Note: Descriptions are shown in the official language in which they were submitted.
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
IN THE UNITED STATES PATENTAND TRADEMARK OFFICE
Utility Patent Application for:
MANAGEMENT OF DERMATITIC SYMPTOMS OF
MAMMALIAN INTEGUMENT WITH EMOLLIENT
DISINFECTANT FORMULATIONS
Inventors: Lawrence A. Rheins (Glendale, AZ); John Reinhardt (Riverside, CA);
John C. Hill (Mesa, AZ); Grace Hastings (Chandler, AZ); James H.
Brown (Scottsdale, AZ); James S. Brown (Gilbert, AZ)
RELATED APPLICATIONS
[0001] This application claims the benefit of United States Provisional Patent
Application Serial
No. 60/920,604 filed in the United States Patent and Trademark Office on March
29,
2007 by Lawrence A. Rheins, John C. Hill, Grace Hastings, James H. Brown, and
John
Reinhardt, and is a continuation-in-part of United States Patent Application
Serial No.
10/611,775 filed in the United States Patent and Trademark office on June 30,
2003 by
John C. Hill and United States Patent Application Serial No. 09/478,071
filed.in the
United States Patent and Trademark Office on January 03, 2000 by James H.
Brown,
Lee Roy Copeland, Robert Kleiman, Sambasivarao Koritala, and Melanie K.
Cummings.
1
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
FIELD OF INVENTION
[0002] The present invention generally relates to emollient and sanitation
compositions; and
more particularly, representative embodiments of the present invention
generally
concern delivery of emollients in topically applied disinfectant formulations.
BACKGROUND OF INVENTION
[0003] The spread of infectious disease due to inadequate hand hygiene is
generally
acknowledged by the scientific community and accepted by the public at large.
As
reported by the United States National Institute of Allergy and Infectious
Diseases in
2006, the escalating incidence of nosocomial acquired infections by patients
lead to
approximately two million (2,000,000) hospital acquired infections per year
and
approximately ninety thousand (90,000) deaths in the United States alone, as
compared
to about thirteen thousand (13,000) deaths in 1992. This is especially
disturbing due to
the rapid development and spread of antibiotic-resistant bacteria, fungi, and
parasites as
well as antiviral, drug-resistant viruses. Antibiotic resistant strains of
disease-causing
bacteria, such as Staphylococcus aureus, are now commonly acquired in hospital
settings
due to close contact of patients who are more susceptible to infection and the
extensive
use of antibiotics, which generally provide selection pressure for these
strains of
bacteria. Consequently, people infected with these microbes are likely to have
longer
hospital stays and may require treatment with second- and third- choice
antibiotics that
may be less effective and more expensive.
2
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0004] Despite the knowledge that frequent hand washing is an effective
preventative measure
against the spread of disease-causing microbes, a significant level of
healthcare worker
non-compliance persists. Although most workers in the healthcare industry are
regulated by policies requiring frequent hand washing and/or the use of liquid
hand
sanitizers, non-compliance with these policies has been reported to be between
45% and
70%. A prominent reason cited for non-compliance is the incidence of acute and
chronic
irritated skin and, to a lesser extent, contact allergic hand dermatitis due
to repeated use
of antibacterial soaps and the use of alcohol-based (either ethanol or
isopropanol, 60%-
95% wt/wt) hand sanitizers. The use of these sanitizers can be as high as
fifty or more
times during each work day.
[0005] A negative side effect of the use of conventional ethanol hand
sanitizers, upon
application to the skin, is that they generally operate to remove various
surface lipids
from the uppermost region of the skin known as the stratum corneum. These
lipids
typically function to maintain homeostatic balance of the skin. The chronic
stripping of
the lipid barrier usually results in xerosis, scaling, erythema, rough skin,
and tight skin.
More serious and painful side effects include inflammation, fissures, allergic
contact
dermatitis, and the harboring of transient pathogenic organisms that may cause
infections. Common sensations associated with de-lipidization include itching,
tingling,
burning, stinging, and the like. Non-compliance that results from experiencing
these
types of side effects with the use of conventional hand sanitizers actually
leads to further
spread of diseases that hand-hygiene guidelines are promulgated with the
intent of
preventing.
3
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0006] As a mechanism for addressing adverse side effects, many individuals
turn to
moisturizers, corticosteroids, and the like; however, these mechanisms for
replenishing
moisture and/or combating dryness and other skin irritations are of limited
efficacy when
multiple hand cleansing cycles throughout the day are required. This is due to
each
cleansing cycle operating to remove the previously applied moisturizers as
they sit on
the uppermost surface of the skin - thereby reducing the exposure of the skin
to the
moisturizer and the moisturizer's overall effectiveness. Accordingly, there is
a need for
alternative sanitizer formulations to reduce the negative effects associated
with frequent
washing while maintaining effective disinfectant function.
SUMMARY OF THE INVENTION
[0007] In a representative aspect, the present invention provides compositions
and methods for
providing botanically-sourced and/or botanically-derived topical emollient
compositions
with disinfectant properties to ameliorate dermatitic symptoms of mammalian
integument. The sanitizing component of the composition may include an anti-
microbial sanitizer. The emollient component of the composition may include
botanical
lipid materials (and/or their derivatives) selected to demonstrate properties
at least
partially analogous to mammalian sebum. The combination of sanitizing and
emollient
components of the resulting formulations may be employed to manage dermatitic
symptoms and sanitize mammalian integument.
[0008] Advantages of the present invention will be set forth in the Detailed
Description which
follows and may be apparent in view of the Detailed Description or may be
learned by
4
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
practice of exemplary embodiments of the invention. Still other advantages of
the
invention may be realized by means of any of the instrumentalities, methods or
combinations particularly pointed out in the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] Representative elements, operational features, applications and/or
advantages of the
present invention reside in the details of construction and operation as more
fully
hereafter depicted, described and claimed - reference being made to the
accompanying
drawings forming a part hereof, wherein like numerals refer to like parts
throughout.
Other elements, operational features, applications and/or advantages may
become
apparent in light of certain exemplary embodiments recited in the Detailed
Description,
wherein:
[0010] FIG. 1 illustrates clinical data relating to transepidermal water loss
(TEWL) associated
with use and non-use of an emollient sanitizing formulation in accordance with
a
representative embodiment of the present invention;
[0011] FIG. 2 illustrates clinical data relating to TEWL associated with use
and non-use of an
emollient sanitizing formulation in accordance with a representative
embodiment of the
present invention;
[0012] FIG. 3 is a photomicrographic representation of a cross-section of
human skin tissue
obtained via punch biopsy prior to application of an emollient sanitizing
composition in
accordance with a representative embodiment of the present invention; and
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0013] FIG. 4 is a photomicrographic representation of a cross-section of
human skin tissue
obtained via punch biopsy after fourteen (14) days of regular application (at
least 8
times/day) of an emollient sanitizing composition in accordance with a
representative
embodiment of the present invention.
[0014] Elements in the Figures are illustrated for simplicity and clarity and
have not necessarily
been depicted to scale. For example, the dimensions of some of the elements in
the
Figures may be exaggerated relative to other elements to help improve
understanding of
various embodiments of the present invention. Furthermore, the terms "first",
"second",
and the like herein, if any, are used inter alia for distinguishing between
similar
elements and not necessarily for describing a sequential or chronological
order.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
[0015] The following representative descriptions of the present invention
generally relate to
exemplary embodiments and the inventors' conception of the best mode, and are
not
intended to limit the applicability or configuration of the invention in any
way. Rather,
the following description is intended to provide convenient illustrations for
implementing various embodiments of the invention. As will become apparent,
changes
may be made in the function and/or arrangement of any of the elements
described in the
disclosed exemplary embodiments without departing from the spirit and scope of
the
invention.
[0016] Various representative implementations of the present invention may be
applied to any
system for providing botanically-sourced (or botanically-derived) topical
emollient
6
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
sanitizing compositions. As used herein, the terms "derivative," "extract,"
"source," or
any combinatorial, variational or contextual equivalent thereof, are generally
intended to
include anything that may be regarded as at least being susceptible to
characterization as,
or generally referring to, one or more compounds as they exist in nature
and/or
chemically altered forms thereof.
[0017] As used herein, the terms "sanitize", "sanitizing", "sanitization", or
any combinatorial,
variational or contextual equivalent thereof, are generally intended to
include anything
that may be regarded as at least being susceptible to characterization as, or
generally
referring to, a material having anti-microbial, bactericidal, antiviral and/or
disinfectant
activity, including the prevention and/or inhibition of growth and/or killing
of bacteria,
viruses, fungi of any kind and by any mechanism of action or system of
activation.
[0018] As used herein, the terms "topical formulation", "topical composition",
or any
combinatorial, variational or contextual equivalent thereof, are generally
intended to
include anything that may be regarded as at least being susceptible to
characterization as,
or generally referring to, a cosmetic, a pharmaceutical, a topical medicament,
a personal
care product, a shampoo, a conditioner, a leave-in conditioner, a hair
product, a hair-
styling product, a mousse, a nail product, a skin product, a moisturizer, a
soap, a body
wash, a shaving product, a gel, a lotion, a cream, an ointment, a fragrance, a
foundation,
a mascara, a gloss, a lip balm, a lip stick, a lip liner, an eye liner, a
cosmetic remover, a
cleanser, a scrub, a wax, a spray, a foam, a paste, a solid, a liquid, a
towelette, a napkin,
a feminine hygiene product, a facial mask, a sanitizer, a balm, a detergent,
an ultraviolet
7
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
radiation absorber, a sunscreen, a suntan lotion, a sun block, a sun tan oil,
a repellant, a
skin astringent, a skin toner, a skin freshener, and/or the like.
[0019] As used herein, the term "topical application" or any combinatorial,
variational or
contextual equivalent thereof, is generally intended to include anything that
may be
regarded as at least susceptible to characterization as, or generally
referring to, use of a
topical composition or topical formulation on or in conjunction with the hair,
skin or a
component layer of the skin, nails and/or any surface of any subject (animate
or
otherwise) or object.
[0020] As used herein, the terms "subject", "user", or any combinatorial,
variational or
contextual equivalent thereof, are generally intended to include anything that
may be
regarded as at least being susceptible to characterization as, or generally
referring to, an
animal, a human, and/or any at least partially porous surface (living or
inanimate)
suitably adapted for receiving a topical application of a topical formulation
or topical
composition.
[0021] As used herein, the term "botanical", including any combinatorial,
variational or
contextual equivalent thereof, generally refers to anything that may be
regarded as at
least being susceptible to characterization as, or generally indicative of, a
material or
combination of materials that may be sourced, liberated or derived (chemically
or
otherwise) from a naturally occurring resource. While the use of the term
"botanical"
(and equivalents thereof) may certainly be intended to reference the
vernacular meaning
ordinarily ascribed to the term as designating properties of or relating to
plant life, the
scope of the term "botanical" (as used herein) should be understood to extend
to various
8
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
other "naturally occurring" materials that may be sourced or otherwise
liberated from
any material that at one time comprised living matter (plant-based or
otherwise; e.g.,
petrolatum, mineral oil, etc.) and/or other mineral resources.
[0022] As used herein, the terms "organic", "organic certification",
"organically derived" or any
combinatorial, variational or contextual equivalent thereof, are generally
intended to
include anything that may be regarded as at least being susceptible to
characterization as,
or generally referring to, materials that have satisfied the criteria of a
certification
process generally imposed on producers of organic agricultural products. In
general, any
business supplying natural- and/or naturally-derived products may be
certified, including
seed suppliers, farmers, food processors, retailers and restaurants.
Requirements vary
from country to country, and generally involve production standards for
growing,
storage, processing, packaging and shipping that include, for example: (i)
avoidance of
most synthetic chemical inputs (e.g., fertilizer, pesticides, antibiotics,
food additives,
etc.), genetically modified organisms, irradiation, and the use of sewage
sludge; use of
farmland that has been free from chemicals for a number of years (e.g., often,
three (3)
or more); keeping detailed written production and sales records (e.g., audit
trail);
maintaining strict physical separation of organic products from non-certified
products;
submitting to periodic on-site inspections; and other procedures/requirements
prescribed
by various organic certifying authorities.
[0023] As used herein, the terms "improvement", "improved", "benefit",
"beneficial", or any
combinatorial, variational or contextual equivalent thereof, may mean an
increased
incidence in observance of a favorable property or a decreased incidence in
observance
9
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
of an unfavorable property. That notwithstanding, these same terms may also
refer to a
decrease in incidence in observance of what may correspond in alternative,
conjunctive
or sequential applications to an otherwise favorable property or the increase
in incidence
in observance of an otherwise unfavorable property.
[0024] A detailed description of a representative embodiment, namely a
composition and
method for providing a botanically-sourced or botanically-derived topical
emollient
sanitizing composition, is provided as a specific enabling disclosure that may
be
generalized to any application of the disclosed compositions and methods in
accordance
with various representative aspects of the present invention.
[0025] The present invention relates to botanically-sourced and botanically-
derived topical
emollient compositions having disinfectant properties. In a representative
embodiment
of the present invention, a composition may comprise a sanitizing component
and a
botanically-sourced or botanically-derived emollient component.
[0026] In accordance with various aspects of the present invention, a suitable
emollient
sanitizing composition may comprise an anti-microbial sanitizer and a
botanically-
sourced or botanically-derived emollient. The emollient sanitizing composition
may
representatively be applied to a topical surface of a subject, such as the
skin of a
mammalian subject. The emollient sanitizing composition may then be rubbed on
the
skin until the emollient components are substantially absorbed and/or the
sanitizing
components are substantially evaporated or otherwise dissipated. This process
may then
be repeated with the subject as frequently as indicated to provide both
sanitizing function
to the applied surface as well as improved moisturizing function not found
with
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
conventional hand sanitizing formulations. Representative benefits may
include, for
example, improved moisture retention, soft-feel, increased substantivity,
and/or the like.
Additionally, in various aspects in accordance with representative embodiments
of the
present invention, the disclosed emollient sanitizing compositions may be
implemented
to at least maintain or otherwise improve lipid profiles of the skin of a
mammalian
subject while concurrently, conjunctively or sequentially sanitizing the
skin's surface
after application.
[0027] In accordance with various aspects of the present invention, an anti-
microbial sanitizer
may comprise any composition suitably adapted to provide an at least partially
disinfecting function when topically applied to a surface. In a representative
embodiment of the present invention, a suitable anti-microbial sanitizer may
at least
partially penetrate cell walls of bacteria and denature proteins within the
cells. This
denaturing generally operates to interrupt the life-cycle of the bacterium,
thereby killing
it.
[0028] In accordance with various aspects of the present invention,
representative sanitizing
compositions may include alcohols and/or other disinfectant/anti-microbial
formulations
and/or botanical extracts (or derivatives thereof) including, but not limited
to,
chlorhexidine gluconate, benzalkonium chloride, iodine, grapeseed oil, lemon
juice, tea
tree oil, citronellol, camphor oil, cade oil, eucalyptol, clove oil, and/or
the like. In a
representative embodiment of the present invention, an anti-microbial
sanitizer may
comprise a lower hydrocarbon chain alcohol, such as a CI-4 alcohol. In another
representative embodiment of the present invention, the alcohol may comprise
ethanol,
11
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
2-propanol, and/or n-propanol. In yet another representative embodiment of the
present
invention, an anti-microbial sanitizer may further comprise a dermatological
active
agent, a pharmaceutical composition, an antibiotic, a bactericidal agent, an
antiseptic
agent, a disinfectant agent, an antiviral agent, a nitrogenous cationic
surface-active
agent, a fruit juice, a fruit extract, and/or the like.
[0029] It should be appreciated that in representative embodiments of the
present invention, a
suitable anti-microbial sanitizer may comprise a combination of water and
alcohol, such
as an ethanol azeotrope. In yet a further representative embodiment of the
present
invention, ethanol may be present in concentrations between about 60%-95%
(wt/wt).
[0030] With respect to various representative aspects of the present
invention, an anti-microbial
sanitizer may be suitably adapted for combination with a botanically-sourced
or
botanically-derived emollient composition to provide both sanitizing and
moisturizing
function.
[0031] A representative emollient composition in accordance with various
aspects of the present
invention may comprise any components that are suitably adapted for providing
moisture retention, reduction of transepidermal water loss (TEWL), smooth
feel,
softness, increased substantivity, and/or the like. Additionally,
representative emollient
compositions may be employed to soften or smooth the skin by reducing
roughness,
cracking, irritation, and/or the like. In representative and exemplary
aspects, a
botanically-source or botanically-derived emollient may be selected to provide
a lipid
profile substantially similar to that of mammalian sebum (e.g., human sebum).
12
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0032] Additionally, in accordance with various aspects of the present
invention, botanical
emollient compositions may include bland, fatty, oleaginous substances that
smooth the
skin by penetration into the surface layers of skin tissue through the action
of rubbing
and massaging after application by the user.
[0033] Sources of representative botanical emollients in accordance with
various aspects of the
present invention include a number of fatty acids, wax esters, sterols, and/or
the like
(e.g., jojoba oil, shea oil, macadamia oil, rice bran wax, African dry zone
mahogany seed
oil, custard apple seed oil, sugar apple seed oil, common seabuckthorn seed
oil, and/or
the like - including derivatives thereof). Fatty acids generally comprise
aliphatic
hydrocarbons or other organic chains with carboxylic substitutes therein,
typically
having between 8 and 24 carbon atoms in the backbone. Fatty acids generally
include at
least one of stearic acid, oleic acid, myristic acid and palmitic acid. Other
typical fatty
acids include linoleic acid, behenic acid, arachidic, lignoceric, and other
common fatty
acids of the general formulae CõH(2r+l)COOH, CnH(2n_I)COOH or CõH(2r_3)COOH
where
"n" is an integer from 8 to 24.
[0034] Fatty alcohols have been found to be less sticky and less heavy than
many other fatty
materials (such as fatty acids), and are frequently used to improve the
viscosity and
stability of lotions and creams. Representative examples of fatty alcohols
which find use
in cosmetics and personal care products are cetyl alcohol, lauryl alcohol,
stearyl alcohol,
and oleyl alcohol.
[0035] Additional examples of representative emollients include fatty esters.
One of the
qualities of fatty esters is that they generally do not feel as oily to the
touch as some
13
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
other types of fatty emollient ingredients. Representative examples include
isopropyl
palmitate, isopropyl myristate, myristyl propionate, ethylhexyl palmitate, and
glyceryl
stearate.
[0036] In a representative embodiment of the present invention, a topical
emollient composition
may be derived or extracted from a botanical source. In another representative
embodiment of the present invention, a botanically-sourced (or botanically-
derived)
emollient composition may include fatty acids, esters of fatty acids,
alkoxylated fatty
acids, fatty alcohols, esters of fatty alcohols, esters of fatty alcohols with
fatty acids,
sugar alcohols, isopropyl esters, wax esters and/or combinations thereof
derived from the
seed oil of the jojoba plant (Simmondsia chinensis), such as, for example: raw
and/or
refined jojoba oil, a jojoba ester, hydrogenated jojoba oil, a jojoba
hydrolysate, a
hydrolyzed jojoba ester, a jojoba alcohol, an alkoxylated jojoba wax, an
alkoxylated and
at least partially hydrogenated jojoba wax, an alkoxylated product of jojoba
oil
interesterified with hydrogenated jojoba oil, an isopropyl jojobate, and/or
the like. In a
representative embodiment of the present invention, a botanical emollient
composition
may include jojoba oil and/or derivatives including hydrogenated jojoba oil,
isopropyl
jojobate, jojoba alcohol, jojoba esters, and/or hydrolyzed jojoba esters.
[0037] Jojoba oil and jojoba derivatives according to the present invention
may comprise about
more than 6% unsaponifiables. The term "unsaponifiable" generally refers to a
portion
of the fat and/or oil (or in the case of jojoba, a wax ester) that is not
susceptible to
saponification. Generally, unsaponifiable materials typically comprise
components that
are naturally found in the fats and/or oils, such as phenols, tocopherols,
triterpenes,
14
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
steroids, sterols, hydrocarbons such as squalene, alcohols, and/or the like.
Unsaponifiable material may be retained with the saponified material through
an in situ
saponification process, in which the unsaponifiable material is generally not
removed
and/or separated from the saponified material.
[0038] A saponification reaction may be accomplished by the hydrolysis of an
ester under basic
conditions, such as in the presence aqueous alkali metal hydroxides (e.g.,
NaOH, LiOH,
KOH, CaOH, MgOH, and/or the like) to form an alcohol and a salt of a
carboxylic acid.
In a representative embodiment of the present invention, in situ
saponification may be
affected through a base-catalyzed hydrolysis reaction between jojoba oil (a
liquid wax
ester at room temperature) and/or jojoba derivatives and an alkyl alcohol.
[0039] The products of the in situ saponification of jojoba oil typically
comprise jojoba
hydrolysates, which include a mixture of: (i) salts of jojoba fatty acids
(saponifiables);
and (ii) non-polar, lipophilic materials (unsaponifiables), with the
possibility of other
materials also present, depending on the source, state and form of the initial
reactant
(include residual jojoba wax ester).
[0040] The in situ production of unsaponifiable materials in tandem with
saponified material
from fats, oils and/or their derivatives having high levels of unsaponifiables
in
accordance with various aspects of the present invention may provide various
benefits in
compositions prepared for topical application to the skin of a subject. These
benefits
may include, for example, moisturization, a desirable texture, substantivity,
resistance to
wear, and water- and/or rinse-resistance. The presence of high unsaponifiables
may also
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
provide occlusive properties to the topical formulation where water is
maintained in the
skin, providing retained softness and smoothness.
(0041] In a representative embodiment of the present invention, botanical
emollient material
comprising in situ products of saponification may function to preserve
superior skin feel
and substantivity generally attributed to the polar hydrophilic properties of,
for example,
jojoba oil components.
[0042] Additionally, emollient materials in accordance with various
representative
embodiments of the present invention may generally form stable emulsions more
readily
than those incorporating naturally occurring jojoba oil. In another
representative
embodiment of the present invention, representative emollient materials may
also impart
an improved lipid profile to the skin of a subject after multiple treatments,
as compared
with conventional skin sanitizers.
[0043] It should further be appreciated that in accordance with various
aspects of the present
invention, botanical emollient components of the disclosed compositions may be
employed to at least partially reconstitute the lipid profile of the stratum
corneum barrier
of the skin by providing lipids and derivatives thereof that chemically
resemble human
sebum. Additionally, in a representative embodiment of the present invention,
botanical
emollient compositions in accordance with the present invention generally
provide
superior smoothness and substantive skin-feel by being absorbing into the skin
and/or
maintaining a persisting presence on the surface of the skin.
[0044] Representative botanical emollient and anti-microbial sanitizer
compositions may be
formulated in any suitable manner. For example, a suitably adapted anti-
microbial
16
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
sanitizer may comprise a substantially transparent, translucent and/or opaque
liquid.
Additionally, a suitably adapted botanical ('sourced' or `derived') emollient
component
of the composition may comprise carrier particles, such as natural and/or
synthetic
emollient beads. Representative carrier particles may comprise any suitable
synthetic
and/or natural components. For example, carrier particles at least partially
comprising
natural emollient beads may be produced from combinations of fatty alcohols,
isopropyl
esters, wax esters, and/or the like, obtained from jojoba oil and/or jojoba
derivatives.
Carrier particles comprising at least partially synthetic beads may also
include
components such as polyethylene, petrolatum, ethylhexyl palmitate, and/or the
like.
[0045] Additionally, it should be appreciated that representative carrier
particles may include
any suitable texture, size, shape, and/or the like. For example, suitably
adapted carrier
particles may comprise visible mono-sized beads having a diameter on the order
of at
least about 50 microns to more than about 5,000 microns. In a representative
embodiment of the present invention, suitably configured carrier particles may
comprise
beads that are generally soft and adapted to rub into the skin while leaving
substantially
no debris behind. In another representative embodiment, suitably configured
carrier
particles may be adapted to carry active ingredients. In yet a further
representative
embodiment of the present invention, carrier particle beads may be comprised
of
materials that are solid at room temperature and configured in various shapes
and/or
sizes.
[0046] Additionally, carrier particle beads may provide color and/or texture
so as to be visible
in product suspension. In another representative embodiment of the present
invention,
17
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
the color and/or texture of carrier particles may at least partially assist
the user in topical
application or delivery of a botanical component of an emollient sanitizing
composition
to a surface via visual verification of deposition.
[0047] Botanical emollient sanitizing compositions for topical use, in
accordance with various
representative aspects of the present invention, may be formulated in any
suitable
manner. For example, in a representative embodiment, an anti-microbial
sanitizer and a
botanical emollient may be combined with one or more additives. Representative
additives, in accordance with various aspects of the present invention, may
include, for
example: a coloring agent, a dye, a color shifting pigment, a preservative, a
pH
adjusting material, a pH buffering agent, a thickening agent/polymer, a
fragrance
material, a polar extract of a fragrance material, water, a polyacrylic acid,
polymer, a
sugar alcohol, glitter, a special effect pigment, a vitamin, a provitamin, an
amino acid, a
protein, a peptide, a peptide complex, an active agent, and/or the like.
[0048] In another representative embodiment of the present invention, a
botanical emollient
sanitizing composition may be formulated with glycerine (alternatively spelled
"glycerin ", but equivalent to glycerine in material respect). As a humectant,
glycerine
generally functions to enhance or at least substantially maintain
substantivity of an
emollient composition. A humectant is generally regarded as a hygroscopic
substance
and is often a molecule with several hydrophilic groups, most often hydroxyl
groups;
however, amines and carboxyl groups (sometimes esterified) may be employed as
well.
Humectants typically demonstrate an affinity to form hydrogen bonds with
molecules of
water. Humectants are often found in many cosmetic products where
moisturization is
18
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
desired, including, for example, moisturizing treatments for the hair.
Representative
examples of humectants include glycerine, propylene glycol (E 1520), butylene
glycol,
polyglycerol, polyglycerol esters, and glyceryl triacetate (E1518) and the
like. Others
may include polyols like sorbitol (E420), xylitol and maltitol (E965), or
polymeric
polyols like polydextrose (E1200) or natural extracts like quillaia (E999), or
lactic acid
or urea, and the like.
[0049] The property of a material demonstrating "substantivity" may generally
be regarded as
its propensity to persist and reside on a surface to which it is applied. With
enhanced
substantivity, the combination of glycerine with an emollient may provide
improved
moisture retention properties.
[0050] In accordance with various representative aspects of the present
invention, a botanical
emollient sanitizing composition may be formulated into one or more commercial
formulations, as generally illustrated by the Examples given below. The
percentages
detailed below should be regarded as approximate.
[0051 ] A representative antibacterial emollient sanitizing composition may be
formulated as a
hand sanitizer gel in accordance with the following:
19
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0052] Example 1
Phase Common Designation INCI Designation %(wt/wt)
A Deionized Water Water 6.70
FLORASOLVS
(International Flora
Technologies, Ltd. Jojoba Oil PEG-150 Esters 0.10
{"Floratech"}, Chandler, AZ,
USA) PEG-150
Hydrogenated Jojoba
CARBOPOL (Lubrizol
Advanced Materials, Inc., Acrylates/C10-30 Alkyl Acrylate
Cleveland, Ohio, USA) ETD Crosspolymer;
2020 (1.0% in water);
KATHON (Rohm & Haas Water; 25.00
Company, Philadelphia, PA, Methylchloroisothiazolinone and
USA) CG at 0.01%; and Methylisothiazolinone; and
Triethanolamine (TEA) Triethanolamine
titrated to pH=6.5
Glycerine Glycerin 0.70
SIMULGEL (Societe Acrylamide/Sodium
D'Exploitation de Produits Acryloyldimethyltaurate Copolymer
Pour Les Industries (and) Isohexadecane (and) 2.00
Chimiques Seppic, Paris,
France) 600 Polysorbate 80
B Ethanol SDA 40-2 (200 Alcohol 61.00
proof)
FLORAESTERS (Floratech, Jojoba Esters (and) lsopropyl Jojobate 2.00
Chandler, AZ, USA) IPJ (and) Jojoba Alcohol
FLORAMAC (Floratech Ethyl Macadamiate 1.40
Chandler, AZ, USA) 10
Bisabolol Bisabolol 0.10
FLORAESTERS K-100 Hydrolyzed Jojoba Esters (and) 0.10
Jojoba Esters (and) Water
Dermolene (Aston Olea Europaea (Olive Oil)
Chemicals, Ltd., Aylesbury, Unsaponiflables 0.10
UK)
C FLORASOMES (Floratech,
Chandler, AZ, USA) Jojoba Jojoba Esters (and) Tocopheryl 0.80
SMS White (natural) 10% Acetate
Vitamin E
TOTAL: 100%
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0053] Formulation Procedure:
[0054] 1. In a suitable vessel, add water of Phase A. Add CARBOPOL
ETD/KATHON/TEA
gel to the water and mix until uniformly dispersed. Add FLORASOLVS PEG-150
Hydrogenated Jojoba and mix with propeller agitation until clear.
[0055] 2. Add glycerin to main mixing vessel and stir until uniform. Add
SIMULGEL 600 to
the main mixing vessel and stir until a uniform texture results.
[0056] Referring now to Example 2, in another representative embodiment of the
present
invention, FLORAMAC 10 and Dermolene are excluded while additional
FLORAESTERS K-100 may be used to modify pH. Fragrance and preservative may
also be added.
21
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0057] Example 2
Common Designation INCI Designation % (wt/wt)
Deionized Water Water 8.65
FLORASOLVS PEG-150 Jojoba Oil PEG-150 Esters 0.10
Hydrogenated Jojoba
CARBOPOL ETD 2020 Acrylates/C10-30 Alkyl Acrylate
Crosspolymer;
(1.0% in water);
KATHON CG at 0.01 %; and Water;
20.00
Methylchloroisothiazolinone and
Triethanolamine titrated to
pH=6.5 Methylisothiazolinone; and
Triethanolamine
Glycerine Glycerin 5.00
Acrylamide/Sodium
SIMULGEL 600 Acryloyldimethyltaurate Copolymer 2.50
(and) lsohexadecane (and)
Polysorbate 80
Ethanol SDA 40-2 (200 Alcohol 61.00
proof)
FLORAESTERS IPJ Jojoba Esters (and) lsopropyl Jojobate 1.50
(and) Jojoba Alcohol
Bisabolol Bisabolol 0.10
FLORAESTERS K-100 Hydrolyzed Jojoba Esters (and) 0.10
Jojoba Esters (and) Water
FLORASOMES Jojoba Jojoba Esters (and) Tocopheryl 0.50
SMS 10% Vitamin E Acetate
FLORASOMES Jojoba Jojoba Esters (and) Tocopheryl
MDS 10% Vitamin E Acetate 0.50
Fragrance fragrance 0.05
TOTAL: 100%
[0058] Another representative embodiment of the present invention may be
illustrated in
Example 3. In this representative formulation, the amount of CARBOPOL,
glycerine,
FLORAESTERS IPJ, SIMULGEL 600, FLORASOMES, TEA and preservative is
reduced as compared to Example 2, while water is added to compensate for the
decreased formulation volume. Optionally, fragrance may be excluded as well.
22
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0059] Example 3
Common Designation INCI Designation % (wt/wt)
Deionized Water Water 18.70
FLORASOLVS PEG-150 Jojoba Oil PEG-150 Esters 0.10
Hydrogenated Jojoba
CARBOPOL ETD 2020 Acrylates/C10-30 Alkyl Acrylate
(1 .0% in water); Crosspolymer;
KATHON CG at 0.01%; and Water; 15.00
Triethanolamine titrated to Methylchloroisothiazolinone and
pH=6.5 Methylisothiazolinone; and
Triethanolamine
Glycerine Glycerin 1.40
Acrylamide/Sodium
SIMULGEL 600 Acryloyldimethyltaurate Copolymer 2.00
(and) Isohexadecane (and)
Polysorbate 80
Ethanol SDA 40-2 (200 Alcohol 61.00
proo fl
FLORAESTERS IPJ Jojoba Esters (and) Isopropyl Jojobate 1.00
(and) Jojoba Alcohol
Bisabolol Bisabolol 0.10
FLORAESTERS K-100 Hydrolyzed Jojoba Esters (and) 0.10
Jojoba Esters (and) Water
FLORASOMES Jojoba Jojoba Esters (and) Tocopheryl 0.30
SMS 10% Vitamin E Acetate
FLORASOMES Jojoba Jojoba Esters (and) Tocopheryl 0.30
MDS 10% Vitamin E Acetate
TOTAL: 100%
[0060] The formulation of Example 3 illustrates a representative embodiment
that may serve to
reduce stickiness and/or "tack" otherwise associated with conventional hand
sanitizers.
The representative formulation of Example 3 may also reduce production costs.
[0061] In the Examples disclosed supra, representative botanical emollients
may comprise
FLORAESTERS IPJ, FLORAMAC 10, FLORAESTERS K100, and FLORASOMES.
FLORAESTERS IPJ are generally obtained from the product of incomplete
23
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
saponification of jojoba oil (Simmondsia chinensis) yielding in approximately
equal
amounts: wax esters, jojoba alcohols, and isopropyl esters of jojoba fatty
acids.
FLORAMAC 10 corresponds to ethyl esters of macadamia oil (Macadamia
integrifolia)
fatty acids. Macadamia oil and FLORAMAC 10 are high in palmitoleic acid
(C16:1) - a
fatty acid know to be present as a significant fraction of human sebum.
FLORAESTERS K100 corresponds to saponification products of jojoba oil in
conjunction with unsaponifiable material produced from that reaction.
Specifically,
FLORAESTERS K100 is generally comprised of potassium salts of jojoba fatty
acids,
the corresponding jojoba free fatty alcohols, and a small amount of residual
jojoba wax
ester. FLORASOMES generally comprise jojoba oil randomized with fully
hydrogenated jojoba oil, yielding wax esters of varying degrees of
unsaturation.
[0062] Unsaturated alcohols of human sebum have not been fully characterized
previously,
however, somewhat similar alcohols have been observed in the seed oil of
Simmondsia
chinsensis. FLORAESTERS K100 provides a significant source of unsaturated
alcohols
derived from botanically-sourced jojoba oil. Human sebum also contains wax
esters,
with more active sebaceous glands producing sebum lipids with a higher
proportion of
C16:1 straight chain fatty acids. Similar wax esters may be obtained from, for
example,
FLORAESTERS IPJ and FLORASOMES - both representing derived compounds from
botanically-sourced jojoba oil. Additionally, a C16:1 lipid profile similar to
that of
mammalian sebum may be obtained with FLORAMAC 10 - a derived material of
botanically-sourced macadamia oil.
24
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0063] Disclosed botanical topical emollient sanitizing compositions, in
accordance with
various representative embodiments of the present invention, may be formulated
for
delivery via in any suitable manner, such as with a towelette, a pre-saturated
towelette, a
wipe, a napkin, a feminine hygiene product, a spray, a liquid, a gel, a cream,
a lotion, a
foam, a paste, a facial mask, a soap, and/or any other suitable formulation
vehicle.
[0064] In accordance with various aspects of the present invention,
representative topical
emollient sanitizing compositions may be formulated in a towelette, where the
towelette
may be suitably adapted to absorb and/or retain the emollient sanitizing
composition.
Additionally, a towelette may be implemented so as to prevent drying or
evaporation of
an emollient sanitizing composition. The material of the towelette may also be
disposable, washable, reusable, and/or the like.
[0065] In a further representative embodiment of the present invention, a
topical emollient
sanitizing composition may be added to the material of a towelette in
sufficient quantity
to dampen the towelette material so that the composition may be transferred to
the skin
or other surface of application upon contact with the towelette. The user may
rub and/or
wipe the towelette on the skin until emollient sanitizing composition is
substantially
absorbed. Debris on the surface of the skin may be further removed by contact
of the
towelette material on the skin.
[0066] In another representative embodiment of the present invention, an
emollient sanitizing
composition may be formulated to produce a sanitizing and moisturizing
detergent for
use as, for example, an anti-microbial soap for the removal of apolar
bacteria, dirt,
grease, oils, and/or the like from skin. Apolar materials may be lifted from
the skin by
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
association with micelles formed with soap molecules for subsequent washing
away with
water.
[0067] The presence of representatively disclose emollient sanitizing
formulations in
conjunction with soap generally provides a soft substantive skin-feel and
reduces
dermatitic symptoms associated with frequent hand washing. In another
representative
embodiment of the present invention, an emollient sanitizing soap may comprise
the
products of the saponification of a variety of botanical and/or synthetic
fats. In a further
representative embodiment, an emollient sanitizing soap may be provided as a
solid,
liquid, foam, spray, gel, cream, lotion, and/or the like.
[0068] Representative botanical emollient sanitizing compositions in
accordance with the
present invention may also be formulated with a skin toner. Skin toners
generally
function to sanitize the skin and diminish the size of pores. Conventional
skin toners
may vary according to their concentration of alcohol. For example, an
astringent is a
type of skin toner that generally comprises alcohol up to about 60%. A Skin
tonic, on
the other hand, is a type of skin toner that generally comprises less alcohol
than
astringents and may have up to about 20% alcohol. Additionally, a skin
refresher is a
type of skin toner that generally comprises the least amount of alcohol - on
the order of
about less than 10% alcohol.
[0069] In accordance with various representative aspects of the present
invention, botanical
emollient sanitizing compositions may increase the range of applications for
skin
sanitizing compositions that may be suitably formulated for use by persons in
which
conventional sanitizing formulations may be contra-indicated. For example,
individuals
26
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
with sensitive skin, eczema, shingles, the skin of infants or young children,
and/or the
like, may experience significant adverse dermatitic symptoms upon repeated use
of
conventional sanitizer products. Individuals with these same dermatological
conditions
generally do not experience such symptoms, or at least observe a reduction of
symptoms,
after use of botanical emollient sanitizing compositions in accordance with
representative embodiments of the present invention. Furthermore, botanical
emollient
sanitizing compositions in accordance with the present invention may also be
suitably
adapted for use on artificial (e.g., inanimate) surfaces that require
sanitization without
drying effects.
[0070] In a representative embodiment of the present invention, an emollient
sanitizing
composition may be employed to alleviate dermatitis, such as acquired
occupational
hand dermatitis, and/or the like. In a recent study of individuals having a
history of
chronic hand dermatitis for an average of thirteen (13) years of duration
prior to the
study due to repeated daily use (> 10 times per day) of conventional
commercial alcohol
gel sanitizing products, all fourteen (14) participants in the study exhibited
substantial
reduction of dermatitic symptoms after topical application of the emollient
sanitizing
composition corresponding to Example 1.
[0071] The study participants used the emollient sanitizing composition a
minimum of eight (8)
times per day over a fourteen (14) day period. For three (3) consecutive days
prior to the
study, the subjects cleansed their hands with CETAPHIL (Galderma Laboratories,
L.P.,
Cham Switzerland) soap, which was used to replace their daily hand soap to
provide a
baseline reference. The use of other hand moisturizers and topical products
(over-the-
27
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
counter or prescription) were not permitted during the duration of the study.
At the
beginning of the study, a physician's assessment was conducted to evaluate
abnormal
skin symptoms. Additionally, bio-instrumental evaluation for evaporative skin
moisture
loss (TEWL) was performed on the dorsal side of the subjects' hands (commonly
referred to as the "back" or "topside" of the hand), as well as on the palmar
side of the
subjects' hands (commonly referred to as the "palm" or the "inner surface" of
the hand).
Subjects thereafter started a course of application of the emollient sanitizer
composition
corresponding to Example 1 a minimum of eight (8) times per day for a duration
of
fourteen (14) days.
[0072] Table 1
Baseline 14 Days Post-Treatment
z Dorsal z Palmer z Dorsal z Palmer
Erythema 2.0 2.0 <1.0 <1.0
Scaling 2.0 2.0 <1.0 <1.0
Fissuring 1.0 1.0 0 (healed) 0 (healed)
Xerosis 2.0 2.0 <1.0 <1.0
Edema 0 (absent) 0 (absent) 0 (no change) 0 (no change)
Vesiculation 0 (absent) 0 (absent) 0 (no change) 0 (no change)
Lichenification 0 (absent) 0 (absent) 0 (no change) 0 (no change)
[0073] Table 1 representatively illustrates a physician's clinical assessments
of the hands of
study participants. The physician evaluated both dorsal and palmar sides of
the hands
before treatment and after fourteen (14) days of treatment. Subjects were
evaluated for
symptoms corresponding to various dermatological abnormalities, including, for
28
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
example: erythema (e.g., redness of the skin caused by capillary congestion);
scaling
(e.g., flaking of the skin); fissuring (e.g., cracks in the skin that may
bleed); xerosis (e.g.,
dry skin); edema (e.g., swelling of the skin); vesiculation (e.g., formation
of blisters);
and lichenification (e.g., the formation of thick, leathery skin, usually the
result of
constant scratching and rubbing). The physician used a scoring system
corresponding to
the following: 0 = an absence of the condition; 1 = mild incidence; 2 =
moderate
incidence; 3 = moderately severe incidence; and 4 = very severe incidence. At
day
fourteen (14), all fourteen (14) subjects demonstrated clinical improvement
(or at least
no change with respect to edema, vesiculation and lichenification). Erythema,
scaling,
and xerosis went from a moderate score of 2 at baseline, to slight improvement
following fourteen (14) days of product use. Notably, following two (2) weeks
of
product use, fissures that subjects presented with at baseline were healed!
[0074] Over a fourteen (14) day period, the hands of study participants were
clinically assessed
for TEWL, an objective measurement of moisture loss from the skin due to, for
example,
evaporation. TEWL values are related to the degree of perturbation of the
stratum
corneum with a decrease in TEWL values denoting improved function of this
barrier
layer of the skin. These measurements were performed using a TM 300 Tewameter
(Courage-Khazaka, Koln, Germany).
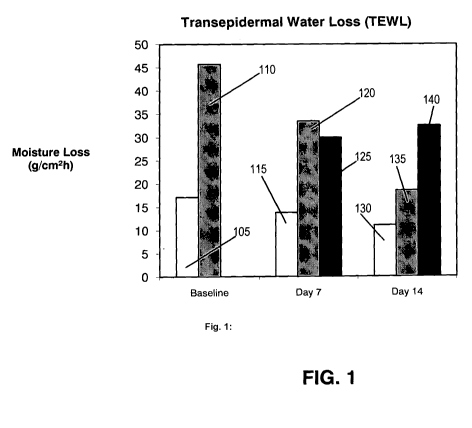
[0075] Referring to Figure 1, differences in TEWL with the use of an emollient
sanitizing
composition (Example 1) measured on dorsal 115, 130 and palmar 120, 135
surfaces of
hands of study participants as compared to baseline 105, 110 (dorsal and
palmar,
respectively) over time were observed. TEWL was measured in units of grams of
water
29
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
lost per square-centimeter per hour (g/cm2h). At baseline (prior to
application of the
emollient sanitizer composition corresponding to Example 1), the mean dorsal
TEWL
105 was 17.12 g/cm2h and the mean palmar TEWL 110 was 45.71 g/cm2h. These
values
decreased to 13.8 g/cm2h and 33.5 g/cm2h after seven (7) days of regular
application of
emollient sanitizer (dorsal 115 and palmar 120, respectively). This
corresponded to a
19.39% reduction of dorsal TEWL and a 26.71% reduction of palmar TEWL from
baseline thru day seven (7).
[0076] After two (2) weeks of application, dorsal 130 and palmar 135 values
decreased again to
11.04 g/cm2h and 18.51 g/emZh, respectively. This corresponded to a 20.00%
reduction
of dorsal TEWL and a 44.75% reduction of palmar TEWL from day seven (7) thru
day
fourteen (14). The aggregate effect observed over the course of the entire
study
corresponded to a 35.51% reduction of dorsal TEWL and a 59.51% reduction of
palmar
TEWL from baseline thru day fourteen (14).
[0077] To account for environmental factors that could potentially result in
errant increases or
decreases in TEWL, as well as to verify that the Tewameter probe was
functioning
properly, untreated sites 125, 140 were measured on medial inner-wrist patches
for each
subject on day seven (7) and day fourteen (14), respectively. The mean value
for TEWL
control readings on wrist patches at seven (7) days 125 corresponded to 30.00
g/cm2h.
The mean value for TEWL control readings on wrist patches at fourteen (14)
days 140
was 32.5 g/cm2h. Notably, even with slight increase in control measurements of
TEWL
over the duration of the study, both dorsal and palmar TEWL readings were
dramatically
reduced.
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0078] In terms of statistical significance, Student's t-distributions were
calculated which
demonstrated: a less than 2% probability that the reduction in mean dorsal
TEWL
values between baseline and seven (7) days occurred as a matter of random
chance; a
less than 0.1% probability that the reduction in mean palmar TEWL values
between
baseline and seven (7) days occurred as a matter of random chance; less than
1%
probability that the reduction in mean dorsal TEWL values between baseline and
fourteen (14) days occurred as a matter of random chance; and less than 0.1%
probability that the reduction in mean palmar TEWL values between baseline and
fourteen (14) days occurred as a matter of random chance.
[0079] Referring to Figure 2, differences in TEWL with the use of an emollient
sanitizing
composition (Example 1) were measured as averages of combined mean dorsal and
mean palmar values 210, 220 compared to baseline 205 over time were observed.
At
baseline (prior to application of the emollient sanitizer composition
corresponding to
Example 1), the average combined mean dorsal and palmar TEWL 205 was 31.41
g/cm2h. This value decreased to 23.68 g/cm2h after seven (7) days of regular
application
of emollient sanitizer (dorsal and palmar combined 210). This corresponded to
a
24.61% reduction of dorsal and palmar TEWL from baseline thru day seven (7).
[0080] After two (2) weeks of application, the combined dorsal and palmar
value 220 decreased
again to 14.78 g/cm2h. This corresponded to a 37.58% reduction of combined
dorsal and
palmar TEWL from day seven (7) thru day fourteen (14). The aggregate effect
observed
over the course of the entire study corresponded to a 52.94% reduction of
combined
dorsal and palmar TEWL from baseline thru day fourteen (14).
31
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
[0081 ] Again, to account for environmental factors that could potentially
result in errant
increases or decreases in TEWL, as well as to verify that the Tewameter probe
was
functioning properly, untreated sites 215, 225 were measured on medial inner-
wrist
patches for each subject on day seven (7) and day fourteen (14), respectively.
The mean
value for TEWL control readings on wrist patches at seven (7) days 215
corresponded to
30.00 g/cm2h. The mean value for TEWL control readings on wrist patches at
fourteen
(14) days 225 was 32.5 g/cmZh. Notably, even with slight increase in control
measurements of TEWL over the duration of the study, the combined averages of
mean
dorsal and palmar TEWL readings were dramatically reduced.
[0082] In terms of statistical significance, Student's t-distributions were
calculated which
demonstrated a less than 0.01% probability that the reduction in combined
average of
mean dorsal and palmar TEWL values between baseline and seven (7) days
occurred as
a matter of random chance, and a less than 0.01% probability that the
reduction in
combined average of mean dorsal and palmar TEWL values between baseline and
fourteen (14) days occurred as a matter of random chance.
[0083] Figures 3 and 4 illustrate histopathology associated with a
representative dermatological
inflammatory process (i.e., contact dermatitis) both before and following
application of
an emollient sanitizer composition corresponding to Example 1. Figure 3
corresponds to
baseline photomicrograph results of a 3mm punch biopsy of untreated skin
stained with
Hematoxylin and Eosin at a magnification of 400x. The histology depicts a
thickening
of the stratum corneum 310 and a mild-moderate inflammatory infiltrate of
polymorphonuclear leukocytes (PMN's; i.e., white blood cells) 320 in the basal
portion
32
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
of the epidermis. This histological evaluation is consistent with common signs
and
symptoms associated with hand irritant contact dermatitis (e.g., redness,
dryness, and
fissuring).
[0084] Figure 4 corresponds to measurement at fourteen (14) days post-
treatment following use
of the emollient sanitizing formulation of Example 1. Again, the
photomicrograph was
made from a 3mm punch biopsy stained with Hematoxylin and Eosin at 400x
magnification. (The vacuolization observed as open areas in the stratum
corneum 310
depicted in Figures 3 and 4 correspond to artifacts of the method employed to
prepare
the cross-sectional samples for photomicrography and, as such, represent no
difference
between the photomicrographs.) The histology of Figure 4 demonstrates a less
thick
stratum corneum 310 and less inflammatory (PMN) infiltrate 420 residing in the
basal
portion of the epidermis. These findings are consistent with resolution of
inflammatory
hand irritant contact dermatitis (e.g., reduction in redness, dryness, and
fissuring).
[0085] Accordingly, emollient sanitizing compositions corresponding to various
representative
embodiments of the present invention generally provide a demonstrated
reduction in
transepidermal water loss, increase in substantivity and smooth skin-feel, as
well as anti-
inflammatory activity useful for the mitigation of adverse dermatitic
symptoms.
[0086] In the foregoing specification, the invention has been described with
reference to
specific exemplary embodiments; however, it will be appreciated that various
modifications and changes may be made without departing from the scope of the
present
invention as set forth herein. The specification is to be regarded in an
illustrative
manner, rather than a restrictive one and all such modifications are intended
to be
33
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
included within the scope of the present invention. Accordingly, the scope of
the
invention should be determined by the claims and their legal equivalents
rather than by
merely the examples described above.
[0087] For example, the steps recited in any method or process embodiment may
be executed in
any order and are not limited to the specific order presented in the claims.
Additionally,
the components and/or elements recited in any apparatus or composition
embodiment
may be assembled or otherwise operationally configured in a variety of
permutations to
produce substantially the same result as the present invention and are
accordingly not
limited to the specific configuration recited in claims.
[0088] Benefits, other advantages and solutions to problems have been
described above with
regard to particular embodiments; however, any benefit, advantage, or solution
to a
problem or any element that may cause any particular benefit, advantage, or
solution to
occur or to become more pronounced are not to be construed as critical,
required, or
essential features or components of the invention.
[0089] As used herein, the terms "comprising", "having", "including" or any
variation thereof,
are intended to reference a non-exclusive inclusion, such that a process,
method, article,
composition or apparatus that comprises a list of elements does not include
only those
elements recited, but may also include other elements not expressly listed or
inherent to
such process, method, article, composition or apparatus. Other combinations
and/or
modifications of the above-described structures, arrangements, applications,
proportions,
elements, materials or components used in the practice of the present
invention, in
addition to those not specifically recited, may be varied or otherwise
particularly adapted
34
CA 02678780 2009-08-18
WO 2008/121355 PCT/US2008/004120
to specific environments, manufacturing specifications, design parameters or
other
operating requirements without departing from the general principles of the
same.