Note: Descriptions are shown in the official language in which they were submitted.
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
MICRO-ARRAY ANALYSIS SYSTEM AND METHOD THEREOF
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to solutions for
detecting cellular material, and involves imaging
techniques, image processing, optics, fluorescence and
biochemistry.
2. Background Art
Micro-arrays are matrices of bio molecules
spots deposited on a substrate that usually has the
appearance of a microscope slide.
Micro-array technology was initially developed
for genomics studies in order to realize massively
parallel genetic assays for research applications such
as human genome sequencing. DNA micro-array is a
collection of microscopic DNA spots attached to a solid
surface, such as glass, plastic or silicon chip forming
an array for the purpose of expression profiling,
monitoring expression levels for thousands of genes
simultaneously. In one such application, molecules
which are immobilized are single-stranded DNA molecules.
A solution of probe molecules, which are fluorescently
labeled single stranded DNA, is then made to interact
with the micro-array. The probed DNA will then
specifically bind to spots that are formed of
complementary strands therefore revealing that those
spots have a sequence that is similar to the probe. The
arrays are then dried and read by a fluorescence
detection system.
-1-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
DNA micro-array fluorescent signals can be
very weak which has led micro-array scanners to evolve
into sophisticated and expensive confocal laser scanning
systems for optimal sensibility, or cooled CCD camera
systems for fast readout. These types of systems
produce an image of the whole micro-array with a
resolution of a few hundred pixels per spot. The imaging
system's capability is used for compensating for
variations in locations of the spots within the micro-
array disposition, and for segmenting the spots from
their surrounding background signal.
Recently, proteins have been immobilized on
substrates to produce protein arrays. The deposited
proteins can be antibodies targeted to specifically bind
to viruses, toxins and micro-organism. The assay
consists in letting a solution of unknown biological
agent interact and specifically bind with the proteins
on the array. If the unknown agents were previously
fluorescently labeled, the micro-array can be analyzed
right away, otherwise the array is revealed with a
solution of fluorescently labeled antibodies that are
also specific to the targeted biological agent.
Antibodies or other types of capture molecules which are
more similar in their nature, such as peptides or
aptmers, can also be used to target agents.
Conventional micro-array scanners designed for
DNA micro-arrays as detailed above, are still used for
the analysis of protein based micro-arrays even though
the requirements for these new applications are very
different. The signal read by a conventional scanner is
a measure of the total signal emanating from one spot,
and can thus be affected by spurious contributions.
Impurities, such as dusts and biological residues from
the host solution, generally fluoresce and therefore
-2-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
contribute to the measured intensity of the spot's
signal, in a way that is not related to the targeted
agent. Furthermore, the efficiency of the fluorescence
labeling of the revealing antibodies can vary greatly
from experiment to experiment, leading to added
variability which impairs the limit of detection.
SUMMARY OF INVENTION
It is therefore an aim of the present
invention to provide a scanning system and method to
overcome the above-mentioned drawbacks found with the
prior art.
Therefore, in accordance with the present
invention, there is provided a method for analyzing
spots in a micro-array, the spots containing targeted
specimen, the method comprising the steps of: providing
a slide with a micro-array of the spots thereon;
illuminating at least one of the spots; directing the
light onto the at least one spot; collecting light
emitted from the at least one spot; forming an image of
the at least one spot using the collected light; and
analyzing the image to distinguish at least one unit of
the targeted specimen located within the at least one
spot from any undesired material.
In accordance with another embodiment of the
present invention, there is provided an apparatus for
imaging at least one spot containing targeted specimen,
the at least one spot located on a micro-array, the
system comprising: a source of light; an optical
apparatus associated to the light source to direct the
light onto the at least one spot, and to collect light
emitted from the at least one spot, the collected light
forming an image of the at least one spot; a digital
-3-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
image acquisition device positioned with respect to the
optical apparatus in a manner to permit the image
acquisition, to acquire the formed image; and a
processing unit operatively connected to the digital
image acquisition device, to analyze the image in order
to distinguish at least a unit of the targeted specimen
located within the at least one spot from any undesired
material.
In this specification, the term "fluorescing
specimen" or "targeted specimen" is intended to refer to
a targeted agent which is optically resolvable, such as
for example a micro-organism or cells, and which has
been made to fluoresce by direct labeling before
interaction with the micro-array, or indirectly by
labeled antibodies added once the targeted specimen are
captured on the micro-array.
Further in this specification, the adjective
"high resolution" is intended to refer to an imaging
resolution that is sufficient to optically resolve each
unit of targeted specimen or an agent targeted by the
assay.
Again in this specification, the term
"fluorescing element" is intended to refer to one
fluorescing specimen or an agglomeration of fluorescing
specimen seen on a captured spot image.
Still further in this specification, the term
"blob" is intended to refer to a group of connected
pixels found on the corresponding binary picture of a
captured spot image. A group of pixels can define at
least one unit of targeted specimen or a cluster of
them.
-4-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
BRIEF DESCRIPTION OF THE DRAWINGS
Having thus generally described the nature of
the invention, reference will now be made to the
accompanying drawings, showing by way of illustration a
preferred embodiment thereof and in which:
Fig. 1 is a schematic view of a micro-array
analysis system in accordance with a first embodiment of
the present invention;
Fig. 2 is a schematic view of an imaging
system of the micro-array analysis system of Fig. 1;
Fig. 3 is a block diagram illustrating a
micro-array analysis method in accordance with a second
embodiment of the present invention;
Fig. 4 is a block diagram illustrating an
image processing method of the micro-array analysis
method of Fig. 3;
Fig. 5a is an image of a top plan view of a
spot acquired with the imaging system of Fig. 1; and
Fig. 5b is the image of Fig. 5a once processed
using the processing method of Fig. 4.
It will be noted that throughout the appended
drawings, like features are identified by like reference
numerals.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The proposed micro-array scanning system can
be used for example, to detect, classify and quantify
units of fluorescing specimen. The spot location onto
which the specimen binds provides for specificity and
identification functionality. The processing of an image
of a sufficiently high resolution, taken at a spot
location on a micro-array, enables such identification
and provides robustness to spurious signal sources such
-5-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
as dust, food residue and culture broth. For example,
morphological features such as size and shape are used
to discriminate targeted specimen from dust and other
agents leading to detection errors. This is in contrast
with known prior art, in which a measure of the total
signal from one spot on a micro-array is taken.
As an example, in a protein micro-array aimed
at the detection of cells such as micro-organisms or
human cells, the fluorescence signal can be
significantly higher than what is generated when reading
common DNA micro-arrays. In addition, when the captured
cells can be optically resolved with a proper optical
system, the enumeration of individual units is made
possible.
The micro-array analysis system herein
described can also be used to account for any variation
in the efficiency of fluorescence labeling by
normalizing the acquired image.
Now referring to the drawings, Fig. 1 is a
schematic view of a micro-array analysis system in
accordance with a first embodiment of the present
invention.
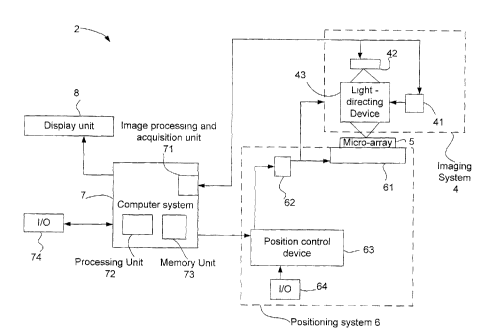
The analysis system 2 has an imaging system 4
for capturing an image of each spot location on a micro-
array 5 being analyzed. The imaging system 4 allows the
acquisition of an image having a resolution sufficiently
high to distinguish the targeted specimen or agent.
The analysis system 2 also has a positioning
system 6 for permitting the capture of images of all the
spot locations on the micro-array 5 being scanned. A
computer system 7 performs the image processing
operations required by the analysis system 2, and the
display unit 8 displays the analysis results to a user.
-6-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
The imaging system 4 and the positioning
system 6 are both controlled by the computer system 7.
Still referring to Fig. 1, the imaging system
4 has a light-emitting device 41, a camera 42, and a
light-directing device 43.
Camera 42 may be any digital image acquisition
device, such as a conventional digital camera. In
addition, the light-directing device 43 is designed to
permit camera 42 to capture an image having a
sufficiently high resolution.
Again referring to Fig. 1, the positioning
system 6 has a mobile stage 61, actuators and
controllers 62, and a position control device 63.
The position control device 63 receives
control signals from the computer system 7.
The actuators and controllers 62, controlled
by the position control device 63, move the mobile stage
61 with respect to the imaging system 4, such that all
the spots on the micro-array 5 can be scanned.
It is also considered possible to displace the
imaging system 4 with respect to a fixed micro-array 5.
Input and output ports 64 of the position control device
63 are optionally provided to override the automatic
computer control of the positioning system 6. A user may
thus directly control the positioning system 6 via
joysticks, mouse or keyboards for example.
Some of the functions of the positioning
system 6 can be achieved by the computer system 7
instead. For example, the position control device 63
could form part of the computer system 7, or its
functions replaced by the operations of the computer
system 7.
-7-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
Still referring to Fig. 1, the computer system
7 has an image processing and acquisition unit 71, any
other processing unit 72 and a memory unit 73.
Among many other function, computer system 7
controls the light-emitting device 41. In one
embodiment, a signal created by the image processing and
acquisition unit 71 can be used as a control signal. For
example, a control signal sent to the light-emitting
device 41 may act as to increase the intensity of the
spot excitation light if lower levels of fluorescent
light intensity are being emitted by the specimen in the
spot.
Computer system 7 also controls the position
system 6 such that any desired spots located on the
micro-array are imaged. The computer can also
automatically evaluate from the captured image if the
spot location is correct. Thus, in the case of a
misalignment, realignment can be done. For example, when
a spot is not located exactly at the predicted place on
the micro-array, a repositioning algorithm based on the
misaligned spot image can correct the situation by
enabling an appropriate realignment of the micro-array 5
with respect to the imaging system 4 until the spot
image is acquired correctly.
All image processing steps are performed by
the computer system 7, which sends the final images,
analysis information and results to the display unit 8
for display to a user.
Particular functions are performed by each
component of computer system 7. For example, the image
processing and acquisition unit 71 receives images and
processes them.
The processing unit 72 performs any other
operations necessary for running computer software,
-8-
CA 02675103 2009-07-09
WO 2008/092226 = PCT/CA2007/000113
implementing control operations, storing and retrieving
data, etc. The processing unit 72 may also perform some
of the image processing steps in conjunction with unit
71.
The memory unit 73 is for storing images
captured and received by unit 71. The storage process
and retrieving can be done according to particular spot
locations corresponding to the captured spot images.
Finally, the computer system 7 may further
have input and output peripherals 74 such as a keyboard,
joysticks, mouse, speakers and the like.
Fig. 2 is a schematic view of the imaging
system 4 of the analysis system 2 of Fig. 1.
In one embodiment illustrated by Fig. 2, the
light-emitting device 41 has a light source 411, a light
transfer apparatus having collimating lenses 412, and an
optical fiber 414.
Other embodiments are also possible. For
example, the light-emitting device 41 can simply be a
light source which is stable enough and emits an
appropriate level of light intensity located within a
range of wavelengths including at least the necessary
excitation wavelengths. The excitation wavelengths
depend on the dye or probe being used in a specific
experiment or analysis. It is the light energy which is
absorbed by the molecules under observation in order for
them to emit fluorescent light.
In the particular embodiment illustrated in
Fig. 2, the light source 411 may be any type of light
source capable of emitting light at least at the
excitation wavelengths. A light-emitting diode (LED) is
an example. Other light-emitting semiconductor devices
can also be used, such as laser diodes.
-9-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
The light transfer apparatus is herein
illustrated as having injection lenses 412 for
directing the light being emitted from light source 411,
into the piece of fiber 414. Any injection lenses or
other apparatus for transferring the light of the image
appropriately can be used.
The optical fiber 414 can be used to render
the light being emitted by the light source 411 uniform.
The optical fiber 414 can also ease the replacement of
the light source 411. The optical fiber 414 can be any
kind of fiber, having various transmission
characteristics, dimensions and lengths. The use of
multi-mode fiber is however well suited for the
applications herein discussed.
Still referring to Fig. 2, the light-directing
device 43 has a collimating lens 431, a first filter
432, a dichroic mirror or filter 433 (such as a beam-
splitting device), another lens 434, a focusing
mechanism 435, a second filter 436 and a last lens 437.
The collimating lens 431 is for collimating
the light emerging from the light-emitting device 41
such that the light passes through filter 432 with
minimum angular spread. The first excitation filter 432
ensures that the excitation light passing through has
the necessary characteristics for the specimen to
generate fluorescent light.
The dichroic mirror 433 directs the excitation
light towards lens 434. A dichroic mirror or filter is
known in the art for being able to selectively pass
light within a determined range of wavelengths and
reflect others within another range of wavelengths.
Lens 434 focuses the excitation light onto the
spot of the micro-array 5 being scanned.
-10-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
The light emitted from the spot then
propagates back through lens 434, as in an upright epi-
illumination scheme. Inverted designs are also
possible.
Lens 434 forms an image of the spot an
infinite distance away (the point of focus is said to be
located at infinity since the emerging light rays are
quasi-parallel).
The focusing mechanism 435 further ensures a
proper focusing of the lens 434 by adjusting its
location relative to the micro-array 5.
The emitted fluorescent light then passes
through the dichroic mirror 433 before reaching the
second emission filter 436.
The second filter 436, an emission or barrier
filter, passes only light at wavelengths for which
fluorescent light is being emitted. Various filters can
be used and their specific characteristics depend on the
dye or probe being used, along with the excitation light
characteristics for a specific analysis.
The lens 437 then focuses the fluorescent
light forming the image of the spot into the plane of
the detector; in this case, into the eyepiece or sensor
of camera 42.
In the above-described embodiment, the focal
lengths of both lenses 431 and 434 are chosen
appropriately, depending on the core diameter of the
fiber 414 and the size of the spot being scanned.
Different image ratios can thus be implemented. In one
embodiment, an image ratio of 1:1 with respect to the
core diameter of fiber 414 and the surface area of the
spot being imaged can be obtained. In such a case, the
surface area being illuminated has a size corresponding
to the core diameter of fiber 414.
-11-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
In addition, the dimensions of the camera's
sensor further determine the ratio of the focal lengths
of lenses 434 and 437. The desired resolution of the
image being taken by camera 42 will further determine
the choice of focal lengths for lenses 434 and 437. In
one embodiment, if the camera 42 has a sensor with pixel
dimensions 10 m by 10 m, the focal lengths of lenses
434 and 437 are chosen such that a spot having a surface
area defined by a diameter of 300 lZm forms an image
having a surface area defined by a diameter of 3 mm once
on the plane of the camera's sensor. The resulting
captured image will thus have a resolution of about
1 pm/pixel.
Further in the above-described embodiment
illustrated in Fig. 2, a skilled person in the art will
understand that all the lenses described, lenses 412,
431, 434 with focusing mechanism 435, and lens 437, can
be implemented using an assembly of lenses instead of a
single lenses. Similarly, it is understood that the
filters 432 and 436, as well as the dichroic mirror 433,
can also be replaced by any other optical apparatus or
assembly of optical items achieving the desired
functionalities.
Fig. 3 is a block diagram illustrating a
micro-array analysis method in accordance with a second
embodiment.
The first step 500 consists in obtaining the
coordinates of a first spot located on the micro-array
5. A referencing technique is used to relate spot
coordinates given with respect to a specific micro-array
being read, to spot coordinates given with respect to a
given reference point in space, such that the position
system 6 may align the spot being read with the imaging
system 4.
-12-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
In one embodiment a referencing system can be
used, such that spot coordinates listed in a computer
file stored in memory unit 73 of the computer system 7,
are read one after the other until the end of the list.
These spot coordinates could be column and row
positions, for example, given with respect to a specific
micro-array layout. The computer system 7 could further
translate these coordinates into physical coordinates
appropriate for controlling the positioning system 6
such that the imaging system 4 is properly aligned with
the spot corresponding to the correct column and row
positions on the micro-array 5. The physical
coordinates could be defined by X and Y coordinates or
control signals. These physical coordinates are given
with respect to a fixed point in space and can depend on
the moving mechanism used by the positioning system 6.
Such spot coordinates can be stored in advance in a
computer readable file defining the design of a specific
micro-array layout.
In step 501, the computer system 7 sends
appropriate control signals to the positioning system 6.
The imaging system 4 is now aligned with the given spot
location on the micro-array.
In step 502, the imaging system 4 captures an
image of the given spot.
In step 503, the captured spot image is sent
to the computer system 7 for storage. For example, a
database located in memory unit 73 can be used to store
the captured spot images according to the spot's
coordinates. Other storage and file naming techniques
can be used as well.
In steps 504 and 505, the analysis system 2
moves on to the next spot in the list of spots to be
-13-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
scanned and analyzed, unless all the spots have been
read or do not require being read.
An alternative embodiment can permit a user to
override the positioning system 6 through the use of
input and output ports 64 of the positioning system 6,
to directly align the imaging system 4 with a desired
spot location.
In step 506, each captured and stored spot
image is processed by the computer system 7, through the
image processing and acquisition unit 71, such that the
cellular material within each spot may be properly
identified and quantified.
In step 507, the final micro-array analysis
results are finally computed and displayed.
Step 506 can also be performed after step 503
and before step 504, since the analysis of a spot can be
completed by the system as soon as the spot to be
analyzed is captured. Once spot analysis results are
available for the spot in process, the system moves to
the next spot,in the micro-array in steps 504 and 505.
Fig. 4 is a block diagram illustrating the
image processing step 506 of the analysis method of
Fig. 3.
The image processing step can be done in
various ways, and such that each unit of cellular
material, or each bacteria (herein referred to as units
of targeted specimen), can be identified and counted
once the image processing method is accomplished. More
particularly, the image processing method is implemented
such as to remove any undesired background effects,
recognize shapes and sizes of fluorescing elements of
interest, and segment any potential clusters into
individual units of targeted specimen.
-14-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
In step 600, a gradient calculation is
performed from the acquired spot image, to obtain a
gradient image and thus enable the detection of blobs
possibly containing one or more units of targeted
specimen. This step can be achieved by convolving the
captured spot image with a discrete differentiation
operator, such as the Sobel operator, to obtain a
gradient image indicative of the captured spot image's
intensity function.
In step 601, the above gradient image is
thresholded to obtain a binary picture. A binary
picture has only two levels of intensity. From this
picture, one may distinguish blobs of varying shapes,
sizes and areas, each formed by possibly one unit or an
agglomeration of targeted specimen with other foreign
and undesired material. Also, in one embodiment, the
thresholding process can be performed by using a
hysteresis technique.
In step 602, each blob found on the binary
picture is labeled with its corresponding blob size or
area.
In step 603, each blob in the binary picture
is further analyzed.
In step 604, if the size of the blob being
analyzed exceeds a threshold value set by a given size
range of one unit of a targeted specimen , the method
proceeds to step 605. If not, the method goes directly
to step 606. This is done until all the blobs found on
the thresholded gradient image are analyzed.
In step 605, the blob being analyzed may
contain more than one unit of targeted specimen. An
image segmentation algorithm is thus used to delimit
each unit of targeted specimen on the captured spot
image. In one embodiment, the segmentation algorithm
-15-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
may involve a bimodal thresholding of the histogram of
the pixels forming the corresponding fluorescing element
in the captured spot image. A bimodal thresholding
technique supposes a preset minimal and a maximal size
for each fluorescing element. Once boundary or
threshold values are found using any segmentation
technique, only the pixels of the blob which belong to
the pale mode (or within the determined range or
boundary limits) are kept. The resulting image thus
shows segmented blobs, from which it is possible to
distinguish units of targeted specimen and other units
of fluorescing material.
In step 606, once all the blobs are analyzed
through steps 603 to 605, any holes or empty pixels
located within each unit of targeted specimen of the
resulting fluorescing elements, or processed and
segmented blobs, are filled to obtain a clearer spot
image. This step further ensures that the proper area
and size calculations are performed in the following
steps.
In step 607, each unit of targeted specimen
within the resulting fluorescing elements on the image
processed is labeled with its corresponding area and
size. In other words, the resulting blobs are labeled
with their area and size.
In step 608, the blobs having areas exceeding
a given maximum threshold value are discarded. These
blobs are elements possibly formed by impurities or
other undesired fluorescing agents and material.
In step 609, the final processed image now
contains blobs corresponding to units of targeted
specimen. The specimen are counted and identified
accordingly. A concentration value may be obtained from
this final count or total signal of the spot.
-16-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
Fig. 5a is an image of a top plan view of a
spot A acquired with the imagining system 4 of Fig. 2.
This is an example of an unprocessed image of a spot A.
Fig. 5b is a final processed image of a spot
A'. The targeted specimen on this processed spot image
can be counted to obtain a total signal for this spot.
The image processing method described above
permits the elimination of background fluorescence
effects and any fluorescent light emanating from
undesired artifacts which are optically differentiable
from the targeted specimen. The variance in the
efficiency of fluorescence marking is also alleviated by
image processing method.
The image processing method implemented by the
analysis system 2 herein described permits the
quantification of various types of specimen. Other
embodiments of the image processing method and analysis
system 2 can be used to identify different specimen such
as micro-organisms, like bacteria, or any other elements
which are made to fluoresce in a way that they are
distinguishable on the captured spot image. The
detection of cells such as micro-organisms is of
particular interest since micro-organisms generally have
a size scale of a micron, which can be easily optically
resolved. Other examples include the detection of
different bacteria such as Salmonella, Citrobacter
freundii, Escherichia coli, Klebsiella pneumoniae and
Serratia marcescens. The micro-organisms can be set in
a pure culture or mixed with other bacteria or food
samples. Targeted specimen can be made to fluoresce
directly by labeling before interaction with the micro-
array, or indirectly by adding labeled antibody once the
targeted specimen are captured in the micro-array. The.
micro-array analysis system 2 herein detailed is capable
-17-
CA 02675103 2009-07-09
WO 2008/092226 PCT/CA2007/000113
of reading fluorescence from any type of micro-array
design. The number of spots can be limited in certain
circumstances due to the speed with which the analysis
system 2 is made to read each spot. Similarly, the
surface area taken by the micro-array on the microscope
slide can be limited depending on the mechanical
restrictions of the chosen specifications of the
positioning system 6. Various schemes may be
implemented to permit the analysis of a larger number of
spots per microscope slide, such as using both ends of
one slide.
It is understood that several other
embodiments of the micro-array scanning system and
method may be implemented, and thus fall within the
scope of the present invention.
-18-