Note: Descriptions are shown in the official language in which they were submitted.
CA 02688206 2009-11-24
NOVEL INDOL-PYRROL DERIVATIVES FOR THE TREATMENT OF
PROLIFERATIVE AND INFLAMMATORY DISEASES
The invention relates to novel indole-pyrrole
derivatives, to pharmaceutical compositions con-
taining such compounds, to the uses of such com-
pounds, and to methods for producing such com-
pounds.
From the documents Sun, L. et al, J. Med.
Chem. 46:1116-1119 (2003), Manley, J. M. et al, J.
Org. Chem. 68:6447-6450 (2003) and Mendel, D.B. et
al, Clin Cancer Res. 9 ( 1 ) : 3 2 7 - 3 3 7 (2003), various
indole-pyrrole derivatives are known in the art.
These are suitable for the treatment of different
types of cancer, mastocytosis, allergy-associated
chronic rhinitis, diabetes, arthritis, angiogene-
sis and various immunologic and cardiovascular
diseases.
In principle, there is a strong demand of new
and improved active substances, which are capable
of inhibiting the proliferation of cancer cells
and thus the growth of neoplastic tumors and of
inhibiting excessive defense reactions of the
body, such as e.g. septic shock, autoimmune dis-
eases, transplant rejections and acute and chronic
inflammation reactions, and that with simultane-
ously only low to no cytotoxicity at all with re-
spect to intact cells. Additionally, it is in-
tended to inhibit the growth of unicellular organ-
isms.
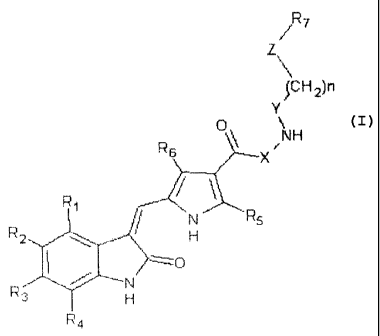
For this purpose, the invention teaches a com-
pound according to Formula I
CA 02688206 2009-11-24
2 -
/ R7
L
(
)CH2)n
(.J .
NH
Y__..._Y =
~r/ t!
R
tq ,. Rs
H
/"~.. / r~""R ~ /
4 Formula I
R
R
wherein R1 to R4 may be identical or different and
may be -H, -F, -Cl, -Br, -I or (C1-C$) oxyalkyl,
wherein R5 and R6 may be identical or different
and may be -H or Cl-C4 alkyl, wherein X, Y and Z
may be identical or different and may be either a
binding or -0- or -S-, at least one of the groups
X, Y and Z being -0- or -S-, wherein n may be 0 to
8, and wherein R7 may be an arbitrary radical, or
a metabolite of such a compound and physiologi-
cally well tolerated salts of such compounds or
metabolites.
Preferably, R1, R3 and R4 are -H, and R2 is -
F, -Cl, -Br, -I or (C1-C$) oxyalkyl. R5 and R6 may
be (C1-C3) alkyl, in particular methyl.
X preferably is a binding, wherein Y and/or Z
is -0-. n = 1 or 2 is preferred.
R7 preferably is an -NR8R9 group, in which R8
and R9 may be identical or different and may be -
H, a(C1-Clo) alkyl group or (C1-C6) oxyalkyl, a
(C1-Clo) alkyl group or, if applicable, a par-
tially or completely halogenated, in particular
fluorinated (C1-Clo) alkyl group, (C3-C-7 ) cycloal-
kyl group, (C2-Clo) alkenyl group, (CZ-Clo) alkinyl
group, (C1-CB) alkyl- (C3-C7) cycloalkyl group, (Cz-
CB) alkenyl-(C3-C7) cycloalkyl group, heterocyclyl
CA 02688206 2009-11-24
3 -
group, (C1-C$) alkylheterocyclyl group, (C2-C8) al-
kenylheterocyclyl group, aryl group, (C1-C8) al-
kylaryl group, (C2-Ca) alkenylaryl group, or (C2-
C$) alkinylaryl group, or a, if applicable, mono-
cyclic or bicyclic heteroaryl group substituted by
1-2 keto groups, 1-2 (C1-C5) alkyl groups, 1-2
(C1-C5) alkoxy groups, 1-3 halogen atoms, 1-2
exomethyl groups, and containing 1-3 nitrogen at-
oms and/or 1-2 oxygen atoms and/or 1-2 sulfur at-
oms, a (C1-C$) alkylheteroaryl group, or a.(C2-C8)
alkenylheteroaryl group, a (C2-C8) alkinylhet-
eroaryl group, wherein these groups may be linked
at an arbitrary position with R2 and, if applica-
ble, may be hydrated at one or several positions.
More specifically, R7 preferably is an -NR8R9
group, in which R8 and R9 may be identical or dif-
ferent and may be -H, methyl, ethyl, methoxy or
ethoxy, or wherein R7 is pyrrolidine-1-yl, mor-
pholine-4-yl, pyrridine-4-yl, 1-methyl-4-pip-
eridyl, or triazole-l-yl, optionally substituted
singly or multiply with (C1-C6) alkyl, -F, -Cl, -
Br, -I, or (C1-C6) oxyalkyl.
The alkyl groups for the described radicals,
in particular R3, may be straight-chained or
branched and may for instance be a methyl, ethyl,
n-propyl, iso-propyl, n-butyl, iso-butyl, tert-bu-
tyl or n-pentyl, 2,2-dimethylpropyl, 2-methylbutyl
or 3-methylbutyl group, and the hexyl, heptyl,
nonyl, decyl group and their arbitrarily branched
derivatives. A methyl or ethyl group is preferred.
The mentioned alkyl groups may be, if applicable,
substituted by 1-5 halogen atoms. A partially or
completely halogenated, in particular fluorinated
C1-C3 alkyl group may for instance be the follow-
ing partially or completely fluorinated groups:
fluoromethyl, difluoromethyl, trifluoromethyl,
fluoroethyl, 1,1-difluoroethyl, 1,2-difluoroethyl,
CA 02688206 2009-11-24
4 -
1,1,1-trifluoroethyl, tetrafluoroethyl, pentaflu-
oroethyl. Among these are preferred the trifluoro-
methyl or the pentafluoroethyl group, while the
completely fluorinated group is also called per-
fluoroalkyl group.
The alkoxy groups (= oxyalkyl) may be
straight-chained or branched and may for instance
be a methoxy, ethoxy, n-propoxy, iso-propoxy, n-
butoxy, iso-butoxy, tert-butoxy or n-pentoxy, 2,2-.
dimethylpropoxy, 2-methylbutoxy or 3-methylbutoxy
group. Cl-C5 alkoxy groups are preferred. A meth-
oxy or ethoxy group is particularly preferred.
The cycloalkyl group is, if applicable, a
saturated cyclic group with 3 to 7 ring carbon at-
oms substituted by one or more halogen atoms, (C1-
C5)alkyl groups, (C1-C5) alkoxy groups, NR10R11
groups, COOR12 groups, CHO, cyano, such as cyclo-
propyl, methylcyclopropyl, cyclobutyl, methylcy-
clobutyl, cyclopentyl, methylcyclopentyl, cyclo-
hexyl, methylcyclohexyl, cycloheptyl, methylcyclo-
heptyl.
A(C1-Cs.) alkyl-(C3-C7) cycloalkyl group is a
cycloalkyl group, which is linked by a straight-
chained or branched (C1-C8) alkyl unit with the
ring system.
A (C2-C8) alkenyl- (C3-C7) cycloalkyl group is a
cycloalkyl group, which is linked by a straight-
chained or branched (C2-C8) alkenyl unit with the
ring system.
The heterocyclyl group is not aromatic and may
for instance be pyrrolidine, imidazolidine, pyra-
zolidine, and piperidine. Perhydrochinoline and
CA 02688206 2009-11-24
- 5 -
perhydroisochinoline also belong to the included
heterocyclyl groups.
Aryl groups in the meaning of the invention
are aromatic or partially aromatic carbocyclic
groups with 6 to 14 carbon atoms, which comprise a
ring, for instance phenyl or phenylene, or several
condensed rings, such as naphthyl or anthranyl.
Examples are phenyl, naphthyl, tetralinyl, an-
thranyl, indanyl, and indenyl.
The aryl groups may be substituted at any
suitable position, which leads to a stable stereo-
isomer, by one or more radicals from the group hy-
droxy or halogen.
A(C1-C8) alkylaryl group is an aryl group,
such as described above, which is linked by a
straight-chained or branched (C1-C8) alkyl unit
with the ring system.
A(C2-C8) alkenylaryl group is an aryl group,
such as described above, which is linked by a
straight-chained or branched (C2-C8) alkenyl unit
with the ring system.
A (C2-C8) alkinylaryl group is an aryl group,
such as described above, which is linked by a
straight-chained or branched (C2-C8) alkinyl unit
with the ring system.
Monocyclic heteroaryl groups may for instance
be pyridine, pyrazine, pyrimidine, pyridazine,
triazine, azaindolizine, 2H and 4H-pyran, 2H and
4H-thiopyran, furan, thiophene, 1H and 4H-
pyrazole, 1H and 2H-pyrrole, oxazole, thiazole,
furazan, 1H and 4H-imidazole, isoxazole,
CA 02688206 2009-11-24
6 -
isothiazole, oxadiazole, triazole, tetrazole,
thiadiazole.
Bicyclic heteroaryl groups may for instance be
phthalidyl, thiophthalidyl, indolyl, isoindolyl,
dihydroindolyl, dihydroisoindolyl, indazolyl,
benzothiazolyl, indolonyl, dihydroindolonyl,
isoindolonyl, dihydroisoindolonyl, benzofuranyl,
benzimidazolyl, dihydroisochinolinyl, dihy-
drochinolinyl, benzoxazinonyl, phthalazinonyl,
dihydrophthalazinonyl chinolinyl, isochinolinyl,
chinolonyl, isochinolonl, chinazolinyl,
chinoxalinyl, cinnolinyl, phthalazinyl,
dihydrophthalazinyl, 1,7 or 1,8-
naphthyridinylcumarinyl, cumarinyl, isocumarinyl,
indolizinyl, isobenzofuranyl, azaindolyl,
azaisoindolyl, furanopyridyl, furanopyrimidinyl,
furanopyrazinyl, furanopyidazinyl,
dihydrobenzofuranyl, dihydrofuranopyridyl,
dihydrofuranopyrimidinyl, dihydrofuranopyrazinyl,
dihydrofuranopyridazinyl, dihydrobenzofuranyl,
chromenyl, isochromenyl, chromenonyl or the
isochromenonyl group.
A(C1-Ca) alkylheteroaryl group is a heteroaryl
group, such as described above, which is linked by
a straight-chained or branched (C1-C$) alkyl unit
with the ring system.
A(C2-C$) alkenylheteroaryl group is a hetero-
aryl group, such as described above, which is
linked by a straight-chained or branched (C2-C8)
alkenyl unit with the ring system.
A(C2-Ca) alkinylheteroaryl group is a hetero-
aryl group, such as described above, which is
linked by a straight-chained or branched (C2-Ca)
alkinyl unit with the ring system.
CA 02688206 2009-11-24
7 -
A(C1-C8) alkylheterocyclyl group is a hetero-
cyclyl group, such as described above, which is
linked by a straight-chained or branched (C1-Ca)
alkyl unit with the ring system.
A(C2-C$) alkenylheterocyclyl group is a het-
erocyclyl group, such as described above, which is
linked by a straight-chained or branched (Cz-Ca)
alkenyl unit with the ring system.
Examples for compounds according to the inven-
tion are:
5-[5-fluoro-2-oxo-1,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[5-bromo-2-oxo-1,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[5-chloro-2-oxo-l,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[6-fluoro-2-oxo-1,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[6-chloro-2-oxo-1,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[6-bromo-2-oxo-1,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[5-methyl-2-oxo-1,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[5-methoxy-2-oxo-1,2-dihydro-indole-(3Z)-ylid-
enemethyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic
acid (2-diethylamino-ethoxy)-amide,
CA 02688206 2009-11-24
- 8 -
5-[6-methyl-2-oxo-l,2-dihydro-indole-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide,
5-[6-methoxy-2-oxo-l,2-dihydro-indole-(3Z)-ylid-
enemethyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic
acid (2-diethylamino-ethoxy)-amide,
or derivatives of the above compounds, wherein the
2-diethylamino-ethoxy group is replaced by one of
the groups selected from "2-(triazole-l-yl) eth-
oxy, 2-triazole-l-yl) methoxy, (pyridine-4-yl)
methoxy, 2-(pyridiny-4-yl) ethoxy, 2-(morpholine-
4-yl) ethoxy, (morpholine-4-yl) methoxy, (1-
methyl-4-piperidyl) methoxy, 2-(1-methyl-4-
piperidyl) ethoxy, 2-(pyrrolidine-l-yl) ethoxy,
(pyrrolidine-l-yl) methoxy, 2-(dimethylamino) eth-
oxy, (dimethylamino) methoxy and (dimethylamino)
methoxy".
In all above specific compounds, X and Z are a
binding, and Y is -0-. In all above specific com-
pounds, Y may also be -S-. Further, in all above
specific compounds, in addition to Y, X and/or Z
may also be -0-, instead of a binding. Further, in
all above specific compounds, -Y- may be a bind-
ing, if X and/or Z is -0- or -S-.
For obtaining pharmaceutical compositions,
compounds according to the invention may be com-
bined with other per se known active substances,
for instance: aldesleukin, amifostine, atrasentan,
bevacizumab, bexaroten, bortezomib, capecitabine,
carboplatin, chlorambucil, cisplatin, cladribine,
cyclophosphamid, cytamid, dacarbazin, docetaxel,
droloxifene, edrecolomab, epothilone, erlotinib,
etoposide, exemestane, flavopiridol, fludarabine,
fuorouracil, formestane, fulvestrant, gefitinib,
gemcitabine, idarubicin, irinotecan, ixabepilone,
CA 02688206 2009-11-24
9 -
lonafarnib, miltefosine, mitomycin, neovastat, ox-
aliplatin, pemetrexed, porfimer, rituximab, tega-
fur, temozolomide, tipifarnib, topotecan, tri-
metrexate, vorozole, vinblastine, and mixtures of
two or more of such active substances. For the
purpose of a single galenic preparation, the com-
pound according to the invention may be mixed with
the active substance. It is however also possible
that the pharmaceutical composition consists of
two (or more) different galenic preparations,
wherein in a first preparation the compound ac-
cording to the invention and in a second prepara-
tion the active substance are contained. In the
first preparation, an active substance being dif-
ferent from the active substance of the second
preparation may be provided.
The invention further relates to a method for
producing compounds according to the invention,
wherein a substance of Formula II
H n
R. }~2:z__O
N~~'R5
f
Formula II
is reacted with a substance of Formula III
Z_R7
~
Y (CH2)n
f.,i 2N F o r m u l a I I I
CA 02688206 2009-11-24
- 10 -
to a substance of Formula IV
R7
1%
z
(CH 2)~
y
HN
R 6
N
Q~ H Formula IV
wherein then the substance of Formula IV is react-
ed with a substance of Formula V
R1
2~ 0
N
R
3 H
R4
Formula V
to a compound of Formula VI
/ Z,R r
0 P-1 2) n
Rb A `f
NI-I
~
, t, c 5
1
N
4
CA 02688206 2009-11-24
- 11 -
Formula VI
wherein the radicals Rl to R7 and the groups Y and
Z have the above meanings, and wherein preferably
at least Y is -0- or -S-.
Substances of Formula II may for instance be
synthesized by a reaction of compounds of Formulas
VII and VIII or Formulas IX and X with each other
and by a subsequent reaction to the substance of
Formula II, wherein at least the respective reac-
tive group Y or Z is -0- or -S-.
H3C Y --H
~- rv
~
H3C Formula VII
(CH2)n p7 Formula VIII
r
ti..
J
H3C Y --(CH2)n
J
H4X
Formula IX
H Rr'' Formula X
Exemplary suitable reaction conditions for the
above reactions can be taken from the examples of
execution.
CA 02688206 2009-11-24
- 12 -
Compounds according to the invention may be
produced for instance according to the following
synthesis scheme:
OH
+'1 FhN O/~ N~~; tiN,i
tJ' --_- r ~ ; H
N~\.~
C H CG1376.4 N CC.1376.7
H C.4}}MN303
C.yHyP4C13 Mol. J4t:; 281,35
Mot ft.: 167.16
u msix~ i:illy atiii>3ahle ! F
,F }W H
C_
!dbi. M. 73:019 cornnxxciatl} available
etxrn;;iMcli4iy [ivi:iitible % CqHr6FPdp
N aid. lht: 151,14 0
H.
N
YOI 0 j't-,;
N
H
Ni' ... N CG1376.3 F
~0 t~H~xFN4f1
p{r:; PM. VLi: 414,47
{::,il c{;c;`d C)}{2D},}20 {;C-13'76.0
Mkl. I.W. 172.10 Atlp;. UVt.: 172.27
corrnr i.ire31 avaiiniiie
Other compounds of the invention may be pro-
duced in an analogous manner by using educts, in
which the radicals Ri to R7 are modified according
to the definition.
The invention further teaches a pharmaceutical
composition containing a compound according to the
invention. Optionally, one or more physiologically
well tolerated auxiliary substances and/or carrier
substances can be mixed with the compound, and the
mixture is galenically prepared for the local or
systemic administration, in particular oral, par-
enteral, for the infusion or infundation in a tar-
get organ, for the injection (e.g. i.v., i.m., in-
tracapsulary or intralumbal), for the application
in tooth pockets (space between root of the tooth
CA 02688206 2009-11-24
- 13 -
and the gum) and/or for inhalation. The selection
of the additional and/or auxiliary substances will
depend on the selected type of administration. The
galenic preparation of the pharmaceutical
composition according to the invention may be
performed in a conventional manner. As counter
ions for ionic compounds may for instance be used
Ca++, CaCl+, Na+, K+, Li+ or cyclohexylammonium or
C1-, Br-, acetate, trifluoroacetate, propionate,
lactate, oxalate, malate, maleate, malonate,
maleinate, citrate, benzoate, salicylate etc.
Suitable solid or liquid galenic forms of
preparation are instance granulates, powders, dra-
gees, tablets, (micro) capsules, suppositories,
syrups, juices, suspensions, emulsions, drops or
injectable solutions (i.v., i.p., i.m., s.c.) or
fine dispersions (aerosols), forms of preparation
for dry powder inhalation, transdermal systems,
and preparations with protracted release of active
substance, for the production of which usual means
are used, such as carrier substances, explosives,
binding, coating, swelling, sliding or lubricating
agents, tasting agents, sweeteners and solution
mediators. It is also possible to encapsulate the
active substance in preferably biologically decom-
posable nanocapsules, for instance for making a
preparation for the inhalation. As auxiliary sub-
stances are named here magnesium carbonate, tita-
nium dioxide, lactose, mannite and other sugars,
talcum, milk protein, gelatin, starch, cellulose
and derivatives, animal and vegetable oils such as
cod-liver oil, sunflower, peanut or sesame oil,
polyethylene glycols and solvents, such as sterile
water and mono or multi-valent alcohols, for in-
stance glycerin. A pharmaceutical composition ac-
cording to the invention can be produced by that
at least one combination of substances used ac-
cording to the invention is mixed in a defined
CA 02688206 2009-11-24
- 14 -
dose with a pharmaceutically suitable and physio-
logically well tolerated carrier and possibly fur-
ther suitable active, additional or auxiliary sub-
stances in a defined dose, and is prepared in the
desired form of administration.
As dilution agents, polyglycols, ethanol, wa-
ter and buffer solutions can be used. Suitable
buffer solutions are for instance N,N'-diben-
zylethylenediamine, diethanolamine, ethylenedia-
mine, N-methylglucamine, N-benzylphenethylamine,
diethylamine, phosphate, sodium bicarbonate, or
sodium carbonate. It is however also possible not
to use any dilution agent at all.
Physiologically tolerated salts are salts with
inorganic or organic acids, such as hydrochloric
acid, sulfuric acid, acetic acid, citric acid, p-
toluolsulfonic acid, and in particular malic acid,
or with inorganic or organic bases, such as NaOH,
KOH, Mg(OH)2, diethanolamine, ethylenediamine, or
with amino acids, such as arginine, lysine,
glutamine acid etc., or with inorganic salts, such
as CaC12, NaCl or the free ions thereof, such as
Ca2+, Na+, Cl , S042 or combinations thereof. They
are also produced by using standard methods. In
addition, reference is made to suitable counter
ions, as mentioned in the context with the galenic
preparation.
The invention is based on the finding that by
the introduction of at least one -0- for one of
the groups X, Y, or Z, an improved efficiency is
obtained, since compounds with -N-O- groups on one
hand competitively bind natural ligands, and on
the other hand cannot be metabolized. The inhibi-
tory effect is thus substantially increased.
CA 02688206 2009-11-24
- 15 -
The invention therefore further teaches the
use of a compound according to the invention for
producing a pharmaceutical composition for treat-
ing one or several diseases from the group con-
sisting of "cancer, such as lung cancer, leukemia,
ovary cancer, sarcoma, meningioma, intestine can-
cer, lymph node cancer, brain tumors, breast can-
cer, pancreas cancer, prostate cancer, skin can-
cer, chronic inflammations, asthma, allergy, rhi-
nitis, uveitis, urticaria, arthritis, osteoarthri-
tis, chronic polyarthritis, rheumatoid arthritis,
inflammatory bowl disease, degenerative joint dis-
eases, diseases of the rheumatic form circle with
cartilage breakdown, sepsis, autoimmune diseases,
type I diabetes, Hashimoto thyreoiditis, autoim-
munethrombozytopenia, multiple sclerosis, myasthe-
nia gravis, chronic inflammatory intestinal dis-
eases, morbus Crohn, oveitis, psoriasis, atypical
dermatitis, collagenoses, Goodpasture syndroma,
diseases with disturbed leukocyte adhesion,
cachexia, diseases by increased TNF-alpha concen-
tration, diabetes, adipositas, bacterial infec-
tions, in particular with resistant bacteria,
heart insufficiency and the chronic cardiac fail-
ure (CCF)". The term treatment also comprises the
prophylaxis.
For the purpose of the invention, various fur-
ther embodiments are possible. For instance, a
pharmaceutical composition according to the inven-
tion may comprise several different compounds fal-
ling under the above Formula I. Further, a pharma-
ceutical composition according to the invention
may in addition comprise an active substance dif-
ferent from the compound of Formula I. Then it is
a combination preparation. The different used ac-
tive substances may be prepared in a single unit
of administration, i.e. the active substances are
CA 02688206 2009-11-24
- 16 -
mixed in the unit of administration. It is however
also possible to prepare the different active sub-
stances in separate units of preparation of the
same or different kind.
The invention also relates to a method for
producing a pharmaceutical composition, wherein at
least one compound according to the invention is
mixed with a pharmaceutically suitable and physio-
logi.cally well-tolerated carrier substance and, if
applicable, further suitable active, additional or
auxiliary substances, and brought into a suitable
form of administration.
Preferably, the pharmaceutical composition is
produced and administered in dosage units, wherein
every unit contains as an active component a de-
fined dose of the compound according to the inven-
tion according to Formula I. For solid dosage
units such as tablets, capsules, dragees or sup-
positories, this dose may be 0.1 to 1,000 mg,
preferably 1 to 300 mg, and for injection solu-
tions in vial form 0.01 to 1,000 mg, preferably 1
to 100 mg.
For treating an adult of 50 to 100 kg, for in-
stance 70 kg, patients are injected for instance
daily doses of 0.1 to 1,000 mg active substance,
preferably 1 to 500 mg. Under certain circum-
stances, however, higher or lower daily doses may
also be suitable. The administration of the daily
dose may be performed once a day in the form of a
single dosage unit or in several smaller dosage
units as well as by multiple administration of
dosage portions in certain intervals.
However, a combination of one or more of the
active substances according to the invention with
CA 02688206 2009-11-24
- 17 -
aminooxyacetate (AOA, NH2 O-CH2-COOH or salts or
esters thereof, for instance C1-C10 alkyl or hy-
droxyalkylester) is also possible. AOA is particu-
larly effective against small tumors (< 0.1 to 1
cm3) or inhibit the generation thereof, in par-
ticular the spreading of metastases, whereas com-
pounds according to the invention are particularly
effective against the large tumors. The reason for
this is the different metabolism in small and
large tumors. The above explanations for combina-
tions apply in an analogous manner.
In the following, synthesis examples for com-
pounds according to the invention are described.
1.1: Synthesis of acetone (3-diethylaminoethyl
oxime (CC-1376.3).
Reaction:
;............__...__.__. _._.___.._ _._.__..,_._._
\-N
I-1C:1 \1
\
OH
(.61115C'12N GafiNO C4W420 L 172,10 Md. Vlk.: 73,09 CC-1376.4:
Mol. Vtit.: 172,27
Batch CC-1376.3-1:
Sodium (EH-122.5-5, 18.4 g, 0.8 mole) is dis-
solved in 600 ml dry ethanol under an argon atmos-
phere. After addition of acetoxime (29.2 g, 0.4
mole) and diethylaminoethylchloride hydrochloride
(EH-873.3-2, 68.8 g, 0.4 mole) it is heated to re-
flux. After 2 h stirring the heating bath is re-
moved and stirring is performed over night at am-
bient temperature. After filtration, acidification
with 2N aqueous HCl (pH 5), and condensation in
CA 02688206 2009-11-24
- 18 -
vacuum is performed. The residue is received in
10% NaOH, extracted twice with 250 ml diethyl
ether each, and the organic phases are condensed
in vacuum. After vacuum distillation (56 - 68 C,
approx. 6 mbar), CC 1376.3-1 (43.94 g, 64 %) is
obtained.
1.2: Synthesis of (3-diethylaminoethoxyamine (CC-
1376.4)
Reaction:
... ........
N N
~ - - ->
C~0F-12CN7O Cr;III [;N<<)
F<,4o1. ti1R.: 172,27 1\1ol. Wt.: 132,20
Batch CC-1376.4-1:
Acetone B-diethylaminoethyl oxime (CC-1376.3-
1, 43.94 g, 255 mmole) is reacted in 3N aqueous
HC1 (450 ml) and brought to reflux. After 3h stir-
ring at this temperature, it is cooled down and
condensed in vacuum. The residue is alkalized with
a 10 % and 50% sodium hydroxide solution. The
aqueous phase is extracted twice with 250 ml di-
ethyl ether each, dried over sodium sulfate and
condensed in vacuum. After vacuum distillation (62
- 66 C, approx. 6 mbar) CC-1376.4-1 (27.2g, 81
slightly contaminated, is obtained, which is
used without further purification in the next
step.
CA 02688206 2009-11-24
- 19 -
1.3: Synthesis of 5-formyl-2,4-dimethyl-lH-pyr-
role-3-carboxylic acid (2-diethylaminoeth-
oxy) amide (CC-1376.7).
Reaction:
J0
~-~ 0
;j- N _ N O ~
- H
C H { CC=1376.4 AJ N(C-13763
H C; 1~r.atdDa
Mos. Wt.: 281.35
tOoi O,t.: 16'1.16
Batch CC-1376.7-1:
Hydroxybenzotriazole (EH-I100.2-2, 300 mg,
2.22 mmole), EDCI (430 mg, 2.24 mmole) and 3,5-di-
methyl-2-formylpyrrole-4 carbonic acid (EH-
1195.10-1, 250 mg, 1.5 mmole) are provided under
argon atmosphere at ambient temperature in 10 ml
dry DMF and reacted with triethylamine (EH-18.3-
13, 0.42 ml, 3 mmole). Then CC-1376.4-1 (400 mg, 3
mmole) is quickly added in drops and stirred over
night at ambient temperature. The reaction solu-
tion is diluted with 5 ml water, 3 ml NaCl and 5
ml NaHCO3 solution, extracted twice with 10 ml
DCM/MeOH 9:1 each, dried over sodium sulfate and
condensed in vacuum. The residue is co-evaporated
with toluene, reacted with 10 ml hexane/diethyl
ether 3:1, and the supernatant solution is de-
canted off. The residue is again co-evaporated
with toluene, and after cleaning over flash silica
gel (DCM/MeOH 1:1) and drying in high vacuum, CC-
1376.7-1 as a brown solid material (114 mg, 28 %)
is obtained.
CA 02688206 2009-11-24
- 20 -
1.4: Synthesis of 5-[5-fluoro-2-oxo-l,2-dihydro-
indole-(3Z)-ylidenemethyl]-2,4-dimethyl-lH-
pyrrole-3-carboxylic acid (2-diethylamino-
ethoxy)-amide (CC-1376.0).
Reaction:
-..-_
' tx 1376.7 O 7
~'.N' b
H
N' N 0 H C-, 41 H
b1rr. W.: 281,35
--=--r~_...~ _~ ~~~`,-'`\
n Gy ti = FN iC3
F ~~ ~N Moi. ~fld.: 414.4?
r~ H
=C CC-1378.0
H
C9Ft~f NCt
tr'Icl.1M,.: 151.14 Batch CC-1376.0-3:
5-fluoroxindole (0.33 g, 2.18 mmole) is dis-
solved in 12 ml EtOH. Then, 5-formyl-2,4-dimethyl-
1H-pyrrole-3-carbonic-acid-(2-diethylaminoethoxy)-
amide (CC-1376.7-2, 620 mg, 2.20 mmole) and pyr-
rolidine (18 p1, 0.22 mmole) are added. After ad-
dition, heating for 3 h to 78 C is performed, and
an orange solid material is deposited. The reac-
tion mixture is cooled down, and the precipitate
is sucked of through a frit, re-washed with etha-
nol and again stirred with ethanol at 78 C. An-
other filtration of the precipitate and drying in
high-vacuum results in CC-1376.0-3 as a yellow
solid material (0.21 g, 23 %).
CA 02688206 2009-11-24
- 21 -
2: Structures and analytical data.
2.1: 5-[5-fluoro-2-oxo-l,2-dihydro-indole-(3Z)-
ylidenemethyl]-2,4-dimethyl-lH-pyrrole-3-
carboxylic acid (2-diethylamino-ethoxy)-
amide.
/-"CHs
N
0 \---CH3
H3C\ hiH
N' 'C H3
F H
T>o
Nf
H
Mol.Wt.: 414.47 g/mol.
Melting point: 216.0 C (Electrothermal IA
9200, heating rate 1 C/min).
1H-NMR (measured with Bruker Avance 400 (400
MHz, dmso-d6, TMS as internal standard): S(ppm) =
0.96 (t, 6H, J = 7.1 Hz, Me), 2.43 (s, 3H, Me),
2.50 (m, 4H, NCH2CH3), 2.68 (t, 2H, J = 6.1 Hz,
NCH2CH2), 3.92 (t, 2H, J = 6.1 Hz, NCH2CH2), 6.83
- 6. 86 (m, 1H, Hindole) r 6.91 - 6.96 (m, 1H, Hin-
dole) r 7.71 (s, 1H, C=CH), 7.75 - 7.79 (m, 1H, Hin-
dole)r 10.92 (bs, 1H, NH), 13,71 (bs, 1H, NH).
13C-NMR (measured with Bruker Avance 400
(100, 6 MHz, dmso-d6, TMS as internal standard) :
b(ppm) = 10.43 (s, 2C, NCH2CH3), 13.21 (s, 1C,
Me), 46.75 (s, 2C, NCH2CH3), 50.4-3 (s, iC,
NCH2CH3) , 73. 69 (s, 1C, NCH2CH3) , 105. 97 (d, 1C, J
CA 02688206 2009-11-24
- 22 -
= 25.5 Hz, CH-indole) i 109. 96 (d, 1C, J= 8. 6 Hz,
CH-indole) , 112.45 (d, 1C, J= 24.1 Hz, CH-indole) i
114.94 (d, 1C, J = 3.8 Hz), 117.51 (s, 1C), 124.72
(s, 1C), 125.85 (s, 1C, C=CH), 126.96 (d, 1C),
130.42 (s, 10), 134.50 (d, 10, J = 1.3 Hz), 136.52
(s, 1C), 156.97 (s, 1C), 159.30 (s, 1C), 169.47
(d, 1C, J = 0.86 Hz).
2.2: N-[2-(diethylamino) ethoxy]-5-[(Z)-(5-
fluoro-1,2-dihydro-2-oxo-3H-indole-3-
ylidene) methyl]-2,4-dimethyl-lH-pyrrole-3-
carboxamide, (2S)-hydroxybutanedionate (1:
0.5) .
>-C H3
/-N
1y
"`
~ C H 3
N H
1~ t
IN C Fi:3
F
H
0.5 malate
N
H
Mol.Wt.: 481,52 g/mol.
Melting point: 203 C, decomposition (Electro-
thermal IA 9200, heating rate 1 C/min).
1H-NMR (measured with Bruker Avance 400 (400
MHz, dmso-d6, TMS as internal standard): b(ppm) =
1.08 (t, 6H, J = 7.0 Hz, Me), 2.33 (dd, 0.5H, J=
4.0 Hz, J = 15. 6 Hz, CH2malic) , 2.41 (B, 3H, Me),
2. 44 (s, 3H, Me), 2.50 (m, 0. 5H, CH2malic) ~ 2- 84
(bs, 4H, NCH2CH3), 2.97 (bs, 2H, NCH2CH2), 3.90
CA 02688206 2009-11-24
- 23 -
(dd, 0.5H, J= 3. 9 Hz, J 10.0 Hz CHmalic) , 4.05
(m, 2H, J = 6.1 Hz, NC2CHZ) , 6.85 (dd, 1H, J = 4. 6
Hz, J = 8.4 Hz, Hindole) i 6.89 - 6. 98 (m, 1H,
Hindole) 7= 73 (s, 1H, C=CH) , 7.78 (dd, 1H, J = 2. 4
Hz, J = 9. 4 Hz, Hindole) , 10. 94 (bs, 1H, NH), 13. 76
(bs, 1H, NH).
2.3: N-[2-(diethylamino) ethoxy]-5-[(Z)-(5-flu-
oro-1,2- dihydro-2-oxo-3H-indole-3-ylidene)
methyl]-2,4-dimethyl-lH-pyrrole-3-carbox-
amide, (2S)-hydroxybutanedionate (1:1).
f""`C I-13
Q 0 ~-`"CH3
~ /
H3Cr. 1JH
{y CH3
F =~~~ ~y H
Ci
N
H 1.0 malate
Mol.Wt.: 548.56 g/mol.
1H-NMR (measured with Bruker Avance 400 (400
MHz, dmso-d6r TMS as internal standard): b(ppm) =
1.12 (t, 6H, J = 7.1 Hz, Me), 2.34 (dd, 1H, J =
4. 0 Hz, J= 15. 6 Hz, CH2malic) r 2. 42 (S, 3H, M e ) ,
2. 45 (s, 3H, M e ) , 2. 50 (m, 1H, CH2malic) i 2. 95 (bs,
4H, NCH2CH3), 3.08 (bs, 2H, NCH2CH2)1 3.95 (dd,
1H, J = 4. 6 Hz, J = 9.1 Hz CHmalic) r 4.09 (m, 2H,
NCH2CH2), 6.85 (dd, 1H, J = 4.6 Hz, J = 8.5 Hz,
Hindole) , 6.89 - 6. 98 (m, 1H, Hindole) r 7.73 (s, 1H,
C=CH) , 7. 78 (dd, 1H, J = 2. 5 Hz, J 9.4 Hz, Hin-
dole) i 10. 94 (bs, 1H, NH), 13.76 (bs, 1H, NH) .
CA 02688206 2009-11-24
- 24 -
13C-NMR (measured with Bruker Avance 400
(100.6 MHz, dmso-d6, TMS as internal standard):
b(ppm) = 9.92 (s, 2C, NCH2CH3), 10.47 (s, 1C, Me),
13.29 (s, 1C, Me), 35.40 (s, 1C, NCH2CH2), 41.05
(s, 1C), 46.62 (s, 2C, NCH2CH3), 49.87 (s, 1C,
NCHZCH2) , 65. 94 (s, 1C, CHmalic) , 71.58 (d, 1C,
NCH2CH2), 106.06 (d, 1C, J = 26.0 Hz, CH-indole) i
110.03 (d, 1C, J = 8.7 Hz, CH-indole) r 112.60 (d,
1C, J = 23. 9 Hz, CH-indole) - 115.30 (d, 1C, J= 3.1
Hz) , 112. 60 .(d, 1C, J = 23. 9 Hz, CH-indole) , 115.30
(d, 1C, J 3.1 Hz), 116.71 (s, 1C), 124.67 (s,
1C, C=CH), 125.94 (s, 1C), 126.90 (d, 1C, J = 9.5
Hz), 130.34 (s, 1C), 134.57 (d, 1C, J = 1.4 Hz),
136.76 (s, 1C), 158.15 (d, 1C, J = 234.3 Hz),
163.75 (s, 1C), 169.47 (d, 1C, J= 0.9 Hz), 171.82
(s, 1C, C0), 175.97 (s, 1C, C0).
3: Biological data.
3.1: Cell culture results.
The substances of Examples 2.1 to 2.3 were
tested for different cell lines in presence and
absence of pyruvate in the nutrient medium for
their proliferation inhibiting effect. The sub-
stances were each dissolved in DMSO. Then, the ac-
tive substance was tested in eight concentration
intervals. The calculation of the IC50 values was
based in each concentration on eight individual
values. The IC50 value is the substance concentra-
tion, at which compared to the control group,
which has been treated exclusively with the sol-
vent DMSO, there was a 50 % inhibition of the cell
proliferation.
The IC50 values for SO 272 were between 1 and
11 pM. The obtained values are shown in Table 1.
CA 02688206 2009-11-24
- 25 -
Table 1
Cell line Substance IC50 (XTT) IC50 (XTT)
(XXT) (from example) with pyruvate without
pyruvate
MCF-7 2.1 11 8
2.2 8 11
2.3 9 10
HT-29 2.1 3.5
2.2 3
2.3 3
BxPC-3 2.1 1.3 2
2.2 2 2
2.3 2 2
MDA-MB-453 2.1 11 7
2.2 8 7
2.2 8 7
NK1 2.1 2
2.2 4
2.3 4
Wi-38 2.1 11
KBV-600 2.1 5
MCF-7: human breast cancer cell line; MDA-MB-
453: human breast cancer cell line; HT29: human
colon carcinoma cell line; BxPC-3: human pancreas
tumor cell line; KBV600: multidrug-resistant de-
scendant of HeLa cells (KB-V1), cultivated with
600 ng/ml Vinblastine; Wi-38: human, fetal cell
line similar to fibroblasts of the lung; NK: No-
vikoff rat hepatoma cell line.
The substances inhibit several receptors of
thyrosinkinases, which are important for the
CA 02688206 2009-11-24
- 26 -
growth and angiogenesis of the tumors. The multi-
thyrosinkinase inhibitor is preferably also suit-
able e.g. for the second therapy of metastasized
renal cell carcinomas (RCC) or metastasized gas-
trointestinal stroma tumors (GIST). In all, the
results show that by means of the substances ac-
cording to the invention not only specific tumor
types can be treated, but that very different tu-
mor types can be subjected to a therapy.
3.2: Colony assays.
In a colony assay, tumor stem cells of differ-
ent human tumor entities were cultivated in soft
agar, and the generation of tumor colonies in
presence and in absence of the substance according
to the invention (Example 2.1, if applicable as a
malate of Example 2.2 or 2.3) was counted. Alto-
gether 25 different tumor models were tested.
Thereto belong colon, pancreas and stomach carci-
nomas, small-cell and non-small-cell lung tumors,
breast, ovary, and kidney carcinomas and melano-
mas. The wide range of different tumor entities
and the large number of models permit after the
cell culture another test for the efficiency of
the substance and give first hints, whether the
substance has a selective effect on certain tumor
entities.
The results are shown in Figure 1 (graphic
analysis of the mean values for the substance
based on the IC70 values). The bars represent the
IC70 concentrations referred to the mean values of
all IC70 data. For bars towards left, the IC70
value is lower than the mean value of all IC70
models, i.e. these models are more sensitive com-
pared to the average of all models. Bars towards
CA 02688206 2009-11-24
- 27 -
right mean higher IC70 values than the average of
the models and thus a lower sensitivity.
Tumor models: CXF = colon carcinomas, GXF =
stomach carcinomas, LXFA = non-small-cell adeno-
carcinomas of the lung, LXFE = squamous cell car-
cinomas of the lung, FXFL = large-cell lung carci-
nomas, LXFS = small-cell lung carcinomas, MAXF =
breast tumors, MEXF = melanomas, OVXF = ovary car-
cinomas, PAXF = pancreas carc.inomas, and RXF =
kidney carcinomas.
It can be seen that the substance in the col-
ony assay was tested with very good results. The
substance led for all 25 models to a dose-depend-
ent inhibition of the colony formation. The mean
IC50 value was 15 M. The mean IC70 value was 20
M. The highest sensitivity had a non-small-cell
lung tumor model with an IC50 value of 4 M and an
IC70 value of 6 M. The highest IC50 value (30 M)
or IC70 value (40 M), respectively, was detected
for a breast tumor model.
These results, too, confirm again that the
substance has a very good spectrum of effects.
There was not a single model, which was not inhib-
ited. The cell culture results of Example 3.1
showed for the six tested tumor models a mean IC50
value of 6 M and comprised values between 1 and
11 M, so that the results in the colony assay
were in the range of results of the cell culture
tests of Example 3.1 and therefore confirmed these
results.
In general, all colony assays showed that the
substance is advantageous over the prior art,
since it has a broad therapeutic potential. The
broad spectrum of effects reflects that the sub-
CA 02688206 2009-11-24
- 28 -
stance influences metabolic processes, which are
common to all tumors, irrespective of the entity.
3.3: Comparison tests.
In a colony assay according to Example 3.2,
for an ovary carcinoma (OVXF 550) the following
values were measured for SU11248, 5-[5-Fluoro-2-
oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-di-
methyl-lH-pyrrole-3-carboxylic acid (2-diethylami-
noethyl) amide, known from literature (PCT WO 01/
60814 A2 and J. Med. Chem. 2003, 46, 1116-1119):
OVXF 550: IC50 = 18.0 M, IC70 = 26.7 M
In the same colony assay and under identical
experimental conditions, the following values were
found for the substance according to the invention
(5-[5-fluoro-2-oxo-l,2-dihydro-indol-(3Z)-ylidene-
methyl]-2,4-dimethyl-lH-pyrrole-3-carboxylic acid
(2-diethylamino-ethoxy)-amide (preferable the salt
of the malic acid, malate):
OVXF 550: IC50 = 16.7 M, IC70 = 21.6 M
It can be seen that the values for the sub-
stance according to the invention are better than
those for the prior art substance.
It has an essential importance that these ad-
vantages are caused by the (single) structural
difference, which is the -NH-O- partial structure
according to the invention. In addition, for sub-
stances according to the invention less side ef-
fects can be expected due to this partial struc-
ture.