Note: Descriptions are shown in the official language in which they were submitted.
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
2,5-DISUBSTITUTED IMIDAZO[4,5-C]PYRIDINES FOR TREATING MUSCULAR DYSTROPHY
[0001] This application claims priority to U.K. Patent Application No.
GB0809314.0,
filed May 22, 2008, the content of which is incorporated by reference herein
by its entirety.
FIELD
[0002] Provided herein are compounds for the treatment of muscular dystrophy
and
related conditions, including Duchenne muscular dystrophy, compositions
comprising the
compounds, and methods of use thereof. Also provided herein is a method for
the treatment
of muscular dystrophy and related conditions, including Duchenne muscular
dystrophy.
BACKGROUND
[0003] Duchenne muscular dystrophy (DMD) is a common, genetic neuromuscular
disease associated with the progressive deterioration of muscle function,
first described over
150 years ago by the French neurologist, Duchenne de Boulogne, after whom the
disease is
named. DMD has been characterized as an X-linked recessive disorder that
affects 1 in 3,500
males caused by mutations in the dystrophin gene. The gene is the largest in
the human
genome, encompassing 2.6 million base pairs of DNA and containing 79 exons.
Approximately 60% of dystrophin mutations are large insertion or deletions
that lead to
frame-shift errors downstream, whereas approximately 40% are point mutations
or small
frame-shift rearrangements. The vast majority of DMD patients lack the
dystrophin protein.
Becker muscular dystrophy is a much milder form of DMD caused by reduction in
the
amount, or alteration in the size, of the dystrophin protein. The high
incidence of DMD (1 in
10,000 sperm or eggs) means that genetic screening will never eliminate the
disease, so an
effective therapy is highly desirable.
[0004] A number of natural and engineered animal models of DMD exist, and
provide a
mainstay for preclinical studies (Allamand et al., Animal models for muscular
dystrophy:
valuable tools for the development of therapies, Hum. Mol. Genet. 9, 2459-2467
(2000).)
Although the mouse, cat and dog models all have mutations in the DMD gene and
exhibit a
biochenlical dystrophinopathy similar to that seen in humans, they show
surprising and
considerable variation in terms of their phenotype. Like humans, the canine
(golden retriever
muscular dystrophy and German short-haired pointer) models have a severe
phenotype; these
dogs typically die of cardiac failure. Dogs offer the best phenocopy for human
disease, and
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
are considered a high benchmark for preclinical studies. Unfortunately,
breeding these
animals is expensive and difficult, and the clinical time course can be
variable among litters.
[0005] The mdx mouse is the most widely used model due to availability, short
gestation
time, time to mature and relatively low cost (Bulfield et al., X chromosome-
linked muscular
dystrophy (mdx) in the mouse, Proc. Natl Acad. Sci. USA 81, 1189-1192
(1984).).
[0006] Since the discovery of the DMD gene about 20 years ago, varying degrees
of
success in the treatment of DMD have been achieved in preclinical animal
studies, some of
which are being followed up in humans. Present therapeutic strategies can be
broadly
divided into three groups: first, gene therapy approaches; second, cell
therapy; and last,
pharmacological therapy. Gene- and cell-based therapies offer the fundamental
advantage of
obviating the need to separately correct secondary defects/pathology (for
example,
contractures), especially if initiated early in the course of the disease.
Unfortunately, these
approaches face a number of technical hurdles. Immunological responses against
viral
vectors, myoblasts and newly synthesized dystrophin have been reported, in
addition to
toxicity, lack of stable expression and difficulty in delivery.
[0007] Pharmacological approaches for the treatment of muscular dystrophy
differ from
gene- and cell-based approaches in not being designed to deliver either the
missing gene
and/or protein. In general, the pharmacological strategies use drugs/molecules
in an attempt
to improve the phenotype by means such as decreasing inflammation, improving
calcium
homeostasis and increasing muscle progenitor proliferation or commitment.
These strategies
offer the advantage that they are easy to deliver systemically and can
circumvent many of the
immunological and/or toxicity issues that are related to vectors and cell-
based therapies.
Although investigations with corticosteroids and sodium cromoglycate, to
reduce
inflammation, dantrolene to maintain calcium homeostasis and clenbuterol to
increase muscle
strength, have produced promising results, none of these potential therapies
has yet been
shown to be effective in treating DMD.
[0008] An alternative pharmacological approach is upregulation therapy.
Upregulation
therapy is based on increasing the expression of alternative genes to replace
a defective gene
and is particularly beneficial when an immune response is mounted against a
previously
absent protein. Upregulation of utrophin, an autosomal paralogue of dystrophin
has been
proposed as a potential therapy for DMD (Perkins et al., Neuromuscul. Disord.,
S 1: S78-S89
(2002); Khurana et al., Nat. Rev. Drug Discov. 2:379-390 (2003).). When
utrophin is over-
expressed in transgenic mdx mice it localizes to the sarcolemma of muscle
cells and restores
-2-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
the components of the dystrophin-associated protein complex (DAPC), which
prevents the
dystrophic development and in turn leads to functional improvement of skeletal
muscle.
Adenoviral delivery of utrophin in the dog has been shown to prevent
pathology.
Commencement of increased utrophin expression shortly after birth in the mouse
model can
be effective, and no toxicity is observed when utrophin is ubiquitously
expressed, which is
promising for the translation of this therapy to humans. Upregulation of
endogenous utrophin
to sufficient levels to decrease pathology might be achieved by the delivery
of small
diffusible compounds.
[0009] In earlier applications, PCT/GB2007/050055, PCT/GB2007/050056, UK
Patent
Application No. 0617739.8, UK Patent Application No. 0619282.7, UK Patent
Application
No. 0623985.9, UK Patent Application No. 0617740.6, UK Patent Application No.
0619283.5, UK Patent Application No. 0714303.5, and UK Patent Application No.
0803906.7, we disclosed compounds which upregulate endogenous utrophin in
predictive
screens, and thus may be useful in the treatment of DMD.
SUMMARY
[0010] Provided herein are compounds for the treatment of muscular dystrophy
and
related conditions, including DMD, compositions comprising the compounds, and
methods of
use thereof. In one embodiment, provided herein are compounds that upregulate
endogenous
utrophin, and are useful in the treatment of muscular dystrophy, including
DMD.
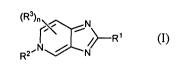
[00111 In one embodiment, provided herein is a compound of formula (I):
(R3)n i
~~--Rl
R2"N / N/
(I)
or a tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof; wherein R1, Rz, R3, and n are defined herein elsewhere, for
use in the
treatment or prophylaxis of Duchenne muscular dystrophy, Becker muscular
dystrophy, or
cachexia.
[0012] In certain embodiments, provided herein is a compound of formula (I),
or a
tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or prodrug
thereof.
-3-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[0013] Also provided herein are pharmaceutical compositions comprising a
compound of
formula (I), including an enantiomer, a mixture of enantiomer, or a mixture of
two or more
diastereomers; or a pharmaceutically acceptable salt, solvate, hydrate, or
prodrug thereof; in
combination with one or more pharmaceutically acceptable carriers or
excipients.
[0014] Also provided herein is a method for the treatment or prophylaxis of
Duchenne
muscular dystrophy, Becker muscular dystrophy, or cachexia. In one embodiment,
the
method treats, prevents, or ameliorates one or more symptoms of Duchenne
muscular
dystrophy, Becker muscular dystrophy, or cachexia. In one embodiment, the
method
comprises administering to a patient in need thereof an effective amount of a
compound of
formula (I). In another embodiment, the method comprises administering to a
patient in need
thereof an effective amount of a pharmaceutically acceptable salt, solvate,
hydrate, or
prodrug, of a compound of formula (I).
[0015] In one embodiment, the compounds of formula (I) are used to treat or
prevent
Duchenne muscular dystrophy, Becker muscular dystrophy, or cachexia. In
another
embodiment, the compounds of formula (I) are used in the treatment or
prophylaxis of
Duchenne muscular dystrophy or Becker muscular dystrophy. In another
embodiment, the
compounds of formula (I) are used in the treatment or prophylaxis of Duchenne
muscular
dystrophy.
DETAILED DESCRIPTION
A. Definitions
[0016] Unless defined otherwise, all technical and scientific terms used
herein generally
have the same meaning as commonly understood by one of ordinary skill in the
art to which
this disclosure belongs. To facilitate understanding of the disclosure set
forth herein, a
number of terms are defined below.
[0017] As used herein, and unless otherwise specified, the term "CI -C6 alkyl"
refers to an
optionally substituted straight or branched saturated hydrocarbon chain having
one to six
carbon atoms. In one embodiment, the optional substituent is halo. Examples
include, but
are not limited to, methyl, ethyl, n-propyl, isopropyl, t-butyl, n-hexyl,
trifluoromethyl, and
1,2-dichloroethyl.
[0018] As used herein, and unless otherwise specified, the terms "Cl-CS
alkyl", "Cl-C4
alkyl", and "Cl-Clo alkyl" have similar meanings except that they contain
respectively from
one to five, from one to four, and from one to ten carbon atoms.
-4-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[0019] As used herein, and unless otherwise specified, the term "C3-C7
cycloalkyl" refers
to an optionally substituted fully saturated carbocyclic ring having from 3 to
7 ring carbon
atoms. Examples include, but are not limited to, cyclopropyl, cyclobutyl,
cyclopentyl, and
cyclohexyl.
[0020] As used herein, and unless otherwise specified, the term "alkylene"
refers to a
linear or branched saturated divalent hydrocarbon radical, wherein the
alkylene may
optionally be substituted as described herein. The term "alkylene" encompasses
both linear
and branched alkylene, unless otherwise specified. In certain embodiments, the
alkylene is a
linear saturated divalent hydrocarbon radical that has 1 to 20 (Ci_20), 1 to
15 (C1_15), 1 to 10
(Cl_lo), 1 to 6(C1_6), or 1 to 5(C1_5) carbon atoms, or branched saturated
divalent
hydrocarbon radical of 3 to 20 (C3.20), 3 to 15 (C3_15), 3 to 10 (C3_jo), 3 to
6(C3_6), or 3 to 5
(C3_5) carbon atoms. As used herein, linear Ci_6 and branched C3_6 alkylene
groups are also
referred as "lower alkylene." Examples of alkylene groups include, but are not
limited to,
methylene, ethylene, propylene (including all isomeric forms), n-propylene,
isopropylene,
butylene (including all isomeric forms), n-butylene, isobutylene, t-butylene,
pentylene
(including all isomeric forms), and hexylene (including all isomeric forms).
For example,
C1_6 alkylene refers to a linear saturated divalent hydrocarbon radical of 1
to 6 carbon atoms
or a branched saturated divalent hydrocarbon radical of 3 to 6 carbon atoms
(0021] As used herein, and unless otherwise specified, the term "carbocyclic"
refers to an
optionally substituted ring system in which all the ring atoms are carbon
atoms.
[0022] As used herein, and unless otherwise specified, the term "heterocyclic"
refers to
an optionally substituted ring system in which one or more of the ring atoms
is a hetero atom
selected from N, 0 and S.
[0023] As used herein, and unless otherwise specified, in the carbocyclic and
heterocyclic
ring systems, one or more ring CH2 groups may be replaced with a C=0 to form a
cyclic
ketone. In certain embodiment, one or more ring CH2 groups may be replaced
with a C=0 to
form a cyclic amide.
[0024] As used herein, and unless otherwise specified, the term "aromatic"
refers to an
optionally substituted carbocyclic or heterocyclic ring system which has
aromatic character.
In one embodiment, the aromatic ring has one or two rings and from 5 to 10
ring atoms. In
bicyclic systems, one of the rings may have aromatic character. Examples of
aromatic ring
systems include, but are not limited to, phenyl, naphthalene, pyridine,
pyrimidine, furan,
thiophene, indole, isoindole, benzofuran, benzimidazole, benzimidazoline,
benzodioxyl,
-5-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
benzodioxane, quinoline, isoquinoline, tetrahydroisoquinoline, quinazoline,
thiazole,
benzthiazole, benzoxazole, indazole, and imidazole ring systems.
[0025] As used herein, and unless otherwise specified, the term "non-aromatic"
refers to
an optionally substituted carbocyclic or heterocyclic ring system which may be
fully or
partially saturated. In one embodiment, the non-aromatic ring is monocyclic
and has from 4
to 7 ring atoms. Examples of non-aromatic ring systems include, but are not
limited to,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, piperidine, piperazine,
morpholine,
tetrahydrofuran, and pyrrolidine.
[0026] As used herein, and unless otherwise specified, carbocyclic or
heterocyclic ring
systems are optionally substituted with one or more -X4R7, wherein:
X4 is a bond, -NR5-, -S-, -0-, -(CR5R5)q-, -(CR5R5)yO-, -O(CRSRS)q-NRSC(O)-,
-NRSC(S)-, -NRSC(O)O-, -NRSSOZ-, -NRSC(O)NRS-, -NRSC(S)NRS-,
-NRSC(NH)NR5-, -NRSC(NH)-, -C(O)-, -C(S)-, -C(O)NRS-, -C(S)NRS-, -SO-,
-SO2-, -SO2NR5-, -OC(O)NR5-, or -P(O)OR5-;
each R5 is independently H or CI-C6 alkyl optionally substituted with one or
more
halo;
qis0, 1,or2;and
R7 is H, CI-C6 alkyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein
the alkyl,
cycloalkyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted
with one
or more halo or -O(CI-C6 alkyl), and wherein the cycloalkyl, heterocyclyl,
aryl, and
heteroaryl are each optionally substituted with one or more CI -C6 alkyl; or
when X4 is a bond, R7 may also be halo, NO2, or CN.
[0027] As used herein, and unless otherwise specified, aromatic or non-
aromatic ring
systems are optionally substituted with one or more -X4R7, wherein X4 and R7
are defined
herein elsewhere.
[0028] As used herein, and unless otherwise specified, the terms "cycloalkyl"
or "non-
aromatic carbocyclic" refer to a cyclic saturated bridged and/or non-bridged
monovalent
hydrocarbon radical, which may be optionally substituted with one or more -
X4R7, wherein
X4 and R7 are defined herein elsewhere. In certain embodiments, the cycloalkyl
has from 3 to
20 (C3_20), from 3 to 15 (C3_15), from 3 to 10 (C3_10), or from 3 to 7(C3_7)
carbon atoms.
Examples of cycloalkyl groups include, but are not limited to, cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, bicyclo[2.1.1]hexyl,
bicyclo[2.2.1]heptyl, decalinyl,
and adamantyl.
-6-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[0029] As used herein, and unless otherwise specified, the terms "aryl" or
"aromatic
carbocyclic" refer to a monocyclic aromatic group and/or multicyclic
monovalent aromatic
group that contain at least one aromatic hydrocarbon ring. In certain
embodiments, the aryl
has from 6 to 20 (C6_20), from 6 to 15 (C6_15), or from 6 to 10 (C6_10) ring
atoms. Examples of
aryl groups include, but are not limited to, phenyl, naphthyl, fluorenyl,
azulenyl, anthryl,
phenanthryl, pyrenyl, biphenyl, and terphenyl. Aryl also refers to bicyclic or
tricyclic carbon
rings, where one of the rings is aromatic and the others of which may be
saturated, partially
unsaturated, or aromatic, for example, dihydronaphthyl, indenyl, indanyl, or
tetrahydronaphthyl (tetralinyl). In certain embodiments, aryl may be
optionally substituted
with one or more -X4R7, wherein X4 and R7 are defined herein elsewhere.
[0030] As used herein, and unless otherwise specified, the term "aralkyl" or
"aryl-alkyl"
refers to a monovalent alkyl group substituted with aryl. In certain
embodiments, the alkyl
and aryl moieties are optionally substituted with one or more substituents.
[0031] As used herein, and unless otherwise specified, the terms "heteroaryl"
or
"aromatic heterocyclic" refer to a monocyclic aromatic group and/or
multicyclic aromatic
group that contain at least one aromatic ring, wherein at least one aromatic
ring contains one
or more heteroatoms independently selected from 0, S, and N. Each ring of a
heteroaryl
group can contain one or two 0 atoms, one or two S atoms, and/or one to four N
atoms,
provided that the total number of heteroatoms in each ring is four or less and
each ring
contains at least one carbon atom. In certain embodiments, the heteroaryl has
from 5 to 20,
from 5 to 15, or from 5 to 10 ring atoms. Examples of monocyclic heteroaryl
groups include,
but are not limited to, furanyl, imidazolyl, isothiazolyl, isoxazolyl,
oxadiazolyl, oxazolyl,
pyrazinyl, pyrazolyl, pyridazinyl, pyridyl, pyrimidinyl, pyrrolyl,
thiadiazolyl, thiazolyl,
thienyl, tetrazolyl, triazinyl, and triazolyl. Examples of bicyclic heteroaryl
groups include,
but are not limited to, benzofuranyl, benzimidazolyl, benzoisoxazolyl,
benzopyranyl,
benzothiadiazolyl, benzothiazolyl, benzothienyl, benzothiophenyl,
benzotriazolyl,
benzoxazolyl, furopyridyl, imidazopyridinyl, imidazothiazolyl, indolizinyl,
indolyl,
indazolyl, isobenzofuranyl, isobenzothienyl, isoindoly], isoquinolinyl,
isothiazolyl,
naphthyridinyl, oxazolopyridinyl, phthalazinyl, pteridinyl, purinyl,
pyridopyridyl,
pyrrolopyridyl, quinolinyl, quinoxalinyl, quinazolinyl, thiadiazolopyrimidyl,
and
thienopyridyl. Examples of tricyclic heteroaryl groups include, but are not
limited to,
acridinyl, benzindolyl, carbazolyl, dibenzofuranyl, perimidinyl,
phenanthrolinyl,
phenanthridinyl, phenarsazinyl, phenazinyl, phenothiazinyl, phenoxazinyl, and
xanthenyl. In
-7-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
certain embodiments, heteroaryl may also be optionally substituted with one or
more -X4R7,
wherein X4 and R' are defined herein elsewhere.
[0032] As used herein, and unless otherwise specified, the terms "non-aromatic
heterocyclyl" or "non-aromatic heterocyclic" refers to a monocyclic non-
aromatic ring
system and/or multicyclic ring system that contains at least one non-aromatic
ring, wherein
one or more of the non-aromatic ring atoms are heteroatoms independently
selected from 0,
S, or N; and the remaining ring atoms are carbon atoms. In certain
embodiments, the
heterocyclyl or heterocyclic group has from 3 to 20, from 3 to 15, from 3 to
10, from 3 to 8,
from 4 to 7, or from 5 to 6 ring atoms. In certain embodiments, the
heterocyclyl is a
monocyclic, bicyclic, tricyclic, or tetracyclic ring system, which may include
a fused or
bridged ring system, and in which the nitrogen or sulfur atoms may be
optionally oxidized,
the nitrogen atoms may be optionally quatemized, and some rings may be
partially or fully
saturated, or aromatic. The heterocyclyl may be attached to the main structure
at any
heteroatom or carbon atom which results in the creation of a stable compound.
Examples of
such heterocyclic radicals include, but are not limited to, azepinyl,
benzodioxanyl,
benzodioxolyl, benzofuranonyl, benzopyranonyl, benzopyranyl,
benzotetrahydrofuranyl,
benzotetrahydrothienyl, benzothiopyranyl, benzoxazinyl, 0-carbolinyl,
chromanyl,
chromonyl, cinnolinyl, coumarinyl, decahydroisoquinolinyl,
dihydrobenzisothiazinyl,
dihydrobenzisoxazinyl, dihydrofuryl, dihydroisoindolyl, dihydropyranyl,
dihydropyrazolyl,
dihydropyrazinyl, dihydropyridinyl, dihydropyrimidinyl, dihydropyrrolyl,
dioxolanyl, 1,4-
dithianyl, furanonyl, imidazolidinyl, imidazolinyl, indolinyl,
isobenzotetrahydrofuranyl,
isobenzotetrahydrothienyl, isochromanyl, isocoumarinyl, isoindolinyl,
isothiazolidinyl,
isoxazolidinyl, morpholinyl, octahydroindolyl, octahydroisoindolyl,
oxazolidinonyl,
oxazolidinyl, oxiranyl, piperazinyl, piperidinyl, 4-piperidonyl,
pyrazolidinyl, pyrazolinyl,
pyrrolidinyl, pyrrolinyl, quinuclidinyl, tetrahydrofuryl,
tetrahydroisoquinolinyl,
tetrahydropyranyl, tetrahydrothienyl, thiamorpholinyl, thiazolidinyl,
tetrahydroquinolinyl,-
and 1,3,5-trithianyl. In certain embodiments, heterocyclic may also be
optionally substituted
with one or more -X4R7, wherein X4 and R7 are defined herein elsewhere.
[0033] As used herein, and unless otherwise specified, the term "halo" refers
to fluoro,
chloro, bromo, or iodo.
[0034] As used herein, and unless otherwise specified, appropriate
pharmaceutically and
veterinarily acceptable salts include basic addition salts such as sodium,
potassium, calcium,
-8-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
aluminum, zinc, magnesium, and other metal salts, as well as choline,
diethanolamine,
ethanolamine, ethyl diamine, and other known basic addition salts.
[0035] Where appropriate, pharmaceutically or veterinarily acceptable salts
may also
include salts of organic acids, especially carboxylic acids, including but not
limited to acetate,
trifluoroacetate, lactate, gluconate, citrate, tartrate, maleate, malate,
pantothenate, adipate,
alginate, aspartate, benzoate, butyrate, digluconate, cyclopentanate,
glucoheptanate,
glycerophosphate, oxalate, heptanoate, hexanoate, fumarate, nicotinate,
pamoate, pectinate,
3-phenylpropionate, picrate, pivalate, proprionate, tartrate, lactobionate,
pivolate,
camphorate, undecanoate and succinate, organic sulfonic acids such as
methanesulfonate,
ethanesulfonate, 2-hydroxyethane sulfonate, camphorsulfonate, 2-
naphthalenesulfonate,
benzenesulfonate, p-chlorobenzenesulfonate and p-toluenesulfonate; and
inorganic acids such
as hydrochloride, hydrobromide, hydroiodide, sulfate, bisulfate, hemisulfate,
thiocyanate,
persulfate, phosphoric and sulfonic acids.
[0036] Salts which are not pharmaceutically or veterinarily acceptable may
still be
valuable as intermediates.
[0037] As used herein, and unless otherwise specified, the term "prodrug"
refers to any
covalently bonded compounds which release the active parent drug according to
formula (I)
in vivo.
[00381 If a chiral center or another form of isomeric center is present in a
compound
provided herein, all forms of such isomer or isomers, including enantiomers
and
diastereoisomers, are intended to be within the scope of this disclosure.
Compounds
provided herein containing a chiral center may be used as a racemic mixture,
an
enantiomerically enriched mixture, or the racemic mixture may be separated
using known
techniques and an individual enantiomer may be used alone.
[0039] As used herein, and unless otherwise specified, the term "subject"
refers to an
animal, including but not limited to, a primate (e.g., human), cow, pig,
sheep, goat, horse,
dog, cat, rabbit, rat, or mouse. The terms "subject" and "patient" are used
interchangeably
herein in reference, for example, to a mammalian subject, such as a human
subject, in one
embodiment, a human.
[0040] As used herein, and unless otherwise specified, the terms "treat,"
"treating," and
"treatment" are meant to include alleviating or abrogating a disorder,
disease, or condition, or
one or more of the symptoms associated with the disorder, disease, or
condition; or
alleviating or eradicating the cause(s) of the disorder, disease, or condition
itself.
-9-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[0041] As used herein, and unless otherwise specified, the terms "prevent,"
"preventing,"
and "prevention" are meant to include a method of delaying and/or precluding
the onset of a
disorder, disease, or condition, and/or its attendant symptoms; barring a
subject from
acquiring a disorder, disease, or condition; or reducing a subject's risk of
acquiring a
disorder, disease, or condition.
[0042] As used herein, and unless otherwise specified, the term
"therapeutically effective
amount" are meant to include the amount of a compound that, when administered,
is
sufficient to prevent development of, or alleviate to some extent, one or more
of the
symptoms of the disorder, disease, or condition being treated. The term
"therapeutically
effective amount" also refers to the amount of a compound that is sufficient
to elicit the
biological or medical response of a biological molecule (e.g., a protein,
enzyme, RNA, or
DNA), cell, tissue, system, animal, or human, which is being sought by a
researcher,
veterinarian, medical doctor, or clinician. -
[0043] As used herein, and unless otherwise specified, the term
"pharmaceutically
acceptable carrier," "pharmaceutically acceptable excipient," "physiologically
acceptable
carrier," or "physiologically acceptable excipient" refers to a
pharmaceutically-acceptable
material, composition, or vehicle, such as a liquid or solid filler, diluent,
solvent, or
encapsulating material. In one embodiment, each component is "pharmaceutically
acceptable" in the sense of being compatible with the other ingredients of a
pharmaceutical
formulation, and suitable for use in contact with the tissue or organ of
humans and animals
without excessive toxicity, irritation, allergic response, immunogenicity, or
other problems or
complications, commensurate with a reasonable benefit/risk ratio. See,
Remington: The
Science and Practice of Pharmacy, 21st Edition, Lippincott Williams & Wilkins:
Philadelphia, PA, 2005; Handbook of Pharmaceutical Excipients, 5th Edition,
Rowe et al.,
Eds., The Pharmaceutical Press and the American Pharmaceutical Association:
2005; and
Handbook of Pharmaceutical Additives, 3rd Edition, Ash and Ash Eds., Gower
Publishing
Company: 2007; Pharmaceutical Preformulation and Formulation, 2nd Edition,
Gibson Ed.,
CRC Press LLC: Boca Raton, FL, 2009.
[0044] As used herein, and unless otherwise specified, the term "about" or
"approximately" means an acceptable error for a particular value as determined
by one of
ordinary skill in the art, which depends in part on how the value is measured
or determined.
In certain embodiments, the term "about" or "approximately" means within 1, 2,
3, or 4
standard deviations. In certain embodiments, the term "about" or
"approximately" means
-10-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
within 50%, 20%, 15%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.5%, or 0.05%
of a
given value or range.
[0045] As used herein, and unless otherwise specified, the terms "active
ingredient" and
"active substance" refer to a compound, which is administered, alone or in
combination with
one or more pharmaceutically acceptable excipients, to a subject for treating,
preventing, or
ameliorating one or more symptoms of a condition, disorder, or disease. As
used herein,
"active ingredient" and "active substance" may be an optically active isomer
of a compound
described herein.
[0046] As used herein, and unless otherwise specified, the terms "drug,"
"therapeutic
agent," and "chemotherapeutic agent" refer to a compound, or a pharmaceutical
composition
thereof, which is administered to a subject for treating, preventing, or
ameliorating one or
more symptoms of a condition, disorder, or disease.
[0047] In certain embodiments, "optically active" and "enantiomerically
active" refer to a
collection of molecules, which has an enantiomeric excess of no less than
about 50%, no less
than about 70%, no less than about 80%, no less than about 90%, no less than
about 91%, no
less than about 92%, no less than about 93%, no less than about 94%, no less
than about 95%,
no less than about 96%, no less than about 97%, no less than about 98%, no
less than about
99%, no less than about 99.5%, or no less than about 99.8%. In certain
embodiments, the
compound comprises about 95% or more of the desired enantiomer and about 5% or
less of
the less preferred enantiomer based on the total weight of the racemate in
question.
[0048] In describing an optically active compound, the prefixes R and S are
used to
denote the absolute configuration of the molecule about its chiral center(s).
The (+) and (-)
are used to denote the optical rotation of the compound, that is, the
direction in which a plane
of polarized light is rotated by the optically active compound. The (-) prefix
indicates that
the compound is levorotatory, that is, the compound rotates the plane of
polarized light to the
left or counterclockwise. The (+) prefix indicates that the compound is
dextrorotatory, that
is, the compound rotates the plane of polarized light to the right or
clockwise. However, the
sign of optical rotation, (+) and (-), is not related to the absolute
configuration of the
molecule, R and S.
[0049] As used herein, and unless otherwise specified, the term "solvate"
refers to a
compound provided herein or a salt thereof, which further includes a
stoichiometric or non-
stoichiometric amount of solvent bound by non-covalent intermolecular forces.
Where the
solvent is water, the solvate is a hydrate.
-11-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
B. Compounds
[0050] Provided herein is a compound of formula (I):
(R3)n\ i\
--R 1
R2' ;CN/
(I)
or a tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof, wherein
R' is optionally substituted 5-10 membered monocyclic or bicyclic aromatic
carbocyclic ring system, optionally substituted 5-10 membered non-aromatic
carbocyclic ring
system, optionally substituted 5-10 membered monocyclic or bicyclic aromatic
heterocyclic
ring system, or optionally substituted 5-10 membered non-aromatic heterocyclic
ring system;
RZ is R6, SO2R6 or LSOZR6;
each R3 is independently halo, CN, NO2, OH, SH, NH2, CI -C4 alkyl, or
-O(CI -C4 alkyl);
R6 is (i) Cl-Clo alkyl optionally substituted with one or more halo, -OH, -
O(CI-C6
alkyl), or optionally substituted 5-10 membered monocyclic or bicyclic
aromatic or 5-10
membered non-aromatic carbocyclic or heterocyclic ring system; or (ii)
optionally substituted
C3-C7 cycloalkyl;
L is CI -C5 alkylene;
nis0, 1,2,or3;
for use in the treatment or prophylaxis of Duchenne muscular dystrophy, Becker
muscular
dystrophy, or cachexia.
[0051] In certain embodiments, provided herein is a compound of formula (I),
or a
tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or prodrug
thereof.
[0052] In one embodiment, R' is optionally substituted 5-10 membered
monocyclic or
bicyclic aromatic carbocyclic ring system. In another embodiment, R' is
optionally
substituted 5-10 membered monocyclic or bicyclic aromatic heterocyclic ring
system. In
another embodiment, R' is optionally substituted monocyclic aromatic
carbocyclic ring
system. In another embodiment, R' is optionally substituted bicyclic aromatic
carbocyclic
ring system. In another embodiment, R' is optionally substituted monocyclic
aromatic
heterocyclic ring system. In another embodiment, R' is optionally substituted
bicyclic
-12-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
aromatic heterocyclic ring system. In another embodiment, R' is optionally
substituted 5-10
membered non-aromatic carbocyclic ring system. In another embodiment, R' is
optionally
substituted 5-10 membered non-aromatic heterocyclic ring system.
[0053] In one embodiment, R' is unsubstituted aromatic carbocyclic ring
system. In
another embodiment, R' is aromatic carbocyclic ring system optionally
substituted with one
or more halo substituents. In certain embodiments, R' is phenyl or naphthyl
optionally
substituted with one or more halo. In another embodiment, R' is phenyl
optionally
substituted with one or more halo. In another embodiment, R' is naphthyl
optionally
substituted with one or more halo. In another embodiment, R' is unsubstituted
phenyl. In
another embodiment, R' is unsubstituted naphthyl. In another embodiment, R' is
phenyl or
naphthyl optionally substituted with one or more chloro. In another
embodiment, R' is
phenyl optionally substituted with one or more chloro. In another embodiment,
R' is
naphthyl optionally substituted with one or more chloro. In another
embodiment, R' is
phenyl substituted with two chloro substituents. Specific examples of R'
include, but are not
limited to, naphthalene-2-yl, phenyl, 3,4-dichlorophenyl and 2,3-
dichlorophenyl.
[0054] In one embodiment, R2 is R6. In another embodiment, R 2 is S02R6. In
another
embodiment, R2 is LSOZR6.
[0055] In one embodiment, R6 is Cl-Clo alkyl optionally substituted with one
or more
halo, -OH, -O(CI-C6 alkyl), or optionally substituted 5-10 membered monocyclic
or bicyclic
aromatic or 5-10 membered non-aromatic carbocyclic or heterocyclic ring
system. In another
embodiment, R6 is optionally substituted C3-C7 cycloalkyl. In another
embodiment, R6 is Ci-
C10 alkyl optionally substituted with one or more halo. In another embodiment,
R6 is CI-CIo
alkyl optionally substituted with one or more -OH. In another embodiment, R6
is C'-C'o
alkyl optionally substituted with one or more -O(CI-C6 alkyl). In another
embodiment, R6 is
Cl-Clo alkyl optionally substituted with optionally substituted 5-10 membered
monocyclic or
bicyclic aromatic carbocyclic ring system. In another embodiment, R6 is Ci-Cio
alkyl
optionally substituted with optionally substituted 5-10 membered monocyclic or
bicyclic
aromatic heterocyclic ring system. In another embodiment, R6 is C~-CIo alkyl
optionally
substituted with optionally substituted 5-10 membered non-aromatic carbocyclic
ring system.
In another embodiment, R6 is C1-Clo alkyl optionally substituted with
optionally substituted
5-10 membered non-aromatic heterocyclic ring system.
[0056] In one embodiment, R2 is Ci-Clo alkyl optionally substituted with one
or more
halo. In another embodiment, R2 is straight or branched CI -C6 alkyl
optionally substituted
-13-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
with one or more halo. In another embodiment, R2 is straight or branched CI -
C6 alkyl
optionally substituted with one or more fluoro: In another embodiment, R 2 is
Cl-Clo alkyl
optionally substituted with one or more phenyl. Specific examples of R 2
include, but are not
limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, any of which may be
optionally
substituted with one or more halo substituents, such as fluoro, or phenyl.
Other examples of
R2 include, but are not limited to, 3,3,3-trifluoro-propyl and benzyl.
[0057] In one embodiment, R2 is optionally substituted C3-C7 cycloalkyl. In
another
embodiment, R 2 is optionally substituted cyclopentyl or cyclohexyl. Specific
examples of R2
include, but are not limited to, cyclopentyl and cyclohexyl.
[0058] In certain embodiments, n is 0 and R3 is absent. In another embodiment,
n is 1. In
another embodiment, n is 2. In another embodiment, n is 3.
[0059] In one embodiment, L is CI -CS alkylene.
[0060] In one embodiment, R3 is halo. In another embodiment, R3 is CN. In
another
embodiment, R3 is NOZ. In another embodiment, R3 is OH. In another embodiment,
R3 is
SH. In another embodiment, R3 is NH2. In another embodiment, R3 is CI-C4
alkyl. In
another embodiment, R3 is -O(CI-C4 alkyl).
[0061] Any combinations of R', R2, R3, R5, R6, R7, X4, L, q, and n are
encompassed by
this disclosure and specifically provided herein.
[0062] In one embodiment, specific examples of compounds of formula (1)
include, but
are not limited to, the following:
1. 5 -benzyl-2-phenyl-5H-imidazo[4,5 -c]pyri dine;
N
~ I N /JN ~ /
2. 5-ethyl-2-(naphthalen-2-yl)-5H-imidazo [4,5-c]pyridine;
N N
3. 5-methyl-2-(naphthalen-2-yl)-5H-imidazo [4,5-c]pyridine;
N - ~
,N /
4. 2-(naphthalen-2-yl)-5-propyl-5H-imidazo [4,5-c]pyridine;
N
N
5. 5 -butyl-2-(naphthalen-2-yl)-5 H-imidazo [4,5 -c]pyridine;
-14-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
N
6. 5-benzyl-2-(naphthalen-2-yl)-SH-imidazo[4,5-c]pyridine;
N
N
7. 5-isopropyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine;
N
8. 2-( 3,4-dichlorophenyl )-5 -i sopropyl-5 H-imidazo [4, 5-c] pyridi ne;
ci
-N
-
N N ci
9. 2-(3,4-dichlorophenyl)-5-propyl-5 H-imidazo [4,5-c] pyridine;
ci
N
~ ~ -
N CI
10. 2-(3,4-dichlorophenyl)-5-methyl-5 H-imidazo [4,5-c] pyridine;
ci
N
/ ~ -
ci
11. 5-cyclopentyl-2-(naphthalen-2-yl)-5 H-imidazo [4,5-c] pyridine;
D,!5 N
NN
~
12. 5-cyclopentyl-2-(3,4-dichlorophenyl)-5H-imidazo [4,5-c]pyridine;
ci
N
/ ~ -
,/N / N \ / CI
v
13. 2-(2, 3-dichlorophenyl)-5-isopropyl-5 H-imidazo [4,5-c] pyridine;
CI cl
N
N N
14. 5-cyclopentyl-2-(2,3-dichlorop henyl)-5H-imidazo[4,5-c]pyridine;
ci ci
/ N
NN C/
-15-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
15. 2-(2, 3-dichlorophenyl)-5-propyl-5H-imidazo [4,5-c]pyridine;
cl cl
N
I-I~N N
16. 2-(3,4-dichlorophenyl)-5-(3,3,3-trifluoropropyl)-5H-imidazo[4,5-
c]pyridine;
ci
N
F N ,IXN \ / CI
F F
or a tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof.
[0063] Provided herein is a compound of formula (I) or a tautomer, enantiomer,
pharmaceutically acceptable salt, hydrate, solvate, complex, or prodrug
thereof.
[0064] Specific examples include, but are not limited to, the following:
5-ethyl-2-(naphthalen-2-yl)-5 H-imidazo [4,5-c]pyridine;
5-methyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine;
2-(naphthalen-2-yl)-5-propyl-5H-imidazo [4,5-c]pyridine;
5-butyl-2-(naphthalen-2-yl)-5 H-imidazo [4,5-c]pyridine;
5-benzyl-2-(naphthalen-2-yJ)-SH-imidazo [4,5-c]pyridine;
5-isopropyl-2-(naphthalen-2-yl)-5H-imidazo [4,5-c] pyridine;
2-(3,4-dichlorophenyl)-5-isopropyl-5H-imidazo[4,5-c]pyridine;
2-(3,4-dichlorophenyl)-5-propyl-5H-imidazo[4,5-c]pyridine;
2-(3,4-dichlorophenyl)-5-methyl-5H-imi dazo[4,5-c]pyri dine;
5-cyclopentyl-2-(naphthalen-2-yl)-5H-imidazo [4,5-c]pyridine;
5-cyclopentyl-2-(3,4-dichlorophenyl)-5 H-imidazo[4,5-c] pyridine;
2-(2,3-dichlorophenyl)-5-isopropyl-5 H-imidazo [4,5-c]pyridine;
5-cyclopentyl-2-(2,3-dichlorophenyl)-5 H-imidazo [4,5-c]pyridine;
2-(2, 3-dichlorophenyl)-5-propyl-5H-imidazo [4,5-c]pyridine;
2-(3,4-dichlorophenyl)-5 -(3,3,3-trifluoropropyl)-5 H-imidazo [4, 5-
c]pyridine;
or a tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof.
[0065] Also provided herein is the use of a compound of formula (1) in the
preparation of
an agent for the treatment or prophylaxis of Duchenne muscular dystrophy,
Becker muscular
dystrophy, or cachexia.
C. Synthesis of the Compounds
-16-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[0066] In one embodiment, provided herein is a method of making a compound of
formula (I). The compounds of formula (I) can be synthesized from commercially
available
starting materials using the following methods.
[0067] Compounds of formula (I) may be prepared by reacting a compound of
formula
(II):
(R3)n' N
~R'
HN N
(II)
wherein R', R3, and n are as defined in herein elsewhere;
by reaction with a compound of formula (III):
R2-X
(III)
wherein R 2 is as defined herein elsewhere; and X is halo, for example bromo
or iodo.
[0068] In one embodiment, when R2 is an alkyl, X may be bromo. In another
embodiment, when R2 is cycloalkyl, X may be iodo. The reaction may be carried
out in the -
presence of an aqueous base such as sodium hydroxide, generally at a
temperature from 15 to
30 C, such as 25 C.
[0069] In one embodiment, when R2 is haloalkyl, for example 3,3,3-
trifluoropropyl, X
may be iodo. The reaction may be carried out under basic conditions. In some
embodiments,
the reaction may be carried out in a polar organic solvent such as
dimethylformamide in the
presence of a base such as sodium hydride. In some embodiments, the reaction
is carried out
at elevated temperature, for example 80 to 150 C.
[0070] Compounds of formula (III) are readily available or may be prepared by
methods
which are known to those of skills in the art.
[0071] Compounds of formula (II) may be prepared by reacting a compound of
general
(IV):
R'-COOH
(IV)
wherein R' is as defined herein elsewhere; with a 3,4-diaminopyridine of
formula (V):
-17-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
NHZ
NH2
(R3~n
N
(V)
wherein R3 and n are as defined herein elsewhere.
[0072] The reaction may be carried out in the presence of an acidic
dehydrating agent
such as polyphosphoric acid and at elevated temperature, for example 150 to
200 C.
[0073] Compounds of general formulae (IV) and (V) are known and are either
readily
available or may be prepared by methods known to those of skills in the art.
[0074] In the syntheses described herein, suitable protecting groups may be
used, as
known to one skilled in the art. One skilled in the art would be able to
choose appropriate
protecting groups and conditions to introduce and remove such protecting
groups.
Information concerning protecting groups is available in "Protecting Groups in
Organic
Synthesis", Theodora W. Greene and Peter G. M. Wuts, published by John Wiley &
Sons
Inc.
D. Pharmaceutical Compositions
[0075] In one embodiment, the compounds of formula (I) for use in the
treatment of
DMD is administered in the form of a pharmaceutical composition. Provided
herein is a
pharmaceutical composition comprising a compound of formula (I), or a
tautomer,
enantiomer, pharmaceutically acceptable salt, hydrate, solvate, complex, or
prodrug thereof;
and one or more pharmaceutically acceptable excipients.
[0076] In one embodiment, the phannaceutical composition comprises less than
80%
w/w, less than 50% w/w, less than 20% w/w, or between 0.1 to 20% w/w, of a
compound of
formula (I), or a pharmaceutically acceptable salt thereof, in admixture with
a
pharmaceutically acceptable diluent or carrier.
[0077] In one embodiment, provided herein is a process for the production of
such a
pharmaceutical composition which comprises mixing the ingredients.
[0078] In one embodiment, examples of pharmaceutical formulations,
compositions, and
suitable diluents or carriers, include, but are not limited to, the following:
for intravenous injection or infusion - purified water or saline solution;
for inhalation compositions - coarse lactose;
-18-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
for tablets, capsules and dragees - microcrystalline cellulose, calcium
phosphate,
diatomaceous earth, a sugar such as lactose, dextrose or mannitol, talc,
stearic acid, starch,
sodium bicarbonate and/or gelatin;
for suppositories - natural or hardened oils or waxes.
[0079] In one embodiment, the compound is used in aqueous solution, for
example in
intravenous infusion, the pharmaceutical composition comprising the compound
may further
comprise one or more excipients. In certain embodiments, the excipients
include, but are not
limited to, chelating or sequestering agents, antioxidants, tonicity adjusting
agents, pH-
modifying agents and buffering agents.
[0080] In one embodiment, solutions containing a compound of formula (I) may,
if
desired, be evaporated, for example, by freeze drying or spray drying, to give
a solid
composition, which may be reconstituted prior to use.
[0081] In one embodiment, the compound of formula (I) is not used as a
solution. In one
embodiment, the compound of formula (I) is in a form having a mass median
diameter of
from 0.01 to 10 m. In certain embodiment, the pharmaceutical composition
comprising a
compound of formula (I) may further contain preserving, stabilizing, and
wetting agents,
solubilizers, for example, a water-soluble cellulose polymer such as
hydroxypropyl
methylcellulose, or a water-soluble glycol such as propylene glycol,
sweetening and coloring
agents and flavorings. In one embodiment, the compositions may be formulated
in sustained
release form.
[0082] In one embodiment, the pharmaceutical composition comprises a compound
of
formula (I) in about 0.01% to about 99.9% w/w, relative to the entire
preparation. In certain
embodiment, the pharmaceutical composition comprises a compound of formula (I)
in about
0.1% to about 50% w/w, relative to the entire preparation.
[0083] Provided herein is a pharmaceutical composition comprising a compound
of
formula (I), and a pharmaceutically acceptable excipient, adjuvant, carrier,
buffer, or
stabiliser.
[0084] In one embodiment, the pharmaceutically acceptable excipient, adjuvant,
carrier,
buffer, or stabiliser is non-toxic and does not interfere with the efficacy of
the active
ingredient. The precise nature of the carrier or other material will depend on
the route of
administration, which may be oral or by injection, such as cutaneous,
subcutaneous, or
intravenous injection.
-19-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[0085] In one embodiment, the pharmaceutical compositions are provided in a
dosage
form for oral administration, which comprise a compound provided herein, and
one or more
pharmaceutically acceptable excipients or carriers. The pharmaceutical
compositions
provided herein that are formulated for oral administration may be in tablet,
capsule, powder,
or liquid form. A tablet may comprise a solid carrier or an adjuvant. Liquid
pharmaceutical
compositions generally comprise a liquid carrier such as water, petroleum,
animal or
vegetable oils, or mineral oil or synthetic oil. Physiological saline
solution, dextrose or other
saccharide solution, or glycols such as ethylene glycol, propylene glycol, or
polyethylene
glycol may be included. A capsule may comprise a solid carrier such as
gelatin.
[0086] In another embodiment, the pharmaceutical compositions are provided in
a dosage
form for parenteral administration, and one or more pharmaceutically
acceptable excipients
or carriers. Where pharmaceutical compositions may be formulated for
intravenous,
cutaneous or subcutaneous injection, the active ingredient will be in the form
of a
parenterally acceptable aqueous solution, which is pyrogen-free and has a
suitable pH,
isotonicity, and stability. Those of relevant skill in the art are well able
to prepare suitable
solutions using, for example, isotonic vehicles, such as Sodium Chloride
injection, Ringer's
injection, or Lactated Ringer's injection. Preservatives, stabilisers,
buffers, antioxidants,
and/or other additives may be included as required.
[0087] In yet another embodiment, the pharmaceutical compositions are provided
in a
dosage form for topical administration, which comprise a compound provided
herein, and one
or more pharmaceutically acceptable excipients or carriers.
[0088] The pharmaceutical compositions can also be formulated as modified
release
dosage forms, including delayed-, extended-, prolonged-, sustained-, pulsatile-
, controlled-,
accelerated- and fast-, targeted-, programmed-release, and gastric retention
dosage forms.
These dosage forms can be prepared according to conventional methods and
techniques
known to those skilled in the art (See, Remington: The Science and Practice of
Pharmacy,
supra; Modified-Release Drug Delivery Technology, 2nd Edition, Rathbone et
al., Eds.,
Marcel Dekker, Inc.: New York, NY, 2008).
[0089] The pharmaceutical compositions provided herein can be provided in a
unit-
dosage form or multiple-dosage form. A unit-dosage form, as used herein,
refers to
physically discrete a unit suitable for administration to a human and animal
subject, and
packaged individually as is known in the art. Each unit-dose contains a
predetermined
quantity of an active ingredient(s) sufficient to produce the desired
therapeutic effect, in
-20-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
association with the required pharmaceutical carriers or excipients. Examples
of a unit-
dosage form include an ampoule, syringe, and individually packaged tablet and
capsule. A
unit-dosage form may be administered in fractions or multiples thereof. A
multiple-dosage
form is a plurality of identical unit-dosage forms packaged in a single
container to be
administered in segregated unit-dosage form. 'Examples of a multiple-dosage
form include a
vial, bottle of tablets or capsules, or bottle of pints or gallons.
[0090] The pharmaceutical compositions provided herein can be administered at
once, or
multiple times at intervals of time. It is understood that the precise dosage
and duration of
treatment may vary with the age, weight, and condition of the patient being
treated, and may
be determined empirically using known testing protocols or by extrapolation
from in vivo or
in vitro test or diagnostic data. It is further understood that for any
particular individual,
specific dosage regimens should be adjusted over time according to the
individual need and
the professional judgment of the person administering or supervising the
administration of the
formulations.
[0091] In another embodiment, the pharmaceutical compositions provided herein
further
comprise one or more therapeutic agents for the treatment of Duchenne muscular
dystrophy,
Becker muscular dystrophy, or cachexia..
[0092] In yet another embodiment, provided herein is the use of a compound of
formula
(I) in the manufacture of a medicament for the treatment of Duchenne muscular
dystrophy,
Becker muscular dystrophy, or cachexia. In certain embodiments, the medicament
is in
tablet, capsule, powder, or liquid form. In certain embodiments, the
medicament is
formulated as described herein.
-21-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
1. Oral Adniinistration
[0093] The pharmaceutical compositions provided herein for oral administration
can be
provided in solid, semisolid, or liquid dosage forms for oral administration.
As used herein,
oral administration also includes buccal, lingual, and sublingual
administration. Suitable oral
dosage forms include, but are not limited to, tablets, fastmelts, chewable
tablets, capsules,
pills, strips, troches, lozenges, pastilles, cachets, pellets, medicated
chewing gum, bulk
powders, effervescent or non-effervescent powders or granules, oral nt.ists,
solutions,
emulsions, suspensions, wafers, sprinkles, elixirs, and syrups. In addition to
the active
ingredient(s), the pharmaceutical compositions can contain one or more
pharmaceutically
acceptable carriers or excipients, including, but not limited to, binders,
fillers, diluents,
disintegrants, wetting agents, lubricants, glidants, coloring agents, dye-
migration inhibitors,
sweetening agents, flavoring agents, emulsifying agents, suspending and
dispersing agents,
preservatives, solvents, non-aqueous liquids, organic acids, and sources of
carbon dioxide.
[0094] Binders or granulators impart cohesiveness to a tablet to ensure the
tablet
remaining intact after compression. Suitable binders or granulators include,
but are not
limited to, starches, such as corn starch, potato starch, and pre-gelatinized
starch (e.g.,
STARCH 1500); gelatin; sugars, such as sucrose, glucose, dextrose, molasses,
and lactose;
natural and synthetic gums, such as acacia, alginic acid, alginates, extract
of Irish moss,
panwar gum, ghatti gum, mucilage of isabgol husks, carboxymethylcellulose,
methylcellulose, polyvinylpyrrolidone (PVP), Veegum, larch arabogalactan,
powdered
tragacanth, and guar gum; celluloses, such as ethyl cellulose, cellulose
acetate,
carboxymethyl cellulose calcium, sodium carboxymethyl cellulose, methyl
cellulose,
hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), hydroxypropyl
methyl
cellulose (HPMC); microcrystalline celluloses, such as AVICEL-PH-101, AVICEL-
PH-103,
AVICEL RC-581, AVICEL-PH-105 (FMC Corp., Marcus Hook, PA); and mixtures
thereof.
Suitable fillers include, but are not limited to, talc, calcium carbonate,
microcrystalline
cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid,
sorbitol, starch, pre-
gelatinized starch, and mixtures thereof. The amount of a binder or filler in
the
pharmaceutical compositions provided herein varies upon the type of
formulation, and is
readily discernible to those of ordinary skill in the art. The binder or
filler may be present
from about 50 to about 99% by weight in the pharmaceutical compositions
provided herein.
[0095] Suitable diluents include, but are not limited to, dicalcium phosphate,
calcium
sulfate, lactose, sorbitol, sucrose, inositol, cellulose, kaolin, mannitol,
sodium chloride, dry
-22-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
starch, and powdered sugar. Certain diluents, such as mannitol, lactose,
sorbitol, sucrose, and
inositol, when present in sufficient quantity, can impart properties to some
compressed tablets
that permit disintegration in the mouth by chewing. Such compressed tablets
can be used as
chewable tablets. The amount of a diluent in the pharmaceutical compositions
provided
herein varies upon the type of formulation, and is readily discernible to
those of ordinary skill
in the art.
[0096] Suitable disintegrants include, but are not limited to, agar;
bentonite; celluloses,
such as methylcellulose and carboxymethylcellulose; wood products; natural
sponge; cation-
exchange resins; alginic acid; gums, such as guar gum and Veegum HV; citrus
pulp; cross-
linked celluloses, such as croscarmellose; cross-linked polymers, such as
crospovidone;
cross-linked starches; calcium carbonate; microcrystalline cellulose, such as
sodium starch
glycolate; polacrilin potassium; starches, such as corn starch, potato starch,
tapioca starch,
and pre-gelatinized starch; clays; aligns; and mixtures thereof. The amount of
a disintegrant
in the pharmaceutical compositions provided herein varies upon the type of
formulation, and
is readily discernible to those of ordinary skill in the art. The amount of a
disintegrant in the
pharmaceutical compositions provided herein varies upon the type of
formulation, and is
readily discernible to those of ordinary skill in the art. The pharmaceutical
compositions
provided herein may contain from about 0.5 to about 15% or from about 1 to
about 5% by
weight of a disintegrant.
[0097] Suitable lubricants include, but are not limited to, calcium stearate;
magnesium
stearate; mineral oil; light mineral oil; glycerin; sorbitol; mannitol;
glycols, such as glycerol
behenate and polyethylene glycol (PEG); stearic acid; sodium lauryl sulfate;
talc;
hydrogenated vegetable oil, including peanut oil, cottonseed oil, sunflower
oil, sesame oil,
olive oil, corn oil, and soybean oil; zinc stearate; ethyl oleate; ethyl
laureate; agar; starch;
lycopodium; silica or silica gels, such as AEROSIL 200 (W.R. Grace Co.,
Baltimore, MD)
and CAB-O-SIL (Cabot Co. of Boston, MA); and mixtures thereof. The
pharmaceutical
compositions provided herein may contain about 0.1 to about 5% by weight of a
lubricant.
[0098] Suitable glidants include, but are not limited to, colloidal silicon
dioxide, CAB-O-
SIL (Cabot Co. of Boston, MA), and asbestos-free talc. Suitable coloring
agents include,
but are not limited to, any of the approved, certified, water soluble FD&C
dyes, and water
insoluble FD&C dyes suspended on alumina hydrate, and color lakes and mixtures
thereof.
A color lake is the combination by adsorption of a water-soluble dye to a
hydrous oxide of a
heavy metal, resulting in an insoluble form of the dye. Suitable flavoring
agents include, but
-23-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
are not limited to, natural flavors extracted from plants, such as fruits, and
synthetic blends of
compounds which produce a pleasant taste sensation, such as peppermint and
methyl
salicylate. Suitable sweetening agents include, but are not limited to,
sucrose, lactose,
mannitol, syrups, glycerin, and artificial sweeteners, such as saccharin and
aspartame.
Suitable emulsifying agents include, but are not limited to, gelatin, acacia,
tragacanth,
bentonite, and surfactants, such as polyoxyethylene sorbitan monooleate
(TWEEN' 20),
polyoxyethylene sorbitan monooleate 80 (TWEEN 80), and triethanolamine
oleate. Suitable
suspending and dispersing agents include, but are not limited to, sodium
carboxymethylcellulose, pectin, tragacanth, Veegum, acacia, sodium
carbomethylcellulose,
hydroxypropyl methylcellulose, and polyvinylpyrrolidone. Suitable
preservatives include,
but are not limited to, glycerin, methyl and propylparaben, benzoic add,
sodium benzoate and
alcohol. Suitable wetting agents include, but are not limited to, propylene
glycol
monostearate, sorbitan monooleate, diethylene glycol monolaurate, and
polyoxyethylene
lauryl ether. Suitable solvents include, but are not limited to, glycerin,
sorbitol, ethyl alcohol,
and syrup. Suitable non-aqueous liquids utilized in emulsions include, but are
not limited to,
mineral oil and cottonseed oil. Suitable organic acids include, but are not
limited to, citric
and tartaric acid. Suitable sources of carbon dioxide include, but are not
limited to, sodium
bicarbonate and sodium carbonate.
[0099] It should be understood that many carriers and excipients may serve
several
functions, even within the same formulation.
[00100] The pharmaceutical compositions provided herein for oral
administration can be
provided as compressed tablets, tablet triturates, chewable lozenges, rapidly
dissolving
tablets, multiple compressed tablets, or enteric-coating tablets, sugar-
coated, or film-coated
tablets. Enteric-coated tablets are compressed tablets coated with substances
that resist the
action of stomach acid but dissolve or disintegrate in the intestine, thus
protecting the active
ingredients from the acidic environment of the stomach. Enteric-coatings
include, but are not
limited to, fatty acids, fats, phenyl salicylate, waxes, shellac, ammoniated
shellac, and
cellulose acetate phthalates. Sugar-coated tablets are compressed tablets
surrounded by a
sugar coating, which may be beneficial in covering up objectionable tastes or
odors and in
protecting the tablets from oxidation. Film-coated tablets are compressed
tablets that are
covered with a thin layer or film of a water-soluble material. Film coatings
include, but are
not limited to, hydroxyethylcellulose, sodium carboxymethylcellulose,
polyethylene glycol
4000, and cellulose acetate phthalate. Film coating imparts the same general
characteristics
-24-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
as sugar coating. Multiple compressed tablets are compressed tablets made by
more than one
compression cycle, including layered tablets, and press-coated or dry-coated
tablets.
[00101] The tablet dosage forms can be prepared from the active ingredient in
powdered,
crystalline, or granular forms, alone or in combination with one or more
carriers or excipients
described herein, including binders, disintegrants, controlled-release
polymers, lubricants,
diluents, and/or colorants. Flavoring and sweetening agents are especially
useful in the
formation of chewable tablets and lozenges.
[00102] The pharmaceutical compositions provided herein for oral
administration can be
provided as soft or hard capsules, which can be made from gelatin,
methylcellulose, starch, or
calcium alginate. The hard gelatin capsule, also known as the dry-filled
capsule (DFC),
consists of two sections, one slipping over the other, thus completely
enclosing the active
ingredient. The soft elastic capsule (SEC) is a soft, globular shell, such as
a gelatin shell,
which is plasticized by the addition of glycerin, sorbitol, or a similar
polyol. The soft gelatin
shells may contain a preservative to prevent the growth of microorganisms.
Suitable
preservatives are those as described herein, including methyl- and propyl-
parabens, and
sorbic acid. The liquid, semisolid, and solid dosage forms provided herein may
be
encapsulated in a capsule. Suitable liquid and semisolid dosage forms include
solutions and
suspensions in propylene carbonate, vegetable oils, or triglycerides. Capsules
containing
such solutions can be prepared as described in U.S. Pat. Nos. 4,328,245;
4,409,239; and
4,410,545. The capsules may also be coated as known by those of skill in the
art in order to
modify or sustain dissolution of the active ingredient.
[00103] The pharmaceutical compositions provided herein for oral
administration can be
provided in liquid and semisolid dosage forms, including emulsions, solutions,
suspensions,
elixirs, and syrups. An emulsion is a two-phase system, in which one liquid is
dispersed in
the form of small globules throughout another liquid, which can be oil-in-
water or water-in-
oil. Emulsions may include a pharmaceutically acceptable non-aqueous liquid or
solvent,
emulsifying agent, and preservative. Suspensions may include a
pharmaceutically acceptable
suspending agent and preservative. Aqueous alcoholic solutions may include a
pharmaceutically acceptable acetal, such as a di(lower alkyl) acetal of a
lower alkyl aldehyde,
e.g., acetaldehyde diethyl acetal; and a water-miscible solvent having one or
more hydroxyl
groups, such as propylene glycol and ethanol. Elixirs are clear, sweetened,
and
hydroalcoholic solutions. Syrups are concentrated aqueous solutions of a
sugar, for example,
sucrose, and may also contain a preservative. For a liquid dosage form, for
example, a
-25-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
solution in a polyethylene glycol may be diluted with a sufficient quantity of
a
pharmaceutically acceptable liquid carrier, e.g., water, to be measured
conveniently for
administration.
[00104] Other useful liquid and semisolid dosage forms include, but are not
limited to,
those containing the active ingredient(s) provided herein, and a dialkylated
mono- or poly-
alkylene glycol, including, 1,2-dimethoxymethane, diglyme, triglyme,
tetraglyme,
polyethylene glycol-350-dimethyl ether, polyethylene glycol-550-dimethyl
ether,
polyethylene glycol-750-dimethyl ether, wherein 350, 550, and 750 refer to the
approximate
average molecular weight of the polyethylene glycol. These formulations can
further
comprise one or more antioxidants, such as butylated hydroxytoluene (BHT),
butylated
hydroxyanisole (BHA), propyl gallate, vitamin E, hydroquinone,
hydroxycoumarins,
ethanolamine, lecithin, cephalin, ascorbic acid, malic acid, sorbitol,
phosphoric acid, bisulfite,
sodium metabisulfite, thiodipropionic acid and its esters, and
dithiocarbamates.
[00105] The pharmaceutical compositions provided herein for oral
administration can be
also provided in the forms of liposomes, micelles, microspheres, or
nanosystems. Micellar
dosage forms can be prepared as described in U.S. Pat. No. 6,350,458.
[00106] The pharmaceutical compositions provided herein for oral
administration can be
provided as non-effervescent or effervescent, granules and powders, to be
reconstituted into a
liquid dosage form. Pharmaceutically acceptable carriers and excipients used
in the non-
effervescent granules or powders may include diluents, sweeteners, and wetting
agents.
Pharmaceutically acceptable carriers and excipients used in the effervescent
granules or
powders may include organic acids and a source of carbon dioxide.
[00107] Coloring and flavoring agents can be used in all of the dosage forms
provided
herein.
[00108] The pharmaceutical compositions provided herein for oral
administration can be
formulated as immediate or modified release dosage forms, including delayed-,
sustained,
pulsed-, controlled, targeted-, and programmed-release forms.
2. Parenteral Administration
[00109] The pharmaceutical compositions provided herein can be administered
parenterally by injection, infusion, or implantation, for local or systemic
administration.
Parenteral administration, as used herein, include intravenous, intraarterial,
intraperitoneal,
-26-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
intrathecal, intraventricular, intraurethral, intrasternal, intracranial,
intramuscular,
intrasynovial, intravesical, and subcutaneous administration.
[00110] The pharmaceutical compositions provided herein for parenteral
administration
can be formulated in any dosage forms that are suitable for parenteral
administration,
including solutions, suspensions, emulsions, micelles, liposomes,
microspheres, nanosystems,
and solid forms suitable for solutions or suspensions in liquid prior to
injection. Such dosage
forms can be prepared according to conventional methods known to those skilled
in the art of
pharmaceutical science (See, Remington: The Science and Practice of Pharmacy,
supra).
[00111] The pharmaceutical compositions intended for parenteral administration
can
include one or more pharmaceutically acceptable carriers and excipients,
including, but not
limited to, aqueous vehicles, water-miscible vehicles, non-aqueous vehicles,
antimicrobial
agents or preservatives against the growth of microorganisms, stabilizers,
solubility
enhancers, isotonic agents, buffering agents, antioxidants, local anesthetics,
suspending and
dispersing agents, wetting or emulsifying agents, complexing agents,
sequestering or
chelating agents, cryoprotectants, lyoprotectants, thickening agents, pH
adjusting agents, and
inert gases.
[00112] Suitable aqueous vehicles include, but are not Iimited to, water,
saline,
physiological saline or phosphate buffered saline (PBS), sodium chloride
injection, Ringers
injection, isotonic dextrose injection, sterile water injection, dextrose and
lactated Ringers
injection. Suitable non-aqueous vehicles include, but are not limited to,
fixed oils of
vegetable origin, castor oil, corn oil, cottonseed oil, olive oil, peanut oil,
peppermint oil,
safflower oil, sesame oil, soybean oil, hydrogenated vegetable oils,
hydrogenated soybean oil,
and medium-chain triglycerides of coconut oil, and palm seed oil. Suitable
water-miscible
vehicles include, but are not limited to, ethanol, 1,3-butanediol, liquid
polyethylene glycol
(e.g., polyethylene glyco1300 and polyethylene glycol 400), propylene glycol,
glycerin, N-
methyl-2-pyrrolidone, N,N-dimethylacetamide, and dimethyl sulfoxide.
[00113] Suitable antimicrobial agents or preservatives include, but are not
limited to,
phenols, cresols, mercurials, benzyl alcohol, chlorobutanol, methyl and propyl
p-
hydroxybenzoates, thimerosal, benzalkonium chloride (e.g., benzethonium
chloride), methyl-
and propyl-parabens, and sorbic acid. Suitable isotonic agents include, but
are not limited to,
sodium chloride, glycerin, and dextrose. Suitable buffering agents include,
but are not
limited to, phosphate and citrate. Suitable antioxidants are those as
described herein,
including bisulfite and sodium metabisulfite. Suitable local anesthetics
include, but are not
-27-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
limited to, procaine hydrochloride. Suitable suspending and dispersing agents
are those as
described herein, including sodium carboxymethylcelluose, hydroxypropyl
methylcellulose,
and polyvinylpyrrolidone. Suitable emulsifying agents are those described
herein, including
polyoxyethylene sorbitan monolaurate, polyoxyethylene sorbitan monooleate 80,
and
triethanolamine oleate. Suitable sequestering or chelating agents include, but
are not limited
to EDTA. Suitable pH adjusting agents include, but are not limited to, sodium
hydroxide,
hydrochloric acid, citric acid, and lactic acid. Suitable complexing agents
include, but are not
limited to, cyclodextrins, including a-cyclodextrin, 0-cyclodextrin,
hydroxypropyl-p-
cyclodextrin, sulfobutylether-p-cyclodextrin, and sulfobutylether 7-(3-
cyclodextrin
(CAPTISOL , CyDex, Lenexa, KS).
[00114] When the pharmaceutical compositions provided herein are formulated
for
multiple dosage administration, the multiple dosage parenteral formulations
must contain an
antimicrobial agent at bacteriostatic or fungistatic concentrations. All
parenteral formulations
must be sterile, as known and practiced in the art.
[00115] In one embodiment, the pharmaceutical compositions for parenteral
administration are provided as ready-to-use sterile solutions. In another
embodiment, the
pharmaceutical compositions are provided as sterile dry soluble products,
including
lyophilized powders and hypodermic tablets, to be reconstituted with a vehicle
prior to use.
In yet another embodiment, the pharmaceutical compositions are provided as
ready-to-use
sterile suspensions. In yet another embodiment, the pharmaceutical
compositions are
provided as sterile dry insoluble products to be reconstituted with a vehicle
prior to use. In
still another embodiment, the pharmaceutical compositions are provided as
ready-to-use
sterile emulsions.
[00116] The pharmaceutical compositions provided herein for parenteral
administration
can be formulated as immediate or modified release dosage forms, including
delayed-,
sustained, pulsed-, controlled, targeted-, and programmed-release forms.
[00117] The pharmaceutical compositions provided herein for parenteral
administration
can be formulated as a suspension, solid, semi-solid, or thixotropic liquid,
for administration
as an implanted depot. In one embodiment, the pharmaceutical compositions
provided herein
are dispersed in a solid inner matrix, which is surrounded by an outer
polymeric membrane
that is insoluble in body fluids but allows the active ingredient in the
pharmaceutical
compositions diffuse through.
-28-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[00118] Suitable inner matrixes include, but are not limited to,
polymethylmethacrylate,
polybutyl-methacrylate, plasticized or unplasticized polyvinylchloride,
plasticized nylon,
plasticized polyethylene terephthalate, natural rubber, polyisoprene,
polyisobutylene,
polybutadiene, polyethylene, ethylene-vinyl acetate copolymers, silicone
rubbers,
polydimethylsiloxanes, silicone carbonate copolymers, hydrophilic polymers,
such as
hydrogels of esters of acrylic and methacrylic acid, coliagen, cross-linked
polyvinyl alcohol,
and cross-linked partially hydrolyzed polyvinyl acetate.
[00119] Suitable outer polymeric membranes include but are not limited to,
polyethylene,
polypropylene, ethylene/propylene copolymers, ethylene/ethyl acrylate
copolymers,
ethylene/vinyl acetate copolymers, silicone rubbers, polydimethyl siloxanes,
neoprene rubber,
chlorinated polyethylene, polyvinylchloride, vinyl chloride copolymers with
vinyl acetate,
vinylidene chloride, ethylene and propylene, ionomer polyethylene
terephthalate, butyl
rubber epichlorohydrin rubbers, ethylene/vinyl alcohol copolymer,
ethylene/vinyl
acetate/vinyl alcohol terpolymer, and ethylene/vinyloxyethanol copolymer.
3. Topical Administration
[00120] The pharmaceutical compositions provided herein can be administered
topically to
the skin, orifices, or mucosa. The topical adnlinistration, as used herein,
includes
(intra)dermal, conjunctival, intracorneal, intraocular, ophthalmic, auricular,
transdermal,
nasal, vaginal, urethral, respiratory, and rectal administration.
[00121] The pharmaceutical compositions provided herein can be formulated in
any
dosage forms that are suitable for topical administration for local or
systemic effect, including
emulsions, solutions, suspensions, creams, gels, hydrogels, ointments, dusting
powders,
dressings, elixirs, lotions, suspensions, tinctures, pastes, foams, films,
aerosols, irrigations,
sprays, suppositories, bandages, and dermal patches. The topical formulation
of the
pharmaceutical compositions provided herein can also comprise liposomes,
micelles,
microspheres, nanosystems, and mixtures thereof.
[00122] Pharmaceutically acceptable carriers and excipients suitable for use
in the topical
formulations provided herein include, but are not limited to, aqueous
vehicles, water-miscible
vehicles, non-aqueous vehicles, antimicrobial agents or preservatives against
the growth of
microorganisms, stabilizers, solubility enhancers, isotonic agents, buffering
agents,
antioxidants, local anesthetics, suspending and dispersing agents, wetting or
emulsifying
-29-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
agents, complexing agents, sequestering or chelating agents, penetration
enhancers,
cryoprotectants, lyoprotectants, thickening agents, and inert gases.
[00123] The pharmaceutical compositions can also be administered topically by
electroporation, iontophoresis, phonophoresis, sonophoresis, or microneedle or
needle-free
injection, such as POWDERJECTTM (Chiron Corp., Emeryville, CA), and BIOJECTTM
(Bioject Medical Technologies Inc., Tualatin, OR).
[00124] The pharmaceutical compositions provided herein can be provided in the
forms of
ointments, creams, and gels. Suitable ointment vehicles include oleaginous or
hydrocarbon
vehicles, including lard, benzoinated lard, olive oil, cottonseed oil, and
other oils, white
petrolatum; emulsifiable or absorption vehicles, such as hydrophilic
petrolatum,
hydroxystearin sulfate, and anhydrous lanolin; water-removable vehicles, such
as hydrophilic
ointment; water-soluble ointment vehicles, including polyethylene glycols of
varying
molecular weight; emulsion vehicles, either water-in-oil (W/O) emulsions or
oil-in-water
(O/W) emulsions, including cetyl alcohol, glyceryl monostearate, lanolin, and
stearic acid
(See, Remington: The Science and Practice of Pharmacy, supra). These vehicles
are
emollient but generally require addition of antioxidants and preservatives.
[00125] Suitable cream base can be oil-in-water or water-in-oil. Suitable
cream vehicles
may be water-washable, and contain an oil phase, an emulsifier, and an aqueous
phase. The
oil phase is also called the "internal" phase, which is generally comprised of
petrolatum and a
fatty alcohol such as cetyl or stearyl alcohol. The aqueous phase usually,
although not
necessarily, exceeds the oil phase in volume, and generally contains a
humectant. The
emulsifier in a cream formulation may be a nonionic, anionic, cationic, or
amphoteric
surfactant.
[00126] Gels are semisolid, suspension-type systems. Single-phase gels contain
organic
macromolecules distributed substantially uniformly throughout the liquid
carrier. Suitable
gelling agents include, but are not limited to, crosslinked acrylic acid
polymers, such as
carbomers, carboxypolyalkylenes, and CARBOPOL ; hydrophilic polymers, such as
polyethylene oxides, polyoxyethylene-polyoxypropylene copolymers, and
polyvinylalcohol;
cellulosic polymers, such as hydroxypropyl cellulose, hydroxyethyl cellulose,
hydroxypropyl
methylcellulese, hydroxypropyl methylcellulose phthalate, and methylcellulose;
gums, such
as tragacanth and xanthan gum; sodium alginate; and gelatin. In order to
prepare a uniform
gel, dispersing agents such as alcohol or glycerin can be added, or the
gelling agent can be
dispersed by trituration, mechanical mixing, and/or stirring.
-30-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[00127] The pharmaceutical compositions provided herein can be administered
rectally,
urethrally, vaginally, or perivaginally in the forms of suppositories,
pessaries, bougies,
poultices or cataplasm, pastes, powders, dressings, creams, plasters,
contraceptives,
ointments, solutions, emulsions, suspensions, tampons, gels, foams, sprays, or
enemas.
These dosage forms can be manufactured using conventional processes as
described in
Remington: The Science and Practice of Pharmacy, supra.
[00128] Rectal, urethral, and vaginal suppositories are solid bodies for
insertion into body
orifices, which are solid at ordinary temperatures but melt or soften at body
temperature to
release the active ingredient(s) inside the orifices. Pharmaceutically
acceptable carriers
utilized in rectal and vaginal suppositories include bases or vehicles, such
as stiffening
agents, which produce a melting point in the proximity of body temperature,
when
formulated with the pharmaceutical compositions provided herein; and
antioxidants as
described herein, including bisulfite and sodium metabisulfite. Suitable
vehicles include, but
are not limited to, cocoa butter (theobroma oil), glycerin-gelatin, carbowax
(polyoxyethylene
glycol), spermaceti, paraffin, white and yellow wax, and appropriate mixtures
of mono-, di-
and triglycerides of fatty acids, and hydrogels, such as polyvinyl alcohol,
hydroxyethyl
methacrylate, and polyacrylic acid;. Combinations of the various vehicles can
also be used.
Rectal and vaginal suppositories may be prepared by compressing or molding.
The typical
weight of a rectal and vaginal suppository is about 2 to about 3 g.
[00129] The pharmaceutical compositions provided herein can be administered
ophthalmically in the forms of solutions, suspensions, ointments, emulsions,
gel-forming
solutions, powders for solutions, gels, ocular inserts, and implants.
[00130] The pharmaceutical compositions provided herein can be administered
intranasally or by inhalation to the respiratory tract. The pharmaceutical
compositions can be
provided in the form of an aerosol or solution for delivery using a
pressurized container,
pump, spray, atomizer, such as an atomizer using electrohydrodynamics to
produce a fine
mist, or nebulizer, alone or in combination with a suitable propellant, such
as 1,1,1,2-
tetrafluoroethane or 1,1,1,2,3,3,3-heptafluoropropane. The pharmaceutical
compositions can
also be provided as a dry powder for insufflation, alone or in combination
with an inert
carrier such as lactose or phospholipids; and nasal drops. For intranasal use,
the powder can
comprise a bioadhesive agent, including chitosan or cyclodextrin.
[00131] Solutions or suspensions for use in a pressurized container, pump,
spray, atomizer,
or nebulizer can be formulated to contain ethanol, aqueous ethanol, or a
suitable alternative
-31-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
agent for dispersing, solubilizing, or extending release of the active
ingredient provided
herein; a propellant as solvent; and/or a surfactant, such as sorbitan
trioleate, oleic acid, or an
oligolactic acid.
[00132] The pharmaceutical compositions provided herein can be micronized to a
size
suitable for delivery by inhalation, such as about 50 micrometers or less, or
about 10
micrometers or less. Particles of such sizes can be prepared using a
comminuting method
known to those skilled in the art, such as spiral jet milling, fluid bed jet
milling, supercritical
fluid processing to form nanoparticles, high pressure homogenization, or spray
drying.
[00133] Capsules, blisters, and cartridges for use in an inhaler or
insufflator can be
formulated to contain a powder mix of the pharmaceutical compositions provided
herein; a
suitable powder base, such as lactose or starch; and a performance modifier,
such as 1-
leucine, mannitol, or magnesium stearate. The lactose may be anhydrous or in
the form of
the monohydrate. Other suitable excipients or carriers include, but are not
limited to, dextran,
glucose, maltose, sorbitol, xylitol, fructose, sucrose, and trehalose. The
pharmaceutical
compositions provided herein for inhaled/intranasal administration can further
comprise a
suitable flavor, such as menthol and levomenthol; and/or sweeteners, such as
saccharin and
saccharin sodium.
[00134] The pharmaceutical compositions provided herein for topical
administration can
be formulated to be immediate release or modified release, including delayed-,
sustained-,
pulsed-, controlled-, targeted, and programmed release.
4. Modified Release
[00135] The pharmaceutical compositions provided herein can be formulated as a
modified release dosage form. As used herein, the term "modified release"
refers to a dosage
form in which the rate or place of release of the active ingredient(s) is
different from that of
an immediate dosage form when administered by the same route. Modified release
dosage
forms include, but are not limited to, delayed-, extended-, prolonged-,
sustained-, pulsatile-,
controlled-, accelerated- and fast-, targeted-, programmed-release, and
gastric retention
dosage forms. The pharmaceutical compositions in modified release dosage forms
can be
prepared using a variety of modified release devices and methods known to
those skilled in
the art, including, but not limited to, matrix controlled release devices,
osmotic controlled
release devices, multiparticulate controlled release devices, ion-exchange
resins, enteric
coatings, multilayered coatings, microspheres, liposomes, and combinations
thereof. The
-32-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
release rate of the active ingredient(s) can also be modified by varying the
particle sizes and
polymorphorism of the active ingredient(s).
[00136] Examples of modified release include, but are not limited to, those
described in
U.S. Pat. Nos.: 3,845,770; 3,916,899; 3,536,809; 3,598,123; 4,008,719;
5,674,533;
5,059,595; 5,591,767; 5,120,548; 5,073,543; 5,639,476; 5,354,556; 5,639,480;
5,733,566;
5,739,108; 5,891,474; 5,922,356; 5,972,891; 5,980,945; 5,993,855; 6,045,830;
6,087,324;
6,113,943; 6,197,350; 6,248,363; 6,264,970; 6,267,981; 6,376,461; 6,419,961;
6,589,548;
6,613,358; and 6,699,500.
(a) Matrix Controlled Release Devices
[00137] The pharmaceutical compositions provided herein in a modified release
dosage
form can be fabricated using a matrix controlled release device known to those
skilled in the
art (See, Takada et al. in "Encyclopedia of Controlled Drug Delivery," Vol. 2,
Mathiowitz
Ed., Wiley, 1999).
[00138] In certain embodiments, the pharmaceutical compositions provided
herein in a
modified release dosage form is formulated using an erodible matrix device,
which is water-
swellable, erodible, or soluble polymers, including, but not limited to,
synthetic polymers,
and naturally occurring polymers and derivatives, such as polysaccharides and
proteins.
[00139] Materials useful in forming an erodible matrix include, but are not
liniited to,
chitin, chitosan, dextran, and pullulan; gum agar, gum arabic, gum karaya,
locust bean gum,
gum tragacanth, carrageenans, gum ghatti, guar gum, xanthan gum, and
scleroglucan;
starches, such as dextrin and maltodextrin; hydrophilic colloids, such as
pectin; phosphatides,
such as lecithin; alginates; propylene glycol alginate; gelatin; collagen;
cellulosics, such as
ethyl cellulose (EC), methylethyl cellulose (MEC), carboxymethyl cellulose
(CMC), CMEC,
hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), cellulose acetate
(CA),
cellulose propionate (CP), cellulose butyrate (CB), cellulose acetate butyrate
(CAB), CAP,
CAT, hydroxypropyl methyl cellulose (HPMC), HPMCP, HPMCAS, hydroxypropyl
methyl
cellulose acettite trimellitate (HPMCAT), and ethyl hydroxyethyl cellulose
(EHEC);
polyvinyl pyrrolidone; polyvinyl alcohol; polyvinyl acetate; glycerol fatty
acid esters;
polyacrylamide; polyacrylic acid; copolymers of ethacrylic acid or methacrylic
acid
(EUDRAGIT , Rohm America, Inc., Piscataway, NJ); poly(2-hydroxyethyl-
methacrylate);
polylactides; copolymers of L-glutamic acid and ethyl-L-glutamate; degradable
lactic acid-
glycolic acid copolymers; poly-D-(-)-3-hydroxybutyric acid; and other acrylic
acid
-33-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
derivatives, such as homopolymers and copolymers of butylmethacrylate, methyl
methacrylate, ethyl methacrylate, ethylacrylate, (2-
dimethylaminoethyl)methacrylate, and
(trimethylaminoethyl)methacrylate chloride.
[00140] In certain embodiments, the pharmaceutical compositions provided
herein are
formulated with a non-erodible matrix device. The active ingredient(s) is
dissolved or
dispersed in an inert matrix and is released primarily by diffusion through
the inert matrix
once administered. Materials suitable for use as a non-erodible matrix device
include, but are
not limited to, insoluble plastics, such as polyethylene, polypropylene,
polyisoprene,
polyisobutylene, polybutadiene, polymethylmethacrylate, polybutylmethacrylate,
chlorinated
polyethylene, polyvinylchloride, methyl acrylate-methyl methacrylate
copolymers, ethylene-
vinyl acetate copolymers, ethylene/propylene copolymers, ethylene/ethyl
acrylate
copolymers, vinyl chloride copolymers with vinyl acetate, vinylidene chloride,
ethylene and
propylene, ionomer polyethylene terephthalate, butyl rubbers, epichlorohydrin
rubbers,
ethylene/vinyl alcohol copolymer, ethylene/vinyl acetate/vinyl alcohol
terpolymer,
ethylene/vinyloxyethanol copolymer, polyvinyl chloride, plasticized nylon,
plasticized
polyethylene terephthalate, natural rubber, silicone rubbers,
polydimethylsiloxanes, and
silicone carbonate copolymers; hydrophilic polymers, such as ethyl cellulose,
cellulose
acetate, crospovidone, and cross-linked partially hydrolyzed polyvinyl
acetate; and fatty
compounds, such as carnauba wax, rnicrocrystalline wax, and triglycerides.
[00141] In a matrix controlled release system, the desired release kinetics
can be
controlled, for example, via the polymer type employed, the polymer viscosity,
the particle
sizes of the polymer and/or the active ingredient(s), the ratio of the active
ingredient(s) versus
the polymer, and other excipients or carriers in the compositions.
[00142] The pharmaceutical compositions provided herein in a modified release
dosage
form can be prepared by methods known to those skilled in the art, including
direct
compression, dry or wet granulation followed by compression, and melt-
granulation followed
by compression.
(b) Osmotic Controlled Release Devices
[00143] The pharmaceutical compositions provided herein in a modified release
dosage
form can be fabricated using an osmotic controlled release device, including,
but not limited
to, one-chamber system, two-chamber system, asymmetric membrane technology
(AMT),
and extruding core system (ECS). In general, such devices have at least two
components: (a)
-34-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
a core which contains an active ingredient; and (b) a semi-permeable membrane
with at least
one delivery port, which encapsulates the core. The semi-permeable membrane
controls the
influx of water to the core from an aqueous environment of use so as to cause
drug release by
extrusion through the delivery port(s).
[00144] In addition to the active ingredient(s), the core of the osmotic
device optionally
includes an osmotic agent, which creates a driving force for transport of
water from the
environment of use into the core of the device. One class of osmotic agents is
water-
swellable hydrophilic polymers, which are also referred to as "osmopolymers"
and
"hydrogels." Suitable water-swellable hydrophilic polymers as osmotic agents
include, but
are not Iimited to, hydrophilic vinyl and acrylic polymers, polysaccharides
such as calcium
alginate, polyethylene oxide (PEO), polyethylene glycol (PEG), polypropylene
glycol (PPG),
poly(2-hydroxyethyl methacrylate), poly(acrylic) acid, poly(methacrylic) acid,
polyvinylpyrrolidone (PVP), crosslinked PVP, polyvinyl alcohol (PVA), PVA/PVP
copolymers, PVA/PVP copolymers with hydrophobic monomers such as methyl
methacrylate
and vinyl acetate, hydrophilic polyurethanes containing large PEO blocks,
sodium
croscarmellose, carrageenan, hydroxyethyl cellulose (HEC), hydroxypropyl
cellulose (HPC),
hydroxypropyl methyl cellulose (HPMC), carboxymethyl cellulose (CMC) and
carboxyethyl,
cellulose (CEC), sodium alginate, polycarbophil, gelatin, xanthan gum, and
sodium starch
glycolate.
[00145] The other class of osmotic agents is osmogens, which are capable of
imbibing
water to affect an osmotic pressure gradient across the barrier of the
surrounding coating.
Suitable osmogens include, but are not limited to, inorganic salts, such as
magnesium sulfate,
magnesium chloride, calcium chloride, sodium chloride, lithium chloride,
potassium sulfate,
potassium phosphates, sodium carbonate, sodium sulfite, lithium sulfate,
potassium chloride,
and sodium sulfate; sugars, such as dextrose, fructose, glucose, inositol,
lactose, maltose,
mannitol, raffinose, sorbitol, sucrose, trehalose, and xylitol; organic acids,
such as ascorbic
acid, benzoic acid, fumaric acid, citric acid, maleic acid, sebacic acid,
sorbic acid, adipic acid,
edetic acid, glutamic acid, p-toluenesulfonic acid, succinic acid, and
tartaric acid; urea; and
mixtures thereof.
[00146] Osmotic agents of different dissolution rates can be employed to
influence how
rapidly the active ingredient(s) is initially delivered from the dosage form.
For example,
amorphous sugars, such as MANNOGEM'm EZ (SPI Pharma, Lewes, DE) can be used to
provide faster delivery during the first couple of hours to promptly produce
the desired
-35-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
therapeutic effect, and gradually and continually release of the remaining
amount to maintain
the desired level of therapeutic or prophylactic effect over an extended
period of time. In this
case, the active ingredient(s) is released at such a rate to replace the
amount of the active
ingredient metabolized and excreted.
[00147] The core can also include a wide variety of other excipients and
carriers as
described herein to enhance the performance of the dosage form or to promote
stability or
processing.
[00148] Materials useful in forming the semi-permeable membrane include
various grades
of acrylics, vinyls, ethers, polyamides, polyesters, and cellulosic
derivatives that are water-
permeable and water-insoluble at physiologically relevant pHs, or are
susceptible to being
rendered water-insoluble by chemical alteration, such as crosslinking.
Examples of suitable
polymers useful in forming the coating, include plasticized, unplasticized,
and reinforced
cellulose acetate (CA), cellulose diacetate, cellulose triacetate, CA
propionate, cellulose
nitrate, cellulose acetate butyrate (CAB), CA ethyl carbamate, CAP, CA methyl
carbamate,
CA succinate, cellulose acetate trimellitate (CAT), CA dimethylaminoacetate,
CA ethyl
carbonate, CA chloroacetate, CA ethyl oxalate, CA methyl sulfonate, CA butyl
sulfonate, CA
p-toluene sulfonate, agar acetate, amylose triacetate, beta glucan acetate,
beta glucan
triacetate, acetaldehyde dimethyl acetate, triacetate of locust bean gum,
hydroxylated
ethylene-vinylacetate, EC, PEG, PPG, PEG/PPG copolymers, PVP, HEC, HPC, CMC,
CMEC, HPMC, HPMCP, HPMCAS, HPMCAT, poly(acrylic) acids and esters and poly-
(methacrylic) acids and esters and copolymers thereof, starch, dextran,
dextrin, chitosan,
collagen, gelatin, polyalkenes, polyethers, polysulfones, polyethersulfones,
polystyrenes,
polyvinyl halides, polyvinyl esters and ethers, natural waxes, and synthetic
waxes.
[00149] Semipermeable membrane can also be a hydrophobic microporous membrane,
wherein the pores are substantially filled with a gas and are not wetted by
the aqueous
medium but are permeable to water vapor, as disclosed in U.S. Pat. No.
5,798,119. Such
hydrophobic but water-vapor permeable membrane are typically composed of
hydrophobic
polymers such as polyalkenes, polyethylene, polypropylene,
polytetrafluoroethylene,
polyacrylic acid derivatives, polyethers, polysulfones, polyethersulfones,
polystyrenes,
polyvinyl halides, polyvinylidene fluoride, polyvinyl esters and ethers,
natural waxes, and
synthetic waxes.
[00150] The delivery port(s) on the semi-permeable membrane can be formed post-
coating
by mechanical or laser drilling. Delivery port(s) can also be formed in situ
by erosion of a
-36-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
plug of water-soluble material or by rupture of a thinner portion of the
membrane over an
indentation in the core. In addition, delivery ports can be formed during
coating process, as
in the case of asymmetric membrane coatings of the type disclosed in U.S. Pat.
Nos.
5,612,059 and 5,698,220.
[00151] The total amount of the active ingredient(s) released and the release
rate can
substantially by modulated via the thickness and porosity of the semi-
permeable membrane,
the composition of the core, and the number, size, and position of the
delivery ports.
[00152] The pharmaceutical compositions in an osmotic controlled-release
dosage form
can further comprise additional conventional excipients or carriers as
described herein to
promote performance or processing of the formulation.
[00153] The osmotic controlled-release dosage forms can be prepared according
to
conventional methods and techniques known to those skilled in the art (See,
Remington: The
Science and Practice of Pharmacy, supra; Santus and Baker, J. Controlled
Release 1995, 35,
1-2 1; Verma et al., Drug Development and Industrial Pharmacy 2000, 26, 695-
708; Verma et
al., J. Controlled Release 2002, 79, 7-27).
[00154] In certain embodiments, the pharmaceutical compositions provided
herein are
formulated as AMT controlled-release dosage form, which comprises an
asymmetric osmotic
membrane that coats a core comprising the active ingredient(s) and other
pharmaceutically
acceptable excipients or carriers. See, U.S. Pat. No. 5,612,059 and WO
2002/17918. The
AMT controlled-release dosage forms can be prepared according to conventional
methods
and techniques known to those skilled in the art, including direct
compression, dry
granulation, wet granulation, and a dip-coating method.
[00155] In certain embodiments, the pharmaceutical compositions provided
herein are
formulated as ESC controlled-release dosage form, which comprises an osmotic
membrane
that coats a core comprising the active ingredient(s), a hydroxylethyl
cellulose, and other
pharmaceutically acceptable excipients or carriers.
-37-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
(c) Multiparticulate Controlled Release Devices
[00156] The pharmaceutical compositions provided herein in a modified release
dosage
form can be fabricated as a multiparticulate controlled release device, which
comprises a
multiplicity of particles, granules, or pellets, ranging from about 10 pm to
about 3 mm, about
50 pm to about 2.5 mm, or from about 100 pm to about 1 mm in diameter. Such
multiparticulates can be made by the processes known to those skilled in the
art, including
wet-and dry-granulation, extrusion/spheronization, roller-compaction, melt-
congealing, and
by spray-coating seed cores. See, for example, Multiparticulate Oral Drug
Delivery; Marcel
Dekker: 1994; and Pharmaceutical Pelletization Technology; Marcel Dekker:
1989.
[00157] Other excipients or carriers as described herein can be blended with
the
pharmaceutical compositions to aid in processing and forming the
multiparticulates. The
resulting particles can themselves constitute the multiparticulate device or
can be coated by
various film-forming materials, such as enteric polymers, water-swellable, and
water-soluble
polymers. The multiparticulates can be further processed as a capsule or a
tablet.
(d) Tareeted Delivery
[00158] The pharmaceutical compositions provided herein can also be formulated
to be
targeted to a particular tissue, receptor, or other area of the body of the
subject to be treated,
including liposome-, resealed erythrocyte-, and antibody-based delivery
systems. Examples
include, but are not limited to, those disclosed in U.S. Pat. Nos. 6,316,652;
6,274,552;
6,271,359; 6,253,872; 6,139,865; 6,131,570; 6,120,751; 6,071,495; 6,060,082;
6,048,736;
6,039,975; 6,004,534; 5,985,307; 5,972,366; 5,900,252; 5,840,674; 5,759,542;
and
5,709,874.
E. Methods of Use
[00159] Provided herein is a method for the treatment or prophylaxis of
Duchenne
muscular dystrophy, Becker muscular dystrophy, or cachexia, the method
comprising
administering to a patient in need thereof an effective amount of a compound
of formula (I),
or tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof.
[00160] In one embodiment, the dose of the compound of formula (I) is
determined in
consideration of age, body weight, general health condition, diet,
administration time,
administration method, clearance rate, combination of drugs, the level of
disease for which
-38-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
the patient is under treatment for, and other factors. The dose varies
depending on the target
disease, condition, subject of administration, administration method, and the
like.
[00161] In one embodiment, the pharmaceutical composition comprising a
compound of
formula (I) is administered orally as a therapeutic agent for the treatment of
Duchenne
muscular dystrophy in a patient suffering from such a disease. In one
embodiment, from
about 0.01 mg to about 10 g of the compound is administered. In another
embodiment, from
about 0.1 mg to about 100 mg of the compound is administered. In one
embodiment, the
compound is administered in a single dose per day. In another embodiment, the
compound is
administered in 2 or 3 portions per day.
[00162] In one embodiment, provided herein is a method for the treatment or
prophylaxis
of Duchenne muscular dystrophy or Becker muscular dystrophy, the method
comprising
administering to a patient in need thereof an effective amount of a compound
of formula (I),
or tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof.
[00163] In one embodiment, provided herein is a method of treating,
preventing, and/or
managing a disorder or symptoms related to Duchenne muscular dystrophy, Becker
muscular
dystrophy, or cachexia, the method comprising administering to a patient in
need thereof an
effective amount of a compound of formula (I), or tautomer, enantiomer,
pharmaceutically
acceptable salt, hydrate, solvate, complex, or prodrug thereof.
[00164] In another embodiment, provided herein is a method of treating,
preventing,
and/or managing a disorder or symptoms related to Duchenne muscular dystrophy
or Becker
muscular dystrophy, the method comprising administering to a patient in need
thereof an
effective amount of a compound of formula (I), or tautomer, enantiomer,
pharmaceutically
acceptable salt, hydrate, solvate, complex, or prodrug thereof.
[00165] In one embodiment, provided herein is a method of treating,
preventing, and/or
managing Duchenne muscular dystrophy. In another embodiment, provided herein
is a
method of treating, preventing, and/or managing Becker muscular dystrophy. In
another
embodiment, provided herein is a method of treating, preventing, and/or
managing cachexia.
[00166] In one embodiment, provided herein is the use of a compound of formula
(I), or
tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or prodrug
thereof, in the manufacturing of a medicament for the treatment of Duchenne
muscular
dystrophy, Becker muscular dystrophy, or cachexia. In another embodiment,
provided herein
is the use of a compound of formula (I), or tautomer, enantiomer,
pharmaceutically
-39-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
acceptable salt, hydrate, solvate, complex, or prodrug thereof, in the
manufacturing of a
medicament for the treatment of Duchenne muscular dystrophy or Becker muscular
dystrophy. In another embodiment, provided herein is the use of a compound of
formula (1),
or tautomer, enantiomer, pharmaceutically acceptable salt, hydrate, solvate,
complex, or
prodrug thereof, in the manufacturing of a medicament for the treatment of
Duchenne
muscular dystrophy. In another embodiment, provided herein is the use of a
compound of
formula (I), or tautomer, enantiomer, pharmaceutically acceptable salt,
hydrate, solvate,
complex, or prodrug thereof, in the manufacturing of a medicament for the
treatment of
Becker muscular dystrophy. In another embodiment, provided herein is the use
of a
compound of formula (I), or tautomer, enantiomer, pharmaceutically acceptable
salt, hydrate,
solvate, complex, or prodrug thereof, in the manufacturing of a medicament for
the treatment
of cachexia.
[00167] In one embodiment, the method comprises administering to a subject
(e.g., a
human) a therapeutically or prophylactically effective amount of a composition
of a
compound of formula (I). In one embodiment, the subject is a human. In another
embodiment, the subject is a mammal. In yet another embodiment, the subject is
a non-
human primate, a farm animal, such as cattle, a sport anirrial, or a pet such
as a horse, dog, or
cat.
[00168] In some embodiment, compound activity can be assessed by functional
assays
described herein elsewhere. In certain embodiments, the efficacious
concentration of the
compounds provided herein is less than 0.1 nM, less than 1 nM, less than 10
nM, less than
100 nM, less than 1 gM, less than 10 M, less than 100 gM, or less than 1 mM.
In other
embodiments, compounds' activity may be assessed in various art-recognized
animal models
as described herein elsewhere.
[00169] In some embodiments, the compounds provided herein are active in at
least one
model, which can be used to measure the activity of the compounds and estimate
their
efficacy in treating Duchenne muscular dystrophy, Becker muscular dystrophy,
or cachexia.
For example, when the model is for Duchenne muscular dystrophy, the compounds
are active
in, for example the mdx mouse model, when compared to vehicle. In some
embodiments, the
compounds provided herein are active in a dose-dependent manner. In some
embodiments,
the compounds provided herein produce a similar disparity in measured endpoint
between
treated animals and animals treated with vehicle.
-40-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
[00170] Depending on the disorder, disease, or condition to be treated, and
the subject's
condition, the compounds or pharmaceutical compositions provided herein can be
administered by oral, parenteral (e.g., intramuscular, intraperitoneal,
intravenous, ICV,
intracistemal injection or infusion, subcutaneous injection, or implant),
inhalation, nasal,
vaginal, rectal, sublingual, or topical (e.g., transdermal or local) routes of
administration and
can be formulated, alone or together, in suitable dosage unit with
pharmaceutically acceptable
excipients, carriers, adjuvants, and vehicles appropriate for each route of
administration.
Also provided is administration of the compounds or pharmaceutical
compositions provided
herein in a depot formulation, in which the active ingredient is released over
a predefined
time period.
[00171] In the treatment, prevention, or amelioration of one or more symptoms
of the
disorders, diseases, or conditions described herein, an appropriate dosage
level generally is
ranging from about 0.001 to about 1000 mg per kg subject body weight per day
(mg/kg per
day), from about 0.001 to about 300 mg/kg per day, from about 0.001 to about
100 mg/kg per
day, from about 0.01 to about 75 mg/kg per day, from about 0.1 to about 50
mg/kg per day,
from about 0.5 to about 25 mg/kg per day, or from about 1 to about 20 mg/kg
per day, which
can be administered in single or multiple doses. Within this range, the dosage
can be ranging
from about 0.005 to about 0.05, from about 0.05 to about 0.5, from about 0.5
to about 5.0,
from about I to about 15, from about 1 to about 20, or from about 1 to about
50 mg/kg per
day.
[00172) In one embodiment, in the treatment, prevention, or amelioration of
one or more
symptoms of Duchenne muscular dystrophy, Becker muscular dystrophy, or
cachexia, an
appropriate dosage level is less than 0.001 mg/kg per day, less than 0.01
mg/kg per day, less
than 0.1 mg/kg per day, less than 0.5 mg/kg per day, less than 1 mg/kg per
day, less than 5
mg/kg per day, less than 10 mg/kg per day, less than 15 mg/kg per day, less
than 20 mg/kg
per day, less than 25 mg/kg per day, less than 50 mg/kg per day, less than 75
mg/kg per day,
less than 100 mg/kg per day, less than 200 mg/kg per day, less than 500 mg/kg
per day, or
less than 1 g/kg per day.
[00173] For oral administration, the pharmaceutical compositions provided
herein can be
formulated in the form of tablets containing from about 1.0 to about 1,000 mg
of the active
ingredient, in one embodiment, about 1, about 5, about 10, about 15, about 20,
about 25,
about 50, about 75, about 100, about 150, about 200, about 250, about 300,
about 400, about
500, about 600, about 750, about 800, about 900, and about 1,000 mg of the
active ingredient
-41-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
for the symptomatic adjustment of the dosage to the patient to be treated. The
pharmaceutical
compositions can be administered on a regimen of 1 to 4 times per day,
including once,
twice, three times, and four times per day.
[00174] It will be understood, however, that the specific dose level and
frequency of
dosage for any particular patient can be varied and will depend upon a variety
of factors
including the activity of the specific compound employed, the metabolic
stability and length
of action of that compound, the age, body weight, general health, sex, diet,
mode and time of
administration, rate of excretion, drug combination, the severity of the
particular condition,
and the host undergoing therapy.
[00175] In another embodiment, the compounds provided herein, e.g., a compound
of
formula (I), or tautomer, enantiomer, pharmaceutically acceptable salt,
hydrate, solvate,
complex, or prodrug thereof, can be combined or used in combination with other
agents or
therapies useful in the treatment, prevention, or amelioration of Duchenne
muscular
dystrophy, Becker muscular dystrophy, or cachexia. Suitable other therapeutic
agents
include, but are not limited to, corticosteroids, such as for example,
prednisone and
deflazacort. In certain embodiments, the other therapies that may be used in
combination
with the compounds provided herein include, but are not limited to, physical
therapy, gene
therapy, or orthopedic appliances, such as braces and wheelchairs.
[00176] Such other agents, or drugs, can be administered, by a route and in an
amount
commonly used therefor, simultaneously or sequentially with the compounds
provided
herein, or tautomer, enantiomer, pharmaceutically acceptable salt, hydrate,
solvate, complex,
or prodrug thereof. When a compound provided herein is used contemporaneously
with one
or more other drugs, a pharmaceutical composition containing such other drugs
in addition to
the compound provided herein can be utilized, but is not required.
Accordingly, the
pharmaceutical compositions provided herein include those that also contain
one or more
other active ingredients or therapeutic agents, in addition to a compound
provided herein.
[00177] The weight ratio of a compound provided herein to the second active
ingredient
can be varied, and will depend upon the effective dose of each ingredient.
Generally, an
effective dose of each will be used. Thus, for example, when a compound
provided herein is
combined with a corticosteroid, the weight ratio of the compound to the
corticosteroid can
range from about 1,000:1 to about 1:1,000, about 200:1 to about 1:200, about
100:1 to about
1:100, or about 10:1 to about 1:10. Combinations of a compound provided herein
and other
-42-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
active ingredients will generally also be within the aforementioned range, but
in each case, an
effective dose of each active ingredient should be used.
[00178] The compounds provided herein can also be provided as an article of
manufacture
using packaging materials well known to those of skill in the art. See, e.g.,
U.S. Pat. Nos.
5,323,907; 5,052,558; and 5,033,252. Examples of pharmaceutical packaging
materials
include, but are not limited to, blister packs, bottles, tubes, inhalers,
pumps, bags, vials,
containers, syringes, and any packaging material suitable for a selected
formulation and
intended mode of administration and treatment.
[00179] Provided herein also are kits which, when used by the medical
practitioner, can
simplify the administration of appropriate amounts of active ingredients to a
subject. In
certain embodiments, the kit provided herein includes a container and a dosage
form of a
compound provided herein, .
[00180] In certain embodiments, the kit includes a container comprising a
dosage form of
the compound provided herein, or tautomer, enantiomer, pharmaceutically
acceptable salt,
hydrate, solvate, complex, or prodrug thereof, in a container comprising one
or more other
therapeutic agent(s) described herein.
[00181] Kits provided herein can further include devices that are used to
administer the
active ingredients. Examples of such devices include, but are not limited to,
syringes, needle-
less injectors drip bags, patches, and inhalers.
[00182] Kits provided herein can further include pharmaceutically acceptable
vehicles that
can be used to administer one or more active ingredients. For example, if an
active ingredient
is provided in a solid form that must be reconstituted for parenteral
administration, the kit can
comprise a sealed container of a suitable vehicle in which the active
ingredient can be
dissolved to form a particulate-free sterile solution that is suitable for
parenteral
administration. Examples of pharmaceutically acceptable vehicles include, but
are not
limited to: aqueous vehicles, including, but not limited to, Water for
Injection USP, Sodium
Chloride Injection, Ringer's Injection, Dextrose Injection, Dextrose and
Sodium Chloride
Injection, and Lactated Ringer's Injection; water-miscible vehicles,
including, but not limited
to, ethyl alcohol, polyethylene glycol, and polypropylene glycol; and non-
aqueous vehicles,
including, but not limited to, corn oil, cottonseed oil, peanut oil, sesame
oil, ethyl oleate,
isopropyl myristate, and benzyl benzoate.
- 43 -
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
EXAMPLES
[00183] Certain embodiments are illustrated by the following non-limiting
examples.
[00184] HPLC-UV-MS was performed on a Gilson 321 HPLC with detection performed
by a Gilson 170 DAD and a Finnigan AQA mass spectrometer operating in
electrospray
ionisation mode. The HPLC column used is a Phenomenex Gemini C18 150x4.6mm.
[00185] Preparative HPLC was performed on a Gilson 321 with detection
performed by a
Gilson 170 DAD. Fractions were collected using a Gilson 215 fraction collector
or a Dionex
Ultimate 3000 system. The preparative HPLC column used is a Phenomenex Gemini
C 18
150 x 10 mm and the mobile phase is acetonitrile/water.
[00186] 'H NMR spectra were recorded on a Bruker instrument operating at 300
MHz.
NMR spectra were obtained as CDC13 solutions (reported in ppm), using
chloroform as the
reference standard (7.25 ppm) or DMSO-D6 (2.50 ppm). When peak multiplicities
are
reported, the following abbreviations are used s (singlet), d (doublet), t
(triplet), m
(multiplet), br (broadened), dd (doublet of doublets), dt (doublet of
triplets), td (triplet of
doublets). Coupling constants, when given, are reported in Hertz (Hz).
[00187] Column chromatography was performed either by flash chromatography (40-
65 m silica gel) or using an automated purification system (SPITM
Purification System from
Biotage or an ISCO Companion). Reactions in the microwave were done in an
Initiator
8T'" (Biotage) or an Explorer 48 (CEM).
[00188] The abbreviations used are DMSO (dimethylsulfoxide), HATU (O-(7-
azabenzotriazol-1 yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate), HCI
(hydrochloric acid), MgSO4 (magnesium sulfate), NaOH (sodium hydroxide),
Na2CO3
(sodium carbonate), NaHCO3 (sodium bicarbonate), STAB (sodium
triacetoxyborohydride),
THF (tetrahydrofuran).
Example 1
Preparation of 5-benzyl-2-phenyl-5H-imidazo[4,5-cluyridine
(Compound 1)
A. 2-Phenyl-5H-imidazo[4,5-c]pyridine
[00189] A mixture of 3,4-diaminopyridine (4.0 g, 3.7 mmol), benzoic acid
(4.477 g, 3.7
mmol) and polyphosphoric acid (100 g) was heated at 190 C for 3 hours with
stirring. The
reaction mixture was allowed to cool to 25 C and poured into ice/water and the
mixture
neutralised by the addition of solid Na2CO3. The crude product was collected
by filtration
-44-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
and washed with water to give the final product (3.467 g, 17.76 mmol, 48%
yield). 'H NMR
(DMSO) 13.34 (1H, s), 8.95 (1H, s), 8.32 (1H, d, J=5.6Hz), 8.21-8.24 (2H, m),
7.53-7.62
(4H, m). LCMS RT = 4.06 mins; MH+ 196.2.
B. 5-Benzyl-2-phenyl-5H-imidazo[4,5-c]pyridine (Compound 1)
[00190] 2-Phenyl-5H-imidazo[4,5-c]pyridine (240 mg, 1.23 mmol) was dissolved
in DMF
(5 mL) and sodium hydroxide (75 mg, 1.85 mmol) dissolved in water (75 L) was
added and
the mixture stirred for 20 minutes. Benzyl bromide (176 L, 1.48 mmol) was
added and the
reaction mixture stirred at ambient temperature for 16 hours. The reaction
mixture was
diluted with water and extracted with dichloromethane. The organic extract was
reduced in
vacuo to give the crude product which was recrystallised from ethyl acetate
and 40-60
petroleum ether to give the title compound (109 mg, 0.38 mmol, 31% yield). 'H
NMR
(DMSO) 9.11 (1 H, s), 8.34-8.38 (2H, m), 8.18 (1 H, dd, J=6.7, 1.6Hz), 7.73 (1
H, d,
J=7.OHz), 7.33-7.50 (8H, m), 5.66 (2H, s). LCMS RT = 4.43 mins; MH+ 286.2.
Example 2
Preparation of 2-(2,3-dichlorophenyl)-5-isonronyl-5H-imidazo[4,5- clpyridine
(Compound 13)
A. 2-(2,3-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine
[00191] A mixture of 3,4-diaminopyridine (2.0 g, 18.35 mmol), 2,3-
dichlorobenzoic acid
(3.505 g, 18.35 nunol) and polyphosphoric acid (48 g) was heated at 190 C for
3 hours with
stirring. The reaction mixture was allowed to cool to 25 C and poured into
ice/water and the
mixture neutralised by the addition of solid Na2CO3. The crude product was
collected by
filtration and washed with water to give the title compound (3.317 g, 12.56
mmol, 70%
yield). 'H N1VIR (DMSO) 13.32 (1H, s), 9.01 (1H, s), 8.36 (1H, d, J=5.5Hz),
8.74-8.79 (2H,
m), 7.64 (1H, s), 7.57 (1H, t, J=7.8Hz).
B. 2-(2,3-dichlorophenyl)-5-isopropyl-5H-imidazo[4,5-c]pyridine (Compound 13)
[00192] 2-(2,3-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (320 mg, 1.21 mmol)
was
dissolved in DMF (8 mL) and sodium hydroxide (291 mg, 7.27 mmol) dissolved in
water (0.4
mL) was added and the mixture stirred for 20 minutes. 2-lodopropane (723 L,
7.27 mmol)
was added and the reaction mixture stirred at 25 C for 16 hours. Water (70 mL)
and ethyl
acetate (70 mL) were added and the organic extract was washed with water (2 x
60 mL) and
brine (60 mL), dried and concentrated in vacuo to give a crude solid.
Recrystallisation from
- 45 -
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
ethyl acetate and petroleum ether gave the title compound (83 mg, 0.27 mmol,
22% yield).
'H NMR (DMSO) 9.24 (1 H, s), 8.27 (IH, d, J=6.3Hz), 7.95 (1 H, d, J=7.6Hz),
7.81 (1 H, d,
J=6.7Hz) 7.70-7.73 (IH, m), 7.46 (1 H, t, J=7.9Hz), 4.88 (1 H, quintet), 1.61
(6H, d,
J=6.6Hz). LCMS RT = 1.56 mins; MH+ 306.1.
Example 3
Preparation of 2-(2,3-dichlorophenyl)-5-propyl-5H-imidazo[4,5-clpyridine
(Compound 15)
[00193] 2-(2,3-Dichlorophenyl)-SH-imidazo[4,5-c]pyridine (320 mg, 1.21 mmol)
was
dissolved in DMF (8 mL) and sodium hydroxide (291 mg, 7.27 mmol) dissolved in
water (0.4
mL) was added and the mixture stirred for 20 minutes. 1-Bromopropane (660 L,
7.27
mmol) was added and the reaction mixture stirred at 25 C for 16 hours. Water
(70 mL) and
ethyl acetate (70 mL) were added and the organic extract was washed with water
(2 x 60 mL)
and brine (60 mL), dried and concentrated in vacuo to give an oil.
Recrystallisation from
ethyl acetate and petroleum ether gave the desired product (78 mg, 0.25 mmol,
21 % yield).
'H NMR (DMSO) 9.10 (1H, s), 8.15 (1H, dd, J=6.8Hz, 1.6Hz), 7.96 (1H, dd,
J=7.7Hz,
1.6Hz), 7.80 (IH, d, J=6.8Hz) 7.70 (IH, dd, J=7.9Hz, 1.8Hz), 7.46 (1H, t,
J=7.9Hz), 4.43
(2H, t, J=7.2Hz), 1.88-2.00 (2H, m), 0.88 (3H, t, J=7.5Hz). LCMS RT = 1.59
mins; MH+
306.1.
Example 4
Preparation of 5-cyclopentyl-2-(2,3-dichlorophenyl)-5H-imidazo[4,5-clpyridine
(Compound 14)
[00194] 2-(2,3-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (320 mg, 1.21 mmol)
was
dissolved in DMF (8 mL) and sodium hydroxide (291 mg, 7.27 mmol) dissolved in
water (0.4
mL) was added and the mixture stirred for 20 minutes. Cyclopentyl iodide (841
L, 7.27
mmol) was added and the reaction mixture stirred at 25 C for 16 hours. Water
(70 mL) and
ethyl acetate (70 mL) were added and the organic extract was washed with water
(2 x 60 mL)
and brine (60 mL), dried and concentrated in vacuo to give a crude solid.
Recrystallisation
from ethyl acetate and petroleum ether gave the desired product (182 mg, 0.55
mmol, 45%
yield). 'H NMR (DMSO) 9.18 (1 H, d, J=1.4 Hz), 8.23 (IH, dd, J=7.OHz, 1.7Hz),
7.96 (1 H,
dd, J=7.8Hz, 1.7Hz), 7.80 (1 H, d, J=6.9Hz) 7.71 (1 H, dd, J=1.6Hz, 8.OHz),
7.45 (1 H, t,
J=7.8Hz), 5.01 (1H, t, J=7.9Hz), 2.27-2.39 (2H, m), 1.92-2.07 (4H, m), 1.69-
1.76 (2H, m).
LCMS RT =-1.63 mins; MH+ 332.1.
-46-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
Example 5
Preparation of 2-(3,4-dichlorophenyl)-5-methyl-5H-imidazof4,5-clpyridine
(Compound 10)
A. 2-(3,4-Dichlorophenyl)-SH-imidazo[4,5-c]pyridine
[00195] A mixture of 3,4-diaminopyridine (2.5 g, 22.94 mmol), 3,4-
dichlorobenzoic acid
(4.374 g, 22.90 mmol) and polyphosphoric acid (60 g) was heated at 190 C for 3
hours with
stirring. The reaction mixture was allowed to cool to 25 C and poured into
ice/water and the
mixture neutralised by the addition of solid Na2CO3. The crude product was
collected by
filtration and washed with water to give the desired product (3.994 g, 15.12
mmol, 66%
yield). ' H NMR (DMSO) 8.96 (1 H, d, J=1.OHz), 8.44 (1 H, d, J=2.OHz), 8.30 (1
H, d,
J=5.6Hz), 8.20 (1H, dd, J=8.4, 2.1Hz), 7.86 (1H, d, J=8.4Hz), 7.62 (1H, dd,
J=5.6, 1.1Hz).
B. 2-(3,4-Dichlorophenyl)-5-methyl-5H-imidazo[4,5-c]pyridine (Compound 10)
[00196] 2-(3,4-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (320 mg, 1.21 mmol)
was
dissolved in DMF (8 mL) and sodium hydroxide (291 mg, 7.27 mmol) dissolved in
water (0.4
mL) was added and the mixture stirred for 20 minutes. Methyl iodide (454 L,
7.27 mmol)
was added and the reaction mixture stirred at 25 C for 16 hours. Water (70 mL)
and ethyl
acetate (70 mL) were added and the organic extract was washed with water (2 x
60 mL) and
brine (60 mL), dried and concentrated in vacuo to give a crude solid.
Recrystallisation from
ethyl acetate and petroleum ether gave the title compound (155 mg, 0.56 mmol,
46% yield).
'H NMR (DMSO) 8.95 (1 H, d, J=1.3Hz), 8.48 (1 H, d, J=1.9Hz), 8.30 (1 H, dd,
J=1.9Hz,
8.3Hz), 8.05 (1H, dd, J=1.5Hz, 6.8Hz), 7.72-7.76 (2H, m) 4.23 (3H, s). LCMS RT
= 3.98
mins; MH+ 278Ø
Example 6
Preparation of 2-(3,4-dichlorophenyl)-5-propyl-5H-imidazo[4,5-clpyridine -
(Compound 9)
[00197] 2-(3,4-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (320 mg, 1.21 mmol)
was
dissolved in DMF (8 mL) and sodium hydroxide (291 mg, 7.27 mmol) dissolved in
water (0.4
mL) was added and the mixture stirred for 20 minutes. 1-Bromopropane (661 L,
7.27
mmol) was added and the reaction mixture stirred at 25 C for 16 hours. Water
(70 mL) and
ethyl acetate (70 mL) were added and the organic extract was washed with water
(2 x 60 mL)
and brine (60 mL), dried and concentrated in vacuo to give a crude solid.
Recrystallisation
-47-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
from ethyl acetate and petroleum ether gave the desired compound (142 mg, 0.46
mmol, 38%
yield). 'H NMR (DMSO) 9.03 (1 H, d, J=1.OHz), 8.48 (1 H, d, J=1.8Hz), 8.30 (1
H, dd,
J=2.OHz, 8.4Hz), 8.13 (1H, dd, J=1.5Hz, 6.7Hz) 7.73-7.77 (2H, m), 4.42 (2H, t,
J=7.OHz), 1.92 (2H, sextet, J=7.3), 0.87 (3H, t, J=7.4Hz). LCMS RT = 4.02
mins; MH+
306.1.
Example 7
Preparation of 2-(3,4-dichlorophenyl)-5-isopropyl-5H-imidazof4,5-clpyridine
(Compound 8)
[00198] 2-(3,4-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (320 mg, 1.21 mmol)
was
dissolved in DMF (8 mL) and sodium hydroxide (291 mg, 7.27 mmol) dissolved in
water (0.4
mL) was added and the mixture stirred for 20 minutes. 2-lodopropane (732 L,
7.27 mmol)
was added and the reaction mixture stirred at 25 C for 16 hours. Water (70 mL)
and ethyl
acetate (70 mL) were added and the organic extract was washed with water (2 x
60 mL) and
brine (60 mL), dried and concentrated in vacuo to give a crude solid.
Recrystallisation from
ethyl acetate and petroleum ether gave the desired product (236 mg, 0.77 mmol,
64% yield).
'H NMR (DMSO) 9.14 (1 H, d, J=1.3Hz), 8.48 (1 H, d, J=1. 8Hz), 8.30 (1 H, dd,
J=2.OHz,
8.4Hz), 8.23 (1H, dd, J=1.6Hz, 7.0Hz), 7.73-7.77 (2H, m), 4.80-4.93 (1H,
septet), 1.60 (6H,
d, J=6.9Hz). LCMS RT = 4.01 mins; MH+ 306.1.
Example 8
Preparation of 5-cyclopentyl-2-(3,4-dichlorophenyl)-5H-imidazo[4,5-clpyridine
(Compound 12)
[00199] 2-(3,4-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (260 mg, 0.98 mmol)
was
dissolved in DMF (7 mL) and sodium hydroxide (236 mg, 5.91 mmol) dissolved in
water
(0.35 mL) was added and the mixture stirred for 20 minutes. Cyclopentyl iodide
(684 L,
5.91 mmol) was added and the reaction mixture stirred at 25 C for 16 hours.
Water (70 mL)
and ethyl acetate (70 mL) were added and the organic extract was washed with
water (2 x 60
mL) and brine (60 mL), dried and concentrated in vacuo to give a crude solid.
Recrystallisation from ethyl acetate and petroleum ether gave the title
compound(57 mg, 0.17
mmol, 18% yield). 'H NMR (DMSO) 9.10 (IH, s), 8.48 (IH, d, J=1.5Hz), 8.30 (1H,
dd,
J=1.5Hz, 8.3Hz), 8.20 (IH, d, J=6.8Hz), 7.73-7.76 (2H, m), 4.94-5.05 (1H, m),
2.30-2.34
(2H, m), 1.95-2.05 (4H, m), 1.70-1.74 (2H, m). LCMS RT = 1.70 mins; MH+ 332.1.
-48-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
Example 9
Preparation of 2-(3,4-dichlorophenyl)-5-(3,3,3-trifluoropropyl)-5H-imidazo[4,5-
clpyridine (Compound 16)
[00200] 2-(3,4-Dichlorophenyl)-5H-imidazo[4,5-c]pyridine (1.60 g, 6.06 mmol)
was
dissolved in DMF (50 mL) and 60% sodium hydride in mineral oil (631 mg, 15.8
mmol) was
added and the mixture stirred for 30 minutes. 1-Iodo-3,3,3- trifluoropropane
(3.55 mL, 30.3
mmol) was added and the reaction mixture stirred at 100 C for 16 hours. Then
additional 1-
iodo-3,3,3-trifluoropropane (355 L, 3.0 mmol) was added and the mixture
stirred at 120 C
for 2 hours. The reaction mixture was diluted with ethyl acetate (140 mL) and
the precipitate
removed by filtration before washing with water (2 x 100 mL). The organic
extract was dried
over magnesium sulfate and concentrated in vacuo to give the crude product
(1.256 g). The
crude product was purified by column chromatography using a 5-10% gradient of
methanol
in dichloromethane to a product (661 mg) which contained residual
dimethylformamide.
This was then dissolved in ethyl acetate (100 mL) and washed with water (5 x
50 mL), then
brine (50 mL), dried over magnesium sulfate and concentrated in vacuo to give
a product
(532 mg) containing a small amount of impurities. This was then purified by
column
chromatography using an isocratic solvent system of 5% methanol in
dichloromethane to give
the title compound (265 mg, 0.74 mmol, 12% yield). 'H NMR (DMSO) 9.10 (1H, s),
8.48
(1 H, d, J=1. 8Hz), 8.30 (1 H, dd, J=1.8Hz, 8.3Hz), 8.20 (1 H, dd, J=1,4,
6.9Hz), 7.74-7.79
(2H, m), 4.76 (2H, t, J=6.8Hz), 3.05-3.21 (2H, m). LCMS RT = 1.61 mins; MH+
359.9.
Example 10
Preparation of 5-methyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-clpyridine
(Compound 3)
A. 2-(Naphthalen-2-yl)-5H-imidazo [4,5-c]pyridine
[00201] A mixture of 3,4-diaminopyridine (6.0 g, 5.5 mmol), 2-napthoic acid
(9.479 g, 5.5
mmol) and polyphosphoric acid (150 g) was heated at 190 C for 3 hours with
stirring. The
reaction mixture was allowed to cool to 25 C and poured into ice/water and the
mixture
neutralised by the addition of solid Na2CO3. The crude product was collected
by filtration
and washed with water to give the title compound (5.523 g, 0.023 mol, 41%
yield). 'H NMR
(DMSO) 8.98 (1H, s), 8.83 (1H, s), 8.30-8.38 (2H, m), 7.99-8.12 (3H, m),
7.60-7.64 (3H, m). LCMS RT = 4.36 mins; MH+ 246.2.
-49-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
B. 5-Methyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine
[00202] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (240 mg, 0.98 mmol) was
dissolved in DMF (8 mL) and sodium hydroxide (118 mg, 2.94 mmol) dissolved in
water
(0.3 mL) was added and the mixture stirred for 20 minutes. Methyl iodide (185
L, 2.94
mmol) was added and the reaction mixture stirred at ambient temperature for 16
hours.
Water (25 mL) was added and the precipitate which formed was filtered off and
washed with
water. The crude product was dried by 16 hours in a vacuum oven and then
recrystallised
from ethyl acetate and petroleum ether to give the title compound (65.2 mg,
0.25 mmol, 26%
yield). 'H NMR (DMSO) 8.91 (2H, m), 8.51 (1H, dd, J=8.6, 1.6Hz), 7.93-8.08
(4H, m), 7.73
(1 H, d, J=6.6Hz), 7.51-7.57 (2H, m), 4.23 (3H, s). LCMS RT = 4.41 mins; MH+
260.1.
Example 11
Preparation of 5-ethyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-clpyridine
(Compound 2)
[00203] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (240 mg, 0.98 mmol) was
dissolved in DMF (8 mL) and sodium hydroxide (59 mg, 1.47 mmol) dissolved in
water (0.15
mL) was added and the mixture stirred for 20 minutes. Ethyl bromide (88 }IL,
1.17 mmol)
was added and the reaction mixture stirred at ambient temperature for 16
hours. Water (25
mL) and ethyl acetate (25 mL) were added and the organic extract was washed
with water (30
mL) and brine (30 mL), dried and concentrated in vacuo to give the crude
solid.
Recrystallisation from ethyl acetate and petroleum ether gave the title
compound (72 mg,
0.26 mmol, 27% yield). ' H NMR (DMSO) 9.00 (1 H, d, J=1.2Hz), 8.93 (1 H, s),
8.53 (1 H,
dd, J=1.5Hz, 8.5Hz), 7.93-8.14 (4H, m), 7.75 (1H, d, J=6.7Hz), 7.51-7.58 (2H,
m), 4.49 (2H,
q, J=7.3Hz), 1.53 (3H, t, J=7.3Hz). LCMS RT = 4.42 mins; MH+ 274.2.
Example 12
Preparation of 2-(naphthalen-2-yl)-5-propyl-5H-imidazo[4,5-clpyridine
(Compound 4)
[00204] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (400 mg, 1.62 mmol) was
dissolved in DMF (6 mL) and sodium hydroxide (194 mg, 4.86 mmol) dissolved in
water
(0.25 mL) was added and the mixture stirred for 30 minutes. 1-Bromopropane
(442 L, 4.86
mmol) was added and the reaction mixture stirred at room temperature for 16
hours. Water
(30 mL) and ethyl acetate (30 mL) were added and the organic extract was
washed with water
(30 mL) and brine (30 mL), dried and concentrated in vacuo to give the crude
solid.
-50-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
Recrystallisation from chloroform and petroleum ether gave the title compound
(28 mg, 0.10
mmol, 6% yield). ' H NMR (DMSO) 8.99 (1 H, d, J=1.2Hz), 8.93 (1 H, s), 8.51 (1
H, dd,
J=1.5Hz, 8.5Hz), 7.93-8.12 (4H, m), 7.74 (1H, d, J=6.7Hz), 7.53-7.56 (2H, m),
4.42 (2H, t,
J=7.OHz), 1.87-2.00 (m, 2H), 0.89 (3H, t, J=7.3Hz). LCMS RT = 4.51 mins; MH+
288.2.
Example 13
Preparation of 5-butyl-2-(naphthalen-2-yl)-5H-imidazof4,5-clpyridine
(Compound 5)
[00205] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (400 mg, 1.62 mmol) was
dissolved in DMF (6 mL) and sodium hydroxide (194 mg, 4.86 mmol) dissolved in
water
(0.25 mL) was added and the mixture stirred for 30 minutes. 1-Bromobutane (522
L, 4.86
mmol) was added and the reaction mixture stirred at room temperature for 16
hours. Water
(30 mL) was added and the precipitate which formed was filtered and washed
with water (50
mL). The crude product was dried in a vacuum oven and then recrystallised from
chloroform
and petroleum ether to give the title compound (148 mg, 0.49 mmol, 30% yield).
'H NMR
(DMSO) 9.00 (1H, d, J=1.2Hz), 8.93 (1H, s), 8.52 (1H, dd, J=1.5Hz, 8.5Hz),
7.93-8.13 (4H,
m), 7.74 (IH, d, J=6.7Hz), 7.53-7.56 (2H, m), 4.45 (2H, t, J=7.OHz), 1.89 (2H,
quintet, J=
7.2Hz) 1.72 (2H, sextet, J=7.2Hz), 0.91 (3H, t, J=7.3Hz). LCMS RT = 4.62 mins;
MH+
302.2.
Example 14
Preparation of 5-benzyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-clnyridine
(Compound 6)
[00206] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (1.0 g, 4.06 mmol) was
dissolved
in DMF (8 mL) and sodium hydroxide (487 mg, 12.18 mmol) dissolved in water
(0.60 mL)
was added and the mixture stirred for 30 minutes. Benzyl bromide (1446 L,
12.18 mmol)
was added and the reaction mixture stirred at room temperature for 16 hours.
Water (30 mL)
and ethyl acetate (30 mL) were added and the organic extract was washed with
water (30 mL)
and brine (30 mL), dried and concentrated in vacuo to give the crude solid.
Recrystallisation
from chloroform and petroleum ether gave the title compound (20 mg, 0.06 mmol,
2% yield).
'H NMR (DMSO) 9.08 (1H, d, J=1.2Hz), 8.86 (1H, s), 8.44 (1H, dd, J=1.5Hz,
8.5Hz), 8.14
(1 H, dd, J=1.6Hz, 6.8Hz) 7.86-8.01 (3H, m), 7.70 (1 H, d, J=6.7Hz), 7.30-7.50
(7H, m), 5.62
(2H, s). LCMS RT = 4.58 mins; MH+ 336.2.
-51-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
Example 15
Preparation of 5-isoprouyl-2-(nauhthalen-2-yl)-5H-imidazo[4,5-clpyridine
(Compound 7)
[00207] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (600 mg, 2.43 mmol) was
dissolved in DMF (8 mL) and sodium hydroxide (292 mg, 7.29 mmol) dissolved in
water
(0.35 mL) was added and the mixture stirred for 30 minutes. 2-lodopropane (725
gL, 7.29
mmol) was added and the reaction mixture stirred at room temperature for 16
hours. Water
(80 mL) and ethyl acetate (80 mL) were added and the organic extract was
washed with water
(2 x 80 mL) and brine (80 mL), dried and concentrated in vacuo to give the
crude solid.
Recrystallisation from chloroform and petroleum ether gave the title compound
(100 mg,
0.35 mmol, 14% yield). 'H NMR (DMSO) 9.10 (1 H, d, J=1.2Hz), 8.93 (1 H, s),
8.51 (1 H,
dd, J=1.5Hz, 8.5Hz), 8.21 (1H, dd, J=1.6Hz, 6.8Hz) 7.93-8.08 (3H, m), 7.74
(1H, d,
J=6.7Hz), 7.53-7.56 (2H, m), 4.82-4.91 (1H, s), 1.62 (6H, d, J=7.lHz). LCMS RT
= 4.48
mins; MH+ 288.2.
Example 16
Preparation of 5-cvclopentyl-2-(naphthalen-2-yl)-5H-imidazo[4,5-clnyridine
(Compound 11)
[00208] 2-(Naphthalen-2-yl)-5H-imidazo[4,5-c]pyridine (260 mg, 1.06 mmol) was
dissolved in DMF (7 mL) and sodium hydroxide (254 mg, 6.34 mmol) dissolved in
water
(0.35 mL) was added and the mixture stirred for 30 minutes. Cyclopentyl iodide
(733 L,
6.34 mmol) was added and the reaction mixture stirred at room temperature for
16 hours.
Water (60 mL) and ethyl acetate (60 mL) were added and the organic extract was
washed
with water (2 x 60 mL) and brine (60 mL), dried and concentrated in vacuo to
give a crude
solid. Recrystallisation from ethyl acetate and petroleum ether gave the
desired product
(111.5 mg, 0.36 mmol, 34% yield). 1H NMR (DMSO) 9.05 (1H, d, J=1.5Hz), 8.93
(1H, s),
8.51 (1 H, dd, J=1.6Hz, 6.9Hz), 8.18 (1 H, dd, J=1.6Hz, 6.8Hz) 7.93-8.08 (3H,
m), 7.73 (1 H,
d, J=6.7Hz), 7.53-7.56 (2H, m), 4.95-5.05 (1H, m), 2.26-2.39 (2H, m), 1.94-
2.09 (4H, m),
1.71-1.75 (2H, m). LCMS RT = 4.04 mins; MH+ 314.2.
Example 17
[00209] The potential activity of the compounds of formula (I) for use in the
treatment of
DMD may be demonstrated in the following predictive assay.
-52-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
Luciferase reporter assay (murine H2K cells)
[00210] The cell line used for the screen is an immortalized mdx mouse H2K
cell line that
has been stably transfected with a plasmid containing = 5kb fragment of the
Utrophin A
promoter including the first untranslated exon linked to a luciferase reporter
gene.
[00211] Under conditions of low temperature and interferon containing media,
the cells
remain as myoblasts. These are plated into 96 well plates and cultured in the
presence of
compound for three days. The level of luciferase is then determined by cell
lysis and reading
of the light output from the expressed luciferase gene utilising a plate
luminometer.
Luciferase Assay for 96 Well Plates
[00212] The H2K/mdx/Utro A reporter cell line cells were plated out into 96
well plates
(Falcon 353296, white opaque) at a density of approximately 5000 cells/well in
190 l
normal growth medium. The plates were then incubated at 33 C in the presence
of 10% COz
for 24 hrs.
[00213] Compounds were dosed by adding 10 l of diluted compound to each well
giving
a final concentration of 10 M (where a different final concentration was
required, the
amount of compound solution added was amended accordingly). The plates were
then
incubated for a further 48 hrs. Cells were then lysed in situ following the
manufacture's
protocols (Promega Steady-Glo Luciferase Assay System(E2520)). Then counted
for 10
seconds using a plate luminometer (Victor1420).
Compound Storage
[00214] Compounds for screening were stored at -20 C as 10 mM stocks in 100%
DMSO
until required.
Results
[00215] Biological activity as assessed using the luciferase reporter assay in
murine H2K
cells, and the results are shown in Table 1, which also lists the
concentration of the test
compound solution in M.
-53-
CA 02688469 2009-10-29
WO 2009/141159 PCT/EP2009/003645
Table 1
Compound Activity Concn / uM
1 + 3
2 + 10
3 ++ 10
4 + 10
++ 10
6 + 10
7 + 10
8 ++ 10
9 ++ 10
+++ 10
11 + 10
12 ++ 10
13 ++ 10
14 +++ 10
++ 10
16 + 10
[00216] The results in Table 1 are classified as follows:
+ Up to 200% relative to control
++ Between 201% and 300% relative to control
+++ Between 301% and 400% relative to control
++++ Above 401% relative to control
[00217] The results in Table 1 show that all of the exemplified compounds had
increased
activity in the luciferase reporter assay relative to the control.
[00218] The examples set forth above are provided to give those of ordinary
skill in the art
with a complete disclosure and description of how to make and use the claimed
embodiments, and are not intended to limit the scope of what is disclosed
herein.
Modifications that are obvious to persons of skill in the art are intended to
be within the
scope of the following claims.
[00219] All publications, patents, and patent applications cited in this
specification are
incorporated herein by reference as if each such publication, patent or patent
application were
specifically and individually indicated to be incorporated herein by
reference.
-54-