Note: Descriptions are shown in the official language in which they were submitted.
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
1
AUTOMATED THERAPY SYSTEM AND METHOD
INCORPORATION BY REFERENCE
[0001] All publications and patent applications mentioned in this
specification are
herein incorporated by reference to the same extent as if each individual
publication or
patent application was specifically and individually indicated to be
incorporated by
reference.
BACKGROUND OF THE INVENTION
[0002] Fluids and other substances are infused into patients for a variety of
reasons.
For example, fluids may be given to a patient intravenously to hydrate the
patient or to
control overall blood volume.
[0003] It is often important to control infusion of fluid into patients in
order to
optimize the therapy being provided. Monitoring of patient parameters can
consume
precious health care time and resources, however. Fluid infusion into patients
is therefore
not always optimized.
[0004] Mantle US 2006/0161107 describes a system that extracts fluid from a
body
cavity, processes the fluid and then recirculates fluid back into the cavity.
Mantle does not
describe infusion of a fluid into a patient without extraction of the fluid
from the patient,
however. In addition, the parameters on which the Mantle system is controlled
are
limited.
SUMMARY OF THE INVENTION
[0005] One aspect of the invention provides an automated therapy system having
an
infusion catheter; a sensor adapted to sense a patient parameter; and a
controller
communicating with the sensor and programmed to control flow output from the
infusion
catheter into a patient based on the patient parameter without removing fluid
from the
patient. In some embodiments, the sensor may be incorporated into the
catheter, and in
other embodiments, the sensor may be separate from the catheter. The sensor
may be,
e.g., an ECG sensor; an EEG sensor; a pulse oximetry sensor; a blood pressure
sensor; a
cardiac output sensor; a thermodilution cardiac output sensor; a cardiac
stroke volume
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
2
sensor; a heart rate sensor; a blood flow sensor; a pH sensor; a blood p02
sensor; an
intracranial pressure sensor; and/or a solute sensor.
[0006] In embodiments of the invention, the catheter may be a peripheral
venous
catheter; a central venous catheter; an arterial catheter; or a peritoneal
catheter (possibly
incorporating an intraperitoneal pressure sensor).
[0007] Another aspect of the invention provides a method of controlling
infusion of a
fluid to a patient. The method includes the following steps: monitoring a
patient
parameter with a sensor to generate a sensor signal; providing the sensor
signal to a
controller; and adjusting fluid flow to the patient based on the sensor signal
without
removing fluid from the patient. In some embodiments, the method includes the
step of
monitoring cardiac output with the sensor and, possibly, adjusting fluid flow
to the patient
based on cardiac output monitored by the sensor. In embodiments of the
invention, the
patient parameter includes an electrocardiogram; an electroencephalogram;
blood oxygen
saturation; blood pressure; cardiac output; cardiac stroke volume; heart rate;
blood flow;
total circulating blood volume; whole body oxygen consumption; pH; blood pOZ;
osmolarity; peritoneal cavity compliance; intrathoracic pressure; bladder
pressure; and/or
rectal pressure.
[0008] In some embodiments, the adjusting step includes the step of adjusting
fluid
flow to achieve or maintain patient euvolumia; adjusting flow of a therapeutic
agent (such
as a chilled medium) to the patient; adjusting fluid flow to the patient
through a peripheral
venous catheter; adjusting fluid flow to the patient through a central venous
catheter;
adjusting fluid flow to the patient through an arterial catheter; and/or
adjusting fluid flow
to the patient's peritoneal cavity.
[0009] Yet another aspect of the invention provides a method of treating
hypotension
in a patient. The method includes the following steps: monitoring a patient
parameter
(such as blood pressure or cardiac output) with a sensor to generate a sensor
signal;
providing the sensor signal to a controller; and adjusting fluid flow to the
patient based on
the sensor signal without removing fluid from the patient.
[00010] Still another aspect of the invention provides a method of treating
sepsis in a
patient. The method includes the following steps: monitoring a patient
parameter (such as
blood pressure, central venous pressure, or cardiac output) with a sensor to
generate a
sensor signal; providing the sensor signal to a controller; and adjusting
fluid flow to the
patient based on the sensor signal without removing fluid from the patient.
Prevention of
hypotension and/or hypovolemia is critical in the care of patients that have
suffered severe
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
3
hemorrhage or are septic. These patients are very difficult to monitor and
treat, taking
significant nursing time and still resulting in suboptimal therapy due to the
intermittent
nature of the blood pressure, central venous pressure and/or cardiac output
checks. The
present invention, then, will optimize fluid flow to the patient while also
freeing up the
already over-taxed nursing staff for other duties.
[00011] Yet another aspect of the invention provides a method of inducing and
reversing therapeutic hypothermia in a patient. The method includes the steps
of:
monitoring intracranial pressure to generate a sensor signal; providing the
sensor signal to
a controller; and adjusting rate of hypothermia induction or rewarming based
on
intracranial pressure (such as by adjusting fluid flow to the patient), or
depth of
hypothermia, based on the sensor signal.
[00012] In some embodiments of the invention, irrigation and/or lavage of
bodily
tissues, cavities or spaces (or other patient interventions) may be optimized
using a sensor
or sensors to report electrical, chemical, acoustic, mechanical properties,
pressure,
temperature, pH or other parameters surrounding the access device in order to
automate
and optimize the irrigation/lavage.
[00013] Embodiments of the invention include a peritoneal catheter containing
one or
more sensors which may detect changes in electrocardiograph monitoring,
electroencephalograph monitoring, pulse oximetry (either internally or
peripherally),
peritoneal cavity compliance, intrathoracic pressure, intraperitoneal
pressure,
intraperitoneal pressure waveforms, bladder pressure, rectal pressure, cardiac
output,
cardiac stroke volume, cardiac rate, blood flow (e.g., in superior mesenteric,
celiac, renal
or other arteries), pressure in veins (particularly the inferior vena cava or
those that empty
into the inferior vena cava, e.g., femoral vein), pressure in arteries
(particularly those distal
to the aorta, e.g., the femoral artery), total circulating blood volume, blood
oxygenation
(e.g., in rectal mucosa, peripheral fingers and toes, etc.), whole body oxygen
consumption,
pH and/or arterial p02 (or any other parameter that shows a measurable change
with
increased peritoneal pressure) to ensure safety of automated or manual
peritoneal lavage.
The invention also includes methods of performing peritoneal lavage using such
devices.
[00014] Embodiments of the invention include an intravascular catheter
containing one
or more sensors which may detect changes in electrocardiograph monitoring,
electroencephalograph monitoring, pulse oximetry (either internally or
peripherally),
partial pressure of oxygen or C02, pH, temperature, blood pressure, central
venous
pressure, cardiac output, cardiac stroke volume, cardiac rate, blood flow
(e.g., in superior
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
4
mesenteric, celiac, renal or other arteries), total circulating blood volume,
pressure in veins
(particularly those that empty into the inferior vena cava, e.g., femoral
vein), pressure in
arteries (particularly those distal to the aorta, e.g., the femoral artery),
blood oxygenation
(e.g., in rectal mucosa, peripheral fingers and toes, etc.), whole body oxygen
consumption,
pH and/or arterial pO2 (or any other parameter that shows a measurable change
with
intravascular volume overload) to ensure safety of manual or automated
intravascular
infusion. The invention also includes methods of using such devices.
[00015] Other embodiments of the invention include control of the rate of
infusion to
minimize negative effects observed by the sensors. The invention may be used
to induce
and/or maintain hypothermia or hyperthermia; maximize hydration and/or
intravascular
volume in a patient receiving intravenous fluids (such as, e.g., post-
operative patients,
post-hemorrhage patients, septic patients or other intensive care patients).
BRIEF DESCRIPTION OF THE DRAWINGS
[00016] The novel features of the invention are set forth with particularity
in the claims
that follow. A better understanding of the features and advantages of the
present invention
will be obtained by reference to the following detailed description that sets
forth
illustrative embodiments, in which the principles of the invention are
utilized, and the
accompanying drawings of which:
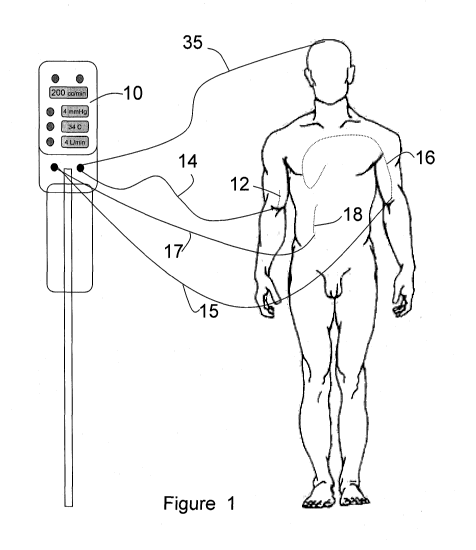
[00017] Figure 1 shows an automated infusion system in which infusion is
controlled
based on patient parameters sensed by multiple sensors.
[00018] Figure 2 shows an automated infusion system in which a sensor
controlling
infusion is separate from the infusion catheter.
[00019] Figure 3 shows an automated infusion system in which sensing and
infusion
are performed with the same catheter.
DETAILED DESCRIPTION OF THE INVENTION
[00020] Figures 1-3 show embodiments of the invention wherein intravenous
fluid
delivery may be automated, or manually adjusted, based on feedback from one or
more
sensors. In these embodiments, the infusion catheter may have a sensor to aid
in insertion,
but this is not necessary for this invention.
[00021] In one embodiment, the infusion catheter also is used to detect the
parameters
used to optimize therapy. Figure 1 shows an infusion system with an infusion
controller
operably connected to an intravenous infusion catheter 12 via an infusion line
14.
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
Infusion catheter 12 also has a sensor (not shown) attached to or associated
with it to
monitor a patient parameter. The sensor also communicates with controller 10
either
through line 14 or via some other communication channel. Suitable patient
parameters
include electrocardiograph monitoring, electroencephalograph monitoring, pulse
oximetry
(either internally or peripherally), blood pressure, central venous pressure,
cardiac output,
cardiac stroke volume, cardiac rate, blood flow (e.g., in superior mesenteric,
celiac, renal
or other arteries), total circulating blood volume, pressure in veins
(particularly those that
empty into the inferior vena cava, e.g., femoral vein), pressure in arteries
(particularly
those distal to the aorta, e.g., the femoral artery), blood oxygenation (e.g.,
in rectal
mucosa, peripheral fingers and toes, etc.), whole body oxygen consumption, pH,
arterial
P02, or any other parameter that shows a measurable change with intravascular
volume
overload.
[00022] As shown in Figure 1, additional catheters, here envisioned as a
peripherally
inserted central catheter (PICC) 16 and/or a peritoneal catheter 18, or
additional sensors on
infusion catheter 12 may be used to monitor these or other parameters, and to
optimize the
infusion rate and achieve euvolemia without fluid overload or dehydration.
Flow of fluid
and/or a fluid/solid mixture (e.g. an ice slurry) to catheters 16 and/or 18 is
controlled by
controller 10 through lines 14, 15 and/or 17, respectively. The information
from the
sensors may then be transmitted to central controller 10, which integrates all
of this
information to determine the flow of intravenous fluid through catheter 12
and/or catheter
16 and flow of peritoneal fluid through catheter 18. This information may be
used to
achieve or maintain euvolemia (e.g., in sepsis, hemorrhagic shock, etc.) or to
maximize
infusion for delivery of a therapeutic agent, e.g., chilled fluid and/or
solids to achieve
hypothermia. Alternatively, catheters 16 and 18 may be used with sensors to
obtain patent
information, and fluid may be infused into the patient solely through catheter
16 or
catheter 18. In yet further embodiments, the depth of hypothermia and/or rate
of
hypothermia induction or rewarming may be tailored based on intracranial
pressure
sensor(s) (not shown) communicating with controller 10 via communication line
35. This
system and method may be used with any method of inducing hypothermia (e.g.
cooling
blankets, intravascular catheters, intravenous fluid infusion, peritoneal
lavage, etc.) so long
as the change in temperature, particularly rewarming, is controlled at least
in part by an
intracranial pressure sensor.
[00023] The sensor or sensors, whether cables/catheters or percutaneous
monitoring
technologies, and whether wired or wireless, may also be separate from the
infusion line
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
6
so long as the information from this sensor or sensors is transferred to the
control unit in
order to optimize fluid flow. Thus, as shown in Figure 2, the patient
parameter sensor may
be associated with PICC 24 and communicate with controller via line 26, and
infusion to
the patient may be via line 22 and infusion catheter 20, as controlled by
controller 10. In
some embodiments, of course, sensing and infusion may be performed through a
single
catheter, such as PICC 30, and controlled by controller 10 through lines 32
and 34, as
shown in Figure 3. In some embodiments, the infusion and monitoring device of
the
current invention may incorporate an access sensor, such as that described in
a
concurrently filed and commonly owned patent application titled "Device and
Method for
Safe Access to a Body Cavity" (Attorney docket number 10729-700.200).
[00024] One example of such a device is a peripheral venous, central venous or
arterial
catheter that is capable of maintaining hydration without causing fluid
overload. The
catheter may incorporate a sensor that may detect central venous pressure,
total circulating
blood volume, peripheral venous pressure, cardiac output or osmolarity, and/or
solute
concentrations (e.g., chloride, sodium, etc.) in order to prevent fluid
overload. The sensor
may also be external to the catheter, so long as the output of said sensor is
capable of
controlling fluid flow through the catheter. In this embodiment, fluid flow is
controlled by
the output of the sensor, which is integrated by a fluid flow control unit
which alters the
rate of fluid flow based on this output. This embodiment may allow the user to
bolus large
volumes of fluids or solids into the vascular space in order to rehydrate,
induce
hypothermia or reverse hypothermia, or deliver a therapeutic agent or maintain
blood
pressure in sepsis.
[00025] In addition, this technology may provide a fully automated mechanism
to
optimize fluid flow into the vessel without fluid overloading the patient.
Without this
automated fluid delivery coupled to hemodynamic parameter monitoring, the
patient is in
danger of dehydration or fluid overload from infusion of fluid into any body
cavity. This
technology may also be applied to liquid or solid infusion into any body
cavity or space in
so long as the fluid flow is automated based on feedback from sensors within
the body
(possibly incorporated into the catheter itself) in order to optimize the
volume of infusion.
[00026] This device and method of automating fluid flow based on hemodynamic
sensor-based feedback may also be used to generate intravenous hypothermia. In
its
current state, IV hypothermia induction is limited due to concerns of fluid
overload. If the
hemodynamic parameters of the patient can be measured and fluid flow directly
or
indirectly controlled based on the output of these measurements, the volume of
fluid can
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
7
be maximized while ensuring hemodynamic instability. In this embodiment, the
sensor
may be incorporated within the catheter, and fluid flow into the vasculature
may be
tailored based on central venous pressure, total circulating blood volume,
peripheral
venous pressure, cardiac output or osmolarity, and/or solute concentrations
(e.g., chloride,
sodium, etc.) in order to prevent fluid overload.
[00027] In one embodiment, the fluid infusion catheter also may function as a
thermodilution cardiac output sensor such that the same fluid that is used to
generate
hypothermia may also be used to detect cardiac output. This information may
then be
relayed, either directly or indirectly, back to the fluid infusion controller
to increase,
decrease or even halt fluid flow based on these parameters. For example, if
cardiac output
is low and venous pressure or total circulating volume is low, the patient has
a low
circulating volume and large volumes of fluid may be safely delivered. If the
cardiac
output is normal, fluid may also be safely delivered, but the cardiac output
must be
monitored to ensure that it does not begin to decrease (an indication of fluid
overload).
Blood flow, as detected by, for instance, thermodilution may determined in a
peripheral
vessel as well. These data, while relatively useless on their own in a
clinical setting due
to variability in peripheral blood flow, may provide a baseline flow profile
which may be
rechecked over time in order to compare flow within that individual vessel to
the baseline
flow. Relatively improved flow may be correlated to improved cardiac output,
while a
relative reduction in flow may be correlated to fluid overload.
[00028] This same system may be used to infuse normal fluids or hypothermic
fluids to
sepsis patients or patients requiring intensive maintenance of their
hemodynamic status.
Sepsis patients that are aggressively monitored do much better than those that
are not.
Aggressive monitoring is very nurse-intensive, however. A system that provides
automated optimal fluid infusion based on sensed parameters to ensure that
fluid overload
does not occur and that fluid infusion is not insufficient would be an
improvement over
current methods of treating sepsis patients. The devices and methods for
automated
sensor-based input to control fluid flow to a patient may be applicable to a
wide range of
conditions and should not be limited to the narrow scope of the conditions
requiring fluid
infusion described here.
[00029] The logic controller of the present invention may provide improved
safety by
monitoring for any of the deleterious changes expected with excess fluid flow,
e.g. into the
peritoneal cavity or vascular space. Examples of monitored parameters that may
signal a
warning or automatically result in an adjustment to rate of fluid
infusion/extraction and/or
CA 02684807 2009-10-02
WO 2008/124644 PCT/US2008/059496
8
fluid temperature include: electrocardiograph monitoring,
electroencephalograph
monitoring, pulse oximetry (either internally or peripherally), peritoneal
cavity
compliance, intrathoracic pressure, intraperitoneal pressure, intraperitoneal
pressure
waveforms, bladder pressure, rectal pressure, cardiac output, cardiac stroke
volume,
cardiac rate, total circulating blood volume, blood flow (e.g., in superior
mesenteric,
celiac, renal or other arteries), pressure in veins (particularly those that
empty into the
IVC, e.g., femoral vein), pressure in arteries (particularly those distal to
the aorta, e.g., the
femoral artery), blood oxygenation (e.g., in rectal mucosa, peripheral fingers
and toes,
etc.), whole body oxygen consumption, pH and arterial p02 and any other
parameter that
shows a measurable change once the peritoneal or vascular spaces have been
overloaded.
[00030] These parameters in particular have been found to change with
increases in
peritoneal pressure, with significantly negative impact on each parameter
found at 40
mmHg. Thus, monitoring for these changes in conjunction with a peritoneal
infusion
catheter of the present invention will allow for even greater safety with
peritoneal
infusion. These parameters may be measured a variety of ways and the data
transmitted
either wirelessly or via wires to the logic controller in order to alert the
healthcare provider
or to automatically adjust the fluid flow/temperature in order to optimize
both the flow of
the peritoneal fluid and patient safety.