Note: Descriptions are shown in the official language in which they were submitted.
CA 02686565 2015-04-14
- I -
UTILIZATION OF LOW BTU GAS GENERATED DURING
IN SITU HEATING OF ORGANIC-RICH ROCK
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Patent
Application
Serial No. 60/931,820, filed May 25, 2007. That application is titled
"Utilization of Low
BTU Gas Generated During In Situ Heating of Organic-Rich Rock".
[0002] This application is related to co-pending, concurrently filed, and
commonly
assigned U. S. Patent Application [Attorney Docket No. 2007EM146] entitled "A
Process for
Producing Hydrocarbon Fluids Combining In Situ Heating, a Power Plant and a
Gas Plant",
which claims the benefit of U. S. Provisional Patent Application Serial No.
60/931,940, filed
May 25, 2007.
BACKGROUND OF THE INVENTION
Field of the Invention
[0003] The present invention relates to the field of hydrocarbon recovery
from
subsurface formations. More specifically, the present invention relates to in
situ recovery of
hydrocarbon fluids from organic-rich rock formations, including, for example,
oil shale
formations, coal formations and tar sands formations.
Background of the Invention
[0004] Certain geological formations are known to contain an organic matter
known
as "kerogen." Kerogen is a solid, carbonaceous material. When kerogen is
imbedded in rock
formations, the mixture is referred to as oil shale. This is true whether or
not the mineral is,
in fact, technically shale, that is, a rock formed from compacted clay.
[0005] Kerogen is subject to decomposing upon exposure to heat over a
period of
time. Upon heating, kerogen molecularly decomposes to produce oil, gas, and
carbonaceous
coke. Small amounts of water may also be generated. The oil, gas and water
fluids are
mobile within the rock matrix, while the carbonaceous coke remains essentially
immobile.
[0006] Oil shale formations are found in various areas world-wide,
including the
United States. Oil shale formations tend to reside at relatively shallow
depths. In the United
States, oil shale is most notably found in Wyoming, Colorado, and Utah. These
formations
CA 02686565 2015-04-14
- 2 -
are often characterized by limited permeability. Some consider oil shale
formations to be
hydrocarbon deposits which have not yet experienced the years of heat and
pressure thought
to be required to create conventional oil and gas reserves.
[0007] The decomposition rate of kerogen to produce mobile hydrocarbons is
temperature dependent. Temperatures generally in excess of 270 C (518 F)
over the course
of many months may be required for substantial conversion. At higher
temperatures
substantial conversion may occur within shorter times. When kerogen is heated,
chemical
reactions break the larger molecules forming the solid kerogen into smaller
molecules of oil
and gas. The thermal conversion process is referred to as pyrolysis or
retorting.
[0008] Attempts have been made for many years to extract oil from oil shale
formations. Near-surface oil shales have been mined and retorted at the
surface for over a
century. In 1862, James Young began processing Scottish oil shales. The
industry lasted for
about 100 years. Commercial oil shale retorting through surface mining has
been conducted
in other countries as well such as Australia, Brazil, China, Estonia, France,
Russia, South
Africa, Spain, and Sweden. However, the practice has been mostly discontinued
in recent
years because it proved to be uneconomical or because of environmental
constraints on spent
shale disposal. (See T.F. Yen, and G.V. Chilingarian, "Oil Shale," Amsterdam,
Elsevier, p.
292.) Further, surface retorting requires mining of the oil shale, which
limits application to
very shallow formations.
[0009] In the United States, the existence of oil shale deposits in
northwestern
Colorado has been known since the early 1900's. While research projects have
been
conducted in this area from time to time, no serious commercial development
has been
undertaken. Most research on oil shale production has been carried out in the
latter half of
the 1900's. The majority of this research was on shale oil geology,
geochemistry, and
retorting in surface facilities.
[0010] In 1947, U.S. Pat. No. 2,732,195 issued to Ljungstrom. That patent,
entitled
"Method of Treating Oil Shale and Recovery of Oil and Other Mineral Products
Therefrom,"
proposed the application of heat at high temperatures to the oil shale
formation in situ to
distill and produce hydrocarbons.
[0011] Ljungstrom coined the phrase "heat supply channels" to describe bore
holes
drilled into the formation. The bore holes received an electrical heat
conductor which
CA 02686565 2015-04-14
- 3 -
transferred heat to the surrounding oil shale. Thus, the heat supply channels
served as heat
injection wells. The electrical heating elements in the heat injection wells
were placed within
sand or cement or other heat-conductive material to permit the heat injection
well to transmit
heat into the surrounding oil Aale while preventing the inflow of fluid.
According to
Ljungstrom, the "aggregate" was heated to between 5000 and 1,000 C in some
applications.
[0012] Along with the heat injection wells, fluid producing wells were also
completed
in near proximity to the heat injection wells. As kerogen was pyrolyzed upon
heat
conduction into the rock matrix, the resulting oil and gas would be recovered
through the
adjacent production wells.
[0013] Ljungstrom applied his approach of thermal conduction from heated
wellbores
through the Swedish Shale Oil Company. A full scale plant was developed that
operated
from 1944 into the 1950s. (See G. Salamonsson, "The Ljungstrom In Situ Method
for Shale-
Oil Recovery," 2nd Oil Shale and Cannel Coal Conference, v. 2, Glasgow,
Scotland, Institute
of Petroleum, London, p. 260-280 (1951).)
[0014] Additional in situ methods have been proposed. These methods
generally
involve the injection of heat and/or solvent into a subsurface oil shale. Heat
may be in the
form of heated methane (see U.S. Pat. No. 3,241,611 to J.L. Dougan), flue gas,
or
superheated steam (see U.S. Pat. No. 3,400,762 to D.W. Peacock). Heat may also
be in the
form of electric resistive heating, dielectric heating, radio frequency (RF)
heating (U.S. Pat.
No. 4,140,180, assigned to the ITT Research Institute in Chicago, Illinois) or
oxidant
injection to support in situ combustion. In some instances, artificial
permeability has been
created in the matrix to aid the movement of pyrolyzed fluids. Permeability
generation
methods include mining, rubblization, hydraulic fracturing (see U.S. Pat. No.
3,468,376 to
M.L. Slusser and U.S. Pat. No. 3,513,914 to J. V. Vogel), explosive fracturing
(see U.S. Pat.
No. 1,422,204 to W. W. Hoover, et al.), heat fracturing (see U.S. Pat. No.
3,284,281 to R.W.
Thomas), and steam fracturing (see U.S. Pat. No. 2,952,450 to H. Purre).
[0015] In 1989, U.S. Pat. No. 4,886,118 issued to Shell Oil Company. That
patent, entitled "Conductively Heating a Subterranean Oil Shale to Create
Permeability and Subsequently Produce Oil," declared that "[c]ontrary to the
implications
of . . . prior teachings and beliefs ... the presently described conductive
heating
process is economically feasible for use even in a substantially
CA 02686565 2015-04-14
- 4 -
impermeable subterranean oil shale." (col. 6, In. 50-54). Despite this
declaration, it is noted
that few, if any, commercial in situ shale oil operations have occurred other
than
Ljungstrom's application. The '118 patent proposed controlling the rate of
heat conduction
within the rock surrounding each heat injection well to provide a uniform heat
front.
[0016] Additional history behind oil shale retorting and shale oil recovery
can be
found in co-owned patent publication WO 2005/010320 entitled "Methods of
Treating a
Subterranean Formation to Convert Organic Matter into Producible
Hydrocarbons," and in
patent publication WO 2005/045192 entitled "Hydrocarbon Recovery from
Impermeable Oil
Shales."
[0017] A need exists for improved processes for the production of shale
oil. In
addition, a need exists for improved methods of producing shale oil with
improved
propertics. Further, a need exists for a process that is able to utilize low
quality gas and/or gas
with a changing quality over time that is produced from in situ heating.
Further, a need exists
for a process that is able to generate electricity from a low quality gas
and/or gas with a
changing quality over time that is produced from in situ heating.
SUMMARY OF THE INVENTION
[0018] In one embodiment, the invention includes a method for utilizing gas
produced
from an in situ conversion process. The method includes heating an organic-
rich rock
formation in situ and producing a production fluid from the organic-rich rock
formation,
where the production fluid is at least partially generated as a result of
pyrolysis of formation
hydrocarbons located in the organic-rich rock formation. The production fluid
may include
hydrocarbon fluids. The method may further include obtaining a gas stream from
the
production fluid, where the gas stream includes a combustible hydrocarbon
fluid. The
method may further include separating the gas stream into a first composition
gas stream and
a second composition gas stream, where the composition of the first
composition gas stream
is maintained in a substantially constant condition and the first composition
gas stream has a
lower heating value less than 800 BTU/SCF. The method may further include
passing the
first composition gas stream through a first gas turbine to form a first gas
turbine exhaust
stream, where the first gas turbine is configured to provide energy to a first
electrical
generator.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 5 -
[0019] In one embodiment, the invention includes a method for utilizing
gas produced
from an in situ conversion process. The method includes heating an organic-
rich rock
formation in situ and producing a production fluid from the organic-rich rock
formation,
where the production fluid is at least partially generated as a result of
pyrolysis of formation
hydrocarbons located in the organic-rich rock formation. The production fluid
may include
hydrocarbon fluids. The method may include obtaining a gas stream from the
production
fluid, where the gas stream includes a combustible hydrocarbon fluid and the
Wobbe Index of
the gas stream changes over time. The method may further include separating
the gas stream
into a first composition gas stream and a second composition gas stream, where
the Wobbe
Index of the first composition gas stream is maintained substantially constant
and the first
composition gas stream has a lower heating value less than 800 BTU/SCF. The
method may
further include passing the first composition gas stream through a first gas
turbine to form a
first gas turbine exhaust stream, the first gas turbine being configured to
provide energy to a
first electrical generator.
[0020] In one embodiment, the invention includes a method for producing a
hydrocarbon fluid. The method includes heating an organic-rich rock formation
in situ and
producing a production fluid from the organic-rich rock formation, where the
production
fluid is at least partially generated as a result of pyrolysis of formation
hydrocarbons located
in the organic-rich rock formation. The production fluid may include
hydrocarbon fluids.
The method may further include obtaining a gas stream from the production
fluid, where the
gas stream includes a combustible hydrocarbon fluid. The method may further
include
separating the gas stream into a first composition gas stream and a second
composition gas
stream, where the composition of the first composition gas stream is
maintained in a
substantially constant condition and the first composition gas stream has a
lower heating
value less than 800 BTU/SCF. The method may further include passing the first
composition
gas stream through a first gas turbine to form a first gas turbine exhaust
stream, where the
first gas turbine is configured to provide energy to a first electrical
generator.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] So that the manner in which the features of the present invention
can be better
understood, certain drawings, graphs and flow charts are appended hereto. It
is to be noted,
however, that the drawings illustrate only selected embodiments of the
inventions and are
therefore not to be considered limiting of scope, for the inventions may admit
to other equally
effective embodiments and applications.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 6 -
[0022] Figure 1 is a cross-sectional isomeric view of an illustrative
subsurface area.
The subsurface area includes an organic-rich rock matrix that defines a
subsurface formation.
[0023] Figure 2 is a flow chart demonstrating a general method of in
situ thermal
recovery of oil and gas from an organic-rich rock formation, in one
embodiment.
[0024] Figure 3 is a cross-sectional side view of an illustrative oil
shale formation that
is within or connected to groundwater aquifers and a formation leaching
operation.
[0025] Figure 4 is a plan view of an illustrative heater well pattern,
around a
production well. Two layers of heater wells are shown.
[0026] Figure 5 is a bar chart comparing one ton of Green River oil
shale before and
= after a simulated in situ, retorting process.
[0027] Figure 6 is a process flow diagram of exemplary surface
processing facilities
for a subsurface formation development.
[0028] Figure 7 is a graph of the weight percent of each carbon number
pseudo
component occurring from C6 to C38 for laboratory experiments conducted at
three different
stress levels.
[0029] Figure 8 is a graph of the weight percent ratios of each carbon
number pseudo
component occurring from C6 to C38 as compared to the C20 pseudo component for
laboratory experiments conducted at three different stress levels.
[0030] Figure 9 is a graph of the weight percent ratios of each carbon
number pseudo
component occurring from C6 to C38 as compared to the C25 pseudo component for
laboratory experiments conducted at three different stress levels.
[0031] Figure 10 is a graph of the weight percent ratios of each
carbon number
pseudo component occurring from C6 to C38 as compared to the C29 pseudo
component for
laboratory experiments conducted at three different stress levels.
[0032] Figure 11 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 for laboratory experiments
conducted
at three different stress levels.
[0033] Figure 12 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 as compared to the normal-C20
hydrocarbon compound for laboratory experiments conducted at three different
stress levels.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 7 -
[0034] Figure 13 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 as compared to the normal-C25
hydrocarbon compound for laboratory experiments conducted at three different
stress levels.
[0035] Figure 14 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 as compared to the normal-C29
hydrocarbon compound for laboratory experiments conducted at three different
stress levels.
[0036] Figure 15 is a graph of the weight ratio of normal alkane
hydrocarbon
compounds to pseudo components for each carbon number from C6 to C38 for
laboratory
experiments conducted at three different stress levels.
[0037] Figure 16 is a bar graph showing the concentration, in molar
percentage, of the
hydrocarbon species present in the gas samples taken from duplicate laboratory
experiments
conducted at three different stress levels.
[0038] Figure 17 is an exemplary view of the gold tube apparatus used in
the
unstressed Parr heating test described in Example 1.
[0039] Figure 18 is a cross-sectional view of the Parr vessel used in
Examples 1-5.
[0040] Figure 19 is gas chromatogram of gas sampled from Example 1.
[0041] Figure 20 is a whole oil gas chromatogram of liquid sampled from
Example 1.
[0042] Figure 21 is an exemplary view of a Berea cylinder, Berea plugs,
and an oil
shale core specimen as used in Examples 2-5.
[0043] Figure 22 is an exemplary view of the mini load frame and sample
assembly
used in Examples 2-5.
[0044] Figure 23 is gas chromatogram of gas sampled from Example 2.
[0045] Figure 24 is gas chromatogram of gas sampled from Example 3.
[0046] Figure 25 is a whole oil gas chromatogram of liquid sampled from
Example 3.
[0047] Figure 26 is gas chromatogram of gas sampled from Example 4.
[0048] Figure 27 is a whole oil gas chromatogram of liquid sampled from
Example 4.
[0049] Figure 28 is gas chromatogram of gas sampled from Example 5.
[0050] Figure 29 is a process flow diagram of exemplary processing
facilities that
may be used in some embodiments of the invention.
[0051] Figure 30 is an alternative process flow diagram of exemplary
processing
facilities that may be used in some embodiments of the invention.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 8 -
[0052] Figure 31 is an alternative process flow diagram of exemplary
processing
facilities that may be used in some embodiments of the invention.
[0053] Figure 32 is a graph of several gaseous species evolved from
laboratory
heating of Colorado oil shale. The left y-axis reports the concentration in
mol% of the
measured gaseous species, including CO2, Hz, methane, ethane, and CO, evolved
over a 12-
hour experiment. The x-axis represents time and is in terms of hours.
[0054] Figure 33 is an alternative process flow diagram of exemplary
processing
facilities that may be used in some embodiments of the invention.
[0055] Figure 34 is an alternative process flow diagram of exemplary
processing
facilities that may be used in some embodiments of the invention.
[0056] Figure 35 is an alternative process flow diagram of exemplary
processing
facilities that may be used in some embodiments of the invention.
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
Definitions
[0057] As used herein, the term "hydrocarbon(s)" refers to organic
material with
molecular structures containing carbon bonded to hydrogen. Hydrocarbons may
also include
other elements, such as, but not limited to, halogens, metallic elements,
nitrogen, oxygen,
and/or sulfur.
[0058] As used herein, the term "hydrocarbon fluids" refers to a
hydrocarbon or
mixtures of hydrocarbons that are gases or liquids. For example, hydrocarbon
fluids may
include a hydrocarbon or mixtures of hydrocarbons that are gases or liquids at
formation
conditions, at processing conditions or at ambient conditions (15 C and 1 atm
pressure).
Hydrocarbon fluids may include, for example, oil, natural gas, coal bed
methane, shale oil,
pyrolysis oil, pyrolysis gas, a pyrolysis product of coal, and other
hydrocarbons that are in a
gaseous or liquid state.
[0059] As used herein, the terms "produced fluids" and "production
fluids" refer to
liquids and/or gases removed from a subsurface formation, including, for
example, an
organic-rich rock formation. Produced fluids may include both hydrocarbon
fluids and non-
hydrocarbon fluids. Production fluids may include, but are not limited to,
pyrolyzed shale
oil, synthesis gas, a pyrolysis product of coal, carbon dioxide, hydrogen
sulfide and water
(including steam). Produced fluids may include both hydrocarbon fluids and non-
hydrocarbon fluids.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 9 -
[0060] As used herein, the term "condensable hydrocarbons" means those
hydrocarbons that condense at 25 C and one atmosphere absolute pressure.
Condensable
hydrocarbons may include a mixture of hydrocarbons having carbon numbers
greater than 4.
[0061] As used herein, the term "non-condensable hydrocarbons" means
those
hydrocarbons that do not condense at 25 C and one atmosphere absolute
pressure. Non-
condensable hydrocarbons may include hydrocarbons having carbon numbers less
than 5.
[0062] As used herein, the term "heavy hydrocarbons" refers to
hydrocarbon fluids
that are highly viscous at ambient conditions (15 C and 1 atm pressure).
Heavy
hydrocarbons may include highly viscous hydrocarbon fluids such as heavy oil,
tar, and/or
asphalt. Heavy hydrocarbons may include carbon and hydrogen, as well as
smaller
concentrations of sulfur, oxygen, and nitrogen. Additional elements may also
be present in
heavy hydrocarbons in trace amounts. Heavy hydrocarbons may be classified by
API
gravity. Heavy hydrocarbons generally have an API gravity below about 20
degrees. Heavy
oil, for example, generally has an API gravity of about 10-20 degrees, whereas
tar generally
has an API gravity below about 10 degrees. The viscosity of heavy hydrocarbons
is generally
greater than about 100 centipoise at 15 C.
[0063] As used herein, the term "solid hydrocarbons" refers to any
hydrocarbon
material that is found naturally in substantially solid form at formation
conditions. Non-
limiting examples include kerogen, coal, shungites, asphaltites, and natural
mineral waxes.
[0064] As used herein, the term "formation hydrocarbons" refers to both
heavy
hydrocarbons and solid hydrocarbons that are contained in an organic-rich rock
formation.
Formation hydrocarbons may be, but are not limited to, kerogen, oil shale,
coal, bitumen, tar,
natural mineral waxes, and asphaltites.
[0065] As used herein, the term "tar" refers to a viscous hydrocarbon
that generally
has a viscosity greater than about 10,000 centipoise at 15 C. The specific
gravity of tar
generally is greater than 1.000. Tar may have an API gravity less than 10
degrees.
[0066] As used herein, the term "kerogen" refers to a solid, insoluble
hydrocarbon
that principally contains carbon, hydrogen, nitrogen, oxygen, and sulfur. Oil
shale contains
kerogen.
[0067] As used herein, the term "bitumen" refers to a non-crystalline
solid or viscous
hydrocarbon material that is substantially soluble in carbon disulfide.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 10 -
[0068] As used herein, the term "oil" refers to a hydrocarbon fluid
containing a
mixture of condensable hydrocarbons.
[0069] As used herein, the term "subsurface" refers to geologic strata
occurring below
the earth's surface.
[0070] As used herein, the term "hydrocarbon-rich formation" refers to
any formation
that contains more than trace amounts of hydrocarbons. For example, a
hydrocarbon-rich
formation may include portions that contain hydrocarbons at a level of greater
than 5 volume
percent. The hydrocarbons located in a hydrocarbon-rich formation may include,
for
example, oil, natural gas, heavy hydrocarbons, and solid hydrocarbons.
[0071] As used herein, the term "organic-rich rock" refers to any rock
matrix holding
solid hydrocarbons and/or heavy hydrocarbons. Rock matrices may include, but
are not
limited to, sedimentary rocks, shales, siltstones, sands, silicilytes,
carbonates, and diatomites.
[0072] As used herein, the term "formation" refers to any finite
subsurface region.
The formation may contain one or more hydrocarbon-containing layers, one or
more non-
hydrocarbon containing layers, an overburden, and/or an underburden of any
subsurface
geologic formation. An "overburden" and/or an "underburden" is geological
material above
or below the formation of interest. An overburden or underburden may include
one or more
different types of substantially impermeable materials. For example,
overburden and/or
underburden may include rock, shale, mudstone, or wet/tight carbonate (i.e.,
an impermeable
carbonate without hydrocarbons). An overburden and/or an underburden may
include a
hydrocarbon-containing layer that is relatively impermeable. In some cases,
the overburden
and/or underburden may be permeable.
[0073] As used herein, the term "organic-rich rock formation" refers to
any formation
containing organic-rich rock. Organic-rich rock formations include, for
example, oil shale
formations, coal formations, and tar sands formations.
[0074] As used herein, the term "pyrolysis" refers to the breaking of
chemical bonds
through the application of heat. For example, pyrolysis may include
transforming a
compound into one or more other substances by heat alone or by heat in
combination with an
oxidant. Pyrolysis may include modifying the nature of the compound by
addition of
hydrogen atoms which may be obtained from molecular hydrogen, water, carbon
dioxide, or
carbon monoxide. Heat may be transferred to a section of the formation to
cause pyrolysis.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 11 -
[0075] As
used herein, the term "water-soluble minerals" refers to minerals that are
soluble in water.
Water-soluble minerals include, for example, nahcolite (sodium
bicarbonate), soda ash (sodium carbonate), dawsonite (NaAl(CO3)(OH)2), or
combinations
thereof. Substantial solubility may require heated water and/or a non-neutral
pH solution.
[0076] As
used herein, the term "formation water-soluble minerals" refers to water-
soluble minerals that are found naturally in a formation.
[0077] As
used herein, the term "migratory contaminant species" refers to species that
are both soluble or moveable in water or an aqueous fluid, and are considered
to be
potentially harmful or of concern to human health or the environment.
Migratory
contaminant species may include inorganic and organic contaminants. Organic
contaminants
may include saturated hydrocarbons, aromatic hydrocarbons, and oxygenated
hydrocarbons.
Inorganic contaminants may include metal contaminants, and ionic contaminants
of various
types that may significantly alter pH or the formation fluid chemistry.
Aromatic
hydrocarbons may include, for example, benzene, toluene, xylene, ethylbenzene,
and tri-
methylbenzene, and various types of polyaromatic hydrocarbons such as
anthracenes,
naphthalenes, chrysenes and pyrenes. Oxygenated hydrocarbons may include, for
example,
alcohols, ketones, phenols, and organic acids such as carboxylic acid. Metal
contaminants
may include, for example, arsenic, boron, chromium, cobalt, molybdenum,
mercury,
selenium, lead, vanadium, nickel or zinc. Ionic contaminants include, for
example, sulfides,
sulfates, chlorides, fluorides, ammonia, nitrates, calcium, iron, magnesium,
potassium,
lithium, boron, and strontium.
[0078] As
used herein, the term "cracking" refers to a process involving
decomposition and molecular recombination of organic compounds to produce a
greater
number of molecules than were initially present. In cracking, a series of
reactions take place
accompanied by a transfer of hydrogen atoms between molecules. For example,
naphtha may
undergo a thermal cracking reaction to form ethene and H2 among other
molecules.
[0079] As
used herein, the term "sequestration" refers to the storing of a fluid that is
a
by-product of a process rather than discharging the fluid to the atmosphere or
open
environment.
[0080] As
used herein, the term "subsidence" refers to a downward movement of a
surface relative to an initial elevation of the surface.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 12 -
[0081] As used herein, the term "thickness" of a layer refers to the
distance between
the upper and lower boundaries of a cross section of a layer, wherein the
distance is measured
normal to the average tilt of the cross section.
[0082] As used herein, the term "thermal fracture" refers to fractures
created in a
formation caused directly or indirectly by expansion or contraction of a
portion of the
formation and/or fluids within the formation, which in turn is caused by
increasing/decreasing the temperature of the formation and/or fluids within
the formation,
and/or by increasing/decreasing a pressure of fluids within the formation due
to heating.
Thermal fractures may propagate into or form in neighboring regions
significantly cooler
than the heated zone.
[0083] As used herein, the term "hydraulic fracture" refers to a fracture
at least
partially propagated into a formation, wherein the fracture is created through
injection of
pressurized fluids into the formation. The fracture may be artificially held
open by injection
of a proppant material. Hydraulic fractures may be substantially horizontal in
orientation,
substantially vertical in orientation, or oriented along any other plane.
[0084] As used herein, the term "wellbore" refers to a hole in the
subsurface made by
drilling or insertion of a conduit into the subsurface. A wellbore may have a
substantially
circular cross section, or other cross-sectional shapes (e.g., circles, ovals,
squares, rectangles,
triangles, slits, or other regular or irregular shapes). As used herein, the
term "well", when
referring to an opening in the formation, may be used interchangeably with the
term
"wellbore."
Description of Specific Embodiments
[0085] The inventions are described herein in connection with certain
specific
embodiments. However, to the extent that the following detailed description is
specific to a
particular embodiment or a particular use, such is intended to be illustrative
only and is not to
be construed as limiting the scope of the invention.
[0086] As discussed herein, some embodiments of the invention include or
have
application related to an in situ method of recovering natural resources. The
natural
resources may be recovered from an organic-rich rock formation, including, for
example, an
oil shale formation. The organic-rich rock formation may include formation
hydrocarbons,
including, for example, kerogen, coal, and heavy hydrocarbons. In some
embodiments of the
invention the natural resources may include hydrocarbon fluids, including, for
example,
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 13 -
products of the pyrolysis of formation hydrocarbons such as shale oil. In some
embodiments
of the invention the natural resources may also include water-soluble
minerals, including, for
example, nahcolite (sodium bicarbonate, or 2NaHCO3), soda ash (sodium
carbonate, or
Na2CO3) and dawsonite (NaA1(CO3)(OH)2)-
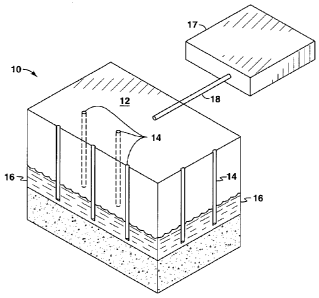
[0087] Figure 1 presents a perspective view of an illustrative oil shale
development
area 10. A surface 12 of the development area 10 is indicated. Below the
surface is an
organic-rich rock formation 16. The illustrative subsurface formation 16
contains formation
hydrocarbons (such as, for example, kerogen) and possibly valuable water-
soluble minerals
(such as, for example, nahcolite). It is understood that the representative
formation 16 may
be any organic-rich rock formation, including a rock matrix containing coal or
tar sands, for
example. In addition, the rock matrix making up the formation 16 may be
permeable, semi-
permeable or non-permeable. The present inventions are particularly
advantageous in oil
shale development areas initially having very limited or effectively no fluid
permeability.
[0088] In order to access formation 16 and recover natural resources
therefrom, a
plurality of wellbores is formed. Wellbores are shown at 14 in Figure 1. The
representative
wellbores 14 are essentially vertical in orientation relative to the surface
12. However, it is
understood that some or all of the wellbores 14 could deviate into an obtuse
or even
horizontal orientation. In the arrangement of Figure 1, each of the wellbores
14 is completed
in the oil shale formation 16. The completions may be either open or cased
hole. The well
completions may also include propped or unpropped hydraulic fractures
emanating
therefrom.
[0089] In the view of Figure 1, only seven wellbores 14 are shown.
However, it is
understood that in an oil shale development project, numerous additional
wellbores 14 will
most likely be drilled. The wellbores 14 may be located in relatively close
proximity, being
from 10 feet to up to 300 feet in separation. In some embodiments, a well
spacing of 15 to 25
feet is provided. Typically, the wellbores 14 are also completed at shallow
depths, being
from 200 to 5,000 feet at total depth. In some embodiments the oil shale
formation targeted
for in situ retorting is at a depth greater than 200 feet below the surface or
alternatively 400
feet below the surface. Alternatively, conversion and production occur at
depths between
500 and 2,500 feet.
[0090] The wellbores 14 will be selected for certain functions and may be
designated
as heat injection wells, water injection wells, oil production wells and/or
water-soluble
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 14 -
mineral solution production wells. In one aspect, the wellbores 14 are
dimensioned to serve
two, three, or all four of these purposes. Suitable tools and equipment may be
sequentially
run into and removed from the wellbores 14 to serve the various purposes.
[0091] A
fluid processing facility 17 is also shown schematically. The fluid
processing facility 17 is equipped to receive fluids produced from the organic-
rich rock
formation 16 through one or more pipelines or flow lines 18. The fluid
processing facility 17
may include equipment suitable for receiving and separating oil, gas, and
water produced
from the heated formation. The fluid processing facility 17 may further
include equipment
for separating out dissolved water-soluble minerals and/or migratory
contaminant species,
including, for example, dissolved organic contaminants, metal contaminants, or
ionic
contaminants in the produced water recovered from the organic-rich rock
formation 16. The
contaminants may include, for example, aromatic hydrocarbons such as benzene,
toluene,
xylene, and tri-methylbenzene.
The contaminants may also include polyaromatic
hydrocarbons such as anthracene, naphthalene, chrysene and pyrene. Metal
contaminants
may include species containing arsenic, boron, chromium, mercury, selenium,
lead,
vanadium, nickel, cobalt, molybdenum, or zinc. Ionic contaminant species may
include, for
example, sulfates, chlorides, fluorides, lithium, potassium, aluminum,
ammonia, and nitrates.
[0092] In
order to recover oil, gas, and sodium (or other) water-soluble minerals, a
series of steps may be undertaken. Figure 2 presents a flow chart
demonstrating a method of
in situ thermal recovery of oil and gas from an organic-rich rock formation
100, in one
embodiment. It is understood that the order of some of the steps from Figure 2
may be
changed, and that the sequence of steps is merely for illustration.
[0093]
First, the oil shale (or other organic-rich rock) formation 16 is identified
within the development area 10. This step is shown in box 110. Optionally, the
oil shale
formation may contain nahcolite or other sodium minerals. The targeted
development area
within the oil shale formation may be identified by measuring or modeling the
depth,
thickness and organic richness of the oil shale as well as evaluating the
position of the
organic-rich rock formation relative to other rock types, structural features
(e.g. faults,
anticlines or synclines), or hydrogeological units (i.e. aquifers). This is
accomplished by
creating and interpreting maps and/or models of depth, thickness, organic
richness and other
data from available tests and sources. This may involve performing geological
surface
surveys, studying outcrops, performing seismic surveys, and/or drilling
boreholes to obtain
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 15 -
core samples from subsurface rock. Rock samples may be analyzed to assess
kerogen
content and hydrocarbon fluid generating capability.
[0094]
The kerogen content of the organic-rich rock formation may be ascertained
from outcrop or core samples using a variety of data. Such data may include
organic carbon
content, hydrogen index, and modified Fischer assay analyses. Subsurface
permeability may
also be assessed via rock samples, outcrops, or studies of ground water flow.
Furthermore
the connectivity of the development area to ground water sources may be
assessed.
[0095]
Next, a plurality of wellbores 14 is formed across the targeted development
area 10. This step is shown schematically in box 115. The purposes of the
wellbores 14 are
set forth above and need not be repeated. However, it is noted that for
purposes of the
wellbore formation step of box 115, only a portion of the wells need be
completed initially.
For instance, at the beginning of the project heat injection wells are needed,
while a majority
of the hydrocarbon production wells are not yet needed. Production wells may
be brought in
once conversion begins, such as after 4 to 12 months of heating.
[0096] It
is understood that petroleum engineers will develop a strategy for the best
depth and arrangement for the wellbores 14, depending upon anticipated
reservoir
characteristics, economic constraints, and work scheduling constraints. In
addition,
engineering staff will determine what wellbores 14 shall be used for initial
formation 16
heating. This selection step is represented by box 120.
[0097]
Concerning heat injection wells, there are various methods for applying heat
to
the organic-rich rock formation 16. The present methods are not limited to the
heating
technique employed unless specifically so stated in the claims. The heating
step is
represented generally by box 130. Preferably, for in situ processes the
heating of a
production zone takes place over a period of months, or even four or more
years. The
formation 16 is heated to a temperature sufficient to pyrolyze at least a
portion of the oil shale
in order to convert the kerogen to hydrocarbon fluids. The bulk of the target
zone of the
formation may be heated to between 270 C to 800 C. Alternatively, the
targeted volume of
the organic-rich formation is heated to at least 350 C to create production
fluids. The
conversion step is represented in Figure 2 by box 135. The resulting liquids
and
hydrocarbon gases may be refined into products which resemble common
commercial
petroleum products. Such liquid products include transportation fuels such as
diesel, jet fuel
and naptha. Generated gases include light alkanes, light alkenes, H2, CO2, CO,
and NH3.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 16 -
[0098] Conversion of the oil shale will create permeability in the oil
shale section in
rocks that were originally impermeable. Preferably, the heating and conversion
processes of
boxes 130 and 135, occur over a lengthy period of time. In one aspect, the
heating period is
from three months to four or more years. Also as an optional part of box 135,
the formation
16 may be heated to a temperature sufficient to convert at least a portion of
nahcolite, if
present, to soda ash. Heat applied to mature the oil shale and recover oil and
gas will also
convert nahcolite to sodium carbonate (soda ash), a related sodium mineral.
The process of
converting nahcolite (sodium bicarbonate) to soda ash (sodium carbonate) is
described
herein.
[0099] In connection with the heating step 130, the rock formation 16 may
optionally
be fractured to aid heat transfer or later hydrocarbon fluid production. The
optional
fracturing step is shown in box 125. Fracturing may be accomplished by
creating thermal
fractures within the formation through application of heat. By heating the
organic-rich rock
and transforming the kerogen to oil and gas, the permeability of portions of
the formation are
increased via thermal fracture formation and subsequent production of a
portion of the
hydrocarbon fluids generated from the kerogen. Alternatively, a process known
as hydraulic
fracturing may be used. Hydraulic fracturing is a process known in the art of
oil and gas
recovery where a fracture fluid is pressurized within the wellbore above the
fracture pressure
of the formation, thus developing fracture planes within the formation to
relieve the pressure
generated within the wellbore. Hydraulic fractures may be used to create
additional
permeability in portions of the formation and/or be used to provide a planar
source for
heating.
[0100] As part of the hydrocarbon fluid production process 100, certain
wells 14 may
be designated as oil and gas production wells. This step is depicted by box
140. Oil and gas
production might not be initiated until it is determined that the kerogen has
been sufficiently
retorted to allow maximum recovery of oil and gas from the formation 16. In
some instances,
dedicated production wells are not drilled until after heat injection wells
(box 130) have been
in operation for a period of several weeks or months. Thus, box 140 may
include the
formation of additional wellbores 14. In other instances, selected heater
wells are converted
to production wells.
[0101] After certain wellbores 14 have been designated as oil and gas
production
wells, oil and/or gas is produced from the wellbores 14. The oil and/or gas
production
process is shown at box 145. At this stage (box 145), any water-soluble
minerals, such as
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 17 -
nahcolite and converted soda ash may remain substantially trapped in the rock
formation 16
as finely disseminated crystals or nodules within the oil shale beds, and are
not produced.
However, some nahcolite and/or soda ash may be dissolved in the water created
during heat
conversion (box 135) within the formation.
[0102] Box 150 presents an optional next step in the oil and gas recovery
method 100.
Here, certain wellbores 14 are designated as water or aqueous fluid injection
wells. Aqueous
fluids are solutions of water with other species. The water may constitute
"brine," and may
include dissolved inorganic salts of chloride, sulfates and carbonates of
Group I and II
elements of The Periodic Table of Elements. Organic salts can also be present
in the aqueous
fluid. The water may alternatively be fresh water containing other species.
The other species
may be present to alter the pH. Alternatively, the other species may reflect
the availability of
brackish water not saturated in the species wished to be leached from the
subsurface.
Preferably, the water injection wells are selected from some or all of the
wellbores used for
heat injection or for oil and/or gas production. However, the scope of the
step of box 150
may include the drilling of yet additional wellbores 14 for use as dedicated
water injection
wells. In this respect, it may be desirable to complete water injection wells
along a periphery
of the development area 10 in order to create a boundary of high pressure.
[0103] Next, optionally water or an aqueous fluid is injected through the
water
injection wells and into the oil shale formation 16. This step is shown at box
155. The water
may be in the form of steam or pressurized hot water. Alternatively the
injected water may
be cool and becomes heated as it contacts the previously heated formation. The
injection
process may further induce fracturing. This process may create fingered
caverns and
brecciated zones in the nahcolite-bearing intervals some distance, for example
up to 200 feet
out, from the water injection wellbores. In one aspect, a gas cap, such as
nitrogen, may be
maintained at the top of each "cavern" to prevent vertical growth.
[0104] Along with the designation of certain wellbores 14 as water
injection wells,
the design engineers may also designate certain wellbores 14 as water or water-
soluble
mineral solution production wells. This step is shown in box 160. These wells
may be the
same as wells used to previously produce hydrocarbons or inject heat. These
recovery wells
may be used to produce an aqueous solution of dissolved water-soluble minerals
and other
species, including, for example, migratory contaminant species. For example,
the solution
may be one primarily of dissolved soda ash. This step is shown in box 165.
Alternatively,
single wellbores may be used to both inject water and then to recover a sodium
mineral
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 18 -
solution. Thus, box 165 includes the option of using the same wellbores 14 for
both water
injection and solution production (Box 165).
[0105] Temporary control of the migration of the migratory contaminant
species,
especially during the pyrolysis process, can be obtained via placement of the
injection and
production wells 14 such that fluid flow out of the heated zone is minimized.
Typically, this
involves placing injection wells at the periphery of the heated zone so as to
cause pressure
gradients which prevent flow inside the heated zone from leaving the zone.
[0106] Figure 3 is a cross-sectional view of an illustrative oil shale
formation that is
within or connected to ground water aquifers and a formation leaching
operation. Four
separate oil shale formation zones are depicted (23, 24, 25 and 26) within the
oil shale
formation. The water aquifers are below the ground surface 27, and are
categorized as an
upper aquifer 20 and a lower aquifer 22. Intermediate the upper and lower
aquifers is an
aquitard 21. It can be seen that certain zones of the formation are both
aquifers or aquitards
and oil shale zones. A plurality of wells (28, 29, 30 and 31) is shown
traversing vertically
downward through the aquifers. One of the wells is serving as a water
injection well 31,
while another is serving as a water production well 30. In this way, water is
circulated 32
through at least the lower aquifer 22.
[0107] Figure 3 shows diagrammatically the water circulation 32 through
an oil shale
volume that was heated 33, that resides within or is connected to an aquifer
22, and from
which hydrocarbon fluids were previously recovered. Introduction of water via
the water
injection well 31 forces water into the previously heated oil shale 33 and
water-soluble
minerals and migratory contaminants species are swept to the water production
well 30. The
water may then processed in a facility 34 wherein the water-soluble minerals
(e.g. nahcolite
or soda ash) and the migratory contaminants may be substantially removed from
the water
stream. Water is then reinjected into the oil shale volume 33 and the
formation leaching is
repeated. This leaching with water is intended to continue until levels of
migratory
contaminant species are at environmentally acceptable levels within the
previously heated oil
shale zone 33. This may require 1 cycle, 2 cycles, 5 cycles 10 cycles or more
cycles of
formation leaching, where a single cycle indicates injection and production of
approximately
one pore volume of water. It is understood that there may be numerous water
injection and
water production wells in an actual oil shale development. Moreover, the
system may
include monitoring wells (28 and 29) which can be utilized during the oil
shale heating phase,
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 19 -
the shale oil production phase, the leaching phase, or during any combination
of these phases
to monitor for migratory contaminant species and/or water-soluble minerals.
[0108] In order to expand upon various features and methods for shale oil
development, certain sections are specifically entitled below.
[0109] In some fields, formation hydrocarbons, such as oil shale, may
exist in more
than one subsurface formation. In= some instances, the organic-rich rock
formations may be
separated by rock layers that are hydrocarbon-free or that otherwise have
little or no
commercial value. Therefore, it may be desirable for the operator of a field
under
hydrocarbon development to undertake an analysis as to which of the
subsurface, organic-
rich rock formations to target or in which order they should be developed.
[0110] The organic-rich rock formation may be selected for development
based on
various factors. One such factor is the thickness of the hydrocarbon
containing layer within
the formation. Greater pay zone thickness may indicate a greater potential
volumetric
production of hydrocarbon fluids. Each of the hydrocarbon containing layers
may have a
thickness that varies depending on, for example, conditions under which the
formation
hydrocarbon containing layer was formed. Therefore, an organic-rich rock
formation will
typically be selected for treatment if that formation includes at least one
formation
hydrocarbon-containing layer having a thickness sufficient for economical
production of
produced fluids.
[0111] An organic-rich rock formation may also be chosen if the thickness
of several
layers that are closely spaced together is sufficient for economical
production of produced
fluids. For example, an in situ conversion process for formation hydrocarbons
may include
selecting and treating a layer within an organic-rich rock formation having a
thickness of
greater than about 5 meters, 10 meters, 50 m, or even 100 meters. In this
manner, heat losses
(as a fraction of total injected heat) to layers formed above and below an
organic-rich rock
formation may be less than such heat losses from a thin layer of formation
hydrocarbons. A
process as described herein, however, may also include selecting and treating
layers that may
include layers substantially free of formation hydrocarbons or thin layers of
formation
hydrocarbons.
[0112] The richness of one or more organic-rich rock formations may also
be
considered. Richness may depend on many factors including the conditions under
which the
formation hydrocarbon containing layer was formed, an amount of formation
hydrocarbons in
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 20 -
the layer, and/or a composition of formation hydrocarbons in the layer. A thin
and rich
formation hydrocarbon layer may be able to produce significantly more valuable
hydrocarbons than a much thicker, less rich formation hydrocarbon layer. Of
course,
producing hydrocarbons from a formation that is both thick and rich is
desirable.
[0113] The kerogen content of an organic-rich rock formation may be
ascertained
from outcrop or core samples using a variety of data. Such data may include
organic carbon
content, hydrogen index, and modified Fischer assay analyses. The Fischer
Assay is a
standard method which involves heating a sample of a formation hydrocarbon
containing
layer to approximately 500 C in one hour, collecting fluids produced from the
heated
sample, and quantifying the amount of fluids produced.
[0114] Subsurface formation permeability may also be assessed via rock
samples,
outcrops, or studies of ground water flow. Furthermore the connectivity of the
development
area to ground water sources may be assessed. Thus, an organic-rich rock
formation may be
chosen for development based on the permeability or porosity of the formation
matrix even if
the thickness of the formation is relatively thin.
[0115] Other factors known to petroleum engineers may be taken into
consideration
when selecting a formation for development. Such factors include depth of the
perceived pay
zone, stratigraphic proximity of fresh ground water to kerogen-containing
zones, continuity
of thickness, and other factors. For instance, the assessed fluid production
content within a
formation will also effect eventual volumetric production.
[0116] In producing hydrocarbon fluids from an oil shale field, it may be
desirable to
control the migration of pyrolyzed fluids. In some instances, this includes
the use of injection
wells, particularly around the periphery of the field. Such wells may inject
water, steam,
CO2, heated methane, or other fluids to drive cracked kerogen fluids inwardly
towards
production wells. In some embodiments, physical barriers may be placed around
the area of
the organic-rich rock formation under development. One example of a physical
barrier
involves the creation of freeze walls. Freeze walls are formed by circulating
refrigerant
through peripheral wells to substantially reduce the temperature of the rock
formation. This,
in turn, prevents the pyrolyzation of kerogen present at the periphery of the
field and the
outward migration of oil and gas. Freeze walls will also cause native water in
the formation
along the periphery to freeze.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 21 -
[0117] The use of subsurface freezing to stabilize poorly consolidated
soils or to
provide a barrier to fluid flow is known in the art. Shell Exploration and
Production
Company has discussed the use of freeze walls for oil shale production in
several patents,
including U.S. Pat. No. 6,880,633 and U.S. Pat. No. 7,032,660. Shell's '660
patent uses
subsurface freezing to protect against groundwater flow and groundwater
contamination
during in situ shale oil production. Additional patents that disclose the use
of so-called freeze
walls are U.S. Pat. No. 3,528,252, U.S. Pat. No. 3,943,722, U.S. Pat. No.
3,729,965, U.S. Pat.
No. 4,358,222, U.S. Pat. No. 4,607,488, and WO Pat. No. 98996480.
[0118] Another example of a physical barrier that may be used to limit
fluid flow into
or out of an oil shale field is the creation of grout walls. Grout walls are
formed by injecting
cement into the formation to fill permeable pathways. In the context of an oil
shale field,
cement would be injected along the periphery of the field. This prevents the
movement of
pyrolyzed fluids out of the field under development, and the movement of water
from
adjacent aquifers into the field.
[0119] As noted above, several different types of wells may be used in
the
development of an organic-rich rock formation, including, for example, an oil
shale field.
For example, the heating of the organic-rich rock formation may be
accomplished through the
use of heater wells. The heater wells may include, for example, electrical
resistance heating
elements. The production of hydrocarbon fluids from the formation may be
accomplished
through the use of wells completed for the production of fluids. The injection
of an aqueous
fluid may be accomplished through the use of injection wells. Finally, the
production of an
aqueous solution may be accomplished through use of solution production wells.
[0120] The different wells listed above may be used for more than one
purpose.
Stated another way, wells initially completed for one purpose may later be
used for another
purpose, thereby lowering project costs and/or decreasing the time required to
perform
certain tasks. For example, one or more of the production wells may also be
used as injection
wells for later injecting water into the organic-rich rock formation.
Alternatively, one or
more of the production wells may also be used as solution production wells for
later
producing an aqueous solution from the organic-rich rock formation.
[0121] In other aspects, production wells (and in some circumstances
heater wells)
may initially be used as dewatering wells (e.g., before heating is begun
and/or when heating
is initially started). In addition, in some circumstances dewatering wells can
later be used as
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 22 -
production wells (and in some circumstances heater wells). As such, the
dewatering wells
may be placed and/or designed so that such wells can be later used as
production wells and/or
heater wells. The heater wells may be placed and/or designed so that such
wells can be later
used as production wells and/or dewatering wells. The production wells may be
placed
and/or designed so that such wells can be later used as dewatering wells
and/or heater wells.
Similarly, injection wells may be wells that initially were used for other
purposes (e.g.,
heating, production, dewatering, monitoring, etc.), and injection wells may
later be used for
other purposes. Similarly, monitoring wells may be wells that initially were
used for other
purposes (e.g., heating, production, dewatering, injection, etc.). Finally,
monitoring wells
may later be used for other purposes such as water production.
[0122] The wellbores for the various wells may be located in relatively
close
proximity, being from 10 feet to up to 300 feet in separation. Alternatively,
the wellbores
may be spaced from 30 to 200 feet or 50 to 100 feet. Typically, the wellbores
are also
completed at shallow depths, being from 200 to 5,000 feet at total depth.
Alternatively, the
wellbores may be completed at depths from 1,000 to 4,000 feet, or 1,500 to
3,500 feet. In
some embodiments, the oil shale formation targeted for in situ retorting is at
a depth greater
than 200 feet below the surface. In alternative embodiments, the oil shale
formation targeted
for in situ retorting is at a depth greater than 500, 1,000, or 1,500 feet
below the surface. In
alternative embodiments, the oil shale formation targeted for in situ
retorting is at a depth
between 200 and 5,000 feet, alternatively between 1,000 and 4,000 ft, 1,200
and 3,700 feet,
or 1,500 and 3,500 feet below the surface.
[0123] It is desirable to arrange the various wells for an oil shale
field in a pre-
planned pattern. For instance, heater wells may be arranged in a variety of
patterns including,
but not limited to triangles, squares, hexagons, and other polygons. The
pattern may include
a regular polygon to promote uniform heating through at least the portion of
the formation in
which the heater wells are placed. The pattern may also be a line drive
pattern. A line drive
pattern generally includes a first linear array of heater wells, a second
linear array of heater
wells, and a production well or a linear array of production wells between the
first and second
linear array of heater wells. Interspersed among the heater wells are
typically one or more
production wells. The injection wells may likewise be disposed within a
repetitive pattern of
units, which may be similar to or different from that used for the heater
wells.
[0124] One method to reduce the number of wells is to use a single well
as both a
heater well and a production well. Reduction of the number of wells by using
single wells for
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 23 -
sequential purposes can reduce project costs. One or more monitoring wells may
be disposed
at selected points in the field. The monitoring wells may be configured with
one or more
devices that measure a temperature, a pressure, and/or a property of a fluid
in the wellbore.
In some instances, a heater well may also serve as a monitoring well, or
otherwise be
instrumented.
[0125] Another method for reducing the number of heater wells is to use
well
patterns. Regular patterns of heater wells equidistantly spaced from a
production well may
be used. The patterns may form equilateral triangular arrays, hexagonal
arrays, or other array
patterns. The arrays of heater wells may be disposed such that a distance
between each
heater well is less than about 70 feet (21 m). A portion of the formation may
be heated with
heater wells disposed substantially parallel to a boundary of the hydrocarbon
formation.
[0126] In alternative embodiments, the array of heater wells may be
disposed such
that a distance between each heater well may be less than about 100 feet, or
50 feet, or 30
feet. Regardless of the arrangement of or distance between the heater wells,
in certain
embodiments, a ratio of heater wells to production wells disposed within a
organic-rich rock
formation may be greater than about 5, 8, 10, 20, or more.
[0127] In one embodiment, individual production wells are surrounded by
at most one
layer of heater wells. This may include arrangements such as 5-spot, 7-spot,
or 9-spot arrays,
with alternating rows of production and heater wells. In another embodiment,
two layers of
heater wells may surround a production well, but with the heater wells
staggered so that a
clear pathway exists for the majority of flow away from the further heater
wells. Flow and
reservoir simulations may be employed to assess the pathways and temperature
history of
hydrocarbon fluids generated in situ as they migrate from their points of
origin to production
wells.
[0128] Figure 4 provides a plan view of an illustrative heater well
arrangement using
more than one layer of heater wells. The heater well arrangement is used in
connection with
the production of hydrocarbons from a shale oil development area 400. In
Figure 4, the
heater well arrangement employs a first layer of heater wells 410, surrounded
by a second
layer of heater wells 420. The heater wells in the first layer 410 are
referenced at 431, while
the heater wells in the second layer 420 are referenced at 432.
[0129] A production well 440 is shown central to the well layers 410 and
420. It is
noted that the heater wells 432 in the second layer 420 of wells are offset
from the heater
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 24 -
wells 431 in the first layer 410 of wells, relative to the production well
440. The purpose is
to provide a flowpath for converted hydrocarbons that minimizes travel near a
heater well in
the first layer 410 of heater wells. This, in turn, minimizes secondary
cracking of
hydrocarbons converted from kerogen as hydrocarbons flow from the second layer
of wells
420 to the production wells 440.
[0130] In
the illustrative arrangement of Figure 4, the first layer 410 and the second
layer 420 each defines a 5-spot pattern. However, it is understood that other
patterns may be
employed, such as 3-spot or 6-spot patterns. In any instance, a plurality of
heater wells 431
comprising a first layer of heater wells 410 is placed around a production
well 440, with a
second plurality of heater wells 432 comprising a second layer of heater wells
420 placed
around the first layer 410.
[0131]
The heater wells in the two layers also may be arranged such that the majority
of hydrocarbons generated by heat from each heater well 432 in the second
layer 420 are able
to migrate to a production well 440 without passing substantially near a
heater well 431 in the
first layer 410. The heater wells 431, 432 in the two layers 410, 420 further
may be arranged
such that the majority of hydrocarbons generated by heat from each heater well
432 in the
second layer 420 are able to migrate to the production well 440 without
passing through a
zone of substantially increasing formation temperature.
[0132]
One method to reduce the number of heater wells is to use well patterns that
are elongated in a particular direction, particularly in the direction of most
efficient thermal
conductivity. Heat convection may be affected by various factors such as
bedding planes and
stresses within the formation. For instance, heat convection may be more
efficient in the
direction perpendicular to the least horizontal principal stress on the
formation. In some
instances, heat convection may be more efficient in the direction parallel to
the least
horizontal principal stress.
[0133] In
connection with the development of an oil shale field, it may be desirable
that the progression of heat through the subsurface in accordance with steps
130 and 135 be
uniform.
However, for various reasons the heating and maturation of formation
hydrocarbons in a subsurface formation may not proceed uniformly despite a
regular
arrangement of heater and production wells. Heterogeneities in the oil shale
properties and
formation structure may cause certain local areas to be more or less
productive. Moreover,
formation fracturing which occurs due to the heating and maturation of the oil
shale can lead
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 25 -
to an uneven distribution of preferred pathways and, thus, increase flow to
certain production
wells and reduce flow to others. Uneven fluid maturation may be an undesirable
condition
since certain subsurface regions may receive more heat energy than necessary
where other
regions receive less than desired. This, in turn, leads to the uneven flow and
recovery of
production fluids. Produced oil quality, overall production rate, and/or
ultimate recoveries
may be reduced.
[0134] To detect uneven flow conditions, production and heater wells may
be
instrumented with sensors. Sensors may include equipment to measure
temperature,
pressure, flow rates, and/or compositional information. Data from these
sensors can be
processed via simple rules or input to detailed simulations to reach decisions
on how to adjust
heater and production wells to improve subsurface performance. Production well
performance may be adjusted by controlling backpressure or throttling on the
well. Heater
well performance may also be adjusted by controlling energy input. Sensor
readings may
also sometimes imply mechanical problems with a well or downhole equipment
which
requires repair, replacement, or abandonment.
[0135] In one embodiment, flow rate, compositional, temperature and/or
pressure data
are utilized from two or more wells as inputs to a computer algorithm to
control heating rate
and/or production rates. Unmeasured conditions at or in the neighborhood of
the well are
then estimated and used to control the well. For example, in situ fracturing
behavior and
kerogen maturation are estimated based on thermal, flow, and compositional
data from a set
of wells. In another example, well integrity is evaluated based on pressure
data, well
temperature data, and estimated in situ stresses. In a related embodiment the
number of
sensors is reduced by equipping only a subset of the wells with instruments,
and using the
results to interpolate, calculate, or estimate conditions at uninstrumented
wells. Certain wells
may have only a limited set of sensors (e.g., wellhead temperature and
pressure only) where
others have a much larger set of sensors (e.g., wellhead temperature and
pressure, bottomhole
temperature and pressure, production composition, flow rate, electrical
signature, casing
strain, etc.).
[0136] As noted above, there are various methods for applying heat to an
organic-rich
rock formation. For example, one method may include electrical resistance
heaters disposed
in a wellbore or outside of a wellbore. One such method involves the use of
electrical
resistive heating elements in a cased or uncased wellbore. Electrical
resistance heating
involves directly passing electricity through a conductive material such that
resistive losses
CA 02686565 2015-04-14
- 26 -
cause it to heat the conductive material. Other heating methods include the
use of downhole
combustors, in situ combustion, radio-frequency (RF) electrical energy, or
microwave
energy. Still others include injecting a hot fluid into the oil shale
formation to directly heat it.
The hot fluid may or may not be circulated. One method may include generating
heat by
burning a fuel external to or within a subsurface formation. For example, heat
may be
supplied by surface burners or downhole burners or by circulating hot fluids
(such as methane
gas or naphtha) into the formation through, for example, wellbores via, for
example, natural
or artificial fractures. Some burners may be configured to perform flameless
combustion.
Alternatively, some methods may include combusting fuel within the formation
such as via a
natural distributed combustor, which generally refers to a heater that uses an
oxidant to
oxidize at least a portion of the carbon in the formation to generate heat,
and wherein the
oxidation takes place in a vicinity proximate to a wellbore. The present
methods are not
limited to the heating technique employed unless so stated in the claims.
[0137] One method for formation heating involves the use of electrical
resistors in
which an electrical current is passed through a resistive material which
dissipates the
electrical energy as heat. This method is distinguished from dielectric
heating in which a
high-frequency oscillating electric current induces electrical currents in
nearby materials and
causes them to heat. The electric heater may include an insulated conductor,
an elongated
member disposed in the opening, and/or a conductor disposed in a conduit. An
early patent
disclosing the use of electrical resistance heaters to produce oil shale in
situ is U.S. Pat. No.
1,666,488. The '488 patent issued to Crawshaw in 1928. Since 1928, various
designs for
downhole electrical heaters have been proposed. Illustrative designs are
presented in U.S.
Pat. No. 1,701,884, U.S. Pat. No. 3,376,403, U.S. Pat. No. 4,626,665, U.S.
Pat. No.
4,704,514, and U.S. Pat. No. 6,023,554).
[0138] A review of application of electrical heating methods for heavy oil
reservoirs
is given by R. Sierra and S.M. Farouq Ali, "Promising Progress in Field
Application of
Reservoir Electrical Heating Methods", Society of Petroleum Engineers Paper
69709, 2001.
[0139] Certain previous designs for in situ electrical resistance heaters
utilized solid,
continuous heating elements (e.g., metal wires or strips). However, such
elements may lack
the necessary robustness for long-term, high temperature applications such as
oil shale
maturation. As the formation heats and the oil shale matures, significant
expansion of the
rock occurs. This leads to high stresses on wells intersecting the formation.
These stresses
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 27 -
can lead to bending and stretching of the wellbore pipe and internal
components. Cementing
(e.g., U.S. Pat. No. 4,886,118) or packing (e.g., U.S. Pat. No. 2,732,195) a
heating element in
place may provide some protection against stresses, but some stresses may
still be transmitted
to the heating element.
[0140] As an alternative, international patent publication WO 2005/010320
teaches
the use of electrically conductive fractures to heat the oil shale. A heating
element is
constructed by forming wellbores and then hydraulically fracturing the oil
shale formation
around the wellbores. The fractures are filled with an electrically conductive
material which
forms the heating element. Calcined petroleum coke is an exemplary suitable
conductant
material. Preferably, the fractures are created in a vertical orientation
along longitudinal,
horizontal planes formed by horizontal wellbores. Electricity may be conducted
through the
conductive fractures from the heel to the toe of each well. The electrical
circuit may be
completed by an additional horizontal well that intersects one or more of the
vertical fractures
near the toe to supply the opposite electrical polarity. The WO 2005/010320
process creates
an "in situ toaster" that artificially matures oil shale through the
application of electric heat.
Thermal conduction heats the oil shale to conversion temperatures in excess of
300 C
causing artificial maturation.
[0141] International patent publication WO 2005/045192 teaches an
alternative
heating means that employs the circulation of a heated fluid within an oil
shale formation. In
the process of WO 2005/045192 supercritical heated naphtha may be circulated
through
fractures in the formation. This means that the oil shale is heated by
circulating a dense, hot
hydrocarbon vapor through sets of closely-spaced hydraulic fractures. In one
aspect, the
fractures are horizontally formed and conventionally propped. Fracture
temperatures of 320
¨ 400 C are maintained for up to five to ten years. Vaporized naptha may be
the preferred
heating medium due to its high volumetric heat capacity, ready availability
and relatively low
degradation rate at the heating temperature. In the WO 2005/045192 process, as
the kerogen
matures, fluid pressure will drive the generated oil to the heated fractures,
where it will be
produced with the cycling hydrocarbon vapor.
[0142] The purpose for heating the organic-rich rock formation is to
pyrolyze at least
a portion of the solid formation hydrocarbons to create hydrocarbon fluids.
The solid
formation hydrocarbons may be pyrolyzed in situ by raising the organic-rich
rock formation,
(or zones within the formation), to a pyrolyzation temperature. In certain
embodiments, the
temperature of the formation may be slowly raised through the pyrolysis
temperature range.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 28 -
For example, an in situ conversion process may include heating at least a
portion of the
organic-rich rock formation to raise the average temperature of the zone above
about 270 C
at a rate less than a selected amount (e.g., about 10 C, 5 C; 3 C, 1 C,
0.5 C, or 0.1 C) per
day. In a further embodiment, the portion may be heated such that an average
temperature of
the selected zone may be less than about 375 C or, in some embodiments, less
than about
400 C. The formation may be heated such that a temperature within the
formation reaches
(at least) an initial pyrolyzation temperature (e.g., a temperature at the
lower end of the
temperature range where pyrolyzation begins to occur.
[0143]
The pyrolysis temperature range may vary depending on the types of
formation hydrocarbons within the formation, the heating methodology, and the
distribution
of heating sources. For example, a pyrolysis temperature range may include
temperatures
between about 270 C and about 900 C. Alternatively, the bulk of the target
zone of the
formation may be heated to between 3.0, = to 600 C. In an alternative
embodiment, a
i
pyrolysis temperature range may include te" peratures between about 270 C to
about 500 C.
[0144]
Preferably, for in situ processes the heating of a production zone takes place
over a period of months, or even four or more years. Alternatively, the
formation may be
heated for one to fifteen years, alternatively, 3 to 10 years, 1.5 to 7 years,
or 2 to 5 years. The
bulk of the target zone of the formation may be heated to between 270 to 800
C. Preferably,
the bulk of the target zone of the formation is heated to between 300 to 600
C.
Alternatively, the bulk of the target zone is ultimately heated to a
temperature below 400 C
(752 F).
[0145] In
certain embodiments of the methods of the present invention, downhole
burners may be used to heat a targeted oil shale zone. Downhole burners of
various design
have been discussed in the patent literature for use in oil shale and other
largely solid
hydrocarbon deposits. Examples include U.S. Pat. No. 2,887,160; U.S. Pat. No.
2,847,071;
U.S. Pat. No. 2,895,555; U.S. Pat. No. 3,109,482; U.S. Pat. No. 3,225,829;
U.S. Pat. No.
3,241,615; U.S. Pat. No. 3,254,721; U.S. Pat. No. 3,127,936; U.S. Pat. No.
3,095,031; U.S.
Pat. No. 5,255,742; and U.S. Pat. No. 5,899,269. Downhole burners operate
through the
transport of a combustible fuel (typically natural gas) and an oxidizer
(typically air) to a
subsurface position in a wellbore. The fuel and oxidizer react downhole to
generate heat.
The combustion gases are removed (typically by transport to the surface, but
possibly via
injection into the formation).
Oftentimes, downhole burners utilize pipe-in-pipe
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 29 -
arrangements to transport fuel and oxidizer downhole, and then to remove the
flue gas back
up to the surface. Some downhole burners generate a flame, while others may
not.
[0146] The use of downhole burners is an alternative to another form of
downhole
heat generation called steam generation. In downhole steam generation, a
combustor in the
well is used to boil water placed in the wellbore for injection into the
formation.
Applications of the downhole heat technology have been described in F.M.
Smith, "A Down-
hole burner ¨ Versatile tool for well heating," 25th Technical Conference on
Petroleum
Production, Pennsylvania State University, pp 275-285 (Oct. 19-21, 1966); H.
Brandt, W.G.
Poynter, and J.D. Hummell, "Stimulating Heavy Oil Reservoirs with Downhole Air-
Gas
Burners," World Oil, pp. 91-95 (Sept. 1965); and C.I. DePriester and A.J.
Pantaleo, "Well
Stimulation by Downhole Gas-Air Burner," Journal of Petroleum Technology, pp.
1297-1302
(Dec. 1963).
[0147] Downhole burners have advantages over electrical heating methods
due to the
reduced infrastructure cost. In this respect, there is no need for an
expensive electrical power
plant and distribution system. Moreover, there is increased thermal efficiency
because the
energy losses inherently experienced during electrical power generation are
avoided.
[0148] Few applications of downhole burners exist. Downhole burner design
issues
include temperature control and metallurgy limitations. In this respect, the
flame temperature
can overheat the tubular and burner hardware and cause them to fail via
melting, thermal
stresses, severe loss of tensile strength, or creep. Certain stainless steels,
typically with high
chromium content, can tolerate temperatures up to ¨700 C for extended
periods. (See for
example H.E. Boyer and T.L. Gall (eds.), Metals Handbook, "Chapter 16: Heat-
Resistant
Materials", American Society for Metals, (1985.) The existence of flames can
cause hot
spots within the burner and in the formation surrounding the burner. This is
due to radiant
heat transfer from the luminous portion of the flame. However, a typical gas
flame can
produce temperatures up to about 1,650 C. Materials of construction for the
burners must be
sufficient to withstand the temperatures of these hot spots. The heaters are
therefore more
expensive than a comparable heater without flames.
[0149] For downhole burner applications, heat transfer can occur in one
of several
ways. These include conduction, convection, and radiative methods. Radiative
heat transfer
can be particularly strong for an open flame. Additionally, the flue gases can
be corrosive
due to the CO2 and water content. Use of refractory metals or ceramics can
help solve these
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 30 -
problems, but typically at a higher cost. Ceramic materials with acceptable
strength at
temperatures in excess of 900 C are generally high alumina content ceramics.
Other
ceramics that may be useful include chrome oxide, zirconia oxide, and
magnesium oxide
based ceramics. Additionally, depending on the nature of the downhole
combustion NO
generation may be significant.
[0150] Heat transfer in a pipe-in-pipe arrangement for a downhole burner
can also
lead to difficulties. The down going fuel and air will heat exchange with the
up going hot
flue gases. In a well there is minimal room for a high degree of insulation
and hence
significant heat transfer is typically expected. This cross heat exchange can
lead to higher
flame temperatures as the fuel and air become preheated. Additionally, the
cross heat
exchange can limit the transport of heat downstream of the burner since the
hot flue gases
may rapidly lose heat energy to the rising cooler flue gases.
[0151] The process of heating formation hydrocarbons within an organic-
rich rock
formation, for example, by pyrolysis, may generate fluids. The heat-generated
fluids may
include water which is vaporized within the formation. In addition, the action
of heating
kerogen produces pyrolysis fluids which tend to expand upon heating. The
produced
pyrolysis fluids may include not only water, but also, for example,
hydrocarbons, oxides of
carbon, ammonia, molecular nitrogen, and molecular hydrogen. Therefore, as
temperatures
within a heated portion of the formation increase, a pressure within the
heated portion may
also increase as a result of increased fluid generation, molecular expansion,
and vaporization
of water. Thus, some corollary exists between subsurface pressure in an oil
shale formation
and the fluid pressure generated during pyrolysis. This, in turn, indicates
that formation
pressure may be monitored to detect the progress of a kerogen conversion
process.
[0152] The pressure within a heated portion of an organic-rich rock
formation
depends on other reservoir characteristics. These may include, for example,
formation depth,
distance from a heater well, a richness of the formation hydrocarbons within
the organic-rich
rock formation, the degree of heating, and/or a distance from a producer well.
[0153] It may be desirable for the developer of an oil shale field to
monitor formation
pressure during development. Pressure within a formation may be determined at
a number of
different locations. Such locations may include, but may not be limited to, at
a wellhead and
at varying depths within a wellbore. In some embodiments, pressure may be
measured at a
producer well. In an alternate embodiment, pressure may be measured at a
heater well. In
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 31 -
still another embodiment, pressure may be measured downhole of a dedicated
monitoring
well.
[0154] The process of heating an organic-rich rock formation to a
pyrolysis
temperature range not only will increase formation pressure, but will also
increase formation
permeability. The pyrolysis temperature range should be reached before
substantial
permeability has been generated within the organic-rich rock formation. An
initial lack of
permeability may prevent the transport of generated fluids from a pyrolysis
zone within the
formation. In this manner, as heat is initially transferred from a heater well
to an organic-rich
rock formation, a fluid pressure within the organic-rich rock formation may
increase
proximate to that heater well. Such an increase in fluid pressure may be
caused by, for
example, the generation of fluids during pyrolysis of at least some formation
hydrocarbons in
the formation.
[0155] Alternatively, pressure generated by expansion of pyrolysis fluids
or other
fluids generated in the formation may be allowed to increase. This assumes
that an open path
to a production well or other pressure sink does not yet exist in the
formation. In one aspect,
a fluid pressure may be allowed to increase to or above a lithostatic stress.
In this instance,
fractures in the hydrocarbon containing formation may form when the fluid
pressure equals
or exceeds the lithostatic stress. For example, fractures may form from a
heater well to a
production well. The generation of fractures within the heated portion may
reduce pressure
within the portion due to the production of produced fluids through a
production well.
[0156] Once pyrolysis has begun within an organic-rich rock formation,
fluid
pressure may vary depending upon various factors. These include, for example,
thermal
expansion of hydrocarbons, generation of pyrolysis fluids, rate of conversion,
and withdrawal
of generated fluids from the formation. For example, as fluids are generated
within the
formation, fluid pressure within the pores may increase. Removal of generated
fluids from
the formation may then decrease the fluid pressure within the near wellbore
region of the
formation.
[0157] In certain embodiments, a mass of at least a portion of an organic-
rich rock
formation may be reduced due, for example, to pyrolysis of formation
hydrocarbons and the
production of hydrocarbon fluids from the formation. As such, the permeability
and porosity
of at least a portion of the formation may increase. Any in situ method that
effectively
produces oil and gas from oil shale will create permeability in what was
originally a very low
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 32 -
permeability rock. The extent to which this will occur is illustrated by the
large amount of
expansion that must be accommodated if fluids generated from kerogen are
unable to flow.
The concept is illustrated in Figure 5.
[0158] Figure 5 provides a bar chart comparing one ton of Green River oil
shale
before 50 and after 51 a simulated in situ, retorting process. The simulated
process was
carried out at 2,400 psi and 750 F on oil shale having a total organic carbon
content of 22 wt.
% and a Fisher assay of 42 gallons/ton. Before the conversion, a total of 15.3
ft3 of rock
matrix 52 existed. This matrix comprised 7.2 ft3 of mineral 53, i.e.,
dolomite, limestone, etc.,
and 8.1 ft3 of kerogen 54 imbedded within the shale. As a result of the
conversion the
material expanded to 26.1 ft3 55. This represented 7.2 ft3 of mineral 56 (the
same number as
before the conversion), 6.6 ft3 of hydrocarbon-liquid 57, 9.4 ft3 of
hydrocarbon vapor 58, and
2.9 ft3 of coke 59. It can be seen that substantial volume expansion occurred
during the
conversion process. This, in turn, increases permeability of the rock
structure.
[0159] In an embodiment, heating a portion of an organic-rich rock
formation in situ
to a pyrolysis temperature may increase permeability of the heated portion.
For example,
permeability may increase due to formation of thermal fractures within the
heated portion
caused by application of heat. As the temperature of the heated portion
increases, water may
be removed due to vaporization. The vaporized water may escape and/or be
removed from
the formation. In addition, permeability of the heated portion may also
increase as a result of
production of hydrocarbon fluids from pyrolysis of at least some of the
formation
hydrocarbons within the heated portion on a macroscopic scale.
[0160] Certain systems and methods described herein may be used to treat
formation
hydrocarbons in at least a portion of a relatively low permeability formation
(e.g., in "tight"
formations that contain formation hydrocarbons). Such formation hydrocarbons
may be
heated to pyrolyze at least some of the formation hydrocarbons in a selected
zone of the
formation. Heating may also increase the permeability of at least a portion of
the selected
zone. Hydrocarbon fluids generated from pyrolysis may be produced from the
formation,
thereby further increasing the formation permeability.
[0161] Permeability of a selected zone within the heated portion of the
organic-rich
rock formation may also rapidly increase while the selected zone is heated by
conduction.
For example, permeability of an impermeable organic-rich rock formation may be
less than
about 0.1 millidarcy before heating. In some embodiments, pyrolyzing at least
a portion of
CA 02686565 2015-04-14
- 33 -
organic-rich rock formation has an initial total permeability less than 1
millidarcy,
alternatively less than 0.1 or 0.01 mil lidarcies, before heating the organic-
rich rock formation.
In one embodiment, the organic-rich rock formation has a post heating total
permeability of
greater than 1 millidarcy, alternatively, greater than 10, 50 or 100
millidarcies, after heating
the organic-rich rock formation.
[0162] In connection with heating the organic-rich rock formation, the
organic-rich
rock formation may optionally be fractured to aid heat transfer or hydrocarbon
fluid
production. In one instance, fracturing may be accomplished naturally by
creating thermal
fractures within the formation through application of heat. Thermal fracture
formation is
caused by thermal expansion of the rock and fluids and by chemical expansion
of kerogen
transforming into oil and gas. Thermal fracturing can occur both in the
immediate region
undergoing heating, and in cooler neighboring regions. The thermal fracturing
in the
neighboring regions is due to propagation of fractures and tension stresses
developed due to
the expansion in the hotter zones. Thus, by both heating the organic-rich rock
and
transforming the kerogen to oil and gas, the permeability is increased not
only from fluid
formation and vaporization, but also via thermal fracture formation. The
increased
permeability aids fluid flow within the formation and production of the
hydrocarbon fluids
generated from the kerogen.
[0163] In addition, a process known as hydraulic fracturing may be used.
Hydraulic
fracturing is a process known in the art of oil and gas recovery where a
fracture fluid is
pressurized within the wellbore above the fracture pressure of the formation,
thus developing
fracture planes within the formation to relieve the pressure generated within
the wellbore.
Hydraulic fractures may be used to create additional permeability and/or be
used to provide
an extended geometry for a heater well. The WO 2005/010320 patent publication
describes
one such method.
[0164] In connection with the production of hydrocarbons from a rock
matrix,
particularly those of shallow depth, a concern may exist with respect to earth
subsidence.
This is particularly true in the in situ heating of organic-rich rock where a
portion of the
matrix itself is thermally converted and removed. Initially, thc formation may
contain
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 34 -
formation hydrocarbons in solid form, such as, for example, kerogen. The
formation may
also initially contain water-soluble minerals.
Initially, the formation may also be
substantially impermeable to fluid flow.
[0165]
The in situ heating of the matrix pyrolyzes at least a portion of the
formation
hydrocarbons to create hydrocarbon fluids. This, in turn, creates permeability
within a
matured (pyrolyzed) organic-rich rock zone in the organic-rich rock formation.
The
combination of pyrolyzation and increased permeability permits hydrocarbon
fluids to be
produced from the formation. At the same time, the loss of supporting matrix
material also
creates the potential for subsidence relative to the earth surface.
[0166] In
some instances, subsidence is sought to be minimized in order to avoid
environmental or hydrogeological impact. In this respect, changing the contour
and relief of
the earth surface, even by a few inches, can change runoff patterns, affect
vegetation patterns,
and impact watersheds. In addition, subsidence has the potential of damaging
production or
heater wells formed in a production area. Such subsidence can create damaging
hoop and
compressional stresses on wellbore casings, cement jobs, and equipment
downhole.
[0167] In
order to avoid or minimize subsidence, it is proposed to leave selected
portions of the formation hydrocarbons substantially unpyrolyzed. This serves
to preserve
one or more unmatured, organic-rich rock zones. In some embodiments, the
unmatured
organic-rich rock zones may be shaped as substantially vertical pillars
extending ,through a
substantial portion of the thickness of the organic-rich rock formation.
[0168]
The heating rate and distribution of heat within the formation may be designed
and implemented to leave sufficient unmatured pillars to prevent subsidence.
In one aspect,
heat injection wellbores are formed in a pattern such that untreated pillars
of oil shale are left
therebetween to support the overburden and prevent subsidence.
[0169] It
is preferred that thermal recovery of oil and gas be conducted before any
solution mining of nahcolite or other water-soluble minerals present in the
formation.
Solution mining can generate large voids in a rock formation and collapse
breccias in an oil
shale development area. These voids and brecciated zones may pose problems for
in situ and
mining recovery of oil shale, further increasing the utility of supporting
pillars.
[0170] In
some embodiments, compositions and properties of the hydrocarbon fluids
produced by an in situ conversion process may vary depending on, for example,
conditions
within an organic-rich rock formation. Controlling heat and/or heating rates
of a selected
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 35 -
section in an organic-rich rock formation may increase or decrease production
of selected
produced fluids.
[0171] In one embodiment, operating conditions may be determined by
measuring at
least one property of the organic-rich rock formation. The measured properties
may be input
into a computer executable program. At least one property of the produced
fluids selected to
be produced from the formation may also be input into the computer executable
program.
The program may be operable to determine a set of operating conditions from at
least the one
or more measured properties. The program may also be configured to determine
the set of
operating conditions from at least one property of the selected produced
fluids. In this
manner, the determined set of operating conditions may be configured to
increase production
of selected produced fluids from the formation.
[0172] Certain heater well embodiments may include an operating system
that is
coupled to any of the heater wells such as by insulated conductors or other
types of wiring.
The operating system may be configured to interface with the heater well. The
operating
system may receive a signal (e.g., an electromagnetic signal) from a heater
that is
representative of a temperature distribution of the heater well. Additionally,
the operating
system may be further configured to control the heater well, either locally or
remotely. For
example, the operating system may alter a temperature of the heater well by
altering a
parameter of equipment coupled to the heater well. Therefore, the operating
system may
monitor, alter, and/or control the heating of at least a portion of the
formation.
[0173] In some embodiments, a heater well may be turned down and/or off
after an
average temperature in a formation may have reached a selected temperature.
Turning down
and/or off the heater well may reduce input energy costs, substantially
inhibit overheating of
the formation, and allow heat to substantially transfer into colder regions of
the formation.
[0174] Temperature (and average temperatures) within a heated organic-
rich rock
formation may vary, depending on, for example, proximity to a heater well,
thermal
conductivity and thermal diffusivity of the formation, type of reaction
occurring, type of
formation hydrocarbon, and the presence of water within the organic-rich rock
formation. At
points in the field where monitoring wells are established, temperature
measurements may be
taken directly in the wellbore. Further, at heater wells the temperature of
the immediately
surrounding formation is fairly well understood. However, it is desirable to
interpolate
temperatures to points in the formation intermediate temperature sensors and
heater wells.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 36 -
[0175] In accordance with one aspect of the production processes of the
present
inventions, a temperature distribution within the organic-rich rock formation
may be
computed using a numerical simulation model. The numerical simulation model
may
calculate a subsurface temperature distribution through interpolation of known
data points
and assumptions of formation conductivity. In addition, the numerical
simulation model may
be used to determine other properties of the formation under the assessed
temperature
distribution. For example, the various properties of the formation may
include, but are not
limited to, permeability of the formation.
[0176] The numerical simulation model may also include assessing various
properties
of a fluid formed within an organic-rich rock formation under the assessed
temperature
distribution. For example, the various properties of a formed fluid may
include, but are not
limited to, a cumulative volume of a fluid formed in the formation, fluid
viscosity, fluid
=
density, and a composition of the fluid formed in the formation. Such a
simulation may be
used to assess the performance of a commercial-scale operation or small-scale
field
experiment. For example, a performance of a commercial-scale development may
be
assessed based on, but not limited to, a total volume of product that may be
produced from a
research-scale operation.
[0177] Some embodiments include producing at least a portion of the
hydrocarbon
fluids from the organic-rich rock formation. The hydrocarbon fluids may be
produced
through production wells. Production wells may be cased or uncased wells and
drilled and
completed through methods known in the art.
[0178] Some embodiments further include producing a production fluid from
the
organic-rich rock formation where the production fluid contains the
hydrocarbon fluids and
an aqueous fluid. The aqueous fluid may contain water-soluble minerals and/or
migratory
contaminant species. In such case, the production fluid may be separated into
a hydrocarbon
stream and an aqueous stream at a surface facility. Thereafter the water-
soluble minerals
and/or migratory contaminant species may be recovered from the aqueous stream.
This
embodiment may be combined with any of the other aspects of the invention
discussed
herein.
[0179] The produced hydrocarbon fluids may include a pyrolysis oil
component (or
condensable component) and a pyrolysis gas component (or non-condensable
component).
Condensable hydrocarbons produced from the formation will typically include
paraffins,
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 37 -
cycloalkanes, mono-aromatics, and di-aromatics as components.
Such condensable
hydrocarbons may also include other components such as tri-aromatics and other
hydrocarbon species.
[0180] In
certain embodiments, a majority of the hydrocarbons in the produced fluid
may have a carbon number of less than approximately 25. Alternatively, less
than about 15
weight % of the hydrocarbons in the fluid may have a carbon number greater
than
approximately 25. The non-condensable hydrocarbons may include, but are not
limited to,
hydrocarbons having carbon numbers less than 5.
[0181] In
certain embodiments, the API gravity of the condensable hydrocarbons in
the produced fluid may be approximately 20 or above (e.g., 25, 30, 40, 50,
etc.). In certain
embodiments, the hydrogen to carbon atomic ratio in produced fluid may be at
least
approximately 1.7 (e.g., 1.8, 1.9, etc.).
[0182]
One embodiment of the invention includes an in situ method of producing
hydrocarbon fluids with improved properties from an organic-rich rock
formation.
Applicants have surprisingly discovered that the quality of the hydrocarbon
fluids produced
from in situ heating and pyrolysis of an organic-rich rock formation may be
improved by
selecting sections of the organic-rich rock formation with higher lithostatic
stress for in situ
heating and pyrolysis.
[0183]
The method may include in situ heating of a section of the organic-rich rock
formation that has a high lithostatic stress to form hydrocarbon fluids with
improved
properties. The method may include creating the hydrocarbon fluid by pyrolysis
of a solid
hydrocarbon and/or a heavy hydrocarbon present in the organic-rich rock
formation.
Embodiments may include the hydrocarbon fluid being partially, predominantly
or
substantially completely created by pyrolysis of the solid hydrocarbon and/or
heavy
hydrocarbon present in the organic-rich rock formation. The method may include
heating the
section of the organic-rich rock formation by any method, including any of the
methods
described herein. For example, the method may include heating the section of
the organic-
rich rock formation by electrical resistance heating. Further, the method may
include heating
the section of the organic-rich rock formation through use of a heated heat
transfer fluid. The
method may include heating the section of the organic-rich rock formation to
above 270 C.
Alternatively, the method may include heating the section of the organic-rich
rock formation
between 270 C and 500 C.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 38 -
[0184] The method may include heating in situ a section of the organic-
rich rock
formation having a lithostatic stress greater than 200 psi and producing a
hydrocarbon fluid
from the heated section of the organic-rich rock formation. In alternative
embodiments, the
heated section of the organic-rich rock formation may have a lithostatic
stress greater than
400 psi. In alternative embodiments, the heated section of the organic-rich
rock formation
may have a lithostatic stress greater than 800 psi, greater than 1,000 psi,
greater than 1,200
psi, greater than 1,500 psi or greater than 2,000 psi. Applicants have found
that in situ
heating and pyrolysis of organic-rich rock formations with increasing amounts
of stress lead
to the production of hydrocarbon fluids with improved properties.
[0185] The lithostatic stress of a section of an organic-rich formation
can normally be
estimated by recognizing that it will generally be equal to the weight of the
rocks overlying
the formation. The density of the overlying rocks can be expressed in units of
psi/ft.
Generally, this value will fall between 0.8 and 1.1 psi/ft and can often be
approximated as 0.9
psi/ft. As a result the lithostatic stress of a section of an organic-rich
formation can be
estimated by multiplying the depth of the organic-rich rock formation interval
by 0.9 psi/ft.
Thus the lithostatic stress of a section of an organic-rich formation
occurring at about 1,000 ft
can be estimated to be about (0.9 psi/ft) multiplied by (1,000 ft) or about
900 psi. If a more
precise estimate of lithostatic stress is desired the density of overlying
rocks can be measured
using wireline logging techniques or by making laboratory measurements on
samples
recovered from coreholes. The method may include heating a section of the
organic-rich
rock formation that is located at a depth greater than 200 ft below the
earth's surface.
Alternatively, the method may include heating a section of the organic-rich
rock formation
that is located at a depth greater than 500 ft below the earth's surface,
greater than 1,000 ft
below the earth's surface, greater than 1,200 ft below the earth's surface,
greater than 1,500 ft
below the earth's surface, or greater than 2,000 ft below the earth's surface.
[0186] The organic-rich rock formation may be, for example, a heavy
hydrocarbon
formation or a solid hydrocarbon formation. Particular examples of such
formations may
include an oil shale formation, a tar sands formation or a coal formation.
Particular formation
hydrocarbons present in such formations may include oil shale, kerogen, coal,
and/or
bitumen.
[0187] The hydrocarbon fluid produced from the organic-rich rock
formation may
include both a condensable hydrocarbon portion (e.g. liquid) and a non-
condensable
hydrocarbon portion (e.g. gas). The hydrocarbon fluid may additionally be
produced together
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 39 -
with non-hydrocarbon fluids. Exemplary non-hydrocarbon fluids include, for
example,
water, carbon dioxide, hydrogen sulfide, hydrogen, ammonia, and/or carbon
monoxide.
[0188] The condensable hydrocarbon portion of the hydrocarbon fluid may
be a fluid
present within different locations associated with an organic-rich rock
development project.
For example, the condensable hydrocarbon portion of the hydrocarbon fluid may
be a fluid
present within a production well that is in fluid communication with the
organic-rich rock
formation. The production well may serve as a device for withdrawing the
produced
hydrocarbon fluids from the organic-rich rock formation. Alternatively, the
condensable
hydrocarbon portion may be a fluid present within processing equipment adapted
to process
hydrocarbon fluids produced from the organic-rich rock formation. Exemplary
processing
equipment is described herein. Alternatively, the condensable hydrocarbon
portion may be a
fluid present within a fluid storage vessel. Fluid storage vessels may
include, for example,
fluid storage tanks with fixed or floating roofs, knock-out vessels, and other
intermediate,
temporary or product storage vessels. Alternatively, the condensable
hydrocarbon portion
may be a fluid present within a fluid transportation pipeline. A fluid
transportation pipeline
may include, for example, piping from production wells to processing equipment
or fluid
storage vessels, piping from processing equipment to fluid storage vessels, or
pipelines
associated with collection or transportation of fluids to or from intermediate
or centralized
storage locations.
[0189] The following discussion of Fig. 7 - 16 concerns data obtained in
Examples 1
- 5 which are discussed in the section labeled "Experiments". The data was
obtained through
the experimental procedures, gas and liquid sample collection procedures,
hydrocarbon gas
sample gas chromatography (GC) analysis methodology, gas sample GC peak
integration
methodology, gas sample GC peak identification methodology, whole oil gas
chromatography (WOGC) analysis methodology, whole oil gas chromatography
(WOGC)
peak integration methodology, whole oil gas chromatography (WOGC) peak
identification
methodology, and pseudo component analysis methodology discussed in the
Experiments
section. For clarity, when referring to gas chromatography chromatograms of
hydrocarbon
gas samples, graphical data is provided for one unstressed experiment through
Example 1,
two 400 psi stressed experiments through Examples 2 and 3, and two 1,000 psi
stressed
experiments through Examples 4 and 5. When referring to whole oil gas
chromatography
(WOGC) chromatograms of liquid hydrocarbon samples, graphical data is provided
for one
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 40 -
unstressed experiment through Example 1, one 400 psi stressed experiments
through
Example 3, and one 1,000 psi stressed experiment through Example 4.
[0190] Fig. 7 is a graph of the weight percent of each carbon number
pseudo
component occurring from C6 to C38 for each of the three stress levels tested
and analyzed in
the laboratory experiments discussed herein. The pseudo component weight
percentages
were obtained through the experimental procedures, liquid sample collection
procedures,
whole oil gas chromatography (WOGC) analysis methodology, whole oil gas
chromatography (WOGC) peak identification and integration methodology, and
pseudo
component analysis methodology discussed in the Experiments section. For
clarity, the
pseudo component weight percentages are taken as a percentage of the entire C3
to pseudo
C38 whole oil gas chromatography areas and calculated weights. Thus the
graphed C6 to
C38 weight percentages do not include the weight contribution of the
associated gas phase
product from any of the experiments which was separately treated. Further, the
graphed
weight percentages do not include the weight contribution of any liquid
hydrocarbon
compounds heavier than (i.e. having a longer retention time than) the C38
pseudo component.
The y-axis 2000 represents the concentration in terms of weight percent of
each C6 to C38
pseudo component in the liquid phase. The x-axis 2001 contains the identity of
each
hydrocarbon pseudo component from C6 to C38. The data points occurring on line
2002
represent the weight percent of each C6 to C38 pseudo component for the
unstressed
experiment of Example 1. The data points occurring on line 2003 represent the
weight
percent of each C6 to C38 pseudo component for the 400 psi stressed experiment
of Example
3. While the data points occurring on line 2004 represent the weight percent
of each C6 to
C38 pseudo component for the 1,000 psi stressed experiment of Example 4. From
Fig. 7 it
can be seen that the hydrocarbon liquid produced in the unstressed experiment,
represented
by data points on line 2002, contains a lower weight percentage of lighter
hydrocarbon
components in the C8 to C17 pseudo component range and a greater weight
percentage of
heavier hydrocarbon components in the C20 to C29 pseudo component range, both
as
compared to the 400 psi stress experiment hydrocarbon liquid and the 1,000 psi
stress
experiment hydrocarbon liquid. Looking now at the data points occurring on
line 2003, it is
apparent that the intermediate level 400 psi stress experiment produced a
hydrocarbon liquid
having C8 to C17 pseudo component concentrations between the unstressed
experiment
represented by line 2002 and the 1,000 psi stressed experiment represented by
line 2004. It is
noted that the C17 pseudo component data for both the 400 psi and 1,000 psi
stressed
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 41 -
experiments are about equal. Further, it is apparent that the weight
percentage of heavier
hydrocarbon components in the C20 to C29 pseudo component range for the
intermediate
stress level experiment represented by line 2003 falls between the unstressed
experiment
(Line 2002) hydrocarbon liquid and the 1,000 psi stress experiment (Line 2004)
hydrocarbon
liquid. Lastly, it is apparent that the high level 1,000 psi stress experiment
produced a
hydrocarbon liquid having C8 to C17 pseudo component concentrations greater
than both the
unstressed experiment represented by line 2002 and the 400 psi stressed
experiment
represented by line 2003. Further, it is apparent that the weight percentage
of heavier
hydrocarbon components in the C20 to C29 pseudo component range for the high
level stress
experiment represented by line 2004 are less than both the unstressed
experiment (Line 2002)
hydrocarbon liquid and the 400 psi stress experiment (Line 2003) hydrocarbon
liquid. Thus
pyrolyzing oil shale under increasing levels of lithostatic stress appears to
produce
hydrocarbon liquids having increasingly lighter carbon number distributions.
[0191] Fig. 8 is a graph of the weight percent ratios of each carbon
number pseudo
component occurring from C6 to C38 as compared to the C20 pseudo component for
each of
the three stress levels tested and analyzed in the laboratory experiments
discussed herein.
The pseudo component weight percentages were= obtained as described for Fig.
7.. The y-
axis 2020 represents the weight ratio of each C6 to C38 pseudo component
compared to the
C20 pseudo component in the liquid phase. The x-axis 2021 contains the
identity of each
hydrocarbon pseudo component ratio from C6/C20 to C38/C20. The data points
occurring on
line 2022 represent the weight ratio of each C6 to C38 pseudo component to C20
pseudo
component for the unstressed experiment of Example 1. The data points
occurring on line
2023 represent the weight ratio of each C6 to C38 pseudo component to C20
pseudo
component for the 400 psi stressed experiment of Example 3. While the data
points
occurring on line 2024 represent the weight ratio of each C6 to C38 pseudo
component to
C20 pseudo component for the 1,000 psi stressed experiment of Example 4. From
Fig. 8 it
can be seen that the hydrocarbon liquid produced in the unstressed experiment,
represented
by data points on line 2022, contains a lower weight percentage of lighter
hydrocarbon
components in the C8 to C18 pseudo component range as compared to the C20
pseudo
component and a greater weight percentage of heavier hydrocarbon components in
the C22 to
C29 pseudo component range as compared to the C20 pseudo component, both as
compared
to the 400 psi stress experiment hydrocarbon liquid and the 1,000 psi stress
experiment
hydrocarbon liquid. Looking now at the data points occurring on line 2023, it
is apparent that
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 42 -
the intermediate level 400 psi stress experiment produced a hydrocarbon liquid
having C8 to
C18 pseudo component concentrations as compared to the C20 pseudo component
between
the unstressed experiment represented by line 2022 and the 1,000 psi stressed
experiment
represented by line 2024. Further, it is apparent that the weight percentage
of heavier
hydrocarbon components in the C22 to C29 pseudo component range as compared to
the C20
pseudo component for the intermediate stress level experiment represented by
line 2023 falls
between the unstressed experiment (Line 2022) hydrocarbon liquid and the 1,000
psi stress
experiment (Line 2024) hydrocarbon liquid. Lastly, it is apparent that the
high level 1,000
psi stress experiment produced a hydrocarbon liquid having C8 to C18 pseudo
component
= concentrations as compared to the C20 pseudo component greater than both
the unstressed
experiment represented by line 2022 and the 400 psi stressed experiment
represented by line
2023. Further, it is apparent that the weight percentage of heavier
hydrocarbon components
in the C22 to C29 pseudo component range as compared to the C20 pseudo
component for
= the high level stress experiment represented by line 2024 are less than
both the unstressed
experiment (Line 2022) hydrocarbon liquid and the 400 psi stress experiment
(Line 2023)
hydrocarbon liquid. This analysis further supports the relationship that
pyrolizing oil shale
under increasing levels of lithostatic stress produces hydrocarbon liquids
having increasingly
lighter carbon number distributions.
[0192] Fig. 9 is a graph of the weight percent ratios of each
carbon number pseudo
component occurring from C6 to C38 as compared to the C25 pseudo component for
each of
the three stress levels tested and analyzed in the laboratory experiments
discussed herein.
The pseudo component weight percentages were obtained as described for Fig. 7.
The y-axis
2040 represents the weight ratio of each C6 to C38 pseudo component compared
to the C25
pseudo component in the liquid phase. The x-axis 2041 contains the identity of
each
hydrocarbon pseudo component ratio from C6/C25 to C38/C25. The data points
occurring on
line 2042 represent the weight ratio of each C6 to C38 pseudo component to C25
pseudo
component for the unstressed experiment of Example 1. The data points
occurring on line
2043 represent the weight ratio of each C6 to C38 pseudo component to C25
pseudo
component for the 400 psi stressed experiment of Example 3. While the data
points
occurring on line 2044 represent the weight ratio of each C6 to C38 pseudo
component to
C25 pseudo component for the 1,000 psi stressed experiment of Example 4. From
Fig. 9 it
can be seen that the hydrocarbon liquid produced in the unstressed experiment,
represented
by data points on line 2042, contains a lower weight percentage of lighter
hydrocarbon
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 43 -
components in the C7 to C24 pseudo component range as compared to the C25
pseudo
component and a greater weight percentage of heavier hydrocarbon components in
the C26 to
C29 pseudo component range as compared to the C25 pseudo component, both as
compared
to the 400 psi stress experiment hydrocarbon liquid and the 1,000 psi stress
experiment
hydrocarbon liquid. Looking now at the data points occurring on line 2043, it
is apparent that
the intermediate level 400 psi stress experiment produced a hydrocarbon liquid
having C7 to
C24 pseudo component concentrations as compared to the C25 pseudo component
between
the unstressed experiment represented by line 2042 and the 1,000 psi stressed
experiment
represented by line 2044. Further, it is apparent that the weight percentage
of heavier
hydrocarbon components in the C26 to C29 pseudo component range as compared to
the C25
pseudo component for the intermediate stress level experiment represented by
line 2043 falls
between the unstressed experiment (Line 2042) hydrocarbon liquid and the 1,000
psi stress
experiment (Line 2044) hydrocarbon liquid. Lastly, it is apparent that the
high level 1,000
psi stress experiment produced a hydrocarbon liquid having C7 to C24 pseudo
component
concentrations as compared to the C25 pseudo component greater than both the
unstressed
experiment represented by line 2042 and the 400 psi stressed experiment
represented by line
2043. Further, it is apparent that the weight percentage of heavier
hydrocarbon components
in the C26 to C29 pseudo component range as compared to the C25 pseudo
component for
the high level stress experiment represented by line 2044 are less than both
the unstressed
experiment (Line 2042) hydrocarbon liquid and the 400 psi stress experiment
(Line 2043)
hydrocarbon liquid. This analysis further supports the relationship that
pyrolizing oil shale
under increasing levels of lithostatic stress produces hydrocarbon liquids
having increasingly
lighter carbon number distributions.
[0193] Fig. 10 is a graph of the weight percent ratios of each carbon
number pseudo
component occurring from C6 to C38 as compared to the C29 pseudo component for
each of
the three stress levels tested and analyzed in the laboratory experiments
discussed herein.
The pseudo component weight percentages were obtained as described for Fig. 7.
The y-axis
2060 represents the weight ratio of each C6 to C38 pseudo component compared
to the C29
pseudo component in the liquid phase. The x-axis 2061 contains the identity of
each
hydrocarbon pseudo component ratio from C6/ C29 to C38/ C29. The data points
occurring
on line 2062 represent the weight ratio of each C6 to C38 pseudo component to
C29 pseudo
component for the unstressed experiment of Example 1. The data points
occurring on line
2063 represent the weight ratio of each C6 to C38 pseudo component to C29
pseudo
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 44 -
component for the 400 psi stressed experiment of Example 3. While the data
points
occurring on line 2064 represent the weight ratio of each C6 to C38 pseudo
component to
C29 pseudo component for the 1,000 psi stressed experiment of Example 4. From
Fig. 10 it
= can be seen that the hydrocarbon liquid produced in the unstressed
experiment, represented
by data points on line 2062, contains a lower weight percentage of lighter
hydrocarbon
= components in the C6 to C28 pseudo component range as compared to the C29
pseudo
component, both as compared to the 400 psi stress experiment hydrocarbon
liquid and the
1,000 psi stress experiment hydrocarbon liquid. Looking now at the data points
occurring on
line 2063, it is apparent that the intermediate level 400 psi stress
experiment produced a
hydrocarbon liquid having C6 to C28 pseudo component concentrations as
compared to the
C29 pseudo component between the unstressed experiment represented by line
2062 and the
1,000 psi stressed experiment represented by line 2064. Lastly, it is apparent
that the high
level 1,000 psi stress experiment produced a hydrocarbon liquid having C6 to
C28 pseudo
component concentrations as compared to the C29 pseudo component greater than
both the
unstressed experiment represented by line 2062 and the 400 psi stressed
experiment
represented by line 2063. This analysis further supports the relationship that
pyrolizing oil
shale under increasing levels of lithostatic stress produces hydrocarbon
liquids having
increasingly lighter carbon number distributions.
[0194] Fig. 11 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from the normal-C6 alkane to the normal-C38 alkane for
each of the
three stress levels tested and analyzed in the laboratory experiments
discussed herein. The
= normal alkane compound weight percentages were obtained as described for
Fig. 7, except
that each individual normal alkane compound peak area integration was used to
determine
each respective normal alkane compound weight percentage. For clarity, the
normal alkane
hydrocarbon weight percentages are taken as a percentage of the entire C3 to
pseudo C38
whole oil gas chromatography areas and calculated weights as used in the
pseudo compound
data presented in Fig. 7. The y-axis 2080 represents the concentration in
terms of weight
percent of each normal-C6 to normal-C38 compound found in the liquid phase.
The x-axis
2081 contains the identity of each normal alkane hydrocarbon compound from
normal-C6 to
normal-C38. The data points occurring on line 2082 represent the weight
percent of each
normal-C6 to normal-C38 hydrocarbon compound for the unstressed experiment of
Example
1. The data points occurring on line 2083 represent the weight percent of each
normal-C6 to
normal-C38 hydrocarbon compound for the 400 psi stressed experiment of Example
3.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 45 -
While the data points occurring on line 2084 represent the weight percent of
each normal-C6
to normal-C38 hydrocarbon compound for the 1,000 psi stressed experiment of
Example 4.
From Fig. 11 it can be seen that the hydrocarbon liquid produced in the
unstressed
experiment, represented by data points on line 2082, contains a greater weight
percentage of
hydrocarbon compounds in the normal-C12 to normal-C30 compound range, both as
compared to the 400 psi stress experiment hydrocarbon liquid and the 1,000 psi
stress
experiment hydrocarbon liquid. Looking now at the data points occurring on
line 2083, it is
apparent that the intermediate level 400 psi stress experiment produced a
hydrocarbon liquid
having normal-C12 to normal-C30 compound concentrations between the unstressed
experiment represented by line 2082 and the 1,000 psi stressed experiment
represented by
line 2084. Lastly, it is apparent that the high level 1,000 psi stress
experiment produced a
hydrocarbon liquid having normal-C12 to normal-C30 compound concentrations
less than
both the unstressed experiment represented by line 2082 and the 400 psi
stressed experiment
represented by line 2083. Thus pyrolyzing oil shale under increasing levels of
lithostatic
stress appears to produce hydrocarbon liquids having lower concentrations of
normal alkane
hydrocarbons.
[0195] Fig. 12 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 as compared to the normal-C20
hydrocarbon compound for each of the three stress levels tested and analyzed
in the
laboratory experiments discussed herein. The normal compound weight
percentages were
obtained as described for Fig. 11. The y-axis 3000 represents the
concentration in terms of
weight ratio of each normal-C6 to normal-C38 compound as compared to the
normal-C20
compound found in the liquid phase. The x-axis 3001 contains the identity of
each normal
alkane hydrocarbon compound ratio from normal-C6/normal-C20 to normal-
C38/normal-
C20. The data points occurring on line 3002 represent the weight ratio of each
normal-C6 to
normal-C38 hydrocarbon compound as compared to the normal-C20 compound for the
unstressed experiment of Example 1. The data points occurring on line 3003
represent the
weight ratio of each normal-C6 to normal-C38 hydrocarbon compound as compared
to the
normal-C20 compound for the 400 psi stressed experiment of Example 3. While
the data
points occurring on line 3004 represent the weight ratio of each normal-C6 to
normal-C38
hydrocarbon compound as compared to the normal-C20 compound for the 1,000 psi
stressed
experiment of Example 4. From Fig. 12 it can be seen that the hydrocarbon
liquid produced
in the unstressed experiment, represented by data points on line 3002,
contains a lower
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 46 -
weight percentage of lighter normal alkane hydrocarbon components in the
normal-C6 to
normal-C17 compound range as compared to the normal-C20 compound and a greater
weight
percentage of heavier hydrocarbon components in the normal-C22 to normal-C34
compound
range as compared to the normal-C20 compound, both as compared to the 400 psi
stress
experiment hydrocarbon liquid and the 1,000 psi stress experiment hydrocarbon
liquid.
Looking now at the data points occurring on line 3003, it is apparent that the
intermediate
level 400 psi stress experiment produced a hydrocarbon liquid having normal-C6
to normal-
C17 compound concentrations as compared to the normal-C20 compound between the
unstressed experiment represented by line 3002 and the 1,000 psi stressed
experiment
represented by line 3004. Further, it is apparent that the weight percentage
of heavier
hydrocarbon components in the normal-C22 to normal-C34 compound range as
compared to
the normal-C20 compound for the intermediate stress level experiment
represented by line
3003 falls between the unstressed experiment (Line 3002) hydrocarbon liquid
and the 1,000
psi stress experiment (Line 3004) hydrocarbon liquid. Lastly, it is apparent
that the high
level 1,000 psi stress experiment produced a hydrocarbon liquid having normal-
C6 to
normal-C17 compound concentrations as compared to the normal-C20 compound
greater
than both the unstressed experiment represented by line 3002 and the 400 psi
stressed
experiment represented by line 3003. Further, it is apparent that the weight
percentage of
heavier hydrocarbon components in the normal-C22 to normal-C34 compound range
as
compared to the normal-C20 compound for the high level stress experiment
represented by
line 3004 are less than both the unstressed experiment (Line 3002) hydrocarbon
liquid and
the 400 psi stress experiment (Line 3003) hydrocarbon liquid. This analysis
further supports
the relationship that pyrolizing oil shale under increasing levels of
lithostatic stress produces
hydrocarbon liquids having lower concentrations of normal alkane hydrocarbons.
[0196] Fig. 13 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 as compared to the normal-C25
hydrocarbon compound for each of the three stress levels tested and analyzed
in the
laboratory experiments discussed herein. The normal compound weight
percentages were
obtained as described for Fig. 11. The y-axis 3020 represents the
concentration in terms of
weight ratio of each normal-C6 to normal-C38 compound as compared to the
normal-C25
compound found in the liquid phase. The x-axis 3021 contains the identity of
each normal
alkane hydrocarbon compound ratio from normal-C6/normal-C25 to normal-
C38/normal-
C25. The data points occurring on line 3022 represent the weight ratio of each
normal-C6 to
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 47 -
normal-C38 hydrocarbon compound as compared to the normal-C25 compound for the
unstressed experiment of Example 1. The data points occurring on line 3023
represent the
weight ratio of each normal-C6 to normal-C38 hydrocarbon compound as compared
to the
normal-C25 compound for the 400 psi stressed experiment of Example 3. While
the data
points occurring on line 3024 represent the weight ratio of each normal-C6 to
normal-C38
hydrocarbon compound as compared to the normal-C25 compound for the 1,000 psi
stressed
experiment of Example 4. From Fig. 13 it can be seen that the hydrocarbon
liquid produced
in the unstressed experiment, represented by data points on line 3022,
contains a lower
weight percentage of lighter normal alkane hydrocarbon components in the
normal-C6 to
normal-C24 compound range as compared to the normal-C25 compound and a greater
weight
percentage of heavier hydrocarbon components in the normal-C26 to normal-C30
compound
range as compared to the normal-C25 compound, both as compared to the 400 psi
stress
experiment hydrocarbon liquid and the 1,000 psi stress experiment hydrocarbon
liquid.
Looking now at the data points occurring on line 3023, it is apparent that the
intermediate
level 400 psi stress experiment produced a hydrocarbon liquid having normal-C6
to normal-
C24 compound concentrations as compared to the normal-C25 compound between the
unstressed experiment represented by line 3022 and the 1,000 psi stressed
experiment
represented by line 3024. Further, it is apparent that the weight percentage
of heavier
hydrocarbon components in the normal-C26 to normal-C30 compound range as
compared to
the normal-C25 compound for the intermediate stress level experiment
represented by line
3023 falls between the unstressed experiment (Line 3022) hydrocarbon liquid
and the 1,000
psi stress experiment (Line 3024) hydrocarbon liquid. Lastly, it is apparent
that the high
level 1,000 psi stress experiment produced a hydrocarbon liquid having normal-
C6 to
normal-C24 compound concentrations as compared to the normal-C25 compound
greater
than both the unstressed experiment represented by line 3022 and the 400 psi
stressed
experiment represented by line 3023. Further, it is apparent that the weight
percentage of
heavier hydrocarbon components in the normal-C26 to normal-C30 compound range
as
compared to the normal-C25 compound for the high level stress experiment
represented by
line 3024 are less than both the unstressed experiment (Line 3022) hydrocarbon
liquid and
the 400 psi stress experiment (Line 3023) hydrocarbon liquid. This analysis
further supports
the relationship that pyrolizing oil shale under increasing levels of
lithostatic stress produces
hydrocarbon liquids having lower concentrations of normal alkane hydrocarbons.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 48 -
[0197] Fig. 14 is a graph of the weight percent of normal alkane
hydrocarbon
compounds occurring from normal-C6 to normal-C38 as compared to the normal-C29
hydrocarbon compound for each of the three stress levels tested and analyzed
in the
laboratory experiments discussed herein. The normal compound weight
percentages were
obtained as described for Fig. 11. The y-axis 3040 represents the
concentration in terms of
weight ratio of each normal-C6 to normal-C38 compound as compared to the
normal-C29
compound found in the liquid phase. The x-axis 3041 contains the identity of
each normal
alkane hydrocarbon compound ratio from normal-C6/normal-C29 to normal-
C38/normal-
C29. The data points occurring on line 3042 represent the weight ratio of each
normal-C6 to
normal-C38 hydrocarbon compound as compared to the normal-C29 compound for the
unstressed experiment of Example 1. The data points occurring on line 3043
represent the
weight ratio of each normal-C6 to normal-C38 hydrocarbon compound as compared
to the
normal-C29 compound for the 400 psi stressed experiment of Example 3. While
the data
points occurring on line 3044 represent the weight ratio of each normal-C6 to
normal-C38
hydrocarbon compound as compared to the normal-C29 compound for the 1,000 psi
stressed
experiment of Example 4. From Fig. 14 it can be seen that the hydrocarbon
liquid produced
in the unstressed experiment, represented by data points on line 3042,
contains a lower
weight percentage of lighter normal alkane hydrocarbon components in the
normal-C6 to
normal-C26 compound range as compared to the normal-C29 compound, both as
compared
to the 400 psi stress experiment hydrocarbon liquid and the 1,000 psi stress
experiment
hydrocarbon liquid. Looking now at the data points occurring on line 3043, it
is apparent that
the intermediate level 400 psi stress experiment produced a hydrocarbon liquid
having
normal-C6 to normal-C26 compound concentrations as compared to the normal-C29
compound between the unstressed experiment represented by line 3042 and the
1,000 psi
stressed experiment represented by line 3044. Lastly, it is apparent that the
high level 1,000
psi stress experiment produced a hydrocarbon liquid having normal-C6 to normal-
C26
compound concentrations as compared to the normal-C29 compound greater than
both the
unstressed experiment represented by line 3042 and the 400 psi stressed
experiment
represented by line 3043. This analysis further supports the relationship that
pyrolizing oil
shale under increasing levels of lithostatic stress produces hydrocarbon
liquids having lower
concentrations of normal alkane hydrocarbons.
[0198] Fig. 15 is a graph of the weight ratio of normal alkane
hydrocarbon
compounds to pseudo components for each carbon number from C6 to C38 for each
of the
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 49 -
three stress levels tested and analyzed in the laboratory experiments
discussed herein. The
normal compound and pseudo component weight percentages were obtained as
described for
Figs. 7 & 11. For clarity, the normal alkane hydrocarbon and pseudo component
weight
percentages are taken as a percentage of the entire C3 to pseudo C38 whole oil
gas
chromatography areas and calculated weights as used in the pseudo compound
data presented
in Fig. 7. The y-axis 3060 represents the concentration in terms of weight
ratio of each
normal-C6/pseudo C6 to normal-C38/pseudo C38 compound found in the liquid
phase. The
x-axis 3061 contains the identity of each normal alkane hydrocarbon compound
to pseudo
component ratio from normal-C6/pseudo C6 to normal-C38/pseudo C38. The data
points
occurring on line 3062 represent the weight ratio of each normal-C6/pseudo C6
to normal-
C38/pseudo C38 ratio for the unstressed experiment of Example 1. The data
points occurring
on line 3063 represent the weight ratio of each normal-C6/pseudo C6 to normal-
C38/pseudo
C38 ratio for the 400 psi stressed experiment of Example 3. While the data
points occurring
on line 3064 represent the weight ratio of each normal-C6/pseudo C6 to normal-
C38/pseudo
C38 ratio for the 1,000 psi stressed experiment of Example 4. From Fig. 15 it
can be seen
that the hydrocarbon liquid produced in the unstressed experiment, represented
by data points
on line 3062, contains a greater weight percentage of normal alkane
hydrocarbon compounds
to pseudo components in the C10 to C26 range, both as compared to the 400 psi
stress
experiment hydrocarbon liquid and the 1,000 psi stress experiment hydrocarbon
liquid.
Looking now at the data points occurring on line 3063, it is apparent that the
intermediate
level 400 psi stress experiment produced a hydrocarbon liquid having normal
alkane
hydrocarbon compound to pseudo component ratios in the C10 to C26 range
between the
unstressed experiment represented by line 3062 and the 1,000 psi stressed
experiment
represented by line 3064. Lastly, it is apparent that the high level 1,000 psi
stress experiment
produced a hydrocarbon liquid having normal alkane hydrocarbon compound to
pseudo
component ratios in the C10 to C26 range less than both the unstressed
experiment
represented by line 3062 and the 400 psi stressed experiment represented by
line 3063. Thus
pyrolizing oil shale under increasing levels of lithostatic stress appears to
produce
hydrocarbon liquids having lower concentrations of normal alkane hydrocarbons
as compared
to the total hydrocarbons for a given carbon number occurring between C10 and
C26.
[0199] From the above-described data, it can be seen that heating and
pyrolysis of oil
shale under increasing levels of stress results in a condensable hydrocarbon
fluid product that
is lighter (i.e., greater proportion of lower carbon number compounds or
components relative
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 50 -
to higher carbon number compounds or components) and contains a lower
concentration of
normal alkane hydrocarbon compounds. Such a product may be suitable for
refining into
gasoline and distillate products. Further, such a product, either before or
after further
fractionation, may have utility as a feed stock for certain chemical
processes.
[0200] In some embodiments, the produced hydrocarbon fluid includes a
condensable
hydrocarbon portion. In some embodiments the condensable hydrocarbon portion
may have
one or more of a total C7 to total C20 weight ratio greater than 0.8, a total
C8 to total C20
weight ratio greater than 1.7, a total C9 to total C20 weight ratio greater
than 2.5, a total C10
to total C20 weight ratio greater than 2.8, a total C11 to total C20 weight
ratio greater than
2.3, a total C12 to total C20 weight ratio greater than 2.3, a total C13 to
total C20 weight ratio
greater than 2.9, a total C14 to total C20 weight ratio greater than 2.2, a
total C15 to total C20
weight ratio greater than 2.2, and a total C16 to total C20 weight ratio
greater than 1.6. In
alternative embodiments the condensable hydrocarbon portion has one or more of
a total C7
to total C20 weight ratio greater than 2.5, a total C8 to total C20 weight
ratio greater than 3.0,
a total C9 to total C20 weight ratio greater than 3.5, a total C10 to total
C20 weight ratio
greater than 3.5, a total C11 to total C20 weight ratio greater than 3.0, and
a total C12 to total
C20 weight ratio greater than 3Ø In alternative embodiments the condensable
hydrocarbon
portion has one or more of a total C7 to total C20 weight ratio greater than
3.5, a total C8 to
total C20 weight ratio greater than 4.3, a total C9 to total C20 weight ratio
greater than 4.5, a
total C10 to total C20 weight ratio greater than 4.2, a total C11 to total C20
weight ratio
greater than 3.7, and a total C12 to total C20 weight ratio greater than 3.5.
As used in this
paragraph and in the claims, the phrase "one or more" followed by a listing of
different
compound or component ratios with the last ratio introduced by the conjunction
"and" is
meant to include a condensable hydrocarbon portion that has at least one of
the listed ratios or
that has two or more, or three or more, or four or more, etc., or all of the
listed ratios.
Further, a particular condensable hydrocarbon portion may also have additional
ratios of
different compounds or components that are not included in a particular
sentence or claim
and still fall within the scope of such a sentence or claim. The embodiments
described in this
paragraph may be combined with any of the other aspects of the invention
discussed herein.
[0201] In some embodiments the condensable hydrocarbon portion has a
total C7 to
total C20 weight ratio greater than 0.8. Alternatively, the condensable
hydrocarbon portion
may have a total C7 to total C20 weight ratio greater than 1.0, greater than
1.5, greater than
2.0, greater than 2.5, greater than 3.5 or greater than 3.7. In alternative
embodiments, the
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 51 -
condensable hydrocarbon portion may have a total C7 to total C20 weight ratio
less than
10.0, less than 7.0, less than 5.0 or less than 4Ø In some embodiments the
condensable
hydrocarbon portion has a total C8 to total C20 weight ratio greater than 1.7.
Alternatively,
the condensable hydrocarbon portion may have a total C8 to total C20 weight
ratio greater
than 2.0, greater than 2.5, greater than 3.0, greater than 4.0, greater than
4.4, or greater than
4.6. In alternative embodiments, the condensable hydrocarbon portion may have
a total C8 to
total C20 weight ratio less than 7.0 or less than 6Ø In some embodiments the
condensable
hydrocarbon portion has a total C9 to total C20 weight ratio greater than 2.5.
Alternatively,
the condensable hydrocarbon portion may have a total C9 to total C20 weight
ratio greater
than 3.0, greater than 4.0, greater than 4.5, or greater than 4.7. In
alternative embodiments,
the condensable hydrocarbon portion may have a total C9 to total C20 weight
ratio less than
7.0 or less than 6Ø In some embodiments the condensable hydrocarbon portion
has a total
C10 to total C20 weight ratio greater than 2.8. Alternatively, the condensable
hydrocarbon
portion may have a total C10 to total C20 weight ratio greater than 3.0,
greater than 3.5,
greater than 4.0, or greater than 4.3. In alternative embodiments, the
condensable
hydrocarbon portion may have a total C10 to total C20 weight ratio less than
7.0 or less than
6Ø In some embodiments the condensable hydrocarbon portion has a total C11
to total C20
weight ratio greater than 2.3. Alternatively, the condensable hydrocarbon
portion may have a
total C11 to total C20 weight ratio greater than 2.5, greater than 3.5,
greater than 3.7, greater
than 4Ø In alternative embodiments, the condensable hydrocarbon portion may
have a total
C11 to total C20 weight ratio less than 7.0 or less than 6Ø In some
embodiments the
condensable hydrocarbon portion has a total C12 to total C20 weight ratio
greater than 2.3.
Alternatively, the condensable hydrocarbon portion may have a total C12 to
total C20 weight
ratio greater than 2.5, greater than 3.0, greater than 3.5, or greater than
3.7. In alternative
embodiments, the condensable hydrocarbon portion may have a total C12 to total
C20 weight
ratio less than 7.0 or less than 6Ø In some embodiments the condensable
hydrocarbon
portion has a total C13 to total C20 weight ratio greater than 2.9.
Alternatively, the
condensable hydrocarbon portion may have a total C13 to total C20 weight ratio
greater than
3.0, greater than 3.1, or greater than 3.2. In alternative embodiments, the
condensable
hydrocarbon portion may have a total C13 to total C20 weight ratio less than
6.0 or less than
5Ø In some embodiments the condensable hydrocarbon portion has a total C14
to total C20
weight ratio greater than 2.2. Alternatively, the condensable hydrocarbon
portion may have a
total C14 to total C20 weight ratio greater than 2.5, greater than 2.6, or
greater than 2.7. In
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 52 -
alternative embodiments, the condensable hydrocarbon portion may have a total
C14 to total
C20 weight ratio less than 6.0 or less than 4Ø In some embodiments the
condensable
hydrocarbon portion has a total C15 to total C20 weight ratio greater than
2.2. Alternatively,
the condensable hydrocarbon portion may have a total C15 to total C20 weight
ratio greater
than 2.3, greater than 2.4, or greater than 2.6. In alternative embodiments,
the condensable
hydrocarbon portion may have a total C15 to total C20 weight ratio less than
6.0 or less than
4Ø In some embodiments the condensable hydrocarbon portion has a total C16
to total C20
weight ratio greater than 1.6. Alternatively, the condensable hydrocarbon
portion may have a
total C16 to total C20 weight ratio greater than 1.8, greater than 2.3, or
greater than 2.5. In
alternative embodiments, the condensable hydrocarbon portion may have a total
C16 to total
C20 weight ratio less than 5.0 or less than 4Ø Certain features of the
present invention are
described in terms of a set of numerical upper limits (e.g. "less than") and a
set of numerical
lower limits (e.g. "greater than") in the preceding paragraph. It should be
appreciated that
ranges formed by any combination of these limits are within the scope of the
invention unless
otherwise indicated. The embodiments described in this paragraph may be
combined with
any of the other aspects of the invention discussed herein.
[0202] In some embodiments the condensable hydrocarbon portion may have
the one
or more of a total C7 to total C25 weight ratio greater than 2.0, a total C8
to total C25 weight
ratio greater than 4.5, a total C9 to total C25 weight ratio greater than 6.5,
a total C10 to total
C25 weight ratio greater than 7.5, a total C11 to total C25 weight ratio
greater than 6.5, a total
C12 to total C25 weight ratio greater than 6.5, a total C13 to total C25
weight ratio greater
than 8.0, a total C14 to total C25 weight ratio greater than 6.0, a total C15
to total C25 weight
ratio greater than 6.0, a total C16 to total C25 weight ratio greater than
4.5, a total C17 to
total C25 weight ratio greater than 4.8, and a total C18 to total C25 weight
ratio greater than
4.5. In alternative embodiments the condensable hydrocarbon portion has one or
more of a
total C7 to total C25 weight ratio greater than 7.0, a total C8 to total C25
weight ratio greater
than 10.0, a total C9 to total C25 weight ratio greater than 10.0, a total C10
to total C25
weight ratio greater than 10.0, a total C11 to total C25 weight ratio greater
than 8.0, and a
total C12 to total C25 weight ratio greater than 8Ø In alternative
embodiments the
condensable hydrocarbon portion has one or more of a total C7 to total C25
weight ratio
greater than 13.0, a total C8 to total C25 weight ratio greater than 17.0, a
total C9 to total C25
weight ratio greater than 17.0, a total C10 to total C25 weight ratio greater
than 15.0, a total
C11 to total C25 weight ratio greater than 14.0, and a total C12 to total C25
weight ratio
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 53 -
greater than 13Ø As used in this paragraph and in the claims, the phrase
"one or more"
followed by a listing of different compound or component ratios with the last
ratio introduced
by the conjunction "and" is meant to include a condensable hydrocarbon portion
that has at
least one of the listed ratios or that has two or more, or three or more, or
four or more, etc., or
all of the listed ratios. Further, a particular condensable hydrocarbon
portion may also have
additional ratios of different compounds or components that are not included
in a particular
sentence or claim and still fall within the scope of such a sentence or claim.
The
embodiments described in this paragraph may be combined with any of the other
aspects of
the invention discussed herein.
[0203] In some embodiments the condensable hydrocarbon portion has a
total C7 to
total C25 weight ratio greater than 2Ø Alternatively, the condensable
hydrocarbon portion
may have a total C7 to total C25 weight ratio greater than 3.0, greater than
5.0, greater than
10.0, greater than 13.0, or greater than 15Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C7 to total C25 weight ratio less than
30.0 or less than
25Ø In some embodiments the condensable hydrocarbon portion has a total C8
to total C25
weight ratio greater than 4.5. Alternatively, the condensable hydrocarbon
portion may have a
total C8 to total C25 weight ratio greater than 5.0, greater than 7.0, greater
than 10.0, greater
than 15.0, or greater than 17Ø In alternative embodiments, the condensable
hydrocarbon
portion may have a total C8 to total C25 weight ratio less than 35.0, or less
than 30Ø In
some embodiments the condensable hydrocarbon portion has a total C9 to total
C25 weight
ratio greater than 6.5. Alternatively, the condensable hydrocarbon portion may
have a total
C9 to total C25 weight ratio greater than 8.0, greater than 10.0, greater than
15.0, greater than
17.0, or greater than 19Ø In alternative embodiments, the condensable
hydrocarbon portion
may have a total C9 to total C25 weight ratio less than 40.0 or less than
35Ø In some
embodiments the condensable hydrocarbon portion has a total C10 to total C25
weight ratio
greater than 7.5. Alternatively, the condensable hydrocarbon portion may have
a total C10 to
total C25 weight ratio greater than 10.0, greater than 14.0, or greater than
17Ø In alternative
embodiments, the condensable hydrocarbon portion may have a total C10 to total
C25 weight
ratio less than 35.0 or less than 30Ø In some embodiments the condensable
hydrocarbon
portion has a total C11 to total C25 weight ratio greater than 6.5.
Alternatively, the
condensable hydrocarbon portion may have a total C11 to total C25 weight ratio
greater than
8.5, greater than 10.0, greater than 12.0, or greater than 14Ø In
alternative embodiments, the
condensable hydrocarbon portion may have a total C11 to total C25 weight ratio
less than
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 54 -
35.0 or less than 30Ø In some embodiments the condensable hydrocarbon
portion has a total
C12 to total C25 weight ratio greater than 6.5. Alternatively, the condensable
hydrocarbon
portion may have a total C12 to total C25 weight ratio greater than 8.5, a
total C12 to total
C25 weight ratio greater than 10.0, greater than 12.0, or greater than 14Ø
In alternative
embodiments, the condensable hydrocarbon portion may have a total C12 to total
C25 weight
ratio less than 30.0 or less than 25Ø In some embodiments the condensable
hydrocarbon
portion has a total C13 to total C25 weight ratio greater than 8Ø
Alternatively, the
condensable hydrocarbon portion may have a total C13 to total C25 weight ratio
greater than
10.0, greater than 12.0, or greater than 14Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C13 to total C25 weight ratio less than
25.0 or less than
20Ø In some embodiments the condensable hydrocarbon portion has a total C14
to total
C25 weight ratio greater than 6Ø Alternatively, the condensable hydrocarbon
portion may
have a total C14 to total C25 weight ratio greater than 8.0, greater than
10.0, or greater than
12Ø In alternative embodiments, the condensable hydrocarbon portion may have
a total C14
to total C25 weight ratio less than 25.0 or less than 20Ø In some
embodiments the
condensable hydrocarbon portion has a total C15 to total C25 weight ratio
greater than 6Ø
Alternatively, the condensable hydrocarbon portion may have a total C15 to
total C25 weight
ratio greater than 8.0, or greater than 10Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C15 to total C25 weight ratio less than
25.0 or less than
20Ø In some embodiments the condensable hydrocarbon portion has a total C16
to total
C25 weight ratio greater than 4.5. Alternatively, the condensable hydrocarbon
portion may
have a total C16 to total C25 weight ratio greater than 6.0, greater than 8.0,
or greater than
10Ø In alternative embodiments, the condensable hydrocarbon portion may have
a total C16
to total C25 weight ratio less than 20.0 or less than 15Ø In some
embodiments the
condensable hydrocarbon portion has a total C17 to total C25 weight ratio
greater than 4.8.
Alternatively, the condensable hydrocarbon portion may have a total C17 to
total C25 weight
ratio greater than 5.5 or greater than 7Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C17 to total C25 weight ratio less than
20Ø In some
embodiments the condensable hydrocarbon portion has a total C18 to total C25
weight ratio
greater than 4.5. Alternatively, the condensable hydrocarbon portion may have
a total C18 to
total C25 weight ratio greater than 5.0 or greater than 5.5. In alternative
embodiments, the
condensable hydrocarbon portion may have a total C18 to total C25 weight ratio
less than
15Ø Certain features of the present invention are described in terms of a
set of numerical
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 55 -
upper limits (e.g. "less than") and a set of numerical lower limits (e.g.
"greater than") in the
preceding paragraph. It should be appreciated that ranges formed by any
combination of
these limits are within the scope of the invention unless otherwise indicated.
The
embodiments described in this paragraph may be combined with any of the other
aspects of
the invention discussed herein.
[0204] In some embodiments the condensable hydrocarbon portion may have
the one
or more of a total C7 to total C29 weight ratio greater than 3.5, a total C8
to total C29 weight
ratio greater than 9.0, a total C9 to total C29 weight ratio greater than
12.0, a total C10 to
total C29 weight ratio greater than 15.0, a total C11 to total C29 weight
ratio greater than
13.0, a total C12 to total C29 weight ratio greater than 12.5, and a total C13
to total C29
weight ratio greater than 16.0, a total C14 to total C29 weight ratio greater
than 12.0, a total
C15 to total C29 weight ratio greater than 12.0, a total C16 to total C29
weight ratio greater
than 9.0, a total C17 to total C29 weight ratio greater than 10.0, a total C18
to total C29
weight ratio greater than 8.8, a total C19 to total C29 weight ratio greater
than 7.0, a total C20
to total C29 weight ratio greater than 6.0, a total C21 to total C29 weight
ratio greater than
5.5, and a total C22 to total C29 weight ratio greater than 4.2. In
alternative embodiments the
condensable hydrocarbon portion has one or more of a total C7 to total C29
weight ratio
greater than 16.0, a total C8 to total C29 weight ratio greater than 19.0, a
total C9 to total C29
weight ratio greater than 20.0, a total C10 to total C29 weight ratio greater
than 18.0, a total
C11 to total C29 weight ratio greater than 16.0, a total C12 to total C29
weight ratio greater
than 15.0, and a total C13 to total C29 weight ratio greater than 17.0, a
total C14 to total C29
weight ratio greater than 13.0, a total C15 to total C29 weight ratio greater
than 13.0, a total
C16 to total C29 weight ratio greater than 10.0, a total C17 to total C29
weight ratio greater
than 11.0, a total C18 to total C29 weight ratio greater than 9.0, a total C19
to total C29
weight ratio greater than 8.0, a total C20 to total C29 weight ratio greater
than 6.5, and a total
C21 to total C29 weight ratio greater than 6Ø In alternative embodiments the
condensable
hydrocarbon portion has one or more of a total C7 to total C29 weight ratio
greater than 24.0,
a total C8 to total C29 weight ratio greater than 30.0, a total C9 to total
C29 weight ratio
greater than 32.0, a total C10 to total C29 weight ratio greater than 30.0, a
total C11 to total
C29 weight ratio greater than 27.0, a total C12 to total C29 weight ratio
greater than 25.0, and
a total C13 to total C29 weight ratio greater than 22.0, a total C14 to total
C29 weight ratio
greater than 18.0, a total C15 to total C29 weight ratio greater than 18.0, a
total C16 to total
C29 weight ratio greater than 16.0, a total C17 to total C29 weight ratio
greater than 13.0, a
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 56 -
total C18 to total C29 weight ratio greater than 10.0, a total C19 to total
C29 weight ratio
greater than 9.0, and a total C20 to total C29 weight ratio greater than 7Ø
As used in this
paragraph and in the claims, the phrase "one or more" followed by a listing of
different
compound or component ratios with the last ratio introduced by the conjunction
"and" is
meant to include a condensable hydrocarbon portion that has at least one of
the listed ratios or
that has two or more, or three or more, or four or more, etc., or all of the
listed ratios.
Further, a particular condensable hydrocarbon portion may also have additional
ratios of
different compounds or components that are not included in a particular
sentence or claim
and still fall within the scope of such a sentence or claim. The embodiments
described in this
paragraph may be combined with any of the other aspects of the invention
discussed herein.
[0205] In some embodiments the condensable hydrocarbon portion has a
total C7 to
total C29 weight ratio greater than 3.5. Alternatively, the condensable
hydrocarbon portion
may have a total C7 to total C29 weight ratio greater than 5.0, greater than
10.0, greater than
18.0, greater than 20.0, or greater than 24Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C7 to total C29 weight ratio less than
60.0 or less than
50Ø In some embodiments the condensable hydrocarbon portion has a total C8
to total C29
weight ratio greater than 9Ø Alternatively, the condensable hydrocarbon
portion may have a
total C8 to total C29 weight ratio greater than 10.0, greater than 18.0,
greater than 20.0,
greater than 25.0, or greater than 30Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C8 to total C29 weight ratio less than
85.0 or less than
75Ø In some embodiments the condensable hydrocarbon portion has a total C9
to total C29
weight ratio greater than 12Ø Alternatively, the condensable hydrocarbon
portion may have
a total C9 to total C29 weight ratio greater than 15.0, greater than 20.0,
greater than 23.0,
greater than 27.0, or greater than 32Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a total C9 to total C29 weight ratio less than
85.0 or less than
75Ø In some embodiments the condensable hydrocarbon portion has a total C10
to total
C29 weight ratio greater than 15Ø Alternatively, the condensable hydrocarbon
portion may
have a total C10 to total C29 weight ratio greater than 18.0, greater than
22.0, or greater than
28Ø In alternative embodiments, the condensable hydrocarbon portion may have
a total C10
to total C29 weight ratio less than 80.0 or less than 70Ø In some
embodiments the
condensable hydrocarbon portion has a total C11 to total C29 weight ratio
greater than 13Ø
Alternatively, the condensable hydrocarbon portion may have a total C11 to
total C29 weight
ratio greater than 16.0, greater than 18.0, greater than 24.0, or greater than
27Ø In
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 57 -
alternative embodiments, the condensable hydrocarbon portion may have a total
C11 to total
C29 weight ratio less than 75.0 or less than 65Ø In some embodiments the
condensable
hydrocarbon portion has a total C12 to total C29 weight ratio greater than
12.5.
Alternatively, the condensable hydrocarbon portion may have a total C12 to
total C29 weight
ratio greater than 14.5, greater than 18.0, greater than 22.0, or greater than
25Ø In
alternative embodiments, the condensable hydrocarbon portion may have a total
C12 to total
C29 weight ratio less than 75.0 or less than 65Ø In some embodiments the
condensable
hydrocarbon portion has a total C13 to total C29 weight ratio greater than
16Ø
Alternatively, the condensable hydrocarbon portion may have a total C13 to
total C29 weight
ratio greater than 18.0, greater than 20.0, or greater than 22Ø In
alternative embodiments,
the condensable hydrocarbon portion may have a total C13 to total C29 weight
ratio less than
70.0 or less than 60Ø In some embodiments the condensable hydrocarbon
portion has a total
C14 to total C29 weight ratio greater than 12Ø Alternatively, the
condensable hydrocarbon
portion may have a total C14 to total C29 weight ratio greater than 14.0,
greater than 16.0, or
greater than 18Ø In alternative embodiments, the condensable hydrocarbon
portion may
have a total C14 to total C29 weight ratio less than 60.0 or less than 50Ø
In some
embodiments the condensable hydrocarbon portion has a total C15 to total C29
weight ratio
greater than 12Ø Alternatively, the condensable hydrocarbon portion may have
a total C15
to total C29 weight ratio greater than 15.0 or greater than 18Ø In
alternative embodiments,
the condensable hydrocarbon portion may have a total C15 to total C29 weight
ratio less than
60.0 or less than 50Ø In some embodiments the condensable hydrocarbon
portion has a total
C16 to total C29 weight ratio greater than 9Ø Alternatively, the condensable
hydrocarbon
portion may have a total C16 to total C29 weight ratio greater than 10.0,
greater than 13.0, or
greater than 16Ø In alternative embodiments, the condensable hydrocarbon
portion may
have a total C16 to total C29 weight ratio less than 55.0 or less than 45Ø
In some
embodiments the condensable hydrocarbon portion has a total C17 to total C29
weight ratio
greater than 10Ø Alternatively, the condensable hydrocarbon portion may have
a total C17
to total C29 weight ratio greater than 11.0 or greater than 12Ø In
alternative embodiments,
the condensable hydrocarbon portion may have a total C17 to total C29 weight
ratio less than
45Ø In some embodiments the condensable hydrocarbon portion has a total C18
to total
C29 weight ratio greater than 8.8. Alternatively, the condensable hydrocarbon
portion may
have a total C18 to total C29 weight ratio greater than 9.0 or greater than
10Ø In alternative
embodiments, the condensable hydrocarbon portion may have a total C18 to total
C29 weight
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 58 -
ratio less than 35Ø In some embodiments the condensable hydrocarbon portion
has a total
C19 to total C29 weight ratio greater than 7Ø Alternatively, the condensable
hydrocarbon
portion may have a total C19 to total C29 weight ratio greater than 8.0 or
greater than 9Ø In
alternative embodiments, the condensable hydrocarbon portion may have a total
C19 to total
C29 weight ratio less than 30Ø Certain features of the present invention are
described in
terms of a set of numerical upper limits (e.g. "less than") and a set of
numerical lower limits
(e.g. "greater than") in the preceding paragraph. It should be appreciated
that ranges formed
by any combination of these limits are within the scope of the invention
unless otherwise
indicated. The embodiments described in this paragraph may be combined with
any of the
other aspects of the invention discussed herein.
[0206] In some embodiments the condensable hydrocarbon portion may have
the one
or more of a total C9 to total C20 weight ratio between 2.5 and 6.0, a total
C10 to total C20
weight ratio between 2.8 and 7.3, a total C11 to total C20 weight ratio
between 2.6 and 6.5, a
total C12 to total C20 weight ratio between 2.6 and 6.4 and a total C13 to
total C20 weight
ratio between 3.2 and 8Ø In alternative embodiments the condensable
hydrocarbon portion
has one or more of a total C9 to total C20 weight ratio between 3.0 and 5.5, a
total C10 to
total C20 weight ratio between 3.2 and 7.0, a total C11 to total C20 weight
ratio between 3.0
and 6.0, a total C12 to total C20 weight ratio between 3.0 and 6.0, and a
total C13 to total
C20 weight ratio between 3.3 and 7Ø In alternative embodiments the
condensable
hydrocarbon portion has one or more of a total C9 to total C20 weight ratio
between 4.6 and
5.5, a total C10 to total C20 weight ratio between 4.2 and 7.0, a total C11 to
total C20 weight
ratio between 3.7 and 6.0, a total C12 to total C20 weight ratio between 3.6
and 6.0, and a
total C13 to total C20 weight ratio between 3.4 and 7Ø As used in this
paragraph and in the
claims, the phrase "one or more" followed by a listing of different compound
or component
ratios with the last ratio introduced by the conjunction "and" is meant to
include a
condensable hydrocarbon portion that has at least one of the listed ratios or
that has two or
more, or three or more, or four or more, etc., or all of the listed ratios.
Further, a particular
condensable hydrocarbon portion may also have additional ratios of different
compounds or
components that are not included in a particular sentence or claim and still
fall within the
scope of such a sentence or claim. The embodiments described in this paragraph
may be
combined with any of the other aspects of the invention discussed herein.
[0207] In some embodiments the condensable hydrocarbon portion has a
total C9 to
total C20 weight ratio between 2.5 and 6Ø Alternatively, the condensable
hydrocarbon
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 59 -
portion may have a total C9 to total C20 weight ratio between 3.0 and 5.8,
between 3.5 and
5.8, between 4.0 and 5.8, between 4.5 and 5.8, between 4.6 and 5.8, or between
4.7 and 5.8.
In some embodiments the condensable hydrocarbon portion has a total C10 to
total C20
weight ratio between 2.8 and 7.3. Alternatively, the condensable hydrocarbon
portion may
have a total C10 to total C20 weight ratio between 3.0 and 7.2, between 3.5
and 7.0, between
4.0 and 7.0, between 4.2 and 7.0, between 4.3 and 7.0, or between 4.4 and
7Ø= In some
embodiments the condensable hydrocarbon portion has a total C11 to total C20
weight ratio
between 2.6 and 6.5. Alternatively, the condensable hydrocarbon portion may
have a total
C11 to total C20 weight ratio between 2.8 and 6.3, between 3.5 and 6.3,
between 3.7 and 6.3,
between 3.8 and 6.3, between 3.9 and 6.2, or between 4.0 and 6.2. In some
embodiments the
condensable hydrocarbon portion has a total C12 to total C20 weight ratio
between 2.6 and
6.4. Alternatively, the condensable hydrocarbon portion may have a total C12
to total C20
weight ratio between 2.8 and 6.2, between 3.2 and 6.2, between 3.5 and 6.2,
between 3.6 and
6.2, between 3.7 and 6.0, or between 3.8 and 6Ø In some embodiments the
condensable
hydrocarbon portion has a total C13 to total C20 weight ratio between 3.2 and
8Ø
Alternatively, the condensable hydrocarbon portion may have a total C13 to
total C20 weight
ratio between 3.3 and 7.8, between 3.3 and 7.0, between 3.4 and 7.0, between
3.5 and 6.5, or
between 3.6 and 6Ø The embodiments described in this paragraph may be
combined with
any of the other aspects of the invention discussed herein.
[0208] In some embodiments the condensable hydrocarbon portion may have
one or
more of a total C10 to total C25 weight ratio between 7.1 and 24.5, a total
C11 to total C25
weight ratio between 6.5 and 22.0, a total C12 to total C25 weight ratio
between 6.5 and 22.0,
and a total C13 to total C25 weight ratio between 8.0 and 27Ø In alternative
embodiments
the condensable hydrocarbon portion has one or more of a total C10 to total
C25 weight ratio
between 10.0 and 24.0, a total C11 to total C25 weight ratio between 10.0 and
21.5, a total
C12 to total C25 weight ratio between 10.0 and 21.5, and a total C13 to total
C25 weight ratio
between 9.0 and 25Ø In alternative embodiments the condensable hydrocarbon
portion has
one or more of a total C10 to total C25 weight ratio between 14.0 and 24.0, a
total C11 to
total C25 weight ratio between 12.5 and 21.5, a total C12 to total C25 weight
ratio between
12.0 and 21.5, and a total C13 to total C25 weight ratio between 10.5 and
25Ø As used in
this paragraph and in the claims, the phrase "one or more" followed by a
listing of different
compound or component ratios with the last ratio introduced by the conjunction
"and" is
meant to include a condensable hydrocarbon portion that has at least one of
the listed ratios or
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 60 -
that has two or more, or three or more, or four or more, etc., or all of the
listed ratios.
Further, a particular condensable hydrocarbon portion may also have additional
ratios of
different compounds or components that are not included in a particular
sentence or claim
and still fall within the scope of such a sentence or claim. The embodiments
described in this
paragraph may be combined with any of the other aspects of the invention
discussed herein.
[0209] In some embodiments the condensable hydrocarbon portion has a
total C10 to
total C25 weight ratio between 7.1 and 24.5. Alternatively, the condensable
hydrocarbon
portion may have a total C10 to total C25 weight ratio between 7.5 and 24.5,
between 12.0
and 24.5, between 13.8 and 24.5, between 14.0 and 24.5, or between 15.0 and
24.5. In some
embodiments the condensable hydrocarbon portion has a total C11 to total C25
weight ratio
between 6.5 and 22Ø Alternatively, the condensable hydrocarbon portion may
have a total
C11 to total C25 weight ratio between 7.0 and 21.5, between 10.0 and 21.5,
between 12.5 and
21.5, between 13.0 and 21.5, between 13.7 and 21.5, or between 14.5 and 21.5.
In some
embodiments the condensable hydrocarbon portion has a total C12 to total C25
weight ratio
between 10.0 and 21.5. Alternatively, the condensable hydrocarbon portion may
have a total
C12 to total C25 weight ratio between 10.5 and 21.0, between 11.0 and 21.0,
between 12.0
and 21.0, between 12.5 and 21.0, between 13.0 and 21.0, or between 13.5 and
21Ø In some
embodiments the condensable hydrocarbon portion has a total C13 to total C25
weight ratio
between 8.0 and 27Ø Alternatively, the condensable hydrocarbon portion may
have a total
C13 to total C25 weight ratio between 9.0 and 26.0, between 10.0 and 25.0,
between 10.5 and
25.0, between 11.0 and 25.0, or between 11.5 and 25Ø The embodiments
described in this
paragraph may be combined with any of the other aspects of the invention
discussed herein.
[0210] In some embodiments the condensable hydrocarbon portion may have
one or
more of a total C10 to total C29 weight ratio between 15.0 and 60.0, a total
C11 to total C29
weight ratio between 13.0 and 54.0, a total C12 to total C29 weight ratio
between 12.5 and
53.0, and a total C13 to total C29 weight ratio between 16.0 and 65Ø In
alternative
embodiments the condensable hydrocarbon portion has one or more of a total C10
to total
C29 weight ratio between 17.0 and 58.0, a total C11 to total C29 weight ratio
between 15.0
and 52.0, a total C12 to total C29 weight ratio between 14.0 and 50.0, and a
total C13 to total
C29 weight ratio between 17.0 and 60Ø In alternative embodiments the
condensable
hydrocarbon portion has one or more of a total C10 to total C29 weight ratio
between 20.0
and 58.0, a total C11 to total C29 weight ratio between 18.0 and 52.0, a total
C12 to total C29
weight ratio between 18.0 and 50.0, and a total C13 to total C29 weight ratio
between 18.0
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 61 -
and 50Ø As used in this paragraph and in the claims, the phrase "one or
more" followed by
a listing of different compound or component ratios with the last ratio
introduced by the
conjunction "and" is meant to include a condensable hydrocarbon portion that
has at least one
of the listed ratios or that has two or more, or three or more, or four or
more, etc., or all of the
listed ratios. Further, a particular condensable hydrocarbon portion may also
have additional
ratios of different compounds or components that are not included in a
particular sentence or
claim and still fall within the scope of such a sentence or claim. The
embodiments described
in this paragraph may be combined with any of the other aspects of the
invention discussed
herein.
[0211] In some embodiments the condensable hydrocarbon portion has a
total C10 to
total C29 weight ratio between 15.0 and 60Ø Alternatively, the condensable
hydrocarbon
portion may have a total C10 to total C29 weight ratio between 18.0 and 58.0,
between 20.0
and 58.0, between 24.0 and 58.0, between 27.0 and 58.0, or between 30.0 and
58Ø In some
embodiments the condensable hydrocarbon portion has a total C11 to total C29
weight ratio
between 13.0 and 54Ø Alternatively, the condensable hydrocarbon portion may
have a total
C11 to total C29 weight ratio between 15.0 and 53.0, between 18.0 and 53.0,
between 20.0
and 53.0, between 22.0 and 53.0, between 25.0 and 53.0, or between 27.0 and
53Ø In some
embodiments the condensable hydrocarbon portion has a total C12 to total C29
weight ratio
between 12.5 and 53Ø Alternatively, the condensable hydrocarbon portion may
have a total
C12 to total C29 weight ratio between 14.5 and 51.0, between 16.0 and 51.0,
between 18.0
and 51.0, between 20.0 and 51.0, between 23.0 and 51.0, or between 25.0 and
51Ø In some
embodiments the condensable hydrocarbon portion has a total C13 to total C29
weight ratio
between 16.0 and 65Ø Alternatively, the condensable hydrocarbon portion may
have a total
C13 to total C29 weight ratio between 17.0 and 60.0, between 18.0 and 60.0,
between 20.0
and 60.0, between 22.0 and 60.0, or between 25.0 and 60Ø The embodiments
described in
this paragraph may be combined with any of the other aspects of the invention
discussed
herein.
[0212] In some embodiments the condensable hydrocarbon portion may have
one or
more of a normal-C7 to normal-C20 weight ratio greater than 0.9, a normal-C8
to normal-
C20 weight ratio greater than 2.0, a normal-C9 to normal-C20 weight ratio
greater than 1.9, a
normal-C10 to normal-C20 weight ratio greater than 2.2, a normal-C11 to normal-
C20 weight
ratio greater than 1.9, a normal-C12 to normal-C20 weight ratio greater than
1.9, a normal-
C13 to normal-C20 weight ratio greater than 2.3, a normal-C14 to normal-C20
weight ratio
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 62 -
greater than 1.8, a normal-C15 to normal-C20 weight ratio greater than 1.8,
and normal-C16
to normal-C20 weight ratio greater than 1.3. In alternative embodiments the
condensable
hydrocarbon portion has one or more of a normal-C7 to normal-C20 weight ratio
greater than
4.4, a normal-C8 to normal-C20 weight ratio greater than 3.7, a normal-C9 to
normal-C20
weight ratio greater than 3.5, a normal-C10 to normal-C20 weight ratio greater
than 3.4, a
normal-C11 to normal-C20 weight ratio greater than 3.0, and a normal-C12 to
normal-C20
weight ratio greater than 2.7. In alternative embodiments the condensable
hydrocarbon
portion has one or more of a normal-C7 to normal-C20 weight ratio greater than
4.9, a
normal-C8 to normal-C20 weight ratio greater than 4.5, a normal-C9 to normal-
C20 weight
ratio greater than 4.4, a normal-C10 to normal-C20 weight ratio greater than
4.1, a normal-
C11 to normal-C20 weight ratio greater than 3.7, and a normal-C12 to normal-
C20 weight
ratio greater than 3Ø As used in this paragraph and in the claims, the
phrase "one or more"
followed by a listing of different compound or component ratios with the last
ratio introduced
by the conjunction "and" is meant to include a condensable hydrocarbon portion
that has at
least one of the listed ratios or that has two or more, or three or more, or
four or more, etc., or
all of the listed ratios. Further, a particular condensable hydrocarbon
portion may also have
additional ratios of different compounds or components that are not included
in a particular
sentence or claim and still fall within the scope of such a sentence or claim.
The
embodiments described in this paragraph may be combined with any of the other
aspects of
the invention discussed herein.
[0213] In some embodiments the condensable hydrocarbon portion has a
normal-C7
to normal-C20 weight ratio greater than 0.9. Alternatively, the condensable
hydrocarbon
portion may have a normal-C7 to normal-C20 weight ratio greater than 1.0, than
2.0, greater
than 3.0, greater than 4.0, greater than 4.5, or greater than 5Ø In
alternative embodiments,
the condensable hydrocarbon portion may have a normal-C7 to normal-C20 weight
ratio less
than 8.0 or less than 7Ø In some embodiments the condensable hydrocarbon
portion has a
normal-C8 to normal-C20 weight ratio greater than 1.7. Alternatively, the
condensable
hydrocarbon portion may have a normal-C8 to normal-C20 weight ratio greater
than 2.0,
greater than 2.5, greater than 3.0, greater than 3.5, greater than 4.0, or
greater than 4.4. In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C8 to
normal-C20 weight ratio less than 8.0 or less than 7Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C9 to normal-C20 weight ratio
greater than
1.9. Alternatively, the condensable hydrocarbon portion may have a normal-C9
to normal-
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 63 -
C20 weight ratio greater than 2.0, greater than 3.0, greater than 4.0, or
greater than 4.5. In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C9 to
normal-C20 weight ratio less than 7.0 or less than 6Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C10 to normal-C20 weight ratio
greater than
2.2. Alternatively, the condensable hydrocarbon portion may have a normal-C10
to normal-
C20 weight ratio greater than 2.8, greater than 3.3, greater than 3.5, or
greater than 4Ø In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C10 to
normal-C20 weight ratio less than 7.0 or less than 6Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C11 to normal-C20 weight ratio
greater than
1.9. Alternatively, the condensable hydrocarbon portion may have a normal-C11
to normal-
C20 weight ratio greater than 2.5, greater than 3.0, greater than 3.5, or
greater than 3.7. In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C11 to
normal-C20 weight ratio less than 7.0 or less than 6Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C12 to normal-C20 weight ratio
greater than
1.9. Alternatively, the condensable hydrocarbon portion may have a normal-C12
to normal-
C20 weight ratio greater than 2.0, greater than 2.2, greater than 2.6, or
greater than 3Ø In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C12 to
normal-C20 weight ratio less than 7.0 or less than 6Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C13 to normal-C20 weight ratio
greater than
2.3. Alternatively, the condensable hydrocarbon portion may have a normal-C13
to normal-
C20 weight ratio greater than 2.5, greater than 2.7, or greater than 3Ø In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C13 to
normal-C20
weight ratio less than 6.0 or less than 5Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C14 to normal-C20 weight ratio greater than
1.8.
Alternatively, the condensable hydrocarbon portion may have a normal-C14 to
normal-C20
weight ratio greater than 2.0, greater than 2.2, or greater than 2.5. In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C14 to
normal-C20
weight ratio less than 6.0 or less than 4Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C15 to normal-C20 weight ratio greater than
1.8.
Alternatively, the condensable hydrocarbon portion may have a normal-C15 to
normal-C20
weight ratio greater than 2.0, greater than 2.2, or greater than 2.4. In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C15 to
normal-C20
weight ratio less than 6.0 or less than 4Ø In some embodiments the
condensable
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 64 -
hydrocarbon portion has a normal-C16 to normal-C20 weight ratio greater than
1.3.
Alternatively, the condensable hydrocarbon portion may have a normal-C16 to
normal-C20
weight ratio greater than 1.5, greater than 1.7, or greater than 2Ø In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C16 to
normal-C20
weight ratio less than 5.0 or less than 4Ø Certain features of the present
invention are
described in terms of a set of numerical upper limits (e.g. "less than") and a
set of numerical
lower limits (e.g. "greater than") in the preceding paragraph. It should be
appreciated that
ranges formed by any combination of these limits are within the scope of the
invention unless
otherwise indicated. The embodiments described in this paragraph may be
combined with
any of the other aspects of the invention discussed herein.
[0214] In
some embodiments the condensable hydrocarbon portion may have one or
more of a normal-C7 to normal-C25 weight ratio greater than 1.9, a normal-C8
to normal-
C25 weight ratio greater than 3.9, a normal-C9 to normal-C25 weight ratio
greater than 3.7, a
normal-C10 to normal-C25 weight ratio greater than 4.4, a normal-C11 to normal-
C25 weight
ratio greater than 3.8, a normal-C12 to normal-C25 weight ratio greater than
3.7, a normal-
C13 to normal-C25 weight ratio greater than 4.7, a normal-C14 to normal-C25
weight ratio
greater than 3.7, a normal-C15 to normal-C25 weight ratio greater than 3.7, a
normal-C16 to
normal-C25 weight ratio greater than 2.5, a normal-C17 to normal-C25 weight
ratio greater
than 3.0, and a normal-C18 to normal-C25 weight ratio greater than 3.4. In
alternative
embodiments the condensable hydrocarbon portion has one or more of a normal-C7
to
normal-C25 weight ratio greater than 10, a normal-C8 to normal-C25 weight
ratio greater
than 8.0, a normal-C9 to normal-C25 weight ratio greater than 7.0, a normal-
C10 to normal-
C25 weight ratio greater than 7.0, a normal-C11 to normal-C25 weight ratio
greater than 7.0,
and a normal-C12 to normal-C25 weight ratio greater than 6Ø In alternative
embodiments
the condensable hydrocarbon portion has one or more of a normal-C7 to normal-
C25 weight
ratio greater than 10.0, a normal-C8 to normal-C25 weight ratio greater than
12.0, a normal-
C9 to normal-C25 weight ratio greater than 11.0, a normal-C10 to normal-C25
weight ratio
greater than 11.0, a normal-C11 to normal-C25 weight ratio greater than 9.0,
and a normal-
C12 to normal-C25 weight ratio greater than 8Ø As used in this paragraph and
in the claims,
the phrase "one or more" followed by a listing of different compound or
component ratios
with the last ratio introduced by the conjunction "and" is meant to include a
condensable
hydrocarbon portion that has at least one of the listed ratios or that has two
or more, or three
or more, or four or more, etc., or all of the listed ratios. Further, a
particular condensable
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 65 -
hydrocarbon portion may also have additional ratios of different compounds or
components
that are not included in a particular sentence or claim and still fall within
the scope of such a
sentence or claim. The embodiments described in this paragraph may be combined
with any
of the other aspects of the invention discussed herein.
[0215] In some embodiments the condensable hydrocarbon portion has a
normal-C7
to normal-C25 weight ratio greater than 1.9. Alternatively, the condensable
hydrocarbon
portion may have a normal-C7 to normal-C25 weight ratio greater than 3.0,
greater than 5.0,
greater than 8.0, greater than 10.0, or greater than 13Ø In alternative
embodiments, the
condensable hydrocarbon portion may have a normal-C7 to normal-C25 weight
ratio less
than 35.0 or less than 25Ø In some embodiments the condensable hydrocarbon
portion has a
normal-C8 to normal-C25 weight ratio greater than 3.9. Alternatively, the
condensable
hydrocarbon portion may have a normal-C8 to normal-C25 weight ratio greater
than 4.5,
greater than 6.0, greater than 8.0, greater than 10.0, or greater than 13Ø
In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C8 to
normal-C25
weight ratio less than 35.0 or less than 25Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C9 to normal-C25 weight ratio greater than
3.7.
Alternatively, the condensable hydrocarbon portion may have a normal-C9 to
normal-C25
weight ratio greater than 4.5, greater than 7.0, greater than 10.0, greater
than 12.0, or greater
than 13Ø In alternative embodiments, the condensable hydrocarbon portion may
have a
normal-C9 to normal-C25 weight ratio less than 35.0 or less than 25Ø In some
embodiments
the condensable hydrocarbon portion has a normal-C10 to normal-C25 weight
ratio greater
than 4.4. Alternatively, the condensable hydrocarbon portion may have a normal-
C10 to
normal-C25 weight ratio greater than 6.0, greater than 8.0, or greater than
11Ø In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C10 to
normal-C25
weight ratio less than 35.0 or less than 25Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C11 to normal-C25 weight ratio greater than
3.8.
Alternatively, the condensable hydrocarbon portion may have a normal-C11 to
normal-C25
weight ratio greater than 4.5, greater than 7.0, greater than 8.0, or greater
than 10Ø In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C11 to
normal-C25 weight ratio less than 35.0 or less than 25Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C12 to normal-C25 weight ratio
greater than
3.7. Alternatively, the condensable hydrocarbon portion may have a normal-C12
to normal-
C25 weight ratio greater than 4.5, greater than 6.0, greater than 7.0, or
greater than 8Ø In
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 66 -
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C12 to
normal-C25 weight ratio less than 30.0 or less than 20Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C13 to normal-C25 weight ratio
greater than
4.7. Alternatively, the condensable hydrocarbon portion may have a normal-C13
to normal-
C25 weight ratio greater than 5.0, greater than 6.0, or greater than 7.5. In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C13 to
normal-C25
weight ratio less than 25.0 or less than 20Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C14 to normal-C25 weight ratio greater than
3.7.
Alternatively, the condensable hydrocarbon portion may have a normal-C14 to
normal-C25
weight ratio greater than 4.5, greater than 5.5, or greater than 7Ø In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C14 to
normal-C25
weight ratio less than 25.0 or less than 20Ø In some embodiments the
condensable
hydrocarbon portion has a. normal-C15 to normal-C25 weight ratio greater than
3.7.
Alternatively, the condensable hydrocarbon portion may have a normal-C15 to
normal-C25
weight ratio greater than 4.2 or greater than 5Ø In alternative embodiments,
the condensable
hydrocarbon portion may have a normal-C15 to normal-C25 weight ratio less than
25.0 or
less than 20Ø In some embodiments the condensable hydrocarbon portion has a
normal-C16
to normal-C25 weight ratio greater than 2.5. Alternatively, the condensable
hydrocarbon
portion may have a normal-C16 to normal-C25 weight ratio greater than 3.0,
greater than 4.0,
or greater than 5Ø In alternative embodiments, the condensable hydrocarbon
portion may
have a normal-C16 to normal-C25 weight ratio less than 20.0 or less than 15Ø
In some
embodiments the condensable hydrocarbon portion has a normal-C17 to normal-C25
weight
ratio greater than 3Ø Alternatively, the condensable hydrocarbon portion may
have a
normal-C17 to normal-C25 weight ratio greater than 3.5 or greater than 4Ø In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C17 to
normal-C25
weight ratio less than 20Ø In some embodiments the condensable hydrocarbon
portion has a
normal-C18 to normal-C25 weight ratio greater than 3.4. Alternatively, the
condensable
hydrocarbon portion may have a normal-C18 to normal-C25 weight ratio greater
than 3.6 or
greater than 4Ø In alternative embodiments, the condensable hydrocarbon
portion may have
a normal-C18 to normal-C25 weight ratio less than 15Ø Certain features of
the present
invention are described in terms of a set of numerical upper limits (e.g.
"less than") and a set
of numerical lower limits (e.g. "greater than") in the preceding paragraph. It
should be
appreciated that ranges formed by any combination of these limits are within
the scope of the
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 67 -
invention unless otherwise indicated. The embodiments described in this
paragraph may be
combined with any of the other aspects of the invention discussed herein.
[0216] In some embodiments the condensable hydrocarbon portion may have
one or
more of a normal-C7 to normal-C29 weight ratio greater than 18.0, a normal-C8
to normal-
C29 weight ratio greater than 16.0, a normal-C9 to normal-C29 weight ratio
greater than
14.0, a normal-C10 to normal-C29 weight ratio greater than 14.0, a normal-C11
to normal-
C29 weight ratio greater than 13.0, a normal-C12 to normal-C29 weight ratio
greater than
11.0, a normal-C13 to normal-C29 weight ratio greater than 10.0, a normal-C14
to normal-
C29 weight ratio greater than 9.0, a normal-C15 to normal-C29 weight ratio
greater than 8.0,
a normal-C16 to normal-C29 weight ratio greater than 8.0, a normal-C17 to
normal-C29
weight ratio greater than 6.0, a normal-C18 to normal-C29 weight ratio greater
than 6.0, a
normal-C19 to normal-C29 weight ratio greater than 5.0, a normal-C20 to normal-
C29 weight
ratio greater than 4.0, a normal-C21 to normal-C29 weight ratio greater than
3.6, and a
normal-C22 to normal-C29 weight ratio greater than 2.8. In alternative
embodiments the
condensable hydrocarbon portion has one or more of a normal-C7 to normal-C29
weight ratio
greater than 20.0, a normal-C8 to normal-C29 weight ratio greater than 18.0, a
normal-C9 to
normal-C29 weight ratio greater than 17.0, a normal-C10 to normal-C29 weight
ratio greater
than 16.0, a normal-C11 to normal-C29 weight ratio greater than 15.0, a normal-
C12 to
normal-C29 weight ratio greater than 12.5, a normal-C13 to normal-C29 weight
ratio greater
than 11.0, a normal-C14 to normal-C29 weight ratio greater than 10.0, a normal-
C15 to
normal-C29 weight ratio greater than 8.0, a normal-C16 to normal-C29 weight
ratio greater
than 8.0, a normal-C17 to normal-C29 weight ratio greater than 7.0, a normal-
C18 to normal-
C29 weight ratio greater than 6.5, a normal-C19 to normal-C29 weight ratio
greater than 5.5,
a normal-C20 to normal-C29 weight ratio greater than 4.5, and a normal-C21 to
normal-C29
weight ratio greater than 4Ø In alternative embodiments the condensable
hydrocarbon
portion has one or more of a normal-C7 to normal-C29 weight ratio greater than
23.0, a
normal-C8 to normal-C29 weight ratio greater than 21.0, a normal-C9 to normal-
C29 weight
ratio greater than 20.0, a normal-C10 to normal-C29 weight ratio greater than
19.0, a normal-
C11 to normal-C29 weight ratio greater than 17.0, a normal-C12 to normal-C29
weight ratio
greater than 14.0, a normal-C13 to normal-C29 weight ratio greater than 12.0,
a normal-C14
to normal-C29 weight ratio greater than 11.0, a normal-C15 to normal-C29
weight ratio
greater than 9.0, a normal-C16 to normal-C29 weight ratio greater than 9.0, a
normal-C17 to
normal-C29 weight ratio greater than 7.5, a normal-C18 to normal-C29 weight
ratio greater
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 68 -
than 7.0, a normal-C19 to normal-C29 weight ratio greater than 6.5, a normal-
C20 to normal-
C29 weight ratio greater than 4.8, and a normal-C21 to normal-C29 weight ratio
greater than
4.5. As used in this paragraph and in the claims, the phrase "one or more"
followed by a
listing of different compound or component ratios with the last ratio
introduced by the
conjunction "and" is meant to include a condensable hydrocarbon portion that
has at least one
of the listed ratios or that has two or more, or three or more, or four or
more, etc., or all of the
listed ratios. Further, a particular condensable hydrocarbon portion may also
have additional
ratios of different compounds or components that are not included in a
particular sentence or
claim and still fall within the scope of such a sentence or claim. The
embodiments described
in this paragraph may be combined with any of the other aspects of the
invention discussed
herein.
[0217] In some embodiments the condensable hydrocarbon portion has a
normal-C7
to normal-C29 weight ratio greater than 18Ø Alternatively, the condensable
hydrocarbon
portion may have a normal-C7 to normal-C29 weight ratio greater than 20.0,
greater than
22.0, greater than 25.0, greater than 30.0, or greater than 35Ø In
alternative embodiments,
the condensable hydrocarbon portion may have a normal-C7 to normal-C29 weight
ratio less
than 70.0 or less than 60Ø In some embodiments the condensable hydrocarbon
portion has a
normal-C8 to normal-C29 weight ratio greater than 16Ø Alternatively, the
condensable
hydrocarbon portion may have a normal-C8 to normal-C29 weight ratio greater
than 18.0,
greater than 22.0, greater than 25.0, greater than 27.0, or greater than 30Ø
In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C8 to
normal-C29
weight ratio less than 85.0 or less than 75Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C9 to normal-C29 weight ratio greater than
14Ø
Alternatively, the condensable hydrocarbon portion may have a normal-C9 to
normal-C29
weight ratio greater than 18.0, greater than 20.0, greater than 23.0, greater
than 27.0, or
greater than 30Ø In alternative embodiments, the condensable hydrocarbon
portion may
have a normal-C9 to normal-C29 weight ratio less than 85.0 or less than 75Ø
In some
embodiments the condensable hydrocarbon portion has a normal-C10 to normal-C29
weight
ratio greater than 14Ø Alternatively, the condensable hydrocarbon portion
may have a
normal-C10 to normal-C29 weight ratio greater than 20.0, greater than 25.0, or
greater than
30Ø In alternative embodiments, the condensable hydrocarbon portion may have
a normal-
C10 to normal-C29 weight ratio less than 80.0 or less than 70Ø In some
embodiments the
condensable hydrocarbon portion has a normal-C11 to normal-C29 weight ratio
greater than
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 69 -
13Ø Alternatively, the condensable hydro-carbon portion may have a normal-
C1l to normal-
C29 weight ratio greater than 16.0, greater than 18.0, greater than 24.0, or
greater than 27Ø
In alternative embodiments, the condensable hydrocarbon portion may have a
normal-C11 to
normal-C29 weight ratio less than 75.0 or less than 65Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C12 to normal-C29 weight ratio
greater than
11Ø Alternatively, the condensable hydrocarbon portion may have a normal-C12
to normal-
C29 weight ratio greater than 14.5, greater than 18.0, greater than 22.0, or
greater than 25Ø
In alternative embodiments, the condensable hydrocarbon portion may have a
normal-C12 to
normal-C29 weight ratio less than 75.0 or less than 65Ø In some embodiments
the
condensable hydrocarbon portion has a normal-C13 to normal-C29 weight ratio
greater than
10Ø Alternatively, the condensable hydrocarbon portion may have a normal-C13
to normal-
C29 weight ratio greater than 18.0, greater than 20.0, or greater than 22Ø
In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C13 to
normal-C29
weight ratio less than 70.0 or less than 60Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C14 to normal-C29 weight ratio greater than
9Ø
Alternatively, the condensable hydrocarbon portion may have a normal-C14 to
normal-C29
weight ratio greater than 14.0, greater than 16.0, or greater than 18Ø In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C14 to
normal-C29
weight ratio less than 60.0 or less than 50Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C15 to normal-C29 weight ratio greater than
8Ø
Alternatively, the condensable hydrocarbon portion may have a normal-C15 to
normal-C29
weight ratio greater than 12.0 or greater than 16Ø In alternative
embodiments, the
condensable hydrocarbon portion may have a normal-C15 to normal-C29 weight
ratio less
than 60.0 or less than 50Ø In some embodiments the condensable hydrocarbon
portion has a
normal-C16 to normal-C29 weight ratio greater than 8Ø Alternatively, the
condensable
hydrocarbon portion may have a normal-C16 to normal-C29 weight ratio greater
than 10.0,
greater than 13.0, or greater than 15Ø In alternative embodiments, the
condensable
hydrocarbon portion may have a normal-C16 to normal-C29 weight ratio less than
55.0 or
less than 45Ø In some embodiments the condensable hydrocarbon portion has a
normal-C17
to normal-C29 weight ratio greater than 6Ø Alternatively, the condensable
hydrocarbon
portion may have a normal-C17 to normal-C29 weight ratio greater than 8.0 or
greater than
12Ø In alternative embodiments, the condensable hydrocarbon portion may have
a normal-
C17 to normal-C29 weight ratio less than 45Ø In some embodiments the
condensable
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 70 -
hydrocarbon portion has a normal-C18 to normal-C29 weight ratio greater than
6Ø
Alternatively, the condensable hydrocarbon portion may have a normal-C18 to
normal-C29
weight ratio greater than 8.0 or greater than 10Ø In alternative
embodiments, the
condensable hydrocarbon portion may have a normal-C18 to normal-C29 weight
ratio less
than 35Ø In some embodiments the condensable hydrocarbon portion has a
normal-C19 to
normal-C29 weight ratio greater than 5Ø Alternatively, the condensable
hydrocarbon
portion may have a normal-C19 to normal-C29 weight ratio greater than 7.0 or
greater than
9Ø In alternative embodiments, the condensable hydrocarbon portion may have
a normal-
C19 to normal-C29 weight ratio less than 30Ø In some embodiments the
condensable
hydrocarbon portion has a normal-C20 to normal-C29 weight ratio greater than
4Ø
Alternatively, the condensable hydrocarbon portion may have a normal-C20 to
normal-C29
weight ratio greater than 6.0 or greater than 8Ø In alternative embodiments,
the condensable
hydrocarbon portion may have a normal-C20 to normal-C29 weight ratio less than
30Ø In
some embodiments the condensable hydrocarbon portion has a normal-C21 to
normal-C29
weight ratio greater than 3.6. Alternatively, the condensable hydrocarbon
portion may have a
normal-C21 to normal-C29 weight ratio greater than 4.0 or greater than 6Ø In
alternative
embodiments, the condensable hydrocarbon portion may have a normal-C21 to
normal-C29
weight ratio less than 30Ø In some embodiments the condensable hydrocarbon
portion has a
normal-C22 to normal-C29 weight ratio greater than 2.8. Alternatively, the
condensable
hydrocarbon portion may have a normal-C22 to normal-C29 weight ratio greater
than 3Ø In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C22 to
normal-C29 weight ratio less than 30Ø Certain features of the present
invention are
described in terms of a set of numerical upper limits (e.g. "less than") and a
set of numerical
lower limits (e.g. "greater than") in the preceding paragraph. It should be
appreciated that
ranges formed by any combination of these limits are within the scope of the
invention unless
otherwise indicated. The embodiments described in this paragraph may be
combined with
any of the other aspects of the invention discussed herein.
[0218] In some embodiments the condensable hydrocarbon portion may have
one or
more of a normal-C10 to total C10 weight ratio less than 0.31, a normal-C 1 1
to total C11
weight ratio less than 0.32, a normal-C12 to total C12 weight ratio less than
0.29, a normal-
C13 to total C13 weight ratio less than 0.28, a normal-C14 to total C14 weight
ratio less than
0.31, a normal-C15 to total C15 weight ratio less than 0.27, a normal-C16 to
total C16 weight
ratio less than 0.31, a normal-C17 to total C17 weight ratio less than 0.31, a
normal-C18 to
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 71 -
total C18 weight ratio less than 0.37, normal-C19 to total C19 weight ratio
less than 0.37, a
normal-C20 to total C20 weight ratio less than 0.37, a normal-C21 to total C21
weight ratio
less than 0.37, a normal-C22 to total C22 weight ratio less than 0.38, normal-
C23 to total C23
weight ratio less than 0.43, a normal-C24 to total C24 weight ratio less than
0.48, and a
normal-C25 to total C25 weight ratio less than 0.53. In alternative
embodiments the
condensable hydrocarbon portion has one or more of a normal-C11 to total C11
weight ratio
less than 0.30, a normal-C12 to total C12 weight ratio less than 0.27, a
normal-C13 to total
C13 weight ratio less than 0.26, a normal-C14 to total C14 weight ratio less
than 0.29, a
normal-C15 to total C15 weight ratio less than 0.24, a normal-C16 to total C16
weight ratio
less than 0.25, a normal-C17 to total C17 weight ratio less than 0.29, a
normal-C18 to total
C18 weight ratio less than 0.31, normal-C19 to total C19 weight ratio less
than 0.35, a
normal-C20 to total C20 weight ratio less than 0.33, a normal-C21 to total C21
weight ratio
less than 0.33, a normal-C22 to total C22 weight ratio less than 0.35, normal-
C23 to total C23
weight ratio less than 0.40, a normal-C24 to total C24 weight ratio less than
0.45, and a
normal-C25 to total C25 weight ratio less than 0.49. In alternative
embodiments the
condensable hydrocarbon portion has one or more of a normal-C11 to total C11
weight ratio
less than 0.28, a normal-C12 to total C12 weight ratio less than 0.25, a
normal-C13 to total
C13 weight ratio less than 0.24, a normal-C14 to total C14 weight ratio less
than 0.27, a
normal-C15 to total C15 weight ratio less than 0.22, a normal-C16 to total C16
weight ratio
less than 0.23, a normal-C17 to total C17 weight ratio less than 0.25, a
normal-C18 to total
C18 weight ratio less than 0.28, normal-C19 to total C19 weight ratio less
than 0.31, a
normal-C20 to total C20 weight ratio less than 0.29, a normal-C21 to total C21
weight ratio
less than 0.30, a normal-C22 to total C22 weight ratio less than 0.28, normal-
C23 to total C23
weight ratio less than 0.33, a normal-C24 to total C24 weight ratio less than
0.40, and a
normal-C25 to total C25 weight ratio less than 0.45. As used in this paragraph
and in the
claims, the phrase "one or more" followed by a listing of different compound
or component
ratios with the last ratio introduced by the conjunction "and" is meant to
include a
condensable hydrocarbon portion that has at least one of the listed ratios or
that has two or
more, or three or more, or four or more, etc., or all of the listed ratios.
Further, a particular
condensable hydrocarbon portion may also have additional ratios of different
compounds or
components that are not included in a particular sentence or claim and still
fall within the
scope of such a sentence or claim. The embodiments described in this paragraph
may be
combined with any of the other aspects of the invention discussed herein.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 72 -
[0219] In some embodiments the condensable hydrocarbon portion has a
normal-C10
to total C10 weight ratio less than 0.31. Alternatively, the condensable
hydrocarbon portion
may have a normal-C10 to total C10 weight ratio less than 0.30 or less than
0.29. In
alternative embodiments, the condensable hydrocarbon portion may have a normal-
C10 to
total C10 weight ratio greater than 0.15 or greater than 0.20. In some
embodiments the
condensable hydrocarbon portion has a normal-C11 to total C11 weight ratio
less than 0.32.
Alternatively, the condensable hydrocarbon portion may have a normal-C11 to
total C11
weight ratio less than 0.31, less than 0.30, or less than 0.29. In alternative
embodiments, the
condensable hydrocarbon portion may have a normal-C11 to total C11 weight
ratio greater
than 0.15 or greater than 0.20. In some embodiments the condensable
hydrocarbon portion
has a normal-C12 to total C12 weight ratio less than 0.29. Alternatively, the
condensable
hydrocarbon portion may have a normal-C12 to total C12 weight ratio less than
0.26, or less
than 0.24. In alternative embodiments, the condensable hydrocarbon portion may
have a
normal-C12 to total C12 weight ratio greater than 0.10 or greater than 0.15.
In some
embodiments the condensable hydrocarbon portion has a normal-C13 to total C13
weight
ratio less than 0.28. Alternatively, the condensable hydrocarbon portion may
have a normal-
C13 to total C13 weight ratio less than 0.27, less than 0.25, or less than
0.23. In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C13 to
total C13
weight ratio greater than 0.10 or greater than 0.15. In some embodiments the
condensable
hydrocarbon portion has a normal-C14 to total C14 weight ratio less than 0.31.
Alternatively,
the condensable hydrocarbon portion may have a normal-C14 to total C14 weight
ratio less
than 0.30, less than 0.28, or less than 0.26. In alternative embodiments, the
condensable
hydrocarbon portion may have a normal-C14 to total C14 weight ratio greater
than 0.10 or
greater than 0.15. In some embodiments the condensable hydrocarbon portion has
a normal-
C15 to total C15 weight ratio less than 0.27. Alternatively, the condensable
hydrocarbon
portion may have a normal-C15 to total C15 weight ratio less than 0.26, less
than 0.24, or less
than 0.22. In alternative embodiments, the condensable hydrocarbon portion may
have a
normal-C15 to total C15 weight ratio greater than 0.10 or greater than 0.15.
In some
embodiments the condensable hydrocarbon portion has a normal-C16 to total C16
weight
ratio less than 0.31. Alternatively, the condensable hydrocarbon portion may
have a normal-
C16 to total C16 weight ratio less than 0.29, less than 0.26, or less than
0.24. In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C16 to
total C16
weight ratio greater than 0.10 or greater than 0.15. In some embodiments the
condensable
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 73 -
hydrocarbon portion has a normal-C17 to total C17 weight ratio less than 0.31.
Alternatively,
the condensable hydrocarbon portion may have a normal-C17 to total C17 weight
ratio less
than 0.29, less than 0.27, or less than 0.25. In alternative embodiments, the
condensable
hydrocarbon portion may have a normal-C17 to total C17 weight ratio greater
than 0.10 or
greater than 0.15. In some embodiments the condensable hydrocarbon portion has
a normal-
C18 to total C18 weight ratio less than 0.37. Alternatively, the condensable
hydrocarbon
portion may have a normal-C18 to total C18 weight ratio less than 0.35, less
than 0.31, or less
than 0.28. In alternative embodiments, the condensable hydrocarbon portion may
have a
normal-C18 to total C18 weight ratio greater than 0.10 or greater than 0.15.
In some
embodiments the condensable hydrocarbon portion has a normal-C19 to total C19
weight
ratio less than 0.37. Alternatively, the condensable hydrocarbon portion may
have a normal-
C19 to total C19 weight ratio less than 0.36, less than 0.34, or less than
0.31. In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C19 to
total C19
weight ratio greater than 0.10 or greater than 0.15. In some embodiments the
condensable
hydrocarbon portion has a normal-C20 to total C20 weight ratio less than 0.37.
Alternatively,
the condensable hydrocarbon portion may have a normal-C20 to total C20 weight
ratio less
than 0.35, less than 0.32, or less than 0.29. In alternative embodiments, the
condensable
hydrocarbon portion may have a normal-C20 to total C20 weight ratio greater
than 0.10 or
greater than 0.15. In some embodiments the condensable hydrocarbon portion has
a normal-
C21 to total C21 weight ratio less than 0.37. Alternatively, the condensable
hydrocarbon
portion may have a normal-C21 to total C21 weight ratio less than 0.35, less
than 0.32, or less
than 0.30. In alternative embodiments, the condensable hydrocarbon portion may
have a
normal-C21 to total C21 weight ratio greater than 0.10 or greater than 0.15.
In some
embodiments the condensable hydrocarbon portion has a normal-C22 to total C22
weight
ratio less than 0.38. Alternatively, the condensable hydrocarbon portion may
have a normal-
C22 to total C22 weight ratio less than 0.36, less than 0.34, or less than
0.30. In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C22 to
total C22
weight ratio greater than 0.10 or greater than 0.15. In some embodiments the
condensable
hydrocarbon portion has a normal-C23 to total C23 weight ratio less than 0.43.
Alternatively,
the condensable hydrocarbon portion may have a normal-C23 to total C23 weight
ratio less
than 0.40, less than 0.35, or less than 0.29. In alternative embodiments, the
condensable
hydrocarbon portion may have a normal-C23 to total C23 weight ratio greater
than 0.15 or
greater than 0.20. In some embodiments the condensable hydrocarbon portion has
a normal-
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 74 -
C24 to total C24 weight ratio less than 0.48. Alternatively, the condensable
hydrocarbon
portion may have a normal-C24 to total C24 weight ratio less than 0.46, less
than 0.42, or less
than 0.40. In alternative embodiments, the condensable hydrocarbon portion may
have a
normal-C24 to total C24 weight ratio greater than 0.15 or greater than 0.20.
In some
embodiments the condensable hydrocarbon portion has a normal-C25 to total C25
weight
ratio less than 0.48. Alternatively, the condensable hydrocarbon portion may
have a normal-
C25 to total C25 weight ratio less than 0.46, less than 0.42, or less than
0.40. In alternative
embodiments, the condensable hydrocarbon portion may have a normal-C25 to
total C25
weight ratio greater than 0.20 or greater than 0.25. Certain features of the
present invention
are described in terms of a set of numerical upper limits (e.g. "less than")
and a set of
numerical lower limits (e.g. "greater than") in the preceding paragraph. It
should be
appreciated that ranges formed by any combination of these limits are within
the scope of the
invention unless otherwise indicated. The embodiments described in this
paragraph may be
combined with any of the other aspects of the invention discussed herein.
[0220] The use of "total C_" (e.g., total C10) herein and in the claims
is meant to refer
to the amount of a particular pseudo component found in a condensable
hydrocarbon fluid
determined as described herein, particularly as described in the section
labeled "Experiments"
herein. That is "total C_" is determined using the whole oil gas
chromatography (WOGC)
analysis methodology according to the procedure described in the Experiments
section of this
application. Further, "total C_" is determined from the whole oil gas
chromatography
(WOGC) peak integration methodology and peak identification methodology used
for
identifying and quantifying each pseudo-component as described in the
Experiments section
herein. Further, "total C_" weight percent and mole percent values for the
pseudo
components were obtained using the pseudo component analysis methodology
involving
correlations developed by Katz and Firoozabadi (Katz, D.L., and A.
Firoozabadi, 1978.
Predicting phase behavior of condensate/crude-oil systems using methane
interaction
coefficients, J. Petroleum Technology (Nov. 1978), 1649-1655) as described in
the
Experiments section, including the exemplary molar and weight percentage
determinations.
[0221] The use of "normal-C_" (e.g., normal-C10) herein and in the claims
is meant
to refer to the amount of a particular normal alkane hydrocarbon compound
found in a
condensable hydrocarbon fluid determined as described herein, particularly in
the section
labeled "Experiments" herein. That is "normal-C_" is determined from the GC
peak areas
determined using the whole oil gas chromatography (WOGC) analysis methodology
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 75 -
according to the procedure described in the Experiments section of this
application. Further,
"total C_" is determined from the whole oil gas chromatography (WOGC) peak
identification
and integration methodology used for identifying and quantifying individual
compound peaks
as described in the Experiments section herein. Further, "normal-C_" weight
percent and
mole percent values for the normal alkane compounds were obtained using
methodology
analogous to the pseudo component exemplary molar and weight percentage
determinations
explained in the Experiments section, except that the densities and molecular
weights for the
particular normal alkane compound of interest were used and then compared to
the totals
obtained in the pseudo component methodology to obtain weight and molar
percentages.
[0222] The following discussion of Fig. 16 concerns data obtained in
Examples 1 - 5
which are discussed in the section labeled "Experiments". The data was
obtained through the
experimental procedures, gas sample collection procedures, hydrocarbon gas
sample gas
chromatography (GC) analysis methodology, and gas sample GC peak
identification and
integration methodology discussed in the Experiments section. For clarity,
when referring to
gas chromatograms of gaseous hydrocarbon samples, graphical data is provided
for one
unstressed experiment through Example 1, two 400 psi stressed experiments
through
Examples 2 and 3, and two 1,000 psi stressed experiments through Examples 4
and 5.
[0223] Fig. 16 is a bar graph showing the concentration, in molar
percentage, of the
hydrocarbon species present in the gas samples taken from each of the three
stress levels
tested and analyzed in the laboratory experiments discussed herein. The gas
compound molar
percentages were obtained through the experimental procedures, gas sample
collection
procedures, hydrocarbon gas sample gas chromatography (GC) analysis
methodology, gas
sample GC peak integration methodology and molar concentration determination
procedures
described herein. For clarity, the hydrocarbon molar percentages are taken as
a percentage of
the total of all identified hydrocarbon gas GC areas (i.e., methane, ethane,
propane, iso-
butane, n-butane, iso-pentane, n-pentane, 2-methyl pentane, and n-hexane) and
calculated
molar concentrations. Thus the graphed methane to normal C6 molar percentages
for all of
the experiments do not include the molar contribution of any associated non-
hydrocarbon gas
phase product (e.g., hydrogen, CO2 or H2S), any of the unidentified
hydrocarbon gas species
listed in Tables 2, 4, 5, 7, or 9 (e.g., peak numbers 2, 6, 8-11, 13, 15-22,
24-26, and 28-78 in
Table 2) or any of the gas species dissolved in the liquid phase which were
separately treated
in the liquid GC's. The y-axis 3080 represents the concentration in terms of
molar percent of
each gaseous compound in the gas phase. The x-axis 3081 contains the identity
of each
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 76 -
hydrocarbon compound from methane to normal hexane. The bars 3082A-I represent
the
molar percentage of each gaseous compound for the unstressed experiment of
Example 1.
That is 3082A represents methane, 3082B represents ethane, 3082C represents
propane,
3082D represents iso-butane, 3082E represents normal butane, 3082F represents
iso-pentane,
3082G represents normal pentane, 3082H represents 2-methyl pentane, and 30821
represents
normal hexane. The bars 3083A-I and 3084A-I represent the molar percent of
each gaseous
compound for samples from the duplicate 400 psi stressed experiments of
Examples 2 and 3,
with the letters assigned in the manner described for the unstressed
experiment. While the
bars 3085A-I and 3086A-I represent the molar percent of each gaseous compound
for the
duplicate 1,000 psi stressed experiments of Examples 4 and 5, with the letters
assigned in the
manner described for the unstressed experiment. From Fig. 16 it can be seen
that the
hydrocarbon gas produced in all the experiments is primarily methane, ethane
and propane on
a molar basis. It is further apparent that the unstressed experiment,
represented by bars
3082A-I, contains the most methane 3082A and least propane 3082C, both as
compared to
the 400 psi stress experiments hydrocarbon gases and the 1,000 psi stress
experiments
hydrocarbon gases. Looking now at bars 3083A-I and 3084A-I, it is apparent
that the
intermediate level 400 psi stress experiments produced a hydrocarbon gas
having methane
3083A & 3084A and propane 3083C & 3084C concentrations between the unstressed
experiment represented by bars 3082A & 3082C and the 1,000 psi stressed
experiment
represented by bars 3085A & 3085C and 3086A & 3086C. Lastly, it is apparent
that the high
level 1,000 psi stress experiments produced hydrocarbon gases having the
lowest methane
3085A & 3086A concentration and the highest propane concentrations 3085C &
3086C, as
compared to both the unstressed experiments represented by bars 3082A & 3082C
and the
400 psi stressed experiment represented by bars 3083A & 3084A and 3083C &
3084C. Thus
pyrolizing oil shale under increasing levels of lithostatic stress appears to
produce
hydrocarbon gases having decreasing concentrations of methane and increasing
concentrations of propane.
[0224] The hydrocarbon fluid produced from the organic-rich rock
formation may
include both a condensable hydrocarbon portion (e.g. liquid) and a non-
condensable
hydrocarbon portion (e.g. gas). In some embodiments the non-condensable
hydrocarbon
portion includes methane and propane. In some embodiments the molar ratio of
propane to
methane in the non-condensable hydrocarbon portion is greater than 0.32. In
alternative
embodiments, the molar ratio of propane to methane in the non-condensable
hydrocarbon
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 77 -
portion is greater than 0.34, 0.36 or 0.38. As used herein "molar ratio of
propane to methane"
is the molar ratio that may be determined as described herein, particularly as
described in the
section labeled "Experiments" herein. That is "molar ratio of propane to
methane" is
determined using the hydrocarbon gas sample gas chromatography (GC) analysis
methodology, gas sample GC peak identification and integration methodology and
molar
concentration determination procedures described in the Experiments section of
this
application.
[0225] In some embodiments the condensable hydrocarbon portion of the
hydrocarbon fluid includes benzene. In some embodiments the condensable
hydrocarbon
portion has a benzene content between 0.1 and 0.8 weight percent.
Alternatively, the
condensable hydrocarbon portion may have a benzene content between 0.15 and
0.6 weight
percent, a benzene content between 0.15 and 0.5, or a benzene content between
0.15 and 0.5.
[0226] In some embodiments the condensable hydrocarbon portion of the
hydrocarbon fluid includes cyclohexane. In some embodiments the condensable
hydrocarbon
portion has a cyclohexane content less than 0.8 weight percent. Alternatively,
the
condensable hydrocarbon portion may have a cyclohexane content less than 0.6
weight
percent or less than 0.43 weight percent. Alternatively, the condensable
hydrocarbon portion
may have a cyclohexane content greater than 0.1 weight percent or greater than
0.2 weight
percent.
[0227] In some embodiments the condensable hydrocarbon portion of the
hydrocarbon fluid includes methyl-cyclohexane. In some embodiments the
condensable
hydrocarbon portion has a methly-cyclohexane content greater than 0.5 weight
percent.
Alternatively, the condensable hydrocarbon portion may have a methly-
cyclohexane content
greater than 0.7 weight percent or greater than 0.75 weight percent.
Alternatively, the
condensable hydrocarbon portion may have a methly-cyclohexane content less
than 1.2 or 1.0
weight percent.
[0228] The use of weight percentage contents of benzene, cyclohexane, and
methyl-
cyclohexane herein and in the claims is meant to refer to the amount of
benzene,
cyclohexane, and methyl-cyclohexane found in a condensable hydrocarbon fluid
determined
as described herein, particularly as described in the section labeled
"Experiments" herein.
That is, respective compound weight percentages are determined from the whole
oil gas
chromatography (WOGC) analysis methodology and whole oil gas chromatography
(WOGC)
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 78 -
peak identification and integration methodology discussed in the Experiments
section herein.
Further, the respective compound weight percentages were obtained as described
for Fig. 11,
except that each individual respective compound peak area integration was used
to determine
each respective compound weight percentage. For clarity, the compound weight
percentages
are taken as a percentage of the entire C3 to pseudo C38 whole oil gas
chromatography areas
and calculated weights as used in the pseudo compound data presented in Fig.
7.
[0229] In some embodiments the condensable hydrocarbon portion of the
hydrocarbon fluid has an API gravity greater than 30. Alternatively, the
condensable
hydrocarbon portion may have an API gravity greater than 30, 32, 34, 36, 40,
42 or 44. As
used herein and in the claims, API gravity may be determined by any generally
accepted
method for determining API gravity.
[0230] In some embodiments the condensable hydrocarbon portion of the
hydrocarbon fluid has a basic nitrogen to total nitrogen ratio between 0.1 and
0.50.
Alternatively, the condensable hydrocarbon portion may have a basic nitrogen
to total
nitrogen ratio between 0.15 and 0.40. As used herein and in the claims, basic
nitrogen and
total nitrogen may be determined by any generally accepted method for
determining basic
nitrogen and total nitrogen. Where results conflict, the generally accepted
more accurate
methodology shall control.
[0231] The discovery that lithostatic stress can affect the composition
of produced
fluids generated within an organic-rich rock via heating and pyrolysis implies
that the
composition of the produced hydrocarbon fluid can also be influenced by
altering the
lithostatic stress of the organic-rich rock formation. For example, the
lithostatic stress of the
organic-rich rock formation may be altered by choice of pillar geometries
and/or locations
and/or by choice of heating and pyrolysis formation region thickness and/or
heating
sequencing.
[0232] Pillars are regions within the organic-rich rock formation left
unpyrolized at a
given time to lessen or mitigate surface subsidence. Pillars may be regions
within a
formation surrounded by pyrolysis regions within the same formation.
Alternatively, pillars
may be part of or connected to the unheated regions outside the general
development area.
Certain regions that act as pillars early in the life of a producing field may
be converted to
producing regions later in the life of the field.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 79 -
[0233] Typically in its natural state, the weight of a formation's
overburden is fairly
uniformly distributed over the formation. In this state the lithostatic stress
existing at
particular point within a formation is largely controlled by the thickness and
density of the
overburden. A desired lithostatic stress may be selected by analyzing
overburden geology
and choosing a position with an appropriate depth and position.
[0234] Although lithostatic stresses are commonly assumed to be set by
nature and
not changeable short of removing all or part of the overburden, lithostatic
stress at a specific
location within a formation can be adjusted by redistributing the overburden
weight so it is
not uniformly supported by the formation. For example, this redistribution of
overburden
weight may be accomplished by two exemplary methods. One or both of these
methods may
be used within a single formation. In certain cases, one method may be
primarily used earlier
in time whereas the other may be primarily used at a later time. Favorably
altering the
lithostatic stress experienced by a formation region may be performed prior to
instigating
significant pyrolysis within the formation region and also before generating
significant
hydrocarbon fluids. Alternately, favorably altering the lithostatic stress may
be performed
simultaneously with the pyrolysis.
[0235] A first method of altering lithostatic stress involves making a
region of a
subsurface formation less stiff than its neighboring regions. Neighboring
regions thus
increasingly act as pillars supporting the overburden as a particular region
becomes less stiff.
These pillar regions experience increased lithostatic stress whereas the less
stiff region
experience reduced lithostatic stress. The amount of change in lithostatic
stress depends upon
a number of factors including, for example, the change in stiffness of the
treated region, the
size of the treated region, the pillar size, the pillar spacing, the rock
compressibility, and the
rock strength. In an organic-rich rock formation, a region within a formation
may be made to
experience mechanical weakening by pyrolyzing the region and creating void
space within
the region by removing produced fluids. In this way a region within a
formation may be
made less stiff than neighboring regions that have not experienced pyrolysis
or have
experienced a lesser degree of pyrolysis or production.
[0236] A second method of altering lithostatic stress involves causing a
region of a
subsurface formation to expand and push against the overburden with greater
force than
neighboring regions. This expansion may remove a portion of the overburden
weight from
the neighboring regions thus increasing the lithostatic stress experienced by
the heated region
and reducing the lithostatic stress experienced by neighboring regions. If the
expansion is
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 80 -
sufficient, horizontal fractures will form in the neighboring regions and the
contribution of
these regions to supporting the overburden will decrease. The amount of change
in lithostatic
stress depends upon a number of factors including, for example, the amount of
expansion in
the treated region, the size of the treated region, the pillar size, the
pillar spacing, the rock
compressibility, and the rock strength. A region within a formation may be
made to expand
by heating it so to cause thermal expansion of the rock. Fluid expansion or
fluid generation
can also contribute to expansion if the fluids are largely trapped within the
region. The total
expansion amount may be proportional to the thickness of the heated region. It
is noted that
if pyrolysis occurs in the heated region and sufficient fluids are removed,
the heated region
may mechanically weaken and thus may alter the lithostatic stresses
experienced by the
neighboring regions as described in the first exemplary method.
[0237] Embodiments of the method may include controlling the composition
of
produced hydrocarbon fluids generated by heating and pyrolysis from a first
region within an
organic-rich rock formation by increasing the lithostatic stresses within the
first region by
first heating and pyrolyzing formation hydrocarbons present in the organic-
rich rock
formation and producing fluids from a second neighboring region within the
organic-rich
rock formation such that the Young's modulus (i.e., stiffness) of the second
region is reduced.
[0238] Embodiments of the method may include controlling the composition
of
produced hydrocarbon fluids generated by heating and pyrolysis from a first
region within an
organic-rich rock formation by increasing the lithostatic stresses within the
first region by
heating the first region prior to or to a greater degree than neighboring
regions within the
organic-rich rock formation such that the thermal expansion within the first
region is greater
than that within the neighboring regions of the organic-rich rock formation.
[0239] Embodiments of the method may include controlling the composition
of
produced hydrocarbon fluids generated by heating and pyrolysis from a first
region within an
organic-rich rock formation by decreasing the lithostatic stresses within the
first region by
heating one or more neighboring regions of the organic-rich rock formation
prior to or to a
greater degree than the first region such that the thermal expansion within
the neighboring
regions is greater than that within the first region.
[0240] Embodiments of the method may include locating, sizing, and/or
timing the
heating of heated regions within an organic-rich rock formation so as to alter
the in situ
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 81 -
lithostatic stresses of current or future heating and pyrolysis regions within
the organic-rich
rock formation so as to control the composition of produced hydrocarbon
fluids.
[0241] Some production procedures include in situ heating of an organic-
rich rock
formation that contains both formation hydrocarbons and formation water-
soluble minerals
prior to substantial removal of the formation water-soluble minerals from the
organic-rich
rock formation. In some embodiments of the invention there is no need to
partially,
substantially or completely remove the water-soluble minerals prior to in situ
heating. For
example, in an oil shale formation that contains naturally occurring
nahcolite, the oil shale
may be heated prior to substantial removal of the nahcolite by solution
mining. Substantial
removal of a water-soluble mineral may represent the degree of removal of a
water-soluble
mineral that occurs from any commercial solution mining operation as known in
the art.
Substantial removal of a water-soluble mineral may be approximated as removal
of greater
than 5 weight percent of the total amount of a particular water-soluble
mineral present in the
zone targeted for hydrocarbon fluid production in the organic-rich rock
formation. In
alternative embodiments, in situ heating of the organic-rich rock formation to
pyrolyze
formation hydrocarbons may be commenced prior to removal of greater than 3
weight
percent, alternatively 7 weight percent, 10 weight percent or 13 weight
percent of the
formation water-soluble minerals from the organic-rich rock formation.
[0242] The impact of heating oil shale to produce oil and gas prior to
producing
nahcolite is to convert the nahcolite to a more recoverable form (soda ash),
and provide
permeability facilitating its subsequent recovery. Water-soluble mineral
recovery may take
place as soon as the retorted oil is produced, or it may be left for a period
of years for later
recovery. If desired, the soda ash can be readily converted back to nahcolite
on the surface.
The ease with which this conversion can be accomplished makes the two minerals
effectively
interchangeable.
[0243] In some production processes, heating the organic-rich rock
formation
includes generating soda ash by decomposition of nahcolite. The method may
include
processing an aqueous solution containing water-soluble minerals in a surface
facility to
remove a portion of the water-soluble minerals. The processing step may
include removing
the water-soluble minerals by precipitation caused by altering the temperature
of the aqueous
solution.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 82 -
[0244] The water-soluble minerals may include sodium. The water-soluble
minerals
may also include nahcolite (sodium bicarbonate), soda ash (sodium carbonate),
dawsonite
(NaAl(CO3)(OH)2), or combinations thereof. The surface processing may further
include
converting the soda ash back to sodium bicarbonate (nahcolite) in the surface
facility by
reaction with CO2. After partial or complete removal of the water-soluble
minerals, the
aqueous solution may be reinjected into a subsurface formation where it may be
sequestered.
The subsurface formation may be the same as or different from the original
organic-rich rock
formation.
[0245] In some production processes, heating of the organic-rich rock
formation both
pyrolyzes at least a portion of the formation hydrocarbons to create
hydrocarbon fluids and
makes available migratory contaminant species previously bound in the organic-
rich rock
formation. The migratory contaminant species may be formed through pyrolysis
of the
formation hydrocarbons, may be liberated from the formation itself upon
heating, or may be
made accessible through the creation of increased permeability upon heating of
the
formation. The migratory contaminant species may be soluble in water or other
aqueous
fluids present in or injected into the organic-rich rock formation.
[0246] Producing hydrocarbons from pyrolyzed oil shale will generally
leave behind
some migratory contaminant species which are at least partially water-soluble.
Depending on
the hydrological connectivity of the pyrolyzed shale oil to shallower zones,
these components
may eventually migrate into ground water in concentrations which are
environmentally
unacceptable. The types of potential migratory contaminant species depend on
the nature of
the oil shale pyrolysis and the composition of the oil shale being converted.
If the pyrolysis
is performed in the absence of oxygen or air, the contaminant species may
include aromatic
hydrocarbons (e.g. benzene, toluene, ethylbenzene, xylenes), polyaromatic
hydrocarbons (e.g.
anthracene, pyrene, naphthalene, chrysene), metal contaminants (e.g. As, Co,
Pb, Mo, Ni, and
Zn), and other species such as sulfates, ammonia, Al, K, Mg, chlorides,
flourides and
phenols. If oxygen or air is employed, contaminant species may also include
ketones,
alcohols, and cyanides. Further, the specific migratory contaminant species
present may
include any subset or combination of the above-described species.
[0247] It may be desirable for a field developer to assess the
connectivity of the
organic-rich rock formation to aquifers. This may be done to determine if, or
to what extent,
in situ pyrolysis of formation hydrocarbons in the organic-rich rock formation
may create
migratory species with the propensity to migrate into an aquifer. If the
organic-rich rock
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 83 -
formation is hydrologically connected to an aquifer, precautions may be taken
to reduce or
prevent species generated or liberated during pyrolysis from entering the
aquifer.
Alternatively, the organic-rich rock formation may be flushed with water or an
aqueous fluid
after pyrolysis as described herein to remove water-soluble minerals and/or
migratory
contaminant species. In other embodiments, the organic-rich rock formation may
be
substantially hydrologically unconnected to any source of ground water. In
such a case,
flushing the organic-rich rock formation may not be desirable for removal of
migratory
contaminant species but may nevertheless be desirable for recovery of water-
soluble
minerals.
[0248] Following production of hydrocarbons from an organic-rich
formation, some
migratory contaminant species may remain in the rock formation. In such case,
it may be
desirable to inject an aqueous fluid into the organic-rich rock formation and
have the injected
aqueous fluid dissolve at least a portion of the water-soluble minerals and/or
the migratory
contaminant species to form an aqueous solution. The aqueous solution may then
be
produced from the organic-rich rock formation through, for example, solution
production
wells. The aqueous fluid may be adjusted to increase the solubility of the
migratory
contaminant species and/or the water-soluble minerals. The adjustment may
include the
addition of an acid or base to adjust the pH of the solution. The resulting
aqueous solution
may then be produced from the organic-rich rock formation to the surface for
processing.
[0249] After initial aqueous fluid production, it may further be
desirable to flush the
matured organic-rich rock zone and the unmatured organic-rich rock zone with
an aqueous
fluid. The aqueous fluid may be used to further dissolve water-soluble
minerals and
migratory contaminant species. The flushing may optionally be completed after
a substantial
portion of the hydrocarbon fluids have been produced from the matured organic-
rich rock
zone. In some embodiments, the flushing step may be delayed after the
hydrocarbon fluid
production step. The flushing may be delayed to allow heat generated from the
heating step
to migrate deeper into surrounding unmatured organic-rich rock zones to
convert nahcolite
within the surrounding unmatured organic-rich rock zones to soda ash.
Alternatively, the
flushing may be delayed to allow heat generated from the heating step to
generate
permeability within the surrounding unmatured organic-rich rock zones.
Further, the flushing
may be delayed based on current and/or forecast market prices of sodium
bicarbonate, soda
ash, or both as further discussed herein. This method may be combined with any
of the other
aspects of the invention as discussed herein
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 84 -
[0250] Upon flushing of an aqueous solution, it may be desirable to
process the
aqueous solution in a surface facility to remove at least some of the
migratory contaminant
species. The migratory contaminant species may be removed through use of, for
example, an
adsorbent material, reverse osmosis, chemical oxidation, bio-oxidation, and/or
ion exchange.
Examples of these processes are individually known in the art. Exemplary
adsorbent
materials may include activated carbon, clay, or fuller's earth.
[0251] In the production of oil and gas resources, it may be desirable to
use the
produced hydrocarbons as a source of power for ongoing operations. This may be
applied to
the development of oil and gas resources from oil shale. In this respect, when
electrically
resistive heaters are used in connection with in situ shale oil recovery,
large amounts of
power are required.
[0252] Electrical power may be obtained from turbines that turn
generators. It may
be economically advantageous to power the gas turbines by utilizing produced
gas from the
field. However, such produced gas must be carefully controlled so to maximize
efficiency
and so not to damage the turbine, cause the turbine to misfire, or generate
excessive
pollutants (e.g., NO).
[0253] One source of problems for gas turbines is the presence of
contaminants
within the fuel. Contaminants include solids, water, heavy components present
as liquids,
and hydrogen sulfide. Additionally, the combustion behavior of the fuel is
important.
Combustion parameters to consider include heating value, specific gravity,
adiabatic flame
temperature, flammability limits, autoignition temperature, autoignition delay
time, and flame
velocity. Wobbe Index (WI) is often used as a key measure of fuel quality. WI
is equal to
the ratio of the lower heating value to the square root of the gas specific
gravity. Control of
the fuel's Wobbe Index to a target value and range of, for example, 10% or
20% can allow
simplified turbine design and increased optimization of performance. In
general gas turbines
are highly optimized machines and variations in the gas feed can result in the
need to
periodically shut-down the turbine for significant overhaul and parts
replacement to re-
optimize the turbine. This is typically a costly operation in terms of both
direct expenses and
lost operational time. It is therefore desirable to minimize the need for such
overhauls.
[0254] Fuel quality control may be useful for shale oil developments
where the
produced gas composition may change over the life of the field and where the
gas typically
has significant amounts of CO2, CO, and H2 in addition to light hydrocarbons.
Commercial
CA 02686565 2015-04-14
- 85 -
scale oil shale retorting is expected to produce a gas composition that
changes with time. The
variation in gas composition over time results from multiple and competing
decomposition
reactions occurring simultaneously when oil shale is pyrolyzed.
[0255] Inert gases in the turbine fuel can increase power generation by
increasing
mass flow while maintaining a flame temperature in a desirable range. Moreover
inert gases
can lower flame temperature and thus reduce NO, pollutant generation. Gas
generated from
oil shale maturation may have significant CO2 content. Therefore, in certain
embodiments of
the production processes, the CO2 content of the fuel gas is adjusted via
separation or
addition in the surface facilities to optimize turbine performance. Total
removal of CO2 is
not necessarily ideal.
[0256] Achieving a certain hydrogen content for low-BTU fuels may also be
desirable to achieve appropriate burn properties. In certain embodiments of
the processes
herein, the H2 content of the fuel gas is adjusted via separation or addition
in the surface
facilities to optimize turbine performance. Adjustment of H2 content in non-
shale oil surface
facilities utilizing low BTU fue's has been discussed in the patent literature
(e.g., U.S. Pat.
No. 6,684,644 and U.S. Pat. No. 6,858,049).
[0257] In certain areas with oil shale resources, additional oil shale
resources or other
hydrocarbon resources may exist at lower depths. Other hydrocarbon resources
may include
natural gas in low permeability formations (so-called "tight gas") or natural
gas trapped in
and adsorbed on coal (so called "coalbed methane"). In some embodiments with
multiple
shale oil resources it may be advantageous to develop deeper zones first and
then sequentially
shallower zones. In this way, wells will need not cross hot zones or zones of
weakened rock.
In other embodiments it may be advantageous to develop deeper zones by
drilling wells
through regions being utilized as pillars for shale oil development at a
shallower depth.
[0258] Simultaneous development of shale oil resources and natural gas
resources in
the same area can synergistically utilize certain facility and logistic
operations. For example,
gas treating may be performed at a single plant. Likewise personnel may be
shared among
the developments.
[0259] Figure 6 illustrates a schematic diagram of an embodiment of surface
facilities 70 that may be configured to treat a produced fluid. The produced
fluid 85 may be
produced from the subsurface formation 84 though a production well 71 as
described herein.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 86 -
The produced fluid may include any of the produced fluids produced by any of
the methods
as described herein. The subsurface formation 84 may be any subsurface
formation,
including, for example, an organic-rich rock formation containing any of oil
shale, coal, or tar
sands for example. A production scheme may involve quenching 72 produced
fluids to a
temperature below 300 F, 200 F, or even 100 F, separating out condensable
components
(i.e., oil 74 and water 75) in an oil separator 73, treating the
noncondensable components 76
(i.e. gas) in a gas treating unit 77 to remove water 78 and sulfur species 79,
removing a
portion of the heavier components from the gas (e.g., propane and butanes) in
a gas plant 81
to form liquid petroleum gas (LPG) 80 for sale, and generating electrical
power 82 in a power
plant 88 from the remaining gas 83. The electrical power 82 may be used as an
energy
source for heating the subsurface formation 84 through any of the methods
described herein.
For example, the electrical power 82 may be fed at a high voltage, for example
132 kV, to a
transformer 86 and let down to a lower voltage, for example 6600 V, before
being fed to an
electrical resistance heater element located in a heater well 87 located in
the subsurface
formation 84. In this way all or a portion of the power required to heat the
subsurface
formation 84 may be generated from the non-condensable portion of the produced
fluids 85.
Excess gas, if available, may be exported for sale.
[0260] Produced fluids from in situ oil shale production contain a number
of
components which may be separated in surface facilities. The produced fluids
typically
contain water, noncondensable hydrocarbon alkane species (e.g., methane,
ethane, propane,
n-butane, isobutane), noncondensable hydrocarbon alkene species (e.g., ethene,
propene),
condensable hydrocarbon species composed of (alkanes, olefins, aromatics, and
polyaromatics among others), CO2, CO, H2, H2S, and NH3.
[0261] In a surface facility, condensable components may be separated
from non-
condensable components by reducing temperature and/or increasing pressure.
Temperature
reduction may be accomplished using heat exchangers cooled by ambient air or
available
water. Alternatively, the hot produced fluids may be cooled via heat exchange
with produced
hydrocarbon fluids previously cooled. The pressure may be increased via
centrifugal or
reciprocating compressors. Alternatively, or in conjunction, a diffuser-
expander apparatus
may be used to condense out liquids from gaseous flows. Separations may
involve several
stages of cooling and/or pressure changes.
[0262] Water in addition to condensable hydrocarbons may be dropped out
of the gas
when reducing temperature or increasing pressure. Liquid water may be
separated from
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 87 -
condensable hydrocarbons via gravity settling vessels or centrifugal
separators. Demulsifiers
may be used to aid in water separation.
[0263] Methods to remove CO2, as well as other so-called acid gases (such
as H2S),
from produced hydrocarbon gas include the use of chemical reaction processes
and of
physical solvent processes. Chemical reaction processes typically involve
contacting the gas
stream with an aqueous amine solution at high pressure and/or low temperature.
This causes
the acid gas species to chemically react with the amines and go into solution.
By raising the
temperature and/or lowering the pressure, the chemical reaction can be
reversed and a
concentrated stream of acid gases can be recovered. An alternative chemical
reaction process
involves hot carbonate solutions, typically potassium carbonate. The hot
carbonate solution
is regenerated and the concentrated stream of acid gases is recovered by
contacting the
solution with steam. Physical solvent processes typically involve contacting
the gas stream
with a glycol at high pressure and/or low temperature. Like the amine
processes, reducing
the pressure or raising the temperature allows regeneration of the solvent and
recovery of the
acid gases. Certain amines or glycols may be more or less selective in the
types of acid gas
species removed. Sizing of any of these processes requires determining the
amount of
chemical to circulate, the rate of circulation, the energy input for
regeneration, and the size
and type of gas-chemical contacting equipment. Contacting equipment may
include packed
or multi-tray countercurrent towers. Optimal sizing for each of these aspects
is highly
dependent on the rate at which gas is being produced from the formation and
the
concentration of the acid gases in the gas stream. Alternatively, a iron redox
process (e.g.,
Low-Cat"') may be useful, especially where appreciable carbon monoxide and low
levels of
sulfur are present in the hydrocarbon gas stream. In liquid redox processes,
an iron
compound containing solution is cyclically reduced and oxidized, such that
during the
reductive part of the cycle, the hydrogen sulfide containing gases react with
the iron
compound to form elemental sulfur. The elemental sulfur is removed by in-line
filters
situated in the solution circuit between the reduction and oxidation portions
of the cycle.
[0264] Acid gas removal may also be effectuated through the use of
distillation
towers. Such towers may include an intermediate freezing section wherein
frozen CO2 and
H2S particles are allowed to form. A mixture of frozen particles and liquids
fall downward
into a stripping section, where the lighter hydrocarbon gasses break out and
rise within the
tower. A rectification section may be provided at an upper end of the tower to
further
facilitate the cleaning of the overhead gas stream. Additional details of such
a process and
CA 02686565 2015-04-14
- 88 -
related processes may be found in United States Patents 3,724,225, 4,511,382,
4,533,372,
4,923,493, 5,120,338, 5,956,971.
[0265] The
hydrogen content of a gas stream may be reduced by removing all or a
portion of the hydrogen or increased by removing all or a portion of the non-
hydrogen
species (e.g., CO2, CI-14, etc.) Separations
may be accomplished using cryogenic
condensation, pressure-swing Or temperature-swing adsorption, or selective
diffusion
membranes. If additional hydrogen is needed, hydrogen may be made by reforming
methane
via the classic water-shift reaction.
[0266] In one
embodiment, a method for utilizing gas produced from an in situ
conversion process is provided. The method may include heating an organic-rich
rock
formation in situ. Further, the method may include producing a production
fluid from the
organic-rich rock formation. The production fluid may include hydrocarbon
fluids and be at
least partially generated as a result of pyrolysis of formation hydrocarbons
located in the
organic-rich rock formation. A gas stream comprising combustible hydrocarbon
fluids may
be obtained from the production fluid. The gas stream may be separated into a
first
composition gas stream and a second composition gas stream, where the
composition of the
first composition gas stream is maintained in a substantially constant
condition. The first
composition gas stream may be passed through a first gas turbine to form a
first gas turbine
exhaust stream. The first gas turbine is configured to provide energy to a
first electrical
generator for the purpose of generating electricity.
[0267] As in other
embodiments described herein the organic-rich rock formation
may be, for example, a heavy hydrocarbon formation or a solid hydrocarbon
formation.
Particular examples of such formations may include an oil shale formation, a
tar sands
formation or a coal formation. Particular formation hydrocarbons present in
such formations
may include heavy hydrocarbons, oil shale, kerogen, coal, and/or bitumen.
[0268] The
production fluid produced from the organic-rich rock formation may
include a hydrocarbon fluid. The hydrocarbon fluid may include both a
condensable
hydrocarbon portion (e.g., liquid) and a non-condensable hydrocarbon portion
(e.g., gas).
The hydrocarbon fluid of the production fluid may additionally be produced
together with
non-hydrocarbon fluids. Exemplary non-hydrocarbon fluids include, for example,
water,
carbon dioxide (CO2), hydrogen sulfide (H2S), hydrogen gas (H2), ammonia
(NH3), and/or
carbon monoxide (CO). In in situ heating operations the composition of the
production fluid
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 89 -
is expected to change over time. Initially, both the production fluid flow
rate and
composition are expected to be different than from after a year or more of
heating. For
example early in pyrolysis of a volume of oil shale, the composition of the
produced gas may
have a high CO2 mole fraction and a low H2 mole fraction. As the pyrolysis
continues, the
composition of the produced gas changes to where the CO2 concentration is low
and the H2
concentration is high. Alkane species (e.g., methane and ethane) may exhibit
maximum
concentrations in the pyrolysis gas at intermediate times. (See for example
"Isothermal
Decomposition of Colorado Oil Shale", DOE/FE/60177-2288.)
[0269] The composition of a gas stream produced from in situ heating of
organic-rich
rock formations is expected to change over time. In particular the proportion
of hydrogen gas
and carbon dioxide is expected to change significantly over the life of a
commercial in situ
heating field development, which may take from 3 to 10 years or more.
Moreover, the
relative proportions of gaseous hydrocarbon species, including methane,
ethane, ethylene,
propane, iso-propane and propylene are expected to change over the life of the
field
development. These changes may be particularly significant for a commercial
oil shale
development. In an oil shale development, the concentration of hydrogen gas in
the produced
gas is expected to increase over time while the concentration of CO2 is
expected to decrease
over time. These expected changes in the overall produced gas composition are
expected to
present processing challenges, particularly where the produced gas or a
portion thereof is
combusted in a gas turbine because of the inability of gas turbines with
specific combustors
to accommodate large changes in feed gas composition while maintaining stable
operation.
Embodiments of the invention include obtaining a gas stream from the
production fluid
where the composition of the gas stream changes over time. The composition of
the gas
stream changing over time may include the averaged daily concentration of one
or more
species in the gas stream changing by greater than 5 mol percent over a 1 year
period. In
alternate embodiments, the averaged daily concentration of one or more species
in the gas
stream may change by greater than 10, 15 or 20 mol percent over a 1 year
period. In alternate
embodiments, the averaged daily concentration of one or more species in the
gas stream may
change by greater than 5, 10, 15 or 20 mol percent over a 2 year period. In
particular
embodiments the species who's concentration changes may be CO2, methane,
hydrogen gas,
or combinations thereof. In alternate embodiments, the averaged daily Wobbe
Index or
Modified Wobbe Index of the gas stream may change by greater than 5, 10, 15 or
20 percent
over a 1 year period. In alternate embodiments, the averaged daily Wobbe Index
or Modified
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 90 -
Wobbe Index of the gas stream may change by greater than 5, 10, 15 or 20
percent over a 2
year period.
[0270] Figure 32 provides a graphical depiction of several gaseous
species evolved
from laboratory heating of Colorado oil shale. The figure is based on data
from Miknis, F.
P.; Conn, P. J.; and Turner, T. F., "Isothermal Decomposition of Colorado Oil
Shale",
DOE/FE/60177-2288 (DE87009043). The experiment consisted of heating and
maintaining a
sample of Colorado oil shale at a constant temperature of 368 C for a period
of at least 12
hours. The left y-axis 351 reports the concentration in mol% of the measured
gaseous
species, including CO2 352, H2 353, methane 354, ethane 355, and CO 356,
evolved over the
12-hour experiment. The x-axis 350 represents time and is in terms of hours.
The data in the
figure represent values obtained by differentiating measured cumulative
compositions so to
obtain estimates of instantaneous gas compositions. The right y-axis 358
reports the Wobbe
Index in units of BTU/SCF. Wobbe Index 357 was calculated based on the non-
sulfur
species in the gas. Lower heating value in BTU/SCF and specific gravity
relative to air were
used in the Wobbe Index calculation. As the graph shows, initially (hour 0-2)
the evolved
gas is primarily CO2, ranging from 70 down to 40 mol% CO2, with smaller
amounts of
methane (12-18 mol%) and ethane (2-6 mol%). As time progresses, hydrogen gas
production
increases with an almost corresponding decrease in CO2 production. Near the
end of the 7.5
hour-period, the CO2 concentration has dropped to about 4 mol% while the
hydrogen
production has increased to over 70 mol%. The hydrocarbon species have also
varied over
the 7-hour period, however to a much lesser extent. The methane concentration
has ranged
from a low of about 12 mol% initially to a high of about 22 mol% at hour 4,
back down to
about 10 mol% by hour 7.5. The ethane concentration has ranged from a low of
about 2-3
mol% initially to a high of about 8 mol% at hour 4.5, back down to about 5
mol% by hour
7.5. The varying composition over time results in a large change in Wobbe
Index over time.
The value is initially about 210 BTU/SCF and steadily increases to about 890
BTU/SCF at
hour 7.5. The data also indicates that the gas produced from heating and
pyrolyzing oil shale
will be a relatively low BTU gas, including for example, large amounts of CO2.
The data
presented in Figure 32 is illustrative of the potential variation in gas
composition over time
for heating oil shale however the time scale in a commercial operation would
be over a much
longer time frame due to lower in situ temperatures, for example 270-350 C.
The use of
lower temperatures reflect the impracticality of rapidly heating large volumes
of rock and
increased efficiencies associated with application of lower average
temperatures in the
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 91 -
subsurface. For example, it may take from 1 to 3 years to gain significant
hydrocarbon
production in a commercial oil shale development depending on the energy
input, heating
rate, target formation density, target formation thickness, heater well
spacing and heater well
geometry. Further it may take from 6 to 10 years or more to fully convert the
kerogen in the
oil shale to producible fluid hydrocarbons in a commercial in situ heating oil
shale
development, again dependent on the specific development parameters mentioned
previously.
[0271] A gas stream comprising combustible hydrocarbon fluids may be
obtained
from the production fluid. The gas stream may be further separated into a
first composition
gas stream and a second composition gas stream. In some embodiments the first
composition
gas stream and the second composition gas stream both contain hydrocarbon
fluids. The
identity of hydrocarbon species in the first composition gas stream may be the
same or
different from the identity of hydrocarbon species in the second composition
gas stream.
Further, the concentration of the same hydrocarbon species in the respective
gas streams may
be similar or very different. Exemplary gaseous hydrocarbon species in the
respective gas
streams may include methane, ethane, ethylene, propane, propylene, butane, iso-
butane,
butene, with potentially lesser amounts of pentane, iso-pentane, pentylene,
C6+ hydrocarbon
species and any combination thereof. In some embodiments, the first gas stream
is greater
than 5 mole percent hydrocarbon gas. In alternative embodiments, the first gas
stream is
greater than 10, 15, 20, 25, 30, 35 or 40 mole percent hydrocarbon gas. In
some
embodiments, the first composition gas stream is greater than 15 mole percent
methane. In
alternative embodiments, the first composition gas stream is greater than 20,
25, 30, 35 or 40
mole percent methane.
[0272] In some embodiments the first composition =gas stream and the
second
composition gas stream both comprise combustible gases. Combustible gas may
include both
hydrocarbon gases and non-hydrocarbon gases, for example, hydrogen gas. In
some
embodiments the first composition gas stream, the second composition gas
stream, or both
are considered low heating value or low BTU gases. The streams are considered
low BTU
gas stream because they have a lower heating value less than the lower heating
value (LHV)
of typical pipeline natural gas, which has a lower heating value of about
1,000 BTU/SCF.
However, the streams may have a high enough heating value to be useful in gas
turbines
and/or fired boilers and are therefore distinguished from gases which are the
byproducts of
combustion or incomplete combustion which may have lower heating values in the
50 to 150
BTU/SCF range. In some embodiments the first composition gas stream, the
second
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 92 -
composition gas stream, or both have a lower heating value greater than 200
BTU/SCF. In
alternative embodiments, the first composition gas stream, the second
composition gas
stream, or both have a lower heating value greater than 300 BTU/SCF, 400
BTU/SCF, 500
BTU/SCF or 600 BTU/SCF. In other embodiments, the first composition gas
stream, the
second composition gas stream, or both have a lower heating value less than
800 BTU/SCF.
In alternative embodiments, the first composition gas stream, the second
composition gas
stream, or both have a lower heating value less than 700 BTU/SCF, 600 BTU/SCF
or 500
BTU/SCF.
[0273] The gas stream, the first composition gas stream, the second
composition gas
stream, or any combination thereof may include at least one inert gas, for
example, CO2. The
presence of CO2, as a zero heating value gas, serves as a diluent for heating
value purposes
and contributes to lowering the overall lower heating value of the gas stream.
In some
embodiments, the invention takes advantage of the pressure and mass flow rate
of CO2
present in the production fluid and makes such CO2 available in the first gas
stream for
eventual processing in the first gas turbine. It is envisioned that the
production fluid may be
produced from the organic-rich rock formation at elevated pressures of, for
example, greater
than 300, 400, 500, 600 or greater than 700 psig. A gaseous portion of the
pressurized
production fluid, after processing, may be sent to a gas turbine to recover
energy from letting
down the pressure of such a pressurized gaseous stream across the gas turbine.
In this way
energy is recovered not only from combustion of the combustible portions of
the first gas
stream but also from the pressurized non-combustible portions of the first gas
stream. In
some embodiments, the gas stream, the first composition gas stream, the second
composition
gas stream, or any combination thereof may have a CO2 content that is greater
than 10 mole
percent. Alternatively, the gas stream, the first composition gas stream, the
second
composition gas stream, or any combination thereof may have a CO2 content that
is greater
than 15, 20, 25, 30, 35 or 40 mole percent. Alternatively, the gas stream, the
first
composition gas stream, the second composition gas stream, or any combination
thereof may
have a CO2 content that is less than 70, 60, 50 or 45 mole percent.
[0274] In one embodiment, the first composition gas stream is greater
than 5 mole
percent hydrogen gas. Alternatively, the first composition gas stream may be
greater than 10,
20, 30, 40, 50, 60, 70, 80 or 90 mole percent hydrogen gas. Alternatively, the
second
composition gas stream may be greater than 30, 50, 70 or 90 mole percent
hydrogen gas. The
hydrogen gas content of the first composition gas stream, the second
composition gas stream,
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 93 -
or both may also be adjusted to compensate for high inert content (e.g., CO2)
in such gas
streams, particularly when such a gas stream is combusted in a gas turbine. As
the CO2
concentration of a gas turbine feed stream increases, there is a corresponding
decrease in such
feed streams flame speed. Hydrogen gas has a relatively high flame speed and
may be used
to compensate for high CO2 concentrations in gas turbine feed streams, thereby
obtaining a
gas turbine feed stream with a flame speed varying within acceptable
parameters for a given
turbine design. Alternatively, if additional hydrogen is needed, hydrogen may
be made by
reforming methane via the classic steam reforming followed by the water-gas
shift reaction,
thereby increasing the hydrogen concentration of a gas turbine feed stream or
making
additional hydrogen gas available for mixing with the gas turbine feed stream.
The water-gas
shift reaction is provided below:
Steam Reforming: CH4 + H20 --+ CO + 3H2
Water-Gas Shift: H20 + CO H2 + CO2
[0275] In one embodiment, the first composition gas stream has a
substantially
constant H2 to CO2 molar ratio, thereby obtaining a relatively constant flame
speed in the gas
turbine. In alternate embodiments, the first composition gas stream has an H2
to CO2 molar
ratio between 0.1 to 2.0, 0.3 to 1.8, 0.5 to 1.6 or between 0.7 to 1.4.
Alternatively, the first
composition gas stream may have a substantially constant ethane to CO2 molar
ratio. In
particular, the ethane to CO2 molar ratio of the first composition gas stream
on an average
daily basis may vary by less than 15 percent over a 7 day period. Further, the
ethane to CO2
molar ratio of the first composition gas stream on an average daily basis
varies by less than
or 5 percent over a 7 day period.
[0276] The method includes passing at least the first composition gas
stream to a gas
turbine. In alternative embodiments the second composition gas stream may also
optionally
be passed to a gas turbine. In either case, a gas turbine includes a means of
combustion that
is a combustor. Generally, combustors include a nozzle or injector for
injecting the gas feed
and mixing the feed with air or an oxygen containing stream. The resulting
mixture is then
combusted prior to entry into the turbine portion of the gas turbine where
energy is extracted
from the hot combustion product stream. Gas combustors and their injectors are
typically
designed for a certain gas composition or range of compositions where the gas
turbine will
operate stably and efficiently. If the gas composition changes outside the
design range then
the gas turbine can experience unstable operation, inefficient operation,
reduced reliability,
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 94 -
and/or increased emissions of environmentally regulated species, including,
for example,
nitrogen oxides (NOõ), carbon monoxide (CO), and/or sulfur (e.g., sulfur
oxides (SO))
emissions. In some embodiments, the first gas turbine is equipped with a dual
gas combustor
comprised of a first gas injector and a second gas injector, where the first
gas injector is
optimized or designed for the first composition gas stream and the second gas
injector
optimized or designed for the second composition gas stream. In alternate
embodiments, the
first gas turbine and/or the second gas turbine may be equipped with variable
geometry
combustors. In still further embodiments, the first gas turbine and/or the
second gas turbine
may be equipped with catalytic combustors. In still further embodiments, the
first gas turbine
and/or the second gas turbine may be equipped with lean pre-mixed
combustor(s), Dry Low
NO (DLN) combustor(s), or Dry Low Emissions (DLE) combustor(s). Further, steam
injection or water injection may be used to reduce NO.
[0277] Gas turbine manufactures typically designate a preferred pressure
or range of
pressures to deliver the gas turbine feed gas stream for combustion in the gas
turbine
combustor and further processing in the turbine of the gas turbine. If the gas
turbine feed gas
stream is delivered to the gas turbine outside the designated pressure range
then the gas
turbine can experience unstable operation, reduced efficiency and/or increased
emissions of
environmentally regulated components. Thus it is useful to operate an organic-
rich rock
formation in situ heating operation to provide not only a gas turbine feed gas
with a
composition that is in a substantially constant condition, but also to provide
the feed gas
within a targeted pressure range to the gas turbines. In some embodiments, the
first
composition gas stream may be provided to the first gas turbine at a
substantially constant
pressure. In some embodiments, the first composition gas stream may be
delivered to the
first gas turbine at a pressure in the range of 200 to 1,000 pounds per square
inch gauge
(psig). Alternatively, the first composition gas stream may be delivered to
the first gas
turbine at a pressure in the range of 300 to 800, 350 to 650 or 400 to 600
psig. In some
embodiments, the pressure of the first composition gas stream on an averaged
daily basis
varies by less than 20 percent gauge pressure over a 7 day period. In
alternate embodiments,
the pressure of the first composition gas stream on an averaged daily basis
varies by less than
15, 10, or 5 percent gauge pressure over a 7 day period.
[0278] One feature of the invention is that the composition of at least
the first
composition gas stream is maintained in a substantially constant condition.
The composition
of the first composition gas stream is maintained in a substantially constant
condition in order
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 95 -
to meet the desired operating parameters of the first gas turbine. Modern gas
turbines are
typically equipped with low emissions combustors in order to meet modern
environmental
regulation. Such turbines require relatively constant fuel compositions and
therefore have
little flexibility to accommodate wide changes in fuel gas composition. This
is due to the fact
that most gas turbines are equipped with fixed geometry combustors, typically
a set of
circular orifices. In order for the gas turbine to reliability operate
according to generally
accepted performance criteria, the fuel gas must be supplied with a well-
regulated flowrate,
pressure ratio, composition, temperature etc. If the fuel composition changes,
and thus the
Modified Wobbe Index changes, then the pressure ratio required to supply
sufficient amounts
of fuel energy to the turbine (in order to maintain load) will change.
However, the pressure
ratio required by the specific combustor geometry is set by the aero-
mechanical design of the
combustor, which is generally a fixed geometry. Therefore, any changes in fuel
gas
composition will force the combustor to operate outside of its optimal design
point. When
desired operation deviates from the optimal design point to a point beyond the
acceptable
design range, then negative consequences are typically encountered. A gas
having a
composition in a substantially constant condition is meant to refer to the
range of fuel gas
composition that a given gas turbine can utilize while maintaining a
sufficiently stable
operational performance. For example, a gas with a substantially constant set
of conditions
is able to be utilized by a given gas turbine without experiencing
unacceptable combustion
dynamics, including pressure pulsations, which may lead to unreliability
caused by flame
extinction, ultimately resulting in a shutdown of the turbine. Further, a gas
with a
substantially constant condition is able to be utilized by a given gas turbine
without
generating emissions (e.g., NO CO, etc.) in excess of specified targets or
environmental
regulations. Further, a gas with a substantially constant condition is able to
be utilized by a
given gas turbine such that the turbine may be operated without need for
frequent overhauls
or replacement of its internal parts that may be caused by wear or fatigue of
components due
to excessive combustion dynamics or the damage of components due to flame
flashback or
flame anchoring in a location that is not designed for the elevated
temperatures cause by such
an event. Further, a gas with a substantially constant condition is able to be
utilized without
the need to shut down the turbine in order to replace the combustion
components with
components that are designed to accommodate a different fuel gas composition
with respect
to the initial fuel gas composition. This component replacement may be
necessary to match
the fuel injection port geometry to the new fuel gas composition in order to
achieve the
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 96 -
necessary pressure ratio of fuel gas supplied into the combustion zone, or it
may be necessary
to alter the geometry of the dilution air holes in the combustor in order to
provide the proper
air split between combustion and dilution.
[0279] There are many ways to evaluate expected gas turbine performance
based on
the quality of the gas. One method includes maintaining a substantially
constant Wobbe
index. One measure of Wobbe Index may be calculated using the following
equation:
L
WI =HV
[0280] Where W/ is the Wobbe Index, LHV is the lower heating value of the
fuel gas
in units of BTU/SCF (or equivalent units), where BTU is a British Thermal Unit
and SCF is
the unit standard cubic feet, and SG is the specific gravity of the gas fuel
relative to air at
standard conditions (e.g., 1 atm and 20 C).
[0281] A related measure is the Modified Wobbe Index, which may be
calculated
using the following equation:
MWI = LHV
111 SG *Tgas
[0282] Where MW/ is the Modified Wobbe Index, LHV is the lower heating
value of
the gas fuel in units of BTU/SCF, where BTU is a British Thermal Unit and SCF
is the unit
standard cubic feet, SG is the specific gravity of the gas fuel relative to
air, and Tgas is the
temperature of the gas fuel in degrees Rankine.
[0283] In some embodiments of the invention the first composition gas
stream may be
maintained in a substantially constant condition by varying the Wobbe Index or
Modified
Wobbe Index of the first composition gas stream on an averaged daily basis by
less than 15
percent over a 7 day period. In alternative embodiments, the Wobbe Index or
Modified
Wobbe Index of the first composition gas stream on an averaged daily basis
varies by less
than 10 percent or 5 percent over a 7 day period. In further alternative
embodiments, the
Wobbe Index or Modified Wobbe Index of the first composition gas stream on an
averaged
hourly basis varies by less than 15 percent over a 1 day period. In further
alternative
embodiments, the Wobbe Index or Modified Wobbe Index of the first composition
gas stream
on an averaged hourly basis varies by less than 10 percent or 5 percent over a
1 day period.
In further embodiments, the Wobbe Index or Modified Wobbe Index of the first
composition
gas stream on a five minute averaged basis varies by less than 15 percent over
a 1 hour
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 97 -
period. In further alternative embodiments, the Wobbe Index or Modified Wobbe
Index of
the first composition gas stream on a five minute averaged basis varies by
less than 10
percent or 5 percent over a 1 hour period. In alternative embodiments, the
Wobbe Index or
Modified Wobbe Index of the first composition gas stream on an averaged daily
basis varies
by less than 15, 10, or 5 percent over a 30 day period.
[0284] In further embodiments, it may be sufficient to maintain a
property of the gas
stream in a substantially constant condition in order to maintain the
composition of the gas
stream in a substantially constant condition. For example, in some embodiments
the specific
gravity of the first composition gas stream on an averaged daily basis varies
by less than 15,
10, or 5 percent over a 7 day period. In alternative embodiments, the specific
gravity of the
first composition gas stream on an averaged daily basis varies by less than
15, 10, or 5
percent over a 30 day period. In alternative embodiments, the specific gravity
of the first
composition gas stream on an averaged hourly basis varies by less than 15, 10
or 5 percent
over a 1 day period. In further alternative embodiments, the lower heating
value of the first
composition gas stream on an averaged daily basis varies by less than 15, 10
or 5 percent
over a 7 day period. In further alternative embodiments, the lower heating
value of the first
composition gas stream on an averaged daily basis varies by less than 15, 10
or 5 percent
over a 30 day period. In alternative embodiments, the lower heating value of
the first
composition gas stream on an averaged hourly basis varies by less than 15, 10,
or 5 percent
over a 1 day period.
[0285] The Modified Wobbe Index may be fine tuned by adjusting the
temperature of
the gas turbine feed stream. Adjusting the temperature of the gas turbine feed
stream will
change the density of the gas, thus the specific gravity of the gas will
change and therefore
also affect the Wobbe Index equation presented above. Thus embodiments of the
invention
include adjusting the temperature of the first composition gas stream, thereby
adjusting the
Wobbe Index or Modified Wobbe Index of the first composition gas stream. The
temperature
of the first composition gas stream may be adjusted by various methods,
including heat
exchanging the first composition gas stream with the production fluid or a
derivative thereof,
heat exchanging the first composition gas stream with steam or boiler feed
water, or heat
exchanging the first composition gas stream with any of the various process
stream included
in the oil shale development surface processing facilities. In particular
embodiments, the
temperature of the first composition gas stream may be adjusted by heat
exchanging the first
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 98 -
composition gas stream with the first gas turbine exhaust stream, the
production fluid, steam,
or combinations thereof.
[0286] There are alternate methods of maintaining the composition of a
gas stream in
a substantially constant condition in order to meet the desired operating
parameters of a gas
turbine. In some embodiments of the invention the composition of the first
composition gas
stream may be maintained in a substantially constant condition by varying the
total
concentration of a particular species or group of species by less than a
specified amount. In
some embodiments of the invention the first composition gas stream may be
maintained in a
substantially constant condition by varying the concentration of inert species
in the first
composition gas stream on an averaged daily basis by less than 15 mole percent
over a 7 day
period. In alternative embodiments, the total concentration of inert species
in the first
composition gas stream on an averaged daily basis varies by less than 10 or 5
mole percent
over a 30 day period. In alternative embodiments, the total concentration of
inert species in
the first composition gas stream on an averaged daily basis varies by less
than 10 or 5 mole
percent over a 7 day period. In further alternative embodiments, the total
concentration of
inert species in the first composition gas stream on an averaged hourly basis
varies by less
than 15, 10 or 5 mole percent over a 1 day period. In one embodiment the
concentration of a
particular inert species, carbon dioxide, may be varied by less than a
specified amount in
order to maintain the gas stream in a substantially constant condition. In one
embodiment the
concentration of CO2 in the first composition gas stream on an averaged daily
basis varies by
less than 15, 10 or 5 mole percent over a 7 day period. In one embodiment the
concentration
of CO2 in the first composition gas stream on an averaged daily basis varies
by less than 15,
or 5 mole percent over a 30 day period. In alternative embodiments, the
concentration of
CO2 in the first composition gas stream on an averaged hourly basis varies by
less than 15, 10
or 5 mole percent over a 1 day period.
[0287] In some embodiments of the invention the composition of the first
gas stream
may be maintained in a substantially constant condition by varying the total
concentration of
hydrogen gas by less than a specified amount. In some embodiments of the
invention the
first gas stream may be maintained in a substantially constant condition by
varying the
concentration of H2 in the first gas stream on an averaged daily basis by less
than 15 mole
percent over a 7 day period. In alternative embodiments, the total
concentration of H2 in the
first gas stream on an averaged daily basis varies by less than 10 or 5 mole
percent over a 7
day period. In alternative embodiments, the total concentration of H2 in the
first gas stream
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 99 -
on an averaged daily basis varies by less than 10 or 5 mole percent over a 30
day period. In
further alternative embodiments, the total concentration of H2 in the first
gas stream on an
averaged hourly basis varies by less than 15, 10 or 5 mole percent over a 1
day period.
[0288] In some embodiments of the invention the composition of the first
composition gas stream may be maintained in a substantially constant condition
by varying
the total concentration of a particular hydrocarbon species or group of
hydrocarbon species
by less than a specified amount. In some embodiments of the invention the
composition of
the first composition gas stream may be maintained in a substantially constant
condition by
varying the concentration of methane in the first composition gas stream on an
averaged daily
basis by less than 15, 10 or 5 mole percent over a 7 day period. In
alternative embodiments,
the concentration of methane in the first composition gas stream on an
averaged daily basis
varies by less than 15, 10 or 5 mole percent over a 30 day period. In
alternative
embodiments, the concentration of methane in the first composition gas stream
on an
averaged hourly basis varies by less than 15, 10 or 5 mole percent over a 1
day period. In
alternative embodiments, the concentration of ethane in the first composition
gas stream on
an averaged daily basis varies by less than 15, 10 or 5mole percent over a 7
day period. In
alternative embodiments, the concentration of ethane in the first composition
gas stream on
an averaged daily basis varies by less than 15, 10 or 5 mole percent over a 30
day period. In
alternative embodiments, the concentration of ethane in the first composition
gas stream on
an averaged hourly basis varies by less than 15, 10, or 5 mole percent over a
1 day period. In
some embodiments of the invention the composition of the first gas stream may
be
maintained in a substantially constant condition by varying the concentration
of propane in
the first gas stream on an averaged daily basis by less than 15, 10 or 5 mole
percent over a 7
day period. In alternative embodiments, the concentration of propane in the
first gas stream
on an averaged daily basis varies by less than 15, 10 or 5 mole percent over a
30 day period.
In alternative embodiments, the concentration of propane in the first gas
stream on an
averaged hourly basis varies by less than 15, 10 or 5 mole percent over a 1
day period.
[0289] In some embodiments, the composition of the second composition gas
stream
is also maintained in a substantially constant condition. Such conditions may
be possible, by
adjusting the relative rates of the first and second composition gas streams
while maintaining
a substantially constant composition of both streams. In such embodiments, the
second
composition gas stream may also be sent to a gas turbine, for example a second
gas turbine,
for power recovery.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 100 -
[0290] In some embodiments of the invention maintaining the composition
of the first
composition gas stream in a substantially constant condition may be aided by
monitoring the
condition of the first composition gas stream. For example the first
composition gas stream
may be monitored for one or more properties. Exemplary properties that may be
monitored
may include one or more properties selected from gas composition, temperature,
heating
value, specific gravity, Wobbe Index, Modified Wobbe Index, dew point,
flammability limit,
flame velocity, and combinations thereof.
[0291] In some embodiments of the invention the first composition gas
stream may be
altered in order to control one or more operating parameters of the first
composition gas
stream. In such embodiments it may be useful to monitor the condition of the
first
composition gas stream and base such alterations or controls, either primarily
or partially, on
the results of such monitoring. Exemplary operating parameters that may be
controlled
include the concentration of one or more of C2 and higher hydrocarbons, C3 and
higher
hydrocarbons, carbon dioxide, inert gases, hydrogen gas, ethane, ethylene,
propane, and
combinations thereof. The chosen operating parameters may be maintained
through altering
the composition of the first composition gas stream through blending a blend
gas stream with
the first composition gas stream. The blend gas stream may include methane or
a mixture of
gaseous hydrocarbons, including a blend gas stream from a source other than
the production
fluid. In other embodiments the inert gas content of the first composition gas
stream may be
altered by adjusting the inert gas content of the first composition gas stream
to maintain a
substantially constant Wobbe Index or Modified Wobbe Index value over time.
[0292] In some embodiments of the invention altering the composition of
the first
composition gas stream includes adding hydrogen, ethane, ethylene, or
combinations thereof
to the first composition gas stream. The addition of such components may be
useful in
increasing the flame speed of the first composition gas stream, adjusting the
burn rate of the
first composition gas stream, stabilizing combustion in the first gas turbine,
or combinations
thereof.
[0293] In further embodiments, the first composition gas stream may be
altered by
reforming at least a portion of the methane in the first composition gas
stream to generate
hydrogen. This may be particularly useful where the gaseous feed to a gas
turbine combustor
contains significant CO2 and therefore requires additional hydrogen in order
to maintain a
substantially constant flame speed in the combustor of the gas turbine. In
some
embodiments, other hydrocarbon compounds, for example ethane and/or propane,
may also
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 101 -
be reformed together with the methane. The hydrogen gas content of the gas
turbine feed
may be increased by different reforming configurations. In one embodiment, at
least a
portion of the methane is removed from the first composition gas stream prior
to the
reforming, the removed methane is reformed in separate processing facilities
to generate
hydrogen gas, and the generated hydrogen gas is returned to the first
composition gas stream
prior to passing the first composition gas stream to the gas turbine.
Alternatively, at least a
portion of the methane is reformed on line while present in the first
composition gas stream
without the need to remove the methane and generate hydrogen gas in separate
reforming
facilities. In either case, the portion of methane reformed into hydrogen may
be controlled to
maintain a chosen operating parameter, including for example a substantially
constant Wobbe
Index or Modified Wobbe Index value over time.
[0294] In the various methods described herein, the composition of the
first
composition gas stream may be altered by adjusting the pressure or temperature
of one or
more oil-gas separators located in the processing facilities. Such a pressure
or temperature
adjustment will thereby change the composition of the off gas from such a
separator. The
processing facilities may include several stages of oil-gas separators,
typically at successively
lower pressures. For a series of separators at similar temperatures, the off
gas from the initial
higher pressure separators will be lighter (e.g., richer in methane and
hydrogen) than the off
gas from the later low pressure separators (e.g., richer in propane and carbon
dioxide). Thus
the first composition gas stream may be comprised of a blend of at least a
first separator gas
from a first oil-gas separator and a second separator gas from a second oil
gas separator.
Further, the method may include altering the composition of the first
composition gas stream
by adjusting the relative amounts of the first separator gas and the second
separator gas
making up the first composition gas stream.
[0295] In the various methods described herein, the composition of the
first
composition gas stream or the second composition gas stream may be altered
through use of
vapor-liquid extraction techniques. In such techniques a gas stream may be
contacted with a
liquid in order to allow for mass transfer of certain components in the gas
stream with
preferential solubility in the liquid stream, typically heavier components, to
move from the
gas stream and dissolve in the liquid stream, thereby altering the composition
of the resulting
gas stream. Thus contacting may be used for example to increase H2 content of
a gas stream
by reducing the amount of heavier components. There are many ways known in the
art for
conduction vapor-liquid extraction, including, for example employing trayed or
packed
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 102 -
columns to carry out contacting the liquid stream with the vapor or gas
stream. Typically, the
gas stream is cooled before contacting to improve solubility. Pressure may
also be increased
to improve solubility. The production fluid or a fraction thereof may be used
as the liquid
stream. In particular, a portion of produced liquids from later in the fluid
processing system
may be recycled back to contact with the gas stream, the first composition gas
stream, the
second composition gas stream, or derivatives thereof to solubilize and remove
a portion of
components in the stream. One method known as the Ryan-Holmes process is
disclosed in
U.S. Pat. No. 4,318,723 to Holmes et al. This patent describes the
distillation of acid gases
from methane using a non-polar addition such as C2 -05 alkanes. For additional
discussion of
the Ryan/Holmes process, see Holmes et al., Hydrocarbon Processing, May 1982,
pp. 131-
136; and Oil and Gas Journal, Jun. 27, 1983, pp. 85-91.
[0296] The method may include generating electricity from a gas turbine
and
optionally, the gas turbine being part of combined cycle power facilities. In
such an
embodiment the method may include, after passing the first composition gas
stream through a
first gas turbine and combusting the first composition gas stream, feeding the
combusted first
gas turbine exhaust stream to a steam boiler, thereby providing heat to the
steam boiler for
producing steam in the steam boiler. In a combined cycle operation the
generated steam may
then be fed to a steam turbine that is configured to provide energy to a
second electrical
generator. The second electrical generator may be the same electrical
generator used by the
first gas turbine or a different electrical generator. In different
embodiments the steam boiler
may be a supplementally fired waste heat boiler or may not include a
supplemental boiler
feed stream. By using a combined cycle power plant, it may be possible to
generate all or
most of the electricity demand of the in situ heating operation where the in
situ heating is
conducted primarily, predominately or exclusively through electrical resistive
heating.
[0297] In some embodiments, it may be desirable to feed the second
composition gas
stream to a steam boiler or fired heater without first passing the second
composition gas
stream to a gas turbine for power recovery. In some embodiments, the second
composition
gas stream will have a varying composition in light of maintaining the first
composition gas
stream composition substantially constant. In other embodiments, the second
composition
gas stream may have a lower heating value that is less than the lower heating
value of the first
composition gas stream. In order to accommodate the varying composition and/or
low
heating value of the second composition gas stream, which may not be
sufficiently constant
for stable processing in a given gas turbine, the second composition gas
stream may be sent
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 103 -
to a fired steam boiler. Steam generated in the steam boiler may be used for
generating
electrical power through use of a steam turbine, thus further contributing to
the overall
process power generation capacity. In alternative embodiments the steam boiler
may be a
supercritical boiler or a steam boiler equipped with catalytic combustors
which are
particularly immune to composition variations in a combusted gas stream.
[0298] In some embodiments the hot production fluid may be heat exchanged
with
water to produce low pressure steam. In such a case, the water stream would be
a boiler feed
water quality water stream.
[0299] In other embodiments the steam generated from waste heat recovery
of the
first gas turbine exhaust stream, from combustion of the second composition
gas stream in a
steam boiler or portions of the steam generated from one or both source may be
used in
surface processing facilities and/or for providing at least a portion of the
heat for heating the
organic-rich rock formation. Depending on the generation method, the steam may
be
generated as a low, medium or high pressure steam stream. A low pressure steam
is
generally at a pressure below 150 psig, a medium pressure steam is generally
in the range of
150-250 psig, while a high pressure steam is generally over 250 psig. In one
embodiment at
least a portion of the steam, particularly where a high pressure steam, is
delivered to the
organic-rich rock formation to assist in heating the formation. Lower pressure
steam,
including for example a medium pressure steam, may also be useful in for
formation heating
through injection. In some embodiments, particularly where the steam is a
steam turbine
exhaust stream of a low or medium pressure, the low pressure steam stream may
be utilized
for process heat in processing of the production fluid or derivatives thereof.
Exemplary
processes where steam may be useful include in the regeneration of an absorber
fluid for
heavy hydrocarbons or acid gases, in a reboiler of a distillation system, or
regeneration of a
solid adsorption system for acid gas and trace contaminant removal. Further
examples
include membrane separation, cryogenic distillation, and pressure swing
adsorption. In any
of the aforementioned applications where steam is used for heating, the first
gas turbine
exhaust stream itself may be utilized for process heat as an alternative to
steam in the
processing of the production fluid or derivatives thereof.
[0300] Depending on the eventual destination of the gas turbine exhaust
streams there
may be a need to control the emissions from the various gas turbines described
herein. For
example, a gas turbine exhaust stream may, in some instances, be vented to the
atmosphere,
either before or after subsequent waste heat recovery and/or further treatment
of the gas
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 104 -
turbine exhaust stream. Certain gas turbine combustors are available in the
market that are
designed to reduce the creation of nitrogen oxide (NO) compounds in the gas
turbine
combustion process. Thus, some embodiments of the invention include equipping
the first
gas turbine with lean pre-mixed combustor(s), Dry Low NO (DLN) combustor(s),
Dry Low
Emissions (DLE) combustor(s), or other similar devices in order to reduce the
formation of
NO. These technologies can be combined with targeting a gas turbine feed gas
stream
composition that is also conducive to low NO generation. NO formation is known
to be
affected by flame temperature and residence time of the nitrogen gas (N2) in
the combustion
zone. Thus NO generation can be reduced by decreasing the combustion zone
temperature
and/or the amount of N2 present in the combustion zone. In one embodiment, the
composition of the first composition gas stream may be altered by reducing the
inert gas
content of the first composition gas stream. In such an embodiment, the inert
gas
concentration of the first composition gas stream may be reduced by reducing
the nitrogen
gas content of the first composition gas stream. In one embodiment, the
composition of the
first composition gas stream may be altered by increasing the inert gas
content of the first
composition gas stream to reduce NO generation in the first gas turbine. In
such an
embodiment, the inert gas concentration may be increased by increasing the CO2
content of
the first composition gas stream. In such an embodiment the inert gas content
of the first
composition gas stream that is passed to the first gas turbine may be between
10-60 mole
percent.
[0301] Additional NO reduction technologies may be used instead of or in
combination with the previously discussed methods. The previously discussed
technologies
strive to reduce the generation of NO, however, there are additional methods
useful in
reducing the NO present in a gas turbine exhaust stream. For example,
generated NO may
be removed from a gas turbine exhaust stream by contacting the gas turbine
exhaust stream
with an ammonia (NH3) treatment stream. The ammonia treatment stream may
optionally be
obtained from a stream derived completely or partially from the production
fluid. One
embodiment includes separating NH3 from the production fluid to form a NH3
treatment
stream and injecting the NH3 treatment stream into the first gas turbine
exhaust stream,
thereby converting a portion of NO components in the first gas turbine exhaust
stream to N2.
Insome embodiments, the NH3 treatment stream has a composition of greater than
50 mole
percent NH3. In alternate embodiments, the NH3 treatment stream has a
composition of
greater than 90 mole percent NH3.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 105 -
[0302] The production fluid may contain sulfur compounds, including, for
example,
hydrogen sulfide. The hydrogen sulfide will be most prevalent in the gas
stream formed from
the production fluid. It is often desirable to remove hydrogen sulfide or
other sulfur
containing compounds from gas streams which are subsequently combusted in
order to
reduce or prevent the formation of sulfur oxides (e.g., S02) which are
environmentally
regulated compounds. The method may include treating the gas stream, the first
composition
gas stream, the second composition gas stream, or any combination thereof to
remove at least
a portion of the sulfur containing compounds present in such stream so that
the resultant
respective gas stream has less than 5 mole percent of sulfur containing
compounds.
Alternatively, the sulfur containing compounds present in such stream may be
reduced so that
the resultant respective gas stream has less than 1 mole percent or 1,000 ppm
of sulfur
containing compounds. In some embodiments, the method may include
substantially
removing H2S from the gas stream to form a rich H2S stream which can be
further processed
in, for example, a sulfur recovery plant. Alternatively, the rich H2S may be
injected into a
coal seam, a deep aquifer, a substantially depleted fractured tight gas zone,
a substantially
depleted oil shale zone, an oil shale zone depleted of sodium minerals or
combinations
thereof.
[0303] As previously discussed there may be synergies between organic-
rich rock
developments and other hydrocarbon recovery developments. For example, in some
instances tight gas deposits are located in close proximity to oil shale
deposits. In such a
case, the produced gas from a tight gas development may be used as a feed for
the first gas
turbine used in the oil shale development. As previously discussed, it may
take a period of
time before achieving full hydrocarbon gas production from an in situ heating
process of an
organic-rich rock development. Thus there may be a need for a supplemental gas
turbine gas
feed source initially. In such a case, a portion of the produced gas from a
tight gas
development may be used as a feed for the first gas turbine for a period of
time until
sufficient hydrocarbon gas is produced from the organic-rich rock development,
for example
in the case of an oil shale development. Thus in one embodiment the produced
gas from the
tight gas development is used as a feed for the first gas turbine for a period
of time beginning
after commencement of heating the organic-rich rock formation until at least
some time
before completion of producing a production fluid comprising hydrocarbon
fluids from the
organic-rich rock. In alternate embodiments, electricity generated from
electrical generators
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 106 -
coupled to the gas turbines of the organic-rich rock formation development may
be used to
power a compressor used to compress produced gas from a tight gas development.
[0304] The methods of heating an organic-rich rock formation described
herein may
result in production of water vapor which may be produced from the organic-
rich rock
formation with the production fluid. The heating of the organic-rich rock
formation in situ
may cause the formation of water vapor. In such cases the water vapor may be
produced to
the surface for further processing. The produced water vapor may be condensed
above-
ground in the surface processing facilities thereby forming a condensed water
stream. This
condensed water stream may be a high purity water stream without significant
mineral
components. There are many possible uses of such a condensed water stream. For
example
the condensed water stream may be used as boiler feed water. In one
embodiment, the
condensed water or a derivative thereof may be fed to a waste heat boiler of a
cogeneration
combined-cycle or combined heat and power system. Moreover, the condensed
water stream
may be fed to a gas turbine to improve the performance of the gas turbine. The
condensed
water stream may be fed to the gas turbine with the gas turbine feed gas, into
the combustion
chamber of the gas turbine, or with the oxidant (e.g., air) feed stream. In
one embodiment,
the condensed water or a derivative thereof may be fed to the first gas
turbine, thereby
augmenting power in the first gas turbine, controlling emissions from the
first gas turbine, or
combinations thereof. In any of the cases described above, the condensed water
or a
derivative thereof may be preheated by heat exchanging it with the production
fluid or
derivatives thereof.
[0305] The various methods of using the gas stream or derivatives thereof
for the
generation of power may include using the generated power as an energy source
for heating
the organic-rich rock formation. As previously discussed, electrical power may
be generated
by coupling the first gas turbine to a first electrical generator, thereby
generating electricity in
the first electrical generator. Further, electrical power may be generated
from steam turbines
coupled to electrical generators as described herein. In any of these cases,
the generated
electricity may be used in combination with electrical resistance heaters to
heat the organic-
rich rock formation. The electrical resistance heaters may be powered
partially, substantially
or completely through use of the generated electricity. In one embodiment, the
generated
electricity accounts for greater than 60 percent of the heat used in heating
the organic-rich
rock formation. In alternate embodiments, the generated electricity accounts
for greater than
70, 80, 90 or 95 percent of the heat used in heating the organic-rich rock
formation.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 107 -
[0306] The methods may in some embodiments utilize heating methods other
than
electrical resistance heating methods. In such cases a portion of the gas
stream may be
combusted in a process furnace to heat a process fluid. In one embodiment, the
second
composition gas stream may be fed to a furnace and combusted in the furnace to
provide heat
to a process stream. The heated process stream or a derivative thereof may
then be used to
heat the organic-rich rock formation. Alternatively, the heated process stream
may be used as
a heat transfer fluid in heating a separate fluid that is used to heat the
organic-rich rock
formation.
[0307] The generated electricity may be utilized for alternate uses in
some
embodiments. For example, the generated electricity, or a portion thereof, may
be sold to a
third party, including for example, an electric utility. Some embodiments may
include
buying electricity from an electricity supplier at selected off-peak demand
times. Some or all
of the purchased electricity may be used to heat the organic-rich rock
formation by, for
example, electrical resistance heating.
[0308] In some embodiments the production fluid or derivatives thereof
may be sold
or used in other processing units. In one embodiment the gas stream or
derivatives thereof
may be used for purposes other than the generation of electricity. For
example, if electricity
demands do not require utilization of the entirety of the gas stream, the
first composition gas
stream and/or the second composition gas stream for electricity generation,
then a portion of
such streams may be sold or used in other processes. The method may include
obtaining a
liquid stream from the production fluid in some embodiments. The liquid stream
may be
comprised of combustible hydrocarbon fluids. Exemplary liquid streams may
include an
LPG stream, a naphtha stream, a distillate stream and heavy oil stream. One or
more of these
streams may be sold or further refined to produce salable hydrocarbon
products.
[0309] Figure 29 depicts a portion of the processing facilities for an
exemplary in situ
heating organic-rich rock formation field development project. An organic-rich
rock
formation 89a located in a portion of the subsurface 89 is penetrated by
wellbore 87a. A
heating element 87 is disposed in the wellbore 87a. The heating element 87 may
be any type
of heating element, for example, an electrical resistance heating element. In
an actual
commercial development, there would be several heater well wellbores 87a with
heating
elements 87 disposed in such wellbores 87a. Electricity is fed to the heating
element 87
through electrically conductive line 318 in order to commence heating of the
organic-rich
rock formation 89a. After a period of heating the organic-rich rock formation
89a, a
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 108 -
production fluid will be produced from the organic-rich rock formation 89a,
for example
through a production well (not shown), and transferred to the processing
facilities by
production line 71. The production fluid, which may be comprised of
hydrocarbon fluids,
including, for example, a condensable (liquid) portion and a non-condensable
(gas) portion,
and non-hydrocarbon fluids, including, for example, water, CO2, H2S, and H2,
is transferred
to an oil-water separator 300 where the production fluid is split into three
streams, a water
stream 75, a hydrocarbon liquid stream 79, and a gas stream 76. Though not
shown the
production fluid may be quenched to reduce its temperature and thereby
condense a portion
of the gaseous components of the production fluid before entry into the oil-
water separator
300. The production fluid may be quenched indirectly in a heat exchanger by
use of a
cooling fluid (e.g., boiler feed water) or may be quenched directly by the
addition and mixing
with a quench water stream. It is understood that oil-water separator 300 may
be comprised
of multiple oil-gas, oil-water, and/or oil-water-gas separators in a
commercial facility,
however only one separator is shown for brevity. The water stream 75 may be
processed in
other facilities (not shown) to remove and/or recover dissolved species before
using or re-
injecting the water into the subsurface. The hydrocarbon liquid stream 79 may
contain a
broad range of hydrocarbon species and have a broad distillation range. The
hydrocarbon
liquid stream 79 may be further processed and refined in other facilities (not
shown) into
useful hydrocarbon products.
[0310] The gas stream 76 is transferred to an acid gas contactor 301
where the gas
stream is contacted with a lean amine stream 326 to facilitate removal of H2S
and, in some
cases, other acid gas species like CO2. In the acid gas contactor 301, H2S and
other acid gas
species may dissolve in the lean amine forming a rich amine solution which
falls to the
bottom of the acid gas contactor 301 and is removed through rich amine stream
319. The rich
amine stream 319 is fed to amine regenerator 320 where the temperature of the
rich amine
stream is raised and the H2S in the rich amine stream is liberated from the
amine and is
recovered in H2S stream 321. The recovered H2S stream 321 may be further
processed in a
sulfur recovery plant to produce elemental sulfur. Alternatively, H2S stream
321 may be
injected into the subsurface as previously discussed. Heat is added to the
amine regenerator
320 by amine reboiler 323 through removal of a first portion 322 of amine
regenerator 320
bottoms stream and subsequently returned hot to amine regenerator 320 by
reboiler return
stream 325. A second portion of amine regenerator 320 bottoms or lean amine
stream 326 is
returned to the amine contactor 301 for reuse in removing acid gases from the
gas stream 76.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 109 -
Further discussion of amine systems has been provided herein previously. In
addition
different methods for removing H2S and/or CO2 have been discussed previously
and may be
substituted for the particular amine system depicted in Figure 29.
[0311] Sweetened gas stream 302 is removed from the amine contactor 301
and
cooled in cooler 303 before entering gas stripper tower 304. A bottoms product
is removed
from the gas stripper 304 to form an LPG product stream 80. Gas stripper 304
includes an
overhead first composition gas stream 83 and a side draw second composition
gas stream
327. It is understood that gas stripper tower 304 may have additional draw
streams (not
shown) but that only two are depicted for simplicity. Further, it is
understood that any of the
depicted draw streams may have a reflux circuit (not shown). It is also
understood that gas
stripper 304 may be comprised of multiple stripping and/or distillation towers
with multiple
draw and reflux streams in a commercial operation, however only one tower is
shown for
brevity.
[0312] In some embodiments, the composition of the first composition gas
stream 83
and second composition gas stream 327 will be different and will require
different gas turbine
configurations as previously discussed. In the depicted embodiment, the first
composition
gas stream 83 is subsequently fed to first gas turbine 305. While only a
single first gas
turbine 305 is depicted in Figure 29, it is understood that there may be
multiple first gas
turbines in a commercial operation. The first composition gas stream 83 is
combusted in the
first gas turbine 305 and energy is recovered from the combusted gas stream in
the turbine
portions of the first gas turbine 305. In the depicted embodiment, the first
gas turbine is
coupled to an electrical generator 317 for the purpose of generating
electricity which is
transported from the electrical generator 317 by electrically conductive line
332a. The
generated electricity may be combined and transported to transformer 86
through electrically
conductive line 82. The transformed electricity may then be used to generate
heat in heating
element 87 as previously discussed. In commercial embodiments there may be
several
transformers, heating elements and wells, though only one of each is depicted
in Figure 29.
[0313] First gas turbine 305 includes a combustor (not shown) that is
optimized for
the first composition gas stream compositions as previously discussed. That
is, first gas
turbine 305 is optimized for a range of compositions expected for the first
composition gas
stream 83. The relative amounts of the first composition gas stream 83 and the
second
composition gas stream 327 may change over the life of the field development
as previously
discussed.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 110 -
[0314] Depending on the embodiment, the second composition gas stream 327
may
be further processed in a variety of ways. In one embodiment, the second
composition gas
stream may be further processed in a second gas turbine (shown in Fig. 34). In
an alternative
embodiment, the second composition gas stream may be further processed to
produce a
salable product. Exemplary salable products include hydrogen gas, methane,
ethane,
ethylene, propane, propylene or combinations thereof. In alternative
embodiments, the second
composition gas stream may be used a fuel for a steam boiler or fired heater
as discussed
herein. In still alternative embodiments, the second composition gas stream
327 may be
partially blended with the first composition gas stream 83, with the unblended
portion of the
second composition gas stream 327 being used in any of the ways specified
above in this
paragraph and the now blended first composition gas stream 83 being combusted
in the first
gas turbine 305.
[0315] The first gas turbine exhaust stream 307 is comprised of combusted
gas and is
fed to a steam boiler 312 for the purpose of generating steam from the hot
combusted turbine
exhaust stream. Gas turbine exhaust stream 307 is passed through steam boiler
312, gives up
heat to the steam boiler 312 system and exits the steam boiler 312 at a
reduced temperature
through stream 309. Boiler feed water 310 is fed to steam boiler 312 where it
is heated by the
hot gas turbine exhaust stream 307, thus generating steam which exits the
steam boiler 312
through steam stream 311. In some embodiments, the steam stream 311 may be a
high
pressure steam stream. In some embodiments, particularly where the steam
stream 311 is a
high pressure steam stream, the generated steam may be fed to a steam turbine
313 for further
power recovery, this type of power cycle, including a gas turbine and a steam
turbine, is
generally referred to as a combined cycle power generation cycle.
Alternatively, the steam
stream 311 may be used for other processing needs (not shown). In the depicted
embodiment
steam stream 311 is fed to steam turbine 313 where energy is recovered from
steam stream
311 in the steam turbine 313, thereby producing a low pressure steam stream
315 which may
be used for other processing uses (not shown) or reheated (not shown) for
eventual reuse in
the steam turbine 313. The steam turbine 313 is coupled to an electrical
generator 314 which
generates electricity which is carried to the transformer 86 by electrically
conductive lines
316 and 82.
[0316] Figure 30 depicts an alternative embodiment that utilizes a second
gas turbine
for processing the second composition gas stream. The process flow of Figure
30 is the
same as that described for Figure 29 for processing occurring up to the gas
stripper 304
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 1 1 1 -
outlet streams. Picking up the description with the gas stripper 304 gas
product streams. The
gas stripper 304 gas product streams include an overhead first composition gas
stream 83 and
a side draw second composition gas stream 327. As in Figure 29, it is
understood that gas
stripper tower 304 may have additional draw streams (not shown) but that only
two are
depicted for simplicity. Further, it is understood that the depicted draw
streams may have a
reflux circuit (not shown). It is also understood that gas stripper 304 may be
comprised of
multiple stripping and/or distillation towers with multiple draw and reflux
streams in a
commercial operation, however only one tower is shown for brevity.
[0317] In the depicted embodiment, both the first composition gas stream
83 and the
second composition gas stream 327 are subsequently fed to gas turbines 305 &
328. While
only a single first gas turbine 305 and second gas turbine 328 are depicted in
Figure 30, it is
understood that there may be multiple gas turbines for each type of gas
turbine in a
commercial operation. The respective gas streams 83 & 327 are combusted in the
respective
gas turbines 305 & 328 and energy is recovered from the combusted gas streams
in the
turbine portions of the respective gas turbines 305 & 328. Though not shown,
alternatively
the two gas turbines could be replaced with one gas turbine with a duel
combustor comprised
on a first gas injector optimized for the first composition gas stream and a
second gas injector
optimized for the second composition gas stream as previously discussed
herein. In the
depicted embodiment, each gas turbine is coupled to an electrical generator
317 & 329 for the
purpose of generating electricity which is transported from the electrical
generators by
electrically conductive lines 332 & 332a. The generated electricity may be
combined and
transported to transformer 86 through electrically conductive line 82. The
transformed
electricity may then be used to generate heat in heating element 87 as
previously discussed.
[0318] Gas turbines 305 & 328 include combustors (not shown) that are
optimized for
feed gasses having different compositions as previously discussed. That is,
first gas turbine
305 is optimized for a range of compositions expected for the first
composition gas stream 83
and second gas turbine 328 is optimized for a range of compositions expected
for the second
composition gas stream 327. The relative amounts of the first composition gas
stream 83 and
the second composition gas stream 327 is expected to change over the life of
the field
development, but the composition may remain in a substantially constant
condition such that
turbine performance remains acceptable.
[0319] The first gas turbine exhaust stream 307 and the second gas
turbine exhaust
stream 330, both comprised of combusted gas, are combined in stream 331 and
fed to a steam
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 112 -
boiler 312 for the purpose of generating steam from the hot combusted turbine
exhaust
streams. Combined stream 331 passed through steam boiler 312, gives up heat to
the steam
boiler 312 system and exits the steam boiler 312 at a reduced temperature
through stream
309. Boiler feed water 310 is fed to steam boiler 312 where it is heated by
the hot combined
stream 331, thus generating steam which exits the steam boiler 312 through
steam stream
311. In some embodiments, the steam stream 311 may be a high pressure steam
stream. In
some embodiments, particularly where the steam stream 311 is a high pressure
steam stream,
the generated steam may be fed to a steam turbine 313 for further power
recovery, this type
of power cycle, including a gas turbine and a steam turbine, is generally
referred to as a
combined cycle power generation cycle. Alternatively, the steam stream 311 may
be used for
other processing needs (not shown). In the depicted embodiment steam stream
311 is fed to
steam turbine 313 where energy is recovered from steam stream 311 in the steam
turbine 313,
thereby producing a low pressure steam stream 315 which may be used for other
processing
uses (not shown) or reheated (not shown) for eventual reuse in the steam
turbine 313. The
steam turbine 313 is coupled to an electrical generator 314 which generates
electricity which
is carried to the transformer 86 by electrically conductive lines 316 and 82.
[0320] Figure 31 depicts an alternative embodiment that utilizes a fired
steam boiler
instead of a second gas turbine for processing the second composition gas
stream. The
process flow of Figure 31 is the same as that described for Figure 29 for
processing
occurring up to the feed inlet for the gas stripper tower 304. Picking up the
description with
the sweetened gas stream 302 exiting the amine contactor 301, sweetened gas
stream 302 is
removed from the amine contactor 301 and cooled in cooler 303 before entering
gas stripper
tower 304. A bottoms product is removed from the gas stripper 304 to form an
LPG product
stream 80 as discussed in Figure 29. Gas stripper 304 includes an overhead
stream 83. As in
Figure 29, it is understood that gas stripper tower 304 may have additional
draw streams (not
shown) but that only one is depicted for simplicity. Further, it is understood
that the depicted
draw stream may have a reflux circuit (not shown). It is also understood that
gas stripper 304
may be comprised of multiple stripping and/or distillation towers with
multiple draw and
reflux streams in a commercial operation, however only one tower is shown for
brevity.
Overhead stream is fed to a second splitter tower 333 for further separation
of the feed
overhead stream 83. Gas splitter tower 333 includes an overhead first
composition gas
stream 334 and a bottom draw second composition gas stream 335. Though
depicted as a
bottoms stream, second composition gas stream 335 may alternatively be a side
draw with a
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 113 -
separate bottoms stream. In the case where the second composition gas stream
335 is a
bottom stream, the second composition gas stream 335 may be regassed (not
shown) through
addition of heat before being combusted in the fired steam boiler 336.
[0321] In some embodiments, the composition of the first composition gas
stream 334
and second composition gas stream 335 will be different and will require
different down
stream processing as previously discussed. In the depicted embodiment, the
first composition
gas stream 334 is subsequently fed to gas turbine 305. The first composition
gas stream 334
is combusted in the first gas turbine 305 and energy is recovered from the
combusted gas
stream in the turbine portion of the first gas turbine 305. The first gas
turbine 305 is coupled
to an electrical generator 317 for the purpose of generating electricity which
is transported
from the electrical generator 317 by electrically conductive line 332a. The
generated
electricity may be transported and used to generate heat in heating element 87
as previously
discussed.
[0322] In the embodiment depicted in Figure 31, the second composition
gas stream
335 is not fed to a gas turbine as described in the embodiment depicted in
Figure 30, but is
instead combusted in a fired steam boiler 336 to generate high pressure steam.
The second
composition gas stream 335 is combusted in the fired steam boiler 336, thereby
providing
heat to the steam boiler system before exiting the fired steam boiler 336
through exhaust
stream 337. Alternatively, the fired steam boiler 336 could be replaced with a
fired heater
(not shown). In such case the fired heater may be used to heat a process fluid
instead of
steam. The heated process fluid could be used for other processing needs,
including, for
example, being used as a heating fluid for heating the organic-rich rock
formation as
discussed previously. Exhaust stream 337 may be vented to the atmosphere as
shown or be a
source of heat for further heat recovery (not shown) or for injection into the
subsurface (not
shown). Boiler feed water 339 is fed to fired steam boiler 336 which is heated
in steam boiler
336 thereby generating a high pressure steam stream 338. High pressure steam
stream 338 is
fed to steam turbine 313 for power recovery as discussed in Figure 29.
[0323] The embodiment depicted in Figure 31 may be useful where it is not
possible
to maintain the composition of both the first composition gas stream and the
second
composition gas stream in a substantially constant condition. In such a case,
the first
composition gas stream may be maintained in a substantially constant condition
while the
second composition gas stream is allowed to vary. Because of the variability
of the second
composition gas stream it may not be suitable for power recovery using a gas
turbine due to
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 114 -
the limited range of compositions under which a gas turbine can maintain
stable operation as
previously discussed. Thus the variable second composition gas stream may be
processed in
a fired stream boiler which is much less affected by compositional changes of
a feed gas
stream. In cases where substantial electricity generation is desired, the
steam produced in the
fired steam boiler may be used for electricity generation through use of a
steam turbine as
depicted in Figure 31.
[0324] In some embodiments the steam generated from waste heat recovery
of the
first gas turbine exhaust stream, from combustion of the second composition
gas stream in a
steam boiler or portions of the steam generated from one or both source may be
used in
surface processing facilities and/or for providing at least a portion of the
heat for heating the
organic-rich rock formation. Referring now to Figure 33 which depicts an
alternative
embodiment that includes various uses of steam generated in the surface
facilities. Figure 33
utilizes a first gas turbine 305 and a fired steam boiler 336 as depicted in
Figure 31. The
process flow of Figure 33 is the same as that described for Figure 31 except
that Figure 33
includes additional process flows for steam streams and additional processing
equipment
related to steam processing and steam use. Picking up the description with the
fired steam
boiler 336 and the steam boiler 312, boiler feed water 339 is fed to fired
steam boiler 336
which is heated in fired steam boiler 336 thereby generating a high pressure
steam stream
338. High pressure steam stream 338 is fed to steam turbine 313 for power
recovery as
discussed in Figure 29. With reference to the steam boiler 312, combined
stream 331 passes
through steam boiler 312, gives up heat to the steam boiler 312 system and
exits the steam
boiler 312 at a reduced temperature through stream 309. Boiler feed water 310
is fed to
steam boiler 312 where it is heated by the hot combined stream 331, thus
generating steam
which exits the steam boiler 312 through steam stream 311. In some
embodiments, the steam
stream 311 may be a high pressure or medium pressure steam stream. In some
embodiments,
particularly where the steam stream 311 is a high pressure steam stream, the
generated steam
may be fed to a steam turbine 313 for further power recovery as depicted. In
addition, the
steam stream 311 from the steam boiler 312 and the high pressure steam stream
338 from the
fired steam boiler 336 may be used for other processing needs, some of which
are depicted in
Figure 33.
[0325] As depicted in Figure 33, a portion of the steam streams 338 & 311
may be
sent to a high pressure steam drum 342 through line 341. A first high pressure
steam stream
343a may be used as a heat source in heating the organic-rich rock formation
89a as
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 115 -
previously discussed herein. Steam from the outlet of the steam turbine 313
forms a low
pressure steam stream 315 which is sent to a low pressure steam drum 345
together with
steam condensate outlet 344 of amine reboiler 323. Low pressure steam may be
used in
various processing facilities, including the depicted first low pressure steam
stream 349 used
in an amine contactor preheater 361. The steam condensate outlet 362 from the
amine
contactor preheater 361 may be recycled for other processing needs or recycled
as a boiler
feed water stream (not shown). A second low pressure steam stream 360 may be
used as a
heat source for a stripper reboiler 363 used as a heat source for gas stripper
tower 304. The
steam condensate outlet 364 from the stripper reboiler 363 may be recycled for
other
processing needs or recycled as a boiler feed water stream (not shown). A
third low pressure
steam stream 343 may be used as a heat source for amine reboiler 323 as
previously
discussed with reference to Figure 29. As previously discussed herein there
are other uses
for high, medium and low pressure steam in the processing facilities and the
forgoing are
described as illustrative examples. Exemplary processes where steam may be
useful include
in the regeneration of an adsorber or absorber for heavy hydrocarbons, in a
reboiler of a
distillation system, or regeneration of a solid adsorption system for acid gas
and trace
contaminant removal. Further examples include membrane separation, cryogenic
distillation,
pressure swing adsorption and sulfur recovery units.
[0326] In the various methods described herein, the composition of the
first
composition gas stream or the second composition gas stream may be altered by
adjusting the
pressure or temperature of one or more oil-gas separators located in the
processing facilities.
Figure 34 depicts an alternative illustrative embodiment including various oil-
gas separators
that may optionally be used in surface processing facilities. Figure 34 picks
up at the
overhead stream from the acid gas contactor. Sweetened gas stream 302 is
removed from the
amine contactor (not shown) and cooled in cooler 303 before entering absorber
feed drum
500. Absorber feed drum 500 includes a liquid stream 501 and a vapor stream
which is split
into a first absorber feed drum gas stream 502 and absorber feed stream 503.
Absorber feed
stream 503 is contacted by oil stream 530 in absorber 504 for the purpose of
removing
heavier hydrocarbon species from absorber feed stream 503 before feeding the
gas stripper
tower 304 with absorber overhead stream 506. The oil stream 530 may be any oil
stream,
including the hydrocarbon liquid stream 79, or a derivative thereof, as
previously discussed
with reference to Figure 29. The absorber bottoms 505 may be sent to a flash
drum 524 after
being heated by heat exchanger 520. In the flash drum the now heated feed 523
can be
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 116 -
separated into a flash drum gas 525 and a flash drum liquid 526. Absorber
overhead stream
506 is fed to gas stripper tower 304 as previously discussed with reference to
Figure 29. The
gas stripper tower 304 depicted in Figure 34 however includes an optional
reflux cooler 507a
and reflux drum 508 as well as an optional overhead cooler 512 and overhead
product drum
513. The reflux drum 508 includes a reflux offgas stream 516, a reflux stream
510 and a
reflux product stream 509. The optional overhead product drum 513 includes
overhead gas
product 515 and overhead liquid product 514. One or more of the first absorber
feed drum
gas stream 502, flash drum gas 525, reflux offgas stream 516, or overhead gas
product 515,
may be used as the first composition gas stream or the second composition gas
stream as
previously described herein. The composition of the first or second
composition gas stream
may therefore be adjusted by adjusting the temperature and/or pressure of the
absorber feed
drum 500, flash drum 524, reflux drum 508, or overhead product drum 513,
thereby changing
the composition of the off gas from such respective oil-gas separator. While
only one oil-gas
separator is depicted in Figure 34 for each service type, it is understood
that the processing
facilities may include several stages of oil-gas separators, typically at
successively lower
pressures. For a series of separators at similar temperatures, the off gas
from the initial
higher pressure separators will be lighter (e.g., richer in methane and
hydrogen) than the off
gas from the later low pressure separators (e.g., richer in propane and carbon
dioxide). Thus
the first composition gas stream may be comprised of a blend of at least a
first separator gas
from a first oil-gas separator and a second separator gas from a second oil
gas separator.
Further, the method may include altering the composition of the first
composition gas stream
by adjusting the relative amounts of the first separator gas and the second
separator gas
making up the first composition gas stream. Particular exemplary streams that
may be
selected for mixing to form the first composition gas stream include, for
example, one or
more of the first absorber feed drum gas stream 502, flash drum gas 525,
reflux offgas stream
516, overhead gas product 515, and LPG product stream 80.
[0327] In the various methods described herein, the composition of the
first
composition gas stream or the second composition gas stream may be altered
through use of
vapor-liquid extraction techniques, such as described for absorber 504. In
such techniques a
gas stream may be contacted with a liquid in order to allow for mass transfer
of certain
components in the gas stream with preferential solubility in the liquid
stream, typically
heavier components, to move from the gas stream and dissolve in the liquid
stream, thereby
altering the composition of the resulting gas stream. The production fluid or
a fraction
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 117 -
thereof may be used as the liquid stream. In particular, a portion of produced
liquids from
later in the fluid processing system may be recycled back to contact with the
gas stream, the
first composition gas stream, the second composition gas stream, or
derivatives thereof to
solubilize and remove a portion of components in the stream.
[0328] The methods of heating an organic-rich rock formation described
herein may
result in production of water vapor which may be produced from the organic-
rich rock
formation with the production fluid. In such cases the water vapor may be
further processed
in the processing facilities. Figure 35 depicts an alternative embodiment that
includes
processing of the produced water and uses for the condensed produced water in
the surface
facilities. Figure 35 utilizes a steam turbine 313 and a fired steam boiler
336 as depicted in
Figure 31. The process flow of Figure 35 is the same as that described for
Figure 31 except
that Figure 35 includes additional process flows for produced water and
additional
processing equipment related to boiler feed water processing and use. Picking
up the
description with the oil-water separator 300 where the production fluid 71 is
split into three
streams, a water stream 75, a hydrocarbon liquid stream 79, and a gas stream
76. Though not
shown the production fluid may be quenched to reduce its temperature and
thereby condense
a portion of the gaseous components of the production fluid before entry into
the oil-water
separator 300. The water stream 75 may be a high purity water stream without
significant
mineral components, especially if water entered the wellbore 87a as a vapor.
In the depicted
embodiment, the water stream 75 is transferred to a boiler feed water drum
550. Optionally,
the water stream may be treated in water treatment facilities 552 before
entering the boiler
feed water drum 550. A first boiler feed water stream 339 feeds fired steam
boiler 336. Fired
steam boiler 336 has been previously discussed with reference to Figure 31. A
second boiler
feed water stream 310 feeds steam boiler 312. Steam boiler 312 has been
previously
discussed with reference to Figure 31. A third boiler feed water stream 551 is
used as a feed
to first gas turbine 305. First gas turbine 305 has been previously discussed
with reference to
Figure 31. As previously discussed herein, a condensed produced water stream
may be fed
to a gas turbine to improve the performance of the gas turbine. The third
boiler feed water
stream 551 may be fed to the first gas turbine 305 with the gas turbine feed
gas 334, into the
combustion chamber of the gas turbine, or with the oxidant (e.g., air) feed
stream.
EXPERIMENTS
[0329] Heating experiments were conducted on several different oil shale
specimens
and the liquids and gases released from the heated oil shale examined in
detail. An oil shale
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 118 -
sample from the Mahogany formation in the Piceance Basin in Colorado was
collected. A
solid, continuous block of the oil shale formation, approximately 1 cubic foot
in size, was
collected from the pilot mine at the Colony mine site on the eastern side of
Parachute Creek.
The oil shale block was designated CM-1B. The core specimens taken from this
block, as
described in the following examples, were all taken from the same
stratigraphic interval. The
heating tests were conducted using a Parr vessel, model number 243HC5, which
is shown in
Fig. 18 and is available from Parr Instrument Company.
Example 1
[0330] Oil shale block CM-1B was cored across the bedding planes to
produce a
cylinder 1.391 inches in diameter and approximately 2 inches long. A gold tube
7002
approximately 2 inches in diameter and 5 inches long was crimped and a screen
7000 inserted
to serve as a support for the core specimen 7001 (Fig. 17). The oil shale core
specimen 7001,
82.46 grams in weight, was placed on the screen 7000 in the gold tube 7002 and
the entire
assembly placed into a Parr heating vessel. The Parr vessel 7010, shown in
Fig. 18, had an
internal volume of 565 milliliters. Argon was used to flush the Parr vessel
7010 several times
to remove air present in the chamber and the vessel pressurized to 500 psi
with argon. The
Parr vessel was then placed in a furnace which was designed to fit the Parr
vessel. The
furnace was initially at room temperature and was heated to 400 C after the
Parr vessel was
placed in the furnace. The temperature of the Parr vessel achieved 400 C
after about 3 hours
and remained in the 400 C furnace for 24 hours. The Parr vessel was then
removed from the
furnace and allowed to cool to room temperature over a period of approximately
16 hours.
[0331] The room temperature Parr vessel was sampled to obtain a
representative
portion of the gas remaining in the vessel following the heating experiment. A
small gas
sampling cylinder 150 milliliters in volume was evacuated, attached to the
Parr vessel and the
pressure allowed to equilibrate. Gas chromatography (GC) analysis testing and
non-
hydrocarbon gas sample gas chromatography (GC) (GC not shown) of this gas
sample
yielded the results shown in Fig. 19, Table 2 and Table 1. In Fig. 19 the y-
axis 4000
represents the detector response in pico-amperes (pA) while the x-axis 4001
represents the
retention time in minutes. In Fig. 19 peak 4002 represents the response for
methane, peak
4003 represents the response for ethane, peak 4004 represents the response for
propane, peak
4005 represents the response for butane, peak 4006 represents the response for
pentane and
peak 4007 represents the response for hexane. From the GC results and the
known volumes
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 119 -
and pressures involved the total hydrocarbon content of the gas (2.09 grams),
CO2 content of
the gas (3.35 grams), and H2S content of the gas (0.06 gram) were obtained.
Table 2. Peak and area details for Fig. 19 - Example 1 - 0 stress - gas GC
Peak RetTime Area
Name
Number [min] [pA*s]
1 0.910 1.46868e4 Methane
2 0.999 148.12119 ?
3 1.077 1.26473e4 Ethane
4 2.528 1.29459e4 Propane
5 4.243 2162.93066 iC4
6 4.922 563.11804 ?
7 5.022 5090.54150 n-Butane
8 5.301 437.92255 ?
9 5.446 4.67394 ?
10 5.582 283.92194 ?
11 6.135 15.47334 ?
12 6.375 1159.83130 iC5
13 6.742 114.83960 ?
14 6.899 1922.98450 n-Pentane
15 7.023 2.44915 ?
16 7.136 264.34424 ?
17 7.296 127.60601 ?
18 7.383 118.79453 ?
19 7.603 3.99227 ?
20 8.138 13.15432 ?
21 8.223 13.01887 ?
22 8.345 103.15615 ?
23 8.495 291.26767 2-methyl pentane
24 8.651 15.64066 ?
25 8.884 91.85989 ?
26 9.165 40.09448 ?
27 9.444 534.44507 n-Hexane
28 9.557 2.64731 ?
29 9.650 32.28295 ?
30 9.714 52.42796 ?
31 9.793 42.05001 ?
32 9.852 8.93775 ?
33 9.914 4.43648 ?
34 10.013 24.74299 ?
35 10.229 13.34387 ?
36 10.302 133.95892 ?
37 10.577 2.67224 ?
38 11.252 27.57400 ?
39 11.490 23.41665 ?
40 11.567 8.13992 ?
41 11.820 32.80781 ?
42 11.945 4.61821 ?
43 12.107 30.67044 ?
44 12.178 2.58269 ?
45 12.308 13.57769 ?
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 120 -
Table 2. (Cont.)
Peak RetTime Area
Name
Number [min] [pA*s]
46 12.403 12.43018 ?
47 12.492 34.29918 ?
48 12.685 4.71311 ?
49 12.937 183.31729 ?
50 13.071 7.18510 ?
51 13.155 2.01699 ?
52 13.204 7.77467 ?
53 13.317 7.21400 ?
54 13.443 4.22721 ?
55 13.525 35.08374 ?
56 13.903 18.48654 ?
57 14.095 6.39745 ?
58 14.322 3.19935 ?
59 14.553 8.48772 ?
60 14.613 3.34738 ?
61 14.730 5.44062 ?
62 14.874 40.17010 ?
63 14.955 3.41596 ?
64 15.082 3.04766 ?
65 15.138 7.33028 ?
66 15.428 2.71734 ?
67 15.518 11.00256 ?
68 15.644 5.16752 ?
69 15.778 45.12025 ?
70 15.855 3.26920 ?
71 16.018 3.77424 ?
72 16.484 4.66657 ?
73 16.559 5.54783 ?
74 16.643 10.57255 ?
75 17.261 2.19534 ?
76 17.439 10.26123 ?
77 17.971 1.85618 ?
78 18.097 11.42077 ?
[0332] The Parr vessel was then vented to achieve atmospheric pressure,
the vessel
opened, and liquids collected from both inside the gold tube and in the bottom
of the Parr
vessel. Water was separated from the hydrocarbon layer and weighed. The amount
collected
is noted in Table 1. The collected hydrocarbon liquids were placed in a small
vial, sealed
and stored in the absence of light. No solids were observed on the walls of
the gold tube or
the walls of the Parr vessel. The solid core specimen was weighed and
determined to have
lost 19.21grams as a result of heating. Whole oil gas chromatography (WOGC)
testing of the
liquid yielded the results shown in Fig. 20, Table 3, and Table 1. In Fig. 20
the y-axis 5000
represents the detector response in pico-amperes (pA) while the x-axis 5001
represents the
retention time in minutes. The GC chromatogram is shown generally by label
5002 with
individual identified peaks labeled with abbreviations.
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 121 -
Table 3. Peak and area details for Fig. 20 - Example 1 - 0 stress - liquid GC
Ret. Time Peak Area Compound
Peak #
[min] [pA*s] Name
1 2.660 119.95327 iC4
2 2.819 803.25989 nC4
3 3.433 1091.80298 iC5
4 3.788 2799.32520 nC5
5.363 1332.67871 2-methyl pentane (2MP)
6 5.798 466.35703 3-methyl pentane (3MP)
7 6.413 3666.46240 nC6
8 7.314 1161.70435 Methyl cyclopentane (MCP)
9 8.577 287.05969 Benzene (BZ)
10 9.072 530.19781 Cyclohexane (CH)
11 10.488 4700.48291 nC7
12 11.174 937.38757 Methyl cyclohexane (MCH)
13 12.616 882.17358 Toluene (TOL)
14 14.621 3954.29687 nC8
18.379 3544.52905 nC9
16 21.793 3452.04199 nCIO
17 24.929 3179.11841 nC11
18 27.843 2680.95459 nC12
19 30.571 2238.89600 nC13
20 33.138 2122.53540 nC14
21 35.561 1773.59973 nC15
22 37.852 1792.89526 nC16
23 40.027 1394.61707 nC17
24 40.252 116.81663 Pristane (Pr)
42.099 1368.02734 nC18
26 42.322 146.96437 Phytane (Ph)
27 44.071 1130.63342 nC19
2845.956 920.52136 nC20
29 47.759 819.92810 nC21
30 49.483 635.42065 nC22
31 51.141 563.24316 nC23
32 52.731 432.74606 nC24
33 54.261 397.36270 nC25
34 55.738 307.56073 nC26
57.161 298.70926 nC27
36 58.536 252.60083 nC28
37 59.867 221.84540 nC29
38 61.154 190.29596 nC30
39 62.539 123.65781 nC31
40 64.133 72.47668 nC32
41 66.003 76.84142 nC33
42 68.208 84.35004 nC34
43 70.847 36.68131 nC35
44 74.567 87.62341 nC36
77.798 33.30892 nC37
46 82.361 21.99784 nC38
Totals: 5.32519e4
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 122 -
Example 2
[0333] Oil shale block CM-1B was cored in a manner similar to that of
Example 1
except that a 1 inch diameter core was created. With reference to Fig. 21, the
core specimen
7050 was approximately 2 inches in length and weighed 42.47 grams. This core
specimen
7050 was placed in a Berea sandstone cylinder 7051 with a 1-inch inner
diameter and a 1.39
inch outer diameter. Berea plugs 7052 and 7053 were placed at each end of this
assembly, so
that the core specimen was completely surrounded by Berea. The Berea cylinder
7051 along
with the core specimen 7050 and the Berea end plugs 7052 and 7053 were placed
in a slotted
stainless steel sleeve and clamped into place. The sample assembly 7060 was
placed in a
spring-loaded mini-load-frame 7061 as shown in Fig. 22. Load was applied by
tightening the
nuts 7062 and 7063 at the top of the load frame 7061 to compress the springs
7064 and 7065.
The springs 7064 and 7065 were high temperature, Inconel springs, which
delivered 400 'psi
effective stress to the oil shale specimen 7060 when compressed. Sufficient
travel of the
springs 7064 and 7065 remained in order to accommodate any expansion of the
core
specimen 7060 during the course of heating. In order to ensure that this was
the case, gold
foil 7066 was placed on one of the legs of the apparatus to gauge the extent
of travel. The
entire spring loaded apparatus 7061 was placed in the Parr vessel (Fig. 18)
and the heating
experiment conducted as described in Example 1.
[0334] As described in Example 1, the room temperature Parr vessel was
then
sampled to obtain a representative portion of the gas remaining in the vessel
following the
heating experiment. Gas sampling, hydrocarbon gas sample gas chromatography
(GC)
testing, and non-hydrocarbon gas sample gas chromatography (GC) was conducted
as in
Example 1. Results are shown in Fig. 23, Table 4 and Table 1. In Fig. 23 the y-
axis 4010
represents the detector response in pico-amperes (pA) while the x-axis 4011
represents the
retention time in minutes. In Fig. 23 peak 4012 represents the response for
methane, peak
4013 represents the response for ethane, peak 4014 represents the response for
propane, peak
4015 represents the response for butane, peak 4016 represents the response for
pentane and
peak 4017 represents the response for hexane. From the gas chromatographic
results and the
known volumes and pressures involved the total hydrocarbon content of the gas
was
determined to be 1.33 grams and CO2 content of the gas was 1.70 grams.
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 123 -
Table 4. Peak and area details for Fig. 23 - Example 2 - 400 psi stress - gas
GC
Peak RetTime Area
Name
Number [min] [pA*s]
1 0.910 1.36178e4 Methane
2 0.999 309.65613 ?
3 1.077 1.24143e4 Ethane
4 2.528 1.41685e4 Propane
5 4.240 2103.01929 iC4
6 4.917 1035.25513 ?
7 5.022 5689.08887 n-Butane
8 5.298 450.26572 ?
9 5.578 302.56229 ?
10 6.125 33.82201 ?
11 6.372 1136.37097 iC5
12 6.736 263.35754 ?
13 6.898 2254.86621 n-Pentane
14 7.066 7.12101 ?
15 7.133 258.31876 ?
16 7.293 126.54671 ?
17 7.378 155.60977 ?
18 7.598 6.73467 ?
19 7.758 679.95312 ?
20 8.133 27.13466 ?
21 8.216 24.77329 ?
22 8.339 124.70064 ?
23 8.489 289.12952 2-methyl pentane
24 8.644 19.83309 ?
25 8.878 92.18938 ?
26 9.184 102.25701 ?
27 9.438 664.42584 n-Hexane
28 9.549 2.91525 ?
29 9.642 26.86672 ?
30 9.705 49.83235 ?
31 9.784 52.11239 ?
32 9.843 9.03158 ?
33 9.904 6.18217 ?
34 10.004 24.84150 ?
35 10.219 13.21182 ?
36 10.292 158.67511 ?
37 10.411 2.49094 ?
38 10.566 3.25252 ?
39 11.240 46.79988 ?
40 11.478 29.59438 ?
41 11.555 12.84377 ?
42 11.809 38.67433 ?
43 11.935 5.68525 ?
44 12.096 31.29068 ?
45 12.167 5.84513 ?
46 12.297 15.52042 ?
47 12.393 13.54158 ?
48 12.483 30.95983 ?
49 12.669 20.21915 ?
50 12.929 229.00655 ?
51 13.063 6.38678 ?
52 13.196 10.89876 ?
53 13.306 7.91553 ?
54 13.435 5.05444 ?
55 13.516 44.42806 ?
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 124 -
Table 4. (Cont.)
Peak RetTime Area
Name
Number [min] [pA*s]
56 13.894 20.61910 ?
57 14.086 8.32365 ?
58 14.313 2.80677 ?
59 14.545 9.18198 ?
60 14.605 4.93703 ?
61 14.722 5.06628 ?
62 14.865 46.53282 ?
63 14.946 6.55945 ?
64 15.010 2.85594 ?
65 15.075 4.05371 ?
66 15.131 9.15954 ?
67 15.331 2.16523 ?
68 15.421 3.03294 ?
69 15.511 9.73797 ?
70 15.562 5.22962 ?
71 15.636 3.73105 ?
72 15.771 54.64651 ?
73 15.848 3.95764 ?
74 16.010 3.39639 ?
75 16.477 5.49586 ?
76 16.552 6.21470 ?
77 16.635 11.08140 ?
78 17.257 2.28673 ?
79 17.318 2.82284 ?
80 17.433 11.11376 ?
81 17.966 2.54065 ?
82 18.090 14.28333 ?
[0335] At this point, the Parr vessel was vented to achieve atmospheric
pressure, the
vessel opened, and liquids collected from inside the Parr vessel. Water was
separated from
the hydrocarbon layer and weighed. The amount collected is noted in Table 1.
The collected
hydrocarbon liquids were placed in a small vial, sealed and stored in the
absence of light.
Any additional liquid coating the surface of the apparatus or sides of the
Parr vessel was
collected with a paper towel and the weight of this collected liquid added to
the total liquid
collected. Any liquid remaining in the Berea sandstone was extracted with
methylene
chloride and the weight accounted for in the liquid total reported in Table 1.
The Berea
sandstone cylinder and end caps were clearly blackened with organic material
as a result of
the heating. The organic material in the Berea was not extractable with either
toluene or
methylene chloride, and was therefore determined to be coke formed from the
cracking of
hydrocarbon liquids. After the heating experiment, the Berea was crushed and
its total
organic carbon (TOC) was measured. This measurement was used to estimate the
amount of
coke in the Berea and subsequently how much liquid must have cracked in the
Berea. A
constant factor of 2.283 was used to convert the TOC measured to an estimate
of the amount
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 125 -
of liquid, which must have been present to produce the carbon found in the
Berea. This
liquid estimated is the "inferred oil" value shown in Table 1. The solid core
specimen was
weighed and determined to have lost 10.29 grams as a result of heating.
Example 3
[0336] Conducted in a manner similar to that of Example 2 on a core
specimen from
oil shale block CM-1B, where the effective stress applied was 400 psi. Results
for the gas
sample collected and analyzed by hydrocarbon gas sample gas chromatography
(GC) and
non-hydrocarbon gas sample gas chromatography (GC) (GC not shown) are shown in
Fig.
24, Table 5 and Table 1. In Fig. 24 the y-axis 4020 represents the detector
response in pico-
amperes (pA) while the x-axis 4021 represents the retention time in minutes.
In Fig. 24 peak
4022 represents the response for methane, peak 4023 represents the response
for ethane, peak
4024 represents the response for propane, peak 4025 represents the response
for butane, peak
4026 represents the response for pentane and peak 4027 represents the response
for hexane.
Results for the liquid collected and analyzed by whole oil gas chromatography
(NOGC)
analysis are shown in Fig. 25, Table 6 and Table 1. In Fig. 25 the y-axis 5050
represents the
detector response in pico-amperes (pA) while the x-axis 5051 represents the
retention time in
minutes. The GC chromatogram is shown generally by label 5052 with individual
identified
peaks labeled with abbreviations.
Table 5. Peak and area details for Fig. 24 ¨ Example 3 ¨ 400 psi stress ¨ gas
GC
Peak RetTime Area
Name
Number [min] [pA*s]
1 0.910 1.71356e4 Methane
2 0.998 341.71646
3 1.076 1.52621e4 Ethane
4 2.534 1.72319e4 Propane
5 4.242 2564.04077 iC4
6 4.919 1066.90942
7 5.026 6553.25244 n-Butane
8 5.299 467.88803
9 5.579 311.65158
10 6.126 33.61063
11 6.374 1280.77869 iC5
12 6.737 250.05510
13 6.900 2412.40918 n-Pentane
14 7.134 249.80679
15 7.294 122.60424
16 7.379 154.40988
17 7.599 6.87471
18 8.132 25.50270
19 8.216 22.33015
20 8.339 129.17023
21 8.490 304.97903 2-methyl pentane
22 8.645 18.48411
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 126 -
Table 5. (Cont.)
Peak RetTime Area
Name
. Number [min] [pA*s]
23 8.879 98.23043 ?
24 9.187 89.71329 ?
25 9.440 656.02161 ?
26 9.551 3.05892 n-Hexane
27 9.645 25.34058 ?
28 9.708 45.14915 ?
29 9.786 48.62077 ?
30 9.845 10.03335 ?
31 9.906 5.43165 ?
32 10.007 22.33582 ?
33 10.219 16.02756 ?
34 10.295 196.43715 ?
35 10.413 2.98115 ?
36 10.569 3.88067 ?
37 11.243 41.63386 ?
38 11.482 28.44063 ?
39 11.558 12.05196 ?
40 11.812 37.83630 ?
41 11.938 5.45990 ?
42 12.100 31.03111 ?
43 12.170 4.91053 ?
44 12.301 15.75041 ?
45 12.397 13.75454 ?
46 12.486 30.26099 ?
47 12.672 15.14775 ?
48 12.931 207.50433 ?
49 13.064 3.35393 ?
50 13.103 3.04880 ?
51 13.149 1.62203 ?
52 13.198 7.97665 ?
53 13.310 7.49605 ?
54 13.437 4.64921 ?
55 13.519 41.82572 ?
56 13.898 19.01739 ?
57 14.089 7.34498 ?
58 14.316 2.68912 ?
59 14.548 8.29593 ?
60 14.608 3.93147 ?
61 14.725 4.75483 ?
62 14.869 40.93447 ?
63 14.949 5.30140 ?
64 15.078 5.79979 ?
65 15.134 7.95179 ?
66 15.335 1.91589 ?
67 15.423 2.75893 ?
68 15.515 8.64343 ?
69 15.565 3.76481 ?
70 15.639 3.41854 ?
71 15.774 45.59035 ?
72 15.850 3.73501 ?
73 16.014 5.84199 ?
74 16.480 4.87036 ?
75 16.555 5.12607 ?
76 16.639 9.97469 ?
77 17.436 8.00434 ?
78 17.969 3.86749 ?
79 18.093 9.71661 ?
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 127 -
Table 6. Peak and area details from Fig. 25 - Example 3 - 400 psi stress -
liquid GC.
RetTime Peak Area Compound
Peak #
[min] [pA*s] Name
1 2.744 102.90978 iC4
2 2.907 817.57861 nC4
3 3.538 1187.01831 iC5
4 3.903 3752.84326 nC5 _
5 5.512 1866.25342 2MP
6 5.950 692.18964 3MP
7 6.580 6646.48242 nC6
8 7.475 2117.66919 MCP
9 8.739 603.21204 BZ
10 9.230 1049.96240 CH
11 10.668 9354.29590 nC7
12 11.340 2059.10303 MCH
13 12.669 689.82861 TOL
14 14.788 8378.59375 nC8
15 18.534 7974.54883 nC9
16 21.938 7276.47705 nCIO
17 25.063 6486.47998 nC11
18 27.970 5279.17187 nC12
19 30.690 4451.49902 nC13
20 33.254 4156.73389 nC14
21 35.672 3345.80273 nC15
22 37.959 3219.63745 nC16
23 40.137 2708.28003 nC17
24 40.227 219.38252 Pr
25 42.203 2413.01929 nC18
26 42.455 317.17825 Ph
27 44.173 2206.65405 nC19
28 46.056 1646.56616 nC20
29 47.858 1504.49097 nC21
30 49.579 1069.23608 nC22
31 51.234 949.49316 nC23
32 52.823 719.34735 nC24
33 54.355 627.46436 nC25
34 55.829 483.81885 nC26
35 57.253 407.86371 nC27
36 58.628 358.52216 nC28
37 59.956 341.01791 nC29
38 61.245 214.87863 nC30
39 62.647 146.06461 nC3 1
40 64.259 127.66831 nC32
41 66.155 85.17574 nC33
42 68.403 64.29253 nC34
43 71.066 56.55088 nC35
44 74.282 28.61854 nC36
45 78.140 220.95929 nC37
46 83.075 26.95426 nC38
Totals: 9.84518e4
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 128 -
Example 4
[0337] Conducted in a manner similar to that of Example 2 on a core
specimen from
oil shale block CM-1B; however, in this example the applied effective stress
was 1,000 psi.
Results for the gas collected and analyzed by hydrocarbon gas sample gas
chromatography
(GC) and non-hydrocarbon gas sample gas chromatography (GC) (GC not shown) are
shown
in Fig. 26, Table 7 and Table 1. In Fig. 26 the y-axis 4030 represents the
detector response
in pico-amperes (pA) while the x-axis 4031 represents the retention time in
minutes. In Fig.
26 peak 4032 represents the response for methane, peak 4033 represents the
response for
ethane, peak 4034 represents the response for propane, peak 4035 represents
the response for
butane, peak 4036 represents the response for pentane and peak 4037 represents
the response
for hexane. Results for the liquid collected and analyzed by whole oil gas
chromatography
(WOGC) are shown in Fig. 27, Table 8 and Table 1. In Fig. 27 the y-axis 6000
represents
the detector response in pico-amperes (pA) while the x-axis 6001 represents
the retention
time in minutes. The GC chromatogram is shown generally by label 6002 with
individual
identified peaks labeled with abbreviations.
Table 7. Peak and area details for Fig. 26 ¨ Example 4 ¨ 1000 psi stress ¨ gas
GC
Peak RetTime Area
Name
Number [min] [pA*s]
1 0.910 1.43817e4 Methane
2 1.000 301.69287 ?
3 1.078 1.37821e4 Ethane
4 2.541 1.64047e4 Propane
4.249 2286.08032 iC4
6 4.924 992.04395 ?
7 5.030 6167.50000 n-Butane
8 5.303 534.37000 ?
9 5.583 358.96567 ?
6.131 27.44937 ?
11 6.376 1174.68872 iC5
12 6.740 223.61662 ?
13 6.902 2340.79248 n-Pentane
14 7.071 5.29245 ?
7.136 309.94775 ?
16 7.295 154.59171 ?
17 7.381 169.53279 ?
18 7.555 2.80458 ?
19 7.601 5.22327 ?
7.751 117.69164 ?
21 8.134 29.41086 ?
22 8.219 19.39338 ?
23 8.342 133.52739 ?
24 8.492 281.61343 2-methyl pentane
8.647 22.19704 ?
26 8.882 99.56919 ?
27 9.190 86.65676 ?
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 129 -
Table 7. (Cont.)
Peak RetTime Area
Name
Number [min] [pA*s]
28 9.443 657.28754 n-Hexane
29 9.552 4.12572 ?
30 9.646 34.33701 ?
31 9.710 59.12064 ?
32 9.788 62.97972 ?
33 9.847 15.13559 ?
34 9.909 6.88310 ?
35 10.009 29.11555 ?
36 10.223 23.65434 ?
37 10.298 173.95422 ?
38 10.416 3.37255 ?
39 10.569 7.64592 ?
40 11.246 47.30062 ?
41 11.485 32.04262 ?
42 11.560 13.74583 ?
43 11.702 2.68917 ?
44 11.815 36.51670 ?
45 11.941 6.45255 ?
46 12.103 28.44484 ?
47 12.172 5.96475 ?
48 12.304 17.59856 ?
49 12.399 15.17446 ?
50 12.490 31.96492 ?
51 12.584 3.27834 ?
52 12.675 14.08259 ?
53 12.934 207.21574 ?
54 13.105 8.29743 ?
55 13.151 2.25476 ?
56 13.201 8.36965 ?
57 13.312 9.49917 ?
58 13.436 6.09893 ?
59 13.521 46.34579 ?
60 13.900 20.53506 ?
61 14.090 8.41120 ?
62 14.318 4.36870 ?
63 14.550 8.68951 ?
64 14.610 4.39150 ?
65 14.727 4.35713 ?
66 14.870 37.17881 ?
67 14.951 5.78219 ?
68 15.080 5.54470 ?
69 15.136 8.07308 ?
70 15.336 2.07075 ?
71 15.425 2.67118 ?
72 15.516 8.47004 ?
73 15.569 3.89987 ?
74 15.641 3.96979 ?
75 15.776 40.75155 ?
76 16.558 5.06379 ?
77 16.641 8.43767 ?
78 17.437 6.00180 ?
79 18.095 7.66881 ?
80 15.853 3.97375 ?
81 16.016 5.68997 ?
82 16.482 3.27234 ?
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 130 -
Table 8. Peak and area details from Fig. 27 - Example 4 - 1000 psi stress -
liquid GC.
Peak RetTime Peak Area Compound
# [min] [pA*s] Name
1 2.737 117.78948 iC4
2 2.901 923.40125 nC4
3 3.528 1079.83325 iC5
4 3.891 3341.44604 nC5
5 5.493 1364.53186 2MP
6 5.930 533.68530 3MP
7 6.552 5160.12207 nC6
8 7.452 1770.29932 MCP
9 8.717 487.04718 BZ
10 9.206 712.61566 CH
II 10.634 7302.51123 nC7
12 11. 1755.92236 MCH
13 12.760 2145.57666 TOL
14 14.755 6434.40430 nC8
15 18.503 6007.12891 nC9
16 21.906 5417.67480 nCIO
17 25.030 4565.11084 nC1 I
18 27.936 3773.91943 nC12
19 30.656 3112.23950 nC I 3
20 33.220 2998.37720 nC14
21 35.639 2304.97632 nC15
22 37.927 2197.88892 nC16
23 40.102 1791.11877 nC17
24 40.257 278.39423 Pr
25 42.171 1589.64233 nC18
26 42.428 241.65131 Ph
27 44.141 1442.51843 nC19
28 46.025 1031.68481 nC20
29 47.825 957.65479 nC21
30 49.551 609.59943 nC22
31 51.208 526.53339 nC23
32 52.798 383.01022 nC24
33 54.329 325.93640 nC25
34 55.806 248.12935 nC26
35 57.230 203.21725 nC27
36 58.603 168.78055 nC28
37 59.934 140.40034 nC29
38 61.222 95.47594 nC30
39 62.622 77.49546 nC31
40 64.234 49.08135 nC32
41 66.114 33.61663 nC33
42 68.350 27.46170 nC34
43 71.030 35.89277 nC35
44 74.162 16.87499 nC36
45 78.055 29.21477 nC37
46 82.653 9.88631 nC38
Totals: 7.38198e4
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 131 -
Example 5
[0338] Conducted in a manner similar to that of Example 2 on a core
specimen from
oil shale block CM-1B; however, in this example the applied effective stress
was 1,000 psi.
Results for the gas collected and analyzed by hydrocarbon gas sample gas
chromatography
(GC) and non-hydrocarbon gas sample gas chromatography (GC) (GC not shown) are
shown
in Fig. 28, Table 9 and Table 1. In Fig. 28 the y-axis 4040 represents the
detector response
in pico-amperes (pA) while the x-axis 4041 represents the retention time in
minutes. In Fig.
28 peak 4042 represents the response for methane, peak 4043 represents the
response for
ethane, peak 4044 represents the response for propane, peak 4045 represents
the response for
butane, peak 4046 represents the response for pentane and peak 4047 represents
the response
for hexane.
Table 9. Peak and area details for Fig. 28 - Example 5 - 1000 psi stress - gas
GC
Peak RetTime Area
Name
Number [min] [pA*s]
1 0.910 1.59035e4 Methane
2 0.999 434.21375 ?
3 1.077 1.53391e4 Ethane
4 2.537 1.86530e4 Propane
4.235 2545.45850 iC4
6 4.907 1192.68970 ?
7 5.015 6814.44678 n-Butane
8 5.285 687.83679 ?
9 5.564 463.25885 ?
6.106 30.02624 ?
11 6.351 1295.13477 iC5
12 6.712 245.26985 ?
13 6.876 2561.11792 n-Pentane
14 7.039 4.50998 ?
7.109 408.32999 ?
16 7.268 204.45311 ?
17 7.354 207.92183 ?
18 7.527 4.02397 ?
19 7.574 5.65699 ?
7.755 2.35952 ?
21 7.818 2.00382 ?
22 8.107 38.23093 ?
23 8.193 20.54333 ?
24 8.317 148.54445 ?
8.468 300.31586 2-methyl pentane
26 8.622 26.06131 ?
27 8.858 113.70123 ?
28 9.168 90.37163 ?
29 9.422 694.74438 n-Hexane
9.531 4.88323 ?
31 9.625 45.91505 ?
32 9.689 76.32931 ?
33 9.767 77.63214 ?
34 9.826 19.23768 ?
9.889 8.54605 ?
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 132 -
Table 9. (Cont.)
Peak RetTime Area
Name
Number [min] [pA*s]
36 9.989 37.74959 ?
37 10.204 30.83943 ?
38 10.280 184.58420 ?
39 10.397 4.43609 ?
40 10.551 10.59880 ?
41 10.843 2.30370 ?
42 11.231 55.64666 ?
43 11.472 35.46931 ?
44 11.547 17.16440 ?
45 11.691 3.30460 ?
46 11.804 39.46368 ?
47 11.931 7.32969 ?
48 12.094 30.59748 ?
49 12.163 6.93754 ?
50 12.295 18.69523 ?
51 12.391 15.96837 ?
52 12.482 33.66422 ?
53 12.577 2.02121 ?
54 12.618 2.32440 ?
55 12.670 12.83803 ?
56 12.851 2.22731 ?
57 12.929 218.23195 ?
58 13.100 14.33166 ?
59 13.198 10.20244 ?
60 13.310 12.02551 ?
61 13.432 8.23884 ?
62 13.519 47.64641 ?
63 13.898 22.63760 ?
64 14.090 9.29738 ?
65 14.319 3.88012 ?
66 14.551 9.26884 ?
67 14.612 4.34914 ?
68 14.729 4.07543 ?
69 14.872 46.24465 ?
70 14.954 6.62461 ?
71 15.084 3.92423 ?
72 15.139 8.60328 ?
73 15.340 2.17899 ?
74 15.430 2.96646 ?
75 15.521 9.66407 ?
76 15.578 4.27190 ?
77 15.645 4.37904 ?
78 15.703 2.68909 ?
79 15.782 46.97895 ?
80 15.859 4.69475 ?
81 = 16.022 7.36509 ?
82 16.489 3.91073 ?
83 16.564 6.22445 ?
84 16.648 10.24660 ?
85 17.269 2.69753 ?
86 17.445 10.16989 ?
87 17.925 2.28341 ?
88 17.979 2.71101 ?
89 18.104 11.19730 ?
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 133 -
Table 1. Summary data for Examples 1-5.
Example 1 Example 2 Example 3 Example 4 Example 5
Effective Stress (psi) 0 400 400 1000 1000
Sample weight (g) 82.46 42.57 48.34 43.61 43.73
Sample weight loss (g) 19.21 10.29 11.41 10.20
9.17
Fluids Recovered:
Oil (g) 10.91 3.63 3.77 3.02 2.10
36.2 gal/ton 23.4 gal/ton 21.0 gal/ton 19.3
gal/ton 13/1 gal/ton
Water (g) 0.90 0.30 0.34 0.39 0.28
2.6 gal/ton 1.7 gal/ton 1.7 gal/ton 2.1
gal/ton 1.5 gaUton
HC gas (g) 2.09 1.33 1.58 1.53 1.66
683 scf/ton 811 scf/ton 862 scf/ton 905
scf/ton 974 scf/ton
CO2 (g) 3.35 1.70 1.64 1.74 1.71
700 scf/ton 690 scf/ton 586 scf/ton 690
scf/ton 673 scf/ton
H2S (g) 0.06 0.0 0.0 0.0 0.0
Coke Recovered: 0.0 0.73 0.79 .47 0.53
Inferred Oil (g) 0.0 1.67 1.81 1.07 1.21
0 gal/ton 10.8 gal/ton 10.0 gal/ton 6.8 gal/ton 7.6
gal/ton
Total Oil (g) 10.91 5.31 5.58 4.09 3.30
36.2 gal/ton 34.1 gal/ton 31.0 gal/ton 26.1
gal/ton 20.7 gal/ton
Balance (g) 1.91 2.59 3.29 3.05 2.91
Analysis
[0339] The gas and liquid samples obtained through the experimental
procedures and
gas and liquid sample collection procedures described for Examples 1-5, were
analyzed by
the following hydrocarbon gas sample gas chromatography (GC) analysis
methodology, non-
hydrocarbon gas sample gas chromatography (GC) analysis methodology, gas
sample GC
peak identification and integration methodology, whole oil gas chromatography
(WOGC)
analysis methodology, and whole oil gas chromatography (WOGC) peak
identification and
integration methodology.
[0340] Gas samples collected during the heating tests as described in
Examples 1-5
were analyzed for both hydrocarbon and non-hydrocarbon gases, using an Agilent
Model
6890 Gas Chromatograph coupled to an Agilent Model 5973 quadrapole mass
selective
detector. The 6890 GC was configured with two inlets (front and back) and two
detectors
(front and back) with two fixed volume sample loops for sample introduction.
Peak
identifications and integrations were performed using the Chemstation software
(Revision
A.03.01) supplied with the GC instrument. For hydrocarbon gases, the GC
configuration
consisted of the following:
a) split/splitless inlet (back position of the GC)
b) HD (Flame ionization detector) back position of the GC
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 134 -
c) HP Ultra-2 (5% Phenyl Methyl Siloxane) capillary columns (two) (25
meters
x 2001.tm ID) one directed to the FID detector, the other to an Agilent 5973
Mass Selective Detector
d) 500 1 fixed volume sample loop
e) six-port gas sampling valve
f) cryogenic (liquid nitrogen) oven cooling capability
g) Oven program -80 C for 2 mins., 20 C/min. to 0 C, then 4 C/min to 20
C,
then 10 C/min. to 100 C, hold for 1 min.
h) Helium carrier gas flow rate of 2.2m1/min
i) Inlet temperature 100 C
Inlet pressure 19.35 psi
k) Split ratio 25:1
1) HD temperature 310 C
[0341] For non-hydrocarbon gases (e.g., argon, carbon dioxide and
hydrogen sulfide)
the GC configuration consisted of the following:
a) PTV (programmable temperature vaporization) inlet (front position of the
GC)
b) TCD (Thermal conductivity detector) front position of the GC
c) GS-GasPro capillary column (30 meters x 0.32mm ID)
d) 100 1 fixed volume sample loop
e) six port gas sampling valve
0 Oven program: 25 C hold for 2 min., then 10 C/min to 200 C, hold
1 min.
g) Helium carrier gas flow rate of 4.1 ml/min.
h) Inlet temperature 200 C
i) Inlet pressure 14.9 psi
Splitless mode
k) TCD temperature 250 C
[0342] For Examples 1-5, a stainless steel sample cylinder containing gas
collected
from the Parr vessel (Fig. 18) was fitted with a two stage gas regulator
(designed for lecture
bottle use) to reduce gas pressure to approximately twenty pounds per square
inch. A septum
fitting was positioned at the outlet port of the regulator to allow withdrawal
of gas by means
of a Hamilton model 1005 gas-tight syringe. Both the septum fitting and the
syringe were
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 135 -
purged with gas from the stainless steel sample cylinder to ensure that a
representative gas
sample was collected. The gas sample was then transferred to a stainless steel
cell (septum
cell) equipped with a pressure transducer and a septum fitting. The septum
cell was
connected to the fixed volume sample loop mounted on the GC by stainless steel
capillary
tubing. The septum cell and sample loop were evacuated for approximately 5
minutes. The
evacuated septum cell was then isolated from the evacuated sample loop by
closure of a
needle valve positioned at the outlet of the septum cell. The gas sample was
introduced into
the septum cell from the gas-tight syringe through the septum fitting and a
pressure recorded.
The evacuated sample loop was then opened to the pressurized septum cell and
the gas
sample allowed to equilibrate between the sample loop and the septum cell for
one minute.
The equilibrium pressure was then recorded, to allow calculation of the total
moles of gas
present in the sample loop before injection into the GC inlet. The sample loop
contents were
then swept into the inlet by Helium carrier gas and components separated by
retention time in
the capillary column, based upon the GC oven temperature program and carrier
gas flow
rates.
[0343] Calibration curves, correlating integrated peak areas with
concentration, were
generated for quantification of gas compositions using certified gas
standards. For
hydrocarbon gases, standards containing a mixture of methane, ethane, propane,
butane,
pentane and hexane in a helium matrix in varying concentrations (parts per
million, mole
basis) were injected into the GC through the fixed volume sample loop at
atmospheric
pressure. For non-hydrocarbon gases, standards containing individual
components, i.e.,
carbon dioxide in helium and hydrogen sulfide in natural gas, were injected
into the GC at
varying pressures in the sample loop to generate calibration curves.
[0344] The hydrocarbon gas sample molar percentages reported in Fig. 16
were
obtained using the following procedure. Gas standards for methane, ethane,
propane, butane,
pentane and hexane of at least three varying concentrations were run on the
gas
chromatograph to obtain peak =area responses for such standard concentrations.
The known
concentrations were then correlated to the respective peak area responses
within the
Chemstation software to generate calibration curves for methane, ethane,
propane, butane,
pentane and hexane. The calibration curves were plotted in Chemstation to
ensure good
linearity (R2 > 0.98) between concentration and peak intensity. A linear fit
was used for each
calibrated compound, so that the response factor between peak area and molar
concentration
was a function of the slope of the line as determined by the Chemstation
software. The
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 136 -
Chemstation software program then determined a response factor relating GC
peak area
intensity to the amount of moles for each calibrated compound. The software
then
determined the number of moles of each calibrated compound from the response
factor and
the peak area. The peak areas used in Examples 1-5 are reported in Tables 2,
4, 5, 7, and 9.
The number of moles of each identified compound for which a calibration curve
was not
determined (i.e., iso-butane, iso-pentane, and 2-methyl pentane) was then
estimated using the
response factor for the closest calibrated compound (i.e., butane for iso-
butane; pentane for
iso-pentane; and hexane for 2-methyl pentane) multiplied by the ratio of the
peak area for the
identified compound for which a calibration curve was not determined to the
peak area of the
calibrated compound. The values reported in Fig. 16 were then taken as a
percentage of the
total of all identified hydrocarbon gas GC areas (i.e., methane, ethane,
propane, iso-butane, n-
butane, iso-pentane, n-pentane, 2-methyl pentane, and n-hexane) and calculated
molar
concentrations. Thus the graphed methane to normal C6 molar percentages for
all of the
experiments do not include the molar contribution of the unidentified
hydrocarbon gas
species listed in Tables 2, 4, 5, 7, or 9 (e.g., peak numbers 2, 6, 8-11, 13,
15-22, 24-26, and
28-78 in Table 2).
[0345] Liquid samples collected during the heating tests as described in
Examples 1,
3 and 4 were analyzed by whole oil gas chromatography (WOGC) according to the
following
procedure. Samples, QA/QC standards and blanks (carbon disulfide) were
analyzed using an
Ultra 1 Methyl Siloxane column (25 m length, 0.32 [tm diameter, 0.52 m film
thickness) in
an Agilent 6890 GC equipped with a split/splitless injector, autosampler and
flame ionization
detector (HI)). Samples were injected onto the capillary column in split mode
with a split
ratio of 80:1. The GC oven temperature was kept constant at 20 C for 5 min,
programmed
from 20 C to 300 C at a rate of 5 C.min-1, and then maintained at 300 C
for 30 min (total
run time = 90 min.). The injector temperature was maintained at 300 C and the
HD
temperature set at 310 C. Helium was used as carrier gas at a flow of 2.1 mL
min-1. Peak
identifications and integrations were performed using Chemstation software
Rev.A.10.02
[1757] (Agilent Tech. 1990-2003) supplied with the Agilent instrument.
[0346] Standard mixtures of hydrocarbons were analyzed in parallel by the
WOGC
method described above and by an Agilent 6890 GC equipped with a
split/splitless injector,
autosampler and mass selective detector (MS) under the same conditions.
Identification of
the hydrocarbon compounds was conducted by analysis of the mass spectrum of
each peak
from the GC-MS. Since conditions were identical for both instruments, peak
identification
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 137 -
conducted on the GC-MS could be transferred to the peaks obtained on the GC-
FID. Using
these data, a compound table relating retention time and peak identification
was set up in the
GC-H1) Chemstation. This table was used for peak identification.
[0347] The gas chromatograms obtained on the liquid samples (Figures 4, 9
and 11)
were analyzed using a pseudo-component technique. The convention used for
identifying
each pseudo-component was to integrate all contributions from normal alkane to
next
occurring normal alkane with the pseudo-component being named by the late
eluting n-
alkane. For example, the C-10 pseudo-component would be obtained from
integration
beginning just past normal-C9 and continue just through normal-C10. The carbon
number
weight % and mole % values for the pseudo-components obtained in this manner
were
assigned using correlations developed by Katz and Firoozabadi (Katz, D.L., and
A.
Firoozabadi, 1978. Predicting phase behavior of condensate/crude-oil systems
using methane
interaction coefficients, J. Petroleum Technology (Nov. 1978), 1649-1655).
Results of the
pseudo-component analyses for Examples 1, 3 and 4 are shown in Tables 10, 11
and 12.
[0348] An exemplary pseudo component weight percent calculation is
presented
below with reference to Table 10 for the C10 pseudo component for Example 1 in
order to
illustrate the technique. First, the C-10 pseudo-component total area is
obtained from
integration of the area beginning just past normal-C9 and continued just
through normal-C10
as described above. The total integration area for the C10 pseudo component is
10551.700
pico-ampere-seconds (pAs). The total C10 pseudo component integration area
(10551.700
pAs) is then multiplied by the C10 pseudo component density (0.7780 g/ml) to
yield an "area
X density" of 8209.22 pAs g/ml. Similarly, the peak integration areas for each
pseudo
component and all lighter listed compounds (i.e., nC3, iC4, nC4, iC5 & nC5)
are determined
and multiplied by their respective densities to yield "area X density" numbers
for each
respective pseudo component and listed compound. The respective determined
"area X
density" numbers for each pseudo component and listed compound is then summed
to
determine a "total area X density" number. The "total area X density" number
for Example 1
is 96266.96 pAs g/ml. The C10 pseudo component weight percentage is then
obtained by
dividing the C10 pseudo component "area X density" number (8209.22 pAs g/m1)
by the
"total area X density" number (96266.96 pAs g/m1) to obtain the C10 pseudo
component
weight percentage of 8.53 weight percent.
[0349] An exemplary pseudo component molar percent calculation is
presented below
with reference to Table 10 for the C10 pseudo component for Example 1 in order
to further
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 138 - =
illustrate the pseudo component technique. First, the C-10 pseudo-component
total area is
obtained from integration of the area beginning just past normal-C9 and
continued just
through normal-C10 as described above. The total integration area for the C10
pseudo
component is 10551.700 pico-ampere-seconds (pAs). The total C10 pseudo
component
integration area (10551.700 pAs) is then multiplied by the C10 pseudo
component density
(0.7780 g/m1) to yield an "area X density" of 8209.22 pAs g/ml. Similarly, the
integration
areas for each pseudo component and all lighter listed compounds (i.e., nC3,
iC4, nC4, iC5 &
nC5) are determined and multiplied by their respective densities to yield
"area X density"
numbers for each respective pseudo component and listed compound. The C10
pseudo
component "area X density" number (8209.22 pAs g/m1) is then divided by the
C10 pseudo
component molecular weight (134.00 g/mol) to yield a C10 pseudo component
"area X
density / molecular weight" number of 61.26 pAs mol/ml. Similarly, the "area X
density"
number for each pseudo component and listed compound is then divided by such
components
or compounds respective molecular weight to yield an "area X density /
molecular weight"
number for each respective pseudo component and listed compound. The
respective
determined "area X density / molecular weight" numbers for each pseudo
component and
listed compound is then summed to determine a "total area X density /
molecular weight"
number. The total "total area X density / molecular weight" number for Example
1 is 665.28
pAs mol/ml. The C10 pseudo component molar percentage is then obtained by
dividing the
C10 pseudo component "area X density / molecular weight" number (61.26 pAs
mol/ml) by
the "total area X density / molecular weight" number (665.28 pAs mol/ml) to
obtain the C10
pseudo component molar percentage of 9.21 molar percent.
Table 10. Pseudo-components for Example 1 - GC of liquid - 0 stress
Molecular
Component Area (cts.) Area % Avg.Boiling Density
(g/m1) Wt. (g/l) Wt. % Mol %
Pl. CF) mo
nC3 41.881 0.03 -43.73 0.5069 44.10 0.02 0.07
iC4 120.873 0.10 10.94 0.5628 58.12 0.07
0.18
nC4 805.690 0.66 31.10 0.5840 58.12 0.49 1.22
iC5 1092.699 0.89 82.13 0.6244 72.15 0.71 1.42 -
nC5 2801.815 2.29 96.93 0.6311 72.15 1.84 3.68
Pseudo C6 7150.533 5.84 147.00 0.6850 84.00 5.09 8.76
Pseudo C7 10372.800 8.47 197.50 0.7220 96.00 7.78 11.73
Pseudo C8 11703.500 9.56 242.00 0.7450 107.00 9.06 12.25
Pseudo C9 11776.200 9.61 288.00 0.7640 121.00 9.35 11.18
Pseudo C10 10551.700 8.61 330.50 0.7780 134.00 8.53 9.21
Pseudo Cu 9274.333 7.57 369.00 0.7890 147.00 7.60 7.48
Pseudo C12 8709.231 7.11 407.00 0.8000 161.00 7.24 6.50
Pseudo C13 7494.549 6.12 441.00 0.8110 175.00 6.31 5.22
Pseudo C14 6223.394 5.08 475.50 0.8220 190.00 5.31 4.05
Pseudo C15 6000.179 4.90 511.00 0.8320 206.00 5.19 3.64
Pseudo C16 5345.791 4.36 542.00 0.8390 222.00 4.66 3.04
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
. - 139 -
Table 10. (Cont.)
Molecular
Component Area (cts.) Area % Avg. Boiling pt. toF) Density
(g/m1) wt. tgimot) Wt. % Mol To
Pseudo C17 4051.886 3.31 572.00 0.8470 237.00 3.57
2.18
Pseudo C18 3398.586 2.77 595.00 0.8520 251.00 3.01
1.73
Pseudo C19 2812.101 2.30 617.00 0.8570 263.00 2.50
1.38
Pseudo C20 2304.651 1.88 640.50 0.8620 275.00 2.06
1.09
Pseudo C21 2038.925 1.66 664.00 0.8670 291.00 1.84
0.91
Pseudo C22 1497.726 1.22 686.00 0.8720 305.00 1.36
0.64
Pseudo C23 1173.834 0.96 707.00 0.8770 318.00 1.07
0.49
Pseudo C24 822.762 0.67 727.00 0.8810 331.00 0.75
0.33
Pseudo Cm 677.938 0.55 747.00 0.8850 345.00 0.62
0.26
Pseudo C26 532.788 0.43 766.00 0.8890 359.00 0.49
0.20
Pseudo C27 459.465 0.38 784.00 0.8930 374.00 0.43
0.16
Pseudo C28 413.397 0.34 802.00 0.8960 388.00 0.38
0.14
Pseudo C29 522.898 0.43 817.00 0.8990 402.00 0.49
0.18
Pseudo C30 336.968 0.28 834.00 0.9020 416.00 0.32
0.11
=
Pseudo C31 322.495 0.26 850.00 0.9060 430.00 0.30
0.10
Pseudo C32 175.615 0.14 866.00 0.9090 444.00 0.17
0.05
Pseudo C33 165.912 0.14 881.00 0.9120 458.00 0.16
0.05
Pseudo C34 341.051 0.28 895.00 0.9140 472.00 0.32
0.10
Pseudo C35 286.861 0.23 908.00 0.9170 486.00 0.27
0.08
Pseudo C36 152.814 0.12 922.00 0.9190 500.00 0.15
0.04
Pseudo C37 356.947 0.29 934.00 0.9220 514.00 0.34
0.10
Pseudo C38 173.428 0.14 947.00 0.9240 528.00 0.17
0.05
Totals 122484.217 100.00 100.00
100.00
Table 11. Pseudo-components for Example 3 - GC of liquid - 400 psi stress
Avg. Boiling Density Molecular Wt.
Component Area Area% Wt. % Mol %
Pt. ( F) (g/m1) (g/mol)
nC3 35.845 0.014 -43.730 0.5069 44.10 0.01
0.03
iC4 103.065 0.041 10.940 0.5628 58.12 0.03
0.07
nC4 821.863 0.328 31.100 0.5840 58.12 0.24
0.62
iC5 1187.912 0.474 82.130 0.6244 72.15 0.37
0.77
nC5 3752.655 1.498 96.930 0.6311 72.15 1.20
2.45
Pseudo C6 12040.900 4.805 147.000 0.6850 84.00 4.17
7.34
Pseudo C7 20038.600 7.997 197.500 0.7220 96.00 7.31
11.26
Pseudo C8 24531.500 9.790 , 242.000 0.7450 107.00
9.23 12.76
Pseudo C9 25315.000 10.103 288.000 0.7640 121.00
9.77 11.94
Pseudo C10 22640.400 9.035 330.500 0.7780 134.00
8.90 9.82
Pseudo CI 1 20268.100 8.089 369.000 0.7890 147.00
8.08 8.13
Pseudo C12 18675.600 7.453 407.000 0.8000 161.00 7.55
6.93
Pseudo C13 16591.100 6.621 441.000 0.8110 175.00
6.80 5.74
Pseudo C14 13654.000 5.449 475.500 0.8220 190.00 5.67
4.41
Pseudo C15 , 13006.300 5.191 511.000 0.8320 206.00 5.47
3.92
Pseudo C16 11962.200 4.774 542.000 0.8390 222.00
5.07 3.38
Pseudo C17 8851.622 3.533 572.000 0.8470 237.00 3.79
2.36
Pseudo C18 7251.438 2.894 595.000 0.8520 251.00 3.12
1.84
Pseudo C19 5946.166 2.373 617.000 0.8570 263.00 2.57
1.45
Pseudo C20 4645.178 1.854 640.500 0.8620 275.00 2.02
1.09
Pseudo C21 4188.168 1.671 664.000 0.8670 291.00 1.83
0.93
Pseudo C22 2868.636 1.145 686.000 0.8720 305.00 1.26
0.61
Pseudo C23 2188.895 0.874 707.000 0.8770 318.00 0.97
0.45
Pseudo C24 1466.162 0.585 727.000 0.8810 331.00 0.65
0.29
Pseudo C25 1181.133 0.471 747.000 0.8850 345.00 0.53
0.23
Pseudo C26 875.812 0.350 766.000 0.8890 359.00 0.39
0.16
Pseudo C27 617.103 0.246 784.000 0.8930 374.00 0.28
0.11
Pseudo C28 538.147 0.215 802.000 0.8960 388.00 0.24
0.09
Pseudo C29 659.027 0.263 817.000 0.8990 402.00 0.30
0.11
Pseudo C30 1013.942 0.405 834.000 0.9020 416.00 0.46
0.16
CA 02686565 2009-11-05
WO 2008/147503
PCT/US2008/006463
- 140 -
Table 11. (Cont.)
Avg. Boiling Density Molecular Wt.
Component Area Area% Wt. % Mol %
(g/n11) (ghnol)
Pseudo C31 761.259 0.304 850.000 0.9060 430.00 0.35
0.12
Pseudo C32 416.031 0.166 866.000 0.9090 444.00 0.19
0.06
Pseudo C33 231.207 0.092 881.000 0.9120 458.00 0.11
0.03
Pseudo C34 566.926 0.226 895.000 0.9140 472.00 0.26
0.08
Pseudo C35 426.697 0.170 908.000 0.9170 486.00 0.20
0.06
Pseudo C36 191.626 0.076 922.000 0.9190 500.00 0.09
0.03
Pseudo C37 778.713 0.311 934.000 0.9220 514.00 0.36
0.10
Pseudo Cm 285.217 0.114 947.000 0.9240 528.00 0.13
0.04
Totals 250574.144 100.000 100.00 100.00
Table 12. Pseudo-components for Example 4 - GC of liquid - 1000 psi stress
Avg. Boiling Density Molecular Wt.
Component Area Area % Wt. % Mol %
Pt. ( F) (0111) (g/mol)
nC3 44.761 0.023 -43.730 0.5069 44.10 0.01
0.05
iC4 117.876 0.060 10.940 0.5628 58.12 0.04
0.11
nC4 927.866 0.472 31.100 0.5840 58.12 0.35
0.87
iC5 1082.570 0.550 82.130 0.6244 72.15 0.44
0.88
nC5 3346.533 1.701 96.930 0.6311 72.15 1.37
2.74
Pseudo C6 9579.443 4.870 147.000 0.6850 84.00 4.24
7.31
Pseudo C7 16046.200 8.158 197.500 0.7220 = 96.00 7.49
11.29
Pseudo C8 19693.300 10.012 242.000 0.7450 107.00 9.48
12.83
Pseudo C9 20326.300 10.334 288.000 0.7640 121.00 10.04
12.01
Pseudo C10 18297.600 9.302 330.500 0.7780 134.00 9.20
9.94
Pseudo C11 16385.600 8.330 369.000 0.7890 147.00 8.36
8.23
Pseudo C12 15349.000 7.803 407.000 0.8000 161.00 7.94
7.14
Pseudo C13 13116.500 6.668 441.000 0.8110 175.00 6.88
5.69
Pseudo C14 10816.100 5.499 475.500 0.8220 190.00 5.75
4.38
Pseudo C15 10276.900 5.225 511.000 0.8320 206.00 5.53
3.88
Pseudo C16 9537.818 4.849 542.000 0.8390 222.00 5.17
3.37
Pseudo C17 6930.611 3.523 572.000 0.8470 237.00 3.79
2.32
Pseudo C18 5549.802 2.821 595.000 0.8520 251.00 3.06
1.76
Pseudo C19 4440.457 2.257 617.000 0.8570 263.00 2.46
1.35
Pseudo C20 3451.250 1.755 640.500 0.8620 275.00 1.92
1.01
Pseudo C21 3133.251 1.593 664.000 0.8670 291.00 1.76
0.87
Pseudo C22 2088.036 1.062 686.000 0.8720 305.00 1.18
0.56
Pseudo C23 1519.460 0.772 707.000 0.8770 318.00 0.86
0.39
Pseudo C24 907.473 0.461 727.000 0.8810 331.00 0.52
0.23
Pseudo C25 683.205 0.347 747.000 0.8850 345.00 0.39
0.16
Pseudo C26 493.413 0.251 766.000 0.8890 359.00 0.28
0.11
Pseudo C27 326.831 0.166 784.000 0.8930 374.00 0.19
0.07
Pseudo C28 272.527 0.139 802.000 0.8960 388.00 0.16
0.06
Pseudo C29 291.862 0.148 817.000 0.8990 402.00 0.17
0.06
Pseudo C30 462.840 0.235 834.000 0.9020 416.00 0.27
0.09
Pseudo C31 352.886 0.179 850.000 0.9060 430.00 0.21
0.07
Pseudo C32 168.635 0.086 866.000 0.9090 444.00 0.10
0.03
Pseudo C33 67.575 0.034 881.000 0.9120 _ 458.00 0.04
0.01
Pseudo C34 95.207 0.048 895.000 0.9140 472.00 0.06
0.02
Pseudo C35 226.660 0.115 908.000 0.9170 486.00 0.13
0.04
Pseudo C36 169.729 0.086 922.000 0.9190 500.00 0.10
0.03
-
Pseudo C37 80.976 0.041 934.000 0.9220_ 514.00 0.05
0.01
Pseudo C38 42.940 0.022 947.000 0.9240 528.00 0.03
0.01
Totals 196699.994 100.000 100.00 100.00
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 141 -
[0350] TOC and Rock-eval tests were performed on specimens from oil shale
block
CM-1B taken at the same stratigraphic interval as the specimens tested by the
Parr heating
method described in Examples 1-5. These tests resulted in a TOC of 21% and a
Rock-eval
Hydrogen Index of 872 mg/g-toc.
[0351] The TOC and rock-eval procedures described below were performed on
the oil
shale specimens remaining after the Parr heating tests described in Examples 1-
5. Results are
shown in Table 13.
[0352] The Rock-Eval pyrolysis analyses described above were performed
using the
following procedures. Rock-Eval pyrolysis analyses were performed on
calibration rock
standards (IFP standard #55000), blanks, and samples using a Delsi Rock-Eval
II instrument.
Rock samples were crushed, micronized, and air-dried before loading into Rock-
Eval
crucibles. Between 25 and 100mg of powdered-rock samples were loaded into the
crucibles
depending on the total organic carbon (TOC) content of the sample. Two or
three blanks
were run at the beginning of each day to purge the system and stabilize the
temperature. Two
or three samples of IFP calibration standard #55000 with weight of 100 +/- 1
mg were run to
calibrate the system. If the Rock-Eval T. parameter was 419 C +/- 2 C on
these
standards, analyses proceeded with samples. The standard was also run before
and after
every 10 samples to monitor the instrument's performance.
[0353] The Rock-Eval pyrolysis technique involves the rate-programmed
heating of a
powdered rock sample to a high temperature in an inert (helium) atmosphere and
the
characterization of products generated from the thermal breakdown of chemical
bonds. After
introduction of the sample the pyrolysis oven was held isothermally at 300 C
for three
minutes. Hydrocarbons generated during this stage are detected by a flame-
ionization
detector (FlD) yielding the S1 peak. The pyrolysis-oven temperature was then
increased at a
gradient of 25 C/minute up to 550 C, where the oven was held isothermally
for one minute.
Hydrocarbons generated during this step were detected by the 1411) and yielded
the S2 peak.
[0354] Hydrogen Index (HI) is calculated by normalizing the S2 peak
(expressed as
Mghydrocarbons/grock) toweight % TOC (Total Organic Carbon determined
independently) as
follows:
HI = (S2 I TOC)* 100
where HI is expressed as InghyctrocarbonsigTOC
CA 02686565 2009-11-05
WO 2008/147503 PCT/US2008/006463
- 142 -
[0355] Total Organic Carbon (TOC) was determined by well known methods
suitable for geological samples - i.e., any carbonate rock present was removed
by acid
treatment followed by combustion of the remaining material to produce and
measure organic
based carbon in the form of c02.
Table 13. TOC and Rock-eval results on oil shale specimens after the Parr
heating tests.
Example 1 Example 2 Example 3 Example 4
Example 5
TOC (To) 12.07 10.83 10.62 11.22 11.63
HI (mg/g-toc) 77 83 81 62 77
[0356] The API gravity of Examples 1-5 was estimated by estimating the
room
temperature specific gravity (SG) of the liquids collected and the results are
reported in Table
14. The API gravity was estimated from the determined specific gravity by
applying the
following formula:
API gravity = (141.5/ SG)-131.5
[0357] The specific gravity of each liquid sample was estimated using the
following
procedure. An empty 50 IA Hamilton Model 1705 gastight syringe was weighed on
a Mettler
AE 163 digital balance to determine the empty syringe weight. The syringe was
then loaded
by filling the syringe with a volume of liquid. The volume of liquid in the
syringe was noted.
The loaded syringe was then weighed. The liquid sample weight was then
estimated by
subtracting the loaded syringe measured weight from the measured empty syringe
weight.
The specific gravity was then estimated by dividing the liquid sample weight
by the syringe
volume occupied by the liquid sample.
Table 14. Estimated API Gravity of liquid samples from Examples 1-5
Example Example 1 Example 2 = Example 3 Example 4 Example 5
API Gravity 29.92 30.00 27.13 32.70 30.00
[0358] The above-described processes may be of merit in connection with
the
recovery of hydrocarbons in the Piceance Basin of Colorado. Some have
estimated that in
some oil shale deposits of the Western United States, up to 1 million barrels
of oil may be
recoverable per surface acre. One study has estimated the oil shale resource
within the
nahcolite-bearing portions of the oil shale formations of the Piceance Basin
to be 400 billion
barrels of shale oil in place. Overall, up to 1 trillion barrels of shale oil
may exist in the
Piceance Basin alone.
[0359] Certain features of the present invention are described in terms
of a set of
numerical upper limits and a set of numerical lower limits. It should be
appreciated that
CA 02686565 2015-04-14
- 143 -
ranges formed by any combination of these limits are within the scope of the
invention unless
otherwise indicated. Although some of the dependent claims have single
dependencies in
accordance with U.S. practice, each of the features in any of such dependent
claims can be
combined with each of the features of one or more of the other dependent
claims dependent
upon the same independent claim or claims.
[0360] While it
will be apparent that the invention herein described is well calculated
to achieve the benefits and advantages set forth above, it will be appreciated
that the
invention is susceptible to modification, variation and change. The scope of
the claims should
not be limited by particular embodiments set forth herein, but should be
construed in a
manner consistent with the specification as a whole.