Note: Descriptions are shown in the official language in which they were submitted.
CA 02677953 2009-09-11
69666-173D
1
COMPOSITE ARRAYS UTILIZING MICROSPHERES
WITH A HYBRIDIZATION CHAMBER
This is a divisional application of Canadian Patent Application No. 2,414,618
filed
June 28, 2001.
FIELD OF THE INVENTION
The invention relates to sensor compositions comprising a composite array of
individual arrays, to allow for simultaneous processing of a number of
samples.
The invention further provides methods of making and using the composite
arrays.
The invention further relates to an apparatus comprising a hybridization
chamber
for holding composite arrays.
The subject matter of this divisional application is directed to an array
system, a
method of decoding an array of nucleic acids using an array system, a method
of
detecting the presence or absence of a target analyte in a sample using an
array
system and a kit comprising an array system and a decoding binding ligand.
The subject matter of the parent application has been restricted to a
hybridization
chamber, a method of decoding an array location using a hybridization chamber,
and a method of detecting the presence or absence of a target analyte in a
sample using a hybridization chamber. However, it should be understood that
the
expression "the invention" and the like, when used herein, encompasses the
subject matter of both the parent and this divisional application.
BACKGROUND OF THE INVENTION
There are a number of assays and sensors for the detection of the presence
and/or concentration of specific substances in fluids and gases. Many of these
rely
on specific ligand/antiligand reactions as the mechanism of detection. That
is,
pairs of substances (i.e. the binding pairs or ligand/antiligands) are known
to bind
to each other, while binding little or not at all to other substances. This
has been
the focus of a number of techniques that utilize these binding pairs for the
detection of the complexes. These generally are done by labeling one component
of the complex in some way, so as to make the entire complex detectable,
using,
for example, radioisotopes, fluorescent and other optically active molecules,
enzymes, etc.
CA 02677953 2009-09-11
69666-173D
2
Of particular use in these sensors are detection mechanisms utilizing
luminescence.
Recently, the use of optical fibers and optical fiber strands in combination
with light
absorbing dyes for chemical analytical determinations has undergone rapid
development, particularly within the last decade. The use of optical fibers
for such
purposes and techniques is described by Milanovich et al., "Novel Optical
Fiber
Techniques For Medical Application", Proceedings of the SPIE 28th Annual
International Technical Symposium On Optics and Electro-Optics, Volume
494,1980;
Seitz, W.R, "Chemical Sensors Based On Immobilized Indicators and Fiber
Optics" in
C.R.C. Critical Reviews In Analytical Chemistry, Vol. 19, 1988, pp. 135-173;
Wolfbeis, 0. S., "Fiber Optical Fluorosensors In Analytical Chemistry" in
Molecular
Luminescence Spectroscopy, Methods and Applications (S. G. Schulman, editor),
Wiley & Sons, New York (1988); Angel, S.M., Spectroscopy 2 (4):38 (1987);
Walt, et
aL, "Chemical Sensors and Microinstrumentation", ACS Symposium Series, Vol.
403,
1989, p. 252, and \Volfbeis, O.S., Fiber Optic Chemical Sensors, Ed. CRC
Press, Boca
Raton, FL, 1991, 2nd Volume.
More recently, fiber optic sensors have been constructed that permit the use
of multiple
dyes with a single, discrete fiber optic bundle. U.S. Pat. Nos. 5,244,636 and
5,250,264
to Walt, et al. disclose systems for affixing multiple, different dyes on the
distal end of
the bundle. The disclosed configurations enable separate optical fibers of the
bundle to
optically access individual dyes. This avoids the problem of deconvolving the
separate
signals in the returning light from each dye, which arises when the signals
from two or
more dyes are combined, each dye being sensitive to a different analyte, and
there is
significant overlap in the dyes' emission spectra.
U.S. Patent Nos. 6,023,540 and 6,327,410 describe array compositions that
utilize
microspheres or beads on a surface of a substrate, for example on a terminal
end of a
fiber optic bundle, with each individual fiber comprising a bead containing an
optical
signature. Since the beads go down randomly, a unique optical signature is
needed to
"decode" the array; i.e. after the array is made, a correlation of the
location of an
individual site on the array with the bead or bioactive agent at that
particular site can
CA 02677953 2009-09-11
69666-173D
3
be made. This means that the beads may be randomly distributed on the array, a
fast
and inexpensive process as compared to either the in situ synthesis or
spotting
techniques of the prior art. Once the array is loaded with the beads, the
array can be
decoded, or can be used, with full or partial decoding occurring after
testing, as is more
fully outlined below.
In addition, compositions comprising silicon wafers comprising a plurality of
probe
arrays in microtiter plates have been described in U.S. Patent No. 5,545,531.
SUMMARY OF THE INVENTION
In accordance with the above objects, the present invention provides composite
array
compositions comprising a first substrate with a surface comprising a
plurality of assay
locations, each assay location comprising a plurality of discrete sites. The
substrate
further comprises a population of microspheres comprising at least a first and
a second
subpopulation, wherein each subpopulation comprises a bioactive agent. The
microspheres are distributed on each of the assay locations.
In a further aspect, the invention provides composite array compositions
comprising a
first substrate with a surface comprising a plurality of assay locations and a
second
substrate comprising a plurality of array locations, each array location
comprising
discrete sites. The compositions further comprise a population of microspheres
comprising at least a first and a second subpopulation, wherein each
subpopulation
comprises a bioactive agent. The microspheres are distributed on each of the
array
locations.
In an additional aspect, the present invention provides methods of decoding an
array
composition comprising providing an array composition as outlined above, and
adding
a plurality of decoding binding ligands to the composite array composition to
identify
the location of at least a plurality of the bioactive agents.
CA 02677953 2009-09-11
69666-173D
4
In a further aspect, the present invention provides methods of determining the
presence
of one or more target analytes in one or more samples comprising contacting
the
sample with a composition as outlined herein, and determining the presence or
absence
of said target analyte.
In a further aspect the invention provides a hybridization chamber. The
hybridization
chamber includes a base plate and a lid. A sealant is localized between the
lid and
base plate to provide for an airtight seal. When a two-component array system
is used,
the chamber also includes component ports in the lid to immobilize the array
components. That is, array components are inserted through the port in the
lid. The
ports may include seals so that an airtight seal is maintained. The chamber
also may
include clamps and alignment pins.
In a further aspect the invention provides a hybridization chamber wherein the
base
plate contains holes. The holes may be in a microplate array format. In one
embodiment at least two holes are joined by a channel. In one embodiment a
flexible
membrane is placed on the base plate. When pressure i.e. a vacuum, is applied
to the
membrane, wells form in the membrane at the location of the holes in the base
plate.
The apparatus also includes a pneumatic device for the delivery of a vacuum or
positive pressure to the membrane.
In a further aspect the invention provides a method of mixing samples in an
array
format. The method includes providing a vacuum to the membrane such that wells
are
formed. A solution is then applied to the membrane such that at least one of
the wells
is filled with liquid. Subsequently, the vacuum is applied intermittently to
the
membrane, which results in mixing of the liquid.
In a further aspect the invention provides an apparatus comprising a
hybridization
chamber as described herein and any of the composite array compositions
described
herein.
CA 02677953 2009-09-11
69666-173D
In a further aspect the invention provides performing methods of decoding an
array composition as described herein in a hybridization chamber as described
herein.
In a further aspect the invention provides performing methods of determining
the
5 presence of one or more target analytes in one or more samples as described
herein in a hybridization chamber as described herein.
According to one aspect of the invention of the present divisional
application, there
is provided an array system comprising: a) a first array component comprising
a
plurality of wells; and b) a second array component comprising a plurality of
projections, each projection having an end comprising a surface, said surface
having a population of nucleic acids distributed thereon, wherein the
plurality of
projections is configured to be dipped into the plurality of wells.
According to another aspect of the invention of the present divisional
application,
there is provided a method of decoding an array of nucleic acids, said method
comprising: a) providing an array system comprising: i) a first array
component
comprising a plurality of wells; and ii) a second array component comprising a
plurality of projections, each projection having an end comprising a surface,
said
surface having a population of nucleic acids distributed thereon, wherein the
plurality of projections is configured to be dipped into the plurality of
wells; and b)
providing a plurality of decoding binding ligands to said surface to identify
the
location of at least one of said nucleic acids.
According to yet another aspect of the invention of the present divisional
application, there is provided a method of detecting the presence or absence
of a
target analyte in a sample, said method comprising: a) providing an array
system
comprising: i) a first array component comprising a plurality of wells; and
ii) a
second array component comprising a plurality of projections, each projection
having an end comprising a surface, said surface having a population of
nucleic
acids distributed thereon, wherein the plurality of projections is configured
to be
dipped into the plurality of wells; b) providing a sample to at least one
well; c)
contacting said sample with at least one surface; and d) determining the
presence
or absence of said target analyte.
CA 02677953 2010-05-11
69666-173D
5a
According to one aspect of the invention of the present divisional
application, there
is provided an array system comprising: a) a base plate adapted to hold a
first
array component comprising a well; and b) a lid comprising a component port
for
immobilizing a second array component, said a second array component
comprising an end comprising a surface, said surface having a population of
nucleic acids distributed thereon, wherein said first array component and said
second array component are adapted to fit together.
According to another aspect of the invention of the present divisional
application,
there is provided a method of decoding an array of nucleic acids, said method
comprising: a) providing an array system comprising: i) a base plate adapted
to
hold a first array component comprising a well; and ii) a lid comprising a
component port for immobilizing a second array component, said a second array
component comprising an end comprising a surface, said surface having a
population of nucleic acids distributed thereon, wherein said first array
component
and said second array component are adapted to fit together; and b) providing
a
plurality of decoding binding ligands to said surface to identify the location
of at
least one of said nucleic acids.
According to yet another aspect of the invention of the present divisional
application, there is provided a method of detecting the presence or absence
of a
target analyte in a sample, said method comprising: a) providing an array
system
comprising: i) a base plate adapted to hold a first array component comprising
a
well; and ii) a lid comprising a component port for immobilizing a second
array
component, said a second array component comprising an end comprising a
surface, said surface having a population of nucleic acids distributed
thereon,
wherein said first array component and said second array component are adapted
to fit together; b) providing a sample to said well; c) contacting said sample
with
said surface; and d) determining the presence or absence of said target
analyte.
According to still another aspect of the invention of the present divisional
application, there is provided a kit comprising an array system as described
herein
and a decoding binding ligand.
CA 02677953 2010-05-11
=
69666-173D
5b
BRIEF DESCRIPTION OF THE FIGURES
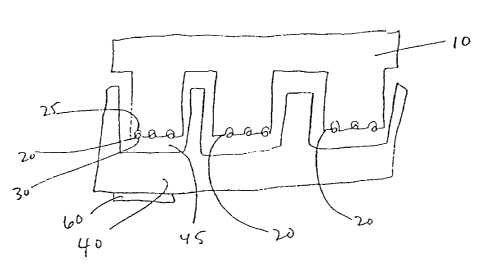
Figures 1 A, 1 B, 1 C, 1 D and 1 E depict several different "two component"
system
embodiments of the invention. In Figure 1A, a bead array is depicted. The
first
substrate 10 has array locations 20 with wells 25 and beads 30. The second
substrate 40 has assay locations 45. An optional lens or filter 60 is also
shown; as
will be appreciated by those in the art, this may be internal to the substrate
as
well. Figure 1 B is similar except that beads are not used; rather, array
locations
20 have discrete sites 21, 22, 23, etc. that may be formed using spotting,
printing,
photolithographic techniques, etc. Figures 1 C-F depict the use of a plurality
of first
substrates. Figure 1 C depicts a "bead of beads" that may have additional use
for
mixing functions. Figure 1 D depicts a plurality of bead arrays and Figure 1 E
depicts a plurality of non-bead arrays. Figure 1F depicts the use of binding
functionalities to "target" first substrates 10 to locations on the second
substrate
40; as will be appreciated by those in the art, this may be done on flat
second
substrates or on compartmentalized second substrates. Figure 1 F utilizes
binding
ligand pairs 70/70', 71/71, 72/72', etc. These may be either chemical
functionalities or biological ones, such as are described for IBUDBL pairs,
such as
oligonucleotides, etc.
Figures 2A and 2B depict two different "one component" systems. Figure 2A
depicts a bead array, with the substrate 50 having assay locations 45 with
wells
comprising beads 30. Figure 2B depicts a non-bead array; each assay location
45 has discrete sites 21, 22, 23, etc.
CA 02677953 2009-09-11
69666-173D
6
Figure 3 depicts clustering in hyperspectral alpha space (a, = I,/EI1, a2 =
I2/EI;, a3 =
13/E11, etc.). A set of 128 different bead types present on a fiber bundle
were decoded
with by hybridizing set of complementary oligonucleotides labeled with four
dyes:
Bodipy-493, Bodipy-R6G, Bodipy-TXR, and Bod-564 (only one dye per
oligonucleotide). Shown is the second stage of a four stage decode in which
4013
beads were decoded. Ovals are drawn around zones of hue clusters.
Figure 4 Illustrates a two color decoding process wherein either FAM-labeled
or
Cy3-labeled oligo complements are use to "paint" (label) the different bead
types on
the array.
Figure 5 depicts the decoding 128 different bead types with four colors and
four
decode stages. (inset shows a single decode stage using four different dyes to
decode
16 bead types.)
Figure 6 depicts grey scale decoding of 16 different bead types. (A)
Combinatorial
pooling scheme for complementary decoding oligos. A (B) Two independent
normalizing images were acquired, and the resulting bead intensities compared.
(C)
The alpha values (ratio of bead intensity in indicated decode stage to
intensity in
normalization image) are plotted for three decodes stage described in (A).
Figure 7 schematically depicts the lid and base plate. A. Depicts the lid 110
and base
plate 160 of the hybridization chamber. Ports 120 in the lid allow for fiber
optic
bundles 130 to be inserted through the lid and contact the sample in the wells
of the
microtiter plate 140 in the base cavity 150 of the base plate 160. B. Depicts
the base
cavity 150 of the base plate 160.
Figure 8 schematically depicts the hybridization chamber including the lid 110
and base
plate 160. Also shown are the peripheral seal 80, the clamp 90 and clamp
receptacle
95, fiber optic bundles 130 inserted through the lid and into the well of the
microtiter
plate 140.
CA 02677953 2009-09-11
69666-173D
7
Figure 9 depicts a base plate with holes 105. A Depicts the holes 105 in the
base plate.
B Depicts channels 100 connecting the holes 105.
Figure 10 depicts variable solution volume and localization on the membrane
caused
by pressure and/or vacuum. A. +P indicates pressure; -P indicates vacuum.
Upward
bending of the membrane in response to pressure in all chambers and holes. B.
Fluid
is moved to the left side of the membrane when vacuum is applied to the left
chambers
and pressure is applied to the middle and right chambers. C. When vacuum is
first
applied to the left section, fluid fills the wells. When vacuum is
subsequently applied
to the middle and right chambers, empty wells are formed. D. Fluid moves to
the
center of the membrane when vacuum is applied to the center and pressure is
applied
to left and right chambers. E. Fluid fills in wells formed by high vacuum in
the
center. Empty wells form on the left and right when low vacuum is applied. F.
Fluid
moves to the right when vacuum is applied to the right chamber and pressure is
applied
to the left and middle chambers.
Figure 11 depicts a flow chart of a representative assay scheme that finds use
with the
hybridization chamber.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to the formation of very high density arrays
that can
allow simultaneous analysis, i.e. parallel rather than serial processing, on a
number of
samples. This is done by forming an "array of arrays", i.e. a composite array
comprising a plurality of individual arrays, that is configured to allow
processing of
multiple samples. For example, each individual array is present within each
well of a
microtiter plate. Thus, depending on the size of the microtiter plate and the
size of the
individual array, very high numbers of assays can be run simultaneously; for
example,
using individual arrays of 2,000 distinct species (with high levels of
redundancy built
in) and a 96 well microtiter plate, 192,000 experiments can be done at once;
the same
arrays in a 384 microtiter plate yields 768,000 simultaneous experiments, and
a 1536
microtiter plate gives 3,072,000 experiments.
CA 02677953 2009-09-11
69666-173D
8
Generally, the array compositions of the invention can be configured in
several ways.
In a preferred embodiment, as is more fully outlined below, a "one component"
system
is used. That is, a first substrate comprising a plurality of assay locations
(sometimes
also referred to herein as "assay wells"), such as a microtiter plate, is
configured such
that each assay location contains an individual array. That is, the assay
location and
the array location are the same. For example, the plastic material of the
microtiter
plate can be formed to contain a plurality of "bead wells" in the bottom of
each of the
assay wells. Beads containing bioactive agents can then be loaded into the
bead wells
in each assay location as is more fully described below. It should be noted
that while
the disclosure herein emphasizes the use of beads, beads need not be used in
any of the
embodiments of the invention; the bioactive agents can be directly coupled to
the array
locations. For example, other types of arrays are well known and can be used
in this
format; spotted, printed or photolithographic arrays are well known; see for
example
WO 95/25116; WO 95/35505; WO 98/050782; U.S. Patent Nos. 5,700,637;
5,807,522 5,445,934, 6,406,845, and 6,482,593; and references cited within. In
one
component systems, if beads are not used, preferred embodiments utilize non-
silicon wafer substrates.
Alternatively, a "two component" system can be used. In this embodiment, the
individual arrays are formed on a second substrate, which then can be fitted
or
"dipped" into the first microliter plate substrate. As will be appreciated by
those in the
art, a variety of array formats and configurations may be utilized. A
preferred
embodiment utilizes fiber optic bundles as the individual arrays, generally
with a
"bead well" etched into one surface of each individual fiber, such that the
beads
containing the bioactive agent are loaded onto the end of the fiber optic
bundle. The
composite array thus comprises a number of individual arrays that are
configured to fit
within the wells of a microtiter plate. Alternatively, other types of array
formats may
be used in a two component system. For example, ordered arrays such as those
made
by spotting, printing or photolithographic techniques can be placed on the
second
substrate as outlined above. Furthermore, as shown in Figures 1 C-F, "pieces"
of
arrays, either random or ordered, can be utilized as the first substrate.
CA 02677953 2009-09-11
69666-173D
9
The present invention is generally based on previous work comprising a bead-
based
analytic chemistry system in which beads, also termed microspheres, carrying
different
chemical functionalities are distributed on a substrate comprising a patterned
surface
of discrete sites that can bind the individual microspheres. The beads are
generally put
onto the substrate randomly, and thus several different methodologies can be
used to
"decode" the arrays. In one embodiment, unique optical signatures are
incorporated
into the beads, generally fluorescent dyes, that could be used to identify the
chemical
functionality on any particular bead. This allows the synthesis of the
candidate agents
(i.e. compounds such as nucleic acids and antibodies) to be divorced from
their
placement on an array, i.e. the candidate agents may be synthesized on the
beads, and
then the beads are randomly distributed on a patterned surface. Since the
beads are
first coded with an optical signature, this means that the array can later be
"decoded",
i.e. after the array is made, a correlation of the location of an individual
site on the
array with the bead or candidate agent at that particular site can be made.
This means
that the beads may be randomly distributed on the array, a fast and
inexpensive process
as compared to either die in situ synthesis or spotting techniques of the
prior art.
These methods are generally outlined in WO 98/040726; WO 99/018434; WO
00/016101; WO 99/067641; and U.S. Patent Nos. 6,023,540; 6,544,732; and
6,327,410. In addition, while the discussion herein is generally directed to
the use of
beads, the same configurations can be applied to cells and other particles;
see for
example WO 99/045357.
In these systems, the placement of the bioactive agents is generally random,
and thus a
coding/decoding system is required to identify the bioactive agent at each
location in
the array. This may be done in a variety of ways, as is more fully outlined
below, and
generally includes: a) the use a decoding binding ligand (DBL), generally
directly
labeled, that binds to either the bioactive agent or to identifier binding
ligands (IBLs)
attached to the beads; b) positional decoding, for example by either targeting
the
placement of beads (for example by using photoactivatible or photocleavable
moieties
to allow the selective addition of beads to particular locations), or by using
either sub-
bundles or selective loading of the sites, as are more fully outlined below;
c) selective
CA 02677953 2009-09-11
69666-173D
decoding, wherein only those beads that bind to a target are decoded; or d)
combinations of any of these. In some cases, as is more fully outlined below,
this
decoding may occur for all the beads, or only for those that bind a particular
target
analyte. Similarly, this may occur either prior to or after addition of a
target analyte_
5 Once the identity (i.e. the actual agent) and location of each microsphere
in the array
has been fixed, the array is exposed to samples containing the target
analytes, although
as outlined below, this can be done prior to or during the analysis as well.
The target
analytes will bind to the bioactive agents as is more fully outlined below,
and results in
a change in the optical signal of a particular bead.
10 In the present invention, "decoding" can use optical signatures, decoding
binding
ligands that are added during a decoding step, or a combination of these
methods. The
decoding binding ligands will bind either to a distinct identifier binding
ligand partner
that is placed on the beads, or to the bioactive agent itself, for example
when the beads
comprise single-stranded nucleic acids as the bioactive agents. The decoding
binding
ligands are either directly or indirectly labeled, and thus decoding occurs by
detecting
the presence of the label. By using pools of decoding binding ligands in a
sequential
fashion, it is possible to greatly minimize the number of required decoding
steps.
Accordingly, the present invention provides composite array compositions
comprising
at least a first substrate with a surface comprising a plurality of assay
locations. By
"array" herein is meant a plurality of candidate agents in an array format;
the size of
the array will depend on the composition and end use of the array. Arrays
containing
from about 2 different bioactive agents (i.e. different beads) to many
millions can be
made, with very large fiber optic arrays being possible. Generally, the array
will
comprise from two to as many as a billion or more, depending on the size of
the beads
and the substrate, as well as the end use of the array, thus very high
density, high
density, moderate density, low density and very low density arrays may be
made.
Preferred ranges for very high density arrays are from about 10,000,000 to
about
2,000,000,000, (with all numbers being per square centimeter) with from about
100,000,000 to about 1,000,000,000 being preferred. High density arrays range
about
CA 02677953 2009-09-11
69666-173D
11
100,000 to about 10,000,000, with from about 1,000,000 to about 5,000,000
being
particularly preferred. Moderate density arrays range from about 10,000 to
about
100,000 being particularly preferred, and from about 20,000 to about 50,000
being
especially preferred. Low density arrays are generally less than 10,000, with
from
about 1,000 to about 5,000 being preferred. Very low density arrays are less
than
1,000, with from about 10 to about 1000 being preferred, and from about 100 to
about
500 being particularly preferred. In some embodiments, the compositions of the
invention may not be in array format; that is, for some embodiments,
compositions
comprising a single bioactive agent may be made as well. In addition, in some
arrays,
multiple substrates may be used, either of different or identical
compositions. Thus for
example, large arrays may comprise a plurality of smaller substrates.
In addition, one advantage of the present compositions is that particularly
through the
use of fiber optic technology, extremely high density arrays can be made. Thus
for
example, because beads of 200 m or less (with beads of 200 nm possible) can
be
used, and very small fibers are known, it is possible to have as many as
40,000 -
50,000 or more (in some instances, 1 million) different fibers and beads in a
1 mm2
fiber optic bundle, with densities of greater than 15,000,000 individual beads
and
fibers (again, in some instances as many as 25-50 million) per 0.5 ctp'
obtainable.
By "composite array" or "combination array" or grammatical equivalents herein
is
meant a plurality of individual arrays, as outlined above. Generally the
number of
individual arrays is set by the size of the microtiter plate used; thus, 96
well, 384 well
and 1536 well microtiter plates utilize composite arrays comprising 96, 384
and 1536
individual arrays, although as will be appreciated by those in the art, not
each
microtiter well need contain an individual array. It should be noted that the
composite
arrays can comprise individual arrays that are identical, similar or
different. That is, in
some embodiments, it may be desirable to do the same 2,000 assays on 96
different
samples, alternatively, doing 192,000 experiments on the same sample (i.e. the
same
sample in each of the 96 wells) may be desirable. Alternatively, each row or
column
of the composite array could be the same, for redundancy/quality control. As
will be
appreciated by those in the art, there are a variety of ways to configure the
system. In
CA 02677953 2009-09-11
69666-173D
12
addition, the random nature of the arrays may mean that the same population of
beads
may be added to two different surfaces, resulting in substantially similar but
perhaps
not identical arrays.
By "substrate" or "solid support" or other grammatical equivalents herein is
meant any
material that can be modified to contain discrete individual sites appropriate
for the
attachment or association of beads and is amenable to at least one detection
method.
As will be appreciated by those in the art, the number of possible substrates
is very
large. Possible substrates include, but are not limited to, glass and modified
or
functionalized glass, plastics (including acrylics, polystyrene and copolymers
of
styrene and other materials, polypropylene, polyethylene, polybutylene,
polyurethanes,
TeflonJ, etc.), polysaccharides, nylon or nitrocellulose, resins, silica or
silica-based
materials including silicon and modified silicon, carbon, metals, inorganic
glasses,
plastics, optical fiber bundles, and a variety of other polymers. In general,
the
substrates allow optical detection and do not themselves appreciably
fluorescese.
Generally the substrate is flat (planar), although. as will be appreciated by
those in the
art, other configurations of substrates may be used as well; for example,
three
dimensional configurations can be used, for example by embedding the beads in
a
porous block of plastic that allows sample access to the beads and using a
confocal
microscope for detection. Similarly, the beads may be placed on the inside
surface of a
tube, for flow-through sample analysis to minimize sample volume. Preferred
substrates include optical fiber bundles as discussed below, and flat planar
substrates
such as glass, polystyrene and other plastics and acrylics. In some
embodiments,
silicon wafer substrates are not preferred. In one embodiment the substrate is
in the
shape of or is a microscope slide.
The first substrate comprises a surface comprising a plurality of assay
locations, i.e.
the location where the assay for the detection of a target analyte will occur.
The assay
locations are generally pl ysically separated from each other, for example as
assay
wells in a micro titer plate, although other configurations
(hydrophobicity/hydrophilicity, etc.) can be used to separate the assay
locations.
CA 02677953 2009-09-11
69666-173D
13
In a preferred embodiment, the second substrate is an optical fiber bundle or
array, as
is generally described in U.S. Patent Nos. 7,115,884 and 6,200,737, WO
98/040726, and
WO 98/050782. Preferred embodiments utilize preformed unitary fiber optic
arrays.
By "preformed unitary fiber optic array" herein is meant an array of discrete
individual fiber optic strands that are co-axially disposed and joined along
their
lengths. The fiber strands are generally individually clad. However, one thing
that
distinguished a preformed unitary array from other fiber optic formats is that
the
fibers are not individually physically manipulatable; that is, one strand
generally cannot
be physically separated at any point along its length from another fiber
strand.
However, in some "two component" embodiments, the second substrate is not a
fiber
optic array.
In a preferred embodiment, the assay locations (of the "one component system")
or the
array locations (of the "two component system") comprise a plurality of
discrete sites.
Thus, in the former case, the assay location is the same as the array
location, as
described herein. In the latter case, the array location is fitted into the
assay location
separately. In these embodiments, at least one surface of the substrate is
modified to
contain discrete, individual sites for later association of microspheres (or,
when
microspheres are not used, for the attachment of the bioactive agents). These
sites may
comprise physically altered sites, i.e. physical configurations such as wells
or small
depressions in the substrate that can retain the beads, such that a
microsphere can rest
in the well, or the use of other forces (magnetic or compressive), or
chemically altered
or active sites, such as chemically functionalized sites, electrostatically
altered sites,
hydrophobically/ hydrophilically functionalized sites, spots of adhesive, etc.
The sites may be a pattern, i.e. a regular design or configuration, or
randomly
distributed. A preferred embodiment utilizes a regular pattern of sites such
that the
sites may be addressed in the X-Y coordinate plane. "Pattern" in this sense
indudes a
repeating unit cell, preferably one that allows a high density of beads on the
substrate.
However, it should be noted that these sites may not be discrete sites. That
is, it is
CA 02677953 2009-09-11
69666-173D
14
possible to use a uniform surface of adhesive or chemical functionalities, for
example,
that allows the attachment of beads at any position. That is, the surface of
the
substrate is modified to allow attachment of the microspheres at individual
sites,
whether or not those sites are contiguous or non-contiguous with other sites.
Thus,
the surface of the substrate may be modified such that discrete sites are
formed that
can only have a single associated bead, or alternatively, the surface of the
substrate is
modified and beads may go down anywhere, but they end up at discrete sites.
In a preferred embodiment, the surface of the substrate is modified to contain
wells,
i.e. depressions in the surface of the substrate. This may be done as is
generally known
in the art using a variety of techniques, including, but not limited to,
photolithography,
stamping techniques, molding techniques and microetching techniques. As will
be
appreciated by those in the art, the technique used will depend on the
composition and
shape of the substrate. When the first substrate comprises both the assay
locations and
the individual arrays, a preferred method utilizes molding techniques that
form the
bead wells in the bottom of the assay wells in a microtiter plate. Similarly,
a preferred
embodiment utilizes a molded second substrate, comprising "fingers" or
projections in
an array format, and each finger comprises bead wells.
In a preferred embodiment, physical alterations are made in a surface of the
substrate
to produce the sites. In a preferred embodiment, for example when the second
substrate is a fiber optic bundle, the surface of the substrate is a terminal
end of the
fiber bundle, as is generally described in U.S. Patent Nos. 6,023,540 and
6,327,410. In
this embodiment, wells are made in a terminal or distal end of a fiber optic
bundle
comprising individual fibers. In this embodiment, the cores of the individual
fibers are
etched, with respect to the cladding, such that small wells or depressions are
formed at
one end of the fibers. The required depth of the wells will depend on the size
of the
beads to be added to the wells.
Generally in this embodiment, the microspheres are non-covalently associated
in the
wells, although the wells may additionally be chemically functionalized as is
generally
CA 02677953 2009-09-11
69666-173D
described below, cross-linking agents may be used, or a physical barrier may
be used,
i.e. a film or membrane over the beads.
In a preferred embodiment, the surface of the substrate is modified to contain
modified
sites, particularly chemically modified sites, that can be used to attach,
either
5 covalently or non-covalently, the microspheres of the invention to the
discrete sites or
locations on the substrate. "Chemically modified sites" in this context
includes, but is
not limited to, the addition of a pattern of chemical functional groups
including amino
groups, carboxy groups, oxo groups and thiol groups, that can be used to
covalently
attach microspheres, which generally also contain corresponding reactive
functional
10 groups; the addition of a pattern of adhesive that can be used to bind the
microspheres
(either by prior chemical functionalization for the addition of the adhesive
or direct
addition of the adhesive); the addition of a pattern of charged groups
(similar to the
chemical functionalities) for the electrostatic attachment of the
microspheres, i.e. when
the microspheres comprise charged groups opposite to the sites; the addition
of a
15 pattern of chemical functional groups that renders the sites differentially
hydrophobic
or hydrophilic, such that the addition of similarly hydrophobic or hydrophilic
microspheres under suitable experimental conditions will result in association
of the
microspheres to the sites on the basis of hydroaffinity. For example, the use
of
hydrophobic sites with hydrophobic beads, in an aqueous system, drives the
association of the beads preferentially onto the sites.
In addition, biologically modified sites may be used to attach beads to the
substrate.
For example, binding ligand pairs as are generally described herein may be
used; one
partner is on the bead and the other is on the substrate. Particularly
preferred in this
embodiment are complementary nucleic acid strands and antigen/antibody pairs.
Furthermore, the use of biological moieties in this manner allows the creation
of
composite arrays as well. This is analogous to the system depicted in Figure
IF,
except that the substrate 10 is missing. In this embodiment, populations of
beads
comprise a single binding partner, and subpopulations of this population have
different
bioactive agents. By using different populations with different binding
partners, and a
CA 02677953 2009-09-11
69666-173D
16
substrate comprising different assay or array locations with spatially
separated binding
partners, a composite array. can be generated. This embodiment also a reuse of
codes,
as generally described below, as each separate array of the composite array
may use the
same codes.
As outlined above, "pattern" in this sense includes the use of a uniform
treatment of
the surface to allow attachment of the beads at discrete sites, as well as
treatment of the
surface resulting in discrete sites. As will be appreciated by those in the
art, this may
be accomplished in a variety of ways.
As will be appreciated by those in the art, there are a number of possible
configurations of the system, as generally depicted in. the Figures. In
addition to the
standard formats described herein, a variety of other formats may be used. For
example, as shown in Figures iC-iF, "pieces" of substrates may be used, that
are not
connected to one another. Again, these may be the same arrays or different
arrays.
These pieces may be made individually, or they may be made as a large unit on
a
single substrate and then the substrate is cut or separated into different
individual
substrates. Thus, for example, Figures 1C and 1D depict a plurality of bead
arrays that
are added to the wells of the second substrate; figure 1 C is a "bead of
beads" that is
configured to maximize mixing. Figure 1D utilizes a plurality of planar first
substrates; as will be appreciated by those in the art, these may or may not
be attached
to the second substrate. In one embodiment, no particular attachment means are
used;
alternatively, a variety of attachment techniques are used. For example, as
outlined for
attachment of beads to substrates, covalent or non-covalent forces may be
used,
including the use of adhesives, chemistry, hydrophobic/hydrophilic
interactions, etc.
In addition, the substrate may be magnetic and held in place (and optionally
mixed)
magnetically as well. Thus, for example, as depicted in Figure IF, binding
moieties
can be used; these can be covalent linkages or non-covalent linkages. They may
be
used simply for attachment, or for targeting the first substrate arrays to
particular
locations in or on the second substrate. Thus, for example, different
oligonucleotides
may be used to target and attach the first substrate to the second.
CA 02677953 2009-09-11
69666-173D
17
In a preferred embodiment, there are optical properties built into the
substrate used for
imaging. Thus, for example, "lensing" capabilities may be built into the
substrate,
either in a one component or two component system. For example, in a one
component system, the bottom of one or more of the assay locations may have
unique
or special optical components, such as lenses, filters, etc.
In addition, preferred embodiments utilize configurations that facilitate
mixing of the
assay reaction. For example, preferred embodiments utilize two component
systems
that allow mixing. That is, in some embodiments, the arrays project from the
block
and can be used as a "stick" that stirs the reaction to facilitate good mixing
of the assay
components, increase the kinetics of the reaction, etc. As will be appreciated
by those
in the art, this may be accomplished in a variety of ways. In a preferred
embodiment,
the first and second substrates are configured such that they can be moved
relative to
one another, either in the X-Y coordinate plane, the X-Z coordinate plane, the
Y-Z
coordinate plane, or in three dimensions (X-Y-Z). Preferred embodiments
utilize a
block jig that allows the block to move freely in either the plane of the
plate or
orthogonal to it. This is particularly useful when the reaction volumes are
small, since
standard mixing conditions frequently do not work well in these situations.
In addition to this, or in place of it, there may be additional mixing
components as part
of the system. For example, there may be exogeneous mixing particles added;
one
embodiment for example utilizes magnetic particles, with a magnet that is
moved to
force mixing; for example small magnetic-mixing bars and magnetic stir plates
may be
used.
Alternatively, mixing in either one or two component systems can be
accomplished by
sealing the system and shaking it using standard techniques, optionally using
mixing
particles.
In a preferred embodiment, the system is configured to reduce evaporation and
facilitate small sample size and handling. That is, the system is closed or
sealed by
enclosing a defined space to maintain control over the small sample volumes.
In this
CA 02677953 2009-09-11
69666-173D
18
regard the invention provides a hybridization chamber that encompasses or
encloses
the array and/or sample. As is more fully outlined below, preferred
embodiments
utilize the hybridization chambers comprising a base plate and alignment
moieties that
find particular use in the two-component system, although they also find use
in the
one-component system.
One advantage of the enclosed system is that it reduces or dampens vibration.
That is,
because of the small sample volume, the arrays may be susceptible to
disturbances
caused by vibration, for example, by platform shaking, motor vibration, or air
circulation. By enclosing the array, and placing the array on the base plate,
the
samples and arrays are less susceptible to disturbances caused by vibration as
the base
plate dampens the vibration.
An additional advantage of this aspect of the invention is that the enclosed
array allows
for the use of increasingly small volumes. In an open array format, small
sample
volumes may evaporate resulting in a variety of problems including sample
variation,
alteration and inconsistent concentration of solutes in the solution. For
example, when
small sample volumes are present in different assay locations, differential
evaporation
of the solution may result in dramatically altered solute concentration. Such
differences may alter hybridization kinetics, for example, and make it
difficult to
interpret and compare results obtained from such open arrays. However, by
enclosing
the array, for example in the hybridization chamber outlined herein, such
sample
variance is minimized thereby rendering the data obtained from the enclosed
array
more reliable. Accordingly, any of the methods described herein, find use with
the
hybridization chamber.
Also, the enclosed array allows for prolonged assay/incubation times relative
to
incubation times in an open array. Again, the sealed or enclosed array
prevents sample
evaporation, allowing for prolonged incubation periods.
In addition, the enclosed array facilitates mixing of the sample, when
necessary. In
general, when using small sample volumes, adequate mixing of the sample may be
CA 02677953 2009-09-11
69666-173D
19
difficult to achieve. However, as is more fully outlined below, in one
embodiment the
hybridization chamber facilitates mixing when flexible membranes are used with
a
pneumatic device that provides vacuum and/or pressure.
When a "two-component" system is used, a hybridization chamber may be used.
That
is, both of the components i.e. the substrate comprising a plurality of assay
locations
and the substrate comprising a plurality of array locations, are enclosed
within the
hybridization chamber. In a preferred embodiment, these components include but
are
not limited to a fiber optic array and a multi-well microtiter plate that are
enclosed in
the hybridization chamber.
In a preferred embodiment the hybridization chamber contains a base plate upon
which
or into which one of the components is placed. By base plate is meant any
platform or
holder onto which one of the array components is placed. The base plate may be
made
of any material including plastic, glass or metal -or any materials outlined
herein for
substrates; when the base plate is metal, it is preferably made of aluminum.
Aluminum provides for a light weight yet durable base plate. In addition,
aluminum
allows for efficient and/or rapid heat transfer to the chamber. However, when
the base
plate is made of plastic or glass, the component is directly contacted with
the base
plate. Alternatively, metal sheets or templates may be inserted into or placed
on the
base plate. The metal sheets or templates can be designed to hold any of a
variety of
shapes to accommodate a variety of component sizes and/or shapes. As
previously
described, metal offers the advantage of being a rapid and efficient heat
conductor.
In one embodiment the base plate contains at least one depression or base
cavity into
which the array component is placed. That is, when a microtiter plate is the
component, for example, the depression or base cavity is formed such that the
microtiter plate is placed directly into it and preferably fits tightly to
avoid extra
vibration and allow efficient heat transfer. The depression may be molded into
the
base plate. In addition, the base plate may contain multiple depressions or
cavities
such that multiple separate array components are placed on a single base
plate.
CA 02677953 2009-09-11
69666-173D
Alternatively, the base plate may be flat, and preferably comprise hooks or
other
attachment moieties to keep the arrays in place.
In addition preferred embodiments utilize a lid with the hybridization
chamber. The
lid can be made of any material (again, as listed for substrates herein), but
glass,
5 plastics or metal is preferred. The lid is preferably matched to the base
plate such that
when the lid is placed on the base plate, a closed chamber is formed.
In another embodiment the lid comprises at least one component placement port
By
component placement port is meant a site in the lid to which a component is
immobilized. That is, the placement port allows for attachment of one of the
10 components to the lid. In a preferred embodiment, the port is a hole in the
lid through
which the component is inserted. For example, when a fiber optic bundle is the
component, the bundle is inserted through the port. In this embodiment, the
port
additionally comprises a sealant surrounding the attachment site, such that an
airtight
seal is formed between the component, i.e_ the distal end of the fiber optic
bundle, and
15 the lid. This sealant may be any material including silicon, rubber,
plastic, etc., as
outlined below. Alternatively, the seal may be a gel-based substance such as
petroleum jelly, or a film based substance such as PARAFILM.
In an additional embodiment, the lid comprises a plurality of ports in the
lid. That is,
when multiple components are to be used, it is necessary to have a separate
port for
20 each component For example, when multiple fiber optic bundles are used,
each fiber
optic bundle is placed in a separate port However, although it is possible for
each
fiber optic bundle to be inserted into one port at a time, it is also possible
for the same
fiber optic bundle to be inserted into different ports successively. That is,
there is
nothing to limit the number of ports into which a component is inserted
successively.
For example, as shown in Figure 7A the lid 110 contains multiple ports 120
into
which fiber optic bundles 130 are placed. The lid is then placed onto a
microtiter
plate 140 in the base cavity 150 of the base plate 160. A base plate 160 is
depicted in
Figure 7b and shows the base plate 160 and base cavity 150.
CA 02677953 2009-09-11
69666-173D
21
In a preferred embodiment, the port seal reduces or prevents solution cross
contamination. That is, the seal surrounding the individual port/component
forms a
seal against the base plate or array component such that the solution from the
sample
corresponding to a particular port/component is separated or sealed from the
other
components.
In an alternative embodiment, not all ports are filled with components at all
times.
When it is appropriate or desired to have less than maximal filling of the
ports, plugs
can be inserted into the ports that do not contain components. In this manner,
the lid
still forms an airtight seal with the base plate, despite the presence of
ports without
components. The plugs can be in the form of a rubber stopper, a gasket, a
film, a gel
and the like.
In a preferred embodiment around the periphery of the chamber between the lid
and
base plate resides a sealant. The sealant may be of any material that results
in an
airtight seal being formed between the lid and base plate. In a preferred
embodiment,
the sealant is formed of rubber, such as a rubber or silicon gasket or O-ring
80 (see Fig
8). The sealant may be fixed to either the lid or baseplate. To this end, the
sealant
may be permanently affixed to the lid or baseplate. Alternatively, the sealant
may fit
into a groove in either the lid or base plate. As such, the sealant is
immobilized to the
lid or base plate, but the immobilization is not necessarily permanent.
Alternatively,
the sealant may be formed from a liquid sealant such as petroleum jelly or
from a
pliable film material such as PARAFILM or other waxes.
In a preferred. embodiment, when a two-component system is used, the
hybridization
chamber further comprises alignment moieties. By alignment moieties is meant a
feature of the chamber that facilitates alignment of the lid with the base
plate. The
importance of the alignment moieties resides not only in the alignment of a
single lid
and base plate, but also reproducible alignment of multiple lids and base
plates. That
is, the alignment moieties facilitate the physical alignment between any array
components and any multiple well microtiter plate configuration. When fiber
optic.
bundles in the lid are to be aligned with a microtiter plate on the base
plate, the
CA 02677953 2009-09-11
69666-173D
22
alignment moieties allow for alignment of the vertical center axis of the
fiber bundle
with their corresponding well center axis. In a preferred embodiment,
alignment is
such that all fiber bundles clear, ie. do not touch, the inner walls of the
wells. This
alignment may be important for sequential imaging.
In one embodiment the alignment moiety is a complementary male/female fitting.
The
male fitting may be affixed to the lid or base plate, although it need not be
permanently
affixed. When a male fitting is used as an alignment moiety in either the base
plate or
lid of the chamber, it is preferable that the opposite chamber piece contain a
slot or
hole (female fitting) into which the male fitting is inserted. One of ordinary
skill in the
art appreciates the variations of this male/female fitting that find use with
the
invention. In this regard, the features may be indexer pins or bumps on one
chamber
piece and holes or complementary grooves on the other piece.
In a preferred embodiment, fiducials are used; see WO 00/047996.
In an alternative embodiment, the chamber may also contain clamp features to
maintain secure contact between the lid and base plate. The advantage of
clamping is
to distribute uniform loading throughout the chamber to accomplish uniform
seal
compression. By "clamp features" or "clamps" is meant any feature that allows
for
the application and maintenance of increased pressure or a seal between the
lid and
base plate. In one embodiment, the claim feature includes a rotating
stud/receptacle
mechanism. That is, a stud 90 is inserted into a receptacle 95 and rotated to
depress
the lid and base plate together (see Fig 8). Alternatively, the mechanism may
include a
hook and latch mechanism. One of ordinary skill in the art appreciates the
number of
clamping mechanisms that find use with the invention. In addition, one of
ordinary
skill in the art appreciates that the method of clamping is not limited to
manual
clamping. As such, it may also be automated.
In an alternative embodiment, the chamber includes features around the
periphery for
handling the chamber. In a preferred embodiment the features are slots that
are wide
CA 02677953 2009-09-11
69666-173D
23
enough to permit a users fingers to manually handle the chamber/array. In an
alternative embodiment, the features are slots, grooves, handles and the like
and may
find particular use in automatic or robotic movement of the chambers. These
additional features may also be distributed asymmetrically to facilitate
robotic
handling.
As described above, an advantage of the hybridization chamber is that small
sample
volumes can be used without the loss of sample solution. In a further
embodiment, the
chamber may contain one or more reservoirs to hold additional solutions. As
such, the
hybridization chamber also functions as a humidity chamber. The inclusion of
additional solution in the reservoir further prevents evaporation of sample.
In an alternative embodiment, for example when no microtiter plate is used,
the sample
may be applied to a membrane that is on the surface of a base plate.
Advantages of
using the membrane include ease of cleaning or even disposing of the membrane
after
each use and the flexible membrane will not damage pipette tips or fiber optic
tips due
to contacting the tips with the bottom of the sample well.
In this embodiment, the base plate contains a series of small openings 105 ,
for
example in microplate format (Figure 9A). Thus, the membrane is depressed into
the
openings forming separate assay locations. A variety of membranes are useful
with the
invention. What is important is that the membrane is flexible. In some
embodiments
it may be desired to have a chemically inert -membrane, while in some
embodiments it
may be desirable to have a membrane to which assay components will interact,
for
example nylon, nitrocellulose membranes and the like.
In a preferred embodiment, channels connect each of the openings (Fig 9B). The
channels 100 may connect to a pneumatic device that produces vacuum and/or
pressure. Thus, when vacuum is applied, the membrane deforms into the openings
105
to form small pockets or wells. The sample can then be applied to the pockets.
By
applying different amounts of vacuum to the membrane through the openings, the
volume of the well formed by the deformed membrane and fluid height can be
CA 02677953 2009-09-11
69666-173D
24
changed. Furthermore, applying intermittent vacuum to the membrane through the
channel can also agitate or mix the liquid in the wells. Such a mixing method
is
advantageous because the entire system does not have to be vibrated and stir
bars or
tumblers are not required. Furthermore, when subsets of openings are connected
to
different channels, different subsets can be mixed independently in the same
base
plate.
When positive pressure is applied, the membrane deforms up or stays flat
depending
on the magnitude of the pressure, whether there is a load on top of the
membrane and
the size and shape of the opening. This has significant advantages
particularly in
washing or cleaning of the chamber.
When pressure and vacuum are applied to different ports in certain sequences,
small
amounts of solutions can be made to migrate to different portions of the
membrane.
That is, as shown in Fig I OA-F, differential application of pressure and
vacuum results
in a membrane that is elevated in some places and depressed in other places.
Thus, a
solution that is applied to the membrane will migrate to the lower sections of
the
membrane. This has the advantage of allowing incubations of a sample on the
membrane to proceed for precise times. That is following the particular time,
vacuum
can be released and if necessary pressure applied to remove the solution. This
will
allow the incubation in small sections to achieve uniform incubation time
between the
first and last wells across an array.
Advantages of regulating sample volume through the application of vacuum or
pressure, include reducing consumption volume of reagents, such as
hybridization
solutions; increasing the ease of mixing small sample volumes and increasing
the ease
of cleaning the membrane.
In a preferred embodiment the channels connect to common fluid handling
devices to
pump in or suck out sample solutions such as hybridization mixtures or wash
fluids.
Again, in one embodiment all openings are connected to a single channel. As
such, all
wells are treated with the same solution. Alternatively, subpopulations of
openings
CA 02677953 2009-09-11
69666-173D
are connected to different channels allowing for differential application of
solutions to
the subpopulations.
When the channels are connected to fluid handling devices, it will be
necessary to
include a feature for the application and removal of the liquid from the
sample. That is,
5 for liquid to be added and removed through the opening in the base plate,
the
membrane must be penetrated to allow the fluid to be moved. In this regard, a
needle,
for example, is useful for puncturing the membrane to apply and remove the
fluid.
When needles are used, it may be necessary to use a resealable membrane, or
apply a
sealant to the puncture location to prevent undesired leakage of the solution.
10 In some embodiments the chamber includes heat transfer features. That is,
when
elevated temperatures are required or desired, the chamber is designed to
maintain
elevated temperatures. In one embodiment, this includes the application of an
insulating material to the chamber. Then, when pre-warmed solution is
introduced
into the chamber, the elevated temperature is maintained. That is, the
solution can be
15 easily heated outside of the chamber prior to being pumped into the
chamber. The
simple chamber geometry will facilitate the maintenance of equal temperatures
between liquid in different wells.
In an alternative embodiment, the chamber includes a heating mechanism to
maintain
the elevated temperature in the chamber. In one embodiment, the chamber is
heated
20 uniformly by the heating apparatus. In an alternative embodiment, the
heating
apparatus heats different sections of the chamber independently.
As described above, the use of metal such as aluminum on the base plate
facilitates
heat transfer because the metal is a fast and efficient conductor of heat.
When a "one-component" system is used, a lid and a sealing mechanism can be
used.
25 That is, as described above, the lid forms an airtight seal with the base
plate. Thus,
like the lid above, the lid of the "one-component" system also includes a
sealant
between the lid and base plate. In one embodiment, the lid and base plate also
include
CA 02677953 2009-09-11
69666-173D
26
alignment moieties as described above for the "two-component" system.
Alternatively, in one embodiment the chamber of the one-component system does
not
include alignment moieties. In this respect, the necessity for stringent
alignment of the
lid and base plate in the one-component system is lower than that for the two-
component system. That is, because the one-component system does not have
array
components in the lid to be aligned with array locations on the base plate,
alignment is
not as stringent. However, alignment may still be important for imaging.
Furthermore, as described above, the lid of the chamber in the one-component
system
can be made of glass, plastic or metal. Again, the use of metal facilitates
the
maintenance of temperature as the metal is a fast and efficient heat
conductor.
In addition, the system may comprise additional elements as well. These
include a
holder or holders for the probes or fiber optic bundles. Such holders are more
fully
described in U.S. Patent No. 6,396,995, and WO 00/071992. In addition, the
system
may include cells as described in U.S. Patent Nos. 6,210,910 and 6,377,721 and
WO
99/04535709. In addition, the system may include fiducials as described in WO
00/047996.
In a preferred embodiment, the methods and compositions of the invention
comprise a
robotic system. Many systems are generally directed to the use of 96 (or more)
well
microtiter plates, but as will be appreciated by those in the art, any number
of different
plates or configurations may be used. In addition, any or all of the steps
outlined herein
may be automated; thus, for example, the systems may be completely or
partially
automated.
As will be appreciated by those in the art, there are a wide variety of
components
which can be used, including, but not limited to, one or more robotic arms;
plate
handlers for the positioning of microplates; automated lid handlers to remove
and
replace lids for wells on non-cross contamination plates; tip assemblies for
sample
CA 02677953 2009-09-11
69666-173D
27
distribution with disposable tips; washable tip assemblies for sample
distribution; 96
well loading blocks; cooled reagent racks; microtitler plate pipette positions
(optionally cooled); stacking towers for plates and tips; and computer
systems.
Fully robotic systems include automated liquid- and particle-handing,
including high
S throughput pipetting to perform all steps of screening applications. This
includes
liquid, and particle manipulations such as aspiration, dispensing, mixing,
diluting,
washing, accurate volumetric transfers; retrieving, and discarding of pipet
tips; and
repetitive pipetting of identical volumes for multiple deliveries from a
single sample
aspiration. These manipulations are cross-contamination-free liquid and
particle
transfers.
In a preferred embodiment, chemically derivatized particles, plates, tubes,
magnetic
particle, or other solid phase matrix with specificity to the ligand or
variant proteins
are used. The binding surfaces of microplates, tubes or any solid phase
matrices
include non-polar surfaces, highly polar surfaces, modified dextran coating to
promote
covalent binding, antibody coating, affinity media to bind fusion proteins or
peptides,
surface-fixed proteins such as recombinant protein A or G, nucleotide resins
or
coatings, and other affinity matrix are useful in this invention.
In a preferred embodiment, platforms for multi-well plates, multi-tubes,
minitubes,
deep-well plates, microfuge tubes, cryovials, square well plates, filters,
chips, optic
fibers, beads, and other solid-phase matrices or platform with various volumes
are
accommodated on an upgradable modular platform for additional capacity. This
modular platform includes a variable speed orbital shaker, and multi-position
work
decks for source samples, sample and reagent dilution, assay plates, sample
and
reagent reservoirs, pipette tips, and an active wash station.
In a preferred embodiment, thermocycler and thermoregulating systems are used
for
stabilizing the temperature of the heat exchangers such as controlled blocks
or
platforms to provide accurate temperature control of incubating samples from 4
C to
100 C.
CA 02677953 2009-09-11
69666-173D
28
In a preferred embodiment, Interchangeable pipet heads (single or multi-
channel ) with
single or multiple magnetic probes, affinity probes, or pipetters robotically
manipulate
the liquid and particles. Multi-well or multi-tube magnetic separators or
platforms
manipulate liquid and particles in single or multiple sample formats.
In some preferred embodiments, the instrumentation will include CCD cameras to
capture and transform data and images into quantifiable formats; and a
computer
workstation. These will enable data analysis.
The flexible hardware and software allow instrument adaptability for multiple
applications. The software program modules. allow creation, modification, and
running of methods. The system diagnostic modules allow instrument alignment,
correct connections, and motor operations. The customized tools, labware, and
liquid
and particle transfer patterns allow different applications to be performed.
The
database allows method and parameter storage. Robotic and computer interfaces
allow
communication between instruments.
In a preferred embodiment, the robotic workstation includes one or more
heating or
cooling components. Depending on the reactions and reagents, either cooling or
heating may be required, which can be done using any number of known heating
and
cooling systems, including Peltier systems.
In a preferred embodiment, the robotic apparatus includes a central processing
unit
which communicates with a memory and a set of input/output devices (e.g.,
keyboard,
mouse, monitor, printer, etc.) through a bus. The general interaction between
a central
processing unit, a memory, input/output devices, and a bus is known in the
art. Thus,
a variety of different procedures, depending on the experiments to be run, are
stored in
the CPU memory.
In a preferred embodiment, the compositions of the invention further comprise
a
population of microspheres. By "population" herein is meant a plurality of
beads as
outlined above for arrays. Within the population are separate subpopulations,
which
CA 02677953 2009-09-11
69666-173D
29
can be a single microsphere or multiple identical microspheres. That is, in
some
embodiments, as is more fully outlined below, the array may contain only a
single bead
for each bioactive agent; preferred embodiments utilize a plurality of beads
of each
type.
By "microspheres" or "beads" or "particles" or grammatical equivalents herein
is
meant small discrete particles. The composition of the beads will vary,
depending on
the class of bioactive agent and the method of synthesis. Suitable bead
compositions
include those used in peptide, nucleic acid and organic moiety synthesis,
including, but
not limited to, plastics, ceramics, glass, polystyrene, methylstyrene, acrylic
polymers,
paramagnetic materials, thoria sol, carbon graphite, titanium dioxide, latex
or cross-
linked dextrans such as Sepharose, cellulose, nylon, cross-linked micelles and
Teflon
may allbe used. `Microsphere Detection Guide" from Bangs Laboratories, Fishers
IN
is a helpful guide.
The beads need not be spherical; irregular particles may be used. In addition,
the
beads may be porous, thus increasing the surface area of the bead available
for either
bioactive agent attachment or IBL attachment. The bead sizes range from
nanometers,
i.e. 100 nm, to millimeters, i.e. 1 mm, with beads from about 0.2 micron to
about 200
microns being preferred, and from about 0.5 to about 5 micron being
particularly
preferred, although in some embodiments smaller beads may be used.
It should be noted that a key component of the invention is the use of a
substrate/bead
pairing that allows the association or attachment of the beads at discrete
sites on the
surface of the substrate, such that the beads do not move during the course of
the
assay.
Each microsphere comprises a bioactive agent, although as will be appreciated
by
those in the art, there may be some microspheres which do not contain a
bioactive
agent, depending on the synthetic methods. By "candidate bioactive agent" or
"bioactive agent" or "chemical functionality" or "binding ligand" herein is
meant as
used herein describes any molecule, e.g., protein, oligopeptide, small organic
CA 02677953 2009-09-11
69666-173D
molecule, coordination complex, polysaccharide, polynucleotide, etc. which can
be
attached to the microspheres of the invention. It should be understood that
the
compositions of the invention have two primary uses. In a preferred
embodiment, as is
more fully outlined below, the compositions are used to detect the presence of
a
5 particular target analyte, for example, the presence or absence of a
particular
nucleotide sequence or a particular protein, such as an enzyme, an antibody or
an
antigen. In an alternate preferred embodiment, the compositions are used to
screen
bioactive agents, i.e. drug candidates, for binding to a particular target
analyte.
Bioactive agents encompass numerous chemical classes, though typically they
are
10 organic molecules, preferably small organic compounds having a molecular
weight of
more than 100 and less than about 2,500 Daltons. Bioactive agents comprise
functional groups necessary for structural interaction with proteins,
particularly
hydrogen bonding, and typically include at least an amine, carbonyl, hydroxyl
or
carboxyl group, preferably at least two of the functional chemical groups. The
15 bioactive agents often comprise cyclical carbon or heterocyclic structures
and/or
aromatic or polyaromatic structures substituted with one or more of the above
functional groups. Bioactive agents are also found among biomolecules
including
peptides, nucleic acids, saccharides, fatty acids, steroids, purines,
pyrimidines,
derivatives, structural analogs or combinations thereof. Particularly
preferred are
20 nucleic acids and proteins.
Bioactive agents can be obtained from a wide variety of sources including
libraries of
synthetic or natural compounds. For example, numerous means are available for
random and directed synthesis of a wide variety of organic compounds and
biomolecules, including expression of randomized oligonucleotides.
Alternatively,
25 libraries of natural compounds in the form of bacterial, fungal, plant and
animal
extracts are available or readily produced. Additionally, natural or
synthetically
produced libraries and compounds are readily modified through conventional
chemical, physical and biochemical means. Known pharmacological agents may be
subjected to directed or random chemical modifications, such as acylation,
alkylation,
30 esterification and/or amidification to produce structural analogs.
CA 02677953 2009-09-11
69666-173D
31
In a preferred embodiment, the bioactive agents are proteins. By "protein"
herein is
meant at least two covalently attached amino acids, which includes proteins,
pogypeptides, oligopeptides and peptides. The protein may be made up of
naturally
occurring amino acids and peptide bonds, or synthetic peptidomimetic
structures.
Thus "amino acid", or "peptide residue", as used herein means both naturally
occurring
and synthetic amino acids. For example, homo-phenylalanine, citrulline and.
norleucine are considered amino acids for the purposes of the invention. The
side
chains may be in either the (R) or the (S) configuration. In the preferred
embodiment,
the amino acids are in the (S) or L-configuration. If non-naturally occurring
side
chains are used, non-amino acid substituents may be used, for example to
prevent or
retard in vivo degradations.
In one preferred embodiment, the bioactive agents are naturally occurring
proteins or
fragments of naturally occuring proteins. Thus, for example, cellular extracts
containing proteins, or random or directed digests of proteinaceous cellular
extracts,
may be used. In this way libraries of procaryotic and eukaryotic proteins may
be made
for screening in the systems described herein. Particularly preferred in this
embodiment are libraries of bacterial, fungal, viral, and mammalian proteins,
with the
latter being preferred, and human proteins being especially preferred.
In a preferred embodiment, the bioactive agents are peptides of from about 5
to about
30 amino acids, with from about 5 to about 20 amino acids being preferred, and
from
about 7 to about 15 being particularly preferred. The peptides may be digests
of
naturally occurring proteins as is outlined above, random peptides, or
"biased" random
peptides. By "randomized" or grammatical equivalents herein is meant that each
nucleic acid and peptide consists of essentially random nucleotides and amino
acids,
respectively. Since generally these random peptides (or nucleic acids,
discussed
below) are chemically synthesized, they may incorporate any nucleotide or
amino acid
at any position. The synthetic process can be designed to generate randomized
proteins or nucleic acids, to allow the formation of all or most of the
possible
combinations over the length of the sequence, thus forming a library of
randomized
bioactive proteinaceous agents.
CA 02677953 2009-09-11
69666-173D
32
In a preferred embodiment, a library of bioactive agents are used. The library
should
provide a sufficiently structurally diverse population of bioactive agents to
effect a
probabilistically sufficient range of binding to target analytes. Accordingly,
an
interaction library must be large enough so that at least one of its members
will have a
structure that gives it affinity for the target analyte. Although it is
difficult to gauge
the required absolute size of an interaction library, nature provides a hint
with the
immune response: a diversity of 107-10' different antibodies provides at least
one
combination with sufficient affinity to interact with most potential antigens
faced by an
organism. Published in vitro selection techniques have also shown that a
library size
of 107 to l08 is sufficient to find structures with affinity for the target.
Thus, in a
preferred embodiment, at least 10", preferably at least 107, more preferably
at least I0'
and most preferably at least 109 different bioactive agents are simultaneously
analyzed
in the subject methods. Preferred methods maxiwize library size and diversity.
In a preferred embodiment, the library is fully randomized, with no sequence
preferences or constants at any position. In a preferred embodiment, the
library is
biased. That is, some positions within the sequence are either held constant,
or are
selected from a limited number of possibilities. For example, in a preferred
embodiment, the nucleotides or amino acid residues are randomized within a
defined
class, for example, of hydrophobic amino acids, hydrophilic residues,
sterically biased
(either small or large) residues, towards the creation of cysteines, for cross-
linking,
prolines for SH-3 domains, serines, threonines, tyrosines or histidines for
phosphorylation sites, etc., or to purines, etc.
In a preferred embodiment, the bioactive agents are nucleic acids (generally
called
``probe nucleic acids" or "candidate probes" herein). By "nucleic acid" or
"oligonucleotide" or grammatical equivalents herein means at least two
nucleotides
covalently linked together. A nucleic acid of the present invention will
generally
contain phosphodiester bonds, although in some cases, as outlined below,
nucleic acid
analogs are included that may have alternate backbones, comprising, for
example,
phosphoramide (Beaucage, et al., Tetrahedron, 49(10):1925 (1993) and
references
therein; Letsinger, J. Org. Chem, 35:3800 (1970); Sprinzl, et al., Eur. J.
Biochem.,
CA 02677953 2009-09-11
69666-173D
33
81:579 (1977); Letsinger, et at, Nucl. Acids Res.. 14:3487 (1986); Sawai, et
at,,
Chem. Lett., 805 (1984), Letsinger, et al., J. Am. Chem. Soc., 110:4470
(1988); and
Pauwels, et at, Chemica Scripta. 26:141 (1986)), phosphorothioate (Mag, et
al.,
Nucleic Acids Res., 19:1437 (1991); and U.S. Patent No. 5,644,048),
S phosphorodithioate (Briu, et at., J. Am. Chem. Soc., 111:2321 (1989)), 0-
methylphophoroamidite linkages (see Eckstein, Oligonucleotides and Analogues:
A
Practical Approach, Oxford University Press), and peptide nucleic acid
backbones and
linkages (see Egholm,l. Am. Chem. Soc.. 114:1895 (1992); Meier, et al., Chem.
Int.
Ed_Engl. . 31:1008 (1992); Nielsen, Nature 365:566 (1993); Carlsson, et al.,
Nature,
380:207 (1996). Other analog nucleic acids include those with positive
backbones
(Denpcy, et al., Proc. Natl. Acad. Sci. USA, 92:6097 (1995)); non-ionic
backbones
(U.S. Patent Nos. 5,386,023; 5,637,684; 5,602,240; 5,216,141; and 4,469,863;
Kiedrowshi, et at., Angew. Chem. Ind. Ed. English, 30:423 (1991); Letsinger,
et
at., 1. Am. Chem. Soc., 110:4470 (1988); Letsinger, et al., Nucleosides &
Nucleotides,
13:1597 (1994); Chapters 2 and 3, ASC Symposium Series 580, "Carbohydrate
Modifications in Antisense Research", Ed. Y.S. Sanghui and P. Dan Cook;
Mesmaeker,
et al., Bioorganic & Medicinal Chem. Lett.. 4:395 (1994); Jeffs, et al., J.
Biomolecular NMR. 34:17 (1994); Tetrahedron Lett., 37:743 (1996)) and non-
ribose
backbones, including those described in U.S. Patent Nos. 5,235,033 and
5,034,506,
and Chapters 6 and 7, ASC Symposium Series 580, "Carbohydrate Modifications in
Antisense Research", Ed. Y.S. Sanghui and P. Dan Cook. Nucleic acids
containing
one or more carbocyclic sugars are also included within the definition of
nucleic
acids (see Jenkins, et al., Chem. Soc. Rev.. (1995) pp. 169-176). Several
nucleic acid
analogs are described in Rawls, C E News, June 2, 1997, page 35. These
modifications of the ribose-phosphate backbone may be done to facilitate the
addition
of additional moieties such as labels, or to increase the stability and half-
life
of such molecules in physiological environments; for example, PNA is
particularly
preferred. In addition, mixtures of naturally occurring nucleic acids and
analogs can
be made. Alternatively, mixtures of different nucleic acid analogs, and
mixtures of
naturally occurring nucleic acids and analogs may be made. The nucleic acids
may be
single stranded or double stranded, as specified, or contain portions of both
double
CA 02677953 2009-09-11
69666-173D
34
stranded or single stranded sequence. The nucleic acid may be DNA, both
genomic
and cDNA, RNA or a hybrid, where the nucleic acid contains any combination of
deoxyribo- and ribo-nucleotides, and any combination of bases, including
uracil,
adenine, thymine, cytosine, guanine, inosine, xanthanine, hypoxanthanine,
isocytosine,
isoguanine, and base analogs such as nitropyrrole and nitroindole, etc.
In a preferred embodiment, the bioactive agents are libraries of clonal
nucleic acids,
including DNA and RNA. In this embodiment, individual nucleic acids are
prepared,
generally using conventional methods (including, but not limited to,
propagation in
plasmid or phage vectors, amplification techniques including PCR, etc.). The
nucleic
acids are preferably arrayed in some format, such as a microtiter plate
format, and
beads added for attachment of the libraries.
Attachment of the clonal libraries (or any of the nucleic acids outlined
herein) may be
done in a variety of ways, as will be appreciated by those in the art,
including, but not
limited to, chemical or affinity capture (for example, including the
incorporation of
derivatized nucleotides such as AminoLink or biotinylated nucleotides that can
then be
used to attach the nucleic acid to a surface, as well as affinity capture by
hybridization), cross-linking, and electrostatic attachment, etc.
In a preferred embodiment, affinity capture is used to attach the clonal
nucleic acids to
the beads. For example, cloned nucleic acids can be derivatized, for example
with one
member of a binding pair, and the beads derivatized with the other member of a
binding pair. Suitable binding pairs are as described herein for IBL/DBL
pairs. For
example, the cloned nucleic acids may be biotinylated (for example using
enzymatic
incorporate of biotinylated nucleotides, for by photoactivated cross-linking
of biotin).
Biotinylated nucleic acids can then be captured on streptavidin-coated beads,
as is
known in the art. Similarly, other hapten-receptor combinations can be used,
such as
digoxigenin and anti-digoxigenin antibodies. Alternatively, chemical groups
can be
added in the form of derivatized nucleotides, that can them be used to add the
nucleic
acid to the surface.
CA 02677953 2009-09-11
69666-173D
Preferred attachments are covalent, although even relatively weak interactions
(i.e.
non-covalent) can be sufficient to attach a nucleic acid to a surface, if
there are
multiple sites of attachment per each nucleic acid. Thus, for example,
electrostatic
interactions can be used for attachment, for example by having beads carrying
the
5 opposite charge to the bioactive agent.
Similarly, affinity capture utilizing hybridization can be used to attach
cloned nucleic
acids to beads. For example, as is known in the art, polyA+RNA is routinely
captured
by hybridization to oligo-dT beads; this may include oligo-dT capture followed
by a
cross-linking step, such as psoralen crosslinking). If the nucleic acids of
interest do
10 not contain a polyA tract, one can be attached by polymerization with
terminal
transferase, or via ligation of an oligoA linker, as is known in the art.
Alternatively, chemical crosslinking may be done, for example by
photoactivated
crosslinking of thymidine to reactive groups, as is known in the art.
In general, special methods are required to decode clonal arrays, as is more
fully
15 outlined below.
As described above generally for proteins, nucleic acid bioactive agents may
be
naturally occurring nucleic acids, random nucleic acids, or "biased" random
nucleic
acids. For example, digests of procaryotic or eukaryotic genomes may be used
as is
outlined above for proteins.
20 In general, probes of the present invention are designed to be
complementary to a
target sequence (either the target analyte sequence of the sample or to other
probe
sequences, as is described herein), such that hybridization of the target and
the probes
of the present invention occurs. This complementarily need not be perfect;
there may
be any number of base pair mismatches that will interfere with hybridization
between
25 the target sequence and the single stranded nucleic acids of the present
invention.
However, if the number of mutations is so great that no hybridization can
occur under
even the least stringent of hybridization conditions, the sequence is not a
CA 02677953 2009-09-11
69666-173D
36
complementary target sequence. Thus, by "substantially complementary" herein
is
meant that the probes are sufficiently complementary to the target sequences
to
hybridize under the selected reaction conditions. High stringency conditions
are
known in the art; see for example Maniatis et at., Molecular Cloning.A
Laboratory
Manual, 2d Edition, 1989, and Short Protocols in Molecular Biology, ed.
Ausubel, et
at.. Stringent conditions are sequence-dependent and will be different in
different
circumstances. Longer sequences hybridize specifically at higher temperatures.
An
extensive guide to the hybridization of nucleic acids is found in Tijssen,
Techniques in
Biochemistry and Molecular Biology--Hybridization with Nucleic Acid Probes,
"Overview of principles of hybridization and the strategy of nucleic acid
assays"
(1993). Generally, stringent conditions are selected to be about 5-10 C lower
than the
thermal melting point (T,,,) for the specific sequence at a defined ionic
strength pH.
The T. is the temperature (under defined ionic strength, pH and nucleic acid
co11celltration) at which 50% of the probes complementary to the target
hybridize to
the target sequence at equilibrium (as the target sequences are present in
excess, at Tm,
50% of the probes are occupied at equilibrium). Stringent conditions will be
those in
which the salt concentration is less than about 1.0 M sodium ion, typically
about 0.01
to 1.0 M sodium ion concentration (or other salts) at pH 7.0 to 8.3 and the
temperature is at least about 30 C for short probes (e.g. 10 to 50
nucleotides) and at
least about 60 C for long probes (e.g. greater than 50 nucleotides). Stringent
conditions
may also be achieved with the addition of destabilizing agents such as
formamide. In
another embodiment, less stringent hybridization conditions are used; for
example,
moderate or low stringency conditions may be used, as are known in the art;
see
Maniatis and Ausubel, supra, and Tijssen, supra.
The term'target sequence" or grammatical equivalents herein means a nucleic
acid
sequence on a single strand of nucleic acid. The target sequence may be a
portion of a
gene, a regulatory sequence, genomic DNA, cDNA, RNA including mRNA and
rRNA, or others. It may be any length, with the understanding that longer
sequences
are more specific. As will be appreciated by those in the art, the
complementary target
sequence may take many forms. For example, it may be contained within a larger
CA 02677953 2009-09-11
69666-173D
37
nucleic acid sequence, i.e. all or part of a gene or mRNA, a restriction
fragment of a
plasmid or genomic DNA, among others. As is outlined more fully below, probes
are
made to hybridize to target sequences to determine the presence or absence of
the
target sequence in a sample. Generally speaking, this term will be understood
by those
skilled in the art.
In a preferred embodiment, the bioactive agents are organic chemical moieties,
a wide
variety of which are available in the literature.
In a preferred embodiment, each bead comprises a single type of bioactive
agent,
although a plurality of individual bioactive agents are preferably attached to
each bead.
Similarly, preferred embodiments utilize more than one microsphere containing
a
unique bioactive agent; that is, there is redundancy built into the system by
the use of
subpopulations of microspheres, each microsphere in the subpopulation
containing the
same bioactive agent.
As will be appreciated by those in the art, the bioactive agents may either be
synthesized directly on the beads, or they may be made and then attached after
synthesis. In a preferred embodiment, linkers are used to attach the bioactive
agents to
the beads, to allow both good attachment, sufficient flexibility to allow good
interaction with the target molecule, and to avoid undesirable binding
reactions.
In a preferred embodiment, the bioactive agents are synthesized directly on
the beads.
As is known in the art, many classes of chemical compounds are currently
synthesized
on solid supports, including beads, such as peptides, organic moieties, and
nucleic
acids.
In a preferred embodiment, the bioactive agents are synthesized first, and
then
covalently attached to the beads. As will be appreciated by those in the art,
this will be
done depending on the composition of the bioactive agents and the beads. The
functionalization of solid support surfaces such as certain polymers with
chemically
reactive groups such as thiols, amines, carboxyls, etc. is generally known in
the art.
CA 02677953 2009-09-11
69666-173D
38
Accordingly, "blank" microspheres may be used that have surface chemistries
that
facilitate the attachment of the desired functionality by the user. Some
examples of
these surface chemistries for blank microspheres include, but are not limited
to, amino
groups including aliphatic and aromatic amines, carboxylic acids, aldehydes,
amides,
chloromethyl groups, hydrazide, hydroxyl groups, sulfonates and sulfates.
These functional groups can be used to add any number of different candidate
agents
to the beads, generally using known chemistries. For example, candidate agents
containing carbohydrates may be attached to an amino-functionalized support;
the
aldehyde of the carbohydrate is made using standard techniques, and then the
aldehyde
is reacted with an amino group on the surface. In an alternative embodiment, a
sulfhydryl linker may be used. There are a number of sulfhydryl reactive
linkers
known in the art such as SPDP, maleimides, cc-haloacetyls, and pyridyl
disulfides (see
for example the 1994 Pierce Chemical Company catalog, technical section on
cross-
linkers, pages 155-200) which can be used to attach
1 S cysteine containing proteinaceous agents to the support. Alternatively, an
amino group
on the candidate agent may be used for attachment to an amino group on the
surface.
For example, a large number of stable bifunctional groups are well known in
the art,
including homobifunctional and heterobifunctional linkers (see Pierce Catalog
and
Handbook, pages 155-200). In an additional embodiment, carboxyl groups (either
from the surface or from the candidate agent) may be derivatized using well
known
linkers (see the Pierce catalog). For example, carbodiimides activate carboxyl
groups
for attack by good nucleophiles such as amines (see Torchilin et al., Critical
Rev.
Therapeutic Drug Carrier Systems, 7(4):275-308 (1991). Proteinaceous candidate
agents may also be attached using other techniques known in the art, for
example for
the attachment of antibodies to polymers; see Slinkin et al., Bioconj. Chem.
2:342-348
(1991); Torchilin et al., supra; Trubetskoy et al., Bioconi. Chem. 3:323-327
(1992);
King et al., Cancer Res. 54:6176-6185 (1994); and Wilbur et al,-Bioconjugate
Chem.
5:220-235 (1994),. It should be understood that the candidate agents may be
attached in
a variety of ways, including those listed above. Preferably, the manner of
attachment
does not significantly alter the functionality of the candidate agent; that
is,
CA 02677953 2009-09-11
69666-173D
39
the candidate agent should be attached in such a flexible manner as to allow
its
interaction with a target. In addition, these types of chemical or biological
functionalities may be used to attach arrays to assay locations, as is
depicted in Figure
IF, or individual sets of beads.
Specific techniques for immobilizing enzymes on microspheres are known in the
prior
art. In one case, NH2 surface chemistry microspheres are used. Surface
activation is
achieved with a 2.5% glutaraldehyde in phosphate buffered saline (10 mM)
providing
a pH of 6.9. (138 mM NaCl, 2.7 mM, KCI). This is stirred on a stir bed for
approximately 2 hours at room temperature. The microspheres are then rinsed
with
ultrapure water plus 0.0 1% tween 20 (surfactant) -0.02%, and rinsed again
with a pH
7.7 PBS plus 0.01% tween 20. Finally, the enzyme is added to the solution,
preferably
after being prefiltered using a 0.45 m amicon micropure filter.
In some embodiments, the microspheres may additionally comprise identifier
binding
ligands for use in certain decoding systems. By "identifier binding ligands"
or "IBLs"
herein is meant a compound that will specifically bind a corresponding decoder
binding ligand (DBL) to facilitate the elucidation of the identity of the
bioactive agent
attached to the bead. That is, the 1BL and the corresponding DBL form a
binding
partner pair. By "specifically bind" herein is meant that the IBL binds its
DBL with
specificity sufficient to differentiate between the corresponding DBL and
other DBLs
(that is, DBLs for other IBLs), or other components or contaminants of the
system.
The binding should be sufficient to remain bound under the conditions of the
decoding
step, including wash steps to remove non-specific binding- In some
embodiments, for
example when the IBLs and corresponding DBLs are proteins or nucleic acids,
the
dissociation constants of the IBL to its DBL will be less than about 10'-10-6
M4, with
less than about 10-5 to 10-9 M' being preferred and less than about 10-' -10-9
M' being
particularly preferred.
IBL-DBL binding pairs are known or can be readily found using known
techniques.
For example, when the IBL is a protein, the DBLs include proteins
(particularly
including antibodies or fragments thereof (FAbs, etc.)) or small molecules, or
vice
CA 02677953 2009-09-11
69666-173D
versa (the IBL is an antibody and the DBL is a protein). Metal ion- metal ion
ligands
or chelators pairs are also useful. Antigen-antibody pairs, enzymes and
substrates or
inhibitors, other protein-protein interacting pairs, receptor-ligands,
complementary
nucleic acids (including nucleic acid molecules that form triple helices), and
5 carbohydrates and their binding partners are also suitable binding pairs.
Nucleic acid -
nucleic acid binding proteins pairs are also useful, including single-stranded
or double-
stranded nucleic acid binding proteins, and small molecule nucleic acid
binding agents.
Similarly, as is generally described in U.S. Patents 5,270,163, 5,475,096,
5,567,588,
5,595,877, 5,637,459, 5,683,867,5,705,337, and related patents, nucleic acid
10 "aptamers" can be developed for binding to virtually any target; such an
aptamer-
target pair can be used as the IBL-DBL pair. Similarly, there is a wide body
of
literature relating to the development of binding pairs based on combinatorial
chemistry methods.
In a preferred embodiment, the IBL is a molecule whose color or luminescence
15 properties change in the presence of a selectively-binding DBL.
In one embodiment, the DBL may be attached to a bead, ie. a "decoder bead",
that
may carry a label such as a fluorophore.
In a preferred embodiment, the 1BL-DBL pair comprise substantially
complementary
single-stranded nucleic acids. In this embodiment, the binding ligands can be
referred
20 to as "identifier probes" and "decoder probes". Generally, the identifier
and decoder
probes range from about 4 basepairs in length to about 1000, with from about 6
to
about 100 being preferred, and from about 8 to about 40 being particularly
preferred.
What is important is that the probes are long enough to be specific, ie. to
distinguish
between different IBL-DBL pairs, yet short enough to allow both a)
dissociation, if
25 necessary, under suitable experimental conditions, and b) efficient
hybridization.
In a preferred embodiment, as is more fully outlined below, the IBLs do not
bind to
DBLs. Rather, the IBLs are used as identifier moieties ("IMs") that are
identified
directly, for example through the use of mass spectroscopy.
CA 02677953 2009-09-11
69666-173D
41
Alternatively, in a preferred embodiment, the IBL and the bioactive agent are
the same
moiety; thus, for example, as outlined herein, particularly when no optical
signatures
are used, the bioactive agent can serve as both the identifier and the agent.
For
example, in the case of nucleic acids, the bead-bound probe (which serves as
the
bioactive agent) can also bind decoder probes, to identify the sequence of the
probe on
the bead. Thus, in this embodiment, the DBLs bind to the bioactive agents.
This is
particularly useful as this embodiment can give information about the array or
the
assay in addition to decoding. For example, as is more fully described below,
the use
of the DBLs allows array calibration and assay development. This may be done
even
if the DBLs are not used as such; for example in non-random arrays, the use of
these
probe sets can allow array calibration and assay development even if decoding
is not
required.
In a preferred embodiment, the microspheres do not contain an optical
signature. That
is, as outlined in U.S. Patent Nos. 6,023,540 and 6,327,410, previous work had
each
subpopulation of microspheres comprising a unique optical signature or optical
tag that
is used to identify the unique bioactive agent of that subpopulation of
microspheres;
that is, decoding utilizes optical properties of the beads such that a bead
comprising
the unique optical signature may be distinguished from beads at other
locations with
different optical signatures. Thus the previous work assigned each bioactive
agent a
unique optical signature such that any microspheres comprising that bioactive
agent
are identifiable on the basis of the signature. These optical signatures
comprised dyes,
usually chromophores or fluorophores, that were entrapped or attached to the
beads
themselves. Diversity of optical signatures utilized different fluorochromes,
different
ratios of mixtures of fluorochromes, and different concentrations
(intensities) of
fluorochromes.
Thus, the present invention need not rely solely on the use of optical
properties to
decode the arrays, although in some instances it may. However, as will be
appreciated
by those in the art, it is possible in some embodiments to utilize optical
signatures as
an additional coding method, in conjunction with the present system. Thus, for
example, as is more fully outlined below, the size of the array may be
effectively
CA 02677953 2009-09-11
69666-173D
42
increased while using a single set of decoding moieties in several ways, one
of which
is the use in combination with optical signatures one beads. Thus,. for
example, using
one "set" of decoding molecules, the use of two populations of beads, one with
an
optical signature and one without, allows the effective doubling of the array
size. The
use of multiple optical signatures similarly increases the possible size of
the array.
In a preferred embodiment, each subpopulation of beads comprises a plurality
of
different IBLs. By using a plurality of different IBLs to encode each
bioactive agent,
the number of possible unique codes is substantially increased. That is, by
using one
unique IBL per bioactive agent, the size of the array will be the number of
unique IBLs
(assuming no "reuse" occurs, as outlined below). However, by using a plurality
of
different IBLs per bead, n, the size of the array can be increased to 2 , when
the
presence or absence of each IBL is used as the indicator. For example, the ass
ggnment
of 10 IBLs per bead generates a 10 bit binary code, where each bit can be
designated as
"1" (IBL is present) or "0" (IBL is absent). A 10 hit binary code has 210
possible
variants However, as is more fully discussed below, the size of the array may
be
further increased if another parameter is included such as concentration or
intensity;
thus for example, if two different concentrations of the IBL are used, then
the array
size increases as 3 . Thus, in this embodiment, each individual bioactive
agent in the
array is assigned a combination of IBLs, which can be added to the beads prior
to the
addition of the bioactive agent, after, or during the synthesis of the
bioactive agent, i.e.
simultaneous addition of IBLs and bioactive agent components.
Alternatively, when the bioactive agent is a polymer of different residues,
i.e. when the
bioactive agent is a protein or nucleic acid, the combination of different
IBLs can be
used to elucidate the sequence of the protein or nucleic acid.
Thus, for example, using two different IBLs (IBLI and IBL2), the first
position of a
nucleic acid can be elucidated: for example, adenosine can be represented by
the
presence of both IBLI and IBL2; thymidine can be represented by the presence
of
IBLI but not IBL2, cytosine can be represented by the presence of IBL2 but not
IBL1,
and guanosine can be represented by the absence of both. The second position
of the
nucleic acid can be done in a similar manner using IBL3 and IBL4; thus, the
presence
CA 02677953 2009-09-11
69666-173D
43
of IBLI, IBL2, IBL3 and IBL4 gives a sequence of AA; IBLI, IBL2, and IBL3
shows
the sequence AT; IBLI, IBL3 and IBL4 gives the sequence TA, etc. The third
position
utilizes IBL5 and IBL6, etc. In this way, the use of 20 different identifiers
can yield a
unique code for every possible 10-mer.
The system is similar for proteins but requires a larger number of different
IBLs to
identify each position, depending on the allowed diversity at each position.
Thus for
example, if every amino acid is allowed at every position, five different IBLs
are
required for each position. However, as outlined above, for example when using
random peptides as the bioactive agents, there may be bias built into the
system; not all
amino acids may be present at all positions, and some positions may be preset;
accordingly, it may be possible to utilize four different IBLs for each amino
acid.
In this way, a sort of "bar code" for each sequence can be constructed; the
presence or
absence of each distinct IBL will allow the identification of each bioactive
agent.
In addition, the use of different concentrations or densities of IBLs allows a
"reuse" of
sorts. If, for example, the bead comprising a first agent has a 1X
concentration of IBL,
and a second bead comprising a second agent has a 10X concentration of IBL,
using
saturating concentrations of the corresponding labelled DBL allows the user to
distinguish between the two beads.
Once the microspheres comprising the candidate agents and the unique IBLs are
generated, they are added to the substrate to form an array. It should be
noted that
while most of the methods described herein add the beads to the substrate
prior to the
assay, the order of making, using and decoding the array can vary. For
example, the
array can be made, decoded, and then the assay done. Alternatively, the array
can be
made, used in an assay, and then decoded; this may find particular use when
only a few
beads need be decoded. Alternatively, the beads can be added to the assay
mixture, i.e.
the sample containing the target analytes, prior to the addition of the beads
to the
substrate; after addition and assay, the array may be decoded. This is
particularly
preferred when the sample comprising the beads is agitated or mixed; this can
increase
CA 02677953 2009-09-11
69666-173D
44
the amount of target analyte bound to the beads per unit time, and thus (in
the case of
nucleic acid assays) increase the hybridization kinetics. This may find
particular use in
cases where the concentration of target analyte in the sample is low;
generally, for low
concentrations, long binding times must be used.
In addition, adding the beads to the assay mixture can allow sorting or
selection. For
example, a large library of beads may be added to a sample, and only those
beads that
bind the sample may be added to the substrate. For example, if the target
analyte is
fluorescently labeled (either directly (for example by the incorporation of
labels into
nucleic acid amplification reactions) or indirectly (for example via the use
of sandwich
assays)), beads that exhibit fluorescence as a result of target analyte
binding can be
sorted via Fluorescence Activated Cell Sorting (FACS) and only these beads
added to
an array and subsequently decoded. Similarly, the sorting may be accomplished
through affinity techniques; affinity columns comprising the target analytes
can be
made, and only those beads which bind are used on the array- Similarly, two
bead
systems can be used; for example, magnetic beads comprising the target
analytes can
be used to "pull out" those beads that will bind to the targets, followed by
subsequent
release of the magnetic beads (for example via temperature elevation) and
addition to
an array.
In general, the methods of making the arrays and of decoding the arrays is
done to
maximize the number of different candidate agents that can be uniquely
encoded. The
compositions of the invention may be made in a variety of ways. In general,
the arrays
are made by adding a solution or slurry comprising the beads to a surface
containing
the sites for association of the beads. This may be done in a variety of
buffers,
including aqueous and organic solvents, and mixtures. The solvent can
evaporate, and
excess beads removed.
In a preferred embodiment, when non covalent methods are used to associate the
beads
to the array, a novel method of loading the beads onto the array is used. This
method
comprises exposing the array to a solution of particles (including
microspheres and
cells) and then applying energy, e.g. agitating or vibrating the mixture. This
results in
CA 02677953 2009-09-11
69666-173D
an array comprising more tightly associated particles, as the agitation is
done with
sufficient energy to cause weakly-associated beads to fall off (or out, is the
case of
wells). These sites are then available to bind a different bead. In this way,
beads that
exhibit a high affinity for the sites are selected. Arrays made in this way
have two
5 main advantages as compared to a more static loading: first of all, a higher
percentage
of the sites can be filled easily, and secondly, the arrays thus loaded show a
substantial
decrease in bead loss during assays. Thus, in a preferred embodiment, these
methods
are used to generate arrays that have at least about 50% of the sites filled,
with at least
about 75% being preferred, and at least about 90% being particularly
preferred.
10 Similarly, arrays generated in this manner preferably lose less than about
20% of the
beads during an assay, with less than about 10% being preferred and less than
about
5% being particularly preferred.
In this embodiment, the substrate comprising the surface with the discrete
sites is
immersed into a solution comprising the particles (beads, cells, etc.). The
surface may
15 comprise wells, as is described herein, or other types of sites on a
patterned surface
such that there is a differential affinity for the sites. This differnetial
affinity results in
a competitive process, such that particles that will associate more tightly
are selected.
Preferably, the entire surface to be "loaded" with beads is in fluid contact
with the
solution. This solution is generally a slurry ranging from about 10,000:1
20 beads:solution (vol:vol) to 1:1. Generally, the solution can comprise any
number of
reagents, including aqueous buffers, organic solvents, salts, other reagent
components,
etc. In addition, the solution preferably comprises an excess of beads; that
is, there are
more beads than sites on the array. Preferred embodiments utilize two-fold to
billion-
fold excess of beads.
25 The immersion can mimic the assay conditions; for example, if the array is
to be
"dipped" from above into a microtiter plate comprising samples, this
configuration can
be repeated for the loading, thus minimizing the beads that are likely to fall
out due to
gravity.
CA 02677953 2009-09-11
69666-173D
46
Once the surface has been immersed, the substrate, the solution, or both are
subjected
to a competitive process, whereby the particles with lower affinity can be
disassociated
from the substrate and replaced by particles exhibiting a higher affinity to
the site.
This competitive process is done by the introduction of energy, in the form of
heat,
somcation, stirring or mixing, vibrating or agitating the solution or
substrate, or both.
A preferred embodiment utilizes agitation or vibration. In general, the amount
of
manipulation of the substrate is minimized to prevent damage to the array;
thus,
preferred embodiments utilize the agitation of the solution rather than the
array,
although either will work. As will be appreciated by those in the art, this
agitation can
take on any number of forms, with a preferred embodiment utilizing microtiter
plates
comprising bead solutions being agitated using microtiter plate shakers.
The agitation proceeds for a period of time sufficient to load the array to a
desired fill.
Depending on the size and concentration of the beads and the size of the
array, this
time may range from about 1 second to days, with from about 1 minute to about
24
hours being preferred.
It should be noted that not all sites of an array may comprise a bead; that
is, there may
be some sites on the substrate surface which are empty. In addition, there may
be
some sites that contain more than one bead, although this is not preferred.
In some embodiments, for example when chemical attachment is done, it is
possible to
associate the beads in a non-random or ordered way. For example, using
photoactivatible attachment linkers or photoactivatible adhesives or masks,
selected
sites on the array may be sequentially rendered suitable for attachment, such
that
defined populations of beads are laid down.
The arrays of the present invention are constructed such that information
about the
identity of the candidate agent is built into the array, such that the random
deposition
of the beads in the fiber wells can be "decoded" to allow identification of
the candidate
CA 02677953 2009-09-11
69666-173D
47
agent at all positions. This may be done in a variety of ways, and either
before, during
or after the use of the array to detect target molecules.
Thus, after the array is made, it is "decoded" in order to identify the
location of one or
more of the bioactive agents, i.e. each subpopulation of beads, on the
substrate surface.
Figure 11 depicts a flow chart exemplifying, but not limiting, the assays that
can be
performed with the arrays and hybridization chamber of the invention.
In a preferred embodiment, a selective decoding system is used. In this case,
only
those microspheres exhibiting a change in the optical signal as a result of
the binding
of a target analyte are decoded. This is commonly done when the number of
"hits",
i.e. the number of sites to decode, is generally low. That is, the array is
first scanned
under experimental conditions in the absence of the target analytes. The
sample
containing the target analytes is added, and only those locations exhibiting a
change in
the optical signal are decoded. For example, the beads at either the positive
or
negative signal locations may be either selectively tagged or released from
the array
(for example through the use of photocleavable linkers), and subsequently
sorted or
enriched in a fluorescence-activated cell sorter (FACS). That is, either all
the negative
beads are released, and then the positive beads are either released or
analyzed in situ,
or alternatively all the positives are released and analyzed. Alternatively,
the labels
may comprise halogenated aromatic compounds, and detection of the label is
done
using for example gas chromatography, chemical tags, isotopic tags, or mass
spectral
tags.
As will be appreciated by those in the art, this may also be done in systems
where the
array is not decoded; i.e. there need not ever be a correlation of bead
composition with
location. In this embodiment, the beads are loaded on the array, and the assay
is run.
The "positives", i.e. those beads displaying a change in the optical signal as
is more
fully outlined below, are then "marked" to distinguish or separate them from
the
"negative" beads. This can be done in several ways, preferably using fiber
optic
arrays. In a preferred embodiment, each bead contains a fluorescent dye. After
the
assay and the identification of the "positives" or "active beads", light is
shown down
CA 02677953 2009-09-11
69666-173D
48
either only the positive fibers or only the negative fibers, generally in the
presence of a
light-activated reagent (typically dissolved oxygen). In the former case, all
the active
beads are photobleached. Thus, upon non-selective release of all the beads
with
subsequent sorting, for example using a fluorescence activated cell sorter
(FACS)
machine, the non-fluorescent active beads can be sorted from the fluorescent
negative
beads. Alternatively, when light is shown down the negative fibers, all the
negatives
are non-fluorescent and the the postives are fluorescent, and sorting can
proceed. The
characterization of the attached bioactive agent may be done directly, for
example
using mass spectroscopy.
Alternatively, the identification may occur through the use of identifier
moieties
("IMs"), which are similar to IBLs but need not necessarily bind to DBLs. That
is,
rather than elucidate the structure of the bioactive agent directly, the
composition of
the IMs may serve as the identifier. Thus, for example, a specific combination
of IMs
can serve to code the bead, and be used to identify the agent on the bead upon
release
from the bead followed by subsequent analysis, for example using a gas
chromatograph or mass spectroscope.
Alternatively, rather than having each bead contain a fluorescent dye, each
bead
comprises a non-fluorescent precursor to a fluorescent dye. For example, using
photocleavable protecting groups, such as certain ortho-nitrobenzyl groups, on
a
fluorescent molecule, photoactivation of the fluorochrome can be done. After
the
assay, light is shown down again either the "positive" or the "negative"
fibers, to
distinguish these populations. The illuminated precursors are then chemically
converted to a fluorescent dye. All the beads are then released from the
array, with
sorting, to form populations of fluorescent and non-fluorescent beads (either
the
positives and the negatives or vice versa).
In an alternate preferred embodiment, the sites of association of the beads
(for example
the wells) include a photopolymerizable reagent, or the photopolymerizable
agent is
added to the assembled array. After the test assay is run, light is shown down
again
either the "positive" or the "negative" fibers, to distinquish these
populations. As a
CA 02677953 2009-09-11
69666-173D
49
result of the irradiation, either all the positives or all the negatives are
polymerized and
trapped or bound to the sites, while the other population of beads can be
released from
the array.
In a preferred embodiment, the location of every bioactive agent is determined
using
decoder binding ligands (DBLs). As outlined above, DBLs are binding ligands
that
will either bind to identifier binding ligands, if present, or to the
bioactive agents
themselves, preferably when the bioactive agent is a nucleic acid or protein.
In a preferred embodiment, as outlined above, the DBL binds to the IBL.
In a preferred embodiment, the bioactive agents are single-stranded nucleic
acids and
the DBL is a substantially complementary single-stranded nucleic acid that
binds
(hybridizes) to the bioactive agent, termed a decoder probe herein. A decoder
probe
that is substantially complementary to each candidate probe is made and used
to
decode the array. In this embodiment, the candidate probes and the decoder
probes
should be of sufficient length (and the decoding step run under suitable
conditions) to
I5 allow specificity; i.e. each candidate probe binds to its corresponding
decoder probe
with sufficient specificity to allow the distinction of each candidate probe.
In a preferred embodiment, the DBLs are either directly or indirectly labeled.
By
"labeled" herein is meant that a compound has at least one element, isotope or
chemical compound attached to enable the detection of the compound. In
general,
labels fall into three classes: a) isotopic labels, which may be radioactive
or heavy
isotopes; b) magnetic, electrical, thermal; and c) colored or luminescent
dyes; although
labels include enzymes and particles such as magnetic particles as well.
Preferred
labels include luminescent labels. In a preferred embodiment, the DBL is
directly
labeled, that is, the DBL comprises a label. In an alternate embodiment, the
DBL is
indirectly labeled; that is, a labeling binding ligand (LBL) that will bind to
the DBL is
used. In this embodiment, the labeling binding ligand-DBL pair can be as
described
above for IBL-DBL pairs. Suitable labels include, but are not limited to,
fluorescent
lanthanide complexes, including those of Europium and Terbium, fluorescein,
CA 02677953 2009-09-11
69666-173D
rhodamine, tetramethylrhodamine, eosin, erythrosin, coumarin, methyl-
couinarins,
pyrene, Malacite green, stilbene, Lucifer Yellow, Cascade Bluemi, Texas Red,
FITC,
PE, cy3, cy5 and others described in the 6th Edition of the Molecular Probes
Handbook by Richard P. Haugland.
5 In one embodiment, the label is a molecule whose color or luminescence
properties
change in the presence of the IBL, due to a change in the local environment
For
example, the label may be: (1) a fluorescent pH indicator whose emission
intensity
changes with pH; (2) a fluorescent ion indicator, whose emission properties
change
with ion concentration; or (3) a fluorescent molecule such as an ethidium salt
whose
10 fluorescence intensity increases in hydrophobic environments.
Accordingly, the identification of the location of the individual beads (or
subpopulations of beads) is done using one or more decoding steps comprising a
binding between the labeled DBL and either the IBL or the bioactive agent
(i.e. a
hybridization between the candidate probe and the decoder probe when the
bioactive
15 agent is a nucleic acid). After decoding, the DBLs can be removed and the
array can
be used; however, in some circumstances, for example when the DBL binds to an
IBL
and not to the bioactive agent, the removal of the DBL is not required
(although it may
be desirable in some circumstances). In addition, as outlined herein, decoding
may be
done either before the array is used in an assay, during the assay, or after
the assay.
20 In one embodiment, a single decoding step is done. In this embodiment, each
DBL is
labeled with a unique label, such that the number of unique labels is equal to
or
greater than the number of bioactive agents (although in some cases, "reuse"
of the
unique labels can be done, as described herein; similarly, minor variants of
candidate
probes can share the same decoder, if the variants are encoded in another
dimension,
25 Le. in the bead size or label). For each bioactive agent or IBL, a DBL is
made that will
specifically bind to it and contains a unique label, for example one or more
fluorochromes. Thus, the identity of each DBL, both its composition (i.e. its
sequence
when it is a nucleic acid) and its label, is known. Then, by adding the DBLs
to the
array containing the bioactive agents under conditions which allow the
formation of
CA 02677953 2009-09-11
69666-173D
51
complexes (termed hybridization complexes when the components are nucleic
acids)
between the DBLs and either the bioactive agents or the IBLs, the location of
each
DBL can be elucidated. This allows the identification of the location of each
bioactive
agent; the random array has been decoded. The DBLs can then be removed, if
necessary, and the target sample applied.
In a preferred embodiment, the number of unique labels is less than the number
of
unique bioactive agents, and thus a sequential series of decoding steps are
used. To
facilitate the discussion, this embodiment is explained for nucleic acids,
although other
types of bioactive agents and DBLs are useful as well. In this embodiment,
decoder
probes are divided into n sets for decoding. The number of sets corresponds to
the
number of unique tags. Each decoder probe is labeled in n separate reactions
with n
distinct tags. All the decoder probes share the same n tags. Each pool of
decoders
contains only one of the n tag versions of each decoder, and no two decoder
probes
have the same sequence of tags across all the pools. The number of pools
required for
this to be true is determined by the number of decoder probes and the n.
Hybridization
of each pool to the array generates a signal at every address comprising an
IBL. The
sequential hybridization of each pool in turn will generate a unique, sequence-
specific
code for each candidate probe. This identifies the candidate probe at each
address in
the array. For example, if four tags are used, then 4 X n sequential
hybridizations can
ideally distinguish 4" sequences, although in some cases more steps may be
required.
After the hybridization of each pool, the hybrids are denatured and the
decoder probes
removed, so that the probes are rendered single-stranded for the next
hybridization
(although it is also possible to hybridize limiting amounts of target so that
the available
probe is not saturated. Sequential hybridizations can be carried out and
analyzed by
subtracting pre-existing signal from the previous hybridization).
As will be appreciated by one of ordinary skill in the art, hybridization or
incubation
times vary. Generally, hybridization or incubation times last from seconds to
minutes
or up to hours or days or more. When the hybridization chamber as described
herein is
utilized, hybridization or incubation times can be increased relative to
incubation times
without the hybridization chamber.
CA 02677953 2009-09-11
69666-173D
52
An example is illustrative. Assuming an array of 16 probe nucleic acids
(numbers 1-
16), and four unique tags (four different fluors, for example; labels A-D).
Decoder
probes 1-16 are made that correspond to the probes on the beads. The first
step is to
label decoder probes 1-4 with tag A, decoder probes 5-8 with tag B, decoder
probes 9-
12 with tag C, and decoder probes 13-16 with tag D. The probes are mixed and
the
pool is contacted with the array containing the beads with the attached
candidate
probes. The location of each tag (and thus each decoder and candidate probe
pair) is
then determined. The first set of decoder probes are then removed. A second
set is
added, but this time, decoder probes 1, 5, 9 and 13 are labeled with tag A,
decoder
probes 2, 6, 10 and 14 are labeled with tag B, decoder probes 3, 7, 11 and 15
are
labeled with tag C, and decoder probes 4, 8, 12 and 16 are labeled with tag D.
Thus,
those beads that contained tag A in both decoding steps contain candidate
probe 1; tag
A in the first decoding step and tag B in the second decoding step contain
candidate
probe 2; tag A in the first decoding step and tag C in the second step contain
candidate
probe 3; etc. As will be appreciated by those in the art, the decoder probes
can be
made in any order and added in any order.
In one embodiment, the decoder probes are labeled in situ; that is, they need
not be
labeled prior to the decoding reaction. In this embodiment, the incoming
decoder
probe is shorter than the candidate probe, creating a 5' "overhang" on the
decoding
probe. The addition of labeled ddNTPs (each labeled with a unique tag) and a
polymerase will allow the addition of the tags in a sequence specific manner,
thus
creating a sequence-specific pattern of signals. Similarly, other
modifications can be
done, including ligation, etc.
In addition, since the size of the array will be set by the number of unique
decoding
binding ligands, it is possible to "reuse" a set of unique DBLs to allow for a
greater
number of test sites. This may be done in several ways; for example, by using
some
subpopulations that comprise optical signatures. Similarly, the use of a
positional
coding scheme within an array; different sub-bundles may reuse the set of
DBLs.
Similarly, one embodiment utilizes bead size as a coding modality, thus
allowing the
reuse of the set of unique DBLs for each bead size. Alternatively, sequential
partial
CA 02677953 2009-09-11
69666-173D
53
loading of arrays with beads can also allow the reuse of DBLs. Furthermore,
"code
sharing" can occur as well.
In a preferred embodiment, the DBLs may be reused by having some
subpopulations
of beads comprise optical signatures. In a preferred embodiment, the optical
signature
is generally a mixture of reporter dyes, preferably fluoroscent. By varying
both the
composition of the mixture (i.e. the ratio of one dye to another) and the
concentration
of the dye (leading to differences in signal intensity), matrices of unique
optical
signatures may be generated. This may be done by covalently attaching the dyes
to the
surface of the beads, or alternatively, by entrapping the dye within the bead.
The dyes
may be chromophores or phosphors but are preferably fluorescent dyes, which
due to
their strong signals provide a good signal-to-noise ratio for decoding.
Suitable dyes for
use in the invention include those listed for labeling DBLs, above.
In a preferred embodiment, the encoding can be accomplished in a ratio of at
least two
dyes, although more encoding dimensions may be added in the size of the beads,
for
example. In addition, the labels are distinguishable from one another; thus
two
different labels may comprise different molecules (i.e. two different fluors)
or,
alternatively, one label at two different concentrations or intensity.
In a preferred embodiment, the dyes are covalently attached to the surface of
the beads.
This may be done as is generally outlined for the attachment of the bioactive
agents,
using functional groups on the surface of the beads. As will be appreciated by
those in
the art, these attachments are done to minimize the effect on the dye.
In a preferred embodiment, the dyes are non-covalently associated with the
beads,
generally by entrapping the dyes in the pores of the beads.
Additionally, encoding in the ratios of the two or more dyes, rather than
single dye
concentrations, is preferred since it provides insensitivity to the intensity
of light used
to interrogate the reporter dye's signature and detector sensitivity.
CA 02677953 2009-09-11
69666-173D
54
In a preferred embodiment, a spatial or positional coding system is done. In
this
embodiment, there are sub-bundles or subarrays (i.e. portions of the total
array) that
are utilized. By analogy with the telephone system, each subarray is an "area
code",
that can have the same labels (i.e. telephone numbers) of other subarrays,
that are
separated by virtue of the location of the subarray. Thus, for example, the
same unique
labels can be reused from bundle to bundle. Thus, the use of 50 unique labels
in
combination with 100 different subarrays can form an array of 5000 different
bioactive
agents. In this embodiment, it becomes important to be able to identify one
bundle
from another; in general, this is done either manually or through the use of
marker
beads; these can be beads containing unique tags for each subarray, or the use
of the
same marker bead in differing amounts, or the use of two or more marker beads
in
different ratios.
In alternative embodiments, additional encoding parameters can be added, such
as
microsphere size. For example, the use of different size beads may also allow
the
reuse of sets of DBLs; that is, it is possible to use microspheres of
different sizes to
expand the encoding dimensions of the microspheres. Optical fiber arrays can
be
fabricated containing pixels with different fiber diameters or cross-sections;
alternatively, two or more fiber optic bundles, each with different cross-
sections of the
individual fibers, can be added together to form a larger bundle; or, fiber
optic bundles
with fiber of the same size cross-sections can be used, but just with
different sized
beads. With different diameters, the largest wells can be filled with the
largest
microspheres and then moving onto progressively smaller microspheres in the
smaller
wells until all size wells are then filled. In this manner, the same dye ratio
could be
used to encode microspheres of different sizes thereby expanding the number of
different oligonucleotide sequences or chemical fiinctionalities present in
the array.
Although outlined for fiber optic substrates, this as well as the other
methods outlined
herein can be used with other substrates and with other attachment modalities
as well.
In a preferred embodiment, the coding and decoding is accomplished by
sequential
loading of the microspheres into the array. As outlined above for spatial
coding, in
this embodiment, the optical signatures can be "reused". In this embodiment,
the
CA 02677953 2009-09-11
69666-173D
library of microspheres each comprising a different bioactive agent (or the
subpopulations each comprise a different bioactive agent), is divided into a
plurality of
sublibraries; for example, depending on the size of the desired array and the
number of
unique tags, 10 sublibraries each comprising roughly 10% of the total library
may be
5 made, with each sublibrary comprising roughly the same unique tags. Then,
the first
sublibrary is added to the fiber optic bundle comprising the wells, and the
location of
each bioactive agent is determined, generally through the use of DBLs. The
second
sublibrary is then added, and the location of each bioactive agent is again
determined.
The signal in this case will comprise the signal from the "first" DBL and the
"second"
10 DBL; by comparing the two matrices the location of each bead in each
sublibrary can
be determined. Similarly, adding the third, fourth, etc. sublibraries
sequentially will
allow the array to be filled.
In a preferred embodiment, codes can be "shared" in several ways. In a first
embodiment, a single code (i.e. IBL/DBL pair) can be assigned to two or more
agents
15 if the target analytes different sufficiently in their binding strengths.
For example, two
nucleic acid probes used in an mRNA quantitation assay can share the same code
if the
ranges of their hybridization signal intensities do not overlap. This can
occur, for
example, when one of the target sequences is always present at a much higher
concentration than the other. Alternatively, the two target sequences might
always be
20 present at a similar concentration, but differ in hybridization efficiency.
Alternatively, a single code can be assigned to multiple agents if the agents
are
functionally equivalent. For example, if a set of oligonucleotide probes are
designed
with the common purpose of detecting the presence of a particular gene, then
the
probes are functionally equivalent, even though they may differ in sequence.
Similarly,
25 if classes or "families" of analytes are desired, all probes for different
members of a
class such as kinases or G-protein coupled receptors could share a code.
Similarly, an
array of this type could be used to detect homologs of known genes. In this
embodiment, each gene is represented by a heterologous set of probes,
hybridizing to
different regions of the gene (and therefore differing in sequence). The set
of probes
30 share a common code. If a homolog is present, it might hybridize to some
but not all
CA 02677953 2009-09-11
69666-173D
56
of the probes. The level of homology might be indicated by the fraction of
probes
hybridizing, as well as the average hybridization intensity. Similarly,
multiple
antibodies to the same protein could all share the same code.
In a preferred embodiment, decoding of self assembled random arrays is done on
the
bases of pH titration. In this embodiment, in addition to bioactive agents,
the beads
comprise optical signatures, wherein the optical signatures are generated by
the use of
pH-responsive dyes (sometimes referred to herein as "pH dyes") such as
fluorophores.
This embodiment is similar to that outlined in WO 98/040726 and U.S. Patent
No.
6,327,410, except that the dyes used in the present invention exhibits changes
in
fluorescence intensity (or other properties) when the solution pH is adjusted
from below
the pKa to above the pKa. (or vice versa). In a preferred embodiment, a set of
pH dyes
is used, each with a different pKa, preferably separated by at least 0.5 pH
units.
Preferred emboditnenis utilize a pH dye set of pKa's of 2.0, 2.5, 3,0, 3.5,
4.0, 4.5, 5.0,
5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0,
9.5, 10.0, 10.5, 11, and 11.5. Each bead can contain any subset of the pH
dyes, and in
this way a unique code for the bioactive agent is generated. Thus, the
decoding of an
array is achieved by titrating the array from pH 1 to pH 13, and measuring the
fluorescence signal from each bead as a function of solution pH.
In a preferred embodiment, there are additional ways to increase the number of
unique
or distinct tags. That is, the use of distinct attributes on each bead can be
used to
increase the number of codes. In addition, sequential decoding allows a reuse
of codes
in new ways. These attributes are independent of each other, thus allowing the
number
of codes to grow exponentially as a function of the number of decoding steps
and the
number of attributes (e.g. distinct codes). However, by increasing the amount
of
decoding information obtained in a single decoding step, the number of
decoding steps
is markedly reduced. Alternatively, the number of distinct codes is markedly
increased. By increasing the number of attributes per decoding step, fewer
decoding
steps are required for a given number of codes. Thus, in a preferred
embodiment, a
variety of methods are used to generate a number of codes for use in the
process of
decoding the arrays, while minimizing the necessary decoding steps. For
example, a
CA 02677953 2009-09-11
69666-173D
57
variety of different coding strategies can be combined: thus, different
"colors",
combinations of colors ("hues"), different intensities of colors or hues or
both, etc. can
all be combined.
In a preferred embodiment DBLs rely on attaching or embedding a quantitative
or
discrete set of physical attributes to the bead, i.e. labeling the bead.
Preferred physical
attributes of a bead include but are not limited to: surface "smoothness" or
"roughness", color (Fluorescent and otherwise), color intensity, size,
detectable
chemical moieties, chemical reactivity, magnetization, pH sensitivity, energy
transfer
efficiency between dyes present, hydrophobicity, hydrophilicity, absorptivity,
charge,
pH sensitivity, etc.
A bead decoding scheme includes assigning/imbuing a single quantifiable
attribute to
each bead type wherein each bead type differs in the quantifiable value of
that
attribute. For instance, one can attach a given number of fluorophores to a
bead and
quantitate the number of attached fluorophores in the decoding process;
however, in
practice, attaching a "given amount" of an attribute to a bead and accurately
measuring
the attribute may be problematic. In general, the goal is to reduce the
coefficient of
variation (CV). By coefficient of variation is meant the variability in
labeling a bead
in successive labelings. This CV can be determined by labeling beads with a
defined
given number of label (fluorophore, for example) in multiple tests and
measuring the
resulting signal emitted by the bead. A large CV limits the number of useable
and
resolvable "levels" for any given attribute.
A more robust decoding scheme employs ratiometric rather than absolute
measurements for segmenting a quantitative attribute into codes. By
ratiometric
decoding is meant labeling a bead with a ratio of labels (i.e. 1:10, 1:1, and
10:1). In
theory any number of ratios can be. used so long as the difference in signals
between
the ratios is detectable. This process produced smaller CVs and allowing more
attribute segmentation within a given dynamic range. Thus, in a preferred
embodiment, the use of ratiometric decoding reduces the coefficient of
variability.
CA 02677953 2009-09-11
69666-173D
58
In addition, as will be appreciated by those in the art, ratiometric decoding
can be
accomplished in a different way. In this embodiment, rather than add a given
number
of DBLs with a first dye (or dye combination) intensity in the first decoding
reaction
and a second number with a second dye intensity in the sequential second
decoding
reaction, this ratiometric analysis may be done by using a ratio of
labelled:unlabelled
DBLs. That is, given a set saturating concentration of decoding beads, for
example
100,000 DBLs/reaction, the first intensity decoding step may be done by adding
100,000 labelled DBLs and the second step can be done by adding 10,000
labelled
DBLs and 90,000 unlabeled DBLs. Equilibrium dictates that the second step will
give
one tenth the signal intensity.
Because of the spread in values of a quantitatively measured attribute value,
the
number of distinct codes is practically limited to less than a dozen or so
codes.
However, by serially "painting" (i.e. temporarily attaching an attribute level
to a bead)
and "stripping" (removing the attribute level) a bead with different attribute
values, the
number of possible codes grows exponentially with the number of serial stages
in the
decoding process.
An example is illustrative. For instance, 9 different bead types and three
distinguishable attribute distributions (Table 1). "Painting" (labeling) the
beads with
different attribute values in a combinatorially distinct pattern in the two
different
stages, generates a unique code for each bead type, i.e. nine distinct codes
are
generated. Thus, in a preferred embodiment beads are labeled with different
attributes
in a combinatorially distinct pattern in a plurality of stages. This generates
unique
codes for each bead type. Examples of different attributes are described
above.
Labeling of beads with different attributes is performed by methods known in
the art.
CA 02677953 2009-09-11
69666-173D
59
stage I stage 2
Bead attribute attribute Code
Type value value
1 L L (L, L)
2 L M (L, M)
3 L H (L, H)
4 M L (M, L) Number of unique codes =
5 M M (M, M) Number of attributes^Number of stages
6 M H (M, H)
7 H L (H, L)
8 H M (H, M)
9 H H (H, H)
Table 1 Serial decode generates unique codes using a small number of attribute
levels.
Fluorescent colors are a particularly convenient attribute to use in a
decoding scheme. {
Fluorescent colors can be attached to any agent that recognizes an IBL to form
a
labeled DBL. The discussion is directed to oligonucleotides (including nucleic
acid
analogs) as the DBLs. A fluorescently labeled oligonucleotide is a
particularly useful
DBL since it can specifically and reversibly "paint" (label) any desired
subset of beads
with a particular color simply by the process of hybridization and
dehybridization (i.e.
to the DBL with a complementary sequence). Moreover, fluorescence is easily
imaged and quantitated using standard optical hardware and software. In order
to
"paint" a given bead type with a particular color, the bead type must be
labeled with a
unique hybridizable DNA sequence (IBL) and the decoding solution must contain
the
color-labeled complement of that sequence.
One consideration in implementing a decoding scheme is to minimize the number
of
images collected. In a color-based scheme, the number of images collected is
the
product of the number of colors and the number of stages. The number of images
can
be reduced by "painting" a bead with multiple colors for each given stage. By
CA 02677953 2009-09-11
69666-173D
assigning multiple colors to a bead, the number of effective codes is
increased. As an
example, in a 24 bit three color scheme (e.g. red, green, blue) coloring
process used by
computers, a total of 256*256*256 = 16.7 million different "hues" can be
generated
from just three colors (red, green, blue).
5 Thus, in a preferred embodiment DBLs are labeled with a combination of
colored
fluorophores. As such, this method finds use in increasing the number of
available
codes for labeling DBLs using only a handful of different dyes (colors).
Increasing the
number of codes available at each decoding step will greatly decrease the
number of
decoding steps required in a given decoding process.
10 In one embodiment a population of oligonucleotides encoding a single DBL is
labeled
with a defined ratio of colors such that each bead to which the DBL binds is
identified
based on a characteristic "hue" formulated from the combination of the colored
fluorophores. In a preferred embodiment two distinct colors are used. In a
preferred
embodiment, three or more distinct dyes (colors) are available for use. In
this instance
15 the number of differentiable codes generated by labeling a population of
oligonucleotides encoding a single DBL with any given color is three. However
by
allowing combinations of colors and color levels in the labeling, many more
codes are
generated.
For decoding by hybridization, a preferred number of distinguishable color
shades is
20 from 2 to 2000; a more preferred number of distinguishable color shades is
from 2 to
200 and a most preferred number of distinguishable color shades is from 2 to
20.
Utilizing three different color shades (intensities) and three colors, the
number of
different hues will be 3 = 81. Combining a hue with sequential decoding
allows a
virtually limitless number of codes to be generated.
25 As previously described, the DBL can be any agent that binds to the IBL. In
a
preferred embodiment, a single DBL is labeled with a pre-determined ratio of
colors.
This ratio is varied for each DBL thus allowing for a unique "hue" for each
DBL
labeled as such. Following treatment of the beads with the DBL, the bead is
analyzed
CA 02677953 2009-09-11
69666-173D
61
to determine the "hue" associated with each bead, thereby identifying the bead
with its
associated bioactive agent.
For instance, with four primary colors and two intensity levels (color is
present or
absent), fifteen different hues/stage are possible. If four dyes and three
different
intensity levels are used (absent, half-present, fully present), then 73
different
hues/stage are possible. In this case, acquisition of only 4 color images is
sufficient to
obtain information on 73 different coding hues.
In a preferred embodiment, the present invention provides array compositions
comprising a first substrate with a surface comprising discrete sites.
Preferred
embodiments utilize a population of microspheres distributed on the sites, and
the
population comprises at least a first and a second subpopulation. Each
subpopulation
comprises a bioactive agent, and, in addition, at least one optical dye with a
given pKa.
The pKas of the different optical dyes are different.
In a preferred embodiment, when for example the array comprises cloned nucleic
acids, there are several methods that can be used to decode the arrays. In a
preferred
embodiment, when some sequence information about the cloned nucleic acids is
known, specific decoding probes can be made as is generally outlined herein.
In a preferred embodiment, "random" decoding probes can be made. By sequential
hybridizations or the use of multiple labels, as is outlined above, a unique
hybridization pattern can be generated for each sensor element. This allows
all the
beads representing a given clone to be identified as belonging to the same
group. In
general, this is done by using random or partially degenerate decoding probes,
that
bind in a sequence-dependent but not highly sequence-specific manner. The
process
can be repeated a number of times, each time using a different labeling
entity, to
generate a different pattern of signals based on quasi-specific interactions.
In this way,
a unique optical signature is eventually built up for each sensor element. By
applying
pattern recognition or clustering algorithms to the optical signatures, the
beads can be
grouped into sets that share the same signature (i.e. carry the same probes).
CA 02677953 2009-09-11
69666-173D
62
In order to identify the actual sequence of the clone itself, additional
procedures are
required; for example, direct sequencing can be done. By using an ordered
array
containing the clones, such as a spotted cDNA array, a "key" can be generated
that
links a hybridization pattern to a specific clone whose position in the set is
known. In
this way the clone can be recovered and further characterized.
Alternatively, clone arrays can be decoded using binary decoding with vector
tags. For
example, partially randomized oligos are cloned into a nucleic acid vector
(e.g.
plasmid, phage, etc.). Each oligonucleotide sequence consists of a subset of a
limited
set of sequences. For example, if the limites set comprises 10 sequences, each
oligonucleotide may have some subset (or all of the 10) sequences. Thus each
of the
10 sequences can be present or absent in the oligonucleotide. Therefore, there
are 210
or 1,024 possible combinations. The sequences may overlap, and minor variants
can
also be represented (e.g. A, C, T and G substitutions) to increase the number
of
possible combinations. A nucleic acid library is cloned into a vector
containing the
random code sequences. Alternatively, other methods such as PCR can be used to
add
the tags. In this way it is possible to use a small number of oligo decoding
probes to
decode an array of clones.
In a preferred embodiment, discriminant analysis and cluster algorithms and
computer
apparatus are used to analyze the decoding data from the arrays of the
invention. The
potentially large number of codes utilized in the invention, coupled with the
use of
different intensities and "hues" of fluorophores in multi-step decoding
processes
requires good classification of the data. The data, particularly intensity
data, is
acquired in a multi-step process during which beads are reversibly labeled
(for
example by hybridizing dye-labeled complementary decoding oligonucleotides to
the
IBL probes on the beads, or the formation of binding ligand pairs for non-
nucleic acid
IBL-DBL pairs) with different colors or mixtures of colors ("hues") at each
stage. The
challenge is to accurately classify a bead as to which color with which it was
painted at
each step. The more closely related the labels are to one another (as
determined by the
optical imaging system), the more difficult the classification.
CA 02677953 2009-09-11
69666-173D
63
The proximity of the dyes as seen by the imaging system is determined by the
spectral
properties of the decoding dyes and the spectral channel separation of the
imaging
system. Better color separation is achieved by employing fluorescent dyes with
narrow emission spectra, and by employing an optical system with narrow band
pass
excitation and emission filters which are designed to excite the dye "on peak"
and
measure its emission "on peak". The process of optically imaging the dyes on
the
beads is similar to the human vision process in which our brain sees color by
measuring the ratio of excitation in the three different cone types within our
eye.
However, with an optical imaging system, the number of practical color
channels is
much greater than the three present in the human eye. CCD based imaging
systems
can "see" color from 350 nm up to 850 nm whereas the cones in the eye are
tuned to
the visible spectrum from 500 - 600 nm.
The problem of decoding bead arrays is essentially a discriminant analysis
classification problem. Thus, in a preferred embodiment, an analysis of
variance in
hyperspectral alpha space is performed on a known set of bead colors or hues.
The
center of the bead clusters in alpha space are termed the centroids of the
clusters, and
the scatter of the points within a cluster determines the spread of the
cluster. A robust
classification scheme requires that the distance between the centroids of the
different
bead classes (hues) is much greater than the spread of any cluster class.
Moreover, the
location of the centroids should remain invariant from fiber to fiber and from
experiment to experiment.
Thus, in a preferred embodiment, a hue "zone" is defined as a region in alpha
space
surrounding the hue.centroid and extending out to the spread radius of the
cluster.
Given a reference set of hue centroids and spread radii, as determined
empirically, the
classification of a new set of data can be accomplished by asking whether a
given bead
point falls closest to or within the "zone" of a hue cluster. This is
accomplished by
calculating the Mahalanobis distance (in this case, it is simply a Euclidean
distance
metric) of the bead point from the centroids of the different hue classes. For
the data
shown in Fig. 3, the location of the centroids and their distances from one
another are
indicated in Table 2.
CA 02677953 2009-09-11
69666-173D
64
Table 2 Centroid position Distance between centroids
dye/channel Blue Green Yello Red Bod- Bod- Bod- Bod-
w 493 R6G 564 TXR
Bod-493 0.63 0.22 0.11 0.03 0.00
Bod-R6G 0.03 0.51 0.37 0.09 0.72 0.00
Bod-564 0.06 0.04 0.57 0.32 0.81 0.55 0.00
Bod-TXR 0.09 0.05 0.04 0.82 0.99 0.93 0.73 0.00
For classifying the different beads into a particular hue class, a Euclidean
distance
cutoff of 0.3 was chosen. The closest two centroids, the Bod-R6G and Bod-564
(dist =
0.55), have a slight overlap in their decoding zones when using a Euclidean or
Mahalanobis distance of 0.3. An improvement in classification can be achieved
by
decreasing this distance, and by weighting the different coordinate axes
appropriately.
Accordingly, the present invention provides computer methods for analyzing and
classifying the color of a bead. The classification of the color of the bead
is done, by
viewing the bead in hyperspectral "alpha" space (a1 = I1/SII, a2 = I2/Sa3 =
I3/Setc.)
in which each coordinate axis represents the fraction of the bead intensity
within a
given imaging channel. For instance, if four imaging channels are used to
image the
beads, the color or hue of a bead can be represented by a point in 3-D alpha
space (the
fourth dimension is not necessary since Sal = 1). Given a set of different
primary dyes
by which to label the beads, the number of hues that can be generated from
these dyes
is unlimited since the dyes can be combined in varying ratios and in varying
combinatorial patterns. The number of practical hues is experimentally
determined by
the separation of the different hue clusters in hyperspectral alpha space.
Fig: 3 shows a hyperspectral alpha plot of beads labeled with four different
hues
imaged in four separate imaging channels. Note that the beads form four
distinct
clusters. The fact that these four clusters are well separated allows a robust
decode
classification scheme to be implemented.
In a preferred embodiment, a quality control analysis of the decoding process
is done.
This is achieved by performing a cluster analysis of alpha space for each
decoding
CA 02677953 2009-09-11
69666-173D
stage. The number of clusters determined will be fixed by the expected number
of
hues. The positions of the cluster centroids will be monitored and any
deviations from
the expected position will be noted.
Thus the invention provides an apparatus for decoding the arrays of the
invention. In
5 addition to the compositions outlined herein, the apparatus includes a
central
processing unit which communicates with a memory and a set of input/output
devices
(e.g., keyboard, mouse, monitor, printer, etc.) through a bus. The general
interaction
between a central processing unit, a memory, input/output devices, and a bus
is known
in the art. One aspect of the present invention is directed toward the
hyperspectral
10 "alpha" space classification system stored in the memory.
The classification system program includes a data acquisition module that
receives
data from the optical reader or confocal microscope (or other imaging system).
In
general, the classification program also includes an analysis module, that can
analyze
the variance in hyperspectral alpha space, calculate the centroids of the
clusters,
15 calculate the scatter of the cluster (the spread) and define the hue zone
and distance
cutoff. In general, the analysis module will further determine whether a data
point
falls within the hue zone by calculating the Mahalanobis distance.
Finally,. the analysis module will analyze the different sequential decoding
information
to finally assign a bioactive agent to a bead location.
20 In this way, sequential decoding steps are run, with each step utilizing
the discriminant
analysis calculations to assign each bead in the array to a hue cluster at
each step. The
buildup of the sequential decoding information allows the correlation of the
location of
a bead and the chemistry contained on it.
Once made, the compositions of the invention find use in a number of
applications. In
25 a preferred embodiment, the compositions are used to probe a sample
solution for the
presence or absence of a target analyte, including the quantification of the
amount of
target analyte present. By "target analyte" or "analyte" or grammatical
equivalents
CA 02677953 2009-09-11
69666-173D
66
herein is meant any atom, molecule, ion, molecular ion, compound or particle
to be
either detected or evaluated for binding partners. As will be appreciated by
those in
the art, a large number of analytes may be used in the present invention;
basically, any
target analyte can be used which binds a bioactive agent or for which a
binding partner
(i.e. drug candidate) is sought.
Suitable analytes include organic and inorganic molecules, including
biomolecules.
When detection of a target analyte is done, suitable target analytes include,
but are not
limited to, an environmental pollutant (including pesticides, insecticides,
toxins, etc.);
a chemical (including solvents, polymers, organic materials, etc.);
therapeutic
molecules (including therapeutic and abused drugs, antibiotics, etc.);
biomolecules
(including hormones, cytokines, proteins, nucleic acids, lipids,
carbohydrates, cellular
membrane antigens and receptors (neural, hormonal, nutrient, and cell surface
receptors) or their ligands, etc); whole cells (including procaryotic (such as
pathogenic
bacteria) and eukaryotic cells, including mammalian tumor cells); viruses
(including
retroviruses, herpesviruses, adenoviruses, lentiviruses, etc.); and spores;
etc.
Particularly preferred analytes are nucleic acids and proteins.
In a preferred embodiment, the target analyte is a protein. As will be
appreciated by
those in the art, there are a large number of possible proteinaceous target
analytes that
may be detected or evaluated for binding partners using the present invention.
Suitable
protein target analytes include, but are not limited to, (1) imrnunoglobulins;
(2)
enzymes (and other proteins); (3) hormones and cytokines (many of which serve
as
ligands for cellular receptors); and (4) other proteins.
In a preferred embodiment, the target analyte is a nucleic acid. These assays
find use
in a wide variety of applications, as is generally outlined in WO 00/63473.
In a preferred embodiment, the probes are used in genetic diagnosis. For
example,
probes can be made using the techniques disclosed herein to detect target
sequences
CA 02677953 2009-09-11
69666-173D
67
such as the gene for nonpolyposis colon cancer, the BRCA1 breast cancer gene,
P53,
which is a gene associated with a variety of cancers, the Apo E4 gene that
indicates a
greater risk of Alzheimer's disease, allowing for easy presymptomatic
screening of
patients, mutations in the cystic fibrosis gene, cytochrome p450s or any of
the others
well known in the art.
In an additional embodiment, viral and bacterial detection is done using the
complexes
of the invention. In this embodiment, probes are designed to detect target
sequences
from a variety of bacteria and viruses. For example, current blood-screening
techniques rely on the detection of anti-HIV antibodies. The methods disclosed
herein
allow for direct screening of clinical samples to detect HIV nucleic acid
sequences,
particularly highly conserved HIV sequences. In addition, this allows direct
monitoring of circulating virus within a patient as an improved method of
assessing
the efficacy of anti-viral therapies. Similarly, viruses associated with
leukemia,
HTLV-I and HTLV-II, may be detected in this way. Bacterial infections such as
tuberculosis, chlamydia and other sexually transmitted diseases, may also be
detected.
In a preferred embodiment, the nucleic acids of the invention find use as
probes for
toxic bacteria in the screening of water and food samples. For example,
samples may
be treated to lyse the bacteria to release its nucleic acid, and then probes
designed to
recognize bacterial strains, including, but not limited to, such pathogenic
strains as,
Salmonella, Campylobacter, Vibrio cholerae, Leishmania, enterotoxic strains of
E.
coil, and Legionnaire's disease bacteria. Similarly, bioremediation strategies
may be
evaluated using the compositions of the invention.
In a further embodiment, the probes are used for forensic "DNA fingerprinting"
to
match crime-scene DNA against samples taken from victims and suspects.
In an additional embodiment, the probes in an array are used for sequencing by
hybridization.
CA 02677953 2009-09-11
69666-173D
68
The present invention also finds use as a methodology for the detection of
mutations or
mismatches in target nucleic acid sequences. For example, recent focus has
been on
the analysis of the relationship between genetic variation and phenotype by
making use
of polymorphic DNA markers. Previous work utilized short tandem repeats (STRs)
as
polymorphic positional markers; however, recent focus is on the use of single
nucleotide polymorphisms (SNPs), which occur at an average frequency of more
than
1 per kilobase in human genomic DNA. Some SNPs, particularly those in and
around
coding sequences, are likely to be the direct cause of therapeutically
relevant
phenotypic variants. There are a number of well known polymorphisms that cause
clinically important phenotypes; for example, the apoE2/3/4 variants are
associated
with different relative risk of Alzheimer's and other diseases (see Cordor et
al.,
Science 261(1993). Multiplex PCR amplification of SNP loci with subsequent
hybridization to oligonucleotide arrays has been shown to be an accurate and
reliable
method of simultaneously genotyping at least hundreds of SNPs; see Wang et
at.,
Science, 280:1077 (1998); see also Schafer et al., Nature Biotechnology 16:33-
39
(1998). The compositions of the present invention may easily be substituted
for the
arrays of the prior art; in particular, single base extension (SBE) and
pyrosequencing
techniques are particularly useful with the compositions of the invention.
In a preferred embodiment, the compositions of the invention are used to
screen
bioactive agents to find an agent that will bind, and preferably modify the
function of,
a target molecule. As above, a wide variety of different assay formats may be
run, as
will be appreciated by those in the art. Generally, the target analyte for
which a
binding partner is desired is labeled; binding of the target analyte by the
bioactive
agent results in the recruitment of the label to the bead, with subsequent
detection.
In a preferred embodiment, the binding of the bioactive agent and the target
analyte is
specific; that is, the bioactive agent specifically binds to the target
analyte. By
"specifically bind" herein is meant that the agent binds the analyte, with
specificity
sufficient to differentiate between the analyte and other components or
contaminants
of the test sample. However, as will be appreciated by those in the art, it
will be
possible to detect analytes using binding which is not highly specific; for
example, the
CA 02677953 2009-09-11
69666-173D
69
systems may use different binding ligands, for example an array of different
ligands,
and detection of any particular analyte is via its "signature" of binding to a
panel of
binding ligands, similar to the manner in which "electronic noses" work. This
finds
particular utility in the detection of chemical analytes. The binding should
be
sufficient to remain bound under the conditions of the assay, including wash
steps to
remove non-specific binding, although in some embodiments, wash steps are not
desired; i.e. for detecting low affinity binding partners. In some
embodiments, for
example in the detection of certain biomolecules, the dissociation constants
of the
analyte to the binding ligand will be less than about 10-10' M"1, with less
than about
10-5 to 10-9 M_1 being preferred and less than about 10-7 -10-9 M.1 being
particularly
preferred.
Generally, a sample containing a target analyte (whether for detection of the
target
analyte or screening for binding partners of the target analyte) is added to
the array,
under conditions suitable for binding of the target analyze to at least one of
the
bioactive agents, i.e. generally physiological conditions. The presence or
absence of
the target analyte is then detected. As will be appreciated by those in the
art, this may
be done in a. variety of ways, generally through the use of a change in an
optical signal.
This change can occur via many different mechanisms. A few examples include
the
binding of a dye-tagged analyte to the bead, the production of a dye species
on or near
the beads, the destruction of an existing dye species, a change in the optical
signature
upon analyte interaction with dye on bead, or any other optical interrogatable
event.
In a preferred embodiment, the change in optical signal occurs as a result of
the
binding of a target analyte that is labeled, either directly or indirectly,
with a detectable
label, preferably an optical label such as a fluorochrome. Thus, for example,
when a
proteinaceous target analyte is used, it may be either directly labeled with a
fluor, or
indirectly, for example through the use of a labeled antibody. Similarly,
nucleic acids
are easily labeled with fluorochromes, for example during PCR amplification as
is
known in the art. Alternatively, upon binding of the target sequences, a
hybridization
indicator may be used as the label. Hybridization indicators preferentially
associate
with double stranded nucleic acid, usually reversibly. Hybridization
indicators include
CA 02677953 2009-09-11
69666-173D
intercalators and minor and/or major groove binding moieties. In a `preferred
embodiment, intercalators may be used; since intercalation generally only
occurs in the
presence of double stranded nucleic acid, only in the presence of target
hybridization
will the label light up. Thus, upon binding of the target analyte to a
bioactive agent,
5 there is a new optical signal generated at that site, which then may be
detected.
Alternatively, in some cases, as discussed above, the target analyte such as
an enzyme
generates a species that is either directly or indirectly optical detectable.
Furthermore, in some embodiments, a change in the optical signature may be the
basis
of the optical signal. For example, the interaction of some chemical target
analytes
10 with some fluorescent dyes on the beads may alter the optical signature,
thus
generating a different optical signal.
As will be appreciated by those in the art, in some embodiments, the presence
or
absence of the target analyte may be done using changes in other optical or
non-optical
signals, including, but not limited to, surface enhanced Raman spectroscopy,
surface
15' plasmon resonance, radioactivity, etc.
The assays may be run under a variety of experimental conditions, as will be
appreciated by those in the art. A variety of other reagents may be included
in the
screening assays. These include reagents like salts, neutral proteins, e.g.
albumin,
detergents, etc which may be used to facilitate optimal protein-protein
binding and/or
20 reduce non-specific or background interactions. Also reagents that
otherwise improve
the efficiency of the assay, such as protease inhibitors, nuclease inhibitors,
anti-microbial agents, etc., may be used. The mixture of components may be
added in
any order that provides for the requisite binding. Various blocking and
washing steps
may be utilized as is known in the art.
25 In a preferred embodiment, two-color competitive hybridization assays are
run. These
assays can be based on traditional sandwich assays. The beads contain a
capture
sequence located on one side (upstream or downstream) of the SNP, to capture
the
CA 02677953 2009-09-11
69666-173D
71
target sequence. Two SNP allele-specific probes, each labeled with a different
fluorophor, are hybridized to the target sequence. The genotype can be
obtained from
a ratio of the two signals, with the correct sequence generally exhibiting
better binding.
This has an advantage in that the target sequence itself need not be labeled.
In
addition, since the probes are competing, this means that the conditions for
binding
need not be optimized. Under conditions where a mismatched probe would be
stably
bound, a matched probe can still displace it. Therefore the competitive assay
can
provide better discrimination under those conditions. Because many assays are
carried
out in parallel, conditions cannot be optimzed for every probe simultaneously.
Therefore, a competitive assay system can be used to help compensate for non-
optimal
conditons for mismatch discrimination.
In a preferred embodiment, dideoxynucleotide chain-termination sequencing is
done
using the compositions of the invention. In this embodiment, a DNA polymerase
is
used to extend a primer using fluorescently labeled ddNTPs. The 3' end of the
primer
is located adjacent to the SNP site. In this way, the single base extension is
complementary to the sequence at the SNP site. By using four different
fluorophors,
one for each base, the sequence of the SNP can be deduced by comparing the
four
base-specific signals. This may be done in several ways. In a first
embodiment, the
capture probe can be extended; in this approach, the probe must either be
synthesized
5'-3' on the bead, or attached at the 5' end, to provide a free 3' end for
polymerase
extension. Alternatively, a sandwich type assay can be used; in this
embodiment, the
target is captured on the bead by a probe, then a primer is annealed and
extended.
Again, in the latter case, the target sequence need not be labeled. In
addition, since
sandwich assays require two specific interactions, this provides increased
stringency
which is particularly helpful for the analysis of complex samples.
In addition, when the target analyte and the DBL both bind to the agent, it is
also
possible to do detection of non-labelled target analytes via competition of
decoding.
In a preferred embodiment, the methods of the invention are useful in array
quality
control. Prior to this invention, no methods have been described that provide
a
CA 02677953 2009-09-11
69666-173D
72
positive test of the performance of every probe on every array. Decoding of
the array
not only provides this test, it also does so by making use of the data
generated during
the decoding process itself. Therefore, no additional experimental work is
required.
The invention requires only a set of data analysis algorithms that can be
encoded in
software.
The quality control procedure can identil' a wide variety of systematic and
random
problems in an array. For example, random specks of dust or other contaminants
might
cause some sensors to give an incorrect signal-this can be detected during
decoding.
The omission of one or more agents from multiple arrays can also be detected.
An
advantage of this quality control procedure is that it can be implemented
immediated
prior to the assay itself, and is a true functional test of each individual
sensor.
Therefore any problems that might occur between array assembly and actual use
can be
detected. In applications where a very high level of confidence is required,
and/or
there is a significant chance of sensor failure during the experimental
procedure,
decoding and quality control can be conducted both before and after the actual
sample
analysis.
In a preferred embodiment, the arrays can be used to do reagent quality
control. In
many instances, biological macromolecules are used as reagents and must be
quality
controlled. For example, large sets of oligonucleotide probes may be provided
as
reagents. It is typically difficult to perform quality control on large
numbers of
different biological macromolecules. The approach described here can be used
to do
this by treating the reagents (formulated as the DBLs) as variable instead of
the arrays.
In a preferred embodiment, the methods outlined herein are used in array
calibration.
For many applications, such as mRNA quantitation, it is desirable to have a
signal that
is a linear response to the concentration of the target analyte, or,
alternatively, if non-
linear, to determine a relationship between concentration and signal, so that
the
concentration of the target analyte can be estimated. Accordingly, the present
invention provides methods of creating calibration curves in parallel for
multiple beads
in an array. The calibration curves can be created under conditions that
simulate the
CA 02677953 2009-09-11
69666-173D
73
complexity of the sample to be analyzed. Each curve can be constructed
independently
of the others (e.g. for a different range of concentrations), but at the same
time as all
the other curves for the array. Thus, in this embodiment, the sequential
decoding
scheme is implemented with different concentrations being used as the code
"labels",
rather than different fiuorophores. In this way, signal as a response to
concentration
can be measured for each bead. This calibration can be carried out' ust prior
to array
use, so that every probe on every array is individually calibrated as needed.
In a preferred embodiment, the methods of the invention can be used in assay
development as well. Thus, for example, the methods allow the identification
of good
and bad probes; as is understood by those in the art, some probes do not
function well
because they do not hybridize well, or because they cross-hybridize with more
than
one sequence. These problems are easily detected during decoding. The ability
to
rapidly assess probe performance has the potential to greatly reduce the time
and
expense of assay development.
Similarly, in a preferred embodiment, the methods of the invention are useful
in
quantitation in assay development. Major challenges of many assays is the
ability to
detect differences in analyte concentrations between samples, the ability to
quantitate
these differences, and to measure absolute concentrations of analytes, AM' the
presence of a complex mixture of related analytes. An example of this problem
is the
quantitation of a specific mRNA in the presence of total cellular mRNA. One
approach that has been developed as a basis of mRNA quantitation makes use of
a
multiple match and mismatch probe pairs (Lockhart et al., 1996). While this
approach is simple, it requires relatively large numbers of probes. In this
approach, a
quantitative response to concentration is obtained by averaging the signals
from a set
of different probes to the gene or sequence of interest. This is necessary
because only
some probes respond quantitatively, and it
is not possible to predict these probes with certainty. In the absence of
prior
knowledge, only the average response of an appropriately chosen collection of
probes
is quantitative. However, in the present invention, this can be applied
generally to
nucleic acid based assays as well as other assays. In essence, the approach is
to
CA 02677953 2009-09-11
69666-173D
74
identify the probes that respond quantitatively in a particular assay, rather
than average
them with other probes. This is done using the array calibration scheme
outlined
above, in which concentration-based codes are used. Advantages of this
approach
include: fewer probes are needed; the accuracy of the measurement is less
dependent
on the number of probes used; and that the response of the sensors is known
with a
high level of certainty, since each and every sequence can be tested in an
efficient
manner. It is important to note that probes that perform well are chosen
empirically,
which avoids the difficulties and uncertainties of predicting probe
performance,
particularly in complex sequence mixtures. In contrast, in experiments
described to
date with ordered arrays, relatively small numbers of sequences are checked by
performing quantitative spiking experiments, in which a known mRNA is added to
a
mixture.
In a preferred embodiment, cDNA arrays are made for RNA expression profiling.
In.
this embodiment, individual cDNA clones are amplified (for example, using PCR)
from cDNA libraries propagated in a host-vector system. Each amplified DNA is
attached to a population of beads. Different populations are mixed together,
to create a
collection of beads representing the cDNA library. The beads are arrayed,
decoded as
outlined above, and used in an assay (although as outlined herein, decoding
may occur
after assay as well). The assay is done using RNA samples (whole cell or mRNA)
that
are extracted, labeled if necessary, and hybridized to the array. Comparative
analysis
allows the detection of differences in the expression levels of individual
RNAs.
Comparison to an appropriate set of calibration standards allows
quantification of
absolute amounts of RNA.
The cDNA array can also be used for mapping, e.g. to map deletions/insertions
or copy
number changes in the genome, for example from tumors or other tissue samples.
This
can be done by hybridizing genomic DNA. Instead of cDNAs (or ESTs, etc.),
other
STS (sequence tagged sites), including random genomic fragments, can also be
arrayed
for this purpose.