments, more negative than -20%o from the alkaline-earth-metal-ion
containing water, wherein
the synthetic carbonate comprises CO2 derived from the gaseous industrial
waste stream; and producing a
roadway component comprising the synthetic carbonate where the carbonate has a
613C value more
negative than -10%0, or, in some embodiments, more negative than -20%o.
In some embodiments the invention provides a method of producing a carbon
sequestration
tradable commodity, the method comprising: producing a roadway component
comprising a CO2
sequestering synthetic carbonate compound; determining a quantified amount of
CO2 sequestered in the
roadway component; and producing a carbon sequestration tradable commodity
based on the determined
quantified amount.
In some embodiments the invention provides a method of obtaining a carbon
sequestration
tradable commodity, the method comprising: (a) generating C02; (b) forwarding
the CO2 to a CO2
sequesterer that: (i) produces a roadway component comprising a CO2
sequestering synthetic carbonate
compound; (ii) determines a quantified amount of CO2 sequestered in the
roadway component; and (iii)
produces a carbon sequestration tradable commodity based on the determined
quantified amount; and (c)
receiving the carbon sequestration tradable commodity from the CO2
sequesterer.
III. Methods
The methods of the invention include methods of manufacturing aggregate,
methods of
sequestering CO2 through manufacturing aggregate, the production of sets of
aggregate to a predetermined
set of characteristics, methods of making settable compositions, methods of
making structures that include
the aggregates of the invention, and business methods.
A. Methods of manufacturing aggregate.
In some embodiments the invention provides methods of manufacturing aggregate.
In one
embodiment, the invention provides a method of manufacturing aggregate by
dissolving carbon dioxide
from an industrial waste stream in an aqueous solution and precipitating one
or more carbonate compounds
from the aqueous solution, dewatering the precipitate, and in some embodiments
further treating the
dewatered precipitate to produce an aggregate. The industrial waste stream may
be any suitable waste
stream, as described herein. In some embodiments the industrial waste stream
is the flue gas from a coal-
fired power plant. Contacting may be by any suitable apparatus and procedure,
also as described herein,
such as by a flat jet contactor, or by aerosol contact. In some embodiments
the CO2 in the industrial waste
stream is contacted with the aqueous solution using a flat stream contactor as
described herein. Protons are
removed from the aqueous solution containing the dissolved CO2 (and
bicarbonate and carbonate, as
dictated by pH) by any convenient means, also as described further herein; in
some embodiments protons
CA 02670049 2010-06-30
are removed by an electrochemical system that may be used to produce base for
proton removal, or may he
used to directly remove protons (e.g., by contact with the solution in which
the CO2 is dissolved); for
further description see this application and U.S. Patent Application
Publication No. 2009-0169452 Al. The
composition of the precipitate depends on the composition of the aqueous
solution; the aqueous solution
contains divalent cations, e.g., magnesium and/or calcium, which may be from
one or more of a variety of
sources, including seawater, brines such as geologic brines, minerals such as
minerals, e.g., serpentine,
olivine, and the like, flyash, slag, other industrial waste such as red mud
from bauxite refining. Thus the
calcium/magnesium ratio in the precipitate may vary and may be one of the
ratios described herein, such as
5/1 to 1/5, or 1/1 to 1/10, or 100/1 to 10/1, or any other ratio depending on
the material used in the aqueous
solution. The precipitate contains calcium and/or magnesium carbonates and
may, in addition, contain
other components of the industrial waste gas contained in the precipitate, as
described herein, e.g., sulfates
or sulfites, precipitated nitrogen-containing compounds, heavy metals such as
mercury, and others as
disclosed herein. In some embodiments the precipitate is dewatercd. Further
treatment can include
treatment by elevated temperature and/or pressure, as described elsewhere
herein, e.g., by means of platen
press, or by extrusion. The dewatered precipitate in some embodiments is
further dried, then water is added
back to the desired percentage, e.g., to 1-20%, or 1-10%, or 3-7% w/w. In some
embodiments the
dewatered precipitate, optionally dried and reconstituted, is treated by being
sent through an extrusion
press, which may produce aggregate of virtually any desired shape and size, as
described further herein. In
some embodiments the dewatered precipitate, optionally dried and
reconstituted, is treated by being
pressing in a platen press, which may produce shaped aggregate or "plates" of
aggregate that may be further
treated. The dewatered precipitate in some embodiments is subjected to high
pressure, e.g., 2000-6000 psi,
or even 2000-20,000 psi, for suitable time, e.g., 0.1 minute to 100 minutes,
or 1-20 minutes, or 1-10
minutes, and at suitable temperature, e.g., 50-150 C, or 70-120 C, or 80-100
C. In souse entbodinients
the product so formed is used as is. In some embodiments, the product contains
carbonate and has a 6"C.
more negative than -10%o, or more negative than -15% 0, or more negative than -
20%.o, or more negative than
-25700. In other embodiments the product is further treated, e.g., through
crushing, grinding, and the like.
In some embodiments, the methods further include combining the aggregate so
produced in a settable
composition.
In some embodiments the invention provides a method of producing an aggregate
comprising a
synthetic carbonate by obtaining a synthetic carbonate; and producing an
aggregate comprising the
synthetic carbonate. Any suitable method, such as those described herein, may
be used to obtain the
synthetic carbonate as long as it is suitable for use in an aggregate. In some
embodiments, the synthetic
carbonate comprises sequestered CO,. In some embodiments, the synthetic
carbonate has a S"C more
negative than -Moo, or more negative than -15%o, or more negative than -20%.,
or more negative than -
25%c. The obtaining step may comprise precipitating the synthetic carbonate
from an alkaline-earth-metal-
ion containing water, for example a salt water such as sea water, or brine, or
water treated to contain
alkaline-earth metals, e.g., from minerals or from industrial waste such as
flyash, slag, or red mud. In some
embodiments, the obtaining step further comprises contacting the alkaline-
earth-metal-ion containing water
to an industrial gaseous waste stream comprising CO2 prior to the
precipitating step; the industrial gaseous
waste stream may he from, e.g., a power plant, foundry, cement plant,
refinery, or smelter; the gas waste
51
CA 02670049 2009-07-02
stream may be, e.g. flue gas, such as flue gas from a coal-fired power plant.
In some embodiments the
obtaining step further comprises raising the pH of the alkaline-earth-metal-
ion containing water to 10 or
higher, during the precipitating step. In some embodiments the producing step
further comprises: generating
a settable composition comprising the synthetic carbonate; and allowing the
settable composition to form a
solid product. In some alternative embodiments the producing step further
comprises subjecting the
precipitate to a combination of temperature and pressure sufficient to produce
an aggregate suitable for the
intended use; such as a temperature of between 35-500 C, or 50-200 C, or 50-
150 C, and a pressure
between 1000 psi to 20,000 psi, or 1000 psi to 10,000 psi, or 1000 psi to 6000
psi, such as 4000 psi to 6000
psi. In some alternative embodiments the generating step comprises mixing the
synthetic carbonate with
one or more of water, Portland cement, fly ash, lime and a binder. The
generating step may further
comprise mechanically refining the solid product, such as by molding,
extruding, pelletizing, crushing, or
some combination thereof. In some embodiments the producing step comprises
contacting the synthetic
carbonate with fresh water, e.g., to convert the synthetic carbonate to a
freshwater stable product. In some
embodiments the contacting step comprises: spreading the synthetic carbonate
in an open area; and
contacting the spread synthetic carbonate with fresh water.
B. Other methods
In some embodiments the invention provides a method comprising: obtaining a
composition
comprising a hydraulic cement and an aggregate comprising a synthetic
carbonate; and producing a settable
composition comprising the obtained composition. The aggregate comprising a
synthetic carbonate may, in
some embodiments, be made by the methods described herein. In some
embodiments, the synthetic
carbonate comprises sequestered CO2. In some embodiments, the synthetic
carbonate has a 513C more
negative than -10%o, or more negative than -15%o, or more negative than -20%o,
or more negative than -
25%o. The method may further comprises allowing the settable composition to
set into a solid product, such
as a structural product, e.g., part of a road, or asphalt, or a building
foundation.
In some embodiments the invention provides a method of sequestering carbon
dioxide, the method
comprising: precipitating a CO2 sequestering carbonate compound composition
from an alkaline-earth-
metal-ion containing water; and producing aggregate comprising the CO2
sequestering carbonate compound
composition. In some embodiments the invention provides a method of
sequestering CO2 by contacting an
alkaline-earth-metal-ion containing water to a gaseous industrial waste stream
comprising C02;
precipitating a synthetic carbonate from the alkaline-earth-metal-ion
containing water, wherein the
synthetic carbonate comprises CO2 derived from the gaseous industrial waste
stream; and producing
aggregate comprising the synthetic carbonate. In some embodiments, the
aggregate is combined in a
settable composition. The aggregate may be used in making manmade structures.
In some embodiments,
the aggregate makes up at least 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, or
95% of the manmade structure. In
some embodiments, the manmade structure is a building. In some embodiments,
the manmade structure is
a roadway, or a component of a roadway. In some embodiments, the manmade
structure is a dam. In other
embodiments, the aggregate is transported to a storage site, such as an
underwater storage site, or an
underground storage site, e.g., a coal mine or other fossil fuel removal site.
The aggregate may be
transported to the site by, e.g., rail, such as the same rail cars that
transported coal to the coal-fired power
52
CA 02670049 2009-07-02
plant at which the aggregate was produced. The aggregate may be produced in a
variety of shapes so as to
make packing in the storage site more efficient and/or give a stronger
packing.
In some embodiments the invention provides a method of producing a CO2
sequestering aggregate
by: obtaining a CO2 sequestering component; and producing an aggregate
comprising the CO2 sequestering
component. The C02-sequestering component may be obtained in some embodiments
by precipitating a
carbonate from an aqueous solution that has been contacted with a C02-
containing industrial waste gas
stream. The aggregate may be produced by any suitable method such as the
methods described herein.
In some embodiments the invention provides a method of producing an aggregate
containing
carbon with a 513C more negative than -10%0, or more negative than -15%o, or
more negative than -20%0, or
more negative than -25%o by: obtaining a component containing carbon with a
S13C more negative than -
10%o, or more negative than -15%o, or more negative than -20%0, or more
negative than -25%o; and
producing an aggregate from the component, thus producing an aggregate
containing carbon with a 813C
more negative than -10%o, or more negative than -15%o, or more negative than -
20%0, or more negative than
-25%0. The component may be obtained in some embodiments by precipitating a
carbonate-containing
precipitate from an aqueous solution that has been contacted with an
industrial waste gas stream that
contains CO2 from combustion of fossil fuel; depending on the type of fossil
fuel, the CO2will contain
carbon with a a 813C more negative than -10%o, or more negative than -15%o, or
more negative than -20%0,
or more negative than -25%o, and the carbonates precipitated from this gas
will also have similar 813C
values. The counterion to the carbonate is in some embodiments calcium,
magnesium or a combination of
calcium and magnesium in any ratio as described herein. In some embodiments,
the aggregate is combined
in a settable composition. The aggregate may be used in making manmade
structures. In some
embodiments, the aggregate makes up at least 1, 5, 10, 20, 30, 40, 50, 60, 70,
80, 90, or 95% of the
manmade structure. In some embodiments, the manmade structure is a building.
In some embodiments, the
manmade structure is a roadway, or a component of a roadway. In some
embodiments, the manmade
structure is a dam. In other embodiments, the aggregate is transported to a
storage site, such as an
underwater storage site, or an underground storage site, e.g., a coal mine or
other fossil fuel removal site.
The aggregate may be transported to the site by, e.g., rail, such as the same
rail cars that transported coal to
the coal-fired power plant at which the aggregate was produced. The aggregate
may be produced in a
variety of shapes so as to make packing in the storage site more efficient
and/or give a stronger packing.
Storage sites also include wave-resistant structures (e.g., artificial reefs),
or other structures resistant to
water currents and motion (such as riprap); thus the invention provides wave-
resistant structures that
contain one or more of the aggregates described herein, and also provides
structures resistant to water
currents and motion containing one or more of the aggregates described herein.
The invention further
provides methods of making wave-resistant structures or water-resistant
structures that include
manufacturing an aggregate as described herein, and forming a wave-resistant
structure or a structure
resistant to water currents and motion using the aggregate.
In some embodiments, the invention provides a method comprising: obtaining a
settable
composition comprising a hydraulic cement and a CO2 sequestering aggregate;
and producing a solid
product from the settable composition.
53
CA 02670049 2009-07-02
In some embodiments the invention provides a method of producing a carbon
negative structure by
using a carbon negative aggregate in the construction of the structure.
"Carbon negative" has the meaning
described herein. In some embodiments, the structure is a building. In some
embodiments, the structure is
a dam. In some embodiments, the structure is a roadway. In some embodiments,
the structure is a
component of a larger structure, e.g., a foundation for a building, or a base
course or other base layer for a
roadway. In some embodiments, the carbon negative aggregate comprises at least
5, 10, 20, 30, 40, 50, 60,
70, 80, or 90% of the structure. In some embodiments, the structure also
includes at least one other C02-
sequestering component. For example, in some embodiments, the structure
further contains a C02-
sequestering supplementary cementitious material, and/or a C02-sequestering
pozzolan, that is used in the
making of cement for the structure. In some embodiments, the structure further
contains a CO2..
cement. In some embodiments, the amount of CO2 sequestered in the making of
the structure
and its components exceeds the amount of CO2 produced in the making of the
structure and its components
by at least 1, 5, 10, 20, 30, 40 50, 60 70, 80, 90, or 95%, where % is
calculated as described for "carbon
negative" elsewhere herein.
In some embodiments, the invention provides a method of producing a carbon
sequestration
tradable commodity by producing an aggregate comprising a synthetic CO2
sequestering carbonate
compound; determining a quantified amount of CO2 sequestered in the aggregate;
and producing a carbon
sequestration tradable commodity based on said determined quantified amount.
In some embodiments the
invention provides method of obtaining a carbon sequestration tradable
commodity by generating C02;
forwarding the CO2 to a CO2 sequesterer that: (i) produces an aggregate
comprising a synthetic CO2
sequestering carbonate compound; (ii) determines a quantified amount of CO2
sequestered in the CO2
aggregate; and (iii) produces a carbon sequestration tradable commodity based
on the determined quantified
amount; and (c) receiving said carbon sequestration tradable commodity from
the CO2 sequesterer.
In some embodiments the invention provides methods of producing lightweight
aggregate by
treating a starting material in such a way that there is no net production of
CO2 during the treatment, to
produce a lightweight aggregate. In some embodiments there is a net
sequestration of CO2 in the
production of the aggregate. The starting material may be an aqueous solution,
a CO27-containing gas
stream, such as industrial waste gas stream, a source of divalent cations, or
a combination thereof. The
starting materials may be treated so as to precipitate a carbonate, where the
carbonate sequesters CO2 in the
process. The process may further include treating the precipitate under
conditions that produce a
lightweight aggregate, e.g., an aggregate with a bulk density (unit weight) of
75 lb/ft3 to 125 lb/ft3, such as
90 lb/ft3 to 115 lb/ft3.
In some embodiments the invention provides a method of manufacturing an
artificial rock without
the use of a binder by subjecting a synthetic carbonate to conditions that
cause it to undergo a physical
transformation, thereby forming an artificial rock, where the formation of the
artificial rock is not
dependent on chemical reactions of the starting material. In some embodiments,
the artificial rock is
formed by dissolution and re-precipitation of compounds in the starting
synthetic carbonate to produce new
compounds or greater quantities of compounds already in the starting material.
In some embodiments the
new or greater quantities of compounds include one or more of dypingite,
hydromagnesite, and/or
nesquehonite. In some embodiments the manufacture of the artificial rock
includes subjecting the synthetic
54
CA 02670049 2009-07-02
carbonate to a combination of elevated temperature and pressure for a period
of time sufficient to product
the artificial rock. In some embodiments the conditions to which the synthetic
carbonate is subjected are
sufficient to produce an artificial rock having a hardness of greater than 2,
or greater than 3, or greater than
4, or 2-7, or 2-6, or 2-5, on the Mohs scale or equivalent on the Rockwell,
Vickers, or Brinell scale. In some
embodiments the conditions to which the synthetic carbonate is subjected are
sufficient to produce an
artificial rock having a bulk density of 50 lb/ft3 to 200 lb/ft3. In some
embodiments the conditions to which
the synthetic carbonate is subjected are sufficient to produce an artificial
rock having a bulk density of 75
lb/ft' o 125 lb/ft .
In some embodiments the invention provides a method of manufacturing an
aggregate comprising
combining waste gases from an industrial process with water containing species
that will react with the
waste gas to form a precipitate and processing the precipitate to form an
aggregate.
The methods of the invention allow the production of virtually any size or
shape of aggregate, as
well as any number of other characteristics of the aggregate, such as
hardness, abrasion resistance, density,
porosity, chemical composition, mineral composition, acid resistance, alkaline
resistance, chloride content,
sodium content, retention of C02, and reactivity (or lack thereof).
Accordingly, in some embodiments the
invention provides methods of manufacturing aggregate by manufacturing the
aggregate to a predetermined
set of characteristics. In some of these embodiments, the aggregate contains
CO2 from an industrial waste
gas stream. In some embodiments, the characteristics include two or more of
size, shape, hardness,
abrasion resistance, density, porosity, chemical composition, mineral
composition, acid resistance, alkaline
resistance, chloride content, sodium content, retention of C02, and reactivity
(or lack thereof). In some
embodiments, the characteristics include three or more of size, shape,
hardness, abrasion resistance, density,
porosity, chemical composition, mineral composition, acid resistance, alkaline
resistance, chloride content,
sodium content, retention of C02, and reactivity (or lack thereof). In some
embodiments, the characteristics
include four or more of size, shape, hardness, abrasion resistance, density,
porosity, chemical composition,
mineral composition, acid resistance, alkaline resistance, chloride content,
sodium content, retention of
CO2, and reactivity (or lack thereof). In some embodiments the characteristics
include size and shape. In
some embodiments the characteristics include size, shape, and at least one of
hardness, abrasion resistance,
density, porosity, chemical composition, mineral composition, acid resistance,
alkaline resistance, chloride
content, sodium content, retention of C02, and reactivity (or lack thereof).
In embodiments where the set of aggregates is made to include aggregates of
predetermined size
and shape, methods of making aggregate to a desired shape or size are as
described herein. Any desired
mixture may be produced, for example, a mixture of aggregate with one, two,
three, four, five, six, seven,
eight, nine, ten, or more than ten sizes of aggregate, in combination with
one, two, three, four, five, six,
seven, eight, nine, ten, or more than ten shapes of aggregate. For example, an
aggregate set may have at
least two sizes and at least two shapes, or exactly two sizes and exactly two
shapes. This is exemplary only,
and any combination of numbers of sizes and shapes may be used. The sizes may
be any desirable size,
e.g., to provide a desired degree of packing and reduce the need for cement in
a concrete, a graded set of
sizes may be used, e.g., selected from the largest of coarse aggregate down to
the finest of fine aggregate, or
any combination in between. Similarly, the shapes may be any desirable shape
that is predetermined, for
example, all one shape, or a variety of shapes. Some sizes of aggregate in the
set may be produced in one
CA 02670049 2009-07-02
shape while others may be produced in one or more other shapes. For example,
the methods of the
invention allow the production of a set of aggregates that include aggregate
of spherical or disk shape in a
set of graded sizes, for packing, as well as a portion of the larger particles
being of elongate shape (i.e.,
having a high aspect ratio, as described elsewhere herein) to improve
flowability and/or to reduce cracking
by acting as "pins." Other possibilities are sets of aggregate with some star-
shaped pieces for interlocking
combined with other, smaller pieces for packing and reducing the need for
cement. These possibilities are
exemplary only and those of skill in the art will recognize that aggregate
sets may be made in virtually any
combination of size and shape depending on the job for which they are
intended; from this job the
characteristics of the aggregate set may be determined and the set may be
"made to order."
Further useful characteristics may be included besides size and shape, such as
reactivity. In some
applications, some degree of reactivity may be useful, or it may be useful to
have a certain percentage of an
aggregate set, but not all of the set, be reactive. In the construction of
roadways, for example, it may be
useful to have a base course composed of some degree of reactive aggregate so
that water seeping through
the roadway surface will cause the underlying aggregate to react and form a
stronger base. The methods of
the invention allow for a calibrated amount of reactive aggregate, e.g.,
aggregate containing siliceous
materials, to be used in a set of aggregate to achieve a desired degree of
overall reactivity. This can be in a
certain percentage of aggregate of a certain size, or all of a particular size
of aggregate, or shape, etc.
Other characteristics that may be varied based on the conditions under which
the aggregate is
manufactured include hardness. While in general harder aggregate is preferred,
certain classes of size or
shape of aggregate in a set may be more useful if somewhat softer, e.g., to
provide deformation in certain
highly packed uses. For example, if aggregate is used to refill mining voids,
e.g., in coal mines, it may be
desirable to make a set of aggregate with a variety of sizes for packing as
well as having a certain
percentage of the smaller aggregate as somewhat softer for deformation as the
aggregate is packed as
tightly as practical in the void left by the mining of the coal.
Further characteristics include stability, e.g., solubility such as solubility
in neutral, acid, or basic
pH. The aggregates of a set may all have the same solubility or different
solubilities. Certain aggregates in
a set may be deliberately manufactured to be soluble under their conditions of
use so that over a period of
time, which may be of any duration, they dissolve, leaving a void space in a
concrete or other material, that
matches the size and shape of the aggregate. This allows for the manufacture
of concrete of controlled
permeability.
Abrasion resistance may also be controlled in the sets of aggregates, thus an
aggregate may be
produced with all of one abrasion resistance or may have sets of different
aggregates of different abrasion
resistance.
IV. Systems
Aspects of the invention further include systems, e.g., processing plants or
factories, for producing
the carbonate compound compositions, e.g., saltwater derived carbonate and
hydroxide mineral
compositions, and aggregates of the invention, as well as concretes and
mortars that include the aggregates
of the invention. Systems of the invention may have any configuration which
enables practice of the
particular production method of interest.
56
CA 02670049 2009-07-02
Aspects of the invention further include systems, for example, processing
plants or factories, for
producing aggregate of the invention from divalent cations and components of
industrial waste gas, as well
as concretes and mortars that include the aggregates of the invention. Systems
of the invention may have
any configuration which enables practice of the particular production method
of interest. Systems of the
invention include a system for producing aggregate where the system includes
an input for a divalent
cation-containing water, a carbonate compound precipitation station that
subjects the water to carbonate
compound precipitation conditions and produces a precipitated carbonate
compound composition; and an
aggregate producer for producing aggregate from the precipitated carbonate
compound composition. In
some embodiments the system further includes an input for a C02-containing
industrial waste gas stream,
which may be in some embodiments a waste gas stream from a power plant,
foundry, cement plant, or
smelter; e.g., in some embodiments, a power plant such as a coal-fired power
plant. The aggregate
producer of the system may be an aggregate producer that uses any suitable
method for producing
aggregate of the desired qualities, e.g., any of the methods described herein,
such as using a combination of
temperature and pressure such as in a platen press, an extruder, or a roller
system. In some embodiments
the aggregate producer is capable of producing an aggregate of a specific size
and/or of a specific shape. In
some embodiments, the aggregate producer is capable of producing aggregates of
a variety of sizes and/or
shapes. The aggregate producer may produce aggregate in one step or in more
than one step, e.g., a step of
producing a solid block optionally followed by one or more steps to produce
aggregate of the desired
properties, e.g., size and/or shape, from the block. In some embodiments a
system of the invention is
capable of producing at least 0.5, 1, 2, 5, 10, 50, 100, 1000, or 10,000 tons
of aggregate per day containing
at least 0.1, 0.2, 0.3, 0.4, or 0.5 tons of CO2 sequestered from a source of
CO2 per ton of aggregate. In some
embodiments a system of the invention is capable of producing at least 1 ton
of aggregate per day
containing at least 0.1 ton of CO2 sequestered from a source of CO2 per ton of
aggregate. In some
embodiments a system of the invention is capable of producing at least 1 ton
of aggregate per day
containing at least 0.2 ton of CO2 sequestered from a source of CO2 per ton of
aggregate. In some
embodiments a system of the invention is capable of producing at least 1 ton
of aggregate per day
containing at least 0.3 ton of CO2 sequestered from a source of CO2 per ton of
aggregate. In some
embodiments a system of the invention is capable of producing at least 10 tons
of aggregate per day
containing at least 0.3 tons of CO2 sequestered from a source of CO2 per ton
of aggregate. In some of these
embodiments the aggregate is suitable for use as a building material.
Figure 2 provides a schematic of a precipitation and aggregate production
system according to one
embodiment of the invention. In Figure 2, system 100 includes divalent cation
source 110. In certain
embodiments, divalent cation source 110 includes a structure having an input
for an aqueous solution of
divalent cations, such as a pipe or conduit from an ocean, etc. Where the
aqueous solution of divalent
cations that is processed by the system to produce the precipitation material,
and, subsequently, aggregate,
is seawater, the input is in fluid communication with the seawater. For
example, the input may be a pipe
line or feed from ocean water to a land based system, or the input may be a
inlet port in the hull of ship(e.g.,
where the system is part of a ocean-faring ship).
Also shown in Figure 2, is gaseous waste stream source 130, which comprises
carbon dioxide and
other components of combustion gases. The waste gas stream may vary as
described above. The divalent
57
CA 02670049 2010-06-30
cation source and the gaseous waste stream source are connected to the charger
and precipitator reactor 120.
The charger and precipitator 120 may include any of a number of different
elements, such as temperature
regulators (e.g., configured to heat the water to a desired temperature),
chemical additive elements(e.g., for
introducing chemical pH-raising agents (such as fly ash) into the water), and
electrolysis elements(e.g.,
cathodes/anodes, etc. Charger and precipitator 120 may operate in a batch
process, semi-batch process, or a
continuous process.
The product of the precipitation reaction(e.g., a slurry) is optionally
processed at a separator 140,
as illustrated in Figure 2. The separator 140 may use a variety of different
water removal processes,
including processes such as continuous centrifugation, centrifugation, filter
centrifugation, gravitational
settling, and the like. The precipitation material may be simply washed with
fresh water and left wet for a
fresh water hardening reaction to proceed. Partial mechanical water removal
may he performedto adjust the
density of the set product, controlling strength and hardness. Effluent liquid
resulting from the processes in the
separator may be sent to a tailings pond, ocean or other liquid repository
142.
The system shown in Figure 2 also includes an optional dryer 160 for drying
the dewatered precipitation material
produced at separator 140. Depending on the particular drying protocol of the
system, the dryer 160 may include a
filtration element, freeze drying structure, oven drying, spray drying
structure, etc., as described above in more detail.
Also shown is optional washing station 150, where bulk dewatered precipitation
material from
separator 140 is washed, for example, to remove salts and other solutes from
the precipitation material prior
to drying in dryer 160.
Dried precipitation material from dryer 160 is then provided to aggregate
production unit 170, where the
precipitation material may be set and mechanically processed to produce a
final aggregate product 180. Effluent
discharge air and fine particulates 162 that cannot be used to make aggregate
may also be discharged by the dryer.
As indicated above, the system may be present on land or sea. For example, the
system may be a land-based system
that is in a coastal region (e.g., close to a source of sea water), or even an
interior location, where water is piped into the
precipitation and aggregate producing system from a divalent cation source
(e.g., ocean). Alternatively, the
precipitation and aggregate producing system may be a water-based system
(i.e., a system that is present on or in
water). Such as system may be present on a boat, ocean-based platform etc., as
desired.
IV. utility
The subject aggregates and settable compositions that include the same, find
use in a variety of
different applications, such as above ground stable CO2 sequestration
products, as well as building or
construction materials. Specific structures in which the settable compositions
of the invention find use
include, but are not limited to: pavements, architectural structures, e.g.,
buildings, foundations,
motorways/roads, overpasses, parking structures, brick/block walls and
footings for gates, fences and poles.
Mortars of the invention find use in binding construction blocks, e.g.,
bricks, together and filling gaps
between construction blocks. Mortars can also be used to fix existing
structure, e.g., to replace sections
where the original mortar has become compromised or eroded, among other uses.
58
CA 02670049 2009-07-02
The following examples are put forth so as to provide those of ordinary skill
in the art with a
complete disclosure and description of how to make and use the present
invention, and are not intended to
limit the scope of what the inventors regard as their invention nor are they
intended to represent that the
experiments below are all or the only experiments performed. Efforts have been
made to ensure accuracy
with respect to numbers used (e.g. amounts, temperature, etc.) but some
experimental errors and deviations
should be accounted for. Unless indicated otherwise, parts are parts by
weight, molecular weight is weight
average molecular weight, temperature is in degrees Centigrade, and pressure
is at or near atmospheric.
EXAMPLES
Example 1. Preparation of precipitation material for use in aggregate
76,000 gallons of seawater was pumped at 40 gallons per minute into a 250,000-
gallon open tank
configured with spargers in the bottom of the tank until the height of
seawater in the tank was six feet above
the spargers. Carbon dioxide was subsequently sparged into the seawater at a
rate that kept the pH above
5.6.
With continued carbon dioxide sparging, a slurry containing 4,500 kg ofjet-
milled (to decrease
particle size and improve dissolution rates) magnesium hydroxide was added
through piping with in-line
mixers. (The magnesium hydroxide for this experiment was waste from a seawater
magnesia (MgO) plant,
the magnesium hydroxide being about 85% Mg(OH)2, about 12% CaCO3, and about 3%
SiO2.) Carbon
dioxide sparging continued after complete addition of magnesium hydroxide and
until 9,400 pounds of
carbon dioxide had been added. Half of the reaction mixture in the tank (Tank
A) was subsequently
transferred to another tank (Tank B). The total time to complete these steps
was approximately 30 hours.
About 300 gallons of a 50% (w/w) sodium hydroxide solution was added to Tank A
over a period
of 4-6 hours until the pH reached 9.5. This mixture was then transferred to
Tank B over the course of about
5 hours and allowed to settle under the action of gravity for 8-12 hours.
The settled precipitation material was removed from the bottom of Tank B and a
portion of the
settled precipitation material was subsequently washed with fresh water,
dewatered in a filter press to
produce a filter cake at approximately 30% solids, and used to make aggregate
(see Example 2).
X-ray fluorescence (XRF) data (Table 3) indicates that the precipitation
material had a high Mg:Ca
weight ratio of 12. Thermogravimetric analysis (TGA) data provided herewith
(Fig. 5 and Fig. 6) indicated
that the precipitation material remained wet. Fig. 5 provides a TGA analysis
of wet precipitation material.
Fig. 6 provides a TGA analysis of precipitation material dried in a
desiccator.
Na Mg Al Si S CI K Ca Fe
Weight % 1.65 19.99 0.00 0.24 0.06 2.09 0.07 1.68 0.04
Table 3: XRF elemental analysis of precipitation material
% H2O % CO2
Weight % 27.38 31.98
59
CA 02670049 2009-07-02
Table 4: Percent C02 content (coulometry) and calculated percent H2O from TGA
X-ray diffraction (XRD) analysis (Fig. 4) of the precipitation material
indicates the presence of
dypingite (Mg5(C03)4(OH)2.5(H2O)) as a major phase, nesquehonite (MgC03.3H2O)
as another phase,
some hydromagnesite (Mg5(CO3)4(OH)2.4(H20)), and calcite as a minor component.
Some halite (NaCl)
was also detected.
A Fourier transform-infrared (FT-IR) spectrum of the precipitation material is
also provided (Fig.
7). Scanning electron microscope (SEM) images of the precipitation material at
1000x (left) and 4000x
(right) magnifications are provided as well (Fig. 8).
Example 2: Preparation of aggregate from precipitation material
The steel molds of a Wabash hydraulic press (Model No.: 75-24-2TRM; ca. 1974)
were cleaned
and the platens were preheated such that the platen surfaces (including mold
cavity and punch) were at 90
C for a minimum of 1 hour.
Some of the precipitation material filter cake from Example 1 was oven-dried
in sheet pans at 40
C for 48 hours and subsequently crushed and ground in a blender such that the
ground material passed a
No. 8 sieve. The ground material was then mixed with water resulting in a
mixture that was 90-95% solids
with the remainder being the added water (5-10%).
A 4" x 8" mold in the Wabash press was filled with the wet mixture of ground
precipitation
material and a pressure of 64 tons (4000 psi) was applied to the precipitation
material for about 10 seconds.
The pressure was then released and the mold was reopened. Precipitation
material that stuck to the sides of
the mold was scraped and moved toward the center of the mold. The mold was
then closed again and a
pressure of 64 tons was applied for a total of 5 minutes. The pressure was
subsequently released, the mold
was reopened, and the pressed precipitation material (now aggregate) was
removed from the mold and
cooled under ambient conditions. Optionally, the aggregate may be transferred
from the mold to a drying
rack in a 110 C oven and dried for 16 hours before cooling under ambient
conditions.
Once cooled to room temperature, the aggregate had the appearance of a
slightly tan to white
limestone. The surface of the aggregate could not be scratched with a coin,
indicating a Mohs hardness of 3
or greater, which is the hardness of most natural limestone. A laminar
structure was observed when the
aggregate was broken in half. When natural limestone from the Calera formation
in Northern California
was fractured, the same laminar structure was observed as for the aggregate.
Flakes of the natural limestone
broke with only a slightly greater force than that needed to break the
aggregate. Rubbing samples of the
natural limestone and the aggregate between one's palms for 5 seconds
indicated that the aggregate was
only slightly more friable than the limestone.
Figs. 9-12 provide spectra and images of the experiment: Fig. 9 provides an
XRD spectrum of the
aggregate; Fig. 10 provides an FT-IR spectrum of the aggregate; Fig. 11
provides TGA data for the
aggregate; and Fig. 12 provides SEM images of the aggregate at 1000x (left)
and 4000x (right)
magnifications.
Example 3: Aggregate from mixture of wollastonite and precipitation material
CA 02670049 2009-07-02
Some of the precipitation material prepared in Example 1 (primarily
nesquehonite rods from an
unwashed filter cake of precipitation material) was dried in an oven to a
consistent weight. The dried
starting precipitation material (5 kg) was subsequently added to a reaction
vessel followed by 1 kg of
commercial grade wollastonite (calcium silicate) and 500 mL of 50% (w/w)
sodium hydroxide (with
stirring). With continued stirring, 12 kg of water was added to the reaction
mixture. The reaction mixture
was subsequently heated at 70 C overnight.
The resulting product material was filtered, spray dried, and used to prepare
aggregate as described
in Example 2, including the optional step of drying the aggregate on an drying
rack in a 110 C oven for 16
hours.
Na Mg Al 11 Si S Cl K Ca Fe
Weight % 0.00 0.48 0.27 22.12 0.00 0.19 0.00 36.18 0.30
Table 5: XRF elemental analysis of wollastonite starting material
Na Mg Al Si S Cl K Ca Fe
Weight % 12.14 13.09 0.12 4.48 0.36 2.47 0.06 7.19 0.07
Table 6: XRF elemental analysis of spray-dried material
%H20 %C02
Weight % 14.67 23.77
Table 7: Percent CO2 content (coulometry) and calculated % H2O from TGA for
spray-dried material
Fig. 13 provides XRD spectra of aggregate (top spectrum), spray-dried material
(middle
spectrum), and wolloastonite starting material (bottom spectrum). The XRD
spectrum for the wollastonite
starting material (top spectrum) indicates that the wollastonite starting
material comprises wollastonite-lA
and possibly wollastonite-2M (two wollastonite polymorphs), wustite (FeO), and
corundum (A1203) phases.
The spray-dried material (middle spectrum) shows phases of hydromagnesite
(Mg5(C03)4(OH)=4H20) and
aragonite (CaCO3). (Coulometry indicates that the spray-dried material has a
%C02 of 24 wt%, supporting
the presence of the observed carbonate phases.) Most of the peaks associated
with the wollastonite starting
material are still visible; however, several peaks show broadening, indicating
that some reactions are
occurring between the starting precipitation material and the wollastonite
during the above procedure.
XRD analysis of the aggregate (top spectrum) indicates that there is little
change in the crystalline phases
between the spray-dried material and the aggregate.
Fig. 14, which provides a TGA analysis of the aggregate (solid line) and the
spray-dried material
(dashed line), indicates that water is lost during pressing (first peak below
100 C), but little other change
occurs as a result of pressing. The peaks around 400 C are indicative of the
magnesium carbonate
hydrates, and the peaks around 650-680 C are indicative of calcium
carbonates.
Fig. 15 provides SEM images of the spray-dried material (top) and the
aggregate (bottom). In the
aggregate, left over crystals of wollastonite (determined by energy-dispersive
X-ray spectroscopy (EDS))
appear to be surrounded by a matrix of leftover starting precipitation
material. Based on the XRD and the
61
CA 02670049 2009-07-02
SEM images, it is unclear whether the matrix has additional networking or if
it is a packing/densification of
the starting precipitation material.
Example 4: Aggregate from precipitation material produced from fly ash
Seawater (900 gallons) in a suitably sized reaction vessel was sparged with a
gaseous CO2 mixture
(comprising 20% CO2 and 80% compressed air) until the pH was consistent at
about pH 5.8. With
continued sparging, 10 kg of NaOH solution (50% (w/w) NaOH (aq)) was added
while the pH was
maintained at pH 8.5 or below. To a separate mixing vessel was added Indian
River fly ash (25 kg) and
water (25 kg) to form a 1:1 mixture of fly ash:water. To the resulting fly ash-
water mixture was then added
60 kg of NaOH solution (50% (w/w) NaOH (aq)) with thorough stirring. With
continued CO2 sparging, the
fly ash-water mixture was added to the reaction mixture in the reaction vessel
while the pH of the reaction
mixture was maintained at about pH 10Ø Any remaining fly ash-water mixture
was flushed from the
separate mixing vessel with 10 L of water, after which CO2 sparging was
stopped. The reaction mixture was
stirred for an additional 10 minutes, and the reaction mixture was transferred
to a settling tank and allowed
to settle under the action of gravity.
The reaction product was filtered, spray dried, and used to prepare aggregate
as described in
Example 2, including the optional step of drying the aggregate on an drying
rack in a 110 C oven for 16
hours.
The XRF data for the fly ash starting material and the resulting spray-dried
material are given
below:
Na Mg Al Si S Cl K Ca Fe
Weight % 0.00 1.12 14.63 23.68 0.24 0.48 1.95 1.17 3.69
Table 8: XRF elemental analysis of fly ash starting material.
Na Mg Al Si S Cl K Ca Fe
Weight % 15.23 6.88 4.76 6.84 0.45 11.91 1.05 3.44 1.17
Table 9: XRF elemental analysis of spray-dried material.
% H2O % C02
Weight % 10.21 12.64
Table 10: % CO2 (coulometry) and calculated % H2O from TGA (Fig. 17) for spray-
dried material.
Fig. 16 provides XRD spectra for the fly ash starting material (top spectrum),
the spray-dried
material (middle spectrum), and the aggregate (bottom spectrum). Fig. 16 also
provides corresponding
phase analysis. The XRD spectrum for the fly ash starting material indicates
standard fly ash crystalline
phases such as quartz (SiO2) and mullite. The XRD spectrum for the spray-dried
material indicates
primarily the crystalline fly ash phases (i.e., quartz and mullite), as well
as shallow peaks that may be
associated with northupite (Na2Mg(C03)2Cl), hydromagnesite
(Mg5(CO3)4(OH)=4H2O), halite (NaCI), and
aragonite (CaCO3). The XRD spectrum for the aggregate shows crystalline phases
(e.g., hydromagnesite,
halite, northupite, and aragonite) present in the spray-dried material as well
as the fly ash phases indicated
62
CA 02670049 2009-07-02
above. The spray-dried material has a %C02 of 13 wt%, indicating that there is
carbonated material, even if
not in crystalline form.
Fig. 18 provides SEM images at 1000X(left) 4000X (right) showing a cleaved
surface of a sample
of aggregate made from fly ash. SEM observations of the aggregate confirm the
presence of fly ash starting
material in the aggregate; however, a matrix appears around the fly ash, in
addition to some crystallites in
the matrix. The sample was easy to grind indicating that the matrix may not be
well formed, or that it might
be a friable material. With the amount of fine fly ash particles dispersed
within the matrix, it was
inconclusive by SEM-EDS as to whether or not there was silica in the matrix or
if the silica contribution
was from these fine fly ash particles.
Fig. 17 provides TGA analyses of the spray-dried material and the aggregate.
As evidenced by the
TGA graphs, water is lost (peaks below 250 C) during aggregate formation, but
there is no notable change
in the nature of the phases present from the spray-dried material to the
aggregate.
Example 5: Aggregate in mortars
In general, aggregate from Example 2 was broken into pieces and the pieces of
aggregate were
sieved to give a #2 sized aggregate, a #4 sized aggregate, #16 sized
aggregate, and a fine sand aggregate (as
below).
Aggregate sizes:
= Size 1: Retained on a sieve #4 (4.75mm) [+ 4]
= Size 2: Passing sieve #4 (4.76mm) but retained on sieve #16 (1.19mm) [- 4 /+
16]
= Size 3: Passing sieve #16 (1.19mm) but retained on sieve #35 (0.5mm) [- 16 /
+ 35]
= Rejected: Passing sieve #35 [- 35]
Portland cement and water were mixed in a water:cement ratio of 0.50 (1:2) for
1 minute.
Aggregate was subsequently added until the mortar reached the correct
consistency (i.e., paste covering all
the aggregate, but remaining fluid enough to be cast in mortar cubes and
finished).
In a first mortar example, the size 3 fraction of aggregate was used to make a
sample comprising 5
g of Portland cement, 2.5g water, and 7.5 g of the size 3 aggregate. The
resulting mortar sample became
warm after 20 min, reaching a temperature of 31.8 C.
In a second mortar example, the aggregate was used to make 2" cubes comprising
309 g of
Portland cement, 155 g of water, and 338 g of aggregate (179 g of the size 1
(coarse) fraction and 159 g of
the size 2 (intermediate) fraction). The cubes were then cast and allowed to
age for about 60 hours in a 98%
relative humidity room at 23 C.
Example 6: Aggregate containing aragonite
A suitably sized reaction vessel was charged with 900 gallons of seawater
collected from Moss
Landing, CA on October 10, 2008, and stirred with an overhead stirrer. A
gaseous mixture of carbon
dioxide (20% CO2 and 80% compressed air) was sparged into the seawater at a
flow rate of 5 scfm for the
CO2 and 20 scfm for the compressed air. With continued sparging, 3.4 kg (dry
weight) magnesium
hydroxide (waste from a seawater magnesia plant, the magnesium hydroxide
comprising 85% Mg(OH)2,
12% CaCO3, and about 3% Si02) was slowly added.
63
CA 02670049 2009-07-02
When the pH dropped to about pH 7.0 ( 0.1), 50% NaOH solution (50% (w/w) NaOH
(aq)) was
added. The pH of the reaction mixture was adjusted to about pH 7.9, after
which the pH was maintained at
about pH 7.9 ( 0.2) by manually controlling the addition of NaOH while
continuously sparging the
reaction mixture with the gas mixture. If the pH was less than pH 7.9, 50%
NaOH solution was added. If
the pH was greater than or equal to 7.9, the addition of 50% NaOH was stopped.
After 43 kg of the 50%
NaOH solution had been added, no further 50% NaOH solution was added; however,
the reaction mixture
was continuously sparged until the pH was about pH 7.4 ( 0.1). At this point,
sparging was stopped.
Additional 50% NaOH was then added to the reaction mixture until the reaction
mixture was pH
8.5 (that of the initial seawater). The overhead stirrer was subsequently
stopped and the contents of the
reaction vessel were transferred to a settling tank. The reaction mixture (a
slurry) was then allowed to sit for
more than 1.5 hours, allowing for the precipitation material to settle under
the action of gravity.
Elapsed Time pH Gas Flow Status C02 Flow Rate (scfm) Air Flow Rate (scfm) Base
Weight (kg) 1
0:00 8.47 On 5 20 0
1:05 7.88 On 5 20 17.5
1:27 7.94 On 5 20 25
1:32 8.04 On 5 20 27.5
1:43 7.95 On 5 20 30.5
1:53 7.94 On 5 20 33.5
2:00 7.00 On 5 20 0
2:00 8.22 On 5 20 40.5
2:16 8.00 On 5 20 43
2:20 7.90 On 5 20 43
2:28 8.00 Off 5 20 45
2:30 8.44 Off 5 20 48.5
Table [[#]]: Detailed data for reaction.
After settling, the precipitation material was separated from the supernatant
and dewatered by
filtration (filter press). A portion of the precipitation material was
subsequently oven-dried in sheet pans at
110 C for 48 hours, crushed by hand, and ground in a blender.
As evidenced in Table 1 below, the precipitation material produced by the
above method had a
Mg:Ca weight ratio of about 1:7.
Na Mg Al Si S Cl K Ca Fe
Weight % 1.30 4.17 0.46 0.87 0.09 1.26 0.09 28.43 0.26
Table 1: XRF elemental analysis of MLD6P00006-204 sample
The XRD analysis (Fig. 19) of the oven-dried precipitation material indicated
the presence of
aragonite (CaCO3) as a major phase, halite (NaCI), and some magnesium calcite
(Mg,,Ca(l_,,)C03 with x-4%
molar) and hydromagnesite (Mg5(C03)4(OH)2.4H20) as minor components.
64
CA 02670049 2009-07-02
% H2O % C02
Weight % 5.58 38.46
Table 2 % CO2 content (coulometry) and calculated % H2O from TGA
Figs. 20-22 provide spectra and images of the precipitation material: Fig. 20
provides a TGA of the
precipitation material; Fig. 21 provides an FT-IR of the precipitation
material; and Fig. 22 provides SEM
images of the precipitation material at 250x (left) and 4000x (right).
As described for Example 2 above, the steel molds of the Wabash hydraulic
press were cleaned
and the platens were preheated such that the platen surfaces were at 90 C for
a minimum of 2 hours.
The oven-dried precipitation material was then crushed and ground in a blender
such that the
ground material passed a No. 8 sieve. The ground material was then mixed with
water resulting in a mixture
that was 90% solids with the remainder being the added water.
A 4" x 8" mold in the Wabash press was filled with the wet mixture of ground
precipitation
material and a pressure of 60 tons was applied to the precipitation material
for about 10 seconds. The
pressure was then released and the mold was reopened. Precipitation material
that stuck to the sides of the
mold was scraped and moved toward the center of the mold. The mold was then
closed again and a pressure
of 60 tons was applied for a total of 5 minutes. The pressure was subsequently
released, the mold was
reopened, and the pressed precipitation material (now aggregate) was removed
from the mold and cooled
under ambient conditions. Optionally, the aggregate may be transferred from
the mold to a drying rack in a
110 C oven and dried for 16 hours before cooling under ambient conditions.
The aggregate was moderately easy to break and grind for analytical
preparation.
Figs. 23-26 provide spectra and images of the aggregate: Fig. 23 provides XRD
spectra for the
aggregate and the precipitation material from which the aggregate was
prepared; Fig. 24 provides an FT-IR
of the aggregate; Fig. 25 provides a TGA of the aggregate; and Fig. 25
provides SEM images of the
aggregate at 1000x (left) and 4000x (right).
As evidenced by Figs. 23-25, a compositional change did not result from
pressing and subsequent
drying of the precipitation material. The SEM images appear to indicate a
packing of the particles in the
aggregate, but limited to absence of formation of a matrix.
Example 7: Aggregate formed by extruding the precipitate
In this example, a sample of precipitated carbonates prepared essentially as
described in Example
1 and comprising nesquehonite and aragonite and containing approximately 60%
by weight water was
placed into a heated, vented 1.5 inch diameter barrel extruder. The extruder
was heated to approximately
220 C, and the material was placed in the extruder for approximately five
seconds. The opening of the
extruder exit die was 0.375 inch. Material was obtained from the extruder
comprising hydromagnesite and
calcite as well as the starting minerals with a water content of less than
10%. However, much of the
material lithified prematurely within the extruder to produce a cake mass.
This caked mass was
subsequently oven-dried at 60 degrees C to produce a hard, friable mass that
was broken into fine
aggregate particles.
Example 8: Aggregate formed by wet milling the precipitate with ethanol
CA 02670049 2009-07-02
In this example an aggregate was prepared by wet-milling the precipitate with
ethanol. In
preparing this example, a sample of precipitated carbonate prepared
essentially as described in
Example 1 above was filtered on a standard industrial filter press to produce
a filter cake that was
approximately 50% solids. A 10% ethanol w/w solution was added to the
precipitate and the
mixture was ball milled for 2-24 hours. The milled precipitate was then dried
in a fume hood in
ambient air overnight The resultant product obtained was a dense, self-
consolidated sheet that was
broken up into fragments suitable for coarse or fine aggregates. The Mohs
hardness of the product
was at least 2.
Example 9: Fine synthetic aggregate from carbonate precipitate
Fine Synthetic Aggregate (FSA) is a synthetic aggregate similar to sand
particles and is prepared
from the present precipitated carbonate using methods as described herein. FSA
is intended to be blended
into concrete mixes and can replace a portion or all of the fine aggregate
(sand) in concrete mixes to
balance with its sequestered carbon content the emitted carbon content of the
Portland cement. Usages are
expected to be several hundred pounds per cubic yard, as each 100 pounds of
portland cement will require
about 200 pounds of FSA to make carbon neutral concrete. A 6 sack mix with 50%
fly ash will require 564
pounds of FSA to be carbon neutral; at 25% flyash 846 pounds; with 100% OPC
1128 pounds will be
required. Typical sand contents of concrete are 1100 - 1600 pounds.
Use of FSA to produce carbon-reduced or carbon-neutral concrete will assist
the concrete industry
in meeting burgeoning greenhouse gases reduction legislation.. Use of FSA
could provide innovation
carbon credits as well as the recycled materials credit. Because FSA is a
filler replacing another filler,
acceptance is expected be much quicker and easier than a product that replaces
a portion of the cementitious
material. FSA can be used in concrete, stucco, gunnite, etc. as a replacement
for sand, in order to reduce or
eliminate the carbon footprint of these products.
Key characteristics of FSA include:
= Calcium and magnesium carbonate composition
= Minimum 45% captured CO2 content
= Particle size range, based on cumulative % passing through the sieve:
o 100 % passing #4 screen (4,750u)
o 95-98 % passing #8 screen (2,360u)
o 65-75 % passing #16 screen (1,180u)
o 40-50% passing #30 screen (600u)
o 10-15% passing #50 screen (300u)
o 0-2% passing the #100 screen (150u)
= Particle size distribution consistent to within 10% lot-to-lot
= Conforms to ASTM C-33
= Flow properties in concrete at carbon-neutral level unchanged or improved
versus sand at similar
water contents
= Strength properties in concrete at carbon-neutral level unchanged or
improved versus sand at
similar water contents
66
CA 02670049 2009-07-02
= Durability properties (ASR, freeze-thaw, etc.) in concrete at carbon-neutral
level unchanged or
improved versus sand at similar water contents
= Shrinkage properties in concrete at carbon-neutral level unchanged or
improved versus sand at
similar water contents
= Finishability of concrete at carbon-neutral level unchanged or improved
versus sand at similar
water contents
= Leachable NaCl content <0.1 %
= Be stable during storage and transport
Example 10: Coarse synthetic aggregate from the present carbonate precipitate
Coarse Synthetic Aggregate (CSA) designates an aggregate with a particle size
range of 1/4" to 1
1/2". CSA prepared by methods as described herein and is intended to be used
where natural coarse
aggregate is currently used. Largest uses will be in road bases, asphalt and
concrete. Use of CSA to
produce carbon-reduced or carbon-neutral concrete assists the concrete
industry in meeting green house
gases reduction legislation such as CA AB32. Use of CSA could provide carbon
credits as well as the
recycled materials credit. Because CSA is a filler replacing another filler,
acceptance is much quicker and
easier than a product that replaces a portion of the cementitious material.
CSA can be used where similarly graded gravel or crushed stone are used.
Silicaceous CSA
produced at plants using flyash or mafic minerals as cation sources may be
restricted to roadbase and
asphalt usage. CSA is intended to be used in any way which natural coarse
aggregate is currently used.
Largest uses will be in road bases, asphalt and concrete.
Based on plant location and cation / base source, two grades of CSA are
available. One is a 100%
carbonate material (Carbonate CSA) that will be suitable for all uses. The
other grade (Silaceous CSA) will
only be used in asphalt and road base due to the potential for Alkali-Silica
reactivity (ASR) if used in
concrete.
Key characteristics of FSA include:
= Meets industry standards (ASTM C033) for coarse limestone aggregate
= Meets Caltrans specifications for coarse aggregate for concrete, asphalt and
road base
= Minimum 44% captured CO2 content in all-carbonate CSA
= Minimum 30% captured CO2 content in silaceous CSA
= Consistent gradation
= Does not reduce workability, mechanical properties, shrinkage or durability
of road base, asphalt
or concrete versus conventional coarse aggregate.
= Leachable NaCI content <0.1% for carbonate CSA used in concrete applications
= Be stable during storage and transport in an uncovered, exposed to the
elements setting
Example 11: Measurement of S13C value for a solid precipitate
A solid precipitate comprising carbonates was produced from seawater by
bubbling commercially
available CO2 (Praxair) through the seawater followed by adjustment of the pH.
Two precipitates were
produced in two different procedures (P00361 and MLD 13). Unlike atmospheric
gases, air separation is
67
CA 02670049 2009-07-02
not the primary source of carbon dioxide in the bottled gas. Though sometimes
it is derived from directly
combusting a fuel, the most economical way to produce carbon dioxide is to
recover it as a byproduct from
other companies' manufacturing processes or from natural wells. Then it is
purified and liquefied and sold
to customers worldwide. In general, 513C = approx. -30%o to -20%o for bottled
gas from fermentation, and
513C = approx. -40%o to -30%o bottled gas from petroleum sources. Thus, the
bottled gas was expected to be
isotopically light (like flue gas) and in the range of -20%o to -40%o. For
comparison, the 513C value for CO2
in seawater is about 0, that of air no more negative than -10%o, and for
carbonates in natural limestone the
513C value is 3%o. If the carbonates in the precipitate contained
predominantly CO2 from the bottled gas,
their 513C values would be expected to be in the -20%o to -40%o range, as
well, not closer to 0 as for CO2
from seawater or air, or carbonates in natural limestone.
813C values for the two precipitates were measured by mass spectrometry.
Duplicate samples were
run for each precipitate. 513C values that do not correspond with typical
values for natural limestone and
seawater, and that correspond to the isotopcally light CO2 expected to be
found in the bottled gas, were
measured in the precipitates, see table below (5180 values were also
measured):
d13C d180 d13C d180
(%o) (%o) (%o) (%o)
Sample ID uncor StDev uncor StDev corr corr
P00361-001 -29.42 0.01 0 -11.51 0.01 1 -31.44 -12.44
P00361-004 -29.73 0.01 0 -7.84 0.01 0 -31.16 -8.32
MLD13P00001-
105 -27.75 0.01 0 -7.25 0.01 0 -28.40 -7.54
MLD13P00001-
006 -27.66 0.01 0 -7.23 0.00 1 -27.42 -7.28
This example demonstrated that 513C values for carbonate-containing
precipitates produced
according to methods included in the invention can be measured with high
precision, and that such 813C
values are in the predicted negative range for CO2 from industrial sources,
which differentiates it from
carbonates in natural limestones or CO2 from air or seawater.
Example 12. Measurement of S13C value for a solid precipitate and starting
materials
This Example demonstrates precipitation of carbonate material from saline
solution using bottled
carbon dioxide (CO2) and a magnesium rich industrial waste material and
determination of 51BC values for
materials and product. The procedure was conducted in a container open to the
atmosphere.
The starting materials were commercially available bottled CO2 gas, seawater,
and brucite tailings
from a magnesium hydroxide production site as the industrial waste source of
base. The brucite tailings
were approximately 85% Mg(OH)2i 12% CaCO3 and 3% Si02.
A container was filled with locally available seawater (around Santa Cruz,
CA). Brucite tailings
were added to the seawater, providing a pH (alkaline) and divalent cation
concentration suitable for
carbonate precipitation and CO2 gas was sparged into the alkaline seawater
solution. Sufficient time was
allowed for interaction of the components of the reaction, after which the
precipitate material was separated
from the remaining seawater solution, also known as the supernatant solution.
Elevated temperature or
68
CA 02670049 2009-07-02
other special procedures were not used to dry the precipitate carbonate
material. The carbonate material
was characterized using 613C analysis, x-ray diffraction (XRD) analysis, and
scanning electron microscopy
(SEM).
513C values for the process starting materials, precipitate carbonate material
and supernatant
solution were measured. The 513C value for the atmospheric air was not
measured, but a value from
literature is given in Table 3. The analysis system used was manufactured by
Los Gatos Research and uses
direct absorption spectroscopy to provide 513C and concentration data for
gases ranging from 2% to 20%
CO2. The instrument was calibrated using standard gases, and measurements of
travertine and IAEA marble
#20 yielded values that were within measurement error of the accepted values
found in literature. The CO2
source gas was sampled using a syringe. The CO2 gas was passed through a gas
dryer, then into the bench-
top commercially available analysis system. Solid samples, such as the brucite
tailings and precipitate,
were first digested with perchloric acid (2M HC1O4). CO2 gas was evolved from
the digestion, and then
passed into the gas dryer. From there, the gas passed into the analysis
system, resulting in carbon isotopic
fractionation data. This digestion process is shown in Figure 27. Similarly,
the supernatant solution was
digested to evolve CO2 gas that was then dried and passed to the analysis
instrument resulting in 613C data.
Measurements from the analysis of the CO2 source, industrial waste (brucite
tailings), carbonate
precipitate, and supernatant solution are listed in Table 3. The S13C values
for the precipitate and
supernatant solution were -31.98%o and -38.59%o, respectively. The 813C values
of both products of the
reaction reflect the incorporation of the CO2 source (S'3C = -41.39 %o) and
the influence of the brucite
tailings that included some calcium carbonate (S13C = -6.73%o). This Example
illustrates that 613C values
may be used to confirm the primary source of carbon in a carbonate
composition.
TABLE 3
EXPERIMENTAL SOURCE MATERIALS AND VALUES MEASURED
FOR ISOTOPIC FRACTIONATION CHARACTERIZATION
ATMOS- C02 SUPER-
PHERE SOURCE BASE NATANT PRECIPI-
EX- PC C02 S13C BASE PC SOLUTION TATE S13C
AMPLE VALUE SOURCE VALUE SOURCE VALUE
1 0 [ /00] 813C VALUE ~ VALUE
00]
[0/00 /001 1/00
bottled Mg(OH)2
10 -8 gas, -41.39 + Ca(CO)3 -6.73 -38.59 -31.98
source 1 tailings
bottled
gas Mg(OH)2
11 -8 conformin -41.56 + Ca(CO)3 -6.73 -34.16 -30.04
g to NIST tailings
RM8563 2
flue gas
Mg(OH)2
12 -8 from -25.00 + Ca(CO)3 -6.73 -24.8 -19.92
propane
tailings
burner
S02/CO2
13 -8 bottled -12.45 fly ash -17.46 -11.70 -15.88
gas mix
69
CA 02670049 2009-07-02
1. Zeebe, R.E. and Wolf-Galdrow, E., CO in Seawater: Equilibrium, Kinetics,
Isotopes (2005)
Elsevier, San Diego, g. 169.
2. FROM NIST SPECIFICATION RM8563, CO2 Light Isotopic Gas Standard
Example 13: Measurement of 813C value for a solid precipitate and starting
materials
This precipitation was conducted in a 250,000 gallon container. The starting
materials were
commercially available bottled CO2 gas, seawater (from around Santa Cruz, CA),
and brucite tailings as the
industrial waste. The brucite tailings were approximately 85% Mg(OH)2i 12%
CaCO3 and 3% Si02.
The 250,000 gallon container was partially filled with locally available
seawater. Brucite tailings
were added to the seawater, providing a pH (alkaline) and divalent cation
concentration suitable for
carbonate precipitation without releasing CO2 into the atmosphere. CO2 gas was
sparged at a rate and time
suitable to precipitate carbonate material from the alkaline seawater
solution. Sufficient time was allowed
for interaction of the components of the reaction, after which the precipitate
material was separated from
the remaining seawater solution, also known as the supernatant solution. The
carbonate material was
characterized using 813C analysis, x-ray diffraction (XRD) analysis, and
scanning electron microscopy
(SEM).
513C values for the process starting materials, resulting materials and
supernatant solution were
measured. The 813C value for the atmospheric air was not measured, but a value
from literature is given in
Table 3. The analysis system used was manufactured by Los Gatos Research as
described in Example 12.
Measurements from the analysis of the CO2 source, industrial waste (brucite
tailings), carbonate
precipitate, and supernatant solution are listed in Table 3. The 513C values
for the precipitate and
supernatant solution are -30.04%o and -34.16%o, respectively. The 513C values
of both products of the
reaction reflect the incorporation of the CO2 source (S13C = -41.56%o) and the
influence of the brucite
tailings that included some calcium carbonate (S1$C = -6.73%o). The
precipitated carbonate material was
more likely to incorporate calcium carbonate from the brucite tailings than
the supernatant solution, so the
813C value of the precipitate reflects that by being less negative than that
of the supernatant solution. This
Example illustrates that 813C values may be used to confirm the primary source
of carbon in a carbonate
composition.
Example 14: Measurement of 813C value for a solid precipitate and starting
materials
This experiment was performed using flue gas resulting from burning propane
and a magnesium
rich industrial waste material. The procedure was conducted in a container
open to the atmosphere.
The starting materials were flue gas from a propane burner, seawater (from
around Santa Cruz,
CA), and brucite tailings as the industrial waste. The brucite tailings were
approximately 85% Mg(OH)2,
12% CaCO3 and 3% SiO2.
A container was filled with locally available seawater. Brucite tailings were
added to the seawater,
providing a pH (alkaline) and divalent cation concentration suitable for
carbonate precipitation without
releasing CO2 into the atmosphere. Flue gas was sparged at a rate and time
suitable to precipitate carbonate
material from the alkaline seawater solution. Sufficient time was allowed for
interaction of the components
of the reaction, after which the precipitate material was separated from the
remaining seawater solution,
also known as the supernatant solution.
CA 02670049 2009-07-02
S13C values for the process starting materials, resulting precipitate
carbonate material and
supernatant solution were measured. The 513C value for the atmospheric air was
not measured, but a value
from literature is given in Table 3. The analysis system used was manufactured
by Los Gatos Research and
uses direct absorption spectroscopy to provide 513C and concentration data for
gases ranging from 2% to
20% C02, as detailed in Example 12.
Measurements from the analysis of the flue gas, industrial waste (brucite
tailings), carbonate
precipitate, and supernatant solution are listed in Table 3. The 813C values
for the precipitate and
supernatant solution are -19.92%o and -24.8%x, respectively. The S1$C values
of both products of the
reaction reflect the incorporation of the flue gas, CO2 source, (613C = -
25.00%o) and the influence of the
brucite tailings that included some calcium carbonate (S13C = -6.73%o). This
Example illustrates that 813C
values may be used to confirm the primary source of carbon in a carbonate
composition.
Example 15. Measurement of S13C value for a solid precipitate and starting
materials
This experiment precipitated carbonated material from saline solution using a
mixture of bottled
SO2 and bottled carbon dioxide (COZ) gases and a fly ash as an industrial
waste material. The procedure
was conducted in a container open to the atmosphere.
The starting materials were a mixture of commercially available bottled SO2
and CO2 gas
(SO2/CO2 gas), seawater (from around Santa Cruz, CA), and fly ash as the
industrial waste.
A container was filled with locally available seawater. Fly ash was added to
the seawater after
slaking, providing a pH (alkaline) and divalent cation concentration suitable
for carbonate precipitation
without releasing CO2 into the atmosphere. SO2/CO2 gas was sparged at a rate
and time suitable to
precipitate carbonate material from the alkaline seawater solution. Sufficient
time was allowed for
interaction of the components of the reaction, after which the precipitate
material was separated from the
remaining seawater solution, also known as the supernatant solution.
513C values for the process starting materials, precipitate carbonate material
and supernatant
solution were measured as detailed in Example 12.
Measurements from the analysis of the SO2/CO2 gas, industrial waste (fly ash),
carbonate
precipitate, and supernatant solution are listed in Table 3. The 813C values
for the precipitate and
supernatant solution are -15.88%o and -11.70%0, respectively. The 813C values
of both products of the
reaction reflect the incorporation of the S02/C02 gas (S18C = -12.45%0) and
the fly ash that included some
carbon that was not fully combusted to a gas (S13C = -17.46%0). Because the
fly ash, itself a product of
fossil fuel combustion, had a more negative 513C than the CO2 used, the
overall 813C value of the precipitate
reflects that by being more negative than that of the CO2 itself. This Example
illustrates that 513C values
may be used to confirm the primary source of carbon in a carbonate
composition.
Although the foregoing invention has been described in some detail by way of
illustration and
example for purposes of clarity of understanding, it is readily apparent to
those of ordinary skill in the art in
light of the teachings of this invention that certain changes and
modifications may be made thereto without
departing from the spirit or scope of the appended claims.
71
CA 02670049 2009-07-02
Accordingly, the preceding merely illustrates the principles of the invention.
It will be appreciated
that those skilled in the art will be able to devise various arrangements
which, although not explicitly
described or shown herein, embody the principles of the invention and are
included within its spirit and
scope. Furthermore, all examples and conditional language recited herein are
principally intended to aid the
reader in understanding the principles of the invention and the concepts
contributed by the inventors to
furthering the art, and are to be construed as being without limitation to
such specifically recited examples
and conditions. Moreover, all statements herein reciting principles, aspects,
and embodiments of the
invention as well as specific examples thereof, are intended to encompass both
structural and functional
equivalents thereof. Additionally, it is intended that such equivalents
include both currently known
equivalents and equivalents developed in the future, i.e., any elements
developed that perform the same
function, regardless of structure. The scope of the present invention,
therefore, is not intended to be limited
to the exemplary embodiments shown and described herein. Rather, the scope and
spirit of present
invention is embodied by the appended claims.
72