Note: Descriptions are shown in the official language in which they were submitted.
CA 02674681 2014-01-17
Medically Active Plaster for Releasing an Active
Compound that is Liquid at Room Temperature
The present invention relates to a medically active plaster
for releasing pharmaceutical active compounds which are
liquid at room temperature to the skin, in particular for
releasing the antiphlogistically active compound
etofenamate.
Medically active plasters for releasing pharmaceutical
active compounds to the skin are known per se. These
plasters as a rule comprise a top layer, a backing layer
containing the pharmaceutical active compound and a
peelable protective layer. However, the production of such
plasters is difficult since the backing layer in contact
with the skin must ensure optimum passage of the active
compound into the skin and at the same time adhere to the
skin less firmly than to the top layer, so that the plaster
can be removed easily and completely from the skin.
In this context, the backing layer should contain the
highest possible concentration of the active compound so
that this can be released from the plaster in a
pharmaceutically active concentration over a comparatively
long period of time. This is difficult to achieve for
active compounds which are liquid at room temperature,
since liquid active compounds must be embedded in the
backing layer in a stable manner and must be compatible
with the material of the backing layer. The active compound
must also be released from the backing material at a
sufficient rate over a relatively long period of time. In
this context, not only the nature of the backing material
but also the chemical structure of the active compound
plays an important role.
CA 02674681 2014-01-17
2
It has now been found that the generally poorly soluble
antiphlogistically active compound etofenamate surprisingly
can be embedded in a pure and uniformly finely distributed
form in a stable manner in a self-adhesive silicone matrix
and forms a finely divided dispersion there, so that a
matrix with very good adhesive properties which releases
the active compound in an active concentration over a
relatively long period of time and can be Used according to
the invention as the backing layer is obtained.
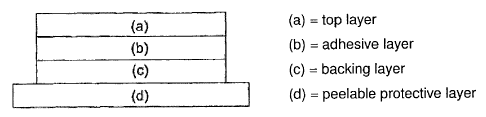
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is an illustration showing the layers of a medical
plaster according to one embodiment of the invention.
Figure 2 is an illustration showing the layers of a medical
plaster according to another embodiment of the invention.
The present invention relates to a medical plaster for releasing
pharmaceutical active compounds which are liquid at room
temperature to the skin, in particular for releasing the
antiphlogistically active compound etofenamate. In a particular
embodiment, this plaster has a structure according to Figure 1,
comprising the top laver (a), the backing layer (c), the
peelable protective layer (d) and optionally an intermediate
layer (b), characterized in that:
- the top layer (a) comprises an inert material,
- the backing layer (c) comrpsies a self-adhesive
polysiloxane in which the antiphlogistically active
compound, preferably etofenamate, optionally together
with an agent which promotes permeation through the
skin and optionally further additives, is embedded in
the form of a dispersion, wherein
- the backing layer (c) adheres directly to the top
layer (a) or is optionally joined to this via the
intermediate layer (b); and
22487869.1
= CA 02674681 2009-07-07
W02008/083508
PCT/CH2008/000010
3
the peelable protective layer (d) comprises an inert
material, adheres to the backing layer (c) and can
easily be peeled off from this.
A preferred medical plaster is that which has a structure
according to Figure 2, comprising the top layer (a), the
backing layer (c) and the peelable protective layer (d),
characterized in that:
the top layer (a) comprises an inert material,
- the backing layer (c) comprises a self-adhesive
polysiloxane in which the antiphlogistically active
compound, preferably etofenamate, optionally together
with an agent which promotes permeation through the
skin and optionally further additives, is embedded in
the form of a dispersion, and this backing layer (c)
adheres directly to the top layer (a), and
the peelable protective layer (d) comprises an inert
material, adheres to the backing layer (c) and can
easily be peeled off from this.
If an adhesive layer (b) is present as an intermediate
layer between the top layer (a) and the backing layer (c),
this adhesive layer (b) either contains no active compound
or is optionally loaded with active compound in various
amounts and has a comparatively high adhesive strength, so
that the adhesive layer (b) adheres firmly both to the top
layer (a) and to the backing layer (c). In this context,
the adhesive strength of the adhesive layer (b) is largely
independent of the backing layer (c) containing the active
compound and higher than the adhesive strength of the
backing layer (c). The adhesive strength of the backing
CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
4
layer (c) is only so high for it to be possible to remove
the plaster easily and completely from the skin.
If the adhesive layer (b) is loaded with active compound,
the active compound is preferably present in the adhesive
layer (b) in at least the same amount as the active
compound is present in the backing layer (c). Preferably,
the adhesive layer (b) is loaded with the active compound
up to the saturation limit.
The present invention also relates to a process for the
production of the plaster according to Figure 1 comprising
an adhesive layer (b), characterized in that the
constituents of the adhesive layer (b), in the liquefied
state, i.e. as a "hot melt" without addition of a solvent,
or as a solution in a suitable organic solvent, are applied
to the top layer (a) and are then freed from the organic
solvent which may be present, or dried; the constituents of
the backing layer (c) are then applied in the liquefied
state, i.e. as a "hot melt" without addition of a solvent,
or as a solution in a suitable organic solvent, to the
adhesive layer (b) and are then freed from the organic
solvent which may be present, or dried; and the peelable
protective layer (d) is applied to the dried backing layer
(c). In this context, the adhesive layer (b), as described
above, can in each case also optionally contain the active
compound.
The present invention also relates to a process for the
production of the plaster according to Figure 2,
characterized in that the constituents of the backing layer
(c) are applied in the liquefied state, i.e. as a "hot
CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
melt" without addition of a solvent, or as a solution in a
suitable organic solvent, to the top layer (a) and are then
freed from the organic solvent which may be present, or
dried; and the peelable protective layer (d) is applied to
5 the dried layer (c).
The production of the plaster according to the invention
can also be started from the peelable protective layer (d).
The process for the production of the plaster, for example
according to Figure 1, is then characterized in that the
constituents of the backing layer (c), in the liquefied
state, i.e. as a "hot melt" without addition of a solvent,
or as a solution in a suitable organic solvent, are applied
to the peelable protective layer (d) and are then freed
from the organic solvent which may be present, or dried; in
a separate step the constituents of the adhesive layer (b),
in the liquefied state, i.e. as a "hot melt" without
addition of a solvent, or as a solution in a suitable
organic solvent, are applied to the top layer (a) and are
then freed from the organic solvent which may be present,
or dried; and the backing layer (c) is then laminated with
the top layer (a), which already contains the adhesive
layer (b).
Analogously, the process for the production of the plaster,
for example according to Figure 2, is characterized in that
the constituents of the backing layer (c), in the liquefied
state, i.e. as a "hot melt" without addition of a solvent,
or as a solution in a suitable organic solvent, are applied
to the peelable protective layer (d) and are then freed
from the organic solvent which may be present, or dried;
= CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
6
and the top layer (a) is applied to the dried backing layer
(c).
Preferably, the adhesive layer (b) and the backing layer
(c) are dried directly after the application if these
contain solvents. However, it is also possible to apply all
the layers in succession and to dry these only at the end.
Preferably, the backing layer is processed as a "hot melt".
For this, the constituents of the backing layer (c) are
mixed intensively in the liquefied state by employing the
active compound without addition of a solvent, or as a
solution in a suitable organic solvent, optionally together
with an agent which promotes permeation through the skin
and optionally further additives. In this context, the
constituents are preferably mixed at a temperature in the
range of 80 C - 190 C, preferably in the range of 140 C -
180 C and in particular in the range of 160 C - 180 C,
until the desired dispersion of the active compound in the
matrix has formed. The mixture is then allowed to cool to
the laminating temperature, i.e. to a temperature in the
range of 120 C - 140 C, preferably in the range of 80 C -
120 C and in particular to about 100 C, the dispersion
being applied to or laminated on the desired substrate at
this temperature in the form of a "hot melt" and processed
to give the backing layer (c). At the laminating
temperature the dispersion is preferably solvent-free, or
is then freed, before or after the lamination, from the
organic solvent which may still be present. Polysiloxanes
which can be applied without a solvent and polysiloxanes
which are preferably applied from a solvent with subsequent
removal of the solvent are known per se.
CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
7
The top layer (a) preferably comprises an elastic textile
planar structure which is coated with a polymeric material.
Textile material which is used is e.g. a textile planar
structure which is produced, for example, from cotton and
is coated with polyethylene terephthalate (PET). The
textile planar structure can also comprise a synthetic
textile fibre, such as, for example, PET, PVC, PU and
further polymeric plastics, or be produced in non-woven
form. Such materials are known per se and commercially
obtainable.
The adhesive layer (b) comprises an organic polymer which
is known per se, is preferably soluble in an organic
solvent and has good adhesive properties. Suitable adhesive
materials are, for example, polymers of isoprene or
copolymers of isoprene with styrene, such as styrene/
isoprene/styrene (S IS); polyalkyl acrylates, prepared from,
for example, amyl, butyl, hexyl, heptyl, octyl, nonyl, 2-
ethylhexyl or 2-methoxyethyl acrylate, or copolymers of
such alkyl acrylates with acrylic acid, methacrylic acid,
methyl or ethyl acrylate, hydroxyethyl acrylate or
hydroxypropyl acrylate. Styrene/butadiene/styrene
copolymers (SBS) or polyisobutylenes and copolymers thereof
are also suitable.
Copolymers prepared from 2-ethylhexyl acrylate and methyl
acrylate, such as, for example, from about 19.9 wt.% of
2-ethylhexyl acrylate and about 79.3 wt.% of methyl
acrylate, with an average molecular weight in the range of
from 350,000 to 550,000 dalton, preferably about 400,000 to
500,000 dalton, and polymerized e.g. in the presence of
' = CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
8
azobisisobutyronitrile, are particularly preferred for the
production of the adhesive layer (b).
The acrylate polymers mentioned are preferably also used in
combination with SBS polymers, it being possible for the
ratio to be optimized by the person skilled in the art.
Thus, for example, the weight ratio of the acrylate polymer
to SBS can be in the range of from 2:1 to 1:2, preferably
in the range of from 5:3 to 3:5.
Styrene/butadiene/styrene block copolymers (SBS),
styrene/butadiene block copolymers (SB) and mixtures of
these copolymers with a glass transition temperature (Tg) of
preferably less than -22 C [Tg<(-22 C)] are also preferred
for the production of the adhesive layer (b). These
polymers can contain additives, such as e.g. the glycerol
ester of hydrogenated colophony or polyterpenes. A
preferred composition contains, for example, 17.0 wt.% of
SB, 11.3 wt.% of SBS, 70.8 wt.% of colophony glycerol ester
and 0.9 wt.% of an antioxidant. Preferred solvents for
these adhesive materials are, for example, saturated and
aromatic hydrocarbons, such as e.g. hexane, heptane,
octane, benzene or toluene.
For the production of the adhesive layer (b), the polymers
and/or copolymers are preferably dissolved in a suitable
solvent. If the adhesive layer (b) contains the active
compound, the active compound is preferably dissolved in
the desired amount in a suitable solvent beforehand and
mixed in the dissolved form with the polymer and/or
copolymer of the adhesive layer (b). The solution of the
adhesive layer (b) is applied to the top layer (a) and the
CA 02674681 2009-07-07
WO 2008/083508
PCT/C112008/000010
9
solvent is removed. Suitable solvents are, for example,
ethyl acetate, propyl acetate and saturated and aromatic
hydrocarbons, such as e.g. hexane, heptane, octane, benzene
or toluene.
Numerous polymers which are suitable for the production of
the adhesive layer (b) can also be applied in the liquefied
state without the addition of a solvent, as a "hot melt".
Such polymers and copolymers which can be used for the
production of the adhesive layer (b) are known per se and
commercially obtainable. Coated planar structures are
obtainable, for example, as Scotchpake, from 3M. For
example, the top layer is coated with about 40 g/m2 of SBS
(as the adhesive layer) in a first step. Scotchpak is used,
for example, as the protective layer (e.g. Scotchpak 1022
from 3M). Whether the adhesive layer (b) is applied to the
top layer (a) from a solvent or without a solvent is not
essential according to the invention. Preferably, the
plaster according to the invention comprises no adhesive
layer (b).
According to the invention, the backing layer (c) comprises
a self-adhesive polysiloxane. Self-adhesive polysiloxanes
are known per se and are prepared in various compositions
and marketed commercially, for example, by Dow Corning
under the trade name BIO-PSA(0 Amine-Compatible Silicone
Adhesives. Such silicone polymers can be used according to
the invention. To optimize the adhesive properties, such
silicone polymers can additionally contain additives known
per se for modification of the adhesive properties, such as
e.g. colophony compounds, such as e.g. dehydrogenated or
CA 02674681 2009-07-07
WO 2008/083508 PCT/C112008/000010
hydrogenated colophony, colophony glycerol ester, terpene
resins, polyterpene resins from alpha- or beta-pinene, or
low-viscosity silicones or polysiloxanes which contain
terminal silanol groups, or polydimethylsiloxanes, such as
5 e.g. dimethiconol.
Self-adhesive polysiloxanes which are suitable for the
production of the backing layer (c) without addition of a
solvent, as a "hot melt", are commercially obtainable, for
10 example known as BIO-PSACO 7-4560 Silicone Adhesive from Dow
Corning. Such siloxane compounds are known e.g. under CAS
number 68440-70-0 and CAS number 63148-62-9, and mixtures
thereof, e.g. compounds of CAS number 68440-70-0 in a
concentration of at least 60 wt.% with compounds of CAS
number 63148-62-9 in a concentration of from 10.0 to
30.0 wt.%.
Self-adhesive polysiloxanes which are suitable for the
production of the backing layer (c) with the addition of a
solvent are commercially obtainable, for example as BIO-
PSAEI 7-4603 or BIO-PSAO 7-4201 from Dow Corning. Suitable
polysiloxanes for the production of the backing layer (c)
can easily be chosen by the person skilled in the art.
Suitable self-adhesive polysiloxanes are obtained, for
example, by condensation of a dimethylpolysiloxane
containing silanol groups with a silicate resin which is
soluble in organic solvents and subsequent reaction of the
remaining silanol groups with a reactive trimethylsilyl
compound. Such polysiloxanes are soluble in organic
solvents, such as, for example, in ethyl acetate, propyl
= CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
11
acetate and saturated and aromatic hydrocarbons, such as
e.g. hexane, heptane, octane, benzene or toluene.
In a preferred embodiment, the self-adhesive polysiloxane
of the backing layer (c) contains a compound or a mixture
of compounds which lower the viscosity of the self-adhesive
polysiloxane containing the active compound, without
adversely influencing the other properties of the self-
adhesive polysiloxane. Such compounds are preferably
glycerol and/or ester compounds of a medium-chain fatty
acid with a monohydric or polyhydric alcohol. Preferred
ester compounds of a medium-chain fatty acid with a
monohydric alcohol are, for example, esters of propyl
alcohol, isopropyl alcohol, butyl alcohol or isopropyl
alcohol with a (C0--C16)-fatty acid, preferably with medium-
chain (C12-C10-fatty acids, preferably with lauric acid,
myristic acid or palmitic acid, such as, for example,
isopropyl myristate. Ester compounds of medium-chain fatty
acids with polyhydric alcohols are, for example, mono-, di-
or triesters of glycerol with medium-chain (C10-C16)-fatty
acids, preferably mono-, di or triesters of glycerol with
medium-chain (C12-C15)-fatty acids, and also natural oils,
preferably olive oil or castor oil. Compounds which can
also be used as compounds which analogously lower the
viscosity of the self-adhesive polysiloxane containing the
active compound are liquid paraffin, polysorbates (i.e.
polyoxyethylene sorbitan fatty acid ester compounds), such
as, for example, Tween0,60 (polyoxyethylene sorbitan
monostearate) or TweenC,80 (polyoxyethylene sorbitan
monooleate), polyethylene glycols, such as e.g.
polyethylene glycol 400, propylene glycol and polypropylene
glycols, esters of polyhydric acids with alcohols, such as
' CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
12
triethyl citrate, and mixtures of these compounds. The
self-adhesive polysiloxanes used according to the invention
preferably contain about 2-15 wt.%, preferably about 5-
wt.% of these compounds, based on the total weight of
5 the backing layer (c). The compounds mentioned can
occasionally also act as permeation accelerators.
- The backing layer (c) containing the active compound
and also the adhesive layer (b) optionally containing
10 the active compound contain the active compound
optionally together with an agent which promotes
permeation through the skin. However, the presence of
an agent which promotes permeation through the skin is
not critical. The active compound can additionally
also be mixed with further active compounds and
additionally contain e.g. stabilizers and odoriferous
substances.
Etofenamate, corresponding to the chemical formula:
rir
o 0.,,,.....----.0,----....,,OH
H
õ, N.....õ...õ..,,-..-Or,
I it-,4
'----...--
etofenamate
i.e. 2-(2-hydroxyethoxy)-ethyl (a,u,a-trifluoro-m-toly1)-
anthranilate (etofenamate), is preferred.
The backing layer (c) contains the active compound,
preferably etofenamate, preferably in a concentration in
the range of from about 1.0 wt.% to about 25.0 wt.%,
preferably 2.0 wt.% to 20 wt.% and preferably 2.5 wt.% to
15 wt.%, preferably in a concentration of from about 5 wt.%
' CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
13
to about 10 wt.%, calculated for the total weight of the
backing layer.
A process for the preparation of the dispersion which can
be used as the backing layer (c) containing at least one
self-adhesive polysiloxane and the active compound,
preferably etofenamate, optionally together with an agent
which promotes permeation through the skin and optionally
further additives, is characterized in that the self-
adhesive polysiloxane which forms the backing layer (c) is
heated together with the active compound, optionally
together with an agent which promotes permeation through
the skin and optionally further additives to a temperature
in the range of 80 C - 200 C, preferably in the range of
140 C - 190 C and in particular in the range of 160 C -
190 C, with intensive stirring, until the desired
dispersion has formed. This dispersion can be processed
further without a solvent in the stated temperature range,
but preferably in the range of 120 C - 140 C, preferably in
the range of 80 C - 120 C and in particular at about 100 C,
and applied as a thin layer in the desired amount to the
envisaged substrate.
A further process for the preparation of the dispersion
which can be used as the backing layer (c) containing at
least one self-adhesive polysiloxane and the active
compound, preferably etofenamate, optionally together with
an agent which promotes permeation through the skin and
optionally further additives, is characterized in that the
self-adhesive polysiloxane which forms the backing layer
(c) is heated together with the active compound, optionally
together with an agent which promotes permeation through
. , CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
14
the skin and optionally further additives in the presence
of a solvent at elevated temperature, preferably in the
range of 40 C - 90 C, preferably in the region of the
boiling point of the solvent, with intensive stirring,
until the desired dispersion has formed. Preferably,
solvent is added in an amount such that the dispersion
obtained can be processed to a plaster at room temperature,
i.e. the dispersion obtained can be applied as a thin layer
in the desired amount to the envisaged substrate at room
temperature.
Alternatively, it is possible to remove the solvent
substantially or completely from the dispersion formed [for
formation of the backing layer (c)] and to further process
the dispersion formed in this manner at elevated
temperature substantially or completely without a solvent
and to apply it as a thin layer in the desired amount to
the envisaged substrate.
Solvents which are used are preferably an organic solvent,
such as, for example, ethyl acetate, propyl acetate and
saturated and/or aromatic hydrocarbons, such as e.g.
hexane, heptane, octane, benzene or toluene. The solvent is
preferably evaporated off at a temperature in the range of
from 50 C to 90 C, depending on the boiling point of the
solvent, preferably at a temperature of from about 60 C to
70 C, during a period of time of from about 30 minutes to
60 minutes, either from the dispersion obtained or from the
layer applied in the plaster.
The backing layer (c) contains the active compound in
highly disperse distribution with an average droplet size
CA 02674681 2009-07-07
WO 2008/083508 PCT/C112008/000010
in the range of from 0.1 pm to 500 pm, preferably from
1.0 pm to 100 pm. The covering of the surface for the
adhesive layer (b) and the backing layer (c) is in each
case in the range of from 30 g/m2 to 300 g/m2, preferably in
5 the range of from about 40 g/m2 to 200 g/m2 and in
particular in the range of from about 40 g/m2 to 100 g/m2.
The adhesive strength of the adhesive layer (b) is
preferably in the range of from 0.8 N/25 mm to 2.0 N/25 mm,
10 preferably in the range of from 0.9 N/25 mm to 1.7 N/25 mm.
The adhesive strength of the backing layer (c) is
preferably in the range of from 0.8 N/25 mm to 1.4 N/25 mm.
Compounds which promote permeation through the skin
15 (permeation enhancers) are additives which promote
administration of the active compound to the skin or
penetration of the stratum corneum. Such compounds are
known per se and also for use in such plasters. Naturally
occurring substances, such as natural oils and fats, or
fatty acids and higher fatty alcohols and esters thereof as
well as glycerol and mixtures of these compounds are
preferred. The weight ratio of active compound : permeation
enhancer is preferably in the range of from 98 : 2 to
2 : 8, preferably in the range of from 9 : 1 to 3 : 7,
preferably about 1 : 2.
Natural oils and fats are, in particular, mono-, di- and
triglycerides, which are glyceride esters with saturated
and/or unsaturated fatty acids; for example esters of fatty
acids having preferably 4 to 22 carbon atoms. Such fatty
acids are preferably butyric, caproic, capric, myristic,
palmitic, stearic, arachic, palmitoleic, oleic, ricinoleic,
CA 02674681 2009-07-07
WO 2008/083508 PCT/CH2008/000010
16
linoleic, linolenic or arachidonic acid. Such fatty acids
having preferably 4 to 22 carbon atoms can also be employed
as accelerators by themselves.
Alcohols are to be understood as meaning the corresponding
alcohols and fatty alcohols having 4 to 22 carbon atoms,
preferably n-, iso- and sec-butyl alcohol; n-, iso- and
tert-amyl alcohol; n-hexyl alcohol, cyclohexyl alcohol;
octyl alcohol, capryl alcohol (2-octanol), n-decyl alcohol,
lauryl alcohol, myristyl alcohol, cetyl alcohol and stearyl
alcohol.
Synthetic fatty acid esters of the fatty acids mentioned
with low or higher alcohols, such as, for example, ethyl
stearate, palmitic acid cetyl ester, isopropyl myristate,
isopropyl palmitate or mixtures of such compounds, are also
suitable. Isopropyl myristate is preferred.
Natural oils are also e.g. castor oil, olive oil, groundnut
oil, maize oil, hazelnut oil, jojoba oil and wheat germ
oil.
The peelable protective layer (d) comprises an inert
material which adheres to the backing layer (c) and can
easily be peeled off from this. Such materials are known in
the form of thin films and are commercially obtainable, for
example, from 3M under the brand name Scotchpak . The
following examples illustrate the invention without
limiting this.
CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
17
Example 1
5.0 parts of pure ethyl acetate are added to 5.0 parts of
pure etofenamate in a glass round-bottomed flask and the
substances are mixed intensively on a magnetic stirrer
plate. 85 parts of a self-adhesive polysiloxane dissolved
in ethyl acetate (BIO-PSA(D 7-4603 or, respectively, BIO-
PSA 7-4560 from Dow Corning) are then added and the
mixture is stirred intensively at room temperature for
2.5 hours. Where appropriate, 10 parts of isopropyl
myristate (IPM) are added to the mixture. A laminate with a
weight per unit area of 100 g/m2, 75 g/m2 and 40 g/m2 (=
backing layer (c) on a peelable layer (d)] is produced from
the resulting mixture using a coating unit, and is dried in
a drying cabinet at a temperature of 60 C for 60 minutes,
until all the solvents are removed. After the drying, the
laminate obtained is laminated with a top layer (a)
comprising PET fabric which is provided with an adhesive
layer (b) (Duro-Tak0 87-6173 from National Starch), the
adhesive layer having a weight per unit area of 40 g/m2. The
additional examples of Table 1 were produced in an
analogous manner.
Table 1
Etofenamate Bio PSA 7-4603 IPM Area mass
5 % 95 % 40 g/m2
5% 95% 75 g/m2
10 % 90 % 40 g/m2
10 % 90 % 75 g/m2
5 % 85 % 10 % 100 g/m2
10 % 80 % 10 % 100 g/m2
. .
CA 02674681 2009-07-07
WO 2008/083508
PCT/CH2008/000010
18
Example 2
5.0 parts of pure etofenamate are stirred intensively with
parts of isopropyl myristate (IPM) and 85 parts of a
self-adhesive polysiloxane (BIO-PSA 4650 from Dow Corning)
5 in a high-speed mixer of the brand name Becomix at a
temperature of 190 C for 2.5 hours. The mixture or
dispersion obtained is allowed to cool and processed with a
coating unit from Hofmann & Schwabe at a laminating
temperature of 100 C to give a laminate with a weight per
10 unit area of 100 g/m2, 75 g/m2 and of 40 g/m2 [= backing
layer (c) on the top layer (a), comprising PET fabric],
which is cooled to room temperature. The laminate obtained
is then laminated with a protective layer (d) (Scotchpak@,
a removable film from 3M coated with a fluoropolymer).