Note: Descriptions are shown in the official language in which they were submitted.
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
REMOTELY TRIGGERED RELEASE FROM HEATABLE SURFACES
Related Applications
[0001] This application claims priority under 35 U.S.C. 119(e) to U.S.
Provisional
Patent Applications 60/873,897, filed December 8, 2006 ("the `897
application"), and
60/969,389, filed August 31, 2007 ("the `389 application"). The entire
contents of the `897
application and the `3 89 application are incorporated herein by reference in
their entirety.
Government Support
[0002] The United States Government has provided grant support utilized in the
development of the present invention. In particular, National Institutes of
Health (contract
numbers N01-C0-37117, R01-CA-124427-01, U54 CA119349, U54 CA119335, and EB
006324) have supported development of this invention. The United States
Government has
certain rights in the invention.
Background of the Invention
[0003] Conventional modes of drug administration include oral, intravenous,
pulmonary,
and transdermal delivery. Typically, materials designed for such methods of
drug delivery
are engineered to have characteristic release or degradation properties given
their in vivo
environments. However, these agents are passive in their delivery and lack the
ability to
deliver payload in response to external commands (Sengupta et al., 2005,
Nature, 436:568;
incorporated herein by reference). Such externally-timed drug delivery is
currently limited
primarily to treatment via repeated administration.
[0004] The primary means of externally timed drug administration is the use of
multiple
injections, a treatment that remains standard practice for multi-step
vaccinations, timed
hormone dosing, and for treatment of diseases such as diabetes. Frequent re-
administration
in inconvenient for patients and caregivers and often leads to patient non-
compliance.
[0005] Implantable microchips with addressable, drug-containing wells are
currently
being developed. These wells are individually opened for programmable or
externally
controlled delivery, allowing multiple drugs to be simultaneously expelled.
However, this
technology requires the additional burden of a permanent implant with a power
supply,
electronic wiring, and non-degradable scaffold.
Page 1 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[0006] Ultrasound can be used either to locally burst vesicle-coated bubbles
that contain
drug or to physically erode a hard, hydrophobic drug-containing polymer
implant. Both
methods have aroused questions about the safety of repeated ultrasound
exposure and are
limited in their ability to delivering protein or hydrophilic drugs.
Additionally, the cavitation
of vesicle-coated bubbles cannot be used to deliver drugs long after
administration and is
more suited to targeted delivery. Furthermore, because the bubble sizes become
unstable
below approximately 100 nm, such methods have limited ability to deliver drug
beyond the
endothelium.
[0007] Several other systems utilize temperature-sensitive, drug-doped
hydrogels for
controlled delivery. One such material can be designed such that at
temperatures beyond a
critical temperature, they collapse and release any soluble materials inside.
Many different
means of obtaining the required temperature increase have been investigated,
ranging from
the use of externally applied heating pads to various internal sources of
heat. Often these
methods lack either sufficient means of controlling the extent of release or
sufficiently
localizing heat so as to avoid heating body tissues. Additionally, these
schemes have limited
ability to delivery hydrophobic drugs.
[0008] Hydrogel approaches are limited by the fact that they have a single
transition
temperature. By restricting a system to one transition temperature, it is not
possible to
controllably deliver a variety of drug combinations by releasing different
drugs at different
temperatures. Additionally, approaches requiring hydrogels are not easily
amenable for
design as injectable, targeted delivery platforms. Furthermore, current means
for thermally
regulated delivery rely on conformation changes in surrounding hydrogels with
micron size
limitations. Additionally, because hydrogels do not physically immobilize
their contents, the
drugs continually diffuse out of the gel over time, preventing strict on/off
modulation of
release.
[0009] To date, implantable devices have been synthesized to facilitate
scheduled release
of multiple payloads via surface degradation (Wood et al., 2006, Proc. Natl.
Acad. Sci., USA,
103:10207; incorporated herein by reference) or via programmable
electronically controlled
microchips. While these approaches provide local release of a bioactive
payload, their
dimensions preclude external activation of release to targeted regions.
[0010] Thus, there is a need in the art for methods which offer the ability to
safely and
precisely release a variety of drugs from a non-permanent carrier in response
to external
signals. There is a need in the art for improved methods for controlled drug
release that
decrease non-specific drug release. There is a need in the art for methods for
drug delivery
Page 2 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
that enable one-time administration of multi-injection treatments to allow for
convenient
dosage modulation for continuous treatment of disease (e.g. diabetes).
Summary of the Invention
[0011] The present invention provides a novel means of remotely and/or
controllably
releasing an agent to be delivered (e.g. therapeutic, diagnostic,
prophylactic, and/or
nutraceutical agent). In general, heatable surfaces which heat in response to
external stimuli
(e.g. electromagnetic (EM) fields, light, etc.) are provided. Heatable
surfaces are typically
associated with one or more agents to be delivered via thermally-responsive
linkers. When
the resulting thermally-responsive conjugate is subjected to an external
stimulus (e.g. EM
field, light), heatable surfaces release a certain amount of heat. The amount
of heat released
may or may not be sufficient to disrupt the function of the thermally-
responsive linker,
resulting in release of the agent to be delivered.
[0012] In some embodiments, a heatable surface comprises any substance that
can be
heated. In some embodiments, a heatable surface comprises any material
experiencing local
or macroscopic temperature change. In some embodiments, a heatable surface
comprises
electromagnetically or optically responsive material. In some embodiments, a
heatable
surface comprises any substance that is heated in electromagnetic (EM) fields.
In some
embodiments, a heatable surface comprises any substance that is heated in
response to light.
[0013] In some embodiments, heatable surfaces are particles (e.g.
nanoparticles,
microparticles, etc.). In some embodiments, a heatable surface comprises a
metal
nanoparticle (e.g. gold) which experiences inductive heating in an EM field.
In some
embodiments, the heatable surface is a magnetic nanoparticle. In general, a
particle in
accordance with the present invention is any entity having a greatest
dimension (e.g.
diameter) of less than 100 microns ( m). In some embodiments, particles have a
greatest
dimension of less than 10 m, 1000 nanometers (nm), 900 nm, 800 nm, 700 nm,
600 nm, 500
nm, 400 nm, 300 nm, 200 nm, or 100 nm. Typically, particles have a greatest
dimension
(e.g., diameter) of 300 nm or less.
[0014] In some embodiments, heatable surfaces are nanorods, nanorings, and/or
nanoshells. In some embodiments, heatable surfaces are macroscopic surfaces
(e.g. sheets or
blocks of metal) which can be heated in response to EM fields and/or other
stimuli.
[0015] In some embodiments, heatable surfaces have detectable properties
and/or are
attached to detectable moieties. Such heatable surfaces allow for detection of
thermally-
Page 3 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
responsive conjugates coincident with or subsequent to therapeutic
administration of the
conjugates. In some embodiments, detectable heatable surfaces are magnetically
detectable.
In some embodiments, detectable heatable surfaces are optically detectable.
[0016] The present invention provides thermally-responsive linkers which
mediate the
association between an agent to be delivered and a heatable surface in a
temperature-sensitive
manner. For example, when exposed to temperatures below a characteristic
temperature or
characteristic temperature range (referred to herein as the "trigger
temperature"), a thermally-
responsive linker can mediate the association between an agent to be delivered
and a heatable
surface. When the thermally-responsive linker and/or a conjugate comprising a
thermally-
responsive linker is exposed to the trigger temperature and/or temperatures
higher than the
trigger temperature, the thermally-responsive linker is no longer capable of
mediating the
association between the two or more entities (i.e. the thermally-responsive
linker is
"disrupted"), and the agent to be delivered is released from the heatable
surface.
[0017] Any substance that is responsive to changes in temperature (e.g.
displays different
properties at different temperatures) may be a thermally-responsive linker in
accordance with
the present invention. In some embodiments, thermally-responsive linkers
comprise at least
two individual components which interact with one another in a temperature-
sensitive
manner. In some embodiments, thermally-responsive linkers mediate the
association of a
conjugate assembly in which disruption of the conjugate assembly results in
release of the
agent to be delivered. In some embodiments, thermally-responsive linkers
comprise a single
component which mediates the association of two or more moieties (e.g.
heatable surfaces) in
a temperature-sensitive manner. In some embodiments, thermally-responsive
linkers
comprise at least one individual component which has a temperature-sensitive
three-
dimensional conformation. In some embodiments, thermally-responsive linkers
comprise
nucleic acids; peptides and/or proteins; carbohydrates; and/or polymers. In
certain
embodiments, thermally-responsive linkers comprise complimentary Watson-Crick
base
pairing of nucleic acid strands (e.g. DNA, RNA, and/or PNA strands). In
certain
embodiments, thermally-responsive linkers comprise nucleic acids whose
properties result
from the three-dimensional structure of the nucleic acid (e.g. an aptamer). In
certain
embodiments, thermally-responsive linkers comprise interactions between
complimentary
peptides, lipids, polymers, and/or carbohydrates. In certain embodiments,
thermally-
responsive linkers comprise proteins which can undergo temperature dependent
conformational changes.
Page 4 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[0018] In certain embodiments, a thermally-responsive linker comprises any
material that
swells and/or shrinks in response to temperature changes. In certain
embodiments, a
thermally-responsive linker comprises any material that swells and/or shrinks
in response to
temperature changes and also that does not break in response to temperature
changes. For
example, such a thermally-responsive linker may include a polymer such as
pNIPAM.
[0019] Disruption of the linker typically occurs at sites where temperature
triggers are
present. For example, when a conjugate comprising a thermally-responsive
linker is exposed
to a trigger temperature, disruption of the linker leads to separation of the
heatable surface
and agent to be delivered. Whereas, without exposure to the trigger
temperature, the agent to
be delivered remains associated with the particle.
[0020] In some embodiments, disruption of the linker occurs at temperatures
higher than
ambient temperature. In some embodiments, disruption of the linker occurs at
temperatures
higher than body temperature.
[0021] The present invention encompasses the recognition that thermally-
responsive
linkers may be modulated such that the agent to be delivered is releases at
different trigger
temperatures. Such modulation enables production of thermally-responsive
linkers having a
specific and/or desired trigger temperature and enables multiplexing of
several different drug
release schemes.
[0022] In some embodiments, thermally-responsive linkers may include nucleic
acid
residues. In some embodiments, the trigger temperature can be modulated by
varying the
number of complimentary hybridizing bases on two or more nucleic acid strands.
In some
embodiments, the duplex region does not comprise any nucleotide mismatches. In
some
embodiments, the duplex region may be interrupted by 1, 2, 3, 4, 5, or more
nucleotide
mismatches. In some embodiments, the nucleotide mismatches may be contiguous
(i.e.
mismatches are adjacent to one another). In some embodiments, the nucleotide
mismatches
may be non-contiguous (i.e. mismatches are separated by one or more base
pairs). In general,
the presence of mismatches decreases the trigger temperature relative to the
absence of
mismatches.
[0023] In some embodiments, a thermally-responsive linker comprises a duplex
region
and at least one single-stranded nucleic acid overhang on either side or both
sides of the
duplex region. In some embodiments, the trigger temperature can be modulated
by varying
the nucleotide content of the nucleic acid strands. For example, increasing
the amount of
guanine and/or cytosine relative to the amount of adenine, thymine, and/or
uracil tends to
raise the trigger temperature of a thermally-responsive linker. Likewise,
increasing the
Page 5 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
amount of adenine, thymine, and/or uracil relative to the amount of guanine
and/or cytosine
tends to lower the trigger temperature of a thermally-responsive linker. In
some
embodiments, the trigger temperature can be modulated by including one or more
modified
nucleotide residues.
[0024] In some embodiments, thermally-responsive linkers include amino acid
residues.
In some embodiments, protein and/or peptide linkers may comprise two or more
moieties that
interact with one another in a heat-sensitive manner. Protein-based
interactions may be heat-
sensitive if their association is at least partially-mediated by hydrogen
bonding. In some
embodiments, thermally-responsive linkers may include any protein-protein
interaction
domains that involve hydrogen bonding. In certain embodiments, thermally-
responsive
linkers may be based on coil geometries (e.g. a-helices, leucine zippers,
collagen helices,
etc.), (3-sheet motifs (e.g. amphiphilic peptides), etc.
[0025] In some embodiments, protein and/or peptide linkers may comprise any
heat-
sensitive affinity interaction. In certain embodiments, protein and/or peptide
linkers may
comprise ligand-receptor interactions (e.g. TGFa-EGF receptor interactions).
In some
embodiments, protein and/or peptide linkers may comprise antibody-antigen
interactions. In
some embodiments, protein and/or peptide linkers may comprise other types of
affinity
interactions (e.g. any two proteins which specifically bind to one another).
[0026] In some embodiments, thermally-responsive linkers include
carbohydrates.
[0027] In some embodiments, thermally-responsive linkers include polymers
(e.g.
synthetic polymers). In some embodiments, polymer-based embodiments encompass
sol-gel
hydrogels whose transition is based on temperature, including natural
polymers,
poly(ethylene oxide)/poly (propylene oxide) block copolymers, N-
isopropylacrylamide
copolymers, etc. In general, a sol-gel hydrogel refers to a class of polymer
that can change
from a solution to a gel under a particular set of conditions that are
specific for the identity of
the given polymer. In some embodiments, polymer-based thermally-responsive
linkers may
comprise multiphase hydrogels (see, e.g., Ehrick et al., 2005, Nat. Mater.,
4:298;
incorporated herein by reference).
[0028] In some embodiments, thermally-responsive linkers are hybrid linkers.
In some
embodiments, the term "hybrid linkers" refers to thermally-responsive linkers
comprise at
least two of the following: nucleic acids, proteins/peptides, carbohydrates,
lipids, polymers,
and small molecules.
[0029] In some embodiments, thermally-responsive linkers comprise at least two
individual components which associate with one another below the trigger
temperature, but
Page 6 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
do not associate with one another at and/or above the trigger temperature.
Typically, one
individual component is associated with the heatable surface, and another
individual
component is associated with the agent to be delivered. In some embodiments,
the
association is covalent. In some embodiments, the association is non-covalent
(e.g. hydrogen
bonding, charge interactions, affinity interactions, van der Waals forces,
etc.).
[0030] In certain embodiments, thermally-responsive linkers comprise at least
two
complementary nucleic acid strands (e.g. DNA, RNA, PNA, and/or combinations
thereof). In
some embodiments, heat labile linkers may comprise interactions among proteins
and/or
peptides having coil geometries (e.g. a-helices, leucine zippers, collagen
helices, etc.), (3-
sheet motifs (e.g. amphiphilic peptides), etc. In some embodiments, heat
labile linkers may
comprise a ligand-receptor interaction. In some embodiments, heat labile
linkers may
comprise an antibody-antigen interaction. In some embodiments, heat labile
linkers may
comprise an enzyme-substrate interaction. In some embodiments, heat labile
linkers may
comprise another type of affinity interaction (e.g. an interaction between any
proteins which
specifically bind to one another).
[0031] In some embodiments, thermally-responsive linkers mediate the
association of a
conjugate assembly for which disruption of the conjugate assembly results in
release of the
agent to be delivered. In some embodiments, conjugate assemblies may enable
triggered
enhancement of component transport or clearance. For example, a conjugate
assembly may
be too large for clearance from the body, but the individual conjugates within
the assembly
may be small enough for clearance from the body.
[0032] The present invention encompasses the recognition that thermally-
responsive
linkers may be modulated such that the agent to be delivered is releases at
different trigger
temperatures, enabling multiplexing of several different drug release schemes.
For example,
the nucleotide content of nucleic acid thermally-responsive linkers may be
modified such that
a set of linkers is generated, in which each member of the set is
characterized by a different
nucleotide content (e.g. nucleotide sequence) and, consequently, a different
trigger
temperature.
[0033] In some embodiments, thermally-responsive linkers comprise at least one
individual component which has a temperature-sensitive three-dimensional
conformation. In
certain embodiments, thermally-responsive linkers comprise proteins and/or
peptides which
can undergo temperature-dependent conformational changes. In some embodiments,
protein
and/or peptide structures containing hydrogen bonds (e.g. a-helices, (3-
sheets, amphiphilic
peptides, etc.) encapsulate hydrophobic agents in the interior of the
structures and, upon
Page 7 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
disassociation (e.g. upon exposure to a trigger temperature), release the
agents to be
delivered. In some embodiments, release can occur because the protein and/or
peptide
structure is no longer able to contain the agent to be delivered (e.g. the
agent to be delivered
can "leak out" of the protein and/or peptide structure).
[0034] In some embodiments, protein and/or peptide structures may associate
with agents
to be delivered in a manner that is dependent on the three-dimensional
structure of the protein
(and/or peptide) and/or the agent to be delivered. In some embodiments,
release can occur
because the protein and/or peptide structure no longer associates with the
agent to be
delivered.
[0035] According to the present invention, thermally-responsive conjugates may
be used
for delivery of any agent, including, for example, therapeutic, diagnostic,
prophylactic, and/or
nutraceutical agents. One of ordinary skill in the art will appreciate that
any agent can be
delivered by the compositions and methods in accordance with the present
invention. In
some embodiments, agents to be delivered may include any molecule, material,
substance, or
construct that may be transported into a cell by conjugation to a nano- or
micro-structure.
Exemplary agents to be delivered in accordance with the present invention
include, but are
not limited to, small molecules, organometallic compounds, nucleic acids (e.g.
DNA, RNA,
peptide nucleic acids, etc.), proteins (including multimeric proteins, protein
complexes, etc.),
peptides, lipids, carbohydrates, hormones, metals, radioactive elements and
compounds,
hydrophobic drugs, hydrophilic drugs, vaccines, immunological agents, organic
constructs,
inorganic constructs, inhibitors, catalysts, nanoparticles, microparticles,
etc., and/or
combinations thereof.
In some embodiments, the agent to be delivered may be a mixture of
pharmaceutically active
agents.
[0036] In some embodiments, thermally-responsive conjugates in accordance with
the
present invention comprise one or more targeting moieties. In general, a
targeting moiety is
any moiety that binds to a component associated with an organ, tissue, cell,
subcellular
locale, and/or extracellular matrix component. A targeting moiety may be a
nucleic acid,
polypeptide, glycoprotein, carbohydrate, lipid, etc. For example, a targeting
moiety can be a
nucleic acid targeting moiety (e.g. an aptamer) that binds to a cell type
specific marker. In
general, an aptamer is an oligonucleotide (e.g., DNA, RNA, or an analog or
derivative
thereof) that binds to a particular target, such as a polypeptide. In some
embodiments, a
targeting moiety may be a naturally occurring or synthetic ligand for a cell
surface receptor,
e.g., a growth factor, hormone, LDL, transferrin, etc. A targeting moiety can
be an antibody,
Page 8 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
which term is intended to include antibody fragments, characteristic portions
of antibodies,
single chain antibodies, etc. Synthetic binding proteins such as affibodies,
etc., can be used.
Peptide targeting moieties can be identified, e.g., using procedures such as
phage display.
This widely used technique has been used to identify cell specific ligands for
a variety of
different cell types. Nanoparticle conjugates comprising targeting moieties
are described in
further detail in co-pending U.S. Patent Application entitled "DELIVERY OF
NANOPARTICLES AND/OR AGENTS TO CELLS," filed December 6, 2007 (the entire
contents of which are incorporated herein by reference and are attached hereto
as Appendix
A).
[0037] In some embodiments, targeting moieties bind to an organ, tissue, cell,
extracellular matrix component, and/or intracellular compartment that is
associated with a
specific developmental stage or a specific disease state (i.e. a "target" or
"marker").
[0038] In some embodiments, populations of thermally-responsive conjugates are
"single-component" systems. In other words, "single component" conjugates
comprise
heatable surfaces, thermally-responsive linkers, and/or agents to be delivered
that are all
identical to one another. In some embodiments, conjugate systems are "two-
component" or
"multi-component" conjugate systems. In other words, "two-component" or "multi-
component" conjugate systems (e.g. conjugate populations, pluralities of
conjugates, etc.)
comprise heatable surfaces, thermally-responsive linkers, and/or agents to be
delivered that
are not all identical to one another.
[0039] In some embodiments, a single thermally-responsive conjugate may
comprise a
particle associated with multiple different thermally-responsive linkers and
multiple different
agents to be delivered. In some embodiments, the multiple different thermally-
responsive
linkers are sensitive to different temperatures. In some embodiments, such a
conjugate may
be used to deliver different therapeutic agents at different points in time
(i.e. a dosage
schedule).
[0040] The present invention provides methods of triggering disassembly of
dendrimer-
like conjugate assemblies connected via heat-liable linkers. Controlled
disassociation of
conjugate assemblies enables timed cargo release from large aggregates for the
purpose of
sensing, MRI, catalysis, delivery of localized high drug dosage, gene therapy,
or facilitating
body clearance of particles in vivo.
[0041] In some embodiments, individual conjugates within a population of
conjugates
interact and/or associate with one another to form assemblies of conjugates.
In some
embodiments, a population of conjugates comprises assemblies of individual
conjugates. In
Page 9 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
some embodiments, conjugate assemblies may be characterized as having an
ordered
structure. In some embodiments, conjugate assemblies may be characterized as
having an
unordered structure.
[0042] In some embodiments, thermally-responsive conjugates may be
manufactured
using any available method. Methods of forming heatable surfaces (e.g.
magnetic particles)
are known in the art. In general, assembly of conjugates involves at least one
chemical
reaction. For example, attaching the agent to be delivered to the thermally-
responsive linker
may take place in one reaction, and attaching the heatable surface to a
thermally-responsive
linker may take place in a second reaction. From this point, the conjugates
are formed by
self-assembly, which can be performed in a controlled manner by dictating the
concentrations
of the individual components (e.g. heatable surfaces, thermally-responsive
linkers, agents to
be delivered, etc.).
[0043] In some embodiments, a heatable surface and a thermally-responsive
linker are
physically associated with one another. In some embodiments, a thermally-
responsive linker
and an agent to be delivered are physically associated with one another. In
some
embodiments, a heatable surface and an agent to be delivered are physically
associated with
one another. In some embodiments, a heatable surface and a targeting moiety
are physically
associated with one another. In some embodiments, a thermally-responsive
linker and a
targeting moiety are physically associated with one another. In some
embodiments, an agent
to be delivered and a targeting moiety are physically associated with one
another. In certain
specific embodiments, a heatable surface, thermally-responsive linker, and
agent to be
delivered are physically associated with one another. In certain specific
embodiments, a
heatable surface, thermally-responsive linker, agent to be delivered, and
targeting moiety are
physically associated with one another.
[0044] Physical association can be achieved in a variety of different ways.
Physical
association may be covalent or non-covalent. In some embodiments, non-covalent
physical
association may be characterized by association with the surface of,
encapsulated within,
surrounded by, and/or distributed throughout the polymeric matrix of a
heatable surface. In
some embodiments, a heatable surface, thermally-responsive linker, and/or
agent to be
delivered may be directly conjugated to one another or may be conjugated by
means of one or
more linkers.
[0045] In some embodiments, a composition in accordance with the invention is
administered to a subject for therapeutic, diagnostic, and/or prophylactic
purposes. In some
embodiments, the amount of thermally-responsive conjugate and/or population of
thermally-
Page l0 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
responsive conjugates is sufficient to treat, alleviate symptoms of, diagnose,
prevent, and/or
delay the onset of a disease, condition, and/or disorder. In some embodiments,
the invention
encompasses "therapeutic cocktails," including, but not limited to, approaches
in which
multiple thermally-responsive conjugates are administered. The present
invention provides
thermally-responsive conjugates that enable delivery of an agent (e.g.
therapeutic, diagnostic,
and/or prophylactic agent) at a specific time. An agent to be delivered, as
described herein,
may be released from conjugates free in the bloodstream, from conjugates in
tissues, from
conjugates in cells, from conjugates within a hydrogel, from conjugates
immobilized onto a
surface, and/or from conjugates behind a membrane. Conjugates may be used in
vitro as well
as in vivo.
[0046] To give but a few examples, applications include intelligent drug
delivery,
controllable drug implants, simplified vaccinations, more potent cancer
treatments, enhanced
sensing capabilities, MRI, gene therapy, monitoring enzyme catalysis of
endogenous and/or
delivered substrates, delivery of high drug or cargo dosages to single points,
reduction of
non-specific drug release, localized release of growth factors to cells,
intracellular cargo
delivery, and/or controlled vehicle disassembly for easing clearance of
particles in vivo.
[0047] Thermally responsive conjugates in accordance with the present
invention and
pharmaceutical compositions thereof may be administered using any amount and
any route of
administration effective for treatment. In some embodiments, pharmaceutical
compositions
in accordance with the present invention are administered by a variety of
routes, including
oral, intravenous, intramuscular, intra-arterial, intramedullary, intrathecal,
subcutaneous,
intraventricular, transdermal, interdermal, rectal, intravaginal,
intraperitoneal, topical (e.g. by
powders, ointments, creams, gels, lotions, and/or drops), mucosal, nasal,
bucal, enteral,
sublingual; by intratracheal instillation, bronchial instillation, and/or
inhalation; and/or as an
oral spray, nasal spray, and/or aerosol.
[0048] In some embodiments, the present invention provides for pharmaceutical
compositions comprising thermally-responsive conjugates as described herein
and one or
more pharmaceutically acceptable excipients. Such pharmaceutical compositions
may
optionally comprise one or more additional therapeutically-active substances.
In accordance
with some embodiments, a method of administering a pharmaceutical composition
comprising thermally-responsive conjugates to a subject in need thereof is
provided.
[0049] The invention provides a variety of kits for conveniently and/or
effectively
carrying out methods in accordance with the present invention. Kits in
accordance with the
invention typically comprise one or more thermally-responsive conjugates. In
some
Page 11 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
embodiments, kits comprise a collection of different thermally-responsive
conjugates to be
used for different purposes (e.g. diagnostics, treatment, and/or prophylaxis).
Kits may
include additional components or reagents. For example, kits may comprise one
or more
tools and/or pieces of equipment for exposing thermally-responsive conjugates
to an EM
field. In some embodiments, such a kit is used in the treatment, diagnosis,
and/or prophylaxis
of a subject suffering from and/or susceptible to a disease, condition, and/or
disorder (e.g.
cancer). In some embodiments, such a kit comprises (i) a thermally-responsive
conjugate
that is useful in the treatment of cancer; (ii) a syringe, needle, applicator,
etc. for
administration of the to a subject; and (iii) instructions for use.
Brief Description of the Drawing
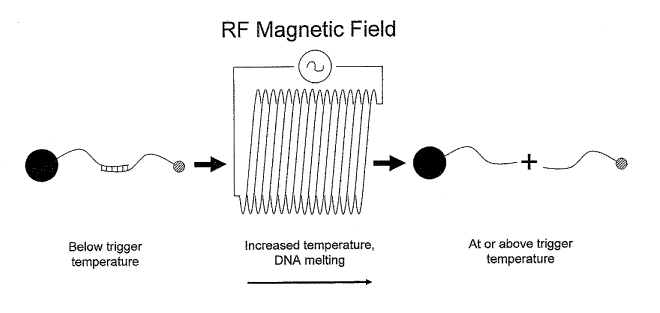
[0050] Figure 1: Schematic diagram of a thermally-responsive linker which
comprises
two complementary nucleic acid strands. One nucleic acid strand is associated
with the
heatable surface, and a second nucleic acid strand is associated with the
agent to be delivered.
A portion of each nucleic acid strand is complementary to the other strand.
When the
temperature is below a characteristic trigger temperature, the complementary
portions anneal
to form a duplex region. When the conjugate is subjected to a radio frequency
(RF) magnetic
field, the conjugate is heated to the trigger temperature or to temperatures
higher than the
trigger temperature. The two strands of the thermally-responsive linker
denature and
dissociate from one another, and the agent to be delivered is released from
the heatable
surface.
[0051] Figure 2: Schematic diagram of a thermally-responsive linker which
mediates the
association of a conjugate assembly for which disruption of the conjugate
assembly results in
release of the agent to be delivered. The agent to be delivered is attached to
a single-stranded
nucleic acid acting as a linker between one single-stranded nucleic acid bound
to one particle
and a second single-stranded nucleic acid bound to a second particle. When the
conjugate is
placed in an EM field capable of heating the particles to and/or above the
trigger temperature,
the nucleic acid duplexes are disrupted, releasing the linker nucleic acid and
the agent to be
delivered while disassociating the particles from each other.
[0052] Figure 3: EM field-triggered release of nanoparticle-tethered dye in
pulsatile and
multistage profiles. Superparamagnetic nanoparticles transduce external
electromagnetic
energy to heat, thereby melting oligonucleotide duplexes that act as thermally-
responsive
tethers to model drugs. (A) Thermally responsive conjugates comprising
particles, linkers,
Page 12 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
and fluorophores were assembled by first covalently linking a 30 bp "parent"
strand, and then
allowing a fluorescent complement (of 12 bp, 18 bp, or 24 bp) to hybridize.
(B) In vitro,
nanoparticles hybridized to fluorescein-conjugated 18 bp were embedded in
hydrogel plugs.
Repeated EM field pulses of 5 minutes resulted in corresponding release of
fluorescein.
Alteration of oligonucleotide duplex length shifts response of thermally-
responsive tether
enabling complex release profiles. Low power EM field exposure results in
release of FAM-
conjugated 12 bp whereas higher power results in simultaneous melting of both
12 bp and 24
bp tethers (C).
[0053] Figure 4: EM field-induced temperature rise varies with particle
concentration
and sample diameter. Experimental data (open circles) were collected by
applying maximum
EM field (3 kW power) to solutions of various diameters (D) containing various
concentration of magnetic particles (p). These data were fit to a conductive
heat transfer
equation (inset), where k is thermal conductivity (for water: 0.64 W/m= C),
and q is the
heating rate (mW/mg). With a threshold of 5 C temperature rise to trigger
release, these
results estimate a minimum of 1.2 mg particles must be delivered to a 1 cm
diameter tumor.
[0054] Figure 5: Triggered release from thermally-responsive conjugates in
vivo.
Conjugates were mixed with matrigel and injected subcutaneously near the
posterior
mammary fat pad of mice, forming tumor phantoms (A). Application of EM field
to
implanted phantoms with 18 bp tethers resulted in release of model drugs and
penetration into
surrounding tissue (B) when compared to unexposed controls (C, scale bar = 100
microns).
These mice were imaged with a 7T MRI scanner, and a transverse section is
shown in (D)
(arrow indicates tumor phantom).
Definitions
[0055] Agent to be delivered: As used herein, the phrase "agent to be
delivered" refers to
any substance that can be delivered to an organ, tissue, cell, subcellular
locale, and/or
extracellular matrix locale. In some embodiments, the agent to be delivered is
a biologically
active agent, i.e., it has activity in a biological system and/or organism.
For instance, a
substance that, when administered to an organism, has a biological effect on
that organism, is
considered to be biologically active. In particular embodiments, where an
agent to be
delivered is a biologically active agent, a portion of that agent that shares
at least one
biological activity of the agent as a whole is typically referred to as a
"biologically active"
portion. In some embodiments, an agent to be delivered is a therapeutic agent.
As used
Page 13 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
herein, the term "therapeutic agent" refers to any agent that, when
administered to a subject,
has a beneficial effect. The term "therapeutic agent" refers to any agent
that, when
administered to a subject, has a therapeutic, diagnostic, and/or prophylactic
effect and/or
elicits a desired biological and/or pharmacological effect. As used herein,
the term
"therapeutic agent" may be a therapeutic, diagnostic, prophylactic, and/or
nutraceutical agent.
[0056] Animal: As used herein, the term "animal" refers to any member of the
animal
kingdom. In some embodiments, "animal" refers to humans, at any stage of
development. In
some embodiments, "animal" refers to non-human animals, at any stage of
development. In
certain embodiments, the non-human animal is a mammal (e.g., a rodent, a
mouse, a rat, a
rabbit, a monkey, a dog, a cat, a sheep, cattle, a primate, and/or a pig). In
some
embodiments, animals include, but are not limited to, mammals, birds,
reptiles, amphibians,
fish, and/or worms. In some embodiments, an animal may be a transgenic animal,
genetically-engineered animal, and/or a clone.
[0057] Approximately: As used herein, the term "approximately" or "about," as
applied
to one or more values of interest, refers to a value that is similar to a
stated reference value.
In certain embodiments, the term "approximately" or "about" refers to a range
of values that
fall within 25%,20%,19%,18%,17%,16%,15%,14%,13%,12%,11%,10%,9%,8%
,
7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction (greater than or less
than) of the
stated reference value unless otherwise stated or otherwise evident from the
context (except
where such number would exceed 100% of a possible value).
[0058] Associated with: As used herein, the terms "associated with,"
"conjugated,"
"linked," "attached," and "tethered," when used with respect to two or more
moieties, means
that the moieties are physically associated or connected with one another,
either directly or
via one or more additional moieties that serves as a linking agent, to form a
structure that is
sufficiently stable so that the moieties remain physically associated under
the conditions in
which structure is used, e.g., physiological conditions. In some embodiments,
the moieties
are attached to one another by one or more covalent bonds. In some
embodiments, the
moieties are attached to one another by a mechanism that involves specific
(but non-covalent)
binding (e.g. streptavidin/avidin interactions, antibody/antigen interactions,
etc.). In some
embodiments, a sufficient number of weaker interactions can provide sufficient
stability for
moieties to remain physically associated.
[0059] Biocompatible: As used herein, the term "biocompatible" refers to
substances that
are not toxic to cells. In some embodiments, a substance is considered to be
"biocompatible"
if its addition to cells in vivo does not induce inflammation and/or other
adverse effects in
Page 14 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
vivo. In some embodiments, a substance is considered to be "biocompatible" if
its addition to
cells in vitro or in vivo results in less than or equal to about 50%, about
45%, about 40%,
about 35%, about 30%, about 25%, about 20%, about 15%, about 10%, about 5%, or
less
than about 5% cell death.
[0060] Biodegradable: As used herein, the term "biodegradable" refers to
substances that
are degraded under physiological conditions. In some embodiments, a
biodegradable
substance is a substance that is broken down by cellular machinery. In some
embodiments, a
biodegradable substance is a substance that is broken down by chemical
processes.
[0061] Heatable surface: As used herein, the term "heatable surface" refers to
any
substance capable of heating upon exposure to an external stimulus. In
general, a heatable
surface is a component of a thermally-responsive conjugate. One of ordinary
skill in the art
will appreciate that any heatable surface can be used in thermally-responsive
conjugates. To
give but a few examples, a heatable surface may be a magnetic, metallic,
semiconductor,
and/or hybrid particle (e.g. nanoparticle, microparticle, etc.). In certain
embodiments, a
heatable surface is a nanoparticle. In certain embodiments, a heatable surface
is a
microparticle. Such particles may have spherical, cubic, rod-like,
ellipsoidal, plate-like, or
other geometries tuned to enhance electromagnetic (EM) properties and/or to
facilitate
targeting and/or delivery. In certain embodiments, a heatable surface is
capable of heating in
response to EM fields. In some embodiments, a heatable surface is capable of
heating in
response to particular frequencies. In certain embodiments, a heatable surface
is capable of
heating in response to light. In some embodiments, a heatable surface is
capable of releasing
a particular amount of heat in response to EM fields and/or light.
[0062] Homology: As used herein, the term "homology" refers to the overall
relatedness
between polymeric molecules, e.g. between nucleic acid molecules (e.g. DNA
molecules
and/or RNA molecules) and/or between polypeptide molecules. In some
embodiments,
polymeric molecules are considered to be "homologous" to one another if their
sequences are
at least 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%,
95%, or 99% identical. In some embodiments, polymeric molecules are considered
to be
"homologous" to one another if their sequences are at least 25%, 30%, 35%,
40%, 45%, 50%,
55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 99% similar.
[0063] Identity: As used herein, the term "identity" refers to the overall
relatedness
between polymeric molecules, e.g. between nucleic acid molecules (e.g. DNA
molecules
and/or RNA molecules) and/or between polypeptide molecules. Calculation of the
percent
identity of two nucleic acid sequences, for example, can be performed by
aligning the two
Page 15 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
sequences for optimal comparison purposes (e.g., gaps can be introduced in one
or both of a
first and a second nucleic acid sequences for optimal alignment and non-
identical sequences
can be disregarded for comparison purposes). In certain embodiments, the
length of a
sequence aligned for comparison purposes is at least 30%, at least 40%, at
least 50%, at least
60%, at least 70%, at least 80%, at least 90%, at least 95% or 100% of the
length of the
reference sequence. The nucleotides at corresponding nucleotide positions are
then
compared. When a position in the first sequence is occupied by the same
nucleotide as the
corresponding position in the second sequence, then the molecules are
identical at that
position. The percent identity between the two sequences is a function of the
number of
identical positions shared by the sequences, taking into account the number of
gaps, and the
length of each gap, which needs to be introduced for optimal alignment of the
two sequences.
The comparison of sequences and determination of percent identity between two
sequences
can be accomplished using a mathematical algorithm. For example, the percent
identity
between two nucleotide sequences can be determined using the algorithm of
Meyers and
Miller (CABIOS, 1989, 4:11-17), which has been incorporated into the ALIGN
program
(version 2.0) using a PAM120 weight residue table, a gap length penalty of 12
and a gap
penalty of 4. The percent identity between two nucleotide sequences can,
alternatively, be
determined using the GAP program in the GCG software package using an
NWSgapdna.CMP matrix.
[0064] In vitro: As used herein, the term "in vitro" refers to events that
occur in an
artificial environment, e.g., in a test tube or reaction vessel, in cell
culture, etc., rather than
within an organism (e.g. animal, plant, and/or microbe).
[0065] In vivo: As used herein, the term "in vivo" refers to events that occur
within an
organism (e.g. animal, plant, and/or microbe).
[0066] Nucleic acid: As used herein, the term "nucleic acid," in its broadest
sense, refers
to any compound and/or substance that is or can be incorporated into an
oligonucleotide
chain. In some embodiments, a nucleic acid is a compound and/or substance that
is or can be
incorporated into an oligonucleotide chain via a phosphodiester linkage. In
some
embodiments, "nucleic acid" refers to individual nucleic acid residues (e.g.
nucleotides
and/or nucleosides). In some embodiments, "nucleic acid" refers to an
oligonucleotide chain
comprising individual nucleic acid residues. As used herein, the terms
"oligonucleotide" and
"polynucleotide" can be used interchangeably. In some embodiments, "nucleic
acid"
encompasses RNA as well as single and/or double-stranded DNA and/or cDNA.
Furthermore, the terms "nucleic acid," "DNA," "RNA," and/or similar terms
include nucleic
Page 16 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
acid analogs, i.e. analogs having other than a phosphodiester backbone. For
example, the so-
called "peptide nucleic acids," which are known in the art and have peptide
bonds instead of
phosphodiester bonds in the backbone, are considered within the scope of the
present
invention. The term "nucleotide sequence encoding an amino acid sequence"
includes all
nucleotide sequences that are degenerate versions of each other and/or encode
the same
amino acid sequence. Nucleotide sequences that encode proteins and/or RNA may
include
introns. Nucleic acids can be purified from natural sources, produced using
recombinant
expression systems and optionally purified, chemically synthesized, etc. Where
appropriate,
e.g., in the case of chemically synthesized molecules, nucleic acids can
comprise nucleoside
analogs such as analogs having chemically modified bases or sugars, backbone
modifications, etc. A nucleic acid sequence is presented in the 5' to 3'
direction unless
otherwise indicated. The term "nucleic acid segment" is used herein to refer
to a nucleic acid
sequence that is a portion of a longer nucleic acid sequence. In many
embodiments, a nucleic
acid segment comprises at least 3, 4, 5, 6, 7, 8, 9, 10, or more residues. In
some
embodiments, a nucleic acid is or comprises natural nucleosides (e.g.
adenosine, thymidine,
guanosine, cytidine, uridine, deoxyadenosine, deoxythymidine, deoxyguanosine,
and
deoxycytidine); nucleoside analogs (e.g., 2-aminoadenosine, 2-thiothymidine,
inosine,
pyrrolo-pyrimidine, 3-methyl adenosine, 5-methylcytidine, C-5 propynyl-
cytidine, C-5
propynyl-uridine, 2-aminoadenosine, C5-bromouridine, C5-fluorouridine, C5-
iodouridine,
C5-propynyl-uridine, C5-propynyl-cytidine, C5-methylcytidine, 2-
aminoadenosine, 7-
deazaadenosine, 7-deazaguanosine, 8-oxoadenosine, 8-oxoguanosine, 0(6)-
methylguanine,
and 2-thiocytidine); chemically modified bases; biologically modified bases
(e.g., methylated
bases); intercalated bases; modified sugars (e.g., 2'-fluororibose, ribose, 2'-
deoxyribose,
arabinose, and hexose); and/or modified phosphate groups (e.g.,
phosphorothioates and 5'-N-
phosphoramidite linkages). In some embodiments, the present invention is
specifically
directed to "unmodified nucleic acids," meaning nucleic acids (e.g.
polynucleotides and
residues, including nucleotides and/or nucleosides) that have not been
chemically modified in
order to facilitate or achieve delivery.
[0067] Particle: As used herein, the term "particle" refers to any entity
having a diameter
of less than 100 microns ( m). Typically, particles have a longest dimension
(e.g. diameter)
of 1000 nm or less (e.g. a "nanoparticle"). In general, particles have
dimensions small
enough to allow their uptake by eukaryotic cells. In some embodiments,
particles have a
diameter of 300 nm or less. In some embodiments, particles have a diameter of
200 nm or
less. In some embodiments, particles have a diameter of 100 nm or less. In
general, particles
Page 17 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
are greater in size than the renal excretion limit, but are small enough to
avoid accumulation
in the liver. In some embodiments, particles are spheres, spheroids, flat,
plate-shaped, cubes,
cuboids, ovals, ellipses, cylinders, cones, or pyramids. In some embodiments,
particles can
comprise one or more heatable surfaces. In some embodiments, magnetic
particles are
among the particles that are used in various embodiments. "Magnetic particles"
refers to
magnetically responsive particles that contain one or more metals or oxides or
hydroxides
thereof Metals of use in the nanoparticles include, but are not limited to,
gold, silver, iron,
cobalt, zinc, cadmium, nickel, gadolinium, chromium, copper, manganese,
palladium, tin, and
alloys and/or oxides thereof.
[0068] Protein: As used herein, the term "protein" refers to a polypeptide
(i.e., a string of
at least two amino acids linked to one another by peptide bonds). Proteins may
include
moieties other than amino acids (e.g., may be glycoproteins) and/or may be
otherwise
processed or modified. Those of ordinary skill in the art will appreciate that
a "protein" can
be a complete polypeptide chain as produced by a cell (with or without a
signal sequence), or
can be a functional portion thereof Those of ordinary skill will further
appreciate that a
protein can sometimes include more than one polypeptide chain, for example
linked by one
or more disulfide bonds or associated by other means. Polypeptides may contain
L-amino
acids, D-amino acids, or both and may contain any of a variety of amino acid
modifications
or analogs known in the art. Useful modifications include, e.g., terminal
acetylation,
amidation, etc. In some embodiments, polypeptides may comprise natural amino
acids, non-
natural amino acids, synthetic amino acids, and combinations thereof. The term
"peptide" is
used to refer to a polypeptide having a length of less than about 100 amino
acids.
[0069] Self-assembly: As used herein, the term "self-assembly" refers to a
process of
spontaneous assembly of a higher order structure that relies on the natural
attraction of the
components of the higher order structure (e.g., molecules) for each other. It
typically occurs
through random movements of the molecules and formation of bonds based on
size, shape,
composition, or chemical properties.
[0070] Similarity: As used herein, the term "similarity" refers to the overall
relatedness
between polymeric molecules, e.g. between nucleic acid molecules (e.g. DNA
molecules
and/or RNA molecules) and/or between polypeptide molecules. Calculation of
percent
similarity of polymeric molecules to one another can be performed in the same
manner as a
calculation of percent identity, except that calculation of percent similarity
takes into account
conservative substitutions as is understood in the art.
Page 18 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[0071] Small molecule: In general, a "small molecule" is understood in the art
to be an
organic molecule that is less than about 5 kilodaltons (Kd) in size. In some
embodiments, the
small molecule is less than about 3 Kd, 2 Kd, or 1 Kd. In some embodiments,
the small
molecule is less than about 800 daltons (D), less than about 600 D, less than
about 500 D,
less than about 400 D, less than about 300 D, less than about 200 D, or less
than about 100 D.
In some embodiments, small molecules are non-polymeric. In some embodiments,
small
molecules are not proteins, peptides, or amino acids. In some embodiments,
small molecules
are not nucleic acids or nucleotides. In some embodiments, small molecules are
not
saccharides or polysaccharides.
[0072] Subject: As used herein, the term "subject" or "patient" refers to any
organism to
which a composition in accordance with the invention may be administered,
e.g., for
experimental, diagnostic, prophylactic, and/or therapeutic purposes. Typical
subjects include
animals (e.g., mammals such as mice, rats, rabbits, non-human primates, and
humans) and/or
plants.
[0073] Substantially: As used herein, the term "substantially" refers to the
qualitative
condition of exhibiting total or near-total extent or degree of a
characteristic or property of
interest. One of ordinary skill in the biological arts will understand that
biological and
chemical phenomena rarely, if ever, go to completion and/or proceed to
completeness or
achieve or avoid an absolute result. The term "substantially" is therefore
used herein to
capture the potential lack of completeness inherent in many biological and
chemical
phenomena.
[0074] Suffering from: An individual who is "suffering from" a disease,
disorder, and/or
condition has been diagnosed with or displays one or more symptoms of a
disease, disorder,
and/or condition.
[0075] Susceptible to: An individual who is "susceptible to" a disease,
disorder, and/or
condition has not been diagnosed with and/or may not exhibit symptoms of the
disease,
disorder, and/or condition. In some embodiments, an individual who is
susceptible to a
disease, disorder, and/or condition (for example, cancer) may be characterized
by one or
more of the following: (1) a genetic mutation associated with development of
the disease,
disorder, and/or condition; (2) a genetic polymorphism associated with
development of the
disease, disorder, and/or condition; (3) increased and/or decreased expression
and/or activity
of a protein associated with the disease, disorder, and/or condition; (4)
habits and/or lifestyles
associated with development of the disease, disorder, and/or condition; (5) a
family history of
the disease, disorder, and/or condition; (6) infection by a microbe associated
with
Page 19 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
development of the disease, disorder, and/or condition. In some embodiments,
an individual
who is susceptible to a disease, disorder, and/or condition will develop the
disease, disorder,
and/or condition. In some embodiments, an individual who is susceptible to a
disease,
disorder, and/or condition will not develop the disease, disorder, and/or
condition.
[0076] Therapeutically effective amount: As used herein, the term
"therapeutically
effective amount" means an amount of an agent to be delivered (e.g., drug,
therapeutic agent,
diagnostic agent, prophylactic agent, etc.) that is sufficient, when
administered to a subject
suffering from or susceptible to a disease, disorder, and/or condition, to
treat, diagnose,
prevent, and/or delay the onset of the disease, disorder, and/or condition.
[0077] Thermally-responsive conjugate: As used herein, the term "thermally-
responsive
conjugate" refers to a composition comprising one or more heatable surfaces,
one or more
thermally-responsive linkers, and one or more agents to be delivered. In
general, a thermally-
responsive conjugate can be used for delivering an agent (e.g. therapeutic,
diagnostic,
prophylactic, and/or nutraceutical agent) to an organ, tissue, cell,
subcellular locale, and/or
extracellular matrix locale. Each thermally-responsive conjugate has a
characteristic "trigger
temperature." The thermally-responsive conjugate releases the agent to be
delivered upon
exposure to temperatures at or higher than the trigger temperature.
[0078] Thermally-responsive linker: As used herein, the term "thermally-
responsive
linker" refers to a moiety which is capable of mediating the association
between two or more
entities in a temperature-sensitive manner. In some embodiments, a thermally-
responsive
linker mediates the association between an agent to be delivered and a
heatable surface in a
temperature-sensitive manner. For example, when exposed to temperatures below
a
characteristic temperature and/or characteristic range of temperatures
(referred to herein as
the "trigger temperature"), a thermally-responsive linker is capable of
mediating the
association between an agent to be delivered and a heatable surface. When the
thermally-
responsive linker and/or a conjugate comprising a thermally-responsive linker
is exposed to
the trigger temperature and/or temperatures higher than the trigger
temperature, the
thermally-responsive linker is no longer capable of mediating the association
between the two
or more entities, and the agent to be delivered is released from the heatable
surface.
[0079] Treating: As used herein, the term "treating" refers to partially or
completely
alleviating, ameliorating, relieving, delaying onset of, inhibiting
progression of, reducing
severity of, and/or reducing incidence of one or more symptoms or features of
a particular
disease, disorder, and/or condition. For example, "treating" cancer may refer
to inhibiting
survival, growth, and/or spread of a tumor. Treatment may be administered to a
subject who
Page 20 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
does not exhibit signs of a disease, disorder, and/or condition and/or to a
subject who exhibits
only early signs of a disease, disorder, and/or condition for the purpose of
decreasing the risk
of developing pathology associated with the disease, disorder, and/or
condition. In some
embodiments, treatment comprises delivery of a therapeutically effective
amount of
thermally-responsive conjugates to a subject.
Detailed Description of Certain Embodiments
[0080] The present invention provides systems and methods for controlled
release of
pharmaceutical cargo for the purpose of remotely actuating drug delivery. One
or more
agents to be delivered (e.g. drugs, therapeutic agents, prophylactic agents,
diagnostic agents,
etc.) are associated with heatable surfaces (e.g. particles) via thermally-
responsive linkers,
yielding thermally-responsive conjugates. When the thermally-responsive linker
is exposed
to a characteristic temperature and/or characteristic temperature range (i.e.
a "trigger
temperature"), the linker is disrupted and the agent is released. Thermally-
responsive linkers
can be designed to be disrupted at different temperatures, enabling delivery
of complex drug
profiles, in specific orders, over variable periods of time. The method may be
used for
delivery of nucleic acids (e.g. DNA, RNA, peptide nucleic acids, etc.),
peptides and proteins,
small molecules, drugs, inhibitors, catalysts, and nano- and micro-particles
using a multitude
of difference heat sources. The present invention provides systems which
incorporate
electromagnetically excitable particles or surfaces to allow remotely actuated
drug release.
Heat-Triggered Release
[0081] The present invention provides a novel means of controllably releasing
an agent to
be delivered (e.g. therapeutic, diagnostic, prophylactic, and/or nutraceutical
agent). In
general, heatable surfaces which heat in response to external stimuli (e.g.
electromagnetic
(EM) fields, light, etc.) are provided. Heatable surfaces are typically
associated with one or
more agents to be delivered via thermally-responsive linkers. When the
resulting thermally-
responsive conjugate is subjected to an external stimulus (e.g. EM field,
light), heatable
surfaces release a certain amount of heat. The amount of heat released may or
may not be
sufficient to disrupt the function of the thermally-responsive linker,
resulting in release of the
agent to be delivered.
[0082] In some embodiments, a heatable surface comprises a porous surface
layer. For
example, a thermally-responsive conjugate may comprise (i) a heatable surface
comprising a
Page 21 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
porous surface layer, (ii) an agent to be delivered, and (iii) a thermally-
responsive linker,
wherein the agent and the linker are located underneath the porous surface
layer. When the
agent is released from the heatable surface upon heating to trigger
temperature, the agent can
diffuse through the porous surface layer.
[0083] In some embodiments, a heatable surface comprises any substance that
can be
heated. In some embodiments, a heatable surface comprises any material
experiencing local
or macroscopic temperature change. In some embodiments, a heatable surface
comprises
electromagnetically or optically responsive material. In some embodiments, a
heatable
surface comprises any substance that is heated in electromagnetic (EM) fields.
In some
embodiments, a heatable surface comprises any substance that is heated in
response to light.
[0084] In some embodiments, a heatable surface is or comprises a particle
(e.g.
nanoparticle, microparticle, etc.). Nanoparticles are attractive heat sources
because they can
obtain tens of degrees of localized, nanoscale temperature increase without
affecting the
macroscopic solution temperature. Particles such as superparamagnetic iron
oxide show
significant heating in magnetic resonance (MR) frequency fields, making them
fully
compatible with the existing clinical practice of MRI.
[0085] In some embodiments, heatable surfaces span radio frequencies (e.g.
magnetic
materials, conductive materials, etc.). In some embodiments, heatable surfaces
span optical
and/or infrared frequencies (e.g. plasmonic materials, such as gold, silver,
copper, and
materials incorporating these elements alongside other semiconductor,
inorganic, or organic
materials). In some embodiments, heatable surfaces comprise nanoscale and
marcoscale
conductive materials, semiconductor materials, and/or organic materials that
absorb radio
frequencies or optical energy. These materials may be tuned to absorb specific
frequencies of
interest by altering material composition or their shape. For example, gold
nanoparticles
absorb at approximately 520 nm when spherical, but rod-shapes or core-shell
architectures
can be tuned to absorb in the near infrared region of light (about 700 nm -
about 1000 nm).
Higher frequencies typically correspond to higher rate of energy deposition.
In some
embodiments, antennas on the scale of microns or macro scale can focus EM
fields or heat
inductively according to Faraday's law.
[0086] In some embodiments, heatable surfaces include materials which heat via
magnetic hysteresis, Neel relaxation, and/or Brownian relaxation in radio
frequency ranges
(i.e. 3 Hz to 3 GHz), including but not limited to iron oxides, cobalt, hybrid
doped magnetic
materials, etc. In some embodiments, heatable surfaces include organic
materials. In certain
embodiments, heatable surfaces include organic molecules such as chromophores,
Page 22 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
fluorophores, and/or nanoparticle carriers of high densities of such
molecules. In certain
embodiments, heatable surfaces include optical polymers. In some embodiments,
heatable
surfaces include carbon nanotubes. In some embodiments, heatable surfaces
include
semiconductor materials (e.g. quantum dots, photonic crystals, etc.). In some
embodiments,
heatable surfaces include metallic materials (e.g., gold, silver, copper,
and/or other plasmonic
materials). In some embodiments, heatable surfaces include combination
materials
comprising plasmonic components and conductive materials for inductive, Joule
heating.
These span excitations from GHz through Infrared frequencies. In some
embodiments,
higher frequency can correlate with a higher temperature released from the
heatable surface.
In some embodiments, heatable surfaces can utilize optical excitation (e.g.
200 nm - 1200
nm) or radio frequency (3 Hz to 3 GHz).
[0087] In some embodiments, heatable surfaces may be tuned to absorb specific
frequencies of interest by altering composition and/or shape of the heatable
surface. For
example, gold nanoparticles absorb at approximately 520 nm when spherical, but
rod-shapes
or core-shell architectures can be tuned to absorb in the near infrared region
of light
(approximately 700 nm - approximately 1000 nm). Higher frequencies indeed
typically
correspond to higher rate of energy deposition.
[0088] In some embodiments, a heatable surface comprises a nanorod for which
heat
release is triggered with light. Conductive nanoparticles (e.g. gold, silver,
etc.) display
plasmon resonances (discussed in further detail below) that are tunable by
manipulating
geometry (e.g. nanorods, cubes, etc.) or particle composition (e.g.
nanoshells). In some
embodiments, geometry may directionally relay and/or focus EM energy into a
releasable
bond, enabling remote-controlled release. Due to the relative deficit of near-
infrared light
absorbing chromophores and scattering agents, shifting this resonance into the
near-infrared
enables particle actuation within biological specimens. In some embodiments,
tunable
linkers may be interfaced with tunable nanoparticles enabling frequency-
specific, and
temperature-specific release of therapeutic agents. In some embodiments,
plasmonic or other
nanoparticles that absorb light strongly may be utilized to efficiently
capture light for
conversion into heat.
[0089] EM fields can be applied using any method known in the art. For
example, in
optical frequencies, EM fields can be applied using a light source. In some
embodiments,
EM fields in optical frequencies can be applied using an endoscope, a laser, a
bulb, a fiber
optic, and/or combinations thereof. In some embodiments, EM fields at
frequencies lower
Page 23 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
than optical frequencies can be applied using a coil, handheld device,
portable source, and/or
combinations thereof.
[0090] In some embodiments, heatable surfaces have detectable properties
and/or are
attached to detectable moieties. Such heatable surfaces allow for detection of
thermally-
responsive conjugates coincident with or subsequent to therapeutic
administration of the
conjugates. In some embodiments, detectable heatable surfaces are magnetically
detectable.
In some embodiments, detectable heatable surfaces are optically detectable.
[0091] In some embodiments, a heatable surface comprises a metal nanoparticle
(e.g.
gold) which experiences inductive heating in an EM field. In some embodiments,
the
heatable surface is a magnetic nanoparticle. "Magnetic particles" refers to
magnetically
responsive particles that contain one or more metals, oxides, and/or
hydroxides thereo Such
particles typically react to magnetic force resulting from a magnetic field. A
magnetic field
can attract or repel particles towards or away from the source of the magnetic
field,
respectively, optionally causing acceleration or movement in a desired
direction in space.
Magnetic particles may experience heating due to Brownian relaxation and
reorientation of
their magnetic poles.
[0092] Magnetic particles may comprise one or more ferrimagnetic,
ferromagnetic,
paramagnetic, and/or superparamagnetic materials. Useful particles may be made
entirely or
in part of one or more materials selected from the group consisting of: iron,
cobalt, nickel,
niobium, magnetic iron oxides, hydroxides such as maghemite (y-Fez03),
magnetite (Fe304),
feroxyhyte (FeO[OH]), double oxides or hydroxides of two- or three-valent iron
with two- or
three-valent other metal ions such as those from the first row of transition
metals such as
Co(II), Mn(II), Cu(II), Ni(II), Cr(III), Gd(III), Dy(III), Sm(III), mixtures
of the afore-
mentioned oxides or hydroxides, and mixtures of any of the foregoing. See,
e.g., U.S. Patents
5,916,539 for suitable synthesis methods for certain of these particles.
Additional materials
that may be used in magnetic particles include yttrium, europium, and
vanadium.
[0093] A magnetic particle may contain a magnetic material and one or more
nonmagnetic materials, which may be a metal or a nonmetal (e.g. quantum dots,
ceramics,
polymers comprising inorganic materials, bone-derived materials, bone
substitutes, viral
particles, etc.). In certain embodiments, a magnetic particle is a composite
particle
comprising an inner core or layer containing a first material and an outer
layer or shell
containing a second material, wherein at least one of the materials is
magnetic. Optionally
both of the materials are metals. In some embodiments, the heatable surface is
a nanoshell
(i.e. nanoparticle coated with metal shell) which typically absorbs specific
wavelengths of
Page 24 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
incident electromagnetic energy by varying particle diameter and shell
thickness. In certain
embodiments, a heatable surface is an iron oxide particle, e.g., the particle
has a core of iron
oxide. Optionally the iron oxide is monocrystalline. In certain embodiments,
the particle is a
superparamagnetic iron oxide particle, e.g., the particle has a core of
superparamagnetic iron
oxide. In certain embodiments, the heatable surface is a gold nanoshell.
[0094] In some embodiments, a heatable surface may be a magnetically
detectable
particle. A magnetically detectable particle is a magnetic particle that can
be detected as a
consequence of its magnetic properties. In some embodiments, a magnetically
detectable
particle can be detected within a living cell as a consequence of its magnetic
properties. Use
of magnetically detectable particles allows for in vivo monitoring of particle
delivery,
movement, migration, uptake by the liver, clearance by the kidney, and/or
degradation. The
present invention provides methods for imaging and/or monitoring a patient
undergoing
therapeutic treatment in real time. The present invention provides methods in
which a
clinician is able to monitor therapeutic pharmacokinetics in real time and
make decisions as
to the timing of drug dosing.
[0095] An optically detectable particle is one that can be detected within a
living cell
using optical means compatible with cell viability. Optical detection is
accomplished by
detecting the scattering, emission, and/or absorption of light that falls
within the optical
region of the spectrum, i.e., that portion of the spectrum extending from
approximately 180
nm to several microns. Optionally a sample containing cells is exposed to a
source of
electromagnetic energy. In some embodiments, absorption of electromagnetic
energy (e.g.,
light of a given wavelength) by the nanoparticle or a component thereof is
followed by the
emission of light at longer wavelengths, and the emitted light is detected. In
some
embodiments, scattering of light by the nanoparticles is detected. In certain
embodiments,
light falling within the visible portion of the electromagnetic spectrum,
i.e., the portion of the
spectrum that is detectable by the human eye (approximately 400 nm to
approximately 700
nm) is detected. In some embodiments, light that falls within the infrared or
ultraviolet
region of the spectrum is detected.
[0096] The optical property can be a feature of an absorption, emission, or
scattering
spectrum or a change in a feature of an absorption, emission, or scattering
spectrum. The
optical property can be a visually detectable feature such as, for example,
color, apparent
size, or visibility (i.e. simply whether or not the particle is visible under
particular
conditions). Features of a spectrum include, for example, peak wavelength or
frequency
(wavelength or frequency at which maximum emission, scattering intensity,
extinction,
Page 25 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
absorption, etc. occurs), peak magnitude (e.g., peak emission value, peak
scattering intensity,
peak absorbance value, etc.), peak width at half height, or metrics derived
from any of the
foregoing such as ratio of peak magnitude to peak width. Certain spectra may
contain
multiple peaks, of which one is typically the major peak and has significantly
greater
intensity than the others. Each spectral peak has associated features.
Typically, for any
particular spectrum, spectral features such as peak wavelength or frequency,
peak magnitude,
peak width at half height, etc., are determined with reference to the major
peak. The features
of each peak, number of peaks, separation between peaks, etc., can be
considered to be
features of the spectrum as a whole. The foregoing features can be measured as
a function of
the direction of polarization of light illuminating the particles; thus
polarization dependence
can be measured. Features associated with hyper-Rayleigh scattering can be
measured.
Fluorescence detection can include detection of fluorescence modes.
[0097] Intrinsically fluorescent or luminescent nanoparticles, nanoparticles
that comprise
fluorescent or luminescent moieties, plasmon resonant nanoparticles, and
magnetic
nanoparticles are among the detectable nanoparticles that are used in various
embodiments.
Such particles can have a variety of different shapes including spheres,
oblate spheroids,
cylinders, shells, cubes, pyramids, rods (e.g., cylinders or elongated
structures having a
square or rectangular cross-section), tetrapods (particles having four leg-
like appendages),
triangles, prisms, etc. In general, the nanoparticles should have dimensions
small enough to
allow their uptake by eukaryotic cells. Typically the nanoparticles have a
longest straight
dimension (e.g., diameter) of 200 nm or less. In some embodiments, the
nanoparticles have a
diameter of 100 nm or less. Smaller nanoparticles, e.g., having diameters of
50 nm or less,
e.g., 5-30 nm, are used in some embodiments. In some embodiments, the term
"nanoparticle"
encompasses atomic clusters, which have a typical diameter of 1 nm or less and
generally
contain from several (e.g., 3-4) up to several hundred atoms.
[0098] Fluorescence or luminescence can be detected using any approach known
in the
art including, but not limited to, spectrometry, fluorescence microscopy, flow
cytometry, etc.
Spectrofluorometers and microplate readers are typically used to measure
average properties
of a sample while fluorescence microscopes resolve fluorescence as a function
of spatial
coordinates in two or three dimensions for microscopic objects (e.g., less
than about 0.1 mm
diameter). Microscope-based systems are thus suitable for detecting and
optionally
quantitating nanoparticles inside individual cells.
[0099] Flow cytometry measures properties such as light scattering and/or
fluorescence
on individual cells in a flowing stream, allowing subpopulations within a
sample to be
Page 26 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
identified, analyzed, and optionally quantitated (see, e.g., Mattheakis et
al., 2004, Analytical
Biochemistry, 327:200; incorporated herein by reference). Multiparameter flow
cytometers
are available. In certain embodiments, laser scanning cytometery is used
(Kamentsky, 2001,
Meth. Cell Biol., 63:51; incorporated herein by reference). Laser scanning
cytometry can
provide equivalent data to a flow cytometer but is typically applied to cells
on a solid support
such as a slide. It allows light scatter and fluorescence measurements and
records the
position of each measurement. Cells of interest may be re-located, visualized,
stained,
analyzed, and/or photographed. Laser scanning cytometers are available, e.g.,
from
CompuCyte (Cambridge, MA).
[00100] In certain embodiments, an imaging system comprising an
epifluorescence
microscope equipped with a laser (e.g., a 488 nm argon laser) for excitation
and appropriate
emission filter(s) is used. The filters should allow discrimination between
different
populations of nanoparticles used in the particular assay. For example, in one
embodiment,
the microscope is equipped with fifteen 10 nm bandpass filters spaced to cover
portion of the
spectrum between 520 and 660 nm, which would allow the detection of a wide
variety of
different fluorescent particles. Fluorescence spectra can be obtained from
populations of
nanoparticles using a standard UV/visible spectrometer.
[00101] In certain embodiments, optically detectable particles are metal
particles. Metals
of use in the particles include, but are not limited to, gold, silver, iron,
cobalt, zinc, cadmium,
nickel, gadolinium, chromium, copper, manganese, palladium, tin, and alloys
thereof Oxides
of any of these metals can be used.
[00102] Certain lanthanide ion-doped particles exhibit strong fluorescence and
are of use
in certain embodiments. A variety of different dopant molecules can be used.
For example,
fluorescent europium-doped yttrium vanadate (YVO4) particles have been
produced
(Beaureparie et al., 2004, Nano Letters, 4:2079; incorporated herein by
reference). Such
particles may be synthesized in water and are readily functionalized with
biomolecules.
[00103] Noble metals (e.g., gold, silver, copper, platinum, palladium, etc.)
are typically
used for plasmon resonant particles, which are discussed in further detail
below. For
example, gold, silver, or an alloy comprising gold, silver, and optionally one
or more other
metals can be used. Core/shell particles (e.g., having a silver core with an
outer shell of gold,
or vice versa) can be used. Particles containing a metal core and a
nonmetallic inorganic or
organic outer shell, or vice versa, can be used. In certain embodiments, the
nonmetallic core
or shell comprises or consists of a dielectric material such as silica.
Composite particles in
which a plurality of metal particles are embedded or trapped in a nonmetal
(e.g., a polymer or
Page 27 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
a silica shell) may be used. Hollow metal particles (e.g., hollow nanoshells)
having an
interior space or cavity are used in some embodiments. In some embodiments, a
nanoshell
comprising two or more concentric hollow spheres is used. Such a particle
optionally
comprises a core, e.g., made of a dielectric material.
[00104] In certain embodiments, at least 1%, or typically at least 5% of the
mass or
volume of the particle or number of atoms in the particle is contributed by
metal atoms. In
certain embodiments, the amount of metal in the particle, or in a core or
coating layer
comprising a metal, ranges from approximately 5% to 100% by mass, volume, or
number of
atoms, or can assume any value or range between 5% and 100%.
[00105] Certain metal particles, referred to as plasmon resonant particles,
exhibit the well
known phenomenon of plasmon resonance. When a metal particle (usually made of
a noble
metal such as gold, silver, copper, platinum, etc.) is subjected to an
external electric field, its
conduction electrons are displaced from their equilibrium positions with
respect to the nuclei,
which in turn exert an attractive, restoring force. If the electric field is
oscillating (as in the
case of electromagnetic radiation such as light), the result is a collective
oscillation of the
conduction electrons in the particle, known as plasmon resonance (Kelly et
al., 2003, J. Phys.
Chem. B., 107:668; Schultz et al., 2000, Proc. Natl. Acad. Sci., USA, 97:996;
and Schultz,
2003, Curr. Op. Biotechnol., 14:13; all of which are incorporated herein by
reference). The
plasmon resonance phenomenon results in extremely efficient wavelength-
dependent
scattering and absorption of light by the particles over particular bands of
frequencies, often
in the visible range. Scattering and absorption give rise to a number of
distinctive optical
properties that can be detected using various approaches including visually
(i.e., by the naked
eye or using appropriate microscopic techniques) and/or by obtaining a
spectrum, such as a
scattering spectrum, extinction (scattering + absorption) spectrum, or
absorption spectrum
from the particle(s).
[00106] Features of the spectrum of a plasmon resonant particle (e.g., peak
wavelength)
depend on a number of factors, including the particle's material composition,
the particle's
shape and size, the surrounding medium's refractive index or dielectric
properties, and the
presence of other particles in the vicinity. Selection of particular particle
shapes, sizes, and
compositions makes it possible to produce particles with a wide range of
distinguishable
optically detectable properties.
[00107] Single plasmon resonant particles of sufficient size can be
individually detected
using a variety of approaches. For example, particles larger than about 30 nm
in diameter are
readily detectable under an optical microscope operating in dark-field. A
spectrum from
Page 28 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
these particles can be obtained, e.g., using a CCD detector or other optical
detection device.
Despite their small dimensions relative to the wavelength of light, metal
particles can be
detected optically because they scatter light very efficiently at their
plasmon resonance
frequency. An 80 nm particle, for example, would be millions of times brighter
than a
fluorescein molecule under the same illumination conditions (Schultz et al.,
2000, Proc. Natl.
Acad. Sci., USA, 97:996; incorporated herein by reference). Individual plasmon
resonant
particles can be optically detected using a variety of approaches including
near-field scanning
optical microscopy, differential interference microscopy with video
enhancement, total
internal reflection microscopy, photo-thermal interference contrast, etc. For
measurements
on a population of cells, a standard spectrometer, e.g., equipped for
detection of UV, visible,
and/or infrared light, can be used. In certain embodiments, particles are
optically detected
with the use of surface-enhanced Raman scattering (SERS) (Jackson et al, 2004,
Proc. Natl.
Acad. Sci., USA, 101:17930; incorporated herein by reference). Optical
properties of metal
particles and methods for synthesis of metal particles have been reviewed
(Link et al., 2003,
Annu. Rev. Phys. Chem., 54:331; and Masala et al., 2004, Annu. Rev. Mater.
Res., 34:41; both
of which are incorporated herein by reference).
[00108] In certain embodiments, particles may comprise a bulk material that is
not
intrinsically fluorescent, luminescent, plasmon resonant, or magnetic, but may
comprise one
or more fluorescent, luminescent, or magnetic moieties. For example, a
particle may
comprise quantum dots, fluorescent or luminescent organic molecules, or
smaller particles of
a magnetic material. In some embodiments, an optically detectable moiety such
as a
fluorescent or luminescent dye, etc., is entrapped, embedded, or encapsulated
by a particle
core and/or coating layer. In some embodiments, an optically detectable moiety
such as a
fluorescent or luminescent dye, etc., is conjugated to a particle.
Physical Properties of Heatable Surfaces
[00109] In some embodiments, heatable surfaces comprise particles that are
biodegradable
and biocompatible. In general, a biocompatible substance is not toxic to
cells. In some
embodiments, a substance is considered to be biocompatible if its addition to
cells results in
less than a certain threshhold of cell death. In some embodiments, a substance
is considered
to be biocompatible if its addition to cells does not induce adverse effects.
In general, a
biodegradable substance is one that undergoes breakdown under physiological
conditions
over the course of a therapeutically relevant time period (e.g., weeks,
months, or years). In
some embodiments, a biodegradable substance is a substance that can be broken
down by
Page 29 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
cellular machinery. In some embodiments, a biodegradable substance is a
substance that can
be broken down by chemical processes.
[00110] In some embodiments, a particle which is biocompatible and/or
biodegradable
may be associated with an agent to be delivered that is not biocompatible, is
not
biodegradable, or is neither biocompatible nor biodegradable. In some
embodiments, a
particle which is biocompatible and/or biodegradable may be associated with a
therapeutic or
diagnostic agent that is also biocompatible and/or biodegradable.
[00111] In general, a particle in accordance with the present invention is any
entity having
a greatest dimension (e.g. diameter) of less than 100 microns ( m). In some
embodiments,
particles have a greatest dimension of less than 10 m. In some embodiments,
particles have
a greatest dimension of less than 1000 nanometers (nm). In some embodiments,
particles
have a greatest dimension of less than 900 nm, 800 nm, 700 nm, 600 nm, 500 nm,
400 nm,
300 nm, 200 nm, or 100 nm. Typically, particles have a greatest dimension
(e.g., diameter)
of 300 nm or less. In some embodiments, particles have a greatest dimension
(e.g., diameter)
of 250 nm or less. In some embodiments, particles have a greatest dimension
(e.g., diameter)
of 200 nm or less. In some embodiments, particles have a greatest dimension
(e.g., diameter)
of 150 nm or less. In some embodiments, particles have a greatest dimension
(e.g., diameter)
of 100 nm or less. Smaller particles, e.g., having a greatest dimension of 50
nm or less are
used in some embodiments. In some embodiments, particles have a greatest
dimension
ranging between 5 nm and 1 m. In some embodiments, particles have a greatest
dimension
ranging between 25 nm and 200 nm.
[00112] In some embodiments, particles have a diameter of approximately 1000
nm. In
some embodiments, particles have a diameter of approximately 750 nm. In some
embodiments, particles have a diameter of approximately 500 nm. In some
embodiments,
particles have a diameter of approximately 450 nm. In some embodiments,
particles have a
diameter of approximately 400 nm. In some embodiments, particles have a
diameter of
approximately 350 nm. In some embodiments, particles have a diameter of
approximately
300 nm. In some embodiments, particles have a diameter of approximately 275
nm. In some
embodiments, particles have a diameter of approximately 250 nm. In some
embodiments,
particles have a diameter of approximately 225 nm. In some embodiments,
particles have a
diameter of approximately 200 nm. In some embodiments, particles have a
diameter of
approximately 175 nm. In some embodiments, particles have a diameter of
approximately
150 nm. In some embodiments, particles have a diameter of approximately 125
nm. In some
embodiments, particles have a diameter of approximately 100 nm. In some
embodiments,
Page 30 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
particles have a diameter of approximately 75 nm. In some embodiments,
particles have a
diameter of approximately 50 nm. In some embodiments, particles have a
diameter of
approximately 25 nm.
[00113] In certain embodiments, particles are greater in size than the renal
excretion limit
(e.g. particles having diameters of greater than 6 nm). In specific
embodiments, particles
have diameters greater than 5 nm, greater than 10 nm, greater than 15 nm,
greater than 20
nm, greater than 50 nm, greater than 100 nm, greater than 250 nm, greater than
500 nm,
greater than 1000 nm, or larger. In certain embodiments, particles are small
enough to avoid
clearance of particles from the bloodstream by the liver (e.g. particles
having diameters of
less than 1000 nm). In specific embodiments, particles have diameters less
than 1500 nm,
less than 1000 nm, less than 750 nm, less than 500 nm, less than 250 nm, less
than 100 nm, or
smaller. In general, physiochemical features of particles, including particle
size, can be
selected to allow a particle to circulate longer in plasma by decreasing renal
excretion and/or
liver clearance. In some embodiments, particles have diameters ranging from 5
nm to 1500
nm, from 5 nm to 1000 nm, from 5 nm to 750 nm, from 5 nm to 500 nm, from 5 nm
to 250
nm, or from 5 nm to 100 nm. In some embodiments, particles have diameters
ranging from
nm to 1500 nm, from 15 nm to 1500 nm, from 20 nm to 1500 nm, from 50 nm to
1500
nm, from 100 nm to 1500 nm, from 250 nm to 1500 nm, from 500 nm to 1500 nm, or
from
1000 nm to 1500 nm. In some embodiments, particles under 100 nm may be easily
endocytosed in the reticuloendothelial system (RES). In some embodiments,
particles under
400 nm may be characterized by enhanced accumulation in tumors. While not
wishing to be
bound by any theory, enhanced accumulation in tumors may be caused by the
increased
permeability of angiogenic tumor vasculature relative to normal vasculature.
Particles can
diffuse through such "leaky" vasculature, resulting in accumulation of
particles in tumors.
[00114] It is often desirable to use a population of particles that is
relatively uniform in
terms of size, shape, and/or composition so that each particle has similar
properties. For
example, at least 80%, at least 90%, or at least 95% of the particles may have
a diameter or
greatest dimension that falls within 5%, 10%, or 20% of the average diameter
or greatest
dimension. In some embodiments, a population of particles may be heterogeneous
with
respect to size, shape, and/or composition.
[00115] Zeta potential is a measurement of surface potential of a particle. In
some
embodiments, particles have a zeta potential ranging between -50 mV and +50
mV. In some
embodiments, particles have a zeta potential ranging between -25 mV and +25
mV. In some
embodiments, particles have a zeta potential ranging between -10 mV and +10
mV. In some
Page 31 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
embodiments, particles have a zeta potential ranging between -5 mV and +5 mV.
In some
embodiments, particles have a zeta potential ranging between 0 mV and +50 mV.
In some
embodiments, particles have a zeta potential ranging between 0 mV and +25 mV.
In some
embodiments, particles have a zeta potential ranging between 0 mV and +10 mV.
In some
embodiments, particles have a zeta potential ranging between 0 mV and +5 mV.
In some
embodiments, particles have a zeta potential ranging between -50 mV and 0 mV.
In some
embodiments, particles have a zeta potential ranging between -25 mV and 0 mV.
In some
embodiments, particles have a zeta potential ranging between -10 mV and 0 mV.
In some
embodiments, particles have a zeta potential ranging between -5 mV and 0 mV.
In some
embodiments, particles have a substantially neutral zeta potential (i.e.
approximately 0 mV).
[00116] Particles can have a variety of different shapes including spheres,
oblate
spheroids, cylinders, ovals, ellipses, shells, cubes, cuboids, cones,
pyramids, rods (e.g.,
cylinders or elongated structures having a square or rectangular cross-
section), dumbbells,
tetrapods (particles having four leg-like appendages), triangles, prisms, etc.
In some
embodiments, particles can be complex aggregates of particles characterized by
any of these
shapes.
[00117] In some embodiments, particles are microparticles (e.g. microspheres).
In
general, a "microparticle" refers to any particle having a diameter of less
than 1000 m. In
some embodiments, particles are nanoparticles (e.g. nanospheres). In general,
a
"nanoparticle" refers to any particle having a diameter of less than 1000 nm.
In some
embodiments, particles are picoparticles (e.g. picospheres). In general, a
"picoparticle" refers
to any particle having a diameter of less than 1 nm. In some embodiments,
particles are
liposomes. In some embodiments, particles are micelles.
[00118] Particles can be solid or hollow and can comprise one or more layers
(e.g.,
nanoshells, nanorings, etc.). Particles may have a core/shell structure,
wherein the core(s)
and shell(s) can be made of different materials. Particles may comprise
gradient or
homogeneous alloys. Particles may be composite particles made of two or more
materials, of
which one, more than one, or all of the materials possesses magnetic
properties, electrically
detectable properties, and/or optically detectable properties.
[00119] In certain embodiments, a particle is porous, by which is meant that
the particle
contains holes or channels, which are typically small compared with the size
of a particle.
For example a particle may be a porous silica particle, e.g., a mesoporous
silica nanoparticle
or may have a coating of mesoporous silica (Lin et al., 2005, J. Am. Chem.
Soc., 17:4570;
incorporated herein by reference). Particles may have pores ranging from about
1 nm to
Page 32 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
about 50 nm in diameter, e.g., between about 1 and 20 nm in diameter. Between
about 10%
and 95% of the volume of a particle may consist of voids within the pores or
channels.
[00120] Particles may have a coating layer. Use of a biocompatible coating
layer can be
advantageous, e.g., if the particles contain materials that are toxic to
cells. Suitable coating
materials include, but are not limited to, natural proteins such as bovine
serum albumin
(BSA), biocompatible hydrophilic polymers such as polyethylene glycol (PEG) or
a PEG
derivative, phospholipid-(PEG), silica, lipids, polymers, carbohydrates such
as dextran, other
nanoparticles that can be associated with nanoparticles etc. Coatings may be
applied or
assembled in a variety of ways such as by dipping, using a layer-by-layer
technique, by self-
assembly, conjugation, etc. Self-assembly refers to a process of spontaneous
assembly of a
higher order structure that relies on the natural attraction of the components
of the higher
order structure (e.g., molecules) for each other. It typically occurs through
random
movements of the molecules and formation of bonds based on size, shape,
composition, or
chemical properties.
[00121] In some embodiments, particles may optionally comprise one or more
dispersion
media, surfactants, release-retarding ingredients, or other pharmaceutically
acceptable
excipient. In some embodiments, particles may optionally comprise one or more
plasticizers
or additives.
[00122] A variety of different particles are of use in accordance with the
invention. In
some embodiments, particles may be intrinsically magnetic particles. In some
embodiments,
fluorescent or luminescent nanoparticles, particles that comprise fluorescent
or luminescent
moieties, and plasmon resonant particles are among the particles that are used
in various
embodiments. In some embodiments, polymeric particles may be used in
accordance with
the present invention if they heat in response to external stimuli (e.g. if
particles absorb radio
frequency and/or optical energy).
Thermally-Responsive Linkers
[00123] The present invention provides thermally-responsive conjugates
comprising one
or more heatable surfaces, thermally-responsive linkers, and agents to be
delivered. In
general, a thermally-responsive linker mediates the association between an
agent to be
delivered and a heatable surface in a temperature-sensitive manner. For
example, when
exposed to temperatures below a characteristic temperature and/or range of
temperatures
(referred to herein as the "trigger temperature"), a thermally-responsive
linker can mediate
the association between an agent to be delivered and a heatable surface. When
the thermally-
Page 33 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
responsive linker and/or a conjugate comprising a thermally-responsive linker
is exposed to
the trigger temperature and/or temperatures higher than the trigger
temperature, the
thermally-responsive linker is no longer capable of mediating the association
between the two
or more entities (i.e. the thermally-responsive linker is "disrupted"), and
the agent to be
delivered is released from the heatable surface.
[00124] Any substance that is responsive to changes in temperature (e.g.
displays different
properties at different temperatures) may be a thermally-responsive linker in
accordance with
the present invention. In some embodiments, thermally-responsive linkers
comprise at least
two individual components which interact with one another in a temperature-
sensitive
manner. In some embodiments, thermally-responsive linkers mediate the
association of a
conjugate assembly in which disruption of the conjugate assembly results in
release of the
agent to be delivered. In some embodiments, thermally-responsive linkers
comprise a single
component which mediates the association of two or more moieties (e.g.
heatable surfaces) in
a temperature-sensitive manner. In some embodiments, thermally-responsive
linkers
comprise at least one individual component which has a temperature-sensitive
three-
dimensional conformation. In some embodiments, thermally-responsive linkers
comprise
nucleic acids; peptides and/or proteins; carbohydrates; and/or polymers. In
certain
embodiments, thermally-responsive linkers comprise complimentary Watson-Crick
base
pairing of nucleic acid strands (e.g. DNA, RNA, and/or PNA strands). In
certain
embodiments, thermally-responsive linkers comprise nucleic acids whose
properties result
from the three-dimensional structure of the nucleic acid (e.g. an aptamer). In
certain
embodiments, thermally-responsive linkers comprise interactions between
complimentary
peptides, lipids, polymers, and/or carbohydrates. In certain embodiments,
thermally-
responsive linkers comprise proteins which can undergo temperature dependent
conformational changes.
[00125] In certain embodiments, a thermally-responsive linker may include a
disulfide
bridge (Oishi et al., 2005, J. Am. Chem. Soc., 127:1624; incorporated herein
by reference). In
some embodiments, a thermally-responsive linker may include a transition metal
complex
that falls apart when the metal is reduced. In specific embodiments, a
thermally-responsive
linker may include an acid-labile thioester. In some embodiments, a thermally-
responsive
linker includes an aminocaproic acid (also termed aminohexanoic acid) linkage.
[00126] In certain embodiments, a thermally-responsive linker comprises any
material that
swells and/or shrinks in response to temperature changes. In certain
embodiments, a
thermally-responsive linker comprises any material that swells and/or shrinks
in response to
Page 34 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
temperature changes and also that does not break in response to temperature
changes. For
example, such a thermally-responsive linker may include a polymer such as
pNIPAM.
[00127] A thermally-responsive linker typically comprises between
approximately 2 to
approximately 1000 atoms, between approximately 2 to approximately 750 atoms,
between
approximately 2 to approximately 500 atoms, between approximately 2 to
approximately 250
atoms, between approximately 2 to approximately 100 atoms, or between about 6
to about 30
atoms. In some embodiments, a thermally-responsive linker suitable for the
practice of the
invention may be a flexible linker. In some embodiments, a thermally-
responsive linker
suitable for the practice of the invention may not be a flexible linker.
[00128] Disruption of the linker typically occurs at sites where temperature
triggers are
present. For example, when a conjugate comprising a thermally-responsive
linker is exposed
to a trigger temperature, disruption of the linker leads to separation of the
heatable surface
and agent to be delivered. Whereas, without exposure to the trigger
temperature, the agent to
be delivered remains associated with the particle.
[00129] In some embodiments, disruption of the linker occurs at temperatures
higher than
ambient temperature. In some embodiments, disruption of the linker occurs at
temperatures
higher than body temperature. In some embodiments, disruption of the linker
occurs at a
precise temperature. In some embodiments, disruption of the linker occurs at
approximately
15 C, approximately 20 C, approximately 25 C, approximately 30 C,
approximately 35
C, approximately 40 C, approximately 45 C, approximately 50 C,
approximately 55 C,
or approximately 60 C. In some embodiments, disruption of the linker occurs
at
approximately 23 C, approximately 24 C, approximately 25 C, approximately
26 C,
approximately 27 C, approximately 28 C, approximately 29 C, approximately
30 C,
approximately 31 C, approximately 32 C, approximately 33 C, approximately
34 C,
approximately 35 C, approximately 36 C, approximately 37 C, approximately
38 C,
approximately 39 C, approximately 40 C, approximately 41 C, approximately
42 C,
approximately 43 C, approximately 44 C, approximately 45 C, or higher. In
some
embodiments, disruption of the linker occurs over a range of temperatures. In
some
embodiments, disruption of the linker occurs at temperatures ranging between
15 C to 20
C, between 20 C to 25 C, between 25 C to 30 C, between 30 C to 35 C,
between 35 C
to 40 C, or between 40 C to 45 C.
Nucleic Acid Linkers
[00130] In some embodiments, thermally-responsive linkers include nucleic acid
residues
and may comprise between approximately 1 to approximately 100, between
approximately 1
Page 35 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
to approximately 50, between approximately 1 to approximately 30, between
approximately 2
to approximately 20, or between approximately 2 to approximately 10 nucleic
acid residues
joined by phosphodiester linkages. In some embodiments, thermally-responsive
linkers
comprise approximately 4, approximately 6, approximately 8, approximately 10,
approximately 12, approximately 14, approximately 16, approximately 18,
approximately 20,
approximately 22, approximately 24, approximately 26, approximately 28,
approximately 30,
or more nucleic acid residues joined by phosphodiester linkages.
[00131] The present invention encompasses the recognition that thermally-
responsive
linkers may be modulated such that the agent to be delivered is releases at
different trigger
temperatures. Such modulation enables production of thermally-responsive
linkers having a
specific and/or desired trigger temperature and enables multiplexing of
several different drug
release schemes (described in further detail below). In some embodiments, the
trigger
temperature can be modulated by varying the number of complimentary
hybridizing bases on
the nucleic acid strands. In this manner, an external stimulus (e.g. an EM
field, light, etc.)
can be introduced such that, to give but one example, a thermally-responsive
linker having a
12 bp duplex region is disrupted, while a thermally-responsive linker having a
longer duplex
region (e.g. 14, 16, 18, 20, 22, 24, or more bp duplex region) is not
disrupted.
[00132] In some embodiments, the duplex region does not comprise any
nucleotide
mismatches. In some embodiments, the duplex region may be interrupted by 1, 2,
3, 4, 5, or
more nucleotide mismatches. In some embodiments, the nucleotide mismatches may
be
contiguous (i.e. mismatches are adjacent to one another). In some embodiments,
the
nucleotide mismatches may be non-contiguous (i.e. mismatches are separated by
one or more
base pairs). In general, the presence of mismatches decreases the trigger
temperature relative
to the absence of mismatches.
[00133] In some embodiments, a thermally-responsive linker comprises a duplex
region
and at least one single-stranded nucleic acid overhang on either side or both
sides of the
duplex region. In some embodiments, the duplex region comprises approximately
4,
approximately 6, approximately 8, approximately 10, approximately 12,
approximately 14,
approximately 16, approximately 18, approximately 20, approximately 22,
approximately 24,
approximately 26, approximately 28, approximately 30, or more base pairs. In
some
embodiments, the single-stranded overhang comprises approximately 1,
approximately 2,
approximately 3, approximately 4, approximately 5, approximately 6,
approximately 7,
approximately 8, approximately 9, approximately 10, approximately 15,
approximately 20,
Page 36 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
approximately 25, approximately 30, approximately 35, approximately 40,
approximately 45,
approximately 50, or more nucleotides.
[00134] In some embodiments, the trigger temperature can be modulated by
varying the
nucleotide content of the nucleic acid strands. For example, increasing the
amount of
guanine and/or cytosine relative to the amount of adenine, thymine, and/or
uracil tends to
raise the trigger temperature of a thermally-responsive linker. Likewise,
increasing the
amount of adenine, thymine, and/or uracil relative to the amount of guanine
and/or cytosine
tends to lower the trigger temperature of a thermally-responsive linker.
[00135] In some embodiments, the trigger temperature can be modulated by
including one
or more modified nucleotide residues, which are described in further detail
below.
[00136] For example, locked nucleic acid (LNA), a bicyclic high-affinity RNA
mimic in
which the sugar ring is locked in the 3'-endo conformation by the introduction
of a methylene
bridge group connecting the 2'-O atom with the 4'-C atom. It has been
regularly
demonstrated that the incorporation of LNA into an oligonucleotide probe
greatly increases
the affinity of that probe for its complementary target. In some embodiments,
this is
expressed as an increase in melting temperature (T,,,) and/or affinity of the
oligonucleotide
probe against its target. For example, whereas a given full-length DNA
oligonucleotide
probe for an miRNA target may have a T,, of 60 C, an LNA-enhanced
oligonucleotide probe
for the same target would have a Tm for target of 74 C. For LNA-enhanced
oligonucleotides, the T,, difference between a perfectly matched target and a
mismatched
target is substantially higher than that observed when a DNA-based
oligonucleotide is used.
See, for example, Roberts et al., Sept. 2006, Nat. Meth., vol. 3 (incorporated
herein by
reference). Therefore, the present invention encompasses the recognition that
LNA-enhanced
oligonucleotides may be used for finely controlling the trigger temperature of
a given nucleic
acid thermally-responsive linker.
[00137] Nucleic acids in accordance with the present invention (including
nucleic acid
targeting moieties and/or functional RNAs to be delivered, e.g., RNAi agents,
ribozymes,
tRNAs, etc., described in further detail above) may be prepared according to
any available
technique including, but not limited to chemical synthesis, enzymatic
synthesis, enzymatic or
chemical cleavage of a longer precursor, etc. Methods of synthesizing RNAs are
known in
the art (see, e.g., Gait, M.J. (ed.) Oligonucleotide synthesis: a practical
approach, Oxford
[Oxfordshire], Washington, DC: IRL Press, 1984; and Herdewijn, P. (ed.)
Oligonucleotide
synthesis: methods and applications, Methods in molecular biology, v. 288
(Clifton, N.J.)
Totowa, N.J.: Humana Press, 2005).
Page 37 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00138] Nucleic acids in accordance with the present invention may comprise
naturally
occurring nucleosides, modified nucleosides, naturally occurring nucleosides
with
hydrocarbon linkers (e.g., an alkylene) or a polyether linker (e.g., a PEG
linker) inserted
between one or more nucleosides, modified nucleosides with hydrocarbon or PEG
linkers
inserted between one or more nucleosides, or a combination of thereof. In some
embodiments, nucleotides or modified nucleotides of a nucleic acid targeting
moiety can be
replaced with a hydrocarbon linker or a polyether linker provided that the
binding affinity and
selectivity of the nucleic acid targeting moiety is not substantially reduced
by the substitution
(e.g., the dissociation constant of the nucleic acid targeting moiety for the
target should not be
greater than about 1 x 10-3 M).
[00139] It will be appreciated by those of ordinary skill in the art that
nucleic acids in
accordance with the present invention may comprise nucleotides entirely of the
types found
in naturally occurring nucleic acids, or may instead include one or more
nucleotide analogs or
have a structure that otherwise differs from that of a naturally occurring
nucleic acid. U.S.
Patents 6,403,779; 6,399,754; 6,225,460; 6,127,533; 6,031,086; 6,005,087;
5,977,089; and
references therein disclose a wide variety of specific nucleotide analogs and
modifications
that may be used. See Crooke, S. (ed.) Antisense Drug Technology: Principles,
Strategies,
and Applications (lst ed), Marcel Dekker; ISBN: 0824705661; 1st edition (2001)
and
references therein. For example, 2'-modifications include halo, alkoxy and
allyloxy groups.
In some embodiments, the 2'-OH group is replaced by a group selected from H,
OR, R, halo,
SH, SRi, NH2, NHR, NR2 or CN, wherein R is Ci-C6 alkyl, alkenyl, or alkynyl,
and halo is F,
Cl, Br, or I. Examples of modified linkages include phosphorothioate and 5'-N-
phosphoramidite linkages.
[00140] Nucleic acids comprising a variety of different nucleotide analogs,
modified
backbones, or non-naturally occurring internucleoside linkages can be utilized
in accordance
with the present invention. Nucleic acids in accordance with the present
invention may
include natural nucleosides (i.e., adenosine, thymidine, guanosine, cytidine,
uridine,
deoxyadenosine, deoxythymidine, deoxyguanosine, and deoxycytidine) or modified
nucleosides. Examples of modified nucleotides include base modified nucleoside
(e.g.,
aracytidine, inosine, isoguanosine, nebularine, pseudouridine, 2,6-
diaminopurine, 2-
aminopurine, 2-thiothymidine, 3-deaza-5-azacytidine, 2'-deoxyuridine, 3-
nitorpyrrole, 4-
methylindole, 4-thiouridine, 4-thiothymidine, 2-aminoadenosine, 2-
thiothymidine, 2-
thiouridine, 5-bromocytidine, 5-iodouridine, inosine, 6-azauridine, 6-
chloropurine, 7-
deazaadenosine, 7-deazaguanosine, 8-azaadenosine, 8-azidoadenosine,
benzimidazole, M1-
Page 38 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
methyladenosine, pyrrolo-pyrimidine, 2-amino-6-chloropurine, 3-methyl
adenosine, 5-
propynylcytidine, 5-propynyluridine, 5-bromouridine, 5-fluorouridine, 5-
methylcytidine, 7-
deazaadenosine, 7-deazaguanosine, 8-oxoadenosine, 8-oxoguanosine, 0(6)-
methylguanine,
and 2-thiocytidine), chemically or biologically modified bases (e.g.,
methylated bases),
modified sugars (e.g., 2'-fluororibose, 2'-aminoribose, 2'-azidoribose, 2'-O-
methylribose, L-
enantiomeric nucleosides arabinose, and hexose), modified phosphate groups
(e.g.,
phosphorothioates and 5'-N-phosphoramidite linkages), and combinations
thereof. Natural
and modified nucleotide monomers for the chemical synthesis of nucleic acids
are readily
available. In some cases, nucleic acids comprising such modifications display
improved
properties relative to nucleic acids consisting only of naturally occurring
nucleotides. In
some embodiments, nucleic acid modifications described herein are utilized to
reduce and/or
prevent digestion by nucleases (e.g. exonucleases, endonucleases, etc.). For
example, the
structure of a nucleic acid may be stabilized by including nucleotide analogs
at the 3' end of
one or both strands order to reduce digestion.
[00141] Modified nucleic acids need not be uniformly modified along the entire
length of
the molecule. Different nucleotide modifications and/or backbone structures
may exist at
various positions in the nucleic acid. One of ordinary skill in the art will
appreciate that the
nucleotide analogs or other modification(s) may be located at any position(s)
of a nucleic acid
such that the function of the nucleic acid is not substantially affected. To
give but one
example, modifications may be located at any position of an aptamer such that
the ability of
the aptamer to specifically bind to the aptamer target is not substantially
affected. The
modified region may be at the 5'-end and/or the 3'-end of one or both strands.
For example,
modified aptamers in which approximately 1-5 residues at the 5' and/or 3' end
of either of
both strands are nucleotide analogs and/or have a backbone modification have
been
employed. The modification may be a 5' or 3' terminal modification. One or
both nucleic
acid strands may comprise at least 50% unmodified nucleotides, at least 80%
unmodified
nucleotides, at least 90% unmodified nucleotides, or 100% unmodified
nucleotides.
[00142] Nucleic acids in accordance with the present invention may, for
example,
comprise a modification to a sugar, nucleoside, or internucleoside linkage
such as those
described in U.S. Patent Publications 2003/0175950, 2004/0192626,
2004/0092470,
2005/0020525, and 2005/0032733 (all of which are incorporated herein by
reference). The
present invention encompasses the use of any nucleic acid having any one or
more of the
modification described therein. For example, a number of terminal conjugates,
e.g., lipids
such as cholesterol, lithocholic acid, aluric acid, or long alkyl branched
chains have been
Page 39 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
reported to improve cellular uptake. Analogs and modifications may be tested
using, e.g.,
using any appropriate assay known in the art, for example, to select those
that result in
improved efficacy of a therapeutic agent, improved specific binding of an
aptamer to an
aptamer target, etc. In some embodiments, nucleic acids in accordance with the
present
invention may comprise one or more non-natural nucleoside linkages. In some
embodiments,
one or more internal nucleotides at the 3'-end, 5'-end, or both 3'- and 5'-
ends of the aptamer
are inverted to yield a linkage such as a 3' - 3' linkage or a 5' - 5'
linkage.
[00143] In some embodiments, nucleic acids in accordance with the present
invention are
not synthetic, but are naturally-occurring entities that have been isolated
from their natural
environments.
Protein Linkers
[00144] In some embodiments, thermally-responsive linkers include amino acid
residues
and may range from about 5 to about 5000, 5 to about 1000, about 5 to about
750, about 5 to
about 500, about 5 to about 250, about 5 to about 100, about 5 to about 75,
about 5 to about
50, about 5 to about 40, about 5 to about 30, about 5 to about 25, about 5 to
about 20, about 5
to about 15, or about 5 to about 10 amino acids in size. As used herein, the
term "peptide"
refers to a polypeptide having a length of less than about 100 amino acids.
Peptides from
panels of peptides comprising random sequences and/or sequences which have
been varied
consistently to provide a maximally diverse panel of peptides may be used.
[00145] Polypeptides may contain L-amino acids, D-amino acids, or both and may
contain
any of a variety of amino acid modifications or analogs known in the art.
Useful
modifications include, e.g., terminal acetylation, amidation, etc. In some
embodiments,
polypeptides may comprise natural amino acids, unnatural amino acids,
synthetic amino
acids, and combinations thereof
[00146] In some embodiments, protein and/or peptide linkers may comprise two
or more
moieties that interact with one another in a heat-sensitive manner. Protein-
based interactions
may be heat-sensitive if their association is at least partially-mediated by
hydrogen bonding.
In some embodiments, thermally-responsive linkers may include any protein-
protein
interaction domains that involve hydrogen bonding. In certain embodiments,
thermally-
responsive linkers may be based on coil geometries (e.g. a-helices, leucine
zippers, collagen
helices, etc.), (3-sheet motifs (e.g. amphiphilic peptides), etc.
[00147] In some embodiments, protein and/or peptide linkers may comprise any
heat-
sensitive affinity interaction. In certain embodiments, protein and/or peptide
linkers may
comprise ligand-receptor interactions (e.g. TGFa-EGF receptor interactions).
In some
Page 40 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
embodiments, protein and/or peptide linkers may comprise antibody-antigen
interactions. In
some embodiments, protein and/or peptide linkers may comprise other types of
affinity
interactions (e.g. any two proteins which specifically bind to one another).
Carbohydrate Linkers
[00148] In some embodiments, thermally-responsive linkers include
carbohydrates.
Carbohydrates may be monosaccharides, disaccharides, and/or polysaccharides.
In some
embodiments, carbohydrate linkers may comprise between approximately 1 to
approximately
100, between approximately 1 to approximately 50, between approximately 1 to
approximately 30, between approximately 2 to approximately 20, or between
approximately
2 to approximately 10 monosaccharides joined by glycosidic linkages.
[00149] A carbohydrate may be natural or synthetic. A carbohydrate may also be
a
derivatized natural carbohydrate. In certain embodiments, a carbohydrate may
be a simple or
complex sugar. In certain embodiments, a carbohydrate is a monosaccharide,
including but
not limited to glucose, fructose, galactose, and ribose. In certain
embodiments, a
carbohydrate is a disaccharide, including but not limited to lactose, sucrose,
maltose,
trehalose, and cellobiose. In certain embodiments, a carbohydrate is a
polysaccharide,
including but not limited to cellulose, microcrystalline cellulose,
hydroxypropyl
methylcellulose (HPMC), methylcellulose (MC), dextrose, dextran, glycogen,
xanthan gum,
gellan gum, starch, and pullulan. In certain embodiments, a carbohydrate is a
sugar alcohol,
including but not limited to mannitol, sorbitol, xylitol, erythritol, malitol,
and lactitol.
Polymer Linkers
[00150] In some embodiments, thermally-responsive linkers include polymers
(e.g.
synthetic polymers). In some embodiments, polymer-based embodiments encompass
sol-gel
hydrogels whose transition is based on temperature, including natural
polymers,
poly(ethylene oxide)/poly (propylene oxide) block copolymers, N-
isopropylacrylamide
copolymers, etc. In some embodiments, polymer-based thermally-responsive
linkers may
comprise multiphase hydrogels (see, e.g., Ehrick et al., 2005, Nat. Mater.,
4:298;
incorporated herein by reference).
Hybrid Linkers
[00151] In some embodiments, thermally-responsive linkers are hybrid linkers.
In some
embodiments, the term "hybrid linkers" refers to thermally-responsive linkers
comprise at
least two of the following: nucleic acids, proteins/peptides, carbohydrates,
lipids, polymers,
and small molecules. To give but one example, in certain embodiments,
thermally-
responsive linkers may comprise affinity interactions based on small
molecules,
Page 41 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
carbohydrates, lipids, polymers, and/or nucleic acids interacting with
peptides, proteins,
glycoproteins, and/or proteoglycans. To give another example, in certain
embodiments,
thermally-responsive linkers may comprise affinity interactions based on small
molecules,
carbohydrates, lipids, polymers, peptides, proteins, glycoproteins, and/or
proteoglycans
interacting with nucleic acids.
Mechanisms of Action of Thermally-Responsive Linkers
[00152] Multi-Component Thermally-Responsive Linkers
[00153] In some embodiments, thermally-responsive linkers comprise at least
two
individual components which associate with one another below the trigger
temperature, but
do not associate with one another at and/or above the trigger temperature.
Typically, one
individual component is associated with the heatable surface, and another
individual
component is associated with the agent to be delivered. In some embodiments,
the
association is covalent. In some embodiments, the association is non-covalent
(e.g. hydrogen
bonding, charge interactions, affinity interactions, van der Waals forces,
etc.).
[00154] In certain embodiments, thermally-responsive linkers comprise at least
two
complementary nucleic acid strands (e.g. DNA, RNA, PNA, and/or combinations
thereof).
To give but one example, one nucleic acid strand may be associated with the
heatable surface
(e.g. covalently), and a second nucleic acid strand is associated with the
agent to be delivered
(e.g. covalently; see, for example, Figure 1). At least a portion of each
nucleic acid strand is
complementary to the other strand, and the complementary portions anneal (i.e.
via hydrogen
bonding, to form a "duplex region") when the temperature is below a
characteristic trigger
temperature. However, when exposed to the trigger temperature or to
temperatures higher
than the trigger temperature (e.g. when an EM field sufficiently heats the
conjugate such that
the trigger temperature is reached), the two strands denature and dissociate
from one another
(i.e. the duplex is disrupted), and the agent to be delivered is released from
the heatable
surface.
[00155] In some embodiments, heat labile linkers may comprise interactions
among
proteins and/or peptides having coil geometries (e.g. a-helices, leucine
zippers, collagen
helices, etc.), (3-sheet motifs (e.g. amphiphilic peptides), etc. For example,
one a-helix of a
leucine zipper motif may be associated with the heatable surface, and the
second a-helix of
the leucine zipper motif may be associated with the agent to be delivered. The
two a-helices
associate with one another when the temperature is below a characteristic
trigger temperature,
forming the leucine zipper moti However, when exposed to the trigger
temperature, the two
Page 42 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
a-helices dissociate from one another, and the agent to be delivered is
released from the
heatable surface.
[00156] In some embodiments, heat labile linkers may comprise a ligand-
receptor
interaction. For example, a ligand (e.g. TGFa) may be associated with the
heatable surface,
and a receptor to which the ligand binds (e.g. EGF receptor) may be associated
with the agent
to be delivered. Alternatively, the ligand may be associated with the agent to
be delivered,
and the receptor may be associated with the heatable surface. The ligand and
receptor
associate with one another when the temperature is below a characteristic
trigger temperature.
However, when exposed to the trigger temperature, the ligand and receptor
dissociate from
one another, and the agent to be delivered is released from the heatable
surface.
[00157] In some embodiments, heat labile linkers may comprise an antibody-
antigen
interaction. For example, an antibody may be associated with the heatable
surface, and an
antigen to which the antibody binds may be associated with the agent to be
delivered.
Alternatively, the antibody may be associated with the agent to be delivered,
and the antigen
may be associated with the heatable surface. The antibody and antigen
associate with one
another when the temperature is below a characteristic trigger temperature.
However, when
exposed to the trigger temperature, the antibody and antigen dissociate from
one another, and
the agent to be delivered is released from the heatable surface.
[00158] In some embodiments, heat labile linkers may comprise an enzyme-
substrate
interaction. For example, glutathione S-transferase (GST) may be associated
with the
heatable surface, and glutathione may be associated with the agent to be
delivered.
Alternatively, GST may be associated with the agent to be delivered, and
glutathione may be
associated with the heatable surface. GST and glutathione associate with one
another when
the temperature is below a characteristic trigger temperature. However, when
exposed to the
trigger temperature, GST and glutathione dissociate from one another, and the
agent to be
delivered is released from the heatable surface.
[00159] In some embodiments, heat labile linkers may comprise another type of
affinity
interaction (e.g. an interaction between any entities which specifically bind
to one another).
For example, streptavidin may be associated with the heatable surface, and
biotin may be
associated with the agent to be delivered. Alternatively, biotin may be
associated with the
agent to be delivered, and streptavidin may be associated with the heatable
surface.
Streptavidin and biotin associate with one another when the temperature is
below a
characteristic trigger temperature. However, when exposed to the trigger
temperature, biotin
and streptavidin dissociate from one another, and the agent to be delivered is
released from
Page 43 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
the heatable surface. In some embodiments, thermally-responsive linkers may be
based upon
Ni-NTA interactions; peptide-metal interactions or peptide-semiconductor
interactions (see,
e.g., Whaley et al., 2000, Nature, 405:665; incorporated herein by reference);
small
molecule-target interactions; and/or adsorbed small molecule interactions.
[00160] In some embodiments, thermally-responsive linkers mediate the
association of a
conjugate assembly for which disruption of the conjugate assembly results in
release of the
agent to be delivered. To give but one example, the agent to be delivered may
be associated
with a thermally-responsive linker which is a single-stranded nucleic acid. A
heatable
surface may be associated with a single-stranded nucleic acid adapter that is
at least partially
complementary to the thermally-responsive linker. The thermally-responsive
linker, thus, is
able to associate with the adapter via Watson-Crick base pairing, thereby
forming a duplex
region. In some embodiments, the thermally-responsive linker is able to
associate with two
or more adapters simultaneously, thereby joining together two or more heatable
surfaces.
When the conjugate is subjected to an external stimulus (e.g. placed in an EM
field) which
heats the particles to and/or above the trigger temperature, nucleic acid
duplexes are
disrupted, releasing the linker nucleic acid and the agent to be delivered
while disassociating
the particles from each other. Figure 2 shows one example of such a conjugate
assembly
containing two particles, but one of ordinary skill in the art will recognize
that the conjugate
assembly may comprise many more particle linkages than one.
[00161] To give another example of a conjugate assembly, the agent to be
delivered may
be associated with an antigen that has multiple binding sites for an antibody
(e.g. several
epitopes in tandem). A heatable surface may be associated with an antibody
that specifically
binds to the antigen. The antigen is able to associate with several antibodies
at once; thus the
agent to be delivered is able to associate with two or more heatable surfaces
simultaneously,
thereby joining together two or more heatable surfaces. When the conjugate is
subjected to
an external stimulus (e.g. placed in an EM field) which heats the particles to
and/or above the
trigger temperature, the antibody-antigen associations are disrupted,
releasing the antigen and
the agent to be delivered while disassociating the particles from each other.
This example
described the use of antibody-antigen interactions to build such a conjugate
assembly
conjugate assembly, but one of ordinary skill in the art will recognize that
any protein-protein
interaction (e.g. affinity interaction, enzyme-substrate interaction, ligand-
receptor interaction,
interactions among proteins and/or peptides having coil geometries, and so
forth) may be
utilized to build such a conjugate assembly.
Page 44 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00162] In some embodiments, conjugate assemblies may enable triggered
enhancement of
component transport or clearance. For example, a conjugate assembly may be too
large for
clearance from the body, but the individual conjugates within the assembly may
be small
enough for clearance from the body.
[00163] Although the specific examples provided herein relate to thermally-
responsive
linkers comprising nucleic acids, peptides, and/or proteins, one of ordinary
skill in the art will
readily recognize that conjugates in accordance with the present invention may
comprise
thermally-responsive linkers comprising any moieties (e.g. nucleic acids,
peptides and/or
proteins, carbohydrates, lipids, polymers, etc.) which associate with one
another in a
temperature-sensitive manner.
[00164] The present invention encompasses the recognition that thermally-
responsive
linkers may be modulated such that the agent to be delivered is releases at
different trigger
temperatures, enabling multiplexing of several different drug release schemes.
For example,
the nucleotide content of nucleic acid thermally-responsive linkers may be
modified such that
a set of linkers is generated, in which each member of the set is
characterized by a different
nucleotide content (e.g. nucleotide sequence) and, consequently, a different
trigger
temperature. Modulation of nucleic acid thermally-responsive linkers is
described in further
detail above, in the section entitled "Nucleic Acid Linkers."
[00165] To give another example, the amino acid sequence of a protein
thermally-
responsive linker may be modified such that a set of linkers is generated in
which each
member of the set is characterized by a different trigger temperature. For
example, for a
linker that is based upon the interaction between an antibody and an antigen,
the amino acid
sequence of the antigen may be modified in several different ways in order to
generate a set
of mutated antigens. Each member of the set of antigens may have a different
binding
affinity for the antibody, and consequently, a different trigger temperature.
[00166] Single-Component Thermally-Responsive Linkers
[00167] In some embodiments, thermally-responsive linkers comprise at least
one
individual component which has a temperature-sensitive three-dimensional
conformation.
Such thermally-responsive linkers may include nucleic acids, peptides,
proteins,
carbohydrates, hybrid biopolymers (e.g. as described in the section entitled
"Hybrid
Linkers"), a-helical motifs, (3-sheet assemblies, sol-gel polymers, etc.
[00168] In certain embodiments, thermally-responsive linkers comprise proteins
and/or
peptides which can undergo temperature-dependent conformational changes. In
some
Page 45 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
embodiments, protein and/or peptide structures containing hydrogen bonds (e.g.
a-helices, R-
sheets, amphiphilic peptides, etc.) encapsulate hydrophobic agents in the
interior of the
structures and, upon disassociation (e.g. upon exposure to a trigger
temperature), release the
agents to be delivered. In some embodiments, release can occur because the
protein and/or
peptide structure is no longer able to contain the agent to be delivered (e.g.
the agent to be
delivered can "leak out" of the protein and/or peptide structure).
[00169] In some embodiments, protein and/or peptide structures may associate
with agents
to be delivered in a manner that is dependent on the three-dimensional
structure of the protein
(and/or peptide) and/or the agent to be delivered. In some embodiments,
release can occur
because the protein and/or peptide structure no longer associates with the
agent to be
delivered.
[00170] In some embodiments, the protein and/or peptide structure is wholly
denatured
upon exposure to the trigger temperature. In some embodiments, the protein
and/or peptide
structure is only partially denatured upon exposure to the trigger
temperature. In some
embodiments, part of the protein and/or peptide structure is wholly denatured
and part of the
protein and/or peptide structure is not denatured upon exposure to the trigger
temperature. In
some embodiments, part of the protein and/or peptide structure is wholly
denatured and part
of the protein and/or peptide structure is only partially denatured upon
exposure to the trigger
temperature. In some embodiments, part of the protein and/or peptide structure
is partially
denatured and part of the protein and/or peptide structure is not denatured
upon exposure to
the trigger temperature.
[00171] In some embodiments, the rate of release of the agent to be delivered
correlates
with the extent to which the protein and/or peptide structure is denatured. In
other words,
more complete denaturation may result in more rapid, more effective, and/or
more complete
release of the agent to be delivered.
[00172] In certain embodiments, thermally-responsive linkers comprise nucleic
acids
whose properties result from the three-dimensional structure of the nucleic
acid (e.g. an
aptamer). An aptamer refers to a polynucleotide that binds to a specific
target structure that
is associated with a particular organ, tissue, cell, subcellular locale,
and/or extracellular
matrix locale. In some embodiments, agents to be delivered (e.g. small
molecule drugs) can
non-covalently associate with aptamers in a temperature-sensitive manner
(Bagalkot et al.,
200, Angew Chem. Int. Ed. Engl., 45:8149; incorporated herein by reference).
In some
embodiments, the agent is released from the aptamer at and/or above the
trigger temperature.
Page 46 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
In some embodiments, release can occur because the aptamer no longer
associates with the
agent to be delivered.
[00173] In some embodiments, binding of the agent to the aptamer depends at
least partly
on the three-dimensional conformation of the aptamer. In some embodiments,
binding of an
aptamer to an agent is mediated by the interaction between the two- and/or
three-dimensional
structures of both the aptamer and the drug. In some embodiments, binding of
an aptamer to
an agent is not solely based on the primary sequence of the aptamer, but
depends on the
three-dimensional structure(s) of the aptamer and/or agent.
[00174] In some embodiments, an aptamer is wholly denatured upon exposure to
the
trigger temperature. In some embodiments, the aptamer is only partially
denatured upon
exposure to the trigger temperature. In some embodiments, part of the aptamer
is wholly
denatured and part of the aptamer is not denatured upon exposure to the
trigger temperature.
In some embodiments, part of the aptamer is wholly denatured and part of the
aptamer is only
partially denatured upon exposure to the trigger temperature. In some
embodiments, part of
the aptamer is partially denatured and part of the aptamer is not denatured
upon exposure to
the trigger temperature.
[00175] In some embodiments, the rate of release of the agent to be delivered
correlates
with the extent to which the aptamer is denatured. In other words, more
complete
denaturation may result in more rapid, more effective, and/or more complete
release of the
agent to be delivered.
[00176] To give but one example, some agents (e.g. doxorubicin) are known to
be capable
of intercalating between the bases of nucleic acid molecules. In some
embodiments, an agent
to be delivered may intercalate between the bases of a nucleic acid thermally-
responsive
linker in a temperature-sensitive manner.
[00177] Although the embodiments described above relate to proteins, peptides,
and/or
nucleic acid single-component thermally-responsive linkers, one of ordinary
skill in the art
will readily recognize that the same principles apply for single-component
thermally-
responsive linkers comprising carbohydrates, lipids, polymers, and/or any
substance having a
temperature-sensitive three-dimensional conformation.
Agent to Be Delivered
[00178] According to the present invention, thermally-responsive conjugates
may be used
for delivery of any agent, including, for example, therapeutic, diagnostic,
prophylactic, and/or
nutraceutical agents. One of ordinary skill in the art will appreciate that
any agent can be
Page 47 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
delivered by the compositions and methods in accordance with the present
invention. In
some embodiments, agents to be delivered may include any molecule, material,
substance, or
construct that may be transported into a cell by conjugation to a nano- or
micro-structure.
Exemplary agents to be delivered in accordance with the present invention
include, but are
not limited to, small molecules, organometallic compounds, nucleic acids (e.g.
DNA, RNA,
peptide nucleic acids, etc.), proteins (including multimeric proteins, protein
complexes, etc.),
peptides, lipids, carbohydrates, hormones, metals, radioactive elements and
compounds,
hydrophobic drugs, hydrophilic drugs, vaccines, immunological agents, organic
constructs,
inorganic constructs, inhibitors, catalysts, nanoparticles, microparticles,
etc., and/or
combinations thereof.
[00179] One of ordinary skill in the art will appreciate that an agent to be
delivered should
retain at least part of its therapeutic effectiveness (e.g. biological and/or
physiological
activity) at or above the trigger temperature of the conjugate with which the
agent is
associated.
[00180] In some embodiments, each particle of a thermally-responsive conjugate
comprises one or more agents to be delivered. In some embodiments, each
particle of a
thermally-responsive conjugate comprises exactly one agent to be delivered. In
some
embodiments, some of the particles of a population of thermally-responsive
conjugates
comprise one or more agents to be delivered. In some embodiments, some of the
particles of
a population of thermally-responsive conjugates do not comprise any agents to
be delivered.
[00181] In some embodiments, conjugates comprise less than 50% by weight, less
than
40% by weight, less than 30% by weight, less than 20% by weight, less than 15%
by weight,
less than 10% by weight, less than 5% by weight, less than 1% by weight, less
than 0.5% by
weight, less than 0.1% by weight, or less than 0.05% by weight of the agent to
be delivered.
[00182] In some embodiments, the agent to be delivered may be a mixture of
pharmaceutically active agents. For example, a local anesthetic may be
delivered in
combination with an anti-inflammatory agent such as a steroid. To give but
another example,
an antibiotic may be combined with an inhibitor of the enzyme commonly
produced by
bacteria to inactivate the antibiotic (e.g., penicillin and clavulanic acid).
[00183] In some embodiments, the agent to be delivered may be useful for
treating growth
deficiencies. For example, the agent to be delivered may be a growth hormone
(e.g. human
growth hormone). In some embodiments, the agent to be delivered may be useful
for treating
diabetes. In some embodiments, the agent to be delivered may be insulin.
Page 48 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00184] In certain embodiments, the drug is an anti-atherosclerotic agent
(e.g., beta-
blockers, cholesterol lowering agents, etc.). In some embodiments, the drug is
a cholesterol
lowering agent (e.g., lovastatin, pravastatin, simvastatin, fluvastatin,
atorvastatin, niacin,
etc.). In some embodiments, the drug is an anti-inflammatory agent (e.g.,
prednisone;
dexamethasone, fluorometholone; prednisolone; methylprednisolone; clobetasol;
halobetasol;
hydrocortisone; triamcinolone; betamethasone; fluocinolone; fluocinonide;
loteprednol;
medrysone; rimexolone; celecoxib; folic acid; diclofenac; diflunisal;
fenoprofen;
flurbiprofen; indomethacin; ketoprofen; meclofenamate; meclofamate; piroxicam;
sulindac;
salsalate; nabumetone; oxaprozin; tolmetin; hydroxychloroquine sulfate;
rofecoxib;
etanercept; infliximab; leflunomide; naproxen; oxaprozin; piroxicam;
salicylates; valdecoxib;
sulfasalazine; methylprednisolone; ibuprofen; budesonide, meloxicam;
methylprednisolone
acetate; gold sodium thiomalate; aspirin; azathioprine; triamcinolone
acetonide; propxyphene
napsylate/apap; folate; nabumetone; diclofenac; ketorolac; piroxicam;
etodolac; diclofenac
sodium; diclofenac potassium; oxaprozin; methotrexate; minocycline; tacrolimus
(FK-506);
sirolimus (rapamycin) and rapamycin analogs; phenylbutazone; diclofenac
sodium/misoprostol; acetaminophen; indomethacin; glucosamine
sulfate/chondroitin;
cyclosporin, etc.). In some embodiments, the drug is an anti-platelet agent
(e.g., aspirin,
clopidogrel, ticlopidine, dipyridamole, glycoprotein IIb/IIIa receptor blocker
[e.g., abciximab,
eptifibatide, tirofiban], cilostazol, etc.). In some embodiments, the drug is
an anti-coagulant
(e.g., warfarin, acenocoumarol, phenprocoumon, phenindione, heparin, low
molecular weight
heparin, fondaparinux, etc.). In some embodiments, the drug is an anti-
proliferative agent
(e.g., alkylating agents, antimetabolites, plant alkaloids, vinca alkaloids,
taxanes,
podophyllotoxin, topoisomerase inhibitors, hormonal therapy, antitumor
antibiotics, etc.). In
some embodiments, the drug is a cytotoxic agent. In certain embodiments, the
drug is an
immunosuppressant (e.g., glucocorticoids, cytostatics [e.g., alkylating
agents, methotrexate,
azathioprine, mercaptopurine], antibodies, cyclosporin, tacrolimus, sirolimus,
interferons,
opiods, TNF binding proteins, mycophenolate, etc.). In certain embodiments,
the agent is a
drug approved by the United States Food and Drug Administration (U.S.F.D.A.)
for human
or veterinary use.
[00185] In some embodiments, the agent to be delivered may be a mixture of
anti-cancer
agents. In some embodiments, thermally-responsive conjugates are administered
in
combination with one or more of the anti-cancer agents described herein.
Combination
therapy is described in further detail below, in the section entitled,
"Administration." To give
but one example, in some embodiments, conjugates comprising an agent to be
delivered may
Page 49 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
be administered in combination with an alkylating agent. To provide another
example,
compositions comprising an anti-cancer agent to be delivered are administered
in
combination with hormonal therapy. The growth of some types of tumors can be
inhibited by
providing or blocking certain hormones. For example, steroids (e.g.
dexamethasone) can
inhibit tumor growth or associated edema and may cause regression of lymph
node
malignancies. In some cases, prostate cancer is often sensitive to
finasteride, an agent that
blocks the peripheral conversion of testosterone to dihydrotestosterone.
Breast cancer cells
often highly express the estrogen and/or progesterone receptor. Inhibiting the
production
(e.g. with aromatase inhibitors) or function (e.g. with tamoxifen) of these
hormones can often
be used in breast cancer treatments. In some embodiments, gonadotropin-
releasing hormone
agonists (GnRH), such as goserelin possess a paradoxic negative feedback
effect followed by
inhibition of the release of follicle stimulating hormone (FSH) and
leuteinizing hormone
(LH), when given continuously.
Small Molecule Agents
[00186] In some embodiments, the agent to be delivered is a small molecule
and/or
organic compound with pharmaceutical activity. In some embodiments, the agent
is a
clinically-used drug. In some embodiments, the drug is an anti-cancer agent,
antibiotic, anti-
viral agent, anti-HIV agent, anti-parasite agent, anti-protozoal agent,
anesthetic,
anticoagulant, enzyme inhibitor, enzyme activator, steroidal agent, steroidal
or non-steroidal
anti-inflammatory agent, antihistamine, immunosuppressant agent, anti-
neoplastic agent,
antigen, vaccine, antibody, decongestant, sedative, opioid, analgesic, anti-
pyretic, birth
control agent, hormone, prostaglandin, progestational agent, anti-glaucoma
agent, ophthalmic
agent, anti-cholinergic, anti-depressant, anti-psychotic, neurotoxin,
hypnotic, tranquilizer,
anti-convulsant, muscle relaxant, anti-Parkinson agent, anti-spasmodic, muscle
contractant,
channel blocker, miotic agent, anti-secretory agent, anti-thrombotic agent,
anticoagulant, anti-
cholinergic, (3-adrenergic blocking agent, diuretic, cardiovascular active
agent, vasoactive
agent, vasodilating agent, anti-hypertensive agent, angiogenic agent,
modulators of cell-
extracellular matrix interactions (e.g. cell growth inhibitors and anti-
adhesion molecules),
inhibitors of DNA, RNA, or protein synthesis, etc.
[00187] In certain embodiments, the therapeutic agent to be delivered is an
anti-cancer
agent (i.e. cytotoxic agents). Most anti-cancer agents can be divided in to
the following
categories: alkylating agents, antimetabolites, natural products, and hormones
and
antagonists.
Page 50 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00188] Anti-cancer agents typically affect cell division and/or DNA
synthesis. However,
some chemotherapeutic agents do not directly interfere with DNA. To give but
one example,
tyrosine kinase inhibitors (imatinib mesylate/Gleevec ) directly target a
molecular
abnormality in certain types of cancer (chronic myelogenous leukemia,
gastrointestinal
stromal tumors, etc.).
[00189] Alkylating agents are so named because of their ability to add alkyl
groups to
many electronegative groups under conditions present in cells. Alkylating
agents typically
function by chemically modifying cellular DNA. Exemplary alkylating agents
include
nitrogen mustards (e.g. mechlorethamine, cyclophosphamide, ifosfamide,
melphalan (1-
sarcolysin), chlorambucil), ethylenimines and methylmelamines (e.g.
altretamine
(hexamethylmelamine; HMM), thiotepa (triethylene thiophosphoramide),
triethylenemelamine (TEM)), alkyl sulfonates (e.g. busulfan), nitrosureas
(e.g. carmustine
(BCNU), lomustine (CCMU), semustine (methyl-CCNU), streptozocin
(streptozotocin)), and
triazenes (e.g. dacarbazine (DTIC; dimethyltriazenoimidazolecarboxamide)).
[00190] Antimetabolites act by mimicking small molecule metabolites (e.g.
folic acid,
pyrimidines, and purines) in order to be incorporated into newly synthesized
cellular DNA.
Such agents also affect RNA synthesis. An exemplary folic acid analog is
methotrexate
(amethopterin). Exemplary pyrimidine analogs include fluorouracil (5-
fluorouracil; 5-FU),
floxuridine (fluorodeoxyuridine; FUdR), and cytarabine (cytosine arabinoside).
Exemplary
purine analogs include mercaptopurine (6-mercaptopurine; 6-MP), azathioprine,
thioguanine
(6-thioguanine; TG), fludarabine phosphate, pentostatin (2'-deoxycoformycin),
cladribine (2-
chlorodeoxyadenosine; 2-CdA), and erythrohydroxynonyladenine (EHNA).
[00191] Natural small molecule products which can be used as anti-cancer
agents include
plant alkaloids and antibiotics. Plant alkaloids and terpenoids (e.g. vinca
alkaloids,
podophyllotoxin, taxanes, etc.) typically block cell division by preventing
microtubule
function. Vinca alkaloids (e.g. vincristine, vinblastine (VLB), vinorelbine,
vindesine, etc.)
bind to tubulin and inhibit assembly of tubulin into microtubules. Vinca
alkaloids are derived
from the Madagascar periwinkle, Catharanthus roseus (formerly known as Vinca
rosea).
Podophyllotoxin is a plant-derived compound used to produce two other
cytostatic
therapeutic agents, etoposide and teniposide, which prevent cells from
entering the GI and S
phases of the cell cycle. Podophyllotoxin is primarily obtained from the
American Mayapple
(Podophyllum peltatum) and a Himalayan Mayapple (Podophyllum hexandrum).
Taxanes
(e.g. paclitaxel, docetaxel, etc.) are derived from the Yew Tree. Taxanes
enhance stability of
microtubules, preventing the separation of chromosomes during anaphase.
Page 51 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00192] Antibiotics which can be used as anti-cancer agents include
dactinomycin
(actinomycin D), daunorubicin (daunomycin; rubidomycin), doxorubicin,
idarubicin,
bleomycin, plicamycin (mithramycin), and mitomycin (mytomycin C).
[00193] Other small molecules which can be used as anti-cancer agents include
platinum
coordination complexes (e.g. cisplatin (cis-DDP), carboplatin),
anthracenedione (e.g.
mitoxantrone), substituted urea (e.g. hydroxyurea), methylhydrazine
derivatives (e.g.
procarbazine (N-methylhydrazine, MIH), and adrenocortical suppressants (e.g.
mitotane
(o,p'-DDD), aminoglutethimide).
[00194] Hormones which can be used as anti-cancer agents include
adrenocorticosteroids
(e.g. prednisone), aminoglutethimide, progestins (e.g. hydroxyprogesterone
caproate,
medroxyprogesterone acetate, megestrol acetate), estrogens (e.g.
diethylstilbestrol, ethinyl
estradiol), antiestrogen (e.g. tamoxifen), androgens (e.g. testosterone
propionate,
fluoxymesterone), antiandrogens (e.g. flutamide), and gonadotropin-releasing
hormone
analog (e.g. leuprolide).
[00195] Topoisomerase inhibitors act by inhibiting the function of
topoisomerases, which
are enzymes that maintain the topology of DNA. Inhibition of type I or type II
topoisomerases interferes with both transcription and replication of DNA by
upsetting proper
DNA supercoiling. Some exemplary type I topoisomerase inhibitors include
camptothecins
(e.g. irinotecan, topotecan, etc.). Some exemplary type II topoisomerase
inhibitors include
amsacrine, etoposide, etoposide phosphate, teniposide, etc., which are
semisynthetic
derivatives of epipodophyllotoxins, discussed herein.
[00196] In certain embodiments, a small molecule agent can be any drug. In
some
embodiments, the drug is one that has already been deemed safe and effective
for use in
humans or animals by the appropriate governmental agency or regulatory body.
For
example, drugs approved for human use are listed by the FDA under 21 C.F.R.
330.5, 331
through 361, and 440 through 460, incorporated herein by reference; drugs for
veterinary use
are listed by the FDA under 21 C.F.R. 500 through 589, incorporated herein
by reference.
All listed drugs are considered acceptable for use in accordance with the
present invention.
[00197] A more complete listing of classes and specific drugs suitable for use
in the
present invention may be found in Pharmaceutical Drugs: Syntheses, Patents,
Applications
by Axel Kleemann and Jurgen Engel, Thieme Medical Publishing, 1999 and the
Merck
Index: An Encyclopedia of Chemicals, Drugs and Biologicals, Ed. by Budavari et
al., CRC
Press, 1996, both of which are incorporated herein by reference.
Nucleic Acid Agents
Page 52 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00198] In certain embodiments, thermally-responsive conjugates are used to
deliver one
or more nucleic acids (e.g. RNA, DNA, functional RNAs, functional DNAs,
peptide nucleic
acids, etc.) to a specific location such as an organ, tissue, cell,
subcellular locale, and/or
extracellular matrix locale.
[00199] Functional RNA
[00200] In general, a "functional RNA" is an RNA that does not code for a
protein but
instead belongs to a class of RNA molecules whose members characteristically
possess one
or more different functions or activities within a cell. It will be
appreciated that the relative
activities of functional RNA molecules having different sequences may differ
and may
depend at least in part on the particular cell type in which the RNA is
present. Thus the term
"functional RNA" is used herein to refer to a class of RNA molecule and is not
intended to
imply that all members of the class will in fact display the activity
characteristic of that class
under any particular set of conditions. In some embodiments, functional RNAs
include
RNAi-inducing entities (e.g. short interfering RNAs (siRNAs), short hairpin
RNAs
(shRNAs), and microRNAs (miRNAs), antagomirs, etc.), ribozymes, tRNAs, rRNAs,
RNAs
useful for triple helix formation, etc.
[00201] RNAi is an evolutionarily conserved process in which presence of an at
least
partly double-stranded RNA molecule in a eukaryotic cell leads to sequence-
specific
inhibition of gene expression. RNAi was originally described as a phenomenon
in which the
introduction of long dsRNA (typically hundreds of nucleotides) into a cell
results in
degradation of mRNA containing a region complementary to one strand of the
dsRNA (U.S.
Patent 6,506,559; and Fire et al., 1998, Nature, 391:806; both of which are
incorporated
herein by reference). Subsequent studies in Drosophila showed that long dsRNAs
are
processed by an intracellular RNase III-like enzyme called Dicer into smaller
dsRNAs
primarily comprised of two approximately 21 nucleotide (nt) strands that form
a 19 base pair
duplex with 2 nt 3' overhangs at each end and 5'-phosphate and 3'-hydroxyl
groups (see,
e.g., PCT Publication WO 01/75164; U.S. Patent Publications 2002/0086356 and
2003/0108923; Zamore et al., 2000, Cell, 101:25; and Elbashir et al., 2001,
Genes Dev.,
15:188; all of which are incorporated herein by reference).
[00202] Short dsRNAs having structures such as this, referred to as siRNAs,
silence
expression of genes that include a region that is substantially complementary
to one of the
two strands. This strand is referred to as the "antisense" or "guide" strand,
with the other
strand often being referred to as the "sense" strand. The siRNA is
incorporated into a
ribonucleoprotein complex termed the RNA-induced silencing complex (RISC) that
contains
Page 53 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
member(s) of the Argonaute protein family. Following association of the siRNA
with RISC,
a helicase activity unwinds the duplex, allowing an alternative duplex to form
the guide
strand and a target mRNA containing a portion substantially complementary to
the guide
strand. An endonuclease activity associated with the Argonaute protein(s)
present in RISC is
responsible for "slicing" the target mRNA, which is then further degraded by
cellular
machinery.
[00203] Considerable progress towards the practical application of RNAi was
achieved
with the discovery that exogenous introduction of siRNAs into mammalian cells
can
effectively reduce the expression of target genes in a sequence-specific
manner via the
mechanism described above. A typical siRNA structure includes a 19 nucleotide
double-
stranded portion, comprising a guide strand and an antisense strand. Each
strand has a 2 nt 3'
overhang. Typically the guide strand of the siRNA is perfectly complementary
to its target
gene and mRNA transcript over at least 17-19 contiguous nucleotides, and
typically the two
strands of the siRNA are perfectly complementary to each other over the duplex
portion.
However, as will be appreciated by one of ordinary skill in the art, perfect
complementarity is
not required. Instead, one or more mismatches in the duplex formed by the
guide strand and
the target mRNA is often tolerated, particularly at certain positions, without
reducing the
silencing activity below useful levels. For example, there may be 1, 2, 3, or
even more
mismatches between the target mRNA and the guide strand (disregarding the
overhangs).
Thus, as used herein, two nucleic acid portions such as a guide strand
(disregarding
overhangs) and a portion of a target mRNA that are "substantially
complementary" may be
perfectly complementary (i.e., they hybridize to one another to form a duplex
in which each
nucleotide is a member of a complementary base pair) or they may have a lesser
degree of
complementarity sufficient for hybridization to occur. One of ordinary skill
in the art will
appreciate that the two strands of the siRNA duplex need not be perfectly
complementary.
Typically at least 80%, preferably at least 90%, or more of the nucleotides in
the guide strand
of an effective siRNA are complementary to the target mRNA over at least about
19
contiguous nucleotides. The effect of mismatches on silencing efficacy and the
locations at
which mismatches may most readily be tolerated are areas of active study (see,
e.g., Reynolds
et al., 2004, Nat. Biotechnol., 22:326; incorporated herein by reference).
[00204] It will be appreciated that molecules having the appropriate structure
and degree
of complementarity to a target gene will exhibit a range of different
silencing efficiencies. A
variety of additional design criteria have been developed to assist in the
selection of effective
siRNA sequences. Numerous software programs that can be used to choose siRNA
Page 54 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
sequences that are predicted to be particularly effective to silence a target
gene of choice are
available (see, e.g., Yuan et al., 2004, Nucl. Acids. Res., 32:W130; and
Santoyo et al., 2005,
Bioinformatics, 21:1376; both of which are incorporated herein by reference).
[00205] As will be appreciated by one of ordinary skill in the art, RNAi may
be effectively
mediated by RNA molecules having a variety of structures that differ in one or
more respects
from that described above. For example, the length of the duplex can be varied
(e.g., from
about 17 - 29 nucleotides); the overhangs need not be present and, if present,
their length and
the identity of the nucleotides in the overhangs can vary (though most
commonly symmetric
dTdT overhangs are employed in synthetic siRNAs).
[00206] Additional structures, referred to as short hairpin RNAs (shRNAs), can
mediate
RNA interference. An shRNA is a single RNA strand that contains two
complementary
regions that hybridize to one another to form a double-stranded "stem," with
the two
complementary regions being connected by a single-stranded loop. shRNAs are
processed
intracellularly by Dicer to form an siRNA structure containing a guide strand
and an
antisense strand. While shRNAs can be delivered exogenously to cells, more
typically
intracellular synthesis of shRNA is achieved by introducing a plasmid or
vector containing a
promoter operably linked to a template for transcription of the shRNA into the
cell, e.g., to
create a stable cell line or transgenic organism.
[00207] While sequence-specific cleavage of target mRNA is currently the most
widely
used means of achieving gene silencing by exogenous delivery of short RNAi
entities to
cells, additional mechanisms of sequence-specific silencing mediated by short
RNA entities
are known. For example, post-transcriptional gene silencing mediated by small
RNA entities
can occur by mechanisms involving translational repression. Certain
endogenously
expressed RNA molecules form hairpin structures containing an imperfect duplex
portion in
which the duplex is interrupted by one or more mismatches and/or bulges. These
hairpin
structures are processed intracellularly to yield single-stranded RNA species
referred to as
known as microRNAs (miRNAs), which mediate translational repression of a
target transcript
to which they hybridize with less than perfect complementarity. siRNA-like
molecules
designed to mimic the structure of miRNA precursors have been shown to result
in
translational repression of target genes when administered to mammalian cells.
[00208] Thus the exact mechanism by which a short RNAi entity inhibits gene
expression
appears to depend, at least in part, on the structure of the duplex portion of
the RNAi entity
and/or the structure of the hybrid formed by one strand of the RNAi entity and
a target
transcript. RNAi mechanisms and the structure of various RNA molecules known
to mediate
Page 55 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
RNAi, e.g., siRNA, shRNA, miRNA and their precursors, have been extensively
reviewed
(see, e.g., Dykxhhorn et al., 2003, Nat. Rev. Mol. Cell Biol., 4:457; Hannon
et al., 2004,
Nature, 431:3761; and Meister et al., 2004, Nature, 431:343; all of which are
incorporated
herein by reference). It is to be expected that future developments will
reveal additional
mechanisms by which RNAi may be achieved and will reveal additional effective
short RNAi
entities. Any currently known or subsequently discovered short RNAi entities
are within the
scope of the present invention.
[00209] A short RNAi entity that is delivered according to the methods in
accordance with
the invention and/or is present in a composition in accordance with the
invention may be
designed to silence any eukaryotic gene. The gene can be a mammalian gene,
e.g., a human
gene. The gene can be a wild type gene, a mutant gene, an allele of a
polymorphic gene, etc.
The gene can be disease-associated, e.g., a gene whose over-expression, under-
expression, or
mutation is associated with or contributes to development or progression of a
disease. For
example, the gene can be oncogene. The gene can encode a receptor or putative
receptor for
an infectious agent such as a virus (see, e.g., Dylxhhorn et al., 2003, Nat.
Rev. Mol. Cell
Biol., 4:457 for specific examples; incorporated herein by reference).
[00210] In some embodiments, shRNAs may be used as molecular sensors. For
example,
shRNAs may serve as molecular beacons. In some embodiments, molecular beacons
comprise nucleic acids that comprise fluorophore-quencher pairs (e.g. so that
fluorescence is
quenched prior to binding of a target mRNA). In certain embodiments,
fluorescence is
quenched when the shRNA is on the particle and bent, but dequenched when it is
released
and bound to its target. In certain embodiments, fluorescence is dequenched
when the
shRNA is on the particle and bent, but quenched when it is released and bound
to its target.
In such embodiments, by externally monitoring fluorescence, both the release
and the
intracellular binding to a target RNA of an shRNA agent may be separately
monitored.
[00211] In some embodiments, tRNAs are functional RNA molecules whose delivery
to
eukaryotic cells can be monitored using the compositions and methods in
accordance with the
invention. The structure and role of tRNAs in protein synthesis is well known
(Soll and
Rajbhandary, (eds.) tRNA: Structure, Biosynthesis, and Function, ASM Press,
1995). The
cloverleaf shape of tRNAs includes several double-stranded "stems" that arise
as a result of
formation of intramolecular base pairs between complementary regions of the
single tRNA
strand. There is considerable interest in the synthesis of polypeptides that
incorporate
unnatural amino acids such as amino acid analogs or labeled amino acids at
particular
positions within the polypeptide chain (see, e.g., K6hrer and RajBhandary,
"Proteins carrying
Page 56 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
one or more unnatural amino acids," Chapter 33, In Ibba et al., (eds.),
Aminoacyl-tRNA
Synthetases, Landes Bioscience, 2004). One approach to synthesizing such
polypeptides is to
deliver a suppressor tRNA that is aminoacylated with an unnatural amino acid
to a cell that
expresses an mRNA that encodes the desired polypeptide but includes a nonsense
codon at
one or more positions. The nonsense codon is recognized by the suppressor
tRNA, resulting
in incorporation of the unnatural amino acid into a polypeptide encoded by the
mRNA
(Kohrer et al., 2001, Proc. Natl. Acad. Sci., USA, 98:14310; and Kohrer et
al., 2004, Nucleic
Acids Res., 32:6200; both of which are incorporated herein by reference).
However, as in the
case of siRNA delivery, existing methods of delivering tRNAs to cells result
in variable
levels of delivery, complicating efforts to analyze such proteins and their
effects on cells.
[00212] The invention contemplates the delivery of tRNAs, e.g., suppressor
tRNAs, and
thermally-responsive conjugates to eukaryotic cells in order to achieve the
synthesis of
proteins that incorporate an unnatural amino acid with which the tRNA is
aminoacylated.
The analysis of proteins that incorporate one or more unnatural amino acids
has a wide
variety of applications. For example, incorporation of amino acids modified
with detectable
(e.g., fluorescent) moieties can allow the study of protein trafficking,
secretion, etc., with
minimal disturbance to the native protein structure. Alternatively or
additionally,
incorporation of reactive moieties (e.g., photoactivatable and/or cross-
linkable groups) can be
used to identify protein interaction partners and/or to define three-
dimensional structural
motifs. Incorporation of phosphorylated amino acids such as phosphotyrosine,
phosphothreonine, or phosphoserine, or analogs thereof, into proteins can be
used to study
cell signaling pathways and requirements.
[00213] In some embodiments, the functional RNA is a ribozyme. A ribozyme is
designed
to catalytically cleave target mRNA transcripts may be used to prevent
translation of a target
mRNA and/or expression of a target (see, e.g., PCT publication WO 90/11364;
and Sarver et
al., 1990, Science 247:1222; both of which are incorporated herein by
reference).
[00214] In some embodiments, endogenous target gene expression may be reduced
by
targeting deoxyribonucleotide sequences complementary to the regulatory region
of the target
gene (i.e., the target gene's promoter and/or enhancers) to form triple
helical structures that
prevent transcription of the target gene (see generally, Helene, 1991,
Anticancer Drug Des.
6:569; Helene et al., 1992, Ann, N. Y. Acad. Sci. 660:27; and Maher, 1992,
Bioassays 14:807;
all of which are incorporated herein by reference).
[00215] RNAs such as RNAi-inducing entities, tRNAs, ribozymes, etc., for
delivery to
eukaryotic cells may be prepared according to any available technique
including, but not
Page 57 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
limited to chemical synthesis, enzymatic synthesis, enzymatic or chemical
cleavage of a
longer precursor, etc. Methods of synthesizing RNA molecules are known in the
art (see,
e.g., Gait, M.J. (ed.) Oligonucleotide synthesis: a practical approach, Oxford
(Oxfordshire),
Washington, DC: IRL Press, 1984; and Herdewijn, P. (ed.) Oligonucleotide
synthesis:
methods and applications, Methods in Molecular Biology, v. 288 (Clifton, N.J.)
Totowa,
N.J.: Humana Press, 2005). Short RNAi entities such as siRNAs are commercially
available
from a number of different suppliers. Pre-tested siRNAs targeted to a wide
variety of
different genes are available, e.g., from Ambion (Austin, TX), Dharmacon
(Lafayette, CO),
Sigma-Aldrich (St. Louis, MO).
[00216] When siRNAs are synthesized in vitro the two strands are typically
allowed to
hybridize before contacting them with cells. It will be appreciated that the
resulting siRNA
composition need not consist entirely of double-stranded (hybridized)
molecules. For
example, an RNAi entity commonly includes a small proportion of single-
stranded RNA.
Generally, at least approximately 50%, at least approximately 90%, at least
approximately
95%, or even at least approximately 99% - 100% of the RNAs in an siRNA
composition are
double-stranded when contacted with cells. However, a composition containing a
lower
proportion of dsRNA may be used, provided that it contains sufficient dsRNA to
be effective.
[00217] Vectors
[00218] In some embodiments, a nucleic acid to be delivered is a vector. As
used herein,
the term "vector" refers to a nucleic acid molecule (typically, but not
necessarily, a DNA
molecule) which can transport another nucleic acid to which it has been
linked. A vector can
achieve extra-chromosomal replication and/or expression of nucleic acids to
which they are
linked in a host cell. In some embodiments, a vector can achieve integration
into the genome
of the host cell.
[00219] In some embodiments, vectors are used to direct protein and/or RNA
expression.
In some embodiments, the protein and/or RNA to be expressed is not normally
expressed by
the cell. In some embodiments, the protein and/or RNA to be expressed is
normally
expressed by the cell, but at lower levels than it is expressed when the
vector has not been
delivered to the cell.
[00220] In some embodiments, a vector directs expression of any of the
proteins described
herein. In some embodiments, a vector directs expression of a protein with
anti-cancer
activity. In some embodiments, a vector directs expression of any of the
functional RNAs
described herein, such as RNAi-inducing entities, ribozymes, etc. In some
embodiments, a
vector directs expression of a functional RNA with anti-cancer activity.
Page 58 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
Protein Agents
[00221] In some embodiments, the agent to be delivered may be a protein or
peptide, as
defined herein. In certain embodiments, peptides range from about 5 to about
5000, 5 to
about 1000, about 5 to about 750, about 5 to about 500, about 5 to about 250,
about 5 to about
100, about 5 to about 75, about 5 to about 50, about 5 to about 40, about 5 to
about 30, about
to about 25, about 5 to about 20, about 5 to about 15, or about 5 to about 10
amino acids in
size. Peptides from panels of peptides comprising random sequences and/or
sequences which
have been varied consistently to provide a maximally diverse panel of peptides
may be used.
[00222] Polypeptides may contain L-amino acids, D-amino acids, or both and may
contain
any of a variety of amino acid modifications or analogs known in the art.
Useful
modifications include, e.g., terminal acetylation, amidation, etc. In some
embodiments,
polypeptides may comprise natural amino acids, unnatural amino acids,
synthetic amino
acids, and combinations thereof, as described herein.
[00223] In some embodiments, the agent to be delivered may be a peptide,
hormone,
erythropoietin, insulin, cytokine, antigen for vaccination, etc. In some
embodiments, the
agent to be delivered may be an antibody and/or characteristic portion thereof
In some
embodiments, antibodies may include, but are not limited to, polyclonal,
monoclonal,
chimeric (i.e. "humanized"), single chain (recombinant) antibodies. In some
embodiments,
antibodies may have reduced effector functions and/or bispecific molecules. In
some
embodiments, antibodies may include Fab fragments and/or fragments produced by
a Fab
expression library, as described in further detail above.
[00224] In some embodiments, the agent to be delivered may be an anti-cancer
agent.
Exemplary protein anti-cancer agents are enzymes (e.g. L-asparaginase) and
biological
response modifiers, such as interferons (e.g. interferon-a), interleukins
(e.g. interleukin 2; IL-
2), granulocyte colony-stimulating factor (G-CSF), and granulocyte/macrophage
colony-
stimulating factor (GM-CSF). In some embodiments, a protein anti-cancer agent
is an
antibody or characteristic portion thereof which is cytotoxic to tumor cells.
Carbohydrate Agents
[00225] In some embodiments, the agent to be delivered is a carbohydrate, such
as a
carbohydrate that is associated with a protein (e.g. glycoprotein,
proteogycan, etc.). A
carbohydrate may be natural or synthetic. A carbohydrate may also be a
derivatized natural
carbohydrate. In certain embodiments, a carbohydrate may be a simple or
complex sugar. In
certain embodiments, a carbohydrate is a monosaccharide, including but not
limited to
glucose, fructose, galactose, and ribose. In certain embodiments, a
carbohydrate is a
Page 59 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
disaccharide, including but not limited to lactose, sucrose, maltose,
trehalose, and cellobiose.
In certain embodiments, a carbohydrate is a polysaccharide, including but not
limited to
cellulose, microcrystalline cellulose, hydroxypropyl methylcellulose (HPMC),
methylcellulose (MC), dextrose, dextran, glycogen, xanthan gum, gellan gum,
starch, and
pullulan. In certain embodiments, a carbohydrate is a sugar alcohol, including
but not limited
to mannitol, sorbitol, xylitol, erythritol, malitol, and lactitol.
Lipid Agents
[00226] In some embodiments, the agent to be delivered is a lipid, such as a
lipid that is
associated with a protein (e.g. lipoprotein). Exemplary lipids that may be
used in accordance
with the present invention include, but are not limited to, oils, fatty acids,
saturated fatty acid,
unsaturated fatty acids, essential fatty acids, cis fatty acids, trans fatty
acids, glycerides,
monoglycerides, diglycerides, triglycerides, hormones, steroids (e.g.,
cholesterol, bile acids),
vitamins (e.g. vitamin E), phospholipids, sphingolipids, and lipoproteins.
[00227] In some embodiments, the lipid may comprise one or more fatty acid
groups or
salts thereof. In some embodiments, the fatty acid group may comprise
digestible, long chain
(e.g., C8-C50), substituted or unsubstituted hydrocarbons. In some
embodiments, the fatty
acid group may be a Cio-Czo fatty acid or salt thereo In some embodiments,
the fatty acid
group may be a C15-C20 fatty acid or salt thereof. In some embodiments, the
fatty acid group
may be a C15-C25 fatty acid or salt thereof. In some embodiments, the fatty
acid group may
be unsaturated. In some embodiments, the fatty acid group may be
monounsaturated. In
some embodiments, the fatty acid group may be polyunsaturated. In some
embodiments, a
double bond of an unsaturated fatty acid group may be in the cis conformation.
In some
embodiments, a double bond of an unsaturated fatty acid may be in the trans
conformation.
[00228] In some embodiments, the fatty acid group may be one or more of
butyric,
caproic, caprylic, capric, lauric, myristic, palmitic, stearic, arachidic,
behenic, or lignoceric
acid. In some embodiments, the fatty acid group may be one or more of
palmitoleic, oleic,
vaccenic, linoleic, alpha-linolenic, gamma-linoleic, arachidonic, gadoleic,
arachidonic,
eicosapentaenoic, docosahexaenoic, or erucic acid.
Diamostic Ments
[00229] In some embodiments, the agent to be delivered is a diagnostic agent.
In some
embodiments, diagnostic agents include gases; commercially available imaging
agents used
in positron emissions tomography (PET), computer assisted tomography (CAT),
single
photon emission computerized tomography, x-ray, fluoroscopy, and magnetic
resonance
imaging (MRI); anti-emetics; and contrast agents. Examples of suitable
materials for use as
Page 60 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
contrast agents in MRI include gadolinium chelates, as well as iron,
magnesium, manganese,
copper, and chromium. Examples of materials useful for CAT and x-ray imaging
include
iodine-based materials.
[00230] In some embodiments, thermally-responsive conjugates may comprise a
diagnostic agent used in magnetic resonance imaging (MRI), such as iron oxide
particles or
gadolinium complexes. Gadolinium complexes that have been approved for
clinical use
include gadolinium chelates with DTPA, DTPA-BMA, DOTA and HP-DO3A (reviewed in
Aime et al., 1998, Chemical Society Reviews, 27:19; incorporated herein by
reference).
[00231] In some embodiments, thermally-responsive conjugates may comprise
radionuclides as therapeutic and/or diagnostic agents. Among the radionuclides
used,
gamma-emitters, positron-emitters, and X-ray emitters are suitable for
diagnostic and/or
therapy, while beta emitters and alpha-emitters may also be used for therapy.
Suitable
radionuclides for forming thermally-responsive conjugates in accordance with
the invention
include, but are not limited to, 123I1125I1130 I, 131I1133I1135I447Sc, 72 As ,
72 Se, 90Y, RRY, 97Ru
,
100Pd 101mRh 1195b 128 Ba 197Hg211At 212Bi 212Pb 109Pd, 68Ga 67Cu 75 Br 77 Br
, , , , , , , , , , ,
99mTc, 14C, 13N, 150, 32P, 33P, and 18F.
[00232] In some embodiments, a diagnostic agent may be a fluorescent,
luminescent, or
magnetic moiety. In some embodiments, a detectable moiety such as a
fluorescent or
luminescent dye, etc., is entrapped, embedded, or encapsulated by a particle
core and/or
coating layer.
[00233] Fluorescent and luminescent moieties include a variety of different
organic or
inorganic small molecules commonly referred to as "dyes," "labels," or
"indicators."
Examples include fluorescein, rhodamine, acridine dyes, Alexa dyes, cyanine
dyes, etc.
Fluorescent and luminescent moieties may include a variety of naturally
occurring proteins
and derivatives thereof, e.g., genetically engineered variants. For example,
fluorescent
proteins include green fluorescent protein (GFP), enhanced GFP, red, blue,
yellow, cyan, and
sapphire fluorescent proteins, reef coral fluorescent protein, etc.
Luminescent proteins
include luciferase, aequorin and derivatives thereo Numerous fluorescent and
luminescent
dyes and proteins are known in the art (see, e.g., U.S. Patent Application
Publication
2004/0067503; Valeur, B., "Molecular Fluorescence: Principles and
Applications," John
Wiley and Sons, 2002; Handbook of Fluorescent Probes and Research Products,
Molecular
Probes, 9th edition, 2002; and The Handbook - A Guide to Fluorescent Probes
and Labeling
Technologies, Invitrogen, 10th edition, available at the Invitrogen web site;
both of which are
incorporated herein by reference).
Page 61 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
Prophylactic Agents
[00234] In some embodiments, the agent to be delivered is a prophylactic
agent. In some
embodiments, prophylactic agents include vaccines. Vaccines may comprise
isolated
proteins or peptides, inactivated organisms and viruses, dead organisms and
virus, genetically
altered organisms or viruses, and cell extracts. Prophylactic agents may be
combined with
interleukins, interferon, cytokines, and adjuvants such as cholera toxin,
alum, Freund's
adjuvant, etc. Prophylactic agents may include antigens of such bacterial
organisms as
Streptococccus pnuemoniae, Haemophilus influenzae, Staphylococcus aureus,
Streptococcus
pyrogenes, Corynebacterium diphtheriae, Listeria monocytogenes, Bacillus
anthracis,
Clostridium tetani, Clostridium botulinum, Clostridium perfringens, Neisseria
meningitidis,
Neisseria gonorrhoeae, Streptococcus mutans, Pseudomonas aeruginosa,
Salmonella typhi,
Haemophilus parainfluenzae, Bordetella pertussis, Francisella tularensis,
Yersinia pestis,
Vibrio cholerae, Legionella pneumophila, Mycobacterium tuberculosis,
Mycobacterium
leprae, Treponema pallidum, Leptospirosis interrogans, Borrelia burgdorferi,
Camphylobacterjejuni, and the like; antigens of such viruses as smallpox,
influenza A and B,
respiratory syncytial virus, parainfluenza, measles, HIV, varicella-zoster,
herpes simplex 1
and 2, cytomegalovirus, Epstein-Barr virus, rotavirus, rhinovirus, adenovirus,
papillomavirus,
poliovirus, mumps, rabies, rubella, coxsackieviruses, equine encephalitis,
Japanese
encephalitis, yellow fever, Rift Valley fever, hepatitis A, B, C, D, and E
virus, and the like;
antigens of fungal, protozoan, and parasitic organisms such as Cryptococcus
neoformans,
Histoplasma capsulatum, Candida albicans, Candida tropicalis, Nocardia
asteroides,
Rickettsia ricketsii, Rickettsia typhi, Mycoplasma pneumoniae, Chlamydial
psittaci,
Chlamydial trachomatis, Plasmodium falciparum, Trypanosoma brucei, Entamoeba
histolytica, Toxoplasma gondii, Trichomonas vaginalis, Schistosoma mansoni,
and the like.
These antigens may be in the form of whole killed organisms, peptides,
proteins,
glycoproteins, carbohydrates, or combinations thereo
Nutraceutical Agents
[00235] In some embodiments, the therapeutic agent to be delivered is a
nutraceutical
agent. In some embodiments, the nutraceutical agent provides basic nutritional
value,
provides health or medical benefits, and/or is a dietary supplement. In some
embodiments,
the nutraceutical agent is a vitamin (e.g. vitamins A, B, C, D, E, K, etc.),
mineral (e.g. iron,
magnesium, potassium, calcium, etc.), or essential amino acid (e.g. lysine,
glutamine, leucine,
etc.).
Page 62 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00236] In some embodiments, nutraceutical agents may include plant or animal
extracts,
such as fatty acids and/or omega-3 fatty acids (e.g. DHA or ARA), fruit and
vegetable
extracts, lutein, phosphatidylserine, lipoid acid, melatonin, glucosamine,
chondroitin, aloe
vera, guggul, green tea, lycopene, whole foods, food additives, herbs,
phytonutrients,
antioxidants, flavonoid constituents of fruits, evening primrose oil,
flaxseeds, fish and marine
animal oils (e.g. cod liver oil), and probiotics.
[00237] Exemplary nutraceutical agents and dietary supplements are disclosed,
for
example, in Roberts et al., (Nutriceuticals: The Complete Encyclopedia of
Supplements,
Herbs, Vitamins, and Healing Foods, American Nutriceutical Association, 2001).
Nutraceutical agents and dietary supplements are also disclosed in Physicians'
Desk
Reference for Nutritional Supplements, 1st Ed. (2001) and The Physicians'
DeskReference
for Herbal Medicines, 1st Ed. (2001).
[00238] Those skilled in the art will recognize that this is an exemplary, not
comprehensive, list of agents that can be delivered using the thermally-
responsive conjugates
in accordance with the present invention. Any agent may be associated with
particles for
remotely controlled delivery in accordance with the present invention.
Targeting Moieties
[00239] In some embodiments, thermally-responsive conjugates in accordance
with the
present invention comprise one or more targeting moieties. In general, a
targeting moiety is
any moiety that binds to a component associated with an organ, tissue, cell,
subcellular
locale, and/or extracellular matrix component. In some embodiments, such a
component is
referred to as a "target" or a "marker," and these are discussed in further
detail below.
[00240] A targeting moiety may be a nucleic acid, polypeptide, glycoprotein,
carbohydrate, lipid, etc. For example, a targeting moiety can be a nucleic
acid targeting
moiety (e.g. an aptamer) that binds to a cell type specific marker. In
general, an aptamer is an
oligonucleotide (e.g., DNA, RNA, or an analog or derivative thereof) that
binds to a
particular target, such as a polypeptide. In some embodiments, a targeting
moiety may be a
naturally occurring or synthetic ligand for a cell surface receptor, e.g., a
growth factor,
hormone, LDL, transferrin, etc. A targeting moiety can be an antibody, which
term is
intended to include antibody fragments, characteristic portions of antibodies,
single chain
antibodies, etc. Synthetic binding proteins such as affibodies, etc., can be
used. Peptide
targeting moieties can be identified, e.g., using procedures such as phage
display. This
Page 63 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
widely used technique has been used to identify cell specific ligands for a
variety of different
cell types.
[00241] In some embodiments, targeting moieties bind to an organ, tissue,
cell,
extracellular matrix component, and/or intracellular compartment that is
associated with a
specific developmental stage or a specific disease state (i.e. a "target" or
"marker"). In some
embodiments, a target is an antigen on the surface of a cell, such as a cell
surface receptor, an
integrin, a transmembrane protein, an ion channel, and/or a membrane transport
protein. In
some embodiments, a target is an intracellular protein. In some embodiments, a
target is a
soluble protein, such as immunoglobulin. In some embodiments, a target is more
prevalent,
accessible, and/or abundant in a diseased locale (e.g. organ, tissue, cell,
subcellular locale,
and/or extracellular matrix component) than in a healthy locale. To give but
one example, in
some embodiments, a target is preferentially expressed in tumor tissues versus
normal tissues.
In some embodiments, a target is more prevalent, accessible, and/or abundant
in locales (e.g.
organs, tissues, cells, subcellular locales, and/or extracellular matrix
components) associated
with a particular developmental state than in locales associated with a
different
developmental state. In some embodiments, targeting moieties facilitate the
passive entry
into target sites by extending circulation time of conjugates, reducing non-
specific clearance
of conjugates, and/or geometrically enhancing the accumulation of conjugates
in target sites.
[00242] In some embodiments, a targeting moiety in accordance with the present
invention
may be a nucleic acid. As used herein, a "nucleic acid targeting moiety"
refers to a nucleic
acid that binds selectively to a target. In some embodiments, a nucleic acid
targeting moiety
is a nucleic acid aptamer. An aptamer is typically a polynucleotide that binds
to a specific
target structure that is associated with a particular organ, tissue, cell,
subcellular locale,
and/or extracellular matrix component. In general, the targeting function of
the aptamer is
based on the three-dimensional structure of the aptamer and/or target.
[00243] In some embodiments, a targeting moiety in accordance with the present
invention
may be a small molecule. In certain embodiments, small molecules are less than
about 2000
g/mol in size. In some embodiments, small molecules are less than about 1500
g/mol or less
than about 1000 g/mol. In some embodiments, small molecules are less than
about 800 g/mol
or less than about 500 g/mol. One of ordinary skill in the art will appreciate
that any small
molecule that specifically binds to a desired target can be used in accordance
with the present
invention.
[00244] In some embodiments, a targeting moiety in accordance with the present
invention
may be a protein or peptide. In certain embodiments, peptides range from about
5 to 100, 10
Page 64 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
to 75, 15 to 50, or 20 to 25 amino acids in size. In some embodiments, a
peptide sequence
can be based on the sequence of a protein. In some embodiments, a peptide
sequence can be
a random arrangement of amino acids.
[00245] The terms "polypeptide" and "peptide" are used interchangeably herein,
with
"peptide" typically referring to a polypeptide having a length of less than
about 100 amino
acids. Polypeptides may contain L-amino acids, D-amino acids, or both and may
contain any
of a variety of amino acid modifications or analogs known in the art. Useful
modifications
include, e.g., terminal acetylation, amidation, lipidation, phosphorylation,
glycosylation,
acylation, farnesylation, sulfation, etc.
[00246] Exemplary proteins that may be used as targeting moieties in
accordance with the
present invention include, but are not limited to, antibodies, receptors,
cytokines, peptide
hormones, proteins derived from combinatorial libraries (e.g. avimers,
affibodies, etc.), and
characteristic portions thereo
[00247] In some embodiments, a targeting moiety may be an antibody and/or
characteristic
portion thereof. The term "antibody" refers to any immunoglobulin, whether
natural or
wholly or partially synthetically produced and to derivatives thereof and
characteristic
portions thereo An antibody may be monoclonal or polyclonal. An antibody may
be a
member of any immunoglobulin class, including any of the human classes: IgG,
IgM, IgA,
IgD, and IgE.
[00248] As used herein, an antibody fragment (i.e. characteristic portion of
an antibody)
refers to any derivative of an antibody which is less than full-length. In
general, an antibody
fragment retains at least a significant portion of the full-length antibody's
specific binding
ability. Examples of antibody fragments include, but are not limited to, Fab,
Fab', F(ab')2,
scFv, Fv, dsFv diabody, and Fd fragments.
[00249] An antibody fragment may be produced by any means. For example, an
antibody
fragment may be enzymatically or chemically produced by fragmentation of an
intact
antibody and/or it may be recombinantly produced from a gene encoding the
partial antibody
sequence. Alternatively or additionally, an antibody fragment may be wholly or
partially
synthetically produced. An antibody fragment may optionally comprise a single
chain
antibody fragment. Alternatively or additionally, an antibody fragment may
comprise
multiple chains which are linked together, for example, by disulfide linkages.
An antibody
fragment may optionally comprise a multimolecular complex. A functional
antibody
fragment will typically comprise at least about 50 amino acids and more
typically will
comprise at least about 200 amino acids.
Page 65 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00250] In some embodiments, antibodies may include chimeric (e.g.
"humanized") and
single chain (recombinant) antibodies. In some embodiments, antibodies may
have reduced
effector functions and/or bispecific molecules. In some embodiments,
antibodies may
include fragments produced by a Fab expression library.
[00251] Single-chain Fvs (scFvs) are recombinant antibody fragments consisting
of only
the variable light chain (VL) and variable heavy chain (VH) covalently
connected to one
another by a polypeptide linker. Either VL or VH may comprise the NH2-terminal
domain.
The polypeptide linker may be of variable length and composition so long as
the two variable
domains are bridged without significant steric interference. Typically,
linkers primarily
comprise stretches of glycine and serine residues with some glutamic acid or
lysine residues
interspersed for solubility.
[00252] Diabodies are dimeric scFvs. Diabodies typically have shorter peptide
linkers
than most scFvs, and they often show a preference for associating as dimers.
[00253] An Fv fragment is an antibody fragment which consists of one VH and
one VL
domain held together by noncovalent interactions. The term "dsFv" as used
herein refers to
an Fv with an engineered intermolecular disulfide bond to stabilize the VH-VL
pair.
[00254] A F(ab')2 fragment is an antibody fragment essentially equivalent to
that obtained
from immunoglobulins by digestion with an enzyme pepsin at pH 4.0 - 4.5. The
fragment
may be recombinantly produced.
[00255] A Fab' fragment is an antibody fragment essentially equivalent to that
obtained by
reduction of the disulfide bridge or bridges joining the two heavy chain
pieces in the F(ab')2
fragment. The Fab' fragment may be recombinantly produced.
[00256] A Fab fragment is an antibody fragment essentially equivalent to that
obtained by
digestion of immunoglobulins with an enzyme (e.g. papain). The Fab fragment
may be
recombinantly produced. The heavy chain segment of the Fab fragment is the Fd
piece.
[00257] In some embodiments, a targeting moiety in accordance with the present
invention
may comprise a carbohydrate (e.g. glycoproteins, proteoglycans, etc.). In some
embodiments, a carbohydrate may be a polysaccharide comprising simple sugars
(or their
derivatives) connected by glycosidic bonds, as known in the art. Such sugars
may include,
but are not limited to, glucose, fructose, galactose, ribose, lactose,
sucrose, maltose, trehalose,
cellobiose, mannose, xylose, arabinose, glucoronic acid, galactoronic acid,
mannuronic acid,
glucosamine, galatosamine, and neuramic acid. In some embodiments, a
carbohydrate may
be one or more of pullulan, cellulose, microcrystalline cellulose,
hydroxypropyl
methylcellulose, hydroxycellulose, methylcellulose, dextran, cyclodextran,
glycogen, starch,
Page 66 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
hydroxyethylstarch, carageenan, glycon, amylose, chitosan, N,O-
carboxylmethylchitosan,
algin and alginic acid, starch, chitin, heparin, konjac, glucommannan,
pustulan, heparin,
hyaluronic acid, curdlan, and xanthan. In some embodiments, the carbohydrate
may be
aminated, carboxylated, acetylated and/or sulfated. In some embodiments,
hydrophilic
polysaccharides can be modified to become hydrophobic by introducing a large
number of
side-chain hydrophobic groups.
[00258] In some embodiments, a targeting moiety in accordance with the present
invention
may comprise one or more fatty acid groups or salts thereof (e.g.
lipoproteins). In some
embodiments, a fatty acid group may comprise digestible, long chain (e.g., C8-
Cso),
substituted or unsubstituted hydrocarbons. In some embodiments, a fatty acid
group may be
a C10-C20 fatty acid or salt thereof. In some embodiments, a fatty acid group
may be a C15-
C20 fatty acid or salt thereof. In some embodiments, a fatty acid group may be
a C15-C25 fatty
acid or salt thereof. In some embodiments, a fatty acid group may be
unsaturated. In some
embodiments, a fatty acid group may be monounsaturated. In some embodiments, a
fatty
acid group may be polyunsaturated. In some embodiments, a double bond of an
unsaturated
fatty acid group may be in the cis conformation. In some embodiments, a double
bond of an
unsaturated fatty acid may be in the trans conformation. In some embodiments,
a fatty acid
group may be one or more of butyric, caproic, caprylic, capric, lauric,
myristic, palmitic,
stearic, arachidic, behenic, or lignoceric acid. In some embodiments, a fatty
acid group may
be one or more of palmitoleic, oleic, vaccenic, linoleic, alpha-linoleic,
gamma-linoleic,
arachidonic, gadoleic, arachidonic, eicosapentaenoic, docosahexaenoic, or
erucic acid.
[00259] In some embodiments, thermally-responsive conjugates are not targeted
to
particular locales (e.g. organs, tissues, cells, subcellular locales, and/or
extracellular matrix
components) by any of the targeting moieties described above. In some
embodiments,
targeting may instead be facilitated by a property intrinsic to a thermally-
responsive
conjugate (e.g. geometry of the conjugate and/or conjugate assembly).
[00260] In some embodiments, an agent to be delivered may function as a
targeting moiety
as described herein. To give but one example, an antibody that is useful for
targeting
conjugates to specific tissues may also serve as a therapeutic agent. In some
embodiments,
the agent to be delivered may be distinct from a targeting moiety.
[00261] Nanoparticle conjugates comprising targeting moieties are described in
further
detail in co-pending U.S. Patent Application entitled "DELIVERY OF
NANOPARTICLES
AND/OR AGENTS TO CELLS," filed December 6, 2007 (the entire contents of which
are
incorporated herein by reference and are attached hereto as Appendix A).
Page 67 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
Single- and Multi-Conjugate Systems
[00262] The present invention provides heatable surfaces (e.g. particles)
which heat in
response to external stimuli (e.g. EM fields, light, etc.) and are associated
with one or more
agents to be delivered via thermally-responsive linkers that mediate release
of the agent
above a trigger temperature. In this manner, exposing the heatable surfaces to
external
stimuli causes them to be heated sufficiently to trigger release of the agent.
In some
embodiments, such systems are designed for a single release by having a
uniform population
of heatable surfaces and linkers. In some embodiments, by using heatable
surfaces that heat
at specific frequencies and linkers with varying temperatures of release,
populations of
particles and linkers can be triggered to emit an unlimited spectrum of
complex dosages in
response to imposed EM fields.
[00263] An advantage of this design is its ability to simultaneously use a
wide variety of
thermally-responsive linkers to release different agents at different
temperatures. This is
accomplished by using a multitude of thermally-responsive linkers designed to
release at
specific local temperatures, enabling delivery of simple or complex drug
mixtures, in specific
orders, over long or short periods of time. By using several different types
of particles, each
with preferential heating at specific frequencies, the population of thermally-
responsive
conjugates can be designed such that it enables release of complex drug
dosages in response
to imposed external stimuli (e.g. EM fields).
[00264] In some embodiments, populations of thermally-responsive conjugates
are
"single-component" systems. In other words, "single component" conjugates
comprise
heatable surfaces, thermally-responsive linkers, and/or agents to be delivered
that are all
identical to one another. To give but a few examples, heatable surfaces that
are suitable for
use in single-component conjugates may include magnetic particles (e.g. gold,
silver, iron,
cobalt, zinc, cadmium, nickel, gadolinium, chromium, copper, manganese,
palladium, tin,
etc., alloys thereof, and/or oxides thereof).
[00265] In some embodiments, conjugate systems are "two-component" or "multi-
component" conjugate systems. In other words, "two-component" or "multi-
component"
conjugate systems (e.g. conjugate populations, pluralities of conjugates,
etc.) comprise
heatable surfaces, thermally-responsive linkers, and/or agents to be delivered
that are not all
identical to one another. In some embodiments, a two-component conjugate
system
comprises two populations of conjugates, wherein each population comprises
different
heatable surfaces. In some embodiments, a multi-component system comprises
more than
Page 68 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
two populations of conjugates, wherein at least two populations comprise
different heatable
surfaces. In some embodiments, a two-component conjugate system comprises two
populations of conjugates, wherein each population comprises different
thermally-responsive
linkers. In some embodiments, a multi-component system comprises more than two
populations of conjugates, wherein at least two populations comprise different
thermally-
responsive linkers. In some embodiments, a two-component conjugate system
comprises two
populations of conjugates, wherein each population comprises different agents
to be
delivered. In some embodiments, a multi-component conjugate system comprises
more than
two populations of conjugates, wherein at least two populations comprise
different agents to
be delivered.
[00266] In some embodiments, a multi-component conjugate system comprises more
than
two populations of conjugates, wherein at least two populations comprise
different heatable
surfaces and different agents to be delivered. In some embodiments, a multi-
component
conjugate system comprises more than two populations of conjugates, wherein at
least two
populations comprise different heatable surfaces and different thermally-
responsive linkers.
In some embodiments, a multi-component conjugate system comprises more than
two
populations of conjugates, wherein at least two populations comprise different
thermally-
responsive linkers and different agents to be delivered. In some embodiments,
a multi-
component conjugate system comprises more than two populations of conjugates,
wherein at
least two populations comprise different heatable surfaces, different
thermally-responsive
linkers, and different agents to be delivered.
[00267] In some embodiments, a single thermally-responsive conjugate may
comprise a
particle associated with multiple different thermally-responsive linkers and
multiple different
agents to be delivered. In some embodiments, the multiple different thermally-
responsive
linkers are sensitive to different temperatures. In some embodiments, such a
conjugate may
be used to deliver different therapeutic agents at different points in time
(i.e. a dosage
schedule). To give but one example, a conjugate may comprise (i) a particle
which heats
upon exposure to EM fields, (ii) a first thermally-responsive linker that is
disrupted at
temperatures of 42 C or greater, (iii) a first therapeutic agent associated
with the first
thermally-responsive linker, (iv) a second thermally-responsive linker that is
disrupted at
temperatures of 50 C or greater, and (v) a second therapeutic agent
associated with the
second thermally-responsive linker. The particle can be subjected first to an
EM field having
a frequency sufficient to cause heating of the particle to a temperature of
equal to or greater
than 42 C, but less than 50 C, thereby causing selective release of the
first therapeutic agent
Page 69 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
associated with the first thermally-responsive linker. The particle can then
be subjected to an
EM field having a frequency sufficient to cause heating of the particle to 50
C or greater,
thereby causing release of the second therapeutic agent associated with the
second thermally-
responsive linker.
[00268] In some embodiments, therapeutic effect may be enhanced by delivering
a first
agent, waiting for a specified period of time, and then delivering a second
agent. In the
previously-described example, therapeutic effect may be enhanced by delivering
the
chemotherapeutic agent, waiting for a specified period of time, and then
delivering the
siRNA. To give a specific example, a thermally-responsive conjugate similar to
what is
described in the previous paragraph can be used for timing the co-delivery of
a
chemotherapeutic agent (e.g. cisplatin) and an siRNA that is known to
sensitize cells to that
particular chemotherapeutic agent or to chemotherapeutic agents in general
(e.g. siRNAs
targeting MAPKAP kinase 2 (Reinhardt et al., 2007, Cancer Cell, 11:175;
incorporated
herein by reference)).
[00269] The present invention provides methods of triggering disassembly of
dendrimer-
like conjugate assemblies connected via heat-liable linkers. Controlled
disassociation of
conjugate assemblies enables timed cargo release from large aggregates for the
purpose of
sensing, MRI, catalysis, delivery of localized high drug dosage, gene therapy,
or facilitating
body clearance of particles in vivo.
[00270] In some embodiments, a population of conjugates comprises multiple
individual
conjugates. In some embodiments, individual conjugates within a population of
conjugates
are physically separated from one another. In some embodiments, individual
conjugates
within a population of conjugates do not interact and/or associate with one
another. In some
embodiments, individual conjugates within a population of conjugates are not
physically
separated from one another. In some embodiments, individual conjugates within
a population
of conjugates interact and/or associate with one another.
[00271] In some embodiments, individual conjugates within a population of
conjugates
interact and/or associate with one another to form assemblies of conjugates.
In some
embodiments, a population of conjugates comprises assemblies of individual
conjugates. In
some embodiments, conjugate assemblies may be characterized as having an
ordered
structure. In some embodiments, conjugate assemblies may be characterized as
having an
unordered structure.
[00272] In some embodiments, conjugate assemblies may range from about 10 nm
to
about 100 m in size (e.g. as measured by diameter and/or greatest dimension).
In some
Page 70 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
embodiments, conjugate assemblies may range from about 10 nm to about 50 m,
about 10
nm to about 10 m, about 10 nm to about 5 m, about 10 nm to about 1 m, about
10 nm to
about 500 nm, or about 10 nm to about 100 nm in size. In some embodiments,
conjugate
assemblies may range from about 100 nm to about 100 m, about 100 nm to about
50 m,
about 100 nm to about 10 m, about 100 nm to about 5 m, about 100 nm to about
1 m, or
about 100 nm to about 500 nm in size.
[00273] In some embodiments, conjugate assemblies may be approximately 10 nm,
approximately 50 nm, approximately 100 nm, approximately 250 nm, approximately
500 nm,
approximately 1 m, approximately 2 m, approximately 3 m, approximately 4
m,
approximately 5 m, approximately 10 m, approximately 25 m, approximately 50
m,
approximately 75 m, approximately 100 m in size, or larger.
[00274] In some embodiments, conjugate assemblies comprise two or more
individual
conjugates. In some embodiments, conjugate assemblies contain approximately 2,
approximately 3, approximately 4, approximately 5, approximately 10,
approximately 25,
approximately 50, approximately 75, approximately 100, approximately 250,
approximately
500, approximately 750, approximately 1000, approximately 2500, approximately
5000,
approximately 7500, approximately 10,000, or more individual conjugates.
[00275] In some embodiments, conjugate assemblies consist of or consist
essentially of
approximately 2, approximately 3, approximately 4, approximately 5,
approximately 10,
approximately 25, approximately 50, approximately 75, approximately 100,
approximately
250, approximately 500, approximately 750, approximately 1000, approximately
2500,
approximately 5000, approximately 7500, approximately 10,000, or more
individual
conjugates.
[00276] Conjugate assemblies may be formed by any method available in the art.
In some
embodiments, conjugate assemblies may be formed by a layer-by-layer coating
process. In
some embodiments, conjugate assemblies may be formed by a single-step
equilibrium
assembly. In some embodiments, conjugate assemblies may be formed by any
aqueous
and/or organic solvent process. In some embodiments, conjugate assemblies are
formed by
serial dilution and introduction of new conjugates to self assemble around the
existing
formations. In this way, assembly can be conducted in a controlled, non-
aggregate manner.
[00277] Therapeutic, diagnostic, and/or prophylactic applications of single-
and multi-
component thermally-responsive conjugate systems and of populations of
conjugates are
described in further detail in the section entitled "Therapeutic Applications"
(below).
Page 71 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
Methods of Manufacturing Thermally-Responsive Conjugates
[00278] Thermally-responsive conjugates may be manufactured using any
available
method. Methods of forming heatable surfaces (e.g. magnetic particles) are
known in the art.
For example, aqueous and organic solvent syntheses for monodisperse
semiconductor,
conductive, magnetic, organic, and other particles have been developed
elsewhere (Pellegrino
et al., 2005, Small, 1:48; Murray et al., 2000, Ann. Rev. Mat. Sci., 30:545;
and Trindade et
al., 2001, Chem. Mat., 13:3843; all of which are incorporated herein by
reference).
Alternatively or additionally, particulate formulations can be formed by
methods as milling,
microfabrication, nanofabrication, sacrificial layers, etc., which are known
in the art (Haynes
et al., 2001, J. Phys. Chem., 105:5599; incorporated herein by reference).
[00279] In general, assembly of conjugates involves at least one chemical
reaction. For
example, attaching the agent to be delivered to the thermally-responsive
linker may take
place in one reaction, and attaching the heatable surface to a thermally-
responsive linker may
take place in a second reaction. From this point, the conjugates are formed by
self-assembly,
which can be performed in a controlled manner by dictating the concentrations
of the
individual components (e.g. heatable surfaces, thermally-responsive linkers,
agents to be
delivered, etc.). For example, if particle A and particle B (each associated
with a thermally-
responsive linker) associate with each other and are mixed together in equal
amounts, they
may bind one another into large aggregates. However, if the ratio of A to B is
1:100, the
particles formed may contain a single A completely surrounded by B particles.
This method
can be repeated for stepwise synthesis of ordered conjugate assemblies.
[00280] In some embodiments, such ratiometric assembly may be utilized to
synthesize
and/or form diverse structures with varying geometries and components.
Conjugate
assemblies may be templated to form spherical assemblies around a central
sphere, worm-like
structures around a central worm, and/or branched morphologies. Individual
conjugates
within conjugate assemblies may serve unique, complimentary, and/or
contradictory
purposes. In some embodiments, individual conjugates within conjugate
assemblies may
have properties when assembled that differ from properties of unassembled,
individual
conjugates (e.g. emergent pharmacokinetics, transport rates, binding
affinities,
electromagnetic properties, etc.).
[00281] In some embodiments, a heatable surface and a thermally-responsive
linker are
physically associated with one another. In some embodiments, a thermally-
responsive linker
and an agent to be delivered are physically associated with one another. In
some
embodiments, a heatable surface and an agent to be delivered are physically
associated with
Page 72 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
one another. In some embodiments, a heatable surface and a targeting moiety
are physically
associated with one another. In some embodiments, a thermally-responsive
linker and a
targeting moiety are physically associated with one another. In some
embodiments, an agent
to be delivered and a targeting moiety are physically associated with one
another. In certain
specific embodiments, a heatable surface, thermally-responsive linker, and
agent to be
delivered are physically associated with one another. In certain specific
embodiments, a
heatable surface, thermally-responsive linker, agent to be delivered, and
targeting moiety are
physically associated with one another.
[00282] Physical association can be achieved in a variety of different ways.
Physical
association may be covalent or non-covalent. In some embodiments, non-covalent
physical
association may be characterized by association with the surface of,
encapsulated within,
surrounded by, and/or distributed throughout the polymeric matrix of a
heatable surface.
[00283] In some embodiments, a heatable surface, thermally-responsive linker,
and/or
agent to be delivered may be directly conjugated to one another, e.g., by one
or more covalent
bonds, or may be conjugated by means of one or more linkers. In some
embodiments, the
linker forms one or more covalent or non-covalent bonds with the heatable
surface and one or
more covalent or non-covalent bonds with the thermally-responsive linker,
thereby attaching
them to one another. In some embodiments, a first linker forms a covalent or
non-covalent
bond with the heatable surface and a second linker forms a covalent or non-
covalent bond
with the thermally-responsive linker. The two linkers form one or more
covalent or non-
covalent bond(s) with each other.
[00284] In some embodiments, the linker forms one or more covalent or non-
covalent
bonds with the heatable surface and one or more covalent or non-covalent bonds
with the
agent to be delivered, thereby attaching them to one another. In some
embodiments, a first
linker forms a covalent or non-covalent bond with the heatable surface and a
second linker
forms a covalent or non-covalent bond with the agent to be delivered. The two
linkers form
one or more covalent or non-covalent bond(s) with each other.
[00285] In some embodiments, the linker is a cleavable linker. To give but a
few
examples, thermally-responsive linkers include protease thermally-responsive
peptide linkers,
nuclease sensitive nucleic acid linkers, lipase sensitive lipid linkers,
glycosidase sensitive
carbohydrate linkers, pH sensitive linkers, hypoxia sensitive linkers, photo-
thermally-
responsive linkers, thermally-responsive linkers, enzyme thermally-responsive
linkers,
ultrasound-sensitive linkers, x-ray thermally-responsive linkers, etc. In some
embodiments,
the linker is not a cleavable linker.
Page 73 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00286] Any of a variety of methods can be used to conjugate a linker (e.g. a
biomolecule
such as a polypeptide, carbohydrate, or nucleic acid) to a particle (e.g.
magnetic particle).
General strategies include passive adsorption (e.g., via electrostatic
interactions), multivalent
chelation, high affinity non-covalent binding between members of a specific
binding pair,
covalent bond formation, etc. (Gao et al., Curr. Op. Biotechnol., 16:63).
[00287] A bifunctional cross-linking reagent can be employed. Such reagents
contain two
reactive groups, thereby providing a means of covalently conjugating two
target groups. The
reactive groups in a chemical cross-linking reagent typically belong to
various classes of
functional groups such as succinimidyl esters, maleimides, and
pyridyldisulfides. Exemplary
cross-linking agents include, e.g., carbodiimides, N-hydroxysuccinimidyl-4-
azidosalicylic
acid (NHS-ASA), dimethyl pimelimidate dihydrochloride (DMP),
dimethylsuberimidate
(DMS), 3,3'-dithiobispropionimidate (DTBP), N-Succinimidyl 3-[2-pyridyldithio]-
propionamido (SPDP), succimidyl a-methylbutanoate, biotinamidohexanoyl-6-amino-
hexanoic acid N-hydroxy-succinimide ester (SMCC), succinimidyl-[(N-
maleimidopropionamido)-dodecaethyleneglycol] ester (NHS-PEO12), etc. For
example,
carbodiimide-mediated amide formation and active ester maleimide-mediated
amine and
sulfhydryl coupling are widely used approaches.
[00288] Common schemes for forming a conjugate involve the coupling of an
amine group
on one molecule to a thiol group on a second molecule, sometimes by a two- or
three-step
reaction sequence. A thiol-containing molecule may be reacted with an amine-
containing
molecule using a heterobifunctional cross-linking reagent, e.g., a reagent
containing both a
succinimidyl ester and either a maleimide, a pyridyldisulfide, or an
iodoacetamide. Amine-
carboxylic acid and thiol-carboxylic acid cross-linking, maleimide-sulfhydryl
coupling
chemistries (e.g., the maleimidobenzoyl-N-hydroxysuccinimide ester (MBS)
method), etc.,
may be used. Polypeptides can conveniently be attached to particles via amine
or thiol
groups in lysine or cysteine side chains respectively, or by an N-terminal
amino group.
Nucleic acids such as RNAs can be synthesized with a terminal amino group. A
variety of
coupling reagents (e.g., succinimidyl 3-(2-pyridyldithio)propionate (SPDP) and
sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC)
may be
used to conjugate the various components of thermally-responsive conjugates.
Heatable
surfaces can be prepared with functional groups, e.g., amine or carboxyl
groups, available at
the surface to facilitate conjugation to a biomolecule.
[00289] Non-covalent specific binding interactions can be employed. For
example, either
a particle or a biomolecule can be functionalized with biotin with the other
being
Page 74 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
functionalized with streptavidin. These two moieties specifically bind to each
other non-
covalently and with a high affinity, thereby conjugating the particle and the
biomolecule.
Other specific binding pairs could be similarly used (e.g. antibody-antigen
pairs).
Alternately, histidine-tagged biomolecules can be conjugated to particles
conjugated with
nickel-nitrolotriaceteic acid (Ni-NTA).
[00290] Any biomolecule to be attached to a heatable surface, thermally-
responsive linker,
and/or agent to be delivered may include a spacer. The spacer can be, for
example, a short
peptide chain, e.g., between 1 and 10 amino acids in length, e.g., 1, 2, 3, 4,
5, or more amino
acids in length, a nucleic acid, an alkyl chain, etc.
[00291] For additional general information on conjugation methods and cross-
linkers, see
the journal Bioconjugate Chemistry, published by the American Chemical
Society, Columbus
OH, PO Box 3337, Columbus, OH, 43210; "Cross-Linking," Pierce Chemical
Technical
Library, available at the Pierce web site and originally published in the 1994
- 1995 Pierce
Catalog, and references cited therein; Wong SS, Chemistry of Protein
Conjugation and
Cross-linking, CRC Press Publishers, Boca Raton, 1991; and Hermanson, G. T.,
Bioconjugate Techniques, Academic Press, Inc., San Diego, 1996.
[00292] It is to be understood that the compositions in accordance with the
invention can
be made in any suitable manner, and the invention is in no way limited to
compositions that
can be produced using the methods described herein. Selection of an
appropriate method
may require attention to the properties of the particular moieties being
conjugated.
[00293] If desired, various methods may be used to separate conjugates with an
attached
thermally-responsive linker and/or agent to be delivered from conjugates with
which the
thermally-responsive linker and/or agent to be delivered has not become
associated, or to
separate conjugates having different numbers of thermally-responsive linkers,
and/or agents
to be delivered attached thereto. For example, size exclusion chromatography,
agarose gel
electrophoresis, or filtration can be used to separate populations of
conjugates having
different numbers of moieties attached thereto and/or to separate conjugates
from other
entities. Some methods include size-exclusion or anion-exchange
chromatography.
[00294] Any method may be used to determine whether aggregates of thermally-
responsive conjugates have formed, including measuring extinction
coefficients, atomic force
microscopy (AFM), etc. An extinction coefficient, generally speaking, is a
measure of a
substance's turbidity and/or opacity. If EM radiation can pass through a
substance very
easily, the substance has a low extinction coefficient. Conversely, if EM
radiation hardly
penetrates a substance, but rather quickly becomes "extinct" within it, the
extinction
Page 75 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
coefficient is high. For example, to determine whether aggregates of thermally-
responsive
conjugates have formed, EM radiation is directed toward and allowed to pass
through a
sample. If the sample contains primarily conjugate aggregates, EM radiation
will deflect and
scatter in a pattern that is different from the pattern produced by a sample
containing
primarily individual conjugates.
[00295] In general, AFM utilizes a high-resolution type of scanning probe
microscope and
attains resolution of fractions of an Angstrom. The microscope has a
microscale cantilever
with a sharp tip (probe) at its end that is used to scan a specimen surface.
The cantilever is
frequently silicon or silicon nitride with a tip radius of curvature on the
order of nanometers.
When the tip is brought into proximity of a sample surface, forces between the
tip and the
sample lead to a deflection of the cantilever according to Hooke's law.
Typically, a feedback
mechanism is employed to adjust the tip-to-sample distance to maintain a
constant force
between the tip and the sample. Samples are usually spread in a thin layer
across a surface
(e.g. mica), which is mounted on a piezoelectric tube that can move the sample
in the z
direction for maintaining a constant force, and the x andy directions for
scanning the sample.
[00296] In general, forces that are measured in AFM may include mechanical
contact
force, Van der Waals forces, capillary forces, chemical bonding, electrostatic
forces,
magnetic forces, Casimir forces, solvation forces, etc. Typically, deflection
is measured
using a laser spot reflected from the top of the cantilever into an array of
photodiodes.
Alternatively or additionally, deflection can be measured using optical
interferometry,
capacitive sensing, or piezoresistive AFM probes.
Applications
[00297] In some embodiments, a composition in accordance with the invention is
administered to a subject for therapeutic, diagnostic, and/or prophylactic
purposes. In some
embodiments, the amount of thermally-responsive conjugate and/or population of
thermally-
responsive conjugates is sufficient to treat, alleviate symptoms of, diagnose,
prevent, and/or
delay the onset of a disease, condition, and/or disorder. In some embodiments,
the invention
encompasses "therapeutic cocktails," including, but not limited to, approaches
in which
multiple thermally-responsive conjugates are administered.
[00298] The present invention provides thermally-responsive conjugates that
enable
delivery of an agent (e.g. therapeutic, diagnostic, and/or prophylactic agent)
at a specific
time. An agent to be delivered, as described herein, may be released from
conjugates free in
the bloodstream, from conjugates in tissues, from conjugates in cells, from
conjugates within
Page 76 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
a hydrogel, from conjugates immobilized onto a surface, and/or from conjugates
behind a
membrane. Thermally-responsive conjugates may be used in vitro as well as in
vivo.
[00299] To give but a few examples, applications include intelligent drug
delivery,
controllable drug implants, simplified vaccinations, more potent cancer
treatments, enhanced
sensing capabilities, MRI, gene therapy, monitoring enzyme catalysis of
endogenous and/or
delivered substrates, delivery of high drug or cargo dosages to single points,
reduction of
non-specific drug release, localized release of growth factors to cells,
intracellular cargo
delivery, and/or controlled vehicle disassembly for easing clearance of
particles in vivo.
Therapeutic Applications
[00300] In some embodiments, thermally-responsive conjugates are used for
delivery of a
therapeutic and/or nutraceutical agent to an organ, tissue, cell, subcellular
locale, and/or
extracellular matrix locale. Any therapeutic and/or nutraceutical agent may be
delivered
using the thermally-responsive conjugates described herein, and examples of
therapeutic
and/or nutraceutical agents that can be delivered using thermally-responsive
conjugates are
described in the section entitled "Agents to be Delivered" (above). Such
agents include, but
are not limited to, chemotherapeutic agents, radiation-sensitizers (e.g., for
radiation therapy),
peptides and/or proteins that affect the cell cycle, protein toxins, vitamins,
and/or any other
therapeutic and/or nutraceutical agent.
[00301] The present invention provides methods for the treatment of a disease,
disorder,
and/or condition. In some embodiments, the treatment of a disease, disorder,
and/or
condition comprises administering a therapeutically effective amount of
thermally-responsive
conjugates to a subject in need thereof, in such amounts and for such time as
is necessary to
achieve the desired result. In certain embodiments, a "therapeutically
effective amount" of
an conjugate is that amount effective for treating, alleviating, ameliorating,
relieving,
delaying onset of, inhibiting progression of, reducing severity of, and/or
reducing incidence
of one or more symptoms or features of a disease, disorder, and/or condition.
[00302] Any disease, disorder, and/or condition may be treated using
conjugates.
Exemplary diseases, disorders, and/or conditions that may be treated include,
but are not
limited to, autoimmune disorders (e.g. diabetes, lupus, multiple sclerosis,
psoriasis,
rheumatoid arthritis); inflammatory disorders (e.g. arthritis, pelvic
inflammatory disease);
infectious diseases (e.g. viral, bacterial, and fungal infections; sepsis);
neurological disorders
(e.g. Alzheimer's disease, autism); cardiovascular disorders (e.g.
atherosclerosis, thrombosis,
clotting disorders, angiogenic disorders such as macular degeneration);
proliferative disorders
(e.g. cancer); respiratory disorders (e.g. chronic obstructive pulmonary
disease); digestive
Page 77 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
disorders (e.g. inflammatory bowel disease, ulcers); musculoskeletal disorders
(e.g.
fibromyalgia, arthritis); endocrine, metabolic, and nutritional disorders
(e.g. diabetes,
osteoporosis); urological disorders (e.g. renal disease); liver disorders
(e.g. hepatocellular
carcinoma; fibrosis/cirrhosis; genetic defects; metabolic and clotting
disorders, such as
diabetes and obesity that are mediated through the liver; hepatitis, such as
hepatitis A, B, C,
and/or D; other infectious diseases, such as malaria, dengue, etc.; etc.);
psychological
disorders (e.g. depression, schizophrenia); skin disorders (e.g. wounds,
eczema); blood and
lymphatic disorders (e.g. anemia, hemophilia); etc. In some embodiments,
conjugates are
used to treat a cell proliferative disorder. In some embodiments, conjugates
are used to treat
cancer. In some embodiments, for example, a therapeutically effective amount
of a
thermally-responsive conjugate and/or conjugate system is that amount
effective for
inhibiting survival, growth, and/or spread of a tumor.
[00303] In some embodiments, the invention provides efficient and effective
methods for
controllable delivery of therapeutic agents utilizing thermally-responsive
conjugates in
accordance with the present invention. In some embodiments, the present
invention provides
methods for delivery of therapeutic agents which permit release of the
therapeutic agent to
the subject only at desired times and/or at desired locations within a subject
(e.g. desired
organ, tissue, cell, subcellular locale, and/or extracellular matrix
component). In some
embodiments, the invention provides methods for delivery of increased
therapeutic dosages
relative to traditional methods of drug delivery. For example, when delivering
therapeutic
agents with adverse side effects (e.g. chemotherapeutic and/or cytotoxic
drugs) using
traditional methods of drug delivery, low doses are typically administered in
order to avoid
adverse side effects. Using methods in accordance with the present invention,
higher doses
of therapeutic agents can be delivered because the therapeutic agents are
released and/or
delivered in a controlled manner. In some embodiments, such methods can be
used to
provide more potent therapies (e.g. cancer treatments, antibiotic treatments,
delivery of
growth factors to cells, etc.) relative to traditional treatments. In some
embodiments, such
methods as those described above comprise administering a therapeutically
effective amount
of thermally-responsive conjugates in accordance with the present invention to
a subject in
need thereof.
[00304] The present invention provides controllable drug implants comprising
thermally-
responsive conjugates in accordance with the present invention. In some
embodiments,
controllable drug implants may be placed at any location within a subject,
including, but not
limited to, subcutaneous implantation. In some embodiments, controllable drug
implants
Page 78 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
may be placed within a subject for a period of days, weeks, months, or even
years. In some
embodiments, controllable drug implants are physically removed from the
subject (e.g.
surgically) after it has been implanted within a subject (e.g. after the
therapeutic purpose has
been served). In some embodiments, controllable drug implants may disintegrate
over time,
thereby not requiring physical removal of the implant. In some embodiments,
controllable
drug implants may be used to administer multiple doses of an agent to be
delivered. For
example, a controllable drug implant comprising an agent to be delivered (e.g.
therapeutic,
diagnostic, prophylactic, and/or other agent) may be heated to a trigger
temperature (e.g.
upon exposure to an EM field or light) at desired points in time in order to
release doses of
the therapeutic agent at the desired points in time.
[00305] The present invention provides methods for delivering agents (e.g.
therapeutic,
prophylactic, diagnostic, nutraceutical, etc., agents) to subcellular locales
(including
intracellular locales and/or cell membranes). For example, in some
embodiments, conjugates
may comprise targeting moieties which specifically bind to a membrane-bound
target. To
give another example, in some embodiments, conjugates may comprise moieties
which
facilitate their entry into cells. In some embodiments, conjugates may
comprise targeting
moieties which specifically bind to an intracellular target. Such conjugates
may be useful for
delivering agents which facilitate gene therapy (e.g. vectors, functional
RNAs, mutagens,
etc.).
[00306] In some embodiments, thermally-responsive conjugates are used for
external
monitoring of drug accumulation in target sites. For example, using MRI
techniques,
location of particles can be detected and monitored. From this information, a
skilled person
can infer where drug will be released.
[00307] In some embodiments, thermally-responsive conjugates are used for
delivery of a
prophylactic agent to an organ, tissue, cell, subcellular locale, and/or
extracellular matrix
locale. Any prophylactic agent may be delivered using the thermally-responsive
conjugates
described herein, and examples of agents that can be delivered using thermally-
responsive
conjugates are described in the section entitled "Agents to be Delivered"
(above). Such
agents include, but are not limited to, vaccines and/or any other prophylactic
agent.
[00308] In some embodiments, the present invention provides simplified methods
for
vaccinating an individual. For example, some types of vaccines require
multiple
administrations of the vaccine in order for the vaccine to be effective (e.g.
adults receiving
the chicken pox vaccine should receive two doses scheduled 4 weeks to 8 weeks
apart).
Some types of vaccines require booster doses of the vaccine at some point in
time after the
Page 79 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
vaccine was initially administered (e.g. a booster dose of tetanus vaccine is
administered to a
subject within 10 years after the previous dose of tetanus vaccine). The
present invention
provides simplified methods for administering multiple administrations of a
vaccine. In some
embodiments, thermally-responsive conjugates in accordance with the present
invention
comprise a vaccine component (e.g. protein/peptide antigen; live, killed, or
attenuated
microbe; etc.) as the agent to be delivered. Such conjugates may be subjected
to an external
stimulus (e.g. EM field, light) at particular points in time corresponding to
the desired
vaccination dosing schedule, thereby releasing the vaccine component from the
conjugate
according to the desired vaccination dosing schedule. In some embodiments,
such methods
can allow an individual to receive multiple doses of a vaccine (e.g. booster
doses) without
multiple visits to a physician and/or without multiple needle pricks.
[00309] In some embodiments, thermally-responsive conjugates are used for
delivery of a
diagnostic agent to an organ, tissue, cell, subcellular locale, and/or
extracellular matrix locale.
Any diagnostic agent may be delivered using the thermally-responsive
conjugates described
herein, and examples of diagnostic agents that can be delivered using
thermally-responsive
conjugates are described in the section entitled "Agents to be Delivered"
(above). Such
agents include, but are not limited to, radioactive moieties, radiopaque
moieties,
paramagnetic moieties, particles, vesicles, markers, marker enzymes (e.g.,
horseradish
peroxidase, (3-galactosidase, and/or any other enzyme suitable for marking a
cell), contrast
agents (e.g., for diagnostic imaging), and/or any other diagnostic agent.
[00310] The present invention provides methods for the diagnosis of a disease,
disorder,
and/or condition. In some embodiments, the diagnosis of a disease, disorder,
and/or
condition comprises administering a therapeutically effective amount of
thermally-responsive
conjugates to a subject in need thereof, in such amounts and for such time as
is necessary to
achieve the desired result. In certain embodiments of the present invention a
"therapeutically
effective amount" of a thermally-responsive conjugate is that amount effective
for detecting,
monitoring, and/or measuring the presence of one or more symptoms or features
of a disease
(e.g. cancer). In some embodiments, for example, a therapeutically effective
amount of a
thermally-responsive conjugate is that amount effective for detecting the
presence and/or
determining the location of a tumor. To give but one example, a thermally-
responsive
conjugate may be capable of being imaged directly or it may be conjugated to a
ligand (e.g.
DPTA) that binds a heavy metal (e.g. yttrium, indium, etc.) and/or other agent
that can be
imaged.
Page 80 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00311] In some embodiments, the present invention provides methods of
enhancing
sensing capabilities. In some embodiments, conjugate assemblies are
characterized by
having optical, magnetic, electric, and/or other type of detectable properties
that are different
from the optical, magnetic, electric, and/or other type of detectable
properties of the
individual conjugates that make up the conjugate assemblies. For example, in
some
embodiments, enhanced sensing capabilities refer to the initiation of an
imaging signal by
disrupting a conjugate assembly by exposure to the trigger temperature. By
specifically
changing an imaging signal at a given time, the sensitivity of detection over
background can
be improved for fluorescence measurements, MRI measurements, luminescence
measurements, and so forth.
[00312] To give but one example, in some embodiments, thermally-responsive
conjugates
can be utilized in magnetic resonance imaging (MRI) applications. MRI utilizes
superparamagnetic nanoparticles to dephase populations of protons and to
enhance proton
relaxation. Such nanoparticles are typically administered as monomeric
particles with
constant imaging signatures. In some embodiments, the present invention
provides methods
in which, by disassembling conjugate assemblies, the specificity of MR
measurements can be
enhanced as conjugate assemblies are deconstructed into individual conjugates,
thereby
changing their MR signatures in predictable ways. Such changes can overcome a
typical
limitation of magnetic particles, wherein particle signatures may be mistaken
for magnetic
field inhomogeneities by removing contrast upon demand.
[00313] In some embodiment s, thermally-responsive conjugates may be used for
external
monitoring of accumulation of agents to be delivered. For example, MRI can be
used to
monitor the distribution of thermally-responsive conjugates within a subject.
Thus, it is
possible to infer where a therapeutic agent may be released or may accumulate.
[00314] In some embodiments, an agent to be delivered may be a substrate for
an enzyme.
In such embodiments, when an agent is released from a thermally-responsive
conjugate in the
presence of an enzyme which acts upon the agent, catalysis may occur. In some
embodiments, catalysis results in production of a detectable product (e.g.
fluorescence, color
change, etc.). Therefore, in some embodiments, thermally-responsive conjugates
may be
used to detect release of an agent from the thermally-responsive conjugate. To
give but one
example, a thermally-responsive conjugate may release a substrate for a tumor-
associated
enzyme that itself provides a detectable signal upon catalysis.
[00315] In some embodiments, such methods involve using an assembly of
conjugates
that, after release of the agent to be delivered, could be cleared from the
body as individual
Page 81 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
conjugates or particles. This could allow for large systems of conjugates (>
100 nm), holding
a quantity of drug, to disassociate into conjugates or particles small enough
for renal
clearance (> 7 nm). One problem with depending on the liver for particle
removal is that
many particulates are not easily cleared from it and can stay for long periods
of time with
unknown effect. Alternatively or additionally, particles larger than
approximately 200 nm
can be non-specifically filtered by the spleen. Thus, the present invention
provides methods
for tailoring of particle delivery schemes for renal clearance.
[00316] The present invention provides methods which offer several advantages
for
treatment of conditions such as cancer, where vascular permeability many
increase to > 200
nm, but full tumor perfusion is more limited. The present invention provides
large
assemblies which can be designed to escape the leaky vasculature and then be
triggered to
disassociate. This could allow individual conjugates to diffuse further into
the tumor before
delivering the agent. Such methods can result in increased drug dosage, and
exposing cells to
higher dosages can increase the pharmacological effect of the drug.
[00317] The present invention encompasses the recognition that aggregate
disassembly
can be used to sequentially disrobe layers from a conjugate assembly. Such
controlled
disassembly may be utilized to release a series of drugs, to expose hidden
binding sites,
and/or to reveal a new type of particle at the assembly surface. To give one
example, such
methods may be used to expose an entity (e.g. an antigenic peptide) that
simulates and/or
inhibits an immune response, inflammatory cascade, localized coagulation etc.
Administration
[00318] Thermally responsive conjugates in accordance with the present
invention and
pharmaceutical compositions thereof may be administered using any amount and
any route of
administration effective for treatment. The exact amount required will vary
from subject to
subject, depending on the species, age, and general condition of the subject,
the severity of
the infection, the particular composition, its mode of administration, its
mode of activity, and
the like. Thermally responsive conjugates are typically formulated in dosage
unit form for
ease of administration and uniformity of dosage. It will be understood,
however, that the
total daily usage of the compositions of the present invention will be decided
by the attending
physician within the scope of sound medical judgment. The specific
therapeutically effective
dose level for any particular subject or organism will depend upon a variety
of factors
including the disorder being treated and the severity of the disorder; the
activity of the
specific compound employed; the specific composition employed; the age, body
weight,
Page 82 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
general health, sex, and diet of the subject; the time of administration,
route of administration,
and rate of excretion of the specific compound employed; the duration of the
treatment; drugs
used in combination or coincidental with the specific compound employed; and
like factors,
well known in the medical arts.
[00319] Pharmaceutical compositions of the present invention may be
administered by any
route. In some embodiments, pharmaceutical compositions of the present
invention are
administered by a variety of routes, including oral, intravenous,
intramuscular, intra-arterial,
intramedullary, intrathecal, subcutaneous, intraventricular, transdermal,
interdermal, rectal,
intravaginal, intraperitoneal, topical (e.g. by powders, ointments, creams,
gels, lotions, and/or
drops), mucosal, nasal, buccal, enteral, vitreal, intratumoral, sublingual; by
intratracheal
instillation, bronchial instillation, and/or inhalation; as an oral spray,
nasal spray, and/or
aerosol, and/or through a portal vein catheter. In some embodiments,
pharmaceutical
compositions are administered by systemic intravenous injection, regional
administration via
blood and/or lymph supply, and/or direct administration to an affected site
(e.g. a therapeutic
implant, such as a hydrogel). In general the most appropriate route of
administration will
depend upon a variety of factors including the nature of the agent (e.g., its
stability in the
environment of the gastrointestinal tract), the condition of the subject
(e.g., whether the
subject is able to tolerate oral administration), etc. In specific
embodiments, thermally-
responsive conjugates in accordance with the present invention and/or
pharmaceutical
compositions thereof may be administered intravenously. In specific
embodiments,
thermally-responsive conjugates in accordance with the present invention
and/or
pharmaceutical compositions thereof may be administered intraperitoneally. In
specific
embodiments, thermally-responsive conjugates in accordance with the present
invention
and/or pharmaceutical compositions thereof may be administered intrathecally.
In specific
embodiments, thermally-responsive conjugates in accordance with the present
invention
and/or pharmaceutical compositions thereof may be administered intratumorally.
In specific
embodiments, thermally-responsive conjugates in accordance with the present
invention
and/or pharmaceutical compositions thereof may be administered
intramuscularly. In
specific embodiments, thermally-responsive conjugates in accordance with the
present
invention and/or pharmaceutical compositions thereof may be administered via
vitreal
administration. In specific embodiments, thermally-responsive conjugates in
accordance
with the present invention and/or pharmaceutical compositions thereof may be
administered
via a portal vein catheter. In specific embodiments, thermally-responsive
conjugates in
accordance with the present invention and/or pharmaceutical compositions
thereof may be
Page 83 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
immobilized into a hydrogel for controlled long-term release of thermally-
responsive
conjugates. However, the invention encompasses the delivery of thermally-
responsive
conjugates and/or pharmaceutical compositions thereof by any appropriate route
taking into
consideration likely advances in the sciences of drug delivery.
[00320] In certain embodiments, compositions in accordance with the invention
may be
administered orally or parenterally at dosage levels sufficient to deliver
from about 0.001
mg/kg to about 100 mg/kg, from about 0.01 mg/kg to about 50 mg/kg, from about
0.1 mg/kg
to about 40 mg/kg, from about 0.5 mg/kg to about 30 mg/kg, from about 0.01
mg/kg to about
mg/kg, from about 0.1 mg/kg to about 10 mg/kg, or from about 1 mg/kg to about
25
mg/kg of subject body weight per day to obtain the desired therapeutic effect.
The desired
dosage may be delivered more than three times per day, three times per day,
two times per
day, once per day, every other day, every third day, every week, every two
weeks, every three
weeks, every four weeks, every two months, every six months, or every twelve
months. In
certain embodiments, the desired dosage may be delivered using multiple
administrations
(e.g., two, three, four, five, six, seven, eight, nine, ten, eleven, twelve,
thirteen, fourteen, or
more administrations).
[00321] It will be appreciated that thermally-responsive conjugates in
accordance with the
present invention and pharmaceutical compositions thereof can be employed in
combination
therapies. The particular combination of therapies (e.g. therapeutics or
procedures) to
employ in a combination regimen will take into account compatibility of the
desired
therapeutics and/or procedures and the desired therapeutic effect to be
achieved. It will be
appreciated that the therapies employed may achieve a desired effect for the
same purpose
(for example, conjugates useful for reducing the size of tumors may be
administered
concurrently with another agent useful for reducing the size of tumors), or
they may achieve
different effects (e.g., control of any adverse effects).
[00322] Pharmaceutical compositions in accordance with the present invention
may be
administered either alone or in combination with one or more other therapeutic
agents. By
"in combination with," it is not intended to imply that the agents must be
administered at the
same time and/or formulated for delivery together, although these methods of
delivery are
within the scope of the invention. The compositions can be administered
concurrently with,
prior to, or subsequent to, one or more other desired therapeutics or medical
procedures. In
general, each agent will be administered at a dose and/or on a time schedule
determined for
that agent. Additionally, the invention encompasses the delivery of
pharmaceutical
compositions in combination with agents that may improve their
bioavailability, reduce
Page 84 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
and/or modify their metabolism, inhibit their excretion, and/or modify their
distribution
within the body.
[00323] The particular combination of therapies (e.g. therapeutics and/or
procedures) to
employ in a combination regimen will take into account compatibility of the
desired
therapeutics and/or procedures and/or the desired therapeutic effect to be
achieved. It will be
appreciated that the therapies employed may achieve a desired effect for the
same disorder
(for example, a thermally-responsive conjugate may be administered
concurrently with
another agent used to treat the same disorder), and/or they may achieve
different effects (e.g.,
control of any adverse effects).
[00324] In will further be appreciated that therapeutically active agents
utilized in
combination may be administered together in a single composition (e.g. a
conjugate which
comprises a particle and two different therapeutic agents associated with two
different
thermally-responsive linkers) or administered separately in different
compositions (e.g. two
pharmaceutical compositions, each comprising a conjugate comprising a
different therapeutic
agent).
[00325] In general, it is expected that agents utilized in combination with be
utilized at
levels that do not exceed the levels at which they are utilized individually.
In some
embodiments, the levels utilized in combination will be lower than those
utilized
individually.
[00326] In some embodiments, thermally-responsive conjugates which are used as
therapeutic agents may be used in combination with other therapeutic agents.
To give but
one example, thermally-responsive conjugates used to treat tumors may be
administered in
combination with other agents useful in the treatment of tumors. For example,
thermally-
responsive conjugates may be administered in combination with traditional
chemotherapy,
radiation treatment, surgical removal of a tumor, administration of biologics
(e.g. therapeutic
antibodies), etc.
Pharmaceutical Compositions
[00327] The present invention provides thermally-responsive conjugates
comprising one
or more heatable surfaces, one or more thermally-responsive linkers, and one
or more agents
to be delivered. In some embodiments, the present invention provides for
pharmaceutical
compositions comprising thermally-responsive conjugates as described herein
and one or
more pharmaceutically acceptable excipients. Such pharmaceutical compositions
may
optionally comprise one or more additional therapeutically-active substances.
In accordance
Page 85 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
with some embodiments, a method of administering a pharmaceutical composition
comprising thermally-responsive conjugates to a subject in need thereof is
provided. In some
embodiments, the compositions are administered to humans. For the purposes of
the present
disclosure, the phrase "active ingredient" generally refers to a thermally-
responsive
conjugate.
[00328] Although the descriptions of pharmaceutical compositions provided
herein are
principally directed to pharmaceutical compositions which are suitable for
ethical
administration to humans, it will be understood by the skilled artisan that
such compositions
are generally suitable for administration to animals of all sorts.
Modification of
pharmaceutical compositions suitable for administration to humans in order to
render the
compositions suitable for administration to various animals is well
understood, and the
ordinarily skilled veterinary pharmacologist can design and/or perform such
modification
with merely ordinary, if any, experimentation. Subjects to which
administration of the
pharmaceutical compositions in accordance with the invention is contemplated
include, but
are not limited to, humans and/or other primates; mammals, including
commercially relevant
mammals such as cattle, pigs, horses, sheep, cats, and/or dogs; and/or birds,
including
commercially relevant birds such as chickens, ducks, geese, and/or turkeys.
[00329] Formulations of the pharmaceutical compositions described herein may
be
prepared by any method known or hereafter developed in the art of
pharmacology. In
general, such preparatory methods include the step of bringing the active
ingredient into
association with an excipient and/or one or more other accessory ingredients,
and then, if
necessary and/or desirable, shaping and/or packaging the product into a
desired single- or
multi-dose unit.
[00330] A pharmaceutical composition in accordance with the invention may be
prepared,
packaged, and/or sold in bulk, as a single unit dose, and/or as a plurality of
single unit doses.
As used herein, a "unit dose" is discrete amount of the pharmaceutical
composition
comprising a predetermined amount of the active ingredient. The amount of the
active
ingredient is generally equal to the dosage of the active ingredient which
would be
administered to a subject and/or a convenient fraction of such a dosage such
as, for example,
one-half or one-third of such a dosage.
[00331] Relative amounts of the active ingredient, the pharmaceutically
acceptable
excipient, and/or any additional ingredients in a pharmaceutical composition
in accordance
with the invention will vary, depending upon the identity, size, and/or
condition of the subject
treated and further depending upon the route by which the composition is to be
administered.
Page86of117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
By way of example, the composition may comprise between 0.1% and 100% (w/w)
active
ingredient.
[00332] Pharmaceutical formulations of the present invention may additionally
comprise a
pharmaceutically acceptable excipient, which, as used herein, includes any and
all solvents,
dispersion media, diluents, or other liquid vehicles, dispersion or suspension
aids, surface
active agents, isotonic agents, thickening or emulsifying agents,
preservatives, solid binders,
lubricants and the like, as suited to the particular dosage form desired.
Remington's The
Science and Practice of Pharmacy, 21st Edition, A. R. Gennaro, (Lippincott,
Williams &
Wilkins, Baltimore, MD, 2006) discloses various excipients used in formulating
pharmaceutical compositions and known techniques for the preparation thereof.
Except
insofar as any conventional excipient medium is incompatible with a substance
or its
derivatives, such as by producing any undesirable biological effect or
otherwise interacting in
a deleterious manner with any other component(s) of the pharmaceutical
composition, its use
is contemplated to be within the scope of this invention.
[00333] In some embodiments, the pharmaceutically acceptable excipient is at
least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100% pure. In some
embodiments,
the excipient is approved for use in humans and for veterinary use. In some
embodiments,
the excipient is approved by United States Food and Drug Administration. In
some
embodiments, the excipient is pharmaceutical grade. In some embodiments, the
excipient
meets the standards of the United States Pharmacopoeia (USP), the European
Pharmacopoeia
(EP), the British Pharmacopoeia, and/or the International Pharmacopoeia.
[00334] Pharmaceutically acceptable excipients used in the manufacture of
pharmaceutical
compositions include, but are not limited to, inert diluents, dispersing
and/or granulating
agents, surface active agents and/or emulsifiers, disintegrating agents,
binding agents,
preservatives, buffering agents, lubricating agents, and/or oils. Such
excipients may
optionally be included in the formulations. Excipients such as cocoa butter
and suppository
waxes, coloring agents, coating agents, sweetening, flavoring, and/or
perfuming agents can
be present in the composition, according to the judgment of the formulator.
[00335] Exemplary diluents include, but are not limited to, calcium carbonate,
sodium
carbonate, calcium phosphate, dicalcium phosphate, calcium sulfate, calcium
hydrogen
phosphate, sodium phosphate lactose, sucrose, cellulose, microcrystalline
cellulose, kaolin,
mannitol, sorbitol, inositol, sodium chloride, dry starch, cornstarch,
powdered sugar, etc.,
and/or combinations thereof
Page 87 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00336] Exemplary granulating and/or dispersing agents include, but are not
limited to,
potato starch, corn starch, tapioca starch, sodium starch glycolate, clays,
alginic acid, guar
gum, citrus pulp, agar, bentonite, cellulose and wood products, natural
sponge, cation-
exchange resins, calcium carbonate, silicates, sodium carbonate, cross-linked
poly(vinyl-
pyrrolidone) (crospovidone), sodium carboxymethyl starch (sodium starch
glycolate),
carboxymethyl cellulose, cross-linked sodium carboxymethyl cellulose
(croscarmellose),
methylcellulose, pregelatinized starch (starch 1500), microcrystalline starch,
water insoluble
starch, calcium carboxymethyl cellulose, magnesium aluminum silicate (Veegum),
sodium
lauryl sulfate, quaternary ammonium compounds, etc., and/or combinations
thereof.
[00337] Exemplary surface active agents and/or emulsifiers include, but are
not limited to,
natural emulsifiers (e.g. acacia, agar, alginic acid, sodium alginate,
tragacanth, chondrux,
cholesterol, xanthan, pectin, gelatin, egg yolk, casein, wool fat,
cholesterol, wax, and
lecithin), colloidal clays (e.g. bentonite [aluminum silicate] and Veegum
[magnesium
aluminum silicate]), long chain amino acid derivatives, high molecular weight
alcohols (e.g.
stearyl alcohol, cetyl alcohol, oleyl alcohol, triacetin monostearate,
ethylene glycol distearate,
glyceryl monostearate, and propylene glycol monostearate, polyvinyl alcohol),
carbomers
(e.g. carboxy polymethylene, polyacrylic acid, acrylic acid polymer, and
carboxyvinyl
polymer), carrageenan, cellulosic derivatives (e.g. carboxymethylcellulose
sodium, powdered
cellulose, hydroxymethyl cellulose, hydroxypropyl cellulose, hydroxypropyl
methylcellulose,
methylcellulose), sorbitan fatty acid esters (e.g. polyoxyethylene sorbitan
monolaurate
[Tween 20], polyoxyethylene sorbitan [Tween 60], polyoxyethylene sorbitan
monooleate
[Tween 80], sorbitan monopalmitate [Span 40], sorbitan monostearate [Span 60],
sorbitan
tristearate [Span 65], glyceryl monooleate, sorbitan monooleate [Span 80]),
polyoxyethylene
esters (e.g. polyoxyethylene monostearate [Myrj 45], polyoxyethylene
hydrogenated castor
oil, polyethoxylated castor oil, polyoxymethylene stearate, and Solutol ),
sucrose fatty acid
esters, polyethylene glycol fatty acid esters (e.g. Cremophor ),
polyoxyethylene ethers, (e.g.
polyoxyethylene lauryl ether [Brij 30]), poly(vinyl-pyrrolidone), diethylene
glycol
monolaurate, triethanolamine oleate, sodium oleate, potassium oleate, ethyl
oleate, oleic acid,
ethyl laurate, sodium lauryl sulfate, Pluronic F 68, Poloxamer 188,
cetrimonium bromide,
cetylpyridinium chloride, benzalkonium chloride, docusate sodium, etc. and/or
combinations
thereo
[00338] Exemplary binding agents include, but are not limited to, starch (e.g.
cornstarch
and starch paste); gelatin; sugars (e.g. sucrose, glucose, dextrose, dextrin,
molasses, lactose,
lactitol, mannitol,); natural and synthetic gums (e.g. acacia, sodium
alginate, extract of Irish
Page 88 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
moss, panwar gum, ghatti gum, mucilage of isapol husks,
carboxymethylcellulose,
methylcellulose, ethylcellulose, hydroxyethylcellulose, hydroxypropyl
cellulose,
hydroxypropyl methylcellulose, microcrystalline cellulose, cellulose acetate,
poly(vinyl-
pyrrolidone), magnesium aluminum silicate (Veegum ), and larch arabogalactan);
alginates;
polyethylene oxide; polyethylene glycol; inorganic calcium salts; silicic
acid;
polymethacrylates; waxes; water; alcohol; etc.; and combinations thereof.
[00339] Exemplary preservatives may include, but are not limited to,
antioxidants,
chelating agents, antimicrobial preservatives, antifungal preservatives,
alcohol preservatives,
acidic preservatives, and/or other preservatives. Exemplary antioxidants
include, but are not
limited to, alpha tocopherol, ascorbic acid, acorbyl palmitate, butylated
hydroxyanisole,
butylated hydroxytoluene, monothioglycerol, potassium metabisulfite, propionic
acid, propyl
gallate, sodium ascorbate, sodium bisulfite, sodium metabisulfite, and/or
sodium sulfite.
Exemplary chelating agents include ethylenediaminetetraacetic acid (EDTA),
citric acid
monohydrate, disodium edetate, dipotassium edetate, edetic acid, fumaric acid,
malic acid,
phosphoric acid, sodium edetate, tartaric acid, and/or trisodium edetate.
Exemplary
antimicrobial preservatives include, but are not limited to, benzalkonium
chloride,
benzethonium chloride, benzyl alcohol, bronopol, cetrimide, cetylpyridinium
chloride,
chlorhexidine, chlorobutanol, chlorocresol, chloroxylenol, cresol, ethyl
alcohol, glycerin,
hexetidine, imidurea, phenol, phenoxyethanol, phenylethyl alcohol,
phenylmercuric nitrate,
propylene glycol, and/or thimerosal. Exemplary antifungal preservatives
include, but are not
limited to, butyl paraben, methyl paraben, ethyl paraben, propyl paraben,
benzoic acid,
hydroxybenzoic acid, potassium benzoate, potassium sorbate, sodium benzoate,
sodium
propionate, and/or sorbic acid. Exemplary alcohol preservatives include, but
are not limited
to, ethanol, polyethylene glycol, phenol, phenolic compounds, bisphenol,
chlorobutanol,
hydroxybenzoate, and/or phenylethyl alcohol. Exemplary acidic preservatives
include, but
are not limited to, vitamin A, vitamin C, vitamin E, beta-carotene, citric
acid, acetic acid,
dehydroacetic acid, ascorbic acid, sorbic acid, and/or phytic acid. Other
preservatives
include, but are not limited to, tocopherol, tocopherol acetate, deteroxime
mesylate,
cetrimide, butylated hydroxyanisol (BHA), butylated hydroxytoluened (BHT),
ethylenediamine, sodium lauryl sulfate (SLS), sodium lauryl ether sulfate
(SLES), sodium
bisulfite, sodium metabisulfite, potassium sulfite, potassium metabisulfite,
Glydant Plus ,
Phenonip , methylparaben, Germall 115, Germaben II, NeoloneTM, KathonT'",
and/or Euxyl .
[00340] Exemplary buffering agents include, but are not limited to, citrate
buffer solutions,
acetate buffer solutions, phosphate buffer solutions, ammonium chloride,
calcium carbonate,
Page 89 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
calcium chloride, calcium citrate, calcium glubionate, calcium gluceptate,
calcium gluconate,
D-gluconic acid, calcium glycerophosphate, calcium lactate, propanoic acid,
calcium
levulinate, pentanoic acid, dibasic calcium phosphate, phosphoric acid,
tribasic calcium
phosphate, calcium hydroxide phosphate, potassium acetate, potassium chloride,
potassium
gluconate, potassium mixtures, dibasic potassium phosphate, monobasic
potassium
phosphate, potassium phosphate mixtures, sodium acetate, sodium bicarbonate,
sodium
chloride, sodium citrate, sodium lactate, dibasic sodium phosphate, monobasic
sodium
phosphate, sodium phosphate mixtures, tromethamine, magnesium hydroxide,
aluminum
hydroxide, alginic acid, pyrogen-free water, isotonic saline, Ringer's
solution, ethyl alcohol,
etc., and/or combinations thereof
[00341] Exemplary lubricating agents include, but are not limited to,
magnesium stearate,
calcium stearate, stearic acid, silica, talc, malt, glyceryl behanate,
hydrogenated vegetable
oils, polyethylene glycol, sodium benzoate, sodium acetate, sodium chloride,
leucine,
magnesium lauryl sulfate, sodium lauryl sulfate, etc., and combinations
thereof.
[00342] Exemplary oils include, but are not limited to, almond, apricot
kernel, avocado,
babassu, bergamot, black current seed, borage, cade, camomile, canola,
caraway, carnauba,
castor, cinnamon, cocoa butter, coconut, cod liver, coffee, corn, cotton seed,
emu, eucalyptus,
evening primrose, fish, flaxseed, geraniol, gourd, grape seed, hazel nut,
hyssop, isopropyl
myristate, jojoba, kukui nut, lavandin, lavender, lemon, litsea cubeba,
macademia nut,
mallow, mango seed, meadowfoam seed, mink, nutmeg, olive, orange, orange
roughy, palm,
palm kernel, peach kernel, peanut, poppy seed, pumpkin seed, rapeseed, rice
bran, rosemary,
safflower, sandalwood, sasquana, savoury, sea buckthorn, sesame, shea butter,
silicone,
soybean, sunflower, tea tree, thistle, tsubaki, vetiver, walnut, and wheat
germ oils.
Exemplary oils include, but are not limited to, butyl stearate, caprylic
triglyceride, capric
triglyceride, cyclomethicone, diethyl sebacate, dimethicone 360, isopropyl
myristate, mineral
oil, octyldodecanol, oleyl alcohol, silicone oil, and/or combinations thereo
[00343] Liquid dosage forms for oral and parenteral administration include,
but are not
limited to, pharmaceutically acceptable emulsions, microemulsions, solutions,
suspensions,
syrups, and/or elixirs. In addition to active ingredients, liquid dosage forms
may comprise
inert diluents commonly used in the art such as, for example, water or other
solvents,
solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol,
ethyl carbonate,
ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene
glycol,
dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ,
olive, castor, and
sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and
fatty acid esters
Page 90 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
of sorbitan, and mixtures thereof. Besides inert diluents, oral compositions
can include
adjuvants such as wetting agents, emulsifying and suspending agents,
sweetening, flavoring,
and/or perfuming agents. In certain embodiments for parenteral administration,
compositions
are mixed with solubilizing agents such an Cremophor , alcohols, oils,
modified oils, glycols,
polysorbates, cyclodextrins, polymers, and/or combinations thereof.
[00344] Injectable preparations, for example, sterile injectable aqueous or
oleaginous
suspensions may be formulated according to the known art using suitable
dispersing agents,
wetting agents, and/or suspending agents. Sterile injectable preparations may
be sterile
injectable solutions, suspensions, and/or emulsions in nontoxic parenterally
acceptable
diluents and/or solvents, for example, as a solution in 1,3-butanediol. Among
the acceptable
vehicles and solvents that may be employed are water, Ringer's solution,
U.S.P., and isotonic
sodium chloride solution. Sterile, fixed oils are conventionally employed as a
solvent or
suspending medium. For this purpose any bland fixed oil can be employed
including
synthetic mono- or diglycerides. Fatty acids such as oleic acid can be used in
the preparation
of injectables.
[00345] Injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter, and/or by incorporating sterilizing agents in the
form of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium prior to use.
[00346] In order to prolong the effect of an active ingredient, it is often
desirable to slow
the absorption of the active ingredient from subcutaneous or intramuscular
injection. This
may be accomplished by the use of a liquid suspension of crystalline or
amorphous material
with poor water solubility. The rate of absorption of the drug then depends
upon its rate of
dissolution which, in turn, may depend upon crystal size and crystalline form.
Alternatively,
delayed absorption of a parenterally administered drug form is accomplished by
dissolving or
suspending the drug in an oil vehicle. Injectable depot forms are made by
forming
microencapsule matrices of the drug in biodegradable polymers such as
polylactide-
polyglycolide. Depending upon the ratio of drug to polymer and the nature of
the particular
polymer employed, the rate of drug release can be controlled. Examples of
other
biodegradable polymers include poly(orthoesters) and poly(anhydrides). Depot
injectable
formulations are prepared by entrapping the drug in liposomes or
microemulsions which are
compatible with body tissues.
[00347] Compositions for rectal or vaginal administration are typically
suppositories
which can be prepared by mixing compositions with suitable non-irritating
excipients such as
Page 91 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
cocoa butter, polyethylene glycol or a suppository wax which are solid at
ambient
temperature but liquid at body temperature and therefore melt in the rectum or
vaginal cavity
and release the active ingredient.
[00348] Solid dosage forms for oral administration include capsules, tablets,
pills,
powders, and granules. In such solid dosage forms, the active ingredient is
mixed with at
least one inert, pharmaceutically acceptable excipient such as sodium citrate
or dicalcium
phosphate and/or fillers or extenders (e.g. starches, lactose, sucrose,
glucose, mannitol, and
silicic acid), binders (e.g. carboxymethylcellulose, alginates, gelatin,
polyvinylpyrrolidinone,
sucrose, and acacia), humectants (e.g. glycerol), disintegrating agents (e.g.
agar, calcium
carbonate, potato or tapioca starch, alginic acid, certain silicates, and
sodium carbonate),
solution retarding agents (e.g. paraffin), absorption accelerators (e.g.
quaternary ammonium
compounds), wetting agents (e.g. cetyl alcohol and glycerol monostearate),
absorbents (e.g.
kaolin and bentonite clay), and lubricants (e.g. talc, calcium stearate,
magnesium stearate,
solid polyethylene glycols, sodium lauryl sulfate), and mixtures thereof. In
the case of
capsules, tablets and pills, the dosage form may comprise buffering agents.
[00349] Solid compositions of a similar type may be employed as fillers in
soft and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high
molecular weight polyethylene glycols and the like. The solid dosage forms of
tablets,
dragees, capsules, pills, and granules can be prepared with coatings and
shells such as enteric
coatings and other coatings well known in the pharmaceutical formulating art.
They may
optionally comprise opacifying agents and can be of a composition that they
release the
active ingredient(s) only, or preferentially, in a certain part of the
intestinal tract, optionally,
in a delayed manner. Examples of embedding compositions which can be used
include
polymeric substances and waxes. Solid compositions of a similar type may be
employed as
fillers in soft and hard-filled gelatin capsules using such excipients as
lactose or milk sugar as
well as high molecular weight polyethylene glycols and the like.
[00350] Dosage forms for topical and/or transdermal administration of a
compound in
accordance with this invention may include ointments, pastes, creams, lotions,
gels, powders,
solutions, sprays, inhalants and/or patches. Generally, the active ingredient
is admixed under
sterile conditions with a pharmaceutically acceptable excipient and/or any
needed
preservatives and/or buffers as may be required. Additionally, the present
invention
contemplates the use of transdermal patches, which often have the added
advantage of
providing controlled delivery of a compound to the body. Such dosage forms may
be
prepared, for example, by dissolving and/or dispensing the compound in the
proper medium.
Page 92 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
Alternatively or additionally, the rate may be controlled by either providing
a rate controlling
membrane and/or by dispersing the compound in a polymer matrix and/or gel.
[00351] Suitable devices for use in delivering intradermal pharmaceutical
compositions
described herein include short needle devices such as those described in U.S.
Patents
4,886,499; 5,190,521; 5,328,483; 5,527,288; 4,270,537; 5,015,235; 5,141,496;
and
5,417,662. Intradermal compositions may be administered by devices which limit
the
effective penetration length of a needle into the skin, such as those
described in PCT
publication WO 99/34850 and functional equivalents thereof. Jet injection
devices which
deliver liquid vaccines to the dermis via a liquid jet injector and/or via a
needle which pierces
the stratum corneum and produces ajet which reaches the dermis are suitable.
Jet injection
devices are described, for example, in U.S. Patents 5,480,381; 5,599,302;
5,334,144;
5,993,412; 5,649,912; 5,569,189; 5,704,911; 5,383,851; 5,893,397; 5,466,220;
5,339,163;
5,312,335; 5,503,627; 5,064,413; 5,520,639; 4,596,556; 4,790,824; 4,941,880;
4,940,460;
and PCT publications WO 97/37705 and WO 97/13537. Ballistic powder/particle
delivery
devices which use compressed gas to accelerate vaccine in powder form through
the outer
layers of the skin to the dermis are suitable. Alternatively or additionally,
conventional
syringes may be used in the classical mantoux method of intradermal
administration.
[00352] Formulations suitable for topical administration include, but are not
limited to,
liquid and/or semi liquid preparations such as liniments, lotions, oil in
water and/or water in
oil emulsions such as creams, ointments and/or pastes, and/or solutions and/or
suspensions.
Topically-administrable formulations may, for example, comprise from about 1%
to about
10% (w/w) active ingredient, although the concentration of the active
ingredient may be as
high as the solubility limit of the active ingredient in the solvent.
Formulations for topical
administration may further comprise one or more of the additional ingredients
described
herein.
[00353] A pharmaceutical composition in accordance with the invention may be
prepared,
packaged, and/or sold in a formulation suitable for pulmonary administration
via the buccal
cavity. Such a formulation may comprise dry particles which comprise the
active ingredient
and which have a diameter in the range from about 0.5 nm to about 7 nm or from
about 1 nm
to about 6 nm. Such compositions are conveniently in the form of dry powders
for
administration using a device comprising a dry powder reservoir to which a
stream of
propellant may be directed to disperse the powder and/or using a self
propelling
solvent/powder dispensing container such as a device comprising the active
ingredient
dissolved and/or suspended in a low-boiling propellant in a sealed container.
Such powders
Page 93 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
comprise particles wherein at least 98% of the particles by weight have a
diameter greater
than 0.5 nm and at least 95% of the particles by number have a diameter less
than 7 nm.
Alternatively, at least 95% of the particles by weight have a diameter greater
than 1 nm and at
least 90% of the particles by number have a diameter less than 6 nm. Dry
powder
compositions may include a solid fine powder diluent such as sugar and are
conveniently
provided in a unit dose form.
[00354] Low boiling propellants generally include liquid propellants having a
boiling point
of below 65 F at atmospheric pressure. Generally the propellant may
constitute 50% to
99.9% (w/w) of the composition, and the active ingredient may constitute 0.1%
to 20% (w/w)
of the composition. The propellant may further comprise additional ingredients
such as a
liquid non-ionic and/or solid anionic surfactant and/or a solid diluent (which
may have a
particle size of the same order as particles comprising the active
ingredient).
[00355] Pharmaceutical compositions in accordance with the invention
formulated for
pulmonary delivery may provide the active ingredient in the form of droplets
of a solution
and/or suspension. Such formulations may be prepared, packaged, and/or sold as
aqueous
and/or dilute alcoholic solutions and/or suspensions, optionally sterile,
comprising the active
ingredient, and may conveniently be administered using any nebulization and/or
atomization
device. Such formulations may further comprise one or more additional
ingredients
including, but not limited to, a flavoring agent such as saccharin sodium, a
volatile oil, a
buffering agent, a surface active agent, and/or a preservative such as
methylhydroxybenzoate.
The droplets provided by this route of administration may have an average
diameter in the
range from about 0.1 nm to about 200 nm.
[00356] The formulations described herein as being useful for pulmonary
delivery are
useful for intranasal delivery of a pharmaceutical composition. Another
formulation suitable
for intranasal administration is a coarse powder comprising the active
ingredient and having
an average particle from about 0.2 m to 500 m. Such a formulation is
administered in the
manner in which snuff is taken, i.e. by rapid inhalation through the nasal
passage from a
container of the powder held close to the nose.
[00357] Formulations suitable for nasal administration may, for example,
comprise from
about as little as 0.1% (w/w) and as much as 100% (w/w) of the active
ingredient, and may
comprise one or more of the additional ingredients described herein. A
pharmaceutical
composition in accordance with the invention may be prepared, packaged, and/or
sold in a
formulation suitable for buccal administration. Such formulations may, for
example, be in
the form of tablets and/or lozenges made using conventional methods, and may,
for example,
Page 94 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
0.1% to 20% (w/w) active ingredient, the balance comprising an orally
dissolvable and/or
degradable composition and, optionally, one or more of the additional
ingredients described
herein. Alternately, formulations suitable for buccal administration may
comprise a powder
and/or an aerosolized and/or atomized solution and/or suspension comprising
the active
ingredient. Such powdered, aerosolized, and/or aerosolized formulations, when
dispersed,
may have an average particle and/or droplet size in the range from about 0.1
nm to about 200
nm, and may further comprise one or more of the additional ingredients
described herein.
[00358] A pharmaceutical composition in accordance with the invention may be
prepared,
packaged, and/or sold in a formulation suitable for ophthalmic administration.
Such
formulations may, for example, be in the form of eye drops including, for
example, a
0.1/1.0% (w/w) solution and/or suspension of the active ingredient in an
aqueous or oily
liquid excipient. Such drops may further comprise buffering agents, salts,
and/or one or more
other of the additional ingredients described herein. Other opthalmically-
administrable
formulations which are useful include those which comprise the active
ingredient in
microcrystalline form and/or in a liposomal preparation. Ear drops and/or eye
drops are
contemplated as being within the scope of this invention.
[00359] General considerations in the formulation and/or manufacture of
pharmaceutical
agents may be found, for example, in Remington: The Science and Practice of
Pharmacy 21st
ed., Lippincott Williams & Wilkins, 2005.
Kits
[00360] The invention provides a variety of kits for conveniently and/or
effectively
carrying out methods in accordance with the present invention. Kits typically
comprise one
or more thermally-responsive conjugates. In some embodiments, kits comprise a
collection
of different thermally-responsive conjugates to be used for different purposes
(e.g.
diagnostics, treatment, and/or prophylaxis). Typically kits will comprise
sufficient amounts
of thermally-responsive conjugates to allow a user to perform multiple
treatments of a
subject(s) and/or to perform multiple experiments. In some embodiments, kits
are supplied
with or include one or more thermally-responsive conjugates that have been
specified by the
purchaser.
[00361] Kits may include additional components or reagents. For example, kits
may
comprise one or more tools and/or pieces of equipment for exposing thermally-
responsive
conjugates to an EM field. In some embodiments, such tools and/or pieces of
equipment are
known to one of ordinary skill in the art and may provide EM radiation in the
kHz to GHz
Page 95 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
range (e.g. UV to infrared). In some embodiments, such tools and/or pieces of
equipment are
known to one of ordinary skill in the art and may provide EM radiation in the
ultraviolet to
infrared range. Kits may comprise one or more control thermally-responsive
conjugates, e.g.,
positive and negative control thermally-responsive conjugates. Other
components of kits
may include cells, cell culture media, tissue, and/or tissue culture media.
[00362] Kits may comprise instructions for use. For example, instructions may
inform the
user of the proper procedure by which to prepare a pharmaceutical composition
comprising
thermally-responsive conjugates and/or the proper procedure for administering
the
pharmaceutical composition to a subject.
[00363] In some embodiments, kits include a number of unit dosages of a
pharmaceutical
composition comprising thermally-responsive conjugates. A memory aid may be
provided,
for example in the form of numbers, letters, and/or other markings and/or with
a calendar
insert, designating the days/times in the treatment schedule in which dosages
can be
administered. Placebo dosages, and/or calcium dietary supplements, either in a
form similar
to or distinct from the dosages of the pharmaceutical compositions, may be
included to
provide a kit in which a dosage is taken every day.
[00364] Kits may comprise one or more vessels or containers so that certain of
the
individual components or reagents may be separately housed. Kits may comprise
a means for
enclosing the individual containers in relatively close confinement for
commercial sale, e.g.,
a plastic box, in which instructions, packaging materials such as styrofoam,
etc., may be
enclosed.
[00365] In some embodiments, kits comprise one or more thermally-responsive
conjugates
in accordance with the present invention. In some embodiments, such a kit is
used in the
treatment, diagnosis, and/or prophylaxis of a subject suffering from and/or
susceptible to a
disease, condition, and/or disorder (e.g. cancer). In some embodiments, such a
kit comprises
(i) a thermally-responsive conjugate that is useful in the treatment of
cancer; (ii) a syringe,
needle, applicator, etc. for administration of the to a subject; and (iii)
instructions for use.
Exemplification
[00366] The representative Examples that follow are intended to help
illustrate the
invention, and are not intended to, nor should they be construed to, limit the
scope of the
invention. Indeed, various modifications of the invention and many further
embodiments
thereof, in addition to those shown and described herein, will become apparent
to those
skilled in the art from the full contents of this document, including the
examples which
Page 96 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
follow and the references to the scientific and patent literature cited
herein. It should further
be appreciated that the contents of those cited references are incorporated
herein by reference
to help illustrate the state of the art.
[00367] The following Examples contain important additional information,
exemplification and guidance that can be adapted to the practice of this
invention in its
various embodiments and the equivalents thereof. It will be appreciated,
however, that these
examples do not limit the invention. Variations of the invention, now known
and/or further
developed, are considered to fall within the scope of the present invention as
described herein
and as hereinafter claimed.
Example 1: Complement Binding and Heat-Triggered Release
[00368] Single-stranded DNAs (ssDNAs) have been attached to gold and iron
oxide
particles. Complement binding was shown, and release was demonstrated with
increased
macroscopic temperature. These experiments were successfully used for
releasing ssDNAs
from aggregates in solution and from separate particles in a gel. For release
from aggregates
in solution, thermal triggering of aggregate disassociation was demonstrated.
Particles may
be released with electromagnetical excitation.
Example 2: Remotely Triggered Release from Magnetic Nanoparticles
Introduction
[00369] Multivalent nanoparticles have tremendous potential in the diagnosis
and
treatment of human disease (Ferrari, 2005, Nat. Rev. Cancer, 5:161;
incorporated herein by
reference). Their multivalency allows simultaneous conjugation of targeting
ligands to
improve nanoparticle homing, polymers (e.g. polyethylene glycol (PEG)) to
improve
nanoparticle pharmacokinetics, as well as therapeutic drug cargo. Drug release
from a
nanoparticle surface has been accomplished by bonds that are sensitive to
hydrolytic
degradation (Gref et al., 1994, Science, 263:1600; incorporated herein by
reference) or pH
(Kohler et al., 2005, Langmuir, 21:8858; incorporated herein by reference);
however,
complex release profiles that can be controlled from large distances (>10 cm)
have not been
achieved. Here, a multifunctional nanoparticle is described that is: (1)
multivalent, (2)
remotely-actuated, and (3) imaged non-invasively by magnetic resonance imaging
(Figure 3).
Page 97 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00370] Superparamagnetic nanoparticles act as transducers to capture external
electromagnetic energy at 350 kHz - 400 kHz, which is not significantly
absorbed by tissue,
to disrupt hydrogen bonding between complementary oligonucleotides on demand.
With a
nucleic acid strand covalently linked to a particle (e.g. a nanoparticle), dye-
labeled single
stranded DNA (i.e., a model antisense therapeutic) self-assembles on the
particle's surface,
forming a tunable, thermally-responsive linker. The resulting multifunctional
nanoparticles
are used to demonstrate remote, pulsatile release of a single species and
multistage release of
two species in vitro, as well as noninvasive imaging and remote actuation upon
implantation
in vivo.
[00371] Release from surfaces or polymers triggered by an external stimulus
(for example,
electric current (Kwon, 1991, Nature, 354:29 1; and Santini et al., 1999,
Nature, 397:335;
both of which are incorporated herein by reference) magnetic fields (Edelman
et al., 1985, J.
Biomed. Mater. Res., 19:67; incorporated herein by reference); temperature
(Chilkoti et al.,
2002, Adv. Drug Deliv. Rev., 54:613; and Jeong et al., 2002, Adv. Drug Deliv.
Rev., 54:37;
both of which are incorporated herein by reference); light (Mathiowitz and
Cohen, 1989, J.
Membrane Sci., 40:67; incorporated herein by reference); ultrasound (Kost et
al., 1989, Proc.
Natl. Acad. Sci., USA, 86:7663; incorporated herein by reference); etc.) has
been extensively
studied (reviewed in Santini et al., 2000, Agnew Chem. Int. Edit., 39:2397;
incorporated
herein by reference). These strategies, however, have been principally applied
to macro- and
micro-scale materials and drug reservoirs. For focal diseases, such as cancer,
these devices
must be implanted at the tumor site (e.g. Gliadel ). Another approach is to
replace these
larger depots with drug-carrying nanoparticles that can be individually
targeted to the tumor.
Heat (Needham and Dewhirst, 2001, Adv. Drug Deliv. Rev., 53:285; incorporated
herein by
reference) and light-sensitive (Shum et al., 2001, Adv. Drug Deliv. Rev.,
53:273; incorporated
herein by reference) liposomes, for example, can be delivered systemically and
their contents
released in response to an external stimulus. The present invention has the
added advantage
of radiofrequency electromagnetic (EM) field activation, which improves
penetration depth
over heat or light (at 400 kHz, field penetration into 15 cm of tissue is >99%
(Young et al.,
1980, Electron Lett., 16:358; incorporated herein by reference). Similarly,
energy absorption,
and thus background heating, of water and tissue is insignificant in the 350
kHz - 400 kHz
frequency regime (Stauffer et al., 1984, Ieee T. Biomed. Eng., 31:235;
incorporated herein by
reference). In contrast, when applied to magnetic materials, these fields
produce heat as the
magnetic dipole of the material aligns with the external field (Jordan et al.,
1993, Int. J.
Page 98 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
Hyperther., 9:51; and Hergt et al., 1998, Ieee T. Magnetics, 34:3745; both of
which are
incorporated herein by reference).
Materials and Methods
Particle Preparation
[00372] Synthetic 30 bp "parent" DNA (5'-Thiol-GAA GTG CGG TTA GTC GGC TTG
AAT CAG CGA-3'; SEQ ID NO: 1) was conjugated to 50 nm aminated magnetite
nanoparticles (dextran-coated, Micromod), using sulfo-SMCC (Sigma) as the
crosslinker. As
the particles were found to contain approximately 104 amine groups by
fluorescamine assay,
a 1000-fold excess of DNA was used in a two step reaction. Particles were
first reacted with
crosslinker for 1 hour, filtered on a magnetic column (Miltenyi Biotec) to
remove excess
crosslinker, added to reduced DNA, and reacted overnight. After filtration of
unconjugated
parent DNA using a magnetic column, fluorescent complement DNA was added to
the
particles (in PBS) and allowed to hybridize overnight. The sequences used in
these
experiments were as follows: 24 base pair (bp) complement (5'-CGC TGA TTC AAG
CCG
ACT AAC CGC-3'; SEQ ID NO: 2), 18 bp complement (5'-TGA TTC AAG CCG ACT
AAC-3'; SEQ ID NO: 3), and 12 bp complement (5'-TCG CTG ATT CAA-3'; SEQ ID NO:
4). Dye conjugations were performed by the DNA supplier and occurred at the 5'
end of the
oligonucleotides. After hybridization, particles were filtered on a magnetic
column at 4 C to
remove unbound complement.
Matrigel Plug Preparation
[00373] Phenol red free, growth factor reduced matrigel (400 l, BD
Biosciences) was
added to 100 l of particles. To obtain 1.05% total concentration of
particles, 75 l of DNA-
conjugated particles (approximately 3.3 mg/ml) were added to 25 l of similar
50 nm
particles (200 mg/ml, Chemicell). Gels (total volume 500 l) were mixed at 4 C
to prevent
gelation.
In Vitro Experiments
[00374] For in vitro experiments, gels were added to polypropylene
microcentrifuge tubes
and incubated at 37 C for 45 minutes to allow gelation. Gel plugs were then
washed three
Page 99 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
times with 500 l of PBS over 15 minutes. Buffer (200 l) was added to the
plugs, and
sampled and replaced with fresh buffer at 10 minute intervals. When treated
with EM fields
during a time interval, fields were switched on for 5 minutes only, preceded
by approximately
2.5 minutes and followed by approximately 2.5 minutes at room temperature.
When fields
were not applied during an interval, samples remained at room temperature.
Supernatant
samples were assayed on a plate-reader fluorometer (Molecular Devices Gemini
XS) and
amount of DNA quantified with standards.
In Vivo Experiments
[00375] Prior to injection of matrigel plugs into mice, approval from the
Burnham Institute
Animal Use Committee was obtained (AUF 05-054). In these experiments, 500 l
volumes
were injected subcutaneous near the posterior mammary fat pad of six athymic
nude mice and
allowed to gel for 45 minutes. Prior to injection, animals were anesthetized
with Avertin
(tribromoethanol) and remained under anesthesia during the remainder of the
experiment.
Three animals were treated with EM fields for two 5 minute doses, with 15
minutes between
field applications (+EMF), while three were not treated (-EMF). For treatment,
mice were
placed inside a plastic tube, which was mounted inside a horizontal two-turn
copper coil.
One hour after EM field treatment, animals were sacrificed. Tumor phantom and
surrounding tissue (fascia and skin) were removed and embedded in OTC for
histology.
Sections were stained with DAPI and an anti-fluorescein antibody (followed by
fluorescein
conjugated secondary) to amplify small signals. To quantify penetration depth,
8 images of
the tissue/phantom boundary were taken for each animal (3 animals per group,
24 images
total). DAPI staining was used to demarcate the boundary between the two
regions. Using
Metamorph software (Universal Imaging), green fluorescence on the tissue side
of the
boundary was quantified. For each fluorescent "object," the area and distance
from the tissue
boundary was measured. An area-weighted average distance was calculated.
Radiofrequency Electromagnetic Field Applicator
[00376] A 3 kW induction heating power supply (Ameritherm Nova 3) was used
with a
remote heating station and custom-made coils. The coil for in vitro
experiments was 2.5-
turns, 12 mm ID, and resonated at 400 kHz. For the heat transfer model and
mice
experiments, a 2-turn, 30 mm OD coil resonating at 338 kHz was used. All coils
were
Page 100 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
constructed from 4.88 mm OD copper tubing and spray-coated with insulating
paint. During
experiments, cooling water (10 C - 16 C) was circulated through the coil.
MR Imaging
[00377] TI-weighted data sets of mice implanted with iron oxide particle
containing gel
plugs were acquired using a horizontal bore 7-Tesla imaging spectrometer
(General Electric).
TI-weighted acquisition was intended to achieve good anatomical detail. Data
were acquired
using a custom small animal imaging coil. Imaging parameters included a spin
echo
sequence, TR 500, TE 12, 40 mm field of view, matrix 256 x 256, slice
thickness 0.5 mm.
Results
In Vitro Release
[00378] A 30 bp DNA was conjugated to dextran-coated iron oxide nanoparticles
and
added a complement of 12 bp, 18 bp, or 24 bp linked to a model drug, a
fluorophore. Excess
fluorescent DNA was removed by trapping the particle on a magnetic column and
washing
with buffer. Particles were trapped in a hydrogel plug as an in vitro model of
tumor tissue,
allowing fluorescent DNA to diffuse out into the surrounding buffer only when
liberated
from the particles. Figure 3B demonstrates pulsatile release of a fluorophore
initiated by EM
field pulses (400 kHz, 1.25 kW) of 5 minute duration every 40 minutes. The
fluorescence of
the surrounding buffer increased markedly in the sampling immediately after EM
field
application, followed by a fluorescence decrease in subsequent samplings.
Because much of
the fluorescent DNA rehybridized to the particles upon cooling of the plug to
room
temperature, subsequent EM field application allowed further release. Such a
profile would
be useful for metronomic dosing of a cytotoxic drug (Hanahan et al., 2000, J.
Clin. Invest.,
105:1045; incorporated herein by reference).
Complex Release Profiles
[00379] The use of a nucleic acid duplex as a thermally-responsive linker adds
the
additional feature of temperature tunability through changes in chain length
and variations in
G/C content. Using a variable-gain RF amplifier to control particle heating,
biomolecules
tethered to these oligonucleotides can be released in multiple stages. In
Figure 3C,
oligonucleotides of two different lengths and corresponding fluorescent
species (12 bp, FAM;
Page 101 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
24 bp, HEX) are used to demonstrate the potential for complex release
profiles. Low power
EM field pulses (0.55 kW) triggered release predominantly of FAM by melting of
the 12 bp
complement whereas higher power (3 kW) led to simultaneous release of both
species. Such
a profile may be used to release multiple drugs in series, synergistic drug
combinations such
as a chemosensitizer and chemotherapeutic, or combination regimens such as
antiangiogenic
and cytotoxic compounds (Sengupta et al., 2005, Nature, 436:568; incorporated
herein by
reference).
Optimization of Release Conditions
[00380] This release scheme relies on sufficient temperature rise in vivo to
initiate the
DNA melting. While heating sufficient to trigger release cannot be attained at
the single
particle level (AT approximately 10-5 C) (Rabin, 2002, Int. J. Hyperthermia,
18:194;
incorporated herein by reference), accumulation of a critical mass at a tumor
site allows
remote triggering through EM field application. It is therefore of interest to
determine the
nanoparticle concentrations required to heat various tumor diameters. Using an
EMF setup
and iron oxide formulation, the relationship between temperature rise,
particle concentration
and sample diameter was determined (Figure 4), and these results were fitted
to a conductive
heat transfer model derived from Fourier's law (Rabin, 2002, Int. J.
Hyperthermia, 18:194;
incorporated herein by reference). Heating to 42 C in vivo was accomplished
using
approximately 1.2 mg particles in a 1 cm diameter spherical tumor. For this
particular
experiment and these particular conditions, these data serve as an upper bound
on the
potential for collateral heating as the model is based on steady-state
temperature rise - shorter
heating intervals can be used to generate steeper temperature gradients
between the tumor
and surrounding tissue. For release to occur, elevated temperatures are only
required for
short time periods, as DNA oligonucleotides disassociate tens of microseconds
after
sufficient heat is applied (Ansari et al., 2001, Proc. Natl. Acad. Sci., USA,
98:7771;
incorporated herein by reference). Our experiments show that reducing heating
interval from
minutes to 30 seconds significantly reduces collateral heating. The present
invention
demonstrates that achieving a similar temperature rise with a shorter heating
interval requires
higher particle concentrations or increased EM field strength.
In Vivo Release
[00381] The use of the multifunctional nanoparticles in vivo was explored by
implantation
of a subcutaneous model tumor consisting of a matrigel plug containing
nanoparticles in
Page 102 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
living mice. The release of a fluorescein-labeled 18 bp oligonucleotide was
examined by EM
field exposure of 3 kW for 5 minutes. After EM field treatment, tissue
surrounding the plug
was removed and examined for the presence of released dye. Fluorescent
micrographs of
histological sections in Figure 5B,C depict a dramatic increase in penetration
depth of the
model cargo into surrounding tissue due to EM field exposure. Image analysis
was
performed on 24 fluorescent images from each group (3 animals, 8 images each).
The
average distance of fluorescence signal from the tissue/phantom boundary in
animals treated
with EM field was approximately six-fold over unexposed controls (250 11 vs.
42 3 m,
mean SEM). Such an increase in penetration depth could prove useful for
treatment of the
tumor periphery - areas often underdosed in hyperthermia generated by thermal
seeds (Hilger
et al., 2002, Invest. Radiol., 37:580; incorporated herein by reference). For
deep-seated
tumors, the use of EM field energy to break bonds remotely is an advantage
over near-
infrared light and other potential triggers that are more efficiently absorbed
or scattered by
tissue (Hirsch et al., 2003, Proc. Natl. Acad. Sci., USA, 100:13549;
incorporated herein by
reference). In addition to facilitating remote actuation, the magnetic
particle core allows
noninvasive visualization by MRI, as depicted in Figure 5D, suggesting the
potential for
simultaneous diagnosis and therapeutic delivery.
Discussion
[00382] The present invention demonstrates the fabrication of integrated,
multifunctional
nanodevices which offer the potential to shift the current paradigm whereby
diagnostics and
therapeutics are sequential elements of patient care. For example,
nanoparticles may be
delivered intravascularly using homing peptides (Akerman et al., 2002, Proc.
Natl. Acad.
Sci., USA, 99:12617; incorporated herein by reference), used to visualize
diseased tissue by
MRI, and then to guide focused application of electromagnetic energy,
ultimately enabling
remote, physician-directed drug delivery with minimal collateral tissue
exposure. The
present invention encompasses the recognition that performance of these
devices can be
improved in the future by new materials and chemistry. Particle cores with
higher
magnetization results in greater heating efficiency, requiring a lower
particle concentration
for release. Additionally, an improved thermally-responsive tether, with a
sharp temperature
transition slightly above 37 C, is obtained by attaching several duplexes in
parallel (Jin et
al., 2003, J. Am. Chem. Soc., 125:1643; incorporated herein by reference), and
non-native
nucleic acids are used to mitigate the effect of nucleases. Nevertheless, the
scheme outlined
Page 103 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
here demonstrates the potential to remotely trigger release of a biomolecule
from the surface
of a nanoparticle.
Equivalents and Scope
[00383] Those skilled in the art will recognize, or be able to ascertain using
no more than
routine experimentation, many equivalents to the specific embodiments of the
invention,
described herein. The scope of the present invention is not intended to be
limited to the
above Description, but rather is as set forth in the appended claims.
[00384] Those skilled in the art will recognize, or be able to ascertain using
no more than
routine experimentation, many equivalents to the specific embodiments of the
invention
described herein. The scope of the present invention is not intended to be
limited to the
above Description, but rather is as set forth in the appended claims.
[00385] In the claims articles such as "a," "an," and "the" may mean one or
more than one
unless indicated to the contrary or otherwise evident from the context. Thus,
for example,
reference to "a nanoparticle" includes a plurality of such nanoparticle, and
reference to "the
cell" includes reference to one or more cells known to those skilled in the
art, and so forth.
Claims or descriptions that include "or" between one or more members of a
group are
considered satisfied if one, more than one, or all of the group members are
present in,
employed in, or otherwise relevant to a given product or process unless
indicated to the
contrary or otherwise evident from the context. The invention includes
embodiments in
which exactly one member of the group is present in, employed in, or otherwise
relevant to a
given product or process. The invention includes embodiments in which more
than one, or
all of the group members are present in, employed in, or otherwise relevant to
a given product
or process. Furthermore, it is to be understood that the invention encompasses
all variations,
combinations, and permutations in which one or more limitations, elements,
clauses,
descriptive terms, etc., from one or more of the listed claims is introduced
into another claim.
For example, any claim that is dependent on another claim can be modified to
include one or
more limitations found in any other claim that is dependent on the same base
claim.
Furthermore, where the claims recite a composition, it is to be understood
that methods of
using the composition for any of the purposes disclosed herein are included,
and methods of
making the composition according to any of the methods of making disclosed
herein or other
methods known in the art are included, unless otherwise indicated or unless it
would be
evident to one of ordinary skill in the art that a contradiction or
inconsistency would arise.
Page 104 of 117
CA 02671806 2009-06-05
WO 2008/073851 PCT/US2007/086863
[00386] Where elements are presented as lists, e.g., in Markush group format,
it is to be
understood that each subgroup of the elements is also disclosed, and any
element(s) can be
removed from the group. It should it be understood that, in general, where the
invention, or
aspects of the invention, is/are referred to as comprising particular
elements, features, etc.,
certain embodiments of the invention or aspects of the invention consist, or
consist essentially
of, such elements, features, etc. For purposes of simplicity those embodiments
have not been
specifically set forth in haec verba herein. It is noted that the term
"comprising" is intended
to be open and permits the inclusion of additional elements or steps.
[00387] Where ranges are given, endpoints are included. Furthermore, it is to
be
understood that unless otherwise indicated or otherwise evident from the
context and
understanding of one of ordinary skill in the art, values that are expressed
as ranges can
assume any specific value or subrange within the stated ranges in different
embodiments of
the invention, to the tenth of the unit of the lower limit of the range,
unless the context clearly
dictates otherwise.
[00388] In addition, it is to be understood that any particular embodiment of
the present
invention that falls within the prior art may be explicitly excluded from any
one or more of
the claims. Since such embodiments are deemed to be known to one of ordinary
skill in the
art, they may be excluded even if the exclusion is not set forth explicitly
herein. Any
particular embodiment of the compositions of the invention (e.g., any heatable
surface, any
thermally-responsive linker, any trigger temperature, any agent to be
delivered, any
pharmaceutical composition, any method of administration, any method of use,
etc.) can be
excluded from any one or more claims, for any reason, whether or not related
to the existence
of prior art.
[00389] The publications discussed above and throughout the text are provided
solely for
their disclosure prior to the filing date of the present application. Nothing
herein is to be
construed as an admission that the inventors are not entitled to antedate such
disclosure by
virtue of prior disclosure.
Page 105 of 117