Note: Descriptions are shown in the official language in which they were submitted.
CA 02671221 2016-01-14
-1-
TITLE OF THE INVENTION
BLOOD FLOW MEASURING APPARATUS AND BRAIN
ACTIVITY MEASURING APPARATUS USING THE SAME
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention generally relates to
a blood flow measuring apparatus configured to
accurately measure a blood supply state without
being influenced by an oxygen saturation
concentration of the blood, and to a brain activity
measuring apparatus using the blood flow measuring
apparatus.
2. Description of the Related Art
As apparatuses to measure a blood flow,
for example, there have been brain activity
measuring apparatuses, which are used by wearing a
probe that forms an optical waveguide on a head,
measuring a blood flow of a brain, and displaying an
image of an activity state of the brain on a monitor
(Patent Document 1).
As another brain activity measuring
apparatus, there has been an apparatus including an
optical source to irradiate a living body with light,
a light measuring unit including an optical
transceiver which detects light with plural
wavelengths emitted from the living body, a change
measuring unit to measure a change over time of a
specific component included in the blood according
to a change in an amount of the transmitted light
with the plural wavelengths, and a blood flow
calculating unit to calculate a blood flow according
to the change over time of the specific comodnent
and a proportion of the specific component in the
blood (for example, see Patent Document 2). The
apparatuses disclosed in Patent Documents I and 2
are also called optical topography apparatuses,
CA 02671221 2009-07-09
-2-
whereby plural light emitting parts and light
receiving parts are mounted on a head and an amount
of transmitted light which has propagated inside a
brain is detected by using near-infrared
spectroscopy, so as to map an activity state of a
brain function.
As blood flow measuring apparatuses to
measure a blood flow of parts other than a brain,
there has been an apparatus to measure a presence or
absence of a blood clot. In this apparatus, the
blood layer is irradiated with light and an amount
of light which has transmitted through the blood
layer is measured to detect the blood clot (for
example, see Patent Document 3).
By the methods to measure a blood flow by
using a light emitting part and a light receiving
part which form an optical waveguide, such as those
employed by the apparatuses disclosed in Patent
Documents 1 to 3, a change in amount of light
transmitted through blood has been measured.
However, an amount or density (hematocrit) of red
blood cells, which varies in accordance with a brain
activity, has not been measured. It is known that
hemoglobin (Hb) included in red blood cells has a
property to absorb and scatteringly reflect light,
and its optical characteristics are influenced by a
Hb density, oxygen saturation, and an optical path
length in the blood. Therefore, by the method of
measuring a blood flow by using the light measuring
unit as described above, a measurement result is
changed depending on two conditions: namely,
hemoglobin included in red blood cells and oxygen
saturation (an oxygen amount carried by the red
blood cells).
Therefore, when oxygen saturation of blood
is constant, a blood flow can be accurately measured
based on an amount of transmitted light that depends
CA 02671221 2009-07-09
-3-
on an amount or density (hematocrit) of red blood
cells in the blood. However, when oxygen
consumption is increased or decreased by activities
of a brain and muscles, the oxygen saturation is
changed by an oxygen partial pressure (Pa02), which
changes an optical absorption factor. As a result,
there is a possibility in that a change of the
amount of transmitted light caused by the change of
oxygen saturation is also measured as a change of
the blood flow.
[Patent Document 1] Japanese Patent
Application Publication No. 2003-149137
[Patent Document 2] Japanese Patent
Application Publication No. 2003-144401
[Patent Document 3] Japanese Patent
Application Publication No. 2002-345787
In the case of measuring a blood flow in a
blood vessel for supplying blood to a brain or
muscles by using the measuring apparatuses disclosed
in Patent Documents 1 through 3, it has been
difficult to accurately measure an activity state of
the brain and muscles since the oxygen saturation
changes depending on the oxygen partial pressure in
the blopd, which changes when the brain or muscles
are highly active.
When the activity of the brain becomes
greater, oxygen consumption of the brain increases.
Therefore, multiple capillaries supply blood to the
brain. Thus, a blood flow of a predetermined region,
where plural capillaries are present, is measured
depending on the size of a sensor (diameter of a
probe which forms an optical waveguide). However,
in the case where blood flows with different oxygen
saturations in the plural capillaries, the
conventional blood flow measuring apparatus and
brain activity measuring apparatus have also
detected a change in an amount of transmitted light
1
CA 02671221 2009-07-09
-4-
that is caused by the change of the oxygen
saturation. Therefore, it has been difficult to
accurately measure an activity state of the brain.
In the case of measuring a blood flow in a
blood vessel of other than a brain, it has been
difficult to accurately measure the blood flow when
the oxygen saturation of blood is not constant. It
is because the amount of transmitted light changes
depending on factors of both the density
(hematocrit) or amount of red blood cells and the
oxygen saturation.
in view of the above-described
circumstances, it is an object of at least one
embodiment of the present invention to provide a
blood fLow measuring apparatus that solves the above
problems and a brain activity measuring apparatus
using the blood measuring apparatus.
To solve the above-described problems, the
present invention provides the following measures.
SUMMARY OF THE INVENTION
According to one aspect of the present
invention, a blood flow measuring apparatus includes
a sensor unit including a light emitting part
configured to emit light onto a measurement area and
a light receiving part configured to receive the
light transmitted through the measurement area; at
least one more light receiving part configured to
receive the light transmitted through the
measurement area; and a control part configured to
measure a blood flow state of the measurement area
according to signals outputted by the light
receiving parts. The light emitted by the light
emitting part is received by the light receiving
parts arranged at different distances from the light
emitting part and the light receiving parts output
the signals responsive to the received light. The
CA 02671221 2009-07-09
-5-
control part measures the blood flow state of the
measurement area by performing an arithmetic process
to cancel a component of oxygen saturation in the
blood, said component being included in the signals
outputted by the light receiving parts.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 illustrates a system configuration
diagram showing an embodiment of a brain activity
measuring apparatus using a blood flow measuring
apparatus of the present invention;
FIG. 2A illustrates an enlarged schematic
diagram showing a longitudinal cross section of
attached sensor units 24;
FIG. 2B illustrates a schematic diagram
showing a Longitudinal cross section of a variation
example of the sensor unit 24;
FIG. 3 illustrates a diagram for
describing a principle of a blood flow measuring
method;
FIG. 4 illustrates a graph showing a
relationship between the wavelength of laser light
and optical absorptance in the case where oxygen
saturation of blood is changed;
FIG. 5 illustrates a diagram of a brain
seen from the left side;
FIG. 6 illustrates a diagram for
describing a principle of measuring brain activity
according to blood flow of the brain;
FIG. 7 illustrates a flowchart for
describLng a blood flow measuring process of a brain,
which is performed by a control part 30 of a brain
activity measuring apparatus 100;
FIG. 8 illustrates a flowchart for
describing a measurement data image display process
performed by a measurement data image display
control device 80 of a data managing device 50;
CA 02671221 2009-07-09
-6-
FIG. 9A illustrates a schematic diagram
showing states of a shoulder motor area 352 and an
elbow motor area 354 before measurement;
FIG. 9B illustrates a schematic diagram
showing image data obtained from measurement data in
the case of raising an arm;
FIG. 9C illustrates a schematic diagram
showing image data obtained from measurement data in
the case of raising an arm with an elbow bent;
FIG. 10A illustrates a schematic diagram
showing an optical propagation path of light emitted
by a light emitting part 120;
FIG. 10B illustrates a longitudinal cross-
sectional diagram taken along a line A-A of FIG. 10A,
showing a state right after (elapsed time ti) light
irradiation by the light emitting part 120;
FIG. 10C illustrates a longitudinal cross-
sectional diagram taken along a line A-A of FIG. 10A,
showing a state after an elapsed time t2 from the
light irradiation by the light emitting part 120;
FIG. 10D illustrates a longitudinal cross-
sectional diagram taken along a line A-A of FIG. 10A,
showing a state after an elapsed time t3 from the
light irradiation by the light emitting part 120;
FIG. 11A illustrates a diagram showing a
mounted brain activity measuring apparatus according
to a variation example 1;
FIG. 11B illustrates a block diagram
showing configurations of devices according to the
variation example 1;
FIG. 12 illustrates a diagram showing a
mounted brain activity measuring apparatus according
to a variation example 2;
FIG. 13 illustrates a diagram showing a
mounted brain activity measuring apparatus according
to a variation example 3;
FIG. 14 illustrates a schematic diagram
CA 02671221 2009-07-09
-7-
showing a longitudinal cross section of a variation
example of a sensor unit;
FIG. 15 illustrates a schematic diagram
showing a configuration of a blood flow measuring
apparatus of embodiment 2;
FIG. 16 illustrates a schematic
configuration diagram showing a longitudinal cross-
section of a sensor unit 820 of embodiment 2; and
FIG. 17 illustrates a schematic diagram
showing a configuration of a blood flow measuring
apparatus of embodiment 3.
DETAILED DESCRIPTION OF THE PREFERED EMBODIMENTS
Hereinafter, preferred embodiments of the
present invention are described with reference to
the drawings.
[Embodiment 1]
FIG. 1 is a system configuration diagram
showing an embodiment of a brain activity measuring
apparatus using a blood flow measuring apparatus
according to the present invention. As shown in FIG.
1, a brain activity measuring system 10 includes a
brain activity measuring apparatus 100 and a data
managing device 50 to manage measurement data
collected by the brain activity measuring apparatus
100. Although FIG. 1 shows only one side of the
brain activity measuring apparatus 100, an opposite
side that corresponds to the back side of the
drawing has a similar configuration.
The brain activity measuring apparatus 100
includes a blood flow measuring apparatus 20 mounted
on a head, a control part 30 to measure the activity
state (distribution of red blood cells) of a brain
according Lo detection signals of an amount of
transmitted light that is measured by the blood flow
measuring apparatus 20, and a wireless communication
device 40 to wirelessly send measurement results
CA 02671221 2009-07-09
-8-
(blood flow data) outputted from the control part 30
to an external device.
The control part 30 stores a control
program that performs such arithmetic processing
(see artthmetic expressions described below) as to
cancel a component of oxygen saturation, which is
included in signals obtained from two or more light
receiving parts.
The blood flow measuring apparatus 20
includes plural optical sensor units 24 (241 through
24) which form an optical waveguide by irradiating
a hat-shapad base 22 with light. In this embodiment,
the sensor unit 24 has a diameter of about 10 to 50
mm. Therefore, about 150 to 300 sensor units 24 are
attached in a predetermined arrangement pattern (at
a predetermined interval) on the semispherical base
22. The plural sensor units 24 are independently
managed in advance by address data corresponding to
measuremen-: positions of a subject to be measured.
Measuremen-L data obtained by the sensor units 24 are
sent with respective address data and stored.
The plural sensor units 24 (241 to 24) are
preferably arranged in a matrix at a constant
interval. However, the shape of a head to be
measured is not constant but varies in size and
curved surface shape. Therefore, the sensor units
24 may be arranged at an irregular interval as well.
The brain activity measuring apparatus 10
includes the wireless communication device 40 as an
output unit. Therefore, in this embodiment, the
brain activity measuring apparatus 10 is used in
combination with a data managing device 50 which
manages blood flow measurement data sent from the
wireless communication device 40. However, the
blood flow measurement data may be sent to another
external device as well (for example, an electronic
device such as a personal computer or a device to be
CA 02671221 2009-07-09
-9-
controlled such as an actuator).
The data managing device 50 includes a
wireless communication device 60 which receives the
blood flow measurement data sent from the wireless
communication device 40, a database 70 which stores
the blood flow measurement data obtained from the
wireless communication device 60, a measurement data
image display control device 80 which forms image
data according to the blood flow measurement data
supplied tnrough the database 70, and a monitor 90
to display the image data of the measurement results,
which are generated by the measurement data image
display control device 80.
The data managing device 50, which can
wirelessly communicate with the brain activity
measuring apparatus 100, can be set apart from the
brain activity measuring apparatus 100. For example,
the data managing device 50 can be set in a place
where a subject cannot see the data managing device
50.
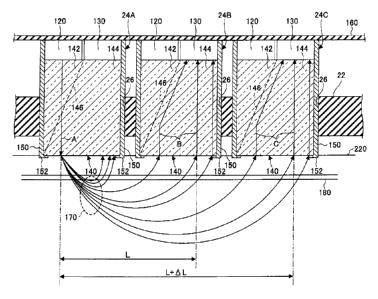
FIG. 2A is an enlarged diagram of an
attachment structure of the sensor units 24. FIG.
2A shows a state where sensor units 24A, 248, and
240 are mounted, among the plural sensor units 24.
As shown in FIG. 2, the sensor units 24A, 248, and
240 are inserted in attachment holes 26 of the
semispherical base 22 which is flexible, and fixed
by an adhesive and the like. Therefore, when the
sensor units 24A, 24B, and 24C are fixed in the
attachment holes 26 of the semispherical base 22,
they are held so that their leading end parts
contact a scalp surface 220 of the subject. The
sensor units 24A, 24B, and 24C have the same
configurations, in which the same components are
denoted by the same reference numerals.
The sensor unit 24 includes a light
emitting part 120 formed of a laser diode for
CA 02671221 2009-07-09
-10-
irradiating the scalp surface 220 with a laser light
(emission light) A, a light receiving part 130
formed Df a light receiving element to output an
electrical signal responsive to an amount of
received transmitted light, and an optical path
separating member 140 formed of a hologram which is
constit-Ated to have different refraction indexes
with respect to the laser light A emitted by the
light emitting part 120 to an area to be measured
(measurement area), and to lights B and C incident
through the measurement area, which proceeds to the
light receiving part 130.
A brain wave measuring electrode 150 for
measuring brain waves is fit on a peripheral surface
of the optical path separating member 140. The
brain wave measuring electrode 150 is formed in a
cylindrical shape over a leading end surface and a
side surface of the optical path separating member
140. A top end of the brain wave measuring
electrode 150 is electrically connected to a wiring
pattern of a flexible wiring board 160.
The top surfaces of the light emitting
part 120 and the light receiving part 130 are
mounted on a bottom surface side of the flexible
wiring board 160. On the flexible wiring board 160,
the wiring pattern connected to the control part 30
is formed. Connecting terminals of the light
emitting part 120 and the light receiving part 130
are electrically connected to the wiring pattern at
positions corresponding to the sensor units 24 by
soldering and the like. The flexible wiring board
160 can be bent in accordance with the shape of a
head when leading ends of the sensor units 24
contact the measurement area. In this manner, the
flexible wiring board 160 is configured so as not to
cause a broken wire when the base 22 is mounted or
detached.
CA 02671221 2009-07-09
-11-
The brain wave measuring electrode 150 has
a contact terminal 152 that is bent inward at a
leading end. The contact terminal 152 protrudes
from an end surface of the optical path separating
member 140. Therefore, when the end surface of the
optical path separating member 140 contacts the
measurement area, the contact terminal 152 also
contacts the measurement area, and can measure the
brain waves. Further, the brain wave measuring
electrode 150 can be also formed over a peripheral
surface and a leading end edge part of the optical
path separating member 140 by a method of applying a
conductive film by a thin film forming method such
as evaporation and plating. Moreover, the brain
wave measuring electrode 150 may be formed of, for
example, a transparent conductive film formed of
indium tin oxide which is called ITO, over the
peripheral surface and leading end edge part of the
optical path separating member 140. When the brain
wave measuring electrode 150 is formed of this
transparen-: conductive film, the brain wave
measuring electrode 150 becomes capable of
transmitting light. Therefore, the entirety of the
peripheral surface and the leading end surface of
the optical path separating member 140 can be
covered with the brain wave measuring electrode 150.
Normally, brain waves cannot be measured
at the same time as measuring the blood flow by
taking a laminagram of the brain and the like.
However, by providing the brain wave measuring
electrode 150 for the sensor unit 24, it becomes
possible to measure the blood flow and brain waves
simultaneously. Thus, it becomes possible to
analyze a correlation between the blood flow and
brain waves of the brain in details.
When measuring the blood flow, the control
part 30 selects an arbitrary sensor unit 24 among
CA 02671221 2009-07-09
-12-
the plural arranged sensor units 24 so as to emit
the laser light A from the light emitting part 120
of the selected sensor unit 24. At this time, the
laser light A emitted from the light emitting part
120 is outputted with a wavelength 805 nm),
which is not influenced by the oxygen saturation.
The sensor units 24 are held with their
leading ends (the end surfaces of the optical path
separating members 140) contacting the measurement
area of a ?lead. The laser light A is incident from
the light emitting part 120 and proceeds through the
optical path separating member 140 toward a scalp of
the head into the brain in an orthogonal direction.
Inside the brain, the laser light A proceeds toward
the center of the brain while the laser light A
propagates toward a periphery along a surface of the
brain from the incident position as a base point.
Optical propagation paths 170 of the laser light A
inside the brain are formed in circular arcs when
seen from a side of the head, pass through a blood
vessel 1180 of the head, and return to the scalp
surface 220.
In this manner, the light which passes
through the optical propagation paths 170 reaches
the sensor units 24B and 24C on a light receiving
side, while changing into transmitted light with an
amount responsive to an amount or density of red
blood cells included in blood which flows through
the blood vessel 180. Further, the laser light A
gradually decreases in the amount of transmitted
light in a process of propagating inside the brain.
Therefore, a light receiving level of the light
receiving part 130 is decreased in proportion to a
distance from the incident position of the laser
light A. Thus, the amount of received transmitted
light also changes depending on the distance from
the incident position of the laser light A.
CA 02671221 2009-07-09
-13-
In FIG. 21\, when the sensor unit 24A
positioned at a left end is used as a base point on
a light emission side, the sensor unit 24A, the
sensor unit 24B adjacent on the right of the sensor
unit 24A, and the sensor unit 24C adjacent on the
right of the sensor unit 24B correspond to base
points on the light receiving side (measurement
points).
The optical path separating member 140 is
formed so as to make the laser light A proceed
straight and guide the incident lights B and C to
the light receiving parts 130 by, for example,
changing the density distribution of a transparent
acrylic resin. Further, the optical path separating
member 140 includes an emission side transmitting
area 142 which lets the laser light A emitted from
the light emitting part 120 transmit from a base end
side (top surface side in FIG. 2A) to a leading end
side (bottom surface side in FIG. 2A), an incident
side transmitting area 144 which lets the light
propagated in the brain transmit from the leading
end side (bottom surface side in FIG. 2A) to the
base end side (top surface side in FIG. 2A), and a
refraction area 146 formed between the emission side
transmitting area 142 and the incident side
transmitting area 144. This refraction area 146 has
a property to transmit the laser light A and reflect
light (incident lights B and C) which has
transmitted through a blood flow. The refraction
area 146 is formed by, for example, changing the
density of the acrylic resin, providing a metal thin
film, and dispersing metal microparticles in this
area. Accordingly, lights incident from the leading
ends of the optical path separating members 140 are
all gathered at the corresponding light receiving
parts 130.
FIG. 2B is a diagram showing a cross
CA 02671221 2009-07-09
-14-
section of a variation example of the sensor unit 24.
As shown in FIG. 2B, a sensor unit 24X of the
variation example is provided with a diffraction
grating 190 at a lower end of the optical path
separating member 140. A bottom surface side
peripheral edge part of the diffraction grating 190
is held by the contact terminal 152 which is formed
by bending the leading end of the brain wave
measuring electrode 150 inward. The diffraction
grating 193 has a pattern with fine protrusions and
recesses on front and back surfaces. The
diffraction grating 190 is an optical element
constit..ited so that incident light from the scalp
surface 223 is diffracted toward the light receiving
part 130 by a diffraction effect when passing
through a border part of the pattern with
protrusions and recesses.
Here, a principle of a blood flow
measuring method is described.
FIG. 3 is a diagram for describing the
principle of the blood flow measuring method. As
shown in FIG. 3, when blood is irradiated with the
laser light A externally, the laser light A incident
into a blood layer 230 transmits through the blood
as light having both components: namely, a normal
light component scatteringly reflected by red blood
cells 210 and a light component scatteringly
reflected by an attached blood clot.
In transmitting through the blood layer
230, the laser light A receives an influence that
constantly changes depending on the state of the
blood. Therefore, by continuously measuring an
amount of transmitted light (may be an amount of
reflected light) to observe the change of the amount
of light, changes of various properties of the blood
can be observed.
When the activity of the brain increases,
CA 02671221 2009-07-09
-15-
the brain consumes more oxygen. Therefore, the
blood flow state, which is changed by the hematocrit
of red blood cells which carry oxygen and oxygen
saturation of the blood, causes a change of the
amount of right.
Here, changes of the hematocrit (Hct: a
volume ratio of red blood cells per unit volume,
that is, a volume concentration of red blood cells
per unit volume, also referred to as Ht) and the
like are also related to a change of the density of
hemoglobin and influence the change of the amount of
light. A basic principle of this embodiment is to
use the laser light A to measure a blood flow state
according to a change of an optical path and an
amount of transmitted light in the blood flow, and
further to measure the activity of a brain according
to the blood flow state of the brain.
1, configuration of the present invention
is described below. Optical characteristics of
blood are determined by blood cell components
(especially hemoglobin in erythroid cells).
Moreover, red blood cells have a property in that
hemoglobin is easily coupled to oxygen. Therefore,
the red blood cells also have a role to carry oxygen
to brain cells. Oxygen saturation in blood is a
value that indicates a percentage of hemoglobin
coupled to oxygen in the blood. The oxygen
saturation which is correlated to an oxygen partial
pressure (Pa02) in arterial blood is an important
index for a respiratory function (gas exchange).
It is known that the oxygen saturation is
increased when the oxygen partial pressure becomes
higher. When the oxygen saturation changes, the
amount of light which transmits through blood
changes as well. Therefore, a blood flow can be
accurately measured by removing the influence of the
oxygen saturation.
CA 02671221 2009-07-09
-16-
As factors having influences on the oxygen
partial pressure (Pa02), there is alveolar
ventilation. Further, there are environmental
factors such as atmospheric pressure and a fraction
of inspiratory oxygen (Fi02), and gas exchange in
alveolar such as a ventilation/blood flow ratio, gas
diffusion capacity, and a shunt rate.
The control part 30 includes an arithmetic
unit which processes signals responsive to the
amounts of transmitted light (light intensities),
which are generated by the light receiving parts 130
of the sensor units 24A, 24B, and 24C. This
arithmetic unit performs an arithmetic process to
detect a blood flow state according to measurement
values outputted by the light receiving parts 130 of
the sensor units 24B and 24C as described below.
The laser light A is emitted by the light
emitting part 120 as a pulsed light that is emitted
intermittently at a predetermined time interval (for
example, 13 Hz to 1 MHz) or a continuous light. In
this case, when the pulsed light is employed as the
laser light A, a pulse frequency at which the pulsed
light flashes is determined by the speed of the
blood flow. In that case, measurement is performed
continuously or at a measurement sampling frequency
which is twice or more of the pulse frequency of the
laser ligh7.. A. When the continuous light is
employed as the laser light A, measurement is
conducted at a measurement sampling frequency
determined by the speed of the blood flow.
Hemoglobin (Hb) in blood chemically reacts
with oxygen in lungs by respiration and become Hb02;
thereby the oxygen can be taken into the blood.
Depending on respiration and the like, however, the
degree of oxygen taken into the blood (oxygen
saturatdon is slightly different. That is, in
connection with the present invention, such a
CA 02671221 2009-07-09
-17-
phenomenon was found that when light is emitted into
blood, optical absorptance of the blood changes
depending on the oxygen saturation. This phenomenon
is a disturbance element in measurement of a blood
flow using the laser light A. Thus, the influence
of the oxygen saturation is to be removed in the
present invention.
FIG. 4 is a graph showing a relationship
between a wavelength of the laser light A and
optical absorptance of the case where the oxygen
saturation of blood is changed. Hemoglobin included
in red blood cells is divided into hemoglobin oxide
coupled to oxygen (Hb02: graph II) and hemoglobin
that is no oxidized (Hb: graph I) in a living body.
Hemoglobin in these two states exhibit quite
different optical absorptances with respect to light.
For example, blood including sufficient oxygen is
bright-colored as fresh blood. On the other hand,
venous blood is dark colored since oxygen is
released. These optical absorptances vary in a wide
optical wavelength range as shown by the graphs I
and II in FIG. 4.
It is found that a blood flow can be
measured by irradiating blood with light without
having an influence on the optical absorptance by
selecting a specific wavelength from the graphs I
and II in FIG. 4 even when the oxygen saturation of
hemoglobin in red blood cells largely changes by
oxygen metabolism in a living body and the like.
Regardless of the oxygen saturation of
hemoglobin in red blood cells, the optical
absorptance is small in a certain wavelength range.
In this manner, it is determined whether the light
at a wavelength 4 easily transmits through a blood
layer. Therefore, when light in a predetermined
wavelength range (for example, 4 = about 800 nm to
about 1300 nm) is used, a blood flow can be measured
CA 02671221 2009-07-09
-18-
by suppressing an influence of the oxygen saturation.
Therefore, the laser light A in a
wavelength range of about 600 nm to about 1500 nm is
used in the present invention. Accordingly, the
optical absorptance of hemoglobin (Hb) can be
practically kept low enough. Moreover, since this
range includes an isosbestic point X, the isosbestic
point can be determined through calculation by using
measurement points of two wavelengths or more. That
is, a specification which is not influenced by the
oxygen saturation can be made. In other wavelength
ranges, S/N (Signal to Noise ratio) is decreased
since the optical absorptance increases when X. =
less than 600 nm. When X = more than 1500 nm, a
light receiving sensitivity of the light receiving
part 130 is not sufficient and there is an influence
of a disturbance such as other components in blood.
Thus, a measurement with high precision cannot be
performed in this case.
Therefore, in this embodiment, a light
emitting element formed of a wavelength variable
semiconduc:or laser is used as the light emitting
part 120. Wavelengths of the laser light A emitted
by the light emitting part 120 are set at ?A - 805
nm (first light) which has the isosbestic point X in
graphs 1 and II and at X2 = 680 nm (second light) at
which the optical absorptance is the lowest in graph
I.
Here, a description is made of a method
for detecting red blood cell concentrations R, Rp,
and Rpw. In this method, the red blood cell
concentrations R, Rp, and Rpw are detected according
to the amounts of transmitted light in the case of
receiving the laser light A propagated through the
optical propagation path 170 (see FIG. 2A).
An arithmetic expression (1) of the red
blood cell concentration R by using a one-point-one-
,
CA 02671221 2009-07-09
-19-
wavelength method employed in a conventional
measuring method can be expressed as the following
expression.
loglO(Iin/Iout) = f(Iin, L, Ht) _ (1)
3y the method as expressed in expression
(1), the red blood cell concentration corresponds to
a function of an amount Iin of incident transmitted
light of the laser light A emitted by the light
emitting part 120, a distance (optical path length)
L between the light emitting part 120 and the light
receiving part 130, and the hematocrit (Ht). lout
denotes an amount of transmitted light of the laser
light A received by the light receiving part 130.
Therefore, it is difficult to accurately calculate
the red blood cell concentration by the method of
expression (1) since the red blood cell
concentration changes depending on the above-
described three factors.
An arithmetic expression (2) of the red
blood cell concentration Rp by using a two-point-
one-wavelengh method according to this embodiment is
expressed as the following expression.
Rp = loglOtIout/(Iout - AIout)1 = oaci (AL,
Ht) (2)
By the method as expressed in expression
(2), propagated light of the laser light A is
received at two points (the light receiving parts
130 of the sensor units 24B and 24C) set at
different distances from the incident point of the
laser light A as shown in FIG. 2. In the expression,
lout denotes an amount of light received by the
light receiving part 130 which is closer to the
light emitting part while (lout - About) denotes an
amount of Light received by the light receiving part
130 which :s further from the light emitting part
120, in which Abut denotes a difference (change) in
the amount of received light between the two light
CA 02671221 2009-07-09
-20-
receiving parts 130. Therefore, the red blood cell
concentration Rp is obtained as a function of a
distance AL between the two light receiving parts
130 and the hematocrit (Ht). Thus, since the
distance AL between the two light receiving parts
130 is known in advance among the two factors, the
red blood cell concentration is measured as a value
having the hematocrit (Ht) as a coefficient, in the
case of using expression (2) to calculate the red
blood cell concentration. Accordingly, by this
calculating method, the red blood cell concentration
can be accurately measured as a measurement value
responsive to the hematocrit (Ht).
Further, an arithmetic expression (3) of
the red blood cell concentration Rpw by using a two-
point-two-wavelength method according to a variation
example of this embodiment can be expressed as the
following expression.
Rpw = (loglOtIout/(Iout -
About)1X1]/[loglO{bout/(Iout - About))X2] = (Ht)
...(3)
By the method of expression (3),
wavelengths of the laser light A emitted by the
light emitting part 120 are set as Xl and X2 (X1 =
805 nm while X2 = 680 nm in this embodiment), which
are different from each other. In the expression,
bout denotes an amount of light received by the
light receiving part 130 which is closer to the
light emitting part while (bout - About) denotes an
amount of light received by the light receiving part
130 which is further from the light emitting part
120, in which Abut denotes a difference (change) in
the amount of received light between the two light
receiving parts 130. Accordingly, the red blood
cell concentration Rpw is calculated as a function
of only the hematocrit (Ht). Therefore, according
to this caiculating method, the red blood cell
CA 02671221 2009-07-09
-21-
concentration can be accurately measured as a
measurement value responsive to the hematocrit (Ht).
Here, a brain to be used as a measurement
area is described. FIG. 5 is a diagram of a brain
seen from its left side. As shown in FIG. 5, a
brain 300 of a human includes a cerebrum 301, a
cerebellum 302, and a brainstem 303. The cerebrum
301 is a nerve center that controls motor functions
of the human body. A cerebral cortex is divided
into motor areas corresponding to the parts of the
human body (joints of hands, elbows, shoulders, back,
knees, ankles, and the like). For example, the
brain 3D0 includes a prefrontal area 330, a premotor
area 340, a motor area 350, a somatic sensory area
360, and the like. Moreover, the brain 300 has a
frontal eye field 332, a Broca's area 334, and an
olfactory area 336. The premotor area 340 has a
motor association area 342.
Further, the motor area 350 manages
movemen-:s of hands and feet. For example, the motor
area 350 includes a shoulder motor area 352 and an
elbow motor area 354. Therefore, by measuring blood
flows of the shoulder motor area 352 and the elbow
motor area 354 and mapping changes of the blood
flows in each area, it can be detected how the
shoulder and elbow are going to be moved.
FIG. 6 is a diagram showing a principle of
measurement of brain activity according to a blood
flow of the brain. As shown in FIG. 6, the brain
300 is covered with spinal fluid 400, a skull bone
410, and a scalp 420. The leading end surfaces of
the optical path separating members 140 of the
sensor units 24 are made to contact the scalp 420 so
as to measure blood flows. The laser light A
emitted by the light emitting part 120 of the sensor
unit 24A proceeds into the brain 300 through the
scalp 420, the skull bone 410, and the spinal fluid
CA 02671221 2009-07-09
-22-
400. The light emitted onto the head propagates in
directions of an arcuate pattern 440 (directions of
depths and radii) as shown by broken lines in FIG. 6.
When an optical propagation path of the
laser light A becomes longer in accordance with a
distance in the direction of the radius from a base
point 450 on which the laser light is emitted, light
transmittance becomes lower. Therefore, the sensor
unit 2413, which is arranged adjacent to and at a
predetermined distance from the sensor unit 24A on a
light emission side, has a high light receiving
level (amount of transmitted light). The sensor
unit which is provided adjacent to and at a
predetermined distance from the sensor unit 24B, has
a light receiving level (amount of transmitted
light) that is lower than that of the sensor unit
24B. Furtner, a light receiving part of the sensor
unit 24A on the light emission side also receives
light from the brain 300. Detection signals
responsive to the intensities of light received by
the plural sensor units 24 undergo a mapping
process; thereby an optical intensity distribution
responsive to the change of blood flow is obtained
in a form of a striped graphic (contour lines).
When the detection signals (signals
responsive to the amount of received transmitted
light) outputted by the sensor units 24 are used as
lout of expression (2) or (3), the red blood cell
concentration can be accurately measured as a
measurement value responsive to the hematocrit (Ht)
(that is, as a value which is not influenced by the
oxygen saturation).
Here, a measurement process of the blood
flow (blood flow measurement process) of a brain,
which is performed by the control part 30 of the
brain activity measuring apparatus 100, is described
with reference to FIG. 7. As shown in FIG. 7, the
CA 02671221 2009-07-09
-23-
control part 30 performs the blood flow measurement
process by dividing the cerebral cortex into
measurement blocks corresponding to motor areas.
For example, the control part 30 performs the blood
flow measurement processes of measurement blocks of
the prefrontal area 330, the premotor area 340, the
motor area 350, and the somatic sensory area 360 in
parallel. Here, for example, a description is made
of the case of performing a blood flow measurement
of the motor area 350 and performing a mapping
process of the activity state of the motor area 350.
First, in step S11, the control part 30
selects an arbitrary sensor unit 24A (sensor unit
with an address number n = 1) among the plural
sensor units 24 and makes the light emitting part
120 of the sensor unit 24A emit a laser light onto a
measurement area (head area containing the motor
area 353). Subsequently, in step S12, a detection
signal (electric signal responsive to an amount of
received transmitted light) outputted by the light
receiving part 130 of the sensor unit 24B with an
address number n n + 1, which is adjacent to the
address number n = 1, is sent from the wireless
communication device 40 to the data managing device
50. The data managing device 50 stores data of the
sensor uni-.1 24B with the address number n = n + 1,
which is obtained from the wireless communication
device 60, in the database 70.
:n subsequent step S13, a detection signal
(electric signal responsive to an amount of received
transmitted light) outputted by the light receiving
part 130 of the sensor unit 240 with an address
number n = n + 2, which is adjacent to the address
number n + 1, is sent from the wireless
communication device 40 to the data managing device
50. The data managing device 50 stores data of the
sensor unit 24C with the address number n = n f' 2,
CA 02671221 2009-07-09
-24-
which is obtained from the wireless communication
device 60, in the database 70.
In this manner, detection signals of all
the sensor units 24 arranged around the sensor unit
24A which emits the laser light A as a base point,
are sent to the data managing device 50.
In step S14, an address of the sensor unit
to serve as a light emission point (base point) is
changed to n -I- 1. In step S15, it is determined
whether all the sensor units 24 have emitted light.
When all the sensor units 24 have not completed
light emission in step S15, the laser light A is
emitted by the light emitting part 120 of the sensor
unit 24B having the address number n 1, and the
processes of steps Sll to S15 are repeated.
In addition, in step S15, when all the
sensor units 24 have completed light emission, the
blood flow measurement process of this measurement
block may be finished, or performed again from the
beginning.
Here, with reference to FIG. 8, a
description is made of an image display process of
measurement data, which is performed by the
measurement data image display control device 80 of
the data managing device 50. The measurement data
image dispLay control device 80 reads in the
measurement data (data of an amount of transmitted
light responsive to a blood flow) stored in the
database 70 in step S21 of FIG. 8. In step S22, the
red blood cell concentration Rp or Rpw is calculated
by using the measurement data and arithmetic
expression (2) or (3).
7n step S23, a distribution map (line map
formed of contour lines) of the red blood cell
concentrations at each measurement point is formed
and image data of the distribution map are stored in
the database 70. In step S24, it is determined
CA 02671221 2009-07-09
-25-
whether the calculations of the red blood cell
concentration Rp or Rpw of all the measurement
points are completed. When the blood cell
concentrations Rp or Rpw of all the measurement
points have not been completed in step S24, the
operation returns to step S21 to repeat the process
from step S21.
When the red blood cell concentrations Rp
or Rpw of all the measurement points are completed
in step S24, the operation proceeds to step S25. In
step S25, a brain activity state view showing a
distribution of the red blood cell concentrations is
displayed on a monitor 90.
In this manner, the red blood cell
concentration Rp or Rpw is calculated from the
measurement data according to the blood flow
measured by the brain activity measuring apparatus
100, and the brain activity state based on a red
blood cell concentration distribution of the
measurement block is displayed on the monitor 90.
Therefore, the brain activity state of the
measurement area can be accurately determined.
Here, a description is made of a display
example of image data displayed by the measurement
data image display control device 80. The image
data are obtained as a measurement result of an
amount of a blood flow (red blood cell
concentration) of a brain by analyzing the
measuremen: data sent from the brain activity
measuring apparatus 100. FIG. 9A is a schematic
diagram of the states of the shoulder motor area 352
and the elbow motor area 354 before measurement.
FIG. 9B is a schematic diagram showing image data
based on measurement data obtained when an arm is
going to be raised. FIG. 9C is a schematic diagram
showing image data based on measurement data
obtained when an arm is going to be raised with an
CA 02671221 2009-07-09
-26-
elbow bent.
As shown in FIG. 9A, the shoulder motor
area 352 (area indicated by a broken line) of the
brain 300 has adductor areas 352a and abductor areas
352b. The elbow motor area 354 (area indicated by a
broken line) has flexion areas 354a and an extension
area 354b of an elbow.
As shown in FIG. 9B, for example, when the
brain 300 makes an order to raise an arm, image data
of an activity area 360, that look like contour
lines having the adductor areas 352a and abductor
areas 352b of the shoulder motor area 352 as centers,
are formed and displayed on the monitor 90. In this
image data of the activity area 360, a dense part
surrounded by many lines indicates high light
intensity, which means that there is much blood flow.
On the other hand, a coarse part surrounded by fewer
lines indicates low light intensity, which means
that there is little blood flow. As shown in the
drawing of FIG. 9B, brain activities of the adductor
areas 352a and the abductor areas 352b of the
shoulder motor area 352 are activated. Thus, it can
be known that the brain 300 is making an order to
raise the arm.
As shown in FIG. 9C, for example, when the
brain 300 makes an order to raise the arm with the
elbow bent, image data of an activity area 370, that
looks like contour lines having the adductor areas
352a and the abductor areas 352b of the shoulder
motor area 352, and the flexion areas 354a of the
elbow motor area 354 as centers, are formed and
displayed on the monitor 90. In this activity area
370, a dense part surrounded by many lines indicates
high light intensity, which means there is much
blood flow. On the other hand, a coarse part
surrounded by less lines indicates low light
intensity, which means that there is little blood
CA 02671221 2009-07-09
-27-
flow. As shown in the drawing of FIG. 9C, brain
activities of the adductor areas 352a and the
abductor areas 352b of the shoulder motor area 352
and the flexion area 354a of the elbow motor area
354 are activated. Thus, it can be known that the
brain 300 is making an order to raise the arm with
the elbow bent.
Here, display examples of the measurement
results of a blood flow in the direction of depth
are described with reference to FIGS. 10A to 10D.
FIG. 10A is a schematic diagram of an optical
propagation path of light emitted by the light
emitting part 120. FIG. 10B is a longitudinal
cross-sectional diagram taken along a line A-A of
FIG. 10A, which shows a state right after (elapsed
time tl) the light irradiation by the light emitting
part 120. FIG. 10C is a longitudinal cross-
sectional diagram taken along the line A-A, which
shows a state after an elapsed time t2 from the
light irradiation by the light emitting part 120.
FIG. 100 is a longitudinal cross-sectional diagram
taken along the line A-A, which shows a state after
an elapsed time t3 from the light irradiation by the
light emitting part 120.
As shown in FIG. 10A, the laser light A
emitted by the light emitting part 120 propagates,
for example, by tracking a substantially arcuate
trajectory as shown by the three optical propagation
paths 170. Moreover, in FIGS. 10B through 10D,
changes of light intensity at measurement points Al,
Al, and A3, where the three optical propagation
paths 170 and the line A-A intersect, are shown as
images.
As shown in FIG. 10B, in the optical
propagalion paths 170 right after (elapsed time tl)
the liOt irradiation by the light emitting part 120,
it is seen that a blood flow amount (intensity of
CA 02671221 2009-07-09
-28-
received light) at the measurement point A3 is
detected to be the most.
As shown in FIG. 10C, in the optical
propagation paths 170 after the elapsed time t2 from
the light irradiation by the light emitting part 120,
it is seen that a blood flow amount (intensity of
received light) at the measurement point A2 is
detected to be the most.
As shown in FIG. 10D, in the optical
propagation paths 170 after the elapsed time t3 from
the light irradiation by the light emitting part 120,
it is seen that a blood flow amount (intensity of
received light) at the measurement point Al is
detected to be the most.
In this manner, a distribution of amounts
of blood flow in the direction of the depth can be
measured according to the amounts of transmitted
light at tie measurement points Al, A2, and A3
arranged in the direction of the depth on the
optical propagation paths 170. For example, in the
cases of FIGS. 108 through 10D, it can be measured
that the point at which there is the most amount of
blood flow moves from inside the brain to a surface
layer part of the brain over time.
Next, variation examples of the brain
activity measuring apparatus 100 are described.
FIG. 11A is a diagram showing a mounted
brain activity measuring apparatus 100A according to
a variation example 1. As shown in FIG_ 11A, a
blood flow measuring apparatus 20A of the brain
activity measuring apparatus 100A according to the
variation example 1 has a spherically formed net-
like base 22A to which plural sensor units 24 are
attached. Although FIG. 11A shows only one side of
the brain activity measuring apparatus 100A, an
opposite side that corresponds to the back side of
the drawing has a similar configuration.
1
CA 02671221 2009-07-09
-29-
The sensor units 24 are held passing
through intersection parts of the net of the base
22A. Further, square-shaped coupling structures of
the net-like base 22A are stretched and deformed
into diamond shapes in accordance with the shape of
a head surface on which the net-like base 22A is
mounted. Therefore, the net-like base 22A can be
deformed into a spherical shape corresponding to the
shape of the head surface.
The net-like base 22A has (four to eight)
net arm parts connected to the intersection parts,
which are formed of a resin material having
elasticity. Due to the elasticity of the material
itself, end parts of the plural sensor units 24 can
be tightly attached onto the head surface on which
the net-like base 22A is mounted. Regardless of the
shape of the head surface, the leading end parts of
the plural sensor units 24 can be made to contact
the head surface which is an object to be measured.
In the variation example 1, the sensor
unit 24 has a diameter of about 10 mm to 50 mm.
Therefore, about 150 to 300 sensor units 24 are
attached on the net-like base 22A in a predetermined
arrangement pattern (at a predetermined interval).
The plural sensor units 24 are independently managed
in advance by address data corresponding to
measurement positions of the object to be measured
in a manner similar to the embodiment 1. The
measurement data obtained by the sensor units 24 are
sent with respective address data to the data
managing device 50 and stored.
The net-like base 22A is partitioned into
plural blocks A through N, which have respective
small wtreless communication devices 400A through
400N (shown as black circles in FIG. 11A). The
measureEent data obtained by the plural sensor units
24 can he sent independently from the wireless
CA 02671221 2009-07-09
-30-
communicatton devices 400A through 400N of the
blocks A through N to the data managing device 50.
FIG. 113 is a block diagram showing
configurations of devices of the variation example 1.
As shown in FIG. 113, the plural sensor units 24 are
classified by, for example, blocks A through N that
partition the brain 300 according to functions. For
example, the sensor units 24 are grouped into sensor
units 24A1 through 24An, 2431 through 24Bn, _ 24N1
through 24Nn. The wireless communication devices
400A through 400N provided in the blocks A through N
send and receive wireless signals to/from the data
managing device 50. Upon receiving an order of
light emission from the data managing device 50, the
wireless communication devices 400A through 400N
output light emission signals to the sensor units 24
of the blocks A through N in parallel. Accordingly,
the light emitting parts 120 of the blocks A through
N sequentially irradiate the head surface
(measurement area) of the blocks with the laser
light. At the same time, measurement data
responsive to the amount of transmitted light
received by the light receiving parts 130 of the
sensor units 24A1 through 24An, 24B1 through 24Bn, _
24N1 through 24Nn provided in the blocks A through N
are sent from the wireless communication devices
400A through 400N to the data managing device 50.
Therefore, in the data managing device 50, the data
of the blocks A through N, which have been measured
by the sensor units 24A1 through 24An, 24BI through
24Bn, _ 24N1 through 24Nn, are processed in parallel.
In this variation example 1, the brain
activity measuring apparatus 100A includes the
plural wireless communication devices 400A through
400N. Therefore, the measurement data measured by
the sensor units 24A1 through 24An, 24B1 through
24Bn, _ 24N1 through 24Nn can be sent in a short
1
CA 02671221 2009-07-09
-31-
time. Moreover, the data managing device 50 can
analyze the measurement data of each of the blocks A
through N and efficiently form image data of each of
the blocks A through N in parallel.
Further, in the net-like base 22A, two
arms of the plural arms connected to the
intersection parts may be formed of a conductive
material and connected to the light emitting part
120 and the light receiving part 130 of the sensor
unit 24 so as to be used for ordering light emission
and detecting the measurement data of the received
light.
FIG. 12 is a diagram showing a mounted
brain activity measuring apparatus 1008 of a
variation example 2. As shown in FIG. 12, a blood
flow measuring apparatus 20B of the brain activity
measuring apparatus 100B according to the variation
example 2 has a flexible wiring board 500 formed of
a resin material. The flexible wiring board 500 has
plural slits 510A through 510N which are provided
radially. Although FIG. 12 shows only one side of
the brain activity measuring apparatus 100B, an
opposite side that corresponds to the back side of
the drawing has a similar configuration. Moreover,
the flexible wiring board 500 holds the plural
sensor units 24 arranged at a predetermined interval
in a manner similar to embodiment 1.
Since the flexible wiring board 500 has
flexibility, it can be easily deformed into a curved
shape corresponding to the shape of the head surface
due to the plural slits 510A though 510N. Moreover,
by providing the plural slits 510A through 510N
directed from an outline side to a central part of
the flexible wiring board 500 which is formed in a
flat shape and adjusting the cutting angles and
cutting lengths of the slits, the flexible wiring
board 500 can assume various curved shapes.
CA 02671221 2009-07-09
-32-
Therefore, in this variation example 2, the flexible
wiring board 500 can be easily mounted on the head
surface by bending the flexible wiring board 500,
and also can be easily detached from the head
surface only by returning the flexible wiring board
500 into the flat shape after the measurement.
The plural sensor units 24 held by the
flexible wiring board 500 are controlled in each
area partitioned by the slits 510A through 510N, and
grouped into, for example, the sensor units 24AI
through 24An, 2431 through 24Bn, 24N1
through 24Nn.
Therefore, since the plural slits 510A through 510N
can be provided at arbitrary positions, the area of
each of the blocks A through N can be set in
accordance with the corresponding measurement area.
In this variation example 2 as well, the
small wireless communication devices 400A through
400N (shown as black circles in FIG. 12) are
provided in the blocks A through N respectively.
Therefore, the measurement data obtained by the
plural sensor units 24 can be independently sent per
blocks A through N from the corresponding wireless
communication devices 400A through 400N to the data
managing device 50.
FIG. 13 is a diagram showing a mounted
brain activity measuring apparatus 100C according to
a variation example 3. As shown in FIG. 13, a blood
flow measuring apparatus 20C of the brain activity
measuring apparatus 1000 of the variation example 3
is formed of a flexible wiring board 600 that is
formed of a resin material in a belt shape and then
wrapped around a head in a spiral manner. Although
FIG. 13 shows only one side of the brain activity
measuring apparatus 1000, an opposite side that
corresponds to the back side of the drawing has a
similar configuration. The flexible wiring board
600 holds the plural sensor units 24 and the
CA 02671221 2009-07-09
-33-
wireless communication devices 400A through 400N
(shown as black circles in FIG. 13) at a
predetermined interval in a manner similar to the
variation example 2.
Since the flexible wiring board 600 is
formed in a belt shape with flexibility, it can be
freely wrapped around the shape of the head surface,
and can be easily mounted on the head so as to be
tightly attached to the shape of the curved surface
of the head. Although there are various shapes of
heads of the subjects, the flexible wiring board 600
can be rounted on the heads of various shapes by
appropriately adjusting a wrapping area of the
flexible wiring board 600.
FIG. 14 is a longitudinal schematic
diagram showing a cross section of a sensor unit 700,
which is a variation example of the sensor unit 24.
In FIG. 14, the same components as those in the
sensor unit 24 in FIG. 2 are denoted by the same
reference numerals and description thereof is
omitted here. In the sensor unit 700, as shown in
FIG. 14, an optical path separating member 720
formed in a tapered shape is inserted and held in a
brain wave measuring electrode 710 formed in a
tapered cylindrical shape. In this embodiment, the
brain wave measuring electrode 710 is fit on an
outer periphery of the optical path separating
member 720 in an integrated manner. Tapered angles
of the brain wave measuring electrode 710 and the
optical path separating member 720 are arbitrarily
set depending on a whole length, areas of top and
bottom end parts, and the like. The optical path
separating member 720 is formed of a hologram in a
manner similar to the embodiment 1. The optical
path separating member 720 transmits the laser light
emitted by the light emitting part 120 to a leading
end part 722, and condenses the light which has
CA 02671221 2009-07-09
-34-
propagated through the brain 300 and reentered from
the leading end part 722 to the light receiving part
130.
A leading end part 712 of the brain wave
measuring electrode 710 protrudes slightly downward
from the leading end part 722 of the optical path
separating member 720. Therefore, a brain wave of
this measurement area can be measured by the leading
end part 712 contacting the scalp surface 220.
A collar part 714 with a large diameter is
provided on a base end side of the brain wave
measuring electrode 710. This collar part 714 is
inserted slidably in an axis direction (vertical
directions) along an inner wall of an external
cylindrical member 730 formed of a conductive
material. The external cylindrical member 730 has a
space 740 in which the brain wave measuring
electrode 710 and the optical path separating member
720 are slid in the axis direction, a top wall part
732 formed so as to surround an upper part of the
space 740, and a lower wall part 734 formed so as to
surround a lower part of the space 740.
A biasing member (coil spring) 750 to bias
the brain wave measuring electrode 710 downward is
provided between the collar part 714 of the brain
wave measuring electrode 710 and the upper wall part
732. When the leading ends of the brain wave
measuring electrode 710 and the optical path
separating member 720 contact the scalp surface 220,
the biasing member 750 is compressed by the pressure
force. By a repulsive force against the compression
force, the front ends of the brain wave measuring
electrode 710 and the optical path separating member
720 are pressed onto the scalp surface 220.
Therefore, by mounting the sensor unit 700
by pressing the external cylindrical member 730
downward, a biasing force of the biasing member 750
CA 02671221 2009-07-09
-35-
acts to tightly attach the leading ends of the brain
wave measuring electrode 710 and the optical path
separating member 720 onto the scalp surface 220.
Therefore, even when there is hair on the measured
area, the front ends of the brain wave measuring
electrode 710 and the optical path separating member
720 can be made to surely contact the scalp surface
220.
On a top end surface 724 of the optical
path separating member 720, the light emitting part
120 and the light receiving part 130 are mounted.
The optical path separating member 720 of this
variation example is formed in a tapered shape so
that its top end has a large diameter. Therefore,
an area of the top end surface 724 can be set in
accordance with the sizes of the light emitting part
120 and the light receiving part 130. Moreover, the
diameter of the leading end part 722 of the optical
path separating member 720 can be reduced to make a
contact area with the scalp surface 220 smaller,
regardless of the sizes of the light emitting part
120 and the light receiving part 130. Accordingly,
when the leading end surface 722 of the optical path
separating member 720 contacts the scalp surface 220,
a possibility of catching the hair is reduced and
the precision of the measurement is enhanced.
In this embodiment, the laser light A
emitted onto the scalp surface 220 and light
received.. at the leading end part 722 of the optical
path separating member 720 form a waveguide while
being reflected on the tapered inner wall of the
brain wave measuring electrode 710. Therefore,
there is nc influence on the amount of light
transmitting through the optical separating member
720.
[Embodiment 2]
FIG. 15 is a systematic diagram showing a
CA 02671221 2009-07-09
-36-
schemat:c configuration of a blood flow measuring
apparatus 800 of embodiment 2. As shown in FIG. 15,
the blood flow measuring apparatus 800 of embodiment
2 measures a blood flow amount in the case of
dialysis treatment. The blood flow measuring
apparatus 800 includes a sensor unit 820 mounted on
a dialysis tube 812 connected to a dialysis device
810 and a control part 830 to control the dialysis
device 10 according to measurement data outputted
by the sensor unit 820.
The dialysis tube 812 is formed of a
translucent resin tube with elasticity. The
dialysis tube 812 is connected to blood vessels 842
and 844 of a patient 840 who takes dialysis
treatment. Blood taken out of the blood vessels 842
and 844 is supplied through the dialysis tube 812 to
the dialysis device 810. The dialysis device 810
includes an artificial kidney (dialyzer) to filter
the blood and supply dialysate, and a pump device to
send the blood.
The control part 830 calculates a blood
flow amount and a red blood cell concentration
according to measurement data measured by the sensor
unit 820, controls the amount of dialysate to be
supplied and a pump rotational speed of the dialysis
device 810 according to the blood flow amount.
Moreover, the control part 830 outputs measurement
results of the sensor unit 820 and dialysis data to
a personal computer 850. The personal computer 850
performs accumulation, analysis, and the like of the
measurement results and dialysis data.
FIG. 16 is a longitudinal schematic
diagram showing a configuration of the sensor unit
820 of embodiment 2. As shown in FIG. 16, the
sensor unit 820 includes a holding member 860 which
holds a part of the dialysis tube 812 so as to be
pressurized from an upper side and a lower side, and
CA 02671221 2009-07-09
-37-
two sets of sensor parts 870 and 880. The first
sensor part 870 includes a first light emitting part
872 arranged above the dialysis tube 812 and first
and second light receiving parts 874 and 876
arranged below the dialysis tube 812. The second
sensor part 880 includes, in a manner similar to the
first sensor part 870, a second light emitting part
882 arranged above the dialysis tube 812 and third
and fourth light receiving parts 884 and 886
arranged below the dialysis tube 812.
In this embodiment, the red blood cell
concentration Rpw is measured by the two-point-two-
wavelengths measuring method by using arithmetic
expression (3). That is, by emitting laser lights
with different wavelengths X1 and X2 (in this
embodiment, X1 = 805 nm and X2 - 680 nm) from the
first and second light emitting parts 872 and 882,
the red blood cell concentration is measured as a
function of only a hematocrit (Ht). Therefore,
according o this calculation method, the red blood
cell concentration can be accurately measured as a
measurement value responsive to the hematocrit (Ht).
:Embodiment 3]
FIG. 17 is a schematic diagram showing a
configuration of a blood flow measuring apparatus
900 of embodiment 3. As shown in FIG. 17, the blood
flow measuring apparatus 900 of embodiment 3
includes a measuring part 920 which contacts a skin
surface 910 of a measurement area, a sensor unit 930
incorporated in the measuring part 920, and a
control part 940 which generates a blood flow
measurement image according to the measurement data
outputted by the sensor unit 930.
The measuring part 920 is formed in such a
size that can be carried by hand. For example, the
measuring part 920 can be moved as required
depending on a part of a human body where a blood
CA 02671221 2009-07-09
-38-
flow is measured. Further, the measuring part 920
has a cone-shaped part 922 of which bottom surface
serves as a measurement surface 924 to be in contact
with the measurement area. A holding part 926
protrudes on an upper part of the cone-shaped part
922. Therefore, a measurer can measure a blood flow
of the measurement area by holding the holding part
926 and making contact with the measurement surface
924 on the skin surface 910 of the measured area as
required.
The sensor unit 930 includes a light
emitting part 950 which emits the laser light A, a
pair of light receiving parts 960 and 962 arranged
with different distances from a light emitting point,
and an optical path separating member 970 formed of
a hologram. The light emitting part 950 and the
pair of light receiving parts 960 and 962 are
mounted on an upper surface of the optical path
separating member 970. A bottom surface of the
optical path separating member 970 serves as the
measurement surface 924.
Therefore, when the laser light A is
emitted by the light emitting part 950 through the
optical path separating member 970 onto the skin
surface 910 of an arbitrary measurement area, the
laser light A transmits through a blood flow in the
blood vessel 912 present below the skin surface 910
and propagates to the measurement surface 924. The
light receiving parts 960 and 962 individually
receive the light which has propagated through the
optical path separating member 970 and output
electrical signals responsive to the amount of
transmitted and received light to the control part
940.
In this embodiment, the red blood cell
concentration Rp of blood flowing through the blood
vessel 912 is measured by the two-point-one-
,
CA 02671221 2009-07-09
-39-
wavelength measuring method by using arithmetic
expression (2). That is, the red blood cell
concentration is a function of a distance AL between
the two light receiving parts 960 and 962 and the
hematocrit (Ht). Therefore, since the distance AL
between the two light receiving parts 960 and 962 is
known in advance, the red blood cell concentration
Rp is measured as a value having the hematocrit (Ht)
as a coefficient. Therefore, by this calculation
method, the red blood cell concentration can be
accurately measured as a measurement value
responsive to the hematocrit (Ht).
The control part 940 is connected to a
monitor 980. The control part 940 generates image
data from the measurement data of the blood flow
measured by the sensor unit 930 of the measuring
part 920, and displays a measurement image 982 based
on the image data on the monitor 980. Accordingly,
a measurer can check whether his/her blood flow is
normal by nolding the measuring part 920 in hand and
making contact with the measurement surface 924 on
the skin surface 910 while seeing the measurement
image 982 displayed on the monitor 980.
The measuring part 920 of the blood flow
measuring apparatus 900 can be moved as required.
Therefore, blood flows of parts other than the head
of a human body can be easily measured. Moreover,
since tne olood flow measuring apparatus 900 is
highly portable, it can be used in any place in
addition to a clinic of a medical institution (for
example, in a temporary clinic, buildings other than
medical institutions, a tent, or outdoors in a
disaster area).
According to at least one embodiment,
light emitted from a light emitting part is received
by two or more light receiving parts arranged at
positions with different distances from the light
CA 02671221 2016-01-14
-40-
emitting part, and a blood flow state of a
measurement area is measured according to signals
obtained by the two or more light receiving parts.
Therefore, a component depending on the oxygen
saturation, which is included in the obtained
signals, can be cancelled. As a result, blood flow
and a brain activity state can be accurately
measured according to a signal responsive to a
proportion of a volume of red blood cells included
TO in blood flowing through the measurement area.