Note: Descriptions are shown in the official language in which they were submitted.
CA 02672854 2013-12-04
-
SURFACE PRETREATMENT FLUID FOR THE METAL TO BE COATED BY
CATIONIC ELECTRODEPOSITION
TECHNICAL FIELD
The present invention relates to a metal surface
treatment liquid, particularly to a metal surface treatment
liquid suited for cation electrodeposition coating, and a
method of metal surface treatment.
BACKGROUND ART
In order to impart anti-corrosion properties to various
metal base materials, surface treatments have thus far been
performed. Particularly, a zinc phosphate treatment has been
generally employed on metal base materials which constitute
automobiles. However, this zinc phosphate treatment has a
problem of sludge generation as a by-product. Accordingly, a
surface treatment without use of zinc phosphate for a next
generation has been demanded, and a surface treatment with
zirconium ions is one of such treatments (see, for example,
Patent Document 1).
Meanwhile, metal base materials which constitute
automobiles and necessitate high anti-corrosion properties are
subjected to cation electrodeposition coating following the
surface treatment. The cation electrodeposition coating is
carried out on the grounds that the coated film obtained by
cation electrodeposition coating has superior anti-corrosion
properties, and it has "throwing power", generally referred to,
CA 02672854 2013-12-04
2
that is a property of allowing automobile bodies having a
complicated shape to be completely coated.
However, it has been recently proven that when a metal base
material which had been surface treated with the zirconium
ions is subjected to the cation electrodeposition coating,
there may be a case in which no significant effect in terms of
the throwing power is achieved, for example, the throwing
power may not be sufficient for cold-rolled steel plates in
some cases. Accordingly, when the cation electrodeposition
coating is carried out, sufficient anticorrosion properties
cannot be achieved if the throwing power is insufficient. See
Japanese Patent Publication No. 2004-218070.
DISCLOSURE OF THE INVENTION
Problems to be Solved by the Invention
An object of the present invention is to provide a surface
treatment with zirconium ions that enables sufficient throwing
power and exhibit superior anti-corrosion properties to be
exhibited, when thus surface treated metal base material is
subjected to cation electrodeposition coating.
Means for Solving the Problems
Aspects of the present invention are as follows.
In a first aspect of the present invention, a metal surface
treatment liquid for cation electrodeposition coating contains
zirconium ions, and tin ions, and has a pH of in the range of
1.5 to 6.5, in which: the concentration of the zirconium ions
CA 02672854 2013-12-04
3
is in the range of 10 to 10,000 ppm; and the concentration
ratio of the tin ions to the zirconium ions is 0.005 to 1 on a
mass basis, and further comprises fluorine ions; and A) a
chelate compound, wherein the chelate compound is selected
from the group consisting of amino acid, aminocarboxylic acid,
ascorbic acid and sulfonic acid, wherein the sulfonic acid is
selected from the group consisting of methanesulfonic acid,
isethionic acid, taurine, naphthalenedisulfonic acid,
aminonaphthalenedisulfonic acid, sulfosalicylic acid, a
naphthalenesulfonic acid-formaldehyde condensate,
alkylnaphthalenesulfonic acid, a salt of any of these and
sodium polystyrenesulfonate, or B) a nitrogenous, sulfur
and/or phenolic rust-preventive agent selected from the group
consisting of hydroquinone, ethyleneurea, quinolinol, thiourea,
benzotriazole, a salt of any of these and
mercaptobenzothiazole; or C) indium ions, wherein the amount
of free fluorine ions at a pH of 3.0 is in the range of 0.1 to
50 ppm.
In a second aspect of the present invention, a metal surface
treatment liquid for cation electrodeposition coating
according to the first aspect further includes a polyamine
compound.
In a third aspect of the present invention, a metal surface
treatment liquid for cation electrodeposition coating
according to the first or second aspect further includes
copper ions.
In a fourth aspect of the present invention, a metal surface
CA 02672854 2013-12-04
4
treatment liquid for cation electrodeposition coating
according to any one of the first to third aspects further
includes an oxidizing agent.
In a fifth aspect of the present invention, a metal surface
treatment liquid for cation electrodeposition coating
according to any one of the first to fourth aspects further
includes aluminum ions.
In a sixth aspect of the present invention, a method of metal
surface treatment includes a step of subjecting a metal base
material to a surface treatment with the metal surface
treatment liquid for cation electrodeposition coating
according to any one of the first to fifth aspects.
In a seventh aspect of the present invention, the method
according to the sixth aspect further includes the step of
subjecting the surface treated metal base material to cation
electrodeposition coating.
In an eighth aspect of the present invention, a metal base
material includes a coating film formed by a surface treatment
obtained by the method according to the sixth or seventh
aspects.
In a ninth aspect of the present invention, a metal base
material according to the eighth aspect includes an element
ratio of zirconium/tin on a mass basis in the coating film is
in the range of 1/10 to 10/1.
Accordingly, the metal surface treatment liquid for cation
electrodeposition coating of the present invention is a
chemical conversion treatment liquid containing zirconium ions
CA 02672854 2013-12-04
. . - .
and tin ions, and having a pH in the range of 1.5 to 6.5, in
which the concentration of zirconium ions in the range of 10
to 10,000 ppm, and the content of the tin ions with respect to
the zirconium ions is 0.005 to 1 on a mass basis. Moreover,
the metal surface treatment liquid for cation
electrodeposition coating may further contain a polyamine
compound, copper ions, fluorine ions, a chelate compound, an
oxidizing agent, and a rust-preventive agent. When the
fluorine ions are included, the amount of free fluorine ions
at a pH of 3.0 may be 0.1 to 50 ppm.
The method of metal surface treatment of the present invention
includes the step of subjecting a metal base material to a
surface treatment with the abovementioned metal
surface treatment liquid.
A coating film obtained by the surface treatment is formed on
the surface treated metal base material of the present
invention. The element ratio of zirconium/tin on mass basis in
the coating film may be 1/10 to 10/1.
The method of cation electrodeposition coating of the present
invention includes a step of subjecting a metal base
CA 02672854 2013-12-04
6
. . . .
material to a surface treatment with the abovementioned metal
surface treatment liquid, and a step of subjecting the surface
treated metal base material to cation electrodeposition
coating.
The metal base material coated by the cation
electrodeposition of the present invention is obtained by the
abevementioned method of coating.
Effects of the Invention
It is believed that the throwing power attained by the
metal surface treatment liquid for cation electrodeposition
coating of the present invention can be improved by including
tin ions in addition to zirconium ions when the cation
electrodeposition coating is carried out after forming a
conversion coating film with this treatment liquid. Although
not clarified, the grounds are conceived as follows.
When zirconium ions are used alone, formation of their
oxide coating film is believed to be executed simultaneously
with etching of the metal base material in an acidic medium.
However, since segregation materials and the like of compounds
containing silicon or carbon in addition to silica may be
present on cold-rolled steel plates, such parts are not
susceptible to etching. Therefore, the coating film cannot be
uniformly formed with zirconium oxide, whereby portions
without coating film formation can be present. Since a
difference in electric current flow is believed to be
generated between the parts with and without formation of the
coating film, the electrodeposition is not uniformly executed,
CA 02672854 2013-12-04
7
and consequently, the throwing power cannot be sufficiently
attained.
When tin ions are additionally present, it is further
considered as in the following. Since the tin ions are less
likely to be affected on the steel plate as compared with the
zirconium ions, their oxide coating film can be more easily
formed on the base material. Although formation of the coating
film of the tin ions is not specific to the parts where the
zirconium ions are not significantly deposited, formation of
the oxide coating film of the tin ions is not restricted to a
specific part while having another part remain without
formation of the film. As a result, the tin ions would form
the coating film such that it covers the part where the
zirconium ion could not form the coating film.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention can improve
adhesiveness to the coated film by cation electrodeposition
through including the polyamine compound, and consequently, it
can pass SDT test under more stringent conditions. In addition,
the metal surface treatment liquid for cation
electrodeposition coating of the present invention can improve
anti-corrosion properties by including the copper ion.
Although the grounds are not clarified, it is believed that
some interaction may be caused between copper and zirconium in
forming the coating film. Furthermore, the metal surface
treatment liquid for cation electrodeposition coating of the
present invention can form a zirconium oxide coating film in a
CA 02672854 2013-12-04
8
stable manner by including a chelate compound when a metal
other than zirconium is included in large quantity. This
occurrence is believed to result from capture by the chelate
compound of metal ions that are more likely to be deposited
than zirconium.
BRIEF DESCRIPTION OF THE DRAWINGS
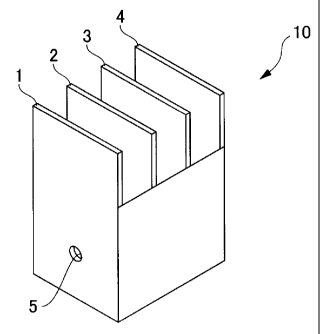
Fig. 1 shows a perspective view illustrating one example
of the box for use in evaluating the throwing power; and
Fig. 2 shows a view schematically illustrating evaluation
of the throwing power.
PREFERRED MODE FOR CARRYING OUT THE INVENTION
The metal surface treatment liquid for cation
electrodeposition coating of the present invention is a
chemical conversion treatment liquid that contains zirconium
ions and tin ions, and has a pH in the range of 1.5 to 6.5.
The zirconium ions are included at a concentration in a
range of 10 to 10,000 ppm. When the concentration is less than
ppm, sufficient anti-corrosion properties cannot be
achieved since deposition of the zirconium coating film is not
enough. In addition, even though the concentration may exceed
10,000 ppm, an effect to justify the amount cannot be
exhibited since the deposition amount of the zirconium coated
film is not increased, and adhesiveness of the coated film may
be deteriorated, thereby leading to inferior anticorrosion
performance such as those in SDT. The lower limit and the
CA 02672854 2013-12-04
9
. ,
upper limit of the concentration are preferably 100 ppm and
500 ppm, respectively.
The concentration of the metal ions herein, when a
complex or oxide thereof was formed, is represented by the
concentration based on the metal element, taking into account
only of the metal atom in the complex or oxide. For example,
the concentration based on the metal element of zirconium of
100 ppm complex ions ZrFe- (molecular weight: 205) is
calculated to be 44 ppm by the formula of 100 x (91/205). In
the metal surface treatment liquid for cation
electrodeposition coating of the present invention, the metal
compound (zirconium compound, tin compound, copper compound
and other metal compounds) is included at just a slight
proportion, if present, in the state of a nonionic state such
as an oxide portion, and is believed to be present almost in
the form of the metal ion. Therefore, the metal ion
concentration referred to herein is, irrespective of the
presence in the form of the nonionic portion, the metal ion
concentration when it is assumed to be present as the metal
ion dissociated at a level of 100%.
The tin ion included in the metal surface treatment
liquid for cation electrodeposition coating of the present
invention is preferably a bivalent cation. When the tin ion
has other valence, the intended effect may not be exhibited.
However, the tin ion is not limited to the bivalent cation,
but can be used in the present invention as long as it can be
deposited on the metal base material. For example, when the
CA 02672854 2013-12-04
. ,
tin ions form a complex, it may be a quadrivalent cation,
which can also be used in the present invention. The
concentration of the tin ions is 0.005 to 1 on a mass basis
with respect to the concentration of the zirconium ions. When
the ratio is less than 0.005, the effect by addition is not
exhibited, while zirconium may not be significantly deposited
when the ratio exceeds 1. The lower limit and the upper limit
of the concentration are preferably 0.02 and 0.2, respectively.
However, when the total amount of the zirconium ion and tin
ion is too small, the effect of the present invention may not
be exhibited. Therefore, the total concentration of the
zirconium ion and the tin ion in the metal surface treatment
liquid of the present invention is preferably no less than 15
ppm.
The content of the tin ions in the metal surface
treatment liquid of the present invention is preferably is
preferably 1 to 100 ppm. When the content is less than 1 ppm,
deposition of tin at the portion where zirconium could not
form the coating film may be insufficient, and the anti-
corrosion properties such as those in SDT are likely to be
inferior. When the content exceeds 100 ppm, deposition of the
zirconium coating film may be difficult, whereby the anti-
corrosion properties and the coating appearance are likely to
be inferior. The concentration is more preferably 5 to 100 ppm,
and still more preferably 5 to 50 ppm.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention has a pH in
CA 02672854 2013-12-04
11
the range of 1.5 to 6.5. When the pH is less than 1.5, the
metal base material cannot be sufficiently etched to decrease
the coating film amount, and sufficient anti-corrosion
properties cannot be achieved. In addition, the stability of
the treatment liquid may not be sufficient. In contrast, when
the pH is higher than 6.5, excessive etching may lead to
failure in formation of sufficient coating film, or an un-
uniform adhesion amount and film thickness of the coating film
may adversely affect the coating appearance and the like. The
lower limit and the upper limit of pH are preferably 2.0 and
5.5, and still more preferably 2.5 and 5.0, respectively.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention may further
include a polyamine compound for improving adhesiveness to the
coated film by cation electrodeposition which is formed after
the surface treatment. The polyamine compound used in the
present invention is believed to be fundamentally significant
in being an organic molecule having an amino group. Although
speculative, the amino group is believed to be incorporated in
the coating film by a chemical action with zirconium oxide
deposited as a coating film on the metal base plate, or with
the metal base plate. In addition, the polyamine compound that
is an organic molecule is believed to be responsible for
adhesiveness with the coated film provided on the metal base
plate having the coating film formed thereon. Therefore, when
the polyamine compound that is an organic molecule having an
amino group is used, adhesiveness between the metal base plate
CA 02672854 2013-12-04
12
. ,
and the coated film is significantly improved, and superior
corrosion resistance can be attained. Examples of the
polyamine compound include hydrolysis condensates of
aminosilane, polyvinylamine, polyallylamine, water soluble
phenolic resins having an amino group, and the like. Since the
amount of amine can be freely adjusted, the hydrolysis
condensate of aminosilane is preferred. Therefore, exemplary
metal surface treatment liquids for cation electrodeposition
coating of the present invention include, for example, the
metal surface treatment liquids for cation electrodeposition
coating which contain zirconium ions, tin ions, and a
hydrolysis condensate of aminosilane; the metal surface
treatment liquids for cation electrodeposition coating which
contain zirconium ions, tin ions, and polyallylamine; and the
metal surface treatment liquids for cation electrodeposition
coating which contain zirconium ions, tin ions, and a water
soluble phenolic resin having an amino group. In addition,
these metal surface treatment liquids for cation
electrodeposition coating may contain fluorine as described
later.
The hydrolysis condensate of aminosilane is obtained by
carrying out hydrolysis condensation of an aminosilane
compound. Examples of the aminosilane compound include
vinyltrichlorosilane, vinyltrimethoxysilane,
vinyltriethoxysilane, 2-(3,4 epoxycyclohexyl)-
ethyltrimethoxysilane, 3-glycidoxypropyltrimethoxysilane, 3-
glycidoxypropylmethyldiethoxysilane, 3-
CA 02672854 2013-12-04
13
glycidoxypropyltriethoxysilane, p-styryltrimethoxysilane, 3-
methacryloxypropylmethyldimethoxysilane, 3-
methacryloxypropyltrimethoxysilane, 3-
methacryloxypropylmethyldiethoxysilane, 3-
methacryloxypropyltriethoxysilane, 3-
acryloxypropyltrimethoxysilane, N-2-(aminoethyl)-3-
aminopropylmethyldimethoxysilane, N-2-(aminoethyl)-3-
aminopropyltrimethoxysilane, N-2-(aminoethyl)-3-
aminopropyltriethoxysilane, 3-aminopropyltrimethoxysilane, 3-
aminopropyltriethoxysilane, 3-triethoxysilyl-N-(1,3-dimethyl-
butylidene)-propylamine, N-pheny1-3-
aminopropyltrimethoxysilane, N-(vinylbenzy1)-2-aminoethyl-3-
aminopropyltrimethoxysilane hydrochloride, 3-
ureidepropyltriethoxysilane, 3-chloropropyltrimethoxysilane,
3-mercaptopropylmethyldimethoxysilane, 3-
mercaptopropyltrimethoxysilane,
bis(triethoxysilylpropyl)tetrasulfide, and 3-isocyanate
propyltriethoxysilane, which are silane coupling agents having
an amino group. In addition, examples of commercially
available products which can be used include "KBM-403", "KBM-
602", "KBM-603", "KBE-603", "KBM-903", "KBE-903", "KBE-9103",
"KBM-573", "KBP-90" (all trade names, manufactured by Shin-
Etsu Chemical Co.,), "XS1003" (trade name, manufactured by
Chisso Corporation), and the like.
The hydrolytic condensation of the aforementioned
aminosilane can be carried out by a method well known to
persons skilled in the art. Specifically, the hydrolytic
CA 02672854 2013-12-04
14
condensation can be carried out by adding water required for
hydrolysis of the alkoxysilyl group to at least one kind of
aminosilane compound, and stirring the mixture while heating
as needed. The degree of condensation can be regulated with
the amount of water used.
A higher degree of condensation of aminosilane hydrolysis
condensate is preferred, since in this case where zirconium is
deposited as an oxide, the above aminosilane hydrolysis
condensate tends to be easily incorporated therein. For
example, the portion on a mass basis of dimer or higher-order
multimers of aminosilane in the total amount of the
aminosilane is preferably no less than 40%, more preferably no
less than 50%, still more preferably no less than 70%, and
even more preferably no less than 80%. Therefore, when
aminosilane is allowed to react in a hydrolytic condensation
reaction, it is preferred to permit the reaction under
conditions in which aminosilane is more likely to be
hydrolysed and condensed such as those in which an aqueous
solvent containing a catalyst such as acetic acid and alcohol
is used as the solvent. In addition, by allowing for a
reaction under conditions with a comparatively high
aminosilane concentration, a hydrolysis condensate having a
high degree of condensation is obtained. Specifically, it is
preferred to allow for the hydrolytic condensation at an
aminosilane concentration falling within the range of 5% by
mass to 50% by mass. The degree of condensation can be
determined by 29Si-NMR measurement.
CA 02672854 2013-12-04
As the polyvinylamine and polyallylamine, commercially
available products can be used. Examples of polyvinylamine
include "PVAMTm-0595B" (manufactured by Mitsubishi Chemical
Corporation) and the like, and examples of the polyallylamine
include "PAATm-01", "PAkm-10C", "PAATm-H-10C", "PAkm-D-41HC1"
(manufactured by Nitto Boseki Co., Ltd.) and the like.
The molecular weight of the polyamine compound is
preferably in the range of 150 to 500,000. When the molecular
weight is less than 150, a conversion coating film having
sufficient adhesiveness may not be obtained. When the
molecular weight exceeds 500,000, formation of the coating
film may be inhibited. The lower limit and the upper limit are
more preferably 5,000 and 70,000, respectively. When the
polyamine compound has the amino group in too large an amount,
it may adversely influence the coating film, while the effect
to improve the adhesiveness with the coating film provided by
the amino group is not significantly achieved when the amount
is too small. Therefore, the polyamine compound preferably has
a primary and/or secondary amino group of no less than 0.1
mmol and no more than 17 mmol per gram of the solid content,
and more preferably a primary and/or secondary amino group of
no less than 3 mmol and no more than 15 mmol per gram of the
solid content.
The number of moles of the primary and/or secondary amino
group per gram of the solid content of the polyamine compound
can be determined according to the following formula (1).
Amount of Amino Group=(mX-nY)/(m+n)..Formula (1)
CA 02672854 2013-12-04
16
in which the mass ratio of solid contents of the
polyamine compound and the compound having a functional group
A and/or a functional group B is defined as m:n; the number of
mmoles of the functional group A and/or the functional group B
per gram of the compound having the functional group A and/or
the functional group B is defined as Y; and the number of
mmoles of the primary and/or secondary amino group included
per gram of the polyamine compound when the compound having
the functional group A and/or the functional group B is not
included in the composition for the metal surface treatment is
defined as X.
The content of the polyamine compound in the metal
surface treatment liquid for cation electrodeposition coating
of the present invention can be in the range of 1 to 200%
based on mass of the zirconium metal included in the surface
treatment liquid. When the content is less than 1%, the
intended effect cannot be exhibited, while the content
exceeding 200% may lead to failure in sufficient formation of
the coating film. The upper limit of the content is more
preferably 120%, more preferably 100%, still more preferably
80%, and even more preferably 60%.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention may further
contain a copper ion for improving the anti-corrosion
properties. With respect to the amount of the copper ions, the
concentration preferably accounts for 10 to 100% with respect
to the concentration of the tin ions. When the concentration
CA 02672854 2013-12-04
17
is less than 10%., the intended effect may not be exhibited,
while deposition of zirconium may be difficult, similarly to
the case of the tin ions when it exceeds the concentration of
the tin ions. Exemplary metal surface treatment liquids for
cation electrodeposition coating of the present invention
include, for example, the metal surface treatment liquids for
cation electrodeposition coating which contain zirconium ions,
tin ions and copper ions. In this case, the fluorine ions
described later can be further included and the aforementioned
polyamine compound can be included.
It is preferred that the metal surface treatment liquid
for cation electrodeposition coating of the present invention
contains fluorine ions. Since the concentration of the
fluorine ions varies depending on the pH, the amount of free
fluorine ions is defined at a specified pH. In the present
invention, the amount of the free fluorine ions at a pH of 3.0
is in the range of 0.1 to 50 ppm. When the amount is less than
0.1 ppm, the metal base material cannot be sufficiently etched
so that the coating film amount is decreased, and sufficient
anticorrosion properties cannot be achieved. In addition, the
treatment liquid may not have enough stability. In contrast,
when the amount is above 50 ppm, excessive etching may lead to
failure in formation of sufficient coating film, or an un-
uniform adhesion amount and film thickness of the coating film
may adversely affect the coating appearance and the like. The
lower limit and the upper limit are preferably 0.5 ppm and 10
ppm, respectively. Exemplary metal surface treatment liquids
CA 02672854 2013-12-04
18
for cation electrodeposition coating of the present invention
include, for example, the metal surface treatment liquids for
cation electrodeposition coating which contain zirconium ions,
tin ions, and fluorine ions.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention may include
a chelate compound. By including the chelate compound,
deposition of metals other than zirconium can be suppressed in
the treatment liquid, and the coating film of zirconium oxide
can be stably formed. As the chelate compound, amino acid,
aminocarboxylic acid, a phenolic compound, aromatic carboxylic
acid, sulfonic acid, ascorbic acid and the like can be
exemplified. Carboxylic acid having a hydroxyl group such as
citric acid and gluconic acid, conventionally known as
chelating agents, cannot exert their function enough in the
present invention.
As the amino acid, a variety of naturally occurring amino
acids and synthetic amino acids, as well as amino acids having
at least one amino group and at least one acid group (carboxyl
group, sulfonic acid group or the like) in one molecule, can
be extensively utilized. Among these, at least one selected
from the group consisting of alanine, glycine, glutamic acid,
aspartic acid, histidine, phenylalanine, asparagine, arginine,
glutamine, cysteine, leucine, lysine, proline, serine,
tryptophan, valine and tyrosine, and a salt thereof can be
preferably used. Furthermore, when there is an optical isomer
of the amino acid, any one can be suitably used irrespective
CA 02672854 2013-12-04
19
,
of the forms, i.e., L-form, D-form, or racemic bodies.
In addition, as the aminocarboxylic acid, a compound
having both functional groups, an amino group and a carboxyl
group in one molecule other than the amino acid described
above can be extensively used. Among these, at least one
selected from the group consisting of diethylenetriamine
pentaacetic acid (DTPA), hydroxyethylethylenediamine triacetic
acid (HEDTA), triethylenetetraamine hexaacetic acid (TTHA),
1,3-propanediamine tetraacetic acid (PDTA), 1,3-diamino-6-
hydroxypropane tetraacetic acid (DPTA-OH), hydroxyethylimino
diacetic acid (HIDA), dihydroxyethylglycine (DHEG),
glycolether diamine tetraacetic acid (GEDTA), dicarboxymethyl
glutamic acid (CMGA), (S,S)-ethylenediamine disuccinic acid
(EDDS), ethylenediamine tetraacetic acid (EDTA),
nitrilotriacetic acid (NTA), and a salt thereof can be
preferably used.
Furthermore, examples of the phenolic compound include
compounds having two or more phenolic hydroxyl groups, and
phenolic compounds including the same as a basic skeleton.
Examples of the former include catechol, gallic acid,
pyrogallol, tannic acid, and the like. Meanwhile, examples of
the latter include flavonoids such as flavone, isoflavone,
flavonol, flavanone, flavanol, anthocyanidin, aurone, chalcone,
epigallocatechin gallate, gallocatechin, theaflavin, daidzin,
genistin, rutin, and myricitrin, polyphenolic compounds
including tannin, catechin and the like, polyvinylphenol,
water soluble resol, novolak resins, lignin, and the like.
CA 02672854 2013-12-04
Among them, tannin, gallic acid, catechin and pyrogallol are
particularly preferred.
As the sulfonic acid, at least one selected from the
group consisting of methanesulfonic acid, isethionic acid,
taurine, naphthalenedisulfonic acid,
aminonaphthalenedisulfonic acid, sulfosalicylic acid, a
naphthalenesulfonic acid-formaldehyde condensate,
alkylnaphthalenesulfonic acid and the like, and a salt thereof
can be preferably used.
When sulfonic acid is used, coating performance and
corrosion resistance of the object following the chemical
conversion treatment can be improved. Although the mechanism
is not clarified, the following grounds are conceived.
First, since there exist silica segregation products and
the like on the surface of the object such as steel plates to
yield an un-uniform surface composition, a portion not
susceptible to etching in the chemical conversion treatment
may be present. However, it is speculated that such a portion
not susceptible to etching can be particularly etched by
adding sulfonic acid, and consequently, a uniform metal oxide
film is likely to be formed on the object surface. In other
words, sulfonic acid is believed to act as an etching
accelerator.
Second, it is possible that in chemical conversion
treatment, hydrogen gas which can be generated by the chemical
conversion reaction inhibits the reaction at the interface,
and sulfonic acid is speculated to remove the hydrogen gas
CA 02672854 2013-12-04
21
,
through a depolarizing action thereby accelerating the
reaction.
Of these, use of taurine is preferred since it has both
an amino group and a sulfone group. The content of sulfonic
acid is preferably in the range of 0.1 to 10,000 ppm, and more
preferably in the range of 1 to 1,000 ppm. When the content is
less than 0.1 ppm, the effect is not significantly exhibited,
while deposition of zirconium can be inhibited when the
content exceeds 10,000 ppm.
Use of ascorbic acid leads to uniform formation of the
metal oxide film such as zirconium oxide, tin oxide and the
like on the object surface by the chemical conversion
treatment, and the coating performance and corrosion
resistance can be improved. Although the mechanism is not
clarified, the etching action in the chemical conversion
treatment is uniformly executed on the object such as steel
plates, and consequently, it is speculated that zirconium
oxide and/or tin oxide is deposited on the etched part to form
an entirely uniform metal oxide film. In addition, tin is
speculated to become apt to be deposited in the form of the
tin metal at the metal interface due to some influence, and as
a consequence, zirconium oxide is deposited at the part where
the tin metal deposited, whereby surface concealability on the
object may be improved as a whole. The content of ascorbic
acid is preferably in the range of 5 to 5,000 ppm, and more
preferably in the range of 20 to 200 ppm. When the content is
less than 5 ppm, the effect is not significantly exhibited,
CA 02672854 2013-12-04
22
while deposition of zirconium can be inhibited when the
content exceeds 5,000 ppm.
When the chelating agent is included, its content is
preferably 0.5 to 10 times the concentration of the total
concentration of other metal ions except for zirconium such as
tin ion and copper ion. When the concentration is less than
0.5 times, the intended effect cannot be exhibited, while a
concentration exceeding 10 times may adversely influence on
formation of the coating film.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention can further
contain a nitrogenous, sulfur and/or a phenolic rust-
preventive agent. The rust-preventive agent can inhibit
corrosion through forming an anti-corrosion coating film on
the metal surface. As the nitrogenous, sulfurous, phenolic
rust-preventive agent, at least one selected from the group
consisting of hydroquinone, ethyleneurea, quinolinol, thioures,
benzotriazole and the like, and a salt thereof can be used.
Use of the nitrogenous, sulfurous, phenolic rust-preventive
agent in the metal surface treatment liquid for cation
electrodeposition coating of the present invention leads to
uniform formation of the metal oxide film such as zirconium
oxide, tin oxide and the like on the object surface by the
chemical conversion treatment, whereby the coating performance,
corrosion resistance can be improved. Although the mechanism
is not clarified, the followings are conceived.
That is, since there exist silica segregation products
CA 02672854 2013-12-04
23
. ,
and the like on the steel plate surface to yield an un-uniform
surface composition, a portion having the conversion coating
film formed by etching in the chemical conversion treatment,
and a portion without formation of the conversion coating film
due to different etching behavior thereby having iron oxide
may be present. The nitrogenous, sulfurous, phenolic rust-
preventive agent improves primary rust-preventive properties
through adsorbing to the portion without formation of the
conversion coating film in the chemical conversion treatment
to cover the metal interface. It is speculated that the
coating performance, corrosion resistance of the object
following the chemical conversion treatment can be
consequently improved.
In addition, when copper is excessively deposited on the
conversion coating film, this copper may serve as a cathode
base point to form an electrically un-uniform conversion
coating film. However, by allowing the rust-preventive agent
to be adsorbed to the portion where an excessive amount of
copper deposited, improvement of the corrosion resistance is
expected to be enabled by attaining a uniform
electrodeposition coating property on the object following the
chemical conversion treatment.
The content of the nitrogenous, sulfurous and/or phenolic
rust-preventive agent is preferably in the range of 0.1 to
10,000 ppm, and more preferably in the range of 1 to 1,000 ppm.
When the content is less than 0.1 ppm, the effect is not
significantly exhibited, while deposition of zirconium can be
CA 02672854 2013-12-04
24
. .
inhibited when the content exceeds 10,000 ppm.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention may further
contain aluminum ions and/or indium ions. Since these cations
have similar functions to the tin ions, they can be used in
combination when the use of the tin ions alone cannot exhibit
the effect. Of these, aluminum is more preferred. The content
of the aluminum ions and/or the indium ions is preferably in
the range of 10 to 1,000 ppm, more preferably in the range of
50 to 500 ppm, and still more preferably in the range of 100
to 300 ppm. The amount of the aluminum ions and indium ions
can be a concentration accounting for, for example, 2 to
1,000% of the zirconium ion concentration. Exemplary metal
surface treatment liquids for cation electrodeposition coating
of the present invention include, for example, the metal
surface treatment liquids for cation electrodeposition coating
which contain zirconium ions, tin ions and aluminum ions.
These can further contain fluorine as described later, and can
also contain the polyamine compound described later.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention may contain
various cations in addition to the aforementioned components.
Examples of the cation include magnesium, zinc, calcium,
gallium, iron, manganese, nickel, cobalt, silver, and the like.
In addition, there exist cations and anions that are derived
from a base or an acid added for adjusting the pH, or are
included as the counter ion of the aforementioned components.
CA 02672854 2013-12-04
The metal surface treatment liquid for cation
electrodeposition coating of the present invention can be
produced by placing each of the components thereof, and/or
compound containing the same into water, followed by mixing.
Examples of the compound for supplying the zirconium ions
include fluorozirconic acid, salts of fluorozirconic acid such
as potassium fluorozirconate and ammonium fluorozirconate,
zirconium fluoride, zirconium oxide, zirconium oxide colloid,
zirconyl nitrate, zirconium carbonate, and the like.
Examples of the compound that supplies the tin ions
include tin sulfate, tin acetate, tin fluoride, tin chloride,
tin nitrate, and the like. On the other hand, as the compound
that supplies the fluorine ions, for example, fluorides such
as hydrofluoric acid, ammonium fluoride, fluoboric acid,
ammonium hydrogen fluoride, sodium fluoride, sodium hydrogen
fluoride, and the like can be exemplified. Additionally, a
complex fluoride can also be used as the source, and examples
thereof include hexafluorosilicic acid salts, specifically,
hydrofluosilicic acid, zinc hydrofluosilicicate, manganese
hydrofluosilicate, magnesium hydrofluosilicate, nickel
hydrofluosilicate, iron hydrofluosilicate, calcium
hydrofluosilicate, and the like. Furthermore, a compound that
supplies zirconium ions, and is a complex fluoride is also
acceptable. Moreover, copper acetate, copper nitrate, copper
sulfate, copper chloride and the like as the compound that
supplies copper ions; aluminum nitrate, aluminum fluoride and
the like as the compound that supplies aluminum ions; and
CA 02672854 2013-12-04
26
. ,
indium nitrate, indium chloride and the like as the compound
that supplies indium ions can be exemplified, respectively.
After mixing these components, the metal surface
treatment liquid for cation electrodeposition coating of the
present invention can be regulated to have a predetermined
value of pH using an acidic compound such as nitric acid or
sulfuric acid, and a basic compound such as sodium hydroxide,
potassium hydroxide or ammonia.
The metal surface treatment liquid for cation
electrodeposition coating of the present invention may contain
an oxidizing agent. The oxidizing agent is particularly
preferably at least one selected from the group consisting of
nitric acid, nitrous acid, hydrogen peroxide, bromic acid, and
salts of the same. The oxidizing agent allows a metal oxide
film to be uniformly formed on the surface of an object,
whereby coatability and corrosion resistance of the object can
be improved.
Although the mechanism is not clarified, it is speculated
that use of the oxidizing agent in a specified amount allows
the etching action in the chemical conversion treatment to be
uniformly executed on an object such as a steel plate, whereby
zirconium oxide and/or tin oxide is deposited at the etched
part to form an entirely uniform metal oxide film. It is also
speculated that the oxidizing agent in the specified amount
renders tin readily deposited as a tin metal at the metal
interface, and thus zirconium oxide is deposited at the
portions of deposition of the tin metal, whereby the surface
CA 02672854 2013-12-04
27
concealability on the entire object is improved.
In order to affect such an action, the content of each
oxidizing agent is as in the following. Accordingly, the
content of nitric acid is preferably in the range of 100 to
100,000 ppm, more preferably in the range of 1,000 to 20,000
ppm, and still more preferably in the range of 2,000 to 10,000
ppm. The content of nitrous acid and bromic acid is preferably
in the range of 5 to 5,000 ppm, and more preferably in the
range of 20 to 200 ppm. The content of nitrous acid and bromic
acid is preferably in the range of 5 to 5,000 ppm, and more
preferably in the range of 20 to 200 ppm. The content of
hydrogen peroxide is preferably in the range of 1 to 1,000 ppm,
and more preferably in the range of 5 to 100 ppm. When content
of each is less than the lower limit, the aforementioned
effect is not significantly exhibited, while the deposition of
zirconium can be inhibited when the content exceeds the upper
limit.
The method of the metal surface treatment of the present
invention includes a step of subjecting a metal base material
to a surface treatment using the metal surface treatment
liquid described above.
The metal base material is not particularly limited as
long as it can be cation electrodeposited, and for example, an
iron-based metal base material, aluminum-based metal base
material, zinc-based metal base material and the like can be
exemplified.
Examples of the iron-based metal base material include
CA 02672854 2013-12-04
28
cold-rolled steel plates, hot-rolled steel plates, soft steel
plates, high-tensile steel plates, and the like. Moreover,
examples of the aluminum-based metal base material include
5,000 series aluminum alloys, 6,000 series aluminum alloys,
and aluminum-coated steel plates treated by aluminum-based
electroplating, hot dipping, or vapor deposition plating.
Furthermore, examples of the zinc-based metal base material
include zinc or zinc-based alloy coated steel plates treated
by zinc-based electroplating, hot dipping, or vapor deposition
plating such as zinc coated steel plate, zinc-nickel coated
steel plate, zinc-titanium coated steel plate, zinc-magnesium
coated steel plate, zinc-manganese coated steel plate, and the
like. There are a variety of grades of the high-tensile steel
plate depending on the strength and manufacture method, and
examples thereof include JSC400J, JSC440P, JSC440W, JSC590R,
JSC590T, JSC590Y, JSC780T, JSC780Y, JSC980Y, JSC1180Y, and the
like.
Metal base materials including a combination of multiple
kinds of metals such as iron-based, aluminum-based, zinc-based
metals and the like (including joint area and contact area of
different kinds of metals) can be simultaneously applied as
the metal base material.
The surface treatment step may be carried out by bringing
the metal surface treatment liquid into contact with the metal
base material. Specific examples of the method include a
dipping method, a spraying method, a roll coating method, a
pouring method, and the like.
CA 02672854 2013-12-04
29
, .
The treatment temperature in the surface treatment step
preferably falls within the range of 20 to 70 C. When the
temperature is lower than 20 C, it is possible to cause
failure in formation of a sufficient coating film, while a
corresponding effect cannot be expected at a temperature above
70 C. The lower limit and the upper limit are more preferably
30 C and 50 C, respectively.
The treatment time period in the surface treatment step
is preferably 2 to 1100 seconds. When the time period is less
than 2 seconds, a sufficient coating film amount may not be
attained, while a corresponding effect cannot be expected even
though it is longer than 1100 seconds. The lower limit and the
upper limit are still more preferably 30 seconds and 120
seconds, respectively. Accordingly, a coating film is formed
on the metal base material.
The surface treated metal base material of the present
invention is obtained by the surface treatment method
described above. On the surface of the metal base material is
formed a coating film that contains zirconium and tin. The
element ratio of zirconium/tin in the coating film is
preferably in the range of 1/10 to 10/1 on a mass basis. When
the ratio is out of this range, the intended performance may
not be attained.
The content of zirconium in the coating film is
preferably no less than 10 mg/m2 in the case of iron-based
metal base materials. When the content is less than 10 mg/e,
sufficient anti-corrosion properties may not be achieved. The
CA 02672854 2013-12-04
content is more preferably no less than 20 mg/m2, and still
more preferably no less than 30 mg/m2. Although the upper
limit is not specifically defined, too large an amount of the
coating film may lead to an increased likelihood of crack
generation of the rust-preventive coating film, and may make
it difficult to obtain a uniform coating film. In this respect,
the content of zirconium in the coating film is preferably no
greater than I g/m2, and more preferably no greater than 800
mg./m2.
When the coating film is formed using the metal surface
treatment liquid which contains copper ions, the content of
copper in the coating film is preferably no less than 0.5 mg/m2
in order to achieve the intended effect.
The method of cation electrodeposition coating of the
present invention includes a step of subjecting a metal base
material to a surface treatment using the metal surface
treatment liquid described above, and a step of subjecting the
surface treated metal base material to cation
electrodeposition coating.
The surface treatment step in the aforementioned cation
electrodeposition coating is same as the surface treatment
step in the surface treatment method described above. The
surface treated metal base material obtained in the surface
treatment step may be subjected to the cation
electrodeposition coating step directly or after washing.
In the cation electrodeposition coating step, the surface
treated metal base material is subjected to the cation
CA 02672854 2013-12-04
31
electrodeposition coating. In the cation electrodeposition
coating, the surface treated metal base material is dipped in
cation electrodeposition coating solution, and a voltage of 50
to 450 V is applied thereto using the same as a cathode for a
certain period of time. Although the application time period
of voltage may vary depending on the conditions of the
electrodeposition, it is generally 2 to 4 minutes.
As the cation electrodeposition coating solution, a
generally well known one can be used. Specifically, such
general coating solutions are prepared by blending: a binder
cationized through adding amine or sulfide to an epoxy group
carried by an epoxy resin or an acrylic resin, followed by
adding thereto a neutralizing acid such as acetic acid; block
isocyanate as a curing agent; and a pigment dispersing paste
including a rust-preventive pigment dispersed in a resin.
After completing the cation electrodeposition coating
step, a hardened coated film can be obtained by baking at a
predetermined temperature directly, or after washing with
water. Although the baking conditions may vary depending on
the type of the cation electrodeposition coating solution used,
usually the baking may be conducted in the range of 120 to
260 C, and preferably in the range of 140 to 220 C. The baking
time period can be 10 to 30 minutes. The resulting metal base
material coated by the cation electrodeposition is also
involved as an aspect of the present invention.
EXAMPLES
CA 02672854 2013-12-04
32
, .
Production Example 1: Production of Hydrolysis Condensate of
Aminosilane, Part 1
As aminosilane, 5 parts by mass of KBE603 (3-aminopropyl-
triethoxysilane, effective concentration: 100%, manufactured
by Shin-Etsu Chemical Co., Ltd.) was added dropwise using a
dropping funnel to a mixed solvent (solvent temperature: 25 C)
containing 47.5 parts by mass of deionized water and 47.5
parts by mass of isopropyl alcohol over 60 minutes to a
homogenous state, followed by allowing for reaction under a
nitrogen atmosphere at 25 C for 24 hours. Then, the reaction
solution was subjected to a reduced pressure to allow for
evaporation of isopropyl alcohol, and deionized water was
further added thereto, whereby a hydrolysis condensate of
aminosilane including 5% of the active ingredient was obtained.
Production Example 2: Production of Hydrolysis Condensate of
Aminosilane, Part 2
In a similar manner to Production Example 1, except that
the amounts were changed to 20 parts by mass of K3E603, 40
parts by mass of deionized water, and 40 parts by mass of
isopropyl alcohol, a hydrolysis condensate of aminosilane
including 20% of the active ingredient was obtained.
Example 1
A metal surface treatment liquid for cation
electrodeposition coating was obtained by: mixing a 40%
aqueous zircon acid solution as a zirconium ion source, tin
sulfate as a tin ion source, and hydrofluoric acid; diluting
the mixture so as to give a zirconium ion concentration of 500
CA 02672854 2013-12-04
33
. ,
ppm, and a tin ion concentration of 30 ppm; and adjusting the
pH to 3.5 using nitric acid and sodium hydroxide. Measurement
of free fluorine ion concentration using a fluorine ion meter
after adjusting the pH of this treatment liquid to 3.0
revealed a value of 5 ppm.
Example 2
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 1 except that: the hydrolysis condensate of
aminosilane obtained in Production Example 1 was further added
to be 200 ppm; tin sulfate was changed to tin acetate so as to
give the tin ion concentration of 10 ppm; and the pH was
adjusted to 2.75. Measurement of the free fluorine ion
concentration using a fluorine ion meter after adjusting the
pH of this treatment liquid to 3.0 revealed a value of 5 ppm.
Example 3
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 1 except that: polyallylamine "PAATm-H-10C"
(manufactured by Nitto Boseki Co., Ltd.) was further added to
be 25 ppm; zirconium ion concentration was changed to 250 ppm;
and the pH was adjusted to 3Ø Measurement of the free
fluorine ion concentration using a fluorine ion meter on this
treatment liquid revealed a value of 5 ppm.
Example 4
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
CA 02672854 2013-12-04
34
. ,
Example 1, except that: copper nitrate was further added so as
to give a copper ion concentration of 10 ppm; the tin ion
concentration was changed to 10 ppm; and the pH was adjusted
to 3Ø Measurement of the free fluorine ion concentration
using a fluorine ion meter on this treatment liquid revealed a
value of 5 ppm.
Example 5
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 4, except that: the hydrolysis condensate of
aminosilane obtained in Production Example 2 was further added
to be 200 ppm; and the tin ion concentration was changed to 30
ppm. Measurement of the free fluorine ion concentration using
a fluorine ion meter on this treatment liquid revealed a value
of 5 ppm.
Example 6
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 2, except that: aluminum nitrate was further added so
as to give an aluminum ion concentration of 200 ppm; and tin
sulfate was changed to tin acetate so as to give the tin ion
concentration of 30 ppm. Measurement of the free fluorine ion
concentration using a fluorine ion meter after adjusting the
pH of this treatment liquid to 3.0 revealed a value of 5 ppm.
Examples 7 and 8
Metal surface treatment liquids for cation
electrodeposition coating were obtained in a similar manner to
CA 02672854 2013-12-04
,
,
Example 6, except that the pH was adjusted to 3.5 and 4Ø The
free fluorine ion concentration measured using a fluorine ion
meter after adjusting the pH of this treatment liquid to 3.0
is shown in Table 1.
Examples 9 to 16
Metal surface treatment liquids for cation
electrodeposition coating were obtained in a similar manner to
Example 7, except that the amount of added 40% aqueous
zirconic acid solution, tin sulfate, and aluminum nitrate was
changed so as to give a zirconium ion concentration, a tin ion
concentration, and an aluminum ion concentration as shown in
Table 1. The free fluorine ion concentration measured using a
fluorine ion meter after adjusting the pH of this treatment
liquid to 3.0 is shown in Table 1.
Example 17
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 2, except that: indium nitrate was further added so as
to give an indium ion concentration of 200 ppm; tin sulfate
was changed to tin fluoride so as to give a tin ion
concentration of 30 ppm; and the pH was adjusted to 3.5.
Measurement of the free fluorine ion concentration using a
fluorine ion meter after adjusting the pH of this treatment
liquid to 3.0 revealed a value of 5 ppm.
Example 18
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
CA 02672854 2013-12-04
36
. ,
Example 2, except that: diethylenetriamine pentaacetic acid
(DTPA) was further added as a chelating agent to give a
concentration of 100 ppm; tin acetate was changed to tin
sulfate, thereby changing the tin ion concentration to 30 ppm;
and further, the zirconium ion concentration was changed to
1,000 ppm. Measurement of the free fluorine ion concentration
using a fluorine ion meter after adjusting the pH of this
treatment liquid to 3.0 revealed a value of 10 ppm.
Example 19
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 2, except that: sodium nitrate was further added so as
to give a sodium ion concentration of 5,000 ppm; and the tin
ion concentration was changed to 30 ppm. Measurement of the
free fluorine ion concentration using a fluorine ion meter
after adjusting the pH of this treatment liquid to 3.0
revealed a value of 5 ppm.
Example 20
A metal surface treatment liquid for cation
electrodeposition coating was obtained in a similar manner to
Example 5, except that: glycine as chelating agents and copper
nitrate further added so as to give a concentration of 50 ppm
and copper ion concentration of 10 ppm, respectively; and the
concentration of polyamine was changed to 100 ppm. Measurement
of the free fluorine ion concentration using a fluorine ion
meter on this treatment liquid revealed a value of 5 ppm.
Examples 21 to 31
CA 02672854 2013-12-04
37
Metal surface treatment liquids for cation
electrodeposition coating were respectively obtained in a
similar manner to Example I, except that: polyamine as
described in Table I was added in a specified amount; and the
concentration of the other component was changed as shown in
Table I. The free fluorine ion concentrations measured using a
fluorine ion meter on these treatment liquids under a
condition of pH 3.0 are shown together in Table 1.
Examples 32 to 50
Metal surface treatment liquids for cation
electrodeposition coating were respectively obtained in a
similar manner to Example 1, except that: sulfonic acid
described in Table 2 was added in a specified amount; and
polyamine and the other component were changed as shown in
Table 2. The free fluorine ion concentrations measured using a
fluorine ion meter on these treatment liquids under a
condition of pH 3.0 are shown together in Table 2. In Table 2,
the used naphthalenesulfonic acid-formaldehyde condensate was
DEMOLTm NL manufactured by Kao Corporation; sodium
alkylnaphthalenesulfonate was PELEXTm NBL manufactured by Kao
Corporation; and sodium polystyrenesulfonate was P-NASS-1
manufactured by Tosoh Corporation.
Examples 51
Metal surface treatment liquids for cation
electrodeposition coating were respectively obtained in a
similar manner to Example 1, except that: ascorbic acid as
described in Table 3 was added in a specified amount; and
CA 02672854 2013-12-04
38
, .
polyamine and the other component were changed as shown in
Table 3. The free fluorine ion concentrations measured using a
fluorine ion meter on these treatment liquids under a
condition of pH 3.0 are shown together in Table 3.
Examples 52 to 59
Metal surface treatment liquids for cation
electrodeposition coating were respectively obtained in a
similar manner to Example 1, except that: the oxidizing agent
described in Table 3 was added in a specified amount; and
polyamine and the other component were changed as shown in
Table 3. The free fluorine ion concentrations measured using a
fluorine ion meter on these treatment liquids under a
condition of pH 3.0 are shown together in Table 3.
Examples 60 to 74
Metal surface treatment liquids for cation
electrodeposition coating were respectively obtained in a
similar manner to Example 1, except that: the nitrogen-based
rust-preventive agent, the sulfur-based rust-preventive agent,
or the phenol-based rust-preventive agent described in Table 3
was added in a specified amount; and polyamine and the other
component were changed as shown in Table 3. The free fluorine
ion concentrations measured using a fluorine ion meter on
these treatment liquids under a condition of pH 3.0 are shown
together in Table 3.
Examples 75 to 77
Metal surface treatment liquids for cation
electrodeposition coating were respectively obtained in a
CA 02672854 2013-12-04
39
similar manner to Example 1, except that: instead of a cold-
rolled steel plate (SPC) a high-tensile steel plate was used
as the base plate that is the object; and polyamine and the
other component described in Table 3 were changed as shown in
Table 3. The free fluorine ion concentrations measured using a
fluorine ion meter on these treatment liquids under a
condition of pH 3.0 are shown together in Table 3.
Examples 78 to 106
With respect to Examples 2, 3, and 5 to 31, metal surface
treatment liquids for cation electrodeposition coating were
obtained in a similar manner to each Example, except that
polyamine was not added. The free fluorine ion concentrations
measured using a fluorine ion meter after adjusting the pH of
the treatment liquids to 3.0 are shown in Table 4.
Comparative Examples 1 to 6: Preparation of Comparative Metal
Surface Treatment Liquid
According to the description in Table 1 and Table 3,
comparative metal surface treatment liquids were obtained,
respectively, based on the aforementioned Examples. Thus
resulting metal surface treatment liquids are summarized in
Table 1 and Table 3.
_
_
IA
Added Component
W
Zr Tin ion Sn sn/Zr (Concentraion in
Parenthesis (ppm)) Free tr
Concentration supplying Concentration pH
Fluorineion 1--.
M
(ppm) compound (ppm) ratio Polyamine Compound
Others Concentration
I-'
Example 1 500 tin sulfate 30 0.06 3.5
absent 5
Example 2 500 tin sulfate 10 0.02
2.75 Production Exapmle 1(200) 5
Example 3 250 tin sulfate 30 0.12
3 poly allylamine(25) 5
Example 4 500 tin sulfate 10 0.02 3
absent copper nitrate(10) 5
Example 5 500 tin sulfate 30 0.06 3
Production Exapmle 2(200) copper nitrate(10) 5
Example 6 500 tin acetate 30 0.06
2.75 Production Exapmle 1(200) aluminum nitrate(200) 5
0
Example 7 500 tin acetate 30 0.06
3.5 Production Exapmle 1(200) aluminum nitrate(200) 5
0
Example 8 500 tin acetate 30 0.06
4 Production Exapmle 1(200) aluminum nitrate(200) 5 n.)
cn
-.3
Example 9 1000 tin acetate 30 0.03
3.5 Production Exapmle 1(200) aluminum nitrate(200) 7 n.)
co
Ln
Example 10 500 tin acetate 30 0.06
3.5 Production Exapmle 1(200) aluminum nitrate(500) 5 4=.
..P.
0
n.)
Example 11 500 tin acetate 30 0.06
3.5 Production Exapmle 1(200) aluminum nitrate(1000) 5 0
I-,
W
Example 12 500 tin acetate 10 0.02
3.5 Production Exapmle 1(200) aluminum nitrate(500) 5 i
i-,
n.)
Example 13 500 tin acetate 200 0.4
3.5 Production Exapmle 1(200) aluminum nitrate(500) 5 i
0
4=.
Example 14 200 tin acetate 10 0.05
3.5 Production Exapmle 1(200) aluminum nitrate(200) 7
Example 15 200 tin acetate 30 0.15
3.5 Production Exapmle 1(200) aluminum nitrate(200) 5
Example 16 200 tin acetate 70 0.35
3.5 Production Exapmle 1(200) aluminum nitrate(200) 5
Example 17 500 tin fluoride 30 0.06 3.5
Production Exapmle 1(200) indium nitrate(50) 5
Example 18 1000 tin sulfate 30 0.03
2.75 Production Exapmle 1(200) DTPA(100) 10
Example 19 500 tin sulfate 30 0.06
2.75 Production Exapmle 1(200) sodium nitrate(5000) 5
Example 20 500 tin sulfate 30 0.06
3 Production Exapmle 2(100) coppoer sulfate(10),
glycine(50)
5
_
Added Component
Zr Tin ion Sn
(Concentraion in Parenthesis (ppm)) Free
Sn/Zr
Concentration supplying Concentration pH
Fluorineion
ratio
Concentration
(ppm) compound (ppm) Polyamine Compound
Others
Example 21 20 tin sulfate 5 0.25 3 Production Exapmle
1(10) 2
Example 22 500 tin sulfate 20 0.04 2 Production Exapmle
1(200) 1
Example 23 500 tin sulfate 30 0.06 5.5
Production Exapmle 1(200) 20
Example 24 5000 tin sulfate 25 0.005 3
Production Exapmle 1(2000) 10
Example 25 50 tin sulfate 10 0.2 3 Production Exapmle
2(50) 3
Example 26 50 tin sulfate 50 1 3 Production Exapmle 2(25)
1
0
Example 27 500 tin sulfate 30 0.06 3 Production Exapmle
1(50) 0
0
Example 28 500 tin sulfate 30 0.06 2.75
Production Exapmle 2(50) 0.1 n.)
01
....1
Example 29 500 tin sulfate 30 0.06 2.75
Production Exapmle 2(50) 0.6 n.)
co
Ln
Example 30 500 tin sulfate 30 0.06 4 Production Exapmle
1(200) 20 IP.
-P.
Example 31 500 tin sulfate 30 0.06 4.5
Production Exapmle 1(200) 50 0
1¨,
Comparative
(A)
500 absent 0 0 3.5 Production Exapmle
1(200) 7 1
Example 1
N.)
Comparative
500 absent 0 0 3 Production Exapmle
1(200) aluminum nitrate(500) 5 1
Example 2
0
IP.
Comparative
50 absent 0 0 3.5 Production Exapmle
1(200) 5
Example 3
Comparative
500 tin sulfate 250 0.5 1 Production Exapmle
1(200) 5
Example 4
Comparative
500 tin sulfate 250 0.5 8 Production Exapmle
1(200) 5
Example 5
H
Added Component
W
Zr Tin ion Sn
Free tr
Sn/Zr
(Concentration in Parenthesis(ppm))
Concentration supplying Concentration pH
Fluorinelon 1--'
ratio '
(ppm) compound (ppm) Polyamine
Other Metal
Others Concentration M
Compounds
t\)
Production
Example 32 500 tin sulfate 30 0.06 3.5
taurine(100) 5
Exapmple 1(200)
Example 33 500 tin sulfate 30 0.06 3 Production
methan sulfonic.5 5
Exapmple 1(200)
acid(100)
Example 34 500 tin sulfate 30 0.06 3 Production.5
isethionic acid(100) 5
Exapmple 1(200)
sodium
Example 35 500 tin sulfate 30 0.06 3 Production.5
naphthalenedisulfonate 5
Exapmple 1(200)
0
(100)
sodium amino
Production
0
Example 36 500 tin sulfate 30 0.06 3.5
naphthalenedisulfonate 5 t\.)
Exapmple 1(200)
M
(100)
t\.)
M
Example 37 500 tin sulfate 30 0.06 3 Production
sulfosalicylic.5 5 Ln
Exapmple 1(200)
acid(100) al.
..P.
naphthalene sulfonic
Production
0
Example 38 500 tin sulfate 30 0.06 3.5 acid
- formaldehyde 5
Exapmple 1(200)
W
condensate (100)
i
I-
Production
sodium alkylnaphthalene ,
Example 39 500 tin sulfate 30 0.06 3.5
5
Exapmple 1(200)
sulfonate(100) 0
al.
Example 40 500 tin sulfate 30 0.06 3 Production
copper.5 taurine(100) 5
Exapmple 1(200) nitrate(10)
_
Added Component
Zr Tin ion Sn
Free
Sn/Zr
(Concentration in Parenthesis (ppm)
Concentration supplying Concentration 101-I
Fluorineion
ratio '
(ppm) compound (ppm) Polyamine
Concentration
Other Metal
Others
Compounds
Example 41 500 tin sulfate 30 0.06 3.5 copper
taurine (100)
5
nitrate (10)
aluminum
methan sulfonic
Example 42 500 tin sulfate 30 0.06 3.5 - nitrate
5
acid (100)
(200)
copper
Example 43 500 tin sulfate 30 0.06 3.5
isethionic acid(100) 5
nitrate (10)
aluminum
sodium
Example 44 500 tin sulfate 30 0.06 3.5 nitrate
naphthalenedisulfonate 5
0
(200)
(100)
sodium
copper 0
Example 45 500 tin sulfate 30 0.06 3.5
aminonaphthalenedisulfo 5 t\.)
nitrate(10)
M
nate ( 100 )
t\.)
aluminum M
sulfosalicylic
Example 46 500 tin sulfate 30 0.06 3.5 nitrate
5 0-1
acid(100)
al.
(200)
(...a
Ni
naphthalene sulfonic
0
Example 47 500 tin sulfate 30 0.06 3.5 copper
acid - formaldehyde
5 I-,
nitrate(10)
W
I
condensate(100)
I-,
aluminum
Ni
Example 48 500 tin sulfate 30 0.06 3.5 nitrate
5
sodium alkylnaphthalene
i
sulfonate(100)
0
(200) al.
Example 49 500 tin sulfate 30 0.06 3.5 copper
sodium styrenesulfonate
nitrate(10)
(100)
aluminum
sodium polystyrene
Example 50 500 tin sulfate 30 0.06 3.5 - nitrate
5
sulfonate(100)
(200)
_
IA
Added Component
W
Zr Tin ion Sn
Free tr
Sn/Zr
(Concentration in Parenthesis(ppm))
Concentration supplying Concentration PH
Fluorineion I¨.
ratio
M
(ppm) compound (ppm) Polyamine
Other Metal
Others Concentration
Compounds
(...)
Production
sodium ascorbate
Example 51 500 tin sulfate 30
0.06 3.5 5
Example 1(200)
(50)
Production as
sodium nitrate
Example 52 500 tin sulfate 30
0.06 3.5 5
Example 1(200)
(10000)
Production
Example 53 500 tin sulfate 30 0.06
3.5 hydrogen peroxide(10) 5
Example 1(200)
Production
Example 54 500 tin sulfate 30 0.06
3.5 sodium nitrite(50) 5
Example 1(200)
0
Production
0
Example 55 500 tin sulfate 30 0.06
3.5 sodium bromate(100) 5 N.)
Example 1(200)
m
.....3
.
N.)
m
copper as
sodium nitrate
'Example 56 500 tin sulfate 30 0.06 3.5 -
nitrate(10)
(10000) 5 Ln
0.
-P.
-P,
Ni
aluminum
0
Example 57 500 tin sulfate 30 0.06 3.5
nitrate hydrogen peroxide(10) 5
w
1
(200)
i¨,
tv
copper
1
Example 58 500 tin sulfate 30 0.06 3.5 _
nitrate(10)
sodium nitrite(50) 5 0
0.
,
aluminum
Example 59 500 tin sulfate 30 0.06 3.5 -
nitrate sodium bromate(100) 5
(200)
_
Added Component
Zr Tin ion Sn
Free
Sn/Zr
(Concentration in Parenthesis(ppm))
Concentration supplying Concentration pH
Fluorineion
ratio '
(ppm) compound (ppm) Polyamine
Other Metal
Others Concentration
Compounds
Production
Example 60 500 tin sulfate 30 0.06 3.5 -
hydroquinone(100) 5
Example 1(200)
Production
Example 61 500 tin sulfate 30 0.06 3.5ethylene urea(100)
5
Example 1(200)
Production
Example 62 500 tin sulfate 30 0.06 3.5
quinolinol(100) 5
Example 1(200)
1
Production
Example 63 500 tin sulfate 30 0.06 3.5 -
thiourea(100) 5
Example 1(200)
0
Production
0
Example 64 500 tin sulfate 30 0.06 3.5 -
benzotriazole(100) 5 t\.)
Example 1(200)
M
...1
\
N)
Production
mercaptobenzothiazole M
Example 65 500 tin sulfate 30 0.06 3.5
5 Ln
Example 1(200)
(100) al.
-P
LA
Ni
Production
0
Example 66 500 tin sulfate 30 0.06 3.5
KBM803(100) 5
Example 1(200)
W
i
I- N)`
Production copper
i
Example 67 500 tin sulfate 30 0.06 3.5
benzotriazole(100) 5
Example 1(200) nitrate(10)
0
al.
copper
Example 68 500 tin sulfate 30 0.06 3.5
nitrate(10)
hydroquinone(100) 5
_
Added Component
Zr Tin ion Sn
Free
Sn/zr
(Concentration in Parenthesis(ppm))
Concentration supplying Concentration
PH Fluorineion
ratio
(ppm) compound (ppm) Polyamine
Other Metal Others Concentration
Compounds
-
Example 69 500 tin sulfate 30 0.06 3.5 - copper
ethylene urea(100)
5
nitrate(10)
. _
Example 70 500 tin sulfate 30 0.06 3.5 - copper
nitrate(10) quinolinol(100) 5
_ .
Example 71 500 tin sulfate 30 0.06 3.5 - copper
thiourea(100)
5
nitrate(10)
Example 72 500 tin sulfate 30 0.06 3.5 - copper
benzotriazole(100)
5
nitrate(10)
C)
_.
copper
mercaptohenzothiazole 0
Example 73 500 tin sulfate 30 0.06 3.5
5
nitrate(10) (100) t\.)
M
...1
IV
copper
CO
Example 74 500 tin sulfate 30 0.06 3.5 -
K5M803(100) 5 Ln
nitrate(10)
Ch
tv
Production copper as
sodium nitrate 0
Example 75 500 tin sulfate 30 0.06 3.5
5 I-,
Example 1(200) nitrate(10) (10000) W
i
I-,
Production copper
N.)
Example 76 500 tin sulfate 30 0.06 3.5
taurine(100) 5 1
Example 1(200) nitrate(10) 0
0.
_
Production copper
Example 77 500 tin sulfate 30 0.06 3.5
benzotriazole(100) 5
Example 1(200) nitrate(10)
Comparative Production
500 -- 3.5 -
5
Example 6 Example 1(200)
_
IA
Added Component
W
Zr Tin ion Sn
(Concentralon in Parenthesis(ppm)) Free tr
Concentration supplying Concentration Sn/Zr H
ratio P Fluorineion I-'
(PPm) compound (ppm)
Concentration M
Polyamine Compound
Others
.
a=
,
Example 78 500 tin sulfate 10 0.02 2.75 -
5
Example 79 250 tin sulfate 30 0.12 3 -
5
. .
Example 80 500 tin sulfate 30 0.06 3 -
copper nitrate(10) 5
_ .
Example 81 500 tin sulfate 30 0.06 2.75 -
aluminum nitarte(200) 5
. _
Example 82 500 tin acetate 30 0.06 3.5 -
aluminum nitarte(200) 5
Example 83 500 tin acetate 30 0.06 4 -
aluminum nitarte(200) 5
Example 84 1000 tin acetate 30 0.03 3.5 -
aluminum nitarte(200) 7 0
Example 85 500 tin acetate 30 0.06 3.5 -
aluminum nitrate(500) 5 0
. ,
Example 86 500 tin acetate 30 0.06 3.5 -
aluminum nitrate(1000) 5 m
....3
Example 87 500 tin acetate 10 0.02 3.5 -
aluminum nitrate(500) 5 m
(.n
Example 88 500 tin acetate 200 0.4 3.5 -
aluminum nitrate(500) 5
.
0
Example 89 200 tin acetate 10 0.05 3.5 -
aluminum nitarte(200) 7
w
.
1
Example 90 200 tin acetate 30 0.15 3.5 -
aluminum nitarte(200) 5
N.)
1
Example 91 200 tin acetate 70 0.35 3.5 -
aluminum nitarte(200) 5 0
0.
,
Example 92 500 tin fluoride 30 0.06 3.5 -
indium nitrate(50) 5
-
Example 93 1000 tin sulfate 30 0.03 2.75 -
DTPA(100) 10
Example 94 500 tin sulfate 30 0.06 2.75 -
sodium nitrate(5000) 5
Added Component
Zr Tin ion Sn
Sn/Zr
(Concentraion in Parenthesis(ppm)) Free
Concentration supplying Concentration pH
Fluorineion
ratio
(ppm) compound (ppm) Polyamine Compound
Others Concentration
-
copper nitrate(l0),
Example 95 500 tin sulfate 30 0.06 3 -
5
glycine(50)
Example 96 20 tin sulfate 5 0.25 3
2
Example 97 500 tin sulfate 20 0.04 2
1
_
Example 98 500 tin sulfate 30 0.06 5.5
20
Example 99 5000 tin sulfate 25 0.005 3 -
10
Example 100 50 tin sulfate 10 0.2 3
3
0
Example 101 50 tin sulfate 50 1 3 -
1
0
Example 102 500 tin sulfate 30 0.06 3
0 t\.)
m
...1
Example 103 500 tin sulfate 30 0.06 2.75
0.1 t\.)
m
Ln
Example 104 500 tin sulfate 30 0.06 2.75 -
0.6
00
t\.)
Example 105 500 tin sulfate 30 0.06 4 -
20 0
1-`
Example 106 500 tin sulfate 30 0.06 4.5
50 w
I
I-
F..,
1
0
41.
CA 02672854 2013-12-04
49
. ,
Surface Treatment
As metal base materials, a commercially available cold-
rolled steel plate (SPC, manufactured by Nippon Testpanel Co.,
Ltd., 70 mm x 150 mm x 0.8 mm) was provided for Examples 1 to
74, Examples 78 to 106, and Comparative Examples 1 to 5, and a
high-tensile steel plate (70 mm x 150 mm x 1.0 mm) was
provided for Examples 75 to 77, and Comparative Example 6.
These plates were subjected to a degreasing treatment using
"SURFCLEANERTM EC92" (manufactured by Nippon Paint Co., Ltd.)
as an alkali degreasing treatment agent at 40 C for 2 minutes.
This plate was dipped and washed in a water washing bath, and
then washed by spraying tap water thereon for approximately 30
seconds.
The metal base material following the degreasing
treatment was subjected to a surface treatment by dipping
thereof in the metal surface treatment liquid prepared in
Examples and Comparative Examples at 40 C for 90 seconds.
However, the treatment time period was 240 seconds and 15
seconds, respectively, in Examples 21 and 22. After completing
the surface treatment, the plate was dried at 40 C for 5
minutes, and the thus surface treated metal base material was
obtained. Unless specifically stated, this surface treated
metal base material was used as a test plate in the following
evaluation.
Measurement of Element Content in Coating Film
The content of each element included in the coating film
was measured using an X-ray fluorescence spectrometer
CA 02672854 2013-12-04
"XRF1700" manufactured by Shimadzu Corporation.
Primary Rust Prevention
After immersing the test plate in pure water at 25 C for
5 hours, the generation state of rust was visually observed.
A: no rust generation observed
B: slightly generated rust observed
C: rust generation clearly identified
Observation of Sludge
With 10 L of the surface treatment liquids of the
Examples and Comparative Examples, 200 test panels were
subjected to the surface treatment and evaluation was made
according to the following standards through visual
observation as to whether the surface treatment liquid became
turbid due to generation of sludge following the lapse of 30
days at room temperature.
A: transparent liquid
B: slightly turbid
C: turbid
D: precipitate (sludge) generated
Evaluation of Throwing Power
The throwing power was evaluated according to a "four-
plate box method" described in Japanese Patent Publication No.
2000-038525. More specifically, as shown in Fig. 1, test
plates 1 to 4 were arranged to stand up in parallel with
intervals of 20 mm to produce a box 10 sealed with an
insulator such as cloth adhesive tape at the underneath of
both side faces and the bottom face. Through-holes 5 having a
CA 02672854 2013-12-04
51
,
diameter of 8 mm were provided underneath the metal materials
1, 2 and 3, except for metal material 4.
This box 10 was dipped into an electrodeposition coating
vessel 20 filled with a cation electrodeposition coating
solution POWERNICSTM 110" (manufactured by Nippon Paint Co.,
Ltd.). In this case, the cation electrodeposition coating
solution entered inside the box 10 only from each through-hole
5.
Each of the test plates 1 to 4 was electrically connected
while stirring the cation electrodeposition coating solution
with a magnetic stirrer, and a counter electrode 21 was
arranged such that the distance from the test plate 1 became
150 mm. Voltage was applied with each of the test plates 1 to
4 as cathodes, and the counter electrode 21 as an anode to
execute cation electrodeposition coating. The coating was
carried out by elevating to the intended voltage (210 V and
160 V) over 30 seconds from initiation of the application, and
thereafter maintaining the voltage for 150 seconds. The bath
temperature in this process was regulated to 30 C.
After washing each of the test plates 1 to 4 with water
after coating, they were baked at 170 C for 25 minutes,
followed by air cooling. The throwing power was then evaluated
by measuring the film thickness of the coated film formed on
side A of the test plate 1 that is the closest to the counter
electrode 21, and the film thickness of the coated film formed
on side G of the test plate 4 that is the farthest from the
counter electrode 21 to determine a ratio of the film
CA 02672854 2013-12-04
52
thickness (side G)/film thickness (side A). As this value
becomes greater, better evaluation of the throwing power can
be decided. The acceptable level was no less than 40%.
Coating Voltage
Using the surface treatment liquids of Examples and
Comparative Examples, cold-rolled steel plates and zinc coated
steel plates were subjected to a surface treatment, whereby
test plates were obtained. Using the cation electrodeposition
coating solution "POWERNICSTm 110" described above on these
test plates, the voltage required for obtaining a 20 pm
electrodeposition coated film was determined. The difference
in coating voltage required for obtaining the 20 pm
electrodeposition coated film was then determined between the
case in which the metal base material was a zinc coated steel
plate, and the case of the cold-rolled steel plate. As the
difference becomes smaller, superiority as a surface treated
coating film is suggested. A difference of no greater than 40
V is acceptable.
The voltage required for obtaining a 20 pm
electrodeposition coated film was determined as in the
following manner. Under the electrodeposition condition, the
voltage was elevated to a specified voltage over 30 seconds,
and thereafter maintaining for 150 seconds. The resulting film
thickness was measured. Such a procedure was conducted for 150
V, 200 V, and 250 V. Thus, a voltage to give a 20 pm film
thickness was derived from the formula of relationship between
the determined voltage and the film thickness.
CA 02672854 2013-12-04
53
Appearance of Coating
The test plate was subjected to cation electrodeposition
coating, and the appearance of the resulting electrodeposition
coated film was evaluated according to the following standards.
The results are shown in Tables 5 to 8.
A: uniform coated film obtained
B: nearly uniform coated film obtained
C: some non-uniformity of the coated film found
D: non-uniformity of the coated film found
Secondary Adhesion Test (SDT)
After forming a 20 pm electrodeposition coated film, the
test plates were incised to provide two parallel cut lines
that ran longitudinally, with the depth to reach to the metal
basis material, and then immersed in a 5% aqueous sodium
chloride solution at 55 C for 240 hours. After water washing
and air drying, an adhesive tape LPACKTM LP-24" (manufactured
by Nichiban Co., Ltd.) was adhered to the portion including
the cuts. Then, the adhesive tape was peeled off abruptly.
The maximum width (one side) of the coating adhered to the
stripped adhesive tape was measured.
A: 0 mm
B: less than 2 mm
C: at least 2 mm to less than 5 mm
D: no less than 5 mm
Cycle Corrosion Test (CCT)
After forming the 20 pm electrodeposition coated film on
the test plate, the edge and back face was sealed with a tape,
CA 02672854 2013-12-04
54
thereby providing cross cuttings that reached to the metal
basis material. A 5% aqueous sodium chloride solution
incubated at 35 C was continuously sprayed for 2 hours onto
this sample in a salt spray tester kept at 35 C, and with a
humidity of 95%. Subsequently, it was dried under conditions
of 60 C and with a humidity of 20 to 30% for 4 hours. Such a
sequence of procedures repeated three times in 24 hours was
defined as one cycle, and 200 cycles were carried out.
Thereafter, the width of the swelling portion of the coated
film (both sides) was measured.
A: less than 6 mm
B: at least 6 mm to less than 8 mm
C: at least 8 mm to less than 10 mm
D: no less than 10 mm
Salt Spray Test (SST)
After forming the 20 pm electrodeposition coated film on
the test plate, the edge and the back face were sealed with a
tape, thereby providing cross cuttings that reached to the
metal basis material. A 5% aqueous sodium chloride solution
incubated at 35 C was continuously sprayed for 840 hours to
this sample in a salt spray tester kept at 35 C, and with a
humidity of 95%. After washing with water and air drying, an
adhesive tape "L-PACKTM LP-24" (manufactured by Nichiban Co.,
Ltd.) was adhered on the portion including the cuts. Then, the
adhesive tape was peeled off quickly. The maximum width (one
side) of the coating adhered to the stripped adhesive tape was
measured.
CA 02672854 2013-12-04
A: less than 2 mm
B: at least 2 mm to less than 5 mm
C: no less than 5 mm
The evaluation results are summarized in Tables 5 to 8.
_
H
Content of Element Primary Throwing Difference in
Appearance of W
Observation
tr
Rust PowerTt.) Coating
SDT CCT SST
of sludge
Coating
Zr Si Sn Cu Prevention 210V
160V Voltage (V) M
Example 1 45 22 A B 60% 52% 30 A
- B A cn
Example 2 51 3.3 13 A B 57% 25=
40 B A B A
Example 3 44 24 A B 57% 44% 40 A
B B A
Example 4 55 16 8 A B 58% 51 40 A
A A A .
Example 5 46 6.2 27 11 A B 61% 55%
20 A A A A
Example 6 42 3.5 19 A B 57-4- 47
40 A A B A
Example 7 56 3.7 15 A B 53% 42%
30 B A a A
0
Example 8 62 4.1 12 - A C 51% 39%
30 B A B A
Example 9 41 2.3 16 A B 53% 41%
30 B B B A 0
(..)
Example 10 72 2.4 15 A c 54% 43%
30 B A B , A 2
1..,
m
Example 11 62 2.4 15 - A c 53% 43
30 B B B A . 01
o.
tif
Example 12 75 3.2 10 A c 49% 40%
30 B A A A
0
Example 13 32 2.1 31 - A B 59% 51%
20 B B B A i-,
w
1
Example 14 52 2.5 12 - A B 58% 30%
40 B A B A
t..)
1
Example 15 38 2.3 18 A B 59% 48%
20 B B B A 0
o.
Example 16 31 2.1 23 A B 62% 50%
20 B B , B A
Example 17 55 3 22 A B 59% 50
20 A A B A
Example 18 51 3.3 19 A A 56% 51%
30 A B B A
Example 19 44 2.5 23 A B 56% 49%
30 A A a A
Example 20 48 4.8 22 6 A A 58% 52%
20 A B A A
Example 21 28 1.8 21 A B 52% 44%
30 B a B A
Example 22 63 4.2 28 A B 55% 49
30 B B B A
Example 23 44 2.9 26 A a 60% 43%
30 B B B A
Example 24 77 5.1 31 A B 52% 52%
20 B A A A
Example 25 34 2.6 26 A B 51% 41%
30 B B B A
,
Content of Element Primary Throwing
Difference in
Observation Power(%)
Rust Coating
Appearance ofSDT CCT SST
of sludge
Coating
Zr Si Sn Cu Prevention 210V 160V Voltage
(V)
Example 26 42 2.6 27 A B 62% 48% 20
B B B A
_
Example 27 38 2.7 18 A B 52% 29% 40
B B B A
Example 28 38 3.5 21 A B 53% 36% 30
B B B A
Example 29 41 3 26 A B 55% 42% 30
B B B A
Example 30 44 3 22 A B 58% 41% 30
B A A A
Example 31 47 3.5 25 A B 57% 48% 20
B A A A
Comparative
52 3.5 B B 21% 12% 80
C B C A 0
Example 1
Comparative
o
55 3.3 B B 36% 15% 50
B D C B t..)
Example 2
m
...3
Comparative
t..)
5.2 0.1 38 A B 60% 55% 30 B D D C
m
Example 3
0-1
o.
Comparative
cn
1.2 0.1 0.2 C D 57% 45% 30 B
D D C
Example 4
o
i-,
Comparative 0
w
0 0 C38% -
B D D C 1
Example 5
i-,
t..)
O
o.
-
IA
W
Content of Element Throwing
tr
Power(%) Difference
I--'
Primary Rust Observation
Appearance
in Coating
SOT CCT SST 0
Prevention of sludge of
Coating
Zr Si Sn Cu 210V
160V Voltage (V) cr,
. .
Example 32 42 3.2 18 A B 69% 61% 10
A A A , A
_
Example 33 45 3.3 16 A B 62% 57% 20
A A A A
. .
Example 34 41 3 15 A B 62% 55% 20
A A A A
Example 35 38 2.9 16 A B 64% 51% 30
A A A A
_
Example 36 44 3.1 19 A B 61% 53% 30
A A A A
_
Example 37 51 3.6 21 A B 59% 52% 30
A A A A
_
Example 38 48 3.5 16 A B 60% 47% 30
A A A , A
. .
_
0
Example 39 , 42 32 22 A B 60% 46%
20 A A A A
Example 40 55 3.8 18 8 A B 69% 62%
10 A A A A 0
_
Iv
Example 41 48 18 8 A B 68% 65% 10
A A A A m
..3_
Iv
Example 42 41 16 A B 65% 60% 20
A B B A m
_
In
Example 43 52 17 7 A B 65% 60% 20
A B A A
oo
Example 44 43 18 A B 62% 55% 30
A B B A n.)
0
_
i-,
Example 45 55 18 , 9 A B 60% 56% 30
A B A , A w
_
1
Example 46 43 16 A B 59% 53% 30
A B B A
N)
Example 47 58 20 6 A B 61% 49% 30
A B A A
0 _
Example 48 45 19 A B 62% 47% 30
A B B , A
Example 49 56 17 7 A B 58% , 44% 40
A B A , A
Example 50 41 , 16 A B 58% 45% 40
A B B A
..
1-3
Throwing
0
Content of Element
primaryDifference tr
Observation of Power(%) Appearance I--'
SDT CCT SST
Rust in
Coating
sludge of
Coating M
Zr Si Sn Cu Prevention 210V
160v Voltage (V)
--A
Example 51 91 5.7 19 _ A B 62% 55% ,
30 A A A A
Example 52 75 5.1 21 A B 57% 50% 30
A A A A
Example 53 61 5.3 18 A B 56% 51% 30
A A A A
,
Example 54 88 5.7 14 A B 59% 47% 30
A A A A
Example 55 72 4.8 17 A B 60% 508 30
A A A A
Example 56 72 18 6 A B 59% 51% 20 A
B B A .
Example 57 85 21 A B 571. 48% 30
A B B A
Example 58 91 20 7 A B 59% 51% 20 A
B B A
Example 59 94 18 A B 60% 52% 30 A
B B A o
P
Example 60 44 3.2 15 A B 62% 55% 30
A A A A o
_
N.)
Example 61 46 3.1 19 A B 61% 51% 30
A A A A m
Example 62 49 3.6 18 A B , 60% 53% , 30
A A A A N.)
m
Example 63 38 3 20 A B 65% 57% 20
A A A A ui
0.
Example 64 44 , 3.2 16 A B 66% 55% 20
A A A A LA
4D N)
Example 65 41 3_5 17 A B 61% 58% 20 A
A A A 0
1-`
_
w
Example 66 49 3.2 16 A B 62% 551 , 30
A A A A i
Example 67 41 3.2 15 7 A B 68% 599, 20
A A A A N)
Example 68 51 18 7 A B 59% 53% 30 A
B A A 0
._
0.
Example 69 52 18 5 A B 63% 511 30 A
B A A
_
Example 70 48 19 9 A B 61% 53% 30 A
B A A
Example 71 55 17 6 A B 65% 55% 30 A
B A A
Example 72 43 16 10 A B 62% 58% 20 A
B A A
_
Example 73 49 20 7 A B 66% 54 20 A
B A A
Example 74 52 _ 17 5 , A B 62% 52% 30
A B A A
Example 75 67 4.7 18 A B 59% 52% 30
A A A A
. .
Example 76 54 3.2 16 A B 62% 58% 20
A A A A
_
Example 77 h 48 2.8 17 A B 59% 50% 30
A A A A
Comparative
58 4.2 B B 22% 10% 80 C
B D B
Example 6
,
IA
W
Content of
tr
Element Primary
Observation Throwing Power(%) Difference
Appearance
1--1
Rust in Coating
SOT
CCT SST M
of sludge of
Coating
Zr Si Sn Cu Prevention 210V 160V Voltage (V)
c0
Example 78 55 13 _ A B 58% 50% 40 B
C B B
Example 79 44 24 A B , 58% 25% 40
B C B B
Example 80 49 21 A B 69% 55% 30 A
C A B .
Example 81 45 18 , A B 59% 50% 20 A
C A B
Example 82 38 26 A B 60% 50% 20 A
C A , B
-
Example 83 45 9 A B 59% 51% 20 A
C A B
Example 84 51 18 A B , 58% 52% 20
A C A B
Example 85 43 21 A B 60% 54% 20 A
C A B 0
Example 86 36 18 A B 61% 53% 10 A
C A B
_
0
Example 87 47 23 A B 59% 51% 10 A
C A B t..)
m
Example 88 32 33 A B 60% 53% 20 A
C A B ...3
t..)
m
Example 89 _52 12 A B 61% 53% 20 A
C A B ul
o.
Example 90 42 21 A B 60% 51% 20 A
C A B C^
CD t..)
Example 91 36 28 A B 59% 53% 20 A
C A B 0
i-,
w
Example 92 50 22 A B , 60% 51% 30
A C B B 1
i-,
Example 93 50 24 A A 55% 48% 30 B
C B B t..)
1
Example 94 46 26 A B , 58% 49% 30
B C B B 0
o.
Example 95 46 15 A A , 60% 51% 20
A C A B
Example 96 _30 21 A B , 56% 48% 40
B C B B
Example 97 65 26 A B 57% 47% 30 A
C B B
Example 98 42 19 A B 53% 50% 30 A
C B B
_
Example 99 72 21 A B 52% 49% 30 A
C B B
Example 100 33 10 A B 56% 32% 40 B
c B B
Example 101 43 22 A B 52% 52% 20 A
C B B
Example 102 40 24 A B 58% 42% 20 A
C B B
Example 103 43 16 A B 57% 48% 30 B
C B B
Example 104 41 17 A B 54% 34% 30
A C B B
Example 105 40 21 A B 57% 33% 30 A
C B B
Example 106 40 11 A B 55% 45% 30 A
C B B
CA 02672854 2013-12-04
,
. 61
_
INDUSTRIAL APPLICABILITY
The metal surface treatment liquid for cation
electrodeposition coating of the present invention is
applicable to metal base materials, such as automobile bodies
and parts to be subjected to cation electrodeposition.