Note: Descriptions are shown in the official language in which they were submitted.
=
CA 02673193 2014-01-15
51440-122
TITLE OF THE INVENTION
HOMOGENEOUS PASTE AND GEL FORMULATIONS
FIELD OF THE INVENTION
This invention provides for oral homogeneous veterinary pastes and gels which
are
used in treating, controlling and preventing of endo- and ectoparasite
infections in warm-
3 0 blooded animals, such as birds, horses and household pets. This
invention further provides
for a process of preparing these veterinary pastes and gels and for a method
for increasing the
bioavailability of the agents contained in the paste or gel in the warm-
blooded animal.
-
CA 02673193 2014-01-15
=
51440422
BACKGROUND OF THE INVENTION
= Therapeutic agents are administered to animals by a variety of routes.
These routes
include, for example, oral ingestion, topical application or parental
administration. The
particular route selected by the practitioner depends upon factors such as the
physiochemical
properties of the pharmaceutical or therapeutic agent, the condition of the
host, and economic
factors.
For example, one method of formulating a therapeutic agent for oral, topical,
dermal
or subdermal administration is to formulate the therapeutic agent as a paste
or as an injectable
formulation and reference is made to US application Ser. No. 09/504,741, filed
February 16,
2000, now U.S. Patent 6,787,342, entitled IMPROVED PASTE FORMULATIONS or to
Ser. No. 09/346,905, filed July 2, 1999, now U.S. Patent 6,239,112; Ser. No.
09/112,690,
filed July 9, 1999 now U.S. Patent 5,958,888 and Ser. No 09/152,775, filed
September 14,
1998, now U.S. Patent 6,174,540, entitled LONG ACTING INJECTABLE
FORMULATIONS CONTAINING HYDROGENATED CASTOR OIL.
Other methods include placing the therapeutic agent in a solid or liquid
matrix for oral delivery.
An important area in veterinary science in the control of endo- and
ectoparasites in
warm-blooded animals, such as equine animals and domestic pets. Infections of
parasites,
including cestodes and nematodes, commonly occur in animals such as horse,
donkeys,
mules, zebras, dogs, cats. Various classes anthelmintic agents have been
developed in the art
to control these infections; see, e.g., U.S, Patents 3,993,682 and 4,032,655,
which disclose
phenylguanidines as anthelmintic agents. Further, the art recognizes that it
is advantageous
to administer combinations of two or more different classes of anthelmintic
agents in order to
improve the spectrum of activity; see, e.g., product disclosure for RMO
Parasiticide-10, an
anthelmintic paste comprising febantel and praziquantel.
Macrolide anthelmintic compounds are known in the art for treating endo- and
ectoparasite infections in warm-blooded animals. Compounds that belong to this
class of
agents include the avermeetin and milbemyein series of compounds. These
compounds are
potent antiparasitic agents against a wide range of internal and external
parasites.
Avermectins and milbemycins share the same common 16-membered macrocyclic
lactone
ring; however, milbemycins do not possess the disaccharide substituent on the
13-position of
the lactone ring. In addition to treating parasitic insects, such as flies,
avermectins and
2
CA 02673193 2014-01-15
51440-122
milbemycins are used to treat endoparasites, e.g., round worm infections, in
warm-blooded
animals.
The avermectin and milbemycin series of compounds either are natural products
or
are semi-synthetic derivatives. The natural product avermectins are disclosed
in U.S. Patent
4,310,519 to Albers-Schonberg, et al., and the 22,23-dihydro avermectin
compounds are
disclosed in Chabala, et al., U.S. Patent 4,199,569. For a general discussion
of avermectins,
which include a discussion of their uses in humans and animals, see
"Ivermectin and
Abamectin," W.C. Campbell, ed., Springer-Verlag, New York (1989). Naturally
occurring
milbemycins are described in Aoki et al., U.S. Patent 3,950,360 as well as in
the various
references cited in "The Merck Index" 12" ed., S. Budavari, Ed., Merck & Co.,
Inc.
Whitehouse Station, New Jersey (1996). Semisynthetic derivatives of these
classes of
compounds are well known in the art and are described, for example, in U.S.
Patent
5,077,308, U.S. Patent 4,859,657, U.S. Patent 4,963,582, U.S. Patent
4,855,317, U.S. Patent
4,871,719, U.S. Patent 4,874,749, U.S. Patent 4,427,663, U.S. Patent
4,310,519, U.S. Patent
4,199,569, U.S. Patent 5,055,596, U.S. Patent 4,973,711, U.S. Patent
4,978,677, and U.S.
Patent 4,920,148.
Avermectins and milbemycins are ineffective against cestodes, such as
tapeworms,
which also are a common parasite in warm-blooded animals (see, U.S. Patent
6,207,179). Of
particular importance in the industry is the treatment of equine tapeworms, in
general, and
Anoplacephala perfoliata, in particular (see, e.g., U.S. Patent 6,207,179 or
U.S. Patent
5,824,653). In order to treat cestode (and trematode) infections in warm-
blooded animals, it =
is know, to administer 2-acy1-4-oxo-pyrazino-isoquinoline derivatives to the
animal (see, e.g.,
U.S. 4,001,441). A compound of this class that is often
used to treat cestode and nematode infections is praziquantel, which has the
following
structure:
140
Wo
ITD
As mentioned above, often it is beneficial to administer a formulation that
contains a
combination of two or more anthelmintics, which possess different activity, in
order to obtain
a composition with a broad spectrum of activity. Further, the combination
allows the user to
3
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
administer one formulation instead of two or more different formulations to
the animal.
Formulations which administer a combination of two or more anthelmintics are
know in the
art. These formulations may be in the form of solutions, suspensions, pastes,
drenches or
pour-on formulations (see, e.g., U.S. Patent 6,165,987 to Harvey or U.S.
Patent 6,340,672 to
Mihalik). For example, U.S. Patent 4,468,390 to Kitano and U.S. Patent
5,824,653 to Beuvry
et al. describe anthelmintic compositions for treating nematode and cestode
infections in
animals, such as horses, that comprise an avermectin or a milbemycin and an
isoquinoline
compounds, such as praziquantel, to the animal. In these formulations, the
avermectin or
milbemycin compound and the isoquinoline compound are not dissolved in a
solvent, which
is then dispersed in a semi-solid matrix. Similarly, U.S. Patent 6,207,179 to
Mihalik relates
to anthelmintic paste formulations wherein the avermectin or milbemycin is
dissolved in a
non-aqueous liquid and pyrantel or morantel, compounds which are in the same
class as
praziquantel, but are said in the art to be far less effective as
praziquantel, are suspended in
the liquid. These prior patents do not describe a formulation wherein the both
the
praziquantel and the avermectin or milbemycin are dissolved in a solvent and
then dispersed
in a carrier matrix. U.S. Patent 6,165,987 relates to anthelmintic
formulations containing
praziquantel and at least one avermectin or milbemycin dissolved in glycerol
formal, benzyl
alcohol and N-methyl-2- pyrrolidone, which may be liquids, pastes or drenches;
the amount
of praziquantel administered to the animal is always at a dose of more that
2.0 mg per kg of
body weight. U.S. Patent 6,165,987 provides for pastes which require the
presence of two
solvents, one for the praziquantel and one for the macrolide compound.
Citation or identification of any document in this application is not an
admission that
such document is available as prior art to the present invention.
SUMMARY OF THE INVENTION
The present invention provides for a stable paste or gel formulation for a
wide range
of veterinary and pharmaceutical products. The present invention also provides
for an
improved process to make the inventive paste and gel products.
This invention provides for oral homogeneous veterinary pastes and gels for
the
treating, controlling and preventing of endo- and ectoparasite infections in
warm-blooded
animals, such as birds, horses and household pets, as well as to a process for
preparing these
formulations. The inventive oral veterinary pastes and gels provide for a more
effective
treatment of parasitic infections in non-human animals since the active
ingredients do not
interfere with each other, hence increasing the bioavailability in the animal,
while still having
the benefits of being administered by as a paste or a gel. Further, the
inventive formulations
4
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
provide for a formulation that exhibits good chemical and physical stability
over the shelf-life
of the product. Thus, the oral veterinary formulations of the invention may
exhibit the
benefits of both a solution and a formulation that is a paste or a gel.
In advantageous embodiment, the pharmaceutical or veterinary paste formulation
of
the present invention may comprise:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is a
COX-2 inhibitor,
(b) a colorant, wherein the colorant is titanium dioxide,
(c) an absorbent, wherein the absorbent is magnesium carbonate,
(d) a thickener, wherein the thickener is colloidal anhydrous silica and
(e) a viscosity modifier, wherein the viscosity modifier is PEG
300.
In another advantageous embodiment, the pharmaceutical or veterinary gel
formulation of the present invention may comprise:
(a) an effective amount of a therapeutic agent,
(b) an antioxidant, wherein the antioxidant is sodium ascorbate,
(c) a preservative, wherein the preservative is potassium sorbate,
(d) a flavoring, wherein the flavoring is a peppermint and/or apple flavor,
(e) a buffering system or thickener, wherein the buffering system or
thickener is
sodium carboxymethylcellulose,
(0 optionally a solvent, wherein the solvent is propylene glycol and
(g) optionally a viscosity modifier, wherein the viscosity
modifier is PEG 400.
Advantageously, the therapeutic agent may comprise an avermectin,
advantageously
eprinomectin or ivermectin. In other advantageous embodiments, the therapeutic
agent may
comprise a COX-2 inhibitor, a proton pump inhibitor, advantageously
omeprazole,
praziquantel or any combination thereof, including one or more avermectins.
It is noted that in this disclosure and particularly in the claims and/or
paragraphs,
terms such as "comprises", "comprised", "comprising" and the like can have the
meaning
attributed to it in U.S. Patent law; e.g., they can mean "includes",
"included", "including",
and the like; and that terms such as "consisting essentially of" and "consists
essentially of"
have the meaning ascribed to them in U.S. Patent law, e.g., they allow for
elements not
explicitly recited, but exclude elements that are found in the prior art or
that affect a basic or
novel characteristic of the invention.
These and other embodiments are disclosed or are obvious from and encompassed
by,
the following Detailed Description.
5
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
BRIEF DESCRIPTION OF THE DRAWINGS
The following detailed description, given by way of example, but not intended
to limit
the invention solely to the specific embodiments described, may best be
understood in
conjunction with the accompanying drawings, in which:
FIG. 1 depicts leverage plots of variables and whole model and
FIG. 2 depicts a prediction profiler.
DETAILED DESCRIPTION
The present invention provides for a stable paste or gel formulation for a
wide range
of veterinary and pharmaceutical products. The present invention also provides
for an
improved process to make the inventive paste or gel products. In particular,
the paste or gel
formulation of the present invention provides for lower percentage of active
ingredient with a
resulting increase in the percentage of solvent,
The present invention provides for a pharmaceutical or veterinary paste or gel
formulation comprising:
(a) an effective amount of a therapeutic agent;
(b) optionally, a fumed silica;
(c) a viscosity modifier;
(d) a carrier;
(e) optionally, an absorbent; and
(f) optionally, a colorant, stabilizer, surfactant, or preservative.
This invention also provides for a process for preparing a paste or gel
formulation
comprising the steps of:
(a) dissolving or dispersing the therapeutic agent into the carrier by mixing;
(b) adding the optional fumed silica and absorbent to the carrier containing
the
dissolved therapeutic agent, mixing at low shear (advantageously 300 rpm) and
maintaining
the temperature at about 25 C until the silica and absorbent is dispersed in
the carrier and
(c) adding the viscosity modifier to the intermediate with mixing to produce a
uniform
paste or gel.
The steps are illustrating, but not limiting. For example, step (a) can be
moved to the
last step.
More preferred are pharmaceutical and veterinary pastes or gels comprising:
(a) a therapeutic agent selected from the group consisting of insecticides,
acaricides,
parasiticides, growth enhancers, oil-soluble NSAIDS or a proton pump
inhibitor;
(b) optionally, a fumed silica;
6
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
(c) a viscosity modifier;
(d) an absorbent;
(e) a colorant; and
(f) a carrier which is triacetin, a monoglyceride, a diglyceride, or a
triglyceride.
Also preferred are pastes or gels comprising:
(a) a therapeutic agent selected from the group consisting of avermectins,
milbemycins, nordulisporic acid and its derivatives, estrogens, progestins,
androgens,
substituted pyridyl methyl derivatives, phenylpyrazoles, COX-2 inhibitors or 2-
(2-
benzimidazoly1)-pyrimidine derivatives;
(b) optionally, a fumed silica;
(c) a viscosity modifier;
(d) an absorbent;
(e) a colorant; and
(f) a hydrophilic carrier which is triacetin, a monoglyceride, a diglyceride,
or a
triglyceride.
The above compositions wherein the fumed silica is a colloidal silicon dioxide
such as
CAB-O-SIL (Cabot, TD11) or AEROSIL (Degussa, Technical Bulletin Pigments, No.
11 and
No. 49), the viscosity modifier is a polyethylene glycol such as PEG 200, PEG
300, PEG
400, PEG 600, monoethanolamine, triethanolamine, glycerol, propylene glycol,
polyoxyethylene (20) sorbitan mono-oleate (polysorbate 80 or Tween 80),
polyoxamers (e.g.,
Pluronic L 81); the absorbent is magnesium carbonate, calcium carbonate,
starch, or cellulose
and its derivatives; and the colorant is titanium dioxide iron oxide, or FD&C
Blue #1
Aluminum Lake are most especially preferred.
In an advantageous embodiment, the fumed silica is a colloidal silicon dioxide
such as
CAB-O-SIL (Cabot, TD11), advantageously at a concentration of 5% w/w, the
viscosity
modifier is PEG 400 and the absorbent is magnesium carbonate.
In an advantageous embodiment, the viscosity modifier is PEG 300.
Advantageously,
the PEG 300 is at a concentration of about 0.4% w/w.
Advantageously, the absorbent is magnesium carbonate, in particular a heavy
magnesium carbonate. In an advantageous embodiment, the magnesium carbonate is
at a
concentration of about 2% w/w.
In another advantageous embodiment, the colorant is titanium dioxide, in
particular
E171 titanium dioxide. Advantageously, the titanium dioxide is at
concentration of about
0.2% w/w.
7
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Advantageously, the carrier is triacetin. In an advantageous embodiment, the
triacetin
is at a concentration of up to about 100% w/w depending on the volume of the
active
substance and other excipients.
The therapeutic agents which are used in the inventive formulations are those
which
are known to the practitioner as agents which may be formulated as pastes or
gels. Classes of
therapeutic agents contemplated by the inventive formulations include
insecticides,
acaricides, parasiticides, growth enhancers, oil-soluble, nonsteroidal anti-
inflammatory drugs
(NSAIDS), proton pump inhibitors and antibacterial compounds. Specific classes
of
compounds which fall within these classes include, for example, avermectins,
milbemycins,
nodulisporic acid and its derivatives, estrogens, progestins, androgens,
substituted
pyridylmethyl derivatives, phenylpyrazoles, COX-2 inhibitors, 2-(2-
benzimidazoly1)-
pyrimidines derivatives, depsipeptides (such as emodepside) and macrolide
antibiotics.
In an advantageous embodiment, the therapeutic agent is a non-steroidal anti-
inflammatory drug (NSAID), in particular a COX-2 inhibitor. In a particularly
advantageous
embodiment, the COX-2 inhibitor is firocoxib. In a most advantageous
embodiment, the
firocoxib is at a concentration of about 0.82% w/w.
Firocoxib is a veterinary COX-2 inhibitor (anti-inflammatory and anti-
arthritic) with a
primary application in canines. Exemplary patents include, but are not limited
to, U.S. Patent
No, 6,677,373, which covers polymorphic Form B of 3-(cyclopropylmethoxy)-4-[4-
(methylsulfonyl)pheny1]-5,5-dimethy1-5H-furan- 2-one and U.S. Patent No.
5,981,576, which
covers the compound 3-(cyclopropylmethoxy)-444-(methylsulfonyl)pheny1]-5,5-
dimethy1-
5H-furan- 2-one. See also, e.g., AU0079218A5, AU0774172B2, AU0774172C,
AU4206979AA, CA2386549AA, EP1090915A1, JP2003511443T2, U520030050337A1,
U56677373 and W00127097A1 and AT0304537E, AU0077944A5, AU0773221B2,
CA2386807AA, DE60022663CO3 DE60022663T2, DK1218366T3, EP1218366A2,
EP1218366B1, EP1218366B8, E52249299T3, FR2799462A1, FR2799462B1,
JP2003511444T2, U520030028036A1, U56541646, U56541646B2, W00127098A2 and
W00127098A3.
The present invention further encompasses other COX-2 inhibitors in addition
to
firocoxib.
The term "cyclooxygenase-2 inhibitor" means any pharmaceutically acceptable
compound or combination of compounds, including salts, tautomers and prodrugs
of such
compound or compounds, that inhibits the enzyme cyclooxygenase-2 in the
arachidonic
acid/prostaglandin pathway. The specific cyclooxygenase-2 inhibitor or
inhibitors used in the
8
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
food composition-are not narrowly critical so long as the inhibitor or
inhibitors are
pharmaceutically acceptable and are compatible with the specific processing
conditions
selected.
The cyclooxygenase-2 inhibitors employed in this invention include, but are
not
limited to, the compounds corresponding to the structural formula:
R4
0
If
r,
1111 A
NN. 3
wherein A is a 5- or 6-member ring substituent selected from partially
unsaturated or
unsaturated heterocyclo and carbocyclic rings;
wherein Rl is cyclohexyl or phenyl optionally substituted with one, two or
three
radicals selected from C1_2 alkyl, C1_2 haloalkyl, cyano, carboxyl, Ci_2
alkoxycarbonyl,
hydroxyl, c12 hydroxyalkyl, c12 haloalkoxy, amino, c12 alkylamino,
phenylamino, nitro,
C1_2 alkoxy-C1_2 alkyl, C1_2 alkylsulfinyl, halo, C1_2 alkoxy and C1_2
alkylthio;
wherein R2 is methyl or amino;
wherein R3 is a radical selected from halo, C1_2 alkyl, alkenyl, alkynyl, oxo,
cyano,
carboxyl, cyanoalkyl, heterocyclyloxy, C1_2 alkyloxy, alkylthio,
alkylcarbonyl, cycloalkyl,
phenyl, C1_2 haloalkyl, heterocyclo, cycloalkenyl, phenylalkyl,
heterocyclylalkyl,
alkylthioalkyl, C1_2 hydroxyalkyl, alkoxycarbonyl, phenylcarbonyl,
phenylalkylcarbonyl,
phenylalkenyl, alkoxyalkyl, phenylylthioalkyl, phenylyloxyalkyl,
phenylalkylthioalkyl,
phenylalkoxyalkyl, alkoxyphenylalkoxyalkyl, alkoxycarbonylalkyl,
aminocarbonyl,
aminocarbonylalkyl, alkylaminocarbonyl, N-phenylaminocarbonyl, N-alkyl-N-
phenylaminocarbonyl, alkylaminocarbonylalkyl, carboxyalkyl, alkylamino, N-
arylamino, N-
aralkylamino, N-alkyl-N-aralkylamino, N-alkyl-N-arylamino, aminoalkyl,
alkylaminoalkyl,
N-phenylaminoalkyl, N-phenylalkylaminoalkyl, N-alkyl-N-phenylalkylaminoalkyl,
N-alkyl-
N-phenylaminoalkyl, phenyloxy, phenylalkoxy, phenylthio, phenylalkylthio,
alkylsulfinyl,
alkylsulfonyl, aminosulfonyl, alkylaminosulfonyl, N-phenylaminosulfonyl,
phenylsulfonyl
and N-alkyl-N-phenylaminosulfonyl; and
wherein R4 is hydrido or fluoro;
or a pharmaceutically-acceptable salt thereof
9
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
A class of cyclooxygenase-2 inhibitors of particular interest consists of
those
compounds with the structural formula:
R4
0
"
R
re-S ------------------------------ =
k\ ........................................
0 3
wherein A is a 5- or 6-member ring substituent selected from partially
unsaturated or
unsaturated heterocyclo and carbocyclic rings selected from the group
consisting of thienyl,
oxazolyl, furyl, pyrrolyl, thiazolyl, imidazolyl, benzofuryl, indenyl,
benzothienyl, isoxazolyl,
pyrazolyl, cyclopentenyl, cyclopentadienyl, benzindazolyl,
benzopyranopyrazolyl, phenyl,
and pyridyl; wherein Rl is cyclohexyl or phenyl optionally substituted with
one, two or three
radicals selected from C1_2 alkyl, halo, and C1_2 alkoxy; wherein R2 is methyl
or amino;
wherein R3 is a radical selected from halo, C1_2 alkyl, oxo, cyano, carboxyl,
Ci_2 alkyloxy,
phenyl, C1_2 haloalkyl, and C1_2 hydroxyalkyl; and wherein R4 is hydrido or
fluoro; or a
pharmaceutically-acceptable salt thereof.
In another advantageous embodiment, the COX-2 inhibitor is 3-
(cyclopropylmethoxy)-5,5-dimethy1-4-[4-(methylsulfonyl)pheny1]-2(5H)-furanone
with a
CAS number of 189954-96-9 which has the structure:
0 0
./"F\ SO2Me
Other COX-2 inhibitors that may be employed in the present invention include,
but
are not limited to, lomoxicam, 1,5-dipheny1-3-substituted pyrazoles,
radicicol, N-benzy1-3-
indoleacetic acids, the inhibitors of GB-02283745, TP-72, indene inhibitors of
COX-2,
carbocyclic diarylmethylene derivatives, 1,2-diarylindole, 1,2-
bisarylcyclobutene derivatives,
novel stilbene derivatives as prodrug forms of the diphenylcyclopentenonees of
US-
05474995, WO-09500501 and WO-09518799, 2,4-diphenylbutenoic acid derivatives
as
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
prodrugs of COX-2 inhibitors claimed in US-05474995, WO-09500501 and WO-
09518799,
1-(4-chlorobenzoy1)-3-[4-(4-fluorophenyl)thiazol-2-ylmethyl]-5-methoxy-2-
methylindole,
sulfonamide substituted diarylthiazole, 4-(4-cyclohexy1-2-methyloxazol-5-y1)-2-
fluorobenzenesulfonamide, 5,6-diarylthiazolo[3,2-B][1,2,4]triazolo,
indometacin-derived
indolalkanoic acid, 1-methylsulfony1-441,1-dimethyl-4-(4-
fluorophenyl)cyclopenta-2,4-dien-
3-yl]benzene, 4,4-dimethy1-2-phenyl-3-[4-(methylsulfonyl)phenyl]cyclobutenone;
1,2-
diarylcyclobutenes, 2-(4-methoxypheny1)-4-methyl-1-(4-sulfamoylphenyl)pyrrole;
1,2-
diphenylpyrrole derivatives, tetrahydrofuranones, N-[5-(4-
fluoro)phenoxyl]thiophene-2-
methanesulfonamide, 5(E)-(3,5-di-tert-buty1-4-hydroxy)benzylidene-2-ethy1-1,2-
isothiazolidine-1,1-dioxide, 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-
1-
benzopyran-4-one, benzenesulfonamide, 4-(5-(4-methylpheny1)-3-
(trifluoromethyl)-1H-
pyrazole-1-y1)-, 2H-1,2-benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-(5-
methy1-2-
thiazoly1)-,1,1-dioxide-, methanesulfonamide, N-(4-nitro-2-phenoxyphenyl) and
methanesulfonamide, N-(4-nitro-2-phenoxyphenty1).
Other COX-2 inhibitors that may be employed in the present invention include,
but
are not limited to, the COX-2 inhibitors disclosed in AT 9700165, AU 9719132 ,
CA
2164559, DE 19518421, DE 19533643, DE 19533644, DE 19753463, EP 714895, EP
799823 , EP 832652, EP 846689, EP 850894, EP 850895, EP 95909447.5, EP
95928164.3,
FR 2751966, GB 2283745, GB 2294879, GB 2319772, GB 2320715, JP 08157361, JP
09048769, JP 09071656, JP 09071657, JP 09077664, JP 09194354, JP 09221422, JP
10175861, PCT Application US97/05497, PCT Application US98/07677, SOFRC
95/1107,
US 5380738, US 5380738, US 5393790, US 5418254, US 5420343, US 5434178, US
5466823, US 5474995, US 5486534, US 5504215, US 5508426, US 5510368, US
5510496,
US 5516907, US 5521207, US 5547975, US 5563165, US 5565482, US 5576339, US
5580985, US 5596008, US 5604260, US 5616458, US 5616601, US 5620999, US
5633272,
US 5643933, US 5663180, US 5663195, US 5668161, US 5670510, US 5670532, US
5672626, US 5677318, US 5677318 , US 5681842, US 5686460, US 5686470, US
5696143,
US 5719163, US 5719163, US 5733909, US 5736579, US 5739166, US 5753688, US
5753688, US 5756530, US 5760068, US 5776967, US 5783597, US Application
08/004/822,
US Application 08/237739, US Application 08/387680, US Application 08/424979,
US
Application 08/425022, US Application 08/425029, US Application 08/457902, US
Application 08/464722, US Application 08/540522, US Application 08/541850, US
Application 08/647911, US Application 08/702417, US Application 08/765,865, US
Application 08/765865, US Application 08/776090, US Application 08/776358, US
11
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Application 08/801768, US Application 08/809318, US Application 08/809475, US
Application 08/822528, US Application 08/849069, US Application 08/894102, US
Application 08/894124, US Application 08/908554, US Application 08/945840, US
Application 08/952156, US Application 08/952661, US Application 08/957345, US
Application 08/969953, US Application 08/987356, US Application 08/992327, US
Application 09/005610, US Application 09/062537 , US Application 09/101493, US
Application 60/032688, US Application 60/044485, WO 9413635, WO 9414977, WO
9420480, WO 9426731, WO 9427980, WO 9427980, WO 9500501, WO 9511883, WO
9515316, WO 9515318, WO 9521817, WO 9530652, WO 9603385, WO 9603385, WO
9603385, WO 9603387, WO 9603387, WO 9603388, WO 9603392, WO 9606840, WO
9608482, WO 960923, WO 9609293, WO 9609304, WO 9609304, WO 9609304, WO
9611676, WO 9612483, WO 9613483, WO 9616934, WO 9619462, WO 9619462, WO
9619463, WO 9619463, WO 9619469, WO 9621667, WO 9623786, WO 9624584, WO
9624585, WO 9624585, WO 9624604, WO 9625405, WO 9625405, WO 9625928, WO
9626921, WO 9631509, WO 9636617, WO 9636623, WO 9637467, WO 9637469, WO
9638418, WO 9638442, WO 9638442, WO 9639144, WO 9640143, WO 9641625 , WO
9641626, WO 9641626, WO 9641645, WO 9703667, WO 9703953, WO 9709977, WO
9710840, WO 9711701, WO 9711701, WO 9711704, WO 9713755, WO 9713767, WO
9714679, WO 9714691, WO 9716435, WO 9725045, WO 9725046, WO 9725047, WO
9725048, WO 9727181, WO 9727181, WO 9728120, WO 9728121, WO 9729774, WO
9729775, WO 9729776, WO 9730030, WO 9731631, WO 9734882, WO 9736497, WO
9736863, WO 9737984, WO 9738686, WO 9738986, WO 9740012, WO 9744027, WO
9744028, WO 9745420, WO 9746524, WO 9746532, WO 9800416, WO 9803484, WO
9804527, WO 9805639, WO 9806708, WO 9806715, WO 9807425, WO 9807714, WO
9811080, WO 9815528, WO 9816227, WO 9816227, WO 9817292, WO 9821195, WO
9821195, WO 9822101, WO 9822101, WO 9822104, WO 9822442, WO 9822457, WO
9824782, WO 9825896 and ZA 9704806.
Still more advantageous COX-2 inhibitors are selected from the group
consisting of:
12
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
1)
0
R i
C N
-,,,,,.
..,---
2)
NH2
i
0=0
N- \\
-....,..
1 ,---'
C.I ;
3)
H2N 0.2S
h CH3
N
i \
CF3
;
4)1
NHSO2CH3
1
1
1 _
..--"" "-----
NO2
;
5)
13
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
H2N025.
\ / \
....
--....õ.
III \\
N
6)
CH3
1
0=S
F
i 0
F' ------i ..
,
7)
NHSO2CH3 F
So ,S
( F
\¨.
8)
r, 0
I
'S
4.1 \\,,
0 0
;
14
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
9)
1
0
WI(
0
........,
1
H2N, ---
S.--'
it \\
,
10)
ON_
1
,
-N
1 )----'-'-'
j)
0 0 F
i
1 1)
H3C0aS
...--
/
\ ---->
\
---,_
¨
. 0 ,
12)
L\--FFIN't
Ft2N,,s,
1110
=
0 0 , ;
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
13)
0 0
1 4)
CH3
0=S
0
,N
3
15)
NH2
0
CF.
I
16)
41111`-,
N
CF
H2Nõ,
0 0
16
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
17)
NH2
1
0=--S
0
1
\- ,N
N \
F"- -----:;
;
18)
NHSO2CH3
NO2'
19)
NHS02C1-13 F
,
20)
NHSO2cH3
a
,s,=o
H2N \\
o ;
17
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
21)
/-
22)
fiHso,cH3
-4j '111
F
23)
P,,IHSO2CH3 CH3
24)
CH3
H3 CH
3
a
\\I
0
25)
NHSO2C H3
1,!)
0 CF13
0
18
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
26)
NHSO2CH3 Cr
CI
,S=0
H7N
0
'27)
CH1
Mk ID
28)
H2Nozs.
cHF2
19
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
29)
C F3
N
H3C '0
30)
C F3
N
H2N
31)
N
0
H2N, C1120H
0 0
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
32)
''"`----;>-
I
H2Ns, ..-----õ,,-,--,-----'
=S
0 0 ,
,
13)
1
-- N
,-- \
0
CF2H
00 ;
34)
NH2
i
0=is
01 lip
N.õ
1
iso ----`
;
35)
CH3
1
0=5
......õ, so
....õ,..
..
,
36)
21
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Nt 112
0=S
o' !
-,õ
1
1
;
37)
0
a 0
...--- OH
0 CF3
CI .
,
38)
NHSO2CH3
1-iN-5-0
1\
0 ;
39)
cp
1 4:
..,,,,..
0 CF3
C1 ;
40)
0
4111
ci 11
0 CF3
M ;
22
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
41)
0
----- t ---- NH2
f
---õ,
0 BF
CI ,,
,
42)
0
ri---)
In another advantageous embodiment, the cyclooxygenase-2 inhibitors are
selected
from the group consisting of:
(1) the compound 4-[5-(4-methylpheny1)-3-(trifluoromethyl)-1H-pyrazole-1-
ylThenzenesulfonamide which has the structure:
.H2N 0
\s,
L.,,,,,,,,
j¨CF3
I :
:
;
(2) the compound 4-[5-((3-fluoro-4-methoxy)pheny1)-3-(difluoromethyl)-1H
pyrazole-1-yl]benzenesulfonamide which has the structure:
23
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
H 2 N 0
OF
I ft
i I
i CF 2H
1 *,-).õ,,--- -------/
F ;
(3) the compound 445-(4-chloropheny1)-3-(trifluoromethyl)-1H-pyrazole-1-
ylThenzenesulfonamide which has the structure:
Ralkl 0
\ --,
S
--=,,,,.õ,,,,,¨,N, _,õ.-N
N Nksõ
'
--õ,...
,
(4) the compound that has the structure:
0
Ç..,
---, OH
00
0 CF 3
CI ;
(5) the compound 4-[4-(methylsulfonyl)pheny1]-3-(4-(methoxy)phenyl)furan-2(5H)-
one which has the structure:
24
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Ha
, -,,,...-.'"-="")
0
It
i I i
,
0 ;
and (6) the compound 445-methy1-3-phenylisoxazol-4-ylThenzenesulfonamide,
which
has the structure:
H-44 0
$
47
0
LNõ pia
..---- \
p
---, /
0------ ----õ, N
1
Compounds which inhibit gastric acid secretion in the stomach or act as proton
pump
inhibitors are well known to the practitioner and are also provided for in the
present
invention. These compounds include 2-(2-benzimidazolyl-pyridines) and their
salts. Such
compounds are described, for example in EP 005 129, U.S. Pat. No. 4,255,431 as
well as in
U.S. Pat. No. 5,629,305. These compounds are also known to treat Helicobacter
infections,
U.S. Pat. No. 5,093,342, and to act as synergists when combined with an acid
degradable
antibiotic, see e.g. U.S. Pat. No. 5,629,305. These synergistic combinations
may also be
formulated in the pastes of the present invention. Omeprazole or its salts is
an especially
preferred compound.
The proton pump inhibitors used in the present invention can include compounds
of
the general formula:
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
0 N ...........................................
q 0 A
R
NH
wherein Ra is
R2 0 C H
0
R
1111
0
S N o r
N
,
where Rl and R3 are independently selected from from hydrogen, lower alkyl,
lower
alkoxy and halogen, R2 is selected from hydrogen, lower alkyl, lower alkoxy-
lower alkoxy,
lower fluoralkoxy and
0
( )
N
I
R4 and R5 are independently selected from lower alkyl,
A is
R6
..,..,..
is 0 C H3
0.- .
N
R7
R6 and R7 are independently selected from hydrogen, lower alkyl, lower alkoxy,
lower
fluoroalkoxy, lower fluoroalkyl, halogen,
0 N.....
11
r)
wherein R8 is lower alkyl or lower alkoxy.
Examples of proton pump inhibitors include:
26
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
CH
CH3 CH
0
*
0 N
N CH2¨!--1. NH mepra zole
7'
OCH2C F3
clxC 113
0 0 N
it _IL
N
CH,- Lansoprazole
NH
QC H3
-
cix0 C H3
C H F2
rµ 1
cP
n ......i.
N 11
1...,H2---b NH ,..õ--- Pantprazole
õ
C H2-0CH-4
/
CH,
cix _
CH
II
N
NH 7
11 II 1
NH 7 - Leminoprazoie
C H3¨Ns'C H2
I '
C ,,
CHI' 'C H3
27
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
4.
Or H3
0
a\ .
......70
0 Nu
11 S
N CH-1-S --ILNP, S-4216
Examples of other PPI include esomeprazole (nee: perprazole), rabeprazole, and
IY-
81149 (distributed by Axican Pharma).
The preferred proton pump inhibitor used in the present invention is the
compound
known as omeprazole.
The proton pump inhibitors used in the present invention are known compounds
in the
art. and methods for their preparation may be found in the literature. For
example,
omeprazole is disclosed in EP 5129, lansoprazole in EP 174.716, pantoprazole
in EP 166,287,
leminoprazole in GB 2,163,747, rabeprazole in U.S. Pat. No. 5,045,552.
The present invention also provides for an oral homogeneous anthelmintic
veterinary
paste or gel, for the treating, controlling and preventing of endo-and
ectoparasite infections in
warm-blooded animal, which comprises an anthelmintic agent, such a
praziquantel, and/or
pyrantel and, as a second agent, at least one macrolide anthelmintic agent, a
solvent which
dissolves both the first anthelmintic agent and the macrolide anthelmintic
agent, and a
thickening agent.
More specifically, this invention provides for an oral homogeneous veterinary
paste or
gel consisting essentially of praziquantel and/or pyrantel and at least one
macrolide
anthelmintic compound, a solvent, which dissolves both the praziquantel and/or
pyrantel and
the macrolide anthelmintic compound, and at least one thickening agent.
Preferred are oral
homogeneous veterinary pastes or gels consisting essentially of praziquantel
and/or pyrantel
and at least one macrolide anthelmintic compound, a solvent, which dissolves
both the
praziquantel and/or pyrantel and the macrolide anthelmintic compound, at least
one
thickening agent, and at least one viscosity modifier. Another embodiment of
the invention is
an oral veterinary composition consisting essentially of the inventive oral
homogeneous
veterinary pastes or gels and an opacifier.
28
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
The inventive oral homogeneous veterinary pastes or gels provide for the
combination
of at least two different anthelmintic agents, one of which is a macrolide
anthelmintic
compound. The classes of compounds encompassed by the first agent are well
known to
practitioners in this art. These compounds include, in addition to
praziquantel and its related
compounds, anthelmintic agents such as pyrantel (see, U.S. Patent 3,502,661
for a description
of pyrantel and its related compounds).
The invention provides for an oral homogeneous veterinary paste or gel
consisting
essentially of praziquantel and/or pyrantel and at least one macrolide
anthelmintic compound,
a solvent, which dissolves both the praziquantel and/or pyrantel and the
macrolide
anthelmintic compound, at least one thickening agent, and at least one
viscosity modifier. In
a preferred embodiment, the macrolide anthelmintic compound is selected from
the group
consisting of doramectin, abamectin, moxidectin, selamectin and ivermectin;
the solvent is
glycerol formal, propylene glycol, n-methylpyrrolidone, or dimethyl sulfoxide;
the thickening
agent is selected from the group consisting of a cellulose, a starch,
monothioglycerol,
polymers or copolymers of polyvinylpyrrolidone, polymers and copolymers of
(meth)acrylate, and a natural gum; and the viscosity modifier is selected from
the group
consisting of vegetable oils, or hydrogenated vegetable oils. In a preferred
embodiment, the
thickening agent is hydroxypropylcellulose, xanthum gum or hydroxyethyl starch
and the
viscosity modifier is hydrogenated castor oil, corn oil or olive oil.
The macrolide anthelmintic compounds contemplated in this invention are also
well
known to a practitioner of this area. These compounds include avermectins and
milbemycins, some of which are discussed above. Non-limiting examples of
compounds
belonging to this class are represented by the following structure:
29
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Ri
CH3 22
R4
13
n R2
0
OH
0 ___________________________________
CH3
R3
where the broken line indicates a single or a double bond at the 22,23-
positions;
R1 is hydrogen or hydroxy provided that R1 is present only when the broken
line
indicates a single bond;
R2 is alkyl of from 1 to 6 carbon atoms or alkenyl of from 3 to 6 carbon atoms
or
cycloalkyl of from 3 to 8 carbon atoms;
R3 is hydroxy, methoxy or = NOR5 where R5 is hydrogen or lower alkyl; and
R4 is hydrogen, hydroxy or
OCH3
R6
OCH3
___________________________________________ C)
__3_
0
H3C
where R6 is hydroxy, amino, mono-or di-lower alkylamino or lower
alkanoylamino.
The preferred compounds are avermectin Bla/Blb (abamectin), 22,23-dihydro
avermectin Bla/Blb (ivermectin) and the 4"-acetylamino-5-ketoximino derivative
of
avermectin Bla/Blb. Both abamectin and ivermectin are approved as broad
spectrum
antiparasitic agents. The structures of abamectin and ivermectin are as
follows:
CA 02673193 2009-06-18
WO 2008/077130 PCT/US2007/088225
OCH3
(io_
OCH3
H
0 R1
H3C 0
2CH3
CH3 22 _.-'
0.______
c/0 0
".
H3 CD2X
T4 1 R2
÷3.._r
I 0 0
L OH
1
0------r
CH3
OH
wherein for abamectin the broken line represents a double bond and R1 is not
present and for
ivermectin the double bond represents a single bond and R1 is hydrogen; and R2
is isopropyl
or sec-butyl.
The 4"-acetyl amino-5-ketoximino derivatives of avermectin Bla/Blb has the
following structural formula:
0 OCH3
043¨ H -N
OCH3
_________________________ CD
H3C 0
CH3
CH3 22
H3 0 0.._______
c/ 0
'.
O'X
R2
H3C .'..''''.')..,..,....
1 0
..--.- 0
IOH
r-------- 1
0 5
31 r\CH3
N_
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
where R2 is isopropyl or sec-butyl.
The avermectin products are generally prepared as a mixture of at least 80% of
the
compound where R2 is sec-butyl and no more than 20% of the compound where R2
is
isopropyl.
Other preferred avermectins, include emamectin, eprinomectin and doramectin.
Doramectin is disclosed in U.S. Patent 5,089,490 and EP 214 738. This compound
has the
following structure:
OCH3
Holl11,........-1,,,
OCH3
0 ()441/4.
H3C H
CH3
CH3...---
=='µµ'µ -
H
______________________________________ 0
T
H3c 0 0 H :
0 E
oo'
17
H3C
1 0 0
OH
= H
0 .
CH3
H
OH
In the present formulations, ivermectin is especially preferred.
A representative structure for a milbemycin is that for milbemycin al:
32
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
CH3 = 3
=
7 0 I
`N
9 =
= `0**.H \ 33
H3Cµµµ ,1H3
0= col
CH3
(LH
t
H3Cs CH3 CH3
o 11
0
1100
H CH3
0 =
CH3
OH
An especially preferred milbemycin is moxidectin, whose structure is as
follows:
The compound is disclosed in U.S. Patent No. 5,089,490.
The monosaccharide avermectin derivatives are also preferred especially where
an
oxime substitution is present on the 5-position of the lactone ring. Such
compounds are
described, for example, in EP 667,054. Selamectin is an especially preferred
compound of
this class of derivatives.
This application contemplates all pharmaceutically or veterinary acceptable
acid or
base salts forms of the anthelmintic compounds, where applicable. The term
"acid"
contemplates all pharmaceutically or veterinary acceptable inorganic or
organic acids.
Inorganic acids include mineral acids such as hydrohalic acids, such as
hydrobromic and
hydrochloric acids, sulfuric acids, phosphoric acids and nitric acids. Organic
acids include all
pharmaceutically or veterinary acceptable aliphatic, alicyclic and aromatic
carboxylic acids,
dicarboxylic acids tricarboxylic acids and fatty acids. Preferred acids are
straight chain or
branched, saturated or unsaturated Ci-C20 aliphatic carboxylic acids, which
are optionally
substituted by halogen or by hydroxyl groups, or C6-C12 aromatic carboxylic
acids.
Examples of such acids are carbonic acid, formic acid, fumaric acid, acetic
acid, propionic
acid, isopropionic acid, valeric acid, a-hydroxy acids, such as glycolic acid
and lactic acid,
chloroacetic acid, benzoic acid, methane sulfonic acid, and salicylic acid.
Examples of
33
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
dicarboxylic acids include oxalic acid, malic acid, succinic acid, tataric
acid and maleic acid.
An example of a tricarboxylic acid is citric acid. Fatty acids include all
pharmaceutically or
veterinary acceptable saturated or unsaturated aliphatic or aromatic
carboxylic acids having 4
to 24 carbon atoms. Examples include butyric acid, isobutyric acid, sec-
butyric acid, lauric
acid, palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid,
and phenylsteric acid.
Other acids include gluconic acid, glycoheptonic acid and lactobionic acid.
The term "base" contemplates all pharmaceutically or veterinary acceptable
inorganic
or organic bases. Such bases include, for example, the alkali metal and
alkaline earth metal
salts, such as the lithium, sodium, potassium, magnesium or calcium salts.
Organic bases
include the common hydrocarbyl and heterocyclic amine salts, which include,
for example,
the morpholine and piperidine salts.
The ester and amide derivatives of these compounds, where applicable, are also
contemplated. Specific compounds which belong to this class of macrolide
antiparasitic
agents are well known to the practitioner of this art.
The solvents provided for in the inventive homogeneous pastes or gels are
those polar
solvent that will dissolve both the first anthelmintic agent and the macrolide
anthelmintic
compound. These solvents include, for example, glycerol formal, 1-
methylpyrrolidone
(NMP), dimethyl sulfoxide (DMSO). Glycerol formal exists in two isomeric
forms, the ct,ce-
form and the a,13-form. These forms are reproduced below:
(o __ ) 0...õ,õ/
OH
OH
o ___________ <..-----
o
a,ce-form a,I3-form
The thickeners contemplated by this invention are well known to a practitioner
of this
art. Compounds which function as thickeners include, for example, celluloses,
starches,
natural gums, monothioglycerol, synthetic polymers, such as polymers and
copolymers of
polyvinylpyrrolidone or (meth)acrylates, etc. Especially preferred thickeners
are sodium
carboxymethylcellulose (CMC), hydroxypropylcellulose, xanthum gum and
hydroxyethyl
starch. Thickeners may be present in amounts of from about 3 % to about 30 %.
Advantageously, the thickener is silica, in particular an anhydrous silica,
even more
particularly a colloidal anhydrous silica. In an advantageous embodiment, the
colloidal
anhydrous silica is at a concentration of about 4.5 % w/w.
Opacifiers may be added to absorb and/or reflect certain light and/or energy
of certain
wavelengths and may thus enhance the stability of the formulations. Opacifiers
include, for
34
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
example, zinc oxide or titanium dioxide and may be present in amounts from
about 0.5 to 2.5
%. Titanium dioxide is especially preferred. These compounds are well known to
practitioners of this art.
Additionally, the inventive formulations may contain other inert ingredients
such as
antioxidants, preservatives, or pH stabilizers. These compounds are well known
in the
formulation art. Antioxidant such as an alpha tocopheral, ascorbic acid,
ascrobyl palmitate,
fumaric acid, malic acid, sodium ascorbate, sodium metabisulfate, n-propyl
gallate, BHA
(butylated hydroxy anisole), BHT (butylated hydroxy toluene) monothioglycerol
and the like,
may be added to the present formulation. The antioxidants are generally added
to the
formulation in amounts of from about 0.01 to about 2.0 %, based upon total
weight of the
formulation, with about 0.05 to about 1.0 % being especially preferred.
Preservatives, such as
the parabens (methylparaben and/or propylparaben), are suitably used in the
formulation in
amounts ranging from about 0.01 to about 2.0 %, with about 0.05 to about 1.0 %
being
especially preferred. Other preservatives include benzalkonium chloride,
benzethonium
chloride, benzoic acid, benzyl alcohol, bronopol, butylparaben, cetrimide,
chlorhexidine,
chlorobutanol, chlorocresol, cresol, ethylparaben, imidurea, methylparaben,
phenol,
phenoxyethanol, phenylethyl alcohol, phenylmercuric acetate, phenylmercuric
borate,
phenylmercuric nitrate, potassium sorbate, sodium benzoate, sodium propionate,
sorbic acid,
thimerosal, and the like. Preferred ranges for these compounds include from
about 0.01 to
about 5 %.
Colorants may be added to the inventive formulations. Colorants contemplated
by the
present invention are those commonly known in the art. Specific colorants
include, for
example, dyes, an aluminum lake, caramel, colorant based upon iron oxide or a
mixture of
any of the foregoing. Especially preferred are organic dyes and titanium
dioxide. Preferred
ranges include from about 0.5 % to about 25 %.
Compounds which stabilize the pH of the formulation are also contemplates.
Again,
such compounds are well known to a practitioner in the art as well as how to
use these
compounds. Buffering systems include, for example, systems selected from the
group
consisting of acetic acid/acetate, malic acid/malate, citric acid/citrate,
tartric acid/tartrate,
lactic acid/lactate, phosphoric acid/phosphate, glycine/glycimate, tris,
glutamic
acid/glutamates, sodium carbonate and sodium carboxymethylcellulose (CMC).
Preferred
ranges for pH include from about 4 to about 6.5.
The present invention also encompasses flavorings. In an advantageous
embodiment,
the flavoring may be a mint flavor, fruit flavor, an herb flavor or a spice
flavor. Mint flavors
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
include, but are not limited to peppermint, spearmint and wintergreen. Fruit
flavors include,
but are not limited to, apple, apricot, banana, blackberry, blueberry, cherry,
clementine,
coconut, cranberry, currant, grape, grapefruit, huckleberry, juniper berry,
kiwifruit, lemon,
lime, mandarin, melon, orange, papaya, peach, pear, pineapple, plum,
pomegranate,
raspberry, strawberry, tangerine and watermelon flavors. Herb and/or spice
flavors include,
but are not limited to, allspice, ancho chile, anise, basil, bay leaf, black
pepper, black walnut,
caraway seed, cardamom, celery seed, chai-tea, chamomile, chervil, chickory,
chipotle chile,
chives, cilantro, cinnamon, citrus ginger, clove, coriander, cumin, dill,
elderflower, fennel,
ginger, ginseng, guarana, hot pepper, jalapeno, jamaica, jasmine, lavender,
lemon grass,
licorice, mace, marjoram, mint, mustard, nutmeg, oregano, paprika, parsley,
poppy seed, pure
vanilla, red pepper, rose, rosemary, saffron, sage, savory, sesame seed,
tamarind, tarragon,
tea, teaberry, thyme, turmeric, vanilla and white pepper. The flavors may be
natural or
artificial.
In an advantageous embodiment, the flavoring is a peppermint flavor or an
apple
flavor, advantageously an artificial apple flavor. In another advantageous
embodiment, the
flavoring is a cinnamon flavor.
The inventive pastes or gels may be administered to warm-blooded animals. Warm-
blooded animals include, for example, all ruminants, equines, canines, felines
and avians.
Especially preferred are birds, cattle, sheep, pigs, dogs, cats, horses and
the like. The amount
of each of anthelmintic compounds is well known to a practitioner of this art.
Preferred
amounts of praziquantel include, for example, from about 0.5 mg/kg to about
7.5 mg/kg of
animal body weight, with a range of about 0.5 mg/kg to about 2 mg/kg or 2.5
mg/kg of body
weight being especially preferred. A most especially preferred amount is about
1.0 mg/kg of
animal body weight. Preferred ranges for the anthelmintic macrolide compounds
include, for
example about 0.01 to about 200 mg/kg of animal body weight, with the ranges
of about 0.1
to about 50 mg/kg and from about 1 to about 30 mg/kg being especially
preferred.
The inventive oral homogeneous pastes may be prepared, for example, by a
process
which comprises:
-- dissolving the at least two different anthelmintic agents, e.g.,
praziquantel or
pyrantel and macrolide anthelmintic compound or compounds, into the solvent;
and
-- adding the thickening agent or agents and stirring until a homogeneous
paste is
formed.
More preferred processes comprise:
36
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
-- dissolving the at least two different anthelmintic agents, e.g.,
praziquantel or
pyrantel, and macrolide anthelmintic compound or compounds, and thickening
agent or
agents into the solvent and forming a thickened solution;
-- cooling the thickened solution to a temperature below about 35 C
-- adding the viscosity modifier agent and stirring until a homogeneous paste
is
formed or
-- dissolving the at least two different anthelmintic agents, e.g.,
praziquantel or
pyrantel, and macrolide anthelmintic compound or compounds, the thickening
agent or
agents and least one compound selected from the group consisting of an
antioxidant, a
colorant, a pH stabilizer and/or a preservative into the solvent and forming a
thickened
solution;
-- cooling the solution to a temperature below about 35 C; and
-- adding the viscosity modifying agent or agents and stirring until a
homogeneous
paste is formed.
A preferred process to prepare the inventive oral veterinary compositions
comprises:
--dissolving the at least two different anthelmintic agents, e.g.,
praziquantel or
pyrantel, and at least one macrolide anthelmintic compound or compounds and
the thickening
agent or agents into the solvent and forming a thickened solution;
--adding the opacifier to the thickened solution and mixing until the
opacifier is
evenly dispersed;
--cooling the thickened solution with the evenly dispersed opacifier to a
temperature
below about 35 C;
--adding the viscosity modifier and stirring until the oral veterinary
composition is
formed.
The inventive oral veterinary formulations may be used to treat a number of
ecto-and
endoparasite infections. The determining of a treatment protocol for an
infection of a specific
parasite or parasites would be well within the skill level of a practitioner
of the veterinary art.
This invention further provides for a method to increase the bioavailability
of the at least two
different anthelmintic agents in the animal.
The invention will now be further described by way of the following non-
limiting
examples.
37
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
EXAMPLES
Example 1: Oral Veterinary Homogeneous Paste
A better understanding of the present invention and of its many advantages
will be
had from the following example, given by way of illustration.
An oral veterinary homogeneous paste, which had the following ingredients:
INGREDIENTS AMOUNT (% w/w)
Praziquantel 7.75
Ivermectin 1.55
Butylated hydroxyanisole (BHA) 0.02
Sunset Yellow (FD&C Yellow No. 6) 0.04
Titanium dioxide 2.0
Hydroxypropylcellulose (HPC) 6.0
Hydrogenated castor oil 4.0
Stabilized glycerol formal QS AD 100
was prepared by the following process:
1. Add some or all of the stabilized glycerol formal to a mixture
followed by the addition of the praziquantel, ivermectin and
BHA. The ingredients are mixed until they are dissolved in the
stabilized glycerol formal at a temperature above 35 C.
2. Add sunset yellow to the solution and mix until dissolved.
3. Add titanium dioxide to the solution and mix until completely
dispersed.
4. Add the remainder of glycerol formal, if necessary.
5. Add HPC to the solution and mix the solution until a
homogeneous, viscous solution is obtained.
6. Cool the solution to a temperature below 35 C.
7. Once the solution is cooled to a temperature below 35 C, add
the hydrogenated castor oil, while mixing, until all the
hydrogenated castor oil is mixed into the solution; the
temperature of the solution is maintained below 35 C.
8. Once the hydrogenated castor oil has been added, increase the
agitation speed of the mixer while heating the mixture.
9. Mix until the product is a paste.
38
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Example 2: Use of Statistically Designed Experiments for Formulation
Optimization of a
Semisolid
A lower percentage of active was desired in a paste formulation with a
resulting
increase in the percentage of solvent. Optimization studies of the formulation
were
undertaken in an effort to eliminate or reduce paste separation and the
results are summarized
below.
It is believed that the structure of the paste formed from hydrogen bonding
between
the colloidal silicon dioxide and the polyethylene glycol (see, e.g., Raghavan
et al., Langmuir
2000, 16, 7920-7930). The main objective of this example was to test the
effect of the
following factors on the physical stability (phase separation) of the paste
formulation:
(a) amount of colloidal silicon dioxide (Cab-O-Sil) at either 4 or 5%
(b) type of magnesium carbonate (light or heavy)
(c) type of polyethylene glycol (PEG 300 or PEG 400)
(d) shear used during manufacture (high or low shear)
(e) temperature of paste during manufacture (25 C or 45 C)
A stock solution of active in triacetin was prepared. To 15 g of triacetin-
drug stock
solution, titanium dioxide, magnesium carbonate (light or heavy), and 4% w/w
or 5% w/w
colloidal silicon dioxide was added using a Lightnin mixer with an appropriate
size impeller.
The mixer speed was set at either 300 rpm (low shear) or 800 rpm (high shear)
and the beaker
was set in a circulating water bath maintained at either 25 C or 45 C
according to the
requirements of the experimental run. As a final step, remaining triacetin and
viscosity
modifier (PEG 300 or PEG 400) were added to the beaker while mixing so as to
make a 50 g
paste. Paste was removed from the beaker and approximately 8 grams was
centrifuged at
15,000 rpm for 15 minutes and the resulting supernatant liquid was weighed.
Table 1: Design of Experiments and their results
Run Colloidal MgCO3 Viscosity Shear Temp. Weight of liquid,
g
No. Si02, type Modifier Intensity (C)
Tube 1 Tube 2 Avg.
% w/w type
1 4 Heavy PEG400 Low 45
3.0726 2.9635 3.018
2 5 Heavy PEG300 Low 45
2.4013 2.2292 2.315
3 4 Heavy PEG400 High 25 3.1701 3.0601 3.115
4 5 Light PEG300 Low 25 1.8128 1.7567 1.785
5 5 Heavy PEG300 High 25 1.7167 1.6695 1.693
6 5 Heavy PEG400 Low 25 1.6302 1.7079 1.669
7 5 Light PEG400 High 25 1.8434 1.8817 1.863
8 4 Heavy PEG300 Low 25
2.8748 2.8450 2.860
9 5 Heavy PEG400 High 45 1.8680 1.8740 1.871
39
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Run Colloidal MgCO3 Viscosity Shear
Temp. Weight of liquid, g
No. Si02, type Modifier Intensity (C)
Tube 1 Tube 2 Avg.
% w/w type
4 Light PEG400 Low 25 2.9016 2.8292
2.865
11 5 Light PEG300 High 45 1.7862 1.8679 1.827
12 4 Light PEG300 High 25
2.7648 2.8402 2.803
13 4 Heavy PEG300 High 45
2.8487 2.6880 2.768
14 5 Light PEG400 Low 45 1.9213 1.9697 1.946
4 Light PEG400 High 45 3.0419 3.0740
3.058
16 4 Light PEG300 Low 45
3.0446 2.8130 2.929
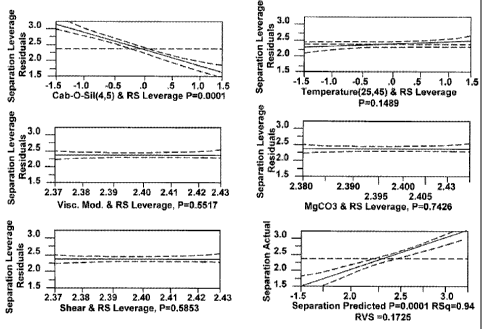
The leverage plots are provided for each of the variable factors as well as
the whole
model as shown in FIG. 1. The strength of the effect is shown by the slope of
the central fit
line. The greater the slope (positive or negative), the greater the effect
that variable has on
the paste separation. The distance from each point to the central fit line is
what the error
5 would be if the variable is taken out of the model. Confidence curves on
the graph show
whether an effect is significant or not. If the 95% confidence curves cross
from the
horizontal reference line, then the effect is significant; if the curves do
not cross, then it is not
significant.
From the plots of FIG. 1, it is evident that only the amount of colloidal
dioxide in the
10 formulation is very significant and the effect of temperature is
marginally significant.
The following table provides the parameters for the variables when the
desirability
function is maximized. Predicted formulation for the least amount of phase
separation is
shown in Table 2.
Table 2: Predicted formulation
Variable Factor Desired Value
Colloidal silicon dioxide 5% w/w
MgCO3 Light
Viscosity Modifier PEG 400
Shear Low
Temperature 25 C
15 Example 3: Oral Paste for Horses
An oral paste for horses contains firocoxib as active substance, a non-
steroidal anti-
inflammatory drug (NSAID) that reduces prostaglandin biosynthesis through the
selective
inhibition of cyclooxygenase-2 (COX-2).
The intended indication in horses is the alleviation of pain and inflammation
in
animals with ortheoarthritis and reduction of associated lameness. The
intended therapeutic
dose in horses is 0.1 mg firocoxib/kg bw/day orally for up to 14 consecutive
days. The 0.82%
oral paste for horses is presented in pre-filled oral syringes containing 7.32
g of oral paste
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
labelled in 100-kg dosing increments. Each syringe contains sufficient product
to treat a 600
kg horse.
Table 3: Qualitative and Quantitative Composition of the Medicinal Product
Qualitative Compositiol Quantitative Functiorr-- Refere ''''''''''
Active Substance In-house
Firocoxib 0.82 % w/w Active substance monograph
Excipients
Titanium Dioxide (E171) 0.20 % w/w Colourant Ph.Eur.
Magnesium Carbonate 2.00 % w/w Adsorbant Ph.Eur.
(heavy) 4.50 % w/w Thickener Ph.Eur.
Colloidal Anhydrous Silica 0.40 % w/w Viscosity modifier Ph.Eur.
Polyethylene Glycol 300 To 100 % w/w Vehicle Ph.Eur.
Triacetin
As detailed above, early formulations contained active substance
concentrations
ranging from 0.41 ¨ 2.05 %. The final active substance concentration was
chosen with
reference to the dose (0.1 mg/kg) and convenience of administration to horses
up to 600 kg
bodyweight in plastic syringes which are widely used for this dosage form.
The active substance is poorly soluble in water and triacetin was the vehicle
of choice
because of its ability to solubilise firocoxib and the stability of the
resultant solutions under
accelerated conditions. Because the active substance is dissolved in the
vehicle, particle size
and polymorphic properties of the active substance are not relevant to the
bioavailability of
the formulation. In order to produce a semi solid formulation, several
viscosity modifying
agents, in combination with colloidal silicon dioxide were investigated.
Formulations
containing combinations of colloidal silicon dioxide, monoethanolamine and
polyethylene
glycol 300 (PEG 300) were investigated for viscosity, manageability and
penetration values.
From experience with previous paste formulations the applicant concludes that
a penetration
value of between 6-40 mm is ideal in order to ensure that the paste is
manageable but does
not drip from the syringe barrel when inverted. Good paste viscosity (with
penetrometer)
was achieved over a wide range of PEG 300 concentrations.
Titanium dioxide was included to improve the appearance of the paste.
Following
stability studies at 60 C it was decided that addition of an adsorbent was
necessary in order to
reduce liquid separation in the syringe on storage. Magnesium carbonate is
widely used to
adsorb liquids in the tabletting process. Its compatability with the
formulation was
established and the differences between heavy and light grades were
investigated during
41
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
development. No significant differences in penetration values were observed
between batches
manufactured using heavy and light grades from the same supplier.
Various development studies were carried out in order to optimise the
formulation, to
ensure satisfactory penetration value, minimise liquid separation and optimise
the
concentration of other components. Triacetin, colloidal silicon dioxide,
polyethylene glycol
300, titanium dioxide and magnesium carbonate are all monographed in the
Ph.Eur. and are
generally regarded as non-toxic and non-irritant materials.
Batch instructions for the final pilot scale formulations stated the level of
colloidal
silicon dioxide to be used as 4.0 - 4.5 %. The amount dispensed for these
batches was 4.25
%. However during storage of in-process samples in the laboratory it was noted
that there
was some accumulation of clear liquid in small concavities on the surface of
the paste. On
testing both the liquid and paste phases were found to contain approximately
equal quantities
of active substance. Because early development batches demonstrated no
differences in
physico-chemical properties of paste produced with 4.25 % and 4.50 % colloidal
silicon
dioxide, commercial batches will be manufactured with 4.50 % colloidal silicon
dioxide in
order to minimise liquid separation.
Magnesium carbonate is widely used to adsorb liquids at a concentration of 0.5-
1.0 %.
The paste contains 2 % of magnesium carbonate, which is already above the
typical
concentration range. The paste contains 4.5 % colloidal silicon dioxide which
is typically
used at concentrations ranging 2-10 % as a suspending and thickening agent in
semi-solid
preparations. Hence it was decided to increase the concentration of colloidal
silicon dioxide
rather than magnesium carbonate. Additionally, formulations with 4 % magnesium
carbonate
showed lower penetration values (higher viscosity) as compared to formulations
with 2 %
magnesium carbonate. It is important that a minimum penetration value
specification of 6
mm be maintained for proper flow characteristics and there is an increased
risk that the
penetration value would not meet this specification throughout shelf life of
the product if
higher levels of magnesium carbonate were utilised.
Example 4: A Palatable Oral Gel dosage Form Containing Eprinomectin for Horses
A clear gel formulation containing eprinomectin was developed as an oral
dosage
form for horses and ponies for the treatment of internal parasites. The gel
formulation can be
administered directly from a syringe onto the horse's tongue.
In addition to elegance, an oral gel formula provides improved consistency in
delivering the dose as compared to suspensions or paste. Manufacturability is
simpler than
suspensions or pastes.
42
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
Table 4: Examples of components and compositions of an oral gel formultation:
Example 1 Example 2
INGREDIENTS % w/w % w/w
Eprinomectin 0.050 0.050
Sodium Ascorbate 1.0 1.0
Potassium Sorbate 0.5 0.5
Propylene glycol 20.0 0.0
Polyethylene glycol 400 0.0 20.0
Peppermint Flavor 0.0 1.0
Art. Apple Flavor 1.5 0.0
Sodium CMC 3.9 3.9
Water 73.0 73.5
Total 100.0 100.0
The formulation was prepared by first dissolving eprinomectin in propylene
glycol or
polyethylene glycol. This solution is added to a dispersion of sodium
carboxymethylcellulose
(Na-CMC) in water. The liquid flavor, sodium ascorbate and potassium sorbate
are added and
mixed until a clear homogenious system is obtained.
While the examples above were carried out with eprinomectin, other active
ingredients would be expected to provide similar results in oral gel
formulation. Other
gelling agents such as xanthan gum, citrus pectin, sodium alginate,
poloxamers, carbomers as
well as other cellulose gums can also be utilized in the formulation. In
addition to horses and
ponies, the oral gel formulation is also suited for use in swine, sheep and
cattle.
The invention is further described by the following numbered paragraphs:
1. An oral homogeneous veterinary paste consisting essentially of
praziquantel
and/or pyrantel and at least one macrolide anthelmintic compound, a solvent,
which dissolves
both the praziquantel and/or pyrantel and the macrolide anthelmintic compound,
and at least
one thickening agent.
2. An oral homogeneous veterinary paste consisting essentially of
praziquantel
and/or pyrantel and at least one macrolide anthelmintic compound, a solvent,
which dissolves
both the praziquantel and/or pyrantel and the macrolide anthelmintic compound,
at least one
thickening agent, and at least one viscosity modifier.
3. An oral homogeneous veterinary paste consisting essentially of
praziquantel
and/or pyrantel and at least one macrolide anthelmintic compound, a solvent,
which dissolves
both the praziquantel and/or pyrantel and the macrolide anthelmintic compound,
at least one
thickening agent, a viscosity modifier and at least one compound selected from
the group
consisting of an antioxidant, a colorant, a pH stabilizer and a preservative.
43
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
4. An oral veterinary composition consisting essentially of an oral
homogeneous
veterinary paste according to any one of paragraphs 1 to 3 and an opacifier.
5. An oral veterinary composition according to paragraph 4, wherein said
composition is non-aqueous.
6. An oral veterinary composition according to any one of paragraphs 1 to 3
wherein said composition is non-aqueous.
7. The oral homogeneous paste according to paragraph 1 wherein the
macrolide
anthelmintic compound is selected from the group consisting of doramectin,
abamectin,
moxidectin, selamectin, eprinomectin and milbemycin; the solvent is glycerol
formal,
propylene glycol, 1-methylpyrrolidone, or dimethyl sulfoxide; and the
thickening agent is
selected from the group consisting of a cellulose, a starch, monothioglycerol,
a natural gum,
a polymer or copolymer of polyvinylpyrrolidone, and a polymer or copolymer of
(meth)acrylate.
8. An oral veterinary composition consisting essentially of an oral
homogeneous
veterinary paste according to paragraph 7 and an opacifier.
9. The oral homogeneous paste according to paragraph 7 wherein the
macrolide
anthelmintic compound is ivermectin.
10. The oral veterinary composition according to paragraph 8 wherein the
macrolide anthelmintic compound is ivermectin.
11. The oral homogeneous paste according to paragraph 2 wherein the
macrolide
anthelmintic compound is selected from the group consisting of doramectin,
abamectin,
moxidectin, selamectin, eprinomectin and milbemycin; the solvent is glycerol
formal,
propylene glycol, n-methylpyrrolidone, or dimethyl sulfoxide; the thickening
agent is
selected from the group consisting of a cellulose, a starch, monothioglycerol,
polymers or
copolymers of polyvinylpyrrolidone, polymers and copolymers of (meth)acrylate,
and a
natural gum; and the viscosity modifier is selected from the group consisting
of vegetable
oils, or hydrogenated vegetable oils.
12. The oral homogeneous paste according to paragraph 11 wherein the
thickening
agent is hydroxypropylcellulose, xanthan gum or hydroxyethyl starch and the
viscosity
modifier is hydrogenated castor oil, corn oil or olive oil.
13. The oral homogeneous paste according to paragraph 12 wherein the
macrolide
anthelmintic compound is ivermectin.
14. An oral veterinary composition consisting essentially of an oral
homogeneous
veterinary paste according to paragraph 11 and an opacifier.
44
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
15. The oral veterinary composition according to paragraph 14
wherein the
thickening agent is hydroxypropylcellulose, xanthum gum or hydroxyethyl starch
and the
viscosity modifier is hydrogenated castor oil, corn oil or olive oil and the
opacifier is selected
from the group consisting of titanium dioxide and zinc oxide.
16. The oral veterinary composition according to paragraph 15 wherein the
macrolide anthelmintic compound is ivermectin and the opacifier is titanium
dioxide.
17. The oral homogeneous paste according to paragraph 3 wherein
-- the macrolide anthelmintic compound is selected from the group consisting
of
doramectin, abamectin, eprinomectin, moxidectin, selamectin and milbemycin;
--the solvent is glycerol formal, propylene glycol, n-methylpyrrolidone, or
dimethyl sulfoxide; the thickening agent is selected from the group consisting
of
a cellulose, a starch, monothioglycerol, polymers or copolymers of
polyvinylpyrrolidone, polymers or copolymers of (meth)acrylate, and a natural
gum;
--the viscosity modifier is selected from the group consisting of vegetable
oils and
hydrogenated vegetable oils.
--the antioxidant is selected from the group consisting of alpha tocopherol,
ascorbic
acid, ascrobyl palmitate, fumaric acid, malic acid, sodium ascorbate, sodium
metabisulfate, n-
propyl gallate, butylated hydroxy anisole, butylated hydroxy toluene,
monothioglycerol;
--the colorant is dye, an aluminum lake, caramel, and colorant based upon iron
oxide;
--the pH stabilizer is a buffering system selected from the group consisting
of acetic
acid/acetate, malic acid/malate, citric acid/citrate, tartric acid/tartrate,
lactic acid/lactate,
phosphoric acid/phosphate, glycine/glycimate, tris, glutamic acid/glutamates
and sodium
carbonate; and
--the preservative is a compound selected from the group consisting of
benzalkonium
chloride, benzethonium chloride, benzoic acid, benzyl alcohol, bronopol,
butylparaben,
centrimide, chlorhexidine, chlorobutanol, chlorocresol, cresol, ethylparaben,
imidurea,
methylparaben, propylparaben, phenol, phenoxyethanol, phenylethyl, alcohol,
phenylmercuric acetate, pheylmecuric borate, phenylmercuric nitrate, potassium
sorbate,
sodium benzoate, sodium propionate, sorbic acid, and thimerosal.
18. The oral homogeneous paste according to paragraph 17 wherein the
macrolide
anthelmintic compound is ivermectin.
19. The oral homogeneous veterinary paste according to paragraph
3, wherein the
macrolide anthelmintic compound is ivermectin, the solvent is glycerol formal,
the thickener
is hydroxypropylcellulose, the viscosity modifier is hydrogenated castor oil,
the colorant is an
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
organic dye, and the preservative is selected from the group consisting of
butylated
hydroxytoluene or butylated hydroxy anisole.
20. The oral homogeneous veterinary paste according to paragraph
19 wherein the
dye is an organic dye which is sunset yellow.
21. The oral veterinary composition consisting essentially of a an oral
homogeneous veterinary paste according to paragraph 17 and an opacifier.
22. The oral veterinary composition consisting essentially of an oral
homogeneous veterinary paste according to paragraph 19 and an opacifier.
23. The oral veterinary composition according to paragraph 20 wherein the
dye is
an organic dye which is sunset yellow
24. The oral veterinary composition according to paragraph 4 which is
praziquantel 7.75 % w/w
ivermectin 1.55 % w/w
butylated hydroxyanisole 0.02 % w/w
sunset yellow (FD&C Yellow No. 6) 0.04 % w/w
titanium dioxide 2.0 % w/w
hydroxypropylcellulose 6.0 % w/w
hydrogenated castor oil 4.0 % w/w
stabilized glycerol formal amount to make 100%.
25. The oral homogeneous paste according to any one of paragraph 1 to 3,
wherein the first anthelmintic agent is praziquantel.
26. The oral homogeneous paste according to paragraph 4, wherein the first
anthelmintic agent is praziquantel.
27. A process for preparing an oral homogeneous veterinary paste according
to paragraph 1 which comprises
-- dissolving the praziquantel and/or pyrantel and macrolide anthelmintic
compound
or compounds into the solvent; and
-- adding the thickening agent or agents and stirring until a homogeneous
paste is
formed.
28. A process for preparing an oral homogeneous veterinary paste according
to
paragraph 2 which comprises
-- dissolving the praziquantel and/or pyrantel and macrolide anthelmintic
compound
or compounds and thickening agent or agents into the solvent and forming a
thickened
solution;
46
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
-- cooling the thickened solution to a temperature below about 35 C; and
-- adding the viscosity modifier agent or agents and stirring until a
homogeneous
paste is formed.
29. A process for preparing an oral homogeneous veterinary paste
according to
paragraph 3 which comprises:
-- dissolving the praziquantel and/or pyrantel and macrolide anthelmintic
compound
or compounds, the thickening agent or agents and least one compound selected
from the
group consisting of an antioxidant, a colorant, a pH stabilizer and/or a
preservative into the
solvent and forming a thickened solution; and
-- cooling the thickened solution to a temperature below about 35 C; and
-- adding the viscosity modifying agent or agents and stirring until a
homogeneous
paste is formed.
30. A process for preparing an oral veterinary composition
according to paragraph
4 which comprises:
--dissolving the parzequantel and at least one macrolide anthelmintic compound
or
compounds and the thickening agent or agents into the solvent and forming a
thickened
solution;
--adding the opacifier to the thickened solution and mixing until the
opacifier is
evenly dispersed;
--cooling the thickened solution with the evenly dispersed opacifier to a
temperature
below about 35 C;
--adding the viscosity modifier and stirring until the oral veterinary
formulation is
formed.
31. A method for increasing the bioavailability of praziquantel and at
least one
macrolide anthelmintic compound in a warm-blooded animal which comprises
administering
the oral homogeneous veterinary paste according to any one paragraphs 1 to 3
said warm-
blooded animal or bird.
32. A method for increasing the bioavailability of praziquantel and at
least one
macrolide anthelmintic compound in a warm-blooded animal or bird which
comprises
administering the oral veterinary composition according to paragraph 4 to said
warm-blooded
animal or bird.
33. An oral veterinary paste consisting essentially of dissolved
praziquantel and
dissolved ivermectin.
47
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
34. The oral veterinary paste of paragraph 33 wherein the praziquantel and
ivermectin are both dissolved in glycerol formal.
35. The oral veterinary paste of paragraph 34 further consisting
essentially of
praziquantel and ivermectin dissolved in glycerol formal and a cellulose.
36. The oral veterinary paste of paragraph 35 further consisting
essentially of
hydrogenated caster oil.
37. The oral veterinary paste of paragraph 35, wherein the cellulose is
hydroxypropyl cellulose.
38. The oral veterinary paste of paragraph 36 further consisting
essentially of
antioxidant, colorant, titanium dioxide.
39. The oral veterinary paste of paragraph 38 wherein the cellulose is
hydroxypropyl cellulose, the antioxidant is butylated hydroxyanisole and the
colorant is
sunset yellow (FD&C Yellow No. 6).
40. The oral veterinary paste of paragraph 33 further consisting
essentially of a
cellulose, hydrogenated castor oil, and glycerol formal.
41. The oral veterinary paste according to paragraph 40 wherein the
cellulose is
hydroxypropylcellulose.
42. The oral veterinary paste of paragraph 33 further consisting
essentially of a
cellulose, hydrogenated castor oil, glycerol formal and one or more compounds
selected from
the group consisting of an antioxidant, an opacifier and a colorant.
43. The oral veterinary paste according to paragraph 42 wherein the
cellulose is
hydroxypropylcellulose.
44. A method for increasing the bioavailability of praziquantel and a
macrolide
anthelmintic compound in a warm-blooded animal which comprises administering
the oral
veterinary paste according to paragraph 33 to said warm-blooded animal.
45. The method of paragraph 44 wherein the warm-blooded animal is bird,
cattle,
sheep, pig, dog, cat or horse.
46. The method of paragraph 45 wherein the warm-blooded animal is a bird.
47. The method of paragraph 45 wherein the warm-blooded animal is a horse.
50. An oral veterinary paste consisting essentially of praziquantel,
ivermectin,
antioxidant, colorant, titanium dioxide, a cellulose, hydrogenated castor oil,
and glycerol
formal.
51. The oral veterinary paste according to paragraph 50 wherein the
cellulose is
hydroxypropylcellulose.
48
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
52. The oral veterinary paste of paragraph 50, wherein the
cellulose is
hydroxypropylcellulose and the glycerol formal is stabilized glycerol formal.
53. The oral veterinary paste of paragraph 50 which is produced by
the process
comprising:
(a) dissolving praziquantel, ivermectin, colorant, titanium dioxide,
antioxidant
and cellulose into glycerol formal to form a thickened solution;
(b) cooling the thickenened solution to a temperature below about 35 C;
(c) adding hydrogenated castor oil to the thickened solution and stirring
until a
homogenous paste is formed,
wherein the cellulose is hydroxypropyl cellulose and wherein the antioxidant
is
butylated hydroxyanisole, the opacifier is titanium dioxide and the colorant
is sunset yellow
(FD&C Yellow No. 6).
54. The oral veterinary paste according to paragraph 50, which is
praziquantel 7.75 (% w/w)
ivermectin 1.55 (% w/w)
butylated hydroxyanisole 0.02 (% w/w)
sunset yellow (FD&C Yellow No. 6) 0.04 (% w/w)
titanium dioxide 2.0 (% w/w)
hydroxypropylcellulose 6.0 (% w/w)
hydrogenated castor oil 4.0 (% w/w)
glycerol formal QS AD 100.
55. An oral veterinary paste comprising:
praziquantel 7.75 (% w/w)
ivermectin 1.55 (% w/w)
hydroxypropylcellulose 6.0 (% w/w)
hydrogenated castor oil 4.0 (% w/w)
glycerol formal QS AD 100
and optionally, one or more compounds selected from the group consisting of an
antioxidant, an opacifier and a colorant.
56. The oral veterinary paste of paragraph 55, wherein the antioxidant is
butylated
hydroxyanisole, the opacifier is titanium dioxide and the colorant is sunset
yellow (FD&C
Yellow No. 6).
49
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
57. A method for increasing the bioavailability of praziquantel and a
macrolide
anthelmintic compound in a warm-blooded animal which comprises administering
the oral
veterinary paste according to paragraph 50 to said warm-blooded animal.
58. The method of paragraph 57 wherein the warm-blooded animal is bird,
cattle,
sheep, pig, dog, cat or horse.
59. The method of paragraph 58 wherein the warm-blooded animal is a bird.
60. The method of paragraph 58 wherein the warm-blooded animal is a horse.
61. A method for preparing a pharmaceutical or veterinary paste formulation
comprising:
dissolving or dispersing a therapeutic agent into a carrier,
adding fumed silica and an absorbent to the carrier containing the dissolved
therapeutic agent,
mixing the fumed silica, absorbent and carrier containing the dissolved
therapeutic
agent at low shear,
maintaining the temperature at about 25 C until the silica and absorbent is
dispersed in
the carrier and
adding a viscosity modifier to the intermediate with mixing to produce a
uniform
pharmaceutical or veterinary paste formulation.
62. The method of paragraph 61 wherein the mixing at low shear is at 300
rpm.
63. The method of paragraph 61 wherein the fumed silica is a colloidal
silicon
dioxide.
64. The method of paragraph 63 wherein the colloidal silicon dioxide is at
a final
concentration of 5% w/w in the pharmaceutical or veterinary paste formulation.
65. The method of paragraph 61 wherein the absorbent is magnesium
carbonate.
66. The method of paragraph 65 wherein the magnesium carbonate is a light
magnesium carbonate.
67. The method of paragraph 61 wherein the viscosity modifier is PEG 400.
68. The method of paragraph 61 wherein
the mixing at low shear is at 300 rpm,
the fumed silica is a colloidal silicon dioxide,
the absorbent is magnesium carbonate and
the viscosity modifier is PEG 400.
69. The method of paragraph 68 wherein
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
the colloidal silicon dioxide is at a final concentration of 5% w/w in the
pharmaceutical or veterinary paste formulation.
70. The method of paragraph 68 wherein
the magnesium carbonate is a light magnesium carbonate.
71. The method of paragraph 68 wherein
the colloidal silicon dioxide is at a final concentration of 5% w/w in the
pharmaceutical or veterinary paste formulation and
the magnesium carbonate is a light magnesium carbonate.
72. A pharmaceutical or veterinary paste formulation comprising:
(a) an effective amount of a therapeutic agent,
(b) a fumed silica, wherein the fumed silica is 5% w/w colloidal silicon
dioxide,
(c) a viscosity modifier, wherein the viscosity modifier is PEG 400
(d) an absorbent, wherein the absorbent is magnesium carbonate.
73. The pharmaceutical or veterinary paste of paragraph 72,
wherein the
magnesium carbonate is a light magnesium carbonate.
74. The pharmaceutical or veterinary paste of paragraph 72 wherein
the
therapeutic agent is selected from the group consisting of avermectins,
milbemycins,
nordulisporic acid and its derivatives, estrogens, progestins, androgens,
substituted pyridyl
methyl derivatives, phenylpyrazoles, COX-2 inhibitors or 2-(2-benzimidazoly1)-
pyrimidine
derivatives.
75. The pharmaceutical or veterinary paste of paragraph 72 wherein
the
therapeutic agent comprises praziquantel and ivermectin.
76. The pharmaceutical or veterinary paste of paragraph 75 wherein
the
therapeutic agent comprises dissolved praziquantel and dissolved ivermectin.
77. The pharmaceutical or veterinary paste of paragraph 72 further
comprising a
carrier.
78. The pharmaceutical or veterinary paste of paragraph 77 wherein the
carrier is a
triacetin, a monoglyceride, a diglyceride, or a triglyceride.
79. The pharmaceutical or veterinary paste of paragraph 78 wherein the
carrier is a
triacetin.
80. The pharmaceutical or veterinary paste of paragraph 72 further
comprising a
colorant, stabilizer, surfactant or preservative.
81. A pharmaceutical or veterinary paste formulation comprising:
51
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is a
COX-2 inhibitor,
(b) a colorant, wherein the colorant is titanium dioxide,
(c) an absorbent, wherein the absorbent is magnesium carbonate,
(d) a thickener, wherein the thickener is colloidal anhydrous silica,
(e) a viscosity modifier, wherein the viscosity modifier is PEG
300.
82. The pharmaceutical or veterinary paste of paragraph 81, wherein the COX-
2
inhibitor is firocoxib.
83. The pharmaceutical or veterinary paste of paragraph 81 or 82, wherein
the
titanium dioxide is an E171 titanium dioxide.
84. The pharmaceutical or veterinary paste of any one of paragraphs 81 to
83,
wherein the magnesium carbonate is a heavy magnesium carbonate.
85. The pharmaceutical or veterinary paste of any one of paragraphs 81 to
84
further comprising a carrier.
86. The pharmaceutical or veterinary paste of paragraph 85 wherein the
carrier is a
triacetin.
87. The pharmaceutical or veterinary paste of any one of
paragraphs 81 to 86,
further comprising a colorant, stabilizer, surfactant or preservative.
88. A pharmaceutical or veterinary paste formulation comprising:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is a
firocoxib,
(b) a colorant, wherein the colorant is titanium dioxide,
(c) an absorbent, wherein the absorbent is magnesium carbonate,
(d) a thickener, wherein the thickener is colloidal anhydrous silica,
(e) a viscosity modifier, wherein the viscosity modifier is PEG 300,
(0 a carrier, wherein the carrier is a triacetin.
89. The pharmaceutical or veterinary paste formulation of
paragraph 88, wherein
the firocoxib is about 0.82%w/w.
90. The pharmaceutical or veterinary paste formulation of
paragraph 88 or 89,
wherein the titanium dioxide is an E171 titanium dioxide.
91. The pharmaceutical or veterinary paste formulation of any one
of paragraphs
88 to 90, wherein the titanium dioxide is about 0.2%w/w.
92. The pharmaceutical or veterinary paste formulation of any one
of paragraphs
88 to 91, wherein the magnesium carbonate is a heavy magnesium carbonate.
52
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
93. The pharmaceutical or veterinary paste formulation of any one of
paragraphs
88 to 92, wherein the magnesium carbonate is about 2%w/w.
94. The pharmaceutical or veterinary paste formulation of any one of
paragraphs
88 to 93, wherein the colloidal anhydrous silica is about 4.5%w/w.
95. The pharmaceutical or veterinary paste formulation of any one of
paragraphs
88 to 94, wherein the PEG 300 is about 0.4%w/w.
96. The pharmaceutical or veterinary paste formulation of any one
of paragraphs
88 to 95, wherein the triacetin is about 92%w/w.
97. A pharmaceutical or veterinary paste formulation comprising:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is
about 0.82%w/w firocoxib,
(b) a colorant, wherein the colorant is about 0.2%w/w titanium dioxide,
(c) an absorbent, wherein the absorbent is about 2%w/w magnesium carbonate,
(d) a thickener, wherein the thickener is about 4.5%w/w colloidal anhydrous
silica,
(e) a viscosity modifier, wherein the viscosity modifier is 0.4%w/w PEG
300,
(0 a carrier, wherein the carrier is about 92%w/w triacetin.
98. A pharmaceutical or veterinary gel formulation comprising:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is an
avermectin,
(b) an antioxidant, wherein the antioxidant is sodium ascorbate,
(c) a preservative, wherein the preservative is potassium sorbate,
(d) a flavoring, wherein the flavoring is a peppermint or apple flavor and
(e) a buffering system or thickener, wherein the buffering system or
thickener is
sodium carboxymethylcellulose.
99. The pharmaceutical or veterinary gel formulation of paragraph
98 further
comprising a solvent, wherein the solvent is propylene glycol.
100. The pharmaceutical or veterinary gel formulation of paragraph 98 further
comprising a viscosity modifier, wherein the viscosity modifier is
polyethylene glycol 400.
101. The pharmaceutical or veterinary gel formulation of any one of paragraphs
98
to 100, wherein the avermectin is eprinomectin.
102. The pharmaceutical or veterinary gel formulation of any one of paragraphs
98
to 101, wherein the flavoring is a peppermint flavor.
53
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
103. The pharmaceutical or veterinary gel formulation of any one of paragraphs
98
to 101, wherein the flavoring is an apple flavor.
104. The pharmaceutical or veterinary gel formulation of paragraph 103,
wherein
the flavoring is an artificial apple flavor.
105. A pharmaceutical or veterinary gel formulation comprising:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is
eprinomectin,
(b) an antioxidant, wherein the antioxidant is sodium ascorbate,
(c) a preservative, wherein the preservative is potassium sorbate,
(d) a flavoring, wherein the flavoring is a peppermint or apple flavor and
(e) a buffering system or thickener, wherein the buffering system
or thickener is
sodium carboxymethylcellulose.
106. The pharmaceutical or veterinary gel formulation of paragraph 105 further
comprising a solvent, wherein the solvent is propylene glycol.
107. The pharmaceutical or veterinary gel formulation of paragraph 105 further
comprising a viscosity modifier, wherein the viscosity modifier is
polyethylene glycol.
108. The pharmaceutical or veterinary gel formulation of any one of paragraphs
105 to 107, wherein the eprinomectin is about 0.050% w/w.
109. The pharmaceutical or veterinary gel formulation of any one of paragraphs
105 to 108, wherein the sodium ascorbate is about 1.0% w/w.
110. The pharmaceutical or veterinary gel formulation of any one of paragraphs
105 to 109, wherein the potassium sorbate is about 0.5% w/w.
111. The pharmaceutical or veterinary gel formulation of any one of paragraphs
106 and 108 to 110, wherein the propylene glycol is about 20.0% w/w.
112. The pharmaceutical or veterinary gel formulation of any one of paragraphs
107 to 110, wherein the polyethylene glycol is about 20.0% w/w.
113. The pharmaceutical or veterinary gel formulation of any one of paragraphs
105 to 112, wherein the peppermint flavor is about 1.0% w/w.
114. The pharmaceutical or veterinary gel formulation of any one of paragraphs
105 to 112, wherein the apple flavor is about 1.5% w/w.
115. The pharmaceutical or veterinary gel formulation of any one of paragraphs
105 to 114, wherein the sodium carboxymethylcellulose is about 3.9% w/w.
116. A pharmaceutical or veterinary gel formulation comprising:
54
CA 02673193 2009-06-18
WO 2008/077130
PCT/US2007/088225
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is
about 0.050% w/w eprinomectin,
(b) an antioxidant, wherein the antioxidant is about 1.0% w/w sodium
ascorbate,
(c) a preservative, wherein the preservative is about 0.5% w/w potassium
sorbate,
(d) a flavoring, wherein the flavoring is about 1.0% w/w peppermint or
about
1.5% artificial apple flavor and
(e) a buffering system or thickener, wherein the buffering system
or thickener is
about 3.9% w/w sodium carboxymethylcellulose.
117. The pharmaceutical or veterinary gel formulation of paragraph 116 further
comprising a solvent, wherein the solvent is about 20.0% w/w propylene glycol.
118. The pharmaceutical or veterinary gel formulation of paragraph 116 further
comprising a viscosity modifier, wherein the viscosity modifier is about 20.0%
polyethylene
glycol 400.
119. A pharmaceutical or veterinary gel formulation comprising:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is
ivermectin,
(b) an antioxidant, wherein the antioxidant is sodium ascorbate,
(c) a preservative, wherein the preservative is potassium sorbate,
(d) a flavoring, wherein the flavoring is a peppermint or apple flavor and
(e) a buffering system or thickener, wherein the buffering system or
thickener is
sodium carboxymethylcellulose.
120. The pharmaceutical or veterinary gel formulation of paragraph 119 wherein
the therapeutic agent further comprises praziquantel.
121. A pharmaceutical or veterinary gel formulation comprising:
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is a
proton pump inhibitor,
(b) an antioxidant, wherein the antioxidant is sodium ascorbate,
(c) a preservative, wherein the preservative is potassium sorbate,
(d) a flavoring, wherein the flavoring is a peppermint or apple flavor and
(e) a buffering system or thickener, wherein the buffering system or
thickener is
sodium carboxymethylcellulose.
122. The pharmaceutical or veterinary gel formulation of paragraph 121 wherein
the proton pump inhibitor is omeprazole.
123. A pharmaceutical or veterinary gel formulation comprising:
CA 02673193 2014-01-15
51440-122
(a) an effective amount of a therapeutic agent, wherein the therapeutic
agent is a
COX-2 inhibitor,
(b) an antioxidant, wherein the antioxidant is sodium ascorbate,
(c) a preservative, wherein the preservative is potassium sorbate,
(d) a flavoring, wherein the flavoring is a peppermint or apple flavor and
(e) a buffering system or thickener, wherein the buffering
system or thickener is
sodium carboxymethylcellulose.
124. =The pharmaceutical or veterinary gel formulation of paragraph 123
wherein
the COX-2 inhibitor is 3-(cyclopropylmethoxy)-5,5-dimethy1-414-
(methylsulfonyl)phenylk
2(5H)-furanone.
125. The pharmaceutical or veterinary gel formulations of any one of
paragraphs
119 to 124 further comprising a solvent, wherein the solvent is propylene
glycol.
126. The pharmaceutical or veterinary gel formulation of any one of paragraphs
119 to 125 further comprising a viscosity modifier, wherein the viscosity
modifier is =
polyethylene glycol 400.
128. =The pharmaceutical or veterinary gel formulation of any one of
paragraphs
= 119 to 127, wherein the flavoring is a peppermint flavor.
129. The pharmaceutical or veterinary gel formulation of any one of paragraphs
119 to 127, wherein the flavoring is an apple flavor.
130. The pharmaceutical or veterinary gel formulation of paragraph 129,
wherein
the flavoring is an artificial apple flavor.
* * *
Having thus described in detail preferred embodiments of the present
invention, it is
to be understood that the invention defined by the above paragraphs is not to
be limited to
particular details set forth in the above description as many apparent
variations thereof are
possible without departing from the scope of the present invention, which is
as defined by the
appended claims.
56