Note: Descriptions are shown in the official language in which they were submitted.
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
1
PHARMACEUTICAL PACKAGING SYSTEMS
FOR IMPROVED PATIENT COMPLIANCE
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of provisional application no.
60/871,601, filed December 22, 2006, which application is herein incorporated
in its entirety.
FIELD OF THE INVENTION
The present invention relates generally to a packaging system for
pharmaceutical products. More particularly, the present"invention relates to a
packaging system designed to be prominently positioned in a location of the
patient's choice, thus improving patient compliance with a recommended
dosing regimen.
BACKGROUND
Patient compliance with recommended dosing regimens is a major
public health concern. The World Health Organization, in a report in 2003,
0
called noncompliance a "worldwide problem of striking magnitude." It is
estimated that in developed nations, half of patients did not take medicines
for
chronic diseases in the prescribed manner. Studies have shown that
noncompliance causes 125,000 deaths annually in the U.S. (Smith, D.,
Compliance Packaging: A Patient Education Tool, American Pharmacy, Vol.
NS29, No. 2 February 1989), leads to 10 to 25 percent of hospital and nursing
home admissions, and is becoming an international epidemic. One study
concluded that 23% of nursing home admissions were due to noncompliance
(Standberg, L.R., Drugs as a Reason for Nursing Home Admissions, American
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
2
Health care Association Journal, 10, 20, 1984). Another study concluded that
10% of hospital admissions were due to noncompliance (Schering Report IX,
The Forgetful Patient: The High Cost of Improper Patient Compliance, March
1981.) It has also been estimated that about 50% of the prescriptions filled
each year are not taken correctly (Hayes, R.B., NCPIE Prescription Month,
October 1989). Thus, there is clearly a need for improved patient compliance.
Pharmaceutical agents in the form of tablets and capsules are generally
dispensed to users in packages of two types. The first type of packaging which
has historically dominated the market is bulk packaging which generally takes
the form of a small vial or bottle having a cap that either twists or pops
away
from the body of the containment article so as to provide access to the
articles
disposed therein. Such caps may include locking structures when engaged with
the package body so as to prohibit access to the contents by children.
One deficiency with bulk packaging is that the user is responsible for
maintaining an independent record by human memory or other means as to
whether or not the proper dosage has actually been administered. This
deficiency is particularly problematic for users who suffer from weak short-
term
memory performance. Thus, one can easily take either too many or too few
doses in a given period of time, thereby either reducing the efficiency of the
medication, or in a more serious situation actually causing damage to one's
system.
In order to address the inherent deficiencies of traditional bulk storage
containers, a second category of storage systems, referred to as blister
packaging, has been developed. Such blister packaging typically consists of a
set of individualized packets, one for each dose or fractional dose of the
pharmaceutical agent, located together on a card. This card is printed with a
designation of dosages and warnings. The tablets themselves are encased
between two materials such as aluminum foil and polyvinyl chloride film such
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
3
that the tablet can be pressed through the aluminum foil backing thereby
leaving a broken blister indicating that that dose has been utilized. While
the
use of a blister pack solves the problem of one being unable to remember
whether or not a dosage has been administered, a traditional blister packaging
system with a necessarily easily opened foil barrier may be susceptible to
damage due to tearing or child intrusion. In lieu of a push-through sheet, the
backing may comprise a sheet of material designed to be pealed away from the
the packaging.
Conventional bulk packaging and blister packaging systems suffer from
the weakness that they may not be visually prominent to the patient, thus
failing
to provide a visual stimulus to trigger the patient's memory to administer the
drug and thus facilitate compliance. Furthermore, by virtue of the fact that
they
are not in any way associated or anchored to any one location, they may be
subject to being misplaced, possibly within reach of a child.
The prior art reflects various attempts to remedy certain of these
shortcomings. U.S. Pat. No. 5,927,500 provides a packaging material which
includes a paperboard or polymer sheet stock reinforced with a fabric
substrate
layer, thereby providing a composite which is resistant to tearing. U.S. Pat.
No.
6,273,260 is directed to a packaging system that incorporates external
reminders for effectively dispensing medication on an irregular basis or
lengthy
periodic basis. This reference also suggests that packaging systems that can
be oriented vertically may, through their prominence, assist in reminding
patients of the need to comply with a dosing regimen regarding the
pharmaceutical product contained within.
However, there remains the need for pharmaceutical packaging systems
that will further improve patient compliance.
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
4
SUMMARY OF THE INVENTION
In some embodiments, the invention is directed to a packaging system
for a pharmaceutical product comprising a blister package containing a
plurality
of medication dosages and a retaining device for attaching said blister
package
to a mounting surface, wherein said retaining device supports said blister
package in a substantially vertical orientation.
In some embodiments, the retaining device comprises a magnet
disposed on the rear surface of said blister, wherein said magnet is suitable
for
suspending said blister package from a ferrous vertical mounting surface.
In some embodiments, the retaining device comprises an adhesive
disposed on the rear surface of said blister package, wherein said adhesive is
suitable for suspending said blister package from a vertical mounting surface.
In some embodiments, the adhesive is a repositionable adhesive.
In some embodiments, the retaining device comprises a fastener
suitable for supporting said blister package from a mounting surface, wherein
said fastener is selected from the group consisting of hooks, pegs, clamps,
clip
springs, hook-and-loop strips, and suction cups. In some embodiments, the
retaining device further comprises a magnetic base portion suitable for
supporting said blister package from a ferrous mounting surface. In some
embodiments, the retaining device further comprises a tether attached at one
end to said blister package and at the other end to said fastener.
In some embodiments, the blister package further comprises a ferrous
surface disposed on the rear surface of said blister package, and said
fastener
comprises a magnet disposed to engage said ferrous surface.
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
In some embodiments, the retaining device comprises a grooved
retaining fastener adapted to supportingly engage an edge of said blister
package.
In some embodiments, the blister package is attached to said retaining
device by a hinge joint allowing said blister package to pivot about said
hinge
joint.
In some embodiments, the retaining device comprises a grooved
retaining fastener for attaching said blister package to said mounting
surface.
In some embodiments, the invention is directed to a packaging system
for a pharmaceutical product comprising a blister package containing a
plurality
of medication dosages and a stand suitable for supporting said medication
storage device from a horizontal surface. In some embodiments, the stand is
integral to said medication storage device. In some embodiments, the stand
comprises at least one fold-out cross-member. In some embodiments, the
stand comprises a horizontal fold-out base.
In some embodiments, the invention is directed to a method of
enhancing patient compliance comprising the use of the packaging system of
claim 1 to store and display a pharmaceutical product.
BRIEF DESCRIPTION OF THE DRAWINGS
The aforementioned and current features and objects of this invention
will be better understood from the following detailed description in view of
the
drawings wherein:
FIGS. 1A and 1B illustrate a packaging system comprising a blister
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
6
package on the rear surface of which are located, alternatively, either
magnetic
or adhesive pads, as edge-mounted strips;
FIGS. 1 C and 1 D illustrate a packaging system comprising a blister
package on the rear surface of which are located, alternatively, either
magnetic
or adhesive pads, as buttons;
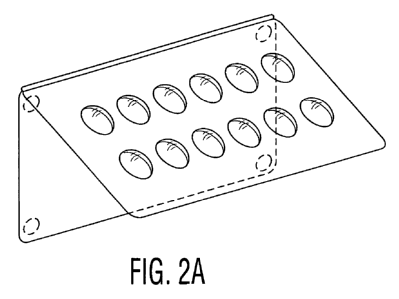
FIGS. 2A and 2B illustrate a packaging system comprising a blister
package that is supported via a hinge joint to a mounting structure, the rear
surface of which comprises, alternatively, either magnetic or adhesive
buttons;
FIGS. 2C and 2D illustrate side views of a packaging system comprising
a blister package that is supported via a hinge joint integral to a mounting
structure;
FIGS. 3A and 3B illustrate a packaging system comprising a blister
package that is mountable by a hook or pin;
FIG. 3C illustrates a packaging system comprising a blister package that
is mounted by a hook;
FIGS. 4A, 4B and 4C illustrate alternate embodiments of a packaging
system comprising a blister package suspended from a grooved retaining
fastener;
FIGS. 4D and 4E illustrate side views of alternative embodiments of a
grooved retaining fastener;
FIGS. 5A, 5B and 5C illustrate embodiments of a packaging system
comprising a blister package supported from its top edge by a retention
component that comprises, alternatively, a clamp or spring clip;
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
7
FIGS. 6A, 6B and 6C illustrate embodiments of a packaging system
comprising a blister package supported from a side edge by a retention
component that comprises a base and, alternatively, a clamp or spring clip,
and
a tether;
FIGS. 7A and 7B illustrate a packaging system comprising a blister
package supported by a freestanding base;
FIGS. 7C and 7D illustrate a packaging system comprising a blister
package supported by an interlocking cross-member;
FIGS. 7E and 7F illustrate a packaging system comprising a blister
package supported by integral fold-out cross-members; and,
FIGS. 7G and 7H illustrate a packaging system comprising a blister
package supported by an integral horizontal fold-out base.
DETAILED DESCRIPTION OF THE INVENTION
The present invention will now be described more fully herein with
reference to the accompanying drawings in which preferred embodiments of
the invention are shown. This invention may, however, be embodied in many
different forms and should not be construed as being limited to the
embodiments set forth herein. Rather, these embodiments are provided so that
this disclosure will be thorough and complete, and will fully convey the scope
of
the invention to those skilled in the art. As will be appreciated by one
skilled in
the art, the present invention may be embodied as a packaging system or
method.
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
8
The present invention is directed to a pharmaceutical packaging system
comprising a substantially planar medication storage device from which one or
more doses of a drug may be dispensed. In particular, this invention is
directed
to such packaging systems that maintain the storage device in a substantially
vertical orientation, and thus provide a visually prominent display to a
patient.
Such a vertically-oriented, visually prominent display of the storage device
is
intended to provide the patient with a visual cue that will serve to remind
him or
her of the need to comply with the prescribed dosing regimen associated with
the medication, and thus reduce the incidence of noncompliance. This
invention encompasses a variety of embodiments in which a medication
storage device is supported in its vertical orientation from any direction,
i.e.,
from the rear surface or front surface, from the top edge, or from either side
edge, a bottom edge, or a corner.
The many options included within the scope of the invention are
intended to provide the degree of flexibility in selection of mounting
location
required to achieve both visual prominence and convenience to the patient,
while also being out of the reach of any children who may be present, thus
avoiding potential pediatric overdoses. All embodiments provide for attachment
of the storage device to a mounting location that is intended to remain
constant
during the course of administration of the doses contained therein. This is
intended to encourage the patient to return the storage device to the same
mounting location after each administration, and thus avoid potential
mislocation of the storage device. In some embodiments, the storage device is
physically anchored or tethered to the mounting location, thus providing a
tangible reminder to the patient of the association between the storage device
and the mounting location and further reducing the possibility of
misplacement.
The storage device is attached to the mounting surface by a retaining
device. The retaining device comprises a mounting surface attachment feature
and a storage device attachment feature, either or both of which attachment
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
9
features may include the use of adhesive, magnet, or a fastener such as a
hook, peg, clamp, clip spring, a hook-and-loop system, base, suction cup and
the like. The fastener(s) must be fixed to a suitable location on the surface
(herein referred to as the "mounting location"). The fastener(s) may be so
fixed
to the mounting location on the surface of choice by use of fastening hardware
including one or more screws, nails, brads, staples, etc. Where the chosen
mounting location comprises a ferrous metal, the fastener(s) may include a
magnet in its base of suitable size to support the fastener and the storage
device supported therefrom. In the case of a clamp or spring clip, the spring
load of the fastener must be sufficient to retain the storage device at rest,
but
not so great as to hinder removal and replacement of the storage device by the
patient. Where the fastener is a hook or peg, there must be a hole suitably
sized and located in the storage device to allow the hook or peg to pass
therethrough, thus allowing the storage device to suspend vertically
therefrom.
Alternatively in the case of a hook, the hook may be sized and disposed to
engage a wire or loop appropriately disposed from the rear surface or edge of
the storage device, similar to a conventional picture-hanging arrangement.
The storage device may alternatively be fastened to the mounting
location by use of a hook-and-loop fabric system, such as that which goes by
the tradename of Velcro . The fabric backing of the two opposed hook-and-
loop strips can be attached to the mounting surface and the rear surface of
the
storage device or mounting structure by means of adhesive.
When adhesive is used to bond the storage device directly to the
mounting surface, the adhesive selected must be capable of retaining the
storage device on the mounting surface after repeated cycles of application to
and removal from the mounting surface. Assuming the storage device is
removed from the mounting surface once each time a dosage is administered,
and only one dosage is administered at a time, the minimum number of such
cycles to be expected will be the number of medication dosages contained in
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
the blister pack when new. Thus, for a fourteen-dose blister pack, the
adhesive
must be able to retain the blister pack to the mounting surface after at least
fourteen cycles of application and removal. Adhesives suitable for this sort
of
service include those categorized as "repositionable adhesives." Such
repositionable adhesives are described and exemplified in the literature,
e.g.,
U.S. Pat. no. 5, 795,636. Ultrastik is an example of a repositionable
adhesive.
The adhesive used to bond the storage device to the mounting surface
may be disposed on a pad that is raised from the remainder of the adjacent
surface of the storage device. The pad may be integral to the storage device,
or may be a distinct component, which is itself permanently bonded to the
storage device. Such a pad may have a relatively soft surface that will
conform
to any irregularities in the mounting surface, and thus maximize the effective
bonding area.
A patient may choose to locate the medication storage device in any
number of locations in the home, at work, etc. In the home, it may be
desirable
to locate the storage device on a convenient and visually accessible vertical
surface, for example a wall or a side of a medicine cabinet, vanity, or
refrigerator. FIGS. 1A, 1 B, 1 C, and 1 D illustrate a number of preferred
embodiments of this invention in which magnets or adhesive pads are fixed to
the rear surface of a blister package. Of course, magnets will only retain the
storage to ferrous surfaces, while the adhesive pads can be used for most flat
surfaces, whether ferrous or non-ferrous.
As illustrated, the magnets or adhesive may be disposed on raised pads
on the rear surface of the blister package locally at corners and/or at mid-
points
or other locations near the edges. The surface area of the magnets or surfaces
to which adhesive is applied should be designed to sufficiently retain the
storage device when loaded with the maximum number of medication doses.
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
11
Alternatively, the magnets or adhesive may be disposed on raised strips that
extend for a portion of, or substantially the entirety of the length, of an
edge of
the blister package on the rear surface. In alternate embodiments, greater
portions of the rear surface of the blister package, up to the entirety of the
surface, are used to dispose a magnet or adhesive.
Alternatively, the rear surface of the blister package may have one or
more ferrous surfaces attached or incorporated therein. One or more magnetic
pads can then be used to detachably engage the ferrous surface on the rear
surface of the blister pack, while being more strongly attached to the
mounting
surface by adhesive or a mechanical fastener, such as a bolt.
FIGS. 2A and 2B illustrate embodiments of the storage device in which a,
blister package is supported from a mounting structure through a hinge
arrangement arranged, alternatively, horizontally or vertically. FIGS. 2C and
2D are side views of a horizontally mounted hinged packaging system. The
mounting structure may be a flat panel that mounts directly to the mounting
surface by adhesive, fastener, or magnet. The hinge joint allows the blister
package to pivot away from the mounting structure, thus allowing access to the
rear surface of the blister package and removal of a dosage form therefrom,
without physically detaching the blister package from the mounting structure.
This is an example of an embodiment in which the storage device is physically
anchored to the mounting location.
In yet another set of embodiments, the blister package is suspended
from a surface mounted fastener such as a hook or a peg. The blister package
has at least one hole sized to accommodate the hook or peg. The fastener
may be mounted to the mounting surface via a magnet or adhesive. FIGS. 3A,
3B and 3C illustrate embodiments in which a blister package is suspended from
one or two hooks.
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
12
In yet another set of embodiments, the blister package is suspended
from a grooved retaining fastener which features a lip or groove sized and
located to accommodate a surface edge of the blister pack. FIGS. 4A, 4B and
4C illustrate the use of a rectangular grooved retaining fastener that has a
lip
disposed on its top edge. The lip is dimensioned to accommodate the upper
edge of the cut out central area of the blister pack, and when so disposed, to
support the blister pack against the vertical surface. The groove may be
dimensioned to allow the blister pack to be easily lifted out. In the
embodiment
illustrated in FIG. 4B, the blister pack is open at one end, allowing for its
removal by laterally sliding it across the mounting surface. The grooved
retaining fastener may be fixed to the mounting surface by magnet, adhesive
clamp, spring clip, hook-and-loop system or other fastening device, as
described above. A tether may be used to tie the blister package to the
grooved retaining fastener. These embodiments have the added advantage of
providing a prominent surface on the grooved retaining fastener to display
information of value to the patient, such as dosing instructions, warnings, or
merely, a reminder to replace the blister package after each administration of
the dosage form.
In some embodiments, the storage device may be suspended from a
horizontal surface, to which a fastener is attached either permanently or
detachably. The horizontal surface may be, for example, a ceiling or a lower
surface of a shelf or a cabinet and the fastener may be a magnet, adhesive
clamp, spring clip, hook-and-loop system or other fastening device, as
described above. FIGS. 5A, 5B and 5C illustrate embodiments of the
suspension of a blister package from a horizontal surface by use of,
alternatively, a clamp or a spring clip.
In other embodiments, the storage device is suspended by one of its
vertical edges. This arrangement allows the storage device to be very
prominently suspended from the edge of a cabinet, refrigerator, or wall, for
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
13
example. This embodiment may include the use of adhesive, one or more
magnets, spring clip, clamp or other fastener disposed on the vertical surface
at
the mounting surface and suitable for supportingly engaging an edge of the
storage device. FIGS. 6A, 6B and 6C illustrate embodiments in which the
fastener comprises, alternatively, a clamp or a spring clip, and the blister
package is attached to the fastener by means of a tether. The tether is long
enough to allow sufficient access to the blister package to allow removal of a
dosage but prevents the patient from removing the blister package to a
distance beyond its length. This is another example of an embodiment in which
the storage device is physically anchored to the mounting location.
In yet other embodiments, the medication storage device is supported in
its vertical orientation by a stand that reposes on a horizontal surface, such
as
a table top or a counter top. The medication storage device may be vertically
supported by a stand that is either integral to, or distinct from, the storage
device.
In embodiments wherein the stand is distinct to the dispensing product,
the stand may clip or pinch the base of the dispensing product. The stand may
freely rest on the horizontal surface without any fixity thereto, or
alternatively,
may be fixed to the mounting surface by any of the fasteners mentioned above.
FIGS. 7A and 7B illustrate an embodiment of a blister package supported from
a horizontal surface by a stand that is distinct to the blister package. FIGS.
7C
and 7D illustrate an embodiment in which the bottom edge of the blister
package has a vertical notch dimensioned to accommodate a distinct cross-
member that has a matching vertical notch. When assembled as shown in the
figures, the blister package and cross-member interlock to form a base.
In embodiments wherein the stand is integral to the storage device,
supporting members may fold out from the base of the storage device to
provide lateral stability, and maintain the vertical orientation of the
storage
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
14
device. The supporting members may be flaps that are integrally bonded or
otherwise attached to the lower part of the storage device. Alternatively, the
cross-members may be formed by perforated segments of the lower edge of
the blister package that can be folded outward, as shown in FIGS. 7E and 7F.
In yet another embodiment, the base can be formed by appropriately folding
the lower edge of the blister package at horizontal locations, as shown in
FIGS.
7G and 7H. The fold locations may be marked and of lesser cross-section or
stiffness to facilitate folding.
As stated above, the present invention is directed to pharmaceutical
storage systems designed to maintain an essentially planar medication storage
device in a vertical orientation. In many preferred embodiments, the
medication storage device is a conventional blister-pack card. A typical
blister
pack card comprises a translucent sheet and a rupturable or pealable backing,
and is mounted atop a sheet having a plurality of cutouts, each cutout
defining
an opening having an area large enough for one dose of medication to pass
through. The cutouts can be perforated or non-perforated. A translucent sheet
is placed over a portion of the first sheet, essentially overlapping it. The
translucent sheet forms a plurality of blisters and is preferably manufactured
from clear plastic, but can be manufactured from any other comparable
material or combination of materials known to those skilled in the art. Each
blister has a hollow cavity upon which one dose of medication is stored. A
rupturable or pealable backing seals the hollow cavity so as to prevent
contamination of dose of medication, each backing positioned to overlap each
opening. In the case of a rupturable backing, the backing is preferably
manufactured using a flexible, rupturable material such as a thin metallic
sheet,
however it can be manufactured using plastic or any other comparable material
or combination of materials known to those skilled in the art. The rupturable
backing is sandwiched in between the translucent sheet and the first sheet.
When a user wishes to take dose of medication from the packaging system, all
the user must do is apply pressure against the blister, causing a dose of the
CA 02673236 2009-06-18
WO 2008/079306 PCT/US2007/026098
medication to break the rupturable backing and exit the packaging system
through the opening.
While in the foregoing specification this invention has been described in
relation to certain preferred embodiments thereof, and many details have been
set forth for purpose of illustration, it will be apparent to those skilled in
the art
that the invention is susceptible to additional embodiments and that certain
of
the details described herein can be varied considerably without departing from
the basic principles of the invention.