Note: Descriptions are shown in the official language in which they were submitted.
CA 02674113 2009-07-27
PATENT APPLICATION
Title
METHOD AND COMPOSITION TO INCREASE VISCOSITY OF CROSSLINKED
POLYMER FLUIDS
Field of the Invention
[0001] This invention relates to compositions and methods for treating
subterranean
formations penetrated by well bores. More particularly, the invention relates
to enhance the
production of oil or gas using a viscosifying agent based upon crosslinked
polymer fluid
showing increased viscosity.
Background
[0002] The statements in this section merely provide background information
related to
the present disclosure and may not constitute prior art.
[0003] Polymers are used in a wide variety of ways to enhance the production
of oil or
gas from underground formations. Usually the function of the polymer is to
control the
viscosity of the aqueous fluids which are injected into the formation. For
example, in water
flooding the efficiency of the water flood is improved by adding a water
soluble polymer
to the aqueous phase and thereby decreasing the mobility difference between
the injected
water and the oil in place. Polymers are also used in acidizing and/or
fracture acidizing in
which acidic compositions are used to stimulate production of hydrocarbon from
underground formations by increasing the formation porosity. A water soluble
or water
dispersible polymer is incorporated to increase the viscosity of the fluid so
that wider
fractures can be developed and live acid can be forced farther into the
formations. This
increases the proppant carrying capacity of the acid solutions and permits
better fluid loss
control.
1
CA 02674113 2009-07-27
[0004] Generally high molecular weight polymers or polymers with various
gelling or
crosslinking agents are used for this purpose. Most commercially available
polymeric
viscosifiers, however, are degraded by the hostile reservoir environment
including high
temperatures, acidity and extreme shear conditions, as well as by the
electrolytes which are
encountered in the oil recovery process. For example, hydrolyzed
polyacrylamides fail in
sea water solution at elevated temperatures due to precipitation of the
polymer in the
presence of calcium ions in the sea water. Xanthan polymers are insensitive to
calcium
ions but these polymers degrade at high temperatures and lose their
viscosifying efficiency.
[0005] Also, conventional crosslinked polymer fracturing fluids have several
inherent
characteristics. The viscosity of a crosslinked polymer fluid with a given
polymer
concentration decreases with time and/or temperature. Hence the polymer
concentration is
increased in order to maintain a given or required viscosity for a longer
period of time or to
achieve the required viscosity at higher temperatures. The fluid loss control
of the
crosslinked polymer fluid in a formation with a given permeability is
dependent to great
extent on the polymer concentration. Increasing the polymer concentration in
general will
improve the fluid loss control as the polymer creates a filter cake on the
face of the
formation. Increasing polymer concentrations in the fluid result in lower
fracture
conductivity and retained permeability in the fracture faces. Both decrease
the productivity
of the final propped fracture. Exposure to high shear tends to degrade the
properties of the
crosslinked polymer fluid: to a lesser or greater degree the viscosity of the
crosslinked
fluid is reduced after it has been exposed to high shear (1000/s) which is
common when
displacing the f]uid in a workstring to the perforations. The time for the
fluid to recover
viscosity after being exposed to high shear may take minutes and it is during
this time that
the fluid/proppant is entering into the hydraulic fracturing. The reduced
viscosity of the
fluid results in a narrower hydraulic fracture and so increase the risk of the
proppant
screening out in the well bore.
[0006] To combat these problems associated with polymeric gelling agents, some
surfactants have been used as gelling agents. In particular cases, some
surfactants, when
mixed with an aqueous fluid having a certain ionic strength, are capable of
forming a
viscous fluid that has certain elastic properties, one of which may be shear
thinning.
Surfactant molecules (or ions) at specific conditions may form micelles (e.g.,
worm-shaped
2
CA 02674113 2009-07-27
micelles, rod-shaped micelles, etc.) in an aqueous fluid. Depending on, among
other
things, the surfactant concentration, and the ionic strength of the fluid,
etc., these micelles
may impart increased viscosity to the aqueous fluid, such that the fluid
exhibits
viscoelastic behavior due, at least in part, to the association of the
surfactant molecules
contained therein.
[0007] As a result, these treatment fluids exhibiting viscoelastic behavior
may be used in a
variety of subterranean treatments where a viscosified treatment fluid may be
useful.
Because the micelles may be sensitive to the pH and hydrocarbons, the
viscosity of these
treatment fluids may be reduced after introduction into the subterranean
formation without
the need for conventional gel breakers (e.g., oxidizers). This may allow a
substantial
portion of the treatment fluid to be produced back from the formation without
the need for
expensive remedial treatments.
100081 In the same way, fracturing fluids with viscoelastic surfactants have
also several
inherent characteristics. As a solids free fluid, they may not create residual
damage in
either proppant pack or the faces of the fractures. As a solids free fluid,
they may have
limited fluid loss control in high permeability formations. No filter cake is
formed so the
fluid loss may be a function of the viscosity of the fluid, permeability of
the formation and
properties of the reservoir fluids. One fluid can easily displace the other in
the porous
medium under reservoir conditions. High concentrations of surfactant arc
required to create
a fluid with sufficient viscosity to create a hydraulic fracture in any
formation with
permeability greater than a few millidarcy. The viscosity of a fluid with a
given
concentration is very sensitive to any change in temperature above 150 Deg F
and in
almost every case drops dramatically. Compatibility with formation crude as
the VES
viscosity is very sensitive to the presence of surfactants or demulsifiers.
[0009] The objective is to create a hybrid fluid which combines a low
concentration of
VES and a crosslinked polymer fluid. The final fluid will overcome to some
degree the
technical and economic disadvantages of crosslinked polymer and VES fluids
taken
separately.
3
CA 02674113 2009-07-27
Summary
[0010] In an embodiment, a well treatment composition for use in a
subterranean
formation includes a carrier fluid, and a viscoelastic surfactant being
present in a
concentration of less than about 1.5% by weight. Optionally, the fluid
comprises a
crosslinked polymer in a thickening amount in the carrier fluid.
[0011] In another embodiment, the viscoelastic surfactant is present in a
concentration of
less than about 1% or less than about 0.8% by weight.
[0012] In a further embodiment, the crosslinked polymer may be one of
polysaccharides,
substituted galactomannans, guar gums, high-molecular weight polysaccharides
composed
of mannose and galactose sugars, guar derivatives, hydroxypropyl guar (HPG),
carboxymethylhydroxypropyl guar (CMHPG), carboxymethyl guar (CMG),
hydrophobically modified guars, guar-containing compounds, synthetic polymers
and
mixtures thereof. The composition may further contain a co-surfactant. The
viscoelastic
surfactant may be a zwitterionic surfactant. In one embodiment, the
viscoelastic surfactant
is betaine. The carrier fluid may be any suitable medium, such as, but not
limited to, an
aqueous based fluid.
[0013] In a further aspect, a method of treating a subterranean formation from
a well
includes providing a carrier fluid comprising a viscoelastic surfactant in a
concentration of
less than about 1.5% by weight based upon total fluid weight, and introducing
the fluid
into the well. Optionally, the fluid comprises a crosslinked polymer in a
thickening amount
in the carrier fluid.
[0014] In one embodiment, the method further involves contacting the fluid and
the
subterranean formation. In a second embodiment, the method further includes
fracturing
the subterranean formation.
[0015] The fluid may have proppant. In another embodiment, the viscoelastic
surfactant is
present in a concentration of less than about 1% or less than about 0.8% by
weight, based
upon total fluid weight.
[0016] In a further aspect, a method to increase the viscosity of a fluid
includes providing
a fluid comprising a thickening amount of a crosslinked polymer, adding a
viscoelastic
4
CA 02674113 2009-07-27
surfactant at a given concentration to the fluid, adjusting the fluid to a
given temperature,
defining a viscosity profile of the fluid depending of the concentration and
the temperature,
comparing said viscosity profile to a viscosity profile of the crosslinked
polymer fluid
alone, and defining an optimum concentration of the viscoelastic surfactant
for each
temperature. -
[0017] In an embodiment, the given temperature is between 45degC and 95degC.
Brief Description of the Drawings
[0018] The following figures form part of the present specification and are
included to
further demonstrate certain aspects of the present invention. The invention
may be better
understood by reference to one or more of these figures in combination with
the detailed
description of specific embodiments presented herein. The components in the
figures are
not necessarily to scale, with the emphasis instead being placed upon clearly
illustrating
principles of the present invention.
[0019] Figure 1 shows graph comparing viscosity of crosslinked polymer fluid
alone and
crosslinked polymer fluid and VES at 54.4 degC.
[0020] Figure 2 shows graph comparing viscosity of crosslinked polymer fluid
alone and
crosslinked polymer fluid and VES at 54.4 degC with shear 2 minutes at 1000/s.
[0021] Figure 3 shows graph comparing viscosity of crosslinked polymer fluid
alone and
crosslinked polymer fluid and VES at 71.1 degC.
[0022] Figure 4 shows graph comparing viscosity of crosslinked polymer fluid
alone and
crosslinked polymer fluid and VES for a different concentration at 71.1 degC.
[0023] Figure 5 shows graph of the viscosity of crosslinked polymer fluid and
VES for a
different concentration at 71.1 degC.
[0024] Figure 6 shows graph of the viscosity of crosslinked polymer fluid and
VES at 82.2
degC.
[0025] Figure 7 shows graph of the viscosity of crosslinked polymer fluid and
VES for a
different concentration at 82.2 degC.
CA 02674113 2009-07-27
[0026] Figure 8 shows fluid loss of the composition according to one
embodiment of the
invention at 71.1 degC.
[0027] Figure 9, Figure 10 and Figure 11 show comparison of fluid loss control
between
composition of crosslinked polymer fluid alone and two compositions of
crosslinked
polymer fluid with different concentration of VES at 71.1 degC.
Detailed Description
[0028] At the outset, it should be noted that in the development of any actual
embodiments, numerous implementation-specific decisions must be made to
achieve the
developer's specific goals, such as compliance with system- and business-
related
constraints, which can vary from one implementation to another. Moreover, it
will be
appreciated that such a development effort might be complex and time consuming
but
would nevertheless be a routine undertaking for those of ordinary skill in the
art having the
benefit of this disclosure.
[0029] The description and examples are presented solely for the purpose of
illustrating
the embodiments of the invention and should not be construed as a limitation
to the scope
and applicability of the invention. While the compositions of the present
invention are
described herein as comprising certain materials, it should be understood that
the
composition could optionally comprise two or more chemically different
materials. In
addition, the composition can also comprise some components other than the
ones already
cited. In the summary of the invention and this detailed description, each
numerical value
should be read once as modified by the term "about" (unless already expressly
so
modified), and then read again as not so modified unless otherwise indicated
in context.
Also, in the summary of the invention and this detailed description, it should
be understood
that a concentration range listed or described as being useful, suitable, or
the like, is
intended that any and every concentration within the range, including the end
points, is to
be considered as having been stated. For example, "a range of from 1 to 10" is
to be read
as indicating each and every possible number along the continuum between about
1 and
about 10. Thus, even if specific data points within the range, or even no data
points within
the range, are explicitly identified or refer to only a few specific, it is to
be understood that
6
CA 02674113 2009-07-27
inventors appreciate and understand that any and all data points within the
range are to be
considered to have been specified, and that inventors possession of the entire
range and all
points within the range.
[0030] The following definitions are provided in order to aid those skilled in
the art in
understanding the detailed description of the invention.
[0031] The term "fracturing" refers to the process and methods of breaking
down a
geological formation and creating a fracture, i.e. the rock formation around a
well bore, by
pumping fluid at very high pressures, in order to increase production rates
from a
hydrocarbon reservoir. The fracturing methods otherwise use conventional
techniques
known in the art.
[0032] The term "surfactant" refers to a soluble or partially soluble compound
that reduces
the surface tension of liquids, or reduces inter-facial tension between two
liquids, or a
liquid and a solid by congregating and orienting itself at these interfaces.
[0033] The term "viscoelastic" refers to those viscous fluids having elastic
properties, i.e.,
the liquid at least partially returns to its original form when an applied
stress is released.
[0034] The phrase "viscoelastic surfactant" or "VES" refers to that class of
compounds
which can form micelles (spherulitic, anisometric, lamellar, or liquid
crystal) in the
presence of counter ions in aqueous solutions, thereby imparting viscosity to
the fluid.
Anisometric micelles can be used, as their behavior in solution most closely
resembles that
of a polymer.
[0035] One embodiment is directed towards a well treatment composition for use
in a
subterranean formation comprising a carrier fluid; a crosslinked polymer; and
a
viscoelastic surfactant being present in a concentration of less than about
1.5% by weight.
[0036] The carrier fluid can generally be any liquid carrier suitable for use
in oil and gas
producing wells. One such liquid carrier is water. The liquid carrier can
comprise water,
can consist essentially of water, or can consist of water. Water will
typically be a major
component by weight of the fluid. The water can be potable or non-potable
water. The
water can be brackish or contain other materials typical of sources of water
found in or
near oil fields.
[0037] A salt may be present in the fluid carrier. The salt can be present
naturally if brine
7
CA 02674113 2009-07-27
is used, or can be added to the fluid carrier. For example, it is possible to
add to water; any
salt, such as an alkali metal or alkali earth metal salt (NaCO3, NaC1, KCI,
etc.). The salt is
generally present in weight percent concentration between about 0.1% to about
5%, from
about 1% to about 3% by weight. One useful concentration is about 2% by
weight.
[0038] The crosslinked polymer can generally be any crosslinked polymers. The
polymer
viscosifier can be a metal-crosslinked polymer. Suitable polymers for making
the metal-
crosslinked polymer viscosifiers include, for example, polysaccharides such as
substituted
galactomannans, such as guar gums, high-molecular weight polysaccharides
composed of
mannose and galactose sugars, or guar derivatives such as hydroxypropyl guar
(HPG),
carboxymethylhydroxypropyl guar (CMHPG) and carboxymethyl guar (CMG),
hydrophobically modified guars, guar-containing compounds, and synthetic
polymers.
Crosslinking agents based on boron, titanium, zirconium or aluminum complexes
are
typically used to increase the effective molecular weight of the polymer and
make them
better suited for use in high-temperature wells.
[0039] Other suitable classes of polymers effective as viscosifiers include
polyvinyl
polymers, polymethacrylamides, cellulose ethers, lignosulfonates, and
ammonium, alkali
metal, and alkaline earth salts thereof. More specific examples of other
typical water
soluble polymers are acrylic acid-acrylamide copolymers, acrylic acid-
methacrylamide
copolymers, polyacrylamides, partially hydrolyzed polyacrylamides, partially
hydrolyzed
polymethacrylamides, polyvinyl alcohol, polyalkyleneoxides, other
galactomannans,
heteropolysaccharides obtained by the fermentation of starch-derived sugar and
ammonium and alkali metal salts thereof.
[0040] Cellulose derivatives are used to a smaller extent, such as
hydroxyethylcellulose
(HEC) or hydroxypropylcellulose (HPC), carboxymethylhydroxyethylcellulose
(CMHEC)
and carboxymethycellulose (CMC), with or without crosslinkers. Xanthan,
diutan, and
scleroglucan, three biopolymers, have been shown to have excellent proppant-
suspension
ability even though they are more expensive than guar derivatives and
therefore have been
used less frequently, unless they can be used at lower concentrations.
[0041] In other embodiments, the crosslinked polymer is made from a
crosslinkable,
hydratable polymer and a delayed crosslinking agent, wherein the crosslinking
agent
comprises a complex comprising a metal and a first ligand selected from the
group
8
CA 02674113 2009-07-27
consisting of amino acids, phosphono acids, and salts or derivatives thereof.
Also the
crosslinked polymercan be made from a polymer comprising pendant ionic
moieties, a
surfactant comprising oppositely charged moieties, a clay stabilizer, a borate
source, and a
metal crosslinker. Said embodiments are described in U.S. Patent Publications
US2008-
0280790 and US2008-0280788 respectively.
[0042] Linear (not cross-linked) polymer systems may be used. However, in some
cases, may not achieve the full benefits because they may require more
concentration, and
may require a breaker. Any suitable crosslinked polymer system may be used,
including
for example, those which are delayed, optimized for high temperature,
optimized for use
with sea water, buffered at various pH's, and optimized for low temperature.
Any
crosslinker may be used, for example boron, titanium, zirconium, aluminum and
the like.
Suitable boron crosslinked polymers systems include by non-limiting example,
guar and
substituted guars crosslinked with boric acid, sodium tetraborate, and
encapsulated borates;
borate crosslinkers may be used with buffers and pH control agents such as
sodium
hydroxide, magnesium oxide, sodium sesquicarbonate, and sodium carbonate,
amines
(such as hydroxyalkyl amines, anilines, pyridines, pyrimidines, quinolines,
and
pyrrolidines, and carboxylates such as acetates and oxalates) and with delay
agents such as
sorbitol, aldehydes, and sodium gluconate. Suitable zirconium crosslinked
polymer
systems include by non-limiting example, those crosslinked by zirconium
lactates (for
example sodium zirconium lactate), triethanolamines, 2,2'-iminodiethanol, and
with
mixtures of these ligands, including when adjusted with bicarbonate. Suitable
titanates
include by non-limiting example, lactates and triethanolamines, and mixtures,
for example
delayed with hydroxyacetic acid. Any other chemical additives may be used or
included
provided that they are tested for compatibility with the viscoelastic
surfactant. For
example, some of the standard crosslinkers or polymers as concentrates usually
contain
materials such as isopropanol, n-propanol, methanol or diesel oil.
[0043] The viscoelastic surfactant can generally be any viscoelastic
surfactant. The
surfactant is present in a low weight percent concentration. Some suitable
concentrations
of surfactant are about 0.001% to about 1.5% by weight, from about 0.01% to
about 0.75%
by weight, or even about 0.25%, about 0.5% or about 0.75% by weight.
[0044] However, it should be noted the effect of increasing VES concentration
is not
9
CA 02674113 2009-07-27
limited to 1.5% by weight. The increase in viscosity due to the addition VES
appears to
increase in an approximately linear manner with increasing concentration of
VES up to
1.5%, the highest concentration tested. While economically it makes less sense
to include
higher VES concentrations it is reasonable to presume that the same linear
increase in
viscosity will occur with higher concentrations of VES.
[0045] The VES may be selected from the group consisting of cationic, anionic,
zwitterionic, aniphoteric, nonionic and combinations thereof. Some non-
limiting examples
are those cited in U.S. Patents 6,435,277 (Qu et al.) and 6,703,352
(Dahayanake et al.).
The viscoelastic surfactants, when used alone or in combination, are capable
of forming
micelles that form a structure in an aqueous environment that contribute to
the increased
viscosity of the fluid (also referred to as "viscosifying micelles"). These
fluids are
normally prepared by mixing in appropriate amounts of VES suitable to achieve
the
desired viscosity. The viscosity of VES fluids may be attributed to the three
dimensional
structure formed by the components in the fluids. When the concentration of
surfactants in
a viscoelastic fluid significantly exceeds a critical concentration, and in
most cases in the
presence of an electrolyte, surfactant molecules aggregate into species such
as micelles,
which can interact to form a network exhibiting viscous and elastic behavior.
[0046] Non-limiting examples of suitable viscoelastic surfactants useful for
viscosifying
some fluids include cationic surfactants, anionic surfactants, zwitterionic
surfactants,
amphoteric surfactants, nonionic surfactants, and combinations thereof.
[0047] In general, particularly suitable zwitterionic surfactants have the
formula:
RCONH- (CH2) a(CH2CH2O) m(CH2) b-N+ (CH3) 2- (CHz) a. (CH2CH2O) m= (CH2) b=COO-
in which R is an alkyl group that contains from about 11 to about 23 carbon
atoms which
may be branched or straight chained and which may be saturated or unsaturated;
a, b, a',
and b' are each from 0 to 10 and m and m' are each from 0 to 13; a and b are
each 1 or 2 if
m is not 0 and (a + b) is from 2 to 10 if m is 0; a' and b' are each 1 or 2
when m' is not 0
and (a' + b') is from 1 to 5 if m is 0; (m + m') is from 0 to 14; and CHZCH2O
may also be
OCH2CH2.
[0048] In an embodiment of the invention, a zwitterionic surfactants of the
family of
betaine is used. Two suitable examples of betaines are BET-O and BET-E. The
surfactant
CA 02674113 2009-07-27
in BET-O-30 is shown below; one chemical name is oleylamidopropyl betaine. It
is
designated BET-O-30 because as obtained from the supplier (Rhodia, Inc.
Cranbury, New
Jersey, U. S. A.) it is called Mirataine BET-O-30 because it contains an oleyl
acid amide
group (including a C17H33 alkene tail group) and contains about 30% active
surfactant; the
remainder is substantially water, sodium chloride, and propylene glycol. An
analogous
material, BET-E-40, is also available from Rhodia and contains an erucic acid
amide group
(including a C21 H41 alkene tail group) and is approximately 40% active
ingredient, with the
remainder being substantially water, sodium chloride, and isopropanol. VES
systems, in
particular BET-E-40, optionally contain about 1% of a condensation product of
a
naphthalene sulfonic acid, for example sodium polynaphthalene sulfonate, as a
rheology
modifier, as described in U. S. Patent Application Publication No. 2003-
0134751. The
surfactant in BET-E-40 is also shown below; one chemical name is
erucylamidopropyl
betaine. As-received concentrates of BET-E-40 were used in the experiments
reported
below, where they will be referred to as "VES". BET surfactants, and other
VES's that are
suitable for the embodiments according to the invention, are described in U.
S. Patent No.
6,258,859. According to that patent, BET surfactants make viscoelastic gels
when in the
presence of certain organic acids, organic acid salts, or inorganic salts; in
that patent, the
inorganic salts were present at a weight concentration up to about 30%. Co-
surfactants
may be useful in extending the brine tolerance, and to increase the gel
strength and to
reduce the shear sensitivity of the VES-fluid, in particular for BET-O-type
surfactants. An
example given in U. S. Patent No. 6,258,859 is sodium dodecylbenzene sulfonate
(SDBS),
also shown below. Other suitable co-surfactants include, for example those
having the
SDBS-like structure in which x = 5 - 15; other co-surfactants are those in
which x = 7 -
15. Still other suitable co-surfactants for BET-O-30 are certain chelating
agents such as
trisodium hydroxyethylethylenediamine triacetate. The rheology enhancers of
the
embodiments according to the invention may be used with viscoelastic
surfactant fluid
systems that contain such additives as co-surfactants, organic acids, organic
acid salts,
and/or inorganic salts.
11
CA 02674113 2009-07-27
H H3C O
I \+.(CH2)p
C17H33 YN Q
\ (CH2 n \ CH3
O
Surfactant in BET-O-30 (when n = 3 and p 1)
H H3C O
\+,-(CH2)p
C21 H41 N \ O
(CH2 r CH3
O
Surfactant in BET-E-40 (when n = 3 and p 1)
SO3
\i
(CH2)xCH3
SDBS (when x = 11 and the counter-ion is Na)
[0049] Some embodiments use betaines; for example BET-E-40. Although
experiments
have not been performed, it is believed that mixtures of betaines, especially
BET-E-40,
with other surfactants are also suitable. Such mixtures are within the scope
of
embodiments of the invention.
[0050) Other betaines that are suitable include those in which the alkene side
chain (tail
group) contains 17 - 23 carbon atoms (not counting the carbonyl carbon atom)
which may
be branched or straight chained and which may be saturated or unsaturated, n =
2 - 10, and
p = 1 - 5, and mixtures of these compounds. Some betaines are those in which
the alkene
12
CA 02674113 2009-07-27
side chain contains 17 - 21 carbon atoms (not counting the carbonyl carbon
atom) which
may be branched or straight chained and which may be saturated or unsaturated,
n = 3 - 5,
and p = 1- 3, and mixtures of these compounds. These surfactants are used at a
concentration of about 0.5 to about 10%, or from about 1 to about 5%, or even
from about
1.5 to about 4.5%.
[0051] Exemplary cationic viscoelastic surfactants include the amine salts and
quatemary amine salts disclosed in U.S. Patent Nos. 5,979,557, and 6,435,277
which have
a common Assignee as the present application. Examples of suitable cationic
viscoelastic
surfactants include cationic surfactants having the structure:
RlN+(R2)(Rs)(Ra) X-
[00521 in which Rl has from about 14 to about 26 carbon atoms and may be
branched or
straight chained, aromatic, saturated or unsaturated, and may contain a
carbonyl, an amide,
a retroamide, an imide, a urea, or an amine; R2 , R3, and R4 are each
independently
hydrogen or a C1 to about C6 aliphatic group which may be the same or
different, branched
or straight chained, saturated or unsaturated and one or more than one of
which may be
substituted with a group that renders the R2, R3, and R4 group more
hydrophilic; the R2, R3
and R4 groups may be incorporated into a heterocyclic 5- or 6-member ring
structure which
includes the nitrogen atom; the R2, R3 and R4 groups may be the same or
different; Rl, R2,
R3 and/or R4 may contain one or more ethylene oxide and/or propylene oxide
units; and X-
is an anion. Mixtures of such compounds are also suitable. As a further
example, Rl is
from about 18 to about 22 carbon atoms and may contain a carbonyl, an amide,
or an
amine, and R2, R3, and R4 are the same as one another and contain from 1 to
about 3 carbon
atoms.
[0053] Cationic surfactants having the structure R1N+(R2)(R3)(R4) X- may
optionally
contain amines. having the structure R1N(R2)(R3). It is well known that
commercially
available cationic quatemary amine surfactants often contain the corresponding
amines (in
which Rt, R2, and R3 in the cationic surfactant and in the amine have the same
structure).
As received commercially available VES surfactant concentrate formulations,
for example
cationic VES surfactant formulations, may also optionally contain one or more
members of
the group consisting of alcohols, glycols, organic salts, chelating agents,
solvents, mutual
solvents, organic acids, organic acid salts, inorganic salts, oligomers,
polymers, co-
13
CA 02674113 2009-07-27
polymers, and mixtures of these members. They may also contain performance
enhancers,
such as viscosity enhancers, for example polysulfonates, for example
polysulfonic acids, as
described in U. S. Patent No. 7,084,095.
[0054] Another suitable cationic VES is erucyl bis(2-hydroxyethyl) methyl
ammonium
chloride, also known as (Z)-13 docosenyl-N-N- bis (2-hydroxyethyl) methyl
ammonium
chloride. It is commonly obtained from manufacturers as a mixture containing
about 60
weight percent surfactant in a mixture of isopropanol, ethylene glycol, and
water. Other
suitable amine salts and quaternary amine salts include (either alone or in
combination in
accordance with the invention), erucyl trimethyl ammonium chloride; N-methyl-
N,N-
bis(2-hydroxyethyl) rapeseed ammonium chloride; oleyl methyl bis(hydroxyethyl)
ammonium chloride; erucylamidopropyltrimethylamine chloride, octadecyl methyl
bis(hydroxyethyl) ammonium bromide; octadecyl tris(hydroxyethyl) ammonium
bromide;
octadecyl dimethyl hydroxyethyl ammonium bromide; cetyl dimethyl hydroxyethyl
ammonium bromide; cetyl methyl bis(hydroxyethyl) ammonium salicylate; cetyl
methyl
bis(hydroxyethyl) ammonium 3,4,-dichlorobenzoate; cetyl tris(hydroxyethyl)
ammonium
iodide; cosyl dimethyl hydroxyethyl ammonium bromide; cosyl methyl
bis(hydroxyethyl)
ammonium chloride; cosyl tris(hydroxyethyl) ammonium bromide; dicosyl dimethyl
hydroxyethyl ammonium bromide; dicosyl methyl bis(hydroxyethyl) ammonium
chloride;
dicosyl tris(hydroxyethyl) ammonium bromide; hexadecyl ethyl bis(hydroxyethyl)
ammonium chloride; hexadecyl isopropyl bis(hydroxyethyl) ammonium iodide; and
cetylamino, N-octadecyl pyridinium chloride.
[0055] Many fluids made with viscoelastic surfactant systems, for example
those
containing cationic surfactants having structures similar to that of erucyl
bis(2-
hydroxyethyl) methyl ammonium chloride, inherently have short re-heal times
and the
rheology enhancers of the embodiments according to the invention may not be
needed
except under special circumstances, for example at very low temperature.
[0056] Amphoteric viscoelastic surfactants are also suitable. Exemplary
amphoteric
viscoelastic surfactant systems include those described in U.S. Patent No.
6,703,352, for
example amine oxides. Other exemplary viscoelastic surfactant systems include
those
described in U.S. Patents Nos. 6,239,183; 6,506,710; 7,060,661; 7,303,018; and
7,510,009
for example amidoamine oxides. Mixtures of zwitterionic surfactants and
amphoteric
14
CA 02674113 2009-07-27
surfactants are suitable. An example is a mixture of about 13% isopropanol,
about 5% 1-
butanol, about 15% ethylene glycol monobutyl ether, about 4% sodium chloride,
about
30% water, about 30% cocoamidopropyl betaine, and about 2%
cocoamidopropylamine
oxide.
[0057] The viscoelastic surfactant system may also be based upon any suitable
anionic
surfactant. In some embodiments, the anionic surfactant is an alkyl
sarcosinate. The alkyl
sarcosinate can generally have any number of carbon atoms. Alkyl sarcosinates
can have
about 12 to about 24 carbon atoms. The alkyl sarcosinate can have about 14 to
about 18
carbon atoms. Specific examples of the number of carbon atoms include 12, 14,
16, 18, 20,
22, and 24 carbon atoms. The anionic surfactant is represented by the chemical
formula:
Rl CON(R2)CH 2X
wherein RI is a hydrophobic chain having about 12 to about 24 carbon atoms, R2
is
hydrogen, methyl, ethyl, propyl, or butyl, and X is carboxyl or sulfonyl. The
hydrophobic
chain can be an alkyl group, an alkenyl group, an alkylarylalkyl group, or an
alkoxyalkyl
group. Specific examples of the hydrophobic chain include a tetradecyl group,
a hexadecyl
group, an octadecentyl group, an octadecyl group, and a docosenoic group.
[0058] To provide the ionic strength for the desired micelle formation, in
some cases, the
treatment fluids of the embodiments according to the invention may comprise a
water-
soluble salt. Adding a salt may help promote micelle formation for the
viscosification of
the fluid in some instances. In some embodiments, the aqueous base fluid may
contain the
water-soluble salt, for example, where saltwater, a brine, or seawater is used
as the aqueous
base fluid. Suitable water-soluble salts may comprise lithium, ammonium,
sodium,
potassium, cesium, magnesium, calcium, or zinc cations, and chloride, bromide,
iodide,
formate, nitrate, acetate, cyanate, or thiocyanate anions. Examples of
suitable water-
soluble salts that comprise the above-listed anions and cations include, but
are not limited
to, ammonium chloride, lithium bromide, lithium chloride, lithium formate,
lithium nitrate,
calcium bromide, calciurim chloride, calcium nitrate, calcium formate, sodium
bromide,
sodium chloride, sodium formate, sodium nitrate, potassium chloride, potassium
bromide,
potassium nitrate, potassium formate, cesium nitrate, cesium formate, cesium
chloride,
cesium bromide, magnesium chloride, magnesium bromide, zinc chloride, and zinc
CA 02674113 2009-07-27
bromide.
[0059] The composition also typically contains proppants. The selection of a
proppant
involves many compromises imposed by economical and practical considerations.
Criteria
for selecting the proppant type, size, and concentration is based on the
needed
dimensionless conductivity, and can be selected by a skilled artisan. Such
proppants can be
natural or synthetic (including but not limited to glass beads, ceramic beads,
sand, and
bauxite), coated, or contain chemicals; more than one can be used sequentially
or in
mixtures of different sizes or different materials. The proppant may be resin
coated, or
pre-cured resin coated, provided that the resin and any other chemicals that
might be
released from the coating or come in contact with the other chemicals of the
Invention are
compatible with them. Proppants and gravels in the same or different wells or
treatments
can be the same material and/or the same size as one another and the term
"proppant" is
intended to include gravel in this discussion. In general the proppant used
will have an
average particle size of from about 0.15 mm to about 2.39 mm (about 8 to about
100 U. S.
mesh), more particularly, but not limited to 0.25 to 0.43 mm (40/60 mesh),
0.43 to 0.84
mm (20/40 mesh), 0.84 to 1.19 mm (16/20), 0.84 to 1.68 mm (12/20 mesh) and
0.84 to
2.39 mm (8/20 mesh) sized materials. Normally the proppant will be present in
the slurry
in a concentration of from about 0.12 to about 0.96 kg/L, or from about 0.12
to about 0.72
kg/L, or from about 0.12 to about 0.54 kg/L. The fluid may also contain other
enhancers or
additives.
[0060) In other embodiments, the composition may further comprise an additive
for
maintaining and/or adjusting pH (e.g., pH buffers, pH adjusting agents, etc.).
For example,
the additive for maintaining and/or adjusting pH may be included in the
treatment fluid so
as to maintain the pH in, or adjust the pH to, a desired range and thereby
maintain, or
provide, the necessary ionic strength to form the desired micellar structures.
Examples of
suitable additives for maintaining and/or adjusting pH include, but are not
limited to,
sodium acetate, acetic acid, sodium carbonate, potassium carbonate, sodium
bicarbonate,
potassium bicarbonate, sodium or potassium diacetate, sodium or potassium
phosphate,
sodium or pota'ssium hydrogen phosphate, sodium or potassium dihydrogen
phosphate,
sodium hydroxide, potassium hydroxide, lithium hydroxide, combinations
thereof,
derivatives thereof, and the like. The additive for adjusting and/or
maintaining pH may be
16
CA 02674113 2009-07-27
present in the treatment fluids of the embodiments according to the invention
in an amount
sufficient to maintain and/or adjust the pH of the fluid. One of ordinary
skill in the art, with
the benefit of this disclosure, will recognize the appropriate additive for
maintaining and/or
adjusting pH and amount thereof to use for a chosen application.
[0061] In some embodiments, the composition may optionally comprise additional
additives, including, but not limited to, acids, fluid loss control additives,
gas, corrosion
inhibitors, scale inhibitors, catalysts, clay control agents, biocides,
friction reducers,
combinations thereof and the like. For example, in some embodiments, it may be
desired to
foam the composition using a gas, such as air, nitrogen, or carbon dioxide. In
one certain
embodiment, the composition may contain a particulate additive, such as a
particulate scale
inhibitor.
[0062] According to the invention, the composition may be used for carrying
out a variety
of subterranean treatments, where a viscosified treatment fluid may be used,
including, but
not limited to, drilling operations, fracturing treatments, and completion
operations (e.g.,
gravel packing). In some embodiments, the treatment fluids may be used in
treating a
portion of a subterranean formation. In certain embodiments, the composition
may be
introduced into a well bore that penetrates the subterranean formation.
Optionally, the
treatment fluid further may comprise particulates and other additives suitable
for treating
the subterranean formation. For example, the treatment fluid may be allowed to
contact the
subterranean formation for a period of time sufficient to reduce the viscosity
of the
treatment fluid. In some embodiments, the treatment fluid may be allowed to
contact
hydrocarbons, formations fluids, and/or subsequently injected treatment
fluids, thereby
reducing the viscosity of the treatment fluid. After a chosen time, the
treatment fluid may
be recovered through the well bore.
[0063] In certain embodiments, the treatment fluids may be used in fracturing
treatments. In the fracturing embodiments, the composition may be introduced
into a well
bore that penetrates a subterranean formation at or above a pressure
sufficient to create or
enhance one or more fractures in a portion of the subterranean formation.
Generally, in the
fracturing embodiments, the composition may exhibit viscoelastic behavior
which may be
due. Optionally, the treatment fluid further may comprise particulates and
other additives
suitable for the fracturing treatment. After a chosen time, the treatment
fluid may be
17
CA 02674113 2009-07-27
recovered through the well bore.
[0064] The composition according to the invention provides the following
benefits when
fracturing permeable formations in the 50 to 90 degC temperature range, or
even the 54 to
82 degC temperature range: higher viscosity at a given temperature with lower
polymer
concentration (71.1 degC at a shear rate of 100s/s and 25 minutes at
temperature - prior art
fluid 130cp, fluid according to the invention 210cp); improved fluid loss
control (static
leakoff test in an 80mD core at7l.ldegC - prior art fluid spurt loss 4.81, Cw
0.006088,
fluid according to the invention spurt loss 2.61, Cw 0.001598); improved shear
recovery
(viscosity at 100/s after 2 minutes shear at 100/s - prior art fluid 100cp,
fluid according to
the invention 175cp); less sensitive to the presence of surfactants and de-
emulsifiers.
[0065] The method of the invention is also suitable for gravel packing, or for
fracturing
and gravel packing in one operation (called, for example frac and pack, frac-n-
pack, frac-
pack, StimPac treatments, or other names), which are also used extensively to
stimulate the
production of hydrocarbons, water and other fluids from subterranean
formations. These
operations involve pumping a slurry of "proppant" (natural or synthetic
materials that prop
open a fracture after it is created) in hydraulic fracturing or "gravel" in
gravel packing. In
low permeability formations, the goal of hydraulic fracturing is generally to
form long,
high surface area fractures that greatly increase the magnitude of the pathway
of fluid flow
from the formation to the wellbore. In high permeability formations, the goal
of a
hydraulic fracturing treatment is typically to create a short, wide, highly
conductive
fracture, in order to bypass near-wellbore damage done in drilling and/or
completion, to
ensure good fluid communication between the rock and the wellbore and also to
increase
the surface area available for fluids to flow into the wellbore.
[0066] Gravel is also a natural or synthetic material, which may be identical
to, or
different from, proppant. Gravel packing is used for "sand" control. Sand is
the name
given to any particulate material from the formation, such as clays, that
could be carried
into production. equipment. Gravel packing is a sand-control method used to
prevent
production of formation sand, in which, for example a steel screen is placed
in the wellbore
and the surrounding annulus is packed with prepared gravel of a specific size
designed to
prevent the passage of formation sand that could foul subterranean or surface
equipment
and reduce flows. The primary objective of gravel packing is to stabilize the
formation
18
CA 02674113 2009-07-27
while causing minimal impairment to well productivity. Sometimes gravel
packing is done
without a screen. High permeability formations are frequently poorly
consolidated, so that
sand control is needed; they may also be damaged, so that fracturing is also
needed.
Therefore, hydraulic fracturing treatments in which short, wide fractures are
wanted are
often combined in a single continuous ("frac and pack") operation with gravel
packing.
For simplicity, in the following we may refer to any one of hydraulic
fracturing, fracturing
and gravel packing in one operation (frac and pack), or gravel packing, and
mean them all.
[0067] To facilitate a better understanding of the invention, the following
examples of
embodiments are given. In no way should the following examples be read to
limit, or
define, the scope of the invention.
Examples
[0068] A series of experiments were conducted to compare viscosity and fluid
loss
control of prior art samples made of crosslinked polymer fluid comprising guar
polymer
and of samples according to the invention at different temperatures. The
temperature range
over which most of the test were conducted was 45 C to 95 C
Rheology experiments
[0069] To illustrate some embodiments according to the invention, a comparison
is made
between a prior art fluid made of guar polymer and a fluid according to the
invention
further comprising a VES made of erucic amidopropyl dimethyl betaine (0.5% by
weight),
ethoxylated linear alcohols (0.2% by weight) and non-emulsifying agent (0.1%
by weight).
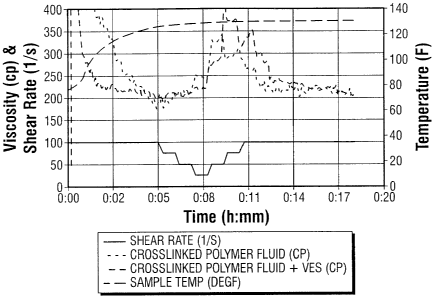
[0070] Figure 1 shows viscosity profile over time for a first fluid made of
guar polymer
and a second fluid made of guar polymer with erucic amidopropyl dimethyl
betaine (0.5%
by weight), ethoxylated linear alcohols (0.2% by weight) and non-emulsifying
agent (0.1 %
by weight). Tests are conducted at temperature of 54.4 C. A small increase in
the viscosity
can be noted compared to prior art fluid.
[0071] Figure 2 shows shear recovery after 2 min of 1000/s shear for a first
fluid made of
guar polymer and a second fluid made of guar polymer with erucic amidopropyl
dimethyl
betaine (0.5% -by weight), ethoxylated linear alcohols (0.2% by weight) and
non-
19
CA 02674113 2009-07-27
emulsifying agent. Tests are conducted at temperature of 54.4 C. Clearly an
increase in the
viscosity can be noted compared to prior art fluid.
[0072] Figure 3 shows viscosity profile over time for a first fluid made of
guar polymer
and a second fluid made of guar polymer with erucic amidopropyl dimethyl
betaine (0.5%
by weight), ethoxylated linear alcohols (0.2% by weight) and non-emulsifying
agent (0.1 %
by weight). Tests are conducted at temperature of 71.1 C. An increase in the
viscosity can
be noted compared to prior art fluid and compared to that one at 54.4 C.
[0073] Figure 4 shows viscosity profile over time for a first fluid made of
guar polymer
and a second fluid made of guar polymer with erucic amidopropyl dimethyl
betaine
(0.75% by weight), ethoxylated linear alcohols (0.2% by weight) and non-
emulsifying
agent (0.1% by weight). Tests are conducted at temperature of 71.1 C. An
increase in the
viscosity can be noted compared to prior art fluid and previous results.
[0074] Figure 5 shows viscosity profile over time for a fluid made of guar
polymer with
erucic amidopropyl dimethyl betaine (0.75% by weight) and non-emulsifying
agent (0.5%
by weight). Tests are conducted at temperature of 71.1 C. An increase in the
viscosity can
be noted for the fluid according to the invention.
[0075] Figure 6 shows viscosity profile over time for a fluid made of guar
polymer with
erucic amidopropyl dimethyl betaine (0.75% by weight). Tests are conducted at
temperature of 82.2 C. An increase in the viscosity can be noted for the fluid
according to
the invention.
[0076] Figure 7 shows viscosity profile over time for a fluid made of guar
polymer with
erucic amidopropyl dimethyl betaine (0.75% by weight) and non-emulsifying
agent (0.5%
by weight). Tests are conducted at temperature of 82.2 C. An increase in the
viscosity can
be noted for the fluid according to the invention.
CA 02674113 2009-07-27
Fluid loss control
[0077] To illustrate some embodiments according to the invention, tests were
run at
71.1 C as follows: establish baseline permeability to 2% KCI, perform static
leakoff test
with fracturing -fluid (30 in. 500 psi) and measure retained permeability to
2% KCl at
increasing differential pressure up to 250 psi. A test was run with 0.25% by
weight of VES
in a core with an effective permeability to water of 18 mD shown on Figure 8.
Despite the
relatively high permeability the values of both spurt loss and CW were much
lower than
what is reported in the specification for crosslinked polymer fluid made of
guar at 65.5 C
in a 1 mD core (see Table 1 below).
T(degC) Permeability (md) CW Spurt (galUS/100 ft2)
(ft/min'/z)
38. 0.76 0.0017 1.62
52 0.77 0.0016 0.15
66 0.73 0.0023 5.17
Table 1
[0078] Series.of tests were run in a high permeability core: 85 mD with no
VES, and
after with 0.1% by weight or 0.5% by weight VES made of erucic amidopropyl
dimethyl
betaine. Results indicate that the fluid loss control of the fluid is improved
with increasing
concentrations of VES as shown on Figures 9, 10 and 11.
[0079] Table 2 shows the viscosity of VES 0.1% versus temperature. This table
clearly
shows that there is a synergistic effect when using guar and VES together,
rather than
simply summing the viscosity of the two components.
21
CA 02674113 2009-07-27
Tempera~..#ur+e Viscosities (cP)
Formutation n, W at Siiear Rates
degF dL-,gC 40a'I 100 sI 170s 1
74 23 0..302E1 0.0139 51 27 18
109 43 0.4420 0:0073 45 27 20
127 53 0..3740 0.0151 72 40 29
147 64 0.,5634 C 0035 33 22 18
VES 109aIiJS?1.G00 172 78 0.1660 0.0134 313 14 9
galus,
197 91-1 0:2230 3 0095 26 13 8
KCI : 16711~1 y~0 g~31.ls 217 103 d~ 2680 U_413a3 20 10 7
238 114 0_1700 0D070 16 7 5
256 124 0.1040 Q.0077 14 6 4
271 133 0_0780 0M82 13 6 3
294 146 0M-60 0.0107 14 6 3
Table 2
Optimization
[0080] At 54.4degC there is little difference in the rheology of the
crosslinked polymer
fluid with or without VES. However, the fluid loss control/efficiency of the
fluid is greatly
improved (shown on Figure 8) as is the shear recovery (shown on Figure 2). At
each
temperature there is an optimum concentration of VES: 0.25% by weight at
54.4degC
(improved fluid loss control), 0.5% by weight at 65.5degC (improved rheology
and fluid
loss control), 0.75% by weight at 82.2degC (improved rheology and fluid loss
control).
[0081] As said before, the temperature range over which most of the test were
conducted
was 45 C to 95 C. However, some tests were run at 121 C with crosslinked guar
that
showed the same viscosity increase with the addition of VES; although the
viscosity of the
fluid system (crosslinked guar + VES) was considerably lower due to the
temperature. The
viscosity at 121 C was 110 cp at 100 s 1 and 160 cp at 100 s t with the
addition of 0.75%
VES. It is not unreasonable to believe that this effect would be seen at even
higher
temperatures.
[0082] It is clear that the invention is well adapted to carry out its
objectives and attain
the ends and advantages mentioned above as well as those inherent therein.
While
embodiments of the invention have been described in varying detail for
purposes of
22
CA 02674113 2009-07-27
disclosure, it will be understood that numerous changes may be made which will
readily
suggest themselves to those skilled in the art and which are encompassed
within the spirit
of the invention disclosed and as defined in the written description and
appended claims.
23