Note: Descriptions are shown in the official language in which they were submitted.
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
PEEL-COAT COMPOSITIONS
BACKGROUND
The present invention relates to compositions for forming protective
coatings. In particular, the present invention relates to compositions for
forming peelable
coatings that protect underlying substrate surfaces.
A variety of manufactured products (e.g., vehicles and appliances) include
metal surfaces that are painted for aesthetic qualities. However, during
storage and
transportation, the manufactured products are often subjected to one or more
potentially
damaging conditions, such as chemical and abrasive conditions. Without
protection, the
painted surfaces of the manufactured products may be chipped, scratched, or
otherwise
damaged during storage and transportation. Furthermore, for manufactured
products that
are subjected to atmospheric conditions during storage and transportation
(e.g.,
automobiles), the elements of nature, such as rain and dust, may also render
the
manufactured products dirty, thereby requiring subsequent cleaning steps.
One technique for protecting painted surfaces from damage involves
applying coatings to the surfaces, where the coatings include polyvinyl
chloride (PVC).
However, vinyl chloride emissions that are produced during the synthesis and
degradation
of PVC are believed to be potential environmental concerns. Thus, due to such
concerns, an
increasing number of consumers are requesting products that are free of PVC-
based
compositions. Accordingly, there is a need for PVC-free coatings that protect
underlying
painted surfaces from damaging conditions during storage and transportation,
and are
readily removable.
SUMMARY
The present invention relates to a peel-coat composition and a peel coat
formed from the composition. The composition includes an aqueous carrier, a
polyvinyl
butyral, and an acrylic latex compound.
BRIEF DESCRIPTION OF THE DRAWINGS
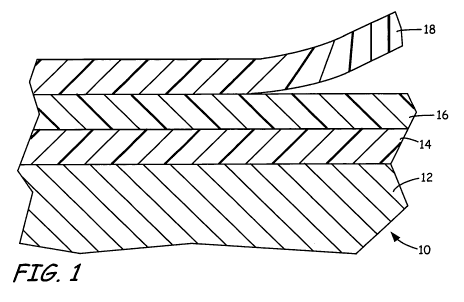
FIG. 1 is a sectional view of a coated article, where the coated article
includes a coating formed from a peel-coat composition of the present
invention.
1
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
FIG. 2 is a flow diagram of a method for forming a coated article containing
the coating formed from the peel-coat composition.
DETAILED DESCRIPTION
FIG. 1 is a sectional view of one embodiment of coated article 10, which
preferably includes substrate 12, primer coating 14, topcoat 16, and peel coat
18, where peel
coat 18 is a removable coating formed from a peel-coat composition of the
present
invention. In this embodiment, substrate 12 is a structure (e.g., a metal
structure) that is
coated with a coating that decorates or protects (or both decorates and
protects) the
substrate. In the embodiment shown, the coating comprises a two-composition
system
comprising primer coating 14 and topcoat 16. Primer coating 14 and topcoat 16
are
preferably surface-treatment coatings that provide protective and/or other
aesthetic qualities
(e.g., color) to coated article 10. As discussed below, peel coat 18 is
suitable for use with a
variety of coatings. As such, primer coating 14 and topcoat 16 may be formed
from a
variety of different compositions (e.g., fluoropolymers and urethanes). In an
alternative
embodiment, primer coating 14 and topcoat 16 may comprise a single layer
(e.g., a paint
that functions as both a primer and a topcoat), or additional compositions or
additional
layers of the compositions may be used.
In one embodiment, the peel-coat composition used to form peel coat 18
preferably includes an aqueous carrier, polyvinyl butyral, and an acrylic
latex compound.
This combination provides a coating that preferably has an adhesive strength
that prevents
the coating from delaminating from the adhered-to surface (e.g., topcoat 16)
during normal
transportation and storage, while also preferably allowing the coating to be
removed without
excessive removal strengths. For example, the adhesive strength desirably
allows the
coating to be removed without damaging the coating (e.g., the required removal
strength is
less than the tensile strength of the coating). Additionally, in preferred
embodiments the
peel-coat composition is substantially free of PVC-containing compounds (i.e.,
less than
about 100 parts-per-million by weight). As a result, the peel coat composition
does not
exhibit the above-discussed environmental concerns that are prevalent with PVC
emissions.
The peel-coat composition may be prepared by mixing (e.g., at high speeds)
the aqueous carrier, the polyvinyl butyral, the acrylic latex compound, and
any additional
optional additives, until the polyvinyl butyral is at least substantially
dispersed, solubilized,
emulsified, or otherwise suspended in the aqueous carrier and the acrylic
latex compound is
2
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
at least substantially dispersed, solubilized, emulsified, or otherwise
suspended in the
aqueous carrier. The peel-coat composition desirably has a percentage of non-
volatile
materials by weight (%NVM) that provides suitable dry film thicknesses for
peel coat 18.
Examples of suitable %NVMs for the peel-coat composition prior to coating
include at least
about 40% by weight, based on the entire weight of the peel-coat composition,
and as
measured pursuant to ASTM D2369-98.
FIG. 2 is a flow diagram of method 20, which is a suitable method for
forming coated article 10 (shown in FIG. 1) with the peel-coat composition.
Method 20
preferably includes steps 22, 24 and 26, and initially involves applying a
coating (e.g.,
primer coating 14 and topcoat 16) onto substrate 12 (step 22). This may be
performed in a
variety of manners using standard coating techniques. For example, primer
coating 14 may
be applied by coating a primer composition onto substrate 12 and then baking
or otherwise
curing the composition to form primer coating 14. Topcoat 16 may be applied on
primer
coating 14 in the same manner. Alternatively, primer coating 14 and topcoat 16
may
comprise a single layer of a coating composition.
The peel-coat composition is then preferably coated onto topcoat 16 (step
24). This may be performed using a variety of coating techniques, such as
sheet coating,
coil coating, roll coating, spray coating, and the like. The peel-coat
composition is then
preferably baked to at least substantially remove the aqueous carrier and to
cure the
remaining composition, thereby forming peel coat 18 on topcoat 16 (step 26).
In one
embodiment, the baking may be performed by passing the coated substrate 12
through an
oven maintained at a temperature suitable for volatilizing the aqueous
carrier. Suitable
baking temperatures of the oven may vary depending on multiple factors, such
as the speed
of the coating process, the coating thickness, and heat transfer conditions.
Examples of
suitable baking conditions include baking temperatures ranging from about 150
C to about
400 C with baking durations ranging from about 30 seconds to about 2 minutes.
Once formed, peel coat 18 is preferably adhered to topcoat 16 with an
adhesive strength that prevents premature peeling during transportation and
storage while
also allowing peel coat 18 to be peeled from topcoat 16 without an undue
removal strength.
Removal strengths required to remove peel coat 18 from topcoat 16 may vary
depending on
the composition of topcoat 16. However, peel coat 18 preferably exhibits
balanced peel
strengths for a variety of topcoat compositions, such as fluoropolymer and
urethane
topcoats. Examples of suitable adhesive strengths between peel coat 18 and
topcoats (e.g.,
3
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
topcoat 16) range from about 5.3 kilograms/centimeter2 (kg/cm2) (about 75
pounds/inch2
(lbs/in 2)) to about 21 kg/cm2 (about 300 lbs/in2), with particularly suitable
adhesive
strengths ranging from about 7.0 kg/cm2 (about 100 lbs/in 2) to about 14
kg/cm2 (about 200
lbs/in2), where the adhesive strengths are measured pursuant to ASTM D903-98.
Peel coat 18 desirably has a dry film thickness that allows peel coat 18 to
protect topcoat 16 from typical chemical and abrasive conditions (e.g.,
scratches and chips
incurred during transportation). Examples of suitable dry film thicknesses for
peel coat 18
range from about 2.5 micrometers (about 0.1 mil) to about 50 micrometers
(about 2 mil),
with particularly suitable dry film thicknesses ranging from about 13
micrometers (about
0.5 mil) to about 25 micrometers (about 1 mil).
As discussed above, the peel-coat composition used to form peel coat 18
preferably includes an aqueous carrier, polyvinyl butyral, and an acrylic
latex compound.
The aqueous carrier of the composition preferably includes water, and may also
include
organic solvents to preferably increase evaporation rates and/or to function
as
coalescent/film-forrning aids.
Examples of organic solvents for use in the aqueous carrier include
methanol, ethanol, isopropyl alcohols, butyl alcohols (e.g., n-butanol), 2-
butoxyethanol, 2-
(2-butoxyethoxy)ethanol (i.e., butyl carbitol), aromatic solvents,
isophorones, glycol ethers,
glycol ether acetates, acetone, methyl-ethyl ketones (MEK), N,N-
dimethylformamides,
ethylene carbonates, propylene carbonates, diglymes, N-methylpyrrolidones
(NMP), ethyl
acetates, ethylene diacetates, propylene glycol diacetates, alkyl ethers of
ethylene,
propylene glycol monoacetates, toluene, xylenes, and combinations thereof.
Examples of particularly suitable organic solvents for use in the aqueous
carrier include coalescent/film-forming aids, such as 2-(2-
butoxyethoxy)ethanol. Suitable
concentrations of organic solvents in the aqueous carrier range from about 0.1
% by weight
to about 30% by weight, based on the total weight of the aqueous carrier. The
term "about"
is used herein with respect to component concentrations due to expected
compositional
variations known to those skilled in the art (e.g., limitations and
variabilities in
measurements).
The polyvinyl butyral component preferably functions to increase the
toughness and weathering resistance of peel coat 18. The polyvinyl butyral
desirably has
good optical clarity and flexibility to provide suitable aesthetic and
physical properties. In
one embodiment, the polyvinyl butyral may be a reaction product of
butyraldehyde and
4
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
polyvinyl alcohol, which desirably provides a polymer having acetal moieties
(e.g.,
butyraldehyde acetyl groups). The polyvinyl butyral may also include one or
more
hydroxyl moieties (e.g., polyvinyl alcohol groups) and one or more ester
moieties (e.g.,
polyvinyl acetate groups) in the polymer chain. Hydroxyl moieties and the
ester moieties
are typically found in commercially available polyvinyl butyral resins.
Suitable concentrations of the hydroxyl moieties in the polyvinyl butyral
range include about 30% by weight or less, with particularly suitable
concentrations to
about 20% by weight or less. Suitable concentrations of the ester moieties in
the polyvinyl
butyral range include about 10% by weight or less, with particularly suitable
concentrations
to about 5% by weight or less. Examples of suitable weight average molecular
weights
(Mw)for the polyvinyl butyral range from about 5,000 to about 300,000, with
particularly
suitable weight average molecular weights ranging from about 50,000 to about
200,000.
The polyvinyl butyral is preferably dispersible, soluble, emulsifiable, or
otherwise suspendable in water, thereby preferably allowing the polyvinyl
butyral to form a
mixture in the aqueous carrier. In one embodiment, the polyvinyl butyral may
form a
storage-stable mixture in the aqueous carrier, in which the mixture has at
least one month
shelf stability at 25 C.
In an additional embodiment, the polyvinyl butyral may also be provided as a
dispersion in water, where the water of the polyvinyl butyral dispersion may
constitute at
least a portion of the aqueous carrier. Examples of suitable commercially
available
polyvinyl butyral dispersions include those under the series "BUTVAR " resin
dispersions
from Solutia, Inc., St. Louis, MO, which are plasticized polyvinyl butyrals
dispersed in
water. Suitable concentrations of the polyvinyl butyral in the peel-coat
composition range
from about 10% by weight to about 40% by weight, with particularly suitable
concentrations ranging from about 15% by weight to about 25% by weight, based
on the
total solids weight of the peel-coat composition.
The acrylic latex compound is preferably an ethylenically-unsaturated
compound that is preferably dispersible, soluble, emulsifiable, or otherwise
suspendable in
water, thereby allowing the acrylic latex compound to form a mixture in the
aqueous carrier.
The acrylic latex compound is more preferably emulsifiable in the aqueous
carrier with the
use of a dispersing agent (e.g., a surfactant). In one embodiment, the acrylic
latex
compound may form a storage-stable emulsion in the aqueous carrier, in which
the
emulsion has at least one month shelf stability at 25 C.
5
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
The ethylenically-unsaturated compound is preferably a compound (e.g.,
monomers, oligomers, polymers, and combinations thereof) having one or more
(meth)acrylate functionalities. The term "(meth)acrylate", as used herein,
refers to an
acrylate and a methacrylate functionality. Examples of suitable compounds
having one or
more (meth)acrylate functionalities for use in the peel-coat composition
include methyl
(meth)acrylates, ethyl (meth)acrylates, propyl (meth)acrylates, butyl
(meth)acrylates, 2-
ethylhexyl (meth)acrylates, hydroxyethyl (meth)acrylates, hydroxybutyl
(meth)acrylates,
cyclohexyl (meth)acrylates, acrylic acids, methacrylic acids, and combinations
thereof.
In one embodiment, the acrylic latex compound may also be provided as an
emulsion in water, where the water of the acrylic emulsion may constitute at
least a portion
of the aqueous carrier. Examples of suitable commercially available acrylic
emulsions
include those under the series "UCAR " latexes from Union Carbide Chemicals &
Plastics
Technology Corporation, Danbury, CT. Suitable concentrations of the acrylic
latex
compound in the peel-coat composition range from about 5% by weight to about
40% by
weight, with particularly suitable concentrations ranging from about 15% by
weight to
about 30% by weight, based on the total solids weight of the peel-coat
composition.
Furthermore, suitable solids weight ratios of the polyvinyl butyral to the
acrylic latex
compound range from about 20:80 (i.e., about 20 parts of polyvinyl butyral to
about 80
parts of the acrylic latex compound, by weight) to about 80:20, with
particularly suitable
solids weight ratios ranging from about 40:60 to about 60:40.
Suitable dispersing agents for forming an emulsion in the aqueous carrier
with the ethylenically-unsaturated compound include one or more nonionic or
anionic
surfactants. Suitable concentrations of the dispersing agents in the peel-coat
composition
range from about 0.1 % by weight to about 10% by weight, with particularly
suitable
concentrations ranging from about 0.5% by weight to about 5% by weight, based
on the
total weight of the peel-coat composition.
Examples of suitable nonionic surfactants for use in the peel-coat
composition include tertoctylphenoxy ethylpoly(39)ethoxyethanol,
dodecyloxypoly-
(10)ethoxyethanol, nonylphenoxyethylpoly(40)ethoxyethanol, polyethylene glycol
2000
monooleate, ethoxylated castor oil, fluorinated alkyl esters and alkoxylates,
polyoxyethylene (20) sorbitan monolaurate, sucrose monococoate, di(2-
butyl)phenoxypoly(20)ethoxyethanol, hydroxyethyl-cellulose polybutyl acrylate
graft
copolymer, dimethyl silicone polyalkylene oxide graft copolymer, poly(ethylene
6
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
oxide)poly(butyl acrylate), block copolymer, block copolymers of propylene
oxide and
ethylene oxide, 2,4,7,9-tetramethyl-5-decyne-4,7-diol ethoxylated with 30
moles of ethylene
oxide, N-polyoxyethylene(20)-lauramide, N-lauryl-N-polyoxyethylene
(3)amine,poly(10)
ethylene glycol dodecyl thioether, and combinations thereof.
Examples of suitable anionic surfactants for use in the peel-coat composition
include sodium lauryl sulfate, sodium dodecylbenzenesulfonate, potassium
stearate, sodium
dioctyl sulfosuccinate, sodium dodecyldiphenyloxide disulfonate,
nonylphenoxyethylpoly-
(I)ethoxyethyl sulfate ammonium salt, sodium styrene sulfonate, sodium dodecyl
allyl
sulfosuccinate, linseed oil fatty acid, sodium or ammonium salts of phosphate
esters of
ethoxylated nonylphenol, sodium octoxynol-3-sulfonate, sodium cocoyl
sarcocinate, sodium
1-alkoxy-2-hydroxypropyl sulfonate, sodium alpha-olefin (C i 4-C 16)
sulfonate, sulfates of
hydroxyalkanols, tetrasodium N-(1,2-dicarboxyethyl)-N-octadecylsulfo-
succinamate,
disodium N-octadecylsulfo- succinamate, disodium alkylamido polyethoxy
sulfosuccinate,
disodium ethoxylated nonylphenol half ester of sulfosuccinic acid, the sodium
salt of tert-
octylphenoxy ethoxy-poly(39)ethoxyethyl sulfate, and combinations thereof.
In addition to the aqueous carrier, the polyvinyl butyral, and the acrylic
latex
compound, the peel-coat composition may also include one or more additional
components,
such as plasticizers, polyols, defoaming agents, flow-control agents, heat
stabilizers,
leveling agents, thickening agents, pH-modifying agents, coalescents, dyes,
pigments,
colorants, ultraviolet-light absorbers, optical brighteners, biocides, and
combinations
thereof.
Plasticizers preferably modify the flow properties of the peel-coat
composition and increase the flexibility of peel coat 18. Examples of suitable
plasticizers
include phthalates, phosphates, adipates, sebacates, epoxidized oils,
polyesters, and
combinations thereof. Suitable concentrations of plasticizers in the peel-coat
composition
range from about 0.1% by weight to about 25% by weight, with particularly
suitable
concentrations ranging from about 1% by weight to about 10% by weight, based
on the total
weight of the peel-coat composition.
Examples of suitable polyols for use in the peel-coat composition include
alcohols having 1 to 6 hydroxy groups, such as ethylene glycol, 1,2-
propanediol (i.e.,
propylene glycol), 1,3-propanediol, 1,2-butanediol, 1,3-butanediol, 1,4-
butanediol, 2-ethyl-
1,3-propanediol, 2-methylpropanediol, 2-butyl-2-ethylpropanediol, 2-ethyl-1,3-
hexanediol,
1,3 neopentyl glycol, 2,2-dimethyl-1,3-pentanediol, 1,6 hexanediol, 1,2-
cyclohexanediol,
7
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
1,4-cyclohexanediol, bisphenol A, 1,2-bis(hydroxymethyl)cyclohexane, 1,4-
bis(hydroxymethyl) cyclohexane, bis(4-hydroxycyclohexyl)-methane, adipic acid
bis-
(ethylene glycol ester), ether alcohols (e.g., diethylene glycol and
triethylene glycol),
dipropylene glycol, perhydrogenated bisphenols, 1,2,4-butanetriol, 1,2,6-
hexanetriol,
trimethylolethane, trimethylolpropane, trimethylolhexane, glycerol,
pentaerythritol,
dipentaerythritol, mannitol, and sorbitol, and combinations thereof. Suitable
concentrations
of polyols in the peel-coat composition range from about 0.1 % by weight to
about 10% by
weight, with particularly suitable concentrations ranging from about 3% by
weight to about
7% by weight, based on the total weight of the peel-coat composition.
Examples of suitable defoaming agents include polysiloxane defoamers (e.g.,
methylalkylpolysiloxanes), polymeric defoamers, and combinations thereof.
Suitable
concentrations of the defoaming agents in the peel-coat composition range from
about 0.1 %
by weight to about 1% by weight, with particularly suitable concentrations
ranging from
about 0.1% by weight to about 0.5% by weight, based on the total weight of the
peel-coat
composition.
PH-modifying agents are beneficial for maintaining desired pH levels for the
peel-coat composition. In general, polyvinyl butyral forms a stable dispersion
in water for
pHs ranging from about 8 to about 10. However, the pHs of acrylic latex
compounds are
typically below a pH of 8. As a result, when the polyvinyl butyral resin and
the acrylic
latex compound are combined in the aqueous carrier, the acrylic latex compound
may lower
the pH of the peel-coat composition, thereby causing a portion of the
polyvinyl butyral resin
to precipitate out of the aqueous carrier. Thus, pH-modifying agents are
beneficial for
maintaining a composition pH of at least about 8. Examples of suitable pH-
modifying
agents include nitrogen-containing bases, such as amines (e.g.,
dimethylolamine). Suitable
concentrations of the pH-modifying agents in the peel-coat composition range
from about
0.1% by weight to about 2% by weight, with particularly suitable
concentrations ranging
from about 0.1% by weight to about 0.5% by weight, based on the total weight
of the peel-
coat composition.
The peel-coat composition used to form peel coat 18 may also contain an
optional coalescent, and many coalescents are known in the art. In one
embodiment, the
optional coalescent may be a low-volatile organic compound (VOC) coalescent.
Examples
of suitable low-VOC coalescents are described in Brandenburger et al., U.S.
Patent No.
6,762,230.
8
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
While discussed above for use with topcoat-covered surfaces (e.g., topcoat
16), the peel-coat composition may alternatively be applied directly to a
variety of
substrates including wood, cement, cement fiber board, wood-plastic
composites, tile,
metal, plastic, glass, optical fibers, and fiberglass. As such, the peel-coat
composition may
be used to form peel coats to protect a variety of structures from weathering,
chemical, and
abrasive conditions.
EXAMPLES
The present invention is more particularly described in the following
examples that are intended as illustrations only, since numerous modifications
and
variations within the scope of the present invention will be apparent to those
skilled in the
art. Unless otherwise noted, all parts, percentages, and ratios reported in
the following
examples are on a weight basis, and all reagents used in the examples were
obtained, or are
available, from the chemical suppliers described below, or may be synthesized
by
conventional techniques.
Examples 1-3
Coated Articles of Examples 1-3 each included a peel coat of the present
invention disposed on a topcoat-coated substrate, where the topcoats varied
between each
coated article. The peel coats for the coated articles were initially prepared
by preparing a
peel-coat composition having the component concentrations listed in Table 1.
Table 1
Component J Percent by Weight
PVB dispersion FP 40.5
Acrylic emulsion 52.6
Propylene glycol 5.3
Defoaming agent 0.2
Butyl carbitol 1.1
Surfactant 0.1
Dimethylolamine 0.2
PVB dispersion FP: A polyvinyl butyral dispersion commercially available
under the trademark "BUTVAR PVB Resin Dispersion FP" from Solutia, Inc., St.
Louis,
MO, which contained a plasticized polyvinyl butyral dispersed in water (about
50% by
weight solids of the polyvinyl butyral resin).
9
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
Acrylic emulsion: An acrylic emulsion commercially available under the
trademark "UCAR 411 Latexes" from Union Carbide Chemicals & Plastics
Technology
Corporation, Danbury, CT, which contained an acrylic latex compound emulsified
in water
(about 45% by weight solids of the acrylic latex compound).
Propylene glycol: Propylene glycol commercially available from Lyondell
Chemical Company, Houston, TX.
Defoaming agent: An anti-foam agent commercially available from
Verichem, Inc., Pittsburgh, PA.
Butyl carbitol: 2-(2-butoxyethoxy)ethanol film-forming aid commercially
available from Eastman Chemical Company, Kingsport, TN.
Surfactant: A water-soluble, anionic phosphate fluorosurfactant
commercially available under the trademark "ZONYL FSP" from E. I. du Pont de
Nemours
and Company.
Dimethylolamine: A pH-modifying agent commercially available from
Huntsman International LLC, Salt Lake City, UT.
The water portions of the polyvinyl butyral dispersion and the acrylic
emulsion constituted the aqueous carrier for the peel-coat composition. The
components
were mixed with a Cowles-type blade at high speeds until substantially
dispersed/emulsified
in the aqueous carrier. After mixing, the peel-coat composition had a solids
ratio of the
polyvinyl butyral to the acrylic latex compound of about 45:55.
The peel-coat composition was then coated onto a topcoat-coated substrate
with a #60 wire-wound rod. The topcoat used for the coated article of Example
1 was a
polyvinylidine difluoride (PVDF) coating commercially available under the
trademark
"FLUROPON " from Valspar Corporation, Minneapolis, MN. The topcoat used for
the
coated article of Example 2 was a fluoroethylene-alkyl vinyl ether (FEVE)
copolymer
commercially available under the trademark "VALFLON " from Valspar
Corporation. The
topcoat used for the coated article of Example 3 was a thick-film urethane
(TFU) coating
commercially available from Valspar Corporation.
Each coated article was then baked in a low-velocity electric oven at a
temperature of 177 C (350 F) for 60 seconds to obtain a peak metal temperature
(PMT)
ranging from 149 C (300 F) to 160 C (320 F). The baking process removed the
aqueous
carrier, thereby forming a peel coat on the topcoat, where the peel coat had a
total dry film
thickness of 33-38 micrometers (1.3-1.5 mils) (measured pursuant to ASTM D5796-
99).
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
Examples 4-6
Coated Articles of Examples 4-6 each included an alternative peel coat of the
present invention disposed on a topcoat-coated substrate, where the topcoats
varied between
each coated article. The peel coats for the coated articles were initially
prepared by
preparing a peel-coat composition having the component concentrations listed
in Table 2.
Table 2
Component Percent by Weight
PVB dispersion FP 46.6
Acrylate emulsion 47.3
Propylene glycol 4.7
Defoaming agent 0.1
Butyl carbitol 1.0
Surfactant 0.1
Dimethylolamine 0.2
The water portions of the polyvinyl butyral dispersion and the acrylic
emulsion constituted =the aqueous carrier for the peel-coat composition. The
components
were mixed with a Cowles-type blade at high speeds until substantially
dispersed/emulsified
in the aqueous carrier. After mixing, the peel-coat composition had a solids
ratio of the
polyvinyl butyral to the acrylic latex compound of about 55:45. The peel-coat
composition
was then coated onto topcoat-coated substrates and baked pursuant to the
procedures
discussed above for the coated articles of Examples 1-3, respectively. This
provided the
coated articles of Examples 4-6:
Comparative Example A-C
Coated Articles of Comparative Examples A-C each included a PVC-based
peel coat disposed on a topcoat-coated substrate. For each coated article, the
PVC-based
peel coat was initially prepared with a PVC-based composition commercially
available
from Valspar Corporation, Minneapolis, MN, and having the component
concentration
listed in Table 3.
11
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
Table 3
Component Percent by Weight
Plasticizer 28.8
Polyvinyl chloride 53.3
Melamine 2.0
Isobutyl alcohol 0.4
Cyclohexanone 1.1
Mineral spirits 14.4
The peel-coat composition was coated onto a topcoat-coated substrate with a
#60 wire-wound rod, where the topcoats used for the coated articles of
Examples A-C were
the same as those discussed above for the coated articles of Examples 1-3,
respectively.
Each coated article was then baked in a low-velocity electric oven at a
temperature of
330 C (625 F) for 20-40 seconds to obtain a PMT of 200 C (390 F). The baking
process
formed a PVC-based peel coat on the topcoat, where the peel coat had a total
dry film
thickness of 25-30 micrometers (1.0-1.2 mils) (measured pursuant to ASTM D5796-
99).
Peel Strength Testing of Examples 1-6 and Comparative Examples A-C
The coated articles of Examples 1-6 and Comparative Examples A-C were
each tested for peel strengths pursuant to ASTM D903-98, which involved
scoring the peel
coat of the coated article and measuring the strength required to peel the
peel coat from the
given topcoat. Table 4 provides the peel strength results for the coated
articles of Examples
1-6 and Comparative Examples A-C.
TABLE 4
Example Peel Coat Topcoat Peel Strength (psi)
Example I PVB/Acrylic PVDF 150
(45:55 solids ratio)
Example 2 PVB/Acrylic FEVE 45
(45:55 solids ratio)
Example 3 PVB/Acrylic TFU 200
(45:55 solids ratio)
Example 4 PVB/Acrylic PVDF 75
(55:45 solids ratio)
Example 5 PVB/Acrylic FEVE 0
(55:45 solids ratio)
Example 6 PVB/Acrylic TFU 150
(55:45 solids ratio)
Comparative Example A PVC-based PVDF 125
Comparative Example B PVC-based FEVE 0
Comparative Example C PVC-based TFU No peel
12
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
The results shown in Table 4 show the adhesive properties of the peel coats
of the present invention. With the exception of Example 5, the coated articles
of the present
invention exhibited suitable peel strengths over the various topcoats. The
peel-coat
composition used with the coated articles of Examples 1-3 exhibited
particularly suitable
peel strengths with all three topcoat compositions.
In comparison, the PVC-based peel coat composition used with the coated
articles of Comparative Examples A-C exhibited no adhesion to FEVE and would
not peel
from the thick film urethane topcoat. Additionally, the use of PVC, as
discussed above,
poses potential environmental concerns with PVC emissions that are not present
with the
peel-coat compositions of the present invention.
Examples 7-18
Peel-coat compositions for Examples 7-18 were prepared pursuant to the
procedure discussed above for the peel-coat composition used in the coated
articles of
Examples 1-3. However, the peel-coat compositions for Examples 7-18 each
included
propylene glycol at 7.0% by weight, the defoaming agent at 0.1% by weight, the
surfactant
at 0.1% by weight, and the dimethylolamine at 0.2% by weight. The balance of
each peel-
coat composition include the acrylic emulsion, the PVB dispersion FP, and a
second
polyvinyl butyral dispersion commercially available under the trademark
"BUTVAR PVB
Resin Dispersion CO24PON", from Solutia, Inc., St. Louis, MO (referred to as
"PVB
Dispersion CO24PON.
Table 5 lists the solids weight ratios of the acrylic latex compound to the
polyvinyl butyral, and the solids weight ratios of the PVB resin FB to the PVB
resin
CO24PON, for each peel-coat composition of Examples 7-18. Table 5 also lists
the %NVM
for each peel-coat composition of Examples 7-18 (measured pursuant to ASTM
D2369-98).
13
CA 02677350 2009-08-04
WO 2008/097415 PCT/US2008/000494
TABLE 5
Solids Ratio of PVB to Solids Ratio of PVB
Example Acrylic Latex Compound Dispersion FP to PVB %NVM
Dispersion CO24PON
Example 7 50/50 50/50 42.9
Example 8 60/40 50/50 44.2
Example 9 70/30 50/50 45.5
Example 10 80/20 50/50 46.9
Example 11 50/50 40/60 42.9
Example 12 60/40 40/60 44.1
Example 13 70/30 40/60 45.4
Example 14 80/20 40/60 46.8
Example 15 50/50 60/40 43.0
Example 16 60/40 60/40 44.3
Example 17 70/30 60/40 45.6
Example 18 80/20 60/40 47.0
The solids ratios listed in Table 5 illustrate suitable ratios of the
polyvinyl
butyral to the acrylic latex compound that may be used to form stable peel-
coat
compositions. The peel-coat compositions of Examples 7-18 are accordingly
suitable for
forming coated articles pursuant to the procedures discussed above for the
coated articles of
Examples 1-3
Although the present invention has been described with reference to
preferred embodiments, workers skilled in the art will recognize that changes
may be made
in form and detail without departing from the spirit and scope of the
invention.
All patents, patent applications and literature cited in the specification are
hereby incorporated by reference in their entirety. In the case of any
inconsistencies, the
present disclosure, including any definitions therein will prevail.
14