Note: Descriptions are shown in the official language in which they were submitted.
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
ELIMINATION OF INTERFERENCE IN IMMUNOASSAYS
CAUSED BY ANTI-CARBOHYDRATE ANTIBODIES
FIELD OF THE INVENTION
[0001] The present invention is directed to assays for the detection of an
anti-
drug antibody in general and in particular to the detection of an anti-drug
antibody
wherein the drug has a carbohydrate moiety. The invention is also directed to
assays for the detection of a drug in general and in particular to the
detection of a
drug wherein the drug has a carbohydrate moiety. The invention is further
directed to
methods for identifying appropriate subjects for treatment with a drug
containing a
carbohydrate moiety.
BACKGROUND OF THE INVENTION
[0002] Glycoproteins are increasingly being used for the treatment and
diagnosis
of cancer, infections, and immunological diseases. In particular, monoclonal
antibodies from various species, as well as chimeric or "humanized" antibodies
are
the kinds of glycoproteins that are finding increasing clinical use. When
administered
to a subject an immune response is sometimes generated by patients against
these
antibodies. The response is defined by the difference between the reactivity
of the
pre-treatment and that of the post-treatment serum or plasma with the infused
antibody.
[0003] Regulatory agencies require the monitoring and quantification of human
immune response to drugs and or proteins in treated patients. However, pre-
dose
reactivity of human serum or plasma to monoclonal antibodies, in some cases,
is
high and may interfere with the monitoring and quantification of the specific
immune
response to the glycoprotein. Furthermore, it has been noted that subjects
that are
naive with respect to exposure to certain antibodies used as a therapeutic
often
demonstrate a pre-treatment serum reactivity with the therapeutic protein.
This
-1-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
reactivity can be attributed to the presence in human serum or plasma of
"natural
antibodies" reactive with the antibody and in particular with the carbohydrate
portion
of the administered glycoprotein. (See, e.g., Robert G. Hamilton and N.
Franklin
Adkinson. Naturally Occurring Carbohydrate Antibodies: Interference in Solid-
Phase
Immunoassays. Journal of Immunological Methods 77 (1985) 95-108.);
"Hamilton").
Furthermore, it has been observed that anti-carbohydrate antibodies can act as
a
source of interference in immunoassays. Justus Adedoyin, S.G.O. Johansson,
Hans
Gr6nlund, Marianne van Hage. Interference in immunoassays by human IgM with
specifi'city for the carbohydrate moiety of animal proteins. Journal of
Immunological
Methods 310 (2006) 117-125).
[0004] Hamilton discloses that interference caused by these natural antibodies
to
carbohydrate in immunoassay analysis of human sera can be removed by pre-
adsorption of the subject sera with soluble or solid phase carbohydrate
reactive with
the natural antibodies. Also, U.S. Patent No. 5,856,106 discloses a method for
determining the levels of specific immune responsiveness to a glycoprotein in
an
individual being treated therewith by contacting a body fluid sample obtained
from the
individual subsequent to glycoprotein treatment with the glycoprotein that has
been
modified to have an oxidized carbohydrate portion.
[0005] Since the pretreatment serum reactivity with a glycoprotein might
either
conceal or augment the real immune response to the glycoprotein when
administered
as a therapeutic, reduction of this reactivity is desired prior to assessing
immunoreactivity of a potential therapeutic glycoprotein.
SUMMARY OF THE INVENTION
[0006] The present disclosure reports the existence of pretreatment serum
reactivity with a drug having a carbohydrate moiety, wherein the carbohydrate
moiety
is not directly associated with a glycoprotein. Drug-carrier conjugates
comprising
the drug calicheamicin conjugated to a monoclonal antibody which targets the
calicheamicin to a particular cell type or types have been found to react with
pre-
treatment or na7ve human and monkey serum. The pre-treatment reactivity is
-2-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
specific for the calicheamicn portion of the drug-carrier conjugate and in
particular is
directed to a carbohydrate portion of the calicheamicin. It has further been
found that
providing additional carbohydrate to pre-treatment sera removes background
reactivity of the sera with calicheamicin.
[0007] Thus, in one aspect, the invention provides an assay to detect the
presence of an antibody to a drug containing a carbohydrate moiety in a fluid
sample
containing an antibody, the assay comprising the following steps: combining
(i) the
sample with (ii) the drug containing a carbohydrate moiety and (iii)
additional
carbohydrate; and detecting whether or not specific binding occurs between an
antibody in the sample and the drug containing a carbohydrate moiety; wherein
the
drug containing a carbohydrate moiety does not comprise a glycoprotein.
[0008] In another aspect, the invention provides an assay to detect the
presence
of a drug containing a carbohydrate moiety in a fluid sample containing an
antibody,
the assay comprising: combining (i) the sample with (ii) a capture reagent
that
specifically binds to the drug containing a carbohydrate moiety and (iii)
additional
carbohydrate; and detecting whether or not specific binding occurs between the
drug
containing a carbohydrate moiety in the sample and the capture reagent;
wherein the
drug containing a carbohydrate moiety does not comprise a glycoprotein.
[0009] In another aspect, the invention provides an assay to detect the
presence
of an antibody to a drug containing a carbohydrate moiety in a fluid sample
containing an antibody, the assay comprising the following steps: a) combining
(i) the
fluid sample with (ii) the drug containing a carbohydrate moiety and (iii)
additional
carbohydrate; and b) detecting whether or not specific binding occurs between
an
antibody in the sample and the drug containing a carbohydrate moiety; wherein
the
additional carbohydrate is not pre-adsorbed with the fluid sample prior to
detecting
whether or not specific binding occurs between an antibody in the sample and
the
drug containing a carbohydrate moiety.
[0010] In another aspect, the invention provides an assay to detect the
presence
of a drug containing a carbohydrate moiety in a fluid sample containing an
antibody,
-3-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
the assay comprising: combining (i) the sample with (ii) a capture reagent
that
specifically binds to the drug containing a carbohydrate moiety and (iii)
additional
carbohydrate; and detecting whether or not specific binding occurs between the
drug
containing a carbohydrate moiety in the sample and the capture reagent;
wherein the
additional carbohydrate is not pre-adsorbed with the fluid sample prior to
detecting
whether or not specific binding occurs between the capture reagent and the
drug
containing a carbohydrate moiety.
[0011] In one embodiment, the invention provides any one or more of the assays
described herein wherein the assay is performed on a fluid sample from a
subject
after exposure of the subject to the drug containing the carbohydrate moiety.
[0012] In another embodiment, the invention provides any one or more of the
assays described herein wherein the assay is performed on a fluid sample prior
to
exposure of the subject to the drug containing the carbohydrate moiety (i.e.,
naive
subjects). In such embodiments, the assay provides information about the
presence
or absence in a naive subject of endogenous pre-existing antibodies that
specifically
bind to the drug containing a carbohydrate moiety.
[0013] In some embodiments, information about the presence or absence in a
naive subject of endogenous pre-existing antibodies that specifically bind to
the drug
containing a carbohydrate moiety can be used to identify subjects that are
more likely
to respond to treatment with the drug containing a carbohydrate moiety.
[0014] In some embodiments wherein the assay is performed on a fluid sample
from a naive subject, the assay can further comprise a comparative analysis
between
the sample from a subject before the subject has been exposed to a drug and a
sample from the same subject after the subject has been exposed to the drug,
wherein the amount of antibody to a drug containing a carbohydrate moiety in a
sample following exposure is indicative of an immune response to the drug.
-4-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0015] In some embodiments wherein the assay is performed on a fluid sample
from a naive subject, the additional carbohydrate is added to a final
concentration
that removes any reactivity with the drug containing a carbohydrate moiety
that is a
result of endogenous pre-treatment anti-carbohydrate antibody present in the
naive
subjecYs sera.
[0016] In another embodiment, the invention provides any one or more of the
assays described herein wherein the fluid sample from the subject comprises at
least
one of: whole blood; serum; mucous, saliva, colostrum and plasma.
[0017] In another embodiment, the invention provides any one or more of the
assays described herein wherein the detecting step comprises one or more of a
sandwich assay, a bridging assay and a competitive binding assay.
[0018] In another embodiment, the invention provides any one or more of the
assays described herein wherein the detecting step comprises at least one of:
detecting or measuring a change in refractive index at a solid optical surface
in
contact with the sample; detecting or measuring a change in luminescence;
detecting
or measuring a change in color; detecting or measuring a change in
radioactivity;
detecting or measuring using biolayer interferometry; detecting or measuring
using
cantilever-detection; detecting or measuring using label-free intrinsic
imaging; and
detecting or measuring using acoustic detection.
[0019] In another embodiment, the invention provides any one or more of the
assays described herein wherein the detecting step comprises an assay selected
from the group consisting of: an enzyme-linked immunosorbent assay (ELISA); an
electro-chemiluminescent assay (ECL); a radioimmunoassay (RIA); solid-phase
radioimmunoassay (SPRIA); immunoblotting; immunoprecipitation; Fluorescent
Activated Cell Sorting (FACS): and immunostaining.
[0020] In another embodiment, the invention provides any one or more of the
assays described herein wherein the additional carbohydrate is one or more of
-5-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
bacterial I ipo-polysaacha ride (LPS), a dextran, a levan, pneumococcal
polysaccharide, agarose, cellulose, carrageenan, and methylglycoside or a
functional
fragment of any one of the foregoing carbohydrates.
[0021] In another embodiment, the invention provides any one or more of the
assays described herein wherein the additional carbohydrate is present in the
combination at a concentration between about 10 ng/ml and about 1 mg/ml. In
another embodiment, the additional carbohydrate is present in the combination
at a
concentration between about 10 ng/ml and about 0.5 mg/ml. In another
embodiment,
the additional carbohydrate is present in the combination at a concentration
between
about 100 ng/mi and about 0.5 mg/mI. In another embodiment, the additional
carbohydrate is present in the combination at a concentration between about
100
ng/ml and about 0.75 mg/mi In another embodiment, the additional carbohydrate
is
present in the combination at a concentration between about 100 ng/ml and
about
0.25 mg/ml and any ranges in between, including fractions thereof.
[0022] In another embodiment, the invention provides any one or more of the
assays described herein wherein the drug containing a carbohydrate moiety
further
comprises a carrier conjugated to the drug. In some embodiments, the carrier
is
selected from the group consisting of: mono- and polyclonal antibodies and
their
chemically or genetically manipulated counterparts; their antigen-recognizing
fragments and their chemically or genetically manipulated counterparts; small
modular immunopharmaceuticals (SMIPs) and their chemically or genetically
manipulated counterparts; nanobodies and their chemically or genetically
manipulated counterparts; soluble receptors and their chemically or
genetically
manipulated counterparts; growth factors and their chemically or genetically
manipulated counterparts; aptamers; liposomes; non-glycosylated proteins; and
nanoparticles. In some embodiments, the carrier specifically binds to an
antigen
expressed on or within cancer cells. In some embodiments the antigen expressed
on
or within the cancer cells is selected from the group consisting of: 5T4;
CD19; CD20;
CD22; CD33; Lewis Y; HER-2; type I Fc receptor for immunoglobulin G (Fc gamma
R1); CD52; epidermal growth factor receptor (EGFR); vascular endothelial
growth
factor (VEGF); DNA/histone complex; carcinoembryonic antigen (CEA); CD47;
-6-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
VEGFR2 (vascular endothelial growth factor receptor 2 or kinase insert domain-
containing receptor, KDR); epithelial cell adhesion molecule (Ep-CAM);
fibroblast
activation protein (FAP); Trail receptor-1 (DR4); progesterone receptor;
oncofetal
antigen CA 19.9; and fibrin.
[0023] In another embodiment, the invention provides one or more of the assays
described herein wherein the carrier is a monoclonal antibody. In some
embodiments, the monoclonal antibody is selected from the group consisting of:
an
anti-CD22 monoclonal antibody; an anti-5T4 monoclonal antibody; anti-CD33
antibody; and an anti-Lewis Y monoclonal antibody.
[0024] In another embodiment, the invention provides one or more of the assays
described herein wherein the drug containing a carbohydrate moiety is selected
from
the group consisting of: a cytotoxic agent; a radiotherapeutic agent; an
immunomodulatory agent; an anti-angiogenic agent; an anti-proliferative agent;
a
pro-apoptotic agent; a chemotherapeutic agent; a therapeutic nucleic acid; an
inhibitor of tubulin polymerization; an alkylating agent that binds to and
disrupt DNA;
an inhibitor of protein synthesis; and a tyrosine kinase inhibitor. The assays
described herein can be performed on fluid samples containing or exposed to
more
than one drug containing a carbohydrate moiety. The assays described herein
can
include portions or functional fragments of the drug containing a carbohydrate
moiety.
[0025] In other embodiments, the invention provides any one or more of the
assays described herein wherein the drug containing a carbohydrate moiety is a
cytotoxic agent is selected from calicheamicins, thiotepa, taxanes,
vincristine,
daunorubicin, doxorubicin, epirubicin, actinomycin, authramycin, azaserines,
bleomycins, tamoxifen, idarubicin, dolastatins/auristatins, hemiasterlins, and
maytansinoids. In some preferred embodiments the cytotoxic agent is a
calicheamicin and in some embodiment can be N-acetyl gamma dimethyl hydrazine
calicheamicin.
-7-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0026] In another embodiment, the invention provides an assay as described
herein wherein the antibodies to a drug having a carbohydrate moiety or the
drug
containing a carbohydrate moiety is detected by binding to a capture agent.
[0027] In some embodiments, the capture agent that specifically binds the anti-
drug antibody comprises the drug having a carbohydrate moiety or a portion or
functional fragment of the drug containing a carbohydrate moiety.
[0028] In some embodiments, the capture agent that specifically binds the anti-
drug antibody is the carbohydrate portion of the drug containing a
carbohydrate
moiety or a functional fragment or portion of the drug containing a
carbohydrate
moiety.
[0029] In some embodiments, the drug containing a carbohydrate moiety and the
capture reagent is S-[(2R,4S,6S)-6-({[(2R,4S,5R)-5-({(2S,4S,5S)-5-
[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-4-hydroxy-6-
methoxy-2-
methyltetrahydro-2H-pyran-3-yl]am ino}oxy)-4-hydroxy-2-methyltetrahydro-2H-
pyran-
3-yl]4-{[(2S, 3R,4R, 5S,6S )-3, 5-d ihyd roxy-4-m ethoxy-6-m ethyltetrahydro-
2H-pyran-2-
yI]oxy}-3-iodo-5,6-dim ethoxy-2-methylbenzenecarbothioate.
[0030] In some embodiments, the capture agent that specifically binds the drug
having a carbohydrate moiety comprises an antibody to the drug containing a
carbohydrate moiety. In some embodiments wherein the drug containing a
carbohydrate moiety comprises a carrier-drug conjugate, the capture agent that
specifically binds the drug having a carbohydrate moiety comprises a target
that
specifically binds the carrier portion of the carrier-drug conjugate.
[0031] In some embodiments wherein the drug containing a carbohydrate moiety
comprises a carrier-drug conjugate, the capture agent that specifically binds
the drug
having a carbohydrate moiety comprises at least a portion of 5T4; CD19; CD20;
CD22; CD33; Lewis Y; HER-2; type I Fc receptor for immunoglobulin G (Fc gamma
R1); CD52; epidermal growth factor receptor (EGFR); vascular endothelial
growth
-8-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
factor (VEGF); DNA/histone complex; carcinoembryonic antigen (CEA); CD47;
VEGFR2 (vascular endothelial growth factor receptor 2 or kinase insert domain-
containing receptor, KDR); epithelial cell adhesion molecule (Ep-CAM);
fibroblast
activation protein (FAP); Trail receptor-1 (DR4); progesterone receptor;
oncofetal
antigen CA 19.9; and fibrin, wherein the portion specifically binds to the
carrier
portion of the carrier-drug conjugate.
[0032] In another embodiment, the bound antibody to the drug containing a
carbohydrate moiety is detected by binding of a labeled compound to the
antibody.
In some preferred embodiments, the labeled compound comprises the capture
reagent further comprising a detectable label; or a second antibody or
antibody
fragment further comprising a detectable label, wherein the second antibody
specifically binds to the bound antibody. In some embodiments, the detectable
label
comprises at least one of an enzyme label, a luminescent label and a
radioisotope
label.
[0033] In some embodiments, the invention provides an assay as described
herein in any one or more of examples one through eight.
[0034] In some embodiments, the presence of drug containing a carbohydrate
moiety bound to the capture reagent is detected with a labeled compound that
also
specifically binds to the drug. In some embodiments, the labeled compound is
selected from the group consisting of: the capture reagent further comprising
a
detectable label; and an antibody further comprising a detectable label,
wherein the
antibody specifically binds to the drug. In some embodiments, the detectable
label
comprises at least one label selected from the group consisting of an enzyme
label; a
luminescent label; and a radioisotope label.
[0035] In some embodiments, the assay to detect the presence of an antibody to
a drug containing a carbohydrate moiety or the presence of a drug containing a
carbohydrate moiety in a fluid sample containing antibodies comprises at least
one
of: measuring a change in refractive index at a solid optical surface in
contact with
-9-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
the sample; measuring a change in luminescence; measuring a change in color;
and
measuring a change in radioactivity.
[0036] In some embodiments, the assay to detect the presence of an antibody to
a drug containing a carbohydrate moiety or the presence of a drug containing a
carbohydrate moiety in a fluid sample containing antibodies comprises an assay
selected from the group consisting of: an ELISA; an ECL; an RIA; a SPRIA;
immunoblotting; immunoprecipitation; and immunostaining.
BRIEF DESCRIPTION OF THE DRAWINGS
[0037] Figure 1 is a graphical representation of the presence of an
interfering
factor in naive serum having a binding specificity for calicheamicin.
[0038] Figure 2 shows a diagrammatic representation of a calicheamicin as
conjugated to an antibody.
[0039] Figure 3 is a graphical representation of the reduction in
calicheamicin
specific binding in sera titrated with various concentrations of detoxified
LPS.
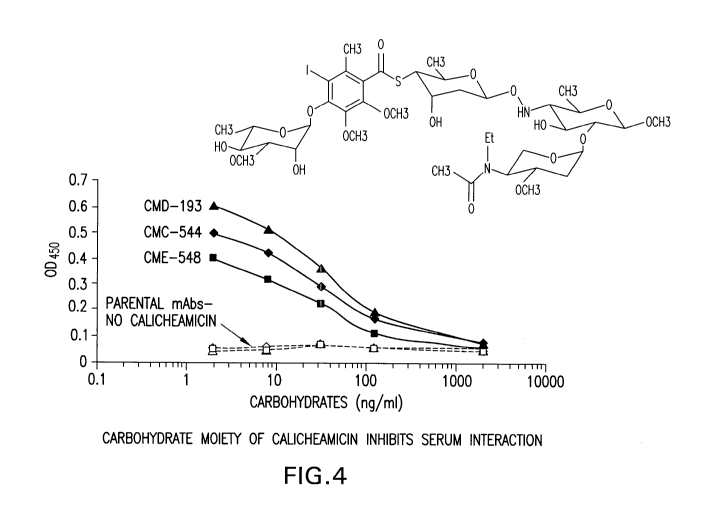
[0040] Figure 4 is a graphical representation of the reduction in
calicheamicin
specific binding in sera titrated with various concentrations of the
carbohydrate
moiety of calicheamicin, methyl glycoside.
[0041] Figure 5 is a graphical representation demonstrating the reduction in
calicheamicin specific binding in sera tftrated with various concentrations of
detoxified
LPS.
[0042] Figure 6 shows a graphical representation of calicheamicin specific IgM
from calicheamicin specific sera binding IgM.
-10-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0043] Figure 7 shows a graphical representation of IgM from Calicheamicin
specific sera binding to calicheamicin.
[0044] Figure 8 shows a graphical representation of the presence of
calicheamicin specific binding factor in naive human sera.
[0045] Figure 9 shows the chemical structure of S-[(2R,4S,6S)-6-({[(2R,4S,5R)-
5-({(2S,4S,5S)-5-[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-
4-
hydroxy-6-methoxy-2-methyltetrahydro-2H-pyran-3-yl]am ino}oxy)-4-hydroxy-2-
methyltetrahyd ro-2H-pyran-3-yl]4-{[(2S,3R,4R,5S,6S)-3,5-dihydroxy-4-methoxy-6-
m ethyltetra hyd ro-2 H-pyra n-2-yl]oxy}-3-iod o-5, 6-d i m ethoxy-2-
m ethyl benzeneca rboth ioate).
DETAILED DESCRIPTION OF THE INVENTION
[0046] Drug conjugates developed for systemic pharmacotherapy are target-
specific cytotoxic agents. The concept involves coupling a therapeutic agent
to a
carrier molecule with specificity for a defined target cell population. As
used herein,
such drug conjugates are also referred to as "carrier-drug conjugates."
[0047] Antibodies with high affinity for antigens are a natural choice as
targeting
moieties or as the "carrier" portion of a carrier-drug conjugate. With the
availability of
high affinity monoclonal antibodies, the prospects of antibody-targeting
therapeutics
have become promising. Toxic substances that have been conjugated to
monoclonal
antibodies include toxins, low-molecular-weight cytotoxic drugs, biological
response
modifiers, and radionuclides. Antibody-toxin conjugates are frequently termed
immunotoxins, whereas immunoconjugates consisting of antibodies and low-
molecular-weight drugs such as methothrexate and Adriamycin are called
chemoimmunoconjugates. Immunomodulators contain biological response modifiers
that are known to have regulatory functions such as lymphokines, growth
factors, and
complement-activating cobra venom factor (CVF). Radioimmunoconjugates consist
of radioactive isotopes, which may be used as therapeutics to kill cells by
their
-11-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
radiation or used for imaging. Antibody-mediated specific delivery of
cytotoxic drugs
to tumor cells is expected to not only augment their anti-tumor efficacy, but
also
prevent nontargeted uptake by normal tissues, thus increasing their
therapeutic
indices.
[0048] A number of antibody-based therapeutics for treating a variety of
diseases
including cancer and rheumatoid arthritis have been approved for clinical use
or are
in clinical trials for a variety of malignancies including B-cell malignancies
such as
Non-Hodgkin's lymphoma. One such antibody-based therapeutic is rituximab
(Rituxan.TM.), an unlabelled chimeric human 71 (+my1V-region) antibody, which
is
specific for cell surface antigen CD20, which is expressed on B-cells. These
antibody
based therapeutics rely either on complement-mediated cytotoxicity (CDCC) or
antibody-dependent cellular cytotoxicity (ADCC) against B cells, or on the use
of
radionuclides, such as 1311 or 90Y, which have associated preparation and use
problems for clinicians and patients. Consequently, there is a need for the
generation
of immunoconjugates that can overcome the shortcomings of current antibody-
based
therapeutics to treat a variety of malignancies including hematopoietic
malignancies
like non-Hodgkin's lymphoma (NHL), which can be produced easily and
efficiently,
and which can be used repeatedly without inducing an immune response.
[0049] Immunoconjugates comprising a member of the potent family of
antibacterial and antitumor agents, known collectively as the calicheamicins
or the
LL-E33288 complex, (see U.S. Pat. No. 4,970,198 (1990)), were developed for
use in
the treatment of myelomas. One of the most potent of the calicheamicins is
designated y, which is herein referenced simply as gamma. These compounds
contain a methyltrisulfide that can be reacted with appropriate thiols to form
disulfides, at the same time introducing a functional group such as a
hydrazide or
other functional group that is useful in attaching a calicheamicin derivative
to a
carrier. (See U.S. Pat. No. 5,053,394).
[0050] The presence of antibodies in naive sera that specifically bind to the
carbohydrate portion of a drug containing a carbohydrate moiety or a carrier
drug
conjugate wherein the drug portion of the conjugate contains a carbohydrate
moiety
-12-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
can interfere with immunoassays to detect (i) antibodies generated in response
to the
carrier drug conjugate or (ii) the drug or carrier drug conjugate having a
carbohydrate. Such interference has now been determined to include endogenous
antibodies that specifically bind to carbohydrates that are not associated
with a
glycoprotein or glycoprotein portion of a carrier drug conjugate.
[0051] As an example, if a drug is used as a target to capture antibodies
specific
for the drug, there may be a false positive reaction (or increased quantity)
if an
endogenous anti-carbohydrate moiety antibody binds to the capture agent. Also,
in
an assay where a capture reagent for the drug is used in conjunction with a
labeled
compound to detect the drug in sera, there may be false negatives (or a
decrease in
quantity) due to anti-carbohydrate antibody binding to the drug, which
prevents or
reduces specific binding of a labeled compound to the drug if the bound
antibody
interferes with binding of the labeled compound.
[0052] It is reported herein that the presence of additional carbohydrate in
an
assay to detect specific binding between an anti-carrier-drug conjugate
antibody and
the can-ier-drug conjugate in a fluid sample containing antibody will enable
the
specific detection of anti-carrier-drug conjugate antibody that is generated
in
response to the exposure of a subject to the carrier-drug conjugate. The
additional
carbohydrate does not have to be pre-adsorbed with the fluid sample prior to
detecting specific binding between the anti-carrier-drug conjugate antibody
and the
carrier-drug conjugate. The specific detection is possible because the
additional
carbohydrate added to the assay will remove any activity that may have been
present
before exposure to the drug.
[0053] Similarly the presence of additional carbohydrate in an assay to detect
specific binding between an capture reagent and a carrier-drug conjugate in a
fluid
sample containing antibody will enable the specific detection of the carrier-
drug
conjugate antibody. The additional carbohydrate does not have to be pre-
adsorbed
with the fluid sample prior to detecting specific binding between the capture
reagent
and the carrier-drug conjugate. The specific detection is possible because the
-13-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
additional carbohydrate added to the assay will remove any masking activity of
pre-
existing anti-drug antibodies present in the sample.
[0054] The additional carbohydrate is added to the serum in order to negate
the
effect of binding of endogenous antibodies in the serum to the drug containing
a
carbohydrate moiety. The amount of additional carbohydrate can be titrated
with
naive serum in order to determine the optimal amount to add when seeking to
reduce
background. If analysis and detection of the drug containing carbohydrate
moiety
will rely on a capture reagent that does not bind to the carbohydrate moiety,
then
additional carbohydrate may be added to excess.
[0055] The development of specific binding assay techniques has provided
extremely useful analytical methods for determining various substances of
diagnostic, medical, environmental and industrial importance which appear in
various
liquid or solid (e.g., tissue) samples at very low concentrations. Specific
binding
assays are based on the specific interaction between a bindable analyte under
determination and a binding partner therefore, i.e. analyte-specific moiety.
The
binding of the analyte-specific moiety and interaction of any additional
reagents, if
necessary, effect a mechanical separation of bound and unbound labeled analyte
or
affect the label in such a way as to modulate the detectable signal. The
former
situation is normally referred to as heterogeneous and the latter as
homogeneous, in
that the latter does not require a separation step.
[0056] MYLOTARG (Sievers, E. L. et al (1999) Blood: 93, 3678-3684), also
referred to as CMA-676 or CMA, is a commercially available drug that comprises
calicheamicin bound to a monoclonal antibody as the carrier. MYLOTARG
(gemtuzumab ozogamicin) is currently approved for the treatment of acute
myeloid
leukemia in elderly patients. The drug consists of an antibody against CD33
that is
bound to calicheamicin by means of an acid-hydrolyzable linker. The disulfide
analog
of the semi-synthetic N-acetyl gamma calicheamicin was used for conjugation
(U.S.
Pat. Nos. 5,606,040 and 5,770,710).
-14-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0057] In conventional label conjugate specific binding assay techniques, a
sample of the liquid medium to be assayed is combined with various reagent
compositions. Such compositions include a label conjugate comprising a binding
component incorporated with a label. The binding component in the conjugate
participates with other constituents, if any, of the reagent composition and
the ligand
in the medium under assay to form a binding reaction system producing two
species
or forms of the conjugate, e.g., a bound-species (conjugate complex) and a
free-
species. In the bound-species, the binding component of the conjugate is bound
by a
corresponding binding partner whereas in the free species, the binding
component is
not so bound. The amount or proportion of the conjugate that results in the
bound
species compared to the free species is a function of the presence (or amount)
of the
analyte to be detected in the test sample.
[0058] An alternative format for specific binding assays is the "sandwich"
assay
protocol in which the target analyte is bound between a first specific binding
partner,
which is fixed directly or through a linkage group to a solid matrix, and a
second
specific binding partner, which is associated with a signal generating or
labeling
system.
[0059] Additional immunoassay formats and protocols are also known in the art
and are applicable to use in the present invention. Various binding assays
using
immobilized or immobilizable materials for the direct immobilization of one of
the
binding participants in a binding assay reaction, e.g., immobilized antigen or
antibody, in order to accomplish the desired separation of the bound and free
forms
of a labeled reagent, have been proposed. In particular, a number of such
binding
assays have been described wherein an antibody to an antigen to be detected is
bound to an immobilizing material such as the inner wall of a test tube or a
plastic or
magnetic bead.
[0060] U.S. Pat. No. 4,243,749 discloses a competitive binding assay wherein a
reaction is carried out in a test tube having a specific antibody to a hapten
under
determination insolubilized or immobilized on the inner wall of the test tube.
The
reaction includes a labeled hapten conjugate wherein the quantity of the
labeled
-15-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
hapten conjugate which becomes bound to the test tube wall is inversely
proportional
to the amount of the hapten under determination.
[0061] Another of such binding assays is described by U.S. Pat. No. 4,230,683
which discloses a method employing a 6 mm polystyrene bead having antigen or
antibody bound thereto wherein the antigen or antibody is reacted with a
hapten-
conjugated antibody to the antigen or antibody. The bound hapten-conjugated
antibody is further reacted with labeled anti-hapten antibody in order to
determine the
amount of antigen or antibody in a test sample.
[0062] Still another of such binding assays is described by U.S. Pat. No.
4,228,237 which discloses a method for the detection and determination of
ligands in
a liquid medium using enzyme labeled avidin and a biotin labeled reagent in a
specific binding process. In this method, the ligand to be detected is
contacted with
an insoluble phase containing a specific binding substance for the ligand.
[0063] In addition to the direct immobilization techniques heretofore
described,
indirect immobilization by marking or labeling a binding assay reaction
participant to
be immobilized with a first binding substance, and then adding an immobilized
second binding substance, has been proposed.
[0064] For example, U.S. Pat. No. 4,298,685 discloses an enzyme immunoassay
wherein a sample containing a biological substance under determination is
mixed
with antibodies to the biological substance tagged with biotin and with an
enzyme-
labeled form of the substance under assay. An immobilized form of avidin is
then
added wherein the avidin binds to the biotin-tagged antibody to immobilize the
antibody-bound fraction of the enzyme-labeled reagent. Similarly, United
Kingdom
Patent Application No. GB 2,084,317A discloses an antigen-linked competitive
enzyme immunoassay using avidin bound to a solid material and a biotin-labeled
antigen. Radiolabeled and enzyme-tagged immunoassays require some type of
separation step. Recently, a different approach was disclosed which does not
require
a separation step and therefore has been referred to as a homogeneous system,
in
contrast to a heterogeneous system in which separation is essential. U.S. Pat.
No.
-16-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
3,817,837 discloses a competitive binding assay method involving the steps of
combining the liquid to be assayed with a soluble complex consisting of an
enzyme
as a labeling substance covalently bound to the ligand to be detected and with
a
soluble receptor, usually an antibody, for the ligand; and analyzing for the
effect of
the liquid to be assayed on the enzymatic activity of the enzyme in the
complex.
[0065] Still further, the labeled reagent can include other conventional
detectable
chemical groups. Such detectable chemical groups can be any material having a
detectable physical or chemical property. Such materials have been well
developed
in the field of immunoassays and in general any label useful in such methods
can be
applied to the present invention. Particularly useful are enzymatically active
groups,
such as enzymes (see Clin. Chem. (1976)22:1243), enzyme substrates (see U.S.
Pat. No. 4,492,751), prosthetic groups or coenzymes (see U.S. Pat. Nos.
4,230,797
and 4,238,565), and enzyme inhibitors (see U.S. Pat. No. 4,134,792); spin
labels;
fluorescers (see Clin. Chem. (1979)25:353); chromophores; luminescers such as
chemiluminescers and bioluminescers (see U.S. Pat. No. 4,380,580);
specifically
bindable ligands (e.g., biotin and haptens); electroactive species; and
radioisotopes
such as 3 H, 35 S, 32 P, 1251, and14 C. Such labels and labeling pairs are
detected on
the basis of their own physical properties (e.g., fluorescers, chromophores
and
radioisotopes) or their reactive or binding properties (e.g., enzymes,
substrates,
coenzymes and inhibitors).
DEFINITIONS
[0066] As used in the specification and the appended claims, the singular
forms
"a," "an" and "the" include reference to the plural unless the context clearly
dictates
otherwise. Thus, for example, reference to "an antibody" includes a plurality
of such
compositions, i.e., "antibodies."
[0067] The term "about" means within 20%, more preferably within 10% and
more preferably within 5% of a stated measurement or concentration.
-17-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0068] The term "additional carbohydrate" as used herein, refers to
carbohydrates that can compete with carbohydrate that binds to endogenous
antibody in serum. The use of bacterial lipopolysaccharide (LPS),
methylglycoside,
dextran, levan, pneumococcal polysaccharide, agarose, cellulose, or
carrageenan in
an immunoassay can block the binding of antibodies generally binding to
carbohydrates. The addition of any of these carbohydrates will allow specific
binding
of other reagents in the assay to bind to non-carbohydrate regions of N-acetyl
gamma dimethyl hydrazide alone or as part of a carrier-drug conjugate, thus
reducing
non-specific background signal generated from anti-carbohydrate antibodies in
serum. In addition, selection of the proper carbohydrate can involve a
carbohydrate
that specifically binds to the drug-carrier conjugate.
[0069] Unless the context specifically indicates otherwise, the term
"antibody" as
used herein is meant to include one or more of mono- and polyclonal antibodies
and
their chemically or genetically manipulated counterparts; their antigen-
recognizing
fragments and their chemically or genetically manipulated counterparts; and
synthetic
molecules comprising their antigen-recognizing fragments.
[0070] As used herein, the phrase "additional carbohydrate" is also meant to
encompass the same carbohydrate that is attached to the drug containing a
carbohydrate moiety or a fragment thereof as well as other carbohydrates or
fragments thereof that specifically mimic the carbohydrate portion of the drug
containing a carbohydrate moiety. To specifically mimic a carbohydrate portion
of a
drug containing a carbohydrate moiety the carbohydrate or fragment thereof
will
specifically bind the same target and receptor compound that binds to the
carbohydrate moiety of the drug.
[0071] A "bridging assay" refers to an immunoassay wherein the capturing
system is an immunocomplex containing the sample analyte formed in solution
and
then captured by a bridging element which links the immunocomplex to a solid
phase. A detectable label covalently coupled to an element binding to the
bridging
element is used for the detection of a binding event.
-18-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0072] As used herein, the term "capture reagent" is meant to include any
composition that specifically binds to a corresponding target. For example, a
capture
reagent for a corresponding target that is a drug containing a carbohydrate
moiety
includes but is not limited to an antibody that specifically binds the drug
containing a
carbohydrate moiety and any specific binding partner for the drug containing a
carbohydrate moiety. For example, if a drug containing a carbohydrate moiety
further comprises an antibody conjugated to the drug, the target of the
antibody's
antigen-binding domain is also a capture reagent for the drug containing a
carbohydrate moiety. Furthermore, if the drug containing a carbohydrate moiety
has
a corresponding receptor binding partner, or comprises a soluble form of a
receptor
that has a corresponding binding partner, the receptor and receptor binding
partner
are capture reagents for each other.
[0073] The term "competition assay' refers to an assay where an unlabeled
analyte in a heterogeneous sample is measured by its ability to compete with
an
element, primarily an antibody or antigen, covalently attached to a detectable
label.
The unlabeled analyte and the labeled molecule will compete with each other
for the
binding to a capture reagent immobilized on a solid phase. The unlabeled
analyte
blocks the ability of the labeled molecule to bind because the binding site on
the
capture reagent is already occupied. Thus, in a competitive immunoassay, less
label
measured in the assay means more of the unlabeled analyte is present. The
amount
of antigen in the heterogenous sample is inversely related to the amount of
label
measured.
[0074] The term "cytotoxin" or "cytotoxic agent" generally refers to an agent
that
inhibits or prevents the function of cells and/or results in destruction of
cells.
Representative cytotoxins include antibiotics, inhibitors of tubulin
polymerization,
tyrosine kinase inhibitors, alkylating agents that bind to and disrupt DNA,
and agents
that disrupt protein synthesis or the function of essential cellular proteins
such as
protein kinases, phosphatases, topoisomerases, enzymes, and cyclins.
Representative cytotoxins include, but are not limited to, doxorubicin,
daunorubicin,
idarubicin, aclarubicin, zorubicin, mitoxantrone, epirubicin, carubicin,
nogalamycin,
menogaril, pitarubicin, valrubicin, cytarabine, gemcitabine, trifluridine,
ancitabine,
-19-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
enocitabine, azacitidine, doxifluridine, pentostatin, broxuridine,
capecitabine,
cladribine, decitabine, floxuridine, fludarabine, gougerotin, puromycin,
tegafur,
tiazofurin, adriamycin, cisplatin, carboplatin, cyclophosphamide, dacarbazine,
vinblastine, vincristine, mitoxantrone, bleomycin, mechlorethamine,
prednisone,
procarbazine, methotrexate, flurouracils, etoposide, taxol, taxol analogs,
platins such
as cis-platin and carbo-platin, mitomycin, thiotepa, taxanes, vincristine,
daunorubicin,
epirubicin, actinomycin, authramycin, azaserines, bleomycins, tamoxifen,
idarubicin,
dolastatins/auristatins, hemiasterlins, esperamicins and maytansinoids.
[0075] As used herein, the term "carrier or carrier molecule includes, but is
not
limited to: mono- and polyclonal antibodies and their chemically or
genetically
manipulated counterparts; their antigen-recognizing fragments and their
chemically
or genetically manipulated counterparts; small modular immunopharmaceuticals
(SMIPs) and their chemically or genetically manipulated counterparts;
nanobodies
and their chemically or genetically manipulated counterparts; soluble
receptors and
their chemically or genetically manipulated counterparts; growth factors and
their
chemically or genetically manipulated counterparts; aptamers; liposomes; non-
glycosylated proteins; and nanoparticles; aptamers; liposomes; non-
glycosylated
proteins; and nanoparticles
[0076] A "detectable label" refers to any substance or group of substances
which
is either directly or indirectly involved in the production of a detectable
signal and
may be covalently attached to any antibody or antigen used in any of the
immunoassays of this invention. Detectable labels conventionally used are, for
example, enzymatic, radioactive, fluorescent, chemiluminescent or
phosphorescent
labels. A preferred label is an enzyme label, and still more preferred is
horseradish
peroxidase (HRP) which results in a colorimetric assay upon addition of the
enzyme
substrate which, in the case of HRP, is a solution of hydrogen peroxide and
3,3',5.5'-
tetramehylbinzidine-2 HCI (TMB). HRP can be used in a direct manner when
covalently attached to an antibody or antigen that will specifically bind to
an already
bound captured analyte. HRP can also be added to an immunoassay in an indirect
manner when added as a secondary reagent, for example, if an antibody or
antigen
is labeled with biotin and avidin-HRP is subsequently added.
-20-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0077] The term "drug containing a carbohydrate moiety" as used herein
refers to any substance having biological or detectable activity, for example
therapeutic agents, detectable labels, binding agents, etc., and prodrugs,
which are
metabolized to an active agent in vivo. The term drug containing a
carbohydrate
moiety also includes drug derivates, wherein a drug containing a carbohydrate
moiety has been functionalized to enable conjugation with an antibody or
another
carrier molecule. Generally, these types of conjugates are referred to as
immunoconjugates. In addition, the term is meant to refer to a portion or
functional
fragment of a drug containing a carbohydrate moiety that is able to bind to
antibodies that specifically bind to the carbohydrate moiety when it is part
of the
drug or carrier-drug conjugate. Thus, as used herein, the term drug containing
a
carbohydrate moiety can be applied to the drug portion of a carrier-drug
conjugate
(e.g., a monoclonal carrier bound to calicheamicin) as well as the
calicheamicin
portion of a drug or the methylglycoside portion of the calicheamicin (i.e., S-
[(2R,4S,6S)-6-({[(2R,4S,5R)-5-({(2S,4S,5S)-5-[acetyl(ethyl)amino]-4-
m eth oxytetra hyd ro-2 H-pyra n-2-yl}oxy)-4-hyd roxy-6-m eth oxy-2-m
ethyltetra hyd ro-2 H-
pyran-3-yl]am ino}oxy)-4-hydroxy-2-methyltetrahydro-2H-pyran-3-yl]4-
{[(2S,3R,4R,5S,6S)-3,5-dihydroxy-4-methoxy-6-methyltetrahydro-2H-pyran-2-
yl]oxy}-3-iodo-5,6-d im ethoxy-2-methylbenzenecarbothioate).
[0078] A drug containing a carbohydrate moiety functionalized to enable
conjugation with an antibody as used herein is also meant to include the
"drug"
portion of a "carrier-drug conjugate". Examples of the antibodies, as part of
the
carrier-drug conjugate, are specific for CD22, 5T4, CD33 and Lewis-Y antigens.
These antibodies are specific for receptors that specifically binds to an
antigen
expressed on cancer cells. When reference is made to a drug containing a
carbohydrate moiety that "does not comprise a glycoprotein," such reference is
meant to include the situation wherein the carrier portion of the carrier-drug
conjugate
can include a glycoprotein.
[0079] Representative non-limiting examples of therapeutic drugs useful as
part
of a carrier-drug conjugate include cytotoxins, radioisotopes,
chemotherapeutic
-21-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
agents, immunomodulatory agents, anti-angiogenic agents, anti-proliferative
agents,
pro-apoptotic agents, and cytostatic and cytolytic enzymes (e.g., RNAses). A
drug
may also include a therapeutic nucleic acid, such as a gene encoding an
immunomodulatory agent, an anti-angiogenic agent, an anti-proliferative agent,
or a
pro-apoptotic agent. These drug descriptors are not mutually exclusive, and
thus a
therapeutic agent may be described using one or more of the above-noted terms.
For
example, selected radioisotopes are also cytotoxins. Therapeutic agents may be
prepared as pharmaceutically acceptable salts, acids or derivatives of any of
the
above. Generally, conjugates having a radioisotope as the drug are referred to
as
radioimmunoconjugates and those having a chemotherapeutic agent as the drug
are
referred to as chemoimmunoconjugates. Examples of suitable drugs for use in
immunoconjugates include the taxanes, maytansines, CC-1065 and the
duocarmycins, the calicheamicins and other enediynes, and the auristatins.
Other
examples include the anti-folates, vinca alkaloids, and the anthracyclines.
Plant
toxins, other bioactive proteins, enzymes (i.e., ADEPT), radioisotopes,
photosensitizers (i.e., for photodynamic therapy) can also be used in
immunoconjugates. In addition, conjugates can be made using secondary carriers
as
the cytotoxic agent, such as liposomes or polymers, for example.
[0080] An "immune response" is meant to refer to any response to an antigen or
antigenic determinant by the immune system of a vertebrate subject, including
humoral immune responses (e.g. production of antigen-specific antibodies) and
cell-
mediated immune responses (e.g. lymphocyte proliferation). Representative
immunomodulatory agents include cytokines, xanthines, interleukins,
interferons, and
growth factors (e.g., TNF, CSF, GM-CSF and G-CSF), and hormones such as
estrogens (diethylstilbestrol, estradiol), androgens (testosterone,
HALOTESTINO(fluoxymesterone)), progestins (MEGACEO (megestrol acetate),
PROVERAO (medroxyprogesterone acetate)), and corticosteroids (prednisone,
dexamethasone, hydrocortisone).
[0081] "Luminescent" labels include labels that involve phosphorescence,
chemiluminescence andfluorescence. "FluorescenY' label refers to a fluorophore
that
when covalently attached to a protein (or other compound) will, when excited
with
-22-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
short wavelengths, show optimal energy transfer between fluorophores and can
be
read at specific wavelengths, primarily ultraviolet. The most common
fluorescent
labels are derivatives of fluorescein and rohodamine, FITC and TRITC
respectively.
These fluorophores are covalently attached to antibodies or antigens and are
used
for detection of a specific binding event. A chemiluminescent label is one in
which it
is induced to emit light by a developing reagent causing the formation of an
excited
state molecule that decays, thereby emitting detectable light. The most
commonly
used chemiluminescent labels are: acrodinium, luminol and dioxetane.
Acrodinium
and luminol are excited by peroxidase enzyme reactions and can be used in
immunoassays that employ an HRP label. A phosphorescent label is one in which
there is an emission of light from a substance exposed to radiation and
persisting as
a glow after the excitatory radiation is removed. An example of a
phosphorescent
detectable label are derivatives of p-isothiocyanatophenyl Pt(ll)-and Pd(II)-
coproporphyrin I.
[0082] A radioactive label refers to a radionuclide that when covalently
attached
to a protein will decay, the presence of a binding event then determined by
the
detection of the radiation that is emitted. Common radionuclides used as
detectable
labels are: 3H, 1251 and 35S. The labels, listed above, are detected using a
detecting
system that is specific to the nature of each of the labels.
[0083] As used herein, the term "portion or functional fragment thereof when
used to identify a capture reagent, antibody, or drug containing a
carbohydrate
moiety is meant to include any portion of the compound in question that is
able to
undergo specific binding with a target partner in a manner the same or similar
to the
parent molecule.
[0084] As used herein, the terms "pre-mixing" or "pre-mixed" refer to mixing
of
two or more components of an assay prior to beginning the assay. For example,
"pre-adsorption of sera and additional carbohydrate" (or "preadsorption of the
sera
and additional carbohydrate") as used in the assays described herein would
means
pre-mixing or pre-incubation of the sera and additional carbohydrate prior to
mixing
-23-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
either the sera or additional carbohydrate with the drug containing a
carbohydrate
moiety.
[0085] The term "sandwich assay" refers to a non-competitive assay format that
provides a high level of sensitivity and specificity. The format is referred
to as a
sandwich due to the fact that the analyte, comprising a heterogeneous sample
of
different antibodies and antigens, is bound (sandwiched) between two highly
specific
reagents. The first specific reagent is a capture reagent that is a purified
antibody or
antigen that is bound to a solid phase typically attached to the bottom of a
plate well.
The heterogeneous analyte is exposed to the capture reagent and any
constituent of
the analyte having affinity for the capture reagent will specifically bind to
it. A
second, highly specific binding element is added. The binding element has a
covalently attached detectable label. The complex, usually antibody-antigen,
can
then be detected. In a non-competitive sandwich assay, the measurement of the
labeled binding event, is directly proportional to the amount of antigen
present in the
sample.
[0086] The term "specific binding" refers to an affinity between two
molecules, for
example specific binding between amolecule and its binding partner results in
preferential binding in a heterogeneous sample comprising the molecule and its
binding partner and one or more different molecules. Binding in IgG
antibodies, e.g.,
is generally characterized by an affinity of at least about 10"' M or higher,
such as at
least about 10$ M or higher, or at least about 10-9 M or higher, or at least
about 10"10
or higher, or at least about 10"" M or higher, or at least about 10"12 M or
higher. IgM
molecules are known in some instances to have affinities as low as 10-4
.
[0087] Representative anti-angiogenic agents include inhibitors of blood
vessel
formation, for example, famesyltransferase inhibitors, COX-2 inhibitors, VEGF
inhibitors, bFGF inhibitors, steroid sulphatase inhibitors (e.g., 2-
methoxyoestradiol
bis-sulphamate (2-MeOE2bisMATE)), interleukin-24, thrombospondin,
metallospondin proteins, class I interferons, interleukin 12, protamine,
angiostatin,
laminin, endostatin, and prolactin fragments.
-24-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0088] Anti-proliferative agents and pro-apoptotic agents include activators
of
PPAR-gamma (e.g., cyclopentenone prostaglandins (cyPGs)), retinoids,
triterpinoids
(e.g., cycloartane, lupane, ursane, oleanane, friedelane, dammarane,
cucurbitacin,
and limonoid triterpenoids), inhibitors of EGF receptor (e.g., HER4),
rampamycin,
CALCITRIOL® (1,25-dihydroxycholecalciferol (vitamin D)), aromatase
inhibitors
(FEMARA® (letrozone)), telomerase inhibitors, iron chelators (e.g., 3-
aminopyridine-2-carboxaldehyde thiosemicarbazone (Triapine)), apoptin (viral
protein
3--VP3 from chicken aneamia virus), inhibitors of Bcl-2 and Bcl-X(L), TNF-
alpha, FAS
ligand, TNF-related apoptosis-inducing ligand (TRAIUApo2L), activators of TNF-
alpha/FAS ligand/TNF-related apoptosis-inducing ligand (TRAIUApo2L) signaling,
and inhibitors of PI3K-Akt survival pathway signaling (e.g., UCN-01 and
geldanamycin).
[0089] In particular embodiments of the invention, the cytotoxic drug having a
carbohydrate moiety is an antibiotic such as a calicheamicin, also called the
LL-
E33288 complex, for example, gamma-calicheamicin,). See U.S. Pat. No.
4,970,198. Early studies with antibody conjugates of gamma calicheamicin
hydrazide
derivatives showed antigen-based cytotoxicity in vitro and activity in
xenograft
experiments. Stabilizing the disulfide bond that is present in all
calicheamicin
conjugates by adding dimethyl substituents made additional improvements.
Additional examples of calicheamicins suitable for use in preparing
antibody/drug
conjugates of the invention are disclosed in U.S. Pat. Nos. 4,671,958;
5,053,394;
5,037,651; 5,079,233; and 5,108,912; which are incorporated herein in their
entirety.
These compounds contain a methyltrisu[fide that may be reacted with
appropriate
thiols to form disulfides, at the same time introducing a functional group
such as a
hydrazide or other functional group that is useful for conjugating
calicheamicin to an
antibody. Stabilizing the disulfide bond that is present in all calicheamicin
conjugates
by adding dimethyl substituents made additional improvements. This led to the
choice of N-acetyl gamma calicheamicin dimethyl hydrazide, or NAc-gamma DMH,
as one of the optimized derivatives for conjugation. Disulfide analogs of
calicheamicin can also be used, for example, analogs described in U.S. Pat.
Nos.
5,606,040 and 5,770,710, which are incorporated herein in their entirety.
-25-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[0090] Representative methods for preparing antibody/drug conjugates include
those described for preparation of CMC-544 in U.S. patent application
Publication
No. 2004-0082764A1 and U.S. patent application Publication No. 2004-0192900,
which are incorporated herein in their entirety. Conjugation may be performed
using
the following conditions: 10 mg/mI antibody, 8.5% (w/w) calicheamicin
derivative,
37.5 mM sodium decanoate, 9% (v/v) ethanol, 50 mM HEPBS (N-(2-
Hydroxyethyl)piperazine-N'-(4-butanesulfonic acid)), pH 8.5, 32° C., 1
hour.
Hydrophobic interaction chromatography (HIC) may be performed using a butyl
sepharose FF resin, 0.65 M potassium phosphate loading buffer, 0.49 M
potassium
phosphate wash buffer, and 4 mM potassium phosphate elution buffer. Buffer
exchange may be accomplished by size exclusion chromatography,
ultrafiltration/diafiltration, or other suitable means.
[0091] As part of the carrier-drug conjugates, mentioned above, N-acetyl gamma
dimethyl hydrazide was conjugated to monoclonal antibodies that specifically
bind to
the CD22 receptor, the 5T4 receptor and the Lewis-Y antigen, all expressed on
cancer cells. These carrier-drug conjugates, as part of the N-acetyl gamma
dimethyl
hydrazide molecule, have methylglycoside (i.e., S-[(2R,4S,6S)-6-({[(2R,4S,5R)-
5-
({(2S,4S,5S)-5-[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-4-
hydroxy-6-methoxy-2-methyltetrahydro-2H-pyran-3-yl]am ino}oxy)-4-hydroxy-2-
methyltetrahydro-2H-pyran-3-yl]4-{[(2S,3R,4R,5S,6S )-3,5-dihydroxy-4-methoxy-6-
methyltetra hyd ro-2H-pyran-2-yl]oxy}-3-iodo-5,6-d imethoxy-2-
methylbenzenecarbothioate) a carbohydrate moiety. This carbohydrate, along
with
other carbohydrates, can interact with endogenous antibodies in serum that are
directed against general carbohydrate moieties.
[0092] All patents, patent applications, and other literature cited herein are
hereby incorporated by reference in their entirety.
[0093] The present invention is further illustrated and supported by the
following
examples. However, these examples should in no way be considered to further
limit
the scope of the invention. To the contrary, one having ordinary skill in the
art would
readily understand that there are other embodiments, modifications, and
equivalents
-26-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
of the present invention without departing from the spirit of the present
invention
and/or the scope of the appended claims.
EXAM PLES
EXAMPLE 1
INTERFERENCE OF SIGNAL IN NAIVE SERA
[0094] During the development of anti-calicheamicin conjugate antibody assays,
initial studies were undertaken on naive human or monkey sera. An ELISA was
performed using calicheamicin as a capture reagent immobilized in the wells of
a
microtiter assay plate and blocked in 4% NFDM/PBST buffer (Non Fat Dry
Milk/Phosphate Buffered Saline Tween; 137mM NaCI, 2.7mM KCI, 4.3 mM
Na2HPO4, I mM KH2PO4, pH7.2). The human or monkey sera was diluted in 1%
NFDM/PBST, added to ELISA wells, and then incubated. The wells were washed in
THST (50 mM Tris-HCI, 0.5 M NaCI, 1 mM glycine and 0.05% (v/v)Tween 20, pH 8;)
and incubated with an anti-species-HRP conjugate. The plates were washed in
THST
before developed for a color reaction by the addition of the TMB
(tetramethylbenzidine) reagent.
[0095] The data during these initial assays revealed a high, interfering
background signal observed after developing the reaction. In subsequent assays
large dilutions, up to 1:160, were made on the sera added to the wells. The
background in these assays decreased, as did the sensitivity of the assay. The
data
suggested that there was an element in the serum contributing to the
interference of
specific signal in the assay wells. This high background was observed in
approximately one-third of the human and 1/3 of the monkey sera that was
tested.
[0096] In order to determine what portion of the calicheamicin conjugate was
interacting with the interfering serum element, a bridging-type ELISA was
perfonned.
Calicheamicin conjugates, CMC-544, CMD-193 and CME-548 and their parental,
-27-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
unconjugated antibodies, anti-CD22 humanized monoclonal antibody G544, anti-
Lewis-Y humanized monoclonal G193 and anti-5T4 humanized monoclonal antibody
huH8 respectively, were immobilized on plates at a concentration of 100 ng/ml,
and
blocked in 4% NFDM/PBST.
[0097] CMD-544 is a calicheamicin-monoclonal antibody drug conjugate
described in U.S. patent application US2004082764. The monoclonal antibody
portion of this carrier drug conjugate has a target specificity for human CD22
antigen,
with the parental antibody designated herein as "G-544."
[0098] CMD-193 is a calicheamicin-monoclonal antibody drug conjugate
described in U.S. patent application Publication No. US20060002942. The
monoclonal antibody portion of this carrier drug conjugate has a target
specificity for
Lewis Y antigen, with the parental antibody designated herein as "G-193."
[0099] CME-548 is a calicheamicin-monoclonal antibody drug conjugate
described in U.S. patent application Publication No. US2006008522. The
monoclonal antibody portion of this carrier drug conjugate has a target
specificity for
human 5T4 receptor antigen, with the parental antibody designated herein as
"huH8."
[0100] Pooled cynomolgus monkey serum was diluted 1:20 in 1% NFDM/PBST
and incubated in wells. Other wells were incubated in buffer without serum as
controls. After washing in THST, the assay plate was incubated with
calicheamicin-
HRP conjugate diluted 1:2000 in PBST. The wells were washed in THST before
development with the TMB reagent.
[0101] As seen in Figure 1, there was high background observed only in wells
containing the calicheamicin conjugate and not the parental antibody,
indicating that
the serum element specifically bound only the conjugates, but not their
parental
antibodies, and "bridged" them to the calicheamicin-HRP. This data suggested
that
the interfering element in the serum is binding to the calicheamicin moiety
and not to
the parental unconjugated antibody. Additionally, the ability of the serum
element to
-28-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
bridge two different calicheamicin moieties, the calicheamicin conjugated
antibodies
and the calicheamicin-HRP, suggested a multivalent molecule, namely an
immunoglobulin as the interfering serum element.
EXAMPLE 2
REDUCTION OF THE INTERFERENCE BY USING ADDITIONAL
CARBOHYDRATES
[0102] In order to determine the nature of the interaction between the
interfering
element in the sera and the calicheamicin, a bridging-type ELISA was
performed.
Calicheamicin has, as part of the molecule, a carbohydrate moiety that could
be
bound by pre-existing anti-carbohydrate antibodies in some serum samples. CMC-
544 was used as a capture reagent, immobilized in assay wells and then blocked
in
4% NFDM/PBST buffer. Sera used in the previously described assays (na7ve) were
diluted in 1 % NFDM/PBST and added to the wells. In an attempt to compete out
any
interactions with the calicheamicin carbohydrates, detoxified
lipopolysaccharide
(LPS) was titrated into the sera in concentrations ranging from 1 ng/ml to 100
ng/ml.
Control wells contained no LPS. The assay was then incubated for 1 hour with
shaking (standard unless otherwise indicated for all examples herein). The
wells
were washed in THST and then incubated with calicheamicin-HRP conjugate at a
dilution of 1:2000 in PBST. The assay was then developed for a color reaction
by the
addition of TMB reagent. As shown in Figure 3, increasing amounts of LPS added
to
the CMC-544 wells caused a decrease in background signal. In the control wells
containing CMC-544 without LPS, there was high background observed in all
samples.
[0103] Similar tests were done with a secondary ELISA, wherein the CMC-544
monoclonal antibody. Antibody was immobilized in assay wells and then blocked
in
4% NFDM/PBST buffer. Sera used in the previously described assays (naive) were
diluted in 1% NFDM/PBST and added to the wells. Interfering amounts of either
the
detoxified portion of LPS or LPS were added to the reaction. CMC-544(biotin)
that
bound to the CMC-544 on plates was bridged via an antibody in the serum. The
-29-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
CMC-544 biotin was detected with Strepravidin bound to an HRP detector.
Incresaing amounts of LPS and detoxified LPS interfered with this specific
binding.
Results are shown in Figure 5.
[0104] These data suggested that the interfering element in the serum
interacted
with the carbohydrate moiety on calicheamicin and not the antibody portion of
the
conjugate. The addition of the LPS was able to compete with the carbohydrate
on the
calicheamicin for the interfering serum element without the need for pre-
adsorption of
the sera with the LPS.
[0105] A further study was performed to demonstrate the specificity of the
serum
element for the calicheamicin carbohydrate moiety. Exogenously added methyl
glycosideS-[(2R,4S,6S)-6-({[(2R,4S,5R)-5-({(2S,4S,5S)-5-[acetyl(ethyl)amino]-4-
m ethoxytetra hyd ro-2 H-pyra n-2-yl}oxy)-4-hyd roxy-6-m ethoxy-2-m ethyltetra
hyd ro-2 H-
pyran-3-yl]am ino}oxy)-4-hydroxy-2-methyltetrahydro-2H-pyran-3-yl]4-
{[( 2 S, 3 R, 4 R, 5S , 6 S)-3 , 5-d i h yd roxy-4-m eth oxy-6-m eth yltetra h
yd ro-2 H-py ra n-2-
yl]oxy}-3-iodo-5,6-dimethoxy-2-methylbenzenecarbothioate) was tested for its
ability
to specifically compete out any interactions with the methylglycoside moiety
on
calicheamicin and therefore to inhibit the interaction with the serum element.
The
same assay, as described above for the addition of LPS was performed in
separate
wells containing CMD-193, CME-548 and CMC-544. Their parental, unconjugated
antibodies were used as a control. The above methylglycoside was substituted
in this
assay for the LPS at various final concentrations ranging from 2 to 2000ng per
ml.
As shown in Figure 4, the above indicated methyl glycoside was proven to be a
potent inhibitor of the interfering element in the sera tested, with an IC50
in the range
of 30-70 nM. These data suggest that the interfering element in sera
specifically
binds to the carbohydrate methylglycoside S-[(2R,4S,6S)-6-({[(2R,4S,5R)-5-
({(2S,4S,5S)-5-[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-4-
hydroxy-6-methoxy-2-methyltetrahydro-2H-pyran-3-yl]am ino}oxy)-4-hydroxy-2-
methyltetrahyd ro-2H-pyran-3-yl]4-{[(2S, 3R,4R, 5S,6S )-3, 5-dihyd roxy-4-
methoxy-6-
m ethyltetra hyd ro-2 H-pyra n-2-yl]oxy}-3-iod o-5, 6-d im ethoxy-2-
methylbenzenecarbothioate) present on calicheamicin.
-30-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
EXAMPLE 3
SERUM IgG IS NOT THE INTERFERING FACTOR
[0106] As mentioned previously, the bridging capability of the factor
indicated an
immunoglobulin. Both IgG and IgM were initially examined as possibilities. For
IgG,
protein A resin was used to purify the IgG fraction from monkey serum known to
contain the interfering factor. SDS-PAGE indicated successful isolation of IgG
(not
shown; L38054-17). Both the IgG-enriched and IgG-depleted fractions of the
serum
were tested for bridging capability after adjusting concentrations to account
for
inevitable dilution of sample during the batch-binding chromatography process.
[0107] The results demonstrated that the purified IgG fractions showed no
bridging activity over the buffer-only background, and the IgG-depleted
fraction
showed the same level of LPS-sensitive bridging activity as untreated serum.
Therefore, IgG does not appear to be responsible for the activity..
EXAMPLE 4
IDENTIFYING SERUM FACTOR AS IGM
[0108] To determine the nature of the interfering molecule in the serum
binding to
the carbohydrate moiety of calicheamicin that could be responsible for the
interference observed in the previously described assays, a bridging-type
ELISA was
performed. Assay wells were coated with the capture reagents: CMC-544, its
unconjugated parental antibody G544, or monkey IgM as the positive control.
Milk
was used as the negative control. The wells were then blocked in 4% NFDM/PBST
buffer. Serum was diluted in 1% NFDM/PBST and added to the wells. LPS was
added to the serum at a concentration of 250 pg/mI and incubated. The wells
were
washed in THST and incubated with anti-IgM-HRP to detect any IgM bound to the
plates. The assay was developed for a color reaction by the addition of the
TMB
reagent. As shown in Figure 6, CMC-544 captured large amounts of IgM from
serum,
-31 -
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
with significantly less signal obtained in the presence of lipopolysaccharide.
Very little
IgM bound to the parental unconjugated antibody G544.
[0109] In an additional assay, wells were coated with anti-monkey IgM. Anti-
mouse IgG was used as a negative control. Dilutions of sera, made in PBST,
ranging
from .001 to 10% were made and added to the wells. After incubation,
calicheamicin-
HRP was added. The plates were washed and developed in the TMB reagent. As
shown in Figure 7, the serum IgM captured on the plate bound the calicheamicin-
HRP in a dose dependent fashion. The wells containing anti-mouse IgG did not.
[0110] These data demonstrate that IgM present in some sera has the ability to
specifically bind to the carbohydrate portion of the calicheamicin molecule.
Such
binding can give rise to high background signal in assays used to detect
antibodies
against calicheamicin conjugates or detecting calicheamicin itself. The
addition of
exogenously added carbohydrates to the assay will aid in the abrogation of the
signal
generated by the presence of the interfering IgM in the serum.
EXAMPLE 5
NAIVE SERUM CONTAINING ANTI-CARBOHYDRATE IGM
[0111] In order to determine the prevalence of anti-carbohydrate antibodies in
a
random panel of naive human sera, a bridge-type ELISA was performed. CMC-544
was used as a capture reagent and immobilized in the assay wells. The wells
were
blocked in 4% NFDM/PBST buffer. The naive serum samples were diluted in 1%
NFDM/PBST, added to the wells, and then incubated. The wells were washed in
THST and then incubated with calicheamicin-HRP. The assay was then developed
for a color reaction by the addition of TMB reagent. No carbohydrate was added
to
this assay in an effort to determine what percentage of the serum samples
examined
contained interfering IgM that could bind to the carbohydrate moiety on
calicheamicin. As shown in Figure 8, approximately 30% of the serum samples
tested showed interference. Similar observations were noted in naive monkey
sera,
-32-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
where 2 out of 20 monkeys showed a high level of anti-carbohydrate antibodies
and
8 out of 20 showed significant levels of anti-carbohydrate antibodies.
EXAMPLE 6
DETECTION OF ANTI CALICHEAMICIN CONJUGATE
ANTIBODIES IN SERUM
[0112] In order to detect the presence of antibodies in serum specifically
generated in response to exposure to a calicheamicin-conjugated monoclonal
antibody, for example CMC-544, a bridging-type ELISA is performed after the
optimal
concentration of methyl glycoside S-[(2R,4S,6S)-6-({[(2R,4S,5R)-5-({(2S,4S,5S)-
5-
[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-4-hydroxy-6-
methoxy-2-
m ethyltetra hyd ro-2 H-pyra n-3-yl]a m i no}oxy)-4-hyd roxy-2-m ethyltetra
hyd ro-2 H-pyra n-
3-yI]4-{[(2S , 3 R,4 R, 5S , 6S )-3, 5-d i hyd roxy-4-m ethoxy-6-m ethyltetra
hyd ro-2 H-pyra n-2-
yl]oxy}-3-iodo-5,6-dimethoxy-2-methylbenzenecarbothioate) present on
calicheamicin
is determined.
[0113] Naive sera is titrated as above to determine the minimum (and thus
optimum) concentration of carbohydrate that will remove endogenous anti-
carbohydrate background noise from the naive sera from a subject being tested
for
production of anti-drug antibody after exposure to the drug.
[0114] Experimental subjects are injected with or otherwise exposed to CMC-544
and serum is collected. CMC-544, as well as the unconjugated parental antibody
G544, are used as capture reagents and immobilized in separate wells at a
concentration of 100 ng/ml. The wells are blocked in 4% NFDM/PBST buffer.
Serum
is collected from the exposed subjects and mixed with 1% NFDM/PBST, added to
the
wells, and then incubated. The wells are washed in THST and incubated with
calicheamicin-HRP conjugate and the optimal concentration of additional
carbohydrate methyl glycoside (S-[(2R,4S,6S)-6-({[(2R,4S,5R)-5-({(2S,4S,5S)-5-
-33-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-4-hydroxy-6-
methoxy-2-
methyltetrahydro-2H-pyran-3-yl]am ino}oxy)-4-hyd roxy-2-methyltetrahydro-2H-
pyran-
3-yI]4-{[(2S,3R,4R, 5S,6S)-3,5-d i hydroxy-4-m ethoxy-6-m ethyltetra hyd ro-2
H-pyran-2-
yI]oxy}-3-iodo-5,6-dimethoxy-2-methylbenzenecarbothioate) present on
calicheamicin). Alternatively, the additional carbohydrate can be added
directly to
the wells with the serum in the initial incubation with the capture reagent
and control
wells. Following washing, plates are then developed for a color reaction by
the
addition of the TMB reagent. The presence of a color reaction after
development will
indicate the presence of antibodies against a calicheamicin conjugate in the
serum
that are not a result of endogenous anti-carbohydrate antibody but are instead
a
result of the production of an immune reaction in response to the carrier-drug
conjugate.
[0115] To determine if the antibody response is generated against the
carbohydrate portion of calicheamicin, the sera from an exposed subject is
then
titrated with the calicheamicin-specific methyl glycoside to determine if the
antibody
reactivity returns to background levels. If the antibody response is titrated
back to
background levels, then the antibody response generated in response to the
carrier-
drug conjugate is due to the carbohydrate moiety of the calicheamicin.
EXAMPLE 7
TESTING OF NAIVE SERUM FOR PRESENCE OF ANTIBODY
TO A CARRIER DRUG CONJUGATE WHEREIN THE DRUG HAS A
CARBOHYDRATE MOIETY
[0116] In order to determine the presence of pre-existing interfering IgM in
the
serum from a candidate subject for treatment with a calicheamicin conjugate, a
bridging- type ELISA is performed. Calicheamicin is used as a capture reagent
and
immobilized in the assay wells. The wells are blocked in 4% NFDM/PBST buffer.
Serum, collected from the drug naive subject is diluted in 1% NFDM/PBST, added
to
-34-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
the assay and incubated. The optimum concentration of methyl glycoside is
determined as described above. A titration of carbohydrates can also include a
no
carbohydrate control test for each serum sample. The wells are washed in THST
and
incubated with calicheamicin-HRP conjugate and the optimal concentration of
additional methyl glycoside (S-[(2R,4S,6S)-6-({[(2R,4S,5R)-5-({(2S,4S,5S)-5-
[acetyl(ethyl)am ino]-4-methoxytetrahydro-2H-pyran-2-yl}oxy)-4-hydroxy-6-
methoxy-2-
methyltetrahydro-2H-pyran-3-yl]am ino}oxy)-4-hydroxy-2-methyltetrahydro-2H-
pyran-
3-yl]4-{[(2S,3R,4R, 5S,6S)-3,5-d ihydroxy-4-methoxy-6-methyltetra hydro-2H-
pyra n-2-
yl]oxy}-3-iodo-5,6-dimethoxy-2-methylbenzenecarbothioate) present on
calicheamicin). Following washing, plates are then developed for a color
reaction by
the addition of the TMB reagent. A color reaction would indicate Ig bound to
the
calicheamicin bound in the wells and also bound to the calicheamicin HRP
subsequently added.
[0117] Alternatively, an initial screen of naive sera would have no additional
carbohydrate added. Sera would then be subjected to a titration of
carbohydrate to
verify that carbohydrates are the target of the sera reactivity.
EXAMPLE 8
TESTING FOR CARRIER-DRUG CONJUGATE WITH
CALICHEAMICN AS THE DRUG
[0118] In order to detect a calicheamicin conjugate, for example CMC-544, in
serum from subjects administered this drug, an ELISA is performed. Subjects
are
injected with or otherwise exposed to CMC-544 and serum is collected. CD22,
the
antigen that is specifically bound by parental antibody G544, is immobilized
in assay
wells and used as a capture reagent. The wells are then blocked in 4%
NFDM/PBST
buffer. Serum is diluted in 1% NFDM/PBST and added to the wells, and then
incubated. Since the additional carbohydrate should not interfere with capture
of
CMC-544 by CD22-specific binding, a vast excess of carbohydrate can be added
-35-
CA 02686206 2009-11-03
WO 2008/137885 PCT/US2008/062739
here to block antibody present in the sera that might bind to the CMC-544.
(The
plate is washed in THST and incubated with anti-calicheamicin-HRP conjugate
and
the plates are then developed for a color reaction by the addition of the TMB
reagent.
A color reaction would indicate the presence of the calicheamicin conjugate in
the
sera.
-36-